Abstract
Obesity is a serious health concern as it may initiate common chronic diseases. Chili pepper is an important spice that brings spiciness and commonly used in cuisines. However, the antioxidant and anti-obesity properties of chili varieties in Malaysia has not yet been fully investigated. Therefore, this study aimed to determine the antioxidant (content and activity) and anti-obesity properties of five different varieties of local chili peppers. The antioxidant activities of the extracts were determined through ferric-reducing antioxidant power (FRAP) and 2, 2′azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid (ABTS) assays. Cell cytotoxicity of the selected chili extracts was determined in 3T3-L1 pre adipocytes using cell viability assay (MTT) assay. Whereas the ability to inhibit oil accumulation in fully differentiated 3T3-L1 adipocytes of the selected chili pepper extracts was assayed using Oil Red O staining. The results showed that Kulai 568 pulp extract had the highest level of total phenolic content (TPC) (47.88 ± 0.220 mg GAE/g), whereas Centil pulp extract had the highest level of total flavonoid content (TFC) (26.60 ± 0.52 mg QE/g). In term of antioxidant activities, Bara pulp extract had the highest value in FRAP (3.058 ± 0.002 mM Fe2+/mg extract) and ABTS (IC50 = 12.411 ± 0.025). High performance liquid chromatography (HPLC) analysis, Bara pulp extract has the highest level of capsaicin (72.271 ± 0.957 µg/ml). In terms of inhibition of oil accumulation Centil seed extract presented the best result (69.09–92.20%), while Bara pulp extract inhibited the most pancreatic lipase activity (IC50 = 4.84 ± 0.57 µg/ml). Thus, it is suggested that Centil seed and Bara pulp extracts can be a potent antioxidant and anti-obesity agents.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04400-x) contains supplementary material, which is available to authorized users.
Keywords: Anti-obesity properties, Chilli pepper, Capsaicin, Antioxidant compounds, Diet interventions
Introduction
Obesity is a complex disease caused by the interaction of a myriad of genetic, dietary, lifestyle, and environmental factors, which favours a chronic positive energy balance, and leads to increased body fat mass (Mohamed et al. 2014). Nowadays, obesity is a worldwide health concern as it is a risk factor for many common chronic diseases including heart disease and stroke, diabetes mellitus, osteoarthritis (OA), and hypertension (Mohamed et al. 2014).
The options for weight management to cure obesity remain quite limited. Some diet interventions for instances, low-carbohydrate and high protein diet, low-carbohydrate and high-fat diet, and low energy diet respectively for obesity treatment are carried out by using established behaviour-change techniques and social support (Lagiou et al. 2012). However, they may work on a short-term basis. Besides, adherence to a ketogenic fad diet has lower sustainability, compared to a non-ketogenic diet (Brouns 2018). The lifestyle changes in the form of dieting do not generally produce marked or sustainable weight loss (LeBlanc et al. 2011). As for anti-obesity drugs, they can be categorized into three main actions, inhibit intestinal fat absorption, suppress appetite, and increase energy consumption or thermogenesis (Zhou et al. 2012). However, most of the anti-obesity drugs such as phentermine, sibutramine, and rimonabant have been withdrawn from the market due to undesirable side effects of consumers such as the increased risk of cardiovascular diseases, hypertension, constipation and depression (Zhou et al. 2012).
Fruits and vegetables are important components of a healthy diet. Fruits and vegetables are rich sources of vitamins and minerals, dietary fibre and a host of beneficial non-nutrient substances including plant sterols, flavonoids and other antioxidants (Zinoviadou et al. 2015). Reduced fruit and vegetable consumption is linked to poor health and increased risk of non-communicable diseases (NCDs). Studies suggests that when consumed as part of a healthy diet low in fat, sugars and salt/sodium, fruits and vegetables may also help to prevent weight gain and reduce the risk of obesity, an independent risk-factor for NCDs (Ozturk et al. 2009; Fadda et al. 2018; Nour et al. 2018; Sarafi et al. 2018).
Nowadays, consumers are gradually demanding for nutritious, and fresh like food products, with high organoleptic properties (Zinoviadou et al. 2015). Chili pepper, a plant that belongs to the genus of capsicum, is an important spice that is widely used in cuisines. Except for its usage as a cooking ingredient, they are also used as an analgesic agent for chronic pains (Bosland and Votava 2000). Some recent studies explored that natural bioactive compounds have been associated with some nutritional functions such as anti-tumor, anti-cancer and anti-obesity agent due to the activities of the unique bioactive components, called capsaicin and capsaicinoids (Abdul Rahman et al. 2017; Deng et al. 2015; Galanakis et al. 2015).
Some recent in-vitro and in vivo studies have showed that capsaicin, the component that contributes to the pungent scent of hot chili pepper, is a potential agent for anti-obesity caused by oxidative-stress and adipogenesis of cells (Leung 2008). Since this is a quite newly emerging issue, there are only a few shreds of evidence about the effect of capsaicin on anti-obesity properties. Additionally, the bioactive compounds content and antioxidant activities in local chilies pepper varieties in Malaysia have not been investigated in detail. Therefore, the present study was carried out to investigate the antioxidant properties (total phenolic content, total flavonoid content) and antioxidant activities in local chili varieties using in-vitro methods.
Materials and methods
Sample preparation
The local fresh chilies sample were collected in triplicate from the various wet and supermarkets in Malaysia (Pasar Borong, and Tesco, Petaling Jaya, Selangor, Malaysia). There are a total of 15 sample extracts (seeds, pulp and whole fruit parts of each variety) used in this study. The varieties are Cili kulai 151, Cili kulai 568, Cili centil, Cili bara, and Cili pelita. All the chilies samples were dried using freeze drier (VirTis BenchTop K, USA) and ground into powder using stainless steel blender (Waring Commercial, Torrington, CT, USA). The resulting chili powder was then stored at − 20 °C (Bruder HF-150T) until experiment time. All chemicals, standards, and solvents in the analytical grade were purchased from Sigma-Aldrich Company (Sigma-Aldrich, Germany).
Extract preparation
The samples were extracted as described in Alam et al. (2014) but also some adjustments were made to obtain better quality extract. Ethanolic extraction of chilies was applied where 4 g of powder of chili sample for each species were weighed. Then, 40 ml of 70% ethanol was mixed with the chili sample in a conical flask. Subsequently, the mixture was left to stand for 24 h in incubator shaker (Heidolph Unimax 1010, Germany) at 25 °C with 175 rpm. Afterward, the mixture was left to sediment for 10–20 min. Then, the supernatant was filtered through Whatmann filter paper (Whatmann, USA). The extraction was repeated twice using combined fresh solvent and the extracted solvent. The extracted samples were concentrated using rotary evaporator (Buchi Rotavapor R-210, Buchi Labortechnik AG, Switzerland) at 37 °C until 70% of total volume was reduced. Finally, the extracted sample was stored at – 20 °C (Bruder HF-150T) for further use.
Determination of bioactive compounds content
Total phenolic content (TPC)
The total phenolic content of chili extracts was carried out according to a method of Taegu et al. (1984) with minor modification. In this analysis, gallic acid was used as a standard and sample was replaced with ethanol (70%) for control. A gallic acid standard curve was constructed to determine the phenolic contents in terms of gallic acid equivalent (μg/ml) of extract. Briefly, 1 ml of each sample was added to 2 ml of 1:3 concentrated Na2CO3. Then the mixture was left to sediment for 2 min for incubation in room temperature. In the next step, 0.1 ml of 50% Folin-Ciacalteu’s phenol reagent was added to the mixture. Then, the mixture was incubated at room temperature in dark condition for 30 min. Finally, the absorbance was read at wavelength 765 nm by using spectrophotometer (Shidmazu UV-1800, UK). The antioxidant potential was calculated from gallic acid standard curve and expressed as Gallic Acid Equivalents (mg GAE/g).
Total flavonoid content (TFC)
The flavonoid content of samples was determined according to a method of Ozkok et al. (2010). Quercetin was used as standard. Briefly, 1 ml of standards/samples were added with 3 ml 95% of ethanol, 0.2 ml of 10% aluminium chloride, 0.2 ml of 1 M potassium acetate and 5.6 ml distilled water. The mixture was vortexed for 30 s. Then, the mixture was left at room temperature for 30 min. Finally, absorbance was read at 415 nm using spectrophotometer (Shidmazu UV-1800, UK). The total flavonoid content of samples (mg/ml) is calculated from quercetin standard curve and expressed as Quercetin Equivalent (QE):
Determination of antioxidant activity
Ferric reducing antioxidant power assay (FRAP)
The ferric reducing power assay was performed to determine antioxidant activities of different chili pepper extract. FRAP reagent was prepared by combining 2.5 ml of 10 mM 2, 4, 6-tri (2-pyridyl acid). In this study, Ferrous sulfate heptahydrate (FeSO4·7H2O) was used as a standard. Standards (Trolox) and chili extracts (0.1 ml) were incubated with 1 ml of FRAP reagent for 30 min at dark room temperature. Absorbance was then read at 593 nm using spectrophotometer (Shimadzu UV-1800, UK). The antioxidant activity was calculated from Ferrous sulfate heptahydrate (FeSO4·7H2O) standard curve and expressed as mM Fe2+ equivalents. The FRAP value for each sample was calculated according to the formula below:
2,2′Azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)/ABTS analysis
The method, based on the ability of antioxidant molecules to quench the long-lived ABTS radical cation (ABTS·+), of Re et al. (1999) was modified. The ABTS·+ was produced by reacting 7 mM ABTS stock solution with 2.45 mM potassium persulphate (final concentration) and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The ABTS·+ solution was diluted with deionized water and 95% ethanol (1:1) to an absorbance of 0.70 (± 0.02) at 734 nm using spectrophotometer (Shimadzu, UV-1800, UK). Next, 20 µl of the extract were mixed with 6 ml of diluted ABTS·+ solution. The decrease in absorbance was recorded at 1 min after mixing. Trolox and synthetic capsaicin was used as a positive control. Absorbance decreases were calculated as ABTS values for comparison with the fresh sample. ABTS value for each sample extract was calculated as the following formula:
Quantification of capsaicin by HPLC
HPLC analysis was performed using a modified method previously carried out by Guo et al. (2015). Chili extracts with the highest antioxidant activities in the category of seed, pulp, and whole fruit were selected. Then, the extracts were analysed by using HPLC machine (Agilent, HPLC 1100), to determine the level of capsaicin. HPLC analysis was performed using the setting provided in the supplementary table S1.
Preparation of standard solution
Exactly, 1 mg of capsaicin (Sigma-Aldrich, Germany) was transferred into 10 ml of a volumetric flask, dissolved, and made up to (10 ml) volume with acetonitrile (Sigma-Aldrich, Germany). The stock solution was further diluted with acetonitrile to yield solution concentration of 900, 150, 100, 80, 60, 40, 20 µg/ml, respectively.
Anti-obesity properties
Cell culture and differentiation of 3T3-L1 preadipocytes
As described previously by Kang et al. (2016). The 3T3-L1 pre adipocytes (ATCC, USA) were cultured in high glucose DMEM (Gibco) (4.5 mg/ml), containing 1% penicillin–streptomycin (PS) (Gibco) and 10% bovine calf serum (Gibco) at 37 °C under 5% CO2 atmosphere. The cells were harvested when approximately 70% of confluency is reached. The 2-day post-confluent pre adipocytes (designated Day 0) were cultured in multi-daily insulin (MDI) differentiation medium (DMEM containing 1% PS, 10% FBS. 0.5 mM IBMX, 0.25 µM dexamethasone and 1 mg/ml insulin) for 2 days. The cells were then cultured for another 2 days in DMEM containing 1% PS, 10% FBS and 5 mg/ml insulin. Lastly, the cells were maintained in post differentiation medium (DMEM containing 1% PS and 10% FBS), with the replacement of the medium every 2 days.
Cell viability assay (MTT assay)
The cell viability of 3T3-L1 pre adipocytes was determined according to the methods described by Mosmann (1983). The 3T3-L1 pre adipocytes were seeded at a density of 2 × 104 cells/well in 96 well plates. The cells were treated with varying doses (100–0.6 mg/ml) of chilies extracts and were incubated for 24, 48, and 72 h, respectively. After completion of the treatment, the cells were incubated with an MTT solution for 3 h at 37 °C. The supernatant was aspirated, DMSO was added to each well, and the plates are agitated to dissolve the purple-coloured crystal product. Absorbance was measured at 570 nm using a multi-well plate reader (Molecular Devices Corp., VERSAmax, Sunnyvale, CA, USA). Subsequently, the cell viability was determined at 25%, 50% and 75% concentration of extracts (mg/ml), respectively. Finally, cell viability (%) was calculated according to the following formula:
Oil Red O staining
Intracellular lipid accumulation in 3T3-L1 adipocytes was evaluated by Oil Red O staining that previously described by Kang et al. (2016). After adipocyte differentiation, the differentiated cells were treated with differentiation media containing varies doses of chilies extracts which contributes to 50% of cell viability (IC50). The cells were stained with Oil Red O, an indicator of cell lipid content with slight modifications. Briefly, adipocytes were washed with phosphate-buffered saline (PBS). Then, they were fixed with 10% buffered formalin and stain with Oil Red O solution (0.5 g in 100 ml isopropanol) for 15 min. After removing the staining solution, the dye retained in the cells was eluted into DMSO and optical density is measured at 520 nm by using multi-well plate reader (Molecular Devices Corp., VERSAmax, Sunnyvale, CA, USA). In this test, distilled water was used as negative control. Percentage of lipid inhibition of sample extracts was calculated using the following equation:
Measurement of pancreatic lipase inhibitory activity
The activity of pancreatic lipase was evaluated colorimetrically through measurement of the hydrolysis of p-NPB. The analysis was carried out based on the methods described by Slanc et al. (2009) with minor modifications. An enzyme buffer was prepared by the addition of 6 μl porcine pancreatic lipase solution (Sigma-Aldrich) in buffer containing 10 mM morpholinepropanesulphonic acid (MOPS) and 1 mM EDTA (at pH 6.8) to 169 μl Tris buffer (100 mM Tris-HC1 and 5 mM CaCl2, pH 7.0). Then, 20 μl of either the chilie extracts at the test concentration (0, 0.625, 1.25, 2.5, 5, 7.5, 10, 50, and 100 μg/ml) or orlistat (Xenical) were mixed with 175 μl enzyme buffer and with 5 μl substrate solution (10 mM p-nitrophenylbutyrate (p-NPB) in dimethyl formamide; the enzymatic reactions were allowed to proceed for 15 min at 37 °C. Lipase activity was determined by measuring the amount of p-nitrophenol at 405 nm using a multi-well plate reader (Molecular Devices Corp, VERSAmax, Sunnyvale, CA, USA). Inhibition of lipase activity was expressed as the percentage decrease in OD when porcine pancreatic lipase was incubated with the test materials. Orlistat, a potential lipase inhibitor, was used as a positive control. Lipase inhibition (%) was calculated according to the following formula:
where A is the activity without inhibitor, a is the negative control without inhibitor, B is the activity with inhibitor, and b is the negative control with inhibitor.
Data analysis and interpretation
All analyses were carried out in triplicate (n = 3) for each species of chilies. Data is stated as mean ± standard deviation, with significance letter (if any). A one-way ANOVA test was performed on all data (Minitab ver.17) to determine the significant difference among samples. The mean comparison was performed by Post Hoc test (Tukey’s test) to show the significance of differences at a level of p < 0.05. Correlation between variables was determined by using Pearson’s correlation.
Results and discussion
Bioactive compounds content
Table 1 demonstrated the examined total phenolic content (TPC) and Total flavonoid content (TFC) in the red chili extracts. According to the results TPC was measured as 16.37–47.88 mg GAE/g of the dried chili sample; respectively. Overall, the TPC level among chili samples is ranked in the following order: Kulai 568 pulp > Centil pulp > Kulai 151 whole fruit > Bara pulp > Kulai 151 pulp > Kulai 568 whole fruit > Pelita pulp > Centil whole > Bara whole fruit > Pelita whole fruit > Bara seed > Pelita seed > Centil seed > Kulai 568 seed > Kulai 151 seed. The TPC in both pulp and whole fruit were not significantly different, except the Bara and Pelita varieties. The pulp of all chili peppers had higher TPC than its whole fruit except for Kulai varieties. Nevertheless, TPC of the Kulai 151 whole fruit was not significantly higher than its pulp.
Table 1.
TPC and TFC in different varieties and parts of chilli peppers
| Sample | TPC (mg GAE/g) | TFC (mg QE/g) |
|---|---|---|
| Centil | ||
| Seed | 26.28 ± 0.81e | 18.05 ± 0.19d,e,f |
| Pulp | 47.69 ± 0.57a | 26.60 ± 0.52a |
| Whole | 45.65 ± 1.40a,b,c | 20.33 ± 0.38b,c,d |
| Kulai 568 | ||
| Seed | 16.37 ± 1.71f | 3.06 ± 0.33h |
| Pulp | 47.88 ± 0.22a | 22.56 ± 0.60b |
| Whole | 46.91 ± 0.57a,b | 15.53 ± 0.08f,g |
| Kulai 151 | ||
| Seed | 7.35 ± 0.78g | 14.02 ± 0.25g |
| Pulp | 47.05 ± 0.91a,b | 19.26 ± 0.95c,d |
| Whole | 47.46 ± 0.44a | 18.70 ± 0.56c,d,e |
| Bara | ||
| Seed | 36.19 ± 2.51d | 19.32 ± 1.06c,d |
| Pulp | 47.44 ± 0.63a | 20.72 ± 0.88b,c |
| Whole | 43.60 ± 0.56b,c | 20.56 ± 1.58b,c,d |
| Pelita | ||
| Seed | 27.86 ± 0.14e | 16.04 ± 1.46e,f,g |
| Pulp | 46.57 ± 1.34a,b | 18.14 ± 1.63c,d.e,f |
| Whole | 42.17 ± 1.96c | 16.28 ± 0.71e,f,g |
Different lowercase superscript letters (a–h) represent significant differences between different varieties and parts of the chilli peppers at p < 0.01. Concentration of sample used: 0.6–100 mg/ml
GAE gallic acid equivalent, QE quercetin equivalent
The seed of all red chili varieties had a significantly lower TPC compared to their pulp and whole fruit extracts at p < 0.01. The results also showed that seed extract from C. frutescens (Bara, Centil, Pelita) has a higher level of TPC than C.annuum such as the Kulai varieties. This finding was aligned with the study reported by Sora et al. (2015), who also determined higher TPC level in seed extract of Capsicum compared to the pulp. Therefore, chili pepper seed was a potential source of a nutraceutical as it contained a good amount of total phenolics. In contrast, the study of C. frustescens conducted by Oboh and Ogunruku (2010), found that the pericarp of the fruit has higher phenolic content compared to seed extract. We also found that there were no significant differences in TPC level among pulp extract of all the studied varieties.
The TFC level of chili extracts ranged from 3.06–26.60 mg QE/g. The TFC level in chilli samples is ranked in the following order: Centil pulp > Kulai 568 pulp > Bara pulp > Bara whole fruit > Centil whole fruit > Bara seed > Kulai 151 pulp > Kulai 151 whole fruit > Pelita pulp > Centil seed > Pelita whole fruit > Pelita seed > Kulai 568 whole > Kulai 151 seed > Kulai 568 seed. The results showed that pulp of most varieties of chili peppers had significantly higher TFC than seed and whole fruit extracts at p < 0.01, except for varieties of Bara and Pelita. Overall, the seed extracts of all chilies varieties have the lowest TFC among the three main parts of chillies (seed, pulp, and whole fruit).
The variation of TPC and TFC in the studied samples may be due to the effect of geographical environments and genetic variations. Different local growing conditions such as the pH value of soil and weather can influence the TPC and TFC synthesis in chili peppers (Moreno-Ramírez et al. 2018). As reported by Kovačević et al. (2018), the temperature, types of solvents used, extraction time, as well as their interactions significantly influenced the bioactive compound extraction yield. They also demonstrated that temperature is the most important parameter for bioactive compound extraction, where the highest phenolic compounds were obtained under higher cycle numbers.
Antioxidant activities
Antioxidants are molecules bearing active hydroxyl groups, such as vitamins E and C, polyphenol and flavanol compounds, are potent radical scavengers (Lu et al. 2010). Free radicals and reactive oxygen species is the main factor that initiates pathological conditions such as inflammation, metabolic disorders, cell aging, and carcinogenesis. Previous literature have found that FRAP and ABTS methods could be used effectively to determine the antioxidant activity, as this method is convenient and easy to use, and also gives reliable results (Nagarajan et al. 2019; Abdul Rahman et al. 2017; Sora et al. 2015). Therefore, the antioxidant capacity of the studied chillies sample were assessed and compared using FRAP and ABTS method.
Ferric reducing ability (FRAP)
Antioxidant activities of different varieties of Malaysian red hot chili peppers are shown in Table 2. The antioxidant activities in different chili varieties were determined by FRAP and ABTS assays. Among the studied chili extracts, Bara pulp has the highest FRAP value (3.058 ± 0.002 mM Fe2 + /mg extract), while Kulai 568 seed extract has the lowest FRAP value (1.203 ± 0.277 mM Fe2 + /mg extract). Overall, the seed from smaller sized chilies such as Centil, Bara and Pelita has significantly higher FRAP value than Kulai varieties at p < 0.01. The results also showed that seed extracts of all chili peppers had significantly lower FRAP values compared with pulp and whole fruit at p < 0.01. Also, no significant difference was found between FRAP values of pulp and whole fruit for all the chili peppers. This may due to the higher TPC and TFC present in pulp and whole fruit extracts. Among all the extracts, Bara pulp extract has the highest FRAP value. This may be caused by the higher level of capsaicin. This finding is aligned with Maksimova et al. (2014), who stated that the antioxidant potential of ethanolic oleoresin extract from chili pepper is dependent on the concentration of capsaicin. Meanwhile, a study conducted by Gougoulias et al. (2017), agreed that the antioxidant activity through FRAP, is strongly correlated by the TPC. However, the same study also proved that there is no relationship between the amount of capsaicin and antioxidant activities.
Table 2.
FRAP values and IC50 of ABTS inhibition of different varieties and parts of chilli pepper
| Sample | FRAP value (mM Fe2+/mg) | IC50 of ABTS inhibition (mg/ml) |
|---|---|---|
| Centil | ||
| Seed | 2.606 ± 0.038c,d | 34.450 ± 0.807e |
| Pulp | 3.049 ± 0.004b | 13.133 ± 0.159h |
| Whole | 2.984 ± 0.002b | 15.283 ± 1.398g,h |
| Kulai 568 | ||
| Seed | 1.203 ± 0.277f | 91.95 ± 2.28a |
| Pulp | 3.039 ± 0.007b | 15.154 ± 1.600g,h |
| Whole | 3.051 ± 0.005b | 18.927 ± 0.282f,g |
| Kulai 151 | ||
| Seed | 1.848 ± 0.197e | 66.703 ± 1.183a |
| Pulp | 3.036 ± 0.002b | 16.601 ± 0.177g,h |
| Whole | 3.037 ± 0.002b | 15.406 ± 0.268g,h |
| Bara | ||
| Seed | 2.389 ± 0.153d | 48.898 ± 1.624c |
| Pulp | 3.058 ± 0.002b | 12.411 ± 0.025h |
| Whole | 2.795 ± 0.048b,c | 23.108 ± 0.439f |
| Pelita | ||
| Seed | 2.348 ± 0.044d | 40.019 ± 0.807d |
| Pulp | 3.016 ± 0.005b | 13.028 ± 0.222h |
| Whole | 3.011 ± 0.007b | 21.939 ± 0.068a |
| Capsaicin | 3.058 ± 0.045b | 0.767 ± 0.024i |
| Trolox | 4.294±0.257a | 0.406±0.014i |
**Correlation is significant at p < 0.01
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)/ABTS assay
According to Lu et al. (2010), ABTS+ is a stabled and coloured radical, which has strong absorption in the visible region. ABTS+ radicals are more reactive than DPPH radicals, and the reactions with ABTS+ radicals involve a single-electron transfer process. It converts into colourless compound upon reception of an electron or active hydrogen atom from the antioxidant containing phenolic. Thus, the radical scavenging capacity of antioxidants can be determined by changes in absorbance.
In this assay, the inhibition power of sample extracts towards ABTS free radicals is expressed as IC50. The lower value of IC50, means stronger the ability of the extract to scavenge free radicals (Nagarajan et al. 2019). There are significant differences in ABTS inhibition levels among the seed, pulp and whole fruit of most Malaysian chili pepper at p < 0.01. Seed extracts of all chili pepper varieties had the lowest ABTS inhibition level compared to pulp and whole fruit extracts. Pulp extract of chilies has the significantly highest ABTS inhibition level among the three parts of chilies extracts, except for the variety of Kulai 568. Comparing among all the chili pepper samples, the extract of smaller chili pepper such as Centil, Bara and Pelita varieties showed significantly higher ABTS inhibition levels compared with the larger chilies such as Kulai varieties (p < 0.01). In brief, the pulp extract of chilli pepper Bara has the strongest free radical scavenging ability as determined by ABTS assay. The similar trend was also demonstrated from the results of Nagarajan et al. (2019), they found that the scavenging activity of DPPH and ABTS radical assays for the fractionated lycopene isolated from the pink guava by product was higher than the standard lycopene, the IC50 values of the fractionated lycopene and the standard lycopene were 83.7 µg/ml and 179.25 µg/ml, respectively.
Correlation between bioactive compounds and antioxidant activities
The antioxidant status of chillies extracts should be based on the estimation of both total phenols and particular fractions such as TPC, TFC and TAC, since the overall activity may be defined by the contribution of the most active compounds and their particular quantities (Galanakis et al. 2015). As shown in Table S2 there are significant correlations between antioxidant content (TPC, TFC) and antioxidant activities (FRAP, ABTS) of the chili extracts. The results showed that there was a very strong and positive correlation between TPC and FRAP value (R = 0.886; p < 0.01). In addition, there was also a strong and negative correlation between ABTS value and TPC (R = − 0.770; p < 0.01). Meanwhile, TFC also has strong correlation to both FRAP (R = 0.786; p < 0.01) and ABTS value (R = − 0.753; p < 0.01) (table S2).
HPLC analysis
After determination of antioxidant activities of all chili extracts, three extracts with the highest antioxidant in the categories of seed (Centil seed), pulp (Bara pulp) and whole fruit (Kulai 568 whole fruit) were selected for further investigation. The capsaicin content on these three selected chili extracts was determined on the basis of standard solutions of capsaicin (Sigma-Aldrich, Schnelldorf, Germany) using absorbance of 280 nm. The results for the concentration of capsaicin in the samples were calculated using the linearity curve and linearity equation, y = 8.6722 x−21.949 (R2 = 1) obtained from the standard solutions of capsaicin (Fig. 1).
Fig. 1.
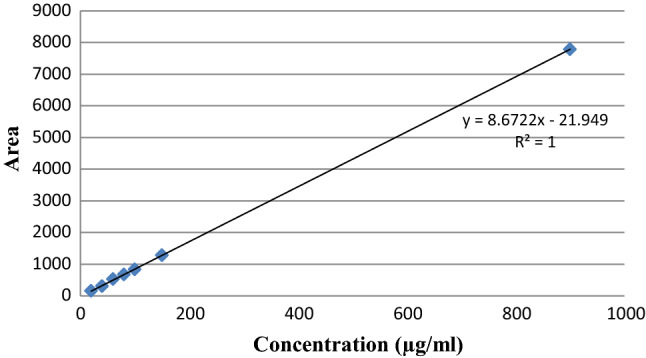
Linearity curve for standard solution of capsaicin (λ = 280 nm)
The concentration of capsaicin in each chili extract is summarized in Table 3. Based on the result obtained, Bara pulp extract has significantly the highest concentration of capsaicin (72.271 ± 0.957 µg/ml) followed by Centil seed extract (22.388 ± 1.271 µg/ml) and Kulai 568 whole fruit extract (7.728 ± 0.436 µg/ml) at p < 0.01.
Table 3.
Capsaicin concentration of selected chili extracts*
| Types of extracts | Capsaicin concentration (µg/ml) |
|---|---|
| Bara pulp | 72.271 ± 0.957a |
| Centil seed | 22.388 ± 1.271b |
| Kulai 568 whole fruit | 7.728 ± 0.436c |
*Different lowercase superscript letters (a–c) represent significant differences between different chilli extracts at p < 0.01
The differences in findings may be due to diverse cultural environment and genotype of chili peppers between foreign countries and Malaysia. The variability in pungency and capsaicin content among the studied chili pepper extracts may also be related to the different amount of pungency alleles present in the genotypes (Bertao et al. 2016). There are studies which proved higher expression of capsaicinoid biosynthesis genes and higher capsaicin level in C. frutescens extract compared to C. annuum extract (Bertao et al. 2016). Besides, the concentration of capsaicin in chili pepper depends on environmental factors such as light intensity, growing temperature, and age of plant and the position of the fruit on the plant (Othman et al. 2011).
Anti-obesity analysis
Cell viability assay
The 3T3-L1 preadipocytes were cultured until 70–80% of confluence, then they were seeded into 96 well-plate. The cells are then treated with different concentrations of chili extracts (1.5–100 mg/ml). Orlistat, as the commercial drug in treating obesity, was used as a positive control in this study. In order to investigate and maximize the effect of extracts towards pre-adipocytes viability, the optimal incubation period was determined. The treated 3T3-L1 cells were then divided into three incubation times, 24 h, 48 h and 72 h respectively. In this test, IC25, IC50, and IC75 for each incubation hours were determined. IC25 was applied in order to determine the suitable concentration of each extract that can be used to kill 25% of cells and remain 75% desirable cell viability in the next anti-obesity analysis. Besides, IC75 and IC50 were applied to determine the lethal concentration of chili extract for 3T3-L1 cells. The result can be used to serve as a reference for future research which involved chili extracts treatment on adipocytes cells. The reason for having an incubation time of 24 h, 48 h and 72 h is to determine the incubation time for the highest efficiency of chili extracts, which can be applied in the next analysis. The lower concentration of chili extracts used to achieve 25%, 50% and 75% of cell viability, the more effective the anti-obesity properties of the extracts. Figure 2 and Tables 4 show the MTT results for each type of chili extracts for different incubation hours. The extract of Bara pulp under 72 h incubation hours showed a significant lowest concentration of IC25 (16.38 ± 0.22 mg/ml), IC50 (8.69 ± 0.15 mg/ml), and IC75 (2.71 ± 0.22 mg/ml). Orlistat, as the control of this assay, has the highest, non-significant difference in concentration of IC25, IC50, and IC75. In the optimal incubation hours (72 h), both Bara pulp and Kulai 568 whole fruit extracts were significantly different compared with Centil seed extract in the categories of IC25 and IC50. Except for the category of IC75, Centil seed extract showed no significant difference with Kulai 568 whole fruit and Bara pulp extracts. Overall, the effect of cell viability for each type of chili extract is dose and time-dependent. The efficiency of chili extracts is affected by concentration used and incubation time. The higher the concentration of chili extracts used in treatment, or the longer the incubation time after the cells has treated, the higher the efficiency of the extracts in terms of adipocytes inhibition.
Fig. 2.
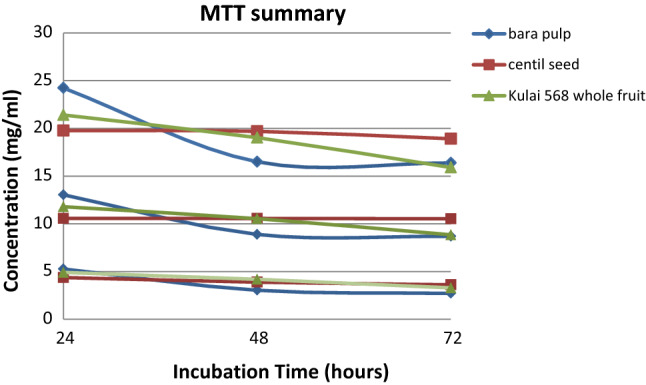
Summarized MTT result of chili extracts for different cell viability and incubation hours
Table 4.
MTT results of different types of chili extracts for 24, 48 and 72 h
| Time (h) | Cell viability | Bara pulp (mg/ml) | Centil seed (mg/ml) | Kulai 568 whole fruit (mg/ml) |
|---|---|---|---|---|
| 24 | IC75 | 24.23 ± 1.43a | 18.91 ± 0.73b | 21.40 ± 1.25a,b |
| IC50 | 13.04 ± 0.27a | 10.57 ± 0.38b | 11.79 ± 1.01a,b | |
| IC25 | 5.27 ± 0.84a | 4.37 ± 0.28a | 4.91 ± 1.07a | |
| 48 | IC75 | 16.50 ± 0.43b | 19.69 ± 0.81a | 19.03 ± 0.87a |
| IC50 | 8.89 ± 0.41b | 10.56 ± 0.28a | 10.54 ± 0.23a | |
| IC25 | 3.04 ± 0.59b | 3.89 ± 0.40a,b | 4.19 ± 0.29a | |
| 72 | IC75 | 16.38 ± 0.39b | 19.77 ± 1.26a | 15.90 ± 0.83b |
| IC50 | 8.69 ± 0.26b | 10.53 ± 0.70a | 8.85 ± 0.43b | |
| IC25 | 2.71 ± 0.39a | 3.61 ± 0.37a | 3.29 ± 0.42a |
*Different lowercase superscript letters (a, b) represent significant differences between different varieties of the chilli peppers at p < 0.01
Lipid inhibition activity
After reaching 100% of confluency, differentiation media is used to induce differentiation of pre adipocytes into mature adipocytes. It took about 7–14 days to complete the differentiation process. Oil red O staining solution (mix with 5% of isopropanol) was used to indicate the lipid droplet remained in adipocyte after treated with sample extracts. From MTT assay, the incubation time for optimal cell viability for each chili extracts was selected as 72 h. The adipocytes were treated with various concentrations of chili extracts (IC25, IC50, IC75) which is obtained from MTT assay. The lipid inhibition activity was expressed as the percentage of lipid remained in adipocytes, with control at 0%. The results are presented in the supplementary data table S3.
The present study showed that Centil seed extract, at the non-cytotoxic concentration (IC25) has significantly highest lipid inhibition percentage (69.09 ± 3.33%) compared to Bara pulp (63.40 ± 2.54%) and Kulai 568 whole fruit (47.33 ± 2.76%) extracts. Both Bara pulp and Centil seed extracts do not show significant differences in the concentration of IC25 and IC50. Overall, both Bara pulp and Centil seed extracts used to treat adipocytes for 72 h incubation in biocompatible concentration (IC25), do not reach 50% of lipid inhibition.
Inhibition of pancreatic lipase activity
Pancreatic lipase inhibition is an important standard to identify potential anti-obesity efficacy among natural products (Bertao et al. 2016). Therefore, pancreatic lipase in vitro model was used to determine the ability of chili extract in pancreatic lipase inhibition. The anti-lipase activity was expressed in IC50, which means inhibit 50% of pancreatic lipase activity. Orlistat is commonly used as an anti-obesity drug that effectively inhibits the activity of pancreatic lipase. Thus, it was used as a positive control in this assay. Water was used as a negative control, with 0% of inhibition percentage. Lower IC50 value corresponds to the higher efficacy of samples in pancreatic lipase inhibition.
In the present study, the results presented in the supplementary data table S4 shows that the IC50 of pancreatic lipase inhibition of Bara pulp extract (4.839 ± 0.57 µg/ml), Kulai 568 whole fruit extract (5.030 ± 0.47 µg/ml) and Centil seed extract (10.97 ± 2.38 µg/ml) were higher than orlistat (2.619 ± 1.16 µg/ml). However, although one-way ANOVA analysis found that, the extract of Bara pulp and Kulai 568 whole fruit showed higher IC50 than orlistat, but there were no significant differences in terms of the ability to inhibit pancreatic lipase activity. Whereas, Centil seed extract showed significant higher IC50 value than orlistat.
Correlation between antioxidant activities and anti-obesity properties
Lipid accumulation in adipocytes is the cause of obesity. As reported by Furukawa et al. (2017), increased formation of oil droplets in adipocytes is closely related to elevated oxidative stress. Oxidative stress initiates the uncontrolled production of free radicals, which leads to various metabolic diseases, including obesity (Mau et al. 2001). In the differentiation process from pre-adipocytes into mature adipocytes, ROS has produced in time-dependent manner. Based on a previous study conducted by Calzadilla et al. (2011), increased production of ROS is found in differentiated 3T3-L1 adipocytes compared to the undifferentiated pre-adipocytes. From this point of view, antioxidant plays an important role to prevent the production of ROS. Natural bioactive products that possess with high antioxidant activities are currently investigated for their ability to control lipid oxidation in oils, lipid systems and obesity prevention, as they act by hydrogen donating and sequentially quenching of radicals (Park et al. 2010; Abdul Rahman et al. 2017). For instances, Park et al. (2010) reported that body weight control is improved with the enhancement of antioxidant enzymes. Instead, Galanakis et al. (2018a, b) also revealed the efficacy of polyphenols extracted from Olive mill wastewater for the prevention of oil oxidation and UV booster in cosmetics. In the current study, due to the higher correlation of ABTS to the anti-obesity activities of chili extracts, they can be suggested as a potential bioactive and anti-obesity agents. Similarly, some studies showed that capsaicin, the component that contributes to the pungent scent of hot chili pepper, is a potential agent for anti-obesity caused by oxidative-stress and adipogenesis of cells (Leung 2008). Therefore the current study is carried out to make further justification on this compound.
The results of this research showed that there were significant correlations between antioxidant activities and anti-obesity properties of the selected chili extracts. The results show an inverse relationship between antioxidant activities (FRAP and ABTS) and anti-obesity properties of chili extracts. There were very strong and inversed correlations between FRAP value and Oil Red O staining at the non-cytotoxic and biocompatible concentration (IC75) of each chili extracts (R = 0.814; p < 0.01). Whereas ABTS value and Oil Red O staining at non-cytotoxic concentration of IC75 (R = 0.908; p < 0.01) also strongly associated. According to the International Organization for Standardization (ISO), a material is considered as toxic and not biocompatible if the cell viability is below 70% (Alam et al. 2014). It is also important to determine the non-cytotoxic concentration in studied materials in order to avoid the threat to the survival of other normal cells in the body system. Usually, it is important to obtain a material which has optimal efficiency with minimum damage to the other cells. In terms of pancreatic lipase inhibition, the FRAP value of studied chili extracts was moderately associated. Whereas their ABTS value was strongly associated with their ability to inhibit lipase activity. Since the antioxidant activities are commonly associated with the presence of bioactive compounds (TPC and TFC). Thus, the strong ABTS value in the current study may be associated due to the higher level of bioactive compounds. The Pearson correlation analysis showed that antioxidant activities in chili extract were strongly and positively associated with the TPC in chili extracts (Table 1). Therefore, to further confirmation, another correlation test is carried out to test the correlation between TPC and anti-obesity properties (Table S5). The result showed that the TPC present in chili extract is positively and strongly associated with pancreatic lipase inhibition (r = 0.936). TPC in chili extracts showed a high and negative association with the inhibition of oil droplet formation in ORO staining test when the 3T3-L1 adipocytes were treated with biocompatible dosage (IC25) of chili extracts. However, there was no significant correlation between total flavonoid content (TFC) in chili extract and their anti-obesity activities (inhibition of oil droplets and lipase) towards 3T3-L1 adipocytes. Our results are in agreement with the previously published literature which suggested that the higher concentrations of phenols especially TFC did not always reflects significantly higher antioxidant capacity as TFC can have either synergistic or antagonistic effect on the overall antioxidant capacity (Galanakis et al. 2015).
Thus this study results suggests that the antioxidant activity of natural products is depending on their bioactive compounds (such as capsaicin, lycopene and pectin) (Nagarajan et al. 2019). In this research, the level of TPC is strongly correlated with the anti-obesity activities, especially inhibition of pancreatic lipase. A similar balance between TPC and inhibitory activity of pancreatic lipase has been reported to exist in some selected medicinal plants (Abdul Rahman et al. 2017). As already mentioned, TPC in chili pepper extracts play a major role to scavenge the free radicals produced. Thus, capsaicinoids, especially capsaicin, which only exists in Capsicum species, may be one of the phenolic contents that contribute to this result.
Conclusion
In conclusion, different parts of different varieties of chili peppers extracts have shown various results of bioactive compounds content and antioxidant activities. The variety of Kulai 568 demonstrated higher TPC and stronger antioxidant activities. Although it contained a lower concentration of capsaicin compared to Centil seed and Bara pulp extract, it does exhibit strong inhibitory ability against lipid accumulation and pancreatic lipase. Thus, this study suggests that Kulai 568 whole fruit exerts favourable anti-obesity effects in 3T3-L1 preadipocytes. However, further and detailed investigations are required to evaluate the full therapeutic potential against obesity and to justify the dosage for safe consumption of chili peppers in obese patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by University Putra Malaysia (UPM) Research Grant no. 9512500 to Associate Professor Azirna Azlan, Department of Nutrition and Dietetics.
Abbreviations
- BHT
Butylated hydroxytoluene
- BHA
Butylated hydroxyanisole
- HPLC
High performance liquid chromatography
- TPC
Total phenolic content
- TFC
Total flavonoids content
- EDTA
Ethylenediaminetetraacetic acid
Compliance with ethical standards
Conflicts of interest
The authors declare that there is no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdul Rahman H, Saari N, Abas F, Ismail A, Mumtaz MW, Abdul Hamid A. Anti-obesity and antioxidant activities of selected medicinal plants and phytochemical profiling of bioactive compounds. Int J Food Prop. 2017;20:2616–2629. doi: 10.1080/10942912.2016.1247098. [DOI] [Google Scholar]
- Alam M, Juraimi AS, Rafii MY, Abdul Hamid A, Aslani F, Hasan MM, Uddin M. Evaluation of antioxidant compounds, antioxidant activities, and mineral composition of 13 collected purslane (Portulaca oleracea L.) accessions. Biomed Res Int. 2014;2014:10. doi: 10.1155/2014/296063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertao MR, Moraes MC, Palmieri DA, SiL P, Goncalves RM. Cytotoxicity, genotoxicity and antioxidant activity of extracts from Capsicum spp. Res J Med Plants. 2016;10:265–275. doi: 10.3923/rjmp.2016.265.275. [DOI] [Google Scholar]
- Brouns F. Overweight and diabetes prevention: is a low-carbohydrate–high-fat diet recommendable? Eur J Nutr. 2018;5:1–12. doi: 10.1007/s00394-018-1636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosland PW, Votava EJ. Peppers: vegetable and spice capsicums. Wallingford: CABI Publishing; 2000. pp. 1–16. [Google Scholar]
- Calzadilla P, Sapochnik D, Cosentino S, Diz V, Dicelio L, Calvo JC, Guerra LN (2011) N-acetylcysteine reduces markers of differentiation in 3T3-L1 adipocytes. Int J Mole Sci 12:6936–6951 [DOI] [PMC free article] [PubMed]
- Deng Q, Zinoviadou KG, Galanakis CM, Orlien V, Grimi N, Vorobiev E, Barba FJ. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng Rev. 2015;7:357–381. doi: 10.1007/s12393-014-9104-9. [DOI] [Google Scholar]
- Fadda A, Virdis A, Barberis A, Melito S. Variation in secondary metabolites contents of Spinoso Sardo artichoke (Cynara cardunculus L.) under different day lengths. Turk J Agric For. 2018;42:372–381. doi: 10.3906/tar-1711-27. [DOI] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Shimomura I (2017) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Cli Invest 114:1752–1761 [DOI] [PMC free article] [PubMed]
- Galanakis CM, Kotanidis A, Dianellou M, Gekas V. Phenolic content and antioxidant capacity of Cypriot wines. Czech J Food Sci. 2015;33:126–136. doi: 10.17221/335/2014-CJFS. [DOI] [Google Scholar]
- Galanakis CM. Separation of functional macromolecules and macromolecules: from ultrafiltration to the border of nanofiltration. Trends Food Sci Tech. 2015;42:44–63. doi: 10.1016/j.tifs.2014.11.005. [DOI] [Google Scholar]
- Galanakis CM, Tsatalas P, Charalambous Z, Galanakis IM. Polyphenols recovered from olive mill wastewater as natural preservatives in extra virgin olive oils and refined olive kernel oils. Environ Sci Tech Inno. 2018;10:62–70. doi: 10.1016/j.eti.2018.01.012. [DOI] [Google Scholar]
- Galanakis CM, Tsatalas P, Galanakis IM. Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind Crop Prod. 2018;111:30–37. doi: 10.1016/j.indcrop.2017.09.058. [DOI] [Google Scholar]
- Guo CL, Chen HY, Cui BL, Chen YH, Zhou YF, Peng XS, Wang Q. Development of a HPLC method for the quantitative determination of capsaicin in collagen sponge. Int J Anal Chem. 2015;2015:6. doi: 10.1155/2015/912631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MC, Kang N, Ko SC, Kim YB, Jeon YJ. Anti-obesity effect of seaweeds of Jeju Island on the differentiation of 3T3 L1-preadipocytes and obese mice fed a high fat diet. Food Chem Toxic. 2016;90:36–44. doi: 10.1016/j.fct.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Dia Metab J. 2012;36:13–25. doi: 10.4093/dmj.2012.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovačević DB, Barba FJ, Granato D, Galanakis CM, Herceg Z, Dragović-Uzelac V, Putnik P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018;254:150–157. doi: 10.1016/j.foodchem.2018.01.192. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Lof M. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:4005–4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc ES, O'connor E, Whitlock EP, Patnode CD, Kapka T (2011) Effectiveness of primary care–relevant treatments for obesity in adults: a systematic evidence review for the US Preventive Services Task Force. Ann Inter Medic 155:434–447 [DOI] [PubMed]
- Leung FW. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008;83:1–5. doi: 10.1016/j.lfs.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Lu JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimova V, Koleva Gudeva L, Ruskovska T, Gulaboski R, Cvetanovska A. Antioxidative effect of Capsicum oleoresins compared with pure capsaicin. IOSR J Pharm. 2014;4:44–48. [Google Scholar]
- Moreno-Ramírez Y, Martínez-Ávila G, González-Hernández V, Castro-López C, Torres-Castillo J. Free radical-scavenging capacities, phenolics and capsaicinoids in Wild Piquin Chili (Capsicum annuum var. Glabriusculum) Molecule. 2018;23:2655. doi: 10.3390/molecules23102655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougoulias N, Wogiatzi E, Vagelas I, Giurgiulescu L, Gogou I, Ntalla MN, Kalfountzos D. Comparative study on polyphenols content, capsaicin and antioxidant activity of different hot pepper varieties (Capsicum annuum L.) under environmental conditions of Thessaly region. Greece J Food Sci Technol. 2017;9:109–116. [Google Scholar]
- Mau JL, Chao GR, Wu KT (2001) Antioxidant properties of methanolic extracts from several ear mushrooms. J Agric Food Chem 49:5461–5467 [DOI] [PubMed]
- Mohamed GA, Ibrahim SR, Elkhayat ES, El Dine RS. Natural anti-obesity agents. Bull Faculty Pharm Cairo Univ. 2014;52:269–284. doi: 10.1016/j.bfopcu.2014.05.001. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nour M, Lutze SA, Grech A, Allman-Farinelli M. The relationship between vegetable intake and weight outcomes: a systematic review of cohort studies. Nutrients. 2018;10(11):16–26. doi: 10.3390/nu10111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan J, Krishnamurthy NP, Ramanan RN, Raghunandan ME, Galanakis CM, Ooi CW. A facile water-induced complexation of lycopene and pectin from pink guava by-product: Extraction, characterization and kinetic studies. Food Chem. 2019;296:47–55. doi: 10.1016/j.foodchem.2019.05.135. [DOI] [PubMed] [Google Scholar]
- Oboh G, Ogunruku OO. Cyclophosphamide-induced oxidative stress in brain:protective effect of hot short pepper (Capsicum frutescens L. var. abbreviatum) Exp Toxicol Pathol. 2010;62:227–233. doi: 10.1016/j.etp.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Othman ZAA, Ahmed YBH, Habila MA, Ghafar AA. Determination of capsaicin and dihydrocapsaicin in capsicum fruit samples using high performance liquid chromatography. Molecules. 2011;16:8919–8929. doi: 10.3390/molecules16108919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkok A, Darcy B, Sorkun K. Total phenolic acid and total flavonoid content of Turkish pine honeydew honey. J Api Prod Api Med Sci. 2010;2:65–71. doi: 10.3896/IBRA.4.02.2.01. [DOI] [Google Scholar]
- Ozturk I, Ercisli S, Kalkan F, Demir B. Some chemical and physico-mechanical properties of pear cultivars. Afr J Biotech. 2009;8:687–693. [Google Scholar]
- Park CH, Jeong SJ, Lee HJ, Lee EO, Bae H, Lee MH, Kim SH (2010) Traditional medicine Taeeumjowitangkagambang exerts antiobesity and hypolipidemic effects via antioxidant enzyme enhancement. Phytotherapy Res 24:1700–1709 [DOI] [PubMed]
- Re R, Pellegrini N, Rroteggente A, Pannala A, Rice-Evans YMC. Antioxidant activity applying an improved ABTS radical cation decolonization assay. Free Radical Bio Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sarafi E, Siomos A, Tsouvaltzis P, Chatzissavvidis C, Therios I. Boron and maturity effects on biochemical parameters and antioxidant activity of pepper (Capsicum annuum L.) cultivars. Turk J Agric Forestry. 2018;42:237–247. doi: 10.3906/tar-1708-31. [DOI] [Google Scholar]
- Slanc P, Doljak B, Kreft S, Lunder M, Janes D, Štrukelj B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phytotherapy Res Int J Devoted Pharmacol Toxicol Eval Natur Prod Deriv Wiley Online Libr. 2009;23:874–877. doi: 10.1002/ptr.2718. [DOI] [PubMed] [Google Scholar]
- Sora GTS, Haminiuk CWI, da Silva MV, Zielinski AAF, Gonçalves GA, Bracht A, Peralta RM. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: an application of chemometrics. J Food Sci Technol. 2015;52:8086–8094. doi: 10.1007/s13197-015-1935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegu MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidants. J Am Oil Chem Soc. 1984;61:1928–1931. [Google Scholar]
- Zinoviadou KG, Galanakis CM, Brnčić M, Grimi N, Boussetta N, Barba MMJ. Fruit juice sonication: implications on food safety and physicochemical and nutritional properties. Food Res Int. 2015;77:743–752. doi: 10.1016/j.foodres.2015.05.032. [DOI] [Google Scholar]
- Zhou YH, Ma XQ, Wu C, Lu J, Zhang SS, Guo J, He J. Effect of anti-obesity drug on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2012;7:39–62. doi: 10.1371/journal.pone.0039062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


