Abstract
The impact of ultraviolet light (UV-C) irradiation on oxidative enzymes [Polyphenol oxidase (PPO) and Peroxidase (POD)], free essential amino acids and sensory profile of coconut water were investigated. PPO and POD activities were lost to 94 and 93%, respectively of its original value at fluence level of 400 mJ/cm2. Inactivation kinetics of both enzymes were fitted to nonlinear Weibull model with an increase in UV dosage with a high coefficient of determination (R2 > 0.97) and low root mean square error (RMSE < 0.06). No significant change was observed in all essential amino acids (p > 0.05) after UV-C treatment up to maximum delivered fluence of 400 mJ/cm2. Sensory attributes of coconut water up to a treated UV-C fluence level of 200 mJ/cm2 were well retained in terms of chosen descriptors (p > 0.05). This study allow to further investigate the development of UV-C light technology for inhibition of spoilage enzymes and prolonged shelf-life of low acid beverages.
Keywords: Ultraviolet irradiation, Coconut water, Polyphenol oxidase, Peroxidase, Amino acids, Sensory attributes
Introduction
Coconut water is a popular beverage consumed widely in Asia, South America, and Northern America. The mineral composition, low acid, and sugar content make coconut water a potential rehydrating drink (Prades et al. 2012; Saat et al. 2002). The increase in demand for coconut water has been growing worldwide because of its sensory and nutritional properties. The demand for coconut water is growing up considerably, global coconut water market was valued at ~ 2.2 billion USD in 2017, and forecast to reach 8.3 billion USD by 2023 (Conway 2019).
Polyphenol oxidase (PPO) and peroxidase (POD), play a major role in discoloration and quality degradation occurring during the preservation of juices (Tomás-Barberán and Espín 2001). PPO can oxidize the phenolic substrates (majorly oxidation of o-dihydroxy phenols to o-quinones) which is responsible for the production of brown colored pigments (Terefe et al. 2014). POD along with hydrogen peroxide can oxidize a wide range of phenolic compounds. These enzymes in coconut water is liable for brown, yellow or pink coloring during storage (Prades et al. 2012).
Thermal inactivation of oxidative enzymes in coconut water was extensively studied (Matsui et al. 2007; Murasaki-Aliberti et al. 2009; Prades et al. 2012). Since PPO and POD have relatively high heat resistance, thermal processes can adversely affects their nutritional quality. As an alternative, non-thermal approaches are attractive since they have the potential to preserve the sensory and physicochemical characteristics of juices (Caminiti et al. 2012).
UV-C light technology has been promising and effective for inactivating vegetative bacteria (Bhullar et al. 2018; Gopisetty et al. 2019), spores (Pendyala et al. 2019) and oxidative enzymes including different fruit juices, such as apple juice (Noci et al. 2008; Manzocco et al. 2009; Müller et al. 2014), grape juice (Müller et al. 2014), orange juice (Sampedro et al. 2014), and Nectraine Juice (Aguilar et al. 2016). However, inactivation of enzymes in coconut water using UV-C light was not studied, even though the optical properties, low fat content and absence of suspended particles of the product is more suitable conditions to treat with UV-C light.
The delivered UV-C dose depends on optical attenuation parameters of the test fluid. Majority of reports on UV-C irradiation considered applied dose (measured at the surface of fluid) which may cause inconsistent inactivation kinetics in various test fluids (Unluturk et al. 2010; Islam et al. 2016). The impact of UV-C irradiation on oxidative enzymes, amino acids content and sensory attributes of coconut water has not been reported to date. The present study addresses the problem and accounts for optics and UV intensity gradients. This study investigates the effectiveness of UV-C irradiation on the PPO and POD activity of coconut water. In addition, we report the effect of UV-C irradiation on the concentration of free essential amino acids (histidine (His), lysine (Lys), methionine (Met), phenylalanine (Phe), threonine (Thr), tryptophan (Trp), leucine (Leu), and isoleucine (Ile)) and sensory quality of coconut water.
Materials and methods
Coconut water preparation
Tender green coconuts (n = 20) were acquired from a local grocery store (Nashville, TN, USA). The coconuts were pierced and the water was removed into a sterile container. A Whatman filter paper (20–25 µm diameter) (Fisher Scientific, Pittsburgh, USA) was used to remove any solid particles that may present in the collected water. The pH of the prepared coconut water was 4.88 ± 0.14.
UV-C irradiation treatment
The absorbance and ultraviolet transmittance (UVT) of the coconut water at 254 nm was measured using UV–Vis Spectrophotometer (Gopisetty et al. 2019). Coconut water was treated with UV-C light at 254 nm at reduction equivalent fluence (REF) ranging from 0 to 400 mJ/cm2 (n = 3) using a flow through UV system (Bhullar et al. 2018; Gopisetty et al. 2018). The system comprises a low pressure mercury UV lamp (40 W) with cooling fan, coiled food grade Teflon tube and a pump. For UV treatment, coconut water was pumped through the tube, which receives UV irradiation from the lamp. During UV-C treatment, Coconut water temperature was maintained at ~ 4 °C by placing the inlet, outlet tanks and samples in ice containers. Dean flow pattern in the curved tube critically improves the efficiency of the fluid mixing by the centripetal forces at the curve, is governed by Dean number (De).
| 1 |
where represents the diameter of the tube (0.003 m), Re denotes Reynolds number, and is curvature diameter (0.1 m).
The experimental flow rates were in the range of 84–420 mL/min with Dean number 116–578. Since the dean muber, which denotes secondary dynamic instability of dean vorices (De > 75) to fully turbulent flow conditions (De > 400) (Ligrani 1994). Validation of UV-C fluence delivery and uniformity of the treatment at similar experimental flow conditions was performed by using bio-dosimetry (Bhullar et al. 2019). A linear relationship between flow rate and reduction equivalent fluence (REF) was observed with R2 > 0.99, which confirm uniform distribution of UV fluence throughout the reactor (Bhullar et al. 2019).
Measurement of PPO and POD activities
The PPO activity was measured using the method described by Falguera et al. (2013) with few modifications. Aliquots of 2.0 mL coconut water and 1.0 mL of 4-methyl-catechol prepared in buffer solution (0.01 M, pH 6.0) (McIlvaine solution) were added in a quartz cuvette. The change in solution absorbance (Abs) at 410 nm was estimated every 20 s for 5 min with a spectrophotometer. The enzyme tyrosinase (Sigma-Aldrich Co., USA) was used as a PPO calibration standard.
The POD activity was estimated by following the method described by Augusto et al. (2015) and Falguera et al. (2013) with few modifications. Coconut water—0.1 mL, Milli Q water—2.1 mL, buffer solution (pH 6.0)—0.32 mL and 5% (m/v) pyrogallol (C6H6O3) (Sigma-Aldrich Co., USA) solution—320 μL was mixed in a cuvette. Following that, hydrogen peroxide (H2O2) solution—160 μL (0.147 M) was mixed to initate the reaction. Then the absorbance (Abs) at 420 nm was taken at 20 s time interval for 3 min using a spectrophotometer. The initial rate was calculated from the slope of the absorbance versus time curve. The enzyme horseradish peroxidase (Sigma-Aldrich Co., USA) was used as a POD calibration standard. For both enzymes, the slope of absorbance versus time curve was used to calculate the rate (Umit Unal et al. 2007). One unit of enzyme activity expressed as the change in absorbance value of 0.01 per min.
Modeling oxidative enzymes inactivation curves
To describe inactivation kinetics of oxidative enzymes at different UV-C REF levels in coconut water, PPO and POD inactivation curves were fitted by the following non-linear Weibull models Eqs. (2) and (3), respectively. These models are available in the GInaFiT tool (a freeware Microsoft Excel add-in) (Geeraerd et al. 2005). Weibull model has extensively been used to describe inactivation kinetics of microorganisms by thermal and non-thermal techniques (Mafart et al. 2002).
| 2 |
| 3 |
In Eq. (2) Weibull model has three parameters (, and , where represents initial relative activity, parameter characterizes the curve shape (dimensionless). corresponds to the for the inactivation of enzymes. In Eq. (3), the parameter indicates tailing.
Estimation of essential amino acids
Essential amino acids (His, Met, Trp, Phe, Lys, Thr, Val and Leu/Ile) was quantified with LC–MS/MS method developed for a Shimadzu Nexera XR UHPLC system (Shimadzu Scientific Instruments, Columbia, MD) An isocratic HPLC method was developed with 0.1% formic acid as the mobile phase (flow rate—0.6 mL/min). Phenomenex Kinetex 2.6 EVO C18 column (50 × 2.1 mm, 2.6 µm) was used to separate compounds maintained at temperature of 40 °C. Sample injection volume was 2µL. For LC–MS/MS detection, an electrospray ionization source in postive ion mode was selected with the following parameters: temperature—250 °C; nebulizing gas flow—3.00 L/min; heat block—450 °C; and drying gas flow—20 L/min. Data were processed with Shimadzu LabSolutions software. Calibration curve was prepared with standards at concentration of 1.0 ng/mL to 2500 ng/mL, and the internal standard was para-aminobenzoic acid (PABA).
Sensory analysis of UV-C treated coconut water
A descriptive sensory analysis was performed to profile the UV-C irradiated coconut water. Among several sensory profiling techniques, descriptive tests are amongst the most refined tools to evaluate the sensory attributes (Lawless and Heymann 1998). It provides information about the detection or discrimination and depiction of qualitative and quantitative sensory attributes of a food product by trained panel (Meilgaard et al. 1999).
Panel Selection
Sensory panel members were screened based on their ability to separate between similar samples and rate products for intensity and recognize tastes and aromas. A sensory pool consisting of 15 members with an age group ranging from 20 to 45 years comprising men and women, participated in several sessions to evaluate the coconut water samples treated with UV-C at two different levels (200 mJ/cm2 and 400 mJ/cm2).
Aliquots of 20 mL coconut water samples were served to the sensory panel using a glass container with a lid which is coded with three digit random number. The samples were served along with crackers and spring water to cleanse the mouth/palate. Quantitative descriptive analysis (QDA) was selected and employed to profile the coconut water treated with UV-C (Stone et al. 2008).
Lexicon generation
Several sensory descriptors were generated based on group discussion and free choice profiling. Following this, common sensory descriptors (color, body (viscosity), aroma, fermented, fresh, cooked, sour, sweet, burnt, off flavors, and pleasantness) were selected on the general consensus and were used in the sample score card. Prior to the sensory analysis, the sensory panel were trained on several training sessions to familiarize the descriptors and score card with appropriate standards/reference samples. The sensory scores were recorded on a 15 cm linear line scale with 1.25 cm as the lowest and 13.75 cm as highest anchor points. Panelists were asked to mark their sensory perceptions (intensity) by crossing the line scale appropriately and indicating their corresponding sample code on the line. Finally the sample scores were decoded and data was screened for any outliers. The final profilogram were generated based on the edited sensory descriptors data and discussed.
Statistical analysis
To evaluate variation between results of samples, one way analysis of variance (ANOVA) with Post-hoc Tukey’s HSD test was conducted with SAS statistical computer environment (SAS 2016), and differences were measured significant at p ≤ 0.05. Experiments were performed in triplicate (n = 3) on different working days.
Results and discussion
Oxidative enzyme inactivation kinetics in UV-C treated coconut water
The optical absorbance of coconut water at 254 nm was measured as 1.01 ± 0.018 which shows that the chemical compounds present in coconut water absorbs UV-C light. To inhibit oxidative enzymes activity in fruit juices, higher doses of UV-C treatment are recommended (Koutchma et al. 2016). For this study, UV-C reduction equivalent fluence (REF) rate up to 400 mJ/cm2 were used and evaluated the impact on selective oxidative enzyme activities polyphenol oxidase (PPO) and Peroxidase (POD).
Polyphenol oxidase (PPO)
Total PPO activity in the untreated raw coconut water was found to be 2.43 U/mL/min. The relative activity of PPO in the coconut water with increasing UV-C REF is shown in Fig. 1. It is evident that relative enzyme activity decreased as UV exposure was increased. Inactivation of PPO activity observed in coconut water was 94% at 400 mJ/cm2. To evaluate inactivation kinetics of UV-C treatment the nonlinear Weibull model [Eq. (2)] was fitted and showed a good fit with RMSE = 0.03 and R2 = 0.99, enabling the study of PPO inactivation in UV-C treated coconut water through the difference in and fitted parameters (Table 1).
Fig. 1.
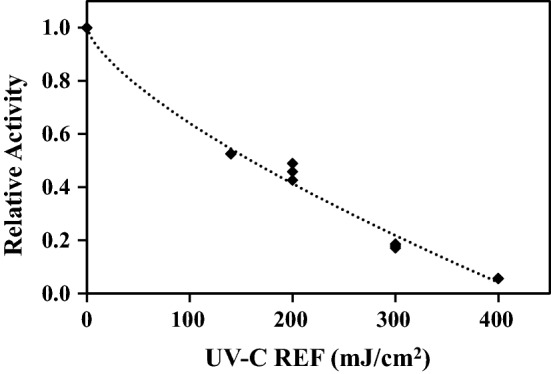
Effect of UV-C treatment on PPO activity in coconut water
Table 1.
Kinetic parameters of enzymes inactivation
| Kinetic parameters | PPO | POD |
|---|---|---|
| R2 | 0.99 | 0.97 |
| RMSE | 0.03 | 0.06 |
| 424 ± 17a | 124 ± 7a | |
| 0.71 ± 0.05a | 2.11 ± 0.35a | |
| NA | 0.16 ± 0.02a |
aIndicates standard error
Peroxidase (POD)
POD activity in the untreated raw coconut water was noticed to be 0.22 U/mL/min. The effect of UV-C irradiation on POD enzyme in coconut water was shown in Fig. 2. It was observed that the POD activity also reduced like PPO with an increase in UV-C REF. POD inactivation was noticed to be 93% at high UV-C REF of 400 mJ/cm2. To assess inactivation kinetics of UV-C treatment the nonlinear Weibull model with tail [Eq. (3)] was fitted and provided a good fit with RMSE = 0.06 and R2 = 0.97, allowing the estimation of POD inactivation in UV-C treated coconut water via difference of and fitted parameters (Table 1). The results demonstrated that in comparison to POD, PPO is considered to be most resistant to UV-C and require higher doses to inactivate this enzyme (Figs. 1 and 2).
Fig. 2.

Effect of UV-C treatment on POD activity in coconut water
Several studies have investigated the effect of photo irradiation on oxidative enzymes in fruit juices (Table 2). Augusto et al. (2015) reported the inactivation of PPO and POD in coconut water model solution. PPO activity was found to be 2% of its original value after 30 min of exposure to photo-irradiation by 400 W medium pressure mercury lamp that emits photons in the range between 250 and 740 nm with a maximum peak at (400–420 nm), the surface of the fluid was located 23.4 cm away from the lamp. However, Falguera et al. (2011, 2013, 2014) noticed complete inactivation of PPO and POD in apple, pear and grape juices (except 20% residual activity of PPO in grape juice), when exposed to photo-irradiation for 100 min with a high-pressure mercury lamp (400 W) that emits light ranging from 250 to 740 nm. According to Noci et al. (2008), UV-C processing has shown no effect on PPO and POD when apple juice was poured in sterile Pyrex dishes, exposed to a 30 W UV-lamp at a distance of 30 cm for 30 min. Haddouche et al. (2015) reported 10% PPO residual activity in buffer with surface exposure at 4800 mJ/cm2. Manzocco et al. (2009) study revealed that in clear apple juice the residual activity of PPO was found to be 10% even at UV dose of 19,841 mJ/cm2. In addition to that, Sampedro et al. (2014) also show inconsistent residual activities in UV-C irradiated phosphate buffer, acetate buffer and orange juice with UV surface exposure of 58.2 kJ/cm2 at maximum emissions of two selectable wavelengths (185 and 254 nm).
Table 2.
Reported remnant activity of PPO and POD in various photo/UV-C irradiated test juices/fluids
| Test juice/fluid | Dose/fluence (mJ/cm2) | PPO activity (remnant) | POD activity (remnant) | References |
|---|---|---|---|---|
| Apple | NC | 99.5 | 97 | Noci et al. (2008) |
| Apple (Sparkling) | NC | 0 | 0 | Falguera et al. (2011) |
| Grape (Dauphine) | NC | 20 | 0 | Falguera et al. (2013) |
| Pear (Flor de Invierno) | NC | 0 | 0 | Falguera et al. (2014) |
| Orange | 58.2 | 75 | 97 | Sampedro et al. (2014) |
| Acetate buffer (pH 4.0) | 58.2 | 31 | 17 | Sampedro et al. (2014) |
| Phosphate buffer (pH 6.8) | 4800 | 10 | NA | Haddouche et al. (2015) |
| Apple (Clear) | 12,483 | 10 | _ | Manzocco et al. (2009) |
| Nectraine Juice | 60 | 40 | Aguilar et al. (2016) | |
| Clear apple juice | 72 | 70 | NA | Akgün and Ünlütürk (2017) |
| Coconut water model solution | NC | 2 | 1 | Augusto et al. (2015) |
| Tiger nuts milk | 4230 | NA | 13.9 | Corrales et al. (2012) |
| Grape | 30.1 kJ/L | 60.9 | NA | Müller et al. (2014) |
| Apple | 30.1 kJ/L | 15.8 | NA | Müller et al. (2014) |
| Apple | 8.1 kJ/L | 86.1 | NA | Gayán et al. (2013) |
| Apple | 1.5 kJ/L | 114.5 | NA | Orlowska et al. (2014) |
| Mango nectar | 108 kJ/L | 19 | NA | Guerrero-Beltran et al. (2006) |
| Coconut water | 400 | 6 | 7 | Current study |
NC not calculated, NA not analyzed
Other studies in continuous UV treatment reported that PPO activity was reduced to 41.7% at a fluence rate of 100.48 kJ/L (25 cycles) in buffer and 44.3% in apple juice (Müller et al. 2014). Whereas Gayán et al. (2013) reported 86% residual PPO activity in UV treated apple juice at a fluence rate of 8.1 kJ/L. Residual PPO activity in UV irradiated mango nectar was estimated as 19% (Guerrero-Beltran et al. 2006). This discrepancy in reported inconsistent enzyme activities per UV dose delivered is due to improper dose calculation without consideration of test fluid absorbance, variation in light irradiation wavelengths.
Studies proposed light photons would affect the activity of proteins by altering their structure via two major mechanisms; (1) direct photo oxidation—aromatic and sulfur containing amino acids (Trp, Tyr, Phe, His, Met and Cys) of proteins, and bound chromophore groups can absorb UV radiation at 254 nm which results in singlet or triplet excited states or radicals via ionization, (2) indirect photo-oxidation via singlet O2 generated by the transfer of energy by protein bound, or other chromophores (photosensitizers) (Davies and Truscott 2001). As a result enzyme loss functional activity due to side chain oxidation, backbone fragmentation and formation of aggregates and crosslinks (Davies 2003). Manzocco et al. (2009) studied molecular changes of PPO by HPLC-gel permeation after UV-C light exposure, and reported PPO inactivation occurred as a consequence of protein aggregates. Augusto et al. (2015) compared change in absorption spectra of untreated and UV-C treated samples, proposed molecular unfolding and formation of aggregates as a possible mechanism for photo-inactivation. Although, further studies are needed to determine the exact mechanism of enzyme inactivation.
Essential amino acids
Nine essential amino acids were analyzed with an LC–MS/MS method. Results are shown in Fig. 3. Of the amino acids tested, Trp was present in coconut water at highest concentration. The concentration of Trp in untreated coconut water was 1288 nM. Trp concentration were 1169, 1170, 1100, and 1101 nM in coconut water samples treated with fluence of 100, 200, 300, and 400 mJ/cm2 respectively. A one-way ANOVA test showed the change in the Trp concentration was not significant at the 95% confidence limit.
Fig. 3.
The effect of UV-C reduction equivalent fluence (REF) on the concentration of essential amino acids
In addition to Trp, other amino acids analyzed were present in untreated coconut water (control) in a range of concentrations from 1.3 nM for Lys to 49.6 nM for Val. For UV treated coconut water samples, the reduction of amino acids at fluence of 100, 200, 300, and 400 mJ/cm2 were not statistically significant for all essential amino acids at tested fluence levels (p > 0.05). Yen et al. (2014) reported 6.9% reduction in Trp content at UV-C dose of 400 mJ/cm2 in cell culture media. Light effect on degradation of compounds vary among various foods. The extent of chemical compounds (For e.g. aminoacids) degradation depends on presence of photo sensitizers (such as vitamins, porphyrins) and single oxygen quenchers (antioxidants) present in the food (Bekbölet 1990).
Sensory attributes
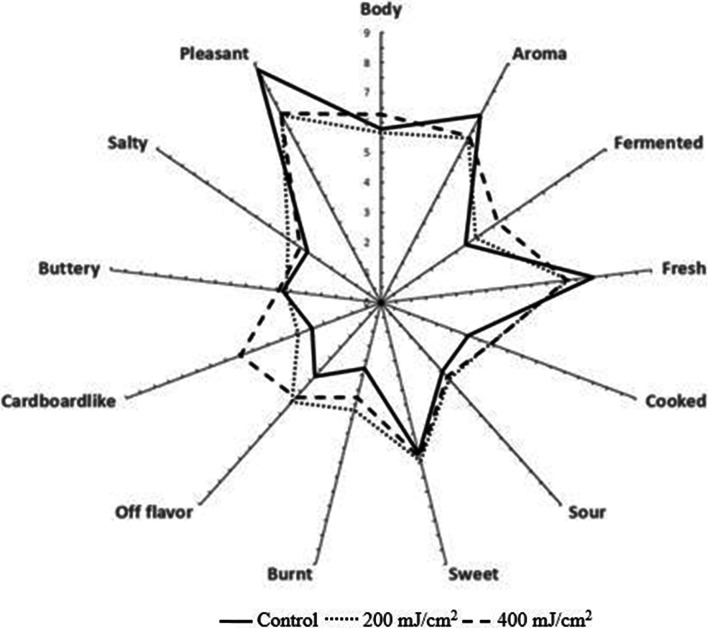
The sensory profilogram generated for UV-C treated samples were depicted as Fig. 4. Control sample had high scores for aroma, fresh, sweet, and pleasant notes, while it had low scores for fermented note, cooked, sour, burnt, off flavor, cardboard like and salty notes. Attributes like aroma, fresh, sweet, and pleasant are more desirable, which are the characteristics of the coconut water and liked by the consumers. In general, attributes like fermented note, cooked, sour, burnt, off flavor, cardboard and salty notes are undesirable. The changes in these attributes due to processing can influence the consumer preference and liking. Samples treated with UV-C (200 mJ/cm2) had slightly lower scores for aroma, fresh, and pleasant sensory attributes when compared to control samples (means comparison). But no statistical difference was observed (p > 0.05) between the means. Comparatively, 400 mJ/cm2 samples showed significant difference (p < 0.05) in cardboard like (p = 0.042) and fermented (p = 0.047) sensory attributes. But the p values revealed slight changes in these sensory attributes. De Marchi et al. (2015) reported that significant sensory differences were perceived between fresh untreated and thermally processed (90 °C, 1 min) coconut water samples. The thermal processed samples shown more cooked, toasted bread and hazelnut notes because of the presence of more ketones and aldehydes produced at that high temperature.
Fig. 4.
Sensory profilogram of coconut water samples treated with UV-C at 200 and 400 mJ/cm2
Non-thermal technologies, such as UV-light (UV), pulsed electric fields (PEF), high hydrostatic pressure (HPP), membrane processes, high pressure carbon dioxide (HPCD) and ultrasound (US), can potentially provide more ideal food products with fresh-like characteristics when compared to thermal counterparts. Among these UV-C processing of coconut water is an ideal choice, due to (1) Efficient light penetration in high transmittance fluids; (2) Effective inactivation of spores (challenging problem in processing of low acid foods) (Pendyala et al. 2019), oxidative enzymes inactivation; (3) Simple equipment which require low capital cost; (4) low operational cost with high energy efficiency; (5) System can be easily retrofitted into current production lines.
Conclusion
The results of this work demonstrated that UV-C irradiation is capable to inactivate PPO and POD enzymes in coconut water without change in essential amino acids and sensory profile. This study demonstrated the potential use of UV-C to suppress oxidative enzymes and thereby browning reactions in coconut water. Further research is needed to investigate the precise mechanism of enzymes inactivation, to study the effect of storage time and temperature on shelf life, and to evaluate safety (cytotoxicity) of UV-C treated coconut water.
Acknowledgements
This work is funded under the Agriculture and Food Research Initiative (Food Safety Challenge Area), United States Department of Agriculture, award number 2015-69003-23117.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ankit Patras, Email: apatras@tnstate.edu.
Brahmaiah Pendyala, Email: bpendyal@tnstate.edu.
References
- Aguilar K, Ibarz R, Garvín A, Ibarz A. Effect of UV–Vis irradiation on enzymatic activities and the physicochemical properties of nectarine juices from different varieties. Food Sci Technol. 2016;65:969–977. [Google Scholar]
- Akgün MP, Ünlütürk S. Effects of ultraviolet light emitting diodes (LEDs) on microbial and enzyme inactivation of apple juice. Int J Food Microbiol. 2017;260:65–74. doi: 10.1016/j.ijfoodmicro.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Augusto PE, Ibarz R, Garvín A, Ibarz A. Peroxidase (POD) and polyphenol oxidase (PPO) photo-inactivation in a coconut water model solution using ultraviolet (UV) Food Res Int. 2015;74:151–159. doi: 10.1016/j.foodres.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Bekbölet M. Light effects on food. J Food Prot. 1990;53(5):430–440. doi: 10.4315/0362-028X-53.5.430. [DOI] [PubMed] [Google Scholar]
- Bhullar MS, Patras A, Kilonzo-Nthenge A, Pokharel B, Yannam SK, Rakariyatham K, Sasges M. Microbial inactivation and cytotoxicity evaluation of UV irradiated coconut water in a novel continuous flow spiral reactor. Food Res Int. 2018;103:59–67. doi: 10.1016/j.foodres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Bhullar MS, Patras A, Kilonzo-Nthenge A, Pokharel B, Sasges M. Ultraviolet inactivation of bacteria and model viruses in coconut water using a collimated beam system. Food Sci Technol. 2019;25(7):121–133. doi: 10.1177/1082013219843395. [DOI] [PubMed] [Google Scholar]
- Caminiti IM, Palgan I, Muñoz A, Noci F, Whyte P, Morgan DJ, Lyng JG. The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol. 2012;5:680–686. doi: 10.1007/s11947-010-0365-x. [DOI] [Google Scholar]
- Conway J (2019) Global coconut water market value from 2017 to 2023. https://www.statista.com/statistics/673554/coconut-water-market-value-worldwide/. Accessed 18 Dec 2019
- Corrales M, de Souza PM, Stahl MR, Fernández A. Effects of the decontamination of a fresh tiger nuts’ milk beverage (horchata) with short wave ultraviolet treatments (UV-C) on quality attributes. Innov Food Sci Emerg Technol. 2012;13:163–168. doi: 10.1016/j.ifset.2011.07.015. [DOI] [Google Scholar]
- Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem Biophys Res Commun. 2003;305(3):761–770. doi: 10.1016/S0006-291X(03)00817-9. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Truscott RJ. Photo-oxidation of proteins and its role in cataractogenesis. J Photoch Photobio B. 2001;63(1–3):114–125. doi: 10.1016/S1011-1344(01)00208-1. [DOI] [PubMed] [Google Scholar]
- De Marchi F, Aprea E, Endrizzi I, Charles M, Betta E, Corollaro ML, Gasperi F. Effects of pasteurization on volatile compounds and sensory properties of coconut (Cocos nucifera L.) water: thermal vs. high-pressure carbon dioxide pasteurization. Food Bioprocess Technol. 2015;8(7):1393–1404. doi: 10.1007/s11947-015-1501-4. [DOI] [Google Scholar]
- Falguera V, Pagán J, Ibarz A. Effect of UV irradiation on enzymatic activities and physicochemical properties of apple juices from different varieties. Food Sci Technol. 2011;44(1):115–119. [Google Scholar]
- Falguera V, Aliguer N, Falguera M. An integrated approach to current trends in food consumption: moving toward functional and organic products. Food Control. 2013;26(2):274–281. doi: 10.1016/j.foodcont.2012.01.051. [DOI] [Google Scholar]
- Falguera V, Garza S, Pagán J, Garvín A, Ibarz A. Effect of UV–Vis irradiation on enzymatic activities and physicochemical properties of four grape musts from different varieties. Food Bioprocess Technol. 2014;6(8):2223–2229. doi: 10.1007/s11947-012-0781-1. [DOI] [Google Scholar]
- Gayán E, Serrano MJ, Monfort S, Álvarez I, Condón S. Pasteurization of apple juice contaminated with Escherichia coli by a combined UV mild temperature treatment. Food Bioprocess Technol. 2013;6(11):3006–3016. doi: 10.1007/s11947-012-0937-z. [DOI] [Google Scholar]
- Geeraerd AH, Valdramidis VP, Van Impe JF. GInaFiT a freeware tool to assess non-log-linear microbial survivor curves. Int J Food Microbiol. 2005;102(1):95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Gopisetty VVS, Patras A, Kilonzo-Nthenge A, Yannam S, Bansode RR, Sasges M, Xiao H. Impact of UV-C irradiation on the quality, safety, and cytotoxicity of cranberry-flavored water using a novel continuous flow UV system. Food Sci Technol. 2018;95:230–239. [Google Scholar]
- Gopisetty VVS, Patras A, Pendyala B, Kilonzo-Nthenge A, Ravi R, Pokharel B, Sasges M. UV-C irradiation as an alternative treatment technique: study of its effect on microbial inactivation, cytotoxicity, and sensory properties in cranberry-flavored water. Innov Food Sci Emerg. 2019;52:66–74. doi: 10.1016/j.ifset.2018.11.002. [DOI] [Google Scholar]
- Guerrero-Beltrán JA, Barbosa-Cánovas GV, Moraga-Ballesteros GE, Moraga-Ballesteros MJ, Swanson BG. Effect of pH and ascorbic acid on high hydrostatic pressure-processed mango puree. J Food Process Preserv. 2006;30(5):582–596. doi: 10.1111/j.1745-4549.2006.00090.x. [DOI] [Google Scholar]
- Haddouche L, Phalak A, Tikekar RV. Inactivation of polyphenol oxidase using 254 nm ultraviolet light in a model system. Food Sci Technol. 2015;62(1):97–103. [Google Scholar]
- Islam MS, Patras A, Pokharel B, Vergne MJ, Sasges M, Begum A, Rakariyatham K, Pan C, Xiao H. Effect of UV irradiation on the nutritional quality and cytotoxicity of apple juice. J Agric Food Chem. 2016;64(41):7812–7822. doi: 10.1021/acs.jafc.6b02491. [DOI] [PubMed] [Google Scholar]
- Koutchma T, Popović V, Ros-Polski V, Popielarz A. Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Compr Rev Food Sci F. 2016;15(5):844–867. doi: 10.1111/1541-4337.12214. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Heymann H. Sensory evaluation of food: principles and practices. New York: Chapman and Hall; 1998. [Google Scholar]
- Ligrani PM (1994) A study of dean vortex development and structure in a curved rectangular channel with aspect ratio of 40 at Dean numbers up to 430”, U.S. Army Research Laboratory (Contractor Report ARL-CR-l44) and Lewis Research Center (NASA Contractor Report 4607)
- Mafart P, Couvert O, Gaillard S, Leguérinel I. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int J Food Microbiol. 2002;72:107–113. doi: 10.1016/S0168-1605(01)00624-9. [DOI] [PubMed] [Google Scholar]
- Manzocco L, Quarta B, Dri A. Polyphenoloxidase inactivation by light exposure in model systems and apple derivatives. Innov Food Sci Emerg Technol. 2009;10(4):506–511. doi: 10.1016/j.ifset.2009.02.004. [DOI] [Google Scholar]
- Matsui KN, Granado LM, De Oliveira PV, Tadini CC. Peroxidase and polyphenol oxidase thermal inactivation by microwaves in green coconut water simulated solutions. Food Sci Technol. 2007;40(5):852–859. [Google Scholar]
- Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. Boca Raton: CRC Press; 1999. [Google Scholar]
- Müller A, Noack L, Greiner R, Stahl MR, Posten C. Effect of UV-C and UV-B treatment on polyphenol oxidase activity and shelf life of apple and grape juices. Innov Food Sci Emerg Technol. 2014;26:498–504. doi: 10.1016/j.ifset.2014.05.014. [DOI] [Google Scholar]
- Murasaki-Aliberti NDC, Da Silva RM, Gut JA, Tadini CC. Thermal inactivation of polyphenoloxidase and peroxidase in green coconut (Cocos nucifera) water. Int J Res Agric Food Sci. 2009;44(12):2662–2668. [Google Scholar]
- Noci F, Riener J, Walkling-Ribeiro M, Cronin DA, Morgan DJ, Lyng JG. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. J Food Eng. 2008;85(1):141–146. doi: 10.1016/j.jfoodeng.2007.07.011. [DOI] [Google Scholar]
- Orlowska M, Koutchma T, Kostrzynska M, Tang J, Defelice C. Evaluation of mixing flow conditions to inactivate Escherichia coli in opaque liquids using pilot-scale Taylor–Couette UV unit. J Food Eng. 2014;120:100–110. doi: 10.1016/j.jfoodeng.2013.07.020. [DOI] [Google Scholar]
- Pendyala B, Patras A, Gopisetty VVS, Sasges M, Balamurugan S. Inactivation of Bacillus and Clostridium spores in coconut water by ultraviolet light. Foodborne Pathog Dis. 2019 doi: 10.1089/fpd.2019.2623. [DOI] [PubMed] [Google Scholar]
- Prades A, Dornier M, Diop N, Pain JP. Coconut water preservation and processing: a review. Fruits. 2012;67(3):157–171. doi: 10.1051/fruits/2012009. [DOI] [Google Scholar]
- Saat M, Singh R, Sirisinghe RG, Nawawi M. Rehydration after exercise with fresh young coconut water, carbohydrate-electrolyte beverage and plain water. J Physiol Anthropol Appl Hum Sci. 2002;21(2):93–104. doi: 10.2114/jpa.21.93. [DOI] [PubMed] [Google Scholar]
- Sampedro F, Phillips J, Pan X. Use of response surface methodology to study the combined effects of UV-C and thermal processing on vegetable oxidative enzymes. Food Sci Technol. 2014;55(1):189–196. [Google Scholar]
- Stone H, Sidel J, Oliver S, Woolsey A, Singleton RC. Sensory evaluation by quantitative descriptive analysis. Descr Sens Anal Pract. 2008;28:23–34. [Google Scholar]
- Terefe NS, Buckow R, Versteeg C. Quality-related enzymes in fruit and vegetable products: effects of novel food processing technologies, part 1: high-pressure processing. Crit Rev Food Sci Nutr. 2014;54(1):24–63. doi: 10.1080/10408398.2011.566946. [DOI] [PubMed] [Google Scholar]
- Tomás-Barberán FA, Espín JC. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric. 2001;81(9):853–876. doi: 10.1002/jsfa.885. [DOI] [Google Scholar]
- Ümit Ünal M, Şener A, Şen K. Characterization of Sultaniye grape (Vitis vinifera L. cv. Sultana) polyphenol oxidase. Int J Food Sci Technol. 2007;42(9):1123–1127. doi: 10.1111/j.1365-2621.2006.01406.x. [DOI] [Google Scholar]
- Unluturk S, Atılgan MR, Baysal AH, Unluturk MS. Modeling inactivation kinetics of liquid egg white exposed to UV-C irradiation. Int J Food Microbiol. 2010;142(3):341–347. doi: 10.1016/j.ijfoodmicro.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Yen S, Sokolenko S, Manocha B, Patras A, Daynouri-Pancino F, Blondeel EJ, Sasges M, Aucoin MG. Treating cell culture media with UV irradiation against adventitious agents: minimal impact on CHO performance. Biotechnol Prog. 2014;30(5):1190–1195. doi: 10.1002/btpr.1942. [DOI] [PubMed] [Google Scholar]