Abstract
Encapsulation in packaging of food ingredients is of great interest at micro and nano levels. It is a distinct process leading to the entrapping of one substance within another material. Lipid oriented encapsulation methods are currently considered as a superior choice for encapsulation of sensitive ingredients, focusing on foods and dietary supplements of hydrophobic and hydrophilic molecules along with bioactive compounds, food ingredients supplementary systems for therapeutic purpose. Liposome and nanoliposome techniques have been widely used in food industry in nutrient enrichment and supplements. It enhances the sensory attributes and shelf life of the food product and serves as an alternative to micro encapsulation. These lipid and water oriented systems have distinguished advantages and provide higher surface area in food processing, which increases product solubility, bioavailability and permits accurate targeting of the encapsulated material to a greater extent in food and nutraceutical production. This review article focuses on nanoliposome, its preparation techniques, advantages and application of nanoliposome in food and nutraceutical process.
Keywords: Encapsulation, Liposome preparation, Nanoliposome application, Food and nutraceutical
Introduction
Encapsulation systems is an excellent choice for protecting bioactive compounds and an additive in food applications. It facilitates site specific controlled release of hydrophobic or lipophilic and hydrophilic or lipophobic molecules to a food preparation system (Toniazzo et al. 2014). New functional food components promoted, require unique encapsulation technologies with low reactive activity or hazards due to reaction of oxidized components, when it reacts with other food ingredients and thereby reducing the bioavailability of components and total acceptability of the product, leading to product deterioration (Mozafari et al. 2008; Schrooyen et al. 2001).
Microencapsulation is defined as the technology of packing solids, liquid and gaseous compounds in a form of miniature, sealed capsules, which can discharge their contents at a controlled rate under specific conditions (Pothakamury and Barbosa-Cánovas 1995; Walde and Ichikawa 2001). The actives or filled spherical shaped microcapsule materials of size ranging from the sub micro to several millimeters, act as membrane, carrier, shell and matrix system (Gibbs et al. 1999; Amoabediny et al. 2018). Essentially, the shape severely influences the encapsulating material, its structure and the ingredients from which the micro or nano capsules are made. These capsules can be carbohydrate, protein and lipid derivatives, phospholipids or synthetic polymer compounds (Gibbs et al. 1999; Mozafari et al. 2008).
In food processing industry, microencapsulation technologies are used to preserve and release the inner components, thereby sustaining the flavour, colour, texture, stability and shelf life of the food components along with its functional benefits. Encapsulation technique in many ways, offer advantages to the food processor by protecting the sensitive food components from degradation as susceptible to environmental factors; securing against nutritional and sensory characteristics loss; favoring the time release mechanisms and converting liquid compounds into easy to handle solid ingredients (Pothakamury and Barbosa-Cánovas 1995; Thompson and Singh 2006; Yang et al. 2013).
A number of encapsulation techniques have been developed on micro and nano scales. Three steps for encapsulation of food components, include (1) the material to be encapsulated around the wall (2) undesired leakage should be prevented and (3) removal of undesired materials (Mozafari et al. 2008). The encapsulated material can be produced by spray drying, spray chilling or spray cooling, fluidized bed drying or coating, extrusion, liposome techniques, centrifugal separation or extrusion, rotational suspension separation and electrostatic deposition (Gibbs et al. 1999; Akbarzadeh et al. 2013). In food colloidal systems, emulsions and its derivatives such as micro emulsion, nano emulsion, micelle and lipid particles represent one of the classes of encapsulation systems, which may be obtained by using lipids as the primary component (McClements and Rao 2011).
Encapsulation techniques are extensively applied to nutraceutical products too. They are found to be functional foods, originating from food substances, which promote beneficial effect to human health (Daud et al. 2017; Montero et al. 2019). In this context, the pharmaceutical fortificants and without scientific approved food ingredients cannot be considered as nutraceuticals. Protection of bioactive components from quality changes and deterioration were investigated extensively by adopting nano encapsulation techniques (Ray et al. 2016).
The properties of nano compounds are different from micro compounds. In nano components the ingredients are very small in size (10−9) and their surface are decreased less than their volume, where the presence of more surface area significantly improve the solubility, bioavailability, controlled releasing capability and permits accurate targeting system. It has high potential reactivity with different elastic, tensile and magnetic properties and also minimizing or avoiding the environmental factors interaction (Fathi et al. 2012; Isailović et al. 2013; Shukla et al. 2017). As far as, the application of nanotechnology in food components is concerned, the quality assurance is required for regulating the physicochemical characteristics of the nano food particles and its concentration (FSAI 2008).
In recent years, liposomes are getting more attention in the field of food application with specific targeted dietary supplements ensuring high encapsulation efficacy and solubility of bioactive compounds and food ingredients with lower toxicity owing to phospholipids content, sensitive ingredients protection against from various degradation factors like pH, light, digestive enzyme and the cells interaction. As per the USFDA (United States Food and Drug Administration) guidance, liposomes are different from emulsions, microemulsions and drug-lipid complexes. On taking this into consideration, promotion of liposomal products demands proper documentation for its chemistry, manufacture and control components, in addition to bioavailability in human pharmacokinetics and labeling (FDA 2018).
Nanoliposome is an innovative technique in encapsulation of food ingredients with enhanced functional properties and health benefits to the human, which needs to be analysed in clear perspective for safe food applications conferring potential health benefits. This article, intents to describe liposome and nanoliposome technologies, their applications in different food production systems and nutraceutical formulations and to provide an overview of the advantages of nanoliposomes in the light of compound protection, promotion and targeted delivery which will confer protection beyond the realm of existing encapsulation and delivery systems.
Liposome and nanoliposome
Nanotechnology is used in the field of food science and nutrition as a means to describe how physicochemical characteristics of food nano scale materials may affect, the physical texture, structure, chemical, nutritional value, quality and quantity of the food ingredients, influencing the absorption of nutrients and also controlled release of bioactive materials with enhanced bioavailability in biological systems. Lipid or phospholipid based nano carrier systems are the most promising encapsulation techniques, which has contributed immensely to the growing field of food and nutraceutical applications in nanotechnology. It includes lipid nanoemulsion, liposome, nanoliposome, nanocochleates, archaeosomes, solid lipid nanoparticles and nanostructured lipid carrier systems (Fathi et al. 2012; Shukla et al. 2017).
The nanoliposome is defined as, “bilayer lipid vesicles possessing and maintaining nanometric size (10−9), which ranges during storage and application”. In relation to micro encapsulation techniques viz, polymeric, chitosan and alginate based carriers, lipid based nano encapsulation possess unique distinguished advantages, including the enhanced ability to entrap material with various range of solubility, improving the possibilities of industrial production by using natural food ingredients and minimal production cost. This can be achieved by reducing the preparatory steps such as reducing the usage of chemicals, solvents and time taken for production of liposome formulations (Fathi et al. 2012; Sercombe et al. 2015).
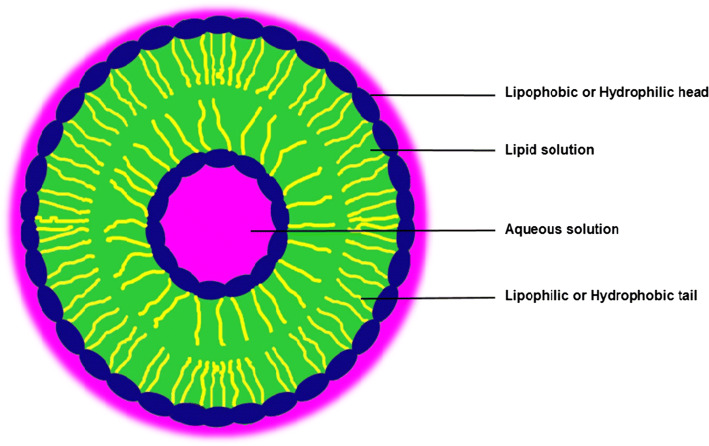
The structure of liposome as shown in Fig. 1 are artificial microscopic vesicles and are formed especially from phospholipids which are attached to water, making it amphiphilic characteristic molecules, containing hydrophilic (interaction with water or polar) and hydrophobic (interaction with lipid or non-polar) substances with different solubility, leading to diverse food and nutritional applications (Khorasani et al. 2018). The liposome materials are normally present in natural form in human food, as in breast milk (Argov et al. 2008; Paula et al. 2019).
Fig. 1.
Structure of liposome
The structural blocks of liposomes and nanoliposomes are phospholipids, which are usually combined with bilayers, double-chained lipids with large head groups such as phosphatidylserine, phosphatidylcholine, phosphatidilinositol, phosphatidylglicerol and phosphatidic acid. This unique property of phospholipids, tends to improve self-sealing characteristics in liposome and in aqueous materials, which helps to improve the number of application in various fields including agriculture, food processing, cosmetics, tissue engineering and pharmaceuticals as an encapsulation structure for the modification, protection and targeted delivery of the required bioactive compounds in cancer therapy (Sercombe et al. 2015).
Purified phosphatidylcholine has been used for entrapping the bioactive peptides from hydrolyzed sheep whey with microbial protease (Paula et al. 2019). Cholesterol is a sterol, which is mostly used in the manufacture of lipid vesicles along with phytosterols (Chen et al. 2012a, b). Cholesterol, when combined with phospholipid membranes in high concentrations at the ratio of 1:1 or 2:1 M, forms phospholipid molecule (Ibarguren et al. 2010). Cholesterol aids to increase the stability characters of the bilayer vesicles in liposomes by modifying the fluidity character of the phospholipid layer and also preventing the formation of crystallization in acyl chain of phospholipid molecule and interpreting the steric movement (Reineccius et al. 2002; Khorasani et al. 2018). Chemically, phospholipid components are most commonly found in soy and egg lecithin, as well as in other sources such as milk fat globule membrane (Thompson and Singh 2006; Jin et al. 2016). The in vitro targeting mechanism of liposome entrapped lysozyme is extended to cheese matrix regions for increasing the beneficiary microbes (Mozafari et al. 2008; Amoabediny et al. 2018). In liposomes, other compounds such as carbohydrate and protein derivatives may be present within the lipid and phospholipid structure, which helps to increase the liposome stability, targeting strategy, improving the mechanism to their vesicles stability and also leading to improved shelf life of liposomal products. The incorporated materials are sterols (cholesterol), vitamin E (antioxidant) and polymers (chitosan) in the structure of the liposome vesicles (Kim et al. 2017; Khorasani et al. 2018).
Nutritionally, most of the bioactive compounds such as unsaturated fatty acids, polyphenols, fat soluble vitamins, aromas and preservatives possess various therapeutic properties with poor stability. They are hydrophobic in nature and less in oral bioavailability. This lipophilic compounds disperse in oil in water as liposomal form and are easily absorbed in intestine and carried out by digestible lipid in the intestine and offers various advantages of protecting the molecules from undesired interaction with intestinal environment, degradation during at digestion and absorption (McClements 2012). As nanoliposomes, provide more surface area, it proportionally increases the yield of absorption and is eco-friendly. It increased the ingredient solubility, enhanced bioavailability of nutrients, improved controlled release and precise targeting, to a greater extent in biological systems (Fathi et al. 2012; Khorasani et al. 2018).
Liposomes are classified into five types based on mechanism of the intracellular compounds (Sharma and Sharma 1997) such as,
Conventional liposomes: The lipid layer of liposomes is negatively and positively charged phospholipids and cholesterol, which are attached to aqueous core. The bilayer of lipid and the aqueous penetrate through the hydrophobic and hydrophilic compounds, respectively.
pH-sensitive liposomes: The lipid composition of liposomes, destabilize when the external pH changes from a neutral or alkaline to an acidic state.
Cationic liposomes: The liposome contains one positive (cationic) charged (phospho)lipid. It interacts with negatively charged compounds or nucleic acids at the time of a simple mixing process.
Immuno liposomes: The liposomes contains antibody molecules or substances in the liposomal surface.
Long circulating liposomes: The hydrophilic layer of oligosaccharides, glycoproteins, polysaccharides and synthetic polymers are coated to the liposome surface, which helps to allow the prolonged circulation of the liposomal material in drug delivery systems.
Based on the lipid composition, method of preparation and based on its diameter, the liposomes can be classified into five types (Lasic 1998; Storm and Crommelin 1998), they are:
MLV—multilamellar vesicles—0.5–5 μm—five to twenty lipid bilayer.
SUV—small unilamellar vesicles—20–200 nm—one lipid bilayer.
LUV—large unilamellar vesicles—> 200 nm—one lipid bilayer.
GUV—giant unilamellar vesicles—> 1 μm—one lipid bilayer.
MVV—multi vesicular vesicles (MVV)—diameters > 1 μm—multi lipid bilayer.
Methods of preparation of nanoliposome
The parameters which need to be considered in the preparation of liposome or nanoliposome compounds include the physicochemical characteristics of the ingredients, nature of the medium, concentration of the encapsulated material, size, polydispersity and shelf life of the liposome.
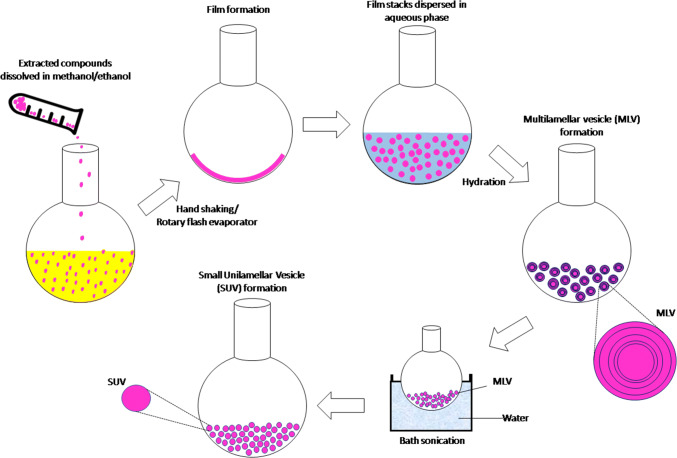
Figure 2 presents the process of preparation of nanoliposome from liposome. The standard protocol is carried out by the vaporization of chloroform and/or methanol solution of amphiphilic ingredients, such as phospholipid, sterol, carbohydrate and protein derivatives and other hydrophobic ingredients, which produces the thin film. An addition of aqueous and/or hydrophilic ingredients to the thin film layer followed by addition of an aqueous phase and/or hydrophilic compounds followed by involvement of sufficient amount of thermal energy, mechanical agitation by shaking and sonication or combination of these techniques causes the formation of bilayer sheets with the inclusion of hydrophobic compounds, which can finally be separated from the bulk materials to form liposome or nanoliposomes (Fathi et al. 2012; Jahadi et al. 2015; Khorasani et al. 2018).
Fig. 2.
The process for preparation of nanoliposome from liposome
Nanoliposome carried encapsulation system can be attained by two other mechanisms which include (1) passive loading encapsulation, which is done by the entrapment of compounds during the process of vesicle formation. Here, hydrophilic compounds are encapsulated within the aqueous phase, while hydrophobic compounds are located in the lipid bilayer of the liposomes. On the other hand, amphiphilic molecules are located in their lipid soluble region between the liposomal lipid bilayers (Daud et al. 2017; Khorasani et al. 2018). (2) Active or remote loading encapsulation, involves the entrapment of bioactive compounds into intact vesicles. Bioactive ingredients are inserted in the liposomes with the additional driving force potential achieved by ammonium sulphate (weak base) and calcium acetate (weak acid). This method was found highly effective in the formulation of higher drug or compounds to lipid ratio by the action of pumping or forcing mechanism. In addition, the product should be controlled by “inside locking of bioactive compounds” to the liposome, which would help to enhance the control releasing activity (Gubernator 2011; Sercombe et al. 2015).
The liposomes and nanoliposomes preparation methods generally involve the utilization of non-food grade or toxic solvents such as hexane, ethanol, ether solution, methanol and chloroform and detergents, such as alkyl benzene sulfonates substances, cholate, alkyl glycoside and triton X-100 for the solubilization of hydrophillic and/or hydrophobic ingredients (Lakkis 2007; Jahadi et al. 2015).
The laboratory and industrial scale methods for the preparing of nanoliposome are given in Table 1. The nanoliposomes have numerous beneficial characters in the preparation, utilization and delivery, with few limiting factors such as poor encapsulation efficiency, lack of processing parameters in continuous industrial production and stability, extreme pH status (highly acidic), high cost of food ingredients, usage of non-food grade solvents, detergents, applying of high force sonication, homogenization and pressure (Khorasani et al. 2018).
Table 1.
Methods for the preparation of nanoliposome in food and nutraceutical ingredients
| Preparation techniques | Food and nutraceutical ingredients | References |
|---|---|---|
| Passive loading techniques | ||
| Mechanical dispersion method | ||
| Lipid film hydration | Hemoglobin | Li et al. (2005) |
| Micro emulsification | Nisoldipine | Nekkanti et al. (2016) |
| Sonication | Resveratrol | Isailović et al. (2013) |
| Membrane extrusion | Hemoglobin | Li et al. (2005) |
| Dried reconstituted vesicles | Ketoprofen | Li and Deng (2004) |
| Solvent dispersion method | ||
| Ethanol or ether injection | Salidroside | Fang and Bhandari (2010) |
| Double emulsion | Flurbiprofen | Liu et al. (2012) |
| Reverse phase evaporation vesicles | Bovine serum albumin | Ko and Bickel (2012) |
| Detergent removal technique | ||
| Dialysis | Poly(γ-benzyl L glutamate) | Marsden et al. (2010) |
| Gel permeation chromatography | Fisetin (3,3′,4′,7-tetrahydroxyflavone) | Mignet et al. (2012) |
| Diffusion | Resveratrol | Isailović et al. (2013) |
| Active loading techniques | ||
| Citrate method | Doxorubicin citrate | Swenson et al. (2001) |
| Ammonium sulfate method | Brucine | Chen et al. (2010) |
| Ionophore generated pH gradient method | Ciprofloxacin | Johnston et al. (2006) |
| EDTA ion grading method | Topotecan | Yang et al. (2012) |
Techniques have been developed to reduce or completely avoid the usage of organic solvents to the liposome formulation, which includes microfluidics techniques, rapid expansion of supercritical solution, supercritical reverse phase evaporation and several dense gas processes (Tsai and Rizvi 2016). The industrialization of this process requires skilled technical knowhow and high investment. To overcome these problems, an improved heating method has been successfully employed in the making of liposome and nanoliposomes in one-step single equipment, with the absence of toxic solvents and preparation time of less than an hour (Jahadi et al. 2015). This method is economical, capable of superior mono dispersity and storage stability for manufacturing bioactive carriers using a simple protocol, and it can be easily adopted in small industrial scales. Another method is the micro fluidization techniques, which are also adopted for the production of liposome without toxic solvents, but it has some disadvantages such as loss of encapsulated material, contamination and difficulty for scale up (Zheng et al. 1999).
Application of liposomes
Interest in liposomes have been greatly aroused in food, pharmaceutical, agriculture and bioprocessing industries, due to its high solubility improvement and favourable characteristics. Examples for liposome techniques, which are applied in food and nutraceutical products are given in Table 2.
Table 2.
Example for application of liposome techniques applied in food and nutraceutical products
| Liposomal ingredients | Benefits |
|---|---|
| Lipase and protease enzymes | Protect the casein from early hydrolysis in cheese making (Pezeshki et al. 2011) |
| Flavourzyme | Protect the casein from early proteolysis and inhibit the pre maturation of curd (Kilcawley et al. 2002; Benech et al. 2003; Jahadi et al. 2015) |
| Neutrase | Improved the cheese ripening process and reduce the economic losses by usage of enzyme concentration (Kirby, Brooker and Law 1987) |
| Vitamin D | Increases the Vitamin D recovery in cheese (Thompson and Singh 2006) |
| Nisin | Inhibit the growth of lactococci cells (Benech et al. 2003; Laridi et al. 2003) |
| Lysozyme | Inhibited the Listeria monocytogenes growth (Were et al. 2004) |
| Curry plants essential oil | Antibacterial activity against Bacillus cereus (Cui et al. 2017) |
| Poly unsaturated fatty acids from fish oil | Decreases the off flavour (Ghorbanzade et al. 2017) |
| Resveratrol | Improved antioxidant activity and minimize the cytotoxicity of resveratrol (Caddeo et al. 2008; Isailović et al. 2013) |
| Tea polyphenol | Enhancing bioavailability (Lu et al. 2011) |
| Beta-carotene | Increases the chemical stability and bioavailability (Toniazzo et al. 2014; Hudiyanti et al. 2018) |
| Vitamin C with medium chain fatty acids (MCFA) | Active dietary supplements and improved antioxidant activity (Yang et al. 2013) |
| Retinal | Reduces the degradation of vitamin A and enhanced the retinol stability (Shukla et al. 2017) |
| Iron | Improved bioavailability (Muñoz and Humeres 2012; Waldvogel-Abramowski et al. 2014) |
| Curcumin | Improved stability and bioavailability (Basniwal et al. 2011; Hasan et al. 2016) |
| Hemoglobin | Improved oxygen affinity and capacity (Li et al. 2005) |
| Peptides | antimicrobial activity against Listeria monocytogenes and Escherichia coli (Cantor et al. 2019) |
Food processing
The liposomal applications have been studied extensively in dairy products (Laridi et al. 2003), in terms of stability of the food components against degradation (Hudiyanti et al. 2018) and enhanced delivery action of the antimicrobial peptides (Paula et al. 2019). In cheese-making, liposome encapsulated enzymes reduced the cheese ripening time up to 50%. The encapsulated sources can enhance the economic profitability for the producers, by reducing the ripening time of cheese and cheddar, compared to conventional methods, which require extensive time of more than one year to maximize the acceptability (Law and King 1985).
In Iranian white brined cheese making, fast proteolysis activity in ripening process, may affect the casein reaction ability, hastening the release of enzymes into the whey and thereby decreasing the loss of cheese components from curd to whey (Pezeshki et al. 2011). In contrast, encapsulation of lipase and protease enzymes, has gained attention in dairy industry (Walde and Ichikawa 2001) as it protects the casein from early hydrolysis in cheese production.
In cheese processing, the enzyme encapsulated by traditional methods to accelerate cheese ripening has an edge over the chemical or detergent induced liposomes as they show low encapsulation efficiency (Jahadi et al. 2015). Flavourzyme is an enzyme, obtained from Aspergillus oryzae. This enzyme is used in the production of cheese, to accelerate the cheese ripening process with the aim of reducing bitter taste and enhancing flavour (Kilcawley et al. 2002). The production of enzyme encapsulated liposome for cheese ripening is produced by heating method. The most favourable formulation of liposomal Flavourzyme was reported to be 4.5% lecithin in 45 °C temperature, 5% of Flavourzyme/lecithin ratio at 30 min stirring time and pH 6.0. The encapsulation efficiency achieved was 9.96% having spherical shaped diameter of 189 nm in liposomal Flavourzyme (Jahadi et al. 2015). The liposomal Flavourzyme did not affect the cheese yield as the encapsulation may protect casein from early proteolysis and also inhibit the pre-maturation of curd (Jahadi et al. 2015).
In baked food products, the use of sweeteners produce undesirable caramelization during the application of heat, which may affect the flavour and nutrient content of the product on consumption. This can be minimized by nanoliposome applications leading to desirable liposomal flavor and nutrient ingredients to the product (Desai and Park 2005).
The study on the antimicrobial activity for the liposomal encapsulated nisin released their active components on 3.8 μg/ml, after 6 h by the incubation in cheddar cheese. This was due to the starter bacteria for partial degradation of nisin active components and immobilization characters in lactococci cell walls. However, the presence of acidic conditions from the starter bacteria may result in increasing encapsulated nisin stability and decreasing its release rate (Laridi et al. 2003). In contrast, Listeria monocytogenes were inhibited by the combined application of low temperature and nanovesicle-encapsulated nisin in whole fat and skim milk (Were et al. 2004).
Curry plant essential oil is a natural preservative and its application is generally restricted owing to its volatile nature and chemical instability in air, light, moisture and high temperature. An optimized curry plant essential oil liposome in the concentration of 5 mg/ml of an average particle size—196.6 nm with a poly dispersity index—0.218, encapsulation efficiency—56.34% and zeta potential—231.1 mV, showed antibacterial activity against Bacillus cereus in rice flour and it may be useful as a natural preservative in the field of food preservation (Cui et al. 2017).
Poly unsaturated fatty acids (PUFA) confer protective effects against cardio vascular diseases, cancer, auto immune disorders and help to improve functioning of the brain and retina. Hence, inclusion of the required amount of PUFA in our diet is important. Marine sources especially fish are rich sources of PUFA (Ghorbanzade et al. 2017). The PUFA incorporated or fortified food with fish oil renders pronounced fish oil flavour. To improve the sensory characteristics of fish oil, the fish oil may be encapsulated by liposome technology. Ghorbanzade et al. (2017) developed nanoencapsulated fish oil by nanoliposome techniques, followed by fortifying the same to yogurt. The overall, results revealed that adding nanoencapsulated fish oil to yogurt gave closer characteristics to unencapsulated fish oil yogurt in terms of sensory parameters.
Antioxidants significantly contributes to human health as well as reducing the degradation of nutrients in foods through retarding the oxidation process (Mozafari et al. 2008). The usage of antioxidants is a trending concept in the food industry to alter the saturated fat into unsaturated fats in their oxidation process (Pothakamury and Barbosa-Cánovas 1995; Hunter 2006). Lipids in food products such as spreads, margarines, deep fried chips and salad dressings, easily deteriorate as these water–lipid phase emulsions enhances oxidative degradation of oxygen and metal ions in food materials (Caddeo et al. 2008; Pothakamury and Barbosa-Cánovas 1995). These problems can be resolved by the addition of antioxidants into lipid vesicles of liposomes to improve the nutritional value and bioavailability.
Beta-carotene (provitamin A) is important for preventing vitamin A deficiency and confers protection against lung cancer and cardiovascular disease (Krinsky and Johnson 2005). In liquid food products, beta carotene has a low chemical stability and low bioavailability due to its hydrophobicity. Introducing liposome techniques may improve the solubility of beta carotene by incorporation of dried phospholipid particles using spray drying (Toniazzo et al. 2014) and active loading techniques (Hudiyanti et al. 2018) in sesame based liposome ingredients.
Nutraceuticals
Suitable dietary supplementary carriers are needed to serve as a delivery system for the compounds to a specific targeted area of the cells, which also requires protecting itself and enhancing bioavailability. This is a challenge for the formulation of suitable encapsulated nutraceutical materials, which possess carrier and delivery properties of nutraceuticals. Liposome can bring significant modification in nano carrier products, by tailored product enabling system, with vide variety of application in target oriented dietary based products to the biological site inside (as incorporation of antibodies) or outside (based on particle or cell size) a human body (Hasan et al. 2016). The micro encapsulated molecules affect the absorption of the targeted delivery sites and does not completely survive the gastric intestinal system due to its physical, chemical and biological properties. The nanoliposome techniques adopted substances, such as low molecular weight compounds, bioactive compounds, proteins, peptides and nucleic acids slowly release their active components for a prolonged time to the target region, in both passive and active targeting delivery pathway for improving the efficacy of the solubility and absorption due to presence of more surface area in the nanoliposome. Therefore, the quantity of the substances are reduced, which permits decreasing the toxicity of the substance and side effects. Hence, the healthy cells are not affected, alternatively unhealthy or damaged cells are affected (Kumar et al. 2010; Tehrany et al. 2012).
The complex nanoliposome products are made from a hydrophilic component such as vitamin C and hydrophobic component as medium chain fatty acids (MCFA) with an addition of sucrose as carbohydrate source. The MCFA and vitamin C complex nanoliposomes are produced from freeze dried complex nanoliposomes, which exhibited high encapsulation efficiency in MCFAs of 44.26 ± 3.34%, diameter of 110.4 ± 7.28 nm and better storage stability under the condition of 4 °C for 60 days. The use of this nanoliposomes in commercial liposomes in food and dietary supplements is suggested by Yang et al. (2013).
Curcumin is a natural polyphenolic compound obtained from the rhizome turmeric. It is widely used in traditional medicine for its antioxidant, anti-inflammatory, antimicrobial and anticancer properties. Curcumin delivery to the target parts by conventional encapsulation techniques has low bioavailability, due to its poor solubility in aqueous phase and low stability against base pH conditions of the digestive systems (Basniwal et al. 2011). In pharmaceutical applications, curcumin encapsulated with salmon purified phospholipid and coating with chitosan (Hasan et al. 2016) yields a lipid based nanostructure with chitosan, which has improved stability and bioavailability of curcumin by long lasting residence time at the site of absorption (Chen et al. 2012a, b). The stability of the curcumin liposomes on milk fat globule membrane (MFGM) phospholipids and soybean lecithin were noted to have slightly higher stability in MFGM liposomes than in soybean lecithin formulated liposome, under the different environmental conditions such as Fe3+, light, oxygen, temperature and relative humidity (Jin et al. 2016).
Tea contains high polyphenol content, which is a sensitive compound towards light and oxygen. This compound present in green tea possess reputable biological and pharmacological actions and is widely used in food products and provides health benefits such as antioxidant properties by donating hydrogen atom, acceptance of free radicals, intruding oxidation chain reactions, chelating of metal ions and lowering blood lipids level (Jomova and Valko 2011). The tea polyphenol liposomal product was prepared by thin film ultrasonic dispersion method for enhancing bioavailability of the components. The optimized parameters are, tea polyphenol to lecithin—0.125:1, lecithin to cholesterol—4:1, phosphate buffered saline pH—6.62, ultra sonication time—3.5 min. The tea polyphenol liposome entrapment efficiency was 60.09 ± 0.69%, with mean size 160.4 nm and zeta potential value—67.2 (Lu et al. 2011).
An encapsulated hemoglobin liposome has a better feasibility of oxygen carrying in blood substitute (Yuan et al. 2013). This liposome is found in a lipid film compound as 5% dimyristoyl phosphatidylcholine, 42% dimyristoyl phosphatidyl glycerol, 40% cholesterol, 2% alpha-tocopherol, 10% distearoyl glycerol phosphatidyl ethanolamine and 1% Kþ ionophore. Microencapsulation was extruded (25 times extrusion) into the plain hemoglobin solution (30 g/dl) and the mixed solution contained 1 mg/ml of actin and 3 g/dl of hemoglobin through polycarbonate membranes with 400 and 600 nm pore sizes, respectively. Thin disk shaped liposome as actin and hemoglobin solution conformed to sizes ranging from 136 to 140 nm and spherical shaped plain hemoglobin liposome conforming in sizes from 115 to 142 nm. Both of these encapsulated hemoglobin materials had an encapsulation efficiency of 30% with similar oxygen affinity and capacity (Li et al. 2005).
Iron performs numerous physiological functions in human systems especially in erythropoiesis, oxidative metabolism and cellular immune responses (Muñoz and Humeres 2012; Waldvogel-Abramowski et al. 2014). Micronized iron encapsulation carried out by using liposome techniques provides newer chances for improving the intake of iron through oral therapy. It is related with the lowest exposure to various gastric contents and digestive enzymes, lesser interface activities with food components and is supplementary with targeted cell delivery mechanism.
Cantor et al. (2019) studied the antibiotic activity of peptide based nanoliposome against Listeria monocytogenes and Escherichia coli by two approaches. One approach involved the structural modification of antimicrobial peptides and the other approach involved the nano-vehiculisation of the modified peptides into polymer-coated liposomes. The results showed that the degree of hydrophilic modification in the peptide leads to different characteristics of amphipathicity and subsequently to different physicochemical behavior of microbes.
Conclusion
Among the various encapsulation system, nanoliposomes are important suitable system for entrapment of sensitive food ingredients, owing to the hydrophilic and hydrophobic nature for safe food consumption and nutraceuticals applications, which confers potential health and functional benefits. Compared to liposome, nanoliposomes have a lower surface area, which helps to maintain the nanometric size during storage and application in food and drug systems. From the point of safety concerns, nanoliposomes are mostly produced using toxic solvents or high shear force treatments on large scale, which further needs research to employ solvent free and mild procedures such as Mozafari method and micro fluidization techniques. In future, biocompatibility and biodegradability characteristics of nanoliposomes are active preservative ingredients against harmful microbial pathogens and food toxins in food processing systems. Nevertheless, the interaction and effectiveness of the nano liposomal ingredients of solid, semi-solid and liquid material and its interaction with human system is not much discovered. However, approaching some novel experiences in in vitro, in vivo and in silico studies in nanoliposome is further needed for new basic research, which begins from aqueous and lipid biotechnology, that will confer better food processing, health benefits and human uses.
Acknowledgements
Thirukkumar Subramani would like to thank the University Grants Commission (UGC), Government of India for the support of Junior Research Fellowship for doctorate study.
Author contributions
Thirukkumar contributed to the design and preparation of this manuscript for his study and Hemalatha has mentored the student and revised the manuscript.
Funding
Funding was provided by University Grants Commission (Grant No. 1543/(OBC)(NET-DEC. 2015)).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akbarzadeh A, Rezaei-sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8:1–9. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoabediny G, Haghiralsadat F, Naderinezhad S, et al. Overview of preparation methods of polymeric and lipid-based (niosome, solid lipid, liposome) nanoparticles: a comprehensive review. Int J Polym Mater Polym Biomater. 2018;67:383–400. doi: 10.1080/0014037.2017.1332623. [DOI] [Google Scholar]
- Argov N, Danielle G, German JB. Milk fat globule structure and function: nanoscience comes to milk production. Trends Food Sci Technol. 2008;19:617–623. doi: 10.1016/j.tifs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basniwal RK, Buttar HS, Jain VK, Jain N. Curcumin nanoparticles: preparation, characterization, and antimicrobial study. J Agric Food Chem. 2011;59:2056–2061. doi: 10.1021/jf104402t. [DOI] [PubMed] [Google Scholar]
- Benech R, Kheadr EE, Lacroix C, Fliss I. Impact of nisin producing culture and liposome-encapsulated nisin on ripening of Lactobacillus added-Cheddar cheese. J Dairy Sci. 2003;86:1895–1909. doi: 10.3168/jds.S0022-0302(03)73776-X. [DOI] [PubMed] [Google Scholar]
- Caddeo C, Teskaˇ K, Sinico C, Kristl J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int J Pharm. 2008;363:183–191. doi: 10.1016/j.ijpharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Cantor S, Vargas L, Rojas A, Yarce CJ, Salamanca CH, Onate-Garzon J. Evaluation of the antimicrobial activity of cationic peptides loaded in surface-modified nanoliposomes against foodborne bacteria. Int J Mol Sci. 2019;20(3):1–15. doi: 10.3390/ijms20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lin A, Chen Z, et al. Ammonium sulfate gradient loading of brucine into liposomes: effect of phospholipid composition on entrapment efficiency and physicochemical properties in vitro. Drug Dev Ind Pharm. 2010;36:245–253. doi: 10.3109/03639040903099736. [DOI] [PubMed] [Google Scholar]
- Chen D, Love KT, Chen Y, et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- Chen H, Wu J, Sun M, et al. N-trimethyl chitosan chloride-coated liposomes for the oral delivery of curcumin. J Liposome Res. 2012;22:100–109. doi: 10.3109/08982104.2011.621127. [DOI] [PubMed] [Google Scholar]
- Cui H, Li W, Lin L. Antibacterial activity of liposome containing curry plant essential oil against Bacillus cereusin rice. J Food Saf. 2017;37:3–7. doi: 10.1111/jfs.12302. [DOI] [Google Scholar]
- Daud M, Jalil JA, Madieha I et al (2017) “Unsafe” nutraceuticals products on the internet : the need for stricter regulation in Malaysia. In: Proceedings of the 5th international conference on information technology for cyber and IT service management at Bali, Indonesia
- Desai KGH, Park HJ. Recent developments in microencapsulation of food ingredients. Dry Technol. 2005;23(7):1361–1394. doi: 10.1081/DRT-200063478. [DOI] [Google Scholar]
- Fang Z, Bhandari B. Encapsulation of polyphenols—a review. Trends Food Sci Technol. 2010;21:510–523. doi: 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci Technol. 2012;23:13–27. doi: 10.1016/j.tifs.2011.08.003. [DOI] [Google Scholar]
- Food and Drug Administration (2018) Liposome drug products—guidance for industry. https://www.fda.gov/media/70837/. Accessed 24 May 2019
- Food Safety Authority of Ireland (2008) The relevance for food safety of applications of nanotechnology in the food and feed industries. http://www.fsai.ie/assets/0/86/204/b81b142b-9ef7-414c-96143a969835b392.pdf. Accessed 24 May 2019
- Ghorbanzade T, Jafari SM, Akhavan S, Hadavi R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017;216:146–152. doi: 10.1016/j.foodchem.2016.08.022. [DOI] [PubMed] [Google Scholar]
- Gibbs F, Kermasha S, Alli I, Mulligan CN. Encapsulation in the food industry: a review. Int J Food Sci Nutr. 1999;50:213–224. doi: 10.1007/s11745-006-5049-y. [DOI] [PubMed] [Google Scholar]
- Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin Drug Deliv. 2011;8:565–580. doi: 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- Hasan M, Ben Messaoud G, Michaux F, et al. Chitosan-coated liposomes encapsulating curcumin: study of lipid-polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016;6:45290–45304. doi: 10.1039/c6ra05574e. [DOI] [Google Scholar]
- Hudiyanti D, Fawrin H, Siahaan P (2018) Simultant encapsulation of vitamin C and beta-carotene in sesame (Sesamum indicum l.) liposomes. In: IOP conference series: materials science and engineering, vol 349, no 1, pp 0129014. IOP Publishing
- Hunter JE. Dietary trans fatty acids: review of recent human studies and food industry responses. Lipids. 2006;41:967–992. doi: 10.1007/s11745-006-5049-y. [DOI] [PubMed] [Google Scholar]
- Ibarguren M, Alonso A, Tenchov BG, Goñi FM. Quantitation of cholesterol incorporation into extruded lipid bilayers. Biochim Biophys Acta Biomembr. 2010;1798:1735–1738. doi: 10.1016/j.bbamem.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Isailović BD, Kostić IT, Zvonar A, et al. Resveratrol loaded liposomes produced by different techniques. Innov Food Sci Emerg Technol. 2013;19:181–189. doi: 10.1016/j.ifset.2013.03.006. [DOI] [Google Scholar]
- Jahadi M, Khosravi-Darani K, Ehsani MR, et al. The encapsulation of flavourzyme in nanoliposome by heating method. J Food Sci Technol. 2015;52:2063–2072. doi: 10.1007/s13197-013-1243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Lu Q, Jiang J. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J Dairy Sci. 2016;99:1–11. doi: 10.3168/jds.2015-10391. [DOI] [PubMed] [Google Scholar]
- Johnston MJ, Semple SC, Klimuk SK, et al. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta Biomembr. 2006;1758(1):55–64. doi: 10.1016/j.bbamem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Khorasani S, Danaei M, Mozafari MR. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci Technol. 2018;79:106–115. doi: 10.1016/j.tifs.2018.07.009. [DOI] [Google Scholar]
- Kilcawley KN, Wilkinson MG, Fox PF. Determination of key enzyme activities in commercial peptidase and lipase preparations from microbial or animal sources. Enzyme Microb Technol. 2002;31:310–320. doi: 10.1016/S0141-0229(02)00136-9. [DOI] [Google Scholar]
- Kim CH, Lee SG, Kang MJ, et al. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J Pharm Investig. 2017;47:203–227. doi: 10.1007/s40005-017-0329-5. [DOI] [Google Scholar]
- Kirby CJ, Brooker BE, Law BA. Accelerated ripening of cheese using liposome-encapsulated enzyme. Int J Food Sci Technol. 1987;22:355–375. doi: 10.1111/j.1365-2621.1987.tb00499.x. [DOI] [Google Scholar]
- Ko YT, Bickel U. Liposome-encapsulated polyethylenimine/oligonucleotide polyplexes prepared by reverse-phase evaporation technique. AAPS PharmSciTech. 2012;13(2):373–378. doi: 10.1208/s12249-012-9757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Kumar A, Badde S, Kamble R, Pokharkar VB. Development and characterization of liposomal drug delivery system for nimesulide. Int J Pharm Pharm Sci. 2010;2(4):87–89. [Google Scholar]
- Lakkis JM. Encapsulation and controlled release technologies in food systems. New York: Blackwell Pub; 2007. [Google Scholar]
- Laridi R, Kheadr EE, Benech R, et al. Liposome encapsulated nisin Z: optimization, stability and release during milk fermentation. Int Dairy J. 2003;13:325–336. doi: 10.1016/S0958-6946(02)00194-2. [DOI] [Google Scholar]
- Lasic DD. Novel applications of liposomes. Trends Biotechnol. 1998;16(7):307–321. doi: 10.1016/S0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- Law BA, King JS. Use of liposomes for proteinase addition to Cheddar cheese. J Dairy Res. 1985;52(1):183–188. doi: 10.1017/S0022029900024006. [DOI] [Google Scholar]
- Li C, Deng Y. A novel method for the preparation of liposomes: freeze drying of monophase solutions. J Pharm Sci. 2004;93(6):1403–1414. doi: 10.1002/jps.20055. [DOI] [PubMed] [Google Scholar]
- Li S, Nickels J, Palmer AF. Liposome-encapsulated actin-hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials. 2005;26:3759–3769. doi: 10.1016/j.biomaterials.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Liu M, Chen L, Zhao Y, et al. Physicochemical and engineering aspects preparation, characterization and properties of liposome-loaded polycaprolactone microspheres as a drug delivery system. Colloids Surf A. 2012;395:131–136. doi: 10.1016/j.colsurfa.2011.12.017. [DOI] [Google Scholar]
- Lu Q, Li DC, Jiang JG. Preparation of a tea polyphenol nanoliposome system and its physicochemical properties. J Agric Food Chem. 2011;59:13004–13011. doi: 10.1021/jf203194w. [DOI] [PubMed] [Google Scholar]
- Marsden HR, Quer CB, Sanchez EY, et al. Detergent-aided polymersome preparation. Biomacromol. 2010;11(4):833–838. doi: 10.1021/bm1001763. [DOI] [PubMed] [Google Scholar]
- McClements DJ. Nanoemulsion versus microemulsion: terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. [DOI] [Google Scholar]
- McClements DJ, Rao J. Food-grade nanoemulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit Rev Food Sci Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- Mignet N, Seguin J, Romano MR, et al. Development of a liposomal formulation of the natural flavonoid fisetin. Int J Pharm. 2012;423:69–76. doi: 10.1016/j.ijpharm.2011.04.066. [DOI] [PubMed] [Google Scholar]
- Montero P, Mosquera M, Marín-peñalver D, et al. Changes in structural integrity of sodium caseinate films by the addition of nanoliposomes encapsulating an active shrimp peptide fraction. J Food Eng. 2019;244:47–54. doi: 10.1016/j.jfoodeng.2018.09.024. [DOI] [Google Scholar]
- Mozafari MR, Johnson C, Hatziantoniou S, Demetzos C. Nanoliposomes and their applications in food nanotechnology. J Liposome Res. 2008;18:309–327. doi: 10.1080/08982100802465941. [DOI] [PubMed] [Google Scholar]
- Muñoz P, Humeres A. Iron deficiency on neuronal function. Biometals. 2012;25:825–835. doi: 10.1007/s10534-012-9550-x. [DOI] [PubMed] [Google Scholar]
- Nekkanti V, Rueda J, Wang Z, Betageri GV. Comparative evaluation of proliposomes and self micro-emulsifying drug delivery system for improved oral bioavailability of nisoldipine. Int J Pharm. 2016;505:79–88. doi: 10.1016/j.ijpharm.2016.03.065. [DOI] [PubMed] [Google Scholar]
- Paula A, Corrêa F, Bertolini D, et al. Characterization of nanoliposomes containing bioactive peptides obtained from sheep whey hydrolysates. LWT—Food Sci Technol. 2019;101:107–112. doi: 10.1016/j.lwt.2018.11.036. [DOI] [Google Scholar]
- Pezeshki A, Hesari J, Ahmadi ZA, Ghambarzadeh B. Influence of withania coagulans protease as a vegetable rennet on proteolysis of Iranian UF white cheese. J Agric Sci Technol. 2011;13(4):567–576. [Google Scholar]
- Pothakamury UR, Barbosa-Cánovas GV. Fundamental aspects of controlled release in foods. Trends Food Sci Technol. 1995;6:397–406. doi: 10.1016/S0924-2244(00)89218-3. [DOI] [Google Scholar]
- Ray S, Raychaudhuri U, Chakraborty R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016;13:76–83. doi: 10.1016/j.fbio.2015.12.009. [DOI] [Google Scholar]
- Reineccius TA, Reineccius GA, Peppard TL. Encapsulation of flavors using cyclodextrins: comparison of flavor retention in alpha, beta, and gamma types. J Food Sci. 2002;67:3271–3279. doi: 10.1111/j.1365-2621.2002.tb09577.x. [DOI] [Google Scholar]
- Schrooyen PMM, van der Meer R, De Kruif CG. Microencapsulation: its application in nutrition. Proc Nutr Soc. 2001;60(4):475–479. doi: 10.1079/pns2001112. [DOI] [PubMed] [Google Scholar]
- Sercombe L, Veerati T, Moheimani F, et al. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:1–13. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154(2):123–140. doi: 10.1016/S0378-5173(97)00135-X. [DOI] [Google Scholar]
- Shukla S, Haldorai Y, Hwang SK, et al. Current demands for food-approved liposome nanoparticles in food and safety sector. Front Microbiol. 2017;8:1–14. doi: 10.3389/fmicb.2017.02398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm G, Crommelin DJA. Liposomes: quo vadis? Pharm Sci Technol Today. 1998;1:19–31. doi: 10.1016/S1461-5347(98)00007-8. [DOI] [Google Scholar]
- Swenson CE, Perkins WR, Roberts P, Janoff AS. Liposome technology and the development of Myocet™ (liposomal doxorubicin citrate) Breast. 2001;10:1–7. doi: 10.1054/brst.2000.0201. [DOI] [PubMed] [Google Scholar]
- Tehrany EA, Kahn CJ, Baravian C, et al. Elaboration and characterization of nanoliposome made of soya; rapeseed and salmon lecithins: application to cell culture. Colloids Surf B. 2012;95:75–81. doi: 10.1016/j.colsurfb.2012.02.024. [DOI] [PubMed] [Google Scholar]
- Thompson AK, Singh H. Preparation of liposomes from milk fat globule membrane phospholipids using a microfluidizer. J Dairy Sci. 2006;89(2):410–419. doi: 10.3168/jds.s0022-0302(06)72105-1. [DOI] [PubMed] [Google Scholar]
- Toniazzo T, Berbel IF, Cho S, et al. β-carotene-loaded liposome dispersions stabilized with xanthan and guar gums: physico-chemical stability and feasibility of application in yogurt. LWT—Food Sci Technol. 2014;59(2):1265–1273. doi: 10.1016/j.lwt.2014.05.021. [DOI] [Google Scholar]
- Tsai WC, Rizvi SSH. Liposomal microencapsulation using the conventional methods and novel supercritical fluid processes. Trends Food Sci Technol. 2016;55:61–71. doi: 10.1016/j.tifs.2016.06.012. [DOI] [Google Scholar]
- Walde P, Ichikawa S. Enzymes inside lipid vesicles: preparation, reactivity and applications. Biomol Eng. 2001;18:143–177. doi: 10.1016/S1389-0344(01)00088-0. [DOI] [PubMed] [Google Scholar]
- Waldvogel-Abramowski S, Waeber G, Gassner C, et al. Physiology of iron metabolism. Transfus Med Hemother. 2014;41:213–221. doi: 10.1159/000362888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Were LM, Bruce B, Davidson PM, Weiss J. Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J Food Prot. 2004;67(5):922–927. doi: 10.4315/0362-028x-67.5.922. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ma Y, Wang S. A novel method to load topotecan into liposomes driven by a transmembrane NH4EDTA gradient. Eur J Pharm Biopharm. 2012;80:332–339. doi: 10.1016/j.ejpb.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Yang SB, Liu CM, Liu W, et al. Preparation and characterization of nanoliposomes entrapping medium-chain fatty acids and vitamin C by lyophilization. Int J Mol Sci. 2013;14:19763–19773. doi: 10.3390/ijms141019763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Geng L, Ge L, et al. Effect of iron liposomes on anemia of inflammation. Int J Pharm. 2013;454:82–89. doi: 10.1016/j.ijpharm.2013.06.078. [DOI] [PubMed] [Google Scholar]
- Zheng S, Alkan-Onyuksel H, Beissinger RL, Wasan DT. Liposome microencapsulations without using any organic solvent. J Dispers Sci Technol. 1999;20:1189–1203. doi: 10.1080/01932699908943844. [DOI] [Google Scholar]