Abstract
Recent data suggest that the transcription factor Zfp148 represses activation of the tumor suppressor p53 in mice and that therapeutic targeting of the human orthologue ZNF148 could activate the p53 pathway without causing detrimental side effects. We have previously shown that Zfp148 deficiency promotes p53-dependent proliferation arrest of mouse embryonic fibroblasts (MEFs), but the underlying mechanism is not clear. Here, we showed that Zfp148 deficiency downregulated cell cycle genes in MEFs in a p53-dependent manner. Proliferation arrest of Zfp148-deficient cells required increased expression of ARF, a potent activator of the p53 pathway. Chromatin immunoprecipitation showed that Zfp148 bound to the ARF promoter, suggesting that Zfp148 represses ARF transcription. However, Zfp148 preferentially bound to promoters of other transcription factors, indicating that deletion of Zfp148 may have pleiotropic effects that activate ARF and p53 indirectly. In line with this, we found no evidence of genetic interaction between TP53 and ZNF148 in CRISPR and siRNA screen data from hundreds of human cancer cell lines. We conclude that Zfp148 deficiency, by increasing ARF transcription, downregulates cell cycle genes and cell proliferation in a p53-dependent manner. However, the lack of genetic interaction between ZNF148 and TP53 in human cancer cells suggests that therapeutic targeting of ZNF148 may not increase p53 activity in humans.
Subject terms: Cell division, Computational biology and bioinformatics, Senescence
Introduction
Activation of the tumor suppressor p53 may have beneficial effects on tumors with wild-type p531. The first drugs targeting the p53 repressor murine double minute 2 (MDM2) have entered clinical trials, but their clinical benefit is still under evaluation2. Because knockout of Mdm2 in mice causes widespread and lethal activation of p53 in normal tissues3, efficient pharmacological inhibition of MDM2 may have adverse effects. Thus, identifying alternative ways to activate the p53 pathway with less adverse effects is warranted.
Zinc finger protein 148 (Zfp148, Zbp-89, BFCOL, BERF1, htβ) is a transcription factor that binds to GC-rich DNA sequences, thus activating or repressing transcription of target genes4–11. Earlier studies have shown that Zfp148 is a potent repressor of p53 activity in mice. Firstly, deletion of Zfp148 caused p53-dependent proliferation arrest of cultured mouse embryonic fibroblasts (MEFs) and prenatal lung tissue that was rescued by reducing oxidative stress12. Moreover, deletion of one copy of Zfp148 in the ApcMin/+ model of intestinal adenomas reduced tumor numbers and increased survival by increasing p53 activity13. In line with this, conditional deletion of one or both alleles of Zfp148 in the gut epithelium of ApcFL/+ mice reduced tumor formation through a mechanism involving β-catenin14. Finally, deletion of one copy of Zfp148 reduced proliferation of macrophages and atherosclerosis in Apoe−/− mice by increasing p53 activity15. These results indicate that deletion of one copy of Zfp148 is sufficient to induce therapeutically meaningful p53 activation. This is important because mice lacking one copy of Zfp148 have a normal life span and appear to be healthy. Therapeutic targeting of Zfp148 could therefore protect against cancer or atherosclerosis by increasing p53 activity without causing detrimental side effects.
The mechanism leading to increased p53 activity is not well understood. Zfp148 interacts physically with p5316 raising the possibility that Zfp148 regulates p53 by protein–protein interaction. However, indirect mechanisms are equally possible. Here, we investigate mechanisms behind cell cycle arrest of Zfp148-deficient (Zfp148gt/gt) MEFs by using global gene expression analysis, targeted inactivation of Cdkn2a genes, genome-wide chromatin immunoprecipitation, and public CRISPR and siRNA screen data.
Results
Zfp148 deficiency downregulates expression of E2F-responsive cell cycle genes
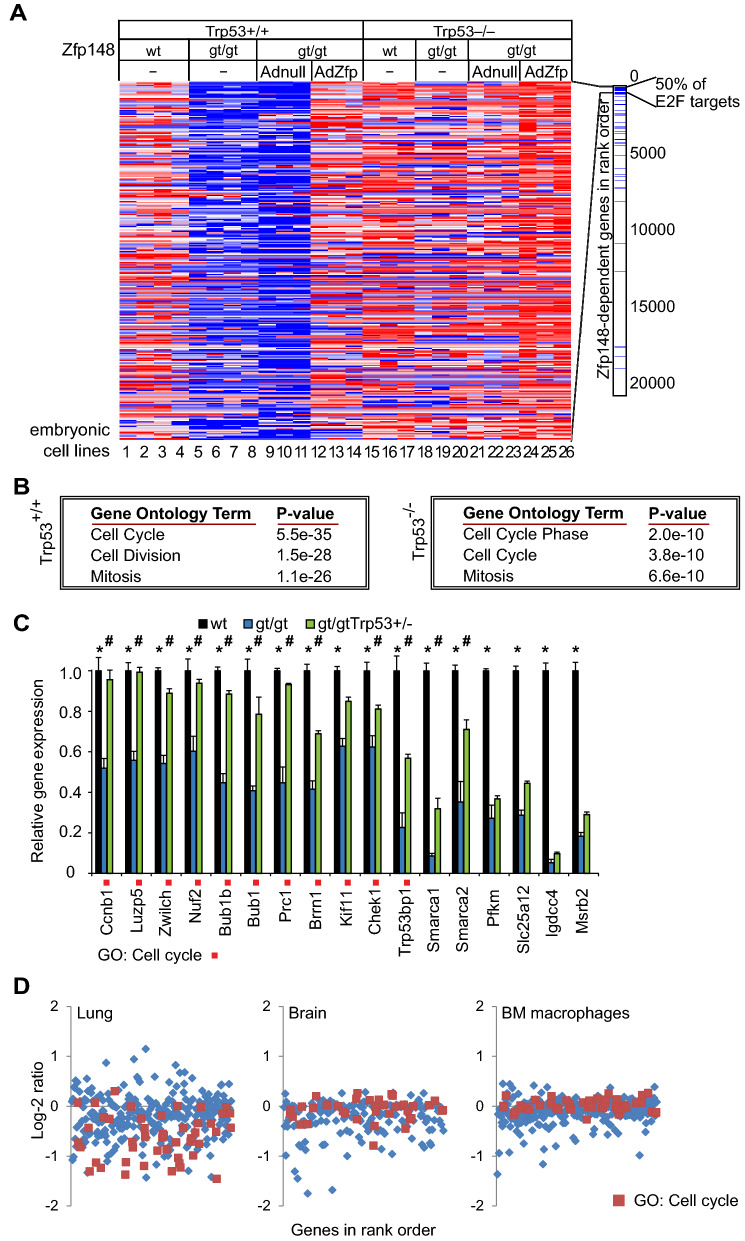
To define mechanisms that cause proliferation arrest of Zfp148gt/gt MEFs, we used transcript profiling to identify genes that were differentially expressed between Zfp148gt/gt MEFs and wild-type controls. We also compared Zfp148gt/gt MEFs infected with adenovirus carrying Zfp148 cDNA or empty vector to verify that differentially expressed genes depended on Zfp148. The analysis revealed a set of more than 300 genes that were downregulated in Zfp148gt/gt MEFs compared to wild-type controls, and rescued in Zfp148gt/gt MEFs after adenoviral expression of Zfp148 cDNA compared to empty vector (Fig. 1A and Supplementary Data S1). Overrepresentation analysis showed strong enrichment of cell cycle-related genes (Fig. 1B). Since this category of genes is regulated by E2F transcription factors, we identified known E2F targets from earlier publications17,19 and investigated their rank in the gene list. The majority of known E2F targets clustered at the top of the list confirming that Zfp148 deficiency downregulated E2F-dependent cell cycle genes (Fig. 1A).
Figure 1.
E2F-responsive cell cycle genes are downregulated in Zfp148-deficient cells. (A) Heat-map showing expression profiles of the top 300 genes that were downregulated in the absence of Zfp148 (blue colour indicates downregulation, red indicates upregulation). Bar showing the rank order of 59 validated E2F target genes according to the heat-map. (B) Table showing enriched GO terms on Trp53+/+ (left) or Trp53−/− (right) genetic background in rank order. (C) Real-time RT-PCR analysis of 17 genes selected from the top 300-set in wild-typeand Zfp148gt/gt MEFs on Trp53+/+ (n = 3) or Trp53+/− (n = 4) genetic background. Red box indicates genes linked to the GO-term “Cell Cycle”. * and # indicate significant differential expression in wild-type versus Zfp148gt/gt and Zfp148gt/gt versus Zfp148gt/gtTrp53+/− MEFs, respectively. (D) Graphs showing log2 expression ratios of the top-300 downregulated genes in lungs (left), brains (middle), and bone marrow-derived macrophages (right) from newborn Zfp148gt/gt mice compared to controls (n = 3). Red colour indicates genes linked to the GO term “Cell Cycle”.
To investigate the impact of p53 on gene expression, we repeated the analysis with RNA extracted from MEFs generated on a Trp53-null genetic background. Downregulation of the cell cycle-enriched gene set was markedly attenuated on this background (Fig. 1A), confirming that p53 plays a dominant role. However, the rank of differentially expressed genes was similar to the rank obtained on Trp53 wild-type background (Spearman correlation, r = 0.56, P < 0.0001) with strong enrichment of cell cycle-related gene ontology (GO) terms among the downregulated genes (Fig. 1B). We therefore conclude that Zfp148 deficiency downregulates cell cycle genes by both p53-dependent and p53-independent mechanisms.
The previous data panels show qualitative changes. To obtain quantitative data, we determined mRNA levels with real-time RT PCR for 17 genes arbitrarily selected from the downregulated gene set. Expression of genes related to the GO term cell cycle was reduced by half in Zfp148gt/gt MEFs compared to controls (Fig. 1C). Deletion of one copy of Trp53 is sufficient to rescue cell proliferation of Zfp148-deficient MEFs12. In line with this, expression of 11 genes was restored to near normal levels in Zfp148gt/gtTrp53+/− compared to Zfp148gt/gt MEFs, and 9 of those genes were linked to the GO-term cell cycle (Fig. 1C). Only 1 of the 6 remaining genes that were not restored on Trp53+/− background was linked to the cell cycle. These results suggest that p53-mediated repression of cell cycle genes causes the proliferative arrest of Zfp148gt/gt MEFs.
To assess whether downregulation of the gene set observed in response to Zfp148 deficiency in MEFs also occurred in vivo, we isolated RNA from neonatal lungs and brains and from bone marrow-derived macrophages of Zfp148gt/gt and wild-type mice. We determined gene expression by microarrays and showed that the gene set was markedly downregulated compared to wild-type controls in Zfp148gt/gt lungs but not in brains or macrophages (Fig. 1D). Previous studies showed that Zfp148 deficiency induces cell cycle arrest in prenatal lungs in a p53-dependent manner12. In contrast, brains and bone marrow derived macrophages from Zfp148 deficient mice displayed no overt phenotypes12,20,21. Thus, the observed downregulation of cell cycle genes in lungs but not brain or bone marrow derived macrophages from Zfp148 deficient mice compared to controls makes sense (Fig. 1D).
The Cdkn2a transcript ARF is required for cell proliferation arrest of Zfp148gt/gt MEFs
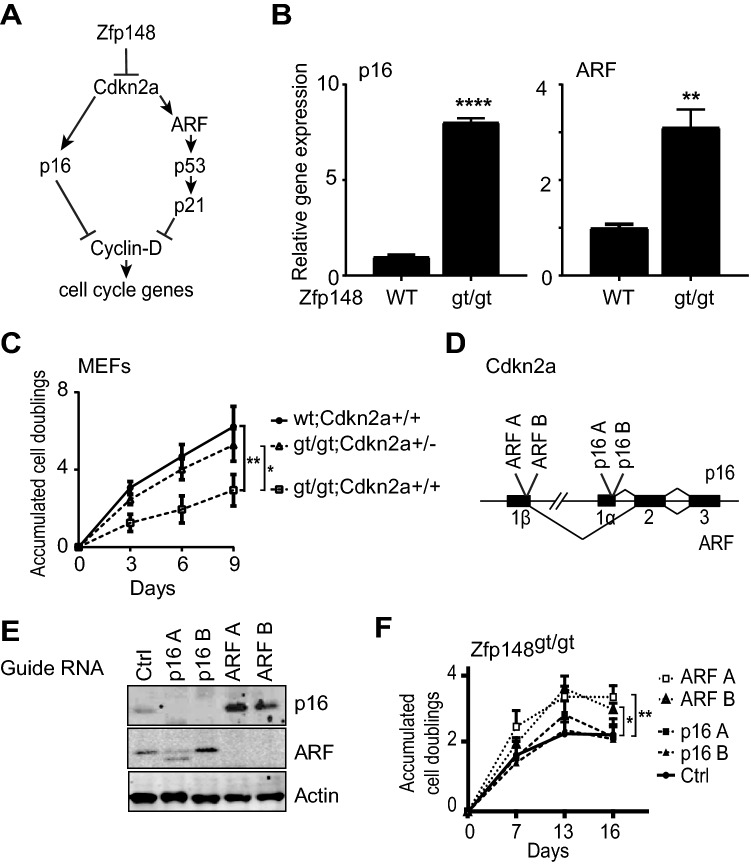
The downregulation of cell cycle genes in Zfp148gt/gt MEFs by p53-dependent and -independent mechanisms prompted us to investigate mRNA levels of the cyclin-dependent kinase inhibitor Cdkn2a. Cdkn2a produces two gene products, ARF and p16, which downregulate cell cycle genes by activating the p53 pathway and inactivating the CDK4/6 complex, respectively (Fig. 2A). ARF and p16 mRNA were markedly increased in Zfp148gt/gt MEFs compared to controls (Fig. 2B), supporting a role for Cdkn2a in the downregulation of cell cycle genes.
Figure 2.
The Cdkn2a gene product ARF reduces proliferation of Zfp148gt/gt MEFs. (A) Schematic of Cdkn2a regulation of cell cycle genes. (B) Graphs showing p16 and ARF mRNA levels in wild-type and Zfp148gt/gt MEFs (n = 6). (C) Growth curves (accumulated cell doublings) of wild-type MEFs and Zfp148gt/gt MEFs on Cdkn2a+/+ and Cdkn2a+/− background (n = 5). (D) Schematic of the mouse Cdkn2a locus showing genome locations of guide-RNA target sites for ARF A, ARF B, p16 A, and p16 B. (E) Western blots showing expression of ARF and p16 in Zfp148gt/gt MEFs infected with guide-RNAs targeting ARF, p16, or scrambled control. Actin was used as loading control. Full-length blots are presented in Supplementary Fig. S1. (F) Graphs showing growth (accumulated cell doublings) of Zfp148gt/gt MEFs infected with the indicated guide-RNAs (n = 5, data are mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001.
We have previously demonstrated that Zfp148 deficiency induces cell proliferation arrest in MEFs after a few passages, and that p53 is required for the arrest12. To further investigate the role of Cdkn2a in the proliferation arrest, Zfp148gt/+ mice were bred on a Cdkn2a+/− background to generate Zfp148gt/gtCdkn2a+/− MEFs. Deletion of one copy of Cdkn2a restored normal proliferation of Zfp148gt/gt MEFs (Fig. 2C), demonstrating that Cdkn2a is required for proliferation arrest of these cells. To determine the relative importance of ARF and p16 for the proliferation arrest, we used lentiviral guide-RNAs and CRISPR to generate Zfp148gt/gt MEFs lacking either ARF or p16 (Fig. 2D, E and Supplementary Fig. S1). Selective ARF knockout rescued growth of Zfp148gt/gt cells (Fig. 2F) despite increased expression of p16, which is an expected consequence of ARF inactivation22. Knockout of p16 had no effect on cell growth (Fig. 2F). Collectively, these data show that ARF but not p16 is required for p53 activation and proliferation arrest of Zfp148gt/gt MEFs.
Zfp148 binds to clustered cytosine-rich DNA elements in basal promoters of other transcription factors
To understand the primary basis of ARF and p53 activation in Zfp148gt/gt MEFs, we used chromatin immunoprecipitation-on-chip (ChIP-chip) assays to identify target sites of FLAG-tagged Zfp148 (AdZfp148FLAG) in Zfp148gt/gtTrp53−/− MEFs. The experiment was performed on a Trp53-null background to overcome senescence and obtain sufficient amounts of starting material. The CMV-driven adenovirus vector used produces Zfp148 protein amounts that are similar to those found in wild-type MEFs12.
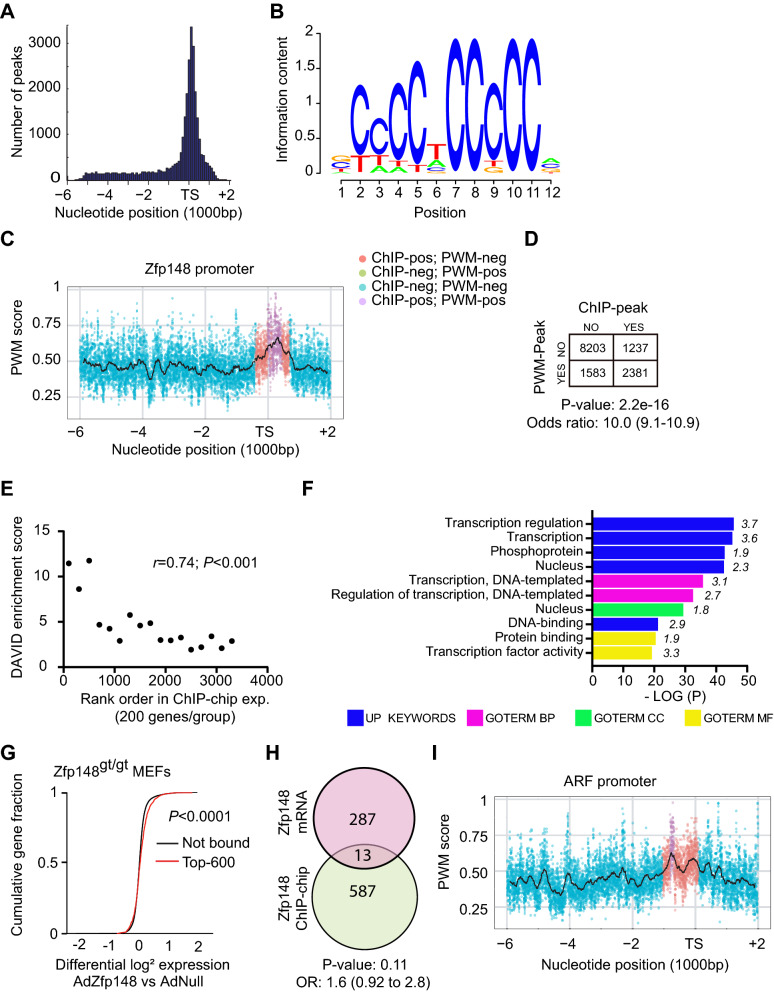
Zfp148 target sites were recorded in four independent experiments with a strong preference for binding close to transcription starts (Fig. 3A and Supplementary Data S2). To determine the DNA-binding preferences of Zfp148, we constructed a position weight matrix (PMW) model from 16 known Zfp148 binding sites (Table 1). As expected, the model predicted a cytosine-rich binding motif (Fig. 3B). We next applied the model to DNA regions surrounding known transcription starts. While there was no correlation between peak positions of ChIP-chip data and PWM-predicted single motifs, Zfp148 showed a strong preference for binding to regions with clustered PWM-predicted motifs (Fig. 3C,D). These data indicate that Zfp148 binds to clustered motifs in cytosine-rich basal promoters.
Figure 3.
Zfp148 binds to clustered cytosine-rich elements in promoters of other transcription factors. (A) Frequency plot showing the location of binding sites of AdZfp148FLAG in relation to transcription starts (TS) for one representative ChIP-chip experiment. (B) Position Weight Matrix (PWM)-logo for Zfp148. (C) A representative gene (Zfp148) with both ChIP-peaks and PWM-peaks. Each point represents the PWM score at the specific position, taking only the best score of the two strands. The black line indicates the mean score over the current position in a 200 bp surrounding window. The intersect of ChIP- and PWM-peaks is indicated by a color code (explained in the figure). (D) Contingency table showing the distribution of promoters with or without ChIP- and/or CWM-peaks. 95% confidence interval in brackets. (E) Graph showing maximum DAVID enrichment score on y-axis and rank order on x-axis for bins of 200 genes based on ChIP-binding score. r indicates Spearman correlation. (F) Graph showing enriched functional terms for the top-600 AdZfp148FLAG binding genes. Fold-enrichment is shown in italics. − LOG10 P-value, negative logarithm with base 10 of the adjusted p-value; UP, UniPRot; GOTERM, gene ontology term; CC, cell compartment; MF, molecular function; BP, biological process. (G) Cumulative distribution function (CDF) of log2-transformed gene expression ratios in Zfp148gt/gt MEFs transduced with AdZfp148 versus AdNull. Red line, genes that bind AdZfp148FLAG with high affinity (n = 600 based on rank order); black line, genes that did not bind AdZfp148FLAG in the ChIP-chip experiments (n = 10,163) (expression analysis, n = 3; ChIP-chip analysis, n = 4). (H) Venn diagram showing the intersect of the top-300 genes that were downregulated in Fig. 1 (red) and the top-600 AdZfp148FLAG binding genes (green). 95% confidence interval in brackets. (I) Graph showing ChIP scores and PWM scores for the ARF promoter. Data representation as in (C).
Table 1.
Zfp148 binding sites used to construct a position weight matrix (PMW) model.
| Symbol | Description | Organism | Genomic site | References |
|---|---|---|---|---|
| TCRB | T cell receptor B | Human | CCACCACCCCCA | Wang5 |
| Ptcra | Pre-T cell receptor A | Mouse | ACCCCACCCCCA | Wang5 |
| Ptcra | Pre-T cell receptor A | Mouse | GCCCCTCCCCCG | Reizis and Leder42 |
| LCK | Lymphocyte-specific tyrosine kinase | Human | TCACCACCCCCA | Yamada43 |
| MMP3 | Matrix metalloproteinase 3 | Human | TTTTTTCCCCCC | Ye44 |
| Col1a1 | Collagen type I alpha 1 | Mouse | CCTCCTCCCCCC | Hasegawa6 |
| Col1a1 | Collagen type I alpha 1 | Mouse | GCCCCCCCTCCC | Hasegawa6 |
| Col1a2 | Collagen type I alpha 2 | Mouse | GTCCCTCCCCCC | Hasegawa6 |
| PDGFRA | Platelet-derived growth factor receptor, alpha | Human | TCCCCTCCCCCG | De Bustos45 |
| ODC1 | Ornithine decarboxylase | Human | GCCCCTCCCCCG | Law46 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | Human | CCTCCTCCCCCA | Bai8 |
| STAT1 | Signal transducer and activator of transcription 1 | Human | GTCCCACCCCCG | Bai47 |
| GAS | Gastrin | Human | CCCACCCCGCCC | Merchant4 |
| CXCL5 | Chemokine, cxc motif, ligand 5 | Human | CCCCCTCCCCCA | Keates48 |
| ENO3 | Beta enolase | Human | CCCCCTCCCCCA | Feo49 |
| Adx1 | Adrenodoxin | Bovine | GCCCCGCCCCCT | Cheng50 |
Overrepresentation analysis of 3,488 genes that bound to Zfp148 at 1% false discovery rate in all experiments showed strong enrichment of functional terms. Binning genes according to their rank in the ChIP-chip experiment revealed a striking correlation between binding scores and the enrichment of functional terms; the enrichment declined abruptly after 600 genes (Spearman correlation r = 0.74, P < 0.001, Fig. 3E). Since functional connectivity is an expected property of co-regulated genes, the number of Zfp148 target genes is likely much lower than the number of binding genes. The top-ranked genes (top 600) were enriched for functional terms related to transcriptional regulation, suggesting that Zfp148 mainly operates upstream of other transcription factors (Fig. 3F). There was no overrepresentation of cell cycle-related terms.
By integrating ChIP-chip and gene expression data, we confirmed a small but significant upregulation of Zfp148 target genes in Zfp148gt/gt MEFs after infection with AdZfp148 (Fig. 3G). However, there was no significant overlap between Zfp148-binding genes and the set of cell cycle-enriched genes that was downregulated in Zfp148gt/gt MEFs (Fig. 3H). Notably, Zfp148 bound to the ARF promoter in all experiments with binding regions centered at 800 base pairs upstream of the transcription start (Fig. 3I) suggesting that Zfp148 may repress ARF transcription. However, the preferential binding of Zfp148 to promoters of other transcription factors indicates that deletion of Zfp148 could have pleiotropic effects that activate ARF and p53 indirectly.
No genetic interaction between ZNF148 and TP53 in human cancer cell lines
Since the integrated transcriptomic and promoter occupancy analysis suggested pleiotropic and indirect mechanisms of p53 and ARF activation in Zfp148gt/gt MEFs, we decided to explore the genetic interaction between ZNF148 (the human orthologue of Zfp148) and TP53 in CRISPR and siRNA data from human cancer cell lines.
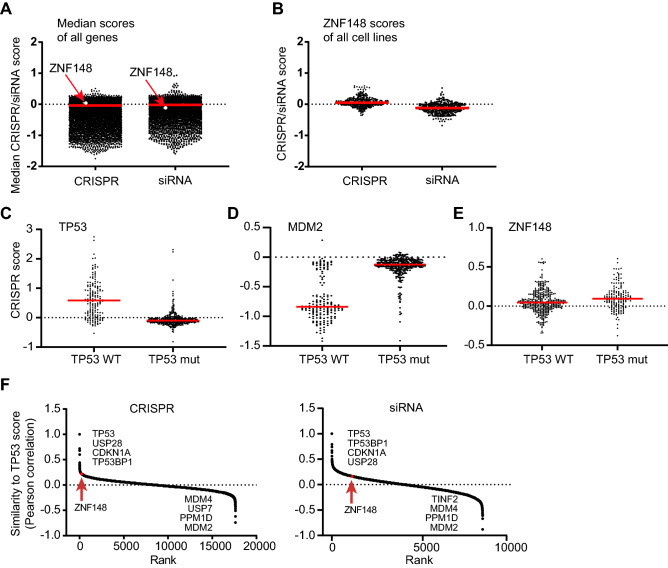
We downloaded CRISPR and siRNA scores for all genes in 487 human cancer cell lines from the Cancer Dependency Map database (https://depmap.org). A negative score indicates that knockout (CRISPR) or knockdown (siRNA) of that gene has a negative impact on cell growth or survival. Genes with CRISPR scores below − 0.6 are considered to be essential23. The median CRISPR and siRNA scores for ZNF148 were 0.05 (± 0.01 95% CI) and − 0.12 (± 0.02 95% CI), suggesting that cancer cells are marginally affected by ZNF148 inactivation (Fig. 4A). ZNF148 was not essential for the growth or survival of any of the tested cell lines (Fig. 4B).
Figure 4.
No genetic interaction between ZNF148 and TP53 in human cancer cell lines. (A) Graph showing the median CRISPR and siRNA scores across the human cancer cell lines for all genes. ZNF148 scores are indicated by arrows. n = 17,631 (CRISPR) and 16,725 (siRNA) genes, respectively. (B) Graph showing the CRISPR and siRNA scores of ZNF148 for all cancer cell lines. n = 432 (CRISPR) and 328 (siRNA) cell lines, respectively. (C–E) Graphs showing the CRISPR scores of TP53 (C) or MDM2 (D) or ZNF148 (E) for cell lines with wild-type (left, n = 354) or mutated (right, n = 164) TP53. (F) Graphs showing Pearson correlation with CRISPR (left) or siRNA (right) scores of TP53 (y-axis) for all genes sorted by rank (x-axis). The position of ZNF148 is indicated by arrows. n = 17,631 (CRISPR) and 8,296 (siRNA) genes, respectively.
We next divided the cell lines into two groups depending on their TP53 genotype. As expected, inactivation of TP53 increased the growth of cells with wild-type TP53 but had no impact on cells with TP53 mutations (Fig. 4C). The p53 repressor MDM2 showed a reverse pattern with low scores in TP53 wild-type cells and scores near zero in cells with TP53 mutations (Fig. 4D). In contrast, there was no marked difference in ZNF148 scores between TP53 wild-type and mutant cell lines (Fig. 4E).
We finally ranked the genes according to their similarity with TP53 as judged by the Pearson correlation of CRISPR or siRNA scores across the set of human cancer cell lines. Canonical activators of the p53 pathway such as USP28, CDKN1A, and TP53BP1 showed strong correlation with TP53 and were positioned at the top of the list, while repressors including MDM2, PPM1D, and MDM4 were positioned at the end (Fig. 4F). In contrast, ZNF148 did not show strong correlation or anti-correlation with TP53 (Fig. 4F), suggesting that there is no genetic interaction between ZNF148 and TP53 in human cancer cell lines under standard culture conditions.
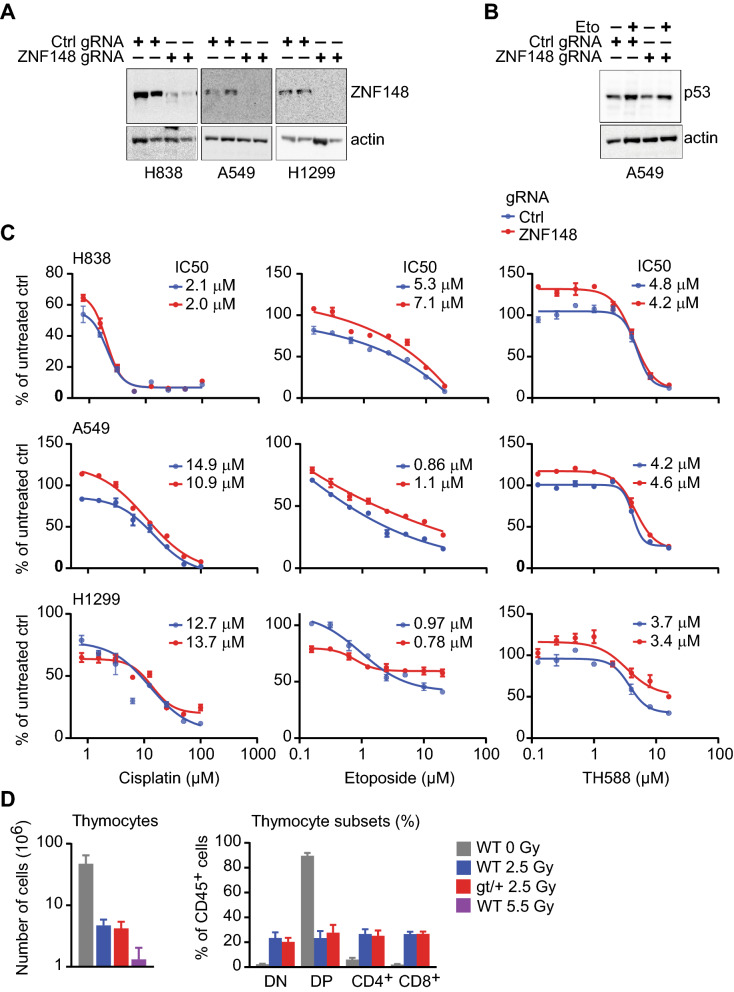
To exclude the possibility that ZNF148 exerts mild regulation of p53 that is compensated under standard conditions, we investigated the role of ZNF148 under DNA damaging conditions. We used CRISPR-Cas9 to delete ZNF148 in H838, A549, and H1299 (TP53 null) human lung cancer cells (Fig. 5A and Supplementary Fig. S3), and confirmed that p53-induction by etoposide was intact (Fig. 5B and Supplementary Fig. S3). To uncover quantitative differences in the DNA damage response, we established dose–response curves for cisplatin and etoposide that activates p53 through ATM/ATR, and for TH588 that activates p53 though USP2824. There was no difference in IC50 values between ZNF148 knockout cells and controls in any of the tested cell lines (Fig. 5C).
Figure 5.
ZNF148 deficiency does not sensitize cells to DNA damage. (A) Protein extracts of H838, A549, and H1299 cells expressing gRNA targeting ZNF148 or non-targeting control, blotted with an antibody against ZNF148. Actin was used as loading control. (B) Protein extracts of etoposide (3uM) or vehicle treated A549 cells expressing gRNA against ZNF148 or non-targeting control, blotted with an antibody against p53. Actin was used as loading control. Full-length blots of (A) and (B) are presented in Supplementary Fig. S3. (C) Drug response curves of H838, A549, and H1299 cells expressing gRNA targeting ZNF148 or non-targeting control, for cisplatin, etoposide, or TH588 (n = 3 replicates per concentration). IC50, the half maximal inhibitory concentration. (D) Graphs showing total number of thymocytes (left), or thymocyte subsets as percent of CD45+ cells (right), in wild-type (WT) or Zfp148gt/+ (gt/+) mice exposed to whole body irradiation at 0, 2.5, or 5 Gy (n = 2 WT at 0 Gy, 12 WT and 7 gt/+ at 2.5 Gy, 4 WT at 5 Gy). DP, double CD4- and CD8-positive cells; DN, double CD4- and CD8-negative cells; error bars, S.E.M.
To assess the role of Zfp148 under DNA damaging conditions in vivo, we exposed Zfp148 heterozygous mice and controls to ionizing radiation and quantified the depletion of immature thymocytes, a process that is entirely p53-dependent25. As expected, ionizing radiation reduced the number of thymocytes in a dose dependent manner (Fig. 5D). However, there was no difference in the total number of thymocytes, or the distribution of different thymocyte subsets, between Zfp148gt/+ and control mice 72 h after exposure to ionizing radiation (Fig. 5D). We conclude that Zfp148 does not regulate the p53-pathway under DNA damaging conditions.
Discussion
We have previously shown that Zfp148 deficiency arrests proliferation in MEFs by activating p5312, and here we investigated the underlying mechanism by analyzing the transcriptional output and promoter occupancy of Zfp148 in MEFs. Our results suggest that Zfp148 deficiency, by derepressing ARF transcription, downregulates cell cycle genes and cell proliferation in a p53-dependent manner. The model is supported by three lines of evidence. Firstly, Zfp148 deficiency downregulated cell cycle genes in a p53-dependent and -independent manner, with p53 playing a dominant role. Secondly, proliferation arrest of these cells required increased expression of the Cdkn2a transcript ARF, which is a major regulator of the p53 pathway. Finally, Zfp148 interacted physically with the ARF promoter, indicating that Zfp148 may regulate ARF transcription.
Zfp148 did not bind to promoters of cell cycle genes more often than predicted by chance, suggesting that regulation of cell cycle genes is indirect. p53 can downregulate cell cycle genes by inducing the cyclin-dependent kinase inhibitor p21, which is also regulated by ZNF1488,9. Although regulation of the p21 promoter by ZNF148 could theoretically explain the p53-independent repression of cell cycle genes, the promoter was negatively regulated by ZNF148 (in response to butyrate) in most published experiments8,9, which would not explain our current finding. Given that the second transcript of the Cdkn2a locus, p16, was markedly increased in Zfp148gt/gt MEFs compared to controls, a more likely explanation is that p53-independent downregulation of cell cycle genes is mediated by p16.
The cell cycle arrest in Zfp148gt/gt MEFs was rescued by heterozygous deletion of Cdkn2a or selective knockout of the ARF transcript, indicating a plausible mechanism for p53 activation by ARF. In contrast to homozygous deletion of Cdkn2a, which causes hyper-proliferation of MEFs and spontaneous escape from senescence, heterozygous deletion does not increase cell proliferation on its own26. Hence, increased expression of ARF is likely part of the mechanism underlying proliferation arrest in Zfp148gt/gt MEFs. We have previously shown that Zfp148gt/gt MEFs are rescued by exogenous antioxidants or culture at reduced oxygen concentration showing that oxidative stress contributes to the proliferation arrest in these cells12. Because ARF suppresses the transcriptional activity of NRF2 (NFE2L2)27—the master regulator of endogenous antioxidants—increased ARF transcription may explain the oxidative stress weakness of Zfp148gt/gt MEFs.
Zfp148 interacted physically with the ARF promoter, raising the possibility that ARF is a direct target of Zfp148. Thus, deletion of Zfp148 may activate p53 by derepressing ARF transcription, similar to knockouts of polycomb members Bmi1, M33, Mel18, and Phc228–31. In line with this, inhibition of ZNF148 potentiates butyrate-induced senescence of human HT116 colon cancer cells by derepressing the p16 promoter32. However, increased expression of ARF could have many underpinnings. The strong enrichment of transcription factors among Zfp148-binding genes suggests that Zfp148 operates at a high level of the transcription factor hierarchy33. Small orchestrated alterations of many transcripts may therefore contribute to the activation of ARF and p53 in Zfp148gt/gt MEFs, possibly by affecting cellular homeostasis. Collectively, our data suggest that Zfp148 deficiency activates p53 indirectly, by derepressing ARF and by altering the activity of other transcription factors.
A previous publication showed that ZNF148 interacts physically with p5316, thus identifying another possible mechanism behind the activation of p53 in Zfp148gt/gt MEFs. However, our current data on ZNF148 and TP53 dependencies in human cancer cells strongly argue against a physical interaction between ZNF148 and p53 playing a significant role. There was no evidence of genetic interaction between ZNF148 and TP53 in CRISPR and siRNA screens of hundreds of human cancer cell lines, performed under conditions that revealed strong dependencies between TP53 and known repressors of p53 activity including MDM2, MDM4, and PPM1D. Moreover, the was no evidence of genetic interaction between ZNF148 and TP53 under DNA damaging conditions in human lung cancer cells or mice. However, it remains possible that regulation of ARF is important. For reasons not well understood, ARF appears to play a more prominent role as a tumor suppressor in mice than in humans34. Moreover, accumulating evidence indicate that ARF has important roles that are independent of p5334. In line with this, we observed that the correlation between CDKN2A and TP53 was weak in the CRISPR screen and absent in the siRNA screen (data not shown).
In the light of these results, we need to reevaluate the role of Zfp148 and its impact on the p53 pathway. Without evidence of genetic interaction between ZNF148 and TP53 in human cancer cells, the incentive for therapeutic targeting of ZNF148 becomes weak. The recent finding that inhibition of ZNF148 potentiates butyrate-induced senescence of human HT116 colon cancer cells32 suggests that core functions of Zfp148 are evolutionarily conserved. Further investigation of the interaction between Zfp148 and Cdkn2a promoters may provide valuable mechanistic insights. However, transcriptomes are shaped by combinatorial interactions of transcription factors forming complex network motifs, including feedforward and feedbackward loops, that cooperatively govern gene expression35. Our current data suggest that Zfp148 operates at a high level of the transcription factor hierarchy. Thus, future studies of Zfp148 warrant a more integrated and comprehensive approach.
Material and methods
Mice
Cdkn2atm1rdb (Cdkn2a−/) and Trp53tm1Tyj (Trp53−/) mice were obtained from The Jackson Laboratory and Zfp148gt/+ mice were produced by us12. The mice were kept on a 129/Bl6 mixed genetic background and all experiments were performed with littermate controls. Genotyping was performed by PCR amplification of genomic DNA from mouse biopsies obtained during earmarking. PCR primers used for genotyping are listed in Supplementary Table S1. Mice were fed on a regular diet and had unlimited supply of food and water. All animal procedures used in this study were approved by The Animal Research Ethics Committee in Gothenburg and performed in accordance with relevant guidelines and regulations.
Cell culture
Primary MEFs were isolated from E13.5–15.5 embryos as described12. Cells were cultured in DMEM low glucose medium with 10% fetal bovine serum, 100 µg/ml penicillin and streptomycin, 1% non-essential amino acids, 1–4 mM glutamine, and 0.5 mM β-mercaptoethanol. Growth curves were established using a modified 3T3 protocol (50,000 cells/well in 6-well plates).
Adenoviral transduction
Cells were incubated with 15 multiplicities of infection of empty control adenoviruses (AdNull) or adenoviruses encoding Zfp148 (AdZfp148) or Zfp148FLAG (AdZfp148FLAG) (Vector Biolabs) for 36–48 h before analyses, as described in12.
Gene expression analysis
Total RNA from MEFs, lungs, brains and macrophages was isolated using the Gene Elute kit (Sigma) and hybridized to Affymetrix Mouse Gene 1.0 ST chips. Data were normalized with the Robust Multi-Array Analysis (RMA) method36. The heat-map was constructed using the Hierarchical Clustering Explorer 3.0 software. Gene ontology statistics was calculated using the DAVID software37,38.
Real-time quantitative PCR
TaqMan and SYBR Green assays were performed as described39 using TaqMan/SYBR Green universal PCR mastermix (A25741, Applied-biosystem) and the pre-designed TaqMan assays (Applied Biosystems) or RT-PCR primers listed in Supplementary Table S1.
CRISPR-Cas9 knockouts
Pre-designed guide-RNA (gRNA) sequences targeting p16 or ARF or ZNF148 or control (does not target a sequence in the genome) were cloned into the Lenticrisprv2 vector, and co-transfected with pCMV-dR8.2 (Addgene # 8455) and pCMV-VSV-G (Addgene # 8454) vectors into HEK293T cells to produce complete Cas9 and gRNA coding lentivirus. Virus infected Zfp148gt/gt MEFs or human lung cancer cells (H838, A549, or H1299) were selected with puromycin for 3 days to obtain batch clones that were used for experiments. The gRNA sequences used are listed in Supplementary Table S1. Lenticrisprv2 was a kind gift from Feng Zhang (Addgene # 52961)40.
Western blot analyses
Protein levels was determined as previously described41 with antibodies against p16ink4a (sc-1207, santa cruz), p19arf (sc-3278, santa cruz), ZNF148 (HPA001656, SigmaAldrich Atlas), p53 (DO-1 (sc126), Santa Cruz), and actin (A2066, Sigma Aldrich Atlas). Secondary antibodies were anti-mouse IRDye 680RD (926-68072, LI-COR Biosciences), anti-rabbit 680RD (926-68071, LI-COR Biosciences), anti-rabbit IgG Conformation Specific (L27A9) HRP Conjugate (5,127, Cell Signaling), and anti-mouse IgG-hl-HRP (ab6728, abcam). Protein bands were detected with the immubilon western chemiluminescent HRP substrate (Millipore) using a Chemi Doc Touch Imaging system (Bio Rad) or the Li-Cor Odyssey Imager. Exposure times were adjusted to the signal intensity of each blot. Images were adjusted for brightness and contrast with the Photoshop tool levels. All changes were applied equally across the entire image.
ChIP-on-chip analysis
MEFs (1 × 108) transduced with AdZfp148FLAG were fixed in 1% formaldehyde. 200–500-bp DNA fragments were precipitated using the anti-FLAG M2 antibody (Sigma) conjugated to sheep anti-mouse IgG Dynabeads (Invitrogen), purified (QIAquick, Qiagen), amplified (WGA2 kit, Sigma), and hybridized to MM8_Deluxe_Promoter_HX1 chips at NimblGen (www.nimblegen.com). The analysis was done on Zfp148gt/gt background to avoid competition with endogenous Zfp148 and Trp53−/− background to overcome senescence and obtain sufficient amounts of starting material. Gene ontology statistics was calculated using the DAVID software with “functional annotation clustering” setting for DAVID enrichment scores and “functional annotation chart” setting for ranking of functional terms37,38.
Motif prediction
A position weight matrix (PWM) model based on 16 known binding sites of ZNF148 (Table 1)4–6,8,42–50 was applied on regions around known transcription starts (TS), following the approach described in Wasserman and Sandelin (2004). The PWM model slides across the region one nucleotide at a time, generating a score between 0 and 1 at each position for both strands. The best score (of the two strands) for each position was then averaged over a 200 bp window. A region was called as a potential binding site (PWM-peak positive) when the average score of the window exceeded 0.6.
Meta-analysis of CRISPR and siRNA data
CRISPR-scores (Achilles_gene_effect), siRNA-scores (D2_combined_gene_dep_scores), and somatic mutation calls (CCLE_mutations) for human cancer cells (cancer cell line encyclopaedia) were downloaded from the Cancer Dependency Map portal at the Broad Institute (https://depmap.org/portal/download/).
Drug response curves
15,000 cells were plated in 12-well plates (per well), incubated with cisplatin (Sigma), etoposide (Sigma), or TH588 (TH588 hydrochloride, Axon Medchem) for three days, and stained for 20 min with a fixing/staining solution containing 0.025% crystal violet (Sigma), 1% formaldehyde, and 1% methanol in PBS. After drying overnight, the crystal violet stained cells were solubilized in 10% acetic acid and the absorbance determined at 590 nm with a Spectramax II (Molecular Devices).
Radiosensitivity
Zfp148gt/+ mice and wild-type controls were radiated using an RS-2000 X-ray irradiator (Rad Source Technologies) at the indicated doses. The thymus was collected after sacrifice on day 4 after radiation. Thymocytes were disseminated into single cell suspension and stained with CD45-BUV737 (clone 104, cat. no. 612778, BD Biosciences), CD4-BV785 (clone RM4-5, cat. no. 1102760, Sony Biotechnology), CD8-FITC (clone 53-6.7, cat. no. 1103525, Sony Biotechnology) and propidium iodide (cat. no. P3566, Life Technologies). Samples were run on a Fortessa X20 (BD Biosciences) and data was analyzed using FlowJo version 10.6.1.
Statistical analyses
Values are mean ± SEM. Statistics were performed with Student’s t-test for comparisons between two groups; one-way ANOVA for multiple groups; Fisher exact test for contingency tables; two-sample Kolgomorov-Smirnov test for cumulative distribution function plots; Spearman correlation coefficient for ranked values; and Pearson correlation coefficient for continuous variables. Differences between groups were considered significant when P < 0.05.
Supplementary information
Acknowledgements
We would like to warmly thank Rosie Perkins for constructive criticism on the manuscript. The project was supported by the Swedish Research Council, Polysackaridforskning AB, the Swedish Cancer Association, the Swedish Heart-Lung Foundation, The Swedish Child Cancer Foundation, the Sahlgrenska University Hospital ALF research grants, and the University of Gothenburg (to P. Lindahl) and Swedish Research Council, Swedish Society for Medicine, BioCARE and Knut and Alice Wallenberg foundation (to V.I. Sayin).
Author contributions
Z.V.Z., V.I.S. and P.L. conceived the study. Z.V.Z., N.G., A.A.B., V.G., E.M.E., A.A.H.P., J.J.D., B.O.T., K.L.G., I.J., A.S., V.I.S. and P.L. performed experiments and analyzed data. M.L, and E.L. performed the transcription factor motif analyses. Å.T., E.F.A., and M.O.B. provided conceptual and technical advice. P.L. and V.I.S. wrote the manuscript. All authors reviewed the text.
Funding
Open Access funding provided by University of Gothenburg.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Volkan I. Sayin, Email: volkan.sayin@wlab.gu.se
Per Lindahl, Email: per.lindahl@wlab.gu.se.
Supplementary information
is available for this paper at 10.1038/s41598-020-70824-2.
References
- 1.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tisato V, Voltan R, Gonelli A, Secchiero P, Zauli G. MDM2/X inhibitors under clinical evaluation: perspectives for the management of hematological malignancies and pediatric cancer. J. Hematol. Oncol. 2017;10:133. doi: 10.1186/s13045-017-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10:501–514. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Merchant JL, et al. ZBP-89, a Kruppel-like zinc finger protein, inhibits epidermal growth factor induction of the gastrin promoter. Mol. Cell. Biol. 1996;16:6644–6653. doi: 10.1128/mcb.16.12.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Kobori JA, Hood L. The ht beta gene encodes a novel CACCC box-binding protein that regulates T-cell receptor gene expression. Mol. Cell. Biol. 1993;13:5691–5701. doi: 10.1128/MCB.13.9.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasegawa T, Takeuchi A, Miyaishi O, Isobe K, de Crombrugghe B. Cloning and characterization of a transcription factor that binds to the proximal promoters of the two mouse type I collagen genes. J. Biol. Chem. 1997;272:4915–4923. doi: 10.1074/jbc.272.8.4915. [DOI] [PubMed] [Google Scholar]
- 7.Passantino R, et al. Negative regulation of beta enolase gene transcription in embryonic muscle is dependent upon a zinc finger factor that binds to the G-rich box within the muscle-specific enhancer. J. Biol. Chem. 1998;273:484–494. doi: 10.1074/jbc.273.1.484. [DOI] [PubMed] [Google Scholar]
- 8.Bai L, Merchant JL. Transcription factor ZBP-89 cooperates with histone acetyltransferase p300 during butyrate activation of p21waf1 transcription in human cells. J. Biol. Chem. 2000;275:30725–30733. doi: 10.1074/jbc.M004249200. [DOI] [PubMed] [Google Scholar]
- 9.Bai L, Kao JY, Law DJ, Merchant JL. Recruitment of ataxia-telangiectasia mutated to the p21(waf1) promoter by ZBP-89 plays a role in mucosal protection. Gastroenterology. 2006;131:841–852. doi: 10.1053/j.gastro.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Zhang X, Salmon M, Zehner ZE. The zinc finger repressor, ZBP-89, recruits histone deacetylase 1 to repress vimentin gene expression. Genes Cells. 2007;12:905–918. doi: 10.1111/j.1365-2443.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, et al. The transcription factor ZBP-89 suppresses p16 expression through a histone modification mechanism to affect cell senescence. FEBS J. 2009;276:4197–4206. doi: 10.1111/j.1742-4658.2009.07128.x. [DOI] [PubMed] [Google Scholar]
- 12.Sayin VI, et al. Zfp148 deficiency causes lung maturation defects and lethality in newborn mice that are rescued by deletion of p53 or antioxidant treatment. PLoS ONE. 2013;8:e55720. doi: 10.1371/journal.pone.0055720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilton A, et al. Targeting Zfp148 activates p53 and reduces tumor initiation in the gut. Oncotarget. 2016 doi: 10.18632/oncotarget.10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Essien BE, et al. Transcription factor ZBP-89 drives a feedforward loop of beta-catenin expression in colorectal cancer. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-15-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayin VI, et al. Loss of one copy of Zfp148 reduces lesional macrophage proliferation and atherosclerosis in mice by activating p53. Circ. Res. 2014 doi: 10.1161/CIRCRESAHA.115.304992. [DOI] [PubMed] [Google Scholar]
- 16.Bai L, Merchant JL. ZBP-89 promotes growth arrest through stabilization of p53. Mol. Cell. Biol. 2001;21:4670–4683. doi: 10.1128/MCB.21.14.4670-4683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong LJ, Chang JT, Bild AH, Nevins JR. Compensation and specificity of function within the E2F family. Oncogene. 2007;26:321–327. doi: 10.1038/sj.onc.1209817. [DOI] [PubMed] [Google Scholar]
- 18.Chong JL, et al. E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature. 2009;462:930–934. doi: 10.1038/nature08677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, et al. A comprehensive ChIP-chip analysis of E2F1, E2F4, and E2F6 in normal and tumor cells reveals interchangeable roles of E2F family members. Genome Res. 2007;17:1550–1561. doi: 10.1101/gr.6783507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sayin VI, et al. Loss of one copy of Zfp148 reduces lesional macrophage proliferation and atherosclerosis in mice by activating p53. Circ. Res. 2014;115:781–789. doi: 10.1161/CIRCRESAHA.115.304992. [DOI] [PubMed] [Google Scholar]
- 21.Nilton A, et al. Zinc finger protein 148 is dispensable for primitive and definitive hematopoiesis in mice. PLoS ONE. 2013;8:e70022. doi: 10.1371/journal.pone.0070022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Wang J, Al-Ahmadie H, Abate-Shen C. ARF regulates the stability of p16 protein via REGgamma-dependent proteasome degradation. Mol. Cancer Res. 2013;11:828–833. doi: 10.1158/1541-7786.MCR-13-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers RM, et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat. Genet. 2017;49:1779–1784. doi: 10.1038/ng.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gul N, et al. The MTH1 inhibitor TH588 is a microtubule-modulating agent that eliminates cancer cells by activating the mitotic surveillance pathway. Sci. Rep. 2019;9:14667. doi: 10.1038/s41598-019-51205-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 26.Serrano M, et al. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/S0092-8674(00)81079-X. [DOI] [PubMed] [Google Scholar]
- 27.Chen D, et al. NRF2 is a major target of ARF in p53-independent tumor suppression. Mol. Cell. 2017;68:224–232. doi: 10.1016/j.molcel.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 29.Core N, Joly F, Boned A, Djabali M. Disruption of E2F signaling suppresses the INK4a-induced proliferative defect in M33-deficient mice. Oncogene. 2004;23:7660–7668. doi: 10.1038/sj.onc.1207998. [DOI] [PubMed] [Google Scholar]
- 30.Isono K, et al. Mammalian polyhomeotic homologues Phc2 and Phc1 act in synergy to mediate polycomb repression of Hox genes. Mol. Cell. Biol. 2005;25:6694–6706. doi: 10.1128/MCB.25.15.6694-6706.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miki J, Fujimura Y, Koseki H, Kamijo T. Polycomb complexes regulate cellular senescence by repression of ARF in cooperation with E2F3. Genes Cells. 2007;12:1371–1382. doi: 10.1111/j.1365-2443.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- 32.Ocadiz-Ruiz R, et al. ZBP-89 function in colonic stem cells and during butyrate-induced senescence. Oncotarget. 2017;8:94330–94344. doi: 10.18632/oncotarget.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerstein MB, et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489:91–100. doi: 10.1038/nature11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontana R, Ranieri M, La Mantia G, Vivo M. Dual role of the alternative reading frame ARF protein in cancer. Biomolecules. 2019 doi: 10.3390/biom9030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alon U. Network motifs: theory and experimental approaches. Nat. Rev. Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- 36.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 37.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.da Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson E, et al. Discovery of microvascular miRNAs using public gene expression data: miR-145 is expressed in pericytes and is a regulator of Fli1. Genome Med. 2009;1:108. doi: 10.1186/gm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ibrahim MX, et al. Targeting isoprenylcysteine methylation ameliorates disease in a mouse model of progeria. Science. 2013;340:1330–1333. doi: 10.1126/science.1238880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reizis B, Leder P. Expression of the mouse pre-T cell receptor alpha gene is controlled by an upstream region containing a transcriptional enhancer. J. Exp. Med. 1999;189:1669–1678. doi: 10.1084/jem.189.10.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada A, et al. Identification and characterization of a transcriptional regulator for the lck proximal promoter. J. Biol. Chem. 2001;276:18082–18089. doi: 10.1074/jbc.M008387200. [DOI] [PubMed] [Google Scholar]
- 44.Ye S, Whatling C, Watkins H, Henney A. Human stromelysin gene promoter activity is modulated by transcription factor ZBP-89. FEBS Lett. 1999;450:268–272. doi: 10.1016/S0014-5793(99)00509-8. [DOI] [PubMed] [Google Scholar]
- 45.De Bustos C, et al. A PDGFRA promoter polymorphism, which disrupts the binding of ZNF148, is associated with primitive neuroectodermal tumours and ependymomas. J. Med. Genet. 2005;42:31–37. doi: 10.1136/jmg.2004.024034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Law GL, et al. Transcription factor ZBP-89 regulates the activity of the ornithine decarboxylase promoter. J. Biol. Chem. 1998;273:19955–19964. doi: 10.1074/jbc.273.32.19955. [DOI] [PubMed] [Google Scholar]
- 47.Bai L, Merchant JL. Transcription factor ZBP-89 is required for STAT1 constitutive expression. Nucleic Acids Res. 2003;31:7264–7270. doi: 10.1093/nar/gkg929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keates AC, et al. ZBP-89, Sp1, and nuclear factor-kappa B regulate epithelial neutrophil-activating peptide-78 gene expression in Caco-2 human colonic epithelial cells. J. Biol. Chem. 2001;276:43713–43722. doi: 10.1074/jbc.M107838200. [DOI] [PubMed] [Google Scholar]
- 49.Feo S, et al. Transcription of the human beta enolase gene (ENO-3) is regulated by an intronic muscle-specific enhancer that binds myocyte-specific enhancer factor 2 proteins and ubiquitous G-rich-box binding factors. Mol. Cell. Biol. 1995;15:5991–6002. doi: 10.1128/mcb.15.11.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng PY, Kagawa N, Takahashi Y, Waterman MR. Three zinc finger nuclear proteins, Sp1, Sp3, and a ZBP-89 homologue, bind to the cyclic adenosine monophosphate-responsive sequence of the bovine adrenodoxin gene and regulate transcription. Biochemistry. 2000;39:4347–4357. doi: 10.1021/bi992298f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.