Abstract
Objective:
To determine the relationship between various parameters of high-frequency biphasic stimulation (HFBS) and the recovery period of post-HFBS block of the pudendal nerve in cats.
Materials and Methods:
A tripolar cuff electrode was implanted on the pudendal nerve to deliver HFBS in ten cats. Two hook electrodes were placed central or distal to the cuff electrode to stimulate the pudendal nerve and induce contractions of external urethral sphincter (EUS). A catheter was inserted toward the distal urethra to slowly perfuse the urethra and record the back-up pressure generated by EUS contractions. After determining the block threshold (T), HFBS (6 or 10 kHz) of different durations (1, 5, 10, 20, 30 min) and intensities (1T or 2T) was used to produce the post-HFBS block.
Results:
HFBS at 10 kHz and 1T intensity must be applied for at least 30 min to induce post-HFBS block. However, 10 kHz HFBS at a higher intensity (2T) elicited post-HFBS block after stimulation of only 10 min; and 10 kHz HFBS at 2T for 30 min induced a longer-lasting (1–3 h) post-HFBS block that fully recovered with time. HFBS of 5-min duration at 6 kHz produced a longer period (20.4 ± 2.1 min, p < 0.05, N = 5 cats) of post-HFBS block than HFBS at 10 kHz (9.5 ± 2.1 min).
Conclusion:
HFBS of longer duration, higher intensity, and lower frequency can produce longer-lasting reversible post-HFBS block. This study is important for developing new methods to block nerve conduction by HFBS.
Keywords: Block, cat, high-frequency, nerve, stimulation
INTRODUCTION
It has been known for almost 80 years that high-frequency (kHz) biphasic stimulation (HFBS) can block nerve conduction (1–3). In recent years, HFBS has been applied successfully to treat chronic pain (4,5) and obesity (6). It has also been proposed to block the pudendal nerve for restoring lower urinary tract function after spinal cord injury (7,8). However, the mechanism underlying HFBS block is still unknown. Studies to better characterize the HFBS block will be critical for understanding the possible mechanism and should be useful for improving clinical applications.
Previous studies (9,10) showed that HFBS block can be initiated quickly and is also fully reversible within seconds after termination of HFBS. However, more recent experiments (11,12) showed that the compound action potentials can be suppressed for many minutes after termination of HFBS, raising the possibility of a post-HFBS block of nerve conduction. It is worth noting that the reduction in the amplitude of compound action potentials in these experiments could have been caused by a slowing of action potential conduction velocity differently in different size axons and not necessarily due to a conduction block. To address this concern, our previous study (13) using frog sciatic nerve-muscle preparation verified the post-HFBS block by recording muscle contractions instead of compound action potentials. Recently, the post-HFBS block was further confirmed by a study using rat sciatic nerve (14), but unfortunately intermittent HFBS was used in this study without quantitively determining the relationship between the recovery period of post-HFBS block and the HFBS parameters (amplitude, duration, and frequency). This relationship is important for understanding the possible mechanisms of post-HFBS block.
Currently, there is no consensus about the mechanism of HFBS block although ion channel activation/inactivation may play an important role in the block (15). Our previous computer simulation studies (16,17) using single axon models have revealed a possible post-HFBS block mechanism. Each stimulus pulse of HFBS can generate an inward sodium current and an outward potassium current across the axonal membrane, which will cumulatively change the intracellular/extracellular ion concentrations as the HFBS continues. Eventually, the disruption of the normal intracellular/extracellular ion concentration gradients reaches a level that is sufficient to block axonal conduction. Therefore, the recovery period of post-HFBS block will depend on the time required for the sodium–potassium pump to restore the normal intracellular/extracellular ion concentrations. If this hypothesis is true, then continuous HFBS of a higher intensity, a longer duration, or a lower frequency that can produce larger changes in intracellular/extracellular ion concentrations should produce a longer period of post-HFBS block. To test this hypothesis, this study in cats investigated the post-HFBS block of the pudendal nerve and determined the relationship between various HFBS parameters and the recovery period of post-HFBS block.
MATERIALS AND METHODS
All protocols involving the use of animals in this study were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Experimental Preparation
Nine cats (five males and four females; 3.3–4.2 kg; Marshall Bio-Resources, North Rose, NY, USA) were anesthetized by isoflurane (2–5% oxygen) during surgery and switched to α-chloralose anesthesia (initial 65 mg/kg i.v. with supplemental doses as needed) during data collection. The right cephalic vein was catheterized for administration of fluid or anesthetics. The airway was kept patent by a tracheotomy. A catheter was inserted into the right carotid artery to monitor the blood pressure. A pulse oximeter (9847V; NONIN Medical, Plymouth, MN, USA) was attached to the tongue to monitor the heart rate and blood oxygen.
Via an abdominal incision a catheter was inserted into the distal urethra to slowly (1 mL/min) perfuse the urethra with saline and record the urethral pressure increase caused by contractions of external urethral sphincter (EUS) that was induced by pudendal nerve stimulation (Fig. 1). The ureters were tied, cut, and drained externally. The left pudendal nerve was exposed via a 3–4 cm incision in the sciatic notch lateral to the tail for implantation of a tripolar cuff electrode (NEC113, MicroProbes Inc., Gaithersburg, MD, USA) to deliver HFBS (Stim.B in Fig. 1). The tripolar cuff electrode had an inner cuff diameter of 1 mm with 1 mm distance between the three stainless-steel wire electrodes. Two stainless-steel hook electrodes were placed central (Stim.C) and distal (Stim.D) to the tripolar cuff electrode. The right pudendal nerve was also exposed and implanted with the same three sets of electrodes. Pudendal nerves were transected centrally to prevent reflex activation of the EUS (Fig. 1). The pudendal nerves and stimulation electrodes were immersed in a warm (35–37°C) mineral oil pool formed by retracting skin flaps. Stimulus pulses (20 Hz, 0.2 msec) generated by a stimulator (Grass S88, Grass Technologies, RI, USA) were delivered via a stimulation isolator (SIU5, Grass Technologies) to the hook electrodes (Stim.C or Stim. D) to induce >30 cmH2O increase in urethral pressure. HFBS (6 or 10 kHz square waveform without a pulse interval, see Fig. 1) generated by a computer running a LabView program (National Instrument, TX, USA) was delivered via a stimulation isolator (A395, World Precision Instruments, FL, USA) to the tripolar cuff electrode to block pudendal nerve conduction and suppress EUS contractions induced by Stim.C (Fig. 1).
Figure 1.
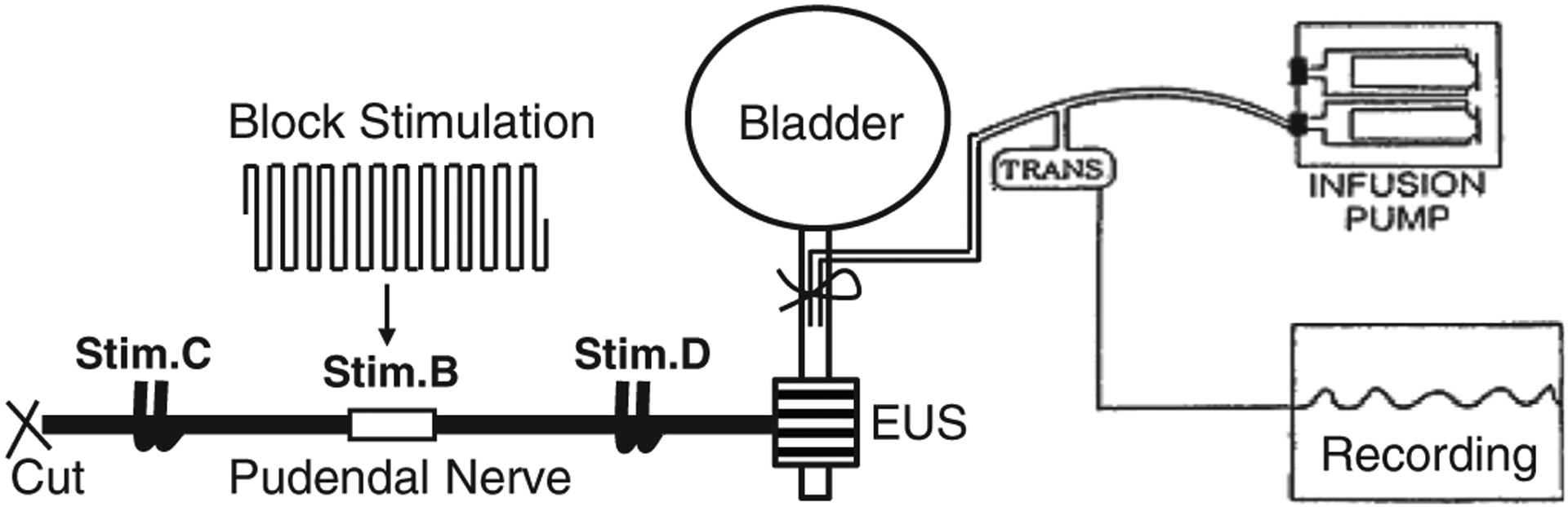
Experimental setup. Block stimulation is applied to the pudendal nerve by a tripolar cuff electrode (Stim.B) to block propagation of the action potentials induced by a bipolar hook electrode at a central site (Stim.C). Another bipolar hook electrode is placed at a distal site (Stim.D) to confirm that the external urethral sphincter (EUS) is not fatigued. The urethra is slowly perfused by an infusion pump so that the EUS contraction can be recorded by the increase in urethral pressure.
Stimulation Protocol
The intensity threshold for HFBS to block pudendal nerve conduction was determined at the beginning of every experiment by applying HFBS (10 kHz) of 30-sec duration via the tripolar cuff electrode at an increasing intensity starting from 1 mA with 1 mA increment (Fig. 2). Based on our previous studies (7,13), the minimal HFBS intensity that induced no increase in urethral pressure at the end of the 30-sec stimulation is the block threshold (T) that varied from 2 to 4 mA (N = 9 cats, Fig. 2) in this study.
Figure 2.
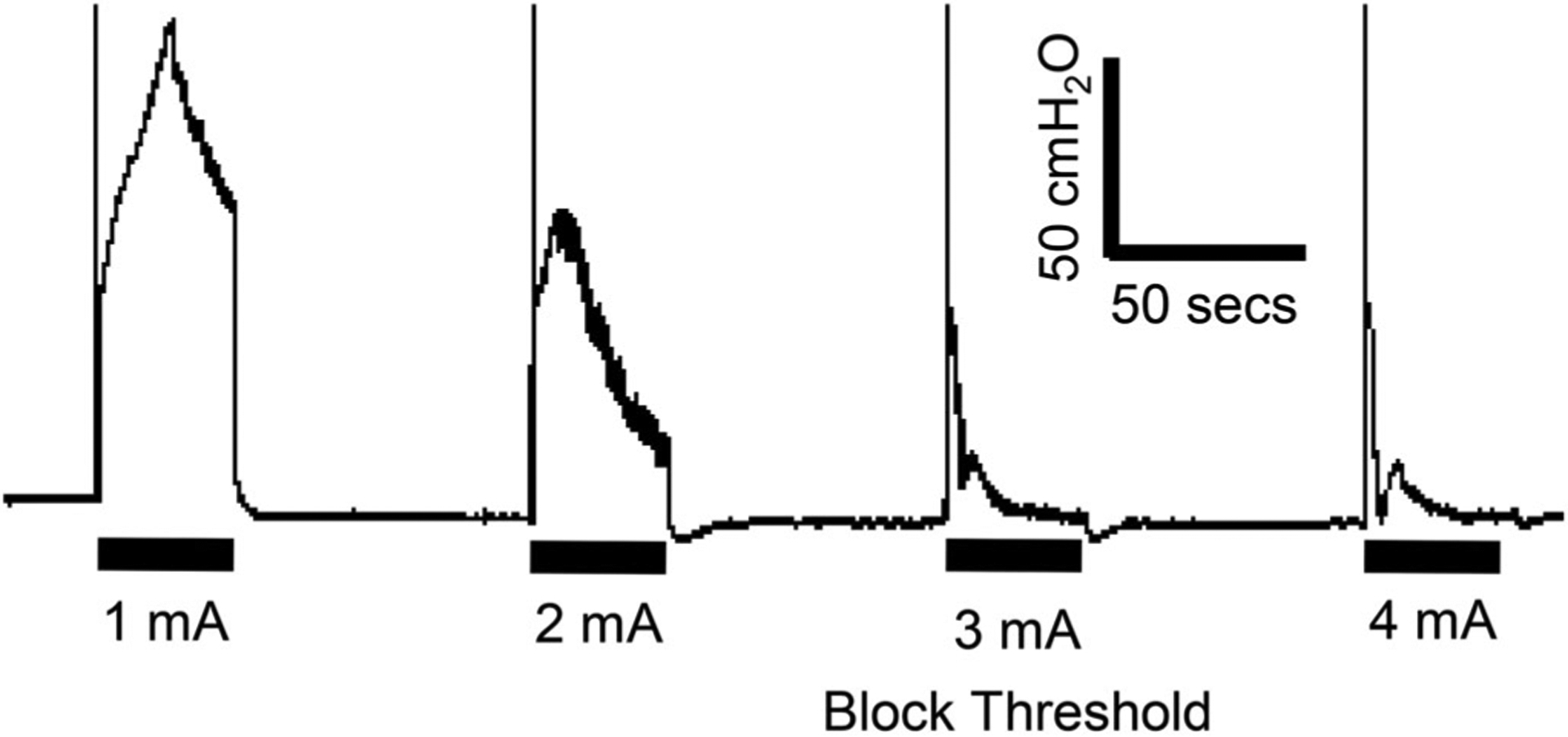
The block threshold is determined as the intensity of the 10 kHz block stimulation that produces no urethral pressure increase at the end of the 30-sec stimulation. The thick black bar under the bladder pressure trace indicates the stimulation duration (30 sec).
In the first three cats HFBS (10 kHz) of various intensities (1–2T) and durations (1–30 min) were tested to confirm that the pudendal nerve was blocked during the post-HFBS period and that fatigue of the EUS did not contribute to the suppression of the evoked responses by HFBS (Fig. 3). In the following six cats HFBS (Stim.B: 10 kHz, 1T) of different durations (1, 5, 10, 20, and 30 min) was applied to block the left pudendal nerve and suppress the urethral pressure increase induced by Stim.C (20 Hz, 0.2 msec, 5 sec on, and 55 sec off). Then, the HFBS intensity was increased to 2T and tested again for different durations (1, 5, 10, 20, and 30 min). After ending each HFBS, the next application of HFBS was delayed for a time period that was long enough to allow a full recovery of the pudendal nerve conduction. The waiting period for the post-HFBS block to recover was variable because HFBS of different intensities and/or durations produced post-HFBS block of variable durations (1–180 min). After testing the left pudendal nerve, the influence of different frequencies (6 or 10 kHz) on post-HFBS block was tested on the right pudendal nerve using HFBS of 2T intensity and 5-min duration in five cats. The 6 kHz is the minimal frequency required for blocking the pudendal nerve (9) while the 10 kHz is most often used in basic science and clinical studies.
Figure 3.
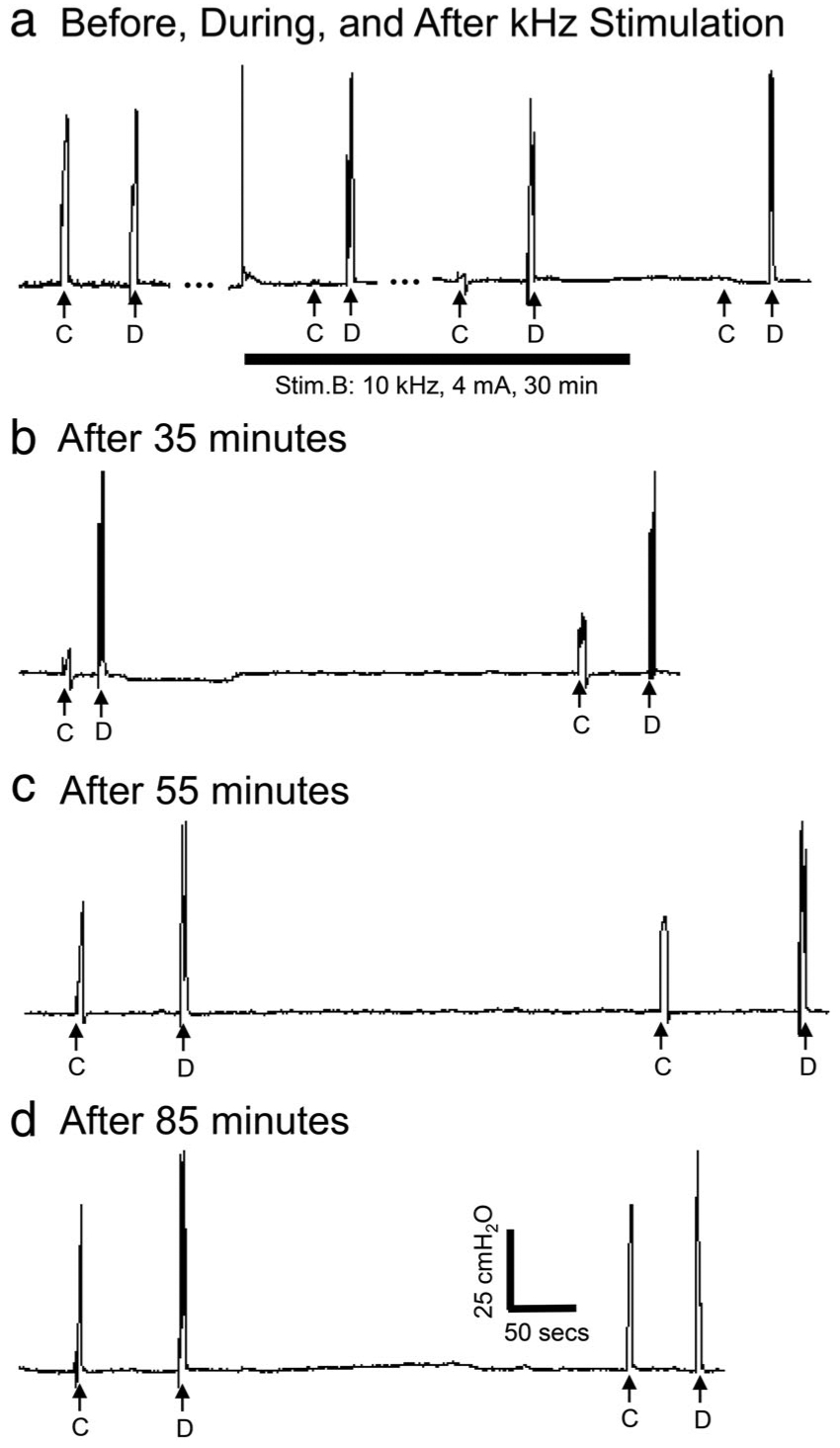
(a) Urethral pressure responses before, during, and after 30 min of 10 kHz block stimulation in a cat. C—Stim.C: 20 Hz, 0.2 msec, 2 V, 5 sec; D—Stim.D: 20 Hz, 0.2 msec, 4 V, 5 sec; Stim.B: 10 kHz, 30 min, 2T =4 mA. T: block threshold. During Stim.B the urethral pressure increase induced by Stim.C is blocked but the increase induced by Stim.D is not blocked, indicating that nerve conduction block occurs locally at the site of Stim.B without fatigue of the external urethral sphincter. (b–d) The block recovers gradually (35–85 min) after termination of 10 kHz block stimulation.
Data Analysis
To quantify the relationship between the recovery period of post-HFBS block and HFBS parameters, the complete block period and total recovery period (Fig. 4a) were measured after each application of HFBS. The total recovery period is defined as the post-HFBS period required for the urethral pressure response to recover to 90–100% of the pre-HFBS level. The data obtained under the same conditions in different animals were averaged and presented as mean ± SE. Paired Student’s t-test and repeated measures ANOVA followed by Dunnett’s or Bonferroni multiple comparison were used to detect a statistical significance (p < 0.05).
Figure 4.
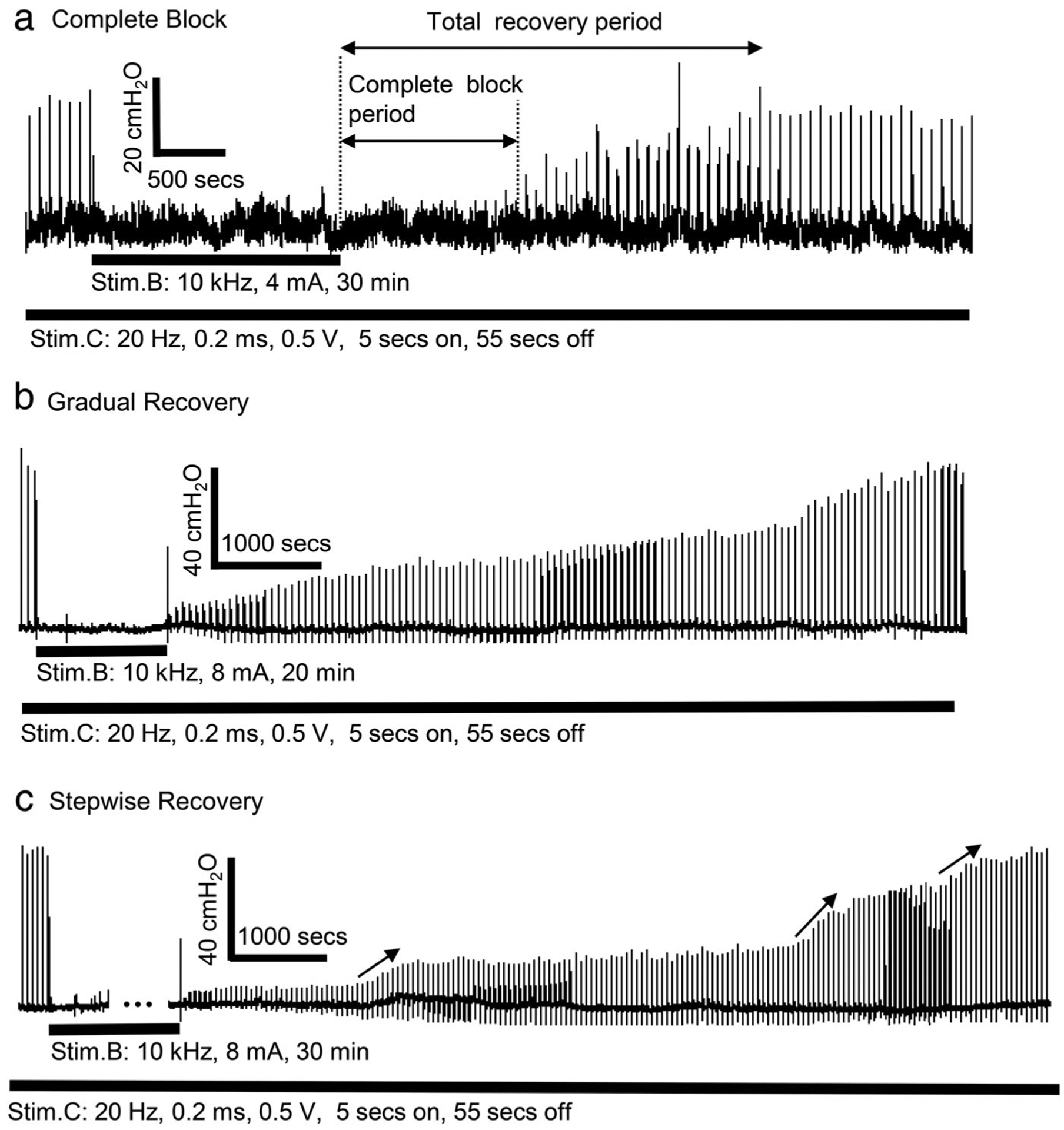
Poststimulation block induced by prolonged (10–30 min) 10 kHz stimulation at the intensity of two times block threshold (2T) in different cats. (a) Complete block of pudendal nerve conduction during the poststimulation period. (b) Gradual recovery of pudendal nerve conduction. (c) Stepwise recovery of pudendal nerve conduction. The thick black bar under the urethral pressure trace indicates the stimulation duration.
RESULTS
Post-HFBS Block of Pudendal Nerve Conduction
As shown in Fig. 2, 10 kHz HFBS at low intensities elicited increases in intraurethral pressure that were maintained throughout the 30-sec period of stimulation; while higher intensities induced transient increases in pressure that rapidly declined. The minimal intensity that produced a decline to zero pressure at the end of stimulation is defined as the block threshold (T =3 mA in Fig. 2).
Test stimulation (20 Hz, for 5 sec at submaximal intensities) at sites central (C) and distal (D) to the blocking electrode elicited brief increases in urethral pressure (Fig. 3a). The intensities of test stimuli were adjusted to elicit responses of approximately equal magnitude. Continuous application of 10 kHz HFBS (2T) for 30 min at site B completely suppressed the response to stimulation at the central site (C) but did not alter the response to stimulation at the distal site (D) (Fig. 3a) indicating axonal block at the site of the blocking electrode (Stim.B, Fig. 1). After termination of the HFBS nerve conduction was still completely blocked (Fig. 3a) and then over the course of 35–85 min the response to Stim.C gradually recovered (Fig. 3b–d) indicating a persistent partial block of axonal conduction induced by prolonged HFBS. The post-HFBS block was completely reversible in all animals (N = 9) after periods of 1–180 min depending on the HFBS intensity and duration (Figs. 4–6). The post-HFBS block was always repeatable after the evoked responses fully recovered.
Figure 6.
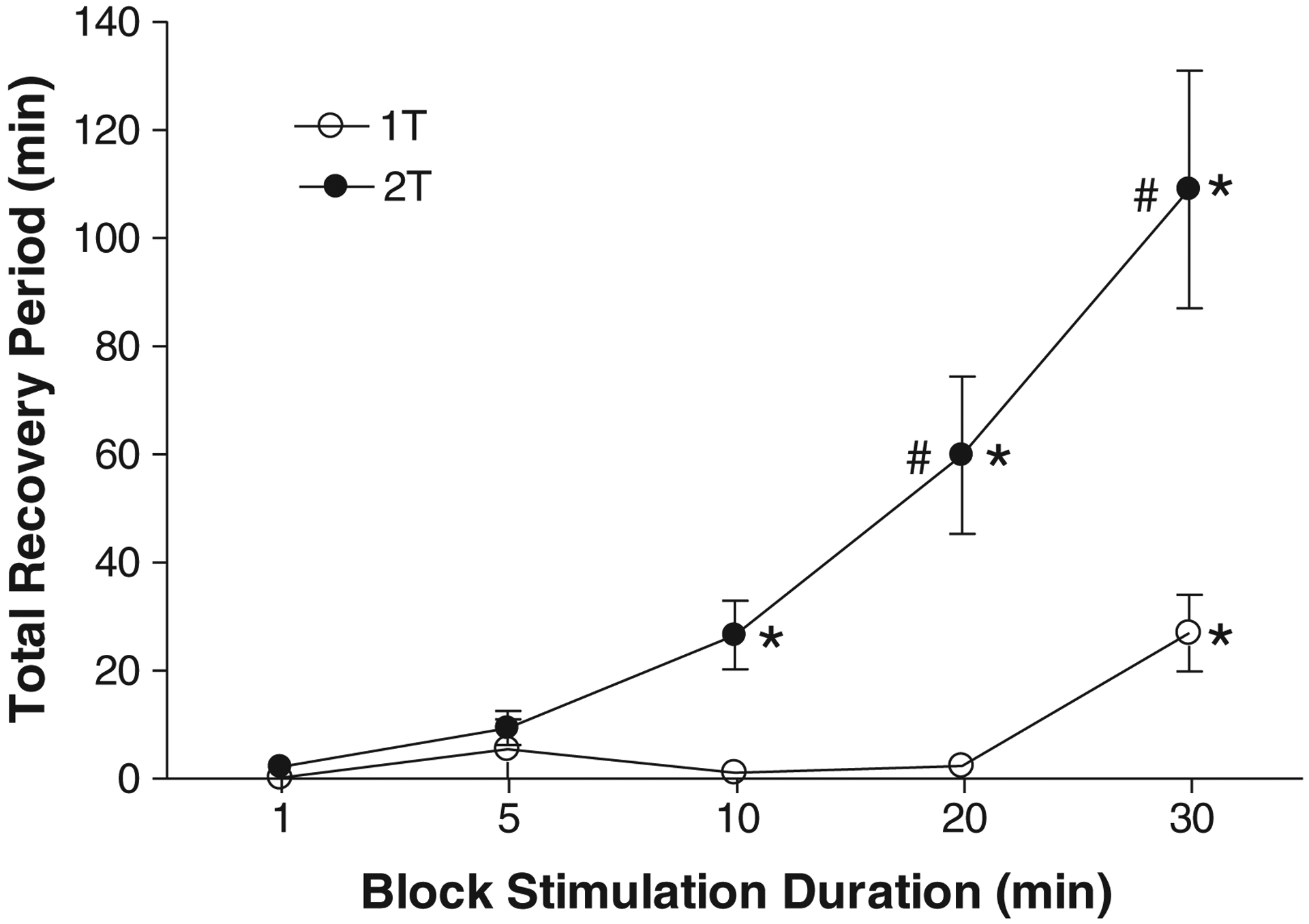
Total recovery period is dependent on the duration (1–30 min) and intensity (1–2T) of 10 kHz block stimulation. Stim.B: 10 kHz, T = 2–4 mA. T: block threshold. * indicates significantly (p < 0.05, one-way ANOVA followed by Dunnett’s multiple comparison) different from 1 min. # indicates significantly (p < 0.05, two-way ANOVA followed by Bonferroni multiple comparison) different from 1T. N = 6 cats.
Relationship Between the Recovery Period of Post-HFBS Block and HFBS Parameters
Post-HFBS block could occur in two distinct phases: an initial complete block followed by a recovery phase characterized by a slow gradual recovery (Fig. 4a). The complete block was only observed after prolonged (20 or 30 min) 10 kHz HFBS at 2T intensity but not at 1T intensity. HFBS (10 kHz) of 20-min duration induced complete post-HFBS block in four of the six cats tested with an average complete block period of 9.2 ± 7.6 min. HFBS (10 kHz) of 30-min duration induced complete post-HFBS block in five of the six cats with an average complete block period of 19.4 ± 12.6 min.
Two types of post-HFBS recovery were identified—a gradual recovery (Fig. 4b) and a stepwise recovery (Fig. 4c) where an increase in urethral pressure occurred more abruptly at several times during the recovery. The stepwise recovery was observed in five of the six cats after 2T HFBS (three cats after 30-min stimulation, one cat after 20-min stimulation, and one cat after both 20-and 30-min stimulation), while it was observed in three of the six cats after 1T HFBS (only after 30-min stimulation). For 1T HFBS of duration ≤20 min, the recovery from post-HFBS block was more rapid (<1–3 min) in most cases (Fig. 5).
Figure 5.
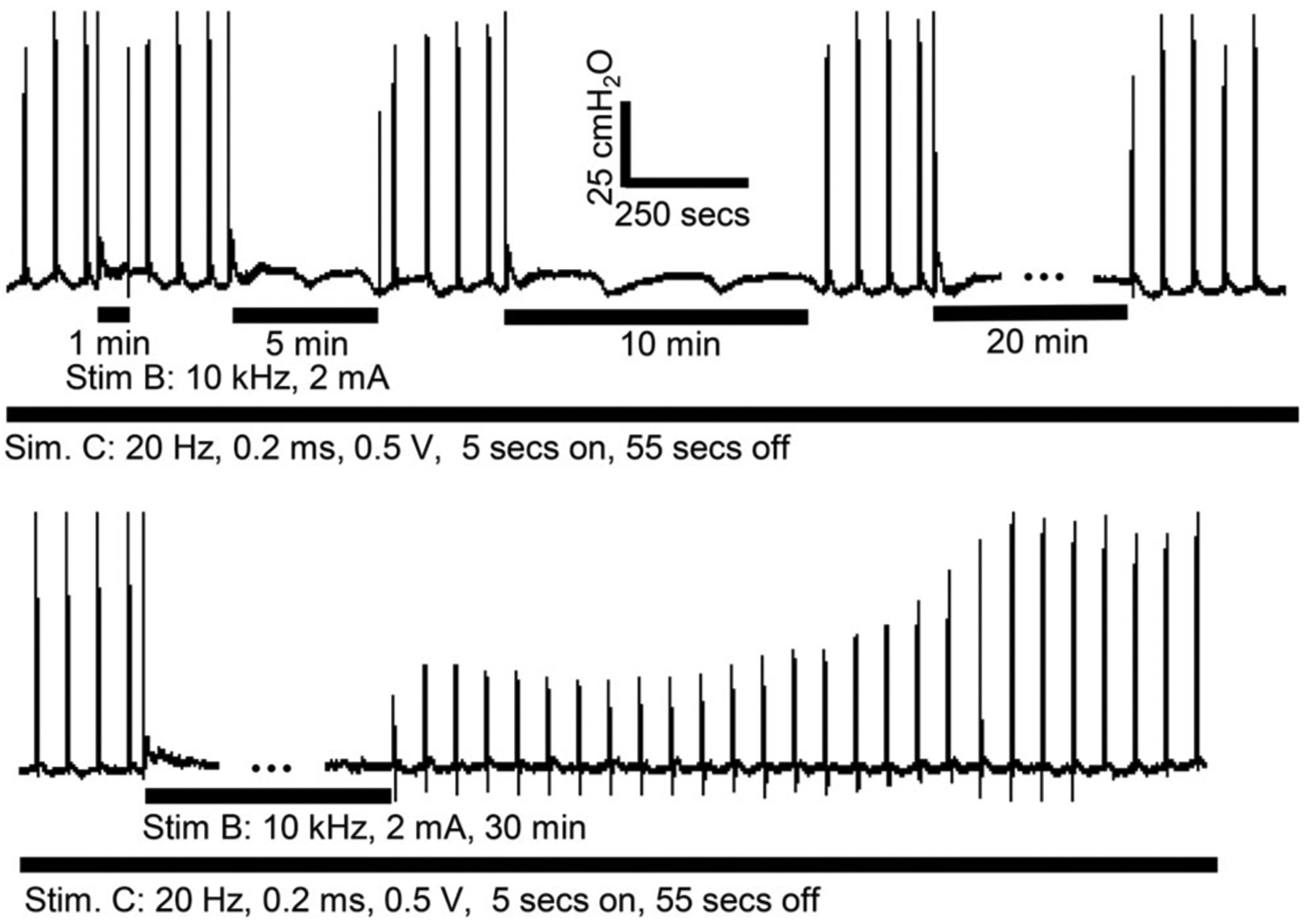
Poststimulation block induced by prolonged (30 min) 10 kHz stimulation at the block threshold (1T) intensity in a cat. The thick black bar under the urethral pressure trace indicates the stimulation duration.
On average, HFBS (10 kHz) at 2T intensity and duration ≥10 min or at 1T intensity and duration ≥30 min produced a significantly (p < 0.05, one-way ANOVA followed by Dunnett’s multiple comparison, N = 6 cats) longer period of post-HFBS block than HFBS of 1-min duration (Fig. 6). HFBS at 2T also induced a significantly (p < 0.05, two-way ANOVA followed by Bonferroni multiple comparison, N = 6 cats) longer period of post-HFBS block than 1T HFBS (Fig. 6). Therefore, HFBS of higher intensity or longer duration can produce longer periods of post-HFBS block. HFBS (10 kHz) of 2T intensity and 30-min duration induced the longest recovery period of 50–181 min (average, 109 ± 11 min, N = 6 cats, Fig. 6).
Influence of HFBS Frequency on Post-HFBS Block
To determine the influence of stimulation frequency on post-HFBS block, 6 or 10 kHz HFBS was tested at a fixed intensity (2T) with a fixed duration (5 min). The 5-min duration of stimulation was selected for this experiment because complete post-HFBS block was not observed with this short duration of stimulation and the recovery time was shorter thereby allowing multiple tests to be completed. Unexpectedly, 6-kHz HFBS produced a significantly (p <0.05, paired Student’s t-test) longer recovery period (20.4 ± 2.1 min) than the recovery period (9.5 ± 2.1 min) induced by 10-kHz HFBS (N = 5 cats, Fig. 7). Although the 6-kHz HFBS was always tested before the 10-kHz HFBS on the right pudendal nerve, this testing order did not influence the recovery period induced by 10-kHz HFBS of 5-min duration because a similar recovery period (9.4 ± 3.2 min, see Fig. 6) was also induced by 10-kHz HFBS alone on the left pudendal nerve.
Figure 7.
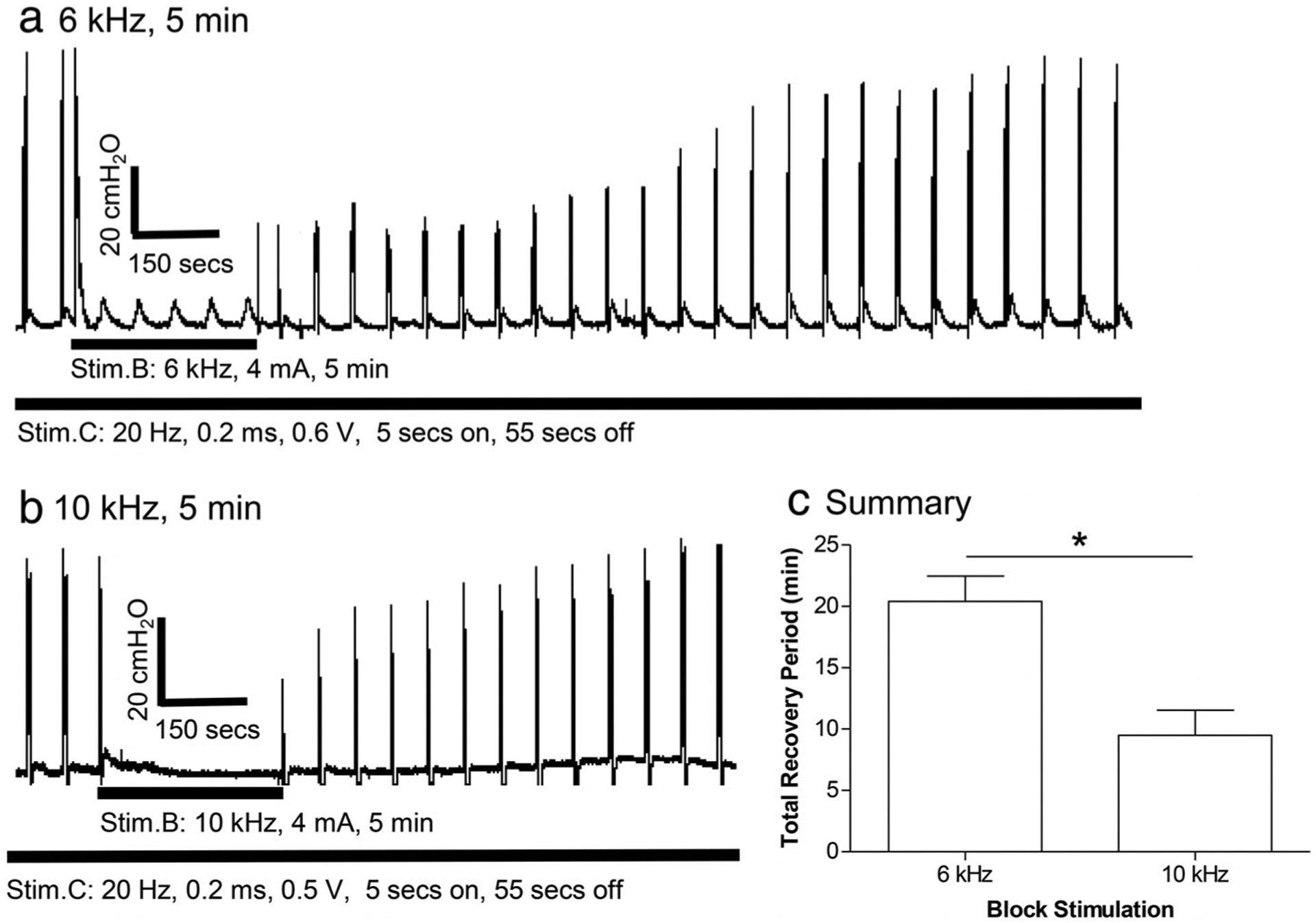
Effect of different kHz frequencies on poststimulation block. (a) Recovery from poststimulation block after 5 min of 6 kHz stimulation in a cat. (b) Recovery from poststimulation block after 5 min of 10 kHz stimulation in another cat. (c) Summary of the total recovery period after 5-min block stimulation at different frequencies. * indicates significantly (p < 0.05) different (paired Student’s t-test). Stim.B: 6 or 10 kHz, 5 min, 2T = 4–8 mA. T: block threshold measured at 10 kHz. N = 5 cats.
DISCUSSION
This study in cats revealed that the duration of post-HFBS block of axonal conduction of myelinated motor axons in the pudendal nerve is directly related to the intensity and duration of 10 kHz HFBS (Fig. 6). High intensity (2T) and prolonged (20–30 min) HFBS produced complete post-HFBS block followed by a recovery with either a gradual or a stepwise increase in EUS contractions (Fig. 4), which can persist for as long as 50–181 min (Figs. 3 and 6). Urethral pressure responses to stimulation of the pudendal nerve distal to the blocking electrode were not affected by HFBS (Fig. 3) indicating that neither a suppression of neuromuscular transmission or fatigue of the urethral sphincter muscle contributed to the initial or persistent HFBS block. When HFBS was applied for a relatively short duration (5 min) a higher frequency (10 kHz) produced a post-HFBS block with a shorter recovery period than that produced by a lower frequency (6 kHz) (Fig. 7). These results provide insights into the possible mechanisms underlying nerve conduction block produced by HFBS.
Our previous computer simulation studies (16,17) employing unmyelinated (Hodgkin–Huxley model) and myelinated (Frankenhaeuser–Huxley model) axonal models have shown that each pulse of the HFBS can induce an inward sodium current and an outward potassium current, which will certainly increase the concentrations of intracellular sodium and extracellular potassium ions. The HFBS used in this study has a continuous waveform without an interval between the square pulses (Fig. 1), which leaves no time for the sodium–potassium pump to restore the normal ion concentration gradients. Therefore, if HFBS is applied for a long time, it will increase the intracellular sodium and extracellular potassium concentrations to the levels that are high enough to block axonal conduction. This block will be maintained after termination of the HFBS until the sodium–potassium pump can restore the normal ion concentrations. Hence, the recovery period for post-HFBS block should depend on the speed of the sodium–potassium pump and the magnitude of the changes in ion concentrations. This hypothesis can fully explain the relationship between the recovery period of post-HFBS block and the HFBS parameters as observed in this study. At a low intensity (1T) HFBS requires at least 30 min to accumulate enough changes in ion concentrations to induce a post-HFBS block (Figs. 5 and 6). At a high intensity (2T) the HFBS duration for inducing a post-HFBS block is shortened to 10 min (Fig. 6), because each HFBS pulse at 2T can generate larger ion currents than the low intensity (1T) pulse thereby producing larger changes in ion concentrations and requiring a shorter time to achieve the block. Furthermore, at the same intensity (2T) HFBS of a longer duration can also produce larger changes in ion concentrations requiring a longer period for the sodium–potassium pump to restore the ion concentrations to normal levels thereby producing a longer duration post-HFBS block (Fig. 6). Since ion concentrations must reach a maximal level, our hypothesis further predicts that the post-HFBS block should also have a maximal recovery period. Therefore, if HFBS (2T) of durations >30 min are tested in future experiments, a maximal post-HFBS block period should be observed (see Fig. 6). It is well known that after periods of activity, the sodium–potassium pump works slowly to restore normal ion concentrations possibly taking hours to normalize membrane excitability depending on the extent of the changes in ion concentrations (18). This may explain the maximal recovery period obtained in this study (1–3 h with an average about 2 h, Fig. 6). The large variation of the maximal recovery period could be due to many factors including the accuracy of the 10 kHz block threshold and the efficacy of ion pumps that can be influenced by the variation in the blood supply to the nerve in different animals.
In one experiment where a short period of HFBS was tested in the same preparation at two frequencies, the lower frequency (6 kHz) induced a longer post-HFBS block period than the higher frequency (10 kHz) (Fig. 7). This can be explained by the fact that the lower frequency has a wider pulse width than the higher frequency because a continuous waveform was used in this study to generate the HFBS (see Fig. 1). A longer pulse can maintain the peak ion current for a longer time. Multiple shorter pulses of the same total pulse width as the single longer pulse can only maintain the peak ion current for a shorter time because the multiple shorter pulses will have to increase the ion current from zero level multiple times thereby reducing the total duration of the maximal ion current. Therefore, the lower frequency (6 kHz) is more effective than the higher frequency (10 kHz) to induce changes in ion concentrations thereby producing a longer post-HFBS block period.
The initial nerve firing induced by HFBS could be prevented if the HFBS starts at an intensity below its excitation threshold. It is possible that the sub-threshold HFBS may, if applied continuously, gradually change axonal ion concentrations and produce a delayed onset increase in the excitation threshold of axons. If this change occurs, the intensity of sub-threshold HFBS could be increased to a new sub-threshold level after minutes or hours of stimulation. In theory repeating this procedure could eventually allow HFBS to reach an intensity high enough to produce large changes in ion concentrations causing a nerve conduction block without producing an initial excitation of the nerve. If the initial nerve firing is not a concern, then the HFBS could be applied at a supra-threshold intensity that would significantly shorten the time to achieve a post-HFBS block. More studies are needed to answer these interesting questions.
Stepwise recovery was observed in five of the six cats (Fig. 4c). Similar recovery was also observed in our previous study using frog sciatic nerve (13), although the stepwise increase occurred more rapidly in frogs than in cats. The stepwise recovery indicates that a group of axons must recover conduction simultaneously during a short period of time. Therefore, the three steps in increasing urethral pressure as shown in Fig. 4c indicates a possibility that three distinct groups of axons recovered conduction at different times as the sodium–potassium pump gradually restored the normal ion concentrations. The different groups of axons could have very different axon diameters because large or small diameter axons might recover differently from changes in ion concentration. This could be due to multiple reasons: 1) activity of sodium–potassium pump is different in different size axons; 2) different groups of axons recover conduction at different ion concentrations; 3) changes in ion concentrations vary in different size axons. These possibilities still need to be investigated.
Post-HFBS block was also reported previously in frog and rat sciatic nerves (13,14). The study in rats (14) showed that post-HFBS block can be observed after about 15 min of HFBS at 1.2–1.3 T intensity, which agrees well with our result that a minimum of 10 min is required at 2T intensity (Fig. 6). It is unfortunate that the rat study did not determine the relationship between the HFBS parameters (intensity, frequency, and duration) and the recovery period of post-HFBS block because the HFBS was applied intermittently (5–10 min on with 5–10 min off). However, the rat study did show that the level of post-HFBS block was increased as the total duration of the intermittent HFBS increased, which supports our hypothesis about the importance of cumulative changes in the ion concentrations in the generation of post-HFBS block. Our previous study using frog sciatic nerve (13) also showed that the recovery period of post-HFBS block is dependent on HFBS intensity and duration, which agrees with our current result. However, post-HFBS block of frog sciatic nerve occurs after a minimum of 4–5 min of HFBS at 1.4–2T intensity, which is less than the HFBS duration (10 min) required in the current study. This difference indicates that amphibian nerve might be more sensitive to post-HFBS block than mammalian nerve. Our previous frog study (13) was performed at room temperature about 20°C, while the temperature of the mineral oil pool in the current cat study was around 35–37°C. Therefore, the difference between our previous frog study and current cat study could also be due to a lower activity of the sodium–potassium pump at a lower temperature, which produces a faster cumulative change in ion concentrations and a slower recovery. In addition, the post-HFBS block of frog sciatic nerve was not dependent on HFBS frequency (5 or 10 kHz), indicating a possible blocking mechanism different from that in mammalian nerve.
It is worth noting that the HFBS was tested using incremental durations of stimulation and stimulus intensities. Although a waiting period long enough for the post-HFBS block to fully recover was used, accumulative effects from previous HFBS application that cannot be detected by EUS pressure measurement could still exist, which might cause the HFBS of longer duration or higher intensity to become more effective in producing post-HFBS block. However, this potential accumulative effect should not change our conclusion that post-HFBS can be more easily induced by kHz stimulation of longer duration and higher intensity. In addition, only large motor A-fibers controlling EUS contraction were investigated in this study. Whether post-HFBS can also occur in small C-fibers that are more relevant to painful sensations still needs to be investigated in the future.
This study using cat pudendal nerve provides evidence consistent with the hypothesis that post-HFBS block is due to the changes in intracellular/extracellular ion concentrations produced by prolonged HFBS. Understanding the mechanisms of HFBS block is important to develop new methods to block nerve conduction or improve current clinical applications of HFBS to treat chronic disorders (4–8).
Source(s) of financial support:
This study is supported by the National Institutes of Health under grants NS109198, DK121698, and DK111382.
Footnotes
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Conflict of Interest: Dr. Tai is the inventor of a patent application related to this study. The other authors declare no conflicts of interest.
REFERENCES
- 1.Cattell M, Gerard RW. The “inhibitory” effect of high-frequency stimulation and the excitation state of nerve. J Physiol 1935;83:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reboul J, Rosenblueth A. The action of alternating currents upon the electrical excitability of nerve. Am J Physiol 1939;125:205–215. [Google Scholar]
- 3.Rosenblueth A, Reboul J. The blocking and deblocking effects of alternating currents on nerve. Am J Physiol 1939;125:251–264. [Google Scholar]
- 4.Soin A, Shah NS, Fang ZP. High-frequency electrical nerve block for postamputation pain: A pilot study. Neuromodulation 2015;18:197–205. [DOI] [PubMed] [Google Scholar]
- 5.Finch P, Price L, Drummond P. High-frequency (10 kHz) electrical stimulation of peripheral nerves for treating chronic pain: a double-blind trial of presence vs absence of stimulation. Neuromodulation 2018;22:529–536. 10.1111/ner.12877. [DOI] [PubMed] [Google Scholar]
- 6.Apovian CM, Shah SN, Wolfe BM et al. Two-year outcomes of vagal nerve blocking (vBloc) for the treatment of obesity in the ReCharge trial. Obes Surg 2017;27:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang G, Wang J, Shen B, Roppolo JR, de Groat WC, Tai C. Pudendal nerve stimulation and block by a wireless-controlled implantable stimulator in cats. Neuromodulation 2014;17:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaunt RA, Prochazka A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil Neural Repair 2009;23:615–626. [DOI] [PubMed] [Google Scholar]
- 9.Tai C, Roppolo JR, de Groat WC. Block of external urethral sphincter contraction by high frequency electrical stimulation of pudendal nerve. J Urol 2004;172:2069–2072. [DOI] [PubMed] [Google Scholar]
- 10.Bhadra N, Kilgore KL. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve 2005;32:782–790. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Zhu L, Sheng S et al. Post stimulus effects of high frequency biphasic electrical current on a fibre’s conductibility in isolated frog nerves. J Neural Eng 2013;10:036024. [DOI] [PubMed] [Google Scholar]
- 12.Waataja JJ, Tweden KS, Honda CN. Effects of high-frequency alternating current on axonal conduction through the vagus nerve. J Neural Eng 2011;8:056013. [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Xiao Z, Wang J et al. Poststimulation block of frog sciatic nerve by high-frequency (kHz) biphasic stimulation. Med Biol Eng Comput 2017;55:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhadra N, Foldes E, Vrabec T, Kilgore K, Bhadra N. Temporary persistence of conduction block after prolonged kilohertz frequency alternating current on rat sciatic nerve. J Neural Eng 2018;15:016012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avendano-Coy J, Serrano-Munoz D, Taylor J, Goicoechea-Garcia C, Gomez-Soriano J. Peripheral nerve conduction block by high-frequency alternating currents: a systematic review. IEEE Trans Neural Syst Rehabil Eng 2018;26:1131–1140. [DOI] [PubMed] [Google Scholar]
- 16.Tai C, de Groat WC, Roppolo JR. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans Neural Syst Rehab Eng 2005;13:415–422. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Roppolo JR, de Groat WC, Tai C. Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Trans Biomed Eng 2006;53:2445–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clausen T Quantification of Na+, K+ pumps and their transport rate in skeletal muscle: functional significance. J Gen Physiol 2013;142:327–345. [DOI] [PMC free article] [PubMed] [Google Scholar]


