Abstract
The endangered black-footed ferret (BFF; Mustela nigripes) is an important example of the benefits of assisted reproduction in species conservation with both semen evaluation and artificial insemination using fresh and frozen sperm being successfully incorporated into the breeding program. Currently, electroejaculation (EE) is routinely utilized for semen collection in BFFs, a technique that requires custom equipment and experienced operators, and does not consistently yield viable samples in this species. In this case study, we evaluated the feasibility of urethral catheterization (UC) for semen collection, a method predominately tested in domestic and non-domestic felids, on four occasions (three BFF males). After general anesthesia with a combination of ketamine, midazolam and α2-agonist dexmedetomidine (thought to promote semen release into the urethra), a lightly lubricated, flexible feeding tube was passed into the urethral opening and advanced ~7–8 cm into the urethra. A syringe attached to the feeding tube was used to apply mild negative pressure to collect sperm. Semen samples were successfully collected on all four attempts. Sperm characteristics ranged as follows: 10.5–26.0 × 106 sperm/ml concentration, 50–90% motility and 36–61% normal sperm morphology. This is the first report of the use of UC as a potential alternative to EE in the BFF, a more field-friendly technique that is less invasive and more consistent for obtaining samples free of urine contamination.
Keywords: Assisted reproductive technologies, electroejaculation, ferret, semen analysis, urethral catheterization
Introduction
Assisted reproductive technologies (ARTs), particularly artificial insemination (AI), have the potential to significantly enhance the genetic management and long-term conservation of threatened wildlife species. ARTs can be used to improve the reproductive performance of captive individuals, ensure the contribution of genetically distinct individuals and mitigate behavioural incompatibilities or other health-related issues (Howard and Wildt, 2009). Furthermore, cryopreserved sperm from genetically valuable males can be stored in biobanks for several generations providing insurance against a sudden or drastic loss of genetic diversity and avoiding genetic drift in small populations (Wildt and Roth, 1997; Wildt and Wemmer, 1999).
Assisted reproduction has played an important role in the current success of the black-footed ferret (BFF; Mustela nigripes) recovery strategy. In 1988, a recovery program was initiated with the remaining 18 BFFs, which emphasized a multi-institutional ex situ breeding program and the development of ARTs for the species (US Fish and Wildlife Service, 1988). From seven founder individuals, the captive breeding population is currently > 300 animals across six institutions, while more than 4300 ferrets have been released back into the wild since 1991 (Santymire and Graves, 2019). More importantly, AI was involved in the production of ~ 140 offspring using fresh and frozen/thawed semen (Howard and Wildt, 2009).
The collection of semen is a fundamental step for fertility assessment, assisted reproduction and genome banking (Lueders et al., 2012). Electroejaculation (EE) has been employed as the method of choice for most non-domestic species due to its ability to collect semen from males that are unable to mate naturally due to physical or behavioural handicaps (Leibo and Songsasen, 2002; Tecirlioglu et al., 2002). EE uses rectal probes that release increasing electrical pulses in a stepwise fashion until ejaculation is achieved (Brindley, 1981). However, EE requires expensive and custom equipment and an experienced operator; furthermore, permission to use the technique is not readily granted in many parts of the world, including European countries (Falk et al., 2001; Zambelli et al., 2008). Lastly, utilization of EE for in situ sperm collection is complicated by the need for electricity that can prove impractical in remote locations.
Urethral catheterization (UC) after medetomidine administration, also known as the ‘Zambelli’ method, is a novel technique that has been successfully performed in domestic cats (Felis catus—Zambelli et al., 2008), African lions (Panthera leo—Lueders et al., 2012), Asiatic golden cats (Catopuma temminckii—Lueders et al., 2014), jungle cats (Felis chaus—Kheirkhah et al., 2017), Amur leopards (Panthera pardus orientalis—Jeong et al., 2018), polar bears (Ursus maritimus—Curry and Roth, 2015) and Asiatic black bears (Ursus thibetanus—Jeong et al., 2019). Zambelli et al. (2008) found that sperm concentration following EE was significantly higher after the administration of medetomidine compared to ketamine. The ductus deferens is thought to contain alpha-adrenoreceptors, and therefore, alpha-2 agonists, such as medetomidine, may enhance smooth muscle contractions of the ductus deferens causing semen to be released into the pelvic urethra (Turner et al., 1995; Johnston and Deluca, 1998). UC has been found to result in lower semen volume, but higher sperm concentration and lower urine contamination in comparison to EE (Zambelli et al., 2008; Jeong et al., 2019). Higher-quality semen samples, minimal invasiveness and lower cost make UC an attractive alternative toEE.
Currently, EE is routinely used for semen collection in BFFs with variable success. The purpose of this case study was to evaluate the feasibility of the ‘Zambelli’ method for reproductive assessment of male BFFs prior to breeding introductions in captivity or for future use in thewild.
Materials and methods
Animals and housing
All protocols and procedures were conducted in accordance with the Toronto Zoo Animal Care and Research Committee guidelines for animal use. Three male BFFs aged 10 months to 4 years were included in the study over two consecutive breeding seasons (2018 and 2019; one animal repeated in both years). Animals were housed indoors at an ambient temperature of 19–22 °C in individual pens (84 cm × 213 cm × 92 cm) illuminated by overhead fluorescence bulbs on a changing light:dark cycle to mimic natural seasonal lighting conditions (US Fish and Wildlife Black-footed Ferret Managed Care Operations Manual). Males were fed 60–70 g of Toronto Zoo Small Carnivore Diet (Milliken Meat Products; Scarborough, ON, Canada) daily, supplemented with a whole animal (mouse or rat) twice weekly. Testicular firmness was manually assessed daily and graded on a scale of 1–3 (1-firm, 3-flaccid). Semen collection was only attempted on animals with a sustained testicular firmness of 1–1.5 for at least 30 days.
Anesthesia and semen collection
Animals were anesthetized using an intramuscular (i.m.) combination of ketamine (Ketaset®; 2 mg/kg; Zoetis Canada Inc. Kirkland, QC, Canada), midazolam (Midazolam sterile injectable solution; 0.2 mg/kg; Sandoz Canada Inc. Boucherville, QC, Canada) and dexmedetomidine (Dexdomitor®; 25 ug/kg; Orion Pharma, Espoo, Finland). Additional ketamine was administered subcutaneously (s.c) throughout the procedure as required. Based on a previous study in cats, semen collection by UC was conducted no sooner than 25 minutes post anesthesia induction to ensure adequate time for the dexmedetomidine to stimulate semen release into the ureter (Swanson et al., 2016).
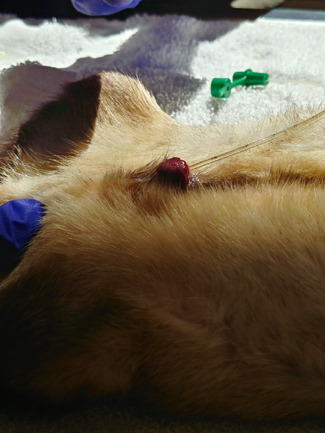
Following Brown and Pollock (2010), the location and distension of the urethral opening was achieved by the insertion of a 24 G intravenous catheter without the needle, using the groove of the baculum as a guide (Fig. 1). The opening is ~4 mm from the distal tip of the J-shaped penis. A lightly lubricated (Priority Care, First Priority, Inc., IL, USA; non-spermicidal lubricant) 3.5 Fr × 31 cm flexible argyle feeding tube (cat# 8888-261206, Sherwood Medical, St. Louis, Missouri, USA or cat#461206, Covidien Ilc, Mansfield, MA, USA) was inserted adjacent to the intravenous catheter. Following successful insertion, the intravenous catheter was slowly removed and the feeding tube was advanced ~7-8 cm into the urethra (Fig. 2). Negative pressure was created by applying a mild suction (0.1–0.2 ml) using a 3 ml syringe attached to the end of the catheter. After 30 seconds in situ, the catheter was retracted 4 cm, and 0.2 ml of negative pressure was applied and held for an additional 30 seconds before retraction of the catheter from the penis. Urine contamination in the catheter was noted. Semen was immediately ejected into a 1.5 ml microcentrifuge tube containing 200 μl of pre-warmed (37 °C) semen extender (TEST yolk refrigeration medium; Irvine Scientific, Santa Ana, CA, USA) and held at 37 C.
Figure 1. Location and distension of the opening of the urethra using an intravenous catheter without the needle and the groove of the baculum as a guide.
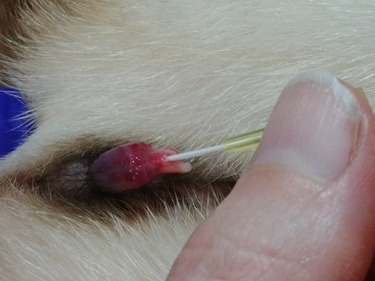
Figure 2. Placement of the feeding tube in the urethra.
Semen evaluation
Samples were microscopically assessed for the presence or absence of spermatozoa, and subsequently evaluated for motility (subjectively; 0–100%), progressive motility (0–5 scale; 0 = non-motile and 5 = rapid forward movement) and concentration using a hemocytometer. Due to the minimal volumes (<10 μl) of semen retrieved from the catheter, sperm concentration and total sperm count were calculated based on the initial 200 μl dilution volume of extender. A small volume was also smeared on a microscope slide, which was then fixed in 1% paraformaldehyde before staining (Spermac Stain; FertiPro NV, Beernem, Belgium) to evaluate sperm morphology.
Results
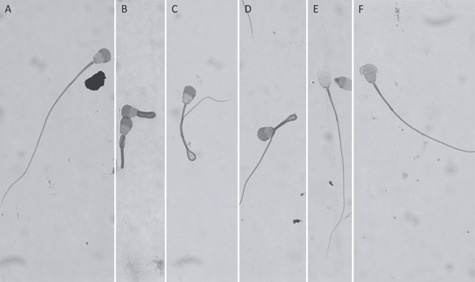
Semen was successfully collected from all three males (four attempts, one male repeated in each collection year). During the procedure, resistance occurred 6 cm post insertion (tip of catheter palpable at the caudal flexion of the penis, immediately caudal to the proximal baculum) but could be easily passed with gentle manipulation. In male ‘1’, a different catheter (Covidien) was attempted and proved to be more difficult to insert, resulting in a delay of 70 minutes from injection of anesthetic to collection. Sperm motility ranged from 50% to 90% with progressive motility ranging from 3–5 (Table 1). Sperm concentration ranged from 10.5–26.0 × 106/ml (Table 1). Normal sperm morphology ranged from 36–61% with coiled tails, bent tails and mid-pieces and damaged or absent acrosomes being the predominant abnormalities (Table 1, Fig. 3). Urine contamination was not observed in any of the samples.
Table 1. Evaluation of sperm characteristics following collection byUC.
| Individual | ||||
|---|---|---|---|---|
| 1 | 2 (2018) | 2 (2019) | 3 | |
| Age (year) | 4 | 1 | 2 | 2 |
| Time from injection to collection (min) | 81 | 30 | 27 | 35 |
| Motility (%) | 90 | 60 | 90 | 50 |
| Progressive motility (0–5) | 4–5 | 2 | 4 | 3–4 |
| Sperm concentration (×106/ml)a | 15 | 10.5 | 26 | 18.5 |
| Total sperm count (×106)a | 3 | 2.1 | 5.2 | 3.7 |
| Normal morphology (%) | 59 | 49 | 61 | 36 |
| Abnormal morphologyb: | ||||
| Coiled tails (%) | 5 | 23 | 12 | 26 |
| Bent tail (%) | 7 | 11 | 16 | 17 |
| Bent midpiece (%) | 2 | 2 | 4 | 8 |
| Damaged acrosome (%) | 32 | NR | 33 | 32 |
| Absent acrosome (%) | 27 | NR | 10 | 18 |
a Calculation based on initial volume of extender present in collectiontube.
b Only commonly observed abnormalities are listed. NR = data not recorded.
Figure 3. Morphological assessment of sperm stained with Spermac® showing normal and predominant abnormalities observed. (A) normal, (B) coiled tail, (C) bent tail, (D) bent mid-piece, (E) absent acrosome, (F) damaged acrosome.
Discussion
To our knowledge, this is the first study to demonstrate the use of UC to successfully obtain semen from BFFs on all four attempts. Currently in BFFs, semen collection is routinely carried out using EE, a protocol that can only be undertaken by facilities having the necessary equipment and trained operators. Furthermore, the technique has been difficult to implement consistently and successfully in this species. Our results indicate that UC can be considered as an alternative method for semen collection and reproductive assessment in BBFs. UC has been described as being less invasive, less expensive and quicker to perform than EE, as well as producing samples with lower urine contamination and higher sperm concentration compared to EE (Zambelli et al., 2008; Lueders et al., 2012; Swanson et al., 2016; Jeong et al., 2018). Additionally, unlike EE, UC does not require electricity, which, combined with the ease of implementation, makes this a more feasible technique for in situ collections. Notably, utilization of medetomidine in the induction cocktail, which is not routinely used with ferrets in our facility, did not have any adverse effects on the animals or the procedure.
Studies collecting semen via EE in BFFs reported an average volume of 26.9 ± 3.1 μl and sperm concentration of 558.6 ± 121.5 × 106/ml (Santymire et al., 2006). Howard et al. (2015) documented similar values with average ejaculate volume of 20 ± 0.2 μl and sperm concentration of 772.7 ± 133.3 × 106/ml. In the current study, samples were immediately diluted into sperm extender (TEST) to conserve sperm viability, preventing accurate volume measurements. Although the UC method resulted in lower semen concentration in comparison to previous reports using EE, these outcomes may have been influenced by training period (i.e. implementation of a new technique), low sample size and individual reproductive status of the males. Furthermore, catheterization was not repeated if an adequate number of sperm were observed, possibly resulting in incomplete collection of all available sperm in the urethra. Reports describing sperm morphology and incidence of abnormalities vary greatly in this species, and appear to be impacted by age (Wolf et al., 2000) and level of inbreeding (Santymire et al., 2018). Sperm morphology following UC was similar to that described previously (0–89%, μ = 33 ± 1.1; Santymire et al., 2018) with the predominant abnormalities including damaged acrosomes, coiled tails and bent tails or midpieces.
Unexpectedly, in this small sample size, the type of catheter greatly impacted the ease of insertion into the urethra. Although both catheters, Sherwood Medical and Covidien, were made of polyvinylchloride, identical in size (3.5 Fr) and lumen opening shape, and labelled as feeding tubes, the Sherwood Medical feeding tube was much easier to insert and advance into the urethra allowing for more rapid sperm collection (<5 minutes) compared to the Covidien feeding tube (>1 hour). We were also unable to discern a palpable difference between the catheters, but we suspect a slight variance in rigidity, despite reassurance from the manufacturer that there is no difference in their composition. Clinically, recommendations for urethral catheters in ferrets to clear urinary blockages include opaque 3.0 Fr silicone catheters known as ‘Slippery Sam’, 3.5 Fr red rubber catheters or 3.5 Fr Tom cat catheters (Marini et al., 1994; Brown and Pollock, 2010; Hoefer, 2013). In our experience, clear flexible tubing is ideal for easy manipulation and visualization allowing the operator to determine a successful collection without needing to fully remove the catheter before repositioning.
Our attempts to perform EE in BFFs have been met with limited success with negligible volume and sperm being obtained during most retrievals. Although the sperm concentration reported in this case study was lower, the samples obtained following UC were a significant improvement to previous attempts with EE. This suggests that further investigation of UC is warranted in this and other small, difficult-to-collect species. Most importantly, development of this minimally invasive and easily applicable technique could be a valuable alternative for researchers working with small mammals in the field.
Funding
This work was supported by the Toronto Zoo.
Acknowledgments
We would like to thank the dedicated Toronto Zoo wildlife care staff, Gerri Mintha and Ayesha Beyersbergen, for their assistance with the BFF semen evaluations and breeding program.
References
- Brindley GS. (1981) Electroejaculation: its technique, neurological implications and uses. J Neurol Neurosurg Psychiatry 44: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C, Pollock C (2010) Urethral catheterization of the male ferret for treatment of urinary tract obstruction. Lab Anim (NY) 40: 19–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry E, Roth T (2015) A rapid, minimally invasive method of collecting semen from polar bears. Reprod Fertil Dev 28: 189. [Google Scholar]
- Falk AJ, Waldner CL, Cotter BS, Gudmundson J, Barth AD (2001) Effects of epidural lidocaine anesthesia on bulls during electroejaculation. Can Vet J 42: 116–120. [PMC free article] [PubMed] [Google Scholar]
- Hoefer H. (2013) Excellence in exotics: practice tip: ferret urinary tract catheterization. Compend Contin Educ Vet 35: E6. [PubMed] [Google Scholar]
- Howard JG, Wildt D (2009) Approaches and efficacy of artificial insemination in felids and mustelids. Theriogenology 71: 130–148. [DOI] [PubMed] [Google Scholar]
- Howard JG, Lynch C, Santymire RM, Marinari PE, Wildt DE (2015) Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Anim Conserv 19: 102–111. [Google Scholar]
- Jeong D, Kim J, Na K (2018) Characterization and cryopreservation of Amur leopard cats (Prionailurus bengalensis euptilurus) semen collected by urethral catheterization. Theriogenology 119: 91–95. [DOI] [PubMed] [Google Scholar]
- Jeong D, Yang J, Seo M, Lee A, Lim Y (2019) Effectiveness of urethral catheterization under ultrasound guidance for semen collection from Asiatic black bears (Ursus thibetanus). Theriogenology 129: 154–159. [DOI] [PubMed] [Google Scholar]
- Johnston P, DeLuca J (1998) Chemical ejaculation of stallions after the administration of oral imipramine followed by intravenous xylazine. AAEP Proceedings 44: 12–15. [Google Scholar]
- Kheirkhah M, Mollapour Sisakht M, Mohammadsadegh M, Moslemi H (2017) Sperm evaluation of jungle cat (Felis chaus) obtained by urethral catheterization (CT) after medetomidine administration. Theriogenology 91: 17–20. [DOI] [PubMed] [Google Scholar]
- Leibo S, Songsasen N (2002) Cryopreservation of gametes and embryos of non-domestic species. Theriogenology 57: 303–326. [DOI] [PubMed] [Google Scholar]
- Lueders I, Luther I, Scheepers G, van der Horst G (2012) Improved semen collection method for wild felids: urethral catheterization yields high sperm quality in African lions (Panthera leo). Theriogenology 78: 696–701. [DOI] [PubMed] [Google Scholar]
- Lueders I, Ludwig C, Schroeder M, Mueller K, Zahmel J, Dehnhard M (2014) Successful nonsurgical artificial insemination and hormonal monitoring in an Asiatic golden cat (catopuma temmincki). J Zoo Wildl Med 45: 372–379. [DOI] [PubMed] [Google Scholar]
- Marini RP, Esteves MI, Fox JG (1994) A technique for catheterization of the urinary bladder in the ferret. Lab Anim-UK 28: 155–157. [DOI] [PubMed] [Google Scholar]
- Santymire RM, Marinari PE, Kreeger JS, Wildt DE, Howard JG (2006) Sperm viability in the black-footed ferret (Mustela nigripes) is influenced by seminal and medium osmolality. Cryobiology 53: 37–50. [DOI] [PubMed] [Google Scholar]
- Santymire RM, Lonsdorf EV, Lynch CM, Wildt DE, Marinari PE, Kreeger JS, Howard JG (2018) Inbreeding causes decreased seminal quality affecting pregnancy and litter size in the endangered black-footed ferret. Anim Conserv 22: 331–340. [Google Scholar]
- Santymire R, Graves G (2019) Black-footed Ferret SAFE Program Action Plan. Association of Zoos and Aquariums. Silver Spring, Maryland, USA. [Google Scholar]
- Swanson WF, Bateman HL, Vansandt LM (2016) Urethral catheterization and sperm vitrification for simplified semen banking in felids. Reprod Domest Anim 52: 255–260. [DOI] [PubMed] [Google Scholar]
- Tecirlioglu R, Hayes E, Trounson A (2002) Semen collection from mice: electroejaculation. Reprod Fert Develop 14: 363. [DOI] [PubMed] [Google Scholar]
- Turner R, McDonnell S, Hawkins J (1995) Use of pharmacologically induced ejaculation to obtain semen from stallion with a fractured radius. J Am Vet Med Assoc 206: 1906–1908. [PubMed] [Google Scholar]
- US Fish and Wildlife Service (1988) Black-footed ferret recovery plan. US Fish and Wildlife Service, Denver, Colorado, USA, p. 154. [Google Scholar]
- Wildt D, Roth T (1997) Assisted reproduction for managing and conserving threatened felids. Int Zoo Yearb 35: 164–172. [Google Scholar]
- Wildt D, Wemmer C (1999) Sex and wildlife: the role of reproductive science in conservation. Biodivers Conserv 8: 965–976. [Google Scholar]
- Wolf KN, Wildt DE, Vargas A, Marinari PE, Kreeger JS, Ottinger MA, Howard JG (2000) Age-dependent changes in sperm production, semen quality and testicular volume in the black-footed ferret (Mustela nigripes). Biol Reprod 63: 179–187. [DOI] [PubMed] [Google Scholar]
- Zambelli D, Prati F, Cunto M, Iacono E, Merlo B (2008) Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 69: 485–490. [DOI] [PubMed] [Google Scholar]


