Abstract
Background
Lack of consensus on how to diagnose sarcopenia has limited the ability to diagnose this condition and hindered drug development. The Sarcopenia Definitions and Outcomes Consortium (SDOC) was formed to develop evidence-based diagnostic cut points for lean mass and/or muscle strength that identify people at increased risk of mobility disability. We describe here the proceedings of a meeting of SDOC and other experts to discuss strategic considerations in the development of evidence-based sarcopenia definition.
Methods
Presentations and panel discussions reviewed the usefulness of sarcopenia as a biomarker, the analytical approach used by SDOC to establish cut points, and preliminary findings, and provided strategic direction to develop an evidence-based definition of sarcopenia.
Results
The SDOC assembled data from eight epidemiological cohorts consisting of 18,831 participants, clinical populations from 10 randomized trials and observational studies, and 2 nationally representative cohorts. In preliminary assessments, grip strength or grip strength divided by body mass index was identified as discriminators of risk for mobility disability (walking speed <0.8 m/s), whereas dual-energy X-ray absorptiometry-derived lean mass measures were not good discriminators of mobility disability. Candidate definitions based on grip strength variables were associated with increased risk of mortality, falls, mobility disability, and instrumental activities of daily living disability. The prevalence of low grip strength increased with age. The attendees recommended the establishment of an International Expert Panel to review a series of position statements on sarcopenia definition that are informed by the findings of the SDOC analyses and synthesis of literature.
Conclusions
International consensus on an evidence-based definition of sarcopenia is needed. Grip strength—absolute or adjusted for body mass index—is an important discriminator of mobility disability and other endpoints. Additional research is needed to develop a predictive risk model that takes into account sarcopenia components as well as age, sex, race, and comorbidities.
Keywords: Sarcopenia, Lean mass cut-points, Grip strength cut-points, Mobility disability, Risk factors for mobility disability
The concept of sarcopenia as a clinical biomarker to identify older adults at risk of physical disability and poor health outcomes is appealing for older adults who are at risk for mobility disability, practicing clinicians who care for these patients, and to the pharmaceutical companies engaged in the development of function promoting therapies to prevent or treat functional limitations (1–3). Although many have proposed that the definition be based on the measurement of lean mass and/or muscle strength (3–14), the lack of consensus on how to diagnose sarcopenia has limited the ability of practicing clinicians to diagnose and treat this condition and has hindered pharmaceutical efforts to develop function promoting therapies. The powerful demographic trends toward aging of human populations across the globe have inspired large investments in the field of sarcopenia by governments, pharmaceutical companies, and professional societies across geographic boundaries. The Sarcopenia Definition and Outcomes Consortium (SDOC) was formed to address this global public health need.
In response to a funding opportunity announcement from the National Institute on Aging (NIA), several investigators representing many cohort studies and clinical populations formed the SDOC. This team has applied data-driven analytical approaches to define sarcopenia. In October 2017, the SDOC held a meeting to discuss important considerations in the development and clinical application of an evidence-based definition of sarcopenia. This meeting also included a keynote lecture to highlight similarities and differences between the definitions of sarcopenia and osteoporosis. The analytical approach used by the SDOC investigators to establish cut points for lean body mass and/ or muscle strength measures to define sarcopenia and its implications were discussed in several presentations and panel discussions. This article provides a summary of the conference proceedings including preliminary findings of the analyses and potential strategies toward the development of a consensus definition of sarcopenia. Several original manuscripts describing the methods and detailed results of these analyses are being prepared for publication.
Overview of the SDOC Goals and Analytic Approach
The primary goal of the SDOC is to develop and evaluate evidence-based diagnostic cut points for low lean mass and/or muscle strength that identify people at increased risk of objectively assessed mobility disability, defined as usual gait speed less than 0.8 m/s over 4–6 m. Secondary goals are to evaluate the identified cut points against incident clinical outcomes such as mortality, falls, fractures, and instrumental activities of daily living disability and to assess performance characteristics (sensitivity, specificity, and predictive value) in population-based studies and clinical populations. These analyses build on those of the related Foundation for the National Institutes of Health Sarcopenia project that preceded this effort and ran from 2009 to 2012. In 2015, the National Institute on Aging funded the SDOC, and the Foundation for the National Institutes of Health provided additional support in March 2016.
The SDOC project represents one of the most ambitious efforts to define sarcopenia using an evidence-based approach informed by the analyses of data from large epidemiological studies, randomized clinical trials and cohorts of clinical populations, and two nationally representative population-based cohorts. The SDOC project not only evaluated the cut points that emerged from the SDOC analyses but also evaluated other competing definitions existing in the literature (3–14).
Sources of Epidemiological Data
The data used by the SDOC came from eight epidemiological cohorts consisting of 18,831 participants (13,683 men and 5,148 women) with a mean age of 75 years (Table 1) (15–24). Of these participants, 3,143 (17%) had self-reported mobility limitation defined as any difficulty walking 2–3 blocks or climbing 10 steps. In addition, SDOC included 10 clinical populations of patients drawn from randomized trials and smaller observational studies in patients with hip fractures and HIV (25–34). Finally, the cut points derived from analyses of the epidemiological studies were applied to two nationally representative population-based cohorts, the Health and Retirement Survey (n = 7,370 with 3,170 men and 4,200 women) and the National Health and Aging Trends Survey (n = 5,614, with 2,460 men and 3,154 women) (35,36) to determine the prevalence of older adults who were below these thresholds.
Table 1.
Studies Participating in the Sarcopenia Outcomes and Definitions Consortium
| Study | Eligibility | N Men | N Women |
|---|---|---|---|
| Epidemiological studies | |||
| Osteoporotic Fractures in Men (MrOS) Study1,2 | Ambulatory community-dwelling men, aged ≥ 65 y | 5,835 | 0 |
| Study of Osteoporotic Fractures (SOF)3,4 | Ambulatory community-dwelling women, aged ≥ 65 y | 0 | 1,246 |
| Health, Aging and Body Composition Study (Health ABC)5 | Nondisabled black and white men and women aged 70–80 | 652 | 746 |
| MrOS Sweden6 | Men aged ≥ 70 y in three Swedish communities | 2,876 | 0 |
| Mr and MsOS Hong Kong7,8 | Men and women aged ≥ 65 y residing in Hong Kong | 2,000 | 2,000 |
| Concord Health and Aging in Men Project (CHAMP)9 | Men aged ≥ 65 y living near Concord, Australia | 1,529 | 0 |
| Cardiovascular Health Study10,11 | Age ≥65 y at original study enrollment | 638 | 871 |
| Johnston County Arthritis Study12 | Rural white and black residents of Johnston County, North Carolina | 153 | 285 |
| Epidemiological studies total | 13,683 | 5,148 | |
| Clinical populations and randomized trials | |||
| LIFE-Pilot Trial | Men and women aged 70–89 y | 72 | 187 |
| VIVE2 Trial | Community-dwelling men and women ≥ 70 y | 80 | 64 |
| TOM Trial | Community-dwelling men ≥ 65 y | 194 | — |
| SECRET Trial | Men and women aged > 60 y with HFPEF | 16 | 71 |
| PIE2 Trial | Men and women aged ≥ 60 y with HFPEF | 17 | 40 |
| IDEA Trial | Men and women aged ≥ 60 y with closed hip fracture | 54 | 130 |
| MACS Bone Strength Substudy (BOSS) | HIV-infected and HIV-uninfected men aged 50–79 y | 370 | — |
| Women’s Interagency HIV Study | HIV-infected and HIV-uninfected women aged 30–55 | — | 187 |
| Baltimore Hip Studies 4 | Community-dwelling women ≥ 65 y | — | 121 |
| Baltimore Hip Studies 7 | Men and women aged ≥ 65 y and admitted for surgical repair of hip fracture | 54 | 71 |
| Clinical populations and randomized trials total | 857 | 871 | |
| Population-based studies | |||
| Health and Retirement Survey (HRS) | Men and women aged 51+; our analyses limited to those ≥ 65 y | 3,170 | 4,200 |
| National Health and Aging Trends Study (NHATS) | Nationally representative sample of Medicare beneficiaries, ≥ 65 y | 2,460 | 3,154 |
| Population-based studies total | 5,630 | 7,354 |
Note: LIFE-Pilot, Lifestyle Intervention for Elders Pilot Study; MrOS Sweden, Osteoporotic Fractures in Men Study in Sweden; MrOS Hong Kong, Osteoporotic Fractures in Men Study in Hong Kong; MsOS Hong Kong, Osteoporotic Fractures in Women Study in Hong Kong; TOM Trial, Testosterone in Older Men with Mobility Limitation Trial; VIVE2 Trial, The Vitality, Independence, and Vigor in the Elderly 2 Study; SECRET Trial, Exercise Intolerance in Elderly Patients With HFpEF (Heart Failure With Preserved Ejection Fraction); PIE2 Trial, Pharmacologic Interventions in the Elderly Study 2; IDEA Trial, Intensive Diet and Exercise for Arthritis study.
Analytical Approach
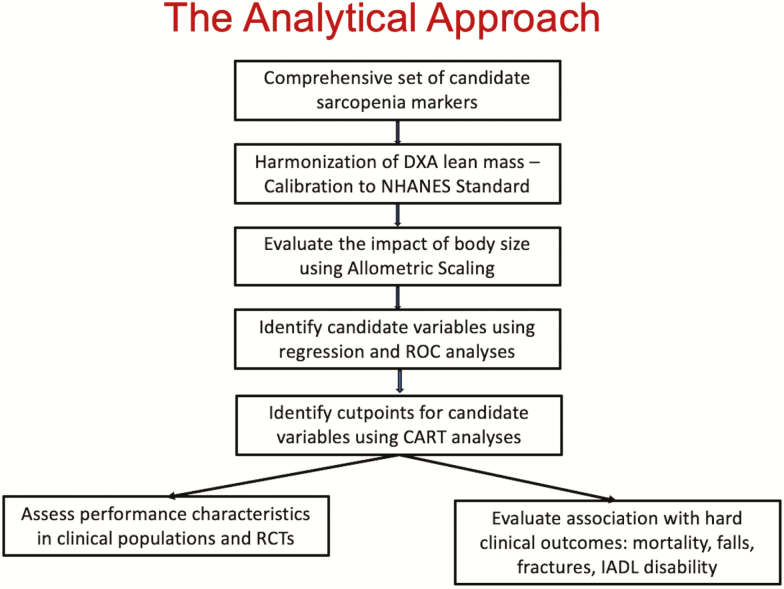
The analytic approach of the SDOC is summarized in Figure 1. As noted later, we first harmonized the measurements of body composition assessed by dual-energy X-ray absorptiometry (DXA), and other variables across the epidemiological studies, thereby removing excess variation resulting from the use of different machines and methods in different studies. We selected candidate markers of the sarcopenia phenotype after assessing the effect of allometric scaling. We used receiver operating characteristic and area under the curve from logistic regression to screen several putative “sarcopenia” variables derived from lean mass, grip strength, body size, and their combinations against the outcome of mobility disability, defined as walking speed less than 0.8 m/s. Those variables with the highest area under the curve that were most consistently associated with mobility disability were entered into Classification and Regression Trees models to derive cut points for low muscle mass and strength as discriminators of mobility disability. Sex-stratified Classification and Regression Trees analyses were conducted with age, several measures of body size (height, weight, and body mass index), grip strength (alone or standardized to body size), and lean mass (total, appendicular, and leg lean mass alone or standardized to body size) as potential discriminators of mobility disability. Finally, we examined the ability of these cut points to predict other incident clinical endpoints (e.g. mortality, falls, mobility, and instrumental activities of daily living disability) and evaluated the performance characteristics of these cut points in clinical populations and nationally representative samples (sensitivity, specificity, predictive value, and prevalence).
Figure 1.
A schematic depiction of the analytic approach.
Harmonization of DXA Data
Owing to the variation between the machines and software, DXA values obtained for a given individual may vary across machines. Therefore, DXA-derived estimates of lean and fat mass were harmonized across the eligible epidemiological cohorts using mathematical equations provided by scientific group at Hologic, Inc (37).
Allometric Scaling
We used allometric scaling to evaluate various coefficients to account for the influence of body size on lean mass and strength (38). For example, the measure of appendicular lean mass/height2 (ALM/ht2) includes the ht2 term to remove the influence of height (as a surrogate measure for body size) from the lean mass measure. However, the squared term is arbitrary, and another power (perhaps x3.1 or x4.2) may more appropriately account for height. Thus, we regressed the log of height on the log of appendicular lean mass and took the intercept of this regression as the allometric coefficient. A total of 73 different measures of low lean mass or grip strength (stratified by gender) allometrically scaled to height, height2, weight, body surface area, body mass index, fat mass, appendicular fat mass, leg lean mass, etc. were evaluated against gait speed cutoffs, using the area under the receiver operating characteristic curve. We performed both ratio-based and regression residual approach with grip strength and appendicular lean mass.
This enabled us to determine the number of subjects below each cut point and to compare the sensitivity and specificity of the cut points to those of leading definitions of sarcopenia.
The performance characteristics of various cut points for the recovery from hip fracture cohorts (Baltimore Hip Studies 4 and 7) were analyzed separately. This was necessary because the hip fracture cohorts differed from other studies in that patients with hip fracture are not ambulatory at the point of fracture and then improve over time, whereas other populations of older adults generally exhibit declining walking performance over time. Data from two cohorts in the Baltimore Hip Studies (n = 226) were used; mobility disability was defined as gait speeds of less than 0.6 m/s; and improvement in gait speed as an increase of at least 0.1 m/s (note: the baseline was measured at 2 months post-fracture). The Classification and Regression Trees-derived cut points were used to derive prevalence estimates and to evaluate the sensitivity and specificity of these cut points in predicting both objectively measured mobility disability 6 months post-hip fracture and improvement in gait speed from 2 to 6 months.
The performance characteristics of various cut points in the HIV cohorts were also analyzed separately. The life span of HIV+ individuals is now almost the same as that for HIV– individuals. In general, HIV+ populations have a higher prevalence of aging-related comorbidities, and older HIV+ populations may have a more rapid decline in physical function with aging and an earlier appearance of physical frailty. The cut points were applied to those with or at risk of HIV infection and their sensitivity, specificity, and predictive value in identifying low gait speed in this population were compared. The data came from the Bone Strength Sub Study of the Multicenter AIDS Cohort Study, a study of men who have sex with men; these men have been evaluated semi-annually since 1984 at four U.S. sites. The data on women were obtained from the Musculoskeletal Sub Study of the Women’s Interagency HIV Study, a cohort of women with or at risk for HIV infection recruited from three Women’s Interagency HIV Study sites. All participants were receiving anti-HIV therapy.
The cut points were applied to two nationally representative studies of older adults—The Health and Retirement Survey and the National Health and Aging Trends Study—to estimate the proportion of adults with values below cut points in the older U.S. population and to describe how prevalence varies by age, sex, and race/ethnicity. In addition, we determined the sensitivity and specificity of various cut points for the identification of mobility disability (<0.8 m/s gait speed). The advantage of these studies is that the sampling frame in the U.S. population is known, thus, the responses can be reweighted to account for missing data from the underlying population to provide true population level estimates for the United States.
Synthesis of the Preliminary Analytical Results
The SDOC investigators presented the conceptual framework of the analytical approach and preliminary results of the analyses. First, the DXA harmonization had little impact on high-level conclusions. This effort has important implications for clinical practice and cross-comparison with other analyses and addressed a major criticism of previous efforts to define sarcopenia. Second, the SDOC found that allometric scaling had minimal impact on the sensitivity and specificity of cut points for identifying mobility disability. Third, the SDOC’s initial analyses consistently identified a small subset of variables, most often a cut point in grip strength or grip strength/body mass index as discriminators for identifying men and women at risk for mobility disability. Body composition measures (e.g. body mass index, lean mass by DXA, or body fat) did not emerge as potential discriminators of older adults with mobility disability. Fourth, many of the candidate definitions that included grip strength were associated with increased risk of mortality, hip fracture, falls, mobility disability, and instrumental activities of daily living disability. However, measures of DXA-based lean mass did not discriminate persons at risk for these outcomes. Fifth, the sensitivity, specificity, and prevalence varied in different populations (e.g. clinical populations, patients with hip fracture, and HIV+ individuals). For patients with hip fracture, the proportion of those with mobility disability at baseline was very high, so a more important outcome in this population may be the recovery of mobility. Finally, the prevalence of sarcopenia as defined by low grip strength increased with age and was high in older Americans. The sensitivity, specificity, and prevalence varied by definition, sex, and race.
Discussion
Historical Perspective From the Osteoporosis Field
Dr. Cummings’ keynote lecture highlighted the similarities between osteoporosis and sarcopenia: both are defined by cut points of a continuous biomarker (39). The establishment of bone mineral density (BMD) cut points to define osteoporosis catalyzed the development and approval of drugs for the treatment of osteoporosis and made it easier for clinicians to diagnose osteoporosis and get reimbursed. The cut point for osteoporosis as a T-score equal to –2.5 was based on consensus of a World Health Organization’s Expert Panel that was informed primarily by the prevalence of “osteoporosis” in women aged at least 50 years at candidate cut points: a prevalence of 15% at a T-score less than –2.5 was considered reasonable (39,40). Eventually, a T-score less than or equal to –2.5 at either the hip or spine became an entry criterion for clinical trials of new drugs and an Food and Drug Administration-approved indication for their prescription.
The World Health Organization Committee defined “osteopenia” as a hip BMD T-score of between –1.0 and –2.5 (39,40). However, most women aged 50–60 years with “osteopenia” had a low absolute risk of fracture. Thus, the definition of “osteopenia” resulted in labeling millions of women who had a low absolute risk of fracture as having a “disease”, sometimes causing anxiety and increasing the prescription of drugs to women with a low risk of fracture. To mitigate this problem, a multivariable index called FRAX (Fracture Risk Assessment Tool) that considers risk factors, such as age, sex, and history of fracture, is now commonly used to estimate a person’s absolute risk of fracture (41).
Dr. Cummings cautioned that a cut point to define sarcopenia, such as that based on grip strength, should not be viewed as a disease diagnosis. Establishing a cut point would increase awareness of this condition, which could lead to recommendations of physical activity interventions or other lifestyle changes that might be beneficial; it also could lead patients to believe that they have a disease and heighten anxiety and increase use of drug therapies by people who have a low risk of mobility disability. Dr. Cummings suggested that absolute risk of an important outcome, such as mobility disability, may be a more rational basis for clinical interventions.
The attendees suggested that SDOC develop a robust risk model of mobility disability or other important clinical outcomes. When therapies become available, treatment thresholds should take into account the treatment’s benefits and costs. In addition, a clinical diagnosis of “sarcopenia” may also include consideration of functional limitations, such as slow walking, difficulty rising from a chair without hands, or walking upstairs; and treatment would be indicated for improving these functions and enabling older individuals to remain independent.
Considerations in Implementation of Sarcopenia Definition in Clinical Practice and Drug Development
The potential hurdles in the implementation of the measures of sarcopenia in assessments of older adults and steps that can be taken to increase awareness among patients and clinicians were discussed. The reimbursement issues could be potential barriers to implementing grip strength in clinical practice. It was suggested that the initial application of cut points may be focused on identifying the most impaired. Outcome measures in health care are best adopted when a new measure adds value in helping a clinician make individual patient decisions. The cost and burden of measuring grip strength are minimal, but the consequences of the diagnosis, in addition to the cost of testing and treatment, should be weighed against potential benefits.
There is limited understanding of sarcopenia among clinicians and patients. Scientific journals can play a big role in raising awareness. Professional societies can also play an important role in maintaining outcomes registries. Patients make especially effective advocates—they can have a substantial impact on policy makers and funding agencies. The panel noted that efforts to raise awareness now are important, as it takes many years to translate knowledge into clinical practice. Some of these efforts have already been initiated by patient advocacy organizations such as the aging in motion (AIM) Coalition, which recently facilitated the approval of an ICD-10 code for sarcopenia. To further advance this effort, the AIM Coalition has submitted an 18-month proposal to the Food and Drug Administration to begin the process of qualifying performance measures such as gait speed tests as functional measures in clinical trials. Partnering with patient organizations such as the American Association of Retired Persons may advance efforts to increase awareness of sarcopenia. Local groups can work with others at the national level to capture input of diverse populations.
The panel discussants noted that drug development for sarcopenia has been much more difficult than expected for several reasons. The lack of regulatory guidance was cited as a major impediment. In addition, sarcopenia affects a population with a lifetime of environmental exposures, multiple diseases, and unknown genetics. It has a complex biology and there are no good preclinical models in which to confirm or predict that a target intervention will have the intended effect in humans with this condition.
Recommendations
Strategies to harmonize a sarcopenia definition and to incorporate it into clinical practice, clinical trials, and treatment guidelines require continued international collaborative effort (Table 1). The attendees urged the formation of an International Expert Panel to review the analytical findings and a synthesis of the published evidence and to develop a series of position statements that would form the basis of a consensus definition of sarcopenia to be discussed at an International Consensus Conference in the Fall of 2018 (Table 2). However, sarcopenia defined by a cut point should not be viewed as a “disease” without a corresponding method for estimating a person’s risk of important outcomes that are the main motivation for interventions. Anticipating a consensus definition of “sarcopenia,” there also is a need to develop potential strategies for the dissemination and implementation of sarcopenia definition in clinical practice. The discussion emphasized the need to simultaneously develop predictive risk model of adverse health outcomes, such as physical disability, that takes into account sarcopenia components as well as age, sex, race, and comorbidities.
Table 2.
A Summary of the Preliminary Findings and of the Recommendations of the Sarcopenia Definition and Outcomes Consortium (SDOC) Conference
| Preliminary findings of the SDOC analyses |
| • Grip strength—either absolute or adjusted for body mass index—is an important discriminator of mobility disability, and a predictor of adverse health-related outcomes, such as falls, instrumental activities of daily living disability, and mortality. |
| • Lean mass, measured using dual-energy X-ray absorptiometry, is not a good discriminator of mobility disability. |
| Major recommendations of the SDOC Conference |
| • Develop a series of position statements on sarcopenia definition that are informed by the findings of the SDOC analyses and synthesis of the literature. |
| • Establish an independent International Expert Panel to review the position statements and the supporting analytical results and the literature synthesis. |
| • Convene an International Sarcopenia Definition and Outcomes Position Statement Conference to solicit a consensus on the position statements that would form the foundation of the evidence-based sarcopenia definition. |
| •Develop a predictive risk model that takes into account sarcopenia components as well as age, sex, race, and comorbidities. |
Funding
This research and the Sarcopenia Definitions and Outcomes Conference was supported by a cooperative agreement from the National Institute on Aging (1UO1AG051421) and by the Foundation for National Institutes of Health. We thank Dr. Lyndon Joseph of the National Institute on Aging, the project’s Program Officer for his guidance and oversight of the project. Dr. Rosaly Correa-De-Arauajo is an employee of the National Institute on Aging. Her participation or the materials should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health or the National Institute on Aging, except where noted. We thank Kevin Wilson and Tom Kelly of Hologic Corporation for their assistance in harmonizing the Dual-Energy X-Ray Absorptiometry (DXA) data. Funding support for the individual studies that comprise the pooled data set is gratefully acknowledged. MrOS (US): The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and National Institute of Health (NIH) Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. MrOS Hong Kong: MrOS in Hong Kong was supported by a U.S. National Institute of Health R01 Grant AR049439-01A1, the Research Grants Council Earmarked Grant CUHK 4101/02M, and a direct grant for research of The Chinese University of Hong Kong (No. 2041657). MrOS Sweden: Financial support was received from the Swedish Research Council (2006-3832), the Swedish Foundation for Strategic Research, the Swedish Research Council grant for the Gothenburg Region of Sweden (ALF/FUA) research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg’s Foundation, Petrus and Augusta Hedlunds Foundation, the Västra Götaland Foundation, the Göteborg Medical Society and the Novo Nordisk Foundation. SOF: The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576. Health Aging and Body Composition Study (Health ABC): This study was funded by the National Institutes of Aging. This research was supported by NIA contracts N01AG62101, N01AG62103, and N01AG62106. Cardiovascular Health Study (CHS): This CHS research was supported by National Institute of Heart, Lung and Blood Institute (NHLBI) contracts N01-HC- 85079, N01-HC-85080, N01-HC-85081, N01-HC-85082, N01-HC-85083, N01-HC-85084, N01-HC-85085, N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, N01-HC-85239, and by HHSN268201200036C and NHLBI grants HL080295, HL087652, HL105756, HL103612 with additional contribution from National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the NIA. Framingham Osteoporosis Study (FOS)/Framingham Heart Study (FHS): The study was funded by grants from the US National Institute for Arthritis, Musculoskeletal and Skin Diseases and National Institute on Aging (R01 AR 41398 and U24AG051129; DPK and R01AR057118. The Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine were supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (N01-HC-25195). Johnson County Study: The Johnston County Osteoarthritis Project is supported in part by cooperative agreements S043, S1734, and S3486 from the Centers for Disease Control and Prevention/Association of Schools of Public Health; the NIAMS Multipurpose Arthritis and Musculoskeletal Disease Center grant 5-P60-AR30701; and the NIAMS Multidisciplinary Clinical Research Center grant 5 P60 AR49465-03. Concord Health and Ageing in Men Project: CHAMP is funded by the National Health and Medical Research Council (project grant number 301916) and the Ageing and Alzheimer’s Institute. LASA: The Longitudinal Aging Study Amsterdam (LASA) is largely supported by a grant from the Netherlands Ministry of Health, Welfare and Sports, Directorate of Long-Term Care. The data collection in 2012–2013 was financially supported by the Netherlands Organization for Scientific Research (NWO) in the framework of the project “New Cohorts of young old in the 21st century” (File Number 480-10-014).
Conflict of interest statement
None declared
References
- 1. Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr. 1993;123(2 Suppl):465–468. doi: 10.1093/jn/123.suppl_2.465 [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner RN, Koehler KM, Gallagher D, et al. . Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 3. Fielding RA, Vellas B, Evans WJ, et al. . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Studenski SA, Peters KW, Alley DE, et al. . The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cawthon PM, Peters KW, Shardell MD, et al. . Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alley DE, Shardell MD, Peters KW, et al. . Grip strength cutpoints for the identification of clinically relevant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:559–566. doi: 10.1093/gerona/glu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alexandre Tda S, Duarte YA, Santos JL, Wong R, Lebrão ML. Sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging. 2014;18:751–756. doi: 10.1007/s12603-014-0450-3 [DOI] [PubMed] [Google Scholar]
- 9. Lee WJ, Liu LK, Peng LN, Lin MH, Chen LK; ILAS Research Group Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc. 2013;14:528.e1–528.e7. doi: 10.1016/j.jamda.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 10. Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. [DOI] [PubMed] [Google Scholar]
- 11. Newman AB, Kupelian V, Visser M, et al. ; Health ABC Study Investigators Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 12. Melton LJ III, Khosla S, Riggs BL. Epidemiology of sarcopenia. Mayo Clin Proc. 2000;75(Suppl):S10–S12; discussion S12. [PubMed] [Google Scholar]
- 13. Melton LJ III, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48: 625–630. [PubMed] [Google Scholar]
- 14. Morley JE, Abbatecola AM, Argiles JM, et al. . Society on Sarcopenia, Cachexia and Wasting Disorders Trialist Workshop. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. doi: 10.1016/j.jamda.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orwoll E, Blank JB, Barrett-Connor E, et al. . Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 16. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. . Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 17. Cummings SR, Nevitt MC, Browner WS, et al. . Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202 [DOI] [PubMed] [Google Scholar]
- 18. Newman AB, Haggerty CL, Goodpaster B, et al. ; Health Aging And Body Composition Research Group Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. [DOI] [PubMed] [Google Scholar]
- 19. Mellström D, Johnell O, Ljunggren O, et al. . Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006;21:529–535. doi: 10.1359/jbmr.060110 [DOI] [PubMed] [Google Scholar]
- 20. Lau EM, Leung PC, Kwok T, et al. . The determinants of bone mineral density in Chinese men–results from Mr. Os (Hong Kong), the first cohort study on osteoporosis in Asian men. Osteoporos Int. 2006;17:297–303. doi: 10.1007/s00198-005-2019-9 [DOI] [PubMed] [Google Scholar]
- 21. Lau EM, Suriwongpaisal P, Lee JK, et al. . Risk factors for hip fracture in Asian men and women: the Asian osteoporosis study. J Bone Miner Res. 2001;16:572–580. doi: 10.1359/jbmr.2001.16.3.572 [DOI] [PubMed] [Google Scholar]
- 22. Cumming RG, Handelsman D, Seibel MJ, et al. . Cohort Profile: the Concord Health and Ageing in Men Project (CHAMP). Int J Epidemiol. 2009;38:374–378. doi: 10.1093/ije/dyn071 [DOI] [PubMed] [Google Scholar]
- 23. Newman AB, Arnold AM, Sachs MC, et al. . Long-term function in an older cohort–the cardiovascular health study all stars study. J Am Geriatr Soc. 2009;57:432–440. doi: 10.1111/j.1532-5415.2008.02152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 25. Jordan JM, Helmick CG, Renner JB, et al. . Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 26. Orwig DL, Hochberg M, Yu-Yahiro J, et al. . Delivery and outcomes of a yearlong home exercise program after hip fracture: a randomized controlled trial. Arch Intern Med. 2011;171:323–331. doi: 10.1001/archinternmed.2011.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292:837–846. doi: 10.1001/jama.292.7.837 [DOI] [PubMed] [Google Scholar]
- 28. Rejeski WJ, Fielding RA, Blair SN, et al. . The lifestyle interventions and independence for elders (LIFE) pilot study: design and methods. Contemp Clin Trials. 2005;26:141–154. doi: 10.1016/j.cct.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 29. Liu CK, Leng X, Hsu FC, et al. . The impact of sarcopenia on a physical activity intervention: the Lifestyle Interventions and Independence for Elders Pilot Study (LIFE-P). J Nutr Health Aging. 2014;18:59–64. doi: 10.1007/s12603-013-0369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirn DR, Koochek A, Reid KF, et al. . The Vitality, Independence, and Vigor in the Elderly 2 Study (VIVE2): design and methods. Eur Geriatr Med, 2014; 5:S202. [DOI] [PubMed] [Google Scholar]
- 31. Greenspan SL, Perera S, Nace D, et al. . FRAX or fiction: determining optimal screening strategies for treatment of osteoporosis in residents in long-term care facilities. J Am Geriatr Soc. 2012;60:684–690. doi: 10.1111/j.1532-5415.2011.03884.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Greenspan S, Nace D, Perera S, et al. . Lessons learned from an osteoporosis clinical trial in frail long-term care residents. Clin Trials. 2012;9:247–256. doi: 10.1177/1740774511430516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. LeBrasseur NK, Lajevardi N, Miciek R, Mazer N, Storer TW, Bhasin S. Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (The TOM Trial): design and methods. Contemp Clin Trials. 2009;30:133–140. doi: 10.1016/j.cct.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Travison TG, Basaria S, Storer TW, et al. . Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–1099. doi: 10.1093/gerona/glr100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goudy WJ. Effects of sample attrition and data analysis in the Retirement History Study. Exp Aging Res. 1985;11:161–167. doi: 10.1080/03610738508259181 [DOI] [PubMed] [Google Scholar]
- 36. Missikpode C, Michael YL, Wallace RB. Midlife occupational physical activity and risk of disability later in life: national health and aging trends study. J Am Geriatr Soc. 2016;64:1120–1127. doi: 10.1111/jgs.14083 [DOI] [PubMed] [Google Scholar]
- 37. Shepherd JA, Fan B, Lu Y, et al. . A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone and Mineral Res. 2012;27(10):2208–2216. doi: 10.1002/jbmr.1654 [DOI] [PubMed] [Google Scholar]
- 38. Garrow JS, Webster J. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147–153. [PubMed] [Google Scholar]
- 39. Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J Intern Med. 2015;277:650–661. doi: 10.1111/joim.12369 [DOI] [PubMed] [Google Scholar]
- 40. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 41. Cauley JA, El-Hajj Fuleihan G, Luckey MM. FRAX(®) Position Development Conference Members. FRAX® International Task Force of the 2010 Joint International Society for Clinical Densitometry & International Osteoporosis Foundation Position Development Conference. J Clin Densitom. 2011;14(3):237–239. doi: 10.1016/j.jocd.2011.05.016 [DOI] [PubMed] [Google Scholar]