Abstract
Study Objectives
Suggested neural correlates of insomnia disorder have been hard to replicate. Even the most consistent finding, altered white matter microstructure in the anterior limb of the internal capsule, is based on handful studies. The urge for replicable targets to understand the underlying mechanisms of insomnia made us study white matter fractional anisotropy (FA) across three samples of cases and controls.
Methods
3-Tesla MRI diffusion tensor imaging data of three independent samples were combined for analysis, resulting in n = 137 participants, of whom 73 were diagnosed with insomnia disorder and 64 were matched controls without sleep complaints. Insomnia severity was measured with the Insomnia Severity Index (ISI). White matter microstructure was assessed with FA. White matter tracts were skeletonized and analyzed using tract-based spatial statistics. We performed a region-of-interest analysis using linear mixed-effect models to evaluate case–control differences in internal capsule FA as well as associations between internal capsule FA and insomnia severity.
Results
FA in the right limb of the anterior internal capsule was lower in insomnia disorder than in controls (β = −9.76e−3; SE = 4.17e−3, p = .034). In the entire sample, a higher ISI score was associated with a lower FA value of the right internal capsule (β = −8.05e− 4 FA/ISI point, SE = 2.60e− 4, p = .008). Ancillary whole brain voxel-wise analyses showed no significant group difference or association with insomnia severity after correction for multiple comparisons.
Conclusions
The internal capsule shows small but consistent insomnia-related alterations. The findings support a circuit-based approach to underlying mechanisms since this tract connects many brain areas previously implicated in insomnia.
Keywords: insomnia disorder, white matter, internal capsule, diffusion tensor imaging, fractional anisotropy
Statement of Significance.
Insomnia disorder is a major burden for individuals and society alike. While the current first-line treatment for insomnia is highly effective on average, it brings insufficient relief to a considerable proportion of people. Knowledge of underlying vulnerability and associated deviations in brain structure and function can provide rational targets for the development of novel treatment. Explorative neuroimaging studies reported equivocal findings on the involvement of white matter tracts. We here confirm altered white matter microstructure in the internal capsule in insomnia disorder across three independent samples. Interestingly, this structure connects brain areas where insomnia-related alterations have previously been reported. It may, therefore, be hypothesized that the risk of insomnia involves a vulnerability anywhere in this circuit, not necessarily showing voxel-wise overlap across individuals. Since the circuit accommodates the so-called salience and limbic networks, it could help direct the development of novel interventions. These might, for example, target coping with altered sensory processing, attention, or emotion regulation.
Introduction
Insomnia disorder is one of the most prevalent mental disorders, affecting about 10% of the general population [1, 2]. Insomnia complaints include difficulty falling asleep, maintaining sleep, early morning waking, and subjectively impaired daily functioning. If insomnia symptoms occur at least three times a week and persist for more than 3 months, a diagnosis of insomnia disorder can be considered [3]. We will here refer to insomnia disorder as “insomnia”. People with insomnia are more likely to experience impaired academic performance or work disability [4, 5]. In addition, insomnia is associated with an increased risk of obesity, cardiovascular disease [6, 7], and psychiatric disorders, such as depressive and anxiety disorders [8, 9]. Neurobiological mechanisms underlying insomnia are still elusive [10]. Neuroimaging studies on insomnia have usually been explorative, with low reproducibility, and remain inconclusive about pathophysiology [11, 12]. Studies demonstrating replicable results in larger samples are direly needed. Because individual differences in objective sleep quality and white matter microstructure are associated [13], several studies investigated white matter microstructure in insomnia but with varying outcomes [14–16]. Currently, the most consistent finding may be that people with insomnia show an altered microstructure in the anterior limb of the internal capsule [14–16]. This structure forms a white matter hub with numerous fiber bundles to and from, for example, the prefrontal cortex [17]. The anterior limb of the internal capsule accommodates connections of brain areas that change their activity in association with wake and sleep states, such as the thalamus, prefrontal cortex, and pontine brain stem [18, 19]. The anterior limb of the internal capsule also connects brain areas that show altered structure or function in insomnia, such as orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), putamen, and the head of the caudate nucleus [20–26]. This makes the anterior internal capsule a white matter region of particular interest in insomnia. White matter microstructure can be quantified by fractional anisotropy (FA) from diffusion tensor images. FA represents the degree of directionality of water diffusion in tissue and ranges from zero (equal diffusion in all directions) to one (diffusion in only one direction). A lower FA in white matter regions indicates an alteration of the microstructure. This could be a lesser degree of myelination, a different proportion of crossing fibers or an alteration in the interstitial space between fibers [27]. The current study, therefore, investigated whether lower FA in the internal capsule in insomnia could robustly be demonstrated across three independent neuroimaging study samples. Furthermore, we conducted whole brain voxel-wise analyses to explore additional white matter differences in insomnia.
Methods
Participants
Participants with insomnia and controls without sleep complaints were recruited through advertisement or through the Netherlands Sleep Registry (www.sleepregistry.org). Applicants were eligible if they were between 18 and 70 years of age. After application, participants were contacted by phone and later face-to-face to evaluate whether they met the inclusion criteria, did not meet exclusion criteria, and whether people reporting insomnia symptoms met the criteria for insomnia disorder. Exclusion criteria were (1) a current diagnosis of sleep apnea, (2) restless leg syndrome, (3) narcolepsy, (4) any other neurological, psychiatric, or somatic disorders, (5) the use of sleep or other psychotropic medication in the 2 months prior to the study, (6) current shift work, or (7) any magnetic resonance imaging (MRI) contraindication such as non-MR compatible metal implants, claustrophobia, or pregnancy. In the case of participants reporting insomnia, we determined whether they fulfilled the diagnosis of insomnia disorder in accordance to the International Classification of Sleep Disorders, Third edition [28], Diagnostic and Statistical Manual of Mental Disorders, Fourth [29] or Fifth edition [3], and included quantification of the duration of the disorder.
Comorbid sleep-disorders were part of the exclusion criteria and assessed in the following way. First, the screening included questions on diagnosed sleep disorders as well as validated questionnaires to detect undiagnosed probable comorbid sleep disorders, including restless legs, sleep apnea, and circadian rhythm disorders. Candidates with a diagnosis or probable diagnosis were excluded. Second, polysomnography (PSG) included video, audio, tibialis electromyography (EMG), and respiratory belts. EMG signal was visually inspected on periodic leg movements and breathing belt signals on phase asynchrony that could reflect respiratory effort to overcome upper airway obstruction. If there were symptoms suggesting sleep disorders other than insomnia, the participant was excluded from the analysis. The procedures led to exclusion of 17 people. For this study, 170 participants were included who had diffusion-weighted images. After quality control, 33 were excluded due to insufficient quality of the diffusion-weighted images. The final sample (see Table 1) included 73 people with ID (age M ± SD = 46.5 years ± 13.8, 16 male) and 64 people without sleep problems (age M ± SD = 43.3 years ± 14.7, 22 male). Assessments were part of more elaborate studies that were approved by the ethical board of the VU Medical Center or the University of Amsterdam. Written informed consent was obtained from all participants.
Table 1.
Group demographics and polysomnographic sleep parameters
| Control (N = 64) | Insomnia (N = 73) | P-value | |
|---|---|---|---|
| Male/female | 22/42 | 16/57 | .152 |
| Age (years) | 43.33 (14.7) | 46.47 (13.8) | .203 |
| Duration of insomnia (years)† | — | 19.20 (15.13) | |
| Insomnia Severity Index | 3.56 (4.1) | 16.42 (5.0) | <.001* |
| Objective sleep (PSG)‡ | |||
| Total sleep time (min) | 431.81 (53.1) | 403.75 (67.9) | .011* |
| Sleep onset latency (min) | 15.13 (18.1) | 13.29 (11.8) | .504 |
| Wake after sleep onset (min) | 29.62 (21.2) | 56.22 (48.2) | <.001* |
| Sleep efficiency (%) | 90.48 (7.0) | 84.67 (11.0) | .001* |
Mean (standard deviation) provided where relevant. Insomnia duration and polysomnography (PSG) measures were not available for all participants. Objective sleep measures are reported in minutes (min) or as percentage (%).
† N insomnia = 62.
‡ N = 125 (Ncontrol = 60, Ninsomnia = 65).
*p < .05.
Procedures
Participants were scanned at different sites: (sample 1) the Amsterdam UMC, location VU (GE Signa HDxt 3T; Ninsomnia = 22, Ncontrol = 16), (sample 2) the Spinoza Centre—Roeterseiland (Philips Achieva 3T; Ninsomnia = 25, Ncontrol = 24), and (sample 3) the Spinoza Centre for Neuroimaging (Philips Achieva 3T; Ninsomnia = 26, Ncontrol =24). Participants were asked to refrain from alcohol and drugs on assessment days and were not allowed to consume caffeinated beverages at least 6 hours before MRI assessments. The majority of participants (Ninsomnia = 65, Ncontrol = 60) completed two nights of PSG in the sleep lab at the Netherlands Institute for Neuroscience to assess objective sleep. Participants adhered to their habitual bedtime and wake-up time. PSG was performed using high-density electroencephalography (HD-EEG, 256-channel Electrical Geodesic Inc., Eugene, OR, Net Amps 300 amplifier, input impedance: 200 MΩ, A/D converter: 24 bits).
MRI acquisition
Diffusion-weighted imaging was performed using an 8-channel head coil (sample 1) or a 32-channel head coil (samples 2 and 3). Head motion during scanning was restricted by foam pads. In sample 1, diffusion-weighted data were acquired using a single-shot echo-planar imaging sequence with 30 diffusion gradient directions (b = 1,000 s/mm2) preceded by five additional images without diffusion weighting (b = 0 s/mm2). The sequence parameters were as follows: 14,000 ms repetition time, 85.5 ms echo time, 256 × 256 matrix, 256 × 256 mm field of view, 2.4 mm section thickness, voxel size 1 mm3, and 48 transverse sections (slices) without gap covering the whole brain. In samples 2 and 3, diffusion-weighted data were acquired using a single-shot echo-planar imaging sequence with 32 diffusion gradient directions (b = 1,000 s/mm2) followed by one additional image without diffusion weighting (b = 0 s/mm2). Scanning parameters: 16,070 ms repetition time, 48 ms echo time (shortest), 112 × 112 matrix, 224 × 224 mm field of view, 2 mm section thickness, voxel size 2 mm3 (isotropic), and 65 transverse sections (slices) without gap covering the whole brain.
MRI preprocessing
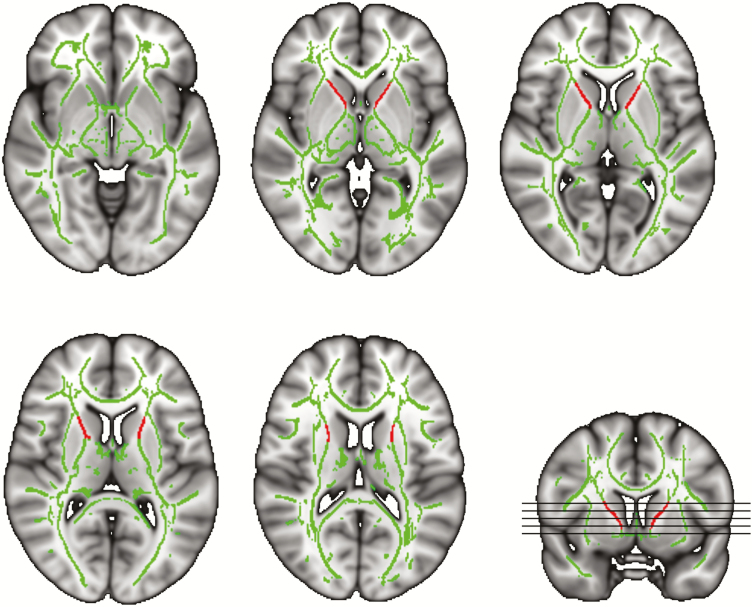
Diffusion-weighted data were analyzed with FMRIB’s Diffusion Toolbox of FMRIB Software Library (FSL) Version 5.0.10 [30]. FSL’s Eddy tool was used to correct for head motion, eddy currents, and inter-slice intensity outliers [31, 32]. Further analysis of FA data was based on tract-based spatial statistics [33]. First, individual FA images were created by fitting a tensor model to the raw diffusion data. After brain extraction [34], all participants’ FA images were aligned to a 1 × 1 × 1 mm target FA image (FMRIB58_FA; FMRIB Software Library) using nonlinear registration [35, 36]. Next, the aligned FA images were transformed into the Montreal Neurologic Institute 152 template using affine registrations. A mean FA image across all participants was generated and thinned to create a mean FA skeleton which represents the centers of all tracts common to the sample. We set an FA threshold of 0.3 to form the skeleton, a spatial representation of the majority of major white matter fiber bundles. In the final step, the aligned FA data of all participants were projected onto this skeleton by searching for the local center of the relevant fiber tract. For the region of interest analysis, we calculated a separate mean FA value for the left and right anterior limb of the internal capsule based on the skeletonized FA images (Figure 1). Both regions were defined by masks based on the JHU ICBM-DTI-81 White Matter labels atlas [37].
Figure 1.
Study-specific FA skeleton used for analysis. Green represents the mean FA skeleton across all participants. Red depicts the left and right mask of the anterior limb of the internal capsule which was used to calculate the mean FA value of the structure. Underlying gray-scale images are the Montreal Neurologic Institute 152-T1 1 mm template.
Sleep measures
Insomnia severity was assessed with the Insomnia Severity Index (ISI, range 0–28) [38, 39], where higher scores indicate more severe insomnia complaints. The second night of PSG was visually scored by experienced raters according to standard procedures [40] to obtain the objective sleep measures: total sleep time (TST, min), sleep onset latency (SOL, min), wake after sleep onset (WASO, min), and sleep efficiency (SE, %). All questionnaires were completed within the measuring period. In practice, this was within a week of the MRI scan.
Statistical analysis
Two-sample two-tailed t-tests were used to determine differences in age, ISI, and objective sleep measures between people with insomnia and controls. A Pearson χ 2 test was performed to determine group differences in the proportion of females and males. We used linear mixed-effects analysis in R (version 3.4.3) [41] with the package lme4 [42] to evaluate multiple models describing FA of the anterior internal capsule. The independent variables that were tested included (1) an indicator variable “insomnia” for the diagnosis of insomnia, (2) ISI score, (3) TST, (4) WASO, (5) SE, and (6) insomnia duration. For each independent variable, we performed separate models to evaluate the mean FA of the left and right anterior limb of the internal capsule (see Table 2). Furthermore, all models included age and sex as covariates and “sample” as random intercept covariate. The random intercept variable “sample” is included to account for the structural differences that might occur between the three original studies due to the scanner site and/or scan acquisition parameters. Visual inspection of residual plots did not reveal deviations from homoscedasticity or normality. Reported p-values are for the fixed effect of ISI or diagnosis of insomnia on FA. Table 2 shows the p-values of the likelihood-ratio tests of the models with versus without the independent variable. Interaction effects between ISI and sample, age, or insomnia duration were tested using linear regression and/or likelihood-ratio tests by comparing the mixed model with interaction term to the model without. Standardized beta’s of the linear mixed models were computed using the “sjstats” package [43].
Table 2.
Likelihood-ratio tests of linear mixed models with different independent variables
| Linear mixed model | Anterior limb of the internal capsule | P-value |
|---|---|---|
| FA ~ insomnia + age + sex + (1|sample) | Right | .021* |
| Left | .219 | |
| FA ~ ISI + age + sex + (1|sample) | Right | .002* |
| Left | .064 | |
| FA ~ TST + age + sex + (1|sample)† | Right | .096 |
| Left | .104 | |
| FA ~ WASO + age + sex + (1|sample)† | Right | .628 |
| Left | .174 | |
| FA ~ SE + age + sex + (1|sample)† | Right | .252 |
| Left | .155 | |
| FA ~ insomnia duration + age + sex + (1|sample)‡ | Right | .505 |
| Left | .748 |
Clinical data were not available for all participants. FA, fractional anisotropy; ISI, Insomnia severity index; TST, total sleep time; WASO, wake after sleep onset; SE, sleep efficiency.
† N = 125 (Ncontrol = 60, NInsomnia = 65).
‡ N insomnia =62.
*p <.05; P-values were obtained by likelihood ratio tests of the full model with the independent variable in question (insomnia, ISI, TST, WASO, SE, or insomnia duration) compared with the model without this variable.
For whole brain voxel-wise case–control comparison of FA and the association of insomnia severity with FA, we combined tract-based spatial statistics [33] with permutation-based nonparametric testing [44] (500 permutations with a “tail approximation” acceleration method [45]). Permutations were made within each sample [46]. Threshold-Free Cluster Enhancement was applied with a significance level set at p < .05 and Family Wise Error correction for multiple comparisons. Age and sex were added as covariates in the model. The clusters in the supplementary were listed using FSL’s “autoaq” function with the JHU ICBM-DTI-81 White Matter Labels and JHU White Matter Tractography Atlases.
Results
Demographic and sleep characteristics
Table 1 presents the characteristics of the insomnia and control group. Demographic characteristics of participants with insomnia did not differ from the controls without sleep complaints. As expected, the ISI score was significantly higher in people with insomnia (M ± SD = 16.4 ± 5.0) compared with controls (3.6 ± 4.1; p < .001). With respect to objective sleep, participants with insomnia had lower TST (p = .011), higher WASO (p = .001), and lower SE (p < .001).
Lower FA in the anterior internal capsule in insomnia
Linear mixed models were used to estimate whether independent variables (insomnia, ISI, TST, WASO, SE, or insomnia duration) were associated with the mean FA of the anterior internal capsule. TST, WASO, SE, and insomnia duration showed no significant association with the mean FA of the right or left anterior internal capsule (see Table 2).
Right internal capsule FA was significantly lower in participants with insomnia (β ID = −9.76e− 3; SE = 4.17e− 3; p = .034). Lower FA in the right internal capsule was significantly associated with higher ISI (β ISI = −8.05e− 4; SE = 2.60e− 4; p = .008, see also Supplementary Figure S1). FA of the left internal capsule showed a similar trend level association with ISI (β ISI = −4.97e− 4; SE = 2.67e−4; p = .08). We found no evidence for interaction effects of either a diagnosis of insomnia, or ISI, with sample, age, or the duration of insomnia. Standardized beta coefficients show that the dimensional model of association of right anterior internal capsule FA with ISI (β ISI = −.148; SE = .048; AIC = −611.3) is somewhat stronger than the alternative, categorical, model of an association of right anterior internal capsule FA with the presence versus absence of the diagnosis of insomnia disorder (β ID = −.114; SE = .049; AIC = −607.3). Although the 4 units difference between these Akaike information criterion (AICs) indicates that a dimensional association of FA with insomnia severity is somewhat more likely than a categorical difference in FA between cases and controls [47], such conclusion would require replication in future studies. Estimated standardized beta coefficients and standard errors of the association of ISI with FA were of a similar order of magnitude: β ISI = −.148, SE = .048 for the whole sample, β ISI = −.120, SE = .081 for the subset of controls, and β ISI = −.098, SE = .064 for the subset of cases with insomnia disorder. As a result of the smaller sample sizes, significance was not reached in the subsamples.
Whole brain cluster analysis showed no significant differences in FA
Voxel-wise comparison using tract-based spatial statistics showed a few clusters with lower FA values in insomnia including in the bilateral anterior internal capsule. After correcting for multiple comparisons, there were no significant differences in FA between insomnia and controls without sleep complaints or an association with ISI (see Supplementary Tables S1and S2 for a list of clusters based on the uncorrected results of the association with ISI).
Discussion
This study set out to evaluate the robustness of the association between insomnia and FA in the anterior limb of the internal capsule. We performed a region-of-interest analysis on the anterior limb of the internal capsule because it may represent the brain region with the least equivocal association with insomnia [14–16]. By combining three samples, we were able to compile a larger dataset than commonly reported in the studies on insomnia disorder. People with insomnia showed significantly lower FA values in the right internal capsule compared with controls without sleep complaints. In line with this finding, higher ISI scores were significantly associated with lower FA values of the right internal capsule. Ancillary subgroup analyses investigated whether the association of white matter microstructure of the right anterior limb of the internal capsule with insomnia severity was specifically driven by either controls or participants with insomnia. Whereas beta coefficients did not reach significance within the subgroups of only controls or participants with insomnia, their order of magnitude was comparable to the standardized beta coefficient of the whole sample. The reductions in significance apparently result from smaller sample sizes. Inspection of the data did not suggest a discontinuity in the slope of ISI, although admittedly the variance would make it difficult to find it by eyeballing (Supplementary Figure S1). Together, this indicates that insomnia is associated with altered white matter microstructure in this area. The ancillary whole brain voxel-wise analyses showed no significant group difference or association with insomnia severity after correction for multiple comparisons.
Alterations in the white matter microstructure of the anterior limb of the internal capsule have been linked to insomnia in several other studies. One previous study found that FA in the right anterior internal capsule was lower in people with insomnia than in controls and that FA in both left and right anterior internal capsule correlated negatively with insomnia severity [15]. A second study found that people with insomnia had lower FA values in several tracts in the right hemisphere, most pronounced in the anterior and posterior limbs of the internal capsule [14]. A third study showed that people with insomnia had lower FA values and lower axial diffusivity values in tracts connecting the left thalamus and the pars triangularis of the prefrontal cortex [16]. Notably, the tractography results reported in that study show that a large proportion of the affected tracts between the thalamus and pars triangularis run through the anterior internal capsule. Lastly, a study in a large sample of community-dwelling older adults reported lower FA in the right anterior internal capsule in participants with a poor sleep quality as compared with those that reported good sleep [48]. Robustness of lower FA in the anterior internal capsule, especially in the right hemisphere, is now supported by our study that included three independent samples of people with insomnia and matched controls without sleep complaints. Together with the previous findings, our results underline and support the role of the anterior internal capsule in insomnia. Its neuroanatomical and functional properties deserve closer attention.
Numerous white matter fibers travel through the anterior limb of the internal capsule. The majority of the fibers serve connection to and from the prefrontal cortex [17]. These connections include thalamocortical projections as well as projections of the prefrontal cortex to the pons and to subcortical structures, notably the head of the caudate nucleus. The anterior limb of the internal capsule also contains fibers that connect the caudate nucleus with other subcortical structures, such as the putamen and globus pallidus [49]. Structures connected through these fibers, such as the thalamus, prefrontal cortex, and pons, are known to modulate sleep [18, 19]. Fronto-subcortical circuits, for example, mediate the disrupting effects of stress on sleep in rodents [50]. Furthermore, previous studies report that people with insomnia show functional alterations in fronto-subcortical networks [51] and structural alterations in the fronto-limbic system as indicated by network properties of its white matter connectivity [25, 52]. Interestingly, the anterior limb of the internal capsule is surrounded by several structures reported to be involved in insomnia and serves their connectivity. These structures include the putamen and the head of the caudate nucleus. Both have been implicated in insomnia [25, 26, 53]. In addition, the anterior internal capsule accommodates fibers that connect more distant areas previously associated with insomnia, notably the OFC [20, 21] and the ACC [23, 24, 54]. Concertedly, the picture emerging from these findings is that insomnia involves a network of gray matter areas, including the OFC, ACC, putamen, caudate, and thalamus of which many interconnecting white matter tracts travel through the anterior limb of the internal capsule. We can only speculate about which aforementioned tracts specifically are involved. One possibility is that the altered microstructure of the anterior internal capsule in insomnia affects the connectivity between the OFC and its primary projection target: the head of the caudate nucleus. Both structures have been associated with insomnia. Previous work, moreover, demonstrated a link between structural and functional deviations. Reduced gray matter volume in the OFC was shown to be associated with insufficient functional recruitment of the head of the caudate nucleus [26]. Since this nucleus plays a key role in cortical inhibition, insufficient recruitment could contribute to the cortical hyperarousal, that is characteristic of insomnia [55, 56]. The anterior limb of the internal capsule accommodates the primary projection of the OFC to the head of the caudate nucleus. It is, therefore, conceivable that alterations in the internal capsule could likewise result in attenuated efferent functional connectivity from the OFC to the head of the caudate nucleus. Possibly, alterations in any of the above-described white matter tracts could result in a suboptimal balance and predispose individuals to hyperarousal, hypersensitivity, hyperreactivity, and insufficient emotion regulation, and consequently develop insomnia [57]. If so, it is understandable that whole brain voxel-based studies are unlikely to always identify the same brain structures across studies [11, 12], as they sample from a population with heterogeneity in the exact site(s) of alteration within the network. Indeed, contrary to a previous study employing tract-based spatial statistics [14], our study did not yield significant differences between insomnia and controls with this approach, in spite of our large number of participants (n = 137). The stringent correction for multiple testing, that is, required for whole-brain voxel-based analysis, in combination with the possibility that alterations might be heterogeneous across participants, could undermine the detection of differences in white matter. Evaluating a predefined network could be an appealing approach as it strongly reduces the number of comparisons, thus increasing statistical power. Another approach would be attempting to reduce heterogeneity by identifying subtypes of insomnia [58] that may have more similar structural or functional deviations. Weak associations of brain structural measures are not only specific to insomnia, but are also characteristic of other mental disorders. The large sample sizes that are required to find reliable small effects benefit from pooling clinical samples from many different sites. The benefits of pooling data transcend the disadvantage of variability due to scan site, as demonstrated in the successful ENIGMA cohorts.
Altered white matter microstructure in the anterior limb of the internal capsule is also reported in other disorders, notably major depression disorder where a meta-analysis reports reduced FA in the anterior limb of the internal capsule and a plausible correlation with depression severity [59]. An interesting question is also whether altered anterior internal capsule FA could be normalized by treatment. At present, cognitive behavioral therapy for insomnia (CBT-I) is the first-line treatment for insomnia with results equivalent to sleep medication and no side effects [60]. As far as we know, the effect of CBT-I on white matter has not been addressed in insomnia. For other disorders, we could only find one study that described cognitive behavioral therapy (CBT) in obsessive–compulsive disorder (OCD) and its effects on FA [61]. OCD patients had significant lower FA in right orbital frontal cortex, left orbital frontal cortex, right cerebellum, left superior parietal gyrus, and higher FA in the right putamen nucleus compared with controls. After 12 weeks of CBT, several FA values were changed specifically in patients who responded, most notable in the left orbital frontal cortex, right cerebellum, and right putamen nucleus. After successful CBT, these brain areas showed no significant difference in FA between OCD and controls, although the authors did not report whether there was a significant interaction effect of time by treatment success on FA. It would be interesting to investigate similar effects in insomnia disorder.
The current study shows significant associations of insomnia with white matter features in the anterior limb of the internal capsule in the right hemisphere. A trend was seen in the left hemisphere. Previous studies have reported associations either or both in the left and right hemispheres [14–16, 25]. Overall, the findings do not support the hemispheric specificity of the association. Due to the cross-sectional design of our study, we can only speculate on the causal interpretation of the altered white matter microstructure in the internal capsule of people with insomnia. We found no association between anterior internal capsule FA and insomnia duration, which is in line with previous work reporting no association between insomnia duration and neuroimaging measures [16, 20, 23, 26, 62]. This hints toward the possibility that individual differences in brain structure may predispose toward insomnia. Testing this hypothesis requires longitudinal studies that commence with symptom-free participants.
This study has several limitations that should be taken into account. Focusing on insomnia, our sleep recordings do not routinely include the (oronasal) airflow and oxygen desaturation sensors that would be required to formally exclude the presence of comorbid sleep apnea. To exclude apnea to the best of our ability, participants were screened extensively both during inclusion and by monitoring throughout the night of PSG. As described in Methods section, any indication of suspect sleep apnea led to exclusion of the participant. Diffusion MRI has well-known limitations. Alterations in diffusion values can be driven by several factors, including fiber geometry (amount of crossing fibers), axon density, axon diameter, and level of myelination. From our results, we can only speculate about which fibers within the internal capsule differ in insomnia. Interestingly, a recent study combining molecular tracing and diffusion MRI showed a relative spatial topology in the anterior internal capsule [63]. This topology within the tract represented different prefrontal cortex areas that are connected with subcortical areas. This finding opens up the possibility for future studies to distinguish between fronto-subcortical fiber bundles.
In summary, our results support that insomnia and insomnia severity are associated with altered microstructure in the right anterior limb of the internal capsule. The anterior internal capsule accommodates several tracts that connect brain areas previously related to sleep in general and insomnia in particular. Future studies could focus on which specific tracts within the anterior limb of the internal capsule are affected. Moreover, future studies might benefit from novel approaches that can account for individual differences in where exactly deviations within a connected circuit occur. Such approaches may be key to improve our understanding of the neurobiological mechanism underlying insomnia.
Supplementary Material
Acknowledgments
This publication reflects the views only of the authors and the European Commission cannot be held responsible for any use which may be made of the information contained therein.
Funding
This work was supported by the European Commission (European Research Council Grant ERC-ADG-2014–671084 INSOMNIA); by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek, The Hague, The Netherlands (ZONMW Neuropsychoanalysis Fund Grant 16.561.0001 and VICI 453.07.001); by the Bial Foundation, S. Mamede do Coronado, Portugal (Grant 253/2012 and 190/2016); and by a University Research Fellowship from the Vrije Universiteit Amsterdam, Amsterdam.
Conflict of interest statement. None declared.
References
- 1. Bassetti CL, et al. Neurology and psychiatry: waking up to opportunities of sleep.: state of the art and clinical/research priorities for the next decade. Eur J Neurol. 2015;22(10):1337–1354. [DOI] [PubMed] [Google Scholar]
- 2. Riemann D, et al. Chronic insomnia: clinical and research challenges–an agenda. Pharmacopsychiatry 2011;44(1):1–14. [DOI] [PubMed] [Google Scholar]
- 3. American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders. 5th ed.Arlington, TX: American Psychiatric Publishing; 2013. [Google Scholar]
- 4. Morin CM. Combined therapeutics for insomnia: should our first approach be behavioral or pharmacological? Sleep Med. 2006;7(Suppl 1):S15–S19. [DOI] [PubMed] [Google Scholar]
- 5. Kyle SD, et al. Insomnia and health-related quality of life. Sleep Med Rev. 2010;14(1):69–82. [DOI] [PubMed] [Google Scholar]
- 6. Hargens TA, et al. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep. 2013;5:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarrin DC, et al. Insomnia and hypertension: a systematic review. Sleep Med Rev. 2018;41:3–38. [DOI] [PubMed] [Google Scholar]
- 8. Hertenstein E, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. [DOI] [PubMed] [Google Scholar]
- 9. Pigeon WR, et al. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):44. [DOI] [PubMed] [Google Scholar]
- 10. Van Someren EJ, et al. Disrupted sleep: from molecules to cognition. J Neurosci. 2015;35(41):13889–13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tahmasian M, et al. Reply to Hua Liu, HaiCun Shi and PingLei Pan: coordinate based meta-analyses in a medium sized literature: considerations, limitations and road ahead. Sleep Med Rev. 2018;42:236–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spiegelhalder K, et al. Neuroimaging insights into insomnia. Curr Neurol Neurosci Rep. 2015;15(3):9. [DOI] [PubMed] [Google Scholar]
- 13. Piantoni G, et al. Individual differences in white matter diffusion affect sleep oscillations. J Neurosci. 2013;33(1):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li S, et al. Reduced integrity of right lateralized white matter in patients with primary insomnia: a diffusion-tensor imaging study. Radiology. 2016;280(2):520–528. [DOI] [PubMed] [Google Scholar]
- 15. Spiegelhalder K, et al. Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp. 2014;35(7):3431–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang JM, et al. Low white-matter integrity between the left thalamus and inferior frontal gyrus in patients with insomnia disorder. J Psychiatry Neurosci. 2018;43(4):170195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zarei M, et al. Two-dimensional population map of cortical connections in the human internal capsule. J Magn Reson Imaging. 2007;25(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saper CB, et al. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saper CB, et al. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. [DOI] [PubMed] [Google Scholar]
- 20. Altena E, et al. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67(2):182–185. [DOI] [PubMed] [Google Scholar]
- 21. Stoffers D, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suh S, et al. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2015;39(1):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Winkelman JW, et al. Increased rostral anterior cingulate cortex volume in chronic primary insomnia. Sleep. 2013;36(7):991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wassing R, et al. Haunted by the past: old emotions remain salient in insomnia disorder. Brain. 2019;142(6):1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jespersen KV, et al. Reduced structural connectivity in insomnia disorder. J Sleep Res. 2020;29(1):e12901. [DOI] [PubMed] [Google Scholar]
- 26. Stoffers D, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137(Pt 2):610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mädler B, et al. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874–888. [DOI] [PubMed] [Google Scholar]
- 28. American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 29. Naftolowitz DF, et al. DSM-IV. CNS Drugs. 1995; 4:1–7 . doi: 10.2165/00023210-199504010-00001 [DOI] [Google Scholar]
- 30. Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- 31. Andersson JLR, et al. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andersson JLR, et al. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016;141:556–572. [DOI] [PubMed] [Google Scholar]
- 33. Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. [DOI] [PubMed] [Google Scholar]
- 34. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andersson JLR, et al. Non-linear registration, aka spatial normalisation. FMRIB Technial Report TR07JA2 2007:1–21. www.fmrib.ox.ac.uk/analysis/techrep. Accessed February 14, 2019.
- 36. Andersson JLR, et al. Non-linear optimisation. FMRIB technical report TR07JA1 2007:1–16. http://fsl.fmrib.ox.ac.uk/analysis/techrep/tr07ja1/tr07ja1.pdf.
- 37. Mori S, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bastien CH, et al. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 39. Morin CM, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silber MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131. [PubMed] [Google Scholar]
- 41. R core team. R: a language and environment for statistical computing. R Found Stat Comput Vienna, Austria: R Foundation for Statistical Computing;2017. doi:http://www.R-project.org [Google Scholar]
- 42. Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 43. Lüdecke D. sjstats: statistical functions for regression models. R package version 0.17.7 2019. https://cran.r-project.org/web/packages/sjstats/index.html. Accessed November 14, 2019.
- 44. Winkler AM, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winkler AM, et al. Faster permutation inference in brain imaging. Neuroimage. 2016;141:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Winkler AM, et al. Multi-level block permutation. Neuroimage. 2015;123:253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burnham KP, et al. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261–304. [Google Scholar]
- 48. Sexton CE, et al. Associations between self-reported sleep quality and white matter in community-dwelling older adults: a prospective cohort study. Hum Brain Mapp. 2017;38(11):5465–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Axer H, et al. Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Methods. 2000;94(2):165–175. [DOI] [PubMed] [Google Scholar]
- 50. Cano G, et al. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28(40):10167–10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nofzinger EA, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161(11):2126–2128. [DOI] [PubMed] [Google Scholar]
- 52. Lu FM, et al. Diffusion tensor imaging tractography reveals disrupted white matter structural connectivity network in healthy adults with insomnia symptoms. Front Hum Neurosci. 2017;11:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jansen PR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51:394–403. [DOI] [PubMed] [Google Scholar]
- 54. Suh S, et al. Cortical thinning and altered cortico-cortical structural covariance of the default mode network in patients with persistent insomnia symptoms. Sleep. 2016;39(1):161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Colombo MA, et al. More severe insomnia complaints in people with stronger long-range temporal correlations in wake resting-state EEG. Front Physiol. 2016;7:576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wei Y, et al. EEG microstates indicate heightened somatic awareness in insomnia: toward objective assessment of subjective mental content. Front Psychiatry. 2018;9:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. [DOI] [PubMed] [Google Scholar]
- 58. Blanken TF, et al. Subtyping insomnia disorder – authors’ reply. Lancet Psychiatry. 2019;6(4):285–286. [DOI] [PubMed] [Google Scholar]
- 59. Chen G, et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: a voxel-based meta-analysis of diffusion tensor imaging. Prog Neuro-Psychopharmacology Biol Psychiatry. 2017;76(March):179–187. [DOI] [PubMed] [Google Scholar]
- 60. Rossman J. Cognitive-Behavioral Therapy for Insomnia: an Effective and Underutilized Treatment for Insomnia. Am J Lifestyle Med. 2019;13(6):544–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhong ZX, et al. Abnormalities of white matter microstructure in unmedicated patients with obsessive–compulsive disorder: changes after cognitive behavioral therapy. Brain Behav. 2019;9(2):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li M, et al. Altered gray matter volume in primary insomnia patients: a DARTEL-VBM study. Brain Imaging Behav. 2018;12(6):1759–1767. [DOI] [PubMed] [Google Scholar]
- 63. Safadi Z, et al. Functional segmentation of the anterior limb of the internal capsule: linking white matter abnormalities to specific connections. J Neurosci. 2018;38(8):2106–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.