Abstract
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid test is currently the gold standard for diagnosing coronavirus disease 2019 (COVID-19). This disease requires high-quality viral nucleic acid tests, and selecting the type of specimen from patients, who are at different disease stages, to use in the nucleic acid test is challenging. This article reports in detail the diagnosis and treatment process for two patients with confirmed COVID-19 and analyzes the results of the SARS-CoV-2 nucleic acid tests that were used for different types of specimens (sputum from deep cough, nasopharyngeal swab, and feces). The nucleic acid testing results of sputum from deep cough showed the best performance for positive detection. Our findings provide a reference for selecting the most suitable specimen for the clinical diagnosis of COVID-19 and improving the positive detection rate.
Keywords: Severe acute respiratory syndrome coronavirus 2, coronavirus disease 2019, nucleic acid test, clinical specimens, case report, positive detection rate
Introduction
Since the outbreak of coronavirus disease 2019 (COVID-19), which was first reported in Wuhan, China in December 2019, the pandemic caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly in China and globally.1,2 By implementing several active measures, the COVID-19 epidemic has been suppressed greatly in China, but the number of confirmed cases has continued to rise in other countries. As of 28 March 2020, there were 82,279 confirmed cases in China and 520,726 confirmed cases in other countries. In China, COVID-19 has been classified as a class B infectious disease and an acute respiratory infection in accordance with the Law of the People’s Republic of China on the Prevention and Treatment of Infectious Diseases, and it has been treated using the control measures that are applicable to class A infectious diseases.
In accordance with the Diagnosis and Treatment Protocol for COVID-19 Pneumonia (Trial revised 7th edition), which was issued by the China National Health Commission and the State Administration of Traditional Chinese Medicine,3 common clinical manifestations of COVID-19 include fever, dry cough, and fatigue. Suspected cases can be confirmed with etiological or serological evidence that are obtained from the following tests: 1) Detection of SARS-CoV-2 RNA using real-time fluorescence-based reverse transcriptase-polymerase chain reaction (RT-PCR); 2) Sequencing of the viral genome to check for its homology to the genome of SARS-CoV-2; and 3) Detection of SARS-CoV-2-specific IgM and IgG antibodies in serum, whether it changes from negative to positive, or whether the number of SARS-CoV-2-specific IgG antibodies increases more than four times in the recovery period compared with the acute period. Therefore, the timely and accurate pathological test of nucleic acids has become a critical component in diagnosing COVID-19.
Specimens that were used in the nucleic acid tests for respiratory diseases include upper respiratory tract specimens and lower respiratory tract specimens. The former includes nasopharyngeal and oropharyngeal swabs, while the latter includes bronchial lavage fluid, alveolar lavage fluid, sputum from deep cough, and lung biopsy specimens. Of note, sputum from a deep cough in our study is obtained as described below. After preliminary oral cleaning in the morning, the patient rinsed their mouth with 0.9% NaCl solution. After inhaling deeply, the patient spit out sputum into a sterile bottle. To date, throat swabs have been the most commonly used specimens in SARS-CoV-2 nucleic acid tests because the collection procedure is easier and less invasive. However, existing SARS-CoV-2 nucleic acid test results show that the positive detection rate in the nucleic acid tests of throat swabs is low.4–6 This study reports the details of the process that was used in the diagnosis and treatment of two patients with confirmed cases COVID-19. This report also analyzes the results of the SARS-CoV-2 nucleic acid tests that used different types of specimens, to provide a reference selecting the most suitable specimen to clinically diagnose COVID-19 and improve the positive detection rate.
Case report
Case 1
The patient was a 67-year-old man from Wuhan, China. The patient’s chief complaint was “cough and shortness of breath that had lasted for more than 1 month.” The patient complained that on 22 January 2020, for no apparent reason, he started to have dry cough with no sputum, which was accompanied by chest tightness, shortness of breath, fatigue, and poor appetite. He did not have chills, fever, nausea, vomiting, or other discomforts. The patient was admitted to Wuhan Hanyang Hospital on 3 February 2020 because of intensified chest tightness. Physical examination upon admission showed that his body temperature was 36.5°C, pulse was 106 beats/minute, respiratory rate was 22 breaths/minute, blood pressure was 120/70 mmHg, and oxygen saturation (SpO2) measured using pulse oximeter was 88%. The white blood cell count was 10.44 × 109/L, platelet count was 97 × 109/L, and the percentage of neutrophils was 91.01%. The absolute lymphocyte count was 750 cells/µL, the total percentage of T cells was 31.4%, and the absolute T cell count was 235/µL. The patient tested negative for influenza A and B, respiratory syncytial virus, human parainfluenza virus, adenovirus, echovirus, group B coxsackievirus, Mycoplasma pneumoniae, Chlamydia pneumoniae, and tuberculosis (using anti-tuberculosis IgM antibodies). The concentration of interleukin-6, interferon gamma, tumor necrosis factor alpha, and interleukin-10 were 167.44 pg/mL, 24.08 pg/mL, 36.36 pg/mL, and 12.06 pg/mL, respectively. The blood gas analysis showed that pH was 7.57 and partial pressure of oxygen was 45.7 mmHg. On 4 February 2020, chest CT scan imaging showed diffuse infections in both lungs and the results of nucleic acid test by nasopharyngeal swab was presumptively positive. The patient was treated with moxifloxacin, tienam combined with linezolid, and piperacillin/tazobactam for anti-infection, methylprednisolone for anti-inflammation, ambroxol for expectoration, and thymalfasin for immune response enhancement. However, his condition did not improve, so he was transferred to our hospital on 26 February 2020.
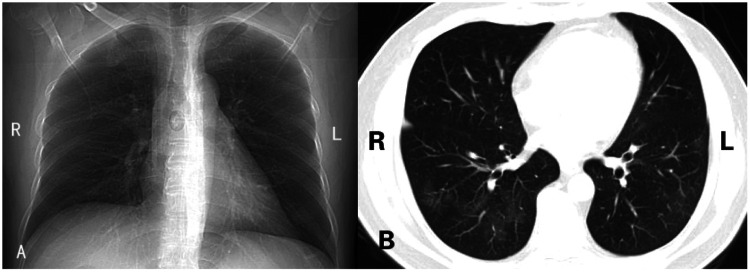
Upon admission to our hospital, the patient had a body temperature of 36.5°C, a pulse of 106 beats/minute, a respiratory rate of 22 breaths/minute, a blood pressure of 120/70 mmHg, and the SpO2 was 89%. Chest physical examinations including inspection, palpation, and percussion indicated no obvious abnormalities, while the auscultation was not performed because of the limitations of the doctor’s protective clothing. The chest CT scan that was performed on 2 March 2020 showed an abnormal opacity in both lungs, which suggested viral pneumonia (Figure 1). The patient’s nasopharyngeal swabs were collected and sent for nucleic acid testing on 27 February and 4 March 2020, and both results were negative. On 5 March 2020, the result of SARS-CoV-2-specific IgM tests was positive. The next day, three different types of specimens (sputum from deep cough, nasopharyngeal swab, and feces) were collected for the nucleic acid test, and the results were positive, negative, and negative, respectively. The timeline of nucleic acid tests, IgM antibody tests, and the corresponding results was presented in Figure 2. The patient was treated with piperacillin/tazobactam for anti-infection, ambroxol for expectoration, and thymalfasin for immune response enhancement. His respiratory symptoms gradually improved afterward, and SpO2 increased to 95% without the assistance of a ventilator. The nasopharyngeal swabs were collected and sent for nucleic acid testing on 14 March and 17 March 2020, respectively, and the results were both negative. The patient was discharged on 18 March 2020 and was isolated for 2 more weeks in a community quarantine center. Additionally, the patient received thymalfasin treatment to enhance the immune response during hospitalization, and follow-up data within 1 month after discharge showed that no complications had occurred.
Figure 1.
CT scans for Case 1. (a) A reconstructed coronal scan; (b) A transverse scan
L, left; R, right; CT, computed tomography.
Figure 2.
Timeline of nucleic acid testing, IgM antibody testing, and the corresponding test results for Case 1.
Case 2
The patient was a 56-year-old man from Wuhan, China. The patient’s chief complaint was “cough and fever that had lasted for more than 1 month.” The patient complained that on 26 January 2020, he started to have the symptom of dry cough with no sputum for no apparent reason. He did not have any discomfort such as chest tightness, shortness of breath, fatigue, or poor appetite. Because the patient had a history of pharyngitis, the condition was considered to be recurrent pharyngitis, and the patient did not receive additional treatment. On 31 January 2020, he started to have a fever, with the highest body temperature was 39.2°C, which was accompanied by chills (without shivering), fatigue, and muscle pain in the extremities. He was admitted to Wuhan No. 9 Hospital the next day and was administered moxifloxacin infusion for anti-infective treatment. The COVID-19 nucleic acid test result was positive on 7 February 2020, so the patient was sent to the mobile cabin hospital. During hospitalization, a nucleic acid tests of nasopharyngeal swabs were performed four times, and the results were presumptive positive, positive, negative, and presumptive positive, respectively. To receive essential treatment, the patient was transferred to our hospital on 7 March 2020.
Upon admission to our hospital, the patient had a body temperature of 36.2°C, a pulse of 92 beats/minute, a respiratory rate of 17 breaths/minute, and a blood pressure of 141/82 mmHg. Chest physical examination including inspection, palpation, and percussion indicated no obvious abnormalities, while the auscultation was not performed because of the limitations of the doctor’s protective clothing. On 8 March 2020, the laboratory test results for whole blood cell counts and percentages, C-reactive protein, and interleukin-6 were all within their normal ranges. On 9 March 2020, chest CT scan results showed abnormal opacities in both lungs, which suggested viral pneumonia (Figure 3). The SARS-CoV-2-specific IgM and IgG test results were both positive and the nucleic acid test results for the nasopharyngeal swab were presumptive positive. The patient received treatment with arbidol hydrochloride tablets and oseltamivir phosphate capsules for antiviral therapy, and lansoprazole for stomach protection. The results of nucleic acid tests of nasopharyngeal swabs were both negative on 12 March and 15 March 2020, and he had no discomfort, which met the discharge criteria. However, considering the patient’s history of multiple positive nucleic acid test results, three types of specimens (sputum from deep cough, nasopharyngeal swab, and feces) were sent for nucleic acid testing on 17 March 2020, and the sputum specimen tested positive, while the others tested negative. On 27 March 2020, nucleic acid tests using sputum, nasopharyngeal, and stool specimens was performed again and the results were positive, negative, and presumptive positive, respectively (Figure 4). The patient continued to be treated in the hospital.
Figure 3.
CT scans for Case 2. (a) A reconstructed coronal scan; (b) A transverse scan
L, left; R, right; CT, computed tomography.
Figure 4.
Timeline of nucleic acid testing, IgM or IgG antibody testing, and the corresponding results for Case 2.
Discussion
In the clinical diagnosis and treatment of the COVID-19, it is not reliable to make diagnoses solely based on symptoms, laboratory, and imaging tests. Detection of SARS-CoV-2 RNA is the gold standard for diagnosing COVID-19. Therefore, the early, timely, and accurate detection of SARS-CoV-2 RNA plays an important role because it allows the early isolation and effective treatment of the patient, prevents further spread of the COVID-19 epidemic, and reduces medical costs that are associated with hiring a larger number of observers for contact tracing.7
Currently, several companies in China and other countries have developed real-time, fluorescence-based quantitative PCR testing kits for SARS-CoV-2 nucleic acid tests. However, the mainstream media and medical experts have recently reported that the positive detection rate of SARS-CoV-2 RNA is low, and that in some cases, the patient has tested positive after repeated negative results. Some doctors also reported cases where the pharyngeal swab showed a negative result multiple times, but the sputum specimen eventually tested positive. There were also cases where the nucleic acid tests showed repeated negative results, while the chest CT scan exhibited typical features of COVID-19.8
In this study, we performed SARS-CoV-2 nucleic acid tests for two patients with COVID-19 using three types of specimens (sputum from deep cough, nasopharyngeal swab, and feces), and we found the following: (1) even when tests using nasopharyngeal swabs were negative repeatedly, when the sputum from deep cough was used, the viral RNA test could still be positive for both patients 1 and 2; (2) stool specimens could also be used for viral RNA testing, and this result was positive for patient 2. Our findings in the two patients are consistent with previous studies. In a study of 1070 specimens that were collected from 205 patients with COVID-19, the positive testing rate of nasal swabs specimens (6/13, 46%) was lower than that of bronchoalveolar lavage fluid (14/15, 93%) and sputum specimens (72/104, 72%), but was higher than that of feces (44/153, 29%).4 In another study of 52 diagnosed COVID-19 patients, the positive testing rates of SARS-CoV-2 from sputum specimens and throat swabs were 76.9% and 44.2%.5 It has been reported that viral RNA concentrations in the sputum decreased more slowly than that in throat swabs specimens.9 These findings indicated that sputum is an appropriate choice as a specimen source for nucleic acid tests to diagnose COVID-19.
Based on our study, we propose the following for considerations: (1) Problems are associated with the type of specimen and method of collection, as described below. The collection of pharyngeal swabs requires highly technical skills, and the standard procedure must be strictly followed. Preferably, two specimens should be collected simultaneously and stored in the same storage tube to improve the positive detection rate. The positive detection rate of sputum specimens is high, but many patients with COVID-19 have no cough or dry cough, which makes it difficult to collect sputum specimens. Stool specimens are easy to collect, where the procedure is less demanding than that of throat swabs and sputum specimens. The value of stool specimens in SARS-CoV-2 nucleic acid tests cannot be ignored. For COVID-19 patients who tested positive for a long time (such as patient 2), the possibility of fecal transmission still requires further research; and (2) The patient’s disease course and severity must be considered. The SARS-CoV-2 viral load varies in patients at different stages and with different disease severities.
Ensuring the safety of laboratory staff while producing reliable nucleic acid test results matters in the fight against COVID-19.10 We recommend that whenever it is possible to collect a lower respiratory tract specimen (such as sputum), this type of specimen should be used for testing. However, it must be clarified that obtaining lower respiratory tract secretions exposes the clinical staff who is collecting specimens to undue risk of infection. Oropharyngeal swabs, nasopharyngeal swabs, and stool specimens would be useful for the SARS-CoV-2 nucleic acid test to reduce the incidence of “re-test” of the SARS-CoV-2 RNA. However, because of the small sample size in this study, our findings cannot yet be considered to be representative, and we are conducting more clinical case studies.
Acknowledgements
We would like to show our great respect to all the workers and volunteers in the fight against COVID-19.
Availability of data and materials
The raw data analyzed during the current study are not publicly available as the data are also part of an ongoing study, but they are available from the corresponding author upon reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
This study was approved by the ethics committee at the Guanggu District of Hubei Province Maternal and Child Health Hospital [Number FYGG(L)2020-004]. Written informed consent was obtained from the patients.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by “the Fundamental Research Funds for the Central Universities” (Project No. 22120180392), “Program of Shanghai Academic/Technology Research Leader” (Project No. 18XD1403000), Shanghai 2018 “Science and Technology Innovation Action Plan” Science and Technology Support Project in Biomedicine (18441903500), and “the Guanggu District of Hubei Province Maternal and Child Health Hospital” (Project No. 2020-FYGG-021).
ORCID iD
Zhongwei Lv https://orcid.org/0000-0003-3370-5560
References
- 1.Wu Y, Ho W, Huang Y, et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet 2020; 395: 949–950. DOI: 10.1016/s0140-6736(20)30557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–269. DOI: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.China National Health Commission. Diagnosis and treatment protocol for COVID-19 pneumonia (Trial revised 7th edition), www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989shtml (2020, accessed 4 March 2020).
- 4.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323: 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C, Xiang J, Yan M, et al. Comparison of throat swabs and sputum specimens for viral nucleic acid detection in 52 cases of novel coronavirus (SARS-Cov-2)-infected pneumonia (COVID-19). Clin Chem Lab Med 2020; 58: 1089–1094. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis Epub ahead of print 29 Feb 2020. DOI: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed]
- 7.Layne SP, Hyman JM, Morens DM, et al. New coronavirus outbreak: framing questions for pandemic prevention. Sci Transl Med 2020; 12: abb1469. DOI: 10.1126/scitranslmed.abb1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Z, Lu Y, Cao Q, et al. Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol Epub ahead of print 14 Mar 2020. DOI: 10.2214/ajr.20.22959. [DOI] [PubMed]
- 9.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581: 465–469. [DOI] [PubMed] [Google Scholar]
- 10.Tang YW, Schmitz JE, Persing DH, et al. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 2020; 58: e00512–e00520. DOI: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data analyzed during the current study are not publicly available as the data are also part of an ongoing study, but they are available from the corresponding author upon reasonable request.