Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on l‐lysine monohydrochloride and l‐lysine sulfate produced using Corynebacterium glutamicum CGMCC 7.266 when used as a nutritional additive in feed and water for drinking for all animal species. The active substance is l‐lysine and it is produced in two different forms (monohydrochloride or sulfate salts). None of those forms pose any safety concern associated with the genetic modification of the production strain. l‐Lysine HCl and l‐lysine sulfate produced by C. glutamicum CGMCC 7.266 are considered safe for the target species, for the consumer and for the environment. For both products, the FEEDAP Panel has concerns regarding the safety for the target species when the additives are administered via feed and water for drinking, simultaneously. In the absence of data, the FEEDAP Panel cannot conclude on the safety of both forms of the additive for the user. The products under assessment are considered efficacious sources of the amino acid l‐lysine for all animal species. For these products to be as efficacious in ruminants as in non‐ruminant species, they require protection against degradation in the rumen.
Keywords: nutritional additive, amino acid, lysine monohydrochloride, lysine sulfate, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Welding GmbH & Co. KG.2 for authorisation of the products l‐lysine monohydrochloride and l‐lysine sulfate, when used as feed additives for all animal species (category: nutritional additives; functional group: amino acids, their salts and analogues).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 3 July 2018.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product l‐lysine monohydrochloride and l‐lysine sulfate produced by fermentation with Corynebacterium glutamicum CGMCC 7.266, when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
l‐Lysine is currently authorised for its use in all animal species as a nutritional additive.3 No maximum content in feedingstuffs is established in the European Union (EU).
l‐Lysine is authorised for use in food,4 cosmetics5 and as a veterinary medicinal product.6 , 7
l‐Lysine hydrochloride is described in a monograph of the European Pharmacopoeia (PhEur 9th edition, 2017) monograph 01/2008:0930.
The scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) has published several opinions on the safety and efficacy of l‐lysine and/or its salts produced by different strains of C. glutamicum or Escherichia coli for all animal species (EFSA, 2007a; EFSA FEEDAP Panel, 2013, 2014, 2015a,b,c, 2016a,b, 2017a, 2019a,b,c,d,e).
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier8 in support of the authorisation request for the use of l‐lysine monohydrochloride and l‐lysine sulfate produced by fermentation with C. glutamicum CGMCC 7.266 as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the l‐lysine monohydrochloride and l‐lysine sulfate produced by fermentation with C. glutamicum CGMCC 7.266 in animal feed. The Executive Summary of the EURL report can be found in Annex A.9
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐lysine monohydrochloride and l‐lysine sulfate produced by fermentation with C. glutamicum CGMCC 7.266 is in line with the principles laid down in Regulation (EC) No 429/2008 and the relevant guidance documents: Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017b), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017c), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017d), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019f).
3. Assessment
The current application is for the authorisation of l‐lysine monohydrochloride (HCl) (minimum 78% l‐lysine on dry matter (DM) basis) and l‐lysine sulfate (minimum 55% l‐lysine on DM basis) produced by fermentation by a genetically modified strain of C. glutamicum (CGMCC 7.266). These products are intended to be used in feed and water for drinking for all animal species as nutritional additives (functional group: amino acids, their salts and analogues). The active substance of both forms of the additive is l‐lysine.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The production microorganism is a genetically modified strain of C. glutamicum which has been deposited in the China General Microbiological Culture Collection Center (CGMCC) with deposition number CGMCC 7.266.10 The identity of the production strain as belonging to C. glutamicum species was demonstrated ■■■■■■■■■■■■■■■■■■■■
The susceptibility of the production strain to antibiotics was tested as recommended by FEEDAP for Corynebacterium and other Gram‐positive organisms (EFSA FEEDAP Panel, 2018) using microdilution.13 The minimum inhibitory concentration (MIC) values were equal (for chloramphenicol) or below (for all others) to the corresponding cut‐off values (EFSA FEEDAP Panel, 2018), therefore the strain is considered to be susceptible to the tested antibiotics.
■■■■■ was interrogated for the presence of antimicrobial resistance (AMR) genes ■■■■■
Similarly, ■■■■■ pathogenicity and virulence factors ■■■■■■■■■■■■■■■
■■■■■
■■■■■
■■■■■■■■■■
■■■■■
■■■■■
■■■■■■■■■■■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
-
■■■■■
■■■■■
■■■■■
3.1.2. Manufacturing process
l‐Lysine is produced by fermentation using C. glutamicum CGMCC 7.266. ■■■■■■■■■■
The applicant stated that no antibiotics are used during the production process.■■■■■
3.1.3. Characterisation of l‐Lysine monohydrochloride
l‐Lysine HCl (IUPAC name: (2S)‐2,6‐diaminohexanoic acid monohydrochloride, synonym l‐lysine hydrochloride, a compound identified with the CAS No 657‐27‐2 and the EINECS No 211‐519‐9), has a molecular weight of 182.65 g/mol. The theoretical content of lysine in lysine monohydrochloride is 80%. The molecular formula is NH2‐(CH2)4‐CH(NH2)‐COOH‐HCl and the molecular structure is given in Figure 1.
Figure 1.

Molecular structure of l‐lysine HCl
The product is specified to contain ≥ 98.5% l‐lysine HCl (representing ≥ 78% l‐lysine), and ≤ 1% moisture.18
The average lysine content analysed in five batches was 78.6% (range 77.5–80.6%) on a DM basis (two out of five batches were below the specification).19 The content of chloride was calculated to be 20%. Loss on drying was on average 1.3% (range 0.8–1.7%, four of the five batches analysed had moisture above the specification). Other amino acids were analysed in one batch and only valine could be detected (0.02%). On a DM basis, the sum of quantified material including calculated chloride was on average 98.6%.20
The specific optical rotation was measured in three batches and ranged from +21.9° to +22.1°.21 This is within the range specified in the European Pharmacopoeia (+21.0 to +22.5°) and confirms that the additive is the l‐stereoisomer of lysine.
3.1.3.1. Impurities
Three batches of l‐lysine HCl were analysed for undesirable substances. As regards heavy metals, cadmium was below the limit of detection (LOD),22 lead ranged from < LOD to 0.14 mg/kg and mercury ranged from 0.005 to 0.13 mg/kg. Arsenic ranged from 0.02 to 0.05 mg/kg.23
Three batches were analysed for the presence of dioxins (polychlorinated dibenzo‐p‐dioxins (PCDD) and polychlorinated dibenzofurans (PCDF)) and dioxin‐like PCBs. PCDD/F ranged from 0.037 to 0.45 ng WHO TEQ/kg additive and dioxin‐like PCBs ranged from 0.0021 to 0.0030 ng WHO TEQ/kg additive on a DM basis.24
In reference to the microbiological contamination, analysis of three batches showed that Salmonella spp., Escherichia coli, coliforms, filamentous fungi and yeasts were not detected.25
Regarding the mycotoxin content, analytical data of the same batches showed levels of ochratoxin, zearalenone, sum of fumonisins (B1 + B2 + B3) and deoxynivalenol (DON) below the LOD.26 Aflatoxins ranged from 5 to 9 μg/kg and citrinin ranged from 32 to 42 μg/kg.23
The production strain meets the Qualified Presumption of Safety (QPS) qualifications and is not expected to have any antimicrobial activity relevant to antimicrobials used in humans and animals.
■■■■■■■■■■■■■■■
■■■■■■■■■■■■■■■
■■■■■■■■■■■■■■■
Considering the results of all the tests done, the Panel concludes that the data indicate that no viable cells of the production strain are present in the additive.
■■■■■■■■■■■■■■■
3.1.3.2. Physical properties
l‐Lysine HCl is a light yellow granular product with an approximate density of 0.6 kg/L,31 pH 5.72 (at 10% solution in water at 20°C) and with a water solubility of about 650 g/L at 20°C.32
The dusting potential analysed by the Stauber–Heubach method in three batches ranged from 0.9 to 10 g/m3.33 Concerning the particle size distribution, three batches were analysed by laser diffraction. The fraction of particles having a diameter smaller than 18, 50 and 100 μm ranged 1–2%, 1–4% and 3–7%, respectively.34
3.1.3.3. Stability and homogeneity
The shelf life of the additive was studied in three batches of the additive kept in closed bags, protected from light, either at room temperature for 12 months or at 40°C for 6 months. No losses were observed.23
The stability of the additive (three batches) in a vitamin/mineral premixture containing choline (16,000 mg/kg) was studied when added at 10% and stored in sealed plastic bags at ambient temperature for 6 months.35 Losses observed ranged from 0 to 4% depending on the batch considered.
The stability of the additive (three batches) in feedingstuffs was tested in meal and pelleted compound feed for pigs for fattening (basal diet containing barley and soybean meal) when supplemented at 0.5% (corresponding to 0.39% lysine).36 The samples were kept at room temperature in individual bags for 3 months. Total (free plus protein‐bound) lysine was analysed. Losses ranged from 1 to 4% in the meal feed and from 1 to 5% in the pelleted feed. The effect of feed processing (e.g. conditioning, pelleting) on the stability of the additive was not reported.
The stability of the additive (three batches) was studied in water at a concentration of about 0.4% and stored at room temperature for 24 h.23 No losses were detected. It is noted that the duration of the stability study is half the recommended in the guidance documents.
The capacity of one batch of l‐lysine HCl to distribute homogeneously in the pelleted feed described above was studied in 10 subsamples. Total lysine was measured in each subsample and the background lysine concentration of the basal diet at the beginning of the study was subtracted from each subsample. The coefficient of variation (CV) was 1.5% when total lysine was considered and 4.4% when the calculated concentration of supplemental lysine was considered.37
3.1.4. Characterisation of l‐lysine sulfate
l‐Lysine sulfate (CAS No 60343‐69‐3) has a molecular weight of 390.38 g/mol. The molecular formula is [NH2‐(CH2)4‐CH(NH2)‐COOH]2 SO4 and the molecular structure is given in Figure 2. The theoretical content of lysine in the lysine sulfate is 75%.
Figure 2.
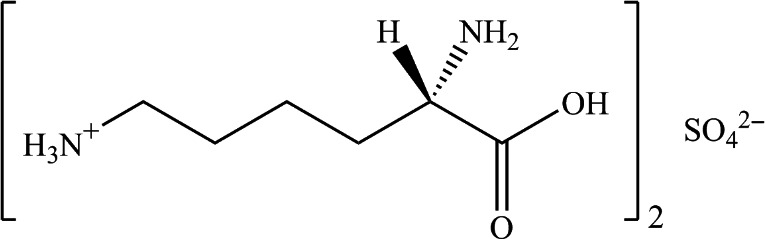
Molecular structure of l‐lysine sulfate
l‐Lysine sulfate contains by specification ≥ 55% of lysine on DM basis, ≤ 3% loss on drying and ≤ 4% residues on ignition.18
The compositional data of five batches showed an average lysine concentration of 53.3% on as is basis (range 52.7–54.0%), corresponding to an average 55.1% lysine (range 54.4–56.0%) on a DM basis (two out of five batches were below the specification). Water content was on average 3.2% (range 2.9–3.5%, two out of five batches were above the specification).38 Sulfate content (analysed in three batches) ranged 22.0–23.1% ‘as is’.39 The calculated proportion of sulfate not associated with lysine in relation to total lysine was about 4% on as is basis. Crude ash was analysed in a single batch and was 1.6%.
Other constituents analysed (three batches) were (on as is basis) lactic acid (0.29–0.34%) formic acid (0.4% in all batches), sulfur (7.3–7.7%), dietary fibre (1.1–1.4%), crude fat (0.9–1.1%), moisture (2.2–2.3%) and cadaverine (0.03–0.09%).39 Amino acids other than lysine were analysed after hydrolysis in three batches and the sum ranged 7.7–8.8% on as is basis.40
3.1.4.1. Impurities
Three batches of l‐lysine sulfate were analysed for undesirable substances. Regarding the levels of heavy metals, cadmium was below the LOD,41 lead ranged 0.018–0.025 mg/kg and mercury 0.41–1.00 mg/kg. Arsenic ranged 0.15–0.21 mg/kg.42 Dioxins (WHO‐PCDD/F) ranged 0.251–0.309 ng TEQ/kg and the sum of dioxin plus dioxin‐like PCBs (WHO‐PCDD/F‐PCB) ranged 0.370–0.429 ng TEQ/kg.43
Microbiological contamination (analysed in three batches) showed that Salmonella spp., E. coli, coliforms, filamentous fungi and yeasts were not detected.44
Regarding the mycotoxin content, analytical data of the same batches showed levels of zearalenone and sum of fumonisins (B1 + B2 + B3) below the LOD.45 Aflatoxins ranged from 3 to 4 μg/kg, ochratoxin A from < LOD to 5 μg/kg, citrinin ranged from 23 to 39 μg/kg and DON from 163 to 224 μg/kg.
■■■■■■■■■■ ■■■■■
■■■■■
■■■■■■■■■■■■■■■
Considering the results of all the tests done, the Panel concludes that the data indicate that no viable cells of the production strain are present in the additive.
The presence of recombinant DNA in l‐lysine sulfate from the production strain was investigated in three batches, each analysed in triplicate. ■■■■■■■■■■■■■■■
3.1.4.2. Physical properties
l‐Lysine sulfate is an odourless light yellow or brown granular product, with a density of 0.6 kg/l at 25°C,48 a pH of 3.88 (10% solution in water at 20°C) and a solubility in water of 120 g/100 mL at 25°C.49
The dusting potential analysed the Stauber–Heubach method in three batches ranged from 0.3 to 0.5 g/m3.50 Concerning the particle size distribution, three batches were analysed by laser diffraction. No particles having a diameter < 100 μm were detected.51
3.1.4.3. Stability and homogeneity
The shelf life of the additive (three batches) was studied when stored in closed bags and protected from light, either at room temperature for 12 months or at 40°C for 6 months. No losses were observed.42
The stability of the additive (three batches) in a vitamin/mineral premixture containing choline (16,000 mg/kg) was studied when added at 100 g/kg and stored in sealed plastic bags at ambient temperature for 6 months. No losses were observed in one batch whereas the other two had losses of 15 and 27%.52
The stability of the additive (three batches) in feedingstuffs was tested in meal and pelleted compound feed for pigs for fattening (basal diet containing barley and soybean meal) when supplemented at 0.5% (corresponding to 0.27% lysine).53 The samples were kept at room temperature in individual bags for 3 months. Total (free plus protein‐bound) lysine was analysed. Losses ranged from 0 to 5% in the meal feed and from 2 to 5% in the pelleted feed, depending on the batch considered. The effect of feed processing (e.g. conditioning, pelleting) on the stability of the additive was not reported.
The stability of the additive (three batches) was studied in water at a concentration of about 0.26% when stored at room temperature for 24 h.42 No losses were detected. It is noted that the duration of the stability study is half that recommended in the guidance documents.
The capacity of l‐lysine sulfate (one batch) to distribute homogeneously in the pelleted feed mentioned above was studied in 10 subsamples. Total lysine was measured in each subsample and the background lysine concentration of the basal diet at the beginning of the study was subtracted from each subsample. The CV was 1.2% when total lysine was considered and 4.5% when the calculated concentration of supplemental lysine was considered.54
3.1.5. Physico‐chemical incompatibilities in feed
No physico‐chemical incompatibilities in feed are expected with other additives, medicinal products or other feed materials.
3.1.6. Conditions of use
According to the applicant, both forms of the additive are intended for all animal species and can be added directly in compound feed or be administered via complementary feed or premixtures.55 No proposed inclusion levels are provided, as the optimal daily allowance in quantitative terms depends on the species, the physiological state of the animal, the performance level and the environmental conditions, and in particular the amino acid composition of the unsupplemented diet.
The applicant states that both forms of the additive can be used in water for drinking but should not be simultaneously administered via water for drinking and feed. No inclusion levels were proposed by the applicant.
3.2. Safety
3.2.1. Safety aspects of the production organism
The recipient organism belongs to a species C. glutamicum considered by EFSA to be suitable for the qualified presumption of safety approach to safety assessment, when used for production purposes (EFSA, 2007a,b; EFSA BIOHAZ Panel, 2019). The production strain CGMCC 7.266 has been identified as C. glutamicum and contains no antibiotic resistance genes, therefore, it is considered to be safe. As compared to the recipient strain, the production strain overproduces lysine. The products l‐lysine monohydrochloride and l‐lysine sulfate produced by fermentation with C. glutamicum CGMCC 7.266 are considered to be safe with regard to the genetic modification of the production strain.
3.2.2. Safety of l‐lysine HCl and l‐lysine sulfate for the target species, consumer and the environment
l‐Lysine requirements of different non‐ruminant species and animal categories, absorption and metabolic fate of l‐lysine, tolerance to l‐lysine excess and the lysine to arginine antagonism have been described in detail in previous opinions. No safety concerns for ruminants would arise from ruminal lysine metabolism (EFSA FEEDAP Panel, 2013, 2014). The use of the amino acid ‘per se’ will not raise safety concerns for the target animals provided it is supplemented in appropriate amounts to satisfy the nutritional requirements of the animals in l‐lysine deficient diets. However, due to the risk of nutritional imbalances and hygienic reasons, associated to the use of amino acids via water for drinking (EFSA FEEDAP Panel, 2010), the FEEDAP Panel has concerns on the safety of the use of the amino acid via water for drinking.
There is a high inherent content of sulfate in l‐lysine sulfate which could be a safety concern for the target species, depending on the supplementation level and the tolerance of the target species. The FEEDAP Panel (2019) already concluded that the formulation of the complete feed should carefully take into account the maximum tolerable level of total sulfur (S), as established by NRC (2005) and set in ruminant diets at 3 g S/kg DM (diet rich in concentrate) and at 5 g S/kg DM (diet rich in roughage) and in non‐ruminant diets at 4 g S/kg DM. Also, the contribution of S/sulfate present in water for drinking to the total S intake should be considered, especially when the content is high. The studies, already published in the scientific literature and provided also by the applicant (Drewnoski et al. (2014) in feedlot cattle; Kerr et al. (2014) and Bobeck et al. (2013) in growing pigs; Kim et al. (2014) in growing/finishing pigs and Spears et al. (2011) in steers), confirm the statement by NRC (2005), as specified above.56 Consequently, no negative effects are to be expected at normal use levels for the target species provided that the total S intake complies with the recommendations of established scientific bodies.
Absorption, distribution, metabolism and excretion of l‐lysine were described in a previous scientific opinion of the FEEDAP Panel (2013). Potential concerns for consumers would arise from the fermentation process. The production strain is considered safe (see Section 3.2.1). The use of the amino acid l‐lysine itself in animal nutrition is considered safe for consumers.
The amino acid l‐lysine is a physiological and natural component of animals and plants. When supplemented to feed, it will be incorporated into proteins of tissues and/or products of animal origin and any potential excess will be catabolised and excreted as urea/uric acid and carbon dioxide. The use of l‐lysine in animal nutrition would not lead to any localised increase in the concentration of l‐lysine or its metabolites in the environment.
The FEEDAP Panel considers that l‐lysine HCl and l‐lysine sulfate produced by C. glutamicum CGMCC 7.266 are safe for the target species, consumer and the environment.
3.2.3. Safety of l‐lysine HCl and l‐lysine sulfate for the user
No specific studies to support the assessment of the safety for the user were submitted.
The dusting potential of l‐lysine HCl was analysed to be up to 10 g/m3 and the product has a significant fraction of particles having a diameter < 100 μm (up to 7%) (see Section 3.2.1), indicating that users can be likely exposed to dust from the additive.
The physical properties of l‐lysine sulfate showed that there were no particles having a diameter < 100 μm and the dusting potential ranged from 0.3 to 0.5 g/m3 (see Section 3.1.4.2). Exposure of users by inhalation is unlikely.
In the absence of data, the FEEDAP Panel cannot conclude on the potential of the l‐lysine HCl and l‐lysine sulfate produced by the strain C. glutamicum CGMCC 7.266 to be toxic by inhalation, irritant to skin or eyes, or on its potential to be a dermal sensitiser.
3.3. Efficacy
Efficacy studies are not required for amino acids naturally occurring in proteins of plants and animals. The nutritional role of the amino acid l‐lysine is well established in the scientific literature. The efficacy of l‐lysine for both non‐ruminant and ruminant species was described in two previous EFSA opinions (EFSA FEEDAP Panel, 2013, 2014). In general, the products l‐lysine HCl and l‐lysine sulfate are considered as efficacious sources of the essential amino acid l‐lysine for non‐ruminant animal species. For the supplemental l‐lysine to be as efficacious in ruminants as in non‐ruminant species, would require protection against degradation in the rumen.
3.4. Post‐marketing monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation57 and Good Manufacturing Practice.
4. Conclusions
The production strain C. glutamicum CGMCC 7.266 and its recombinant DNA were not detected in the final products. The products l‐lysine HCl and l‐lysine sulfate do not pose any safety concern associated with the production strain.
l‐Lysine HCl and l‐lysine sulfate produced by C. glutamicum CGMCC 7.266 are considered safe for the target species. When using l‐lysine sulfate, the background sulfur/sulfate content in the compound feed should be taken into account. l‐Lysine HCl and K‐lysine sulfate produced by C. glutamicum CGMCC 7.266 are safe for the consumer and for the environment.
In the absence of data, the FEEDAP Panel cannot conclude on the potential of the l‐lysine HCl and l‐lysine sulfate produced by the strain C. glutamicum CGMCC 7.266 to be toxic by inhalation, irritant to skin or eyes, or on their potential to be a dermal sensitiser.
l‐Lysine HCl and l‐lysine sulfate produced by C. glutamicum CGMCC 7.266 are considered as efficacious sources of the essential amino acid l‐lysine for non‐ruminant animal species. For the supplemental l‐lysine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
5. Recommendation
The specifications of both forms of the additive should reflect their batch‐to‐batch variation: l‐lysine HCl minimum 77.5% l‐lysine on a DM basis and maximum 1.7% loss on drying; l‐lysine sulfate minimum 54.5% l‐lysine on a DM basis and maximum 3.5% loss on drying.
Chronology
| Date | Event |
|---|---|
| 13/05/2018 | Dossier received by EFSA L‐lysine formulated as L‐lysine monohydrochloride and L‐lysine sufate. Submitted by Welding GmbH & Co. KG |
| 22/05/2018 | Reception mandate from the European Commission |
| 03/07/2018 | Application validated by EFSA – Start of the scientific assessment |
| 06/08/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation of the production strain, conditions of use, characterisation of both forms of the additive, safety for the target species and safety for the user. |
| 03/10/2018 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 03/10/2018 | Comments received from Member States |
| 08/04/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 21/06/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the production strain and characterisation of both forms of the additive. |
| 25/10/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 28/01/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AFC
EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food
- CAS
Chemical Abstracts Service
- CCTCC
China Center for Type Culture collection
- CFU
colony forming unit
- CG
chemical group
- CV
coefficient of variation
- DM
dry matter
- DON
deoxynivalenol
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EURL
European Union Reference Laboratory
- FCC
Food Chemical Codex
- FEEDAP
EFSA Scientific Panel on additives and products or substances used in animal feed
- IEC‐VIS/FLD
ion exchange chromatography coupled to visible or fluorescence detection
- IUPAC
International Union of Pure and Applied Chemistry
- LOD
limit of detection
- MIC
minimum inhibitory concentration
- PCB
polychlorinated biphenyl
- PCDD/F
polychlorinated dibenzo‐p‐dioxin/dibenzofuran
- QPS
qualified presumption of safety
- RSDr
relative standard deviation for repeatability
- RSDR
relative standard deviation for reproducibility
- TEQ
Toxic equivalents
- WHO
World Health Organization
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for l‐lysine monohydrochloride and l‐lysine sulfate from Corynebacterium glutamicum CGMCC 7.266
1.
In the current application authorisation is sought under Article 4(1) for l‐lysine monohydrochloride and l‐lysine sulfate produced by Corynebacterium glutamicum CGMCC 7.266, under the category/functional group 3(c) ‘nutritional additives’/’amino acids, their salts and analogues’, according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for all animal species.
According to the Applicant l‐lysine monohydrochloride has a minimum purity (mass fraction) of 98.5% (minimum of 78% of l‐lysine) while l‐lysine sulfate contains a minimum of 55% of l‐lysine.
For the quantification of lysine in the feed additive the Applicant submitted the ring‐trial validated method EN ISO 17180:2013 based on ion exchange chromatography coupled to visible or fluorescence detection (IEC‐VIS/FLD). This standard method does not distinguish between the salts of amino acids and it cannot differentiate between enantiomers. It applies for products containing more than 10% of amino acid. The following performance characteristics are reported: a relative standard deviation for repeatability (RSDr) ranging from 0.7 to 1.7% and a relative standard deviation for reproducibility (RSDR) ranging from 1.5 to 2.5%. In addition, the EURL identified the “l‐lysine monohydrochloride monograph” of the Food Chemical Codex (FCC) for the identification of l‐lysine monohydrochloride in the feed additive and the generic European Pharmacopoeia monograph on sulfates (Ph. Eur. 20301) for the identification of sulfate in l‐lysine sulfate.
For the quantification of l‐lysine in premixtures, feedingstuffs and water the Applicant submitted the ring‐trial validated Community method (Commission Regulation (EC) No 152/2009) based on IEC coupled with photometric detection (VIS). This method, designed only for the analysis of amino acids in premixtures and feedingstuffs, does not distinguish between the salts and the amino acid enantiomers. The following performance characteristics were reported for the quantification of total lysine: RSDr ranging from 2.1 to 2.8% and RSDR ranging from 3.0 to 6.7%. In the frame of the stability studies the Applicant presented experimental data obtained analysing lysine in water with the Community method and demonstrated its applicability for the determination of lysine in water.
In the frame of this authorisation the EURL recommends for official control (i) the “l‐lysine monohydrochloride monograph” of the Food Chemical Codex (FCC) based on infrared absorption for the identification of l‐lysine monohydrochloride in the feed additive; (ii) the European Pharmacopoeia monograph (Ph. Eur. 01/2008:20301) for the identification of the sulfate ion in l‐lysine sulfate; (iii) the ring‐trial validated method EN ISO 17180:2013 based on ion exchange chromatography coupled to visible or fluorescence detection (IEC‐VIS/FLD) to quantify free lysine in the feed additive and premixtures (containing more than 10% lysine); (iv) the ring‐trial validated Community method based on IEC‐VIS for the quantification of lysine in premixtures, feedingstuffs and water.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Cocconcelli PS, Dierick N, Glandorf B, Herman L, Mantovani A, Prieto Maradona M, Saarela M, Wallace RJ, Anguita M, Pettenati E, Tarrés‐Call J and Ramos F, 2020. Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride and l‐lysine sulfate produced using Corynebacterium glutamicum CGMCC 7.266 for all animal species. EFSA Journal 2020;18(2):6019, 17 pp. 10.2903/j.efsa.2020.6019
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00427
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria Bastos, Henrik Christensen, Birgit Dusemund, Mojca Kos Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: Rosella Brozzi, Jaume Galobart, Yolanda García Cazorla, Matteo Lorenzo Innocenti and Niovi Kordali.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Adopted: 28 January 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Welding GmbH & Co. KG. Esplanase 39, 20354 Hamburg, Germany.
Commission Directive 88/485/EEC of 26 July 1988 amending the Annex to Council Directive 82/471/EEC concerning certain products used in animal nutrition. OJ L 239, 30.8.88, pp. 36–39.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009, OJ L 181, 29.6.2013, p.35.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, pp. 1–528.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OL L 152, 16.6.2009, p. 11.
FEED dossier reference: FAD‐2018‐0019.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep_fad-2018-0019_l-lysinehcl_sulphate.pdf
Technical dossier/Section II/Annex 2.2.1.2a.
■■■■■
■■■■■
Technical dossier/Section II/Annex 2.2.1.2c and supplementary information April 2019/Annex 4.
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
Technical dossier/Section 2.1.3.
Technical dossier/Section II/Annex 2.1.3a. Lysine analysed using VDLUFA method.
Technical dossier/Section II/Annex 2.1.3a and supplementary information April 2019/Annex 5.
Technical dossier/Supplementary information April 2019/Annexes 6a to 6c.
LOD in mg/kg was 0.002 for cadmium and 0.01 for lead.
Technical dossier/Section II/Annex 2.1.3a.
Technical dossier/Supplementary information April 2019/Annexes 8a to 8c.
Technical dossier/Section II/Annex 2.1.3a and supplementary information April 2019/Annex 7.
LOD in µg/kg was 5 for ochratoxin A, 17 for zearalenone, 25 for the sum of fumonisins B1 + B2 + B3 and 134 for DON.
■■■■■
■■■■■
■■■■■
■■■■■
Technical dossier/Section 2.1.5.
Technical dossier/Section II/Annex 2.5.2a.
Technical dossier/Section II/Annexes 2.1.5 g to 2.1.5i.
Technical dossier/Section II/Annexes 2.1.5.d2 to 2.5.1.f2.
Technical dossier/Section II/Annexes 2.4.1a and 2.4.1b; supplementary information Sin April 2019/FAD‐2018‐0019 SIn 060818 Answers 190329.
Technical dossier/Section II/Annex 2.4.1d and 2.4.1b.
Technical dossier/Section II/Annex 2.4.1b.
Technical dossier/Section II/Annex 2.1.3b and supplementary information April 2019/FAD‐2018‐0019 SIn 060818 Answers 190329. Lysine analysed by the VDLUFA method.
Technical dossier/Section II/Annex 2.1.3e.
Technical dossier/Section II/Annex 2.1.3d and supplementary information April 2019/Annexes 10a and 10b.
LOD of cadmium was 0.002 mg/kg.
Technical dossier/Section II/Annex 2.1.3b.
Technical dossier/Section II/Annexes 2.1.4a to c.
Technical dossier/Section II/Annex 2.1.3b and supplementary information April 2019/Annex 11.
Technical dossier/Section II/Annex 2.1.3b. LOD in µg/kg was 5 for ochratoxin A, 17 for zearalenone and 25 for sum of fumonisines B1, B2 and B3.
Technical dossier/Supplementary information April 2019/Annex 12.
■■■■■
■■■■■
Technical dossier/Section II/2.1.5.
Technical dossier/Section II/Annex 2.5.2b and supplementary information September 2019/Annex answers 190911.
Technical dossier/Section II/Annexes 2.1.5j to l.
Technical dossier/Section II/Annexes 2.1.5.a2 to c2.
Technical dossier/Section II/Annexes 2.4.1c and 2.4.1a; supplementary information April 2019/ FAD‐2018‐0019 SIn 060818 Answers 190329.
Technical dossier/Section II/Annex 2.4.1c and 2.4.1d; supplementary information April 2019/ FAD‐2018‐0019 SIn 060818 Answers 190329.
Technical dossier/Section II/Annex 2.4.1c.
Technical dossier/Section 2.5.1 and supplementary information April 2019/FAD‐2018‐0019 SIn 060818 Answers 190329.
Technical dossier/Supplementary information April 2019/Annex 14 Literature review.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Bobeck EA, Payne RL, Kerr BJ and Persia ME, 2013. Supplemental lysine sulfate does not negatively affect the performance of broiler chicks fed dietary sulfur from multiple dietary and water sources. Journal of Applied Poultry Research, 22, 461–468. [Google Scholar]
- Drewnoski ME, Doane P and Hansen LS, 2014. Ferric citrate decreases ruminal hydrogen sulphide concentrations in feedlot cattle fed diets high in sulfate. British Journal of Nutrition, 111, 261–269. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2007a. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) on a request from the European Commission on the safety and efficacy of l‐lysine sulfate (Vitalys® Liquid and Vitalys® Dry) for all animal species. EFSA Journal 2007;5(9), 522, 26 pp. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2007.522 [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(12), 587, 16 pp. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2007.587 [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernandez Escamez PS, Maradona MP, Querol A, Suarez JE, Sundh I, Vlak J, Barizzone F, Correia S and Herman L, 2019. Statement on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 9: suitability of taxonomic units notified to EFSA until September 2019. EFSA Journal 2019;17(1):5555, 46 pp. 10.2903/j.efsa.2019.5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances Used in Animal Feed), 2010. Scientific Opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2013. Scientific Opinion on the safety and efficacy of concentrated liquid l‐lysine (base), concentrated liquid l‐lysine monohydrochloride and l‐lysine monohydrochloride produced by Escherichia coli (FERM BP‐10941) for all animal species, based on three dossiers submitted by Ajinomoto Eurolysine SAS. EFSA Journal 2013;11(10):3365, 22 p. 10.2903/j.efsa.2013.3365 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of concentrated liquid l‐lysine (base), concentrated liquid l‐lysine monohydrochloride and l‐lysine monohydrochloride technically pure produced using Escherichia coli (FERM BP‐11355) for all animal species based on a dossier submitted by Ajinomoto Eurolysine S.A.S. EFSA Journal 2014;12(11):3895, 19 pp. 10.2903/j.efsa.2014.3895 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015a. Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride produced by fermentation with Escherichia coli for all animal species based on a dossier submitted by HELM AG on behalf of Meihua Holdings Group Co. Ltd. EFSA Journal 2015;13(3):4052, 16 pp. 10.2903/j.efsa.2015.4052 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015b. Scientific Opinion on the safety and efficacy of l‐lysine sulfate produced by fermentation with Escherichia coli CGMCC 3705 for all animal species. EFSA Journal 2015;13(7):4155, 22 pp. 10.2903/j.efsa.2015.4155 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015c. Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride, technically pure, produced with Escherichia coli CGMCC 3705 and l‐lysine sulphate produced with Corynebacterium glutamicum CGMCC 3704 for all animal species, based on a dossier submitted by HELM AG. EFSA Journal 2015;13(7):4156, 25 pp. 10.2903/j.efsa.2015.4156 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016a. Scientific opinion on the safety and efficacy of concentrated liquid l‐lysine (base), l‐lysine monohydrochloride and l‐lysine sulfate produced using different strains of Corynebacterium glutamicum for all animal species based on a dossier submitted by AMAC/EEIG. EFSA Journal 2016;14(3):4346, 3 pp. 10.2903/j.efsa.2016.4346 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016b. Scientific opinion on the safety of l‐lysine monohydrochloride produced by fermentation with Escherichia coli CGMCC 7.57 for all animal species based on a dossier submitted by Feedway Europe NV. EFSA Journal 2016;14(5):4471, 9 pp. 10.2903/j.efsa.2016.4471 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wester P, Costa L, Dierick N, Leng L and Wallace RJ, 2017a. Scientificopinion on the safety of l‐lysine sulfate produced by fermentation with Escherichia coli CGMCC 3705 for all animal species. EFSA Journal 2017;15(2):4714, 7 pp. 10.2903/j.efsa.2017.4714 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in animal feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017b. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in animal feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017c. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017d. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Karenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Dierick N, Flachowsky G, Glandorf B, Herman L, Karenlampi S, Leng L, Mantovani A, Wallace RJ, Aguilera J, Tarres‐Call J and Ramos F, 2019a. Scientific Opinion on the safety of concentrated l‐lysine (base), l‐lysine monohydrochloride and l‐lysine sulfate produced using different strains of Corynebacterium glutamicum for all animal species based on a dossier submitted by FEFANA asbl. EFSA Journal 2019;17(1):5532, 24 pp. 10.2903/j.efsa.2019.5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Costa L, Dierick N, Flachowsky G, Glandorf B, Herman L, Karenlampi S, Mantovani A, Aguilera J, Anguita M, Tarres‐Call J and Wallace RJ, 2019b. Scientific opinion on the safety and efficacy of l‐lysine monohydrochloride and concentrated liquid l‐lysine (base) produced by fermentation using Corynebacterium glutamicum strain NRRL B‐50775 for all animal species based on a dossier submitted by ADM. EFSA Journal 2019;17(1):5537, 18 pp. 10.2903/j.efsa.2019.5537 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Dierick N, Flachowsky G, Mantovani A, Wallace RJ, Tarres‐Call J and Ramos F, 2019c. Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride and l‐lysine sulfate produced using Corynebacterium glutamicum CCTCC M 2015595 for all animal species. EFSA Journal 2019;17(3):5643,19 pp. 10.2903/j.efsa.2019.5643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Flachowsky G, Mantovani A, Wallace RJ, Tarres‐Call J and Ramos F, 2019d. Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride and concentrated liquid l‐lysine(base) produced by fermentation using Corynebacterium glutamicum strain KCCM 10227 for all animal species. EFSA Journal 2019;17(5):5697, 15 pp. 10.2903/j.efsa.2019.5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Flachowsky G, Glandorf B, Herman L, Mantovani A,Anguita M, Tarres‐Call J and Ramos F, 2019e. Scientific Opinion on the safety and efficacy of l‐lysine monohydrochloride and concentrated liquid l‐lysine (base) produced by fermentation using Corynebacterium glutamicum strains NRRL‐B‐67439 or NRRL B‐67535 for all animal species. EFSA Journal 2019;17(11):5886, 22 pp. 10.2903/j.efsa.2019.5886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos M, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, de Knecht J, Kolar B, van Beelen P, Padovani L, Tarres‐Call J, Vettori MV and Azimonti G, 2019f. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Pharmacopeia (PhEur) , 2017. Lysine hydrochloride, Monograph 01/2008:0930, 9th Edition. Strasbourg, France. Council of Europe (COE) – European Directorate for the Quality of Medicines.
- Kerr BJ, Weber TE, Ziemer CJ, Spence C, Cotta MA and Whitehead TR, 2014. Effect of dietary inorganic sulfur level on growth performance, fecal composition, and measures of inflammation and sulfate‐reducing bacteria in the intestine of growing pigs. Journal of Animal Science, 89, 426–437. [DOI] [PubMed] [Google Scholar]
- Kim BG, Kil DY, Mahan DC, Hill GM and Stein HH, 2014. Effects of dietary sulfur and distillers dried grains with solubles on carcass characteristics, loin quality and tissue concentrations of sulfur, selenium, and copper in growing‐finishing pigs. Journal of Animal Science, 92, 4486–4493. [DOI] [PubMed] [Google Scholar]
- NRC , 2005. Sulfur In: Mineral tolerance of animals. 7th rev. Edition, National Academy Press, Washington. [Google Scholar]
- Spears JW, Lloyd KE and Fry RS, 2011. Tolerance of cattle to increased dietary sulfur and effect of dietary cation‐anion balance. Journal of Animal Science, 89, 2502–2509. [DOI] [PubMed] [Google Scholar]


