Abstract
Introduction
We investigated the hypothesis that retinal capillary perfusion is a biomarker of early cognitive decline and cerebrovascular perfusion associated with small vessel disease in a pilot data set of Latinx adults at high risk for vascular cognitive impairment and dementia.
Methods
High‐resolution optical coherence tomography angiography (OCTA) images were acquired from dilated eyes of Latinx subjects using a 3 × 3 mm2 scan pattern from a commercially available device. A previously validated method was used to quantify the density of perfused retinal capillaries as the retinal vessel skeleton density (VSD). The association of VSD with Clinical Dementia Rating Sum of Boxes, total Montreal Cognitive Assessment (MoCA) score, and individual MoCA test elements were analyzed using multivariate statistics that adjusted for confounders. VSD was also compared with magnetic resonance imaging (MRI) measures of cerebrovascular reactivity (CVR) and perfusion in the middle cerebral artery perforator (MCA‐Perf) territory.
Results
The mean (± SD) age of the subjects was 68 (± 6) years. For every 0.01‐unit lower VSD, the risk of having a CDR‐SOB >0 was 20% higher (95%CI = 5%–90%; P = .031). Similarly, a lower VSD was associated with lower total MoCA score (r = 0.3; P = .038). The Visuospatial/Executive domain of the MoCA assessment showed the strongest association with VSD (β = 0.02; P = .022). Lower retinal VSD was associated with worse MRI measure of CVR (r = 0.7, P = .04) and less perfusion in the MCA‐Perf territory (r = 0.45, P = .02).
Discussion
Impaired retinal capillary perfusion is associated with cognitive impairment and abnormalities in cerebrovascular perfusion and function. OCTA‐based retinal capillary assessment holds promise for identifying and quantifying retinal correlates of neurovascular abnormalities associated with vascular cognitive impairment.
Keywords: capillary, clinical dementia rating (CDR), cerebrovascular reactivity, Fazekas scale, Montreal Cognitive Assessment (MoCA), optical coherence tomography angiography (OCTA), retina, vascular cognitive impairment and dementia (VCID), white matter hyperintensity
1. BACKGROUND
It is estimated that by 2025 there will be 1.2 billion people >60 years of age, with the prevalence of dementia estimated to be >30% for those >80 years of age. 1 Vascular contributions to cognitive impairment and dementia are common, especially later in life, and represent a significant unmet medical need in terms of accurate diagnosis and treatment. Epidemiologic studies demonstrate that >50% of subjects with Alzheimer's disease (AD) pathology have significant comorbid vascular pathology. 2 , 3 , 4 , 5 In 2011 and 2013, consensus statements from several leading groups highlighted the importance of vascular contributions to cognitive impairment and dementia (VCID). 1 , 6 These consensus statements called for development of novel, clinically feasible biomarkers of vascular cognitive impairment and dementia.
The retinal vasculature provides one potential biomarker for VCID because it shares many similarities with central nervous system (CNS)tissue and is optically accessible by high‐resolution imaging that is not feasible in the brain. Clinical studies have shown that retinal arteriolar narrowing, arteriosclerosis, and exudates correlate with cerebral white matter lesions (WMLs). 7 , 8 , 9 Epidemiologic studies showed that clinically detectable retinopathy such as microaneurysms, retinal hemorrhages, and exudates are most strongly associated with WMLs, cerebral atrophy, 10 and declines in executive and psychomotor function. 10 , 11 , 12 Therefore, macroscopic retinal vascular changes (≈100 μm) correlate with both cognitive decline and magnetic resonance imaging (MRI) findings of small vessel disease (SVD). These macroscopic findings are essentially end‐stage retinal capillary pathology and are very likely preceded by subclinical changes that should be present in the retinal capillaries. This hypothesis is supported by high‐resolution imaging methods that have demonstrated subclinical changes in capillary density and morphology preceding clinically detectable retinopathy in several diseases such as hypertension and diabetes. 13 , 14 , 15 , 16
Optical coherence tomography angiography (OCTA) is an U.S. Food and Drug Administration (FDA)–approved, low‐cost, and noninvasive method of assessing retinal capillary perfusion and morphology. 13 Although several studies have demonstrated changes in retinal capillary perfusion or reactivity in subjects with clinical AD, 17 , 18 , 19 there is a paucity of evidence demonstrating the changes associated with VCID. 5 In this study, we hypothesize that retinal capillary perfusion can serve as a potential biomarker of early cerebral SVD and VCID, and that there is a significant association between cognitive and structural markers of SVD and retinal capillary perfusion. We test this hypothesis in a pilot data set of a prospective community‐based study of Latinx subjects 20 who are at high risk for VCID. Cerebrovascular disease sufficient to contribute to dementia has been demonstrated in more than half of demented subjects with Hispanic origin, compared to Blacks and non‐Hispanic Whites with 40% and 28% prevalence, respectively. 21 Because the Latinx population is the fastest‐growing group of older adults in the United States and have a high prevalence of cardiovascular disease associated with higher risks of developing vascular dementia and AD, 22 , 23 , 24 it is an ideal and understudied population in which retinal manifestations of VCID may be particularly informative.
2. METHODS
The methods used by the study adhered to the tenets of the Declaration of Helsinki, and the study protocol has been approved by the University of Southern California Institutional Review Board. Written informed consent was obtained from all participants before enrollment.
2.1. Subjects
Study participants were prospectively recruited from subjects who had participated in the Los Angeles Latino Eye Study (LALES), a longitudinal population‐based cohort study of eye health initiated in 2000. 20 LALES participants who were older than 60 years of age were re‐approached for enrollment in the current study. Latinx were defined as individuals who were born into or have descended from a Spanish‐speaking community, regardless of race. 20 To be recruited, the subject must have the capacity to sign consent or give assent and have an appropriate surrogate (as determined by California law) to sign consent. Subjects with a history of major psychiatric illness (eg, schizophrenia, bipolar disorder) were excluded.
The study participants received a screening eye exam by a board‐certified ophthalmologist and fellowship‐trained retina specialist (AHK) and a neurocognitive evaluation by a board‐certified neurologist and a neuropsychologist (JR and LD). The eye exam consisted of comprehensive clinical assessments including ocular and medical history, visual acuity, autorefraction, pupillary assessment, tonometry, slit‐lamp, and dilated fundus examination to identify ocular morbidities that confound OCTA retinal microvascular assessment. Pupillary dilation for fundus exams and OCTA assessment was achieved using one drop each of proparacaine (0.5%), tropicamide (1%), and phenylephrine hydrochloride (2.5%) eye drops.
This current analysis was based on data collected between 04/12/2017 and 12/1/2019 from participants who had OCTA imaging and underwent assessment using the Clinical Dementia Rating (CDR) scale 25 and/or Montreal Cognitive Assessment (MoCA). 26 Eyes with history of co‐morbid retinal pathology including glaucoma, pathologic myopia, age‐related macular degeneration, or macular edema, or with history of retinal or laser surgery procedures were excluded. Pathologic myopia was defined conservatively as myopic refraction >6D or with clinical signs of myopic degeneration (eg, posterior staphyloma, peripapillary atrophy, tilted‐optic nerve head insertion, atrophy of the retinal pigment epithelium) or both. Individuals with significant media opacities including cataract or asteroid hyalosis were also excluded. In all, 67 subjects met the inclusion/exclusion criteria. Another six of these were excluded due to poor OCTA quality (from motion and shadow artifacts) in both eyes. The mean (± SD) age of the 61 subjects analyzed was 68 (± 6) years, and the number of years of formal education ranged between 0 and 17 years (Table 1).
TABLE 1.
The table shows the demographic, medical, and clinical status of the subjects
| Subset of subjects with clinical status defined (N = 50) | |||
|---|---|---|---|
| All subjects (N = 61) | Cognitively normal (N = 35) | Cognitively abnormal (N = 15) | |
| Age, years ‐ mean (SD) | 68 (6) | 68.35 (7) | 68.46 (6) |
| Refractive error ‐ median (range) | +1.00 (−1.25 ± 4.50) | +1.00 (−1.00 ± 4.50) | +1.00 (−1.00 ± 4.50) |
| Male gender | 21% | 14 % | 40 % |
| Years of education ‐ mean (SD) | 8 (4) | 9 (5) | 6 (3)* |
| MoCA score ‐ mean (SD) | 22 (4) | 23 (4) | 20 (4)* |
| % with diabetes | 33% | 31% | 33% |
| % with hypertension | 58% | 55% | 80% |
| % with hyperlipidemia | 58% | 59% | 73% |
| Suspected etiology | NA | NA |
7 (47%) with AD 2 (13%) with VCID 6 (40%) with other causes |
| VSD ‐ mean (SD) | 0.145 (0.006) | 0.15 (0.006) | 0.147 (0.006) |
| OCTA signal strength ‐ median range | 9 (8–10) | 9 (8–10) | 9 (8–10) |
The refractive error measures reported were computed from equivalent sphere. Clinical status was defined by a neurologist and neuropsychologist after a comprehensive review of the subject's medical and clinical history. Eleven subjects did not make the follow‐up visit at which the cognitive status of the subjects was clinically assessed.
Indicates the comparisons between the cognitively normal and cognitively abnormal group which are significant at P = .05. Other etiologies include learning disorder, depression, and anxiety.
Abbreviations: AD, Alzheimer's disease; NA, not applicable; VCID, vascular contribution to cognitive impairment and dementia.
2.2. Retinal vascular assessment
The retinal microvasculature was assessed using an 840 nm light source Spectral Domain OCTA ‐ Cirrus HD‐OCTA (Carl Zeiss Meditec, Inc., Dublin, California, USA). The 3×3 mm2 foveal centered scan pattern had an estimated acquisition time of 5 seconds but can vary based on several variables including a subject's ability to fixate. 13 , 27 Each B‐scan consists of 245 A‐scans with an inter A‐scan distance of ~12 μm. Each eye received at least two scans to enhance the chances of obtaining images with minimal artifacts for analysis. The commercially reported axial and transverse resolution of the scan pattern is 5 and 15 μm, respectively. The commercially available segmentation software was used to generate a superficial retinal layer vascular slab (comprising the retinal nerve fiber layer, ganglion cell layer, and inner plexiform layer) for all analyses.
Images were acquired in each eye after pharmacologic dilation and were graded for image quality. Only images with signal strength greater than 7/10 were considered. When images from both eyes met the inclusion criteria, the best image (fewest artifacts) was selected as the representative image for the participant. The rater was blinded to the cognitive and cerebrovascular data when making the selection. A previously validated semiautomated software written in MATLAB (R2018b; MathWorks, Inc., Natick, Massachusetts, USA) was used to determine retinal capillary perfusion and termed retinal vessel skeleton density (VSD). In brief, the OCTA image acquired was converted into a binarized image using a combination of methods consisting of a global threshold, hessian filter, and adaptive threshold. The binarized images were then skeletonized. VSD estimated the length of perfused vessels from the binarized and skeletonized angiographic images and was computed as the ratio of skeletonized vessel length to the total area of the image 14 , 16 , 28 (Figure 1).
FIGURE 1.

Optical coherence tomography angiography (OCTA) images and quantification. The figure shows two 3 × 3 mm 2 parafoveal OCTA images (signal strength 10/10) of two subjects with CDR‐SOB scores = 0 and CDR‐SOB = 1, and ages 76 and 73 years, respectively. Neither subject has diabetes but both have hypertension. Images on the second and third columns show the corresponding skeletonized vessel images and pseudo‐colored maps of the vessel density. The subject with CDR‐SOB = 0 has more localized areas of higher retinal vessel density than the age and medical condition similar subject with CDR‐SOB >0
HIGHLIGHTS
Retinal capillary perfusion is readily assessed using optical coherence tomography angiography
Retinal capillary perfusion is altered in subjects with mild or preclinical cognitive impairment
Retinal capillary perfusion correlates with MRI measures of cerebrovascular reactivity and perfusion
RESEARCH IN CONTEXT
Systematic review: On June 16, 2020, a Web of Science search for articles that applied optical coherence tomography angiography (OCTA) imaging in dementia found 13 articles. Six of these are related to our study and have been cited.
Interpretation: Consistent with previous findings, we show an association between lower retinal capillary perfusion and mild cognitive impairment in a cohort of subjects at high risk for vascular cognitive impairment and dementia (VCID). We demonstrate a significant, positive, and cross‐sectional association of retinal capillary perfusion with cognitive assessments, and abnormal cerebrovascular perfusion (cerebrovascular reactivity and middle cerebral artery‐perforator perfusion). These findings support the notion that retinal vascular changes are indicators of cerebrovascular structure and function and are potentially useful for assessment of CNS changes in subjects at high risk for VCID.
Future directions: Future work will investigate longitudinal changes in retinal capillary perfusion associated with aging, declining cognitive status, and impaired cerebrovascular health.
2.3. Clinical assessment
Clinical status was assessed using the CDR Sum of Boxes (CDR‐SOB) and MoCA scores. The CDR was performed by a board‐certified neurologist trained in its administration and is a structured interview of the subject and informant in which subjects are rated: 0 (asymptomatic), 0.5 (equivocal impairment), 1 (mild), 2 (moderate), or 3 (severe dementia) in the domains of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. 25 CDR‐SOB scores range from 0 to 18 at 0.5 interval gradings and offer a wider dynamic range for staging the severity of the clinical dysfunction due to cognitive decline, especially in the milder stages of disease. 29 The MoCA was performed by a licensed neuropsychologist and is a brief screening test in which scores range from 0 to 30. 26 As was determined by the comfort level of the subject, testing was administered in English or Spanish. Eight (≈13%) cognitive assessments were done in English and 53 (≈87%) were in Spanish.
Clinical status was defined according to the standard guidelines stipulated in the 3rd version of the Uniform Data Set used in the National Alzheimer's Coordinating Center (NACC). 29 The clinical status of participants was based on a comprehensive review of neuropsychological testing results, taking into account demographic and medical data available to the clinical team. In the NACC database, subjects are categorized as being cognitively normal or abnormal. If abnormal, the degree of cognitive impairment (eg, subtype of mild cognitive impairment, dementia, and so on) and suspected etiologies were identified.
2.4. MRI assessment of brain structure and cerebral hemodynamic status
MRI was performed on a 3T Siemens Prisma scanner with a 20‐channel head‐coil including a T1‐weighted multi‐echo Magnetization Prepared ‐ RApid Gradient Echo (MPRAGE) scan (resolution = 1 × 1 × 1 mm3, inversion time/TR = 1100/2530 ms, echo time (TE) 1/2/3/4 = 1.69/3.55/5.41/7.27 ms), a T2‐weighted fluid‐attenuated inversion recovery (FLAIR) scan (resolution = 1 × 1 × 1 mm3, inversion time/TE/TR = 1800/388/5000 ms), a T2*‐weighted multi‐echo gradient‐echo scan (resolution = 1.2 × 1.2 × 1.2 mm3, TR = 24 ms, TE1/2/3/4/5/6/7/8 = 2.98/5.51/8.04/10.57/13.10/15.63/18.16/20.69 ms), a cerebrovascular reactivity (CVR) with 5% CO2 and two‐dimensional (2D) echo planar (EPI) scan (resolution = 3.4 × 3.4 × 3.8 mm3, TE/TR = 21/1500 ms, 7 min 17 sec), 30 and a 3D g radient and spin echo (GRASE) pseudo‐continuous arterial spin labeled (pCASL) scan (2.5 mm 3 isotropic resolution, 48 slices, TR/TE 4300 ms/36.76 ms, label time 1500 ms, post label delay 2000 ms) 31 . The MRI data were processed with standard methods to obtain an assessment of white matter hyperintensity, cerebral perfusion, and CVR. 30 , 32 , 33 , 34 , 35 , 36
2.5. Statistical analysis
We investigated the association between VSD and clinical measures of cognitive function. Due to the skewed distribution of CDR‐SOB scores (shown in the results) among normal and mildly impaired individuals, the CDR‐SOB scores were dichotomized based on definitions proposed by the Texas Alzheimer's Research Consortium study 37 —CDR‐SOB of 0 representing normal clinical function and CDR‐SOB >0 representing questionable impairment or worse. An analysis of covariance (ANCOVA) model that adjusted for the effects of covariates (years of education, age, gender, and testing language) was used to investigate the difference in the mean VSD between the subjects with CDR‐SOB scores = 0 and CDR‐SOB >0. The underlying assumptions of the test statistics were assessed using Schapiro‐Wilk test and Levene test for assessing normality and equality of error variance, respectively. We also computed the proportion of subjects with CDR‐SOB score >0 for each 0.01‐unit change of VSD, and the accompanying odds ratio using a logistic regression model that accounted for the same covariates.
The relationship between VSD and total MoCA score was assessed using multivariate linear regression models that adjusted for covariates (age, gender, and testing language) and reported the partial correlation associated with the VSD metrics. The effect of years of education on total MoCA scores was compensated using an adjustment model developed by Chertkow et al 38 and refined by Zhou et al. 39 Specifically, 1, 2, and 3 points were added to the raw MoCA score when the years of education was 10–12, 4–9, and <4, respectively. Assumptions for multiple linear regression models including normality of data and homoscedasticity were assessed. Following this analysis, we investigated the contribution of retinal VSD for assessing VCID. The VSD comparison with total MoCA score was repeated for the subset of subjects with cardiovascular risk factors (diabetes, hypertension, and hypercholesterolemia). In addition, we explored the association between VSD and MoCA scores for individual cognitive domains as defined by the MoCA subtask assessment. 40 , 41 When there was more than one subtask in a domain, the total score of the subtasks were computed; example: trails (scored over 1), copy cube (scored over 1), and clock drawing (scored over 3) were combined as executive/visuospatial domains. 42 In this subtask analysis, however, years of education were accounted for as a covariate because the previously applied model for adjusting education (from Zhou et al) was only applicable to total MoCA score. We report on the beta coefficients and P‐values of the trend analysis.
In a subset of the subjects with available MRI data, we explored the association between VSD and measures of brain perfusion and cerebrovascular hemodynamics. Specifically, we assessed the relation between the VSD and perfusion in the middle cerebral artery perforator territory (MCA‐Perf [N = 32]) by pCASL using a multiple regression model and report the partial correlation. 30 , 33 , 34 , 35 , 36 Reduced cerebral blood flow in small perforating arteries has been commonly affected in arteriolosclerosis, a prevalent form of cerebral SVD. 43 The analyses adjusted for the effects of age and gender and global cerebral blood flow. Assumptions of normality and homoscedasticity were met. The association between VSD and CVR (N = 11) as well as Fazekas scale grading of WML (N = 32) were also assessed using multiple regression models for simplicity, adjusting for age and gender. Statistical significances were defined at an alpha level of 0.05 (two tailed) and were performed using SPSS version 25 (IBM SPSS Statistics for Windows, Version 25.0. Armonk, New York, USA)
3. RESULTS
Table 1 shows the distribution of demographic, medical history, and neurologic exam findings of the study participants. Cerebrovascular risk factors appeared to be more common in the cognitively abnormal group. However, the differences did not reach significance. As expected, the MoCA scores showed worse cognitive abilities in the cognitively abnormal group.
Although subtle, qualitative comparison of OCTA images of participants matched for age and medical conditions show that subjects with CDR‐SOB scores = 0 generally have a higher vessel density than subjects with CDR‐SOB >0. This difference is better observed on the quantitative pseudo‐colored VSD maps (Figure 1). ANCOVA found significant difference in the mean VSD of subjects with CDR‐SOB = 0 and CDR‐SOB >0 with moderate effect size (F[1,43] = 5.23, P = .03; partial eta 2 = 0.11). The proportion of subjects with CDR‐SOB >0 was 20% higher (95% confidence interval [CI] 5%–90%; P = .03) for every 0.01 lower VSD score (Figure 2). Similar trends were found when the VSD was categorized based on tertiles. These associations were present and significant even when the presence of cardiovascular risk factors (diabetes, hypertension, and cholesterol) was co‐varied.
FIGURE 2.

Association between retinal vessel skeleton density (VSD) and cognitive dementia rating sum of box score (CDR‐SOB). (A) Plot of VSD against crude CDR‐SOB scores. Small amounts of random vertical noise up to 0.05 units were added to the vertical axis to reduce the extent of overlap. (B) Box (25th–75th percentile) and whisker (5th–95th percentile) distribution of VSD versus CDR‐SOB after binarization. (C) Distribution of the proportion of subjects with CDR‐SOB >0 based on 0.01‐unit binning of VSD. (D) The odds ratio of having CDR‐SOB > 0 based on a tertile binning of VSD
MoCA scores were lower among individuals with lower VSD (r = 0.37; P = .008). This association was more pronounced among subjects with cardiovascular disease (r = 0.43, P = .008), and the significance of the association was maintained after adjusting for the effects of covariates (Figure 3). The association between VSD and individual domains of MoCA varied. A lower VSD was associated with lower visuospatial/executive domain scores (Figure 4; β = 0.02, P = .022), but was not associated with the other domains (naming [β = 0.00, P = .9], attention [β = ‐0.00, P = .7], language [β = 0.001, P = .5], abstraction [β = 0.002, P = .14], delayed recall [β = 0.001, P = .12], or orientation [β = 0.00, P = .90]). Similar trends were found when specific subtasks representing domains with more than one subtask were assessed separately (Figure S1.)
FIGURE 3.
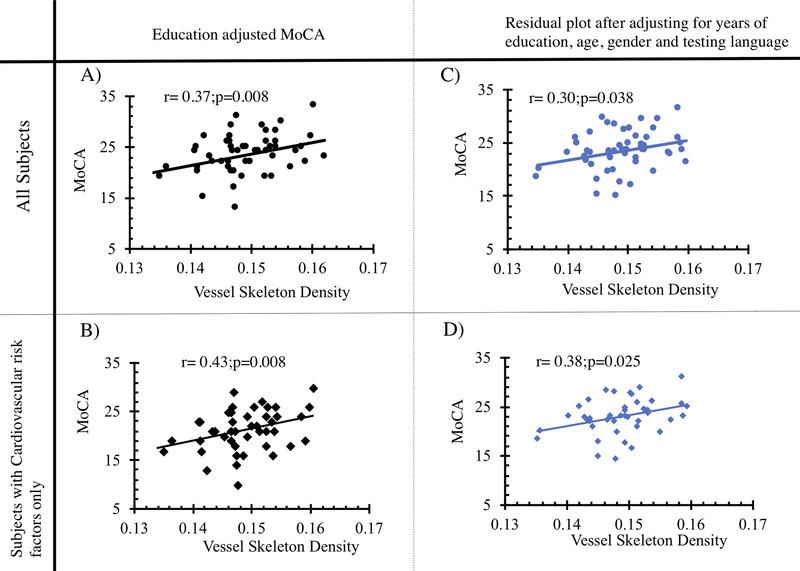
Association between VSD and Montreal Cognitive Assessment (MoCA). (A and B) Plot of raw VSD versus education‐adjusted MoCA for all subjects and subjects with cardiovascular risk factors—diabetes, hypertension, and hypercholesteremia—respectively. (C)and D) Plot of residuals from left panels after adjusting for age, gender, and testing language (English or Spanish)
FIGURE 4.

Association between retinal vessel skeletal density (VSD) and MoCA subtask scores for different cognitive domains. The score range reflects the sum of the findings of subtasks. The boxes show the 25th and 75th percentile distribution and the whiskers 5th–95th of the VSD measures. The “X” is the mean of the VSD distribution
Complementing the association between lower VSD and cognitive function assessment, VSD also showed notable associations with the measures of cerebrovascular perfusion. Specifically, VSD was positively correlated with perfusion in the middle cerebral artery perforator territory (r = 0.45, P = .02) and cerebrovascular reactivity (r = 0.67, P = .046). Higher VSD trended negatively with Fazekas scale (r = ‐0.25, P = .2); however, this association was not statistically significant (Figure 5).
FIGURE 5.

Association between retinal vessel skeletal density (VSD) and measures of cerebrovascular health. (A) Plot of cerebrovascular reactivity (CVR) and VSD adjusted for covariates. (B) Raw data plot of Fazekas scale grading of white matter lesions against VSD. Small amounts of random vertical noise up to 0.3 were added to the Fazekas scale to reduce the number of overlapping points. (C) Plot of perfusion in the middle cerebral artery perforator territory (MCA‐Perf) against VSD adjusted for covariates
4. DISCUSSION
We demonstrate significant and complementary associations between retinal capillary perfusion and several independent measures suggestive of early cerebral SVD in an understudied Hispanic population at high risk for VCID. We observe a significant association of retinal capillary perfusion with two separate measures of cognitive function, CDR‐SOB and MoCA, suggestive of subtle functional deficits and cognitive impairment. These clinical findings are complemented by a significant positive association between retinal capillary perfusion and intracranial cerebrovascular reactivity, suggesting global cerebrovascular dysfunction. Finally, anatomic evidence of impaired perfusion in the middle cerebral artery perforator territory and a trend toward worse Fazekas scores supports the notion of early cerebral SVD in this same population. Collectively these clinical, functional, and anatomic findings suggest that OCTA‐based retinal capillary perfusion is an indicator of several components of early vascular cognitive impairment.
The significant association between retinal capillary perfusion and MoCA subtasks assessing the visuospatial/executive domain (assessed using trails, clock drawing, and copy cube subtasks) is consistent with the literature reporting that executive function deficits are among the most prevalent domain impairments in cerebral SVD and VCID. 40 , 41 , 44 , 45 It is also noteworthy that our results do not show nonspecific correlations between other domains of the MoCA such as attention, language, or naming. The grading of the MoCA subtask performance, such as trails tracing, lack important components such as time‐to‐perform the task but provide compelling data for future prospective studies using more comprehensive neuropsychological assessment. The separate and significant correlation of retinal capillary perfusion with CDR‐SOB and MoCA scores reinforce the notion that there is a true correlation between retinal vascular impairment and early subclinical VCID. Prospective analysis of this in similar cohorts is underway and will be very informative.
In our study, we hypothesize that retinal capillary perfusion is a biomarker of SVD and VCID. Our data suggest that both impaired perfusion of the middle cerebral artery perforator region and impaired cerebrovascular reactivity are significantly correlated with lower retinal capillary density. One interpretation of these concurrent findings is that there is global impairment in cerebral vascular function at multiple levels of the vascular tree. This is consistent with the hypothesis that SVD impairs vasodilatory function of larger cerebral vessels leading to impaired blood flow at the capillary level. Although it is not possible to directly assess capillary level changes within the CNS, the negative correlation of retinal capillary perfusion with the Fazekas score is at least consistent with the hypothesis that retinal capillary changes may mirror concomitant cerebrovascular disease at the capillary level. Due to the cross‐sectional nature of this study, it is not possible to determine causality or temporal sequence of these findings. However, it is reasonable to hypothesize that retinal capillary level changes could be the earliest detectable vascular changes associated with SVD in future longitudinal studies.
Several studies have demonstrated impaired retinal capillary perfusion in AD but without attention to contributions from SVD and mostly with mini‐mental status exam (or MMSE) as the measure of cognition and/or function. 17 , 18 , 19 , 46 , 47 , 48 , 49 , 50 , 51 Therefore, it is not clear to what degree vascular comorbidity may have confounded the reported associations. Of note, O'Bryhim et al demonstrate smaller foveal avascular zone size in cognitively normal subjects who are biomarker positive for AD using either positron emission tomography (PET) or cerebral spinal fluid (CSF) 18 and Querques et al demonstrate impaired retinal vascular reactivity in subjects with mild cognitive impairment. 17 Retinal vessel density is also reported to be decreased in subjects with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, (CADASIL). 52 Although the proposed mechanism of these findings may be different, it supports the notion that OCTA‐derived measures of retinal capillaries are sufficiently sensitive to reflect CNS disease, particularly in the early stages of cognitive impairment. Our study adds unique data, conclusions, and perspective to these previous reports by exploring the understudied effect of early small vessel disease on early cognitive impairment and dementia.
Our study is limited by the relatively small sample size and the accompanying restricted spectrum of the CDR‐SOB scores inherent in early VCID. Nonetheless, we observed significant associations between multiple clinical and radiologic measures highly suggestive of VCID and retinal capillary perfusion, potentially indicating an early onset of retinal vascular attenuation in cognitive impairment. We recognize that it will be important to characterize the OCTA measures across more varying levels of cognitive impairment, and ongoing studies are aimed at addressing that point. Another limitation is the relatively small proportion of subjects with suspected cognitive decline. Despite this small proportion, our data still show consistent and significant findings across multiple domains of VCID assessment. Finally, we were unable to directly correct for axial‐length–induced magnification error in our VSD measures. However, we performed several analyses to estimate the relative change of vessel density findings adjusted for ocular magnification based on refraction data that were available. Specifically, (1) the results are unchanged when we controlled for refractive error as covariate; (2) there was no significant difference between the VSD of the 15 most hyperopic and 15 most myopic eyes, supporting the minimal effect of the axial magnification in this cohort; and (3) an estimate of the axial length magnification on the VSD data from the refractive measurements suggested at most a negligible effect (<5%) on our analysis. Future work in a larger longitudinal cohort will help address these limitations and extend the findings of this study.
In conclusion, our preliminary investigation has shown promising results for the complementary role of OCTA‐based retinal vessel density measures for assessing the vascular contribution to acquired cognitive impairment in an elderly Latinx population. Furthermore, due to the relatively rapid and non‐invasive methodology of OCTA image acquisition, OCTA retinal assessment in dementia may create the opportunity for longitudinal evaluation of retinal capillary perfusion in vivo in ways that can otherwise not yet be attained using the currently available brain imaging technologies.
GRANT SUPPORT
The study was supported by grants from the NIH: K08EY027006, UH2NS100614, UH3NS100614, P30AG066530, and unrestricted department funding from Research to Prevent Blindness (New York, NY, ,USA), Bright Focus Foundation CA2020004, and research grants from Carl Zeiss Meditec Inc (Dublin, California, USA). Carl Zeiss Meditec, Inc was not, however, consulted in the design, implementation or analysis of the study data.
CONFLICTS OF INTEREST
The authors Bright S. Ashimatey, Lina M. D'Orazio, Samantha J. Ma, Kay Jann, Hanzhang Lu, Xuejuan Jiang, Danny J. J. Wang, and John M. Ringman have no conflicts of interest to disclose. Amir H. Kashani is a consultant, and has received financial support from Carl Zeiss Meditec, Inc, a manufacturer of the OCTA platform used for the study.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
We would like to acknowledge Mrs. Anoush Shahidzadeh and Mrs. Marlene Casey for their help in coordination and logistics of the study.
Ashimatey BS, D'Orazio LM, Ma SJ, et al. Lower retinal capillary density in minimal cognitive impairment among older Latinx adults. Alzheimer's Dement. 2020;12:e12071 10.1002/dad2.12071
REFERENCES
- 1. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18(3):691‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology. 2007;69(24):2197‐2204. [DOI] [PubMed] [Google Scholar]
- 5. Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62(7):1148‐1155. [DOI] [PubMed] [Google Scholar]
- 6. Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11(6):710‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanton AV, Wasan B, Cerutti A, et al. Vascular network changes in the retina with age and hypertension. J Hypertens. 1995;13(12 Pt 2):1724‐1728. [PubMed] [Google Scholar]
- 8. Wong TY, Hubbard LD, Klein R, et al. Retinal microvascular abnormalities and blood pressure in older people: the cardiovascular health study. Br J Ophthalmol. 2002;86(9):1007‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong TY, Klein R, Sharrett AR, et al. Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA. 2002;288(1):67‐74. [DOI] [PubMed] [Google Scholar]
- 10. Wong TY, Klein R, Sharrett AR, et al. Retinal microvascular abnormalities and cognitive impairment in middle‐aged persons: the atherosclerosis risk in communities study. Stroke. 2002;33(6):1487‐1492. [DOI] [PubMed] [Google Scholar]
- 11. Gatto NM, Varma R, Torres M, et al. Retinal microvascular abnormalities and cognitive function in Latino adults in Los Angeles. Ophthalmic Epidemiol. 2012;19(3):127‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lesage SR, Mosley TH, Wong TY, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14‐year follow‐up study. Neurology. 2009;73(11):862‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kashani AH, Chen CL, Gahm JK, et al. Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral‐domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9). Oct362‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Carlo TE, Chin AT, Bonini Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35(11):2364‐2370. [DOI] [PubMed] [Google Scholar]
- 16. Koulisis N, Kim AY, Chu Z, et al. Quantitative microvascular analysis of retinal venous occlusions by spectral domain optical coherence tomography angiography. PLoS One. 2017;12(4):e0176404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Querques G, Borrelli E, Sacconi R, et al. Functional and morphological changes of the retinal vessels in Alzheimer's disease and mild cognitive impairment. Sci Rep. 2019;9(1):63‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol. 2018;136(11):1242‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer's disease on optical coherence tomography angiography. PLoS One. 2019;14(4):e0214685‐e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino eye study: design, methods, and baseline data. Ophthalmology. 2004;111(6):1121‐1131. [DOI] [PubMed] [Google Scholar]
- 21. Filshtein TJ, Dugger BN, Jin L‐W, et al. Neuropathological diagnoses of demented hispanic, black, and non‐Hispanic white decedents seen at an Alzheimer's disease center. J Alzheimers Dis. 2019;68:145‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vega IE, Cabrera LY, Wygant CM, Velez‐Ortiz D, Counts SE. Alzheimer's disease in the Latino community: intersection of genetics and social determinants of health. J Alzheimers Dis. 2017;58(4):979‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pew Research Center . Key facts about how the U.S. Hispanic population is changing. 2020; https://www.pewresearch.org/fact-tank/2016/09/08/key-facts-about-how-the-u-s-hispanic-population-is-changing/, Feb 7th, 2020.
- 24. Balfour PC, Jr. , Ruiz JM, Talavera GA, Allison MA, Rodriguez CJ. Cardiovascular disease in Hispanics/Latinos in the United States. J Lat Psychol. 2016;4(2):98‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173‐178. [DOI] [PubMed] [Google Scholar]
- 26. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 27. Rosenfeld PJ, Durbin MK, Roisman L, et al. ZEISS angioplex spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol. 2016;56:18‐29. [DOI] [PubMed] [Google Scholar]
- 28. Chu Z, Lin J, Gao C, et al. Quantitative assessment of the retinal microvasculature using optical coherence tomography angiography. J Biomed Opt. 2016;21(6):66008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Besser L, Kukull W, Knopman DS, et al. Version 3 of the national Alzheimer's coordinating center's uniform data set. Alzheimer Dis Assoc Disord. 2018;32(4):351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lu H, Liu P, Yezhuvath U, Cheng Y, Marshall O, Ge Y. MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J Vis Exp. 2014(94):52306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu P, De Vis JB, Lu H. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. Neuroimage. 2019;187:104‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shao X, Ma SJ, Casey M, D'Orazio L, Ringman JM, Wang DJJ. Mapping water exchange across the blood‐brain barrier using 3D diffusion‐prepared arterial spin labeled perfusion MRI. Magn Reson Med. 2019;81(5):3065‐3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen JJ. Cerebrovascular‐reactivity mapping using MRI: considerations for Alzheimer's disease. Front. Aging Neurosci. 2018;10:170‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lahme L, Esser EL, Mihailovic N, et al. Evaluation of ocular perfusion in Alzheimer's disease using optical coherence tomography angiography. J Alzheimers Dis. 2018;66(4):1745‐1752. [DOI] [PubMed] [Google Scholar]
- 36. Jann K, Shao X, Ma SJ, et al. Test‐retest reproducibility and associations with cognitive impairment of 3D PCASL in elderly subjects at risk of small vessel disease. Proc ISMRM 27, (abstract). 2019. [Google Scholar]
- 37. O'Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008;65(8):1091‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chertkow H, Nasreddine Z, Johns E, Phillips N, McHenry C, P1‐143: The Montreal cognitive assessment (MoCA): Validation of alternate forms and new recommendations for education corrections. Alzheimer's Dementia. 2011;7(4S_Part_5):S157‐S157. [Google Scholar]
- 39. Zhou Y, Ortiz F, Nuñez C, et al. Use of the MoCA in detecting early Alzheimer's disease in a Spanish‐speaking population with varied levels of education. Dement Geriatr Cogn Dis Extra. 2015;5(1):85‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75(1):61‐71. [PMC free article] [PubMed] [Google Scholar]
- 41. Ying H, Jianping C, Jianqing Y, Shanquan Z. Cognitive variations among vascular dementia subtypes caused by small‐, large‐, or mixed‐vessel disease. Arch Med Sci. 2016;12(4):747‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Julayanont P, Phillips N, Chertkow H, Nasreddine ZS. The Montreal cognitive assessment (MoCA): concept and clinical review In: LA J., ed. Cognitive Screening Instruments: A Practical Approach. London: Springer; 2013:111‐152. [Google Scholar]
- 43. Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2016;36(1):6‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89‐98. [DOI] [PubMed] [Google Scholar]
- 45. Ramirez‐Gomez L, Zheng L, Reed B, et al. Neuropsychological profiles differentiate Alzheimer disease from subcortical ischemic vascular dementia in an autopsy‐defined cohort. Dement Geriatr Cogn Disord. 2017;44(1‐2):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoon SP, Grewal DS, Thompson AC, et al. Retinal microvascular and neurodegenerative changes in Alzheimer's disease and mild cognitive impairment compared with control participants. Ophthalmol Retina. 2019;3(6):489‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grewal DS, Polascik BW, Hoffmeyer GC, Fekrat S. Assessment of differences in retinal microvasculature using OCT angiography in Alzheimer's disease: a twin discordance report. Ophthalmic Surg Lasers Imaging Retina. 2018;49(6):440‐444. [DOI] [PubMed] [Google Scholar]
- 48. Bulut M, Kurtulus F, Gozkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer's type dementia. Br J Ophthalmol. 2018;102(2):233‐237. [DOI] [PubMed] [Google Scholar]
- 49. Zabel P, Kaluzny JJ, Wilkosc‐Debczynska M, et al. Comparison of retinal microvasculature in patients with Alzheimer's disease and primary open‐angle glaucoma by optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2019;60(10):3447‐3455. [DOI] [PubMed] [Google Scholar]
- 50. Jiang H, Wei Y, Shi Y, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018;38(3):292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu J, Zhang X, Azhati G, Li T, Xu G, Liu F. Retinal microvascular attenuation in mental cognitive impairment and Alzheimer's disease by optical coherence tomography angiography. Acta Ophthalmol (Copenh). 2020. [DOI] [PubMed] [Google Scholar]
- 52. Nelis P, Kleffner I, Burg MC, et al. OCT‐Angiography reveals reduced vessel density in the deep retinal plexus of CADASIL patients. Sci Rep. 2018;8(1):8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.


