Abstract
Brewers’ spent grain (BSG) is a low‐cost by‐product of the brewing process. BSG liquor names the liquid components of BSG, mainly glucose, maltose, and long‐chain α‐1,4‐glycosidic bond glucose oligomers. These substances should be separated in existing BSG biorefineries, as they might lead to an increased formation of microbe‐inhibiting compounds in well‐established hydrothermal/enzymatic saccharification processes. In most cases, this liquid fraction is discarded. The present study presents for the first time an optimized process with BSG liquor for the purpose of producing bulk chemicals (e.g., lactate) in relevant concentrations. The process comprises the application of yeast extract, produced from own brewing processes, as the sole supplemented complex constituent in a simultaneous fermentation and saccharification process. Kinetic parameters for the final optimized process conditions with the organism Lactobacillus delbrueckii subsp. lactis were: maximum specific growth rate µmax = 0.47 h−1, maximum lactate concentration cLac, max = 79.06 g L−1, process yield YPS = 0.89 gLac gSugar −1, lactate production rate qP = 4.18 gLac gCDW −1 h−1, and productivity P 15 h = 4.93 gLac L−1 h−1. BSG liquor, linked with yeast extract from Brewers’ yeast, can be a promising substrate for further bioprocess engineering tasks and contribute to a holistic and sustainable usage of Brewers’ spent grain.
Keywords: biorefinery, brewers’ spent grain (BSG) liquor, fermentation, lactate, Lactobacillus delbrueckii subsp. lactis
Abbreviations
- BSG
brewers’ spent grain
- CDW
cell dry weight
- MRS medium
cultivation medium by De Man, Rogosa, Sharpe
1. INTRODUCTION
Brewers’ spent grain (BSG) is the most abundant by‐product of the brewing process and available in large quantities since the worldwide annual beer production was 1.95 million hL in 2017 1. As 0.2 kg of BSG accumulate from the production of 1 L beer 2, this resulted in about 39 million metric tons of BSG 3, whereof ∼10 million tons were produced in the European Union 4. After malting, milling and mashing, the lautering step of brewing separates the solid particles of the mash from the liquid wort. Thereby BSG with a moisture content of 70–80% accumulates 2, 3. BSG has a market value of approximately 39 US$ per metric ton and contains high amounts of nutrients. Therefore, it is mainly used as animal feed 5. Otherwise, BSG is proposed as a sustainable feedstock for biorefineries 6. The dry matter of BSG contains the solid components cellulose, hemicellulose, lignin, proteins, lipids, and an ash fraction 5, but also has adhered soluble compounds—mainly sugar like glucose, maltose, and different malto oligomers 3. Recent work on BSG mainly focusses on different pretreatment strategies to break the structural integrity of the lignocellulosic biomass and increase steric accessibility of these structural‐bond polymers for enzymatic saccharification. The pretreatment can be performed, among others, by diluted sulfuric acid hydrolysis 7, 8, diluted phosphoric acid hydrolysis 9, alkali treatment 10, 11, 12, and liquid hot water (LHW) pretreatment 13, 14, 15. The following enzymatic saccharification is carried out with different cellulase and hemicellulase mixtures, obtaining a sugar‐rich fraction, which can serve as a fermentation medium for the production of bulk chemicals.
The soluble components of BSG, called BSG liquor, can be removed by pressing (present work), washing 8, 9 or by autoclave‐assisted pretreatment at 100°C, as high temperatures increase the solubility of the adherent molecules 15, 16. In most studies, dealing with the solid BSG residue, BSG liquor is not used any further. Few studies report potential applications of BSG liquor, without reaching high product concentrations: In 1975, BSG liquor was first mentioned as a growth‐promoting medium for Aspergillus niger 17 and for the production of single‐cell protein, implicating a simultaneous reduction of the biological oxygen demand (BOD) with fungi like Calvatia gigantea and Candida steatolytica 18, 19. In 1976, BSG liquor was described as an antifoaming‐agent for bioreactors 20. One decade later, it was proposed as a cultivation medium for the production of citric acid with Aspergillus niger 21 and in 1999 for the production of pullulan by Aureobasidium pullulans 22. Recently, BSG liquor was used for a screening on growth‐promoting effects for Bacillus strains 23 and for the production of microbial lipids for biodiesel production and high‐value carotenoids by Rhodotorula glutinis 24.
PRACTICAL APPLICATION
BSG liquor, which can be obtained from BSG by pressing, is a suitable basis for a fermentation medium. This was demonstrated for a process with the organism Lactobacillus delbrueckii subsp. lactis for the purpose of lactate production including a potential use for the Brewers’ yeast side stream. As it is beneficial to remove BSG liquor from the solid residue, before applying further pretreatment strategies, this study might contribute to a holistic use of brewery residues.
In the present work, BSG liquor is used to develop a fermentation process with the organism Lactobacillus delbrueckii subsp. lactis, which can be put into the context of a BSG biorefinery (Figure 1). It is important to emphasize that BSG liquor is not identical to hydrolysates, obtained by hydrothermal/enzymatic pretreatment. Processes, using BSG liquor, are not in competition to the mentioned concepts, but can be seen as an addition. After separating the liquid components, it is still possible, to use the BSG residue in a more conventional field of application, e.g., as cattle feed, energy source, or as a feedstock for hydrothermal/enzymatic saccharification and subsequent fermentation processes.
Figure 1.
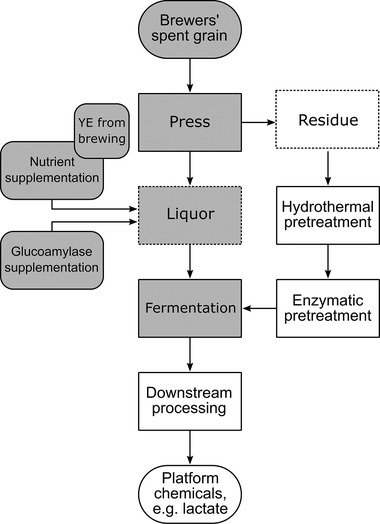
Proposed biorefinery concept for Brewers’ spent grain. The process steps highlighted in grey are subject of this work. Strongly rounded fields represent starting material and product, rectangles with solid lines visualize process steps, rectangles with dotted lines show obtained fractions, slightly rounded rectangles represent supplements; YE, yeast extract
On the one hand, compositional analysis of BSG liquor is described in this publication. On the other hand, optimization strategies of the fermentation process are presented. The mentioned strategies include statistical experimental design, supplementation of enzymes, and substitution of commercial yeast extract by yeast extract produced from Brewers’ yeast. In a second step, these results are applied to improve the final product concentration of the fermentation. Furthermore, the advantages of separating liquid components from the solid residue, are highlighted in this work.
2. MATERIALS AND METHODS
The sections Analysis of BSG liquor, HPLC analysis, as well as Chemicals and enzyme specification are provided in the Supporting Information.
2.1. Raw material
Four brewing processes were carried out in a 100 L pilot plant in order to produce BSG, namely: Wheat bock, Wheat, Helles, and May bock. Grain usage and brewing conditions are provided in Supporting Information Table 1. A small amount of industrial BSG was kindly provided by a local brewery (Privatbrauerei Bischoff GmbH & Co. KG, Winnweiler, Germany), namely Bischoff, for comparative purposes.
2.2. Production of BSG liquor
Brewers’ spent grain was directly pressed after the lautering process by a friction press (ENOL OP 20, Wein GmbH, Bönnigheim, Germany) generating ∼17.6 kg of BSG liquor and ∼14.8 kg residual BSG from ∼34 kg wet spent grain. The moisture content of BSG decreased from ∼75 wt% to ∼65 wt% and was measured by a gravimetrical moisture analyzer (MLB50‐3N, KERN & SOHN GmbH, Balingen, Germany). Obtained BSG liquor was centrifuged at 4200 × g (Z383K, Hermle Labortechnik GmbH, Wehingen, Germany) to remove small solid particles. The supernatant was stored at −20°C until further use.
2.3. Production of yeast extract
Yeast extract was produced by autolysis in a reactor with a working volume of 5 L (RALF, Bioengineering AG, Wald, Switzerland) at 55°C for 24 h, as described previously 25. Therefore, 750 g of fresh Brewers’ yeast slurry (SafAle US‐05, Lesaffre, Marcq‐en‐Baroeul, France), fermented for 5 days, were suspended in 5 L of sterile, deionized water. The autolysate was concentrated by using a rotation evaporator (Laborota 4003, Heidolph Instruments, Schwabach, Germany) and dried in an oven at 50°C for 48 h. In total, 62 g of yeast extract were obtained.
2.4. Strain, cryoconservation and seed train
The organism Lactobacillus delbrueckii subsp. lactis 20072 was obtained by German Collection of Microorganisms and Cellcultures (DSMZ GmbH, Braunschweig, Germany). The cells were grown in anaerobic MRS medium 26 with the following composition (g L−1): glucose (20), casein peptone (10), meat extract (10), yeast extract (5), Tween 80 (1), K2HPO4 (2), sodium acetate (5), (NH4)3 citrate (2), MgSO4·7 H2O (0.2), and MnSO4·H2O (0.05). The pH was adjusted to 7 with 5 M NaOH. Incubation occurred in a shaking incubator (Ecotron, Infors AG, Bottmingen, Switzerland) at a temperature of 45°C, a frequency of 100 rpm and a lift of 25 mm. All media components were sterilized in an autoclave (V‐150, Systec GmbH, Linden, Germany). Sugar and additional supplements were autoclaved separately. To verify anaerobic conditions, oxygen indicator Resazurin was added in a concentration of 1 mg L−1. If no color change was visible, reducing agent Cysteine‐HCl was used in a concentration of 1 g L−1.
For the production of frozen stock cultures, aliquots of the cell suspension were harvested in the mid‐exponential growth phase. An equal volume of 80 vol% glycerol was added and the samples were stored at −80°C (Freezer Model AV039P, Labotec GmbH, Göttigen, Germany) until further use.
A seed train was used composed of two pre‐cultures and grown in anaerobic MRS medium.
Pre‐culture 1 was grown in 50 mL of medium in a 100 mL shaking flask (Pressure Plus, DWK Life Science GmbH, Mainz, Germany). After 24 h, the cell suspension was used to inoculate pre‐culture 2, which was grown in 300 mL MRS medium in a 500 mL shaking flask. Cells were harvested after 16 h of growth, centrifuged at 4200 × g and resuspended in BSG liquor. The main culture was inoculated to OD600 nm = 0.5, as described in Sections 2.5 and 2.6.
2.5. Statistical experimental design
The software (Visual‐XSel, CRGRAPH GbR, Starnberg, Germany) was used to generate a design of experiments (DoE) test plan. The approach was set up as described earlier 27. The overall optimization task was to enhance the lactate production, while minimizing the addition of nutrients to the BSG liquor. Therefore, the lactate concentration was set as the target value. The MRS ingredients, excluding glucose, represented the influencing parameters. Quantitative designs use parameter ranges, e.g., concentration ranges. In contrast, categorical designs distinguish whether a partial experiment of the test plan incorporates or lacks an influencing parameter. In this study a categorical design with a d‐optimal test plan was applied, as the concentrations in the MRS medium were optimized previously 26. D‐optimal test plans are recommended for two‐level interaction studies, if the quantity of experiments prohibits other test plans. Nevertheless, 52 single experiments were carried out with a fermentation medium based on Wheat bock liquor in 100 mL shaking flasks with a working volume of 50 mL. BSG liquor was diluted according to Section 2.6. Shaking flasks were closed directly after autoclaving with septa (Butyl plug‐massive, Glasgerätebau Ochs Laborfachhandel e.K., Bovenden, Germany) and anaerobized by parallel chilling on ice and fumigating with nitrogen through a hollow needle. The nutrient supplementation to the BSG liquor (various combinations of MRS ingredients) was defined by the test plan from Visual‐XSel. The concentration of the target value lactate (Supporting Information Section 1.3) and the optical density at a wavelength of 600 nm (UV spectrophotometer LAMBDA Bio+, Perkin Elmer Corporation, Waltham, United States) were measured after 24 and 28 h of fermentation to ensure that the cells were in the stationary phase. The mean value of these two lactate measurements was used for a multiple regression, which was carried out considering linear interactions between the influencing parameters and parameter‐to‐parameter interactions. Nonsignificant terms were removed by the autostepwise function according to their p‐value (p > 0.05).
2.6. Optimization of the fermentation
Five fermentations were chosen to explain the optimization of the bioprocess. These fermentations are designated in the following by the capital roman numerals (I) to (V). The process conditions, using BSG liquor, are also visualized in Figure 2. Two reference fermentations were carried out in duplicate. The first reference process (I) was conducted with MRS medium according to literature 26. In the second reference fermentation (II), all nine MRS components excluding glucose were added to BSG liquor Wheat bock. Therefore, BSG liquor was diluted to a sugar concentration (sum parameter maltotriose, maltose, glucose) similar to MRS medium for comparative purposes. The concentration of malto oligomers was not considered, as the bacterial consumption of these polysaccharides was very low. Nutrients of the MRS medium were added in the reported concentration 26. The third fermentation (III) contained MRS components according to the results from section 2.5 (Casein peptone, meat extract, and (NH4)3 citrate were not added). In an additional fermentation (IV) 100 µL of a sterile‐filtered 1,4‐α‐glucoamylase mix (Attenuzyme® Core, Novozymes A/S, Bagsvaerd, Denmark) were added to the fermentation broth in order to degrade oligomers to the preferred substrate glucose. The nutrient supplementation was equal to fermentation (III). In the final optimization step (V), commercial yeast extract was exchanged by yeast extract produced from Brewers’ yeast of own brewing processes (2.3). Nutrient supplementation and enzyme application were adopted from fermentation (IV).
Figure 2.
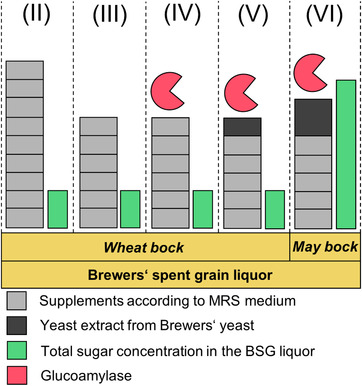
Process conditions for fermentation media based on diluted BSG liquor Wheat bock with complete supplementation (II), optimized supplementation (III), optimized supplementation with glucoamylase (IV), optimized supplementation with glucoamylase and yeast extract from brewing (V) as well as for the scale‐up fermentation with BSG liquor May bock (VI). Stacked, wide bars show the nine supplements according to MRS medium. Light grey blocks stand for commercial supplements and dark grey blocks represent yeast extract, produced from own Brewers’ yeast. Tiny, green bars represent the total sugar concentration. Red symbols characterize the addition of glucoamylase
The cells were grown in a bioreactor with a working volume of 1 L (RALF, Bioengineering AG, Wald, Switzerland). The bioreactor was fumigated with nitrogen at a flow rate of 10 L h−1 directly after autoclaving to obtain anaerobic conditions. Resazurin and Cysteine‐HCl were applied, as reported previously (2.4). Fumigation was carried out until the end of the process. The stirrer speed was adjusted to 400 rpm, temperature was set to 45°C, and the pH was regulated at 6.0 by the addition of 5 M NaOH with a peristaltic pump. Samples were taken hourly in the growth phase and analyzed for optical density at a wavelength of 600 nm. Centrifuged aliquots were stored at −20°C for further analytical measurements (Supporting information Section 1.3).
In order to increase the final lactate concentration, an additional fermentation was carried out with BSG liquor May bock (VI), which is also visualized in Figure 2. With two exceptions, this fermentation was designed as previously reported in fermentation (V). On the one hand, BSG liquor was not diluted. Therefore, a final sugar concentration (sum parameter malto oligomers, maltotriose, maltose, glucose) of ∼88 g L−1 was achieved. On the other hand, yeast extract was deployed in a concentration, which was adjusted proportional to the higher sugar concentration (22 g L−1). The remaining supplements, were applied in the concentrations reported by MRS medium 26.
2.7. Determination of cell dry weight
The correlation between cell dry weight (CDW) and optical density at a wavelength of 600 nm was determined separately for each reactor run. Therefore, 5 mL cell suspension were sampled during the exponential growth phase. The cell suspension was centrifuged three times for 20 min at 4200 × g and washed with deionized water. The cell pellet was dried at 50°C for 48 h and determined gravimetrically (scale LA 214, VWR LLC, Radnor, United States).
3. RESULTS AND DISCUSSION
In order to underline similarities between BSG (and the adherent sugar of BSG) from industrial and self‐made brewing processes, the dry weight percentage of the contained sugar is shown in Supporting Information Figure 1A for industrial BSG Bischoff and in Supporting Information Figure 1B for BSG Helles, obtained from own brewing processes.
3.1. Analysis of BSG liquor
As BSG liquor is proposed as a fermentation medium in the present work, a detailed knowledge about substances like sugars, proteins, amino acids, and trace elements is necessary. Thus, the composition of three BSG liquors in terms of pH, total sugar, protein, and amino acid as well as trace element concentration is provided in Table 1.
Table 1.
BSG liquor composition originated from Wheat bock, Wheat, and Helles brewing recipe
| Wheat bock | Wheat | Helles | ||
|---|---|---|---|---|
| pH value | 5.83 | 5.13 | 5.98 | |
| Sugar (g L−1) | Malto oligomers | 20.84 | 19.27 | 15.57 |
| Maltotriose | 18.20 | 7.87 | 4.15 | |
| Maltose | 47.10 | 23.71 | 12.74 | |
| Glucose | 12.92 | 3.24 | 2.40 | |
| Total | 104.63 | 57.55 | 37.16 | |
| Protein (mg L−1) | Total | 662 | 233 | 50 |
| Amino acids (mg L−1) | Aspartic acid | 6.08 | 1.85 | 1.59 |
| Glutamine | 10.59 | 4.71 | 2.50 | |
| Serine | 5.89 | 2.10 | 1.37 | |
| Histidine | 4.81 | 1.71 | 1.71 | |
| Glycine | 3.98 | 1.20 | 0.75 | |
| Threonine | 4.76 | 1.67 | 1.43 | |
| Arginine | 21.25 | 7.14 | 4.70 | |
| Alanine | 5.88 | 2.28 | 2.05 | |
| Tyrosine | 7.07 | 1.99 | 2.90 | |
| Methionine | 3.28 | 1.19 | 0.74 | |
| Valine | 9.60 | 3.40 | 2.81 | |
| Tryptophan | 6.94 | 2.65 | 1.84 | |
| Phenylalanine | 10.73 | 4.13 | 3.30 | |
| Isoleucine | 6.17 | 2.23 | 1.57 | |
| Leucine | 14.17 | 4.85 | 3.67 | |
| Lysine | 10.38 | 3.36 | 3.07 | |
| Total | 131.59 | 46.42 | 36.01 | |
| Cation (mg L−1) | Calcium | 14 | 38 | 19 |
| Magnesium | 63 | 48 | 26 | |
| Sodium | 25 | 17 | 11 | |
| Potassium | 450 | 206 | 160 | |
| Zinc | 0.3 | 1.2 | 0.22 | |
| Iron | 0.08 | 0.06 | 0.05 | |
| Manganese | 0.22 | 0.19 | 0.36 | |
| Copper | 0.1 | 0.1 | 0.13 | |
| Nickel | 0.01 | 0.01 | 0.01 | |
| Aluminium | 0.1 | 0.5 | 0.23 | |
| Ammonium | 0.02 | < 0.01 | < 0.01 | |
| Anion (mg L−1) | Chloride | 78 | 42 | 24 |
| Sulfate | 99 | 48 | 22 | |
| Nitrate | 8.3 | 11 | 5.5 | |
| Phosphate | 228 | 108 | 95 |
The pH value of BSG liquor was already described as 6.0 21, 5.96 19, 5.5 22, and 4.1 17, respectively. Similar observations were made in this study.
Total sugar concentration of BSG liquor was described as 23 17, 25 21, 23 22, and 30 g L−1 19 obtained as leakage water or press liquor from BSG. The produced BSG liquor contained a higher sugar concentration for all three investigated brewing recipes. Differences can be explained by the method of determination. The sugar concentration in literature was expressed as reducing sugar content without previous hydrolysis as described by Clark or Nelson and Somogyi 17, 18, 19. Using this analytical method, polymeric sugars tend to be determined too low. Liquors from BSG, containing wheat malt (Wheat bock, Wheat), showed higher sugar concentrations than barley containing liquors (Helles). This difference was observed as the starch degradability is higher in wheat than in barley malt 28 and reinforced by the fact that wheat has a higher content of starch than barley (∼78% vs. ∼54%) 29.
Total protein concentration was determined in a range of 50 to 662 mg L−1. Kjeldahl nitrogen in BSG liquor was described in literature as 336 17 and 434 mg L−1 19, respectively. This led, with a correction factor of 6.25 30, to a protein concentration of 2.1 and 2.7 g L−1, respectively. Discrepancies can be explained by differences in protein rest and other nitrogen‐containing compounds in addition to proteins like anthocyanogenes and polyphenols 31. These compounds are determined in Kjeldahl analysis (literature) but not in the Bradford assay (present study). Barley malt contains 9–11% of protein whereas wheat malt holds 11.5–12.5% 31. Thus, wheat malt containing liquors included higher protein concentrations than liquors resulting only from barley grain.
The amino acid and trace element concentrations in BSG liquor were not reported in literature, yet. For this reason, the measured values in BSG liquor were compared with reported data of the brewing process. During the lautering process, soluble, adhering components of the spent grains are stepwise removed by feeding water through the BSG filter cake into the wort kettle. This iterative procedure decreases the concentration of adherent components of the BSG. At the same time, the concentrations in the wort kettle are reduced by dilution 31, 32. BSG liquor can be considered as the liquid product of a final lautering step, which contains lower concentrations of all components than the final wort. The total amino acid concentration in the beer wort lies in the range of 200–250 mg L−1 33. In the present work, concentrations ranging from 36–132 mg L−1 were measured, but only 16 amino acids could be determined. The composition of the beer wort, referring to trace elements, is known for the following minerals (mg L−1): calcium (23–60), magnesium (80–100), sodium (10–25), potassium (500–550), zinc (0.1–0.25), chloride (100–200), sulfate (40–200), nitrate (10–50), and phosphate (600–850) 31. The determined values, shown in Table 1, fit well to the literature values of the beer wort.
Based on the presented results, the potential of BSG liquor as a cultivation medium is easily recognizable. It contains numerous ingredients that are part of complex media like MRS medium 26 and provide carbon sources, nitrogen sources, as well as trace elements. In general, BSG liquor composition is varying from batch to batch. Nevertheless, fermentations using BSG liquor from diverse brewing recipes, supplemented with additional nutrients and the organism L. delbrueckii subsp. lactis revealed, that the final lactate titer is basically in dependence on the initial sugar concentration. Concentrations of amino acids and trace elements enhanced the final lactate concentration (compared to MRS medium), but did not significantly alter the lactate formation when different liquors were used (data not shown). Therefore, BSG liquor can be a promising substrate for further bioprocess engineering tasks and a broad applicability in terms of a controlled biorefinery process seems to be in sight with this organism.
3.2. Decreased supplementation of nutrients
The aim of the statistical experimental design was the reduction of supplements, as around 68% of the production costs for lactate are contributed by raw material substrates 34. Therefore, the influence and necessity of the nine MRS supplements on the production process was investigated, using a fermentation medium based on BSG liquor.
The effect strengths of the nine tested influencing parameters are shown in Figure 3A. This effect strength reflected the maximum increase in lactate in grams per liter that could be achieved by adding the component in a concentration equal to MRS medium 26 to the BSG liquor. As a categorical design was applied, a high effect strength does not mean, that an increased concentration of the investigated component automatically results in a further increase of the target size. It is important to clarify, that a high effect strength only postulates a beneficial effect for the given concentration, according to MRS medium 26. Especially the high effect strengths of 12.6 g L−1 for Tween 80 and 10.7 g L−1 for sodium acetate were remarkable. Long‐chain unsaturated fatty acids are essential for the metabolism of Lactobacillus strains, as they lack enzymes for de novo synthesis. Tween 80 contains 92% of oleic acid (C18:1) and therefore provides the organism with the desired fatty acids 35. Furthermore, Tween 80 increases the permeability of the cell membrane and accordingly enhances the uptake of additional nutrients 34. The positive effect of Tween 80 on the growth of Lactobacillus strains has also been reflected in other literature references 36, 37. Sometimes, sodium acetate is reported as an antifungal agent in culture media 38 but not as growth‐enhancing for Lactobacillus strains 34. Some publications also report a positive effect of sodium acetate supplementation on final cell density 39 and the final lactate concentration 40. Regarding the high effect strength, a positive effect of sodium acetate on the lactate production was investigated in this study. Casein peptone, meat extract, and yeast extract represent the complex media constituents, which are described in MRS medium 26. These supplements provide amino acids, B vitamins, trace elements, and showed effect strengths of 5.0, 4.5, and 4.4 g L−1, respectively. As three complex constituents contained these ingredients, the individual effect strengths were smaller than might be expected. In addition, BSG liquor also provides amino acids, vitamins, and trace elements 31, reducing the necessity of complex media constituents. This reflects the relation between ingredients of the BSG liquor, as presented in Section 3.1, and the supplementation strategy, shown in this section. The effect strengths of MnSO4, K2HPO4, (NH4)3 citrate, and MgSO4 had less influence on the target value, with 4.3, 3.5, 3.3, and 1.8 g L−1, respectively. In Figure 3B, observed values (experimental data) are plotted versus calculated values (model data). The closer values lie to the 45° line, the higher is the quality of the model. The deviations from the 45° line are called residues and should be normally distributed, as it was the case for this model (data not shown). The high quality of this model was also reflected by the high coefficient of determination with R 2 = 0.976. As the adjusted coefficient of determination R 2 adjust = 0.955 was also high, it could be assumed that the model did not contain unnecessary terms 41.
Figure 3.
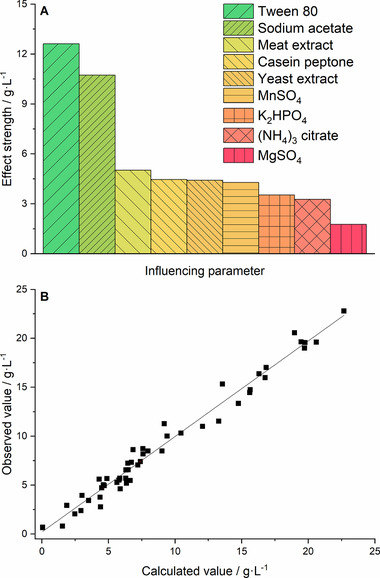
Results of statistical experimental design for the purpose of nutrient optimization. (A) Effect strength for the target value of lactate concentration in terms of the nine influencing parameters (MRS components). (B) Observed versus calculated lactate values for all 52 single experiments of the D‐optimal test plan
Evaluating the supplementation with the model, the following supplementation was chosen (g L−1): yeast extract (5), Tween 80 (1), K2HPO4 (2), sodium acetate (5), MgSO4·7H2O (0.2), and MnSO4·H2O (0.05). In fact, the supplementation was decreased by (g L−1): casein peptone (10), meat extract (10), and (NH4)3 citrate (2). Casein peptone and meat extract represent high‐cost supplements in the MRS medium, which were also applied in high concentrations. Therefore, only one complex media constituent (yeast extract) was necessary, which could also be made from Brewers’ yeast. The model calculated a lactate concentration of 16.4 g L−1 for this setting. The comparative shaking flask experiment revealed 16.3 g L−1.
3.3. Lactate production in batch cultures
BSG can be obtained at low cost throughout the year 42 and makes up around 85% of total brewery residues. Hot trub and yeast contribute to 15% of the by‐products, which are produced during brewing 42. On top, most biorefinery concepts lack a use for BSG liquor 8, 9, 16, 43. Therefore, a process was developed, which proposes a potential use for BSG liquor and comprises an application for the Brewers’ yeast side stream.
Separating BSG liquor and solid residue before applying hydrothermal and enzymatic pretreatment strategies to the solid residue, might be advantageous due to the following reasons: (i) Hydrothermal pretreatment leads to the formation of compounds like hydroxymethylfurfural (HMF), furfural, acetic acid, formic acid, and levulinic acid. These compounds are well described as inhibitors for microbial growth 7, 9, 32, 43. Higher HMF yields were observed for microwave‐induced conversions, when glucose was used instead of cellulose. This indicates that inhibitors are formed more easily from low‐chain sugars (in the liquid fraction) compared to sugars coming from structural‐bound polymers (in the solid residue) 44. Similar observations were made for the hydrothermal pretreatment of pressed and non‐pressed BSG 45. (ii) The removal of the liquid fraction reduces up to 50% of the BSG weight. This diminishes transport costs, if the BSG residue has to be delivered to cattle farms or bio‐based chemical companies 46. (iii) The shelf life of fresh BSG is very short, as a butyric acid fermentation starts after a few hours, initiated by naturally occurring bacteria, like Clostridium butyricum. The removal of adherent sugar increases the shelf‐life of BSG 47. (iv) If BSG is used for energy generation, the moisture content has to be reduced to 55 wt% 3. The obtained BSG liquor is in this case a useful by‐product, which can be used for fermentation (as demonstrated below). (v) Sugar of low chain length can be converted directly by microbial fermentation to valuable products without applying energy‐ and cost‐effective pretreatment strategies. (vi) The evaporation enthalpy of water (4.19 kJ kg−1 K−1) leads to a high energy demand in thermal drying. Therefore, pressing is a cost‐effective and widely used process step in biomass drying and superior to thermal drying 46, 48. Furthermore, screw extrusion (at elevated temperature) could fulfill two tasks in parallel by (a) obtaining BSG liquor and (b) increasing enzymatic accessibility of the solid residue 48.
The proposed concept could increase the economic feasibility of BSG biorefineries in general. On the one hand, the mentioned advantages of the separation process (i)–(vi) suggest that monetary advantages could arise. Moreover, the costs for the supplements appear to be relatively low compared to a potential profit for the produced amount of lactate. On the other hand, it is difficult to estimate the process and downstream costs for fermentation and yeast extract production. In addition, a potential revenue is dependent on the initial substrate concentration in the BSG liquor, which may vary from batch to batch. Therefore, no economic evaluation was performed so far.
Cell dry weight, lactate formation, and substrate consumption for fermentation (I) to (V), which were conducted to optimize the process, are presented in Figure 4. In order to draw a comparison between the processes, compare the fermentations in terms of literature values and to the reference fermentation with MRS medium (I), various kinetic parameters were determined. These values are listed in Table 2.
Figure 4.
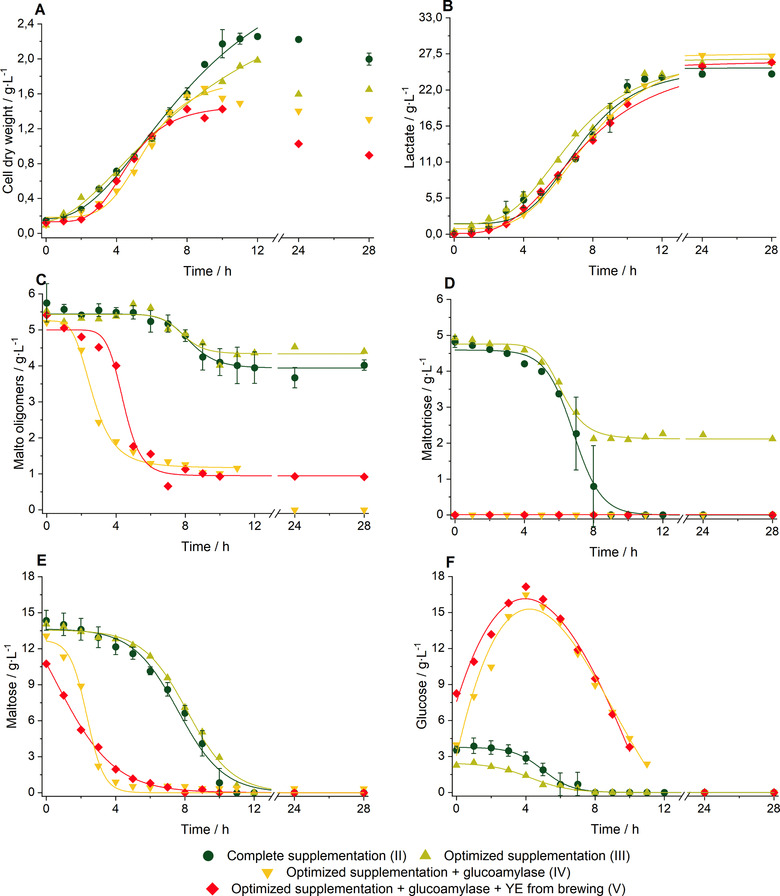
Fermentations with the organism L. delbrueckii subsp. lactis and BSG liquor Wheat bock at T = 45 °C, N = 400 rpm and pH = 6. The values of BSG liquor with complete supplementation (fermentation (II), dark green circles) show the mean values of two independent biological replicates, the error bars represent standard deviations. The fermentations with BSG liquor and optimized conditions (fermentation (III), light green upright triangles), optimized conditions and glucoamylase (fermentation (IV), yellow inverted triangles) and optimized conditions, glucoamylase and YE from brewing (fermentation (V), red diamonds) represent single experiments. (A) Cell dry weight. Concentration of (B) lactate, (C) malto oligomers, (D) maltotriose, (E) maltose and (F) glucose. Glucose data points of the fermentations including glucoamylase were fitted by polynomials of second degree, all other data points were fitted by sigmoidal fits; N, stirrer speed, YE, yeast extract
Table 2.
Kinetic parameters of the fermentations with L. delbrueckii subsp. lactis. Maximum specific growth rate (µmax), correlation between optical density and cell dry weight (OD600 nm ∼ CDW), maximum lactate concentration (cLac, max), process yield (YPS), lactate production rate (qP), substrate consumption rates (qS1,S2,S3), and productivity in the first 11 h (I–V) or 15 h (VI) of fermentation (P). The reference fermentations with MRS medium (I) and BSG liquor with complete supplementation (II) were conducted in biological duplicates, the error represents the standard deviation; n.a., not available as the component was not present in the approach; n.d., not determinable due to the superposition of enzymatic and microbial conversion; YE, yeast extract
| Medium composition | MRS medium | Complete supplementation | Optimized supplementation | Optimized supplementation + glucoamylase | Optimized supplementation + glucoamylase + YE from brewing | Scale‐up fermentation |
|---|---|---|---|---|---|---|
| Fermentation No. | (I) | (II) | (III) | (IV) | (V) | (VI) |
| µmax (h−1) | 0.55 ± 0.01 | 0.52 ± 0.02 | 0.24 | 0.38 | 0.49 | 0.47 |
| OD600 nm ∼ CDW (g L−1) | 0.23 ± 0.02 | 0.28 ± 0.05 | 0.30 | 0.26 | 0.22 | 0.24 |
| CDWmax (g L−1) | 1.60 ± 0.09 | 2.25 ± 0.01 | 1.98 | 1.67 | 1.42 | 5.07 |
| cLac, max (g L−1) | 26.56 ± 1.61 | 24.43 ± 0.44 | 26.07 | 28.54 | 26.22 | 79.06 |
| YPS (gLac gSugar −1) | 1.28 ± 0.16 | 0.83 ± 0.02 | 1.18 | 1.04 | 1.02 | 0.89 |
| qP (gLac gCDW −1 L−1) | 4.58 ± 0.20 | 5.93 ± 0.16 | 3.33 | 3.68 | 5.14 | 4.18 |
| qS1 (gMaltotriose gCDW −1 L−1) | n.a. | −0.91 ± 0.01 | −0.56 | n.d. | n.d. | n.d. |
| qS2 (gMaltose gCDW −1 L−1) | n.a. | −2.99 ± 0.32 | −1.06 | n.d. | n.d. | n.d. |
| qS3 (gGlucose gCDW −1 L−1) | −2.79 ± 0.78 | −1.55 ± 0.14 | −0.54 | n.d. | n.d. | n.d. |
| P (gLac L−1 h−1) | 2.16 ± 0.18 | 2.13 ± 0.05 | 2.18 | 2.05 | 1.98 | 4.93 |
The reference fermentation with MRS medium (I) revealed a growth rate of 0.55 h−1, which was also mentioned in literature for bioreactor cultivations of L. delbrueckii NRRL B445 49. The yield was reported to be 0.8 gLac gSugar −1, using a glucose concentration of 120 g L−1 and achieving only incomplete consumption of the substrate in the medium 50. The lactate formation rate and glucose consumption rate were described as 0.72 gLac gCDW h−1 and 0.96 gGlucose gCDW h−1, respectively 50, and therefore were lower than determined in the present work. The maximum lactate concentration reported in literature varies widely, mainly due to different substrate concentrations. Using a sugar concentration of approximately 20 g L−1 in a complex medium with additional carbon sources, the final lactate concentration was reported to be in the range of 25–30 g L−1 51. This is comparable to the values measured in this study.
The second reference fermentation with BSG liquor and complete supplementation (II) according to MRS medium 26 showed a similar growth rate and final lactate concentration, comparable lactate production rate, and an increased final cell density by 41% compared to the fermentation with MRS medium (I). Growth rates using other natural substrates and the organism L. delbrueckii were reported to be 0.2–0.35 h−1 for rice bran 51 and 0.31 h−1 for casein whey permeate 52. Accordingly, the determined growth rate with BSG liquor was higher (µmax = 0.52 h−1). Glucose, maltose, maltotriose and malto oligomers were consumed in parallel and no distinct diauxic growth was observed. L. delbrueckii was reported to metabolize glucose, maltose as well as long‐chain α‐1,4‐glycosidic bond glucose oligomers, previously 53. Concentration of malto oligomers dropped slightly by ∼1.5 g L−1 in this experiment. Glucose, maltose and maltotriose were consumed completely. The process yield was decreased significantly by 35% compared to the experiment with MRS medium (I), which can be explained by a higher biomass formation. Both reference fermentations showed small deviations between the biological replicates. Thus, the replicability was assumed good.
The fermentation using the optimized supplementation (III) according to Section 3.2, resulted in a decreased growth rate by 54% and a decreased final cell density by 12%. The lower growth rate and cell density can be explained by lower nutrient availability, as no casein peptone and meat extract were applied. The yield was increased, compared to the fermentation with BSG liquor and complete supplementation (II), by 42%. This was caused by an incomplete usage of maltotriose as shown in Figure 4D. All substrate consumption and lactate production rates were reduced between 38 and 65% compared to the fermentation with complete supplementation (II), which was mainly due to the lower growth rate in this experiment. However, the final lactate concentration (26.07 g L−1) was comparable to the fermentation with complete supplementation (II) and significantly increased to non‐pH regulated cultures in shaking flasks (16.3 g L−1). Thus, the target value of lactate concentration was maintained at a constant level, even though the supplementation of nutrients was decreased.
If additional glucoamylase was added (IV) in a simultaneous saccharification and fermentation process (SSF), maltose, maltotriose and malto oligomers were degraded to monomeric glucose as shown in Figure 4C–F in <5 h. Maltotriose was degraded rapidly, so that it could not be detected at the beginning of the fermentation process. The higher availability of glucose significantly increased the growth rate by 58% and slightly increased the maximum lactate concentration from 26.07 to 28.54 g L−1 compared to the fermentation with optimized conditions but without glucoamylase (III). No effect was observed for maximum cell density. The concentration of malto oligomers decreased in total by ∼4.5 g L−1 and by ∼3 g L−1 neglecting the bacterial degradation of malto oligomers without glucoamylase supplementation. This might indicate, that additional sugar coming from enzymatic digestion caused the increase in lactate concentration by ∼2.5 g L−1, but a proof on statistical significance could not be made.
If yeast extract, produced from Brewers’ yeast, was applied instead of commercial yeast extract (V), no significant changes were observed compared to fermentation (IV) besides an increase in the growth rate by 29%. The slight decrease in maximum lactate concentration could be explained by slightly different nutrient supply of homemade yeast extract compared to commercial yeast extract. These findings are very important, as they enable the opportunity to incorporate not only the BSG liquor but also the yeast side stream of brewing into one bioprocess. This can contribute to a holistic usage of brewery residues.
Cell dry weight, lactate formation, and substrate consumption of the scale‐up fermentation are presented in Figure 5. Associated kinetic parameters are presented in Table 2. The maximum specific growth rate was comparable to the previous fermentation (V). Final cell dry weight and lactate concentration (79.06 g L−1) were increased, compared to fermentation (V), by 357 and 302%, respectively. For all optimization fermentations (I) to (V), the productivities, calculated for the exponential phase of 11 h, were in the range of 2 gLac L−1 h−1. The scale‐up fermentation (VI) showed an extended exponential growth phase. The productivity in the 15 h of growth was 4.93 gLac L−1 h−1 and therefore increased by ∼250% compared to all other fermentation scenarios. This enhancement means, that the developed process is not only feasible with higher sugar concentrations (20 g L−1 vs. 88 g L−1) but also with BSG liquor originated from different brewing recipes (Wheat bock vs. May bock).
Figure 5.
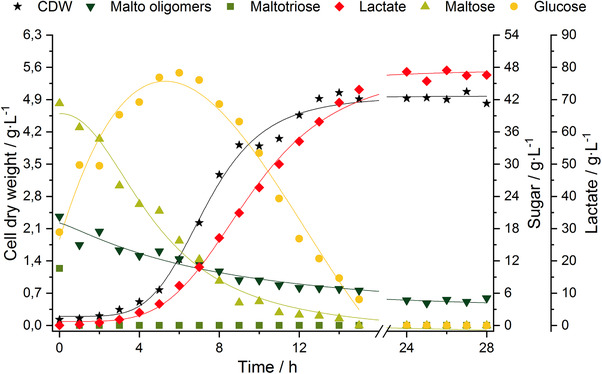
Scale‐up fermentation (VI) with the organism L. delbrueckii subsp. lactis and BSG liquor May bock at T = 45°C, N = 400 rpm, and pH 6. Cell dry weight (black stars), malto oligomers (inverted dark green triangles), maltotriose (medium green squares), maltose (light green upright triangles), glucose (yellow circles), and lactate concentration (red diamonds) during the fermentation. Glucose data points were fitted by polynomials of second degree; all other data points were fitted by sigmoidal fits; N, stirrer speed
4. CONCLUDING REMARKS
In existing BSG biorefineries, it is advantageous to remove the adherent, soluble components of BSG (called BSG liquor) before applying hydrothermal pretreatment methods. For the first time, a comprehensive analysis of the ingredients of BSG liquor was carried out. Due to the provision of carbon and nitrogen sources as well as trace elements, BSG liquor seems to be a promising substrate for biotechnology. Therefore, it was used as the basis to develop a fermentation medium for the cultivation of L. delbrueckii subsp. lactis. BSG liquor can be fermented in almost every plant, which is capable of running anaerobic submerse fermentations (this might even be the case for brewing vessels). Thus, the present infrastructure probably does not need to be significantly changed, when implementing the process into an existing biorefining approach. The fermentation converted nearly 100% of the sugar to lactate independent of the applied BSG liquor. However, the maximum achievable lactate concentration is limited by the available sugar concentration in the BSG liquor. On top of that, a potential use for the Brewers’ yeast side stream was illustrated. Yeast extract, produced from Brewers’ yeast, was incorporated into the fermentation process as the sole complex media constituent. This approach appears to be advantageous, if the process is placed in the context of a complete biorefinery, as it can contribute to a holistic usage of brewery residues.
In future work, it could be possible to improve the presented fermentation by further increasing the product concentration (e.g., by feeding glucose in a fed‐batch approach) or to expand the product range of fermentations with BSG liquor in general (e.g., by screening for other suitable microorganisms).
NOMENCLATURE
| BOD | (%) | biochemical oxygen demand |
| DSMZ | German Collection of Microorganisms and Cellcultures | |
| DoE | design of experiments | |
| g | (m s−2) | gravity acceleration |
| HMF | 5‐(hydroxymethyl)furfural | |
| LHW | liquid hot water | |
| p | (bar) | pressure |
| rpm | (min−1) | revolutions per minute |
| SSF | simultaneous saccharification and fermentation | |
| US$ | ($) | United States Dollar |
| vol% | volume percent | |
| wt% | weight percent | |
| YE | yeast extract | |
| Indices | ||
| CDWmax | (g L−1) | maximum cell dry weight |
| cLac, max | (g L−1) | maximum lactate concentration |
| gCDW | (g) | mass of cells |
| gGlucose | (g) | mass of glucose |
| gLac | (g) | mass of lactate |
| gMaltose | (g) | mass of maltose |
| gMaltotriose | (g) | mass of maltotriose |
| gSugar | (g) | mass of sugar |
| OD 600 nm | absorbance at a wavelength of 600 nm | |
| P | (gLac L−1 h−1) | productivity |
| qP | (gLac gCDW −1 h−1) | product (lactate) formation rate |
| qS1 | (gMaltotriose gCDW −1 h−1) | substrate 1 (maltotriose) uptake rate |
| qS2 | (gMaltose gCDW −1 h−1) | substrate 2 (maltose) uptake rate |
| qS3 | (gGlucose gCDW −1 h−1) | substrate 3 (glucose) uptake rate |
| R2 | (%) | coefficient of determination |
| R2 adjust | (%) | adjusted coefficient of determination |
| YPS | (gLac gSugar −1) | process yield |
| µmax | (h−1) | maximum specific growth rate |
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
This research was performed as a part of the European Union project BIOVAL, which is funded by the European Regional Development Fund EFRE‐interreg (018‐4‐09‐021). We wish to express our gratitude for financial support. Special thanks go to the Novozymes A/S for suppling the glucoamylase mixture Attenuzyme® Core and Privatbrauerei Bischoff GmbH & Co. KG for suppling Brewers’ spent grain.
Akermann A, Weiermüller J, Christmann J, et al. Brewers’ spent grain liquor as a feedstock for lactate production with Lactobacillus delbrueckii subsp. lactis . Eng Life Sci. 2020;20:168–180. 10.1002/elsc.201900143
REFERENCES
- 1. Bart, S. J. , Barth, R. , Barth, A. W. , Der Barth‐Bericht. Hopfen 2018/2019. [Google Scholar]
- 2. Buffington, J. , The economic potential of brewer's spent grain (BSG) as a biomass feedstock. Adv. Chem. Eng. Adn Sci. 2014, 4, 308–318. [Google Scholar]
- 3. Mussatto, S. I. , Brewer's spent grain: a valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [DOI] [PubMed] [Google Scholar]
- 4. Wilkinson, S. , Smart, K. A. , James, S. , Cook, D. J. , Bioethanol production from brewers spent grains using a fungal consolidated bioprocessing (CBP) approach. Bioenergy Res. 2017, 10, 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lynch, K. M. , Steffen, E. J. , Arendt, E. K. , Brewers’ spent grain: a review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar]
- 6. Taherzadeh, M. J. , Karimi, K. , Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mussatto, S. I. , Fernandes, M. , Dragone, G. , Mancilha, I. M. , et al., Brewer's spent grain as raw material for lactic acid production by Lactobacillus delbrueckii . Biotechnol. Lett. 2007, 29, 1973–1976. [DOI] [PubMed] [Google Scholar]
- 8. Mussatto, S. I. , Fernandes, M. , Mancilha, I. M. , Roberto, I. C. , Effects of medium supplementation and pH control on lactic acid production from brewer's spent grain. Biochem. Eng. J. 2008, 40, 437–444. [Google Scholar]
- 9. Rojas‐Chamorro, J. A. , Cara, C. , Romero, I. , Ruiz, E. , et al., Ethanol production from brewers’ spent grain pretreated by dilute phosphoric acid. Energy and Fuels 2018, 32, 5226–5233. [Google Scholar]
- 10. Xiros, C. , Topakas, E. , Katapodis, P. , Christakopoulos, P. , Evaluation of fusarium oxysporum as an enzyme factory for the hydrolysis of brewer's spent grain with improved biodegradability for ethanol production. Ind. Crops Prod. 2008, 28, 213–224. [Google Scholar]
- 11. Xiros, C. , Christakopoulos, P. , Enhanced ethanol production from brewer's spent grain by a Fusarium oxysporum consolidated system. Biotechnol. Biofuels 2009, 2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xiros, C. , Katapodis, P. , Christakopoulos, P. , Factors affecting cellulose and hemicellulose hydrolysis of alkali treated brewers spent grain by Fusarium oxysporum enzyme extract. Bioresour. Technol. 2011, 102, 1688–1696. [DOI] [PubMed] [Google Scholar]
- 13. Michelin, M. , Teixeira, J. A. , Liquid hot water pretreatment of multi feedstocks and enzymatic hydrolysis of solids obtained thereof. Bioresour. Technol. 2016, 216, 862–869. [DOI] [PubMed] [Google Scholar]
- 14. Pierre, G. , Sannier, F. , Goude, R. , Nouviaire, A. , et al., Evaluation of thermomechanical pretreatment for enzymatic hydrolysis of pure microcrystalline cellulose and cellulose from Brewers’ spent grain. J. Cereal Sci. 2011, 54, 305–310. [Google Scholar]
- 15. Carvalheiro, F. , Garrote, G. , Parajó, J. C. , Pereira, H. , et al., Kinetic modeling of brewer's spent grain autohydrolysis. Biotechnol. Prog. 2005, 21, 233–243. [DOI] [PubMed] [Google Scholar]
- 16. Carvalheiro, F. , Duarte, L. C. , Lopes, S. , Parajó, J. C. , et al., Evaluation of the detoxification of brewery's spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar]
- 17. Hang, Y. D. , Splittstoesser, D. F. , Woodams, E. E. , Utilization of brewery spent grain liquor by Aspergillus niger . Appl. Microbiol. 1975, 30, 879–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shannon, L. J. , Stevenson, K. E. , Growth of Calvatia gigantea and Candida steatolytica in brewery wastes for microbial protein production and bod reduction. J. Food Sci. 1975, 40, 830–832. [Google Scholar]
- 19. Shannon, L. J. , Stevenson, K. E. , Growth of fungi and BOD reduction in selected brewery wastes. J. Food Sci. 1975, 40, 826–829. [Google Scholar]
- 20. Roberts, R. T. , Use of an extract of spent grains as an antifoaming agent in fermentors. J. Inst. Brew. 1976, 82, 96–96. [Google Scholar]
- 21. Roukas, T. , Kotzekidou, P. , Production of citric acid from brewery wastes by surface fermentation using Aspergillus niger . J. Food Sci. 1986, 51, 225–228. [Google Scholar]
- 22. Roukas, T. , Pullulan production from brewery wastes by Aureobasidium pullulans . World J. Microbiol. Biotechnol. 1999, 15, 447–450. [DOI] [PubMed] [Google Scholar]
- 23. Płaza, G. A. , Pacwa‐Płociniczak, M. , Piotrowska‐Seget, Z. , Jangid, K. , et al., Agroindustrial wastes as unconventional substrates for growing of Bacillus strains and production of biosurfactant. Environ. Prot. Eng. 2011, 37, 63–71. [Google Scholar]
- 24. Schneider, T. , Graeff‐Hönninger, S. , French, W. T. , Hernandez, R. , et al., Lipid and carotenoid production by oleaginous red yeast Rhodotorula glutinis cultivated on brewery effluents. Energy 2013, 61, 34–43. [Google Scholar]
- 25. Tanguler, H. , Erten, H. , Utilisation of spent brewer's yeast for yeast extract production by autolysis: the effect of temperature. Food Bioprod. Process. 2008, 86, 317–321. [Google Scholar]
- 26. De Man, J. C. , Rogosa, M. , Sharpe, M. E. , A medium used for the cultivation of Lactobacilli . J Appl Bacteriol 1960, 23, 130–135. [Google Scholar]
- 27. Hein, J. , Hippeli, J. , Eibert, T. F. , Efficient EMC parameter analysis for the verification of complex automotive simulation models by the utilization of design of experiments. IEEE Trans. Electromagn. Compat. 2018, 60, 1965–1973. [Google Scholar]
- 28. Herrera‐Saldana, R. E. , Huber, J. , Poore, M. H. , Dry matter, crude protein, and starch degradability of five cereal grains. J. Dairy Sci. 1990, 73, 2386–2393. [Google Scholar]
- 29. Ragaee, S. , Abdel‐Aal, E. S. M. , Noaman, M. , Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar]
- 30. Mussatto, S. I. , Roberto, I. C. , Chemical characterization and liberation of pentose sugars from brewer's spent grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar]
- 31. Narziß, L. , Back, W. , Die Bierbrauerei, Band 2: Technologie der Würzezubereitung, 8th ed., Wiley‐VCH, Weinheim: 2009. [Google Scholar]
- 32. Xiros, C. , Christakopoulos, P. , Biotechnological potential of brewers spent grain and its recent applications. Waste Biomass Valor. 2012, 3, 213–232. [Google Scholar]
- 33. Schwill‐Miedaner, A. , Verfahrenstechnik im Brauprozess (BRAUWELT Wissen), Fachverlag Hans Carl, Einbeck 2011. [Google Scholar]
- 34. Coelho, L. F. , De Lima, C. J. B. , Rodovalho, C. M. , Bernardo, M. P. , et al., Lactic acid production by new Lactobacillus plantarum LMISM6 grown in molasses: optimization of medium composition. Brazilian J. Chem. Eng. 2011, 28, 27–36. [Google Scholar]
- 35. Partanen, L. , Marttinen, N. , Alatossava, T. , Fats and fatty acids as growth factors for Lactobacillus delbrueckii . Syst. Appl. Microbiol. 2001, 506, 500–506. [DOI] [PubMed] [Google Scholar]
- 36. Qi, B. K. , Yao, R. S. , Lai, M. , Deng, S. S. , Effect of Tween 80 on production of lactic acid by Lactobacillus casei . Songklanakarin J. Sci. Technol. 2009, 31, 85–89. [Google Scholar]
- 37. Yu, L. , Lei, T. , Ren, X. , Pei, X. , et al., Response surface optimization of L‐(+)‐lactic acid production using corn steep liquor as an alternative nitrogen source by Lactobacillus rhamnosus CGMCC 1466. Biochem. Eng. J. 2008, 39, 496–502. [Google Scholar]
- 38. Stiles, J. , Penkar, S. , Plockova, M. , Chumchalova, J. , et al., Antifungal activity of sodium acetate and Lactobacillus rhamnosus . J. Food Prot. 2002, 65, 1188–1191. [DOI] [PubMed] [Google Scholar]
- 39. Hébert, E. M. , Raya, R. R. , De Giori, G. S. , Nutritional requirements of Lactobacillus delbrueckii subsp. lactis in a chemically defined medium. Curr. Microbiol. 2004, 49, 341–345. [DOI] [PubMed] [Google Scholar]
- 40. Iino, T. , Uchimura, T. , Komagata, K. , The effect of sodium acetate on the growth yield , the production of L ‐ and D ‐lactic acid , and the activity of some enzymes of the glycolytic pathway of Lactobacillus sakei NRIC 1071 T and Lactobacillus plantarum NRIC 1067 T. J. Gen. Appl. Microbiol. 2002, 102, 91–102. [DOI] [PubMed] [Google Scholar]
- 41. Bender, R. , Ziegler, A. , Lange, S. , Multiple regression. Dtsch. Medizinische Wochenschrift 2007, 132(Suppl), e30‐32. [DOI] [PubMed] [Google Scholar]
- 42. Mussatto, S. I. , Dragone, G. , Roberto, I. C. , Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar]
- 43. Ravindran, R. , Jaiswal, S. , Abu‐Ghannam, N. , Jaiswal, A. K. , A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2017. [DOI] [PubMed] [Google Scholar]
- 44. Li, C. , Zhang, Z. , Zhao, Z. K. , Direct conversion of glucose and cellulose to 5‐hydroxymethylfurfural in ionic liquid under microwave irradiation. Tetrahedron Lett. 2009, 50, 5403–5405. [Google Scholar]
- 45. Akermann, A. , Weiermüller, J. , Ulber, R. , Development of a biorefinery concept for brewers’ spent grain with a preliminary pressing step. Chemie Ing. Tech. 2019, cite.201900017. [Google Scholar]
- 46. Bajwa, D. S. , Peterson, T. , Sharma, N. , Shojaeiarani, J. , et al., A review of densified solid biomass for energy production. Renew. Sustain. Energy Rev. 2018, 96, 296–305. [Google Scholar]
- 47. El‐Shafey, E. I. , Gameiro, M. L. F. , Correia, P. F. M. , de Carvalho, J. M. R. , Dewatering of brewer's spent grain using a membrane filter press: a pilot plant study. Sep. Sci. Technol. 2004, 39, 3237–3261. [Google Scholar]
- 48. Zheng, J. , Rehmann, L. , Extrusion pretreatment of lignocellulosic biomass: a review. Int. J. Mol. Sci. 2014, 15, 18967–18984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gonçalves, L. M. D. , Xavier, A. M. R. B. , Almeida, J. S. , Carrondo, M. J. T. , Concomitant substrate and product inhibition kinetics in lactic acid production. Enzyme Microb. Technol. 1991, 13, 314–319. [Google Scholar]
- 50. Demirci, A. , Pometto, A. L. , Enhanced production of d(‐)‐lactic acid by mutants of Lactobacillus delbrueckii ATCC 9649. J. Ind. Microbiol. 1992, 11, 23–28. [Google Scholar]
- 51. Tanaka, T. , Hoshina, M. , Tanabe, S. , Sakai, K. , et al., Production of d‐lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour. Technol. 2006, 97, 211–217. [DOI] [PubMed] [Google Scholar]
- 52. Prasad, S. , Srikanth, K. , Limaye, A. M. , Sivaprakasam, S. , Homo‐fermentative production of d‐lactic acid by Lactobacillus sp. employing casein whey permeate as a raw feed‐stock. Biotechnol. Lett. 2014, 36, 1303–1307. [DOI] [PubMed] [Google Scholar]
- 53. Carr, J. G. , Cutting, C. V. , Whiting, G. C. , Lactic acid bacteria in beverages and food: proceedings of a symposium held at Long Ashton Research Station, University of Bristol. J. Genereal Appl. Microbilogy 1975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


