Abstract
EFSA was asked to deliver a scientific opinion on the risks to public health related to the presence of aflatoxins in food. The risk assessment was confined to aflatoxin B1 (AFB1), AFB2, AFG1, AFG2 and AFM1. More than 200,000 analytical results on the occurrence of aflatoxins were used in the evaluation. Grains and grain‐based products made the largest contribution to the mean chronic dietary exposure to AFB1 in all age classes, while ‘liquid milk’ and ‘fermented milk products’ were the main contributors to the AFM1 mean exposure. Aflatoxins are genotoxic and AFB1 can cause hepatocellular carcinomas (HCCs) in humans. The CONTAM Panel selected a benchmark dose lower confidence limit (BMDL) for a benchmark response of 10% of 0.4 μg/kg body weight (bw) per day for the incidence of HCC in male rats following AFB1 exposure to be used in a margin of exposure (MOE) approach. The calculation of a BMDL from the human data was not appropriate; instead, the cancer potencies estimated by the Joint FAO/WHO Expert Committee on Food Additives in 2016 were used. For AFM1, a potency factor of 0.1 relative to AFB1 was used. For AFG1, AFB2 and AFG2, the in vivo data are not sufficient to derive potency factors and equal potency to AFB1 was assumed as in previous assessments. MOE values for AFB1 exposure ranged from 5,000 to 29 and for AFM1 from 100,000 to 508. The calculated MOEs are below 10,000 for AFB1 and also for AFM1 where some surveys, particularly for the younger age groups, have an MOE below 10,000. This raises a health concern. The estimated cancer risks in humans following exposure to AFB1 and AFM1 are in‐line with the conclusion drawn from the MOEs. The conclusions also apply to the combined exposure to all five aflatoxins.
Keywords: aflatoxin, liver, cancer, occurrence, exposure, food, margin of exposure (MOE)
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1798/full
Summary
Following a request from the European Commission, the Panel on Contaminants in the Food Chain (CONTAM Panel) has provided a scientific opinion on the human health risks related to the presence of aflatoxins in food. The opinion evaluates the toxicity of aflatoxins to humans, estimates the dietary exposure of the European Union (EU) population to aflatoxins and assesses the human health risks to the EU population due to the estimated dietary exposure. Aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2) and aflatoxin M1 (AFM1) are considered in the risk assessment. Aflatoxin total typically refers to the sum of AFB1, AFB2, AFG1 and AFG2. The risk assessment carried out by the CONTAM Panel of EFSA in 2007 was used as a starting point.
AFB1, AFB2, AFG1 and AFG2 are mycotoxins produced primarily by toxigenic strains of the fungi Aspergillus flavus and Aspergillus parasiticus. In addition to the above‐mentioned four aflatoxins, these fungi also form other substances such as aflatoxicol and sterigmatocystin. The most frequently found aflatoxin in contaminated food samples is AFB1 and the three others are generally not found in the absence of AFB1. Aflatoxin‐producing fungi are found in areas with a hot, humid climate and aflatoxins in food are a result of both pre‐ and post‐harvest fungal contamination. Climate change is anticipated to impact on the presence of aflatoxins in food in Europe. AFM1 is the hydroxylated metabolite of AFB1 and is found in milk and dairy products obtained from livestock that have ingested contaminated feed, and also in human milk.
AFB1 is absorbed in the small intestine and distributed to the liver where it undergoes first pass metabolism. The metabolism of AFB1 in humans and laboratory animals has been well‐characterised with CYP1A2, 2B6, 3A4, 3A5, 3A7, 2A13 and GSTM1 all catalysing aflatoxin metabolism in humans. AFB1, AFG1 and AFM1 are converted to their respective epoxides, which can bind covalently to both DNA and proteins. AFB2 and AFG2 cannot form the 8,9‐epoxide. AFB1 and its metabolites are both excreted via the faecal and the urinary route. The percentage excreted via both routes varies according to the species. AFM1 is also excreted in milk. A limited amount of new information has become available regarding the toxicokinetics of AFB1 in humans since the previous assessment by the CONTAM Panel in 2007. The new data on humans show that absorption of AFB1 and/or its metabolites into the systemic circulation is rapid and high.
In short‐term studies (7–90 days), AFB1 had multiple negative effects on rodents including inhibition of normal growth, liver and kidney damage, as well as sustained alterations in the intestinal microbiota. For AFG1, AFG2, AFB2 or AFM1, no new short‐term toxicity or gut microbiota studies were identified. AFB1 affects reproductive and developmental parameters and aflatoxins, especially AFB1, can produce an immunotoxic effect in rodents. The no‐observed‐adverse‐effect‐levels (NOAELs) for these effects were around 30 μg/kg body weight (bw) per day.
AFB1 is a genotoxic and carcinogenic substance. CYP3A and CYP1A2 activity is important for AFB1 genotoxicity. Upon epoxidation, DNA adducts such as AFB1‐N7‐gua and AFB1‐FAPY are formed and can lead to G‐to‐T transversions. In addition to DNA adduct formation, a broad spectrum of cellular effects has been reported in response to AFB1 exposure. In humans living in areas where hepatitis B virus (HBV) infection and AFB1 exposure are prevalent, hepatocellular carcinoma (HCC) samples show a mutational hotspot (G‐to‐T transversion) at codon 249 of the TP53 gene, which is considered to be a signature mutation for aflatoxin‐induced HCC.
There is evidence for genotoxic effects of AFB1 in pregnant mice, fetuses and young animals. Pregnancy appears to enhance the sensitivity to the genotoxicity of AFB1 for the mothers, possibly due to elevated levels of CYP1A2 and CYP3A enzymes. A study with in utero exposure showed a greater mutational impact of the lesions in the fetus. Early postnatal exposure resulted in higher adduct levels in the liver compared to adult animals.
Besides DNA adduct formation, AFB1 induces oxidative stress including modulation of antioxidant defence systems. Considering the potential sequence of events towards HCC, oxidative stress might compromise critical AFB1 detoxification pathways (e.g. glutathione (GSH) conjugation) and/or induce additional DNA lesions.
In contrast to AFB1, fewer studies are available regarding the genotoxicity of the other aflatoxins. When comparing the genotoxicity of the different aflatoxins, most studies have indicated that AFB1 is the most genotoxic compound. AFG1 is slightly less genotoxic than AFB1; AFB2 and AFG2 are less genotoxic than AFB1. It is not possible, based on these data, to make a quantitative comparison of the genotoxic potency of these compounds. The genotoxic potency can be summarised as AFB1 > AFG1 ≈ aflatoxicol » AFM1 based on the γH2AX in‐cell western technique in cultured human liver cells, while AFB2 and AFG2 showed no effects.
AFB1, AFG1 and AFM1 are carcinogenic when delivered orally via the diet or by gavage. There is limited evidence for the carcinogenicity of AFB2 and inadequate evidence for carcinogenicity of AFG2. AFB1 is more potent than AFG1 with respect to liver carcinogenicity but AFG1 induced a higher incidence of kidney tumours than AFB1. AFB1 is also more potent than AFM1 with respect to liver carcinogenicity by approximately 10‐fold.
AF‐alb (AFB1‐lys), urinary AF‐N7‐gua and urinary AFM1 are all biomarkers of exposure that have been validated against dietary intake of aflatoxin. However, the levels of these biomarkers cannot be converted reliably into dietary exposures in individuals. As AF‐alb (AFB1‐lys) better reflects longer‐term exposure (i.e. several weeks), it tends to be most widely used, while urinary AFM1 and AF‐N7‐gua are suitable biomarkers for recent exposure.
The epidemiological studies reported since 2006 have added to the weight of evidence that aflatoxin exposure is associated with a risk of developing HCC, with a higher risk for people infected with either HBV or hepatitis C virus (HCV). Data suggest that HBV infection of the liver alters the expression of the genes coding for the enzymes, which metabolise/detoxify aflatoxins such as an induction of CYP enzymes or decrease in glutathione S‐transferase (GST) activity. This may provide one mechanistic basis for the higher risk of liver cancer among HBV‐infected individuals exposed to aflatoxins.
Child health is an emerging area of interest for the field of aflatoxin‐related health outcomes but not yet suitable for use in risk assessment. Child growth has been assessed in a growing body of evidence outside European populations but with limited replicability in the observed associations. The evidence related to the remaining child health outcomes is sparse, heterogeneous and with methodological limitations.
The CONTAM Panel considers that liver carcinogenicity of aflatoxins remains the pivotal effect for the risk assessment. In view of the genotoxic properties of aflatoxins, the CONTAM Panel considered that it was not appropriate to establish a tolerable daily intake. Based on studies in animals, the CONTAM Panel selected a BMDL10 of 0.4 μg/kg bw per day for the incidence of HCC in male rats following AFB1 exposure to be used in a margin of exposure (MOE) approach. The calculation of a BMDL from the human data was not appropriate; instead, the cancer potencies estimated by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 2016 were used.
Differences in carcinogenic potency are reported for the different aflatoxins. For AFM1, JECFA concluded, based on a study in Fischer rats, that AFM1 induces liver cancer with a potency one‐tenth that of AFB1. No new evidence has become available that necessitates a change to this conclusion and a potency factor of 0.1 was used in this assessment for AFM1. For the other aflatoxins, the available in vivo data are not sufficient to derive potency factors. In the absence of such potency factors, the CONTAM Panel applied equal potency factors for AFB1, AFB2, AFG1 and AFG2 as used in previous assessments.
Chronic dietary exposure to AFB1, AFM1 and AFT (the sum of AFB1, AFB2, AFG1 and AFG2) +AFM1 was estimated using a data set comprising 209,802 analytical results from 69,166 samples. The highest AFB1 and AFT mean concentrations were obtained for the food category ‘legumes, nuts and oilseeds’ (in particular for pistachios, peanuts and ‘other seeds’). As expected, the highest AFM1 mean concentrations were reported for ‘milk and dairy products’ and milk‐based foods belonging to the food category ‘food for infants and small children’. For adults, the mean lower bound (LB) exposure to AFB1 ranged from 0.22 to 0.49 ng/kg bw per day and the mean UB exposure from 1.35 to 3.25 ng/kg bw per day. For the younger age groups, the mean LB exposure to AFB1 ranged from 0.08 to 1.78 ng/kg per day and the mean upper bound (UB) exposure from 0.58 to 6.95 ng/kg per day. The LB P95 exposure to AFB1 ranged from 0.62 to 1.36 ng/kg bw per day for adults and from 0.35 to 6.22 ng/kg bw per day for the younger age groups. The UB P95 exposure to AFB1 ranged from 2.76 to 6.78 ng/kg bw per day and from 2.79 to 14.01 ng/kg bw per day, respectively. The highest estimated exposure to AFM1 was in infants with a mean exposure of 1.6/2.0 ng/kg bw per day (LB/UB) and a P95 exposure of 6.2/7.9 ng/kg bw per day. Overall, ‘grains and grain‐based products’ made the largest contribution to the LB mean chronic dietary exposure to AFB1 in all age classes. The main subcategories driving the contribution of this food category were ‘grains for human consumption’ (in particular corn grain), ‘bread and rolls’ and ‘fine bakery wares’. The food categories ‘liquid milk’ and ‘fermented milk products’ were the main contributors to the overall AFM1 mean exposure throughout all age groups.
Based on a BMDL10 of 0.4 μg/kg bw per day for the induction of HCC by AFB1 in male rats, MOE values (minimum to maximum) range from 5,000 to 225 for the mean LB exposure to AFB1 and from 690 to 58 for the mean UB exposure to AFB1 across dietary surveys and age groups. The MOE values range from 1,143 to 64 for the P95 LB exposure to AFB1 and from 145 to 29 for the P95 UB exposure to AFB1 across dietary surveys and age groups. The calculated MOEs are below 10,000, which raises a health concern. For AFM1, based on the BMDL10 of 0.4 μg/kg bw per day derived for AFB1 and a potency factor of 0.1, MOE values that range from 100,000 to 2,564 for the mean LB exposure estimates, from 66,667 to 2,020 for the mean UB exposure estimates, from 33,333 to 642 for the P95 LB exposure estimates, and from 25,000 to 508 for the P95 UB exposure estimates across dietary surveys and age groups have been calculated. The CONTAM Panel noted that the calculated MOEs are less than 10,000 for some surveys, particularly for the younger age groups, which raises a health concern. The estimated cancer risks in humans following exposure to AFB1 are in‐line with the conclusion drawn from the animal data. This conclusion also applies to AFM1 and AFT + AFM1.
The CONTAM Panel recommends that data that would allow the derivation of potency factors are generated. Research designed to quantify the relationship between biomarker levels and dietary intake at the individual level, integrating exposure over time with biomarker levels, is recommended. Such study would be performed in populations with an indigenous dietary exposure to aflatoxin resulting in measurable biomarker levels. More data are needed regarding the occurrence of aflatoxicol and aflatoxin M2 (AFM2), to clarify whether these substances should be included in the risk assessment. There is a need to continue to monitor aflatoxin occurrence in the light of potential increases due to climate change using methods with high levels of sensitivity for detection.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
BACKGROUND
In the Codex Alimentarius and, more specifically, in the Codex Committee on Contaminants in Food (CCCF), discussions on maximum levels (MLs) and an associated sampling plan for aflatoxins in different foodstuffs are ongoing.
At the 12th session of the CCCF in March 2018 (CCCF, 2018), discussions on MLs for aflatoxin total (AFT) in ready‐to‐eat peanuts (§103 – §115 of the report) and spices (§116 – §119 of the report) were held but were suspended because of divergent views. The EU could not agree on the discussed MLs for AFT in ready‐to‐eat peanuts (European Commission, 2018a), taking into account the outcome of the EFSA risk assessment (EFSA CONTAM Panel, 2018), nor could it agree on the MLs discussed for certain spices (European Commission, 2018b). New work was agreed at the 12th session of the CCCF on setting MLs for aflatoxins in cereals and cereal‐based food, including food for infants and young children.
In view of the future discussions at the CCCF on MLs for aflatoxins in food and taking into account the recommendations in the last above‐mentioned Opinion of EFSA on the effect on public health of a possible increase of the ML for AFT in peanuts (EFSA CONTAM Panel, 2018), it is necessary that EFSA performs a comprehensive risk assessment related to the presence of aflatoxins in food.
TERMS OF REFERENCE
In accordance with Article 29 (1) of Regulation (EC) No 178/20021, the European Commission asks the European Food Safety Authority for a Scientific Opinion on the human health risks related to the presence of aflatoxins in food.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) concluded that this Opinion should comprise the:
evaluation of the toxicity of aflatoxins for humans, considering all relevant toxicological endpoints;
estimation of the dietary exposure of the EU population to aflatoxins from food, including the consumption patterns of specific groups of the population;
assessment of the human health risks to the EU population, including specific (vulnerable) groups of the population, as a consequence of the estimated dietary exposure.
This risk assessment is confined to aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2) and aflatoxin M1 (AFM1). The inclusion of aflatoxin M2 (AFM2) in the risk assessment was not possible due to the limited data available. Occurrence data for food of animal origin were included in the assessment. However, the transfer from feed into food of animal origin was not within the scope of the assessment. Although aflatoxin‐producing fungi produce other mycotoxins such as aflatoxicol, versicolorin and sterigmatocystin, these mycotoxins are not the subject of the present assessment. This Scientific Opinion is an update of the Scientific Opinion on the potential increase of consumer health risk by a possible increase of the existing MLs for aflatoxins in almonds, hazelnuts and pistachios and derived products adopted by the CONTAM Panel of EFSA in January 2007 (EFSA, 2007a). Therefore, papers published from 2006 onwards were taken into account for the current risk assessment when not yet included in the previous opinion.
1.3. Supporting information for the assessment
This section is an adapted and amended version of the corresponding section in the recently published statement of the CONTAM Panel (2018).
Aflatoxins are bisfuranocoumarin compounds produced primarily by toxigenic strains of the fungi Aspergillus flavus and Aspergillus parasiticus. A. parasiticus produces AFB1, AFB2, AFG1 and AFG2, whereas A. flavus mainly produces AFB1 and AFB2. A. flavus favours the aerial parts of the plants (e.g. leaves and flowers) while A. parasiticus is more adapted to a soil environment and is of more limited distribution than A. flavus (EFSA, 2007a). Many other species closely related to A. flavus (A. minisclerotigenes, A. korhogoensis, A. aflatoxiformans and A. texensis) or to A. parasiticus (A. novoparasiticus and A. arachidicola) also produce aflatoxins B and G (Pildain et al., 2008; Adjovi et al., 2014; Carvajal‐Campos et al., 2017; Singh et al., 2018; Frisvad et al., 2019). In addition to the above‐mentioned four aflatoxins, these fungi also form other substances such as aflatoxicol, versicolorin and sterigmatocystin (Yu, 2012s).
When concentrations or maximum limits mention ‘total’, it typically refers to the sum of AFB1, AFB2, AFG1 and AFG2. The most frequently found aflatoxin in contaminated food samples is AFB1 and the three others are generally not reported in the absence of AFB1 (FAO/WHO, 2018).
The aflatoxin‐producing fungi are found especially in areas with a hot, humid climate and aflatoxins are found in food as a result of both pre‐ and post‐harvest fungal contamination. The rate and degree of contamination depends on temperature, humidity, soil and storage conditions (EFSA, 2007a). Climate change is expected to have an impact on the presence of AFB1 in maize in Europe. Battilani et al. (2016) used a modelling approach to predict aflatoxin contamination in maize under increasing temperatures and showed that a +2°C climate change scenario would increase the probability of aflatoxin contamination from low to medium in European countries in which maize cultivation is common (e.g. France, Italy and Romania). This is in line with the reports of an outbreak of A. flavus in maize in 2012 caused by high temperature and drought in Serbia (Lević et al., 2013) and increased levels of AFM1 in milk due to high levels of AFB1 in maize in northern Italy in 2003 (Piva et al., 2006; Battilani et al., 2008). The year 2003 had a hot and dry summer; with mean temperatures in the period June–August that were about 2.5°C higher than the previous and following year.
AFM1 and AFM2 are the hydroxylated metabolites of AFB1 and AFB2 and are found in milk and dairy products obtained from livestock that have ingested contaminated feed. AFM1 occurrence is also reported in human milk (e.g. Kunter et al., 2017; Radonić et al., 2017; Bogalho et al., 2018; Valitutti et al., 2018).
1.3.1. Chemistry
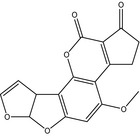
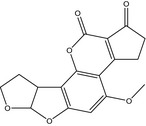
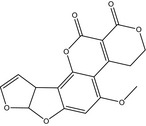
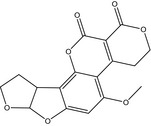
The structures of AFB1, AFB2, AFG1, AFG2, AFM1 and AFM2 are shown in Table 1. Aflatoxins are colourless to pale yellow crystals and they fluoresce in ultraviolet (UV) light: blue for AFB1 and AFB2, green for AFG1 and AFG2 and blue violet for AFM1 (IARC, 2012). They are unstable in UV light in the presence of oxygen, extreme pH (< 3 or > 10) and in the presence of oxidising agents (IARC, 2012). Under alkaline conditions the lactone ring opens; however, the reaction is reversible. The lactone ring also opens and results in decarboxylation when treated with ammonia at high temperatures and high pressure (IARC, 2012). Aflatoxins are insoluble in non‐polar solvents while they are freely soluble in moderately polar organic solvents as chloroform and methanol. The solubility in water is 10–20 mg/L (IARC, 2012).
Table 1.
Chemical structures, CAS number, molecular formula and molecular weight of aflatoxins B1, B2, G1, G2, M1 and M2
| Name | Aflatoxin B1 (AFB1) | Aflatoxin B2 (AFB2) | Aflatoxin G1 (AFG1) |
|---|---|---|---|
| Structure |
|
|
|
| CAS number | 1162‐65‐8 | 7220‐81‐7 | 1165‐39‐5 |
| Molecular formula | C17H12O6 | C17H14O6 | C17H12O7 |
| Molecular weight | 312.3 g/mol | 314.3 g/mol | 328.3 g/mol |
| Log Pa | 1.23 | 1.45 | 0.5 |
| Name | Aflatoxin G2 (AFG2) | Aflatoxin M1 (AFM1) | Aflatoxin M2 (AFM2) |
| Structure |
|
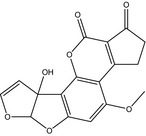
|
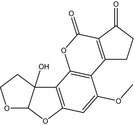
|
| CAS number | 7241‐98‐7 | 6795‐23‐9 | 6885‐57‐0 |
| Molecular formula | C17H14O7 | C17H12O7 | C17H14O7 |
| Molecular weight | 330.3 g/mol | 328.3 g/mol | 330.3 g/mol |
| Log P | 0.71 | 1.21 | 1.16 |
The predicted Log P values for AFB1, AFB2, AFG1 and AFG2 were extracted from the Hazardous Substances Data Bank (HSDB), a database of the National Library of Medicine's TOXNET system (http://toxnet.nlm.nih.gov) on 28 August, 2019. The predicted Log P value for AFM1 and AFM2 were extracted from the Metabolomics Innovation Centre (https://www.metabolomicscentre.ca) and had been calculated with ALOGPS (http://www.vcclab.org/lab/alogps/).
1.3.2. Analytical methods
A wide range of methods have been used for the analysis of aflatoxins (Wacoo et al., 2014; Gacem and Ould El Hadj‐Khelil, 2016; Shephard, 2016; Danesh et al., 2018; FAO/WHO, 2018). The text below describes examples of commonly used analytical methods and does not aim to be exhaustive. Methods using older analytical techniques such as thin‐layer chromatography are not included in this section.
For the analysis of AFB1, AFB2, AFG1 and AFG2, the most widely applied methods for quantitative analysis are liquid chromatography (LC) combined with fluorescence detection (FD) or mass spectrometry (MS) (EFSA, 2007a; FAO/WHO, 2018).
For analysis using LC‐FD, samples are typically extracted with methanol or mixtures of methanol and water or hexane. The latter is used in the case of oil samples. Samples may be cleaned using an immunoassay column specific for aflatoxins before separation with LC, post‐column derivatisation and quantification by FD. Limits of detection (LODs) and limits of quantification (LOQs) are typically reported to be in the range of 0.001–0.20 μg/kg, depending on the matrix and the aflatoxin.
Mass spectrometry determination of aflatoxins has the advantage that no post‐column derivatisation is needed. Aflatoxins are typically extracted with acetonitrile, sometimes in mixtures with water, formic acid, or hexane before analysis by liquid chromatography coupled to mass spectrometry (LC–MS) or liquid chromatography coupled to tandem mass spectrometry (LC–MS/MS). The use of LC–MS or LC–MS/MS also had a great impact on the development of multi‐mycotoxin methods and several papers describe the simultaneous determination of several mycotoxins (e.g. García‐Moraleja et al., 2015; Saladino et al., 2017; Škrbić et al., 2012, 2017; Cunha et al., 2018). According to Shephard (2016), methods have been developed that determine over 100 mycotoxins in a single analysis. Limits of quantification are typically reported to be in the range of 0.007–3 μg/kg, generally with the highest LOQs for the multi‐mycotoxin methods. The MS techniques have also been used to determine mycotoxins together with pesticides, plant toxins, veterinary drugs, and cyanogenic glycosides (Shephard, 2016). However, the inclusion of a high number of substances in an analytical method may lead to a reduced sensitivity.
For the determination of AFM1, the most common analytical method described in the literature is enzyme‐linked immunosorbent assay (ELISA). Commercially available kits specific for AFM1 in milk typically have an LOD of about 0.005 μg/L. LC–MS and LC‐FD methods are also used for the determination AFM1 (e.g. Gomez‐Arranz and Navarro‐Blasco, 2010; Škrbić et al., 2014). The reported LOQs for AFM1 are typically between 0.0007 and 0.014 μg/kg.
ELISA kits are also commercially available for the determination of aflatoxin total (AFT) and AFB1. Other immunochemical‐based methods have been developed for the analysis of aflatoxins and the advantages and disadvantages of the different methods are discussed by Matabaro et al. (2017).
Proficiency testing in different matrices and certified reference materials are available. Standard methods (EN‐methods) also exist for the determination of aflatoxins. As described in the legislation (see Section 1.3.4) there are requirements for performance and quality assurance of the methods used for official control.
1.3.3. Previous assessments
Aflatoxins were previously evaluated by EFSA's CONTAM Panel in 2007 when EFSA was asked to advise on the potential increase in the risk to consumers’ health associated with a proposed change of the existing EU ML in almonds, hazelnuts and pistachios (EFSA, 2007a). In 2009, the CONTAM Panel issued a statement on the effects on public health of an increase in the levels for ‘aflatoxin total’ from 4 μg/kg to 10 μg/kg for tree nuts other than almonds, hazelnuts and pistachios (EFSA, 2009a), and in 2012, EFSA published a technical report ‘Effect on dietary exposure of an increase of the levels for aflatoxin total from 4 to 10 μg/kg for dried figs’ (EFSA, 2012). Finally, in 2018, a statement from the CONTAM Panel was published on the ‘Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs’ (EFSA CONTAM Panel, 2018).
Aflatoxins were also evaluated at several meetings of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (i.e. at its 46th, 49th, 56th, 68th meetings and last time at its 83rd meeting, in 2016) (FAO/WHO, 2018). The International Agency for Research on Cancer's (IARC) latest evaluation of aflatoxins was in 2012 (IARC, 2012).
Carcinogenicity and mode of action
The available toxicological knowledge on aflatoxins is mostly related to AFB1. Aflatoxins are genotoxic and the critical effect of aflatoxins in all the previous assessments was liver cancer. Following absorption, aflatoxins undergo first pass metabolism in the liver where they exert their toxicity due to the formation of toxic metabolites.
AFB1, AFB2 and AFG1 are mutagenic and induce DNA damage in bacteria and bind covalently to isolated DNA as well as to DNA in cells of rodents treated in vivo. AFB1 and AFG1 also cause chromosomal aberrations in mammalian cells both in vitro and in vivo (IARC, 1993). In addition, AFB1 induces point mutations, mitotic recombination in mammalian cells and genetic instability (IARC, 2012). AFM1 is mutagenic to bacteria and binds to DNA in vitro while AFG2 gave conflicting results regarding mutagenicity in bacteria and did not cause DNA damage.
In experimental animals, AFB1, AFG1 and AFM1 are carcinogenic, whereas there is limited evidence for carcinogenicity of AFB2 and inadequate evidence for carcinogenicity of AFG2 (IARC, 2012). There is strong evidence that the carcinogenicity is due to a genotoxic mode of action (IARC, 2012). AFB1 is more potent than AFG1 both with respect to mutagenicity and liver carcinogenicity (Wong and Hsieh, 1976), but AFG1 induced a higher incidence of kidney tumours than AFB1 (EFSA, 2007a). AFB1 is also more potent than AFM1 (Cullen et al., 1987).
Co‐exposure to hepatitis viruses, in particular hepatitis B, has a strong influence on the carcinogenic risk of aflatoxins to humans. In epidemiological studies, there is an interaction between aflatoxin exposure and hepatitis B infection, and subjects positive for hepatitis B surface antigen (HBsAg) show a multiplicative risk for liver cancer when present together with aflatoxin exposure (FAO/WHO, 2018). IARC (2012) classified aflatoxins as a group as carcinogenic to humans (Group 1) causing hepatocellular carcinomas (HCCs).
The double bond in the furan ring of AFB1 and AFG1 can be oxidised and forms an 8,9‐exo‐epoxide that readily reacts with DNA and other nucleophiles (FAO/WHO, 2018). AFB1 forms DNA adducts by covalent binding to N7‐guanine, resulting in persistent DNA lesions. These lesions may subsequently lead to transversion mutations (IARC, 2012).
Detoxification of AFB1 8,9‐exo‐epoxide can take place by several pathways such as hydrolysis, and enzyme‐mediated conjugations with glutathione (GSH), glucuronic acid and sulfate, and excretion. In particular, GSH conjugation of the reactive epoxide catalysed by glutathione S‐transferase (GST) isoforms in the liver appears to be critical and accounts for interspecies susceptibility to AFB1 toxicity. While mice with high GST activity are relatively resistant, hepatic GST activity is much lower in rats, trout and humans and these species are therefore more susceptible to the adverse effects of aflatoxins. Monkeys show intermediate activity (IARC, 2012; FAO/WHO, 2018). AFB1 is also directly detoxified by oxidation. Due to human polymorphisms (e.g. in cytochrome P450 (CYP) enzymes responsible for the activation of AFB1 and the inactivation of AFB1‐8,9‐exo‐epoxide by GST isoforms), there is interindividual variability in susceptibility to AFB1 among humans (EFSA, 2007a; IARC, 2012; FAO/WHO, 2018).
AFB1 dihydrodiol, a hydrolytic product of AFB1 8,9‐epoxide, may bind to lysine residues of proteins forming adducts, i.e. in serum albumin, which is used as a biomarker of aflatoxin exposure in many studies (Guengerich et al., 2002; EFSA, 2007a; FAO/WHO, 2018).
Dose–response considerations
At its 49th meeting, JECFA (FAO/WHO, 1999) performed the first detailed risk assessment and evaluated a large number of epidemiological studies and identified the Chinese study on mortality from liver cancer by Yeh et al. (1989) as the pivotal study. In this study, the mortality from liver cancer associated with exposure to aflatoxins both in HBsAg‐positive and negative individuals was examined. JECFA estimated AFB1 potencies, which corresponded to 0.3 cancer cases/year per 100,000 subjects per ng AFB1/kg body weight (bw) per day (uncertainty range: 0.05–0.5) in HBsAg‐positive individuals. For HBsAg‐negative individuals the potency estimate was 0.01 cancer cases/year per 100,000 subjects per ng AFB1/kg bw per day (uncertainty range: 0.002–0.03). At this meeting, JECFA also concluded that AFM1 has a potency of inducing liver cancer approximately one order of magnitude less than that of AFB1. The Committee based this potency estimate on a comparative carcinogenicity study in male Fischer rats (i.e. Cullen et al., 1987, 2).
At its 56th meeting, JECFA (FAO/WHO, 2001) noted that there were no adequate epidemiological studies on the dose–response relationships between the intake of AFM1, exposure to hepatitis B or C virus, and liver cancer. JECFA therefore assumed that AFM1 acts similarly to AFB1 with hepatitis B (and possibly) C virus. JECFA used the comparative figure for carcinogenic potency derived at its 49th meeting and assumed that the potency of AFM1 was one tenth of AFB1 in the Fischer rat.3 The carcinogenic potency of AFM1 was estimated to be 0.001/100,000 person‐years per ng/kg bw per day in HBsAg‐negative individuals and 0.03/100,000 per year per ng/kg bw per day in HBsAg‐positive individuals.
In 2007, EFSA's CONTAM Panel also considered a large number of epidemiological studies on aflatoxin exposure and HCC and identified the liver carcinogenicity of aflatoxins as the pivotal effect for the risk assessment (EFSA, 2007a). In its assessment of the cancer risk, the CONTAM Panel conducted benchmark dose (BMD) analyses of the Chinese study on mortality from liver cancer (Yeh et al., 1989) and of a group of studies from Africa on the risk of liver cancer (Peers et al., 1976 as corrected by Carlborg, 1979; Van Rensburg et al., 1985; Peers et al., 1987). The prevalence of HBsAg‐positive was 23% in the Chinese cohort, between 21% and 28% for two studies from Africa, and unknown for one study. The CONTAM Panel calculated a BMD lower confidence limit for an extra cancer risk of 10% (BMDL10) on a background risk of 10.5% of 870 ng/kg bw per day from the study by Yeh et al. (1989). From the other studies cited above (not including the Yeh et al. (1989) study), a BMD lower confidence limit for an extra cancer risk of 1% (BMDL01) on a background risk of 0.17–0.50% of 78 ng/kg bw per day was calculated. The CONTAM Panel used these values for the risk characterisation. In addition, cancer rates for adults with a high AFB1 intake were estimated based on cancer potency estimates made by JECFA as referenced above for HBsAg‐negative and positive populations with 0.2% and 7% prevalence of HBsAg.
The CONTAM Panel also considered many studies on aflatoxin and liver cancer in rats and decided to use in its hazard characterisation the two‐year carcinogenicity study by Wogan et al. (1974), in which male Fischer rats were given AFB1 in their diet. A BMDL10 of 170 ng/kg bw per day was calculated.
At its 83rd meeting in 2016, JECFA reviewed and updated the toxicological evidence on aflatoxin hepatocarcinogenicity. JECFA confirmed its previous conclusion that the lifetime dietary study in male F344 rats (Wogan et al., 1974) is the most suitable study in experimental animals for modelling toxicity. Male F344 rats appear to be particularly susceptible, and in this study, AFB1 as low as 1 μg/kg diet produced liver tumours. Rainbow trout exposed for 4 weeks showed a hepatotumourigenic response over a dose range of 0.05–110 μg/kg diet after 1 year (Williams et al., 2009; Williams, 2012). JECFA (FAO/WHO, 2018) noted that the dose‐related tumourigenesis did not seem to deviate from a log‐linear relationship and that a similar relationship was observed between the dose of AFB1 and AFB1–DNA adducts in trout and rat liver (Bailey et al., 1998; Pottenger et al., 2014). These observations with doses approaching human exposures lend support to the application of a linear non‐threshold model in AFB1 cancer risk assessment.
JECFA (FAO/WHO, 2018) concluded at its 83rd meeting that the prospective Chinese study by Yeh et al. (1989), which demonstrated a close to linear relationship between aflatoxin exposure and mortality from HCC, was still the pivotal study for the risk assessment. The risk was recalculated using a Bayesian model averaging approach, as model uncertainty was a concern. Potency estimates of 0.017 (mean) and 0.049 (95% upper bound (UB)) per 100,000 person‐years per ng/kg bw per day were calculated for HBsAg‐negative individuals. For HBsAg‐positive individuals, potency estimates of 0.269 (mean) and 0.562 (95% UB) per 100,000 person‐years per ng/kg bw per day were calculated (FAO/WHO, 2018). The resulting central potency estimates were practically identical to those calculated by the 49th JECFA (i.e. 0.01 and 0.3 per 100,000 person‐years per ng/kg bw per day for HBsAg‐negative and positive individuals, respectively, see above). These recalculated cancer potencies were also used by the CONTAM Panel for the risk characterisation in its statement on ‘Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 μg/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs’ (EFSA CONTAM Panel, 2018).
JECFA at its 83rd meeting also modelled the rat studies of Wogan et al. (1974) using model averaging. The dose that increased the probability of tumours by 1 in 1,000 was calculated. Using linear extrapolation of the potency to a risk associated with an AFB1 exposure of 1 ng/kg bw per day and using a conversion factor for body weight of 0.75 to extrapolate from rats to humans, a unit risk for humans of 4.7 per 100,000 person‐years per ng/kg bw (95% confidence interval (CI): 1.3–74.9) was calculated (FAO/WHO, 2018).
Risk characterisation
In 2007, the CONTAM Panel calculated margins of exposure (MOEs) based on both BMDL10 and BMDL01 values derived from the epidemiological data and the BMDL10 value derived from the animal data. When evaluating AFT (i.e. the sum of AFB1, AFB2, AFG1 and AFG2), the CONTAM Panel took into account that AFG1 and AFB2 were also shown to be carcinogenic in rodents and assumed that the carcinogenic potency of AFT would be similar to that of AFB1. The Panel (EFSA, 2007a) considered this to be a conservative approach. The MOEs based on the BMDL10 from the animal data and estimated dietary exposure in adults (see Section 3.3.4) were considered to indicate a potential concern for human health. The BMDLs from the epidemiological studies on populations with a high rate of HBsAg indicated a sensitivity similar to that of the rats. However, other subgroups were considered likely to be less sensitive.
JECFA calculated, at its 83rd meeting, the cancer risk associated with estimated aflatoxin exposure in different regions and concluded that the lowest cancer risks were estimated for clusters G07 and G08, which include European and other developed countries. The cancer risk estimates for these clusters ranged from < 0.01 to 0.1 aflatoxin‐induced cancers per year and per 100,000 subjects. The highest cancer risk was estimated for cluster G13 (sub‐Saharan African countries and Haiti) and ranged from 0.21 to 3.94 aflatoxin‐induced cancers per year and per 100,000 subjects (FAO/WHO, 2018).
1.3.4. Legislation
In this Opinion, where reference is made to Regulations, the reference should be understood as relating to the most recent amendment, unless otherwise stated.
In order to protect public health, Article 2 of Council Regulation (EEC) No 315/934 of 8 February 1993, laying down Community procedures for contaminants in food, stipulates that, where necessary, maximum tolerances for specific contaminants shall be established. Subsequently, a number of MLs for aflatoxins and other mycotoxins in various foodstuffs were laid down in the Annex, Section 2 of Commission Regulation (EC) No 1881/20065 of 19 December 2006 setting MLs for certain contaminants in foodstuffs. The MLs for aflatoxins are set following the principle of ‘as low as reasonably achievable’ (ALARA), derived from the frequency distribution of the respective food classes (usually at the 90–95th percentile), taking into account the outcome of the risk assessment and the analytical capabilities.
Maximum levels are set for AFB1 and the sum of AFB1, AFB2, AFG1 and AFG2 in tree nuts, apricot kernels, ground nuts (peanuts) and other oilseeds, dried fruit, cereals, and some species of spices as well as processed products thereof. For AFB1, MLs are also set for baby food and processed cereal‐based food for infants and young children as well as in dietary foods for special medical purposes intended specially for infants. In ruminants fed with contaminated feed, AFB1 is metabolised to AFM1 and therefore MLs are set for AFM1 in raw milk, heat‐treated milk and milk used in milk‐based products, infant formula and follow‐on formula for children as well as in dietary foods for special medical purposes intended specially for infants.
According to Article 1 of Commission Regulation (EC) No 1881/2006, foodstuffs shall not be placed on the market when they contain aflatoxins at a level exceeding the MLs. Article 3 of the Regulation stipulates that foodstuffs not complying with the MLs shall not be used as food ingredients and/or shall not be mixed with foodstuffs complying with the MLs.
Criteria for sampling and analysis of aflatoxins are specified in Commission Regulation (EC) No 401/20066 of 23 February 2006. In addition, specific import conditions have been put in place for certain feed and food commodities from certain third countries related to the presence of aflatoxins (i.e. Commission Regulation (EC) No 669/20097 and Commission Implementing Regulation (EU) No 884/20148).
2. Data and methodologies
2.1. Supporting information for the assessment
The CONTAM Panel used its previous risk assessments on aflatoxins issued in 2007 and 2018 as a starting point for drafting the supporting information. The data were summarised in a narrative way based on expert knowledge/judgement and updated when new information became available as identified in reviews and relevant scientific evaluations by national or international bodies. Following a request from the European Commission to look into the effect of roasting on aflatoxin levels in nuts, a literature search was conducted as outlined in Appendix I, Section I.3.
In addition, the draft scientific opinion underwent a public consultation from 4 October 2019 until 15 November 2019. The comments received and how they were taken into account when finalising the scientific opinion were published in an EFSA Technical Report (EFSA, 2020).
2.2. Hazard identification and characterisation
2.2.1. Collection and selection of evidence
A comprehensive search for literature was conducted for peer‐reviewed original research pertaining to adverse health effects in experimental animals and humans. The search strategy was designed to identify scientific literature dealing with toxicokinetics, toxicity and mode of action. Since this Scientific Opinion is an update of the Scientific Opinion on the potential increase of consumer health risk by a possible increase of the existing MLs for aflatoxins in almonds, hazelnuts and pistachios and derived products adopted in January 2007, the literature search was restricted to papers published in 2006 and after.
The literature search was not restricted to publications in English. A first literature search was performed in May 2018 and has been updated to include publications up to the end of May 2019. Web of Science9, PubMed10, SciFinder and Scopus were identified as databases appropriate for retrieving literature for the present evaluation. An overview of the search terms is given in Appendix I, Section I.1. The references obtained from the literature search were imported and saved using a software package (EndNote11). The references obtained were screened based on title and abstract using Distiller SR to identify the relevant literature, and the exclusion criteria are shown in Appendix I, Section I.2.
Additionally, relevant scientific evaluations by national or international bodies and reviews were considered for the current risk assessment.
2.2.2. Appraisal of evidence
The information retrieved has been screened and evaluated by relevant domain experts from the CONTAM working group on aflatoxins in food and has been used for the present assessment. Limitations in the information used are documented in this Scientific Opinion.
Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results.
2.3. Occurrence data submitted to EFSA
2.3.1. Data collection and validation
Following a European Commission mandate to EFSA, a call for the annual collection of data on the occurrence of chemical contaminants in food, including aflatoxins, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit)12 in December 2010.13 European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on aflatoxins in food. The data for the present assessment were provided by organisations from 29 European countries.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
Data on aflatoxins in food submitted to EFSA by the end of December 2018 were considered for the present assessment. Data received after that date were not included.
2.3.2. Data analysis
Following EFSA's SOP on ‘Data analysis of food consumption and occurrence data’ to guarantee an appropriate quality of the data used in the exposure assessment, the initial data set was carefully evaluated by applying several data cleaning and validation steps. Special attention was paid to the identification of duplicates and to the accuracy of different parameters such as ‘Sampling country’, ‘Sampling year’, ‘Sampling strategy’, ‘Analytical methods’, ‘Result express’, ‘Reporting unit’, ‘Limit of detection/quantification’, and the codification of analytical results under FoodEx classification (EFSA, 2011a). The outcome of the data analysis is presented in Section 3.2.1.
The left‐censored data (results below the LOD or below the LOQ) were treated by the substitution method as recommended in ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). The same method is indicated in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option for the treatment of left‐censored data. The guidance suggests that the lower bound (LB) and UB approach should be used for chemicals likely to be present in the food (e.g. naturally occurring contaminants, nutrients and mycotoxins). The LB is obtained by assigning a value of zero (minimum possible value) to all samples reported as lower than the LOD (< LOD) or LOQ (< LOQ). The UB is obtained by assigning the numerical value of LOD to values reported as < LOD and LOQ to values reported as < LOQ (maximum possible value), depending on whether the LOD or LOQ is reported by the laboratory. Additionally, the middle bound is calculated by assigning a value of LOD/2 or LOQ/2 to the left‐censored data. The middle bound was only used to calculate the relative contribution of AFB1, AFB2, AFG1 and AFG2 to AFT (see Section 3.2.1). The substitution method was applied only to individual aflatoxins (AFB1, AFB2, AFG1, AFG2 and AFM1) while for the AFT a specific approach, as described below, was followed.
The mean concentration of any aflatoxin for a given food was calculated based on the analytical results from all samples analysed for that aflatoxin.
The occurrence data for AFT were calculated from the analytical results of the individual aflatoxins considering only the samples for which all four (AFB1, AFB2, AFG1 and AFG2) aflatoxins were analysed and reported. In practice, analytical results for AFT were generated by summing up the available individual concentrations of the four aflatoxins for each sample. Since AFB1 is the aflatoxin most frequently found and at the highest concentration, and that not all aflatoxin‐producing moulds produce all four aflatoxins, simply adding the four LOQs for samples in which none of the aflatoxins are quantified, would overestimate the UB AFT level. Therefore, the concentration of AFT was calculated for each sample as follows:
when quantified results were available for all four aflatoxins, the concentration of AFT was calculated as the sum of all concentrations;
when the results for all four aflatoxins were left‐censored, the UB concentration of AFT was calculated as twice the LOD/LOQ for AFB1 (the main contributor) unless the sum of the four LODs/LOQs was lower;
when there were both quantified and left‐censored results, the UB concentration of AFT was calculated as the sum of quantified values and twice the LOD/LOQ for AFB1, unless the sum of the quantified values and the LODs/LOQs of the left‐censored aflatoxins was lower.
Recovery rates were reported for only 12% of the data. Nevertheless, the analytical results were submitted to EFSA as corrected for recovery in approximately 64% of cases. The results were not corrected for the recovery in 14% of the cases and for the remaining results this information was not provided. For results which were submitted as not corrected for recovery, the results were corrected either by using the recovery rate reported, if available, or the mean of recovery rates retrieved from the data set, which was 92%.
2.4. Food consumption data
The EFSA Comprehensive European Food Consumption Database (hereinafter referred to as the Comprehensive Database) provides a compilation of existing national information on food consumption at the individual level. It was first built in 2010 (EFSA, 2011b; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used have been published in the Guidance of EFSA (EFSA, 2011b). The latest version of the Comprehensive Database, updated in 2018,14 contains results from a total of 60 different dietary surveys carried out in 25 different Member States covering 119,458 individuals.
Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Two additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old, Latvia) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old, Greece).
Overall, the food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐h dietary recalls or dietary records covering 3–7 days per subject. Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
Detailed information on the different dietary surveys used in this report is given in Annex A Table A.1, including the number of subjects and days available for each age class.
2.5. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011a). FoodEx is a food classification system that was developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. The system consists of a large number of individual food items aggregated into food groups and broader food categories in a hierarchical parent–child relationship. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 endpoints (food names or generic food names) at the fourth level.
2.6. Exposure assessment
The CONTAM Panel estimated chronic dietary exposure to aflatoxins. As suggested by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were not considered as they are not adequate for assessing repeated exposure. Similarly, subjects who participated in the dietary studies for only 1 day when the protocol prescribed more reporting days per individual were also excluded for the chronic exposure assessment. When, for one particular country and age class, two different dietary surveys were available, only the most recent one was used.
Thus, for the chronic exposure assessment, food consumption data were used from 38 different and most recent dietary surveys carried out in 22 different European countries present in the latest version of the Comprehensive Database (Annex A, Table A.1).
To calculate chronic dietary exposure to aflatoxins, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the relevant FoodEx level. In addition, the different food commodities were grouped within each food category to better explain their contribution to the total dietary exposure to aflatoxins. The food categories represented by either a very low number of samples (< 6 samples) or for which all data were below the LOD or LOQ were considered not suitable and were not used for the exposure calculation.
The mean and the high (95th percentile) chronic dietary exposures were calculated by combining aflatoxin mean occurrence values for food samples collected in different countries (pooled European occurrence data) with the average daily consumption for each food at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposure were calculated per survey and per age class. Dietary exposure was calculated using the overall European LB and UB mean occurrence of aflatoxins.
Before linking the consumption data to the corresponding occurrence data, the following adjustments to the occurrence and consumption data were made to reduce uncertainty and reach more accurate exposure estimates:
Consumption events for cereal‐based food for infants and young children were adjusted by a factor of 0.25 (when reconstituted with water) or 0.15 (when reconstituted with milk) when the eating occasions were reported as consumed (liquid) since the occurrence data mainly referred to the analysis of the food as purchased. This correction is based on the information given by the data provider whether the product is reconstituted with milk or water.
Occurrence and consumption events for solid forms of certain foods (tea leaves, cocoa powder, cocoa powder preparations and cocoa beans) were adjusted by an appropriate dilution factor and these consumption events were reclassified to the liquid forms as this is considered more appropriate for the current assessment.
Occurrence data and consumption events for solid forms of infant formula and follow‐on formula were adjusted by a dilution factor of 8 and reclassified to the liquid forms (as ready for feed) as this is considered more appropriate in the context of the current assessment (EFSA, 2018b).
In addition, the CONTAM Panel considered that it is of interest to also estimate a short‐term exposure. AFB1 affects reproductive and developmental parameters in rodents and these effects may occur following a short‐term exposure (see Section 3.1.2.5). To evaluate whether these effects should be considered in the risk characterisation of aflatoxins in humans in the EU, the CONTAM Panel decided to compare the identified doses with a scenario of short‐term exposure. A scenario was developed to estimate the short‐term exposure to AFB1 among peanut butter consumers. The CONTAM Panel selected peanut butter as a type of food product that has a relatively homogeneous AFB1 concentration and that might be eaten on a daily basis. The short‐term dietary exposure was calculated on a per day basis (only consuming days considered). The exposure was assessed by multiplying the total consumption amount of each consumption day by the 95th percentile UB occurrence level (2.25 μg/kg) of peanut butter.
The calculations were based on 43 different dietary surveys carried out in 25 European countries present in the latest version of the Comprehensive Database (Annex A, Table A.1). Finally, for each age group and dietary survey, the mean and 95th percentile of exposure were estimated.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 9.4).
2.7. Risk characterisation
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO/IPCS (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. In addition to the principles described by WHO/IPCS (2009), EFSA guidance pertaining to risk assessment has been applied for the present assessment. EFSA guidance documents applied for this risk assessment are the guidance on the approach for risk assessment of substances which are both genotoxic and carcinogenic (EFSA, 2005), on uncertainties in dietary exposure assessment (EFSA, 2007b), on transparency in the scientific aspects of risk assessments (EFSA, 2009b), on standard sample description for food and feed (EFSA, 2010a), on management of left‐censored data in dietary exposure assessments (EFSA, 2010b), on use of the EFSA comprehensive food consumption database in intakes assessment (EFSA, 2011b), on genotoxicity testing (EFSA Scientific Committee, 2011), on selected default values to be used in the absence of data (EFSA Scientific Committee, 2012a), on risk assessment terminology (EFSA Scientific Committee, 2012b) and on the BMD approach (EFSA Scientific Committee, 2017).
3. Assessment
3.1. Hazard identification and characterisation
3.1.1. Toxicokinetics
The toxicokinetics of AFB1 in humans and experimental animals have been detailed by different risk assessment bodies. Since the previous assessment by the CONTAM Panel in 2007 (EFSA, 2007a), limited new information has become available regarding the toxicokinetics. The text below gives a description of previous knowledge complemented with new data. In general, human data on the toxicokinetics of aflatoxins are not as abundant as in animal species.
3.1.1.1. Absorption, distribution, metabolism and excretion
3.1.1.1.1. Absorption
Animal data
Kumagai (1989) injected [3H]‐AFB1 into the stomach and small intestine of Wistar rats. The results suggest that AFB1 is absorbed mainly from the small intestine, most efficiently from the duodenum by passive diffusion (AFB1 has a low molecular weight and is lipophilic). The author demonstrated that the lipophilicity of the aflatoxins determines the rate of absorption (by comparing the rate of disappearance of the aflatoxin from the perfusion medium). For AFB1, the rate of absorption was considerably higher than for AFG1, which is a less lipophilic analogue. As shown in Table 1, the lipophilicity of the aflatoxins differs between compounds, and could explain the difference in absorption.
Wogan et al. (1967) showed that the distribution of radioactivity after oral or intraperitoneal (i.p.) injection of [14C]‐labelled AFB1 in male Fischer (F344) rats was similar, suggesting an efficient absorption following oral exposure.
Human data
Few data are reported in the literature regarding human absorption of AFB1. The relative contribution of various sites of the gastrointestinal (GI) tract to aflatoxin absorption remains unknown.
Since the previous assessment, a TK study with human male volunteers (n=3) was published. The volunteers received orally a low dose of [14C]‐labelled AFB1 (30 ng, 185 Bq). The maximum radioactivity in plasma was observed at 1 h after exposure, suggesting rapid absorption through the GI tract. According to the authors, 95% of the radioactivity was eliminated by urinary excretion, suggesting high oral absorption (Jubert et al., 2009).
3.1.1.1.2. Distribution
It has been shown in three studies with [14C]‐labelled AFB1 that the liver was the major site for accumulation of radioactivity, especially at low doses in the rhesus monkey and the rat (Holeski et al., 1987; Wogan et al., 1967; Wong and Hsieh, 1980). It is also a site of accumulation in the mouse (Wong and Hsieh, 1980; Wogan, 1969). Wogan et al. (1967) showed after a single i.p. dose of [14C]‐labelled AFB1 (0.07 (n = 4), 2.13 (n = 1) or 4.95 (n = 1) mg/kg bw), that the radioactivity in the liver of Fischer rats was 5‐ to 15‐fold higher than in other tissues at 30 min after administration. Between 8 and 24 h, the liver contained as much radioactivity as the remainder of the carcass, and at the end of 24 h the liver retained nearly 10% of the administered radioactivity.
In a distribution study in pigs (n = 2), Lüthy et al. (1980) found after oral administration of [14C]‐labelled AFB1 that the highest radioactivity was found in the liver, followed by the kidney and then the lung, 1 and 2 days after dosage.
Cupid et al. (2004) showed that after oral administration of [14C]‐labelled AFB1 in Fischer rats both AFB1‐albumin adduct and AFB1‐DNA adduct formation were linear over a wide dose range (0.16 ng/kg bw to 12.3 μg/kg bw). The order of adduct formation within the tissues studied was liver > kidney > colon > lung = spleen.
Placenta/fetus transfers
In humans, the transfer of aflatoxins into the placenta has been confirmed by showing the presence of aflatoxin and/or its metabolites in cord serum and in placenta (De Vries et al., 1989; Denning et al., 1990; Turner et al., 2007; Partanen et al., 2010). Although several metabolites have been identified in cord serum, it is not clear whether they are formed in the placenta or are of maternal origin.
In animals, after a single dose of AFB1 (5 mg/kg bw dissolved in dimethyl sulfoxide (DMSO)) either i.p. or orally on gestation day (GD) 14 to gpt delta B6C3F1 mice, the AFB1‐N7‐guanine (AFB1‐N7‐gua) and AFB1 formamidopyrimidine (AFB1‐FAPY) adducts were found in the livers of both the mothers and the fetuses, with the range in the fetuses being about 1% that of the mothers (Chawanthayatham et al., 2015; see Section 3.1.2.3).
3.1.1.1.3. Metabolism
Intestinal metabolism
AFB1 is metabolised during its passage through the GI tract but the main site and the extent of metabolism remains unknown. The absorption rate and the extent of the metabolism in the GI tract determine the concentrations of AFB1 and its metabolites in the hepatic portal flow and therefore the degree of hepatic exposure (Hsieh and Wong, 1994).
In the study by Kumagai (1989) where [3H]‐AFB1 was injected into the stomach and small intestine of Wistar rats, the author reported metabolism of AFB1 by the duodenum and jejunum, but the metabolic activity was not quantified or compared with that of the liver.
Patients (n = 7) undergoing GI tract surgery for cancer received 1 μg [14C]‐AFB1 orally in a gelatine capsule 3.5–7 h prior to surgery (Cupid et al., 2004). The authors reported the formation of AFB1‐DNA adducts in the colon of two out of seven patients. In similar experiments on Fischer rats, with similar dose ranges, the authors found that humans form fewer AFB1‐DNA adducts in the colon than rats.
Lung metabolism
Some studies have shown that CYP2A13, which is predominantly expressed in human respiratory tissues, was able to metabolise AFB1 (He et al., 2006) and AFG1 (Zhang et al., 2013). He et al. (2006) incubated in vitro different concentrations of AFB1 with CYP2A13. At both 15 and 150 μM of AFB1, the formation of AFM1‐8,9‐epoxide was equivalent for CYP2A13 and CYP1A2, but the activity of CYP2A13 was approximately one‐third of CYP1A2 in the formation of AFB1‐8,9‐epoxide.
Liver metabolism
In the liver, aflatoxins are substrates for CYP monooxygenases, including CYP3A4, 3A5 and 1A2. A critical activation step represents the formation of AFB1‐exo‐8,9‐epoxide. The exo‐epoxide is prone to adduct formation with macromolecules like DNA or proteins. However, there is no evidence identified that the respective endo‐epoxide binds to DNA (see Figure 1). The predominant site for DNA adduct formation by AFB1‐exo‐8,9‐epoxide is N7‐gua, resulting in trans‐8,9‐dihydro‐8‐(N7‐guanyl)‐9‐hydroxyaflatoxin B1 (AFB1‐N7‐gua), which in turn can be transformed into the ring‐opened, and more stable and therefore more persistent, AFB1‐FAPY adduct (Figure 2). Since only AFB1, AFG1 and AFM1 have a double bond at the 8,9‐position, only these compounds are activated by CYPs to the reactive 8,9‐epoxide. In comparison with AFB1, less AFG1‐N7‐guanine DNA adducts are formed for a given dose. This is due to a reduced ability of the AFG1‐8,9‐epoxide to intercalate into the DNA helix because of the reduced planarity of the ring structure (EFSA, 2007a).
Figure 1.
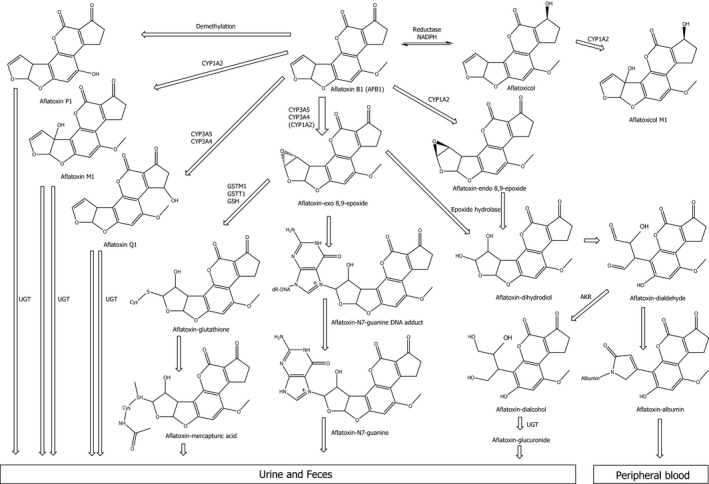
Metabolism and disposition of AFB1 (adapted from FAO/WHO, 2018)
- AKR: NADPH‐dependent aldo‐keto‐reductase; CYP: cytochrome P450; GSH: glutathione; GST: glutathione S‐transferase; NADPH: nicotinamide adenine dinucleotide phosphate; UGT: uridine 5’‐diphospho‐glucuronosyltransferase.
Figure 2.
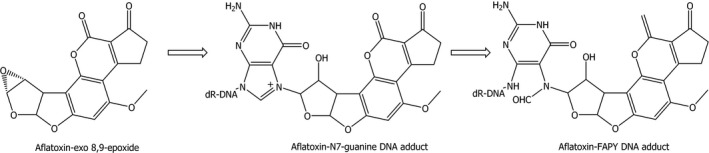
Formation of the aflatoxin‐FAPY DNA adduct
AFB1 can be converted to aflatoxicol in the liver by the reduction of AFB1 mediated by a (NADPH)‐dependent reductase. CYP3A4 and 1A2 oxidise AFB1 to various metabolites other than epoxides, the major ones being the hydroxylated metabolites AFM1 and aflatoxin Q1 (AFQ1) (see Figure 1). In addition, aflatoxin P1 (AFP1) is formed by demethylation. The oxidation products (AFQ1 and AFM1) as well as AFP1 are potential detoxification products, since they represent weaker substrates for epoxidation than AFB1. AFB1‐8,9‐dihydrodiol, resulting from hydrolysis of the 8,9‐epoxide, is unstable and undergoes base‐catalysed rearrangement to a dialdehyde, primarily reacting with proteins such as albumin, but may not reach the DNA. Members of the NADPH‐dependent aldo‐keto‐reductase (AKR) family play a key role in the reduction of the reactive AFB1 dialdehyde to the less reactive AFB1‐dialcohol. Enzymatic hydrolysis by epoxide hydrolase is discussed in the literature, but according to the fast rate of non‐enzymatic hydrolysis, the contribution in vivo of this pathway remains unclear (EFSA, 2007a).
In 2007, the CONTAM Panel acknowledged an ongoing discussion concerning the relevance of the different CYPs with regards to aflatoxin metabolism in humans. CYP3A4, one of the CYP isoforms usually highly expressed in liver tissue, predominantly forms the reactive AFB1‐exo‐8,9‐epoxide, whereas CYP1A2 has been reported to catalyse the formation of both the exo and the endo forms (Pottenger et al., 2014). In a study using human liver microsomes (n = 13), 12‐fold variability in the production rate of AFB1‐exo‐8,9‐epoxide and 22‐fold variability in the formation of the detoxification product AFQ1 was observed. In individuals with low CYP3A4 expression, CYP3A5 appears to play an important role, exclusively generating the AFB1‐exo‐8,9‐epoxide (Kamdem et al., 2006). The CONTAM Panel noted the reported variability in the activity of human CYP3A4 which in part can be due to polymorphisms (Klein and Zanger, 2013) The contribution of CYP1A2 is not fully clarified. Kamdem et al. (2006) suggest that CYP1A2 is ‘negligible’ for the formation of the reactive AFB1‐exo‐8,9‐epoxide. In contrast, in a study with a lower number of samples of human liver microsomes (n = 3) Gallagher et al. (1996) concluded that CYP1A2 dominates the activation of AFB1.
A major pathway for detoxification of the 8,9‐epoxides is GST‐mediated conjugation with GSH (Pottenger et al., 2014). The extent of GSH conjugation differs between species (mouse > rat > human) with humans exhibiting comparatively low conjugation rates (EFSA, 2007a). The relevance of GSH conjugation for detoxification of aflatoxins relates to the levels of individual GST expression in the human liver. The interindividual variation is known to be considerable, presumably reflecting differences in inducibility of GSTs and genetic polymorphisms of the relevant genes (EFSA, 2007a). There are two types of GSTs that are involved in AFB1 detoxification: GST‐μ encoded by the GSTM1 gene and GST‐θ encoded by GSTT1. Except for GSTM1‐1, human GST enzymes are poor catalysts for the conjugation of AFB1 8,9‐epoxide. In several studies, it has been shown that only the GSTM1‐null genotype carriers are at increased risk of HCC in populations exposed to aflatoxins (Wild et al., 2000; see also Section 3.1.4.6.2).
AFM1, AFP1, AFQ1 and aflatoxin‐dialcohol can be conjugated with glucuronic acid and excreted in faeces and urine.
In comparison to AFB1, the information on the metabolism of other aflatoxins is limited. Only a short communication on the metabolism of AFB2 was identified (Groopman et al., 1981).
Neal et al. (1998) incubated in parallel [3H]‐labelled AFM1 and [3H]‐labelled AFB1 with human liver microsomes during 9 h and 6 h, respectively, in order to compare both compound's metabolism. For [3H]‐labelled AFM1, the authors detected the formation of a metabolite (probably AFM1‐dihydrodiol). Compared to AFB1, the authors suggested its limited production was probably explained by a low level of epoxidation of AFM1. No AFB1‐GSH was detected.
3.1.1.1.4. Excretion
Human data
Jubert et al. (2009) analysed blood and 24 h urine samples collected until 72 h after administration of a low dose of [14C]‐labelled AFB1 (30 ng, 185 Bq) from human volunteers. The faeces were not analysed. According to the authors, the kinetic profile of AFB1 and its metabolites fits with a two‐compartment model, with a rapid distribution/elimination phase (half‐life (t1/2) α= 2.9 h) followed by a slower elimination phase (t1/2β = 64.4 h). The authors did not discriminate between free AFB1 and its various metabolites or conjugates. According to the authors, urinary elimination of AFB1 was 95% complete by 24 h.
Previous studies have reported the presence of AFM1 in human urine. Zhu et al. (1987) analysed 252 human urine samples from people exposed to AFB1. They found a strong correlation (R = 0.66, p‐value not provided) between dietary AFB1 intake and excretion of AFM1 in human urine.
AFM1, AFQ1, AFP1, AFB1‐N7‐gua and AFM1‐N7‐guanine are excreted through the urinary route (Egner et al., 2003; Groopman et al., 1985; Mykkänen et al., 2005). Other metabolites (e.g. mercapturic acids arising from GSH conjugation) are also excreted in the urine (Scholl et al., 1997).
AFM1, the hydroxylated metabolite of AFB1, is excreted in human milk. Zarba et al. (1992) estimated that 0.1–0.4% of the amount of AFB1 ingested via the diet is excreted in human milk as AFM1.
Animal data
Aflatoxins are excreted in the faeces in two ways, biliary excretion to the intestine from the absorbed fraction and excretion of the unabsorbed fraction from the lumen of the GI tract.
In Fisher rats (n = 1–4), Wogan et al. (1967) showed that after i.p. administration of [14C]‐labelled AFB1 (ring or methoxy‐labelled), 70–90% of the radioactivity was eliminated during the first 24 h. Biliary excretion into faeces accounted for 54–57% of the administered [14C]‐ring‐labelled AFB1, whereas excretion through the urinary route was 22–34%. After administration of [14C]‐methoxy‐labelled AFB1, biliary excretion into faeces accounted for 24% or the recovered radioactivity, and excretion through the urinary route for 20%.
In another study, Degen and Neumann (1978) described that within 6 h of an i.p. administration of [14C]‐labelled AFB1, more than 50% of total radioactivity was excreted in the bile of Wistar rats (n = 6), mostly as hydrophilic metabolites and a GSH conjugate was the main metabolite. Less than 30% of the total radioactivity was excreted in the bile after oral administration (the authors studied only biliary excretion).
Dalezios et al. (1973) conducted a study in male rhesus monkeys with a single oral dose of 0.4 mg/kg bw (n = 3), or 0.015 mg/kg bw (n = 3), and showed that approximately 40% was excreted in the faeces and 40% was excreted in urine during days 1–4 (excretion was not affected by the dose). Total excretion of radioactivity during days 1–4 amounted to 80–85% of the administered dose.
Holeski et al. (1987) showed that the major biliary metabolite was AFB1‐glutathione, accounting for more than 50% of the total biliary excretion, and AFP1‐glucuronide accounts for 4–15% of total biliary radioactivity in Sprague–Dawley rats (the authors studied only biliary excretion).
Coulombe and Sharma (1985) showed that after an oral dose of [3H]‐labelled AFB1 (0.6 mg/kg bw) in Sprague–Dawley rats, 55% of the total radioactivity is excreted in the faeces and urinary excretion accounted for 15% after oral exposure.
Raj and Lotlikar (1984) showed that approximately 10–16% of a single dose of AFB1 was excreted in urine 24 h after i.p. administration to the rat and hamster. Glucuronide and sulfate conjugates of hydroxy‐metabolites were approximately 60% of the total excretion. In addition, various thiol conjugates were observed and of these AFB1‐GSH and AFB1‐cysteinglycine were the major thiol conjugates
Hsieh and Wong (1994) estimated that the glucuronidated aflatoxin metabolites can be excreted both by biliary and. urinary routes.
As in humans, AFM1 is excreted in animal milk. More information regarding the transfer of aflatoxins into milk from livestock can be found in EFSA (2004) and in the review by Fink‐Gremmels (2008).
Enterohepatic circulation
Hsieh and Wong (1994) suggested that the released AFB1 metabolites in the bile could be reabsorbed (enterohepatic circulation), intestinal microbiota of rats can hydrolyse some glucuronide metabolites leading to a reabsorption of aflatoxin. To asses this hypothesis, the authors injected bile from [14C]‐labelled AFB1 treated rat in to ligated small intestine. They found that the radioactivity remained in the small intestine. They concluded that there was no reabsorption of the [14C]‐labelled AFB1 metabolites from the bile.
3.1.1.1.5. Summary
New information on humans shows that absorption of AFB1 and/or its metabolites into the systemic circulation is rapid, with peak plasma concentrations reached within approximately 1 h. This study shows that AFB1 and/or its metabolites follow a biphasic kinetic profile: they are first rapidly eliminated from the plasma with a first half‐life (t1/2α = 2.86), followed by a second more longer excretion pattern with a terminal half‐life (t1/2β = 64.4 h). According to the authors, urinary elimination was 95% complete by 24 h.
Following administration of [14C]‐labelled AFB1, radioactivity is highest in the liver in different species (rats, monkeys), irrespective of the route of exposure. The relative contribution of metabolism of aflatoxin within the GI tract compared with the liver remains unknown. The metabolism of AFB1 in humans and laboratory animals has been well characterised: CYP1A2, 2B6, 3A4, 3A5, 3A7, 2A13 and GSTM1 are enzymes that catalyse aflatoxin metabolism in humans. CYP enzymes convert AFB1, AFG1 and AFM1 to their respective epoxide, which is capable of binding covalently to both DNA and proteins. AFB2 and AFG2 cannot form the 8,9‐epoxide.
AFB1 and its metabolites are both excreted via the faecal and the urinary route. Nevertheless, the percentage excreted via both routes varies according to the species. AFM1 is also excreted in milk.
3.1.1.2. Kinetic modelling
No physiologically based pharmacokinetic (PBPK) model has been developed for humans. Qian et al., 2013 developed a PBPK model in the Fischer rat on AFB1 and evaluated the toxicokinetics of serum AFB‐lys adduct with different scenarios and doses relevant to acute or chronic human exposure. Nevertheless, this model cannot be extrapolated to humans due to lack of human data for calibration and validation of the model.
3.1.2. Toxicity in experimental animals
3.1.2.1. Acute toxicity (single exposure)
In 2007, the CONTAM Panel concluded that AFB1 causes acute hepatotoxicity in experimental animals. No conclusions could be drawn for other aflatoxins in 2007. In rodents, oral LD50 values for AFB1 have been reported between 1 and 18 mg/kg bw, while for other species LD50 values < 1 mg/kg bw have been reported (Dhanasekaran et al., 2011; Eaton et al., 2018). Wogan et al. (1971) measured LD50 values of 1.16 and 1.5–2.0 mg/kg bw for AFB1 and AFG1, respectively, in male Fischer rats. Neither AFB2 nor AFG2 showed any lethality in rats at single doses up to 200 mg/kg bw.
For the current assessment, the CONTAM Panel identified two recent studies describing the acute toxicity of AFB1, but no studies were identified for the other aflatoxins that are the subject of this Opinion.
Rat
McKean et al. (2006a,b) orally administrated AFB1 (0, 1, 2.15, 4.64 and 10 mg/kg bw) dissolved in DMSO to male F344 rats. Within 72 and 96 h post‐treatment, all animals treated with 4.64 and 10 mg/kg bw AFB1 died, respectively. In animals treated with 2.15 mg/kg bw, 20% died during the 1‐week study period. No mortality was observed in animals treated with the lowest dose (1.0 mg/kg bw) or in the DMSO control group. An LD50 of 2.71 mg/kg AFB1 was determined.
Qian et al. (2013) orally exposed male F344 rats to a single dose of AFB1 (0, 50, 250 or 1,000 μg/kg bw) in DMSO by gavage. Animals were sacrificed at 2 h, 1, 3, 5, 7, 14 and 21 days after the gavage. Biochemical and histological changes were assessed together with the formation of AFB1‐lysine adduct (AFB1‐lys) and liver foci positive for the placental form of GST, a specific and reliable preneoplastic marker. Serum aspartate transaminase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) showed dose‐related elevation, with maximal changes observed (> 100‐fold) at day 3 after treatment. Animals that received 250 μg/kg AFB1 showed concurrent bile duct proliferation, liver necrosis and hepatocytes positive for the placental form of GST at day 3, followed by the appearance of liver foci positive for the placental form of GST at 1 week. Animals that received 1,000 μg/kg AFB1 also showed concurrent bile duct proliferation at day 3, and at this time point they also displayed massive periportal necrotic foci with inflammatory cell infiltration, excessive red blood cells appearing around hepatocytes, and destruction of liver lobes. All animals exposed to the highest dose died at day 7.
Mice
Ishikawa et al. (2017) performed an acute toxicity study in male C57BL/6 mice. AFB1 diluted with a mixture of saline and ethanol (95:5) was administered to groups of five mice by oral gavage at single doses of 44, 442 and 663 μg/kg bw. The animals were sacrificed 5 days later and liver function (ALT, γ‐glutamyl transpeptidase (GGT) and total protein), cytokines (interleukin‐4 (IL‐4), interferon‐gamma (IFN‐γ) and IL‐17), histopathology and the spleen lymphoproliferative response to concanavalin A and lipopolysaccharide were evaluated. AFB1 did not induce significant changes in the biochemical parameters. The highest dose of 663 μg/kg bw induced a hepatic upregulation of IL‐4 and IFN‐γ, along with liver tissue injury and suppression of the lymphoproliferative response to concanavalin A (p < 0.05).
3.1.2.2. Short‐term toxicity (7–90 days)
A number of studies were identified that covered subacute and short‐term toxicity. These are described below. Appendix II, Table II.1 summarises the identified short‐term toxicity studies.
Table II.1.
Summary of oral short‐term toxicity studies for aflatoxin B1
| Species (n) | Route of administration dosage (mg/kg bw per day) | Duration/time of observation | Outcome | NOAEL (LOAEL) | Reference |
|---|---|---|---|---|---|
| Inbred Fischer F344 rats (newly weaned) | Diet 0, 0.12, 0.6, 1.2, 2.4 mg/kg bw per day | 6 weeks | Hepatotoxicity and liver injury but not liver failure. Stunting and wasting and suppression of GH signalling | (0.12 mg/kg bw per day) | Knipstein et al. (2015) |
| Male Albino rat (100–150 g) |
Gavage 0, 0.25, 0.5, 1.0 mg/kg bw per day In olive oil |
7 days |
Altered lipid metabolism: increased plasma and liver cholesterol, triglycerides and phospholipids 0.5 mg/kg and 1.0 mg/kg downregulation of hepatic Cpt1a and increased plasma FFA and triglycerides Dose‐dependent decrease in relative expression of Ahr, Lipc and Lcat and increase in Scarb1 |
(0.25 mg/kg bw per day) | Rotimi et al. (2017) |
| Male Wistar rats (190–220 g) |
Gavage 0, 0.5, 1.0, 2.0 mg/kg bw per day In 8% alcoholic solution |
7 days | Decrease TAS value being most at highest dose. Increase in uric acid, second line antioxidant defence | (0.5 mg/kg bw per day) | Wójtowicz‐Chomicz et al. (2011) |
| Male Sprague–Dawley rats |
Gavage 0, 0.5, 1.0 mg/kg bw per day In corn oil +/−cypermethrin |
Daily for 10, 20, 30, 40 days |
Depression, decreased body weight and feed intake, loose faeces and toxicity in liver and kidney Potentiation of toxic response with combination |
(0.5 mg/kg bw per day) | Hussain et al. (2009) |
| Male Swiss Albino mice (30–35 g) (n = 10) |
Gavage 0.75 and 1.5 mg/kg bw per day In olive oil |
30 days |
Decreased bw and increased organ and kidney weight At higher dose increased ALT, AST, acid phosphatase and serum creatinine Decreased ATPase, ALP, succinate dehydrogenase and serum protein |
(0.75 mg/kg bw per day) | Jha et al. (2014) |
| Male Fischer F344 rats | Gavage 0, 0.005, 0.025, 0.075 mg/kg bw per day in DMSO | 4 weeks 5 days/week | 12 samples analysed (3/group) Controls – phylogenetically diverse microbiota, increasing AFB1 doses decreased diversity but increased evenness of community composition. Some lactic acid bacteria were significantly depleted by AFB1. AFB1 modifies gut microbiota in a dose‐dependent manner | (0.005 mg/kg bw per day) | Wang et al. (2016a) |
| Male Fischer F344 rats | Gavage 0, 0.005, 0.025, 0.075 mg/kg bw per day in DMSO | Daily for 4 weeks | The levels of faecal short‐chain fatty acids were significantly reduced after a 2‐week exposure in all treated groups. The reduction was >70% in the highest dose group. In addition, data on levels of organic acids in the faeces show that aflatoxin exposure affects the gut‐dependent metabolism | (0.005 mg/kg bw per day) | Zhou et al. (2018) |
| Male Wistar rats (240–300 g) | Gavage 150 mg/kg, 300 mg/kg | Twice per week, 5 weeks (cumulative dose 1.5 mg/kg and 3 mg/kg) | Decreased bw and dose‐related decreases in expression of NPY, POMC, SgII and orexin mRNA. AgRP, MCH, CART and TRH expression decreased. Number of EM66‐IR neurons decreased | (1.5 mg/kg) | Trebak et al. (2015) |
| Kunming mice | Gavage 0, 0.1, 0.16 and 0.4 mg/kg bw per daya In ethanol/water mixture | 2 months (twice per day) | AFB1 decreased both genera and phyla of intestinal bacteria. Lactobacillus and Bacteroides were the dominant flora. Significant differences in the relative abundance of intestinal bacterial flora among groups. Most bacteria decreased, but several beneficial and pathogenic bacterial species increased significantly | (0.1 mg/kg bw per day) | Yang et al. (2017) |
| Male Fischer rats (100–120 kg) | Gavage 0, 5, 10, 25, 75 μg/kg bw | 5 days/week for 5 weeks | Decreased body weight, GST‐P+ cells and foci, bile duct proliferation and periportal necrosis | (5 μg/kg bw) | Qian et al. (2013) |
| Kunming mice SPF pathogen‐free |
Gavage 0, 0.1, 0.16 and 0.4 mg/kg bw per daya In ethanol/water mixture |
2 months (twice per day) | Number of bacteria increased in all dose groups. Bifidobacterium increased in the highest dose group. Amylase activity increased in all groups and zylanase and cellulose increased in the highest dose group | (0.1 mg/kg bw per day) | He et al. (2018) |
NOAEL: no‐observed‐adverse‐effect‐level; LOAEL: lowest‐observed‐adverse‐effect‐level; bw: body weight; n: number of animals per group; ATPase: adenosine triphosphatase; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate transaminase; SOD: superoxide dismutase; AFB1: aflatoxin B1; DMSO: dimethyl sulfoxide.
0, 2.5, 4 and 10 μg/mL; 0.4 mL was given twice a day. This is equivalent to 0, 0.05, 0.08 and 0.2 mg/kg bw per shot based on a body weight of 20 g.
Newly weaned inbred F344 Fischer rats were fed AFB1‐supplemented diets (0, 1, 5 10 and 20 mg/kg feed) for 6 weeks. Using a default factor of 0.12 for a subacute study in rats (EFSA Scientific Committee, 2012a), these concentrations correspond to doses of 0, 0.12, 0.6, 1.2 and 2.4 mg/kg bw per day. All doses decreased body weight significantly, while a concentration of 5 mg/kg or greater shortened tibia length. The concentration of AFB1‐alb (ng/mg protein) in the serum increased with an increasing dose of AFB1. Changes in liver function parameters (serum ALT and hepatocellular bromodeoxyuridine staining) increased with dose, indicating liver injury. Dose‐related changes in gut morphology (decreased villous length) suggested that gut absorption might be affected by AFB1. The authors concluded that while dietary AFB1 caused stunting, wasting, suppression of hepatic targets of growth hormone signalling and dose‐dependent liver injury, it did not induce liver failure (Knipstein et al., 2015). A second study comparing outbred Sprague Dawley with the inbred Fischer rats on body weight and levels of AFB1‐albumin adducts showed no difference in sensitivity of the two rat strains for the effect of AFB1 on growth impairment (Knipstein et al., 2015).
Rotimi et al. (2017) showed that oral administration of AFB1 induced liver damage and dysregulation of lipid and lipoprotein metabolising genes. Inbred male albino rats were exposed to AFB1 in olive oil (0, 0.25, 0.5, 1 mg/kg bw per day) by gavage for 7 days. Histological damage was observed in the liver at 0.5 mg/kg bw and above. Plasma cholesterol, triglycerides and free fatty acids increased in a dose‐dependent manner after treatment while plasma phospholipid was not affected. Liver triglycerides and phospholipids also increased. AFB1 decreased expression of genes for Ahr, Cpt1, LCAT and LIPC while SCARB1 gene expression increased, all of which are associated with lipid and lipoprotein metabolism. The largest changes were observed in the Cpt1 and SCARB1 genes identified as the most sensitive.
Administration of AFB1 to Wistar male rats (0, 0.5, 1, 2 mg/kg bw per day) by gavage for 7 days caused a decrease in total blood antioxidant status (TAS) in all AFB1‐treated animals. The two highest doses also increased plasma uric acid concentration. These data indicate significant oxidative stress caused by AFB1 exposure (Wójtowicz‐Chomicz et al., 2011).
Hussain et al. (2009) showed that treatment with AFB1 by gavage (0, 0.5, 1.0 mg/kg bw per day) for up to 40 days decreased feed intake and body weight in adult male rats. These changes were accompanied by liver toxicity including fatty change, necrosis and increased size of both hepatocytes and their nuclei. Toxicity was also observed in the kidney, with tubular necrosis, serum ALT and creatinine increasing while total protein, albumin, serum cholesterol and triglycerides decreased.
Nephrotoxicity was studied in male Albino Swiss mice, after exposure to AFB1 (0, 0.75, 1.5 mg/kg bw per day) by gavage for 30 days (Jha et al., 2014). The exposure resulted in decreased body weight and an increase in relative kidney weight. Increases in a number of enzyme activities were observed in kidney homogenates including AST, ALT, acid phosphatase as well as an increase in serum creatinine. There were decreases in the activities of ALP, succinate dehydrogenase and adenosine triphosphatase and serum protein content. Histopathology showed massive disorganisation in glomerular and tubular structures.
Little is known so far about the potential endocrine or neuroendocrine effects of AFB1. A study with adult male Wistar rats, treated by oral gavage twice a week for 5 weeks with AFB1 (cumulative dose either 1.5 or 3 mg/kg bw) observed reduced body weight gain and suggested that this was associated with a dose‐related decrease in the expression of hypothalamic neuropeptides. Thus, consumption of AFB1 can disrupt the hypothalamic regulation of orexigenic or anorexigenic neuropeptides involved in feeding behaviour, leading to decreased body weight (Trebak et al., 2015).
Qian et al. (2013) carried out an integrated toxicopathological evaluation of Fischer rats exposed to repeated doses (0, 5, 10, 25 and 75 μg/kg bw given by gavage) of AFB1. Over the 5 weeks of the study, the authors observed the changes on a weekly basis. There were no changes in the biochemical profile after week 1 or 3 at all doses and serum AST and ALT increased only at week 5. Bile duct proliferation was found from week 3 onwards in all animals from the highest dose group and at 5 weeks in all animals exposed to 25 μg/kg bw. Periportal necrosis was found with doses higher than 10 μg/kg bw and was observed from week 3 at the highest dose tested. At the highest dose tested, serum AFB‐lys adducts were increased and reached a peak at week 2. Thereafter, the adducts declined slowly. In the two highest dose groups, liver cells positive for the placental form of GST were detected after week 1, developing into foci by week 3. In the 10 μg/kg bw dose group, this was after weeks 2 and 5, respectively. In the lowest dose group, liver cells positive for the placental form of GST were found after week 3 but no foci positive for the placental form of GST over the 5 weeks of the study. A decrease in body weight was observed from week 2 onwards.
In summary, AFB1 at all doses tested has multiple negative effects on rodents including inhibition of normal growth, liver and kidney damage. No studies on short‐term toxicity caused by AFG1, AFG2, AFB2 or AFM1 were identified.
Effects on the gut microbiota
The gut microbiota is critical for healthy development of the gut. In humans and animals, changes in the gut microbial population are associated with multiple health problems. In humans, this includes obesity and inflammatory bowel disease.
Wang et al. (2016a) explored the effects of AFB1 on the gut microbiota in Fischer F344 rats treated by gavage (0, 0.005, 0.025, 0.075 mg/kg bw per day) for 4 weeks (5 days/week) and found that AFB1 modified the gut microbiota in a dose‐dependent manner. Microbial communities were assessed by 16S rRNA sequence analysis. Increasing the dose of AFB1 decreased the diversity of the microbial community and increased the evenness of the resulting community. Specifically, some lactic acid bacteria were depleted. The same group, using the same rat strain and exposure protocol, later showed that exposure for up to 4 weeks affected the gut‐dependent metabolism. In particular, faecal short‐chain fatty acids were decreased with all treatments after only 2 weeks (Zhou et al., 2018).
Yang et al. (2017a) exposed Kunming mice (average weight at start 20 g) for 2 months by gavage to AFB1 (0, 0.1, 0.16 and 0.4 mg/kg bw per day). The dominant flora were Lactobacillus and Bacteroides and all treatments decreased the genera and phyla of the gut microbiota from control to the highest dose indicating a reduction in microbial diversity in response to AFB1 exposure in the colon. In the higher dose groups, increases in some beneficial and in some pathogenic bacteria were observed.
In another study on Kunming mice with the same dosing protocol and duration as described for Yang et al. (2017a), He et al. (2018) showed that the number of bacteria increased in all dose groups with Bifidobacterium species increasing significantly in the highest dose group. Intestinal enzyme activities also increased (amylase, xylanase and cellulose). Despite these changes, there was no effect on the body weight of the mice. The composition of the communities of microbiota is important as it has been observed that some bacterial species can detoxify AFB1 (Wu et al., 2009b).
In summary, AFB1 at all doses tested altered the microbial communities generally decreasing the diversity of the community. No studies on AFG1, AFG2, AFB2 or AFM1 for this endpoint were identified.
3.1.2.3. Genotoxicity
The genotoxicity of AFB1 has been the subject of comprehensive reviews and is well‐established (IARC, 1987, 1993, 2002; EFSA, 2007a; FAO/WHO, 1999, 2018). AFB1 is mutagenic in Salmonella Typhimurium strains TA98 and TA100, and mutagenicity is enhanced about 1,000‐fold by the presence of S9 (IARC, 2002), thus underlining the role of the bioactivation system. The double bond in the furan ring of AFB1 and AFG1 can be oxidised by CYPs to the reactive AFB1‐exo‐8,9‐epoxide that readily reacts with DNA and other nucleophiles (FAO/WHO, 2018). Covalent binding at the N7 position of guanine (AFB1‐N7‐gua adduct) causes primarily G‐to‐T transversions in Escherichia coli, although at a low frequency (4%) (EFSA, 2007a). Based on the available information, the CONTAM Panel concluded in 2007 that the ring‐opened AFB1‐FAPY adduct is likely to be responsible for AFB1 mutagenicity. Indeed, in E. coli the persistent AFB1‐FAPY adduct causes a higher frequency of G‐to‐T mutations than the short‐lived AFB1‐N7‐gua (EFSA, 2007a). In rodent and human cells, gene mutations, chromosomal aberrations and micronuclei, sister chromatid exchanges and unscheduled DNA synthesis are increased by incubation with AFB1 (Pottenger et al., 2014). In rats, AFB1 exposure increases mutations at the HPRT‐locus in circulating T lymphocytes (FAO/WHO, 2018). In humans living in areas where hepatitis B virus (HBV) infection and AFB1 exposure are prevalent, HCC samples show a mutational hotspot (G‐to‐T transversion) at codon 249 of the TP53 gene. This is considered to be a signature mutation for aflatoxin‐induced HCC (Hussain et al., 2007).
In contrast to AFB1, fewer studies were available to the previous assessments regarding the genotoxicity of the other aflatoxins and a few studies examined all the compounds simultaneously. It is not possible, based on these data, to make a quantitative comparison of the genotoxic potency of these compounds. However, in general the order is considered to be AFB1 > AFG1 > AFB2 > AFG2 (Wogan et al., 1971).
Since the previous assessment by the CONTAM Panel, new studies have become available that support the previous conclusions. New information regarding the hotspot at codon 249, the role of different CYP isoenzymes in the bioactivation of AFB1 and the genotoxicity of AFB1 in pregnant mice, fetuses and young animals is described below.
In vitro genotoxicity
Recent studies addressed the role of different CYP isoenzymes in the bioactivation of AFB1. Following AFB1 exposure, DNA adduct formation and increased recombination levels were observed in the DNA repair‐deficient Saccharomyces cerevisiae rad4 rad51 strain only when this one expressed the human CYP3A4 (Fasullo et al., 2017). AFB1 exposure induced micronuclei in differentiated HepaRG cells which express high levels of CYP3A4 (Le Hégarat et al., 2010). Inhibition of CYP3A4 or CYP1A/1B activity by ketoconazole significantly suppressed AFB1 genotoxicity in HepG2 cells as measured by the activation of p53 (Boehme et al., 2010). Similarly, in a co‐culture system of TK6 and Caco‐2 cells, ketoconazole inhibition of CYP3A4 suppressed cytotoxicity and micronuclei induced in both cell lines by AFB1 exposure (Le Hégarat et al., 2010).
A recent study compared the in vitro genotoxic potential of AFB1, AFG1, AFB2, AFG2, AFM1 and aflatoxicol in the HepG2 HCC, the LS‐174T colorectal carcinoma and the ACHN renal carcinoma cell lines applying a γH2AX In‐Cell Western technique (Theumer et al., 2018). This assay assesses the phosphorylation of histone H2AX which is an early, sensitive genotoxic biomarker induced by various types of DNA damage, including DNA double‐strand breaks, DNA bulky adducts, DNA single‐strand breaks (SSBs), DNA replication or transcription blocking lesions (DNA oxidation and alkylation). The relative genotoxic potency in vitro of these aflatoxins was in the following order: AFB1 > AFG1 ≈ aflatoxicol » AFM1. AFG1 resulted in a 10‐fold less genotoxicity than AFB1 in all the cell lines. Aflatoxicol showed some variation in the effect depending on the tested cell line. Thus, in comparison to AFB1, aflatoxicol was 10‐fold less potent in HepG2 cells, equally genotoxic in LS‐174T cells and devoid of genotoxicity in ACHN cells. AFM1 increased genotoxicity only in one cell line (LS‐174T cells). AFB2 and AFG2 did not cause genotoxicity in the three human cell lines.
An in vitro study with AG1521 human diploid fibroblasts exposed to AFB1 reported an increase in mutation frequency at the TP53 gene. Several missense mutations occurred in well‐known human tumour hotspots (codons 175, 245 and 282), with G‐to‐A transitions being the most prevalent class (35%) followed by G‐to‐T transversions (22%) and A‐to‐G transitions (22%). No G‐to‐T transversions were found at codon 249 which is described as a hotspot for AFB1 exposure in HCC (Paget et al., 2008, 2012).
In summary, in a study with human cell lines the order of aflatoxins to induce genotoxicity was AFB1 > AFG1 ≈ aflatoxicol » AFM1. Recent reports on AFB1 are in accord with numerous earlier publications concerning the genotoxic potential of AFB1. The recent literature highlights the importance of CYP3A4 activity for AFB1 genotoxicity.
Genotoxicity in vivo
Information on the experimental design of the in vivo genotoxicity studies, including details on the outcome are presented in Appendix II, Table II.2.
Table II.2.
Experimental design of in vivo genotoxicity studies, including details of the outcome
| Test system | Animals | Concentration/treatment | Details of the outcome not specified in the text | Reference |
|---|---|---|---|---|
| Micronuclei in the bone marrow; single strand breaks | Male Fischer rats | Single oral dose of 0.25 mg/kg bw | Corcuera et al. (2015) | |
| Mutation frequency analysis | Pregnant gpt delta B6C3F1 mice | Single dose either i.p. or orally on GD 14: 6 mg/kg bw in DMSO | Chawanthayatham et al. (2015) | |
| Adduct formation | Pregnant gpt delta B6C3F1 mice | Single dose i.p. on GD 14: 5 mg/kg bw in DMSO |
Level of AFB1‐N7‐gua and AFB1‐FAPY in the liver tissue 6 h after application: – mother: 18.8 ± 2.5 and 45 ± 6 adducts/106 nucleotides (mean ± SD), respectively – fetus: 0.31 ± 0.25 and 0.30 ± 0.19 adducts/106 nucleotides, respectively |
Chawanthayatham et al. (2015) |
| Single dose orally on GD 14: 5 mg/kg bw in DMSO |
Level of AFB1‐N7‐gua and AFB1‐FAPY in the liver tissue 6 h after application: – mother: 6.2 ± 0.8 and 19.1 ± 0.4 adducts/106 nucleotides, respectively – fetus: 0.07 ± 0.04 adducts/106 nucleotides and < LOD, respectively |
|||
| Adduct formation | C57BL/6 J mice (pregnant and non‐pregnant controls) | Single i.p. dose of 6 mg/kg on GD 14 in DMSO | Sriwattanapong et al. (2017) | |
| Mutational patterns | Four‐day old male gpt delta B6C3F1 mice | 6 mg/kg bw by i.p | Chawanthayatham et al. (2017) | |
| DNA adduct formation and mutational patterns | Four‐day old gpt delta B6C3F1 mice | Single dose (6 mg/kg bw, i.p.) | Woo et al. (2011) | |
| Mutational patterns | Four‐day old gpt delta B6C3F1 mice | Single dose (6 mg/kg bw, i.p.) + post‐dosing period of 3 and 10 weeks | Wattanawaraporn et al. (2012) |
GD: gestation day; LOD: limit of detection; i.p.: intraperitoneal; SD: standard deviation; AFB1: aflatoxin B1; DMSO: dimethyl sulfoxide; bw: body weight.
In a study aiming to investigate the consequences of a combined treatment of AFB1 and ochratoxin A, a significant increase of micronuclei in the bone marrow and SSBs in the liver was observed in male Fischer rats treated with a single oral administration of AFB1 (0.25 mg/kg bw) (Corcuera et al., 2015). In particular, SSBs, as measured by Comet assay, were significantly enhanced only following cleavage with the bacterial formamidopyrimidine‐DNA glycosylase (Fpg). This indicates that AFB1 induces oxidative damage to DNA. The authors speculate that these Fpg‐sensitive sites might derive from Fpg recognition and cleavage of AFB1‐FAPY lesions.
In a series of publications, researchers focused on the impact of AFB1 exposure during pregnancy and early life (Chen et al., 2010; Woo et al., 2011; Wattanawaraporn et al., 2012; Chawanthayatham et al., 2015, 2017; Sriwattanapong et al., 2017). Pregnant gpt delta B6C3F1 mice received a single dose of AFB1 either i.p. or orally on GD 14. Measurements of AFB1‐N7‐gua and AFB1‐FAPY (by ultra performance liquid chromatography (UPLC)–MS) were performed 6 h post‐dosing in liver DNA of mothers and embryos. A parallel cohort gave birth and mutations in the livers of this F1 were analysed at the gpt gene at 3 and 10 weeks of age. When AFB1 was administered via i.p., fetal liver adduct levels were 100‐fold lower than in the maternal liver. A similar effect on DNA adduct levels in fetal liver was also observed after gavage, although total DNA adduct formation was about 2.5‐fold lower. The relative risk of gpt mutations in fetuses and adult livers from AFB1‐DNA adducts indicates that there is a higher mutational impact of the lesions in the fetus. Namely, 1% of DNA adducts in comparison to the mothers, but 20% in mutation frequency (Chawanthayatham et al., 2015).
The impact of pregnancy on AFB1 exposure was also investigated in C57BL/6 J mice at GD 14 and matched non‐pregnant controls administered a single i.p. dose of 6 mg/kg AFB1. A twofold higher level of AFB1‐N7‐gua adducts was observed in the liver of pregnant C57BL/6J mice in comparison to non‐pregnant counterparts. The enhanced adduct levels were accompanied by elevated expression levels of CYP1A2 and the mouse equivalent of CYP3A4 (Sriwattanapong et al., 2017).
Four‐day‐old male gpt delta B6C3F1 mice were treated with a regimen of AFB1 that induces HCC within 72 weeks (6 mg/kg bw by i.p.). High‐resolution mutational spectra were acquired in the liver, 10 weeks after birth (in the absence of neoplasia) as well as in tumour DNA (after 72 weeks). The spectrum of mutations at 10 weeks in non‐tumour cells of the liver represents the mutagenic imprint of AFB1 exposure (hotspot of G‐to‐T transversions at CGC sequence). This 10‐week spectrum persisted in the tumour tissue, although accompanied by a more heterogeneous set of mutations that emerged during tumour outgrowth (Chawanthayatham et al., 2017).
In an attempt to explain the higher incidence of HCC in males vs females when treated as infants, Woo et al. (2011) investigated DNA adduct formation and mutational patterns in gpt delta B6C3F1 mice treated 4 days after birth with a single dose of AFB1 (6 mg/kg bw, i.p.). Similar levels of DNA damage and mutations were observed in the liver of newborn males and females. At 21 days, no significant differences were found in the types of mutations between males and females, with the main mutational classes being G‐to‐T transversions and G‐to‐A transitions.
Using a similar AFB1 exposure protocol as in the paragraph above and a post‐dosing period of 3 and 10 weeks, AFB1‐induced mutational spectra were investigated at a second locus, the red/gam genes in the λEG10 transgene of gpt delta B6C3F1 mice (Spi– phenotype). Although some small insertions and deletions were observed, the Spi– spectrum was still dominated by G‐to‐T transversions. Similarly to the gpt mutations, no significant gender differences were observed (Wattanawaraporn et al., 2012).
Big Blue B6C3F transgenic mice were treated at postnatal ages of 4, 7 and 10 days with a dose of AFB1 (6 mg/kg bw by i.p.), while adult animals were treated at day 120, 123 and 126 with 6 or 60 mg/kg bw. All animals were sacrificed 6 weeks later. In the liver of the neonatal mice, the mutation frequency at the cII gene was 22‐fold higher in AFB1‐treated compared with control animals (82‐fold increase in G‐to‐T transversions). Although in AFB1‐treated adult animals no increase in mutation frequency was observed, molecular analysis of the mutants identified a significant increase (fivefold) in G‐to‐T transversions (Chen et al., 2010). These results are in line with an earlier report on lower levels of GST in the liver of neonatal mice, associated with enhanced formation of AFB1‐DNA adducts (Shupe and Sell, 2004).
Taken together, pregnancy appears to enhance sensitivity to the genotoxicity of AFB1 for the mothers, presumably resulting from elevated levels of CYP1A2 and CYP3A4. A study with in utero exposure showed a lower frequency of DNA adducts formed in the fetus compared with the mothers (about 1%), while the mutation frequency differed only by a factor of about five, indicating a greater mutational impact of the lesions in the fetus. Early postnatal exposure resulted in higher adduct levels in the liver compared with treatment of adult animals.
Mutational signatures of aflatoxin B1 exposure
Several studies investigated the role of chronic infections with HBV or hepatitis C virus (HCV), and exposure to dietary AFB1 in the aetiology of HCC (see also Section 3.1.3). Codon 249 in the TP53 tumour suppressor gene represents a hotspot for AFB1‐mediated mutagenesis, predominantly via a G‐to‐T transversion (AGG to AGT, R249S). In HCC cell lines, the R249S variant was found to lack the capacity to bind to p53 response elements and to transactivate p53 target genes (Gouas et al., 2010). Studies indicate a strong association between high levels of R249S and HBV‐related HCC, whereas low to intermediate levels of R249S were detectable in asymptomatic subjects exposed to AFB1 (Ortiz‐Cuaran and Hainaut, 2011).
Two recent studies investigated the potential genome‐wide mutational signatures of AFB1 exposure (Huang et al., 2017; Zhang et al., 2017). Sequencing of the genomes of 4915 HCC cases collected in the AFB1 high‐risk region Qidong, China, (Zhang et al., 2017) were compared with 1,072 HCCs in the general population without known exposure to aflatoxin (obtained from China, the United States, France and Japan). The dominant mutational signature was characterised by increased G‐to‐T transversions, in the sequence motif GGC and preferential localisation in the non‐transcribed strand. The genes most frequently mutated were TP53, TERT, AXIN1, CTNNB1 and ADGRB1 (Zhang et al., 2017). The authors calculated that HCC with aflatoxin signature in the general population were up to 9.8% in China, 3.5% in the United States, 1.7% in France and 0.4% in Japan.
In the other study, whole genome sequencing was applied to analyse the mutation spectra of two human cell lines, liver tumours in wild‐type mice and mice carrying an HBV surface antigen transgene. There was considerable agreement between mutation patterns observed in the different experimental systems. There was also considerable overlap with mutational spectra from HCCs from known high aflatoxin exposure regions, providing confirmatory evidence that such mutational spectra can be used as signatures for aflatoxin exposure. These HCC samples were preselected for the presence of somatic TP53 R249S mutations (Huang et al., 2017). Based on comparison of the genome‐wide analysis of mutational signatures including previously published data, the authors estimated the proportion of AFB1 exposure related to HCCs to be 0.7% in North America, 1% in Japan and 16% in Hong Kong (Huang et al., 2017). Thus, the analysis of mutational spectra by whole genome sequencing appear to provide a useful tool to identify AFB1 exposures in western countries.
3.1.2.4. Long‐term toxicity (including carcinogenicity)
In 2007, the CONTAM Panel concluded that AFB1 is clearly carcinogenic in a variety of animal species. In rodents, the principal tumours were in the liver, primarily HCC, but tumours have also been observed in the lung, kidney and colon. The CONTAM Panel noted that the male Fischer rat is the most sensitive species. The CONTAM Panel selected the study by Wogan et al. (1974) as the pivotal study. In this chronic exposure study, male Fischer rats (approximately 80 g) were fed a semi‐synthetic diet containing AFB1 at concentrations of 0, 1, 5, 15, 50 and 100 μg/kg for up to 105 weeks. A clear dose–response relationship was observed between AFB1 and the incidence of HCC at the two highest doses (Table 2). In 2007, the CONTAM Panel converted the dietary concentrations of AFB1 into daily doses using a factor of 0.04 and by adjusting16 for the shorter study duration in some of the groups. However, EFSA currently uses a default factor of 0.05 for chronic studies in the rat (EFSA Scientific Committee, 2012a), which was also applied by JECFA. In 2016, JECFA used a dose‐correction factor reflecting the squared dependence17 of dose on time. This approach was recommended by Peto et al. (1984) to avoid overcorrection, but this may lead to under‐correction if the study is substantially shorter than the ‘standard lifespan’ of 104 weeks (e.g. due to high mortality). The CONTAM Panel noted that this approach resulted in an inconsistency between the time‐adjusted dose and the incidence of tumours at the highest concentration. Therefore, this correction is not used by the CONTAM Panel for the calculation of time‐adjusted doses in the paper by Wogan et al. (1974) and instead the time adjustment was made as in 2007.
Table 2.
Incidence of hepatocellular carcinomas in male Fischer rats after dietary administration of AFB1 (Wogan et al., 1974)
| Concentration in feed (μg/kg)a | Time of appearance of earliest tumour (weeks)a | Duration of experiment (weeks)a | Tumour incidencea | Dose (μg/kg bw per day)b | Time‐adjusted dose (μg/kg bw per day)c |
|---|---|---|---|---|---|
| 0 | – | 74–109 | 0/18 | 0 | 0 |
| 1 | 104 | 78–105 | 2/22 | 0.05 | 0.05 |
| 5 | 93 | 65–93 | 1/22 | 0.25 | 0.22 |
| 15 | 96 | 69–96 | 4/21 | 0.75 | 0.69 |
| 50 | 82 | 71–97 | 20/25 | 2.5 | 1.97 |
| 100 | 54 | 54–88 | 28/28 | 5 | 2.60 |
bw: body weight.
As reported by Wogan et al. (1974).
Dose calculated by the CONTAM Panel by using a default factor of 0.05 for chronic studies in the rat (EFSA Scientific Committee, 2012a) without adjustment for study duration.
Time adjustment based on time of appearance of earliest tumour as performed by the CONTAM Panel in 2007 (i.e. if a 1‐year exposure is corrected to a 2‐year exposure, then the dose is multiplied by a factor or 0.5).
In 2007, the CONTAM Panel concluded that AFB1 and AFG1 can be considered to be equally potent regarding carcinogenicity. This conclusion was based on the higher potency of AFB1 to cause tumours in the liver versus the higher potency of AFG1 to cause tumours in the kidney. Butler et al. (1969) exposed male and female MRC rats (8–9 weeks old) to AFB1, AFG1 and AFB2 via drinking water. AFG1 caused about six times fewer liver tumours than AFB1 (Table 3). However, AFG1 caused a higher incidence of kidney tumours in male rats in the middle‐dose group than AFB1 (Table 3). AFB2 did not cause liver or kidney tumours, but 6 out of the 15 animals showed other neoplasms (unspecified). Further, Wogan et al. (1971) reported the occurrence of renal adenocarcinomas in 4 out of 26 male Fischer rats exposed to AFG1 via gavage, while this tumour was not reported for the animals exposed to AFB1. No dose–response information was provided for this tumour. HCCs were observed after treatment with AFB1 over a timeline of 14–45 weeks whereas with AFG1 tumours were seen at 20–64 weeks. In the same study, AFB2 was reported to cause HCC following i.p. exposure (total dose 150 mg) but no HCC were observed following gavage treatment (total dose 1 mg).
Table 3.
Incidence of liver and kidney tumours in MRC rats exposed to AFB1, AFG1 and AFB2 via drinking water (Butler et al., 1969)
| Total dose (mg per rat)a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1b | 2 | 6 | |||||||
| M | F | total | M | M | F | total | M | F | total | |
| Liver tumours | ||||||||||
| AFB1 | 0/15 | 0/15 | 0/30 | 3/10 | 8/15 | 11/15 | 19/30 | – | – | – |
| AFG1 | 0/15 | 0/15 | 0/30 | 1/10 | 2/15 | 1/15 | 3/30 | 9/11 | 12/15 | 21/26 |
| AFB2 | 0/15 | 0/15 | 0/30 | 0/10 | – | – | – | – | – | – |
| Kidney tumours | ||||||||||
| AFB1 | 0/15 | 0/15 | 0/30 | 0/10 | 2/15 | 0/15 | 2/30 | – | – | – |
| AFG1 | 0/15 | 0/15 | 0/30 | 0/10 | 5/15 | 0/15 | 5/30 | 6/11 | 0/15 | 6/26 |
| AFB2 | 0/15 | 0/15 | 0/30 | 0/10 | – | – | – | – | – | – |
F: female; M: male; –: not tested; AFB1: aflatoxin B1; AFG1: aflatoxin G1; AFB2: aflatoxin B2.
The total dose refers to the dose during the entire exposure which was 10 weeks for the dose of 1 mg and 20 weeks for the doses of 2 and 6 mg.
Dose of 1 mg/rat was tested in male rats only.
No risk assessment for AFM1 has been carried out by the CONTAM Panel. At its 49th and 56th meetings, JECFA decided on a potency factor for AFM1 based on the study by Cullen et al. (1987). They evaluated the carcinogenicity of AFM1 in male Fischer rats and compared this with AFB1. AFM1 (0, 0.5, 5.0 and 50 μg/kg feed) and AFB1 (50 μg/kg feed) were fed to rats over 22 months. AFB1 induced HCC (19/20 rats) at 17 months of treatment while AFM1 resulted in no tumours until 21 months. At the highest dose tested, AFM1 induced HCC in 2/18 rats at 21 months. No tumours were observed at 0.5 and 5.0 μg/kg of AFM1. AFM1 was therefore found to be a hepatic carcinogen but with lower potency than AFB1.
No new long‐term toxicity studies in rodents were identified since the previous assessment by the CONTAM Panel in 2007.
Studies in rainbow trout ( Oncorhynchus mykiss )
Halver (1968) first suggested the rainbow trout as a test animal for oncology. He tested 13 potential carcinogens including AFB1 and observed liver tumours in all animals fed the carcinogen‐containing diet over 6–9 months.
In a study of 7,200 trout fry, Bailey et al. (1998) found relative tumourigenic potencies of aflatoxins as individual compounds in the liver as AFB1 = 1; aflatoxicol = 0.936; AFM1 = 0.086; aflatoxicol M1 = 0.041. Fish were exposed to aflatoxins for 2 weeks in diet and monitored at 1 year. The dose–response curves for AFB1 and aflatoxicol were similar. The authors suggested that the differences in tumourigenicity were due to alterations in uptake and metabolism and the resultant DNA adduct formation.
The same group carried out a number of other studies with large numbers of fish (Williams, 2012) including ultra‐low dose studies to determine the virtually safe dose. The data available at this time indicated that for AFB1 at low concentrations in the feed (0–110 μg/kg) there was a linear dose–response (Williams, 2012; Williams et al., 2009).
In summary, a group at Oregon State University has used the rainbow trout as a model for liver cancer, testing a range of potential carcinogens including AFB1. The trout are sensitive to AFB1 and showed a linear dose–response across a wide range of concentrations.
3.1.2.5. Developmental and reproductive toxicity
The CONTAM Panel identified several developmental and reproductive toxicity studies that employed multiple dose groups and used the oral exposure route. The in vivo studies in rodents are summarised in Appendix II, Table II.3.
Table II.3.
Summary of in vivo developmental and reproductive toxicity studies for aflatoxin B1
| Reference | Species | Treatment | Effects |
|---|---|---|---|
| Hasanzadeh and Amani (2013) | Female Wistar rats | 0, 4, 8 or 16 μg/kg bw per day by gavage for 25 days | Reduction in the population of healthy primordial, primary, secondary and tertiary ovarian follicles; dose‐dependent at all doses |
| Hasanzadeh et al. (2011) | Male Wistar rats | 0, 4, 8 or 16 μg/kg bw per day by gavage for 48 days | Decreased LH and testosterone; increased FSH and prolactin; dose‐dependent effects at all doses |
| Hasanzadeh and Rezazadeh (2013) | Male Wistar rats | 0, 4, 8 or 16 μg/kg bw per day by gavage for 48 days | Spermatogonia and spermatozoa decreased in all test groups (p < 0.001); primary spermatocytes and spermatids decrease (p < 0.01) only in high‐dose group |
| Mohammadi et al. (2014) | NMRI mice | 0, 100 or 700 μg/kg bw per day by gavage for 35 days | DNA damage and chromatin abnormalities of sperm cells with low fertilisation rate and retarded embryonic development; effects at all doses |
| Murad et al. (2015) | Adult rats |
15, 30 or 45 μL of AFB1/kg (three times/week) orally for 40 days Available information does not make it possible to calculate the dose |
Dose‐dependent increase in testicular and sperm abnormalities |
| Tanaka et al. (2015) | SD female rats |
Dietary exposure to AFB1 at 0, 0.1, 0.3, or 1.0 mg/kg from GD 6 to day PND 21. Examination at PND 21 and 77 Dose during gestation period: 0, 7.1, 20.7 or 66.7 μg/kg bw per day Dose during lactation period: 0, 13.6, 41.7 and 132.7 μg/kg bw per day |
Maternal AFB1 exposure reversibly affects hippocampal neurogenesis targeting type‐3 progenitor cells; NOAEL for offspring neurogenesis was 7.1–13.6 mg/kg bw per day (corresponding concentration in the diet: 0.1 mg/kg) |
| Wang et al. (2016b) | ICR female mice | 0, 50, 500, 5,000 μg/kg bw by gavage for 4 days (from GD 13.5 to 16.5) | Shortened time to delivery and low birth weight in mice treated with 0.5 and 5 mg /kg bw; NOAEL at 50 μg/kg bw |
NOAEL: no‐observed‐adverse‐effect‐level; bw: body weight; AFB1: aflatoxin B1; PND: postnatal day; GD: gestation day; LH: luteinising hormone; FSH: follicle‐stimulating hormone
In a pre‐ and postnatal developmental toxicity study, Sprague Dawley rats (n = 12 per dose group) were exposed daily from GD 6 to postnatal day (PND) 21 to 0, 0.1, 0.3 or 1.0 mg AFB1/kg diet. Offspring were examined at PND 21 and PND 77. Dose levels equalled 0, 7.1, 20.7 or 66.7 μg AFB1/kg bw per day during the gestation period, and 0, 13.6, 41.7 and 132.7 μg/kg bw per day during the lactation period. Maternal AFB1 exposure affected hippocampal neurogenesis targeting type‐3 progenitor cells at PND 21 which the CONTAM Panel considered to be adverse, whereas no changes in neurogenesis‐related parameters were observed at PND 77, implying that this effect is reversible. The no‐observed‐adverse‐effect‐level (NOAEL) for offspring neurogenesis was 0.1 mg/kg feed (7.1–13.6 μg/kg bw per day) (Tanaka et al., 2015; Shibutani, 2019).
In a prenatal developmental toxicity study, ICR mice (n = 10 per dose group) were dosed daily during GD 13.5–16.5 by gavage with AFB1 administered in ethanol/corn oil (1:9 v/v) at 0, 50, 500 and 5,000 μg/kg bw. A shortened time to delivery and low birth weight were observed at 500 and 5,000 μg AFB1/kg bw. The NOAEL was 50 μg/kg bw (Wang et al., 2016b).
Groups of seven male or female adult Wistar rats were exposed to 0, 4, 8 or 16 μg AFB1/kg bw per day via sterile distilled water by gavage, for either 25 days (f) or 48 days (m) (Hasanzadeh and Amani, 2013; Hasanzadeh and Rezazadeh, 2013). Dose‐related effects were observed at all doses, being reduction in the population of healthy primordial, primary, secondary and tertiary follicles (f), and reduced spermatogonia types A, B, and spermatozoa. Primary spermatocytes and spermatids were decreased only at the highest dose. Fertility by mating was not tested in these studies. Considering that effects were observed at the lowest dose tested (4 μg/kg bw per day), only a lowest‐observed‐adverse‐effect‐level (LOAEL) was identified from these studies. In an earlier study in male adult Wistar rats (n = 5 per group) from the same researchers (Hasanzadeh et al., 2011), the same dosing regimen was shown to result in decreased serum concentrations of luteinising hormone and testosterone, and increased follicle‐stimulating hormone (FSH) and prolactin, with dose‐dependent effects at all doses.
In a male fertility study, adult NMRI mice were treated daily for 35 days with AFB1 (100 or 700 μg/kg bw) by gavage. Both dose levels reduced sperm viability and motility and caused sperm DNA damage. Upon mating treated males, fertility rate was reduced, and embryo arrest increased at both dose levels (Mohammadi et al., 2014).
A study on the impact of AFB1 on spermatozoa obtained from bull ejaculate reported enhanced levels of DNA fragmentation and an increased proportion of dead sperm. These effects were observed at 10–100 μM (Komsky‐Elbaz et al., 2018). In porcine oocytes, impairment of maturation was observed in vitro at 50 μM AFB1 (Liu et al., 2015).
In a metabolomics study, zebrafish embryos were exposed to AFB1 in DMSO at concentrations of 0, 0.25, 0.5, 1 and 2 μM. Embryos were exposed to AFB1 for 24 h at 4, 24, 72 and 96 h post‐fertilisation (hpf). AFB1 was more toxic to embryos when exposed at 96 hpf compared to 24 hpf with LC50 of 0.5 and 2.1 μM, respectively. Concentrations of AFB1 below the LC50 values resulted in deformities such as malformation of the head and bending of the tail. Using high‐resolution NMR and principal components analysis 28 metabolites were identified and quantified from AFB1‐treated zebrafish. Of these metabolites, 19 were shown to be altered after 24 h exposure including increases in several amino acids (phenylalanine, tryptophan, tyrosine, isoleucine, glutamate, glutamine and glycine) while cysteine decreased. Increases were also noted in many metabolites associated with carbohydrate metabolism and in the neurotransmitter, gamma aminobutyric acid. Increases in fatty acids and cholesterol were observed but GSH decreased. All of these changes were statistically significant (Zuberi et al., 2019). This study was consistent with ex vivo metabolomics studies in mammals.
In summary, exposure to AFB1 was shown to cause effects on brain development in rats with a NOAEL of 7.1–13.6 μg/kg bw per day. It also caused shortened time to delivery and low birth weight in mice, with a NOAEL of 50 μg/kg. In addition, adverse effects have been found on spermatogenesis and folliculogenesis at the lowest dose tested, 4 μg/kg bw, but fertility was not tested. Thus, AFB1 affects reproductive and developmental parameters at low doses in rodents and these effects may occur following a short‐term exposure.
3.1.2.6. Immunotoxicity
The immuno‐modulatory effects of aflatoxins have been studied both in vitro and in vivo and their immunotoxic potential was shown in several animal species (review in Meissonnier et al., 2006; EFSA, 2007a; Bondy, 2008; Bondy, 2008). In rodents, the NOAELs for AFB1 for impaired immune response were mostly in the region of 30 μg/kg bw per day (EFSA, 2007a).
AFB1 reduces complement activity (EFSA, 2007a). In several animal species, AFB1 has also been demonstrated to inhibit macrophage functions such as phagocytosis, oxygen radical production and cytokine secretion, but also neutrophils chemotaxis and natural killer (NK) cell activity (EFSA, 2007a; Bondy, 2008).
Many studies conducted in poultry, pigs and rats showed that exposure to aflatoxins, mainly from naturally contaminated feed that may also contain other mycotoxins, resulted in suppression of the cell‐mediated immune response with lymphocyte depletion, atrophy of the lymphoid organs, decreasing delayed‐type hypersensitivity response to mitogens and modifying cytokine production (Meissonnier et al., 2006; EFSA 2007a; Bondy, 2008; Jolly et al., 2008). Recent studies also described an effect of AFB1 on target dendritic cells of porcine and human origin (Mehrzad et al., 2014, 2015, 2018a). In human monocyte‐derived dendritic cells exposed in vitro to 10 ng/mL (0.03 μM) of AFB1, an impairment of their phagocytic capacity was observed. The toxin also upregulated the expression level of mRNA encoding for several CYPs, MyD88, nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB), tumour necrosis factor‐alpha (TNF‐α), toll‐like receptor 2 (TLR2), TLR4, COX‐2, HLA‐DR, CCR7, CD209, lymphocyte function‐associated antigen 3 (LFA3) and CD16 and downregulated the expression of AhR, transforming growth factor β (TGF‐β), CD11c and CD64 within 2–12 h post‐exposure (Mehrzad et al., 2018a). In human microglia cells (CHME5 cell line), in vitro exposure to 20 ng/mL AFB1, a low concentration of AFB1, upregulates the mRNA expression of many proinflammatory molecules, such as TLRs, MyD88, NF‐κB and CxCr4, and increases the protein secretion of IFN‐γ and GM‐CSF (Mehrzad et al., 2018b).
Qian et al. (2014) exposed rats to 0, 5, 25 or 75 mg AFB1/kg bw, by daily gavage, for 1 or 5 weeks. At both time points no histological changes were observed in spleen tissue. However, after 1 week of exposure, a dose‐dependent decrease in the percentage of splenic CD8+ T cells and CD3‐CD8a+ NK cells was observed. An inhibition of the expression of IL‐4 and IFN‐γ by CD4+ T cells, IL‐4 and IFN‐γ by CD8a+ cells, and TNF‐α expression by NK cells was also observed. Five‐week exposure with AFB1 significantly increased the percentages of CD3+ and CD8+ T cells, especially at low doses (5 and 25 mg/kg bw). At this time point, a significant decrease of IL‐4 expression by CD4+ T cells and a significant increase of IFN‐γ expression by CD4+ T cells and TNF‐α expression by NK cells was also observed.
As far as humoral immunity is concerned, experiments mainly carried out with naturally contaminated feed, gave less consistent results; only a prolonged exposure to high doses of aflatoxins led to a significant reduction in plasma antibody concentrations in both rodents and farm animals (Jolly et al., 2008).
Data concerning the immunotoxicity of AFM1 are scarce. An in vitro study, on the human lymphoblastoid T‐cell line Jurkat, indicates that AFM1 significantly decreases cell proliferation. Only minor effects were noted on IL‐2 and IFN‐γ cytokines mRNA expression in stimulated cells that had been pre‐incubated with AFM1 (Luongo et al., 2014). Another in vitro study from the same team, performed on the human hepatoblastoma HepG2 cell line, demonstrated a decreased cell viability, an increase in the concentration of three pro‐inflammatory cytokines, IL‐6, IL‐8 and TNF‐α, and a decrease of the anti‐inflammatory interleukin, IL‐4 (Marchese et al., 2018). An in vivo study performed with an i.p. administration of AFM1 (25 and 50 μg/kg bw) for 28 days also demonstrated an effect on some immune parameters including proliferative response to lipopolysaccharide and phytohemagglutinin‐A, hemagglutination titre, delayed type of hypersensitivity, serum haemolytic activity, serum immunoglobulin G level and cytokine production (Shirani et al., 2018).
In mice, i.p. injection of AFB1 (daily injection of 10, 20 or 40 μg/kg bw for 15 days) increased the susceptibility of intranasal infection with Swine influenza virus as demonstrated by viral replication, lung inflammation and damage (Sun et al., 2018a,b). This increased susceptibility is associated with a macrophage polarisation from M1 to M2 as indicated by the decreased level of mRNA encoding for TNF‐α and the increased amount of IL‐10 (Sun et al., 2018b) and involved a TLR4‐NF‐κB signalling mechanism (Sun et al., 2018a). In several other studies performed in farm and laboratory animal species, the immunosuppressive effects of aflatoxins has been correlated with an increased susceptibility to microbial infections (bacterial, parasitic and viral) and to an impaired efficacy of vaccination (Meissonnier et al., 2006).
In conclusion, an immunotoxic effect of aflatoxins, especially of AFB1, has been described. The toxin mainly acts on the cellular immune response. As already mentioned, the NOAELs for these effects were around 30 μg/kg bw in rodents.
3.1.3. Observations in humans
3.1.3.1. Biomarkers of exposure
Several biomarkers of exposure have been used to investigate aflatoxin exposure and three of them have been validated against dietary intake of aflatoxins. These will be discussed in the following order: DNA adducts in urine, AF‐alb adducts in serum and AFM1 excreted in urine and breast milk.
Aflatoxin epoxide binds to DNA to form N7‐guanine adducts (AF‐N7‐gua), which are mutagenic if not repaired (see Section 3.1.2.3 Genotoxicity). AF‐N7‐gua adducts excised from DNA are excreted in urine. These urinary DNA adducts have been used as biomarkers in a number of early studies, employing HPLC with UV detection and using standard curves to quantify AF‐N7‐gua from the urine of exposed individuals. A correlation between dietary intake of aflatoxin and urinary AF‐N7‐gua was reported for populations in China (Groopman et al., 1992a; correlation coefficient 0.8, p < 0.00000001) and Gambia (Groopman et al., 1992b; correlation coefficient 0.82, p < 0.0001) and so urinary AF‐N7‐gua is considered a validated biomarker of exposure. This adduct, together with other urinary metabolites of AFB1, was used as a measure of aflatoxin exposure in a pivotal nested case–control study from a large prospective liver cancer study among middle‐aged men in Shanghai (Qian et al., 1994). In this study, the urinary aflatoxin metabolites revealed a strong association for risk of subsequently diagnosed liver cancer, especially among individuals also infected by HBV.
Aflatoxin epoxide in liver cells and aflatoxin dialdehyde in blood can bind covalently to lysine in albumin to form aflatoxin albumin adducts. As albumin has a serum half‐life of around 20 days, levels of aflatoxin albumin adducts reflect exposure over the previous 6–8 weeks. Methods to quantify aflatoxin albumin adducts require isolation and digestion of albumin from serum. The three main methods that have been applied to quantify these adducts are competitive inhibition ELISA, LC‐FD and LC–MS/MS. In this Opinion, where the LC–MS or LC‐FD is applied, then AFB1‐lys is used as these methods measure this amino acid adduct. Where the ELISA method is applied, AF‐alb is used because the ELISA method is not specific for the AFB1‐lys adduct. Isotope dilution LC–MS/MS can be considered to be the gold standard method for quantification of this adduct. A comparison of the three methods showed that there was an excellent correlation between them, but on average ELISA gave a value 3.2‐fold higher than isotope dilution LC–MS/MS (McCoy et al., 2008). In an earlier comparison across a lower range of adducted samples, the ratio between ELISA and LC–MS/MS was 2.6 (Scholl et al., 2006). The LC‐FD method gave slightly lower values than the LC–MS/MS method, which was attributed to the lack of adjustment for recovery in the LC‐FD method that is part of the isotope dilution LC–MS/MS method. The ELISA method used in these studies has shown good correlation between dietary aflatoxin intake and AF‐alb levels in adults in Gambia (Wild et al., 1992) and children in Tanzania (Routledge et al., 2014). Recently, a modified form of isotope dilution LC–MS, termed high‐resolution MS (HRMS) has been applied to measuring AF‐lys in a case–control study (McMillan et al., 2018).
Wild et al. (1992) measured AF‐alb levels in the serum of Gambian adults (n = 20), for whom aflatoxin intake was assessed by testing food samples from each meal over a 7‐day period. The biomarker sample was taken on day 8. There was a correlation between aflatoxin intake and AF‐alb levels (correlation coefficient = 0.55; p < 0.05). This study found that on average 1 μg aflatoxin intake per day was equivalent to about 30 pg AF‐alb/mg alb. As the average body weight of the individuals was 50 kg, this equates to 20 ng aflatoxin/kg bw per day giving 30 pg AF‐alb/mg alb.
Routledge et al. (2014) measured AF‐alb adducts in 148 children (aged 12–22 months) exposed to dietary aflatoxin in Tanzania. Aflatoxin intake was estimated by measuring aflatoxin contamination levels in maize flour samples from the households in which the children lived and calculating intake based on this and the amount of food eaten as recorded in a 24‐h dietary recall questionnaire. A correlation was seen between the two measurements (correlation coefficient = 0.43; p < 0.01), with a lot of interindividual variation which could reflect differences in absorption, metabolism, detoxification and/or measurement error.
Aflatoxin M1 is a hydroxylated metabolite of AFB1 that can be used as a biomarker for aflatoxin exposure as it is present in the urine and breast milk of exposed individuals. Most AFM1 is excreted in urine within 24 h of exposure, which means that AFM1 is a good biomarker of very recent exposure. In published reports, methods used for measuring AFM1 in human urine include commercial direct ELISA kits (Chen et al., 2018a; Kang'ethe et al., 2017; Schwartzbord et al., 2017), LC‐FD after immunoaffinity column purification (Piekkola et al., 2012) and LC–MS/MS multi‐biomarker approaches (Solfrizzo et al., 2014; Warth et al., 2012). In breastmilk, similar methods are used but with different sample preparation and clean‐up (Omar, 2012; Kang'ethe et al., 2017; Sadeghi et al., 2009; Ghiasian and Maghsood, 2012; Diaz and Sánchez, 2015; Polychronaki et al., 2006). The multi‐mycotoxin biomarker methods enable the rapid detection of biomarkers of several mycotoxins simultaneously. However, this advantage tends to come with a loss of sensitivity for some biomarkers, which may be important for some study populations exposed to lower levels of AFB1.
A correlation between dietary AFB1 intake and urinary AFM1 was reported by Zhu et al. (1987) in a study in the Guangxi Region of China. Analysis of AFB1 contamination of corn and peanut oil samples collected from 32 households each day for 6 days, coupled with careful recording of corn and peanut oil consumption was used to assess AFB1 intake in 52 individuals from whom total urine was collected on days 4–7 of the study. A correlation coefficient of 0.66 was observed. A correlation between dietary intake and AFM1 was also reported for another Chinese adult population (Groopman et al., 1992a). More recently, Chen et al. (2018a) collected urine and blood samples from 84 children aged 6–14 months in Tanzania, with AFB1 intake estimated from analysis of food samples and a 24 h dietary recall questionnaire administered to parents on the day the urine was collected. A correlation between urinary AFM1 levels and dietary intake of AFB1 in maize (r = 0.442, p < 0.001), as well as between AFM1 in urine and AF‐alb in serum of the children (r = 0.468, p < 0.001) was observed. Hence, AFM1 has been validated against dietary intake for short‐term exposure, although the correlation is not as strong as reported for urinary N7‐guanine (see above). AFM1 in breastmilk has not been validated against dietary intake.
A few studies have measured AFB1 in urine or serum as a measure of exposure, but these have not been validated against dietary intake.
In summary, AF‐alb (AFB1‐lys), urinary AF‐N7‐gua and urinary AFM1 are all validated biomarkers of dietary exposure to aflatoxin. However, the levels of these biomarkers cannot be converted reliably into dietary exposures in individuals, owing to the large interindividual variation, and the fact that all sources of dietary intake and/or chronic exposure to aflatoxin have not always been taken into account when determining the correlations. As AF‐alb (AFB1‐lys) better reflects longer‐term exposure (i.e. several weeks), it tends to be most widely used, while urinary AFM1 and AF‐N7‐gua are suitable biomarkers for recent exposure.
3.1.3.2. Liver disease
The CONTAM Panel identified 31 studies on liver disease published since 2006. Of these, 14 were selected as relevant for the risk assessment including 9 on primary HCC and 4 on other liver disease. Altogether, these include two reports of the same cohort study (reported at two time points), three nested case–control studies, seven case–control studies and two cross‐sectional studies, which are detailed below and summarised in Table 5.
Table 5.
Overview of epidemiological studies on the association between exposure to aflatoxin and liver disease
| Reference country | Study type (duration) | N | Age (years) (mean ± SD) | Biomarker (matrix) | Method (LOD/LOQ) | Levels of exposure | Outcome |
|---|---|---|---|---|---|---|---|
|
Afum et al. (2016) Ghana |
Case–control | 38 cases; 102 HBV/HCV +ve controls; 136 –ve controls | > 18 | Urinary AFM1 | HPLC‐FD (LOD = 0.5 pg/mg creatinine) |
68.5 pg/mg creatinine (cases) 67 pg/mg (−ve con) 65.3 pg/mg (+ve con) |
Liver disease |
| Anitha et al. (2014) India | Case–control |
138 cases 108 controls |
> 18 | AF‐alb (s) | ELISA (LOD ?) |
Con 19.25 pg/mg albumin Child's class A 18.1 pg/mg Child's class B 71.25 pg/mgChild's class C 575 pg/mg |
Decompensated liver disease |
|
Chen et al. (2007) Taiwan |
Case–control | 72 cirrhosis, 13 HCC, 229 controls | 66.9 ± 9.7 | AF‐alb (s) | ELISA (LOD 39.8 ng/mL) | 10.5 (ALD) vs. 5.5 (CON) ng/mg alb | ALD |
|
Chu et al., (2017) Taiwan |
Nested case–control |
232 cirrhosis cases 262 HCC cases 577 controls |
R: 30–65 | AF‐alb (s) | ELISA (LOD 2 fmol/mg albumin) |
Median of 21.5 fmol/mg (equivalent to about 9.8 pg/mg) in controls Median for cases not given |
Cirrhosis and HCC in HBV carriers |
| Chu et al. (2018), Taiwan | Nested case–control |
506 cases 2,636 controls |
R: 30–65 | AF‐alb (s) | ELISA (LOD 2 fmol/mg albumin) | Median of 21.5 fmol/mg (equivalent to about 9.8 pg/mg) in controls | HCC in HCV carriers |
|
Habibi et al. (2018) Iran |
Case–control |
41 cases 41 controls |
57.5 ± 10.8 (cases) 44.8 ± 15.1 (controls) |
AF‐alb (s) | ELISA (LOD 0.054 pg/mL serum) |
Cases: Median 3.87 pg/mL, IQR 3.46 Controls: Median 2.63 pg/mL, IQR 3.14c |
HCC |
|
Jolly et al. (2007) Ghana |
Cross‐sectional | 162 | 40.8 (R: 19–86) | AF‐alb (s) | Radioimmunoassay | Mean 0.89 ± 0.46 pmol/mg albumin (equivalent to 407 ± 211 pg/mg albumin) | Liver disease |
|
Kuniholm et al. (2008) Gambia |
Case–control |
97 cases 397 controls |
Controls 44.8 ± 15.2 Cases 42.5 ± 14.1 |
TP53 249ser | FFQ for aflatoxin exposure | NA | Cirrhosis |
|
Liu et al. (2008) China |
Case–control |
71 cases 695 controls |
36.6 ± 15.6 (controls). Not given for cases | AF‐alb (p) | ELISA (LOQ 10 fmol/mg albumin) |
Cases: mean 15.11 pmol/mL plasma Control: mean 10.02 pmol/mL plasmad |
HCC |
|
Lu et al. (2010) China |
Longitudinal (21 years) | 515 HBV +ve (123 went on to develop HCC) | R: 20–60 at recruitment | AFM1 (24 h u) Collected monthly for 8 months at outset | HPLCb following immunoaffinity column concentration (LOD 0.5 ng/mL) |
Mean of 48.46 ng/mL and a median of 24.90 ng/mL, range 5.7–243 ng/mL among AFM1 +ve samples Association with HCC based on +ve vs. −ve AFM1 results |
HCC |
|
Lu et al. (2012) China |
Longitudinal (23 years) |
148 Absolute number of cases not given |
R: 20–60 at recruitment |
AFM1 (24 h u) Collected monthly for 8 months at outset |
HPLCb following immunoaffinity column concentration (LOD 0.5 ng/mL) |
Mean of 48.46 ng/mL and a median of 24.90 ng/mL, range 5.7–243 ng/mL among AFM1 +ve samples Association with HCC based on +ve vs −ve AFM1 results |
HCC |
|
Manda et al. (2018) Côte d'Ivoire |
Case–control |
33 HCC 66 controls (33 HBV +ve & 33 HBV −ve) |
49.84 ± 15.34 (R: 24–77) | AFB1‐lys (s) | HPLC‐FD (LOQ 2.3 pg/mg albumin) | Mean of 36.57 pg/mg albumin in HCC patients, 34.95 pg/mg albumin in HBV patients and 25.63 pg/mg albumin in blood donors | Liver disease |
|
Mohd‐Redzwan et al. (2014) Malaysia |
Cross‐sectional | 71 aflatoxin exposed subjectsa | 34.34 ± 9.7 (R: 23–57) |
AFB1‐lys (s) AF metabolites (u) |
HPLC‐FD (LOQ 0.17 ng/mL) | Mean 6.85 +/−3.2 pg/mg | Liver disease |
|
Wu et al. (2009a) Taiwan |
Nested case–control | 241 cases and 1,052 controls from an initial cohort of 24,000 | 53.8 ± 7.9 |
AF‐alb (s) AF metabolites (u) |
ELISA (LOD 0.01 fmol/μg) ELISA (LOD 1 fmol/mL urine) |
Mean 59.8 fmol/mg albumin (equivalent to about 27 pg/mg) Mean 55.2 fmol/mL urine |
HCC |
|
Zheng et al. (2017) China |
Case–control |
214 cases 214 controls |
50.7 ± 9.7 (cases) 51.2 ± 9.9 (controls) |
AF‐alb (s) AF‐N7‐gua (u) |
ELISA (LOD 0.1 ng/mL) ELISA (LOD 0.1 ng/mL) |
Median; Cases 146.23 pg/mg albumin, controls 74.42 pg/mg albumin Median; cases 0.17 ng/mg creatinine controls 0.14 ng/mg creatinine |
HCC |
+ve: positive; −ve: negative; AF‐alb: aflatoxin albumin adduct; AFB1‐lys: aflatoxin B1 lysine adduct; AF‐N7‐gua: aflatoxin‐N7‐guanine; ALD: advanced liver disease; ELISA: enzyme‐linked immunosorbent assay; FD: fluorescence detection; FFQ: food frequency questionnaire; HBV: hepatitis B virus; HCC: hepatocellular carcinoma; HCV: hepatitis C virus; HPLC: high‐performance liquid chromatography; LOD: limit of detection; LOQ: limit of quantification; IQR: interquartile range; p: plasma; R: range; s: serum; SD: standard deviation; u: urine.
Identified by screening for AFM1 in urine.
Detector not reported.
Concentration reported as pg/mL serum without correcting for albumin levels which may be different depending on health status.
No correction by the authors for the albumin level which was about 10% higher in controls than in cases. Consequently, the CONTAM Panel could not convert the concentration into pg/mg albumin.
3.1.3.2.1. Primary liver cancer
Prior to 2006, it was established that AFB1 exposure was an independent risk factor for primary HCC, with aflatoxin enhancing risk among HBV carriers (see Section 1.3.3). A previous Opinion (EFSA, 2007a) considered the study by Yeh et al. (1989) on the role of aflatoxin exposure and HBV infection in the southern Guanxi Provence, a high‐risk region for liver cancer in China, as the pivotal study for the risk assessment. That study established a linear relationship between estimated mean annual dietary intake of aflatoxin at the community level and primary liver cancer mortality in a cohort of 7,917 men. The estimated aflatoxin intake per person per year was calculated by multiplying the aflatoxin contamination determined by regular testing of food samples by the annual intake of food commodities within a community, divided by the number of people in the community. Of note, in these data is that even communities with the lowest estimated aflatoxin intake, i.e. 12 ng/kg bw per day (see Table 4) had high liver cancer mortality. Prevalence of HBV was high within the population but whereas estimated aflatoxin intake varied 3.5‐fold across the five communities in the study, HBV prevalence did not. This suggests that variations in the incidence of HCC between communities was not driven by HBV prevalence, even though HBV‐positive status was a high‐risk factor for the development of HCC. Yeh et al. (1989) reported that an intake of 60 mg/person per year was associated with primary liver cancer mortality of 600/100,000 person‐years. For HBsAg‐positive and negative subjects, the mortality rates of primary liver cancer were 953.8 and 17.5/100,000 person‐years, respectively. More detailed information on the dose–response relationship for HBsAg‐positive and negative subjects is provided in the paper by Wu‐Williams et al. (1992) and is presented in Table 4.
Table 4.
Aflatoxin dose, number of primary hepatocellular carcinoma cases and adjusted person‐years of follow‐up from the cohort studied by Yeh et al. (1989) and reported by Wu‐Williams et al. (1992)
| Estimated dose of AFB1 (ng/kg bw per day) | Number of primary hepatocellular carcinoma cases | Total person‐years | Number of primary hepatocellular carcinoma cases per total person‐years | |||
|---|---|---|---|---|---|---|
| HBsAg positive | HBsAg negative | HBsAg positive | HBsAg negative | HBsAg positive | HBsAg negative | |
| 12 | 12 | 0 | 2,737 | 9,932 | 0.0044 | 0.0000 |
| 90 | 7 | 1 | 2,017 | 6,114 | 0.0035 | 0.0002 |
| 705 | 12 | 4 | 2,537 | 7,733 | 0.0047 | 0.0005 |
| 2028 | 23 | 2 | 1743 | 5,803 | 0.0132 | 0.0003 |
AFB1: aflatoxin B1; HBsAg: hepatitis B surface antigen; bw: body weight.
The nine studies on aflatoxin and HCC published since 2006 include one cohort study (reported at two time points), three nested case–control studies and five case–control studies. Between 2007 and 2018, three nested case–control studies from Taiwan and one cohort study from China have been reported that used validated biomarkers to assess aflatoxin exposure prior to cancer development. The Chinese 21‐year prospective cohort study (1988–2009) involved collecting monthly 24 h urine samples over an eight month period at the beginning of the study for a cohort of high‐risk individuals who were all positive for HBV infection (n = 515) from Qidong City, Jiangsu Province, an area of China with a high prevalence of HCC (Lu et al., 2010). Aflatoxin exposure was assessed by analysing monthly urinary AFM1 levels, averaged over an eight‐month urine collection period. During the follow‐up 21‐year period, 109 of the 515 patients died of liver cancer. Hepatitis B infection was strongly associated with a risk of liver cancer (relative risk = 7.79) in comparison with the uninfected population. Within this cohort of HBV‐positive individuals, the relative risk for liver cancer in aflatoxin‐exposed vs non‐exposed individuals was 2.23 (p = 0.008). The observations in this study were extended to 148 patients over a 23‐year follow‐up period (Lu et al., 2012). This paper reports an increased relative risk for new incident liver cancer associated with aflatoxin exposure of 2.37 (95% CI: 1.29–4.33).
A nested case–control study (241 cases and 1,052 controls from an initial cohort of 24,000) in Taiwan (Wu et al., 2009a) measured serum AF‐alb and urinary aflatoxin metabolites stored at enrolment. The authors grouped individuals based on whether their biomarker levels were higher or lower than the mean for the study. An increased risk of HCC was found in non‐HBV carrier individuals; for AF‐alb the odds ratio (OR) was 1.54 (95% CI: 1.01–2.36) and for urinary AF metabolites the OR was 1.76 (95% CI: 1.18–2.58). HBV status had a much stronger effect, with an OR of 7.49 (95% CI: 5.13–10.93) for HBV carriers independent of aflatoxin exposure. The association increased to an OR of 10.38 (95% CI: 5.73–18.82) for HBV carriers with above‐mean AF‐alb and an OR of 15.13 (95% CI: 7.83–29.25) for HBV carriers with above‐mean AF urinary metabolite levels.
Two later nested case–control studies in Taiwan investigated the risk of HCC in individuals infected with either HBV (Chu et al., 2017) or HCV (Chu et al., 2018). Serum AF‐alb was measured in samples collected at enrolment, which was up to nine years before the disease outcome was assessed. In the first study, Chu et al. (2017) looked at chronically infected HBV subjects (232 cirrhosis cases, 262 HCC cases and 577 controls from an initial cohort of 24,000). High AF‐alb levels compared with undetectable AF‐alb were associated with an increased risk of cirrhosis at entry (OR 2.45, 95% CI: 1.51–3.98), cirrhotic HCC nine years after entry (OR 5.47, 95% CI: 2.20–13.63) and non‐cirrhotic HCC 4 years after entry (OR 5.39, 95% CI: 1.11–26.18). In the second study (Chu et al., 2018), the role of HCV and alcohol as risk factors were considered and included 506 HCC cases and 2,636 controls. High vs low serum AF‐alb levels were associated with HCC risk in habitual alcohol consumers (OR = 4.22, 95% CI: 1.16–15.37) and in HCV‐infected participants (OR = 3.39, 95% CI: 1.31–8.77).
Four case–control studies examining the association of validated aflatoxin biomarkers with HCC were published between 2007 and 2018. However, it should be noted that all the assessed cancer case–control studies examined exposure biomarkers in samples that were collected after disease occurrence. Liu et al. (2008) reported significantly higher levels of AF‐alb adducts in the serum of HCC patients (n = 71) from Guanxi Zhang Autonomous Region, compared with controls in four HBV categories (n = 71 for each) from the same region. All cases were HBV‐positive, compared with 75/694 controls from three regions of China including high and low HCC risk regions. Various markers of HBV infection were not associated with AF‐alb levels in controls. In a study on newly diagnosed HCC cases (n = 214) and controls (n = 214) in Chongqing, China, Zheng et al. (2017) found an OR of 1.9 (95% CI: 1.1–3.4) for AF‐alb adduct, and 2.1 (95% CI: 1.0–4.2) for AF‐N7‐gua adduct, after adjustment for various confounders including HBV. HBV was the most important risk factor associated with HCC in this population, and there was an interaction between both aflatoxin biomarker levels and HBV, alcohol consumption and diabetes. Habibi et al. (2018) measured AF‐alb in 41 hepatitis‐related HCC patients and 41 controls with HBV or HCV and reported higher mean levels of AF‐alb in the cancer patients (3.87 pg/mL) than in the controls (2.63 pg/mL). However, they reported AF‐alb as pg/mL serum and did not correct for albumin levels, which could be different for cases and controls. Manda et al. (2018) examined AFB1‐lys adducts in the serum of HCC patients (n = 33) and controls (n = 33 HBV‐positive controls and 33 blood donor controls) from Côte d'Ivoire. They did not find any significant difference (p > 0.05) between AFB1 adduct levels in HCC patients (mean: 36.57 pg/mg albumin) and control patient groups. Mean adduct levels were lower, but not statistically different (p > 0.05), in the 33 blood donor controls (25.63 pg/mg albumin) compared to 33 HBV‐positive controls (34.95 pg/mg albumin).
In summary, the studies reported since 2006 have added to the weight of evidence that aflatoxin exposure is associated with a risk of developing HCC, with a higher risk for people infected with either HBV or HCV.
3.1.3.2.2. Other liver disease
Since 2006, four case–control studies and two cross‐sectional studies have investigated liver diseases other than cancer as endpoints associated with aflatoxin exposure. In an endemic HCV area of Taiwan, Chen et al. (2007) found an association between AF‐alb levels and HCV infection (> 8 ng/mg vs. < 8 ng/mg, OR = 2.019 (95% CI: 1.09–4.0)) and between AF‐alb levels and advanced liver disease in HCV‐infected subjects (> 8 ng/mg vs. < 8 ng/mg, OR = 2.29 (95% CI: 1.23–1.47)). Taking all subjects (HCV positive and negative), mean levels of AF‐alb were 10.5 ng/mg alb in advanced liver disease versus 5.5 ng/mg in mild/no liver disease. The CONTAM Panel noted that the biomarker levels are about 1,000 times higher than those in other studies and that there is only an abstract given as a reference for the method. Jolly et al. (2007) examined AFB1‐lys and various measures of liver function and illness in a cross‐sectional study in Ghana (n = 140). There was a positive association between AFB1‐lys and both serum total protein and ALT. Kuniholm et al. (2008) examined the role of aflatoxin as a risk factor for cirrhosis in a Gambian population. Using a questionnaire to estimate lifetime peanut consumption as a proxy for aflatoxin exposure, there was an association between high exposure and cirrhosis (OR = 2.8, 95% CI: 1.1–7.7). Using the mutation at codon 249 in the TP53 gene in circulating DNA as a biomarker of past exposure, the association with cirrhosis increased (OR = 3.8, 95% CI: 1.5–9.6). There was a stronger association for HBV infection (HBsAg, OR = 8.0, 95% CI: 4.4–14.7; hepatitis B e antigen (HBeAg), OR = 10.3, 95% CI: 2.0–53.9). In a study in Malaysia (n = 71), Mohd‐Redzwan et al. (2014) observed a higher total bilirubin and creatinine level, but no differences for other markers of liver function, in subjects with above‐the‐mean AFB1‐lys measured in this study (6.85 pg/mg alb). Anitha et al. (2014) found an association between HBV infection and AFB1‐lys levels with liver cirrhosis in a case–control study in India. AFB1‐lys was detected in 8/108 controls (mean 19.25 pg/mg alb) compared with 18/18 with initial liver disease and 11/112 with cirrhosis. Of these the mean AFB1‐lys increased with severity of disease up to 575 pg/mg. No controls were positive for HBV, whereas 128/130 patients were HBV‐positive. Patients with both HBV‐ and AFB1‐lys‐positive results had more severe liver disease. Afum et al. (2016) found no significant difference in urinary AFM1 levels between cases with liver disease and controls (n = 276) in a study in Ghana. While increased levels of AF biomarkers have been associated with HBV or HCV infection, it is not clear whether the toxin exposure makes infection more likely (e.g. due to liver damage or suppressed immune function) or hepatitis leads to increased levels of biomarker formation. Wild and Montesano (2009) reviewed the interactions between hepatitis virus and aflatoxin in 2009, including discussion of some animal and cell experiments supporting the hypothesis that aflatoxin exposure could increase HBV infection or viral genome integration, but did not come to a conclusions regarding the mechanism underlying the interaction.
AFB1 can cause acute toxicity in humans (acute aflatoxicosis) exposed to high levels of dietary AFB1 in a short time period. Symptoms of acute aflatoxicosis include GI distress, jaundice, hepatitis and liver failure and such outbreaks often have a high mortality rate. Because symptoms may be similar to some types of bacterial food poisoning or infectious disease, confirmation of acute aflatoxicosis has not always been possible. However, several cases have been confirmed by measuring aflatoxin in food or aflatoxin biomarkers in affected individuals. In an outbreak in India in 1974, cases were associated with an estimated consumption of 2–6 mg aflatoxin/day. Out of 397 patients, 106 died (Krishnamachari et al., 1975). In 1981, 12/20 affected adults died in Kenya after consuming high levels of aflatoxin in family food (Ngindu et al., 1982). AF‐lys levels in serum were measured to confirm aflatoxin as the cause of 125 deaths from 317 cases in Kenya in 2004 (Azziz‐Baumgartner et al., 2005). Based on three reported cases of acute aflatoxicosis in India and Kenya, Wild and Gong (2010) calculated that an intake of more than 20 μg/kg bw per day was associated with acute aflatoxicosis in adults. This required maize aflatoxin contamination levels of more than 1,000 μg/kg. It is not clear whether repeated exposure is required, but symptoms of hepatitis may occur up to 3 weeks following acute symptoms such as vomiting (Kamala et al., 2018). In this more recent acute aflatoxicosis outbreak in Tanzania (Kamala et al., 2018), AF‐alb levels of > 1,000 pg/mg albumin in cases versus controls (OR = 13.5, 95% CI: 1.5–165.3) were associated with acute aflatoxicosis. In this outbreak, there were 20 deaths among 68 cases. Maize samples collected about 5 weeks after the outbreak began showed higher levels of contamination from case households versus controls (geometric mean 354.5 μg/kg vs 44.1 μg/kg; p = 0.04). Such contamination levels are far higher than any seen in the EU, and the CONTAM Panel therefore considers the risk of acute aflatoxicosis to be highly unlikely in the EU.
In summary, high AFB1 exposure causes acute aflatoxicosis with a high mortality rate. Lower levels of chronic exposure to AFB1 are associated with cirrhosis and indicators of liver dysfunction. There appears to be an interaction between AFB1 exposure and HBV or HCV infection and consequently the risk of non‐HCC liver disease.
3.1.3.3. Other cancers
There are two case–control studies showing an association between aflatoxin and other cancers. In a case–control study in a Shanghai population of gall bladder cancer (GBC) patients and controls (gallstones) (Koshiol et al., 2017), an association with GBC was found for the presence of AFB1‐lys adducts assessed in serum taken after diagnosis (AFB1‐lys detected in 32% of the cases vs 15% of the controls), as well as the level of AFB1‐lys and GBC (5.4 vs. 1.2 pg/mg alb in cases vs controls). The authors acknowledged weaknesses in the study design.
In a case–control study in Korea (n = 477), Eom et al. (2013) used a structured interview to estimate aflatoxin intake in relation to stomach cancer risk. There were no direct measurements of aflatoxin levels. They observed an elevated risk of stomach cancer associated with higher aflatoxin intake; OR = 1.94, 95% CI: 1.43–2.63.
There is currently insufficient evidence to associate aflatoxin exposure with GBC and stomach cancer.
3.1.3.4. Kidney disease
In a pilot study investigating the possible impact of aflatoxin exposure on kidney disease in indigenous Mexican women (n = 34), de León Martinez et al. (2019) reported a geometric mean of 3.48 (95% CI: 2.4–5.0) pg/mg albumin. Statistically significant correlations were reported between AFB1‐lys and both kidney injury molecule 1 (Rho = 0.498, p = 0.007) and cystatin‐C (Rho = 0.431, p = 0.014), suggesting a possible role for aflatoxin exposure in kidney damage.
3.1.3.5. Anaemia in pregnancy
Only one study was identified that assessed in a cross‐sectional fashion the correlation between aflatoxin exposure (AF‐alb) and anaemia in 755 pregnant women in Ghana (Shuaib et al., 2010b). Although the odds of being anaemic increased statistically significantly with each quartile of AF‐alb, the study design per se and the lack of replication do not support anaemia in pregnancy as an endpoint of interest for the risk assessment at present.
3.1.3.6. HIV‐related outcomes
There is a growing body of evidence assessing how aflatoxin exposure affects biomarkers and health‐related outcomes in populations infected with HIV. As far as observational studies are concerned, five publications were identified spanning 2007 to 2013 and all recruiting populations from Ghana. Only one cohort study was identified (Keenan et al., 2011); 141 HIV‐positive Ghanaians were assessed for aflatoxin exposure (AF‐alb, median 0.94 pmoL/mg albumin equivalent to 430 pg/mg albumin) and followed up for a median of 208 days for the development of symptomatic opportunistic infections. At each visit, a maximum of four symptomatic diseases were recorded in the patient records and the five most frequent diseases were chosen for outcomes (malaria, herpes, tuberculosis, pneumonia and hepatitis). A statistically significant increased hazard ratio was observed for symptomatic tuberculosis (hazard ratio 3.30, 95% CI: 1.34–8.11) for those in the highest AF‐alb quartile compared with the lowest. The remaining four observational studies were cross‐sectional with various degrees of overlap (Jiang et al., 2008; Jolly et al., 2011, 2013; Obuseh et al., 2011).
The presence of only one small longitudinal study pertaining to this group of endpoints does not justify the use of HIV‐related endpoints in the risk assessment at present.
3.1.3.7. Child health
Nineteen studies assessed 31 associations between aflatoxin exposure and outcomes related to mother and child health (Table 6; stillbirth, n = 2; prematurity, n = 1; growth, n = 18; autism, n = 1; nodding syndrome, n = 1; organomegaly, n = 1; hepatitis B surface antibodies, n = 1). Only one study assessed a European population (Italy, autism) while the remaining studies assessed African populations (n = 15), South American populations (n = 2) and Asian populations (n = 1) in settings of higher exposure than European populations.
Table 6.
Overview of epidemiological studies on the association between exposure to aflatoxin and child health
| Reference Country | Study type (months) | N | Age (months) | Biomarker (matrix) | Method (LOD/LOQ) | Levels of exposure (mean ± SD) | Outcome |
|---|---|---|---|---|---|---|---|
| Hoffmann et al. (2018)Kenya | Cluster RCT (24) | 1,230 | NA | AFB1‐lys (s) | HPLC‐FD (0.2 pg/mg alb) | LAZ, stunting | |
| Lauer et al. (2019)Uganda | Birth cohort | 220 | < 2 days | AFB1‐lys (s) | HPLC‐FD (0.2 pg/mg alb) | 5.83 (IQR 3.53–9.62) | Birth weight, birth length, bLAZ, bWAZ, bWLZ, bHC, bHCAZ |
| Voth‐Gaeddert et al. (2018)Gambia | Birth cohort, Cross‐Sectional (5) | 320 | 30.2 years (mothers) | Putative aflatoxin exposure (maize) | ELISA kit for AFT (2 μg/kg) | 48.1 (95% CI: 30.6–65.7) | HAZ |
| Watson et al. (2018)Routledge (2019a)Gambia | Birth cohort (12) | 374 | NA | AF‐alb (s) | ELISA (3 pg/mg alb) | NR | WAZ, LAZ, WLZ, IGFBP‐3, IGF‐1 |
| Mitchell et al. (2017)Nepal | Birth cohort (36) | 85 | 15 | AFB1‐lys (p) | LC–MS (0.4 pg/mg alb) | 3.85 (15.75)c | WAZ, HAZ, WHZ |
| Magoha et al. (2014)Tanzania | Birth cohort (5) | 143 | < 5 | AFM1 (bm) | LC‐FD (0.005 ng/mL) | 11.08 (10.13)b | WAZ, HAZ, WHZ |
| Turner et al. (2007)Gambia | Birth cohort (12) | 138 | NR | AF‐alb (s) | ELISA (5 pg/mg alb) | 40.4 (4.8–260.8)c | WAZ, HAZ, birth weight, birth length |
| Chen et al. (2018b)Tanzania | Cohort (12) | 60 | 24–36 | AFB1‐lys (p) | LC–MS (0.4 pg/mg alb) | 5.1 (95% CI: 3.5–6.6) | WAZ, HAZ, WHZ |
| Leroy et al. (2018)Mexico | Cohort (6) | 347 | 12 | AFB1‐lys (s) | LC‐FD (0.2 pg/mg alb) | 0.82 ± 0.72 | Height, HAD |
| Shirima et al. (2015)Tanzania | Cohort (12) | 166 | 6–14 | AF‐alb (p) | ELISA (3 pg/mg of alb) | 4.7 (95% CI: 3.9–5.6) | WAZ, HAZ, WHZ, growth velocity |
| Carlos et al. (2014)Mexico | Case–control (NA) | 342 | >30 | Aflatoxigenic food intake | FFQ | NA | Stillbirth, LBW |
| Echodu et al. (2018)Uganda | Cross‐sectional (NA) | 84d | NR | Total aflatoxin (food) | ELISA (NR) | 3.8 (0–7.8)a , e | Nodding syndrome |
| Githangá et al. (2019)Kenya | Cross‐sectional (NA) | 205 | 1–14 years | AFB1‐lys (s) | HPLC‐FD (0.4 pg/mg alb) | 45.38 (87.03); g.mean, 20.4 | Low hepatitis B surface antibody titre |
| McMillan et al. (2018)Nigeria | Cross‐sectional (NA) | 58 | 0.5–2 | AFB1‐lys (p) | LC–MS/MS, IDMS (22 pg/mL) | 2.6 (0.2–59.2)a | Severe acute malnutrition, stunting, HAZ, WHZ |
| De Santis et al. (2017)Italy | Cross‐sectional (NA) | 233 | 24–144 | AFB1 (s, u) | LC–MS/MS (LOQ 0.03 ng/mL) | s, 0.01 ± 0.05; u, 0.12 ± 0.12 | Autism |
| Castelino et al. (2015)Kenya | Cross‐sectional (NA) | 199 | 144 | AF‐alb (s) | ELISA (LOD 3 pg/mg alb) | 110.5 (95.4–127.9)c | Height |
| Shouman et al. (2012)Egypt | Cross‐sectional (NA) | 46 | < 52 | AFB1 (s) | TLC (NR) | 51.6 (30.6–62.8)a | WAZ, HAZ |
| Shuaib et al. (2010a)Ghana | Cross‐sectional (NA) | 785 | 26.8 years (mother) | AFB1‐lys (s) | LC‐FD (0.5 pg/mL) | 10.9 ± 19.0 | Stillbirth, prematurity, SGA, LBW |
| Gong et al. (2012)Kenya | Cross‐sectional | 249 | 6–17 years | AF‐alb (s) | ELISA (3 pg/mg alb) | 114.5 (99.7, 131.4)c | Hepatomegaly, splenomegaly, hepatosplenomegaly |
AFB1: aflatoxin B1; bm: breast milk; FFQ: food frequency questionnaire; g. mean: geometric mean; HAD: height‐for‐age difference; HAZ: height‐for‐age z‐score; HPLC: high‐performance liquid chromatography; LC–MS: liquid chromatography coupled to mass spectrometry; LC–MS/MS: liquid chromatography coupled to tandem mass spectrometry; LBW: low birth weight; LOD: level of detection; LOQ: level of quantification; NA: not applicable; NR: not reported; p: plasma; s: serum; SD: standard deviation; SGA: small for gestational age; u: urine; WAZ: weight‐for‐age z‐score; WHZ: weight‐for‐height z‐score; y: year.
Median, range/IQR.
Estimated.
Geometric mean (SD or range or 95% CI).
Households.
In food.
3.1.3.7.1. Pre‐ and postnatal growth
A total of 15 studies published from 2007 to 2019 assessed the association between aflatoxin exposure and indices of child growth (Table 6). One cluster randomised controlled trial in Kenya was identified. Of the remaining 14 observational studies, 5 studies were birth cohorts with a follow‐up for 18 months after birth, 3 were prospective cohorts with a follow‐up ranging from 6 to 36 months, 1 was a case–control study, 5 were cross‐sectional studies and 1 study implemented both a cross‐sectional and cohort study design. The sample sizes of the included observational studies ranged from 46 to 785 participants. All the evaluated populations came from non‐European, low‐ and middle‐income countries: Mexico (n = 2), Guatemala (n = 1), Nepal (n = 1), Egypt (n = 1), Ghana (n = 1), Gambia (n = 2), Kenya (n = 2), Nigeria (n = 1), Uganda (n = 1) and Tanzania (n = 3). All but one of the assessed studies evaluated children younger than 3 years, while Castelino et al. (2015) evaluated schoolchildren.
Most of the studies (80%, n = 12) used biomarkers for exposure assessment; AFB1‐lys was measured in six studies, AF‐alb was measured in four studies, and AFB1 and AFM1 were measured in one study each. The remaining two studies estimated aflatoxin exposure by using a food frequency questionnaire including food items prone to aflatoxin contamination (Carlos et al., 2014) or estimated the putative aflatoxin exposure level defined as the estimate of the average amount of aflatoxins consumed in a single day by a child (Voth‐Gaeddert et al., 2018). All the included studies pertained to populations with considerably higher exposures than those found in European populations.
Child growth was measured using the same indices but with a range of statistical analyses. Intra‐uterine growth was assessed either as a continuous trait using birth weight and length at birth (Turner et al., 2007) or as a dichotomous variable using the small for gestational age (Shuaib et al., 2010a) or low birth weight categorisation (Carlos et al., 2014; Shuaib et al., 2010a). Child growth was measured in most cases using the well‐established weight‐for‐age z‐scores (WAZ) and height‐for‐age z‐scores (HAZ). Stunting or wasting was captured as a baseline characteristic or as a covariate but not commonly used as a study outcome, possibly due to the high prevalence of these conditions in the populations under study. Due to the known complex nature of child growth and its dependence on numerous parameters (Bundy et al., 2017) the included studies used a wide variety of adjustments in the final statistical analyses.
Height or HAZ were statistically significantly and negatively associated or correlated with aflatoxin exposure in six publications (7 studies: 1 birth cohort, 1 cohort, 5 cross‐sectional studies) using various biomarkers. WAZ and birth WAZ were statistically significantly and negatively associated with aflatoxin exposure in 3 and 1 birth cohorts, respectively, again using various biomarkers.
In summary, child growth appears to be an emerging area of research in the field of adverse events related to aflatoxin exposure. However, the currently available body of evidence is characterised by small sample sizes, considerable heterogeneity in the assessed populations and biomarkers, varying methodological quality, and effect inconsistency. Thus, the potential for using these studies for risk assessment is limited. In the following section, prospective studies using a biomarker are reported in detail by study design (RCT, cohort), continent, country, biomarker and year of publication. Information about all assessed studies, including case–control and cross‐sectional studies, is provided in Table 6.
Hoffmann et al. (2018) conducted the only cluster randomised trial in rural Kenya to assess the effectiveness of an intervention that reduced aflatoxin exposure on child linear growth. They enrolled women in the fifth to final month of pregnancy (1,230 unborn children). The intervention consisted of swapping contaminated maize with safe maize and encouraging household purchases from a stock list supplied with clean maize. The primary outcomes were child length‐for‐age z‐score (LAZ), the prevalence of stunting and child serum AFB1‐lys level after 24 months (endline follow‐up); the secondary outcomes included child LAZ, the prevalence of stunting and child serum AFB1‐lys levels at 11 to 19 months (midline follow‐up). At baseline, the observed aflatoxin exposure in the mothers corresponded to 14.7 and 15.5 pg/mg albumin in the intervention and control groups, respectively. Attrition was 28% for LAZ and 35% for the serum AFB1‐lys levels at 24 months with comparable attrition rates between the intervention and control groups. Interestingly, aflatoxin exposure showed a decreasing trend in both groups over the course of the study. At 24 months, the intervention significantly reduced serum AFB1‐lys levels (5.9 vs. 7.5 pg/mg albumin), but had no effect on LAZ or stunting. Conversely, at the intermediate follow‐up points, the intervention statistically significantly increased LAZ and reduced stunting without affecting serum AFB1 levels (4.7 vs. 5 pg/mg albumin). The authors note that this could be due to seasonal variation or differences in response to the intervention and avoid proposing an association between reductions in exposure and improvements in linear growth.
Cohort studies
Lauer et al. (2019) assessed in a birth cohort the association between maternal aflatoxin exposure during pregnancy and birth‐related outcomes in 220 mother–infant pairs in Uganda. AFB1‐lys was measured using HPLC‐FD and the median AFB1‐lys levels in the mothers were 5.83 pg/mg albumin (range: 0.71–95.60 pg/mg albumin, interquartile range: 3.53–9.62 pg/mg albumin). In the adjusted analysis, higher maternal AFB‐lys levels were significantly associated with lower birth weight, lower birth WAZ, and lower head circumference‐for‐age z‐score at birth.
Leroy et al. (2018) report on a cohort study nested within a cluster randomised controlled trial on the efficacy of three micronutrient supplements in Mexico. The cohort study population comprised 347 children with archived samples collected at the trial 4‐month follow‐up and corresponding to about one third of children who participated in the efficacy trial. Aflatoxin exposure was assessed using AFB1‐lys adduct and the baseline exposure was 0.82 (SD 0.72) pg/mg alb, which is lower compared with other studies looking at child growth. Higher serum AFB1‐lys adduct levels at baseline were statistically significantly associated with greater children's linear growth from the trial's baseline. The CONTAM Panel notes that this is an effect in the opposite direction to previous reports. At the 10‐month trial follow‐up point (6‐month cohort follow‐up period, 12% attrition), there was no statistically significant association between aflatoxin exposure and height‐for‐age difference (HAD).
Chen et al. (2018b) assessed the association between aflatoxin exposure and weight and length in a Tanzanian setting with a high reported prevalence of growth impairment. Using a cohort study design, they included a subsample (53%) of 60 children who were assessed for aflatoxin exposure at the age of 24 months (AFB1‐lys) and were followed up for 12 months. At baseline, 17% of the children were underweight, 72% had detectable AFB1‐lys exposure, and the mean level of AFB1‐lys was 5.1 (95% CI: 3.5–6.6) pg/mg alb. There were no statistically significant associations observed between aflatoxin exposure and WAZ or weight‐for‐height z‐score (WHZ).
Mitchell et al. (2017) conducted an extension of the Chen et al. (2018b) study in Nepal. This cohort study included 85 children followed up for 36 months and assessed aflatoxin exposure at 15, 24, and 36 months of age (AFB1‐lys). There were no associations found between AFB1‐lys and WAZ and weight‐for‐length z‐score (WLZ).
Watson et al. (2018) using a birth cohort in Gambia (n = 374) assessed the association between aflatoxin exposure (AF‐alb) at 6 months and growth indices (WAZ, WLZ, length, LAZ) at 6, 12, and 18 months. At 6, 12 and 18 months of age, 48%, 98%, and 99% of available plasma samples had detectable AF‐alb concentrations, respectively. After adjustment for covariates (season, mother's household quality, supplementation group, and age of introduction of non‐breastmilk food), higher average AF‐alb levels were statistically significantly associated with decreased LAZ, WAZ and WLZ scores during follow‐up. Aflatoxin exposure was also statistically significantly associated with change in length, LAZ and WLZ. Moreover, aflatoxin exposure was statistically significantly correlated with insulin‐like growth factor‐binding protein 3 (IGFBP3). Statistically significant associations were not reported for change in WAZ and no statistically significant correlation was reported between aflatoxin exposure and insulin‐like growth factor 1 (IGF‐1).
Turner et al. (2007) reported on a birth cohort with 138 mother–infant pairs in Gambia. The assessed association pertained to in utero aflatoxin exposure (AF‐alb) and birth weight as well as weight and length with a 52‐week follow‐up. The geometric means of AF‐alb levels were 40.4 pg/mg (range 4.8–260.8 pg/mg), 10.1 pg/mg (range 5.0–189.6 pg/mg) and 8.7 pg/mg (range 5.0–30.2 pg/mg) in maternal, cord and infant blood, respectively, with a seasonal variation present for maternal and cord blood measurements. Neither maternal nor cord blood AF‐alb was significantly associated with lower birth weight or birth length. After adjustment for covariates (gender, age, placental weight, maternal weight, gestation time, season) a higher average maternal AF‐alb was significantly related to lighter WAZ (‐0.249 SD; p=0.012). In contrast, cord AF‐alb was not associated with WAZ. No statistically significant associations were reported for HAZ. Besides assessing intra‐uterine exposure through measurements in maternal and cord blood, Turner et al. (2007) also assessed the association between aflatoxin exposure at 16 weeks and WLZ at the 52‐week follow‐up (4% attrition). There were no statistically significant results observed for weight but a statistically significant association was found for length.
Shirima et al. (2015) conducted a multi‐site cohort study on the association between aflatoxin exposure and weight and length in infants in Tanzania (three sites) in settings with a high prevalence of growth impairment. They recruited 166 infants (6–14 months old) and followed them for 12 months (interim assessment at 6 months, 12% attrition). The proportion of underweight children was 8% at baseline. Aflatoxin exposure was measured using plasma AF‐alb adducts. Statistically significant differences were observed between sites at baseline for both the percentage of positive samples and the mean concentrations. Although the results between mean AF‐alb levels and WAZ and WHZ at 12 months were not reported in the published report of the study, communication with the authors confirmed that there were no statistically significant associations observed (Routledge, 2019b).
Magoha et al. (2014) studied the association between AFM1 exposure and growth for 143 mother–infant pairs in a high‐exposure setting in Tanzania using a cohort study design. AFM1 exposure was estimated through AFM1 levels measured in breast milk (age 1, 3 and 5 months), the breast milk intake recorded by the United States Environmental Protection Agency for infants of his/her age, and the infant's body weight. All the breast milk samples were contaminated by AFM1 at levels ranging from 0.01 to 0.55 ng/mL (> 90% of samples above the EU limit for infant food; > 76% above the EU limit for dairy milk and milk products). Exclusive breastfeeding decreased considerably during the follow‐up (19% at month 3, 3% at month 5). The mean estimated AFM1 exposure was 11.08 (± 10.13) ng/kg bw per day) and ranged from 1.13 to 66.79 ng/kg bw per day. Due to the observed decrease in exclusive breastfeeding, the highest exposure levels were observed at baseline. A small but significant inverse association was observed between AFM1 exposure levels and WAZ (adjusted beta −0.009; CI: −0.016 to −0.001) and HAZ (beta −0.013; 95% CI: −0.024 to ‐0.002), but not for WHZ (adjusted beta −0.020; CI: −0.028 to 0.068).
3.1.3.7.2. Effects other than growth
A small number of studies assessed a diverse group of outcomes in children including stillbirth, prematurity, autism, nodding syndrome, anti‐HBs titres and organomegaly. All were of small to moderate sample size and none was longitudinal. Due to the small number and the limitations of the assessed studies, the available evidence does not support any of these endpoints as eligible for the risk assessment. Detailed information on study characteristics for this group is provided in Table 6. In addition, other symptoms have been reported by Voth‐Gaeddert et al. (2018) but these were not specific for aflatoxin exposure.
3.1.3.7.3. Summary
Child health is an emerging area of interest for the field of aflatoxin‐related health outcomes but not yet suitable for use in risk assessment. Child growth is assessed in a growing body of evidence outside European populations with limited replicability in the observed associations. The evidence related to the remaining child health outcomes is sparse, heterogeneous and with methodological limitations.
3.1.4. Mode of action
There is convincing evidence from numerous publications that AFB1 has a genotoxic mode of action. Thereby the formation of pro‐mutagenic DNA adducts can be regarded as a molecular initiating event (Moore et al., 2018). Subsequently, misreplication or mis‐repair of adducted DNA might result in mutations of critical genes. In addition to DNA adduct formation, a broad spectrum of cellular effects have been reported in response to AFB1 exposure.
3.1.4.1. DNA adduct formation
The reactive AFB1‐exo‐8,9‐epoxide can covalently bind to N7 of guanine in DNA, yielding the AFB1‐N7‐gua. DNA adduct formation is > 2,000 times greater in DNA than in aqueous solution with free 2′dG, presumably due to intercalation (Bren et al., 2007; Brown et al., 2009). Under physiological conditions, spontaneous depurination or rearrangement to the more persistent ring‐opened AFB1‐FAPY adduct might occur. In DNA, AFB1‐N7‐gua and AFB1‐FAPY intercalate above the 5′‐face of the respective guanine. In vitro studies indicate sequence specificity with preferential formation of DNA adducts in guanine‐containing sequences (Besaratinia et al., 2009). Both AFB1‐N7‐gua and AFB1‐FAPY produce G‐to‐T transversions in E. coli, with the AFB1‐FAPY being more mutagenic (Banerjee et al., 2011; Stone et al., 2011). A site‐specific mutagenesis assay in mammalian cells (COS‐7) showed a replication error frequency of the AFB1‐FAPY adduct of 97% with G‐to‐T transversions as the predominant effect (Lin et al., 2014, 2016).
Recent studies identified the translesion synthesis DNA polymerase polζ to be able to bypass the AFB1‐FAPY lesion and might account for the commonly occurring G‐to‐T transversions (Lin et al., 2013, 2014; McCullough and Lloyd, 2019).
3.1.4.2. Factors affecting DNA damage and repair
DNA adduct formation depends on the production rate of AFB1‐exo‐8,9‐epoxide and its detoxification by three main pathways: (i) spontaneous or epoxide hydrolase‐mediated hydrolysis; (ii) GSH conjugation; (iii) further oxidation by CYPs. Several of the involved enzymes, particularly GSTs and CYP3A4, are known potential sources of interindividual variation in susceptibility to aflatoxins (EFSA, 2007a; see Sections 3.1.1 and 3.1.4.6).
Hydrolysis of the 8,9‐epoxides leads to the unstable AFB1‐8,9‐dihydrodiol, which is prone to base‐catalysed rearrangement, thus generating AFB1 dialdehyde (Figure 1) that may react with proteins (Guengerich, 2005). Members of the NADPH‐dependent AKR play a key role in the reduction of the reactive AFB1 dialdehyde to the less reactive AFB1‐dialcohol. Expression of the human isoform AKR7A3 in mammalian cells was found to decrease the cytotoxicity of AFB1 and its dialdehyde (Bodreddigari et al., 2008), supporting the role of AKR in detoxification.
In Fischer F344 rats, application of a potent nuclear factor erythroid 2‐related factor 2 (Nrf2) activator (CDDO‐Im) for 5 weeks (three doses of 30 μmol/L by oral gavage every other day) before administration of AFB1 (daily 200 μg/kg by gavage for 4 weeks) was found to suppress the level of AFB‐FAPY‐adducts and prolonged the mean life span of the animals from 74 to 90 weeks (Johnson et al., 2014). Among others, the Nrf2 pathway regulates the expression of GSTs and key enzymes of GSH biosynthesis, which might at least contribute to the reported effects. Furthermore, Nrf2 knockout rats display higher sensitivity to AFB1 toxicity (Taguchi et al., 2016). The effect of AFB1 on antioxidant key enzymes regulated by the Nrf2 pathway is not only limited to the liver but has also been described in the kidney (Wójtowicz‐Chomicz et al., 2013). Taken together, the available studies indicate an important role for the Nrf2 pathway in the suppression of AFB1 adduct formation via regulation of GSH biosynthesis and GST expression.
Base excision repair (BER) was investigated in a study in male mice (heterozygous p53 knock out and control wild‐type) exposed to 0, 0.2 and 1.0 mg/kg AFB1 in the diet for 26 weeks (Mulder et al., 2015). Exposure to AFB1 did not alter BER either in the liver or lungs of p53 knock out (+/−) mice. In p53 (+/+) control livers repair activity was decreased in the 1.0 mg/kg AFB1 treatment group (compared to 0.2 mg/kg), an effect that was not seen in the p53 (+/−) knock out livers. A previous study from the same group using the same dosing protocol, observed the opposite effect on nucleotide excision repair. In that study AFB1 treatment increased global nucleotide excision repair in p53 (+/+) tissues, and this effect was attenuated in p53 (+/−) tissues (Mulder et al., 2014).
In an attempt to induce liver carcinomas in Wistar rats upon i.p. treatment with AFB1, differentially expressed genes were predominately observed for cell proliferation, cell adhesion and vasculature development, thus reflecting tumour development. Downregulation was observed in the gene group involved in apoptosis regulation and DNA repair (Shi et al., 2016).
3.1.4.3. Induction of oxidative stress
There is increasing evidence that AFB1 is not only able to generate DNA adducts, but also induce oxidative stress (da Silva et al., 2018). Imbalance in cellular redox systems might arise from: a) direct pro‐oxidative chemical features of a compound; or b) impact on antioxidative defence systems on the transcriptional (gene expression) or posttranscriptional level (e.g. protein adduct formation; enzyme inhibition).
Recent studies demonstrate that AFB1 enhances reactive oxygen species (ROS) formation and causes oxidative damage (Marin and Taranu, 2012; Zhou et al., 2019). In several animal models, AFB1 was found, among others, to uncouple mitochondrial oxidative phosphorylation, induce mitochondrial permeability, enhance lipid peroxidation and decrease the level of GSH (Liu and Wang, 2016; Shi et al., 2015; da Silva et al., 2018). However, it remains to be clarified whether this is due to interactions on the protein level or impact on gene expression.
Direct impact on cellular proteins has been shown in vitro. Under cell‐free conditions as well as in cell culture (HepG2) AFB1 inhibits the activity of the 20S proteasome, which is involved in the cellular defence against oxidative stress (Amici et al., 2007). Moreover, in the low micromolar range, AFB1 was reported to act as a moderate competitive inhibitor of serine proteases, thus potentially interfering with the removal of damaged proteins (Cuccioloni et al., 2009). AFB1 was reported to induce autophagy in macrophages (An et al., 2017). Autophagy is a central intracellular process, delivering cytoplasmic components to the autophagosomes and lysosomes for degradation. Present studies indicate that autophagy induction by AFB1 occurs downstream of ROS production (An et al., 2017). In 3D4/21 cells18 incubation with AFB1 induced oxidative stress, enhanced the expression levels of the DNA methyltransferases DNMT1 and 3a and activated the JAK2/STAT3 signalling pathway (Zhou et al., 2019). These results might provide an additional link between the induction of oxidative stress by AFB1 and its immunological properties.
Studies in vitro and in vivo argue that AFB1 has an impact on enzymes of the antioxidant defence. Prolonged incubation of HepG2 cells (24 or 48 h) with AFB1 resulted in a decrease of glutathione reductase and catalase activity, whereas an increase in GST activity was apparent (Amici et al., 2007). Male albino Charles Foster rats showed significantly enhanced ROS levels in the liver 4 weeks after AFB1 application (i.p., 1 mg/kg bw, twice on consecutive days) together with declining immunostaining for superoxide dismutase, indicative of a decrease in the antioxidant defence (Singh et al., 2015). These data are in line with reports on the importance of the Nrf2 pathway to suppress AFB1 toxicity (see Section 3.1.4.2).
Recent studies demonstrate that the onset of oxidative stress by AFB1 leads to oxidative DNA damage. In HepG2 human HCC cells, incubation with AFB1 resulted not only in the formation of respective DNA adducts but also > 30‐fold higher amounts of cyclic α‐methyl‐γ‐hydroxy‐1,N 2‐propano‐dG arising from lipid peroxidation (Weng et al., 2017). In adolescents (n = 84) from an area in China at high risk for HCC, urinary AFB1 levels were positively associated with the urinary excretion of 8‐hydroxydeoxyguanosine (8‐OHdG) as well as 8‐OHdG and hOGG1 levels in peripheral lymphocytes indicative for the presence of oxidative stress (Peng et al., 2007). In a case–control study in Taiwan, (74 HCC cases, 290 matched controls) an association between urinary AFB1 metabolites and 8‐oxo‐dG with urinary 15‐F2t‐isoprostan, a lipid oxidation marker, was observed (Wu et al., 2007, 2008). Furthermore, it has to be taken into account that HBV infection has also been shown to induce oxidative stress (Liu et al., 2008).
In summary, besides DNA adduct formation, AFB1 induces oxidative stress including modulation of antioxidant defence systems. Considering the potential sequence of events towards HCC, oxidative stress might compromise critical AFB1 detoxification pathways (e.g. GSH conjugation) and/or induce additional DNA lesions.
3.1.4.4. Gene transcription and epigenetic mechanisms
Toxicogenomic in vitro studies show the clear impact of AFB1 exposure at the transcription level. A spectrum of cellular transcriptional response results from the DNA adduct formation of AFB1. Nevertheless, several studies also argue for non‐genotoxic mechanisms such as the binding of AFB1 to nuclear receptors as a modulating factor for gene regulation. In cultured primary human hepatocytes, a 24 h treatment with AFB1 at non‐cytotoxic concentrations (0.001 μM) upregulates the gene transcription of several nuclear receptors: the aryl hydrocarbon receptor (AhR), the pregnane X receptor (PXR) and the constitutive androstane receptor (CAR). Furthermore, in concentrations up to 1 μM, enhanced transcript levels of the CYP1A1, 1A2, 2B6, 3A5, 3A4 and 2C9 were observed (Ayed‐Boussema et al., 2012), indicating the activation of respective nuclear receptors. In lymphocytes and monocytes from healthy volunteers (n = 10, male), CYP1A1, CYP1B1, CYP3A4, CYP3A5 and CYP3A7 were found to be expressed. In monocytes AFB1 treatment highly induced CYP1A1, CYP1B1 and CYP3A4, but only CYP1A1 was induced in lymphocytes, arguing for a different response in myeloid and lymphoid lineage cells (Bahari et al., 2014, 2015). The partly planar and bulky structure of AFB1 might indeed favour binding to nuclear receptors, thus affecting gene expression.
Several epigenetic mechanisms have been associated with AFB1 exposure and the development of HCC. Overall, a decline of global DNA methylation together with hypermethylation of several tumour suppressor genes has been observed in vitro and in vivo (Zhang et al., 2006, 2012; Wu et al., 2013; Dai et al., 2017; Martin and Fry, 2018). In primary human hepatocytes AFB1 treatment (0.3 μM, repetitive daily treatment for 5 days, followed by 3 days washout) has been reported to affect the DNA methylation pattern (Rieswijk et al., 2016). Upregulation of TXNRD1 diminishes the expression of AFB1‐aldehyde reductase and GST which play an important role in the detoxification of the genotoxic AFB1‐8,9‐epoxide. Feng et al. (2012) reported a statistically significant association between AFB1‐DNA adducts and RASSF1A methylation in human HCC.
Human immortal hepatocytes expressing one oncogenic H‐Ras allele, L02R cells, were treated weekly with 0.3 μM AFB1, leading to a malignant phenotype showing anchorage‐independent grow and the formation of tumours in immunodeficient mice at week 17 post‐treatment. Seven genes were identified as downregulated by DNA hypermethylation (Wang et al., 2017). Among others, transformation was associated with hypermethylation of the RUNX3 gene. In 20 pairs of HCC and their adjacent tissues, hypermethylation of RUNX3 was found in 70% of the HCC samples, downregulation of respective mRNA in 95% (19/20) (Wang et al., 2017).
Furthermore, in utero exposure to AFB1 has been associated with a modified DNA methylation pattern in the offspring (2–8 months), measured in white blood cells of the infants (Hernandez‐Vargas et al., 2015).
A recent study using skin‐ (HaCaT) and lung‐derived cells (L‐132), reported upregulation of both the maintenance (DNMT1) and de novo DNA methyltransferases (DNMT3a and DNMT3b) after incubation with AFB1 (24 h, 1 μM) on the transcription and on the protein level. AFB1‐treatment was found to decrease HAT activity and increases HDAC expression and activity (Soni et al., 2018). However, the underlying mechanism for this impact on epigenetic key enzymes remains to be elucidated.
In Balb/c mice exposure to AFM1 was found to decrease the expression of the microRNA (miRNA) miR‐155 in T cells, which is discussed to contribute to immunotoxicity (Shirani et al., 2019). So far, modulation of the expression levels of several microRNAs (miRNA) have been associated with effects caused by AFB1 exposure including liver carcinogenesis (Zeng et al., 2010; Herceg and Paliwal, 2011; Fang et al., 2013; Liu et al., 2014; Marrone et al., 2016; Dai et al., 2017). In patients with HCC related to AFB1 exposure, differences in expression of several miRNA either in tumour tissue (e.g. miRNA‐24, Liu et al., 2014) or serum (e.g. miRNA‐4651, Wu et al., 2017), have been identified as potentially relevant. However, it is unclear whether AFB1 directly affects miRNA expression (e.g. via binding to CpG‐rich promoters) or whether changes in miRNA expression arise from secondary cellular responses to the genotoxicity of AFB1.
3.1.4.5. Other potential targets
Lung
In air–liquid interface cultures of primary human sinonasal and bronchial cells, AFB1 and AFB2 were found to reduce ciliary beat frequency, a mechanism involved in mucociliary immunity (Lee et al., 2016). In lung cancer cells AFB1 affects several signalling pathways involved in carcinogenesis and tumour cell migration (Cui et al., 2015). In human bronchial epithelial cells (BEAS‐2B), incubation with AFB1 (1.5 μM, 30 min) resulted in a decrease of the p53 level, persisting for 12 h (Van Vleet et al., 2006). In the respiratory tract CYP2A13 is discussed to play a central role for metabolic activation of AFB1 (Yang et al., 2013). But AFB1 also appears to play a role for the onset of oxidative stress in the lung. Treatment of female A/J mice with a single i.p. dose of 50 mg/kg bw AFB1 resulted in an increase of 8‐OHdG formation in alveolar macrophages and Clara cells (Guindon et al., 2007; Guindon‐Kezis et al., 2014).
For AFG1, the presence of TNF‐α as a proinflammatory stimulus was associated with an upregulation of CYP2A13 and enhanced oxidative DNA damage in murine AT‐II cells and human AT‐II like cells (A549) (Shao et al., 2019).
Development and reproduction
In Leydig cells, isolated from 35‐day‐old male Long–Evans rats, incubation with AFB1 decreased the secretion of testosterone in a dose‐dependent manner. Significant effects were observed after 3 h at a concentration of ≥ 1 μM. After 18 h of incubation a significant decrease of testosterone secretion was already measured with 0.01 μM of AFB1. Furthermore, the expression of cholesterol transporter steroidogenic acute regulatory protein (StAR), 3β‐hydroxysteroid dehydrogenase (HSD3B) and 17β‐hydroxysteroid dehydrogenase enzyme (HSD17B3) was suppressed (Adedara et al., 2014). In Sprague Dawley rats, treatment with AFB1 (gavage postnatal days 49–70) at 15 and 150 μg/kg bw per day resulted in a decrease of serum testosterone, luteinising hormone and FSH levels together with a downregulation of testosterone biosynthesis‐related genes and a decrease of the Leydig cell number. In vitro, treatment of isolated adult Leydig cells with AFB1 inhibited the expression of testosterone biosynthesis genes, enhanced ROS production and induced apoptosis. Apoptosis induction was associated with the suppression of the AMPK/mTOR‐mediated autophagy flux pathway (Chen et al., 2019).
In studies with trophoblastic JEG‐3 cells, the placental transporters ABCC2 and OAT4 were increased about fivefold at 2 and 6 μM AFB1, whereas the expression of ABCG2 was suppressed. Several enzymes involved in steroid homeostasis were upregulated including CYP19A1, HSD3B1, HSD17B1 and members of the UGT1A‐family (Huuskonen et al., 2013).
In porcine parthenotes, ex vivo treatment with AFB1 impaired the development of blastocytes at concentrations ≥ 1 nM indicative for impact on early embryonic development (Shin et al., 2018).
Mature male Swiss albino mice were treated i.p. for 7, 14 and 21 days, receiving a daily dose of 20 μg/kg bw AFB1. In addition to the impact on cell cycle regulation by downregulation of CDK1, cyclin D4 and induction of p21, a decrease of estrogen receptor alpha (ERα) expression was observed (Zamir‐Nasta et al., 2018). Male mice (4 weeks of age) receiving a daily dose of 50 μg/kg bw AFB1 i.p. for 45 days prior to potential mating, showed no significant differences in the number and viability of the offspring. The relevance of an apparent increase in transcripts for Renin in the AFB1‐treated males remains to be clarified (Austin et al., 2012).
Taken together, the results argue for impact of AFB1 on key enzymes in hormone homeostasis which may lead to the disturbance of regulatory mechanisms in fertility. Transport processes across the placenta may also be affected.
3.1.4.6. Factors influencing susceptibility in humans
This section focuses on factors influencing susceptibility of humans. The CONTAM Panel notes that in animal studies at high doses, substances that causes regenerative hyperplasia may exacerbate the incidence of tumours.
3.1.4.6.1. Co‐occurrence with viral infections
Hepatitis B virus
It is well‐established that co‐exposure to HBV has a strong influence on the carcinogenic risk of aflatoxins to humans (see Sections 1.3.3 and 3.1.3.2.1). In epidemiological studies, there is an interaction between aflatoxin exposure and hepatitis B infection, and subjects positive for HBsAg show a multiplicative risk for liver cancer when present together with aflatoxin exposure (FAO/WHO, 2018).
At the molecular level, some data suggest that HBV infection of the liver alters the expression of the genes coding for the enzymes which metabolise/detoxify aflatoxins such as an induction of CYP enzymes or decrease in GST activity. This may provide one mechanistic basis for the higher risk of liver cancer among HBV‐infected individuals exposed to aflatoxins (EFSA, 2007a).
Hepatitis C virus
Aflatoxin B1 exposure has also been shown to increases the risk of HCC in patients with HCV infection (see Section 3.1.3.2.1). In a nested case–control study in Taiwan, high serum AF‐alb levels were associated with HCC risk in HCV–infected participants (Chu et al., 2018).
Jeannot et al. (2012) also demonstrated that transgenic mice expressing several HCV proteins (core, E1, E2 and p7, nucleotides 342–2771) were prone to hepatocarcinogenesis when exposed to AFB1. No liver lesions were observed in 7‐day‐old mice (wild‐type or HCV‐transgenic) treated. with a single dose of tricaprylin administered i.p, used as vehicle. Upon treatment with 6 μg/g bw AFB1, tumours (adenomas or carcinomas) and preneoplastic lesions (hyperplasia or foci) were observed in 22.5% (9 of 40) and 50% (18 of 36) of wild‐type and HCV‐transgenic mice, respectively; the difference being largely due to the incidence of adenomas (30.5 vs 12.5%). Although oxidative stress and steatohepatitis were observed in both AFB1‐treated groups, molecular changes indicative of the enhanced inflammatory response and altered lipid metabolism were more pronounced in HCV‐transgenic mice.
Epstein–Barr virus
Epstein–Barr virus (EBV) is a member of the gamma herpes virus family. Although mostly asymptomatic, EBV infection has been associated with several human B‐cell malignancies, e.g. endemic Burkitt's lymphoma in children in sub‐Saharan Africa. The EBV life cycle in B cells comprises latent stages, where only a few viral genes are expressed, and the lytic stage, characterised by expression of all viral genes and rapid replication until lysis of the host cell occurs. In vitro and in animal models, exposure of B cells to AFB1 leads to the alteration of cellular gene expression that in turn reactivates EBV towards the lytic cycle. AFB1 is considered to be a cofactor in EBV‐mediated carcinogenesis (Accardi et al., 2015).
3.1.4.6.2. Genetic polymorphisms
Some genetic polymorphisms have previously been identified as being associated with increased risk of aflatoxin‐related liver cancer, including the GSTM1 null polymorphism and the XRCC1 gene codon 399 AG/GG variants (Kirk et al., 2005).
So far, a spectrum of genetic polymorphisms has been identified affecting the susceptibility of individuals to AFB1‐mediated liver carcinogenesis. A study involving 966 healthy adults (Guangxi, China) reported an association of the GSTM1‐null genotype and XRCC3 genotypes (i.e. threonine/methionine and methionine‐methionine variants) with higher levels of AFB1‐DNA adducts in peripheric blood lymphocytes (measured by ELISA) (Long et al., 2009b). In a case–control study based in the same region with 1,499 liver cancer cases and 2,045 controls, an association between genetic polymorphisms in the DNA repair gene XRCC4 (codon 247 alanine>serine), higher AFB1‐DNA adduct levels and increased risk for HCC (Long et al., 2013). A study with 2,558 healthy adults of the Guangxi Region found XPC genotypes with codon 939 glutamine alleles (XPC‐lysine‐glutamine and XPC‐glutamine‐glutamine variants) to be associated with higher levels of AFB1‐DNA adducts in leucocytes (ELISA) (Long et al., 2015).
Eight single nucleotide polymorphisms (SNPs), including SLCO1B1, SLCO1B3, GSTT1, GSTM1, GSTA1, GSTP1, CYP2E1 and CYP3A4, were determined in a case–control study in a rural Chinese area with 475 patients with liver damage and 475 controls. For SLCO1B1 (T521C), a member of the solute carrier transporter family, the OR of genotype TC vs TT was 0.743, indicating a reduced risk. No clear associations were observed for the other SNPs (Yang et al., 2017b). A study located in the Guangxi region (China), comprising 181 cases of HCC and 641 probands without carcinoma, found an increased risk for HCC in individuals with the GSTM1‐null or GSTT1‐null genotype (Wei et al., 2012).
Between 2006 and 2018 there have been several reports on the role of genetic polymorphisms in DNA repair genes in aflatoxin‐associated HCC from the Guangxi Zhang Autonomous Region of China, a high‐risk region for aflatoxin exposure and HCC. In a case–control study on 491 HCC cases and 862 controls, Long et al. (2008) reported an increased risk of HCC in individuals with the XRCC3 codon 241 methionine/threonine or methionine/methionine variants compared with those homozygous for threonine at codon 241. The adjusted OR for HCC among met homozygotes versus threonine homozygotes was 7.19 (95% CI: 4.52–11.42). Having high levels of AFB1 DNA adducts was also a risk factor for HCC (OR 5.58, 95% CI: 4.19–7.44). In a separate case–control study in the same region, Long et al. (2009a) reported an increased risk of HCC associated with the codon 751 glutamine heterozygous and homozygous variants of XPD compared with the lysine homozygotes, with a higher risk in women than men. No association with HCC risk was observed for the XPD codon 312 polymorphism in this population. In a separate investigation with 1,156 HCC cases and 1,402 controls, the same group (Long et al., 2010) found an association between HCC risk and the glutamine variants of XPC codon 939 versus the lysine homozygotes, although the effect was not large, with an OR of 1.25 (95% CI: 1.03–1.92) for the heterozygotes and 1.81 (95% CI: 1.36–2.40) for the glutamine homozygotes. The XRCC7 rs#7003908 polymorphism was also found to modify HCC risk in the region (Long et al., 2011), with increased risk associated with ‐TG or ‐GG variants compared with ‐TT; OR 3.45 (95% CI: 2.40–4.94) and OR 5.04 (95% CI: 3.28–7.76), respectively.
3.1.4.6.3. Others
A nested case–control study in Taiwan investigated the risk of HCC in relation to aflatoxin exposure and alcohol consumption; high versus low serum AF‐alb levels were associated with HCC risk in habitual alcohol consumers (OR 4.22, 95% CI: 1.16–15.37). It was suggested that alcohol consumption modifies the hepatocarcinogenic effect of AFB1 via the increased hepatocyte vulnerability to AFB1‐induced DNA damage and mutations (Chu et al., 2018).
3.1.5. Considerations of critical effects and dose–response analysis
3.1.5.1. Considerations of critical effects
It is clear from in vitro and animal studies that AFB1, AFB2, AFG1 and AFM1 are mutagenic and also AFB1, AFG1 and AFM1 are carcinogenic when delivered orally via the diet or by gavage with the evidence being most abundant for AFB1. There is limited evidence for the carcinogenicity of AFB2 and inadequate evidence for carcinogenicity of AFG2. Based on evidence for AFB1, it can be concluded that absorption occurs in the small intestine with as much as 50% of the dose reaching the liver where it is activated. A critical step in the activation is the formation of AFB1‐exo‐8,9‐epoxide which is known to form adducts with DNA and proteins. Studies have shown that DNA lesions and DNA adducts such as AFB1‐N7‐gua and AFB1‐FAPY are formed and that these can lead to G‐to‐T transversions.
Effects in experimental animals
A clear dose–response relationship was observed between AFB1 and the incidence of HCC in experimental animals, with the dose–response being linear over a wide concentration range, at least, in rainbow trout. While AFG1 produces fewer liver tumours than AFB1, it induces more kidney tumours in animal models. Studies in vitro have shown a difference in genotoxic potency between AFB1 and AFG1 with the indication that AFG1 is less toxic than AFB1 by about a factor of 10 in liver cells (see Section 3.1.2.3). While the absolute potency of AFB2 and AFG2 is not known, the literature suggests that they are less potent than AFB1. AFM1 is known to be less effective, with a potency 0.1 times that of AFB1 based on carcinogenicity in rats (see Section 1.3.3).
The liver is also the most sensitive organ, with AFB1 causing acute hepatotoxicity in experimental animals. Several indicators of liver damage are altered after AFB1 exposure including biochemical changes (upregulation of enzymes known to indicate damage), histological changes (bile duct proliferation), increases in GST‐P+, a marker of pre‐neoplastic damage and formation of AFB1 adducts. Short‐term toxicity studies reported changes in liver function and gut morphology, and there is also evidence of growth effects, with stunting and wasting being noted.
There is clear evidence for oxidative stress occurring in animals exposed to AFB1 but this was considered secondary to the effects on the liver. Changes in the gut microbiota and immunotoxic effects have been noted, but these occur at higher doses and are therefore not considered critical.
Exposure to AFB1 was shown to cause a number of effects on reproduction and development. These included a shortened time to delivery and low birth weight in mice (NOAEL of 0.05 mg/kg bw per day), effects on brain development in rats (NOAEL of 0.007–0.014 mg/kg bw per day) and adverse effects on spermatogenesis at the lowest dose tested (0.004 mg/kg bw per day) and following a short‐term exposure. To evaluate whether these effects should be considered in the risk characterisation of aflatoxins in humans, the CONTAM Panel compared the identified doses with a scenario of short‐term exposure and noted that calculated exposure is three orders of magnitude lower than the LOAEL of 4 μg/kg bw per day. Therefore, the CONTAM Panel concluded that reproductive and developmental toxicity should not be considered further.
Effects in humans
AFB1 can cause acute aflatoxicosis with a high mortality rate. However, this effect is observed following high AFB1 exposure and is not expected to occur in the EU population (see Section 3.1.3.2.2).
There is clear evidence from the studies reported since the 1970s that aflatoxin exposure is associated with a risk of HCC, with a higher risk for people infected with HBV. The studies on aflatoxin and HCC published since 2006 have added to this evidence and a higher risk is now also reported for HCV. However, there is currently insufficient evidence to associate aflatoxin exposure with other cancers such as gall bladder cancer and stomach cancer.
Child health is an emerging area of interest among aflatoxin‐related health outcomes in humans. Adverse effects on child growth related to aflatoxin exposure have been reported from a growing body of evidence from populations outside of Europe and this is supported by data from experimental animal studies. However, the currently available evidence is weak, being characterised by small sample sizes, heterogeneity in the assessed populations and biomarkers, varying methodological quality, and effect inconsistency. Thus, at present, the potential for using these studies for risk assessment is limited.
Aflatoxin adducts, AF‐alb (AFB1‐lys), urinary AF‐N7‐gua and urinary AFM1 are all validated biomarkers of dietary exposure to aflatoxin. However, these biomarkers cannot be converted reliably into dietary exposures in individuals and can, therefore, presently not be used in dietary risk assessments. Consequently, the new epidemiological studies which used biomarkers of exposure cannot be used to identify a reference point.
Overall, the CONTAM Panel considers that liver carcinogenicity of aflatoxins remains the pivotal effect for the risk assessment, both in experimental animals and in humans. The epidemiological study by Yeh et al. (1989) on mortality from liver cancer in several provinces in China, and the two‐year carcinogenicity study by Wogan et al. (1974), are still considered the most suitable studies for performing a dose–response analysis.
3.1.5.2. Dose–response analysis (including BMD modelling)
As described in Section 3.1.5.1, the CONTAM Panel considered liver carcinogenicity to be the critical effect following oral exposure to aflatoxins. The CONTAM Panel selected the chronic study by Wogan et al. (1974) in male rats for dose–response modelling of the incidence of HCC (see Table 2). A BMD analysis was performed using the EFSA web tool, which is based on the R‐package PROAST 66.38. The BMD analysis performed followed the updated guidance of the Scientific Committee on BMD modelling (EFSA Scientific Committee, 2017) and a detailed description of the BMD analysis performed by the Panel can be found in Appendix III. The default benchmark response (BMR) for quantal data was selected, i.e. an extra risk of 10%. Using model averaging, the resulting BMDL10 for the incidence of HCC was 0.4 μg/kg bw per day (see Appendix III.1).
From the human studies, the CONTAM Panel selected the study by Yeh et al. (1989) as the pivotal study. In 2018, the CONTAM Panel also used this study as the pivotal study and concluded, based on wide BMD confidence intervals, that it was not appropriate to use BMD analysis to identify a reference point for risk assessment (see EFSA CONTAM Panel, 2018, for further details). Instead, the CONTAM Panel decided to use the cancer potency estimates reported by JECFA (FAO/WHO, 2018; see Section 1.3.3). No new information has become available that changes the previous conclusion and the same approach was followed in the current assessment.
3.1.6. Possibilities for derivation of a health‐based guidance value (HBGV)
In view of the genotoxic properties of aflatoxins, the CONTAM Panel considered that it was not appropriate to establish a tolerable daily intake and considered the possibility of applying an MOE approach. Based on studies in animals, the CONTAM Panel selected a BMDL10 of 0.4 μg/kg bw per day for the incidence of HCC to be used in an MOE approach for the risk characterisation. The calculation of a BMDL from the human data was not appropriate and no MOE approach could be used for these data; instead, the cancer potency estimates reported by JECFA were used (see Section 1.3.3 for further details).
Differences in carcinogenic potency are reported for AFB2 and AFG2 compared with AFB1 and AFG1. However, in vivo there is insufficient evidence to derive potency factors for AFB2 and AFG2. There are indications of differences in the cancer potency between AFB1 and AFG1 in the liver with AFB1 being more potent. In the kidney, AFG1 has a higher cancer potency than AFB1. Again, the available data are not sufficient to be able to derive an individual potency factor that can be used in the risk assessment. Therefore, in the absence of new in vivo data to quantify differences between the individual aflatoxins the CONTAM Panel applied equal potency factors for AFB1, AFB2, AFG1 and AFG2 as used in previous assessments. The CONTAM Panel considers that this conservative approach is appropriate in this case, but notes the uncertainty arising from the insufficient data available on AFB2 and AFG2. For AFM1, JECFA (FAO/WHO, 1999, 2001, 2018) concluded, based on a study in Fischer rats, that AFM1 induces liver cancer with a potency one tenth that of AFB1. No new evidence has become available that necessitates a change to this conclusion and a potency factor of 0.1 was used in this assessment for AFM1.
3.2. Occurrence data
3.2.1. Occurrence data on food as submitted to EFSA
An initial number of 533,953 analytical results (analysed from 153,091 samples) for food and beverage samples on aflatoxins from 29 European countries were available in the EFSA database. Analytical results were reported either as individual results for AFB1, AFB2, AFG1, AFG2, aflatoxin G5 (AFG5), AFM1 and AFM2 or as AFT (the sum of AFB1, AFB2, AFG1, AFG2). AFG5 and AFM2 were not included in the present assessment due to the limited number of analytical results for them. In addition, a part of the data was classified as ‘Aflatoxins’ without further specification given (Annex B, Table B.1). Data were reported on samples collected between the years 2003 and 2018 with most of the data collected after 2007. However, in order to reflect the current contamination levels, only the most recent data were used in the assessment (from 2013 onwards).
The occurrence data were carefully evaluated, and a list of validation steps was applied before being used to estimate dietary exposure (see Annex B, Table B.2 for further details). The final data set comprised 209,802 analytical results (analysed from a total of 69,166 samples) on AFB1 (n = 57,983), AFB2 (n = 49,093), AFG1 (n = 49,325), AFG2 (n = 45,534) and AFM1 (n = 7, 867).
An evaluation of the LOQs was performed. As a first step, analytical results were excluded for which the LOQ was higher than the ML in force. This concerned the data for AFB1, AFB2,19 AFG1 and AFG2 reported for processed cereal‐based foods and baby foods for infants and young children and the data for AFM1 reported for infant formulae, while for other food categories the LOQs were lower than MLs. Considering the large amount of left‐censored data present in the data set (around 90%), the presence of relatively high LODs/LOQs may have a significant influence on the UB scenario. In order to reduce this impact, but without compromising the number of analytical results available on food categories mainly contributing to the exposure to aflatoxins, a careful evaluation of LOQs was performed. This evaluation was based on the EFSA internal guidance on the application of LOD/LOQ cut‐offs (EFSA, 2018a). Special attention was paid to those food categories that are considered to be potentially important contributors to the dietary exposure to aflatoxins and for which the difference between the LB and UB mean concentration was larger than 30%. Four main food categories, including ‘Grains and grain‐based products’, ‘Vegetables and vegetable products’, ‘Legumes, nuts and oilseeds’ and ‘Fruit and fruit products’ were identified for AFB1, AFB2, AFG1 and AFG2 and the food category ‘Milk and dairy products’ for AFM1. To identify the most appropriate LOQ cut‐off values, the distributions of quantified values (values above LOQ) as well as the reported LOQs were evaluated. A percentile (75th or 90th) derived from the quantified values was selected as a cut‐off value and subsequently applied to the LOQs reported (Annex B, Table B.3).
Approximately 95% of the data were obtained for samples collected within the official monitoring programmes, while the remaining samples from unspecified surveys, surveillance and monitoring programmes. Regarding the sampling strategy, a part of the analytical results (12%) was obtained by suspect sampling. There were no differences observed between mean concentrations of samples collected via different sampling strategies. Therefore, the CONTAM Panel decided not to exclude any samples on the basis of the sampling strategy.
The analytical results included in the final data set and considered for the dietary exposure to aflatoxins were collected in 26 different European countries, most of them in Germany and the Netherlands (27% of analytical results for each), followed by France (11% of analytical results). Figure 3 shows the distribution of analytical results for AFB1, AFB2, AFG1, AFG2 and AFM1 collected. It should be noted that the origin of the samples was not always the European country reporting the data, i.e. the data set also contained samples originating from North and South America, Africa, Asia and Australia. The samples were collected between 2013 and 2018 and the number of samples per year is presented in Figure 4 for AFB1, AFB2, AFG1, AFG2 and AFM1.
Figure 3.
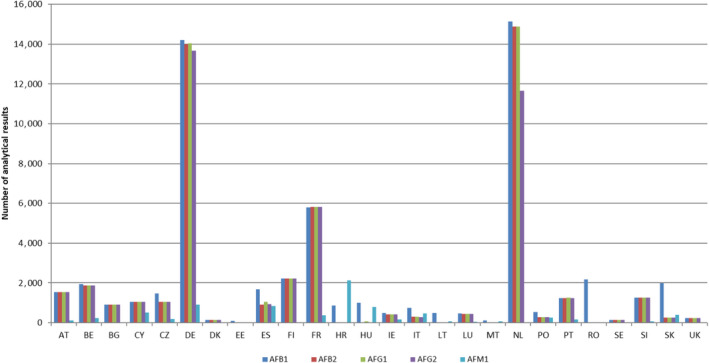
Distribution of analytical results for AFB1, AFB2, AFG1, AFG2 and AFM1 collected from European countries (after excluding non‐qualifying data)
- AT: Austria; BE: Belgium; BG: Bulgaria; CY: Cyprus; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; ES: Spain; FI: Finland; FR: France; HR: Croatia; HU: Hungary; IE: Ireland; IT: Italy; LT: Lithuania; LU: Luxembourg; MT: Malta; NL: Netherlands; PO: Poland; PT: Portugal; RO: Romania; SE: Sweden; SI: Slovenia; SK: Slovakia; UK: United Kingdom.
Figure 4.
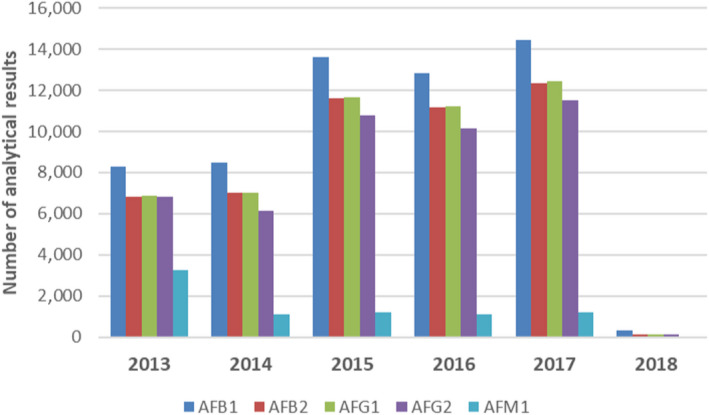
Distribution of analytical results for AFB1, AFB2, AFG1, AFG2 and AFM1 by sampling year (after excluding non‐qualifying data)
Table 7 shows the number of analytical results and the percentage of left‐censored data per substance and food category at FoodEx level 1. Most of the analytical results were available for AFB1 (n = 57,983). About 50,000 analytical results were available for AFB2, AFG1 and AFG2 while results for AFM1 were far fewer (n = 7,867). For each substance, a high proportion of left‐censored data was observed, ranging from 78% for AFM1 to 98% for AFG2 (Table 7).
Table 7.
Distribution of analytical results per toxin and food category
| FoodEx level 1 food category | AFB1 | AFB2 | AFG1 | AFG2 | AFM1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | LCD | N | LCD | N | LCD | N | LCD | N | LCD | |
| Grains and grain‐based products | 8,979 | 94% | 6,617 | 98% | 6,793 | 99% | 4,868 | 99% | 2 | 100% |
| Vegetables and vegetable products | 777 | 90% | 867 | 97% | 867 | 98% | 395 | 98% | – | – |
| Starchy roots and tubers | 53 | 91% | 50 | 96% | 50 | 96% | 50 | 96% | – | – |
| Legumes, nuts and oilseeds | 27,772 | 86% | 24,839 | 94% | 24,877 | 94% | 24,870 | 98% | 1 | 100% |
| Fruit and fruit products | 9,577 | 88% | 8,532 | 95% | 8,554 | 91% | 7,037 | 98% | – | – |
| Meat and meat products | 671 | 99% | 121 | 98% | 121 | 98% | 121 | 98% | – | – |
| Fish and other seafood | 89 | 94% | 34 | 88% | 34 | 94% | 34 | 94% | – | – |
| Milk and dairy products | 22 | 73% | 17 | 76% | 17 | 76% | 17 | 76% | 6,878 | 76% |
| Eggs and egg products | – | – | – | – | – | – | – | – | 2 | 100% |
| Sugar and confectionery | 878 | 78% | 779 | 94% | 779 | 86% | 781 | 95% | 1 | 100% |
| Animal and vegetable fats and oils | 836 | 81% | 807 | 94% | 806 | 93% | 806 | 98% | 26 | 96% |
| Fruit and vegetable juices | 146 | 99% | 145 | 100% | 145 | 99% | 145 | 100% | – | – |
| Non‐alcoholic beverages | 41 | 98% | 38 | 97% | 38 | 100% | 38 | 100% | – | – |
| Alcoholic beverages | 383 | 100% | 381 | 100% | 381 | 100% | 381 | 100% | – | – |
| Herbs, spices and condiments | 5,548 | 71% | 4,712 | 92% | 4,711 | 90% | 4,838 | 95% | – | – |
| Food for infants and small children | 1,433 | 97% | 443 | 99% | 441 | 99% | 442 | 99% | 895 | 89% |
| Products for special nutritional use | 116 | 97% | 101 | 100% | 101 | 100% | 101 | 100% | 4 | 100% |
| Composite food | 101 | 90% | 93 | 94% | 93 | 95% | 93 | 95% | 2 | 50% |
| Snacks, desserts, and other foods | 561 | 85% | 517 | 96% | 517 | 92% | 517 | 99% | 56 | 96% |
| Total | 57,983 | 87% | 49,093 | 95% | v49,325 | 94% | 45,534 | 98% | 7,867 | 78% |
N: number of analytical results; LCD: left‐censored data; AFB1: aflatoxin B1; AFB2: aflatoxin B2; AFG1: aflatoxin G1; AFG2 aflatoxin G2; AFM1: aflatoxin M1.
The most frequently analysed food categories were ‘legumes, nuts and oilseeds’, ‘fruit and fruit products’ and ‘grains and grain‐based products’. A substantial amount of data was also available for many other food categories, while some of them, e.g. ‘eggs and egg products’, ‘fish and other seafood’ and ‘non‐alcoholic beverages’, were much less represented (Table 7).
3.2.1.1. Analytical methods
As specified in Section 3.2.1 (for more detail see Annex B, Tables B.2 and B.3), some of the analytical results obtained by analytical methods with high LOD/LOQ were not included in the final data set. Most results were obtained by LC‐FD (34%) and LC–MS‐based methods (33%). Gas chromatography‐based methods and immunochemical tests, particularly ELISAs, were also used. For the remaining samples, no information on the analytical method was reported.
The distribution of the LOQs for the individual AFB1, AFB2, AFG1, AFG2 and AFM1 across the FoodEx level 1 food categories is summarised in Annex B, Table B.4. No particular variability of LOQs was observed across the food categories with the median LOQs being up to 1 μg/kg for AFB1 and AFB2, up to 2 μg/kg for AFG1 and AFG2 and up to 0.02 μg/kg for AFM1.
3.2.1.2. Occurrence data considered for dietary exposure assessment
The text below describes the occurrence data for AFB1, AFM1 and AFT. Detailed statistical description of the AFB1, AFB2, AFG1, AFG2, AFM1 and AFT occurrence data according to FoodEx levels 1, 2 and 3 are reported in Annex B, Tables B.5 (level 1), B.6 (level 2) and B.7 (level 3). In addition, the summary of occurrence data including the number of results, percentage of left‐censored data and the mean LB and UB concentrations for AFB1, AFM1, AFT and AFT+M1 according to the FoodEx food categories as used for exposure assessment are reported in Annex B, Table B.8.
Occurrence data on AFB1
Table 8 provides a summary of occurrence data on AFB1 across the FoodEx level 1 food categories including the number of results, percentage of left‐censored data and statistical descriptors of the results (mean, median, and 95th percentile). More detail on statistical description and according to lower FoodEx levels are reported in Annex B, Tables B.5–B.7.
Table 8.
Summary of the AFB1 occurrence data by food category (μg/kg)
| Food category, FoodEx level 1 | N | %LCD | Mean | Mediana | P95b | |||
|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |||
| Grains and grain‐based products | 8,979 | 94 | 0.15 | 0.57 | 0 | 0.42 | 0.17 | 1.00 |
| Vegetables and vegetable products | 777 | 90 | 0.34 | 0.95 | 0 | 1.00 | 1.26 | 1.26 |
| Starchy roots and tubers | 53 | 91 | 0.53 | 0.87 | 0 | 0.30 | – | – |
| Legumes, nuts and oilseeds | 27,772 | 86 | 1.72 | 2.18 | 0 | 0.60 | 3.60 | 3.60 |
| Fruit and fruit products | 9,577 | 88 | 0.64 | 0.97 | 0 | 0.20 | 1.63 | 1.63 |
| Meat and meat products | 671 | 99 | 0.01 | 0.17 | 0 | 0.10 | 0.00 | 0.60 |
| Fish and other seafood | 89 | 94 | 0.05 | 0.22 | 0 | 0.10 | 0.28 | 1.50 |
| Milk and dairy products | 22 | 73 | 0.07 | 0.23 | 0 | 0.20 | – | – |
| Sugar and confectionery | 878 | 78 | 0.25 | 0.47 | 0 | 0.20 | 0.90 | 1.00 |
| Animal and vegetable fats and oils | 836 | 81 | 0.80 | 1.02 | 0 | 0.20 | 2.10 | 2.10 |
| Fruit and vegetable juices | 146 | 99 | 0.02 | 1.00 | 0 | 1.00 | 0.00 | 1.00 |
| Non‐alcoholic beverages | 41 | 98 | 0.02 | 0.78 | 0 | 1.00 | – | – |
| Alcoholic beverages | 383 | 100 | 0.00 | 0.88 | 0 | 1.00 | 0.00 | 1.00 |
| Herbs, spices and condiments | 5,548 | 71 | 1.29 | 1.74 | 0 | 0.62 | 4.10 | 4.20 |
| Food for infants and small children | 1,433 | 97 | 0.00 | 0.06 | 0 | 0.03 | 0.00 | 1.00 |
| Products for special nutritional use | 116 | 97 | 0.07 | 0.48 | 0 | 0.20 | 0.00 | 1.00 |
| Composite food | 101 | 90 | 0.04 | 0.73 | 0 | 1.00 | 0.18 | 1.00 |
| Snacks, desserts, and other foods | 561 | 85 | 0.37 | 0.58 | 0 | 0.20 | 1.20 | 1.66 |
N: number of analytical results; % LCD: proportion of left‐censored data; P95: 95th percentile; LB: lower bound; UB: upper bound; AFB1: aflatoxin B1.
Due to the high proportion of left‐censored data, the distribution of the LB concentrations is right‐skewed. Therefore, the LB median results to be zero.
The 95th percentiles obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and are therefore not reported in the table.
The occurrence data on AFB1 were available for 18 FoodEx level 1 food categories. The data set was characterised by a high proportion of left‐censored data. The highest number of available data points corresponded to the food category ‘legumes, nuts and oilseeds’, in particular to different tree nuts (e.g. pistachios, hazelnuts, walnuts, etc.) and to peanuts. The highest AFB1 mean concentrations were obtained for the food category ‘legumes, nuts and oilseeds’, in particular for pistachios, peanuts and ‘other seeds’ and for the food category ‘herbs, spices and condiments’, in particular for anise pepper and flavourings and essences.
Comparison of the occurrence of AFB1 in selected food categories over the sampling years
The CONTAM Panel considered that it might be of interest to evaluate the contamination frequency and concentrations of AFB1 over the last decade. For this purpose, the proportion of left‐censored data and the mean concentrations of the quantified analytical results of AFB1 for selected food categories (i.e. pistachios, hazelnuts, other tree nuts, peanuts and dried figs) sampled between 2008 and 2017 were evaluated (Annex C and Figures C.1–C.5). A low variability of contamination frequency over the years was observed for pistachios and peanuts, while for other food categories the proportion of left‐censored data showed wider ranges (e.g. for hazelnuts it ranged from 17% in 2014 to 42% in 2008). Generally, the proportion of left‐censored data seems to have increased over the time period. The mean AFB1 concentrations did not show a clear trend within any food category over the last 10 years. It should be noted that for some years only a limited number of data was available which may have influenced the results.
Comparison of the occurrence of AFB1 in foods from conventional and organic farming
A total of 14,733 analytical results of AFB1 with a clear specification of the production method were available in the data set. The food categories with a sufficient number of results (n ≥ 40) were selected and a comparison of the AFB1 concentrations between conventional and organic farming was carried out (Annex D, Table D.1). Using conventional farming, for several food categories, in particular for peanuts, tree nuts and vegetable fat, higher mean LB AFB1 concentrations were observed, while for other food categories the mean LB AFB1 concentrations were similar or lower (e.g. cereal‐based food for infants and young children). Since the number of samples of the organic food products was considerably lower than for the conventional ones and the sampling countries and sampling years were not the same, it was not possible to draw a firm conclusion.
Occurrence data on AFT
The occurrence data for AFT were calculated from the analytical results of the individual aflatoxins (for more detail see Section 2.3.2). By this approach, a total of 44,327 samples were available for the assessment of the AFT.
Table 9 provides a summary of occurrence data on AFT across the FoodEx level 1 food categories including the number of results and statistical descriptors of the results (mean, median and 95th percentile). More details on statistical description and according to lower FoodEx levels are reported in Annex B, Tables B.5–B.7.
Table 9.
Summary of the AFT occurrence data by food category (μg/kg)
| Food category, FoodEx level 1 | N | Mean | Mediana | P95b | |||
|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | ||
| Grains and grain‐based products | 4,860 | 0.10 | 0.65 | 0.00 | 0.40 | 0.50 | 2.00 |
| Vegetables and vegetable products | 389 | 0.40 | 1.21 | 0.00 | 0.40 | 1.60 | 2.00 |
| Starchy roots and tubers | 50 | 0.95 | 1.68 | 0.00 | 0.60 | – | – |
| Legumes, nuts and oilseeds | 24,507 | 2.39 | 3.47 | 0.00 | 1.20 | 5.90 | 6.90 |
| Fruit and fruit products | 6,254 | 1.09 | 1.53 | 0.00 | 0.40 | 3.40 | 3.80 |
| Meat and meat products | 120 | 0.05 | 0.25 | 0.00 | 0.20 | 0.00 | 0.55 |
| Fish and other seafood | 34 | 0.20 | 0.43 | 0.00 | 0.20 | – | – |
| Milk and dairy products | 17 | 0.35 | 0.65 | 0.00 | 0.40 | – | – |
| Sugar and confectionery | 778 | 0.44 | 0.85 | 0.00 | 0.40 | 2.17 | 2.94 |
| Animal and vegetable fats and oils | 802 | 1.16 | 1.68 | 0.00 | 0.40 | 2.73 | 3.70 |
| Fruit and vegetable juices | 145 | 0.04 | 2.01 | 0.00 | 2.00 | 0.00 | 2.00 |
| Non‐alcoholic beverages | 38 | 0.03 | 1.68 | 0.00 | 2.00 | – | – |
| Alcoholic beverages | 381 | 0.00 | 1.76 | 0.00 | 2.00 | 0.00 | 2.00 |
| Herbs, spices and condiments | 4,809 | 1.74 | 2.77 | 0.00 | 1.40 | 5.30 | 6.66 |
| Food for infants and small children | 441 | 0.00 | 0.19 | 0.00 | 0.10 | 0.00 | 1.20 |
| Products for special nutritional use | 101 | 0.08 | 0.96 | 0.00 | 0.40 | 0.00 | 2.00 |
| Composite food | 93 | 0.12 | 1.58 | 0.00 | 2.00 | 0.77 | 2.00 |
| Snacks, desserts, and other foods | 508 | 0.52 | 0.89 | 0.00 | 0.40 | 1.70 | 2.20 |
N: number of samples; P95: 95th percentile; LB: lower bound; UB: upper bound; AFT: aflatoxin total.
Due to the high proportion of left‐censored data, the distribution of the LB concentrations is right‐skewed. Therefore, the LB median results to be zero.
The 95th percentiles obtained on occurrence data with fewer than 60 analytical results may not be statistically robust (EFSA, 2011b) and are therefore not reported in the table.
The occurrence data on AFT covered 18 FoodEx level 1 food categories with the majority of samples available for ‘legumes, nuts and oilseeds’ (n = 24,507) belonging mostly to different tree nuts (e.g. pistachios, hazelnuts, walnuts, etc.) and to peanuts. The highest AFT mean concentrations were observed for the same FoodEx level 1 food category, in particular for pistachios, peanuts and ‘other seeds’. High mean AFT levels were also measured for the food category ‘herbs, spices and condiments’ (i.e. anise pepper and flavourings and essences) and ‘animal and vegetable fats and oils’ (i.e. vegetable fat with the majority of samples being hazelnut and other unspecified nut pâté/paste).
The relative contribution of the individual AFB1, AFB2, AFG1 and AFG2 to the AFT MB concentration was calculated for the food categories for which a sufficient number of samples were available (i.e. tree nuts, peanuts and dried figs). Only samples for which at least one aflatoxin was quantified were included. Based on these individual contributions within each sample, the distribution of the contributions, including the mean, median, 5th, 25th 75th and 95th percentiles, was calculated (Table 10). On average, AFB1 contributed about 60% to the MB concentration of AFT and therefore can be considered as the major contributor to the AFT MB concentration. However, it should be noted that for some samples AFG1 contributed considerably as indicated by a relatively high contribution observed at the 95th percentile (68% for tree nuts and 75% for dried figs) (Table 10).
Table 10.
Contribution (%) of the individual AFB1, AFB2, AFG1 and AFG2 to the AFT middle‐bound concentration in all samples of tree nuts, peanuts and dried figs where quantified amounts of at least one aflatoxin were reported
| Substance | Food category | N | Mean | Percentile | ||||
|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||||
| AFB1 | Tree nuts | 2,442 | 60 | 17 | 37 | 63 | 84 | 93 |
| Peanuts | 1,188 | 69 | 25 | 62 | 75 | 83 | 90 | |
| Dried figs | 1,087 | 56 | 13 | 38 | 59 | 79 | 92 | |
| AFB2 | Tree nuts | 2,442 | 8 | 2 | 4 | 7 | 10 | 19 |
| Peanuts | 1,188 | 13 | 3 | 9 | 13 | 18 | 24 | |
| Dried figs | 1,087 | 9 | 2 | 4 | 6 | 11 | 21 | |
| AFG1 | Tree nuts | 2,442 | 27 | 0 | 5 | 19 | 48 | 68 |
| Peanuts | 1,188 | 22 | 1 | 6 | 17 | 36 | 58 | |
| Dried figs | 1,087 | 30 | 1 | 8 | 24 | 49 | 75 | |
| AFG2 | Tree nuts | 2,442 | 8 | 0 | 2 | 6 | 10 | 26 |
| Peanuts | 1,188 | 16 | 0 | 4 | 11 | 27 | 45 | |
| Dried figs | 1,087 | 6 | 0 | 2 | 4 | 7 | 17 | |
N: number of samples; AFB1: aflatoxin B1; AFB2: aflatoxin B2; AFG1: aflatoxin G1; AFG2 aflatoxin G2; AFT: aflatoxin total.
Occurrence data on AFM1
Quantified analytical results on AFM1 were obtained only for milk‐based foods. The highest AFM1 mean concentrations were reported for the food category ‘milk and dairy products’ and milk‐based food belonging to the category ‘food for infants and small children’. In particular, Parmigiano‐Reggiano cheese was the milk‐based food with the highest reported mean AFM1 concentration.
Table 11 provides a summary of the occurrence data on AFM1 across the FoodEx level 1 and 2 food categories, including the number of results, percentage of left‐censored data and statistical descriptors of the results (mean, median and 95th percentile). More detail on the statistical description and according to lower FoodEx levels are reported in Annex B, Tables B.5–B.7.
Table 11.
Summary of the AFM1 occurrence data by food category (μg/kg)
| Food category | N | %LCD | Mean | Mediana,b | P95b | |||
|---|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |||
| Milk and dairy products (Level 1) | 6,878 | 76 | 0.023 | 0.035 | 0 | 0.014 | 0.092 | 0.092 |
| Milk and dairy products | 70 | 89 | 0.001 | 0.012 | 0 | 0.010 | 0.013 | 0.023 |
| Liquid milk | 6,020 | 76 | 0.018 | 0.031 | 0 | 0.015 | 0.087 | 0.087 |
| Milk‐based beverages | 28 | 93 | 0.001 | 0.011 | 0 | 0.007 | – | – |
| Concentrated milk | 168 | 81 | 0.037 | 0.044 | 0 | 0.005 | 0.018 | 0.036 |
| Whey and whey products (excluding whey cheese) | 13 | 92 | 0.003 | 0.006 | 0 | 0.005 | – | – |
| Cream and cream products | 114 | 96 | 0.000 | 0.009 | 0 | 0.010 | 0.000 | 0.020 |
| Fermented milk products | 96 | 94 | 0.052 | 0.069 | 0 | 0.011 | 1.000 | 1.000 |
| Milk derivatives | 8 | 25 | 0.044 | 0.045 | 0.028 | 0.028 | – | – |
| Cheese | 359 | 53 | 0.097 | 0.107 | 0 | 0.050 | 0.415 | 0.415 |
| Milk and milk product imitates | 2 | 100 | 0.000 | 0.007 | – | – | – | – |
| Food for infants and small children (Level 1) | 895 | 89 | 0.036 | 0.053 | 0 | 0.010 | 0.023 | 0.050 |
| Food for infants and small children | 85 | 53 | 0.124 | 0.204 | 0 | 0.018 | 1.000 | 1.000 |
| Infant formula, powder | 354 | 90 | 0.060 | 0.071 | 0 | 0.010 | 1.000 | 1.000 |
| Follow‐on formula, powder | 243 | 100 | 0 | 0.010 | 0 | 0.010 | 0 | 0.025 |
| Cereal‐based food for infants and young children | 43 | 100 | 0 | 0.010 | 0 | 0.012 | – | – |
| Ready‐to‐eat meal for infants and young children | 7 | 86 | 0.003 | 0.018 | 0 | 0.020 | – | – |
| Yoghurt, cheese and milk‐based dessert for infants and young children | 3 | 100 | 0.000 | 0.040 | – | – | – | – |
| Fruit juice and herbal tea for infants and young children | 1 | 100 | 0.000 | 0.013 | – | – | – | – |
| Infant formula, liquid | 54 | 78 | 0.005 | 0.015 | 0 | 0.018 | – | – |
| Follow‐on formula, liquid | 105 | 92 | 0.002 | 0.007 | 0 | 0.003 | 0.023 | 0.023 |
N: number of analytical results; % LCD: proportion of left‐censored data; P95: 95th percentile; LB: lower bound; UB: upper bound; AFM1: aflatoxin M1.
Due to the high proportion of left‐censored data, the distribution of the LB concentrations is right‐skewed. Therefore, the LB median results to be zero.
The 95th percentiles obtained on occurrence data with fewer than 60 analytical results and the median obtained on occurrence data with fewer than six analytical results may not be statistically robust (EFSA, 2011b) and are therefore not reported in the table.
Comparison of the occurrence of AFM1 in foods from conventional and organic farming
A sufficient number of samples for which the production method was reported was only available for the food category ‘liquid milk’. In total, 76 and 143 analytical results coming from organic and conventional farming, respectively, were retrieved from the data set. It should be noted that this sub‐data set comprised a large amount of left‐censored data and the AFM1 concentrations are lower than those in the data set as a whole of AFM1 concentrations in liquid milk (see Table 11). Bearing this limitation in mind, the mean LB AFM1 level in liquid milk was much lower in samples obtained from organic farming than from conventional farming (Annex D, Table D.2).
3.2.2. Levels of biomarkers of exposure in the European population
A limited number of studies have been published that measure the presence of aflatoxin biomarkers in urine and serum for the European population. However, only results on validated biomarkers (see Section 3.1.3.1) are reported in this Scientific Opinion (Appendix IV, Table IV.1).
Table IV.1.
Overview of aflatoxin biomarker concentrations in the European population in urine samples collected in 2006 or later
| Country | Sample | Compound | Sampling period | Population | n | Concentration (ng/mL) | %LC | Analytical method | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Range | μ | |||||||||
| Belgium | Morning urine | AFM1 | n.r. | Adults | 239 | < LOD | / | 100 |
LC–MS/MS LOD: 0.002 ng/mL |
Heyndrickx et al. (2015) |
| Children | 155 | < LOD | / | 100 | ||||||
| Germany | Urine | AFM1 | n.r. | Healthy volunteers | 101 | < LOQ | / | 100 |
LC–MS/MS LOQ: 0.0013 ng/mL |
Gerding et al. (2014) |
| Germany | Urine | AFM1 | 2013–2014 | Healthy adults | 50 | < LOQ | / | 100 |
LC–MS/MS LOQ: 0.01 ng/mL |
Gerding et al. (2015) |
| Italy | Urine | AFM1 | March–April 2014 | Workers occupationally exposed | 29 | < LOD–0.399 | 0.035a | 29 |
HPLC‐FLD LOD: 0.002 ng/mL |
Ferri et al. (2017) |
| Control group | 30 | < LOD–0.259 | 0.027a | 23 | ||||||
| Italy | Morning urine | AFM1 | April 2011 | Not specified | 52 | < LOQ–0.146 | 0.068b | 94 |
LC–MS/MS LOQ: 0.02 ng/mL |
Solfrizzo et al. (2014) |
| Portugal | Serum | AFB‐alb | January–February 2015 | Workers occupationally exposed | 30 | < LOD–4.03 | 1.73b | 53 |
ELISA LOD: 1 ng/mL |
Viegas et al. (2016) |
| Control group | 30 | < LOD | / | 100 | ||||||
AFM1: aflatoxin M1; LC: left‐censored; LOD: limit of detection; LOQ: limit of quantification; LC–MS/MS: Liquid chromatography coupled to tandem mass spectrometry; HPLC: high‐performance liquid chromatography.
Calculated as lower bound.
Mean of the samples with concentrations > LOD/LOQ.
Although AFM1 in human milk is not a validated biomarker against dietary exposure, it is a useful indicator of exposure of infants. Globally, large regional differences in AFM1 levels in human milk have been reported (Degen et al., 2013; Cherkani‐Hassani et al., 2016) and therefore the CONTAM Panel only reports detailed information for European countries. Appendix IV, Table IV.2, gives an overview of the concentrations of AFM1 in human milk reported in scientific literature for the European population. Concentrations are highly variable and range from < LOD to 570 ng/L. The percentage of samples that contained detectable AFM1 concentrations also varied widely (from 5% to 100%) among the studies.
Table IV.2.
Overview of AFM1 concentrations in human milk collected in Europe in 2006 or later
| Country | Sampling period | N mothers | % LC | Concentration (ng/L) | Comment | Analytical method | Reference | |
|---|---|---|---|---|---|---|---|---|
| Range | Mean | |||||||
| Cyprus | March–May 2015 | 50 | 20 | < 5–28.44 | 7.84b |
ELISA (commercial kit) LOD: 5 ng/L |
Kunter et al. (2017) | |
| Italy | 2006 | 82 | 95 | < 7–140 | 55.35b |
HPLC‐FLD LOD: 3 ng/L; LOQ: 7 ng/L |
Galvano et al. (2008) | |
| Italy | 2011–2013 | 35a | 63 | < 7–340 | 12c | Mothers with coeliac disease; gluten‐free diet |
HPLC‐FLD LOQ: 7 ng/L |
Valitutti et al. (2018) |
| 23a | 76 | < 7–67 | 9c | Healthy mothers (control) | ||||
| Portugal | 2015–2016 | 67 | 67 | < 5–10.6 | 7.4d |
ELISA (commercial kit) LOD: 5 ng/L |
Bogalho et al. (2018) | |
| Serbia | 2013–2014 | 55 | 64 | < 5–503 | 175d | Colostrum |
ELISA (commercial kit) LOD: 4.3 ng/L LOQ: 5 ng/L |
Radonić et al. (2017) |
| 5 | 0 | 58–570 | n.r. | Collected 4–8 months after delivery | ||||
| Serbia | April 2013 | 10 | 40 | < 5–22e | 10 e |
ELISA (commercial kit) LOD: 1.5 ng/kg LOQ: 5 ng/kg |
Kos et al. (2014) | |
AFM1: aflatoxin M1; LC: left‐censored; LOD: limit of detection; LOQ: limit of quantification; ELISA: enzyme‐linked immunosorbent assay; LC–MS/MS: Liquid chromatography coupled to tandem mass spectrometry; HPLC: high‐performance liquid chromatography; LOD: limit of detection; LOQ: limit of quantification.
About nine samples/mother).
Mean of the samples with concentrations > LOD/LOQ.
Calculated as middle bound.
Not specified how mean is calculated.
concentration reported as ng/kg.
3.2.3. Processing
Food processing may influence the concentration of aflatoxins in food products. Milling of cereals distributes the aflatoxins among the different milling products but does not destroy them. Grain sorting and cleaning, on the other hand, may lead to a reduction by the removal of contaminated kernels. Heat treatments such as roasting and baking can reduce the concentration of aflatoxins, but a complete reduction is not achieved (FAO/WHO, 2018).
Levels of aflatoxins in nuts are reduced during roasting and the effect increases with increased duration and temperature. Yazdanpanah et al. (2005) reported a reduction of the AFB1 concentration in pistachios of more than 95% following a roasting step of 120 min at 150°C. However, the product was no longer edible. Ariño et al. (2009) on the other hand applied a commercial roasting process of 20 min at 120°C to naturally contaminated raw pistachios but no reduction was noted. It should be noted that the initial AFB1 concentration was low (< 0.2 μg/kg). Martins et al. (2017) applied different time–temperature combinations for the roasting of peanuts and achieved reductions of up to 90%. However, colour analysis with roasted peanut samples available on the market showed that only roasting at 160°C for 5 min gave a similar darkness and this roasting process only led to a reduction of 15%.
In addition, some authors report that the percentage of aflatoxin reduction during roasting of nuts also depends on the initial aflatoxin concentration, with a higher reduction percentage for more contaminated samples (Yazdanpanah et al., 2005; Zivoli et al., 2014; Martins et al., 2017). For example, Martins et al. (2017) reported reductions of 55, 64 and 81% for initial aflatoxin concentrations of 35, 332 and 695 μg/kg, respectively, following 20 min roasting at 180°C. However, this observation was not confirmed by Arzandeh and Jinap (2011). They noted a decrease in the aflatoxin reduction when the initial aflatoxin concentration exceeded 200 μg/kg.
3.3. Dietary exposure assessment for humans
3.3.1. Current dietary exposure assessment
The CONTAM Panel assessed the dietary chronic exposure (following the methodology described in Section 2.6) to the individual AFB1 and AFM1, and the overall AFT and AFT+AFM1 exposure. Analytical results for AFT were generated by summing up the available individual concentrations of all four aflatoxin forms (AFB1, AFB2, AFG1 and AFG2) for each sample as explained in Section 2.3.2. Analytical results for AFT+AFM1 were generated by combining AFT concentrations and AFM1 concentrations multiplied by a factor of 0.1 based on differences in carcinogenic potency (for more detail see Section 3.1.6).
A summary of the occurrence data on AFB1, AFM1, AFT and AFT + AFM1 including the number of results, percentage of left‐censored data and mean concentrations across the FoodEx Level food categories as used for exposure assessment is presented in Annex B, Table B.8.
Overall, it should be kept in mind that a high proportion of left‐censored data has a major impact on the exposure estimates; the exposure is likely to be underestimated with the LB approach and overestimated with the UB approach.
3.3.1.1. Mean and high chronic dietary exposure
Mean and high dietary chronic exposure to AFB1
Table 12 shows summary statistics for the assessment of chronic dietary exposure to AFB1. Detailed mean and 95th percentile dietary exposure estimates calculated for each of the 38 dietary surveys are presented in Annex E, Table E.1.
Table 12.
Summary statistics for the chronic dietary exposure to AFB1 (ng/kg bw per day) across European countries
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (ng/kg bw per day) | ||||||
| Infants | 0.08 | 0.58 | 0.18 | 2.01 | 0.60 | 4.87 |
| Toddlers | 0.43 | 3.15 | 0.64 | 5.35 | 1.05 | 6.95 |
| Other children | 0.47 | 3.46 | 0.76 | 4.93 | 1.78 | 6.12 |
| Adolescents | 0.27 | 1.99 | 0.40 | 2.98 | 1.24 | 4.27 |
| Adults | 0.22 | 1.35 | 0.33 | 2.15 | 0.49 | 3.25 |
| Elderly | 0.19 | 1.32 | 0.26 | 1.90 | 0.31 | 2.91 |
| Very elderly | 0.18 | 1.41 | 0.26 | 2.07 | 0.41 | 2.93 |
| 95th percentile dietary exposure in total population (ng/kg bw per day) | ||||||
| Infantsa | 0.35 | 2.79 | 0.74 | 5.18 | 1.84 | 13.03 |
| Toddlersa | 0.77 | 6.80 | 1.46 | 9.69 | 2.88 | 14.01 |
| Other children | 1.17 | 6.03 | 1.58 | 8.71 | 6.22 | 11.88 |
| Adolescentsa | 0.70 | 3.25 | 0.98 | 5.61 | 4.62 | 8.62 |
| Adults | 0.62 | 2.76 | 0.87 | 4.24 | 1.36 | 6.78 |
| Elderly | 0.47 | 2.76 | 0.62 | 3.72 | 1.10 | 5.26 |
| Very elderlya | 0.42 | 2.81 | 0.56 | 3.86 | 0.93 | 5.05 |
AFB1: aflatoxin B1; bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with fewer than 60 observations may not be statistically robust (EFSA, 2011b) and are therefore not included in this table.
The highest estimated chronic dietary exposure to AFB1 was in the young population groups. Concerning the mean dietary exposure, the highest estimated LB exposure levels were in other children with a maximum exposure of 1.78 ng/kg bw per day, while the highest UB exposure was observed for toddlers (6.95 ng/kg bw per day). The highest LB 95th percentile exposure was for other children with estimates of 6.22 ng/kg bw per day and the highest UB 95th percentile exposure was estimated for toddlers (14.01 ng/kg bw per day).
Dietary exposure in specific groups of the population, namely ‘Pregnant women’ and ‘Lactating women’, were within the range of exposure estimates for the adult population.
Mean and high chronic dietary exposure to AFM1
Table 13 shows summary statistics for the assessment of chronic dietary exposure to AFM1. Detailed mean and 95th percentile dietary exposure estimates calculated for each of the 38 dietary surveys are presented in Annex E, Table E.2.
Table 13.
Summary statistics for the chronic dietary exposure to AFM1 (ng/kg bw per day) across European countries
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (ng/kg bw per day) | ||||||
| Infants | 0.14 | 0.21 | 0.57 | 0.81 | 1.56 | 1.98 |
| Toddlers | 0.45 | 0.64 | 0.68 | 1.05 | 1.42 | 1.81 |
| Other children | 0.18 | 0.28 | 0.35 | 0.52 | 0.78 | 1.00 |
| Adolescents | 0.08 | 0.12 | 0.15 | 0.23 | 0.25 | 0.37 |
| Adults | 0.05 | 0.06 | 0.08 | 0.12 | 0.14 | 0.20 |
| Elderly | 0.04 | 0.06 | 0.08 | 0.12 | 0.14 | 0.18 |
| Very elderly | 0.04 | 0.06 | 0.08 | 0.11 | 0.15 | 0.22 |
| Pregnant women | 0.09 | 0.11 | 0.11 | 0.15 | 0.13 | 0.20 |
| Lactating women | 0.14 | 0.20 | 0.18 | 0.25 | 0.22 | 0.29 |
| 95th percentile dietary exposure in total population (ng/kg bw per day) | ||||||
| Infantsa | 0.66 | 0.98 | 1.48 | 2.06 | 6.23 | 7.88 |
| Toddlersa | 1.05 | 1.49 | 1.47 | 2.18 | 3.80 | 4.85 |
| Other children | 0.43 | 0.62 | 0.80 | 1.26 | 2.16 | 2.73 |
| Adolescentsa | 0.22 | 0.31 | 0.37 | 0.58 | 0.48 | 0.69 |
| Adults | 0.13 | 0.16 | 0.25 | 0.32 | 0.39 | 0.54 |
| Elderly | 0.12 | 0.16 | 0.24 | 0.32 | 0.38 | 0.48 |
| Very elderlya | 0.17 | 0.25 | 0.25 | 0.31 | 0.34 | 0.45 |
| Pregnant women | 0.21 | 0.27 | 0.28 | 0.38 | 0.34 | 0.49 |
| Lactating women | 0.34 | 0.46 | 0.38 | 0.51 | 0.41 | 0.56 |
AFM1: aflatoxin M1; bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with fewer than 60 observations may not be statistically robust (EFSA, 2011b) and are therefore not included in this table.
The highest estimated chronic dietary exposure to AFM1 was in infants and toddlers, which can be explained by their specific consumption patterns that are mostly based on milk and milk products. Concerning the mean dietary exposure, the highest estimated LB and UB exposure levels were in infants with a maximum LB/UB exposure of 1.56/1.98 ng/kg bw per day. The highest LB/UB 95th percentile exposure was also observed for infants, with estimates of 6.23/7.88 ng/kg bw per day.
Dietary exposure in the specific population group ‘Pregnant women’ was within the range of exposure estimates observed for the adult population. ‘Lactating women’ showed higher exposure levels than those estimated for the adult population, with the median mean exposure levels being twice as high. This outcome is driven by an increased consumption of milk and milk products during the lactating period.
Mean and high chronic dietary exposure to AFT+AFM1
Table 14 shows summary statistics for the assessment of chronic dietary exposure to AFT+AFM1. Detailed mean and 95th percentile dietary exposure estimates calculated for each of the 38 dietary surveys are presented in Annex E, Table E.3.
Table 14.
Summary statistics for the chronic dietary exposure to AFT+AFM1b (ng/kg bw per day) across European countries
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Mean dietary exposure in total population (ng/kg bw per day) | ||||||
| Infants | 0.18 | 0.88 | 0.42 | 2.58 | 1.01 | 9.92 |
| Toddlers | 0.74 | 5.09 | 1.23 | 9.14 | 2.05 | 12.51 |
| Other children | 0.87 | 5.32 | 1.22 | 8.63 | 1.92 | 12.54 |
| Adolescents | 0.43 | 2.87 | 0.64 | 5.19 | 1.05 | 6.98 |
| Adults | 0.35 | 2.29 | 0.55 | 4.00 | 0.80 | 6.60 |
| Elderly | 0.28 | 2.11 | 0.46 | 3.68 | 0.58 | 6.51 |
| Very elderly | 0.31 | 2.53 | 0.43 | 3.75 | 0.60 | 6.61 |
| 95th percentile dietary exposure in total population (ng/kg bw per day) | ||||||
| Infantsa | 0.65 | 4.03 | 1.16 | 7.43 | 3.27 | 29.56 |
| Toddlersa | 1.29 | 8.25 | 2.33 | 15.33 | 4.45 | 27.05 |
| Other children | 1.70 | 7.50 | 2.30 | 16.08 | 4.40 | 22.92 |
| Adolescentsa | 1.06 | 4.81 | 1.41 | 11.54 | 2.56 | 13.15 |
| Adults | 0.83 | 4.36 | 1.25 | 9.16 | 2.30 | 14.20 |
| Elderly | 0.65 | 4.40 | 1.01 | 7.58 | 1.59 | 12.34 |
| Very elderlya | 0.73 | 5.11 | 0.95 | 8.85 | 1.44 | 13.34 |
AFT: aflatoxin total; AFM1: aflatoxin M1; bw: body weight; LB: lower bound; UB: upper bound.
The 95th percentile estimates obtained on dietary surveys/age classes with fewer than 60 observations may not be statistically robust (EFSA, 2011b) and are therefore not included in this table.
Dietary exposure to AFT+AFM1 was calculated by applying the potency factor of 0.1 to the concentrations of AFM1 (see Section 3.1.6).
The highest estimated chronic dietary exposure to AFT+AFM1 was in the young population groups. Among the mean dietary exposures calculated for toddlers, the highest LB estimate amounted to 2.05 ng/kg bw per day while the highest UB estimate amounted to 12.54 ng/kg bw per day in other children. The highest LB 95th percentile exposure was for toddlers with estimates of 4.45 ng/kg bw per day and the highest UB 95th percentile exposure was estimated for infants (29.56 ng/kg bw per day). Overall, the chronic dietary exposure estimates for AFT+AFM1 are higher than those calculated for the individual AFB1, with the exception of the highest LB 95th percentile in other children and adolescents. This is explained by the high consumption of candy recorded in several dietary surveys and for which an AFB1 mean occurrence level was higher than the mean occurrence level obtained by summing the individual aflatoxins (due to the loss of one sample not being analysed for all four aflatoxins). Moreover, the food category ‘candies with sugar’ was considered as a separate food category in the exposure assessment for AFB1 while for the AFT+AFM1 exposure it was merged with the upper level food category ‘confectionery (non‐chocolate)’ due to the limited amount of data.
For AFT, detailed mean and 95th percentile dietary exposure estimates calculated for each of the 38 dietary surveys are presented in Annex E, Table E4. The exposure estimates for AFT showed, in general, lower levels than the exposure levels estimated for AFT+AFM1, particularly for population groups of small children.
3.3.1.2. Contributions of different food groups
The contribution (%) of each of the FoodEx level 1 food categories to total mean exposure of AFB1, AFM1, AFT and AFT+M1 was calculated for each age group and dietary survey. Estimations of exposure using the LB approach, which is considered to be less influenced by the value of the LOD/LOQ, were used to explain the contribution of the different food categories. The contribution of individual food categories to the LB mean chronic dietary exposure to AFB1, AFM1, AFT and AFT+M1 varied between the dietary surveys. This is explained by the specific food consumption patterns in the individual European countries and even in different regions of one country.
Contribution of individual food categories to the LB mean chronic dietary exposure to AFB1
The food category ‘grains and grain‐based products’ was the most important contributor to the overall LB mean chronic dietary exposure to AFB1 across all age groups. The LB median contribution among surveys ranges from 38% for adults to 50% for the very elderly, with contributions reaching up to 75% in one survey of toddlers. Grains for human consumption, in particular corn grain, bread and rolls and fine bakery wares had the highest contribution among the food subcategories. The AFB1 concentrations for ‘bread and rolls’ were low; therefore the contribution is driven by high consumption rather than the presence of AFB1. It should be noted that the majority of quantified results (i.e. above the LOQ) of ‘fine bakery wares’ contained nut‐based fillings and therefore it is likely that the high contribution to the exposure to AFB1 is influenced by ingredients other than cereals.
Another very important contributor to the overall LB mean chronic dietary exposure to AFB1 was the food category ‘legumes, nuts and oilseeds’ (contributing up to 29% for adults). In most surveys, this high contribution was driven by peanuts (up to 24% in adults). Despite relatively high AFB1 concentrations measured in almonds, pistachios and other seeds, the exposure to AFB1 from these foods was small, which is explained by low consumption. Similarly, different products within the food category ‘herbs, spices and condiments’ reported with a high AFB1 level (e.g. flavourings or essences) did not make a major contribution to the overall AFB1 LB mean exposure because of the low consumption recorded within the dietary surveys.
Specifically, in other children and adolescents, ‘sugar and confectionary’ made an important contribution to the overall AFB1 LB mean exposure (up to 74% in both population groups). It should be noted that this was driven by a high consumption of candies reported for a limited number of dietary surveys. This food category had high AFB1 concentrations reported, which may be due to nuts present in the candies.
Among young population groups, particularly in toddlers and other children, ‘animal and vegetable fats and oils’ was also an important contributor to the overall AFB1 LB mean exposure. This was mostly driven by a contribution of peanut butter, which is widely consumed by children in several European countries (contributing up to 39% for toddlers).
Other food categories noted as important contributors to the overall AFB1 mean exposure were ‘fruit and fruit products’, ‘vegetables and vegetable products’ and ‘products for special nutritional use’.
The detailed contribution of the different food categories at FoodEx level 1 and grouped by age classes is shown in Annex E, Table E.5. The detailed contribution of the different food categories at FoodEx level as used for exposure assessment and grouped by age classes is shown in Annex E, Table E.5 bis (A).
In addition, the CONTAM Panel calculated the main contributors to the overall AFB1 LB mean exposure for highly exposed individuals identified as subjects having an individual AFB1 exposure above the 75th percentile of the overall exposure calculated for the total population. In the highly exposed adult population groups, peanuts were the major source of AFB1 exposure. Grain‐based food products also contributed considerably, however their contribution is lower as compared to the assessment carried out for the total adult population. In the young population groups, the main contributors are in line with those observed for the total population. The detailed contribution of the different food categories at FoodEx level as used for the exposure assessment and grouped by age classes calculated for highly exposed subjects is shown in Annex E, Table E.5 bis (B).
Contribution of individual food categories to the LB mean chronic dietary exposure to AFM1
The food category ‘milk and dairy products’ was the main contributor to the overall AFM1 LB mean exposure throughout all age groups. The median LB contribution among surveys accounted for almost 100% in all age groups except in infants, where the ‘food for infants and small children’ food category also made an important contribution. The main subcategories driving the contribution of the ‘milk and dairy products’ food category were liquid milk, contributing up to 89% (other children), and fermented milk products (e.g. yoghurt), contributing up to 87% (infants). ‘Cheese’ contributed up to 72% (adults). The contribution of ‘snacks, desserts, and other foods’ (represented only by starchy pudding) was negligible (less than 1% for all age groups).
The food group ‘Animal and vegetable fats and oils’ was not considered for the exposure assessment. The 26 samples consisted of 25 samples of butter (all left‐censored) and 1 sample of butter oil (quantified concentration of AFM1). Considering the large influence of one positive sample on a broadly consumed food group, these samples were not included in the dietary exposure assessment.
The detailed contribution of the different food categories at the FoodEx level as used for exposure assessment and grouped by age classes is shown in Annex E, Table E.6 (A). The detailed contribution of the different food categories at FoodEx level as used for exposure assessment and grouped by age classes calculated for highly exposed subjects is shown in Annex E, Table E.6 (B). No considerable differences were noted when compared the main contributors to the overall AFM1 LB mean exposure between the total population and highly exposed subjects.
Contribution of individual food categories to the LB mean chronic dietary exposure to AFT+AFM1
Overall, the main contributor to the LB mean chronic dietary exposure to AFT+AFM1 was the food category ‘grains and grain‐based products’ (contributing up to 62% in the very elderly). The main subcategories driving the contribution of this food category were fine bakery wares, contributing up to 37% for the very elderly, bread and rolls, contributing up to 31% for the elderly and breakfast cereals, contributing up to 27% for infants.
The ‘milk and dairy products’ food category was only an important source of AFT+AFM1 exposure for infants, toddlers and other children. The LB contribution of this food category accounted up to 40, 26 and 19%, respectively, while for other age groups it was below 15%.
Other important contributors to the LB mean chronic dietary exposure to AFT+AFM1 were sugar and confectionery’ (up to 43% in adolescents), mainly driven by non‐chocolate confectionery, and ‘legumes, nuts and oilseeds’ (up to 32% in adults), mainly driven by peanuts.
The food categories ‘animal and vegetable fats and oils’, ‘herbs, spices and condiments’ and ‘alcoholic beverages’ were also identified as important contributors to the LB mean chronic dietary exposure to AFT+AFM1, but it was mostly specifically related to a high or specific consumption of certain products (e.g. peanut butter, flavourings or essences, beer and beer‐like beverages unspecified).
The contribution of other food categories was minor.
The detailed contribution of the different food categories at FoodEx level 1 and grouped by age classes to the LB mean chronic dietary exposure to AFT+AFM1 is shown in Annex E, Table E.7 and to AFT in Table E.8. The detailed contribution of the different food categories at FoodEx level as used for exposure assessment and grouped by age classes to the LB mean chronic dietary exposure to AFT+AFM1 is shown in Annex E, Table E.7 bis (A).
The evaluation of the main contributors to the LB mean chronic dietary exposure to AFT+AFM1 among the highly exposed subjects showed that beside the grain‐based food products, peanut and tree nuts made the most important contribution. The detailed contribution of the different food categories, at the FoodEx level as used for exposure assessment and grouped by age classes, to the LB mean chronic dietary exposure to AFT+AFM1 for highly exposed subjects is shown in Annex E, Table E.7 bis (B).
3.3.1.3. Scenario for short‐term dietary exposure to AFB1 from peanut butter
As described in Section 3.1.2.5, AFB1 affects reproductive and developmental parameters in rodents and these effects may occur following a short‐term exposure. To evaluate whether these effects should be considered in the risk characterisation of aflatoxins in humans in the EU, the CONTAM Panel decided to compare the identified doses with a scenario of short‐term exposure (for more detail see Section 2.6). Given the limited number of consuming days available in the Comprehensive Database, the Panel focused only on the surveys where the peanut butter consumption was recorded for at least 60 consuming days. Finally, calculations were based on seven different dietary surveys carried out in four European countries.
The UB mean estimates for short‐term dietary exposure to AFB1 from peanut butter across dietary surveys and age groups ranged from 0.24 ng/kg bw per day to 1.53 ng/kg bw per day. The UB 95th percentile short‐term dietary exposure estimates across dietary surveys and age groups ranged from 0.57 ng/kg bw per day to 3.98 ng/kg bw per day. Detailed UB mean and UB 95th percentile dietary exposure estimates calculated for each of the selected dietary surveys are presented in Annex E, Table E.9.
3.3.2. Exposure of infants through breastfeeding
For the exposure assessment for breastfed infants under 6 months of age, a median age of 3 months was selected, equivalent to a body weight of about 6.1 kg, with an estimated average daily milk consumption of about 800 mL and a high consumption of 1,200 mL. The mean occurrence levels were taken from the scientific literature (see Appendix IV, Table IV.1). However, it should be noted that some mean concentrations were calculated using only the samples with concentration > LOD/LOQ. The calculated dietary exposure ranged from 1 to 23 ng/kg bw per day for average milk consumers and from 1.5 to 34 ng/kg bw per day for high milk consumers (Table 15). Some authors also calculated daily exposures based on the detected levels and they are also reported in Table 15.
Table 15.
Overview of AFM1 concentrations in human milk collected in Europe in 2006 or later
| Country reference | N mothers | Mean concentration (ng/L) | Daily exposure (ng/kg bw) calculated by the CONTAM Panel | Daily exposure (ng/kg bw) reported by the authors | |
|---|---|---|---|---|---|
| Average milk consumption | High milk consumption | ||||
|
Cyprus Kunter et al. (2017) |
50 | 7.84b | 1.0 | 1.5 | |
|
Italy Galvano et al. (2008) |
82 | 55.35b | 7.3 | 10.9 | |
|
Italy Valitutti et al. (2018) |
35a | 12c , f | 1.6 | 2.4 | 1.6 |
| 23a | 9c , g | 1.2 | 1.8 | 1.2 | |
|
Portugal Bogalho et al. (2018) |
67 | 7.4d | 1.0 | 1.5 | 0.9–1.1 |
|
Serbia Radonić et al. (2017) |
55 | 175b , h | 23.0 | 34.4 | 2.65i |
|
Serbia Kos et al. (2014) |
10 | 10e | 1.3 | 2.0 | |
AFM1: aflatoxin M1; bw: body weight.
About nine samples/mother).
Mean of the samples with concentrations > LOD/LOQ.
Calculated as middle bound.
Not specified how mean is calculated.
Concentration reported as ng/kg.
Mothers with coeliac disease; gluten‐free diet.
Healthy mothers (control).
Colostrum.
Daily intake was calculated by the authors using a milk consumption of 60 mL per day and body weight of 3.5 kg.
3.3.3. Previously reported dietary exposure
As summarised in the recently published statement on aflatoxins (EFSA CONTAM Panel, 2018), the CONTAM Panel identified several dietary exposure assessments carried out by international risk assessment bodies. The text below describing these dietary exposure assessments is an adapted version of the corresponding section in the recently published statement on aflatoxins. In addition, three total diet studies (TDS) carried out by European Member States and several scientific papers reporting dietary exposure from one or a few food groups in Europe were identified.
International risk assessment bodies
No comprehensive dietary exposure assessment for aflatoxins is available in the EU. In 2007, the CONTAM Panel assessed the average dietary exposure to AFT, truncating the occurrence data to the current EU MLs and using GEMS/Food Consumption Cluster diets data and data from individual surveys (EFSA, 2007a). This assessment included exposure from almonds, hazelnuts, pistachios, other nuts, maize, oilseeds, dried fruit and spices. For adults, this exposure ranged from 0.35 to 1.93 ng/kg bw per day (minimum LB–maximum UB) and for children from 0.56 to 1.91 ng/kg bw per day (minimum LB–maximum UB). Similar dietary exposure assessments have been carried out over the years for different food commodities.
In 2016, JECFA calculated international estimates of chronic dietary exposure using the food consumption data from the GEMS/Food cluster diets and a standard body weight of 60 kg (FAO/WHO, 2018). The calculations covered the exposure from cereals, nuts, spices, and other foods such as figs and soy. The mean UB dietary AFT exposure ranged from 1.3 ng/kg bw per day (cluster G08, comprising Austria, Germany, Poland and Spain) to 34.8 ng/kg bw per day (cluster G13, comprising African countries and Haiti). JECFA reported that a similar pattern of exposure was observed under the LB scenario. The dietary exposure for a high consumer was considered to be twice the mean dietary exposure. Wheat was the main contributor to the UB dietary AFT exposure (range 37–76.5%) for several countries, including many European countries. However, for cluster G10 (comprising European countries such as Italy, Bulgaria, Estonia, Latvia and Lithuania), rice was the main contributor to the UB dietary AFT exposure (range 34.5–80.3%). No information was provided regarding the major contributors to the LB dietary AFT exposure. Based on these calculations and on national estimates, JECFA concluded that with the exception of very high estimates of dietary exposure to AFT for some African countries (105–850 ng/kg bw), all mean dietary AFT exposure were in the range < 0.01–58 ng/kg bw per day with high consumer estimates in the range < 0.01–200 ng/kg bw per day. Considering the different foods included in the exposure assessment, a direct comparison with the results generated by the CONTAM Panel in 2007 is not appropriate.
Both EFSA and JECFA performed impact assessments of the implementation of different MLs for specific food commodities on the dietary exposure. Such assessments are outside the scope of the current Scientific Opinion and are therefore not reported in detail.
Total diet studies carried out by EU Member States
In 2006–2007, the French Agency for Food, Environmental and Occupational Health Safety (ANSES; Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail) conducted a TDS including AFB1, AFB2, AFG1, AFG2 and AFM1. AFB1 was detected in dark chocolate samples, but in none of the other food matrices. The other aflatoxins were not detected in any of the tested food matrices. For French adults, the mean and P95 dietary exposure to AFB1 were 0.002–0.22 and 0.01–0.39 ng/kg bw per day (LB–UB), respectively. For French children (3–17 years) the mean and P95 dietary exposure was 0.001–0.39 and 0.008–0.74 ng/kg bw per day (LB–UB), respectively (Sirot et al., 2013).
In 2011–2012, ANSES conducted a TDS particularly targeting children under 3 years old. AFB1 and AFG2 were only detected in one sample of chocolate and AFB2, AFG1 and AFM1 were not detected in any of the samples. The exposure to the sum of the five aflatoxins20 was the highest for the youngest age group (1–4 months), namely 0–4.46 and 0–8.28 ng/kg bw per day (LB–UB) for the mean and P95 dietary exposure, respectively (ANSES, 2016).
A TDS was carried out by the Italian Istituto Superiore di Sanità in 2012–2014 that included AFB1 and AFM1. For AFB1 the LB–UB dietary exposure was 0.020–0.507 ng/kg bw for the whole population. It should be noted that AFB1 was only detected in three food groups: ‘wheat and flours’, ‘chocolate’, and ‘cocoa’. AFM1 was only detected in the food group ‘meat, meat products and substitutes’ and the LB–UB dietary exposure was 0.17–0.23 μg/kg bw. However, the percentage of left‐censored data was high for both substances and the uncertainty in the dietary exposure assessment is consequently substantial (Cubadda, 2018).
A mycotoxin‐dedicated TDS was conducted in the Netherlands in 2013 which included AFB1, AFB2, AFG1, AFG2 and AFM1. Only AFB1 was detected in two composite samples that contained peanuts but at a concentration below the LOQ of 0.2 μg/kg. Based on the collected occurrence data, dietary exposure was calculated for Dutch children aged 2–6 years and the Dutch population aged 7–69 years old. For Dutch children, the P50 and P95 dietary exposure to AFB1 were 0–0.93 and 0.07–1.67 ng/kg bw per day (LB–UB), respectively. For the rest of the Dutch population the dietary exposure was lower (0–0.42 and 0.033–1.03 ng/kg bw per day (LB–UB), respectively) (López et al., 2016; Sprong et al., 2016a,b).
The Panel noted that the UB exposures reported in these TDS are primarily driven by the LOQs due to the high percentage of left‐censored data.
Dietary exposure for one or a limited number of food groups
Appendix IV, Table IV.3 shows examples of estimated dietary exposures reported in the scientific literature. Several papers estimated the dietary exposure to AFM1 in Serbia from milk consumption. Large differences in dietary exposure were observed between years, due to the large variability in AFM1 concentrations in milk between sampling years (Torović, 2015). In Serbian children, a mean dietary exposure to AFM1 up to 6.5 ng/kg bw per day was reported. For all other aflatoxins, the calculated exposures were typically lower than 0.1 ng/kg bw per day.
Table IV.3.
Examples of dietary exposure assessments of the European population published in the scientific literature since 2013
| Population | Country | Food | Exposure (ng/kg bw per day) | Analytical method; Treatment of left‐censored data if reported | Reference | |
|---|---|---|---|---|---|---|
| Mean | High | |||||
| AFM1 | ||||||
| Children 1–3 years | Portugal | Breakfast cereals, infant cereals, biscuits | 0.052–0.069 | 0.203a | HPLC‐FLD; LB‐UB | Assunção et al. (2018) |
| Children 1–5 syears | Serbia | milk | 6.26–6.45 | ELISA (commercial kit) | Kos et al. (2014) | |
| Children 5–15 years | Serbia | milk | 1.86–2.34 | ELISA (commercial kit) | Kos et al. (2014) | |
| 15–25 years | Serbia | milk | 0.42–1.26 | ELISA (commercial kit) | Kos et al. (2014) | |
| 25–55 years | Serbia | milk | 0.49–0.56 | ELISA (commercial kit) | Kos et al. (2014) | |
| > 55 years | Serbia | milk | 0.51–0.69 | ELISA (commercial kit) | Kos et al. (2014) | |
| Adults | Serbia | milk | 0.5–1.4 | LC–MS/MS | Škrbić et al. (2014) | |
| Adult | Serbia | heat‐treated milk; sampling 2013 | 0.54–0.6 | HPLC‐FLD | Torović (2015) | |
| Adults | Serbia | heat‐treated milk; sampling 2014 | 0.06 | HPLC‐FLD | Torović (2015) | |
| AFB1 | ||||||
| Children 1–3 years | Portugal | Breakfast cereals, infant cereals, biscuits | 0.011–0.012 | 0.055a | HPLC‐FLD; LB‐UB | Assunção et al. (2018) |
| Children | Serbia | Biscuits with fruit fillings | 0.05–0.09 | LC–MS/MS; LB‐UB | Škrbić et al. (2017)b | |
| Adolescents | Serbia | Biscuits with fruit fillings | 0.04–0.08 | LC–MS/MS; LB‐UB | Škrbić et al. (2017)b | |
| Adolescents | Spain | Coffee | 0.001 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| Adults | Portugal | Nuts | 0.0069–0.089 | LC–MS/MS; LB‐UB | Cunha et al. (2018) | |
| Adults | Spain | Bread | 0.03–0.035 | LC–MS/MS; LB‐UB | Saladino et al. (2017) | |
| Adults | Serbia | Biscuits with fruit fillings | 0.03–0.06 | LC–MS/MS; LB‐UB | Škrbić et al. (2017)b | |
| Adults | Spain | Coffee | 0.003 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| AFB2 | ||||||
| Children 1–3 years | Portugal | Breakfast cereals, infant cereals, biscuits | 0.001–0.003 | 0.01a | HPLC‐FLD; LB‐UB | Assunção et al. (2018) |
| Adolescents | Spain | Coffee | < 0.001 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| Adults | Portugal | Nuts | 0.0002–0.0643 | LC–MS/MS; LB‐UB | Cunha et al. (2018) | |
| Adults | Spain | Bread | 0.022–0.026 | LC–MS/MS; LB‐UB | Saladino et al. (2017) | |
| Adults | Spain | Coffee | 0.001 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| AFG1 | ||||||
| Children 1–3 years | Portugal | Breakfast cereals, infant cereals, biscuits | 0.002–0.016 | 0.048 a | HPLC‐FLD; LB‐UB | Assunção et al. (2018) |
| Adolescents | Spain | Coffee | 0.001 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| Adults | Portugal | Nuts | 0–0.0529 | LC–MS/MS; LB‐UB | Cunha et al. (2018) | |
| Adults | Spain | Bread | 0.008–0.018 | LC–MS/MS; LB‐UB | Saladino et al. (2017) | |
| Adults | Spain | Coffee | 0.006 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| AFG2 | ||||||
| Adolescent | Spain | Coffee | 0.003 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| Adult | Portugal | Nuts | 0.0273–0.095 | LC–MS/MS; LB‐UB | Cunha et al. (2018) | |
| Adults | Spain | Coffee | 0.014 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| AFT | ||||||
| Adolescent | Spain | Coffee | 0.008 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
| Adults | Spain | Bread | 0.021–0.078 | LC–MS/MS; LB‐UB | Saladino et al. (2017) | |
| Adults | Spain | Coffee | 0.036 | LC–MS/MS; LB | García‐Moraleja et al. (2015) | |
AFM1: aflatoxin M1; AFB1: aflatoxin B1; AFB2: aflatoxin B2; AFG1: aflatoxin G1; AFG2 aflatoxin G2; AFT: aflatoxin total; bw: body weight; ELISA: enzyme‐linked immunosorbent assay; HPLC: high‐performance liquid chromatography; LB: lower bound; UB: upper bound.
AFT: sum of AFB1, B2, G1 and G2.
P95 calculated via a probabilistic approach in which the left‐censored data were replaced by random values from a uniform distribution with zero as minimum and the LOD as maximum.
Calculated exposures for AFB2, G1 and G2 are not shown since all samples were left‐censored.
3.3.4. Non‐dietary sources of exposure
In addition to dietary exposure, people might be exposed to aflatoxins from the environment, e.g. occupational exposure. Depending on the working conditions, individuals can be exposed by inhalation and potentially dermal and oral routes (e.g. Saad‐Hussein et al., 2016; Rushing and Selim, 2019). While occupational exposure may contribute significantly for individual workers, this is not considered further in this Scientific Opinion.
3.4. Risk characterisation
3.4.1. Risk characterisation based on animal data
The CONTAM Panel selected the BMDL10 of 0.4 μg/kg bw per day for the induction of HCC by AFB1 in male rats as a reference point for the risk characterisation of aflatoxins.
Comparison of the chronic dietary exposure to AFB1 across dietary surveys and age groups reported above (Table 13) to this BMDL10, results in MOE values (Table 16) (minimum ‐ maximum) that range from 5,000 to 225 for the mean LB exposure to AFB1 and from 690 to 58 for the mean UB exposure to AFB1 across dietary surveys and age groups. The MOE values range from 1,143 to 64 for the P95 LB exposure to AFB1 and from 145 to 29 for the P95 UB exposure to AFB1 across dietary surveys and age groups.
Table 16.
Margin of exposure (MOE) values based on dietary exposure to AFB1 for the incidence of HCC across dietary surveys and age groups
| Age groups | MOE calculated from mean dietary exposure to AFB1 | MOE calculated from P95 dietary exposure to AFB1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | Minimum | Median | Maximum | |||||||
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |
| Infants | 5,000 | 690 | 2,222 | 199 | 667 | 82 | 1,143 | 143 | 541 | 77 | 217 | 31 |
| Toddlers | 930 | 127 | 625 | 75 | 381 | 58 | 519 | 59 | 274 | 41 | 139 | 29 |
| Other children | 851 | 116 | 526 | 81 | 225 | 65 | 342 | 66 | 253 | 46 | 64 | 34 |
| Adolescents | 1,481 | 201 | 1,000 | 134 | 323 | 94 | 571 | 123 | 408 | 71 | 87 | 46 |
| Adults | 1,818 | 296 | 1,212 | 186 | 816 | 123 | 645 | 145 | 460 | 94 | 294 | 59 |
| Elderly | 2,105 | 303 | 1,538 | 211 | 1,290 | 137 | 851 | 145 | 645 | 108 | 364 | 76 |
| Very elderly | 2,222 | 284 | 1,538 | 193 | 976 | 137 | 952 | 142 | 714 | 104 | 430 | 79 |
AFB1: aflatoxin B1; HCC: hepatocellular carcinoma; LB: lower bound; UB: upper bound.
For substances that are both genotoxic and carcinogenic, the EFSA Scientific Committee stated that an MOE of 10,000 or higher, if based on the BMDL10 from an animal carcinogenicity study, would be of low concern from a public health point of view (EFSA, 2005). The CONTAM Panel noted that the calculated MOEs are below 10,000, which raises a health concern.
The available data do not make it possible to calculate a BMDL10 for AFM1. However, the CONTAM Panel agreed to use a potency factor of 0.1 in combination with the BMDL10 of 0.4 μg/kg bw per day for the induction of HCC by AFB1 for the AFM1 risk assessment. Table 17 shows the calculated MOE values for AFM1. They range (minimum–maximum) from 100,000 to 2,564 for the mean LB exposure estimates, from 66,667 to 2,020 for the mean UB exposure estimates, from 33,333 to 642 for the P95 LB exposure estimates, and from 25,000 to 508 for the P95 UB exposure estimates across dietary surveys and age groups have been calculated. The CONTAM Panel noted that the calculated MOEs are below 10,000 for some surveys, particularly for the younger age groups, which raises a health concern, albeit the high exposure to AFM1 from milk and dairy products may be limited to a short period in life.
Table 17.
Margin of exposure (MOE) values based on dietary exposure to AFM1 and a potency factor of 0.1 for the incidence of HCC across dietary surveys and age groups
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| MOE calculated from mean dietary exposure to AFM1 | ||||||
| Infants | 28,571 | 19,048 | 7,018 | 4,938 | 2,564 | 2,020 |
| Toddlers | 8,889 | 6,250 | 5,882 | 3,810 | 2,817 | 2,210 |
| Other children | 22,222 | 14,286 | 11,429 | 7,692 | 5,128 | 4,000 |
| Adolescents | 50,000 | 33,333 | 26,667 | 17,391 | 16,000 | 10,811 |
| Adults | 80,000 | 66,667 | 50,000 | 33,333 | 28,571 | 20,000 |
| Elderly | 100,000 | 66,667 | 50,000 | 33,333 | 28,571 | 22,222 |
| Very elderly | 100,000 | 66,667 | 50,000 | 36,364 | 26,667 | 18,182 |
| Pregnant women | 44,444 | 36,364 | 36,364 | 26,667 | 30,769 | 20,000 |
| Lactating women | 28,571 | 20,000 | 22,222 | 16,000 | 18,182 | 13,793 |
| MOE calculated from P95 dietary exposure to AFM1 | ||||||
| Infants | 6,061 | 4,082 | 2,703 | 1,942 | 642 | 508 |
| Toddlers | 3,810 | 2,685 | 2,721 | 1,835 | 1,053 | 825 |
| Other children | 9,302 | 6,452 | 5,000 | 3,175 | 1,852 | 1,465 |
| Adolescents | 18,182 | 12,903 | 10,811 | 6,897 | 8,333 | 5,797 |
| Adults | 30,769 | 25,000 | 16,000 | 12,500 | 10,256 | 7,407 |
| Elderly | 33,333 | 25,000 | 16,667 | 12,500 | 10,526 | 8,333 |
| Very elderly | 23,529 | 16,000 | 16,000 | 12,903 | 11,765 | 8,889 |
| Pregnant women | 19,048 | 14,815 | 14,286 | 10,526 | 11,765 | 8,163 |
| Lactating women | 11,765 | 8,696 | 10,526 | 7,843 | 9,756 | 7,143 |
AFM1: aflatoxin M1; HCC: hepatocellular carcinoma; LB: lower bound; UB: upper bound.
MOE values based on the exposure to the sum of AFT and AFM1 and the BMDL10 of 0.4 μg/kg bw per day are presented in Appendix V. The calculated MOE values were below 10,000, which raises a health concern.
3.4.2. Risk characterisation based on human data
The CONTAM Panel also used the cancer potency estimates reported by JECFA for the risk characterisation. Using model averaging, JECFA calculated potency estimates of 0.017 (mean) and 0.049 (95% UB) per 100,000 person‐years per ng/kg bw per day for HBsAg‐negative individuals and 0.269 (mean) and 0.562 (95% UB) per 100,000 person‐years per ng/kg bw per day for HBsAg‐positive individuals (FAO/WHO, 2018; see Section 1.3.1 for further details). Considering the new evidence regarding HCV as a risk factor, the CONTAM Panel decided to take also the prevalence of HCV into account in the risk characterisation.
In 2016, the European Centre for Disease Prevention and Control (ECDC) published a systematic review on hepatitis B and C prevalence in the EU/EEA (European Economic Area). Studies were included that measured HBV and HCV markers (HBsAg and anti‐HCV antibodies). Based on data from 13 countries, the reported prevalence of HBV for the general population ranged from 0.1 (Ireland) to 4.4% (Romania). For HCV, the prevalence ranged from 0.1 (Belgium, Ireland and the Netherlands) to 5.9% (Italy). Overall, the prevalence of HBV and HCV in the EU/EEA was estimated to be around 0.9 and 1.1%, respectively, corresponding to 4.7 million chronic HBV cases and 5.6 million HCV‐infected subjects (ECDC, 2016). However, no overall data are available regarding the co‐infection with HBV and HCV in the EU/EEA. In some studies on the prevalence of HBV and HCV, none of the subjects were found to be co‐infected (Bulgaria: Kevorkyan et al., 2015; Italy: Fabris et al., 2008 and Cozzolongo et al., 2009; Spain: Calleja‐Panero et al., 2013) while other studies reported a low number of co‐infected persons. In France, two persons out of 14,413 persons were co‐infected (Meffre et al., 2010) and Pendino et al. (2005) reported that 2 persons were co‐infected out of 1645. One study reported a higher co‐infection prevalence of 1.53%. However, it was noted that the overall HBV infection rate was high in this study (4% compared to 0.7% as the average for Italy) (Squeri et al., 2006). Based on this information, the CONTAM Panel concluded that the available data are too limited to estimate the prevalence of co‐infection of HBV and HCV in Europe and followed a conservative approach by adding up the prevalence of HBV and HCV. This sum ranges from 0.2 (Ireland) to 7.6% (Romania) across the 12 European countries for which data were available.
As described in FAO/WHO (2018), the aflatoxin‐related hepatocellular carcinoma risk is estimated from the cancer potency estimates using the following equation:
where R is the cancer risk
PHBV+ is the potency estimates P for the HBV+ fraction of the population
PHBV− is the potency estimates P for the HBV− fraction of the population
HBV+ is the population fraction of chronic HBV cases
Based on the mean potency estimates and a prevalence of 0.2%, the CONTAM Panel estimated the cancer risk from the mean dietary exposure to AFB1 to be between 0.001 and 0.122 aflatoxin‐induced cancers per 100,000 person‐years, across dietary surveys and age groups (Table 18). In adults, the estimated cancer risk ranged between 0.004 and 0.057 aflatoxin‐induced cancers per 100,000 person‐years. The highest exposure and consequent cancer risk were calculated for toddlers. For this age class, the cancer risk was estimated to be between 0.008 and 0.122 aflatoxin‐induced cancers per 100,000 person‐years. Based on the 95th percentile dietary exposure, the estimated cancer risk ranged between 0.006 and 0.245.
Table 18.
Cancer risk estimatesa calculated from the chronic dietary exposure to AFB1, the mean potency estimates of the cancer risk and a HBV/HCV prevalence of 0.2%
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Based on mean dietary exposure in total population | ||||||
| Infants | 0.001 | 0.010 | 0.003 | 0.035 | 0.011 | 0.085 |
| Toddlers | 0.008 | 0.055 | 0.011 | 0.094 | 0.018 | 0.122 |
| Other children | 0.008 | 0.061 | 0.013 | 0.086 | 0.031 | 0.107 |
| Adolescents | 0.005 | 0.035 | 0.007 | 0.052 | 0.022 | 0.075 |
| Adults | 0.004 | 0.024 | 0.006 | 0.038 | 0.009 | 0.057 |
| Elderly | 0.003 | 0.023 | 0.005 | 0.033 | 0.005 | 0.051 |
| Very elderly | 0.003 | 0.025 | 0.005 | 0.036 | 0.007 | 0.051 |
| Based on 95th percentile dietary exposure in total population | ||||||
| Infants | 0.006 | 0.049 | 0.013 | 0.091 | 0.032 | 0.228 |
| Toddlers | 0.013 | 0.119 | 0.026 | 0.170 | 0.050 | 0.245 |
| Other children | 0.020 | 0.106 | 0.028 | 0.152 | 0.109 | 0.208 |
| Adolescents | 0.012 | 0.057 | 0.017 | 0.098 | 0.081 | 0.151 |
| Adults | 0.011 | 0.048 | 0.015 | 0.074 | 0.024 | 0.119 |
| Elderly | 0.008 | 0.048 | 0.011 | 0.065 | 0.019 | 0.092 |
| Very elderly | 0.007 | 0.049 | 0.010 | 0.068 | 0.016 | 0.088 |
AFB1: aflatoxin B1; HBV: hepatitis B virus; HCV: Hepatitis C virus; LB: lower bound; UB: upper bound.
Expressed per 100,000 person‐years.
Based on the UB potency estimates and a prevalence of 7.6%, the CONTAM Panel estimated the cancer risk from the mean dietary exposure to AFB1 to be between 0.007 and 0.612 aflatoxin‐induced cancers per 100,000 person‐years, across dietary surveys and age groups (Table 19). In adults, the estimated cancer risk ranged between 0.019 and 0.286 aflatoxin‐induced cancers per 100,000 person‐years. The highest exposure and consequent cancer risk were calculated for toddlers. For this age class, the cancer risk was estimated to be between 0.038 and 0.612 aflatoxin‐induced cancers per 100,000 person‐years. Based on the 95th percentile dietary exposure, the estimated cancer risk ranged between 0.031 and 1.233.
Table 19.
Cancer risk estimatesa calculated from the chronic dietary exposure to AFB1, the upper bound potency estimates of the cancer risk and a HBV/HCV prevalence of 7.6%
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Based on mean dietary exposure in total population | ||||||
| Infants | 0.007 | 0.051 | 0.016 | 0.177 | 0.053 | 0.429 |
| Toddlers | 0.038 | 0.277 | 0.056 | 0.471 | 0.092 | 0.612 |
| Other children | 0.041 | 0.304 | 0.067 | 0.434 | 0.157 | 0.538 |
| Adolescents | 0.024 | 0.175 | 0.035 | 0.262 | 0.109 | 0.376 |
| Adults | 0.019 | 0.119 | 0.029 | 0.189 | 0.043 | 0.286 |
| Elderly | 0.017 | 0.116 | 0.023 | 0.167 | 0.027 | 0.256 |
| Very elderly | 0.016 | 0.124 | 0.023 | 0.182 | 0.036 | 0.258 |
| Based on 95th percentile dietary exposure in total population | ||||||
| Infants | 0.031 | 0.245 | 0.065 | 0.456 | 0.162 | 1.146 |
| Toddlers | 0.068 | 0.598 | 0.128 | 0.853 | 0.253 | 1.233 |
| Other children | 0.103 | 0.531 | 0.139 | 0.766 | 0.547 | 1.045 |
| Adolescents | 0.062 | 0.286 | 0.086 | 0.494 | 0.407 | 0.758 |
| Adults | 0.055 | 0.243 | 0.077 | 0.373 | 0.120 | 0.597 |
| Elderly | 0.041 | 0.243 | 0.055 | 0.327 | 0.097 | 0.463 |
| Very elderly | 0.037 | 0.247 | 0.049 | 0.340 | 0.082 | 0.444 |
AFB1: aflatoxin B1; HBV: hepatitis B virus; HCV: Hepatitis C virus; LB: lower bound; UB: upper bound.
Expressed per 100,000 person‐years.
To put the cancer risk estimates into context, the WHO Guideline for drinking‐water quality (WHO, 2011) was used. According to this guideline, an excess lifetime cancer risk of 10−5 or less is considered to be of low risk for health concern.21 Assuming a lifetime expectancy of 70 years, this corresponds to a yearly excess cancer risk of 0.014 additional cancer cases22 per 100,000 subjects. Comparing the estimated AFB1‐induced cancers calculated with this yearly excess cancer risk, a higher risk is identified in several surveys when using the mean dietary exposure and in most surveys when using the P95 dietary exposure.
The calculated cancer risk calculated from the chronic dietary exposure to AFM1 and AFT+AFM1 are presented in Appendix V.
Overall, the estimated cancer risks in humans following exposure to AFB1, AFM1 and AFT+M1 are in line with the conclusion drawn from the animal data.
3.5. Uncertainty analysis
The evaluation of the inherent uncertainties in the assessment of exposure to aflatoxins in food has been performed following the guidance of the Opinion of the Scientific Committee related to uncertainties in dietary exposure assessment (EFSA, 2007b). In addition, the report ‘Characterizing and communicating uncertainty in exposure assessment’ has been considered (WHO/IPCS, 2008). The CONTAM Panel took note of the new guidance on uncertainties of the Scientific Committee (EFSA Scientific Committee, 2018), but it was not implemented for this Opinion.
3.5.1. Assessment objectives
The objectives of the assessment were clearly specified in the terms of reference.
3.5.2. Exposure scenario/exposure model
The exposure assessment was based on aflatoxin occurrence data collected in numerous EU countries; however, most of them (~ 65%) were collected in only three Member States while some other countries submitted only a limited number of data. Most of the imported foods, such as nuts and fruits, were sampled in harbour areas and afterwards transported throughout Europe, therefore it is believed that the data for these foods properly covers the EU market. This seems not to be the case for the other food categories largely contributing to the exposure to aflatoxins, in particular ‘grains and grain‐based products’ and ‘milk and milk products’. For these food categories, there is uncertainty around possible regional differences in aflatoxin contamination and the data set is likely not to be fully representative of food for the EU market.
The available occurrence data have been in part collected via a risk‐based monitoring strategy and this may overestimate the background aflatoxin levels.
The CONTAM Panel noted high mean concentrations in fresh figs. However some samples may have been miscodified, resulting in uncertainty whether these samples were actually fresh or dried.
When considered appropriate, occurrence data and consumption events for solid forms of certain foods (e.g. tea leaves, cocoa powder, etc.; for more detail see Section 2.6) were adjusted by an appropriate dilution factor. Assumptions applied for this conversion may, however, not be accurate and representative for all possible commercial products. This may lead to an overestimation or underestimation of exposure.
Processing was not considered in the dietary exposure assessment since the relevant information as to whether the samples were taken from batches subject to sorting, other physical treatment, or from batches intended for direct human consumption was only provided for a limited number of samples. In this context, processing includes milling, sorting, cleaning, heat treatment of cereals and roasting of nuts. This may lead to an overestimation of the exposure, bearing in mind that processing may reduce the aflatoxin concentration.
The large proportion of analytical results with left‐censored data (values below LOD/LOQ) introduced considerable uncertainties to the exposure estimates. The use of the LB in this Opinion tends to underestimate, while UB tends to overestimate the dietary exposure. The limited number of available analytical results for some food categories adds uncertainty to the representativeness of the mean concentration values used to estimate the exposure. The occurrence data for AFT were calculated from the analytical results of the individual aflatoxins (for more detail see Section 2.3.2). For the left‐censored data, the UB AFT sum concentrations were in most of the cases based on the LOQ values reported for AFB1. This approach has introduced uncertainty to the calculated UB AFT occurrence values. This may lead to an overestimation or underestimation of exposure.
Uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database have already been described by EFSA (EFSA, 2011b) and are not further detailed in this Opinion.
3.5.3. Model input (parameters)
There are no prescribed fixed official methods for the analysis of aflatoxins and laboratories can use any appropriate method of analysis, provided it can be demonstrated in a traceable manner that they fulfil the requirements according to Commission Regulation (EC) No 401/2006. This may have added to the uncertainty in the analytical results but only to a minor extent.
3.5.4. Other uncertainties
The CONTAM Panel selected the study by Yeh et al. (1989) as the pivotal study. Nevertheless, considerable uncertainty is due to the fact that the exposure assessment was done at the community level and not at the individual level. Regarding the presence of confounders, Yeh et al. (1989) investigated the association between aflatoxin exposure and hepatocellular cancer while also taking into consideration the role of HBV infection (through HBsAg) which can function as a major confounder. However, no other confounding factors that could impact the liver (e.g. HCV, alcohol consumption) were taken into account. These limitations of the study add to the uncertainty in the hazard and risk characterisation.
The CONTAM Panel used the cancer potencies calculated by JECFA at its 83rd meeting. The liver cancer potencies were calculated using an epidemiological study where the lowest exposure group had an estimated exposure of 12 ng AFB1/kg bw (FAO/WHO, 1999, 2018). The potencies were expressed as the liver cancer cases per 100,000 person‐years per ng aflatoxin. Applying these potency estimates for AFB1 exposure of around 1 ng/kg bw per day and below implies an extrapolation outside the dose‐range. However, considering that AFB1 is a carcinogen showing a linear dose–response in the range of low doses tested in experimental studies (FAO/WHO, 2018), the CONTAM Panel concluded that this extrapolation is appropriate, but is uncertain at very low doses and might overestimate the risk.
The cancer potencies were calculated by JECFA for both HBsAg‐positive and HBsAg‐negative individuals. The cancer potency for HBsAg‐negative individuals is based on relatively few cases and is therefore more uncertain than the estimated potency for HBsAg‐positive subjects.
The use of UB cancer potencies may cause an overestimation of the cancer incidence.
There are limited data regarding the prevalence of co‐infection with HBV and HCV in the EU, that do not allow to estimate a reliable prevalence of co‐infection of HBV and HCV in Europe. However, considering that the prevalence of co‐infection seems low, the CONTAM Panel followed a conservative approach and assumed no co‐infection.
Despite the accumulating evidence, the relevance for human risk assessment of endpoints related to child growth is not clear due to the methodological constrains of the currently available evidence.
In experimental animals, most studies use AFB1. Therefore, it is uncertain whether the other aflatoxins also exhibit short‐term toxicity such as inhibition of growth, liver and kidney damage and changes in the microbiota. An uncertainty linked to the use of animal data is the fact that the HBV and HCV status cannot be taken into account as a risk factor.
The CONTAM Panel also characterised the risk based on animal data and selected the study by Wogan et al. (1974). In this study, different study durations were applied for different dose groups and a time adjustment of the doses was made. However, a BMD analysis of the non‐adjusted doses resulted in the same BMDL10 value of 0.4 μg/kg bw per day (when rounded to one significant number; data not shown) as when time‐adjusted doses were used. Therefore, the uncertainty caused by the time adjustment is low. Despite the fact that this study was carried out before OECD test guidelines were put in place, full histological examinations and detailed autopsies were performed. Highly purified crystalline AFB1 was used and diets were prepared under controlled conditions. A clear dose‐response relationship was observed confirming previous reports of AFB1 as a potent liver carcinogen. No study performed in accordance with current OECD guidelines is available.
For the calculation of the terminal half‐life, it is recommended to use a period of collection (of blood and urine sample) of at least five times the estimated half‐life. In the study by Jubert et al. (2009), the follow‐up period was 72 h, while the calculated terminal half‐life was 64 h. This introduces uncertainty in the calculated terminal half‐life and consequently may influence the conclusion regarding possible accumulation in the longer term.
Although the available evidence suggests differences in potencies between AFB1, AFB2, AFG1 and AFG2, the available data do not make it possible to identify potency factors. The CONTAM Panel assumed equal potencies for the four compounds, which leads to an overestimation of the risk for AFT. In addition, there is inadequate evidence about the interaction of AFB2, AFG1, and AFG2 with HBV and HCV as most studies have used biomarkers of exposure that relate to AFB1 exposure. However, the CONTAM Panel noted that the conclusions regarding the risk based on AFB1 alone and on the AFT+AFM1 are in‐line, showing that the influence of these assumptions on the conclusion regarding the risk related to the presence of aflatoxins in food is small.
This risk assessment is confined to AFB1, AFB2, AFG1, AFG2 and AFM1. However, also other mycotoxins such as aflatoxicol and AFM2 may add to the risk for public health related to the presence of aflatoxins in food.
3.5.5. Summary of uncertainties
In Table 20, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 20.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of aflatoxins in food
| Sources of uncertainty | Direction(a) |
|---|---|
| Extrapolation of the occurrence data to the whole of Europe for certain food categories | +/− |
| Potential reduction of the aflatoxin concentration due to processing not considered for some samples | + |
| Use of analytical data from targeted sampling | + |
| Large proportion of left‐censored data in the data set | +/− |
| Assumptions from the summing of the individual aflatoxins at the level of sample | +/− |
| Uncertainty in the exposure assessment in the study by Yeh et al. (1989) | +/− |
| Estimated cancer potency for hepatitis B surface antigen negative subjects is more uncertain because based on relatively few cases | +/− |
| Use of upper bound cancer potencies | + |
| Assumption on the co‐infection of HBV and HCV in Europe | + |
| The HBV and HCV status cannot be taken into account when using animal data for the risk characterisation | +/− |
| Cancer potency and reference point for aflatoxin B1 applied to ‘aflatoxin total’ | + |
+ : uncertainty with potential to cause overestimation of exposure/risk; −: uncertainty with potential to cause underestimation of exposure/risk.
The CONTAM Panel considered that the impact of the uncertainties on the risk assessment of aflatoxins in food is moderate and that the assessment is likely to be conservative.
4. Conclusions
Hazard identification and characterisation:
Most of the available data are on AFB1 and information on the other aflatoxins is scarce and mentioned when available.
AFB1 is readily absorbed and distributed to the liver.
CYP enzymes convert AFB1, AFG1 and AFM1 to the equivalent 8,9‐epoxides, which are capable of binding to both DNA and proteins while AFB2 and AFG2 cannot form the 8,9‐epoxide.
AFB1 and its metabolites are both excreted via the faecal and urinary routes. AFM1 is also excreted in breast milk.
In short‐term toxicity studies, AFB1 has multiple negative effects on rodents including inhibition of normal growth, and liver and kidney damage as well as sustained alterations in the intestinal microbiota.
The new studies reported in this opinion add to the weight of evidence that AFB1 is genotoxic and limited new information has become available regarding the genotoxicity of the other aflatoxins.
In liver cells (HepG2), the genotoxic potency can be summarised as AFB1 > AFG1 ≈ aflatoxicol > AFM1. AFB2 and AFG2 did not induce genotoxicity in three human cell lines (i.e. HepG2, colorectal carcinoma and renal carcinoma).
Pregnancy appeared to enhance the sensitivity of mother mice to the genotoxicity of AFB1.
In utero exposure in mice resulted in lower DNA adduct levels in the fetus than the mothers, but to a higher relative mutation frequency in the fetus.
In humans, a mutational signature for aflatoxin exposure has been identified in HCC.
AFB1 affects reproductive and developmental parameters (i.e. brain development, shortened time to delivery, low birth weight and adverse effects on spermatogenesis and folliculogenesis) at low doses (≥ 4 μg/kg bw per day) in rodents and these effects may occur following a short‐term exposure.
Aflatoxins impair the immune response, particularly at the cellular level. NOAELs for these effects are around 30 μg/kg bw per day in rodents.
AF‐alb (AFB1‐lys), urinary AF‐N7‐gua and urinary AFM1 are all biomarkers of exposure that have been validated against dietary intake of aflatoxin. However, the levels of these biomarkers cannot be converted reliably into dietary exposures in individuals.
The studies reported since 2006 have added to the weight of evidence that aflatoxin exposure is associated with a risk of HCC, with a higher risk for people infected with either HBV or HCV.
High AFB1 exposure causes acute aflatoxicosis with a high mortality rate. Lower levels of chronic exposure to AFB1 are associated with cirrhosis and indicators of liver dysfunction.
There is currently insufficient evidence to causally associate aflatoxin exposure with gall bladder cancer and stomach cancer. Likewise, there is insufficient evidence for a possible interaction between HIV and aflatoxin exposure.
Child health is an emerging area of interest for aflatoxin‐related hazard identification. There is currently insufficient evidence to support the use of child growth as an endpoint in risk assessment.
AFB1 induces oxidative stress, which might compromise critical AFB1 detoxification pathways and/or induce DNA oxidation. The Nrf2 signalling pathway plays a role in the suppression of AFB1 toxicity.
In vitro and in vivo studies provide evidence that AFB1 exposure results in a decline of global DNA methylation together with hypermethylation of several tumour suppressor genes.
There is increasing evidence that AFB1 affects the expression of key enzymes in hormone homeostasis, particularly steroid hormone homeostasis, which may lead to disturbance of regulatory mechanisms in fertility. Transport processes across the placenta may also be affected.
Some genetic polymorphisms are associated with increased risk of aflatoxin‐related liver cancer, such as GSTM1.
Liver carcinogenicity of aflatoxins, both in experimental animals and in humans, remains the critical effect for the risk assessment. The epidemiological study by Yeh et al. (1989) on mortality from liver cancer in several provinces in China, and the two‐year carcinogenicity study in male Fischer rats by Wogan et al. (1974), remain the most suitable studies for dose–response analysis.
Based on the study in rats, the CONTAM Panel used model averaging to calculate a BMDL10 of 0.4 μg/kg bw per day for the incidence of HCC to be used in an MOE approach for the risk characterisation.
For human data, the CONTAM Panel used the cancer potencies estimated by JECFA in 2016. The cancer potencies were 0.017 (mean) and 0.049 (95% UB) per 100,000 person‐years per ng/kg bw per day for HBsAg‐negative individuals and 0.269 (mean) and 0.562 (95% UB) per 100,000 person‐years per ng/kg bw per day for HBsAg‐positive individuals.
Differences in carcinogenic potency are reported for AFB2 and AFG2 compared with AFB1 and AFG1.
In vivo there is insufficient evidence to derive potency factors for AFB2 and AFG2.
There are indications of differences in the cancer potency between AFB1 and AFG1 in the liver with AFB1 being more potent. In the kidney, AFG1 has a higher cancer potency than AFB1. Again, the available data are not sufficient to be able to derive an individual potency factor that can be used in the risk assessment.
In the absence of new in vivo data to quantify differences between the individual aflatoxins the CONTAM Panel applied equal potency factors for AFB1, AFB2, AFG1 and AFG2 as used in previous assessments.
No new evidence has become available that necessitates a change of the potency factor of 0.1 for AFM1.
Occurrence/exposure for the EU population:
The highest AFB1 and AFT mean concentrations were obtained for the food category ‘legumes, nuts and oilseeds’ (in particular for pistachios, peanuts and ‘other seeds’). As expected, the highest AFM1 mean concentrations were reported for ‘milk and dairy products’ and milk‐based foods belonging to the food category ‘food for infants and small children’.
The highest LB mean exposure to AFB1 was estimated in other children with a maximum exposure of 1.8 ng/kg bw per day, while the highest UB exposure was observed for toddlers (7.0 ng/kg bw per day). The highest LB 95th percentile exposure to AFB1 was for other children with estimates of 6.2 ng/kg bw per day and the highest UB 95th percentile exposure was estimated for toddlers (14 ng/kg bw per day).
The highest estimated mean LB and UB exposure to AFM1 was in infants with a maximum LB/UB exposure of 1.6/2.0 ng/kg bw per day. The highest LB/UB 95th percentile exposure to AFM1 was also observed for infants with estimates of 6.2/7.9 ng/kg bw per day.
Overall, ‘grains and grain‐based products’ made the largest contribution to the LB mean chronic dietary exposure to AFB1 in all age classes. The subcategories driving the contribution of this food category were ‘grains for human consumption’ (in particular corn grain), ‘bread and rolls’ and ‘fine bakery wares’.
The food categories ‘liquid milk’ and ‘fermented milk products’ were the main contributors to the overall AFM1 mean exposure throughout all age groups.
Risk characterisation
Based on a BMDL10 of 0.4 μg/kg bw per day for the induction of HCC by AFB1 in male rats, MOE values (minimum–maximum) that range from 5,000 to 225 for the mean LB exposure to AFB1 and from 690 to 58 for the mean UB exposure to AFB1 across dietary surveys and age groups. The MOE values range from 1,143 to 64 for the P95 LB exposure to AFB1 and from 145 to 29 for the P95 UB exposure to AFB1 across dietary surveys and age groups. The calculated MOEs are below 10,000, which raises a health concern.
For AFM1, based on the BMDL10 of 0.4 μg/kg bw per day derived for AFB1 and a potency factor of 0.1, MOE values that range from 100,000 to 2,564 for the mean LB exposure estimates, from 66,667 to 2,020 for the mean UB exposure estimates, from 33,333 to 642 for the P95 LB exposure estimates, and from 25,000 to 508 for the P95 UB exposure estimates across dietary surveys and age groups have been calculated. The CONTAM Panel noted that the calculated MOEs are less than 10,000 for some surveys particularly for the younger age groups, which raises a health concern. However, the high exposure to AFM1 from milk and dairy products may be limited to a short period in life.
Based on the mean potency estimates of the cancer risk in humans and a HBV/HCV prevalence of 0.2%, the cancer risk was estimated to range from 0.001 to 0.122 aflatoxin‐induced cancers per year per 100,000 persons based on the mean dietary exposure to AFB1 and from 0.006 to 0.245 based on the 95th percentile exposure to AFB1. Based on the UB potency estimates of the cancer risk in humans and a HBV/HCV prevalence of 7.6%, the cancer risk was estimated to range from 0.007 to 0.612 aflatoxin‐induced cancers per year per 100,000 persons based on the mean dietary exposure to AFB1 and from 0.031 to 1.233 based on the 95th percentile exposure to AFB1.
The estimated cancer risks in humans following exposure to AFB1 are in‐line with the conclusion drawn from the animal data. This conclusion also applies to AFM1 and AFT+AFM1.
5. Recommendation
Data are needed to clarify the genotoxic and carcinogenic potential of AFG2.
In order to derive potency factors for AFG1 and AFB2 relative to AFB1, and for AFG2 if required, more data are needed.
Research designed to quantify the relationship between biomarker levels and dietary intake at the individual level, integrating dietary exposure over time with biomarker levels, is recommended. Such study would be performed in populations with an indigenous dietary exposure to aflatoxin resulting in measurable biomarker levels.
More data are needed regarding the occurrence of aflatoxicol and AFM2.
Aflatoxin occurrence should continue to be monitored in the light of potential increases due to climate change using methods with high levels of sensitivity for detection.
Abbreviations
- 8‐OHdG
8‐hydroxydeoxyguanosine
- ACHN
renal carcinoma cells
- AIC
Akaike information criterion
- AF‐alb
aflatoxin albumin adduct
- AFB1
aflatoxin B1
- AFB1‐FAPY
aflatoxin B1 formamidopyrimidine adduct
- AFB1‐lys
aflatoxin B1 lysine adduct
- AFB1‐N7‐gua
aflatoxin B1‐N7‐guanine
- AFB2
aflatoxin B2
- AFG1
aflatoxin G1
- AFG2
aflatoxin G2
- AFG5
aflatoxin G5
- AFM1
aflatoxin M1
- AFM2
aflatoxin M2
- AFP1
aflatoxin P1
- AFQ1
aflatoxin Q1
- AFT
aflatoxin total
- AhR
aryl hydrocarbon receptor
- AKR
NADPH‐dependent aldo‐keto‐reductase
- ALARA
as low as reasonably achievable
- ALP
alkaline phosphatase
- ALD
advanced liver disease
- ALT
alanine aminotransferase
- ANSES
French Agency for Food, Environmental and Occupational Health Safety
- AST
aspartate transaminase
- ATPase
adenosine triphosphatase
- BER
base excision repair
- BMD
benchmark dose
- BMDL
benchmark dose lower confidence limit
- bm
breast milk
- BMR
benchmark response
- bw
body weight
- CAR
constitutive activated/androstane receptor
- CCCF
Codex Committee on Contaminants in Food
- CI
confidence interval
- CONTAM
EFSA Panel on Contaminants in the Food Chain
- CYP
cytochrome P450
- DATA Unit
EFSA former EFSA Dietary and Chemical Monitoring Unit
- DMSO
dimethyl sulfoxide
- DNMT
DNA methyltransferase
- EBV
Epstein–Barr virus
- EEC
European Economic Community
- ELISA
enzyme‐linked immunosorbent assay
- ERα
estrogen receptor alpha
- FAO
Food and Agriculture Organization
- FD
fluorescence detection
- FFQ
food frequency questionnaire
- Fpg
formamidopyrimidine‐DNA glycosylase
- FSH
follicle‐stimulating hormone
- GBC
gall bladder cancer
- GC
gas chromatography
- GDc
gestation day
- GGT
γ‐glutamyl transpeptidase
- GI
gastrointestinal
- GSH
glutathione
- GST
glutathione S‐transferase
- HAD
height‐for‐age difference
- HAZ
height‐for‐age z‐score
- HBeAg
hepatitis B e antigen
- HBGV
health‐based guidance value
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- hpf
hours post‐fertilisation
- HPLC
high‐performance liquid chromatography
- HSD3B
3β‐hydroxysteroid dehydrogenase
- HSD17B3
17β‐hydroxysteroid dehydrogenase enzyme
- IARC
International Agency for Research on Cancer
- IFN
interferon
- IGFBP3
insulin‐like growth factor‐binding protein 3
- IGF‐1
insulin‐like growth factor 1
- IL
interleukin
- i.p.
intraperitoneal
- IQR
interquartile range
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LAZ
length‐for‐age z‐score
- LB
lower bound
- LBW
low birth weight
- LCD
left‐censored data
- LC–MS
liquid chromatography coupled to mass spectrometry
- LC–MS/MS
liquid chromatography coupled to tandem mass spectrometry
- LC‐FD
liquid chromatography coupled to fluorescence detector
- LFA3
lymphocyte function‐associated antigen 3
- LD50
lethal dose killing 50% of the animals
- LH
luteinising hormone
- LOAEL
lowest‐observed‐adverse‐effect‐level
- LOD
limit of detection
- LOQ
limit of quantification
- ML
maximum level
- miRNA
microRNA
- MOE
margin of exposure
- MS
mass spectrometry
- N/A
not applicable
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NK
natural killer (cell)
- NOAEL
no‐observed‐adverse‐effect‐level
- Nrf2
nuclear factor erythroid 2‐related factor 2
- OR
odds ratio
- PBPK
physiologically based pharmacokinetic
- PND
postnatal day
- PXR
pregnane X receptor
- ROS
reactive oxygen species
- RUNX3
runt domain‐relator factors 3
- SD
standard deviation
- SGA
small for gestational age
- SOP
standard operational procedure
- SNP
single nucleotide polymorphism
- StAR
steroidogenic acute regulatory protein
- TAS
total blood antioxidant status
- TDS
total diet study
- TGFβ
transforming growth factor β
- TK
toxicokinetics
- TLR
Toll‐like receptor
- TNF
tumour necrosis factor
- UB
upper bound
- UGT
uridine 5’‐diphospho‐glucuronosyltransferase (UGT)
- UPLC
ultra performance liquid chromatography
- UV
ultraviolet
- WHO
World Health Organization
- WAZ
weight‐for‐age z‐score
- WHZ
weight‐for‐height z‐score
- WLZ
weight‐for‐length z‐score
Appendix I – Identification and selection of evidence relevant for the risk assessment of aflatoxins in food
I.1. Literature search for hazard identification and characterisation
A. Web of Science
Used search string: TOPIC: (aflatoxin*); Time span=2006–2018; Search language=Auto
Results: 8,741
B. PubMed
Used search string: (“aflatoxins”[MeSH Terms] OR “aflatoxins”[All Fields] OR “aflatoxin”[All Fields]) AND (“2006/01/01”[PDAT] : “3000”[PDAT])
Results: 4,126
C. Sci Finder
Aflatoxin B1; year 2006‐; refined for adverse effect including toxicity; 2,116 results
Aflatoxin B2; year 2006‐; refined for adverse effect including toxicity; 322 results
Aflatoxin G1; year 2006‐; refined for adverse effect including toxicity; 318 results
Aflatoxin G2; year 2006‐; refined for adverse effect including toxicity; 275 results
Aflatoxin M1; year 2006‐; refined for adverse effect including toxicity; 273 results
Aflatoxin M2; year 2006‐; refined for adverse effect including toxicity; 16 results
D. Scopus
Used search string: TOPIC: (aflatoxin*); Time span=2006–2018; 8,805 results
E. Total
After removal of all duplicates, 11,981 papers were screened for relevance based on title and abstract.
I.2. Exclusion criteria for abstracts
The titles and abstracts of the references retrieved from the literature search described in Section I.1 were screened to identify the relevant papers for the sections on hazard identification and characterisation. Papers on the following subjects were excluded:
Papers not related to hazard identification and characterisation.
Papers reporting only levels of biomarkers for populations outside Europe.
Studies in experimental animals using routes of exposure other than oral or in which only one dose was tested. This criterion was not applied for genotoxicity and mechanistic studies.
Studies in which experimental animals are exposed to mixtures that include substances other than aflatoxins.
Studies designed to evaluate substances or extracts for anticancer therapy.
Studies in which aflatoxins are solely used for the purpose of a positive control.
I.3. Literature search for processing
In addition, a literature search was conducted in June 2019 to identify papers regarding the effect of roasting on nuts. The following search string was used:
TOPIC: (aflatoxin*) AND TOPIC: (roasting) AND TOPIC: (nut); Time span: All years. Indexes: SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH, BKCI‐S, BKCI‐SSH, ESCI, CCR‐EXPANDED, IC.; 29 results were obtained.
Appendix II – Summary tables hazard identification and characterisation
1.
Appendix III – Benchmark dose analysis of the incidence of HCC in male Fisher rats
1.
The text below describes the benchmark dose (BMD) analysis of the incidence of HCC in male rats using model averaging. BMD analysis was done according to the EFSA guidance (EFSA Scientific Committee, 2017).
III.1. Data description
Data from male Fischer rats treated with AFB1 in feed for up to 105 weeks (Wogan et al., 1974). Doses used in this BMD analysis were corrected for the shorter study duration in some groups.
Table III.1.
Data on the incidence of HCC used for BMD analysis
| Time‐adjusted dose (μg/kg bw per day)a | N | N total |
|---|---|---|
| 0 | 0 | 18 |
| 0.05 | 2 | 22 |
| 0.22 | 1 | 22 |
| 0.69 | 4 | 21 |
| 1.97 | 20 | 25 |
| 2.60 | 28 | 28 |
bw: body weight; N: number of animals.
Time adjustment based on time of appearance of earliest tumour as performed by the CONTAM Panel in 2007 (i.e. if a 1‐year exposure is corrected to a 2‐year exposure, then the dose is multiplied by a factor or 0.5).
III.2. Selection of the benchmark response
A default benchmark response (BMR) of 10% (extra risk) and a 90% interval around the BMD were selected as recommended by EFSA Scientific Committee (2017).
III.3. Software used
Results are obtained using the EFSA web tool for BMD analysis
Fitting benchmark dose models is based on the R‐package PROAST, version 66.38.
Averaging results from multiple fitted benchmark dose models is based on the methodology in Wheeler and Bailer (2008).
III.4. Specification of deviations from default assumptions
General assumptions
No deviation from the recommended defaults (e.g. gamma distributional assumption instead of log‐normal, heteroscedasticity instead of homoscedasticity) was made.
Dose–response models
No deviation from the recommended defaults. Default set of fitted models:
| Model | Number of parameters | Formula | |
|---|---|---|---|
| Null | 1 | y = a | |
| Full | No. of groups |
|
|
| Logistic | 2 |
|
|
| Probit | 2 |
|
|
| Log‐logistic | 3 |
|
|
| Log‐probit | 3 |
|
|
| Weibull | 3 |
|
|
| Gamma | 3 |
|
|
| Two‐stage | 3 |
|
|
| Exp model 3 | 3 |
|
|
| Exp model 5 | 4 |
|
|
| Hill model 3 | 3 |
|
|
| Hill model 5 | 4 |
|
For the Exp and Hill family, models were fit with 3 and 4 parameters as listed in the table. The 3‐parameter model is selected if the difference in AIC is smaller than 5, otherwise the 4‐parameter model is selected.
Procedure for selection of BMDL
There was no deviation from the procedure described in the flow chart to obtain the final BMD confidence interval.
Figure III.1.
Flowchart for selection of BMDL
III.5. Results
Table III.2.
Result for the incidence of HCC in male Fisher rats using model averaging
| Model | Number of parameters | Log‐likelihood | AIC | Accepted AIC | BMDL | BMDU | BMD | Converged |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −91.77 | 185.54 | NA | NA | NA | NA | |
| full | 6 | −33.51 | 79.02 | NA | NA | NA | NA | |
| two.stage | 3 | −36.25 | 78.50 | No | NA | NA | 0.471 | Yes |
| log.logist | 3 | −36.77 | 79.54 | No | NA | NA | 0.649 | Yes |
| Weibull | 3 | −35.67 | 77.34 | Yes | 0.371 | 1.680 | 0.674 | Yes |
| log.prob | 3 | −36.50 | 79.00 | No | NA | NA | 0.653 | Yes |
| gamma | 3 | −36.15 | 78.30 | No | NA | NA | 0.647 | Yes |
| logistic | 2 | −35.96 | 75.92 | Yes | 0.410 | 0.730 | 0.552 | Yes |
| probit | 2 | −35.72 | 75.44 | Yes | 0.377 | 0.649 | 0.497 | Yes |
| LVM: Expon. m3‐ | 3 | −35.40 | 76.80 | Yes | 0.324 | 1.360 | 0.690 | Yes |
| LVM: Hill m3‐ | 3 | −35.69 | 77.38 | Yes | 0.353 | 1.290 | 0.700 | Yes |
AIC: Akaike information criterion; BMD: benchmark dose; BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit.
Estimated model parameters
two.stage
estimate for a‐ : 0.03904
estimate for BMD‐ : 0.4706
estimate for c : 1e+12
log.logist
estimate for a‐ : 0.04983
estimate for BMD‐ : 0.6495
estimate for c : 3.659
Weibull
estimate for a‐ : 0.05056
estimate for BMD‐ : 0.6742
estimate for c : 2.673
log.prob
estimate for a‐ : 0.0501
estimate for BMD‐ : 0.6525
estimate for c : 2.157
gamma
estimate for a‐ : 0.04974
estimate for BMD‐ : 0.6467
estimate for cc : 4.933
logistic
estimate for a‐ : ‐3.296
estimate for BMD‐ : 0.5515
probit
estimate for a‐ : ‐1.866
estimate for BMD‐ : 0.4972
EXP
estimate for a‐ : 1.507
estimate for CED‐ : 0.69
estimate for d‐ : 1.432
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 1.5
estimate for CED‐ : 0.7001
estimate for d‐ : 1.744
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Table III.3.
Mode weights used in model averaging
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.06 | 0.03 | 0.1 | 0.04 | 0.06 | 0.21 | 0.26 | 0.13 | 0.1 |
Confidence intervals for the BMD are based on 5,000 bootstrap data sets. the BMDL is the 5th percentile of all parametric bootstrap BMD values and the BMDU is the 95th percentile.
Table III.4.
Calculated BMDL and BMDU values (μg/kg bw per day) for the incidence of hepatocellular carcinomas reported by Wogan et al. (1974)
| BMDL | BMDU |
|---|---|
| 0.37 | 1.2 |
Visualisation
Appendix IV – Summary tables occurrence and exposure
1.
Appendix V – Risk characterisation
1.
Table V.1.
Margin of exposure (MOE) values based on dietary exposure to AFT+AFM1 for the incidence of HCC across dietary surveys and age groups
| Age groups | MOE calculated from mean dietary exposure to AFT+AFM1 | MOE calculated from P95 dietary exposure to AFT+AFM1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | Minimum | Median | Maximum | |||||||
| LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | LB | UB | |
| Infants | 2,222 | 455 | 952 | 155 | 396 | 40 | 615 | 99 | 345 | 54 | 122 | 14 |
| Toddlers | 541 | 79 | 325 | 44 | 195 | 32 | 310 | 48 | 172 | 26 | 90 | 15 |
| Other children | 460 | 75 | 328 | 46 | 208 | 32 | 235 | 53 | 174 | 25 | 91 | 17 |
| Adolescents | 930 | 139 | 625 | 77 | 381 | 57 | 377 | 83 | 284 | 35 | 156 | 30 |
| Adults | 1,143 | 175 | 727 | 100 | 500 | 61 | 482 | 92 | 320 | 44 | 174 | 28 |
| Elderly | 1,429 | 190 | 870 | 109 | 690 | 61 | 615 | 91 | 396 | 53 | 252 | 32 |
| Very elderly | 1,290 | 158 | 930 | 107 | 667 | 61 | 548 | 78 | 421 | 45 | 278 | 30 |
AFM1: aflatoxin M1; AFT: aflatoxin total; HCC: hepatocellular carcinoma; LB: lower bound; UB: upper bound.
Table V.2.
Cancer risk estimatesa calculated from the chronic dietary exposure to AFM1, the mean potency estimates of the cancer risk and a HBV/HCV prevalence of 0.2%
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Based on mean dietary exposure in total population | ||||||
| Infants | 0.002 | 0.004 | 0.010 | 0.014 | 0.027 | 0.035 |
| Toddlers | 0.008 | 0.011 | 0.012 | 0.018 | 0.025 | 0.032 |
| Other children | 0.003 | 0.005 | 0.006 | 0.009 | 0.014 | 0.018 |
| Adolescents | 0.001 | 0.002 | 0.003 | 0.004 | 0.004 | 0.006 |
| Adults | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.004 |
| Elderly | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 |
| Very elderly | 0.001 | 0.001 | 0.001 | 0.002 | 0.003 | 0.004 |
| Pregnant women | 0.002 | 0.002 | 0.002 | 0.003 | 0.002 | 0.004 |
| Lactating women | 0.002 | 0.004 | 0.003 | 0.004 | 0.004 | 0.005 |
| Based on 95th percentile dietary exposure in total population | ||||||
| Infants | 0.012 | 0.017 | 0.026 | 0.036 | 0.109 | 0.138 |
| Toddlers | 0.018 | 0.026 | 0.026 | 0.038 | 0.067 | 0.085 |
| Other children | 0.008 | 0.011 | 0.014 | 0.022 | 0.038 | 0.048 |
| Adolescents | 0.004 | 0.005 | 0.006 | 0.010 | 0.008 | 0.012 |
| Adults | 0.002 | 0.003 | 0.004 | 0.006 | 0.007 | 0.009 |
| Elderly | 0.002 | 0.003 | 0.004 | 0.006 | 0.007 | 0.008 |
| Very elderly | 0.003 | 0.004 | 0.004 | 0.005 | 0.006 | 0.008 |
| Pregnant women | 0.004 | 0.005 | 0.005 | 0.007 | 0.006 | 0.009 |
| Lactating women | 0.006 | 0.008 | 0.007 | 0.009 | 0.007 | 0.010 |
AFM1: aflatoxin M1; HBV: hepatitis B virus; HCV: Hepatitis C virus; LB: lower bound; UB: upper bound.
Expressed per 100,000 person‐years.
Table V.3.
Cancer risk estimatesa calculated from the chronic dietary exposure to AFM1, the upper bound potency estimates of the cancer risk and a HBV/HCV prevalence of 7.6%
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Based on mean dietary exposure in total population | ||||||
| Infants | 0.012 | 0.018 | 0.050 | 0.071 | 0.137 | 0.174 |
| Toddlers | 0.040 | 0.056 | 0.060 | 0.092 | 0.125 | 0.159 |
| Other children | 0.016 | 0.025 | 0.031 | 0.046 | 0.069 | 0.088 |
| Adolescents | 0.007 | 0.011 | 0.013 | 0.020 | 0.022 | 0.033 |
| Adults | 0.004 | 0.005 | 0.007 | 0.011 | 0.012 | 0.018 |
| Elderly | 0.004 | 0.005 | 0.007 | 0.011 | 0.012 | 0.016 |
| Very elderly | 0.004 | 0.005 | 0.007 | 0.010 | 0.013 | 0.019 |
| Pregnant women | 0.008 | 0.010 | 0.010 | 0.013 | 0.011 | 0.018 |
| Lactating women | 0.012 | 0.018 | 0.016 | 0.022 | 0.019 | 0.026 |
| Based on 95th percentile dietary exposure in total population | ||||||
| Infants | 0.058 | 0.086 | 0.130 | 0.181 | 0.548 | 0.693 |
| Toddlers | 0.092 | 0.131 | 0.129 | 0.192 | 0.334 | 0.427 |
| Other children | 0.038 | 0.055 | 0.070 | 0.111 | 0.190 | 0.240 |
| Adolescents | 0.019 | 0.027 | 0.033 | 0.051 | 0.042 | 0.061 |
| Adults | 0.011 | 0.014 | 0.022 | 0.028 | 0.034 | 0.048 |
| Elderly | 0.011 | 0.014 | 0.021 | 0.028 | 0.033 | 0.042 |
| Very elderly | 0.015 | 0.022 | 0.022 | 0.027 | 0.030 | 0.040 |
| Pregnant women | 0.018 | 0.024 | 0.025 | 0.033 | 0.030 | 0.043 |
| Lactating women | 0.030 | 0.040 | 0.033 | 0.045 | 0.036 | 0.049 |
AFT: aflatoxin total; AFM1: aflatoxin M1; HBV: hepatitis B virus; HCV: Hepatitis C virus; LB: lower bound; UB: upper bound.
Expressed per 100,000 person‐years.
Table V.4.
Cancer risk estimatesa calculated from the chronic dietary exposure to AFT+AFM1, the mean potency estimates of the cancer risk and a HBV/HCV prevalence of 0.2%
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Based on mean dietary exposure in total population | ||||||
| Infants | 0.003 | 0.015 | 0.007 | 0.045 | 0.018 | 0.174 |
| Toddlers | 0.013 | 0.089 | 0.022 | 0.160 | 0.036 | 0.219 |
| Other children | 0.015 | 0.093 | 0.021 | 0.151 | 0.034 | 0.220 |
| Adolescents | 0.008 | 0.050 | 0.011 | 0.091 | 0.018 | 0.122 |
| Adults | 0.006 | 0.040 | 0.010 | 0.070 | 0.014 | 0.116 |
| Elderly | 0.005 | 0.037 | 0.008 | 0.064 | 0.010 | 0.114 |
| Very elderly | 0.005 | 0.044 | 0.008 | 0.066 | 0.011 | 0.116 |
| Based on 95th percentile dietary exposure in total population | ||||||
| Infants | 0.011 | 0.071 | 0.020 | 0.130 | 0.057 | 0.517 |
| Toddlers | 0.023 | 0.144 | 0.041 | 0.268 | 0.078 | 0.473 |
| Other children | 0.030 | 0.131 | 0.040 | 0.281 | 0.077 | 0.401 |
| Adolescents | 0.019 | 0.084 | 0.025 | 0.202 | 0.045 | 0.230 |
| Adults | 0.015 | 0.076 | 0.022 | 0.160 | 0.040 | 0.249 |
| Elderly | 0.011 | 0.077 | 0.018 | 0.133 | 0.028 | 0.216 |
| Very elderly | 0.013 | 0.089 | 0.017 | 0.155 | 0.025 | 0.234 |
AFT: aflatoxin total; AFM1: aflatoxin M1; HBV: hepatitis B virus; HCV: Hepatitis C virus; LB: lower bound; UB: upper bound.
Expressed per 100,000 person‐years.
Table V.5.
Cancer risk estimatesa calculated from the chronic dietary exposure to AFT+AFM1, the upper bound potency estimates of the cancer risk and a HBV/HCV prevalence of 7.6%
| Age group | Minimum | Median | Maximum | |||
|---|---|---|---|---|---|---|
| LB | UB | LB | UB | LB | UB | |
| Based on mean dietary exposure in total population | ||||||
| Infants | 0.016 | 0.077 | 0.037 | 0.227 | 0.089 | 0.873 |
| Toddlers | 0.065 | 0.448 | 0.108 | 0.804 | 0.180 | 1.101 |
| Other children | 0.077 | 0.468 | 0.107 | 0.759 | 0.169 | 1.103 |
| Adolescents | 0.038 | 0.253 | 0.056 | 0.457 | 0.092 | 0.614 |
| Adults | 0.031 | 0.201 | 0.048 | 0.352 | 0.070 | 0.581 |
| Elderly | 0.025 | 0.186 | 0.040 | 0.324 | 0.051 | 0.573 |
| Very elderly | 0.027 | 0.223 | 0.038 | 0.330 | 0.053 | 0.582 |
| Based on 95th percentile dietary exposure in total population | ||||||
| Infants | 0.057 | 0.355 | 0.102 | 0.654 | 0.288 | 2.601 |
| Toddlers | 0.114 | 0.726 | 0.205 | 1.349 | 0.392 | 2.380 |
| Other children | 0.150 | 0.660 | 0.202 | 1.415 | 0.387 | 2.017 |
| Adolescents | 0.093 | 0.423 | 0.124 | 1.015 | 0.225 | 1.157 |
| Adults | 0.073 | 0.384 | 0.110 | 0.806 | 0.202 | 1.249 |
| Elderly | 0.057 | 0.387 | 0.089 | 0.667 | 0.140 | 1.086 |
| Very elderly | 0.064 | 0.450 | 0.084 | 0.779 | 0.127 | 1.174 |
AFT: aflatoxin total; AFM1: aflatoxin M1; HBV: hepatitis B virus; HCV: Hepatitis C virus; LB: lower bound; UB: upper bound.
Expressed per 100,000 person‐years.
Annex A – Dietary surveys per country and age group available in the EFSA Comprehensive Database, considered in the exposure assessment
1.
The excel file containing the dietary surveys per country and age group is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3607186
Annex B – Occurrence data on aflatoxins
1.
The excel file containing the occurrence data on aflatoxins is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3607186
Annex C – Proportion of left‐censored data and the mean concentrations of the quantified analytical results of AFB1 for pistachios, hazelnuts, peanuts, other nuts and dried figs
1.
The excel file containing the proportion of left‐censored data and the mean concentrations of the quantified analytical results of AFB1 for pistachios, hazelnuts, peanuts, other nuts and dried figs is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3607186
Annex D – AFB1 and AFM1 concentrations reported for organic farming and conventional farming in selected food categories
1.
The excel file containing the AFB1 and AFM1 concentrations reported for organic farming and conventional farming in selected food categories is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3607186
Annex E – Mean and high chronic dietary exposure to aflatoxins per survey and the contribution of different food groups to the dietary exposure
1.
The excel file containing the mean and high chronic dietary exposure to aflatoxins per survey and the contribution of different food groups to the dietary exposure is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3607186
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Marko D, Oswald IP, Piersma A, Routledge M, Schlatter J, Baert K, Gergelova P and Wallace H, 2020. Scientific opinion – Risk assessment of aflatoxins in food. EFSA Journal 2020;18(3):6040, 112 pp. 10.2903/j.efsa.2020.6040
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00698
Panel members: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Elena Rovesti. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Competing interest: Michael Routledge declared previous scientific research on aflatoxins funded by private institutions. In accordance with EFSA's Policy on Independence and the Decision of theExecutive Director on Competing Interest Management, a waiver was granted to Michael Routledge regarding his participation to the CONTAM Working Group on aflatoxins in food in accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management. Pursuant to Article 21(6) of the above‐mentioned Decision, the concerned expert was allowed to take part in the discussion and in the drafting phase of the EFSA Scientific Opinion on the risks for public health related to the presence of aflatoxins in food, and has not been allowed to be, or act as, a chairman, a vice‐chairman or rapporteur of the Working Group.
Adopted: 30 January 2020
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1798/full
Notes
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Cullen et al. (1987) estimated a potency of AFM1 of 2–10% of that of AFB1, based on a comparison of the tumour incidence induced in male Fischer rats by AFM1 in their study (0.5, 5.0, and 50.0 µg/kg of AFM1 or 50 µg/kg of AFB1 in the diet) with the tumour incidence induced by AFB1 in the Wogan et al. (1974) study (1, 5, 15, 50 and 100 µg/kg in the diet).
The 56th JECFA also calculated the relative potency of AFB1 and AFM1 from the data in Cullen et al. (1987) by time extrapolation of the tumour incidence of the respective 50 µg/kg dietary dose groups (6 µg/kg bw vs 0.57 µg/kg bw).
Council Regulation (EEC) No 315/93 of 8 February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1–3.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. OJ L 70, 9.3.2006, p. 12–34.
Commission Regulation (EC) No 669/2009 of 24 July 2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of nonanimal origin and amending Decision 2006/504/EC. OJ L 194, 25.7.2009, p. 11–21.
Commission Implementing Regulation (EU) No 884/2014 of 13 August 2014 imposing special conditions governing the import of certain feed and food from certain third countries due to contamination risk by aflatoxins and repealing Regulation (EC) No 1152/2009. OJ L 242, 14.8.2014, p. 4–19.
Web of Science (WoS), formally ISI Web of Knowledge, Thomson Reuters. Available at: http://thomsonreuters.com/thomson-reuters-web-of-science/
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available at: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X5, Thomson Reuters. Available at: http://endnote.com/
From 1 January 2014 onwards, Evidence Management Unit (DATA).
n = 36 whole genome sequencing; n = 13 whole‐exome sequencing.
If a 1‐year exposure is corrected to a 2‐year exposure, then the dose is multiplied by a factor of 0.5.
If a 1‐year exposure is corrected to a 2‐year exposure, then the dose is multiplied by a factor of 0.52.
Porcine alveolar macrophages immortalised with SV40 large T antigen transformed with pSV3‐neo.
Assuming the same ML for AFB2, AFG1 and AFG2 as for AFB1.
= AFM1/10 + AFB1+AFB2+AFG1+AFG2.
An excess lifetime cancer risk of 10_5 is equivalent to one additional case of cancer per 100,000 of the population ingesting drinking water containing the substance at the guideline value for 70 years (WHO, 2011). This risk level is used by the WHO to set guidance values for chemicals in drinking water.
The yearly extra risk was calculated by dividing the excess lifetime cancer risk of 10_5 by the lifetime expectancy of 70 years and expressing it per 100,000 subjects.
References
- Accardi R, Sirand C, Gheit T, Hernandez‐Vargas H, Le C‐KF, Wild CP, Tommasino M, Gruffat H, Fusil F, Cosset F‐L, Manet E and Traverse‐Glehen A, 2015. The mycotoxin aflatoxin B1 stimulates Epstein‐Barr virus‐induced B‐cell transformation in in vitro and in vivo experimental models. Carcinogenesis, 36, 1440–1451. [DOI] [PubMed] [Google Scholar]
- Adedara IA, Nanjappa MK, Farombi EO and Akingbemi BT, 2014. Aflatoxin B1 disrupts the androgen biosynthetic pathway in rat Leydig cells. Food and Chemical Toxicology, 65, 252–259. [DOI] [PubMed] [Google Scholar]
- Adjovi YC, Bailly S, Gnonlonfin BJ, Tadrist S, Querin A, Sanni A, Oswald IP, Puel O and Bailly JD, 2014. Analysis of the contrast between natural occurrence of toxigenic Aspergilli of the Flavi section and aflatoxin B1 in cassava. Food Microbiology, 38, 151–159. 10.1016/j.fm.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Afum C, Cudjoe L, Hills J, Hunt R, Padilla LA, Elmore S, Afriyie A, Opare‐Sem O, Phillips T and Jolly PE, 2016. Association between Aflatoxin M‐1 and liver disease in HBV/HCV infected persons in Ghana. International Journal of Environmental Research and Public Health, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici M, Cecarini V, Pettinari A, Bonfili L, Angeletti M, Barocci S, Biagetti M, Fioretti E and Eleuteri AM, 2007. Binding of aflatoxins to the 20S proteasome: effects on enzyme functionality and implications for oxidative stress and apoptosis. Biological Chemistry, 388, 107–117. [DOI] [PubMed] [Google Scholar]
- An Y, Shi X, Tang X, Wang Y, Shen F, Zhang Q, Wang C, Jiang M, Liu M and Yu L, 2017. Aflatoxin B1 induces reactive oxygen species‐mediated autophagy and extracellular trap formation in macrophages. Frontiers in Cellular and Infection Microbiology, 7, 53 10.3389/fcimb.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha S, Raghunadharao D, Waliyar F, Sudini H, Parveen M, Rao R and Kumar PL, 2014. The association between exposure to aflatoxin, mutation in TP53, infection with hepatitis B virus, and occurrence of liver disease in a selected population in Hyderabad, India. Mutation Research ‐ Genetic Toxicology and Environmental Mutagenesis, 766, 23–28. [DOI] [PubMed] [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire de l'alimentation, de l'environnement et du travail), 2016. Étude de l'alimentation totale infantile. Tome 2 – Partie 2. Composés inorganiques. Rapport d'expertise collective. Édition scientifique.
- Ariño A, Herrera M, Estopanan G, Rota MC, Carraminana JJ, Juan T and Herrera A, 2009. Aflatoxins in bulk and pre‐packed pistachios sold in Spain and effect of roasting. Food Control, 20, 811–814. [Google Scholar]
- Arzandeh S and Jinap S, 2011. Effect of initial aflatoxin concentration, heating time and roasting temperature on aflatoxin reduction in contaminated peanuts and process optimisation using response surface modelling. International Journal of Food Science and Technology, 46, 485–491. 10.1111/j.1365-2621.2010.02514.x [DOI] [Google Scholar]
- Assunção R, Martins C, Vasco E, Jager A, Oliveira C, Cunha SC, Fernandes JO, Nunes B, Loureiro S and Alvito P, 2018. Portuguese children dietary exposure to multiple mycotoxins – an overview of risk assessment under MYCOMIX project. Food and Chemical Toxicology, 118, 399–408. [DOI] [PubMed] [Google Scholar]
- Austin KJ, Cockrum RR, Jons AM, Alexander BM and Cammack KM, 2012. Renin mRNA is upregulated in testes and testicular cells in response to treatment with aflatoxin B1. Theriogenology, 77, 331–337. [DOI] [PubMed] [Google Scholar]
- Ayed‐Boussema I, Pascussi J‐M, Maurel P, Bacha H and Hassen W, 2012. Effect of aflatoxin B1 on nuclear receptors PXR, CAR, and AhR and their target cytochromes P450 mRNA expression in primary cultures of human hepatocytes. International Journal of Toxicology, 31, 86–93. [DOI] [PubMed] [Google Scholar]
- Azziz‐Baumgartner E, Lindblade K, Gieseker K, Rogers HS, Kieszak S, Njapau H, Schleicher R, McCoy LF, Misore A, DeCock K, Rubin C and Slutsker L, 2005. Case‐control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environmental Health Perspectives, 113, 1779–1783. 10.1289/ehp.8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahari A, Mehrzad J, Mahmoudi M, Bassami MR and Dehghani H, 2014. Cytochrome P450 isoforms are differently up‐regulated in aflatoxin B1‐exposed human lymphocytes and monocytes. Immunopharmacology and Immunotoxicology, 36, 1–10. [DOI] [PubMed] [Google Scholar]
- Bahari A, Mehrzad J, Mahmoudi M, Bassami MR and Dehghani H, 2015. GST‐M1 is transcribed moreso than AKR7A2 in AFB(1)‐exposed human monocytes and lymphocytes. Journal of Immunotoxicology, 12, 194–198. 10.3109/1547691X.2014.925994 [DOI] [PubMed] [Google Scholar]
- Bailey GS, Dashwood R, Loveland PM, Pereira C and Hendricks JD, 1998. Molecular dosimetry in fish: quantitative target organ DNA adduction and hepatocarcinogenicity for four aflatoxins by two exposure routes in rainbow trout. Mutation Research, 399, 233–244. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Brown KL, Egli M and Stone MP, 2011. Bypass of Aflatoxin B1 Adducts by the Sulfolobus solfataricus DNA Polymerase IV. Journal of the American Chemical Society, 133, 12556–12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battilani P, Barbano C and Piva G, 2008. Aflatoxin B1 contamination in maize related to the aridity index in North Italy. World Mycotoxin Journal, 1, 449–456. 10.3920/WMJ2008.x043 [DOI] [Google Scholar]
- Battilani P, Toscano P, Van der Fels‐Klerx HJ, Moretti A, Camardo Leggieri M, Brera C, Rortais A, Goumperis T and Robinson T, 2016. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Scientific Reports, 6, 24328 10.1038/srep24328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besaratinia A, Kim S‐I, Hainaut P and Pfeifer GP, 2009. In vitro recapitulating of TP53 mutagenesis in hepatocellular carcinoma associated with dietary aflatoxin B1 exposure. Gastroenterology, 137, 1127–1137, 1137.e1121–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodreddigari S, Jones LK, Egner PA, Groopman JD, Sutter CH, Roebuck BD, Guengerich FP, Kensler TW and Sutter TR, 2008. Protection against aflatoxin B‐1‐induced cytotoxicity by expression of the cloned aflatoxin B‐1‐aldehyde reductases. Rat AKR7A1 and human AKR7A3. Chemical Research in Toxicology, 21, 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme K, Dietz Y, Hewitt P and Mueller SO, 2010. Activation of P53 in Hep G2 cells as surrogate to detect mutagens and promutagens in vitro. Toxicology Letters, 198, 272–281. [DOI] [PubMed] [Google Scholar]
- Bogalho F, Duarte S, Cardoso M, Almeida A, Cabeças R, Lino C and Pena A, 2018. Exposure assessment of portuguese infants to Aflatoxin M1 in breast milk and maternal social‐demographical and food consumption determinants. Food Control, 90, 140–145. [Google Scholar]
- Bondy GS, 2008. Immunological toxicity of mycotoxins. Stewart Postharvest Review, 4. [Google Scholar]
- Bren U, Guengerich FP and Mavri J, 2007. Guanine alkylation by the potent carcinogen aflatoxin B‐1: quantum chemical calculations. Chemical Research in Toxicology, 20, 1134–1140. [DOI] [PubMed] [Google Scholar]
- Brown KL, Bren U, Stone MP and Guengerich FP, 2009. Inherent stereospecificity in the reaction of aflatoxin B(1) 8,9‐epoxide with deoxyguanosine and efficiency of DNA catalysis. Chemical Research in Toxicology, 22, 913–917. 10.1021/tx900002g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundy D, Silva N, Horton A, Patton G, Schultz L and Jamison D, 2017. Child and adolescent health and development: realizing neglected potential In: rd, Bundy DAP, Silva N, Horton S, Jamison DT, Patton GC. (eds.). Child and Adolescent Health and Development. The International Bank for Reconstruction and Development/The World Bank, Washington, DC. [PubMed] [Google Scholar]
- Butler WH, Greenblatt M and Lijinsky W, 1969. Carcinogenesis in rats by aflatoxins B1, G1, and B2. Cancer Research, 29, 2206–2211. [PubMed] [Google Scholar]
- Calleja‐Panero JL, Llop‐Herrera E, Ruiz‐Moraga M, de ‐la‐Revilla‐Negro J, Calvo‐Bonacho E, Pons‐Renedo F, Martinez‐Porras JL, Vallejo‐Gutierrez D, Arregui C and Abreu‐Garcia L, 2013. Prevalence of viral hepatitis (B and C) serological markers in healthy working population. Revista Española de Enfermedades Digestivas, 105, 249–254. [DOI] [PubMed] [Google Scholar]
- Carlborg FW, 1979. Cancer, mathematical models and aflatoxin. Food and Cosmetics Toxicology, 17, 159–166. [DOI] [PubMed] [Google Scholar]
- Carlos RLJ, Leticia IG, Efrain FSE and Miguel RA, 2014. Aflatoxigenic feeding and its possible implications after pregnancy. Biomedical and Pharmacology Journal, 7, 183–193. [Google Scholar]
- Carvajal‐Campos A, Manizan AL, Tadrist S, Akaki DK, Koffi‐Nevry R, Moore GG, Fapohunda SO, Bailly S, Montet D, Oswald IP, Lorber S, Brabet C and Puel O, 2017. Aspergillus korhogoensis, a novel aflatoxin producing species from the cote d'Ivoire. Toxins (Basel), 9, 10.3390/toxins9110353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino JM, Routledge MN, Wilson S, Dunne DW, Mwatha JK, Gachuhi K, Wild CP and Gong YY, 2015. Aflatoxin exposure is inversely associated with IGF1 and IGFBP3 levels in vitro and in Kenyan schoolchildren. Molecular Nutrition & Food Research, 59, 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CCCF (Codex Committee on Contaminants in Foods), 2018. Report of the 12th session of the Codex Committee on Contaminants in foods. FAO/WHO. Available online: http://www.fao.org/fao-who-codexalimentarius/committees/committee/en/?committee=CCCF
- Chawanthayatham S, Thiantanawat A, Egner PA, Groopman JD, Wogan GN, Croy RG and Essigmann JM, 2015. Prenatal exposure of mice to the human liver carcinogen aflatoxin B‐1 reveals a critical window of susceptibility to genetic change. International Journal of Cancer, 136, 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawanthayatham S, Valentine CC, Iii F, Bogdan IF, Edward JL, Lawrence AL, Stuart SS, Stephen LW, Gerald NC and Robert GE, 2017. Mutational spectra of aflatoxin B1 in vivo establish biomarkers of exposure for human hepatocellular carcinoma. Proceedings of the National Academy of Sciences of the United States of America, 114, E3101–E3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C‐H, Wang M‐H, Wang J‐H, Hung C‐H, Hu T‐H, Lee S‐C, Tung H‐D, Lee C‐M, Changchien C‐S, Chen P‐F, Hsu M‐C and Lu S‐N, 2007. Aflatoxin exposure and hepatitis C virus in advanced liver disease in a hepatitis C virus‐endemic area in Taiwan. American Journal of Tropical Medicine and Hygiene, 77, 747–752. [PubMed] [Google Scholar]
- Chen T, Heflich RH, Moore MM and Mei N, 2010. Differential mutagenicity of aflatoxin B‐1 in the liver of neonatal and adult mice. Environmental and Molecular Mutagenesis, 51, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gong YY, Kimanya ME, Shirima CP and Routledge MN, 2018a. Comparison of urinary aflatoxin M1 and aflatoxin albumin adducts as biomarkers for assessing aflatoxin exposure in Tanzanian children. Biomarkers, 23, 131–136. 10.1080/1354750x.2017.1285960 [DOI] [PubMed] [Google Scholar]
- Chen C, Mitchell NJ, Gratz J, Houpt ER, Gong Y, Egner PA, Groopman JD, Riley RT, Showker JL, Svensen E, Mduma ER, Patil CL and Wu F, 2018b. Exposure to aflatoxin and fumonisin in children at risk for growth impairment in rural Tanzania. Environmental International, 115, 29–37. 10.1016/j.envint.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li C, Chen Y, Ni C, Chen X, Zhang L, Xu X, Chen M, Ma X, Zhan H, Xu A, Ge R and Guo X, 2019. Aflatoxin B1 impairs leydig cells through inhibiting AMPK/mTOR‐mediated autophagy flux pathway. Chemosphere, 233, 261–272. 10.1016/j.chemosphere.2019.05.273 [DOI] [PubMed] [Google Scholar]
- Cherkani‐Hassani A, Mojemmi B and Mouane N, 2016. Occurrence and levels of mycotoxins and their metabolites in human breast milk associated to dietary habits and other factors: a systematic literature review, 1984–2015. Trends in Food Science & Technology, 50, 56–69. [Google Scholar]
- Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, Lee MH, Jen CL, You SL, Santella RM and Chen CJ, 2017. Aflatoxin B‐1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. International Journal of Cancer, 141, 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu YJ, Yang HI, Wu HC, Lee MH, Liu J, Wang LY, Lu SN, Jen CL, You SL, Santella RM and Chen CJ, 2018. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. European Journal of Cancer, 94, 37–46. 10.1016/j.ejca.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcuera L‐A, Vettorazzi A, Arbillaga L, Perez N, Gil AG, Azqueta A, Gonzalez‐Penas E, Garcia‐Jalon JA and Lopez de Cerain A, 2015. Genotoxicity of Aflatoxin B1 and Ochratoxin A after simultaneous application of the in vivo micronucleus and comet assay. Food and Chemical Toxicology, 76, 116–124. [DOI] [PubMed] [Google Scholar]
- Coulombe RA Jr and Sharma RP, 1985. Clearance and excretion of intratracheally and orally administered aflatoxin B1 in the rat. Food and Chemical Toxicology, 23, 827–830. [DOI] [PubMed] [Google Scholar]
- Cozzolongo R, Osella AR, Elba S, Petruzzi J, Buongiorno G, Giannuzzi V, Leone G, Bonfiglio C, Lanzilotta E, Manghisi OG, Leandro G, Donnaloia R, Fanelli V, Mirizzi F, Parziale L, Crupi G, Detomaso P, Labbate A, Zizzari S, Depalma M, Polignano A, Lopinto D and Daprile G, 2009. Epidemiology of HCV infection in the general population: a survey in a southern Italian town. American Journal of Gastroenterology, 104, 2740–2746. 10.1038/ajg.2009.428 [DOI] [PubMed] [Google Scholar]
- Cubadda F, 2018. Re: TDS_results on aflatoxins. Message to Baert K. 9 December 2018. Email.
- Cuccioloni M, Mozzicafreddo M, Barocci S, Ciuti F, Re L, Eleuteri AM and Angeletti M, 2009. Aflatoxin B1 misregulates the activity of serine proteases: possible implications in the toxicity of some mycotoxins. Toxicology in Vitro, 23, 393–399. [DOI] [PubMed] [Google Scholar]
- Cui A, Hua H, Shao T, Song P, Kong Q, Luo T and Jiang Y, 2015. Aflatoxin B1 induces Src phosphorylation and stimulates lung cancer cell migration. Tumour Biology, 36, 6507–6513. [DOI] [PubMed] [Google Scholar]
- Cullen JM, Ruebner BH, Hsieh LS, Hyde DM and Hsieh D, 1987. Carcinogenicity of dietary aflatoxin M in male Fischer rats compared to aflatoxin B1. Cancer Research, 47, 1913–1917. [PubMed] [Google Scholar]
- Cunha SC, Sa SVM and Fernandes JO, 2018. Multiple mycotoxin analysis in nut products: occurrence and risk characterization. Food and Chemical Toxicology, 114, 260–269. 10.1016/j.fct.2018.02.039 [DOI] [PubMed] [Google Scholar]
- Cupid BC, Lightfoot TJ, Russell D, Gant SJ, Turner PC, Dingley KH, Curtis KD, Leveson SH, Turteltaub KW and Garner RC, 2004. The formation of AFB1‐macromolecular adducts in rats and humans at dietary levels of exposure. Food and Chemical Toxicology, 42, 559–569. 10.1016/j.fct.2003.10.015 [DOI] [PubMed] [Google Scholar]
- Dai Y, Zhang B, Zhu L, Huang K and Xu W, 2017. Aflatoxin B1‐induced epigenetic alterations: an overview. Food and Chemical Toxicology, 109, 683–689. [DOI] [PubMed] [Google Scholar]
- Dalezios JI, Hsieh DPH and Wogan GN, 1973. Excretion and metabolism of orally administered aflatoxin b1 by rhesus monkeys. Food and Cosmetics Toxicology, 11, 605–616. 10.1016/S0015-6264(73)80331-1 [DOI] [PubMed] [Google Scholar]
- Danesh NM, Bostan HB, Abnous K, Ramezani M, Youssefi K, Taghdisi SM and Karimi G, 2018. Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors: a review. Trac‐Trends in Analytical Chemistry, 99, 117–128. [Google Scholar]
- De Santis B, Raggi ME, Moretti G, Facchiano F, Mezzelani A, Villa L, Bonfanti A, Campioni A, Rossi S, Camposeo S, Soricelli S, Moracci G, Debegnach F, Gregori E, Ciceri F, Milanesi L, Marabotti A and Brera C, 2017. Study on the Association among Mycotoxins and other Variables in Children with Autism. Toxins (Basel), 9 10.3390/toxins9070203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries HR, Maxwell SM and Hendrickse RG, 1989. Foetal and neonatal exposure to aflatoxins. Acta paediatrica Scandinavica, 78, 373–378. [DOI] [PubMed] [Google Scholar]
- Degen GH and Neumann H‐G, 1978. The major metabolite of aflatoxin B1 in the rat is a glutathione conjugate. Chemico‐Biological Interactions, 22, 239–255. 10.1016/0009-2797(78)90129-1 [DOI] [PubMed] [Google Scholar]
- Degen GH, Muñoz K and Hengstler JG, 2013. Occurrence of mycotoxins in breast milk In: Zibadi S, Watson RR. and Preedy VR. (eds.). Handbook of Dietary and Nutritional Aspects of Human Breast Milk. Human Health Handbook no. 5. Wageningen, The Netherlands, Wageningen Academic Publishers; pp. 813–831. [Google Scholar]
- Denning DW, Allen R, Wilkinson AP and Morgan MRA, 1990. Transplacental transfer of aflatoxin in humans. Carcinogenesis, 11, 1033–1035. 10.1093/carcin/11.6.1033 [DOI] [PubMed] [Google Scholar]
- Dhanasekaran D, Shanmugapriya S, Thajuddin N and Panneerselvam A, 2011. Aflatoxins and Aflatoxicosis in human and animals. Aflatoxins ‐ Biochemistry and Molecular Biology, 221–254. [Google Scholar]
- Diaz GJ and Sánchez MP, 2015. Determination of aflatoxin M1 in breast milk as a biomarker of maternal and infant exposure in Colombia. Food Additives and Contaminants ‐ Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 32, 1192–1198. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Beima KM, Bammler TK, Riley RT and Voss KA, 2018. 2.20 ‐ Hepatotoxic Mycotoxins In: McQueen CA. (ed.). Comprehensive Toxicology, 3rd Edition. Elsevier, Oxford: pp. 483–521. [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2016. Systematic review on hepatitis B and C prevalence in the EU/EEA. Stockholm: ECDC. 10.2900/24396 [DOI]
- Echodu R, Edema H, Malinga GM, Hendy A, Colebunders R, Moriku Kaducu J, Ovuga E and Haesaert G, 2018. Is nodding syndrome in northern Uganda linked to consumption of mycotoxin contaminated food grains? BMC Res Notes, 11, 678 10.1186/s13104-018-3774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on contaminants in the food chain (CONTAM) related to Aflatoxin B1 as undesirable substance in animal feed. EFSA Journal 2004;2(3):239, 27 pp. 10.2903/j.efsa.2004.239 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to a harmonized approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA Journal 2005;3(5):282, 31 pp. 10.2903/j.efsa.2005.282 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007a. Opinion of the scientific panel on contaminants in the food chain (CONTAM) related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts and pistachios and derived products. EFSA Journal 2007;5(3):446, 127 pp. 10.2903/j.efsa.2007.446 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009a. Statement of the Scientific Panel on Contaminants in the Food Chain on a request from the European Commission on the effects on public health of an increase of the levels for aflatoxin total from 4 μg/kg to 10 μg/kg for tree nuts other than almonds, hazelnuts and pistachios. EFSA Journal 2009;7(6):1168, 11 pp. 10.2903/j.efsa.2009.1168 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(5):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Evaluation of the FoodEx, the food classification system applied to the development of the EFSA Comprehensive European Food Consumption Database. EFSA Journal 2011;9(3):1970, 27 pp. 10.2903/j.efsa.2011.1970 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011b. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Effect on dietary exposure of an increase of the levels for aflatoxin total from 4 μg/kg to 10 μg/kg for dried figs. EFSA Journal 2012;10:EN‐311 10.2903/sp.efsa.2012.EN-311 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Arcella D and Gómez Ruiz JA, 2018a. Technical report on use of cut‐off values on the limits of quantification reported in datasets used to estimate dietary exposure to chemical contaminants. EFSA supporting publication 2018:EN‐1452, 11 pp. 10.2903/sp.efsa.2018.EN-1452 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Arcella D, Loannidou S and Sousa R, 2018b. Internal report on the harmonisation of dilution factors to be used in the assessment of dietary exposure. EFSA Journal 2018; 10.5281/zenodo.1256085 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Outcome of a public consultation on the draft risk assessment of aflatoxins in food. EFSA supporting publication 2020:EN‐1798. 51 pp. 10.2903/sp.efsa.2020.EN-1798 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2018. Effect on public health of a possible increase of the maximum level for ‘aflatoxin total’ from 4 to 10 mu g/kg in peanuts and processed products thereof, intended for direct human consumption or use as an ingredient in foodstuffs. EFSA Journal 2018;16(2):5175, 32 pp. 10.2903/j.efsa.2018.5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011:9(9);2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Risk Assessment Terminology. EFSA Journal, 2012;10(5):2664, 43 pp. 10.2903/j.efsa.2012.2664 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):5123, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner PA, Yu X, Johnson JK, Nathasingh CK, Groopman JD, Kensler TW and Roebuck BD, 2003. Identification of aflatoxin M1‐N7‐guanine in liver and urine of tree shrews and rats following administration of aflatoxin B1. Chemical Research in Toxicology, 16, 1174–1180. 10.1021/tx034106u [DOI] [PubMed] [Google Scholar]
- Eom S‐Y, Yim D‐H, Zhang Y, Yun J‐K, Moon SI, Yun H‐Y, Song Y‐J, Youn S‐J, Hyun T, Park J‐S, Kim BS, Lee J‐Y, Kim Y‐D and Kim H, 2013. Dietary aflatoxin B1 intake, genetic polymorphisms of CYP1A2, CYP2E1, EPHX1, GSTM1, and GSTT1, and gastric cancer risk in Korean. Cancer Causes & Control, 24, 1963–1972. [DOI] [PubMed] [Google Scholar]
- European Commission , 2018a. European Union comments for the Codex Committee on Contaminants in Food 12th session. Proposed draft maximum level for total aflatoxins in ready‐to‐eat peanuts and associated sampling plan. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/codex_cccf_12_agenda_item_10.pdf
- European Commission , 2018b. European Union comments for the Codex Committee on Contaminants in Food 12th session. Proposed draft maximum levels for total aflatoxins and ochratoxin A in nutmeg, chilli and paprika, ginger, pepper and turmeric and associated sampling plans. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/codex_cccf_12_agenda_item_11.pdf
- Fabris P, Baldo V, Baldovin T, Bellotto E, Rassu M, Trivello R, Tramarin A, Tositti G and Floreani A, 2008. Changing epidemiology of HCV and HBV infections in Northern Italy: a survey in the general population. Journal of Clinical Gastroenterology, 42, 527–532. 10.1097/MCG.0b013e318030e3ab. [DOI] [PubMed] [Google Scholar]
- Fang Y, Feng Y, Wu T, Srinivas S, Yang W, Fan J, Yang C and Wang S, 2013. Aflatoxin B1 negatively regulates Wnt/β‐catenin signaling pathway through activating miR‐33a. PLoS ONE, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 1999. Aflatoxins. Safety evaluation of certain food additives and contaminants. Prepared by the sixty‐eighth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additive Series 40.
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2001. Aflatoxin M1. Evaluation of certain mycotoxins in food. WHO Food Additives Series, No. 47/FAO Food and Nutrition Paper 74. Prepared by the Fifty‐sixth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2018. Aflatoxins. Safety evaluation of certain contaminants in food: prepared by the eighty‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO Food Additives Series, No 74; 2‐280.
- Fasullo M, Freedland J, St JN, Cera C, Hartog M, Ding X, Fasullo M and Egner P, 2017. An in vitro system for measuring genotoxicity mediated by human CYP3A4 in Saccharomyces cerevisiae. Environmental and Molecular Mutagenesis, 58, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xue W‐J, Li P, Sha Z‐Y, Huang H, Rui L, Li H‐X and Mao Q‐S, 2012. RASSF1A hypermethylation is associated with aflatoxin B1 and polycyclic aromatic hydrocarbon exposure in hepatocellular carcinoma. Hepato‐Gastroenterology, 59, 1883–1888. [DOI] [PubMed] [Google Scholar]
- Ferri F, Brera C, De Santis B, Fedrizzi G, Bacci T, Bedogni L, Capanni S, Collini G, Crespi E, Debegnach F, Ferdenzi P, Gargano A, Gattei D, Luberto F, Magnani I, Magnani MG, Mancuso P, Menotta S, Mozzanica S, Olmi M, Ombrini G, Sala O, Soricelli S, Vicentini M and Giorgi Rossi P, 2017. Survey on urinary levels of aflatoxins in professionally exposed workers. Toxins (Basel), 9 10.3390/toxins9040117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink‐Gremmels J, 2008. Mycotoxins in cattle feeds and carry‐over to dairy milk: a review. Food Additives and Contaminants. Part B‐Surveillance, 25, 172–180. 10.1080/02652030701823142 [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Hubka V, Ezekiel CN, Hong SB, Novakova A, Chen AJ, Arzanlou M, Larsen TO, Sklenar F, Mahakarnchanakul W, Samson RA and Houbraken J, 2019. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Studies in Mycology, 93, 1–63. 10.1016/j.simyco.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gacem MA and Ould El Hadj‐Khelil A, 2016. Toxicology, biosynthesis, bio‐control of aflatoxin and new methods of detection. Asian Pacific Journal of Tropical Biomedicine, 6, 808–814. [Google Scholar]
- Gallagher EP, Kunze KL, Stapleton PL and Eaton DL, 1996. The kinetics of aflatoxin B1 oxidation by human cDNA‐expressed and human liver microsomal cytochromes P450 1A2 and 3A4. Toxicology and Applied Pharmacology, 141, 595–606. 10.1006/taap.1996.0326 [DOI] [PubMed] [Google Scholar]
- Galvano F, Pietri A, Bertuzzi T, Gagliardi L, Ciotti S, Luisi S, Bognanno M, La Fauci L, Iacopino AM, Nigro F, Li Volti G, Vanella L, Giammanco G, Tina GL and Gazzolo D, 2008. Maternal dietary habits and mycotoxin occurrence in human mature milk. Molecular Nutrition and Food Research, 52, 496–501. 10.1002/mnfr.200700266 [DOI] [PubMed] [Google Scholar]
- García‐Moraleja A, Font G, Manes J and Ferrer E, 2015. Analysis of mycotoxins in coffee and risk assessment in Spanish adolescents and adults. Food and Chemical Toxicology, 86, 225–233. [DOI] [PubMed] [Google Scholar]
- Gerding J, Cramer B and Humpf HU, 2014. Determination of mycotoxin exposure in Germany using an LC‐MS/MS multibiomarker approach. Molecular Nutrition and Food Research, 58, 2358–2368. 10.1002/mnfr.201400406 [DOI] [PubMed] [Google Scholar]
- Gerding J, Ali N, Schwartzbord J, Cramer B, Brown DL, Degen GH and Humpf H‐U, 2015. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC‐MS/MS approach. Mycotoxin Research, 31, 127–136. [DOI] [PubMed] [Google Scholar]
- Ghiasian S and Maghsood A, 2012. Infants’ exposure to Aflatoxin M1 from mother's breast milk in Iran. Iranian Journal of Public Health, 41, 119–126. [PMC free article] [PubMed] [Google Scholar]
- Githanga D, Wangia RN, Mureithi MW, Wandiga S, Mutegi C, Ogutu B, Agweyu A, Wang JS and Anzala O, 2019. The effects of aflatoxin exposure on Hepatitis B‐induced immunity in Kenyan children. Curr Probl Pediatr Adolesc Health Care. 10.1016/j.cppeds.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Arranz E and Navarro‐Blasco I, 2010. Aflatoxin M1 in Spanish infant formulae: occurrence and dietary intake regarding type, protein‐base and physical state. Food Additives and Contaminants: Part B Surveillance, 3, 193–199. 10.1080/19393210.2010.503353 [DOI] [PubMed] [Google Scholar]
- Gong YY, Wilson S, Mwatha JK, Routledge MN, Castelino JM, Zhao B, Kimani G, Kariuki HC, Vennervald BJ, Dunne DW and Wild CP, 2012. Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environmental Health Perspectives, 120, 893–896. 10.1289/ehp.1104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouas DA, Shi H, Hautefeuille AH, Ortiz‐Cuaran SL, Legros PC, Szymanska KJ, Galy O, Egevad LA, Abedi‐Ardekani B, Wiman KG, Hantz O, Caron dFC, Chemin IA and Hainaut PL, 2010. Effects of the TP53 p.R249S mutant on proliferation and clonogenic properties in human hepatocellular carcinoma cell lines: interaction with hepatitis B virus X protein. Carcinogenesis, 31, 1475–1482. [DOI] [PubMed] [Google Scholar]
- Groopman JD, Fowler KW, Busby WF Jr and Wogan GN, 1981. Interaction of aflatoxin B2 with rat liver DNA and histones in vivo. Carcinogenesis, 2, 1371–1373. 10.1093/carcin/2.12.1371 [DOI] [PubMed] [Google Scholar]
- Groopman JD, Donahue PR, Zhu JQ, Chen JS and Wogan GN, 1985. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proceedings of the National Academy of Sciences of the United States of America, 82, 6492–6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman JD, Zhu JQ, Donahue PR, Pikul A, Zhang LS, Chen JS and Wogan GN, 1992a. Molecular dosimetry of urinary aflatoxin‐DNA adducts in people living in Guangxi Autonomous Region, People's Republic of China. Cancer Research, 52, 45–52. [PubMed] [Google Scholar]
- Groopman JD, Hall AJ, Whittle H, Hudson GJ, Wogan GN, Montesano R and Wild CP, 1992b. Molecular dosimetry of aflatoxin‐N7‐guanine in human urine obtained in The Gambia, West Africa. Cancer Epidemiology, Biomarkers and Prevention, 1, 221–227. [PubMed] [Google Scholar]
- Guengerich F, 2005. Cytochrome P450s and other enzymes in Drug Metabolism and Toxicity. The AAPS Journal. Theme issue: NIDA/AAPS symposium on Drugs of Abuse: Mechanisms of Toxicity, Toxicokinetics and Medical consequences, November 4–5, 2005 8, Article 12. [DOI] [PMC free article] [PubMed]
- Guengerich FP, Arneson KO, Williams KM, Deng Z and Harris TM, 2002. Reaction of aflatoxin B(1) oxidation products with lysine. Chemical Research in Toxicology, 15, 780–792. [DOI] [PubMed] [Google Scholar]
- Guindon KA, Bedard LL and Massey TE, 2007. Elevation of 8‐hydroxydeoxyguanosine in DNA from isolated mouse lung cells following in vivo treatment with aflatoxin B(1). Toxicological Sciences, 98, 57–62. [DOI] [PubMed] [Google Scholar]
- Guindon‐Kezis KA, Mulder JE and Massey TE, 2014. In vivo treatment with aflatoxin B‐1 increases DNA oxidation, base excision repair activity and 8‐oxoguanine DNA glycosylase 1 levels in mouse lung. Toxicology, 321, 21–26. [DOI] [PubMed] [Google Scholar]
- Habibi N, Nassiri‐Toosi M, Sharafi H, Alavian SM, Shams‐Ghahfarokhi M and Razzaghi‐Abyaneh M, 2018. Aflatoxin B1 exposure and the risk of hepatocellular carcinoma in Iranian carriers of viral hepatitis B and C. Toxin Reviews, Ahead of Print. [Google Scholar]
- Halver JE, 1968. Aflatoxicosis and trout hepatoma. Bulletin office international des epizooties, 69, 1249–1278. [PubMed] [Google Scholar]
- Hasanzadeh S and Amani S, 2013. Aflatoxin B1 effects on ovarian follicular growth and atresia in the rat. Comparative Clinical Pathology, 22, 563–572. [Google Scholar]
- Hasanzadeh S and Rezazadeh L, 2013. Effects of aflatoxin B1 on the growth processes of spermatogenic cell series in adult male rats. Comparative Clinical Pathology, 22, 555–562. [Google Scholar]
- Hasanzadeh S, Hosseini E and Rezazadeh L, 2011. Effects of aflatoxin B‐1 on profiles of gonadotropic (FSH and LH), steroid (testosterone and 17 beta‐estradiol) and prolactin hormones in adult male rat. Iranian Journal of Veterinary Research, 12, 332–336. [Google Scholar]
- He XY, Tang LL, Wang SL, Cai QS, Wang JS and Hong JY, 2006. Efficient activation of aflatoxin B‐1 by cytochrome P450 2A13, an enzyme predominantly expressed in human respiratory tract. International Journal of Cancer, 118, 2665–2671. [DOI] [PubMed] [Google Scholar]
- He L, Liu Y, Guo Y, Xiao N and Tan Z, 2018. Influences of Aflatoxin B1 on main intestinal bacteria communities and enzyme activities in mice. Toxin Reviews, 1–6. [Google Scholar]
- Herceg Z and Paliwal A, 2011. Epigenetic mechanisms in hepatocellular carcinoma: how environmental factors influence the epigenome. Mutation Research, 727, 55–61. 10.1016/j.mrrev.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Hernandez‐Vargas H, Cros M‐P, Herceg Z, Castelino J, Routledge MN, Silver MJ, Dominguez‐Salas P, Prentice AM, Hennig BJ, Durand G, Le C‐KF, Wild CP, Moore SE and Gong YY, 2015. Exposure to aflatoxin B1 in utero is associated with DNA methylation in white blood cells of infants in The Gambia. International Journal of Epidemiology, 44, 1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S and De Saeger S, 2015. Human biomonitoring of multiple mycotoxins in the Belgian population: results of the BIOMYCO study. Environment International, 84, 82–89. 10.1016/j.envint.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Hoffmann V, Jones K and Leroy JL, 2018. The impact of reducing dietary aflatoxin exposure on child linear growth: a cluster randomised controlled trial in Kenya. BMJ Global Health, 3, e000983 10.1136/bmjgh-2018-000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holeski CJ, Eaton DL, Monroe DH and Bellamy GM, 1987. Effects of phenobarbital on the biliary excretion of aflatoxin P 1‐glucuronide and aflatoxin B1‐S‐glutathione in the rat. Xenobiotica, 17, 139–153. 10.3109/00498258709043924 [DOI] [PubMed] [Google Scholar]
- Hsieh D and Wong J, 1994. Pharmacokinetics and excretion of aflatoxins In: Eaton DL, Groopman JD. (eds). The Toxicology of Aflatoxins: Human health, veterinary, and agricultural significance. Academic Press, London: pp. 73–88. [Google Scholar]
- Huang MN, Yu W, Teoh WW, Ardin M, Jusakul A, Ng AWT, Boot A, Abedi‐Ardekani B, Villar S, Myint SS, Othman R, Poon SL, Heguy A, Olivier M, Hollstein M, Tan P, Teh BT, Sabapathy K, Zavadil J and Rozen SG, 2017. Genome‐scale mutational signatures of aflatoxin in cells, mice, and human tumors. Genome research, 27, 1475–1486. 10.1101/gr.220038.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Schwank J, Staib F, Wang XW and Harris CC, 2007. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene, 26, 2166–2176. [DOI] [PubMed] [Google Scholar]
- Hussain S, Khan MZ, Khan A, Javed I and Asi MR, 2009. Toxico‐pathological effects in rats induced by concurrent exposure to aflatoxin and cypermethrin. Toxiconology, 53, 33–41. [DOI] [PubMed] [Google Scholar]
- Huuskonen P, Myllynen P, Storvik M and Pasanen M, 2013. The effects of aflatoxin B1 on transporters and steroid metabolizing enzymes in JEG‐3 cells. Toxicology Letters, 218, 200–206. [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, Serra‐Majem L, Volatier JL, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren JD, 2011. Dietary exposure assessments for children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4 10.1186/0778-7367-69-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1987. Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC monographs on the Evaluation of Carcinogenic Risks to Humans, Suppl, 7, 1–440 PMID:3482203. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1993. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC monographs on the Evaluation of Carcinogenic Risks to Humans, 56, 1–599. [Google Scholar]
- IARC (International Agency for Research on Cancer), 2002. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC monographs on the Evaluation of Carcinogenic Risks to Humans, 82, 1–556 PMID:12687954. [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2012. Aflatoxins. Chemical Agents and Related Occupations. A review of Human Carcinogens. IARC monographs on the evaluation of carcinogenic risks to humans, 100F, 225–248. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa AT, Hirooka EY, Silva P, Bracarense A, Flaiban K, Akagi CY, Kawamura O, Costa MC and Itano EN, 2017. Impact of a single oral acute dose of aflatoxin B‐1 on liver function/cytokines and the lymphoproliferative response in C57Bl/6 mice. Toxins, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannot E, Boorman GA, Kosyk O, Bradford BU, Shymoniak S, Tumurbaatar B, Weinman SA, Melnyk SB, Tryndyak V, Pogribny IP and Rusyn I, 2012. Increased incidence of aflatoxin B1‐induced liver tumors in hepatitis virus C transgenic mice. International Journal of Cancer, 130, 1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Saha S and Verma R, 2014. Renoprotective effect of black tea against aflatoxin induced toxicity in mice. Toxicology and Environmental Health Sciences, 6, 25–32. [Google Scholar]
- Jiang Y, Jolly PE, Preko P, Wang JS, Ellis WO, Phillips TD and Williams JH, 2008. Aflatoxin‐related immune dysfunction in health and in human immunodeficiency virus disease. Clinical and Developmental Immunology, 2008, 790309 10.1155/2008/790309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NM, Egner PA, Baxter VK, Sporn MB, Wible RS, Sutter TR, Groopman JD, Kensler TW and Roebuck BD, 2014. Complete protection against aflatoxin B(1)‐induced liver cancer with a triterpenoid: DNA adduct dosimetry, molecular signature, and genotoxicity threshold. Cancer prevention research (Philadelphia, Pa.), 7, 658–665. 10.1158/1940-6207.CAPR-13-0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly PE, Jiang Y, Ellis WO, Awuah RT, Appawu J, Nnedu O, Stiles JK, Wang J‐S, Adjei O, Jolly CM and Williams JH, 2007. Association between aflatoxin exposure and health characteristics, liver function, hepatitis and malaria infections in Ghanaians. Journal of Nutritional & Environmental Medicine, 16, 242–257. [Google Scholar]
- Jolly PE, Jiang Y, Ellis WO, Sheng‐Wang J, Afriyie‐Gyawu E, Phillips TD and Williams JH, 2008. Modulation of the human immune system by aflatoxin. Detection Methods, Management, Public Health and Agricultural Trade, Mycotoxins: pp. 41–52. [Google Scholar]
- Jolly PE, Shuaib FM, Jiang Y, Preko P, Baidoo J, Stiles JK, Wang JS, Phillips TD and Williams JH, 2011. Association of high viral load and abnormal liver function with high aflatoxin B1‐albumin adduct levels in HIV‐positive Ghanaians: preliminary observations. Food Additives and Contaminants, Part A, 28, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly PE, Inusah S, Lu B, Ellis WO, Nyarko A, Phillips TD and Williams JH, 2013. Association between high aflatoxin B1 levels and high viral load in HIV‐positive people. World Mycotoxin Journal, 6, 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubert C, Mata J, Bench G, Dashwood R, Pereira C, Tracewell W, Turteltaub K, Williams D and Bailey G, 2009. Effects of chlorophyll and chlorophyllin on low‐dose aflatoxin B(1) pharmacokinetics in human volunteers. Cancer Prevention Research (Philadelphia, PA), 2, 1015–1022. 10.1158/1940-6207.CAPR-09-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamala A, Shirima C, Jani B, Bakari M, Sillo H, Rusibamayila N, De Saeger S, Kimanya M, Gong Y and Simba A, 2018. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin Journal, 11, 311–320. 10.3920/WMJ2018.2344 [DOI] [Google Scholar]
- Kamdem L, Meineke I, Godtel‐Armbrust U, Brockmoller J and Wojnowski L, 2006. Dominant contribution of P450 3A4 to the hepatic carcinogenic activation of aflatoxin B‐1. Chemical Research in Toxicology, 19, 577–586. [DOI] [PubMed] [Google Scholar]
- Kang'ethe EK, Gatwiri M, Sirma AJ, Ouko EO, Mburugu‐Musoti CK, Kitala PM, Nduhiu GJ, Nderitu JG, Mungatu JK, Hietaniemi V, Joutsjoki V and Korhonen HJ, 2017. Exposure of Kenyan population to aflatoxins in foods with special reference to Nandi and Makueni counties. Food Quality & Safety, 1, 131–137. [Google Scholar]
- Keenan J, Jolly P, Preko P, Baidoo J, Wang JS, Phillips TD, Williams JH and McGwin G Jr, 2011. Association between aflatoxin B1 albumin adduct levels and tuberculosis infection among HIV+ Ghanaians. Archives of Clinical Microbiology, 2, 3 https://doi.org/10:3823/230 [PMC free article] [PubMed] [Google Scholar]
- Kevorkyan A, Teoharov P, Lernout T, Petrova N, Raycheva R, Ivanov I, van Damme P and Kojouharova M, 2015. Prevalence of HBV and HCV among outpatients in the Plovdiv region of Bulgaria, 2010–2011. Journal of Medical Virology, 87, 401–406. 10.1002/jmv.24065 [DOI] [PubMed] [Google Scholar]
- Kirk GD, Lesi OA, Mendy M, Szymanska K, Whittle H, Goedert JJ, Hainaut P and Montesano R, 2005. 249(ser) TP53 mutation in plasma DNA, hepatitis B viral infection, and risk of hepatocellular carcinoma. Oncogene, 24, 5858–5867. 10.1038/sj.onc.1208732 [DOI] [PubMed] [Google Scholar]
- Klein K and Zanger UM, 2013. Pharmacogenomics of cytochrome P450 3A4: recent progress toward the “missing heritability” problem. Frontiers in Genetics, 4, 12–12. 10.3389/fgene.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipstein B, Huang J, Barr E, Sossenheimer P, Dietzen D, Egner PA, Groopman JD and Rudnick DA, 2015. Dietary aflatoxin‐induced stunting in a novel rat model: evidence for toxin‐induced liver injury and hepatic growth hormone resistance. Pediatric Research, 78, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komsky‐Elbaz A, Saktsier M and Roth Z, 2018. Aflatoxin B1 impairs sperm quality and fertilization competence. Toxicology, 393, 42–50. [DOI] [PubMed] [Google Scholar]
- Kos J, Lević J, Đuragić O, Kokić B and Miladinović I, 2014. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control, 38, 41–46. 10.1016/j.foodcont.2013.09.060 [DOI] [Google Scholar]
- Koshiol J, Gao Y‐T, Dean M, Egner P, Groopman J, Nepal C, Andersen JB, Jones K, Luo W, Malasky M, Wang B, Rashid A, Van DAL, Hildesheim A, Ferreccio C, Shen M‐C, Zhu B and Hsing AW, 2017. Association of aflatoxin and gallbladder cancer. Gastroenterology, 153, 488–494.e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachari KA, Bhat RV, Nagarajan V and Tilak TB, 1975. Hepatitis due to aflatoxicosis: an outbreak in Western India. Lancet, 1, 1061–1063. 10.1016/s0140-6736(75)91829-2 [DOI] [PubMed] [Google Scholar]
- Kumagai S, 1989. Intestinal absorption and excretion of aflatoxin in rats. Toxicology and Applied Pharmacology, 97, 88–97. [DOI] [PubMed] [Google Scholar]
- Kuniholm MH, Lesi OA, Mendy M, Akano AO, Sam O, Hall AJ, Whittle H, Bah E, Goedert JJ, Hainaut P and Kirk GD, 2008. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in the Gambia, West Africa. Environmental Health Perspectives, 116, 1553–1557. 10.1289/ehp.11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunter I, Hurer N, Gulcan HO, Ozturk B, Dogan I and Sahin G, 2017. Assessment of aflatoxin M1 and Heavy metal levels in mothers breast milk in famagusta, cyprus. Biological Trace Element Research, 175, 42–49. 10.1007/s12011-016-0750-z [DOI] [PubMed] [Google Scholar]
- Lauer JM, Duggan CP, Ausman LM, Griffiths JK, Webb P, Wang JS, Xue KS, Agaba E, Nshakira N and Ghosh S, 2019. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Maternal and Child Nutrition, 15, e12701 10.1111/mcn.12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hégarat L, Dumont J, Josse R, Huet S, Lanceleur R, Mourot A, Poul J‐M, Guguen‐Guillouzo C, Guillouzo A and Fessard V, 2010. Assessment of the genotoxic potential of indirect chemical mutagens in HepaRG cells by the comet and the cytokinesis‐block micronucleus assays. Mutagenesis, 25, 555–560. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Workman AD, Carey RM, Chen B, Rosen PL, Doghramji L, Adappa ND, Palmer JN, Kennedy DW and Cohen NA, 2016. Fungal aflatoxins reduce respiratory mucosal ciliary function. Scientific Reports, 6, 33221 10.1038/srep33221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de León Martinez LD, Diaz‐Barriga F, Barbier O, Ortiz DLG, Ortega‐Romero M, Perez‐Vazquez F and Flores‐Ramirez R, 2019. Evaluation of emerging biomarkers of renal damage and exposure to aflatoxin‐B‐1 in Mexican indigenous women: a pilot study. Environmental Science and Pollution Research, 26, 12205–12216. [DOI] [PubMed] [Google Scholar]
- Leroy JL, Sununtnasuk C, García‐Guerra A and Wang JS, 2018. Low level aflatoxin exposure associated with greater linear growth in southern Mexico: a longitudinal study. Maternal and Child Nutrition, 14, e12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lević J, Gošić‐Dondo S, Ivanović G, Stanković S, Krnjaja V, Bočarov‐Stančić A and Stepanić A, 2013. An outbreak of aspergillus species in response to environmental conditions in serbia. Pesticides and Phytomedicine (Belgrade), 28, 167–179. 10.2298/PIF1303167L [DOI] [Google Scholar]
- Lin YC, Li L, Burgers PM, Stone MP and Lloyd RS, 2013. Molecular mechanisms underlying aflatoxin‐induced mutagenesis. Faseb Journal, 27. [Google Scholar]
- Lin YC, Li L, Makarova AV, Burgers PM, Stone MP and Lloyd RS, 2014. Error‐prone replication bypass of the primary aflatoxin B‐1 DNA adduct, AFB(1)‐N7‐Gua. Journal of Biological Chemistry, 289, 18497–18506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Owen N, Minko IG, Lange SS, Tomida J, Li L, Stone MP, Wood RD, McCullough AK and Lloyd RS, 2016. DNA polymerase zeta limits chromosomal damage and promotes cell survival following aflatoxin exposure. Proceedings of the National Academy of Sciences of the United States of America, 113, 13774–13779. 10.1073/pnas.1609024113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y and Wang W, 2016. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Animal Science Journal, 87, 1490–1500. 10.1111/asj.12550 [DOI] [PubMed] [Google Scholar]
- Liu Z‐M, Li L‐Q, Peng M‐H, Liu T‐W, Qin Z, Guo Y, Xiao K‐Y, Ye X‐P, Mo X‐S, Qin X, Li S, Yan L‐N, Shen H‐M, Wang L, Wang Q, Wang K‐b, Liang R‐x, Wei Z‐l, Ong CN, Santella RM and Peng T, 2008. Hepatitis B virus infection contributes to oxidative stress in a population exposed to aflatoxin B1 and high‐risk for hepatocellular carcinoma. Cancer Letters, 263, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y‐X, Xi Z‐F, Xing T‐Y, Xia Q, Long X‐D, Ma Y, Huang X‐Y, Yao J‐G and Wang C, 2014. MicroRNA‐24 modulates aflatoxin B1‐related hepatocellular carcinoma prognosis and tumorigenesis. BioMed Research International, 2014, 482926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang Q‐C, Han J, Xiong B and Sun S‐C, 2015. Aflatoxin B1 is toxic to porcine oocyte maturation. Mutagenesis, 30, 527–535. [DOI] [PubMed] [Google Scholar]
- Long XD, Ma Y, Qu DY, Liu YG, Huang ZQ, Huang YZ, Lin ZH, Wei NB and Zhou SC, 2008. The polymorphism of XRCC3 codon 241 and AFB1‐related hepatocellular carcinoma in Guangxi population, China. Annals of Epidemiology, 18, 572–578. [DOI] [PubMed] [Google Scholar]
- Long XD, Ma Y, Zhou YF, Yao JG, Ban FZ, Huang YZ and Huang BC, 2009a. XPD codon 312 and 751 polymorphisms, and AFB1 exposure, and hepatocellular carcinoma risk. BMC Cancer, 9, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X‐d, Ma Y and Deng Z‐l, 2009b. GSTM1 and XRCC3 polymorphisms: effects on levels of aflatoxin B1‐DNA adducts. Chinese Journal of Cancer Research, 21, 177–184. [Google Scholar]
- Long X‐D, Ma Y, Zhou Y‐F, Ma A‐M and Fu G‐H, 2010. Polymorphism in xeroderma pigmentosum complementation group C codon 939 and aflatoxin B1‐related hepatocellular carcinoma in the Guangxi population. Hepatology, 52, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Long X‐D, Yao J‐G, Huang Y‐Z, Huang X‐Y, Ban F‐Z, Yao L‐M and Fan L‐D, 2011. DNA repair gene XRCC7 polymorphisms (rs#7003908 and rs#10109984) and hepatocellular carcinoma related to AFB1 exposure among Guangxi population, China. Hepatology Research, 41, 1085–1093. [DOI] [PubMed] [Google Scholar]
- Long X‐D, Zhao D, Wang C, Huang X‐Y, Yao J‐G, Ma Y, Wei Z‐H, Liu M, Zeng L‐X, Mo X‐Q, Zhang J‐J, Xue F, Zhai B and Xia Q, 2013. Genetic polymorphisms in DNA repair genes XRCC4 and XRCC5 and aflatoxin B1‐related hepatocellular carcinoma. Epidemiology, 24, 671–681. [DOI] [PubMed] [Google Scholar]
- Long XD, Huang HD, Huang XY, Yao JG and Xia Q, 2015. XPC codon 939 polymorphism is associated with susceptibility to DNA damage induced by aflatoxin B1 exposure. International Journal of Clinical and Experimental Medicine, 8, 1197–1204. [PMC free article] [PubMed] [Google Scholar]
- López P, de Rijk T, Sprong R, Mengelers M, Castenmiller J and Alewijn M, 2016. A mycotoxin‐dedicated total diet study in the Netherlands in 2013: part II – occurrence. World Mycotoxin Journal, 9, 89–108. [Google Scholar]
- Lu PX, Wang JB, Zhang QN, Wu Y, Sun Y and Chen TY, 2010. Longitudinal study of aflatoxin exposure in the development of primary liver cancer in patients with chronic hepatitis. Zhonghua Yi Xue Za Zhi, 90, 1665–1669. [PubMed] [Google Scholar]
- Lu PX, Wang JB, Zhang QN, Wu Y, Sun Y and Chen TY, 2012. Study of relationship between aflatoxin exposure and incidence of primary liver cancer among HBsAg carriers. Chinese Journal of Cancer Prevention and Treatment, 19, 324–327. [Google Scholar]
- Luongo D, Russo R, Balestrieri A, Marzocco S, Bergamo P and Severino L, 2014. In vitro study of AFB1 and AFM1 effects on human lymphoblastoid Jurkat T‐cell model. Journal of Immunotoxicology, 11, 353–358. 10.3109/1547691X.2013.848250 [DOI] [PubMed] [Google Scholar]
- Lüthy J, Zweifel U and Schlatter C, 1980. Metabolism and tissue distribution of [14C]aflatoxin B1 in pigs. Food and Cosmetics Toxicology, 18, 253–256. 10.1016/0015-6264(80)90103-0 [DOI] [PubMed] [Google Scholar]
- Magoha H, Kimanya M, De Meulenaer B, Roberfroid D, Lachat C and Kolsteren P, 2014. Association between aflatoxin M exposure through breast milk and growth impairment in infants from northern Tanzania. World Mycotoxin Journal, 7, 277–284. [Google Scholar]
- Manda P, Adépo AJB, Kouassi M'bengue A, Konan M, Verdier N'gbe J, Doumbia M, Toutou T and Djédjé Dano S, 2018. Assessment of the role of aflatoxin B1 in the development of hepatocellular carcinoma in Côte d'Ivoire (Ivory Coast): Preliminary study. Toxicologie Analytique et Clinique, 30, 85–95. [Google Scholar]
- Marchese S, Sorice A, Ariano A, Florio S, Budillon A, Costantini S and Severino L, 2018. Evaluation of aflatoxin M1 effects on the metabolomic and cytokinomic profiling of a hepatoblastoma cell line. Toxins (Basel), 10 10.3390/toxins10110436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin DE and Taranu I, 2012. Overview on aflatoxins and oxidative stress. Toxin Reviews, 31, 32–43. 10.3109/15569543.2012.730092 [DOI] [Google Scholar]
- Marrone AK, Tryndyak V, Beland FA and Pogribny IP, 2016. MicroRNA responses to the genotoxic carcinogens aflatoxin B1 and benzo[a]pyrene in human HepaRG cells. Toxicological Sciences, 149, 496–502. [DOI] [PubMed] [Google Scholar]
- Martin EM and Fry RC, 2018. Environmental influences on the epigenome: exposure‐ associated DNA methylation in human populations. Annual Review of Public Health, 39, 309–333. 10.1146/annurev-publhealth-040617-014629 [DOI] [PubMed] [Google Scholar]
- Martins LM, Sant'Ana AS, Iamanaka BT, Berto MI, Pitt JI and Taniwaki MH, 2017. Kinetics of aflatoxin degradation during peanut roasting. Food Research International, 97, 178–183. 10.1016/j.foodres.2017.03.052 [DOI] [PubMed] [Google Scholar]
- Matabaro E, Ishimwe N, Uwimbabazi E and Lee BH, 2017. Current immunoassay methods for the rapid detection of aflatoxin in milk and dairy products. Comprehensive Reviews in Food Science and Food Safety, 16, 808–820. [DOI] [PubMed] [Google Scholar]
- McCoy LF, Scholl PF, Sutcliffe AE, Kieszak SM, Powers CD, Rogers HS, Gong YY, Groopman JD, Wild CP and Schleicher RL, 2008. Human aflatoxin albumin adducts quantitatively compared by ELISA, HPLC with fluorescence detection, and HPLC with isotope dilution mass spectrometry. Cancer Epidemiology, Biomarkers & Prevention, 17, 1653–1657. [DOI] [PubMed] [Google Scholar]
- McCullough AK and Lloyd RS, 2019. Mechanisms underlying aflatoxin‐associated mutagenesis ‐ implications in carcinogenesis. DNA Repair (Amsterdam), 77, 76–86. 10.1016/j.dnarep.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean C, Tang L, Tang M, Billam M, Wang Z, Theodorakis CW, Kendall RJ and Wang JS, 2006a. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food and Chemical Toxicology, 44, 868–876. [DOI] [PubMed] [Google Scholar]
- McKean C, Tang L, Billam M, Tang M, Theodorakis CW, Kendall RJ and Wang JS, 2006b. Comparative acute and combinative toxicity of aflatoxin B1 and T‐2 toxin in animals and immortalized human cell lines. Journal of Applied Toxicology, 26, 139–147. [DOI] [PubMed] [Google Scholar]
- McMillan A, Renaud JB, Burgess KMN, Orimadegun AE, Akinyinka OO, Allen SJ, Miller JD, Reid G and Sumarah MW, 2018. Aflatoxin exposure in Nigerian children with severe acute malnutrition. Food and Chemical Toxicology, 111, 356–362. [DOI] [PubMed] [Google Scholar]
- Meffre C, Le Strat Y, Delarocque‐Astagneau E, Dubois F, Antona D, Lemasson JM, Warszawski J, Steinmetz J, Coste D, Meyer JF, Leiser S, Giordanella JP, Gueguen R and Desenclos JC, 2010. Prevalence of hepatitis B and hepatitis C virus infections in France in 2004: social factors are important predictors after adjusting for known risk factors. Journal of Medical Virology, 82, 546–555. 10.1002/jmv.21734 [DOI] [PubMed] [Google Scholar]
- Mehrzad J, Devriendt B, Baert K and Cox E, 2014. Aflatoxin B‐1 interferes with the antigen‐presenting capacity of porcine dendritic cells. Toxicology in Vitro, 28, 531–537. [DOI] [PubMed] [Google Scholar]
- Mehrzad J, Bahari A, Dehghani H, Bassami MR and Mahmoudi M, 2015. Immunotoxicological concerns of mycotoxins with emphasis on permissble levels of aflatoxin B‐1 and human dendritic cells. Mycoses, 58, 28. [Google Scholar]
- Mehrzad J, Bahari A, Bassami MR, Mahmoudi M and Dehghani H, 2018a. Data on environmentally relevant level of aflatoxin B1‐induced human dendritic cells’ functional alteration. Data in Brief, 18, 1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrzad J, Hosseinkhani S and Malvandi AM, 2018b. Human microglial cells undergo proapoptotic induction and inflammatory activation upon in vitro exposure to a naturally occurring level of aflatoxin B1. NeuroImmunoModulation, 25, 176–183. 10.1159/000493528 [DOI] [PubMed] [Google Scholar]
- Meissonnier GM, Marin DE, Galtier P, Bertin G and Oswald IP, 2006. Modulation of the immune response by a group of fungal food contaminant, the aflatoxins In: Mengheri E, Roselli M, Britti MS, Finamore A. (Eds.), Nutrition and Immunity. Research Signpost, Kerala: pp. 147–166. [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty A, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food additives & contaminants Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Mitchell NJ, Hsu HH, Chandyo RK, Shrestha B, Bodhidatta L, Tu YK, Gong YY, Egner PA, Ulak M, Groopman JD and Wu F, 2017. Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: an extension of the MAL‐ED study. PLoS ONE, 12, e0172124. 10.1371/journal.pone.0172124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi T, Pousty I and Gilanpour H, 2014. The effect of aflatoxin b1 on the sperm characteristics, sperm dna damage, fertility potential and early embryonic development in NMRI mice. Advances in Environmental Biology, 8, 1186–1191. [Google Scholar]
- Mohd‐Redzwan S, Rosita J, Mohd Sokhini AM, Nurul ‘Aqilah AR, Wang J‐S, Kang M‐S and Zuraini A, 2014. Detection of serum AFB1‐lysine adduct in Malaysia and its association with liver and kidney functions. International Journal of Hygiene and Environmental Health, 217, 443–451. [DOI] [PubMed] [Google Scholar]
- Moore MM, Schoeny RS, Becker RA, White K and Pottenger LH, 2018. Development of an adverse outcome pathway for chemically induced hepatocellular carcinoma: case study of AFB1, a human carcinogen with a mutagenic mode of action. Critical Reviews in Toxicology, 48, 312–337. 10.1080/10408444.2017.1423462 [DOI] [PubMed] [Google Scholar]
- Mulder JE, Bondy GS, Mehta R and Massey TE, 2014. Up‐regulation of nucleotide excision repair in mouse lung and liver following chronic exposure to aflatoxin B1 and its dependence on p53 genotype. Toxicology and Applied Pharmacology, 275, 96–103. [DOI] [PubMed] [Google Scholar]
- Mulder JE, Bondy GS, Mehta R and Massey TE, 2015. The impact of chronic Aflatoxin B‐1 exposure and p53 genotype on base excision repair in mouse lung and liver. Mutation Research‐Fundamental and Molecular Mechanisms of Mutagenesis, 773, 63–68. [DOI] [PubMed] [Google Scholar]
- Murad AF, Ahmed S and Abead S, 2015. Toxicity effect of aflatoxin B1 on reproductive system of albino male rats. Pakistan Journal of Biological Sciences, 18, 107–114. 10.3923/pjbs.2015.107.114 [DOI] [Google Scholar]
- Mykkänen H, Zhu H, Salminen E, Juvonen RO, Ling W, Ma J, Polychronaki N, Kemilainen H, Mykkanen O, Salminen S and El‐Nezami H, 2005. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB‐N7‐guanine) in young Chinese males. International Journal of Cancer, 115, 879–884. 10.1002/ijc.20951 [DOI] [PubMed] [Google Scholar]
- Neal GE, Eaton DL, Judah DJ and Verma A, 1998. Metabolism and toxicity of aflatoxins M1 and B1 in human‐derived in vitro systems. Toxicology and Applied Pharmacology, 151, 152–158. 10.1006/taap.1998.8440 [DOI] [PubMed] [Google Scholar]
- Ngindu A, Johnson BK, Kenya PR, Ngira JA, Ocheng DM, Nandwa H, Omondi TN, Jansen AJ, Ngare W, Kaviti JN, Gatei D and Siongok TA, 1982. Outbreak of acute hepatitis caused by aflatoxin poisoning in Kenya. Lancet, 1, 1346–1348. 10.1016/s0140-6736(82)92411-4 [DOI] [PubMed] [Google Scholar]
- Obuseh FA, Jolly PE, Kulczycki A, Ehiri J, Waterbor J, Desmond RA, Preko PO, Jiang Y and Piyathilake CJ, 2011. Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: a cross‐sectional study. Journal of the International AIDS Society, 14, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar SS, 2012. Incidence of aflatoxin M1 in human and animal milk in Jordan. Journal of Toxicology and Environmental Health, Part A, 75, 1404–1409. 10.1080/15287394.2012.721174 [DOI] [PubMed] [Google Scholar]
- Ortiz‐Cuaran S and Hainaut P, 2011. Molecular signatures of environmental mutagens in hepatocellular carcinoma. Genes and Environment, 33, 141–148. [Google Scholar]
- Paget V, Sichel F, Garon D and Lechevrel M, 2008. Aflatoxin B1‐induced TP53 mutational pattern in normal human cells using the FASAY (Functional Analysis of Separated Alleles in Yeast). Mutation Research, 656, 55–61. [DOI] [PubMed] [Google Scholar]
- Paget V, Lechevrel M, Andre V, Le Goff J, Pottier D, Billet S, Garcon G, Shirali P and Sichel F, 2012. Benzo[a]pyrene, aflatoxin B1 and acetaldehyde mutational patterns in TP53 gene using a functional assay: relevance to human cancer aetiology. PLoS ONE, 7, e30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen HA, El‐Nezami HS, Leppanen JM, Myllynen PK, Woodhouse HJ and Vahakangas KH, 2010. Aflatoxin B1 transfer and metabolism in human placenta. Toxicological Sciences, 113, 216–225. [DOI] [PubMed] [Google Scholar]
- Peers FG, Gilman GA and Linsell CA, 1976. Dietary aflatoxins and human liver cancer. A study in Swaziland. International Journal of Cancer, 17, 167–176. [DOI] [PubMed] [Google Scholar]
- Peers F, Bosch X, Kaldor J, Linsell A and Pluijmen M, 1987. Aflatoxin exposure, hepatitis B virus infection and liver cancer in Swaziland. International Journal of Cancer, 39, 545–553. [DOI] [PubMed] [Google Scholar]
- Pendino GM, Mariano A, Surace P, Caserta CA, Fiorillo MT, Amante A, Bruno S, Mangano C, Polito I, Amato F, Cotichini R, Stroffolini T and Mele A, 2005. Prevalence and etiology of altered liver tests: a population‐based survey in a Mediterranean town. Hepatology, 41, 1151–1159. 10.1002/hep.20689 [DOI] [PubMed] [Google Scholar]
- Peng T, Li L‐Q, Peng M‐H, Liu Z‐M, Liu T‐W, Guo Y, Xiao K‐Y, Qin Z, Ye X‐P, Mo X‐S, Yan L‐N, Lee B‐L, Shen H‐M, Tamae K, Wang LW, Wang Q, Khan KM, Wang K‐B, Liang R‐X, Wei Z‐L, Kasai H, Ong CN and Santella RM, 2007. Evaluation of oxidative stress in a group of adolescents exposed to a high level of aflatoxin B1‐a multi‐center and multi‐biomarker study. Carcinogenesis, 28, 2347–2354. [DOI] [PubMed] [Google Scholar]
- Peto R, Pike MC, Bernstein L, Gold LS and Ames BN, 1984. The TD50: a proposed general convention for the numerical description of the carcinogenic potency of chemicals in chronic‐exposure animal experiments. Environmental Health Perspectives, 58, 1–8. 10.1289/ehp.84581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekkola S, Turner PC, Abdel‐Hamid M, Ezzat S, El‐Daly M, El‐Kafrawy S, Savchenko E, Poussa T, Woo JC, Mykkanen H and El‐Nezami H, 2012. Characterisation of aflatoxin and deoxynivalenol exposure among pregnant Egyptian women. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 29, 962–971. 10.1080/19440049.2012.658442 [DOI] [PubMed] [Google Scholar]
- Pildain MB, Frisvad JC, Vaamonde G, Cabral D, Varga J and Samson RA, 2008. Two novel aflatoxin‐producing Aspergillus species from Argentinean peanuts. International Journal of Systematic and Evolutionary Microbiology, 58, 725–735. 10.1099/ijs.0.65123-0 [DOI] [PubMed] [Google Scholar]
- Piva G, Battilani P and Pietri A, 2006. Emerging issues in Southern Europe: Aflatoxins in Italy In: Barug D, Bhatnagar D, van Egmond HP, van der Kamp JW, van Osenbruggen WA. and Visconti A. (eds.). The mycotoxin factbook. Food & feed topics. Wageningen Academic Publishers, Wageningen, The Netherlands: pp. 139–153. [Google Scholar]
- Polychronaki N, Turner PC, Mykkanen H, Gong YY, Amra H, Abdel‐Wahhab M and El‐Nezami H, 2006. Determinants of aflatoxin M‐1 in breast milk in a selected group of Egyptian mothers. Food Additives and Contaminants, 23, 700–708. [DOI] [PubMed] [Google Scholar]
- Pottenger LH, Andrews LS, Bachman AN, Boogaard PJ, Cadet J, Embry MR, Farmer PB, Himmelstein MW, Jarabek AM, Martin EA, Mauthe RJ, Persaud R, Preston RJ, Schoeny R, Skare J, Swenberg JA, Williams GM, Zeiger E, Zhang F and Kim JH, 2014. An organizational approach for the assessment of DNA adduct data in risk assessment: case studies for aflatoxin B‐1, tamoxifen and vinyl chloride. Critical Reviews in Toxicology, 44, 348–391. [DOI] [PubMed] [Google Scholar]
- Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, Wogan GN and Groopman JD, 1994. A follow‐up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiology, Biomarkers & Prevention, 3, 3–10. [PubMed] [Google Scholar]
- Qian G, Wang F, Tang L, Massey ME, Mitchell NJ, Su J, Williams JH, Phillips TD and Wang J‐S, 2013. Integrative toxicopathological evaluation of aflatoxin B1 exposure in F344 rats. Toxicologic Pathology, 41, 1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian GQ, Tang LL, Guo X, Wang F, Massey ME, Su JJ, Guo TL, Williams JH, Phillips TD and Wang JS, 2014. Aflatoxin B‐1 modulates the expression of phenotypic markers and cytokines by splenic lymphocytes of male F344 rats. Journal of Applied Toxicology, 34, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonić JR, Kocic Tanackov SD, Mihajlovic IJ, Grujic ZS, Vojinovic Miloradov MB, Skrinjar MM and Turk Sekulic MM, 2017. Occurrence of aflatoxin M1 in human milk samples in Vojvodina, Serbia: estimation of average daily intake by babies. Journal of Environmental Science and Health, Part B, 52, 59–63. 10.1080/03601234.2016.1229454 [DOI] [PubMed] [Google Scholar]
- Raj HG and Lotlikar PD, 1984. Urinary excretion of thiol conjugates of aflatoxin B1 in rats and hamsters. Cancer Letters, 22, 125–133. 10.1016/0304-3835(84)90109-5 [DOI] [PubMed] [Google Scholar]
- Rieswijk L, Claessen SMH, Bekers O, van Herwijnen M, Theunissen DHJ, Jennen DGJ, e Kok T, Kleinjans JCS and van Breda SGJ, 2016. Aflatoxin B1 induces persistent epigenomic effects in primary human hepatocytes associated with hepatocellular carcinoma. Toxicology, 350, 31–39. [DOI] [PubMed] [Google Scholar]
- Rotimi OA, Rotimi SO, Duru CU, Ebebeinwe OJ, Abiodun AO, Oyeniyi BO and Faduyile FA, 2017. Acute aflatoxin B1 ‐ induced hepatotoxicity alters gene expression and disrupts lipid and lipoprotein metabolism in rats. Toxicology Reports, 4, 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge M, 2019a. Missing data. Message to Ntzani Evangelia. 1 May 2019. Email.
- Routledge M, 2019b. Shirima et al 2015. Message to Baert K. 14 June 2019. Email.
- Routledge MN, Kimanya ME, Shirima CP, Wild CP and Gong YY, 2014. Quantitative correlation of aflatoxin biomarker with dietary intake of aflatoxin in Tanzanian children. Biomarkers, 19, 430–435. 10.3109/1354750X.2014.924998 [DOI] [PubMed] [Google Scholar]
- Rushing BR and Selim MI, 2019. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food and Chemical Toxicology, 124, 81–100. 10.1016/j.fct.2018.11.047 [DOI] [PubMed] [Google Scholar]
- Saad‐Hussein A, Taha MM, Mahdy‐Abdallah H, Aziz H, Fadl NN, El‐Shamy KA, Awad AH and Moubarz G, 2016. Effects of airborne Aspergillus on serum aflatoxin B1 and liver enzymes in workers handling wheat flour. Human & Experimental Toxicology, 35, 3–9. [DOI] [PubMed] [Google Scholar]
- Sadeghi N, Oveisi MR, Jannat B, Hajimahmoodi M, Bonyani H and Jannat F, 2009. Incidence of aflatoxin M‐1 in human breast milk in Tehran, Iran. Food Control, 20, 75–78. [Google Scholar]
- Saladino F, Quiles JM, Manes J, Fernandez‐Franzon M, Luciano FB and Meca G, 2017. Dietary exposure to mycotoxins through the consumption of commercial bread loaf in Valencia, Spain. Lwt‐Food Science and Technology, 75, 697–701. [Google Scholar]
- Scholl PF, Musser SM and Groopman JD, 1997. Synthesis and characterization of aflatoxin B1 mercapturic acids and their identification in rat urine. Chemical Research in Toxicology, 10, 1144–1151. 10.1021/tx960161+ [DOI] [PubMed] [Google Scholar]
- Scholl PF, Turner PC, Sutcliffe AE, Sylla A, Diallo MS, Friesen MD, Groopman JD and Wild CP, 2006. Quantitative comparison of aflatoxin B1 serum albumin adducts in humans by isotope dilution mass spectrometry and ELISA. Cancer Epidemiology, Biomarkers & Prevention, 15, 823–826. 10.1158/1055-9965.EPI-05-0890 [DOI] [PubMed] [Google Scholar]
- Schwartzbord J, Severe L and Brown D, 2017. Detection of trace aflatoxin M1 in human urine using a commercial ELISA followed by HPLC. Biomarkers, 22, 1–4. 10.1080/1354750X.2016.1203998 [DOI] [PubMed] [Google Scholar]
- Shao P, Guo N, Wang C, Zhao M, Yi L, Liu C, Kang L, Cao L, Lv P, Xing L, Zhang X and Shen H, 2019. Aflatoxin G1 induced TNF‐alpha‐dependent lung inflammation to enhance DNA damage in alveolar epithelial cells. Journal of Cellular Physiology, 234, 9194–9206. 10.1002/jcp.27596 [DOI] [PubMed] [Google Scholar]
- Shephard GS, 2016. Current status of mycotoxin analysis: a critical review. Journal of AOAC International, 99, 842–848. 10.5740/jaoacint.16-0111 [DOI] [PubMed] [Google Scholar]
- Shi D, Liao S, Guo S, Li H, Yang M and Tang Z, 2015. Protective effects of selenium on aflatoxin B1 ‐induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling liver. Biological Trace Element Research, 163, 162–168. [DOI] [PubMed] [Google Scholar]
- Shi J, He J, Sun F, Jiang C, Shi J, Lin J, Sun X, Jiang C and Ou C, 2016. Distinct response of the hepatic transcriptome to Aflatoxin B1 induced hepatocellular carcinogenesis and resistance in rats. Scientific Reports, 6, 31898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M, 2019. Re: AFB1 and hippocompal neurogenesis. Message to Baert K. 19.06.2019. Email.
- Shin KT, Guo J, Niu YJ and Cui XS, 2018. The toxic effect of aflatoxin B1 on early porcine embryonic development. Theriogenology, 118, 157–163. 10.1016/j.theriogenology.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Shirani K, Zanjani BR, Mahmoudi M, Jafarian AH, Hassani FV, Giesy JP and Karimi G, 2018. Immunotoxicity of aflatoxin M1: as a potent suppressor of innate and acquired immune systems in a subacute study. Journal of the Science of Food and Agriculture, 98, 5884–5892. 10.1002/jsfa.9240 [DOI] [PubMed] [Google Scholar]
- Shirani K, Riahi Zanjani B, Mehri S, Razavi‐Azarkhiavi K, Badiee A, Hayes AW, Giesy JP and Karimi G, 2019. miR‐155 influences cell‐mediated immunity in Balb/c mice treated with aflatoxin M1. Drug and Chemical Toxicology, 1–8. 10.1080/01480545.2018.1556682 [DOI] [PubMed] [Google Scholar]
- Shirima CP, Kimanya ME, Routledge MN, Srey C, Kinabo JL, Humpf HU, Wild CP, Tu YK and Gong YY, 2015. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environmental Health Perspectives, 123, 173–178. 10.1289/ehp.1408097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouman BO, El Morsi D, Shabaan S, Abdel‐Hamid AH and Mehrim A, 2012. Aflatoxin B1 level in relation to child's feeding and growth. Indian Journal of Pediatrics, 79, 56–61. 10.1007/s12098-011-0493-y [DOI] [PubMed] [Google Scholar]
- Shuaib FMB, Jolly PE, Ehiri JE, Yatich N, Jiang Y, Funkhouser E, Person SD, Wilson C, Ellis WO, Wang JS and Williams JH, 2010a. Association between birth outcomes and aflatoxin B‐1 biomarker blood levels in pregnant women in Kumasi, Ghana. Tropical Medicine & International Health, 15, 160–167. [DOI] [PubMed] [Google Scholar]
- Shuaib FMB, Jolly PE, Ehiri JE, Jiang Y, Ellis WO, Stiles JK, Yatich NJ, Funkhouser E, Person SD, Wilson C and Williams JH, 2010b. Association between Anemia and Aflatoxin B1 Biomarker Levels among Pregnant Women in Kumasi, Ghana. American Journal of Tropical Medicine and Hygiene, 83, 1077–1083. 10.4269/ajtmh.2010.09-0772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupe T and Sell S, 2004. Low hepatic glutathione S‐transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicology Letters, 148, 1–9. 10.1016/j.toxlet.2003.11.008 [DOI] [PubMed] [Google Scholar]
- da Silva E, Bracarense APFL and Oswald IP, 2018. Mycotoxins and oxidative stress: where are we? World Mycotoxin Journal, 11, 113–134. 10.3920/WMJ2017.2267 [DOI] [Google Scholar]
- Singh KB, Maurya BK and Trigun SK, 2015. Activation of oxidative stress and inflammatory factors could account for histopathological progression of aflatoxin‐B1 induced hepatocarcinogenesis in rat. Molecular and Cellular Biochemistry, 401, 185–196. [DOI] [PubMed] [Google Scholar]
- Singh P, Orbach MJ and Cotty PJ, 2018. Aspergillus texensis: a novel aflatoxin producer with S morphology from the United States. Toxins (Basel), 10, 10.3390/toxins10120513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot V, Fremy JM and Leblanc JC, 2013. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food and Chemical Toxicology, 52, 1–11. 10.1016/j.fct.2012.10.036 [DOI] [PubMed] [Google Scholar]
- Škrbić B, Zivancev J, Durisic‐Mladenovic N and Godula M, 2012. Principal mycotoxins in wheat flour from the Serbian market: levels and assessment of the exposure by wheat‐based products. Food Control, 25, 389–396. [Google Scholar]
- Škrbić B, Zivancev J, Antic I and Godula M, 2014. Levels of aflatoxin M1 in different types of milk collected in Serbia: assessment of human and animal exposure. Food Control, 40, 113–119. [Google Scholar]
- Škrbić B, Antic I and Cvejanov J, 2017. Determination of mycotoxins in biscuits, dried fruits and fruit jams: an assessment of human exposure. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 34, 1012–1025. 10.1080/19440049.2017.1303195 [DOI] [PubMed] [Google Scholar]
- Solfrizzo M, Gambacorta L and Visconti A, 2014. Assessment of multi‐mycotoxin exposure in Southern Italy by urinary multi‐biomarker determination. Toxins, 6, 523–538, 516 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni P, Ghufran MS and Kanade SR, 2018. Aflatoxin B1 induced multiple epigenetic modulators in human epithelial cell lines. Toxiconology, 151, 119–128. 10.1016/j.toxicon.2018.07.011 [DOI] [PubMed] [Google Scholar]
- Sprong RC, De Wit‐Bos L, Zeilmaker MJ, Alewijn M, Castenmiller JJM and Mengelers MJB, 2016a. A mycotoxin‐dedicated total diet study in the Netherlands in 2013: part I ‐ Design. World Mycotoxin Journal, 9, 73–87. [Google Scholar]
- Sprong RC, e Wit‐Bos L, te Biesebeek JD, Alewijn M, Lopez P and Mengelers MJB, 2016b. A mycotoxin‐dedicated total diet study in the Netherlands in 2013: part III ‐ exposure and risk assessment. World Mycotoxin Journal, 9, 109–127. [Google Scholar]
- Squeri R, La Fauci V, Sindoni L, Cannavo G and Ventura Spagnolo E, 2006. Study on hepatitis B and C serologic status among municipal solid waste workers in Messina (Italy). Journal of Preventive Medicine and Hygiene, 47, 110–113. [PubMed] [Google Scholar]
- Sriwattanapong K, Slocum SL, Chawanthayatham S, Fedeles BI, Egner PA, Groopman JD, Satayavivad J, Croy RG and Essigmann JM, 2017. Editor's highlight: pregnancy alters aflatoxin B1 metabolism and increases DNA damage in mouse liver. Toxicological Sciences, 160, 173–179. 10.1093/toxsci/kfx171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MP, Banerjee S, Brown KL and Egli M, 2011. Chemistry and biology of aflatoxin‐DNA adducts. Frontiers in Nucleic Acids, 1082, 147–166. [Google Scholar]
- Sun Y, Su J, Liu Z, Liu D, Gan F, Chen X and Huang K, 2018a. Aflatoxin B1 promotes influenza replication and increases virus related lung damage via activation of TLR4 signaling. Frontiers in Immunology, 9, 2297 10.3389/fimmu.2018.02297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu Z, Liu D, Chen J, Gan F and Huang K, 2018b. Low‐level aflatoxin B1 promotes influenza infection and modulates a switch in macrophage polarization from M1 to M2. Cellular Physiology and Biochemistry, 49, 1110–1126. 10.1159/000493294 [DOI] [PubMed] [Google Scholar]
- Taguchi K, Takaku M, Egner PA, Morita M, Kaneko T, Mashimo T, Kensler TW and Yamamoto M, 2016. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin b1 toxicity. Toxicological Sciences, 152, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Mizukami S, Hasegawa‐Baba Y, Onda N, Sugita‐Konishi Y, Yoshida T and Shibutani M, 2015. Developmental exposure of aflatoxin B‐1 reversibly affects hippocampal neurogenesis targeting late‐stage neural progenitor cells through suppression of cholinergic signaling in rats. Toxicology, 336, 59–69. [DOI] [PubMed] [Google Scholar]
- Theumer MG, Henneb Y, Khoury L, Snini SP, Tadrist S, Canlet C, Puel O, Oswald IP and Audebert M, 2018. Genotoxicity of aflatoxins and their precursors in human cells. Toxicology Letters, 287, 100–107. [DOI] [PubMed] [Google Scholar]
- Torović L, 2015. Aflatoxin M1 in processed milk and infant formulae and corresponding exposure of adult population in Serbia in 2013–2014. Food Additives and Contaminants: Part B Surveillance, 8, 235–244. [DOI] [PubMed] [Google Scholar]
- Trebak F, Alaoui A, Alexandre D, El Ouezzani S, Anouar Y, Chartrel N and Magoul R, 2015. Impact of aflatoxin B1 on hypothalamic neuropeptides regulating feeding behavior. NeuroToxicology, 49, 165–173. [DOI] [PubMed] [Google Scholar]
- Turner PC, Collinson AC, Cheung YB, Gong Y, Hall AJ, Prentice AM and Wild CP, 2007. Aflatoxin exposure in utero causes growth faltering in Gambian infants. International Journal of Epidemiology, 36, 1119–1125. 10.1093/ije/dym122 [DOI] [PubMed] [Google Scholar]
- Valitutti F, De Santis B, Trovato CM, Montuori M, Gatti S, Oliva S, Brera C and Catassi C, 2018. Assessment of mycotoxin exposure in breastfeeding mothers with celiac disease. Nutrients, 10 10.3390/nu10030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rensburg SJ, Cook‐Mozaffari P, Van Schalkwyk DJ, Van der Watt JJ, Vincent TJ and Purchase IF, 1985. Hepatocellular carcinoma and dietary aflatoxin in Mozambique and Transkei. British Journal of Cancer, 51, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vleet TR, Watterson TL, Klein PJ and Coulombe RA, 2006. Aflatoxin B‐1 alters the expression of p53 in cytochrome P450‐expressing human lung cells. Toxicological Sciences, 89, 399–407. [DOI] [PubMed] [Google Scholar]
- Viegas S, Veiga L, Almeida A, os Santos M, Carolino E and Viegas C, 2016. Occupational exposure to Aflatoxin B1 in a portuguese poultry slaughterhouse. Annals of Occupational Hygiene, 60, 176–183. [DOI] [PubMed] [Google Scholar]
- Voth‐Gaeddert LE, Stoker M, Torres O and Oerther DB, 2018. Association of aflatoxin exposure and height‐for‐age among young children in Guatemala. International Journal of Environmental Research and Public Health, 1–13. 10.1080/09603123.2018.1468424 [DOI] [PubMed] [Google Scholar]
- Wacoo AP, Wendiro D, Vuzi PC and Hawumba JF, 2014. Methods for detection of aflatoxins in agricultural food crops. Journal of Applied Chemistry, 2014, 15 10.1155/2014/706291 [DOI] [Google Scholar]
- Wang JC, Tang LL, Glenn TC and Wang JS, 2016a. Aflatoxin B‐1 induced compositional changes in gut microbial communities of male F344 rats. Toxicological Sciences, 150, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tan W, Wang CC and Leung LK, 2016b. Exposure to aflatoxin B1 in late gestation alters protein kinase C and apoptotic protein expression in murine placenta. Reproductive Toxicology, 61, 68–74. [DOI] [PubMed] [Google Scholar]
- Wang S, He Z, Li D, Zhang B, Li M, Li W, Zhu W, Xing X, Zeng X, Wang Q, Dong G, Xiao Y, Chen W and Chen L, 2017. Aberrant methylation of RUNX3 is present in Aflatoxin B1‐induced transformation of the L02R cell line. Toxicology, 385, 1–9. 10.1016/j.tox.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Warth B, Parich A, Atehnkeng J, Bandyopadhyay R, Schuhmacher R, Sulyok M and Krska R, 2012. Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC‐MS/MS multitoxin method. Journal of Agricultural and Food Chemistry, 60, 9352–9363. 10.1021/jf302003n [DOI] [PubMed] [Google Scholar]
- Watson S, Moore SE, Darboe MK, Chen G, Tu YK, Huang YT, Eriksen KG, Bernstein RM, Prentice AM, Wild CP, Xu Y, Routledge MN and Gong YY, 2018. Impaired growth in rural Gambian infants exposed to aflatoxin: a prospective cohort study. BMC Public Health, 18, 1247 10.1186/s12889-018-6164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanawaraporn R, Woo LL, Belanger C, Chang S‐C, Adams JE, Trudel LJ, Bouhenguel JT, Egner PA, Groopman JD, Croy RG, Essigmann JM and Wogan GN, 2012. A single neonatal exposure to aflatoxin b1 induces prolonged genetic damage in two loci of mouse liver. Toxicological Sciences, 128, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Long X, Liu Z, Ma Y and Deng Z, 2012. Genetic polymorphism of glutathione‐S‐transferase M1 and T1 in associated with carcinogenesis of hepatocellular carcinoma and nasopharyngeal carcinoma. Chinese‐German Journal of Clinical Oncology, 11, 138–141. [Google Scholar]
- Weng M‐W, Lee H‐W, Choi B, Wang H‐T, Hu Y, Mehta M, Zheng Y, Tang M‐S, Desai D and Amin S, 2017. AFB1 hepatocarcinogenesis is via lipid peroxidation that inhibits DNA repair, sensitizes mutation susceptibility and induces aldehyde‐DNA adducts at p53 mutational hotspot codon 249. Oncotarget, 8, 18213–18226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MW and Bailer AJ, 2008. Model averaging software for dichotomous dose response risk estimation. Journal of Statistical Software, 26, 1–15.19777145 [Google Scholar]
- WHO (World Health Organization), 2011. Guideline for Drinking‐water Quality. Available online: http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2008. Uncertainty and data quality in exposure assessment. Available online: http://www.who.int/iris/handle/10665/44017
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food, International Programme on Chemical Safety, Environmental Health Criteria 240. Chapter 6: Dietary Exposure Assessment of Chemicals in Food. Available online: http://www.who.int/ipcs/food/principles/en/index1.html
- Wild CP and Montesano R, 2009. A model of interaction: Aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Letters, 286, 22–28. [DOI] [PubMed] [Google Scholar]
- Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R and Groopman JD, 1992. Dietary intake of aflatoxins and the level of albumin‐bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiology, Biomarkers & Prevention, 1, 229–234. [PubMed] [Google Scholar]
- Wild CP and Gong YY, 2010. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis, 31, 71–82. 10.1093/carcin/bgp264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP, Yin F, Turner PC, Chemin I, Chapot B, Mendy M, Whittle H, Kirk GD and Hall AJ, 2000. Environmental and genetic determinants of aflatoxin‐albumin adducts in The Gambia. International Journal of Cancer, 86, 1–7. [DOI] [PubMed] [Google Scholar]
- Williams DE, 2012. The rainbow trout liver cancer model: response to environmental chemicals and studies on promotion and chemoprevention. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 155, 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DE, Orner G, Willard KD, Tilton S, Hendricks JD, Pereira C, Benninghoff AD and Bailey GS, 2009. Rainbow trout (Oncorhynchus mykiss) and ultra‐low dose cancer studies. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 149C, 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogan GN, Edwards GS and Shank RC, 1967. Excretion and tissue distribution of radioactivity from aflatoxin B1‐14‐C in rats. Cancer Research, 27, 1729–1736. [PubMed] [Google Scholar]
- Wogan GN, 1969. Chapter VI Metabolism and biochemical effects of aflatoxins In: Goldblatt L. (ed.). Aflatoxin: Scientific Background, Control, and Implications. New York and London, Academic Press, 486 pp. [Google Scholar]
- Wogan GN, Edwards GS and Newberne PM, 1971. Structure‐activity relationships in toxicity and carcinogenicity of aflatoxins and analogs. Cancer Research, 31, 1936–1942. [PubMed] [Google Scholar]
- Wogan GN, Paglialunga S and Newberne PM, 1974. Carcinogenic effects of low dietary levels of aflatoxin B1 in rats. Food and Cosmetics Toxicology, 12, 681–685. 10.1016/0015-6264(74)90239-9 [DOI] [PubMed] [Google Scholar]
- Wójtowicz‐Chomicz K, Stadnik A, Kowal M, Sztanke K, Sztanke M and Borzecki A, 2011. Disturbances of anti‐oxidative balance in rats caused by aflatoxin B1. Bulletin of the Veterinary Institute in Pulawy, 55, 145–148. [Google Scholar]
- Wójtowicz‐Chomicz K, Stadnik A, Huk‐Wieliczuk E, Czeczuk A and Borzecki A, 2013. Influence of aflatoxin B1 on oxidoreductive balance in renal tissue of rats. Bulletin of the Veterinary Institute in Pulawy, 57, 103–106. [Google Scholar]
- Wong JJ and Hsieh DP, 1976. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proceedings of the National Academy of Sciences of the United States of America, 73, 2241–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ZA and Hsieh DPH, 1980. The comparative metabolism and toxicokinetics of aflatoxin B1 in the monkey, rat, and mouse. Toxicology and Applied Pharmacology, 55, 115–125. 10.1016/0041-008X(80)90227-6 [DOI] [PubMed] [Google Scholar]
- Woo LL, Egner PA, Belanger CL, Wattanawaraporn R, Trudel LJ, Croy RG, Groopman JD, Essigmann JM and Wogan GN, 2011. Aflatoxin B‐1‐DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1 mice. Toxicological Sciences, 122, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H‐C, Wang Q, Wang L‐W, Yang H‐I, Ahsan H, Tsai W‐Y, Wang L‐Y, Chen S‐Y, Chen C‐J and Santella RM, 2007. Urinary 8‐oxodeoxyguanosine, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis, 28, 995–999. [DOI] [PubMed] [Google Scholar]
- Wu H‐C, Wang Q, Yang H‐I, Ahsan H, Tsai W‐Y, Wang L‐Y, Chen S‐Y, Chen C‐J and Santella RM, 2008. Urinary 15‐F2t‐isoprostane, aflatoxin B1 exposure and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Carcinogenesis, 29, 971–976. 10.1093/carcin/bgn057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ and Santella RM, 2009a. Aflatoxin B‐1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiology Biomarkers & Prevention, 18, 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Jezkova A, Yuan Z, Pavlikova L, Dohnal V and Kuca K, 2009b. Biological degradation of aflatoxins. Drug Metabolism Reviews, 41, 1–7. 10.1080/03602530802563850 [DOI] [PubMed] [Google Scholar]
- Wu HC, Wang Q, Yang HI, Tsai WY, Chen CJ and Santella RM, 2013. Global DNA methylation in a population with aflatoxin B1 exposure. Epigenetics, 8, 962–969. 10.4161/epi.25696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XM, Xi ZF, Liao P, Huang HD, Huang XY, Wang C, Ma Y, Xia Q, Yao JG and Long XD, 2017. Diagnostic and prognostic potential of serum microRNA‐4651 for patients with hepatocellular carcinoma related to aflatoxin B1. Oncotarget, 8, 81235–81249. 10.18632/oncotarget.16027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu‐Williams AH, Zeise L and Thomas D, 1992. Risk assessment for aflatoxin B1: a modeling approach. Risk Analysis, 12, 559–567. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang Z, Wang X, Wang Y, Zhang X, Lu H and Wang S‐L, 2013. Cytochrome P450 2A13 enhances the sensitivity of human bronchial epithelial cells to aflatoxin B1‐induced DNA damage. Toxicology and Applied Pharmacology, 270, 114–121. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu L, Chen J and Xiao A, 2017a. Response of intestinal bacterial flora to the long‐term feeding of aflatoxin B1 (AFB1) in mice. Toxins (Basel), 9 10.3390/toxins9100317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Liu WY, Lin H, Zeng H, Zhang RP, Pu CW, Wang LQ, Zheng CF, Tan Y, Luo Y, Feng XB, Tian YQ, Xiao GS, Wang J, Huang YJ, Luo JH, Qiu ZQ, Chen JA, Wu LP, He LX and Shu WQ, 2017b. Interaction effects of AFB1 and MC‐LR co‐exposure with polymorphism of metabolic genes on liver damage: focusing on SLCO1B1 and GSTP1. Scientific Reports, 7, 10.1038/s41598-017-16432-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanpanah H, Mohammadi T, Abouhossain G and Cheraghali AM, 2005. Effect of roasting on degradation of Aflatoxins in contaminated pistachio nuts. Food and Chemical Toxicology, 43, 1135–1139. 10.1016/j.fct.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Yeh FS, Yu MC, Mo CC, Luo S, Tong MJ and Henderson BE, 1989. Hepatitis B virus, aflatoxins, and hepatocellular carcinoma in southern Guangxi, China. Cancer Research, 49, 2506–2509. [PubMed] [Google Scholar]
- Yu J, 2012. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins (Basel), 4, 1024–1057. 10.3390/toxins4111024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir‐Nasta T, Razi M, Shapour H, Malekinejad H and Malekinejad H, 2018. Roles of p21, p53, cyclin D1, CDK‐4, estrogen receptor α in aflatoxin B1‐induced cytotoxicity in testicular tissue of mice. Environmental Toxicology, 33, 385–395. 10.1002/tox.22524 [DOI] [PubMed] [Google Scholar]
- Zarba A, Wild CP, Hall AJ, Montesano R, Hudson GJ and Groopman JD, 1992. Aflatoxin M1 in human breast milk from The Gambia, west Africa, quantified by combined monoclonal antibody immunoaffinity chromatography and HPLC. Carcinogenesis, 13, 891–894. [DOI] [PubMed] [Google Scholar]
- Zeng C, Wang R, Li D, Lin XJ, Wei QK, Yuan Y, Wang Q, Chen W and Zhuang SM, 2010. A novel GSK‐3 beta‐C/EBP alpha‐miR‐122‐insulin‐like growth factor 1 receptor regulatory circuitry in human hepatocellular carcinoma. Hepatology, 52, 1702–1712. 10.1002/hep.23875 [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Rossner P Jr, Chen Y, Agrawal M, Wang Q, Wang L, Ahsan H, Yu MW, Lee PH and Santella RM, 2006. Aflatoxin B1 and polycyclic aromatic hydrocarbon adducts, p53 mutations and p16 methylation in liver tissue and plasma of hepatocellular carcinoma patients. International Journal of Cancer, 119, 985–991. 10.1002/ijc.21699 [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Wu HC, Yazici H, Yu MW, Lee PH and Santella RM, 2012. Global hypomethylation in hepatocellular carcinoma and its relationship to aflatoxin B(1) exposure. World Journal of Hepatology, 4, 169–175. 10.4254/wjh.v4.i5.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang X, Wang Y, Wang X, Lu H, Zhang X, Xiao X, Li S, Wang X and Wang S‐L, 2013. Cytochrome P450 2A13 is an efficient enzyme in metabolic activation of aflatoxin G1 in human bronchial epithelial cells. Archives of Toxicology, 87, 1697–1707. [DOI] [PubMed] [Google Scholar]
- Zhang W, He H, Zang M, Wu Q, Zhao H, Lu LL, Ma P, Zheng H, Wang N, Zhang Y, He S, Chen X, Wu Z, Wang X, Cai J, Liu Z, Sun Z, Zeng YX, Qu C and Jiao Y, 2017. Genetic features of aflatoxin‐associated hepatocellular carcinoma. Gastroenterology, 153, 249–262.e242. 10.1053/j.gastro.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Zheng CF, Zeng H, Wang J, Lin H, Feng XB, Chen JA, Qiu ZQ, Luo JH, Xu AW, Wang LQ, Tan Y, Chen S, Jiang P and Shu WQ, 2017. The association between aflatoxin exposure and primary hepatocellular carcinoma risks: a case‐control study in Chongqing. Zhonghua Yu Fang Yi Xue Za Zhi, 51, 539–545. [DOI] [PubMed] [Google Scholar]
- Zhou J, Tang L, Wang J and Wang JS, 2018. Aflatoxin B1 disrupts gut‐microbial metabolisms of short‐chain fatty acids, long‐chain fatty acids, and bile acids in male F344 rats. Toxicological Sciences, 164, 453–464. 10.1093/toxsci/kfy102 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jin Y, Yu H, Shan A, Shen J, Zhou C, Zhao Y, Fang H, Wang X, Wang J, Fu Y, Wang R, Li R and Zhang J, 2019. Resveratrol inhibits aflatoxin B1‐induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrf2 signaling pathway. Toxiconology, 164, 10–15. 10.1016/j.toxicon.2019.03.022 [DOI] [PubMed] [Google Scholar]
- Zhu JQ, Zhang LS, Hu X, Xiao Y, Chen JS, Xu YC, Fremy J and Chu FS, 1987. Correlation of dietary aflatoxin B1 levels with excretion of aflatoxin M1 in human urine. Cancer Research, 47, 1848–1852. [PubMed] [Google Scholar]
- Zivoli R, Gambacorta L, Perrone G and Solfrizzo M, 2014. Effect of almond processing on levels and distribution of aflatoxins in finished products and byproducts. Journal of Agricultural and Food Chemistry, 62, 5707–5715. 10.1021/jf5018548 [DOI] [PubMed] [Google Scholar]
- Zuberi Z, Eeza MNH, Matysik J, Berry JP and Alia A, 2019. NMR‐based metabolic profiles of intact zebrafish embryos exposed to aflatoxin B1 recapitulates hepatotoxicity and supports possible neurotoxicity. Toxins (Basel), 11 10.3390/toxins11050258 [DOI] [PMC free article] [PubMed] [Google Scholar]


