Abstract
Introduction
Adults with Down syndrome (DS) are at high risk for early onset Alzheimer's disease (AD), characterized by a progressive decline in multiple cognitive domains including language, which can impact social interactions, behavior, and quality of life. This cross‐sectional study examined the relationship between language skills and dementia.
Methods
A total of 168 adults with DS (mean age = 51.4 years) received neuropsychological assessments, including Vineland Communication Domain, McCarthy Verbal Fluency, and Boston Naming Test, and were categorized in one of three clinical groups: cognitively stable (CS, 57.8%); mild cognitive impairment (MCI‐DS, 22.6%); and probable/definite dementia (AD‐DS, 19.6%). Logistic regression was used to determine how well language measures predict group status.
Results
Vineland Communication, particularly receptive language, was a significant predictor of MCI‐DS. Semantic verbal fluency was the strongest predictor of AD‐DS.
Discussion
Assessment of language skills can aid in the identification of dementia in adults with DS. Clinically, indications of emerging language problems should warrant further evaluation and monitoring.
Keywords: Alzheimer's disease, Down syndrome, language skills, mild cognitive impairment, semantic verbal fluency
1. BACKGROUND
Down syndrome (DS), caused by triplication of chromosome 21, is the most common genetic cause of intellectual disability (ID), with an estimated incidence of one in 700 live births. 1 Adults with DS are at high risk for early onset Alzheimer's disease (AD), possibly due to overexpression of the gene on chromosome 21 that produces amyloid precursor protein, which likely contributes to the high levels of amyloid beta (Aβ) protein in the brain that are characteristic of AD. 2 , 3 Nearly all DS adults develop the neuropathology associated with AD by the age of 40 years, 4 , 5 and by age 65, the risk of AD‐related dementia has been reported to be 80% or more, with a mean age at diagnosis of 55 years. 3 , 6 However, there is wide variation in age at onset of dementia, and progression of cognitive decline to the preclinical and clinical stages of AD is not well characterized.
The importance of AD for the DS population has grown dramatically with recent increases in life expectancy due to improved health care, advocacy, and services. 7 Where mean life expectancy was 12 years in the 1940s, 8 today it is ≈60 years. 9 , 10 Planning for, treating, and managing AD should now be part of clinical care for every individual with DS.
For adults in the general population, AD is marked by progressive decline in multiple cognitive domains, including memory, language, and executive function. Disease progression is insidious and formal diagnosis is often preceded by a prodromal phase, termed mild cognitive impairment (MCI), in which clinical decline is evident without meeting full criteria for dementia. The established diagnostic guideline for MCI has been cognitive test scores 1.5 standard deviations below the mean in one or more domains. 11 For adults with DS, detection of early AD‐related cognitive changes can be difficult because of preexisting cognitive impairments 12 : The majority of individuals with DS have baseline cognitive abilities below the MCI diagnostic guideline, 13 motivating the definition of “MCI‐DS” for that population, based on evidence of decline in abilities either obtained through direct observations over time or inferred from performance below expectations with reference to the subpopulation of individuals with comparable severity of cognitive impairment. 14 , 15
Language impairment is an inherent part of the progressive cognitive decline leading to AD‐related dementia, which can negatively impact multiple domains of functioning, including social relationships, behavior, and quality of life, while increasing caregiver burden. 16 , 17 In the general population, early AD‐related deficits can be seen in semantic access of information, 18 followed by declines in naming and verbal fluency 19 and in comprehension of spoken and written language. In the DS population, detection of early cognitive change is complicated by deficits that appear in childhood in language and communication, particularly in expressive language and verbal working memory. 20 , 21 , 22 However, verbal confrontation naming tests appear to be sensitive to decline in early stages of AD, whereas verbal fluency measures appear to be sensitive to decline in later stages, 23 , 24 and one study of relatively young (<34 years) subjects not diagnosed with dementia found that poorer semantic verbal fluency was associated with both poorer outcome on a dementia screening measure and increased plasma levels of Aβ42 peptide, a biomarker for AD. 25
The present cross‐sectional analyses examined language skills in a large cohort of adults with DS participating in the Biomarkers of Alzheimer's Disease in Down Syndrome (ADDS) study, a multi‐site consortium established to identify biomarkers associated with risk of AD in older adults with DS as part of a broader Alzheimer's Biomarker Consortium. 26 The goal of these analyses was to characterize language deficits associated with group dementia status and to determine if language measures can be early indicators of MCI‐DS and AD.
RESEARCH IN CONTEXT
Systematic review: A literature review using traditional (eg, PubMed) sources found that language impairment might be associated with prodromal and fully developed dementia related to Alzheimer's disease (AD) in adults with Down syndrome (DS). However, there have been few studies identifying specific language skills that might indicate the onset of AD‐related cognitive decline.
Interpretation: Using a regression model with a group of 168 adults with DS (mean age 51 years), language measures predictive of early and advanced dementia in adults with DS were identified.
Future directions: This article is the initial report of an ongoing longitudinal study that will provide a long‐term profile of language skills during the progression of AD in DS.
2. METHODS
2.1. Participants
Study participants were all qualifying individuals participating in the ADDS longitudinal study with baseline visit between 2016 and 2019. Study inclusion criteria were: (1) age ≥40 years, (2) karyotype of full trisomy 21, (3) estimated preexisting IQ ≥30, and (4) English speaker. Evaluations were conducted at three U.S. sites: Massachusetts General Hospital; New York State Institute for Basic Research in Developmental Disabilities; and University of California, Irvine. All procedures were reviewed and approved by the institutional review boards at each participating institution. Informed consent was obtained from participants or their legally authorized representative along with participant assent.
2.2. Procedures
Participants received a comprehensive evaluation at study baseline that included: (1) a detailed review of medical records; (2) informant interviews focused on functional and vocational abilities, neuropsychiatric status, health status, and life events that might cause significant stress; and (3) direct one‐on‐one tests, commonly used with individuals with intellectual disabilities, that cover the breadth of cognitive and functional abilities expected to be affected by AD. The instruments used in the present analysis are described below.
Following the evaluation, AD‐related clinical status was determined at a Consensus Review Conference that included all senior staff members and those research assistants who had direct contact with the participant. Determination was based on current evaluation results, the preexisting level of cognitive functioning (LOF) as obtained from prior testing or care provider report, and informant concerns. 14 , 15
Clinical status was classified into five categories: (1) cognitively stable (CS), indicating absence of clinically significant impairment; (2) MCI‐DS, indicating mild cognitive impairment and/or functional decline beyond that expected with aging per se, but of insufficient severity to suggest frank dementia; (3) possible dementia, indicating some signs and symptoms of dementia, but with declines over time not judged to be fully convincing; (4) definite dementia, indicating with high confidence the presence of dementia, including substantial decline over time; and (5) status uncertain due to complications, indicating that clinically significant declines were observed but symptoms might be caused by a medical condition or other factor unrelated to a dementing disorder. For the current analyses, the first four categories were condensed into three clinical status groups: CS, MCI‐DS, and AD‐DS, the last group including both the possible dementia and definite dementia categories. Individuals in the uncertain due to complications classification (N = 4) were excluded from the present analyses.
2.3. Language assessment
Language assessment included both direct testing and informant reports from care providers of participants. All measures have been found to be appropriate for assessment of MCI and AD in individuals with DS. 27
2.3.1. Direct measures
The McCarthy Scales of Children's Abilities 28 Verbal Fluency subtest assesses semantic fluency. Participants are given 20 seconds to produce as many unique words as possible in a given category in each of two trials: “things to eat” and “things to wear.” Total score is the number of unique correct words. Repetitions (eg, boat, sailboat) and synonyms (eg, taxi, cab) are scored as additional words.
The Boston Naming Test (BNT) 29 assesses word retrieval and confrontational naming abilities. A modified version of the BNT was administered that consisted of 30 black and white line drawings of objects. Participants were given 20 seconds to name each picture. Total score (range 0 to 30) is the number of correct spontaneous responses.
2.3.2. Informant report measure
The Vineland Adaptive Behavior ScalesThird Edition (Vineland‐3), Comprehensive Interview Form 30 was administered to care providers; from that measure, the Communication domain was used to assess participants’ everyday language skills in three subdomains: Receptive, Expressive, and Written. The present analyses used the Communication domain standard score (SS; mean =100, standard deviation = 15, range 20 to 140) and raw scores for the Receptive (range 0 to 78), Expressive (range 0 to 98), and Written (range 0 to 76) subdomains.
2.4. Statistical analysis
Descriptive statistics, chi‐square analyses, and one‐way between‐group analysis of variance (ANOVA) were used to summarize the sample and its characteristics. Two‐way between‐group analysis of covariance (ANCOVA) was completed with LOF and clinical status as independent variables and the three primary language measures (Vineland, McCarthy, BNT) as dependent variables, with covariates of age, gender, and race. A similar two‐way between‐group ANCOVA was conducted using the three Vineland language subdomains and the three covariates. Post hoc comparisons using Bonferroni correction were performed when multiple comparisons were significant. Using clinical status group as a binary variable (CS vs MCI‐DS; CS vs AD‐DS; MCI‐DS vs AD‐DS), logistic regressions were performed to determine the strongest predictors of clinical status group using the primary language measures as independent variables, with the four covariates, age, gender, race, and LOF, in the model. A similar logistic regression was conducted using the three Vineland language subdomains as independent variables with the four covariates. Receiver‐operating characteristic (ROC) curves were plotted and the area under the curve (AUC) was calculated to determine sensitivity and specificity of language measures by clinical status group, seeking sensitivity rates of 70%, and specificity rates of 70% to 80%. 31 SPSS version 24 and R version 3.6.3 were used for analyses. All reported P values used two‐tailed significance with α (alpha) set at 0.05.
3. RESULTS
3.1. Demographics and clinical characteristics
Demographics and clinical characteristics of the sample are summarized in Table 1. About half of participants were in the CS group; the remainder exhibited signs of AD‐related impairment (MCI‐DS or AD‐DS groups). The AD‐DS group (N = 33) includes 14 individuals classified as possible dementia and 19 classified as definite dementia. Pre‐existing LOF was in the mild range for ≈50% and was severe for only 6% of the sample.
TABLE 1.
Demographics and clinical characteristics of the sample by clinical status group (N = 168)
| CS (N = 97) Mean (± SD) or % | MCI‐DS (N = 38) Mean (± SD) or % | AD‐DS (N = 33) Mean (± SD) or % | Total Sample (N = 168) Mean (± SD) or % | |
|---|---|---|---|---|
| Clinical status group | 57.8% | 22.6% | 19.6% | 100% |
| Age, years | 49.00 (6.59) | 53.63 (6.94) | 55.64 (5.87) | 51.35 (7.09) |
| Age range, years | 40–72 | 44–81 | 44–68 | 40–81 |
| Gender, Male | 56.7% | 68.4% | 45.5% | 57.1% |
| Race/ethnicity | ||||
| White/Caucasian | 83.5% | 89.5% | 87.9% | 85.7% |
| Other | 16.5% | 10.5% | 12.1% | 14.3% |
| Preexisting level of functioning | ||||
| Mild (N = 85) | 54.6% | 44.7% | 45.5% | 50.6% |
| Moderate (N = 73) | 41.2% | 50.0% | 42.2% | 43.4% |
| Severe (N = 10) | 4.1% | 5.3% | 12.1% | 6.0% |
Abbreviations: CS, cognitively stable; MCI‐DS, mild cognitive impairment‐Down syndrome; AD‐DS, Alzheimer's disease‐Down syndrome; SD, standard deviation.
The three clinical status groups were significantly different in mean age (F(2, 165) = 15.66, P < .001). Post hoc analyses using Bonferroni correction found that the mean age of the CS group was significantly (P ≤.001) lower than that of the MCI‐DS and AD‐DS groups, whereas the MCI‐DS and AD‐DS groups did not differ significantly in age (P = .598). Clinical status groups were not significantly different by LOF (χ2(4, N = 168) = 3.92, P = .417), gender (χ2(2, N = 168) = 3.82, P = .148), or race (χ2(2, N = 168) = .95, P = .621).
3.2. Language skills
Figure 1 shows the mean scores for the primary language measures for each clinical status group, broken down by LOF. As indicated, Vineland scores in the severe LOF group were all near or at the instrument's floor (SS = 20).
FIGURE 1.
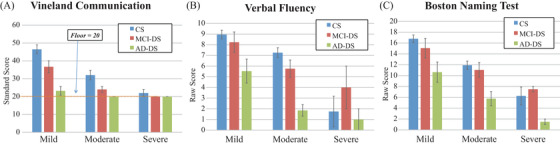
Language scores by clinical status group and level of functioning: (A) Vineland Communication, (B) McCarthy Verbal Fluency, (C) Boston Naming Test
Descriptive statistics are presented in Table 2. Two‐way ANCOVA with age, race, and gender as covariates revealed statistically significant main effects for LOF for Vineland Communication (F(2, 156) = 9.00, P < .001), Verbal Fluency (F(2, 150) = 14.69, P < .001), BNT (F(2, 147) = 16.16, P < .001), and for the Receptive (F(2, 156) = 14.14, P < .001), Expressive (F(2, 156) = 26.28, P < .001), and Written (F(2, 156) = 32.12, P < .001) subdomains. For clinical status group, statistically significant main effects were found for Vineland Communication (F(2, 156) = 4.69, P = .011), Verbal Fluency (F(2, 150) = 4.52, P = .012), BNT (F(2, 147) = 3.89, P = .023), and for the Receptive (F(2, 156) = 54.44, P < .001), Expressive (F(2, 156) = 19.99, P < .001), and Written (F(2, 156) = 7.82, P = .001) subdomains. There was no statistically significant interaction between LOF and clinical status group for Vineland Communication (F(4, 156) = 1.46, P = .220), Verbal Fluency (F(4, 150) = 1.07, P = .380), BNT (F(4, 147) = 0.10, P = .980), or the Expressive F(4, 156) = 2.35, P = .057) or Written subdomains (F(4, 156) = 0.051, P = .995). However, a statistically significant interaction was seen between LOF and clinical status group for the Receptive subdomain (F(4, 156) = 2.89, P = .024).
TABLE 2.
Mean language test scores and covariate adjusted means by level of functioning (LOF) and clinical status group
| LOF | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||||||
| N | M (SE) | Adjusted M (SE) | N | M (SE) | Adjusted M (SE) | N | M (SE) | Adjusted M (SE) | |
| Vineland communication SS | 85 | 40.44 (1.98) | 35.29 (1.88) | 73 | 27.63 (1.62) | 25.30 (1.90) | 10 | 20.80(0.80) | 20.21 (4.95) |
| Expressive RS | 85 | 88.88 (1.44) | 84.64 (1.97) | 73 | 74.62 (2.58) | 70.61 (2.00) | 10 | 45.60 (8.90) | 49.76 (5.19) |
| Receptive RS | 85 | 68.24 (1.16) | 64.24 (1.20) | 73 | 61.05 (1.86) | 56.84 (1.22) | 10 | 47.30 (6.19) | 50.57 (3.16) |
| Written RS | 85 | 39.28 (1.40) | 36.79 (1.44) | 73 | 25.68 (1.43) | 24.19 (1.46) | 10 | 8.90 (2.00) | 10.72 (3.24) |
| Verbal Fluency RS | 85 | 8.21 (0.40) | 7.61 (0.41) | 69 | 5.87 (0.42) | 5.03 (0.43) | 8 | 2.13 (0.90) | 2.37 (1.22) |
| BNT RS | 83 | 15.40 (0.69) | 14.43 (0.68) | 68 | 10.60 (0.65) | 9.83 (0.72) | 8 | 5.38 (1.16) | 5.86 (1.98) |
| Clinical Status | ||||||||
|---|---|---|---|---|---|---|---|---|
| CS | MCI‐DS | AD‐DS | ||||||
| N | M (SE) | Adjusted M (SE) | N | M (SE) | Adjusted M (SE) | N | M (SE) | Adjusted M (SE) |
| 97 | 39.53 (1.92) | 33.44 (2.64) | 38 | 29.47 (1.99) | 26.42 (3.91) | 33 | 21.45 (1.10) | 20.94 (3.13) |
| 97 | 85.86 (1.85) | 65.64 (1.69) | 38 | 85.03 (1.89) | 65.75 (2.5) | 33 | 57.55 (4.31) | 40.26 (2.00) |
| 97 | 70.11 (0.81) | 72.67 (2.77) | 38 | 65.68 (1.36) | 81.17 (4.10) | 33 | 43.42 (3.06) | 51.17 (3.28) |
| 97 | 35.61 (1.40) | 28.09 (2.02) | 38 | 31.55 (2.12) | 27.29 (2.99) | 33 | 19.70 (2.59) | 16.32 (2.39) |
| 96 | 7.97 (0.34) | 5.95 (0.58) | 36 | 6.83 (0.63) | 6.08 (0.86) | 30 | 3.63 (0.70) | 2.98 (0.89) |
| 95 | 14.34 (0.58) | 11.48 (0.94) | 36 | 12.75 (1.12) | 11.67 (1.40) | 28 | 7.89 (1.20) | 6.97 (1.45) |
Abbreviations: CS, cognitively stable; MCI‐DS, mild cognitive impairment‐Down syndrome; AD‐DS, Alzheimer's disease‐Down syndrome; SS, standard score; RS, raw score; BNT, Boston Naming Test.
Multiple comparison results are shown in Table 3. Post hoc analysis for the three primary language measures revealed that Vineland Communication, Verbal Fluency, and BNT scores were significantly lower for the moderate and severe LOF groups than for the mild group. By clinical status group, Vineland Communication and BNT scores were significantly lower for AD‐DS than the CS group, and Verbal Fluency scores were significantly lower for AD‐DS than both the CS and MCI‐DS groups.
TABLE 3.
Multiple comparisons and mean differences in measure by clinical status and level of functioning, controlling for age, gender, and race
| Measure | Clinical status group comparison | M Diff. | Std. Error | P | Bonferroni adjusted 95% CI |
|---|---|---|---|---|---|
| Vineland communication SS | CS versus MCI‐DS | 7.02 | 4.71 | .414 | −4.37, 18.42 |
| CS versus AD‐DS | 12.51** | 4.11 | .008 | 2.55, 22.46 | |
| MCI‐DS versus AD‐DS | 5.48 | 4.94 | .807 | −6.48, 17.44 | |
| Expressive RS | CS versus MCI‐DS | −8.51 | 4.94 | .262 | −20.47, 3.46 |
| CS versus AD‐DS | 21.50*** | 4.32 | <.001 | 11.05, 31.95 | |
| MCI‐DS versus AD‐DS | 30.00*** | 5.19 | <.001 | 17.45, 42.55 | |
| Receptive RS | CS versus MCI‐DS | −0.11 | 3.01 | 1.000 | −7.40, 7.18 |
| CS versus AD‐DS | 25.37*** | 2.63 | <.001 | 19.01, 31.74 | |
| MCI‐DS versus AD‐DS | 25.49*** | 3.16 | <.001 | 17.84, 33.14 | |
| Written RS | CS versus MCI‐DS | 0.80 | 3.60 | 1.000 | −7.92, 9.52 |
| CS versus AD‐DS | 11.76** | 3.15 | .001 | 4.15, 19.38 | |
| MCI‐DS versus AD‐DS | 10.96* | 3.78 | .013 | 1.82, 20.11 | |
| Verbal fluency RS | CS versus MCI‐DS | −0.13 | 1.04 | 1.000 | −2.64, 2.39 |
| CS versus AD‐DS | 2.98* | 1.06 | .017 | 0.40, 5.55 | |
| MCI‐DS versus AD‐DS | 3.10* | 1.22 | .036 | 0.15, 6.05 | |
| BNT RS | CS versus MCI‐DS | −0.19 | 1.69 | 1.000 | −4.27, 3.89 |
| CS versus AD‐DS | 4.51* | 1.74 | .031 | 0.31, 8.71 | |
| MCI‐DS versus AD‐DS | 4.70 | 1.99 | .058 | −0.12, 9.51 | |
| LOF group comparison | |||||
| Vineland communication SS | Mild versus moderate | 9.98** | 2.66 | .001 | 3.54, 16.43 |
| Mild versus severe | 15.07* | 5.25 | .014 | 2.36, 27.78 | |
| Moderate versus severe | 5.09 | 5.25 | 1.000 | −7.62, 17.80 | |
| Expressive RS | Mild versus moderate | 14.03*** | 2.79 | <.001 | 7.27, 20.80 |
| Mild versus severe | 34.88*** | 5.51 | <.001 | 21.54, 48.22 | |
| Moderate versus severe | 20.84** | 5.51 | .001 | 7.50, 34.19 | |
| Receptive RS | Mild versus moderate | 7.39*** | 1.70 | <.001 | 3.27, 11.52 |
| Mild versus severe | 13.67*** | 3.36 | <.001 | 5.54, 21.80 | |
| Moderate versus severe | 6.27 | 3.36 | .192 | −1.86, 14.40 | |
| Written RS | Mild versus moderate | 12.61*** | 2.04 | <.001 | 7.68, 17.54 |
| Mild versus severe | 26.08*** | 4.02 | <.001 | 16.36, 35.80 | |
| Moderate versus severe | 13.47** | 4.02 | .003 | 3.76, 23.19 | |
| Verbal fluency RS | Mild versus moderate | 2.57*** | 0.59 | <.001 | 1.14, 4.01 |
| Mild versus severe | 5.24*** | 1.27 | <.001 | 2.16, 8.32 | |
| Moderate versus severe | 2.66 | 1.28 | .118 | −0.44, 5.77 | |
| BNT RS | Mild versus moderate | 4.60*** | 0.98 | <.001 | 2.23, 6.97 |
| Mild versus severe | 8.57*** | 2.07 | <.001 | 3.57, 13.58 | |
| Moderate versus severe | 3.97 | 2.08 | .176 | −1.08, 9.02 |
Note: * P < .05; ** P < .01; *** P < .001
Abbreviations: CS, cognitively stable; MCI‐DS, mild cognitive impairment‐Down syndrome; AD‐DS, Alzheimer's disease‐Down syndrome; SS, standard score; RS, raw score; BNT, Boston Naming Test.
Post hoc analyses for the Vineland Communication subdomains revealed that the severe LOF group had significantly lower Expressive, Receptive, and Written scores than the mild group, and significantly lower Expressive and Written scores than the moderate group. The moderate LOF group had significantly lower Expressive, Receptive, and Written scores than the mild group. By clinical status group, Expressive, Receptive, and Written scores were significantly lower for the AD‐DS group than both the CS and the MCI‐DS groups.
Logistic regression data are shown in Table 4. A logistic regression model for the CS and MCI‐DS groups with the three primary language measures as predictors and age, gender, race, and LOF as covariates was able to distinguish between groups with 77.0% accuracy, with the only significant predictors being Vineland Communication and age. If the Vineland Communication score decreased by 1 point, holding other variables constant, the likelihood of MCI‐DS group status was 1.04 times greater. A CS and MCI‐DS logistic regression model with the three subdomain language measures as predictors and the same covariates was able to distinguish between groups with 76.3% accuracy, with Receptive score and age the only significant predictors. If the Receptive score decreased by 1 point, holding other variables constant, the likelihood of MCI‐DS group status was 1.11 times greater.
TABLE 4.
Logistic regression model parameters
| β | S.E. | Wald | P | Exp(β) | 95.0% CI exp(β) lower upper | |
|---|---|---|---|---|---|---|
| CS versus MCI‐DS | – | – | – | – | – | – |
| Primary language measures | – | – | – | – | – | – |
| Age | 0.12 | 0.04 | 3.24 | .001 | 1.13 | (1.05, 1.22) |
| Level of functioning | ||||||
| Moderate | −0.270 | 0.49 | −0.55 | .582 | 0.76 | (0.29, 2.00) |
| Severe | −1.70 | 1.18 | −1.44 | .150 | 0.18 | (0.02, 1.85) |
| Race | 0.52 | 0.75 | 0.69 | .489 | 1.68 | (0.39, 7.37) |
| Gender | −0.36 | 0.46 | −0.78 | .433 | 0.70 | (0.28, 1.72) |
| Vineland communication SS | −0.05 | 0.02 | −2.68 | .007 | 0.96 | (0.93, 0.99) |
| Verbal fluency RS | −0.08 | 0.07 | −1.13 | .260 | 0.92 | (0.80, 1.06) |
| BNT RS | 0.05 | 0.05 | 0.98 | .329 | 1.05 | (0.95, 1.15) |
| Vineland subdomains | – | – | – | – | – | – |
| Age | 0.09 | 0.04 | 2.66 | .008 | 1.10 | (1.03, 1.18) |
| Level of functioning | ||||||
| 0.31 | 0.50 | 0.63 | .526 | 1.37 | (0.52, 3.62) | |
| −0.25 | 1.19 | −0.21 | .832 | 0.78 | (0.08, 8.03) | |
| Race | 0.32 | 0.67 | 0.47 | .638 | 1.37 | (0.37, 5.08) |
| Gender | −0.46 | 0.45 | −1.01 | .311 | 0.63 | (0.26, 1.53) |
| Receptive RS | −0.100 | 0.04 | −2.81 | .005 | 0.90 | (0.84, 0.97) |
| Expressive RS | 0.04 | 0.02 | 1.74 | .081 | 1.04 | (1.00, 1.08) |
| Written RS | 0.001 | 0.02 | 0.04 | .970 | 1.00 | (0.96, 1.05) |
| CS versus AD‐DS | – | – | – | – | – | – |
| Primary language measures | – | – | – | – | – | – |
| Age | 0.15 | 0.06 | 2.61 | .009 | 1.17 | (1.04, 1.31) |
| Level of functioning | ||||||
| Moderate | −1.85 | 0.85 | −2.18 | .030 | 0.16 | (0.03, 0.83) |
| Severe | −4.24 | 1.46 | −2.90 | .004 | 0.01 | (8×10−4, 0.25) |
| Race | −1.71 | 1.21 | −1.41 | .158 | 0.18 | (0.02, 1.94) |
| Gender | 0.92 | 0.71 | 1.29 | .196 | 2.51 | (0.62, 10.12) |
| Vineland communication SS | −0.11 | 0.05 | −2.51 | .012 | 0.89 | (0.82, 0.98) |
| Verbal fluency RS | −0.35 | 0.12 | −3.03 | .002 | 0.71 | (0.56, 0.88) |
| BNT RS | −0.05 | 0.08 | −0.62 | .537 | 0.96 | (0.82, 1.11) |
| Vineland subdomains | – | – | – | – | – | – |
| Age | 0.17 | 0.08 | 2.12 | .034 | 1.18 | (1.01, 1.38) |
| Level of functioning | ||||||
| Moderate | −2.69 | 1.42 | −1.90 | .058 | 0.07 | (4×10−4, 1.10) |
| Severe | −5.68 | 2.45 | −2.32 | .021 | 0.003 | (2.8×10−5, 0.42) |
| Race | −3.90 | 1.90 | −2.06 | .040 | 0.02 | (4.8×10−4, 0.83) |
| Gender | 1.38 | 0.96 | 1.45 | .148 | 3.98 | (0.61, 25.89) |
| Receptive RS | −0.37 | 0.09 | −4.01 | <.001 | 0.69 | (0.58, 0.83) |
| Expressive RS | −0.01 | 0.04 | −0.13 | .896 | 1.00 | (0.92, 1.07) |
| Written RS | 0.07 | 0.06 | 1.18 | .240 | 1.07 | (0.96, 1.19) |
| MCI vs. AD‐DS | – | – | – | – | – | – |
| Primary language measures | – | – | – | – | – | – |
| Age | 0.03 | 0.05 | 0.50 | .621 | 1.03 | (0.93, 1.13) |
| Level of functioning | ||||||
| Moderate | −1.40 | 0.78 | −1.80 | .073 | 0.25 | (0.05, 1.14) |
| Severe | −1.81 | 1.32 | −1.37 | .171 | 0.16 | (0.01, 2.19) |
| Race | 0.23 | 1.44 | 0.16 | .873 | 1.26 | (0.08, 20.98) |
| Gender | 1.06 | 0.68 | 1.57 | .117 | 2.90 | (0.77, 10.97) |
| Vineland communication SS | −0.11 | 0.04 | −2.48 | .013 | 0.90 | (0.82, 0.98) |
| Verbal fluency RS | −0.27 | 0.12 | −2.20 | .028 | 0.76 | (0.60, 0.97) |
| BNT RS | 0.02 | 0.07 | 0.30 | .763 | 1.02 | (0.89, 1.17) |
| Vineland subdomains | – | – | – | – | – | – |
| Age | −0.01 | 0.07 | −0.07 | .944 | 1.00 | (0.87, 1.14) |
| Level of functioning | ||||||
| Moderate | −1.85 | 1.06 | −1.75 | .080 | 0.16 | (0.02, 1.25) |
| Severe | −2.28 | 2.57 | −0.89 | .375 | 0.10 | (6×10‐4, 1.57) |
| Race | −1.26 | 1.59 | −0.79 | .428 | 0.28 | (0.01, 6.41) |
| Gender | 1.34 | 0.92 | 1.46 | .145 | 3.81 | (0.63, 23.04) |
| Receptive RS | −0.14 | 0.06 | −2.45 | .014 | 0.87 | (0.78, 0.97) |
| Expressive RS | −0.15 | 0.06 | −2.49 | .013 | 0.86 | (0.77, 0.97) |
| Written RS | 0.10 | 0.06 | 1.61 | .109 | 1.11 | (0.98, 1.25) |
Abbreviations: CS, cognitively stable; MCI‐DS, mild cognitive impairment‐Down syndrome; AD‐DS, Alzheimer's disease‐Down syndrome; SS, standard score; RS, raw score; BNT, Boston Naming Test.
A logistic regression model for the CS and AD‐DS groups with the three primary language measures as predictors and age, gender, race, and LOF as covariates was able to distinguish between groups with 87.7% accuracy, with the only significant predictors being Verbal Fluency, Vineland Communication, LOF, and age. Holding other variables constant, if the Vineland Communication score decreased by 1 point, the likelihood of AD‐DS group status was 1.12 times greater; if the Verbal Fluency score decreased by 1 point, the likelihood of AD‐DS group status was 1.40 times greater. A CS and AD‐DS logistic regression model with the three subdomain language measures and the same covariates was able to distinguish between groups with 93.8% accuracy, with Receptive score and the covariates LOF, age, and race the only significant predictors. If the Receptive score decreased by 1 point and holding other variables constant, the likelihood of AD‐DS group status was 1.44 times greater.
A logistic regression model for the MCI‐DS and AD‐DS groups with the three primary language measures as predictors and age, gender, race, and LOF as covariates was able to distinguish between groups with 80.3% accuracy, with Verbal Fluency score and the Vineland Communication the only significant predictors. Holding other variables constant, if the Verbal Fluency score decreased by 1 point, the likelihood of AD‐DS group status was 1.31 times greater; if the Vineland Communication score decreased by 1 point, the likelihood of AD‐DS group status was 1.12 times greater. A MCI‐DS and AD‐DS logistic regression model with the three subdomain language measures and the same covariates was able to distinguish between groups with 85.9% accuracy, with Expressive score and Receptive score the only significant predictors. Holding other variables the same, if the Receptive score decreased by 1 point, the likelihood of AD‐DS group status was 1.14 times greater; if the Expressive score decreased by 1 point, the likelihood of AD‐DS group status was 1.16 times greater.
As shown in Figure 2A and B, ROC analyses comparing CS versus AD‐DS found that a raw score of 5.5 or lower on semantic verbal fluency provided 80% sensitivity and a 21% false‐positive rate. A cut score of 23 on the Vineland Communication domain provided 94% sensitivity with a 31% false‐positive rate, although this cut score is near the floor of the scale.
FIGURE 2.
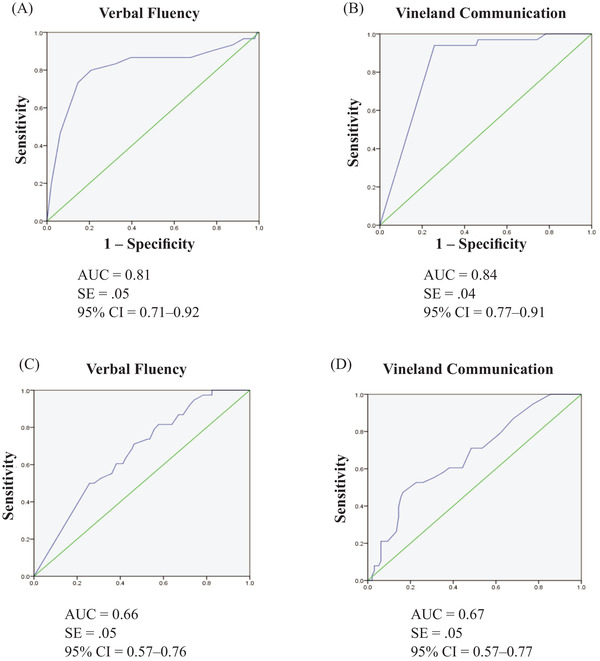
ROC curves for (A) McCarthy Verbal Fluency raw scores and (B) Vineland Communication domain standard scores for cognitively stable (CS) versus Alzheimer's Disease‐Down Syndrome (AD‐DS) clinical status groups. (C) Vineland Communication standard scores and (D) Vineland Receptive Language subdomain raw scores for CS versus mild cognitive impairment‐Down syndrome (MCI‐DS) clinical status groups
As shown in Figure 2C and D, ROC analyses comparing CS versus MCI‐DS indicated that a Vineland Communication SS of 35.0 or lower provided 71% sensitivity but a 46% false‐positive rate. A raw score of 72.5 on the Vineland Receptive subdomain provided 71% sensitivity with a 49% false‐positive rate. Given the high false‐positive rates, no cut scores distinguished AD‐DS from MCI‐DS or MCI‐DS from CS with sensitivity/specificity sufficiently high to inform diagnosis in clinical practice.
4. DISCUSSION
The present study of 168 adults with DS with mean age of 51 years (slightly younger than the mean age of diagnosis of AD‐related dementia 3 , 6 ) found evidence of age‐related decline in nearly half, with 22.6% judged to have mild cognitive impairment (MCI‐DS) and 19.6% to have either possible or definite AD‐related dementia (AD‐DS). Clinical status group was not significantly related to preexisting LOF, indicating that the consensus classification system has clinical promise for use with adults with DS. However, test scores in the most impaired groups were close to the floor of the Vineland Communication domain, preventing meaningful discrimination between clinical status groups for those with severe LOF; and even when raw scores were used (for the Vineland subdomains and the direct measures), low scores and small numbers in the most impaired groups resulted in minimal differences between clinical categories. The mean age of the clinical status groups increased consistently with increasing impairment, with the CS group significantly younger than the more impaired groups, consistent with a model of age‐related vulnerability to AD‐related clinical progression.
All of the language measures were uniformly significantly lower in the more impaired clinical status groups, indicating that decline in language is associated with severity of impairment. This is not surprising, given the importance of language in cognitive functioning, but it was not an assured result because of the other cognitive factors, including memory and executive functioning, that were considered in the Consensus Review committee's determination of clinical status.
Logistic regression identified several language skills that were significant predictors of clinical status, and certain tests were found to have cut scores that could be clinically useful for identification of AD‐related dementia, but cut scores differentiating CS and MCI‐DS had an unacceptably high false‐positive rate.
In early stage AD (CS to MCI‐DS), only scores on the Vineland Communication domain and its Receptive subdomain differed significantly between the two clinical groups. In logistic models of CS versus MCI‐DS status, Vineland Communication was the only one of the three primary language measures that significantly predicted MCI‐DS, and further analysis within the Vineland Communication domain showed the Receptive language subdomain to be the only significant predictor. However, ROC curves indicated that both measures had a high false‐positive rate. Nonetheless, low scores on those language measures should be cause for concern and in a clinical context should warrant further evaluation.
Looking at late stage AD (CS to AD‐DS), all language measures differed significantly between the two clinical groups. In logistic models of CS versus AD‐DS status, Verbal Fluency and Vineland Communication were the significant primary language measure predictors, and within the Vineland Communication domain, Receptive language was the only significant subdomain predictor of AD‐DS. ROC curve analysis showed that a Verbal Fluency cut score ≤5.5 was an indication of AD‐DS, and a Vineland Communication cut score of 23 was also sensitive and specific for AD‐DS status, although that score is near the test floor (SS = 20).
The observed decline in Verbal Fluency with increasing age and clinical status is consistent with and similar in magnitude to observations of other similar studies. 25 , 32 Receptive language and the Vineland Communication scale have not been as well studied as expressive language, but there have been reports of a similar disproportionate impairment in verbal comprehension with age in non‐demented DS individuals up to 66 years of age. 24
4.1. Limitations
Several limitations need to be considered when interpreting the present results. Foremost is the cross‐sectional nature of the study, with predictive relationships inferred through a regression model. Ongoing research should be able to provide a longitudinal follow‐up to this report as the ADDS study proceeds.
Limitations in test sensitivity are a concern with low‐functioning individuals. All of the language measures used have been recommended for use for individuals with DS, 27 but some groups scored at or near the floor on the Vineland Communication domain standard scores. Raw scores were used for the Vineland Communication subdomains, where floor effects were not observed, but overall, our findings suggest that these measures may be most effective for tracking AD progression in adults with less‐severe DS phenotypes.
There is a possibility of selection bias in the recruiting process: The present study, conducted by prominent medical institutions, might disproportionately attract participants with concerns about dementia or other health issues. However, study participants appear to be representative of the greater DS population in terms of LOF and clinical characteristics, and the relative distribution of clinical status groups was comparable to that reported elsewhere for a group of similar age distribution. 10 , 32 Race/ethnicity of participants was predominately White/Caucasian, but not greatly different from that of the overall DS population of comparable age in the United States. 33
4.2. Clinical implications
The present study found that assessment of language skills can aid early identification of cognitive decline in adults with DS, with an informant report of communication skills (Vineland) best identifying early stages (CS to MCI‐DS), and both communication skills and a direct measure of verbal fluency (McCarthy) best identifying later stages (CS to AD‐DS). Although confrontational naming performance (BNT) significantly declined with increasing level of dementia, it did not prove to be a significant predictor of clinical status. In a clinical setting, communication problems relative to preexisting functioning that are reported in everyday life, particularly those in receptive language, should raise concerns of cognitive decline and indicate referral for further evaluation, monitoring, and consideration for increased treatment and interventions.
FUNDING
Data collection and sharing for this project were supported by The Alzheimer's Disease Biomarker Consortium on Down Syndrome (ABC‐DS) funded by the National Institute on Aging (NIA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1 AG051406 and UO1 AG051412). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
The authors thank the adults with Down syndrome volunteering as participants in this study for their invaluable contributions to this work, along with their care providers and families. Finally, the authors thank the staff contributing their many hours of support to this work, including Courtney Jordan, Nusrat Jahan, Eric Doran, Deborah Pang, Tracey Listwan, Cynthia Kovacs, and Haley Duncanson, as well as the other investigators of the ADDS study group.
Pulsifer MB, Evans CL, Hom C, et al. Language skills as a predictor of cognitive decline in adults with Down syndrome. Alzheimer's Dement. 2020;12:e12080 10.1002/dad2.12080
REFERENCES
- 1. Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated national birth prevalence estimates for selected birth defects in the United States, 2004‐2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008‐1016. [DOI] [PubMed] [Google Scholar]
- 2. Robakis NK, Wisniewski HM, Jenkins EC, et al. Chromosome 21q21 sublocalisation of gene encoding beta‐amyloid peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet. 1987;1:384‐385. [DOI] [PubMed] [Google Scholar]
- 3. Sinai A, Mokrysz C, Bernal J, et al. Predictors of age of diagnosis and survival of Alzheimer's disease in Down syndrome. J Alzheimers Dis. 2018;61(2):717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17:278‐282. [DOI] [PubMed] [Google Scholar]
- 5. Mann DMA, Esiri M. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J Ann Neurol Sci. 1989;89:169‐179. [DOI] [PubMed] [Google Scholar]
- 6. McCarron M, McCallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20‐year longitudinal follow‐up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61:843‐852. [DOI] [PubMed] [Google Scholar]
- 7. Yang Q, Rasmussen SA, Friedman J. Mortality associated with Down's syndrome in the USA from 1983‐1997: a Population‐Based Study. Lancet. 2002;359:1019‐1025. [DOI] [PubMed] [Google Scholar]
- 8. Penrose L. The incidence of mongolism in the general population. J Ment Sci. 1949;95:683‐688. [DOI] [PubMed] [Google Scholar]
- 9. Torr J, Strydom A, Patti P, Jokinen N. Aging in Down syndrome: morbidity and mortality. J Policy Pract Intell Disabil. 2010;7:70‐81. [Google Scholar]
- 10. Carr J, Collins S. 50 Years with Down syndrome: a Longitudinal Study. J Appl Res Intellect Disabil. 2018;31:743‐750. [DOI] [PubMed] [Google Scholar]
- 11. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:279‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ball SL, Holland AJ, Hon J, Huppert FA, Treppner P, Watson PC. Personality and behaviour changes mark the early stages of Alzheimer's disease in adults with down syndrome: findings from a prospective population‐based study. Int J Geriat Psychiatry. 2006;21:661‐673. [DOI] [PubMed] [Google Scholar]
- 13. Antonarakis SE, Skotko BG, Rafii MS, et al. Down Syndrome. Nat Rev Dis Primers. 2020;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krinsky‐McHale SJ, Silverman W. Dementia and mild cognitive impairment in adults with intellectual disability: issues of diagnosis. Dev Disabil Res Rev. 2013;18:31‐42. [DOI] [PubMed] [Google Scholar]
- 15. Silverman W, Schupf N, Zigman W, et al. Dementia in adults with mental retardation: assessment at a single point in time. Am J Ment Retard. 2004;109:111‐125. [DOI] [PubMed] [Google Scholar]
- 16. Ferris SH, Farlow M. Language impairment in Alzheimer's disease and benefits of acetylcholinesterase inhibitors. Clin Interv Aging. 2013;8:1007‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kempler D. Language changes in dementia of the Alzheimer type Dementia and Communication. Philadelphia: B.C. Decker; 1991. [Google Scholar]
- 18. Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492‐498. [DOI] [PubMed] [Google Scholar]
- 19. Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer's type: a meta‐analysis. Neuropsychologia. 2004;42:1212‐1222. [DOI] [PubMed] [Google Scholar]
- 20. Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with Down's syndrome. Lancet Neurol. 2010;9:623‐633. [DOI] [PubMed] [Google Scholar]
- 21. Grieco JA, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet. 2015;169:135‐149. [DOI] [PubMed] [Google Scholar]
- 22. Silverman W. Down syndrome: cognitive phenotype. Ment Retard Dev Disabil Res Rev. 2007;13:228‐236. [DOI] [PubMed] [Google Scholar]
- 23. Firth NC, Startin CM, Hithersay R, et al. Aging related cognitive changes associated with Alzheimer's disease in Down syndrome. Ann Clin Transl Neurol. 2018;5:741‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghezzo A, Salvioli S, Solimando MC, et al. Age‐related changes in adaptive and neuropsychological features in persons with Down Syndrome. PLoS One. 2014;9:e113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Del Hoyo L, Xicota L, Sánchez‐Benavides G, et al. Semantic verbal fluency pattern, dementia rating scores and adaptive behavior correlate with plasma Aβ42 concentrations in Down syndrome young adults. Front Behav Neurosci. 2015;9:301‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Handen BL. The search for biomarkers of Alzheimer's disease in Down syndrome. Am J Intellect Dev Disabil. 2020;125:97‐99. [DOI] [PubMed] [Google Scholar]
- 27. Esbensen AJ, Hooper SR, Fidler D, Hartley SL, Edgin J, d'Ardhuy XL, et al. Outcome measures for clinical trials in down syndrome. Am J Intellect Dev Disabil. 2017;122:247‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCarthy D. The McCarthy Scales of Children's Abilities. New York: Psychological Corp; 1972. [Google Scholar]
- 29. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 30. Sparrow SS, Saulnier CA, Cicchetti DC. Vineland Adaptive Behavior Scales. 3rd ed. Bloomington, MN: NCS Pearson; 2016. [Google Scholar]
- 31. Goldstein G, Beers SR, Hersen M. Comprehensive Handbook of Psychological Assessment. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 32. Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer's disease in Down syndrome. D Alzheimers Dement. 2019;15:245‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Graf G, Buckley F, Skotko B. Examination of the number of people with Down syndrome in the United States. Genet Med. 2017;19:439‐447. [DOI] [PubMed] [Google Scholar]


