Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of propyl gallate as feed additive for all animal species. Propyl gallate is neither genotoxic nor carcinogenic. Propyl gallate a is safe for veal calves, cattle for fattening, dairy cows, sheep, goats, sows, horses and salmonids at the proposed maximum use level of 40 mg/kg and for ornamental fish at the proposed maximum use level of 100 mg/kg. The following concentrations (mg/kg complete feed) are considered safe for the other target species: 15 for chickens for fattening; 20 for turkeys for fattening, laying hens and rabbits; 27 for piglets and pigs for fattening and 71 for dogs. The Panel cannot conclude on a safe level for cats. The exposure of the consumer to propyl gallate and its metabolites cannot be estimated owing to the absence of reliable data on residues of propyl gallate and its metabolites in edible tissues and products. Therefore, the FEEDAP Panel is not in the position to conclude on the safety for the consumer of propyl gallate, when used as a feed additive for all food‐producing animal species. Propyl gallate is irritant to skin and eyes and a dermal sensitiser. Exposure via inhalation is possible and it is considered a hazard. The use of the additive in animal nutrition does not pose a risk for the environment. The FEEDAP Panel concludes that propyl gallate has the potential to act as an antioxidant in feedingstuffs. The Panel did not see a reason for the use of propyl gallate as an antioxidant in water for drinking.
Keywords: Propyl gallate, technological additive, antioxidants, safety, efficacy, all animal species
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7 and Article 10(2) of that Regulation also specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, at the latest one year before the expiry date of the authorisation given pursuant to Directive 70/524/EEC for additives with a limited authorisation period, and within a maximum of seven years after the entry into force of this Regulation for additives authorised without a time limit or pursuant to Directive 82/471/EEC.
The European Commission received a request from FEFANA asbl2 for re‐evaluation of the product propyl gallate, when used as a feed additive for all animal species (category: technological additives; functional group: antioxidants).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive) and 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 09 August 2011.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product propyl gallate, when used under the proposed conditions of use (see Section 3.1.3).
1.2. Additional information
Propyl gallate (E310) is included in the European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003. It is currently authorised in application of Article 9t (b) of Council Directive 70/524/EEC3 concerning additives in feedingstuffs (2004/C 50/01) for use in all animal species as a technological additive and foreseen for re‐evaluation with a maximum content of 100 mg/kg complete feedingstuffs alone or together with E 311 (octyl gallate) and/or E 312 (dodecyl gallate). The applicant has asked for the re‐evaluation of the use of propyl gallate as an antioxidant in feed for all animal species and categories and for the authorisation for its use in water for drinking.
Propyl gallate is authorised for use in food as N antioxidant (Directive 95/2/EC)4 up to a maximum level of 400 mg/kg.
The Scientific Committee on Food (SCF, 1989) has established a group acceptable daily intake (ADI) for propyl, octyl and dodecyl gallate of 0–0.2 mg/kg body weight (bw) per day. The Joint FAO/WHO Expert Committee on Food Additives (JECFA, 1997) has set an ADI for propyl gallate of 1.4 mg/kg bw and day based on a no observed adverse effect level (NOAEL) of 1,910 mg/kg feed (equivalent to 135 mg/kg bw per day) derived from a 90‐day toxicity study in rats. The EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel) has established an ADI of 0.5 mg/kg bw per day for propyl gallate (EFSA ANS Panel, 2014) based on the same NOAEL and applying an uncertainty factor (UF) of 300 for extrapolation from subchronic to chronic data and due to the limitations in the reproductive toxicity database.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier5 in support of the authorisation request for the use of product as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the active substance in animal feed. The Executive Summary of the EURL report can be found in Annex A.6
In addition, the EURL also evaluated the methods of analysis for residues of propyl gallate, gallic acid and 4‐O‐methyl gallic acid in edible tissues and products of animal origin (see Section 3.2.4.1).
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of propyl gallate is in line with the principles laid down in Regulation (EC) No 429/20087 and the relevant guidance documents: Guidance on technological additives (EFSA FEEDAP Panel, 2012a,b), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017a,b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel et al., 2017a,b), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The additive under assessment is propyl gallate, intended to be used as a technological additive (functional group: antioxidants) in feedingstuffs and water for drinking for all animal species.
3.1. Characterisation
3.1.1. Characterisation of the additive
Propyl gallate is produced by the esterification of gallic acid with n‐propanol. Gallic acid (1,3,5‐trihydroxy‐benzoic acid n‐propyl ester) is produced by alkaline and acid hydrolysis of tannins (gallotannin, tannic acid, gallotannic acid) from gallnuts. The esterification of gallic acid to propyl gallate is activated by the presence of a strong acid (e.g. sulfuric acid, hydrochloric acid) in anhydrous form. The water produced and the unreacted n‐propanol are removed via azeotropic distillation with benzene. The product is isolated, decolorised and purified several times by crystallisation. The final product is dried, sieved and packed.
Propyl gallate (International Union of Pure and Applied Chemistry (IUPAC) name: n‐propyl 3,4,5‐trihydroxybenzoate, Chemical Abstracts Service (CAS) number 121‐79‐9, the European Inventory of Existing Commercial Chemical Substances (EINECS) number 204‐498‐2, molecular weight 212.20 g/mol) has chemical formula C10H12O5. The structural formula is shown in Figure 1. Propyl gallate solubility in water is 3.5 g/L, in alcohol 103 g/100 mL, in ether 83 g/100 mL, in lard 1.14 g/100 g (45°C) and in cottonseed oil 1.23 g/100 g (30°C).
Figure 1.
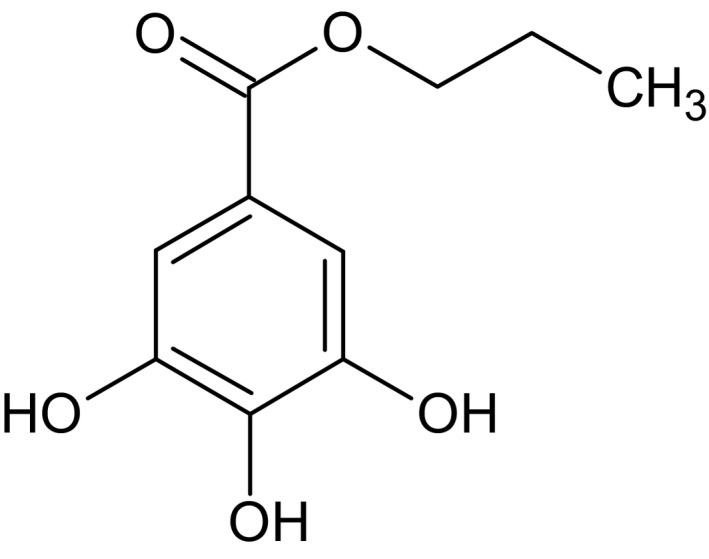
Structural formula of propyl gallate
The additive is a crystalline powder and is specified to contain ≥ 97.0% propyl gallate. The analysis of seven batches of the additive from three producers showed concentrations of propyl gallate ≥ 98.6 %.8 The impurities analysed (sulfated ash, gallic acid, chlorinated organic compounds, lead, mercury and arsenic)9 in minimum three batches of the additive (at least one from each producer) showed concentrations in compliance with the specifications for the food additive10 (sulfated ash < 0.1%, gallic acid < 0.5%, chlorinated organic compounds < 100 mg/kg, lead < 2 mg/kg, mercury < 1 mg/kg, arsenic < 3 mg/kg). Solvents (n‐propanol in four batches, benzene and toluene in two batches, cyclohexane in one batch) were below the respective limits of detection (LODs, 1 mg solvent/kg). The sum of some selected pesticides11 (four batches) < 0.01 mg/kg.9 Dioxins and dioxin‐like polychlorinated biphenyls (PCBs) were reported as not detected in two batches12 (no LOD indicated), dioxins were 0.30 ng WHO PCDD/F‐TEQ/kg in another batch.13 No microbial contamination was detected in three batches of the additive.14
Five batches of additive (from three companies)15 were analysed by laser diffraction for particle size distribution. Three samples from two producers showed approximately 1.7 to 2.2% of particles < 50 μm. Two batches for a third company identified the same fraction with approximately 79 to 82%. The latter sample was analysed according to the Stauber–Heubach method (four replicate analysis),16 showing a dusting potential of 1.36 g/m3.
3.1.2. Stability and homogeneity
The analysis of propyl gallate concentration in three batches of the additive stored in double polyethylene bags for 24 months at 25°C showed no losses of the active substance.17
Three types of mash feed (Feed A: 50/50 wheat/corn feed, Feed B: 50/50 wheat/corn feed with 5% vegetable oil and Feed C: commercial poultry feed) where supplemented with 27 or 100 mg propyl gallate/kg feed.18 Propyl gallate concentrations were analysed immediately after mixing and after 1, 2 and 3 months of storage. No losses of propyl gallate were observed in the feeds A and C after 3 months of storage. In the feed B, a loss of propyl gallate was observed after 3 months, with recoveries of 45% and 38% in the feeds supplemented with 27 and 100 mg/kg, respectively.
For additives intended to have an effect in feed, the stability and homogeneous distribution in feed, water and premixture may also be demonstrated by the maintenance of the effects over time. Propyl gallate was added at a concentration of 1 g/L to a liquid vitamin premixture for chickens (water content 76.3%), labelled to contain 50,000,000 IU vitamin A/L (not analysed) with the scope to preserve vitamin A from oxidation. Two batches analysed after 10 and 12 months of storage showed the same dimension of vitamin A levels as initially labelled.19 However, in the absence of analytical determination of the initial concentration of vitamin A and of a control, these results are of limited value.
3.1.3. Conditions of use
Propyl gallate is intended to be used as an antioxidant in feedingstuffs for all animal species and categories an in water for drinking with a maximum content of 40 mg/kg complete feed for food‐producing animals and 100 mg/kg for non‐food‐producing animals.20
3.2. Safety
The toxicological profile of propyl gallate has been assessed by several bodies (SCF, 1989; BIBRA, 1989a,b,c; JECFA, 1993, 2006, Cosmetic Ingredient Review (CIR), 2007). The toxicological assessment of propyl gallate (also including other gallate esters) has been performed by the ANS Panel in 2014 (EFSA ANS Panel, 2014). The main toxicological effects, as reported by the ANS Panel, are summarised below, together with some more recent studies.
3.2.1. Absorption, distribution, metabolism and excretion
The metabolic fate of propyl gallate in different mammalian species has been reviewed by EFSA ANS Panel (EFSA, 2013). The main features can be summarised as follows:
Propyl gallate is extensively (no precise quantification available) absorbed in rat at the intestinal level as such. In the gut, the microbial esterases of the intestinal microbiota can hydrolyse propyl gallate in gallic acid and the propyl moiety.
The rat and the rabbit metabolise gallic acid by 4‐O‐methylation. 4‐O‐Methyl‐gallic acid is then conjugated to glucuronic acid and excreted as the major metabolite in the urine, aside minor quantities of the unconjugated phenols: gallic acid, 4‐O‐methylgallic acid and pyrogallol.
The metabolic fate of propyl gallate is similar in the rat, pig and human.
The metabolic fate of n‐propyl gallate was investigated in the isolated perfused rat liver. At low portal concentrations (< 50 μM) the gallic acid ester was extracted entirely during a single passage through the liver. The hydrolytic release of gallic acid from n‐propyl gallate was very fast compared with the subsequent transformations of the gallic acid moiety; transport of the propyl ester was fast and flow‐limited in contrast to the slow and barrier‐limited transport of gallic acid (Eler et al., 2013).
No data are available concerning the metabolic pathways of propyl gallate in birds and fishes. However, it has been shown that esterases (aryl, ‘A’ and ‘B’) are expressed in the intestinal tract and the liver of birds (Walker and Mackness, 1987; Fossi et al., 1996) and fish, including salmonids and cyprinidae (Salamastrakis and Haritos, 1988; Li and Fan, 1997; Vimala and Rajaiah, 2014; Nahar and Rashid, 2013), which would strongly suggest that propyl gallate is de‐esterified to gallic acid in birds and fishes too.
The propyl moiety will enter the tricarboxylic acid cycle.
3.2.2. Toxicological studies
3.2.2.1. Genotoxicity studies
Weak mutagenic activity was reported for propyl gallate in a preincubation assay in Salmonella Typhimurium strain TA102 (sensitive to oxidising mutagens) in the presence and absence of an S9 mix. This activity was observed only at the top dose of 100 μg/plate. In the same experimental conditions, the test article was negative in TA 97 strain (Fujita et al., 1988). Propyl gallate was found to be negative in several bacterial reverse mutation studies performed with other bacterial strains (S. Typhimurium TA98, TA100, TA1535, TA1537, TA1538 and Escherichia coli WP2).
Propyl gallate, tested in Chinese hamster fibroblast cells at concentrations up to 0.04 mg/ml in saline, induced chromosomal gaps, breaks, exchanges, and fragmentations in 20% of the cells at a concentration of 0.023 mg/ml (Ishidate et al., 1978). Propyl gallate was tested for the induction of chromosome aberrations and sister chromatid exchanges in a diploid human embryo fibroblast cell line up to a concentration of 0.0021 and 0.0212 mg/mL for 26 to 48 h, with a negative outcome (Sasaki et al. 1980). In a study by Kawachi et al. (1980), propyl gallate was reported to induce chromosomal aberration in hamster lung fibroblasts but not in human embryo fibroblasts. The assays were conducted only without metabolic activation and the tested concentrations are not reported. Tayama and Nakagawa (2001) reported that propyl gallate induced sister chromatid exchanges and chromosomal aberrations in the presence of metabolic activation in Chinese hamster ovary (CHO‐K1) cells at 0.25 to 1.5 mM (corresponding to 0.0531 and 0.318 mg/mL, respectively). At the same concentrations endoreduplications and cell cycle delay were reported, indicating concomitant cytotoxicity.
Propyl gallate was tested for the mutagenic potential in the L5178Y tk+/− mouse lymphoma cell forward mutation assay (McGregor et al., 1988). Cultures were exposed to the chemicals for 4 h, and then cultured for 2 days before plating in soft agar with or without trifluorothymidine (TFT), 3 μg/mL. Significant mutagenic responses were observed in the absence of metabolic activation at all doses tested; however, the dose‐related response in the five higher doses was inverted. The authors considered the outcome of the study positive, but were not able to explain the mechanism of dose–response inversion. No information on colonies size is provided in this study.
An in vivo chromosome aberrations assay in rat bone marrow was available. Five animals per experimental group were given up to 5,000 mg/kg bw propyl gallate as single or for 5 consecutive days administration by gastric intubation. Sampling times were 6, 24, and 48 h after the single administration, and 6 h after the last dosing in the repeated administration. No genotoxic effect was reported in any experimental condition. The Panel notes that no information on local or systemic toxicity is given; therefore, no demonstration of target cells exposure was provided.
Propyl gallate was tested in a mouse bone marrow micronucleus test employing 3 daily exposures by intraperitoneal injection. Bone marrow samples were obtained 24 hours after the final exposure. The percentage of polychromatic erythrocytes (PCE) in the bone marrow was not significantly affected, but the route of administration gives insurance of systemic exposure to the test item. Propyl gallate was negative when administered in doses of 0, 75, 150, and 300 mg/kg bw, lethality was noted at the two high doses (Shelby et al., 1993). The FEEDAP Panel considers this study reliable, but notes that in compliance with OECD guideline 474 two sampling times should be used also after repeated treatments.
3.2.2.2. Acute and subchronic toxicity studies
Propyl gallate has a low acute toxicity with an oral LD50 in mice, rats, hamsters, and rabbits varying from 2,000 to 3,800 mg/kg bw.
As reported by Van der Heijden et al. (1986), the subchronic toxicity has been investigated in mice, rats, guinea pigs and dogs with doses ranging from 177 to 5,000 mg propyl gallate/kg feed; no adverse effects were observed. In a 13‐week study (Speijers et al., 1993), SPF‐derived Wistar RIVM:Tox rats (10 animals/group per sex) were fed a semisynthetic diet containing 0, 490, 1,910 or 7,455 mg propyl gallate/kg feed. Adverse effects of propyl gallate were observed in the high‐dose group involving the haematopoietic system as reflected by the haematological parameters (haemoglobin, haematocrit, red blood cell) and the morphological changes (extramedullary haematopoiesis) in the spleen. Some gender‐related differences were noted, as growth retardation, increase in the adrenal weight and in urinary proteins and decrease in Hb content were observed in males only. By contrast, males and females displayed a reduction in liver 7‐ethoxyresorufin O‐deethylase (EROD), a CYP1A‐mediated enzyme activity, together with an increase in both hepatic uridindiphospho‐glucuronyltransferase (UGT) and glutathione S‐transferase (GST) activities. In the intermediate dose group (1,910 mg propyl gallate/kg feed), statistically significant increases in UGT and GST were also recorded in males and females, respectively (Speijers et al., 1993). As the latter effects were not considered as adverse, a NOAEL of 1,910 mg propyl gallate/kg feed, corresponding to approximately 135 mg of the additive/kg bw per day could be identified.
3.2.2.3. Long‐term toxicity and carcinogenicity studies
Several of the old studies that employed long‐term oral exposure to propyl gallate were not performed or reported to modern standards. Lehman et al. (1951) reported that a dose of 5% in the diet (7,450 mg/kg bw per day) given for 2 years caused patchy hyperplasia of the stomach in mice, with a NOAEL of 1% (1,490 mg/kg bw per day). Orten et al. (1948) reported growth retardation in rats given 608 mg/kg bw per day for 2 years, with a NOAEL of 60 mg/kg bw per day. Another rat study (Lehman et al., 1951, as cited by JECFA, 1993, and van den Heijden et al., 1986) reported that 2,600 mg/kg bw per day given for 2 years caused stomach hyperplasia, with a NOAEL of 520 mg/kg bw per day. No adverse effects were seen in guinea‐pigs given 4.68 mg/kg bw per day for 1 year or dogs given 2,925 mg/kg bw and day for 14 months (Orten et al., 1948).
A study performed in B6C3F1 mice by the US National Toxicology Program (NTP) showed no adverse effects on toxicological endpoints including carcinogenicity at 0.6 or 1.2% (equivalent to 900 or 1,800 mg/kg bw per day) in the diet for 103 weeks (Abdo et al., 1983). Another study (Dacre, 1974) found no effects on chronic toxicity in mice given 0.5 or 1% in the diet (equal to up to 1490 mg/kg bw per day).
The NTP also performed a study on F344 rats given 0.6 or 1.2% (equivalent to 300 or 600 mg/kg bw/day) propyl gallate in their diet for 103 weeks (Abdo et al., 1983). No adverse effects were seen at the low dose, whereas hepatocyte vacuolation and prostate inflammation were seen in males given the high dose. Propyl gallate was not carcinogenic under the experimental conditions.
The results of these studies show that propyl gallate is not carcinogenic in mice and rats, and dietary levels of up to 1.2% (equivalent to 1,800 mg/kg bw per day in mice and 600 mg/kg bw per day in rats) did not cause chronic toxicity.
3.2.2.4. Other toxicity studies
The relationship between the metabolism and the cytotoxic effects of propyl gallate has been studied in freshly isolated rat hepatocytes. Addition of propyl gallate (0.5‐2.0 mM) to the hepatocytes elicited concentration‐dependent cell death, accompanied by decreases in intracellular ATP, adenine nucleotide pools, glutathione, and protein thiols. Propyl gallate was converted to gallic acid, 4‐O‐methyl‐gallic acid, and other minor products over time. In addition, propyl gallate was converted to a dimer (dipropyl‐4,4’,5,5’,6,6’‐hexahydroxydiphenate) and ellagic acid via auto‐oxidation. The order of potency for impairment of mitochondrial function was propyl gallate > propyl gallate‐dimer > gallic acid = 4‐O‐methyl gallic acid = ellagic acid > propyl alcohol. More recently, propyl gallate was found the most active out of 52 different antioxidants in the in vitro ability of generating H2O2; H2O2 production was directly related to cytotoxicity in DU145 cells (IC50 around 100 μM) (Grzesik et al., 2019).
Propyl gallate was found to act as an oestrogen receptor antagonist in the concentration range 1 nM–10 μM when tested in a B17 clone of MCF‐7 cells transfected with a plasmid containing the luciferase gene (Amadasi et al., 2009).
Ter Veld et al. (2006) found that propyl gallate was oestrogenic in the ERα expressing cells as well as in ERβ expressing cells in two different human osteoblastic reporter gene cell lines which stably express either ERα or ERβ in addition to 3xERE‐tata‐Luc as reporter gene. The estradiol equivalency factor (EEF10= EC10estradiol/EC10 propylgallate) for ERα or ERβ cell lines was 6.5x 10−7 and 3.9 x 10−6M, respectively. A decrease in relative uterine weight and in endometrial epithelium cell height was reported in prepubescent female Wistar rats orally dosed with propyl gallate (405 mg/kg bw for three consecutive days), pointing to an anti‐oestrogenic activity of the additive (Pop et al., 2013).
More recently (Pop et al., 2018), using proliferation tests (two different cell lines) and gene reporter assays, propyl gallate was found to behave as either estrogenic or anti‐oestrogenic compound (in the presence of E2) both alone or in binary mixtures with BHA, BHT or butylparaben, thereby confirming its endocrine disrupting properties. The available data do not allow the FEEDAP Panel to conclude on a dose relationship between propyl gallate and its pro‐ and/or anti‐oestrogenic activity.
3.2.2.5. Conclusions on toxicology
Propyl gallate was weakly positive in a bacterial reverse mutation assays on the strain TA 102 of Salmonella Typhimurium, sensitive to oxidising mutagens, and negative in all the other strains. Clastogenic activity was reported in three out of four in vitro cytogenetic studies. The substance was negative in two in vivo studies: a chromosome aberrations assay in rat bone marrow after oral administration and a micronucleus assay in mouse bone barrow after i.p. administration. Propyl gallate, as any antioxidant, can exert pro‐oxidant activity under particular conditions, like during an in vitro experiment, when high local concentrations of test item can be reached in the presence of the high atmospheric concentration of molecular oxygen. Considering that these conditions are not expected to be met in vivo, the FEEDAP Panel concludes that propyl gallate is of no concern for genotoxic activity.
Propyl gallate is not carcinogenic and it has a low acute toxicity. A NOAEL of 1,910 mg propyl gallate/kg feed, corresponding to approximately 135 mg of the additive/kg bw per day was identified, based on effects on haematopoietic system at higher doses in a 13‐week study in rats.
3.2.3. Safety for the target species
No specific tolerance studies with the additive were made available. The maximum feed concentration which can be considered safe for the target animals can be derived from the lowest NOAEL or BMDL10 identified, if suitable data are available (EFSA FEEDAP Panel, 2017a,b).
Toxicological data derived from a subchronic study were available for propyl gallate (see Section 3.2.2.2), for which a NOAEL of 135 mg/kg bw per day was derived. Applying an UF of 100 to the NOAEL of 135 mg/kg bw, the maximum safe intake for the target species was derived, following the EFSA Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017a,b), and thus the maximum safe feed concentration was calculated (see Table 1).
Table 1.
Maximum safe concentration in feed for propyl gallate
| Body weight (kg) | Feed intake (g DM/day) | Daily feed intake (g DM/kg bw) | Maximum safe concentration (mg/kg feed)1 | |
|---|---|---|---|---|
| Chickens for fattening | 2 | 158 | 79 | 15 |
| Laying hens | 2 | 106 | 53 | 22 |
| Turkeys for fattening | 3 | 176 | 59 | 20 |
| Piglets | 20 | 880 | 44 | 27 |
| Pigs for fattening | 60 | 2,200 | 37 | 32 |
| Sows lactating | 175 | 5,280 | 30 | 39 |
| Veal calves (milk replacer) | 100 | 1,890 | 19 | 62 |
| Cattle for fattening | 400 | 8,000 | 20 | 59 |
| Dairy cows | 650 | 20,000 | 31 | 38 |
| Sheep/goats | 60 | 1,200 | 20 | 59 |
| Horses | 400 | 8,000 | 20 | 59 |
| Rabbits | 2 | 100 | 50 | 23 |
| Salmons | 0.12 | 2.1 | 18 | 67 |
| Dogs | 15 | 250 | 17 | 71 |
| Ornamental fish | 0.012 | 0.054 | 5 | 264 |
DM: dry matter; bw: body weight.
Complete feed containing 88% DM, milk replacer 94.5% DM.
The cat is not included in the calculation based on the NOAEL derived from laboratory animal toxicological study. Cats are particularly inefficient in glucuronidation of many phenolic and aromatic compounds due to the known lack of at least two functional UGTs isoforms (e.g. UGT 1A6 and 1A9) (Court, 2013). Although the clearance of few phenolic compounds by glucuronidation and generally by sulfation was shown in the cat, in the absence of specific data it cannot be concluded that the efficiency of both pathways is sufficient to clear gallic acid and 4‐O‐methyl‐gallic that are formed from propyl gallate. No specific information on metabolic fate of propyl gallate has been made available for the feline species. The lack of knowledge is of particular relevance considering the additional load of phenolic compounds by dietary propyl gallate for the full lifetime expectancy of cats.
3.2.3.1. Conclusions on safety for the target species
The FEEDAP Panel concludes that propyl gallate is safe for veal calves, cattle for fattening, dairy cows, sheep, goats, sows, horses and salmonids at the proposed maximum use level of 40 mg/kg and for ornamental fish at the proposed maximum use level of 100 mg/kg. The following maximum use level are considered safe for the other target species:
15 mg/kg complete feed for chickens for fattening,
20 mg/kg complete feed for turkeys for fattening, laying hens and rabbits,
27 mg/kg complete feed for piglets and pigs for fattening,
71 mg/kg complete feed for dogs,
A safe level for cats cannot be established.
3.2.4. Safety for the consumer
The ANS Panel proposed an ADI of 0.5 mg/kg bw per day for propyl gallate (EFSA, 2013) based on the NOAEL of 135 mg/kg bw per day derived from a 90‐day toxicity study in rats and applying an UF of 300 for extrapolation from subchronic to chronic data and due to the limitations in the reproductive toxicity database. The ANS Panel concluded that ‘The high level of exposure exceeded the ADI in adults and the elderly. However, given the conservatism of the exposure assessment, the Panel concluded that the use of propyl gallate as food additive at the current uses and use levels is not of safety concern.’ The ANS Panel noted that additional analytical data would be needed to refine the exposure assessment and that in case the ‘refined exposure assessment remained greater than the ADI, or if additional uses and use levels were proposed, the Panel considered that given the uncertainties identified, additional toxicological data would be requested’. Consequently, the safety for the consumer must be assessed taking into consideration the additional exposure resulting from the use of the product as a feed additive. The application being for all animal species, residue data in edible tissues from poultry and mammals and products (milk and eggs) are considered necessary.
3.2.4.1. Residues
The study of the residues of propyl gallate and its two metabolites gallic acid and 4‐O‐methyl gallic acid was performed in tissues of cattle for fattening, eggs and tissues of laying hens and milk of cows fed diets supplemented with propyl gallate at 70, 45 and 25 mg/kg complete feed, respectively.
Propyl gallate, gallic acid and 4‐O‐methyl gallic acid were analysed in all tissues and products using a liquid Chromatography with tandem mass spectrometry (LC–MS/MS) internally validated method with LOQs determined. However, these methods showed several limitations related to the performance characteristics, precision and recovery. They were evaluated by the EURL who concluded that:
In its current state, the method of analysis used is not fit for purpose to determine reliably propyl gallate in all the tissues of cattle and laying hens;
The limited validation study in eggs and milk samples does not allow the full estimation of the method performance to determine propyl gallate in these matrices.
Therefore, in the absence of proper analytical methods, no reliable residue data are available, for the assessment of the consumer exposure.
Therefore, the additional exposure of the consumer by the use of propyl gallate in animal nutrition cannot be estimated.
3.2.4.2. Conclusions on the safety for the consumer
In the absence of data on consumer exposure, the FEEDAP Panel is not in the position to conclude on the safety for the consumer of propyl gallate, when used as a feed additive for all animal species.
3.2.5. Safety for user
No specific studies performed with the additive were available. In its ‘Final Report on the Amended Safety Assessment of Propyl Gallate’, the CIR reported the effects of propyl gallate in cosmetic products (propyl gallate concentration in the products: 0.003–10%) on skin and eyes. Based on this review, the FEEDAP Panel considers propyl gallate as an irritant to skin and eyes and a dermal sensitiser. No reports on potential respiratory toxicity were referenced.
The additive can contain a high number of particles of respirable size, and considering its dusting potential (1.36 g/m3), exposure via inhalation is possible and it is therefore considered a hazard.
3.2.6. Safety for the environment
The metabolism of propyl gallate indicates that it will be extensively de‐esterified to gallic acid, which will be O‐methylated and glucuronised, leading essentially to the excretion of gallic acid and derived metabolites (see Section 3.2.1). The additive as such will not be excreted in relevant quantities. Gallic acid is a natural component of many plants (e.g. tea leaves, oak bark, witch hazel), where it may be found free or as part of hydrolysable tannins. The use of the additive in animal nutrition is not expected to contribute to a substantial increase of the concentration of gallic acid and derived metabolites in the environment.
3.3. Efficacy
Propyl gallate is authorised to be added as an antioxidant to food with a wide range of moisture content at concentrations of 40 to 400 mg/kg. It could reasonably be assumed that the protective effects against oxidation observed when used in food would occur also in feed, at the recommended inclusion levels.
In addition, the applicant also provided some in vitro studies in which peroxide value and thiobarbituric acid were analysed in three feedingstuffs treated with propyl gallate at 0, 27 or 100 mg kg feed21 and one in a liquid vitamin premixture with propyl gallate at a concentration of 1 g/L in which level s of vitamin A were measured. However, the studies showed several weaknesses (e.g. the absence of statistical analysis, no analytical determination of vitamin A concentration at the start of the study) and were therefore not further considered in this assessment.
Based on the known effects in food, the FEEDAP Panel concludes that propyl gallate has the potential to act as antioxidant in feedingstuffs. As already mentioned in the statement from the FEEDA Panel on the use of feed additives in water, to the knowledge of the FEEDAP Panel there are no reasons to support the use of this additive in water for drinking for all animal species.
4. Conclusions
Propyl gallate a is safe for veal calves, cattle for fattening, dairy cows, sheep, goats, sows, horses and salmonids at the proposed maximum use level of 40 mg/kg and for ornamental fish at the proposed maximum use level of 100 mg/kg. The following concentrations (mg/kg complete feed) are considered safe for the other target species: 15 for chickens for fattening; 20 for turkeys for fattening, laying hens and rabbits; 27 for piglets and pigs for fattening and 71 for dogs. The Panel cannot conclude on a safe level for cats.
The exposure of the consumer to propyl gallate and its metabolites cannot be estimated owing the absence of reliable data on residues of propyl gallate and its metabolite in edible tissues and products. Therefore, the FEEDAP Panel is not in the position to conclude on the safety for the consumer of propyl gallate, when used as a feed additive for all food‐producing animal species.
Propyl gallate is irritant to skin and eyes and a dermal sensitiser. Exposure via inhalation is possible and it is considered a hazard.
The use of the additive in animal nutrition does not pose a risk for the environment.
The FEEDAP Panel concludes that propyl gallate has the potential to act as an antioxidant in feedingstuffs for all animal species. The Panel does not see a reason for the use of propyl gallate as an antioxidant in water for drinking.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 19/08/2010 | Dossier received by EFSA. Propyl gallate for all animal species. Submitted by FEFANA asbl |
| 13/08/2010 | Reception mandate from the European Commission |
| 09/08/2011 | Application validated by EFSA – Start of the scientific assessment |
| 10/11/2011 | Comments received from Member States |
| 24/11/2011 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: charaterisation of the additive, safety, efficacy |
| 09/12/2011 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 16/07/2013 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 08/10/2013 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: efficacy |
| 14/10/2013 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 27/02/2014 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety |
| 28/09/2015 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 29/07/2016 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: safety |
| 12/03/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 17/03/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ADI
acceptable daily intake
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstracts Service
- CHO
Chinese hamster ovary
- CIR
Cosmetic Ingredient Review
- CV
coefficient of variation
- DM
dry matter
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EROD
7‐ethoxyresorufin O‐deethylase and
- EURL
European Unin Reference Laboratory
- FAO
Food Agricultural Organization
- GST
glutathione S‐transferase
- IUPAC
International Union of Pure and Applied Chemistry
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- LC–MS/MS
liquid Chromatography with tandem mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- MRL
maximum residue limit
- NOAEL
no observed adverse effect level
- NTP
National Toxicology Program
- PCB
polychlorinated biphenyl
- PCDD/F
polychlorinated dibenzo‐p‐dioxins and dibenzofurans
- RP‐HPLC‐UV(DAD)
reversed phase high‐performance liquid chromatographic method with ultraviolet‐diode‐array detection
- RSDr
standard deviation for repeatability
- RSDR
standard deviation for reproducibility
- RRec
recovery rate
- SCAN
Scientific Committee on Animal Nutrition
- SCF
Scientific Committee on Food
- TEQ
toxic equivalent
- TFT
trifluorothymidine
- UF
uncertainty factor
- UGT
uridindiphospho‐glucuronyltransferase
- WHO
World Health Organization
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for propyl gallate
1.
In the current application authorisation is sought for Propyl Gallate under Articles 4 and 10, category ‘technological additives’, functional group ‘antioxidants’, according to the classification system of Annex I of Regulation (EC) No 1831/2003. Propyl Gallate (E310) is already authorised as feed additive under Commission Directive 70/524/EEC. According to the Applicant the feed additive contains minimum 97% of Propyl Gallate. Specifically, authorisation is sought for the use of the feed additive for all animal species and categories. The feed additive is intended to be mixed in premixtures or added directly in complete feedingstuffs or water. The Applicant proposed 100 mg/kg as specific maximum level in the daily ration of feedingstuffs for the feed additive alone or together with Octyl Gallate (E311) and/or Dodecyl Gallate (E312).
For the determination of Propyl Gallate in the feed additive the Applicant proposed to apply the internationally recognised European Phamacopoeia ultraviolet‐visible spectrophotometric method (Ph. Eur. 6.0, method 01/2008:1039). Even though no performance characteristics of this method are provided, the EURL considers this method suitable to determine Propyl Gallate in the feed additive within the frame of official control.
For the determination of Propyl Gallate in premixtures and feedingstuffs the Applicant submitted a single laboratory validated and further verified multi‐analyte Reversed Phase High Performance Liquid Chromatographic method with UltraViolet‐Diode‐Array Detection (RP‐HPLC‐UV(DAD)). The following correspondent performance characteristics were reported for concentrations in premixtures ranging from 5 to 120 g/kg and concentrations in feedingstuffs ranging from 35 to 226 mg/kg:
-
–
a standard deviation for repeatability (RSDr) ranging from 1.7 to 7%;
-
–
a standard deviation for reproducibility (RSDR) ranging from 3.0 to 12%;
-
–
a recovery rate (RRec) ranging from 84.4 to 112%; and
-
–
a limit of quantification (LOQ) of 6 mg/kg.
Based on the performance characteristics presented, the EURL recommends for official control, the single laboratory validated and further verified RP‐HPLC‐UV(DAD) method, submitted by the Applicant, to determine Propyl Gallate in premixtures and feedingstuffs.
The Applicant provided no experimental data for the determination of Propyl Gallate in water. Therefore, the EURL could not evaluate nor recommend a method for official control to determine Propyl Gallate in water.
The Applicant provided no experimental data for the determination of Octyl Gallate (E311) and/or Dodecyl Gallate (E312) in feedingstuffs. The EURL identified a method characterised by the “Association of Official Analytical Chemists” (AOAC 983.15) “Phenolic Antioxidants in oils, fats and butter oil”. The method has been ring‐trial validated and it is applicable in a range from 10 to 100 mg/kg. However, the analytical method is specifically designed for the determination of antioxidants in food matrices. Further experiments would be necessary in order to eventually extend the scope of this method to different matrices such as feedingstuffs. Therefore, the EURL could not evaluate nor recommend a method for official control to determine Octyl Gallate (E311) and/or Dodecyl Gallate (E312) in feedingstuffs.
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Aquilina G, Bories G, Gropp J, Nebbia C and Innocenti ML, 2020. Scientific Opinion on the safety and efficacy of propyl gallate for all animal species. EFSA Journal 2020;18(4):6069, 16 pp. 10.2903/j.efsa.2020.6069
Requestor: European Commission
Question number: EFSA‐Q‐2010‐01043
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Kos Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Name and address of the applicant (FEFANA asbl, Rue de Treves 45, 1040 Brussels, Belgium).
Commission List of the authorised additives in feedingstuffs published in application of Article 9t (b) of Council Directive 70/524/EEC concerning additives in feedingstuffs (2004/C 50/01). OJ C 50, 25.2.2004, p. 1.
European Parliament and Council Directive No 95/2/EC of 20 February 1995 on food additives other than colours and sweeteners. OJ No L 61, 18.3.1995, p. 1.
FEED dossier reference: FAD‐2010‐0078.
The full report is available on the EURL website: https://ec.europa.eu/jrc/en/eurl/feed-additives/evaluation-reports/fad-2010-0078?search&form-return
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II and Supplementary Information February 2013/Appendix_Qiiib_Propyl gallate_example 3_solvents and pesticides.pdf and Supplementary Information February 2013/Appendix_Qiiib_Propyl gallate_example 4_solvents and pesticides.pdf.
Supplementary Information February 2013/Appendix_Qiiib_Propyl gallate_example (1 to10).
Commission Regulation (EU) No 231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council.
As described in the 1999 edition of the British Pharmacopeia.
Supplementary Information February 2013/Appendix_Qiiib_Propyl gallate_example 2 and Appendix_Qiiib_gallic acid_example 2.
Supplementary Information February 2013/Appendix_Qiiib_Propyl gallate_example 7.
Supplementary Information February 2013/Appendix_Qiiib_Propyl gallate_example 8, 9 and Appendix_Qiiib_Propyl gallate_example_microbio.
Supplementary Information February 2013/Appendix_Qiv_Propyl gallate_PSD.
Supplementary Information February 2013/Appendix_Qiv_Propyl gallate_Dusting potential.
Technical dossier/Section II/Stability.
Supplementary Information February 2013/Annex_Qv stability and efficacy.pdf.
Supplementary Information February 2013/Annex_Qvi_Propyl Gallate_stability water.pdf.
Supplementary Information April 2014/FAD‐2010‐0078_AppRqst_Clarifications_300414.
Supplementary Information February 2013/Appendix_Qv_Propyl gallate_stability and efficacy complete.
References
- Abdo KM, Huff JE, Haseman JK, Dieter MP, Boorman GA, Hildebrandt P, Prejean JD and Farnell DR, 1983. Carcinogenesis bioassay of propyl gallate in F344 rats and B6C3F1 mice. Journal of the American College of Toxicology, 2, 425–433. [Google Scholar]
- Amadasi A, Mozzarelli A, Meda C, Maggi A and Cozzini P, 2009. Identification of Xenoestrogens in Food Additives by an Integrated in Silico and in vitro Approach. Chemical Research in Toxicology, 22, 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmetic Ingredient Review Expert Panel (CIR) , 2007. Final report on the amended safety assessment of Propyl Gallate. International Journal of Toxicology, 26, 89–118. [DOI] [PubMed] [Google Scholar]
- Court M, 2013. Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms. Veterinary Clinics of North America: Small Animal Practice, 43, 1039–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacre JC, 1974. Long‐term toxicity study of n‐propyl gallate in mice. Food Cosmetic Toxicology, 12, 125–129. [DOI] [PubMed] [Google Scholar]
- Bibra WG, 1989a. Propyl gallate. Toxicity profile, The British Industrial Biological Research Association: 9 pp. [Google Scholar]
- Bibra WG, 1989b. Octyl gallate. Toxicity profile, The British Industrial Biological Research Association: 5 pp. [Google Scholar]
- Bibra WG, 1989c. Dodecyl gallate. Toxicity profile, The British Industrial Biological Research Association: 4 pp. [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2014. Scientific opinion on the re‐evaluation of propyl gallate (E 310) as a food additive. EFSA Journal 2014;12(4):3642, 46 pp. 10.2903/j.efsa.2014.3642 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for technological additives. EFSA Journal 2012;10(1):2528, 23 pp. 10.2903/j.efsa.2012.2528 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017a. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017b. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesik M, Bartosz G, Stefaniuk I, Pichla M, Namieśnik J and Sadowska‐Bartosz I, 2019. Dietary antioxidants as a source of hydrogen peroxide. Food Chemistry, 25(278), 692–699. [DOI] [PubMed] [Google Scholar]
- Fossi MC, Lari L and Casini S, 1996. Interspecies variation of “B” esterases in birds: the influence of size and feeding habits. Archives of Environmental Contamination and Toxicology, 31, 525–532. [DOI] [PubMed] [Google Scholar]
- Fujita H, Nakano M and Sasaki M, 1988. Mutagenicity test for food additives using Salmonella typhimurium TA97 and TA102. Annual Report of Tokyo Metropolitan Research Laboratory of Public Health, 39, 343–350. [Google Scholar]
- Ishidate MJ, Hayashi M, Sawada M, Matsuoka A, Yoshikawa K, Ono M and Nakadate M, 1978. Cytotoxicity test on medical drugs. chromosome aberration tests with Chinese hamster cells in vitro. Eisei Shikenjo Hokoku, 96, 55–61. [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1993. Evaluation of certain food additives and contaminants. Forty‐first Report of the Joint FAO/WHO Expert Committee on Food Additives, WHO Technical Report Series, No 837. [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Gallates (Propyl‐, Octyl‐ and dodecyl‐). Evaluation of certain food additives and contaminants. Forty‐sixth Report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6‐15 February 1996. Published 1997. WHO Food Additives Series: 868.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Propyl Gallate. Prepared at the 46th JECFA (1996), published in FNP 52 Add 4 (1996) superseding specifications prepared at the 41st JECFA (1993), published in FNP 52 Add 2 (1993). Metals and arsenic specifications revised at the 61st JECFA (2003). Online Edition: “Combined Compendium of Food Additive specifications”. Available online: http://www.fao.org/ag/agn/jecfaadditives/specs/Monograph1/Additive-357.pdf
- Kawachi T, Komatsu T, Kada T, Ishidate M, Sasaki M, Sugiyama T and Tazima Y, 1980. Results of recent studies on the relevance of various short‐term screening tests in japan, in: the predictive value of short‐term screening tests in carcinogenicity evaluation. Applied Methods Oncology, 3, 253–267. [Google Scholar]
- Lehman AJ, Fitzhugh OG, Nelson AA and Woodard G, 1951. The pharmacological evaluation of antioxidants. Advances in Food Research, 3, 197–208. [DOI] [PubMed] [Google Scholar]
- Li SN and Fan DF, 1997. Activity of esterases from different tissues of freshwater fish and responses of their isoenzymes to inhibitors. Journal of Toxicology and Environmental Health, 51, 149–157. [DOI] [PubMed] [Google Scholar]
- McGregor DB, Brown A, Cattanach P, Edwards I, McBride D, Riach C and Caspary WJ, 1988. Responses of the L5178Y TK+ TK‐ mouse lymphoma cell forward mutation assay.3. 72 coded chemicals. Environmental and Molecular Mutagenesis, 12, 85–154. [DOI] [PubMed] [Google Scholar]
- Nahar S and Rashid M, 2013. Tissue specific esterase isozyme variation in ocellated Pufferfish. Tetraodon cutcutia Journal of Entomology and Zoology Studies, 1, 38–42. [Google Scholar]
- Orten JM, Kuyper AC and Smith AH, 1948. Studies on the toxicity of propyl gallate and of antioxidant mixtures containing propyl gallate. Food Technology, 2, 308–316. [Google Scholar]
- Pop A, Berce C, Bolfa P, Nagy A, Catoi C, Dumitrescu IB, Silaghi‐Dumitrescu L and Loghin F, 2013. Evaluation of the possible endocrine disruptive effect of butylated hydroxyanisole, butylated hydroxytoluene and propyl gallate in immature female rats. Farmacia, 61, 202–211. [Google Scholar]
- Pop A, Drugan T, Gutleb AC, Lupu D, Cherfan J, Loghin F and Kiss B, 2018. Estrogenic and anti‐estrogenic activity of butylparaben, butylated hydroxyanisole, butylated hydroxytoluene and propyl gallate and their binary mixtures on two estrogen responsive cell lines (T47D‐Kbluc, MCF‐7). Journal of Applied Toxicology, 38(7), 944–957. [DOI] [PubMed] [Google Scholar]
- Salamastrakis SS and Haritos AA, 1988. Physicochemical characterization and tissue distribution of multiple molecular forms of fish (Trachurus trachurus) esterases. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 91, 741–750. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Sugimura K, Yoshida MA and Abe S, 1980. Cytogenic effects of 60 chemicals on cultured human and chinese hamster cells. Senshokutai, 20, 574–584. [Google Scholar]
- SCF (Scientific Committee on Food), 1989. Gallates ‐ Propyl‐, octyl‐, dodecyl‐. Reports of the Scientific Committee for Food (Twenty‐second series). Available online: http://ec.europa.eu/food/fs/sc/scf/reports/scf_reports_22.pdf
- Shelby MD, Erexson GL, Hook GJ and Tice RR, 1993. Evaluation of a three‐exposure mouse bone marrow micronucleus protocol: results with 49 chemicals. Environmental and Molecular Mutagenesis, 21, 160–179. [DOI] [PubMed] [Google Scholar]
- Speijers GJA, Janssen GB, Wallbrink‐de Drieu Y, van Leeuwen FXR, van Loenen HA, KrajncFranken MAM, Vaessen HAMG and Wester PW, 1993. Subchronic toxicity of propyl‐gallate. RIVM Report No. 618311002 (Draft) Rijksinstituut voor Volksgezondheid en Milieuhygiëne. Bilthoven, The Netherlands. [Google Scholar]
- Tayama S and Nakagawa Y, 2001. Cytogenetic effects of propyl gallate in CHO‐K1 cells. Mutation Research, 498, 117–127. [DOI] [PubMed] [Google Scholar]
- Ter Veld MG, Schouten B, Louisse J, van Es DS, van der Saag PT, Rietjens IM and Murk AJ, 2006. Estrogenic potency of food‐packaging‐associated plasticizers and antioxidants as detected in ERalpha and ERbeta reporter gene cell lines. Journal of Agricultural Food Chemistry, 54, 4407–4416. [DOI] [PubMed] [Google Scholar]
- Van der Heijden CA, Janssen P and Strik J, 1986. Toxicology of gallates: a review and evaluation. Food and Chemical Toxicology, 24, 1067–1070. [DOI] [PubMed] [Google Scholar]
- Vimala V and Rajaiah V, 2014. Tissue esterase polymorphism of Cyprinus carpio and Puntius sarana of cypriniformes order. International Journal of Current Microbiology Applied Science, 3, 851–858. [Google Scholar]
- Walker CH and Mackness M, 1987. “A” esterases and their role in regulating thetoxicity of organophosphates. Archives of Toxicology, 60, 30–33. [DOI] [PubMed] [Google Scholar]


