Abstract
The European Commission asked EFSA for a scientific opinion on the risks for animal and human health related to the presence of chlorinated paraffins in feed and food. The data for experimental animals were reviewed and the CONTAM Panel identified the liver, kidney and thyroid as the target organs for the SCCP and MCCP mixtures tested in repeated dose toxicity studies. Decreased pup survival and subcutaneous haematoma/haemorrhage were also identified as critical effects for an MCCP mixture. For the LCCP mixtures tested, the liver was identified as the target organ. The Panel selected as reference points a BMDL 10 of 2.3 mg/kg bw per day for increased incidence of nephritis in male rats, and of 36 mg/kg bw per day for increased relative kidney weights in male and female rats for SCCPs and MCCPs, respectively. For LCCPs, a reference point relevant for humans could not be identified. Due to the limitations in the toxicokinetic and toxicological database, the Panel concluded that derivation of a health‐based guidance value was not appropriate. Only limited data on the occurrence of SCCPs and MCCPs in some fish species were submitted to EFSA. No data were submitted for LCCPs. Thus, a robust exposure assessment and consequently a complete risk characterisation could not be performed. A preliminary risk characterisation based only on the consumption of fish was performed, and the calculated margins of exposure suggested no health concern for this limited scenario. The Panel noted that dietary exposure will be higher due to the contribution of CPs from other foods. The Panel was not able to identify reference points for farm animals, horses and companion animals. No occurrence data for feed were submitted to EFSA. Therefore, no risk characterisation could be performed for any of these animal species.
Keywords: chlorinated paraffins, SCCP, MCCP, LCCP, food, feed, risk assessment
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1815/full
Summary
The European Commission asked the European Food Safety Authority (EFSA) for a scientific opinion on the risks for animal and human health related to the presence of chlorinated paraffins (CPs) in feed and food. The Panel developed the draft scientific opinion which underwent a public consultation from 6 August 2019 to 17 September 2019. The comments received and how they were taken into account when finalising the scientific opinion were published in an EFSA Technical Report (EFSA, 2020).
CPs are complex technical mixtures of polychlorinated alkanes, with varying chain length and degree of chlorination. The commercially available CPs are generally divided into three groups: short‐chain CPs (SCCPs) comprising 10 to 13 carbon atoms, medium‐chain CPs (MCCPs) comprising 14 to 17 carbon atoms and long‐chain CPs (LCCPs) with 18 or more carbon atoms. Some technical mixtures may contain CPs from more than one of these three groups, and some newer CPs do not confine themselves to these groups. Technically, the terms ‘SCCP’, ‘MCCP’ and ‘LCCP’ refer to the commercial mixtures of CPs. In this opinion, the nomenclature of SCCP(s), MCCP(s) and LCCP(s) will be used to refer to any CP mixture(s) that falls within the range of carbon chain lengths indicated above.
There is a vast number of possible combinations of chain length, position and degree of chlorination, and the exact composition of technical mixtures can vary in terms of the amounts of different congeners present. This means that the potential array of compounds that are of relevance is of many thousands and that the analytical challenge for measuring these compounds is considerable.
Analysis of CPs is highly demanding and current methods have been shown to lack precision and accuracy. Congener specific analysis is not currently possible. Several different approaches to analysis exist, and lead to differences in results obtained. Only a limited number of analytical standards are available, and these represent only a tiny fraction of the total number of individual CP congeners that may be found. Care is therefore needed when using occurrence data to perform exposure estimates.
Human risk assessment
Information on the toxicokinetics and toxicity of CPs was identified for a limited number of mixtures.
No studies on the toxicokinetics in humans of relevance for the risk assessment of CPs within the scope of this opinion were identified. However, detection of CPs in human blood and milk samples indicates that CPs are absorbed to some extent in humans and detection of CPs in umbilical cord blood indicates that CPs can be transferred to the fetus.
Data on toxicokinetics for rats and mice were identified, although in several cases from studies not designed as dedicated ADME studies.
Absorption to some extent is indicated in various animal species by excretion of a small amount of radiolabelled CPs and or metabolites in the urine, distribution of radiolabelled CPs and or metabolites to different organs and tissues, and systemic effects observed in toxicity studies. Accumulation in adipose tissue and fat rich organs and tissues is reported and elimination from these tissues appeared to be slower than from other organs and tissues. The available information is insufficient to derive elimination half‐lives. Studies in mice suggested that highly chlorinated SCCPs and MCCPs are metabolised and excreted via faeces whereas lower chlorinated SCCPs and MCCPs can be partly metabolised and exhaled as carbon dioxide. Following oral administration of CPs to rats and mice, the major route of excretion was the faeces with only a small proportion excreted in urine and expired air.
No studies on observations in humans of relevance for the risk assessment of CPs within the scope of this opinion were identified.
In experimental animals, CPs are of low acute toxicity with oral LD50 values all exceeding 4 g/kg body weight (bw).
In repeated dose toxicity studies in rats, mice and dogs, the liver, kidney and thyroid were identified as the target organs for the two SCCPs (C10–12, 58% chlorination; C12, 60% chlorination) and the single MCCP (C14–17, 52% chlorination) tested in the subchronic and chronic toxicity studies. For the LCCPs tested in the subchronic and chronic toxicity studies, only the liver was identified as a target organ. Liver toxicity observed consistently in rats and mice is considered secondary to an adaptive response and the associated energy costs, but could be relevant to humans at high‐dose levels. For an SCCP and an MCCP, changes in thyroid hormone levels in rats were observed at a lower dose level than histopathological effects in the thyroid.
For the SCCPs tested, a no‐observed adverse effect level (NOAEL) of 10 mg/kg bw per day was identified, based on increased kidney weights and histopathological changes in the kidney and thyroid.
For the MCCP tested, a NOAEL of 10 mg/kg bw per day was identified, based on increased kidney weights.
For the LCCPs, a lowest‐observed adverse effect level (LOAEL) of 100 mg/kg bw per day was considered for the two low chlorinated LCCPs (43%) tested and a NOAEL of 900 mg/kg bw per day for the high chlorinated LCCP (70%) tested, based on liver effects, including increased weight and histopathological changes.
An SCCP (carbon chain length not specified, 58% chlorination) caused teratogenic effects such as absence of digits or shortened digits in rats at a high‐dose level (2,000 mg/kg bw per day) in the presence of maternal toxicity (high mortality and decreased body weight gain; the NOAEL for developmental toxicity was 500 mg/kg bw per day, and for maternal toxicity 100 mg/kg bw per day. In the developmental toxicity study in rabbits with the same SCCP, the NOAEL for developmental toxicity was 10 mg/kg bw per day, based on increased number of resorptions, in the absence of maternal toxicity.
For the only MCCP tested (C14–17, 52% chlorination), no developmental toxicity was observed in rats and rabbits at the highest dose levels tested, 5,000 mg/kg bw per day for rats and 100 mg/kg bw per day for rabbits. No parental toxicity or effects on fertility were observed for the same MCCP in a dose‐range finding study for a two‐generation dietary rat study. In this study, postnatal effects in the form of decreased pup survival and subcutaneous haematoma/haemorrhage were observed; a NOAEL of 9 mg/kg bw per day was identified for these effects.
For the two LCCPs tested (C22–26, 43% chlorination; C22–26, 70% chlorination), no developmental toxicity was observed in rats and rabbits at the highest dose levels tested (1,000 mg/kg bw per day for rabbits with the 70% chlorinated LCCP and 5,000 mg/kg bw per day in the three other studies with the 43% or the 70% chlorinated LCCP).
The overall weight of evidence indicates that CPs are not genotoxic.
The SCCP tested (C12, 60% chlorination) caused increased incidences of tumours in rats and mice, i.e. in the liver (rats, mice), kidneys (male rats only) and thyroid (female rats and mice). The LCCP tested (C23, 43% chlorination) also caused increased incidences of tumours in mice, i.e. malignant lymphomas (male mice), and to a marginal extent of hepatocellular neoplasms (female mice). No carcinogenicity study on MCCPs was identified.
Regarding the mode of action, rodent hepatic enzyme induction and proliferation of the smooth endoplasmic reticulum leading to hypertrophy (and associated increases in liver size) is considered as an adaptive physiological response to CPs. In addition, proliferation of rodent peroxisomes occurs, mediated by peroxisome proliferator activated receptor α (PPARα). These responses are considered important for rodent liver carcinogenesis. However, based on the available information, the rodent liver tumours induced by an SCCP, and associated with constitutive androstane receptor (CAR) or PPARα transactivation, appear not to be relevant to humans provided cytotoxicity does not occur. These responses could lead to toxicity if the energy balance to support this compensatory response becomes sufficiently perturbed so as to compromise the cell viability.
SCCP‐induced kidney tumours in male rats appear to relate to sustained nephropathy (induced partly, but not necessarily entirely, by α2u‐microglobulin accumulation), compensatory regenerative hyperplasia and increased S‐phase.
Thyroid effects caused by CPs in rodents can arise due to stimulation of the thyroid via a negative feedback mechanism. This includes a potential increase in hepatic uptake of thyroid hormones, increased hepatic uridine 5’‐diphospho‐glucuronosyltransferase (UDPGT) levels and conjugation of T4, consequent decrease in plasma T4/T3, compensatory release of pituitary thyroid‐stimulating hormone (TSH) and a compensatory increase in T4 production in the thyroid. This eventually leads to hypertrophy, hyperplasia and thyroid tumours. Additional mechanisms that may contribute to carcinogenesis by CPs include oxidative stress and inhibition of intercellular communication.
The CONTAM Panel decided not to use changes in thyroid hormone levels as a basis for a reference point since there were inconsistencies in the hormonal changes between the two available studies on SCCPs, as well as gender differences in the available study with the MCCP. Overall, there is uncertainty about the validity of extrapolating changes related to the thyroid from rats to humans when these changes are mediated by induction of hepatic transporters and hepatic metabolism. However, despite these uncertainties, the thyroid histopathology is used as a basis for the reference point. Potential neurodevelopmental effects from thyroid hormone changes could not be assessed because of a lack of developmental neurotoxicity studies.
In rats, an MCCP perturbed the clotting system in lactating neonates of treated mothers. The haemorrhaging effects in the lactating neonates appear to result as a consequence of vitamin K deficiency.
The CONTAM Panel performed benchmark dose (BMD) modelling on data from different rodent studies. For one tested SCCP (C10–12, 58% chlorination), a BMDL10 of 2.3 mg/kg bw per day for increased incidence of nephritis in male rats was selected as the reference point for the risk characterisation. For the tested MCCP (C14–17, 52% chlorination), a BMDL10 of 36 mg/kg bw per day for increased relative kidney weights in male and female rats was selected as the reference point.
For the tested LCCPs, only liver effects were observed in the available studies. As the effects in the liver are considered secondary to an adaptive response and the associated energy costs, and only relevant to humans at high‐dose levels, a reference point was not identified.
Due to the limitations and uncertainties in the current database on SCCPs, MCCPs and LCCPs the CONTAM Panel did not consider it appropriate to derive health‐based guidance values (HBGVs). Instead, a margin of exposure (MOE) approach was applied to assess a possible health concern. The Panel concluded that an MOE higher than 1,000 might indicate that there is no health concern, taking into account the variability between species (a factor of 10), the variability within human individuals (a factor of 10) and extrapolation from subchronic studies to chronic exposure duration (a factor of 2). The CONTAM Panel considered an additional factor of 5 to take into account the limitations in the database, i.e. toxicity data are only available for a few CPs whereas the risk assessment is covering a large number of CPs, the impact of the degree of chlorination, chlorine position and carbon chain length on toxicokinetics and toxicity cannot be evaluated, and no two‐generation reproductive toxicity study is available for any CP.
Only limited data on the occurrence of SCCPs and MCCPs, and only for some fish species, were submitted to EFSA, while no data were submitted on the occurrence of LCCPs. Due to the unavailability of sufficient occurrence data on foodstuffs other than fish, a robust exposure assessment and consequently a complete risk characterisation could not be performed.
A preliminary exposure assessment of the chronic exposure to SCCPs and MCCPs was made based only on consumption of ‘Fish meat’ and for fish consumers only. A data set consisting of 422 analytical results from 184 samples of ‘Fish meat’ collected in Germany between 2014 and 2017 was used for this purpose. CPs were quantified in most of the samples, with 15% and 14% of left‐censored data for SCCPs and MCCPs, respectively. The mean and P95 occurrence levels were 7.5 and 18 μg/kg wet weight (ww) (lower bound (LB) = upper bound (UB)) for SCCPs, respectively, and 13 and 44 μg/kg ww (LB=UB) for MCCPs, respectively.
For SCCPs, the mean LB and UB exposure estimates across surveys and age groups ranged from 1.9 to 35 ng/kg bw per day. At the 95th percentile exposure, the LB and UB estimates ranged from 4.9 to 87 ng/kg bw per day. For MCCPs, the mean LB and UB exposure estimates ranged from 3.2 to 59 ng/kg bw per day. At the 95th percentile exposure, the LB and UB estimates ranged from 8.5 to 148 ng/kg bw per day. The lowest exposures were found in the Adult groups whilst the highest were for Toddlers.
Comparison of the SCCP dietary exposures from fish consumption only with the BMDL10 of 2.3 mg/kg bw per day resulted in MOEs of about 7 × 104 and 3 × 104 or higher for the mean and 95th percentile exposure, respectively.
Comparison of the MCCP dietary exposures from fish consumption only with the BMDL10 of 36 mg/kg bw per day resulted in MOEs of about 6 × 105 and 3 × 105 or higher for the mean and 95th percentile exposure, respectively.
The CONTAM Panel concluded that these MOEs for this limited scenario do not suggest a health concern, while noting uncertainties because the dietary exposure will be higher due to the contribution of CPs from other foods, the lack of toxicokinetic data for humans and that only a few CPs have been tested in the available toxicity studies.
For breastfed infants, an exposure estimate was made based on data from pooled human milk samples from 11 European countries between 2014 and 2016 and analysed within the WHO/UNEP Coordinated Survey of Human Milk for POPs. For SCCPs, the exposure ranged from 60 to 445 ng/kg bw per day for average consumption of human milk, and from 90 to 668 ng/kg bw per day for high consumption of human milk, respectively. For MCCPs, the exposure ranged from < 25 to 514 ng/kg bw per day, and from < 38 to 771 ng/kg bw per day, respectively, for average and high consumption of human milk. The Panel noted that since these were pooled samples, it was not possible to estimate specific values for highly exposed individuals.
Comparison of the exposure estimates for SCCPs with the BMDL10 of 2.3 mg/kg bw per day, resulted in MOEs of about 5 × 103 and 3 × 103 or higher for average and high human milk consumption, respectively. For MCCPs, comparison of the exposure estimates with the BMDL10 of 36 mg/kg bw per day resulted in MOEs of about 7 × 104 and 5 × 104 or higher for average and high human milk consumption, respectively. The CONTAM Panel concluded that these MOEs do not suggest a health concern.
In addition to dietary exposure, CPs in house dust can be an important additional source of exposure. For children, crawling and hand‐to‐mouth habits should be considered as habits possibly increasing the ingestion of dust. The CONTAM Panel made a scenario to estimate the ingestion from dust in adults and toddlers considering an average dust ingestion of 20 and 50 mg/day, respectively. Using the highest median values found in dust from four European countries, the resulting daily exposures via dust ingestion would be 26, 150 and 11 ng/kg bw per day for toddlers and 1.8, 10 and 0.77 ng/kg bw per day for adults, for SCCPs, MCCPs and LCCPs, respectively. The CONTAM Panel noted that this is only a very crude estimate of the exposure via dust and has a large associated uncertainty, but it shows that for toddlers exposure from dust could be in the same order of magnitude as the dietary exposure estimated from fish. For adults, the exposure from dust was lower than for toddlers both in absolute terms and when compared with the exposure estimated from fish consumption, but could nevertheless still be an important source of exposure to CPs.
The CONTAM Panel considered that the impact of the uncertainties on the risk assessment of exposure to CPs in food is substantial, and due to the limited data on occurrence of CPs in food the dietary exposure is considered to be underestimated.
Farm animals, horses and companion animals risk assessment
Only limited toxicokinetic data were available and only for some farm animal species. In broiler chickens and laying hens given diets supplemented with an SCCP, the highest concentration was recovered in abdominal fat. In the laying hens, a high concentration was also found in egg yolk and liver. A similar distribution pattern was seen in laying Japanese quail given an SCCP or an MCCP orally. The fish studies with rainbow trout indicated that CPs were rapidly accumulated and had high assimilation efficiencies from food and that the half‐lives increased with increasing carbon chain length and chlorination degree. The studies also indicated that the CPs have the potential to biomagnify from food to fish in aquatic food chains with increasing trends with increasing carbon chain length and greater biotransformation of the short‐chain CPs compared to the longer chain CPs. Studies with other fish species support the potential of CPs to accumulate in fish tissues.
For ruminants, pigs, horses, cats or fur animals, no studies on the adverse effects of CPs were identified.
For poultry, a limited number of studies have been conducted and some of these were designed to assess the performance and capacity for food production. The CONTAM Panel concluded that none of them was robust enough for the identification of a NOAEL or a LOAEL as a reference point.
For rabbits, no studies have been located other than the developmental toxicity studies in experimental animals. For an SCCP (carbon chain length not specified, 58% chlorination), the NOAEL for developmental toxicity was 10 mg/kg bw per day, based on increased number of resorptions; the no‐observed effect level (NOEL) for maternal toxicity was 100 mg/kg bw per day (the highest dose tested). For an MCCP (C14–17, 52% chlorination), the NOEL for developmental and maternal toxicity was 100 mg/kg bw per day (the highest dose tested). For a low chlorinated LCCP (C22–26, 43% chlorination; C22–26), the NOEL for developmental and maternal toxicity was 5,000 mg/kg bw per day (the highest dose tested). For a high chlorinated LCCP (C22–26, 70% chlorination), the NOEL for developmental and maternal toxicity was 1,000 mg/kg bw per day (the highest dose tested).
For fish, most studies showed no or minor effects. Based on the available information, the CONTAM Panel could not identify a NOAEL or a LOAEL as reference point.
For dogs, a NOEL of 10 mg/kg bw per day was identified for an MCCP (C14–17, 52% chlorination) from a dietary study in Beagle dogs, based on an increase of hepatic smooth endoplasmic reticulum.
No exposure assessment was possible for any of the farm animal species as no occurrence data for feed were submitted to EFSA. The data for feed reported in the literature were too limited to attempt an exposure scenario.
Due to the lack of occurrence data for feed and the lack of, or limited, data on adverse effects of CPs in farm animals, horses and companion animals, a risk characterisation could not be performed.
Recommendations
In order to improve the risk assessment for both humans and animals, and reduce the uncertainties, the CONTAM Panel recommends the following:
There is a need for validated analytical methods for the determination of CPs, as well as suitable standards and reference materials.
Research is needed to identify which specific CP congeners are more relevant in terms of occurrence in food and of relevance for human health.
There is a need for occurrence data in food for SCCPs, MCCPs and LCCPs to enable a robust human exposure assessment as well as data on the possible transfer of CPs from, e.g. kitchen equipment, during food preparation.
More data on variation of occurrence of CPs in human milk are needed to enable a more robust exposure assessment for breastfed infants.
More information is needed on the toxicokinetics in humans and experimental animals, with respect to the impact of the degree of chlorination, chlorine position and carbon chain length. The development of TK models would facilitate a body burden approach.
There is a need for chronic toxicity studies for relevant CP mixtures.
A better understanding of the relevance of SCCP and MCCP thyroid hormone changes in rodents and of SCCP‐induced rodent thyroid tumours to humans is needed.
There is a need for developmental neurotoxicity studies with SCCP and MCCP because of the reported changes in rodent thyroid hormone levels.
There is a need for occurrence data in feed.
Data on the transfer of CPs from feed to the food of animal origin are needed.
There is a need for data on adverse effects of CPs in ruminants, pigs, poultry and fish. Data in horses, companion animals and fur animals would also be needed to perform a risk assessment on these species.
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
Background
Chlorinated paraffins (CPs) are complex mixtures of polychlorinated n‐alkanes. The chlorination degree of CPs can vary between 30 and 70 weight percent (wt%). CPs are typically subdivided according to their carbon chain length into short chain CPs (SCCPs, C10‐13), medium chain CPs (MCCPs, C14‐17) and long chain CPs (LCCPs, C>17).
CPs may be released into the environment during product use and though improper disposal. There is also potential for contamination of the feed and food chain. CPs, in particular SCCPs and to a lesser extent MCCPs, bioconcentrate in fish and molluscs. Food is considered the main source of human exposure to CPs.
SCCPs are considered to be persistent toxic substances. CPs with an average carbon‐chain length C12 and an average degree of chlorination of approximately 60% are classified by the International Agency for Research on Cancer (IARC) as possibly carcinogenic to humans (Group 2B).
Terms of reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority for a scientific opinion on the risks for animal and human health related to the presence of chlorinated paraffins in feed and food.
1.2. Interpretation of the Terms of Reference
Technically, the terms ‘SCCP’, ‘MCCP’, and ‘LCCP’ refer to the commercial mixtures of CPs. However, most literature uses this terminology for CPs within one of these specific groups, even when the CP patterns deviate from the commercial mixtures due to for instance weathering or biotransformation.
In this opinion the nomenclature of SCCP(s), MCCP(s), and LCCP(s) will be used to refer to any CP mixture(s) that falls within the range of carbon chain lengths indicated above.
This opinion focuses on chlorinated CPs only and not on brominated CPs, chloro/bromo CPs, or sulpho‐chlorinated CPs. Also chlorinated olefins (i.e. chlorinated paraffins with some degree of unsaturation) are not assessed, but it should be mentioned that they can interfere with the analysis of CPs using certain analytical techniques.
1.3. Supporting information for the assessment
1.3.1. Physicochemical properties
Chlorinated paraffins (CPs) are complex technical mixtures of polychlorinated alkanes (PCAs), with varying chain length and degree of chlorination with the general formula CxH(2x + 2) − yCly. The commercially available CPs are generally divided into three groups: short‐chain CPs (SCCPs) comprising 10–13 carbon atoms, medium‐chain CPs (MCCPs) comprising 14–17 carbon atoms and long‐chain CPs (LCCPs) with 18 or more carbon atoms. Some technical mixtures may contain CPs from more than one of these three groups, and some newer CPs do not confine themselves to these classes (see Section 1.3.2). CPs with 9 carbon atoms are becoming more widely used and are known as very short‐chain CPs or vSCCPs (Xia et al., 2019; Zhou et al., 2019a). There are a vast number of possible combinations of chain length, position and degree of chlorination, and the exact composition of technical mixtures can vary in terms of the amounts of different congeners present. This means that the potential array of compounds that are of relevance is many thousands and that the analytical challenge for measuring these compounds is considerable.
Other terms commonly used to refer to CPs include: chlorinated alkanes, chlorinated hydrocarbon waxes, chlorinated paraffin waxes, chlorinated waxes, chloroalkanes, chlorocarbons, chloroparaffin waxes, ‘paraffin, chlorinated’, ‘paraffins, chloro’, paraffin waxes, ‘chlorinated; paroils, chlorinated’; polychlorinated alkanes and polychloro alkanes. Figure 1 shows the general structure of CPs and the CAS numbers for some CPs are shown in Table 1 .
Figure 1.
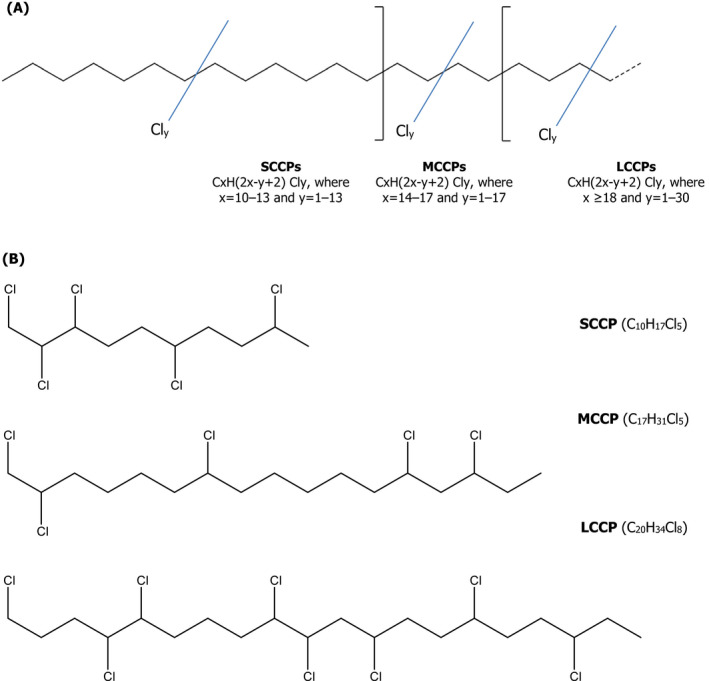
(A) General structure of chlorinated paraffins (CPs) and (B) example of a short‐chain chlorinated paraffin, SCCP (C10H17Cl5), a medium‐chain chlorinated paraffin, MCCP (C17H31Cl5) and a long‐chain chlorinated paraffin, LCCP (C20H34Cl8) of the many possible CP structures
Table 1.
CAS numbers for some chlorinated paraffins
| Chemical name | CAS number |
|---|---|
| Alkanes, C6–18, chloro | 68920‐70‐7 |
| Alkanes, C10–13, chloro | 85535‐84‐8 |
| Alkanes, C12–13, chloro | 71011‐12‐6 |
| Alkanes, C14–17, chloro | 85535‐85‐9 |
| Alkanes, C10–21, chloro | 84082‐38‐2 |
| Alkanes, C18–20, chloro | 106232‐85‐3 |
| Alkanes, C18–28, chloro | 85535‐86‐0 |
| Alkanes, C10–14, chloro | 85681‐73‐8 |
| Paraffins (petroleum), normal C > 10, chloro | 97553‐43‐0 |
| Alkanes, C10–26, chloro | 97659‐46‐6 |
| Alkanes, C10–22, chloro | 104948‐36‐9 |
| Alkanes, C10–12, chloro | 108171‐26‐2 |
| Alkanes, chloro | 61788‐76‐9 |
| Paraffin waxes and hydrocarbon waxes, chloro | 51990‐12‐6 |
| Paraffin waxes and hydrocarbon waxes, chloro | 63449‐39‐8 |
| Alkanes, C21–34‐branched and linear, chloro | 1417900‐96‐9 |
| Alkanes, C22–30‐branched and linear, chloro | 1401974‐24‐0 |
CAS: Chemical Abstracts Service.
Most CPs are viscous colourless or yellowish dense oils, but some LCCPs (C20‐ to C30‐ with a chlorine content > 70%) are solid (Van Mourik et al., 2015). The physicochemical properties vary with the carbon chain length and chlorine content. An extensive review and discussion of the physicochemical properties of CPs is given in previous assessments (WHO/IPCS, 1996; EU‐RAR, 2000, 2005, 2011; Health Canada, 2008) and some of these CPs are shown in Table 2 . More details for the log Kow of 40 synthesised CPs along with a discussion of the influence of chain length and degree of chlorination have been reported (Hilger et al., 2011).
Table 2.
Some physicochemical properties of chlorinated paraffins (from Nielsen and Ladefoged, 2013; EU‐RAR, 2000, 2005, 2011)
| SCCPs | MCCPs | LCCPs | |
|---|---|---|---|
| Description | Clear to yellowish mobile to highly viscous oily liquid | Liquid | Liquid or solida |
| Additivesb | Epoxidised vegetable oil, glycidyl ether, organophosphorous compounds | ||
| Boiling point | > 200°Cc | ||
| Solubility |
Practically insoluble in: Water (< 0.5 mg/L at 20°C), lower alcohols, glycerol and glycols Soluble in: chlorinated solvents, aromatic hydrocarbons, ketones, esters, ethers, mineral oil |
||
| Vapour pressure |
Chlorine content 50%: 1.6 × 10−4 mmHg (0.0213 Pa) (at 40°C) |
Chlorine content 45%: 1.7 × 10−5 mmHg (0.0023 Pa) (at 40°C) Chlorine content 52%: 1.0 × 10−6–2.0 × 10−6 mmHg (0.00013–0.00027 Pa) (at 20°C) |
– |
| Log Po/w |
Chlorine content 49%: 4.39–6.93 Chlorine content 71%: 5.68–8.69 |
Chlorine content 45%: 5.52–8.21 Chlorine content 52%: 5.47–8.01 |
Chlorine content 42%: 9.29–> 12.83 Chlorine content 48%: 8.69–12.83 |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins.
Depending on the carbon chain length and chlorine content.
As stabilisers, to improve thermal and photo stability.
Around 200°C decomposition with release of hydrogen chloride occurs.
1.3.2. Production and industrial use
The many theoretical combinations for the position of chlorine atoms combined with the presence of chiral centres leads to a large number of potential positional isomers, enantiomers and diastereoisomers. For example, there are 122,161 positional isomers for MCCPs, based on the assumption that no more than one chlorine atom binds to any carbon atom. In practice, there are fewer because it is mainly the linear n‐alkanes that are used to produce CPs. The degree of chlorination typically varies between 30% and 72% by weight (Van Mourik et al., 2015). A similar complex situation exists for SCCPs and LCCPs, and results in difficulties in terms of analysis of these compounds, especially in environmental and food matrices.
There have been at least 200 CP formulations commercially available on the market (Alcock et al., 1999). In recent years, production has decreased in Europe and North America, but it has increased significantly in Asia (e.g. India, China, Taiwan and Japan). Current production in Asia has not been confined to the traditional classification of short‐, medium‐ and long‐chained mixtures, but also includes more diverse products containing congeners from the short‐ to the long‐chained CPs (Shen et al., 2016; Glϋge et al., 2018), with greater attention being paid to chlorine content of the products and less attention being given to carbon chain length. This is because the chlorine content is the most important factor to consider for the intended use of the CPs. A list of some of the manufacturers is available from http://www.eurochlor.org. There can be a wide variation in distribution of carbon chain lengths and chlorine content between different CPs. Commercial names for CPs including historic production, are for example: Arubren CP®, Cereclor®, Cloparin®, Chlorowax®, Essechlor®, FLX‐0012®, Hordaflex®, Paroil®, Ribeclor®, Uniclor® and Witaclor®. The letters and figures used in the commercial names differ from country to country and describe both the average chain length and the chlorine content.
Total world production of all CPs was estimated at 300,000 tonnes in 1985 (WHO/IPCS, 1996) and production has increased considerably since then (Bayen et al., 2006). In the late 1990s, world annual production of SCCPs was about 50,000 tonnes (Marvin et al., 2003). Production of all types of CPs in the European Union (EU) had reached between 1,500 and 2,500 tonnes in 2006 and between 7,500 and 11,300 tonnes per year in the USA. Production of all types of CPs in China was around 100,000 tonnes per year before 2005 but had reached over 600,000 tonnes by 2007 (Fiedler, 2010). Figure 2 shows total estimated production of CPs between 1935 and 2012 (Glϋge et al., 2016).
Figure 2.
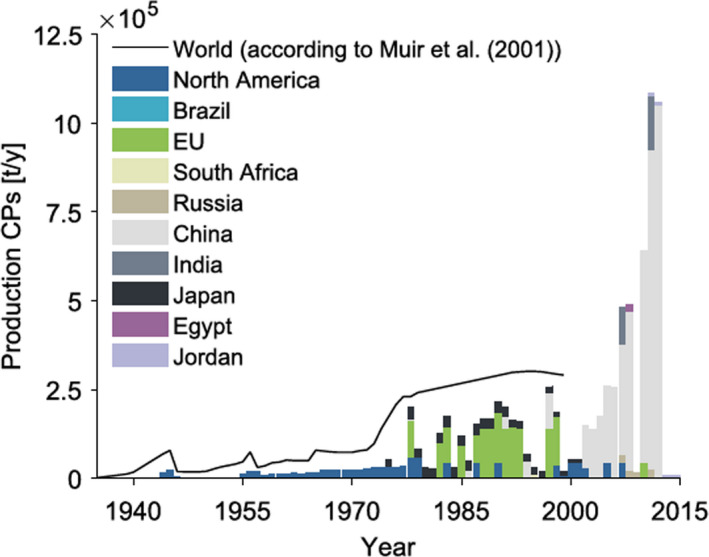
Production of chlorinated paraffins between 1935 and 2012 (from Glϋge et al., 2016, 1)
More recent accurate data is not readily available. Because of this, Glϋge et al. (2016) evaluated an extensive amount of data and reported emission factors, and performed some extrapolations in order to provide estimates. At that time, they concluded that globally, SCCP production and use was increasing. At the same time, worldwide production was estimated as at least 165,000 tonnes per year with global production of total CPs exceeding 1 million tonnes per year. Emissions due to the disposal of waste SCCPs could not be estimated accurately due to lack of essential information.
Glϋge et al. (2018) reported an annual production of 17,800 tonnes of MCCPs in North America (year 1998), 40,000 tonnes in the UK (year 1991) and 27,000 tonnes in Russia (year 2011). No production amounts were reported for Asia since manufacturers do not differentiate between chain lengths, but the authors estimated that the production of MCCPs might have been around 600,000 tonnes in China for the year 2013 (Glϋge et al., 2018).
An estimate derived from the addition of each individual registrant volumes from the European Chemicals Agency (ECHA) shows that the amount of CPs manufactured and/or imported in the European Economic Area is around 10,000–100,000 tonnes per year, but it is difficult to give more precise estimates (C Howick, 2019, Personal Communication).
CPs have been produced since the 1930s for a variety of purposes (Fiedler, 2010; Van Mourik et al., 2015). They are still used as additives in lubricants and cutting fluids in the metal industry and are also used as flame retardants in the rubber industry and in PVC plastics and in sealants (e.g. polysulfide, polyurethane, acrylic and other polymer sealants/adhesives) for use in building, automotive and industrial applications, and the longer chain mixtures are also used as plasticisers in paints and other materials. CPs occupy free volume in the polymer, and screen polar sites on the polymer chains rather than acting as a conventional plasticiser (C Howick, 2019, Personal Communication). They are used in the leather industry as fat liquoring agents, due to the fact that they show better adhesion to the animal skin than natural oils, and yet have similar fattening and softening properties. In the textile industry, highly chlorinated SCCPs can be used in the production of flame‐resistant, water repellent and rot‐preventing textile finishes. These are used for applications such as the production of sail cloths, industrial protective clothing and lorry tarpaulins. CPs with higher chlorine content are more effective as flame retardants as they start to decompose at temperatures above 300°C.
As a result of regulations and concerns about SCCPs, the use of MCCPs has increased and has represented the majority of production over the last decades (Stern and Tomy, 2000; Glϋge et al., 2016, 2018). MCCPs are now being found to dominate some environmental compartments, e.g. in Australian sewage sludge (Brandsma et al., 2017). Glϋge et al. (2018) reported that congeners with a chain length of C14 with medium degree of chlorination (Cl7−8) are the most prevalent MCCPs in the environment followed by MCCPs with C16–17 which can make up to 30% of the total concentrations in the environmental samples in which they could be measured.
1.3.3. Environmental fate and levels
CPs can enter the environment either as a result of production and usage by industry or consumers, or at end of life of products that contain CPs. Largely due to the analytical complexity of measuring these compounds, there is little knowledge about the environmental fate and transport of CPs, in spite of the fact they have been in production and use for so long. The paragraphs below, which do not claim to be complete, give an overview of some aspects related to the environmental fate and levels of CPs.
1.3.3.1. Biodegradation
Biodegradation of SCCPs by soil organisms has been shown for various strains of microorganisms (e.g. Rhodococcus, Pseudomonas) (Wischnak et al., 1998; Allpress and Gowland, 1999; Heath et al., 2006). In general, the biodegradation decreases with increasing chlorine content and is dependent on the carbon chain length (Madeley and Birtley, 1980; Omori and Alexander, 1987; Heath et al., 2006). Madeley and Birtley (1980) used activated sludge to study the biodegradation of SCCPs (49–70% chlorination), MCCPs (40–58% chlorination) and LCCPs (C20–30, 42% chlorination) using an extended biochemical oxygen demand test. SCCPs were shown to be the most biodegradable. Anaerobic microorganisms are less able to perform the biodegradation (Madeley and Birtley, 1980).
Heath et al. (2006) studied the aerobic biodegradation of single mono‐ to hexachlorinated compounds ((1‐chlorodecane, 1,6‐dichlorohexane, 1,10‐dichlorodecane, 1‐chlorohexadecane, 1,2,5,6,9,10‐hexachlorodecane, 1,2,9,10‐tetrachlorodecane) and a MCCP mixture with 52% chlorine (Cereclor S52, C14–17) using a Pseudomonas sp. strain 273. In addition, the authors studied the biodegradation in relationship to the chlorine position, chlorination degree and carbon chain length. The main biodegradation process was dechlorination. The position of the chlorines was of great influence on the biodegradation, being more favourable for CPs that were chlorinated at one of the terminal positions. Substitution with chlorine atoms away from the ends of the molecule reduced the biodegradability, and when chlorine atoms were present at both ends of the molecule, no dechlorination occurred (Heath et al., 2006).
In the study by Omori and Alexander (1987), the biodegradation of SCCP (Toyoparax CP‐265, 63% chlorination), MCCP (Toyoparax C‐145, 43.5% chlorination, Toyoparax CP‐150, 50% chlorination) and LCCP (Toyoparax A‐400, 40.5% chlorination, Toyoparax A‐50, 50% chlorination and Toyoparax A‐70, 70% chlorination) was investigated using different bacterial strains (HK‐3, HK‐6, HK‐8 and HK‐10). The HK‐3 strain showed dechlorination of SCCP (CP‐150), MCCP (CP‐265) and LCCP (A‐400), and the HK‐8 strain only biotransformed SCCP (CP‐150). Mixed strain cultures showed dechlorination of SCCP, MCCP and LCCP (CP‐145, CP‐150, CP‐265 and A‐400). Activated sludge acclimatised to CPs showed little biodegradation.
In the study of Wischnak et al. (1998), the biodegradation of a CP congener (1,10‐dichlorodecane) was studied using the Pseudomonas sp. strain 273. Dechlorination did not occur under anaerobic conditions. The Pseudomonas sp. strain 273 showed biodegradation of 1,10‐dichlorodecane as well as for C9 to C12 chloroalkanes under aerobic conditions.
Li et al. (2019a) studied the uptake, translocation and transformation of four SCCP isomers using whole pumpkin and soybean seedlings via hydroponic exposure. All tested isomers underwent dechlorination and chlorine rearrangement, and carbon chain decomposition products were found for isomers with trichlorinated carbon atoms (CCl3 groups) in both pumpkin and soybean seedlings.
1.3.3.2. Bioaccumulation
An overview of the bioconcentration factors (BCFs) in fish for SCCP and MCCP made by Glϋge et al. (2018) showed no trend between the CP carbon chain length or chlorination degree and BCF. The BCF values for SCCPs in rainbow trout were reported to range from 574 to 7,273 based on parent compound analysis (Madeley et al., 1983a), and in another study with rainbow trout and SCCPs (14C‐labelled study) the BCF was reported to range from 3,600 to 5,300 (Madeley et al., 1983b). A Japanese study reported lipid normalised BCFs values for SCCPs (C13 48.7% chlorination) in common carp. Three main components were determined: C13H23Cl5 (49.8% chlorination), C13H22Cl6 (54.5% chlorination) and C13H21Cl7 (58.4% chlorination) and the BCF values were 1,962 to 2,150, 2,100 to 2,530 and 3,000 and 3,630, respectively (POPRC, 2009).
Fisk et al. (1996, 1998, 2000) performed a number of biomagnification studies in rainbow trout with CPs based on 14C‐radiolabelled compounds (C12H20Cl6, C12H16Cl10, C16H31Cl3, C16H21Cl13). The biomagnification calculations were based on the 14C activity of the whole fish, which therefore include the metabolites and provide maximum biomagnification factors (BMFs). BMFs were corrected for growth dilution and lipid normalisation. The BMFs in the Fisk et al. (1996) study ranged from 0.6 to 0.93 for C12H20Cl6, from 1.76 to 2.15 for C12H16Cl10, from 0.9 to 1.07 for C16H31Cl3 and from 0.44 to 0.72 for C16H21Cl13. No clear trends between the carbon chain length and BMFs were found due to a combination of metabolism and efficiency of uptake. The BMFs for MCCPs ranged from 0.3 to 5 (median 0.99), without a clear trend. The BMFs of LCCPs (C18H31Cl7) was studied by Fisk et al. (2000) using rainbow trout and showed a lipid normalised BMF from 2.1 to 2.8.
Bioaccumulation factors (BAF) from a fish food‐web study in the Great Lakes (US) showed lipid corrected logBAF values for SCCPs from 4.1 to 7.0 for SCCPs, and for MCCPs from 6.3 to 6.8 (Houde et al., 2008). In this study also, the trophic magnification of CPs was studied and showed trophic magnification factors (TMF) for the food webs for SCCP from 0.41 to 2.4, and for MCCP between 0.06 and 0.36. Other TMF studies showed also that the TMF for MCCP is below 1 (NIVA, 2015a,b, 2017; Huang et al., 2017).
In field studies, SCCPs and MCCPs have been found in various organisms ranging from low to higher trophic levels. Limited information is available on the occurrence of LCCPs in food webs. Recent information on the occurrence of CPs in terrestrial birds and mammals in Sweden showed that LCCPs can be found in specific predatory birds, especially peregrine falcons, and CPs up to C30 can be found in the terrestrial environment (Yuan and de Wit, 2018).
A study by Cui et al. (2019) investigated the bioaccessibility of SCCPs in meat and seafood. The bioaccessibility percentages ranged from 33% to 84%, and the authors suggested that factors such as protein and lipid content had an influence on the observed values.
1.3.3.3. Occurrence in the outdoor environment and wildlife
Only few data exist and it is difficult to draw trends and/or conclusions for differences at a congener level. Most data exist for SCCPs, with few data for MCCPs and very little if any data at all in some matrices for LCCPs.
Chen et al. (2019) studied the global environmental fate of SCCPs. The environmental concentrations and compartmental distribution of SCCPs were simulated using a mechanistic fugacity‐based multimedia model. A discrepancy between modelled concentrations based on a single and multiple sets of properties was observed, and was more evident in regions with colder climate and lower pollution levels, such as the Arctic. The model tended to underestimate the environmental occurrence and environmental risk in remote and cold regions, and did not allow conclusions on which SCCP component is of greatest concern. The authors found that treating tens of SCCP homologues as a whole and representing them using a single set of properties minimised the information required for modelling, but resulted in a bias when estimating the environmental occurrence of SCCPs in remote background areas. It was therefore not possible to determine which SCCP homologue poses the greatest concern.
Outdoor air
SCCPs and MCCPs have been measured in air, and levels have been found to be highest in Asia, with peak concentrations of MCCPs measured in China with up to 360,000 pg/m3 (Wang et al., 2013). Lowest concentrations have been found in polar regions with levels in Europe somewhere between the two. However, data is scarce and not available at all for some global regions and therefore, care needs to be taken with this conclusion (Glϋge et al., 2018).
Zhou et al. (2019b) analysed 66 outdoor air samples with particulate matters (PMs) of 100, 10 and 2.5 μm. The samples were taken from 24 homes located near the Pearl River Delta, China. The smallest particles were found to be dominant (93.0%) and CPs were mainly present in that fraction. The mean concentrations of MCCPs exceeded the SCCPs (22.0 vs. 9.2 ng/m3) in all particle samples.
Li et al. (2019b) investigated seasonal variations and inhalation of SCCPs in PM2.5 in Jinan, China. Concentrations of SCCPs ranged from 9.80 to 105 ng/m3, with a mean value of 38.7 ng/m3. The highest concentrations of SCCPs were found in winter months, and the lowest values were found in summer.
Surface water, effluents and sludge of municipal waste water treatment plants (WWTPs)
For surface water, there are a few data from Norway and Canada (Lake Ontario and the Arctic) with levels of both SCCPs and MCCPs typically from around 10 to over 100 ng/L (Glϋge et al., 2018).
Effluents and sludge of municipal WWTPs can contain CPs. In general, the concentrations of MCCPs are dominant in sludge from WWTPs. Sewage sludge from Swedish WWTPs collected between 2004 and 2010 showed a median concentration for SCCPs of 1,100 ng/g dry weight (dw) and for MCCPs a median concentration of 3,800 ng/g dw (Olofsson et al., 2012). Also in sludge from WWTPs in the Czech Republic, collected near a chemical company (Přibylová et al., 2006), Switzerland (Zürich) (Bogdal et al., 2015) and Australia (Brandsma et al., 2017), the MCCP concentrations were higher than SCCPs. Higher SCCP concentrations than MCCPs have been found in sludge from municipal and industrial WWTPs in China (800–52,700 ng/g dw, Zeng et al., 2012) and the UK (SCCPs: 6,900–200,000 ng/g dw, MCCPs: 30–9,700 ng/g dw, Stevens et al., 2003). Nicholls et al. (2001) reported for several regions in the UK levels for the sum of SCCP and MCCPs ranging from 1,800 to 93,100 ng/g dw.
Two studies analysed LCCPs in sludge (Olofsson et al., 2012; Brandsma et al., 2017). Samples from Swedish sewage sludge contained a median LCCP concentration of 31,000 ng/g dw, and in the Australian study the median concentration of LCCPs was about 300 times lower.
Soil and sediments
For soil and sediment, levels of SCCPs and MCCPs are typically from around 10 to over 100 ng/g dw, although higher levels tend to be present in sediment from Asia, and in samples taken from close to known sources where concentrations up to around 10,000 ng/g dw have been found (Glϋge et al., 2018).
Birds
Concentrations of SCCPs and MCCPs in bird eggs and bird tissue have been reported to be broadly similar to those collected from the Arctic and Norway, and contained similar levels of SCCPs and MCCPs, ranging from around 2 to 170 ng/g fat, but lower than concentrations in samples from China (Glϋge et al., 2018). Levels in blood and muscle/liver were also similar for both SCCPs and MCCPs and were around an order of magnitude higher (20−1,700 ng/g fat).
Mammals
A few studies exist on CPs in mammals, again showing similar concentrations for SCCPs and MCCPs. Data is available for ringed seals and polar bears from the Arctic, and finless porpoise, Indo‐Pacific humpback dolphins and yellow weasels from China. Levels are typically an order of magnitude higher in samples from China when compared with the Arctic, and are higher for the yellow weasel followed by dolphin species, porpoises, polar bear and lowest concentrations in the ringed seal (Glϋge et al., 2018). Samples taken in 2014 were significantly higher than those taken 10 years earlier, and some samples exceeded 50,000 ng/g fat for SCCPs and MCCPs.
Fish
Concentrations of SCCPs and MCCPs in fish from the Arctic, Canada, Europe and north‐eastern China are all similar and range between around 10 and 700 ng/g fat. SCCP and MCCP levels are higher in samples from the South China Sea and from paddy fields in the Yangtze River Delta in South East Asia, where concentrations were up to around 2,600 ng/g fat. MCCP concentrations in Arctic fish from a lake on Bear Island were in general higher than those found in fish elsewhere in Canada and in Europe. Given the remote location of this island, the contamination was explained by two possible reasons (Evenset et al., 2004). The lake is located in mountains and receives heavy rainfall, and large seabird colonies use the lake as resting area, resulting in large amounts of guano that can cause an increase in concentrations of persistent organic pollutants (POPs) in general. The contamination of this remote area supports the conclusions that MCCPs are able to undergo long‐range atmospheric transport (Glϋge et al., 2018).
The occurrence of CPs in fish samples from European markets is reported in Section 3.2.2 . Krätschmer et al. (2019) determined the levels of SCCPs and MCCPs in salmon samples from German supermarkets and reported the SCCP and MCCPs homologue profiles of wild and farmed salmon samples from different geographic origins (see Figure 3 ). The homologue profiles in samples from Europe (Norway, Denmark and Scotland, n = 95) were similar and showed different CP profiles compared to farmed salmon from Chile and wild catch salmon from the Pacific. While European farmed salmon was characterised mainly by high‐chlorinated C11Cl9–11 (13% of the total SCCP and MCCP content), Chilean farmed salmon showed higher levels of lower chlorinated C10Cl5–7 (27.6% of the total SCCP and MCCP content). In Chilean salmon, the contribution of MCCPs to the total CP content was lower than in the European samples. Wild salmon presented CP profiles similar to the Chilean farmed salmon. Differences were also observed between the two types of wild salmon analysed: hump salmon (higher contribution and Cl range of C11) and wild chum salmon (higher abundance of C10). The authors reported that the CP profiles can be influenced by habitat and feed, and wild catch can be subject to several more influences including age, season of catch or local contamination hotspots (Krätschmer et al., 2019).
Figure 3.
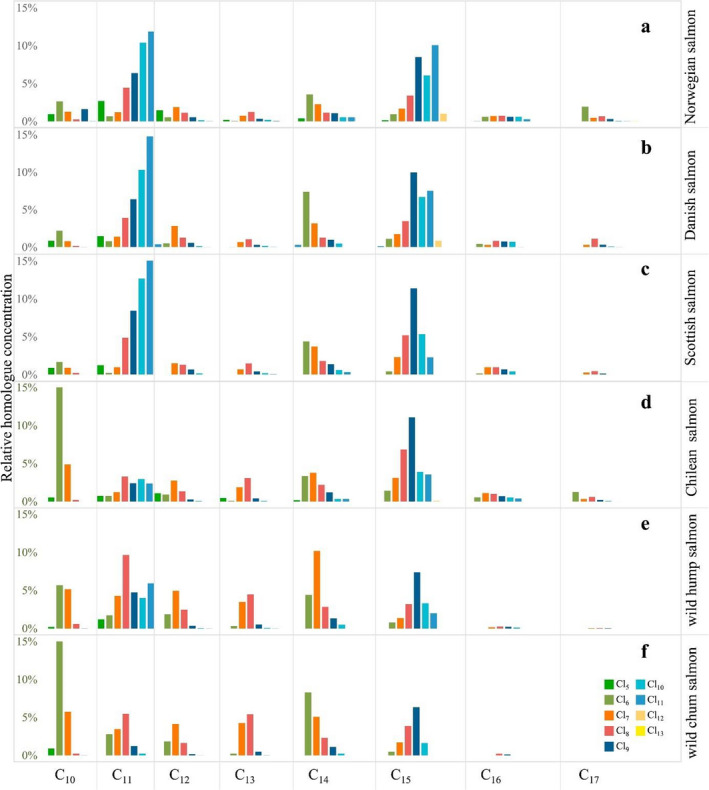
Arithmetic means of the relative homologue group concentration for short‐chain chlorinated paraffins and medium‐chain chlorinated paraffins in Norwegian, Danish, Scottish and Chilean farmed salmon and in different Eastern Pacific wild salmon (from Krätschmer et al., 2019, 2)
The data generated for the above studies were obtained in some cases with techniques using low‐resolution mass spectrometry (LRMS). Congener profiles are therefore considered as more qualitative than quantitative, making congener profiles difficult to assess.
1.3.3.4. Occurrence in the indoor environment
Indoor air
In a Swedish study, indoor air and house dust was sampled in apartments and SCCPs and MCCPs were analysed (Fridén et al., 2011). Concentrations of the sum of SCCPs and MCCPs ranged from < 5 to 210 ng/m3 (Fridén et al., 2011).
Zhou et al. (2019b) analysed 72 indoor air samples with particulates classed as PM100, PM10 and PM2.5. They were taken from 24 homes located near the Pearl River Delta, China. The smallest particles were found to be dominant (89.1%), and CPs were mainly present in that fraction. The mean concentrations of MCCPs exceeded the SCCPs (22.0 vs. 9.2 ng/m3) in all particle samples. Both SCCPs and MCCPs in the indoor particles (mean 13.4 and 30.9 ng/m3) were approximately twice as high as in the outdoor environment (mean 6.1 and 15.2 ng/m3). C11 and C14 chain lengths and six and seven chlorine containing CPs were found to be predominant.
Dust
Levels of CPs in indoor dust were reviewed by Coelhan and Hilger (2014). Concentrations of SCCPs were reported ranging from < 5 to > 2,000 μg/g from a number of different countries and environments. Concentrations of MCCPs ranged from 36 to 892 μg/g but were only measured in a few of the studies. Other reports of CPs in dust include one study where CPs were found in house dust from Germany, Taiwan and Sweden. Concentrations ranged from 1,200 to 892,000 ng/g house dust (Fridén et al., 2011; Hilger et al., 2013; Chen et al., 2016). The German study showed that the concentrations of SCCPs were lower (4,000–27,000 ng/g) than the concentrations of MCCPs (8,000–892,000 ng/g). Wong et al. (2017) determined SCCPs, MCCP and also LCCPs in dust samples from offices homes and non‐residential buildings from Australia, UK, Canada, Sweden and China. The highest CP levels were found in dust from China (mean of 3,044 μg/g). The levels in the other countries ranged from 280 to 1,330 μg/g. MCCPs were the predominant in all samples (accounting between 41% to 65% of the total CPs), except in the Swedish samples, in which LCCPs accounted for 84% of the total CP content. Compared to other flame retardants analysed in the same samples, the levels of CPs were significantly higher. CPs were also detected in house dust sample from Canada with median (range) concentrations of 6.2 (4.0–57) μg/g and 19 (5.9–901) μg/g for SCCPs and MCCPs, respectively (Shang et al., 2019).
Within the SHINE project,3 SCCPs, MCCPs and LCCPs in office and house dust collected between 2016 and 2017 from the Netherlands, Belgium, Sweden and Ireland were analysed by atmospheric pressure chemical ionisation‐mass spectrometry (APCI‐MS) (P Leonards, Personal communication, 2019). Concentrations of SCCPs ranged from 27 to around 55,000 ng/g, with the highest levels found in the Swedish house and kindergarten dust samples. The levels of MCCPs were higher in all dust samples, and ranged from 190 to around 349,000 ng/g. LCCPs were reported at lower concentrations, ranging from < 1 to around 9,000 ng/g. The highest median values found in dust from the four European countries were around 6,300 ng/g SCCPs; 35,000 ng/g for MCCPs and 2,700 ng/g for LCCPs.
Kitchen equipment
A limited number of studies considered the occurrence of CPs in the kitchen (Bendig et al., 2013; Gallistl et al., 2018). In fat of kitchen hoods, CPs have been found with a median concentration of 2,500 ng/g fat). SCCPs were found in all samples (n = 15), and MCCPs were found in 20% of the samples. The sum of SCCP and MCCPs ranged from 140 to 15,000 ng/g fat. In a follow up study, fat collected in the inner surface of 21 baking ovens from Germany showed that CPs in the mg/g fat range were detected in about 50% of the ovens. MCCP concentrations exceeded those for SCCPs, which were found in only three out of the 21 ovens contained SCCPs. The source of MCCP was found to be the components of the inner site of the oven casing. The mean concentration of MCCP was 12.6 mg/g fat (expressed here as 12,600,000 ng/g fat in order to be consistent with units used for occurrence data), with the highest concentration of 93,200,000 ng/g fat.
1.3.4. Sampling and methods of analysis
Sample preparation
The sample preparation of CPs consists of extraction, clean‐up and fractionation steps before the final analysis. The extraction step should be able to extract the CPs which have a wide range of physicochemical properties (e.g. log Kow 5–12). Extraction is often performed with a Soxhlet or accelerated organic solvent extraction (ASE) for solid materials (e.g. tissues, sediment), and for dust also ultrasonic extraction can be used (Van Mourik et al., 2015). Solid phase extraction (SPE) or liquid–liquid (LLE) extraction is mainly used for water samples, and polyurethane foam in combination with a filter to extract CPs from air (Van Mourik et al., 2015). For the sediment, soil and biological samples, a clean‐up step is needed to remove extract matrix such as lipids, humic acid and sulfur‐containing compounds that interfere with the final analysis. For lipid removal, various adsorbents can be used such as sulfuric acid silica gel, sulfuric acid treatment, or aluminium oxide. For sediments and soil, sulfur‐containing compounds can be present in large amounts and should be removed using, for instance, activated copper or gel permeation chromatography. Some studies use a multi‐layer column consisting of activated silica gel and/or florisil and silica gel with sulfuric acid, and/or silica gel with sodium hydroxide. A highly important step in the sample treatment is the fractionation step to remove interfering compounds such as polychlorinated biphenyls (PCBs) and some pesticides (e.g. toxaphene) that can have similar retention times or mass‐to‐charge (m/z) ratios as the CPs if for instance electron capture detection (ECD) or LRMS are used for the detection of CPs. For the fractionation, mainly adsorption chromatography is used such as silica gel and/or alumina. For high‐resolution MS (HRMS), the fractionation step is not necessary as the high‐resolution power of the MS is able to separate the interfering compounds from the CPs.
A lack of analytical standards is a key factor in hindering the development of improved methods (Schinkel et al., 2018).
Analytical techniques
Due to the complexity of the mixtures, there are a number of challenges in the analysis of CPs, especially their characterisation and determination. An overview of different techniques used for the analysis of CPs is given in Table 3 . An example of a chromatogram illustrating the complexity of the separation and measurement of SCCPs and MCCPs is shown in Figure 4 (Krätschmer, 2019).
Table 3.
Different separation and detection techniques for chlorinated paraffins. Information on the sensitivity, selectivity, detection of isomers and calibration are provided
| Technique | Sensitivity | Selectivity | Detection of SCCP, MCCP, LCCP | Chlorine content of isomers | Carbon length chlorine distribution | Calibration (response factors) |
|---|---|---|---|---|---|---|
| GC‐ECNI‐LRMS | High | Moderate | SCCP, MCCP | ≥ Cl5 | Yes | Depending on chlorination degree |
| GC‐ECNI‐HRMS | High | High | SCCP, MCCP | ≥ Cl5 | Yes | Depending on chlorination degree |
| GC‐MS/MS (EI mode) | High | Low, much fragmentation | SCCP, MCCP | No congener‐ and homologue‐specific information | Not possible | Less critical for differences in chlorine content and carbon chain length |
| LC‐APCI‐qTOF‐MS (HRMS) | High | High | SCCP, especially sensitive for MCCP and LCCP | Cl2–Clx | Yes | Less critical for differences in chlorine content |
| GCxGC‐TOF‐MS (ECNI) | High | High | SCCP, MCCP | ≥ Cl5 | Yes | Depending on chlorination degree |
| GCxGC‐ECD | High | High | SCCP, MCCP, (LCCP) | Cl2–Clx | Yes | Depending on chlorination degree |
| GC‐FID and GC‐MS (carbon skeleton method) | Moderate | High – assuming alkanes are removed during sample clean‐up | SCCP, MCCP, LCCP | No congener‐ and homologue‐specific information | No, only carbon length | Good calibration based on hydrocarbon number only |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins; APCI: atmospheric pressure chemical ionisation; ECD: electron capture detector; ECNI: electron capture negative ionisation; FID: flame ionisation detector; GC: gas chromatography; LRMS: low‐resolution MS; GC×GC: comprehensive two‐dimensional gas chromatography; HRMS: high‐resolution MS; MS: mass spectrometry; TOF‐MS: time‐of‐flight MS.
Figure 4.
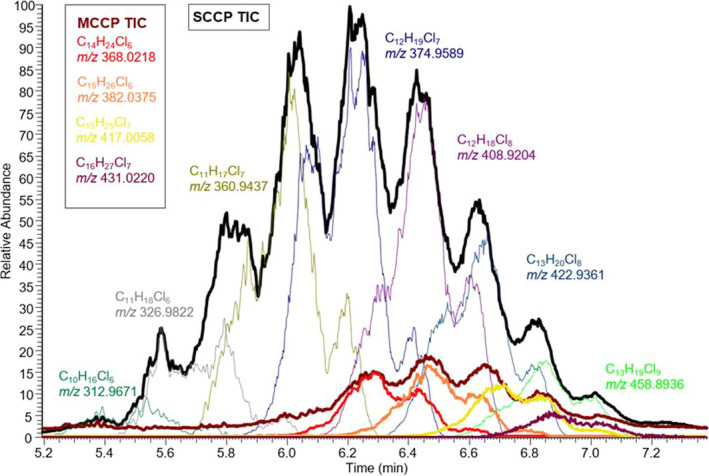
Example of a GC‐ECNI‐Orbitrap‐HRMS chromatogram illustrating the total ion count (TIC, black line) and mass‐to‐charge ratios (m/z, coloured lines) for a few selected short‐chain chlorinated paraffins (SCCPs) and medium‐chain chlorinated paraffins (MCCPs) homologues (Krätschmer, 2019)
In general, the techniques are not able to completely separate and detect isomeric groups, and individual congeners cannot be reported. There is no ideal technique that can be used for analysis of CPs; all available methods have limitations and are a compromise in some aspect, and thus can generate different results.
The most commonly used technique is gas chromatography (GC) combined with LRMS in the electron capture negative ionisation (ECNI) mode (e.g. Reth et al., 2005) or high‐resolution MS (HRMS) (e.g. Tomy et al., 1997). The ECNI technique is a sensitive technique for the detection of CP congeners with ≥ Cl5; however lower chlorinated congeners are hardly detected (Zencak et al., 2005). In practice, only congeners with ≥ Cl5 are reported with this technique. It should be noted that some CP products can have a relatively large number of congeners with < Cl5, which shows the importance of other detection techniques to provide information on the chlorine distribution. Another drawback of ECNI is the decrease in sensitivity from SCCPs, to MCCPs to LCCPs, where LCCPs are almost undetectable. If LRMS is used, interfering compounds such as PCBs with the same nominal mass, should be separated from the CPs using fractionation before the final analysis.
Other less frequently used techniques such as ECD especially in combination with comprehensive two‐dimensional gas chromatography (GC×GC) (Korytár et al., 2005; Xia et al., 2014) are able to detect the lower chlorinated congeners. The advantage of GC×GC‐ECD is the high separation power of CP isomers and the detection of lower chlorinated CPs (Cl2–Cl4). Another advantage is the group separation of CP isomeric, and the analysis of SCCPs, MCCPs and LCCPs simultaneously in one run. This technique is not used by many laboratories because of the relative complicated optimisation and time‐consuming quantification (Van Mourik et al., 2015). The disadvantage of the ECD is that SCCP, MCCP and LCCP groups can overlap if single GC is used, or other halogenated compounds can interfere with the analysis. GC×GC also has been combined successfully with low to moderate resolution time of flight (TOF)‐MS for the analysis of CPs in the ECNI mode (Korytár et al., 2005; Xia et al., 2016). However, GC×GC‐ECNI‐TOF‐MS has insufficient sensitivity to measure the lower chlorinated CPs (< Cl5). An alternative method is the carbon skeleton method coupled with GC‐flame ionisation detector (FID) (Cooke et al., 1980) and MS detector (Pellizzato et al., 2009a). This method dechlorinates the CPs to alkanes and quantification is performed with n‐alkanes. This reduces the calibration problems found with ECNI as the FID detector response is independent of the chlorine content (see below). The disadvantage is that no information on the chlorine content can be provided, and this is important for the hazard and risk assessment.
Other methods have been developed including GC combined with triple quadrupole MS (GC‐MS/MS), novel APCI‐TOF‐MS using CP pattern matching and deconvolution algorithms, and GC‐Orbitrap. A promising analytical method using liquid chromatography (LC) coupled to APCI–TOF‐HRMS was developed (Bogdal et al., 2015). This enables the use of an analytical column to be avoided, since the extract is directly injected into a qTOF‐MS system followed by quantification of CPs by mathematical deconvolution using technical CPs. This technique can also detect the lower chlorinated congers (Cl2–Cl4), and SCCPs, MCCPs and LCCPs can simultaneously be analysed in one single run. In recent interlaboratory studies, the APCI‐TOF method showed good results (see below).
Another challenge in CP analysis is the reliable determination of CPs, which is important for assessing the persistence, bioaccumulation and hazard assessment of these compounds. Currently, only semi‐quantitative analysis is possible due to a lack of proper analytical standards and the complexity of data analysis and processing. Using the novel techniques described above and using pattern matching and deconvolution, accuracy and precision are improving. One significant problem with the determination of CPs is that the only available analytical standards do not match the chlorine configuration of the congeners present in the technical CPs mixtures. Commercial standards contain chlorine atoms at the end positions of the carbon chain while the CPs present in the technical mixtures mainly have chlorines in the middle of the chain. For many years, commercial companies have tried to synthesise individual CPs with chlorine atoms in the mid position and some of them have been made available. No information was available concerning the response factors of these congeners.
In contrast to the low number of analytical standards, over the last 10 years globally more than 200 CP formulations have been used in various applications globally (Zeng et al., 2015). Each of these formulations contain CPs with different carbon length and chlorination degree.
Also because of the fact that during weathering, biotransformation, metabolism and uptake, the carbon length and chlorination degree of the technical CP mixtures that are present may change as degradation occurs (see Section 1.3.3 ). There are no suitable analytical standards available and it is challenging even to find commercial technical CPs that may be suitable as an inferior method for the quantification of CPs in environmental and food samples.
Analytical quality assurance
Several interlaboratory studies have been carried out using samples of different complexity (analytical standards, sample extracts and real samples) (Tomy et al., 1999; Pellizzato et al., 2009b; Van Mourik et al., 2015). These studies show that the accuracy and precision is improving. The variation between laboratories can be more than 100% when the most commonly used technique, GC‐ECNI‐MS, is used (Van Mourik et al., 2015). The results of a first interlaboratory study showed that the variation in the analysis of a standard solution was between 22 and 56%. If real life samples (unspiked fish extract) were analysed the variation increased to 137%, and the results fell in two groups which had a difference of about 10‐fold in concentration. This variation is probably due to the different types of analytical techniques used to quantify CPs (Van Mourik et al., 2015).
An overview of the SCCP 2011–2017 Quasimeme worldwide interlaboratory studies is given in Table 4 . In the last round, five different analytical techniques were used (Van Mourik et al., 2018). The participants (overall 33 laboratories from 14 countries) analysed test solutions to various matrices and the relative standard deviations (RSDs) varied between 23 and 137% of these materials. The RSD of the test solution in the last round (25%) was acceptable. Interestingly, the results with GC‐ECNI‐MS varied the most, and those obtained with newer techniques (APCI‐qTOF‐MS) showed good agreement between the consensus values and the true value.
Table 4.
Overview of Quasimeme interlaboratory studies (from Van Mourik et al., 2018)
| Round (year) | Provided sample (extract) | Provided sample preparation | Exercise |
|---|---|---|---|
| 1 (2012) | Test solution | SCCPs in isooctane | Quantify |
| 2 (2014) | Fish | PLE + Al2O3 + silica | Quantify |
| 3 (2015) |
a. Sediment b. Sediment |
a. PLE + acid silica + SILICA b. PLE only |
a. Quantify b. Clean‐up and Quantify |
| 4 (2017) |
a. Test solution b. House dust c. Soil d. Fish |
a. SCCPs in isooctane b. PLE + acid silica + silica 2x c. PLE + acid silica + silica 2x d. PLE + acid silica + silica 2x |
a. Quantify b. Quantify c. Quantify d. Quantify |
PLE: pressurised liquid extraction; SCCPs: short‐chain chlorinated paraffins.
Between 2017 and 2019, the two first rounds of an interlaboratory study on the determination of CPs in food samples was organised by the EU Reference Laboratory (EU‐RL) for Halogenated POPs in Feed and Food (Krätschmer and Schächtele, 2019). The first round consisted of samples of coconut fat spiked with SCCP and MCCP standards, and the second round consisted of lard samples spiked with SCCPs and MCCPs mimicking homologue profiles commonly found in fish, and to which PCBs and PBDEs were added to some of the test samples to reflect the content of real food samples. More than 55% of the participating laboratories (18 and 9 participants in the first and second round, respectively) had a satisfactory overall performance. The authors reported that the assigned z‐scores for the different samples suggest difficulties in the correct quantification of SCCPs in the presence of MCCPs, a situation likely to reflect real food samples. HRMS and LRMS were both reported to perform satisfactorily. The authors identified as the main factors influencing the quality of the results, the choice of calibration standards, the instrument and type of quantification/data processing method. Further rounds of this interlaboratory study are planned for 2019–2020.
1.3.5. Previous assessments
Several international bodies have carried out hazard or risk assessments related to CPs. These assessments evaluated in a number of cases the environmental risks as well as the risk for human health. The latter are summarised below and in Tables 5 (for SCCPs), 6 (for MCCPs), 7 (for LCCPs) and 8 (all three groups combined). Existing classifications for SCCPs, MCCPs and LCCPs are summarised in Table 9 .
Table 5.
Previous assessments on short‐chain chlorinated paraffins (C10–13)
| Organisation | TDI (μg/kg bw per day) | NOAEL (mg/kg bw per day) | Critical endpoint (s) | Relevant Study | Study reference | Uncertainty factor | Remarks |
|---|---|---|---|---|---|---|---|
| WHO/IPCS (1996) |
100 (for non‐neoplastic effects) |
10 | ↑ liver and kidney weights and hypertrophy of the liver and thyroid | 90‐day rat study | IRDC (1984)a | 100 | Uncertainty factor for intra‐ and interspecies variation (10 × 10) |
| WHO/IPCS (1996) |
11 (for neoplastic effects) |
11 (the estimated dose associated with a 5% increase in tumour incidence) | hepatocellular adenomas or carcinomas (combined) | 2‐year mouse study | NTP (1986a), Bucher et al. (1987) | 1,000 | Uncertainty factor for non‐genotoxic carcinogen |
| OECD (2000) | Not derived | 100 | ↓ body weight gain and increased kidney weight | Rat studies | n.a. | n.a. | – |
| EU‐RAR (2000) | Not derived |
100 500 |
General non‐specific toxicity Developmental effects |
Rat and mouse studies |
n.a. n.a. |
n.a. | – |
| Health Canada (2008) | Not derived | 100 (LOAEL) |
↑ liver and kidney weights and hypertrophy of the liver and thyroid |
90‐day rat study | IRDC (1984)b | n.a. | – |
| UK‐COT (2009) | 30 | 10 | ↑ kidney weight, mild nephritis in males and brown pigmentation in renal tubules in females | 90‐day rat studies | As cited in EU‐RAR (1999)c | 300 | Uncertainty factor for intra‐ (10) and interspecies (10) variations, and an additional uncertainty factor of 3 for a non‐chronic study |
NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; TDI: tolerable daily intake; bw: body weight.
IRDC, 1984. 13‐week oral (gavage) toxicity study in rats with combined excretion, tissue level and elimination studies: determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 58% chlorination of short‐chain length n‐paraffins; 14C‐labelled CP. Mattawan, Michigan, International Research and Development Corporation, 350 pp (Report No. 438‐029/022). As cited in WHO‐IPCS (1996). Note: Study report also provided to EFSA (IRDC, 1984b, see Documentation provided to EFSA).
IRDC, 1984. 13‐week dietary toxicity study in rats with combined excretion, tissue level and elimination studies/determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 70% chlorination of long‐chain length n‐paraffins; 14C‐labelled CP. Mattawan, Michigan, International Research and Development Corporation, 316 pp (Report No. 438‐027/024). As cited in Health Canada (2008). Note: Study report also provided to EFSA (IRDC, 1984e, see Documentation provided to EFSA).
ECB (European Chemicals Bureau), 1999. Alkanes, C10–13, chloro. European Union Risk Assessment Report. As cited in COPT (2009). Note: report cited in this opinion as EU‐RAR (2000).
Table 6.
Previous assessments on medium‐chain chlorinated paraffins (C14–17)
| Organisation | TDI(μg/kg bw per day) | NOAEL(mg/kg bw per day) | Critical endpoint(s) | Relevant study | Study reference | Uncertainty factor | Remarks |
|---|---|---|---|---|---|---|---|
| WHO/IPCS (1996) | 100 | 10 | ↑ liver and kidney weight | 90‐day rat study | IRDC (1984)a | 100 | Uncertainty factor for intra‐ and interspecies variations (10 × 10) |
| EU‐RAR (2011) | Not derived | 2310047 | ↑ kidney weight,neoplastic effectsMaternal effects – for risk characterisation of haemorrhaging effectsMaternal effects – for risk characterisation of breastfeeding mothers | Rat studies | n.a. | ||
| Health Canada (2008) | Not derived | 4.2 (LOAEL) | Mild effects on the kidney and thyroid of female rats | 90‐day rat study | Poon et al. (1995) | n.a. | |
| UK‐COT (2009) | 4 | 4 | Changes in relative liver weight and minimal changes in the inner cortex of the kidney in female rats | 90‐day rat study | As cited in EU‐RAR (2004)b | 1,000 | Uncertainty factor for intra‐ and interspecies variations (10 × 10), and an additional uncertainty factor of 10 to allow for the absence of longer term studies and the lack of a NOAEL for reproductive and developmental effects |
| BauA (2011) | Not derived | 23 | ↑ kidney weight | 90‐day rat study | CXR (2005)c | n.a. | For derivation of the NOAEL: Uncertainty factor for intra‐ and interspecies variations (5 × 1), and an additional uncertainty factor of 2 for subchronic to chronic duration. Allometric scaling applied for conversion of dose in the rat study to the human situation |
| US‐EPA (2015) | not derived | 23 | ↑ kidney weight | 90‐day rat study | CXR (2005)d | n.a. |
NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; TDI: tolerable daily intake; bw: body weight.
IRDC, 1984. 13‐week oral (dietary) toxicity study in rats with combined excretion, tissue level and elimination studies: determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 52% chlorination of intermediate‐chain length n‐paraffins, 14C‐labelled CP. Mattawan, Michigan, International Research and Development Corporation, 328 pp (Report No. 438‐023/026). As cited in WHO/IPCS (1996). Note: Study report also provided to EFSA (IRDC, 1984c, see Documentation provided to EFSA).
ECB (European Chemicals Bureau), 2004. Alkanes, C14–17, chloro. Draft European Union Risk Assessment Report. Note: report cited in this opinion as EU‐RAR (2011).
CXR, 2005. A dietary study to determine the 90 day NOAEL of medium‐chain chlorinated paraffins *Cereclor S52(in male and female Fisher 344 rats. CXR0273. CXR Bioscience Ltd, Dundee, UK. Unpublished report. As cited by BauA (2011). Note: Study report also provided to EFSA (CXR, 2005b, see Documentation provided to EFSA).
Table 7.
Previous assessments on long‐chain chlorinated paraffins (C18–26)
| Organisation | TDI (μg/kg bw per day) | NOAEL (mg/kg bw per day) | Critical endpoint(s) | Relevant study | Study reference | Uncertainty factor | Remarks |
|---|---|---|---|---|---|---|---|
| WHO/IPCS (1996) | 100 | 100 (LOAEL) | Multifocal granulomatous hepatitis and increased liver weight in female rats | 2‐year rat study | NTP (1986b); Serrone et al. (1987); Bucher et al. (1987) | 1,000 | Uncertainty factor for intra‐ and interspecies variations (10 × 10), and an additional factor of 10 for LOAEL instead of NOAEL |
| NRC (2000) | 300 (RfD) | 900 |
C22–26, 70% chlorination: liver toxicity (↑ relative liver weight, hepatocellular hypertrophy, cytoplasmic fat vacuolation, ↑ in serum ALT and AST activities) and kidney toxicity (a slight ↑ in the incidence of nephritis in males) |
90‐day rat study | Serrone et al. (1987) | 3,000 | A factor of 3 (instead of 10) for interspecies variation because toxicokinetics and dynamics of LCCPs were anticipated to be similar in rodents and humans, a factor of 10 for intraspecies variation, a factor of 10 to account for less‐than‐lifetime toxicity data, a factor of 10 because the toxicity database for the LCCPs (C22–26, 70% chlorination) was incomplete |
| Health Canada (2008, 2012) | 71 | 100 (LOAEL) | Diffuse lymphohistiocytic inflammation in the liver and in the pancreatic and mesenteric lymph nodes in female rats | 2‐year rat study | NTP (1986b) | 1,000 | Uncertainty factor for intra‐ and interspecies variations (10 × 10), an additional factor of 10 for LOAEL instead of NOAEL and a factor 5/7 for conversion of 5 days/week administration to daily exposure |
| UK‐Environment Agency (2009) | Not derived |
C22–26, 43% chlorination: 100 (LOAEL) C23, 43% chlorination: 100 (LOAEL) C20–26, 70% chlorination: 900 (NOAEL) C18–20: 23 (NOAEL), based on read‐across from MCCP |
Liver changes in females at all treatment levels Microscopic changes in the liver and lymph nodes Effects on body weight gain, food consumption and the liver |
90‐day rat study 2‐year rat study 90‐day rat study |
IRDC (1984)a NTP (2016) IUCLID (2003) |
n.a. | For the risk characterisation, it was suggested to divide the LOAEL by 3 to provide an estimate of the NOAEL |
| US‐EPA (2015) | Not derived | 100 (LOAEL) | Granulomatous inflammation of the liver in female rats | 2‐year rat study | NTP (1986b) | n.a. |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; TDI: tolerable daily intake; bw: body weight.
IRDC, 1984. Chlorinated paraffin: 43 per cent chlorination of long‐chain length n‐paraffins. 13‐week day oral toxicity study in rats, with combined excretion, tissue level and elimination study. IRDC report 438‐028/438‐021. As cited in UK‐Environment Agency (2009). Note: Study report also provided to EFSA (IRDC, 1984d, see Documentation provided to EFSA).
Table 8.
Group assessment of short‐chain chlorinated paraffins, medium‐chain chlorinated paraffins and long‐chain chlorinated paraffins
| Organisation | TDI (μg/kg bw per day) | NOAEL (mg/kg bw per day) | Critical endpoint | Relevant study | Study reference | Uncertainty factor | |
|---|---|---|---|---|---|---|---|
| Nielsen and Ladefoged (2013)a | 100 (group TDI) | 10 | Effects in the liver, kidney and thyroid as well as in developing offspring | – | several studies | 100 | For intra‐ and interspecies variations |
NOAEL: no‐observed adverse effect level; TDI: tolerable daily intake; bw: body weight.
The assessment was finalised in 2006 and was published in 2013.
Table 9.
Existing carcinogenicity evaluations for short‐chain chlorinated paraffins (SCCPs) and long‐chain chlorinated paraffins (LCCPs)
| Organisation | Outcome | Remarks |
|---|---|---|
| SCCPs | ||
| NTP (1986a, 2016) |
C12, 60% chlorination: Clear evidence of carcinogenicity for F344/N rats and for B6C3F1 mice (NTP, 1986a) Reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals (NTP, 2016) |
|
| IARC (1990) |
C12, 60% chlorination: Group 2B (possibly carcinogenic to humans) |
There is sufficient evidence for the carcinogenicity in experimental animals. No data were available from studies in humans |
| LCCPs | ||
| Organisation | Outcome | Remarks |
| NTP (1986b) |
C23, 43% chlorination: No evidence of carcinogenicity for male F344/N rats, equivocal evidence of carcinogenicity for female F344/N rats, clear evidence of carcinogenicity for male B6C3F1 mice, equivocal evidence of carcinogenicity for female B6C3F1 mice |
|
| IARC (1990) |
C23, 43% chlorination: No IARC Group assigned |
There is limited evidence for the carcinogenicity in experimental animals. No data were available from studies in humans |
No assessments related to the risk for farm animals, horses and companion animals have been identified.
1.3.5.1. SCCPs
The National Toxicology Program (NTP) has conducted toxicology and carcinogenesis studies of SCCPs (C12, 60% chlorination) in rats and mice (NTP, 1986a). NTP concluded that there was clear evidence of carcinogenicity for F344/N rats. This was based on increased incidences of hepatocellular neoplasms (primarily neoplastic nodules) in male and female rats, of adenomas or adenocarcinomas (combined) of the kidney tubular cells in male rats, and of follicular cell adenomas or carcinomas (combined) of the thyroid gland in female rats; and for B6C3F1 mice as shown by increased incidences of hepatocellular adenomas and of adenomas or carcinomas (combined) in male and female mice and increased incidences of adenomas and of adenomas or carcinomas (combined) of thyroid gland follicular cells in female mice. NTP concluded that ‘SCCPs (C12, 60% chlorination) are reasonably anticipated to be human carcinogens based on sufficient evidence of carcinogenicity from studies in experimental animals’ (NTP, 2016).
The International Agency for Research on Cancer (IARC) concluded that there was sufficient evidence for the carcinogenicity of SCCPs (C12, 60% chlorination) in experimental animals and classified it as Group 2B ‘possibly carcinogenic to humans’ (IARC, 1990). IARC also noted that no data were available from studies in humans on the carcinogenicity of CPs.
In 1993, Health Canada assessed the CPs in terms of ‘Toxic’ under the Canadian Environmental Protection Act (CEPA) for Environment (CEPA Paragraph 11a), for Environment on which human life depends (CEPA Paragraph 11b) and for human Life or health (CEPA Paragraph 11c) (Health Canada, 1993). SCCPs were considered to be ‘toxic’ as defined under Paragraph 11(c) of the CEPA due to their carcinogenicity.
The WHO/IPCS reviewed the information available in relation to effects of CPs on human health and the environment (WHO/IPCS, 1996). For SCCPs, a tolerable daily intake (TDI) of 100 μg/kg body weight (bw) per day was derived for non‐neoplastic effects based on the lowest reported no‐observed effect level (NOEL) of 10 mg/kg bw per day and applying an uncertainty factor of 100 to account for inter‐ and intraspecies variations (10 × 10). Increases in liver and kidney weights and hypertrophy of the liver and thyroid were observed at the next highest dose level in a 13‐week study on rats (IRDC, 1984, as cited in WHO/IPCS, 1996, 4) For neoplastic effects, it was recommended that daily doses of SCCPs for the general population should not exceed 11 μg/kg bw per day. This recommendation was based on the estimated dose associated with a 5% increase in tumour incidence (hepatocellular adenomas or carcinomas (combined) in male mice) of 11 mg/kg bw per day and an uncertainty factor of 1,000 for a non‐genotoxic carcinogen (not further detailed in the WHO/IPCS document). Compared to the estimated worst case, daily intake of dairy products and mussels of 4.3 and 25 μg/kg bw per day, respectively (see Section 3.3.1.2 for further details on the exposure assessment), the Committee concluded that, in general, the calculated daily intakes are below the TDIs estimated for non‐neoplastic effects of the SCCPs, as well as the recommended value for neoplastic effects for SCCPs.
In the context of the programme on high production volume (HPV) chemicals, the Organisation for Economic Co‐operation and Development (OECD) has reviewed the toxicological data on SCCPs (C10–13, chloro) and published the results in a Screening Information Dataset (SIDS) Initial Assessment Profile (OECD, 2000). It was concluded that SCCPs are of low toxicity with no‐observed adverse effect levels (NOAELs) for general toxicity (decreased body weight gain and increased kidney weight) of 100 and 1,000 mg/kg bw per day for rats and mice, respectively, following repeated exposure.
A risk assessment report (RAR) for SCCPs (C10–13, chloro) performed under the remit of the European Chemicals Bureau was finalised in 1999 and published in 2000 (EU‐RAR, 2000). The assessment concluded on the risk for worker's exposure, consumer's exposure from products containing SCCPs (e.g. from their use in textiles or leather treatment), and also the general population from indirect exposure via the environment, including food. A NOAEL of 100 mg/kg bw per day was identified based on general non‐specific toxicity from repeated dose toxicity studies in rats and mice, and of 500 mg/kg bw per day for developmental effects. Based on the NTP studies in rodents (NTP, 1986a) it was noted that the SCCPs tested produced toxicologically significant, dose‐related increases in the incidence of several tumour types. For the liver, the chronic tissue damage was considered to be caused by peroxisome proliferation and for the thyroid by long‐term hormonal stimulation, and not relevant to human health. For the kidney, the benign tumours observed in male rats were not considered likely to be relevant for human health. An estimated human intake of 20 μg/kg bw per day from indirect exposure via the environment (including food) was considered to be a reasonable worst‐case prediction based on real data (see Section 3.3.1.2 for further details on the exposure assessment). A ‘margin of safety’ of 5,000 between the NOAEL for repeated dose toxicity of 100 mg/kg bw per day and the estimated human intake of 20 μg/kg bw per day indicates that there is no significant risk to man exposed via the environment’, including food. In an updated version of the RAR (EU‐RAR, 2008), risk characterisation for exposure of infants exposed via milk was included, based on the information from the MCCP EU‐RAR (see below) that internal haemorrhaging leading to deaths has been observed in suckling rat pups from dams treated with MCCPs. Exposure of infants via human milk and cow's milk was estimated based on available published data. Large margins between the estimated infant intake (of both human milk and cow's milk) and the levels at which adverse effects mediated via lactation have been seen in animals exposed to MCCPs indicate there is no significant risk to infants from intake of human milk and cow's milk.
The Commonwealth of Australia has published an assessment of SCCPs in 2001, which was carried out under the ‘National Industrial Chemicals Notification and Assessment Scheme (NICNAS)’ (NICNAS, 2001). The assessment was based on information from a number of sources. The purpose of the assessment was to: (1) determine manufacture/importation volumes; (2) identify likely or potential uses of SCCPs in Australia; (3) review the properties of SCCPs; (4) determine the potential for public and occupational exposure and exposure to the environment resulting from use; (5) review and summarise the health and environmental effects; and (6) determine whether or not the significance for risk is such that a full (risk) assessment should be undertaken. NICNAS recommended that a full risk assessment of the use of SCCPs in metal work was needed for the environment, if new data indicating acceptable releases to sewer was not identified. Whereas, further assessment work for workers and the public was not recommended.
Upon request from the Danish Environmental Protection Agency, Nielsen and Ladefoged (2013) evaluated the health hazards from exposure to CPs to propose a health‐based quality criterion for CPs in ambient air. The assessment was finalised in 2006 and published in 2013. The assessment of SCCPs was based on the EU‐RARs for SCCPs (EU‐RAR, 2000), as well as on the WHO/IPCS (1996) and IARC (1990) evaluations. The authors identified an overall NOAEL for CPs of 10 mg/kg bw per day for effects in the liver, kidney and thyroid as well as for the effects observed in developing offspring. A TDI of 100 μg/kg bw per day was calculated by applying an uncertainty factor of 100 to account for inter‐ and intraspecies variations (10 × 10).
In 2008, Health Canada published an assessment of SCCPs (C10–13) (Health Canada, 2008). An exposure assessment of the Canadian population was done considering uptake via ambient air, indoor air, drinking water, food and soil based on measured concentrations from published studies, although a high degree of uncertainty was acknowledged in the food estimates. Health Canada considered that the lowest‐observed adverse effect level (LOAEL) of 100 mg/kg bw per day for increases in liver and kidney weights and hypertrophy of the liver and thyroid, identified from a 90‐day gavage study in rats (IRDC, 1984, as cited by Health Canada, 2008, 5) was the most sensitive LOAEL for mammals. Regarding carcinogenic effects, Health Canada referred to the WHO/IPCS (1996) estimated dose associated with a 5% increase in tumour incidence (tumourigenic dose05 [TD05]) of 11 mg/kg bw per day. The adult (20–59 years old) upper bound (UB) exposure was estimated at 7.18 μg/kg bw per day (see Section 3.3.1.2 for further details on the exposure assessment). The highest exposure estimates were for younger age groups, with infants not formula fed showing the highest estimate (25.97 μg/kg bw per day), while breastfed infants and formula fed infants were estimated to have an intake of 1.7 and 0.01 μg/kg bw per day, respectively (Health Canada, 2008). The committee did not establish a TDI, but based on the TDI for non‐neoplastic effects of 100 μg/bw per day established by WHO/IPCS (1996), Health Canada concluded that the highest exposure estimate of 25.97 μg/kg bw per day was within the range of the TDI. For the carcinogenic effects, Health Canada concluded that the margin (440) between the WHO/IPCS TD05 of 11 mg/kg bw per day and the highest estimate of exposure was inadequate, considering the uncertainty in the mode of induction of tumours.
The UK Committee on Toxicity (UK‐COT) published in 2009 a statement on SCCPs (C10–13) (UK‐COT, 2009). The Committee established a TDI of 30 μg/kg bw per day, based on a NOAEL of 10 mg/kg bw per day for increased kidney weight, mild nephritis in males and brown pigmentation in renal tubules in females from a 90 day gavage and dietary study described in the EU‐RAR (1999, as cited by UK‐COT, 2009, 6). An uncertainty factor of 3 was applied to take into account that the NOAEL was not from a long‐term study, as well as uncertainty factors of 10 for interspecies variation and 10 for intraspecies variation. For the exposure assessment, and due to the scare data available, the Committee made the conservative assumption that all solid food consumed contained SCCPs at the level of 6.0 μg/kg (as the highest value reported in freshwater eel) (see Section 3.3.1.2 for further details on the exposure assessment). The exposure of a 97.5th percentile adult consumer would be 0.12 μg/kg bw per day while for children 4–6 years old it would be 0.29 μg/kg bw per day. The Committee concluded that the large margin between the TDI and the exposure estimates indicated no cause for concern regarding dietary exposure to SCCPs.
1.3.5.2. MCCPs
In 1993, Health Canada assessed the CPs in terms of ‘Toxic’ under the CEPA for Environment (CEPA Paragraph 11a), for Environment on which human life depends (CEPA Paragraph 11b) and for human Life or health (CEPA Paragraph 11c) (Health Canada, 1993). It could not be assessed whether MCCPs were ‘toxic’ as defined under Paragraph 11(c) of the CEPA. This was due to that the available data were considered inadequate for an assessment of whether current concentrations of MCCPs present in the environment could constitute a danger in Canada to human life or health.
The WHO/IPCS reviewed the information available in relation to effects of MCCPs on human health and the environment (WHO/IPCS, 1996). A TDI of 100 μg/kg bw per day was derived for non‐neoplastic effects based on a NOAEL of 10 mg/kg bw per day and applying an uncertainty factor of 100 to account for inter‐ and intraspecies variations (10 × 10). Increases in liver and kidney weight were observed at the next highest dose level in a 13‐week study on rats (IRDC, 1984, as cited in WHO/IPCS, 1996, 7) Compared to the estimated worst‐case daily intake of dairy products and mussels of 4.3 and 25 μg/kg bw per day, respectively (see Section 3.3.1.2 for further details on the exposure assessment), the Committee concluded that in general, the calculated daily intakes are below the TDI estimated for MCCPs.
A RAR on human health by the European Chemicals Bureau for MCCPs (C14–17, chloro) was finalised in 2008 and published in 2011 (EU‐RAR, 2011). The assessment concluded on the risk for worker's exposure, consumer's exposure from products containing MCCPs (e.g. from their use in textiles or leather treatment), and also the general population from indirect exposure via the environment, including food. A NOAEL of 23 mg/kg bw per day was identified for increased kidney weight from repeated dose toxicity studies in rats. In the absence of carcinogenicity studies, it could not be ruled out that the observed kidney toxicity might lead to kidney tumours through a non‐genotoxic mode of action; therefore, it was decided to apply the same NOAEL of 23 mg/kg bw per day for neoplastic effects for the risk characterisation. From studies on effects in lactating rat pups (internal haemorrhaging and deaths) of dams treated with MCCPs, a maternal NOAEL of 100 mg/kg bw per day was selected for the risk characterisation of haemorrhaging effects potentially occurring in pregnant women at the time of parturition. A maternal NOAEL of 47 mg/kg bw per day was selected for the risk characterisation of an adult population of breastfeeding mothers that might be exposed to MCCPs via the environment, including food. Overall, 50% absorption by the oral route was used for risk characterisation purposes. The total daily human exposure from regional sources has been estimated to 1.3 × 10−4 mg/kg bw per day and the highest continuous local exposure was estimated to be 0.016 mg/kg bw per day. The margins between the NOAELs and the estimated human exposures from regional and local sources indicated that there was no significant risk to man exposed via the environment, including food. Exposure of infants via human milk and cow's milk was estimated based on available published data. Very large margins between the estimated infant intake (of both human milk and cow's milk) and the levels at which adverse effects mediated via lactation have been seen in animals indicated that there was no significant risk to infants from intake of human milk and cow's milk.
Upon request from the Danish Environmental Protection Agency, Nielsen and Ladefoged (2013) evaluated the health hazards from exposure to CPs to propose a health‐based quality criterion for CPs in ambient air. The assessment was finalised in 2006 and published in 2013. The assessment of MCCPs was based on the EU‐RAR for MCCPs (unpublished at the time of the Nielsen and Ladefoged assessment, later published as EU‐RAR, 2011), as well as on the WHO/IPCS (1996) and IARC (1990) evaluations. The authors identified an overall NOAEL for CPs of 10 mg/kg bw per day for effects in the liver, kidney and thyroid as well as for the effects observed in developing offspring. A TDI of 100 μg/kg bw per day was calculated by applying an uncertainty factor of 100 to account for inter‐ and intraspecies variations (10 × 10).
In 2008, Health Canada published an assessment of MCCPs (C14–17) (Health Canada, 2008). An exposure assessment of the Canadian population was done considering uptake via ambient air, indoor air, drinking water, food and soil based on measured concentrations from published studies, although a high degree of uncertainty was acknowledged in the food estimates. Health Canada noted that the lowest effect level observed for mammals is the LOAEL of 4.2 mg/kg bw per day (NOAEL = 0.4 mg/kg bw per day) for mild effects on the kidney and thyroid of female rats from a 13‐week feeding study (Poon et al., 1995). Health Canada indicated that a TDI based on the NOAEL from the Poon et al. (1995) study would be similar to the TDI of 6 μg/kg bw per day as previously established. Regarding the exposure estimates, the adult (20–59 years old) UB exposure was 4.69 μg/kg bw per day (see Section 3.3.1.2 for further details on the exposure assessment). The largest total exposure was found for infants not formula fed (25.51 μg/kg bw per day). No exposure estimate was reported for breastfed infants, while for formula fed infants it was 0.05 μg/kg bw per day (Health Canada, 2008). The Committee concluded that these estimates exceed the TDI (6 μg/kg bw per day), for infants not formula fed up to fourfold.
The UK‐COT published in 2009 a statement on MCCPs (C14–17) (UK‐COT, 2009). The Committee established a TDI of 4 μg/kg bw based on a NOAEL of 4 mg/kg bw per day identified for changes in relative liver weight and minimal changes in the inner cortex of the kidney in female rats from a 90‐day dietary study described in the EU‐RAR (2004, as cited by UK‐COT, 2009, 8). Besides the uncertainty factors of 10 for interspecies and 10 for intraspecies variation, an additional uncertainty factor of 10 was applied to account for the absence of longer term studies and the lack of a NOAEL for reproductive and developmental effects. For the exposure assessment, and due to the scarce data available, the Committee made the conservative assumption that all solid food consumed contained MCCPs at the level of 31.9 μg/kg (as the highest value reported in freshwater eel). The exposure of a 97.5th percentile adult consumer would be 0.63 μg/kg bw per day while for children 4–6 years old it would be 1.6 μg/kg bw per day. The Committee concluded that the margin between the TDI and the exposure estimates indicated no cause for concern regarding dietary exposure to MCCPs.
In 2011, the German Federal Institute for Occupational Safety and Health (BauA) summarised the information on MCCPs (BauA, 2011). An occupational exposure limit of 6 mg/m3 (8‐hour time‐weighted average) was proposed based on the NOAEL of 23 mg/kg bw per day for increased kidney weight identified from a 90‐day rat study (CXR, 2005, as cited in BauA, 2011, 9).
In 2015, US‐Environmental Protection Agency (US‐EPA) reviewed the toxicity of MCCPs in the frame of a pre‐manufacture notice submission to assess the occupational and non‐occupational risks (US‐EPA, 2015). The lowest NOAEL of 23 mg/kg bw per day for increased kidney weight identified from a 90‐day rat study (CXR, 2005, as cited in US‐EPA, 2015, 10) was used to assess the risk. This oral NOAEL was converted to a human equivalent dose (HED). The margin between the HED and the exposure estimates were considered by US‐EPA to indicate a low risk to the general population from environmental releases of MCCPs via exposure to drinking water or fish ingestion.
1.3.5.3. LCCPs
The NTP has conducted toxicology and carcinogenesis studies of LCCPs (C23, 43% chlorination) (NTP, 1986b). NTP concluded that there was no evidence of carcinogenicity for male F344/N rats, equivocal evidence of carcinogenicity for female F344/N rats as shown by an increased incidence of adrenal gland medullary pheochromocytomas, clear evidence of carcinogenicity for male B6C3F1 mice as shown by an increase in the incidence of malignant lymphomas, and equivocal evidence of carcinogenicity for female B6C3F1 mice as shown by a marginal increase in the incidence of hepatocellular neoplasms.
IARC (1990) concluded that there is limited evidence for the carcinogenicity of LCCPs (C23, 43% chlorination) in experimental animals.
In 1993, Health Canada assessed the CPs in terms of ‘Toxic’ under the CEPA for Environment (CEPA Paragraph 11a), for Environment on which human life depends (CEPA Paragraph 11b) and for human Life or health (CEPA Paragraph 11c) (Health Canada, 1993). It could not be assessed whether LCCPs were ‘toxic’ as defined under Paragraph 11(c) of the CEPA. This was due to that the available data were considered inadequate for an assessment of whether current concentrations of LCCPs present in the environment could constitute a danger in Canada to human life or health.
The WHO/IPCS reviewed the information available in relation to effects of LCCPs on human health and the environment (WHO/IPCS, 1996). A TDI of 100 μg/kg bw per day was derived for non‐neoplastic effects based on a LOAEL of 100 mg/kg bw per day for multifocal granulomatous hepatitis and increased liver weight in female rats (NTP, 1986b; Bucher et al., 1987; Serrone et al., 1987). An uncertainty factor of 1,000 was applied to account for inter‐ and intraspecies variations (10 × 10) and because the TDI was derived based on a LOAEL instead of a NOAEL (a factor of 10). Compared to the estimated worst‐case daily intake of dairy products and mussels of 4.3 and 25 μg/kg bw per day, respectively (see Section 3.3.1.2 for further details on the exposure assessment), the Committee concluded that in general, the calculated daily intakes are below the TDI estimated for LCCPs.
The National Research Council (NRC, 2000) reviewed the toxicological risks of selected flame retardants, including LCCPs (C20–30, with high chlorine content, i.e. 70% chlorination) used in residential furniture. A NOAEL of 900 mg/kg bw per day was identified for an LCCP (C22–26, 70% chlorination) from a 13‐week study in rats (Serrone et al., 1987). This was based on liver toxicity (increased relative liver weight, hepatocellular hypertrophy, cytoplasmic fat vacuolation, increases in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities) and kidney toxicity (a slight increase in the incidence of nephritis in males). Based on this NOAEL, a reference dose (RfD) of 300 μg/kg bw per day was derived for LCCPs (C20–30, 70% chlorination). Uncertainty factors were applied to account for interspecies variation (a factor of 3 instead of 10 was used because the toxicokinetics and toxicodynamics of LCCPs were anticipated to be similar in rodents and humans) and intraspecies variation (factor of 10), as well as two additional factors (10 to account for less‐than‐lifetime toxicity data and 10 because the toxicity database for LCCPs (C22–26, 70% chlorination) was incomplete).
Upon request from the Danish Environmental Protection Agency, Nielsen and Ladefoged (2013) evaluated the health hazards from exposure to CPs to propose a health‐based quality criterion for CPs in ambient air. The assessment was finalised in 2006 and published in 2013. The assessment of LCCPs was based on the WHO/IPCS (1996) and IARC (1990) evaluations. The authors identified an overall NOAEL for CPs of 10 mg/kg bw per day for effects in the liver, kidney and thyroid as well as for the effects observed in developing offspring. A TDI of 100 μg/kg bw per day was calculated by applying an uncertainty factor of 100 to account for inter‐ and intraspecies variations (10 × 10).
In 2008, Health Canada published an assessment of LCCPs (C≥18), with an update in 2012 (Health Canada, 2008, 2012). An exposure assessment of the Canadian population was done considering uptake via ambient air, indoor air, drinking water, food and soil based on measured concentrations from published studies, although a high degree of uncertainty was acknowledged in the food estimates. Health Canada considered that the lowest effect level was 100 mg/kg bw per day (NTP, 1986b; Bucher et al., 1987; Serrone et al., 1987); the main effects were seen on the liver. A TDI of 71 μg/kg bw per day is mentioned for non‐neoplastic effects; however, there is no information on how this TDI has been established (Health Canada, 2008). According to the updated assessment on LCCPs (Health Canada, 2012), the TDI of 71 μg/kg bw per day was based on the LOAEL of 100 mg/kg bw per day for adverse effects (diffuse lymphohistiocytic inflammation in the liver and in the pancreatic and mesenteric lymph nodes in female rats) from a carcinogenicity study (NTP, 1986b; Bucher et al., 1987). An uncertainty factor of 1,000 (10 for intraspecies variation, 10 for interspecies variation, 10 for use of a LOAEL rather than a NOAEL) was applied and multiplied by a factor of 5/7 (for conversion of 5 days per week administration to daily exposure). Regarding the exposure estimates, the adult (20–59 years old) UB exposure was 2.12 μg/kg bw per day. The largest total exposure was found for infants not formula fed (16.83 μg/kg bw per day). No exposure estimate was reported for breastfed infants, while for formula fed infants it was 0.07 μg/kg bw per day (Health Canada, 2008). The Committee concluded that none of the highly uncertain exposure estimates for the general population exceeds the TDI (71 μg/kg bw per day), whereas for infants not formula fed, it is noted that the exposure is within the same order of magnitude as the TDI. In the updated assessment on LCCPs (Health Canada, 2012), the adult (20–59 years old) UB exposure was 0.009 μg/kg bw per day. The largest total exposure was found for the 0.5‐ to 4‐year age group (0.024 μg/kg bw per day). The total exposure for infants not formula fed was 0.018 μg/kg bw per day and for formula fed infants it was below 0.01 μg/kg bw per day; no estimate was reported for breastfed infants. The Committee concluded that the exposure estimates are well below the established TDI.
In 2009, the UK Environment Agency issued a science report on the environmental risks of LCCPs (UK‐Environment Agency, 2009). A LOAEL of 100 mg/kg bw per day was identified for one LCCP (C22–26, 43% chlorination) based on the presence of liver changes in female rats at all treatment levels (IRDC, 1984, as cited in UK‐Environment Agency, 2009, 11). A LOAEL of 100 mg/kg bw per day was identified for another LCCP (C23, 43% chlorination) for non‐neoplastic changes, based on the presence of microscopic changes in the liver and lymph nodes at the lowest dose level (NTP, 1986b). And a NOAEL 900 mg/kg bw per day for a third LCCP (C20–26, 70% chlorination) based on the observation of effects on body weight gain, food consumption and the liver (IUCLID, 2003). The LOAEL of 100 mg/kg bw per day was taken forward to the risk assessment for the C20–30 LCCPs, regardless of the chlorination degree, owing to the uncertainties regarding the influence of the chlorination state on the toxicity of LCCPs. It was suggested to divide the LOAEL of 100 mg/kg bw per day by three to provide an estimate of the NOAEL (i.e. assumed NOAEL = 33.3 mg/kg bw per day). For the C18–20 LCCPs, a NOAEL of 23 mg/kg bw per day was assigned, based on the repeated dose toxicity data for MCCPs.
In 2015, US‐EPA reviewed the toxicity of LCCPs in the frame of a pre‐manufacture notice submission to assess the occupational and non‐occupational risks (US‐EPA, 2015). A LOAEL of 100 mg/kg bw per day identified for granulomatous inflammation of the liver in female rats from a 2‐year rat study (NTP, 1986b) for one LCCP (C23, 43% chlorination) was used to assess the risk. This oral LOAEL was converted to a HED. The margin between the HED and the exposure estimates were considered by US‐EPA to indicate a low risk to the general population from environmental releases of LCCPs via exposure to drinking water or fish ingestion.
1.3.5.4. CPs
In 1990, the German MAK Commission summarised the information on CPs (German MAK Commission, 1990). The Commission concluded that a MAK value could not be established for the CPs and therefore, the CPs could not be classified in one of their pregnancy groups. Regarding carcinogenicity, the CPs were placed in Section III B12 of the ‘List of MAK Values’.
1.3.6. Legislation
In order to protect public health, Article 2 of Council Regulation (EEC) No 315/9313 of 8 February 1993 laying down Community procedures for contaminants in food stipulates that, where necessary, maximum tolerances for specific contaminants shall be established. Thus, maximum levels for certain contaminants, e.g. polychlorinated dibenzo‐p‐dioxins and furans, dioxin‐like and non‐dioxin‐like PCBs and several polycyclic aromatic hydrocarbons in foodstuffs are currently laid down in Commission Regulation (EC) No. 1881/200614 of 19 December 2006 (and further amendments). However, none of the CPs considered in this opinion are regulated under this Regulation or under any other specific EU regulation for food.
Council Directive 2002/32/EC15 regulates undesirable substances in animal feed. While maximum levels are set for a number of inorganic and organic contaminants in various feed materials, none of the CPs considered in this opinion are regulated under this Directive or any other specific EU regulation for feed.
Regulation (EC) No 850/200416 on POPs implements the commitments of the EU under the Stockholm Convention on POPs. In 2012, it was amended by Commission Regulation (EU) No 519/201217 to include SCCPs to its Annex I, which lists substances subject to prohibitions. SCCPs (Alkanes C10–13, chloro, CAS No 85535‐84‐8, EC no 287‐476‐5) were listed with specific exemptions on intermediate use or other specifications. The exemptions allowed (i) the production, placing on the market and use of substances or mixtures containing SCCPs in concentrations < 1% by weight, and (ii) the production, placing on the market and use of certain applications provided the MS reported on the progress made to eliminate SCCPs. These derogations were to be reviewed with a view to phasing out the remaining uses of SCCPs as soon as new information on details of uses and safer alternatives or technologies became available. In 2015, Commission Regulation (EU) 2015/203018 clarified that articles that contain SCCP in concentrations lower than 0.15% by weight were allowed to be placed on the market and used (considering this as the amount of SCCP that may be present as an impurity in an article produced with MCCP).
According to ANNEX VI of Regulation (EC) No 1272/2008 (Classification, Labelling and Packaging (CLP) Regulation), SCCPs are classified Carc. 2 H 351 (Suspected of causing cancer), Aquatic Acute 1 H 400 (Very toxic to aquatic life) and Aquatic Chronic 1 H 410 (Very toxic to aquatic life with long lasting effects). MCCPs are classified Lact. H 362 (May cause harm to breastfed children), Aquatic Acute 1 H 400 (Very toxic to aquatic life) and Aquatic Chronic 1 H 410 (Very toxic to aquatic life with long lasting effects). For LCCPs there is no harmonised classification in the EU.
The placing on the market and use of SCCPs is restricted in the EU by Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). ‘Alkanes C10–13, chloro’ (CAS No 85535‐84‐8, SCCPs) are included in the 2008 candidate list of substances of very high concern for Authorisation on the basis of PBT (persistent, bioaccumulative and toxic) and vPvB (very persistent and very bioaccumulative) properties, for eventual inclusion in Annex XIV. The substance was registered but the registration is no longer active.19 ‘Alkanes C10–12, chloro’ (CAS No 108171‐26‐2, SCCPs) was in the pre‐registration process with an envisaged registration deadline 31 May 2018. The final registration deadline passed, and no registration dossier was received. ‘Alkanes C14–17, chloro’ (CAS No 85535‐85‐9, MCCPs) appear as manufactured and/or imported in the European Economic Area in 10,000–100,000 tonnes per year. This substance is under the substance evaluation process, as additional data were requested in order to clarify, among others, whether the substance is a PBT substance.20 ‘Paraffin waxes and Hydrocarbon waxes, chloro’ (CAS No 63449‐39‐8, LCCPs) appears as manufactured and/or imported in the European Economic Area in 10,000–100,000 tonnes per year.21 There has not been any formal authority assessment of the PBT/vPvB status under REACH, but there was an assessment under the Existing Substances Regulation.22
CPs have been listed in other international conventions as described by Fiedler (2010), which is giving increased global concern on CPs. SCCPs are regulated in the global Stockholm Convention and in the United Nations Economic Commission for Europe (UNECE) Protocol to the 1979 Convention on Long‐Range Transboundary Air Pollution on POPs (LRTAP POPs). Under the Stockholm Convention, SCCPs are listed in Annex A for chemicals where measures must be taken to eliminate their production and use.23 SCCPs (alkanes C10–13 chloro, chlorine content > 48%) were listed with specific exemptions in production and use. Due to their inclusion in Annex A of the Stockholm Convention, it is anticipated that the production and use of SCCPs will decrease in future years, and SCCPs will be replaced in most applications.
Regarding MCCPs, the Swedish Chemicals Agency is proposing that the use of MCCPs in electrical and electronic equipment is restricted,24 and the Norwegian authorities are proposing to prohibit the production, import, export and selling of consumer products containing more than 0.1% MCCPs (CAS No 85535‐85‐9) by weight. This will not apply to products with special flame‐retardant (fire‐safety) requirements and where no satisfactory alternatives exist.25
Some regulatory agencies including the US‐EPA26 and Environment and Health Canada27 have undertaken risk assessments for MCCPs, but to date no production or restrictions in terms of use have been formally established. Canada set Federal Environmental Quality Guidelines (FEQGs) for water, sediments and mammalian wildlife under the Canadian Environmental Protection Act, 1999. These guidelines are not effluent limits or never‐to‐be‐exceeded values, but provide benchmarks for the quality of the ambient environment to protect aquatic life and mammalian (non‐human) consumers of aquatic life from adverse effects of CPs (Environment and Climate Change Canada, 2016). In water, the FEQG is 2.4 μg/L for SCCPs, MCCPs and LCCPs. For fish tissues,28 the FEQGs are 1.8 and 0.76 μg/g fat for SCCPs and MCCPs, respectively. No value is reported for LCCPs. For sediments, the FEQGs are 1.8, 5.4 and 100 mg/kg dw for SCCPs, MCCPs and LCCPs (C18–20 liquid), respectively. For mammalian wildlife diet,29 the FEQGs are 18, 0.54 and 18 or 770 mg/kg food wet weight (ww), for SCCPs, MCCPs and LCCPs (C>20 liquid or C>20 solid).
2. Data and methodologies
The CONTAM Panel applied the general principles for the assessment of chemicals in food as described by WHO/IPCS (2009). In addition, EFSA guidances pertaining to risk assessment were followed for the development of the risk assessment when necessary (EFSA, 2009, 2010a,b, 2011a; EFSA Scientific Committee, 2011, 2012a,b, 2017a,b,c).
2.1. Supporting information for the assessment and hazard identification and characterisation
2.1.1. Identification, collection and selection of evidence
The identification of the evidence used to inform the sections on supporting information and hazard identification and characterisation was done through a literature search that interrogated two bibliographic databases or scientific citation research platforms: Web of ScienceTM 30 and PubMed.31 Details about the search strings are shown in Table 10 .
Table 10.
Search strings and other relevant data for the identification of evidence to inform the sections on supporting information and hazard identification and characterisation
| Search strings | (chlorinated paraffin*) OR (short‐chain chlorinated paraffin*) OR (medium‐chain chlorinated paraffin*) OR (long‐chain chlorinated paraffin*) OR (short‐chained chlorinated paraffin*) OR (medium‐chained chlorinated paraffin*) OR (long‐chained chlorinated paraffin*) OR (polychlorinated n‐alkane*) OR (polychlorinated alkane*) OR (SCCP*) OR (MCCP*) OR (LCCP*) OR (chlorinated alkane*) OR (chlorinated paraffin waxes) OR (chlorinated hydrocarbon waxes) OR (chlorinated waxes) OR (chlorocarbons) OR (chloroparaffin waxes) OR (paraffin, chlorinated) OR (paraffins, chloro) OR (paraffin waxes, chlorinated) OR (paroils, chlorinated) OR (poly‐chlorinated alkanes) OR (polychloro alkanes) |
| Publication year | 1950–September 2017 |
| Language | All |
| Type of documents | All (peer reviewed articles, reviews, books, reports, patents, editorials, thesis, letters, abstracts) |
The outcomes of the searches in the two databases were saved in EndNote,32 and duplicates automatically detected. The resulting references were transferred to the web‐based systematic review software DistillerSR® (Evidence Partners, Ottawa, Canada), where an additional automatic duplicate detection was made. The selection for relevance was performed in DistillerSR®. Each reference underwent a series of questions to identify studies relevant to the risk assessment of CPs in food and feed.
The first literature search was done in September 2017 and updates of the search were done on 21 September 2018 and on 11 March 2019 in Web of Science and PubMed, respectively, using the same search string. Since that date, the literature was monitored to identify studies relevant for the risk assessment until the time of endorsement.
Additionally, relevant scientific evaluations by national or international bodies were considered for the current risk assessment, e.g. EU‐RAR, ECHA, IARC and NTP. A dedicated search in the Organohalogen Compounds database (extended abstracts from DIOXIN conferences) was also performed.
When relevant papers were identified during the risk assessment process (e.g. from other studies or reviews), they were also considered.
In addition, the draft scientific opinion underwent a public consultation from 6 August 2019 to 17 September 2019. The comments received and how they were taken into account when finalising the scientific opinion were published in an EFSA Technical Report (EFSA, 2020).
2.1.2. Appraisal of evidence
The information retrieved was screened and evaluated by relevant domain experts from the CONTAM Working Group on CPs in food and was used for the present assessment. Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results. Major limitations in the information used are documented in this scientific opinion.
2.2. Occurrence data submitted to EFSA
2.2.1.
Data collection and validation
Following a European Commission mandate to EFSA, a call for annual collection of chemical contaminant occurrence data in food and feed, including CPs, was issued by the former EFSA Dietary and Chemical Monitoring Unit (now DATA Unit) in December 2010 with a closing date of 1 October of each year. European national authorities and similar bodies, research institutions, academia, food business operators and other stakeholders were invited to submit analytical data on CPs in food and feed.
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description (SSD) for Food and Feed (EFSA, 2010a); occurrence data were managed following the EFSA standard operational procedures (SOPs) on ‘Data collection and validation’ and on ‘Data analysis of food consumption and occurrence data’.
Two data sets were submitted to EFSA containing data on SCCPs and MCCPs in food. The data sets were the following.
-
–
Data set A: 88 analytical results of 44 samples from the year 2007.
-
–
Data set B: 422 analytical results of 184 fish samples from 2014 onwards to 2017, which were used for the present assessment.
The CONTAM Panel noted that Data set A corresponded to the same occurrence data reported in UK‐COT (2009), and that Data set B was partly reported in the study by Krätschmer et al. (2019).
In addition, data on the occurrence of CPs in oil‐based dietary supplements was submitted to EFSA. These data could not be included on time in the EFSA Data warehouse for the current risk assessment, but the data were published by Sprengel et al. (2019) and the outcome is described in Sections 3.2.2 and 3.3.2 .
No data were submitted on LCCPs for food, and there were not data submitted on any CPs for feed materials.
Data analysis
The data received were carefully evaluated by EFSA in view of cleaning and validating. Special attention was paid to the identification of duplicates and to the accuracy of different parameters, such as ‘Analytical methods’, ‘Reporting unit’ and the coding of the different samples under FoodEx classification. Upon identification of potential inconsistencies, data providers were contacted to provide further clarification.
The left‐censored data (analytical data below the limit of detection (LOD) or limit of quantification (LOQ)) were treated by the substitution method as recommended in the ‘Principles and Methods for the Risk Assessment of Chemicals in Food’ (WHO/IPCS, 2009). The same method is described in the EFSA scientific report ‘Management of left‐censored data in dietary exposure assessment of chemical substances’ (EFSA, 2010b) as an option for the treatment of left‐censored data. The guidance suggests that the lower bound (LB) and upper bound (UB) approach should be used for chemicals likely to be present in the food. At the LB, results below the LOQ or LOD were replaced by zero; at the UB, the results below the LOD were replaced by the numerical values of the LOD and those below the LOQ were replaced by the value reported as LOQ. The use of different cut‐off values on the reported LOQs was also evaluated in order to reduce the uncertainty associated to the exposure estimations.
2.3. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of existing national information on food consumption at individual level. It was first built in 2010 (EFSA, 2011a; Huybrechts et al., 2011; Merten et al., 2011). Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a). The latest version of the Comprehensive Database updated in 2018 contains results from a total of 60 different dietary surveys carried out in 25 different Member States covering 119,458 individuals.
Within the dietary studies, subjects are classified in different age classes as follows:
Infants: < 12 months old
Toddlers: ≥ 12 months to < 36 months old
Other children: ≥ 36 months to < 10 years old
Adolescents: ≥ 10 years to < 18 years old
Adults: ≥ 18 years to < 65 years old
Elderly: ≥ 65 years to < 75 years old
Very elderly: ≥ 75 years old
Four additional surveys provided information on specific population groups: ‘Pregnant women’ (≥ 15 years to ≤ 45 years old, Latvia; 17 years old to 46 years old, Portugal) and ‘Lactating women’ (≥ 28 years to ≤ 39 years old, Greece; 18 years old to 45 years old, Estonia).
For chronic exposure assessment, food consumption data were available from 53 different dietary surveys carried out in 22 different European countries. When for one particular country and age class two different dietary surveys were available, only the most recent one was used. This resulted in a total of 38 dietary surveys selected to estimate chronic dietary exposure.
In a separate Excel document (Annex A , Table 1), these dietary surveys and the number of subjects available for the acute and chronic exposure assessment are described.
The food consumption data gathered by EFSA in the Comprehensive Database are the most complete and detailed data currently available in the EU. Consumption data were collected using single or repeated 24‐ or 48‐hour dietary recalls or dietary records covering from three to seven days per subject. Because of the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.4. Food classification
Consumption data were classified according to the FoodEx classification system (EFSA, 2011b). FoodEx is a food classification system developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances. The system consists of a large number of individual food items aggregated into food groups and broader food categories in a hierarchical parent‐child relationship. It contains 20 main food categories (first level), which are further divided into subgroups having 140 items at the second level, 1,261 items at the third level and reaching about 1,800 end‐points (food names or generic food names) at the fourth level.
2.5. Exposure assessment
The CONTAM Panel considered that only chronic dietary exposure had to be assessed.
Due to the unavailability of up‐to‐date data on foodstuff other than fish, the Panel decided to only estimate the exposure using the newer data on the 211 analytical results of 184 fish samples for the consumer only population. The Panel noted that dietary exposure will be higher due to the contribution of CPs from other foods.
As suggested by the EFSA Working Group on Food Consumption and Exposure (EFSA, 2011a), dietary surveys with only 1 day per subject were not considered for chronic exposure as they are not adequate to assess repeated exposure. Similarly, subjects who participated only 1 day in the dietary studies, when the protocol prescribed more reporting days per individual, were also excluded for the chronic exposure assessment. Not all countries provided consumption information for all age groups, and in some cases the same country provided more than one consumption survey.
For calculating chronic dietary exposure to SCCPs and MCCPs, food consumption and body weight data at the individual level were accessed in the Comprehensive Database. Occurrence data and consumption data were linked at the relevant FoodEx level. In this case, as only data on fish were available, the data were linked on FoodEx level2 of the generic ‘Fish meat’.
The mean and the high (95th percentile) chronic dietary exposures were calculated by combining mean occurrence values for the fish samples collected in different countries (pooled European occurrence data) with the average daily consumption of ‘Fish meat’ at individual level in each dietary survey and age class. Consequently, individual average exposures per day and body weight were obtained for all individuals. On the basis of distributions of individual exposures, the mean and 95th percentile exposure were calculated per survey and per age class. Dietary exposure was assessed using overall European LB and UB mean occurrence of CPs.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 7.15).
2.6. Risk characterisation
The general principles of the risk characterisation for chemicals in food as described by WHO/IPCS (2009) will be applied as well as the different EFSA guidance documents relevant to this step of the risk assessment (see above). For the farm animal, horses and companion animals risk characterisation, the same principles were applied.
3. Assessment
3.1. Hazard identification and characterisation
Some of the original study reports (unpublished) cited in this section were not available to the CONTAM Panel and thus the information could not be verified. In such cases, the second source from where the information was retrieved from is indicated.
3.1.1. Toxicokinetics
3.1.1.1. Laboratory animals
A number of studies on the toxicokinetics of CPs in rodents (rats and mice) have been retrieved in the literature search. The main outcomes of these studies are described below while a more detailed description of each individual study can be found in Appendix A .
3.1.1.1.1. Studies in rats
Twelve studies on toxicokinetics in rats are available, three studies on SCCPs, six on MCCPs and three on LCCPs. Administration of CPs was both via diet (repeated exposure) and gavage (repeated exposure and single dose).
Following administration of a single oral dose of a 14C‐labelled SCCP (C10–12, 58% chlorination, 10 or 625 mg/kg bw) to rats, either after 13 weeks of dietary administration of the SCCP or control diet, radioactivity was predominantly detected in the faeces with 54–66% recovered in 7 days; approximately 14% was recovered in the urine and less than 1% in exhaled air as carbon dioxide (IRDC, 1984a, unpublished study, see Documentation provided to EFSA). Blood and tissue levels were generally proportional to the dose level. The highest initial concentrations of radioactivity were found in the liver, adipose tissue, kidneys and ovaries. In general, the elimination of radioactivity from the adipose tissue was slower than for the other three tissues. Only small differences were observed between sexes, dose levels or dosing regimen. Similar results were reported in an identical gavage study (IRDC, 1984b, unpublished study, see Documentation provided to EFSA), except for a slight difference in the percentage of the administered radioactivity recovered in faeces (approximately 48–65%) and urine (approximately 13%). A recent study (Geng et al., 2016) in male rats showed that SCCPs (mixture of C10‐, C11‐, C12‐ and C13‐CPs, 56.5% chlorination) was rapidly absorbed following a single oral administration (1,000 mg/kg bw). Also in this study, the main route of excretion was via faeces (about 28%) with urine as a minor route (about 3.5%). Congener group abundance profiles indicated a relative increase of Cl5‐SCCPs in blood and urine in the elimination phase and a higher accumulation of Cl8–10‐SCCPs in faeces indicating that the distribution discrepancies of SCCPs congener groups in blood and excreta were more dependent on chlorination degree than on carbon chain lengths.
In a dietary study with an MCCP (Cereclor S52, C14–17, 52% chlorination, 10 or 625 mg/kg bw) (IRDC, 1984c, unpublished study, see Documentation provided to EFSA) performed as for the dietary and gavage studies with SCCP described above, faecal excretion was also the main route of elimination (40–48% and 53–61% in low‐ and high‐dose males, respectively; 28–31% and 62–74% in low‐ and high‐dose females) with only a small proportion excreted in urine (0.8–3%) and expired air (0.1–0.3%). As for the SCCPs, there was an indication that blood and tissue levels were proportional to the administered dose although not as clear as for the SCCP. As for the SCCPs, the highest initial concentrations of radioactivity were found in the liver, ovaries and kidneys, whereas for adipose tissue, the concentration of radioactivity was highest at 7 days after the last dose and then declined more slowly than for the other tissues. Distribution of the same MCCP (C14–17, 52% chlorination) to the liver and adipose tissue was also reported in two other dietary studies (Birtley et al., 1980; Poon et al., 1995). One study (doses from 0.4 to 400 mg/kg bw per day) reported a dose‐dependent increase in the concentration of the MCCP in both liver and abdominal fat (Poon et al., 1995) and the other study (doses of 0.4 and 40 mg/kg diet) reported a rapid elimination from the liver (within one week) and slow elimination from the abdominal fat (half‐life of approximately 8 weeks) (Birtley et al., 1980). In addition, a dietary study (CXR, 2005a, unpublished study, see Documentation provided to EFSA) with the same MCCP (C14–17, 52% chlorination, approximately 200 mg/kg bw per day) reported that the content in the white adipose tissue increased with time until the steady‐state concentration was achieved at week 13; then there was an initial rapid elimination where after the elimination rate decreased markedly. Also following a single oral dose of an MCCP (C15, 52% chlorination, 525 mg/kg bw) by gavage (CXR, 2005, as cited in EU‐RAR, 2011) faecal excretion was the main route of elimination (approximately 50% excreted within the first 24 h after dosing and approximately 70% after 5 days) and only a minor proportion in the urine (approximately 5% of the administered radioactivity was eliminated in the urine by after 5 days). Similarly, the initial highest concentrations of radioactivity were detected in the liver, kidney and fat and elimination half‐life was rapid for most tissues (approximately 2–5 days) and slower for white adipose tissue (about 2 weeks).
Only one study (Åhlman et al., 1986) has examined metabolism, i.e. formation of sulfur‐containing metabolites in female bile duct‐cannulated rats given an MCCP (1‐chloro‐polychloro[U‐14C]hexadecane, 65% chlorination, 5–6 mg/kg) by injection into the portal vein. The MCCP was conjugated to mercapturic acid and glutathione and less than 3% of the total radioactivity excreted in the bile was due to the parent compound.
In two studies with LCCPs (both C22–26, either 43% or 70% chlorination, 100 or 3,750 mg/kg bw) (IRDC, 1984d,e, unpublished studies, see Documentation provided to EFSA), performed as for the dietary and gavage studies with SCCPs described above, faecal excretion was also the main route of elimination with only insignificant amounts of radioactivity detected in urine and expired air. For the lower chlorinated LCCP, the concentrations in blood were similar between the two dose levels regardless of sex and dosing regimen, whereas for the higher chlorinated LCCP, the concentrations of radioactivity in blood were greater in relatively high‐dose animals. As for the SCCP and MCCP, the highest initial concentration of radioactivity was found in the liver for both LCCPs. In adipose tissue, the concentration increased between 7 and 28 days and then declined only slightly by 90 days for the lower chlorinated LCCP, whereas for the higher chlorinated LCCP, the concentration increased slowly after dosing with only a small decline by 90 days. For the lower chlorinated LCCP, the concentration in ovaries was higher than in the adipose tissue after 12 h and 7 days, but was comparable with the that in the adipose tissue at 28 days, whereas for the higher chlorinated LCCP, the concentration in ovaries were generally similar or lower than in other tissues. Similarly, following a single oral radiolabelled dose of a shorter chain LCCP (C18, 50–53% chlorination) by gavage (Yang et al., 1987), the highest proportion of radioactivity was excreted in the faeces (76%), and approximately 20% of which was identified as the parent LCCP.
3.1.1.1.2. Studies in mice
Five studies on toxicokinetics in mice are available, two studies on three SCCPs with the same carbon chain length (C11) but different degrees of chlorination (17.4%, 55.9% and 68.5%), and three studies on MCCPs with the same carbon chain length (C16) but different degrees of chlorination (34% and 69%). These studies were all performed by the same Swedish group and applied the same methodology, i.e. single oral dose by gavage or by i.v. injection of radiolabelled test compounds predominantly to female mice followed by visual determination of tissue distribution by whole body autoradiography (WBA), and evaluation of tissue retention and elimination in excreta by measuring radioactivity levels of test compounds and metabolites. The CONTAM Panel noted that the WBA is a semiquantitative measure.
The WBA part of the study with three different C11 SCCPs (SCCP‐I: (C11H18.1Cl4.9)‐14CH2Cl, 55.9% chlorination; SCCP‐II (C11H14.2Cl8.8)‐14CH2Cl, 68.5% chlorination; SCCP‐III: H3C‐(CH2)10‐14CH2Cl, 17.4% chlorination, chlorinated only at the 14C‐atom) (Darnerud et al., 1982), showed highest initial radioactivities in tissues with high cell turnover/high metabolic activity (e.g. intestinal mucosa, bone marrow, salivary glands and thymus), which was more pronounced for SCCP‐III, less for SCCP‐I and weak for SCCP‐II, as well as in the liver and white fat (oral administration). Radioactivity was also observed in the central nervous system for SCCP‐III and SCCP‐I, but not for SCCP‐II (30–60 days after i.v. injection). Distribution of radioactivity in fetuses was similar to the distribution in adult females (late gestation) and was most pronounced for SCCP‐III and only weak for SCCP‐II. A similar picture was seen following administration of a low chlorinated MCCP (1‐14C‐C16H30.7Cl3.3, 34.1% chlorination) (Darnerud and Brandt, 1982), i.e. initial distribution to tissues with high cell turnover/high metabolic activity (intestinal mucosa, bone marrow, exocrine glands and brown fat), but only a low distribution to the white fat depots (oral administration); radioactivity in the brain (30 days after i.v. injection); and distribution of radioactivity in fetuses partly similar to that observed in adult females (late gestation). However, following oral administration of a higher chlorinated radiolabelled MCCP (14C‐labelled C16H20.6Cl13.4, 69% chlorination) (Biessmann et al., 1983), the WBA showed initial high levels of radioactivities in bile, liver, kidney and intestinal contents, as well as in corpora lutea up to 30 days after dosing. A long retention in fat was seen. High levels of radioactivity in adipose tissue, adrenals and in myelinated areas of the brain were seen in autoradiograms from pre‐weaning (10‐day‐old) mice orally dosed with a highly chlorinated MCCP (C16, 69% chlorination) (Eriksson and Darnerud, 1985).
The tissue retention part of the SCCPs study (Darnerud et al., 1982) showed a marked labelling in the liver and fat at 24 h after oral administration of SCCP‐I and SCCP‐II; at 30 days, the radioactivity was minimal in the liver but still high in the fat. For the low chlorinated MCCP (Darnerud and Brandt, 1982), measurable radioactivity was present in all tissues examined (i.e. blood, liver, kidney, white and brown fat and brain). In pre‐weaning mice given the highly chlorinated MCCP (Eriksson and Darnerud, 1985), the level of radioactivity in the brain was approximately 3% of the dose administered in 3‐day‐old mice, and approximately 0.5% in 20‐day‐old mice (24 h after administration). At seven days the level of radioactivity was lower in both brain and liver samples.
The elimination part of the SCCPs study (Darnerud et al., 1982) showed that 67% of the administered radioactivity was recovered 12 h after oral administration of SCCP‐I with 33% as 14CO2 in exhaled air, 29% in the urine and 5% in the faeces, whereas for SCCP‐II, only 33% was recovered 12 h after oral administration with 8% as 14CO2 in exhaled air, 4% in the urine and 21% in the faeces. For the low chlorinated MCCP (Darnerud and Brandt, 1982), about 33% of the administered radioactivity was exhaled as 14CO2 within 12 h after oral administration with 6.5% of the radioactivity in the urine and 14% in the faeces. For the higher chlorinated MCCP (Biessmann et al., 1983), the elimination of radioactivity in urine was about 3% and about 66% in the faeces during 96 h; the elimination in exhaled air was about 1%.
A study in female mice given the two different SCCPs above mentioned (SCCP‐I and SCCP‐II) by i.v. injection (Darnerud, 1984) after pretreatment with cytochrome P450 (CYP450) inducers or inhibitors showed that pretreatment with inducers (phenobarbital, 3‐methylcholanthrene, Aroclor 1254) had limited effects on the degree of 14CO2‐exhalation, whereas pretreatment with inhibitors (piperonyl butoxide, metyrapone) decreased the exhalation of 14CO2 considerably. The inhibitor effects increased with increasing degree of chlorination of the CPs. The results suggest a CYP450‐dependent degradation of SCCPs to 14CO2 in vivo and that the degradation seems to be more important for the higher chlorinated SCCP. For the low chlorinated MCCP (Darnerud and Brandt, 1982), TLC of samples taken from the liver, kidney and brown fat revealed a radiolabelled substance with the same retention value as the reference parent compound suggesting that this MCCP was distributed to these tissues without further metabolism.
According to the authors of the article on the three different SCCPs in mice (Darnerud et al., 1982), the uptake of radioactivity in metabolically active tissues and the formation of 14CO2 were inversely related to the degree of chlorination of the SCCPs and the retention of radioactivity in liver and fat was most marked for the highest chlorinated SCCP (i.e. SCCP‐II). This means, according to the authors, that the metabolic fate of CPs depends on the chlorine contents (and probably also chain length) and that results from one CP preparation cannot be considered valid for CPs in general. Similarly, it was stated by the authors of the article on the low chlorinated MCCP in mice (Darnerud and Brandt, 1982), that the results in this specific study are valid only for the MCCP studied and should not be taken as general for all CP preparations.
3.1.1.2. Humans
No studies on the toxicokinetics in humans of relevance for the risk assessment of CPs within the scope of this opinion were retrieved from the literature search.
One study was identified reporting levels of SCCPs and MCCPs in paired samples of maternal and umbilical cord blood serum collected in China (Beijing) in 2013 (Qiao et al., 2018) and several studies have reported levels in human milk (see Section 3.2.4 ). Detection of CPs in human blood and milk samples indicates that CPs are absorbed to some extent in humans and detection of CPs in umbilical cord blood indicates that CPs can be transferred to the fetus.
3.1.1.3. Farm animals, horses and companion animals
A number of studies on the toxicokinetics of CPs in farm animals (poultry and fish) have been retrieved from the literature search. The main outcomes of these studies are described below while a more detailed description of each individual study can be found in Appendix B .
3.1.1.3.1. Studies in poultry
In broiler chickens (Ueberschär and Matthes, 2004) and laying hens (Ueberschär et al., 2007) given diets supplemented with an SCCP (C10–13, 60% chlorination) at different dietary concentrations, the SCCP concentrations in tissues were related linearly to the SCCP concentration of the feed with the highest concentration recovered in abdominal fat. In the laying hens, a high concentration was also found in abdominal fat, egg yolk and liver, whereas levels in breast muscle were low. In the laying hens, the elimination was biphasic with a rapid phase (4–40 minutes) followed by a slower phase.
In female laying Japanese quail orally given an SCCP (14C‐labelled C12H20.1Cl5.9, 55.9% chlorination) or an MCCP (14C‐labelled C16H30.7Cl3.3, 34.1% chlorination) (Biessmann et al., 1982), the autoradiography distributions showed initial high radioactivities in tissues with high metabolic activity and/or high cell turnover rate, e.g. liver, intestinal mucosa, spleen, bone marrow and oviduct. High levels were also seen in the gall bladder and the kidney, as well as in lipid‐rich tissues such as the outer layers of the yolk of the growing follicles, in the uropygial gland epithelium and fat. There was also a high uptake of radioactivity in the eggshells and, to a lesser extent, in the albumen. After exposure times of 4 and 8 days, the highest radioactivity was observed in fat, the yolk of the follicles and in the uropygial gland. The distribution patterns of the SCCPs and the MCCPs were almost identical after all exposure times: in the egg yolk and albumen the concentration of the SCCP was about half of the MCCP. However, the distribution was somewhat different following administration of a higher chlorinated MCCP (14C‐labelled C16H20.6Cl13.4, 69% chlorination) (Biessmann et al., 1983) with initial high radioactivities in bile, liver, kidney and intestinal contents; high radioactivity was also seen in the hypophysis, retina, blood and egg yolk. After 12 days, radioactivity was only observed in the lipid‐rich tissues (fat, uropygial gland, egg yolk) as for the SCCP and the lower chlorinated MCCP; and in the liver. In the elimination part of the study with the SCCP and the lower chlorinated MCCP (Biessmann et al., 1982), the exhalation of 14CO2 during 8 hours following dosing with the MCCP was about twice (38.8%) that of the SCCP (21.6%); the excretion of radioactivity in urine and faeces (combined, during 8 hours) was also higher following dosing with the MCCP (13.5%) compared with SCCP (9.8%). For the higher chlorinated MCCP (Biessmann et al., 1983), the elimination of radioactivity in urine and faeces (combined) was about 58% during 96 hours after administration; the elimination in exhaled air was about 1%.
3.1.1.3.2. Studies in fish
Several studies were performed in bleak (Alburnus alburnus L.). Although it is not usually farmed, the CONTAM Panel noted it belongs to the carp family (Cyprinidae) and studies might be relevant to farmed species. Studies in which the fish were exposed via the tank water were also included, although this exposure route is not entirely relevant to dietary exposure.
Two different LCCPs (Cereclor 42, C22–30, 42% chlorination; Chlorez 700, C20–28, 70% chlorination) given to juvenile Atlantic salmon (Salmo salar) with dry fish food for up to 181 days followed by a control diet for 74 days were not reported to accumulate in the fish (Zitko, 1974).
An SCCP (Chlorowax 500C, C10–12, 50% chlorination) was found in fingerling rainbow trout (Salmo gairdneri Richardson) following administration in the feed for up to 82 days (Lombardo et al., 1975).
In juvenile rainbow trout (Oncorhynchus mykiss) (Fisk et al., 1996) fed diets with two different SCCPs (C12H20Cl6, 56% chlorination; C12H16Cl10, 69% chlorination) and two different MCCPs (C16H31Cl3, 35% chlorination; C16H21Cl13, 69% chlorination) for 40 days and followed by 160 days (C12H20Cl6, C12H16Cl10, C16H31Cl3) or 173 days (C16H21Cl13) of depuration, all four CPs accumulated in the fish by day 5 of the uptake phase without reaching steady state; the depuration rate in fish exposed to C16H31Cl3 was significantly more rapid than the depuration rates in fish exposed to the other three CPs. The highest percentages of radioactivity were found in the carcass for all four CPs (ranging from 50% to higher than 70%). Relative amounts in the liver were low (about 1.5% of the total fish weight). The metabolism of the CPs was most pronounced for the lower chlorinated SCCP. According to the authors, the highly chlorinated SCCP and the lower chlorinated MCCP appear to have the greatest potential for biomagnification among CP components. The authors also stated that reduced accumulation of the lower chlorinated SCCP should be attributed to metabolism, while uptake from the GI of the highly chlorinated MCCP may have been hindered because of its large molecular size.
In another study (Fisk et al., 1998) with juvenile rainbow trout (Oncorhynchus mykiss) fed diets containing 19 different CPs with varying carbon chain length (C10, C11 and C14) and chlorine content (4–8 chlorine atoms) for 40 days followed by 80 days of depuration, all of the CPs were detected in the fish after 5 days of exposure. With the exception of the C14–CPs, most compounds reached steady state between food and fish within 30 or 40 days. Differences in bioaccumulation parameters between CPs with the same molecular formula, but different chlorine positions were observed for some of the CPs. The half‐lives of the CPs ranged from 7 to 53 days. There was a large range in the assimilation efficiencies (from 13–130%). These results showed that all of the CPs were rapidly accumulated and had high assimilation efficiencies from food, that the half‐lives increased with increasing carbon chain length and chlorine content, indirectly, that the CPs were metabolised in the rainbow trout with the susceptibility to metabolism decreasing with greater carbon chain length and chlorine content, and that higher chlorinated C10‐ and C11‐CPs, and all C14‐CPs, would biomagnify from food to fish in aquatic food chains.
In a third study (Fisk et al., 2000) with juvenile rainbow trout (Oncorhynchus mykiss) fed diets with three different 14C‐CPs (C10H15.3Cl6.7, C14H23.3Cl6.7 and C18H31.4Cl6.6) at two different concentrations for 40 days followed by 160 days of depuration, all three CPs accumulated readily from the food, but without reaching steady state; the uptake curves were similar for all three CPs, and for the two concentrations. The assimilation efficiencies varied from 10 to 22%, except for the high‐concentration SCCP group (approximately 72%). The half‐lives of the LCCP (79 and 91 days) were significantly greater than those of the two shorter chain CPs (26–58 days). The BMFs calculated assuming assimilation efficiencies of 50 and 90% exhibited increasing trends with increasing carbon chain length and indicate that the CPs have the potential to biomagnify in aquatic food chains. These results showed that all three CPs have the potential to biomagnify in aquatic food chains with increasing trends with increasing carbon chain length and greater biotransformation of the short‐chain compared to the longer chain CPs.
A high uptake was initially observed (WBA) in the liver, olfactory organs, gills, skin and kidneys in rainbow trout (Salmo gairdneri) exposed during 7 days to a highly chlorinated SCCP (1‐chloro‐polychloro‐[1‐14C]dodecane (PCDD‐H), 68.5% chlorination) added to tank water (Darnerud et al., 1989). Then the radioactivity decreased in the olfactory organs, gills and skin, while the radioactivity in fat steadily increased and at the end of the experiment radioactivity was mainly present in fat, intestinal contents, liver and bile. Following exposure to a low chlorinated MCCP (1‐chloro‐polychloro‐[U‐14C]hexadecane (PCHD‐L), 23.1% chlorination), the initial highest radioactivities were seen in the contents of the gall bladder, pyloric caeca and intestines; a pronounced uptake was also seen in the olfactory organs and gills. After 7 days in contaminated water, the uptake in the olfactory organs and gills was less marked whereas the concentration of radioactivity in fat tissue had increased. After 7 and 21 days in clean water, the distribution pattern were similar to that found after seven days in contaminated water; the olfactory organs and the gills still retained radioactivity. Following exposure to a higher chlorinated MCCP (1‐chloro‐polychloro‐[U‐14C]hexadecane (PCHD‐M), 51.4% chlorination), the initial distribution pattern was almost similar to that observed for PCHD‐L; however, the liver was comparably more labelled after PCHD‐M exposure. After seven days in contaminated water, the uptake was also generally the same as for PCHD‐L; however, the uptake in fat seemed more accentuated for PCHD‐M and the accumulation of radioactivity in fatty tissues was even more prominent at later time points.
In carp (Cyprinus carpio) given an MCCP (polychloro‐1‐14C‐hexadecane (PCHD), 34% chlorination) by injection, the cumulated excretion of 14CO2 during 96 h amounted to 6.4% of the injected radioactivity (Darnerud et al., 1983). WBA showed initial highest radioactivity in bile, intestinal contents, kidney, liver, nasal mucosa and fat; a marked uptake was also observed in gills, testis and brain. Tissue levels were generally lower 13 days after dosing. In bleak (Alburnus alburnus) exposed for 14 days to this MCCP added to the tank water, a marked initial uptake of radioactivity was noted in gills, nasal cavity, skin, liver and fat; a high level was also present in the brain, bile and intestinal contents. A similar distribution pattern was seen after 14 days of exposure, as well as in fish allowed to recover for 1, 7 and 35 days.
A direct correlation was observed between the amount of an SCCP in contaminated feed (Witaclor 149, C10–13, 49% chlorination, three different concentrations: 590, 2,500 and 5,800 μg/g feed) and the level of organic chlorine found in bleak (Alburnus alburnus) tissues during the first 56 days of exposure; the increase continued in the low‐ and mid‐dose groups, while levels in the high‐dose group were unchanged in the remaining part of the exposure duration (91 days) (Bengtsson and Ofstad, 1982). The uptake was about 45% in the low‐dose group, about 10% in the mid‐dose group and about 5% in the high‐dose group at the end of the exposure period. The levels of Witaclor 149 decreased rapidly during the elimination period (316 days). For a higher chlorinated SCCP (Witaclor 171P, C10–13, 71% chlorination, 3,180 μg/g feed), the bioaccumulation pattern was similar to the mid‐dose Witaclor 149 group with a fairly constant uptake during the exposure period; the uptake was around 6% at the end of the exposure period. This SCCP remained in the fish tissues on a steady level until the end of the elimination period. The uptake was inefficient for an LCCP (Witaclor 549, C18–26, 49% chlorination, 3,400 μg/g feed) with only about 2% by the end of the exposure period. About 50% was excreted within 4–5 weeks, whereas the remaining 50% did not seem to be excreted during the subsequent 40 weeks.
A clear relationship between the structure of CPs and their uptake was seen in bleak (Alburnus alburnus L.) when added to tank water (Bengtsson et al., 1979). A short‐chain length, i.e. SCCPs and a low level of chlorination showed the most effective form for uptake among the five tested CPs. The CP test solutions included Witaclor 149 (C10–13, 49% chlorination), Witaclor 159 (C10–13, 9% chlorination), Witaclor 171P (C10–13, 71% chlorination), Witaclor 350 (C14–17, 50% chlorination) and Witaclor 549 (C18–26, 49% chlorination).
Madeley and Birtley (1980) reported that an LCCP (C20–30, 42% chlorination) was found in the tissues of rainbow trout (Salmo gairdneri) and mussels (Mytilus edulis) when mixed with the feed. Comparison of the LCCP concentration in tissues suggested that mussels eliminated the LCCP as the parent compound whereas the fish appeared to metabolise the test compound.
3.1.1.4. Summary on toxicokinetics
No studies on the toxicokinetics in humans of relevance for the risk assessment of CPs within the scope of this opinion were identified from the literature search.
Data on toxicokinetics for various animal species were identified, although in several cases from studies not designed as dedicated absorption, distribution, metabolism and elimination (ADME) studies.
Following oral administration (diet, gavage) of CPs (single 14C‐labelled dose) to rats the major route of excretion was the faeces with only a small proportion excreted in urine and expired air. The highest initial concentrations of radioactivity were found in the liver (SCCPs, MCCPs, LCCPs), kidneys (SCCPs, MCCPs) and ovaries (SCCPs, MCCPs, LCCPs). Initial high concentrations of radioactivity were also seen in the adipose tissue following administration of SCCPs whereas for MCCPs the concentration of radioactivity was highest at 7 days and for LCCPs the concentration of radioactivity increased up to 28 days. In general, the elimination of radioactivity from the adipose tissue was slower than for the other three tissues for all three CP groups; the elimination rate from the adipose tissue decreased with increasing carbon chain length and chlorination degree. One study in rats demonstrated that differences between the distribution of SCCP congeners in blood, urine and faeces were more dependent on chlorination degree than on carbon chain lengths.
A similar picture regarding distribution and elimination was seen in mice administered radiolabelled SCCPs or MCCPs orally (gavage) and/or by i.v. injection followed by visual determination of tissue distribution by WBA and evaluation of tissue retention and elimination in excreta by measuring radioactivity levels. One study with two relatively highly chlorinated SCCPs suggested that CYP450‐dependent degradation seems to be more important for the higher chlorinated SCCPs. The studies in mice suggested that highly chlorinated SCCPs and MCCPs are metabolised and excreted via faeces whereas lower chlorinated SCCPs and MCCPs can be partly metabolised and exhaled as carbon dioxide. The studies in mice also suggested that results from one CP are valid only for the CP studied and should not be taken as general for all CPs.
In broiler chickens and laying hens given diets supplemented with an SCCP the highest concentration was recovered in abdominal fat. In the laying hens, a high concentration was also found in egg yolk and liver. A similar distribution pattern was seen in laying Japanese quail given an SCCP or an MCCP orally.
The fish studies with rainbow trout indicated that CPs were rapidly accumulated and had high assimilation efficiencies from food and that the half‐lives increased with increasing carbon chain length and chlorination degree. The studies also indicated that the CPs have the potential to biomagnify from food to fish in aquatic food chains with increasing trends with increasing carbon chain length and greater biotransformation of the short‐chain CPs compared to the longer chain CPs. Studies with other fish species support the potential of CPs to accumulate in fish tissues.
3.1.1.5. Physiologically based kinetic (PBK) modelling
No PBK model publications for the risk assessment of CPs within the scope of this opinion were identified from the literature search.
3.1.2. Toxicity in experimental animals
3.1.2.1. Acute toxicity studies
In studies with rats and mice, the oral LD50 values for CPs were always greater than the highest administered dose, indicating that CPs are of low acute oral toxicity. The LD50 values all exceeded 4 g/kg bw (Table 11 ).
Table 11.
Oral acute toxicity studies of chlorinated paraffins (CPs)
| CP category | CP specification | Species/strain | LD50 (g/kg bw) | Reference |
|---|---|---|---|---|
| SCCPs | C12, 60% chlorination | F344/N rat | > 13.6 | NTP (1986a); Bucher et al. (1987) |
| C12, 59% chlorination | Rat | > 21.5 | Diamond Shamrock Chem. Co (1975, unpublished study, as cited by Howard et al. (1975) and WHO/IPCS (1996) | |
| C12, 60% chlorination | B6C3F1 mouse | > 27.2 | NTP (1986a), Bucher et al. (1987) | |
|
Cereclor 50LV, C10–13, 50% chlorination |
Wistar rat | > 4 | Birtley et al. (1980) | |
| MCCPs | C14–17, 40–52% chlorination | Rat | > 15 | Kuhnert (1986a,b), Chater (1978), as cited by EU‐RAR (2011) |
|
Cereclor S52, C14–17, 52% chlorination |
Wistar rat | > 4 | Birtley et al. (1980) | |
| LCCPs | C23, 43% chlorination | F344/N rat | > 11.7 | NTP (1986b); Bucher et al. (1987) |
| C23, 43% chlorination | B6C3F1 mouse | > 23.4 | NTP (1986b); Bucher et al. (1987) | |
|
Cereclor 42, C20–30, 42% chlorination |
Wistar rat | > 4 | Birtley et al. (1980) |
LCCPs: long‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; SCCPs: short‐chain chlorinated paraffins; LD50: lethal dose, median.
Single oral doses of SCCP (C12, 60% chlorination) or LCCP (C23, 43% chlorination) up to 13.6 g/kg bw in rats and 27.2 g/kg bw in mice by gavage caused no mortality. The animals were inactive and ataxic. Rats manifested diarrhoea, while mice had ruffled fur 2–6 days after dosing (NTP, 1986a,b,Bucher et al., 1987).
In a single‐administration study, rats were given three different chlorinated SCCPs (C10–13; 41–50% chlorination, 51–60% chlorination or 61–70% chlorination) by gavage, with a range of maximum doses of 4–13 g/kg bw. Piloerection, muscular incoordination, faecal and urinary incontinence were observed in rats that received doses of 2 g/kg bw or more, generally independent of the chlorine content. No deaths were noted except for one rat treated with 13 g/kg bw of SCCP (C10–13, 63% chlorination) (Birtley et al., 1980; WHO/IPCS, 1996). MCCP (C14–17, 51–60% chlorination) and LCCPs (C20–30; 41–51% chlorination, 51–61% chlorination or 61–70% chlorination) caused similar clinical symptoms to those reported for SCCPs (Birtley et al., 1980).
3.1.2.2. Repeated dose toxicity studies
3.1.2.2.1. SCCPs
Ten repeated dose toxicity studies on SCCPs in rats and five in mice were identified. They are summarised in Table 12 and discussed below.
Table 12.
Repeated dose toxicity studies on SCCPs in rats and mice
| Species/ strain | Number of animals per group | SCCP specification | Duration | Administration | Dose levels (mg/kg bw per day) | Most sensitive endpoints | NOAEL (mg/kg bw per day) | LOAEL (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Studies in rats | |||||||||
| F344 rat | 5/sex per group |
C10–12, 58% chlorination |
14 days | Diet | 0, 100, 300, 1,000, 3,000 | ↑ absolute and relative liver weights, ↑ incidence of hepatocellular hypertrophy | – | 100 | IRDC (1983a)a |
| F344 rat | 5/sex per group |
C10–12, 58% chlorination |
14 days | Gavage (corn oil) | 0, 30, 100, 300, 1,000, 3,000 | ↑ relative liver weights in males | 30 | 100 | IRDC (1981a)a |
| Alpk:APfSD rat | 5/males per group |
Cereclor 56L, C10–13, 56% chlorination |
14 days | Gavage (corn oil) | 0, 10, 50, 100, 250, 500, 1,000 | ↑ relative liver weightsb | 10 | 50 | Wyatt et al. (1993) |
| Alpk:APfSD rat | 5/males per group | Chlorowax 500C, C10–13, 58% chlorination | 14 days | Gavage (corn oil) | 0, 10, 50, 100, 250, 500, 1,000 | ↑ absolute and relative liver weights | 50 | 100 | Wyatt et al. (1993) |
| F344 rat | 5/sex per group |
C12, 60% chlorination |
16 days | Gavage (corn oil) | 0, 469, 938, 1,875, 3,750, 7,500 | Liver enlargement | – | 469 | NTP (1986a), Bucher et al. (1987) |
| Sprague–Dawley rat | 9/males per group |
C10–13, 56.5% chlorination |
28 days | Gavage (corn oil) | 0, 1, 10, 100 | ↓ plasma FT3, ↑ plasma TSH | 1 (NOEL) | 10 (LOEL) | Gong et al. (2018) |
| F344 rat |
15/sex per group |
C10–12, 58% chlorination |
90 days | Diet | 0, 10, 100, 625 | ↑ absolute and relative liver and kidney weights; histopathological changes such as hepatocellular hypertrophy (both sexes), mild nephritis (males only), and thyroid hypertrophy and hyperplasia (males only) | 10 | 100 | IRDC (1984a)a, Serrone et al. (1987) |
| F344 rat | 15/sex per group |
C10–12, 58% chlorination |
90 days |
Gavage (corn oil) |
0, 10, 100, 625 | ↑ absolute and relative liver and kidney weights; histopathological changes such as hepatocellular hypertrophy (males only), mild nephropathy (males only) | 10 | 100 | IRDC (1984b)a, Serrone et al. (1987) |
| F344 rat | 10/sex per group |
C12, 60% chlorination |
90 days | Gavage (corn oil) | 0, 313, 625, 1,250, 2,500, 5,000 | ↑ relative liver weights | – | 313 | NTP (1986a), Bucher et al. (1987) |
| F344 rat |
50/sex per group 20/sex per group |
C12, 60% chlorination |
2 years | Gavage (corn oil) | 0, 312, 625 | ↑ liver and kidney weights; histopathological finding in the liver, kidney, stomach, forestomach and parathyroid | – | 312 | NTP (1986a), Bucher et al. (1987) |
| Studies in mice | |||||||||
| Alpk:APfCD‐1 mouse | 5/males per group | Cereclor 56L, C10–13, 56% chlorination | 14 days | Gavage (corn oil) | 0, 10, 50, 100, 250, 500, 1,000 | ↑ absolute and relative liver weights | 50 | 100 | Wyatt et al. (1993) |
| Alpk:APfCD‐1 mouse | 5/males per group | Chlorowax 500C, C10–13, 58% chlorination | 14 days | Gavage (corn oil) | 0, 10, 50, 100, 250, 500, 1,000 | ↑ relative liver weights | 100 | 250 | Wyatt et al. (1993) |
| B6F3C1 mouse | 5/sex per group |
C12, 60% chlorination |
16 days | Gavage (corn oil) | 0, 938, 1,875, 3,750, 7,500, 15,000 | liver enlargement | – | 938 | NTP (1986a), Bucher et al. (1987) |
| B6F3C1 mouse | 10/sex per group |
C12, 60% chlorination |
90 days | Gavage (corn oil) | 0, 125, 250, 500, 1,000, 2,000 | ↑ relative liver weights (females), hepatocellular hypertrophy (both sexes) | 125 | 250 | NTP (1986a), Bucher et al. (1987) |
| B6F3C1 mouse | 50/sex per group |
C12, 60% chlorination |
2 years | Gavage (corn oil) | 0, 125, 250 | Histopathological findings in the kidney (females only) and in the thyroid (both sexes) | – | 125 | NTP (1986a), Bucher et al. (1987) |
SCCPs: short‐chain chlorinated paraffins; NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; bw: body weight; T3: triiodothyronine; TSH: thyroid‐stimulating hormone; FT3: free T3.
Unpublished study report, see Documentation provided to EFSA.
For absolute liver weight increase, the lowest significant effect was at 10 mg/kg bw per day but followed an irregular dose response. As consequence, it was not used for the determination of NOAEL and LOAEL.
Studies in rats
In a 14‐day rat study, SCCP (C10–12, 58% chlorination) was administered in the diet at dose levels of 0, 100, 300, 1,000 or 3,000 mg/kg bw per day (IRDC, 1983a, unpublished study, see Documentation provided to EFSA). No animals died and no clinical signs of toxicity could be observed. Body weight and food consumption was decreased by approximately 50% at the highest dose after 2 weeks. The absolute and relative liver weights were increased in all treatment groups; the relative liver weights in females by 20% (lowest dose) up to 240% (highest dose) compared to controls. The incidence of hepatocellular hypertrophy increased in all treatment groups in a dose‐dependent manner. Liver microsomal aminopyrine N‐demethylase (APND) activity and total CYP450 content was increased in all dose groups. These effects were significant in females from 300 mg/kg bw per day and in males from 1,000 mg/kg bw per day. The LOAEL of this study was 100 mg/kg bw per day, based on increased relative liver weight and increased incidence of hepatocellular hypertrophy in all treated groups.
SCCP (C10–12, 58% chlorination) was given to rats in doses of 0, 30, 100, 300, 1,000 or 3,000 mg/kg bw per day in corn oil by gavage for 14 days (IRDC, 1981a, unpublished study, see Documentation provided to EFSA). No deaths occurred that were related to the treatment with the test substance. Several clinical signs of toxicity were reported including staining around mouth, nose and anogenital region, decreased motor activity, laboured breathing and excessive lachrymation. Twenty per cent reduction of body weight could be observed at top‐dose females at day 14 compared to the control group. Average food consumption was reduced in top‐dose males and females. A significant increase (> 10%) in average water consumption was noted for the males at doses of 1,000 mg/kg bw per day or more. The absolute and relative liver weight was increased at doses of 300 mg/kg bw per day or more in both sexes. At 100 mg/kg bw per day, there was already a 18% increase of relative liver weight in males compared to controls. Diffuse mild hepatocellular hypertrophy was observed at the two highest dose levels in all animals. The authors also reported ‘trace’ (not further specified) centrilobular hypertrophy in 2 out of 5 animals of each sex at 300 mg/kg bw per day. Dose‐related increase in hepatic microsomal enzyme activity (APND) was noted in females treated with 300 mg/kg bw per day and above. At 3,000 mg/kg bw per day, relative and absolute ovary weights were decreased. Absolute ovary weights were decreased to 42% of the control value. At the same dose level, a significant reduction in absolute and relative thymus weights of both sexes was noted. Absolute thymus weights of males were decreased to 19% of the control value at 3,000 mg/kg bw per day. The NOAEL of this study was 30 mg/kg bw per day, based on relative liver weight increase (18%) in males at 100 mg/kg bw per day.
Male rats (5 per group) were administered two different SCCPs (Cereclor 56L, C10–13, 56% chlorination, or Chlorowax 500C, C10–13, 58% chlorination) in corn oil in doses of 0, 10, 50, 100, 250, 500 or 1,000 mg/kg bw per day per gavage for 14 days (Wyatt et al., 1993). For Cereclor 56L, absolute liver weights were significantly increased at 10 mg/kg bw per day and from 100 mg/kg bw per day or greater (irregular dose response). Relative liver weights were significantly increased at 50 mg/kg bw per day (approximately 17%) or greater in a dose‐related manner. For Chlorowax 500C, both absolute and relative liver weights were significantly and dose‐relatedly increased from 100 mg/kg bw per day (approximately 15%). Palmitoyl coenzyme A (CoA) oxidation, an indicator for peroxisome proliferation, was significantly increased by Cereclor 56L at 1,000 mg/kg bw per day and by Chlorowax 500C from 250 mg/kg bw per day. For Cereclor 56L and Chlorowax 500C, palmitoyl CoA oxidation was 2.5‐fold and 3‐fold higher than the control values at the top dose. Free thyroxine (FT4) and total thyroxine (TT4) levels in plasma were significantly decreased and thyroid‐stimulating hormone (TSH) levels in plasma were significantly increased by both SCCPs at 1,000 mg/kg bw per day, the only dose at which these parameters had been determined. No differences in free triiodothyronine (FT3) or total triiodothyronine (TT3) levels in plasma could be observed for either SCCPs at 1,000 mg/kg bw per day. The capacity of liver microsomes to glucuronidate T4 was markedly increased (approximately 2‐fold) by Cereclor 56L and Chlorowax 500C (1,000 mg/kg bw per day). Glucuronidation of p‐nitrophenol was only significantly increased by Chlorowax 500C but not by Cereclor 56L. The NOAEL for Cereclor 56L was 10 mg/kg bw per day based on the increase of relative liver weights (> 17% at 50 mg/kg bw per day) in a dose‐related manner. The NOAEL for Chlorowax 500C was 50 mg/kg bw per day based on increased absolute and relative liver weights (> 15% increase in relative liver weight at 100 mg/kg bw per day). For more information on the mode of action from this study see also in Section 3.1.5.1 and 3.1.5.3 .
Rats were treated with SCCP (C12, 60% chlorination) in doses of 0, 469, 938, 1,875, 3,750 or 7,500 mg/kg bw per day in corn oil by gavage for 16 days 5 days per week (NTP, 1986a; Bucher et al., 1987). Three deaths occurred in top‐dose animals (1 male, 2 females). Livers were enlarged in all dose groups except the 469 mg/kg bw per day group of females. Histological examinations were not performed. The LOAEL was 469 mg/kg bw per day based on enlarged livers observed at all dose levels in males.
To investigate the effects on thyroid hormones and mode of actions, male rats (9 per group) were administered SCCP (C10–13, 56.5% chlorination; mass ratio of C10:C11:C12:C13 = 1:1:1:1) at doses of 0, 1, 10 or 100 mg/kg bw per day in corn oil by gavage for consecutive 28 days (Gong et al., 2018). Relative thyroid weights were not affected at any dose level. No effects could be observed on thyroid histology at 100 mg/kg bw per day, the only dose examined. At 10 and 100 mg/kg per day, plasma FT3 levels were significantly and dose‐relatedly decreased (20% at 10 mg/kg bw per day), while plasma TSH levels were significantly and dose‐relatedly increased (15% at 10 mg/kg bw per day). At 100 mg/kg bw per day, plasma levels of FT4 and hepatic T4 were significantly decreased, while hepatic T3 levels were significantly increased. Various changes in gene expression were observed, and those relevant to the mode of action are described in Sections 3.1.5.1 and 3.1.5.3 . The NOEL was 1 mg/kg bw per day, based on effects on thyroid hormones (decrease of plasma FT3 and increase of plasma TSH).
In a 90‐day dietary study, SCCP (C10–12, 58% chlorination) was administered to rats in doses of 0, 10, 100 or 625 mg/kg bw per day (Serrone et al., 1987; IRDC, 1984a, unpublished study, see Documentation provided to EFSA). No mortality and no clinical signs of toxicity were observed. There was a slight reduction in body weight gain (high‐dose males). In the blood biochemistry analyses, significant increases in total protein and serum cholesterol in the highest dose, and glucose levels in mid‐ and high‐dose animals were also observed. Slight dose‐related increases in liver protein content were noted in the treated males with corresponding increases in total CYP450 content and APND activity, particularly in top‐dose males. Absolute and relative weights of liver and kidney were significantly increased in the mid‐ and high‐dose group (both sexes). Histopathological findings included increased incidences of hepatocellular hypertrophy in the mid‐ and high‐dose group (both sexes). An increased incidence and severity of trace‐to‐mild thyroid hypertrophy and hyperplasia was observed in mid‐ and high‐dose males. An increased incidence of thyroid hypertrophy was also observed in high‐dose females. An increased incidence and severity of trace‐to‐mild chronic nephritis33 was also observed in kidney at the mid‐dose and high‐dose males. Increased pigmentation of the renal tubules occurred in the high‐dose females. The NOAEL was 10 mg/kg bw per day, based on increased liver and kidney weights and histopathological changes in liver, kidney and thyroid at 100 mg/kg bw per day.
In an accompanying gavage study, SCCP (C10–12, 58% chlorination) was administered to rats for 90 days at doses of 0, 10, 100 or 625 mg/kg bw per day in corn oil (Serrone et al., 1987; IRDC, 1984b, unpublished study, see Documentation provided to EFSA). Similar findings were observed as in the dietary study. No mortality occurred and no clinical signs of toxicity were observed. There was a slight increase in body weight gain in high‐dose females. There was an increase in food consumption in the high‐dose groups of both sexes. Absolute and relative liver and kidney weights were increased in the mid‐ and high‐dose groups of both sexes. Absolute and relative thyroid weights were increased in the high‐dose males. In the males of the mid‐ and high‐dose groups, increased incidences of hepatocellular hypertrophy were observed. The degree of severity in the mid‐dose group was trace and in the high‐dose group it was mild‐to‐moderate. An increased incidence and severity of thyroid hypertrophy and hyperplasia was observed in high‐dose males. Trace and trace‐to‐mild nephropathy occurred in males of the mid‐ and high‐dose groups, respectively. In the kidneys of high‐dose females, a greater degree than normal of brown pigments in the cytoplasm of cortical tubular epithelial cells was noted. The NOAEL was 10 mg/kg bw per day, based on the increase in liver and kidney weights, as well as histopathological changes in liver (hepatocellular hypertrophy, males) and kidney (nephropathy, males) at 100 mg/kg bw per day.
In a 90‐day rat study, SCCP (C12, 60% chlorination) was given in doses of 0, 313, 625, 1,250, 2,500 or 5,000 mg/kg bw per day in corn oil by gavage 5 days per week (NTP, 1986a; Bucher et al., 1987). No mortality was observed. The final body weights of the males that received 2,500 and 5,000 mg/kg bw per day were 11 and 12% lower than the control group. Relative liver weight was significantly increased at all dose levels, the increase ranging from 25% (lowest dose) to 100% (highest dose). Hepatocellular hypertrophy was observed in all top‐dose animals, and nephropathy in all top‐dose males, in 3/10 top‐dose females and in 8/10 male controls. Histopathological examination was performed in high‐dose and control animals only. No histopathological findings were reported in the heart, thymus, thyroid or spleen. The LOAEL of this study was 313 mg/kg bw per day, based on increased relative liver weights observed at all dose levels.
Groups of 50 male and 50 female F344/N rats were given SCCP (C12, 60% chlorination) in doses of 0, 312 or 625 mg/kg bw per day in corn oil by gavage 5 days per week for 2 years (NTP, 1986a; Bucher et al., 1987). Additional groups (20/sex per group) were included in the 6‐ and 12‐month studies. In the main study, survival of the low‐ and high‐dose males and low‐dose females was lower than that of the control (control: 27/34; low‐dose: 6/23; high‐dose: 3/29 for males/females, respectively). Body weight gain was reduced by 12% at 6 and 12 months, and by 23% in the main study. Liver and kidney weights (absolute and relative) were increased in a dose‐related manner; the increase was significant at both 6 and 12 months (up to 124% for liver and up to 46% for kidney), but there was no difference in weight at the two time points. In the main study, the organ weights were not reported. Several non‐neoplastic histopathological changes were found. These included effects in the liver (hepatocellular hypertrophy, necrosis, focal cellular changes and gross dilation of the blood vessels), kidney (cysts; in the cortex of males), tubular cell hyperplasia (in males), nephropathy (incidence and severity in females, severity in males), parathyroid (fibrous osteodystrophy in males), forestomach (ulcers, inflammation, epithelial hyperplasia and hyperkeratosis in males) and glandular stomach (oedema and erosion in males). The LOAEL was 312 mg/kg bw per day, based on the increase in liver and kidney weight and non‐neoplastic lesions observed at both doses. The neoplastic lesions observed in this study are addressed in the carcinogenicity subsection (see Section 3.1.2.7 ).
Studies in mice
Two different SCCPs (Cereclor 56L, C10–13, 56% chlorination or Chlorowax 500C, C10–13, 58% chlorination) in corn oil were administered to male mice (5 per group) in doses of 0, 10, 50, 100, 250, 500 or 1,000 mg/kg bw per day per gavage for 14 days (Wyatt et al., 1993). For Cereclor 56L, absolute and relative liver weights were significantly increased from 100 mg/kg bw per day (approximately 26 and 28%, respectively). For Chlorowax 500C, relative liver weight was increased from 250 mg/kg bw per day (approximately 23%), while absolute liver weight from 500 mg/kg bw per day (approximately 73%). Palmitoyl CoA oxidation as an indicator for peroxisome proliferation was significantly increased from 250 mg/kg bw per day for both Cereclor 56L and Chlorowax 500C. The NOAEL was 50 mg/kg bw per day for Cereclor 56L based on increased relative and absolute liver weights, while it was 100 mg/kg bw per day for Chlorowax 500C based on increased relative liver weights.
In a 16‐day study in mice, SCCP (C12, 60% chlorination) was administered in doses of 0, 938, 1,875, 3,750, 7,500 or 15,000 mg/kg bw per day in corn oil by gavage 5 days per week (NTP, 1986a; Bucher et al., 1987). All animals died that received doses from 3,750 mg/kg bw per day or more. At 1,875 mg/kg bw per day, 4/5 males and 2/5 females died. Diarrhoea occurred in all dose groups except in low‐dose females. Enlargement of the livers were reported in all treated mice that survived. No histopathological examination was performed in this study. The LOAEL was 938 mg/kg bw per day based on liver enlargement.
SCCP (C12, 60% chlorination) was administered to mice in doses of 0, 125, 250, 500, 1,000 or 2,000 mg/kg bw per day in corn oil by gavage 5 days per week for 90 days (NTP, 1986a; Bucher et al., 1987). No substance‐related deaths occurred. Body weight gain was reduced by 13% in high‐dose males. Relative liver weights were increased in a dose‐related manner in both sexes. This effect was significant in females from 250 mg/kg bw per day (21%) and in males from 500 mg/kg bw per day (38%). Hepatocellular hypertrophy was reported from 250 mg/kg bw per day in both sexes, and focal hepatic necrosis from 500 mg/kg bw per day in males and at 2,000 mg/kg bw per day in females. No changes were observed in the thyroid. The NOAEL was 125 mg/kg bw per day, based on hepatocellular hypertrophy (both sexes) and increase in relative liver weight (21%) (females only) at 250 mg/kg bw per day.
In a 2‐year study with B6F3C1 mice, SCCP (C12, 60% chlorination) was administered in doses of 0, 125 or 250 mg/kg bw per day in corn oil by gavage 5 days per week (NTP, 1986a; Bucher et al., 1987). Reduced survival of high‐dose females occurred after week 100 (surviving from original 50 animals, control: 34/35; low‐dose: 30/31; high‐dose: 30/25 for males/females, respectively). Body weights were decreased by about 10% in treated females compared to the controls. Non‐neoplastic histopathological changes were found in the thyroid (follicular cell lesions in all groups ranging from early hyperplasia to multi‐layered projections that extended into the lumen, 10%/32%, 12%/55%, 24%/45% in control, mid‐ and high‐dose males/females, respectively) and in the kidney (increased incidence of nephropathy in females). No non‐neoplastic lesions were reported in the liver. The LOAEL for non‐neoplastic lesions was 125 mg/kg bw per day based on the effects in the kidney (females) and thyroid at both doses. The neoplastic lesions observed in this study are addressed in the carcinogenicity subsection (see Section 3.1.2.7 ).
3.1.2.2.2. MCCPs
Six repeated dose toxicity studies on MCCPs in rats, one study in mice and one study in dogs were identified. They are summarised in Table 13 and discussed below.
Table 13.
Repeated dose toxicity studies on MCCPs in rats, mice and dogs
| Species/ strain | Number of animals per group | MCCP specification | Duration | Administration | Dose levels (mg/kg bw per day) | Most sensitive endpoints | NOAEL (mg/kg bw per day) | LOAEL (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Studies in rats | |||||||||
| F344 rat | 5/sex per group |
Cereclor S52, C14–17, 52% chlorination |
14 days | Diet |
0, 17.3, 57.5, 173, 547, 1,537 (males); 0, 18.1, 57.9, 180, 576, 1,287 (females) |
↑ relative liver weight (females) | 58 | 180 | IRDC (1981b)a |
| Alpk:APfSD rat | 5/males per group | Chlorparaffin 40G, C14–17, 40% chlorination | 14 days | Gavage (corn oil) | 0, 10, 50, 100, 250, 500, 1,000 | ↑ absolute and relative liver weightsb | 50 | 100 | Wyatt et al. (1993) |
| F344 rat |
10/sex per treated group; 20/sex per control |
Cereclor S52, C14–17, 52% chlorination |
90 days | Diet |
0, 2.4, 9.3, 23, 222 (males); 0, 2.5, 9.7, 24.6, 242 (females) |
↓ plasma FT3 in males ↑ plasma TSH in females |
9.3/9.7 (NOEL) | 23/24.6 (LOEL) | CXR (2005b, draft study)a |
| Sprague–Dawley rat | 10/sex per group |
C14–17, 52% chlorination |
90 days | Diet |
0, 0.4, 4, 36, 360 (males); 0, 0.4, 4, 42, 420 (females) |
Histopathological changes in liver, kidney and thyroid | – | –c | Poon et al. (1995) |
| F344 rat | 15/sex per group |
Cereclor S52, C14–17, 52% chlorination |
90 days | Diet | 0, 10, 100, 625 | ↑ absolute and relative liver and kidney weights | 10 | 100 | IRDC (1984c)a |
| Wistar rat | 24/sex per group |
Cereclor S52, C14–17, 52% chlorination; containing 0.2% (w/v) epoxidised vegetable oil as stabiliser |
90 days | Diet |
0, 33, 167, 333 (males); 0, 32, 160, 320 (females) 0, 16.5 (males; 0, 16 (females) |
↑ relative liver weights (females), proliferation of smooth endoplasmic reticulum No significant changes in relative liver weights |
–16 |
32 – |
Birtley et al. (1980) |
| Studies in mice | |||||||||
| Alpk:APfCD‐1 mouse | 5/males per group | Chlorparaffin 40G, C14–17, 40% chlorination | 14 days | Gavage (corn oil) | 0, 10, 50, 100, 250, 500, 1,000 | ↑ palmitoyl CoA oxidation (indicator for peroxisome proliferation) | 250 | 500 | Wyatt et al. (1993) |
| Studies in dogs | |||||||||
| Beagle dog | 4/sex per group | Cereclor S52, C14–17, 52% chlorination; containing 0.2% (w/v) epoxidised vegetable oil as stabiliser | 90 days | Diet | 0, 10, 30, 100 | ↑ hepatic smooth endoplasmic reticulum (males) | 10 (NOEL) | 30 (LOEL) | Birtley et al. (1980) |
MCCPs: medium‐chain chlorinated paraffins; NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; bw: body weight; NOEL: no‐observed effect level; LOEL: lowest‐observed effects level; T3: triiodothyronine; TSH: thyroid‐stimulating hormone; FT3: free T3.
Unpublished study report, see Documentation provided to EFSA.
Relative liver weights were significantly increased at 10 mg/kg bw per day but did not follow a regular dose response. LOAEL of 100 mg/kg bw per day was determined since both absolute and relative livers weights were significantly increased at this dose level.
The CONTAM Panel was not able to interpret the histopathological findings in the liver, kidney and thyroid, and thus not able to identify a NOAEL from this study.
Studies in rats
In a 14‐day dietary study with rats, MCCP (Cereclor S52, C14–17, 52% chlorination) was administered at doses of 0, 17.3, 57.5, 173, 547 or 1,537 mg/kg bw per day to males and 0, 18.1, 57.9, 180, 576 or 1,287 mg/kg bw per day to females (IRDC, 1981b, unpublished study, see Documentation provided to EFSA). No mortality and no clinical signs were observed. Food consumption was reduced by up to 31% (in top‐dose females). Absolute and relative liver weights were increased by up to 80% at the two highest dose levels in both sexes. Relative liver weights in females were already significantly increased by 19% at 180 mg/kg bw per day. Also, absolute and relative ovary weights were decreased (by 38%) at the top dose in females. Diffuse mild hepatocellular hypertrophy was noted at the two highest dose levels in males. No histopathological findings were observed in the ovary. The NOAEL of this study was 58 mg/kg bw per day based on an increased relative liver weight (19% at 180 mg/kg bw per day) in females.
MCCP (Chlorparaffin 40G, C14–17, 40% chlorination) in corn oil was administered to male rats (5 per group) in doses of 0, 10, 50, 100, 250, 500 or 1,000 mg/kg bw per day per gavage for 14 days (Wyatt et al., 1993). Significant increase in relative liver weights was observed at 10 mg/kg bw per day, at 100 mg/kg bw per day and from 500 mg/kg bw per day (irregular dose response). Absolute liver weight was significantly increased at 100 mg/kg bw per day and from 500 mg/kg bw per day (irregular dose response). Palmitoyl CoA oxidation was increased from 500 mg/kg bw per day. The NOAEL was 50 mg/kg bw per day based on increased absolute and relative liver weights (18 and 19%, respectively) at 100 mg/kg bw per day.
In a 90‐day dietary study, F344 rats received MCCP (Cereclor S52, C14–17, 52% chlorination) at doses of 0, 2.4, 9.3, 23 or 222 mg/kg bw per day to males and 0, 2.5, 9.7, 24.6 or 242 mg/kg bw per day to females (CXR, 2005b, unpublished draft study, see Documentation provided to EFSA). No adverse effects on body weight, body weight gain or food consumption were measured. At the highest dose level, absolute and relative liver and kidney weights were significantly increased by 13–31% and by 9–13%, respectively. A small but statistically significant increase by 7% in relative kidney weight was already observed at 24.6 mg/kg bw per day only in females. Such a small change was not considered of toxicological relevance. Thyroid, liver and kidney histopathology was performed in all samples of both sexes. Minimal centrilobular hepatocellular hypertrophy could be found in the top‐dose males. There was a slight increase of the incidence of ultimobranchial cysts in thyroids of males at 23 mg/kg bw per day (3 out of 10 vs. 1 out of 10 in the control group). Significant decreases in plasma triglycerides (by 28–39%) and cholesterol (by 14–23%) were observed in top‐dose animals. Hepatic microsomal T4‐uridine 5’‐diphospho‐glucuronosyltransferase (UDPGT) activity was increased in high‐dose males (by 82%) and in females at the three highest dose levels (30, 30 and 252%). There was no increase on palmitoyl CoA oxidation that would be indicative for peroxisome proliferation. A significant decrease of plasma FT3 levels was detected in males at 23 and 222 mg/kg bw per day by 26 and 22%, respectively. In contrast, TT3 levels, FT4 and TT4 levels were not changed. A significant decrease of plasma FT4 levels could be found in high‐dose females (by 41%) while TT4 levels, or FT3 and TT3 levels were not affected. An increase in plasma TSH levels was reported in females at the two highest dose levels (by 20 and 39%) and in high‐dose males (by 17%). The NOEL was 9.3/9.7 mg/kg bw per day, for males/females, respectively, based on effects on thyroid hormones (decrease of plasma FT3 of males and increase of plasma TSH in females).
Sprague–Dawley rats were fed diets containing MCCP (C14–17, 52% chlorination) at doses of 0, 0.4, 3.6, 36.2 or 362.9 mg/kg bw per day in males and 0, 0.4, 4.2, 42.2 or 418.9 mg/kg bw per day in females for a period of 90 days (Poon et al., 1995). No mortality and no clinical signs of toxicity were reported. Body weight, body weight gain and food consumption were not affected by the treatment. Relative liver weights were increased in females at the two highest dose levels (by 7 and 47%) and in high‐dose males (by 27%). Relative kidney weights were increased in high‐dose animals (by 8–11%). Serum cholesterol was increased in females by about 22, 20 and 28% at 4, 42 and 420 mg/kg bw per day, respectively. However, standard deviations were large but not given in numbers so that no statement on the statistical significance of the cholesterol effect can be made. Such a trend was not observable in males. In males at 360 mg/kg bw per day, AST activity was about 20% higher than control, and inorganic phosphate about 13% higher than control. A significant increase in hepatic UDPGT activity was detected in top‐dose males and females (by 58 and 70%). In the liver, minimal to mild anisokaryosis and vesiculation of the nuclei were observed at the two highest dose levels. There was an increase in perivenous hepatocyte homogeneity in high‐dose males and in females at the two highest dose levels. The CONTAM Panel noted that it is not clear from the paper what ‘perivenous hepatocyte homogeneity’ refers to as this was not further described. Single cell necrosis occurred in high‐dose animals. In the kidney of all treated males, hyaline‐droplet like cytoplasmic inclusions were observed, but these findings were only significant in the high‐dose group. Inner medullary tubular dilation of minimal severity occurrence in a dose‐related manner with incidences of 0/10, 0/10, 1/10, 4/10 and 8/10 in females at 0, 0.4, 4.2, 42 and 420 mg/kg bw per day, respectively. In the thyroid of males from 36 mg/kg bw per day and of females from 4 mg/kg bw per day, reduced follicle sizes, collapsed angularity, increased follicle height, cytoplasmic vacuolation and nuclear vesiculation were observed. The changes were generally minimal to mild in nature. Mild cytoplasmic vacuolation of the pituitary gland and sinus hyperplasia of the spleen were observed at the highest doses. It was noted that the average scorings in females at 4.2 mg/kg bw per day were minimal to mild. It was not possible for the CONTAM Panel to obtain the scorings for individual animals from the study authors. Therefore, the Panel was not able to interpret the histopathological findings in the liver, kidney and thyroid, and thus not able to identify a NOAEL from this study.
In a 90‐day dietary study in F344 rats, MCCP (Cereclor S52, C14–17, 52% chlorination) was given at dose levels of 0, 10, 100 or 625 mg/kg bw per day (IRDC, 1984c, unpublished study, see Documentation provided to EFSA). Two female rats died during the conduct of this study. The deaths were reported to be unrelated to the treatment. Body weight gain was slightly reduced (< 5%) and could be observed in males and females. There was an increase (by 25%) in serum cholesterol in high‐dose females. Absolute and relative liver weights were increased in mid‐dose (up to 28%) and high‐dose animals (up to 100%) of both sexes. Absolute and relative kidney weights were increased in animals of both sexes up to 11% at 100 mg/kg bw per day and up to 25% at 625 mg/kg bw per day. Increased absolute thyroid weights (50%) in males only, and increased adrenals weights in both sexes (up to 39%) were observed at the top dose. Histopathological changes were noted in the liver (hepatocellular hypertrophy of mild severity) in high‐dose males and females (13/15 in both sexes), with males (1/15) at 100 mg/kg bw per day also affected. In the thyroid of almost all control and treated males, trace to moderate hypertrophy and hyperplasia were observed. There was a trend towards increasing severity with increasing dose. At 625 mg/kg bw per day, thyroid hypertrophy was observed in mild form (9/15) and in moderate form (3/15). At the same dose level, thyroid hyperplasia occurred in mild form (9/15). Kidney effects were reported including chronic nephritis in males (1/15, 3/15, 4/15, 10/15) and as renal tubular pigmentation in high‐dose females. The NOAEL was 10 mg/kg bw per day, based on an increase in absolute and relative liver and kidney weights at higher dose levels.
In a 90‐day dietary study with Wistar rats, an MCCP (Cereclor S52, C14–17, 52% chlorination, containing epoxidised vegetable oil stabiliser) was administered at dose levels of 0, 33, 167 or 333 mg/kg bw per day in males and of 0, 32, 160 or 320 mg/kg bw per day in females (Birtley et al., 1980). No animals died and no clinical signs of toxicity were reported. Body weight gain was reduced by 17 and 25%. Relative liver weight was increased in males from 167 mg/kg bw per day (by 15 and 22%) and in females from 32 mg/kg bw per day (by 11, 21 and 48%). Using electron microscopy, histopathological changes in the liver included a dose‐related proliferation of smooth endoplasmic reticulum could be found, starting from the lowest dose. The LOAEL was 32 mg/kg bw per day, based on increased relative liver weights (in females) and proliferation of smooth endoplasmic reticulum. In the same dietary study performed with a time shift of several weeks, rats were administered dose levels of 0, 16 and 16.5 mg/kg bw per day in males and females, respectively, for 13 weeks. No changes could be observed in the relative liver weights between treated animals and the control group and no histopathological abnormalities could be found. Combining these findings, it can be concluded that the NOAEL for the endpoint of relative liver weight is 16 mg/kg bw per day.
Studies in mice
Male mice (5 per group) were administered MCCP (Chlorparaffin 40G, C14–17, 40% chlorination) in corn oil in doses of 0, 10, 50, 100, 250, 500 or 1,000 mg/kg bw per day per gavage for 14 days (Wyatt et al., 1993). Absolute and relative liver weights were significantly increased at 1,000 mg/kg bw per day. Palmitoyl CoA oxidation was significantly increased from 500 mg/kg bw per day (270%). The NOAEL was 250 mg/kg bw per day based on increased palmitoyl CoA oxidation.
Studies in dogs
Beagle dogs were fed MCCP (Cereclor S52, C14–17, 52% chlorination, containing epoxidised vegetable oil stabiliser) in the diet at dose levels of 0, 10, 30 or 100 mg/kg bw per day during a period of 90 days (Birtley et al., 1980). Serum alkaline phosphatase activity and relative liver weight in males were increased at 100 mg/kg bw per day. In addition, smooth endoplasmic reticulum of hepatocytes as measured by electron microscopy, was increased in males at 30 and 100 mg/kg bw per day. The NOEL was 10 mg/kg bw per day, based on an increase of hepatic smooth endoplasmic reticulum in males at higher dose levels.
3.1.2.2.3. LCCPs
Seven repeated dose toxicity studies on LCCPs in rats and three studies in mice were identified. They are summarised in Table 14 and discussed below.
Table 14.
Repeated dose toxicity studies on LCCPs in rats and mice
| Species/ strain | N animals per group | LCCP specification | Duration | Administration | Dose levels (mg/kg bw per day) | Most sensitive endpoints | NOAEL (mg/kg bw per day) | LOAEL (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Studies in rats | |||||||||
| F344 rat | 5/sex per group | C22–26, 43% chlorination | 14 days | Gavage (corn oil) | 0, 30, 100, 300, 1,000, 3,000 | Nephrolithiasis (females) | 1,000 | 3,000 | IRDC (1981c)a |
| F344 rat | 5/sex per group | C23, 43% chlorination | 16 days | Gavage (corn oil) | 0, 235, 469, 938, 1,875, 3,750 | No effects observed | 3,750 | – | NTP (1986b), Bucher et al. (1987) |
| F344 rat | 15/sex per group | C22–26, 43% chlorination | 90 days | Gavage (corn oil) | 0, 100, 900, 3,750 | ↑ absolute liver weight (females), liver histopathology (females) | – | 100 | IRDC (1984d)a |
| F344 rat | 10/sex per group | C23, 43% chlorination | 90 days | Gavage (corn oil) | 0, 235, 469, 938, 1,875, 3,750 | Liver histopathology (females) | – | 235 | NTP (1986b), Bucher et al. (1987) |
| F344 rat |
50/sex per group; 20/sex per group |
C23, 43% chlorination | 2 years | Gavage (corn oil) |
0, 1,875, 3,750 (males); 0, 100, 300, 900 (females) |
↑relative liver weight (females), histopathological changes (lymphohistiocytic inflammation in liver, pancreatic and mesenteric lymph nodes) (females) | – | 100 | NTP (1986b), Bucher et al. (1987) |
| F344 rat | 5/sex per group | Electrofine S70, C22–26, 70% chlorination | 14 days | Diet | 0, 17.1, 55, 169, 565, 1,715 | No effects observed | 1,715 | – | IRDC (1981d)a |
| F344 rat | 15/sex per group | Electrofine S70, C22–26, 70% chlorination | 90 days | Diet | 0, 100, 900, 3,750 | ↑relative liver weight, liver histopathology | 900 | 3,750 | IRDC (1984e)a |
| Studies in mice | |||||||||
| B6C3F1 mouse | 5/sex per group | C23, 43% chlorination | 16 days | Gavage (corn oil) | 0, 469, 938, 1,875, 3,750, 7,500 | No effects observed | 7,500 | – | NTP (1986b), Bucher et al. (1987) |
| B6C3F1 mouse | 10/sex per group | C23, 43% chlorination | 90 days | Gavage (corn oil) | 0, 469, 938, 1,875, 3,750, 7,500 | No effects observed | 7,500 | – | NTP (1986b), Bucher et al. (1987) |
| B6C3F1 mouse | 50/sex per group | C23, 43% chlorination | 2 years | Gavage (corn oil) | 0, 2,500, 5,000 | No non‐neoplastic effects observed | 5,000 | – | NTP (1986b), Bucher et al. (1987) |
LCCPs: long‐chain chlorinated paraffins; NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; bw: body weight.
Unpublished study report, see Documentation provided to EFSA.
Studies in rats
LCCP (43% chlorination)
Rats were treated with LCCP (C22–26, 43% chlorination) at doses of 0, 30, 100, 300, 1,000 or 3,000 mg/kg bw per day in corn oil by gavage during 14 days (IRDC, 1981c, unpublished study, see Documentation provided to EFSA). No compound‐related effects were observed except the increase in kidney nephrolithiasis at 3,000 mg/kg bw per day in females (3 out of 5 animals compared to 1 out of 5 animals in the control group). The NOAEL was 1,000 mg/kg bw per day, based on the kidney nephrolithiasis at the highest dose.
F344/N rats were exposed to LCCP (C23, 43% chlorination) at doses of 0, 235, 469, 938, 1,875 or 3,750 mg/kg bw per day in corn oil by gavage 5 days per week over a 16‐day period (NTP, 1986b; Bucher et al., 1987). No compound‐related clinical signs or gross pathologic effects were observed. The NOAEL was 3,750 mg/kg bw per day (the highest dose in the study).
LCCP (C22–26, 43% chlorination) was administered in corn oil by gavage for 90 days to F344 rats at doses of 0, 100, 900 or 3,750 mg/kg bw per day (IRDC, 1984d, unpublished study, see Documentation provided to EFSA). There was an increase in absolute liver weights (13–15%) in all exposed females and in relative liver weights in females at 900 and 3,750 mg/kg bw per day, but not in males. Multifocal granulomatous hepatitis involving inflammatory changes and necrosis was observed in all groups of treated females. Mineralisation in the kidneys was observed in high‐dose female rats and mild nephropathy was observed in high‐dose male rats. The LOAEL was 100 mg/kg bw per day (the lowest dose tested), based on liver effects in females.
F344/N rats were exposed to LCCP (C23, 43% chlorination) at doses of 0, 235, 469, 938, 1,875 or 3,750 mg/kg bw per day for 90 days (NTP, 1986b; Bucher et al., 1987). LCCP was administered in corn oil by gavage 5 days per week. No clinical signs of toxicity were recorded and no changes in body weight or in organ weights were observed when compared to the control group. A dose‐related increase in the incidence of granulomatous inflammation was reported in the liver in all groups of treated females. Lesions were characterised by multiple, randomly distributed accumulations of histiocytes within the liver sinusoids, which usually compressed the adjacent liver parenchyma. The smaller lesions were usually nodular, whereas the larger, more extensive accumulations appeared to result from the coalescence of adjacent smaller nodules. Lymphoid cells were present in the centre of some histiocytic foci but were most commonly seen at the periphery of the histiocytic accumulations. Large epithelioid cells were present near the centre of some nodules. Numerous lesions contained a small number of hyaline acidophilic cells. The LOAEL was 235 mg/kg bw per day (the lowest dose tested), based on increased incidence of granulomatous inflammation in the liver of females.
Groups of 50 male and 50 female F344/N rats were given LCCP (C23, 43% chlorination) at doses of 0, 1,875 or 3,750 mg/kg bw per day in males and 0, 100, 300 or 900 mg/kg bw per day in females for 2 years (NTP, 1986b; Bucher et al., 1987). LCCP was administered in corn oil by gavage 5 days per week. Additional groups (20/sex per group) were included for concurrent 6‐ and 12‐month studies. Survival was not affected. Relative liver weights were significantly increased in males at 3,750 mg/kg bw per day after 12 months and in females at 900 mg/kg bw per day after 6 months and at 300 and 900 mg/kg bw per day after 12 months. Histopathological changes were found in form of diffuse lymphohistiocytic inflammation in the liver and in the pancreatic and mesenteric lymph nodes in all exposed groups of males and females. Congestion in the spleens that occurred in all dose groups was reported to be a secondary effect of the granulomatous liver lesion, and was reported to impair the hepatic blood flow by obstruction of the sinusoids. In females, ALT was slightly elevated in serum at the medium and high dose after six months, while AST and sorbitol dehydrogenase were slightly elevated at the medium and high dose after 12 months. In males, all three serum enzyme levels were increased at low and high dose after 6 and 12 months. Leukocyte and lymphocyte counts in high‐dose females after 6 months and mid‐dose and high‐dose females at 12 months, neutrophil counts in mid‐dose and high‐dose females after 12 months, and lymphocyte counts in mid‐dose males were significantly greater than those of the control groups. The LOAEL for non‐neoplastic effects was 100 mg/kg bw per day, based on increased relative liver weight and histopathological changes (lymphohistiocytic inflammation in the liver and in the pancreatic and mesenteric lymph nodes) observed at all dose levels in females. The neoplastic lesions observed in this study are addressed in the carcinogenicity subsection (see Section 3.1.2.7 ).
LCCP (70% chlorination)
In a 14‐day dietary study with rats, LCCP (Electrofine S70, C22–26, 70% chlorination) was administered at doses of 0, 17.1, 55, 169, 565 or 1,715 mg/kg bw per day (IRDC, 1981d, unpublished study, see Documentation provided to EFSA). No compound‐related effects were noted. The NOAEL was 1,715 mg/kg bw per day (the highest dose tested).
LCCP (Electrofine S70, C22–26, 70% chlorination) was administered to F344 rats in the diet for 90 days at doses of 0, 100, 900 and 3,750 mg/kg bw per day (IRDC, 1984e, unpublished study, see Documentation provided to EFSA). A slight decrease in body weight gain was observed in males at 3,750 mg/kg bw per day compared to the control group. Increased relative liver weights were observed in high‐dose males (13%) and in high‐dose females (23%). Hepatocellular hypertrophy, cytoplasmic fat vacuolation and increased occurrence of fat droplets, revealed by Oil Red O staining, were noted in the liver at the highest dose level of both sexes. In the blood chemistry analyses, ALT activity was increased in both sexes at the highest dose level, and AST activity was also increased in females of this group. Glucose levels were slightly decreased in the high‐dose females at week 13. There was a slight reduction in serum cholesterol at weeks 8 and 13 in males and at weeks 5 and 13 in females at the high‐dose level. The NOAEL was 900 mg/kg bw per day, based on increased relative liver weight and histopathological changes in the liver.
Studies in mice
Mice were exposed to LCCP (C23, 43% chlorination) at doses of 0, 469, 938, 1,875, 3,750 or 7,500 mg/kg bw per day during 16 days (NTP, 1986b; Bucher et al., 1987). LCCP was administered in corn oil by gavage 5 days per week. No clinical signs or gross pathological effects were observed. The NOAEL was 7,500 mg/kg bw per day (the highest dose tested in this study).
LCCP (C23, 43% chlorination) was administered to B6C3F1 mice by gavage for 90 days at doses of 0, 469, 938, 1,875, 3,750 or 7,500 mg/kg bw per day in corn oil (NTP, 1986b; Bucher et al., 1987). No effects on body or organ weights, no clinical signs of toxicity and no histopathological effects were noted. The NOAEL was 7,500 mg/kg bw per day (the highest dose tested in this study).
In a 2‐year mice study, LCCP (C23, 43% chlorination) was administered at doses of 0, 2,500 or 5,000 mg/kg bw per day (NTP, 1986b; Bucher et al., 1987). LCCP was given in corn oil by gavage 5 days per week. Survival was impacted by the treatment until week 65. No clinical signs of toxicity were reported. However, a Klebsiella oxytoca infection strongly affected the females after week 65: 60–70% of the early deaths in each treatment group of females were attributed to this infection. No significant non‐neoplastic lesions occurred. The NOAEL for non‐neoplastic effects was 5,000 mg/kg bw per day (the highest dose tested in this study). The neoplastic lesions reported in this study are addressed in the carcinogenicity subsection (see Section 3.1.2.7 ).
3.1.2.2.4. Summary on repeated dose toxicity (non‐neoplastic effects)
A number of repeated dose toxicity studies has been performed using a few CPs of different carbon chain length and different degrees of chlorination. These studies have shown that the liver, kidney and thyroid are the target organs for SCCPs and MCCPs, and liver for LCCPs.
SCCPs
For the SCCP (C10–13, 56.5% chlorination; mass ratio of C10:C11:C12:C13 = 1:1:1:1) used in the 28‐day male rat gavage study by Gong et al. (2018), changes in thyroid hormone plasma levels were observed at 10 mg/kg bw per day. No histopathological changes were observed in the thyroid at 100 mg/kg bw per day, the only dose examined. The NOEL was 1 mg/kg bw per day, based on effects on thyroid hormones (decrease of plasma FT3 and increase of plasma TSH).
In two 90‐day rat studies with the same SCCP (C10–12, 58% chlorination), one with dietary administration (IRDC, 1984a, unpublished study, see Documentation provided to EFSA) and the other with gavage (IRDC, 1984b, unpublished study, see Documentation provided to EFSA) the NOAEL was 10 mg/kg bw per day. In the dietary study, increased liver and kidney weights and histopathological changes in liver, kidney (males) and thyroid (males) were observed at 100 mg/kg bw per day. In the gavage study, increased liver and kidney weights and histopathological changes in liver (males) and kidneys (males) were observed at 100 mg/kg bw per day.
In two chronic gavage studies in rats and mice with an SCCP (C12, 60% chlorination) (NTP, 1986a), effects were observed at the lowest tested dose levels of 312 mg/kg bw per day for rats (liver and kidney) and 125 mg/kg bw per day for mice (kidney and thyroid).
MCCPs
In a dietary 90‐day rat study with an MCCP (C14–17, 52% chlorination), a NOEL of 9.3/9.7 mg/kg bw per day for males/females, respectively, was observed, based on decreased plasma FT3 in males and increased plasma TSH in females. A small increase in histopathological changes (ultimobranchial cysts) was observed in the thyroid of male rats at 23 mg/kg per day; this was not seen at higher dose levels and therefore, not considered of toxicological relevance (CXR, 2005b, unpublished study, see Documentation provided to EFSA). In another dietary rat study with the same MCCP, a NOAEL of 10 mg/kg bw per day was observed, based on an increase in liver and kidney weights (IRDC, 1984c, unpublished study, see Documentation provided to EFSA). A NOEL of 10 mg/kg bw per day was observed in a 90‐day dietary study in dogs with the same MCCP, based on an increase of hepatic smooth endoplasmic reticulum in males (Birtley et al., 1980).
LCCPs
For an LCCP (C22–26, 43% chlorination), the LOAEL was 100 mg/kg bw per day (the lowest dose level tested), based on increased liver weight and histopathological changes in the liver (inflammatory changes and necrosis) of female rats in a 90‐day gavage study (IRDC, 1984d, unpublished study, see Documentation provided to EFSA). For another LCCP of a higher chlorination degree (C22–26, 70% chlorination), the NOAEL was 900 mg/kg bw per day, based on liver effects in a 90‐day rat dietary study (IRDC, 1984e, unpublished study, see Documentation provided to EFSA). In a chronic gavage study in rats with an LCCP (C23, 43% chlorination), effects (increased liver weight and histopathological changes such as diffuse lymphohistiocytic inflammation in liver, pancreatic and mesenteric lymph nodes) were observed at 100 mg/kg bw per day for female rats (the lowest dose level tested) (NTP, 1986b). No effects were observed in a chronic gavage study in mice with the same LCCP at dose levels up to 5,000 mg/kg bw per day (the highest dose level tested) (NTP, 1986b).
3.1.2.3. Developmental and reproductive toxicity studies
The developmental and reproductive studies on CPs are summarised in Table 15 .
Table 15.
Developmental and reproductive toxicity studies on chlorinated paraffins
| Species/ strain | Number of animals per group | CP specification | Duration | Administration | Dose levels (mg/kg bw per day) | Most sensitive endpoints | NOAEL (mg/kg bw per day) | LOAEL (mg/kg bw per day) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| SCCPs | |||||||||
| Charles River COBS CD rats | 25/group | Carbon chain length not specified, 58% chlorination | GD6–19 | Gavage (corn oil) | 0, 100, 500, 2,000 |
M: clinical signs (decreased activity, matted, stained or oily fur, emaciated appearance, excessive salivation); D: shortened digits and/or adactyly, post‐implantation losses, decreased number of viable fetuses per dam |
M: 100 D: 500 |
M: 500 D: 2,000 |
IRDC (1982a)a, Serrone et al. (1987) |
| Dutch belted rabbits | 16/group | Carbon chain length not specified, 58% chlorination | GD6–27 | Gavage (corn oil) | 0, 10, 30, 100 |
M: no adverse effects D: increased number of resorptions |
M: 100 (NOEL) D: 10 |
M: – D: 30 |
IRDC (1983b)a, Serrone et al. (1987) |
| MCCPs | |||||||||
| Wistar rats |
5 males per group; 10 females per group |
Cereclor S52, C14–17, 52% chlorination | From 28 days prior do mating, to PND 21c | Diet | 0, 9, 90, 560 |
P: no adverse effects F: no adverse effects PN: decreased pup survival, subcutaneous haematoma/haemorrhage |
P: 560 (NOEL) F: 560 (NOEL) PN: 9 |
P: – F: – PN: 90 |
IRDC (1985)a, Serrone et al. (1987) |
| Sprague–Dawley rats | 12 or 17 animals/sex per group | Cereclor S52, C14–17, 52% chlorination | From 28 days prior to mating to PND 21d | Diet |
0, 21, 44, 84 (males); 0, 23, 47, 99 (females) |
P: ↑ relative liver weight (females), no effects (males) F: no adverse effects D: no adverse effects |
P: 47 (females), 84 (males) F: 84 (males), 99 (females) (NOEL) D: 99b (NOEL) |
P: 99 (females), – (males) F: – D: – |
CXR (2006)a |
| Charles River COBS CD rats | 25/group | Cereclor S52, C14–17, 52% chlorination | GD6–19 | Gavage (corn oil) | 0, 500, 2,000, 5,000 |
M: clinical signs (wet and/or matted fur anogenital region with staining, increased incidence of soft stool) D: no adverse effects |
M: 500 D: 5,000 |
M: 2,000 D: – |
IRDC (1984f), Serrone et al. (1987) |
| Dutch Belted rabbits | 16/group | Cereclor S52, C14–17, 52% chlorination | GD6–27 | Gavage (corn oil) | 0, 10, 30, 100 | M, D: no adverse effects |
M: 100 (NOEL) D: 100 (NOEL) |
M: – D: – |
IRDC (1983c), Serrone et al. (1987) |
| LCCPs | |||||||||
| Charles River COBS CD rats | 25/group | C22–26, 43% chlorination | GD6–19 | Gavage (corn oil) | 0, 500, 2,000, 5,000 | M, D: no adverse effects |
M: 5,000 (NOEL) D: 5,000 (NOEL) |
M: – D: – |
IRDC (1983d), Serrone et al. (1987) |
| Dutch belted rabbits | 16/group | C22–26, 43% chlorination | GD6–27 | Gavage (corn oil) | 0, 500, 2,000, 5,000 | M, D: no adverse effects |
M: 5,000 (NOEL) D: 5,000 (NOEL) |
M: – D: – |
IRDC (1982b), Serrone et al. (1987) |
| Charles River COBS CD rats | 25/group | Electrofine S70, C22–26, 70% chlorination | GD6–19 | Gavage (corn oil) | 0, 500, 2,000, 5,000 | M, D: no adverse effects |
M: 5,000 (NOEL) D: 5,000 (NOEL) |
M: – D: – |
IRDC (1984 g), Serrone et al. (1987) |
| Dutch belted rabbits | 16/group | Electrofine S70, C22–26, 70% chlorination | GD6–27 | Gavage (1% carboxymethyl cellulose in water) | 0, 100, 300, 1,000 | M, D: no adverse effects |
M: 1,000 (NOEL) D: 1,000 (NOEL) |
M: – D: – |
IRDC (1983e), Serrone et al. (1987) |
D: developmental toxicity; F: fertility effects; GD: gestational day; M: maternal toxicity; P: parental toxicity; PN: postnatal toxicity; PND: postnatal day; NOAEL: no‐observed adverse effect level; LOAEL: lowest‐observed adverse effect level; LCCPs: long‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; SCCPs: short‐chain chlorinated paraffins.
Unpublished study report, see Documentation provided to EFSA.
As maternal dose.
Observation until PND70.
Observation until PND21.
3.1.2.3.1. SCCPs
No studies specifically investigating effects of SCCPs on fertility in mammals have been identified, however, examination of reproductive organs was performed in two repeated dose toxicity studies. Two developmental toxicity studies on SCCPs were identified (one in rats and one in rabbits).
In a 14‐day gavage study in female rats with SCCP (C10–12, 58% chlorination) (see Section 3.1.2.2.1 ), reduced absolute (48%) and relative (35%) ovary weight compared to controls at 3,000 mg/kg bw per day were observed (IRDC, 1981a, unpublished study, see Documentation provided to EFSA).
No changes were seen in the seminal vesicles, prostate, testes, ovaries or uterus when rats and mice were treated for 90 days with up to 5,000 and 2,000 mg SCCP (C12, 60% chlorination)/kg bw per day, respectively (NTP, 1986a; Bucher et al., 1987) (see Section 3.1.2.2.1 ).
In a developmental toxicity study, pregnant Charles River COBS CD rats (25 per group) were administered SCCP (carbon chain length not specified, 58% chlorination) at doses of 0, 100, 500 or 2,000 mg/kg bw per day of in corn oil by gavage from day 6 to 19 of gestation (IRDC, 1982a, unpublished study, see Documentation provided to EFSA, also described in Serrone et al., 1987). The mortality in pregnant rats of the high‐dose group was 32%. Clinical signs of maternal toxicity, such as decreased activity, matted, stained or oily fur, emaciated appearance, and excessive salivation, occurred in the mid‐ and high‐dose groups. A decrease in body weight gain by 35% was reported in the high‐dose group. Due to both early and later resorptions in the high‐dose groups, the number of post‐implantation loss was significantly increased. Significant decreases in viable feti per dam were observed in the high‐dose group. Shortened digits and/or adactyly (absence of digits) were noted in 19 feti from 3/15 litters in the high‐dose group. There were no changes in developmental parameters in the mid‐dose group. Dams and feti in the low‐dose group were also not affected. The NOAEL for maternal toxicity was 100 mg/kg bw per day, based on several clinical symptoms (matted, stained or oily haircut, emaciated appearance, excessive salivation and decreased activity) at 500 mg/kg bw per day. The NOAEL for developmental toxicity was 500 mg/kg bw per day, based on teratogenic effects, post‐implantation loss and decreased viable fetuses at 2,000 mg/kg bw per day. The role of maternal toxicity, as manifested in high mortality and decreased body weight gain, for the induction of fetal malformations remained unclear.
In another developmental toxicity study, pregnant Dutch Belted rabbits (16 per group) were given doses of 0, 10, 30 or 100 mg/kg bw per day of SCCP (carbon chain length not specified, 58% chlorination) in corn oil by gavage from day 6 to 27 of gestation (IRDC, 1983b, unpublished study, see Documentation provided to EFSA, also described in Serrone et al., 1987). No mortality in pregnant rabbits and no signs of maternal toxicity were observed. Early whole litter resorptions occurred in two dams of the high‐dose group and in one dam of the medium‐dose group. This effect is seldom observed in this species and therefore, these findings were considered to represent significant embryotoxicity. Because both females in the high‐dose group had only two implantations, the net effect on the group mean post‐implantation loss was negligible. SCCP showed also some degree of fetotoxicity at 30 mg/kg bw per day. In addition to the early whole litter loss in one dam mentioned above, the number of early and late resorptions was slightly increased (8 and 5 at 30 mg/kg bw per day compared to 3 and 1 at 0 mg/kg bw per day and at 10 mg/kg bw per day, while 5 and 0 at 100 mg/kg bw per day). No effect was observed in the occurrence of malformations at any dose levels. The NOEL for maternal toxicity was 100 mg/kg bw per day (the highest dose tested in this study). The NOAEL for developmental toxicity was 10 mg/kg bw per day based on increased number of resorptions that are regarded as embryotoxic effects.
3.1.2.3.2. MCCPs
Two rat studies were identified that investigated reproductive and postnatal effects of MCCPs. Three studies were found that dealt with developmental effects (two studies in rats and one study in rabbits).
In a dose‐range finding study for a two‐generation study (that was never conducted or reported), Wistar rats (5 males and 10 females per group) were administered MCCP (Cereclor S52, C14–17, 52% chlorination) at concentrations of 0, 100, 1,000 or 6,250 mg/kg diet for 28 days prior to mating, during mating and up to post‐natal day 21 (females only) (IRDC, 1985, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). The average doses received were 0, 9, 90 or 560 mg/kg bw per day for males and females.34 From the F1 pups, five males and 10 females from each group were selected and retained on the same diet as their parents from weaning, for up to 70 days after birth. No animals among the parental F0 generation died and no macroscopic abnormalities were observed. A significant decrease in food consumption was found in high‐dose F0 females during lactation days 7–21. There were no treatment‐related effects on fertility indices. Survival of F1 treated groups on day 0 and on lactation day 4 was not affected. F1 pup survival was significantly decreased in the high‐dose group on lactation day 10 (survival 40%) and no pups survived until weaning. Decreased F1 pup survival was also noted in the mid‐dose group on lactation day 21 (survival 89%), although not significantly different from the control group. Dose‐related clinical observations in mid‐ and high‐dose F1 pups included bruised areas, decreased activity, laboured breathing, pale discolouration and/or blood around the orifices. At the external necropsy of pups that died or were sacrificed during the lactation period, subcutaneous haematoma/haemorrhage were observed in the mid‐dose group (in 9 of 17 pups) and in the high‐dose group (in all 64 pups examined), but not in the control group or the low‐dose group. Pale liver, kidney and lungs, and blood in the cranial cavity, brain, stomach and intestines and/or the abdominal or thoracic cavities were also observed in mid‐ and high‐dose pups. There were no macroscopic lesions in the majority of mid‐dose F1 pups surviving until sacrifice on lactation day 21; blood in the brain or cranial cavity was only observed in one mid‐dose litter. Erythrocyte counts, haemoglobin concentration and haematocrit were decreased in high‐dose F1 pups on lactation day 6 compared to the control group (only one single litter examined from each group); clinical pathology was not performed in low‐ and mid‐dose F1 pups. Decreased F1 pup weights were observed on lactation days 7 and 14 in all treated groups, and in the low‐ and mid‐dose groups on lactation day 21. The F1 pup weights in the treated groups were not significantly different from the pup weights in the control group at any time point. The decreases in F1 pup weights in the low‐ and mid‐dose groups were almost similar at all time points (4/5% and 7/6% in low‐/mid‐dose males and females, respectively, on lactation day 7; 12/9% and 15/14% in low‐/mid‐dose males and females, respectively, on lactation day 14; 8/12% and 12/15% in low‐/mid‐dose males and females, respectively, on lactation day 21). After weaning (day 21), the body weights in F1 males were lower in low‐ and mid‐dose groups compared to the control group, but almost similar at all time points in the two dose groups (11/13% at postnatal day (PND) 28 to 4/7% at PND 70 in low‐/mid‐dose, respectively. Slight decreases (4–7%) were observed in F1 female body weights after weaning in low‐ and mid‐dose groups compared to the control group and were almost similar at all time points in the two dose groups. The NOEL for parental toxicity and fertility was 560 mg/kg bw per day (the highest dose tested). The NOAEL for postnatal effects is considered to be 9 mg/kg bw per day, based on decreased pup survival at the two higher dose levels although not being statistically significant in the mid‐dose group, but survival of only 89% is considered as adverse because of the severity of the effect, as well as subcutaneous haematoma/haemorrhage at the two higher dose levels.
In a reproduction/developmental screening toxicity test (OECD Test Guideline 421, but did not comply with all the requirements of this Test Guideline), Sprague–Dawley rats were administered MCCP (Cereclor S52, C14–17, 52% chlorination) at concentrations of 0 (17 animals/sex), 300 (12 animals/sex), 600 (12 animals/sex) or 1,200 (17 animals/sex) mg/kg diet for 28 day before mating, throughout gestation and lactation (CXR, 2006, unpublished study, see Documentation provided to EFSA). The mean dosages for males prior to mating were 0, 21, 44 and 84 mg/kg bw per day and for females 0, 23, 47 and 99 mg/kg bw per day. No clinical signs of toxicity were recorded and no changes in body weight, body weight gain or food consumption were observed in treated groups of F0 males and females prior to mating or for females during gestation or lactation when compared to the control group. In addition, oestrus cycles, mating performance, precoital interval, fertility and gestation lengths were similar in the treated groups and in the control group. In the F0 females at the highest dose, increased absolute and relative liver weights (by 12% and 10%, respectively) were observed when compared to controls. There were no treatment‐related effects on the number of implantations, litter size, sex ratio or pup survival. No clinical signs of toxicity could be observed in the treated male and female offspring. Offspring body weights, body weight gains to weaning and macropathology35 findings were also unaffected by treatment. The absolute liver weights in female offspring were increased by 21%, 18% and 11% at 23, 47 and 99 mg/kg bw per day as maternal dose, respectively. In this study, the offspring was only observed until PND21 instead until PND70 in the dose‐range finding study for a two‐generation study (IRDC, 1985, unpublished study, see Documentation provided to EFSA). The NOAEL for parental toxicity was 47 mg/kg bw per day, based on the increased relative liver weights (10%) in females. The NOELs for fertility were 84 and 99 mg/kg bw per day (the highest dose tested), for males and females, respectively. The NOEL for developmental toxicity was 99 mg/kg bw per day as maternal dose (the highest dose tested in this study).
Besides the study by CXR (2006), other follow‐up studies were conducted to investigate the effect on survival on offspring, the mode of action of haemorrhages (ICI, 1985; CXR, 2003, CXR, 2004, unpublished studies, see Documentation provided to EFSA). For further details, see Section 3.1.5.5 .
In a developmental toxicity study, doses of 0, 500, 2,000 or 5,000 mg/kg bw per day of MCCP (Cereclor S52, C14–17, 52% chlorination) in corn oil were administered by gavage to pregnant Charles River COBS CD rats (25 per group) from day 6 to 19 of gestation (IRDC, 1984f, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). One female from the mid‐dose group died. There were no effects on body weight gain or uterine weight. Clinical signs of toxicity such as wet and/or matted fur in the anogenital region with red or yellow staining, increased incidence of soft stool prior to sacrifice, occurred in mid‐ and high‐dose animals. Developmental indices, including malformations at dose levels up to 5,000 mg/kg bw per day, were not affected. The NOAEL for maternal toxicity was 500 mg/kg bw per day based on several clinical signs. The NOEL for developmental toxicity was 5,000 mg/kg bw per day (the highest dose tested in this study).
In another developmental toxicity study, doses of 0, 10, 30 or 100 mg/kg bw per day of MCCP (Cereclor S52, C14–17, 52% chlorination) in corn oil were administered by gavage to pregnant Dutch Belted rabbits (16 per group) from day 6 to 27 of gestation (IRDC, 1983c, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). Three dams died during the study period: one in the control group, one in the 10 mg/kg bw per day group, and one in the 30 mg/kg bw per day group. Congestion of the lobes of the lungs was observed with an increased frequency when compared to the controls. However, this effect was not clearly dose‐related and its toxicological significance remained unclear. Abortions occurred in the control group (1 dam), in the 30 mg/kg bw per day group (2 dams), and in the 100 mg/kg bw per day group (2 dams). No changes in developmental parameters, including malformations, could be observed. The NOEL for maternal and developmental toxicity was 100 mg/kg bw per day (the highest dose tested in this study).
3.1.2.3.3. LCCPs
No studies could be identified that specifically investigated the effects of LCCPs on fertility. Four studies could be identified that analysed the developmental effects of LCCPs (two studies in rats and two studies in rabbits).
LCCP (43% chlorination)
In a developmental toxicity study, doses of 0, 500, 2,000 or 5,000 mg/kg bw per day of LCCP (C22–26, 43% chlorination) in corn oil were administered by gavage to pregnant Charles River COBS CD rats (25 per group) from day 6 to 19 of gestation (IRDC, 1983d, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). No maternal effects were observed. One female in the high‐dose group died late in gestation; the cause of death could not be ascertained. There were no treatment‐related effects on pre‐ and post‐implantation loss, in fetal weights, or in the incidences of external, visceral and skeletal variations, and malformations in the treated groups when compared to the control group. The NOEL for maternal and developmental toxicity was 5,000 mg/kg bw per day (the highest dose tested in this study).
In a developmental toxicity study, pregnant Dutch Belted rabbits (16 animals per group) were administered by gavage doses of 0, 500, 2,000 or 5,000 mg/kg bw per day of LCCP (C22–26, 43% chlorination) in corn oil from day 6 to 27 of gestation (IRDC, 1982b, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). One high‐dose female died due to a gavage error. One mid‐dose dam and two high‐dose dams aborted. The post‐implantation loss was slightly higher (not statistically significant) in the high‐dose group when compared to the control group, and correspondingly, the mean number of viable fetuses was slightly lower (not statistically significant) in this dose group. No changes in fetal weights or in the incidences of external, visceral and skeletal variations and malformations were observed in the treated groups when compared to the control group. The NOEL for maternal and developmental toxicity was 5,000 mg/kg bw per day (the highest dose tested in this study).
LCCP (70% chlorination)
In a developmental toxicity study, LCCP (Electrofine S70, C22–26, 70% chlorination) was administered to pregnant Charles River COBS CD rats (25 per group) at doses of 0, 500, 2,000 or 5,000 mg/kg bw per day in corn oil by gavage from day 6 to 19 of gestation (IRDC, 1984g, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). No treatment‐related maternal or developmental effects were observed. The NOEL for maternal and developmental toxicity was 5,000 mg/kg bw per day (the highest dose tested in this study).
In a developmental toxicity study, LCCP (Electrofine S70, C22–26, 70% chlorination) was administered by gavage to groups of pregnant Dutch Belted rabbits (16 per group) in the vehicle 1% carboxymethyl cellulose in water at dose levels of 0, 100, 300 or 1,000 mg/kg bw per day from day 6 to 27 of gestation (IRDC, 1983e, unpublished study, see Documentation provided to EFSA; also described in Serrone et al., 1987). Two high‐dose dams died due to gavage errors and one mid‐dose dam died; the cause of death could not be ascertained. No treatment‐related clinical signs of toxicity were recorded in dams and no changes in bodyweight gain were observed when compared to the control group. There were no treatment‐related effects on pre‐ and post‐implantation loss, in fetal weights, or in the incidences of external, visceral and skeletal variations and malformations in the treated groups when compared to the control group. The NOEL for maternal and developmental toxicity was 1,000 mg/kg bw per day (the highest dose tested in this study).
3.1.2.3.4. Summary on developmental and reproductive toxicity
Fertility:
No specific studies on the reproductive effects of SCCPs and LCCPs were identified.
For an MCCP (C14–17, 52% chlorination), the NOEL for parental toxicity and fertility was 560 mg/kg bw per day (the highest dose tested), in the dose‐range finding study for a two‐generation study in rats; the two‐generation study was never conducted or reported. In a reproduction/developmental screening toxicity study in rats with the same MCCP, the NOEL for fertility was 84 and 99 mg/kg bw per day (the highest dose tested), for males and females, respectively. The NOAEL for maternal toxicity was 47 mg/kg bw per day, based on the increased relative liver weights. The NOEL for paternal toxicity was 84 mg/kg bw per day (the highest dose tested).
Developmental toxicity:
An SCCP (carbon chain length not specified, 58% chlorination) caused teratogenic effects in the form of absence of digits (adactyly) or shortened digits in rats at 2,000 mg/kg bw per day. Post‐implantation loss and decreased number of viable fetuses were also observed at this dose level. The NOAEL for developmental toxicity was 500 mg/kg bw per day, and for maternal toxicity 100 mg/kg bw per day, based on several clinical signs of toxicity. The NOAEL for developmental toxicity was 10 mg/kg bw per day in the rabbit study, based on increased number of resorptions and the NOEL for maternal toxicity was 100 mg/kg bw per day (the highest dose tested).
For an MCCP (C14–17, 52% chlorination), no malformations were observed in the developmental toxicity studies in rats and rabbits. The NOELs for developmental toxicity were 5,000 and 100 mg/kg bw per day (the highest doses tested) in rats and rabbits, respectively. The NOAELs for maternal toxicity in rats was 500 mg/kg bw per day (based on several clinical signs). The NOEL for maternal toxicity in rabbits was 100 mg/kg bw per day (the highest dose tested). In the dose‐range finding study for a two‐generation study in rats, the NOEL for parental toxicity and fertility was 560 mg/kg bw per day (the highest dose tested). F1 pup survival was significantly decreased in the high‐dose group on lactation day 10 and no pups survived until weaning. Decreased F1 pup survival was also noted in the mid‐dose group on lactation day 21 (survival 89%), although not significantly different from the control group. Subcutaneous haematoma/haemorrhage were observed in the mid‐ and high‐dose groups, but not in the control group or the low‐dose group. The NOAEL for postnatal effects is considered to be 9 mg/kg bw per day, based on decreased pup survival and subcutaneous haematoma/haemorrhage at the two higher dose levels.
In the developmental toxicity studies with an LCCP (C22–26, 43% chlorination) in rats and rabbits, the NOEL was 5,000 mg/kg bw per day for developmental and maternal toxicity. For another LCCP (C22–26, 70% chlorination), the NOEL for maternal and developmental toxicity was 5,000 mg/kg bw per day in the rat study, and 1,000 mg/kg bw per day in the rabbit study. These values were the highest doses tested.
3.1.2.4. Neurotoxicity studies
3.1.2.4.1. SCCPs
No oral studies specifically investigating the neurotoxicity of SCCPs have been identified.
3.1.2.4.2. MCCPs
The effects of an MCCP (C16, unknown % chlorination) on muscarinic receptors and sodium‐dependent choline uptake were investigated in the central nervous system of immature mice (Eriksson and Nordberg, 1986). Ten‐day‐old NMRI mice of both sexes were administered a single dose of 1 mg MCCP/kg bw by gavage. The CONTAM Panel noted that the dose is not clear as 1) the dose of 1.4 μmol/kg bw corresponds to 1 mg according to the abstract and to 1 mg/kg bw according to Methods, and 2) there is no information on the identity/structure/composition of the test compound and thus, no information on the molecular weight to be applied for conversion of the dose in μmol/kg bw. The mice were sacrificed at 24 hours and 7 days after treatment. Crude synaptosomal fractions (pooled samples) were prepared from the cerebral cortex and the hippocampus and the density of the muscarinic receptors was determined. No changes were observed between treated and control mice. The presynaptically sodium‐dependent choline uptake system was also examined. There was a significant decrease in Vmax (to 65% of the control value) in treated mice at 7 days after treatment, but not at 24 hours after treatment; there were no differences in Km between the two groups at 24 h of 7 days after treatment.
3.1.2.4.3. LCCPs
No studies on the neurotoxicity of LCCPs have been identified.
3.1.2.4.4. Neurobehavioural effects in zebrafish larvae
Effects of CPs on locomotion, path angle and social interactions were studied in zebrafish larvae (Yang et al., 2019). Five days after fertilisation they were exposed to four different commercial CPs at concentrations of 0, 10, 100 or 1,000 μg/L. CP‐52b is a SCCP (C10–13, 52% chlorination) and CP‐42 is a LCCP (C21–24, 42% chlorination). CP‐70 is dominated by LCCP (C21–24, 70% chlorination) with 20% composition of SCCP (C11). CP‐52a consists mainly of LCCP (C21–25, 52% chlorination) with < 10% composition of SCCP (C10–11). In the locomotion test, the swimming distance is measured which is a good indicator for the background activities of the larvae. As a general trend, the higher the chlorine content the more significant was larval locomotion inhibition, particularly at higher CP concentrations. CP‐42 (LCCP) had no effect on the larval locomotion. CP‐52b (SCCP dominant) was more active compared to CP‐52a (LCCP dominant). In the path angle test, CP‐70 significantly inhibited the larval turning activities at the two highest concentrations with the most impact at 100 μg/L. CP‐52b caused significant turning inhibition, especially at 1,000 μg/L in the ranges between −90° to −10° and +10° to +90°. In contrast to that, exposure to 10 and 100 μg/L CP‐52a stimulated hyperactivity of the larvae in the ranges between ‐90° to ‐10° and +10° to +90°. In the two‐fish interaction activity test at the highest concentration, CP‐70 and CP‐52b significantly stimulated the social activity, while CP‐52a significantly shortened the social duration per contact. CP‐42 (LCCP) had no effect on social activity.
3.1.2.5. Immunotoxicity studies
3.1.2.5.1. SCCPs
To study immunomodulatory effects, an SCCP (C9:C10:C11:C12:C13 = 1:1:1:1:1, 52% chlorination) was administered to adult male C57BL/6 mice at doses of 0, 1, 10 and 100 mg/kg bw per day in corn oil via gavage for 28 days (Wang et al., 2019a). Absolute and relative spleen weights were significantly increased at 10 and 100 mg/kg bw per day. Massive delimited and atrophic germinal centres with disperse cell distribution and disorganised boundaries were observed in the spleen at 100 mg/kg bw per day. Treatment with 100 mg/kg bw per day caused significant increases in white blood cell count, neutrophil count, lymphocyte count, monocyte count and platelet count. At the same dose level, significant increases in the blood serum levels of interleukin 12 and tumour necrosis factor α were measured compared to the control group. In the spleen, adenosine triphosphate (ATP) level and phosphofructokinase activity were increased at 100 mg/kg bw per day and pyruvate kinase activity at 10 and 100 mg/kg bw per day, indicating disturbance of energy metabolism. Treatment with 100 mg/kg bw per day caused 424 differentially expressed genes in the spleen. Many of these genes are immune‐related such as ly75, mcl1, runx1, ccr9, tcf4, nfatc2, nfat5, tnfaip3, stat1, ptk2b or involved in immune‐related pathways such as the chemokine signalling pathway, NF‐κB signalling pathway, Jak‐STAT signalling pathway or Th17 cell differentiation. In conclusion, these results suggest that SCCP could cause immunomodulatory effects in mice.
3.1.2.5.2. MCCPs
No studies specifically investigating the immunotoxicity of MCCPs have been identified.
3.1.2.5.3. LCCPs
No studies specifically investigating the immunotoxicity of LCCPs have been identified.
3.1.2.6. Genotoxicity studies
3.1.2.6.1. SCCPs
SCCPs have been tested in six in vitro tests, four reverse mutation assays and two gene mutation tests, as well as in four in vivo studies. These studies are described in Table 16 and a summary of the results are presented below.
Table 16.
Genotoxicity studies on short‐chain chlorinated paraffins
| Compound | Type of test | Experimental system | Exposure conditions | Result | Comments | Reference |
|---|---|---|---|---|---|---|
|
C10–13, 50% chlorination (containing 1% epoxy stabiliser) |
Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 E. coli WP2uvrA |
Up to 10,000 μg/plate +/− S9 (rat liver) |
Around a twofold increase in revertants in TA 98 (− S9) and in TA 100 (+/− S9) from 500 μg/plate and above Negative |
No toxicity observed No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Hoechst (1986) Unpublished study, as cited by EU‐RAR (2000) and WHO/IPCS (1996) |
| C12, 57% chlorination | Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 E. coli WP2uvrA |
Up to 5,000 μg/plate +/− S9 (rat liver) |
Negative | No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Hoechst (1988) Unpublished study, as cited by EU‐RAR (2000) and WHO/IPCS (1996) |
| C12, 60% chlorination | Reverse mutation assay |
S. Typhimurium TA 97, TA 98, TA 100, TA 1535 |
Up to 3,333 μg/plate +/− S9 (rat and hamster liver) |
Negative |
|
NTP (1986a) |
|
Cereclor 50LV (C10–13, 50% chlorination) |
Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1538 |
Up to 2,500 μg/plate +/− S9 (rat liver) Neg. control: DMSO Pos. control: 1,3‐propanesultone and 2AA |
Negative |
|
Birtley et al. (1980) |
| C10–13, 56% chlorination |
In vitro Gene mutation assay (hprt locus) |
Chinese hamster V79 cells |
Up to cytotoxic concentrations +/− S9 (rat liver) |
No significant increased numbers of mutant colonies Negative |
– Well‐conducted, performed to modern protocols (EU‐RAR, 2000) No further details: the original study report was not available to the CONTAM Panel and the information could therefore, not be verified |
Hoechst (1987) Unpublished study, as cited by EU‐RAR (2000) and WHO/IPCS (1996) |
| C12, 60% chlorination |
In vitro Gene mutation assay (TK+/− locus) |
Mouse lymphoma L5178Y cells |
0, 24, 36, 48, 60, 72 μg/mL Without metabolic activation Neg. control: acetone Pos. control: MMS |
Experiment I: average mutant frequency statistically significantly higher at 48, 60 and 72 μg/mL, clear concentration relationship Experiment II: average mutant frequency statistically significantly higher only at 60 μg/mL Positive |
Experiment I: decrease in cloning efficiency in 1 of 2 replicates at ≥ 60 μg/mL Precipitation at 60 μg/mL Experiment II: decrease in cloning efficiency at 60 μg/mL, toxic at 72 μg/mL Size of colonies not reported |
Myhr et al. (1990) |
| C12, 60% chlorination |
In vivo UDS |
Male rats Hepatocytes |
4 animals/group Gavage, single dose 0, 500, 1,000, 2,000 mg/kg bw Exposure: 2 h or 12 h Neg. Control: corn oil Pos. Control: NDMA, 4AB, MMS, 2AAF, 6BT, Benzidine |
Negative | Ashby et al. (1990) | |
| C10–12, 58% chlorination |
In vivo Chromosomal aberration Bone‐marrow cells |
Male F344 rats |
8 animals/group Gavage, daily for 5 days 0, 250, 750, 2,500 mg/kg bw per day Neg. control: corn oil Pos. control: cyclophosphamide |
7 high‐dose animals died Decreased bw in mid‐dose animals No increased frequency of chromosomal aberrations, excluding gaps at 250 or 750 mg/kg bw per day, or in the one surviving at 2,500 mg/kg bw per day, detected in samples taken at day 6 Negative |
|
IRDC (1983f) Unpublished study, see Documentation provided to EFSA |
| C10–13, 58% chlorination |
In vivo Mouse micronucleus assay |
NMRI mice and Hoe NMRKF SPF 71 bone‐marrow cells |
5 animals/sex per group Gavage, single dose 0, 50, 5,000 mg/kg bw |
No differences in polychromatic cells with micronuclei or in the ratio of polychromatic erythrocytes to normocytes detected at 24, 48 and 72 h after administration (high‐dose), or at 24 h after administration (low‐dose) compared to control Negative |
No further details: the original study report was not available to the CONTAM Panel and the information could therefore, not be verified No indication if bone marrow was exposed |
Hoechst (1989) Unpublished study, as cited by WHO/IPCS (1996) |
| C10–12, 58% chlorination |
In vivo Germ cell mutagenicity Dominant lethal assay |
Male rats |
15 animals/group Gavage, daily for 5 days 0, 250, 750, 2,000 mg/kg bw per day Two days after the final treatment, M were paired with two F for 5 days, and after a 2‐day break with another two F, until each M had been paired with 20 F Neg. control: corn oil Pos. control: cyclophosphamide Uterine examinations were conducted in F 15 days after the introduction of the M |
Slight decrease in bw in high‐dose animals during treatment Slight decrease in bw gain in mid‐dose animals Mean bw were then comparable throughout the remainder of the study No mutagenic effects on the post‐meiotic stage of spermatogenesis at any dose level, as demonstrated by the absence of effect on the mean number of viable embryos during the first 4 weeks of mating Negative |
IRDC (1983 g) Unpublished study, see Documentation provided to EFSA |
bw: body weight; F: female; M: male; UDS: unscheduled DNA synthesis.
SCCPs are not mutagenic in bacterial test systems.
In mammalian cells in vitro, an SCCPs (C12, 60% chlorination) showed a mutagenic response in mouse lymphoma cells in the absence of metabolic activation; no mutagenic response was observed with another SCCP (C10–12, 56% chlorination) in Chinese hamster V79 cells with and without metabolic activation.
Negative results were noted in three in vivo assays, i.e. in a rat bone marrow chromosomal aberration test, in a mouse bone marrow micronucleus test, and in a dominant lethal test in rats, after exposure by gavage. A negative result was also reported in an in vivo unscheduled DNA synthesis (UDS) assay in rat hepatocytes; this is considered as a supplementary result because of the low sensitivity of the UDS test in detecting in vivo genotoxicants, and therefore, the use of the UDS test is no longer recommended (EFSA Scientific Committee, 2017c).
3.1.2.6.2. MCCPs
MCCPs have been tested in four in vitro tests, all reverse mutation assays, as well as in three in vivo studies. These studies are described in Table 17 and a summary of the results are presented below.
Table 17.
Genotoxicity studies on MCCPs
| Compound | Type of test | Experimental system | Exposure conditions | Result | Comments | Reference |
|---|---|---|---|---|---|---|
| C14–17, 40% chlorination | Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 |
Up to 5,000 μg/plate +/− S9 |
Negative |
No data presented relating to positive controls No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Wiegand (1989) As cited by EU‐RAR (2005) |
| C14–17, 42% chlorination | Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 |
Up to 5,000 μg/plate +/− S9 |
Negative | No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Conz and Fumero (1988a) As cited by EU‐RAR (European Union‐Risk Assessment Report) (2011) and WHO/IPCS (1996) |
| C14–17, 45% chlorination | Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1537, TA 1538 |
Up to 5,000 μg/plate +/− S9 |
Negative | No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Elliott (1989a) As cited by EU‐RAR (European Union‐Risk Assessment Report) (2011) and WHO/IPCS (1996) |
|
Cereclor S52 (C14–17, 52% chlorination) With or without 0.2% epoxidised vegetable oil stabiliser |
Reverse mutation assay |
S. Typhimurium TA 98, TA 100, TA 1535, TA 1538 |
4, 20, 100, 500, 2500 μg/plate +/− S9 (rat liver) Neg. control: DMSO Pos. control: 1,3‐propanesultone and 2AA |
About a threefold increase in the number of revertants in TA 1538 (+ S9) only at the two lowest concentrations without stabiliser No increases under any other test conditions Negative |
No cytotoxicity observed | Birtley et al. (1980) |
|
Cereclor S52 (C14–17, 52% chlorination) |
In vivo Chromosomal aberration Bone‐marrow cells |
Male F344 rats |
8 animals/group Gavage, daily for 5 days 0, 500, 1,500 or 5,000 mg/kg bw per day |
No animals died and no signs of toxicity No increased frequency of chromosomal aberrations, including and excluding gaps detected in samples taken at day 6 Negative |
– No other sampling times – Cytotoxicity not assessed and therefore no direct measure of whether or not the test substance reached the bone marrow |
IRDC (1983 h) Unpublished study, see Documentation provided to EFSA |
| C14–17, 42% chlorination |
In vivo Mouse micronucleus assay |
Mice (CD‐1) |
5 animals/sex Gavage, single dose 5,000 mg/kg bw Neg. control: corn oil |
Frequency of micronuclei not increased when examined at 18, 43 and 66 hours after administration Negative |
No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Conz and Fumero (1988b) As cited by EU‐RAR (European Union‐Risk Assessment Report) (2011) and WHO/IPCS (1996) |
| C14–17, 45% chlorination |
In vivo Mouse micronucleus assay |
Mice (C57B1/6JFCD‐1/Alpk) |
5 animals/sex per group Gavage, single dose 0, 3,125, 5,000 mg/kg bw Examination at 24, 48 and 72 h after administration (high‐dose), or 24 h after administration (low dose) |
No increases in micronucleus formation Negative |
No further details: the original study report was not available to the CONTAM Panel and the information could therefore not be verified |
Elliott (1989b) |
bw: body weight.
MCCPs are not mutagenic in bacterial test systems.
Negative results were also reported in three in vivo assays, i.e. in a rat bone marrow chromosomal aberration test and in two mouse bone marrow micronucleus tests, after exposure by gavage.
3.1.2.6.3. LCCPs
LCCPs have been tested in four in vitro tests, two reverse mutation assays, a chromosomal aberration test and an SCE test, as well as in two in vivo studies. These studies are described in Table 18 and a summary of the results is presented below.
Table 18.
Genotoxicity studies on long‐chain chlorinated paraffins
| Compound | Type of test | Experimental system | Exposure conditions | Result | Comments | Reference |
|---|---|---|---|---|---|---|
| C23, 43% chlorination | Reverse mutation assay | S. TyphimuriumTA 97, TA 98, TA 100, TA 1535 | Up to 10,000 μg/plate+/− S9 (rat and hamster liver) | Negative |
|
NTP (1986b) |
| Cereclor 42(C20–30, 42% chlorination) | Reverse mutation assay | S. TyphimuriumTA 98, TA 100, TA 1535, TA 1538 | Up to 2,500 μg/plate+/− S9 (rat liver)Neg. control: DMSOPos. control: 1,3‐propanesultone and 2AA | Negative |
|
Birtley et al. (1980) |
| C23, 43% chlorination | Chromosomal aberration test | Chinese hamster ovary cells | −S9:First experiment: 0, 1,250, 2,500, 5,000 μg/mL Exposure: 8 h, harvesting: 2–2.5 h laterSecond experiment: 0, 3,784, 4,400, 5,000 μg/mL+S9 (rat liver):0, 2.513, 3,750, 5,000 μg/mLExposure: 2 h, harvesting: 8–10 h laterNeg. control: acetonePos. control: −S9: MMC, +S9: cyclophosphamide |
−S9:First experiment:Significant increase at 5,000 μg/mL, no concentration response relationshipSecond experiment:Significant increase at 5,000 μg/mL, clear concentration response relationship+S9:No increase CA Positive without metabolic activation |
Cytotoxicity not reported,Oily droplets formed at doses > 157 μg/mL | Anderson et al. (1990) |
| C23, 43% chlorination | SCE | Chinese hamster ovary cells | −S9:First experiment: 0, 0.5, 5.0, 5,000 μg/mL,Second experiment:0, 5.0, 1,700 and 5,000 μg/mL+S9 (rat liver):0, 5.0, 500, 1,700 and 5,000 μg/mL |
First experiment:−S9:Significant increase at 5,000 μg/mL, concentration response relationshipSecond experiment:Significant increase at all three concentrations, concentration response relationship+S9:Significant increase at all four concentrations, concentration response relationship up to 500 μg/mL, at the two highest concentrations induction of SCE was lower than at 500 μg/mL Positive |
Cytotoxicity not reported | Anderson et al. (1990) |
| C22–26, 43% chlorination | In vivoChromosomal aberrationBone‐marrow cells | Male F344 rats | 8 animals/groupGavage, daily for 5 days0, 500, 1,500 or 5,000 mg/kg bw per dayNegative control: corn oil. Positive control: cyclophosphamide |
No signs of toxicityNo increased frequency of chromosomal aberrations, excluding gaps detected in samples taken at day 6 Negative |
|
IRDC (1983i)Unpublished study, see Documentation provided to EFSA |
| C20–30, 70% chlorination | In vivo Chromosomal aberration Bone‐marrow cells | F344 rats | 8 animals/groupGavage, daily for 5 days0, 500, 1,500 or 5,000 mg/kg bw per day |
bw gain decreased in high‐dose animalsNo increased frequency of chromosomal aberrations Negative |
Cytotoxicity not reported and therefore, no indication of whether or not the test substance reached the bone marrow | IRDC (1983, as cited by WHO/IPCS, 1996) |
bw: body weight; SCE: sister chromatid exchange.
LCCPs are not mutagenic in bacterial test systems.
Sister chromatid exchanges (SCEs) were induced by an LCCP (C23, 43% chlorination) in Chinese hamster ovary cells in vitro with and without metabolic activation.
Chromosomal aberrations were only observed in the absence of metabolic activation at a high concentration.
In two in vivo rat bone marrow chromosomal aberration tests using oral administration of LCCPs (gavage), no clastogenic effects were reported.
3.1.2.6.4. Summary on genotoxicity
CPs have been tested in vitro in bacterial and mammalian cells, as well as in in vivo studies. A number of the original study reports were, however, not available to the CONTAM Panel and the information could therefore, not be verified.
Negative results were reported in gene mutation assays in bacterial test systems (SCCPs, MCCPs, LCCPs) and in Chinese hamster V79 cells (an SCCP).
A mutagenic response was reported in an in vitro mouse lymphoma test with an SCCP, and chromosomal aberration and sister chromatid exchanges were induced by an LCCP.
In several in vivo studies using oral administration of CPs, including rat bone marrow cell chromosomal aberration studies (SCCPs, MCCPs, LCCPs), micronucleus tests in mice (SCCPs and MCCPs), UDS in rat hepatocytes (SCCPs) and a germ cell mutagenicity study in rats (SCCPs), neither mutagenic nor clastogenic effects have been reported.
The overall weight of evidence indicates that CPs are not genotoxic.
3.1.2.7. Carcinogenicity
3.1.2.7.1. SCCPs
In a 2‐year study with F344 rats, SCCP (C12, 60% chlorination) was administered in doses of 0, 312 or 625 mg/kg bw per day (50/sex per group) in corn oil by gavage 5 days per week (NTP, 1986a; Bucher et al., 1987). Survival in low‐ and high‐dose males and in low‐dose females was significantly lower than in controls (control: 27/34; low‐dose: 6/23; high‐dose: 3/29 for males/females, respectively). Significantly increased incidences were observed for tumours in the liver (both sexes), kidney (only male) and thyroid (only females) (Table 19 ). In treated males, there was a slight increase in the incidences of pancreatic acinar cell carcinomas (control: 0/50; low‐dose: 0/50; high‐dose: 2/49), pancreatic acinar adenomas (control: 11/50; low‐dose: 22/50; high‐dose: 15/49) and squamous cell papillomas in the forestomach (control: 0/50; low‐dose: 0/50; high‐dose: 3/49).
Table 19.
Incidence of tumours in rats administered short‐chain chlorinated paraffins (C12, 60% chlorination) (NTP, 1986a)
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Dose (mg/kg bw per day) | Control | 312 | 625 | Control | 312 | 625 |
| Hepatocellular neoplastic nodules | 0/50 (0%) | 10/50 (20%)a , b | 16/48 (33%)a , b |
0/50 (0%) |
4/50 (8%)a |
7/50 |
| Hepatocellular carcinomas | 0/50 (0%) |
3/50 |
2/48 (4%) |
0/50 (0%) |
1/50 (2%) |
1/50 (2%) |
| Hepatocellular neoplastic nodules or carcinomas | 0/50 (0%)c | 13/50 (26%)a , b | 16/48 (33%)a , b |
0/50 (0%)d |
5/50 (10%)a |
7/50 |
| Renal tubular‐cell adenomas or adenocarcinomas | 0/50 (0%)e | 9/50 (18%)a , b |
3/49 (6%) |
0/50 (0%) |
0/50 (0%) |
0/50 (0%) |
| Mononuclear cell leukaemia | 7/50 (14%)f | 12/50 (24%)a | 14/50 (28%)a |
11/50 (22%)g |
22/50 (44%)a |
16/50 (32%) |
| Thyroid follicular cell adenomas or carcinomas | 3/50 (6%) |
3/50 (6%) |
3/50 (6%) |
0/50 (0%)h |
6/50 (12%)a , b |
6/50 |
bw: body weight.
Life table analysis, p < 0.05, increase relative to control.
Incidental tumour test for trend, p < 0.05, increase relative to control.
Historical incidence at the study laboratory (mean ± SD): 8/299 (3% ± 3%); historical incidence in NTP studies: 43/1,098 (4% ± 4%).
Historical incidence at the study laboratory (mean ± SD): 2/300 (0.7% ± 1%); historical incidence in NTP studies: 21/1,098 (2% ± 3%).
Historical incidence at the study laboratory (mean): 1/300 (0.3%); historical incidence in NTP studies: 5/1,098 (0.5%).
Historical incidence at the study laboratory (mean ± SD): 19/300 (6% ± 6%); historical incidence in NTP studies: 152/1,100 (14% ± 8%).
Historical incidence at the study laboratory (mean ± SD): 36/300 (12% ± 6%); historical incidence in NTP studies: 196/1,100 (18% ± 9%).
Historical incidence at the study laboratory (mean ± SD): 3/297 (1% ± 1%); historical incidence in NTP studies: 15/1,076 (1% ± 2%).
In a 2‐year study with B6C3F1 mice, SCCP (C12, 60% chlorination) was given in doses of 0, 125 or 250 mg/kg bw per day in corn oil by gavage 5 days per week to 50 males and 50 females per dose (NTP, 1986a; Bucher et al., 1987). Reduced survival of high‐dose females was observed after week 100 (control: 34/35; low‐dose: 30/31; high‐dose: 30/25 for males/females, respectively). Incidences of tumours in the liver (both sexes) and thyroid (only females) were significantly increased (Table 20 ). Incidence of Harderian gland adenomas in females was significantly increased, but not dose‐related. No effects were seen in males. Significantly increased incidences of alveolar/bronchiolar carcinomas were observed in high‐dose males; the trend with dose was also significant. There was no increased incidence of lung tumours in females.
Table 20.
Incidence of tumours in mice administered short‐chain chlorinated paraffin (C12, 60% chlorination) (NTP, 1986a)
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Dose (mg/kg bw per day) | Control | 125 | 250 | Control | 125 | 250 |
| Hepatocellular adenomas | 11/50 (22%) | 20/50 (40%)a , b |
29/50 |
0/50 (0%) |
18/50 |
22/50 |
| Hepatocellular carcinomas | 11/50 (22%) | 15/50 (30%) |
17/50 (34%) |
3/50 (6%) |
4/50 (8%) |
9/50 (18%)a |
| Hepatocellular adenomas or carcinomas | 20/50 (40%)c | 34/50 (68%)a , b |
38/50 |
3/50 (6%)d |
22/50 |
28/50 |
| Thyroid follicular cell adenomas or carcinomas |
3/49 (6%) |
4/50 (8%) |
3/49 (6%) |
8/50 (16%)e |
12/49 (24%) |
15/49 |
| Harderian gland adenomas | – | – | – |
1/50 (2%)f |
6/50 |
2/50 (4%) |
| Alveolar/bronchiolar carcinomas |
0/50 (0%)g |
3/50 (6%) |
6/50 |
– | – | – |
bw: body weight.
Life table analysis, p < 0.05, increase relative to control.
Incidental tumour test for trend, p < 0.05, increase relative to control.
Historical incidence at the study laboratory (mean ± SD): 109/298 (37% ± 12%); historical incidence in NTP studies: 357/1,091 (33% ± 10%).
Historical incidence at the study laboratory (mean ± SD): 18/300 (6% ± 3%); historical incidence in NTP studies: 74/1,092 (7% ± 4%).
Historical incidence at the study laboratory (mean ± SD): 19/291 (7% ± 3%); historical incidence in NTP studies: 40/1,009 (4% ± 3%).
Historical incidence at the study laboratory (mean ± SD): 5/300 (2% ± 2%); historical incidence in NTP studies: 21/1,096 (2% ± 3%).
No specific historical incidence data reported for alveolar/bronchiolar carcinomas. Historical incidence of the alveolar/bronchiolar tumours (adenomas and carcinomas) at the study laboratory (mean ± SD): 54/298 (18% ± 7%); historical incidence of the alveolar/bronchiolar tumours in NTP studies: 169/1,093 (15% ± 6%).
3.1.2.7.2. MCCPs
No in vivo carcinogenicity studies on MCCPs have been identified.
3.1.2.7.3. LCCPs
In a 2‐year study with F344 rats, LCCP (C23, 43% chlorination) was administered at doses of 0, 1,875 or 3,750 mg/kg bw per day in males and 0, 100, 300 or 900 mg/kg bw per day in females (NTP, 1986b; Bucher et al., 1987). Survival was not affected. In female rats, pheochromocytomas occurred with a significant positive trend, and the incidence in the high‐dose group was significantly greater than in the control group and in the historical control (Table 21 ). The incidence of endometrial stromal polyps in the uterus in the low‐dose female rats was significantly greater than in the controls and in the mid‐ and high‐dose group. Two endometrial stromal sarcomas were observed in the low‐dose group (one in an animal with a polyp), but none was noted in any other dose group or in the control. The incidence of endometrial stromal polyps or sarcomas was significantly increased in the female low‐dose group, but not in the mid‐ and high‐dose groups.
Table 21.
Incidence of tumours in female rats administered long‐chain chlorinated paraffin (C23, 43% chlorination) (NTP, 1986b)
| Dose (mg/kg bw per day) | Control | 100 | 300 | 900 |
|---|---|---|---|---|
|
Adrenal medulla, pheochromocytomas (all types) |
1/50 (2%)d |
4/50 (8%)a |
6/50 (12%) |
7/50 |
| Uterus, endometrial stromal polyps |
9/50 (18%) |
17/50 |
10/50 (20%) |
10/50 (20%) |
| Uterus, endometrial stromal sarcomas |
0/50 (0%) |
2/50 (4%) |
0/50 (0%) |
0/50 (0%) |
| Uterus, endometrial stromal polyps and/or sarcomas |
9/50 (18%)e |
18/50 |
10/50 (20%) |
10/50 (20%) |
bw: body weight.
One female rat had a malignant pheochromocytoma.
Life table analysis, p < 0.05, increase relative to control.
Incidental tumour test for trend, p < 0.05, increase relative to control.
Historical incidence at the study laboratory (mean ± SD): 16/300 (5% ± 3%); historical incidence in NTP studies: 65/1,093 (6% ± 3%).
Historical incidence at the study laboratory (mean ± SD): 76/300 (25% ± 6%); historical incidence in NTP studies: 252/1,089 (23% ± 6%).
In a 2‐year study with B6C3F1 mice, LCCP (C23, 43% chlorination) was given in doses of 0, 2,500 or 5,000 mg/kg bw per day in corn oil by gavage 5 days per week to 50 males and 50 females per dose (NTP, 1986b; Bucher et al., 1987). Survival in females was lower than in males (control: 29/21; low‐dose: 36/22; high‐dose: 28/20 for males/females, respectively). Survival in females was strongly influenced after week 65 by a Klebsiella oxytoca infection; 60–70% of the early deaths in each female group were attributed to this utero‐ovarian infection and the low survival may have decreased the sensitivity to detect carcinogenic effects. In male mice, malignant lymphomas occurred with a dose‐related positive trend, the incidence in the high‐dose group was significantly greater than that in the control group and in the historical control (Table 22 ). In female mice, hepatocellular carcinomas and hepatocellular adenomas or carcinomas (combined) occurred with positive trends, but the incidences in the dosed groups were, according to the authors, not significantly greater than the controls. The CONTAM Panel noted that the infection of the females might impact the reliability of the study.
Table 22.
Incidence of tumours in male mice administered long‐chain chlorinated paraffin (C23, 43% chlorination) (NTP, 1986b)
| Dose (mg/kg bw per day) | Males | Females | ||||
|---|---|---|---|---|---|---|
| Control | 2,500 | 5,000 | Control | 2,500 | 5,000 | |
| Malignant lymphomas |
6/50 (12%)c |
12/50 (24%) | 16/50 (32%)a , b |
15/50 (30%) |
12/49 (24%) |
20/50 (40%) |
| Hepatocellular carcinomas |
9/50 (18%) |
12/50 (24%) | 12/50 (24%) |
1/50 (2%) |
1/49 (2%) |
6/50 (12%) |
| Hepatocellular adenomas and/or carcinomas | 18/50 (36%) | 21/50 (42%) | 23/50 (46%) |
4/50 (8%)d |
3/49 (6%) |
10/50 (20%) |
bw: body weight.
Life table analysis, p < 0.05, increase relative to control.
Incidental tumour test for trend, p < 0.05, increase relative to control.
Historical incidence at the study laboratory (mean ± SD): 35/299 (12% ± 5%); historical incidence in NTP studies: 132/1,097 (12% ± 4%).
Historical incidence at the study laboratory (mean ± SD): 18/300 (6% ± 3%); historical incidence in NTP studies: 74/1,092 (7% ± 4%).
3.1.2.7.4. Summary on carcinogenicity
Oral gavage dosing of an SCCP (C12, 60% chlorination) caused tumours at several different tissue sites in rats and mice. Administration by gavage increased the incidence of benign and malignant liver tumours (hepatocellular adenomas and carcinomas) in mice of both sexes, the thyroid gland (follicular‐cell adenoma and carcinoma) in female mice and rats, and the kidney (tubular‐cell adenomas and carcinomas) in male rats. It also caused benign liver tumours (hepatocellular neoplastic nodules) in rats of both sexes and mononuclear‐cell leukaemia in male rats. Therefore, this SCCP (C12, 60% chlorination) is carcinogenic in rats and mice.
Oral dosing of an LCCP (C23, 43% chlorination) caused tumours in different tissues in rats and mice. Administration by gavage increased the incidence of malignant lymphomas in male mice, of adrenal gland medullary pheochromocytomas in female rats, and to a marginal extent of hepatocellular neoplasms in female mice. Therefore, there is some evidence for this LCCP (C23, 43% chlorination) to be carcinogenic in mice and rats at very high‐dose levels (2,500 and 5,000 mg/kg bw per day).
The relevance of these tumours for humans is discussed in Section 3.1.5 .
3.1.3. Observations in humans
Only very limited information on observations in humans have been retrieved from the literature search.
A case report of acute renal toxicity following ingestion of a CP containing lava light liquid has been reported (Erickson et al., 1996). A 65‐year‐old man was hospitalised with acute kidney failure after having ingested a red lava light liquid 12–24 h before admission to the hospital. The lava light liquid contained water (38%), CP (36%; no information on the identity of the CP), polyethylene glycol (molecular weight 200; 13%), kerosene (7%) and microcrystalline wax (6%). According to the authors, the renal toxicity was most probably related to the polyethylene glycol in the lava light liquid. This case story could not be used for this risk assessment because of the mixed exposure and because the observed effects were most probably associated with polyethylene glycol, not CPs. In addition, no information on the identity of the CP in the lava light liquid was provided.
A registry‐based case control study nested in a cohort assessed the association between hepatobiliary cancer (cancer in the liver and biliary tract) and occupational exposure to metalworking fluids (MWF) and additives thereof (Bardin et al., 2005). For an exposure window between 1920 and 1994 and in addition to different types of MWFs used at the three automobile manufacturing plants, exposure to several MWF additives, including CPs used in extreme pressure operations with straight oils, was evaluated. Exposures to CPs were reported to be limited; however, no information was provided regarding the identity of the CPs. In total, 63 cases of hepatobiliary cancer (39 liver cancers and 24 biliary tract cancers) were reported. Of these, one liver cancer patient and five biliary track cancer patients were exposed to CPs. Exposure to CPs was associated with a non‐statistically significant fourfold increased risk for biliary tract cancer. Due to the small number of exposed individuals, the primary focus on MWFs and the lack of characterisation of individual CPs, this cohort study could not be used for this risk assessment.
3.1.4. Adverse effects in farm animals, horses and companion animals
3.1.4.1. Ruminants
No studies investigating effects of CPs in ruminants have been identified.
3.1.4.2. Pigs
No studies investigating effects of CPs in pigs have been identified.
3.1.4.3. Poultry
A limited number of studies have been conducted in poultry, and some of these were designed to assess the performance and capacity for food production. These studies are summarised below.
The acute oral LD50 values of MCCP (Cereclor S52, C14–17, 52% chlorination) were reported to be > 24,606 mg/kg bw per day for ring‐necked pheasants and > 10,280 mg/kg bw per day for mallard ducks. After 5‐day dietary treatment, the LC50 values of MCCP (C14–17, 52% chlorination) for ring‐necked pheasants and also for mallard ducks were reported to be > 24,063 mg/kg diet (Madeley and Birtley, 1980).
SCCP (Cereclor 50LV, C10–13, 49% chlorination), SCCP (Cereclor 70L, C10–13, 70% chlorination) or LCCP (Cereclor 42, C22–26, 42% chlorination) were injected into the yolks of hens’ eggs (White Leghoorn) after 4 days of incubation at sublethal doses of 0 or 300 mg/kg egg, respectively, (Brunström, 1985). The liver weight, the CYP450 concentration in the liver and the liver microsomal activity of APND, aryl hydrocarbon (benzo[a]pyrene) hydroxylase (AHH) and 7‐ethoxycoumarin O‐deethylase (ECOD) were determined in the chick embryos after incubation for 20 days. SCCP (C10–13, 70% chlorination) was the most potent in causing significant increases in liver weight, CYP450 concentration and APND activity. LCCP (C22–26, 42% chlorination) was the least potent in these respects, even causing significantly reduced APND activity. A significant decrease in AHH activity occurred in chick embryos treated with SCCP (C10–13, 49% chlorination), and a significant reduction in ECOD activity was observed as a result of treatment with LCCP (C22–26, 42% chlorination) and SCCP (C10–13, 49% chlorination).
Effects of SCCPs on growth rate were investigated in broiler chickens (Ueberschär and Matthes, 2004). Male ‘Lohmann meat’ broiler chickens (1 day old) were given diets supplemented with SCCP (C10–13, 60% chlorination) in concentrations of 0 (48 animals), 2 (eight animals), 20 (eight animals), 45 (eight animals), 70 (eight animals) and 100 mg/kg feed (48 animals) for 31 days (corresponding to 0, 0.095, 0.95, 2.1, 3.3 and 4.7 mg/kg bw per day36). Feed consumption and live weights were determined weekly in groups of eight animals. At the end of the study, all animals were weighed before slaughtering. The weights of inner organs (liver, kidneys, pancreas, thyroid, heart, gizzard, spleen, bursa of Fabricius) were determined for the control group and the high‐dose group, and the weights were calculated as a percentage of live weights. The mean of six groups of eight animals each were used for the statistical analysis of the performance data. Live weight gain was also based on values for single animals obtained after 4 weeks or 31 days. Seven animals died during the study period, three in the control group, one in the 20 mg/kg group and three in the 100 mg/kg group. No treatment‐related effects between the control group and the high‐dose group were observed on performance (feed intake, live weight gain, feed to gain ratio) for up to 31 days. No indications of lesions, inflammation or other pathological anomalies were observed in the gross macroscopic examination of inner organs and glands. Significantly lower mean live weights and higher relative spleen weights were observed in the high‐dose group compared to the control group. The highest concentration of 100 mg/kg feed (corresponding to 4.7 mg/kg bw per day) is considered as a LOAEL. A NOAEL cannot be evaluated as results have only been presented for the control group and the high‐dose group in the article. The CONTAM Panel noted discrepancies between text in the abstract and in the results section, as well as at other places in the article, which hamper the reliability of this study.
Effects of SCCPs on laying performance were investigated in laying hens (Ueberschär et al., 2007). Laying hens of the ‘Lohmann selected Leghorn’ strain (24 weeks old) were given diets supplemented with SCCP (C10–13, 60% chlorination) in concentrations of 0 (21 animals), 2 (nine animals), 20 (nine animals), 45 (nine animals), 70 (nine animals) and 100 mg/kg feed (36 animals) for 8 weeks (corresponding to 0, 0.2, 1.5, 3.4, 5.3 and 7.6 mg/kg bw per day37). Feed consumption of all animals in the control group and the high‐dose group was recorded weekly. Laying performance was calculated over two periods of 28 days and the egg weights were calculated as the mean weights of eggs collected for 4 days in the 4th or 8th week of experiment, for the control group and the high‐dose group. At the end of the study (32 weeks of age), all animals were weighed. Nine birds from each group were sacrificed and the inner organs (liver, kidneys, pancreas, thyroid, heart, gizzard, spleen) were weighed and the weights were calculated as a percentage to the live weights of the individual birds. No mortality was observed in the study. No treatment‐related effects between the control group and the high‐dose group were noted on the performance (feed intake, egg weight, laying intensity, daily egg mass, feed conversion), live weight and relative organ weights during the 8‐week study period. The highest concentration of 100 mg/kg feed (corresponding to 7.6 mg/kg bw per day) is considered as a NOAEL.
In a reproductive study in mallard ducks (Wildlife International Ltd, 1984, unpublished study, as cited in Serrone et al., 1987), SCCP (C10–13, 58% chlorination) was fed at levels of 0, 28, 166 or 1,000 mg/kg diet (corresponding to 0, 1.4, 8.3 or 50 mg/kg bw per day38) for 22 weeks to young adult mallard ducks approaching their first breeding season. Each group consisted of 20 pens, each containing one pair (a drake and a hen). Eggs were collected daily during the 10‐week egg‐laying period, they were incubated and hatched, and the offspring were maintained on basal diet for 14 days. The authors reported no effects of the SCCP in adults or in hatchlings at any dose level. At the highest dose a reduction in egg fertility was reported. This effect was not reported to be statistically significant when the number of viable embryos of eggs set was compared to controls over the entire study. However, when weekly comparisons were made, a significant difference was found for two of the 10‐week collection periods (week 3 and 6). Also at the highest dose, the authors reported a slight decrease in eggshell thickness (control 0.375 mm v. high dose 0.355 mm). Although the difference was statistically significant, the authors considered the biological significance was questionable, as the mean was within the range of normal values and no increase in cracked shells was observed in the study. No treatment‐related differences in embryo viability or eggshell thickness were noted at 1.4 or 8.3 mg/kg bw per day groups when compared with the control. No effects on egg weight, eggshell cracks, eggs laid, 21‐day embryos or hatchability were observed in any of the treatment groups. Serrone et al. (1987) considered that the NOEL was 166 mg/kg feed, corresponding to 8.3 mg/kg bw per day, for reduction in egg fertility. The CONTAM Panel could not identify a NOAEL based on the summary of this study as reported by Serrone et al. (1987).
In conclusion, the CONTAM Panel did not consider any of the studies sufficiently robust for risk assessment in poultry.
3.1.4.4. Rabbits
No oral CP toxicity studies in rabbits have been identified other than the developmental toxicity studies described already in Section 3.1.2.3.
For an SCCP (carbon chain length not specified, 58% chlorination), the NOAEL for developmental toxicity was 10 mg/kg bw per day, based on increased number of resorptions. The NOEL for maternal toxicity was 100 mg/kg bw per day (the highest dose tested).
For an MCCP (C14–17, 52% chlorination), the NOEL for developmental and maternal toxicity was 100 mg/kg bw per day (the highest dose tested).
For an LCCP (C22–26, 43% chlorination), the NOEL for developmental and maternal toxicity was 5,000 mg/kg bw per day (the highest dose tested). For another LCCP (C22–26, 70% chlorination), the NOEL for developmental and maternal toxicity was 1,000 mg/kg bw per day (the highest dose tested).
3.1.4.5. Fish
Several studies were performed in bleak (Alburnus alburnus L.). Although it is not usually farmed, the CONTAM Panel noted it belongs to the carp family (Cyprinidae) and studies might be relevant to farmed species. Studies in which the fish were exposed via the tank water were also included, although this exposure route is not entirely relevant to dietary exposure.
The acute toxicity of several CPs was examined in two different fish species. For SCCP (C10–12, 58% chlorination), the 96‐h LC50 in rainbow trout (Oncorhynchus mykiss) was reported to be > 300 mg/L (Howard et al., 1975). In bleak (Alburnus alburnus), the 96‐h LC50 values were either > 5,000 mg/L (C10–13, 49% chlorination; C10–13, 63% chlorination; C10–13, 71% chlorination) or > 10,000 mg/L (C10–13, 56% chlorination; C11.5, 70% chlorination) (Lindén et al., 1979). For MCCPs, the 96‐h LC50 values in bleak (Alburnus alburnus) were found to be > 5,000 mg/L (C15.5, 40% chlorination; C14–17, 50% chlorination) or > 10,000 mg/L (C14–17, 52% chlorination) (Lindén et al., 1979). For LCCPs, the 96‐h LC50 values in rainbow trout (Oncorhynchus mykiss) were either > 300 mg/L (C>20, 40–42% chlorination; C>20, 48–54% chlorination; C>20, 70% chlorination) or > 700 mg/L (C20–30, 42% chlorination). In bleak (Alburnus alburnus), the 96‐h LC50 of LCCP (C22–26, 42% chlorination) was > 5,000 mg/L for (Lindén et al., 1979). The CONTAM Panel noted that effect concentrations were given as nominal concentrations without further considering the water solubility of the CPs.
The toxicity following dietary administration for 40 days of two SCCPs (20 and 200 ng/g feed) and two MCCPs (20 and 200 ng/g feed, one of the high chlorinated MCCPs at 2,000 ng/g) was evaluated in rainbow trout (Oncorhynchus mykiss) (Fisk et al., 1996) (see Section 3.1.1.3.2 and Appendix B for details). The doses of 20, 200 and 2,000 ng/g feed corresponded to 0.0003, 0.003 and 0.03 mg/kg bw per day.39 No reduced growth rates or hepatic monooxygenase enzyme induction were seen in any of the CP groups when compared with the control group indicating no toxicity in the juvenile rainbow trout to the four CPs tested under the conditions of this study.
The toxicity following dietary administration of 19 different CPs with varying carbon chain length (C10, C11 and C14) and chlorination pattern was studied in rainbow trout (Oncorhynchus mykiss) (Fisk et al., 1998) (see Section 3.1.1.3.2 and Appendix B for details). Only a single dose level was used for each CP precluding the identification of NOELs. Exposure to the CPs did not appear to influence the health of the rainbow trout as growth rates were not significantly different between the CP exposed and control populations. Lipid percentages and liver somatic indices were also similar between treatments. No deaths occurred in any of the treatments.
The toxicity following dietary administration of three different CPs (C10H15.3Cl6.7, C14H23.3Cl6.7 and C18H31.4Cl6.6) for 40 days followed by 160 day of clean food, was studied in the rainbow trout (Oncorhynchus mykiss) (Fisk et al., 2000) (see Section 3.1.1.3.2 and Appendix B for details). The fish were fed with feed spiked with C10H15.3Cl6.7 at 1.4 and 15 μg/g feed (corresponding to 0.02 and 0.23 mg/kg bw per day40), with C14H23.3Cl6.7 at 1.3 and 13 μg/g feed (corresponding to 0.02 and 0.20 mg/kg bw per day41) and with C18H31.4Cl6.6 at 1.6 and 15 μg/g feed (corresponding to 0.02 and 0.23 mg/kg bw per day41). No mortalities occurred in any treatment group. Body and liver growth rates and liver somatic indices did not vary between CP‐exposed and control rainbow trout showing that exposure to CPs at the concentrations used in this study did not affect the health of the rainbow trout.
Juvenile Atlantic salmon (Salmo salar) (20 fish per tank) were fed with dry fish feed containing two different CPs (Cereclor 42, 42% chlorination; Chlorez 700, 70% chlorination) at concentrations of 10 and 100 mg/kg feed (corresponding to 0.25 and 2.5 mg/kg bw per day41) for up to 181 days followed by a control diet for 74 days (Zitko, 1974). Mortalities occurred both among fish fed the CPs and among fish on the control diet. The LT50 42 values of fish fed the CPs were lower (39–80 days) compared to that of fish on the control diet (138 days). Cereclor 42 was more toxic at 10 than at 100 mg/kg feed (LT50 values of 47 and 80 days, respectively), whereas the opposite was seen for Chlorez 700 (LT50 values of 71 and 39 days, respectively). The amount of feed ingested by the fish was not determined in the study and, according to the authors, it is possible that fish could taste Cereclor 42 and fed less on feed containing the higher concentration of this CP.
Bioaccumulation of a CP from feed was studied in rainbow trout (Lombardo et al., 1975). Fingerling rainbow trout (Salmo gairdneri Richardson) were fed a diet containing Chlorowax 500C at 10 mg/kg feed (corresponding to 0.15 mg/kg bw per day43) for up to 82 days. The treated fish gained significantly less weight than the controls. Adverse effects were also observed, but both the controls and the experimental fish were similarly affected. No gross toxicological effects were noted in the experimental fish.
Bleak (Alburnus alburnus L.) caught in the Baltic Sea and acclimated to laboratory conditions during three to five months (15 fish per group) were exposed during 14 days to the CP test solutions (125 μg/L) added to the tank water (natural Baltic Sea water) and renewed every second or third day (Bengtsson et al., 1979) (see Section 3.1.1.3.2 and Appendix B for details). The CP test solutions included Witaclor 149 (C10–13, 49% chlorination), Witaclor 159 (C10–13, 9% chlorination), Witaclor 171P (C10–13, 71% chlorination), Witaclor 350 (C14–17, 50% chlorination) and Witaclor 549 (C18–26, 49% chlorination). The fish were not fed during the experiments. No mortality occurred in any of the groups. Changes in the behaviour (sluggish movements, absence of shoaling behaviour and abnormal vertical postures) were observed after about seven days in fish exposed to Witaclor 149, 159 and 171P. The abnormalities were most pronounced in fish exposed to Witaclor 149, followed by Witaclor 159 and the by Witaclor 171P. The behaviour became normal again after the fish had been kept in clean water for a couple of days. No effects on behaviour were observed for Witaclor 350 and Witaclor 549.
Uptake and elimination of CPs was studied in bleak (Alburnus alburnus, 30 fish/group) (Bengtsson and Ofstad, 1982) (see Section 3.1.1.3.2 and Appendix B for details). The CPs included Witaclor 149 (C10–13, 49% chlorination, at 590, 2,500, 5,800 mg/kg feed, corresponding to 15, 63 and 145 mg/kg bw per day44), Witaclor 171P (C10–13, 71% chlorination, 3,180 mg/kg feed, corresponding to 80 mg/kg bw per day44) and Witaclor 549 (C18–26, 49% chlorination, 3,400 mg/kg feed, corresponding to 85 mg/kg bw per day44). No significant increase in mortality was observed in the exposed groups. Changes in the behaviour (sluggishly swimming and closer to the bottom than usual, generally accompanied by folded dorsal fins and minor balance problems) were observed in three of the exposed groups and were first noted in the high‐dose Witaclor 149 group after 5 weeks of the accumulation period. After 7 weeks of the accumulation period, the same changed behaviour was also observed in the mid‐dose Witaclor 149 group, and after 12 weeks also in the group exposed to Witaclor 171. These effects gradually disappeared within a couple of weeks of the subsequent elimination period.
SCCP (C12, 49% chlorination; Witaclor 149) or SCCP (C12, 70% chlorination, Hüls 70C) were administered to flounder (Platichthys flesus L.) of both sexes orally in gelatine capsules via stomach tube (Haux et al., 1982). The animals were dosed twice, at day 1 and 4, to obtain a total exposure of 1,000 mg/kg bw. Fish were caught in bays from the Swedish East and West coast. They were placed in tanks of brackish water (7.4‰ salinity) and in marine water (25–30‰ salinity) in groups of 15–30 fish. With the exception of the dry fish food in capsules, the animals were starved throughout the experiment. Sampling was performed 13 and 27 days after the first SCCPs administration. SCCP (C12, 49% chlorination) caused a 21% reduction in haematocrit in the brackish water female fish after 13 days compared to the control group. In marine female flounders, SCCP (C12, 49% chlorination) caused a 13% increase in both haematocrit and number of red blood cells observed after 13 days, while after 27 days no effects were seen. After 27 days, a hypoglycaemia was observed for marine female fish exposed to SCCP (C12, 49% chlorination; Witaclor 149). The liver somatic index was increased in the SCCP (C12, 70% chlorination, Hüls 70C)‐treated brackish water male group after 13 days. SCCP (C12, 70% chlorination, Hüls 70C) changed activities of two steroid metabolising enzymes. There was a decrease in the 6β‐hydroxylase activity in marine female fish after 13 days as well as an increase in the 5α,β‐reductase activity in brackish water female fish after 13 days. Based on the information in this study the CONTAM Panel could not identify a NOAEL.
Juvenile rainbow trout (Oncorhynchus mykiss) were exposed to CPs (C10‐, C11‐, C12‐ and C14) for either 21 or 85 days (Cooley et al., 2001). Commercial fish feed was spiked with one of four SCCPs (C10H15.5Cl6.5, 63% chlorination45; 14C‐C10H15.3Cl6.7, 63.7% chlorination; C11H18.4Cl5.6, 56.9% chlorination; 14C‐C12H19.5Cl6.5, 58.5% chlorination) or one of two MCCPs (C14H24.9Cl5.1, 48% chlorination; 14C‐C14H23.3Cl6.7, 55% chlorination) separately. The juvenile rainbow trout (10/group; initial weights approx. 2 g) were exposed to each CP at three different CP concentrations, ranging from 0.082 to 290 mg/kg feed. In many of the trouts, no or diminished startle response could be observed. Failure to feed on certain days were also observed, and animals from the highest dose groups in many cases stopped feeding. Many trouts showed loss of equilibrium and developed dark coloration. In each exposure group, histopathological liver lesions were seen. Most severe lesions were observed in livers of fish exposed to 14C‐C10H15.3Cl6.7 (C10, 63.7% chlorination) and C11H18.4Cl5.6 (C11, 56.9% chlorination) (whole fish concentrations 0.92 and 5.5 mg/kg, respectively) in which extensive fibrous lesions were present that were not present in any other exposure group. Other effects in the treatment groups included sites of inflammation, hepatocyte necrosis and depletion of glycogen/lipids. The mean hepatocyte volume index of trout treated with C10H15.5Cl6.5 (C10, 63% chlorination), C12H19.5Cl6.5 (C12, 58.5% chlorination) and C14H24.9Cl5.1 (C14, 48% chlorination) were smaller than the controls. No lesions or abnormalities were seen in the thyroids of fish exposed to SCCPs or MCCPs. The authors noted that the toxicity of terminal chlorinated CPs was not different than that of CPs which are not chlorinated on the terminal carbons (Cooley et al., 2001). The CONTAM Panel concluded that the study indicates adversity in the liver based largely on descriptive endpoints that could not be quantified to demonstrate a dose response. It is uncertain if the effects in the liver are direct or secondary to changes in food intake. Although effects were reported in all treatment groups, a LOAEL could not be identified because of inconsistencies in the reporting of the feed concentrations.
In conclusion, most studies showed no or minor effects. Based on the available information, the CONTAM Panel could not identify a NOAEL or a LOAEL for fish.
3.1.4.6. Horses
No studies investigating effects of CPs in horses have been identified.
3.1.4.7. Companion animals (cats and dogs)
No studies investigating effects of CPs in cats have been identified.
One dietary study in Beagle dogs investigating effects of an MCCP (Cereclor S52, C14–17, 52% chlorination, containing epoxidised vegetable oil stabiliser) is described in Section 3.1.2.2.2 . The NOEL was 10 mg/kg bw per day, based on an increase of hepatic smooth endoplasmic reticulum at higher dose levels.
3.1.4.8. Fur animals
No studies investigating effects of CPs in fur animals have been identified.
3.1.5. Mode of action
Studies in experimental animals indicate that the target organs for toxic effects are the liver, kidneys and thyroid. Tumours have been observed in these and other tissues. In addition, effects of MCCP were observed in rat offspring indicative of internal haemorrhaging. The mode of action regarding these effects in each target organ, and the relevance to humans are considered in this chapter. The overall data indicate that CPs do not cause genotoxic effects in vivo (see Section 3.1.2.6 ) and thus a genotoxic mechanism of carcinogenesis does not appear to be relevant. The observed tumours for SCCPs and LCCPs (see Section 3.1.2.7 ) are likely to arise by non‐genotoxic mechanism(s) which differ between tissues and which are likely to arise only at dose levels at and above those in which the responsible non‐genotoxic perturbation occurs.
3.1.5.1. Effects in the liver
Repeated dose toxicity studies with CPs in rats and mice have revealed effects in the liver, including hepatic enzyme induction, increased liver weight, non‐neoplastic hepatic lesions and cancer. Necrosis occurred in mouse liver following dosage of 500 mg/kg bw (see Sections 3.1.2.2 and 3.1.2.7 ).
3.1.5.1.1. In vivo
Male Sprague–Dawley rats were administered i.p. with 1,000 mg/kg bw per day of SCCPs (C10–13, 49% chlorination or 59% or 71% chlorination), MCCP (C14–17, 50% chlorination) or LCCP (C18–26, 49% chlorination), daily for 4 days (Nilsen et al., 1980, 1981; Nilsen and Toftgard, 1981). Treatment with SCCPs, but not with CPs of longer chain lengths, caused an increase in liver weight associated with an induction of various CYP450 enzymes. The activity of O‐deethylation of 7‐ethoxyresorufin was decreased by the SCCPs with higher chlorine content. In contrast, the metabolism of benzo[a]pyrene was induced by each of the CPs. SCCP (C10–13, 49% chlorination) caused a proliferation of the smooth endoplasmic reticulum (twofold as assessed by electron microscopy) whereas LCCP (C18–26, 49% chlorination) caused a smaller increase. All three SCCPs produced an increase in the occurrence and size of lipid droplets in the cytoplasm. SCCP (C10–13, 49% chlorination) moderately increased the number of mitochondria and occasionally caused the formation of enlarged mitochondria. MCCP (C14–17, 50% chlorination) and LCCP (C18–26, 49% chlorination) also increased the number and size of peroxisomes.
An increase in hepatic cytosolic and microsomal epoxide hydrolase activities (130% and 250% increase compared to the control for cytosolic and microsomal hydrolases, respectively) was observed in male C57Bl/6 mice after 5 daily i.p. injection of 400 mg/kg bw SCCP (C12, 70% chlorination) (Meijer and DePierre, 1987). In addition, the amount of microsomal CYP450 was increased by 50%, and cytosolic DT‐diaphorase activity was increased two‐ to threefold. This was associated with enlargement of the liver.
Effects on microsomal enzymes in liver from male Sprague–Dawley rats, after i.p. injection of 1,000 mg/kg bw per day SCCP (C10–13, 70% chlorination), MCCP (C14–17, 58% chlorination), LCCP (C22–26, 42% chlorination) or LCCP (C23, 70% chlorination), once daily for 5 days, was observed (Meijer et al., 1981). Microsomal epoxide hydrolase and glutathione‐S‐transferase activities were also increased by all administered CPs except LCCP (C22–26, 42% chlorination).
As described in Section 3.1.2.2 , SCCPs and MCCPs induced hepatic peroxisome proliferation in rats and mice. Male rats and male mice (5 per group) were administered SCCPs (Chlorowax 500C, C10–13, 56% chlorination or Cereclor 56L, C10–13, 58% chlorination) or MCCP (Chlorparaffin 40G, C14–17, 40% chlorination) at doses of 0, 10, 50, 100, 250, 500 or 1,000 mg/kg bw per day in corn oil per gavage for 14 days (Wyatt et al., 1993). Palmitoyl CoA oxidation as indicator for peroxisome proliferation was increased in rats from 250 mg/kg bw per day (SCCP, C10–13, 56% chlorination) and from 500 mg/kg bw per day (MCCP, C14–17, 40% chlorination). The mice proved to be more responsive than the rats showing increased palmitoyl CoA oxidation from 250 mg/kg bw per day (SCCP, C10–13, 56% chlorination or 58% chlorination) and from 500 mg/kg bw per day (MCCP, C14–17, 40% chlorination). In general, higher dose levels were required for induction of peroxisome proliferation compared to those required for an elevation of liver weight. SCCPs caused a greater increase in palmitoyl CoA oxidation (up to approximately a 10‐fold increase) compared to the increase caused by MCCPs (up to approximately a 4‐fold increase). SCCP (C10–13,56.5% Chlorination) given to rats by gavage daily for 28 days (100 mg/kg bw per day) also caused an upregulation of 11 genes in the liver that are activated by peroxisome proliferator activated receptor α (PPARα) and are involved in fatty acid metabolism. This was associated with enhanced fatty acid oxidation (Gong et al., 2019).
As mentioned in Section 3.1.2.2 , MCCP (C14–17, 52% chlorination) significantly increased UDPGT activity in two 90‐day rat studies (Poon et al., 1995; CXR Biosciences Ltd, 2005b, unpublished study, see Documentation provided to EFSA). The induction of UDPGT (see Section 3.1.5.3 ) in the rat liver is mediated by interaction of SCCPs with the constitutive androstane receptor (CAR) (Gong et al., 2018). The altered liver metabolism of T4 via elevation of UDPGT is considered to be an important component of the mode of action for disturbance of thyroid physiology and pathology leading to thyroid gland follicular‐cell adenoma and carcinoma in the rat. Details of these responses and the relevance to human risk assessment of the role of altered hepatic metabolism of T3/T4 is discussed in Section 3.1.5.3 .
The effects of CPs in the liver have been studied in F344 rats, B6C3F1 mice and male Alpk:Dunkin Hartley guinea pigs. The responses were compared with those produced by a range of known inducers of hepatic enzymes (ICI, 1997, unpublished study, see Documentation provided to EFSA). CPs were given to animals by gavage in corn oil for 14 days (4–5 animals per group). Dose levels were 1,000 to 2,000 mg/kg bw per day of the following CPs: SCCP (C500C, C10–13, 58% chlorination; C56L, C10–13, 56% chlorination), MCCP (C40G, C14–17, 40% chlorination) and LCCP (C20–30, 43% chlorination). Relative liver weight was increased (approximately 1.5‐fold) by SCCPs and MCCP, but not by LCCP. The increased liver weight was associated with hepatocellular hypertrophy, peroxisome proliferation (assessed by electron microscopy and by increase in palmitoyl CoA oxidation) and proliferation of hepatic cell smooth endoplasmic reticulum in both rats and mice. SCCPs and MCCPs also caused induction of CYP450 4A1 in rat and mouse liver and CYP450 2B1/2B2 in the rat liver. In guinea pigs administered 1,000 mg/kg bw per day of SCCP and MCCP liver weight was also increased by 1.5‐fold but no histological or biochemical parameters measured were altered.
In a study by Elcombe et al. (2000) (abstract only), male and female F344 rats were administered SCCP (Chlorowax 500C (C500C), C10–13, 58% chlorination) or MCCP (Chlorparaffin 40G (C40G), C14–17, 40% chlorination) in corn oil by gavage in doses of 0, 312 or 625 mg/kg bw per day for up to 90 days. In addition, male Dunkin Hartley Guinea pigs were administered SCCP (Chlorparaffin 40G (C40G), C10–13, 58% chlorination) in corn oil by gavage in doses of 0, 500 or 1,000 mg/kg bw per day for 14 consecutive days. Liver weight, liver palmitoyl CoA oxidation, UDPGT activity were increased in male and female rats by SCCP and MCCP. Therefore, no effect of SCCP on liver weights and no observable hepatic histopathology in male guinea pigs could be observed.
A recent study by Geng et al. (2019) confirmed in vitro studies (see Section 3.1.5.1.2 ) in demonstrating oxidative stress, PPARα activation and inhibition of energy metabolism produced by SCCPs in the liver of male rats. There was suppression of oxidative phosphorylation, glycolysis and gluconeogenesis, reducing the levels of amino acids and nucleotides. Some of these effects on energy metabolism were produced at the lowest dose studied (0.01 mg/kg bw per day for 28 days). The authors noted the need for further studies to verify the impact on the energy metabolism. While these findings demonstrate a mode of action in hepatotoxicity, the CONTAM Panel could not conclude on a dose level whereby these changes lead to adversity.
3.1.5.1.2. In vitro
Information on a range of parameters altered by an SCCP (C12, 60% chlorination) in human liver cells (not specified) has been reported in the ToxCast database (US‐EPA, 2019). The results are given in the section relating to the kidney below (see Section 3.1.5.2 ). In support of the role of PPARα in mediating the proliferation of peroxisomes in rodent liver, Gong et al. (2019) found that SCCP activated luciferase expression in an in vitro dual‐luciferase reporter gene linked to the rat PPARα gene response element. Moreover, an in silico molecular docking analysis using LeDock46 simulated the interaction of SCCP with PPARα.
A cell viability assay and targeted metabolomic approach were used to evaluate the effects of SCCPs of different chain lengths and chlorine contents (C10, C11, C12 or C13; e.g. C10 chlorine content of 40.4%, 57.9% or 64.4%) at environmentally relevant concentrations (0, 1, 10 or 100 μg/L) on human hepatoma HepG2 cells (Geng et al., 2015). Viable cell number (measured by the MTT assay) was found to be decreased with increases in exposure concentrations of SCCPs. Exposure for 48 h to SCCPs resulted in a significant reduction in cell number compared with 24 h, even at 1 μg/L, while exposure with 100 μg/L SCCP resulted a potent decrease down to 60% after 48 h. The CONTAM Panel noted that the MTT assay does not necessarily indicate cell death since cell number may be reduced by inhibition of cell proliferation. SCCPs exposure caused oxidative stress, as shown with increased catalase and superoxide dismutase activities and altered the intracellular redox status as shown with decreased glutathione contents at 10 ng/L and above. Furthermore, SCCPs exposure caused significant metabolic disruption. SCCPs specifically stimulated the β‐oxidation of unsaturated fatty acids and long‐chain fatty acids. Moreover, SCCPs exposure disturbed glycolysis and amino acid metabolism, and led to the upregulation of glutamate metabolism and urea cycle. Thus, the toxic effects of SCCPs appear mainly to involve the perturbation of energy production, protein biosynthesis, fatty acid metabolism and ammonia recycling.
The effects of SCCP, MCCP and LCCP (1, 10, 100 and 1000 μg/L for 24 h) on viable cell number and disturbance of metabolism was also compared in HepG2 cells (Ren et al., 2019). Viable cell number was reduced at the lowest concentration tested (1 μg/L) for all CPs. ATP levels were reduced and levels of reactive oxygen species and malondialdehyde levels (indicative of lipid peroxidation and oxidative stress) were elevated by all CPs at 100 μg/L. The potency of all three CPs was similar with respect to overall effect on perturbation of various aspects of metabolism, particularly in relation to phospholipid and fatty acid metabolism, however LCCP produced a relatively greater suppression of amino acid transport than seen with SCCP and MCCP. Furthermore, the marked elevation of purines by SCCP and MCCP was not seen with LCCP which reduced the levels of various purines. The CONTAM Panel noted an inability to extrapolate the concentrations of CPs applied in all of the above cell culture studies to cellular concentrations or to tissue concentrations achievable in vivo and hence no attempt was made to interpret the effects in vitro on a quantitative basis.
3.1.5.1.3. Summary on liver toxicity
Regarding the mode of action for liver toxicity, it is considered that enzyme induction and proliferation of the smooth endoplasmic reticulum leading to hypertrophy (and associated increases in liver size) is an adaptive physiological response to CPs. In addition, proliferation of rodent peroxisomes occurs mediated by PPARα. These responses could lead to rodent hepatocellular carcinogenicity (see below) and also to toxicity if the energy balance to support this compensatory response becomes sufficiently perturbed so as to compromise the cell viability. This mode of action of toxicity appears to be achievable in vitro based on the evidence presented above for perturbation by SCCP of energy production in human hepatoma HepG2 cells (Geng et al., 2015). Furthermore, a metabolomic study in these cells showed a clear disturbance by an SCCP of the TCA cycle as well as perturbation of glycerophospholipid and linoleic acid metabolism (Wang et al., 2018a).
3.1.5.1.4. Mode of action in liver carcinogenesis
Regarding the MOA for carcinogenicity of SCCPs in the rodent liver, the Panel considered the following as potentially involved: (1) CAR activation, (2) PPAR alpha activation, (3) cytotoxicity; in the absence of evidence for genotoxicity. The relevance of these rodent liver effects to humans was considered.
Regarding CAR activation, the key associative events in rodents comprise increased hepatocyte replication, induction of cytochrome P450 CYP2B subfamily enzymes and these lead to liver hypertrophy and hepatocellular adenomas/carcinomas. It is well established that various activators of CAR in the rat do not stimulate replicative DNA synthesis in cultured human hepatocytes or in chimeric mice with humanised livers (see Lake (2018) and Felter et al. (2018)). The lack of mitogenic response to activators of CAR in human hepatocytes is shared by other species (hamster and guinea pig). The conclusion regarding the human liver being refractory to the hepatocarcinogenicity of CAR activators is supported by epidemiological studies in which humans chronically exposed to the CAR activator phenobarbital show no evidence of an increased incidence of liver tumours (La Vecchia and Negri, 2014).
The histological and biochemical evidence for peroxisome proliferation (see Sections 3.1.2.2 and 3.1.5.1.1 ) is considered a toxic response for the rat and mouse and is likely to be a key factor in the development of rat and mouse hepatocellular adenoma and carcinoma. However, it is well documented that, as with guinea pigs, primates, including humans, are insensitive to peroxisome proliferator‐induced hepatic effects (Bentley et al., 1993). The susceptibility of rodents to hepatic tumours produced by peroxisome proliferators has been linked to the activation of the PPARα and the associated liver cell proliferation and inhibition of apoptosis. Although PPARα activation can occur in both rodents and humans, the responses leading to tumourigenesis are unique to mice and rats, human hepatocytes being refractory to the cell proliferative response as discussed in detail by González et al. (1998), Roberts (1999), Bentley et al. (1993) and Corton et al. (2018).
Of the responses listed above as potentially contributing to rodent liver carcinogenesis by SCCPs, cytotoxicity could be relevant to human carcinogenesis involving a threshold dose response (Felter et al., 2018).
Based on the available information, the relevance to humans of the rodent liver tumours can be excluded provided cytotoxicity does not occur.
3.1.5.2. Effects in the kidney
Information on a range of parameters altered by an SCCP (C12, 60% chlorination) in human kidney (and liver) cells has been reported in the ToxCast database (US‐EPA, 2019). Particularly sensitive responses in the activation of the nuclear transcription factors pregnane X receptor (PXR), renin/prorenin receptor (RER), vitamin D3 receptor (VDR) and nuclear receptor family 1 group H member 4 (NR1H4) were observed at concentrations below those required for loss of mitochondrial membrane potential. Effects on PPARs were only seen at similar or even higher concentrations of SCCP required for changes in membrane potential. It is noted that the data points suggesting an apparent very high potency for cell death in kidney cells, based on DNA staining, are not considered reliable since it is based on a single replicate and at a concentration far below that causing effects on mitochondrial membrane potential and expression of nuclear transcription factors.
Repeated dose toxicity studies in rodents have revealed nephrotoxicity caused by CPs such as increased kidney weight, pigmentation in tubules, nephritis and nephropathy. This includes tumours in male (but not female) rats, but not in mice treated with SCCPs (see Sections 3.1.2.2 and 3.1.2.7 ).
In the study by Elcombe et al. (2000) (only abstract available), SCCPs and MCCPs produced chronic nephropathy in male rats only, associated with regenerative hyperplasia and increased S‐phase in the proximal tubules. No histopathological changes were seen in kidneys of the SCCP‐treated guinea pigs.
In the 90‐day and 2‐year rat studies (NTP, 1986a), and in the 2‐year mouse study (NTP, 1986b) nephropathy was observed in both males and females.
A study was performed to elucidate the mechanisms for the induction of renal tumours in male rats by SCCPs (Warnasuriya et al., 2010). SCCP (C12, 60% chlorination) was administered to male F344 rats at doses of 0 and 625 mg/kg bw per day in corn oil by gavage for 28 consecutive days. SCCP (C12, 60% chlorination) caused downregulation of α2u‐microglobulin synthesis in the liver of male rats. Specific binding of radiolabelled SCCP to the α2u‐microglobulin could be demonstrated followed by a slow accumulation of α2u‐microglobulin in the kidney followed by delayed onset of α2u‐nephropathy. As a result, the authors suggested a complex, yet uncharacterised mechanism for the carcinogenic effects of SCCP in the kidney of male rats.
Kidney tumours caused by SCCPs in male rats cannot be ascribed to a classic profile of α2u‐microglobulin‐induced nephropathy. While SCCPs bind specifically to α2u‐microglobulin and slow accumulation of the protein in the kidney occurs followed by delayed nephropathy, SCCPs do not induce, but significantly downregulate, α2u‐microglobulin synthesis in the liver which complicates the interpretation of α2u‐microglobulin‐induced nephropathy (see Section 3.1.2.2 ). Overall, although histological evidence for classical hyaline droplet nephropathy is limited, it appears that α2u‐microglobulin‐induced nephropathy is the most likely contributing factor in kidney tumourigenesis involving the reported increase in kidney tubular hyperplasia produced by SCCP in male rats (NTP, 1986a). An area of uncertainty is the evidence also seen in female rats and mice (both sexes) of nephropathy produced by SCCPs (see Section 3.1.2.2 ). However, the importance of this effect in females is questionable since kidney tumours were only found in male rats treated with SCCP.
Since a genotoxic mechanism has been ruled out, the mode of action therefore may relate to sustained cellular damage (at least in part related to nephropathy induced by α2u‐microglobulin in the male rat), compensatory regenerative hyperplasia and increased S‐phase possibly also involving inhibition of intercellular communication (see below) as non‐genotoxic influences. However, there is insufficient evidence to conclude that nephropathy responsible for carcinogenesis is solely male rat specific event mediated by α2u‐microglobulin and, for that reason, the CONTAM Panel concluded that the relevance of renal carcinogenesis to humans cannot be ruled out.
3.1.5.3. Effects in the thyroid
Repeated dose toxicity studies revealed diverse effects of SCCPs and MCCPs in the thyroid such as increased thyroid weight, increased incidences of thyroid hypertrophy and hyperplasia, as well as follicular cell lesions, reduced follicle sizes and cytoplasmic vacuolation. In carcinogenicity studies, SCCP caused thyroid follicular cell adenomas and carcinomas in female rats and female mice. No carcinogenicity studies with MCCPs have been conducted so far.
As described in Section 3.1.2.2 , in two 90‐day rat studies with MCCP (C14–17, 52% chlorination), liver enzyme UDPGT activity was significantly increased (Poon et al., 1995; CXR Biosciences Ltd, 2005b, see Documentation provided to EFSA). In one of these studies, FT3 in plasma was found to be decreased (CXR, 2005b, see Documentation provided to EFSA).
In another study, male F344 rats (5 per group) received 0 or 1,000 mg/kg bw per day of two different SCCPs (Cereclor 56L, C10–13, 56% chlorination or Chlorowax 500C, C10–13, 58% chlorination) or MCCPs (Chlorparaffin 40G, C14–17; 40% chlorination) by oral gavage for 14 days (Wyatt et al., 1993) (see Section 3.1.2.2 ). No females were examined in this study. Significant reductions in both free and total plasma T4 levels (by 44 and 53%, respectively) and an almost twofold increase in UDPGT activity was noted at the treated group. T3 levels remained unaffected, while TSH levels showed an approximate 1.5‐fold increase at 1,000 mg/kg bw per day.
In the study by Elcombe et al. (2000) (abstract only), SCCPs and MCCPs decreased plasma T4 and increased plasma TSH concentrations and thyroid follicular cell hypertrophy and hyperplasia could be observed. No effect on any of the plasma thyroid hormones or plasma TSH concentration could be observed in SCCP‐treated guinea pigs.
Gong et al. (2018) found that an SCCPs (C10–13, 56.52% chlorination; mass ratio of C10:C11:C12:C13 = 1:1:1:1) was able to reduce the plasma FT3 level significantly in rats treated by gavage with 10 and 100 mg/kg bw per day for 28 days. SCCPs did not show significant effects on the expression of thyroid hormone synthesis genes. However, various changes in hepatic metabolism were noted that help to explain the modulation of plasma thyroid hormone levels. The relative hepatic mRNA levels of Dio3 (gene for type III deiodinase ID3), Slc22a7 (gene for organic anion transporter 2 (OAT2)), Slco1a4 (gene for organic anion transporting polypeptide 1a4 (OATP1A4)) and Slc10a1 (gene for sodium taurocholate cotransport polypeptide (NTCP)) were significantly and dose‐relatedly increased at 10 and 100 mg/kg bw per day. The relative mRNA levels of Cyp2b1 (gene for CYP450 2B1) and Ugt1a1 (gene for UDPGT1A1) were significantly increased at 100 mg/kg bw per day. UDPGT protein (which conjugates T4) was also increased at 100 mg/kg bw per day and this is likely to contribute to the observed reduction of the plasma FT4 level at the same dose level. However, this induction was not seen at 10 mg/kg bw per day or below suggesting that an additional mechanism contributes to the perturbation of plasma thyroid hormone levels. In this study, liver OAT2 protein and its mRNA (Slc22a7) were found to be elevated at 1 mg/kg bw per day. The authors referred to OAT2 as being involved in T3 transport into the liver, but the CONTAM Panel could not ratify this conclusion since organic anion transporting polypeptide 2 (OATP2) (which was not measured) is responsible for T3 transport. No evidence in the literature could be found for a role of OAT2 in T3 transport. However, it is likely that OATP2 is also elevated by SCCP because this protein is induced by CAR which Gong et al. (2018) showed was activated by SCCP (see Section 3.1.5.1.1 ) and was responsible for induction of UDPGT. Therefore, it is likely that the reduction of plasma FT3 is influenced by OATP2 induction.
Thus, thyroid tumours in rodents, as caused by SCCPs in female rats and female mice, can arise due to stimulation of the thyroid via a negative feedback mechanism. This includes a potential increase in uptake of thyroid hormones, increased hepatic UDPGT levels and conjugation of T4, consequent decrease in plasma T4/T3, compensatory release of pituitary TSH, and a compensatory increase in T4 production in the thyroid. This eventually leads to hypertrophy, hyperplasia and an increased sensitivity to develop thyroid tumours. The effect was not evident in guinea pigs.
Rodents appear to be particularly susceptible to thyroid changes partly due to having T4‐binding proteins other than the high affinity T4‐binding globulin (TBG), which is present in humans. TBG is expressed in rats, but varies according to age. In adult rats, its levels are low between 2 and 7 months of age and transthyrethin (TTR) is the dominant T4 plasma carrier in adult rats. TTR has a lower affinity for T4 and is therefore less efficient in binding T4 than TBG. The primary function of carrier proteins is extra‐thyroidal storage of thyroid hormones to ensure sustained hormone action across tissues, and this contributes to the control of thyroid hormone homeostasis and protection from peripheral elimination. Thus, in species in which TBG is not the major T4 carrier, the free fraction of T4 is larger than in humans and TT4 half‐life is shorter (around 24 h in rats, versus 5–6 days in humans) (Lewandowski et al., 2004). However, even though TBG is the major and specific transport protein of thyroid hormones in humans, it has recently been shown not to be the physiologically most relevant (Alshehri et al., 2015). The most physiologically relevant carrier protein in humans is also TTR. Furthermore, the differences in half‐lives of the active hormone T3 between rats and humans are much smaller than for T4, as T3 half‐lives for humans are 22–24 h and around 6 h in rats. Based on these more recent findings, the previous assumptions about large qualitative species differences in thyroid hormones homeostasis between rats and humans may be incorrect, and should not be used to dismiss the rat as a predictor for all thyroid related effects in humans. This is despite the fact that, although some studies have shown drug‐induced changes in thyroid hormones in humans, elevations of thyroid stimulating hormone have not been reported in humans for a range of drugs (including delavirdine, fluvastatin, nicardipine, phenobarbital, simvastatin and spironolactone), all of which cause hyperplasia or tumours in rats (Wu and Farrelley, 2006). In addition, there are various reasons why changes affecting the thyroid that are mediated through altered hepatic metabolism may be more pronounced than in humans as discussed in detail by Bartsch et al. (2018).
Overall, this raises uncertainty about the validity of extrapolating changes related to the thyroid from rats to humans when these changes are mediated by induction of hepatic transporters and hepatic metabolism.
Using the current understanding of thyroid physiology and toxicology, the ECHA/EFSA Guidance for the identification of endocrine disruptors (ECHA/EFSA, 2018) “proposed that the following be applied when interpreting data from experimental animals: 1) Substances inducing histopathological changes (i.e. follicular cell hypertrophy and/or hyperplasia and/or neoplasia) in the thyroid, with or without changes in the circulating levels of thyroid hormones, would pose a hazard for human thyroid hormone insufficiency in adults as well as pre‐ and post‐natal neurological development of offspring. 2) Substances that alter the circulating levels of T3 and/or T4 without histopathological findings would still present a potential concern for neurodevelopment.”
Irrespective of the uncertainties regarding extrapolation of liver‐mediated effects on changes in thyroid hormone levels in rodents to humans as discussed above, the Panel noted that in the three rat studies on SCCPs and MCCPs in which thyroid hormone levels have been examined, there are inconsistencies in the hormonal changes seen amongst these studies, as well as between the two genders. Consequently, because of these inconsistencies, the Panel considered that changes in thyroid hormone levels could not be used as a basis for deriving a reference point. However, despite uncertainties regarding extrapolation of liver‐mediated effects from rat to human (discussed above), the thyroid histopathology is used as a basis for deriving a reference point.
There is a potential for modulation of thyroid hormone levels to have an effect in rodents on pre‐ and postnatal neurological development of offspring. It has also been shown that developmental neurotoxicity can arise via thyroid disruption in humans (Fan and Wu, 2016; Ghassabian et al., 2014). In addition, the human fetus and neonate has a shorter T4 half‐life than adults, which may make them more susceptible to a lowering of serum T4 levels (Li et al., 2019c). The CONTAM Panel could not identify any studies on potential neurodevelopmental effects of CPs in mammals. Because of the reported changes in thyroid hormone levels produced by SCCP and MCCP in rodents, the Panel concludes that an assessment of potential neurodevelopmental toxicity is needed.
3.1.5.3.1. Additional thyroid effects investigated in zebrafish
Thyroid hormones play an important role in the development, growth, differentiation and metabolism in early life stage in fish. Zebrafish embryos were exposed to seven different SCCPs, namely from the C10‐group (C10H18Cl4, 1,2,5,6,9,10‐C10H16Cl6 and C10H15Cl7), from the C12‐group (C12H22Cl4, C12H19Cl7 and 1,1,1,3,10,12,12,12‐C12H18Cl8) and the commercial Cereclor 63L (C10–13, 63% chlorination)47 (Liu et al., 2016). The TT3 and TT4 were measured after exposure from 2 to 96 h post fertilisation to 0, 0.5 and 100 μg/L SCCPs. A dose‐dependent decrease of TT3 was observed after exposure to the three C10‐group compounds. However, the effects varied among different congeners. 1,2,5,6,9,10‐C10H16Cl6 and C10H15Cl7 decreased TT3 significantly by 32.4% and 65.3%, respectively, at 0.5 μg/L and by 70.8% and 82.9%, respectively, at 100 μg/L. In comparison, C10H18Cl4 showed no effects on TT3 at 0.5 μg/L, but decreased TT3 significantly by 48.9% at 100 μg/L. The C12‐group compounds and Cereclor 63L caused no effects on TT3. For TT4, significant effects were only observed in two of the three C10‐SCCP treated groups at the low dose of 0.5 μg/L. 1,2,5,6,9,10‐C10H16Cl6 and C10H15Cl7 showed significant decreases of TT4 by 48.5% and 31.6%, respectively, at 0.5 μg/L. No significant change was observed after exposure to the three C10‐SCCP treated groups at 100 μg/L and after exposure to the C12‐group compounds and Cereclor 63L at 0.5 and 100 μg/L. The mRNA levels of the six genes tyr, ttr, dio2, dio3, oatp3a1 and thraa were measured in exposed zebrafish larvae (0, 0.5 and 100 μg/L of all 7 SCCP compounds) at 96 h post fertilisation. Significant changes in gene expression were only found in the C10H18Cl4‐ and C12H22Cl4‐treated groups. Compared to the control, the mRNA of tyr, ttr, dio2 and dio3 were significantly downregulated by C10H18Cl4 in a dose‐dependent manner while thraa was only significantly upregulated at 0.5 μg/L by 2.1‐fold. Significant reduction of ttr expression was observed in larvae treated with C12H22Cl4 at 0.5 and 100 μg/L. For other SCCPs treatment groups, there were no effects on the mRNA expression. 1,2,5,6,9,10‐C10H16Cl6 showed an apparent but non‐significant dose‐related expression increment of dio2 and dio3 by 25.1 and 15.6‐fold at 100 μg/L. SCCPs from the C10‐group were found to be more potent to disrupt thyroid hormone homeostasis than those from the C12‐group. C10H18Cl4 might cause a disturbance of thyroid status in zebrafish by inhibiting T3 production through a mechanism of dio2 inhibition effect. In zebrafish, T3 is produced in plasma and local tissues from T4 conversion mainly by type II iodothyronine deiodinase (Dio2). In the same study, SCCPs caused malformations in zebrafish embryo/larvae at higher concentrations of 1,000 and 10,000 μg/L. Again, SCCPs from the C10‐group were more toxic for this endpoint than C12‐group compounds and Cereclor 63L.
3.1.5.4. Other tumour incidences in rodents treated with CPs
In addition to the tumourogenic effects in liver, kidney and thyroid of rodents, SCCPs were found to induce additional tumour types (Section 3.1.2.7 ) which are not considered relevant to humans. Mononuclear cell leukaemia seen in both male and female rats is a uniquely common spontaneous disorder in F344 rats. The occurrence of such tumours in F344 rats following treatment with non‐genotoxic chemicals is considered not relevant to humans (Caldwell, 1999). The incidence of pancreatic acinar cell tumours in male rats only showed no dose response and was also seen in controls. In addition, there were Harderian gland tumours at the lowest dose of SCCPs in female mice. The absence of this gland in humans coupled with the lack of genotoxicity renders this not relevant to human.
In addition, malignant lymphomas were observed in male mice treated with LCCPs (Section 3.1.2.6 ). However, genotoxicity is not implicated in the mode of action of LCCPs and the lymphomas were only observed at very high‐dose levels being statistically significant at 5,000 mg/kg bw per day only.
Adrenal gland medullary pheochromocytomas in high‐dosed LCCP female rats were also observed. However, this a common tumour in rats but rare in humans (Haschek et al., 2001).
3.1.5.5. Potential additional non‐genotoxic influences of CPs in carcinogenesis
In addition to the specific modes of action described above for the three target organs for carcinogenesis, the recorded effects of CPs on inhibition of intercellular gap junctional communication and on oxidative stress may also be involved. Indeed, these effects are features of many chemicals that cause peroxisome proliferation (Chipman et al., 2003).
In addition to studies on the genotoxicity of CPs as detailed above (Section 3.1.2.5 ), cell transformation assays in vitro have also been reported. In baby hamster kidney cells (BHK21/C13), no increase in cell transformation frequency was detected after treatment with SCCP (Cereclor 50LV, C10–13, 50% chlorination) in the absence of metabolic activation up to cytotoxic concentrations (up to 2,500 μg/mL) (Birtley et al., 1980). However, in contrast, increases in transformation frequency were measured when cells were treated with SCCP (C12, 58% chlorination) with and without metabolic activation, at both cytotoxic and non‐cytotoxic concentrations (Richold et al., 1982a, unpublished study, as cited by WHO/IPCS (1996)).
Cell transformation frequency was not increased after treatment with MCCP (Cereclor S52, C14–17, 52% chlorination) in the absence of metabolic activation at concentrations up to 2,500 μg/mL (Birtley et al., 1980).
No evidence of increased cell transformation frequency could be found after treatment with LCCP (Cereclor 42, C20–30, 42% chlorination) in the absence of metabolic activation at concentrations up to 2,500 μg/mL (Birtley et al., 1980). In contrast, transformation frequency was increased when cells were exposed to LCCP (C20–28, 70% chlorination) or LCCP (C22–28, 70% chlorination) with and without metabolic activation, at both cytotoxic and non‐cytotoxic concentrations (Richold et al., 1982b, unpublished study, as cited by WHO/IPCS (1996)).
Inhibition of cell communication is suggested to play an important role in the carcinogenic process since many tumour promoters including 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA) have been shown to inhibit gap junctional communication in vitro (Warngard et al., 1996). The effects of six different CPs on cell‐cell communication were investigated in rat liver epithelial cells IAR 20 in vitro (Kato and Kenne, 1996). Communication was completely inhibited at 20 μg/mL SCCP (C10–13, 50% chlorination), 15 μg/mL SCCP (C10–13, 60% chlorination), 80 μg/mL MCCP (C14–17, 45% chlorination) and 35 μg/mL MCCP (C14–17, 52% chlorination), but not at concentrations up to 200 μg/mL of LCCP (C22–26, 42% chlorination or 48% chlorination). All these concentrations were non‐cytotoxic. SCCPs and MCCPs were shown to alter the phosphorylation pattern of connexin 43 which is different from that in reaction to TPA. In conclusion, both tested SCCPs and MCCPs, but no LCCPs were found to be potent inhibitors of gap junction intercellular communication. SCCPs and MCCPs may therefore act as tumour promoters in combination with other potentially relevant non‐genotoxic mechanisms discussed above for each tissue type.
3.1.5.6. Conclusions on the relevance of the carcinogenic modes of action to human risk assessment
The overall weight of evidence indicates that CPs are not genotoxicants. The CONTAM Panel concluded that the rodent hepatocarcinogenicity of SCCPs (as shown with C12, 60% chlorination) is not relevant to humans because activation of CAR or PPARα is rodent specific. Furthermore, the additional potential role of liver toxicity in rodent hepatocarcinogenicity was considered irrelevant to human risk based on the dose levels required for rodent liver necrosis (500 mg/kg bw SCCP in the mouse).
The tumourogenicity of SCCPs in the male rat kidney and the female thyroid involve modes of actions that cannot be clearly eliminated for their relevance to humans.
Thus, SCCPs are reasonably anticipated to be human carcinogens via non‐genotoxic mechanisms in the absence of evidence for genotoxicity in vivo. This conclusion is in accordance with the CLP classification as Carc Cat 2 (H351), as well with the NTP Report on Carcinogens (NTP, 2016).
The malignant lymphoma in male mice caused by LCCP were only seen at very high‐dose levels and considered not relevant to humans.
3.1.5.7. Haemorrhaging effect in offspring
Reduced pup survival during lactation, but not at birth, have been observed in offspring of rats administered an MCCP (C14–17, 52% chlorination) (see Section 3.1.2.3 ). Effects observed in the dead pups were indicative of internal haemorrhaging and included an increased occurrence and severity of subcutaneous haematoma/haemorrhage, bruised areas, discolouration and blood around the orifices, pale liver, kidneys and lungs, blood in the cranial cavity, brain, stomach and intestines and/or the abdominal or thoracic cavities. Three studies were performed with the aim of investigating the possible haemorrhagic mode of action of MCCPs.
Groups of male and female Wistar rats were exposed to 0 or 6,250 mg/kg MCCP (C14–17, 52% chlorination) in the diet for 4 weeks before mating (ICI, 1985, unpublished study, see Documentation provided to EFSA). This is equivalent to a dose averaged over the first 4 week of treatment of 0 or 560 mg/kg bw per day.34 After confirmation of mating, the pregnant females were allocated to five treatment groups: (1) 16 females fed control diet rearing their own pups; (2) 26 females fed MCCP diet rearing pups fostered from group 3 control females; (3) 26 females fed control diet rearing pups fostered from group 2 treated females; (4) 16 females fed MCCP diet rearing their own pups; and (5) 16 females fed MCCP diet up to day 10 of pregnancy, rearing their own pups while fed control diets.
During the pre‐mating period and pregnancy, no deaths or clinical signs of toxicity were observed in the parental animals. During days 12–22 post‐partum, pup mortality was significantly increased in group 2 (control pups fostered to treated mothers) and group 4 (treated pups reared by their own treated mothers). Pup mortality was 77% in group 2 and 67% in group 4 compared to 4% in control group 1. Mortality was not increased in pups of groups 3 and 5. Of the total number of pups found dead between days 1 and 22, haemorrhages were seen in 17% and 8%, respectively, of group 2 and group 4 offspring. The observations of dark red bulging eyes, blood clots within the membranes lining the cranium and pale livers were indicative of haemorrhages. In group 4, animals a statistically significant 21% reduction of pup body weight was observed on day 22 post‐partum.
Throughout lactation, a statistically significant reduction in the concentration of clotting factor X in groups 2 and 4 was observed (up to approx. 70% relative to pups in group 1). The concentration of factor X in groups 3 and 5 was similar to control group 1.
Prothrombin times were prolonged in groups 2 and 4 becoming greater with time up to day 11. At day 22, they were still increased but more similar to the controls. No changes were seen in the levels of factor VIII. The high incidence of death specifically in group 2 and 4 pups compared to controls indicate mother‐to‐pup transfer during weaning. Continued availability of MCCP appeared to be necessary for effects. Based on these findings, it can be suggested that the pups received less vitamin K in the breast milk due to treatment‐related effects upon their mothers and as a consequence the vitamin K dependent clotting pathway was impaired, or alternatively that MCCP or a metabolite was either transferred via breast milk, causing interference of the clotting system in the pups.
To test the first hypothesis, i.e. a reduction of vitamin K in the breast milk, 6 female adult Sprague–Dawley rats fed on either a normal diet or a diet deficient in vitamin K3 were administered by oral gavage 0, 500 or 1,000 mg/kg bw per day MCCP (C14–17, 52% chlorination) for 21 days (CXR, 2003, unpublished draft study, see Documentation provided to EFSA). Factor X levels were not affected by treatment. However, plasma vitamin K1 levels were lower at termination (by 34%) in the high‐dose animals fed vitamin K3‐deficient diet. In animals on the normal diet, a statistically significant, dose‐related decrease (by 18% and 42% at the low and high dose of MCCP, respectively) in Factor VII levels was observed. A marked decrease in Factor VII levels was also reported in both control and MCCP‐treated animals fed the vitamin K3‐deficient diet (by 25%, 24% and 44% of the normal diet control group levels at 0, 500 and 1,000 mg/kg per day, respectively). Prothrombin clotting times were not affected in a biologically relevant magnitude. Thus, the haemorrhaging effects on the offspring are unlikely to be caused by reduced levels of vitamin K in breast milk under the conditions in this study.
Another study was conducted to test the second hypothesis, whether MCCP is transferred to the pups through breast milk and causes disruption of the clotting system in the pups (CXR, 2004, unpublished draft study, see Documentation provided to EFSA). Groups of 16 male and 32 female Sprague–Dawley rats were administered 0 or 6,250 mg/kg MCCP (C14–17, 52% chlorination) in the diet for 4 weeks prior to mating, and during mating, gestation and lactation. This equates to an average dose (over the first 4 week of treatment) of 0 or 560 mg/kg bw per day.34 However, the study was terminated prematurely at around day 12 as a result of excessive mortality (and evidence of haemorrhages at necropsy) in pups derived from treated animals. At day 1 of lactation, MCCP was measured in the milk of three MCCP‐treated dams and compared to controls. A mean (± SD) level of 1,057 ± 530 mg/L was detected in milk from treated dams compared to non‐detectable levels in milk from control dams. There was a significant decrease in the plasma vitamin K concentration measured on day 12 of lactation in the treated dams (0.03 ± 0.05 ng/mL) compared to the control dams (0.41 ± 0.14 ng/mL) (MCCP and vitamin K levels in dam milk not mentioned in the CXR (2004) report; but as cited by EU‐RAR, 2011). This finding conflicts with the results discussed above (CXR, 2003) in which plasma vitamin K concentrations were not affected by treatment. One possible explanation for this inconsistency is length of treatment time (7–8 weeks compared to 3 weeks in the CXR (2004) study). Furthermore, in the CXR (2004) study, in which treated females continued through pregnancy and lactation, vitamin K was decreased in maternal plasma and in the milk of treated dams. Vitamin K was not detected in pooled samples from treated dams on days 1 and 4 compared to 0.28 ng/mL (SD = 0.10) detected in samples from 5 control dams. This finding was further substantiated by pooling samples from days 9 and 12 from treated dams in which the vitamin K concentrations were approximately 50% of the concentration in control dams (0.61 ± 0.29 ng/L)
The plasma concentration of vitamin K of adult females following pregnancy and lactation was markedly decreased by treatment with MCCP. This was associated with a decrease in the activity of the plasma clotting factors VII and X in the treated dams compared to controls on day 12 of lactation. However, prothrombin times in the dams were not affected, suggesting a sufficient reserve.
Although insufficient plasma was available from pups to measure vitamin K, clotting factor activities were measured as a surrogate. Treatment with MCCPs decreased clotting factor VII and X activities after day 4 post‐partum.
Overall, these studies suggest that MCCPs perturb the clotting system in lactating neonates of treated mothers. In adult animals, although concentrations of vitamin K and of the clotting factors VII and X were reduced, their prothrombin times were not biologically significantly affected indicating that the functional reserve in these adult animals is sufficient. The fetus in utero appears to receive sufficient vitamin K via the placenta, but after birth becomes deficient in vitamin K and related clotting factors when reliant of these factors via the mother's milk. A further reduction of vitamin K in neonates may occur through receiving MCCP via the milk. In conclusion, haemorrhaging appears to result in neonates as a consequence of vitamin K deficiency. The CONTAM Panel concluded that the haemorrhagic effects are considered as being relevant to humans.
3.1.5.8. Additional endocrine effects
3.1.5.8.1. In vitro
The endocrine effects of three SCCPs (C10, 40.4% chlorination; C10, 66.1% chlorination and C11, 43.2% chlorination) were investigated in two in vitro models: (i) the dual‐luciferase reporter gene assay reporter gene assays for oestrogen receptor α (ERα), glucocorticoid receptor (GR) and thyroid receptor β (TRβ) with Chinese hamster ovary CHO‐K1 cells and (ii) the human adrenal corticocarcinoma cell line H295R (Zhang et al., 2016). In the dual‐luciferase reporter gene assay, all three SCCPs significantly induced oestrogenic activities, which were ERα‐mediated in the following order: SCCP (C11, 43.2% chlorination; effective concentration by 20% induction (EC20) = 6.5 × 10−9 mol/L) > SCCP (C10, 66.1% chlorination; EC20 = 2.7 × 10−8 mol/L) > SCCP (C10, 40.4% chlorination; EC20 = 2.87 × 10−7 mol/L). In addition, both SCCPs (C10, 40.4% chlorination and C10, 66.1% chlorination) showed anti‐oestrogenic activities. Only SCCP (C11, 43.2% chlorination) induced GR‐mediated antagonistic activity, with a 20% inhibitory concentration (IC20) value of 2.6 × 10−8 mol/L. None of the SCCPs showed any agonistic or antagonistic activities against TRβ. All three SCCPs stimulated the secretion of 17β‐oestradiol. Both SCCPs (C10, 66.1% chlorination and C11, 43.2% chlorination) increased the production of cortisol at a high level in H295R cell lines. Possible mechanisms underlying the endocrine effects of SCCPs through the non‐receptor mediated pathway were explored by the measurement of mRNA levels of 9 steroidogenic genes in H295R by real‐time polymerase chain reaction. All three SCCPs upregulated StAR, 17βHSD, CYP11A1, CYP11B1, CYP19 and CYP21 in a concentration‐dependent manner.
3.1.5.8.2. In vivo
There are indications for thyroidal effects from the repeated toxicity and carcinogenicity studies with SCCPs and from the repeated toxicity study with MCCPs that are mediated by alteration of hepatic metabolism (see Sections 3.1.2.2 , 3.1.2.7 and 3.1.4.3 ), but there was no evidence for interaction with TRβ (see above) and there is no evidence to implicate an endocrine‐mediated mode of action for thyroid effects (Gong et al., 2018).
3.1.6. Consideration of critical effects and dose–response analysis for the human risk assessment
3.1.6.1. Consideration of critical effects
No studies on observations in humans of relevance for the risk assessment of CPs within the scope of this opinion were available. Therefore, the human risk assessment is based on data from studies in experimental animals.
A number of repeated dose toxicity studies have been performed in experimental animals using only a few CPs of different carbon chain length and different degrees of chlorination. These studies have shown that the liver, kidney and thyroid are target organs in experimental animals. The effects observed in the kidneys and the thyroid are considered as being relevant to humans (see Section 3.1.5.2 and 3.1.5.3 , respectively). Liver toxicity observed consistently in rats and mice is considered secondary to an adaptive physiological response and the associated energy costs, but could be relevant to humans at high‐dose levels (see Section 3.1.5.1 ). In addition, decreased pup survival and incidence of subcutaneous haematoma/haemorrhage were identified as critical effects for an MCCP in a dose‐range finding study for a two‐generation study; these effects are also considered as being relevant to humans (see Section 3.1.5.7 ). The toxicokinetic studies in rats and mice indicate that the toxicokinetics vary depending on carbon chain length, as well as on position and degree of chlorination (see Section 3.1.1.4 ). Therefore, the toxicokinetic and toxicity studies performed with only a few CPs can in principle only provide information on the CPs investigated. Read‐across to other CPs, both within the same class as well as in other classes, is therefore problematic and will have high uncertainty.
SCCPs
In two chronic gavage studies in rats and mice with an SCCP (C12, 60% chlorination) (NTP, 1986a), effects were observed at the lowest tested dose levels of 312 mg/kg bw per day for rats (liver and kidney) and 125 mg/kg bw per day for mice (kidney and thyroid). This, combined with the fact that only two dose levels were used, precluded these studies from being used to perform dose–response analysis. A NOAEL could, however be identified from subchronic studies with SCCPs (see below).
From two 90‐day rat studies with the same SCCP (C10–12, 58% chlorination), one with dietary administration (IRDC, 1984a, see Documentation provided to EFSA) and the other with gavage (IRDC, 1984b, see Documentation provided to EFSA), a NOAEL of 10 mg/kg bw per day was identified. In the dietary study, increased liver and kidney weights and histopathological changes in liver, kidney (males) and thyroid (males) were observed at ≥ 100 mg/kg bw per day. In the gavage study, increased liver and kidney weights and histopathological changes in liver (males) and kidneys (males) were observed at ≥ 100 mg/kg bw per day; effects in the thyroid (increased weight and histopathological changes) were only observed in males at 625 mg/kg bw per day.
A NOEL for effects in the thyroid was identified for an SCCP (C10–13, 56.5% chlorination; mass ratio of C10:C11:C12:C13 = 1:1:1:1) in a 28‐day gavage study with male rats (Gong et al., 2018). The NOEL was 1 mg/kg bw per day, based on decreased free T3 (20% at the next dose level of 10 mg/kg bw per day) and increased TSH levels (15% at 10 mg/kg bw per day) in the plasma. No histopathological changes were observed in the thyroid at 100 mg/kg bw per day, the only dose examined. In a 14‐day study with male rats (Wyatt et al., 1993) plasma FT4 and TT4 levels were decreased and the plasma TSH level was significantly increased at 1,000 mg/kg bw per day (the only dose at which these parameters were examined). No differences in plasma FT3 or TT3 were observed at 1,000 mg/kg bw per day. The CONTAM Panel noted the inconsistencies in the hormonal changes between the two studies (FT3 decreased in the 28‐day study, FT4 and TT4 decreased in the 14‐day study). Consequently, the Panel decided that changes in thyroid hormone levels could not be used as a basis for deriving a reference point. Therefore, only the histopathological changes have been considered as a basis for deriving a reference point.
An SCCP (carbon chain length not specified, 58% chlorination) caused post‐implantation loss and decreased number of viable fetuses, as well as teratogenic effects in the form of absence of digits (adactyly) or shortened digits at 2,000 mg/kg bw per day (gavage) in a developmental toxicity study in rats, in the presence of maternal toxicity (high mortality and decreased body weight gain from 500 mg/kg bw per day). In a rabbit developmental toxicity study, the NOAEL for developmental toxicity was 10 mg/kg bw per day, based on increased number of resorptions, in the absence of maternal toxicity.
Following administration by gavage of an SCCP (C12, 60% chlorination) (NTP, 1986a) increased incidences of tumours were observed in several organs in rats and mice, i.e. liver (rats, mice), kidneys (male rats only) and thyroid (female rats and mice). Mononuclear‐cell leukaemia was also observed in rats. The relevance to humans of the rodent liver tumours can be excluded provided liver cell toxicity does not occur (see Section 3.1.5.1.4 ). Tumours in the male rat kidney and the female thyroid involve modes of actions that cannot be clearly eliminated for their relevance to humans (see Sections 3.1.5.2 and 3.1.5.3 , respectively). Mononuclear‐cell leukaemia is a uniquely common spontaneous disorder in the rat strain used by NTP (the F344 rat) and therefore, not relevant to humans (see Section 3.1.5.4 ). SCCPs are reasonably anticipated to be carcinogens via non‐genotoxic mechanisms (see Section 3.1.2.6 ). The CONTAM Panel considered that there is a threshold for the carcinogenic effects of this SCCP. Therefore, tumours in the kidneys and thyroid would not be relevant to humans provided that the exposure is insufficient to bring about the relevant non‐genotoxic changes, i.e. nephropathy and thyroid hyperplasia, respectively.
Overall, the CONTAM Panel identified a NOAEL of 10 mg/kg bw per day, based on increased kidney weights, histopathological changes in the kidney and thyroid, and developmental effects.
MCCPs
No chronic/carcinogenicity studies have been identified for MCCPs.
The studies summarised below have all been performed with the same MCCP (C14–17, 52% chlorination).
In a 90‐day dietary rat study (IRDC, 1984c, see Documentation provided to EFSA), a NOAEL of 10 mg/kg bw per day was identified, based on an increase in liver and kidney weights at 100 mg/kg bw per day; histopathological changes in the liver and the kidney (males) were noted at the next higher dose level (625 mg/kg bw per day). Effects in the thyroid (increased absolute weight) were noted in male rats at 625 mg/kg bw per day; trace to moderate hypertrophy and hyperplasia were observed in almost all control and treated males with a trend towards increasing severity with increasing dose (10, 100, 625 mg/kg bw per day).
A NOEL for effects in the thyroid was identified from another 90‐day dietary rat study (CXR, 2005b, see Documentation provided to EFSA). The NOEL was 9.3/9.7 mg/kg bw per day for males/females, respectively, based on decreased plasma FT3 in males and increased plasma TSH in females (see Section 3.1.2.2.2 ). A small increase in histopathological changes (ultimobranchial cysts) was observed in the thyroid of male rats at 23 mg/kg bw per day; this was not seen at higher dose levels and therefore, not considered of toxicological relevance. The Panel noted the inconsistencies in the hormonal changes between the two genders (FT3 decreased only in males, TSH increased only in females). Consequently, the Panel decided that the changes in thyroid hormone levels could not be used as a basis for deriving a reference point. Absolute and relative liver and kidneys weights were increased at the highest dose level (222/242 mg/kg bw per day for males/females, respectively) and minimal centrilobular hepatocellular hypertrophy was observed in males.
The NOAEL for postnatal effects is considered to be 9 mg/kg bw per day, based on decreased pup survival and subcutaneous haematoma/haemorrhage at the two higher dose levels (90 and 560 mg/kg bw per day) in a dose‐range finding study for a two‐generation dietary rat study (IRDC, 1985, see Documentation provided to EFSA). Decreased pup survival was observed at 90 mg/kg bw per day and above, achieving statistical significance at 560 mg/kg bw per day (100% mortality before PND21). The NOEL for parental toxicity and fertility was 560 mg/kg bw per day (the highest dose level tested).
No developmental toxicity, including malformations were observed in developmental toxicity studies in rats and rabbits at the highest dose levels tested, 5,000 mg/kg bw per day for rats (IRDC, 1984f, see Documentation provided to EFSA) and 100 mg/kg bw per day for rabbits (IRDC, 1983c, see Documentation provided to EFSA).
Overall, the CONTAM Panel identified a NOAEL of 10 mg/kg bw per day, based on increased kidney weights and postnatal effects (decreased pup survival and subcutaneous haematoma/haemorrhage).
LCCPs
In a chronic gavage study in rats with an LCCP (C23, 43% chlorination) (NTP, 1986b), effects (increased liver weight and histopathological changes such as diffuse lymphohistiocytic inflammation in liver, pancreatic and mesenteric lymph nodes) were observed at 100 mg/kg bw per day for female rats (the lowest dose level tested). No effects were observed in a chronic gavage study in mice with the same LCCP at dose levels up to 5,000 mg/kg bw per day (the highest dose level tested) (NTP, 1986b).
For another LCCP (C22–26, 43% chlorination), the LOAEL was 100 mg/kg bw per day (the lowest dose level tested), based on increased liver weight and histopathological changes in the liver of female rats in a 90‐day oral gavage study (IRDC, 1984d, see Documentation provided to EFSA). For an LCCP of the same carbon chain length, but with a higher chlorination degree (C22–26, 70% chlorination), the NOAEL was 900 mg/kg bw per day, based on liver effects at 3,750 mg/kg bw per day in a 90‐day rat dietary study (IRDC, 1984e, see Documentation provided to EFSA).
No developmental toxicity, including malformations, was observed for two LCCPs (C22‐26, 43% or 70% chlorination) in developmental toxicity studies in rats and rabbits at the highest dose levels tested, 1,000 mg/kg bw per day for rabbits with the 70% chlorinated LCCP (IRDC, 1982b) and 5,000 mg/kg bw per day in the three other studies (IRDC, 1983d, 1981e, 1983e, see Documentation provided to EFSA).
Oral gavage dosing with an LCCP (C23, 43% chlorination) caused increased incidence of tumours in different organs and tissues in rats and mice, i.e. malignant lymphomas (male mice), adrenal gland medullary pheochromocytomas (female rats), and to a marginal extent of hepatocellular neoplasms (female mice). Therefore, there is some evidence for this LCCP to be carcinogenic in mice and rats at very high‐dose levels (mice: 2,500 and 5,000 mg/kg bw per day; female rats: 900 mg/kg bw per day). The possible carcinogenic effects at very high‐dose levels are reasonably anticipated to be exerted via non‐genotoxic mechanisms in the absence of evidence for genotoxicity in vivo. Therefore, the CONTAM Panel considered that there is a threshold for the possible carcinogenic effects of this LCCP.
Overall, the LOAEL of 100 mg/kg bw per day for two low chlorinated LCCPs (43%) and the NOAEL of 900 mg/kg bw per day for a high chlorinated LCCP (70%) are based on liver effects.
3.1.6.2. Dose–response analysis (including BMD modelling)
In view of the critical effects identified in Section 3.1.6.1 , the CONTAM Panel performed Benchmark dose (BMD) modelling on the effects observed in the kidneys and the thyroid as these effects are considered as being relevant to humans. BMD modelling was performed for changes in kidney weights (SCCPs and MCCPs), and histopathological changes in the kidneys (SCCPs) and thyroid (SCCPs). BMD modelling was also performed for decreased pup survival and incidences of subcutaneous haematoma/haemorrhage identified as critical effects for an MCCP in a dose‐range finding study for a two‐generation study.
No dose–response modelling was performed for LCCPs, as only liver effects were observed in the available studies. The effects in the liver are considered secondary to an adaptative response and the associated energy costs, and only relevant to humans at high‐dose levels.
The EFSA guidance on the use of BMD in risk assessment (EFSA Scientific Committee, 2017a) was used. The results of the BMD modelling are summarised in Table 23 and 24 and the details of the BMD analysis are reported in Appendix C for the different studies. For the selection of the benchmark response (BMR), the CONTAM Panel deviated from the default BMRs in the following cases: (i) for kidney weight, the CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights. (ii) For pup survival, considering the severity of the effect, the CONTAM Panel selected a BMR of 5%, lower than the default BMR of 10% recommended for quantal data. From a statistical point of view, the high study population (> 100 pups per dose group) supported the selection of a lower BMR. (iii) For subcutaneous haematoma/haemorrhage, considering the association of this effect with the decreased pup survival observed in the study, the CONTAM Panel considered applying the same BMR of 5% selected for the dose–response analysis of pup survival. The Panel noted that in this case the selection of a lower BMR is less justified from a statistical point of view considering the low study number of animals evaluated in some dose groups.
Table 23.
Benchmark dose modelling for the critical effects of short‐chain chlorinated paraffins in experimental animals
| Critical effect | Model | BMDL10 | BMDU10 | Reference |
|---|---|---|---|---|
| (mg/kg bw per day) | ||||
| Nephritis a – M rats | Model averaging | 2.29 | 76.4 | IRDC (1984a) |
| Thyroid hypertrophy – M rats | Model averaging | 15.5 | 210 | IRDC (1984a) |
| Increased absolute left kidney weights – M + F rats |
Hill m3‐a Exp m3‐a |
38.0 | 186 | IRDC (1984a) |
| Increased relative left kidney weights – M rats |
Hill m3‐ab Exp m3‐ab |
32.5 | 92.5 | IRDC (1984a) |
| Increased relative left kidney weights – F rats |
Hill m3‐ab Exp m3‐ab |
71.0 | 180 | IRDC (1984a) |
| Nephropathy – M rats | Model averaging | 87.2 | 410 | IRDC (1984b) |
| Thyroid hypertrophy – M rats | Model averaging | 14.7 | 195 | IRDC (1984b) |
| Thyroid hyperplasia – M rats | Model averaging | 45.1 | 266 | IRDC (1984b) |
| Increased absolute kidney weights – M rats |
Hill m5‐ab Exp m5‐ab |
31.4 | 111 | IRDC (1984b) |
| Increased absolute kidney weights – F rats |
Hill m5‐ab Exp m5‐ab |
83.7 | 345 | IRDC (1984b) |
| Increased relative kidney weights – M rats |
Hill m3‐abv Exp m3‐abv |
5.55 | 68.6 | IRDC (1984b) |
| Increased relative kidney weights – F rats |
Hill m3‐abv Exp m3‐abv |
40.7 | 268 | IRDC (1984b) |
bw: body weight; M: males; F: females.
For details of the effects observed see Section 3.1.2.2.1.
Table 24.
Benchmark dose modelling for the critical effects of medium‐chain chlorinated paraffins in experimental animals
| Critical effect | Model | BMDL10 | BMDU10 | Reference |
|---|---|---|---|---|
| (mg/kg bw per day) | ||||
| Increased absolute kidney weight – M + F rats |
Expon m3‐a Hill m3‐a |
81.5 | 364 | CXR (2005b) |
| Increased relative kidney weight – M + F rats |
Expon m3‐a Hill m3‐a |
67.9 | 182 | CXR (2005b) |
| Increased absolute kidney weight (left) – M + F rats |
Expon m3‐a Hill m3‐a |
101 | 418 | IRDC (1984c) |
| Increased relative kidney weight (left) – M + F rats |
Expon m3‐a Hill m3‐a |
36.4 | 224 | IRDC (1984c) |
| Critical effects | Model | BMDL 5 | BMDU 5 | Reference |
| (mg/kg bw per day) | ||||
| Combined incidence of subcutaneous haematoma/haemorrhage – rats | Model averaging | 48.5 | 63.8 | IRDC (1985) |
| Pup mortality – rats | Model averaging | 53.0 | 96.5 | IRDC (1985) |
bw: body weight; BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit; F: females; M: males.
For one tested SCCP (C10–12, 58% chlorination), a BMDL10 of 2.29 mg/kg bw per day (rounded to 2.3 mg/kg bw per day) was selected as the reference point for the risk characterisation, based on increased incidence of nephritis in male rats. This BMDL10 is considered to cover other critical effects of the tested SCCPs (see Section 3.1.6.1 ).
For the tested MCCP (C14–17, 52% chlorination), a BMDL10 of 36.4 mg/kg bw per day (rounded to 36 mg/kg bw per day) was selected as the reference point for the risk characterisation, based on increased relative kidney weight in male and female rats. This BMDL10 is considered to cover other critical effects of the tested MCCP (see Section 3.1.6.1 ).
3.1.6.3. Derivation of a health‐based guidance value/margin of exposure approach
The CONTAM Panel concluded that due to the limitations and uncertainties in the current database on SCCPs, MCCPs and LCCPs (see Section 3.1.6.1 ), the derivation of a health‐based guidance value (HBGV) was not appropriate. Instead, the Panel used a margin of exposure (MOE) approach to assess a possible health concern.
The available toxicity studies in experimental animals have been performed with only a few CPs of different carbon chain length and different degrees of chlorination. The toxicokinetic studies in rats and mice indicate that read‐across from one specific CP to other CPs, both within the same class as well as to other classes is problematic and will have high uncertainty.
For the tested SCCP (C10–12, 58% chlorination; C12, 60% chlorination; C10–13, 56.5% chlorination; carbon chain length not specified, 58% chlorination), the BMDL10 of 2.3 mg/kg bw per day was selected as the reference point for the risk characterisation (see Section 3.1.6.2 ).
For the only tested MCCP (C14–17, 52% chlorination), the BMDL10 of 36 mg/kg bw per day was selected as the reference point for the risk characterisation (see Section 3.1.6.2 ).
For LCCPs, the LOAEL for the lower chlorinated LCCP (C22–26, 43% chlorination) of 100 mg/kg bw per day and the NOAEL for the higher chlorinated LCCP (C22–26, 70% chlorination) of 900 mg/kg bw per day are based on liver effects. As effects in the liver observed consistently in rats and mice are considered secondary to an adaptative response and the associated energy costs and only being relevant to humans at high‐dose levels, and as no other non‐neoplastic effects were observed in the studies with LCCPs, a reference point has not been identified.
An MOE higher than 1,000 might indicate that there is no health concern, taking into account the variability between species (a factor of 10), the variability within human individuals (a factor of 10), and extrapolation from subchronic studies to chronic exposure duration (a factor of 2). It could be questioned whether possible bioaccumulative properties are covered by the general default factor of 10 for interspecies differences; however, in case of no substance specific information, as is the case for the CPs, the default factor of 10 is applied. According to the EFSA Scientific Committee Guidance on selected default values (EFSA Scientific Committee, 2012a) the default factor of 2 for extrapolating from subchronic toxicity studies to chronic exposure duration should not be applied when toxicokinetic data show that the substance under consideration can accumulate, e.g. has a long biological half‐life. However, because of the limited substance specific information on bioaccumulative properties for the CPs, the Panel decided to apply the default factor of 2. According to the EFSA Scientific Committee Guidance on selected default values (EFSA Scientific Committee, 2012a) an additional factor can be considered in case of deficiencies in the database on a case‐by‐case basis. A default value has not been proposed, as it will be directly dependent on the data set available. WHO/IPCS (1994, 1999) has recommended a factor of 3 or 5 if there are minor deficiencies in the database and a factor of 10 if there are major deficiencies in the database. The CONTAM Panel considered a factor of 5 to take into account the limitations in the database, e.g. toxicity data are only available for a few CPs whereas the risk assessment is covering a large number of CPs, the impact of the degree of chlorination, chlorine position and carbon chain length on toxicokinetics and toxicity cannot be evaluated, the limited information on possible bioaccumulative properties and no two‐generation reproductive toxicity study is available for any CP.
3.1.7. Consideration of critical effects and reference points for farm animals, horses and companion animals risk assessment
The CONTAM Panel reviewed the available studies in order to derive a possible NOAEL or LOAEL for farm animals, horses and companion animals. No studies were identified in ruminants, pigs, horses, cats or fur animals.
For poultry, a limited number of studies have been conducted and some of these were designed to assess the performance and capacity for food production. The CONTAM Panel concluded that none of them was robust enough for the identification of a NOAEL/LOAEL as reference point.
For rabbits, no studies have been located other than the developmental toxicity studies in experimental animals. For an SCCP (carbon chain length not specified, 58% chlorination), the NOAEL for developmental toxicity was 10 mg/kg bw per day, based on increased number of resorptions. The NOEL for maternal toxicity was 100 mg/kg bw per day (the highest dose tested). For an MCCP (C14–17, 52% chlorination), the NOEL for developmental and maternal toxicity was 100 mg/kg bw per day (the highest dose tested). For an LCCP (C22–26, 43% chlorination), the NOEL for developmental and maternal toxicity was 5,000 mg/kg bw per day (the highest dose tested). For another LCCP (C22–26, 70% chlorination), the NOEL for developmental and maternal toxicity was 1,000 mg/kg bw per day (the highest dose tested).
For fish, most studies showed no or minor effects. Based on the available information, the CONTAM Panel could not identify a NOAEL or a LOAEL.
For dogs, a NOEL of 10 mg/kg bw per day was identified for an MCCP (Cereclor S52, C14–17, 52% chlorination) from a dietary study in Beagle dogs, based on an increase of hepatic smooth endoplasmic reticulum.
3.2. Occurrence data
3.2.1. Occurrence data submitted to EFSA
Occurrence data on food
A total of two data sets were submitted to EFSA consisting of 510 analytical results of 228 samples.
Data set A
Data set A consisted of a total of 88 analytical results for 44 samples taken in 2007 in the United Kingdom on various foods. The samples were analysed by GC‐HRMS for both SCCPs and MCCPs. The results are summarised on Foodex1 Level 2 in Appendix D .
For SCCPs, the highest UB mean level per category or one single food sample of animal origin was found in crustaceans (one sample with 39.2 μg/kg fat weight), sausages (one sample with 27.9 μg/kg fat weight), fish oil (one sample with 25.0 μg/kg fat weight) and fish meat (12 samples with a mean of 23.8 μg/kg fat weight). For foods with plant origin, SCCPs were detected in one composite bread and one composite fruit sample.
For MCCPs the highest UB mean level or that from one single sample of animal origin was found in livestock meat (four samples with a mean of 91.8 μg/kg fat weight), fish oil (one sample with 72.0 μg/kg fat weight), sausages (one sample with 53.6 μg/kg fat weight) and edible offals (four samples with a mean of 40.3 μg/kg fat weight). For foods with plant origin, MCCPs were detected in one composite bread sample.
No CP contamination was found in vegetable, liquid milk and egg samples.
The CONTAM Panel noted these data, but due to the fact that they were taken over a decade ago, included pooled samples and consisted of only a limited number of samples, the Panel considered that it was not appropriate to use them for a formal current exposure assessment.
Data set B
Data set B consisted of a total of 422 analytical results from 184 samples of fish meat collected in Germany between 2014 and 2017, and analysed using GC‐Orbitrap‐HRMS.
Some of the samples were analysed and reported more than once. In these cases, the mean of the analytical results related to each sample was calculated and used in the assessment in order to avoid bias.
Samples of Data set B were analysed for both SCCPs and MCCPs and their results are summarised on Foodex1 Level 2 and 3 in Tables 25 and 26 . All results are expressed in whole weight (ww).
Table 25.
Mean and P95 levels of ‘Alkanes, C10–13, chloro’ (SCCPs) (μg/kg ww)
| FOODEX1 Level 3 | N | % LC | MEAN (LB) | MEAN (UB) | P95(LB)a | P95 (UB)a |
|---|---|---|---|---|---|---|
| Salmon and trout (Salmo spp.) | 153 | 3 | 8.8 | 8.9 | 18 | 18 |
| Tuna (Thunnus) | 18 | 78 | 0.4 | 0.6 | – | – |
| Sea catfish and wolffish (Anarhichas) | 13 | 77 | 0.6 | 0.8 | – | – |
| FOODEX1 Level 2 | N | % LC | MEAN (LB) | MEAN (UB) | P95 (LB) | P95 (UB) |
| Fish meat | 184 | 15 | 7.5 | 7.5 | 18 | 18 |
SCCPs: short‐chain chlorinated paraffins; LB: lower bound; UB: upper bound; N: number of samples; % LC: Percentage of left‐censored data.
The 95th percentile estimates obtained with less than 60 observations may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table 26.
Mean and P95 levels of ‘Alkanes, C14–17, chloro’ (MCCPs) (μg/kg ww)
| FOODEX1 Level 3 | N | % LC | MEAN (LB) | MEAN (UB) | P95 (LB)a | P95 (UB)a |
|---|---|---|---|---|---|---|
| Salmon and trout (Salmo spp.) | 153 | 5 | 15 | 15 | 49 | 49 |
| Tuna (Thunnus) | 18 | 67 | 1.7 | 2.0 | – | – |
| Sea catfish and wolffish (Anarhichas) | 13 | 54 | 2.5 | 2.8 | – | – |
| FOODEX1 Level 2 | N | % LC | MEAN (LB) | MEAN (UB) | P95 (LB) | P95 (UB) |
| Fish meat | 184 | 14 | 13 | 13 | 44 | 44 |
MCCPs: medium‐chain chlorinated paraffins; LB: lower bound; UB: upper bound; N: number of samples; % LC: Percentage of left‐censored data.
The 95th percentile estimates obtained with less than 60 observations may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
No occurrence data were submitted on LCCPs in food.
Production method of fish
Regarding the different production methods of various fish species (Tables 27 and 28 ), it was found that all samples of tuna were wild caught while all sampled sea catfish and wolffish were coming from farmed production.
Table 27.
Production methods of fish for ‘Alkanes, C10–13, chloro’ (SCCPs) (μg/kg ww)
| FOODEX1 Level 3 | Production method | N | MEAN(LB/UB) | Min (LB/UB) | Max (LB/UB) | ||
|---|---|---|---|---|---|---|---|
| Salmon and trout (Salmo spp.) | Organic production | 15 | 7.5 | 7.5 | 2.7 | 2.7 | 14 |
| Salmon and trout (Salmo spp.) | Wild | 13 | 4.1 | 4.2 | 0 | 0.3 | 13 |
| Salmon and trout (Salmo spp.) | Farmed | 125 | 9.5 | 9.6 | 0 | 0.6 | 172 |
| Tuna (Thunnus) | Wild | 18 | 0.4 | 0.6 | 0 | 0.2 | 3.6 |
| Sea catfish and wolffish (Anarhichas) | Farmed | 13 | 0.6 | 0.8 | 0 | 0.2 | 5.3 |
LB: lower bound; UB: upper bound; N: number of samples.
Table 28.
Production methods of fish for ‘Alkanes, C14–17, chloro’ (MCCPs) (μg/kg ww)
| FOODEX1 Level 3 | Production method | N | MEAN(LB/UB) | Min (LB/UB) | Max (LB/UB) | ||
|---|---|---|---|---|---|---|---|
| Salmon and trout (Salmo spp.) | Organic production | 15 | 29 | 29 | 6.2 | 6.2 | 55 |
| Salmon and trout (Salmo spp.) | Wild | 13 | 4.5 | 4.7 | 0 | 0.3 | 16 |
| Salmon and trout (Salmo spp.) | Farmed | 125 | 14 | 14 | 0 | 0.3 | 70 |
| Tuna (Thunnus) | Wild | 18 | 1.7 | 2.0 | 0 | 0.3 | 14 |
| Sea catfish and wolffish (Anarhichas) | Farmed | 13 | 2.5 | 2.8 | 0 | 0.3 | 19 |
LB: lower bound; UB: upper bound; N: number of samples.
For salmon, organic‐, wild caught‐ and farmed production methods were reported. Considering the SCCP levels, the highest mean contamination was reported in farmed salmon (9.55 μg/kg ww) while in case of MCCPs, organic production showed the highest mean (29.07 μg/kg ww). In both cases wild caught salmon had the lowest levels, though in case of SCCPs the differences between the mean of reported values of the respective production methods were smaller than in case of MCCPs.
Occurrence data in feed
No occurrence data for feed were submitted to EFSA.
3.2.2. Previously reported occurrence data in the open literature in food and feed
3.2.2.1. Food
Several studies were identified in the open literature reporting the levels of CPs in individual food categories or as part of duplicate or market basket studies. A larger number of studies reporting the levels of SCCPs in foodstuff were identified, while less data were available on MCCPs and especially limited data were available on LCCPs. Two studies reporting levels for feed materials were identified. Details about these studies are described below and in Table 29 , including the analytical method used for the determination of the CPs.
Table 29.
Occurrence data on short‐chain chlorinated paraffins (SCCPs), medium‐chain chlorinated paraffins (MCCPs) and long‐chain chlorinated paraffins (LCCPs) in food reported in the open literature
| Reference | Country | Number of samples | Year | Analytical method | Compounds | ||
|---|---|---|---|---|---|---|---|
| SCCPs | MCCPs | LCCPs | |||||
| Lahaniatis et al. (2000) | Fish samples consumed in Europe, fish oil and cod liver oil from Europe and America |
Fish (n = 8): sprat, redfish, salmon, herring, mackerel, halibut, sardine and trout Fish oil (n = 4) Cod liver oil (n = 4) |
n.r. | GC‐ECNI‐MS |
μg/kg fat Fish: 88–237 Fish oil: 44–284 Cod liver oil: 57–385 |
μg/kg fat Fish: 29–99 Fish oil: 63–191 Cod liver oil: 105–248 |
– |
| Parera et al. (2013) |
Spain (Ebro River delta) |
Fish (1 kg of muscle meat for each species): sardine, eel, flounder, conger, gilthead sea bream Shellfish (1 kg of muscle meat for each species): mussel, carpet shell, murex |
2012 | GC‐ion trap‐MS |
μg/kg ww Fish: 33.2–141 Conger: 141 Seabream: 60.5 Flounder: 33.2 Sardine: 104 Eel: 80.4 Shellfish: 3.1–34.6 Mussel: 12.0 Carpet shell: 3.1 Murex: 34.6 |
– | – |
| Krätschmer et al. (2019) | Germany | Fish (n = 133): farmed salmon (n = 122), wild salmon (n = 11) | 2014–2017 | GC‐Orbitrap‐MS |
μg/kg ww Mean (range): 14 (0.97–170) |
μg/kg ww Mean (range): 19 (1.1–79) |
– |
| Labadie et al. (2019) |
France (Rhone River basin) |
Fish (n = 22): common barbel | 2009, 2011 | GC‐ECNI‐TOF‐MS |
μg/kg ww 0.3–10.6 μg/kg fat 63–1,492 |
μg/kg ww 1.3–72.7 μg/kg fat 99–11,300 |
– |
| Yuan et al. (2017) | Sweden | Food market basket study: 13 food categories, up to 31 items per category | 2015 | Direct injection APCI‐qTOF‐MS |
μg/kg ww Cereals: < 1.1 Pastries: < 1.8 Meat: < 1.0 Fish: 4.6 Dairy (fluent): < 2.0 Dairy (solids): < 2.0 Eggs: < 1.3 Fat/oils: < 5.7 Vegetables: < 0.57 Fruit: 0.49 Potatoes: < 0.75 Sugar/sweets: < 0.90 Beverages: < 0.51 |
μg/kg ww Cereals: < 1.1 Pastries: 4.3 Meat: < 1.0 Fish: 5.1 Dairy (fluent): < 2.0 Dairy (solids): < 2.1 Eggs: < 1.4 Fats/oils: 14.5 Vegetables: < 0.58 Fruit: 0.90 Potatoes: 0.98 Sugar/sweets: 6.3 Beverages: < 0.52 |
μg/kg ww Cereals: < 0.04 Pastries: < 0.07 Meat: < 0.04 Fish: < 0.08 Dairy (fluent): < 0.08 Dairy (solids): < 0.08 Eggs: < 0.05 Fat/oils: < 0.22 Vegetables: < 0.02 Fruit: 0.14 Potatoes: 0.12 Sugar/sweets: 0.54 Beverages: < 0.02 |
| Krätschmer et al. (2019a, see Documentation provided to EFSA) | Germany | Raw food samples (n = 49): meat and offal (n = 17), eggs (n = 5), milk and dairy (n = 6), fish (n = 13), fats and oils (n = 8) | 2018–2019 | GC‐ECNI‐Orbitrap‐HRMS |
Total CPs μg/kg ww Meat and offal: 1.4–12 Eggs: 14–23 Milk and dairy: 0.5–17 Fish: 1.8–360 Fats and oils: 15–800 |
||
| Krätschmer et al. (2019a, see Documentation provided to EFSA) | Germany |
Full meals (n = 10): meat‐based (n = 8), vegetarian (n = 2) Total diet samples (n = 5): light diet (n = 3), full diet (n = 2) |
2018–2019 | GC‐ECNI‐Orbitrap‐HRMS |
μg/kg ww Full means: Meat‐based: 1.8–8.9 Vegetarian: 1.2–1.9 Total diet samples: Light diet: 1.0–2.1 Full diet: 4.0–4.7 |
μg/kg ww Full meals: Meat‐based: 1.0–7.8 Vegetarian: 0.6–2.0 Total diet samples: Light diet: 0.4–1.1 Full diet: 4.3–4.8 |
n.a. |
| Sprengel et al. (2019) | Germany (available from the German online market) | Oil‐based supplements (n = 25): vitamin E supplements (n = 14), marine oil supplements (n = 7), other oil supplements (n = 4) | n.r. | GC‐ECNI‐MS |
μg/kg fat mean (min–max) 3,810 (< LOD–61,100) |
μg/kg fat mean (min–max) 15,200 (< LOD–151,000) |
n.a. |
| Yuan et al. (2012) | China (Bohai Sea) | Molluscs (91 composite samples): bivalves (9 species), gastropods (2 species) | 2009 | GC‐ECNI‐MS |
μg/kg dw Molluscs: overall range: 64.9−5,510 Median (min, max) Bivalves: 1,150–2,910 (64.9–5,510) Gastropods: 506–640 (135–1,660) |
n.a. | n.a. |
| Iino et al. (2005) | Japan | Market basket study (11 food categories: grain crops, seeds/potatoes, sugar, fats, vegetables, fruit, fish, shellfish, meat, eggs, milk) | 2003 | GC‐ECNI‐HRMS |
μg/kg ww Mean (SD) Grain crops: 2.5 (3.1) Seeds/potatoes: 1.4 (2.0) Sugar/sweets/snacks/seasoning/beverages: 2.4 (3.0) Fats: 140 (150) Legumes/vegetables: 1.7 (2.3) Fruit: 1.5 (1.9) Fish: 16 (17) [or 170 μg/kg fat] Shellfish: 18 (19) [or 2,500 μg/kg fat] Meat: 7.0 (6.6) Eggs: 2.0 (2.7) Milk: 0.75 (1.6) |
n.a. | n.a. |
| Harada et al. (2011) |
China Korea Japan |
24 h food composite samples (a total of 200 24 h duplicate samples) | 1990s, 2007–2009 | GC‐ECNI‐HRMS |
μg/kga Samples China: < LOD–28.0 Year 1990s: < 0.20–0.60 Year 2009: 8.5–28.0 Samples Japan: < LOD–1.10 Year 1990s: < 50–760 Year 2009: < 50–1.100 Samples Seoul: < 0.050–0.056 Year 2009: 1 sample with trace levels Year 1990s: all < LOD |
n.a. | n.a. |
| Huang et al. (2018) | China | Meat and meat products (n = 20): pork, mutton, beef, chicken, duck, rabbit, pork liver, swine blood | 2011 | GC×GC‐ECNI‐TOF‐MS |
μg/kg ww Mean (SD): 129 (4.1) |
μg/kg ww Mean (SD): 5.7 (0.59) |
– |
| Wang et al. (2019b) | China |
Cereals (n = 19 pooled samples, consisting of 1,710 individual samples) Legumes (n = 19 pooled samples, consisting of 1,710 individual samples) |
2011 | GC×GC‐TOF‐MS |
μg/kg ww Cereals: 343 Legumes: 328 |
μg/kg ww Cereals: 213 Legumes: 184 |
– |
| Zeng et al. (2016) |
China (collection site close to an e‐waste recycling area or far) |
Home‐produced eggs Chicken eggs (n = 17) Goose eggs (n = 23) |
2013 | GC‐ECNI‐MS |
μg/kg fat mean, range Chicken eggs: Close to e‐waste site: 3,400 (2,300–5,500) Far from e‐waste site: 4,000 (2,600–6,800) Goose eggs Close to e‐waste site: 60,000 (< LOD–150,000) Far from e‐waste site: 4,600 (< LOD–11,000) |
– | – |
| Zeng et al. (2018) | China (collection area used to be an e‐waste site) | Home‐produced eggs (n = 68): year 2013 (n = 38), year 2016 (n = 30) | 2013, 2016 | GC‐ECNI‐MS |
μg/kg fat Median: 2013: 926 2016: 1,490 Overall range: 477–111,000 |
μg/kg fat Median: 2013: 1,030 2016: 999 Overall range: 125–91,100 |
– |
| Cao et al. (2015) | China |
Cooking oil (n = 49) Fried confectionary products (n = 20) Raw seeds (n = 13) |
2010, 2012 | GC‐ECNI‐HRMS |
μg/kga Cooking oil: (detected in 48 samples): < 9–7,500 Fried confectionary products: (detected in all samples): 11–1,000 Raw seeds: (detected in 11 samples): < 2–68 |
– | – |
| Sun et al. (2017) | China (collection site e‐waste contaminated area) |
Fish and invertebrates Oriental river prawn (n = 50), Chinese mitten crab (n = 16), crucian carp (n = 16), mud carp (n = 5–60), catfish (n = 2), snakehead (n = 5) |
2014 | GC‐ECNI‐MS |
μg/kg fat 1,700–95,000 Highest mean: Chinese mitten crab: 44,000 Lowest mean: Large mud carp: 2,100 |
– | – |
| Gao et al. (2018) | China |
Duplicate Diet study Part I: fast food outlets (n = 15) Part II: collected by 6 participants who provided duplicate portions of what they consumed for their three meals per day (n = 18) Milk powder (n = 6) |
2016 | GC‐TOF‐HRMS |
μg/kg dw Mean (range) Duplicate diet samples: 113 (24.4–546) Milk power samples: 18.3 (16.2–20.5) |
μg/kg dw Mean (range) Duplicate diet samples: 55 (17.3–384) Milk power samples: 8.87 (17.0–384) |
– |
| Chen et al. (2018) | China (collected from an e‐waste recycling industrial park and its surrounding villages) | Locally produced staple foods (n = 92): 9 food categories: fish, shellfish, shrimp, meat, poultry, egg, vegetable, cereal, culinary oil | 2016–2017 | GC‐ECNI‐MS |
μg/kg fat Fish: 94.3–487 Shellfish: 37.1–66.9 Shrimp: 76.5–118 Meat (pork): 37.2–58.6 Poultry: 25.3–88.3 Eggs: 1.62–6.84 Vegetables: 132–605b Cereals: 37.2–88.6 Culinary oil: 0.892–1.32 |
μg/kg fat Fish: 116–563 Shellfish: 35.5–59.9 Shrimp: 76.5–118 Meat (pork): 48.3–69.3 Poultry: 31.9–111 Eggs: 207–10.6 Vegetables: 245–747b Cereals: 55.4–108 Culinary oil: 1.22–1.76 |
– |
| Gao et al. (2019) | China | Food samples (n = 122): meat (n = 22), offals (n = 10), fish (n = 10), vegetables (n = 27), fruit (n = 15), seafood (n = 21), oil (n = 6), flour (n = 5), rice (n = 6) | 2014–2016 | GC‐ECNI‐LRMS |
μg/kg ww Overall range: 0.67–5,100 Mean Meat: 117 Seafood: 60.5 Fish: 46 Offals: 27.8 Fruit: 16.4 Vegetables: 11.7 Oils: 15.7–5,100 |
– | – |
n.a.: not analysed; n.r.: not reported; GC: gas chromatography; ECNI: electron capture negative ionisation; MS: mass spectrometry; HRMS: high‐resolution MS; TOF‐MS: time‐of‐flight MS.
Not clear whether the results were expressed on a wet, fat or dry basis.
The CONTAM Panel noted that it is uncommon to express levels in vegetables on fat weight basis.
Comparisons between studies should be done carefully due to the differences in and reliability of methods used for the determination of the CPs and the sampling and geographical origin of the samples.
3.2.2.1.1. SCCPs
The EU‐RARs on SCCPs (EU‐RAR, 2000, 2008) (see Section 1.3.5 ) gave a summary of concentrations of SCCPs in aquatic biota and in other biota. The earlier report (EU‐RAR, 2000) showed that SCCPs were found in fish at concentrations up to 1,600 μg/kg fat (unspecified chain length), marine mammals (up to ~ 500 μg/kg ww) and mussels (up to 12,000 μg/kg ww, sum of SCCPs and MCCPs). In other biota, SCCPs were found to be present in various terrestrial mammals, bird livers and muscle, and various foodstuffs. The 2008 report (EU‐RAR, 2008) added to these data for fish taken from close to industrial areas where CP production was known to take place, and SCCPs (60–70% weight chlorination) in white suckers from the St. Lawrence River, were present at up to 3,500 μg/kg dw. Other data for fish were also summarised in the report, but were at concentrations closer to those reported in the earlier assessment in 2000. The updated report mentioned that SCCPs were not found in cows’ milk, but were found in butter samples from several European countries at concentrations up to 2.7 μg/kg fat.
UK‐COT (2009) reported the levels in 45 samples, including composites, of various food types on sale in the UK and sampled in 2007. These data have been submitted to EFSA (see Section 2.2 ). SCCPs were detected in fish at concentrations of < 1.0–6.0 μg/kg ww. SCCPs were also detected in butter (1.23 μg/kg ww), pork sausage (3.9 μg/kg ww), bread (2.1 μg/kg ww), fruit (2.0 μg/kg ww), beef (< 1.0–1.2 μg/kg ww) and lamb (< 1.0–2.4 μg/kg ww). They were rarely or never detected in milk products, eggs and poultry samples. They were rarely or never detected in milk products, eggs and poultry samples. The highest concentrations were in a cod liver oil sample (25 μg/kg ww).
A number of studies reporting the concentrations of SCCPs in fish and seafood collected in different geographic areas in Europe and Asia were identified.
In Europe, Lahaniatis et al. (2000) determined the levels of SCCPs (and MCCPs) in fish, fish oil and cod liver oil samples originating from Europe except one sample from Chile. For fish the concentrations ranged from 88 to 237 μg/kg fat. In fish oil and cod liver oil, similar concentrations were reported (range 44–284 and 57–385 μg/kg fat, respectively). Parera et al. (2013) reported the levels of SCCPs in fish (conger, seabream, flunder, sardine and eels) and shellfish from the Ebro Delta in Spain. The levels in fish ranged from 33.2 and 141 μg/kg ww, while in shellfish the values ranged from 3.1 to 34.6 μg/kg ww. Labadie et al. (2019) reported the concentrations of CPs in tissues of common barbel (Barbus), a fish species frequently used for water quality monitoring purposes in Europe, that is also sometimes consumed. SCCPs were found to be widespread within the Rhone river basin (France), with concentrations ranging from 0.3 to 10.6 μg/kg ww which were lower than concentrations of MCCP at all sites. The authors reported that variations of SCCP composition profiles were generally greater than for MCCPs, suggesting a greater influence of local sources. Krätschmer et al. (2019) determined the levels of SCCPs (and MCCPs) in farmed and wild salmon bought at German supermarkets and collected between 2014 and 2017. These data have been submitted to EFSA (see Section 2.2 ). Samples consisted of farmed salmon from Norway, Scotland, Denmark, Chile and other locations, and wild salmon from different Eastern Pacific locations. The mean (range) SCCP level was 14 (0.97–170) μg/kg ww. The C11H13Cl11 was the predominant SCCP homologue in all salmon samples.
In Asia, several studies have reported the levels of SCCPs in different fish and shellfish species collected from different rivers, lakes or estuaries, especially in China. Wei et al. (2016) reviewed the occurrence and distribution of SCCPs. The concentrations varied widely considering the influence of point sources in some areas. For most biota samples the SCCP congeners were dominated by C10 and C11 and Cl5 and Cl7. Yuan et al. (2012) determined SCCPs in molluscs collected in nine coastal cities along the Bohai Sea. The authors reported concentrations in the range 64.9−5,510 μg/kg dw with an average chlorine content of 61.1%. The predominant chain length was C10 and C11, which accounted for about 30% of the total SCCP content. The predominant substitution was Cl6 and Cl7. In a study designed to calculate bioconcentration factors, Sun et al. (2017) reported concentrations between 1,700 and 95,000 μg/kg fat in several aquatic species including fish and invertebrates in South China.
Levels of CPs in foodstuff other than fish have also been reported.
Sprengel et al. (2019) investigated the levels of SCCPs (and MCCPs) in oil‐based dietary supplements available on the German online market. Fourteen were Vitamin E dietary supplements, 12 containing palm oil and two soybean oil, seven were marine oil supplements, and four were other oil supplements. The mean (min–max) SCCP concentrations were 3,810 (< LOQ–61,100) μg/kg fat. In 13 out of the 25 samples analysed CPs were not detected (< 100 μg/kg fat). Six of the Vitamin E dietary supplements containing palm oil showed the highest SCCP levels ranging from < LOD to 61,100 μg/kg fat, while the levels in the remaining six samples ranged from < LOD to 1,9 μg/kg fat.
Zeng et al. (2016) analysed CPs in home‐produced eggs from China. The eggs (17 chicken and 23 goose eggs) were collected in 2013 from two sites, one close to an e‐waste recycling area and the other far from the e‐waste site. Chicken eggs collected close to the e‐waste site showed mean (range) SCCP levels of 3,400 (2,300–5,500) μg/kg fat, while those collected far way were 4,000 (2,600–6,800) μg/kg fat. For the goose eggs, the concentrations were 60,000 (< LOD–150,000) and 4,600 (< LOD–11,000) μg/kg fat, respectively, for those collected close and far to the e‐waste site. The authors suggested that differences between the two type of eggs could be due to species‐specific differences in habitat preference, and the fact that levels in chicken eggs were higher in those eggs collected far from the e‐waste site could be due the high migration ability of SCCPs (Zeng et al., 2016). The same authors reported the levels of SCCPs (and MCCPs) in home‐produced eggs collected in 2013 and 2016 in a village form an area that was once a typical e‐waste recycling site (Zeng et al., 2018). SCCPs were detected in all samples, with median concentrations of 926 ng/g fat (year 2013) and 1,490 ng/g fat (year 2016). The authors reported no significant differences in the levels between the two sampling years, but in the homologue group profiles. While in eggs collected in 2013 an almost equal abundance of C10, C11, C12 and C13 was observed, in the samples from 2016, C10 and C11 homologues were significantly more abundant than C12 and C13.
Huang et al. (2018) assessed the dietary exposure for the general Chinese population through meat and meat products collected in 2011 from 20 Chinese provinces. SCCPs were determined in pork, mutton, beef, chicken, duck, rabbit, pork liver and swine blood samples purchased from local markets, grocery stores and rural households, and prepared according to local cooking methods of steaming, frying, boiling and pan‐frying. The mean (SD) concentration across provinces was 129 (4.1) μg/kg ww. The authors noted that the geographical distribution of SCCP concentrations was similar to the distribution of CPs manufacturing plants in China. The most abundant SCCP congeners were C10 and C11, and the Cl6 and Cl7 congeners dominated the profile.
The levels of SCCPs in cooking oil, fried confectionary products and raw seeds collected in China in 2010 and 2012 were reported by Cao et al. (2015). SCCPs were found in 48 out of the 49 cooking oil samples analysed with SCCP concentrations ranging from < 9 to 7,500 μg/kg. C13 followed by C11 were the predominant homologue groups, and Cl6 were the most abundant ones. In fried confectionary products, the levels ranged from 11 to 1,000 μg/kg, with the C11 and Cl6 homologues as the most abundant ones. The raw seeds analysed showed lower levels than the cooking oils and confectionery products, with concentrations of < 2–68 μg/kg. The C11 and Cl5 homologues were the dominant ones.
Wang et al. (2019b) analysed SCCPs (and MCCPs) on cereals and legumes as important foodstuff in the Chinese diet. A total of 19 pooled cereal samples and 19 pooled legume samples were collected in 2011 from different provinces in China. Mean SCCP levels were 343 and 328 μg/kg ww in cereals and legumes, respectively. The dominant SCCP congener groups were C10 and Cl5 Cl6 and Cl7 in all samples.
Several studies have determined the levels in more than one food category and estimated the corresponding dietary exposure. Few of these were carried out with samples collected in Europe, while more are available from Asian countries. In this section the levels in the foodstuff are summarised, while in Section 3.3.2 the exposure estimated in these studies is reported.
In Europe, Yuan et al. (2017) measured the levels of SCCPs (and MCCPs and LCCPs) in Swedish food market baskets and estimated the exposure to compare it then to the estimated exposure from hand blender use (see also Section 3.2.3 ). The market baskets consisted of 13 food categories (up to 31 items per category) purchased from five different Swedish food grocery chains in 2015. The highest SCCP levels were found in ‘fish’ (4.6 μg/kg ww), while in ‘fruits’ a value of 0.49 μg/kg ww was reported. The remaining food categories presented levels below the method LOD for each matrix.
Krätschmer et al. (2019a, see Documentation provided to EFSA) analysed raw food samples (meat and offal, eggs, milk and dairy, and fats and oils) collected from supermarkets, full meal samples (meat‐ and vegetarian‐based) collected from gastronomy and caterers, and total diet samples from a hospital (light and full diet). All samples were collected in Germany between 2018 and 2019. Regarding the raw food samples, the highest levels of total CPs were found in oils and fats (15–800 μg/kg ww), followed by fish (1.8–360 μg/kg ww). Lower levels were reported in meat and offal, eggs and milk and dairy (see Table 29 ). The authors found lower levels in the full meal samples analysed compared to the raw food samples, with SCCPs ranging from 1.2 to 8.9 μg/kg ww. In the total diet samples, the SCCP levels ranged from 1.0 to 4.7 μg/kg ww.
In Asia, Iino et al. (2005) carried out a Market Basket Study in Japan with samples collected in 2003. Eleven food categories were considered with 18% of the products coming from countries other than Japan. The food category ‘fats’ was the one showing the higher mean levels (140 μg/kg ww), followed by ‘fish’ (16 μg/kg ww, or 170 μg/kg fat) and ‘shellfish’ (18 μg/kg ww, or 2,500 μg/kg fat). ‘Milk’ was the group with the lowest levels (0.75 μg/kg ww).
Harada et al. (2011) estimated the exposure to SCCPs (C10–13) of Chinese, Japanese and Korean adults via duplicate 24 h food composite samples. The food composite samples were collected in China (Beijing), Japan (Hokkaido, Kyoto and Okinawa) and Korea (Seoul) at two time points: in the 1990s (archived samples) and between 2007 and 2009. The food samples collected in China showed the highest levels (range: < LOD to 28.0 μg/kg), and the concentrations in 2009 were higher than those in the 1990s. In the samples from Japan, SCCP levels ranged from < LOD to 1.10 μg/kg with no substantial changes between the two time points. The samples collected in Seoul showed the lowest levels (range: < 0.050–0.056 μg/kg). Regarding the congener profile, the C12 congeners (especially the Cl7 to Cl9) were the most frequently detected in samples from Japan (both time periods) and in Beijing in 1993. A different profile was reported for the Beijing samples in 2009, where C10 to C13 congeners dominated. Although the samples from China showed high total SCCP concentrations (in 2009) than the Japanese ones, highly chlorinated dodecanes (i.e. C12H18Cl8 and C12H17Cl9) showed comparable levels to those in Japanese samples.
Gao et al. (2018) estimated the exposure of the general population in Beijing (China) through a duplicate diet study that consisted of two parts: one part where food was collected from fast food outlets and the other part where food was collected by 6 participants who provided duplicate portions of what they consumed for their three meals per day. In addition, three different brands of milk powder purchased in supermarkets were analysed. The authors reported a mean (min–max) concentration of SCCPs in the duplicate diet samples of 113 (24.4–546) μg/kg dw, and in the milk power samples of 18.3 (16.2–20.5) μg/kg dw. The C13 and C11 were the predominant congeners (contributing 35 and 31%, respectively) followed by C12 and C10 congeners (23 and 12%, respectively). The Cl7 and Cl8 congeners were the most abundant ones.
Chen et al. (2018) determined SCCPs (and MCCPs) in four types of dust samples and nine categories of locally produced staple foods collected from an e‐waste recycling industrial park and its surrounding villages in China. When expressed on a fat weight basis, the highest SCCP levels were found in vegetables (local lettuce, 519,000 μg/kg fat, or 3,540 μg/kg ww) and fish (mrigal carp, 408,000 μg/kg fat), followed by shrimp, rice, duck, pork, shellfish, chicken and eggs. In foods of animal origin, the C10, C11, C12 and C13 contributed almost equally, although some differences were found for some food categories. Congeners with Cl7 and Cl8 were predominant.
Gao et al. (2019) analysed SCCPs in raw foodstuff and cooked food collected from markets in Beijing (China) between 2014 and 2016. A total of 122 food samples including different meats, offals, fish, vegetables, fruit, seafood, oil, flour and rice were analysed. SCCPs ranged from 0.67 to 5,100 μg/kg ww. The highest mean concentrations were found in meat samples (117 μg/kg ww), followed by seafood (60.5 μg/kg ww) and fish (46 μg/kg ww). Lower levels were found in fruit (16.4 μg/kg ww) and vegetables (11.7 μg/kg ww). The concentrations in oils varied widely from 15.7 to 5,100 μg/kg ww for corn blend oil and peanut oil, respectively. The main carbon homologues were C10 and C11, and the chlorination pattern was dominated by Cl7, Cl6 and Cl8. In addition, the authors analysed seven types of raw foodstuff (chicken eggs, potato, pork, mutton, shrimps, cabbage, rice and oil) after cooking by frying, stewing, boiling or steaming (see Section 3.2.3 for further details on the effects of cooking).
3.2.2.1.2. MCCPs
The EU‐RAR (2011) did not report any occurrence data for food or feed except for cows’ milk (highest value of 63 μg/kg fat based on published data), but estimates of total exposure (including dietary) were made on the basis of predicted concentrations, made from models and experiments conducted to assess bioavailability (see Section 3.3.2 ).
UK‐COT (2009) reported the levels of MCCPs in 45 samples of various food types on sale in the UK and sampled in 2007. These data have been submitted to EFSA (see Section 2.2 ). The levels in fish ranged from < 1.0 to 31.9 μg/kg ww and were higher than those in butter (2.84 μg/kg ww), pork sausage (7.5 μg/kg ww), bread (4.0 μg/kg ww), fruit (< 1.0 μg/kg ww), beef (< 1.0–8.3 μg/kg ww) and lamb (< 1.0–12.6 μg/kg ww). As for SCCPs, they were rarely or never detected in milk products, eggs and poultry samples. The highest concentrations were in the cod liver oil sample (72 μg/kg ww). MCCPs were usually found in these samples at a higher concentration than SCCPs.
Several of the studies reporting the levels of SCCPs (see previous Section 3.2.2.1 ) also determined MCCPs in the same samples.
Lahaniatis et al. (2000) reported MCCP (C14–17) levels in fish samples ranging from 29 to 99 μg/kg fat. For the fish oil and cod liver oil samples, the concentration ranges were 63 to 191 μg/kg fat and 105 to 248 μg/kg fat, respectively. For most of the fish samples, SCCPs showed higher concentrations than MCCPs, while in the fish oil and cod liver oil samples the contribution varied depending on the sample. In the study mentioned above for SCCPs, Labadie et al. (2019) also analysed MCCPs in the common barbel. MCCPs were widespread with concentrations ranging from 1.3 to 72.7 μg/kg ww, which are higher than the values found for SCCPs. Krätschmer et al. (2019) reported mean (range) MCCP levels of 19 (1.1–79) μg/kg ww in the 122 farmed and 11 wild salmon samples bought at German supermarkets. The C15H23Cl9 and C15H21Cl11 were the predominant MCCPs homologues. Based on mean and median concentration, MCCPs were predominant over SCCPs in the salmon samples. A slight decrease of the mean CP concentrations in the Norwegian salmon samples analysed from 2014 to 2017 was observed, with some high SCCP levels in samples from 2015. The authors could not however conclude on a decreasing trend due to the number of samples analysed and comparatively wide range of concentrations measured. The authors also determined PCBs48 and HBCDDs in the same salmon samples. The sum of SCCPs and MCCPs was higher than the sum of PCBs and that of HBCDDs in most of the samples.
Sprengel et al. (2019) reported levels of MCCPs in oil‐based dietary supplements. The mean (min, max) MCCP concentrations were higher than that found for SCCPs, and were 15,200 (< LOQ, 151, 000) μg/kg fat. In 13 out of the 25 samples analysed, CPs were not detected (< 100 μg/kg fat). Six of the palm‐oil‐containing Vitamin E dietary supplements showed again the highest levels, ranging from < LOD to 15,200 μg/kg fat, while the levels in the remaining six samples ranged from < LOD to 1.9 μg/kg fat.
Yuan et al. (2017), in a Swedish food market basket study, found the highest MCCP levels in the category ‘fat/oil’ (14.5 μg/kg ww), followed by ‘sugar/sweets’ (6.3 μg/kg ww), ‘fish’ (5.1 μg/kg ww) and ‘pastries’ (4.3 μg/kg). ‘The categories ‘fruit’ and ‘potatoes’ showed values of 0.90 and 0.98 μg/kg ww, respectively, and the remaining food categories contained levels below the method LOD for each matrix.
Krätschmer et al. (2019) reported MCCP levels in full meal samples ranging from 0.6 to 7.8 μg/kg ww, and in total diet samples ranging from 0.4 to 4.8 μg/kg ww.
Huang et al. (2018) also reported levels of MCCPs in the meat and meat products collected in 20 Chinese provinces. The concentration was lower than for SCCPs, with a mean (SD) level across provinces of 5.7 (0.59) μg/kg ww. As for SCCPs, the authors noted that the geographical distribution of MCCP concentrations was similar to the distribution of CPs manufacturing plants in China. The most abundant MCCP congeners were the C14, and the Cl7 and Cl8 congeners dominated the profile.
Zeng et al. (2018) reported the levels of MCCPs in home produced eggs in 2013 and 2016 collected from an area that used to be an e‐waste site. Median values of 1,030 ng/g fat (year 2013) and 999 ng/g fat (year 2016) were reported. The homologues profile was similar between the two time periods (unlike those for SCCPs), with C14 and C15 as the most abundant groups.
Wang et al. (2019b) also analysed MCCPs in pooled samples of cereals and legumes and the mean levels were 213 and 184 μg/kg ww, respectively. The values were lower than those found for SCCPs.
Gao et al. (2018), in their duplicate diet study, reported that the mean (min–max) concentration of MCCPs in the duplicate diet samples was 55.5 (17.3 to 384) μg/kg dw, and in the milk powder samples it was 8.87 (17.0 to 384) μg/kg dw. In this case, the C14 congeners dominated the profile, and Cl8 CPs were the most abundant ones.
Chen et al. (2018) also reported the levels of MCCPs in the nine categories of locally produced staple foods collected from an e‐waste recycling industrial park and its surrounding villages in China. As for SCCPs, when expressed on a fat weight basis, the highest MCCP levels were found in vegetables (local lettuce, 635,000 μg/kg fat, or 4,360 μg/kg ww) and fish (mrigal carp, 443,000 μg/kg fat), followed by shrimp, rice, duck, pork, shellfish, chicken and eggs. In all food samples, MCCPs were found at higher levels than SCCPs. The C14 homologues dominated the profile for most food samples, and Cl8−10 groups were predominant.
3.2.2.1.3. LCCPs
Yuan et al. (2017), besides SCCPs and MCCPs also measured the levels of LCCPs in their Swedish food market basket study. The highest LCCP levels were found in the category ‘sugar/sweets’ (0.54 μg/kg ww), followed by ‘fruit’ (0.14 μg/kg ww) and ‘potatoes’ (0.12 μg/kg ww). The remaining food categories presented levels below the LOD for each matrix.
3.2.2.2. Feed
3.2.2.2.1. SCCPs
Two reports of CPs in animal feed were identified. Lahaniatis et al. (2000) determined the levels of SCCPs (and MCCPs, see below) by GC‐ECNI‐MS in one fish feed sample. The concentration of C10–13 congeners was 169 μg/kg fat.
Dong et al. (2019) measured SCCPs (and MCCPs) in 16 samples of animal feed material (pools, each of them consisting of at least 8 individual samples), collected in China in 2016, by GC×GC‐ECNI‐TOF. Thirteen of the samples were of animal origin and three were of plant origin. The feed materials analysed were sampled at feed factory warehouses in China: four of the six fish meal samples analysed were imported (one from Chile, two from Peru, and one from the USA), all other samples were produced in China, but the geographical origins of the raw materials used in these products was reported to be uncertain. SCCP concentrations ranged from 120 to 1,700 μg/kg whole product basis with a mean of 640 μg/kg. Fish meal had the highest SCCP concentrations (up to 1,700 μg/kg whole product basis (range 400–1,700)), and peanut meal the lowest (120 μg/kg whole product basis). The predominant congener groups were C10–11Cl6–7.
3.2.2.2.2. MCCPs
The two studies of SCCPs in animal feed materials also determined MCCPs. Lahaniatis et al. (2000) reported the levels of C14–C17 congeners in one fish feed samples to be 1,550 μg/kg fat, while Dong et al. (2019) reported concentrations in the 16 feed materials ranging from 6.4 to 260 μg/kg whole product basis (mean 78 μg/kg whole product basis). As for SCCPs, fish meal showed the highest levels and whey powder the lowest. The C14Cl7 congeners dominated the MCCP profile. The MCCP levels were lower than those of SCCPs.
3.2.2.2.3. LCCPs
No studies reporting the levels of LCCPs for feed materials were identified.
3.2.2.3. Conclusions on the occurrence data reported in the literature
Most of the limited data reported in the scientific literature are for SCCPs and MCCPs, with only one study identified reporting concentrations of LCCPs in food. The majority of information originates for samples taken and analysed in China, which is where much of the global production takes place. The studies in China reported higher concentrations (tens of micrograms) than in other Asian countries (few nanograms).
SCCP and MCCP concentrations can vary dramatically from region to region and between different food types. As is the case for other POPs, highest concentrations may be found in fish and marine mammals, followed by liver and fatty tissues of terrestrial animals. Highest levels have been observed in foods produced in areas of known contamination.
The variation between regions, and the impact of local contamination sources makes it difficult to assess overall typical levels. Some studies report higher values for SCCPs while others report higher values for MCCPs. Both are likely to be within the same order of magnitude. Very little data are available for LCCPs. Occurrence is likely to be shifting away from SCCPs towards the MCCPs and LCCPs as production and usage moves in this direction.
There are some conflicts in reports of the most dominant congeners within each group, which may reflect the contaminant profile within the samples, or may reflect the analytical methodology used. For this reason, it is always recommended to be cautious when comparing data or drawing conclusions about contamination profiles.
The concentrations of SCCPs and MCCPs in the data on fish submitted to EFSA are in line with some of the data reported in the scientific literature, where samples were taken from the same geographical region i.e. Europe and where no specific contamination source was identified. Fish from Asia, and near known production or contamination sites can contain much higher levels of SCCPs and MCCPs for the reasons suggested above.
There are very few data reported in the literature about animal feed, and the few data that are available are not from Europe. It is therefore not possible to make any general conclusion about the concentrations of CPs in animal feed.
Great care needs to be taken when comparing data, and factors to consider include proximity to a known or suspected contamination source, the analytical methods used to measure CPs and the way the results are expressed (e.g. wet, fat or dry weight).
3.2.3. Food processing
Two studies were identified on the effects of cooking on the levels of CPs. Gao et al. (2019) analysed SCCPs in seven types of raw foodstuff (chicken eggs, potato, pork, mutton, shrimps, cabbage, rice and oil) before and after cooking by frying, stewing, boiling or steaming. Lower concentrations were found in the cooked food compared to the raw food in the case of eggs, potato, shrimps, mutton and pork (overall from 3.26–52.1 μg/kg ww in the raw food to 0.67–10.8 μg/kg ww in the cooked food). The highest loss was reported for cooked mutton (raw vs fried), shrimp (raw vs boiled) and eggs (raw vs boiled). For those foods the authors reported elimination of 82, 85 and 93% SCCPs, respectively. In rice and cabbage, SCCP concentrations were lower in the raw food (3.60 and 3.26 μg/kg ww, respectively) than when cooked (6.38 and 5.14 μg/kg ww, respectively). The authors concluded that at low concentrations, water and cookware may introduce SCCPs during the cooking process.
Perkons et al. (2019) analysed SCCPs and MCCPs in unprocessed pastry dough (e.g. pies, buns, strudels, croissants, cinnamon rolls) and in oven‐baked pastry products (e.g. puff pastry, shortcrust pastry, pizza dough and yeast dough). The mean level in the unprocessed dough (12.9 ng/g dw) was twofold higher than those determined in the pastry products (6.3 ng/g dw). An additional experiment with unprocessed dough spiked with SCCPs, subsequently baked for 15 min at 180°C showed a 24% lower concentration in the baked product compared to the dough. The authors also reported a higher proportion of CPs with lower chlorination degree in baked products compared to the unprocessed dough samples. The authors indicated that it is most likely that such transformations are caused by thermally induced dehydrochlorination of CPs and carbon chain cleavage of MCCPs and LCCPs. In this respect, thermal decomposition of a CP technical product was studied by Xin et al. (2019), reporting significant amount of MCCPs, SCCPs and aromatic and chlorinated polycyclic aromatic hydrocarbons (chlorinated PAHs) being formed during the thermal decomposition of CP‐52 (10.9% SCCPs and 85.5% MCCPs in weight, 52% chlorination degree). After an initial dehydrochlorination and carbon chain cleavage, the chlorinated and unsaturated hydrocarbons formed undergo cyclisation and aromatisation at temperatures of 200–400°C, producing mostly aromatic hydrocarbons. At higher temperature, radical‐induced reactions were reported to be the predominant pathways, leading to the formation of chlorinated‐PAHs.
Because CPs can be used in the manufacturing of kitchen utensils, there exists the potential for migration from these products into food. This can either be direct, or via practices such as cleaning using dish cloths. CPs are not authorised for use in plastic food contact materials in Europe.
Yuan et al. (2017) investigated levels of CPs from hand blender use and compared this with background CP exposure from diet. The study quantified 6 SCCPs, 9 MCCPs and 6 LCCPs with details of the individual compounds given in the paper (Yuan et al., 2017). CPs were analysed in food market baskets, in cooking oil/water samples (1 g oil/100 mL water) mixed using 16 different hand blenders, and in dismantled components of the hand blenders. Background dietary intake of CPs was estimated from a food market basket survey and was calculated to be 4.6 μg per day per person for Swedish adults (see Section 3.3.2 ). Total CP amounts in oil/water leakage samples from the hand blenders ranged from < 0.09 to 120 μg (determined by direct injection into an APCI‐qTOF‐MS after sample preparation). Migration of CPs did not decrease even after 20 times of hand blender usage. The CP profiles in the leakage samples matched those of self‐lubricating bearings and/or polymer components disassembled from the hand blenders. The authors concluded that most hand blenders tested had significant levels of CPs, that were estimated to increase the exposure when used to prepare food. The additional dietary intake from the hand blenders was calculated assuming that only one person eats the prepared food and that everything was consumed, resulting in a possible overestimation of exposure due to the fact that some foods may be shared between several people. Also, some preparation methods may require multiple application of the blender, which could result in additional CP migration into foodstuffs. The median intake of SCCPs from one‐time use based on results from all hand blenders (0.10 μg/day) was reported to contribute an additional 4% and 7% to the dietary exposure for breastfed infants and adults in Sweden, respectively. For other countries (China, Japan and the UK), the authors reported that hand blender use would contribute less due to higher dietary intakes from food. The median intake of ∑MCCPs from one‐time hand blender use per day was calculated to contribute an additional 19%–160% to total dietary exposure for Swedish infants and adults. For China and the UK, the authors reported that the increase in exposure of infants to ∑MCCPs would be 253 and 69 times higher, respectively, than the median dietary intake.
The German National reference Laboratory for Materials for Food Contact carried out a study to investigate the release of CPs from hand blenders (BfR, 2019, see Documentation provided to EFSA). The release test was carried out at room temperature using 19 hand blenders with isooctane as food simulant. The release test was repeated three times. The CP determination was carried out by GC‐NCI‐MS. In six out of the 19 hand blenders tested, CPs were found in the isooctane, while for the remaining blenders, concentrations in the isooctane used were below the LOQ. In most the cases, the concentrations in the first release were the highest, with values up to 690 μg/kg isooctane. For one of the hand blenders, the release of total CPs increased with subsequent uses, with a value of 3,000 μg/kg total CPs in isooctane released on the third repeat use.
Gallistl et al. (2017) investigated CPs and other contaminants in dish cloths after their regular use in a conventional household setting in Germany, over a 14‐day period. The individual dish cloths (n = 19) showed a highly variable pattern of contribution from the various classes of polyhalogenated compounds. When they were detected, MCCPs were by far the most prominent compound class with up to 55,400 ng per dish cloth (determined by GC‐ECNI‐MS). In addition to the potential for dermal exposure, there is a potential for transfer from the dish cloth directly or indirectly into foods in the kitchen environment (see Section 3.3.3 ).
Gallistl et al. (2018) also reported SCCPs and MCCPs (and polybrominated diphenyl ethers (PBDEs)) in household oven components, where it is suggested they are used because of their flame retarding properties. Fat obtained by wipe tests on the inner surface ovens was analysed for several halogenated flame retardants including PBDEs, decabromodiphenyl ethane (DBDPE), dechlorane plus (DP), polychlorinated biphenyls (PCBs) in addition to SCCPs and MCCPs (determined by GC‐ECNI‐MS). In about 50% of the samples, CPs were present in the mg/g fat range, i.e. three to four orders of magnitude higher concentrated than the sum of all other compounds measured. The other half of the samples were <LOD, while the other flame retardants were comparable in CP‐positive and CP‐negative samples. The high concentrations of CPs in half of the samples produced strong evidence that these compounds were released from the oven that was used. This hypothesis was further supported by the presence of MCCPs at even higher concentrations in the inner components of one dismantled oven. The authors concluded that the release of substantial amounts of CPs from the oven casing during use may make a significant contribution to human exposure to these compounds. In a previous study, Bendig et al. (2013) analysed CPs and other halogenated contaminants in fat residues in kitchen hoods (n = 15). CPs showed the highest concentrations among all compounds analysed, with a mean of 3,500 μg/kg, and for most samples represented around 70% of the total halogenated contaminants determined in the samples. The authors reported that SCCPs were detected in all samples, while MCCPs were detected in 3 out of 15 samples.
CPs have also been analysed in domestic polymeric products, and the migration from plastic food packaging into food simulants have also been reported in one study.
Wang et al. (2018b) analysed SCCPs and MCCPs in domestic polymeric products, such as plastics, rubber and food packaging (chips, dried fruit and pie packaging). Mean SCCP concentrations in polyethylene terephthalate (PET), polypropylene (PP), polyethylene (PE) and food packaging were 234, 3,968, 150 and 188 μg/kg, respectively. The concentrations of MCCPs were in general lower, with mean values of 37.4, 2,537, 208 and 644 μg/kg, respectively. The C11 and C13 congeners dominated the SCCP profile, while the C14 congeners dominated the MCCP profile. The Cl6, Cl7 and Cl8 congener groups were predominant in all samples. The same authors studied the migration of CPs from six plastic food packaging materials into four food simulants to simulate the migration of CPs into aqueous, acidic, alcoholic and fatty food (Wang et al., 2019c). The overall migration concentrations of SCCPs were < LOD–350, < LOD–702, < LOD–276 and 164–4,412 ng/L in aqueous (water), acidic (3% acetic acid), alcoholic (15% ethanol) and fatty food (hexane) simulants. The total migration concentrations of CPs in hexane food simulant were significantly higher than in other food simulants (one‐way ANOVA, p < 0.05). The authors also reported that the average migration efficiencies of SCCPs (12.15%) were significantly higher than those of MCCPs (1.51%) except in hexane food simulants (ANOVA, p < 0.05), and that the shorter chain length and lower chlorinated congener groups the higher migration efficiency.
In conclusion, limited information is available on the effects of food processing on the concentrations of CPs. The one study identified on the effects of different cooking practices indicates a reduction of the SCCP contents in the case of meat (mutton and pork) after frying and stewing, respectively.
CPs have been found in some common kitchen equipment, e.g. dish cloths, hand blenders and household oven components. This suggests that food could become more contaminated at the preparation stage as a result of transfer during contact (direct or secondary), and this is an important consideration when making exposure estimates.
CPs have also been found in domestic plastic and food packaging, and the possibility that those migrate into the food has been studied. Based on the studies available, migration from plastic and kitchen equipment's can contribute to the total CP exposure. However, because of the limited available information exposure levels cannot be estimated.
3.2.4. Levels in humans
Several studies have been found in the literature reporting the levels of SCCPs, MCCPs and LCCPs in human milk, blood and other human tissues. These studies are described below and in Tables 30 , 31 and 32 for human milk, blood and other tissues, respectively, in which details about the analytical methods used for the CP determination and detection frequency are provided.
Table 30.
Concentrations of chlorinated paraffins in human milk samples. The detection frequency is indicated when provided in the study
| Reference | Country | Number of samples | Sampling year | Analytical method | Concentration (μg/kg fat) | ||
|---|---|---|---|---|---|---|---|
| SCCPs | MCCPs | LCCPs | |||||
| EUROPEAN COUNTRIES | |||||||
| Thomas and Jones (2002), as cited in EU‐RAR (2011) | UK |
22 Lancaster n = 8, London n = 14 |
n.r | n.r. | n.a. |
61 (detected in 1 sample) |
– |
| Thomas et al. (2006) | UK |
25 (18 individuals at different times) London area n = 20 Lancaster area n = 5 |
2001–2002 | GC‐ECNI‐HRMS |
Median (range) 180 (49–820) (detected in 21 samples, range corresponds to the samples > LOD) |
Median (range) 21 (6.2–320) (detected in all samples) |
– |
| Darnerud et al. (2012) | Sweden |
12 pooled samples (438 individual samples) |
1996–2010 | GC‐ECNI‐HRMS |
Median (range) 107 (45–157) (detected in all samples) |
Median (range) 14 (< 1.1–30) (detected in 10 samples, the range corresponds to all samples analysed) |
– |
| Reth et al. (2005) | Germany | 6 | 2004–2005 | GC‐EI‐MS/MS |
Total CPs: Range: 52–275 |
||
| Hilger et al. (2011) | Germany | 60 | n.r. | GC‐ECNI‐LRMS |
Median (range) 15.9 (< LOD–59.9) (detected in 43% of the samples, the median refers to the detectable samples) |
Median (range) 115.4 (9.6–903) (detected in 58% of the samples, the values correspond to the detectable samples) |
– |
| Krätschmer et al. (2019b, see Documentation provided to EFSA) | 11 European countries a | 11 pooled samples (each from about 50 first‐time mothers) | 2014–2016 | GC‐ECNI‐Orbitrap‐HRMS, GC‐EI‐MS/MS |
Range across countries: 13–97 |
Range across countries: < LOQ (5.5)–112 | – |
| OTHER COUNTRIES | |||||||
| Tomy (1997) | Canada | 3 (samples from Inuit women in Northern Quebec) | n.r. | GC‐MS |
Total CPs: Mean (SD) (range) 12.8 (3.2) (10.6–16.5) |
||
| Cao et al. (2017) |
China (Beijing) Korea (Busan, Seoul) Japan (Kyoto, Sendai) |
Pooled samples China (n = 17) Korea (n = 16) Japan (n = 44) |
2007–2010 | GC‐ECNI‐HRMS |
China: < 20–54 (detected in 8 samples) Korea: < 20 (undetected in all samples) Japan: < 20 (undetected in all samples) |
– | – |
| Xia et al. (2017a) |
China (urban areas) |
28 pooled samples (1,370 individual samples) |
2007, 2011 |
GC×GC‐ECNI‐TOF‐MS |
Median (min–max) Year 2007: 681 (170–6,150) Year 2011: 733 (131–16,100) (detected in all samples) |
Median (min–max) Year 2007: 60.4 (18.7–350) Year 2011: 64.3 (22.3–1,501) (detected in all samples) |
– |
| Xia et al. (2017b) |
China (rural areas) |
24 pooled samples (1,412 individual samples) |
2007, 2011 |
GC×GC‐ECNI‐TOF‐MS |
Median Year 2007: 303 (68.0–1,580) Year 2011: 360 (65.6–2,310) (detected in all samples) |
Median Year 2007: 35.7 (9.05–139) Year 2011: 45.4 (9.51–146) (detected in all samples) |
– |
| Yang et al. (2018) |
China (Hebei Province) |
86 samples from 55 women at different nursing periods (from childbirth to 6 months) | 2014–2015 | GC‐ECNI‐MS |
Median (range) 1460 (210–16,200) (detected in all samples) |
– | – |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins; n.r.: not reported; GC: gas chromatography; MS: mass spectrometry; HRMS: high‐resolution MS; ECNI: electron negative chemical ionisation; TOF‐MS: time‐of‐flight MS; bw: body weight.
Bulgaria, Croatia, the Czech Republic, Georgia, Romania, the Netherlands, Moldova, Lithuania, Belgium, Austria, Switzerland.
Table 31.
Concentrations of chlorinated paraffins in human blood samples. The detection frequency is indicated when provided in the study
| Reference | Country | Number of samples | Sampling year | Analytical method | Concentration (μg/kg fat) | ||
|---|---|---|---|---|---|---|---|
| SCCPs | MCCPs | LCCPs | |||||
| OTHER COUNTRIES | |||||||
| Li et al. (2017) |
China (urban area) |
50 | 2012 | UPLC‐qTOF‐MS |
Median (range) 3,500 (370–35,000) (for samples with > 50% detection frequency) |
Median (range) 740 (130–3,200) (for samples with > 50% detection frequency) |
Median (range) 150 (22–530) (for samples with > 50% detection frequency) |
| Xu et al. (2019) | China | 50 | 2015 | GC‐ECNI‐LRMS |
Mean (range) (ww) 32.0 (< LOD–203) (detected in 49 samples) |
– | – |
| Qiao et al. (2018) | China (Beijing) | 21 paired samples of maternal and umbilical cord serum | 2013 | GC×GC‐ECNI‐TOF |
Range Maternal serum 2,570–57,700 Umbilical cord blood 3,750–40,500 (detected in all samples) |
Range Maternal serum 478–6,400 Umbilical cord blood 522–4,600 (detected in all samples) |
– |
| Van Mourik (2018) | Australia | 9 pooled samples (100 individual samples each, males < 65 years old) | 2004–2015 | APCI‐TOF‐HRMS |
Mean (range) 300 (< LOD–360) (detected in 4 samples) |
Mean (range) 610 (< LOD–930) (detected in 8 samples) |
Mean (range) LCCPs: < LOD (undetected in all samples) |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins; n.r.: not reported; GC: gas chromatography; MS: mass spectrometry; HRMS: high‐resolution MS; ECNI: electron negative chemical ionisation; TOF‐MS: time‐of‐flight MS; bw: body weight.
Table 32.
Concentrations of chlorinated paraffins in human samples other than human milk and blood. The detection frequency is indicated when provided in the study
| Reference | Country | Number of samples | Sampling year | Analytical method | Concentration (μg/kg fat) | ||
|---|---|---|---|---|---|---|---|
| SCCPs | MCCPs | LCCPs | |||||
| Schmid and Muller (1985) | Switzerland |
Adipose tissue (n = 1) |
n.r. | GC‐ECNI‐MS |
Total CPs (equivalents to Witaclor‐352): 200 |
||
| Campbell and McConnell (1980) | UK |
Adipose tissue, kidney, liver, brain Adults (n = 20, 21–92 years old) Adolescent (n = 1, 17 years old) Child (n = 1, 5 years old) Infants (n = 2, 20 h, 5 months) |
n.r. | TLC with argentation |
Total CPs (as C10–20, 45–52% chlorination) ‐ reported as mg/kg ww Fat: Adults: < 50–540 (analysed and detected in all samples) Adolescent: < 50 Child: 100 Infants: n.a. Kidney: Adults: ND–400 (analysed in 17 samples and detected in 13 samples) Adolescent: < 50 Child: 60 Infants: 160 (analysed and detected in 1 sample) Liver: Adults: n.d.–1,500 (analysed in all samples, detected in 16 samples) Adolescent: < 50 Child: < 50 Infants: 50 (analysed and detected in all samples) Brain: Adults: < 50–80 (analysed in 19 samples, detected in 9 samples) Adolescent: < 50 Child: n.a. Infants: n.a. Total CPs (as C20–30) ‐ reported as mg/kg ww All samples below the LOD except for the following subjects identified with a number as in the study: Subjects (1), (2) and (3): Fat: 0.1, Kidney: not measured (insufficient tissue available). Subject (3): Liver 0.08 Subject (20): Fat: 3.5, Liver: 0.2 Subject (15): liver: n.d., Other tissues: not measured (insufficient tissue available). Subjects (22), (23) and (24): Brain: not measured (insufficient tissue available) Subject (24): Fat: not measured (insufficient tissue available) |
||
| Wang et al. (2018c) | China |
Placenta (n = 54) |
2016 | GC‐qTOF‐HRMS |
Mean (range) 593 (98.5–3,771) (detected in all samples) |
Mean (range) 316 (80.8–954) (detected in 38 samples) |
‐ |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins; n.d.: not detected; n.r.: not reported; TLC: thin‐layer chromatography; GC: gas chromatography; MS: mass spectrometry; HRMS: high‐resolution MS; qTOF: quadrupole time‐of‐flight; bw: body weight.
3.2.4.1. Human milk
The EU‐RAR (2011) reported about an industry sponsored study that determined the levels of MCCPs in breast milk samples from the UK (Thomas and Jones, 2002, as cited in EU‐RAR, 2011). A total of 22 breast milk samples were analysed from Lancaster and London. MCCPs were found in one sample at 61 μg/kg fat while the remaining 21 samples were below the LOD. The method LOD ranged from 16 to 740 ng/g fat.
Thomas et al. (2006) also reported the levels of SCCPs and MCCPs in 25 human milk samples from UK women from the London area and from the more rural Lancaster area collected between 2001 and 2002. The median SCCP (C10–13) level was 180 μg/kg fat (range of detected values: 49–820 μg/kg fat) while the median MCCP (C14–17) level was 21 μg/kg fat (range: 6.2 to 320 μg/kg fat). The authors reported no significant differences in the levels between the samples from London and Lancaster areas. The C11 and C12 homologues groups contributed most to the total SCCP profile, dominated by the Cl6, Cl7 and Cl8 congeners. The C14 homologues were the highest contributor for the MCCPs, dominated by the Cl5 and Cl6 congeners.
Samples from human milk collected in Sweden between 1996 and 2010 were analysed for SCCPs and MCCPs (Darnerud et al., 2012). A total of 12 pooled milk samples from primiparae women were analysed. The mean (range) level of SCCPs was 107 (45–157) μg/kg fat and of MCCPs was 14 (< 1.1–30) μg/kg fat. The authors reported that although notable differences were observed in the levels between years, no trend was obvious.
Tomy (1997) analysed CPs (reported as ‘polychlorinated alkanes’) in three milk samples from Inuit women from Northern Québec. The mean (range) concentrations were 12.8 (10.6–16.5) μg/kg fat.
In Germany, Reth et al. (2005) determined the levels of total CPs in six human milk samples from South Germany. The concentrations ranged from 55 to 275 μg/kg fat. As part of the Bavarian Monitoring Breast Milk Study, Hilger et al. (2011) reported levels of SCCPs and MCCPs in 60 human milk samples. For SCCPs only three samples showed levels above the LOQ, with a median concentration of 15.9 μg/kg fat for the samples with detectable levels, and a maximum of 59.9 μg/kg fat. MCCPs were quantifiable in 2/3 of the samples, with a mean (range) value of 115.4 (9.6–903) μg/kg fat.
As part of the WHO/UNEP Coordinated Survey of Human Milk for Persistent Organic Pollutants, the EU‐RL for Halogenated POPs in Feed and Food has determined the levels of SCCPs and MCCPs in pooled human milk samples from 11 European countries collected between 2014 and 2016. The levels of SCCPs across countries ranged from 13 to 97 μg/kg fat. The levels of MCCPs across countries ranged from < LOQ (5.5 μg/kg fat) to 112 μg/kg fat (Krätschmer et al., 2019b; see Documentation provided to EFSA).
Several studies have reported the levels of CPs in human milk samples from China and other Asian countries.
Cao et al. (2017) analysed SCCPs in pooled human milk samples from Chinese, Korean and Japanese primiparae or multiparous women. Samples were collected between 2007 and 2010. In the samples from China, the SCCPs ranged from < LOD (< 20 μg/kg fat) to 54 μg/kg fat. In these samples, the C13 homologues were the ones most frequently detected, and mainly the Cl7 and Cl8 substituted ones. The Korean and Japanese samples showed levels < LOD, and the authors reported that in the samples that showed trace levels, the C11 and C12 homologues were the most frequently detected, respectively.
Xia et al. (2017a) analysed pooled human milk samples from urban areas from 28 Chinese provinces in 2007 and in 2011. Median concentrations of SCCPs were 681 and 733 μg/kg fat in 2007 and 2011, respectively, while the median levels of MCCPs were 60.4 and 64.3 μg/kg fat, respectively. The authors reported variations of more than 2 orders of magnitude in the SCCP and MCCP levels between the different provinces, although the congener group profiles were similar among all samples. The most abundant SCCP congener groups were C10 (47%) and C11 (31%), while for MCCPs it was C14 that accounted for around 70% of the total. The Cl6 and Cl7 congeners dominated the SCCP profile, while the Cl7 and Cl8 dominated the MCCP profile.
The same authors reported the concentrations of SCCPs and MCCPs in pooled human milk samples this time from rural areas from 24 Chinese provinces, for the same time frame 2007 and 2011 (Xia et al., 2017b). The levels were lower than those reported in the urban areas, with median SCCP and MCCP concentrations in 2007 of 303 and 35.7 μg/kg fat, respectively, and in 2011 of 360 and 45.4 μg/kg fat. The most abundant congener groups in the rural areas were similar to those reported for the urban areas, with the C10 and C11 congener groups being the most abundant ones for SCCPs (around 50% and 28% of the total, respectively) and the Cl6 and Cl7 congeners dominating the profile. For the MCCPs, the C14 congener group was the most abundant (around 80%), as well as the Cl7 and Cl8 congeners. In both studies the levels reported in 2011 were higher than those in 2007.
Yang et al. (2018) analysed 86 human milk samples from 55 healthy Chinese mothers during different nursing periods in 2014 and 2015. The samples were collected in the Hebei province, a CP‐manufacturing area. The median (range) SCCP level was 1,460 (210–16,200) μg/kg fat. The authors reported a decrease in the levels according to the nursing period, with the highest levels at childbirth (median: 770 μg/kg fat) followed by those collected at later periods (370, 140 and 170 μg/kg fat at 1, 3 and 6 months, respectively). The most abundant SCCP congener group was C12 (34.9%) followed by C10 (29.1%) and C11 (28.8%). This pattern was constant throughout nursing periods. However, differences were observed between nursing periods on the chlorination degree: the proportion of lower chlorinated congener groups (Cl6 and Cl7) decreased from childbirth to 3 months, while those with Cl8 increased. The relevance of age, body weight, place of residence, occupation and lipid content of the human milk samples, as potential factors affecting the concentrations of SCCPs in human milk, was also analysed. The authors reported that women working indoors had higher levels than those who worked outdoors, and that no differences in SCCP concentrations were observed between the urban and rural residents (most residences were near the central city area). It was also reported that no significant linear relationships existed between the concentration of SCCPs and age, body weight before pregnancy, or lipid content. However, significant linear relationships were reported between SCCP levels and body weight or body mass index at the end of pregnancy. A positive linear relationship was reported between the concentrations of most individual SCCP congeners and body mass index at the end of pregnancy. The authors concluded that increased body weight during pregnancy is a key factor in the accumulation of SCCPs in human milk, and that the internal exposure to SCCPs is higher in obese people than in the general population. In addition, a significant positive linear correlation was reported between the number of carbon atoms and the body mass index–concentration slope. The authors also suggested based on the data that the bioaccumulation in human milk of SCCP congeners with Cl7 or Cl8 is most likely to be affected by BMI.
3.2.4.2. Blood
Four studies reporting the levels of CPs in blood were identified, with samples collected in China and Australia. Details are given in Table 31 .
Li et al. (2017) determined SCCPs, MCCPs and LCCPs in 50 human blood samples collected in 2012 from an urban area in China. The mean (range) levels were 3,500 (370–35,000), 740 (130–3,200) and 150 (22–530) μg/kg fat for SCCPs, MCCPs and LCCPs, respectively. The SCCP profile in the blood samples was dominated by the C13 congeners (59%) with a major contribution of the Cl7 to Cl9 congeners. For MCCPs, C14 (42%) and C15 (23%) congeners were dominant with major contributions from Cl8 to Cl10 congeners. The LCCP profile was dominated by C18 (28%) and C19 (15%) congeners with major contributions of the Cl8 and Cl9 congeners.
Xu et al. (2019) analysed SCCPs in 50 plasma samples from the general adult population in China. SCCPs were detected in all but one plasma samples, and the mean (range) SCCP concentrations were 32.0 (< LOD–203) μg/kg ww. The C10 and C11 homologues dominated the congener profile, with major contribution from the Cl6 and Cl7 congeners.
Qiao et al. (2018) reported the levels of SCCPs and MCCPs in 21 paired samples of maternal and umbilical cord blood serum collected in China (Beijing) in 2013. The SCCP concentrations in maternal serum ranged from 2,570 to 57,700 μg/kg fat, while MCCPs ranged from 478 to 6,400 μg/kg fat. For SCCPs, the C10 homologues were the most abundant ones (77.3–85.6%), with the Cl6 and Cl7 congeners dominating the profile. For MCCPs, C14 was the major carbon chain length (54.1–62.4%), dominated by the Cl7 and Cl8 congeners. In cord blood, the levels ranged from 3,750 to 40,500 μg/kg fat for SCCPs, and from 522 to 4,600 μg/kg fat for MCCPs. As in maternal serum, the C10 homologues were the predominant ones.
Van Mourik (2018) analysed SCCPs, MCCPs and LCCPs in serum samples of Australian adult males covering the period 2004–2015. Five time points were used to investigate the time trend of CP levels (2004–2005, 2008–2009, 2010–2011, 2012–2013 and 2014–2015), and for the time point 2012–2013 pools were collected for different age groups from 0–4 to 46 years old. Two pools consisting of 100 individual samples each were analysed per time point (except for the time point 2014–2015 where one pool was analysed). LCCPs were below the LOD in all samples, while SCCPs and MCCPs were detected in 4 and 8 pooled samples, respectively. The mean (range) levels of SCCPs and MCCPs were 300 (< LOD–360) μg/kg fat and 610 (< LOD–930) μg/kg fat, respectively, across time points. The authors reported no age trend for SCCPs due to the low number of samples >LOD, while for MCCPs an increased age trend in males above 16 years old was observed, although not significant. Regarding the time trend, SCCPs were < LOD in the pool samples until 2011. Between 2012 and 2015, the SCCP levels were above the LOD. The author indicated this might have been due to a shift to indoor applications of SCCPs causing a higher exposure. For MCCPs, a positive time trend correlation was observed since 2005, explained by the authors as in line with the increasing global production of MCCPs. In all these Australian samples MCCPs showed higher concentrations than SCCPs. A comparison of the congener profile in the serum samples across time points was performed, showing that the abundance pattern in samples collected before 2009 was different from that in samples collected in the later years. The author also compared the congener profile with air samples from Australia, showing also in this case a different abundance pattern in both matrices.
3.2.4.3. Other human tissues
In 1985, Schmid and Muller (1985) determined the levels of CPs in one sample of human adipose tissue. The sample was obtained from a 65‐year‐old male from Switzerland and the concentration was 200 μg/kg adipose tissue.
Campbell and McConnell (1980) determined the levels of total CPs as C10–20 in human post‐mortem organs and tissues from 20 adults, one adolescent, one child and two infants. In fat, kidney, liver and brain the levels ranged from < 0.05–0.54, < 0.05–0.4, < 0.05–1.5 and < 0.05–0.08 ppm (i.e. mg/kg) ww, respectively. CPs as C20–30 showed concentrations below the LOD in most of the samples.
In 2016, Wang et al. (2018c) analysed 54 human placentas collected from a hospital in China (Henan province). SCCPs were detected in all 54 samples, while MCCPs were found at detectable levels in 38 of the placentas. Mean (range) SCCP concentrations were 593 (98.5–3,771) μg/kg fat, and MCCPs were 316 (80.8–954) μg/kg fat. For SCCPs, C10 and C11 were the major carbon chain lengths (59 and 36%, respectively), dominated by the Cl6 (39%) and Cl7 (52%) congeners. For MCCPs, C15 was the major carbon chain length, dominated by Cl7. The authors reported that C15H25Cl7 accounted for approximately 80% of the total MCCP content, followed by C14Cl7–8.
3.2.4.4. Conclusions on the levels in human samples
Comparisons between studies should be done carefully due to the differences in and reliability of methods used for the determination of the CPs. From the few available studies in European countries, the median SCCPs levels in human milk samples ranged from 15.9 to 180 μg/kg fat, with maximum values reported at 820 μg/kg fat. MCCPs levels in those studies were lower, with median values ranging from 14 to 115.4 μg/kg fat, with maximum values of 903 μg/kg fat.
These levels were lower than those reported in human milk samples from Asia (mainly China), in which the median levels ranged from 303 to 1,460 μg/kg fat with maximum reported values of 16,200 μg/kg fat. MCCPs showed again lower levels, with median concentrations ranging from 35.7 to 64.3 μg/kg fat, with maximum values of 1,501 ng/g fat. As for the food samples, there are some differences in reports of the most dominant congeners within each group, which may reflect the contaminant profile within the samples, or may reflect the analytical methodology used.
Levels in blood samples were only identified for three Chinese and one Australian studies. In China, SCCP levels ranged from 370 to 57,700 μg/kg fat (median values of 3,500 μg/kg fat in one study). In the Australian samples the levels were lower, with a median of 300 μg/kg fat. For MCCPs, the levels in the Chinese samples ranged from 13 to 6,400 μg/kg fat while in the Australian samples, levels up to 930 μg/kg fat were found (median of 610 μg/kg fat). In the Chinese samples the levels of SCCPs were higher than those of MCCPs, while in the Australian samples MCCPs showed higher levels than those of SCCPs.
3.3. Dietary exposure assessment
3.3.1. Current dietary exposure assessment for humans
3.3.1.1. Mean and high dietary exposure
Chronic dietary exposure resulting from ‘Fish meat’ consumption was estimated for the consumers only population across Europe following the methodology described in Section 2.5 . A total of 38 dietary surveys, carried out in 22 different Member States, were selected for this assessment. These dietary surveys and the number of subjects available per age class are described in Annex A (Tables 2, 3, 4).
Two exposure assessments were carried out for SCCPs and for MCCPs. The mean and 95th percentile of chronic exposure estimates at the LB and UB, obtained for different age groups, are shown in Tables 33 and 34 , respectively. Detailed mean and 95th percentile dietary exposure estimates calculated for each dietary survey are presented in Annex A (Tables 2, 3).
Table 33.
Mean and 95th percentile estimates of chronic exposure resulting from fish consumption (for fish consumers only) at the LB and UB for SCCPs
| Age classa | Number of surveys | Mean dietary exposure (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||||
| LB | UB | LB | UB | LB | UB | ||
| Infants | 11 | 4.7 | 4.7 | 13 | 13 | 33 | 34 |
| Toddlers | 14 | 3.2 | 3.2 | 13 | 13 | 34 | 35 |
| Other children | 19 | 4.0 | 4.1 | 12 | 12 | 19 | 19 |
| Adolescents | 18 | 2.0 | 2.0 | 6.6 | 6.6 | 12 | 12 |
| Adults | 19 | 1.9 | 1.9 | 6.1 | 6.1 | 8.2 | 8.3 |
| Elderly | 18 | 2.2 | 2.2 | 6.5 | 6.5 | 8.1 | 8.1 |
| Very elderly | 14 | 3.3 | 3.3 | 6.3 | 6.4 | 14 | 15 |
| Age class a | Number of surveys | 95th percentile dietary exposure (ng/kg bw per day) b | |||||
| Minimum | Median | Maximum | |||||
| LB | UB | LB | UB | LB | UB | ||
| Infants | 5 | 10 | 10 | 25 | 25 | 77 | 77 |
| Toddlers | 8 | 9.8 | 9.8 | 32 | 32 | 86 | 87 |
| Other children | 17 | 11 | 12 | 28 | 28 | 52 | 52 |
| Adolescents | 15 | 5.8 | 5.9 | 16 | 16 | 33 | 33 |
| Adults | 18 | 4.9 | 5.0 | 15 | 16 | 20 | 21 |
| Elderly | 14 | 5.6 | 5.6 | 16 | 16 | 20 | 20 |
| Very elderly | 7 | 10 | 10 | 16 | 16 | 17 | 17 |
SCCPs: short‐chain chlorinated paraffins; bw: body weight; LB: lower bound; SCCPs: short‐chain chlorinated paraffins; UB: upper bound.
Section 2.3 describes the age range within each age class.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
Table 34.
Mean and 95th percentile estimates of chronic exposure resulting from fish consumption (for fish consumers only) at the LB and UB for MCCPs
| Age classa | Number of surveys | Mean dietary exposure (ng/kg bw per day) | |||||
|---|---|---|---|---|---|---|---|
| Minimum | Median | Maximum | |||||
| LB | UB | LB | UB | LB | UB | ||
| Infants | 11 | 8.0 | 8.0 | 22 | 22 | 57 | 57 |
| Toddlers | 14 | 5.5 | 5.5 | 22 | 22 | 59 | 59 |
| Other children | 19 | 6.9 | 6.9 | 21 | 21 | 33 | 33 |
| Adolescents | 18 | 3.4 | 3.4 | 11 | 11 | 20 | 20 |
| Adults | 19 | 3.2 | 3.2 | 10 | 10 | 14 | 14 |
| Elderly | 18 | 3.8 | 3.8 | 11 | 11 | 14 | 14 |
| Very elderly | 14 | 5.6 | 5.6 | 11 | 11 | 25 | 25 |
| Age class a | Number of surveys | 95th percentile dietary exposure (ng/kg bw per day) b | |||||
| Minimum | Median | Maximum | |||||
| LB | UB | LB | UB | LB | UB | ||
| Infants | 5 | 18 | 18 | 42 | 43 | 132 | 132 |
| Toddlers | 8 | 17 | 17 | 54 | 55 | 147 | 148 |
| Other children | 17 | 19 | 20 | 47 | 48 | 89 | 89 |
| Adolescents | 15 | 10 | 10 | 27 | 28 | 57 | 57 |
| Adults | 18 | 8.5 | 8.5 | 26 | 27 | 35 | 35 |
| Elderly | 14 | 9.5 | 9.6 | 27 | 27 | 34 | 34 |
| Very elderly | 7 | 17 | 17 | 27 | 27 | 29 | 29 |
MCCPs: medium‐chain chlorinated paraffins; bw: body weight; LB: lower bound; MCCPs: medium‐chain chlorinated paraffins; UB: upper bound.
Section 2.3 describes the age range within each age class.
The 95th percentile estimates obtained on dietary surveys/age classes with less than 60 observations may not be statistically robust (EFSA, 2011a). Those estimates were not included in this table.
For SCCPs, the mean UB exposure ranged from 1.9 to 35 ng/kg bw per day across dietary surveys and age groups. At the 95th percentile exposure, the UB estimates ranged from 5.0 to 87 ng/kg bw per day. For MCCPs, the mean UB exposure ranged from 3.2 to 59 ng/kg bw per day. At the 95th percentile exposure, the UB estimates ranged from 8.5 to 148 ng/kg bw per day. The lowest exposures were found in the Adult groups while the highest were for Toddlers.
3.3.1.2. Exposure of infants through breastfeeding
For the exposure assessment of breastfed infants under 6 months of age, a median age of three months was selected, equivalent to a body weight of about 6.1 kg, with an estimated average daily milk consumption of about 800 mL and a high consumption of 1,200 mL, each with a mean fat content of 3.5%.
The occurrence levels were taken from the information provided to EFSA on the levels in human milk pools collected from 11 European countries between 2014 and 2016 and analysed within the WHO/UNEP Coordinated Survey of Human Milk for POPs (see Section 3.2.4 ).
The exposure to SCCPs for breastfed infants with average and high human milk consumption was estimated to range from 60 to 445 ng/kg bw per day, and from 90 to 668 ng/kg bw per day, respectively. The exposure to MCCPs for breastfed infants with average and high human milk consumption was estimated to range from < 25 to 514 ng/kg bw per day, and from < 38 to 771 ng/kg bw per day, respectively.49
The Panel noted that since these were pooled samples, it was not possible to estimate specific values for highly exposed individuals.
3.3.2. Previously reported dietary exposure assessments
Some international bodies have estimated the dietary exposure to CPs while performing their risk assessments related to the presence of CPs in food and the environment (see Section 1.3.5 ). Other studies have described occurrence data in different foodstuff (see Section 3.2.2.1 ) or in breast milk samples (see Section 3.2.4 ) and have then estimated the exposure for the adult population or breastfed infants, respectively.
A summary of these studies is presented below and in Tables 35 (dietary exposure) and 36 (breastfeeding) for SCCPs, MCCPs and LCCPs, respectively. Interpretation and comparison between these exposure assessments should be done with care due to different sampling methodologies, the different consumption habits between countries, and due to the differences in and reliability of the methods used for the determination of the CPs. Levels reported in the studies have been expressed as ng/kg bw per day for comparison.
Table 35.
Dietary exposure assessments to chlorinated paraffins reported in the literature
| Reference | Country | Sample type Number of samples | Year | Analytical method | Estimate of exposure (ng/kg bw per day) | Main contributors to the exposurea | ||
|---|---|---|---|---|---|---|---|---|
| SCCPs | MCCPs | LCCPs | ||||||
| EUROPEAN COUNTRIES | ||||||||
| EU‐RAR (2000) | As reported by Campbell and McConnell (1980) and other reports | Butter, margarine, cheese, beef meat, sheep offals, oils of plant and animal origin, coffee, tea, beer, wine, fruitsa | 1977–1978 |
Thin‐layer chromatography with argentation |
Adults: 20,000 | – | – | Fish, shellfish |
| EU‐RAR (2011) | Europe | Cows’ milk (from Thomas and Jones, 2002) | n.r. | n.r. | – |
Regional sources: 130 Highest local: 16,000 Infants fed cow milk: 145 |
– | – |
| UK‐COT (2009)c | UK | Food (n = 45): fish, butter, pork sausage, bread, fruit, beef, lamb, milk products, eggs, poultry, cod liver oil | 2007 | GC‐HRMS |
97.5th percentile consumers: 4–6 years old: 290 Adults: 120 |
97.5th percentile consumer 4–6 years old: 1,600 Adults: 630 |
– | – |
| Yuan et al. (2017) | Sweden | Food market basket study | 2015 | APCI‐qTOF‐MS |
Adults: 18 |
Adults: 39 |
Adults: 2.0 |
Sugar/sweets, dairy products, fats/oil, fishb |
| Krätschmer (2019a, see Documentation provided to EFSA) | Germany |
Meat and offals, eggs, milk and dairy, fish, fats and oils Full meals (meat based and vegetarian) Total diet samples |
2018–2019 | GC‐ECNI‐Orbitrap‐HRMS |
Total CPs Adults: 240–860 Full meals: Meat based: 4.3–22 Vegetarian: 2.4–3.5 Total diet samples: 47–190 |
Processed vegetable oils | ||
| Krätschmer et al. (2019) | Germany | Fish (salmon) | 2018 | GC‐Orbitrap‐MS |
Adult females: 1.22–19.1 ng/kg bw/week Adult males: 1.98–20.3 ng/kg bw/week |
Adult females: 1.72–15.9 ng/kg bw/week Adults males: 2.79–16.8 ng/kg bw/week |
– | – |
| Sprengel et al. (2019) | Germany (available from the German online market) | Oil‐based supplements: vitamin E supplements, marine oil supplements, other oil supplements | n.r. | GC‐ECNI‐MS |
Adults: 77.7 |
Adults: 537 |
– | – |
| OTHER COUNTRIES | ||||||||
| Health Canada (2008) | As reported by Campbell and McConnell (1980) and other studies | Based on concentrations in food reported in the literature for dairy, fats, fruits, vegetables, cereal products, meat and poultry, fish, eggs, sugar, soft drinks, alcohol, coffee, tea | 1977–1978 | Liquid–solid adsorption chromatography |
Infants Formula fed: 10 Non‐formula fed: 25,970 0.5–4 years old: 24,260 5–11 years old: 16,440 12–19 years old: 9,020 20–59 years old: 7,180 > 60 years old: 5,140 |
Infants Formula fed: 50 Non‐formula fed: 25,480 0.5–4 years old: 18,480 5–11 years old: 11,640 12–19 years old: 6,300 20–59 years old: 4,690 > 60 years old: 3,470 |
– | – |
| Health Canada (2012) | – |
Farfield Human Exposure (FHX) model to calculate steady‐state concentrations in the multi‐media compartments including various food groups, including fruits, vegetables, milk, dairy, fish and meats based on the unit emission rate |
– | – | – | – |
C18H37Cl1 (UB estimates): >60 years old: 7 0.5–4 years old: 24 C18H22Cl16 and C18H30Cl8 : an order of magnitude lower than C18H37Cl1 C>20: >60 years old: 13 0.5–4 years old: 40 |
– |
| Iino et al. (2005) | Japan | Market basket study (11 food categories: grain crops, seeds/potatoes, sugar, fats, vegetables, fruit, fish, shellfish, meat, eggs, milk) | 2003 | GC‐ECNI‐HRMS |
Range of median intakes 1 year old: 340–370 5–15 years old: 120–200 20–60 years old: 110–120 >60 years old: 89–97 |
– | – | Fats, fish, shellfish |
| Harada et al. (2011) |
China Korea Japan |
24 h food composite samples China: 10 adults Korea: 10 adults Japan: 40 adults |
1990s, 2007–2009 | GC‐ECNI‐HRMS |
Range of intakes China 1993: n.d.–36 2009: 390–1,000 Japan 1990s: n.d.–76 2009: n.d–93 Korea 1994: ND 2007: ND–50 |
– | – | – |
| Huang et al. (2018) | China | Meat and meat products from 20 Chinese provinces | 2011 | GC×GC‐ECNI‐TOF |
Median (range) 130 (20–560) |
Median (range) 4.7 (3–31) |
– | – |
| Cao et al. (2015) | China |
Cooking oil (n = 49) Fried confectionery products (n = 20) |
2010–2012 | GC‐ECNI‐HRMS |
Mean (range) Cooking oil: < 780 to 38,000 ng/day Fried confectionery products: 590 to 49,700 ng/day |
– | – | – |
| Chen et al. (2018) |
China (e‐waste recycling industrial park and its surrounding villages) |
Locally produced staple food samples (n = 92) | 2016–2017 | GC/ECNI‐LRMS |
Median: Adults: 15,400 Children: 34,100 |
Median: Adults: 19,500 Children: 43,300 |
– | Vegetables, Cereals, Fish |
| Gao et al. (2018) | China | Duplicate Diet study | 2016 | GC‐TOF‐HRMS |
Mean (range) Adults: 611 (316–1,101) |
mean (range) Toddlers: 705 (164–1,465) Adults: n.r. (153–1,307) |
– | – |
| Gao et al. (2019) | China |
122 food samples 7 food samples cooked (duplicate food approach) |
2014–2016 | GC‐ECNI‐LRMS |
Estimate without cooking oil Mean Males: 221 Females 227 P95 Males: 747 Females: 725 Estimate with cooking oil: Mean Males: 1,106 Females: 1,136 P95 Males: 3,544 Females: 3,594 Duplicate food approach (cooked food): 145 |
– | – | |
| Zeng et al. (2018) | China (collection area used to be an e‐waste site) |
Home‐produced eggs (n = 68): year 2013 (n = 38), year 2016 (n = 30) |
2013, 2016 | GC‐ECNI‐MS |
Mean: Adults: Year 2013: 65.4 Year 2016: 274 Children: Year 2013: 281 Year 2016: 1,180 |
Mean: Adults: Year 2013: 151 Year 2016: 193 Children: Year 2013: 651 Year 2016: 829 |
– | – |
| Wang et al. (2019b) | China |
Cereals and legumes (n = 19 pool samples each) |
2011 | GC×GC‐TOF |
Male adult: Cereals: 5,185 Legumes: 529 |
Adult male: Cereals: 3,093 Legumes: 295 |
– | – |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins; n.r.: not reported; GC: gas chromatography; MS: mass spectrometry; HRMS: high‐resolution MS; ECNI: electron negative chemical ionisation; TOF‐MS: time‐of‐flight MS; LCCPs: long‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; SCCPs: short‐chain chlorinated paraffins; bw: body weight.
For studies with several food categories analysed and when the information was available from the study.
The authors noted that the contribution from the dairy group is entirely based on values below LOQ (Yuan et al., 2017).
The authors noted that since the concentrations were measured and reported in a small number of samples, they may not be representative of these foods and it is not possible to calculate a reliable estimate of UK dietary exposure. The authors estimated an extreme worst‐case basis by considering that all solid food consumed by an adult (1.5 kg per day for the 97.5th percentile consumer) contained SCCPs or MCCPs at the levels determined in freshwater eel (UK‐COT, 2009).
Table 36.
Exposure to chlorinated paraffins for breastfed infants reported in the literature
| Reference | Country | Number of samples | Year | Analytical method | Estimate of exposure (ng/kg bw per day) | ||
|---|---|---|---|---|---|---|---|
| SCCPs | MCCPs | LCCPs | |||||
| EUROPEAN COUNTRIES | |||||||
| EU‐RAR (2011) | Europe | Breast milk (from Thomas et al., 2003) | n.r. | n.r. | – | 350 | – |
| OTHER COUNTRIES | |||||||
| Health Canada (2008) | As reported by Tomy (1997) | Breast milk | 1977–1978 | Liquid–solid adsorption chromatography | 1,700 | – | – |
| Xia et al. (2017b) | China |
Breast milk 24 pooled samples (1,412 individual samples) |
2007, 2011 | GC×GC‐ECNI‐TOF‐MS |
Median (range) Year 2007: 1,310 (357–6,320) Year 2011: 1,520 (344–8,650) |
Median (range) Year 2007: 152 (43.0–554) Year 2011: 212 (43.1–450) |
– |
| Cao et al. (2017) |
China Korea Japan |
Breast milk, pooled samples China (n = 17) Korea (n = 16) Japan (n = 44) |
2007–2010 | GC‐ECNI‐HRMS |
Mean (max) China: 337 (468) Korea: 242 (246) Japan: 247 (290) |
– | – |
| Yang et al. (2018) | China |
Breast milk (n = 86) |
2014–2015 | GC–ECNI‐MS |
1‐month infant: 13,000 3‐month infant: 7,100 6‐month infant: 2,500 |
– | – |
SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; LCCPs: long‐chain chlorinated paraffins; n.r.: not reported; GC: gas chromatography; MS: mass spectrometry; HRMS: high‐resolution MS; ECNI: electron negative chemical ionisation; TOF‐MS: time‐of‐flight MS; LCCPs: long‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; SCCPs: short‐chain chlorinated paraffins; bw: body weight.
No studies were identified in the literature that estimated the exposure to CPs for farm animals, horses and companion animals.
3.3.2.1. SCCPs
The EU‐RAR (2000) estimated the daily human intake of SCCPs from various sources using the predictive EUSES (EU System for the Evaluation of Substances) model to estimate concentrations in various food, air and drinking water. However, uncertainty was reported in the concentrations of SCCPs estimated by this model in the different matrices. Based on data reported in published studies, e.g. from Campbell and McConnell (1980), a maximum human intake from food of 20,000 ng/kg bw per day (assuming a 70‐kg bw person and 100% absorption via the oral route) was estimated. The major contributor was fish and shellfish. In its updated risk assessment report of 2008 in relation to human health (EU‐RAR, 2008), exposure of infants via breast milk and cow's milk was considered. Intake estimates were not provided in the updated report on SCCPs, but it is mentioned that the exposure via breast milk and cow's milk for SCCPs was estimated as it was done for MCCPs (EU‐RAR, 2011). Regarding breast milk, it is mentioned that the 95th percentile level of 680 μg/kg fat from a very well conducted survey was used for the risk characterisation. Regarding cow's milk, a concentration of 16 μg/kg fat is mentioned as the worst‐case estimate to be used for the risk characterisation.
Health Canada (2008) estimated the exposure of the Canadian population divided in six age groups, from 0 to 60 + years old, considering uptake via ambient air, indoor air, drinking water, food and soil based on measured concentrations from published studies. For all age groups, food was the major source contributing virtually to 100% of the total intake. For breastfed infants, an intake of 1,700 ng/kg bw per day was estimated, based on the levels in human milk reported by Tomy (1997) and assuming a consumption of 0.75 kg of human milk per day. For formula fed infants, the intake was estimated at 10 ng/kg bw per day, assuming a consumption on 0.8 L per day of reconstituted formula. The intake estimated for the remaining age groups ranged from 5,140 ng/kg bw per day for adults > 60 years of age, to 25,970 ng/kg bw per day for infants who were not formula fed (i.e. those being introduced solid foods) (Health Canada, 2008).
UK‐COT (2009) analysed the levels of SCCPs in various food samples in 2007 on sale in the UK market. Due to the limited number of samples analysed, the authors estimated an extreme worst case as if all solid food consumed by an adult contained SCCPs at the level of 6.0 μg/kg, the highest level determined in a sample of freshwater eel. The exposure was estimated at 120 ng/kg bw per day for the 97.5th percentile adult consumer. For the age group 4–6 years old, the exposure was estimated at 290 ng/kg bw per day for the 97.5th percentile child.
Yuan et al. (2017) measured the levels of SCCPs (and MCCPs and LCCPs) in Swedish food market baskets. The mean dietary intake of SCCPs from all food groups considered for Swedish consumers was 18 ng/kg bw per day (based on a mean body weight of 76.6 kg).
Krätschmer et al. (2019) estimated the exposure of SCCPs (and MCCPs) via the consumption of salmon only available at German supermarkets (see Section 3.2.2.1 for details of the study). Based on a fish and seafood consumption of 2.5–5 g per day in Germany, the SCCP exposure ranged from 1.22–19.1 and from 1.98–20.3 ng/kg bw per week for adult females and males, respectively (that would correspond on a daily basis to 0.17–2.7 and 0.28–2.9 ng/kg bw per day, respectively).
Sprengel et al. (2019) also estimated the exposure to CPs from the ingestion of oil‐based dietary supplements available from the German online market assuming a consumption according to label (1–4 capsules of different size per day) and based on the concentration of the palm‐oil based supplements that contained the highest CP levels (see Section 3.2.2 ). The authors estimated an SCCP intake of 5.5 μg/day, corresponding to 77.7 ng/kg bw per day considering a 70.8 kg bw. The CONTAM Panel noted that exposure from these dietary supplements could be in the same order of magnitude as from the consumption of fish meat (see Section 3.3.1 ).
Several studies have estimated the dietary intake of SCCPs in Japan, China and Korea and are summarised below. Details on the occurrence of SCCPs in these studies are provided in Section 3.2.2.1 (for food) or Section 3.2.4 (for human milk).
In a Market Basket Study in Japan (Iino et al., 2005), the median intakes were higher for infants (1‐year‐old males, 340–370 ng/kg bw per day), than for children/adolescents (5–15 years old, 120–200 ng/kg bw per day) and adults (20–60 years old, 110–120 ng/kg bw per day). In adults > 60 years old the exposure was estimated to be the lowest, at 89–97 ng/kg bw per day. Congeners C10 and C11 were the most abundant ones, as well as the Cl6 to Cl8 congeners. The categories ‘fats’, ‘fish’ and ‘shellfish’ were the highest contributors to the dietary intake.
Harada et al. (2011) estimated the exposure to SCCPs (C10–C13) via duplicate 24 h food composite samples from Chinese, Japanese and Korean adults collected in the 1990s (archived samples) and between 2007 and 2009. The estimated daily intake (in ng/kg bw per day) in Beijing was much higher in 2009 (range: 390–1,000) than in 1993 (range: ND–36). In Japan, the estimated intakes were lower than those in Beijing, and were similar between the two time periods studied (ND–76 vs. ND–93, in the 1990s and in 2009, respectively). In Korea, SCCPs were not detected in the 1994 samples, and in 2009 the exposure was reported to range from ND to 50 ng/kg bw per day (based on detection in one sample).
Gao et al. (2018) estimated the SCCP exposure of the general population in Beijing (China) through a duplicate diet study. The mean (range) exposure for adults was 611 (316–1,101) ng/kg bw per day. The same authors estimated the dietary intake of SCCPs by the Chinese population based on the analysis of 122 food samples from markets in Beijing (China) collected between 2014 and 2016 (Gao et al., 2019). The mean dietary exposure, excluding the oil samples, were 221 to 227 ng/kg bw per day for adult male and females, respectively. The 95th percentile intakes were 747 and 725 ng/kg bw per day, respectively. When the oil samples were included, the mean intake raised to 1,106 and 1,136 ng/kg bw per day and the 95th percentile intake from 3,544 and 3,594 ng/kg bw per day, for males and females, respectively. In a second analysis, the authors estimated the dietary intake based on the occurrence data from seven foodstuff that had been previously cooked (see Section 3.2.3 for further details). The dietary exposure was estimated to be 145 ng/kg bw per day, lower than that estimated using the raw food concentrations.
Huang et al. (2018) assessed the dietary exposure to SCCPs for the general Chinese population through meat and meat products that were prepared according to local cooking methods, steaming, frying, boiling and pan‐frying before analysis and collected in 2011 from 20 Chinese provinces. The exposure was estimated considering the data on meat consumption of a standard Chinese adult male (average body weight of 63 kg). The intake estimates ranged from 20 to 560 ng/kg bw per day across provinces.
Dietary exposure to SCCPs from Chinese cooking oil and fried confectionery products was estimated by Cao et al. (2015). The estimated SCCP dietary intakes from the cooking oil ranged from < 780 to 38,000 ng per day. The estimated mean dietary intake of SCCPs was 14,800 ng per day for residents of Beijing. The estimated dietary intakes in fried confectionery ranged from 590 to 49,700 ng per day. The authors concluded that cooking oil might be one of the main sources of SCCPs in the Chinese diet (Cao et al., 2015).
The estimated intakes of SCCPs (and MCCPs) by adults and children via the consumption of home‐produced chicken eggs in two time periods from an area that was once an e‐wasted site was reported by Zeng et al. (2018). Mean intakes of SCCPs for adults were 65.4 (year 2013) and 274 (years 2016) ng/kg bw per day. For children, higher estimates were reported, of 281 (years 2013) and 1,180 (year 2016) ng/kg bw per day.
Wang et al. (2019b) estimated the SCCP dietary intake from cereals and legumes to be 5,185 and 529 ng/kg bw per day, respectively, for an adult Chinese male.
Chen et al. (2018) estimated the dietary intake of SCCPs based on the analysis of several locally produced foods collected from an e‐waste recycling industrial park and its surrounding villages in China. For adult local residents, the median dietary intake was estimated at 15,400 ng/kg bw per day, while in children it was 34,100 ng/kg bw per day. The main contributors to the dietary intake were vegetables, fish and rice, followed by poultry and pork meat.
Three studies estimated the lactational exposure to SCCPs in infants from China, Korea and Japan.
Xia et al. (2017b) estimated the intake based on the levels in human milk samples from China collected in 2007 and in 2011. The authors assumed an average breast milk intake of 750 mL per day and a body weight of 6 kg. The median (range) estimated daily intake (in ng/kg bw per day) was 1,310 (357–6,320) in 2007 and 1,520 (344–8,650) in 2011.
Lower mean intakes were reported by Cao et al. (2017), that estimated the intake based on human milk samples from China, Korea and Japan collected the same years. The authors assumed a human milk consumption of 600 g per day and a body weight of 7.3 kg for a 1‐year‐old infant. In Japan and Korea, the mean exposure via breast milk was similar (247 and 242 ng/kg bw per day, respectively), while in China it was higher (337 ng/kg bw per day).
Higher estimates were reported by Yang et al. (2018), that estimated the breastfed infant SCCP intake based on the analysis of 86 human milk samples collected at different nursing periods in the period 2014 and 2015 from a CP‐manufacturing area in China. The mean intakes were 13,000, 7,100 and 2,500 ng/kg bw per day for infants at 1, 3 and 6 months of age, respectively (considering a body weight of 4.9, 7.37 and 8.41 kg, and intakes of 673, 702 and 756 mL human milk per day, respectively).
3.3.2.2. MCCPs
The EU‐RAR (2011) estimated the exposure to MCCPs. The total daily human exposure from regional sources (most relevant for population exposure estimates) was estimated to be 130 ng/kg bw per day, and the highest local daily human exposure was estimated to be 16,000 ng/kg bw per day. For breastfed infants, a daily intake for the first three months of life was estimated at 305 ng/kg bw per day. Based on levels in cow's milk reported in the literature, an exposure of 145 ng/kg bw per day was estimated for infants fed cow's milk.
As for SCCPs, Health Canada (2008) also estimated the exposure to MCCPs from different sources. In this case, exposure from ambient or indoor air was not considered since concentration in those matrices in Canada or elsewhere were not identified at the time of the report. As for SCCPs, food was virtually the major source of the total intake. No estimate was reported for breastfed infants, while for formula fed infants an intake of 50 ng/kg bw per day was estimated. The largest total exposure was found for non‐formula fed infants (25,480 ng/kg bw per day), while the lowest was for adults above 60 years (3,470 ng/kg bw per day).
UK‐COT (2009) analysed the levels of MCCPs in various food samples in 2007 on sale in the UK market. Due to the limited number of samples analysed, the authors estimated an extreme worst case as if all solid food consumed by an adult contained MCCPs at the level of 31.9 μg/kg, the highest level determined in freshwater eel samples. The exposure was estimated at 630 ng/kg bw per day for the 97.5th percentile adult consumer. For the age group 4–6 years old, the exposure was estimated at 1,600 ng/kg bw per day for the 97.5th percentile child.
Yuan et al. (2017) measured the levels of MCCPs in Swedish food market baskets and estimated the exposure to compare it then to the estimated exposure from hand blender use (see also Section 3.2.3 ). The mean dietary intakes of MCCPs from all food groups considered for Swedish consumers was 39 ng/kg bw per day (based on a mean body weight of 76.6 kg).
Krätschmer et al. (2019) estimated the exposure of MCCPs via the consumption of salmon available at German supermarkets to range from 1.72–15.9 and from 2.79–16.8 ng/kg bw per week for adult females and males, respectively.
Sprengel et al. (2019) also estimated the exposure to CPs from the ingestion of oil‐based dietary supplements assuming a consumption according to label (1–4 capsules of different size per day) and based on the concentration of the palm‐oil based supplements that contained the highest CP levels (see Section 3.2.2 ). The authors estimated an MCCP intake of 38 μg/day, corresponding to around 537 ng/kg bw per day considering a 70.8 kg bw. The intake of MCCPs was higher than that of SCCPs, and the authors noted that these intakes were on average around 4 times higher or 13 times higher (for SCCPs and MCCPs, respectively) compared to that estimated from market basket studies.
Gao et al. (2018) estimated the MCCP exposure of the general population in Beijing (China) through a duplicate diet study (see Section 3.2.2.2 for the description of the study and details on the occurrence). The exposure levels to MCCPs for adults ranged from 153 to 1,307 ng/kg bw per day, and for toddlers ranged from 164 to 1,465 ng/kg bw per day.
As for SCCPs, Huang et al. (2018) estimated the dietary intake to MCCPs through meat and meat products in China. The intake estimates ranged from 3 to 31 ng/kg bw per day across provinces, with 50th and 95th percentile estimated daily intakes of 4.7 and 31 ng/kg bw per day.
The estimated intakes of MCCPs by adults and children via the consumption of home‐produced chicken eggs in two time periods from an area that was once an e‐wasted site was reported by Zeng et al. (2018). Mean intakes of MCCPs for adults were 151 (year 2013) and 193 (years 2016) ng/kg bw per day. For children, higher estimates were reported, of 651 (year 2013) and 829 (year 2016) ng/kg bw per day.
Chen et al. (2018) also estimated the dietary intake of MCCPs based on the analysis of several locally produced food collected from an e‐waste recycling industrial park and its surrounding villages. For adult local residents, the median dietary intake was estimated at 19,500 ng/kg bw per day, while in children it was 43,300 ng/kg bw per day. The MCCP intakes were higher than those for SCCPs.
Wang et al. (2019b) estimated the MCCP dietary intake of cereals and legumes to be 3,093 and 295 ng/kg bw per day, respectively, for an adult Chinese male.
Regarding lactation exposure, Xia et al. (2017b) also estimated the exposure to MCCPs based on milk samples collected in 2007 and in 2011. The median (range) estimated intake was 152 (43.0–554) ng/kg bw per day in 2007 and 212 (43.1–450) ng/kg bw per day in 2011. Those values were lower than those estimated for SCCPs.
3.3.2.3. LCCPs
Yuan et al. (2017) measured the levels of LCCPs (and SCCPs and MCCPs, see above) in Swedish food market baskets and estimated the exposure to compare it then to the estimated exposure from hand blender use (see also Section 3.2.3 ). The mean dietary intakes of LCCPs from all food groups considered for Swedish consumers was 2.0 ng/kg bw per day.
In 2012, Health Canada performed an update of its previous exposure assessment of the Canadian population to LCCPs (C18H37Cl1, C18H22Cl16 and C18H30Cl8) from different sources (Health Canada, 2008, 2012). Exposure was estimated using a Farfield Human Exposure model (FHX 2008). This model brings together information on chemical partitioning, degradation, environmental fate and transport, and food web bioaccumulation for assessing human age‐class‐specific exposure from chemicals released to the environment, to calculate steady‐state concentrations in, e.g. air, water and soil and various food groups, including fruits, vegetables, milk, dairy, fish and meat based on the unit emission rate. Food was again the major source of the total intake. For C18H37Cl1, no estimate was reported for breastfed infants, while for formula fed infants an intake of 0.000226 ng/kg bw per day was estimated. The largest total exposure was found for infants 0.5–4 years old (24 ng/kg bw per day) while the lowest was for adults above 60 years (7 ng/kg bw per day). The intake estimates for C18H22Cl16 and C18H30Cl8 were reported at an order of magnitude lower than C18H37Cl1. The authors predicted UB intakes for the C>20 type to range from 13 ng/kg bw per day (> 60 years old) to 40 ng/kg bw per day (0.5–4 years old).
3.3.2.4. Total CPs
An estimate of the dietary intake for adults in Germany based on the occurrence data in raw food samples (meat and offal, eggs, milk and dairy, and fats and oils) collected from supermarkets in Germany has been reported by Krätschmer et al. (2019). The exposure to total CPs (sum of SCCPs and MCCPs) based on raw food for adults was 240 or 860 ng/kg bw per day, considering mean and maximum total CP concentrations of each food or sample group, respectively. The highest contributor to the exposure was from processed vegetable oils. For the exposure assessment, the results for mean and maximum total CP contamination level of each food or samples group, and summary statistics from the EFSA Comprehensive Food Consumption Database were used, resulting in conservative estimates. The mean exposure for the general adult population considering the full meals ranged from 4.3 to 22 ng/kg bw per day (meat based) and from 2.4 to 3.5 ng/kg bw per day (vegetarian), although a possible underestimation by using the consumption data referring only to ‘composite food’ for the exposure estimation was reported. The mean exposure from the total diet samples analysed ranged from 47 to 190 ng/kg bw per day.
3.3.2.5. Impact of bioaccessibility on exposure estimates
Cui et al. (2109) suggested that studies that used the total concentrations of SCCPs for exposure estimates without considering their bioaccessibility may overestimate dietary intakes. Factors such as lipid and protein content of the food may have an influence but bioaccessibility was in the range of 33% to 84%, meaning that only about half of the SCCPs in foods are bioavailable.
3.3.2.6. Conclusions on the dietary exposure assessments reported in the literature
The studies available have estimated the intake of CPs via food based on the analysis of specific food categories or via food market basket or duplicate diet studies. The majority of the studies were carried out in Asia and only few were carried out in European countries. Interpretation and comparison between these exposure assessments should be done with care due to different sampling methodologies, different consumption habits between countries, and due to the differences in and reliability of the methods used for the determination of the CPs.
In Europe, from a food market basket study carried out in Sweden, the dietary intake of SCCPs, MCCPs and LCCPs in adults was estimated to be 18, 39 and 2.0 ng/kg bw per day, respectively. From another more recent study in Germany, the total SCCP and MCCPs exposure was estimated to be 240–860 ng/kg bw per day. From a study on dietary supplements available from the German online market, the CONTAM Panel noted that exposure from these products could be in the same order of magnitude as from the consumption of fish meat as estimated by EFSA.
In market basket and duplicate diet studies carried out in China, higher median intakes were reported, ranging from 110 to 1,101 ng/kg bw per day for SCCPs, and from 153 to 1,307 ng/kg bw per day for MCCPs (no data on LCCPs). Thus, the few available studies suggest that MCCPs might contribute more than SCCPs to the dietary intake.
Regarding breastfeeding, studies on SCCPs were only available for China, Japan and Korea. In those studies, mean intakes of 242 ng/kg bw per day were reported from Korean breast milk samples and up to 2,500 ng/kg bw per day from Chinese breast milk samples. For MCCPs, the one study identified in China reported medians of 212 ng/kg bw per day, and the EU‐RAR (2011) estimated a daily intake for the first three months of life at 305 ng/kg bw per day of MCCPs from breast milk.
3.3.3. Non‐dietary sources of exposure
Humans can be exposed to CPs via the environment (from e.g. dust or air), via consumer products treated with CPs, and from the working environment (i.e. occupational exposure).
In occupational exposure, skin contact and inhalation are the major routes of occupational exposure in the production, formulation and use of SCCPs. Bardin et al. (2005) reported health effects in workers in the automobile industry in the United States that had been exposed to metalworking fluids and CPs (see Section 3.1.3 ). Mean exposure to CPs was estimated to be 0.6–2.2 mg/m3.
Non‐occupational exposure can arise from the use of products treated with CPs such as leather, textiles and others, such as metal working fluids available to consumers (EU‐RAR, 2000, see Section 1.3.2 on uses). In addition, the presence of CPs in kitchen dish cloths has been reported by Gallistl et al. (2017) (see Section 3.2.3 ). The authors conducted a dermal uptake assessment to study the relevance of CP skin uptake by dish cloths and daily dermal uptake values were calculated using the concentrations determined in the dish cloth samples studied. The authors assumed even distribution of the contaminants on the dish cloths and a constant level of the contaminants during the whole usage time. The estimated skin uptake of SCCPs and MCCPs due to contact with dish cloths and considering skin absorption factors was 0.07 and 64 ng per day, respectively. For MCCPs, this value corresponded to ~26% of the total daily uptake of all polyhalogenated compounds determined in the study (240 ng per day). The authors concluded that dermal absorption of these compounds by contaminated dish cloths represents a potential uptake route and contributes to the total human exposure, noting that dish cloths are used daily without hand‐protecting gloves.
Cherri and Semple (2010) concluded that dermal and inhalation exposure may be of comparable magnitude for those who are regularly exposed to metal working fluids. The estimated dermal exposure from oil‐based products was 520 mg/day and in a worst‐case scenario 35,000 mg/day. The estimated exposure from using water‐based products was lower (30 and 520 mg/day, respectively).
In addition, environmental (background) exposure due to diffuse emission sources affects the entire general population which may be exposed by several routes, including inhalation of air including particulates in the air as well as intake of house dust. For children, crawling and hand‐to‐mouth habits should be considered as habits possibly increasing the ingestion of dust.
Several studies have estimated the relative contribution of different source of exposure to the total CP intake.
Cao et al. (2019) reported occurrence data and made a human exposure assessment of SCCPs and MCCPs in dusts from plastic sports courts and synthetic turf in Beijing, China. The mean concentration of SCCPs and MCCPs in dusts from plastic tracks were 5,429 and 15,157 μg/g and in basketball courts the mean values were 5,139 and 11,878 μg/g. These concentrations were significantly higher than those found in plastic tennis and badminton courts, and in synthetic turf, and were 1−3 orders of magnitude higher than in dusts from other indoor environments. It was proposed that the friction between the soles of footwear and the plastic track materials may lead to the wear and decomposition of the surface which may be an important source of CPs. The authors concluded that the mean estimated daily intakes of CPs from plastic tracks and basketball courts are generally higher than those estimated from dietary, breast milk, or other indoor dust sources.
Concentrations and specific congener profiles of SCCPs in various environmental matrices, including air, soil and dust, from locations within and nearby a plant producing CPs were analysed to explore SCCP pollution and transportation behaviours (Wang et al., 2018d). SCCP concentrations in air (129–1,442 ng/m3) and soil (28–554 μg/g) found in samples taken inside the facility were higher than those in air (91–333 ng/m3) and soil (102–441 ng/g) samples taken from nearby locations outside the plant. Based on the congener profiles, lighter homologues (C10–11 and Cl5–6) had greater long‐range transport potential, whereas heavier fractions (C12–13 and Cl7–10) were more likely to deposit from the atmosphere. The authors found that large amounts of SCCPs were released from the CP production plant, which resulted in contamination of the environment. The daily occupational exposure of employees in the production facility to SCCPs was estimated as 21.8 μg/kg bw per day).
Concentrations, particle size distributions and inhalation exposure characteristics of CPs were investigated in indoor air particulate matter by Zhou et al. (2019b) (see Section 1.3.3.4 ). The estimated daily CP intakes from 2.5 μm particles by inhalation were 8.1–24.6 and 25.1–76.0 ng/kg bw per day for all age subgroups based on both mean and 95th percentile concentrations of CPs. It was noted that estimated daily uptakes decreased to 4.4–16.4 and 13.5–50.6 ng/kg bw per day, respectively, when the deposition fractions of these particles in the human lung were considered.
Gao et al. (2018) estimated exposure sources for SCCPs and MCCPs for the population in Beijing (China) from four exposure pathways: (i) food (described in Sections 3.2.2.1 and 3.3.2.1 ), (ii) indoor air, (iii) indoor dust and (iv) drinking water. The overall daily intakes of SCCPs and MCCPs for adults were estimated to be 1.01 and 0.83 μg/kg bw per day, respectively and for toddlers 2.31 and 1.32 μg/kg bw per day, respectively. For adults, the predominant exposure source was dietary intake which accounted for 88% for SCCPs and 93% for MCCPs. Indoor dust was the next highest contributor at 9.3% and 6.9% for MCCPs. However, for toddlers, dust ingestion was the predominant exposure route, accounting for 59% for SCCPs and 51% for MCCPs. Dietary intake was the second most relevant source, accounting for 38% for SCCPs and 49% for MCCPs. Exposure via indoor air and drinking water contributed to a minor extent to the overall exposure.
The contribution of dust ingestion and dermal permeation of dust contact to the toddlers could be as high as 70% according to a study in malls in China (Shi et al., 2017).
Chen et al. (2018) determined SCCPs and MCCPs in four types of dust samples and nine categories of locally produced staple foods collected from an e‐waste recycling industrial park and its surrounding villages (see Sections 3.2.2 and 3.3.2 for further details on the food samples). Dust mean concentrations of SCCPs and MCCPs from the surrounding residential homes near the park were 580 and 1,760 μg/g, respectively, which were about 10 times lower than those found in the e‐waste workshops, but higher than those in the control homes (59.0 and 185 μg/g, respectively). For SCCPs dietary and dust intakes in adult local residents were 15.4 and 0.21 μg/kg bw per day, while in children they were 34.1 and 1.79 μg/kg bw per day, respectively. For MCCPs, dietary and dust intakes in adults were 19.5 and 0.54 μg/kg bw per day while in children they were 43.3 and 4.64 μg/kg bw per day. For both adults and children local residents dietary intake was the predominant exposure route over dust ingestion (and dermal absorption).
CPs were measured in Canadian house dust by Shang et al. (2019). A comparison was made between CP concentrations in paired dust samples collected using two different methods from the same homes: fresh or ‘active’ dust (FD) collected by technicians and a sample taken from the household vacuum cleaner (HD). There was a significant positive correlation (p < 0.01) between FD and HD samples for both MCCPs and SCCPs. CPs were detected in every house dust sample (n = 48 HD samples), with median (range) concentrations of 6.2 (4.0–57) μg/g and 19 (5.9–901) μg/g for SCCPs and MCCPs, respectively. The authors reported a wide variability in Canadian household exposures to CPs from dust.
Fridén et al. (2011) estimated the exposure of the sum of CPs via the indoor environment, considering (i) indoor air inhalation and (ii) dust ingestion, based on samples collected in domestic apartments in Stockholm (Sweden). The exposure was estimated considering mean inhalation rates, and mean and high dust ingestion rates. For a 25‐year‐old male adult, mean and high dust intakes of 4 and 55 mg/day were considered, while for toddlers (12–24 months) these were 100 and 200 mg/day, respectively. Based on this, for adults, median exposure considering mean and high dust ingestion was 1 and 1.3 μg/day, respectively. For toddlers, median exposure was higher, estimated at 1.1 and 16 μg/day, respectively for mean and high dust ingestion. The authors concluded that for adults, the exposure via indoor environment is dominated by the inhalation pathway that accounts for 98% or 76% depending on the dust intake rate. Dust ingestion could be important for adult individuals exposed to highlight contaminated dust and with high dust ingestion rate. For toddlers, inhalation and dust ingestion contribute more equally, pointing to dust as a more important source for toddlers than for adults. The authors compared those estimates with exposure estimates via the diet available at the time of the study, and concluded that diet might be the dominant pathway for adults, while in toddlers indoor environment might be the dominant one.
The CONTAM Panel made a scenario to estimate the ingestion from dust in toddlers and adults. The US‐EPA assumed that while an adult ingests an average of 20 mg/day, a child of 1–2 years old ingests an average of 50 mg/day (US‐EPA, 2017). The highest median values found in dust from four European countries were 6,300 μg/kg for SCCPs, 35,000 μg/kg for MCCPs and 2,700 μg/kg for LCCPs (see Section 1.3.3 ). Considering a body weight of 12 kg for a 1‐ to 3‐year‐old child and 70 kg for adults (EFSA Scientific Committee, 2012a), the resulting daily exposures via dust ingestion would be 26, 150 and 11 ng/kg bw per day for toddlers and 1.8, 10 and 0.77 ng/kg bw per day for adults, for SCCPs, MCCPs and LCCPs, respectively. The CONTAM Panel noted that this is only a very crude estimate of the exposure via dust and has a large associated uncertainty, but it shows that for toddlers exposure from dust could be in the same order of magnitude as the dietary exposure estimated from fish. For adults the exposure from dust is lower both in absolute terms and in comparison with the exposure estimated from fish consumption.
3.3.4. Dietary exposure assessment for farm animals, horses and companion animals
No exposure assessment was possible for any of the farm animal species as no occurrence data in feed were submitted to EFSA. The data for feed reported in the literature were too limited to attempt an exposure scenario.
3.4. Risk characterisation
3.4.1. Human health risk characterisation
Due to the lack of occurrence data for CPs in food, the CONTAM Panel was not able to carry out a robust dietary exposure estimate for the general population. This means that a complete risk characterisation could not be performed.
In order to provide a rough indication of whether there could be a possible health concern with respect to dietary exposure to CPs, the CONTAM Panel made a preliminary exposure assessment to SCCPs and MCCPs resulting from ‘Fish meat’ consumption (for fish consumers only) across Europe, noting that exposure will be higher due to the contribution of other foods. MOEs for this limited scenario were calculated for the mean and 95th percentile exposures across surveys and age groups (see Section 3.1.1 ). For LCCPs, in the absence of occurrence data and as no reference point has been identified, a risk characterisation could not be performed.
For SCCPs, the mean UB exposure resulting from ‘Fish meat’ consumption ranged from 1.9 to 35 ng/kg bw per day, and the 95th percentile UB exposure ranged from 4.9 to 87 ng/kg bw per day. Comparison of these dietary exposures with the BMDL10 of 2.3 mg/kg bw per day, which was identified as a reference point for the risk characterisation (see Section 3.1.6 ), resulted in MOEs of about 7 × 104 and 3 × 104 or higher for the mean and 95th percentile exposure, respectively.
For MCCPs, the mean UB exposure resulting from ‘Fish meat’ consumption ranged from 3.2 to 59 ng/kg bw per day, and the 95th percentile UB exposure ranged from 8.5 to 148 ng/kg bw per day. Comparison of these dietary exposures with the BMDL10 of 36.4 mg/kg bw per day, rounded to 36 mg/kg bw per day (see Section 3.1.6 ), which was identified as a reference point for the risk characterisation (see Section 3.1.6 ), resulted in MOEs of about 6 × 105 and 3 × 105 or higher for the mean and 95th percentile exposure, respectively.
The CONTAM Panel concluded that these MOEs for this limited scenario do not suggest a health concern, while noting uncertainties because the dietary exposure will be higher due to the contribution of CPs from other foods, the lack of toxicokinetic data for humans and that only a few CPs have been tested in the available toxicity studies.
For breastfed infants, comparison of the exposure estimates for SCCPs from 11 European countries based on pooled samples collected between 2014 and 2016 (See Section 3.3.1 ) with the BMDL10 of 2.3 mg/kg bw per day, resulted in MOEs of about 5 × 103 and 3 × 103 or higher for average and high human milk consumption, respectively. For MCCPs, comparison of the exposure estimates with the BMDL10 of 36 mg/kg bw per day resulted in MOEs of about 7 × 104 and 5 × 104 or higher for average and high human milk consumption, respectively. The CONTAM Panel concluded that these MOEs do not suggest a health concern. The Panel noted that since these were pooled samples, it was not possible to estimate specific values for highly exposed individuals.
3.4.2. Farm animals, horses and companion animal health risk characterisation
Due to the lack of occurrence data in feed, an exposure assessment for farm and companion animals could not be carried out, and thus a risk characterisation could not be performed. In addition, for ruminants, pigs, horses and fur animals no studies on the adverse effects of CPs were identified. Limited data on the adverse effects in poultry, rabbits, fish and dogs were identified (see Section 3.1.7 ).
3.5. Uncertainty analysis
The evaluation of the inherent uncertainties in the assessment of exposure to CPs has been performed following the guidance of the Opinion of the Scientific Committee related to Uncertainties in Dietary Exposure Assessment (EFSA, 2007). In addition, the report on ‘Characterizing and Communicating Uncertainty in Exposure Assessment’ has been considered (WHO/IPCS, 2008). The CONTAM Panel took note of the new guidance on uncertainties of the Scientific Committee (EFSA Scientific Committee, 2018), but it was not implemented in this opinion.
3.5.1. Assessment objectives
The objectives of the assessment are clarified in Section 1.2 Interpretation of the Terms of Reference.
3.5.2. Exposure scenario/exposure model
Occurrence data
In response to EFSA's request to submit occurrence data on CPs only limited data on the occurrence of SCCPs and MCCPs in food were received. No data were submitted on the presence of LCCPs in food. The majority of the results on SCCPs and MCCPs were obtained from the analysis of samples in the food category ‘Fish meat’ submitted by one European country. Thus, there is uncertainty whether the occurrence data used in the present assessment are representative for all European countries.
There is no information on specific SCCP or MCCP congeners detected in the fish samples.
Much of the data available in the literature originate from Asia, in particular China and regions where CPs are manufactured or thought to have a potential for environmental pollution. The levels documented in these studies may therefore not be representative to the situation found in Europe.
Changes in the CP composition of the technical products due to market requirements and restrictions, as well as the use of recycled materials result in different CP congener profiles being found in the environment and food. The congener profile, or ‘fingerprint’ of CP mixtures changes between production of technical mixtures for industrial use, to the profiles that are found in the environment and in food that are the result of the effects of weathering, breakdown, metabolism and time. Further changes can occur as CPs pass as contaminants through the food chain. The impact of these changes is difficult to estimate as there is little information on the exposure pathways, as well as on the CP congener profile to which humans are exposed.
Consumption data
Uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database were described by EFSA (2011a) and are not further detailed in this Scientific Opinion. These relate to the use of different dietary survey methodologies, standard portion sizes, representativeness of samples included in surveys, or to the inclusion of consumption surveys covering only few days to estimate high percentiles of chronic exposure.
Dietary exposure
Due to the limited number of samples analysed on food commodities other than fish, a robust exposure assessment for the general population could not be performed. In order to obtain an estimate of the potential magnitude of exposure, the data submitted to EFSA on SCCPs and MCCPs in few fish species (salmon, tuna and catfish) were used for a tentative estimation of exposure resulting from ‘Fish meat’ consumption (for fish consumers only). No exposure could be estimated for LCCPs due to the lack of occurrence data.
Since it is expected that other food categories contribute to the exposure (as shown in Section 3.3.2 ), the exposure levels to SCCPs and MCCPs calculated in this opinion are underestimated. A Swedish market basket study including a wider range of food types was carried out in 2015 (Yuan et al., 2017, see Section 3.3.2 ). The mean total CP exposure for adults was estimated to be up to 59 ng/kg bw per day with fish contributing only about 10%, thus the overall exposure of the European population will be higher than the one estimated in the present document due to the contribution of foods other than fish. In addition, exposure estimates presented in this opinion did not consider the exposure to LCCPs. Mean exposure for the German general adult population to the sum of SCCPs and MCCPs for total diet using mean occurrence was estimated to be 170 ng/kg bw per day by Krätschmer et al. (2019, see Documentation provided to EFSA) which also indicates that the exposure only based on fish samples is underestimated.
The exposure of breastfed infants was estimated based on pooled samples from 11 European countries, showing a wide range of concentrations. Since these were pooled samples, it was not possible to estimate specific values for highly exposed individuals.
Limited information is available on the effects of food processing on the concentrations of CPs. The presence of CPs in some common kitchen equipment (dish cloths, hand blenders, household oven components) suggests that food could become more contaminated at the preparation stage, due to either direct or secondary contact, and thus add to the dietary exposure. CPs have also been found in domestic plastic and food packaging and shown to migrate into the food. Based on the studies available, migration from plastic and kitchen equipments can contribute to the total CP exposure.
It was established that dust could be an important source of exposure, but only a crude estimate was made for intake of CPs from dust. Concentrations of CPs in dust cover several orders of magnitude which gives rise to uncertainty in the values used to estimate exposure from this source. No estimates were made for intake of CPs from other non‐dietary sources meaning that total non‐dietary exposure will be underestimated.
No data were available on the occurrence of CPs for feed materials and thus an exposure assessment for farm animals, horses and companion animals could not be performed.
3.5.3. Model input (parameters)
There are no analytical criteria or standard methods for the analysis of CPs in food and feed. The choice of analytical instrument and quantification method strongly influences the results. Depending on the analytical method used the results can vary as there are differences in the performance of the different analytical and deconvolution methods used for homologue pattern and quantitative determination. There is a lack of analytical standards and reference materials. All these points introduce a high degree of uncertainty into the occurrence data and exposure assessment.
3.5.4. Other uncertainties
Studies in experimental animals
Toxicity data were retrieved only for a limited number of mixtures (SCCPs: C10–12, 58% chlorination; C10–13, 56% chlorination; C10–13, 58% chlorination; C12, 60% chlorination; C10–13, 56.5% chlorination; carbon chain length not specified, 58% chlorination; MCCPs: C14–17, 52% chlorination; C14–17, 40% chlorination; LCCPs: C22–26, 43% chlorination; C23, 43% chlorination; C22–26, 70% chlorination) and there is uncertainty on the representativeness of the tested mixtures towards the pattern of CPs present in food. The approach followed in the present opinion was to consider read‐across within and between the SCCP, MCCP and LCCP classes and to use the information available from the tested CPs to conclude on the risks for the general population exposed to an unknown mixture of CPs in food. The available toxicokinetic data indicate that differences among CP congeners, in particular in relation to the degree of chlorination, suggest that read‐across to other CPs, both within the same class as well as to other classes is problematic and will have high uncertainty. In addition, the physicochemical properties of the CPs indicate a potential for accumulation in organs and tissues and the available toxicokinetic studies in experimental animals confirm accumulation in adipose tissue/fat rich organs and tissues. The available information suggests that elimination from these tissues is slower than from other organs and tissues, but the information is insufficient to derive elimination half‐lives from these tissues.
The possible presence in food of CPs with higher toxicity than those for which toxicity data are available and possible accumulation of CPs in humans were considered in the MOE approach followed in the opinion (see Section 3.1.6.3 ) to minimise the possibility of underestimating risks. However, the lack of data on the CP mixtures of relevance for human dietary exposure adds to the overall uncertainty of the assessment.
For SCCPs, the reference point was derived based on histopathological changes in the thyroid. Changes in thyroid hormone levels (decreased FT3, increased TSH) were observed in one study in rats at a lower dose than the selected reference point. The Panel noted inconsistencies in the hormonal changes between the two available studies, which prevented these effects being used as a basis for deriving a reference point albeit with uncertainty. Although there is uncertainty about the validity of extrapolating changes related to the thyroid from rats to humans when these changes are mediated by induction of hepatic transporters and hepatic metabolism, histopathological changes in the rat thyroid were still taken as a reference point. There is a potential for modulation of thyroid hormone levels to have an effect in rodents on pre‐ and postnatal neurological development of offspring. However, the Panel could not identify any studies on potential neurodevelopmental effects of CPs in mammals.
Farm animals, horses and companion animals
No studies investigating effects of CPs in ruminants, pigs, horses, cats and fur animals have been identified. Only limited data are available for other animal species. For poultry, none of the studies were sufficiently robust for hazard characterisation. For rabbits, only developmental toxicity studies are available; these studies are of a very short exposure duration and therefore, not adequate for a chronic risk characterisation.
3.5.5. Summary of uncertainties
In Table 37 , a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the respective source of uncertainty might have led to an over‐ or underestimation of the exposure or the resulting risk.
Table 37.
Summary of qualitative evaluation of the impact of uncertainties on the risk assessment of exposure to chlorinated paraffins from food and feed
| Sources of uncertainty | Directiona |
|---|---|
| Difference in analytical results arising from the application of different methods and due to the lack of available analytical standards | +/− |
| Exposure | |
| Exposure is estimated by considering only occurrence levels in fish due to unavailability of occurrence data on other foodstuffs | − |
| Extrapolation of the occurrence data in fish reported by one country to the whole of Europe | +/− |
| Extrapolation of occurrence data on salmon, tuna and catfish to all other kinds of fish meat | +/− |
| Consumption data: different methodologies/representativeness/underreporting/misreporting/no portion size standard | +/− |
| Methodology used to assess long‐term exposure based on food consumption surveys covering only a few days to estimate high percentiles | + |
| No estimates for exposure to CPs from other non‐dietary sources than dust | − |
| Hazard identification and characterisation | |
| Uncertainty in the read‐across approach followed for SCCPs and MCCPs, in the absence of toxicological data on the CP mixtures relevant for human dietary exposure | +/− |
| Uncertainty in the accumulation potential of CPs | − |
| Uncertainty about the validity of extrapolating changes related to the thyroid from rats to humans when these changes are mediated by induction of hepatic transporters and hepatic metabolism | +/− |
| Toxicity database for farm animals, horses and companion animals very limited | +/− |
CPs: chlorinated paraffins; SCCPs: short‐chain chlorinated paraffins; MCCPs: medium‐chain chlorinated paraffins; UDPGT: uridine 5’‐diphospho‐glucuronosyltransferase.
+ = uncertainty with potential to cause over‐estimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk.
The CONTAM Panel considered that the impact of the uncertainties on the risk assessment of exposure to CPs in food is substantial, and due to the limited data on occurrence of CPs in food the dietary exposure is considered to be underestimated.
Due to the lack of occurrence and toxicity data, a risk assessment of CPs for farm animals, horses and companion animals was not possible.
4. Conclusions
CPs are complex technical mixtures of polychlorinated alkanes, with varying chain length and degree of chlorination. The commercially available CPs are generally divided into three groups: short‐chain CPs (SCCPs) comprising 10 to 13 carbon atoms, medium‐chain CPs (MCCPs) comprising 14 to 17 carbon atoms and long‐chain CPs (LCCPs) with 18 or more carbon atoms. Some technical mixtures may contain CPs from more than one of these three groups, and some newer CPs do not confine themselves to these groups. Technically, the terms ‘SCCP’, ‘MCCP’ and ‘LCCP’ refer to the commercial mixtures of CPs. In this opinion the nomenclature of SCCP(s), MCCP(s) and LCCP(s) will be used to refer to any CP mixture(s) that falls within the range of carbon chain lengths indicated above.
4.1. Analytical methods
Analysis of CPs is highly demanding and current methods have been shown to lack precision and accuracy. Congener specific analysis is not currently possible. Several different approaches to analysis exist, and lead to differences in results obtained. Only a limited number of analytical standards are available, and these represent only a tiny fraction of the total number of individual CP congeners that may be found. Care is therefore needed when using occurrence data to perform exposure estimates.
4.2. Hazard identification and characterisation
4.2.1. Toxicokinetics
No data are available on toxicokinetics in humans, horses and companion animals and only limited data are available for experimental animals and some farm animals.
Absorption to some extent is indicated in various animal species by excretion of a small amount of radiolabelled CPs and or metabolites in the urine, distribution of radiolabelled CPs and or metabolites to different organs and tissues, and systemic effects observed in toxicity studies.
Detection of CPs in human blood and milk samples indicates that CPs are absorbed to some extent in humans and detection of CPs in umbilical cord blood indicates that CPs can be transferred to the fetus.
Accumulation in adipose tissue and fat rich organs and tissues is indicated and elimination from these tissues seems to be slower than from other organs and tissues. The available information is insufficient to derive elimination half‐lives from these tissues.
Studies in mice suggested that highly chlorinated SCCPs and MCCPs are metabolised and excreted via faeces whereas lower chlorinated SCCPs and MCCPs can be partly metabolised and exhaled as carbon dioxide.
Following oral administration of CPs to rats and mice, the major route of excretion was the faeces with only a small proportion excreted in urine and expired air.
There are very limited data on the transfer of CPs from feed to the food of animal origin.
4.2.2. Toxicity in experimental animals
CPs are of low acute toxicity with oral LD50 values all exceeding 4 g/kg bw.
The liver, kidney and thyroid were identified as the target organs for the two SCCPs and the single MCCP tested in the subchronic and chronic toxicity studies in experimental animals. For the LCCPs tested in the subchronic and chronic toxicity studies in rats and mice, only the liver was identified as a target organ.
For the SCCPs tested, a NOAEL of 10 mg/kg bw per day was identified, based on increased kidney weights, and histopathological changes in the kidney and thyroid.
For the MCCP tested, a NOAEL of 10 mg/kg bw per day was identified, based on increased kidney weights.
For the LCCPs tested, a LOAEL of 100 mg/kg bw per day was considered for the two LCCPs with a 43% chlorination degree, and a NOAEL of 900 mg/kg bw per day for the LCCP with a 70% chlorination degree, based on liver effects, including increased weight and histopathological changes.
An SCCP caused teratogenic effects such as absence of digits or shortened digits in rats at 2,000 mg/kg bw per day (gavage) in the presence of maternal toxicity (high mortality and decreased body weight gain); the NOAEL for developmental toxicity was 500 mg/kg bw per day, and for maternal toxicity 100 mg/kg bw per day. In the developmental toxicity study in rabbits with the same SCCP; the NOAEL for developmental toxicity was 10 mg/kg bw per day, based on increased number of resorptions, in the absence of maternal toxicity.
For the MCCP tested, no developmental toxicity was observed in rats and rabbits at the highest dose levels tested, 5,000 mg/kg bw per day for rats and 100 mg/kg bw per day for rabbits. No parental toxicity or effects on fertility were observed for the same MCCP in a dose‐range finding study for a two‐generation dietary rat study. In this study, postnatal effects in the form of decreased pup survival and subcutaneous haematoma/haemorrhage were observed; a NOAEL of 9 mg/kg bw per day was identified for these effects.
For the two LCCPs tested, no developmental toxicity was observed in rats and rabbits at the highest dose levels tested, 1,000 mg/kg bw per day for rabbits with the 70% chlorinated LCCP and 5,000 mg/kg bw per day in the three other studies with the 43% or the 70% chlorinated LCCP.
The overall weight of evidence indicates that CPs are not genotoxic.
The SCCP tested caused increased incidences of tumours in rats and mice, i.e. in the liver (rats, mice), kidneys (male rats only) and thyroid (female rats and mice). The LCCP tested also caused increased incidences of tumours in mice, i.e. malignant lymphomas (male mice), and to a marginal extent of hepatocellular neoplasms (female mice). No carcinogenicity study on MCCPs was identified.
4.2.3. Observations in humans
No studies on observations in humans of relevance for the risk assessment of CPs within the scope of this opinion were identified.
4.2.4. Adverse effects in farm animals, horses and companion animals
No data on adverse effects of CPs in ruminants, pigs, horses, cats and fur animals were identified.
In poultry, none of the available studies were robust enough for the identification of a reference point.
For rabbits, no other studies than the developmental toxicity studies described for experimental animals have been identified.
For fish, most studies showed no or minor effects. Based on the available information, the CONTAM Panel could not identify a NOAEL or a LOAEL.
For dogs, no other studies than the toxicity study described for experimental animals have been identified.
4.2.5. Mode of action
Rodent hepatic enzyme induction and proliferation of the smooth endoplasmic reticulum leading to hypertrophy (and associated increases in liver size) is considered as an adaptive physiological response to CPs which can contribute to hepatocarcinogenicity in rodents. Interference with the TCA cycle and energy balance leads to liver cell toxicity.
The rodent liver tumours induced by an SCCP, and associated with CAR or PPARα transactivation, appear not relevant to humans. Human hepatocarcinogenicity is not anticipated provided hepatic cell toxicity does not occur.
SCCP‐induced kidney tumours in male rats appear to relate to sustained nephropathy (induced partly, but not necessarily entirely, by α2u‐microglobulin accumulation), compensatory regenerative hyperplasia and increased S‐phase.
Thyroid effects caused by CPs in rodents can arise due to stimulation of the thyroid via a negative feedback mechanism. This includes a potential increase in hepatic uptake of thyroid hormones, increased hepatic UDPGT levels and conjugation of T4, consequent decrease in plasma T4/T3, compensatory release of pituitary TSH and a compensatory increase in T4 production in the thyroid. This eventually leads to hypertrophy, hyperplasia and thyroid tumours. Additional mechanisms that may contribute to carcinogenesis by CPs include oxidative stress and inhibition of intercellular communication.
The CONTAM Panel decided not to use changes in thyroid hormone levels as a basis for a reference point since there were inconsistencies in the hormonal changes between the two available studies on SCCPs, as well as gender differences in the available study with the MCCP. Overall, there is uncertainty about the validity of extrapolating changes related to the thyroid from rats to humans when these changes are mediated by induction of hepatic transporters and hepatic metabolism. However, despite these uncertainties, the thyroid histopathology is used as a basis for the reference point. Potential neurodevelopmental effects from thyroid hormone changes could not be assessed because of a lack of developmental neurotoxicity studies.
In rats, the tested MCCP perturbed the clotting system in lactating neonates of treated mothers. The haemorrhaging effect in lactating neonates appears to result as a consequence of vitamin K deficiency.
4.2.6. Margin of exposure (MOE) approach
Due to the limitations and uncertainties in the current database on SCCPs, MCCPs and LCCPs the derivation of HBGVs was not considered appropriate. Instead, an MOE approach was applied to assess a possible health concern.
For one tested SCCP, a BMDL10 of 2.3 mg/kg bw per day was derived from BMD modelling, and selected as the reference point for the risk characterisation, based on increased incidence of nephritis in male rats.
For the tested MCCP, a BMDL10 of 36 mg/kg bw per day was derived from BMD modelling, and selected as the reference point for the risk characterisation, based on increased relative kidney weight in male and female rats.
For the tested LCCPs, only the liver was identified as a target organ. As effects in the liver observed consistently in rats and mice are considered as an adaptive physiological response and the associated energy costs, a reference point has not been identified.
An MOE higher than 1,000 might indicate that there is no health concern, taking into account the variability between species (a factor of 10), the variability within human individuals (a factor of 10), and extrapolation from subchronic studies to chronic exposure duration (a factor of 2). An additional factor of 5 was considered to take into account the limitations in the database.
4.3. Occurrence and exposure
4.3.1. Food
Only limited data on the occurrence of SCCPs and MCCPs, and only for some fish species, were submitted to EFSA, while no data were submitted on the presence of LCCPs.
Due to the lack of sufficient occurrence data on foodstuffs other than fish, it was not possible to carry out a robust exposure assessment. A preliminary exposure assessment of the chronic exposure to SCCPs and MCCPs was made based on the consumption of ‘Fish meat’ and for fish consumers only.
A data set consisting of 422 analytical results from 184 samples of ‘Fish meat’ collected in Germany between 2014 and 2017 was used for this purpose. CPs were quantified in the majority of the samples, with 15% and 14% of left‐censored data for SCCPs and MCCPs, respectively. The mean and P95 occurrence levels were 7.5 and 18 μg/kg ww (LB = UB) for SCCPs, respectively, and 13 and 44 μg/kg ww (LB = UB) for MCCPs, respectively.
For SCCPs, the mean LB and UB exposure estimates ranged from 1.9 to 35 ng/kg bw per day. At the 95th percentile exposure, the LB and UB estimates ranged from 4.9 to 87 ng/kg bw per day.
For MCCPs, the mean LB and UB exposure estimates ranged from 3.2 to 59 ng/kg bw per day. At the 95th percentile exposure, the LB and UB estimates ranged from 8.5 to 148 ng/kg bw per day.
The lowest exposures were found in the Adult groups while the highest were for Toddlers.
For breastfed infants, exposure levels were estimated considering data on pooled human milk samples from 11 European countries based on samples collected between 2014 and 2016. Exposure to SCCPs ranged from 60 to 445 ng/kg bw per day for average consumption of human milk and from 90 to 668 ng/kg bw per day for high consumption of human milk, respectively. For MCCPs, the exposure ranged from < 25 to 514 ng/kg bw per day and from < 38 to 771 ng/kg bw per day, respectively, for average and high consumption of human milk.
The highest median values found in dust from four European countries were 6,300 μg/kg for SCCPs, 35,000 μg/kg for MCCPs and 2,700 μg/kg for LCCPs. For toddlers, exposure from ingestion of dust could be in the same order of magnitude as the dietary exposure estimated from fish. For adults, the estimated exposure from dust was lower than for toddlers both in absolute terms and when compared with the exposure estimated from fish consumption, but could nevertheless still be an important source of exposure to CPs. No estimates were made for intake of CPs from other non‐dietary sources.
4.3.2. Feed
No data for feed were submitted to EFSA. The data for feed reported in the literature were too limited to attempt an exposure scenario.
4.4. Risk characterisation
4.4.1. Human health risk characterisation
A complete risk characterisation could not be performed due to lack of occurrence data for CPs in food.
A preliminary risk assessment could only be performed for SCCPs and MCCPs based on a limited exposure assessment related to the consumption of ‘Fish meat’ (for fish consumers only).
For LCCPs, no risk characterisation could be performed in the absence of occurrence data and identification of a reference point.
For SCCPs, MOEs of about 7 × 104 and 3 × 104 or higher for the mean and 95th percentile exposure, respectively. For MCCPs, MOEs of about 6 × 105 and 3 × 105 or higher for the mean and 95th percentile exposure, respectively. The Panel concluded that these MOEs for this limited scenario do not suggest a health concern, while noting uncertainties because the dietary exposure will be higher due to the contribution of CPs from other foods, the lack of toxicokinetic data for humans and that only a few CPs have been tested in the available toxicity studies.
For breastfed infants, MOEs for SCCPs of about 5 × 103 and 3 × 103 or higher for average and high human milk consumption, respectively, were obtained. For MCCPs, MOEs were about 7 × 104 and 5 × 104 or higher for average and high human milk consumption, respectively. The CONTAM Panel concluded that these MOEs do not suggest a health concern. The Panel noted that since these were pooled samples, it was not possible to estimate specific values for highly exposed individuals.
4.4.2. Farm animals, horses and companion animal health risk characterisation
No risk characterisation could be performed due to the lack of occurrence data of CPs for feed and due to the lack of, or limited, data on the adverse effects of CPs in farm animals, horses and companion animals.
5. Recommendations
There is a need for validated analytical methods for the determination of CPs, as well as suitable standards and reference materials.
Research is needed to identify which specific CP congeners are more relevant in terms of occurrence in food and of relevance for human health.
There is a need for occurrence data in food for SCCPs, MCCPs and LCCPs to enable a robust human exposure assessment, as well as data on the possible transfer of CPs from, e.g. kitchen equipment, during food preparation.
More data on variation of occurrence of CPs in human milk are needed to enable a more robust exposure assessment for breastfed infants.
More information is needed on the toxicokinetics in humans and experimental animals, with respect to the impact of the degree of chlorination, chlorine position and carbon chain length. The development of TK models would facilitate a body burden approach.
There is a need for chronic toxicity studies for relevant CP mixtures.
A better understanding of the relevance of SCCP and MCCP thyroid hormone changes in rodents and of SCCP‐induced rodent thyroid tumours to humans is needed.
There is a need for developmental neurotoxicity studies with SCCP and MCCP because of the reported changes in rodent thyroid hormone levels.
There is a need for occurrence data in feed.
Data on the transfer of CPs from feed to the food of animal origin are needed.
There is a need for data on adverse effects of CPs in ruminants, pigs, poultry and fish. Data in horses, companion animals and fur animals would also be needed to perform a risk assessment on these species.
Documentation provided to EFSA
Krätschmer K, Schächtele A, Malisch R and Vetter W, 2019a. Data provided to EFSA by Kerstin Krätschmer, Alexander Schächtele, Rainer Malisch and Walter Vetter on a study of CPs exposure through food in Southern Germany. Results submitted as an extended abstract to the DIOXIN2019 conference for publication in Organohalogen Compounds. Used in Section 3.2.2 and 3.3.2 .
Krätschmer K, Schächtele A, Malish R and Vetter W, 2019b. Data provided to EFSA by the EU‐Reference Laboratory (EU‐RL) for Halogenated POPs in Feed and Food on the occurrence of CPs in human milk samples from European countries analysed as part the WHO/UNEP Coordinated Survey of Human Milk for Persistent Organic Pollutants. Used in Section 3.2.4 and 3.3.1 .
BfR (Bundesinstitűt fűr Risikobewertung), 2019. Data provided to EFSA on the release of CPs from hand blenders, performed by the National Reference laboratory for Food Contact Materials, BfR, Germany. Used in Section 3.2.3 .
EuroCHLOR, 2018. Data provided to EFSA on unpublished study reports on CPs, and used in Section 3.1 :
ICI (Imperial Chemical Industries), 1985. Hart D, Wickramaratne G, De S, Banham P, Chart I and Gaskell B. Chlorinated paraffin (52% chlorination of intermediate chain length n‐paraffins): Investigation into the possible mechanism of haemorrhage in offspring rats. Report Number CTL/P/1293. ICI Central Toxicology Laboratory, Alderley Park, Cheshire, UK.
ICI (Imperial Chemical Industries), 1997. Elcombe CR, Bars RG, Watson SC and Foster JR. Hepatic effects of chlorinated paraffins in mice, rats and guinea pigs: species differences and implications for hepatocarcinogenicity. ICI Central Toxicology Laboratory, Alderley Park, Cheshire, UK.
IRDC (International Research and Development Corporation), 1981a. 14‐Day oral toxicity study in rats. Chlorinated Paraffin: 58% chlorination of Short‐Chain Length n‐Paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐006.
IRDC (International Research and Development Corporation), 1981b. 14‐Day dietary administration to rats. Chlorinated Paraffin: 52% Chlorination of Intermediate Chain Length n‐Paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No. 438‐003.
IRDC (International Research and Development Corporation), 1981c. 14‐Day oral toxicity study in rats. Chlorinated Paraffin: 43% Chlorination of Long Chain Length n‐Paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐005.
IRDC (International Research and Development Corporation), 1981d. 14‐Day oral toxicity study in rats. Chlorinated Paraffin: 70% Chlorination of Long Chain Length n‐Paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐004.
IRDC (International Research and Development Corporation), 1982a. Teratology study in rats. Chlorinated Paraffin: 58% chlorination of short chain length n‐paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐016.
IRDC (International Research and Development Corporation), 1982b. Teratology Study in Rabbits. Chlorinated Paraffin: 43% Chlorination of Long Chain Length n‐paraffin. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐030.
IRDC (International Research and Development Corporation), 1983a. 14‐Day Dietary Range‐Finding Study in Rats. Chlorinated Paraffin: 58% chlorination of short chain length n‐paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No. 438‐002.
IRDC (International Research and Development Corporation), 1983b. Teratology Study in Rabbits. Chlorinated Paraffin: 58% chlorination of short chain length n‐paraffin. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐031.
IRDC (International Research and Development Corporation), 1983c. Teratology Study in Rabbits. Chlorinated Paraffin: 52% chlorination of intermediate chain length n‐paraffin. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐032.
IRDC (International Research and Development Corporation), 1983d. Teratology Study in Rats. Chlorinated Paraffin (43% Chlorination of Long Chain Length n‐Paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐015.
IRDC (International Research and Development Corporation), 1983e. Teratology Study in Rabbits. Chlorinated Paraffin (70% Chlorination of Long Chain Length n‐Paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐039.
IRDC (International Research and Development Corporation), 1983f. In vivo Cytogenetic Evaluation by Analysis of Rat Bone Marrow Cells. Chlorinated Paraffin: 58% Chlorination of Short Chain Length n‐Paraffins; Cyclophosphamide (positive control agent). Sponsor: Chlorinated Paraffin Program. Report No 438‐013.
IRDC (International Research and Development Corporation), 1983 g. Dominant Lethal Study in Rats. Chlorinated Paraffin: 58% chlorination of short chain length n‐paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐011.
IRDC (International Research and Development Corporation), 1983 h. In vivo Cytogenetic Evaluation by Analysis of Rat Bone Marrow Cells. Chlorinated Paraffin: 52% Chlorination of Intermediate Chain Length n‐Paraffins; Cyclophosphamide (positive control agent). Sponsor: Chlorinated Paraffin Program. Report No 438‐014.
IRDC (International Research and Development Corporation), 1983i. In vivo Cytogenetic Evaluation by Analysis of Rat Bone Marrow Cells. Chlorinated Paraffin: 43% Chlorination of Long Chain Length n‐Paraffins; Cyclophosphamide (positive control agent). Sponsor: Chlorinated Paraffin Program. Report No 438‐012.
IRDC (International Research and Development Corporation), 1984a. 13‐Week Dietary Toxicity Study in Rats with Combined Excretion, Tissue Level and Elimination Study/Determination of Excretion, Tissue Level and Elimination After Single Oral (Gavage) Administration to Rats. Chlorinated Paraffin: 58% chlorination of Short Chain Length n‐Paraffins; 14C labeled CP. Sponsor: Chlorinated Paraffin Program. Report No. 438‐035/438‐022.
IRDC (International Research and Development Corporation), 1984b. 13‐Week Oral Toxicity Study in Rats with Combined Excretion, Tissue Level and Elimination Studies/Determination of Excretion, Tissue Level and Elimination after Single Oral (Gavage) Administration to Rats. Chlorinated Paraffin: 58% Chlorination of Short Chain Length n‐Paraffins; 14C labeled CP. Sponsor: Chlorinated Paraffin Program. Report No. 438‐029/438‐022.
IRDC (International Research and Development Corporation), 1984c. 13‐Week Dietary Toxicity Study in Rats with Combined Excretion, Tissue Level and Elimination Studies/Determination of Excretion, Tissue Level and Elimination after Single Oral (Gavage) Administration to Rats. Chlorinated Paraffin: 52% Chlorination of Intermediate Chain Length n‐Paraffins, 14C labeled CP. Sponsor: Chlorinated Paraffin Program. Report No. 438‐026/438‐023.
IRDC (International Research and Development Corporation), 1984d. 13‐week oral (gavage) toxicity study in rats with combined excretion, tissue level and elimination study/Determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 43% chlorination of long chain length n‐paraffins; 14C labeled CP. Sponsor: Chlorinated Paraffin Program. Report No. 438‐028/438‐021.
IRDC (International Research and Development Corporation), 1984e. 13‐week dietary toxicity study in rats with combined excretion, tissue level and elimination studies/Determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 70% chlorination of long chain length n‐paraffins; 14C labeled CP. Sponsor: Chlorinated Paraffin Program. Report No. 438‐027/438‐024.
IRDC (International Research and Development Corporation), 1984f. Teratology Study in Rats. Chlorinated Paraffin: 52% chlorination of intermediate chain length n‐paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐017.
IRDC (International Research and Development Corporation), 1984 g. Teratology Study in Rats. Chlorinated Paraffin: 70% chlorination of long chain length n‐paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐045.
IRDC (International Research and Development Corporation), 1985. Reproduction Range‐Finding Study in Rats. Chlorinated Paraffin: 52% chlorination of intermediate chain length n‐paraffins. Sponsor: Chlorinated Paraffin Consortium. Report No 438‐049.
CXR, 2003. CXR Bioscience Ltd. Effects of medium chain chlorinated paraffins (MCCPs) on vitamin K concentrations and clotting factors in female Sprague Dawley rats. CXR0179. Unpublished report.
CXR, 2005. CXR Bioscience Ltd. A dietary study to investigate the toxicokinetics of medium chain chlorinated paraffins (Cereclor S52) in male and female Fisher 344 rats. CXR0282. Biosciences Ltd, Dundee, UK. Unpublished interim report.
CXR, 2005. CXR Bioscience Ltd. A Dietary study to determine the 90 day NOAEL of medium chain chlorinated paraffins (Cereclor S52) in male and female FischeR 344 rats. CXR0273. Unpublished draft report.
CXR, 2006. Huntingdon Life Sciences Ltd. C14‐17 n‐alkane, 52% chlorinated study of post‐natal offspring mortality following dietary administration to CD rats. DAR0001/062390. Unpublished report.
CXR, 2004. CXR Bioscience Ltd. MCCP – Study to assess maternal milk and neonate plasma. Inveresk Project N 493569. Inveresk report Number 23697. Unpublished report.
Abbreviations
- ADME
absorption, distribution, metabolism, elimination
- AHH
aryl hydrocarbon (benzo[a]pyrene) hydroxylase
- ALT
alanine aminotransferase
- APCI
atmospheric pressure chemical ionisation
- APND
aminopyrine N‐demethylase
- ASE
accelerated solvent extraction
- AST
aspartate aminotransferase
- ATP
adenosine triphosphate
- BAF
bioaccumulation factor
- BauA
German Federal Institute for Occupational Safety and Health
- BCF
bioconcentration factor
- BMD
benchmark dose (modelling)
- BMDL
benchmark dose lower confidence limit
- BMDU
benchmark dose upper confidence limit
- BMF
biomagnification factor
- BMR
benchmark response
- bw
body weight
- CAR
constitutive androstane receptor
- CAS (number)
Chemical Abstracts Service registration number
- CEPA
Canadian Environmental Protection Act
- CLP
classification, labelling and packaging
- CONTAM Panel
Panel on Contaminants in the Food Chain
- CPs
chlorinated paraffins
- CYP450
cytochrome P450
- dw
dry weight
- ECD
electron capture detection
- ECHA
European Chemicals Agency
- ECNI
electron capture negative ionisation
- ECOD
7‐ethoxycoumarin O‐deethylase
- ERα
oestrogen receptor α
- EU‐RL
EU‐reference laboratory
- FEQGs
Federal Environmental Quality Guidelines (Canada)
- FID
flame ionisation detector
- FT3
free triiodothyronine
- FT4
free thyroxine
- GC
gas chromatography
- GC×GC
comprehensive two‐dimensional gas chromatography
- GD
gestational day
- GR
glucocorticoid receptor
- HBGV
health‐based guidance value
- HED
human equivalent dose
- HPV
high production volume
- HRMS
high‐resolution mass spectrometry
- i.p.
intraperitoneal
- i.v.
intravenous
- IARC
International Agency for Research on Cancer
- IC20
20% inhibitory concentration
- IPCS
International Programme on Chemical Safety
- IRDC
International Research and Development Center
- Jak‐STAT
Janus kinase‐signal transducer and activator of transcription
- LB
lower bound
- LC50
lethal concentration, median
- LCCPs
long‐chain chlorinated paraffins
- LD50
lethal dose, median
- LLE
liquid–liquid extraction
- LOAEL
lowest‐observed adverse effect level
- LOEL
lowest‐observed effects level
- LOD
limit of detection
- LOQ
limit of quantification
- LRMS
Low‐resolution mass spectrometry
- LRTAP
Long‐range transboundary air pollution
- LT50
time required for 50% of the animals to die following exposure to a known concentration of the test agent
- MCCPs
medium‐chain chlorinated paraffins
- MOE
margin of exposure
- MS
mass spectrometry
- MTT assay
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide for assay
- MWF
metal working fluids
- m/z
mass‐to‐charge ratio
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- NICNAS
National Industrial Chemicals Notification and Assessment Scheme
- NIVA
Norwegian Institute for Water Research
- NOAEL
no‐observed adverse effect level
- NOEL
no‐observed effect level
- NR1H4
nuclear receptor family 1 group H member 4
- NTCP
sodium taurocholate cotransport polypeptide
- NTP
National Toxicology Programme
- OAT2
organic anion transporter 2
- OATP2
organic anion transporting polypeptide 2
- OECD
Organisation for Economic Co‐operation and Development
- Palmitoyl CoA
palmitoyl coenzyme A
- PBK
physiologically based kinetic
- PBT
persistent, bioaccumulative and toxic
- PCAs
polychlorinated alkanes
- PCBs
polychlorinated biphenyls
- PLE
pressurised liquid extraction
- PM
particulate matter
- PND
postnatal day
- POPs
persistent organic pollutants
- PPARα
peroxisome proliferator activated receptor α
- PXR
pregnane X receptor
- RAR
risk assessment report
- RER
renin/prorenin receptor
- REACH
registration, evaluation, authorisation and restriction of chemicals
- RfD
reference dose
- RSD
relative standard deviation
- SCCPs
short‐chain chlorinated paraffins
- SD
standard deviation
- SIDS
Screening Information Dataset
- SOP
standard operational procedures
- SPE
solid‐phase extraction
- SSD
standard sample description
- StAR
steroidogenic acute regulatory protein
- T3
triiodothyronine
- T4
Thyroxine
- TBG
T4‐binding globulin
- TD05
tumourigenic Dose05
- TDI
tolerable daily intake
- Th17
T helper 17 cells
- TIC
total ion count
- TMF
trophic magnification factors
- TOF‐MS
time‐of‐flight mass spectrometry
- TPA
12‐O‐tetradecanoylphorbol‐13‐acetate
- TRβ
thyroid receptor β
- TSH
thyroid‐stimulating hormone
- TTR
transthyrethin
- UB
upper bound
- UDPGT
uridine 5’‐diphospho‐glucuronosyltransferase
- UDS
unscheduled DNA synthesis
- UK‐COT
UK‐Committee on Toxicity
- UNECE
United Nations Economic Commission for Europe
- UNEP
United Nations Environment Programme
- US‐EPA
US‐Environmental Protection Agency
- VDR
vitamin D3 receptor
- vPvB
very toxic very bioaccumulative
- vSCCP
very short‐chain CP
- WBA
whole body autoradiography
- WHO
World Health Organization
- ww
wet weight
- WWTP
municipal waste water treatment plant
Appendix A – Detailed description of the toxicokinetic studies in laboratory animals
1.
The studies on the toxicokinetics of CPs in laboratory animals (rats and mice) included in Section 3.1.1.1 are described in more detail in this Appendix.
A.1. Studies in rats
A.1.1. SCCPs
The absorption, distribution and excretion of SCCPs have been investigated in a study in rats (Geng et al., 2016). Male Sprague–Dawley rats (8 weeks old, 339–407 g, four animals/group) were administered either a single oral dose of a mixture of C10‐, C11‐, C12‐ and C13‐CPs (1:1:1:1, chlorine content of the mixture as determined to be 56.5%, 1,000 mg/kg bw in corn oil) or corn oil (the vehicle control group). The animals were placed in metabolic cages just before the dosing and kept there for 28 days. A blood sample was taken from the eye socket vein at 1, 2, 3, 6, 7 and 15 days after dosing, and on day 28 blood was collected by the abdominal aortic method. Urine and faeces samples were collected at 1, 3, 6, 7, 10, 14, 17, 20, 24 and 28 days after dosing. A total of 20 urine samples and 20 faeces samples were obtained by mixing the excreta from four rats at each sampling time. Blood, urine and faeces samples were analysed using a solvent extraction followed by a multilayer silica gel clean up to separate the SCCPs from metabolites and interfering sample matrix. The final measurements were performed with GC‐ECNI‐MS. The absorption was rapid with a half‐life of 1 day. The peak blood concentration of total SCCPs was attained at 2.8 day with a Cmax value of 2.3 mg/L. The half‐lives of total SCCPs in blood for distribution and elimination phases were calculated to 1.7 and 6.6 days, respectively. AUC0‐t and AUC0‐∞ were 25.0 and 26.4 mg/L per day, respectively. The CONTAM Panel noted that the SCCP mixture in this study consists of a mixture of many congeners each with different individual half‐lives, resulting in a change in the congener group profile with time. Consequently, the persistence of each congener is not reflected in the overall half‐life reported. The ‘mean residence time’ (usually defined as the average time a molecule stays in the body) of total SCCPs in rat was calculated to be 9.6 days. The total SCCPs in urine and faeces decreased rapidly at the beginning, and then reached a steady state after the sixth day. About 28% and about 3.5% of the administered SCCPs were excreted without metabolism in the faeces and urine, respectively. Congener group abundance profiles of SCCPs in blood, urine and faeces samples indicated that Cl6‐SCCPs were the most abundant chlorine homologues in the blood and excreta sampled in the earlier stage, whereas Cl5‐SCCPs were the predominant chlorine homologues in all samples collected in the later stage. The relative abundances of Cl7–10‐SCCPs were increased in all faeces samples, but were decreased in urine samples collected in the later stage. The carbon chain congener group C10‐CPs showed a decrease in blood, urine and faeces in the earlier stage, and then a slight increase in the later stage. The increasing tendency was also seen for C11‐CPs in blood and urine in the later stage. C12‐CPs and C13‐CPs tended to decrease in blood in the later stage, whereas C13‐CPs in faeces were increased. The authors concluded that the distribution discrepancies in blood and excreta between SCCPs congener groups were more dependent on chlorine contents than on carbon chain lengths.
As part of a dietary repeated dose toxicity study in rats with an SCCP, there was an assessment of toxicokinetics (IRDC, 1984a, unpublished study, see Documentation provided to EFSA) (see also Section 3.1.2.2.1 ). F344 rats (25 animals/sex per group) were administered 10 or 625 mg/kg bw per day of an SCCP (C10–12, 58% chlorination) daily in the diet for 13 weeks. After 13 weeks, groups of animals received a single oral dose of the 14C‐labelled SCCP by gavage, the same dose level as received daily in the previous weeks. Other groups of animals (18 animals/sex per group), which were not treated previously with the SCCP, received a single oral dose of the 14C‐labelled SCCP by gavage at 10 or 625 mg/kg bw. Following administration of the radiolabelled test substance, three animals per sex from each dose level were placed in metabolism cages and urine, faeces and exhaled air were collected for 12 h or 7 days. Additional groups of three animals per sex from each dose level were kept for 24 h or 48 h, 28 or 90 days following administration of the radiolabelled test substance and at the end of 12 h, or 7, 28 or 90 days samples of tissues (adipose, brain, gonads, heart, kidney and liver) were collected from designated surviving animals. In addition, samples of whole blood were collected from the orbital sinus prior to sacrifice of animals at 12, 24 and 48 h and 7 days. Overall, only small differences in excretion were observed between sexes, dose levels or dosing regimen (single or repeated). Approximately 54–66% of the administered radioactivity was recovered in the faeces in 7 days, approximately 14% in the urine, and less than 1% in exhaled air as carbon dioxide. Blood levels were generally proportional to the dose level and the decline over 7 days was found to be similar between sexes and dosing regimen. Tissue levels of radioactivity were also generally proportional to the administered dose and were similar, irrespective of dosing regimen. The highest initial concentrations of radioactivity were found in the liver, adipose tissue, kidneys and ovaries. In general, the elimination of radioactivity from the adipose tissue was slower, particularly through day 28, than for the other three tissues.
As part of a gavage repeated dose toxicity study in rats with an SCCP, there was an assessment of toxicokinetics (IRDC, 1984b, unpublished study, see Documentation provided to EFSA) (see also Section 3.1.2.2.1 ). The test substance, animal species, dosing regimens and sample collection were identical to those described above for the dietary study. Also, the results were similar to those described above for the dietary study, except for a slight difference in the percentage of the administered radioactivity recovered in faeces (approximately 48–65%) and urine (approximately 13%).
A.1.2. MCCPs
As part of a dietary repeated dose toxicity study in rats with an MCCP, there was an assessment of toxicokinetics (IRDC, 1984c, unpublished study, see Documentation provided to EFSA) (see also Section 3.1.2.2.2 ). F344 rats (25 animals/sex per group) were administered 10 or 625 mg/kg bw per day of Cereclor S52 (C14–17, 52% chlorination) daily in the diet for 13 weeks. After 13 weeks, groups of animals received a single oral dose of a 14C‐labelled MCCP (about equal amounts of an 8‐14C‐labelled chlorinated n‐pentadecane (C15) and Cereclor S52) by gavage, the same dose level as received daily in the previous weeks. Other groups of animals (18 animals/sex per group), which were not treated previously with the MCCP, also received a single oral dose of the 14C‐labelled MCCP by gavage at 10 or 625 mg/kg bw. Following administration of the radiolabelled test substance, three animals per sex from each dose level were placed in metabolism cages and urine, faeces and exhaled air were collected for 12 h or 7 days. Additional groups of three animals per sex from each dose level were kept for 24 or 48 hours, 28 or 90 days following administration of the radiolabelled test substance and at the end of 12 hours, 7, 28 or 90 days samples of tissues (adipose, brain, gonads, heart, kidney and liver) were collected from designated surviving animals. In addition, samples of whole blood were collected from the orbital sinus prior to sacrifice of animals at 12, 24 and 48 h and 7 days. Faecal excretion was the main route of elimination with 40–48% and 53–61% of the dose in low‐ and high‐dose males, respectively, and 28–31% and 62–74% of the dose in low‐ and high‐dose females, respectively; most of the radioactivity (11–75%) was excreted during the first 2 days. Only a small proportion of the dose was excreted in urine (0.8–3%) and exhaled air (0.1–0.3%) within 7 days. Due to large interanimal variability in blood levels, a relationship between blood levels and dosing was difficult to quantify, but there was some evidence of a proportional relationship and decline over 7 days irrespective of sex and dosing regimen. There was also an indication that tissue levels were proportional to the administered dose. The highest initial concentrations of radioactivity were found in the liver, ovaries and kidneys. In adipose tissue, the concentration of radioactivity was highest at 7 days and then declined by a rate slower than that in liver and kidney.
In a dietary study briefly summarised by Birtley et al. (1980), distribution of an MCCP was examined. Male Wistar rats were administered 0.4 or 40 mg/kg of a 36Cl‐labelled Cereclor S52 (C14–17, 52% chlorination) in the diet for 10 or 8 weeks, respectively. At both dose levels, equilibrium levels of radioactivity were reached within 1 week for the liver and within 7 weeks for adipose tissue. No radioactivity was found in the brain or the adrenals. When the diet was changed from the 0.4 mg/kg radioactive MCCP containing diet to untreated diet, the half‐life for elimination of radioactivity from the abdominal fat was approximately 8 weeks; no radioactivity was detected in the liver 1 week post‐dosing.
As part of a dietary repeated dose toxicity study in rats with an MCCP, distribution to the liver and abdominal fat was examined (Poon et al., 1995, see also Section 3.1.2.2.2 ). Samples of liver and adipose tissue were taken at termination of the study from groups of Sprague–Dawley rats that had received a diet containing 0, 5, 50, 500 or 5,000 mg/kg (corresponding to 0, 0.4, 3.6, 36.2 or 362.9 mg/kg bw per day for males, and 0, 0.4, 4.2, 42.2 or 418.9 mg/kg bw per day for females) of an MCCP (C14–17, 52% chlorination) in the diet for 13 weeks. There was a dose‐dependent increase in the concentration of the MCCP in both liver and abdominal fat. The concentration in the abdominal fat was similar to that in the diet and was approximately 20–60 times higher than the concentration in the liver.
A study has been conducted to determine the elimination half‐life of MCCPs in the rat following a single oral administration (CXR, 2005, unpublished study, as cited in EU‐RAR, 2011). Male F344 rats received a single oral dose of an 8‐14C‐labelled CP (C15, 52% chlorination) at 525 mg/kg bw in corn oil by gavage. The highest concentrations of radioactivity were detected in the liver, kidney, fat and skin/fur at 24 h post‐dosing. The elimination half‐life was approximately 2–5 days for most tissues, and about 2 weeks for white adipose tissue. At termination of the study (day 89) approximately 2% of the administered radioactivity remained in the tissues, primarily in the skin/fur. Faecal excretion was the main route of elimination with approximately 50% of the administered radioactivity eliminated within the first 24 h after dosing, and approximately 70% at day 5 after dosing. Approximately 5% of the administered radioactivity was eliminated in the urine at day 5 after dosing.
A dietary study has been performed to investigate the bioaccumulation of MCCPs in rats (CXR, 2005a, interim report, see Documentation provided to EFSA). One group of F344 rats (48 animals of each sex, 6–9 weeks old at study start) was administered Cereclor S52 (C14–17 MCCP, 52% chlorination) in the diet ad libitum at 3,000 mg/kg ‘of supplied chemical, without any correction for purity’ corresponding to approximately 201 mg/kg bw per day for males and 234 mg/kg bw per day for females for 13 weeks. The animals were fed the diet until a steady‐state tissue (white adipose) level of the test compound was achieved which occurred after 14 weeks. The steady‐state tissue level was evaluated by sacrificing eight animals (four of each sex) at approximately 3‐week intervals and analysing the white adipose tissue level. After the steady‐state tissue level was attained, the remaining animals were fed a control diet. After the steady‐state tissue level was attained, the remaining animals were fed a control diet and groups of eight animals (four of each sex) were sacrificed at week 14, 15, 16, 18, 22 and 30 in order to determine the toxicokinetic elimination of the test compound by analysing blood, liver, kidney and white adipose tissue. In this interim report, only the content of the test compound in the white adipose tissue was reported. The average ingested doses of the test compound from the start of the study to the end of week 14 were approximately 201 mg/kg bw per day for males and 234 mg/kg bw per day for females. The content of the test compound in the white adipose tissue increased with time until the steady‐state concentration was achieved at week 13; females had an almost twofold higher content than males (males: 961 ± 395 μg MCCP/g adipose tissue; females: 1726 ± 236 μg MCCP/g adipose tissue). After the switch to the control diet, there was an initial (weeks 15–18) rapid elimination of the test compound from the white adipose tissue; initial half‐life of approximately 4 weeks. Then the elimination rate decreased markedly between weeks 18 and 22, and appeared to be almost stable between weeks 22 and 30.
The formation of sulfur‐containing metabolites of an MCCP has been studied in rats (Åhlman et al., 1986). Bile duct‐cannulated Sprague–Dawley rats (four females) were injected with 1‐chloro‐polychloro[U‐14C]hexadecane (14C‐PCHD; 65% chlorine by weight) into the portal vein (5–6 mg/kg bw). Bile was collected for 2 (3 animals) or 3 days (one animal), and the rat was then sacrificed and tissues (liver, kidney, fat, muscle, adrenal, ovary) were removed for measurement of radioactivity; radioactivity was also determined in bile, urine and faeces. Besides radioactivity determinations also thin‐layer chromatography was carried out to detect potential metabolites. The radioactivity was rapidly excreted in the bile (9–12% of the administered dose in 24 hours); the excretion rate was constant during 24–48 hours after administration of the dose, and only declined slightly on day 3 (one rat). The excretion of radioactivity in urine and faeces (three rats) was low (urine: 0.3–0.5%, faeces: 0.1–0.3% of the administered dose in 48 hours). In the tissues, the liver contained most of the radioactivity, but high concentrations were also found in the ovary and adrenals. Less than 3% of the total radioactivity excreted in the bile was due to the parent compound and it was indicated that the MCCP was conjugated to glutathione and N‐acetylcysteine (mercapturic acid). According to the authors, the study indicates that the mercapturic acid pathway is important in the metabolism of this MCCP, but the final identification of the metabolites requires further studies.
A.1.3. LCCPs
As part of an oral repeated dose toxicity study in rats with an LCCP, there was an assessment of toxicokinetics (IRDC, 1984d, see also Section 3.1.2.2.3 ). F344 rats (25 animals/sex per group) were administered 100 or 3,750 mg/kg bw per day of an LCCP (C22–26, 43% chlorination) daily by gavage for 13 weeks. After 13 weeks, groups of animals received a single oral dose of a 14C‐labelled LCCP (a 43.2% chlorinated pentacosane C25) by gavage, same dose level as received daily in the previous weeks. Other groups of animals (18 animals/sex per group), which were not treated previously with the LCCP, also received a single oral dose of the 14C‐labelled LCCP by gavage at 100 or 3,750 mg/kg bw. Following administration of the radiolabelled test substance, three animals per sex from each dose level were placed in metabolism cages and urine, faeces and exhaled air were collected for 12 h or 7 days. Additional groups of three animals per sex from each dose level were kept for 24 or 48 hours, 28 or 90 days following administration of the radiolabelled test substance and at the end of 12 hours, 7, 28 or 90 days samples of tissues (adipose, brain, gonads, heart, kidney and liver) were collected from designated surviving animals. In addition, samples of whole blood were collected from the orbital sinus prior to sacrifice of animals at 12, 24 and 48 h and 7 days. Faecal excretion was the main route of elimination and mainly during the first two days with only insignificant amounts thereafter. Only insignificant amounts of radioactivity were detected in urine and exhaled air. The concentrations of radioactivity in blood were similar between the two dose levels regardless of sex and dosing regimen. The highest initial concentration of radioactivity was found in the liver. In adipose tissue, the concentration increased between 7 and 28 days and then declined only slightly by 90 days. The concentration in ovaries was higher than in the adipose tissue after 12 h and 7 days, but was comparable with that in the adipose tissue at 28 days.
As part of a dietary repeated dose toxicity study in rats with a higher chlorinated LCCP, there was also an assessment of toxicokinetics (IRDC, 1984e, see also Section 3.1.2.2.3 ). F344 rats were administered 100 or 3,750 mg/kg bw per day of Electrofine S70 (C22–26, 70% chlorination) daily in the diet for 13 weeks. The animal species, dosing regimens and sample collection in the TK part of the study were identical to those described above for the 43.2% chlorinated LCCP, except for that the radiolabelled test material was a 70% chlorinated pentacosane. Almost all the radioactivity was excreted in the faeces irrespective of the sex, dose and dosing regimen. Only insignificant amounts of radioactivity were detected in urine and exhaled air. The concentrations of radioactivity in blood were greater in high‐dose animals. A similar difference was also noted for tissue concentrations of radioactivity. The highest initial concentration of radioactivity was found in the liver. In adipose tissue, the concentration increased slowly after dosing with only a little decline by 90 days. The concentration in ovaries was generally similar or lower than in other tissues.
An oral absorption study was carried out with an LCCP (Yang et al., 1987). Three female Sprague–Dawley rats (3–6 months old) were given a single oral dose of a 14C‐labelled C18‐chlorinated paraffin (50–53% chlorination) at 500 mg/kg bw. Urine and faeces were collected at 24, 48, 72 and 96 h after dosing and the animals were then sacrificed four days after dosing and tissues (liver, kidneys, stomach, small intestine, large intestine, urinary bladder, brain, heart, lung, spleen, muscle, bone (femur), gonads, retroperitoneal fat and residual carcass) were removed for measurement of radioactivity. Additionally, faeces were extracted and subjected to thin‐layer chromatography to separate the parent compound from the metabolites; the metabolites were not characterised. A total of 24.7% of the applied dose was recovered in the excreta at 24 h after dosing followed by more gradual increases to reach an average of 86.2% at 96 h after dosing. Significantly higher radioactivity was excreted in the faeces (76.4% of the dose) than in exhaled air (4.5%) and urine (1.9%) at 96 h after dosing; approximately 20% of the radioactivity in the faeces (approximately 16% of the dose) was identified as the parent LCCP. Approximately 3.3% of the dose was recovered in the tissues at 96 hours after dosing, primarily in the liver, intestines and fat.
A.2. Studies in mice
A.2.1. SCCPs
The disposition of three SCCP was studied in mice (Darnerud et al., 1982). Female C57B1 mice were administered a single oral dose or a single dose by i.v. injection of two different terminally 14C‐labelled 1‐chloro‐polychloro‐1‐14C‐dodecanes (SCCP‐I, chlorinated at the 14C‐atom but otherwise randomly, (C11H18.1Cl4.9)‐14CH2Cl, 55.9% chlorination by weight, number of chlorine atoms/mol = 5.9; and SCCP‐II, chlorinated at the 14C‐atom but otherwise randomly, (C11H14.2Cl8.8)‐14CH2Cl, 68.5% chlorination by weight, number of chlorine atoms/mol = 9.8) or a monochlorododecane (SCCP‐III, chlorinated only at the 14C‐atom, H3C‐(CH2)10‐14CH2Cl, 17.4% chlorination by weight, number of chlorine atoms/mol = 1). Distribution of the three SCCPs following oral administration was investigated using WBA in one mouse (SCCP‐I, SCCP‐III) or two mice (SCCP‐II), and following i.v. injection in three to five mice and in either one or two pregnant mice. Determination of radioactivity in liver and fat, as well as in exhaled air, urine and faeces was performed in four animals per SCCP. In the autoradiography part of the study, uptake of radioactivity at 24 h after administration (oral and i.v. injection) was seen in tissues with high cell turnover/high metabolic activity, e.g. intestinal mucosa, bone marrow, salivary glands and thymus (most pronounced for SCCP‐III, less for SCCP‐I and weak for SCCP‐II). The liver and intestinal contents contained most radioactivity in animals administered SCCP‐II. Radioactivity was also observed in white fat, as well as in bile and urine. At 4–12 days after i.v. injection, the radioactivity was most prominent in the liver and white fat; radioactivity was still present in bile and urine. At 30–60 days after i.v. injection, the authors reported that a considerable retention of radioactivity was seen in the central nervous system for SCCP‐III and SCCP‐I, but not for SCCP‐II. Radioactivity was still present in the liver and white fat, in the fat marked for SCCP‐II and weak for SCCP‐III. Autoradiograms from pregnant mice of late gestation (day 16 or 17) showed that the distribution of radioactivity within the fetuses was similar with the distribution in adult mice. The radioactivity in the fetuses was most pronounced for SCCP‐III and only weak for SCCP‐II. The tissue retention part of the study showed a marked labelling in the liver and fat at 24 h after oral administration of SCCP‐I and SCCP‐II; at 30 days, the radioactivity was minimal in the liver but still high in the fat. For SCCP‐I, 67% of the administered radioactivity was recovered 12 h after oral administration with 33% as 14CO2 in exhaled air, 29% in the urine and 5% in the faeces, whereas for SCCP‐II, only 33% was recovered 12 h after oral administration with 8% as 14CO2 in exhaled air, 4% in the urine and 21% in the faeces. According to the authors, the uptake of radioactivity in metabolically active tissues and the formation of 14CO2 are inverse to the degree of chlorination of the chloroalkanes. And the retention of radioactivity in liver and fat was most marked for the highest chlorinated CP, i.e. SCCP‐II. This means that the metabolic fate of CPs should be discussed on the basis of chlorine contents (and probably also chain length) and that results from one CP preparation cannot be considered valid for CPs in general.
The degradation of SCCPs (1‐14C‐chlorododecanes) to carbon dioxide was studied in mice (Darnerud, 1984). Before the administration of 1‐14C‐chlorododecanes), female C57B1 mice were administered the following substances by i.p. injection for three consecutive days with the last dose 24 h before the start of the study: phenobarbital (60 mg/kg bw, in 0.9% sodium chloride), 3‐methylcholanthrene (80 mg/kg bw, in corn oil), Cereclor S52 (C14–17, 52% chlorination by weight, 1,000 mg/kg bw, in corn oil), Witaclor 149 (C10–13, 49% chlorination by weight, 1,000 mg/kg bw, in corn oil) and Witaclor 171P (C10–13, 70–72% chlorination by weight, 1,000 mg/kg bw, in corn oil). Aroclor 1254 (PCB mixture, 54% chlorination by weight, 500 mg/kg bw, in corn oil) was administered as a single dose 4 days before the administration of 1‐14C‐chlorododecanes. Piperonyl butoxide (400 mg/kg bw, in corn oil) and metyrapone (100 mg/kg bw, in 0.9% sodium chloride) were administered as single doses 30 minutes before the administration of 1‐14C‐chlorododecanes. The vehicles as above were given to control mice. Groups of mice, pretreated as described above, were then given by i.v. injection the following radiolabelled substances: two different 1‐chloro‐polychloro‐1‐14C‐dodecanes (PCDD‐I, chlorinated at the 14C‐atom but otherwise randomly, (C11H18.1Cl4.9)‐14CH2Cl, 55.9% chlorination by weight, number of chlorine atoms/mol = 5.9; and PCDD‐II, chlorinated at the 14C‐atom but otherwise randomly, (C11H14.2Cl8.8)‐14CH2Cl, 68.5 chlorination by weight, number of chlorine atoms/mol = 9.8)), a 1‐monochloro‐1‐14C‐dodecane (MCDD, chlorinated only at the 14C‐atom, H3C‐(CH2)10‐14CH2Cl, 17.4% chlorination by weight, number of chlorine atoms/mol = 1), or an unchlorinated 1‐14C‐dodecane (DD, H3C‐(CH2)10‐14CH3). Pretreatment with inducers of CYP450 (phenobarbital, 3‐methylcholanthrene, Aroclor 1254) or technical grade CPs (Cereclor S52, Witaclor 149, Witaclor 171 P) generally had limited effects on the degree of 14CO2‐exhalation (results are only tabulated for PCDD‐II in the article, the number of animals per group was in general four). Pretreatment with CYP450 inhibitors (piperonyl butoxide, metyrapone) decreased the exhalation of 14CO2 considerably when measured as peak 14CO2‐exhalation rate (results are only tabulated for PCDD‐II in the article). Presentation of data with piperonyl butoxide (the most effective of the two inhibitors) showed that the inhibitory effect increased with increasing degree of chlorination of the paraffins. According to the authors, the results indicate a CYP450‐dependent degradation of C12‐chloroalkanes to 14CO2 in vivo and that the degradation seems to be more important for higher chlorinated paraffins.
A.2.2. MCCPs
The distribution and metabolism of a 14C‐labelled MCCP was studied in mice (Darnerud and Brandt, 1982). C57B1 mice were administered a terminally 14C‐labelled polychlorinated hexadecane (PCHD, 1‐14C‐C16H30.7Cl3.3, chlorinated at random, 34.1% chlorination by weight. The distribution was investigated by WBA. Eight females, two males and five pregnant females were given a single dose by i.v. injection and three females received a single dose orally. Sacrifice of the animals varied in the groups, from 5 minutes to 30 days. Tissue retention of radioactivity was determined in five groups given a single dose by i.v. injection (four females per group) and in five groups given a single dose orally (four females per group). One group from each administration route was sacrificed at 1 h, 4 h, 24 h, 4 days and 12 days after administration, respectively; blood and tissue samples from the liver, kidney, white (abdominal) fat, brown fat and the brain were collected and the radioactivity was measured. Ether extracts of tissues taken at one hour were separated by thin‐layer chromatography (TLC) and localised autoradiographically. The exhalation of 14C‐carbon dioxide was determined in four females given PCHD by i.v. injection and four females administered PCHD orally. Carbon dioxide was sampled from 15 minutes and until 12 h after administration of the test compounds; urine and faeces were collected at the end of the experiment (12 h after administration of the test compounds) and the radioactivity was measured. The autoradiography part of the study showed an initial high uptake of PCHD in tissues with a high cell turnover rate and/or high metabolic capacity such as the brown fat, intestinal mucosa, bone marrow and exocrine glands and only a low distribution to the white fat depots independent of the administration route (no autoradiogram presented in the article). Twenty‐four hours after i.v. injection, the highest radioactivity was seen in brown fat, liver, kidneys, spleen, adrenals, bone marrow, Harderian gland, salivary glands, pancreas and intestinal mucosa. At 12 days after i.v. injection, a high concentration of radioactivity was seen in the adrenal cortex, abdominal fat and the bile. At 30 days after i.v. injection, a high concentration of radioactivity was seen in the brain at a similar level to that in the liver (no autoradiogram presented in the article). Radioactivity was seen in fetuses on GD10 and at GD17 the distribution pattern was partly similar to that observed in adult mice. The tissue retention part of the study showed measurable radioactivity in all tissues examined (blood, liver, kidney, white and brown fat, and brain). The radioactivity in the tissues was generally higher following i.v. injection. For liver and kidneys, peak levels were observed at four hours. The highest levels were measured in brown fat and to a lesser extent in white fat and these tissue levels declined much more slowly than in the liver and kidney. No data were presented for the brain. TLC of samples taken from the liver, kidney and brown fat revealed a radiolabelled substance with the same retention value as the reference parent compound (PCHD), indicating that the PCHD was distributed to these tissues without further metabolism. The exhalation part of the study showed that 33% of the administered radioactive dose was eliminated as 14C‐carbon dioxide within 12 hours after oral administration; about 6.5% was eliminated in the urine and about 14% in the faeces. According to the authors, the PCHD is, at least partly, metabolised and eliminated in mice. But it is also emphasised that the results in this specific study are valid only for this PCHD and should not be taken as general for all CP preparations.
The disposition of a highly chlorinated MCCP was investigated in mice (Biessmann et al., 1983). Female C57B1 mice (22 g) were given a single dose of a uniformly 14C‐labelled 1‐chloro‐polychlorohexadecane (PCHD, C16H20.6Cl13.4, 69% chlorination by weight) orally (4 animals) or by i.v. injection (3 animals). Exhalation of 14CO2 was during 8 h after administration of PCHD, and urine and faeces were collected every 8th hour for 4 days, and assayed for radioactivity. For autoradiography one animal was sacrificed at 4 hours, and 1, 4, 12 and 30 days after the administration of PCHD. The autoradiography part of the study showed high radioactivities in bile, liver, kidney and intestinal contents up to 24 h after oral administration. In addition, the corpora lutea showed a high uptake of radioactivity up to 30 days after dosing. A long retention in fat was also seen. The elimination of radioactivity in urine was about 1% during 8 h and about 3% during 96 h after both oral and i.v. administration. The elimination in faeces during 8 h was about 22% and 2% after oral and i.v. administration, respectively, and about 66% and 43% during 96 h. The elimination in exhaled air was about 1%.
The distribution of radioactivity in the brain of a highly chlorinated MCCP has been studied in pre‐weaning mice (Eriksson and Darnerud, 1985, as cited in EU‐RAR, 2008). NMRI mice (aged 3, 10 or 20 days old; six and two animals per group for tissue retention and autoradiography, respectively) were administered a single oral dose of a 14C‐labelled PCHD (C16, 69% chlorination) by gavage. Animals were sacrificed one and seven days after administration of PCHD. The only quantitative determinations of levels of radioactivity made were for the brain and liver. The autoradiography part of the study showed high levels of radioactivity in adipose tissue, adrenals and in myelinated areas of the brain in 10‐day‐old mice (no data were presented for 3‐ or 20‐day‐old mice). The tissue retention part of the study showed a level of radioactivity in the brain of approximately 3% of the dose administered in 3‐day‐old mice and approximately 0.5% in 20‐day‐old mice, at 24 hours after administration of PCHD. For mice of each age, the level of radioactivity was lower at seven days compared to 24 h in both brain and liver samples.
A.2.3. LCCPs
No studies on the toxicokinetics of LCCPs in mice have been identified.
Appendix B – Detailed description of the toxicokinetic studies in farm animals
1.
The studies on the toxicokinetics of CPs in farm animals (poultry and fish) included in Section 3.1.1.3 are described in more detail in this Appendix.
B.1. Studies in poultry
Chickens/hens
SCCPs
The tissue distribution of an SCCP was investigated in broiler chickens (Ueberschär and Matthes, 2004). Male ‘Lohmann meat’ broiler chickens (1 day old) were given diets supplemented with an SCCP (C10–13, 60% chlorination) in concentrations of 0 (48 animals), 2 (eight animals), 20 (eight animals), 45 (eight animals), 70 (eight animals) and 100 mg/kg bw (48 animals) for 31 days. The SCCP concentrations in abdominal fat (0.36, 1.32, 10.0, 19.4, 27.3, 38.5 mg/kg ww), breast meat (0.02, 0.07, 0.06, 0.16, 0.15, 0.20 mg/kg ww), liver (0.03, 0.10, 0.22, 0.35, 0.54, 0.60 mg/kg ww) and kidneys (0.05, 0.11, 0.28, 0.42, 0.67. 0.79 mg/kg ww) were related linearly to the SCCP concentration of the feed. The highest contents were recovered in abdominal fat with lower contents in liver and kidneys and the lowest contents in blood, meat and bile.
The tissue distribution, accumulation and elimination kinetics of an SCCP were investigated in laying hens (Ueberschär et al., 2007). Laying hens of the ‘Lohmann selected Leghorn’ strain (24 weeks old) were given diets supplemented with an SCCP (C10–13, 60% chlorination) in concentrations of 0 (21 animals), 2 (nine animals), 20 (nine animals), 45 (nine animals), 70 (nine animals) and 100 mg/kg bw (36 animals) for 8 weeks. To follow‐up the decrease of the SCCP concentrations in the tissues, and to describe the elimination kinetics, a withdrawal study was started with additional 15 birds from the high‐dose group at the end of experiment (8th week of the experiment), in total 24 hens. Three animals were slaughtered on days 1, 2, 4, 8, 14, 21, 28 and 42 after the study started; in the control group three animals were slaughtered on days 14, 28 and 42. At the end of the experiment (32 weeks of age), tissue samples were pooled from nine animals of each group, a mixed sample of eggs (yolk and albumen) from nine animals of each group was prepared, and excreta from five birds of each group were collected. To follow the increase in SCCP concentrations in eggs, samples of yolk and albumen pooled from ten eggs from different birds of the control group and the high‐dose group were analysed on nine specific dates from the beginning until the end of the experiment. The SCCP concentrations in the tissues were linearly related to the SCCP concentration of the feed. The highest SCCP concentrations were found in abdominal fat (0.13, 0.74, 2.02, 4.25, 7.92, 11.1 mg/kg ww), egg yolk (0.03, 0.14, 1.00, 1.98, 4,43, 6,37 mg/kg ww) and liver (0.02, 0.07, 0.17, 0.37, 0.73, 0.97 mg/kg ww), whereas breast muscle (0.03, 0.04, 0.07, 0.06, 0.10, 0.45 mg/kg ww), egg albumen and bile contained minimal or no residues. The kinetic part of the study revealed a biphasic elimination with half‐lives of 4–40 minutes in the liver, kidneys, legs, fat and blood for the initial rapid phase, and 15–30 days in blood, fat, liver, yolk, kidneys and legs for the terminal slow phase.
MCCPs
No studies on the toxicokinetics of MCCPs in broiler chicken/hens have been identified.
LCCPs
No studies on the toxicokinetics of LCCPs in broiler chicken/hens have been identified.
Quail
SCCPs and MCCPs
The distribution and metabolism of an SCCP and an MCCP were studied in Japanese quail (Biessmann et al., 1982). Female laying Japanese quail (165 g) were given a single oral dose by gavage of either a 14C‐labelled SCCP (C12H20.1Cl5.9, 55.9% chlorination by weight, chlorinated at random but all 14C positions chlorinated) or a 14C‐labelled MCCP (C16H30.7Cl3.3, 34.1% chlorination by weight, chlorinated at random). For whole‐body autoradiography, individual animals were sacrificed at 1 and 4 hours, and 1, 4 and 12 days after dosing. Four animals at each survival time were taken for radioactivity measurements of 14C in selected organs (not further specified). Additionally, the first 10 eggs laid by the quail from the 12‐day group were collected, separated into shell, albumen and yolk, and assayed for radioactivity. Solvent partitioning was carried out with pooled tissue samples (fat, yolk, uropygial gland, kidney, oviduct) of each quail of each survival time. Exhaled air was collected from four quail after 15 minutes, 30 minutes and then every hour up to 8 h and measured for 14CO2; urine and faeces were collected after 8 h. WBA showed high radioactivity levels in tissues with high metabolic activity and high cell turnover rate (liver, intestinal mucosa, spleen, bone marrow and oviduct) up to 1 day after exposure; high levels were also seen in the gall bladder and the kidney, as well as in lipid‐rich tissues such as the outer layers of the yolk of the growing follicles and in the uropygial gland epithelium. There was also a high uptake of radioactivity in the eggshells and, to a lesser extent, in the albumen. In the fat, radioactivity increased with time. At 4 and 8 days after exposure, the highest radioactivity was observed in fat, the yolk of the follicles and in the uropygial gland. The distribution patterns of the SCCPs and the MCCPs were almost identical after all exposure times. The quantitative radioactivity measurements in selected organs revealed a higher amount of radioactivity in the gall bladder and kidney with the SCCP compared with the MCCP, whereas the opposite was seen in the yolk of the follicles. The radioactivity in the tissues declined steadily during 12 days after dosing, whereas the decline in the lipid‐rich tissues (e.g. fat, uropygial gland, follicle yolk) was comparably slower. In the egg yolk and albumen, the concentration of the SCCP was about half of the MCCP. The partition quotients in heptane/water of fat, yolk and uropygial gland showed a similar increase during the exposure time for both CPs, whereas the quotients in dimethyl formamide/heptane were considerably higher with SCCP compared with MCCP, particularly in the body fat. Following dosing with the MCCP, the exhalation of 14CO2 was about twice (38.8%) that of the SCCP (21.6%). The excretion of radioactivity in urine and faeces (combined) was also higher following dosing with MCCP (13.5%) compared with SCCP (9.8%). In eggs, the radioactivity in yolk, albumen and eggshells following dosing with MCCP was about twice that of the SCCP. The results of this study showed a higher distribution of the CPs to lipid‐rich tissues. Quantitative differences were observed in the rate of metabolism of the CPs depending on the chain length and/or the degree of chlorination and high chlorination seemed to favour the retention in fatty tissues as the parent compound.
The disposition of a highly chlorinated MCCP was investigated in Japanese quail (Biessmann et al., 1983). Female Japanese quail were given a single dose of a uniformly 14C‐labelled 1‐chloro‐polychlorohexadecane (PCHD, C16H20.6Cl13.4, 69% chlorination by weight) orally or by i.v. injection. For assessment of elimination in excreta, urine and faeces were collected every 8th hour during 4 days, and assayed for radioactivity. For whole‐body autoradiography, one animal was sacrificed at 4 hours, and 1, 4, 12 and 30 days after the administration. The elimination of radioactivity in urine and faeces combined was about 2% and 17% during 8 h after oral and i.v. injection, respectively, and about 58% during 96 hours after oral administration (elimination after i.v. administration was not determined). The elimination in exhaled air was about 1%. The autoradiographic distributions showed high radioactivities in bile, liver, kidney and intestinal contents up to 24 h after dosing. In addition, high radioactivity was observed in the hypophysis, retina, blood and egg yolk. After 12 days, radioactivity was only observed in the lipid‐rich tissues (fat, uropygial gland, egg yolk) and in the liver.
LCCPs
No studies on the toxicokinetics of LCCPs in quail have been identified.
B.2. Studies in fish
SCCPs
Bioaccumulation of an SCCP from food was studied in rainbow trout (Salmo Gairdneri Richardson) (Lombardo et al., 1975). Fingerling rainbow trout were fed a diet containing Chlorowax 500C50 at 10 mg/kg for up to 82 days. Samples of 20 experimental and 10 control fish were collected at 22, 30, 58, 72 and 82 days for residue analysis. The analysis was carried out on the whole fish, but without head, tail and viscera. SCCP residues ranging from 0.5 to 1.1 mg/kg were observed when expressed on a tissue basis, 11 to 18 mg/kg when expressed on a fat basis.
MCCPs
Distribution of an MCCP was studied in carp and bleak (Darnerud et al., 1983). Carp (Cyprinus carpio) (body weight 370–415 g) and bleak (Alburnus alburnus) (body weight 4.5–5.5 g) were injected (intra‐arterial) with a polychloro‐1‐14C‐hexadecane (PCHD, 34% chlorination by weight). Excreted 14CO2 was collected from three carp at 4, 24, 72 and 96 hours after injection. Whole‐body autoradiography was performed in one carp sacrificed 1, 5 and 13 days after dosing. Ten bleaks were exposed during 14 days to PCHD added to the tank water and two fish were removed after 24 hours and 14 days of exposure; the remaining fish were transferred to clean water and two of these fish were removed after 24 hours, and 7 and 35 days. In carp, the cumulated excretion of 14CO2 during 96 hours amounted to 6.4% of the injected radioactivity. Autoradiography of the carp showed a high concentration of labelled substance in the bile and intestinal contents. One day after dosing, the highest radioactivity appeared in the kidney, liver, nasal mucosa and fat and was still dominating 5 days after dosing and a marked uptake was also observed in gills, testis and brain; tissue levels were generally lower 13 days after dosing. In the bleak, a marked uptake of radioactivity was noted in gills, nasal cavity, skin, liver and fat after one day of exposure; a high level was also present in the brain, bile and intestinal contents. A similar distribution pattern was seen after 14 days of exposure, as well as in fish allowed to recover for 1, 7 and 35 days.
LCCPs
Uptake of two different LCCPs from food was studied in the Atlantic salmon (Zitko, 1974). Juvenile Atlantic salmon (Salmo salar) (20 fish per tank) were fed with dry fish food containing Cereclor 4251 and Chlorez 70052 at concentrations of 10 and 100 μg/g for up to 181 days followed by a control diet for 74 days. At times one fish from each tank was sacrificed and analysed. The analysis was carried out on the whole fish. The two CPs did not accumulate in the fish.
Bioaccumulation of an LCCP was studied in rainbow trout and mussels (Madeley and Birtley, 1980). Rainbow trout (Salmo gairdneri) and mussels (Mytilus edulis) were administered diets uniformly mixed with Cereclor 42 (C20–30, 42% chlorination). A 14C centrally labelled pentacosane (C25, 42% chlorination) allowed concentrations of CP to be followed in food and animal tissues. Fish (40 individuals) were fed diets containing average concentrations for 47 and 385 mg/kg for 35 days; a control group was fed the same but uncontaminated diet. Subsequently, all fish were fed the control diet for a further 49 days. Mussels received dry baker's yeast dosed with a mean concentration of 524 mg/kg before suspension in freshwater for addition to the test tanks for 47 days; a control group received untreated yeast. Subsequently, all mussels received untreated yeast for a further 56 days. Test and control animals were sampled regularly during the feeding with dosed food, as well as after substitution with uncontaminated diet (six mussels and three fish at each sampling time) and dissected into appropriate tissue and organs for determination of radioactivity. The LCCP was found in the tissues of both fish and mussels. Comparison of the LCCP concentration in tissues suggested that mussels eliminated the LCCP as the parent compound whereas the fish appeared to metabolise the test compound.
Studies with SCCPs, MCCPs and LCCPs
Structure related uptake of three SCCPs, one MCCP and one LCCP when added to tank water was studied in bleak (Bengtsson et al., 1979). Bleak (Alburnus alburnus L) were caught in the Baltic Sea and acclimated to laboratory conditions during three to five months. The fish (15 fish/group, average weight of 4.5 g) were exposed during 14 days to the CP test solutions (125 μg/L) added to the tank water (natural Baltic Sea water) and renewed every second or third day. The CP test solutions included Witaclor 149 (C10–13, 49% chlorination), Witaclor 159 (C10–13, 9% chlorination), Witaclor 171P (C10–13, 71% chlorination), Witaclor 350 (C14–17, 50% chlorination) and Witaclor 549 (C18–26, 49% chlorination). The fish were not fed during the experiments. Five fish were sampled from each of the six groups and placed in uncontaminated Baltic Sea water, which was renewed with the same frequency as the test solutions. Additional samples of fish were taken after one and seven days in the uncontaminated water. A clear relation between the structure of the CPs and their uptake in the fish was seen with a short‐chain length and a low level of chlorination being the most effective form for uptake among the tested CPs.
Uptake and elimination of two SCCPs and one LCCP from food was studied in bleak (Bengtsson and Ofstad, 1982). Bleak (Alburnus alburnus) were caught in the Baltic Sea and kept in brackish water from the bay. The fish (30 fish/group, average weight of 4 g) were exposed to CP‐treated food every day for 91 days (accumulation period) and then the same food, without the CPs, was given for 316 days (elimination period). The CPs included Witaclor 149 (C10–13, 49% chlorination, three different concentrations: 590, 2,500, 5,800 μg/g food), Witaclor 171P (C10–13, 71% chlorination, 3,180 μg/g food) and Witaclor 549 (C18–26, 49% chlorination, 3,400 μg/g food). The content of CPs in the fish was determined in three fish (pooled sample, whole‐body measurements) from each of the test groups and three fish from the control group. Samples were taken on days 14, 28, 56 and 91 of the accumulation period and on days 7, 35 and 133 of the elimination period. For Witaclor 149, there was a direct correlation between the amount of CP in the food and the level of organic chlorine found in the fish tissues during the first 56 days. During the next 5 weeks, a steep increase in tissue levels of organic chlorine was observed in the low‐dose and mid‐dose, while levels in the high‐dose fish were unchanged. When the data were transformed to indicate efficiency in uptake and retention about 45% of the CP given to the low‐dose fish was found in the fish tissues after 91 days, whereas for the mid‐dose and high‐dose fish retention was about 10% and 5%, respectively, indicating a less effective uptake and/or a more effective metabolism of the CP with increasing concentrations in food. The levels of Witaclor 149 decreased rapidly during the elimination period. For Witaclor 171P, the bioaccumulation pattern was similar to the mid‐dose Witaclor 149 group. The uptake was fairly constant during the whole accumulation period. At the end of the accumulation period the uptake from food was around 6%. A high retention was observed and this CP remained in the fish tissues on a steady level until the end of the elimination period. For Witaclor 549, the uptake was inefficient with only about 2% by the end of the accumulation period. During the elimination period, about 50% was excreted within 4–5 weeks, whereas the remaining 50% did not seem to be excreted during the subsequent 40 weeks.
Accumulation of one SCCP and two MCCPs when added to tank water was studied in rainbow trout (Darnerud et al., 1989). Rainbow trout (Salmo gairdneri) (15–25 g, four fish per group) were exposed during 7 days to PCHD‐L (1‐chloro‐polychloro‐[U‐14C]hexadecane, 23.1% chlorination by weight, 3.64 μmol), PCHD‐M (1‐chloro‐polychloro‐[U‐14C]hexadecane), 51.4% chlorination by weight, 3.64 μmol) and PCDD‐H (1‐chloro‐polychloro‐[1‐14C]dodecane, 68.5% chlorination by weight, 1.74 μmol) added to the newly changed water. Fish were removed for whole‐body autoradiography after 24 hours and 7 days of exposure. The remaining fish were transferred to clean water and removed after 7 or 21 days. After 24 hours exposure to PCHD‐L, the highest radioactivities were found in the contents of the gall bladder, pyloric caeca and intestines; a pronounced uptake of radioactivity was also observed in the olfactory organs and gills whereas the labelling of the liver, kidney and skin was low. At 7 days, the uptake in the olfactory organs and gills was less marked; the concentration of radioactivity in fat tissue had increased. After 7 and 21 days in clean water, the distribution pattern were similar to that found after 7 days in contaminated water; the olfactory organs and the gills still retained radioactivity. After 24 hours exposure to PCHD‐M, the distribution pattern was almost similar to that observed for PCHD‐L, including the selective uptake in the olfactory organs and gills; however, the liver was comparably more labelled after PCHD‐M exposure. After 7 days in PCHD‐M‐containing water the uptake in the fish was also generally the same as for PCHD‐L. However, the uptake in fat seemed more accentuated for PCHD‐M and the accumulation of radioactivity in fatty tissues was even more prominent at later time points, although the highest radioactivity was still observed in the bile and intestinal contents, while the olfactory organs and gills only contained low concentrations of radioactivity. For PCDD‐H, high uptake was initially observed in the liver, olfactory organs, gills, skin and kidneys. At longer exposure times the radioactivity decreased in the olfactory organs, gills and skin, while the radioactivity in fat steadily increased. At the end of the experiment radioactivity was mainly present in fat, intestinal contents, liver and bile.
Bioaccumulation, metabolism and tissue distribution following dietary administration of two SCCPs and two MCCPs was studied in the rainbow trout (Fisk et al., 1996). Juvenile rainbow trout (Oncorhynchus mykiss) (initial weights of 2–7 g) were fed diets with two different SCCPs (C12H20Cl6, 56% chlorination by weight; C12H16Cl10, 69% chlorination by weight) and two different MCCPs (C16H31Cl3, 35% chlorination by weight; C16H21Cl13, 69% chlorination by weight) for 40 days. The daily rate of feeding was equal to 1.5% of the mean weight of the rainbow trout, corrected after each sampling period. The dietary concentrations were 20 and 200 ng/g for each CP, and also 2,000 ng/g for C16H21Cl13. The feeding period was followed by 160 days (C12H20Cl6, C12H16Cl10, C16H31Cl3) or 173 days (C16H21Cl13) of depuration. Fifty fish were used in the control tank and the three C16H21Cl13 treatments, and 36 fish were used in the other treatments. From each treatment group three fish were taken for determination 14C radioactivity on days 5, 10, 20, 30 and 40 of the uptake period and on days 5, 10, 20, 40, 80 and 160 (or 173 for C16H21Cl13 treatments) of the depuration period. In addition, six fish from the C16H21Cl13 high‐concentration treatment group were given the MCCP‐spiked food for 80 days to follow the uptake for a longer exposure period. The fish were separated into liver, gastrointestinal (GI) tract and carcass (i.e. whole fish minus liver and GI tract) and each part was weighed and analysed for 14C radioactivity. All four CPs accumulated in the fish by day 5 of the uptake phase without reaching steady state; the depuration rate in fish exposed to C16H31Cl3 was significantly more rapid than the depuration rates in fish exposed to the other three CPs. Whole‐body half‐lives varied from 37 ± 2 days in the high‐concentration C16H31Cl3 group to 87 ± 11 days in the low‐concentration C12H16Cl10 group. Assimilation efficiencies based on whole‐body concentrations ranged from 9.4 ± 1.1% in the mid‐concentration C16H21Cl13 group to 37.6 ± 1.1% in the low‐concentration C12H16Cl10 group. Whole‐body biomagnification factors varied from 0.44 in the mid‐concentration C16H21Cl13 group to 2.15 in the low concentration C12H16Cl10 group. It should be noted that the biomagnification factors are maximum values as they include 14C‐labelled formed metabolites. The highest percentages of radioactivity were found in the carcass for all four CPs (ranging from 50% to higher than 70%. Relative amounts in the liver were low (about 1.5% of the total fish weight). HPLC chromatograms of extracts indicate that the CPs are metabolised, most pronounced for C12H20Cl6. The more highly chlorinated SCCP and MCCP showed lower degree of metabolism. According to the authors, the results of this study indicate that the highly chlorinated SCCP and the lower chlorinated MCCP have the greatest potential for bioaccumulation by aquatic organisms; however, uptake from the GI of the highly chlorinated MCCP may have been hindered because of its large molecular size.
Bioaccumulation following dietary administration of CPs was studied in the rainbow trout (Fisk et al., 1998). Juvenile rainbow trout (Oncorhynchus mykiss) (initial weights of 2–7 g) were fed diets containing one of 19 different CPs with varying carbon chain length (C10, C11 and C14) and chlorine content (4–8 chlorine atoms) for 40 days followed by 80 days of depuration. The CONTAM Panel noted that the CPs used in this study are with known carbon chain length, chlorine content and chlorine position, but also that the authors clearly stated that although the CPs included in the study have the same carbon chain lengths and chlorine content as some CPs, all were 1,2‐chlorine substituted and would not likely be prevalent in commercial CPs. Three fish were sampled from each treatment for 14C determination on days 5, 10, 20, 30 and 40 of the uptake period and on days 5, 10, 20, 40 and 80 of the depuration period. Sampled fish were separated into liver, GI tract and carcass (i.e. whole fish minus liver and GI tract). Each tissue (including the carcass) was weighed and analysed for 14C radioactivity. All of the CPs were detected in the fish after 5 days of exposure. With the exception of the C14‐CPs, most compounds reached steady state between food and fish within 30 or 40 days. Differences in bioaccumulation parameters between CPs with the same molecular formula (C10H15Cl7) but different chlorine positions were observed for some of the CPs (e.g. C10H15Cl7(a) and C10H15Cl7(b)), but not for others (C10H17Cl5(a) and C10H17Cl5(b)). The half‐lives of the CPs ranged from 7 days for C10H17Cl5 to 53 days for C14H25Cl5, The influence of adjacent and terminal chlorine substitution (1,2,9,10‐C10, 1,2,10,11‐C11 and 1,2,13,14‐C14, common to all CPs used in this study) on the half‐lives were, according to the authors, difficult to interpret. There was a large range in the assimilation efficiencies, from 13% for C10H17Cl5(a) to 130% for C10H16Cl6(a) and C14H24Cl6(a). According to the authors, these results showed that all of the CPs were rapidly accumulated and had high assimilation efficiencies from food, that the half‐lives increased with increasing carbon chain length and chlorine content, indirectly, that the CPs were metabolised in the rainbow trout with the susceptibility to metabolism decreasing with greater carbon chain length and chlorine content, and that higher chlorinated C10‐ and C11‐CPs, and all C14‐CPs, would biomagnify from food to fish in aquatic food chains.
Bioaccumulation and biotransformation following dietary administration of an SCCP, an MCCP and an LCCP was studied in the rainbow trout (Fisk et al., 2000). Juvenile rainbow trout (Oncorhynchus mykiss) (initial weights of 1–5 g) were fed diets containing one of three 14C‐CPs (C10H15.3Cl6.7, C14H23.3Cl6.7 and C18H31.4Cl6.6). The daily rate of feeding was equal to 1.5% of the mean weight of the rainbow trout, corrected after each sampling period. Thirty‐six fish were used in each group. Three fish were sampled from each treatment for 14C determination on days 5, 10, 20, 30 and 40 of the uptake period and on days 5, 10, 20, 40, 80 and 160 of the depuration period. Sampled fish were separated into liver, GI tract and carcass (i.e. whole fish minus liver and GI tract). Each tissue was weighed, but only the carcass was analysed for 14C radioactivity. Biotransformation of CPs was examined by comparing toluene extractable and non–toluene extractable 14C radioactivity in the carcass on day 40 of uptake and on day 40 of depuration. All three CPs accumulated readily from the food, but without reaching steady state. The uptake curves were similar for all three CPs, and for the two concentrations. The assimilation efficiencies varied from 10 to 22%, except for the high‐concentration C10H15.3Cl6.7 group (approximately 72%) and were, according to the authors, much lower than reported assimilation efficiencies for other CPs in rainbow trout. Depuration of the CPs appeared to be a first‐order process. The half‐lives of C18H31.4Cl6.6 (79 and 91 days) were significantly greater than those of the two shorter chain CPs (26–58 days) although a clear pattern was not seen between the shorter chain CPs or for the concentrations. The biomagnification factors (BMFs) were between 0.1 and 1 when calculated from the feeding and depuration rates indicating that these CPs may not biomagnify in the aquatic food chains, but that food‐chain transfer remains an important route of accumulation in aquatic organisms. The BMFs calculated assuming assimilation efficiencies of 50 and 90% resulted in BMFs that were greater than 1 (0.9–2.8) and both exhibited increasing trends with increasing carbon chain length; these BMFs indicate that the CPs have the potential to biomagnify in aquatic food chains. The biotransformation evaluation of the CPs showed that on day 40 of uptake, the percentage of extractable 14C in rainbow trout exposed to the C10H15.3Cl6.7 was less than that in rainbow trout exposed to longer‐chain CPs, indicating greater biotransformation of C10H15.3Cl6.7; the differences were not as obvious on day 40 of depuration. According to the authors, these results showed that all three CPs have the potential to biomagnify in aquatic food chains with increasing trends with increasing carbon chain length and greater biotransformation of the short‐chain compared to the longer chain CPs.
Appendix C – BMD modelling of rodent studies
C.1. Introduction
This Appendix contains the details of the BMD modelling performed on experimental animal data. In this introduction a general description of the approach followed in the modelling is given.
Selection of the BMR
The benchmark dose (BMD) is defined as the estimated dose that corresponds with a predefined change in response compared with the background response. The benchmark response (BMR) is the response corresponding with the estimated BMD of interest.
The CONTAM Panel considered the default BMRs of 5% and 10% for continuous and quantal data, respectively, as indicated in the EFSA guidance on BMD in risk assessment (EFSA Scientific Committee, 2017a). Deviations from the default BMR were selected on a case‐by‐case basis and are justified in the specific modelling reports in this Appendix.
A 90% confidence interval around the BMD was estimated, the lower bound is reported by BMDL and the upper bound by BMDU.
Software used
Results were obtained using the EFSA web‐tool for BMD analysis, which used the R‐package PROAST, version 66.38, for the underlying calculations.
Averaging results from multiple fitted benchmark dose models (used only for modelling of quantal data) is based on the methodology in Wheeler and Bailer (2008).
Specification of deviations from default assumptions
No deviations from general assumptions were introduced.
The CONTAM Panel selected the following default models:
Default set of fitted models:
| Model | Number of parameters | Formula | |
|---|---|---|---|
| Null | 1 | y = a | |
| Full | no. of groups |
|
|
| Logistic | 2 |
|
|
| Probit | 2 |
|
|
| Log‐logistic | 3 |
|
|
| Log‐probit | 3 |
|
|
| Weibull | 3 |
|
|
| Gamma | 3 |
|
|
| Two‐stage | 3 |
|
|
| Exp model 3 | 3 |
|
|
| Exp model 5 | 4 |
|
|
| Hill model 3 | 3 |
|
|
| Hill model 5 | 4 |
|
In the case of quantal data, for the Exp and Hill family, models were fit with 3 and 4 parameters as listed in the table. The 3‐parameter model is selected if the difference in AIC is smaller than 5, otherwise the 4‐parameter model is selected.
Procedure for selection of BMDL
BMDL was selected applying the following flow chart given in the EFSA Scientific Committee (2017a,b,c) guidance:
Flow chart for selection of BMDL
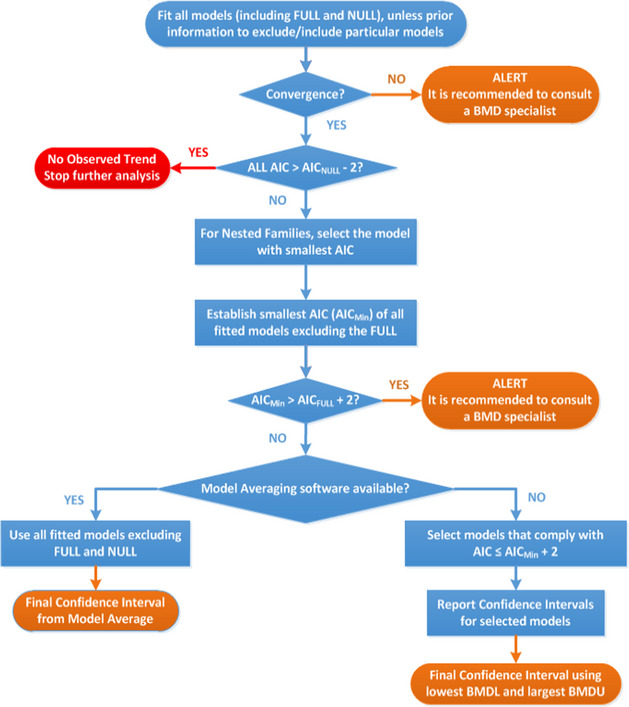
C.2. Incidence of nephritis in male rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984a)
Data description
The endpoint to be analysed is: increased incidence of nephritis.
Data used for analysis:
| Concentration (mg/kg bw per day) | Incidence of nephritis | N |
|---|---|---|
| 0 | 3 | 15 |
| 10 | 2 | 15 |
| 100 | 9 | 15 |
| 625 | 14 | 15 |
Selection of the BMR
A default BMR of 10% was selected (EFSA Scientific Committee, 2017a).
Results
Response variable: increased incidence of nephritis
Fitted Models:
| Model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| Null | 1 | −41.46 | 84.92 | NA | NA | NA | NA | |
| full | 4 | −27.17 | 62.34 | NA | NA | NA | NA | |
| two.stage | 3 | −27.99 | 61.98 | yes | 12.800 | 39.2 | 21.50 | yes |
| log.logist | 3 | −27.44 | 60.88 | yes | 4.110 | 62.3 | 22.60 | yes |
| Weibull | 3 | −27.84 | 61.68 | yes | 1.550 | 58.3 | 13.10 | yes |
| log.prob | 3 | −27.42 | 60.84 | yes | 4.470 | 63.4 | 22.10 | yes |
| gamma | 3 | −27.90 | 61.80 | yes | 0.842 | 64.5 | 14.00 | yes |
| logistic | 2 | −29.37 | 62.74 | yes | 32.000 | 85.2 | 52.90 | yes |
| probit | 2 | −29.51 | 63.02 | no | NA | NA | 57.90 | yes |
| LVM: Expon. m3‐ | 3 | −28.12 | 62.24 | yes | 0.592 | 58.9 | 8.53 | yes |
| LVM: Hill m3‐ | 3 | −27.97 | 61.94 | yes | 0.976 | 56.9 | 10.50 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 0.1702
estimate for BMD‐ : 21.46
estimate for c : 1e‐06
log.logist
estimate for a‐ : 0.1593
estimate for BMD‐ : 22.58
estimate for c : 1.464
Weibull
estimate for a‐ : 0.1552
estimate for BMD‐ : 13.1
estimate for c : 0.8471
log.prob
estimate for a‐ : 0.1618
estimate for BMD‐ : 22.14
estimate for c : 0.8319
gamma
estimate for a‐ : 0.1585
estimate for BMD‐ : 14
estimate for cc : 0.8121
logistic
estimate for a‐ : ‐1.12
estimate for BMD‐ : 52.94
probit
estimate for a‐ : ‐0.6793
estimate for BMD‐ : 57.94
EXP
estimate for a‐ : 1.291
estimate for CED‐ : 8.53
estimate for d‐ : 0.4994
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 1.292
estimate for CED‐ : 10.5
estimate for d‐ : 0.6013
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.17 | 0.12 | 0.18 | 0.11 | 0.07 | 0.06 | 0.09 | 0.1 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 2.29 | 76.4 |
Confidence intervals for the BMD are based on 500 bootstrap data sets.
Visualisation:
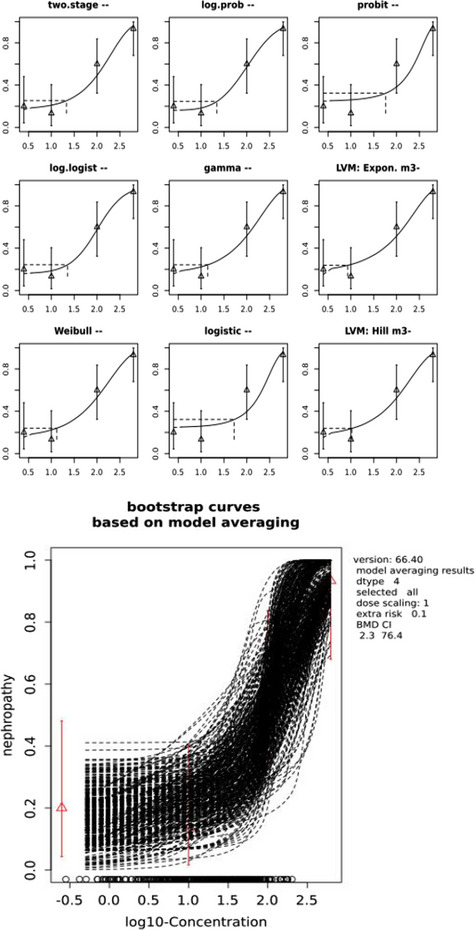
C.3. Incidence of thyroid hypertrophy in male rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984a)
Data description
The endpoint to be analysed is: incidence of thyroid hypertrophy.
Data used for analysis:
|
Concentration (mg/kg bw per day) |
Incidence of thyroid hypertrophy | N |
|---|---|---|
| 0 | 3 | 15 |
| 10 | 4 | 15 |
| 100 | 5 | 15 |
| 625 | 14 | 15 |
Selection of the BMR
A default BMR of 10% was selected (EFSA Scientific Committee, 2017a).
Results
Response variable: incidence of thyroid hypertrophy.
Fitted Models:
| model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −41.05 | 84.10 | NA | NA | NA | NA | |
| full | 4 | −29.43 | 66.86 | NA | NA | NA | NA | |
| two.stage | 3 | −29.50 | 65.00 | yes | 19.70 | 181.0 | 73.0 | yes |
| log.logist | 3 | −29.52 | 65.04 | no | NA | NA | 87.4 | yes |
| Weibull | 3 | −29.51 | 65.02 | no | NA | NA | 79.9 | yes |
| log.prob | 3 | −29.52 | 65.04 | no | NA | NA | 89.0 | yes |
| gamma | 3 | −29.52 | 65.04 | no | NA | NA | 83.5 | yes |
| logistic | 2 | −29.50 | 63.00 | yes | 41.50 | 102.0 | 65.6 | yes |
| probit | 2 | −29.50 | 63.00 | yes | 45.70 | 98.7 | 66.7 | no |
| LVM: Expon. m3‐ | 3 | −29.50 | 65.00 | yes | 4.09 | 395.0 | 70.1 | yes |
| LVM: Hill m3‐ | 3 | −29.50 | 65.00 | yes | 5.77 | 370.0 | 74.4 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 0.2266
estimate for BMD‐ : 72.98
estimate for c : 3.585
log.logist
estimate for a‐ : 0.2328
estimate for BMD‐ : 87.36
estimate for c : 2.311
Weibull
estimate for a‐ : 0.2304
estimate for BMD‐ : 79.92
estimate for c : 1.528
log.prob
estimate for a‐ : 0.2333
estimate for BMD‐ : 89.04
estimate for c : 1.355
gamma
estimate for a‐ : 0.2319
estimate for BMD‐ : 83.51
estimate for cc : 2.034
logistic
estimate for a‐ : ‐1.253
estimate for BMD‐ : 65.55
probit
estimate for a‐ : ‐0.763
estimate for BMD‐ : 66.74
EXP
estimate for a‐ : 1.208
estimate for CED‐ : 70.07
estimate for d‐ : 1.027
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 1.205
estimate for CED‐ : 74.39
estimate for d‐ : 1.182
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging:
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.22 | 0.22 | 0.08 | 0.08 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 15.5 | 210 |
Confidence intervals for the BMD are based on 500 bootstrap data sets.
Visualisation:
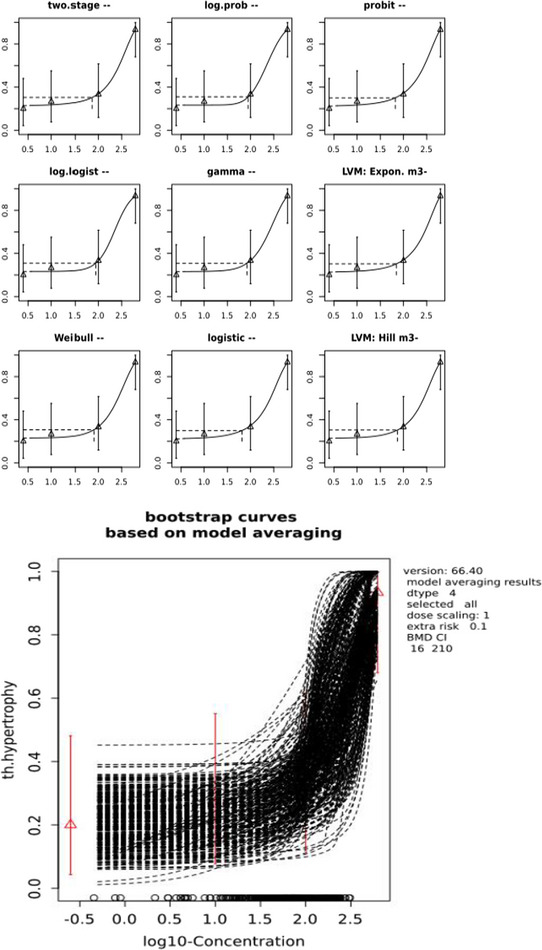
C.4. Increased absolute kidney weight in male and female rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984a)
Data description
The endpoint to be analysed is: increased absolute kidney weight
Data used for analysis:
| Dose(mg/kg bw per day) | Absolute kidney weight (g) | SD1 | N1 | Sexa |
|---|---|---|---|---|
| 0 | 1.04 | 0.080 | 15 | 1 |
| 10 | 1.04 | 0.104 | 15 | 1 |
| 100 | 1.14 | 0.127 | 15 | 1 |
| 625 | 1.33 | 0.104 | 15 | 1 |
| 0 | 0.60 | 0.066 | 15 | 2 |
| 10 | 0.63 | 0.048 | 15 | 2 |
| 100 | 0.67 | 0.055 | 15 | 2 |
| 625 | 0.75 | 0.062 | 15 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased absolute kidney weight.
Fitted models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 122.06 | 9 | −226.12 |
| full‐v | yes | 122.12 | 10 | −224.24 |
| null model | yes | −24.98 | 2 | 53.96 |
| null model‐a | yes | 76.82 | 3 | −147.64 |
| Expon. m3‐ | yes | −19.01 | 4 | 46.02 |
| Expon. m3‐a | yes | 120.48 | 5 | −230.96 |
| Expon. m3‐ab | yes | 121.00 | 6 | −230.00 |
| Expon. m5‐a | yes | 120.58 | 6 | −229.16 |
| Expon. m5‐ab | yes | 121.01 | 7 | −228.02 |
| Hill m3‐a | yes | 120.48 | 5 | −230.96 |
| Hill m3‐ab | yes | 121.00 | 6 | −230.00 |
| Hill m5‐a | yes | 120.58 | 6 | −229.16 |
| Hill m5‐ab | yes | 121.01 | 7 | −228.02 |
Estimated Model Parameters:
EXP
estimate for var‐ : 0.00786
estimate for a‐1 : 1.027
estimate for a‐2 : 0.5994
estimate for CED‐ : 92.29
estimate for d‐ : 0.4817
HILL
estimate for var‐ : 0.00786
estimate for a‐1 : 1.027
estimate for a‐2 : 0.5994
estimate for CED‐ : 92.27
estimate for d‐ : 0.4825
Final BMD Values (mg/kg bw per day):
| model | BMDL | BMDU | BMD |
|---|---|---|---|
| Expon. m3‐a | 38.0 | 186 | 92.29 |
| Hill m3‐a | 38.1 | 186 | 92.27 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| all | 38.0 | 186 |
Visualisation:
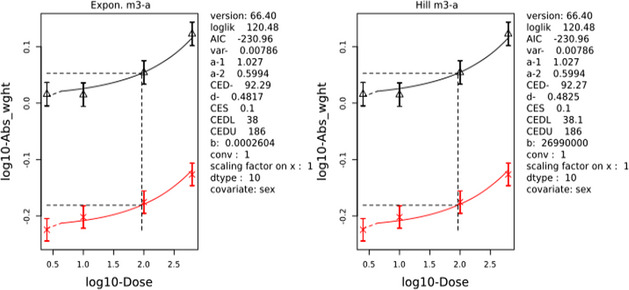
C.5. Increased relative kidney weight in male and female rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984a)
Data description
The endpoint to be analysed is: increased relative kidney weight.
Data used for analysis:
| Dose(mg/kg bw per day) | Relative kidney weight (%) | SD2 | N2 | Sexa |
|---|---|---|---|---|
| 0 | 0.36 | 0.020 | 14 | 1 |
| 10 | 0.36 | 0.023 | 15 | 1 |
| 100 | 0.40 | 0.018 | 15 | 1 |
| 625 | 0.50 | 0.028 | 15 | 1 |
| 0 | 0.38 | 0.033 | 15 | 2 |
| 10 | 0.39 | 0.024 | 15 | 2 |
| 100 | 0.43 | 0.020 | 15 | 2 |
| 625 | 0.48 | 0.030 | 15 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased relative kidney weight.
Fitted models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 168.24 | 9 | −318.48 |
| full‐v | yes | 169.12 | 10 | −318.24 |
| null model | yes | 73.11 | 2 | −142.22 |
| null model‐a | yes | 74.44 | 3 | −142.88 |
| Expon. m3‐ | yes | 151.91 | 4 | −295.82 |
| Expon. m3‐a | yes | 157.60 | 5 | −305.20 |
| Expon. m3‐b | yes | 151.92 | 5 | −293.84 |
| Expon. m3‐ab | yes | 164.51 | 6 | −317.02 |
| Expon. m5‐ | yes | 153.08 | 5 | −296.16 |
| Expon. m5‐a | yes | 158.86 | 6 | −305.72 |
| Expon. m5‐b | yes | 157.93 | 6 | −303.86 |
| Expon. m5‐ab | yes | 164.51 | 7 | −315.02 |
| Hill m3‐ | yes | 151.92 | 4 | −295.84 |
| Hill m3‐a | yes | 157.61 | 5 | −305.22 |
| Hill m3‐b | yes | 151.93 | 5 | −293.86 |
| Hill m3‐ab | yes | 164.51 | 6 | −317.02 |
| Hill m5‐ | yes | 153.08 | 5 | −296.16 |
| Hill m5‐a | yes | 158.86 | 6 | −305.72 |
| Hill m5‐b | yes | 157.32 | 6 | −302.64 |
| Hill m5‐ab | yes | 164.51 | 7 | −315.02 |
Estimated Model Parameters:
EXP
estimate for var‐ : 0.003688
estimate for a‐1 : 0.3524
estimate for a‐2 : 0.3825
estimate for CED‐1 : 56.72
estimate for CED‐2 : 116.4
estimate for d‐ : 0.5373
HILL
estimate for var‐ : 0.003688
estimate for a‐1 : 0.3524
estimate for a‐2 : 0.3825
estimate for CED‐1 : 56.71
estimate for CED‐2 : 116.5
estimate for d‐ : 0.5385
Final BMD Values (mg/kg bw per day):
| model | subgroup | BMDL | BMDU | BMD |
|---|---|---|---|---|
| Expon. m3‐ab | 1 | 32.6 | 92.5 | 56.72 |
| Expon. m3‐ab | 2 | 71.0 | 180.0 | 116.40 |
| Hill m3‐ab | 1 | 32.5 | 92.4 | 56.71 |
| Hill m3‐ab | 2 | 71.0 | 180.0 | 116.50 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| CED‐1 | 32.5 | 92.5 |
| CED‐2 | 71.0 | 180 |
Visualisation:
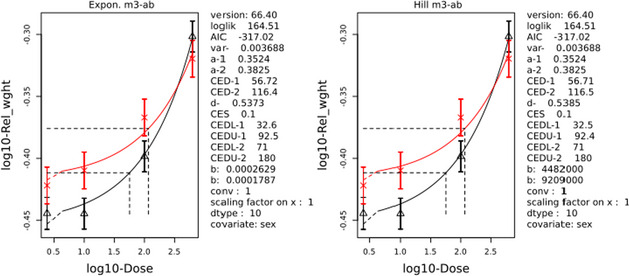
C.6. Incidence of kidney nephropathy in male rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984b)
Data description
The endpoint to be analysed is: kidney nephropathy incidence.
Data used for analysis:
| Concentration (mg/kg bw per day) | Incidence of kidney nephropathy | N |
|---|---|---|
| 0 | 0 | 15 |
| 10 | 0 | 11 |
| 100 | 1 | 15 |
| 13 | 15 |
Selection of the BMR
A default BMR of 10% was selected (EFSA Scientific Committee, 2017a).
Results
Response variable: kidney nephropathy incidence
Fitted models:
| model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −31.49 | 64.98 | NA | NA | NA | NA | |
| full | 4 | −9.56 | 27.12 | NA | NA | NA | NA | |
| two.stage | 3 | −9.59 | 25.18 | yes | 47.5 | 184 | 132 | yes |
| log.logist | 3 | −9.57 | 25.14 | yes | 57.3 | 537 | 120 | yes |
| Weibull | 3 | −9.57 | 25.14 | yes | 50.1 | 611 | 127 | yes |
| log.prob | 3 | −9.56 | 25.12 | yes | 59.5 | 509 | 117 | yes |
| gamma | 3 | −9.57 | 25.14 | yes | 50.8 | 452 | 122 | yes |
| logistic | 2 | −10.10 | 24.20 | yes | 133.0 | 341 | 222 | yes |
| probit | 2 | −9.98 | 23.96 | yes | 123.0 | 293 | 195 | yes |
| LVM: Expon. m3‐ | 3 | −9.57 | 25.14 | yes | 51.3 | 445 | 121 | yes |
| LVM: Hill m3‐ | 3 | −9.56 | 25.12 | yes | 54.1 | 430 | 119 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 1e‐06
estimate for BMD‐ : 132.5
estimate for c : 268.3
log.logist
estimate for a‐ : 1e‐06
estimate for BMD‐ : 119.9
estimate for c : 2.466
Weibull
estimate for a‐ : 1e‐06
estimate for BMD‐ : 127.3
estimate for c : 1.855
log.prob
estimate for a‐ : 1e‐06
estimate for BMD‐ : 116.7
estimate for c : 1.425
gamma
estimate for a‐ : 1e‐06
estimate for BMD‐ : 122.5
estimate for cc : 2.574
logistic
estimate for a‐ : ‐4.254
estimate for BMD‐ : 221.9
probit
estimate for a‐ : ‐2.307
estimate for BMD‐ : 195.1
EXP
estimate for a‐ : 4.482
estimate for CED‐ : 121.3
estimate for d‐ : 0.2503
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 4.482
estimate for CED‐ : 118.7
estimate for d‐ : 0.4698
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging:
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.09 | 0.1 | 0.1 | 0.1 | 0.1 | 0.15 | 0.17 | 0.1 | 0.1 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 87.2 | 410 |
Confidence intervals for the BMD are based on 500 bootstrap data sets.
Visualisation:
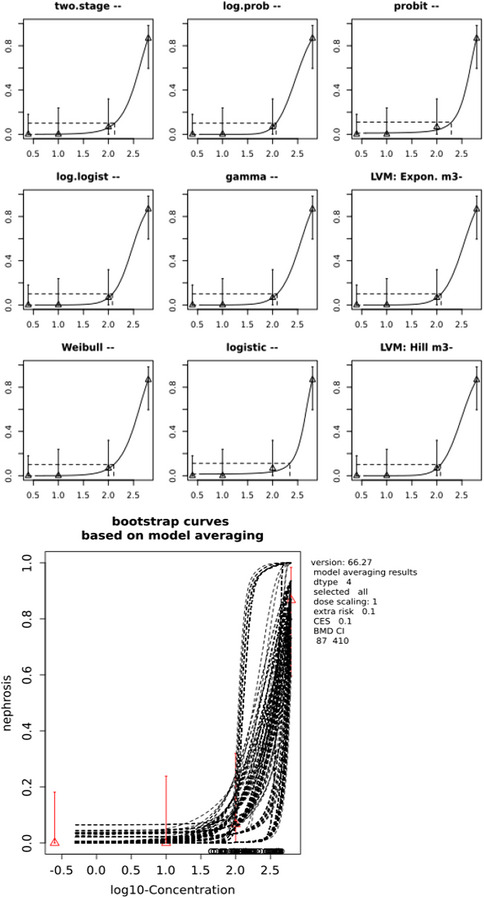
C.7. Incidence of thyroid hypertrophy in male rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984b)
Data description
The endpoint to be analysed is: incidence of thyroid hypertrophy.
Data used for analysis:
| Concentration(mg/kg bw per day) | Incidence of thyroid hypertrophy | N |
|---|---|---|
| 0 | 7 | 15 |
| 10 | 8 | 11 |
| 100 | 8 | 15 |
| 625 | 15 | 15 |
Selection of the BMR
A default BMR of 10% was selected (EFSA Scientific Committee, 2017a).
Results
Response variable: incidence of thyroid hypertrophy.
Fitted Models:
| model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −35.16 | 72.32 | NA | NA | NA | NA | |
| full | 4 | −27.17 | 62.34 | NA | NA | NA | NA | |
| two.stage | 3 | −28.31 | 62.62 | yes | 41.40 | 165.0 | 89.7 | yes |
| log.logist | 3 | −28.11 | 62.22 | yes | 41.50 | 529.0 | 172.0 | yes |
| Weibull | 3 | −28.11 | 62.22 | yes | 18.30 | 608.0 | 320.0 | yes |
| log.prob | 3 | −28.11 | 62.22 | yes | 42.00 | 550.0 | 230.0 | yes |
| gamma | 3 | −28.11 | 62.22 | yes | 17.10 | 504.0 | 242.0 | yes |
| logistic | 2 | −28.97 | 61.94 | yes | 15.80 | 66.8 | 31.1 | yes |
| probit | 2 | −28.78 | 61.56 | yes | 17.00 | 68.3 | 33.6 | yes |
| LVM: Expon. m3‐ | 3 | −28.12 | 62.24 | yes | 9.47 | 345.0 | 253.0 | yes |
| LVM: Hill m3‐ | 3 | −28.12 | 62.24 | yes | 13.70 | 334.0 | 225.0 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 0.543
estimate for BMD‐ : 89.69
estimate for c : 1e+12
log.logist
estimate for a‐ : 0.561
estimate for BMD‐ : 172.2
estimate for c : 31.68
Weibull
estimate for a‐ : 0.561
estimate for BMD‐ : 320.5
estimate for c : 7.311
log.prob
estimate for a‐ : 0.561
estimate for BMD‐ : 229.7
estimate for c : 7.444
gamma
estimate for a‐ : 0.561
estimate for BMD‐ : 241.6
estimate for cc : 40.43
logistic
estimate for a‐ : 0.02905
estimate for BMD‐ : 31.15
probit
estimate for a‐ : 0.02583
estimate for BMD‐ : 33.58
EXP
estimate for a‐ : 0.9626
estimate for CED‐ : 252.8
estimate for d‐ : 4
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 0.9627
estimate for CED‐ : 225.1
estimate for d‐ : 4
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging:
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.09 | 0.11 | 0.11 | 0.11 | 0.11 | 0.12 | 0.15 | 0.11 | 0.11 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 14.7 | 195 |
Confidence intervals for the BMD are based on 1000 bootstrap data sets.
Visualisation:
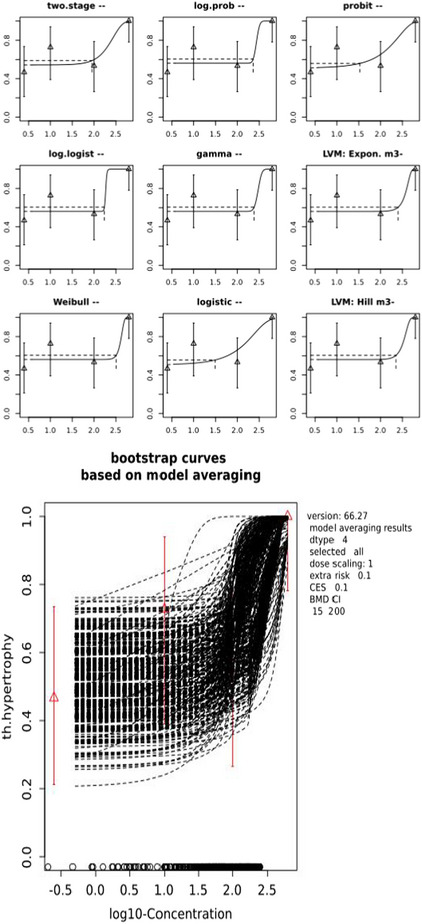
C.8. Incidence of thyroid hyperplasia in male rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984b)
Data description
The endpoint to be analysed is: incidence of thyroid hyperplasia.
Data used for analysis:
| Concentration (mg/kg bw per day) | Incidence of thyroid hyperplasia | N |
|---|---|---|
| 0 | 3 | 15 |
| 10 | 1 | 11 |
| 100 | 2 | 15 |
| 625 | 14 | 15 |
Selection of the BMR
A default BMR of 10% was selected (EFSA Scientific Committee, 2017a).
Results
Response variable: incidence of thyroid hyperplasia
Fitted Models:
| model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −36.50 | 75.00 | NA | NA | NA | NA | |
| full | 4 | −20.42 | 48.84 | NA | NA | NA | NA | |
| two.stage | 3 | −20.93 | 47.86 | yes | 95.7 | 177 | 129.0 | no |
| log.logist | 3 | −20.74 | 47.48 | yes | 64.2 | 603 | 399.0 | yes |
| Weibull | 3 | −20.74 | 47.48 | yes | 53.8 | 606 | 441.0 | yes |
| log.prob | 3 | −20.74 | 47.48 | yes | 66.6 | 611 | 304.0 | yes |
| gamma | 3 | −20.74 | 47.48 | yes | 56.1 | 515 | 337.0 | yes |
| logistic | 2 | −21.24 | 46.48 | yes | 60.1 | 145 | 94.4 | yes |
| probit | 2 | −21.29 | 46.58 | yes | 60.4 | 130 | 89.5 | yes |
| LVM: Expon. m3‐ | 3 | −20.74 | 47.48 | yes | 46.5 | 411 | 372.0 | yes |
| LVM: Hill m3‐ | 3 | −20.74 | 47.48 | yes | 49.7 | 388 | 345.0 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 0.1326
estimate for BMD‐ : 129.1
estimate for c : 1e+12
log.logist
estimate for a‐ : 0.1463
estimate for BMD‐ : 399.1
estimate for c : 10.4
Weibull
estimate for a‐ : 0.1463
estimate for BMD‐ : 441.4
estimate for c : 9.161
log.prob
estimate for a‐ : 0.1463
estimate for BMD‐ : 304.1
estimate for c : 3.747
gamma
estimate for a‐ : 0.1463
estimate for BMD‐ : 336.9
estimate for cc : 19.26
logistic
estimate for a‐ : ‐2.05
estimate for BMD‐ : 94.38
probit
estimate for a‐ : ‐1.204
estimate for BMD‐ : 89.47
EXP
estimate for a‐ : 1.301
estimate for CED‐ : 371.6
estimate for d‐ : 4
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 1.301
estimate for CED‐ : 345.1
estimate for d‐ : 4
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging:
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.08 | 0.1 | 0.1 | 0.1 | 0.1 | 0.16 | 0.16 | 0.1 | 0.1 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 45.1 | 266 |
Confidence intervals for the BMD are based on 1000 bootstrap data sets.
Visualisation:
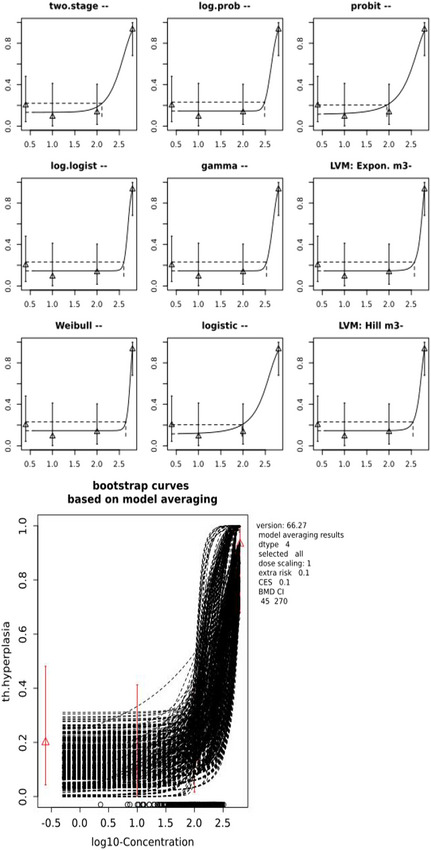
C.9. Increased absolute kidney weights in male and female rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984b)
Data description
The endpoint to be analysed is: increased absolute kidney weight
Data used for analysis:
| Concentration(mg/kg bw per day) | Absolute kidney weight(g) | Sexa |
|---|---|---|
| 0 | 1.82 | 1 |
| 0 | 1.91 | 1 |
| 0 | 2.00 | 1 |
| 0 | 1.74 | 1 |
| 0 | 1.93 | 1 |
| 0 | 1.51 | 1 |
| 0 | 1.79 | 1 |
| 0 | 1.97 | 1 |
| 0 | 2.07 | 1 |
| 0 | 2.06 | 1 |
| 0 | 1.91 | 1 |
| 0 | 1.81 | 1 |
| 0 | 1.93 | 1 |
| 0 | 1.77 | 1 |
| 0 | 1.85 | 1 |
| 10 | 1.78 | 1 |
| 10 | 1.96 | 1 |
| 10 | 1.74 | 1 |
| 10 | 1.88 | 1 |
| 10 | 1.86 | 1 |
| 10 | 1.90 | 1 |
| 10 | 1.78 | 1 |
| 10 | 1.93 | 1 |
| 10 | 1.90 | 1 |
| 10 | 1.97 | 1 |
| 10 | 1.97 | 1 |
| 10 | 1.94 | 1 |
| 10 | 1.83 | 1 |
| 10 | 2.07 | 1 |
| 10 | 1.92 | 1 |
| 100 | 2.00 | 1 |
| 100 | 2.18 | 1 |
| 100 | 1.89 | 1 |
| 100 | 1.98 | 1 |
| 100 | 2.12 | 1 |
| 100 | 2.19 | 1 |
| 100 | 2.36 | 1 |
| 100 | 2.13 | 1 |
| 100 | 2.22 | 1 |
| 100 | 2.22 | 1 |
| 100 | 2.05 | 1 |
| 100 | 1.94 | 1 |
| 100 | 2.15 | 1 |
| 100 | 2.17 | 1 |
| 100 | 2.36 | 1 |
| 625 | 2.22 | 1 |
| 625 | 2.60 | 1 |
| 625 | 2.63 | 1 |
| 625 | 2.43 | 1 |
| 625 | 2.34 | 1 |
| 625 | 2.21 | 1 |
| 625 | 2.43 | 1 |
| 625 | 2.14 | 1 |
| 625 | 2.04 | 1 |
| 625 | 2.60 | 1 |
| 625 | 2.76 | 1 |
| 625 | 2.22 | 1 |
| 625 | 2.23 | 1 |
| 625 | 2.02 | 1 |
| 625 | 2.74 | 1 |
| 0 | 1.10 | 2 |
| 0 | 1.16 | 2 |
| 0 | 1.25 | 2 |
| 0 | 1.02 | 2 |
| 0 | 1.15 | 2 |
| 0 | 1.21 | 2 |
| 0 | 1.22 | 2 |
| 0 | 1.28 | 2 |
| 0 | 1.39 | 2 |
| 0 | 1.29 | 2 |
| 0 | 1.24 | 2 |
| 0 | 1.11 | 2 |
| 0 | 1.25 | 2 |
| 0 | 1.23 | 2 |
| 0 | 1.14 | 2 |
| 10 | 1.08 | 2 |
| 10 | 1.28 | 2 |
| 10 | 1.17 | 2 |
| 10 | 1.17 | 2 |
| 10 | 1.15 | 2 |
| 10 | 1.29 | 2 |
| 10 | 1.35 | 2 |
| 10 | 1.38 | 2 |
| 10 | 1.30 | 2 |
| 10 | 1.25 | 2 |
| 10 | 1.14 | 2 |
| 10 | 1.13 | 2 |
| 10 | 1.34 | 2 |
| 10 | 1.43 | 2 |
| 10 | 1.12 | 2 |
| 100 | 1.34 | 2 |
| 100 | 1.29 | 2 |
| 100 | 1.33 | 2 |
| 100 | 1.38 | 2 |
| 100 | 1.15 | 2 |
| 100 | 1.34 | 2 |
| 100 | 1.11 | 2 |
| 100 | 1.22 | 2 |
| 100 | 1.33 | 2 |
| 100 | 1.22 | 2 |
| 100 | 1.35 | 2 |
| 100 | 1.43 | 2 |
| 100 | 1.16 | 2 |
| 100 | 1.29 | 2 |
| 100 | 1.31 | 2 |
| 625 | 1.38 | 2 |
| 625 | 1.41 | 2 |
| 625 | 1.65 | 2 |
| 625 | 1.38 | 2 |
| 625 | 1.54 | 2 |
| 625 | 1.43 | 2 |
| 625 | 1.50 | 2 |
| 625 | 1.60 | 2 |
| 625 | 1.48 | 2 |
| 625 | 1.55 | 2 |
| 625 | 1.17 | 2 |
| 625 | 1.56 | 2 |
| 625 | 1.42 | 2 |
| 625 | 1.58 | 2 |
| 625 | 1.59 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: absolute kidney weight
Fitted Models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 138.87 | 9 | −259.74 |
| full‐v | yes | 138.98 | 10 | −257.96 |
| null model | yes | −7.95 | 2 | 19.90 |
| null model‐a | yes | 87.70 | 3 | −169.40 |
| Expon. m3‐ | yes | −0.78 | 4 | 9.56 |
| Expon. m3‐a | yes | 136.29 | 5 | −262.58 |
| Expon. m3‐ab | Yes | 136.81 | 6 | −261.62 |
| Expon. m5‐a | Yes | 136.53 | 6 | −261.06 |
| Expon. m5‐ab | Yes | 138.53 | 7 | −263.06 |
| Hill m3‐a | Yes | 136.29 | 5 | −262.58 |
| Hill m3‐ab | Yes | 136.81 | 6 | −261.62 |
| Hill m5‐a | Yes | 136.53 | 6 | −261.06 |
| Hill m5‐ab | Yes | 138.43 | 7 | −262.86 |
Estimated Model Parameters:
EXP
estimate for var‐ : 0.005819
estimate for a‐1 : 1.863
estimate for a‐2 : 1.212
estimate for CED‐1 : 63.5
estimate for CED‐2 : 183.2
estimate for c‐ : 1.271
estimate for d‐ : 1.006
HILL
estimate for var‐ : 0.005828
estimate for a‐1 : 1.867
estimate for a‐2 : 1.214
estimate for CED‐1 : 65.35
estimate for CED‐2 : 175.3
estimate for c‐ : 1.295
estimate for d‐ : 1.296
Final BMD Values (mg/kg bw per day):
| model | BMDL | BMDU | BMD | Subgroupa |
|---|---|---|---|---|
| Expon. m5‐ab | 31.9 | 109 | 63.50 | 1 |
| Expon. m5‐ab | 85.7 | 345 | 183.20 | 2 |
| Hill m5‐ab | 31.4 | 111 | 65.35 | 1 |
| Hill m5‐ab | 83.7 | 317 | 175.30 | 2 |
1: males. 2: females.
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| Subgroupa | bmdl.lowest | bmdu.highest |
|---|---|---|
| CED‐1 | 31.4 | 111 |
| CED‐2 | 83.7 | 345 |
1: males. 2: females.
Visualisation:
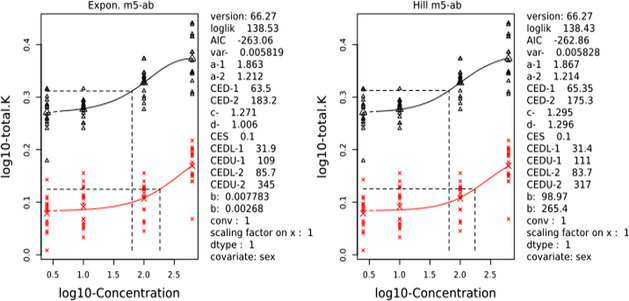
C.10. Increased relative kidney weights in male and female rats exposed to SCCP (C10–12, 58% chlorination) (IRDC, 1984b)
Data description
The endpoint to be analysed is: increased relative kidney weight.
Data used for analysis:
| Concentration (mg/kg bw per day) | Relative kidney weight (%) | Sexa |
|---|---|---|
| 0 | 0.68 | 1 |
| 0 | 0.66 | 1 |
| 0 | 0.73 | 1 |
| 0 | 0.65 | 1 |
| 0 | 0.70 | 1 |
| 0 | 0.63 | 1 |
| 0 | 0.63 | 1 |
| 0 | 0.66 | 1 |
| 0 | 0.72 | 1 |
| 0 | 0.73 | 1 |
| 0 | 0.67 | 1 |
| 0 | 0.68 | 1 |
| 0 | 0.73 | 1 |
| 0 | 0.64 | 1 |
| 0 | 0.67 | 1 |
| 10 | 0.95 | 1 |
| 10 | 0.71 | 1 |
| 10 | 0.74 | 1 |
| 10 | 0.65 | 1 |
| 10 | 0.66 | 1 |
| 10 | 0.67 | 1 |
| 10 | 0.66 | 1 |
| 10 | 0.68 | 1 |
| 10 | 0.68 | 1 |
| 10 | 0.71 | 1 |
| 10 | 0.69 | 1 |
| 10 | 0.87 | 1 |
| 10 | 0.69 | 1 |
| 10 | 0.66 | 1 |
| 10 | 0.69 | 1 |
| 100 | 0.75 | 1 |
| 100 | 0.78 | 1 |
| 100 | 0.75 | 1 |
| 100 | 0.70 | 1 |
| 100 | 0.76 | 1 |
| 100 | 0.75 | 1 |
| 100 | 0.82 | 1 |
| 100 | 0.77 | 1 |
| 100 | 0.81 | 1 |
| 100 | 0.75 | 1 |
| 100 | 0.76 | 1 |
| 100 | 1.28 | 1 |
| 100 | 0.80 | 1 |
| 100 | 0.81 | 1 |
| 625 | 0.94 | 1 |
| 625 | 0.95 | 1 |
| 625 | 0.94 | 1 |
| 625 | 0.88 | 1 |
| 625 | 1.26 | 1 |
| 625 | 0.87 | 1 |
| 625 | 0.93 | 1 |
| 625 | 0.80 | 1 |
| 625 | 0.86 | 1 |
| 625 | 0.89 | 1 |
| 625 | 1.02 | 1 |
| 625 | 0.92 | 1 |
| 625 | 1.40 | 1 |
| 625 | 0.83 | 1 |
| 625 | 1.00 | 1 |
| 0 | 0.64 | 2 |
| 0 | 0.73 | 2 |
| 0 | 0.74 | 2 |
| 0 | 0.70 | 2 |
| 0 | 0.76 | 2 |
| 0 | 0.76 | 2 |
| 0 | 0.77 | 2 |
| 0 | 0.73 | 2 |
| 0 | 0.83 | 2 |
| 0 | 0.80 | 2 |
| 0 | 0.78 | 2 |
| 0 | 0.62 | 2 |
| 0 | 0.78 | 2 |
| 0 | 0.76 | 2 |
| 0 | 0.76 | 2 |
| 10 | 0.71 | 2 |
| 10 | 0.77 | 2 |
| 10 | 0.74 | 2 |
| 10 | 0.71 | 2 |
| 10 | 0.72 | 2 |
| 10 | 0.84 | 2 |
| 10 | 0.86 | 2 |
| 10 | 0.80 | 2 |
| 10 | 0.82 | 2 |
| 10 | 0.76 | 2 |
| 10 | 0.74 | 2 |
| 10 | 0.76 | 2 |
| 10 | 0.81 | 2 |
| 10 | 0.86 | 2 |
| 10 | 0.74 | 2 |
| 100 | 0.94 | 2 |
| 100 | 1.01 | 2 |
| 100 | 0.84 | 2 |
| 100 | 0.82 | 2 |
| 100 | 0.75 | 2 |
| 100 | 0.80 | 2 |
| 100 | 0.72 | 2 |
| 100 | 0.81 | 2 |
| 100 | 0.86 | 2 |
| 100 | 0.74 | 2 |
| 100 | 0.88 | 2 |
| 100 | 0.82 | 2 |
| 100 | 0.78 | 2 |
| 100 | 0.81 | 2 |
| 625 | 0.84 | 2 |
| 625 | 0.84 | 2 |
| 625 | 0.99 | 2 |
| 625 | 0.86 | 2 |
| 625 | 0.88 | 2 |
| 625 | 0.84 | 2 |
| 625 | 0.83 | 2 |
| 625 | 0.95 | 2 |
| 625 | 0.91 | 2 |
| 625 | 1.00 | 2 |
| 625 | 0.76 | 2 |
| 625 | 1.02 | 2 |
| 625 | 0.87 | 2 |
| 625 | 0.97 | 2 |
| 625 | 0.93 | 2 |
| NA | NA | NA |
1: males. 2: females.
The following outliers were identified for Expon. m3‐abv:
| Concentration | total.K.rel | Sea | |
|---|---|---|---|
| 42 | 100 | 1.28 | 1 |
| 57 | 625 | 1.40 | 1 |
The following outliers were identified for Hill m3‐abv:
| Concentration | total.K.rel | Sexa | |
|---|---|---|---|
| 42 | 100 | 1.28 | 1 |
| 57 | 625 | 1.40 | 1 |
The following outliers were identified for Inv.Expon. m3‐abv:
| Concentration | total.K.rel | Sexa | |
|---|---|---|---|
| 42 | 100 | 1.28 | 1 |
| 57 | 625 | 1.40 | 1 |
The following outliers were identified for LN m3‐abv:
| Concentration | total.K.rel | Sexa | |
|---|---|---|---|
| 42 | 100 | 1.28 | 1 |
| 57 | 625 | 1.40 | 1 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased relative kidney weight
Fitted Models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 106.72 | 9 | −195.44 |
| full‐v | yes | 111.17 | 10 | −202.34 |
| null model‐v | yes | 66.78 | 3 | −127.56 |
| null model‐a‐v | yes | 67.71 | 4 | −127.42 |
| Expon. m3‐v | yes | 103.58 | 5 | −197.16 |
| Expon. m3‐av | yes | 105.38 | 6 | −198.76 |
| Expon. m3‐bv | yes | 103.83 | 6 | −195.66 |
| Expon. m3‐abv | yes | 110.89 | 7 | −207.78 |
| Expon. m5‐v | yes | 103.65 | 6 | −195.30 |
| Expon. m5‐av | yes | 105.45 | 7 | −196.90 |
| Expon. m5‐bv | no | 103.82 | 7 | −193.64 |
| Expon. m5‐abv | no | 110.85 | 8 | −205.70 |
| Hill m3‐v | yes | 103.58 | 5 | −197.16 |
| Hill m3‐av | yes | 105.38 | 6 | −198.76 |
| Hill m3‐bv | yes | 103.83 | 6 | −195.66 |
| Hill m3‐abv | yes | 110.89 | 7 | −207.78 |
| Hill m5‐v | yes | 103.65 | 6 | −195.30 |
| Hill m5‐av | yes | 105.45 | 7 | −196.90 |
| Hill m5‐bv | yes | 103.82 | 7 | −193.64 |
| Hill m5‐abv | no | 110.85 | 8 | −205.70 |
Estimated Model Parameters:
EXP
estimate for var‐1 : 0.01325
estimate for var‐2 : 0.006029
estimate for a‐1 : 0.6713
estimate for a‐2 : 0.7455
estimate for CED‐1 : 22.67
estimate for CED‐2 : 110.7
estimate for d‐ : 0.394
HILL
estimate for var‐1 : 0.01325
estimate for var‐2 : 0.006029
estimate for a‐1 : 0.6713
estimate for a‐2 : 0.7455
estimate for CED‐1 : 22.66
estimate for CED‐2 : 110.8
estimate for d‐ : 0.3948
The following outliers were identified:
| model | Concentration | total.K.rel | Sexa | |
|---|---|---|---|---|
| 42 | Expon. m3‐abv | 100 | 1.28 | 1 |
| 57 | Expon. m3‐abv | 625 | 1.40 | 1 |
| 421 | Hill m3‐abv | 100 | 1.28 | 1 |
| 571 | Hill m3‐abv | 625 | 1.40 | 1 |
1: males. 2: females.
Final BMD Values (mg/kg bw per day):
| Model | subgroup | BMDL | BMDU | BMD |
|---|---|---|---|---|
| Expon. m3‐abv | 1 | 5.55 | 68.6 | 22.67 |
| Expon. m3‐abv | 2 | 40.70 | 250.0 | 110.70 |
| Hill m3‐abv | 1 | 5.55 | 68.3 | 22.66 |
| Hill m3‐abv | 2 | 40.80 | 250.0 | 110.80 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| CED‐1 | 5.55 | 68.6 |
| CED‐2 | 40.7 | 250 |
Visualisation:
C.11. Increased absolute kidney weight in male and female rats exposed to Cereclor S52 (C14–17, 52% chlorination) (CXR Biosciences Ltd, 2005b)
Data description
The endpoint to be analysed is: increased absolute kidney weight
Data used for analysis:
| Dose(mg/kg bw per day) | Absolute kidney weight(g) | SD3 | N | Sexa |
|---|---|---|---|---|
| 0.0 | 1.913 | 0.207 | 20 | 1 |
| 2.4 | 1.881 | 0.162 | 10 | 1 |
| 9.3 | 1.890 | 0.162 | 10 | 1 |
| 23.0 | 1.970 | 0.145 | 10 | 1 |
| 222.0 | 2.076 | 0.201 | 10 | 1 |
| 0.0 | 1.217 | 0.108 | 20 | 2 |
| 2.5 | 1.197 | 0.116 | 10 | 2 |
| 9.7 | 1.260 | 0.097 | 10 | 2 |
| 24.6 | 1.271 | 0.111 | 10 | 2 |
| 242.0 | 1.380 | 0.078 | 10 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased absolute kidney weight
Fitted Models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 125.36 | 11 | −228.72 |
| full‐v | yes | 125.78 | 12 | −227.56 |
| null model | yes | 2.64 | 2 | −1.28 |
| null model‐a | yes | 112.54 | 3 | −219.08 |
| Expon. m3‐ | yes | 4.04 | 4 | −0.08 |
| Expon. m3‐a | yes | 123.78 | 5 | −237.56 |
| Expon. m3‐ab | yes | 124.11 | 6 | −236.22 |
| Expon. m5‐a | yes | 124.14 | 6 | −236.28 |
| Expon. m5‐ab | yes | 124.36 | 7 | −234.72 |
| Hill m3‐a | yes | 123.78 | 5 | −237.56 |
| Hill m3‐ab | yes | 124.11 | 6 | −236.22 |
| Hill m5‐a | yes | 124.17 | 6 | −236.34 |
| Hill m5‐ab | yes | 124.41 | 7 | −234.82 |
Estimated Model Parameters:
EXP
estimate for var‐ : 0.00744
estimate for a‐1 : 1.878
estimate for a‐2 : 1.215
estimate for CED‐ : 172.8
estimate for d‐ : 0.5665
HILL
estimate for var‐ : 0.00744
estimate for a‐1 : 1.878
estimate for a‐2 : 1.215
estimate for CED‐ : 172.8
estimate for d‐ : 0.5668
Final BMD Values (mg/kg bw per day):
| model | BMDL | BMDU | BMD |
|---|---|---|---|
| Expon. m3‐a | 81.5 | 364 | 172.8 |
| Hill m3‐a | 81.5 | 364 | 172.8 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| all | 81.5 | 364 |
Visualisation:
C.12. Increased relative kidney weight in male and female rats exposed to Cereclor S52 (C14–17, 52% chlorination) (CXR Biosciences Ltd, 2005b)
Data description
The endpoint to be analysed is: increased relative kidney weight
Data used for analysis:
| Dose(mg/kg bw per day) | Relative kidney weight(%) | SD4 | N | Sexa |
|---|---|---|---|---|
| 0.0 | 0.559 | 0.035 | 20 | 1 |
| 2.4 | 0.567 | 0.027 | 10 | 1 |
| 9.3 | 0.565 | 0.029 | 10 | 1 |
| 23.0 | 0.576 | 0.026 | 10 | 1 |
| 222.0 | 0.627 | 0.028 | 10 | 1 |
| 0.0 | 0.584 | 0.025 | 20 | 2 |
| 2.5 | 0.612 | 0.045 | 10 | 2 |
| 9.7 | 0.607 | 0.036 | 10 | 2 |
| 24.6 | 0.622 | 0.037 | 10 | 2 |
| 242.0 | 0.679 | 0.026 | 10 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased relative kidney weight
Fitted Models
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 186.37 | 11 | −350.74 |
| full‐v | yes | 186.38 | 12 | −348.76 |
| null model | yes | 138.84 | 2 | −273.68 |
| null model‐a | yes | 151.05 | 3 | −296.10 |
| Expon. m3‐ | yes | 165.25 | 4 | −322.50 |
| Expon. m3‐a | yes | 184.54 | 5 | −359.08 |
| Expon. m3‐ab | yes | 184.94 | 6 | −357.88 |
| Expon. m5‐a | yes | 184.53 | 6 | −357.06 |
| Expon. m5‐ab | yes | 184.94 | 7 | −355.88 |
| Hill m3‐a | yes | 184.54 | 5 | −359.08 |
| Hill m3‐ab | yes | 184.94 | 6 | −357.88 |
| Hill m5‐a | yes | 184.53 | 6 | −357.06 |
| Hill m5‐ab | yes | 184.94 | 7 | −355.88 |
Estimated Model Parameters:
EXP
estimate for var‐ : 0.002703
estimate for a‐1 : 0.5536
estimate for a‐2 : 0.5902
estimate for CED‐ : 116.3
estimate for d‐ : 0.4601
HILL
estimate for var‐ : 0.002703
estimate for a‐1 : 0.5536
estimate for a‐2 : 0.5902
estimate for CED‐ : 116.2
estimate for d‐ : 0.4605
Final BMD Values (mg/kg bw per day):
| model | BMDL | BMDU | BMD |
|---|---|---|---|
| Expon. m3‐a | 68.0 | 182 | 116.3 |
| Hill m3‐a | 67.9 | 182 | 116.2 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| all | 67.9 | 182 |
Visualisation:
C.13. Increased absolute kidney weight (left) in male and female rats exposed to Cereclor S52 (C14–17, 52% chlorination) (IRDC, 1984c)
Data description
The endpoint to be analysed is: increased absolute kidney weight
Data used for analysis:
| Dose(mg/kg bw per day) | Absolute kidney weight(g) | SD1 | N | Sexa |
|---|---|---|---|---|
| 0 | 0.95 | 0.066 | 15 | 1 |
| 10 | 0.93 | 0.153 | 15 | 1 |
| 100 | 1.01 | 0.065 | 15 | 1 |
| 625 | 1.08 | 0.098 | 15 | 1 |
| 0 | 0.61 | 0.057 | 14 | 2 |
| 10 | 0.62 | 0.042 | 15 | 2 |
| 100 | 0.65 | 0.047 | 14 | 2 |
| 625 | 0.72 | 0.055 | 15 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased absolute kidney weight
Fitted Models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 117.67 | 9 | −217.34 |
| full‐v | yes | 120.26 | 10 | −220.52 |
| null model‐v | yes | 17.74 | 3 | −29.48 |
| null model‐a‐v | yes | 94.59 | 4 | −181.18 |
| Expon. m3‐v | yes | 33.81 | 5 | −57.62 |
| Expon. m3‐av | yes | 118.83 | 6 | −225.66 |
| Expon. m3‐abv | yes | 118.95 | 7 | −223.90 |
| Expon. m5‐av | yes | 119.34 | 7 | −224.68 |
| Expon. m5‐abv | yes | 119.59 | 8 | −223.18 |
| Hill m3‐av | yes | 118.83 | 6 | −225.66 |
| Hill m3‐abv | yes | 118.95 | 7 | −223.90 |
| Hill m5‐av | yes | 119.34 | 7 | −224.68 |
| Hill m5‐abv | yes | 119.59 | 8 | −223.18 |
Estimated Model Parameters:
EXP
estimate for var‐1 : 0.01072
estimate for var‐2 : 0.005635
estimate for a‐1 : 0.928
estimate for a‐2 : 0.609
estimate for CED‐ : 231.5
estimate for d‐ : 0.525
HILL
estimate for var‐1 : 0.01071
estimate for var‐2 : 0.005635
estimate for a‐1 : 0.928
estimate for a‐2 : 0.609
estimate for CED‐ : 231.4
estimate for d‐ : 0.5255
Final BMD Values (mg/kg bw per day):
| Model | BMDL | BMDU | BMD |
|---|---|---|---|
| Expon. m3‐av | 101.0 | 418 | 231.5 |
| Hill m3‐av | 101.0 | 417 | 231.4 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| all | 101 | 418 |
Visualisation:
C.14. Increased relative kidney weight (left) in male and female rats exposed to Cereclor S52 (C14–17, 52% chlorination) (IRDC, 1984c)
Data description
The endpoint to be analysed is: increased relative kidney weight.
Data used for analysis:
| Dose(mg/kg bw per day) | Relative kidney weight(%) | sd2 | n | Cova |
|---|---|---|---|---|
| 0 | 0.35 | 0.021 | 15 | 1 |
| 10 | 0.35 | 0.054 | 15 | 1 |
| 100 | 0.38 | 0.031 | 15 | 1 |
| 625 | 0.42 | 0.022 | 15 | 1 |
| 0 | 0.36 | 0.054 | 14 | 2 |
| 10 | 0.38 | 0.026 | 15 | 2 |
| 100 | 0.40 | 0.025 | 14 | 2 |
| 625 | 0.45 | 0.027 | 15 | 2 |
1: males. 2: females.
Selection of the BMR
The CONTAM Panel considered the biological relevance of increased absolute and relative kidney weights. It concluded that the selection of the default BMR of 5% for continuous data is not appropriate for these endpoints considering also that histopathological changes of only minimal to mild severity were observed only in some studies and generally at higher doses than those at which increased kidney weights started to be observed. The Panel concluded that a BMR of 10% is sufficient to protect towards adverse changes in kidney weights.
Results
Response variable: increased relative kidney weight
Fitted Models:
| model | converged | loglik | npar | AIC |
|---|---|---|---|---|
| full model | yes | 116.04 | 9 | −214.08 |
| full‐v | yes | 116.09 | 10 | −212.18 |
| null model | yes | 77.55 | 2 | −151.10 |
| null model‐a | yes | 80.96 | 3 | −155.92 |
| Expon. m3‐ | yes | 109.00 | 4 | −210.00 |
| Expon. m3‐a | yes | 114.67 | 5 | −219.34 |
| Expon. m3‐b | yes | 113.01 | 5 | −216.02 |
| Expon. m3‐ab | yes | 114.81 | 6 | −217.62 |
| Expon. m5‐ | yes | 109.09 | 5 | −208.18 |
| Expon. m5‐a | yes | 114.80 | 6 | −217.60 |
| Expon. m5‐b | yes | 113.63 | 6 | −215.26 |
| Expon. m5‐ab | yes | 114.95 | 7 | −215.90 |
| Hill m3‐ | yes | 109.00 | 4 | −210.00 |
| Hill m3‐a | yes | 114.67 | 5 | −219.34 |
| Hill m3‐b | yes | 113.01 | 5 | −216.02 |
| Hill m3‐ab | yes | 114.81 | 6 | −217.62 |
| Hill m5‐ | yes | 109.09 | 5 | −208.18 |
| Hill m5‐a | yes | 114.80 | 6 | −217.60 |
| Hill m5‐b | yes | 113.67 | 6 | −215.34 |
| Hill m5‐ab | yes | 114.95 | 7 | −215.90 |
Estimated Model Parameters:
EXP
estimate for var‐ : 0.008384
estimate for a‐1 : 0.342
estimate for a‐2 : 0.3625
estimate for CED‐ : 102.5
estimate for d‐ : 0.4394
HILL
estimate for var‐ : 0.008384
estimate for a‐1 : 0.342
estimate for a‐2 : 0.3625
estimate for CED‐ : 102.5
estimate for d‐ : 0.4401
Final BMD Values (mg/kg bw per day):
| model | BMDL | BMDU | BMD |
|---|---|---|---|
| Expon. m3‐a | 36.4 | 224 | 102.50 |
| Hill m3‐a | 36.4 | 223 | 102.50 |
Lowest BMDL and highest BMDU Values (mg/kg bw per day):
| subgroup | bmdl.lowest | bmdu.highest |
|---|---|---|
| all | 36.4 | 224 |
Visualisation:
C.15. Pup mortality in rats, from dams exposed to Cereclor S52 (C14–17, 52% chlorination) (IRDC, 1985)
Data description
The endpoint to be analysed is: pup mortality at post‐natal day (PND) 21.
The following data on pup survival at PND 21 were considered:
| Dose(mg/kg bw per day) | Number of born pups at PND 0 | Number of pups surviving at PND 21 |
|---|---|---|
| 0 | 127 | 99 |
| 9 | 131 | 125 |
| 90 | 136 | 116 |
| 560 | 141 | 0 |
Data used for analysis:
17 pups/group were euthanised on PND10 and were therefore excluded from the analysis.
Selection of the BMR
Considering the severity of the effect, the CONTAM Panel selected a BMR of 5%, lower than the default BMR of 10% recommended for quantal data. From a statistical point of view the high study population (> 100 pups per dose group) supported the selection of a lower BMR.
Results
Response variable: pup mortality
Fitted Models:
| model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −314.57 | 631.14 | NA | NA | NA | NA | |
| full | 4 | −116.91 | 241.82 | NA | NA | NA | NA | |
| two.stage | 3 | −119.61 | 245.22 | no | NA | NA | 55.8 | yes |
| log.logist | 3 | −118.25 | 242.50 | yes | 69.1 | 101.0 | 86.2 | yes |
| Weibull | 3 | −118.25 | 242.50 | yes | 53.4 | 112.0 | 83.8 | yes |
| log.prob | 3 | −118.25 | 242.50 | yes | 69.4 | 100.0 | 84.6 | yes |
| gamma | 3 | −118.25 | 242.50 | yes | 61.9 | 104.0 | 82.4 | yes |
| logistic | 2 | −120.04 | 244.08 | yes | 37.9 | 57.9 | 46.8 | yes |
| probit | 2 | −120.04 | 244.08 | yes | 35.5 | 53.3 | 43.6 | yes |
| LVM: Expon. m3‐ | 3 | −118.25 | 242.50 | yes | 44.4 | 123.0 | 81.3 | yes |
| LVM: Hill m3‐ | 3 | −118.25 | 242.50 | yes | 49.2 | 117.0 | 81.0 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 0.06357
estimate for BMD‐ : 55.77
estimate for c : 1e+12
log.logist
estimate for a‐ : 0.07054
estimate for BMD‐ : 86.25
estimate for c : 12.53
Weibull
estimate for a‐ : 0.07054
estimate for BMD‐ : 83.82
estimate for c : 7.245
log.prob
estimate for a‐ : 0.07054
estimate for BMD‐ : 84.62
estimate for c : 4.142
gamma
estimate for a‐ : 0.07054
estimate for BMD‐ : 82.38
estimate for cc : 11.63
logistic
estimate for a‐ : ‐2.865
estimate for BMD‐ : 46.82
probit
estimate for a‐ : ‐1.609
estimate for BMD‐ : 43.58
EXP
estimate for a‐ : 1.445
estimate for CED‐ : 81.32
estimate for d‐ : 4
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 1.445
estimate for CED‐ : 80.96
estimate for d‐ : 4
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging:
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.04 | 0.14 | 0.14 | 0.14 | 0.14 | 0.06 | 0.06 | 0.14 | 0.14 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 53 | 96.5 |
Confidence intervals for the BMD are based on 500 bootstrap data sets.
Visualisation:
C. 16. Combined incidence of haematoma/haemorrhage in rats, from dams exposed to Cereclor S52 (C14–17, 52% chlorination) (IRDC, 1985)
Data description
The endpoint to be analysed is: Combined incidence of haematoma/haemorrhage on pups found dead by post‐natal day (PND) 21.
Data used for analysis:
| Dose(mg/kg bw per day) | Combined incidence of haematoma/haemorrhage | Na |
|---|---|---|
| 0 | 0 | 15 |
| 9 | 0 | 4 |
| 90 | 9 | 17 |
| 560 | 64 | 64 |
Necropsy performed on dead pups at PND10. Additional pups from the control and high‐dose groups were euthanised on PND 10 and included in the necropsy analysis.
Selection of the BMR
Considering the association of this effect with the decreased pup survival observed in the study, the CONTAM Panel considered applying the same BMR of 5% selected for the dose–response analysis of pup mortality (see Section C.11). The Panel noted that in this case the selection of a lower BMR is less justified from a statistical point of view considering the low study number of animals evaluated in some dose groups.
Results
Response variable: Combined incidence of haematoma/haemorrhage
Fitted Models:
| model | No.par | loglik | AIC | accepted | BMDL | BMDU | BMD | conv |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −58.33 | 118.66 | NA | NA | NA | NA | |
| full | 4 | −11.75 | 31.50 | NA | NA | NA | NA | |
| two.stage | 3 | −11.78 | 29.56 | no | NA | NA | 23.5 | yes |
| log.logist | 3 | −11.75 | 29.50 | yes | 16.10 | 84.5 | 63.8 | yes |
| Weibull | 3 | −11.75 | 29.50 | yes | 3.05 | 82.2 | 62.9 | yes |
| log.prob | 3 | −11.75 | 29.50 | yes | 12.60 | 87.5 | 53.4 | yes |
| gamma | 3 | −11.75 | 29.50 | yes | 2.34 | 79.3 | 51.7 | yes |
| logistic | 2 | −11.75 | 27.50 | yes | 18.40 | 79.1 | 76.0 | yes |
| probit | 2 | −11.75 | 27.50 | yes | 16.00 | 77.6 | 55.5 | no |
| LVM: Expon. m3‐ | 3 | −11.75 | 29.50 | yes | 2.30 | 84.6 | 64.5 | yes |
| LVM: Hill m3‐ | 3 | −11.75 | 29.50 | yes | 4.33 | 80.8 | 50.8 | yes |
Estimated Model Parameters:
two.stage
estimate for a‐ : 1e‐06
estimate for BMD‐ : 23.52
estimate for c : 1e+12
log.logist
estimate for a‐ : 1e‐06
estimate for BMD‐ : 63.81
estimate for c : 8.904
Weibull
estimate for a‐ : 1e‐06
estimate for BMD‐ : 62.91
estimate for c : 7.505
log.prob
estimate for a‐ : 1e‐06
estimate for BMD‐ : 53.42
estimate for c : 3.295
gamma
estimate for a‐ : 1e‐06
estimate for BMD‐ : 51.7
estimate for cc : 11.57
logistic
estimate for a‐ : ‐19.53
estimate for BMD‐ : 75.98
probit
estimate for a‐ : ‐4.399
estimate for BMD‐ : 55.46
EXP
estimate for a‐ : 4.482
estimate for CED‐ : 64.53
estimate for d‐ : 1
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
HILL
estimate for a‐ : 4.48
estimate for CED‐ : 50.81
estimate for d‐ : 1.037
estimate for th(fixed) : 0
estimate for sigma(fixed) : 0.25
Weights for Model Averaging:
| two.stage | log.logist | Weibull | log.prob | gamma | logistic | probit | EXP | HILL |
|---|---|---|---|---|---|---|---|---|
| 0.08 | 0.08 | 0.08 | 0.08 | 0.08 | 0.22 | 0.22 | 0.08 | 0.08 |
Final BMD Values (mg/kg bw per day):
| subgroup | BMDL | BMDU |
|---|---|---|
| 48.5 | 63.8 |
Confidence intervals for the BMD are based on 500 bootstrap data sets.
Visualisation:
Appendix D – Summary statistics of the occurrence submitted to EFSA
1.
Data set A 53
Table D.1.
Mean levels of ‘Alkanes, C10–13, chloro’ (SCCP) (μg/kg fat weight)
| FOODEX1 Level 2 | N | % LC | MEAN (LB) | MEAN (UB) |
|---|---|---|---|---|
| Bread and rolls | 1 | 0 | 88 | 88 |
| Vegetables and vegetable products | 1 | 100 | 0.0 | 500 |
| Fruit and fruit products | 1 | 0 | 1,000 | 1,000 |
| Livestock meat | 4 | 50 | 13 | 21 |
| Poultry | 3 | 67 | 3.3 | 11 |
| Edible offal, farmed animals | 4 | 75 | 5.9 | 22 |
| Sausages | 1 | 0 | 28 | 28 |
| Fish meat | 12 | 42 | 6.9 | 24 |
| Crustaceans | 1 | 0 | 39 | 39 |
| Liquid milk | 3 | 100 | 0.0 | 24 |
| Cheese | 5 | 100 | 0.0 | 4.0 |
| Eggs, fresh | 6 | 100 | 0.0 | 9.1 |
| Animal fat | 1 | 0 | 1.4 | 1.4 |
| Fish oil | 1 | 0 | 25 | 25 |
SCCPs: short‐chain chlorinated paraffins; LB: lower bound; UB: upper bound; N: number of samples; % LC: percentage of left‐censored data.
Table D.2.
Mean levels of ‘Alkanes, C14–17, chloro’ (MCCP) (μg/kg fat weight)
| FOODEX1 Level 2 | N | % LC | MEAN (LB) | MEAN (UB) |
|---|---|---|---|---|
| Bread and rolls | 1 | 0 | 167 | 167 |
| Vegetables and vegetable products | 1 | 100 | 0.0 | 500 |
| Fruit and fruit products | 1 | 100 | 0.0 | 500 |
| Livestock meat | 4 | 50 | 83 | 92 |
| Poultry | 3 | 67 | 5.3 | 13 |
| Edible offal, farmed animals | 4 | 50 | 31 | 40 |
| Sausages | 1 | 0 | 54 | 54 |
| Fish meat | 12 | 33 | 23 | 38 |
| Crustaceans | 1 | 0 | 19 | 19 |
| Liquid milk | 3 | 100 | 0.0 | 24 |
| Cheese | 5 | 60 | 3.2 | 5.3 |
| Eggs, fresh | 6 | 100 | 0.0 | 9.1 |
| Animal fat | 1 | 0 | 3.4 | 3.4 |
| Fish oil | 1 | 0 | 72 | 72 |
MCCPs: medium‐chain chlorinated paraffins; LB: lower bound; UB: upper bound; N: number of samples; % LC: percentage of left‐censored data.
Annex A – Occurrence data in food submitted to EFSA and dietary exposure assessment for humans
1.
Annex A can be found as a separate document and also available on the EFSA Knowledge Junction community on Zenodo at: http://doi.org/10.5281/zenodo.3689718
Description: This Annex is an excel file which presents tables on chlorinated paraffins on occurrence data in food and dietary exposure assessment for humans.
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc J‐C, Nebbia CS, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Wallace H, Brüschweiler B, Leonards P, Rose M, Binaglia M, Horváth Z, Bordajandi LR and Nielsen E, 2020. Scientific Opinion – Risk assessment of chlorinated paraffins in feed and food. EFSA Journal 2020;18(3):5991, 220 pp. 10.2903/j.efsa.2020.5991
Requestor: European Commission
Question number: EFSA‐Q‐2016‐00801
Panel members: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace.
Acknowledgements: The Panel wishes to thank the hearing expert: Kerstin Krätschmer and EFSA staff member: Kelly Niermans for the support provided to this scientific output. The CONTAM Panel acknowledges all European competent institutions and other stakeholders that provided occurrence data in food and human milk and data on the toxicity of CPs, and supported the data collection for the Comprehensive European Food Consumption Database.
Adopted: 17 December 2019
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figures 2 and 3: © Elsevier
Pim Leonards was a member of the Working Group on Chlorinated Paraffins in food and feed from 28 August 2017 until 18 October 2018. Due to the revision of EFSA's Policy on Independence of 1 July 2018, he was reclassified as an EFSA Hearing Expert.
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1815/full
Notes
Reprinted from Science of the Total Environment, 573, Glüge J, Wang Z, Bogdal C, Scheringer M and Hungerbühler K, Global production, use, and emission volumes of short‐chain chlorinated paraffins – A minimum scenario, 1132‐1146, © Elsevier B.V. (2016), with permission from Elsevier.
Reprinted from Chemosphere, 227, Krätschmer K, Schächtele A, Malisch R and Vetter W, Chlorinated paraffins (CPs) in salmon sold in southern Germany: Concentrations, homologue patterns and relation to other persistent organic pollutants, 630‐637, © Elsevier Ltd. (2019), with permission from Elsevier.
SHINE Project: Target and non‐target screening of chemicals in the indoor environment for human exposure assessment. CEFIC‐Long‐range Research Activity B17.
IRDC, 1984. 13‐week oral (gavage) toxicity study in rats with combined excretion, tissue level and elimination studies: determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 58% chlorination of short‐chain length n‐paraffins; 14C‐labelled CP. Mattawan, Michigan, International Research and Development Corporation, 350 pp (Report No. 438‐029/022). As cited in WHO/IPCS (1996). Note: Study report also provided to EFSA (IRDC, 1984b, see Documentation provided to EFSA).
IRDC, 1984. 13‐week dietary toxicity study in rats with combined excretion, tissue level and elimination studies/determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 70% chlorination of long‐chain length n‐paraffins; 14C‐labelled CP. Mattawan, Michigan, International Research and Development Corporation, 316 pp (Report No. 438‐027/024). As cited in Health Canada (2008). Note: Study report also provided to EFSA (IRDC, 1984e, see Documentation provided to EFSA).
ECB (European Chemicals Bureau), 1999. Alkanes, C10‐13, chloro. European Union Risk Assessment Report. As cited in UK‐COT (2009). Note: report cited in this opinion as EU‐RAR (2000).
IRDC, 1984. 13‐week oral (dietary) toxicity study in rats with combined excretion, tissue level and elimination studies: determination of excretion, tissue level and elimination after single oral (gavage) administration to rats. Chlorinated paraffin: 52% chlorination of intermediate‐chain length n‐paraffins, 14C‐labelled CP. Mattawan, Michigan, International Research and Development Corporation, 328 pp (Report No. 438‐023/026). As cited in WHO/IPCS (1996). Note: Study report also provided to EFSA (IRDC, 1984c, see Documentation provided to EFSA).
ECB (European Chemicals Bureau), 2004. Alkanes, C14–17, chloro. Draft European Union Risk Assessment Report. Note: report cited in this opinion as EU‐RAR (European Union‐Risk Assessment Report) (2011).
CXR, 2005. A dietary study to determine the 90‐day NOAEL of medium chain chlorinated paraffins (Cereclor S52) in male and female Fisher 344 rats. CXR0273. CXR Bioscience Ltd, Dundee, UK. Unpublished report. As cited by BauA (2011). Note: Study report also provided to EFSA (CXR, 2005b, see Documentation provided to EFSA).
CXR, 2005. Study to Investigate the Elimination of Medium Chain Chlorinated Paraffins in Male F344 Rats. CXR0204. Elcombe, B.M., Dundee, UK. As cited by US‐EPA (2015).
IRCD, 1984. Chlorinated paraffin: 43 per cent chlorination of long chain length paraffins. 13‐week day oral toxicity study in rats, with combined excretion, tissue level and elimination study. IRDC report 438‐028/438‐021. As cited in UK‐Environment Agency (2009). Note: Study report also provided to EFSA (IRDC, 1984d, see Documentation provided to EFSA).
The section of the List of MAK and BAT Values previously called ‘III B’ was renamed as Category 3B of Section III, which includes substances that cause concern that they could be carcinogenic for man but cannot be assessed conclusively because of lack of data. The classification in Category 3 is provisional. Classification in Category 3B is for substances for which in vitro or animal studies have yielded evidence of carcinogenic effects that is not sufficient for classification of the substance in one of the other categories. Further studies are required before a final decision can be made. A MAK or BAT value can be established provided no genotoxic effects have been detected (from https://onlinelibrary.wiley.com/doi/pdf/10.1002/9783527812127.oth).
Council Regulation (EEC) No 315/93 of 8 February 1993 laying down Community procedures for contaminants in food. OJ L 37, 13.2.1993, p. 1.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs, OJ L 364, 20.12.2006, p. 5‐24.
Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in food. OJ L 140, 30.2.2002, p. 10.
Regulation (EC) No 850/2004 of the European Parliament and of the Council of 29 April 2004 on persistent organic pollutants and amending Directive 79/117/EEC. OJ L 158, 29.04.2004. p.7.
Commission Regulation (EU) No 519/2012 of 19 June 2012 amending Regulation (EC) No 850/2004 of the European Parliament and of the Council on persistent organic pollutants as regards Annex I. OJ L 159, 19.06.2012. p.1.
Commission Regulation (EU) 2015/2030 of 13 November 2015 amending Regulation (EC) No 850/2004 of the European Parliament and of the Council on persistent organic pollutants as regards Annex I. OJ L 298, 13.11.2015. p. 1.
US‐EPA, TSCA New chemicals review program standard review risk assessment on medium‐chain chlorinated paraffins and long‐chain chlorinated paraffins; EPA: Washington, DC, 2015. And CPIA. MCCP and LCCP US Regulatory Status Update – March 2017; CPIA: Washington, DC, 2017.
Environment and Climate Change Canada. Proposed Risk Management Approach for Chlorinated Paraffins. http://ec.gc.ca/lcpecepa/default.asp?lang=En&n=D048964A-1. And Environment and Climate Change Canada. Follow‐up Report on a PSL1 Assessment for Which Data Were Insufficient to Conclude Whether the Substances Were ‘Toxic’ to the Environment and to the Human Health: Chlorinated Paraffins; 2008 https://www.ec.gc.ca/lcpe-cepa/09F567A7-B1EE-1FEE-73DB-8AE6C1EB7658/cps_followup-eng.pdf
According to Environment and Climate Change Canada (2016), the fish tissue guidelines are benchmarks for aquatic ecosystems that are intended to protect fish themselves from direct adverse effects.
According to Environmental and Climate Change Canada (2016), the wildlife dietary guidelines are intended to protect non‐human mammalian consumers of aquatic biota. These are benchmark concentrations of substances in aquatic biota (whole body, wet‐weight) that are consumed by terrestrial and semi‐aquatic wildlife.
Web of Science (WoS), formerly ISI Web of Knowledge, Thomson Reuters. Available online: http://thomsonreuters.com/thomson-reuters-web-of-science/. It encompasses the following databases: Web of ScienceTM Core Collection, BIOSIS Citation IndexSM, CABI: CAB Abstracts®, Current Contents Connect®, Data Citation IndexSM, FSTA® – the food science resource, MEDLINE®, SciELO Citation Index, Zoological Record®.
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available online: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X8, Thomson Reuters. Available online: http://endnote.com/
Described in the study report as ‘Characterised by the infiltration of mixed inflammatory cells into the interstitium and tubular regeneration with minimal tubular degeneration. The regenerated tubules contained prominent brown granular pigment’. IRDC (1984a, unpublished study, see Documentation provided to EFSA).
Conversion of the concentration in feed into doses calculated using a conversion factor of 0.09 as recommended by the Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data to be used for subchronic studies (EFSA Scientific Committee, 2012a).
It was noted that no histopathology but only a macroscopic examination was carried out in this study. However, haemorrhagic effects in the pups should have been likely visible macroscopically as red or dark staining at the organ surface.
Considering a body weight of 1,490 g and a feed intake of 70.6 g per day (Ueberschär and Matthes, 2004).
Considering a live weight of 1,521 g and a feed intake of 115 g/day (Ueberschär et al., 2007).
Assuming a body weight of 1,200 g and a feed intake of 60 g/day.
Considering a body weight of 69 g (from Fisk et al., 2000) and a feed intake of 1.5% of the body weight.
Considering a fish weight of 69 g and a feed intake of 1.5% of the body weight.
Considering 7 g as the body weight of the Juvenile Atlantic salmon at the start of the study, and a feed intake of 2.5% of the body weight.
The LT50 is the time required for 50% of the animals to die following exposure to a known concentration of the test agent.
Considering 30.3 g as body weight and a feed intake of 1.5% of the body weight (Fisk et al., 2000).
Considering 4 g as body weight and a feed intake of 2.5% of the body weight.
No detailed composition of the commercial Cereclor 63L could be found but it may have similar composition as Cereclor 60L. The latter was reported to contain 15.5% C10, 32.3% C11, 35.2% C12, and 17.0% C13 (Reth et al., 2006).
ICES‐6: PCB‐28, ‐52, ‐101, ‐138, ‐153, ‐180.
Using the LOQ value of 5.5 ng/g fat as the numerical value for the estimation.
Chlorowax 500C was an SCCP product with a predominant carbon chain length range of C10‐12 and an average of 50% chlorine by weight (information provided by EuroChlor to EFSA).
Cereclor 42 is an LCCP product with a predominantly C20‐30 range and an average chlorination level of 42% by weight (information provided by EuroChlor to EFSA).
Chlorez 700 is an LCCP product with a predominant range of C20‐28 and an average of 70% chlorine by weight (information provided by EuroChlor to EFSA).
References
- Åhlman M, Bergman A, Darnerud PO, Egestad B and Sjovall J, 1986. Chlorinated paraffins: formation of sulphur‐containing metabolites of polychlorohexadecane in rats. Xenobiotica, 16, 225–232. 10.3109/00498258609043525 [DOI] [PubMed] [Google Scholar]
- Alcock RE, Sweetman A and Jones KC, 1999. Assessment of organic contaminant fate in waste water treatment plants. I: Selected compounds and physicochemical properties. Chemosphere, 38, 2247–2262. 10.1016/S0045-6535(98)00444-5 [DOI] [PubMed] [Google Scholar]
- Allpress and Gowland , 1999. Biodegradation of chlorinated paraffins and long‐chain chloroalkanes by Rhodococcus sp. S45‐1. International Biodeterioration and Biodegradation, 43, 173–179. 10.1016/S0964-8305(99)00050-5 [DOI] [Google Scholar]
- Alshehri BD, D'Souza G, Lee JY, Petratos S and Richardson SJ, 2015. The diversity of mechanisms Influenced by transthyretin in neurobiology: development, disease and endocrine disruption. Journal of Neuroendocrinology, 27, 303–323. 10.1111/jne.12271 [DOI] [PubMed] [Google Scholar]
- Anderson BE, Zeiger E, Shelby MD, Resnick MA, Gulati DK, Ivett JL and Loveday KS, 1990. Chromosome aberration and sister chromatid exchange test‐results with 42 chemicals. Environmental and Molecular Mutagenesis, 16, 55–137. 10.1002/em.2850160505 [DOI] [PubMed] [Google Scholar]
- Ashby J, Lefevre PA and Elcombe CR, 1990. Cell replication and unscheduled DNA synthesis (UDS) activity of low molecular weight chlorinated paraffins in the rat liver in vivo. Mutagenesis, 5, 515–518. 10.1093/mutage/5.5.515 [DOI] [PubMed] [Google Scholar]
- Bardin JA, Gore RJ, Wegman DH, Kriebel D, Woskie SR and Eisen EA, 2005. Registry‐based case‐control studies of liver cancer and cancers of the biliary tract nested in a cohort of autoworkers exposed to metalworking fluids. Scandinavian Journal of Work Environmental Health, 31, 205–211. 10.5271/sjweh.870 [DOI] [PubMed] [Google Scholar]
- Bartsch R, Brinkmann B, Jahnke G, Laube B, Lohmann R, Michaelsen S, Neumann I and Greim H, 2018. Human relevance of follicular thyroid tumors in rodents caused by non‐genotoxic substances. Regulatory Toxicology and Pharmacology, 98, 199–208. 10.1016/j.yrtph.2018.07.025 [DOI] [PubMed] [Google Scholar]
- BauA (Bundesanstalt für Arbeitsschutz und Arbeitsmedizin), 2011. Ausschuss für Gefahrstoffe ‐ AGS‐Geschäftsführung. Begründung zu Chlorierte Paraffine in TRGS 900. Chloralkane, C14‐17 (Chlorierte Paraffine C14‐17) (CAS Nr.: 85535‐85‐9).
- Bayen S, Obbard JP and Thomas GO, 2006. Chlorinated paraffins: a review of analysis and environmental occurrence. Environment International, 32, 915–929. 10.1016/j.envint.2006.05.009 [DOI] [PubMed] [Google Scholar]
- Bendig P, Hägele F and Vetter W, 2013. Widespread occurrence of polyhalogenated compounds in fat from kitchen hoods. Analytical and Bioanalytical Chemistry, 405, 7485–7496. 10.1007/s00216-013-7194-5 [DOI] [PubMed] [Google Scholar]
- Bengtsson BE and Ofstad EB, 1982. Long‐term studies on uptake and elimination of some chlorinated paraffins in the bleak, Alburnus‐alburnus. Ambio, 11, 38–39. [Google Scholar]
- Bengtsson BE, Svanberg O, Linden E, Lunde G and Ofstad EB, 1979. Structure related uptake of chlorinated paraffins in bleaks (Alburnus‐alburnus L). Ambio, 8, 121–122. [Google Scholar]
- Bentley P, Calder I, Elcome C, Grasso P, Stringer D and Wiegand HJ, 1993. Hepatic peroxisome proliferation in rodents and its significance for humans. Food and Chemical Toxicology, 31, 857–907. 10.1016/0278-6915(93)90225-N [DOI] [PubMed] [Google Scholar]
- Biessmann A, Brandt I and Darnerud PO, 1982. Comparitive distribution and metabolism of two 14C‐labelled chlorinated paraffins in Japanese quail Coturnix coturnix japonica. Environmental Pollution Series A, Ecological and Biological, 28, 109–120. 10.1016/0143-1471(82)90097-6 [DOI] [Google Scholar]
- Biessmann A, Darnerud PO and Brandt I, 1983. Chlorinated paraffins: disposition of a highly chlorinated polychlorohexadecane in mice and quail. Archives of Toxicology, 53, 79–86. 10.1007/BF01460004 [DOI] [PubMed] [Google Scholar]
- Birtley RDN, Conning DM, Daniel JW, Ferguson DM, Longstaff E and Swan AAB, 1980. Toxicological effects of chlorinated paraffins in mammals. Toxicology and Applied Pharmacology, 54, 514–525. 10.1016/0041-008x(80)90179-9 [DOI] [PubMed] [Google Scholar]
- Bogdal C, Alsberg T, Diefenbacher PS, MacLeod M and Berger U, 2015. Fast quantification of chlorinated paraffins in environmental samples by direct injection high‐resolution mass spectrometry with pattern deconvolution. Analytical Chemistry, 87, 2852–2860. 10.1021/ac504444d [DOI] [PubMed] [Google Scholar]
- Brandsma SH, van Mourik L, O'Brien JW, Eaglesham G, Leonards PEG, de Boer J, Gallen C, Mueller J, Gaus C and Bogdal C, 2017. Medium‐Chain Chlorinated Paraffins (CPs) Dominate in Australian Sewage Sludge. Environmental Science and Technology, 51, 3364–3372. 10.1021/acs.est.6b05318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunström B, 1985. Effects of chlorinated paraffins on liver weight, cytochrome P‐450 concentration and microsomal enzyme activities in chick embryos. Archives of Toxicology, 57, 69–71. 10.1007/BF00286579 [DOI] [PubMed] [Google Scholar]
- Bucher JR, Alison RH, Montgomery CA, Huff J, Haseman JK, Farnell D, Thompson R and Prejean JD, 1987. Comparative toxicity and carcinogenicity of two chlorinated paraffins in F344/N rats and B6C3F1 mice. Fundamental and Applied Toxicology, 9, 454–468. 10.1093/toxsci/9.3.454 [DOI] [PubMed] [Google Scholar]
- Caldwell D, 1999. Review of mononuclear cell leukemia in F‐344 rat bioassays and its significance to human cancer risk: A case study using alkyl phthalates. Regulatory Toxicology Pharmacology, 30, 45–53. 10.1006/rtph.1999.1305 [DOI] [PubMed] [Google Scholar]
- Campbell I and McConnell G, 1980. Chlorinated paraffins and the environment. 1. Environmental occurrence. Environmental Science and Technology, 14, 1209–1214. 10.1021/es60170a001 [DOI] [Google Scholar]
- Cao Y, Harada KH, Liu W, Yan J, Zhao C, Niisoe T, Adachi A, Fujii Y, Nouda C, Takasuga T and Koizumi A, 2015. Short‐chain chlorinated paraffins in cooking oil and related products from China. Chemosphere, 138, 104–111. 10.1016/j.chemosphere.2015.05.063 [DOI] [PubMed] [Google Scholar]
- Cao Y, Harada KH, Hitomi T, Niisoe T, Wang P, Shi Y, Yang HR, Takasuga T and Koizumi A, 2017. Lactational exposure to short‐chain chlorinated paraffins in China; Korea, and Japan. Chemosphere, 173, 43–48. 10.1016/j.chemosphere.2016.12.078 [DOI] [PubMed] [Google Scholar]
- Cao D, Gao W, Wu J, Lv K, Xin S, Wang Y and Jiang G, 2019. Occurrence and human exposure assessment of short‐ and medium‐chain chlorinated paraffins in dusts from plastic sports courts and synthetic turf in Beijing, China. Environmental Science and Technology, 53, 443–451. 10.1021/acs.est.8b04323 [DOI] [PubMed] [Google Scholar]
- Chater B, 1978. Acute oral toxicity, skin and eye irritation and skin sensitisation. Report No. CTL/T/1168. ICI Central Toxicology Laboratory, Alderley Park, Cheshire, UK. As cited by EU‐RAR (2011).
- Chen Y‐H, Chang C‐Y and Ding W‐H, 2016. Vortex‐homogenized matrix solid‐phase dispersion for the extraction of short chain chlorinated paraffins from indoor dust samples. Journal of Chromatography A, 1472, 129–133. 10.1016/j.chroma.2016.10.048 [DOI] [PubMed] [Google Scholar]
- Chen H, Lam JCW, Zhu M, Wang F, Zhou W, Du B, Zeng L and Zeng EY, 2018. Combined Effects of Dust and Dietary Exposure of Occupational Workers and Local Residents to Short‐ and Medium‐Chain Chlorinated Paraffins in a Mega E‐Waste Recycling Industrial Park in South China. Environmental Science and Technology, 52, 11510–11519. 10.1021/acs.est.8b02625 [DOI] [PubMed] [Google Scholar]
- Chen CK, Li L, Liu JZ and Kiu JG, 2019. Global environmental fate of short‐chain chlorinated paraffins: Modeling with a single vs. multiple sets of physicochemical properties. Science of the Total Environment, 666, 423–430. 10.1016/j.scitotenv.2019.02.157 [DOI] [PubMed] [Google Scholar]
- Cherri JW and Semple S, 2010. Dermal exposure to metalworking fluids and medium‐chain chlorinated paraffin (MCCP). The Annals of Occupational Hygene, 54, 228–235. [DOI] [PubMed] [Google Scholar]
- Chipman JK, Mally A and Gareth OE, 2003. Disruption of Gap Junctions in Toxicity and Carcinogenicity. Toxicological Sciences, 71, 146–153. 10.1093/toxsci/71.2.146 [DOI] [PubMed] [Google Scholar]
- Coelhan M and Hilger B, 2014. Chlorinated paraffins in indoor dust samples: a review. Current Organic Chemistry, 18, 2209–2217. 10.2174/1385272819666140804230914 [DOI] [Google Scholar]
- Conz A and Fumero S, 1988a. Study of the Capacity of Solvocaffaro C1642 to Induce Gene Mutations in Strains of Salmonella Typhimurium. RBM Exp. No. 880367. Turin, Istituto di Recerche Biomediche “Antoine Marxer”, Torino, Italy. As cited by EU‐RAR (2011) and WHO/IPCS (1996).
- Conz A and Fumero S, 1988b. Micronucleus induction in bone marrow cells of mice treated by oral route with the test article Solvocaffaro C1642. Turin, Istituto di Recerche Biomediche “Antoine Marxer”, 20 pp (RBM Exp. No. 880368). As cited by EU‐RAR (2011) and WHO/IPCS (1996).
- Cooke M, Nickless G and Roberts DJ, 1980. Carbon skeleton gas‐chromatographic techniques and their applications. Journal of Chromatography, 187, 47–55. 10.1016/S0021-9673(00)87872-3 [DOI] [Google Scholar]
- Cooley HM, Fisk AT, Wiens SC, Tomy GT, Evans RE and Muir DC, 2001. Examination of the behavior and liver and thyroid histology of juvenile rainbow trout (Oncorhynchus mykiss) exposed to high dietary concentrations of C(10)‐, C(11)‐, C(12)‐ and C(14)‐polychlorinated n‐alkanes. Aquatic Toxicology, 54, 81–99. 10.1016/S0166-445X(00)00172-7 [DOI] [PubMed] [Google Scholar]
- Corton JC, Peters JM and Klaunig JE, 2018. The PPAR alpha‐dependent rodent liver tumor response is not relevant to humans: addressing misconceptions. Archives of Toxicology, 92, 83–119. 10.1007/s00204-017-2094-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Gao L, Zheng M, Li J, Zhang L, Wu Y, Qiao L, Xua C, Wang K and Huang D, 2019. Bioaccessibility of short chain chlorinated paraffins in meat and seafood. Science of the Total Environment, 668, 996–1003. 10.1016/j.scitotenv.2019.03.043 [DOI] [PubMed] [Google Scholar]
- CXR Biosciences Ltd , 2005. Elcombe BM. Study to investigate the elimination of medium chain chlorinated paraffins in male F344 rats. CXR0204. CXR Biosciences Ltd, Dundee, UK. Unpublished report. As cited by EU‐RAR, 2011.
- Darnerud PO, 1984. Chlorinated paraffins: effect of some microsomal enzyme inducers and inhibitors on the degradation of 1‐14C‐chlorododecanes to 14CO2 in mice. Acta Pharmacologica et Toxicologica, 55, 110–115. icol (Copenh), 55, 110‐115. 10.1111/j.1600-0773.1984.tb01971.x [DOI] [PubMed] [Google Scholar]
- Darnerud PO and Brandt I, 1982. Studies on the distribution and metabolism of a C‐14‐labeled chlorinated alkane in mice. Environmental Pollution Series a‐Ecological and Biological, 27, 45–56. 10.1016/0143-1471(82)90062-9 [DOI] [Google Scholar]
- Darnerud PO, Biessmann A and Brandt I, 1982. Metabolic fate of chlorinated paraffins: degree of chlorination of [1‐14C]‐chlorododecanes in relation to degradation and excretion in mice. Archives of Toxicology, 50, 217–226. 10.1007/BF00310853 [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Bengtsson BE, Bergman A and Brandt I, 1983. Chlorinated paraffins: disposition of a polychloro‐[1‐14C]‐hexadecane in carp (Cyprinus carpio) and bleak (Alburnus alburnus). Toxicology Letters, 19, 345–351. 10.1016/0378-4274(83)90141-8 [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Bergman A, Lund BO and Brandt I, 1989. Selective accumulation of chlorinated paraffins (C‐12 and C‐16) in the olfactory organ of rainbow‐trout. Chemosphere, 18, 1821–1827. 10.1016/0045-6535(89)90466-9 [DOI] [Google Scholar]
- Darnerud PO, Aune M, Glynn A and Borgen A, 2012. Chlorinated paraffins in Swedish breast milk. Swedish chemicals Agency, KemI. PM 18/12. Available online: https://www.kemi.se/global/pm/2012/pm-18-12.pdf
- Diamond Shamrock Chem. Co , 1975. Unpublished study. As cited by Howard et al. (1975) and WHO/IPCS (1996).
- Dong S, Li X, Su X and Wang P, 2019. Concentrations and congener group profiles of short‐ and medium‐chain chlorinated paraffins in animal feed materials. Science of the Total Environment, 647, 676–681. 10.1016/j.scitotenv.2018.08.017 [DOI] [PubMed] [Google Scholar]
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311,135 pp. 10.2903/j.efsa.2018.5311.ECHA-18-G-01-EN [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(5): 1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Intakes Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2011bThe food classification and description system FoodEx 2 (draft revision1). EFSA Supporting Publications 2011;8(12):215, 438 pp. 10.2903/sp.efsa.2011.EN-215 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2007. Guidance of the Scientific Committee on a request from EFSA related to Uncertainties in Dietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Outcome of a public consultation on the draft risk assessment of chlorinated paraffins in feed and food. EFSA supporting publication 2020:EN‐1815. 43 pp. 10.2903/sp.efsa.2020.EN-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012a. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012b. Scientific Opinion on Risk Assessment Terminology. EFSA Journal 2012;10(5):2664, 43 pp. 10.2903/j.efsa.2012.2664 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017a. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017bScientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Younes M, Aquilina G, Crebelli R, Gurtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, van Benthem J, Carfi M, Georgiadis N, Maurici D, Parra Morte J and Schlatter J, 2017. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017c;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elcombe CR, Warnasuriya GD and Foster JR, 2000. Chlorinated paraffins: Mechanisms of non‐genotoxic carcinogenesis. Organohalogen Compounds, 47, 143–146. [Google Scholar]
- Elliott BM, 1989a. Meflex DC029 (fully formulated) ‐ Ames test. Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 1 p (Report No. CTL/L/2668). As cited by EU‐RAR (2011) and WHO/IPCS (1996).
- Elliott BM, 1989b. Meflex DC029 (fully formulated) – Mouse micronucleus test. Macclesfield, Cheshire, Imperial Chemical Industries Ltd, 1 p (Report No. CTL/L/2693). As cited by EU‐RAR (2011), WHO/IPCS (1996).
- Environment and Climate Change Canada , 2016. Canadian Environmental Protection Act, 1999. Federal Environmental Quality Guidelines for Chlorinated Alkanes. Available online: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=C4148C43-1
- Erickson TB, Aks SE, Zabaneh R and Reid R, 1996. Acute renal toxicity after ingestion of Lava light liquid. Annals of Emergency Medicine, 27, 781–784. 10.1016/S0196-0644(96)70202-0 [DOI] [PubMed] [Google Scholar]
- Eriksson P and Darnerud PO, 1985. Distribution and retention of some chlorinated hydrocarbons and a phthalate in the mouse brain during the pre‐weaning period. Toxicology, 37, 185–203. 10.1016/0300-483X(85)90082-4 [DOI] [PubMed] [Google Scholar]
- Eriksson P and Nordberg A, 1986. The effects of DDT, DDOH‐palmitic acid, and a chlorinated paraffin on muscarinic receptors and the sodium‐dependent choline uptake in the central nervous system of immature mice. Toxicology and Applied Pharmacology, 85, 121–127. 10.1016/0041-008X(86)90105-5 [DOI] [PubMed] [Google Scholar]
- EU‐RAR (European Union‐Risk Assessment Report), 2000. Alkanes, C10‐13, chloro. CAS No.: 85535‐84‐8. EINECS No: 287‐476‐5. European Chemicals Bureau. Institute for Health and Consumer Protection. Volume 4.
- EU‐RAR (European Union‐Risk Assessment Report), 2005. Alkanes, C14‐17, chloro. Part I – environment. CAS No: 85535‐85‐9. EINECS No: 287‐477‐0. European Chemicals Bureau. Institute for Health and Consumer Protection. Volume 58. Available online: https://echa.europa.eu/documents/10162/584faee8-d13a-41dd-8b33-959ad1a81171
- EU‐RAR (European Union‐Risk Assessment Report), 2008. Alkanes, C10‐13, chloro. CAS No: 85535‐84‐8. EINECS No: 287‐476‐5. European Chemicals Bureau. Institute for Health and Consumer Protection. Volume 81. Available online: https://echa.europa.eu/documents/10162/c157d3ab-0ba7-4915-8f30-96427de56f84
- EU‐RAR (European Union‐Risk Assessment Report), 2011. Alkanes, C14‐17, chloro (MCCP). Part II – human health. CAS No: 85535‐85‐9. EINECS No: 287‐477‐0. European Chemicals Bureau. Institute for Health and Consumer Protection. Available online: http://publications.jrc.ec.europa.eu/repository/bitstream/JRC66049/lbna25202enn.pdf
- Evenset A, Christensen GN, Skotvold T, Fjeld E, Schlabach M, Wartena E and Gregor D, 2004. A comparison of organic contaminants in two high Arctic lake ecosystems, Bjornoya (Bear Island), Norway. Science of the Total Environment, 318, 125–141. 10.1016/S0048-9697(03)00365-6 [DOI] [PubMed] [Google Scholar]
- Fan X and Wu L, 2016. The impact of thyroid abnormalities during pregnancy on subsequent neuropsychological development of the offspring: a meta‐analysis. Journal of Maternal‐Fetal and Neonatal Medicine, 29, 3971–3976. 10.3109/14767058.2016.1152248 [DOI] [PubMed] [Google Scholar]
- Felter SP, Foreman JE, Boobis A, Corton JC, Doi AM, Flowers L, Goodman J, Haber LT, Jacobs A, Klaunig JE, Lynch AM, Moggs J and Pandiri A, 2018. Human relevance of rodent liver tumors: Key insights from a Toxicology Forum workshop on nongenotoxic modes of action. Regulatory Toxicology and Pharmacology, 92, 1–7 10.1016/j.yrtph.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler H, 2010. The Handbook of Environmental Chemistry. Founded by Otto Hutzinger Editors‐in‐Chief: Damià Barceló l Andrey G. Kostianoy, 10. [Google Scholar]
- Fisk AT, Bergman A, Cymbalisty CD and Muir DCG, 1996. Dietary accumulation of C‐12‐ and C‐16‐chlorinated alkanes by juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 15, 1775–1782. 10.1002/etc.5620151019 [DOI] [Google Scholar]
- Fisk AT, Cymbalisty CD, Tomy GT and Muir DCG, 1998. Dietary accumulation and depuration of individual C‐10‐, C‐11‐ and C‐14‐polychlorinated alkanes by juvenile rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology, 43, 209–221. 10.1016/S0166-445X(97)00103-3 [DOI] [Google Scholar]
- Fisk AT, Tomy GT, Cymbalisty CD and Muir DCG, 2000. Dietary accumulation and quantitative structure‐activity relationships for depuration and biotransformation of short (C10), medium (C14), and long (C18) carbon‐chain polychlorinated alkanes by juvenile rainbow trout (Oncorhynchus mykiss). Environmental Toxicology and Chemistry, 19, 1508–1516. 10.1002/etc.5620190606 [DOI] [Google Scholar]
- Fridén UE, McLachlan MS and Berger U, 2011. Chlorinated paraffins in indoor air and dust: concentrations, congener patterns, and human exposure. Environment International, 37, 1169–1174. 10.1016/j.envint.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Gallistl C, Lok B, Schlienz A and Vetter W, 2017. Polyhalogenated compounds (chlorinated paraffins, novel and classic flame retardants, POPs) in dishcloths after their regular use in households. Science of the Total Environment, 595, 303–324. 10.1016/j.scitotenv.2017.03.217 [DOI] [PubMed] [Google Scholar]
- Gallistl C, Sprengel J and Vetter W, 2018. High levels of medium‐chain chlorinated paraffins and polybrominated diphenyl ethers on the inside of several household baking oven doors. Science of the Total Environment, 615, 1019–1027. 10.1016/j.scitotenv.2017.09.112 [DOI] [PubMed] [Google Scholar]
- Gao W, Cao D, Wang Y, Wu J, Wang Y, Wang Y and Jiang G, 2018. External Exposure to Short‐ and Medium‐Chain Chlorinated Paraffins for the General Population in Beijing, China. Environmental Science and Technology, 52, 32–39. 10.1021/acs.est.7b04657 [DOI] [PubMed] [Google Scholar]
- Gao W, Cao D, Lv K, Wu J, Wang Y, Wang C, Wang Y and Jiang G, 2019. Elimination of short‐chain chlorinated paraffins in diet after Chinese traditional cooking‐a cooking case study. Environment International, 122, 340–345. 10.1016/j.envint.2018.11.025 [DOI] [PubMed] [Google Scholar]
- Geng N, Zhang H, Zhang B, Wu P, Wang F, Yu Z and Chen J, 2015. Effects of short‐chain chlorinated paraffins exposure on the viability and metabolism of human HepG2 cells. Environmental Science and Technology, 49, 3076–3083. 10.1021/es505802x [DOI] [PubMed] [Google Scholar]
- Geng N, Zhang H, Xing L, Gao Y, Zhang B, Wang F, Ren X and Chen J, 2016. Toxicokinetics of short‐chain chlorinated paraffins in Sprague‐Dawley rats following single oral administration. Chemosphere, 145, 106–111. 10.1016/j.chemosphere.2015.11.066 [DOI] [PubMed] [Google Scholar]
- Geng N, Ren X, Gong Y, Zhang H, Wang F, Xing L, Cao R, Xu J, Gao Y, Giesy JP and Chen J, 2019. Integration of metabolomics and transcriptomics reveals short‐chain chlorinated paraffin‐induced hepatotoxicity in male Sprague‐Dawley rat. Environment International, 133, 105231 10.1016/j.envint.2019.105231 [DOI] [PubMed] [Google Scholar]
- German MAK Commission , 1990. Available online: https://onlinelibrary.wiley.com/doi/pdf/10.1002/3527600418.mb6344939bspe0007
- Ghassabian A, El Marroun H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H and White T, 2014. Downstream Effects of Maternal Hypothyroxinemia in Early Pregnancy: Nonverbal IQ and Brain Morphology in School‐Age Children. Journal of Clinical Endocrinology and Metabolism, 99, 2383–2390. 10.1210/jc.2013-4281 [DOI] [PubMed] [Google Scholar]
- Glϋge J, Wang Z, Bogdal C, Scheringer M and Hungerbuhler K, 2016. Global production, use, and emission volumes of short‐chain chlorinated paraffins ‐ A minimum scenario. Science of the Total Environment, 573, 1132–1146. 10.1016/j.scitotenv.2016.08.105 [DOI] [PubMed] [Google Scholar]
- Glϋge J, Schinkel L, Hungerbϋhler K, Cariou R and Bogdal C, 2018. Environmental risks of medium‐chain chlorinated paraffins (MCCPs): A Review. Environmental Science and Technology, 52, 6743–6760. 10.1021/acs.est.7b06459 [DOI] [PubMed] [Google Scholar]
- Gong Y, Zhang H, Geng N, Xing L, Fan J, Luo Y, Song X, Ren X, Wang F and Chen J, 2018. Short‐chain chlorinated paraffins (SCCPs) induced thyroid disruption by enhancement of hepatic thyroid hormone influx and degradation in male Sprague Dawley rats. Science of the Total Environment, 625, 657–666. 10.1016/j.scitotenv.2017.12.251 [DOI] [PubMed] [Google Scholar]
- Gong Y, Zhang H, Geng N, Ren X, Giesy JP, Luo Y, Xing L, Wu P, Yu Z and Chen J, 2019. Short‐chain chlorinated paraffins (SCCPs) disrupt hepatic fatty acid metabolism in liver of male rat via interacting with peroxisome proliferatoractivated receptor α (PPARα). Ecotoxicology and Environmental Safety, 181, 461–171. 10.1016/j.ecoenv.2019.06.003 [DOI] [PubMed] [Google Scholar]
- González FJ, Peters JM and Cattley RC, 1998. Mechanism of action of the nongenotoxic peroxisome proliferators: role of the peroxisome proliferator‐activator receptor alpha. Journal of the National Cancer Institute, 90, 1702–1709. 10.1093/jnci/90.22.1702 [DOI] [PubMed] [Google Scholar]
- Harada KH, Takasuga T, Hitomi T, Peiyu W, Matsukami H and Koizumi A, 2011. Dietary exposure to short‐chain chlorinated paraffins has increased in Beijing, China. Environmental Science and Technology, 45, 7019–7027. 10.1021/es200576d [DOI] [PubMed] [Google Scholar]
- Haschek WM, Rousseaux CG and Wallig MA, 2001. Handbook of Toxicologic Pathology. Second edition. Academic Press. 2001 ISBN 0 12 330215 3.
- Haux C, Larsson A, Lidman U, Forlin L, Hansson T and Johansson‐Sjobeck ML, 1982. Sublethal physiological effects of chlorinated paraffins on the flounder, Platichthys flesus L . Ecotoxicology and Environmental Safety, 6, 49–59. 10.1016/0147-6513(82)90080-X [DOI] [PubMed] [Google Scholar]
- Health Canada , 1993. Canadian Environmental Protection Act. Priority Substances List Assessment Report. Chlorinated Paraffins. Government of Canada. Environment Canada. Health and Welfare Canada.
- Health Canada , 2008. Follow‐up Report on a PSL1 Assessment for Which Data Were Insufficient to Conclude Whether the Substances Were “Toxic” to the Environment and to the Human Health Chlorinated Paraffins. Canadian Environmental Protection Act, 1999. August 2008. Available online: https://www.canada.ca/content/dam/eccc/migration/main/lcpe-cepa/documents/substances/pc-cp/cps_followup-eng.pdf
- Health Canada , 2012. Update on the Human Health Assessment of Long‐Chain Chlorinated Alkanes. Health Canada. May 2012. Available online: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=14B8724F-1
- Heath E, Brown WA, Jensen SR and Bratty MP, 2006. Biodegradation of chlorinated alkanes and their commercial mixtures by Pseudomonas sp. strain 273. Journal of Industrial Microbiology and Biotechnology, 33, 197–207. 10.1007/s10295-004-0186-x [DOI] [PubMed] [Google Scholar]
- Hilger B, Coelhan M, Fromme H and Völkel W, 2011. Determination of chlorinated paraffins in human breast milk by HRGC‐ECNI‐LRMS. Organohalogen Compounds, 1611–1613. [Google Scholar]
- Hilger B, Fromme H, Voelkel W and Coelhan M, 2013. Occurrence of chlorinated paraffins in house dust samples from Bavaria, Germany. Environmental Pollution, 175, 16–21. 10.1016/j.envpol.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Hoechst , 1986. Hordalub 80 ‐ Study of the mutagenic potential in strains of Salmonella typhimurium (Ames Test) and Escherichia coli. Frankfurt/Main, Hoechst AG, 28 pp (Report No. 86.1078). As cited by EU‐RAR (2000) and WHO/IPCS (1996).
- Hoechst , 1987. Chloroparaffin 56 liquid ‐ Detection of gene mutations in somatic cells in culture. HGPRT‐test with V79 cells. Frankfurt/Main, Hoechst AG, 23 pp (Report No. 87.1719). As cited by EU‐RAR (2000) and WHO/IPCS (1996).
- Hoechst , 1988. Chloroparaffin 56 liquid (unstabilized) ‐ Study of the mutagenic potential in strains of Salmonella typhimurium (Ames Test) and Escherichia coli. Frankfurt/Main, Hoechst AG, 30 pp (Report No. 88.0099). As cited by EU‐RAR (2000) and WHO/IPCS (1996).
- Hoechst , 1989. Chlorowax 500C micronucleus test in male and female NMRI mice after oral administration. Frankfurt/Main, Hoechst AG, 24 pp (Report No. 89.0253). As cited by WHO/IPCS (1996).
- Houde M, Muir DCG, Tomy GT, Whittle DM, Teixeira C and Moore S, 2008. Bioaccumulation and Trophic Magnification of Short‐ and Medium‐Chain Chlorinated Paraffins in Food Webs from Lake Ontario and Lake Michigan. Environmental Science and Technology, 42, 3893–3899. 10.1021/es703184s [DOI] [PubMed] [Google Scholar]
- Howard PH, Santodonato J and Saxena J, 1975. Investigations of selected potential environmental contaminants: chlorinated paraffins. Syracuse, New York, Syracuse University Research Corporation, 107 pp (Report No. 68‐01‐3101). As cited by WHO/IPCS (1996).
- Howick C, 2019. From a presentation given at the Workshop ‘State‐of‐the‐art chloro alkane detection’, 26 February 2019.
- Huang H, Gao L, Xia D and Qiao L, 2017. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, North China. Scientific Reports, 7, 1–9. 10.1038/s41598-017-06148-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gao L, Zheng M, Li J, Zhang L, Wu Y, Wang R, Xia D, Qiao L, Cui L, Su G, Liu W and Liu G, 2018. Dietary exposure to short‐ and medium‐chain chlorinated paraffins in meat and meat products from 20 provinces of China. Environmental Pollution, 233, 439–445. 10.1016/j.envpol.2017.10.022 [DOI] [PubMed] [Google Scholar]
- Huybrechts I, Sioen I, Boon PE, Ruprich J, Lafay L, Turrini A, Amiano P, Hirvonen T, De Neve M, Arcella D, Moschandreas J, Westerlund A, Ribas‐Barba L, Hilbig A, Papoutsou S, Christensen T, Oltarzewski M, Virtanen S, Rehurkova I, Azpiri M, Sette S, Kersting M, Walkiewicz A, SerraMajem L, Volatier JL, Trolle E, Tornaritis M, Busk L, Kafatos A, Fabiansson S, De Henauw S and Van Klaveren J, 2011. Dietary Exposure Assessments for Children in Europe (the EXPOCHI project): rationale, methods and design. Archives of Public Health, 69, 4 10.1186/0778-7367-69-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1990. Chlorinated paraffins. Available online: http://www.inchem.org/documents/iarc/vol48/48-02.html
- Iino F, Takasuga T, Senthilkumar K, Nakamura N and Nakanishi J, 2005. Risk assessment of short‐chain chlorinated paraffins in Japan based on the first market basket study and species sensitivity distributions. Environmental Science and Technology, 39, 859–866. 10.1021/es049221l [DOI] [PubMed] [Google Scholar]
- IUCLID (International Uniform Chemical Information Database), 2003. IUCLID Dataset for paraffin waxes and hydrocarbon waxes, chloro (CAS No: 63449‐39‐8). INEOS Chlor Limited, 19th September 2003. Submitted to the Health and Safety Executive. As cited by UK‐Environment Agency (2009).
- Kato Y and Kenne K, 1996. Inhibition of cell‐cell communication by commercial chlorinated paraffins in rat liver epithelial IAR 20 cells. Pharmacology and Toxicology, 79, 23–28. 10.1111/j.1600-0773.1996.tb00236.x [DOI] [PubMed] [Google Scholar]
- Korytár P, Parera J, Leonards PE, Santos FJ, de Boer J and Brinkman UA, 2005. Characterization of polychlorinated n‐alkanes using comprehensive two‐dimensional gas chromatography‐electron‐capture negative ionisation time‐of‐flight mass spectrometry. Journal of Chromatography A, 1086, 71–82. 10.1016/j.chroma.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Krätschmer K, 2019. From a presentation given at the 10th Working group CPs meeting where she participated as Hearing Expert. 5 March 2019.
- Krätschmer K and Schächtele A, 2019. Interlaboratory studies on chlorinated paraffins: Evaluation of different methods for food matrices. Chemosphere, 234, 252–259. 10.1016/j.chemosphere.2019.06.022 [DOI] [PubMed] [Google Scholar]
- Krätschmer K, Schächtele A, Malisch R and Vetter W, 2019. Chlorinated paraffins (CPs) in salmon sold in southern Germany: Concentrations, homologue patterns and relation to other persistent organic pollutants. Chemosphere, 227, 630–637. 10.1016/j.chemosphere.2019.04.016 [DOI] [PubMed] [Google Scholar]
- Kuhnert R, 1986a. Acute oral toxicity of Chloroparaffin 40G (with 1% stabiliser B74) for rats. Huls AG Report Number 0568. Huls AG, Marl, Germany. As cited in EU‐RAR (2011).
- Kuhnert R, 1986b. Acute oral toxicity of Chloroparaffin 52G (with 1% stabiliser B74) on rats. Huls AG Report Number 0571. Huls AG, Marl, Germany. As cited in EU‐RAR (2011).
- La Vecchia C and Negri E, 2014. A review of epidemiological data on epilepsy, phenobarbital and risk of liver cancer. European Journal of Cancer Prevention, 23, 1–7. 10.1097/CEJ.0b013e32836014c8 [DOI] [PubMed] [Google Scholar]
- Labadie P, Blasi C, Le Menach K, Geneste E, Babut M, Perceval O and Budzinski H, 2019. Evidence for the widespread occurrence of short‐ and medium‐chain chlorinated paraffins in fish collected from the Rhône River basin (France). Chemosphere, 223, 232–239. 10.1016/j.chemosphere.2019.02.069 [DOI] [PubMed] [Google Scholar]
- Lahaniatis M, Coelhan M and Parlar H, 2000. Clean‐up and quantification of short and medium Chain polychlorinated n‐alkanes in fish, fish oil and fish feed. Organohalogen Compounds, 47, 276–279. [Google Scholar]
- Lake BG, 2018. Human relevance of rodent liver formation by constitutive androsterone receptor (CAR) activators. Toxicology Research, 7, 697–717. 10.1039/c8tx00008e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonards P, 2019. Personal communication. Information provided via email, 27 May 2019.
- Lewandowski TA, Seeley MR and Beck BD, 2004. Interspecies differences in susceptibility to perturbation of thyroid homeostasis: a case study with perchlorate. Regulatory Toxicology and Pharmacology, 39, 348–362. 10.1016/j.yrtph.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Li T, Wan Y, Gao S, Wang B and Hu J, 2017. High‐Throughput Determination and Characterization of Short‐, Medium‐, and Long‐Chain Chlorinated Paraffins in Human Blood. Environmental Science and Technology, 51, 3346–3354. 10.1021/acs.est.6b05149 [DOI] [PubMed] [Google Scholar]
- Li Y, Hou X, Chen W, Liu J, Zhou Q, Schnoor JL and Jiang G, 2019a. Carbon Chain Decomposition of Short Chain Chlorinated Paraffins Mediated by Pumpkin and Soybean Seedlings. Environmental Science and Technology, 53, 6765–6772. 10.1021/acs.est.9b01215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li J, Li H, Yu H, Yang L, Chen X and Cai Z, 2019b. Seasonal variations and inhalation risk assessment of short‐chain chlorinated paraffins in PM2.5 of Jinan. China. Environmental Pollution, 245, 325–330. 10.1016/j.envpol.2018.10.133 [DOI] [PubMed] [Google Scholar]
- Li AA, Makris SL, Marty MS, Strauss V, Gilbert ME, Blacker A, Zorrilla LM, Coder PS, Hannas B, Lordi S and Schneider S, 2019c. Practical considerations for developmental thyroid toxicity assessments: What's working, what's not, and how can we do better? Regulatory Toxicology and Pharmacology, 106, 111–136. 10.1016/j.yrtph.2019.04.010 [DOI] [PubMed] [Google Scholar]
- Lindén E, Bengtsson BE, Svanberg O and Sundström G, 1979. The acute toxicity of 78 chemicals and pesticide formulations against two brackish water organisms, the bleak (Alburnus alburnus) and the harpacticoid Nitocra spinipes . Chemosphere, 8, 843–851. 10.1016/0045-6535(79)90015-8 [DOI] [Google Scholar]
- Liu L, Li Y, Coelhan M, Chan HM, Ma W and Liu L, 2016. Relative developmental toxicity of short‐chain chlorinated paraffins in Zebrafish (Danio rerio) embryos. Environmental Pollution, 219, 122–130. 10.1016/j.envpol.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Lombardo P, Dennison JW and Johnson WW, 1975. Bioaccumulation of chlorinated paraffin residues in fish fed chlorowax 500C. Journal of the Association of Official Analytical Chemists, 58, 707–710. [PubMed] [Google Scholar]
- Madeley JR and Birtley RDN, 1980. Chlorinated paraffins and the environment. 2. Aquatic and avian toxicology. Environmental Science and Technology, 14, 1215–1221 10.1021/es60170a004 [DOI] [Google Scholar]
- Madeley JR and Maddock BG, 1983a. Toxicity of a chlorinated paraffin over 60 days. (IV) Chlorinated paraffin – 58% chlorination of short chain chlorinated paraffins. ICI Confidential Report BL/B/2291. As cited in Glűge et al. (2018).
- Madeley JR and Maddock BG, 1983b. The bioconcentration of a chlorinated paraffin in the tissues and organs of rainbow trout (Salmo gairdneri). ICI Confidential Report BL/B/2310. As cited in Glűge et al. (2018).
- Marvin CH, Painter S, Tomy GT, Stern GA, Braekevelt E and Muir DCG, 2003. Spatial and temporal trends in short‐chain chlorinated paraffins in Lake Ontario sediments. Environmental Science and Technology, 37, 4561–4568. 10.1021/es0345064 [DOI] [PubMed] [Google Scholar]
- Meijer J and DePierre JW, 1987. Hepatic levels of cytosolic, microsomal and ‘mitochondrial’ epoxide hydrolases and other drug‐metabolizing enzymes after treatment of mice with various xenobiotics and endogenous compounds. Chemico‐Biological Interactions, 62, 249–269. 10.1016/0009-2797(87)90026-3 [DOI] [PubMed] [Google Scholar]
- Meijer J, Rundgren M, Astrom A, DePierre JW, Sundvall A and Rannug U, 1981. Effects of chlorinated paraffins on some drug‐metabolizing enzymes in rat liver and in the Ames test. Advances in Experimental Medicine and Biology, 136, 821–828. 10.1007/978-1-4757-0674-1_64 [DOI] [PubMed] [Google Scholar]
- Merten C, Ferrari P, Bakker M, Boss A, Hearty Á, Leclercq C, Lindtner O, Tlustos C, Verger P, Volatier JL and Arcella D, 2011. Methodological characteristics of the national dietary surveys carried out in the European Union as included in the European Food Safety Authority (EFSA) Comprehensive European Food Consumption Database. Food Additives and Contaminants, Part A, 28, 975–995. 10.1080/19440049.2011.576440 [DOI] [PubMed] [Google Scholar]
- Myhr B, McGregor D, Bowers L, Riach C, Brown AG, Edwards I, McBride D, Martin R and Caspary WJ, 1990. 151 78Y Mouse lymphoma cell mutation assay results with 41 compounds. Environmental and Molecular Mutagenesis, 16, 138–167. 10.1002/em.2850160506 [DOI] [PubMed] [Google Scholar]
- Nicholls CR, Allchin CR and Law RJ, 2001. Levels of short and medium chain length polychlorinated n‐alkanes in environmental samples from selected industrial areas in England and Wales. Environmental Pollution, 114, 415–430. 10.1016/S0269-7491(00)00230-X [DOI] [PubMed] [Google Scholar]
- NICNAS (National Industrial Chemicals Notification and Assessment Scheme), 2001. Short chain chlorinated paraffins (SCCPs). Priority Existing Chemical Assessment Report No. 16.
- Nielsen E and Ladefoged O, 2013. Evaluation of health hazards by exposure to Chlorinated paraffins and proposal of a health‐based quality criterion for ambient air. Environmental Project No. 1491, 2013. The Danish Environmental Protection Agency. Available online: https://www2.mst.dk/Udgiv/publications/2013/08/978-87-93026-28-5.pdf
- Nilsen OG and Toftgard R, 1981. Effects of polychlorinated terphenyls and paraffins on rat‐liver microsomal cytochrome P‐450 and in vitro metabolic‐activities. Archives of Toxicology, 1, 1–11. [DOI] [PubMed] [Google Scholar]
- Nilsen OG, Toftgard R and Glaumann H, 1980. Changes in rat liver morphology and metabolic activities after exposure to chlorinated paraffins. Developments in Toxicology and Environmental Science, 8, 525–528. [PubMed] [Google Scholar]
- Nilsen OG, Toftgard R and Glaumann H, 1981. Effects of chlorinated paraffins on rat liver microsomal activities and morphology. Importance of the length and the degree of chlorination of the carbon chain. Archives of Toxicology, 1, 1–13. 10.1007/BF00352066 [DOI] [PubMed] [Google Scholar]
- NIVA (Norwegian Institute for Water Research), 2015a. Contaminants in coastal waters of Norway 2014, M‐433. Report Number 6917‐2015. Study funded by the Norwegian Environment Agency. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/M433/M433.pdf
- NIVA (Norwegian Institute for Water Research), 2015b. Environmental contaminants in an urban fjord, 2014, M‐375. Report number 6884‐2015. Study funded by the Norwegian Environment Agency. Available online: https://niva.brage.unit.no/niva-xmlui/bitstream/handle/11250/2357438/6884-2015_200dpi.pdf?sequence=4&isAllowed=y
- NIVA (Norwegian Institute for Water Research), 2017. Environmental Contaminants in an Urban Fjord, 2016, M‐812. Report number 7199‐2017. Study funded by the Norwegian Environment Agency. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/M812/M812.pdf
- NRC (National Research Council), 2000. National Research Council (US) Subcommittee on Flame‐Retardant Chemicals. Toxicological Risk of selected flame‐retardant chemicals. Washington (DC): National Academies Press (US); 2000. [PubMed]
- NTP (National Toxicology Programme), 1986a. Toxicology and carcinogenesis studies of chlorinated paraffins (C12, 60% chlorine) (CAS No. 63449‐39‐8) in F344/N rats and b6c3fi mice (gavage studies). U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health. NTP TR 308.
- NTP (National Toxicology Programme), 1986b. Toxicology and carcinogenesis studies of chlorinated paraffins (C23, 43% chlorine) (CAS NO. 63449‐39‐8) in F344/N rats and B6C3Ft mice (gavage studies). U.S. Department of Health and Human Services. Public Health Service. National Institutes of Health. NTP TR 305.
- NTP (National Toxicology Program), 2016. Report on carcinogens, fourteenth edition. Chlorinated paraffins (C12, 60% chlorine) CAS No. 108171‐26‐2. Available online: https://ntp.niehs.nih.gov/pubhealth/roc/index-1.html
- OECD (Organisation for Economic Cooperation and Development), 2000. Environment directorate joint meeting of the chemicals committee and the working party on chemicals, pesticides and biotechnology. ENV/JM/MONO(2012)4/PART2. SIDS Initial Assessment Profiles agreed in the course of the OECD HPV Chemicals Programme from 1993 to 2011. Series on Testing & Assessment No. 166. CAS No. 855‐35‐84‐8. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2012)4/part2&doclanguage=en
- Olofsson U, Bignert A and Haglund P, 2012. Time‐trends of metals and organic contaminants in sewage sludge. Water Research, 46, 4841–4851. 10.1016/j.watres.2012.05.048 [DOI] [PubMed] [Google Scholar]
- Omori T and Alexander M, 1987. Bacterial dehalogenation of halogenated alkanes and fatty‐acids. Applied and Environmental Microbiology, 35, 867–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parera J, Ábalos M, Santos FJ, Galceran MT and Abad E, 2013. Polychlorinated dibenzo‐p‐dioxins, dibenzofurans, biphenyls, paraffins and polybrominated diphenyl ethers in marine fish species from Ebro River Delta (Spain). Chemosphere, 93, 499–505. 10.1016/j.chemosphere.2013.06.022 [DOI] [PubMed] [Google Scholar]
- Pellizzato F, Ricci M, Held A and Emons H, 2009a. Validation of a method for the determination of short‐chain chlorinated paraffins in soil and sediments. Accreditation and Quality Assurance, 14, 529–540. 10.1007/s00769-009-0559-y [DOI] [Google Scholar]
- Pellizzato F, Ricci M, Held A, Emons H, Böhmer W, Geiss S, Iozza S, Mais S, Petersen M and Lepom P, 2009b. Laboratory intercomparison study on the analysis of short‐chain chlorinated paraffins in an extract of industrial soil. Trends in Analytical Chemistry, 28, 1029–1035. 10.1016/j.trac.2009.05.0021029 [DOI] [Google Scholar]
- Perkons I, Pasecnaja E and Zacs D, 2019. The impact of baking on chlorinated paraffins: Characterization of C10–C17 chlorinated paraffins in oven‐baked pastry products and unprocessed pastry dough by HPLC–ESI–Q–TOF–MS. Food Chemistry, 298, 10.1016/j.foodchem.2019.125100 [DOI] [PubMed] [Google Scholar]
- Poon R, Lecavalier P, Chan P, Viau C, Hakansson H, Chu I and Valli VE, 1995. Subchronic toxicity of a medium‐chain chlorinated paraffin in the rat. Journal of Applied Toxicology, 15, 455–463. 10.1002/jat.2550150607 [DOI] [PubMed] [Google Scholar]
- POPRC (Persistent Organic Pollutants Review Committee), 2009. Additional information on short‐chained chlorinated paraffins: Bioconcentration study of a chlorinated paraffin (UNEP/POPS/POPRC.5?INF/23). Technical report. Persistent Organic Pollutants Review Committee, 2009.
- Přibylová P, Holoubek I and Klanova J, 2006. Screening of short‐ and medium‐chain chlorinated paraffins in selected sediments and sludge from the Czech Republic. Environmental Pollution, 144, 248–254. 10.1016/j.envpol.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Qiao L, Gao L, Zheng M, Xia D, Li J, Zhang L, Wu Y, Wang R, Cui L and Xu C, 2018. Mass Fractions, Congener Group Patterns, and Placental Transfer of Short‐ and Medium‐Chain Chlorinated Paraffins in Paired Maternal and Cord Serum. Environmental Science and Technology, 52, 10097–10103. 10.1021/acs.est.8b02839 [DOI] [PubMed] [Google Scholar]
- Ren X, Geng N, Zhang H, Wang F, Gong Y, Song X, Luo Y, Zhang B and Chen J, 2019. Comparing the disrupting effects of short‐, medium‐ and long chain chlorinated paraffins on cell viability and metabolism. Science of the Total Environment, 685, 297–307. 10.1016/j.scitotenv.2019.05.388 [DOI] [PubMed] [Google Scholar]
- Reth M, Kypke K, Schächtele J and Oehme M, 2005. Chlorinated paraffins in human milk from Germany analyzed by HRGC‐EI‐MS/MS. Organohalogen Compounds, 1671–1673. [Google Scholar]
- Reth M, Ciric A, Christensen GN, Heimstad ES and Oehme M, 2006. Short‐ and medium‐chain chlorinated paraffins in biota from the European Arctic ‐ differences in homologue group patterns. Science of the Total Environment, 367, 252–260. 10.1016/j.scitotenv.2005.12.014 [DOI] [PubMed] [Google Scholar]
- Richold M, Allen JA, Williams A and Ransome SJ, 1982a. Cell transformation test for potential carcinogenicity of chlorinated paraffin (58% chlorination of short chain length n‐paraffins). Huntingdon, Huntingdon Research Centre, 40 pp (Report No. ICI 399C/81468). As cited by WHO/IPCS (1996).
- Richold M, Allen JA, Williams A and Ransome SJ, 1982b. Cell transformation test for potential carcinogenicity of chlorinated paraffin (70% chlorination of long chain length n‐paraffins). Huntingdon, Huntingdon Research Centre, 30 pp (Report No. ICI 399A/415/82313). As cited by WHO/IPCS (1996).
- Roberts RA, 1999. Peroxisome proliferators: mechanisms of adverse effects in rodents and molecular basis for species differences. Archives of Toxicology, 73, 413–418. 10.1007/s002040050629 [DOI] [PubMed] [Google Scholar]
- Schinkel L, Bogdal C, Canonica E, Cariou R, Bleiner D, McNeill K and Heeb NV, 2018. Analyses of medium‐chain and long‐chain chlorinated paraffins: The urgent need for more specific analytical standards. Environmental Science and Technology Letters, 5, 708–717. 10.1021/acs.estlett.8b00537 [DOI] [Google Scholar]
- Schmid PP and Muller MD, 1985. Trace level detection of chlorinated paraffins in biological and environmental‐samples, using gas‐chromatography mass‐spectrometry with negative‐ion chemical ionization. Journal of the Association of Official Analytical Chemists, 68, 427–430. [PubMed] [Google Scholar]
- Serrone DM, Birtley RD, Weigand W and Millischer R, 1987. Toxicology of chlorinated paraffins. Food and Chemical Toxicology, 25, 553–562. 10.1016/0278-6915(87)90209-2 [DOI] [PubMed] [Google Scholar]
- Shang H, Fan X, Kubwabo C and Rasmussen PE, 2019. Short‐chain and medium‐chain chlorinated paraffins in Canadian house dust and NIST SRM 2585. Environmental Science and Pollution Research, 26, 7453–7462. 10.1007/s11356-018-04073-2 [DOI] [PubMed] [Google Scholar]
- Shen L, Luo P and Zhang Y, 2016. The industry situation and environment behaviours of short chain chlorinated paraffins. Modern Chemical Industry, 36, 1–5. [Google Scholar]
- Shi L, Goa Y, Zhang H, Geng N, Xu J, Zhan F, Ni Y, Hou X and Chen J, 2017. Concentrations of short‐ and medium‐chain chlorinated paraffins in indoor dusts from malls in China: Implications for human exposure. Chemosphere, 172, 103–110. 10.1016/j.chemosphere.2016.12.150 [DOI] [PubMed] [Google Scholar]
- Sprengel J, Wieselmann S, Kröpfl A and Vetter W, 2019. High amounts of chlorinated paraffins in oil‐based vitamin E dietary supplements on the German market. Environment International, 128, 438–445. 10.1016/j.envint.2019.04.065 [DOI] [PubMed] [Google Scholar]
- Stern GA and Tomy G, 2000. An Overview of the Environmental Levels and Distribution of Polychlorinated Paraffins. Organohalogen Compounds, 47, 135–138. [Google Scholar]
- Stevens JL, Northcott GL, Stern GA, Tomy GT and Jones KC, 2003. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n‐alkanes in UK sewage sludge: Survey results and implications. Environmental Science and Technology, 37, 462–467. 10.1021/es020161y [DOI] [PubMed] [Google Scholar]
- Sun R, Luo X, Tang B, Chen L, Liu Y and Mai B, 2017. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e‐waste in south china: Bioaccumulation factors, tissue distribution, and trophic transfer. Environmental Pollution, 222, 165–174. 10.1016/j.envpol.2016.12.060 [DOI] [PubMed] [Google Scholar]
- Thomas GO and Jones KC, 2002. Chlorinated paraffins in human and bovine milk‐fat. A report on a research project funded by the Eurochlor Chlorinated Paraffin Sector Group. Department of Environmental Sciences, Lancaster University. As cited in EU‐RAR (2011).
- Thomas GO, Braekevelt E, Stern G, Martin FL and Jones KC, 2003. Further Work on Chlorinated Paraffins in Human Milk‐Fat. A report on a research project funded by the Eurochlor Chlorinated Paraffin Sector Group. Department of Environmental Sciences, Lancaster University, 10th November 2003. As cited by EU‐RAR (2011).
- Thomas GO, Farrar D, Braekevelt E, Stern G, Kalantzi OI, Martin FL and Jones KC, 2006. Short and medium chain length chlorinated paraffins in UK human milk fat. Environment International, 32, 34–40. 10.1016/j.envint.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Tomy GT, 1997. The Mass Spectrometric Characterization of Polychlorinated n‐Alkanes and the Methodology for their Analysis in the Environment. June 1997. PhD thesis.
- Tomy GT, Stern GA, Muir DCG, Fisk AT, Cymbalisty CD and Westmore JB, 1997. Quantifying C‐10‐C‐13 polychloroalkanes in environmental samples by high‐resolution gas chromatography electron capture negative ion high resolution mass spectrometry. Analytical Chemistry, 69, 2762–2771. 10.1021/ac961244y [DOI] [Google Scholar]
- Tomy GT, Westmore JB, Stern GA, Muir DCG and Fisk AT, 1999. Interlaboratory Study on Quantitative Methods of Analysis of C10‐C13 Polychloro‐n‐alkanes. Analytical Chemistry, 71, 446–451. 10.1021/ac9807215 [DOI] [Google Scholar]
- Ueberschär KH and Matthes S, 2004. Dose‐response feeding study of chlorinated paraffins in broiler chickens: effects on growth rate and tissue distribution. Food Additives and Contaminants, 21, 943–948. 10.1080/02652030400006833 [DOI] [PubMed] [Google Scholar]
- Ueberschär KH, Daenicke S and Matthes S, 2007. Dose‐response feeding study of short chain chlorinated paraffins (SCCPs) in laying hens: Effects on laying performance and tissue distribution, accumulation and elimination kinetics. Molecular Nutrition and Food Research, 51, 248–254. 10.1002/mnfr.200600168 [DOI] [PubMed] [Google Scholar]
- UK‐COT (United Kingdom‐ Committee on Toxicity of Chemicals in Food, Consumer products and the Environment), 2009. COT Statement 2009/06. Available online: https://cot.food.gov.uk/sites/default/files/cot/cotstatementcps200906.pdf
- UK‐Environment Agency , 2009. Science report. Using science to create a better place. Environmental risk assessment: long‐chain chlorinated paraffins. SCHO0109BPGR‐E‐E. Published by: Environmental agency, rio House, Waterside Drive, Aztec West, Bristol. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/290855/scho0109bpgr-e-e.pdf
- US‐EPA (United States Environmental Protection Agency), 2015. TSCA new chemicals review program standard review risk assessment on medium‐chain chlorinated paraffins (PMNP‐12‐0282, P‐12‐0283) and long‐chain chlorinated paraffins (PMNP‐12‐0284). Premanufacture Notification Number: P‐12‐0282‐0284. Office of Chemical Safety and Pollution Prevention.
- US‐EPA (United States Environmental Protection Agency), 2017. Update for Chapter 5 of the Exposure Factors Handbook. Soil and Dust Ingestion. EPA/600/R‐17/384F, September 2017.
- US‐EPA (United States Environmental Protection Agency), 2019. Chemistry Dashboard. Chlorinated paraffins: C12, 60% chlorine. https://comptox.epa.gov/dashboard/DTXSID2024824 (accessed April 18, 2019).
- Van Mourik L, 2018. Optimising analytical methods for chlorinated paraffins to evaluate their levels in Australia. University of Queensland, Australia; PhD thesis. [Google Scholar]
- Van Mourik LM, Leonards PE, Gaus C and de Boer J, 2015. Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A review. Chemosphere, 136, 259–272. 10.1016/j.chemosphere.2015.05.045 [DOI] [PubMed] [Google Scholar]
- Van Mourik LM, van der Veen I, Crum S and de Boer J, 2018. Developments and interlaboratory study of the analyses of short‐chain chlorinated paraffins. Trac‐Trends in Analytical Chemistry, 102, 32–40. 10.1016/j.trac.2018.01.004 [DOI] [Google Scholar]
- Wang Y, Li J, Cheng Z, Li Q, Pan X, Zhang R, Liu D, Luo C, Liu X, Katsoyiannis A and Zhang G, 2013. Short‐ and medium‐chain chlorinated paraffins in air and soil of subtropical terrestrial environment in the pearl river delta, south china: distribution, composition, atmospheric deposition fluxes, and environmental fate. Environmental Science and Technology, 47, 2679–2687. 10.1021/es304425r [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang H, Geng N, Ren X, Zhang B, Gong Y and Chen J, 2018a. A metabolomics strategy to assess the combined toxicity of polycyclic aromatic hydrocarbons (PAHs) and short‐chain chlorinated paraffins (SCCPs). Environmental Pollution, 234, 573–580. 10.1016/j.envpol.2017.11.073 [DOI] [PubMed] [Google Scholar]
- Wang C, Gao W, Liang Y, Wang Y and Jiang G, 2018b. Concentrations and congener profiles of chlorinated paraffins in domestic polymeric products in China. Environmental pollution, 238, 326–335. 10.1016/j.envpol.2018.02.078 [DOI] [PubMed] [Google Scholar]
- Wang Y, Gao W, Wang Y and Jiang G, 2018c. Distribution and Pattern Profiles of Chlorinated Paraffins in Human Placenta of Henan Province, China. Environmental Science and Technology Letters, 5, 9–13. 10.1021/acs.estlett.7b00499 [DOI] [Google Scholar]
- Wang P, Zhao N, Cui Y, Jiang W, Wng L, Wang Z, Chen X, Jiang L and Ding L, 2018d. Short‐chain chlorinated paraffin (SCCP) pollution from a CP production plant in China: Dispersion, congener patterns and health risk assessment. Chemosphere, 211, 456–646. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhu J, Kong B, He B, Wei L, Jin Y, Shan Y, Wang W, Pan C and Fu Z, 2019a. C9–13 chlorinated paraffins cause immunomodulatory effects in adult C57BL/6 mice, Science of the Total Environment, 675, 110–121. 10.1016/j.scitotenv.2019.04.199 [DOI] [PubMed] [Google Scholar]
- Wang R, Gao L, Zheng M, Li J, Zhang L, Wu Y, Wang G, Xiong L, Ding D, Lu D, Qiao L, Cui L and Xu C, 2019b. Characterization of short‐ and medium‐chain chlorinated paraffins in cereals and legumes from 19 Chinese provinces. Chemosphere, 226, 282–289. 10.1016/j.chemosphere.2019.03.148 [DOI] [PubMed] [Google Scholar]
- Wang C, Gao W, Liang Y, Jiang Y, Wang Y, Zhnag Q and Jiang G, 2019c. Migration of chlorinated paraffins from plastic food packaging into food simulants: Concentrations and differences in congener profiles. Chemosphere, 225, 557–564. 10.1016/j.chemosphere.2019.03.039 [DOI] [PubMed] [Google Scholar]
- Warnasuriya GD, Elcombe BM, Foster JR and Elcombe CR, 2010. A mechanism for the induction of renal tumours in male Fischer 344 rats by short‐chain chlorinated paraffins. Archives of Toxicology, 84, 233–243. 10.1007/s00204-009-0489-9 [DOI] [PubMed] [Google Scholar]
- Warngard L, Bager Y, Kato Y, Kenne K and Ahlborg UG, 1996. Mechanistic studies of the inhibition of intercellular communication by organochlorine compounds. Archives of Toxicology Suppl, 18, 149–159. 10.1007/978-3-642-61105-6_16 [DOI] [PubMed] [Google Scholar]
- Wei GL, Liang XL, Li DQ, Zhuo MN, Zhang SY, Huang QX, Liao YS, Xie ZY, Guo TL and Yuan ZJ, 2016. Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review. Environment International, 92–93, 373–387. 10.1016/j.envint.2016.04.002 [DOI] [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 1994. Assessing human health risks of chemicals: Derivation of guidance values for health‐based exposure limits. Environmental Health Criteria 170. Geneva, WHO.
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 1996. Environmental Health Criteria 181. Chlorinated Paraffins. International Programme on Chemical Safety. Available online: http://www.inchem.org/documents/ehc/ehc/ehc181.htm
- WHO/IPCS ((World Health Organization/International Programme on Chemical Safety), 1999. Principles for the assessment of risks to human health from exposure to chemicals. Environmental Health Criteria 210. Geneva, WHO.
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2008. Uncertainty and data quality in exposure assessment. Harmonisation project document No. 6. ISBN 978924156376. Available online: https://apps.who.int/iris/handle/10665/44017
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Principles and methods for the risk assessment of chemicals in food. Environmental Health Criteria 240. International Programme on Chemical Safe. ISBN: 9789241572408. Available online: https://www.who.int/foodsafety/publications/chemical-food/en/
- Wiegand W, 1989. Determination of the Mutagenic Effects of Chloroparaffin 40 g. AM‐89/08. Huls AG, Huls AG, Marl, Germany. As cited by EU‐RAR (2005).
- Wildlife International Ltd , 1984. Project No. 188‐101 (1984). A one‐generation reproduction study in mallard ducks with chlorinated paraffin. As cited in Serrone et al. (1987).
- Wischnak C, Löffler FE, Jieran L, Urbance JW and Műller R, 1998. Pseudomonas sp. Strain 273, an aerobic α, ω‐dichloroalkane‐degrading bacterium. Applied and Environmental Microbiology, 64, 3507–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Suzuki G, Michinaka C, Yuan B, Takigami H and de Wit CA, 2017. Dioxin‐like activities, halogenated flame retardants, organophosphate esters and chlorinated paraffins in dust from Australia, the United Kingdom, Canada, Sweden and China. Chemosphere, 168, 1248–1256. 10.1016/j.chemosphere.2016.10.074 [DOI] [PubMed] [Google Scholar]
- Wu KM and Farrelley JG, 2006. Preclinical development of new drugs that enhance thyroid hormone metabolism and clearance: inadequacy of using rats as an animal model for predicting human risks in an IND and NDA. American Journal of Therapeutics, 13, 141–144. 10.1097/01.mjt.0000209673.01885.b0 [DOI] [PubMed] [Google Scholar]
- Wyatt I, Coutts CT and Elcombe CR, 1993. The effect of chlorinated paraffins on hepatic enzymes and thyroid hormones. Toxicology, 77, 81–90. 10.1016/0300-483X(93)90139-J [DOI] [PubMed] [Google Scholar]
- Xia D, Gao L, Zhu S and Zheng M, 2014. Separation and screening of short‐chain chlorinated paraffins in environmental samples using comprehensive two‐dimensional gas chromatography with micro electron capture detection. Analytical and Bioanalytical Chemistry, 406, 7561–7570. 10.1007/s00216-014-8209-6 [DOI] [PubMed] [Google Scholar]
- Xia D, Gao L, Zheng M, Tian Q, Huang H and Qiao L, 2016. A novel method for profiling and quantifying short‐ and medium‐chain chlorinated paraffins in environmental samples using comprehensive two‐dimensional gas chromatography‐electron capture negative ionization high‐resolution time‐of‐flight mass spectrometry. Environmental Science and Technology, 50, 7601–7609. 10.1021/acs.est.6b01404 [DOI] [PubMed] [Google Scholar]
- Xia D, Gao LR, Zheng MH, Li JG, Zhang L, Wu YN, Tian QC, Huang H and Qiao L, 2017a. Human exposure to short‐ and medium‐chain chlorinated paraffins via mothers’ milk in Chinese urban population. Environmental Science and Technology, 51, 608–615. 10.1021/acs.est.6b04246 [DOI] [PubMed] [Google Scholar]
- Xia D, Gao LR, Zheng MH, Li JG, Zhang L, Wu YN, Qiao L, Tian QC, Huang HT, Liu WB, Su GJ and Liu GR, 2017b. Health risks posed to infants in rural China by exposure to short‐ and medium‐chain chlorinated paraffins in breast milk. Environment International, 103, 1–7. 10.1016/j.envint.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Xia D, Gao L, Zheng M, Sun Y, Qiao L, Huang H, Zhang H, Fu J, Wu Y, Li J and Zhang L, 2019. Identification and evaluation of chlorinated nonane paraffins in the environment: A persistent organic pollutant candidate for the Stockholm Convention? Journal of Hazard Material, 371, 449–455. 10.1016/j.jhazmat.2019.02.089 [DOI] [PubMed] [Google Scholar]
- Xin S, GaoW Cao D, Lv K, Liu Y, Zhao C, Wang Y and Jiang G, 2019. The thermal transformation mechanism of chlorinated paraffins: An experimental and density functional theory study. Journal of Environmental Sciences, 75, 378–387. 10.1016/j.jes.2018.05.022 [DOI] [PubMed] [Google Scholar]
- Xu J, Guo W, Wei L, Gao Y, Zhang H, Zhang MS and Chen J, 2019. Validation of a HRGC–ECNI/LRMS method to monitor short‐chain chlorinated paraffins in human plasma. Journal of Environmental Science, 75, 289–295. 10.1016/j.jes.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Yang JJ, Roy TA, Neil W, Krueger AJ and Mackerer CR, 1987. Percutaneous and oral absorption of chlorinated paraffins in the rat. Toxicology and Industrial Health, 3, 405–412. 10.1177/074823378700300312 [DOI] [PubMed] [Google Scholar]
- Yang L, Wang Z, Li J, Ma Y, Kong L, Yang H, Wang L, Liu Y, Lu Y and Zhang J, 2018. Evaluation of short chain chlorinated paraffins in human milk and their intake by infants in Hebei Province, China. Food Additives and Contaminants Part A, 35, 2011–2021. 10.1080/19440049.2018.1492155 [DOI] [PubMed] [Google Scholar]
- Yang X, Zhang B, Gao Y, Chen Y, Yin D and Xu T, 2019. The chlorine contents and chain lengths influence the neurobehavioral effects of commercial chlorinated paraffins on zebrafish larvae. Journal of Hazardous Materials, 377, 172–178. 10.1016/j.jhazmat.2019.05.047 [DOI] [PubMed] [Google Scholar]
- Yuan B and de Wit C, 2018. Screening chlorinated paraffins in Swedish terrestrial birds and mammals (2012‐2017). Stockholm University. Report Dnr 2219‐17‐011. Ärendernr NV‐04762‐17. Available online: https://www.diva-portal.org/smash/get/diva2:1190147/FULLTEXT01.pdf
- Yuan B, Wang T, Zhu N, Zhang K, Zeng L, Fu J, Wang Y and Jiang G, 2012. Short chain chlorinated paraffins in mollusks from coastal waters in the Chinese Bohai sea. Environmental Science and Technology, 46, 6489–6496. 10.1021/es203839h [DOI] [PubMed] [Google Scholar]
- Yuan B, Strid A, Darnerud PO, de Wit CA, Nyström J and Bergman Å, 2017. Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environment International, 109, 73–80. 10.1016/j.envint.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Zencak Z, Borgen A, Reth M and Oehme M, 2005. Evaluation of four mass spectrometric methods for the analysis of polychlorinated n‐alkanes. Journal of Chromatography A, 1067, 295–301. 10.1016/j.chroma.2004.09.098 [DOI] [PubMed] [Google Scholar]
- Zeng L, Wang T, Ruan T, Liu Q, Wang Y and Jiang G, 2012. Levels and distribution patterns of short chain chlorinated paraffins in sewage sludge of wastewater treatment plants in China. Environmental Pollutution, 160, 88–94. 10.1016/j.envpol.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Zeng L, Lam JCW, Wang Y, Jiang G and Lam PKS, 2015. Temporal trends and pattern changes of short‐ and medium‐chain chlorinated paraffins in marine mammals from the South China Sea over the past decade. Environmental Science and Technology, 49, 11348–11355. 10.1021/acs.est.5b02473 [DOI] [PubMed] [Google Scholar]
- Zeng Y‐H, Luo X‐J, Tang B and Mai B‐X, 2016. Habitat‐ and species‐dependent accumulation of organohalogen pollutants in home‐produced eggs from an electronic waste recycling site in South China: Levels, profiles, and human dietary exposure. Environmental Pollution, 216, 64–70. 10.1016/j.envpol.2016.05.039 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Huang C, Luo X, Liu Y, Ren Z and Mai B, 2018. Polychlorinated biphenyls and chlorinated paraffins in home‐produced eggs from an e‐waste polluted area in South China: Occurrence and human dietary exposure. Environment International, 116, 52–59. 10.1016/j.envint.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang J, Zhu J, Liu J, Zhang J and Zhao M, 2016. Assessment of the endocrine‐disrupting effects of short‐chain chlorinated paraffins in in vitro models. Environment International, 94, 43–50. 10.1016/j.envint.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Zhou Y, de Wit CA, Yin G, Du X and Yuan B, 2019a. Shorter than short‐chain: Very short‐chain chlorinated paraffins (vSCCPs) found in wildlife from the Yangtze River Delta. Environment International, 130, 104955 10.1016/j.envint.2019.104955 [DOI] [PubMed] [Google Scholar]
- Zhou M, Ma S, Li G, Yu Y and An T, 2019b. Chlorinated paraffins in the indoor and outdoor atmospheric particles from the Pearl River Delta: Characteristics, sources, and human exposure risks. Science of the Total Environment, 350, 1041–1049. 10.1016/j.scitotenv.2018.09.107 [DOI] [PubMed] [Google Scholar]
- Zitko V, 1974. Uptake of chlorinated paraffins and PCB from suspended solids and food by juvenile Atlantic salmon. Bulletin of Environmental Contaminants and Toxicology, 12, 406–412. 10.1007/BF01684974 [DOI] [PubMed] [Google Scholar]


