Abstract
Introduction
Medin, an aging‐associated amyloidogenic protein, induces cerebrovascular dysfunction and inflammation. We investigated the relationship between cerebrovascular medin and Alzheimer's disease (AD) and vascular dementia (VaD).
Methods
Cerebral arteriole medin was quantified from 91 brain donors with no dementia (ND), AD, VaD, or combined AD and VaD. Correlation analyses evaluated the relationship between arteriole medin, and plaques, tangles, or white matter lesions (WML). Receiver operating characteristic and regression analyses assessed whether medin is predictive of AD or VaD versus other cerebrovascular pathologies (circle of Willis [CoW] atherosclerosis and cerebral amyloid angiopathy [CAA]).
Results
Arteriole medin was higher in those with AD, VaD, or combined AD/VaD versus ND (P < .05), and correlated with tangle, plaque, and WML, but not CAA or CoW atherosclerosis. Among cerebrovascular pathologies, medin was the strongest predictor of AD diagnosis, whereas CoW atherosclerosis and arteriole medin were predictors of VaD.
Discussion
Cerebral arteriole medin is associated with and could be a potential novel risk factor or biomarker for AD and VaD.
Keywords: aging, Alzheimer's disease, amyloid, cerebrovascular disease, dementia, medin, vascular dementia
1. INTRODUCTION
Age is the most important independent risk factor for dementing illnesses such as Alzheimer's disease (AD) and vascular dementia (VaD). 1 Vascular risk factors, which increase in incidence with aging, are also strongly associated with AD. 2 , 3 , 4 , 5 , 6 , 7 The expanding body of epidemiologic and clinical evidence shows that cerebrovascular disease may be a critical etiology of sporadic (non‐autosomal dominant) AD and that it may precede the neurodegenerative process. 8 We previously showed the association between circle of Willis (CoW) atherosclerosis and the following: AD, neuritic plaques, neurofibrillary tangles, and VaD. 9 The mechanisms underlying the tight links among aging, vascular disease, or vascular risk factors and AD remain poorly understood. There is emerging evidence that medin, a 50 amino acid protein that results from cleavage of parent protein milk fat globule‐EGF factor 8, is a mediator of vascular aging pathology. 10 Medin is the component of one of the most common forms of human amyloidosis accumulating in the vasculature with aging. 11 , 12 We recently showed that medin is found in cerebral arteries in elderly brain donors and that it causes endothelial dysfunction and pro‐inflammatory activation that could modulate neuroinflammation. 13 , 14 The role of cerebrovascular medin in AD and VaD is not known. The aim of this study is to determine the association between cerebral arteriole medin and AD and VaD.
2. METHODS
2.1. Brain tissue sources and diagnostic criteria
Participants volunteered for the Banner‐Sun Health Research Institute Brain Donation Program, a longitudinal clinicopathologic study of aging and neurodegenerative disease with catchment area around retirement communities in the northwest metropolitan Phoenix Arizona region. 15 The study was approved by the Sun Health Research Institute and Phoenix Veterans Affairs Institutional Review Boards and written informed consent was obtained from all subjects or their legal representatives. The research has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Subjects comprised donors who met standardized clinicopathologic diagnostic criteria for AD, VAD, or non‐demented (ND) elderly subjects from 1997 to 2017 and who had available biobanked brain tissues for analysis. Final neuropathologic diagnoses were adjudicated by an expert neuropathologist (TGB). AD diagnosis was made when a subject clinically diagnosed with dementia met National Institutes of Aging/Reagan Institute rating of “high” or “intermediate.” 16 Participants clinically diagnosed with dementia were classified as VaD when they met diagnostic criteria using National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherche et l'Enseignement en Neurosciences criteria. 17 Elderly ND participants comprised donors without clinical diagnosis of dementia and only age‐consistent neuropathology. 15
2.2. Histopathologic methods
Histopathologic methods were previously described 9 , 15 and done on standard blocks of brain tissue fixed in 4% formaldehyde and then either dehydrated and paraffin‐embedded or cryopreserved and cut on a freezing, sliding microtome.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional PubMed sources and Google Scholar. The pathobiology of medin is not well studied especially in dementia disorders, despite medin being one of the most common amyloidogenic proteins that accumulate with aging. The relevant citations are appropriately cited.
Interpretation: Our findings suggest a strong relationship between cerebral arteriole medin and Alzheimer's disease (AD), and cerebral arteriole medin and vascular dementia (VaD). In light of medin's previously published effects of inducing cerebral arteriole endothelial function and inflammation that could modulate neuroinflammation, our findings support the potential role of medin as a novel risk factor or biomarker for AD and VaD.
Future directions: The results justify the conduct of additional studies to establish the causal nature and underlying mechanisms underpinning the relationship between cerebrovascular medin and AD and medin and VaD that would support medin as a novel treatment target to prevent or treat AD and VaD.
For quantification of cerebral arteriole medin, 5 μm sections from paraffin blocks of middle frontal gyrus were stained with anti‐medin primary antibody (18G1; 1:1000; generously provided by Prothena Biosciences Limited, Dublin, Ireland), horseradish peroxidase‐conjugated secondary antibody, and 3,3‐diaminobenzidine staining. Imaging was performed on a Biotek Cytation 5 (Winooski, Vermont, USA) or EVOS FL (Thermo Fisher, Waltham Massachusetts, USA) and parenchymal cerebral arterioles (resistance arteries, luminal diameter 114 ± 37 μm, wall thickness 48 ± 18 μm) were visualized (Figure 1). Vessels were considered arteries when the tunica media layer is prominent and the vessel assumes a more uniform, less irregular shape. In some, contiguous slices were stained with hematoxylin‐eosin, which assisted in confirming arterioles and visualizing vessel wall layers. Vessels not meeting this criteria were not considered for quantification. Vessels could be cut in cross‐section, longitudinal, or oblique manner. For each arteriole, arteriole medin score was assigned from 0 to 3 according to the following criteria: 0–none, 1–sparse (single spot or region), 2–moderate (>1 spot or region but <50% of arteriole wall/perivascular diameter involvement), 3–frequent (>1 spotor region, >50% arteriole wall/perivascular diameter involvement). Scoring was performed by two investigators (NK and ST) blind as to neuropathologic diagnoses. If arteriole score differed by 2 or greater, the arteriole image was reviewed by both and a consensus score was assigned. For each participant, arteriole medin score comprised the average scores of all the arterioles in the brain section.
FIGURE 1.
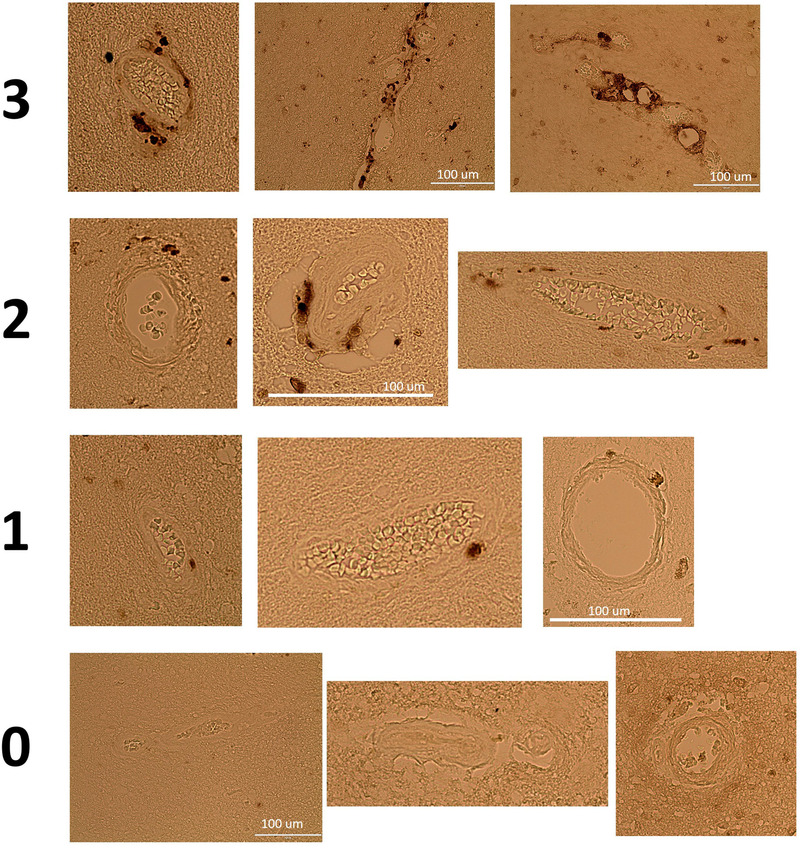
Cerebral arteriole medin and scoring system. Representative images of parenchymal cerebral arterioles after staining with anti‐medin antibody (DAB stain) and scoring system from 0 to 3 (none, sparse, moderate, and frequent)
The classic histopathologic markers for AD are neuritic plaques and neurofibrillary tangles. For plaques and tangles, 40 μm‐thick frozen sections (coronal plane) were taken at the level of the genu of the corpus callosum, amygdala, lateral geniculate nucleus, splenium of the corpus callosum, and occipital pole, which would include frontal, temporal, parietal, occipital lobes; hippocampus; basal ganglia; and thalamus. These were mounted on 5 × 7.5 cm glass slides and stained with Thioflavin S, Campbell‐Switzer, and Gallyas as per methods described by Braak et al. 18 and our prior work. 15 Neuritic plaque scores, neuritic plaque density scores, and Braak stage for neurofibrillary tangle density and distribution were obtained.
Total plaque score is the arithmetic sum of plaque density scores from frontal, temporal, parietal, hippocampal CA1, and entorhinal/transentorhinal regions. Neuritic plaque density score was assigned as none, sparse, moderate, or frequent (0 to 3) as per Consortium to Establish a Registry for AD protocol. 19 All plaque types (neuritic, cored, diffuse) are included. This scoring method was shown to have significant correlation with amyloid beta (Aβ) deposits (Aβ40 and Aβ42) and postmortem autoradiography for florbetapir. 15 , 20
Total tangle is the arithmetic sum of neurofibrillary tangle density scores from the same regions in plaque scoring. Scoring was semiquantitative from 0 to 3 (none, sparse, moderate, or frequent). Braak stage is the extent of neurofibrillary tangles defined from established protocol. 21
Cerebral amyloid angiopathy (CAA) score is a density score for amyloidotic blood vessels in standard brain regions. CAA is scored from 0 to 3 (none, sparse, moderate, and frequent) and the scores in the frontal, temporal, parietal, and occipital regions are added to make the total score. Large vessel CAA and capillary CAA are considered together.
Cerebral white matter lesions (CWMLs) are caused, in part, by cerebral small vessel disease and are classic lesions found in VaD. 22 Cerebral white matter lesion scoring has previously been described. 23 A score of 1 denotes CWMLs restricted to the immediate periventricular area occupying less than one third of the centrum semiovale, 2 denotes CWMLs in one third to two thirds of the centrum semiovale, and 3 denotes involvement in more than two thirds of the centrum semiovale. The sum of the scores in the frontal, temporal, parietal, and occipital regions were used as the total CWML score.
CoW atherosclerosis grading was previously reported 9 and validated to correlate with cross‐sectional measurements of arterial luminal narrowing. 24 The grading is based on gross visual inspection and scored as 0–none, 1–mild, 2–moderate, and 3–severe. Histopathologic scoring was performed by an investigator (TB) blind to final diagnoses.
2.3. Data and statistical analyses
Sample size consideration was based on comparison of two means (non‐demented controls versus AD or VaD) and assuming a 1:2 prevalence ratio between ND and AD donors. To detect a 50% or greater increase in arteriole medin score between the groups assuming a standard deviation of 0.66, 80% power and α = 0.05 would require N = 15 or greater for ND participants and N = 30 or greater for AD or VaD participants.
Data are presented as means ± standard deviation. Significant P‐value was set at 0.05 (two‐sided). Analyses involved comparison of cerebral arteriole medin among ND controls, pure AD, pure VaD, and AD/VaD donors using one‐way analysis of variance (ANOVA) with post‐hoc pairwise analysis using Tukey‐Kramer test. We also analyzed separately ND versus all AD and ND versus all VaD. Comparison of two groups used independent samples t‐test (equal variance) or Welch test (unequal variance). For non‐normally distributed data, we performed non‐parametric analyses (ANOVA on ranks using Kruskal‐Wallis test with post‐hoc pairwise analyses using Conover test for >2 groups). Correlation analyses used Spearman rank correlation. Proportions were compared using chi‐square test or Fisher's exact test (when sample N ≤ 5).
To assess the association or prediction of cerebrovascular pathologic lesions to AD or VaD diagnoses, we performed two types of analyses. First, area under the curve receiver operating characteristic (AUC‐ROC) analyses compared the predictive values of cerebrovascular pathologies (arteriole medin, CAA, or CoW atherosclerosis scores) in predicting AD (pure AD and all AD) and VaD (pure VaD and all AD). Second, we evaluated the independent association of cerebrovascular pathologies to AD or VaD diagnoses using stepwise logistic regression analyses with pure AD, all AD, pure VaD, and all VaD as dependent variables in separate analyses, and the following as independent variables: arteriole medin score, CoW atherosclerosis score, CAA score, expired age, and sex. Presence of apolipoprotein E genotype ε4 (APOEε4) allele was included in separate regression analyses together with variables found to be independently associated with AD or VaD.
To evaluate the independent association of cerebrovascular lesions to AD or VaD pathologic lesions, stepwise multiple regression was performed with the following as dependent variables in separate analyses: total tangle, total plaque, and CMWL score, using the following independent variables: arteriole medin score, CoW atherosclerosis score, CAA score, and expired age. Because plaques are known to be strongly associated or causally linked to development of tangles, we also performed regression analysis with total tangle as dependent variable and the following as independent variables: total plaque, arteriole medin score, CoW atherosclerosis score, CAA score, and expired age to further assess whether arteriole medin score is independently associated with neurofibrillary tangle. Statistical analyses were performed (RQM) using MedCalc version 18.6 (Ostend, Belgium).
3. RESULTS
There were 91 participants including 24 ND, 34 AD, 12 VaD, and 21 with combined AD/VaD (Table 1). ND and AD were significantly younger at expiration than VaD or AD/VaD patients. There were 39 females (43%) with greater proportion in AD/VaD cohort versus ND or AD patients.
TABLE 1.
Cerebrovascular and neural pathology in non‐demented (ND), Alzheimer's disease (AD), vascular dementia (VaD), and AD/VaD participants
| ND (N = 24) | AD (N = 34) | VaD (N = 12) | AD/VaD (N = 21) | Groupwise P | Pairwise P < .05 | |
|---|---|---|---|---|---|---|
| Expired age years | 77 (IQR 69.5, 84.5) | 80 (IQR 71, 86) | 86 (IQR 83.5, 90.5) | 94 (IQR 88.8, 96.2) | <.001 |
(ND) versus (VaD) (AD/VaD) (AD) versus (VaD) (AD/VaD) |
| Sex (% female) | 33 | 38 | 33 | 67 | .04 |
(ND) versus (AD/VaD) (AD) versus (AD/VaD) |
| Arteriole medin score (A.U.) | 1.26 ± 0.7 | 2.03 ± 0.5 | 1.80 ± 0.5 | 2.05 ± 0.5 | <.001 | (ND) versus (AD) (VaD) (AD/VaD) |
| Circle of Willis Atherosclerosis score (A.U.) | 1.38 ± 1.3 | 1.56 ± 0.9 | 2.75 ± 0.6 | 2.52 ± 0.7 | <.001 |
(ND) versus (VaD) (AD/VaD) (AD) versus (VaD) (AD/VaD) |
| CAA score (A.U.) | 0 (IQR 0,2) | 4.5 (IQR 1, 8) | 0 (IQR 0, 0.5) | 4 (IQR 0.75, 7) | <.001 |
(ND) versus (AD) (AD/VaD) (AD) versus (VaD) (VaD) versus (AD/VaD) |
| Total plaque (A.U.) | 0.875 (IQR 0, 5.5) | 14 (IQR 13, 15) | 0 (IQR 0, 4.25) | 12.5 (IQR 11.75, 13.5) | <0.001 |
(ND) versus (AD) (AD/VaD) (AD) versus (VaD) (AD/VaD) (VaD) versus (AD/VaD) |
| Total tangle (A.U.) | 2.0 ± 1.6 | 13.9 ± ± 1.7 | 5.0 ± 3.2 | 10.3 ± 3.4 | <.001 |
(ND) versus (AD) (VaD) (AD/VaD) (AD) versus (VaD), (AD/VaD) (VaD) versus (AD/VaD) |
| CWML score (A.U.) | 1.5 ± 1.7 | 4.7 ± 3.7 | 6.0 ± 3.5 | 7.8 ± 2.3 | <.001 |
(ND) versus (AD) (VaD) (AD/VaD) (AD) versus (AD/VaD) |
| APOEε4 allele presence (%) | 34.8 | 61.8 | 16.7 | 45.0 | 0.03 |
(ND) versus (AD) (AD) versus (VaD) (VaD) versus (AD/VaD) |
Note: Normally distributed data are presented as mean ± standard deviation and non‐normally distributed data are presented as median with interquartile range (IQR).
Abbreviations: AD, Alzheimer's disease; A.U., arbitrary units; CAA, cerebral amyloid angiopathy; CWML, cerebral white matter lesion; IQR, interquartile range; ND, non‐demented; VaD, vascular dementia.
Arteriole medin scores were higher in AD, VaD, and AD/VaD versus ND participants (Figure 2A‐C, Table 1) with distributions of scores seen in Figure 2D‐G. There was no significant difference in arteriole medin among those with AD, VaD and AD/VaD. CoW atherosclerosis scores were higher in VaD or AD/VaD versus ND or AD (Table 1). CAA scores, total plaque, and total tangle were higher in AD or AD/VaD versus ND or VaD. CWML score was lower in ND patients compared to other groups and was lower in AD versus AD/VaD patients. The proportion of donors with at least one APOEε4 allele was significantly higher in AD compared to ND or VaD (Table 1). The causes of death are tabulated in Table S1 in supporting information and co‐morbidities are tabulated in Table S2 in supporting information.
FIGURE 2.
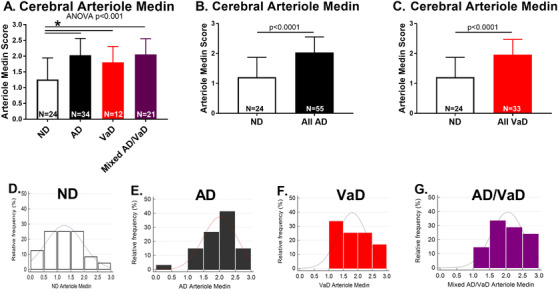
Cerebral arteriole medin. A, Cerebral arteriole medin scores are higher in Alzheimer's disease (AD), vascular dementia (VaD), or mixed AD/VaD compared to non‐demented (ND) donors. There was no difference in medin among AD, VaD, and AD/VaD patients. B–C, All donors with AD and all donors with VaD had higher arteriole medin compared to ND. D–G, Distribution of arteriole medin scores in ND, AD, VaD, and mixed AD/VaD. *P < .05 versus ND
There was significant correlation between arteriole medin and total tangle scores (Figure S1A1, Table S3 in supporting information). There were significant differences in arteriole medin scores among Braak stages, with more advanced stages having greater amount (Figure S1A2). Arteriole medin score correlated with total plaque, with greater plaque density in donors with higher arteriole medin (Figure S1B1‐2, Table S3). Arteriole medin also correlated with CWML scores and participants with CWMLs had more arteriole medin (Figure S1C1‐2, Table S3). Cerebral arteriole medin showed no correlation with CoW atherosclerosis score and CAA score. CoW score correlated with expired age and CWML score and CAA score correlated with total tangle, total plaque, and CWML scores (Table S3).
AUC‐ROC analyses show that cerebral arteriole medin is predictive of AD diagnosis (model including pure AD cases and model including all AD cases, both AUC 0.82, P < .001, Figure 3A‐B). Analyses also show arteriole medin is predictive of VaD diagnosis (model including pure VaD cases, AUC 0.74, P = .005, and model including all VaD cases, AUC 0.79, P < .001, Figure 3C‐D). CAA but not CoW atherosclerosis was also predictive of AD diagnosis while CoW atherosclerosis was predictive of VaD (Figure 3). CAA was predictive of VaD only when all VaD cases were included in the model.
FIGURE 3.
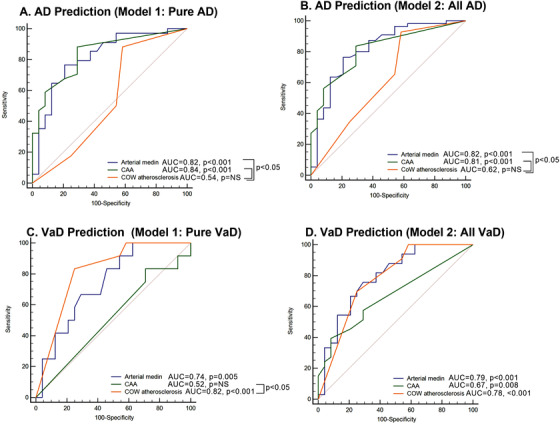
Receiver operating characteristic curves for prediction of diagnosis of Alzheimer's disease (AD) or vascular dementia (VaD) by cerebrovascular lesions. A–B, Prediction of AD diagnosis by cerebrovascular lesions. Model 1 included pure AD cases and non‐demented (ND) controls and Model 2 included all AD cases and ND controls. Both cerebral arterial medin and cerebral amyloid angiopathy (CAA), but not circle of Willis (CoW) atherosclerosis, are predictive of AD diagnosis. C–D, Prediction of VaD diagnosis by cerebrovascular lesions. Model 1 included pure VaD cases and ND controls and Model 2 included all VaD cases and ND controls. Arterial medin and CoW atherosclerosis are predictive of VaD. CAA was predictive only if AD/VaD are included, but not when only pure VaD are included
Regression analysis showed that among cerebrovascular pathologies, arteriole medin was the strongest independent predictor of AD diagnosis, whether modeling pure AD (odds ratio [OR] 16.7, 95% confidence interval [CI] 2.9 to 95.1, P = .002; Table 2A) or all AD (OR 12.2, 95% CI 2.9 to 46.6, P < .001; Table 2B), followed by CAA score. CoW atherosclerosis score was not independently associated with AD diagnosis. Analyses also showed that only CoW atherosclerosis score (OR 4.2, 95% CI 1.3 to 13.4, P = .02) was independently associated with pure VaD (Table 3A), but when including all VaD cases, only arteriole medin (OR 4.6, 95% C. 1.3 to 15.8, P = .02) and expired age (OR 1.2, CI 1.04 to 1.3, P = .0045) were independently associated with VaD. Adding APOEε4 allele presence to the regression models did not change the independent association of arteriole medin in AD and VaD, and APOEε4 variable ended up not included in the final models for AD or VaD.
TABLE 2.
Logistic regression model to predict diagnosis of Alzheimer's disease
| Chi‐squared | Degree of freedom | P | Cox and Snell R2 | |
|---|---|---|---|---|
| (A). Model 1: Pure AD and non‐demented (ND) control | ||||
| Individual variables | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Overall model fit | 40.3 | 2 | <.0001 | 0.50 |
| Chi‐squared | Degree of freedom | P | Cox and Snell R2 | |
|---|---|---|---|---|
| Arteriole medin | 16.7 | 2.9–95.1 | .002 | |
| CAA score | 1.8 | 1.3–2.7 | .001 | |
| Not included in model: Circle of Willis atherosclerosis, expired age, sex. Hosmer and Lemeshow test P = .2. | ||||
| (B). Model 2: All AD (Pure AD and AD/vascular dementia [VaD]) and ND control | ||||
| Individual variables | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Overall model fit | 44.3 | 2 | <.0001 | 0.43 |
| Arteriole medin | 12.2 | 2.9–46.6 | .0003 | |
| CAA score | 1.7 | 1.2–2.3 | .0009 |
Not included in model: circle of Willis atherosclerosis, expired age, sex. Hosmer and Lemeshow test P = .8.
TABLE 3.
Logistic regression model to predict diagnosis of vascular dementia
| Chi‐squared | Degree of freedom | P | Cox and Snell R2 | |
|---|---|---|---|---|
| (A). Model 1: Pure VaD and non‐demented (ND) control | ||||
| Individual variables | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Overall model fit | 12.0 | 1 | .0005 | 0.28 |
| Chi‐squared | Degree of freedom | P | Cox and Snell R2 | |
|---|---|---|---|---|
| Circle of Willis atherosclerosis | 4.2 | 1.3–13.4 | .02 | |
| Not included in model: Arteriole medin, CAA score, expired age, sex. Hosmer and Lemeshow test P = 1.0. | ||||
| (B). Model 2: All VaD (Pure VaD and AD/VaD) and ND control | ||||
| Individual variables | Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Overall model fit | 32.8 | 2 | <.0001 | 0.44 |
| Arteriole medin | 4.6 | 1.3–15.8 | .02 | |
| Expired age | 1.2 | 1.04–1.3 | .0045 |
Not included in model: circle of Willis atherosclerosis, CAA score, sex. Hosmer and Lemeshow test P = .12.
Abbreviations: AD, Alzheimer's disease; CAA, cerebral amyloid angiopathy; ND, non‐demented; VaD, vascular dementia.
Linear regression analysis showed that CAA score and arteriole medin were independently associated with total tangle (Table S4A in supporting information). When total plaque was included in the model, arteriole medin remained independently associated with total tangle (Table S4B). Similarly, CAA score and arteriole medin were independently associated with total plaque (Table S5 in supporting information). Among variables tested, only arteriole medin and expired age were independently associated with CWML scores (Table S6 in supporting information).
4. DISCUSSION
The study presents the following novel findings. First, cerebral arteriole medin is significantly higher in AD, VaD, and combined AD/VaD when compared to non‐demented participants. Second, cerebral arteriole medin is associated with AD, VaD and their pathologic lesions. The findings support the potential role of cerebrovascular medin as a novel risk factor or biomarker for AD and VaD that should be investigated further.
The biologic correlates explaining the strong links among aging, cardiovascular disease, and dementia remain poorly understood. Age is the most important risk factor for AD, VaD, and cerebrovascular disease. 1 , 25 It is now established that in addition to, but independent of, cardiovascular risk factors, aging results in well‐defined phenotypic changes leading to the vascular aging phenotype characterized by endothelial dysfunction and chronic indolent vascular inflammation. 10 , 26 , 27 One of the proposed mediators of the vascular aging phenotype is medin, the protein comprising one of the most common human amyloids that accumulate in the vasculature with aging. 11 , 12 We showed medin to be present in the aorta in 88% of patients more than 50 years old versus 17% of patients less than 50 years old. 13 Medin was shown to be present in multiple arterial beds, including the basilar, collateral, and parenchymal cerebral arteries. 14 , 28
We are just beginning to understand the biologic effects of medin. In aortic aneurysms, medin presence is associated with altered structural wall micromechanical properties. 29 In ex vivo experiments of cannulated/pressurized brain donor collateral cerebral arteries, a physiologic dose of medin induced endothelial dysfunction with 35% reduction in dilator response to acetylcholine versus baseline response; the impairment was attributed to oxidative stress and reduced nitric oxide bioavailability. 13 Endothelial dysfunction from exogenous medin exposure was seen in ND and AD/VaD cerebral arteries. 30 In vitro, human brain microvascular endothelial cells exposed to medin increased gene expressions of interleukin (IL)‐8 (∼5‐fold), IL‐6 (∼6‐fold), and intercellular adhesion molecule‐1 (∼10‐fold). 14 In both 2D and 3D chip cell culture models, exposure of astrocytes to medin‐treated endothelial cells or endothelial cell media caused pro‐inflammatory activation with increased astrocyte production of IL‐8. 14 Similar to Aβ, medin oligomers were recently found to form membrane pores that could disrupt cellular homeostasis. 31 Medin's in vivo effects have not been studied as far as we know and much remains to be learned, including whether the association found in this current study between medin on the one hand and AD, VaD, and pathologic lesions associated with AD and VaD on the other hand represent causal or incidental relationships. A potential causal relationship is supported by studies showing that endothelial dysfunction and inflammation, known pathologic responses to medin, are prominent in AD and VaD. AD and VaD patients showed elevated markers of endothelial dysfunction, 32 and AD patients who were free of cardiovascular risk factors or vascular disease nevertheless had impaired endothelial function proportional to AD severity when compared to healthy elderly controls. 33 Chronic inflammation is a hallmark of both AD and VaD 34 , 35 and AD microvessels showed higher levels of inflammatory cytokines compared to age‐matched controls. 36 , 37 Future studies are needed to confirm the nature of the associational relationship between cerebrovascular medin and AD or VaD. For example, exogenous administration of medin or transgenic manipulation to artificially express medin in animal models to assess development of AD or VaD pathologies would be logical next steps to ascertain medin's causal role and establish its utility as a novel treatment target. Similarly, additional studies are needed to determine whether medin can be used as a novel biomarker of risk for AD and VaD based on our findings. The association between arteriole medin and neurofibrillary tangles, as well as medin and neuritic plaques, represent fertile grounds for exploring potential mechanistic linkages.
It was previously shown that CoW atherosclerosis, when adjusted for age, sex, and APOEε4 status, was a predictor of AD and VaD diagnoses, including AD‐related pathologies of total plaque and Braak stage. 9 However, in this study, CoW atherosclerosis was found not to be independently predictive of AD diagnosis when adjusted for arteriole medin and CAA score, demonstrating that arteriole medin may be a stronger predictor of AD than intracranial atherosclerosis. Indeed, in this cohort, unlike medin, there was no significant correlation between CoW atherosclerosis and pathologic lesions of AD such as total plaque and total tangle. Alternatively, the sample size in the former study was greater, allowing greater ability to detect associational relationships between intracranial atherosclerosis and AD. CAA, a marker of Aβ deposition in cerebral arteries, as expected was a predictor of AD diagnosis, with significant correlations with total plaque and total tangle. In the CERAD (Consortium to Establish a Registry for Alzheimer's Disease) cohort, 83% of autopsy‐confirmed AD participants had CAA, 38 showing that the Aβ dysmetabolism in neural tissue frequently involves cerebral microcirculation. In our linear regression model, arteriole medin remained an independent predictor of AD diagnosis even when adjusted for CAA and CoW atherosclerosis, pointing to a novel vascular pathology that could underlie AD. Although ND participants died at a younger age than AD or VaD, age was included in the multivariable model to account for this possible confounder and results showed arteriole medin to independently predict AD diagnosis with age in the model.
Results reinforce our prior findings 14 on a smaller number of participants that cerebrovascular medin is higher in VaD versus ND. The relationship between vascular medin and VaD is further supported by the significant correlation between medin and CWMLs, which are hallmark lesions of VaD with small vessel disease. CWMLs often represent microvascular ischemic changes with glial and vascular inflammation, 39 changes seen when endothelial cells and astrocytes were exposed to medin in 2D and 3D co‐culture cell models. 14 AUC‐ROC analyses showed arteriole medin and CoW atherosclerosis to be predictive of VaD diagnosis, whether using pure VaD or all VaD cases. However, logistic regression analysis using data from pure VaD donors failed to show arteriole medin as an independent predictor of VaD diagnosis with CoW atherosclerosis as the only predictive cerebrovascular pathology. In the model involving all VaD donors, arteriole medin and expired age (the latter having significant correlation with CoW atherosclerosis), but not CoW atherosclerosis, were independent predictors of VaD diagnosis. The small sample size of pure VaD cases mandates future studies with increased cohort size to better delineate the relative contributions of vascular atherosclerosis and arterial medin to VaD.
Our cohort included a significant proportion of participants meeting neuropathologic criteria for both AD and VaD, consistent with real‐world conditions, described in other large series, showing co‐existence of vascular pathology in 28% of AD patients. 40 , 41 Because of this, we performed separate analyses, AUC‐ROC, and regression models assessing the relationship of arteriole medin with AD or VaD, by pure AD or VaD cases, and all AD or all VaD cases for better delineation of effects. Our results demonstrated associations between arteriole medin, and AD or VaD with both approaches. The results support arteriole medin as a possible shared vascular pathology between AD and VaD, more so than CoW atherosclerosis.
The study has important limitations. Despite having 91 clinicopathologically well‐characterized participants, the sample size remains small and findings need to be confirmed in a larger cohort. Future studies should expand on our findings to assess regional distribution of vascular medin, including areas of interest such as the hippocampus, internal/external capsule, and corpus callosum, as well as probe the origins of medin accumulation and clearance mechanisms.
In conclusion, cerebral arteriole medin is increased in AD, VaD, and combined AD/VaD compared to ND and is associated with neuropathologic markers of AD and VaD. Arteriole medin is associated with and predictive of AD and VaD diagnosis. The findings suggest that cerebrovascular medin could be a novel vascular risk factor or biomarker for AD and VaD.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
We would like to acknowledge Michael Hansen, Gail Farrell, Arizona Veterans Research and Education Foundation, and the Phoenix VA Office of Research. The contents of the work do not represent the views of the Department of Veterans Affairs or the United States government.
Migrino RQ, Karamanova N, Truran S, et al. Cerebrovascular medin is associated with Alzheimer's disease and vascular dementia. Alzheimer's Dement. 2020;12:e12072 10.1002/dad2.12072
Funding information: U.S. Department of Veterans Affairs (Merit BX007080), Department of Defense (W81XWH‐17‐1‐0473), National Institutes of Health (NIARO1 AG019795, NIAP30AG19610, NINDSU24NS072026), Arizona Department of Health Services (211002), Arizona Biomedical Research Commission (4001, 0011, 05‐901, 10010), Michael J. Fox Foundation, British Heart Foundation (FS/12/61/29877).
REFERENCES
- 1. Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45(6):1161‐1168. [DOI] [PubMed] [Google Scholar]
- 2. Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004;6(4):261‐266. [DOI] [PubMed] [Google Scholar]
- 4. Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53(9):1937‐1942. [DOI] [PubMed] [Google Scholar]
- 5. Skoog I, Lernfelt B, Landahl S, et al. 15‐year longitudinal study of blood pressure and dementia. Lancet. 1996;347(9009):1141‐1145. [DOI] [PubMed] [Google Scholar]
- 6. Merchant C, Tang MX, Albert S, Manly J, Stern Y, Mayeux R. The influence of smoking on the risk of Alzheimer's disease. Neurology. 1999;52(7):1408‐1412. [DOI] [PubMed] [Google Scholar]
- 7. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18‐year follow‐up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163(13):1524‐1528. [DOI] [PubMed] [Google Scholar]
- 8. de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33(4):1152‐1162. [DOI] [PubMed] [Google Scholar]
- 9. Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113(1):13‐21. [DOI] [PubMed] [Google Scholar]
- 10. Lakatta EG. The reality of aging viewed from the arterial wall. Artery Res. 2013;7(2):73‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Häggqvist B, Näslund J, Sletten K, et al. Medin: an integral fragment of aortic smooth muscle cell‐produced lactadherin forms the most common human amyloid. Proc Natl Acad Sci U S A. 1999;96(15):8669‐8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson A, Söderberg L, Westermark GT, et al. Unwinding fibril formation of medin, the peptide of the most common form of human amyloid. Biochem Biophys Res Commun. 2007;361(4):822‐828. [DOI] [PubMed] [Google Scholar]
- 13. Migrino RQ, Davies HA, Truran S, et al. Amyloidogenic medin induces endothelial dysfunction and vascular inflammation through the receptor for advanced glycation endproducts. Cardiovasc Res. 2017;113(11):1389‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karamanova N, Truran S, Serrano GE, et al. Endothelial immune activation by medin: potential role in cerebrovascular disease and reversal by monosialoganglioside‐containing nanoliposomes. J Am Heart Assoc. 2020;9(2):e014810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beach TG, Adler CH, Sue LI, et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35(4):354‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(10):1095‐1097. [DOI] [PubMed] [Google Scholar]
- 17. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology. 1993;43(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 18. Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1(3):213‐216. [DOI] [PubMed] [Google Scholar]
- 19. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479‐486. [DOI] [PubMed] [Google Scholar]
- 20. Dugger BN, Clark CM, Serrano G, et al. Neuropathologic heterogeneity does not impair florbetapir‐positron emission tomography postmortem correlates. J Neuropathol Exp Neurol. 2014;73(1):72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol. 1991;82(4):239‐259. [DOI] [PubMed] [Google Scholar]
- 22. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. 2015;11(3):157‐165. [DOI] [PubMed] [Google Scholar]
- 23. Choi S‐A, Evidente VGH, Caviness JN, et al. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol. 2010;119(1):147‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roher AE, Esh C, Kokjohn TA, et al. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer's disease. Arterioscler Thromb Vasc Biol. 2003;23(11):2055‐2062. [DOI] [PubMed] [Google Scholar]
- 25. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139‐146. [DOI] [PubMed] [Google Scholar]
- 26. Ungvari Z, Buffenstein R, Austad SN, Podlutsky A, Kaley G, Csiszar A. Oxidative stress in vascular senescence: lessons from successfully aging species. Front Biosci. 2008;13:5056‐5070. [DOI] [PubMed] [Google Scholar]
- 27. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65(10):1028‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng S, Glennert J, Westermark P. Medin‐amyloid: a recently characterized age‐associated arterial amyloid form affects mainly arteries in the upper part of the body. Amyloid. 2005;12(2):96‐102. [DOI] [PubMed] [Google Scholar]
- 29. Davies HA, Caamaño‐Gutiérrez E, Chim YH, et al. Idiopathic degenerative thoracic aneurysms are associated with increased aortic medial amyloid. Amyloid. 2019;26(3):148‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Migrino RQ, Truran S, Karamanova N, et al. Human cerebral collateral arteriole function in subjects with normal cognition, mild cognitive impairment, and dementia. Am J Physiol Heart Circ Physiol. 2018;315(2):H284‐H290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Younger S, Jang H, Davies HA, et al. Medin oligomer membrane pore formation: a potential mechanism of vascular dysfunction. Biophys J. 2020;118(11):2769‐2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zuliani G, Cavalieri M, Galvani M, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J Neurol Sci. 2008;272(1‐2):164‐170. [DOI] [PubMed] [Google Scholar]
- 33. Dede DS, Yavuz B, Yavuz BB, et al. Assessment of endothelial function in Alzheimer's disease: is Alzheimer's disease a vascular disease? J Am Geriatr Soc. 2007;55(10):1613‐1617. [DOI] [PubMed] [Google Scholar]
- 34. Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer's disease. J Neuroinflammation. 2011;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging. 2001;22(6):837‐842. [DOI] [PubMed] [Google Scholar]
- 37. Grammas P, Ovase R. Cerebrovascular transforming growth factor‐beta contributes to inflammation in the Alzheimer's disease brain. Am J Pathol. 2002;160(5):1583‐1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, part XV. Neurology. 1996;46(6):1592‐1596. [DOI] [PubMed] [Google Scholar]
- 39. Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37(6):1391‐1398. [DOI] [PubMed] [Google Scholar]
- 40. Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part X. neuropathology confirmation of the clinical diagnosis of Alzheimer's disease. Neurology. 1995;45(3 Pt 1):461‐466. [DOI] [PubMed] [Google Scholar]
- 41. Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292(23):2901‐2908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


