Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on l‐histidine monohydrochloride (HCl) monohydrate produced by fermentation using Escherichia coli NITE SD 00268 in the context of the renewal of the authorisation for salmonids when used as a nutritional additive. In addition, the applicant requested the extension of use of the additive for other fin fish. The applicant has provided evidence that the composition of the additive currently in the market complies with the conditions of authorisation. The production strain has been modified by conventional mutagenesis and it does not raise safety concerns. The use of l‐histidine HCl monohydrate produced by fermentation using E. coli NITE SD 00268 is safe for salmonids and other fin fish when used as a nutritional additive to supplement the diet in appropriate amounts to cover the nutritional requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions, the background amino acid composition of the unsupplemented diet and the status of some essential trace elements such as copper and zinc. The FEEDAP Panel considers the maximum total concentration of 1.7% histidine in feed for salmonids proposed by the applicant as safe. For other fin fish species, the level of 1.7% appears to cause adverse effects. Therefore, it is not possible to define a maximum concentration of histidine in fish other than salmonids as it depends on histidine nutritional requirements in the different fish species. The use of the authorised additive in salmonids production does not pose a risk for consumers, and the proposed maximum total concentration of 1.7% histidine in feed is considered safe for the consumer. l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268 supplemented at levels appropriate to cover the nutritional requirements of fish other than salmonids is considered safe for the consumer. The additive under assessment is not a skin irritant. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or a skin sensitiser. The amino acid l‐histidine is a natural component of plants and animals. The use of the additive under assessment in animal nutrition does not represent a risk to the environment. The additive is considered an efficacious source of the amino acid l‐histidine for fish species.
Keywords: nutritional additive, amino acid, l‐histidine monohydrochloride monohydrate, Escherichia coli NITE SD 00268, safety
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7. In addition, Article 14(1) of that Regulation lays down that an application for renewal shall be sent to the Commission at the latest one year before the expiry date of the authorisation.
The European Commission received a request from Kyowa Hakko Europe GmbH2 for renewal of the authorisation of the product l‐histidine monohydrochloride monohydrate produced by Escherichia coli NITE SD 00268 when used as a feed additive for salmonids (category: nutritional additive; functional group: amino acids, their salts and analogues) and for authorisation when used as feed additive for fish other than salmonids (category: nutritional additive; functional group: amino acids, their salts and analogues).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive) and under Article 14(1) (renewal of the authorisation). The particulars and documents in support of the application were considered valid by EFSA as of 22 July 2016 for FAD‐2016‐0020 and as of 20 August 2018 for FAD‐2018‐0039.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product l‐histidine monohydrochloride monohydrate produced by E. coli NITE SD 00268, when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
l‐Histidine monohydrochloride monohydrate minimum 98% produced by E. coli NITE SD 00268 is the subject of the present assessment. The active substance of the product under application is l‐histidine. This additive has been previously assessed as nutritional feed additive (amino acid) for salmonids by the FEEDAP Panel (EFSA, 2005, 2006a) but as a product ■■■■■ of E. coli ATCC 21318. l‐Histidine monohydrochloride monohydrate minimum 98% produced by E. coli ATCC 9637 is currently listed in the European Union Register of Feed Additives, and thus authorised in the European Union for use in feed for salmonids.3
l‐Histidine, ■■■■■ is currently listed in the European Union Register of Feed Additives, and authorised in the European Union as a feed flavouring for all animal species.4 The FEEDAP Panel (EFSA FEEDAP Panel, 2014) assessed the safety and efficacy of l‐histidine as feed flavouring compound. The FEEDAP Panel has published several opinions on the safety and efficacy of l‐histidine monohydrochloride monohydrate produced by fermentation for all animal species (EFSA FEEDAP Panel, 2019a,b,c). l‐Histidine as a flavouring agent was assessed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA, 2004) and was considered safe for use in food. The EFSA's Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) evaluated l‐histidine and considered it safe for use as flavours in food (EFSA, 2006b, 2008a,b; EFSA CEF Panel 2011).
l‐Histidine is authorised for use in food,5 cosmetics6 and as a veterinary medicinal product.7 , 8
The Panel on nutrition, dietetic products, novel food and allergy of the Norwegian Scientific Committee for Food Safety (VKM, 2016) issued a scientific opinion on l‐histidine in food supplements and energy drinks sold in Norway and concluded that doses 550 and 600 mg/day l‐histidine in food supplements are unlikely to cause adverse health effects in humans ≥ 10 years of age.
l‐Histidine HCl monohydrate is described in a monograph of the European Pharmacopoeia (PhEur 9th edition, 2017): monograph 01/2005:0910.
l‐Histidine monohydrochloride monohydrate that is currently authorised for salmonids is produced by E. coli ATCC 21318 (inaccurately identified with the parental strain ATCC 9637 in Regulation (EC) 244/2007 and derived from it by one mutagenesis step). The applicant has requested for a change in the production strain from E. coli ATCC 21318 to E. coli NITE SD 00268.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier9 in support of the renewal of the authorisation for the use of l‐histidine monohydrochloride monohydrate produced by E. coli NITE SD 00268 as a feed additive for salmonids, and the extension of the authorisation to other fin fish.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
The European Union Reference Laboratory (EURL) considered that the conclusions and recommendations reached in the previous assessment regarding the methods used for the control of l‐histidine monohydrochloride monohydrate in animal feed are valid and applicable for the current application.10
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐histidine monohydrochloride monohydrate minimum 98% produced using E. coli NITE SD 00268 is in line with the principles laid down in Regulation (EC) No 429/200811 and the relevant guidance documents: Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019d) and Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018b).
3. Assessment
This assessment regards the renewal of the authorisation of the additive l‐histidine monohydrochloride monohydrate (minimum 98%) produced by E. coli NITE SD 00268 as nutritional additive (functional group: amino acids, their salts and analogues) for salmonids. The applicant is also requesting the extension of the authorisation to other fin fish as a nutritional feed additive (functional group: amino acids, their salts and analogues). The FEEDAP Panel notes that the production strain has been changed; therefore, this assessment deals also with the characterisation of the new strain.
3.1. Characterisation
3.1.1. Characterisation of the production organism
According to Regulation (EC) 244/2007, l‐histidine monohydrochloride monohydrate approved for use in salmonids is produced by E. coli ATCC 9637. The strain ATCC 9637 was inaccurately identified as the production strain in the 2007 authorisation because it was actually the parental strain. The proper production strain of the authorised additive was E. coli ATCC 21318■■■■■
The applicant has now requested to use another E. coli l‐histidine production strain – as compared to the production strain of the l‐histidine already authorised – that has been deposited in the Biological resource Centre of the Japanese National Institute of Technology and Evaluation with the deposition number NITE SD 00268.12 ■■■■■
■■■■■
The morphological characteristics of the production strain are provided in the technical dossier.13 The production strain, ■■■■■ does not raise concerns regarding its capacity to produce substances with antimicrobial activity ■■■■■ or toxins.14 In addition, supporting evidence on the absence of antimicrobial activity in the end product was provided in a test demonstrating no growth inhibition of a Bacillus subtilis strain used as the test organism.15
3.1.2. Manufacturing process
The applicant stated that no antimicrobial compounds are used during the production process.20
3.1.3. Characterisation of the active substance/additive
l‐Histidine monohydrochloride monohydrate (International Union of Pure and Applied Chemistry (IUPAC) name: (2S)‐2‐amino‐3‐(1H‐imidazol‐5‐yl)propanoic acid hydrate hydrochloride), is a compound identified with the Chemical Abstract Service (CAS) No 5934‐29‐2, and the European Inventory of Existing Commercial Chemical Substances (EINECS) No 211‐438‐9. It has a molecular weight of 209.63 Da. The chemical formula of l‐histidine monohydrochloride monohydrate is C6H9N3O2.HCl.H2O. The structural formula is given in Figure 1.
Figure 1.
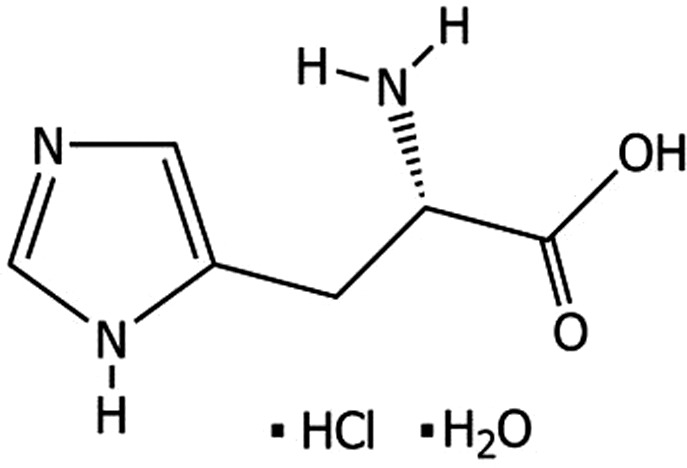
Structural formula of l‐histidine monohydrochloride monohydrate
The additive is currently authorised with a minimum content of 98% l‐histidine HCl monohydrate and < 1% moisture. (Regulation (EC) 244/2007). Analysis of five batches confirmed compliance with these specifications and showed an average l‐histidine content of 74.1% as is (range 72.3–74.7%),21 equivalent to approximately 100% l‐histidine HCl monohydrate (the proportion of HCl and water by stoichiometric calculation is of 26%). The amount of unidentified substances on dry matter basis was < 1%.
The specific optical rotation of the additive was measured in three batches and it was +9.9 in all cases.22 It is within the range (+9.2 to +10.6˚) of the European Pharmacopoeia (2017) and confirms the l‐stereoisomer of histidine.
Impurities
The analyses of three batches of the additive for heavy metals (lead, mercury and cadmium) and arsenic (three batches analysed) showed values below the limits of detection (LODs).23 Sodium ranged from 0.5 to 2 g/kg, potassium from 4.7 to 5.9 mg/kg and iron from 1.0 to 1.6 mg/kg. Dioxins (PCDD/F) and dioxin‐like PCBs were measured in three batches of the final product. In all cases, the measured value was 0.1 pg bioanalytical equivalents (BEQ)/g fat, corresponding to the limit of detection.24
■■■■■■■■■■
■■■■■
The absence of viable cells of the production strain was demonstrated in 1.2 g sample from three batches (tested in triplicate) by spreading on selective agar plates and incubation for three days at 32°C. The positive control (spiked with bacterial broth) yielded a positive result.26
The absence of DNA from the production strain in the additive■■■■■ was confirmed by polymerase chain reaction (PCR) ■■■■■■■■■■
Physico‐chemical characteristics
The additive is a greyish white to greyish brown crystalline powder or granules.28 Its tapped bulk density (three batches analysed) ranged from 0.92 to 1.04 g/cm3.29 Its pH ranged from 3.5 to 4.5 (10% solution in water).30
The dusting potential of the additive (Stauber–Heubach method) analysed in three batches ranged from 4.7 to 5.8 g/m3.31 Particle size distribution (volume based, three batches) was measured by laser diffraction and ranged from about 200 to 900 μm of diameter; no particles of < 100 μm of diameter were found.32 The particle size of the dust of the additive ranged from 2 μm to 26–32 μm diameter (10th and 99th percentiles).33
3.1.4. Stability and homogeneity
The shelf‐life of three batches of the additive was studied when stored at 40°C for 6 months;34 packaging was not described. Only tabulated results were provided (no certificates of analysis). At the end of the storage period, losses of l‐histidine ranged from 0 to 4%.
The shelf‐life of three batches of the additive was studied when stored in polyethylene bags at 25 ± 2°C for three years;35 losses ranged from 0 to 0.6%.
The stability of the additive (one batch) when supplemented at 0.8% (calculated) was estimated in a salmon feed (based on fish meal, soy protein concentrate, wheat, rapeseed oil and fish oil) in mash, extruded feed (20 bar, 110–130°C) and fat coated extruded feed, stored in polyethylene bottles at room temperature for 3 months.36 Total histidine was measured and corrected for basal levels of histidine HCl present in the corresponding sample. No losses were observed at the end of the storage period in any of those feed forms.
The capacity of the additive to distribute homogeneously in feed was studied in the two types of extruded feed for salmon described above by analysing 10 subsamples.37 The coefficient of variation of the calculated added histidine (total histidine after supplementation minus histidine in basal diet) was 5.6% in the pelleted feed and 2.4% in the fat coated pelleted feed.
3.1.5. Physico‐chemical incompatibilities in feed
No physico‐chemical incompatibilities in feed are expected when used with other additives, medicinal products or other feed materials.
3.1.6. Conditions of use
l‐Histidine monohydrochloride monohydrate is currently authorised as a nutritional additive, functional group ‘amino acids, their salts and analogues’ for salmonids with no minimum or maximum content specified. The applicant proposes a maximum total concentration of histidine in feed for salmonids of 1.7%.38
In addition, the applicant is proposing the extension of use to feed for fin fish other than salmonids with a maximum content of 1.7% total histidine in feed. The need of supplementing l‐histidine HCl monohydrate generally depends on the requirement of the animal species/categories and the background content of this essential amino acid in feedingstuffs. The additive is proposed to be supplemented directly into feedingstuffs (complete or complementary feed) or via premixtures.38
3.2. Safety
3.2.1. Safety of the production microorganism
The production strains E. coli NITE SD 00268 (under assessment) and E. coli ATCC 21318 (previously assessed) are derived from the same parental strain E. coli ATCC 9637, which is considered to be safe. The identity of the production strain E. coli NITE SD 00268 ■■■■■ has been established, the strain does not harbour acquired antimicrobial resistant genes and no viable cells or DNA were detected in the final product. Therefore, the use of E. coli NITE SD 00268 to produce l‐histidine monohydrochloride monohydrate does not give rise to safety concerns.
3.2.2. Absorption, distribution, metabolism and excretion
The applicant provided a review of the metabolic fate of l‐histidine.39 It is rapidly and extensively absorbed by animals. Transport across membranes by intestinal enterocytes can occur by diffusion or by active transport, and these processes are usually sodium‐dependent (Webb, 1990; WHO, 2006). l‐Histidine mostly enters cells unchanged.
l‐histidine may be incorporated into proteins or biologically important dipeptides by the animal (WHO, 2006; Bellia et al., 2008). The FEEDAP Panel notes that histidine is different from other essential amino acids in that substantial quantities exist in haemoglobin and in the form of free imidazol derivatives (dipeptides) in animal muscle tissues such as carnosine (β‐alanyl histidine), anserine (β‐alanyl‐1‐methyl histidine) and balenine (β‐alanyl‐3‐methyl histidine) leading to the difficulty of establishing conclusive indispensability. Furthermore, these dipeptides have anti‐inflammatory and antioxidant effects; it is believed that they interact with oxygen radicals and lipid peroxidation products to prevent membrane damage (Buttery and D'Mello, 1994; Onodera, 2003).
Where l‐histidine is not required for protein or peptide synthesis, it can be degraded by oxidation and transamination (Wade and Tucker, 1998; Nelson and Cox, 2008). α‐Ketoglutarate is formed as a degradation product which enters the citric acid cycle leading to further metabolic intermediates. Following l‐histidine loading, unchanged histidine as well as these normal metabolic intermediates are detected in the urine. l‐Histidine is also a precursor for histamine, produced by the enzyme histidine decarboxylase, present in e.g. brain and gut tissues and in gut microbiota.
3.2.3. Safety for the target species
The product under assessment is produced by fermentation using a non‐genetically modified strain of E. coli W type derivative that is considered safe (see Section 3.2.1). No cells of the production strain were found in the final product. Moreover, endotoxin activity of the product (< 100 IU/g) is considered low and of no concern for the target species. The FEEDAP Panel considers that no safety concerns would derive from the fermentation process. The additive contains ≥ 98% l‐histidine HCl monohydrate and the amount of unidentified material is < 1%.
The FEEDAP Panel notes that l‐histidine chelates divalent metal ions; and is necessary for the regulation and catabolism of trace elements such as zinc, copper, iron, manganese and molybdenum. High levels of histidine may therefore theoretically cause deficiencies of the free forms of these metal ions due to increased excretion (Ketola, 1979; Aoyama et al., 1992; Aoyama et al., 2000; Glover and Hogstrand, 2002; Breck et al., 2003; VKM, 2016).
3.2.3.1. Safety for salmonids
In its previous opinion, the FEEDAP Panel noted that there was limited scientific information on the use of excess dietary crystalline histidine in salmonids (EFSA, 2005). Based on a 6‐month study on Atlantic salmon where dietary histidine levels up to 1.7% showed no adverse effects on fish growth and health the FEEDAP Panel concluded that the additive was safe for salmonids up to 1.7%.
The National Research Council (NRC) has established histidine requirements (% of the diet) for Atlantic salmon (0.8%), Pacific salmon (0.7%) and Rainbow trout (0.8%) (NRC, 2011).
The applicant reviewed the scientific literature aiming to identify studies in which l‐histidine HCl monohydrate was supplemented into feed as confirmation of the suitability of the additive as available histidine source.40 The literature search was performed using Google Scholar and the keywords applied were ‘histidine’ combined with ‘fish, salmon, trout, bass, shellfish and shrimp’; the inclusion criterium was ‘studies in which histidine was supplemented to fish feed’. The FEEDAP Panel notes that the literature search had some limitations: only a single database was searched, the keywords did not include the terms ‘safety’ or ‘toxicity’ and the total number of hits and exclusion criteria were not reported; these hindrances cast doubts on the adequacy of the literature search performed to support the safety for the target species.
Nine scientific reports testing the effects of l‐histidine in fish diets (mainly Atlantic salmon but also carp, rainbow trout and Nile tilapia) were selected by the applicant. Only those performed in salmonids were assessed by the FEEDAP Panel: Breck et al. (2003, 2005a,b), Remø et al. (2014); Roland et al. (2015) and Taylor et al. (2015).41
Breck et al. (2003, 2005a) showed that low levels of histidine and N‐acetylhistidine in eye lens are associated with cataract development in Salmo salar L. and confirmed that it could be significantly mitigated by dietary histidine (1.4% histidine versus a control diet with 0.9% histidine) when fed for 23 weeks. In a subsequent experiment (Breck et al., 2005b), the effect of dietary histidine and iron on cataract development of two strains of Atlantic salmon monitored through parr‐smolt transformation was studied. Histidine diets (1.8% histidine vs. 1.2% histidine in control diet) fed for 26 weeks significantly reduced cataract development and significantly increased body weight (BW) and feed intake (FI). A significant genetic influence on the development of cataracts was also detected.
Remø et al. (2014) investigated if dietary histidine requirements to reduce cataract development were higher than those to grow in Atlantic salmon (Salmo salar L) smolts after seawater transfer. Diets containing increasing histidine concentrations (1, 1.2, 1.4, 1.5 and 1.8%) were fed for 13 weeks after seawater transfer. Dietary histidine concentration did not affect weight gain. Lowest severity of cataracts was achieved at 1.3% histidine.
Taylor et al. (2015) studied the effect of increasing levels of dietary histidine (1.3 or 1.7%) on cataract development of diploid or triploid Atlantic salmon when growing in seawater (post‐moults). Experimental diets were administered for 3 months. Dietary histidine did not significantly affect weight or growth rate within ploidy, but improved feed conversion ratio (FCR) in triploid fish fed 1.7% histidine.
Roland et al. (2015) studied the effect of plant proteins and amino acid supplementation on plasma amino acid profiles (monitored from 2 to 42 h after feed intake) and metabolism of rainbow trout (Oncorhynchus mykiss) of 470 g initial BW. Levels of histidine were 0.9% (fish meal), 0.8% (pea protein concentrate) and 1.4% (pea protein concentrate supplemented with histidine, lysine, methionine and threonine to mimic fish meal). The diet with supplemental amino acids resulted in greater and faster rises of plasmatic concentrations followed by sharp decreases compared with the diet containing fish meal. The effect on performance was not studied.
From these studies, it can be concluded that high levels of histidine in feed (up to 1.7%, representing about twice the requirements) are needed to prevent cataract in Atlantic salmon and these levels have no adverse effects on performance in this species. There is a positive relationship between dietary histidine concentration and histidine, anserine and carnosine deposition in fish flesh of Atlantic salmon. Supplemental histidine may result in a faster absorption compared to diets containing protein‐bound histidine, resulting in peaks in the plasmatic amino acid profile. An additional issue that was not considered by the applicant is related to the possibility that high l‐histidine supplementation may lead to interaction with essential metals such as zinc with possible adverse metabolic consequences for the salmonids (see Section 3.2.3).
Notwithstanding the limitations on the literature search described and the data therein reported, the FEEDAP Panel considers that the data provided in the studies reviewed support the conclusions reached in the previous opinion of the FEEDAP Panel that the additive is safe for salmonids up to 1.7% histidine in the diet (EFSA, 2005). The maximum total content of histidine in feed proposed by the applicant is in line with this conclusion.
3.2.3.2. Safety for fin fish other than salmonids
The requirements of histidine (% of the diet) established by NRC (2011) for fish other than salmonids are limited to some species: 0.5% for the common carp (Cyprinus carpio), 0.9% for mrigal carp (Cirrhinus mrigala), 0.9% for rohu (Labeo rohita), 1.0% for Nile tilapia (Oreochromis niloticus) and 0.6% for channel catfish (Ictalurus punctatus).
The applicant conducted a literature search in Web of Knowledge database to identify adverse effects related to histidine in diets of fish other than salmonids.42 Key words used were ‘histidine’ in combination with ‘fish NOT salmon NOT trout’, carp, Cyprinus, tilapia, Oreochromis, bass, Micropterus, tuna, Thunnini, mackerel or scomber. A total of 806 hits were retrieved and title/abstracts screened for relevance. Fifteen studies were selected: seven for carp (two of them, however, were available only in abstract form and were not further considered), four for catfish, and one for each of the following fish species: flounder (Paralichthys olivaceous), croaker (Pseudosciaena crocea), Nile tilapia and yellowtail fish. Those studies are described below.
Regarding African catfish (Clarias gariepinus [Burchel]), Khan and Abidi (2009) tested the effect of increasing levels of dietary histidine (0.25–0.50% histidine in diet on dry matter (DM)) on performance of fries when fed for 12 weeks. Best performance was achieved at 0.40% histidine in diet. The highest level resulted in significant differences increasing FCR and decreasing absolute weight gain, protein efficiency ratio (PER) and specific growth ratio (SGR) in comparison to the group treated with 0.40% histidine.
As per Indian catfish (Heteropneustes fossilis [Bloch]), three studies (fingerlings or fry) were submitted (Ahmed, 2013; Farhat and Kan, 2013; Khan and Abidi, 2014) studying the effect of increasing levels of histidine in the diet (0.25–0.75%; 0.5–1.25%; or 1–2%, respectively) on performance and blood parameters when fed for 8–12 weeks. The best results were obtained within a wide range of dietary histidine concentrations (0.5–1.6%, depending on the study considered) with performance parameters significantly worsening at the highest histidine level of the respective study.
Regarding Asian carp (Cirrhinus mrigala [Hamilton]), Ahmed and Khan (2005) studied the dietary requirements of the fingerlings by surveying the effect of increasing dietary histidine levels (0.25–1.5%) on performance parameters (final BW, body weight gain (BWG), SGR, FCR and PER), when fed for 8 weeks. Performance parameters were optimised at 0.75% histidine and significantly decreased (except FCR that increased) from 1% histidine dietary concentration.
As per juvenile Asian grass carp (Ctenopharyngodon Idella), Gao et al. (2016) studied the effects of increasing dietary histidine levels (0.45–1.78% DM feed) fed during 10 weeks on performance parameters, enzymatic intestinal activity, erythrocyte osmolar fragility and hypoxia tolerance. Histidine concentration in fish flesh increased in a concentration‐dependent manner (2.4% and 3.3% at lowest and highest dietary histidine concentrations respectively). Performance (final BW and BWG) was optimised at 1.02% dietary histidine and significantly decreased in the next higher histidine concentration tested (1.6%).
Zhera and Khan (2014) studied the effects of increasing dietary histidine levels (0.25–0.96%) in fingerlings of South Asian carp (Catla catla [Hamilton]) fed for 12 weeks on performance parameters, carcass composition and haematological parameters. Optimised results on final BW, FCR, FI and protein gain were obtained at the range of 0.63–0.68% histidine in DM diet and no significant differences were observed at the highest dose tested (0.96%).
The effect of histidine supplementation of juvenile (9 g initial BW) European carp (Cyprinus carpio var. Jian) on performance parameters and intestinal enzymatic activity was studied by Zhao et al. (2012) by administering increasing dietary levels of histidine (0.23–1.27%) for 60 d. The concentration optimising final BW, SGR, FI, PER and FCR was 0.78% histidine. The next higher concentration tested (1.1%) significantly decreased those parameters (except FCR that increased). In a very similar study Feng et al. (2013) – using the same fish species and category, histidine levels and duration – showed that levels of histidine around 0.86% improved the antioxidant capacity and inhibit lipid peroxidation and protein oxidation of juvenile Jian carp.
Han et al. (2013) studied the effect of increasing dietary histidine (1.0, 1.5 and 1.9%) and arginine (1.71 and 2.75%) in a two‐factorial study on performance and blood parameters of Japanese flounder (Paralichthys olivaceus) juveniles when fed for 60 days. When arginine was low, BWG, final BW and SGR were higher at the highest level of histidine; and when arginine was high, these parameters were higher at the medium level of histidine, 1.9% histidine resulting in a reduction of these three performance parameters when compared with the medium level of histidine (1.5%). Serum glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) were affected by the different combinations of the two amino acids.
Li et al. (2014) tested the effect of increasing dietary histidine (0.45–1.40%, six treatments with three replicates each) on performance of juvenile large yellow croaker (Pseudosciaena crocea R.) when fed for 51 days. Final weight and WG increased up to a dietary histidine concentration of 0.78%, decreasing significantly at the highest concentration.
Michelato et al. (2016) studied the effect of increasing dietary histidine (0.42–1.15% dry diet. six treatments with three replicates each) on performance and blood parameters of juvenile (5 g initial BW) Nile tilapia (Oreochromis niloticus) when fed for 100 days. Final BW, BWG, FCR, PER and net protein utilisation were optimised in fish fed 0.89% histidine and those parameters significantly decreased (except for FCR that increased) at the highest histidine level. No differences were observed in blood parameters.
Although high levels of l‐histidine in feed (1.7%) are considered safe in Atlantic salmon (Breck et al., 2003, 2005a,b), the data above shows that levels even below this 1.7% can have adverse effects in other fish species.
Although there is limited evidence from the published literature on the effects of supplementing histidine levels above the requirements, the FEEDAP Panel considers that adverse effects might occur with levels of histidine in feeds exceeding the requirements, depending on the balance with other amino acids and the status of some essential trace elements such as copper and zinc (see Section 3.2.3).
The FEEDAP Panel notes that the number of fish species used in aquaculture is growing and is greater than the species reviewed above. The Panel further notes that for fish species other than salmonids, in very few cases there are histidine requirements established by internationally recognised scientific bodies (i.e. common carp, mrigal carp, rohu, Nile tilapia and channel catfish; NRC, 2011).
3.2.3.3. Conclusions on safety for the target species
The use of l‐histidine monohydrochloride monohydrate produced by fermentation using E. coli NITE SD 00268 is safe for salmonids and other fin fish when used as a nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions, the background amino acid composition of the unsupplemented diet and the status of some essential trace elements such as copper and zinc.
The FEEDAP Panel considers the maximum total concentration of 1.7% histidine in feed for salmonids proposed by the applicant as safe. For other fin fish species, the level of 1.7% appears to cause adverse effects. It is not possible to define a maximum safe concentration of histidine in fish other than salmonids as it depends on histidine nutritional requirements in the different fish species.
3.2.4. Safety for the consumer
In the former opinion, the FEEDAP Panel considered that the use of the additive in animal nutrition would not represent a risk for the consumer.
No specific literature review was performed for the safety of the authorised additive for the consumer. The literature search conducted by the applicant to support the safety of the use of the authorised additive in salmonids did not provide any significant information on potential new issues related to safety for the consumer except for concentrations of histidine and related compounds in flesh reported in two studies (Breck et al., 2005b; Remø et al., 2014) that are described in Section 3.2.4.1.43
The product under assessment is produced by fermentation using a non‐genetically modified strain of E. coli W type derivative that is considered safe (see Section 3.1.1). No cells of the production strain were found in the final product. The FEEDAP Panel considers that no safety concerns would derive from the fermentation process. The additive contains ≥ 98% l‐histidine HCl monohydrate and the amount of unidentified material is < 1%.
The FEEDAP Panel, however, is aware that the intake of histamine, a metabolic by‐product of histidine, through fish flesh following microbial spoilage is a serious concern for consumers (EFSA BIOHAZ Panel, 2011). Histamine poisoning from fish flesh has been called ‘scombroid’ poisoning because of the edible fish species (e.g. tuna, mackerel) more liable to histamine formation due to the high content of histidine in their flesh. Commission Regulation (EC) No 2073/2005 sets a maximum limit of 200 mg histamine/kg flesh for sea fishery products (raw fish at the point of the first sale) of fish species associated with a high amount of histidine, in particular fish species of the families Scombridae, Clupeidae, Engraulidae, Coryfenidae, Pomatomidae and Scombresosidae.44
Histamine is a biogenic amine that can be synthesised endogenously from histidine by a l‐histidine decarboxylase, and from histidine released from carnosine via carnosinase. Histamine can be metabolised either extracellularly by a diamino oxidase (DAO) present in the gut mucosa, or intracellularly by a histamine‐N‐methyltranferase (HNMT). As regards the exogenous production of histamine, many different bacterial species are known to possess histidine decarboxylase and have the ability to convert free histidine into histamine (Kanki et al., 2004; Emborg et al., 2006; Dalgaard et al., 2008).
The applicant reviewed the literature retrieved in relation to the search performed to support the safety of the additive for fish other than salmonids (see Section 3.2.3.2) to identify (a) studies in which levels of histidine and related substances were analysed in edible tissues of fish after supplementation with the amino acid and (b) information on histamine formation in fish flesh and the risk posed to consumers.45 The outcome of the literature review and other relevant data are described and assessed below.
3.2.4.1. Histidine intake and histidine deposition in animal tissues
In a study with Atlantic cod (Gadus morhua L.) of 1.5 years of age (950 g initial BW), the effects of three dietary histidine levels (1.54, 2.74 and 6.34% of total histidine in diet, 2 replicates of 4 fish/treatment) on carcass characteristics (liquid leakage and fillet gaping during storage) were tested after feeding for 3 weeks (Førde‐Skjærvik et al., 2006). Histidine concentration in muscle significantly increased (260, 490 and 1,370 mg/kg muscle) in a dose dependent manner with histidine content in the diet. Anserine (1,620, 1,700 and 1,590 mg/kg muscle) was not significantly influenced by the diet. Carnosine was < 50 mg/kg muscle for all groups.
Breck et al. (2005b) studied the effect of dietary histidine and iron on cataract development of two strains of Atlantic salmon monitored through parr‐smolt transformation. Histidine diets (1.8% histidine in two treatments vs 1.2% histidine in control diet) fed for 26 weeks significantly increased histidine (2.7 vs 0.1 μmol/g of muscle, respectively), carnosine (0.60 vs 0.06 μmol/g of muscle, respectively) and anserine (16.1 vs 9.7 μmol/g of muscle, respectively) concentrations in fish flesh.
Remø et al. (2014) fed diets containing increasing histidine concentrations (1, 1.2, 1.4, 1.6 and 1.8%) to Atlantic salmon (Salmo salar L.) smolts for 13 weeks after seawater transfer. Muscle histidine concentrations (at week 9: 0.1, 0.2, 1, 2.2 and 3.1 μmol/g of muscle, respectively)46 reflected the increase of dietary histidine concentrations; and anserine (at week 9: 6, 12, 13.8, 14.2 and 14.3 μmol/g of muscle, respectively)46 and carnosine (values per treatment not given) reached a plateau at different times.
There is a positive relationship between dietary histidine concentration and histidine and anserine deposition (Breck et al., 2005b; Remø et al., 2014), and carnosine deposition (Breck et al., 2005b) in fish flesh of Atlantic salmon. The scarce evidence in other fish species is limited to Atlantic cod (Førde‐Skjærvik et al., 2006) and suggests that supplementation with histidine significantly increases histidine deposition in flesh but the same effect was not observed in anserine concentrations and no data was available on carnosine concentrations.
3.2.4.2. Histamine concentration in animal tissues in relation to dietary histidine
As review by the BIOHAZ Panel (2011), histamine poisoning is mainly related to fermented food of animal origin. The threshold for adverse health effects (acute reference dose) is 50 mg histamine per consumption for healthy individuals, and below detectable limits for individuals with histamine intolerance.
Limited data are available on the relationship between dietary histidine and histamine concentration in animal tissues. Although histidine is a precursor of histamine, the main factors influencing histamine formation in fish are storage time, temperature, pH, hygienic conditions (e.g. bacterial contamination, time to evisceration) or starter cultures of fermented foods, which have been reviewed in previous publications (EFSA BIOHAZ Panel, 2011; FAO, 2013, 2018; Technical report of EFSA, 2017). The literature search performed by the applicant retrieved scientific studies confirming that, more than the histidine content in diet, it is the amount of free histidine and carnosine in fish flesh and the bacterial spoilage that can increase histamine concentration in edible tissues/products from fish (Taylor et al., 1989; Lopez‐Sabater et al., 1996; Ababouch et al., 1991; Zarei et al., 2010; Boldyrev et al., 2013).47
As pointed out by FAO (2018), ‘the available evidence highlights that under appropriate time × temperature control, and within the sensory shelf‐life of the product, histamine development in Salmonidae to the levels that cause scombroid fish poisoning is unlikely to occur’.
In view of the above, the FEEDAP Panel considers that supplementing the diets of the target species with histidine to cover the requirements is unlikely to result in the increase of histamine formation provided that appropriate handling and storage of fish are ensured. Although there is no evidence from other aquaculture species, the Panel considers that the above conclusions can be extrapolated to other commonly farmed fish. For fish species associated with high levels of histidine in flesh,48 the Panel notes that supplemental histidine may increase histidine concentration in fish flesh and the possibility to have higher levels of histamine in fish flesh following improper storage. However, there are limits established for histamine to protect the consumer, in particular for Scombroid fish species.
3.2.4.3. Conclusions on safety for the consumer
No new evidence on consumer safety has been provided that would make the FEEDAP Panel reconsider its previous conclusion. Thus, the FEEEDAP Panel confirms that the use of l‐histidine in salmonids production does not pose a risk for consumers.
l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268 supplemented at levels appropriate to cover the nutritional requirements of fish other than salmonids is considered safe for the consumer.
3.2.5. Safety for user
In the former opinion, no specific studies to support the safety of the additive for users/workers were submitted; the FEEDAP Panel noted that due to the long‐term use of comparable products as food supplements and cosmetic ingredients, specific risks were not expected. However, workers and users should wear protective clothes as recommended by the applicant (EFSA, 2005).
The applicant provided an in vitro study on skin irritation where histidine HCl monohydrate minimum 98% was tested. Additionally, the applicant performed a literature search for reports on toxicity of histidine by inhalation exposure.
3.2.5.1. Safety for the respiratory system
The particle size distribution showed that the additive has no particles < 100 μm. However, the additive has high dusting potential (up to 5.8 g/m3). The particle size of the dust of the additive ranged from 2 to 32 μm diameter. The applicant reviewed the available literature in two adequate database platforms and searching for ‘histidine AND inhalation toxicity’ and no reports could be retrieved in relation to the toxicity of histidine by inhalation exposure.49 The users are exposed by inhalation but due to lack of information on the potential inhalation toxicity of the additive, the FEEDAP Panel cannot draw a conclusion.
The bacterial endotoxin activity (analysed in three batches) was < 100 IU/g. The scenario used to estimate the exposure of persons handling the additive to endotoxins in the dust, based on the EFSA guidance on user safety (EFSA FEEDAP Panel, 2012), is described in Appendix A. The threshold for the quantity of inhaled endotoxins per working day is 900 IU, derived from the provisional occupational exposure limits given by the Dutch Expert Committee on Occupational Safety (Health Council of the Netherlands, 2010) and the UK Health and Safety Executive (HSE, 2013). Based on calculations of the content of endotoxins in dust, assuming a worst‐case scenario in which the amount of bacterial endotoxin activity is 99 IU/g, the estimated exposure would be 320 IU per eight‐hour working day, indicating no risk by inhalation due to exposure to endotoxins for people handling the additive.
3.2.5.2. Safety for skin and eyes
No information was made available to support the potential of the additive to be a skin sensitiser or irritant to eyes.
In an in vitro skin irritation reconstructed human epidermis test in accordance with OECD Guideline 439,50 16 mg of test sample were applied uniformly to cover the epidermis surface. After 42 min exposure at room temperature, the reconstructed skin models were rinsed and incubated for 42 h at 37°C. Subsequently each sample was incubated with 1 mg/mL 3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide (MTT) for 3 h at 37°C. The blue formazan generated was extracted and its optical density measured. Positive and negative controls performed as expected. The test item was classified as non‐irritant.
3.2.5.3. Conclusions on safety for the user
The additive is not a skin irritant. The additive is not hazardous by inhalation due to exposure to endotoxins for people handling the additive. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or a skin sensitiser.
3.2.6. Safety for the environment
In the previous opinion, the FEEDAP Panel concluded that the availability of the product would not adversely influence the environment because (i) the product replaces natural histidine sources, (ii) histidine from the product is highly absorbed and (iii) faecal histidine is rapidly degraded in the environment (EFSA, 2005).
The applicant did not provide any literature search to support the safety of the additive for the environment.
The amino acid l‐histidine is a physiological and natural component of animal and plant proteins. It is not excreted as such (but as urea/uric acid and carbon dioxide). The use of l‐histidine in animal nutrition would not lead to any localised increase in its concentration in the environment. The use of l‐histidine HCl monohydrate produced by E. coli NITE SD 00268 in animal nutrition is not expected to represent a risk to the environment.
3.3. Efficacy
Efficacy studies are not required for amino acids naturally occurring in proteins of plants and animals. The nutritional role of the amino acid l‐histidine HCl monohydrate is well established in the scientific literature (NRC 1994, 2011, 2012).
In general, the product l‐histidine HCl monohydrate is considered an efficacious source of the essential amino acid l‐histidine for salmonids and other fin fish.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation51 and Good Manufacturing Practice.
4. Conclusions
The applicant has provided evidence that the composition of the additive currently in the market complies with the conditions of authorisation. The production strain has been modified by conventional mutagenesis and it does not raise safety concerns.
The use of l‐histidine monohydrochloride monohydrate produced by fermentation using E. coli NITE SD 00268 is safe for salmonids and other fin fish when used as a nutritional additive to supplement the diet in appropriate amounts to cover the requirements, depending on the species, the physiological state of the animal, the performance level, the environmental conditions, the background amino acid composition of the unsupplemented diet and the status of some essential trace elements such as copper and zinc. The FEEDAP Panel considers the maximum total concentration of 1.7% histidine in feed for salmonids proposed by the applicant as safe. For other fin fish species, the level of 1.7% appears to cause adverse effects. Therefore, it is not possible to define a maximum concentration of histidine in fish other than salmonids as it depends on histidine nutritional requirements in the different fish species.
The use of the authorised additive in salmonids production does not pose a risk for consumers, and the proposed maximum total concentration of 1.7% histidine in feed is considered safe for the consumer. l‐Histidine HCl monohydrate produced using E. coli NITE SD 00268 supplemented at levels appropriate to cover the nutritional requirements of fish other than salmonids is considered safe for the consumer.
The additive under assessment is not a skin irritant. In the absence of data, it is not possible to conclude on the potential of the additive to be toxic by inhalation, irritant to eyes or a skin sensitiser.
The amino acid l‐histidine is a natural component of plants and animals. The use of the additive under assessment in animal nutrition does not represent a risk to the environment.
The additive is considered an efficacious source of the amino acid l‐histidine for fish species.
5. Recommendation
Further research and monitoring activities on the levels of free histidine and dipeptides (carnosine) – which are precursors for the biogenic amine histamine – is recommended, aiming to set the safety criteria for fish and fishery products from farmed species other than salmonids, not covered in the Commission Regulation No 2073/2005.
6. Documentation as provided to EFSA / Chronology
| Date | Event |
|---|---|
| 21/03/2016 | Dossier received by EFSA: L‐Histidine monohydrochloride monohydrate – 98%, produced by E. coli. Submitted by Kyowa Hakko Europe GmbH. |
| 22/04/2016 | Reception mandate from the European Commission |
| 22/07/2016 | Application validated by EFSA – Start of the scientific assessment |
| 14/10/2016 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the additive and the production strain, production process, stability, safety. |
| 21/07/2017 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 22/09/2017 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the production strain. |
| 16/10/2017 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 29/11/2017 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the additive and of the production strain; safety for the target species. |
| 23/01/2018 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 18/03/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
| Date | Event |
|---|---|
| 19/07/2018 | Dossier received by EFSA: L‐Histidine monohydrochloride monohydrate (98%) for minor species –other fin fish. Submitted by Kyowa Hakko Europe GmbH. |
| 06/07/2018 | Reception mandate from the European Commission |
| 20/08/2018 | Application validated by EFSA – Start of the scientific assessment |
| 12/10/2018 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation of the additive, conditions of use, safety for the target species, safety for the consumer, safety for the user, characterisation of the production strain. |
| 20/11/2018 | Comments received from Member States |
| 26/03/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 04/08/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation of the production strain |
| 03/01/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 14/02/2020 | Spontaneous submission of supplementary information |
| 19/02/2020 | Clarifications on the conditions of use provided by e‐mail |
| 18/03/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AFC
EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- BEQ
bioanalytical equivalents
- BW
body weight
- BWG
body weight gain
- CAS
Chemical Abstracts Service
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CFU
colony forming unit
- CV
coefficient of variation
- DAO
diamino oxidase
- DM
dry matter
- EINECS
European Inventory of Existing Commercial Chemical Substances
- EURL
European Union Reference Laboratory
- FCR
feed conversion ratio
- FI
feed intake
- GOT
glutamic oxaloacetic transaminase
- GPT
glutamic pyruvic transaminase
- HNMT
histamine‐N‐methyltranferase
- IUPAC
International Union of Pure and Applied Chemistry
- JECFA
the Joint FAO/WHO Expert Committee on Food Additives
- LOD
limit of detection
- LOQ
limit of quantification
- MIC
minimum inhibitory concentration
- MTT
3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2H‐tetrazolium bromide
- NITE
Japanese National Institute of Technology and Evaluation
- NRC
National Research Council
- PCB
polychlorinated biphenyl
- PCDD/F
polychlorinated dibenzo‐p‐dioxins and dibenzofurans
- PCR
polymerase chain reaction
- PER
protein efficiency ratio
- SGR
specific growth ratio
Appendix A – Safety for the user
1.
The effects of the endotoxin inhalation and the exposure limits have been described in a previous opinion (EFSA FEEDAP Panel, 2015).
Calculation of maximum acceptable levels of exposure from feed additives
The likely exposure time according to EFSA guidance (EFSA FEEDAP Panel, 2012) for additives added in premixtures assumes a maximum of 40 periods of exposure per day, each comprising 20 s, equal to = 800 s/day. With an uncertainty factor of 2, maximum inhalation exposure would occur for 2 × 800 = 1,600 s (0.444 h per day). Again, assuming a respiration volume of 1.25 m3/h, the inhalation volume providing exposure to potentially endotoxin‐containing dust would be 0.444 × 1.25 = 0.556 m3/day. This volume should contain no more than 900 IU endotoxin, so the dust formed from the product should contain no more than 900/0.556 = 1,619 IU/m 3.
Calculation of endotoxin content of dust
Two key measurements are required to evaluate the potential respiratory hazard associated with endotoxin content of the product (the dusting potential of the product, expressed in g/m3; the endotoxin activity of the dust, determined by the Limulus amoebocyte lysate assay (expressed in IU/g)). If data for the dust are not available, the content of endotoxins of the product can be used instead. If the content of endotoxins of the relevant additive is a IU/g and the dusting potential is b g/m3, then the content of endotoxins of the dust, c IU/m3, is obtained by the simple multiplication a × b. This resulting value is further used for calculation of potential inhalatory exposure by users to endotoxin from the additive under assessment (Table A.1) (EFSA FEEDAP Panel, 2012).
Table A.1.
Estimation of user exposure to endotoxins from the additive l‐histidine HCl monohydrate produced by Escherichia coli NITE SD 00268, including consideration of using filter half mask (FF P2 or FF P3)52 as a preventative measure
| Calculation | Identifier | Description | Amount | Source |
|---|---|---|---|---|
| a | Endotoxin content IU/g product | 99 | Technical dossier | |
| b | Dusting potential (g/m3) | 5.84 | Technical dossier | |
| a × b | c | Endotoxin content in the air (IU/m3) | 578 | |
| d | No of premixture batches made/working day | 40 | EFSA FEEDAP Panel (2012) | |
| e | Time of exposure (s)/production of one batch | 20 | EFSA FEEDAP Panel (2012) | |
| d × e | f | Total duration of daily exposure/worker (s) | 800 | |
| g | Uncertainty factor | 2 | EFSA FEEDAP Panel (2012) | |
| f × g | h | Refined total duration of daily exposure (s) | 1,600 | |
| h/3 600 | i | Refined total duration of daily exposure (h) | 0.44 | |
| j | Inhaled air (m3)/eight‐hour working day | 10 | EFSA FEEDAP Panel (2012) | |
| j/8 × i | k | Inhaled air during exposure (m3) | 0.56 | |
| c × k | l | Endotoxin inhaled (IU) during exposure/eight‐hour working day | 321 | |
| m | Health‐based recommended exposure limit of endotoxin (IU/m3)/eight‐hour working day | 90 | Health Council of the Netherlands (2010) | |
| m × j | n | Health‐based recommended exposure limit of total endotoxin exposure (IU)/eight‐hour working day | 900 | |
| l /10 | Endotoxins inhaled (IU)/eight‐hour working day reduced by filter half mask FF P2 (reduction factor 10) | 32 | ||
| l /20 | Endotoxins inhaled (IU)/eight‐hour working day reduced by filter half mask FF P3 (reduction factor 20) | 16 |
References
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012 Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. https://doi.org/10.2903/j.efsa.2012.253
EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of L‐lysine monohydrochloride produced by fermentation with Escherichia coli for all animal species based on a dossier submitted by HELM AG on behalf of Meihua Holdings Group Co. Ltd. EFSA Journal 2015;13(3):4052, 16 pp. https://doi.org/10.2903/j.efsa.2015.4052
Health Council of the Netherlands, 2010. Endotoxins. Health‐based recommended occupational exposure limit. Publication no 2010/04OSH, 100 pp.
Suggested citation: EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Glandorf B, Herman L, Mantovani A, Saarela M, Svensson K, Tosti L, Anguita M, Pettenati E, Tarrés‐Call J and Ramos F, 2020. Scientific Opinion on the assessment of the application for renewal of authorisation of l‐histidine monohydrochloride monohydrate produced with Escherichia coli NITE SD 00268 for salmonids and its extension of use to other fin fish. EFSA Journal 2020;18(4):6072, 23 pp. 10.2903/j.efsa.2020.6072
Requestor: European Commission
Question numbers: EFSA‐Q‐2016‐00305 and EFSA‐Q‐2018‐00546
Panel members: Giovanna Azimonti, Vasileios Bampidis Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Kos Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgments: The Panel wishes to acknowledge the contribution of Yolanda Garcia Cazorla to this opinion.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Example: Figure 1: © ECDC
Adopted: 18 March 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Kyowa Hakko Europe GmbH, Am Wehrhaln 50, D‐40211 Düsseldorf, Germany.
Commission Regulation (EC) No 244/2007 of 7 March 2007 concerning the authorisation of L‐histidine monohydrochloride monohydrate as feed additive. OJ L 73/6, 13.3.2007, p. 2.
Commission List of the authorised additives in feedingstuffs published in application of Article 9th of Council Directive 70/524/EEC concerning additives in feedingstuffs (2004/C 50/01). OJ C 50/1, 25.2.2004.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006/141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC) No 41/2009 and (EC) No 953/2009, OJ L 181, 29.6.2013, p.35.
Commission Decision of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97, 5.4.2006, pp. 1–528.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1.
Regulation (EC) No 470/2009 of the European Parliament and of the Council of 6 May 2009 laying down Community procedures for the establishment of residue limits of pharmacologically active substances in foodstuffs of animal origin, repealing Council Regulation (EEC) No 2377/90 and amending Directive 2001/82/EC of the European Parliament and of the Council and Regulation (EC) No 726/2004 of the European Parliament and of the Council. OL L 152, 16.6.2009, p. 11.
FEED dossier reference: FAD‐2016‐0020 for the renewal of the authorization for salmonids and FAD‐2018‐0039 for the extension of the authorization to other fin fish.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/FinRep-FAD-2006-0022.pdf.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II.2.2 and Annex II.14.
Technical dossier FAD‐2018‐0039/Supplementary information January 2020/Annex K.
Technical dossier FAD‐2018‐0039/Section II.2.2.1.1.
Technical dossier FAD‐2018‐0039/Section II/Annexes II.17 and ■■■■■
Technical dossier FAD‐2018‐0039/Supplementary information January 2020/Annex M.
■■■■■
■■■■■
■■■■■
Technical dossier FAD‐2018‐0039/Section II/Annex II.25.
Technical dossier FAD‐2018‐0039/Section II.2.3 and ■■■■■ and II.25.
Technical dossier/Section II.2.2.1.1.
Technical dossier FAD‐2016‐0020/Section II/Annex II.2. Histidine analysed using the EU official method (Regulation (EU) 152/2009)
Technical dossier FAD‐2018‐0039/Section II/Annex II.13.
Technical dossier FAD‐2018‐0039/Section II/Annexes II.6 and ■■■■■ . LOD in µg/kg was 50 for lead, mercury, cadmium and arsenic.
Technical dossier FAD‐2018‐0039/Section II/Annex II.8, Calux method.
■■■■■
Technical dossier FAD‐2018‐0039/Section II/Annex II.19. European Pharmacopoeia method
Technical dossier FAD‐2018‐0039/Supplementary information March 2019/Annex A.
■■■■■
Technical dossier FAD‐2018‐0039/Section II.2.1.3.
Technical dossier FAD‐2018‐0039/Section II.2.1.5/Annex II.12.
Technical dossier FAD‐2018‐0039/Section II/Annex II.11.
Technical dossier FAD‐2016‐0020/Section II.2.1.5 and Annex II.8.
Technical dossier FAD‐2018‐0039/Section II.2.1.5 and Annex II.9.
Technical dossier FAD‐2018‐0039/Supplementary information Mach 2019/Annexes D and E.
Technical dossier FAD‐2018‐0039/Section II2.4.1.2/Annex II.29.
Technical dossier FAD‐2018‐0039/Section II/Annex II.31.
Technical dossier FAD‐2018‐0039/Section II.2.4.1.2 and Annex II.30.
Technical dossier FAD‐2018‐0039/Section II/Annex II.30.
Technical dossier FAD‐2018‐0039/Supplementary information March 2019/His‐HCl response March 2019 and clarification by e‐mail of 18 February 2020.
Technical dossier FAD‐2018‐0039/Section III/Annex III.1.
Technical dossier FAD‐2016‐0020/Supplementary information July 2017/His‐HCl response and Appendixes J and K; supplementary information January 2018/FAD‐2016‐0020‐SIn 291117 Applicant's response, Appendix C.
Technical dossier FAD‐2016‐0020/Supplementary information July 2017/Appendix K.
Technical dossier FAD‐2018‐0039/Supplementary information March 2019/Appendix 3A and Annex B.
Technical dossier FAD‐2016‐0020/Supplementary information July 2017/His‐HCl response and Appendixes J and K; supplementary information January 2018/FAD‐2016‐0020‐SIn 291117.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ 22.12.2005, L 338/1 26 pp.
Technical dossier FAD‐2018‐0039/Supplementary information March 2019/Annex C and Appendix 4A.
Values estimated from a graph.
Technical dossier FAD‐2018‐0039/Supplementary information March 2019/Annex C.
According to the Commission Regulation (EC) No 2073/2005, Fishery products from fish species associated with a high amount of histidine and Fishery products which have undergone enzyme maturation treatment in brine, manufactured from fish species associated with a high amount of histidine. In particular fish species of the families Scombridae, Clupeidae, Engraulidae, Coryfenidae, Pomatomidae and Scombresosidae.
Technical dossier FAD‐2018‐0039/Supplementary information March 2019/Appendix 5B.
Technical dossier/Supplementary information March 2019/Annex H.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
Filtering face piece or filtering half mask according to European standard EN 149. They are graded from 1 to 3 depending on their filtering capacity.
References
- Ababouch L, Afilal ME, Benabdeljelil H and Busta FF, 1991. Quantitative changes in bacteria, amino acids and biogenic amines in sardine (Sardina pilchardus) stored at ambient temperature (25–28 C) and in ice. International Journal of Food Science & Technology, 26, 297–306. [Google Scholar]
- Ahmed I, 2013. Dieatary amino acid L‐histidine requirement of fingerling Indian catfish, Heteropneustes fossilis (Bloch), estimated by growth and whole body protein and fat composition. Journal of Applied Ichthyology, 29, 602–609. [Google Scholar]
- Ahmed I and Khan MA, 2005. Dietary histidine requirement of fingerling Indian major carp, Cirrhinus mrigala (Hamilton). Aquaculture Nutrition, 11, 359–366. [PubMed] [Google Scholar]
- Aoyama Y, Mori M, Hitomi‐Ohmura E and Yoshida A, 1992. Effects of dietary excess histidine and varying levels of copper on the metabolism of lipids and minerals in rats. Bioscience, Biotechnology and Biochemistry, 56, 335–337. [Google Scholar]
- Aoyama Y and Kato C, 2000. Suppressive effect of excess dietary histidine on the expression of hepatic metallothionein‐1 in rats. Bioscience Biotechnology Biochemistry, 64, 588–591. [DOI] [PubMed] [Google Scholar]
- Archer CT, Kim JF, Jeong H, Park JY, Vickers CE, Lee SY and Nielsen LK, 2011. The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome‐scale reconstruction of E. coli. BMC Genomics, 12, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer AP, Ludwig W and Schleifer KH, 2008. A novel DNA microarray design for accurate and straightforward identification of Escherichia coli safety and laboratory strains. Systematic and Applied Microbiology., 31, 50–61. 10.1016/j.syapm.2008.01.001 [DOI] [PubMed] [Google Scholar]
- Bellia F, Amorini AM, La Mendola D, Vecchio G, Tavazzi B, Giardina B, Di Pietro V, Lazzrino G and Rizzarelli E, 2008. New glycosidic derivatives of histidine‐containing dipeptides with antioxidant properties and resistant to carnosinase activity. European Journal of Medicinal Chemistry, 43, 373–380. [DOI] [PubMed] [Google Scholar]
- Boldyrev AA, Aldini G and Derave W, 2013. Physiology and pathophysiology of carnosine. Physiology Review, 93, 1803 –1845. Available online: https://journals.physiology.org/doi/pdf/10.1152/physrev.00039.2012 [DOI] [PubMed] [Google Scholar]
- Breck O, Bjerkås E, Campbell P, Arnesen P, Haldorsen P and Waagbø R, 2003. Cataract preventative role of mammalian blood meal, histidine, iron and zinc in diets for Atlantic salmon (Salmo salar L.). Aquaculture Nutrition, 9, 341–350. [Google Scholar]
- Breck O, Bjerkas E, Sanderson J, Waagbo R and Capmpbell P, 2005a. Dietary histidine affects lens protein turnover and synthesis of N‐acetylhistidine in Atlantic salmon (Salmo salar L.) undergoing parr‐smolt transformation. Aquaculture Nutrition, 11, 321–332. [Google Scholar]
- Breck O, Bjerkas E, Capmpbell P, Rohdes JD, Sanderson J and Waagbo R, 2005b. Histidine nutrition and genotype affect cataract development in Atlantic salmon, Salmo salar L. Journal of Fish Diseases, 28, 357–371. [DOI] [PubMed] [Google Scholar]
- Buttery P and D'Mello J, 1994. Amino acid metabolism in farm animals In: D'Mello J. (ed). Amino acids in farm animal nutrition. Cab International, Wallingford, UK: pp. 1–61. [Google Scholar]
- Dalgaard P, Emborg J, Kjolby A, Sorensen N and Ballin N, 2008. Histamine and biogenic amines ‐ formation and importance in seafood In: Borrensen T. (ed). Improving seafood products for the consumer. Woodhead Publishing Ltd., Cambridge, UK. [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal feed on the safety and the bioavailability of product L‐Histidine monohydrochloride monohydrate for salmonids. EFSA Journal 2005;3(4):195, 10 pp. 10.2903/j.efsa.2005.195 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006a. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal feed on L‐histidine monohydrochloride monohydrate as feed additive for use in salmonids. EFSA Journal 2006;4(11):407, 5 pp. 10.2903/j.efsa.2006.407 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006b. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on the Flavouring Group Evaluation 26 (FGE.26). Consideration of Amino acids from chemical group 34 (Commission Regulation (EC) No 1565/2000 of 18 July 2000). EFSA Journal 2006;8(12):373, 48 pp. 10.2903/j.efsa.2006.373 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008a. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on the Flavouring Group Evaluation 79 (FGE. 79). Consideration of amino acids and related substances evaluated by JECFA (63rd meeting). EFSA Journal 2008;6(11):870, 46 pp. 10.2903/j.efsa.2008.870 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008b. Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with Food (AFC) on amino acids from chemical group 34 Flavouring Group Evaluation 26, Revision 1. EFSA Journal 2008;6(11);790, 51 pp. 10.2903/j.efsa.2008.870 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Assessment of the incidents of histamine intoxication in some EU countries. EFSA supporting publication 2017:EN‐1301, 37 pp. 10.2903/sp.efsa.2017.en-1301 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on risk‐based control of biogenic amine formation in fermented foods. EFSA Journal 2011;9(10):2393, 93 pp. 10.2903/j.efsa.2011.2393. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2011. Scientific Opinion on Flavouring Group Evaluation 96 (FGE.96): Consideration of 88 flavouring substances considered by EFSA for which EU production volumes/anticipated production volumes have been submitted on request by DG SANCO. Addendum to FGE. 51, 52, 53, 54, 56, 58, 61, 62, 63, 64, 68, 69, 70, 71, 73, 76, 77, 79, 80, 83, 84, 85 and 87. EFSA Journal 2011;9(12):1924, 60 pp. 10.2903/j.efsa.2011.1924 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of the use of amino acids (chemical group 34) when used as flavourings for all animal species. EFSA Journal 2014;12(5):3670, 19 pp. 10.2903/j.efsa.2014.3670 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018a. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018b. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Flachowsky G, Glandorf B, Herman L, Mantovani A, Saarela M, Svensson K, Tosti L, Wallace RJ, Anguita M, Tarrés‐Call J and Ramos F, 2019a. Safety and efficacy of L‐histidine monohydrochloride monohydrate produced using Corynebacterium glutamicum KCCM 80172 for all animal species. EFSA Journal 2019;17(7):5783, 20 pp. 10.2903/j.efsa.2019.5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Sanz Y, Villa RE, Woutersen R, Costa L, Cubadda F, Dierick N, Flachowsky G, Mantovani A, Wallace RJ, Manini P, Tarrés‐Call J and Ramos F, 2019b. Safety and efficacy of L‐histidine monohydrochloride monohydrate produced using Corynebacterium glutamicum KCCM 80179 for all animal species based on a dossier submitted by CJ Europe GmbH. EFSA Journal 2019;17(7):5784, 14 pp. 10.2903/j.efsa.2019.5784 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances usedin Animal Feed), Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Glandorf B, Herman L, Maradona Prieto M, Saarela M, Tosti L, Anguita M, Galobart J, Holczknecht O, Manini P, Tarres‐Call J, Pettenati E and Pizzo F, 2019c. Scientific Opinion on the safety and efficacy of L‐histidine monohydrochloride monohydrate produced by fermentation with Escherichia coli (NITE BP‐02526) for all animal species. EFSA Journal 2019;17(8):5785, 22 pp. 10.2903/j.efsa.2019.5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019d. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg J, Dalgaard P and Ahrens P, 2006. Morganella psychrotolerans sp. nov., a histamine producing bacterium isolated from various seafoods. International Journal of Systematic and Evolutionary Microbiology., 56, 2473–2479. [DOI] [PubMed] [Google Scholar]
- European Pharmacopoeia , 9th Edition, 2017. European Directorate for the Quality of Medicines and Health, Monograph 01/2005:0910.
- FAO (Food and Agriculture Organisation of the United Nations), 2013. Joint FAO/WHO Expert Meeting on the Public Health Risks of Histamine and other biogenic ammines from fish and fishery products. Available online: http://www.fao.org/food/food-safety-quality/a-z-index/histamine/en/
- FAO (Food and Agriculture Organization of the United Nations), 2018. Joint FAO/WHO Literature Review Histamine in Salmonids. Available online: http://www.fao.org/3/CA1207EN/ca1207en.pdf
- Farhat M and Kan A, 2013. Effects of varying levels of dietary l‐histidine on growth, feed conversion, protein gain, histidine retention, haematological and body composition in fingerling stinging catfish Heteropneustes fossilis (Bloch).Aquaculture, 404, 130–138. [Google Scholar]
- Feng L, Zhao B, Chen G, Jiang W, Liu Y, Jiang J, Hu K, Li S and Zhou X, 2013. Effects of dietary histidine on antioxidant capacity in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiology and Biochemistry, 39, 559–571. [DOI] [PubMed] [Google Scholar]
- Førde‐Skjærvik O, Skjærvik O, Mørkøre T, Thomassen MS and Rørvik KA, 2006. Dietary influence on quality of farmed Atlantic cod (Gadus morhua): effect on glycolysis and buffering capacity in white muscle. Aquaculture, 252(2–4), 409–420. [Google Scholar]
- Gao Y‐J, Liu Y‐J, Chen X‐Q, Yang H‐J, Li X‐F and Tian L‐X, 2016. Effects of graded levels of histidine on growth performance, digested enzymes activities, erythrocyte osmotic fragility and hypoxia‐tolerance of juvenile grass carp Ctenopharyngodon idella . Aquaculture, 452, 388–394. [Google Scholar]
- Glover CN and Hogstrand C, 2002. Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. Journal of Exploratory Biology, 205, 151–158. [DOI] [PubMed] [Google Scholar]
- Han Y, Koshio S, Ishikawa M and Yokoyama S, 2013. Interactive effects of dietary arginine and histidine on the performances of Japanese flounder Paralichthys olivaceus juveniles. Aquaculture, 414, 173–182. [Google Scholar]
- Health Council of the Netherlands , 2010. Endotoxins. Health‐based recommended occupational exposure limit. The Hague: Health Council of the Netherlands. Publication No 2010/04OSH.
- HSE (Health and Safety Executive), 2013. Occupational hygiene implications of processing waste at materials recycling facilities (MRFs). RR977 Research Report, HSE, London, UK, 41 pp.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2004. Evaluation of certain food additives. Sixty‐third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 928. Geneva, 8–17 June 2004.
- Kanki M, Yoda T, Ishibashi M and Tsukamoto T, 2004. Photobacterium phosphoreum caused a histamine fish poisoning incident. International Journal of Food Microbiology., 92, 79–87. [DOI] [PubMed] [Google Scholar]
- Ketola HG, 1979. Influence of dietary zinc on cataracts in rainbow trout (Salmo gairdneri). Journal of Nutrition, 109, 965–969. [DOI] [PubMed] [Google Scholar]
- Khan MA and Abidi SF, 2009. Optimum histidine requirement of fry African catfish, Clarias gariepinus (Burchell). Aquaculture Research, 40, 1000–1010. [Google Scholar]
- Khan MA and Abidi SF, 2014. Dietary histidine requirement of Singhi, Heteropneustes fossilis fry (Bloch). Aquaculture Research, 45, 1341–1354. [Google Scholar]
- Li Y, Cheng Z, Mai K and Ai Q, 2014. Dietary Histidine Requirement for Juvenile Large Yellow Croaker, Pseudosciaena crocea R. The Israeli Journal of Aquaculture‐Bamidgeh. [Google Scholar]
- López‐Sabater EI, Rodríguez‐Jerez JJ, Hernández‐Herrero M, Roig‐Sagués AX and Mora‐Ventura MT, 1996. Sensory quality and histamine forming during controlled decomposition of tuna (Thunnus thynnus). Journal of Food Protection, 59, 167–174. [DOI] [PubMed] [Google Scholar]
- Michelato M, Zaminhan M, Boscolo W, Nogaroto V, Vicari M, Artoni R, Furuya V and Furuya W, 2016. Dietary histidine requirement of Nile tilapia juveniles based on growth performance, expression of muscle‐growth‐related genes and haematological responses. Aquaculture, 467, 63–70. [Google Scholar]
- Nelson DL and Cox MM, 2008. Lehninger Principles of Biochemistry (5th Edition). New York: WH Freeman.
- NRC (National Research Council), 1994. Nutrient requirements of poultry. 9th revised edn. The National Academies Press, Washington, DC, 176 pp.
- NRC (National Research Council), 2011. Nutrient requirements of fish and shrimp. The National Academies Press, Washington, DC, USA, 376 pp.
- NRC (National Research Council), 2012Nutrient requirement of swine. Eleventh revised edition. The National Academies Press, Washington, DC. 10.17226/13298 [DOI]
- Onodera R, 2003. Essentiality of histidine in ruminants and other animals including human beings. Asian‐Australasian Journal of Animal Science, 16, 445–454. [Google Scholar]
- Remø SC, Hevrøy EM, Olsvik PA, Fontanillas R, Breck O and Waagbø R, 2014. Dietary histidine requirement to reduce the risk and severity of cataracts is higher than the requirement for growth in Atlantic salmon smolts, independently of the dietary lipid source. British Journal on Nutrition., 111, 1759–1772. [DOI] [PubMed] [Google Scholar]
- Roland M, Larsen BK, Holm J, Dalsgaard J and Skov PV, 2015. Effect of plant proteins and crystalline amino acid supplementation on postprandial plasma amino acid profiles and metabolic response in rainbow trout (Oncorhynchus mykiss). Aquaculture International, 23, 1071–1087. [Google Scholar]
- Taylor SL, Stratton JE and Nordlee JA, 1989. Histamine poisoning (scombroid fish poisoning): an allergy‐like intoxication. Journal of Toxicology: Clinical Toxicology, 27, 225–240. [DOI] [PubMed] [Google Scholar]
- Taylor J, Waagbo R, Diez‐Padrisa M, Campbell P, Walton J, Hunter D, Matthew C and Migaud H, 2015. Adult triploid Atlantic salmon (Salmo salar) have higher dietary histidine requirements to prevent cataract development in seawater. Aquaculture Nutrition, 21, 18–32. [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety), 2016. Risk assessment of “other substances” – L‐histidine. Opinion of the Panel on Nutrition, dietetic products, Novel Food an Allergy of the Norwegian Scientific Committee for Food Safety, ISBN: 978‐82‐8259‐214‐7, Oslo, Norway. Available online: https://vkm.no/download/18.645b840415d03a2fe8f2600f/1502800356013/Risk%20assessment%20of%20%22other%20substances%22%20%E2%80%93%20L-histidine.pdf
- Wade AM and Tucker HN, 1998. Antioxidant characteristics of L‐histidine. The Journal of Nutritional Biochemistry, 9, 308–315. [Google Scholar]
- Webb KE, 1990. Intestinal absorption of protein hydrolysis products: a review. Journal of Animal Science, 68, 3011–3022. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2006. Safety evaluation of certain food additives/prepared by the sixty‐third meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA). WHO food additives series: 54. Geneva, Switzerland.
- Zarei M, Mollaie A, Eskandari MH, Pakfetrat S and Shekarforoush S, 2010. Histamine and heavy metals content of canned tuna fish. Global Veterinaria, 5, 259–263. [Google Scholar]
- Zehra S and Khan MA, 2014. Dietary histidine requirement of fingerling Catla catla (Hamilton) based on growth, protein gain, histidine gain, RNA/DNA ratio, haematological indices and carcass composition. Aquaculture Research, 47, 1028–1039. [Google Scholar]
- Zhao B, Feng L, Liu Y, Kuang SY, Tang L, Jiang J, Hu K, Jiang WD, Li SH and Zhou XQ, 2012. Effects of dietary histidine levels on growth performance, body composition and intestinal enzymes activities of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture Nutrition, 18, 220–232. [Google Scholar]


