Abstract
Cattle need physically effective fiber to promote rumination and maintain rumen health, but economics favor the use of low-roughage feedlot diets. The study investigated the optimum barley silage proportion in barley-based finishing diets. Apparent total-tract digestibility (4-d total fecal collection), chewing behavior (6-d video recording), ruminal pH (6-d indwelling pH recording), and fermentation (1 day, sampling 0, 3, 6, 12, and 18 h postfeeding), short-chain fatty acid (SCFA) absorption (washed reticulo-rumen technique), gastrointestinal tract barrier function (marker infusion), and blood variables (catheters) were measured. Eight ruminally fistulated crossbred beef heifers (653 ± 44.2 kg; mean starting body weight [BW] ± SD) were used in a replicated 4 × 4 Latin square design with 28-d periods. Dietary treatments were 0%, 4%, 8%, and 12% of dietary dry matter (DM) as barley silage, with diets containing 80%, 76%, 72%, and 68% barley grain, respectively. Increasing silage proportion decreased dietary starch content from 49.0% to 43.1% DM, while neutral detergent content increased from 22.7% to 25.1% DM. Silage proportion had no effect on DM intake, but apparent DM digestibility decreased quadratically (86.0%, 82.1%, 81.1%, 79.5% for the four diets, respectively; P < 0.001). Although, silage proportion had no effect on eating activity, rumination time increased quadratically (246, 289, 302, 316 min/d; P = 0.04). Increased silage proportion increased minimum (5.07, 5.27, 5.29, 5.41; quadratic, P = 0.011) and mean (5.61, 5.87, 5.93, 5.95; quadratic, P = 0.007) ruminal pH, and there was a quadratic (P ≤ 0.047) decrease in duration and area under the pH acidosis threshold curves of 5.8, 5.5, and 5.2. Although increasing silage proportion decreased ruminal acidosis, it was not completely eliminated even with a diet containing 12% silage DM. SCFA concentration in ruminal fluid was not affected by diet, but silage proportion quadratically (P ≤ 0.088) increased ruminal acetate:propionate. There was no effect of diet on absolute or fractional rates of absorption of acetate, propionate, butyrate or total SCFA, and no effect on gastrointestinal barrier function or blood measurements. In conclusion, responses to roughage level were mostly quadratic with greatest improvements in acidosis variables between 0% and 4% barley silage, with incremental improvements with further increases in silage levels. The study showed a trade-off between maximizing digestibility and energy intake to promote animal performance and minimizing the risk of acidosis.
Keywords: chewing, feedlot, roughage, rumen pH, rumination, short-chain fatty acids
Introduction
The stimulatory effect that roughage has on saliva secretion during eating and rumination is important as it avails bicarbonate and phosphate buffers required for stabilizing ruminal pH in cattle and helps maintain ruminal health (Allen, 1997). Yet roughage inclusion levels in feedlot cattle diets are typically low because economics and management favor the use of grain. The price of roughage is often greater than that of grain when adjusted for digestible energy content. The lower dietary energy concentration of higher roughage diets decreases growth rate and lowers feed conversion efficiency and increases the number of days to market (Stock et al., 1990; Turgeon et al., 2010; Gentry et al., 2016). Increased use of roughage augments the need for feed preparation and handling and requires large investment in silage and hay inventories. Furthermore, increased fecal output due to feeding less digestible diets over a longer feeding period increases the amount of manure that must be handled (Crawford et al., 2008). Consequently, most large feedlots minimize the use of roughage in finishing diets. Feedlots in the United States use mainly corn-based diets containing 6% to 12% roughage (dry matter [DM] basis; Samuelson et al., 2016) while similar proportions of roughage are used by western Canadian feedlots where barley is the main source of grain (Koenig and Beauchemin, 2011).
Low inclusion of roughage in feedlot cattle diets increases the risk of ruminal acidosis, which can compromise animal health and welfare of animals (Galyean and Hubbert, 2014). Consequently, ionophore antibiotics, tetracyclines, and macrolide antibiotics have been used by feedlots to minimize the incidences of digestive disorders and liver abscesses (Reinhardt and Hubbert, 2015; Drouillard, 2018). However, scrutiny of in-feed antimicrobials has increased pressure to prohibit sub-therapeutic feeding of medically important antibiotics. Loss of these technologies may require a change in management, and specifically an increase in the dietary roughage proportion to prevent digestive disease.
While numerous studies have examined roughage inclusion in corn-based finishing diets (Stock et al., 1990; Crawford et al., 2008; Turgeon et al., 2010; Gentry et al., 2016), the optimum proportion of roughage may depend upon source of grain and inclusion of byproduct feeds such as distillers grains (Galyean and Hubbert, 2014). Ruminal pH is typically lower in feedlot cattle fed barley compared with corn because of the greater extent of starch digestion in the rumen for barley (Plascencia et al., 2018). However, few studies have examined optimum levels of roughage in barley-based feedlot diets. Koenig and Beauchemin (2011) reported that increasing the proportion of barley silage from 5% to 20% DM increased mean rumen pH from 5.72 to 5.95, and decreased the duration of pH < 5.8 by 2 h/d. Dried distillers grains with solubles (DDGS) is used in feedlot finishing diets as a protein and energy supplement, but the optimum level of roughage in diets containing DDGS is variable depending upon diet fermentability and particle size (Klopfenstein et al., 2008).
We hypothesized that increasing the proportion of roughage in a barley-based diet containing DDGS would increase rumination time and decrease ruminal acidosis. We further hypothesized that increasing dietary roughage proportion would decrease diet digestibility because of the addition of less digestible forage neutral detergent fiber (NDF), indicating a trade-off between digestible energy intake and healthy rumen function. Therefore, the objective of our study was to evaluate the optimum proportion of roughage (barley silage) in barley grain-based diets containing DDGS, but without inclusion of medically important antibiotics (but with monensin). The responses to roughage inclusion were evaluated by assessing total-tract apparent digestibility of nutrients, chewing behavior, ruminal pH and fermentation, absorptive function of the rumen, gastrointestinal tract barrier function, and blood variables.
Materials and Methods
Animal use for this experiment was approved by the Institutional Animal Care and Use Committee of the Lethbridge Research and Development Centre (Lethbridge, AB; ACC 1404), and was in accordance with the guidelines of the Canadian Council on Animal Care (2009).
Animals, experimental design, and diets
Eight ruminally fistulated crossbred beef heifers (653 ± 44.2 kg; mean body weight [BW] ± SD at the start) were randomly assigned to 1 of 4 treatment diets in a double 4 × 4 Latin square design balanced for residual effects. Experimental periods were 28 d in length with 9 d for dietary adaptation and 19 d for measurements. The sequence of measurements was: days 10 to 16, chewing and ruminal pH; day 17, blood and rumen sampling; days 20 to 22, barrier function; days 20 to 24, total tract digestibility; and day 28, short-chain fatty acid (SCFA) absorption. Prior to the start of the study, the animals were transitioned from diets containing either 70% or 30% silage (DM basis; Chibisa et al., 2015) to a diet containing 12% barley silage DM.
The dietary treatments were four proportions of barley silage: 0%, 4%, 8%, or 12% of dietary DM (Table 1). The four diets also contained 80%, 76%, 72%, or 68% dry-rolled barley grain, respectively, corn DDGS (15% of diet DM) from a single lot, and a pelleted (diameter 6.35 mm) supplement (5% of diet DM) to supply minerals and vitamins. The barley grain was purchased commercially (minimum specified test weight, 57.5 kg/hL) and dry rolled using a processing index (PI) of 82% (defined as the weight of 500 mL of grain after processing/weight of 500 mL of grain before processing × 100%). Monensin (Rumensin premix, Elanco Animal Health, Ontario, Canada) was added to all diets (28 mg/kg DM). The diets were formulated to provide sufficient energy, protein, minerals, and vitamins to exceed the nutrient requirements of animals gaining 1.5 kg/d (NASEM, 2016). Total mixed rations (TMR) were prepared (Data Ranger; American Calan Inc., Northwood, NH) and offered once daily at 1100 hours for ad libitum intake. During the 9-d dietary adaptation in each period, the proportion of barley silage was adjusted upward or downward as appropriate using 3-d steps.
Table 1.
Ingredients and chemical and particle size analysis of the experimental diets
| Barley silage, % of dietary DM | ||||
|---|---|---|---|---|
| Item | 0 | 4 | 8 | 12 |
| Ingredient, % of DM | ||||
| Barley silage | — | 4 | 8 | 12 |
| Barley grain, dry rolled | 80 | 76 | 72 | 68 |
| Corn distillers grains | 15 | 15 | 15 | 15 |
| Supplement (pelleted) | 5 | 5 | 5 | 5 |
| Barley grain | 3.36 | 3.36 | 3.36 | 3.36 |
| Calcium carbonate | 0.15 | 0.15 | 0.15 | 0.15 |
| Salt | 0.05 | 0.05 | 0.05 | 0.05 |
| Feedlot premix1 | 1.31 | 1.31 | 1.31 | 1.31 |
| Vitamin E (500,000 IU/kg) | 0.006 | 0.006 | 0.006 | 0.006 |
| Rumensin premix2 | 0.015 | 0.015 | 0.015 | 0.015 |
| Flavoring agent | 0.003 | 0.003 | 0.003 | 0.003 |
| Chemical analysis, % of DM | ||||
| DM, % as-is | 90.7 ± 0.80 | 86.1 ± 0.63 | 81.2 ± 0.69 | 76.3 ± 0.68 |
| OM | 95.6 ± 0.80 | 96.1 ± 0.33 | 95.1 ± 0.68 | 95.4 ± 0.49 |
| Starch | 49.0 ± 2.24 | 48.2 ± 1.54 | 46.8 ± 1.99 | 43.1 ± 3.19 |
| NDF | 22.7 ± 1.27 | 22.4 ± 1.21 | 23.9 ± 1.31 | 25.1 ± 1.25 |
| ADF | 6.3 ± 0.72 | 6.4 ± 0.48 | 7.3 ± 0.40 | 8.1 ± 0.65 |
| CP | 14.8 ± 0.76 | 14.6 ± 0.67 | 14.8 ± 0.60 | 14.8 ± 0.62 |
| Ether extract | 3.5 ± 0.23 | 3.0 ± 0.09 | 3.0 ± 0.16 | 2.3 ± 0.08 |
| Particle size analysis3 | ||||
| pef8.0 | 0.037 ± 0.0053 | 0.045 ± 0.0053 | 0.055 ± 0.0105 | 0.057 ± 0.0093 |
| peNDF8.0, % of DM | 0.84 ± 0.145 | 1.01 ± 0.114 | 1.32 ± 0.285 | 1.43 ± 0.250 |
| pef1.18 | 0.927 ± 0.0120 | 0.921 ± 0.0165 | 0.925 ± 0.0091 | 0.925 ± 0.0027 |
| peNDF1.18, % of DM | 21.0 ± 1.11 | 20.6 ± 1.10 | 22.1 ± 1.27 | 23.2 ± 1.13 |
| DM retained on sieve4, % | ||||
| 3.35 mm | 24.5 ± 4.27 | 27.6 ± 3.82 | 29.6 ± 3.63 | 32.2 ± 3.80 |
| 2.36 mm | 44.9 ± 2.47 | 42.6 ± 1.95 | 39.9 ± 2.10 | 38.9 ± 2.59 |
| 1.70 mm | 16.4 ± 2.08 | 15.0 ± 1.54 | 14.4 ± 1.46 | 13.9 ± 1.07 |
| 1.18 mm | 5.9 ± 0.74 | 6.0 ± 0.64 | 6.4 ± 0.66 | 6.2 ± 0.50 |
| 850 µm | 3.3 ± 0.70 | 3.4 ± 1.00 | 3.7 ± 0.76 | 3.6 ± 0.46 |
| Pan | 5.2 ± 1.77 | 5.3 ± 2.38 | 6.0 ± 1.76 | 5.2 ± 0.76 |
1Feedlot premix provided an additional 14 g/kg Ca, 103 mg/kg Zn, 26 mg/kg Cu, 47 mg/kg Mn, 1 mg/kg I, 0.50 mg/kg Se, 0.33 mg/kg Co, 17,187 IU/kg vitamin A, 859 IU/kg vitamin D3, and 24 IU/kg vitamin E to the diet DM.
2Rumensin premix (Elanco Animal Health, Ontario, Canada) supplied 28 mg monensin/kg dietary DM.
3Particle size distribution of TMR measured using the Penn State Particle Separator; pef8.0 and pef1.18 = physical effectiveness factor (ranging from 0 to 1) determined as the total proportion of particles retained on 2 sieves (19 and 8 mm) and 3 sieves (19, 8, and 1.18 mm, respectively; peNDF8.0 and peNDF1.18 = physically effective NDF determined as NDF content of TMR multiplied by pef8.0 and pef1.18, respectively.
4Determined by dry sieving through a series of sieves arranged in descending mesh size.
The barley silage was chopped at a theoretical cut length of 5 mm and stored in a bunker silo. The silage was sampled on two consecutive days each week to determine DM content (oven drying at 55 °C for 48 h), and the barley silage inclusion level (as-fed basis) was adjusted if the DM content of the silage deviated by >3% from the average. Fresh water was available at all times throughout the experiment. All heifers were housed in individual tie-stalls that contained rubber mattresses bedded with wood shavings. However, bedding was not provided during total urine and fecal sample collection days. When weather and sampling permitted, heifers were released to an outdoor pen for 2 to 3 h of exercise daily. Heifers were weighed on two consecutive days (prior to feeding, nonfasted) at the beginning of each period and at the end of the study.
Intake
Feed intake was measured daily by recording the amounts of feed offered and refusals remaining at the end of each day. Total mixed ration and refusal samples were collected daily and composited for each 3-d dietary step during the 9-d adaptation period. Thereafter, weekly TMR and refusal samples were collected over two and five consecutive days, respectively, with a proportion of the collected weekly TMR composited by period. Barley silage, barley grain, and supplement samples were also collected once weekly, and composited by period. All samples were stored at −20 °C for later DM determination by oven drying at 55 °C for 72 h to calculate DMI.
Apparent total tract nutrient digestibility
Total collection of urine and feces was conducted over four consecutive days (days 20 to 24) during each period. On day 20, heifers were fitted with urinary indwelling catheters (Bardex Lubricath Foley catheter, balloon size: 75 cm3, catheter diameter: 8.7 mm; Bard Canada Inc., Oakville, ON, Canada) at 0800 hours to collect the urine and prevent contamination of feces. The bladder catheters were connected (1000 hours) through tubing to 20-liter plastic buckets containing a sufficient quantity of acid (~0.5 liter of 4 N H2SO4) to reduce pH to < 2.0. The excreted feces were collected using containers placed behind each heifer and weighed daily. The feces were then thoroughly mixed for each heifer before collection of a 1-kg sub-sample that was stored at −20 °C for later chemical analysis. In addition, a 50-g subsample of feces was also collected daily, composited by period, and stored at –20 °C until particle size distribution analysis.
Chewing behavior and activity
Animal behavior was continuously monitored for 6 d using a digital video recording system (model SRD-470D, Samsung Techwin Co, Ltd., Changwon, Gyengsangnam-do, South Korea) starting at 1100 hours on day 10 of each period and ending at 1100 hours on day 16. A color CCTV camera (model WV-CP484, Panasonic Corp., Kadoma, Osaka, Japan) mounted on a tripod was placed on a wall shelf ~3 m in front of each tie-stall at a height of 2 m, which allowed full view of each heifer. To enable video recording at night, three 1,400 lumen lights were placed in front of the stalls. All video footages were downloaded and reviewed by a trained observer to eliminate interobserver discrepancies. To determine intra-observer reliability, the trained observer watched a subset of the data (eight cows for 5 h) before repeating the observations from the same video 28 d later. Intra-observer correlation (PROC CORR; SAS Institute Inc., Cary, NC) was high (r = 0.99, P < 0.001) for all behavioral observations. Each tape was reviewed continuously and the start and stop times of the different behavioral events for each heifer were recorded. The total time spent performing each behavioral activity over 24 h was then expressed in minutes. Behavioral activities were classified according to Dong et al. (2018) as follows: eating, muzzle in the bunk or over the bunk chewing or swallowing; drinking, muzzle in the water bowl or swallowing water; rumination, regurgitation, chewing, and swallowing of a bolus; standing, upright body supported by at least three legs; and lying, body contact with the ground on the left or right side. The time spent eating, ruminating, and chewing (eating + ruminating) per kilogram of DM, NDF, and physically effective NDF (peNDF) intake, and time spent drinking, standing, and lying are reported.
Ruminal pH
Ruminal pH was measured continuously from days 10 to 16 of each period using indwelling pH data loggers (Lethbridge Research and Development Centre pH data logger system, Dascor, Escondido, CA; Penner et al., 2006). At the beginning of each measurement period, the loggers were standardized in pH 4 and 7 buffers and programmed to record pH every minute. The loggers were then placed in the ventral sac of the rumen. Upon retrieval, electrodes were checked and cleaned, pH data were downloaded, and loggers were re-standardized to account for any signal drift. The recordings were adjusted to reflect the pre- and poststandardization values, assuming any drift that occurred during the measurement period was linear.
Ruminal fermentation characteristics
Approximately 0.5 liter of ruminal contents were collected from four different sites (cranial, caudal, ventral, and dorsal) at 0, 3, 6, 12, and 18 h postfeeding on day 17. Samples were immediately strained through four layers of sterile cheesecloth. Thereafter, 5-mL subsamples of filtrate were preserved with either 1 mL of 25% (w/v) metaphosphoric acid for SCFA and lactate or 1 mL of 1% (v/v) H2SO4 for ammonia-nitrogen (NH3-N) analysis. Samples were then stored at −20 °C until analyzed.
SCFA absorption
The temporarily isolated and washed reticulo-rumen (WRR) technique as described by Care et al. (1984) was used to determine ruminal SCFA absorption on day 28 of each period. Briefly, reticulo-ruminal contents were completely evacuated through the cannula at 0700 hours into insulated and covered plastic containers and weighed. Warm water bottles were placed in the insulated containers to maintain digesta temperature. The digesta was thoroughly mixed and two 1-kg subsamples were collected and oven-dried (55 °C for 72 h) to calculated ruminal liquid volume.
The evacuated reticulo-rumen was washed three times with water (25 liter; 38 °C), followed by three washes using a buffer solution (15 liter; 38 °C). The wash buffer contained (mM) NaCl (100), NaHCO3 (25), sodium acetate (30), and sodium propionate (15). Theoretical osmolality of the buffer was 310 mOsm/kg and its pH was adjusted to 6.2 by adding HCl. After each wash, the wash buffer was removed from the reticulo-rumen using a wet-dry vacuum. Isolation of the reticulo-rumen was achieved by placement of a custom-made esophageal-occluding device (University of Leipzig, Leipzig, Germany) in the distal esophagus. Once in place, an inflatable cuff on the occluding device prevented the passage of saliva into the reticulo-rumen, but allowed its aspiration into a plastic container using a vacuum pump (UN86KT.45P; KNF Neuberger Inc., Trenton, NJ). To prevent passage of experimental buffer from the reticulo-rumen, a bladder catheter (Bardex Lubricath Foley catheter, balloon size: 75 cm3, catheter diameter: 8.7 mm; Bard Canada Inc.) was placed in the omasal orifice before the balloon was inflated. Following isolation, a final wash (5 liters of wash buffer) was conducted and the remaining buffer was then removed completely from the reticulo-rumen. Subsequently, 15 liters of buffer (38 °C; adjusted to pH 6.2) containing (mM) Cr-EDTA (1) as a volume marker, CaCl2 · 2H2O (2), MgCl2 (2), NaCl (5), KCl (5), Na-acetate (30), K-acetate (35), Na-propionate (35), Na-butyrate (8), butyric acid (7), l-lactic acid (5), and NaHCO3 (25) were placed in the reticulo-rumen. Experimental buffer samples (35 mL) were collected into 50-mL centrifuge tubes containing 7 mL of 25% (w/v) H2PO4 prior to, and at 5 and 50 min after incubation in the reticulo-rumen. Samples were immediately stored at −20 °C for later Cr and SCFA analyses. After collection of the last sample, residual ruminal buffer was removed by vacuuming it out. Total saliva secreted during buffer incubation was mixed with the stored reticulo-ruminal contents and transferred back to the rumen.
Total gastrointestinal barrier function
Two liters of 180 mM Cr-EDTA solution were dosed into the rumen on day 20 and used as a paracellular permeability marker for assessment of barrier function (Saunders et al., 1994). Following infusion, 24-h urine output was measured, and daily 40-mL subsamples were collected over the next 48 h (days 21 and 22) for later Cr analysis. The 48-h total Cr output was then calculated based on urine output and Cr concentration.
Blood metabolites
On day 17 of each period, heifers were fitted with jugular catheters (V-CATH Peripherally Inserted Central Catheter; NeoMedical Inc., Fremont, CA) using a peel away introducer over the needle (Mila International Inc., Erlanger, KY). Thereafter, blood was collected at 0, 3, 6, 12, and 18 h postfeeding into one 6-mL tube containing 10.8 mg K2-EDTA (Becton Dickinson, Franklin Lakes, NJ), two 10-mL tubes containing 158 IU lithium heparin (Becton Dickinson), and one 10-mL tube with no preservative (Becton Dickinson). Following sampling, catheters were immediately filled with heparinized (20 IU/mL) 0.9% saline to prevent clotting.
Prior to processing, the K2-EDTA and lithium heparin tubes were stored briefly on ice while the tubes with no preservative were first incubated at room temperature for 20 min. The lithium heparin tubes were centrifuged at 3,000 × g for 20 min at 4 °C before harvest and subsequent storage (–20 °C) of 1-mL plasma aliquots in pyrogen-free tubes for later analysis of glucose, Immunoglobin (Ig)A, IgG, and IgM, and osmolality. Following centrifugation (3,000 × g; 20 min; 4 °C), 1-mL aliquots of serum were also harvested from the tubes with no preservative and stored in pyrogen-free tubes at –20 °C until analyzed for β-hydroxybutyrate (BHBA) and nonesterified fatty acids (NEFA).
Laboratory analyses
At the end of the study, 1-kg subsamples of composited ingredient and TMR samples were used for particle size distribution analysis using the Penn State Particle Separator (PSPS) with three sieves (19, 8, and 1.18 mm; Lammers et al., 1996) and by dry sieving through a series of sieves (apertures of 3.35, 2.36, 1.70, 1.18, and 0.85 mm; W. S. Tyler, Inc., Mentor, OH) arranged in descending mesh size (Ro-Tap Sieve Shaker, Laval Lab, Laval, QC). Using the same series, particle size distribution of fecal samples was determined by wet sieving with a fine spray of water applied to each sieve. Following drying, the weight of particles retained on each screen was then expressed as a proportion of the original sample dry weight.
Composited TMR and refusals (collected during total urine and feces collection) and daily fecal samples were oven dried at 55 °C for 72 h for chemical analysis. Dried fecal samples were pooled based on their respective DM contents to obtain a representative composite sample by animal within period, which together with the dried TMR and refusals samples were sequentially ground through 4- and 1-mm screens (Wiley mill, standard model 4, Arthur H. Thomas Co., Philadelphia, PA). All ground samples were then analyzed for analytical DM by drying at 135 °C for 2 h (AOAC, 2005; method 930.15). Samples were combusted at 600 °C for at least 5 h to determine ash content, and the organic matter (OM) content was calculated by difference (DM – ash; AOAC, 2005; method 942.05). Samples were also analyzed for acid detergent fiber (ADF) and NDF, with amylase and sodium sulfite used during NDF determination (AOAC, 2005; method 2002.04). In preparation for crude protein (CP) (N × 6.25) and starch analyses, samples were pulverized using a ball mill (Mixer Mill MM2000; Retsch, Haan, Germany). For quantification of total N, flash combustion with gas chromatography and thermal conductivity detection (Carlo Erba Instruments, Milan, Italy) were used. To measure total starch, an enzymatic method that involved hydrolysis of starch using amylase, reaction of the resultant free glucose with glucose oxidase/peroxidase (No. P7119, Sigma, St. Louis, MO) and dianisidine dihydrochloride, and measurement of absorbance at 540 nm using a plate reader (SpectraMax 190, Molecular Devices Corp., Sunnyvale, CA) was used. Crude fat was determined according to AOAC (2005; method 920.39) using ether extraction (Extraction Unit E-816 HE; Büchi Labortechnik AG, Flawil, Switzerland).
The concentration of glucose in plasma was determined colorimetrically using the glucose oxidase method (Procedure No. 1070; Stanbio Laboratory, Boerne, TX). To quantify serum BHBA concentration, BHBA dehydrogenase (No. H6501; Roche, Mississauga, Ontario, Canada) was used to catalyze a reaction that oxidized BHBA to acetoacetate and reduced NAD to NADH. The resultant color change was measured using a plate reader at 340 nm. The IgA, IgM, and IgG concentrations in blood plasma were measured using bovine IgA, IgM, and IgG solid-phase sandwich ELISA quantitation kits (Bethyl Laboratories Inc., Montgomery, TX) according to the manufacturer`s instructions. A commercial kit was used to determine serum NEFA concentration (HR Series NEFA-HR2; Wako Chemical, Atlanta, GA). Plasma osmolality (mOsm/kg) was determined by freezing-point depression (Advanced Instruments 3250; Advanced Instruments, Norwood, MA), with standards (290 and 500 mOsm/kg) analyzed at the beginning and end of each day for quality control.
Concentrations of SCFA and lactate in ruminal fluid and SCFA in buffer samples were determined using a Hewlett Packard model 5890A Series Plus II gas liquid chromatograph (Hewlett Packard Co., Palo Alto, CA) fitted with a Zebron capillary column (ZB-FFAP; 30 M × 0.32 mm i.d. × 1.0 μm phase thickness; Phenomenex, Torrance, CA). Crotonic acid was used as an internal standard. The salicylate-nitroprusside-hypochlorite method using a flow injection analyzer (Rhine et al., 1998) was used for measurement of ruminal NH3-N concentration. Ruminal fluid, urine, and buffer Cr concentration were determined using atomic absorption spectroscopy (iCE 3000 series, Thermo Fisher Scientific Inc., Waltham, MA).
Calculations
Physically effective NDF contents of barley silage and TMR were expressed as peNDF1.18 and peNDF8, calculated as NDF content (DM basis) multiplied by the respective measured physically effectiveness factor (ranging from 0 to 1; pef1.18, sum of the proportions of material retained on 19, 8, and 1.18 mm sieves, Kononoff et al., 2003; pef8, sum of the proportions of material retained on 19 and 8 mm sieves, Lammers et al., 1996).
Ruminal pH data for each heifer were summarized by period as daily minimum, mean, maximum, and range. Additionally, the duration (h/d), area under the curve (AUC; pH × h), and acidosis index (pH × h/kg DMI; Chibisa et al., 2015) were calculated using pH thresholds of 5.2 (acute acidosis; Owens et al., 1998), 5.5 (subacute acidosis; Schwaiger et al., 2013), and 5.8 (mild acidosis; Zebeli et al., 2012).
To determine the actual volume of ruminal buffer during the WRR procedure, the Cr concentration of buffer samples collected following 5 and 50 min of incubation were used. The disappearance or absorption rates of acetate, propionate, and butyrate were then calculated according to the following formulas:
where V is the volume of buffer and C is the concentration of acetate, propionate, or butyrate at the respective time.
Statistical analysis
Data were analyzed as a replicated 4 × 4 Latin square using the mixed procedure of SAS (Proc mixed; SAS Institute Inc. ). For all variables that did not have repeated measures over time, the following model was used:
where Yijkl is the dependent variable, μ is the overall mean, Si is the fixed effect of square i, Pj is the fixed effect of periodj, Ck(i) is the random effect of cow k (within square i), Tl is the fixed effect of dietary treatment l, STil is the interaction between square i and treatment l, and Eijkl is the error term assumed to be normally distributed, with mean = 0 and constant variance. Variables that were measured over time (feeding behavior, ruminal pH, SCFA absorption and clearance, nutrient digestibility, barrier function, and blood metabolites) were analyzed accounting for repeated measures by including the variable time or day in the repeated statement, as well as terms for time (hour or day), and interaction (treatment × time or day) in the model described previously. For each response variable, the variance–covariance structure of the repeated measures was modeled separately, with an appropriate structure fitted using the lowest values of the fit statistics based on the Bayesian information criteria. The interaction terms were removed from the model when P ≥ 0.25. Orthogonal contrasts were used to test for linear and quadratic effects of level of barley silage in the diet. Statistical differences were declared at P < 0.05 and differences between treatments at 0.05 < P ≤ 0.10 were considered as tendencies toward significance. All reported values are least squares means ± SEM.
Results
Diet composition
Dietary ingredient and chemical composition are presented in Table 1. Although statistical analysis was not conducted, diets differed numerically in composition and physical characteristics. By substituting barley grain with increasing proportions of barley silage (0%, 4%, 8%, and 12%; DM basis), dietary starch content decreased from 49.0% to 43.1% DM due to the lower starch content of the barley silage compared with barley grain (53.3 vs. 14.1% DM; Table 2). Although the NDF content of barley silage was more than twice that of barley grain, there was only a small increase (from 22.7% to 25.1% DM) in dietary NDF content with increasing proportion of barley silage (Table 1). Similarly, there was only a small increase in dietary peNDF8 (from 0.84% to 1.43% DM) and peNDF1.18 (from 21.0% to 23.2% DM) contents with increasing proportion of silage. The greatest change in particle size due to increased proportion of barley silage occurred for the proportion of DM retained on the 3.35 mm sieve, which increased from 24.5% for control to 32.2% for 12% silage DM (+31%).
Table 2.
Chemical and particle size analysis of dietary ingredients
| Item | Barley silage | Corn DDGS | Barley grain (PI-82)1 | Supplement |
|---|---|---|---|---|
| Chemical analysis, % of DM | ||||
| DM, % | 42.1 | 92.0 | 91.9 | 93.6 |
| OM | 92.0 | 95.2 | 97.5 | 59.1 |
| CP | 12.4 | 29.5 | 12.8 | 7.50 |
| NDF | 52.7 | 36.6 | 21.1 | 11.2 |
| ADF | 29.3 | 12.1 | 5.5 | 3.9 |
| Starch | 14.1 | 2.01 | 53.3 | 31.8 |
| Ether extract | 1.7 | 9.3 | 1.6 | 1.1 |
| Penn State Particle Separator2 | ||||
| Retained on sieve, % as-is | ||||
| 19 mm | 1.70 | |||
| 8 mm | 56.7 | |||
| 1.18 mm | 40.8 | |||
| pan | 0.81 | |||
| pef8.0 | 0.584 | |||
| peNDF8.0, % of DM | 30.8 | |||
| pef1.18 | 0.992 | |||
| peNDF1.18, % of DM | 52.3 | |||
| DM retained on sieve3, % | ||||
| 3.35 mm | 40.7 | 10.2 | 26.8 | |
| 2.36 mm | 29.0 | 17.0 | 56.0 | |
| 1.70 mm | 17.4 | 17.0 | 14.4 | |
| 1.18 mm | 8.70 | 19.4 | 1.90 | |
| 850 µm | 3.52 | 15.0 | 0.512 | |
| Pan | 0.68 | 21.4 | 0.48 |
1PI, processing index defined as the weight of 500 mL of grain after processing/weight of 500 mL of grain before processing ×100%
2Particle size distribution of barley silage measured using the Penn State Particle Separator; pef8.0 and pef1.18 = physical effectiveness factor determined as the total proportion of particles retained on 2 sieves (19 and 8 mm) and 3 sieves (19, 8, and 1.18 mm), respectively; peNDF8.0 and peNDF1.18 = physically effective NDF determined as NDF content of TMR multiplied by pef8.0 and pef1.18, respectively.
3Determined by dry sieving through a series of sieves arranged in descending mesh size.
Nutrient intake and apparent total tract digestibility
Increasing dietary silage proportion had no effect on intakes of DM, OM, NDF, or CP, but intake of ADF increased (P = 0.011) while starch intake tended (P = 0.052) to decrease (Table 3). There was a quadratic decrease (P ≤ 0.033) in apparent total tract DM, OM, NDF, CP, and starch digestibility as silage proportion increased from 0% to 12% of dietary DM. In addition, there was a tendency (quadratic; P = 0.085) for decreased ADF digestibility as proportion of barley silage increased.
Table 3.
BW, nutrient intake and total-tract apparent digestibility in feedlot cattle fed diets varying in percentage of barley silage
| Barley silage, % of dietary DM | P-value1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| BW, kg | 726 | 724 | 720 | 745 | — | — | — | — |
| Intake, kg/d | ||||||||
| DM | 10.5 | 10.9 | 11.1 | 10.7 | 0.47 | 0.52 | 0.15 | 0.96 |
| OM | 9.7 | 10.0 | 9.4 | 10.1 | 0.57 | 0.51 | 0.54 | 0.17 |
| ADF | 0.655 | 0.675 | 0.725 | 0.864 | 0.0604 | 0.011 | 0.92 | 0.077 |
| NDF | 2.36 | 2.37 | 2.38 | 2.71 | 0.155 | 0.12 | 0.60 | 0.15 |
| CP | 1.50 | 1.50 | 1.45 | 1.58 | 0.093 | 0.64 | 0.49 | 0.37 |
| Starch | 5.09 | 5.18 | 4.71 | 4.54 | 0.289 | 0.052 | 0.51 | 0.81 |
| Digestibility, % | ||||||||
| DM | 86.0 | 82.1 | 81.1 | 79.5 | 1.01 | <0.001 | <0.001 | <0.001 |
| OM | 87.2 | 83.7 | 82.4 | 81.1 | 0.93 | <0.001 | <0.001 | <0.001 |
| ADF | 53.5 | 40.2 | 41.9 | 44.3 | 3.45 | 0.023 | 0.009 | 0.085 |
| NDF | 68.9 | 59.5 | 59.3 | 59.0 | 2.50 | 0.010 | 0.008 | 0.033 |
| CP | 81.7 | 76.8 | 77.3 | 75.9 | 1.59 | 0.018 | 0.038 | 0.020 |
| Starch | 98.9 | 98.0 | 98.0 | 96.8 | 0.28 | <0.001 | 0.067 | <0.001 |
1 P-values indicate overall silage, linear, and quadratic effects.
Fecal particle size
Increasing the silage proportion quadratically (P = 0.047) increased the proportion of longer particles (3.35-mm sieve) in feces at the expense of medium particles (2.36 and 1.70 mm sieve; P ≤ 0.025), which tended to linearly decrease (P ≤ 0.072; Table 4).
Table 4.
Physical characteristics of feces from feedlot cattle fed diets varying in percentage of barley silage as determined by wet sieving
| Barley silage, % of dietary DM | P-value1 | |||||||
|---|---|---|---|---|---|---|---|---|
| DM retained on sieve2, % | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| 3.35 mm | 24.5 | 27.6 | 29.6 | 32.2 | 1.37 | 0.004 | 0.088 | 0.047 |
| 2.36 mm | 44.9 | 42.6 | 39.9 | 38.9 | 0.81 | <0.001 | 0.005 | 0.058 |
| 1.70 mm | 16.4 | 15.0 | 14.4 | 13.9 | 0.56 | 0.025 | 0.072 | 0.11 |
| 1.18 mm | 5.85 | 6.01 | 6.36 | 6.22 | 0.227 | 0.41 | 0.20 | 0.96 |
| 850 µm | 3.28 | 3.37 | 3.71 | 3.57 | 0.267 | 0.67 | 0.38 | 0.93 |
| Pan | 5.16 | 5.32 | 5.97 | 5.20 | 0.624 | 0.78 | 0.37 | 0.63 |
1 P-values indicate overall silage, linear, and quadratic effects.
2Determined by applying a fine spray of water to each sieve arranged in descending mesh size.
Chewing behavior and activity
Increasing the proportion of silage in the diet had no effect on eating activity but rumination time increased quadratically (P = 0.04; Table 5). Expressing rumination time relative to intake of DM, NDF, and peNDF1.18 showed a tendency for linear increase (P ≤ 0.100) with increasing proportion of barley silage, while minutes per kilogram of peNDF8 intake (P = 0.091) tended to differ among treatments, but not in a consistent manner. Total chewing time was not affected by treatment, even when expressed relative to DM, NDF, or peNDF1.18 intake, although there was a tendency (P = 0.08) for chewing per kilogram of peNDF8 intake to differ among treatments, but not in a linear or quadratic pattern. Time spent drinking, standing, and lying were not affected by diet (P ≥ 0.67).
Table 5.
Chewing behavior and activity of feedlot cattle fed diets varying in percentage of barley silage observed for 6 days using a digital video recording system
| Barley silage, % of dietary DM | P-value1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item2 | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| Eating | ||||||||
| Min/d | 128 | 111 | 121 | 118 | 17.2 | 0.91 | 0.72 | 0.56 |
| Min/kg of DMI | 13.0 | 10.9 | 12.8 | 11.3 | 1.84 | 0.81 | 0.90 | 0.34 |
| Min/kg of NDFI | 54.0 | 47.7 | 52.2 | 44.1 | 6.91 | 0.74 | 0.96 | 0.31 |
| Min/kg of peNDF8.0 intake | 1,689 | 1,100 | 994 | 826 | 288.6 | 0.19 | 0.17 | 0.20 |
| Min/kg of peNDF1.18 intake | 63.0 | 54.4 | 60.4 | 50.4 | 8.35 | 0.71 | 0.92 | 0.28 |
| Ruminating | ||||||||
| Min/d | 246 | 289 | 302 | 316 | 13.5 | 0.008 | 0.024 | 0.04 |
| Min/kg of DMI | 25.1 | 29.0 | 32.8 | 30.1 | 2.31 | 0.068 | 0.019 | 0.75 |
| Min/kg of NDFI | 106 | 125 | 133 | 118 | 9.2 | 0.100 | 0.017 | 0.76 |
| Min/kg of peNDF8.0 intake | 3,052 | 2,880 | 2,584 | 2,176 | 265.9 | 0.091 | 0.52 | 0.24 |
| Min/kg of peNDF1.18 intake | 123 | 143 | 154 | 135 | 10.7 | 0.090 | 0.014 | 0.96 |
| Chewing | ||||||||
| Min/d | 373 | 399 | 423 | 434 | 23.3 | 0.28 | 0.28 | 0.42 |
| Min/kg of DMI | 38.0 | 39.8 | 41.6 | 45.5 | 3.34 | 0.43 | 0.17 | 0.76 |
| Min/kg of NDFI | 159 | 173 | 185 | 162 | 12.8 | 0.46 | 0.13 | 0.71 |
| Min/kg of peNDF8.0 intake | 4,743 | 3,981 | 3,579 | 3,011 | 482.6 | 0.081 | 0.23 | 0.16 |
| Min/kg of peNDF1.18 intake | 185 | 197 | 214 | 186 | 15.1 | 0.46 | 0.14 | 0.57 |
| Drinking | 12.6 | 13.4 | 13.1 | 12.5 | 1.70 | 0.92 | 0.61 | 0.91 |
| Standing | 388 | 387 | 383 | 414 | 34.7 | 0.67 | 0.62 | 0.44 |
| Lying | 1,052 | 1,053 | 1,057 | 1,026 | 34.7 | 0.67 | 0.62 | 0.44 |
1 P-values indicate overall silage, linear, and quadratic effects.
2Intakes were for the days on which behaviors were monitored.
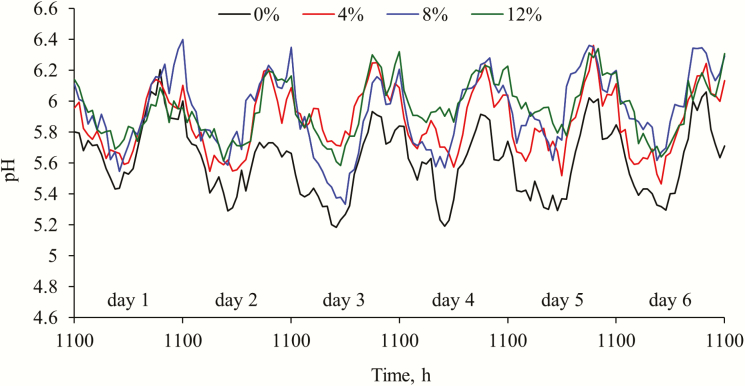
Ruminal pH
Diurnal changes in ruminal pH during the 6-d measurement period are shown in Figure 1. An increase in dietary silage proportion resulted in an increase in minimum (quadratic; P = 0.011), mean (quadratic; P = 0.007), and maximum (linear; P = 0.004) ruminal pH, but did not affect pH range (Table 6). Additionally, there was a decrease in duration and AUC for pH < 5.8, 5.5, and 5.2 (quadratic; P ≤ 0.047), as well as the acidosis index (quadratic; P ≤ 0.035) as dietary barley silage content increased.
Figure 1.
Diurnal changes in ruminal pH during the 6-d measurement period for feedlot cattle fed diets containing 0%, 4%, 8%, and 12% barley silage (DM basis). Feeding time was 1100 hours each day. Data were plotted by hour to smooth the curves. There was an increase in minimum (quadratic; P = 0.01), mean (quadratic; P = 0.01), and maximum pH (linear; P < 0.01) as dietary silage proportion increased. However, there was no day (P ≥ 0.26) or day × diet (P ≥ 0.13) effect on minimum, mean, and maximum pH. Ruminal pH data averaged across the 6-d measurement period to represent a 24-h feeding cycle is reported in Table 6.
Table 6.
Ruminal pH in feedlot cattle fed diets varying in percentage of barley silage measured for 6 days using indwelling pH data loggers
| Barley silage, % of dietary DM | P-value1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| Ruminal pH | ||||||||
| Minimum | 5.07 | 5.27 | 5.29 | 5.41 | 0.082 | 0.003 | 0.043 | 0.011 |
| Mean | 5.61 | 5.87 | 5.93 | 5.95 | 0.144 | <0.001 | <0.001 | 0.007 |
| Maximum | 6.38 | 6.50 | 6.57 | 6.56 | 0.143 | 0.005 | 0.004 | 0.15 |
| Range | 1.31 | 1.23 | 1.28 | 1.15 | 0.091 | 0.33 | 0.99 | 0.12 |
| Duration, h/d | ||||||||
| pH < 5.8 | 16.7 | 11.4 | 9.8 | 8.3 | 3.64 | <0.001 | <0.001 | 0.002 |
| pH < 5.5 | 11.3 | 4.2 | 4.2 | 4.0 | 2.41 | <0.001 | <0.001 | 0.002 |
| pH < 5.2 | 3.66 | 0.51 | 0.88 | 1.00 | 0.684 | 0.001 | 0.001 | 0.009 |
| Area, pH × h/d | ||||||||
| pH < 5.8 | 6.99 | 2.98 | 2.84 | 2.67 | 1.456 | <0.001 | <0.001 | 0.001 |
| pH < 5.5 | 2.72 | 0.64 | 0.77 | 0.85 | 0.527 | <0.001 | <0.001 | 0.002 |
| pH < 5.2 | 0.503 | 0.064 | 0.105 | 0.148 | 0.1018 | 0.011 | 0.006 | 0.047 |
| Acidosis index, (pH × h/d)/kg DMI | ||||||||
| pH < 5.5 | 0.263 | 0.060 | 0.067 | 0.074 | 0.0497 | <0.001 | <0.001 | 0.001 |
| pH < 5.2 | 0.049 | 0.006 | 0.009 | 0.013 | 0.0098 | 0.006 | 0.003 | 0.035 |
1 P-values indicate overall silage, linear, and quadratic effects. There was no day (P ≥ 0.26) or day × diet (P ≥ 0.13) effect on pH variables.
Ruminal fermentation characteristics
There was no diet effect (P > 0.19) on ruminal total SCFA, isobutyrate, lactate, or NH3-N concentration (Table 7). However, increasing barley silage content of the diets quadratically (P ≤ 0.05) increased ruminal acetate, butyrate, and the acetate:propionate ratio and tended (P = 0.088) to increase isovalerate concentrations. In contrast, ruminal concentration of propionate and valerate decreased with increasing proportions of silage (quadratic; P ≤ 0.007).
Table 7.
Mean ruminal fermentation characteristics in feedlot cattle fed diets varying in percentage of barley silage for samples taken 0, 3, 6, 12, and 18 h postfeeding on day 17 of the study
| Barley silage, % of dietary DM | P-value1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| SCFA | ||||||||
| Total3, mM | 133 | 137 | 137 | 127 | 5.4 | 0.29 | 0.24 | 0.44 |
| Acetate3,4, % | 42.6 | 46.6 | 46.4 | 49.5 | 1.32 | 0.005 | 0.074 | 0.005 |
| Propionate3, % | 44.3 | 39.8 | 38.8 | 35.3 | 1.95 | 0.002 | 0.052 | 0.007 |
| Butyrate3, % | 7.3 | 8.7 | 9.2 | 10.5 | 0.86 | 0.003 | 0.077 | 0.013 |
| Isobutyrate3, % | 0.740 | 0.803 | 0.826 | 0.916 | 0.0980 | 0.19 | 0.56 | 0.18 |
| Valerate, % | 3.37 | 2.38 | 2.26 | 1.77 | 0.300 | 0.002 | 0.045 | 0.003 |
| Isovalerate3, % | 1.24 | 1.56 | 1.78 | 2.23 | 0.268 | 0.029 | 0.35 | 0.088 |
| Caproate3, % | 0.335 | 0.211 | 0.259 | 0.231 | 0.0433 | 0.019 | 0.047 | 0.009 |
| Acetate:propionate3 | 0.98 | 1.22 | 1.29 | 1.50 | 0.109 | 0.005 | 0.12 | 0.020 |
| Lactate3, mM | 0.519 | 0.531 | 0.533 | 0.478 | 0.0895 | 0.76 | 0.60 | 0.60 |
| NH3-N3, mg/dL | 6.81 | 6.17 | 7.03 | 6.75 | 1.070 | 0.93 | 0.98 | 0.63 |
1 P-values indicate overall silage, linear, and quadratic effects.
3There were significant effects of sampling time (P < 0.001).
4There was a significant silage × time of sampling interaction (P < 0.05).
Digesta weight, ruminal SCFA absorption, and barrier function
There was a 4 kg increase (P = 0.024) in ruminal liquid for animals fed the 12% compared with 0% barley silage diet, with intermediate weights for cattle fed 4% and 8% silage diets (Table 8). Increased liquid resulted in a tendency (P = 0.064) for an increase in the total fresh weight of ruminal contents, but the DM pool was not affected by diet. There was no diet effect (P ≥ 0.10) on the absolute (mmol/h) or fractional (%/h) rates of absorption of acetate, propionate, butyrate, and total SCFA. In addition, increasing the proportion of silage in the diet had no effect (P = 0.96) on urinary Cr excretion, an indicator of gastrointestinal barrier function.
Table 8.
Ruminal digesta weight and absorptive capacity in feedlot cattle fed diets varying in percentage of barley silage
| Barley silage, % of dietary DM | P-value1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| Digesta, kg | ||||||||
| Total | 41.4 | 42.5 | 43.2 | 45.6 | 2.21 | 0.064 | 0.60 | 0.10 |
| DM pool | 6.55 | 6.88 | 6.40 | 6.50 | 0.570 | 0.71 | 0.97 | 0.50 |
| Liquid pool | 34.9 | 35.6 | 36.8 | 39.1 | 1.75 | 0.024 | 0.56 | 0.11 |
| Absolute absorption, mmol/h | ||||||||
| Total SCFA2 | 728 | 686 | 758 | 804 | 41.0 | 0.20 | 0.87 | 0.96 |
| Acetate | 389 | 370 | 405 | 423 | 22.4 | 0.35 | 0.96 | 0.98 |
| Propionate | 240 | 225 | 249 | 268 | 13.8 | 0.14 | 0.77 | 0.90 |
| Butyrate | 99 | 91 | 104 | 113 | 6.5 | 0.11 | 0.78 | 0.92 |
| Fractional absorption, %/h | ||||||||
| Total SCFA | 43.8 | 41.7 | 46.4 | 49.3 | 2.60 | 0.17 | 0.99 | 0.85 |
| Acetate | 41.2 | 39.6 | 43.5 | 46.1 | 2.37 | 0.25 | 0.97 | 0.83 |
| Propionate | 47.4 | 45.1 | 50.2 | 53.3 | 3.05 | 0.18 | 0.98 | 0.88 |
| Butyrate | 46.5 | 43.2 | 50.1 | 53.7 | 3.28 | 0.10 | 0.99 | 0.96 |
| Urinary Cr output, mg/48 h | 436 | 415 | 411 | 392 | 54.7 | 0.96 | 0.83 | 0.71 |
1 P-values indicate overall silage, linear, and quadratic effects.
2Total SCFA = acetate + propionate + butyrate.
Blood metabolites and immunoglobulins
Increasing silage proportion had no effect on blood osmolality, but quadratically (P = 0.050) affected BHBA concentration in serum, with no effect on glucose or NEFA concentrations (Table 9). Serum IgG, IgM, and IgA were also not affected (P ≥ 0.59) by silage proportion.
Table 9.
Blood metabolite and immunoglobulin concentrations in finishing cattle fed diets containing 15% DDGS (DM basis) and different amounts (0%, 4%, 8%, and 12%; DM basis) of barley silage1
| Barley silage, % of dietary DM | P-value2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 0 | 4 | 8 | 12 | SEM | Silage | Linear | Quadratic |
| Plasma osmolality, mOsm/kg | 300 | 299 | 302 | 298 | 5.2 | 0.89 | 0.74 | 0.49 |
| Serum, mmol/L | ||||||||
| BHBA | 0.256 | 0.341 | 0.320 | 0.346 | 0.0339 | 0.097 | 0.13 | 0.050 |
| Glucose | 4.49 | 4.34 | 4.28 | 4.29 | 0.260 | 0.38 | 0.18 | 0.47 |
| NEFA | 0.086 | 0.088 | 0.089 | 0.100 | 0.0072 | 0.19 | 0.88 | 0.20 |
| Serum, g/L | ||||||||
| IgG | 23.0 | 22.4 | 24.3 | 23.7 | 1.48 | 0.64 | 0.61 | 0.57 |
| IgM | 1.65 | 1.71 | 1.96 | 1.86 | 0.218 | 0.59 | 0.33 | 0.92 |
| IgA | <0.5 | <0.5 | <0.5 | <0.5 | — | — | — | — |
1Blood was sampled on day 17 of each period at 0, 3, 6, 12, and 18 h postfeeding. Means are averaged over sampling times.
2 P-values indicate overall silage, linear, and quadratic effects.
Discussion
The barley-based diets used in the study were typical of those consumed by cattle in western Canadian feedlots (Cowley et al., 2019). The PI of the barley (82%) was relatively coarse, but consistent with recommendations for feedlot cattle (78 to 86%; Mathison, 2000; Beauchemin et al., 2001; Koenig et al., 2003). Degree of processing grain can affect the need for physically effective fiber, as coarse processing of barley grain promotes chewing and helps prevent rumen acidosis, while the opposite is true for fine processing of grain (Koenig et al., 2003). The barley silage used in the study was relatively fine with a pef8 of 0.584 (Soita et al., 2000; Einarson et al., 2004), but consistent with cereal silage chopped using a theoretical cut length of 5 mm (Yang and Beauchemin, 2006). In addition to grain processing and roughage particle length, inclusion of DDGS can influence the ruminal environment although the effects on ruminal pH can be highly variable depending upon how diet fermentability and particle size are altered (Klopfenstein et al., 2008). Substituting barley grain with corn DDGS in finishing diets reportedly helped attenuate (Hünerberg et al., 2013), or had no effect (Walter et al., 2012), on ruminal acidosis. Thus, the use of DDGS in the present study likely had either minor effects on the response to barley silage proportion in the study or may have slightly reduced the need for roughage. Despite widespread use of barley-based diets by the feedlot industry in western Canada, few studies have examined optimum levels of roughage in these types of diets (Koenig and Beauchemin, 2011). Bunk management can also affect the risk of acidosis and the need for roughage in diets (Schwartzkopf-Genswein et al., 2003). Castillo-Lopez et al. (2014) proposed that the severity of ruminal acidosis is greater in individually fed cattle compared with group-housed cattle, and in short-term rather than long-term studies. Taken together, the above factors (grain processing, use of ionophore, inclusion of DDGS, particle size of the barley silage, individual feeding, management, and study duration) may have affected animal responses to dietary roughage proportion. While the controlled nature of the study allowed measurements of complex physiological responses to changes in dietary roughage proportion, the results should be interpreted in context of the conditions employed.
Replacing barley grain with barley silage increased the chemical (ADF, NDF) and physical (peNDF) fiber contents of the diet at the expense of starch content, without affecting DMI even at the highest (12% DM) level of roughage. Although animal performance was not measured in this study, the lack of effect of silage proportion on DMI suggests that weight gain of cattle would be negatively affected with increased roughage use due to a decrease in diet digestibility, and consequently metabolizable energy intake. In contrast to the present study, most studies report that inclusion of roughage in feedlot diets increases DMI because animals eat more to maintain metabolizable energy intake (Galyean and Dafoor, 2003). This compensatory effect is possible until the fiber level restricts rumen fill (NDF intake of ~1.1% to 1.3% of BW; Mertens 1987). When ruminants are fed high-energy diets, fuel-sensing tissues tend to control feed intake, whereas ruminal distention tends to limit feed intake of low-energy diets (Allen, 2014). As NDF intake (<0.5% of BW) in the present study was low, the lack of treatment effect on DMI was unexpected and may have been due to the fine particle size of the silage used. Additionally, the lack of change in DMI with increasing silage proportion may have been due to the use of individually penned cattle in a noncompetitive feeding environment, as these cattle may respond differently than group-housed cattle that experience competition at the feed bunk. It is important to highlight that the DMI of cattle in the study was ~1.5% of BW, which is lower than 2% to 3% of BW that would typically be consumed by feedlot cattle in a commercial feedlot. The small shift in the components of DMI toward increased ADF and decreased starch intakes with increased dietary roughage level was a reflection of the changes in diet composition.
The observed increase in the percentage of long particles (≥3.35 mm) in feces with increasing silage proportion is consistent with the increase in the percentage of ≥3.35-mm particles in the diet. There were consistent and substantial decreases in diet digestibility (DM, OM, ADF, NDF, CP, and starch) with increased inclusion of barley silage. While the responses were quadratic, the highest roughage diet was least digestible. The decrease in diet digestibility was attributed to replacement of a highly digestible, low fiber ingredient (barley grain) with a less digestible, high-fiber ingredient (barley silage). Thus, the decrease in diet digestibility due to supplemental roughage occurred because the silage was less digestible than grain. A decrease in total tract digestibility can also result from a decrease in ruminal digestion of fiber as a result of unfavorable conditions (low ruminal pH) for cellulolytic microorganisms, but that was likely not a factor in this study. Ruminal pH increased with increasing roughage (as discussed below), which would have enhanced, or at the minimum, not worsened the environment for cellulolytic microorganisms (Russell and Wilson, 1996).
The observed small (maximum of 2.1 percentage unit) decrease in apparent total-tract starch digestibility of higher roughage diets indicates that the starch in barley silage was less digestible than that of processed barley grain. Because barley silage is usually not kernel processed during harvesting, the hulls provide a physical barrier to digestion of the whole kernel, which can impede digestion in the gastrointestinal tract of cattle (Eun et al., 2004). Supporting the concept that barley grain in silage may not be fully utilized, Johnson et al. (2020) reported that heifers fed a diet consisting of 10% barley silage with dry rolled corn excreted whole barley kernels. Furthermore, addition of roughage to diets is often associated with a partial shift in the site of starch digestion from the rumen to the small intestine (Yang et al., 2001), which can lower starch digestibility in the total-tract. This shift is thought to be caused by increased liquid passage rate due to greater salivary flow and increased reticulo-ruminal motility due to enhanced raft formation, which move small particles and soluble material from the rumen (Beauchemin, 2018). However, others have proposed the decrease in ruminal starch digestibility with added roughage is due to less amylolytic activity in rumen fluid (Oba and Allen, 2003).
The increase in rumination time (minutes/day) with increasing silage proportion was consistent with the increase in dietary NDF and peNDF (peNDF8, peNDF1.18) contents and particles ≥3.35 in the ration (Gentry et al., 2016). The quadratic response in rumination time indicated that the greatest benefit of added roughage occurred at the lowest level, with an increase of 43 min/day between 0% and 4% silage, and an additional 13 to 14 min/d with each 4% increment of roughage. Expressing rumination time relative to dietary NDF intake showed a linear increase indicating that the ability of NDF to promote rumination increased as silage proportion increased. Thus, total dietary NDF was not a good indicator of rumination potential of the diet, which was expected because NDF content does not account for particle size. However, expressing rumination time relative to peNDF8 or peNDF1.18 intake also did not demonstrate a treatment effect, suggesting that these combined measures of NDF content and particle size (i.e., peNDF) may not be predictive of rumination time for feedlot cattle. The peNDF1.18 values for the diets in the present study were relatively high (21.0% to 23.2% DM), despite low proportions of silage, because much of the pelleted supplement and rolled grain was retained on the sieves of the PSPS. However, the peNDF8 values were exceptionally low (0.84% to 1.43% DM), because only barley silage particles were trapped on the 8 and 19 mm sieves of the PSPS. Fox and Tedeschi (2002) recommended 7% to 10% peNDF in the dietary DM of feedlot cattle to keep mean ruminal pH above 5.7, using book values for pef rather than values obtained by actual sieving of particles. Commercial feedlot finishing diets are often below this recommendation for peNDF when sieved (NASEM, 2016).
Physically effective NDF has been widely adopted by dairy nutritionists, but the concept has limited value for highly fermentable diets even for dairy cattle (White et al., 2017), particularly diets with a high dietary DM content. Hence, the concept of peNDF has not been widely adopted by feedlots (Meyer and Bryant, 2017), and the most effective way of expressing roughage inclusion in feedlot finishing diets is not clear. Based on meta-analysis, Galyean and Defoor (2003) and Arelovich et al. (2008) concluded that total dietary NDF was adequate for exchanging fiber sources in feedlot cattle diets to optimize DM and energy intakes, but rumen function was not considered in those studies. Characterization of diets for their ability to promote rumination and minimize ruminal acidosis in beef cattle is an area that requires additional research, particularly in view of the future direction of minimizing use of in-feed antimicrobials. Based on our results, proportion of dietary DM retained on a 3.35-mm sieve may be more useful for assessing adequacy of particle length of feedlot diets than 1.18, 8, and 19 mm sieves of the PSPS, although this remains to be confirmed in subsequent studies.
There is a positive association between eating time and DMI as well as with dietary particle size for cattle (Beauchemin, 2018). Thus, the lack of treatment effect on eating time is consistent with the lack of effect on DMI. Furthermore, the relatively small changes in dietary particle size with the addition of finely chopped silage did not increase the need for mastication during eating. The finding that low inclusion rate of silage in a barley-based diet did not increase eating time of cattle is important because most feedlots provide limited bunk space per animal (22 to 30 cm; Meyer and Bryant, 2017). An increase in eating time may increase the number of animals wanting to consume feed at a given time, which might promote binge-eating events thereby increasing the prevalence of digestive disorders (Schwartzkopf-Genswein et al., 2003; Meyer and Bryant, 2017).
The observed increase in minimum (+0.34 units) and mean (+0.34) pH, and concomitant decreases in duration and area of pH below the acidosis thresholds and acidosis index clearly demonstrate beneficial effects on the ruminal environment conferred by adding a small amount of roughage (i.e., 4% to 12% of DM) to a feedlot diet. For all pH variables (except maximum pH), the responses were quadratic with the greatest increase between 0% and 4% roughage, which is consistent with the quadratic increase in rumination time. There are many interacting factors that account for an increase in ruminal pH when roughage is added to a diet as discussed by Galyean and Hubbert (2014). In the present study, the observed increased in rumination time would be expected to increase salivation and consequently buffering capacity of the rumen (Beauchemin, 2018). Increased salivary secretion can also shift the site of starch digestion from the rumen as previously discussed, which would decrease the rate of SCFA production. Dilution of starch with NDF also slows the rate of diet fermentation in the rumen, while increasing the acetate:propionate ratio. While SCFA production was not measured in the study, the similar concentration of SCFA in ruminal fluid among treatments was likely the net effect of an increase in ruminal liquid pool size, a shift in site of starch digestion, and a decrease in total-tract digestibility (and presumably ruminal digestion) with increasing roughage levels. Decreased total tract digestibility and a shift in site of starch digestion from the rumen would be expected to decrease SCFA production, while greater liquid volume generally increases SCFA concentrations, although effects are relatively inconsistent within study (Hall et al., 2015). As discussed by Hall et al. (2015), total SCFA concentration in ruminal fluid is not an appropriate indicator of ruminal fermentation when treatments cause great variability in rumen liquid volume. The observed increased molar proportions of acetate and decreased proportions of propionate with increased silage proportion are consistent with a shift towards cellulolytic fermentation pathways with higher fiber diets.
A rumen pH of 5.8 to 7.0 is needed to promote cellulose digestion (Russell and Wilson, 1996), thus a threshold pH value of 5.6 to 5.8 is used for dairy cows and backgrounding cattle fed high forage diets to indicate subacute ruminal acidosis (e.g., <5.6 for 3 h/d or more, Gozho et al., 2006; <5.8 for 5 h/d or more; Zebeli et al., 2012). However, for feedlot cattle fed low fiber diets, fiber digestion is less important, and a pH threshold of 5.5 has been used to indicate subacute ruminal acidosis (Schwaiger et al., 2013). Exposure of the ruminal epithelium to pH of 5.2 to 5.5 has been shown to impair its barrier function (Aschenbach et al., 2011), cause ruminitis (Nagaraja and Lechtenberg, 2007), and trigger an inflammatory response (Gozho et al., 2006). Damage to the rumen wall can allow endotoxins (lipopolysaccharides), amines (histamines), and pathogens to cross the epithelial barrier and enter systemic or lymphatic circulation (Meyer and Bryant, 2017). Acute acidosis, which can lead to death if not treated, is usually diagnosed using a benchmark pH < 5.2 coupled with elevated lactate concentrations (Owens et al., 1998). Despite prolonged durations and areas below the acidosis thresholds, the concentration of lactic acid in ruminal fluid was < 1 mM, suggesting that acute acidosis did not occur on average for any of the dietary treatments.
Despite the relatively low DMI (1.5% of BW) in the present study, subacute ruminal acidosis was extensive in animals that were not fed roughage, with a pH < 5.5 for almost 50% of each day. Provision of 4% to 12% barley silage reduced the duration of subacute acidosis to about 17% of each day, but subacute acidosis was not completely abolished even with 12% roughage added to the diet. The lack of treatment differences for absorption of SCFA from the rumen (mol/h or %/h) indicated that the change in severity and duration of subacute acidosis due to added roughage did not affect the absorptive ability of the ruminal epithelium. Low pH would be expected to increase ruminal SCFA absorption although acidotic conditions also cause parakeratosis and reduce epithelial permeability (Aschenbach et al., 2011, 2019). For example, Schwaiger et al. (2013) observed that an acute bout of ruminal acidosis temporarily decreased the absorption of acetate, propionate, and butyrate. The lack of treatment effect on SCFA absorption despite changes in individual SCFA proportions was consistent with Chibisa et al. (2015) who, using the WRR technique, reported no difference in fractional absorption of individual SCFA in beef cattle fed high- and low-forage diets. Given that fractional absorption was not affected by treatment in the present study, the greater incidence of ruminal acidosis observed for the control cattle likely occurred due to accumulation of SCFA in ruminal fluid. Hence, greater fermentability of the diet without roughage was not compensated for by increased absorption, and thus ruminal pH remained low.
Forty-eight hour urinary Cr appearance, which was used as an indicator of total tract barrier function (Zhang et al., 2013), did not differ among diets in the present study. This was despite the duration pH < 5.5 being 2.7 times longer for the 0 compared with 4%, 8%, and 12% silage diets (11.3 h/d vs. an average of 4.1 h/d). The minimum amount of time that pH must remain below a certain threshold for compromised barrier function to occur (e.g., pH 5.5 to 5.2; Aschenbach, et al., 2011), and how that duration is possibly influenced by other ruminal factors including SCFA concentration, is yet to be fully studied. In an ex vivo experiment using Ussing chambers (Meissner et al., 2017), incubation of ruminal epithelial tissue at pH 5.1 for only 2 h in the presence of SCFA decreased the expression of tight junction proteins, and increased tissue conductance and fluorescein flux rates, which were all suggestive of perturbations in barrier function. Moreover, ruminal acidosis alters the luminal pH in intestinal regions (Gressley et al., 2011; Pederzolli et al., 2018) that are likely more permeable than the rumen (Penner et al., 2014). Therefore, although feeding increasing proportions of silage increased ruminal pH in the present study, this improvement was possibly not substantial enough to result in differences in paracellular permeability across the whole gastrointestinal tract, given that the duration pH < 5.5 was longer than 2 h across diets. The Cr-EDTA method is a total tract measurement approach, thus we cannot exclude the possibility of effects in the rumen. However, further studies evaluating factors that influence both ruminal and hindgut epithelial permeability, including the interactions between pH, SCFA concentration, and exposure time, are still needed.
Blood parameters were measured to indicate the nutrient and health status of the animals, as affected by roughage level. The quadratic increase in BHBA with increased silage inclusion is consistent with the shift in ruminal fermentation toward increased molar proportion of acetate and butyrate, the main ketogenic SCFA. Glucose is synthesized in the liver of cattle from SCFA (Puppel and Kuczyńska, 2016). In beef cattle, high NEFA concentration is driven largely by excessive adipose tissue mobilization, which was not the case in the present study. The lack of effect of treatments on serum glucose and NEFA concentrations was expected given that the animals were gaining weight and lipid reserves were not being mobilized. Differences in subacute acidosis and potential inflammatory response (Gozho et al., 2006; Khafipour et al., 2009) among treatments were not detected in serum IgA, IgG, and IgM concentrations. A similar lack of effect was reported for dairy cows by Iqbal et al. (2014) following repeated oronasal administration of lipopolysaccharide, a cell-wall component of Gram-negative bacteria.
In summary, adding up to 12% barley silage (DM basis) to a barley-based feedlot diet containing DDGS had no effect on DMI of cattle, but decreased diet digestibility. However, rumination time and ruminal pH increased and subacute acidosis decreased quadratically with added roughage, although acidosis was not completely eliminated even when feeding a diet containing 12% barley silage DM. Based on the results of this metabolism study, we speculate that animal performance of cattle in a commercial feedlot would likely decline as proportion of roughage is increased despite improvements in rumen health. Therefore, we accept our hypothesis of a trade-off between maximizing animal performance and promoting animal health and welfare in feedlot cattle. In the present study, responses to roughage level were mostly quadratic with greatest improvements in rumen acidosis variables (rumination, ruminal pH) between 0% and 4% barley silage, with small incremental improvements at higher roughage concentrations. In addition to level of roughage, incidence of subclinical and acute acidosis in feedlot cattle can depend upon a wide range of factors, and those risk factors need to be considered when formulating low-roughage diets for feedlot cattle. Our study suggests that that the optimum roughage level in a feedlot diet depends on the target variable, with the optimum roughage level to minimize ruminal acidosis being greater than the optimum level required to promote animal performance. The results from this short-term study with cattle in a noncompetitive feeding environment need to be validated in longer term studies with group-housed cattle representative of commercial feedlots consuming greater quantities of feed relative to BW.
Acknowledgments
Funding was from the Alberta Meat and Livestock Agency (Edmonton, AB) and Agriculture and Agri-Food Canada. The authors thank K. Andrews, B. Farr, and D. Vedres for excellent technical support and staff of the Beef Metabolism Facility for care of the animals.
Glossary
Abbreviations
- ADF
acid detergent fiber
- AUC
area under pH threshold curve
- BHBA
β-hydroxybutyrate
- BW
body weight
- CP
crude protein
- DDGS
dried distillers grains with solubles
- DM
dry matter
- Ig
immunoglobulin
- NDF
neutral detergent fiber
- NEFA
nonesterified fatty acids
- NH3-N
ammonia-nitrogen
- OM
organic matter
- PSPS
Penn State Particle Separator
- SCFA
short-chain fatty acids
- TMR
total mixed ration
- WRR
washed reticulo-rumen
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Allen M. S. 1997. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 80:1447–1462. doi: 10.3168/jds.S0022-0302(97)76074-0 [DOI] [PubMed] [Google Scholar]
- Allen M. S. 2014. Drives and limits to feed intake in ruminants. Anim. Prod. 54:1513–01524. doi: 10.1071/AN14478 [DOI] [Google Scholar]
- AOAC. 2005. Official methods of analysis, 18th ed. Gaithersburg, MD:Association. Off Analytic. Chemistry. [Google Scholar]
- Arelovich H. M., Abney C. S., Vizcarra J. A., and Galyean M. L.. 2008. Effects of dietary neutral detergent fiber on intakes of dry matter and net energy by dairy and beef cattle: analysis of published data. Prof. Anim. Sci. 24:375–383. doi: 10.15232/S1080-7446(15)30882-2 [DOI] [Google Scholar]
- Aschenbach J. R., Penner G. B., Stumpff F., and Gäbel G.. 2011. Ruminant nutrition symposium: role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 89:1092–1107. doi: 10.2527/jas.2010-3301 [DOI] [PubMed] [Google Scholar]
- Aschenbach J. R., Zebeli Q., Patra A. K., Greco G., Amasheh S., and Penner G. B.. 2019. Symposium review: the importance of the ruminal epithelial barrier for a healthy and productive cow. J. Dairy Sci. 102:1866–1882. doi: 10.3168/jds.2018-15243. [DOI] [PubMed] [Google Scholar]
- Beauchemin K. A. 2018. Invited review: current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 101:4762–4784. doi: 10.3168/jds.2017-13706 [DOI] [PubMed] [Google Scholar]
- Beauchemin K. A., Yang W. Z., and Rode L. M.. 2001. Effects of barley grain processing on the site and extent of digestion of beef feedlot finishing diets. J. Anim. Sci. 79:1925–1936. doi: 10.2527/2001.7971925x [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care (CCAC). 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing.Ottawa, ON, Canada:CCAC. [Google Scholar]
- Care A. D., Brown R. C., Farrar A. R., and Pickard D. W.. 1984. Magnesium absorption from the digestive tract of sheep. Q. J. Exp. Physiol. 69:577–587. doi: 10.1113/expphysiol.1984.sp002844 [DOI] [PubMed] [Google Scholar]
- Castillo-Lopez E., Wiese B. I., Hendrick S., McKinnon J. J., McAllister T. A., Beauchemin K. A., and Penner G. B.. 2014. Incidence, prevalence, severity, and risk factors for ruminal acidosis in feedlot steers during backgrounding, diet transition, and finishing. J. Anim. Sci. 92:3053–3063. doi: 10.2527/jas.2014-7599 [DOI] [PubMed] [Google Scholar]
- Chibisa G. E., Beauchemin K. A., and Penner G. B.. 2015. Relative contribution of ruminal buffering systems to pH regulation in feedlot cattle fed either low- or high-forage diets. Animal 10:1164–1172. doi: 10.1017/S1751731115002888 [DOI] [PubMed] [Google Scholar]
- Cowley F., Jennings J., Cole A., and Beauchemin K.. 2019. Review: recent advances to improve nitrogen efficiency grain-finishing in North American and Australian feedlots. Anim. Prod. Sci. 59:2082–2092. doi: 10.1071/AN19259 [DOI] [Google Scholar]
- Crawford G. I., Keeler C. D., Wagner J. J., Krehbiel C. R., Erickson G. E., Crombie M. B., and Nunnery G. A.. 2008. Effects of calcium magnesium carbonate and roughage level on feedlot performance, ruminal metabolism, and site and extent of digestion in steers fed high-grain diets. J. Anim. Sci. 86:2998–3013. doi: 10.2527/jas.2007-0070 [DOI] [PubMed] [Google Scholar]
- Dong R. L., Chibisa G. E., and Beauchemin K. A.. 2018. Estimating optimal observational sampling frequency of behaviors for cattle fed high- and low-forage diets. J. Anim. Sci. 96:783–796. doi: 10.1093/jas/skx073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouillard J. S. 2018. Current situation and future trends for beef production in the United States of America - a review. Asian-Australas. J. Anim. Sci. 31:1007–1016. doi: 10.5713/ajas.18.0428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarson M. S., Plaizier J. C., and Wittenberg K. M.. 2004. Effects of barley silage chop length on productivity and rumen conditions of lactating dairy cows fed a total mixed ration. J. Dairy Sci. 87:2987–2996. doi: 10.3168/jds.S0022-0302(04)73430-X [DOI] [PubMed] [Google Scholar]
- Eun J. S., Beauchemin K. A., Hong S. H., and Yang W. Z.. 2004. Effects of mechanical processing on the nutritive value of barley silage for lactating dairy cows. J. Dairy Sci. 87:4170–4177. doi: 10.3168/jds.S0022-0302(04)73560-2 [DOI] [PubMed] [Google Scholar]
- Fox D. G., and Tedeschi L. O.. 2002. Application of physically effective fiber in diets for feedlot cattle. Proc. Plains Nutrition Council Spring Conf., Amarillo, TX, April 25–26; p. 67–81. https://theplainsnutritioncouncil.com/past-proceedings/ – [accessed April 1, 2020] [Google Scholar]
- Galyean M. L., and Defoor P. J.. 2003. Effects of roughage source and level on intake by feedlot cattle. J. Anim. Sci. 81:E8–E16. doi: 10.2527/2003.8114_suppl_2E8x [DOI] [PubMed] [Google Scholar]
- Galyean M. L., and Hubbert M. E.. 2014. Review: traditional and alternative sources of fiber – Roughage values, effectiveness, and levels in starting and finishing diets. Prof. Anim. Sci. 30:571–584. doi: 10.15232/pas.2014-01329 [DOI] [Google Scholar]
- Gentry W. W., Weiss C. P., Meredith C. M., McCollum F. T., Cole N. A., and Jennings J. S.. 2016. Effects of roughage inclusion and particle size on performance and rumination behavior of finishing beef steers. J. Anim. Sci. 94:4759–4770. doi: 10.2527/jas.2016-0734 [DOI] [PubMed] [Google Scholar]
- Gozho G. N., Krause D. O., and Plaizier J. C.. 2006. Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. J. Dairy Sci. 89:4404–4413. doi: 10.3168/jds.S0022-0302(06)72487-0 [DOI] [PubMed] [Google Scholar]
- Gressley T. F., Hall M. B., and Armentano L. E.. 2011. Ruminant nutrition symposium: productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 89:1120–1130. doi: 10.2527/jas.2010-3460 [DOI] [PubMed] [Google Scholar]
- Hall M. B., Nennich T. D., Doane P. H., and Brink G. E.. 2015. Total volatile fatty acid concentrations are unreliable estimators of treatment effects on ruminal fermentation in vivo. J. Dairy Sci. 98:3988–3999. doi: 10.3168/jds.2014-8854 [DOI] [PubMed] [Google Scholar]
- Hünerberg M., McGinn S. M., Beauchemin K. A., Okine E. K., Harstad O. M., and McAllister T. A.. 2013. Effect of dried distillers’ grains with solubles on enteric methane emissions and nitrogen excretion from finishing beef cattle. Can. J. Anim. Sci. 93:373–385. doi: 10.4141/CJAS2012-151 [DOI] [PubMed] [Google Scholar]
- Iqbal S., Zebeli Q., Mansmann D. A., Dunn S. M., and Ametaj B. N.. 2014. Repeated oronasal exposure to lipopolysaccharide induced mucosal IgA responses in periparturient dairy cows. PLoS One 9:e103504. doi: 10.1371/journal.pone.0103504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Sutherland B. D., McKinnon J. J., McAllister T. A., and Penner G. B.. 2020. Effect of feeding barley or corn silage with dry-rolled barley, corn, or a blend of barley and corn grain on rumen fermentation, total tract digestibility, and nitrogen balance for finishing beef heifers. J. Anim. Sci. 98. doi: 10.1093/jas/skaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khafipour E., Krause D. O., and Plaizier J. C.. 2009. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 92:1060–1070. doi: 10.3168/jds.2008-1389 [DOI] [PubMed] [Google Scholar]
- Klopfenstein T. J., Erickson G. E., and Bremer V. R.. 2008. Board-invited review: use of distillers by-products in the beef cattle feeding industry. J. Anim. Sci. 86:1223–1231. doi: 10.2527/jas.2007-0550 [DOI] [PubMed] [Google Scholar]
- Koenig K. M., and Beauchemin K. A.. 2011. Optimum extent of barley grain processing and barley silage proportion in feedlot cattle diets: growth, feed efficiency, and fecal characteristics. Can. J. Anim. Sci. 91:411–422. doi: 10.1139/CJAS2010-03 [DOI] [Google Scholar]
- Koenig K. M., Beauchemin K. A., and Rode L. M.. 2003. Effect of grain processing and silage on microbial protein synthesis and nutrient digestibility in beef cattle fed barley-based diets. J. Anim. Sci. 81:1057–1067. doi: 10.2527/2003.8141057x [DOI] [PubMed] [Google Scholar]
- Kononoff P. J., Heinrichs A. J., and Buckmaster D. R.. 2003. Modification of the penn state forage and total mixed ration particle separator and the effects of moisture content on its measurements. J. Dairy Sci. 86:1858–1863. doi: 10.3168/jds.S0022-0302(03)73773-4 [DOI] [PubMed] [Google Scholar]
- Lammers B. P., Buckmaster D. R., and Heinrichs A. J.. 1996. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 79:922–928. doi: 10.3168/jds.S0022-0302(96)76442-1 [DOI] [PubMed] [Google Scholar]
- Mathison G. W. 2000. Alberta feedlot management guide, 2nd ed. Edmonton, Alberta: Alberta Agriculture Food and Rural Development; p. 1B1–1B11. [Google Scholar]
- Meissner S., Hagen F., Deiner C., Günzel D., Greco G., Shen Z., and Aschenbach J. R.. 2017. Key role of short-chain fatty acids in epithelial barrier failure during ruminal acidosis. J. Dairy Sci. 100:6662–6675. doi: 10.3168/jds.2016-12262 [DOI] [PubMed] [Google Scholar]
- Mertens D. R. 1987. Predicting intake and digestibility using mathematical models of ruminal function. J. Anim. Sci. 64:1548–1558. doi: 10.2527/jas1987.6451548x [DOI] [PubMed] [Google Scholar]
- Meyer N. F., and Bryant T. C.. 2017. Diagnosis and management of rumen acidosis and bloat in feedlots. Vet. Clin. North Am. Food Anim. Pract. 33:481–498. doi: 10.1016/j.cvfa.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., and Lechtenberg K. F.. 2007. Acidosis in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23:333–50, viii. doi: 10.1016/j.cvfa.2007.04.002 [DOI] [PubMed] [Google Scholar]
- NASEM. 2016. Nutrient requirements of beef cattle, 8th ed. Washington, DC:The National Academies Press. doi: 10.17226/19014 [DOI] [Google Scholar]
- Oba M., and Allen M. S.. 2003. Effects of corn grain conservation method on ruminal digestion kinetics for lactating dairy cows at two dietary starch concentrations. J. Dairy Sci. 86:184–194. doi: 10.3168/jds.S0022-0302(03)73599-1 [DOI] [PubMed] [Google Scholar]
- Owens F. N., Secrist D. S., Hill W. J., and Gill D. R.. 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275–286. doi: 10.2527/1998.761275x [DOI] [PubMed] [Google Scholar]
- Pederzolli R. A., Van Kessel A. G., Campbell J., Hendrick S., Wood K. M., and Penner G. B.. 2018. Effect of ruminal acidosis and short-term low feed intake on indicators of gastrointestinal barrier function in Holstein steers. J. Anim. Sci. 96:108–125. doi: 10.1093/jas/skx049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner G. B., Aschenbach J. R., Wood K., Walpole M. E., Kanafany-Guzman R., Hendrick S., and Campbell J.. 2014. Characterising barrier function among regions of the gastrointestinal tract in Holstein steers. Anim. Prod. Sci. 54:1282–1287. doi: 10.1071/AN14285 [DOI] [Google Scholar]
- Penner G. B., Beauchemin K. A., and Mutsvangwa T.. 2006. An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. J. Dairy Sci. 89:2132–2140. doi: 10.3168/jds.S0022-0302(06)72284-6 [DOI] [PubMed] [Google Scholar]
- Plascencia A., González-Vizcarra V. M., and Zinn R. A.. 2018. Comparative effects of grain source on digestion characteristics of finishing diets for feedlot cattle: steam-flaked corn, barley, wheat, and oats. Can. J. Anim. Sci. 98:794–800. doi: 10.1139/cjas-2018-0018 [DOI] [Google Scholar]
- Puppel K., and Kuczyńska B.. 2016. Metabolic profiles of cow’s blood; a review. J. Sci. Food Agric. 96:4321–4328. doi: 10.1002/jsfa.7779 [DOI] [PubMed] [Google Scholar]
- Reinhardt C. D., and Hubbert M. E.. 2015. Control of liver abscesses in feedlot cattle: a review. Prof. Anim. Sci. 31:101–108. doi: 10.15232/pas.2014-01364 [DOI] [Google Scholar]
- Rhine E. D., Sims G. K., Mulvaney R. L., and Pratt E. J.. 1998. Improving the Berthelot reaction for determining ammonium in soil extracts and water. Soil Sci. Soc. Am. J. 62:473–480. doi: 10.2136/sssaj1998.03615995006200020026x [DOI] [Google Scholar]
- Russell J. B., and Wilson D. B.. 1996. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 79:1503–1509. doi: 10.3168/jds.S0022-0302(96)76510-4 [DOI] [PubMed] [Google Scholar]
- Samuelson K. L., Hubbert M. E., Galyean M. L., and Löest C. A.. 2016. Nutritional recommendations of feedlot consulting nutritionists: the 2015 New Mexico State and Texas Tech University survey. J. Anim. Sci. 94:2648–2663. doi: 10.2527/jas2016-0282 [DOI] [PubMed] [Google Scholar]
- Saunders P. R., Kosecka U., McKay D. M., and Perdue M. H.. 1994. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am. J. Physiol. 267(5 Pt 1):G794–G799. doi: 10.1152/ajpgi.1994.267.5.G794 [DOI] [PubMed] [Google Scholar]
- Schwaiger T., Beauchemin K. A., and Penner G. B.. 2013. The duration of time that beef cattle are fed a high-grain diet affects the recovery from a bout of ruminal acidosis: dry matter intake and ruminal fermentation. J. Anim. Sci. 91:5729–5742. doi: 10.2527/jas.2013-6471 [DOI] [PubMed] [Google Scholar]
- Schwartzkopf-Genswein K. A., Beauchemin K. A., Gibb D. J., Crews D. H. Jr., Hickman D. D., Streeter M., and McAllister T. A.. 2003. Effect of bunk management on feeding behavior, ruminal acidosis and performance of feedlot cattle: a review. J. Anim. Sci. 81(E. Suppl. 2): E149–E158. doi: 10.2527/2003.8114_suppl_2E149x [DOI] [Google Scholar]
- Soita H. W., Christensen D. A., and McKinnon J. J.. 2000. Influence of particle size on the effectiveness of the fiber in barley silage. J. Dairy Sci. 83:2295–2300. doi: 10.3168/jds.S0022-0302(00)75116-2 [DOI] [PubMed] [Google Scholar]
- Stock R. A., Sindt M. H., Parrott J. C., and Goedeken F. K.. 1990. Effects of grain type, roughage level and monensin level on finishing cattle performance. J. Anim. Sci. 68:3441–3455. doi: 10.2527/1990.68103441x [DOI] [PubMed] [Google Scholar]
- Turgeon O. A., Szasz J. I., Koers W. C., Davis M. S., and Vander Pol K. J.. 2010. Manipulating grain processing method and roughage level to improve feed efficiency in feedlot cattle. J. Anim. Sci. 88:284–295. doi: 10.2527/jas.2009-1859 [DOI] [PubMed] [Google Scholar]
- Walter L. J., McAllister T. A., Yang W. Z., Beauchemin K. A., He M., and McKinnon J. J.. 2012. Comparison of wheat or corn dried distillers grains with solubles on rumen fermentation and nutrient digestibility by feedlot heifers. J. Anim. Sci. 90:1291–1300. doi: 10.2527/jas.2011-3844 [DOI] [PubMed] [Google Scholar]
- White R. R., Hall M. B., Firkins J., and Kononoff P. J.. 2017. Physically adjusted neutral detergent fiber system for lactating dairy cow rations. I: deriving equations that identify factors that influence effectiveness of fiber. J. Dairy Sci. 100:9551–9568. doi: 10.3168/jds.2017-12765 [DOI] [PubMed] [Google Scholar]
- Yang W. Z., and Beauchemin K. A.. 2006. Physically effective fiber: method of determination and effects on chewing, ruminal acidosis, and digestion by dairy cows. J. Dairy Sci. 89:2618–2633. doi: 10.3168/jds.S0022-0302(06)72339-6 [DOI] [PubMed] [Google Scholar]
- Yang W. Z., Beauchemin K. A., and Rode L. M.. 2001. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. J. Dairy Sci. 84:2203–2216. doi: 10.3168/jds.S0022-0302(01)74667-X [DOI] [PubMed] [Google Scholar]
- Zebeli Q., Aschenbach J. R., Tafaj M., Boguhn J., Ametaj B. N., and Drochner W.. 2012. Invited review: role of physically effective fiber and estimation of dietary fiber adequacy in high-producing dairy cattle. J. Dairy Sci. 95:1041–1056. doi: 10.3168/jds.2011-4421 [DOI] [PubMed] [Google Scholar]
- Zhang S., Albornoz R. I., Aschenbach J. R., Barreda D. R., and Penner G. B.. 2013. Short-term feed restriction impairs the absorptive function of the reticulo-rumen and total tract barrier function in beef cattle. J. Anim. Sci. 91:1685–1695. doi: 10.2527/jas.2012-5669 [DOI] [PubMed] [Google Scholar]