Abstract
Following a request from the European Commission, the Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of l‐glutamine (≥ 98.0%) produced by fermentation using a genetically modified strain of Corynebacterium glutamicum (NITE BP‐02524). It is intended to be used in feed for all animal species and categories as nutritional additive (amino acid) and as sensory additive (flavouring compound). Viable cells of the production strain and its recombinant DNA were not detected in the additive. l‐Glutamine manufactured by fermentation using C. glutamicum NITE BP‐02524 does not give rise to any safety concern with regard to the genetic modification of the production strain. The use of l‐glutamine produced by fermentation using C. glutamicum NITE BP‐02524 in animal nutrition is considered safe for all animal species when applied as a nutritional additive to achieve an adequate amino acid profile in feed and to overcome potential glutamine shortages during critical periods of life. The proposed use level (25 mg/kg feed) when used as sensory additive (flavouring compound) is safe for all animal species. The uses of l‐glutamine produced using C. glutamicum NITE BP‐02524 as nutritional additive or as flavouring compound are considered safe for the consumer. l‐Glutamine produced using C. glutamicum NITE BP‐02524 is not toxic by inhalation, is non‐irritant to skin and eyes and is not a skin sensitiser. l‐Glutamine produced using C. glutamicum NITE BP‐02524 is considered safe for the environment. l‐glutamine is a non‐essential amino acid and it plays a physiological role as such. Recent evidence shows that glutamine may act as conditionally essential amino acid mainly in growing animals and has some specific effects e.g. in improving intestinal development and immune response. This amino acid produced by fermentation using C. glutamicum NITE BP‐02524 is regarded as an efficacious source of glutamine for all animal species. For supplemental l‐glutamine to be as efficacious in ruminants as in non‐ruminants, it would require protection against degradation in the rumen. The use of l‐glutamine as sensory additive at 25 mg/kg feed is considered efficacious.
Keywords: glutamine, amino acid, flavouring, Corynebacterium glutamicum, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from Ajinomoto Animal Nutrition Europe2 for authorisation of the product l‐glutamine produced using Corynebacterium glutamicum NITE BP‐02524, when used as a feed additive for all animal species (category: nutritional additives; functional group: amino acids, their salts and analogues; and category: sensory additives; functional group: flavouring compounds.
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). The particulars and documents in support of the application were considered valid by EFSA as of 19 November 2018.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product l‐glutamine produced using C. glutamicum NITE BP‐02524, when used under the proposed conditions of use (see Section 3.1.6).
1.2. Additional information
l‐Glutamine (minimum 98.0% on dry matter basis) produced by fermentation using C. glutamicum NITE BP‐02524 is the object of the present assessment. It has not been previously authorised as feed additive in the European Union.
Glutamine is authorised for use in food for nutritional purposes,3 and as flavouring (FL No 17.007),4 for use in cosmetics5 and as a veterinary medicinal product.6
The EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA Panel) issued a scientific opinion on the substantiation of health claims related to l‐glutamine and concluded that a cause and effect relationship had not been established (EFSA NDA Panel, 2011).
l‐Glutamic acid (minimum 98%, produced by chemical synthesis or protein hydrolysis) is currently authorised as sensory additive (flavouring compound) in the EU register of feed additives.7
The EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS Panel) set an acceptable daily intake (ADI) of 30 mg/kg body weight (bw) per day for glutamic acid (E620) when used as food additive (EFSA ANS Panel, 2017). The Norwegian Scientific Committee for Food Safety (Vitenskapskomiteen for mattrygghet, VKM, 2016) has assessed the risk of l‐glutamine in food supplements.
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier8 in support of the authorisation request for the use of l‐glutamine produced using C. glutamicum NITE BP‐02524 as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers and other scientific reports, to deliver the present output.
The applicant performed two literature searches that are described in Sections 3.2.3 and 3.3.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the l‐glutamine produced by fermentation using C. glutamicum NITE BP‐02524 in animal feed. The Executive Summary of the EURL report can be found in Annex A.9
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of l‐glutamine produced using C. glutamicum NITE BP‐02524 is in line with the principles laid down in Regulation (EC) No 429/200810 and the relevant guidance documents: Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance for assessing the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019a), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018b).
3. Assessment
l‐Glutamine (minimum 98.0% on dry matter basis) produced using C. glutamicum NITE BP‐02524 for all animal species is the object of the present assessment. It is proposed as nutritional feed additive (functional group: amino acids, their salts and analogues) and as sensory additive (functional group: flavouring compounds) in feed for all animal species.
l‐Glutamine and L‐glutamate (together with l‐arginine, l‐threonine and l‐cysteine) are involved in maintenance functions as immune surveillance system and gut mucosal repair processes. They are considered non‐essential amino acids. The nutritional supply of l‐glutamine, however, may become limited at weaning or during intestinal stress due to the rapid turnover and replacement of mucosal cells (D'Mello, 2003). In such situations, dietary supplementation may become relevant.
3.1. Characterisation of the active substance
l‐Glutamine (International Union of Pure and Applied Chemistry (IUPAC)) name: (2S)‐2,5‐diamino‐5‐oxopentanoic acid; synonyms: 2‐Amino‐4‐carbamoylbutanoic acid, (levo)glutamide, l‐2‐aminoglutaramic acid), a compound identified by Chemical Abstracts Service (CAS) No 56‐85‐9 and European Inventory of Existing Commercial Chemical Substances (EINECS) No 200‐292‐1, has a molecular weight of 146.15 g/mol; the molecular formula is C5H10N2O3 and its structural formula is given in Figure 1.
Figure 1.
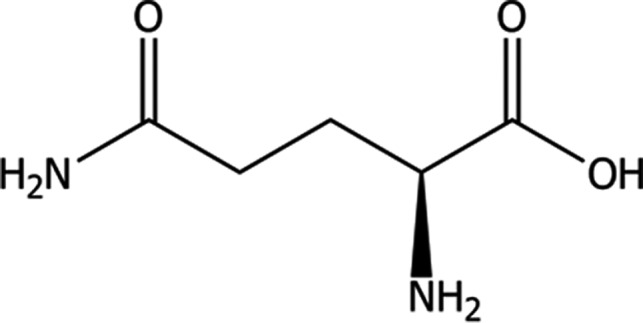
Molecular structure of l‐glutamine
3.1.1. Characterisation of the production organism
l‐Glutamine is produced by a genetically modified strain of C. glutamicum which is deposited in the Japanese National Institute of Technology and Evaluation (NITE) with accession number NITE BP‐02524.11
The identity of the strain as belonging to the species C. glutamicum was confirmed ■■■■■■■■■■
3.1.1.1. Information related to the genetically modified microorganism
Characterisation of the parental microorganism
■■■■■
■■■■■ No antimicrobial resistance genes of concern were detected in the databases used.
The production strain was tested for its susceptibility to the antimicrobials listed for ‘Corynebacterium and other Gram‐positive’ in the Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a).13 ■■■■■ All minimum inhibitory concentration values were below the cut‐off values set in that guidance and the strain is considered susceptible to those antimicrobials.
■■■■■
■■■■■■■■■■
■■■■■
■■■■■
■■■■■
3.1.2. Manufacturing process
■■■■■
The applicant stated that no antibiotics are used during the manufacturing process of the additive.
3.1.3. Characterisation of the additive
The additive is specified to contain a minimum of 98% glutamine on a dry matter basis.
Analysis of 10 batches showed an average of 99.7% glutamine (range 99.2–99.9%) on an ‘as is’ basis, and a loss on drying of 0.05%.15 Sum of quantifiable free amino acids (five out of the 10 batches) was 0.9% ■■■■■.16 The sum of identified material is above 99%.
The specific optical rotation (European Pharmacopoeia method) measured in five batches ranged from 6.5 to 7.2°.16 The values fall within the range given by the US Food Chemicals Codex (FCC, 2012)17 of +6.3 to +7.3° and confirm the presence of the L‐enantiomer of glutamine.
Impurities and microbial contamination
Three batches of the additive were analysed for heavy metals (cadmium, lead and mercury), arsenic, fluorine, melamine and hydrocyanic acid. All analysed values for the above were below the respective limit of quantification (LOQ).18 The sum of polychlorinated dibenzodioxins/dibenzofurans (PCDD/F) and dioxin‐like polychlorinated biphenyls (PCB) was analysed in three batches and ranged from 0.11 to 0.12 ng WHO‐PCDD/F‐PCB TEQ/kg. Non dioxin‐like PCBs (three batches analysed) ranged from 0.07 to 0.08 μg/kg additive.
The detected amounts of these undesirable substances do not raise safety concerns.
In relation to mycotoxins, aflatoxins (B1, B2, G1 and G2), ochratoxin A, zearalenone, deoxynivalenol, zearalenone, T‐2 toxin, HT‐2 toxin and fumonisins (B1, B2 and B3) were analysed in three batches of the additive and found below the LOQ.19
Three batches were analysed for the presence of organochlorinated pesticides, organophosphate pesticides and pyrethroid pesticides. All analytical values were below the LOQ.20
Biogenic amines (cadaverine, spermine, tyramine, tryptamine, 2‐phenylethylamine, histamine, putrescine and spermidine) were analysed in five batches of the additive and all concentrations were below the LOQ.21
The microbial contamination was tested in three batches. Total aerobic counts were up to 2.1 × 103 CFU/g; Salmonella spp. was negative in 25 g samples; Enterobacteriaceae, coliforms, Bacillus cereus, coagulase‐positive Staphylococcus, yeast and moulds were < 10 CFU/g.22
The antimicrobial activity of the final product was assessed according to the EFSA guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a).23 Only inhibition against E. faecalis ATCC 29212, at concentrations of 4.5% and 1.1% after 24 h was detected. However, there was no inhibition after 48 h of incubation. This finding is considered not relevant for the safety given the usual supplementation levels in feed (up to 0.3%); the fact that only one of the five tested strains was inhibited; and that inhibition lasted only for 24 h.
The absence of viable cells of the production strain was tested ■■■■■■■■■■■■■■■ No viable cells of the production organism were detected.
The absence of DNA of the production strain was tested ■■■■■■■■■■■■■■■ No DNA was detected.
Physico‐chemical properties
The additive is a white crystalline powder with a solubility in water (at 30°C) of 4.8 g/100 mL, a pH ranging from 5.18 to 5.21 (solution of 0.5 g in 100 mL), pka of 2.17 and 9.13, a log Kow of 0.8516 and a packed bulk density (measured in three batches) ranging from 589 to 591 kg/m3.
The dusting potential was analysed (Stauber‐Heubach method) in three batches of the final product. The values ranged from 4.2 to 5.6 g/m3.26 The particle size distribution of three batches of the final product was measured (laser diffraction). About 10% of the particles had a size < 10 μm of diameter; 50% of particles had a diameter of ≤ 23 μm; and 90% of the particles had a diameter < 69 μm. In all three batches, 80% of the particles had a size of 9–69 μm diameter.27
3.1.4. Stability and homogeneity
The shelf‐life of the additive (three batches) was tested at 25 °C and 40 °C when stored in nylon‐sealed bags for 1 year.28 No losses were observed at 25°C and losses up to 1% were observed after storage at 40°C.
The stability of the additive (three batches) in three vitamin–mineral premixtures (one for piglets, a second for gestating sows and a third for chickens for fattening, a different batch for each premixture) containing choline chloride (0.8, 1.6 and 2%, respectively) was studied when supplemented at inclusion rates of 5% except for the chickens for fattening that were supplemented at 7%.29 The samples were stored in sealed nylon polyethylene bags at 25 °C and 40 °C for 6 months. A loss of 2% was seen in the premixtures for piglets and for chickens for fattening at 25 °C (no loss in the premix for sows). Losses ranging from 6 to 7% were observed in all three premixtures when stored at 40°C.
The stability of the additive (three batches) was studied in a complete pelleted feed for pigs for fattening, another for gestating sows and a third one for chickens for fattening (a different batch of additive was used for each species) when supplemented at 0.38, 0.38 and 0.3%, respectively.30 The diet of piglets was based on wheat, soy and barley; that of gestating sows on barley, wheat and maize; and the diet of chicken for fattening on wheat and maize. The compound feed was preconditioned at 59–65°C and subsequently pelleted at 70, 76 or 88°C, respectively. Pelleting caused a loss of 3–5%, depending on the feed considered. Samples were stored at 25°C and at 40°C in sealed plastic bags for 3 months. Losses observed in pelleted feed stored at 25°C were 6, 1 and 17%, respectively. Those observed in pelleted feed stored at 40°C were 36, 30 and 69%, respectively.
The premixtures and feedingstuffs described above were used to study the capacity of the additive to distribute homogeneously in feed.31 Glutamine was analysed in 10 subsamples (nine in the case of feed for chicken for fattening) of each feed. The coefficients of variation (CV) of the premixtures were 1, 2 and 2% for piglet, gestating sow and chicken for fattening, respectively. The CVs of the pelleted feeds were 4, 4 and 3%, respectively.
3.1.5. Physico‐chemical incompatibilities
No physico‐chemical incompatibilities in feed are expected with other additives, medicinal products or feed materials.
3.1.6. Conditions of use
l‐Glutamine is intended to be used in feeds to achieve an adequate amino acid profile and to overcome potential glutamine shortages related to reduced capacity of synthesis during critical periods of life of all animal species.32 It can be added directly to complete and complementary feedingstuffs, or via premixtures. No inclusion levels have been proposed, as the use, in quantitative terms, depends on the species, the physiological state of the animal, the performance level, the environmental conditions and the amino acid composition of the unsupplemented diet.
As regards the use of the additive as feed flavouring, l‐glutamine is intended to be used in combination with other flavouring substances as constituents of flavouring premixtures to mash feed or other premixtures for all animal species. The recommended level of inclusion is 25 mg/kg complete feed. No specific restrictions are proposed.
3.2. Safety
3.2.1. Absorption, distribution, metabolism and excretion
In mammals, l‐glutamine is synthetised from glutamate and ammonia in a reaction catalysed by glutamine synthetase. This reaction occurs primarily in muscle, lungs, adipose tissue and liver.
Less than one‐third of the dietary glutamine intake appears in portal vein blood because of extensive intestinal utilisation (Stoll and Burrin, 2006). In neonatal, gestating and lactating pigs, the half‐life of glutamine in blood is relatively short (i.e. 0.65–0.7 h) (Wu et al., 2010a), and orally administered glutamine almost completely disappears from the circulation within 4 h.
3.2.2. Safety of the production microorganism
The recipient strain ■■■■■ is considered to be safe. The strain was modified ■■■■■ resulting in an increased production of glutamine.
C. glutamicum is considered by EFSA to be suitable for the QPS approach to safety assessment for amino acid production purposes (EFSA, 2007; EFSA BIOHAZ Panel, 2019b).
The production strain C. glutamicum NITE BP‐02524 has been properly identified at species level, does not contain ■■■■■ antibiotic resistance genes and no safety concerns were identified related to the genetic modification. The production strain fulfils the qualification to be recommended as QPS for production purposes.
3.2.3. Safety for the target species
No nutritional requirements of glutamine have been described for poultry, pigs, ruminants, cats, dogs, horses, fish or crustaceans. Glutamine and glutamate are considered non‐essential amino acids. Together with arginine, threonine and cysteine, they are involved in maintenance functions like immune surveillance system and gut mucosal repair processes. The nutritional supply of glutamine, however, may become limited at weaning or during intestinal stress due to the rapid turnover and replacement of mucosal cells (D'Mello, 2003; NRC, 2012). In such situations, dietary supplementation may become relevant.
Glutamine is usually present in high proportion in feedingstuffs in comparison to other amino acids (e.g. calculated as percentage of the crude protein, glutamine represents 1.6% in alfalfa meal, 2.6% in rye, 2.5% in barley, 2.1% in oats, 6% in soybeans, 4.2% in Phaseolus beans, 3.1% in chick peas, 5.1% in brewers grains, 3.7% in maize meal, 4.2% in cotton seed, 7.2% in blood meal, 8.4% in feather meal, 7.9% in fish meal, 7.1% in skim milk powder) (NRC, 2012).
When metabolised, l‐glutamine, generates ammonia that is usually excreted in the kidney. When glutamine intake is given in excess, it may result in increased ammonia concentration in blood. If the ammonia production exceeds the capacity of detoxification of the organism, it may cause adverse effects in the nervous system.
The additive under assessment is highly purified (> 98% glutamine and < 1% of unidentified material on dry matter basis), and the production strain qualifies for the QPS approach to safety assessment. No safety concerns would derive from the additive itself. However, as is the case for other amino acids, excess l‐glutamine may have adverse effects in target species. The applicant provided a literature review (the literature review process was not described)33 in support to the safety for the target species that is summarised below. The Panel notes that the concentration of glutamine in the basal diets was analysed in a limited number of the experimental studies. This background glutamine concentration has been estimated by the applicant from the feed ingredients according to the database published by Li et al. (2011) and when that was not possible, using the method described by Lenders et al. (2009).
In a review (Wu and Watford 2018, unpublished) of 14 studies performed in weanling piglets, gestating sows or lactating sows fed diets containing up to 3.7%, 3.1% or 3.2% total glutamine (respectively) during different periods of time (14–30 days), no adverse effects were reported on performance, intestinal development, immune response or reproductive performance in the studies considered, the endpoints investigated depending on the study design (Wu et al., 1996, 2011, 2013; Bignell, 2014; Pardo et al., 2014; Santos de Aquino et al., 2014; Teixeira et al., 2014; Athorn and Hermann, 2016; He et al., 2016a,b; Zhang et al., 2017; Yang et al., 2018).34
In a trial in 1‐day‐old chickens for fattening, Bartell and Batal (2007) supplemented a basal diet of maize and soybean meal (glutamine concentration in the basal diet not measured, estimated 2% glutamine) with 1 or 4% glutamine for 21 days. No adverse effects were seen in growth performance, intestinal development and humoral immune response in birds receiving glutamine at 1% but body weight gain was significantly depressed in the group receiving 4% glutamine compared to the 1% supplemented group. In another trial with 1‐day‐old chickens for fattening, Soltan (2009) supplemented a diet based on maize, soybean meal and fish meal (glutamine concentration in the basal diet not measured, estimated in 1.7–1.9% glutamine) with up to 2% glutamine for 21 days. Performance parameters were optimised at 1% glutamine supplementation and a significant decrease on body weight gain, or blood parameters (RBC count, WBC count, %HB or PCV) was observed in the group treated with 4% histidine compared with the group receiving 1%.35 In a study in helmeted Guineafowl (Numida meleagris) of 38 weeks of age, basal diets containing maize and soybean meal or maize, soybean meal and wheat (concentration of glutamine in basal diet not described, estimated in 1.6–2% glutamine) were supplemented at 0, 0.5% and 1% with glutamine and birds were fed for 6 weeks. No adverse effects were seen in performance or reproductive parameters. When blood parameters were analysed, an increase of white blood cells, of the fraction of heterophils and of the heterophil/lymphocyte ratio was associated with the supplementation of glutamine. Serum chemistry showed significant decreased glucose concentration and TSH (thyroid‐stimulating hormone) concentration in at least one of the supplemented groups of both basal diets tested. Serum concentration of FSH (follicle‐stimulating hormone) and LH (luteinising hormone) significantly increased in both supplemented groups in both basal diets tested. T4 (thyroxine) serum concentration was less consistent showing a significant decrease at 1% glutamine in the maize and soybean meal diet; and a significant increase at 1% glutamine in the diet containing wheat in addition (Gholipour et al., 2017).
Calves of 3 days of age can tolerate well dietary supplementation of milk replacer containing 60% soy protein concentrate with 1% glutamine for 4 weeks as no differences were observed in final BW, average daily gain (ADG), dry matter (DM) intake and gain to feed in comparison to the group not supplemented with glutamine (Drackley et al., 2006). Weaned calves (8w of age) receiving a daily intake of 0.67 g/d of glutamine and 0.67 g/d of glutamate for 2 weeks showed no adverse effects on performance, faecal scores or metabolism (Da Silva et al., 2018). In a study by Plaizier et al. (2001), four Holstein cows in a 4 × 4 Latin square design with 2‐week periods were infused in the abomasum with 200 or 300 g glutamine/day. The highest dose caused decrease of phenylalanine in blood although there were no adverse effects on dry matter feed intake, milk yield and milk composition. Multiparous Holstein cows (crossover design involving eight animals, basal diet consisting on TMR containing maize, grass hay, high moisture maize and protein supplement based on soybean meal) could tolerate well as much as intra‐abomasal infusions of 300 g glutamine/day for 21 days, equivalent to approximately 1% of dry matter intake, as it did not adversely affect DM intake, milk yield or milk composition (Doepel et al., 2007). Italian Frisian dairy cows fed a diet containing maize, oat hay and concentrate supplemented with 160 g/day rumen protected glutamine for 60 days did not show adverse effects on milk production (Caroprese et al., 2012).
In reference to small ruminants, Ocheja et al. (2017) reported that during the hot‐dry season, red Sokoto Nigerian goats fed a diet of maize offal, groundnut hay and smuts finger grass (Digitaria smutsi) hay could tolerate well oral administration of 0.2 g glutamine/kg bw per day for 21 days and no negative effects were seen on rectal temperature, erythrocyte osmotic fragility, serum antioxidant enzyme activities and serum malondialdehyde concentration.
As regards fish, juvenile Channel catfish (Ictalurus punctatus) of 6 g body weight fed a basal diet consisting of casein, gelatine, crystalline amino acids premix (without glutamine), dextrin and lipids of maize and menhaden fish oil can tolerate well dietary supplementation with up to 3% glutamine for up to 70 days (Pohlenz et al., 2012a,b) as no differences in growth‐related parameters were seen. Juvenile turbots (Scophthalmus maximus) of 8 g body weight grew well when a basal diet made up of soybean meal, fish meal and wheat was supplemented with 1.5% glutamine for 8 weeks and no adverse effects on growth parameters were seen (Gu et al., 2017). Also, juvenile grass carp (Cyprinus carpio var. Jian) of 8 g body weight did not exhibit any adverse effects in growth parameters when a diet based on fish meal, rice gluten meal and starch was supplemented with up to 2% glutamine for 80 days (Yan and Qiu‐Zhou, 2006). Similar results were obtained for juvenile red drum, hybrid striped bass (Morone chrysops×Morone saxatilis) when a basal diet formulated from menhaden fishmeal and soybean meal was supplemented up to 2% with glutamine for 8 weeks (Cheng et al., 2012). Half‐smooth tongue sole (Cynoglossus semilaevis) post larvae (11 mg body weight) fed a basal diet of fish meal, meat and bone meal, starch and fish oil were supplemented up to 2% with glutamine for 30 days and no adverse effects were seen in growth parameters (Liu et al., 2015). In a study in juvenile Gilthead seabream (Sparus aurata) of 13 g body weight, Coutinho et al. (2016) showed that a basal diet consisting on soybean meal, fish meal, wheat meal and fish oil supplemented up to 2% with glutamine for 6 weeks had no adverse effects on growth performance parameters. Assuming a glutamine content of 1.5–2% in the basal diets, the different studies reviewed suggest that various species of fish can tolerate up to 4% of total glutamine in diets. Available evidence shows that dietary supplementation with at least 2% glutamine does not result in adverse or undesirable effects on fish.
In a 90‐day oral toxicity study in rats, concentrations 1.25, 2.5 and 5% glutamine supplementation were tested (Tsubuku et al., 2004). Infrequent changes were observed in some urinalysis and haematological parameters in the 2.5% and 5.0% groups. The FEEDAP Panel considers these changes of no toxicological relevance and considered the 5% glutamine supplementation, the highest dose tested, as the NOAEL for both genders (males 3,320 mg/kg bw per day and females 3,840 mg/kg bw per day).
Overall, pigs did not show adverse effects when fed a diet containing an estimated total glutamine concentration up to 3%. A total glutamine concentration of 3% glutamine in the diet appears to be safe for chicken for fattening but concentrations above 3% in the diet adversely affect body weight gain. A total of 3% glutamine in the diet was safe for Guinea fowl. There are more uncertainties in the case of ruminants, fish and rats, as there were practically no estimates of glutamine content on the experimental basal diets in the studies described above.
Overall, the use of l‐glutamine produced by fermentation using C. glutamicum NITE BP‐02524 in levels suitable to achieve an adequate amino acid profile and to overcome potential glutamine shortages related to reduced capacity of synthesis during critical periods of life is considered safe for the target species. However, as seen with other amino acids, there is evidence in the scientific literature of possible adverse effects at some supplementation levels for the target species.
Since the recommended use level proposed for the use of l‐glutamine as flavouring compound (25 mg/kg complete feed) is substantially lower than natural background contents of glutamine in feed ingredients, the FEEDAP Panel considers l‐glutamine produced with C. glutamicum NITE BP‐02524 is safe for the target species when used as a flavouring compound.
3.2.3.1. Conclusions on safety for the target species
The use of l‐glutamine produced by fermentation using C. glutamicum NITE BP‐02524 in animal nutrition is considered safe for all animal species when applied as a nutritional additive to achieve an adequate amino acid profile in feed and to overcome potential glutamine shortages during critical periods of life.
The proposed use level (25 mg/kg feed) when used as sensory additive (flavouring compound) is safe for all animal species.
3.2.4. Safety for the consumer
l‐Glutamine is produced endogenously by the human organism and it is present in human plasma at high concentrations (up 20% of the free α‐amino acid pool of human blood plasma) (Watford, 2008).
Glutamine is authorised for use in food for nutritional purposes,36 and as flavouring (FL No 17.007) without maximum levels,37 and for use in cosmetics.38
When glutamine exceeds the nutritional needs, it undergoes oxidative degradation which results in the elimination of the excess nitrogen in the form of urea (mammals) or uric acid (birds).
It is not expected that the use of l‐glutamine as nutritional additive or as flavouring compound will modify the exposure of the consumer to l‐glutamine via the normal diet.39
The product under assessment is produced by fermentation. Concerns for the consumer would not derive from the amino acid itself, which will be incorporated into proteins, but from possible residues from fermentation. The production strain meets the QPS qualifications and the absence of viable cells of the production strain and of its recombinant DNA in the final product has been demonstrated. Considering the above and the fact that l‐glutamine originating from C. glutamicum NITE BP‐02524 is highly purified (> 99% l‐glutamine and <1% unidentified material on a dry matter basis), its use as nutritional additive or as flavouring compound is considered safe for the consumer.
3.2.4.1. Conclusions on safety for the consumer
The use of l‐glutamine produced using Corynebacterium glutamicum NITE BP‐02524 as nutritional additive or as feed flavouring compound is considered safe for consumers of food derived from animals.
3.2.5. Safety for the user
The applicant submitted an acute inhalation toxicity test, an in vitro skin irritation test, an in vitro acute eye irritation test and a skin sensitisation test performed with the additive under assessment.
3.2.5.1. Effects on the respiratory system
The dusting potential is up to 5.6 g/m3 The data on particle size distribution indicate that about 10% and 90% of the particles had a size < 10 and < 69 μm of diameter, respectively (see Section 3.1.3). So, exposure of users by inhalation is likely to occur.
In an acute inhalation toxicity study in rats in accordance with OECD Guideline 403,40 10 Wistar outbred (Crl:WI(Han)) rats (5 of each sex) were exposed to a concentration of 5.13 g glutamine/m3 for 4 h. Mortality did not occur and no relevant adverse effects were observed. Based on those results, it was concluded that the 4 h LC50 of l‐glutamine in rats is above 5.13 g/m3.
3.2.5.2. Effects on skin and eyes
In an in vitro skin irritation test using reconstructed skin membranes in accordance with OECD Guideline 439,41 skin membranes were exposed to 25 mg of additive for 60 min. Viability of the epidermal cells was assessed using the yellow tetrazolium salt (MTT) test at ca. 42 h post‐exposure. Negative and positive controls were run in parallel and performed as expected. Based on the results obtained, l‐glutamine was considered as non‐irritant (UN GHS No Category).
In an in vitro acute eye irritation study using the isolated chicken eye test in accordance with OECD Guideline 438,42 isolated chicken eyes were exposed to a single application of 30 mg l‐glutamine for 10 seconds followed by a 20 mL saline rinse. Corneal thickness (expressed as corneal swelling), corneal opacity and fluorescein retention of damaged epithelial cells were surveyed. No corneal effects were observed macroscopically and/or microscopically. The positive and negative controls performed as expected. The results indicate that the additive does not need to be classified because of its irritancy to eyes.
In a skin sensitisation test using Local Lymph Node Assay (LLNA) in mice in accordance with OECD Guideline 429,43 the test item was dissolved in 1,2‐propylene glycol. The positive control (α‐hexylcinnamic aldehyde) was dissolved (25%) in DMSO. A pre‐screen test was performed using a dose of 100% (1 g/mL). Based on the results of the pre‐screen test, the dose 100% was selected as top dose for the main test. Five female mice (CBA/Ca) per group were topically exposed (dorsum of both ears) to the test item at concentrations of 25%, 50% and 100%, to the positive control and to the vehicle only. The animals did not show visible clinical symptoms of either local irritation or systemic toxicity when exposed to the test item or the negative control. The additive is not considered a skin sensitiser under the test conditions of this study.
3.2.5.3. Conclusions on safety for the user
l‐Glutamine produced using Corynebacterium glutamicum NITE BP‐02524 is not toxic by inhalation, non‐irritant to skin and eyes, and is not a skin sensitiser.
3.2.6. Safety for the environment
Viable cells of the production strain and its DNA were confirmed not to be present in the final product. The final product does not pose any environmental safety concern associated with the genetic modification of the production strain.
l‐Glutamine is an amino acid present in animals and plants proteins. The amino acid l‐glutamine, when supplemented to feed, will be incorporated into proteins of tissues and/or products of animal origin and any potential excess will be catabolised and excreted as urea/uric acid and carbon dioxide. Therefore, the addition of the additive under assessment to feedingstuffs will not lead to an increase of its concentration in the excreta of animals and in the environment.
3.3. Efficacy
The amino acid l‐glutamine is an amide of glutamic acid. It is a non‐essential amino acid and it plays a physiological role as such. For instance, it is an amino‐group donor, for the synthesis of amino acids, purines and pyrimidines. It can be synthesised endogenously, and therefore, no quantitative requirements have been established. Under practical feeding conditions of most animal species, the requirement is met by the surplus of crude protein.
There is no evidence for a different metabolic role of glutamine in the different animal species.
More recent literature considers glutamine as conditionally essential (it has been recognised as such in pig by NRC (2012)). Alternatively, it is considered semi‐essential for growing animals where the body synthesis would not provide enough glutamine to meet all the nutritional needs. There is recent scientific evidence of extra‐nutritional effects in improving intestinal development (Wu et al., 1996; Domeneguini et al., 2004; Yi et al., 2005; Bartell, 2006a,b; Domeneguini et al., 2006; Bartell and Batal, 2007; Soltan, 2009; Scanes, 2014; He et al., 2016a; Namroud et al., 2017; Johnson and Lay, 2017) and immune response (Domeneguini et al., 2004; Bartell, 2006a,b; Bartell and Batal, 2007; Lee et al., 2003; Soltan, 2009; Duttlinger et al., 2019; He et al., 2016b) in young and growing chickens and piglets. Glutamine is important for highly proliferative cells such as T cells, B cells and macrophages and it serves as fuel for enterocytes (Domeneguini et al., 2004, 2006). In adult rat, more than 60% of dietary glutamine is used in the gut itself (Wu, 1998).
Requirement data or recommendations for practical feeding are not available neither in absolute (percentage of diet) nor in relative figures (e.g. to crude protein, to essential amino acids (EAAs), to a certain amino acid or to non‐essential amino acids (NEAA)); the feedstuffs data bank for glutamine is small and the mode of action of glutamine is not fully understood. Since about 90% of the body's own glutamine synthesis is made in the muscle tissue, it may be hypothesised that the effect of glutamine in growing and adult animals may be different.
It is difficult to demonstrate an effect for non‐essential amino acids because principally the NEAA can replace each other. However, for glutamine, this principle might not apply since the effect of glutamine on growth cannot be reproduced to the same extent by glutamic acid as shown in chicken (Bartell, 2006a,b; confirmed by Ebadiasl, 2011, see Section 3.3.1) or in weaned piglet (He et al., 2016b, see Section 3.3.2).
In general, the product l‐glutamine is considered an efficacious source of the non‐essential amino acid l‐glutamine for non‐ruminant animal species. For the supplemental l‐glutamine to be as efficacious in ruminants as in non‐ruminant species, it would require protection against degradation in the rumen.
As l‐glutamine is used in food as flavouring compound (FLAVIS No 17.007), it is expected that it can provide a similar function in feed and no further demonstration of efficacy is necessary.
The applicant performed a literature search to support the efficacy of the additive in chickens for fattening and in weaned piglets. PubMed and Google Scholar were searched using combinations of the search terms ‘glutamine’, ‘dose response’, ‘supplementation’, ‘pig’ and ‘poultry’. Due to the high number of hits retrieved in Google Scholar, the search was limited to the first 50 references listed. Inclusion criteria (articles or thesis before June 2017, interventional studies with suckling piglets and chickens for fattening, studies focusing on effects of glutamine on performance/gut development) and exclusion criteria (not in English or French language, glutamine not administered orally) were considered. As a result, 12 publications in young pigs and seven in chickens for fattening were selected. From a manual search, six additional records were added. The applicant estimated glutamine and glutamic acid content in the experimental diets based on data provided by Li et al. (2011) and when that was not possible, using the method described by Lenders et al. (2009).
3.3.1. Studies in chickens for fattening
A total of nine publications describing 11 studies in chickens for fattening were submitted. One study was not considered further due to the limitations in the study design which related basically to the lack of a proper control diet (Namroud et al., 2017 [experiment 2]).
A summary of the study design of the studies included in the assessment is presented in Appendix A (Table A.1). All basal diets were made of maize and soybean meal except in one study (Ebadiasl, 2011) which used a wheat‐ and soybean meal‐based diet. Birds were 1‐day‐old chicks of commercial breeds, and the glutamine supplementation (0–4%) and the duration (7–45 days) of the studies varied. Only three studies had a duration of at least 35 days (Bartell, 2006a,b; Soltan, 2009; Ebadiasl, 2011). The publications reported in most of the cases the crude protein content of the experimental diets (19–23%) and the applicant provided an estimation of the content of glutamine (1.6–3%) and glutamic acid (1.3–2%). One study tested different levels of vitamin E and glutamine in a two‐factorial design (Sakamoto et al. (2006), no interaction found regarding performance parameters). The parameters measured varied with the study and included body weight and feed intake (data not shown), histometric measurements of the intestinal mucosa (e.g. villus height and crypt depth, see Appendix A, Table A.2), immune response parameters (e.g. serum IgA and IgG, intestinal IgA, phagocytic activity, see Appendix A, Table A.3), as well as growth performance (see Appendix A, Table A.4). The study design in some of the studies considered treatments other than glutamine but are not presented in the tables/text below, unless considered necessary.
Table A.1.
Details of the designs of the studies conducted with glutamine in chickens for fattening
| Source | Basal diet | CPa (%) | Gln/Glub (%) | Gln supplementation (duration in days) | Replicates/treatment (birds/replicate) |
|---|---|---|---|---|---|
| Bartell, 2006a, PhD Chapter 4 |
Maize and soybean. Gln supplemented at expense of soybean |
23 | 1.6/2.0 |
Control, 1% glutamine (s 1)c 2% glutamine (s 1) 1% glutamine (s 2) (21 d) |
6 (10) |
| Bartell, 2006a, PhD Chapter 5 |
Maize and soybean. Gln supplemented at expense of soybean |
23 (19d) or 18–23 (40d) |
2.0/2.0 (19d) or 1.7–2/1.5–2 (40d) |
Control 1% glutamine (19d) 1% glutamine (40d) |
|
| Sakamoto et al. (2006) | Maize and soybean meal. Gln supplemented at the expense of soybean | 19–22 | 1.87/1.79 |
Vit E 10 mg/kg + 0 Gln Vit E 10 mg/kg + 1 Gln 7d Vit E 10 mg/kg + 1 Gln 14d Vit E 500 mg/kg + 0 Gln Vit E 500 mg/kg + 1 Gln 7d Vit E 500 mg/kg + 1 Gln 14d |
5 (50) |
| Bartell and Batal, 2007. Study 1 |
Maize and soybean. Gln supplemented at expense of soybean |
23 | 2.0/2.0 |
Control 1% feed 4% feed (21 d) |
6 (10) |
| Bartell and Batal, 2007. Study 2 |
Maize and soybean. Gln supplemented at expense of soybean |
23 | 2.0/2.0 |
Control 1% (4 d) 1% (7 d) 1% (14 d) 1% (21 d) |
6 (10) |
| Soltan, 2009; |
Maize, soybean, fish meal |
19–22 | 1.7–1.9/1.6–1.8 |
Control 0.5% Gln 1.0% Gln 1.5% Gln 2.0% Gln (42 d) |
2 (25) |
| Fasina et al. (2010) | Maize and soybean meal. Gln sup. at expense of soybean | 22 | 1.7/1.63 |
Control 1% Gln |
4 (16) |
| Ebadiasl (2011) | Wheat and soybean meal. Gln supplemented at expense of soybean | 19 | 3/1.3 |
Control 0.5% Gln 1% Gln (35d) |
5 (8) |
| Luquetti et al. (2016) | Maize and soybean meal. Gln supplemented at expense of kaolin | 22 | 2.0/1.9 |
Control 1% Gln (28 d) |
4 (25) |
|
Namroud et al. (2017) Study 1 |
Maize, soybean. Gln supplemented at the expense of cellulose | 23 | 2.0/1.95 |
Control 1% Gln 2% Gln |
5 (16) |
Crude protein in the basal diet.
Glutamine/glutamate content estimated in the basal diet.
s1: source 1 of glutamine; s2: source 2 of glutamine.
Table A.2.
Effects of glutamine on intestinal development in chickens for fattening: villi height and crypt depth (μm)
| Source | Day | Supplemental glutamine in the diet (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | 4.0 | ||
| Bartell, 2006a,b. Chapt. 42 | 21 | ||||||
| Duodenal villi height | 336c | 557a/518a, 1 | 585a | ||||
| Bartell, 2006a,b. Chapt. 5 | 25 | ||||||
| Duodenal villi height | 287a | 606b | |||||
| 40 | |||||||
| Duodenal villi height | 572a | 807b | |||||
| Bartell and Batal, 2007. Study 1 | 21 | ||||||
| Duodenal villi height | 739b | 908a | 937a | ||||
| Jejunal villi height | 447c | 750b | 784a | ||||
| Bartell and Batal, 2007. Study 2 | 21 | ||||||
| Duodenal villi height | 746b | 1,091a | |||||
| Jejunal villi height | 591b | 1,049a | |||||
| Soltan (2009) | 21 | ||||||
| Duodenal villi height | 891b | 993ab | 1023a | 1047a | 1090a | ||
| Duodenal crypt depth | 173a | 150ab | 141ab | 135b | 129b | ||
| Jejunal villi height | 457a | 621b | 730a | 747a | 785a | ||
| Jejunal crypt depth | 101a | 95a | 92a | 90a | 87a | ||
| Ebadiasl (2011) | 34 | ||||||
| Villi height mid intestine | 1482 | 1614 | 1,713 | ||||
| Crypt depth | 166 | 190 | 153 | ||||
| Luquetti et al. (2016) | 28 | ||||||
| Duodenal villi height | 1,481 | 1,545 | |||||
| Duodenal crypt depth | 293 | 288 | |||||
| Jejunal villi height | 1,101a | 1,238b | |||||
| Jejunal crypt depth | 251 | 218 | |||||
| Ileal villi height | 1,028 | 1,060 | |||||
| Ileal crypt depth | 187 | 203 | |||||
| Namroud et al. (2017) | 7 | ||||||
| Duodenal villi height | 606a | 688b | 695b | ||||
| Duodenal crypt depth | 155 | 153 | 156 | ||||
| Jejunal villi height | 531a | 616b | 608b | ||||
| Jejunal crypt depth | 128a | 107b | 104b | ||||
| Ileal villi height | 251a | 304b | 310b | ||||
| Ileal crypt depth | 80.2a | 75.1b | 74.0b | ||||
| 14 | |||||||
| Duodenal villi height | 1,218 | 1,232 | 1,227 | ||||
| Jejunal villi height | 936a | 1,105b | 958a | ||||
| Ileal villi height | 690a | 720b | 680a | ||||
First value with the same Gln source as Bartel and Batal; second value with another Gln source.
Ch: chapter.
a,b,c Means within a row with different superscript letters are significantly different (p ≤ 0.05).
Table A.3.
Effect of glutamine supplementation in immune response of chickens for fattening
| Source | Day | Supplemental glutamine in the diet (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | 4.0 | ||
| Bartell, 2006a,b. Chapt. 41 | 21 | ||||||
| Serum IgA (ng/mL) | 0.808b | 1.806a/1.694a | 1.784a | ||||
| Serum IgG (ng/mL) | 1.579b | 1.971a/1.874a | 1.953a | ||||
| Intestinal IgA (ng/mL) | 0.505b | 1.310a/1.380a | 1.144a | ||||
| Bartell, 2006a,b. Chapt. 5 | 25 | ||||||
| Serum IgA (ng/mL) | 1.324a | 2.262b | |||||
| Intestinal IgA (ng/mL) | 1.898a | 2.235b | |||||
| 40 | |||||||
| Serum IgA (ng/mL) | 1.626a | 2.311b | |||||
| Intestinal IgA (ng/mL) | 1.716a | 2.317b | |||||
| Bartell and Batal, 2007. Study 1 | 21 | ||||||
| Serum IgA (ng/mL) | 1.853b | 2.808a | 1.276c | ||||
| Serum IgG (ng/mL) | 1.829c | 2.506a | 2.065b | ||||
| Intestinal IgA (ng/mL) | 2.784b | 3.509a | 2.455b | ||||
| Bartell and Batal, 2007. Study 2 | 21 | ||||||
| Serum IgA (ng/mL) | 1.293b | 2.914a | |||||
| Serum IgG (ng/mL) | 2.290b | 3.475a | |||||
| Intestinal IgA (ng/mL) | 1.905b | 3.267a | |||||
| Soltan (2009) | 42 | ||||||
| Phagocytic activity2 | 18.2b | 19.2b | 22.1a | 19.8ab | 17.8b | ||
| HI3 | 4.35b | 4.62b | 5.34a | 4.42b | 3.98b | ||
For supplemental level 1% Gln, first value obtained with Gln source 1 and second value with Gln source 2.
According to Kawadara et al., 1991: (macrophages containing yeast/total number of macrophages) × 100.
Hemagglutination inhibition test. Geometric mean antibody titre (log2) against Newcastle disease virus vaccine.
a,b,c: Means within a row with different superscript letters are significantly different (p ≤ 0.05).
Table A.4.
Effect of glutamine supplementation on growth. Relative change in body weight gain (total or daily), unsupplemented control = 100
| Source | Day | Supplemental glutamine in the diet (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | 4.0 | ||
| Bartell, 2006a,b. Chapter 41 | 21 | 100b | 111c/93a1 | 91a | |||
| Bartell, 2006a,b. Chapter 52 | 25 | 100 | 114 | ||||
| Sakamoto et al., 2006; | 14 | 100 | 101 | ||||
| Bartell and Batal, 2007. Study 1 | 21 | 100 | 109 | 90 | |||
| Bartell and Batal, 2007. Study 2 | 21 | 100a | 109b | ||||
| Soltan (2009) | 42 | 100b | 103b | 108a | 99b | 98b | |
| Fasina et al., 2010. Study 13 | 14 | 100 | 95 | ||||
| Fasina et al., 2010. Study 2 | 14 | 100a | 110b | ||||
| Fasina et al., 2010. Study 24 | 14 | 100 | 104 | ||||
| Ebadiasl (2011) | 35 | 100 | 104 | 104 | |||
| Luquetti et al. (2016)5 | 28 | 100 | 99 | ||||
| Namroud et al., 2017. Study 1 | 14 | 100b | 107 | 97 | |||
For supplemental level 1% Gln, first value obtained with Gln source ‘A’ and second value with Gln source ‘B’.
Means of four groups: untreated, challenged by Eimeria infection, vaccinated against coccidiosis and challenged + vaccinated.
Birds were challenged with S. Typhimurium (3.6 × 106 cfu/mL) at day 3 of age.
Birds were challenged with S. Typhimurium (7.4 × 107 cfu/mL) at day 3 of age.
Means of birds non‐vaccinated and vaccinated against E. acervulina, E. Maxima, E. Mivati and E. tenella at day 1.
a,b Means within a row with different superscript letters are significantly different (p ≤ 0.05).
Glutamine supplementation shows effects on intestinal development (Bartell, 2006a,b; Bartell and Batal, 2007; Soltan, 2009; Namroud et al., 2017) and immune response (Bartell, 2006a,b; Bartell and Batal, 2007; Soltan, 2009) in young and growing animals. The effects on body weight, duodenal villi height and serum and intestinal IgA as well as serum IgG seen after glutamine (1%) administration cannot be reproduced with application of other NEAA as glutamic acid, aspartic acid and histidine (Bartell, 2006a,b ‐ chapter 4, data not shown). These findings are confirmed by Ebadiasl (2011) at glutamine concentrations of 0.5 and 1.0% on body weight gain and villi height (data not shown). It should also be considered that, in contrast to the small effective dose range of glutamine on body weight gain, in general (with the exception of Namroud et al., 2017 [measurements at 14d]) villus height of enterocytes increase dose dependently until the highest dose tested (2%, Soltan, 2009; and 4%, Bartell and Batal, 2007; [study 1]).
Overall, l‐glutamine is considered to be an amino acid effective in chickens for fattening in increasing the resorptive surface of the small intestine and providing some positive effects on immune response and improving body weight. The dose range providing positive effects on zootechnical performance is rather small; supplements above 1% tend to reduce body weight gain (Bartell, 2006a,b – Chapter 4; Soltan, 2009).
3.3.2. Studies in weaned piglets
The search retrieved a total of 16 publications including 21 studies in piglets. Four of them were not considered further due to the limitations in the study design which related basically to the lack of a proper control diet (Wang et al., 2008, 44; Wang et al., 2015, 45; Zhong et al., 2014, 46; and Pulske et al., 1996).47
A summary of the study design of the studies considered is presented in Appendix B (Table B.1). The studies were in all cases, except one (Cabrera et al., 2013), restricted to the weaning period. In the studies conducted during the weaning phase, the supplementation and follow‐up of the piglets was for short periods of time, supplementation for 7–28 days duration. In one of the studies, Duttlinger et al., 2019, the piglets were treated in the weaning phase but were followed up until slaughter. In the study of Cabrera et al., 2013, the animals started receiving glutamine at the lactation phase (7 days of creep feeding) and were followed until the weaning phase, some treatment groups received glutamine from 7 days preweaning up to 21 days after weaning, and another treatment group only during the weaning period (21 days). Three of the studies challenged the piglets in order to see the effect of glutamine after challenging: in Yi et al. (2005), the animals were challenged with Escherichia coli K 88+; in Kitt et al. (2003), the piglets were challenged with lipopolysaccharides from E. coli; and in Duttlinger et al. (2019), the piglets were weaned and subject to a transportation of 12 h travel prior to starting the experimental phase. Supplementation of the diets differed between the studies but was done in the range 0.2–5%. The publications reported, in most of the cases, the crude protein content of the diets and the applicant provided an estimation of the content of glutamine and glutamic acid. The study design in some of the studies considered treatments other than the glutamine but are not presented in the tables/text below, unless considered necessary.
Table B.1.
Details on the designs of the studies conducted with glutamine in weaned piglets
| Source | Basal diet | CP (%) Gln/Glu | Gln suppl. (%) | animals/replicate Replicates/treatment | Initial body weight (kg) start day of life Duration of study (d) |
|---|---|---|---|---|---|
| Wu et al., 1996. Study 2 | Maize, soybean meal, soybean oil, Gln at expense of starch |
21.1 2.0/1.9 |
0 1 |
5 2 |
– 21 14 |
| Wu et al., 1996. Study 3 | Maize, soybean meal, soybean oil, Gln at expense of starch |
21.1 2.0/1.9 |
0 0.2 0.6 1 |
2 5 |
– 21 14 |
| Kitt et al. (2003)1 | Maize, lactose, sucrose, maize oil, Gln at expense of maize. |
– 10.8 Glx2 |
0 5 |
12 1 |
– 20 14 |
| Domeneguini et al. (2004) | Maize, soybean meal, barley, whey powder, wheat bran, milk powder, soybean oil |
18.1 1.8/1.3 |
0 0.5 |
4 1 |
5 21 28 |
| Yi et al. (2005)3 | Maize, soybean meal, dried whey, fish meal Gln at expense of starch |
21.0 – |
0 2 |
4 10 |
5.3 17 14 |
| Domeneguini et al. (2006) | Maize, soybean meal, barley, whey, wheat bran and milk powder |
18.8 1.8/1.3 |
0 0.5 |
4 2 |
5.0 21 28 |
| Lee et al., 2003. Study 1 | Maize, soybean meal, soy protein and whey, Gln at expense of starch |
23.7 1.9/1.8 |
0 0.5 1 1.5 |
4 3 |
6.0 21 28 |
| Lee et al., 2003. Study 2 | Maize, soybean meal, soy protein and whey, Gln at expense of starch |
23.7 1.9/1.8 |
0 0.5 1 1.5 |
4 3 |
– 21 14 |
| Hsu et al. (2010) | Maize, soybean meal, dried skim milk, whey |
20.3 2/1.8 |
1 2 |
4 4 |
6.7 28 21 |
| Cabrera et al. (2013)4 | Maize, soybean meal, choice white grease. Gln at expense of maize |
20‐22 1.7‐1.9/1.7‐2 |
0 1 |
24 6 |
6.5 – 42 |
| He et al. (2016a) | Maize, soya bean meal, fish meal, rice bran |
20.0 1.6/1.7 |
0 1 |
1 7 |
6.0 28 28 |
| He et al. (2016b) | Maize, soybean meal, extruded maize, fish meal and soybean oil. Gln at expense of maize |
19.4 1.6/1.7 |
0 1 |
10 5 |
9 31 28 |
| Johnson and Lay (2017) | Maize, soybean meal, dried whey, soybean oil |
22.8 1.5/– |
0 0.2 |
20 1 |
5.5 18 14 |
| Duttlinger et al. (2019)5 | Nursery: Maize, dried whey, soybean meal, soybean oil, fish meal |
24‐21 1.5/‐ |
0 0.2 |
10 8 |
5.6 18 14 |
On day 7 of the study, the animals were challenged with lypolysaccharides from E. coli. The study considered the effects of challenging and glutamine supplementation.
Only the sum of Gln and Glu was available, expressed as Glx.
The trial included a challenge of the piglets on day 11 of study with Escherichia coli K88+. The study followed an incomplete design with two control groups with/without challenge, but glutamine treatment was considered only in challenged animals.
The study considered the effect of glutamine when administered prior to weaning and the effect of glutamine after weaning.
The study considered a challenge with 12 h transport and a follow up period until the slaughter age.
The parameters measured varied with the study, but the following were included: histomorphologic measurements of the intestinal mucosa (e.g. villus height and crypt depth) in 10 studies (see Appendix B, Table B.2), immune response parameters in six studies (see Appendix B, Table B.3), as well as body weight in all 12 studies (see Appendix B, Table B.4). Other parameters were also described in the scientific reports but are reported in those tables.
Table B.2.
Effects of glutamine on the intestinal histomorphology including villi height (μm) and crypt depth (μm) of weaned piglets
| Source Parameter | Day | Glutamine supplemented (%) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.2 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| Wu et al. (1996)1 | |||||||
| Duodenal villi height | 7 | 365 | 363 | ||||
| Jejunal villi height | 270a | 358b | |||||
| Duodenal crypt depth | 305 | 312 | |||||
| Jejunal crypt depth | 237 | 256 | |||||
| Duodenal villi height | 14 | 423 | 455 | ||||
| Jejunal villi height | 477 | 447 | |||||
| Duodenal crypt depth | 423 | 421 | |||||
| Jejunal crypt depth | 301a | 279b | |||||
| Domeneguini et al. (2004) | 28 | ||||||
| Ileal villi height | 148a | 200b | |||||
| Ileal crypt | 80b | 152a | |||||
| Yi et al. (2005)2 | 14 | ||||||
| Jejunal villi height | 230b | 297a | |||||
| Ileal villi height | 187b | 248a | |||||
| Jejunal crypt depth | 88 | 82 | |||||
| Ileal crypt depth | 94 | 80 | |||||
| Domeneguini et al. (2006) | 28 | ||||||
| Ileal villi height | 168a | 208b | |||||
| Ileal crypt | 110a | 166b | |||||
| Lee et al. (2003)3 | |||||||
| Duodenal villi height | 7 | 250a | 406b | 349ab | |||
| Jejunal villi height | 221 | 266 | 286 | ||||
| Ileal villi height | 209 | 256 | 293 | ||||
| Duodenal crypt depth | 197 | 230 | 250 | ||||
| Jejunal crypt depth | 194 | 200 | 223 | ||||
| Ileal crypt depth | 210 | 203 | 186 | ||||
| Duodenal villi height | 14 | 282a | 379b | 430b | |||
| Jejunal villi height | 321 | 401 | 376 | ||||
| Ileal villi height | 296 | 353 | 361 | ||||
| Duodenal crypt depth | 254 | 243 | 248 | ||||
| Jejunal crypt depth | 208 | 213 | 222 | ||||
| Ileal crypt depth | 223 | 221 | 207 | ||||
| Hsu et al. (2010) | 21 | ||||||
| Duodenal villi height | 282 | 345 | 332 | ||||
| Jejunal villi height | 306 | 377 | 397 | ||||
| Ileal villi height | 260 | 349 | 302 | ||||
| Duodenal crypt depth | 266 | 282 | 260 | ||||
| Jejunal crypt depth | 202 | 242 | 211 | ||||
| Ileal crypt depth | 193 | 192 | 196 | ||||
| Cabrera et al. (2013) | 21 | ||||||
| Jejunal villi height | 560 | 576/568 | |||||
| Jejunal crypt depth | 192 | 218/225 | |||||
| He et al. (2016a)3 | 28 | ||||||
| Jejunal villi height | 150a | 280b | |||||
| Ileal villi height | 190 | 160 | |||||
| Jejunal crypt depth | 100a | 225b | |||||
| Ileal crypt depth | 120 | 100 | |||||
| He et al. (2016b) | 28 | ||||||
| Duodenal villi height | 326 | 407 | |||||
| Jejunal villi height | 328 | 341 | |||||
| Ileal villi height | 300 | 315 | |||||
| Duodenal crypt | 310 | 312 | |||||
| Jejunal crypt | 286 | 266 | |||||
| Ileal crypt | 254 | 231 | |||||
| Johnson and Lay (2017)4 | 14 | ||||||
| Duodenal villi height | 255 | 270 | |||||
| Jejunal villi height | 185a | 245b | |||||
| Ileal villi height | 225a | 255b | |||||
| Duodenal crypt depth | 140 | 135 | |||||
| Jejunal crypt depth | 135 | 155 | |||||
| Ileal crypt depth | 125 | 135 | |||||
Estimated from a figure.
The treatment with glutamine was applied to E.coli K88+ challenged animals and is compared with values of a challenged control group. For jejunum, values are for proximal jejunum, the study reports also mid jejunum values which showed the same trend as the one identified in the proximal section.
The data refers to the second experiment presented in the study.
Values estimated from figures.
a,b values within one row with different superscript are significantly different (p < 0.05).
Table B.3.
Effects of glutamine on the immune response of weaned piglets
| Lee et al. (2003)1 | Concentration of IgA in bile was significantly increased at d7 post‐weaning (0.5% Gln) but not at day 14 post‐weaning. |
| Domeneguini et al. (2004) |
Increases in the number of macrophages and intra‐epithelial lymphocytes in the ileum and a reduction in the apoptosis in the gut‐associated lymphoid tissue
|
| Domeneguini et al. (2006) |
Enterocytes showed significantly decreased number of nuclei with apoptosis and significantly increased mitosis. The medulla of lymphatic follicles showed significantly decreased number of cells showing nuclei with apoptosis. |
| Yi et al. (2005) | Serum IL‐6 was not affected by treatment (p = 0.42) or time (p = 0.28) within 48 h following E. coli infection, and there was no treatment × time interaction (p = 0.12; data not shown; overall mean 72.1 ± 14.8 pg/mL). |
| He et al. (2016b) | At 14 and at 28 days post‐weaning, the levels of immunoglobulin G (IgG) and immunoglobulin M (IgM) in the serum were increased in the 1% Gln group (p < 0.05), whereas the percentage of the cluster of differentiation eight receptors (CD8+) was reduced (p < 0.05) in the 1% Gln group compared with control group. |
| Duttlinger et al. (2019) | Pigs weaned of 18 d of age at the start of the study and treated for 14 d. On day 13 post‐weaning, plasma tumour necrosis factor alpha (TNF‐α) was reduced (p = 0.02) GLN pigs (40.9 ± 6.9 pg/mL) vs. control pigs (63.2 ± 6.9 pg/mL). There was no difference in TNF‐a concentrations on day 33 post‐weaning. |
Table B.4.
Effect of glutamine on the body weight/daily weight gain of weaned piglets in relative terms1
| Weaned piglets | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Day | Glutamine supplemented (%) | |||||||
| 0 | 0.2 | 0.5 | 0.6 | 1.0 | 1.5 | 2.0 | 5.0 | ||
| Wu et al. (1996)2 | 14 |
100 (0.150) |
103 (0.155) |
110 (0.160) |
100 (0.150) |
||||
|
No challenged |
14 |
100 (0.260) |
96 (0.250) |
||||||
|
Challenged |
14 |
100 (0.150) |
180 (0.270) |
||||||
| Yi et al. (2005) | 14 |
100/974 (9.2/8.9) |
96/994 (8.8) |
||||||
| Domeneguini et al. (2006) | 28 |
100 (11) |
105 (11.6) |
||||||
| Lee et al. (2003)5 | 28 |
100 (15.3) |
98 (15.0) |
98 (15.0) |
101 (15.4) |
||||
| Hsu et al. (2010) | 21 |
100 (9.8) |
106 (10.4) |
106 (10.4) |
|||||
| He et al. (2016a) | 28 |
100 (13.9) |
102 (14.9) |
||||||
| He et al. (2016b) | 28 |
100b (19.6b) |
113a (22.2a) |
||||||
| Johnson and Lay (2017)6 | 14 |
100b (5.9) |
120a (7.1) |
||||||
| Supplementation in pre‐weaning and/or weaning | |||||||||
| Cabrera et al. 2013 | weaning |
100 (6.4) |
100 (6.4) |
||||||
| 42 |
100 (22.2) |
103/977 (22.9/21.7) |
|||||||
| Supplementation in weaning and follow‐up until slaughter | |||||||||
| Duttlinger et al. (2019) | |||||||||
| Weaners | 14 d |
100b (8.2) |
104a (8.5) |
||||||
|
Weaner – (follow‐up after 14 days treatment in weaning phase) |
34 d |
100 (17.0) |
103 (17.5) |
||||||
|
Slaughter (follow‐up after 14 days treatment in weaning phase) |
125 d |
100 (122.3) |
99.5 (121.7) |
||||||
Values within brackets are body weight (in kg) or daily body weight gain (in kg).
The study reports only the average daily weight gain for two periods 0–7 and 7–18.
The treatment with glutamine was applied to E.coli K88+ challenged animals only, in the non‐supplemented diet the first value represents the control not challenged and the second the control challenged, in the supplemented diet the first value is relative to the non‐challenged and non‐supplemented group and the second to the challenged and non‐supplemented group.
The mean value was calculated for the periods and the relative percent is presented in the table. Data are derived from the third experiment presented in the study.
Data is from the first experiment presented in the study.
The study reported only the average daily weight gain, animals were challenged on day 7 with LPS from E. coli.
The values have been extrapolated from the graphs provided in the study.
a,b values within one row with different superscript are significantly different (p < 0.05).
As per the effect of l‐glutamine on the development of the intestine, the results were less consistent than in chickens for fattening: they showed significant increases in the villus height in duodenum (Lee et al., 2003), jejunum (Wu et al., 1996; Yi et al., 2005; He et al., 2016a; Johnson and Lay, 2017) or ileum (Domeneguini et al., 2004; Domeneguini et al., 2006; Johnson and Lay, 2017; Yi et al., 2005) of the piglets fed with glutamine in 10 out of the 26 measurements reviewed. Increase of crypt depth was restricted to only three measurements out of 26 (Domeneguini et al., 2004, 2006; He et al., 2016a) and in another study a reduction of crypt depth was seen (Wu et al., 1996).
Numerical increases in the body weight of the piglets that received glutamine as compared to control were observed in most of the studies, reaching significant values in two studies from dosages as low as 0.2% in weaned piglets of 18 d of age (Johnson and Lay, 2017; Duttlinger et al., 2019) and in a third study at 1% glutamine supplementation in piglets weaned at 35 d of age (He et al., 2016b). The results from Duttlinger et al. (2019) suggest also that the effect of the supplementation on the body weight may not be seen after the supplementation is stopped. The study of He et al. (2016b) included treatments with glutamic acid which did not allow to reproduce the effect seen with glutamine on average daily gain or feed efficiency.
Regarding the effects on the immune system of weaned piglets, concentrations of bile IgA significantly increased at day 7 postweaning with 0.5% glutamine supplementation although the effect was not seen at day 14 postweaning (Lee et al., 2003). In another study, He et al. (2016b) found levels of serum IgG and IgM significantly increased in the 1% glutamine group (p < 0.05) at 14 and at 28 days post weaning, whereas the percentage of the cluster of differentiation eight receptors (CD8+) was significantly reduced (p < 0.05) in the 1% glutamine group compared with control group. Yi et al. (2005) found no effect of 2% glutamine supplementation on serum IL‐6 48 h following E. coli infection (p = 0.12, overall mean 72.1 ± 14.8 pg/mL). Duttlinger et al. (2019), using weaned pigs of 18 d of age treated with 0.2% glutamine for 14 d found that plasma tumour necrosis factor alpha (TNF‐α) was significantly reduced (p = 0.02) in glutamine‐treated pigs (40.9 ± 6.9 pg/mL) vs. control pigs (63.2 ± 6.9 pg/mL), although there was no difference in TNF‐α concentrations on day 33 post weaning. The studies of Domeneguini et al. (2004) detected a significant reduction of number of apoptotic nuclei in enterocytes and in lymphocytes of the medulla of lymphatic follicles at 0.5% glutamine supplementation (p < 0.05) compared with the control, and a significant increase of the mitotic index of the enterocytes.
Overall, the use of l‐glutamine increased the resorptive surface of the small intestine and showed some positive effects on immune response and growth of weaned piglets.
3.3.3. Conclusions on the efficacy
l‐glutamine is an amino acid, non‐essential and it plays a physiological role as such. Recent evidence shows that glutamine may act as conditionally essential amino acid mainly in growing animals and has some specific effects e.g. in improving intestinal development and immune response. This amino acid produced by fermentation using Corynebacterium glutamicum NITE BP‐02524 is regarded as an efficacious source of glutamine for all animal species.
Degradation in the rumen would reduce the delivery of the amino acid to the abomasum, and therefore, measures to ensure a more efficient delivery should be considered.
As l‐glutamine is used in food as flavouring compound (FLAVIS No 17.007), it is expected that it can provide a similar function in feed and no further demonstration of efficacy is necessary.
3.4. Post‐market monitoring
The FEEDAP Panel considers that there is no need for specific requirements for a post‐market monitoring plan other than those established in the Feed Hygiene Regulation48 and Good Manufacturing Practice.
4. Conclusions
Viable cells of the production strain and its recombinant DNA were not detected in the additive. l‐Glutamine manufactured by fermentation using Corynebacterium glutamicum NITE BP‐02524 does not give rise to any safety concern with regard to the genetic modification of the production strain.
The use of l‐glutamine produced by fermentation using C. glutamicum NITE BP‐02524 in animal nutrition is considered safe for all animal species when applied as a nutritional additive to achieve an adequate amino acid profile in feed and to overcome potential glutamine shortages during critical periods of life. The proposed use level (25 mg/kg feed) when used as sensory additive (flavouring compound) is safe for all animal species.
The use of l‐glutamine produced using C. glutamicum NITE BP‐02524 as nutritional additive or as flavouring compound is considered safe for the consumer.
l‐Glutamine produced using C. glutamicum NITE BP‐02524 is not toxic by inhalation, is non‐irritant to skin and eyes and is not a skin sensitiser.
l‐Glutamine produced using C. glutamicum NITE BP‐02524 is considered safe for the environment.
l‐Glutamine is an amino acid, non‐essential and it plays a physiological role as such. Recent evidence shows that glutamine may act as conditionally essential amino acid mainly in growing animals and has some specific effects e.g. in improving intestinal development and immune response. This amino acid produced by fermentation using Corynebacterium glutamicum NITE BP‐02524 is regarded as an efficacious source of glutamine for all animal species as a nutritional additive. For supplemental l‐glutamine to be as efficacious in ruminants as in non‐ruminants, it would require protection against degradation in the rumen.
The use of l‐glutamine as sensory additive at 25 mg/kg feed is considered efficacious.
Documents provided to EFSA/Chronology
| Date | Event |
|---|---|
| 09/08/2018 | Dossier received by EFSA. l‐ Glutamine produced using Corynebacterium glutamicum NITE BP‐02524. Submitted by Ajinomoto Eurolysine S.A.S |
| 07/09/2018 | Reception mandate from the European Commission |
| 19/11/2018 | Application validated by EFSA – Start of the scientific assessment |
| 07/02/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the production strain, characterisation of the additive, manufacturing process. |
| 19/02/2019 | Comments received from Member States |
| 04/03/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 13/03/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: characterisation of the production strain, characterisation of the additive, manufacturing process. |
| 19/03/2019 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 08/04/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 26/07/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Conditions of use, safety, efficacy. |
| 30/09/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 18/03/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- ADG
average daily gain
- ADI
average daily intake
- ANS
EFSA Scientific Panel on Additives and Nutrient Sources added to Food
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony‐forming unit
- CV
coefficient of variation
- DM
dry matter
- EURL
European Union Reference Laboratory
- FAO
Food Agricultural Organization
- FLAVIS
The EU Flavour Information System
- FL‐no
FLAVIS number
- LOD
limit of detection
- LOQ
limit of quantification
- LLNA
Local Lymph Node Assay
- PCB
polychlorinated biphenyls
- TSH
thyroid‐stimulating hormone
- WHO
World Health Organization
Appendix A – Tabulated effects of l‐glutamine on intestinal development, immune response and growth of chickens for fattening
1.
Appendix B – Tabulated effects of l‐glutamine on intestinal development, growth and immune response of weaned piglets
1.
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for L‐glutamine produced using strain NITE BP‐02524
1.
In the current application, authorisation is sought under Article 4(1) for l‐glutamine produced using strain NITE BP‐02524, under the category/functional groups 3(c) ‘nutritional additives’/‘amino acids, their salts and analogues’ and 2(b) ‘sensory additives/flavouring compounds’ according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for all animal species.
According to the Applicant, l‐glutamine has a minimum purity (mass fraction) of 98%. As nutritional feed additive, l‐glutamine is intended to be added directly into feedingstuffs or through premixtures. As sensory feed additive, glutamine is intended to be added into feedingstuffs through flavouring premixtures. The Applicant did not propose any minimum or maximum content of glutamine in feedingstuffs but, when authorised as sensory additive, recommended to use glutamine at a level of 25 mg/kg complete feed.
For the quantification of glutamine in the feed additive, premixtures and feedingstuffs, the Applicant submitted the ring‐trial validated method EN ISO 17180:2013 specifically designed for lysine, methionine and threonine in products containing more than 10% of amino acid. This standard method is based on ion exchange chromatography coupled to visible or fluorescence detection (IEC‐VIS/FLD). It does not distinguish between the salts of amino acids and cannot differentiate between enantiomers. The Applicant presented results from validation and verification studies demonstrating the extension of the scope of the above‐mentioned ISO method for the determination of glutamine in the feed additive, premixtures and feedingstuffs. The following performance characteristics were reported: a relative standard deviation for repeatability (RSDr) ranging from 0.4 to 1.9%, a relative standard deviation for intermediate precision (RSDip) ranging from 0.7 to 2.2%, a recovery rate from 95 to 105% and a limit of quantification (LOQ) of 300 mg/kg feedingstuffs.
However, the proposed method is not applicable for the determination of glutamine in feedingstuffs when the feed additive is used as sensory additive as the LOQ in this case is above the maximum recommended inclusion level of glutamine in feedingstuffs.
In addition, the EURL identified the ‘l‐glutamine monograph’ of the Food Chemical Codex (FCC) for the identification of l‐glutamine in the feed additive.
In the frame of this authorisation, the EURL recommends for official control (i) the ‘l‐glutamine monograph’ of the Food Chemical Codex (FCC) based on infrared absorption for the identification of l‐glutamine in the feed additive; (ii) the ring‐trial validated method EN ISO 17180:2013 based on ion exchange chromatography coupled to visible or fluorescence detection (IEC‐VIS/FLD) to quantify free glutamine in the feed additive, premixtures and feedingstuffs (only as ‘nutritional additive’).
Further testing or validation of the methods to be performed through the consortium of National Reference Laboratories as specified by Article 10 (Commission Regulation (EC) No 378/2005) is not considered necessary.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Glandorf B, Gropp J, Herman L, Rychen G, Saarela M, Anguita M, Galobart J, Holczkecht O, Manini P, Pettenati E, Pizzo F and Tarrés‐Call J, 2020. Scientific Opinion on the safety and efficacy of l‐glutamine produced using Corynebacterium glutamicum NITE BP‐02524 for all animal species. EFSA Journal 2020;18(4):6075, 29 pp. 10.2903/j.efsa.2020.6075
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00693
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Angelica Amaduzzi, Rosella Brozzi, Yolanda Garcia Cazorla, Gloria Lopez Galvez and Lucilla Gregoretti.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Adopted: 18 March 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
Ajinomoto Animal Nutrition Europe, Rue de Courcelles 153, 75817 Paris Cedex 17, France.
Regulation (EU) No 609/2013 of the European Parliament and of the Council of 12 June 2013 on food intended for infants and young children, food for special medical purposes and total diet replacement for weight control and repealing Council Directive 92/52/EEC, Commission Directives 96/8/EC, 1999/21/EC, 2006/125/EC and 2006141/EC, Directive 2009/39/EC of the European Parliament and of the Council and Commission Regulations (EC No 41/2009 and (EC) No 953/2009. OJ L 181, 29.6.2013, p. 35–56.
Commission implementing regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267/1, 02.10.2012. p. 161.
Commission Decision 2006/257/EC of 9 February 2006 amending Decision 96/335/EC establishing an inventory and a common nomenclature of ingredients employed in cosmetic products. OJ L 97/1, 5.4.6006. p. 598.
Commission Regulation (EC) No 37/2010 of 22 December 2009, on pharmacologically active substances and their classification regarding maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. OJ L 15, 20.1.2010, pp. 1.
Commission implementing regulation (EU) 2018/249 of 15 February 2018 concerning the authorisation of taurine, beta‐alanine, l‐alanine, l‐arginine, l‐aspartic acid, l‐histidine, d,l‐isoleucine, l‐leucine, l‐phenylalanine, l‐proline, d,l‐serine, l‐tyrosine, l‐methionine, l‐valine, l‐cysteine, glycine, monosodium glutamate and l‐glutamic acid as feed additives for all animal species and l‐cysteine hydrochloride monohydrate for all species except cats and dogs. OJ L 53, 23.02.2018. p. 32.
FEED dossier reference: FAD‐2018‐0059.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep_fad-2018-0059_l-glutamine.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex Supp II/Annex Supp II.1, and supplementary information March 2019/Supp Info An 2.
■■■■■
Technical dossier/Section II/Annex Supp II/Annex Supp II.17 and supplementary information March 2019/GLN NITE BP‐02524.
■■■■■
Technical dossier/Section II/■■■■■ and II.10. Glutamine analysed by a method derived from EN ISO 17180.
■■■■■
Technical dossier/Section II/Annex II.13.
Technical dossier/Section II/Annex II.5. LOQ in mg/kg was 0.005 for mercury, 0.01 for cadmium, 0.05 for lead and melamine, 0.1 for arsenic, 1.5 for hydrocyanic acid and 20 for fluorine.
Technical dossier/Section II/Annex II.5. LOD/LOQ (in µm/kg) was 0.1 for aflatoxins B1, B2 and G1; 0.2 for aflatoxin G2 and ochratoxin A; 20 for zearalenone, T‐2 toxin, HT‐2 toxin and fumonisins B1, B2 and B3; and 50 for deoxynivalenol.
Technical dossier/Section II/Annex II.5. LOQ (in mg/kg) ranged from 0.1 to 0.002 depending on the pesticide considered.
Technical dossier/Section II/Annex II.5. LOQ (in mg/kg) was 1 for all the analysed biogenic amines except for tryptamine in which it was 5.
Technical dossier/Section II/Annex II.7.
Technical dossier/Section II/Annex II.8 and II.38.
■■■■■
■■■■■
Technical dossier/Section II/Annex II.35.
Technical dossier/Section II/Annex II.6.
Technical dossier/Section II/Annex II.53.
Technical dossier/Section II/Annex II.55.
Technical dossier/Section II/Annex II.57.
Technical dossier/Section II/Annexes II.55 and II.57.
Technical dossier/Section II and Supplementary information September 2019/GLN NITE BP‐02524 Supp Info 26079.
Technical dossier/Section III/Annex III.1.
Technical dossier/Section III/Annex III.1. Basal diets of weanling piglets contained 1.5–1.8% glutamine, the ones of gestating sows 1.2–1.8% glutamine and the ones of lactating sows 1.7–2.1% glutamine.
Blood parameters surveyed as red or white blood cells count, percentage of haemoglobin, packed cell volume, total protein, albumin, globulin or albumin to globulin ratio.
OJ L 181, 29.6.2013, p. 35–56.
OJ L 267/1, 02.10.2012. p. 161.
OJ L 97/1, 5.4.6006. p. 598.
Technical dossier/Section III/Subsection 3.2.3.1.
Technical dossier/Section III/Annex III.44.
Technical dossier/Section III/Annex III.46.
Technical dossier/Section III/Annex III.45.
Technical dossier/Section III/Annex III.47.
Technical dossier/Section IV/Annex IV.21
Technical dossier/Section IV/Annex IV.22.
Technical dossier/Section IV/Annex IV.23.
Technical dossier/Section IV/Annex IV.25.
Regulation (EC) No 183/2005 of the European Parliament and of the Council of 12 January 2005 laying down requirements for feed hygiene. OJ L 35, 8.2.2005, p. 1.
References
- Athorn R and Hermann D, 2016. Effect of L‐Glutamine in late gestation sow diets on survivability and growth of piglets. Final report of the Australian Pork Limited Project 2014/463. Available online: https://australianpork.infoservices.com.au/downloads/2014-463
- Bartell SM, 2006a. Thesis, Chapter 4. The effect of supplementing glutamine, glutamic acid, histidine and asparagine on growth performance, development of the gastrointestinal tract, and immune response of borilers. In: The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and immune response of broiler chicks. Doctoral thesis. University of Georgia. Available online: https://getd.libs.uga.edu/pdfs/bartell_shoshana_m_200605_ms.pdf
- Bartell SML, 2006b. Theses, Chapter 5. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract and immune response of broilers vaccinated and challenged with Eimeria acervulina and Eimeria maxima. In: The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and immune response of broiler chicks. Doctoral thesis. University of Georgia. Available online: https://getd.libs.uga.edu/pdfs/bartell_shoshana_m_200605_ms.pdf
- Bartell SM and Batal AB, 2007. The Effect of Supplemental Glutamine on Growth Performance, Development of the Gastrointestinal Tract, and Humoral Immune Response of Broilers. Poultry Science, 86, 1940–1947. [DOI] [PubMed] [Google Scholar]
- Bignell H, 2014. Maternal ingestion of glutamine and glutamate during sow pregnancy and lactation: lipid profile analysis of milk and neonatal adipose tissues. Thesis. Graduate School‐New Bruswick, New Jersey. Available online: https://pdfs.semanticscholar.org/6c79/2e096cfe452feac0b43239d41b74933dedbe.pdf?_ga=2.150787248.769399963.1583491162-2093015833.1560428886
- Cabrera RA, Ursy JL, Arrellano C, Nogueira ET, Kutschenko M, Moeser AJ and Odle J, 2013. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (Aminogut) on pre‐ and post‐weaning growth performance and intestinal health of piglets. Journal of Animal Science and Biotechnology, 4, 12pp. 10.1186/2049-1891-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroprese M, Albenzio M, Marino R, Santillo A and Sevi A, 2012. Dietary glutamine enhances immune responses of dairy cows under high ambient temperature. Journal of Dairy Science, 96, 3002–3011. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Gatlin Ill DM and Buentello A, 2012. Dietary supplementation of arginine and/or glutamine influences growth performance, immune responses and intestinal morphology of hybrid striped bass (Morone chrysops×Morone saxatilis). Aquaculture, 362, 39–43. [Google Scholar]
- Coutinho F, Castro C, Rufino‐Paolmares E, Ordonez‐Grande B, Gallardo MA, Oliva‐Teles A and Peres H, 2016. Dietary glutamine supplementation effects on amino acid metabolism, intestinal nutrient absorption capacity and antioxidant response of gilthead sea bream (Sparus aurata) juveniles. Comparative Biochemisty and Physiology, Part A, 191, 9–17. [DOI] [PubMed] [Google Scholar]
- Da Silva JT, Manzoni T, Rocha NB, Santos G, Miqueo E, Slanzon GS and Bittar CMM, 2018. Evaluation of milk replacer supplemented with lysine and methionine in combination with glutamate and glutamine in dairy calves. Journal of Applied Animal Research, 46, 960–966. [Google Scholar]
- D'Mello JPF, 2003. An outline of pathways in amino acid metabolism (chapter 4). In: Amino acids in animal nutrition/edited by J.P.F. D'Mello.‐ 2nd ed. CAB International. Wallingford, UK. ISBN 0‐85199‐654‐X
- Doepel L, Lobley GE, Bernier JF, Dubreuil P and Lapierre H, 2007. Effect of glutamine supplementation on splanchnic metabolism in lactating dairy cows. Journal of Dairy Science, 90, 4325–4333. [DOI] [PubMed] [Google Scholar]
- Domeneguini C, di Giancamillo A, Savoini G, Paratte R, Bontempo V and Dell'Orto V, 2004. Structural patterns of swine ileal mucosa following L‐glutamine and nucleotide administration during the weaning period. An Histochemical and Histometrical Study. Histology and Histopathology, 19, 49–58. [DOI] [PubMed] [Google Scholar]
- Domeneguini C, di Giancamillo A, Bosi G and Arrighi S, 2006. Can Nutraceuticals affect the structure of intestinal mucosa? Qualitative and Quantitative microanatomy in L‐glutamine diet‐supplemented weaned piglets. Veterinary Research Communications, 30, 331–342. [DOI] [PubMed] [Google Scholar]
- Drackley JK, Blome RM, Bartlett KS and Bailey KL, 2006. Supplementation of 1% L‐glutamine to milk replacer does not overcome the growth depression in calves caused by soy protein concentrate. Journal of Dairy Science, 89, 1688–1693. [DOI] [PubMed] [Google Scholar]
- Duttlinger AW, Kpodo KR, Lay DC, Richert BT and Johnson JS, 2019. Replacing dietary antibiotics with 0.20% L‐glutamine in swine nursery diets: impact on heath and productivity of pigs following weaning and transport. Journal of Animal Science, 97, 2035–2052. 10.1093/jas/skz098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadiasl G, 2011. Effects of supplemental glutamine and glutamate on growth performance, gastrointestinal development, jejunum morphology and Clostridium perfringens count in caecum of broilers. Swedish University of Agricultural Science. Department of Animal Nutrition and Management. Available online: https://core.ac.uk/download/pdf/11987364.pdf
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA, 2007. EFSA Journal 2007;5(12):587, 16 pp. 10.2903/j.efsa.2007.587 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, Gürtler R, Tobback P, Altieri A, Rincon AM and Lambré C, 2017. Scientific Opinion on the re‐evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA Journal 2017;15(7):4910, 90 pp. 10.2903/j.efsa.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordonez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernandez Escamez PS, Maradona MP, Querol A, Suarez JE, Sundh I, Vlak J, Barizzone F, Correia S and Herman L, 2019b. Statement on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 9: suitability of taxonomic units notified to EFSA until September 2019. EFSA Journal 2019;17(1):5555, 46 pp. 10.2903/j.efsa.2019.5555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in animal feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in animal feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Karenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018a. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances usedin animal feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, Lopez‐Alonso M, Lopez Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018b. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos M, Christensen H, Dusemund B, Kouba M, Kos Durjava M, Lopez‐Alonso M, Lopez Puente S, Marcon F, Mayo B, Pechova A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, de Knecht J, Kolar B, van Beelen P, Padovani L, Tarres‐Call J, Vettori MV and Azimonti G, 2019a. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2011. Scientific Opinion on the substantiation of health claims related to L‐glutamine and growth or maintenance of muscle mass (ID 719, 722, 3185), faster restoration of muscle glycogen stores after strenuous exercise (ID 434, 699, 701, 723, 1569), skeletal muscle tissue repair (ID 721), maintenance of normal neurological function (ID 662, 700), increased attention (ID 700, 1570), improvement of working memory (ID 700, 1570), maintenance of defence against pathogenic gastro‐intestinal microorganisms (ID 452), gut protein synthesis (ID 701), decreasing gut permeability (ID 701), and stimulating immunological responses (ID 1568) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal 2011;9(6):2225, 27 pp. 10.2903/j.efsa.2011.2225. Available online: www.efsa.europa.eu/efsajournal.htm [DOI] [Google Scholar]
- Fasina YO, Bowers JB, Hess JB and McKee SR, 2010. Effect of dietary glutamine supplementation on Salmonella colonisation in the ceca of young broiler chicks. Poultry Science, 89, 1042–1048. [DOI] [PubMed] [Google Scholar]
- Gholipour V, Camani M, Aghdam Shahryar H, Sadeghi AA and Aminafshar M, 2017. Effects of dietary L‐glutamine supplemented on performance, egg quality, fertility and some blood biochemical parameters in Guinea fowls (Numida meleagridis). Kafkas Univ Vet Fak Derg, 23, 903–910. 10.9775/kvfd.2017.17963 [DOI] [Google Scholar]
- Gu M, Bai N, Xu B, Xu X, Jia Q and Zhang Z, 2017. Protective Effect of Glutamine and Arginine Against Soybean Meal‐Induced Enteritis in the Juvenile Turbot (Scophthalmus Maximus). Fish and Shellfish Immunology., 70, 95–105. [DOI] [PubMed] [Google Scholar]
- He L, Li H, Huang N, Tian J, Liu Z, Zhou X, Yao K, Li T and Yin Y, 2016a. Effects of Alpha‐Ketoglutarate on Glutamine Metabolism in Piglet Enterocytes in Vivo and in Vitro. Journal of Agricultural and Food Chemistry, 64, 2668–2673. [DOI] [PubMed] [Google Scholar]
- He J, Feng GD, Ao X, Li YF, Qian HX, Liu JB, Bai GY and He ZZ, 2016b. Effects of L‐glutamine on growth performance, antioxidant ability, immunity and expression of genes related to intestinal health in weanling pigs. Livestock Science, 189, 102–109. [Google Scholar]
- Hsu CB, Huang HJ, Wang CH, Yen HT and Yu B, 2010. The effect of glutamine supplement on small intestinal morphology and xylose absorptive ability of weaned piglets. African Journal of Biotechnology, 9(41), 7003–7008. 10.5897/AJB10.1036 [DOI] [Google Scholar]
- Johnson JS and Lay DC Jr, 2017. Evaluating the behavior, growth performance, immune parameters, and intestinal morphology of weaned piglets after simulated transport and heat stress when antibiotics are eliminated from the diet or replaced with L‐glutamine. Journal of Animal Science, 95, 91–102. 10.2527/jas2016.1070 [DOI] [PubMed] [Google Scholar]
- Kawadara E, Ueda T and Nomura S, 1991. In vitro phagocytic activity of white spotted shark cells after injection with Aeromonas salmonicida extracelular products. Gyobyo, Kenkyu, Japan, 26, 213–214. [Google Scholar]
- Kitt SJ, Miller PS and Fischer RL, 2003. Effect of glutamine on growth performance and intestinal development of immune challenged weanling pigs fed chemically defined diets. Nebraska Swine Reports., 61, 34–37. Available online: https://digitalcommons.unl.edu/coopext_swine/61 [Google Scholar]
- Lee DN, Cheng YH, Wu FY, Sato H, Shinzato I, Chen SP and Yen HT, 2003. Effect of dietary glutamine supplement on performance and intestinal morphology of weaned piglets. Asian‐Australasian Journal of Animal Science, 16, 1770–1776. [Google Scholar]
- Lenders CM, Liu S, Wilmore DW, Sampson L, Dougherty LW, Spiegelman D and Willet WC, 2009. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. European Journal of Clinical Nutrition, 63, 1433–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rezaei R, Li P and Wu G, 2011. Composition of amino acids in feed ingredients for animal diets. Amino Acids, 40, 1159–1168. [DOI] [PubMed] [Google Scholar]
- Liu J, Mai K, Xu W, Zhang Y, Zhou H and Ai Q, 2015. Effects of dietary glutamine on survival, growth performance, activities of digestive enzyme, antioxidant status and hypoxia stress resistance of half‐smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture, 446, 48–56. [Google Scholar]
- Luquetti BC, Fernandez Alarcon MF, Lunedo R, Borges Campos DM, Furlan RL and Macari M, 2016. Effects of glutamine on performance and intestinal mucosa morphometry of broiler chickens vaccinated against coccidiosis. Scientia Agricola, 73, 322–327. 10.1590/0103-9016-2015-0114 [DOI] [Google Scholar]
- Namroud NF, Shivazad M, Zaghari M, Madadgar O and Nourijelyani K, 2017. Impact of dietary glutamine on amino acid digestibility values and intestinal morphometric parameters in neonate chicks. South African Journal of Animal Science, 47, 440–453. 10.4314/sajas.v47i4.3 [DOI] [Google Scholar]
- NRC (National Research Council), 2012. Nutrient requirement of swine. The National Academies Press, Washington, DC: Eleventh revised edition. 10.17226/13298 [DOI] [Google Scholar]
- Ocheja OB, Ayo JO, Aluwong T and Minka NS, 2017. Effects of L‐glutamine on rectal temperature and some markers of oxidative stress in Red Sokoto goats during the hot‐dry season. Tropical Animal Health and Production., 49, 1273–1280. [DOI] [PubMed] [Google Scholar]
- Pardo AL, Poveda A, da Silva C, dos Santos A, Venancio E, Arantes V and Nogueira E, 2014. Effect of L‐glutamine levels in piglets diets challenged with Escherichia coli lipopolysaccharides. Revsita MVZ Córdoba, 19, 4328–4337. [Google Scholar]
- Plaizier JC, Walton JP and McBride BW, 2001. Effect of post‐ruminal infusion of glutamine on plasma amino acids, milk yield and composition in lactating dairy cows. Canadian Journal of Animal Science, 81, 229–235. [Google Scholar]
- Pohlenz C, Buentello A, Bakke AM and Gatlin Ill DM, 2012a. Free dietary glutamine improves intestinal morphology and increases enterocyte migration rates, but has limited effects on plasma amino acid profile and growth performance of channel catfish Ictalurus punctatus . Aquaculture, 370–371, 32–39. [Google Scholar]
- Pohlenz C, Buentello A, Mf Criscitiello, Mwangi W, Smith R and Gatlin Ill DM, 2012b. Synergies between vaccination and dietary arginine and glutamine supplementation improve the immune response of channel catfish against Edwardsiella ictalurid . Fish and Shellfish Immunology, 33, 543–551. [DOI] [PubMed] [Google Scholar]
- Pulske JR, Williams IH and Aherne FX, 1996. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Animal Science, 61, 131–144. [Google Scholar]
- Sakamoto MI, Murakami AE, Silveira TGV, Fernandes JIM and de Oliveira CAL, 2006. Influence of Glutamine and Vitamin E on the Performance and the Immune Responses of Broiler Chickens. Brazilian Journal of Poultry Science, 8, 243–349. [Google Scholar]
- Santos de Aquino R, Dutra Junior WM, Manso HECC, Manso Filho HC, Kutschenko M, Nogueira ET and Watford M, 2014. Glutamine and glutamate (AminoGut) supplementation influences sow colostrum and mature milk composition. Livestock Science, 169, 112–117. 10.1016/j.livsci.2014.07.009 [DOI] [Google Scholar]
- Scanes CG, 2014. Chapter 20: Protein Metabolism. In: Sturkie's Avian Physiology, 6th Edition. Editor CG Scanes. Academic Press. ISBN: 9780124071605. 455 – 467.
- Soltan MA, 2009. Influence of dietary glutamine supplementation on growth performance, small intestine morphology, immune response and some blood parameters of broiler chickens. International Journal of Poultry Science, 8, 60–68. [Google Scholar]
- Stoll B and Burrin DG, 2006. Measuring splanchnic amino acid metabolism in vivo using stable isotopic tracers. Journal of Animal Science, 84, E60–E72. [DOI] [PubMed] [Google Scholar]
- Teixeira AdO, Nogueira ET, Kutschenko M, Rostagno HS and Lopes DC, 2014. Inclusion of glutamine associated with glutamic acid in the diet of piglets weaned at 21 days of age. Revista Brasileira de Saúde e Produção Animal, 15, 881–896. Oct/Dec 2014. [Google Scholar]
- Tsubuku S, Hatayama K, Mawatari K, Smriga M and Kimura T, 2004. Thirteen‐week oral toxicity study of L‐Glutamine in rats. International Journal of Toxicology, 23, 107–112. [DOI] [PubMed] [Google Scholar]
- VKM (Norwegian Scientific Committee for Food Safety), 2016. Risk assessment of “other substances” – L‐glutamine and L‐glutamic acid. Opinion of the Panel on Nutrition, dietetic products, Novel Food an Allergy ofthe Norwegian Scientific Committee for Food Safety, ISBN: 978‐82‐8259‐215‐4, Oslo, Norway.
- Wang J, Chen L, Li P, Li X, Zhou H, Wang f, Li D, Yin Y and Wu G, 2008. Gene Expression Is Altered in Piglet Small Intestine by Weaning and Dietary Glutamine Supplementation. The Journal of Nutrition, 138, 1025–1032. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Ch, Wu G, Sun Y, Wang B, He B, Dai Z and Wu Z, 2015. Glutamine enhances tight junction protein expression and modulates corticotropin‐reseasing factor signalling in the jejunum of weanling piglets. The Journal of Nutrition, 145, 25–31. [DOI] [PubMed] [Google Scholar]
- Watford M, 2008. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. 7th Amino Acid Assessment Workshop. The Journal of Nutrition, 138, 2003–2007. [DOI] [PubMed] [Google Scholar]
- Wu G, 1998. Intestinal mucosal amino acid catabolism. The Journal of Nutrition, 128, 1249–1252. [DOI] [PubMed] [Google Scholar]
- Wu G, Meier SA and Knabe DA, 1996. Dietary Glutamine Supplementation Prevents Jejunal Atrophy in Weaned Pigs. Journal of Nutrition, 126, 2578–2584. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li XL, Satterfield MC, Smith SB and Spencer TE, 2010a. Functional amino acids in swine nutrition and production In: Doppenberg J. (ed). Dynamics in Animal Nutrition. Wageningen Academic Publishers, The, Netherlands: pp. 69–98. [Google Scholar]
- Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL and Wang JJ, 2011. Triennial Growth Symposium: Important Roles for L‐glutamine in Swine Nutrition and Production. Journal of Animal Science, 89, 2017–2030. [DOI] [PubMed] [Google Scholar]
- Wu L, Wang W, Yao K, Zhou T, Yin J, Li T, Yang L, He L, Yang X, Zhang H, Wang Q, Huang R and Yin Y, 2013. Effects of Dietary Arginine and Glutamine on Alleviating the Impairment Induced by Deoxynivalenol Stress and Immune Relevant Cytokines in Growing Pigs. PLoS ONE, 8, e69502 10.1371/journal.pone.0069502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L and Qiu‐Zhou X, 2006. Dietary glutamine supplementation improves structure and function of intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture, 256, 389–394. [Google Scholar]
- Yang XF, Qin JF, Wang L, Gao KG, Zheng CT, Huang L and Jiang ZY, 2018. Improved Milk Glutamine Level and Growth Performance of Suckling Piglets by Glutamine Supplementation in Maternal Diet. American Animal Science, 18, 441–452. [Google Scholar]
- Yi GF, Carrol JA, Allee GL, Gaines AM, Kendall DC, Usry JL, Toride Y and Izuru S, 2005. Effect of glutamine and spray‐dried plasma on growth performance, small intestinal morphology, and immune responses of Escherichia coli K88+‐challenged weaned pigs. Journal of Animal Science, 83, 634–643. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu T, Han L, Zhao L, Niu Y and Chen H, 2017. L‐Glutamine Supplementation Alleviates Constipation during Late Gestation of Mini Sows by Modifying the Microbiota Composition in Feces. Biomed Research International, 2017, 4862861 10.1155/2017/4862861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Zhang XH, Li XM, Zhou YM, Li W, Huang XX, Zhang LL and Wang T, 2014. Intestinal growth and morphology is associated with the increase in heat shock protein 70 expression in weaning piglets through supplementation with glutamine. Journal of Animal Science, 89, 3634–3642. 10.2527/jas.2010-3751 [DOI] [PubMed] [Google Scholar]


