Abstract
The Panel received a mandate from the European Commission to assess the genotoxic potential of triazine amine based on available information submitted by the applicants. Available information includes experimental genotoxicity data on triazine amine, Quantitative Structure–Activity Relationship (QSAR) analysis and read across with structurally similar compounds. Based on the overall weight of evidence, the Panel, in agreement with the cross‐cutting Working Group Genotoxicity, concluded that there is no concern for the potential of triazine amine to induce gene mutations and clastogenicity; however, the potential to induce aneugenicity was not adequately investigated. For a conclusion, an in vitro micronucleus assay performed with triazine amine would be needed.
Keywords: IN‐A4098, CGA 150 829, Aminotriazine, AE F059411, chromosome aberration, mutagenicity, residue, groundwater, pesticide
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
In 2018, the Panel on Plant Protection Products and their Residues (PPR) was requested by the European Commission (EC) to develop a Scientific Opinion on the genotoxic potential of triazine amine (metabolite common to several sulfonylurea active substances) under Article 29 of Regulation (EC) No 178/2002.
Triazine amine (also referred to as aminotriazine) is a metabolite that can form during the metabolism and breakdown of active substances in the triazinylsulfonylurea herbicide group. Several active substances in this group are currently approved for use in plant protection products in the European Union: metsulfuron‐methyl, prosulfuron, iodosulfuron‐methyl, thifensulfuron‐methyl, tribenuron‐methyl, triflusulfuron and chlorsulfuron. The renewal assessments of the first four substances have been completed whereas the assessment of triflusulfuron is ongoing. An application for renewal of approval of chlorsulfuron was not submitted and therefore the approval of this substance will expire in December 2019. A further substance is pending a decision on first approval in the European Union, ethametsulfuron‐methyl.
Following the renewal review of metsulfuron‐methyl, prosulfuron, iodosulfuron‐methyl and thifensulfuron‐methyl, the approval of these substances were renewed subject to a requirement to submit further information on the genotoxic potential of the metabolite triazine amine.
Confirmatory data were submitted by the applicants within the deadlines prescribed in the relevant Implementing Regulations. The data submitted by the applicants were evaluated by the Rapporteur Member States. The assessments, in compliance with Guidance Document 5634/2009‐rev.6.1 (European Commission, 2013), were circulated to the applicant, the other Member States and the European Food Safety Authority (EFSA) for comments, all of which were collated in Technical Reports.
For metsulfuron‐methyl Slovenia evaluated the data submitted. A Technical Report (EFSA, 2017a) summarising the outcome of the evaluation and comments received was published in 2017.
For prosulfuron and iodosulfuron, a combined assessment of the data made available by the applicants was carried out by Sweden and France. A Technical Report (EFSA, 2018) summarising the outcome of the evaluation and comments received was published in August 2018.
The review of the data provided in the context of thifensulfuron‐methyl is ongoing by the United Kingdom. However, no additional studies are available for that evaluation compared to those provided for the other substances. Nevertheless, the assessment by the United Kingdom, once available, can also be taken into account by the Panel.
The Technical Report published in August 2018 indicated that EFSA and Member States agreed that triazine amine does not show potential for clastogenicity/aneugenicity,1 but that a conclusion on whether triazine amine may have gene mutation potential could not be excluded on the basis of the confirmatory information submitted since some issues were identified with regard to the quality and the interpretation of the results of two in vitro gene mutation studies. However, some Member States did not agree and considered that the existing data confirms that there is no concern for genotoxicity for triazine amine.
In the light of the continued divergence of views between Member States and the experts involved in the peer review process, and the need to avoid unnecessary testing on vertebrate animals, the Commission asked the Panel on Plant Protection Products and their Residues to:
Assess the available evidence in relation to the genotoxic potential of the metabolite triazine amine;
Consider the issues identified in the EFSA Technical Reports and whether they could be addressed in light of the current state of the art on assessment of genotoxicity, read‐across and other alternative in silico and non‐testing methods and tools;
Confirm if it is possible to conclude that there is no concern for genotoxicity for the metabolite triazine amine (taking into account all genotoxicity endpoints).
EFSA had access to all of the available information submitted during the evaluation of the aforementioned active substances and provided this to the Panel for their work.
In 2019, the applicants informed the European Commission and EFSA that two new in vitro studies were ongoing for which the final reports were ready on 30 April 2019. Furthermore, an updated weight of evidence document was submitted by the applicants on 11 April 2019.
The deadline for finalising the Scientific Opinion is 10 April 2020.
1.2. Interpretation of the Terms of Reference
The European Food Safety Authority (EFSA) PPR Panel will develop a Scientific Opinion on the genotoxic potential of triazine amine. It interpreted the terms of reference as follows:
The PPR Panel is asked to conclude, based upon experimental data on triazine amine, if there is any concern for genotoxicity (considering all genotoxicity endpoints, i.e. gene mutation, clastogenicity and aneugenicity).
In the event that some genotoxicity endpoints are not adequately investigated by experimental data on triazine amine, the PPR Panel will consider additional information as Quantitative Structure–Activity Relationship (QSAR) and read‐across with structurally related compounds.
1.3. Additional information
After receiving the mandate by the Commission, the PPR panel requested the cross‐cutting Working Group (WG) Genotoxicity to review the evidence for genotoxicity of triazine amine. The PPR panel provided regularly comments on the draft opinion to the cross‐cutting WG Genotoxicity.
Given that a conclusion on whether triazine amine may have gene mutation potential could not be excluded on the basis of the confirmatory information available during the peer review (EFSA, 2017a, 2018), the cross‐cutting WG genotoxicity reassessed the data and provided a more extensive summary of the available in vitro gene mutation assays in mammalian cells in Annex III.
For this assessment, the Opinion on genotoxicity testing strategies (EFSA, 2012) and the Statement on Clarifications of some aspects related to genotoxicity assessment (EFSA, 2017b) were applied.
2. Data and methodologies
2.1. Data
The data available to the PPR panel were shared with the cross‐cutting WG Genotoxicity. The experimental genotoxicity data on triazine amine include the results of bacterial reversion (Ames) assays, in vitro mammalian gene mutation assays, in vitro and in vivo chromosomal aberration assays and in vitro unscheduled DNA Synthesis (UDS) assays. In addition, data from two in vivo toxicity studies on triazine amine with rats (acute and 28‐day toxicity) were made available. QSAR analysis on triazine amine was done by the cross‐cutting WG Genotoxicity using the Organisation for Economic Co‐operation and Development (OECD) QSAR toolbox (version 4.3).
The updated weight of evidence (WoE) document submitted by the applicants included summaries of experimental data on triazine amine and structurally related metabolites and QSARs analysis. The updated WoE document was scrutinised by the cross‐cutting WG Genotoxicity to retrieve relevant information that could address the aneugenic potential of triazine amine. From the updated WoE document read‐across data on genotoxicity from a structurally related (downstream) metabolite, IN‐B5528, were considered. Genotoxicity and toxicokinetic data on structurally related (precursor) metabolite IN‐L5296 were also considered.
The cross‐cutting WG Genotoxicity discussed the data in its meetings between December 2018 and January 2020.
2.2. Methodologies
The compliance of the studies was checked against the OECD Test Guidelines (TGs) in force at the time of the study. The evaluation of the results and the conclusions has been performed according to the OECD TGs in force at present.
3. Assessment
3.1. Identity
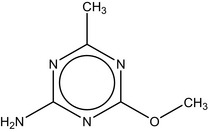
4‐Methoxy‐6‐methyl‐1,3,5‐triazin‐2‐amine is the international union of pure and applied chemistry (IUPAC) name for metabolite codified or named as triazine amine, IN‐A4098, CGA 150 829, Aminotriazine or AE F059411. The purity of the test material in the genotoxicity studies ranged from 97% to 99.6% (Table 1).
Table 1.
Substance identity
| Code/Trivial name | Chemical name (IUPAC) Smiles/InChI Key/CAS Register number | Structure |
|---|---|---|
| triazine amine, IN‐A4098; CGA 150 829; Aminotriazine; or AE F059411 |
4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐amine Cc1nc(N)nc(OC)n1 NXFQWRWXEYTOTK‐UHFFFAOYSA‐N 1668‐54‐8 |
|
3.2. Experimental data on triazine amine
3.2.1. Ames test
Samples of triazine amine of different purity have been tested in the bacterial reversion assays (Ames test) in six independent studies performed under Good Laboratory Practise (GLP) compliance (Table 2). Due to the low solubility in different solvents, the test article was formulated in different vehicles (dimethyl sulfoxide (DMSO) or water + 0.15% agar): after sonication, it was soluble up to 10 mg/mL, and formed workable suspension up to 100 mg/mL. All studies provided clearly negative results, both in the presence and absence of metabolic activation (S9) and applying different experimental protocols (plate incorporation and pre‐incubation method). Overall, triazine amine did not induce reverse mutations in bacteria under the conditions of these studies.
Table 2.
Ames test results
| Type of test | Experimental test system | Test substance | Exposure conditions | Result | WG considerations and conclusion | Reference |
|---|---|---|---|---|---|---|
| Ames test | S. typhimurium TA98, TA100, TA1535, TA1537, E. coli WP2 uvrA |
triazine amine IN‐A4098‐005 (4‐Methoxy‐6‐methyl‐1,3,5‐triazin‐2‐amine) (98.7%) Vehicle: DMSO |
Plate incorporation assay +/− rat liver S9 First exp. 1.5, 5, 15, 50, 150, 500, 1,500, 5,000 μg/plate; Confirmatory exp.: 50, 150, 500, 1,500, 5,000 μg/mL |
No treatment related increase in revertant colonies; no visible precipitation of the test substance, and no evidence of toxicity to bacteria |
GLP compliant According to OECD TG 471 (1997) Negative |
Wagner and Van Dyke (2009) |
| Ames test | S. typhimurium TA98, TA100, TA1535, TA1537, E. coli WP2 uvrA |
triazine amine Aminotriazine, batch 040101 (99%) Vehicle: suspended in DMSO |
Plate incorporation and pre‐incubation assay Six doses tested, from 31.6 to 5,000 μg/plate (+/− rat liver S9) |
No treatment related increase in revertant colonies; no visible precipitation of test substance, no evidence of toxicity to bacteria |
GLP compliant According to OECD TG 471 (1997) Negative |
Donath (2011) |
| Ames test | S. typhimurium TA98, TA100, TA1535, TA1537, TA102 |
triazine amine IN‐A4098 (2‐amino‐4‐methoxy‐6‐methyl‐1,3,5‐triazine) (99.5%) Vehicle: dissolved in DMSO (at 10 mg/mL) |
Six doses tested (+/− rat liver S9) Plate incorporation from 31.6 to 5,000 μg/plate Pre‐incubation assay from 10 to 3,160 μg/plate |
No increased revertant colonies at any dose; signs of toxicity to bacteria (reduced background lawn) at the top dose |
GLP compliant According to OECD TG 471 (1997) Negative |
Flügge (2011a) |
| Ames test |
S. typhimurium TA98, TA100, TA1535, TA1537 E. coli WP2uvrA |
triazine amine CGA 150829 tech (99.4%) Vehicle: suspended in DMSO |
Plate incorporation assay Five doses tested, from 312.5 to 5,000 μg/plate +/− rat liver S9 in two separate assays |
No treatment related increase in revertant colonies; no evidence of toxicity to bacteria |
GLP compliant According to OECD TG 471 (1983) Negative |
Geleick (1991) |
| Ames test |
S. typhimurium TA98, TA100, TA1535, TA1537 E. coli WP2uvrA |
triazine amine IN‐A4098 (2‐amino‐4‐methoxy‐6‐methyl‐1,3,5‐triazine) (97%) Vehicle: suspended in water + 0.15% agar |
Plate incorporation and pre‐incubation assay Five doses tested, from 50 to 5,000 μg/plate +/− rat liver S9 |
No treatment related increase in revertant colonies; no evidence of toxicity to bacteria |
GLP compliant According to OECD TG 471 (1997) Negative |
May (2011) |
| Ames test |
S. typhimurium TA98, TA100, TA1535, TA1537 E. coli WP2uvrA |
triazine amine AE F059411 tech (2‐amino‐4‐methoxy‐6‐methyl‐1,3,5‐triazine) (99.6%) Vehicle: suspended in DMSO |
Plate incorporation and pre‐incubation assay Five doses tested, from 50 to 5,000 μg/plate +/− rat liver S9 |
No treatment related increase in revertant colonies; no evidence of toxicity to bacteria |
GLP compliant According to OECD TG 471 (1997) Negative |
Stammberger and Braun (1988) |
DMSO: dimethyl sulfoxide; S9: metabolic activation.
3.2.2. In vitro mammalian gene mutation studies
During the peer review of sulfonylurea herbicides (EFSA, 2017b, 2018), four in vitro mammalian gene mutation studies were available for their common metabolite triazine amine: two gene mutation studies at the hprt gene (Clarke, 2009; Flügge, 2011b) following the OECD TG 476 (1997)2 and two gene mutation studies at the thymidine kinase (TK) locus using Mouse Lymphoma Assay (Woods, 2011a; Lloyd, 2016) complying with the OECD TG 476 (1997) and 490 (2016), respectively (Table 3 and Annex III). In all the studies, the compound was tested up to the limit of solubility. The difference in the ranges of concentrations tested is due to applied concentration of the solvent (DMSO) and to the methods used to enhance the solubilisation of the compound. No signs of cytotoxicity were noted up to the top concentrations assessed. The evaluation and the interpretation of the results from these studies were carried out following the most recent criteria and recommendations expressed in the OECD TG 476 (2016) and 490 (2016) as the result of the most updated scientific evidence on the biological relevance of the increased mutation frequencies.
Table 3.
In vitro mammalian gene mutation results
| Type of test | Experimental test system | Test substance | Exposure conditions | Result | WG considerations and conclusion | Reference |
|---|---|---|---|---|---|---|
| In vitro mammalian gene mutation | CHO/hprt assay |
Triazine amine IN‐A4098 (98.7%) Vehicle DMSO 1% |
5 h treatment +/− S9 10, 25, 50, 150 μg/mL |
+S9: no increase in mutation frequency −S9: Increase in mutation frequency with large variability between replicate cultures |
GLP study compliant with OECD TG 476 (1997) Insufficient number of treated cells with respect to the recommendations by the OECD 476 (2016) Inconclusive |
Clarke (2009) |
| In vitro mammalian gene mutation | V79/HGPRT assay |
Triazine amine IN‐A4098 (99.5%) Vehicle DMSO 2% |
4 and 24 h treatment +/− S9 at 12.5, 25, 50, 100, 200 μg/mL |
No increase in mutation frequency at any concentration tested Precipitation at 200 μg/mL |
GLP study compliant with OECD TG 476 (1997) Deviation from OECD 476 (2016): Final DMSO concentration 2% instead of 1% Negative |
Flügge (2011b) |
| In vitro mammalian gene mutation | Mouse Lymphoma L5178Y assay |
Triazine amine IN‐A4098 (97%) Vehicle DMSO 2% |
3 and 24 h treatment +/− S9 at 38.5, 77, 154, 308 μg/mL |
3 h +/−S9: no increase in mutation frequency 24 h −S9: Increase in mutation frequency not exceeding the GEF of 126 × 10−6 mutants above the mean concurrent vehicle control mutant frequency |
GLP study compliant with OECD TG 476 (1997) Deviation from OECD 490 (2016): Final DMSO concentration 2% instead of 1% Negative |
Woods (2011a) |
| In vitro mammalian gene mutation | Mouse Lymphoma L5178Y assay |
Triazine amine IN‐A4098 (98.7%) Vehicle DMSO 1% |
3 and 24 h treatment +/− S9 at 2.5, 5, 10, 20, 30, 35, 40 μg/mL | No increase in mutation frequency at any concentration tested |
GLP study compliant with OECD TG 490 (2016) Negative |
Lloyd (2016) |
| In vitro mammalian gene mutation | CHO/hprt assay |
Triazine amine IN‐A4098 (98.7%) Vehicle DMSO (1%) |
5 h treatment − S9 10, 25, 50, 100, 150 μg/mL |
No increase in mutation frequency at any concentration tested |
GLP study compliant with OECD TG 476 (2016) Negative |
Pant (2019) |
| In vitro mammalian gene mutation | Mouse Lymphoma L5178Y assay |
Triazine amine IN‐A4098 (98.7%) Vehicle DMSO 1% |
3 and 24 h treatment +/− S9 at 18,75, 37.5, 75, 150, 300 μg/mL | No increase in mutation frequency at any concentration tested |
GLP study compliant with OECD TG 490 (2016) Negative |
Woods (2019) |
DMSO: dimethyl sulfoxide; S9: metabolic activation; GEF: global evaluation factor.
In V79/hprt assay, no increase in mutation frequency was detected for short‐ (4 h) and long‐term (24 h) treatment at any concentration tested (12.5, 25, 50, 100 and 200 μg/mL) compared with the solvent control in the presence and absence of metabolic activation (Flügge, 2011b). In CHO/hprt assay (Clarke, 2009), no increase in mutation frequency was observed for 5 h treatment in the presence of metabolic activation at 10, 25, 50, 150 μg/mL, but an increase was seen at the highest concentration tested (150 μg/mL) without metabolic activation. The increase (5.4‐fold higher than in the vehicle control group) is outside the range of the historical solvent control data. However, a large variability was observed between replicate cultures both in solvent control and in treated samples. The insufficient number of treated cells with respect to the most recent recommendations by the OECD TG 476 (2016) reduces the power of the assay with the possibility that the observed increases in mutant frequencies may be induced by chance. The results were considered inconclusive.
No increase in the mean mutant frequencies at the thymidine kinase locus was observed in a study using a mouse lymphoma assay following 3 h of exposure at the range of concentrations: 38.5, 77, 154 and 308 μg/mL in the presence and absence of metabolic activation (Woods, 2011a). In the absence of metabolic activation, following a continuous 24 h of exposure, an increase in the mean mutant frequencies was observed at 308 μg/mL (160 × 10−6 mutants corresponding to 2.4‐fold increase with respect to the control value), but the observed increase did not exceed the global evaluation factor (GEF) of 126 × 10−6 mutants above the mean concurrent vehicle control mutant frequency. On the basis of these results, the compound was considered to be ‘negative’ complying the criteria of the OECD TG 490 (2016) where ‘a test chemical is considered to be clearly negative if there is no concentration related response or, if there is an increase in mutant frequency, it does not exceed the GEF’ (Woods, 2011a).
No increase in the mean mutant frequencies that exceeded the GEF at the thymidine kinase locus was observed in a second mouse lymphoma assay at a range of concentrations selected on the basis of the solubility profile: 2.5, 5, 10, 20, 30, 35 and 40 μg/mL (Lloyd, 2016).
Two additional gene mutation studies were received in April 2019 (Pant, 2019; Woods, 2019) (see details in the Annex III).
The Pant, 2019 study is a repeat of the CHO/hprt mammalian cell gene mutation assay by Clarke (2009), without metabolic activation. The test substance triazine amine was tested following the OECD TG 476 (2016) at five concentrations dissolved in DMSO: 0, 10, 25, 50, 100 and 150 μg/mL for 5 h without S9 activation, reaching 1% DMSO in culture medium. No statistically significant dose‐related increase in mutant frequency was observed, as compared to the concurrent vehicle controls, at any concentration evaluated.
In the Woods, 2019 study, the test substance triazine amine was tested for mutagenicity at the thymidine kinase locus in L5178Y cells following the OECD TG 490 (2016). Triazine amine was tested at five concentrations dissolved in DMSO: 18.75, 37.5, 75, 150, 300 μg/mL for either 3 or 24 h in the absence of metabolic activation or 3 h in the presence of metabolic activation, reaching 1% concentration of DMSO in culture medium. No increases in the mean mutant frequencies was observed at any concentration tested for any condition of treatment that exceeded the sum of the mean concurrent vehicle control mutant frequency and the GEF.
In conclusion, five available in vitro gene mutation assays (Flügge, 2011b; Woods, 2011a, 2019; Lloyd, 2016; Pant, 2019) were considered acceptable and negative. The results of the sixth study (Clarke, 2009) were considered inconclusive.
The EFSA cross‐cutting WG genotoxicity concluded that triazine amine did not induce gene mutation in vitro in mammalian cells.
3.2.3. In vitro unscheduled DNA synthesis (UDS) test
Triazine amine was tested for its ability to induce unscheduled DNA synthesis in two cell types with different metabolic capacities in two studies using adequate experimental protocols and under GLP compliance (Table 4). Both studies were clearly negative, indicating that the exposure to the test article did not elicit detectable DNA repair synthesis in mammalian cells in vitro. The cross‐cutting WG genotoxicity noted the in vitro UDS assay is not included in the EFSA strategy for genotoxicity testing (EFSA, 2012) and the OECD TG 482 has been withdrawn.
Table 4.
In vitro UDS test results (DMSO=dimethyl sulfoxide)
| Type of test | Experimental test system | Test substance | Exposure conditions | Result | WG considerations and conclusion | Reference |
|---|---|---|---|---|---|---|
| UDS in vitro | Primary rat hepatocytes |
triazine amine CGA 150 829 tech (97.6%) |
Six concentrations tested from 1 to 100 μg/mL in replicated experiments 100 μg/mL highest usable concentration (1% of DMSO solution at 10 mg/mL) |
No increase in net nuclear grain counts at any concentration in replicated experiments Net positive response with positive control |
GLP compliant According to OECD TG 482 (1987) Negative |
Hertner (1988) |
| UDS in vitro | Human fibroblasts |
triazine amine CGA 150 829 tech (97.6%) |
Six concentrations tested from 1 to 100 μg/mL in replicated experiments 100 μg/mL highest usable concentration (1% of DMSO solution at 10 mg/mL) |
No increase in net nuclear grain counts at any concentration in replicated experiments Net positive response with positive control |
GLP compliant According to OECD TG 482 (1987) Negative |
Meyer (1988) |
3.2.4. In vitro chromosomal aberration
Two in vitro chromosomal aberration studies (Dollenmeier, 1987; Meyer, 1991) were not considered reliable due to major limitations and were not acceptable according to the criteria specified in the OECD TG. Based on the negative results of the three reliable chromosomal aberration studies (Gudi and Rao, 2009; Flügge, 2011c; Woods, 2011b), the EFSA cross‐cutting WG genotoxicity concluded that triazine amine did not induce chromosome aberrations in mammalian cells in vitro.
It is to be noted that only the potential of triazine amine to induce structural chromosomal aberrations has been assessed. In the chromosomal aberration tests performed in vitro, no increase in polyploidy was reported.
Table 5.
In vitro chromosomal aberration results
| Type of test | Experimental test system | Test substance | Exposure conditions | Result | WG considerations and conclusion | Reference |
|---|---|---|---|---|---|---|
| In vitro chromosomal aberration | Human lymphocytes (pooled blood from healthy male non‐smoking donors) |
Triazine amine IN‐A4098 (97%) Negative control: solvent DMSO (2% v/v) |
1st exp.: −S9: 3h expo., harvesting after 18 h, 0 (DMSO), 110.88, 184.8 and 308 μg/mL; +S9 (2% v/v): 3 h expo., harvesting after 18 h, 0 (DMSO), 110.88, 184.8 and 308 μg/mL 2nd exp.: −S9: 21 h expo., harvesting at end of treatment, 0 (DMSO), 66.33, 184.8 and 308 μg/mL; +S9 (5% v/v): 3h expo., harvesting after 18 h, 0 (DMSO), 110.88, 184.8 and 308 μg/mL Duplicate cultures |
No stat. significant increase in % aberrant metaphases No stat. significant increase in proportion of polyploid cells 1st exp: No reduction in MI at 308 μg/ml with or without S9 2nd exp: decrease of MI at the two high doses w/o S9 (MI: 75% and 59% of neg. control, respectively) |
GLP compliant OECD TG 473 (1997) Solubility of IN‐A4098 in DMSO: up to 15.4 mg/mL (308 μg/mL = limit solubility) 2% v/v DMSO added to the culture medium instead of 1% v/v No precipitation, no fluctuation in osmolality or change in pH Cytotoxicity: calculation of MI (not for pos. control treated cultures or cultures where no signs of cytotoxicity) 200 metaphases analysed/dose (according to OECD TG 473, 2016: at least 300 metaphases should be analysed) Minor deviations from current OECD requirements which do not impact the conclusion Negative |
Woods (2011b) |
| In vitro chromosomal aberration | Human lymphocytes (1 healthy non‐smoking 23‐year‐old female donor) |
Triazine amine IN‐A4098 (98.7%) Negative control: solvent DMSO |
Preliminary toxicity test: max. dose tested: 1,400 μg/mL, with or without S9 −S9: expo. 4 h and 20 h, +S9: 4 h Main test: 1st exp.: −S9: 4 h expo., harvesting 20 h after exposure, 0 (DMSO), 500, 1,000 and 1,400 μg/mL; +S9 (2% v/v): 4 h expo., harvesting 20 h after exposure, 0 (DMSO), 500, 1,000 and 1,400 μg/mL 2nd exp.: −S9: 20 h expo., harvesting at end of treatment, 0 (DMSO), 500, 1,000 and 1,400 μg/mL; Duplicate cultures |
Visible precipitate at ≥ 420 μg/mL. Dose levels ≤ 140 μg/mL were soluble in treatment medium. Absence of toxicity No stat. significant increase in the % of cells with structural or numerical aberrations Visible precipitate at ≥ 250 μg/mL, dose level of 125 μg/mL was soluble in treatment medium at beginning of treatment, visible; precipitate at conclusion of treatment at ≥ 500 μg/mL, doses ≤ 250 μg/mL were soluble MI: decrease up to 53% in 1st exp. ‐S9, up to 61% in 1st exp +S9, up to 57% in 2nd exp. –S9 |
GLP compliant OECD TG 473 (1997) Solubility of IN‐A4098 in DMSO: at 150 mg/mL Only conc. above the solubility limit of TA in the culture medium have been tested 200 metaphases analysed/dose (according to OECD 473, 2016: at least 300 metaphases should be analysed) Minor deviations from current OECD requirements which do not impact the conclusion Negative |
Gudi and Rao (2009) |
| In vitro chromosomal aberration | Human lymphocytes (healthy donors) |
Triazine amine IN‐A4098 (99.5%) Negative control: solvent DMSO |
Preliminary cytotoxicity: 0.1–100 μg/mL Main test: 1st exp.: −S9: 4 h expo., harvesting 20 h later, 0 (DMSO), 25, 50, 100 and 200 μg/mL; + S9 (10% v/v): 4 h expo., harvesting 20 h later, 0 (DMSO), 25, 50, 100 and 200 μg/mL 2nd exp.: −S9: 24 h expo., harvesting at end of treatment, 0 (DMSO), 25, 50, 100 and 200 μg/mL; + S9 (10% v/v): 4 h expo., harvesting 20 h later, 0 (DMSO), 25, 50, 100 and 200 μg/mL Duplicate cultures |
MI: −S9: 65% of negative control at HD, +S9: 85% of negative control at HD No stat. significant increase in the % of cells with aberrations excluding gaps No test item‐related polyploidy or endoreduplication Precipitation at the HD 200 μg/mL, no signs of cytotoxicity 1st exp: −S9 MI: 134% of negative control at HD, +S9: 90% of negative control at HD; 2nd exp: −S9 MI: 98% of negative control at HD, +S9: 107% of negative control at HD |
GLP compliant OECD TG 473 (1997) Solubility of IN‐A4098 in DMSO: 10 mg/mL Max. conc. Achieved: 200 μg/mL 200 metaphases analysed/dose (according to OECD 473, 2016: at least 300 metaphases should be analysed) Minor deviations from current OECD requirements which do not impact the conclusion Negative |
Flügge (2011c) |
| In vitro chromosomal aberration | Human lymphocytes |
Triazine amine CGA 150 829 tech. (97%) Negative control: solvent DMSO (1% v/v) |
Preliminary cytotoxicity: 0.0122–100 μg/mL Main test: − S9: 3 h expo., harvesting 44 h later, 0 (DMSO), 0.625, 1.25, 2.5, 5 & 10 μg/mL; +S9 (10% v/v): 3 h expo., harvesting 44 h later, 0 (DMSO), 6.25, 12.5, 25, 50 & 100 μg/mL |
−S9 MI: 37% of negative control at HD, +S9 MI: 75% of negative control at HD −S9: No increase in the % of aberrant metaphases +S9: no dose‐related increase in the % of aberrant cells No increase in polyploidy or aneuploid metaphases Positive control: no clear increase in the % aberrant cells in test +S9 |
Solubility in DMSO: 10 mg/mL No determination of solubility limit in culture medium Cytotoxicity not concurrently measured 100 metaphases analysed/dose (according to OECD TG 473, 2016: at least 300 metaphases should be analysed) No repetition of the experiment No experiment with an extended treatment period No statistical evaluation Major deviations from current OECD requirements which impact the conclusion Negative but not reliable |
Dollenmeier (1987) |
| In vitro chromosomal aberration | CHO cells (CCL 61) |
Triazine amine CGA 150 829 tech. (99.4%) Negative control: solvent DMSO (1% v/v) |
Cytotoxicity: 0.78–100 μg/mL Main test: Original study: Exp 1 −S9: 25, 50 & 100 μg/mL, expo: 18 h, harvesting at end of treatment; Exp 2 +S9 (1.5%): 25, 50 & 100 μg/mL, expo: 3 h, recovery: 15 h later Confirmatory study: Exp 1 −S9: 25, 50 & 100 μg/mL, expo: 18 h, harvesting at end of treatment; Exp 2 +S9 (1.5%): 25, 50 & 100 μg/mL, expo: 3 h, recovery: 15 h later Exp 3 −S9: 25, 50 & 100 μg/mL, expo: 42 h, harvesting at end of treatment; Exp 4 +S9 (1.5%): 25, 50 & 100 μg/mL, expo: 3 h, recovery: 39 h later |
No increase in the % of cells with aberrations Original study: Exp 1: HD: MI = 97% of negative control Exp 2: HD: MI = 73% of negative control Confirmatory study: Exp 3 HD: MI = 35% of negative control Exp 4 HD: MI = 98% of negative control |
GLP compliant OECD TG 473 (1983) Substance dissolved in DMSO for 2 h by ultrasonic treatment. Neither the solubility limit of triazine amine in DMSO nor the solubility limit of triazine amine in culture medium were determined 200 metaphases analysed/dose and only 50 metaphases in the positive control (according to OECD TG 473, 2016: at least 300 metaphases should be analysed) No statistical evaluation Absence of data allowing to determine if the concentrations used were sufficiently high or not Major deviations from current OECD requirements which impact the conclusion Negative but not reliable |
Meyer (1991) |
DMSO: dimethyl sulfoxide; S9: metabolic activation; MI: mitotic index; HD: high dose.
3.2.5. In vivo chromosomal aberrations
The in vivo cytogenetic test in Chinese hamster exposed by gavage to triazine amine (Report 871187) did not show an increase in chromosomal aberrations in the bone marrow. The cross‐cutting WG genotoxicity noted that no demonstration of bone marrow exposure was provided as not clearly required at that time.
Some studies with triazine amine in rats (Report 901504 and Report 38415) are available which could potentially be taken into account to demonstrate bone marrow exposure in Chinese hamster study.
The oral LD50 of triazine amine (Report 901504) was > 2,000 mg/kg body weight (bw) in male rats and approximately 1,000 mg/kg bw in female (2/5 females died). Clinical signs of toxicity (piloerection, hunched posture, dyspnoea, reduced motor activity, muscular hypertonus) were seen in all animals. Ataxia was observed in the 2000 mg/kg bw males. The surviving animals recovered within 6–9 days post‐dosing. All surviving animals gained body weight during the study.
In a 28‐day feeding study (Report 38415), rats were exposed to 0, 50, 150, 500 and 1,000 mg/kg feed (0, 3.6, 11, 37 and 73 mg/kg bw per day in males and 0, 3.8, 11, 36 and 77 mg/kg bw per day in females). No treatment‐related deaths or clinical signs of toxicity were observed. The only effects reported were a decrease in body weight and body weight gain as well as a reduced food consumption and food efficiency in males at all doses and in females from 500 mg/kg feed. There were no treatment‐related effects on haematology, clinical chemistry, urinalysis, neurobehavioural parameters, gross pathology or histopathology.
No indication of bone marrow exposure in Chinese hamster can be extrapolated from these studies in rats. This is because acute toxicity was higher in rats than in hamsters as no mortality was observed in the in vivo chromosomal aberration test at 3200 mg/kg bw. Furthermore, in the 28‐day study decrease in body weight in itself cannot be considered as evidence of systemic toxicity.
However, as triazine amine did not induce structural chromosome aberrations in mammalian cells in vitro, an in vivo follow‐up test is not required according to the genotoxicity testing strategies (EFSA, 2012).
Table 6.
In vivo chromosomal aberration results
| Type of test | Experimental test system | Test substance | Exposure conditions | Result | WG considerations and conclusion | Reference |
|---|---|---|---|---|---|---|
| In vivo chromosomal aberration | BM cells of Chinese hamster (negative control: 8M + 8F, treated animals: 24M + 24F) |
Triazine amine CGA 150 829 tech. (97.6%) Vehicle: 0.5% CMC = negative control |
Preliminary tolerability test up to 5,000 mg/kg bw Gavage 0 & 3,200 mg/kg bw Sampling time: 16, 24 and 48 h after treatment |
3,200 mg/kg bw = highest applicable dose No increase in the number of metaphases with specific aberrations |
GLP compliant OECD 475 (1984) Slides of 5 males & 5 females selected for scoring 100 metaphases analysed/slide No information reported about the toxic effects observed in the preliminary tolerability test Major deviations from OECD TG 475 (2016) requirements Negative but no information on BM exposure |
Report 871187 |
BM: bone marrow; M: male; F: Female; CMC: Carboxymethylcellulose.
3.2.6. Conclusion based on experimental data on triazine amine
Based on the experimental evidence, the cross‐cutting WG genotoxicity concluded that there is no concern for the potential of triazine amine to induce gene mutations and clastogenicity. The cross‐cutting WG genotoxicity noted that the potential to induce numerical chromosomal aberrations (i.e. aneugenicity) was not adequately investigated.
Because potential aneugenicity of triazine amine was not adequately assessed by experimental data, the cross‐cutting WG genotoxicity performed a QSAR analysis and considered the information provided by the applicant on structurally related metabolites (Bentley et al., 2019).
3.3. Quantitative Structure–Activity Relationships (QSARs)
The application of the OECD QSAR toolbox (version 4.3) to triazine amine showed structural alerts for in vitro and in vivo mutagenicity and for carcinogenicity, associated with the nature of primary aromatic amine, but this alert is overruled by the available experimental data set, where no mutagenic effect was reported. However, it should be noted that structural alerts for aneugenicity are not at present addressed by the OECD QSAR toolbox or any other QSAR model. No read‐across analysis for triazine amine has been conducted in OECD QSAR Toolbox by the cross‐cutting WG genotoxicity.
3.4. Information on structurally related compounds
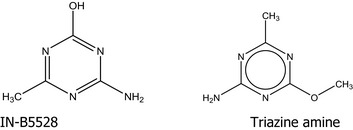
The cross‐cutting WG genotoxicity considered read‐across from the in vitro micronucleus assay on the downstream metabolite IN‐B5528 and the available in vivo micronucleus assay and toxicokinetic study conducted with the precursor metabolite IN‐L5296.
3.4.1. Read‐across from in vitro micronucleus assay conducted with the downstream metabolite IN‐B5528
The applicant considered that metabolite IN‐B5528 and triazine amine show strong structural similarity as regards the core structure and the presence of functional groups and that read‐across could be potentially applied for different endpoints (Bentley et al., 2019). However, the cross‐cutting WG genotoxicity noted that as regards potential aneugenicity, the read‐across was not considered justified as (Q)SARs for aneugenicity are currently not sufficiently clarified. Therefore, the negative in vitro micronucleus assay on IN‐B5528 (Lloyd, 2017) is not considered useful to draw conclusion on triazine amine aneugenicity potential.
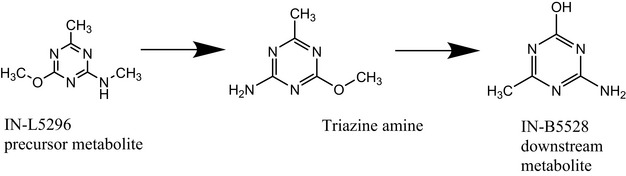
3.4.2. In vivo micronucleus assay and toxicokinetic study with the precursor IN‐L5296
IN‐L5296 is a precursor of triazine amine and of IN‐B5528 (Figure 1).
Scheme for transformation between IN‐L5296 to triazine amine and IN‐B5528 (Bentley et al., 2019).
IN‐L5296 has been evaluated in a battery of in vitro and in vivo genotoxicity studies. However, the cross‐cutting WG Genotoxicity considered only the in vivo micronucleus test (Report 1378/85) relevant for evaluation of aneugenicity.
The bone marrow micronucleus test (Report 1378/85, see Table 7) in CD 1 mice was performed according to OECD TG 474 (OECD, 1997). Based on the results of a preliminary toxicity study, showing mortality at 300 mg/kg bw, animals were treated by gavage at doses of 62.5, 125 and 250 mg/kg bw for two consecutive days. The frequency of micronuclei was comparable between treated and negative control groups. No direct evidence of toxicity to the bone marrow was observed, since no reduction of the polychromatic erythrocytes (PCE)/normochromatic erythrocytes (NCE) ratio was observed after treatment. However, the cross‐cutting WG genotoxicity noticed that clinical signs of toxicity related to central nervous systems were reported at the highest dose tested (e.g. lethargy). Based on the opinion of the EFSA Scientific Committee on ‘Clarification of some aspects related to genotoxicity assessment’ (2017b), this is considered as a line of evidence showing systemic bioavailability of the test item and indicates bone marrow exposure.
Table 7.
In vivo Micronucleus results on the precursor IN‐L5296
| Type of test | Experimental test system | Test substance | Exposure conditions | Result | WG considerations and conclusion | Reference |
|---|---|---|---|---|---|---|
| In vivo Micronucleus test |
BM cells of CD‐1 mice M+F in range finding study (3M+3F at 250, 3M at 300, 1M + 1F at 350, 1M + 3 F at 500 and 3 F at 600 mg/kg bw) 6 M/group in main study |
IN‐L5296 (99.7% pure) Vehicle: 0.5% CMC = negative control |
Range‐finding study: 250, 300, 350, 500 and 600 mg/kg bw (gavage), administration once daily on 2 consecutive days Main study: 0, 62.5, 125 and 250 mg/kg bw (gavage) Sampling time: 24 after second administration |
1 mice/group died within 2 days at doses ≥ 300 mg/kg Lethargy in 2 mice at the high dose No (statistical) increase in frequency of MN PCE No effect on PCE/NCE (no cytotoxicity) |
GLP compliant OECD TG 474 (1997) At least 12,000 PCE analysed Deviations from OECD TG 474 (2016) (Historical positive control data not provided) Negative |
Report 1378/85 |
BM: bone marrow; M: male; F, Female; CMC, Carboxymethylcellulose; PCE, polychromatic erythrocytes; NCE, normochromatic erythrocytes.
Systemic exposure to the test item was confirmed in a follow‐up study investigating in mice the presence and concentration of IN‐L5296 and identified metabolites in plasma and urine (Report 48754). The study was performed in accordance with OECD TG 417 (2010). IN‐L5296 was administered to mice once per day by oral gavage for 2 consecutive days at the dose of 62.5 mg/kg bw, which matched to the lowest single oral dose tested in the previous in vivo micronucleus assay. Blood samples were collected at a single time point (day 2) and pooled into a single sample and quantified by UHPLC (Ultra‐high‐pressure liquid chromatography) with LC/MS/MS (Liquid chromatography tandem‐mass spectrometry) detection. The following metabolites were analysed together with the precursor IN‐L5296: IN‐UZJ04, IN‐QHP91, IN‐R9805, IN‐A4098 (triazine amine), IN‐37739, IN‐L9622 and IN‐B5528. The plasma concentration of IN‐L5296 (662 ng/mL, Limit of Quantification (LOQ) 20 ng/mL) was found to be 1.7% of the concentration of all eight compounds.
In contrast, the concentration of IN‐37739 was 70.3% of the total and the concentration of triazine amine (7,530 ng/mL, LOQ 20 ng/mL) accounted for 21.6% of the total. IN‐37739 is the immediate precursor of triazine amine (see Annex II).
On the basis of the Report 1378/85 and Report 48754 studies, the cross‐cutting WG genotoxicity noted that aneugenicity of triazine amine was not observed in mice at experimental conditions resulting in a plasma concentration of at least 7 μg/ml; however, it is not possible to conclude on higher plasma concentrations or potential site of contact effects through direct exposure to triazine amine. In order to reach a conclusion on the aneugenic potential of triazine amine, an in vitro micronucleus assay with triazine amine is needed.
4. Conclusions
Based on the overall weight of evidence, the cross‐cutting WG genotoxicity concluded that there is no concern for the potential of triazine amine to induce gene mutations and clastogenicity. The cross‐cutting WG genotoxicity noted that the potential to induce numerical chromosomal aberrations (aneugenicity) was not adequately investigated. For a conclusion, an in vitro micronucleus assay performed with triazine amine would be needed.
The PPR Panel agreed with the assessment of the cross‐cutting WG genotoxicity.
5. Recommendation
For a conclusion on potential for aneugenicity, an in vitro micronucleus assay performed with triazine amine would be needed.
Abbreviations
- BM
bone marrow
- CAS
chemical abstracts service
- CHL
Chinese hamster lung
- CHO
Chinese hamster ovary
- DMSO
dimethyl sulfoxide
- GEF
global evaluation factor
- GLP
good laboratory practise
- HD
highest dose
- HGPRT
hypoxanthine‐guanine phosphoribosyltransferase
- IUPAC
international union of pure and applied chemistry
- LC/MS/MS
Liquid chromatography tandem‐mass spectrometry
- LOQ
limit of quantification
- MI
mitotic index
- MN
micronuclei
- NCE
normochromatic erythrocytes
- OECD
Organisation for Economic Co‐operation and Development
- PCE
polychromatic erythrocytes
- PPR
Plant Protection Products and their Residues
- QSAR
Quantitative Structure–Activity Relationship
- RTG
relative total growth
- S9
metabolic activation
- TG
Test Guideline
- TK
thymidine kinase
- UDS
unscheduled DNA Synthesis
- UHPLC
Ultra‐high‐pressure liquid chromatography
- WG
Working Group
- WoE
weight of evidence
Annex I – Names and structures of the metabolites considered for the evaluation
1.
| Code/Trivial name | Chemical name/SMILES notation/InChI Key* | Structural formula* |
|---|---|---|
|
IN‐B5528 4‐amino‐6‐methyl‐1,3,5‐triazin‐2‐ol O‐demethyl triazine amine |
4‐amino‐6‐methyl‐1,3,5‐triazin‐2‐ol Nc1nc(C)nc(O)n1 UUTHDVPZNWJUFV‐UHFFFAOYSA‐N |
|
|
IN‐L9622 Hydroxymethyl triazine amine |
(4‐amino‐6‐methoxy‐1,3,5‐triazin‐2‐yl)methanol COc1nc(CO)nc(N)n1 KHTKRVNPOLBKLX‐UHFFFAOYSA‐N |
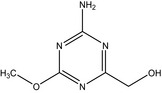
|
| IN‐L5296 |
4‐methoxy‐N,6‐dimethyl‐1,3,5‐triazin‐2‐amine Cc1nc(NC)nc(OC)n1 MNDSUSQBIDHEJU‐UHFFFAOYSA‐N |
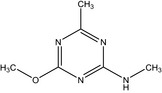
|
| IN‐QHP91 |
[4‐methoxy‐6‐(methylamino)‐1,3,5‐triazin‐2‐yl]methanol COc1nc(CO)nc(NC)n1 JFQHYXLCVRKNOC‐UHFFFAOYSA‐N |
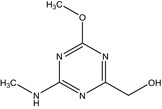
|
| IN‐37739 |
[(4‐methoxy‐6‐methyl‐1,3,5‐triazin‐2‐yl)amino]methanol Cc1nc(NCO)nc(OC)n1 GFYFBMBGFNSSET‐UHFFFAOYSA‐N |
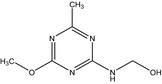
|
| IN‐R9805 |
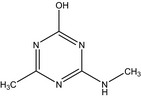
4‐methyl‐6‐(methylamino)‐1,3,5‐triazin‐2‐ol Cc1nc(NC)nc(O)n1 RSBYIKCVMXJHEA‐UHFFFAOYSA‐N |
|
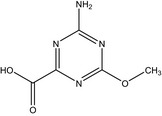
| IN‐UZJ04 |
4‐amino‐6‐methoxy‐1,3,5‐triazine‐2‐carboxylic acid COC1=NC(C(O)=O)=NC(N)=N1 PWWLZHMZBKQGQQ‐UHFFFAOYSA‐N |
|
Names, SMILES codes and InChI Keys are generated by ChemBioDraw Version 13.0.2.3021.
Annex II – Proposed Metabolic Pathway Showing Metabolites Observed in the Mouse Plasma and Urine (Report 48754)
1.
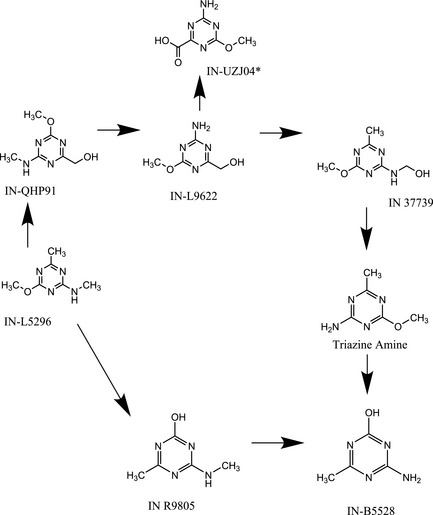
* IN‐UZJ04 was observed as sodium salt.
Annex III – Detailed evaluation of available In vitro mammalian gene mutation studies with triazine amine
1.
Clarke, 2009 . CHO cells In vitro mammalian cell gene mutation test (CHO/ hprt assay)
The test substance IN‐A4098 (purity 98.7%) was tested in CHO/hprt assay following the OECD TG 476 (1998).
In a preliminary range finding test, the substance, dissolved in DMSO, was tested at nine different concentrations from 0.15 to 1425 μg/mL (10 mM) in the presence and absence of metabolic activation (S9). Visible precipitate was detected at concentrations ≥ 150 μg/mL. The cloning efficiency relative to the solvent control at 1425 μg/mL was 63% and 26% without and with S9, respectively.
The range of concentrations selected for the mutation test were 10, 25, 50, 150 μg/mL, mainly based on the precipitation profile. A compensation factor was applied to adjust for percent of purity of the final concentrations tested. The duration of the exposure was 5 h. The relative cloning efficiency was 103 and 86% at the highest concentration (150 μg/mL) without and with S9, respectively.
No increase in mutation frequency was observed in the experiment in the presence of S9.
An increase in mutant frequency was seen in the experiment without S9, with a large variability of mutant frequencies between replicate cultures, both in solvent control and in treated samples. The mutant frequency at the highest concentration was 5.4‐fold higher than in the vehicle control group and outside the range of the historical solvent control data.
However, the authors concluded that the observed increases are not relevant, considering that the mutation frequencies in the treated samples did not exceed 40 mutants/106 clonable cells, referring to the published spontaneous mutation frequency for CHO cells of 2‐50/106 clonable cells. This criterion cannot be applied for this study considering the low spontaneous mutation frequency in controls reported for the laboratory (historical controls mutant frequency (MF)= 3.7±4.3, range 0–16.7).
The statistical analysis was not provided in the report. A statistical analysis, carried out by Bentley et al. (2019) but not reported in detail, using log‐transformation of the data which takes into account the variability, did not find statistical differences between treated groups and vehicle control groups either in the experiment with metabolic activation or the experiment without metabolic activation. The P values for the comparisons with test groups 10, 25, 50, 100 and 150 μg/mL were 0.84, 0.60, 0.42, 0.13 and 0.47, respectively, in the experiment with metabolic activation, and the corresponding values in the experiment without metabolic activation were 0.95, 0.67, 0.93, 0.80 and 0.24, respectively. Neither were statistically significant linear trends established in these experiments (p = 0.18 and 0.19 for the experiments with metabolic activation and without metabolic activation, respectively). No statistically significant increases were also reported using the Poisson analysis.
The study generally complies with OECD TG 476 (1997). A main deviation from the OECD TG 476 (2016) is the low number of cells treated in the study. The TG suggests to maintain 10 spontaneous mutants for every culture in each phase of the assay. In this study, the mutant plate counts for duplicate cultures were 13/3 and 2/6 in the experiment with and without metabolic activation, respectively.
The insufficient number of cells reduces the power of the assay with the possibility that increases in mutant frequencies may be induced by chance. The large variability observed in the study between replicate cultures both in solvent control and in treated samples is the proof of this.
On the basis of these considerations, the study is considered as inconclusive.
Flügge, 2011b . V79 cells in vitro mammalian cell gene mutation test (V79/HGPRT assay)
The test substance IN‐A4098 (purity 99.5%) was tested in V79/HGPRT assay following the OECD TG 476 (1997).
In a preliminary range finding test, the substance, dissolved in DMSO, was tested at seven different concentrations from 0.10 to 200 μg/mL at 4 h in the presence and at 24 h in the absence of S9. The final DMSO concentration in culture medium was 2%. Visible precipitate was detected at 200 μg/mL. No signs of cytotoxicity were noted up to the top concentration of 200 μg/mL. The cloning efficiency was comparable in control and treated samples.
The range of concentrations selected for the mutation test was 12.5, 25, 50, 100 and 200 μg/mL, mainly based on the precipitation profile. In the absence of S9, the cells were exposed to the test compound for 4 h in the first experiment and 24 h in the second experiment. In the presence of S9, the cells were exposed for 4 h in both the experiments. Precipitation in the culture medium was observed at 200 μg/mL. No cytotoxic effects were observed at any concentration tested.
No increase in the mutation frequency was detected in any treated sample compared with the solvent control. The study complies with the OECD TG 476 (2016). The only deviation from the OECD TGs is the concentration of DMSO (2% instead of 1%).
The study is acceptable and is considered as negative.
Woods, 2011a . In Vitro Mutation Test Using Mouse Lymphoma Assay
The test substance IN‐A4098 (purity 97%) was tested for mutagenicity at the thymidine kinase locus in L5178Y cells following the OECD TG 476 (1997).
In a preliminary range finding test, the substance, dissolved in DMSO, was tested up to the limit of solubility, at 10 different concentrations from 0.6 to 308 μg/mL at 3 h exposure in the presence and absence of S9 and at 24 h in the absence of S9. The final DMSO concentration in culture medium was 2%. Only limited toxicity was found.
The range of concentrations selected for the mutation test was 38.5, 77, 154 and 308 μg/mL at all the conditions tested. In the absence of S9 following 3 h of exposure, the relative total growth (RTG) values relative to the vehicle control were from 108 to 77%. No increase in the mean mutant frequencies was observed of any of the test concentrations assessed.
In the presence of S9 following 3 h of exposure, the RTG values relative to the vehicle control were from 117 to 86%. No increase in the mean mutant frequencies was observed of any of the test concentrations assessed.
In the absence of S9 following a continuous 24 h of exposure, the RTG values obtained were from 101 to 54%. An increase in the mean mutant frequencies was observed at 308 μg/mL (160 × 10−6 mutants corresponding to 2.4‐fold increase with respect to the control value), but the observed increases did not exceed the GEF of 126 × 10−6 mutants above the mean concurrent vehicle control mutant frequency. No concentration‐related response was evident, even though the trend test was not reported.
The only deviation from the OECD TG 490 (2016) is the concentration of DMSO (2% instead of 1%). The mutant frequency obtained with the solvent control is included in the range of historical controls; therefore, the study is acceptable. On the basis of these results, the compound is considered to be ‘negative’ complying with the criteria of the OECD TG 490 (2016) where ‘a test chemical is considered to be clearly negative if there is no concentration related response or, if there is an increase in MF, it does not exceed the GEF’.
This study was evaluated ‘equivocal’ by EFSA in 2015 presumably referring to the criteria of the OECD TG 476 (1997) which did not consider the GEF.
Lloyd, 2016 . In Vitro Mutation Test Using Mouse Lymphoma Assay
The test substance IN‐A4098 (purity 98.7%) was tested for mutagenicity at the thymidine kinase locus in L5178Y cells following the OECD TG 490 (2016).
In a preliminary range finding test, the substance, dissolved in DMSO, was tested up to the limit of solubility, at six different concentrations from 1.25 to 40 μg/mL at 3 h exposure in the presence and at nine concentrations from 0.1563 to 40 μg/mL at 24 h in the absence of S9. The maximum concentration tested was 40 μg/mL, limited by the solubility (4 mg/mL) of the test article using 1% DMSO v/v additions of the test article formulations, as recommended by the TG.
The range of concentrations selected for the mutation test was 2.5, 5, 10, 20, 30, 35 and 40 μg/mL at all the conditions tested. In the first experiment, at the highest concentration tested (40 μg/mL) at 3 h of exposure, the RTG values were 129 and 117% in the absence and presence of S9, respectively. In the second experiment, the highest concentration tested (40 μg/mL) gave 82% RTG at 3 h treatment in the presence of S9 and 81% RTG at 24 h treatment in the absence of S9.
No increase in the mean mutant frequencies that exceeded the GEF was observed in any treated culture.
No deviation from the OECD TGs was observed. The study is acceptable and is considered as negative.
Pant, 2019 . CHO/hprt mammalian cell gene mutation assay
In the Pant, 2019 study, the test substance IN‐A4098‐005 (purity 98.7%) was tested in CHO/hprt assay following the OECD TG 476 (2016). The study is a repeat of the CHO/hprt mammalian cell gene mutation assay without metabolic activation by Clarke (2009) in which a slight increase in mutation frequency was observed at the two highest concentrations. Since the results obtained with S9 in the same study were clearly negative, the segment with S9 was not included in this study.
CHO cells were treated at five concentrations of triazine amine dissolved in DMSO: 0, 10, 25, 50, 100 and 150 μg/mL for 5 hours without S9 activation, reaching 1% DMSO in culture medium. No precipitation of the test substance was observed in the culture medium at the beginning and in the end of the treatment period. The average adjusted relative survival was 90.82% at the highest concentration of 150 μg/mL. No statistically significant dose‐related increase was observed in mutant frequency, as compared to the concurrent vehicle controls, at any concentration evaluated. Three test substance concentrations (i.e. 10, 50 and 150 μg/mL) and one of the duplicate vehicle control cultures had mutant frequencies slightly exceeding the 95% historical vehicle control limit.
In a confirmatory experiment performed with the same concentrations, no statistically significant increase in mutant frequency at any concentration evaluated was observed, as compared to the concurrent vehicle controls. All test substance concentrations were within the 95% historical vehicle control range. The test substance is considered negative in this in vitro mammalian cell gene mutation assay.
Woods, 2019 . In Vitro Mutation Test Using Mouse Lymphoma Assay
In the Woods, 2019 study, the test substance IN‐A4098‐005 (purity 98.7%) was tested for mutagenicity at the thymidine kinase locus in L5178Y cells following the OECD TG 490 (2016).
This study was conducted to clarify the results of a previous study in which an increase in the mean mutant frequencies was reported at the highest concentration tested (308 μg/mL) following 24 h of exposure in the absence of S9 mix (Woods, 2011a).
The cells were exposed at five concentrations of triazine amine dissolved in DMSO: 18.75, 37.5, 75, 150, 300 μg/mL for either 3 hours or 24 hours in the absence of metabolic activation (S9 mix) or 3 hours in the presence of S9 mix, reaching 1% concentration of DMSO in culture medium. No reduction in relative total growth (RTG) was observed. No increases in the mean mutant frequencies was observed at any concentration tested for any condition of treatment that exceeded the sum of the mean concurrent vehicle control mutant frequency and the GEF. However, the mean mutation frequency for the vehicle control and for the highest concentration of triazine amine (300 μg/mL) at 3 hour exposure in the absence of S9 mix were above the 95% confidence limits of the historical vehicle control data. A confirmatory experiment carried out at the same conditions did not detect any increase of mutation frequency outside of the 95% confidence limits of the historical vehicle control data. The test substance is considered negative in this in vitro mammalian cell gene mutation assay.
Suggested citation: EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , Hernandez‐Jerez AF, Adriaanse P, Aldrich A, Berny P, Coja T, Duquesne S, Gimsing AL, Marinovich M, Millet M, Pelkonen O, Pieper S, Tiktak A, Topping CJ, Tzoulaki I, Widenfalk A, Wolterink G, Benford D, Aquilina G, Bignami M, Bolognesi C, Crebelli R, Guertler R, Marcon F, Nielsen E, Schlatter JR, Vleminckx C, Maurici D and Parra Morte JM, 2020. Scientific Opinion of the Scientific Panel on Plant Protection Products and their Residues (PPR Panel) on the genotoxic potential of triazine amine (metabolite common to several sulfonylurea active substances). EFSA Journal 2020;18(3):6053, 24 pp. 10.2903/j.efsa.2020.6053
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00830
Panel members: Antonio F Hernandez‐Jerez, Paulien Adriaanse, Annette Aldrich, Philippe Berny, Tamara Coja, Sabine Duquesne, Anne Louise Gimsing, Marina Marinovich, Maurice Millet, Olavi Pelkonen, Silvia Pieper, Aaldrik Tiktak, Christopher J Topping, Ioanna Tzoulaki, Anneli Widenfalk and Gerrit Wolterink.
Adopted: 12 February 2020
Notes
EFSA noted that according to Technical Report (2018) it is quoted that “there was general agreement that triazine amine does not induce chromosome aberration in vitro”. However, it would have been more accurate to mention in the Technical Report (2018) that triazine amine does not induce structural chromosome aberrations (i.e. clastogenicity).
In the past a single OECD guideline 476 (1997) was available for the In Vitro Mammalian Cell Gene Mutation Test, including the evaluation of mutation at thymidine kinase (tk) and hypoxanthine‐guanine phosphoribosyl transferase (Hprt) genes, and a transgene of xanthine guanine phosphoribosyl transferase (xprt). In 2016, two separate OECD guidelines were developed: OECD guideline 476 (2016) for the In Vitro Mammalian Cell Gene Mutation Tests using the Hprt and xprt genes and OECD guideline 490 (2016) for the In Vitro Mammalian Cell Gene Mutation Tests Using the tk Gene.
References
- Bentley K, Malley LA, O'Neal FO and Gaddamidi V, 2019. Triazine Amine (Aminotriazine): Weight of Evidence Assessment Excluding Genotoxicity. Not published. FMC‐50183 EU, Revision No. 1.
- Clarke JJ, 2009. IN‐A4098: In vitro Mammalian Cell Gene Mutation Test (CHO/HGPRT Assay). Not published: DuPont; 2009. Contract No.: DuPont‐28083.
- Dollenmeier P, 1987. CGA150829 tech: Chromosome studies on human lymphocytes in vitro. Not published: Syngenta; 1987. Contract No.: Ciba‐Geigy report 860159.
- Donath C, 2011. Reverse mutation using bacteria (Salmonella typhimurium and Escherichia coli) with aminotriazine. Not published: Cheminova; 2011. Contract No.: report 203 TIM.
- European Comission , 2013. Guidance document on the procedures for submission and assessment of confirmatory information following approval of an active substance in accordance with Regulation (EC) No 1107/2009. SANCO/5634/2009 rev 6.1. Dated December 2013.
- EFSA (European Food Safety Authority), 2012. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379. Available online: www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017a. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for metsulfuron‐methyl in light of confirmatory data. EFSA supporting publication 2017;EN‐1257, 44 pp. 10.2903/sp.efsa.2017.EN-1257 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. EFSA Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger M, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Younes M, Aquilina G, Crebelli R, G€urtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, van Benthem J, Carfı M, Georgiadis N, Maurici D, Parra Morte J and Schlatter J, 2017. Scientific Opinion on the clarification of some aspects related to genotoxicity assessment. EFSA Journal 2017;15(12):5113, 25 pp. 10.2903/j.efsa.2017.5113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Technical report on the outcome of the consultation with Member States, the applicant and EFSA on the pesticide risk assessment for iodosulfuron and prosulfuron in light of confirmatory data. EFSA supporting publication 2018 ;EN‐1470. 56 pp. 10.2903/sp.efsa.2018.en-1470 [DOI] [Google Scholar]
- Flügge RNC, 2011a. Mutagenicity Study of IN‐A4098 in the Salmonella typhimurium Reverse Mutation Assay (In Vitro). Not published: Helm AG 2011a. Contract No.: report 27010.
- Flügge RNC, 2011b. Mutagenicity Study of IN‐A4098 in Mammalian Cells (V79) in the In Vitro Gene Mutation Assay (HPRT Test). Not published: Helm AG; 2011b. Contract No.: report 27012.
- Flügge RNC, 2011c. In Vitro Assessment of the Clastogenic Activity of IN‐A4098 in Cultured Human Peripheral Lymphocytes. Not published: Helm AG; 2011c. Contract No.: report 27011.
- Geleick D, 1991. CGA150829 tech: Salmonella and Escherichia liver microsome test. Not published: Syngenta; 1991. Contract No.: Ciba‐Geigy report 901510.
- Gudi R and Rao M, 2009. IN‐A4098: In vitro Mammalian Chromosome Aberration Test. Not published: DuPont; 2009. Contract No.: DuPont‐28082 Revision 1.
- Hertner T, 1988. CGA150829 tech: Autoradiographic DNA‐repair test on rat hepatocytes. Not published: Syngenta; 1988. Contract No.: Ciba‐Geigy report 871186.
- Lloyd M, 2016. IN‐A4098: In vitro L5178Y Gene Mutation Assay at the tk locus. Not published: DuPont; 2016. Contract No.: DuPont‐42500 Revision 1.
- Lloyd M, 2017. IN‐B5528: In Vitro Human Lymphocyte Micronucleus Assay. Not published: DuPont; 2017. Contract No.: DuPont‐47605.
- May K, 2011. IN‐A4098 bacterial Reverse Mutation Test. Not published: Rotam; 2011. Contract No.: report PII0019.
- Meyer A, 1988. CGA150829 tech: Autoradiographic DNA repair test on human fibroblasts. Not published: Syngenta; 1988. Contract No.: Ciba‐Geigy report 871188.
- Meyer A, 1991. CGA150829 technical: Cytogenetic test on Chinese hamster cells in vitro. Not published: Syngenta; 1991. Contract No.: Ciba‐Geigy report 901511.
- Pant K, 2019. In Vitro Mammalian Cell Forward Gene Mutation (CHO/HPRT) Assay with Duplicate Cultures with IN‐A4098. Not published: FMC Corporation, 2019. Contract No.: FMC‐51667.
- Report 1378/85 .L5296: induction of micronuclei in the bone marrow od treated mice. Not published. DuPont, 2000. Report 1378/85.
- Report 38415 . IN‐A4098: Repeated‐Dose Oral Toxicity 28‐Day Feeding Study in Rats. Not published: DuPont; 2014. Contract No.: DuPont‐report 38415.
- Report 48754 . IN‐L5296: Bone Marrow Exposure Evaluation in the Mouse by Targeted Metabolite Analysis of Plasma and Urine. Not published: DuPont, 2018. Contract No.: DuPont‐Report 48754.
- Report 871187 . Chromosome studies on somatic cells of Chinese Hamster. Not published: Syngenta; 1988. Contract No.: Ciba‐Geigy report 871187.
- Report 901504 . CGA150829 tech: Acute Oral Toxicity in the Rat. Not published: Syngenta; 1991. Contract No.: Ciba‐Geigy report 901504.
- Stammberger I and Braun K, 1988. AE F059411; Substance Technical, Code AE F059411 00 1C99 0001, Bacterial Reverse Mutation Test. Not published: Bayer; 1998. Contract No.: report HMR 98.0717 (M‐181601‐01‐1).
- Wagner VO and Van Dyke MR, 2009. IN‐A‐4098 Bacterial reverse mutation assay. Not published: DuPont; 2009. Contract No.: report DuPont 28277.
- Woods I, 2011a. IN‐A4098: In vitro mutation test using mouse lymphoma L5178Y cells. Not published: Rotam; 2011a. Contract No.: EU TSM Task Force (Cheminova) report PII00021.
- Woods I, 2011b. IN‐A4098: In vitro mammalian chromosome aberration test in human lymphocyte test. Not published: Rotam; 2011b. Contract No.: EU TSM Task Force (Cheminova) report PII00020.
- Woods I, 2019. In Vitro Mutation Test using Mouse Lymphoma L5178Y Cells with IN‐A4098. Not published: FMC Corporation. 2019. Contract No.: FMC‐51668.


