Abstract
A retrospective chronic cumulative risk assessment of dietary exposure to pesticide residues, supported by an uncertainty analysis based on expert knowledge elicitation, was conducted for two effects on the thyroid, hypothyroidism and parafollicular cell (C‐cell) hypertrophy, hyperplasia and neoplasia. The pesticides considered in this assessment were identified and characterised in the scientific report on the establishment of cumulative assessment groups of pesticides for their effects on the thyroid. Cumulative exposure assessments were conducted through probabilistic modelling by EFSA and the Dutch National Institute for Public Health and the Environment (RIVM) using two different software tools and reported separately. These exposure assessments used monitoring data collected by Member States under their official pesticide monitoring programmes in 2014, 2015 and 2016 and individual consumption data from 10 populations of consumers from different countries and different age groups. This report completes the characterisation of cumulative risk, taking account of the available data and the uncertainties involved. For each of the 10 populations, it is concluded with varying degrees of certainty that cumulative exposure to pesticides that have the chronic effects on the thyroid mentioned above does not exceed the threshold for regulatory consideration established by risk managers.
Keywords: pesticide residues, thyroid, cumulative risk assessment, probabilistic modelling, expert knowledge elicitation
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1836/full http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1835/full
This publication is linked to the following EFSA Journal articles: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2020.6087/full
Summary
A retrospective chronic cumulative risk assessment (CRA) of dietary exposure to pesticide residues in 2014, 2015 and 2016 was conducted for two effects on the thyroid: hypothyroidism and C‐cell hypertrophy, hyperplasia and neoplasia.
The first step of the process was to establish cumulative assessment groups (CAG) of pesticides for the effects of relevance so as to assess their combined toxicity on the thyroid. This step is reported in the EFSA scientific report on the establishment of CAGs for their effects on the thyroid (EFSA, 2019a). In that report, all effects of pesticides on the thyroid were reviewed and two were found to meet the criteria established by EFSA's scientific panel on Plant Protection Products and their Residues (PPR Panel) and to be specific for consideration in CRA (hypothyroidism and C‐cell hypertrophy, hyperplasia and neoplasia).
In total, 128 active substances were included in the CAG for hypothyroidism and 17 in the CAG for C‐cell hypertrophy, hyperplasia and neoplasia. All active substances included in the CAGs were characterised by no observed adverse effect levels (NOAELs) for long‐term cumulative exposure/risk assessment, derived from the most sensitive indicator, using all available information across studies, species and sexes.
The number and identity of the active substances included in the CAGs, as well as the allocated NOAELs, are subject to uncertainties. Sources of uncertainty resulting from the methods used to collect and assess toxicological data and from the limitations in the available data and scientific knowledge were therefore identified for appropriate consideration during the CRA conducted with these CAGs. The identified sources of uncertainty were related to the composition of the CAGs, the toxicological characterisation of the active substances, the slope and shape of the dose–response relationship, the contribution of metabolites and degradation products, the adequacy of the dose‐addition model and the inter‐ and intra‐species differences in toxicological sensitivity.
With respect to the composition of the CAGs, the EFSA scientific report used weight of evidence and expert knowledge elicitation (EKE) techniques to address the uncertainty about the total number of active substances in the CAG for hypothyroidism that cause the effect. In this process, active substances were allocated in subgroups of varying levels of evidence and a median estimate of 71 was derived for the number of active substances causing hypothyroidism. A similar exercise was not conducted with the CAG for C‐cell hypertrophy, hyperplasia and neoplasia because the cumulative risk of C‐cell hypertrophy, hyperplasia and neoplasia was anticipated to be very likely lower than the cumulative risk of hypothyroidism.
In a second step, cumulative exposure assessments were conducted of all pesticides included in the CAGs for hypothyroidism (128 active substances) and C‐cell hypertrophy, hyperplasia and neoplasia (17 active substances). These were carried out by EFSA and the Dutch National Institute for Public Health and Environment (RIVM) using probabilistic modelling and with different software tools. The results are reported in the EFSA scientific report on cumulative dietary exposure assessment to pesticides that have chronic effects on the thyroid using SAS® software (EFSA 2019b) and in the external scientific report on cumulative dietary exposure assessment of pesticides that have chronic effects on the thyroid using Monte Carlo Risk Assessment (MCRA) software. The two tools produced nearly identical results and any observed differences are mainly attributed to the random effect of probabilistic modelling. These minor differences do not impact on the outcome of the exposure assessment.
The exposure calculations used monitoring data collected by EU Member States (MSs) under their official monitoring programmes in 2014, 2015 and 2016 and individual food consumption data from 10 populations of consumers from different countries and from different age groups, including vulnerable ones: four populations of adults, three populations of children (3–9 years) and three populations of toddlers (1–3 years).
As agreed by risk managers in the Standing Committee on Plants, Animals, Food and Feed (SC PAFF), the exposure estimates were conducted in a tiered approach. The first‐tier calculations (tier I) use very conservative assumptions for an efficient screening of the exposure with low risk for underestimation; the second‐tier assessment (tier II) includes assumptions that are more refined but still intended to be conservative and therefore likely to overestimate the actual exposure.
For each scenario, exposure estimates relied on the principle of dose addition. They were obtained for different percentiles of the exposure distribution and percentiles were expressed as total margin of exposure (MOET). In accordance with the threshold agreed at the SC PAFF, further regulatory consideration would be required when the MOET calculated at the 99.9th percentile of the exposure distribution is below 100.
The lowest MOET estimates were obtained for pesticides associated with hypothyroidism. In Tier II, MOET estimates at the 50th, 95th and 99th percentile of the exposure distribution were all well above 100. At the 99.9th percentile, estimates were equal or above 100, ranging from 100 to 199 in toddlers and other children. For adults, the MOETs were higher, ranging from 267 to 314. The exposure to this group of pesticides was predominantly driven by the occurrence of bromide ion. Other important drivers were propineb, thiabendazole, ziram, mancozeb, pyrimethanil, chlorpropham and cyprodinil.
For pesticides associated with hypertrophy, hyperplasia and neoplasia of C‐cells, MOETs calculated at the 99.9th percentile of the exposure distribution were higher, ranging from 1,468 to 3,978 in all populations. In this case, the difference between adults and children was less evident and the main drivers for the exposure were identified as thiram and ziram.
The third and last step of the exercise was a cumulative risk characterisation, which is documented in detail in this report. This was based on the outcome of the first two steps and included an uncertainty analysis, performed following the guidance of the EFSA Scientific Committee in order to take account of the limitations in the scientific knowledge and data and of the assumptions used in all steps of the assessment.
Thirty‐one sources of uncertainty affecting the input data, model assumptions and the assessment methodology were identified. The impact of each source of uncertainty on the MOETs at the 99.9th percentile of exposure was quantified. This showed that uncertainties had variable effects, with some tending to overestimate the MOET (e.g. the metabolites were not considered in the assessment) and others tending to underestimate it (e.g. limited availability of processing factors; when such data are missing, it is assumed that all pesticide residues in the raw primary commodity will reach the end consumer without any loss during household or industrial processing). The combined impact of the uncertainties, and their dependencies, on the assessment was then quantified in a sequential approach using EKE techniques and 1‐D Monte Carlo simulations.
As a result of this process, the MOETs at the 99.9th percentile and their confidence intervals were adjusted to take account of the identified uncertainties. For both CAGs, the adjusted MOETs were around two to four times higher compared to those calculated in tier II by the probabilistic tools. This is consistent with the intention of MSs, when selecting the parameters and assumptions to be used, to ensure that the tier II calculations are sufficiently conservative.
Taking into account all uncertainties identified by experts, for hypothyroidism, it was concluded that, with varying degrees of certainty, cumulative exposure does not reach the threshold for regulatory consideration for all the population groups considered. This certainty exceeds 99% for all four adult populations, 95% for two children populations, 90% for one children population and one toddler population and 85% for the remaining two toddler populations. For C‐cell hypertrophy, hyperplasia and neoplasia, the same conclusion was drawn with a certainty exceeding 99% for all 10 populations.
1. Introduction
Cumulative risk assessment (CRA) has been defined as the analysis, characterisation and possible quantification of the combined risks to health or the environment from multiple agents or stressors (US EPA, 2003). It differs from most assessments which consider the effects of one agent or stressor in isolation.
Regulation (EC) No 396/2005 on Maximum Residue Levels (MRLs) of pesticides in or on food and feed states that cumulative and synergistic effects of pesticides should be taken into account for dietary risk assessment, when appropriate methodologies are available. Regulation (EC) No 1107/2009 concerning the placing of plant protection products on the market also states that the residues of the plant protection products shall not have any harmful effects on human health, taking into account known cumulative and synergistic effects where the scientific methods accepted by EFSA to assess such effects are available.
For this reason, EFSA and the Panel on plant protection products and their residues (PPR panel) started in 2007 the development of the necessary methodologies to carry out CRA of pesticide residues. This methodological development included a tiered approach for the assessment of cumulative risks of pesticides residues (EFSA PPR Panel, 2008), a guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues (EFSA PPR Panel, 2012) and a procedure to establish cumulative assessment groups (CAGs) of pesticides on the basis of their toxicological profile (EFSA PPR Panel, 2013a).
1.1. Background and Terms of Reference
In 2014, EFSA started a pilot programme of activities aiming at implementing the CRA of pesticides, using the methodologies developed by the PPR Panel. The objectives of this pilot programme were to evaluate the cumulative effects of pesticide residues on two organs which are known to be sensitive to pesticides (the thyroid and the nervous system), and to test the methodologies over the entire risk assessment process (hazard identification and characterisation, exposure assessment and risk characterisation) for acute and chronic effects.
As part of this programme, the Pesticides Unit (nowadays Pesticides Residues and Pesticides Peer Review units) has been requested by EFSA to prepare a scientific report on the CRA of pesticides residues regarding two chronic effects on the thyroid.
In accordance with article 32 of Regulation (EC) No 396/2005, EFSA has to draw up annual reports on pesticide residues taking into account the results of official control of pesticide residues in food and feed commodities carried out by Member States (MSs), and including an analysis of the acute and chronic risks to the health of consumers from pesticide residues. The present report falls under this article and investigates cumulative risks resulting from the actual exposure to multiple residues.
Based on methodologies adopted by the PPR Panel (EFSA PPR Panel, 2013a,b), EFSA has identified two effects of pesticides on the thyroid which are relevant for CRA, hypothyroidism and C‐cell hypertrophy, hyperplasia and neoplasia.
Therefore, the assessment questions to be addressed by the present report were defined as follows:
What was the chronic cumulative risk of hypothyroidism for European consumers resulting from dietary exposure to pesticide residues from 2014 to 2016?
What was the chronic cumulative risk of C‐cell hypertrophy, hyperplasia and neoplasia for European consumers resulting from dietary exposure to pesticide residues from 2014 to 2016?
In the first assessment question, ‘hypothyroidism’ is defined as an altered function of the thyroid gland resulting in follicular cell hypertrophy, hyperplasia and neoplasia (EFSA, 2019a).
These retrospective assessments were conducted based on official monitoring data collected in 2014, 2015 & 2016, and reported in the respective EFSA annual monitoring reports (EFSA 2016a, 2017a, 2018a). This corresponds to the latest cycle of 3 years of the EU coordinated programme (EUCP) for which data were available when the assessments started.
Thirty plant commodities were selected by EFSA, based on their importance in the diet (EFSA, 2015a), to perform the assessments. Ten populations of consumers, from various MSs and various age groups were selected from the EFSA comprehensive food consumption database: Belgian adults (18–64 years), Czech Republic adults (18–64 years), German adults (18–64 years), Italian adults (18–65 years), Bulgarian children (3–5 years), Dutch children (3–6 years), French children (3–9 years), Danish toddlers (1–3 years), Dutch toddlers (2 years) and United Kingdom toddlers (1–2 years).
It should be noted that:
Non‐dietary routes of exposure are not included in the assessments.
Only pesticide residues are considered in the assessments.
Due the lack of consistent data on effects on hormone levels across substances in current regulatory dossiers, these have not been considered specifically in the establishment of CAGs and in the present assessments. This implies that these assessments do not cover thyroid‐mediated developmental neurotoxicity.
In the absence of a thorough and quantitative understanding of the interactions between the various mechanisms/pathways leading to thyroid toxicity as well as robust substance specific mechanistic data, CAGs and CRAs based on individual mode/mechanisms of action are currently not envisaged.
Only risks resulting from the actual exposure to pesticide residues are assessed. For prospective cumulative assessments (e.g. assessments which would be conducted in the context of applications for MRLs), an approach is currently under development.
1.2. Input from Risk Managers and threshold for regulatory consideration
During the Standing Committee on Plants, Animals, Food and Feed of 11–12 June 2015 (European Commission, 2015), MSs agreed on the use of the combined margin of exposure (MOET, also known as Total Margin of Exposure) concept as the mode of calculation and expression of cumulative risks.
Furthermore, during the Standing Committee on Plants, Animals, Food and Feed of 18–19 September 2018 (European Commission, 2018), MSs agreed on an MOET of 100 at 99.9th percentile of exposure in whole populations as the threshold for regulatory consideration, as an indicative target of safety by analogy to the safety margin currently used for establishing the toxicological reference values (a factor 10 for inter‐species variability and a factor of 10 for intra‐species variability).
The uniform principles for evaluation and authorisation of plant protection products further specify that in interpreting the results of evaluations, MSs shall take into consideration possible elements of uncertainty in order to ensure that the chances of failing to detect adverse effects or of underestimating their importance are reduced to a minimum, and Article 1 of Regulation (EC) No 1107/2009 states that MSs shall not be prevented from applying the precautionary principle where there is scientific uncertainty. Estimates of cumulative risk are necessarily subject to a degree of scientific uncertainty, due to limitations in the data and to assumptions used to address those limitations. In this context, the Standing Committee on Plants, Animals, Food and Feed stated (European Commission, 2018) that the MOET of 100 at 99.9th percentile of exposure would be acceptable provided that the assumptions are sufficiently conservative. This assessment therefore includes a rigorous analysis of the assumptions and uncertainties involved, leading to a quantitative assessment of the degree of certainty that the MOET at the 99.9th percentile of exposure is above 100. This provides a measure of the degree to which the assumptions in the assessment are conservative.
2. Methodology, data and uncertainty analysis
2.1. Methodology
The CRAs conducted in this report were carried out under the assumption of dose addition as recommended by the PPR Panel (EFSA PPR Panel, 2008, 2013b) and the Scientific Committee of EFSA (EFSA Scientific Committee, 2019).
The threshold for regulatory consideration specified by the MSs is expressed in terms of the MOET. Two options are possible to calculate MOET:
Directly, by calculating the reciprocal of the sum of the reciprocals of individual MOEs to each chemical1 contributing to the risk (EFSA PPR Panel, 2008): where MOEi is the margin of exposure for the i th chemical, and RfPi is the toxicological reference point (no observed adverse effect levels (NOAEL) in the present report) for chemical i and Ei its exposure. The MOET is then obtained by taking the reciprocal of 1/MOET.
Indirectly, by determining the sum of potency‐normalised individual exposures as total Index Compound (IC) equivalents and translating the IC equivalents into the MOET to the reference point of the IC. This approach however requires additional work to select an IC and calculate a Relative Potency Factor (RPFi) for each chemical. where RfPIC and RfPi are the reference points for the IC and chemical i, where the denominator sums over all chemicals including the IC.
It should be noted that direct or indirect calculations lead exactly to the same results. This is demonstrated as follows: inverting the previous equation and substituting for RPFi cancelling out RfPIC in numerator and denominator. So: as in the direct calculation above.
An important consequence of this is that the choice of the IC has no influence at all on the result of the assessment, nor on the uncertainties affecting the MOET. This is because any change in RfPIC, e.g. through choosing a different IC or errors in the RfP of the IC, affects both the numerator and denominator of the equation and cancels out, as shown above.
To perform the cumulative exposure assessments for CAG‐TCF and CAG‐TCP, EFSA used the direct calculation method (EFSA, 2019b) and RIVM used the method based on ICs (Van Klaveren et al., 2019).
2.2. Data
The outcome of the hazard assessment (CAGs) and exposure assessment supporting the CRAs of the effects of pesticides on the thyroid are presented in the respective EFSA and RIVM reports (EFSA, 2019a,b, Van Klaveren et al., 2019). They represent the input to the uncertainty analyses and risk characterisations performed in the present report.
2.2.1. Cumulative assessment groups (CAGs)
Two CAGs were used to perform the chronic CRA of dietary exposure to pesticides for the thyroid.
The CAG for hypothyroidism (CAG‐TCF2) includes 128 active substances, metabolites or degradation products (EFSA, 2019a). For 14 of these active substances, strong evidence of the existence for a mode of action (MoA) directly related to the effect is present. For most of the other active substances, varying levels of evidence are available to support a hypothesised MoA, which is liver enzyme induction in most cases. There is a probability that some of the active substances in this CAG do not produce hypothyroidism as a primary effect, which was assessed by expert knowledge elicitation (EKE) and Monte Carlo simulations.3 The median estimate of the total number of active substances that actually produce the effect was 71 with a 90% probability interval of 65–77 assuming independence between subgroups of active substances with similar levels of evidence (EFSA, 2019a). This uncertainty on whether the CAG contains only active substances causing the effect was taken into account in the uncertainty analysis (see Section 3.1 and note 26) as all active substances were kept in the CAG to perform the cumulative exposure assessments described in the following section.
The CAG for C‐cell hypertrophy, hyperplasia and neoplasia (CAG‐TCP4) includes 17 active substances (EFSA, 2019a). A number of MoAs are known to produce this effect but were not investigated experimentally in regulatory studies for any of the active substances included in the CAG.
2.2.2. Cumulative exposure assessments
Cumulative exposure assessments with CAG‐TCF (128 active substances) and CAG‐TCP (17 active substances) have been conducted probabilistically, on whole population basis, by EFSA using SAS® software (EFSA, 2019b) and by RIVM, using the Monte Carlo Risk Assessment (MCRA) software (Van Klaveren et al., 2019). In order to derive long‐term individual intakes from the dietary surveys, which recorded consumption during 2–7 days only, both software tools used the Observed Individual Mean (OIM) model. Very similar results were obtained, despite minor methodological differences between the two software tools, identified and commented in section 3.3 of EFSA (2019b) and in section 5.3 of Van Klaveren et al. (2019).
The assessments included two tiers5 and were based on all parameters and assumptions agreed by MSs for the assessment of cumulative exposure to pesticide residues (European Commission, 2018). While the first‐tier calculations (Tier I) used very conservative assumptions, the second‐tier assessment (Tier II) included assumptions that are more refined but still intended to be conservative. They produced distributions of MOET estimates, from which the one corresponding to the 99.9th percentile of the cumulative exposure, e.g. the threshold for regulatory consideration established by the European Commission and MSs, was specially considered. At that percentile, an MOET of at least 100 is of interest as explained in Section 1.2.
The calculation model and input data, including sample size and statistics, are included in EFSA (2019b) and Van Klaveren et al. (2019). For both CAG‐TCF and CAG‐TCP, calculations showed median MOETs equal or above 100 in Tier II in all populations at the 99.9th percentile of the exposure. MOETs for CAG‐TCP were about 1 order of magnitude larger than MOETs for CAG‐TCF. Confidence intervals (95%) were generated by outer loop execution6 addressing the sampling variability of the consumption and occurrence data as well as some uncertainties associated with probabilities applied in the model.
The MOET estimates at various percentiles of the exposure distribution, and their respective confidence intervals are reported in Tables 1A and 2A.
Table 1A.
CAG‐TCF: Estimates of the MOET and their corresponding 95% confidence intervals at the 50th, 99th and 99.9th percentiles7 of the exposure at Tier II for 10 European populations in 2014–2016
| Country | Population class (no. of individuals) | 50th percentile SAS® a | 99th percentile SAS® a | 99.9th percentile SAS® a | 99.9th percentile MCRAb |
|---|---|---|---|---|---|
| Belgium (BE) | Adults (1,356) | 984 [915–1,048] | 401 [317–481] | 307 [198–387] | 316 [210–391] |
| Czech Republic (CZ) | Adults (1,666) | 1,040 [980–1,110] | 377 [302–446] | 269 [186–366] | 280 [200–349] |
| Germany (DE) | Adults (10,419) | 1,020 [960–1,090] | 362 [298–421] | 259 [205–313] | 266 [228–302] |
| Italy (IT) | Adults (2,313) | 776 [731–844] | 362 [311–411] | 295 [252–330] | 302 [274–335] |
| Bulgaria (BG) | Other children (434) | 328 [307–356] | 155 [131–166] | 127 [114–151] | 130 [118–154] |
| France (FR) | Other children (482) | 523 [492–556] | 229 [203–270] | 201 [187–216] | 200 [193–227] |
| Netherlands (NL) | Other children (957) | 466 [436–501] | 210 [183–234] | 176 [159–197] | 177 [162–196] |
| Denmark (DK) | Toddlers (917) | 328 [313–346] | 183 [158–197] | 127 [102–175] | 128 [110–172] |
| Netherlands (NL) | Toddlers (322) | 360 [335–391] | 160 [111–184] | 103 [86.3–165] | 102 [89–160] |
| United Kingdom (UK) | Toddlers (1,314) | 421 [394–448] | 192 [172–209] | 124 [104–176] | 149 [108–177] |
Results obtained by EFSA with SAS® software.
Results obtained by RIVM with MCRA.
Table 2A.
CAG‐TCP: Estimates of the MOET and their corresponding 95% confidence intervals at the 50th, 95th, 99th and 99.9th percentiles7 of the exposure at Tier II for 10 European populations in 2014–2016
| Country | Population class (no. of individuals) | 50th percentile SAS® a | 99th percentile SAS® a | 99.9th percentile SAS® a | 99.9th percentile MCRAb |
|---|---|---|---|---|---|
| Belgium (BE) | Adults (1,356) | 23,900 [19,500–30,500] | 4,570 [2,120–7,720] | 3,030 [1,150–5,040] | 2,849 [1,389–4,592] |
| Czech Republic (CZ) | Adults (1,666) | 37,600 [31,700–45,500] | 5,010 [2,260–7,990] | 2,620 [1,130–5,600] | 2,532 [1,401–4,017] |
| Germany (DE) | Adults (10,419) | 16,100 [13,000–20,200] | 3,320 [1,900–4,490] | 2,290 [1,210–3,250] | 2,241 [1,496–2,868] |
| Italy (IT) | Adults (2,313) | 16,900 [12,300–22,700] | 4,760 [2,440–6,970] | 3,400 [1,780–5,030] | 3,401 [2,144–4,731] |
| Bulgaria (BG) | Other children (434) | 13,400 [11,600–15,800] | 2,590 [2,170–3,100] | 2,250 [1,840–2,760] | 2,307 [1,860–2,627] |
| France (FR) | Other children (482) | 143,00 [12,200–17,100] | 4,370 [3,470–5,330] | 3,870 [3,100–4,460] | 3,978 [3,337–4,430] |
| Netherlands (NL) | Other children (322) | 9,020 [7,480–11,160] | 2,350 [1,760–3,040] | 1,760 [1,340–2,300] | 1,778 [1,491–2,187] |
| Denmark (DK) | Toddlers (917) | 8,360 [7,240–9,850] | 2,660 [2,220–3,110] | 2,080 [1,210–2,460] | 2,072 [1,516–2,538] |
| Netherlands (NL) | Toddlers (322) | 6,600 [5,450–8,050] | 1,740 [1,280–2,390] | 1,480 [990–1,900] | 1,468 [1,148–1,783] |
| United Kingdom (UK) | Toddlers (1,314) | 11,500 [9,600–13,300] | 3,060 [2,510–3,480] | 2,360 [1,810–2,940] | 2,488 [2,077–2,913] |
Results obtained by EFSA with SAS® software.
Results obtained by RIVM with MCRA.
2.2.2.1. Cumulative exposure assessment for CAG‐TCF
The results of the cumulative exposure assessment at Tier II to pesticides associated with hypothyroidism are presented in Table 1A. The median estimate of the MOET at the 99.9th percentile of exposure is equal or greater than 100 in all cases. In two cases, however, the confidence interval includes values below 100.
Pesticide/commodity combinations contributing significantly to the cumulative risk were identified. Risk drivers were defined as pesticide/commodity combinations, which, under the precise modelling conditions and assumptions at Tier II, contribute on average, in at least one out of the 10 populations, at least 5% of the cumulative exposures exceeding the 99th percentile estimate. To identify these combinations, the cumulative exposure assessments conducted by both EFSA and RIVM were considered (EFSA, 2019b; Van Klaveren et al., 2019).
The risk drivers identified in the 10 populations are reported in Table 1B and ordered according to their contribution level to the cumulative exposure.
Table 1B.
CAG‐TCF: Risk drivers identified in the 10 populations of consumers
| Risk drivers | Contribution to the cumulative exposure per population | ||
|---|---|---|---|
| From 5% to 10% | From 10% to 20% | Exceeding 20% | |
| Bromide ion/wheat |
Adults: BE, CZ, DE Other children: BG, NL Toddlers: DK, NL |
Adults: IT Other children: FR Toddlers: UK |
|
| Bromide ion/oats | Toddlers: UK | ||
| Bromide ion/tomatoes |
Adults: DE, IT Other children: FR Toddlers: UK |
||
| Bromide ion/rye | Toddlers: DK | ||
| Bromide ion/rice | Toddlers: NL, UK | ||
| Thiabendazole/oranges | Other children: NL |
Other children: BG, FR Toddlers: DK, NL |
|
| Propineb/wine grapes | Adults: DE, IT | Adults: BE, CZ | |
| Propineb/apples | Other children: NL | ||
| Ziram/wine grapes | Adults: DE, IT | Adults: BE, CZ | |
| Ziram/apples |
Other children: NL Toddlers: DK |
||
| Mancozeb/oranges |
Other children: BG Toddlers: NL |
||
| Chlorpropham/potatoes |
Other children: BG Toddlers: UK |
||
| Cyprodinil/wine grapes | Adults: BE, CZ | ||
| Pyrimethanil/oranges |
Other children: BG, FR Toddlers: NL |
||
This table shows that the most important risk drivers involve bromide ion, as its residues in five commodities contribute 5 to more than 20% of the cumulative exposure in one or several populations. Thiabendazole, propineb and ziram are the next prominent risk drivers, with residues in certain commodities causing at least 10% of the cumulative exposure.
This table also indicates differences between adult and children populations: certain commodities, when combined with some active substances, emerge as risk drivers in toddlers and other children populations, but not in adult populations. This is the case of apples, oranges, oats, rice and potatoes. The contrary is observed with wine grapes.
2.2.2.2. Cumulative exposure assessment for CAG‐TCP
The results of the cumulative exposure assessment at Tier II to pesticides associated with C‐cell hypertrophy, hyperplasia and neoplasia are presented in Table 2A. The MOET at the 99.9th percentile of exposure is largely greater than 100 in all cases.
Risk drivers were identified as described in previous section, and are reported in Table 2B, ordered according to their contribution level to the cumulative exposure:
Table 2B.
CAG‐TCP: Risk drivers identified in the 10 populations of consumers
| Risk drivers | Contribution to the cumulative exposure per population | ||
|---|---|---|---|
| From 5% to 10% | From 10% to 20% | Exceeding 20% | |
| Thiram/apples | Adults: IT |
Adults: BE Other children: BG |
Adults: DE Other children: FR, NL Toddlers: DK, NL, UK |
| Thiram/wine grapes | Adults: BE, CZ, DE, IT | ||
| Thiram/strawberries | Toddlers: NL, UK |
Other children: BG, FR Toddlers: DK |
|
| Thiram/peaches |
Adults: CZ Toddlers: DK |
Adults: IT Other children: FR, NL Toddlers: NL |
Other children: B |
| Thiram/pears |
Adults: IT Other children: FR, NL Toddlers: DK |
Toddlers: UK | |
| Thiram/table grapes |
Other children: FR Toddlers: DK, NL |
Toddlers: UK | |
| Thiram/lettuce |
Adults: CZ Other children: BG |
Adults: DE, IT Other children: NL |
|
| Ziram/apples |
Adults: DE Other children: FR Toddlers: DK, UK |
Other children: NL Toddlers: NL |
|
| Ziram/wine grapes | Adults: DE | Adults: BE, CZ, IT | |
All risk drivers identified for CAG‐TCP, involved thiram and ziram, the main contributor being thiram.
2.2.2.3. Sensitivity analyses
In addition, for both CAG‐TCF and CAG‐TCP, sensitivity analyses8 were conducted to test the impact of assumptions applied to the left‐censored data9 and missing information on the effect of processing. For a complete understanding of the modalities of these sensitivity analyses, EFSA (2019b) or Van Klaveren et al. (2019) should be consulted.
In the case of CAG‐TCF, when left‐censored data were imputed with 1/2 of the limit of quantification (LOQ) assuming 100% of use in case of authorisation, the MOETs at the 99.9th percentile of the exposure distribution dropped by three to four times. When all left‐censored data were assumed to be at 0, the MOETs increased by a factor of 1.1–1.8. The impact of missing processing factors was important. Assuming that no residue would be transferred to any processed food when a processing factor is missing, the MOETs increased by a factor of 2.3–5.7.
In the case of CAG‐TCP, when left‐censored data were imputed with 1/2 LOQ assuming 100% of use in case of authorisation, the MOETs dropped by four to eight times. When all left‐censored data were assumed to be at 0, the MOETs increased by a factor of 1.1–1.4. Regarding missing processing factors, and assuming that no residue would be transferred to any processed food when a processing factor is missing, the MOETs increased by a factor of 1.3–2.6.
It must be noted that sensitivity analyses related to missing processing factors do not consider the effect of washing and/or peeling of commodities with edible peel consumed without further processing.
2.3. Uncertainty analysis
There are several limitations in the available knowledge and data that affect the capacity of risk assessors to provide a precise answer to the assessment questions mentioned in Section 1.
Therefore, an uncertainty analysis was conducted for each assessment question in order to provide an answer to the following:
If all the uncertainties in the model, 10 exposure assessment, hazard identification and characterisation and their dependencies could be quantified and included in the calculation, what would be the probability that the MOET for the 99,9th percentile of exposure in 2014–2016 is below 100?
This question was considered separately for each of the 10 consumer populations addressed in the probabilistic modelling (EFSA 2019b, Van Klaveren et al., 2019).
This uncertainty analysis was conducted following the guidance of the EFSA Scientific Committee on uncertainty analysis in scientific assessments for case‐specific assessments (EFSA Scientific Committee, 2018a). Specifically, it followed an approach based on the guidance and flow charts for case‐specific assessments, in the sequence summarised below:
When planning the assessment and uncertainty analysis, it was decided to quantify some sources of uncertainty within the probabilistic models for cumulative exposure, rather than assess all sources of uncertainty collectively outside the models. This choice is part of the first phase of uncertainty analysis, depicted in Figure 5 of the Guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a). Specifically, it was decided to quantify sampling variability of occurrence and consumption data in the probabilistic models, since methods for this are included in the guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues (EFSA PPR Panel, 2012).
Since the cumulative assessment involves quantities that are variable as well as uncertain, the probabilistic modelling of uncertainty involved the choices summarised in Figure 10 of EFSA Scientific Committee (2018a). It was decided to model variability and uncertainty using distributions, rather than probability bounds, because methods for this were already established (EFSA PPR Panel, 2012) and implemented in existing software (Van Klaveren et al., 2019). Specifically, variability and sampling variability for some of the model inputs (consumption and occurrence) was quantified by bootstrapping11 as described by EFSA (2019b) and RIVM (Van Klaveren et al., 2019).
The final phase is characterisation of overall uncertainty, summarised in Figures 15–17 of EFSA Scientific Committee (2018a). First, following Figure 15 (ibid.), it was decided to make separate probability judgements for the additional uncertainties and then combine them by calculation with those quantified in the probabilistic models. This process is summarised briefly in Figure 17 of EFSA Scientific Committee (2018a) and requires defining and implementing an appropriate model to combine the probability distribution from the probabilistic modelling with another one for the collective contribution of the additional uncertainties. The model developed for this process and the methods used to implement it are described in the following sections, after first describing the approach taken to identify sources of uncertainty.
Figure 5.
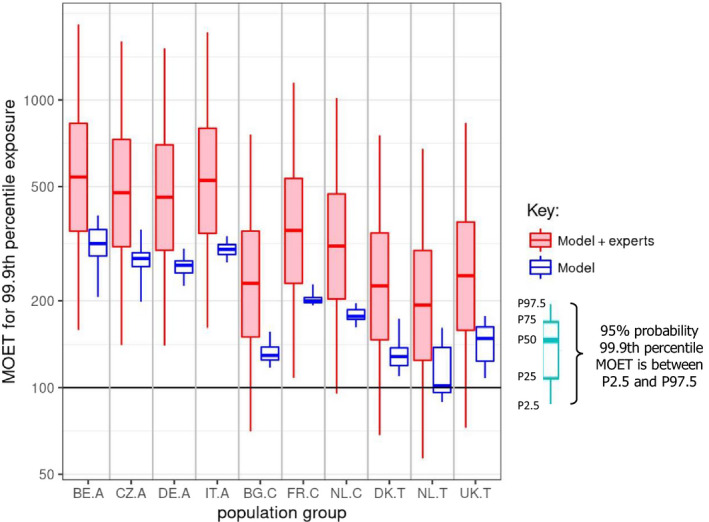
CAG‐TCF: ‘Model’ boxplots show the unadjusted output of the MCRA Tier II model for the MOET at the 99.9th percentile of exposure in each consumer population in 2014–2016. ‘Model+experts’ boxplots show the result of combining the output of the Tier II model with the elicited distributions quantifying additional sources of uncertainty. Note that the vertical axis is plotted on a logarithmic scale; the values plotted for ‘model + experts’ are shown numerically in Table 7. A key to the populations and explanation of the boxplots are provided in the footnote below the graph.
- Key: Population groups: BE.A (Belgian adults), CZ.A (Czech Republic adults), DE.A (German adults), IT.A (Italian adults), BG.C (Bulgarian children), FR.C (French children), NL.C (Dutch children), DK.T (Danish toddlers), NL.T (Dutch toddlers), UK.T (United Kingdom toddlers). The lower and upper edges of each boxplot represent the quartiles (P25 and P75) of the uncertainty distribution for each estimate, the horizontal line in the middle of the box represents the median (P50) and the ‘whiskers’ above and below the box show the 95% probability interval (P2.5 and P97.5).
2.3.1. Identification of sources of uncertainty affecting the assessment
Sources of uncertainty affecting the assessment were identified as recommended by EFSA Scientific Committee (2018a).
The sources of uncertainty were first identified by expert discussion using a systematic approach, reviewing each part of the assessment for potential sources of uncertainty. Specifically, the Working Group experts examined each input data (e.g. occurrence date, processing factors…) and each part of the assessment model (e.g. Dose‐Addition model, Observed Individual Means (OIM) model for the calculation of long‐term exposure…) and considered whether it was affected by any of the types of uncertainty listed in Table 1 the EFSA Scientific Committee (2018a) (ambiguity, accuracy, sampling uncertainty, missing data, missing studies, assumptions, excluded factors, use of fixed values…).
Afterwards, the identified uncertainties were further discussed and precisely defined/described in such a way that they were unambiguously understood by the experts participating to the uncertainty analysis and not overlapping with each other. For instance, three distinct sources of uncertainty were identified regarding the handling of left‐censored occurrence data, corresponding to three associated assumptions: assumption of the authorisation status for pesticide/commodity combinations, assumption of the use frequency for authorised pesticide/commodity combinations and assumption of the residue level (1/2 LOQ) to be imputed to the commodity when a use is assumed.
The sources of uncertainty included those related to the identification of substances to be included in each CAG, which had already been described and assessed in the EFSA Scientific report on the establishment of CAGs of pesticides for their effects on the thyroid (EFSA, 2019a).
All the identified sources of uncertainty were listed in tables, which are presented in Section 3.1 and Appendix A. The Working Group experts then collected and discussed further information that would be helpful to evaluate their impact on the assessment. The results of these discussions and investigations were then summarised in a series of notes, which are also included in Appendix B and cross‐referenced to the list of uncertainties.
The identified sources of uncertainty were subsequently divided into two groups: those relating to exposure and those relating to toxicology (hazard identification and characterisation). In subsequent steps of the uncertainty analysis (EKE Questions 1 and 2, see next Section 2.3.2), the uncertainties relating to exposure were evaluated by the exposure experts in the Working Group and the uncertainties relating to toxicology were evaluated by the toxicology experts.
2.3.2. Model and process for characterising overall uncertainty
The approach developed for characterising overall uncertainty in this assessment is summarised graphically in Figure 1. The whole approach is based upon taking the output of the probabilistic modelling – specifically the uncertainty distribution produced by the modelling for the MOET at the 99.9th percentile of exposure, represented diagrammatically at the top left of Figure 1 – as the starting point for the rest of the uncertainty analysis. The rest of the analysis comprised five key steps, listed on the right hand side of Figure 1, as follows:
Figure 1.
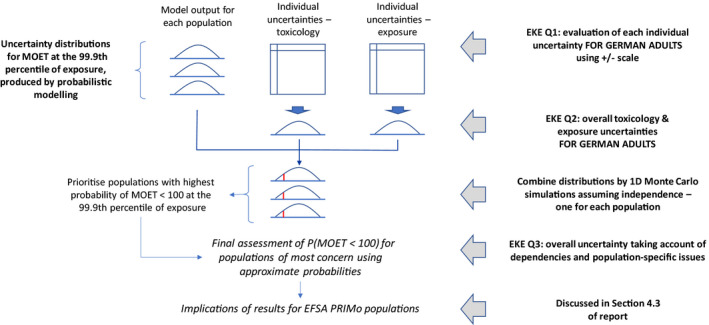
Overview of the approach to characterising overall uncertainty in the CRA, which was conducted separately for each CAG
EKE Question 1 (EKE Q1): This was the first of three steps where the impact of uncertainties on the assessment was quantified by expert judgement. EKE Q1 required the experts to consider each source of uncertainty separately, and quantify its impact on the assessment in terms of how much the median estimate of the MOET at the 99.9th percentile of exposure calculated by the probabilistic model for the German adult population would change if that source of uncertainty was resolved (e.g. by obtaining perfect information on the input or assumption affected by the uncertainty). Focussing on a single population avoided repeating the assessment for each population, which would take 10 times as long and be more vulnerable to biases in judgement due to progressive expert fatigue; German adults were chosen because the consumption survey used in the modelling for this population is larger than that for other populations and therefore the model estimates are less affected by sampling variability. The experts expressed their judgements as multiplicative factors, e.g. a factor of 1 would represent no change in the MOET at the 99.9th percentile of exposure, factors greater than 1 represent an increase, factors less than 1 represent a decrease. The scale and methods used for this step are described in Section 2.3.5 and the results are reported in the tables in Appendix A.
EKE Question 2 (EKE Q2): For this question, the experts were asked to consider all the sources of uncertainty relating to exposure or toxicology (according to their expertise), and quantify their combined impact on the assessment in terms of how much the median estimate of the MOET at the 99.9th percentile of exposure calculated by the probabilistic model for the German adult population would change if all those sources of uncertainty were resolved. This focussed on German adults for the same reason as EKE Q1 (see above) and the degree of change was again expressed as a multiplicative factor. When answering EKE Q2, the experts took account of their evaluations of the individual uncertainties, as assessed in EKE Q1, and combined them by expert judgement. The experts’ uncertainty about the combined impact was elicited in the form of a distribution for the multiplicative factor. The methods used for this step are described in Section 2.3.5 and the results are reported in Section 3.3.
Combine distributions using Monte Carlo simulations: In this step, the distributions for the multiplicative factors quantifying the exposure and toxicology uncertainties, elicited in EKE Q2, were combined by multiplication with the uncertainty distribution for the MOET at the 99.9th percentile of exposure produced by the probabilistic model. Since each of the distributions from EKE Q2 is for a multiplicative adjustment to the MOET at the 99.9th percentile of exposure, multiplying the three distributions together results in a new distribution for the MOET at the 99.9th percentile of exposure which incorporates the experts’ assessment of the impact of the exposure and toxicology uncertainties. This was repeated for each of the 10 modelled populations (see Section 2.3.6).
EKE Question 3 (EKE Q3): For reasons of practicality, the preceding steps involved two important simplifications. In EKE Q2, the uncertainties were assessed with reference to only one of the 10 modelled populations (German adults). Then, in the Monte Carlo simulations, the distributions elicited for German adults were applied to all 10 populations, and it was that assumed that the model distributions and the distributions for exposure and toxicology uncertainties are independent of one another. These simplifications introduce additional uncertainties into the assessment. Therefore, EKE Q3 asked the experts to consider the calculated probability of the MOET at the 99.9th percentile of exposure being less than 100 (derived from the distribution produced by the Monte Carlo simulation for each population) and judge how that probability would change if it was adjusted for any dependencies between the exposure and toxicology uncertainties, for differences in uncertainty between German adults and each of the other populations, and also for any other remaining uncertainties (including differences between the MCRA and EFSA probabilistic models, see following section). In recognition of the difficulty of this judgement, the experts’ response to this question was elicited as an approximate probability (range of probabilities) for each population.
Extrapolation to EFSA PRIMo populations: The recommendation of European Commission and MSs that the MOET at the 99.9th percentile of exposure should be assessed for each of the consumer populations included in the EFSA PRIMo model is addressed in Section 4.3, where the implications of the results for the PRIMo populations are discussed.
Note that different sources of uncertainty were combined by expert judgement in EKE Q2, whereas the two distributions resulting from that (one for exposure and the other for toxicology) were combined by Monte Carlo simulation. This combination of methods for combining uncertainties was considered more practical than combining all the individual uncertainties by Monte Carlo simulation, which would have required eliciting distributions for each of them in EKE Q1 and specification of a suitable model to combine them. It was also considered more rigorous and reliable than combining all the uncertainties in a single expert judgement, since that would have required simultaneous consideration of both the exposure and toxicology uncertainties while each expert was specialised in either exposure or toxicology.
The experts addressing the EKE Questions were the following:
Toxicology uncertainties: Antonio Hernandez‐Jerez, Susanne Hougaard Bennekou, Carsten Kneuer (EKE Q2 and EKE Q3 only) and Gerrit Wolterink (EKE Q2 and EKE Q3 only);
Exposure uncertainties: Bruno Dujardin, Luc Mohimont and Bernadette Ossendorp.
All experts involved in the exercise had thorough knowledge of the CRA methodologies and of uncertainty analysis. Experts in exposure were furthermore very familiar with probabilistic methodology and all were trained to the practice of the EKE technique.
2.3.3. Choice of probabilistic model output for use in the uncertainty analysis
It was decided that EKE Q1 and Q2 would elicit judgements expressed as adjustments to the results from one of the two implementations of the probabilistic model (MCRA or SAS®). The two implementations used the same inputs and were designed to implement the same model, but in practice there were minor differences between their results. These differences are reported and discussed in detail in EFSA (2019b) and Van Klaveren et al. (2019). Part of the difference was attributed to not being able to implement the handling of unspecific residue definitions as intended in the EFSA model (see section 3.3 of EFSA, 2019b). For EKE Q1 and for the individual judgements on EKE Q2 (see below), the elicitation was based on the results of the EFSA model, since at that time the sample data needed for combining with the elicited distributions was not available from MCRA. However, when those data became available, it was decided to use the MCRA results for the subsequent steps of the process, starting with the consensus judgements on EKE Q2 (see Section 2.3.5). The MCRA model was preferred at that stage because it implemented the unspecific residue definitions as intended. To take account of the uncertainty implied by the differences between the two models, the experts were asked to consider this when making their judgements on EKE Q3 (Section 3.1).
2.3.4. Evaluation of individual uncertainties (EKE Question 1)
EKE Question 1 comprised two subquestions, both of which were addressed for each of the sources identified by the Working Group. The subquestions were specified as follows:
EKE Q1A: If this source of uncertainty was fully resolved (e.g. by obtaining perfect information on the issue involved) and addressed in the modelling, by what multiplicative factor would this change the median estimate of the MOET for [CAG name] at the 99.9th percentile of exposure in the German adult population at Tier II?
EKE Q1B: Is the impact of this source of uncertainty the same for the other populations that were assessed? If not, list those populations for which the impact would be smaller, and those for which it would be larger.
The role of these questions in the uncertainty analysis (depicted in Figure 1) and the detailed wording of the questions was explained to and discussed with the experts to ensure a common understanding. Examples were provided to illustrate the meaning of a source of uncertainty being ‘fully resolved’: e.g. if the cause of a source of uncertainty is that there are very few data available for one of the model inputs, or that the data are biased or unreliable, then EKE Q1A asks the experts to consider how the estimated MOET would change if the current data were replaced with a very large sample of perfectly reliable data, such that this source of uncertainty was removed. It was also explained that when assessing the impact of an uncertainty, the experts needed to consider the extent to which the active substances affected by it are ‘risk drivers’ (i.e. the magnitude of their contribution to the estimated MOET).
The meaning of ‘multiplicative factor’ was carefully explained to the experts, and they were asked to assess the factor using the scale shown in Figure 2. They were asked to express their uncertainty by giving a range of factors that they judged has at least a 90% probability of containing the true factor (i.e. the change in estimated MOET that would actually occur if the uncertainty was really resolved). For example: ‘− − −/•’ means at least a 90% chance the true factor is between x1/10 and +20%; ‘++/++’ means ≥ 90% chance between 2x and 5x etc.
Figure 2.
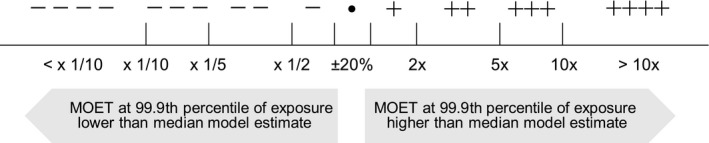
Scale used by the experts when assessing EKE Question 1
It was explained that some sources of uncertainty were already quantified to some extent in the probabilistic modelling: specifically, sampling variability for occurrence and consumption data was quantified by bootstrapping. It was decided not to further consider them under EKE Q1, and it was agreed to consider that the magnitude of the 95% confidence interval obtained by bootstrapping was sufficiently representative of the sampling variability of occurrence and consumption data (see also note 7 in Appendix B.2).
When making their assessments, the experts were provided with the agreed description/definition of each of the uncertainties (Appendix A), the detailed notes summarising the information collected to support the assessment (Appendix B.2) and the list of risk drivers for the CAG under assessment.
Five experts participated in answering EKE Q1: three exposure experts and two toxicology experts. The questions were first addressed separately by each expert, working individually. Each expert was asked to answer both questions for each of the uncertainties that related to their area of expertise (exposure or toxicology), for each of the two CAGs addressed in this report. The answers provided by the experts were then collated and, where there were differences between the assessment of the same uncertainty by different experts, these were resolved remotely to arrive at a consensus judgement. The final judgements for EKE Q1A and Q1B for each source of uncertainty are reported in Appendix A.
2.3.5. Evaluation of combined uncertainties relating to exposure and toxicology (EKE Question 2)
The EKE Q2 was specified as follows: If all the identified sources of uncertainty relating to [exposure/hazard identification and characterisation] were fully resolved (e.g. by obtaining perfect information on the issues involved) and addressed in the modelling, by what multiplicative factor would this change the median estimate for the MOET at the 99.9th percentile of exposure for [name of CAG] in the German adult population at Tier II?
This question was addressed twice for each CAG: once for the uncertainties relating to exposure and once for those relating to toxicology. As for EKE Q1, the experts’ assessment of the impact of the uncertainties was elicited as a multiplicative factor relative to median estimate of the MOET at the 99.9th percentile of exposure for German adults.
The elicitation was conducted in two stages. In the first stage, six experts (the same experts as for EKE Q1 plus one additional toxicology expert) worked separately to make individual judgements. In the second stage, the same experts, plus a further toxicologist, worked together to develop consensus judgements. The meaning of ‘perfect information’ in the EKE question was discussed and defined during the second stage (see below). For the reasons explained in Section 2.3.3, the individual judgements were made relative to the MOET estimates from the EFSA model, but the consensus judgements were made relative to MCRA estimates.
The experts’ uncertainty about the multiplicative factor required by the question was elicited in the form of a probability distribution using the ‘Sheffield’ protocol12 described in EFSA's guidance document on expert knowledge elicitation (EFSA, 2014a). Application of this to EKE Q2 was guided and facilitated by a member of the Working Group who has extensive experience with the Sheffield protocol. The facilitator also provided training to the experts in each step of the process, including how to make probability judgements and interpret fitted distributions, before they applied it to the thyroid assessment.
The individual judgements were elicited using the quartile method (EFSA, 2014a): experts were asked first for their lower and upper plausible bounds for the multiplicative factor, then for their median estimate and finally for their lower and upper quartile estimates. The individual judgements were elicited in this order to mitigate psychological biases known to affect expert judgement, especially anchoring and adjustment, and overconfidence (EFSA, 2014a). Since the individual judgements were made remotely by experts working on their own, they were asked to enter them in the MATCH software,13 view the best fitting distribution and feedback statistics (33rd and 66th percentiles) provided by MATCH, and adjust their judgements until they were satisfied that the final distribution appropriately represented their judgement.
The experts were asked to take account of the following evidence when making their judgements, together with any other relevant information they were aware of: the evaluations of the individual uncertainties from EKE Q1 (Appendix A) and detailed supporting notes on them (Appendix B.2); the lists of risk drivers and supporting information on them (Appendix B.1); the draft reports of RIVM and EFSA modelling and sensitivity analyses for CAG‐TCF and CAG‐TCP (Van Klaveren et al., 2019; EFSA, 2019b); detailed graphics and tables on the model outputs and contributions of risk drivers; tabulated data for the simulated individuals in the 99–100th percentile of total normalised exposure, showing the extent to which they were comprised of one or multiple substances and commodities; and the draft of the EFSA Scientific report on the establishment of CAGs of pesticides for their effects on the thyroid (EFSA, 2019a).
The experts were provided with a template document in which to record their judgements and reasoning. These were then used by the facilitator to produce graphs14 in which the best‐fitting distributions obtained from the judgements of different experts for the same question were plotted together.
While the experts were making their individual judgements, the results of the EFSA probabilistic model were subjected to further analysis. This was designed to provide additional information on two sources of uncertainty identified in EKE Q1: the effect of limitations in sample size (for the consumption and occurrence data) on the performance of the bootstrap method for quantifying uncertainty of the MOET at the 99.9th percentile of exposure (see note 7 in Appendix B.2), and the potential transformation of dithiocarbamates into ethylene thiourea (ETU) and propylene thiourea (PTU) (see note 11 in Appendix B.2).
The consensus judgements were elicited during a face‐to‐face meeting attended by all seven experts, following the guidance for facilitation of consensus judgements in the Sheffield protocol provided by EFSA (2014a,b) and in the SHELF framework.15 EFSA (2014a,b) recommends no more than six to eight experts for the Sheffield protocol. The first half day of the meeting was used to provide the experts with additional information relevant to their judgements: a presentation reviewing recent research (including the Euromix project, https://www.euromixproject.eu/) on the applicability of the dose addition model, a detailed presentation of the final results of the EFSA model and comparison with the MCRA results, and the analysis of the performance of the bootstrap method. The facilitator then explained the form of consensus judgement required by the Sheffield method: not an average or compromise between the individual judgements, but the experts’ collective assessment of what a rational impartial observer would judge (“RIO” concept), having seen the evidence, the list of uncertainties and the individual judgements and having heard the experts’ discussion (EFSA, 2014a; Oakley and O'Hagan, 2016). The experts reviewed the wording of EKE Q2 and for both CAG‐TCF and CAG‐TCP agreed to define ‘perfect information’ for exposure assessment as ‘actual consumption, occurrence, processing methods, processing factors etc.’ and for toxicology as ‘the lowest BMDL1016 , 17 from a perfect set of toxicity studies and perfect knowledge of CAG membership, the toxicity‐exposure relationship and how substances combine’.
The consensus judgements were developed by facilitated discussion between the experts. First, the experts discussed the distributions fitted to their individual judgements and the evidence and reasoning that their judgements were based on. Next, the experts worked towards agreement on shared judgements, which they considered to be a consensus in the sense defined by the RIO concept (see above). The experts were first asked for their consensus judgement for the plausible range for the multiplicative factor. Then, three further consensus judgements were elicited using the probability method, to reduce the tendency of experts to anchor on their individual judgements for medians and quartiles (Oakley and O'Hagan, 2016). In the probability method (described in EFSA (2014a,b) as the fixed interval method), the experts are asked to judge the probability that the quantity of interest lies above (or below) some specified value. For this purpose, the facilitator chose three values in different parts of the plausible range, favouring regions where differences between the individual distributions were most marked. The experts’ consensus judgements for these three values, together with their consensus for the plausible range, were entered into the SHELF Shiny app for eliciting a single distribution18 and the best‐fitting distribution provided by the app was displayed for review by the experts.
A series of checks were then made and discussed with the experts: first, how closely the resulting distribution fitted the consensus judgements, then the values of the median, tertiles and 95% probability interval for that distribution. If any of these, or the visual shape of the distribution, were not judged by the experts as appropriate to represent their consensus, then alternative distributions fitted by the app were considered or, if necessary, the experts made adjustments to one or more of their judgements, until they were satisfied with the final distribution.
2.3.6. 1‐D Monte Carlo simulation to combine distributions quantifying uncertainties related to exposure and toxicology
In this step, the two distributions elicited to quantify uncertainties relating to exposure and toxicology, respectively, were combined by Monte Carlo simulation with an uncertainty distribution for the MOET at the 99.9th percentile of exposure generated by MCRA model. The latter distribution comprised, for each modelled population, the 100 estimates of the MOET at the 99.9th percentile of exposure generated in the 100 outer loops of the MCRA Tier II model. A computer programme to carry out this calculation was prepared in advance using the R software, assuming independence between the three distributions, and this programme was then run for each combination of the two CAGs and 10 consumer populations as the consensus EKE Q2 distributions became available. This was done during the 3‐day meeting referred to in the preceding section, so that the results could be used as the starting point for EKE Q3 which was addressed in the same meeting.
Specifically, for each CAG, the same process was followed:
Draw a sample of 105 values from the experts’ exposure‐factor distribution
Draw a sample of 105 values from the experts’ toxicity‐factor distribution
Multiply corresponding pairs of exposure‐factor and toxicity‐factor values to produce a sample of 105 values for the combined toxicity and exposure factor.
-
For each consumer group:
-
○
Multiply each of the 100 values for the estimates of the MOET at 99.9th percentile of exposure generated by the MCRA model by each of the 105 values from the previous bullet. This results in 107 values for the MOET at 99.9th percentile of exposure, adjusted for combined uncertainties (MOET adjusted for uncertainties).
-
○
From these 107 values, the MOETs at 2.5th, 25th, 50th, 75th and 97.5th percentiles of the exposure as well as the probability of the MOET at the 99.9th percentile of exposure being less than 100 were calculated for graphical presentation and tabulation (Figures 5 and 8, Tables 7 and 10).
-
○
Figure 8.
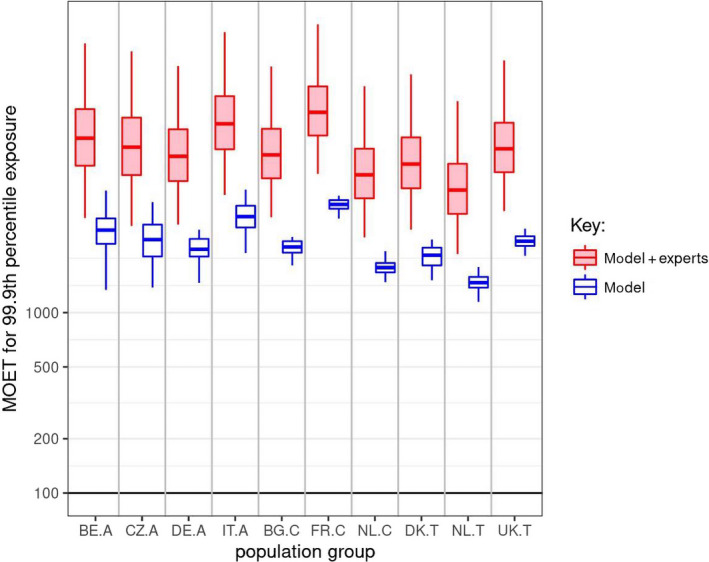
CAG‐TCP: ‘Model’ boxplots show the unadjusted output of the MCRA Tier II model for the MOET at the 99.9th percentile of exposure in each consumer population in 2014–2016. ‘Model + experts’ boxplots show the result of combining the output of the Tier II model with the elicited distributions quantifying additional sources of uncertainty. Note that the vertical axis is plotted on a logarithmic scale; the values plotted for ‘model + experts’ are shown numerically in Table 10. A key to the populations and explanation of the boxplots are provided in the footnote below Figure 5
Table 7.
CAG‐TCF: Statistics for the MOET at the 99.9th percentile of exposure in each consumer population in 2014–2016, calculated by combining the elicited distributions for uncertainties related to exposure and toxicology with the output of the MCRA Tier II model. P2.5, P25 etc. refer to the percentiles plotted in the ‘model+experts’ boxplots in Figure 5
| Population group | MOET at the 99.9th percentile of exposure distribution combining model and elicited uncertainties (from ‘model+expert’ boxplots in Figure 5) | |||||
|---|---|---|---|---|---|---|
| P2.5 | P25 | P50 | P75 | P97.5 | Probability of 99.9%ile MOET < 100 (%) | |
| Belgian adults | 159 | 349 | 540 | 830 | 1,833 | 0.3 |
| Czech Rep. adults | 141 | 308 | 475 | 729 | 1,601 | 0.5 |
| German adults | 140 | 300 | 459 | 698 | 1,513 | 0.5 |
| Italian adults | 161 | 343 | 525 | 797 | 1,722 | 0.2 |
| Bulgarian children | 70 | 150 | 230 | 350 | 757 | 8.9 |
| French children | 108 | 230 | 351 | 533 | 1,149 | 1.8 |
| Dutch children | 95 | 203 | 310 | 471 | 1,016 | 3.1 |
| Danish toddlers | 68 | 147 | 225 | 344 | 753 | 9.6 |
| Dutch toddlers | 57 | 125 | 193 | 299 | 676 | 15.4 |
| United Kingdom toddlers | 73 | 159 | 245 | 376 | 832 | 7.8 |
Table 10.
CAG‐TCP: Statistics for the MOET at the 99.9th percentile of exposure in each consumer population in 2014–2016, calculated by combining the elicited distributions for uncertainties related to exposure and toxicology with the output of the MCRA Tier II model. P2.5, P25 etc. refer to the percentiles plotted in the ‘model + experts’ boxplots in Figure 8
| Population group | MOET at the 99.9th percentile of exposure distribution combining model and elicited uncertainties (from ‘model + expert’ boxplots in Figure 8) | |||||
|---|---|---|---|---|---|---|
| P2.5 | P25 | P50 | P75 | P97.5 | Probability of 99.9% ile MOET < 100 (%) | |
| Belgian adults | 3,344 | 6,523 | 9,269 | 13,434 | 31,084 | 0.00 |
| Czech Rep. adults | 3,023 | 5,787 | 8,266 | 12,046 | 28,097 | 0.00 |
| German adults | 3,076 | 5,361 | 7,352 | 10,383 | 23,285 | 0.00 |
| Italian adults | 4,489 | 8,042 | 11,139 | 15,849 | 35,882 | 0.00 |
| Bulgarian children | 3,378 | 5,554 | 7,488 | 10,443 | 23,112 | 0.00 |
| French children | 5,874 | 9,589 | 12,896 | 17,953 | 39,644 | 0.00 |
| Dutch children | 2,612 | 4,300 | 5,804 | 8,105 | 17,978 | 0.00 |
| Danish toddlers | 2,884 | 4,888 | 6,660 | 9,363 | 20,917 | 0.00 |
| Dutch toddlers | 2,112 | 3,527 | 4,778 | 6,688 | 14,866 | 0.00 |
| United Kingdom toddlers | 3,655 | 6,003 | 8,094 | 11,289 | 24,992 | 0.00 |
The Working Group was provided with output from the Monte Carlo simulations in two forms: first, boxplots showing the median, quartiles and 95% probability interval for the quantified uncertainty of the MOET at the 99.9th percentile of exposure for each of the 10 consumer populations in each CAG; and second, tables containing the numerical values used in the boxplots plus, for each CAG and population, the calculated probability of the MOET at the 99.9th percentile of exposure being less than 100. The latter probabilities were then used as the starting point for judgements on EKE Q3 (see below).
2.3.7. Overall uncertainty analysis (EKE Question 3)
Two versions of EKE Question 3 were defined, one for the German adults and one for the other populations. This was necessary because the aim of EKE Q3 was to take account of all remaining uncertainties. For the German population, this comprised mainly the potential impact of dependencies between the distributions combined in the Monte Carlo simulations (described in the preceding section) while, for the other populations, EKE Q3 also included the uncertainty of extrapolating the results of those simulations for German adults to the other nine consumer populations.
For German adults, EKE Q3 was specified as follows: If all the uncertainties in the model, exposure assessment, hazard identification and characterisation and their dependencies were fully resolved (e.g. by obtaining perfect information on the issues involved) and addressed in the modelling, what is your probability that this would result in the estimated MOET at 99.9th percentile of exposure for the German adult population in 2014–2016 being below 100?
For the other nine consumer populations, EKE Q3 was specified as follows: If all the uncertainties in the model, exposure assessment, hazard identification and characterisation and their dependencies, and differences in these between populations, were fully resolved (e.g. by obtaining perfect information on the issues involved) and addressed in the modelling, what is your probability that this would result in the estimated MOET at 99.9th percentile of exposure for [name of population] in 2014–2016 being below 100?
For both versions of the question, it was agreed that ‘perfect information’ had the same meaning as that defined for EKE Q2 (Section 2.3.5, combining the definitions for the exposure and toxicology versions of Q2).
Elicitation for EKE Q3 was conducted in two stages: partly during the same 3‐day meeting as the consensus judgements for EKE Q2 and partly after the meeting, by email correspondence. This was necessary because Q3 was not completed within the scheduled meeting time; however, this also provided the opportunity for additional simulations to help inform judgements on Q3, as described below.
Before eliciting EKE Q3, the Working Group reviewed the issues to be considered. The facilitator explained that a dependency would exist between the toxicology and exposure uncertainty distributions if having perfect information on toxicology would alter the experts’ assessment of the uncertainties on exposure, or vice versa. The experts considered that dependencies could be expected if resolving some uncertainties led to a change in the risk drivers, which might alter their assessment of the remaining uncertainties. The facilitator also explained that any additional uncertainties, which the experts considered had not been fully accounted for earlier, including any arising from the EKE process itself, should also be taken into account when making judgements for EKE Q3.
The facilitator asked the experts to consider, as their starting point for answering Q3 for each CAG and population, the calculated probability of the MOET at the 99.9th percentile of exposure being less than 100 provided by the Monte Carlo simulations in the preceding step. In addition, the experts were advised to consider the following evidence: other quantiles of the calculated MOET distribution for each population (as shown in the tables and graphics described in the preceding section), considerations identified in the group discussion of dependencies and population differences, notes from EKE Q1B (see Section 2.3.5) on country differences for individual sources of uncertainty (included in the tables in Appendix A), and their personal knowledge and reasoning about the issues involved.
Judgements for EKE Q3 were elicited using the Approximate Probability Scale (APS, Figure 2), which is recommended in EFSA's guidance on uncertainty analysis for harmonised use in EFSA assessments (EFSA Scientific Committee, 2018a,b). The experts were advised to focus on the numeric probability ranges, not the verbal terms, and to consider which range (or, if appropriate, set of ranges) described their judgement on EKE Q3 for each combination of CAG and population. First, the experts were asked to work separately and record their individual judgements in spreadsheet templates provided by the facilitator. The completed templates were collected, and the judgements were collated in a table for each CAG, showing the number of experts who selected each probability range for each population, which was displayed on screen for review by the group. The facilitator then led a discussion to develop consensus judgements (applying the RIO concept, see Section 2.3.5).
The steps described above were not all completed during the 3‐day meeting. All seven experts completed their judgements for CAG‐TCF (hypothyroidism), but none of them did it for TCP because the probability of the MOET at 99.9th percentile of exposure being less than 100, calculated in the preceding step, was extremely low. In view of the limited time remaining, the facilitator focussed discussion on seeking consensus for the upper bound of probability for those populations where the individual probability judgements were highest (infants and other children). The process was completed remotely.
During the meeting, the experts found it difficult to assess the potential impact of dependencies between the uncertainties relating to exposure and toxicology. Therefore, before continuing the EKE Q3 assessment after the meeting, the opportunity was taken to extend the Monte Carlo simulations described in Section 2.3.6, to explore the impact of different degrees of dependence between the uncertainties relating to exposure and toxicology (specifically, rank correlations of −1, −0.75, −0.5, −0.25, 0.25, 0.5, 0.75 and 1). The results of these simulations, together with the individual judgements, discussions and evidence considered in the meeting, were used to develop proposed consensus judgements on EKE Q3 for all of populations for each CAG. This was done by one of the exposure experts, who also had a good knowledge of the toxicological evidence. The other experts were then given the opportunity to review, comment on and revise the proposed judgements by email until a consensus judgement was achieved.
3. Results of uncertainty analyses
3.1. Sources of uncertainty
Twenty‐eight sources of uncertainty related to the assessment inputs were identified as affecting the CRAs for CAG‐TCF and CAG‐TCP. They are listed in Table 3. In this table, each source of uncertainty is associated with a group number. This refers to the area of expertise they relate to (exposure or toxicology), as explained in Section 2.3.1.
Table 3.
Sources of uncertainty concerning the input data and affecting the CRA of hypothyroidism (CAG‐TCF) and C‐cell hypertrophy, hyperplasia and neoplasia (CAG‐TCP). Uncertainties relating to exposure were included in group 1 and uncertainties relating to toxicology were included in group 2
| Assessment input | Type of uncertainty | Description of the uncertainty |
|---|---|---|
| Consumption data (all group 1) | Excluded data | Consumption data of animal commodities and plant commodities not in the list of the 30 selected commodities and their processed derivatives have not been used |
| Ambiguity | The consumption data do not always discriminate between different commodities of a same group (e.g. tomatoes and cherry tomatoes are considered as tomatoes) | |
| Accuracy | The accuracy of the reported amount of food consumed in surveys may be affected by methodological limitations or psychological factors | |
| Sampling variability | Sample size (number of consumers in the 10 populations). A small number of consumers may affect the reliability of risk estimates at the 99.9th percentile of exposure | |
| Sampling bias | Representativeness of the consumption data (selection bias) for the whole population | |
| Use of fixed values | One invariable recipe and conversion factor are used to convert the amount of food consumed into the respective amount of raw primary commodity (RPC) | |
| Occurrence data (all group 1) | Missing data | Active substance/commodity combinations, for which occurrence data are missing and extrapolation from another commodity is not possible, were excluded |
| Excluded data | The contribution of all metabolites and degradation products to the effect has not been considered | |
| Ambiguity | The occurrence data do not always discriminate between different commodities of a same group (e.g. tomatoes and cherry tomatoes are considered as tomatoes) | |
| Accuracy | Laboratory analytical uncertainty | |
| Sampling variability | Sample size (number of occurrence data). A small number of occurrence data may affect the reliability of risk estimates at 99.9th percentile of exposure. This number varies from one pesticide/commodity combination to the other | |
| Sampling bias | Representativeness of the monitoring data (selection bias) | |
| Extrapolation uncertainty | Extrapolation of occurrence data between crops | |
| Extrapolation uncertainty | Extrapolation of occurrence data between countries | |
| Assumption | Assumption of the active substance present on the commodity in case of unspecific residue definition for monitoring | |
| Assumption | Left‐censored data: Assumption of the authorisation status of all pesticide/commodity combinations | |
| Assumption | Left‐censored data: Assumption of the use frequency for authorised pesticide/commodity combinations | |
| Assumption | Left‐censored data: Assumption on the residue level (½ LOQ as imputed value) when an active substance is used, and its residues are below the LOQ | |
| Assumption | Occurrence of residues in drinking water | |
| Processing factors (all group 1) | Assumption | Pesticide residues are transferred without any loss to processed commodities when processing factors are not available |
| Ambiguity | Application of processing factors, derived from a limited number of standardised studies, to the EFSA food classification and description system (FoodEx) | |
| Accuracy | Laboratory analytical uncertainty | |
| Accuracy | Calculation of processing factors is affected by residue levels below the LOQ | |
| Use of fixed values | The value of processing factors used in the calculations is the median value of a limited number of independent trials | |
| Excluded data | Some processing factors are not considered (e.g. peeling and washing of commodities with edible peel) | |
| NOAELs (all group 2) | Adequacy of the CAG | Uncertainty on whether the CAG contains all the active substances causing the effect |
| Adequacy of the CAG | Uncertainty on whether the CAG contains only the active substances causing the effect | |
| Accuracy | Uncertainties affecting the characterisation of active substances included in the CAG (quality of data and NOAEL setting process). This includes uncertainties affecting:
|
Three additional sources of uncertainty were found to be associated with the assessment methodology and are listed in Table 4. All three are related to toxicology (group 2).
Table 4.
Sources of uncertainty concerning the assessment methodology and affecting the CRA of hypothyroidism (CAG‐TCF) and C‐cell hypertrophy, hyperplasia and neoplasia (CAG‐TCP)
| Element of the assessment methodology | Description of the uncertainty |
|---|---|
| Dose addition (group 2) | Uncertainty about the actual mode of combined toxicity |
| Dose–response relationship (group 2) | Uncertainty about the slope and the shape of the dose–response, and consequently about the effect size of individual active substances at the actual exposure levels |
| Use of the OIM model for the exposure assessment (group 2) | Uncertainty about the fitness of the chronic exposure calculation model to human toxicokinetic and toxicodynamic processes involved in the effect |
Extensive information describing the sources of uncertainty listed in Tables 3 and 4 is given as technical notes in Appendix B, Section B.2.
3.2. Evaluation of individual uncertainties (EKE Question 1)
The elicitation questions to be addressed here for each source of uncertainty were expressed as follows:
EKE Q1A: ‘If this source of uncertainty was fully resolved (e.g. by obtaining perfect information on the issue involved) and addressed in the modelling, by what multiplicative factor would this change the median estimate of the MOET for [hypothyroidism/C‐cells hypertrophy, hyperplasia and neoplasia] for the 99.9th percentile of exposure of the German adult population at Tier II?’
EKE Q1B: ‘Is the impact of this source of uncertainty the same for the other populations that were assessed? If not, list those populations for which the impact would be smaller, and those for which it would be larger’
The 31 sources of uncertainty were divided into two groups as indicated in Tables 3 and 4. Group 1 included sources of uncertainty pertaining to the exposure assessment step of CRA and were evaluated by three exposure experts as described in Section 2.3.5. Group 2 included sources of uncertainty pertaining to the hazard identification and characterisation step of CRA and were evaluated by two toxicology experts.
EKE Q1A was addressed using the notes compiled in Appendix B2 and the resulting assessments (ranges of multiplicative factors of MOET at 99.9th percentile of exposure distribution at Tier II) are reported in Tables A.1 and A.2 of Appendix A for CAG‐TCF and CAG‐TCP.
Table A.1.
CAG‐TCF and CAG‐TCP: Assessment of individual sources of uncertainty affecting the input data with respect to EKE Q1A and EKE Q1B
| Assessment input | Type of uncertainty | Description of the uncertainty | Range of multiplicative factor of MOET at 99.9th percentile of tier IIa | Multiplicative factor identical for all populationsb | Informative notes |
|---|---|---|---|---|---|
| Consumption data | Excluded data | Animal commodities and plant commodities not in the list of the 30 selected commodities and their processed derivatives were excluded | −/● | No |
Note 1 Note 2 Note 3 |
| Ambiguity | The consumption data do not always discriminate between different commodities of a same group (e.g. tomatoes and cherry tomatoes are considered as tomatoes) | ● | Yes | Note 4 | |
| Accuracy | The accuracy of the reported amount of food consumed in surveys may be affected by methodological limitations or psychological factors | ● | Yes |
Note 5 Note 6 |
|
| Sampling variability | Small population size (number of consumers in the 10 populations) may affect the reliability of risk estimates at 99.9th percentiles | See confidence intervals of MOET estimates at 99.9th percentile of exposure distribution (Tables 1A and 2A) | Note 7 | ||
| Sampling bias | Representativeness of the consumption data | −/+ | Yes | Note 8 | |
| Use of fixed values | One invariable recipe and conversion factor are used to convert the amount of food consumed into the respective amount of RPC | ● | Yes | Note 9 | |
| Occurrence data | Missing data | Active substance/commodity combinations, for which occurrence data are missing and extrapolation from another commodity is not possible, were excluded | ● | Yes | Note 10 |
| Excluded data | The contribution of metabolites and degradation products has not been considered | −/● | Yes | Note 11 | |
| Ambiguity | The occurrence data do not always discriminate between different commodities of a same group (e.g. tomatoes and cherry tomatoes are considered as tomatoes) | ● | Yes | Note 4 | |
| Accuracy | Laboratory analytical uncertainty | ● | Yes | Note 12 | |
| Sampling variability | A small number of occurrence data may affect the reliability of risk estimates at 99.9th percentile. This number varies from one pesticide/commodity combination to the other | See confidence intervals of MOET estimates at 99.9th percentile of exposure distribution (Tables 1A and 2A) | Note 7 | ||
| Sampling bias | Representativeness of the monitoring data | ●/+ | Yes | Note 13 | |
| Extrapolation uncertainty | Extrapolation of occurrence data between crops | ● | Yes | Note 14 | |
| Extrapolation uncertainty | Extrapolation of occurrence data between countries | ● | Yes | Note 15 | |
| Assumption | Assumption of the active substance present on the commodity in case of unspecific residue definition for monitoring |
−/+ (CAG‐TCF) −−/++ (CAG‐TCP) |
Yes | Note 16 | |
| Assumption | Assumption of the authorisation status of all pesticide/commodity combinations | −/● | Yes | Note 17 | |
| Assumption | Assumption of the use frequency for authorised pesticide/commodity combinations | −/+ | Yes | Note 18 | |
| Assumption | Assumption on the residue level (½ LOQ) when an active substance is used, and its residues are below the LOQ | ● | Yes | Note 31 | |
| Assumption | Occurrence of residues in drinking water | ● | Yes | Note 19 | |
| Processing factors | Assumption | Pesticide residues are transferred without any loss to processed commodities when processing factors are not available | +/++ | Yes | Note 20 |
| Ambiguity | Application of processing factors, derived from a limited number of standardised studies, to the EFSA food classification and description system (FoodEx) | ● | Yes | Note 21 | |
| Accuracy | Laboratory analytical uncertainty | ● | Yes | ||
| Accuracy | Calculation of processing factors is affected by residue levels below the LOQ | ● | Yes | Note 22 | |
| Accuracy | The value of processing factors used in the calculations is the median value of a limited number of independent trials | ● | Yes | Note 23 | |
| Excluded data | Some processing factors are not considered (e.g. peeling and washing of commodities with edible peel) | ●/+ | Yes | Note 24 | |
| NOAELs | Adequacy of the CAG | Uncertainty on whether the CAG contains all the active substances causing the effect | −/● | Yes | Note 25 |
| Adequacy of the CAG | Uncertainty on whether the CAG contains only the active substances causing the effect |
● (CAG‐TCF) ●/+ (CAG‐TCP) |
Yes | Note 26 | |
| Accuracy | Uncertainties affecting the characterisation of active substances included in the CAG (quality of data and NOAEL setting process) |
−/● (CAG‐TCF) −/+ (CAG‐TCP) |
Yes | Note 27 |
The range shown is the same for CAG‐TCF and CAG‐TCP unless otherwise indicated.
The experts considered that only the first source of uncertainty was expected, on its own, to have an impact varying between populations, as indicated in this column, but noted that more differences might be expected if multiple uncertainties were considered together.
Table A.2.
CAG‐TCF and CAG‐TCP: Assessment of individual sources of uncertainty affecting the assessment methodology with respect to EKE Q1A and EKE Q1B
| Element of the assessment methodology | Description of the uncertainty | Range of mulitplicative factor of MOET at 99.9th percentile of tier IIa | Multiplicative factor identical for all populations | Informative notes |
|---|---|---|---|---|
| Dose addition | Uncertainty about the actual mode of combined toxicity | ● | Yes | Note 28 |
| Dose–response relationship | Uncertainty about the slope and the shape of the dose–response, and consequently about the effect size of individual active substances at the actual exposure levels | −/+ | Yes | Note 29 |
| Use of the OIM model for the exposure assessment | Uncertainty about the fitness of the chronic exposure calculation model to human toxicokinetic and toxicodynamic processes related to the effect | ●/+ | Yes | Note 30 |
The range shown is the same for CAG‐TCF and CAG‐TCP unless otherwise indicated.
The experts judged that resolving uncertainty would lead to similar multiplicative factors applicable to hypothyroidism and C‐cells hypertrophy, hyperplasia and neoplasia for most sources of uncertainty.
Multiplicative factors above 1 and increase of MOET estimates would result from perfectly representative monitoring data and perfect information on the effect of food processing and of washing and peeling of commodities consumed without further processing. The same would result from information solving the undue contribution of pesticides included in the CAG without causing the effect (CAG‐TCP only) and the uncertainty affecting the combination of the chronic exposure model with the actual human toxicokinetic and toxicodynamic processes.
Multiplicative factors below 1 and decrease of MOET estimates are expected if perfect information was available to take account of the contribution of excluded commodities, omitted active substances and metabolites relevant for the effect. The same would result from perfect information on the authorisation status, and solving uncertainties in NOAEL‐setting (CAG‐TCF only).
Multiplication factors either above or below 1 would result from perfectly representative consumption data and perfect information about the use frequency and the nature of residues quantified following unspecific residue definition. The same would result from information solving the uncertainty in NOAEL‐setting (CAG‐TCP only).
EKE Q1B was found to be particularly difficult to address for individual sources of uncertainty, because any difference would result from their combination (e.g. differences in consumption of some commodities have significant impact if this is accompanied by exposure to different pesticides, different residues levels or different behaviour in food processing). Only one source of uncertainty (excluded commodities) was noted as a source of possible difference between populations on its own (Appendix A).
3.3. Combined impact of uncertainties (EKE Question 2)
The combined impact of individual uncertainties was evaluated as described in Section 2.3.5, addressing the following elicitation question:
‘If all the identified sources of uncertainty in group [1/2] were fully resolved (e.g. by obtaining perfect information on the issues involved) and addressed in the modelling, by what multiplicative factor would this change the median estimate of the EFSA MOET for the 99.9th percentile of exposure for [hypothyroidism/C‐cells hypertrophy/hyperplasia/neoplasia] in the German adult population at Tier II?’
3.3.1. Impact of uncertainties on the MOET estimates at the 99.9th percentile of exposure in the German adult population for CAG‐TCF
-
a)
Combined impact of uncertainties related to exposure
Following discussion, the experts identified the most important contributors to the combined uncertainty as follows19:
Lack of data on the effect of processing for many pesticide/commodity combinations and lack of information about processing type in consumption data (+, see note 20).
Biases in occurrence data due to selective sampling (+, see note 13).
Missing contribution of commodities not included in modelling (−, see notes 1, 2, 3).
Exclusion of metabolites from the modelling, including the important metabolites ETU and PTU (−, see note 11).
Assumption of the active substance present on the commodity in case of unspecific residue definition. Three risk drivers are concerned (+/−, see note 16).
Assumption of the authorisation status and use frequency, which tends to underestimate the use frequency of pesticides not leading to quantifiable residues and with MRLs below the LOQ. This is particularly the case of herbicides, some of them being highly potent (e.g. amitrole), which constitute an important fraction of CAG‐TCF (−, see notes 17 and 18).
Other contributors to the combined uncertainty have a limited effect:
Non‐consideration of peeling and washing on the residue levels for commodities consumed without further processing (+, see note 24).
Uncertainty in the assignment of foods to commodities (RPC model) (+, see note 9).
Overall, the experts judged it most likely that the MOET at the 99.9th percentile of exposure in the German adult population would increase if all the uncertainties affecting exposure were resolved, because the most influential source of uncertainty was the lack of information about the effect of processing. A decrease of the MOET at the 99.9th percentile of exposure also had some probability, due to the joint contribution of uncertainties acting in the opposite direction. Their consensus judgements are shown in Table 5 together with the parameters for their final consensus distribution, which is also presented in Figure 3.
Table 5.
CAG‐TCF: Consensus judgements and distribution of the experts for the combined impact of the quantified uncertainties affecting exposure (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016. The impact is expressed as a multiplicative factor f to be applied to the Tier II median estimate (shown in Table 1A). The bottom row of the table gives the parameters for the consensus distribution, which is shown graphically in Figure 3
| Experts’ exposure multiplicative factor (f) | |
|---|---|
| Lower plausible bound | f = 0.5 (experts judged there to be < 1% probability that f would be < 0.5) |
| Upper plausible bound | f = 3 (experts judged there to be < 1% probability that f would be > 3) |
| Probability 1 | p (f < 1) = 10% (experts’ probability that f would be less than 1) |
| Probability 2 | p (f > 2) = 25% (experts’ probability that f would be more than 2) |
| Probability 3 | p (f > 1.5) = 55% (experts’ probability that f would be more than 1.5) |
| Consensus distribution | Gamma distribution with shape 3.84 and rate 3.28, offset to start from 0.5 (the experts’ lower plausible bound) instead of 0.0 |
Figure 3.
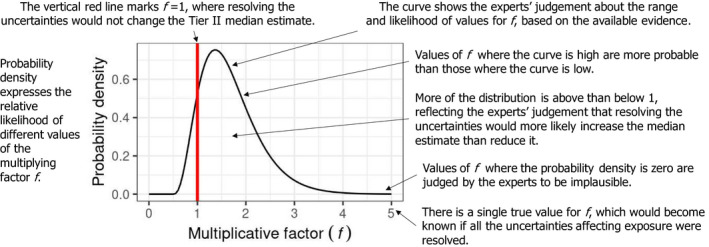
CAG‐TCF: Consensus distribution of the experts for the combined impact of the quantified uncertainties affecting exposure (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate shown in Table 1A. The probability distribution is shown by the curve, which represents the probability density (relative likelihood) for different values of the multiplicative factor f. Distribution parameters are shown in Table 5
-
b)
Combined impact of uncertainties related to toxicology
Following discussion, the experts identified the most important contributors to the combined uncertainty as follows19:
-
–
NOAEL‐setting:
-
–
The NOAEL for bromide ion is based on an incomplete set of toxicity studies (EFSA, 2019a). If the full set of studies was available, testing for all indicators of hypothyroidism, the NOAEL might be significantly lower (−, see note 27).
-
–
Use of the OIM model for chronic exposure calculation: this model tends to overestimate the upper tail of the exposure distribution (+, see note 30).
Other contributors to the combined uncertainty have a limited effect:
-
–
NOAEL‐setting:
-
–
The critical studies for three of the eight risk drivers (ziram, pyrimethanil and chlorpropham) have large dose intervals (factor 10 or more) between the NOAEL and the LOAEL: with narrower dose intervals, their NOAELs would probably be higher and closer to a BMDL10. This is, however, mitigated by the fact that thiabendazole, one of the two main risk drivers, shows a rather narrow dose interval (factor of 3) between the NOAEL and the LOAEL. (+, see note 27).
-
–
Uncertainty regarding dose addition:
-
–
For an important fraction of the substances included in CAG‐TCF, including four risk drivers (thiabendazole, pyrimethanil, cyprodinil and chlorpropham), liver enzyme induction is postulated to be the MOA responsible of hypothyroidism. If this is the case, their contribution by dose addition to hypothyroidism, at dose levels not triggering significant enzyme induction, is unlikely (EFSA, 2019a) (+).
-
–
The experts noted that mixtures of substances causing hypothyroidism by dissimilar modes of action showed combined toxicity compatible with the dose addition model (Crofton, 2008). Similar results have been obtained in most other studies examining mixtures for effects other than hypothyroidism, including recent results from the Euromix project (Kneuer, pers. comm.) The experts concluded there was almost no uncertainty regarding dose addition for this CAG (negligible, see note 28).
-
–
Adequacy of the CAG:
-
–
The median estimate of the total number of active substances in CAG‐TCF that actually cause hypothyroidism is 71, representing 55% of substances (EFSA, 2019a). This means that the contribution of a number or substances is unduly accounted for. However, all the risk drivers are substances which were assessed as having high probabilities of causing hypothyroidism, so the impact of this uncertainty is limited. (+, see note 26).
-
–
There is however a possibility that the CAG is incomplete, because of inaccuracies in the data collection and assessment and because all substances present in food were not screened for hypothyroidism (−, see 25).
Overall, the experts judged it most likely that the MOET at the 99.9th percentile of exposure in the German adult population would increase if all the uncertainties affecting toxicology were resolved, although a decrease was also plausible, especially due to the uncertainty about the NOAEL for bromide ion. Their consensus judgements are shown in Table 6 together with the parameters for their final consensus distribution, which is also presented in Figure 4.
Table 6.
CAG‐TCF: Consensus judgements and distribution of the experts for the combined impact of the quantified uncertainties affecting toxicology (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate (shown in Table 1A). For more explanation, see Table 5
| Experts’ toxicology multiplicative factor (f) | |
|---|---|
| Lower plausible bound | 0.3 |
| Upper plausible bound | 2.5 |
| Probability 1 | p (f < 1) = 40% |
| Probability 2 | p (f > 2) = 10% |
| Probability 3 | p (f < 0.5) = 10% |
| Consensus distribution (see Figure 4) | Gamma distribution with shape 2.13 and rate 2.24, offset to start from 0.3 |
Figure 4.
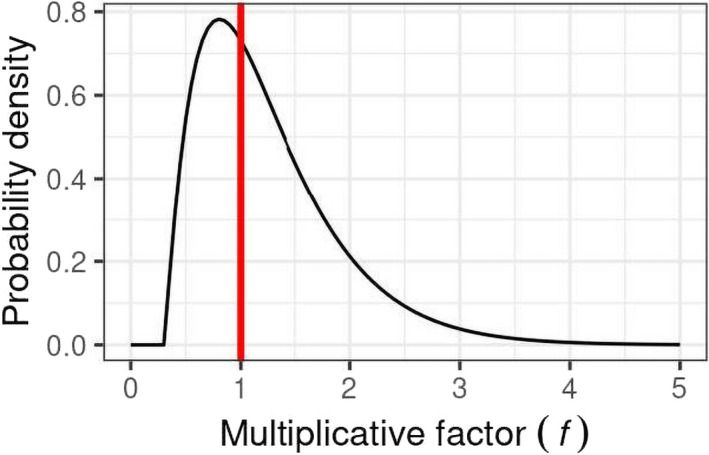
CAG‐TCF: Consensus distribution of the experts for the combined impact of the quantified uncertainties affecting toxicology (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate shown in Table 1A. Distribution parameters are shown in Table 6. Graph content is explained in Figure 3
-
c)
Combined impact of uncertainties related to exposure and toxicology
The elicited distributions for the uncertainties related to toxicology and exposure were combined with the output of the MCRA Tier II model for the MOET at the 99.9th percentile of exposure in each consumer population (see Section 2.2.2.1), using the Monte Carlo calculation described in Section 2.3.6. The elicited distributions were combined with the median 99.9th percentile and uncertainty due to sampling variability (mainly on the occurrence data and consumption data) quantified in the Tier II model and reflected in confidence intervals calculated by outer loop execution (referred to as ‘model’ in Figure 5). These calculations were conducted assuming perfect independence between the elicited distributions for uncertainties affecting exposure and toxicology. The results of combining distributions are shown as ‘model+experts’ in Figure 5.
The boxplots for ‘model+experts’ in Figure 5 are much wider than those for ‘model’. This shows that the combined impact of the sources of uncertainty quantified by elicitation is much larger than the sampling variability that was quantified within the model.
It can also be seen in Figure 5 that the median estimates for ‘model + experts’ are markedly higher than those for ‘model’ (Figure 5). This is to be expected, because the uncertainties quantified in the expert elicitation include the impact of assumptions in the model that were intentionally conservative (overestimating exposure and hence underestimating MOETs). However, the much larger magnitude of the additional uncertainties leads to the 95% probability intervals for the MOET at the 99.9th percentile of exposure extending below 100 for five of the six populations of toddlers and other children, whereas this occurred for only one population in the ‘model’ results.
In summary, taking account of the additional uncertainties increased the median estimate of the MOET at the 99.9th percentile but also, for most populations, increased the assessed probability that the MOET at the 99.9th percentile is below 100. This result emphasises the importance of considering uncertainties that are not quantified in the MCRA and EFSA probabilistic models. Calculated probabilities for the MOET at the 99.9th percentile of exposure being below 100 in each population group are shown in Table 7. Note that these results do not take account of dependencies and population differences in uncertainty, which are addressed in EKE Q3 (see below).
3.3.2. Impact of uncertainties on the MOET estimates at the 99.9th percentile of exposure in the German adult population for CAG‐TCP
The assessment of exposure and toxicology uncertainties was conducted at a quicker rate for CAG‐TCP than for other CAGs due to time constraints and because it was clear from the model results that the MOETs at 99.9th percentile of the exposure distribution for this CAG were at least one order of magnitude above 100 (See Table 2A).
-
a)
Combined impact of uncertainties related to exposure
Following discussion, the experts identified the most important contributors to the combined uncertainty as follows20:
Uncertainty due to the unspecific residue definition for the monitoring of substances producing the common analyte CS2, including thiram and ziram. CS2 residues were allocated at random with equal probability to the different substances authorised to be used on the respective commodities. This is thought to exaggerate the contribution of thiram, which is rarely used in the EU (Garthwaite et al., 2015). Resolving this uncertainty would be expected to increase the MOETs as thiram is a risk driver (+, see note 16).
Lack of data on the effect of processing for many combinations of substance and commodity and lack of information about processing type in consumption data (+, see note 20).
Biases in occurrence data due to selective sampling (+, see note 13).
Uncertainty about the potential contribution of excluded commodities (−, see notes 1, 2, 3).
Other contributors to the combined uncertainty have a limited effect:
Non‐consideration of peeling and washing on the residue levels for commodities consumed without further processing (+, see note 24).
Uncertainty in the assignment of foods to commodities (RPC model) (+, see note 9).
Overall, the experts judged that the MOET at the 99.9th percentile of exposure in the German adult population would increase if all the uncertainties affecting exposure were resolved; for this CAG, they considered that a decrease was not plausible. Their consensus judgements are shown in Table 8 together with the parameters for their final consensus distribution, which is also presented in Figure 6.
Table 8.
CAG‐TCP: Consensus distribution of the experts for the combined impact of the quantified uncertainties affecting exposure (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate (shown in Table 2A). For more explanation, see Table 5
| Experts’ exposure multiplicative factor | |
|---|---|
| Lower plausible bound | 1 |
| Upper plausible bound | 6 |
| Probability 1 | p (f < 3) = 50% |
| Probability 2 | p (f < 2) = 10% |
| Probability 3 | p (f > 4) = 25% |
| Consensus distribution (see Figure 6) | Log‐normal (mean and standard deviation of underlying normal 0.705 and 0.566, respectively), offset to start from 1.0 |
Figure 6.
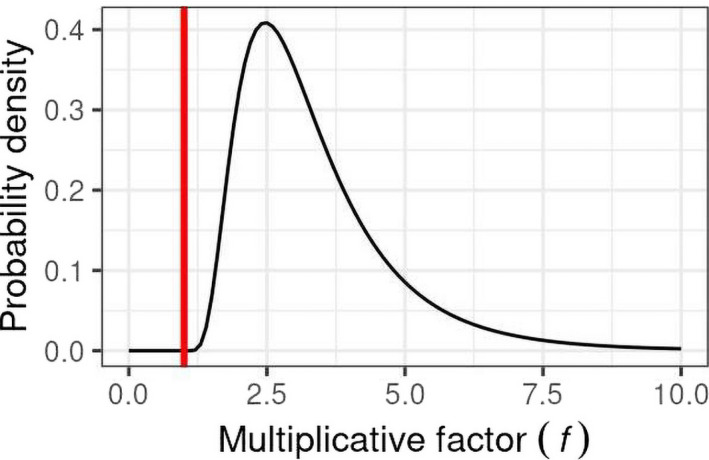
CAG‐TCP: Consensus distribution of the experts for the combined impact of the quantified uncertainties affecting exposure (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate shown in Table 2A. Distribution parameters are shown in Table 8. Graph content is explained in Figure 3
-
b)
Combined impact of uncertainties related to toxicology
Following discussion, the experts identified the most important contributors to the combined uncertainty as follows19:
Uncertainties in NOAEL‐setting, which can lead to either under‐ or overestimation of the MOET (+/−, note 27).
Uncertainty about the composition of the CAG: The identification of indicators of this effect is rather difficult. When observed the effects are specific, and therefore, the chance of including a substance not causing the effect in the CAG is low. The chance of omitting substances causing the effect is higher, due to the lack of biochemical indicators of the effect (−, note 25).
Other contributors to the combined uncertainty have a limited effect:
Uncertainty regarding the applicability of dose addition, due to the very limited knowledge about MoA involved in this effect. However, dose addition is anticipated for thiram and ziram considering their similarity, and as these are the main contributors to the risk, this source of uncertainty has a limited impact (+, note 28).
Use of the OIM model for chronic exposure calculation: this model tends to overestimate the upper tail of the exposure distribution (+, see note 30).
Overall, the experts judged it plausible that the MOET at the 99.9th percentile of exposure in the German adult population could either increase or decrease if all the uncertainties affecting toxicology were resolved. Their consensus judgements are shown in Table 9 together with the parameters for the final consensus distributions, which are also presented in Figure 7.
Table 9.
CAG‐TCP: Consensus distribution of the experts for the combined impact of the quantified uncertainties affecting toxicology (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate (shown in Table 2A). For more explanation, see Table 5
| Experts’ exposure multiplicative factor | |
|---|---|
| Lower plausible bound | 0.5 |
| Upper plausible bound | 2 |
| Probability 1 | p (f < 1) = 40% |
| Probability 2 | p (f > 1.5) = 10% |
| Probability 3 | p (f < 0.8) = 10% |
| Consensus distribution (see Figure 7) | Log Students t (location and scale of underlying 3df t 0.593 and 0.367, respectively), offset to start from 0.5 |
Figure 7.
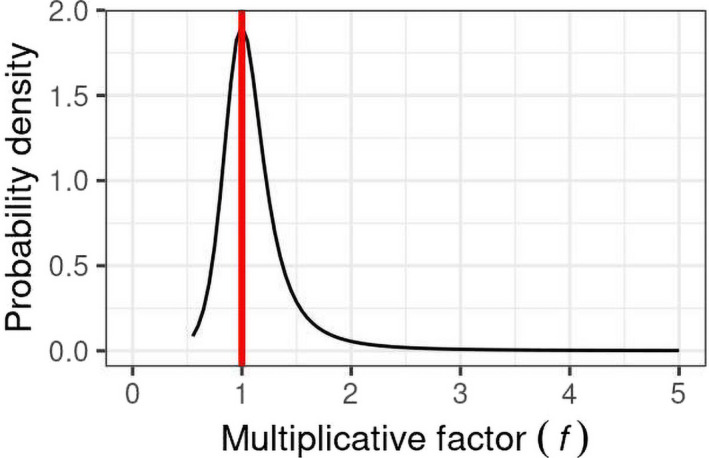
CAG‐TCP: Consensus distribution of the experts for the combined impact of the quantified uncertainties affecting toxicology (if resolved) on the MOET at the 99.9th percentile of exposure for the German adult population in 2014–2016, expressed as a multiplicative factor f to be applied to the Tier II median estimate shown in Table 2A. Distribution parameters are shown in Table 9. Graph content is explained in Figure 3
-
c)
Combined impact of uncertainties related to exposure and toxicology
The elicited distributions for the uncertainties related to toxicology and exposure were combined with the output of the MCRA Tier II model for the MOET at the 99.9th percentile of exposure in each consumer population (see Section 2.2.2.2), using the Monte Carlo calculation described in Section 2.3.6. The elicited distributions were combined with the median 99.9th percentile and uncertainty due to sampling variability (mainly on the occurrence data and consumption data) quantified in the Tier II model and reflected in confidence intervals calculated by outer loop execution (referred to as ‘model’ in Figure 8). These calculations were conducted assuming perfect independence between the elicited distributions for uncertainties affecting exposure and toxicology. The results of combining the distributions are shown as ‘model+experts’ in Figure 8.
Similar to the results for CAG‐TCF, the boxplots for ‘model + experts’ in Figure 8 are much wider than those for ‘model’. This shows that the combined impact of the sources of uncertainty quantified by elicitation is again much larger than the sampling variability that was quantified within the model.
As for CAG‐TCF, the median estimates for ‘model + experts’ are markedly higher than those for ‘model’ (Figure 5), because the uncertainties quantified in the expert elicitation include model assumptions that were intentionally conservative. However, in contrast to CAG‐TCF, none of the 95% probability intervals for any of the populations extend below 100.
Calculated probabilities for the MOET at the 99.9th percentile of exposure being below 100 were zero for every population group, as shown in Table 10. Note that these results do not take account of dependencies and population differences in uncertainty, which are addressed in EKE Q3 (see below).
3.4. Accounting for dependencies, population differences and additional uncertainties (EKE Question 3)
EKE was used to evaluate how much the calculated probabilities for the MOETs at the 99.9th percentile of exposures should be adjusted to take account of (a) dependencies between the elicited distributions for exposure and toxicology and the uncertainties quantified in the model (which were assumed in the calculation to be independent), (b) differences between the uncertainties affecting exposure and toxicology for the German adults population (which were quantified by the elicited distributions) and the uncertainties for other population groups (which were assumed in the ‘model+experts’ calculation to be the same as for German adults) and (c) any other uncertainties which were not yet accounted for.
These factors were addressed by considering the following elicitation questions, repeated for each CAG:
For the German adults population: ‘If all the uncertainties in the model, exposure assessment, hazard identification and characterisation and their dependencies were fully resolved (e.g. by obtaining perfect information on the issues involved) and addressed in the modelling, and also the differences between the EFSA and RIVM models, what is your probability that this would result in the MOET for the 99.9th percentile of exposure for the German adult population in 2014–2016 being below 100?’
For each of the other nine modelled populations: ‘If all the uncertainties in the model, exposure assessment, hazard identification and characterisation and their dependencies, the differences between the EFSA and RIVM models, and differences in these between populations, were fully resolved (e.g. by obtaining perfect information on the issues involved) and addressed in the modelling, what is your probability that this would result in the MOET for the 99.9th percentile of exposure for the [name of the population] in 2014–2016 being below 100?’
Seven experts participated to these assessments and provided independent replies to the elicitation questions for each CAG. Later, they considered differences in their judgements and developed a consensus assessment of the probability of the MOET for the 99.9th percentile of exposure in 2014–2016 being below 100 in each of the 10 populations under consideration. The consensus process was conducted partly during a physical meeting and completed remotely as described in Section 3.1.
It was very difficult to assess the impact of the dependencies referred to in the EKE Q3 elicitation questions by expert judgement. Therefore, it was decided to facilitate this assessment by repeating the 1‐D Monte Carlo simulations described in Section 2.3.6 with the addition of varying levels of dependency between the EKE Q2 distributions for uncertainties affecting exposure and toxicology for the same CAG. This quantified the range of possible effects resulting from dependencies. The worst‐case simulation, assuming perfect positive dependency, was used to define a maximum upper bound for the impact of this dependency on the probability that the MOET is below 100. Conversely, the best‐case simulation, assuming perfect negative dependency, was used to define the minimum lower bound for the impact of this dependency on the probability that the MOET is below 100. In practice, the real level of dependency would be between these two extremes. The maximum effect of dependency was found to differ significantly depending on the nominal probability calculated from the EKE Question 2 process. Under the assumption of perfect positive dependency, the increase in probability exceeds a factor 7 for initial probabilities below 0.25% but does not exceed a factor 2 for initial probabilities above 5% (see Table 11).
Table 11.
CAG‐TCF: Results of EKE Q3. The central five columns give the calculated % probability that the MOET for the 99th percentile of exposure for each population in 2014–2016 is below 100 assuming no dependence (column 2) or varying degrees of dependence (columns 3–6) between the experts’ assessments of uncertainties relating to exposure and toxicology, i.e. between the distributions in Figures 3 and 4 above. The degree of dependency is shown as a correlation coefficient (Corr.). The right hand column shows the consensus judgement of the experts for the probability that the MOET for the 99th percentile of exposure for each population in 2014–2016 is below 100, taking into account both dependencies and differences in uncertainties between populations
| Population | Q2 probability assuming independence (%) | Q2 probability with negative dependency | Q2 probability with positive dependency | Consensus probability for Q3, including dependency and population differences | ||
|---|---|---|---|---|---|---|
| Corr. −1 | Corr. −0.25 | Corr. +1 | Corr. +0.25 | |||
| Belgian adults | 0.25a | 0.00 | 0.08 | 2.2 | 0.54 | < 1% |
| Czech Rep. adults | 0.50 | 0.00 | 0.19 | 3.4 | 0.97 | < 1% |
| German adults | 0.46 | 0.00 | 0.14 | 3.5 | 0.95 | < 1% |
| Italian adults | 0.18 | 0.00 | 0.04 | 2.2 | 0.45 | < 1% |
| Bulgarian children | 9.0 | 0.00 | 6.0 | 17 | 12 | 5–10% |
| French children | 1.8 | 0.00 | 0.77 | 7.3 | 3.0 | < 5% |
| Dutch children | 3.1 | 0.00 | 1.6 | 9.8 | 4.7 | 1< 5% |
| Danish toddlers | 9.7 | 0.00 | 6.7 | 18 | 12 | 5–15% |
| Dutch toddlers | 15 | 0.00 | 12 | 23 | 18 | 10–15% |
| United Kingdom toddlers | 7.9 | 0.00 | 5.3 | 16 | 10 | 5–10% |
The probabilities in this column differ slightly from those shown in Table 7 because they were obtained after repeating the Monte Carlo calculations. This commonly occurs in Monte Carlo calculations unless the number of Monte Carlo samples is very large. In the present case, the overall relative error in a calculated probability is very unlikely to be more than 10% (e.g. ± 0.79 for UK toddlers) except for probabilities calculated to be less than 1% where the relative error may be as much as 50% in some cases and probabilities calculated to be less than 0.1% where it is unlikely that the probability actually exceeds 0.2%.
For the German adults population, only the dependencies and differences between the EFSA and RIVM probabilistic models need to be considered when addressing EKE Question 3, since EKE Question 2 refers specifically to the German adults population. For the other nine consumer populations, EKE Question 3 requires considering, in addition, how the uncertainties relating to exposure and toxicology differ between them and the German adults population. This was done by expert judgement, taking into account the probabilities calculated for each population from EKE Question 2, the results of the calculations for the impact of dependencies, and differences in uncertainties between the populations which are summarised in the following sections.
By capturing these residual uncertainties and combining them to those previously evaluated, the EKE Q3 elicitation questions conclude the uncertainty analysis process and allow the assessment of the overall uncertainty affecting CAG‐TCF and CAG‐TCP.
3.4.1. Overall uncertainty affecting the cumulative risk assessment of hypothyroidism
The experts first discussed their individual judgements for EKE Q3 for the German adults population. The key considerations were as follows:
EKE Q2 was considered with high emphasis to risk drivers. This is introducing a systematic methodological uncertainty which was taken into consideration in EKE Q3. If resolving toxicology uncertainties might cause the risk drivers to be different, then the exposure uncertainty judgement might change and the resulting overall probability interval combining all uncertainties be impacted. Similarly, if resolving exposure uncertainties changes the risk drivers, then the toxicology uncertainty might change. This is a form of dependency between the exposure and toxicology uncertainties. Differences in the risk drivers may result in higher probability of less‐than‐additive effects. This would tend to shift the overall distribution of the multiplicative factor towards higher values. This is, however, mitigated by the fact that the identification of risk drivers at the upper end of the exposure distribution in case of chronic effects/exposure is not sensitive to extreme values of occurrence data. The experts considered that the dependency of uncertainties might increase the probability that the MOET at 99.9th percentile of the exposure distribution was below 100 by a factor corresponding to a rank correlation of about 0.25 or less (see Table 11).
No obvious cause or risk of any other dependency between the exposure and toxicology judgements was identified.
The contribution of uncertainty due to differences between results from the MCRA and EFSA probabilistic models is negligible, given the much larger magnitude of the uncertainties quantified in EKE Q2 and Q3.
Based on these considerations, the experts agreed on a consensus upper bound of 1% for their probability that the MOET for the 99.9th percentile of exposure for the German adult population in 2014–2016 is less than 100 (see right hand column of Table 11).
The experts next discussed their individual judgements for the other populations, and identified the following key considerations:
The degree of dependency between uncertainties in the other populations was considered to be similar to that for German adults. Therefore, similar to German population, the increased probabilities calculated for a rank correlation of 0.25 were considered as an upper bound for the contribution of dependencies in the other population groups.
The differences between populations are essentially induced by differences in food consumption. In this respect, it was assumed that the effect of food processing on residues is expected to have a higher effect for Dutch toddlers and Bulgarian children than for German adults (oranges contribute about 30% of total exposure above the 99th percentile). This would tend to shift the overall distribution of the multiplicative factor towards higher values. It was judged that the estimated 99.9th percentile of the MOET distribution would increase by possibly 20% in Dutch toddlers and Bulgarian children populations.
The difference in occurrence of pesticide residues in food commodities between populations and countries are expected to have a low impact, due to the common market.
For TCF, no element related to the hazard characterisation process was identified as source of difference between populations in different countries or different age ranges.
The vast majority of sources of uncertainty were considered during the EKE Q1 process as impacting the multiplicative factor in the same way for all populations.
It was also considered that the impact of differences between populations, summarised in the preceding bullets, did not contribute to further increase the probability of the MOET for the 99.9th percentile of exposure in 2014–2016 being below 100.
Based on these considerations, the experts agreed on a consensus upper bound for their probabilities for each of the nine other populations and, in addition, a lower bound probability for five populations (see right hand column of Table 11). In making these judgements, the experts considered that the probability calculated for a rank correlation of 0.25 was their upper bound for the impact of dependency, and therefore could be regarded as also allowing for part of the impact of differences between populations, and that for some populations the uncertainty was expected to be less than for German adults (see above). For example, in the case of Bulgarian children, combining the uncertainty distributions from Q2 with a rank correlation of 0.25 gives a probability of 12% for MOET < 100 (Table 11). However, the differences in exposure uncertainties compared to German adults (especially regarding processing effects, see above) tend to increase the MOETs for Bulgarian children and therefore reduce the calculated probabilities. Therefore, even allowing for dependency, the probability that the MOET at the 99.9th percentile of exposure for Bulgarian children in 2014–2016 is below 100 was judged to be less than 10%, but more than 5% (right hand column in Table 11). The width of the final probability ranges in Table 11 reflects the difficulty of assessing the issues addressed in EKE Q3.
3.4.2. Overall uncertainty affecting the cumulative risk assessment of C‐cell hypertrophy, hyperplasia and neoplasia
Due to limitations of time, individual judgements for the EKE Q3 were not produced.
However, 1‐D Monte Carlo simulations showed that, even with full positive dependency between the experts’ assessments of uncertainties relating to exposure and toxicology, the probability for the MOET at the 99th percentile of exposure being below 100, was 0 for all populations (Table 12).
Table 12.
CAG‐TCP: Results of EKE Question 3. The central five columns give the calculated % probability that the MOET for the 99th percentile of exposure for each population in 2014–2016 is below 100 assuming no dependence (column 2) or varying degrees of dependence between the experts’ assessments of uncertainties relating to exposure and toxicology, i.e. between the distributions in Figures 6 and 7 above. The degree of dependency is shown as a correlation coefficient (Corr.). The right hand column shows the consensus judgement of the experts for the probability that the MOET for the 99th percentile of exposure for each population in 2014–2016 is below 100, taking into account both dependencies and differences in uncertainties between populations
| Population | Q2 probability assuming independence (%) | Q2 probability with negative dependency | Q2 probability with positive dependency | Consensus probability for Q3, including dependency and population differences | ||
|---|---|---|---|---|---|---|
| Corr. −1 | Corr. −0.25 | Corr. +1 | Corr. +0.25 | |||
| Belgian adults | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| Czech Rep. adults | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| German adults | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| Italian adults | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| Bulgarian children | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| French children | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| Dutch children | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| Danish toddlers | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| Dutch toddlers | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
| United Kingdom toddlers | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | < 1% |
As differences between populations are not expected to impact significantly this probability, the experts agreed on a consensus upper bound of 1% for their probability that the MOET for the 99.9th percentile of exposure for the German adult population in 2014–2016 is less than 100 (see right hand column of Table 12).
4. Cumulative risk characterisation
4.1. Hypothyroidism
Based on the above finding and analyses, the cumulative risks for hypothyroidism can be summarised as follows:
Very similar results in probabilistic modelling of cumulative exposure were obtained by EFSA and RIVM and showed that all populations had median estimates of the MOETs above 100 at the 99.9th percentile of the exposure (Table 1A, second column of Table 13), which has been identified by the European Commission as a threshold for regulatory consideration. Toddlers and children had similar levels of exposure, which were higher than the exposure levels observed for adults.
Uncertainties in the assessment of toxicology and exposure were quantified by a formal process of expert judgement and combined with the MCRA model results by calculation. For all population groups, this increased the median estimate of the MOET at the 99.9th percentile of exposure by about a factor of 2 (Table 7, third column Table 13), reflecting the effect of purposely conservative assumptions in the assessment, as called for by the legislation (see Section 1.2).
The 95% probability intervals for the adjusted results are much wider than those calculated by the models, because only a small proportion of the uncertainties is quantified in the MCRA and EFSA models. Calculated probabilities for the MOET at the 99.9th percentile of exposure being below the threshold for regulatory consideration were less than 1% for the four adult populations and 2–10% for the children and toddlers, except for Dutch toddlers (15%) (right hand column of Table 7).
Finally, the experts considered the impact of dependencies between the toxicology and exposure uncertainties, differences between populations and additional uncertainties not considered earlier. This results in their overall assessment (fourth column of Table 13). For all four adult populations (BE, CZ, DE and IT), the probability of the MOET at the 99.9th percentile of exposure being below the threshold for regulatory consideration was assessed to remain below 1%. For French children and Dutch children, the probability was assessed to be less than 5%. For Bulgarian children and UK toddlers, the probability was assessed to be between 5% and 10%. For Danish toddlers, it was assessed to be between 5% and 15%. For Dutch toddlers, it was assessed to be between 10% and 15%.
EFSA's guidance on communicating uncertainty (EFSA, 2019c) recommends that for the purpose of communication, probabilities quantifying uncertainty should be expressed as ‘percentage certainty’ of the more probable outcome (in this case, that the MOET at the 99.9th percentile of exposure in 2014–2016 is equal or greater than 100, rather than less). This advice is applied in the right hand column of Table 13. Also shown in the same column are verbal probability terms associated with the assessed range of percent certainty, based on the APS recommended for harmonised use in EFSA assessments.
Table 13.
CAG‐TCF: Outcome of the CRA of hypothyroidism resulting from dietary exposure to pesticides for each population in 2014–2016
| Population | MOET at the 99.9th percentile of exposure | |||
|---|---|---|---|---|
| Median and 95% confidence interval following model and agreed assumptions | Median and 95% probability interval after adjustment for uncertainties | Probability for MOET < 100 | Corresponding percent certainty that the MOET is equal to or greater than 100, and associated probability terma | |
| Belgian adults | 316 [210–391] | 540 [159–1,833] | < 1% | > 99% certainty (almost certain) |
| Czech Rep. adults | 280 [200–349] | 475 [141–1,601] | < 1% | > 99% certainty (almost certain) |
| German adults | 266 [228–302] | 459 [140–1,513] | < 1% | > 99% certainty (almost certain) |
| Italian adults | 302 [274–335] | 525 [161–1,722] | < 1% | > 99% certainty (almost certain) |
| Bulgarian children | 130 [118–154] | 230 [70–757] | 5–10% | 90–95% certainty (very likely) |
| French children | 200 [193–227] | 351 [108–1149] | < 5% | > 95% certainty (extremely likely to almost certain) |
| Dutch children | 177 [162–196] | 310 [95–1,016] | 1–5% | 95–99% certainty (extremely likely) |
| Danish toddlers | 128 [110–172] | 225 [68–753] | 5–15% | 85–95% certainty (likely to very likely) |
| Dutch toddlers | 102 [89–160] | 193 [57–676] | 10–15% | 85–90% certainty (likely) |
| United Kingdom toddlers | 149 [108–177] | 245 [73–832] | 5–10% | 90–95% certainty (very likely) |
The probability terms used in this table are recommended in the EFSA guidance on communication of uncertainty (EFSA, 2019c).
Regulation (EC) 1107/2009 concerning the placing of plant protection products on the market requires in annex II, chapter 3.6.1, that when a health‐based guidance value is established a ‘safety margin of at least 100 shall be ensured taking into account the type and severity of effects and the vulnerability of specific groups of the population’. The factor of 100 comprises a factor of 10 for interspecies differences and a factor of 10 for human interindividual differences. The MOET of 100 required as threshold for regulatory consideration is coherent with this principle.
However, in certain circumstances, information on toxicokinetics or toxicodynamics may be available indicating that the default factors for interspecies and/or human interindividual differences would be more or less conservative.
Regarding the applicability of an MOET of 100 for hypothyroidism, it is noted in the Guidance for the identification of endocrine disruptors in the context of Regulation (EU) No 528/2012 and (EC) No 1107/2009 (ECHA and EFSA, 2018), that there are notable quantitative difference in the systemic regulation of thyroid hormone levels between rodents and humans, where the former can be more sensitive, due to liver enzyme induction. In this regard, when the input data are based on a rodent NOAEL, the inter‐species uncertainty factor of 10 might overestimate the risk. For about 60 active substances, including two (minor) risk drivers, liver enzyme induction is the hypothesised MoA. On the other hand, it is also noted that there is uncertainty regarding intra‐species sensitivities such as age‐related differences, especially during development.
In the present risk assessment, the conservatism of the default MOET of 100 was not considered.
4.2. C‐cell hypertrophy, hyperplasia and neoplasia
Based on the above findings and analyses, the cumulative risks for C‐cell hypertrophy, hyperplasia and neoplasia can be summarised as follows:
Very similar results in probabilistic modelling of cumulative exposure were obtained by EFSA and RIVM and showed that all populations had median estimates of the MOETs greatly exceeding 100 at 99.9th percentile of the exposure (Table 2A, second column Table 14). Toddlers and children had similar levels of exposure, which were higher than the exposure levels observed for adults.
Uncertainties in the assessment of toxicology and exposure were quantified by a formal process of expert judgement and combined with the MCRA model results by calculation. For all population groups, this increased the median estimate of the MOET at the 99.9th percentile of exposure by about a factor of 3–4 (Table 10, third column Table 14), reflecting the effect of purposely conservative assumptions in the assessment, as called for by the legislation (see Section 1.2).
The 95% probability intervals for the adjusted results are much wider, because only a small proportion of the uncertainties is quantified in the MCRA and EFSA models. Calculated probabilities for the MOET at the 99.9th percentile of exposure being below the threshold for regulatory consideration were nevertheless zero for all population groups (right hand column of Table 10).
The impact of dependencies between the toxicology and exposure uncertainties and differences between populations was not assessed formally for this CAG. However, even in case of perfect positive dependency, which is virtually impossible, the probability for the MOET at the 99.9th percentile of exposure being below the threshold for regulatory consideration (100) would still be below 1% for all populations (fourth column of Table 14).
As was the case for CAG‐TCF, the results for CAG‐TCP are expressed in terms of percent certainty in the right hand column of Table 14.
Table 14.
CAG‐TCP: Outcome of the CRA of C‐cell hypertrophy, hyperplasia and neoplasia resulting from dietary exposure to pesticides for each population in 2014–2016
| Population | MOET at the 99.9th percentile of exposure | |||
|---|---|---|---|---|
| Median and 95% confidence interval following model and agreed assumptions | Median and 95% probability interval after adjustment for uncertainties | Probability for MOET < 100 | Corresponding percent certainty that the MOET is equal to or greater than 100, and associated probability terma | |
| Belgian adults | 2,849 [1,389–4,592] | 9,269 [3,344–31,084] | < 1% | > 99% certainty (almost certain) |
| Czech Rep. adults | 2,532 [1,401–4,017] | 8,266 [3,023–28,097] | < 1% | > 99% certainty (almost certain) |
| German adults | 2,241 [1,496–2,868] | 7,352 [3,076–23,285] | < 1% | > 99% certainty (almost certain) |
| Italian adults | 3,401 [2,144–4,731] | 11,139 [4,489–35,882] | < 1% | > 99% certainty (almost certain) |
| Bulgarian children | 2,307 [1,860–2,627] | 7,488 [3,378–23,112] | < 1% | > 99% certainty (almost certain) |
| French children | 3,978 [3,337–4,430] | 12,896 [5,874–39,644] | < 1% | > 99% certainty (almost certain) |
| Dutch children | 1,778 [1,491–2,187] | 5,804 [2,612–17,978] | < 1% | > 99% certainty (almost certain) |
| Danish toddlers | 2,072 [1,516–2,538] | 6,660 [2,884–20,917] | < 1% | > 99% certainty (almost certain) |
| Dutch toddlers | 1,468 [1,148–1,783] | 4,778 [2,112–14,866] | < 1% | > 99% certainty (almost certain) |
| United Kingdom toddlers | 2,488 [2,077–2,913] | 8,094 [3,655–24,992] | < 1% | > 99% certainty (almost certain) |
The probability terms used in this table are recommended in the EFSA guidance on communication of uncertainty (EFSA, 2019c).
4.3. Risks for other European populations
During the Standing Committee on Plants, Animals, Food and Feed of 18–19 September 2018 (European Commission, 2018), MSs recommended considering, in CRA, all population subgroups of consumers included in the EFSA PRIMo model (EFSA, 2018b). For reasons of resources, it was however not possible to extend the calculations for the 10 selected populations to all consumer subgroups of the PRIMo model. However, from the information given in note 1 of Appendix B.2, Dutch toddlers, constituting one of the populations selected for the calculations, have the highest consumption of food of plant origin of the PRIMo model. This suggests that the populations selected for the calculations are representative of European populations with highest vulnerability in terms of dietary exposure potential, and cover teenagers from 9 to 18 year of age and adults above 65 years old, although not represented by any of the assessed populations.
In contrast, infants from 16 weeks to 1 year of age have a higher consumption rate per kg body weight than any of the toddlers and other children populations used for the calculations. However, in its scientific opinion on pesticides in foods for infants and young children (EFSA PPR Panel, 2018), the PPR panel has shown through five case studies that their acute and chronic exposure to pesticide residues in post‐marketing scenario was similar to the exposure of toddlers (12–36 months).
5. Conclusions
As a result of the uncertainty analysis, the MOETs at the 99.9th percentile of exposure and their confidence intervals were adjusted to take account of all identified uncertainties. For both CAGs, the adjusted MOETs were around two to four times higher compared to those calculated in tier II by the probabilistic tools. This is consistent with the intention of MSs, when selecting the parameters and assumptions to be used, to ensure that the tier II calculations are sufficiently conservative.
Considering all uncertainties identified by experts, for hypothyroidism, it was concluded that, with varying degrees of certainty, cumulative exposure does not reach the threshold for regulatory consideration for all the population groups considered. This certainty exceeds 99% for all four adult populations, 95% for two children populations, 90% for one children population and one toddler population and 85% for the remaining two toddler populations. For C‐cell hypertrophy, hyperplasia and neoplasia, the same conclusion was drawn with a certainty exceeding 99% for all 10 populations. These populations can be considered as representative of the European populations with the highest vulnerability in terms of potential exposure.
6. Recommendations
Despite the considerable amount of data used to perform it, CRA suffers from important uncertainties. To reduce the impact of these uncertainties, it is recommended to:
With respect to the toxicological assessment:
Include in probabilistic calculations the sources of uncertainty which can be modelled (e.g. CAG membership).
Use Benchmark Dose (BMD) modelling to characterise the active substances included in the CAG for the specific effect.
Consider other recommendations listed in the scientific report on the establishment of CAGs of pesticides for their effects on the thyroid (EFSA, 2019a), in particular regarding thyroid‐mediated developmental neurotoxicity and the regular update of the CAGs.
With respect to the exposure assessment:
Identify commodities not included in the calculations performed in this report which may significantly contribute to the intake of residues and consider incorporating them in future CRAs.
Consolidate the list of processing factors available for CRAs.
Collect information from competent organisations on national authorisations, use statistics of plant protection products and pesticide residues in drinking water, on risk‐based criteria.
Assess the contribution of metabolites to the effects under consideration, through the application of the guidance of the PPR Panel on the establishment of the residue definition for dietary risk assessment (EFSA PPR Panel, 2016).
Furthermore, it is recommended to evaluate the scientific strategy and technical processes used to perform the different steps (hazard characterisation/identification, exposure assessment, risk characterisation and uncertainty analysis) of the reported assessment and identify options for optimization and/or alternative simpler ways to perform future assessments. This includes consideration of the criteria to identify the effects of relevance for CRA and to group substances into CAGs. This may also encompass the evaluation of the magnitude of the impact of assumptions elaborated to compensate missing data or information (e.g. impact of assumptions for left‐censored occurrence data, pesticides in drinking water…). It is recognised that some CAGs may require more or less detailed analysis than the others.
Abbreviations
- ADI
Acceptable Daily Intake
- AOP
Adverse Outcome Pathway
- APS
Approximate Probability Scale
- ARfD
Acute Reference Dose
- BMD
Benchmark Dose
- BMDL
Lower confidence limit of the benchmark dose
- Bw
body weight
- CAG
Cumulative Assessment Group
- CAG‐TCF
CAG for the chronic assessment of hypothyroidism
- CAG‐TCP
CAG for the chronic assessment of C‐cell hypertrophy, hyperplasia and neoplasia
- CRA
Cumulative Risk Assessment
- EKE
Expert Knowledge Elicitation
- ETU
Ethylene thiourea
- EUCP
European Coordinated Programme
- IC
Index Compound
- KE
Key Event
- LOQ
Limit of Quantification
- MCRA
Monte Carlo Risk Assessment (software)
- MIE
Molecular Initiating Event
- MoA
Mode of Action
- MOE
Margin of Exposure
- MOET
Combined Margin of Exposure
- MRLs
Maximum Residue Levels
- MS
Member State
- NOAEL
No Observed Adverse Effect Level
- PPR
EFSA Panel on Plant Protection Products and their Residues
- PTU
Propylene thiourea
- RfP
(toxicological) Reference Point
- RIO
Rational Impartial Observer
- RIVM
(Dutch) National Institute for Public Health and the Environment
- RPC
Raw Primary Commodity
- SC
PAFF Standing Committee on Plants, Animals, Food and Feed
- SSD
(ESFA) Standard Sample Description
- WHO
World Health Organisation
Appendix A – Assessment of the individual sources of uncertainty affecting the cumulative risk assessment for active substances causing hypothyroidism (CAG‐TCF) and C‐cell hypertrophy, hyperplasia and neoplasia (CAG‐TCP)
1.
The ranges for the values of multiplicative factors that would adjust the median estimate of the MOET for CAG‐TCF and CAG‐TCP at the 99.9th percentile of exposure at Tier II were estimated for each source of uncertainty identified in Section 3.1, assuming that it was fully resolved and addressed in the modelling.
These estimations were first conducted for the German population (EKE Q1A), based on information specific to the cumulative exposure of this population (see EFSA 2019b and Van Klaveren et al., 2019). The scale and methods used for this estimation are described in Section 2.3.5. For example: ‘− − −/●’ means at least a 90% chance the true factor is between x1/10 and +20%; ‘++/++’ means ≥ 90% chance between 2x and 5x, etc. These estimations are reported in the fourth column of Tables A.1 and the third column of Table A.2. The estimated ranges were in most cases the same for CAG‐TCF and CAG‐TCP. When this was not the case, this was explicitly indicated.
It was secondly assessed whether the same multiplicative factor would apply to the other populations for which a CRA is performed (EKE Q1B). The reply to this question (Yes/No) is given in the fifth column of Table A.1 and the fourth column of Table A.2. It was ‘yes’ for all sources of uncertainty except one (excluded consumption data).
In the last column of Table A.2, reference is given to notes in Section B.2 of Appendix B which summarise information used to address EKE Q1A and QIB.
Appendix B – Information used in the uncertainty analysis
B.1. Risk drivers
The assessment of the impact of several sources of uncertainty depends on which active substance/commodity combinations contribute the most to the cumulative risk. Therefore, information of interest on the active substances identified as risk drivers was collected and summarised in Tables B.1 and B.2:
Table B.1.
Risk drivers for CAG‐TCF
| Active substance | CAG membership probability | PFs used | Water solubility pH 7 | Log Kow | Residue definitions |
|---|---|---|---|---|---|
| Bromide ion | Almost certain (> 99%) | None | High | N.A. | Monitoring: Risk assessment: |
| Propineb | Almost certain (> 99%) | None | Instable in water (EFSA conclusions 2016) | Instable in water (EFSA conclusions 2016) | Monitoring: CS2 Risk assessment (pending): CS2 X 2.01 (correction for molecular weight) |
| Ziram | Almost certain (> 99%) | None | 1 mg/L (EC review report 2004) | 1.65 (EC review report 2004) |
Monitoring: CS2 Risk assessment: CS2 X 2.01 (correction for molecular weight) |
| Thiabendazole | Almost certain (> 99%) | Orange and mandarin: peeling | 0.03 g/L (EFSA conclusions 2014) | 2.39 (EFSA conclusions 2014) |
Monitoring: thiabendazole Risk assessment: thiabendazole for post‐harvest use (citrus fruits) (MRL review 2016) |
| Pyrimethanil | Moderate: Subgroup 5 | Apple pears: juicing, pulping/mashing; peas without pods: cooking in water | 0.121 g/L (EFSA conclusions 2006) | 3.0 (EFSA conclusions 2014) |
Monitoring: pyrimethanil Risk assessment: pyrimethanil (MRL review 2011) |
| Mancozeb | Almost certain (> 99%) | None | 6 mg/L (EC review report 2009) | 1.33 (indicative, EC review report 2009) |
Monitoring: CS2 Risk assessment: CS2 X 1.78 (correction for molecular weight) |
| Cyprodinyl | Moderate, subgroup 4 | Strawberries: Cooking and similar thermal preparation processes | 13 mg/L (EFSA conclusions 2006) | 4.0 (EFSA conclusions 2006) |
Monitoring: cyprodinil Risk assessment: cyprodinil (MRL review 2013) |
| Chlorpropham | Almost certain (> 99%) | Potatoes: frying | 110 mg/L (EFSA conclusions 2017) | 3.76 (EFSA conclusions 2017) |
Monitoring: chlorpropham Risk assessment: Chlorpropham and 4′‐hydoxychlorpropham (free and conj.) (EFSA conclusions 2017) |
Table B.2.
Risk drivers for CAG‐TCF
| Active substance | CAG membership probability | PFs used | Water solubility pH 7 | Log Kow | Residue definitions |
|---|---|---|---|---|---|
| Thiram | EKE not performed | None | 18 mg/L (EFSA conclusions 2017) | 1.84 (EFSA conclusions 2017) |
Monitoring: CS2 Risk assessment: CS2 X 1.58 (correction for molecular weight) |
| Ziram | EKE not performed | None | 1 mg/L (EC review report 2004) | 1.65 (EC review report 2004) |
Monitoring: CS2 Risk assessment: CS2 X 2.01 (correction for molecular weight) |
B.2. Notes supporting the assessment of individual uncertainties
Note 1 (Contribution of the selected 30 commodities to the overall diet of plant origin)
The contribution of the selected 30 commodities to the overall long‐term diet of plant origin has been calculated for the 30 population groups of the EFSA PRIMo (Revision 3.1) Model (EFSA, 2018b) in Table B.3. In populations of infant/children/toddlers, the selected 30 commodities contribute to 62–96% of the overall diet of plant origin with a median of 80%. In adult populations, this contribution varies from 49% to 94%, with a median of 80.5%. In Germany, for the general population and for the population of women of child‐bearing age, this contribution is 61% and 63%, respectively.
Table B.3.
Contribution (in percent) of the selected 30 commodities to the overall diet of plant origin in population groups of the EFSA PRIMo Model
| Long‐term dieta | Subgroup of population/age group | Mean body weight (kg) | Total average consumption of the selected 30 commodities (g/kg bw per d) | Total average consumption of plant commodities (g/kg bw per d) | Contribution of selected 30 commodities to overall diet of plant origin (%) |
|---|---|---|---|---|---|
| BE adults | 18–64 | 77 | |||
| BG children | 3–5 | 72 | |||
| CZ adults | 18–64 | 77 | |||
| DE child | Children between 2 and 5 years | 16.2 | 33.42 | 38.26 | 87 |
| DE adults | 18–64 | 78 | |||
| DE general | General population | 76.4 | 11.63 | 18.87 | 61 |
| DE women 14–50 years | Women of child‐bearing age | 67.5 | 12.49 | 19.96 | 63 |
| DK adult | 15–74 years | 75.1 | 8.19 | 8.71 | 94 |
| DK toddlers | 1–3 | 83 | |||
| DK child | 4–6 years | 21.8 | 22.25 | 23.08 | 96 |
| ES adult | Adults > 17 years | 68.5 | 10.00 | 12.19 | 82 |
| ES child | 7–12 years | 34.5 | 15.19 | 17.77 | 85 |
| FI adult | Adults | 77.1 | 6.19 | 12.59 | 49 |
| FI child 3 years | Children up to 3 years | 15.2 | 14.44 | 16.73 | 86 |
| FI child 6 years | Children up to 6 years | 22.4 | 11.05 | 13.08 | 84 |
| FR infant | 7–18 months | 9.1 | 8.62 | 11.06 | 78 |
| FR toddler 2–3 years | 25–36 months | 13.6 | 15.47 | 20.87 | 74 |
| FR child 3 to < 15 years | Children from 3 to less than 15 years | 18.9 | 17.36 | 24.88 | 70 |
| FR children | 3–9 | 82 | |||
| FR adult | Adults > 15 years | 66.4 | 9.29 | 12.48 | 74 |
| IE adult | Adults 18–64 years | 75.2 | 14.05 | 25.60 | 55 |
| IE child | 5–12 years | 20.0 | 3.22 | 3.61 | 89 |
| IT adults | 18–65 | 86 | |||
| IT adult | 18–64 years | 66.5 | 9.86 | 12.12 | 81 |
| IT toddler | 1–17 years | 41.6 | 13.44 | 16.42 | 82 |
| LT adult | 19–64 years | 70.0 | 9.57 | 10.20 | 94 |
| NL child | 2–6 years | 18.4 | 23.09 | 37.27 | 62 |
| NL children | 3–6 | 80 | |||
| NL general | General population, 1–97 years | 65.8 | 10.72 | 17.31 | 62 |
| NL toddlers | 2 | 81 | |||
| NL toddler | 8 to 20 months | 10.2 | 40.50 | 60.61 | 67 |
| PL general | General population, 1–96 years | 62.8 | 8.31 | 9.71 | 86 |
| PT general | General population | 60.0a | 18.23 | 20.84 | 87 |
| RO general | General population | 60.0a | 18.08 | 23.13 | 78 |
| SE general | General population, 1–74 years | 60.0a | 16.65 | 19.65 | 85 |
| UK infant | 6 months–1 year | 8.7 | 14.36 | 18.58 | 77 |
| UK toddlers | 1–2 | 83 | |||
| UK toddler | 18 months–4 years | 14.6 | 15.76 | 21.04 | 75 |
| UK adult | 19–64 years | 76.0 | 7.41 | 8.97 | 82 |
| UK vegetarian | No information | 66.7 | 8.69 | 10.80 | 80 |
Shaded rows refer to the 10 population groups selected for modelling cumulative exposure.
Similar calculations were conducted based on the RPC model (EFSA, 2019d) with the survey data of the 10 population groups used in this report and are also reported in Table B.3 as shaded rows. To improve comparability with the calculations conducted for the diets of the EFSA PRIMo diets, sugar plants were excluded. However, considering the extensive processing that is applied to sugar plants, this does not alter the value of this information for the uncertainty analysis. The calculations showed that the contribution of the 30 commodities used for the cumulative exposure assessments to the overall diet of plant origin ranges from 72 (BG children) to 86% (IT adults). The contribution was 78% in the case of the German adult population.
The calculations for the population groups of the EFSA PRIMo model used the entries for mean consumption of individual products, as they were provided by MSs from national food surveys. These data were provided before the RPC model became available. For this reason, there are differences for the calculated contribution of the selected commodities between the population groups used for the cumulative exposure assessments and those reported in PRIMo model, even if derived from the same survey.
Note 2 (Contribution of animal commodities to the pesticide residues intake)
The contribution of animal commodities to the dietary exposure to pesticide residues is expected to be much lower than the contribution of plant commodities, because the occurrence of pesticide residues in animal commodities is less frequent and at lower levels than in plant commodities. Although no direct comparison of chronic dietary intake of pesticide residues from plant and animal commodities is available, the results of the short‐term risk assessments conducted by EFSA for pesticide/crop combinations covered by the 2014, 2015 and 2016 EUCP clearly show much higher acute exposures resulting from the consumption of plant commodities (EFSA 2016a, 2017a, 2018a).
Over this 3‐year cycle, during which liver of ruminants, swine and poultry, poultry meat, butter, chicken eggs, swine meat and cow's milk were part of the EUCP, only chlordane (in poultry meat, swine meat and cow's milk), heptachlor (in poultry meat and swine meat), dieldrin (in butter and cow's milk) and deltamethrin (in cow's milk) were found at level exceeding 1% of the ARfD. Heptachlor and chlordane, which are included in the CAG‐TCF, were present at levels corresponding to 10–25% of their respective ARfDs, which in both cases are based on effects occurring at lower exposure level than hypothyroidism.
Note 3 (Contribution of the selected 30 commodities to the overall intake of pesticide residues)
Based on the occurrence data collected under the 2016 EUCP on pesticide residues, long‐term exposures to residues of individual pesticides were calculated deterministically with the PRIMo model using either the full diet or only the food products (31 food products) covered by the 3‐year cycle of the EUCP (2014, 2015 and 2016 monitoring years). These calculations used samples taken by ‘surveillance’ sampling strategies only and were performed for all the substances included in the 2016 EUCP programme and for which an ADI was available (162 active substances). They were done with two alternative assumptions for samples at the LOQ (upper and lower bound approaches). In the upper bound approach, a level equal to the LOQ was assumed for samples reported below the LOQ, if at least one sample of the respective substance/commodity combination had quantifiable residues. In contrast, when all samples of a substance/commodity combination were reported to be below the LOQ, the contribution of this combination to the total dietary intake was considered as being nil. In the lower bound approach, the assumption was that residues in samples reported to be below the LOQ were in all cases true zeros.
The calculations were conducted with EFSA PRIMo model for all populations included in the model and the results for the population with the highest intake can be found in Table B.4.
Table B.4.
Calculated contribution of the EUCP 31 commodities to total long‐term exposure
| Active substance | Total long‐term exposure (% of ADI) | Long‐term exposure EUCP 31 commoditiesa (% of ADI) | Contribution of EUCP 31 commodities to total long‐term exposure (%) | |||
|---|---|---|---|---|---|---|
| Upper bound | Lower bound | Upper bound | Lower bound | Upper bound | Lower bound | |
| 2‐phenylphenol | 0.30 | 0.22 | 0.29 | 0.21 | 97 | 98 |
| Abamectin (RD) | 3.6 | 0.016 | 3.4 | 0.012 | 95 | 75 |
| Acephate | 0.22 | 0.011 | 0.21 | 0.007 | 95 | 64 |
| Acetamiprid (RD) | 1.2 | 0.31 | 1.0 | 0.24 | 87 | 76 |
| Acrinathrin | 2.6 | 0.17 | 2.6 | 0.17 | 98 | 100 |
| Azinphos‐methyl | 0.15 | 0.000 | 0.15 | 0.000 | 100 | 100 |
| Azoxystrobin | 0.23 | 0.057 | 0.21 | 0.052 | 90 | 91 |
| Bifenthrin | 1.8 | 0.07 | 1.7 | 0.069 | 95 | 99 |
| Biphenyl | 0.03 | 0.015 | 0.02 | 0.000 | 69 | 1 |
| Bitertanol | 1.7 | 0.05 | 1.7 | 0.05 | 100 | 100 |
| Boscalid (RD) | 1.7 | 0.98 | 1.5 | 0.83 | 87 | 84 |
| Bromopropylate | 0.52 | 0.001 | 0.51 | 0.001 | 98 | 100 |
| Bupirimate | 0.40 | 0.017 | 0.39 | 0.017 | 97 | 100 |
| Buprofezin | 2.8 | 0.2 | 2.7 | 0.19 | 95 | 95 |
| Captan (RD) | 0.85 | 0.69 | 0.80 | 0.66 | 94 | 95 |
| Carbaryl | 2.7 | 0.003 | 2.6 | 0.002 | 98 | 72 |
| Carbendazim (RD) | 1.6 | 0.22 | 1.4 | 0.16 | 89 | 75 |
| Carbofuran (RD) | 27 | 1.1 | 26 | 0.017 | 95 | 2 |
| Chlorantraniliprole | 0.02 | 0.003 | 0.02 | 0.003 | 93 | 93 |
| Chlordane (RD) | 34 | 0.066 | 34 | 0.055 | 99 | 84 |
| Chlorfenapyr | 0.68 | 0.029 | 0.61 | 0.010 | 91 | 34 |
| Chlormequat | 2.4 | 2.0 | 1.9 | 1.5 | 78 | 75 |
| Chlorothalonil (RD) | 2.4 | 0.19 | 2.3 | 0.12 | 94 | 65 |
| Chlorpropham (RD) | 3.7 | 3.3 | 3.6 | 3.3 | 99 | 100 |
| Chlorpyrifos | 46 | 12 | 42 | 11 | 91 | 90 |
| Chlorpyrifos‐methyl | 3.6 | 0.77 | 3.5 | 0.70 | 95 | 91 |
| Clofentezine (RD) | 0.98 | 0.009 | 0.95 | 0.009 | 97 | 98 |
| Clothianidin | 0.27 | 0.002 | 0.26 | 0.002 | 97 | 83 |
| Cyfluthrin | 9.4 | 0.049 | 9.1 | 0.037 | 97 | 76 |
| Cymoxanil | 0.38 | 0.002 | 0.38 | 0.002 | 99 | 100 |
| Cypermethrin | 1.4 | 0.18 | 1.3 | 0.15 | 94 | 81 |
| Cyproconazole | 0.59 | 0.004 | 0.56 | 0.003 | 94 | 75 |
| Cyprodinil (RD) | 1.7 | 0.69 | 1.5 | 0.68 | 91 | 99 |
| DDT (RD) | 9.5 | 0.073 | 9.3 | 0.036 | 99 | 49 |
| Deltamethrin | 7.1 | 1.0 | 6.8 | 0.46 | 96 | 45 |
| Diazinon | 22 | 0.90 | 21 | 0.14 | 94 | 15 |
| Dichlorvos | 145 | 2.8 | 145 | 2.8 | 100 | 100 |
| Dicloran | 1.5 | 0.61 | 0.26 | 0.004 | 17 | 1 |
| Dicofol (RD) | 2.9 | 0.006 | 2.9 | 0.006 | 99 | 100 |
| Dieldrin (RD) | 518 | 1.6 | 509 | 0.55 | 98 | 35 |
| Diethofencarb | 0.01 | 0.000 | 0.01 | 0.000 | 100 | 100 |
| Difenoconazole | 3.3 | 0.36 | 2.9 | 0.18 | 88 | 49 |
| Diflubenzuron (RD) | 0.18 | 0.006 | 0.17 | 0.005 | 94 | 89 |
| Dimethoate (RD) – dimethoate assumption | 33 | 2.0 | 30 | 1.6 | 93 | 82 |
| Dimethoate (RD) – omethoate assumption | 101 | 6.1 | 94 | 4.9 | 93 | 82 |
| Dimethomorph | 0.66 | 0.16 | 0.61 | 0.15 | 93 | 98 |
| Diniconazole | 0.15 | 0.000 | 0.15 | 0.000 | 100 | 100 |
| Diphenylamine | 0.29 | 0.038 | 0.28 | 0.038 | 99 | 99 |
| Dithianon | 4.7 | 2.6 | 4.6 | 2.6 | 97 | 97 |
| Dithiocarbamates (RD) – mancozeb assumption | 11 | 2.7 | 10 | 2.2 | 93 | 81 |
| Dithiocarbamates (RD) – maneb assumption | 10 | 2.6 | 9.7 | 2.1 | 93 | 81 |
| Dithiocarbamates (RD) – metiram assumption | 73 | 18 | 67 | 14 | 93 | 81 |
| Dithiocarbamates (RD) – propineb assumption | 76 | 19 | 71 | 15 | 93 | 81 |
| Dithiocarbamates (RD) – thiram assumption | 30 | 7.5 | 28 | 6.0 | 93 | 81 |
| Dithiocarbamates (RD) – ziram assumption | 101 | 25 | 94 | 20 | 93 | 81 |
| Dodine | 0.25 | 0.10 | 0.23 | 0.099 | 93 | 98 |
| Endosulfan (RD) | 0.90 | 0.007 | 0.80 | 0.001 | 89 | 11 |
| Epoxiconazole | 1.3 | 0.022 | 1.3 | 0.021 | 99 | 99 |
| Ethephon | 3.0 | 0.58 | 2.9 | 0.39 | 95 | 68 |
| Ethion | 0.81 | 0.010 | 0.72 | 0.010 | 88 | 98 |
| Ethirimol | 0.46 | 0.006 | 0.45 | 0.006 | 97 | 97 |
| Etofenprox | 0.96 | 0.17 | 0.87 | 0.15 | 91 | 88 |
| Famoxadone | 1.5 | 0.051 | 1.3 | 0.050 | 91 | 98 |
| Fenamiphos (RD) | 6.6 | 0.073 | 5.3 | 0.012 | 80 | 16 |
| Fenarimol | 0.48 | 0.000 | 0.47 | 0.000 | 98 | 88 |
| Fenazaquin | 4.0 | 0.015 | 3.9 | 0.013 | 97 | 87 |
| Fenbuconazole | 3.8 | 0.078 | 3.6 | 0.031 | 94 | 40 |
| Fenbutatin oxide | 0.36 | 0.046 | 0.30 | 0.035 | 84 | 76 |
| Fenhexamid | 0.25 | 0.21 | 0.24 | 0.20 | 97 | 97 |
| Fenitrothion | 3.4 | 0.006 | 3.4 | 0.006 | 100 | 98 |
| Fenoxycarb | 0.33 | 0.004 | 0.31 | 0.004 | 94 | 100 |
| Fenpropathrin | 0.71 | 0.007 | 0.70 | 0.002 | 99 | 23 |
| Fenpropidin (RD) | 0.48 | 0.004 | 0.48 | 0.004 | 100 | 100 |
| Fenpropimorph (RD) | 3.0 | 0.10 | 3.0 | 0.10 | 99 | 97 |
| Fenpyroximate | 2.2 | 0.036 | 2.1 | 0.030 | 95 | 85 |
| Fenthion (RD) | 0.06 | 0.000 | 0 | 0 | 0 | 0 |
| Fenvalerate (RD) | 1.4 | 0.013 | 1.3 | 0.011 | 93 | 82 |
| Fipronil (RD) | 45 | 0.23 | 43 | 0.11 | 96 | 50 |
| Fludioxonil (RD) | 0.35 | 0.30 | 0.18 | 0.098 | 50 | 33 |
| Flufenoxuron | 0.40 | 0.001 | 0.40 | 0.001 | 100 | 100 |
| Fluopyram (RD) | 2.1 | 0.65 | 1.8 | 0.51 | 88 | 79 |
| Fluquinconazole | 11 | 0.018 | 11 | 0.018 | 100 | 100 |
| Flusilazole (RD) | 1.6 | 0.012 | 1.0 | 0.012 | 65 | 98 |
| Flutriafol | 2.5 | 0.016 | 2.3 | 0.011 | 94 | 67 |
| Folpet (RD) | 0.95 | 0.69 | 0.94 | 0.69 | 98 | 100 |
| Formetanate | 1.4 | 0.044 | 1.4 | 0.044 | 100 | 100 |
| Fosthiazate | 3.8 | 0.043 | 3.6 | 0.043 | 97 | 100 |
| Glyphosate | 0.33 | 0.14 | 0.32 | 0.035 | 100 | 25 |
| Heptachlor (RD) | 8.5 | 0.021 | 0 | 0 | 0 | 0 |
| Hexaconazole | 1.8 | 0.043 | 1.7 | 0.043 | 94 | 100 |
| Hexythiazox | 0.85 | 0.021 | 0.80 | 0.020 | 93 | 93 |
| Imazalil | 17 | 16 | 16 | 15 | 94 | 94 |
| Imidacloprid | 0.65 | 0.057 | 0.58 | 0.047 | 89 | 82 |
| Indoxacarb | 4.2 | 0.26 | 3.9 | 0.23 | 92 | 90 |
| Iprodione (RD) | 3.5 | 1.5 | 3.2 | 1.3 | 89 | 87 |
| Iprovalicarb | 0.54 | 0.18 | 0.51 | 0.18 | 93 | 100 |
| Kresoxim‐methyl (RD) | 0.05 | 0.001 | 0.05 | 0.001 | 98 | 80 |
| Lambda‐cyhalothrin (RD) | 11 | 0.72 | 9.9 | 0.61 | 91 | 84 |
| Lindane | 4.4 | 0.003 | 4.4 | 0.003 | 100 | 100 |
| Linuron | 1.7 | 0.26 | 1.6 | 0.19 | 91 | 74 |
| Lufenuron | 1.4 | 0.005 | 1.3 | 0.004 | 99 | 70 |
| Malathion (RD) | 0.80 | 0.012 | 0.78 | 0.011 | 97 | 95 |
| Mandipropamid | 0.08 | 0.015 | 0.08 | 0.012 | 92 | 80 |
| Mepanipyrim | 0.32 | 0.041 | 0.32 | 0.041 | 99 | 100 |
| Mepiquat | 0.17 | 0.050 | 0.16 | 0.047 | 98 | 95 |
| Metalaxyl | 0.22 | 0.031 | 0.19 | 0.031 | 86 | 97 |
| Methamidophos | 4.9 | 0.089 | 4.7 | 0.042 | 96 | 47 |
| Methidathion | 19 | 0.066 | 18 | 0.062 | 98 | 94 |
| Methiocarb (RD) | 0.48 | 0.029 | 0.41 | 0.007 | 85 | 24 |
| Methomyl (RD) | 1.3 | 0.262 | 0.88 | 0.014 | 70 | 5 |
| Methoxychlor | 0.02 | 0.000 | 0 | 0 | 0 | 0 |
| Methoxyfenozide | 0.23 | 0.025 | 0.22 | 0.0248 | 94 | 98 |
| Monocrotophos | 1.7 | 0.033 | 1.7 | 0.033 | 99 | 100 |
| Myclobutanil (RD) | 1.4 | 0.13 | 1.2 | 0.12 | 92 | 94 |
| Oxadixyl | 0.07 | 0.000 | 0.06 | 0.000 | 87 | 44 |
| Oxamyl | 4.3 | 0.40 | 4.3 | 0.006 | 100 | 1 |
| Oxydemeton‐methyl (RD) | 0.09 | 0.000 | 0 | 0 | 0 | 0 |
| Paclobutrazol | 0.59 | 0.005 | 0.58 | 0.005 | 98 | 100 |
| Parathion | 1.7 | 0.002 | 1.7 | 0.001 | 100 | 70 |
| Penconazole | 0.63 | 0.021 | 0.60 | 0.021 | 95 | 100 |
| Pencycuron | 0.04 | 0.004 | 0.04 | 0.002 | 98 | 42 |
| Pendimethalin | 0.23 | 0.002 | 0.22 | 0.002 | 95 | 100 |
| Permethrin | 0.49 | 0.01 | 0.46 | 0.010 | 93 | 95 |
| Phosmet (RD) | 2.9 | 0.24 | 2.7 | 0.24 | 95 | 97 |
| Pirimicarb (RD) | 0.64 | 0.10 | 0.59 | 0.095 | 92 | 93 |
| Pirimiphos‐methyl | 17 | 13 | 15 | 11 | 87 | 91 |
| Procymidone (RD) | 3.4 | 0.014 | 3.0 | 0.008 | 88 | 56 |
| Profenofos | 0.25 | 0.038 | 0.23 | 0.004 | 91 | 10 |
| Propamocarb (RD) | 0.19 | 0.13 | 0.18 | 0.13 | 97 | 98 |
| Propargite | 0.84 | 0.016 | 0.77 | 0.013 | 92 | 79 |
| Propiconazole | 1.2 | 0.59 | 1.1 | 0.54 | 93 | 91 |
| Propyzamide (RD) | 0.04 | 0.002 | 0.03 | 0.002 | 67 | 100 |
| Pymetrozine (RD) | 0.25 | 0.015 | 0.19 | 0.015 | 77 | 100 |
| Pyraclostrobin | 1.0 | 0.31 | 0.93 | 0.27 | 89 | 88 |
| Pyridaben | 2.3 | 0.045 | 2.2 | 0.026 | 95 | 58 |
| Pyrimethanil (RD) | 1.1 | 0.92 | 1.0 | 0.89 | 96 | 97 |
| Pyriproxyfen | 0.23 | 0.013 | 0.22 | 0.011 | 94 | 87 |
| Quinoxyfen | 0.08 | 0.008 | 0.07 | 0.002 | 93 | 24 |
| Spinosad | 0.93 | 0.11 | 0.84 | 0.11 | 91 | 97 |
| Spirodiclofen | 1.4 | 0.14 | 1.3 | 0.14 | 94 | 98 |
| Spiromesifen | 0.64 | 0.047 | 0.62 | 0.044 | 97 | 95 |
| Spiroxamine (RD) | 0.68 | 0.043 | 0.65 | 0.043 | 95 | 100 |
| tau‐Fluvalinate | 5.1 | 0.039 | 4.7 | 0.034 | 93 | 87 |
| Tebuconazole (RD) | 1.4 | 0.23 | 1.2 | 0.14 | 88 | 63 |
| Tebufenozide | 1.2 | 0.074 | 1.1 | 0.074 | 93 | 100 |
| Tebufenpyrad | 2.7 | 0.065 | 2.6 | 0.057 | 94 | 87 |
| Teflubenzuron | 2.0 | 0.006 | 2.0 | 0.006 | 99 | 100 |
| Tefluthrin | 0.69 | 0.018 | 0.61 | 0.008 | 89 | 46 |
| Terbuthylazine | 2.5 | 0.007 | 2.5 | 0.006 | 100 | 96 |
| Tetraconazole | 6.7 | 0.14 | 6.5 | 0.13 | 96 | 96 |
| Tetradifon | 0.33 | 0.000 | 0.33 | 0.000 | 98 | 77 |
| Thiabendazole (RD) | 1.9 | 1.8 | 1.6 | 1.3 | 81 | 73 |
| Thiacloprid | 2.6 | 0.40 | 2.3 | 0.31 | 90 | 77 |
| Thiamethoxam (RD) | 1.1 | 0.032 | 1.0 | 0.021 | 94 | 64 |
| Thiophanate‐methyl (RD) | 0.46 | 0.024 | 0.42 | 0.017 | 91 | 71 |
| Tolclofos‐methyl | 0.13 | 0.002 | 0.12 | 0.002 | 93 | 100 |
| Tolylfluanid (RD) | 0.01 | 0.000 | 0.01 | 0.000 | 79 | 69 |
| Triadimenol (RD) | 0.66 | 0.073 | 0.57 | 0.007 | 87 | 9 |
| Triazophos | 2.3 | 0.34 | 2.0 | 0.34 | 89 | 100 |
| Trifloxystrobin (RD) | 0.28 | 0.045 | 0.25 | 0.040 | 91 | 88 |
| Triflumuron | 1.1 | 0.032 | 1.1 | 0.022 | 96 | 69 |
| Vinclozolin | 0.13 | 0.014 | 0.11 | 0.000 | 90 | 1 |
Apples, aubergines (egg plants), bananas, bean (with pods), bovine liver, broccoli, carrots, chicken eggs, cucumbers, head cabbages, leeks, lettuce, mandarins, olives for oil production, oranges, peaches, pears, peas (without pods), peppers, potatoes, poultry meat, rice, rye, spinach, strawberries, swine meat, milk, table grapes, tomatoes, wheat, wine grapes.
This table shows that, in the lower bound approach, the contribution of commodities covered by the 3‐year cycle EUCP exceeds 80% of the total intake of about 65% of the active substances and is below 20% of the total intake of 9% of active substances. The median value for the contribution of commodities covered by the 3‐year cycle EUCP is 88.3%. In the cases where the contribution of commodities of the EUCP is low, the overall exposure to the respective active substance is also very low (around or below 1% of their ADI).
Regarding CAG‐TCF, the contribution of the commodities under the EUCP to the total exposure ranges from 73% to 100% for the seven active substances identified as risk drivers. Although such information is not available for bromide ion, it results that no major additional contribution is expected from the excluded RPCs for the risk drivers. There are only three active substances included in the CAG on hypothyroidism for which minor commodities (not covered by the EUCP) contribute to at least 50% of the total long‐term intakes include three substances (dicloran, fenbuconazole and vinclozolin). The overall chronic exposure to these active substances is very low, and therefore, the resulting underestimation is only marginal.
Regarding CAG‐TCP, the contribution of the commodities under the EUCP to the total exposure to CS2 is 81%.
In the upper bound approach, the contribution of foods covered the 3‐year cycle EUCP exceeds 80% of the total intake of 92% of the active substances and is below 20% of the total intake for 3% of active substances.
The list of the 31 food products covered by the 3‐year cycle of the EUCP used for the above calculations is not the same as the list of commodities used in the cumulative exposure assessments, but the similarity is sufficient to ensure a reasonable insight into the degree of underestimation of the cumulative risk resulting from the excluded commodities. Indeed:
The cumulative exposure assessments reported in EFSA (2019b) included the contribution of four additional food products of plant origin: courgettes, melons, cauliflower and oats. The inclusion of these four commodities in the calculations presented in Table B.4 would tend to increase the percentages given in the last two columns.
In contrast, they did not include animal commodities while the EUCP programme included six animal commodities (bovine liver, poultry meat, eggs, milk and swine meat). The exclusion of these 5 commodities from the calculations presented in Table B.4 would tend to decrease the percentages given in the last two columns. However, this decrease would be in practice limited because, despite the high consumption of animal commodities by EU consumers, the occurrence rate and levels of pesticide residues in animal commodities are much lower than in plant commodities. Out of the 173 pesticide residues covered by the EUCP, the number of pesticides detected at or above the LOQ in at least one sample of each of the animal matrices were: eggs, 12 pesticides; milk, none; poultry meat, six pesticides; bovine liver, eight pesticides; and swine meat, seven pesticides. Generally, the contribution of these five animal commodities to the total chronic exposure is very marginal. Residues found in animal commodities are essentially chlorinated, obsolete pesticides (DDT, dieldrin, endosulfan, lindane, chlordane, heptachlor hexachlorocyclohexane (alpha and beta) and hexachlorobenzene) and pyrethroids used as veterinary drugs (i.e. antiparasitic agents).
Note 4 (Ambiguity of consumption and occurrence data)
Part B of annex I to Regulation (EC) No 396/2005 defines groups of commodities containing a main product (e.g. tomatoes) and other similar products to which the same MRL applies (e.g. ground cherries, cape gooseberries, cherry tomatoes etc.). Each group has a code number.
The EFSA Standard Sample Description (SSD) (EFSA, 2014b) defines a matrix code ProdCode, derived from the group code number of the Regulation, and requires this code to be used for the sample description in the reporting of occurrence data. For the monitoring data from 2014, 2015 and 2016, a mechanism to differentiate commodities listed in part B of Annex I from the leading commodity was not in place, and therefore, the occurrence data of all commodities of the group were merged and reported under the same code.
The EFSA comprehensive food consumption database contains similar ambiguities and is based on the same level of aggregation of RPCs as for occurrence data. The practical consequence of this is that probabilistic modelling will combine indiscriminately occurrence and consumption data for the different commodities of this group, although the residue profiles and consumption level may differ between these commodities.
The proportion of occurrence data that are as allocated to one of the 30 RPCs in the scope of this CRA, but which are in fact different commodities, is expected to be low (less than 5%, based on rough estimation), but precise information about the exact cases and proportions is not available.
In chronic exposure assessment, as both the occurrence and consumption data are averaged, this source of uncertainty is not expected to have a significant effect. In acute exposure assessments, this may lead to overestimated intakes of pesticide residues in the upper part of the distribution.
Note 5 (Quality check of consumption data)
Food consumption data provided to EFSA are subject to a validation process upon reception (EFSA, 2011). First the food classification is compared to the food descriptions reported by the data provider. Any inconsistency identified is reported to the data provider for confirmation or correction. Furthermore, the amounts of food reported are validated against several maximum limits, which are derived from the food consumption data already available to EFSA. These limits are defined for each food category per eating occasion and per day. If one of these limits is exceeded, the data provider is requested to provide a justification or to correct the amount reported if necessary.
Note 6 (Impact of psychological factors in consumption surveys)
There is not enough information substantiating the impact of psychological factors in collection of food consumption data (e.g. tendency to under‐report unhealthy food and to over‐report healthy food etc.) to draw reliable conclusions.
Note 7 (Population size and sampling variability – Consumption and occurrence data)
With respect to consumption data, the number of subjects in the 10 populations used to perform CRA ranges from 322 (NL, toddlers, 2 years old) to 10,419 (Germany, adults). The Guidance on the use of the comprehensive food consumption database contains a section on the reliability of high percentiles in food consumption. The minimum number of subjects in a population needed to achieve a 95% confidence interval (significance level (α) at 0.05) increases with the percentile to be computed. This is achieved for n ≥ 59 and n ≥ 298 for the 95th or 99th percentiles, respectively (EFSA, 2011). The number of subjects needed to achieve similar statistical robustness at the 99.9th percentile is approximately 3000.
With respect to occurrence data, the number of data (measurements) for each pesticide/commodity combination in the scope of the conducted CRAs varies widely, from zero to several thousands.
Therefore, cases where occurrence data for authorised substance/commodity combinations were poor were identified:
Pesticide/commodity combinations for which no data is available and for which no extrapolation is possible are addressed in Note 10.
Pesticide/commodity combinations with less than 10 occurrence data in the original data set and for which extrapolations were not possible include azadirachtin/olives for oil production, penflufen/peas (without pods) and pyriofenone/oats. These three combinations concern CAG‐TCF only and represents less than 0.3% of the total number of authorised combinations concerned by this CAG.
Pesticide/commodity combinations with less than 10 occurrence data in the original data set and for which extrapolations were possible are addressed in note 14.
The overall sampling variability associated with consumption and occurrence data were quantified by outer loop execution and the resulting confidence intervals can be found in Sections 2.2.2.1 and 2.2.2.2. However, it is acknowledged that bootstrapping performs less well for small data sets (EFSA Scientific Committee, 2018a,b, annex B.11), especially when the focus is on the tail of the variability distribution as is the case here (99.9th percentile). Therefore, to address the additional uncertainty associated with the use of the bootstrap method, given the sample sizes involved in the exposure assessment, model outputs for different populations were compared to look for any indication of influence of sample size on the estimates obtained. It was noted that:
Boxplots of 99.9th percentiles from MCRA bootstrap samples for the different consumer groups show no sign of instability for the groups having smaller numbers of participants in consumption surveys (Figure B.1).
Boxplots of the ratio of the 99.9th percentile to the 50th percentile from MCRA bootstrap samples for the different consumer groups also shows no sign of instability for the groups having smaller numbers of participants in consumption surveys (Figure B.2).
Figure B.1.
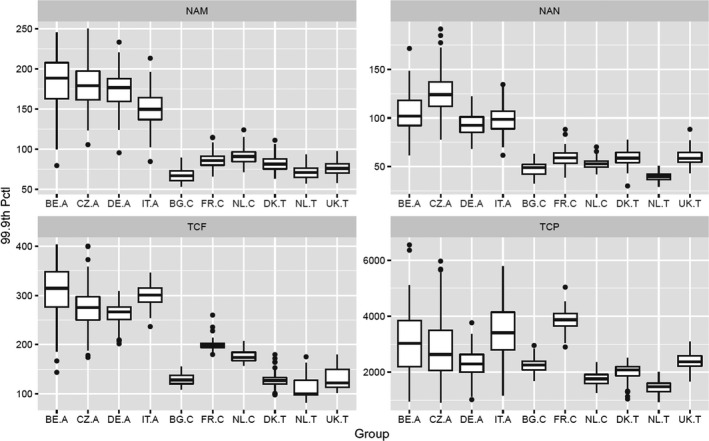
Boxplots of 99.9th percentiles from MCRA bootstrap samples for the different consumer groups. NAM, NAN, TCF and TCP refer to CAG‐NAM, CAG‐NAN,21 CAG‐TCF and CAG‐TCP, respectively
Figure B.2.
Boxplots of the ratio of the 99.9th percentile to the 50th percentile from MCRA bootstrap samples for the different consumer groups. NAM, NAN, TCF and TCP refer to CAG‐NAM, CAG‐NAN, CAG‐TCF and CAG‐TCP, respectively
Overall, this supports the results from the probabilistic models showing estimates of different percentiles which indicate that estimating the 99.9th percentile is not particularly problematic but that estimating the 99.99th percentile is often problematic, giving very similar outcomes as for the 99.9th percentile. The interesting question is why this should be, given the expectation that bootstrapping would perform less well for the smaller consumption surveys. It may indicate that occurrence data are a much bigger driver of uncertainty than consumption data. However, it may also be driven by the fact that for a sample of just 700 values, the median value of the percentile corresponding to the sample maximum is the 99.9th percentile. Even with only 300 values, the median percentile corresponding to the maximum of the sample is the 99.7th percentile.
Note 8 (Representativeness of consumption data)
Biases can arise from a survey sample that does not represent the population group at national level. The EFSA guidance on the use of the comprehensive European food consumption database gives information about the sampling strategy in dietary surveys (EFSA, 2011) for six of the 10 populations used in the present report (Table B.5).
Table B.5.
Sampling information for six dietary surveys included in the EFSA Comprehensive European food consumption database
| Country and name of the dietary survey (Acronym) | Sampling method | Stratification variable (yes/no) | % record according to the day of the week | % of record according to the season | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age | Region | Weekday | Weekend | Spring | Summer | Autumn | Winter | ||
| BE, Diet National 2004 | Random from national population register | Yes | Yes | Yes | 76 | 24 | 26 | 25 | 27 | 23 |
| BG, NUTRICHILD | Random from register of general practitioner's practices | Yes | Yes | Yes | 54 | 46 | 60 | 40 | 0 | 0 |
| CZ, SISP04 | Random from the address register | Yes | Yes | Yes | 74 | 26 | 34 | 23 | 12 | 31 |
| FR, INCA2 | Random from the general population census | Yes | Yes | Yes | 71 | 29 | 20 | 17 | 24 | 39 |
| DE, NATIONAL NUTRITION SURVEY II | Random from national population register | Yes | Yes | Yes | 75 | 25 | 2 | 27 | 40 | 13 |
| IT, INRAN SCAI 2005‐06 | Random from the telephone book | No | No | Yes | 78 | 22 | 26 | 24 | 25 | 25 |
Another factor affecting the representativeness of consumption data is the temporal gap between the period of the surveys and the reference period of the assessment (2014–2016). Depending on the survey, the consumption data used in this CRA were collected from 2001 to 2007. Possible changes in food consumption practices over a period of 10 years needed to be considered. In Netherland, the evolution in food consumption was reported by RIVM by comparing the results of surveys conducted from 2007 to 2010 and from 2010 to 2016.22
Over this period of 5 years, the consumption of cereal products and vegetables was rather stable, while a slight increase of the fruit consumption, including nuts and olives were noted. Decrease in the consumption of potatoes, milk products and meat products were also noted. These observations are supported by the observation of a positive trend in daily fruit and vegetable consumption between 2002 and 2010 by adolescents in 33 countries (Vereecken et al., 2015).
Note 9 (RPC model)
In order to perform cumulative exposure assessments, the new EFSA RPC model (EFSA, 2019d) was used to convert the consumption data for composite foods and RPC derivatives into the equivalent quantities of RPCs.
The main sources of uncertainty of the RPC model result from the following:
Although the FoodEx classification system has been expanded to include intermediate codes, the specificity of the RPC model is still limited by the FoodEx classification system applied in the comprehensive European food consumption database at the time of the model's development. Food consumption data in the comprehensive database have since been updated to include dietary surveys coded with the revised FoodEx2 system (EFSA, 2015b). Meanwhile, RPC consumption data resulting from composite foods that could not be assigned with a more accurate classification code may either be over‐ or underestimated.
The assignment of foods and food components using probabilities introduces an element of uncertainty. Although foods are selected based on the reported consumption records in the food consumption database, a food which is not representative of what was actually consumed may be selected. Some sensitivity tests demonstrated that results obtained through the RPC model may be very variable when low probabilities are considered. This instability was addressed by excluding foods and food components that had probabilities below 10%. This approach increased the reliability of the RPC model. However, the exclusion of certain foods also implies that consumption data for frequently consumed RPCs (e.g. apples) may be slightly overestimated. Likewise, RPCs that are not frequently consumed (e.g. cherries) are likely to be underestimated. In practice, in the present case, as we are using only major commodities, the exclusion or rare food components result in possible overestimation of the consumption/exposure related to the 30 commodities leading to lower MOET than they should be.
The probability table and the disaggregation table do not incorporate inter‐country variation, consumer habits, personal preferences and product or recipe variation. Furthermore, differences between commercial products and household prepared foods are not accounted for. This may lead to either over‐ or underestimations of the RPC consumption data.
As there is currently no harmonised list of reverse yield factors available on either an EU or worldwide level, reverse yield factors sourced in the conversion table of the model may not be accurate. Furthermore, the RPC model uses one single factor for each processing technique. In reality, yields will vary among households and industrial manufacturers. This uncertainty is not expected to have a major impact on average consumption/exposure, but it is expected to underestimate upper tail consumption/exposure.
Populations with the highest consumption of RPC derivatives and composite foods are the most sensitive to this source of uncertainty. The identification of these populations was not conducted for reasons of resources.
Note 10 (Missing occurrence data)
For CAG‐TCF, based on the original occurrence data set (without bootstrapping), the following authorised pesticide/commodity combinations did not have any occurrence data:
Thiencarbazone: olives for oil production, oat, rice, rye, wheat
Penflufen: olives for oil production, rice
Pyriofenone: table grapes
8‐hydroxyquinoline: tomatoes
Topramezone: olives for oil production
Bromide ion: olives for oil production
Mancozeb: olives for oil production
Propineb: olives for oil production
For these cases, it was not possible to extrapolate occurrence data from any other commodities. Therefore, their contribution to the cumulative risk has not been accounted for. This represents 13 active substances/commodity combinations, corresponding to 0.3% of the total number of combinations concerned by the cumulative exposure assessments.
For CAG‐TCP, based on the original occurrence data set (without bootstrapping), all authorised pesticide/commodity combinations were supported by data.
Note 11 (Contribution of metabolites)
The residue definition for monitoring is not always suitable for risk assessment. It is estimated that in 30–40% of cases, the residue definition for risk assessment includes more compounds than the residue definition for monitoring. When this is the case and when enough data are available, a conversion factor is determined to translate residues expressed following the residue definition for monitoring into its counterpart for risk assessment. In the present exercise, the occurrence data were used without any correction for reason of resources, and because the existing residue definition for RA established with respect to the critical effect (e.g. leading to the ADI) is not necessarily valid for the specific effect related to the CAG.
CAG‐TCF: Assuming that the residue definition for risk assessment established with respect to the critical effect would also be valid for hypothyroidism, the residue definition for risk assessment would the same as the respective residue definition for monitoring in all cases but two. The exception is the chlorpropham/potatoes combination, where the residue definition for risk assessment includes 4′‐hydoxychlorpropham (free and conj.) with a tentative conversion factor of 1.85 (EFSA conclusions on chlorpropham, 2017). For propineb, no conclusion could be drawn during the peer review. Pending submission of additional information, propineb‐DIDT might be included in the residue definition for risk assessment (See Table B.1).
The impact of potential degradation of mancozeb, maneb and metiram into ETU and of propineb in PTU under processing on the MOET at 99.9th percentile of exposure was investigated with a sensitivity analysis where it was assumed that all residues of these substances were totally degraded into ETU or PTU in food products derived from a process involving a heating step. The results are given in Table B.6:
Table B.6.
Sensitivity analysis testing the potential degradation of mancozeb, maneb and metiram into ETU and of propineb in PTU under processing involving a heating step
| Country | Population class | 99.9th Percentile Tier II SAS® | 99.9th Percentile Tier II SAS® assuming degradation into ETU and PTU |
|---|---|---|---|
| Belgium | Adults | 314 [176–388] | 248 [145–312] |
| Czech Republic | Adults | 275 [183–366] | 227 [127–281] |
| Germany | Adults | 267 [208–297] | 215 [171–244] |
| Italy | Adults | 301 [259–334] | 266 [206–294] |
| Bulgaria | Other children | 128 [110–152] | 97.6 [86.3–108] |
| France | Other children | 199 [184–221] | 145 [131–165] |
| Netherlands | Other children | 174 [158–199] | 139 [119–159] |
| Denmark | Toddlers | 127 [99.4–164] | 95.7 [69.8–137] |
| Netherlands | Toddlers | 100 [84–161] | 67.4 [51.6–133] |
| United Kingdom | Toddlers | 122 [103–176] | 111 [88.3–134] |
This sensitivity analysis shows that the MOET can decrease by about 30% due to the transformation of dithiocarbamates into ETU and PTU. This is, however, to be considered as a worst case, which does not assume any loss during the processing.
CAG‐TCP: Assuming that the residue definition for risk assessment established with respect to the critical effect would also be valid for C‐cell hypertrophy, hyperplasia and neoplasia, the residue definition for risk assessment of the two risk drivers is the same as the respective residue definition for monitoring (after adjustment for molecular weight differences).
Note 12 (laboratory analytical uncertainty)
The guidance on the use of the EFSA SSD (EFSA, 2014b) provides official laboratories in MSs with a standardised model for the reporting of harmonised data on analytical measurements of chemical substances occurring in food, feed and water.
It provides that laboratories have to always analyse and quantify the residue according to the harmonised EU residue definition which is available from the pesticide legislation in Annexes II and III of Regulation (EC) No 396/2005, even for those residues for which an unspecific residue definition applies. In reporting the results, and for the sake of comparability of data, the analytical uncertainty shall not be taken into account, but can be reported as a separated information under a dedicated field of the reporting model.
The Guidance document of the European Commission on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed proposes a default measurement uncertainty of 50% (corresponding to a 95% confidence level and a coverage factor of 2), calculated from EU proficiency tests (European Commission, 2017a). In general, this 50% value covers the interlaboratory variability between the European laboratories and is recommended to be used by regulatory authorities in cases of enforcement decisions (MRL exceedances).
Note 13 (sampling strategy and representativeness of occurrence data)
Various sampling strategies are used by MSs (objective sampling, selective sampling, suspect sampling, convenient sampling and census). These types of sampling are described in the EFSA Guidance on the use of the EFSA SSD (EFSA, 2014b). To perform the CRAs reported in the present document, EFSA and RIVM used samples collected under the official monitoring programmes of MSs for years 2014, 2105 and 2016 and coded following sampling strategies ST10A or ST20A. The sampling strategies corresponding to these codes are defined as follows:
ST10A (objective sampling): Strategy based on the selection of a random sample from a population on which the data are reported. Random sample is a sample which is taken under statistical consideration to provide representative data.
ST20A (selective sampling): Strategy based on the selection of a random sample from a subpopulation (or more frequently from subpopulations) of a population on which the data are reported. The subpopulations may or may not be determined on a risk basis. The sampling from each subpopulation may not be proportional: the sample size is proportionally bigger for instance in subpopulations considered at high risk.
Under the selective sampling strategy, it is common that some food products, production methods, producers or countries are more targeted than others, and this affects the overall representativeness of the monitoring data. Information about the sampling strategy at MS level can be found in the EFSA technical reports compiling the yearly national summary reports of MSs (EFSA 2016b, 2017b, 2018c).
Although a representative sampling of occurrence data includes lots of commodities pertaining to various distribution channels (e.g. products for local consumption, bioproducts…) and a representative survey of consumption data includes consumers adhering to the respective distribution channels, occurrence data are pooled and averaged before being combined with consumption data, losing therefore existing relationships. This was considered and it was concluded that this has a very minor impact at overall population level, especially at the percentile of interest of the cumulative exposure distribution.
Note 14 (extrapolation of occurrence data between crops)
Crop to crop extrapolation of occurrence data was conducted between specific pairs of commodities as foreseen in the guidance document SANCO 7525/VI/95 (European Commission, 2017b), for cases where the last application of the pesticide takes place after forming of the edible part of the crop, when the number of occurrence data for the ‘minor’ commodity was less than 10. The conditions for extrapolation were supposed to be met when the MRLs were the same for the two crops and when the use of the pesticide was authorised on both crops.
For CAG‐TCF, based on the original data set, occurrence data were extrapolated for thiencarbazone in wine grapes (from table grapes), penflufen in aubergines (from tomatoes) and cauliflower (from broccoli), and for bromide ion in wine grapes (from table grapes). This represents four active substances/commodity combinations, corresponding to 0.1% of the total number of combinations, and 0.3% of the authorised combinations concerned by the exposure assessments.
For CAG‐TCP, based on the original occurrence data set (without bootstrapping), all authorised pesticide/commodity combinations were supported by data and no extrapolations were carried out.
Note 15 (Pooling of occurrence data from all EU MSs)
Occurrence data from all countries were pooled into one single data set that was used to calculate the cumulative risk for the 10 populations. This was done to increase the statistical robustness of the outcomes. Although this leads to losing the country specificity of the residue concentration in commodities, this is not considered to be a major issue since most of the EU population is purchasing and consuming a mixture of local and imported commodities that is drawn from, and similar to, the mixture that is represented by the single data set with pooled occurrence data (‘common market’).
It should be noted that samples analysed and reported in a national monitoring programme are not only taken from lots intended for the internal market (local produce or imported commodities), but also from lots which are in transit or intended for export. This makes very difficult to make national risk assessments based on occurrence data reflecting exactly the residue level in commodities consumed in this country. This would require reporting specific information and/or applying specific data extraction.
Note 16 (Unspecific residue definitions for monitoring)
In tier II, in the absence of information related to the use frequency of pesticides, occurrence data for unspecific residue definition for monitoring were randomly allocated to one of the active substances included in the residue definition and authorised to be used on the respective commodity. All details of the implementing procedure are given in Appendix A of EFSA (2019b).
The residue definitions for monitoring concerning the active substances included in CAG‐TCF and CAG‐TCP are given annexes A.2.03 and A.1.03, respectively, of EFSA (2019b).
CAG‐TCF: The unspecific residue definitions which include at least one active substance included in the CAG are the following:
Benalaxyl including other mixtures of constituent isomers including benalaxyl‐M (sum of isomers).
Captan/Folpet (sum).
Clethodim (sum of Sethoxydim and Clethodim including degradation products calculated as Sethoxydim).
Dazomet (Methylisothiocyanate resulting from the use of dazomet and metam).
Dithiocarbamates (dithiocarbamates expressed as CS2, including maneb, mancozeb, metiram, propineb, thiram and ziram).
Haloxyfop (Sum of haloxyfop, its esters, salts and conjugates expressed as haloxyfop (sum of the R‐ and S‐isomers at any ratio)).
MCPA and MCPB (MCPA, MCPB including their salts, esters and conjugates expressed as MCPA)
Quizalofop (including Quizalofop‐P).
Thiamethoxam (sum of thiamethoxam and clothianidin expressed as thiamethoxam).
Triadimefon and triadimenol (sum of triadimefon and triadimenol).
This source of uncertainty is relevant for three risk drivers:
Propineb (apples and wine grapes).
Ziram (apples and wine grapes).
Mancozeb (orange).
All these substances fall under the same residue definition.
In apples, the following active substances are authorised: mancozeb, maneb, metiram, propineb, thiram, ziram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 16.7% per substance.
In wine grapes, the following active substances are authorised: mancozeb, maneb, metiram, propineb, thiram, ziram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 16.7% per substance.
In oranges, the following active substances are authorised: mancozeb only. All occurrence data were therefore allocated to mancozeb.
In a first cumulative exposure assessment performed with unfinalised CAGs (van Klaveren, 2017), sensitivity analyses conducted in Tier 1 showed an increase by a factor 3 of the MOET from worst‐case (all occurrence data allocated to the most potent authorised substance) to best‐case (all occurrence data allocated to the less potent authorised substance) assumptions for the residue definition.
CAG‐TCP: The unspecific residue definitions which include at least one active substance included in the CAG are the following:
Captan/Folpet (sum).
Dithiocarbamates (dithiocarbamates expressed as CS2, including maneb, mancozeb, metiram, propineb, thiram and ziram).
This source of uncertainty is relevant for the two risk drivers:
Thiram (apples, pears, peaches, table and wine grapes, strawberries and lettuce).
Ziram (apples and wine grapes).
In apples, the following active substances are authorised: mancozeb, maneb, metiram, propineb, thiram, ziram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 16.7% per substance.
In pears, the following active substances are authorised: mancozeb, maneb, metiram, propineb, thiram, ziram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 16.7% per substance.
In peaches, the following active substances are authorised: mancozeb, thiram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 50% per substance.
In table grapes, the following active substances are authorised: mancozeb, maneb, metiram, propineb, thiram, ziram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 16.7% per substance.
In wine grapes, the following active substances are authorised: mancozeb, maneb, metiram, propineb, thiram, ziram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 16.7% per substance.
In strawberries, the following active substances are authorised: thiram. Therefore, all occurrence data were allocated to thiram.
In lettuce, the following active substances are authorised: mancozeb, metiram, thiram. Therefore, the occurrence data were randomly allocated to these substances, with a proportion of 33.3% per substance.
In the first cumulative exposure assessment performed with unfinalised CAGs (van Klaveren, 2017), sensitivity analyses conducted in Tier 1 showed that replacing worst‐case assumption (all occurrence data allocated to the most potent authorised substance) by best‐case (all occurrence data allocated to the most potent authorised substance) assumption for the residue definition has no effect on the MOET at 99.9th percentile. However, thiram was not in the CAG and the assumed authorised uses for ziram were different (only one use on peaches.)
Note 17 (Authorisation status of pesticide/commodity combinations)
In the absence of country‐specific information on authorised used of pesticides, it was assumed that an authorisation exists in all EU countries for an active substance/commodity combination when a use has been reported to EFSA in the context of article 12 and subsequent article 10 reasoned opinions. For active substances not reviewed yet under article 12, an authorised use was assumed in commodities for which the MRL in place on 31 December 2016 was above the LOQ (See sections 2.3.1 and 2.3.2 of EFSA (2019b)). This source of uncertainty is only affecting the treatment of occurrence data below the LOQ, i.e. the decision to consider or not a residue present at a level equal to 1/2 LOQ.
The consequence might be an overestimation of the risk when an authorisation for an active substance/commodity combination exists in certain MSs, but not in all MSs.
The consequence might also be an underestimation of the risk when the assumption of the authorisation status is based on the MRL in place because authorised uses are not necessarily resulting in MRLs above the LOQ. The relevance of this source of uncertainty is, however, depending on the toxicological potency of the active substance: if a child with a body weight of 20 kg consumes within one day 200 g of a commodity containing 0.01 mg/kg (common LOQ level) of a substance with an NOAEL of 0.1 mg/kg body weight (bw) per d, the MOE associated with the intake of this substance would still be 1,000.
In CAG‐TCF, one substance only has an NOAEL for hypothyroidism below 0.1 mg/kg bw per day: fipronil. This substance was, however, reviewed under article 12, and therefore, reliable information on its authorised use was available to EFSA. In CAG‐TCP, all substances have an NOAEL for C‐cell hypertrophy, hyperplasia and neoplasia above 0.1 mg/kg bw per day.
Note 18 (Use frequency of pesticides)
Statistics on the use frequency of pesticides in crops are not available to EFSA.
Therefore, the proportion of the samples of a commodity which might have been treated, and for which residues might be present below the LOQ, needs to rely entirely on assumptions. The European Commission and MSs have defined the assumptions to be made in tiers I and II of probabilistic modelling (European Commission, 2018). All details of the implementing procedure are given in Appendix C of EFSA (2019b).
This source of uncertainty has been subject to sensitivity analyses in the EFSA report on cumulative assessment of the chronic dietary exposure to pesticides that affect the thyroid using SAS® software (EFSA, 2019b). These sensitivity analyses are briefly summarised in Section 2.3.1.
Note 19 (Drinking water)
EFSA does not have access to monitoring data on pesticides in drinking water. Therefore, assumptions were used, which are based on Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. This regulation sets an MRL of 0.1 μg/L to each individual pesticide, and of 0.5 μg/L to the sum of all individual pesticides detected and quantified. In tier I, it was assumed that the five most potent pesticides of the CAG were at a level of 0.1 μg/L. This corresponds to the worst exposure possible complying with the legal provisions. In tier II, it was assumed that the five most potent pesticides of the CAG were at 50% of the allowed level (0.05 μg/L).
In a first cumulative exposure assessment performed with unfinalised CAGs (van Klaveren, 2017), sensitivity analyses were conducted in Tier I and did not show any significant difference between worst‐ (residues of the five most potent substances of the CAG present at 0.1 μg/L) and best‐case (no residue present in drinking water) assumptions for drinking water.
Note 20 (Missing information about the effect of processing)
When consumption data are reported for the raw agricultural commodity, information on the type of processing that was applied prior to consumption is not always available and therefore remains unknown to EFSA. This is particularly true for household processes such as peeling and washing. Furthermore, even when that information is available, processing factors are not available for all active substances and all types of industrial or household processing. Hence, in the absence of processing factors or information on the type of processing, it was assumed in the model that all residues in the raw commodity are transferred to the processed commodity and will reach the end consumer.
This source of uncertainty was subject to sensitivity analyses in EFSA (2019b), which are briefly summarised in Section 2.3.1. In average, assuming that residues will not be present in any processed food when a processing factor is not available increased the MOET at 99.9th percentile of the exposure by factors ranging from 2.3 to 5.7 and from 1.3 to 2.6 for CAG‐TCF and CGA‐TCP, respectively. In practice, the actual transfer to processed commodities will result from many factors and properties of the active substance, including their capacity of being absorbed and translocated in plants, hydrolysis stability, solubility in water, partition coefficient…
Information regarding the availability of processing factors for risk drivers, as well as about their properties, is available in Section B.1.
Although not related to the effect of processing, it is known that residue levels decline between the market distribution and the time of consumption. The impact of this decline is an overestimation of the risk, which was considered and estimated to be minor compared to the effect of missing information on the effect of processing and to the effect of washing and peeling (note 24). There are theoretical reasons to this: this decline is governed by photolysis, volatilisation and to some extent to chemical degradation, but these processes start directly after treatment in field. When they are major degradation/dissipation routes (e.g. volatility of dichlorvos), residues decline shortly after harvest and are low at any other point of the distribution channel and later at point of consumption. When the substance is more stable, these processes are expected to play a minor role and to be much less efficient than industrial or household processing with hydrolysis conditions involving heating or simple physical treatments such as fractionation of commodities, peeling or washing. Collecting factual information on this source of uncertainty would be cumbersome, due to the complexity of this phenomenon, its substance specificity and the multiple influencing factors.
Note 21 (Use of processing factors under the EFSA food classification and description system)
The new database of processing techniques and processing factors compatible with the EFSA food classification and description system FoodEx 2 (Scholz, 2018) defines how the information from regulatory processing studies can be used within the EFSA food classification and description system, and the inherent limitations. The accompanying report23 describes the underlying methodological approach and rationales.
Note 22 (Accuracy of processing factors)
Processing factors are calculated as the ratio between the residue concentrations in the processed commodity and in the RAC. A new database has been produced (Scholz, 2018). When residues in the processed commodity are below the LOQ, the calculation assumes as worst case that the actual residue concentration in the processed commodity is equal to the LOQ, and in this case, the calculated processing factor represents a maximum value. When residues in the raw commodity are below the LOQ, the calculation assumes as best‐case scenario that the actual residue concentration in the raw commodity is equal to the LOQ, and in this case, the calculated processing factor represents a minimum values. In such case, the processing factor was not considered reliable in the processing factor database and therefore not considered in the calculations.
The new database of processing factors (Scholz, 2018) identifies these cases by using ‘<’ and ‘>’ signs.
This source of uncertainty has a limited impact because the overall amount of processing factors used in the exposure calculations, especially regarding risk drivers is low.
Note 23 (Use of a fixed value for processing factors)
Only one value of processing factor is used for each pesticide/commodity/processing type, corresponding to the median of the distribution of values derived from the available processing studies considered as reliable or indicative by Scholz (2018). Information on the number of independent trials performed to determine processing factors and individual results can be found in Scholz (2018).
Note 24 (Effect of washing and peeling of commodities)
The effects of peeling and washing on pesticide residue levels for fruits and vegetables with edible peel and which are consumed raw is not normally considered in deterministic risk assessments because the default worst‐case assumption used is that these commodities may also be consumed unwashed including the peel. Consequently, the available processing factors for peeling and washing of commodities with edible peel are not included in the standard regulatory data set of processing factors (Scholz, 2018). In the absence of these processing factors, it was assumed in all cases that all residues in the raw commodity are transferred to the commodity as eaten, even if it is washed or peeled. This assumption leads to an overestimation of the levels of pesticide residue in the exposure assessment. For fruits and vegetables which are mainly consumed cooked, the effect of washing is covered by the available processing factors for cooking techniques.
Information on the effects of washing of fruits and vegetables on pesticide active substance residue levels from published literature was combined and quantified in a meta‐analysis review (Keikotlhaile et al., 2010); however, the analysis did not distinguish different types of active substances or different commodity types, and therefore, only a generalised conclusion can be drawn. It was reported that overall, washing leads to a combined reduction of pesticide residue levels by a weighted mean response ratio of 0.68.
Information from published literature on the effects of washing and peeling was recently reviewed for specific identified pesticide/commodity combinations (Chung, 2018). A correlation between water solubility of the active substance and pesticide decrease after washing could not be observed. The reduced effect of washing on residue levels for some pesticide/commodity combinations was reported to be attributed to penetration of active substances into the waxy surface of some fruits or translocation of the active substance into plant tissues. It was reported that the partition coefficient (Kow) of active substances may be an indicative factor of the residues partitioning into the waxy surface of some fruits, although a correlation with pesticide decrease after washing was not demonstrated. The time after pesticide spray application was reported to be a contributing factor for a variety of crops, with the decline in time in the proportion of residues reduced by washing being attributed to translocation of residues deeper into the crop surface. The mode of action in terms of whether an active substance is systemic or non‐systemic (contact) was one of several factors used to explain the differences in processing factors for various household processing conditions, including washing and peeling, for various pesticide/commodity combinations.
The effect of peeling and washing on residue levels is not normally reported in EFSA Conclusions or EFSA Article 12 MRL Reviews. However, this information is sometimes available as supplementary information in the EU evaluation DRARs and in JMPR evaluations. For the active substance/commodity combinations identified as risk drivers for CAG‐TCF and CAG‐TCP, these sources were consulted to retrieve reported processing factors for peeling and washing for commodities with edible peel which are consumed raw. The findings are summarised in Tables B.7 and B.8, which also includes information available in published literature. The processing factors for peeling and washing are not considered for active substances where the mode of action is reported to be systemic.
Table B.7.
Processing factors (PF) for peeling and washing commodities with edible peel consumed raw for active substance/commodity combinations identified as risk drivers for CGA‐TCF
| Active substance | Systemic/contact pesticide | Risk driver commodities with edible peel consumed raw | Contribution to the MOET | PFs washing | PFs peeling |
|---|---|---|---|---|---|
| Bromide ion | Systemic | Tomatoes | > 5% | Not considered where the mode of action is reported to be systemic | Not considered where the mode of action is reported to be systemic |
| Propineb | Contact action with protective properties and long residual activity (IUPAC) | Apples | > 5% |
DRAR Italy, 2016 Apple, washed fruit Number of studies: 2 Propineb (determined as CS2) Proposed median processing factor (PF): < 0.37 Propineb (determined as PDA) Proposed PF: 0.46 PTU: Proposed PF: 0.46 (provisional). Data gap PTU residues where the samples are analysed within a time frame where PTU residues are demonstrated to be stable JMPR Evaluation, 2004 Apple, washed fruit Number of studies: 3 Propineb (determined as CS2) median processing factor (PF): < 0.4 Propineb (determined as PDA) median processing factor (PF): 0.4 PTU: Processing Factor could not be calculated as the residues were below the LOQ |
DRAR Italy, 2016 Apple, peeled fruit Number of studies: 2 Propineb (determined as CS2) Proposed median processing factor (PF): < 0.03 Propineb (determined as PDA) Proposed PF: 0.07 PTU: Proposed PF: 0.02 (provisional). Data gap PTU residues where the samples are analysed within a time frame where PTU residues are demonstrated to be stable JMPR Evaluation, 2004 No data available |
| Ziram | Contact action with protective properties (IUPAC) | Apples | > 5% |
DRAR Italy, 2018 Apple, washed fruit: processing studies available, no processing factor proposed JMPR Evaluation 1996 No data available |
DRAR Italy, 2018 No data available JMPR Evaluation 1996 No data available |
Table B.8.
Processing factors (PF) for peeling and washing commodities with edible peel consumed raw for active substance/commodity combinations identified as risk drivers for CAG‐TCP
| Active substance | Systemic/contact pesticide | Risk driver commodities with edible peel consumed raw | Contribution to the MOET | PFs washing | PFs peeling |
|---|---|---|---|---|---|
| Thiram | Contact action with protective properties (IUPAC) | Apples | > 20% |
DRAR France, 2015 Apple, washed fruit Number of studies: 3 Thiram (determined as CS2) individual transfer factors (PF): 0.58; 0.79; 1.38 JMPR Evaluation 1996 No data available |
DRAR France, 2015 Apple, peeled fruit Indicative information from metabolism study Number of studies: 1 Residue in Peel: 37.7% (1.135 mg eq./kg) Residue in Pulp: 59.6% (1.792 mg eq./kg) Indicative transfer factor: 0.6 (apple, pulp) JMPR Evaluation 1996 No data available |
| Strawberries | > 20% |
DRAR France, 2015 Strawberry, washed fruit Number of studies: 1 Thiram (determined as CS2) individual transfer factor (PF): 0.49 Thiram (determined as specific method) individual transfer factor (PF): 0.71 Metabolite M1 (2‐(dimethylamino)‐4,5‐dihydro‐1,3‐thiazole‐4‐carboxylic acid) individual transfer factor (PF): 0.43 JMPR Evaluation 1996 No data available |
Not relevant | ||
| Pears | > 10% |
DRAR France, 2015 No data available JMPR Evaluation 1996 No data available |
DRAR France, 2015 No data available JMPR Evaluation 1996 No data available |
||
| Peaches | > 20% |
DRAR France, 2015 No data available JMPR Evaluation 1996 No data available |
Not relevant | ||
| Table grapes | > 10% |
DRAR France, 2015 No data available JMPR Evaluation 1996 No data available |
Not relevant | ||
| Lettuce | > 10% |
DRAR France, 2015 No data available JMPR Evaluation 1996 No data available |
Not relevant | ||
| Ziram | Contact action with protective properties (IUPAC) | Apples | > 5% |
DRAR Italy, 2018 Apple, washed fruit: processing studies available, no processing factor proposed JMPR Evaluation 1996 No data available |
DRAR Italy, 2018 No data available JMPR Evaluation 1996 No data available |
Information on the occurrence of peeling and washing prior to consumption of commodities with edible peel which are consumed raw is not available to EFSA.
Note 25 (Active substances missing from the CAGs)
If the CAG does not contain ASs contributing to the risk, the outcome of the risk assessment might be underestimated.
Four hundred and twenty‐two active substances were under the scope of the initial data collection (EFSA, 2019a). These substances were all substances approved until 31 May 2013 and additional non‐approved present in the EU consumer's diet as evidenced in the 2011 annual report on the Rapid Alert System for Food and Feed (European Commission, 2011) and/or in the 2010 annual report on pesticide residues in food (EFSA, 2013). However, from one year to the other, different non‐approved may be found by EU control laboratories. It is therefore likely that substances affecting the thyroid, but not in CAG‐TCF or CAG‐TCP, were present in food. This is, however, concerning substances with very low level of occurrence and intake.
There is also some probability that ASs causing hypothyroidism or C‐cell hypertrophy, hyperplasia and neoplasia might have not been identified during the data collection procedure (possibility that information of relevance in original toxicological studies is omitted or misreported in summary documents used as source of information) or during the assessment of collected data.
Based on a check of the information collected to establish the CAG on hypothyroidism, four active substances were identified as meeting the conditions of being included in the CAG, but were not included: cyhalofop‐butyl, lenacil, picloram and oxadiargyl. Three of them are approved. Oxadiargyl is not approved. Only lenacil was found at levels above the LOQ from 2014 to 2016 (39 cases for 91,814 determinations). The other three substances were never found at levels above the LOQ during the same period (20,946, 15,558 and 33,542 determinations, for cyhalofop‐butyl, picloram and oxadiargyl, respectively).
Note 26 (Active substances wrongly assigned to CAGs)
If an active substance, not causing the effect, is included in the respective CAG, the cumulative exposure and risk will be overestimated.
Regarding CAG‐TCF, Section 4.2.1 of the scientific report on the establishment of CAGs for the effects of pesticides on the thyroid provides an estimation of the number of active substances in the CAG which are actually causing hypothyroidism (EFSA, 2019a). The median estimate for this number was 71 ASs (55% of the ASs in the CAG), with a 90% confidence interval of 65–77 ASs (51–60%, see Figure 2).
Based on further details of the assessment of CAG membership concerning the risk drivers identified after exposure assessment, it was considered that this source of uncertainty did not affect the MOET at 99.9th percentile of exposure by more than 20%. The reasons are:
Three risk drivers (thiabendazole, mancozeb and ziram) are in subgroup 1 (almost certain that they cause hypothyroidism).
Two risk drivers (propineb and bromide) have a known MoA, and therefore considered as actually causing hypothyroidism, despite not being included in subgroup 1.
Multiple lines of evidence are available for chlorpropham (included in subgroup 2).
Fewer lines of evidence are available for cyprodinil (subgroup 4) and pyrimethanil (subgroup 5), but these are less prominent risk drivers.
The assessment of CAG membership was not conducted for CAG‐TCP.
Regarding CAG‐TCP, one active substance (bixafen), initially included in this CAG, was withdrawn from the CAG after consideration of the comments submitted during the public consultation on the draft EFSA scientific report on the establishment of CAGs of pesticides for their effects on the thyroid. As the exposure calculations had already been conducted, this caused an overestimation of the risk (nevertheless of low magnitude, considering the potency of bixafen and its detection rate in commodities (from 0.02% to 0.07%).
Information on CAG membership of each risk driver is given in Section B.1.
Note 27 (Uncertainties regarding the NOAEL‐setting)
There are uncertainties affecting the characterisation of active substances included in the CAG. NOAELs can be either under‐ or overestimated.
The robustness of the establishment of the NOAELs in the successive steps of the data collection and assessment may be affected by several factors: initial dossier quality/completeness, variation in the interpretation or analysis of raw data by laboratories performing guideline studies and/or regulatory reviewers, transfer of information from the original toxicological studies to the source documents (DARs, JMPR evaluations), transfer of information from the source documents to the excel spreadsheets and principles and expert judgement used in the NOAEL‐setting. These different phases of the process are described in the EFSA scientific report dealing with the establishment of CAGs for the thyroid.
In general, the database of active substances is complying with the EU regulation and long‐term studies, which are the most appropriate to capture effects on the thyroid, are available. For risk drivers, it is noted that the database for bromide ion is limited to 90‐day rat studies. Under Directive 91/414, 90‐day and 2‐year studies with histopathological investigation and thyroid weight measurements were already required. The most sensitive indicator was used define the NOEAL. Information from different studies were combined when possible. In four cases, an LOAEL was used to derive an NOAEL using an UF of 10. This concerned the active substances chlordane, dicloran, lufenuron, trifluralin, but none of these emerged from the cumulative exposure assessment as a risk driver. Two substances were left uncharacterised in CAG‐TCF because only hormonal alteration was observed (prothioconazole and pyridate).
Another significant weakness of scientific nature is the fact that the current practice consists in using NOAELs for the toxicological characterisation of pesticides. The NOAEL‐setting is influenced by several factors including group size, between‐animals variability (strains, sex), experimental errors, and, importantly, dose spacing in toxicological studies. As this relies on one single point (i.e. a single experimental dose), when the doses are widely spaced in relation to the slope of the dose–response curve, important differences can be observed in the NOAEL across experiments with varying dose levels.
Furthermore, as the NOAEL is a dose level where no statistically significant differences in response are observed compared with background response, it cannot be considered as a ‘no adverse effect dose’. In the update of the guidance of the Scientific Committee on the use of the benchmark dose approach in risk assessment (EFSA Scientific Committee, 2017), it was found that the size of the estimated effect at NOAEL is, on average over a number of studies, close to 10% (quantal responses) or 5% (continuous responses). For this reason, the EFSA scientific Committee proposed, for animal studies, that a default benchmark response value of 5% (change in mean response) be used for continuous data and 10% (extra risk24) for quantal data when toxicological reference values are established by BMD modelling. This was considered to define the meaning of ‘perfect information’ in the EKE Q2 with respect to uncertainties relating to hazard characterisation.
Note 28 (Adequacy of the dose‐addition model)
The rationale behind the use of dose addition has been given by the PPR panel in its opinions on the establishment of CAGs (EFSA PPR Panel, 2013a) and on the relevance of dissimilar modes of action (EFSA PPR Panel, 2013b).
Adequacy of the dose addition model as the default assumption was, amongst other aspect of CRA, recently investigated in the EuroMix collaborative EU research project. Although toxicity to the thyroid was not addressed specifically, the results of a range of bioassays for steatosis, craniofacial malformations and endocrine‐related effects were in agreement with the dose addition model. This applies to test mixtures containing substances eliciting the common adverse effect through both, similar and dissimilar modes of action. Confidence intervals of the dose–response curves for the mixtures overlapped with those of the single substances when all were scaled to the IC using relative potency factors. Confirmatory evidence was drawn from the comparison of relative potency factors calculated based on tests with the substance alone or in combination with the IC (Final (3rd) periodic Technical Report Part B, EuroMix Collaborative Project H2020‐SFS‐2014‐2). As part of the EuroMix project, an Expert Panel Meeting was organised involving eight EU and four non‐EU scientists on 16–18 April 2019 at WHO, Geneva. The Panel agreed that the available information supports the application of the dose addition assumption for risk characterisation of chemicals of an established group or of those with sufficient similarity to that group also when there are differences in the molecular initiating events (MIEs) or some of the key events (KEs) in the respective adverse outcome pathways (AOPs) of those substances (FAO/WHO, 2019).
The possible MoAs intervening in hypothyroidism have been reviewed by EFSA (EFSA, 2019a), and known or hypothesised MoAs have been allocated to a number of active substances included in the CAG. The MoA of risk drivers includes NIS inhibition (bromide ion), TPO inhibition (mancozeb, propineb and ziram) and liver enzyme induction (hypothesised: thiabendazole, pyrimethanil, cyprodinil and chlorpropham). As shown by detailed records in Table C.2.03 (CAG‐TCF) of the EFSA (2019b), the cumulative exposure of consumers above the 99th percentile is, in the vast majority of cases, associated with several substances, essentially the risk drivers that were observed, in varying proportions from one individual to the other.
In experimental studies, Crofton et al. (2005) investigated the cumulative effects of thyroid‐hormone disrupting chemicals on serum total T4 levels. Eighteen polyhalogenated aromatic hydrocarbons known to affect thyroid hormone clearance via at least two mechanisms were tested as individual chemicals and as a mixture at six dose levels using a short‐term (4 day) dosing model in rat. At the highest dose level, concentrations of chemicals in the mixture were at least an order of magnitude lower than individual NOELs with the exception of one chemical, where there was a 16% decrease in T4 at the concentration found in the highest dose. The predicted mixture response using the dose‐additivity model based on single‐chemical data was compared with the experimentally observed mixture response (empirical mixture model).
At the lower dose levels, the experimentally observed response for the mixture did not deviate significantly from the dose‐additivity model, but at the three highest dose levels, the experimentally observed response was significantly greater‐than‐additive in accordance with synergism. The results suggest that the cumulative effect of the mixture was predicted by dose addition at the lower doses (up to a factor of about 10 lower than the highest concentration tested) and that at the higher dose region that the cumulative effect was underpredicted by the dose‐addition model. However, the estimation of cumulative effect by the dose‐addition model in the higher dose region was still reasonably close to the empirical data, and the difference was approximately 15% in terms of T4 concentrations or two‐ to threefold on a dose basis. In the lower dose range deviation from the dose‐addition model would be more difficult to detect statistically.
In a subsequent review article, Crofton (2008) considered the uncertainty regarding the potential for additive, antagonistic or synergistic effects following exposure to mixtures of thyroid‐disrupting chemicals that act through a variety of mechanisms. The review reported that the availability of robust studies on mixtures of thyroid‐disrupting chemicals which undertake concurrent characterisation of individual chemicals and statistical approaches to test cumulative effect models is limited. The effect‐addition model was considered not a tenable hypothesis for mixtures of polyhalogenated aromatic hydrocarbon thyroid‐disrupting chemicals which have been shown to result in effects that were greater than predicted by the effect‐addition model. The dose‐addition model was considered to predict the effects of the mixture (decreased T4 concentration) with a fair degree of accuracy. Although the cumulative effects for these chemicals were reported to be greater‐than‐dose‐additive at high doses (Crofton et al., 2005), the review concluded that the magnitude of underestimation by the additivity model is small even in the high‐dose region. In the lower dose region, departure from additivity was not observed suggesting that dose additivity predicts effects on T4 concentrations at low exposures although this conclusion is constrained by the low statistical power considerations in this dose range. The review article concluded that the dose addition model is reasonably accurate in predicting the effects of mixtures of thyroid‐disrupting chemicals on serum T4 concentrations. These conclusions are in general accordance with the recommendations of the PPR Panel to use dose addition methods for the CRA of mixtures of pesticides with dissimilar modes of action, provided they produce a common adverse outcome (EFSA PPR Panel, 2013b).
In the case of C‐cell hypertrophy, hyperplasia and neoplasia, only two risk drivers were identified (thiram and ziram), which, considering their structural similarity, are assumed to combine their effects by dose addition.
Note 29 (Dose/response at the actual exposure levels)
At low doses of exposure, around or below the NOAEL, there is uncertainty regarding the shape and the slope of the dose–response curve, particularly in poor quality studies from which the NOAEL is derived. The use of a limited number of animals, the inconsistent dose‐spacing and the sensitivity of the test system contributes to increase the uncertainty at such low‐dose levels. The lack of knowledge on the dose–response curve at doses around and below the NOAEL makes it uncertain to assess magnitude of the effect per unit change in dose, which can be larger or smaller than expected under the common assumption of proportionality.
On the other hand, the risk assessment model, using an MOET of 100 as the protection target, implies the assumption that the sensitive human is 100 times more susceptible to the effect than the tested animals. This implies that, for the sensitive human, the dose–response curve is shifted to the left by two orders of magnitude and that the tested doses would be similar to dietary exposure levels causing concern.
Note 30 (Adequacy of the OIM model)
The long‐term exposure distributions were calculated with the OIM model. In this simple model, for each individual of the population, the daily consumption of each food commodity, averaged over the number of days of the survey, is multiplied by the mean concentration of each substance in the food commodity. As the duration of food consumption surveys is relatively short in all cases (2–7 days), the calculated exposure for each individual may significantly differ from the real long‐term exposure, e.g. over years or a lifetime. For this reason, it is acknowledged that the exposures calculated with this method are about right in the middle of the exposure distribution but are expected to overestimate the upper tail and underestimate the lower tail of real long‐term exposures (EFSA PPR Panel, 2012; Goedhart et al., 2012, RIVM letter report 2015‐0191, 2015).
As the threshold for regulatory consideration looks at the 99.9th percentile of the exposure distribution, the use of the OIM model is consequently per se a source of overestimation of the exposure (and of underestimation of the MOET). To assess the impact of this source of uncertainty, one needs to consider how long a long‐term exposure to chemicals needs to be to trigger hypothyroidism (as defined for the purpose of the assessment under consideration) and C‐cell hypertrophy, hyperplasia and neoplasia. For the purpose of the assessments under consideration, it was estimated that the onset of these disorders requires at least a few weeks, and for this reason, the use of the OIM model leads to an underestimation of the MOET at 99.9th percentile of the exposure distribution.
It is difficult to quantify the impact of this source of uncertainty. To assist with this, the experts considered the differences between MOETs at 50th and 99.9th of the exposure distribution, based on the data in Tables 1A, 1B and 2A, 2B. Ratios were generally ranging from 2.6 to 3.9 and from 3.7 to 8 for hypothyroidism and C‐cell hypertrophy, hyperplasia and neoplasia, respectively. The difference in ratios observed between these two effects suggests that the impact of this source of uncertainty is less important when the number of pesticide/commodity combinations contributing to the overall exposure is high, as is the case for the CAG on hypothyroidism. A tentative explanation would be that, at individual respondent level, the impact of overestimated exposures for certain pesticide/commodity combinations could be mitigated by underestimated exposures for other pesticide/commodity combinations.
Another difficulty in the assessment of chronic risks stems from the fact that the usual models for chronic risk assessment compare NOAELs with average exposures whereas in reality exposures to individual substances vary around that average over time. This is not captured by toxicological studies where the substance is administered at constant daily dose levels. In the context of effects resulting from exposure to multiple chemicals, a more continuous overall exposure is however expected. Moreover, in the specific case of hypothyroidism, exposure oscillations to chemicals are expected to be of low relevance considering the internal feedback mechanisms of the thyroid system. The experts judged that this source of uncertainty has only a minor impact on the lower and upper tails of the MOET distribution.
Note 31 (Assumption on the residue level (1/2 LOQ) when an active substance is used, and its residues are below the LOQ)
For CAG‐TCF, This source of uncertainty has a minor impact, because all identified risk drivers are characterised by a high rate of quantifiable measurements and a mean residue level significantly higher than standard LOQs (0.01 mg/kg):
bromide ion: wheat (17%, mean 3.5 mg/kg), oats (19%, mean 3.3 mg/kg), tomatoes (35%, mean 4.5 mg/kg), rye (14%, mean 4.1 mg/kg), rice (10%, mean 6.5 mg/kg).
thiabendazole: orange (35%, mean 0.66 mg/kg).
propineb (measured as CS2): wine grapes (28%, mean 0.10 mg/kg), apples (16%, mean 0.09 mg/kg).
mancozeb (measured as CS2): oranges (15%, mean 0.10 mg/kg).
ziram (measured as CS2): apples (16%, mean 0.09 mg/kg), wine grapes (28%, mg/kg, mean 0.10 mg/kg).
chlorpropham: potatoes (27%, mean 0.29 mg/kg).
cyprodinil: wine grapes (21%, mean 0.05 mg/kg).
pyrimethanil: oranges (21%, mean 0.18 mg/kg).
This suggest that the contribution of samples with residues imputed at a level below the LOQ is minor, and consequently that the level at which these residues are imputed is of minor impact.
Suggested citation: EFSA (European Food Safety Authority) , Craig PS, Dujardin B, Hart A, Hernandez‐Jerez AF, Hougaard Bennekou S, Kneuer C, Ossendorp B, Pedersen R, Wolterink G and Mohimont L, 2020. Scientific report on the cumulative dietary risk characterisation of pesticides that have chronic effects on the thyroid. EFSA Journal 2020;18(4):6088, 71 pp. 10.2903/j.efsa.2020.6088
Requestor: EFSA
Question number: EFSA‐Q‐2018‐00346
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: Olavi Pelkonen, Joanna Tzoulaki and Anneli Widenfalk, and the other members of the Panel on Plant Protection Products and their Residues, for the scientific review and endorsement, respectively, of this scientific output; EFSA staff members Daniela Brocca and Paula Medina for the support provided to this scientific report.
Approved: 20 March 2020
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1836/full http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2020.EN-1835/full
This publication is linked to the following EFSA Journal articles: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2020.6087/full
Notes
In EFSA (2019b), 1/MOET is referred to as the total normalised exposure, reflecting that the exposures are normalised relative to the potency of each substance.
CAG‐TCF stands for ‘Cumulative Assessment Groups ‐ Thyroid/Chronic/Follicular cells’.
Monte Carlo analysis is a computer‐based method of analysis developed in the 1940s that uses statistical sampling techniques in obtaining a probabilistic approximation to the solution of a mathematical equation or model (US EPA, 1997).
CAG‐TCP stands for ‘Cumulative Assessment Groups ‐ Thyroid/Chronic/Parafollicular cells’.
The cumulative exposure assessments were conducted in two steps. The most refined, TIER II, is underlying the risk characterisation performed in the present report.
Sampling variability of consumption and occurrence data was addressed by repeating the inner loop (nominal modelling run) 100 times, each time replacing the original consumption and occurrence data sets with bootstrap data sets, obtained by resampling, with replacement, the same number of observations from the original data sets.
Results at 50th and 99th percentiles of the exposure distribution are given for the SAS®model only, for reason of space.
Additional model runs with different combinations of assumptions from the basic simulations.
Low measured levels of pesticide residues for which an accurate value is not available, because these levels have been reported as being below a Limit of Reporting.
Conceptual mathematical model on which probabilistic modelling is based.
Outer loop execution, bootstrapping: Sampling variability of consumption and occurrence data was addressed by repeating the inner loop (nominal modelling run) 100 times, each time replacing the original consumption and occurrence data sets with bootstrap data sets, obtained by resampling, with replacement, the same number of observations from the original data sets.
Sheffield method: The Sheffield method uses the Sheffield Elicitation Framework (SHELF) to structure the moderated group discussion during a face‐to‐face workshop to reach an appropriate expert consensus.
Available online at http://optics.eee.nottingham.ac.uk/match/uncertainty.php
Using the Shiny app software available at https://jeremy-oakley.shinyapps.io/SHELF-multiple/. This fits a selection of parametric distributions (normal, lognormal, student t, log student t, gamma, beta or the best‐fitting of these) to the judgements of each expert using least squares on the cumulative distribution function.
The BMDL is the benchmark dose's (BMD) lower confidence bound (usually with 95% confidence). This value, rather than the BMD estimated from the fitted dose‐response curve, is normally used as reference point to derive an acceptable daily intake (ADI) or an acute reference dose (ARfD).
The critical response level should normally be defined by risk managers, because it relates to protection goals. In practice, this was not available, and therefore, the 10% response level was chosen by the Working Group as suggested by the EFSA guidance on the use of the benchmark dose approach in risk assessment (EFSA Scientific Committee, 2017) in case of quantal data.
https://jeremy-oakley.shinyapps.io/SHELF-single/. This offers the same choice of distributions as the SHELF‐multiple app (see footnote 15).
Symbols indicate those uncertainties which, if resolved (e.g. by obtaining perfect information), would tend predominantly to increase (+) or decrease (−) the modelled estimate of the MOET at 99.9th percentile of exposure for the German adult population, and those which could change it in either direction (+/−). The notes cited for each source of uncertainty are presented in Annex B2.
Symbols indicate those uncertainties which, if resolved (e.g. by obtaining perfect information), would tend predominantly to increase (+) or decrease (−) the modelled estimate of the MOET at the 99.9th of exposure for the German adult population, and those which could change it in either direction (+/−)
CAG‐NAM and CAG‐NAN are CAGs related to acute effects on the nervous system and used for a CRA reported in a separate scientific report.
Absolute change in frequency of response (additional risk in %) divided by the non‐affected fraction in the control population (100 minus the background response in %).
References
- Chung SW, 2018. How effective are common household preparations on removing pesticide residues from fruit and vegetables? A review. Journal of the Science of Food and Agriculture, 98, 2857–2870. 10.1002/jsfa.8821 [DOI] [PubMed] [Google Scholar]
- Crofton KM, 2008. Thyroid disrupting chemicals: mechanisms and mixtures. International Journal of Andrology, 31, 209–223. [DOI] [PubMed] [Google Scholar]
- Crofton KM, Craft ES, Hedge JM, Gennings C, Simmons JE, Carchman RA, Carter Jr WH and DeVito MJ, 2005. Thyroid‐hormone–disrupting chemicals: evidence for dose‐dependent additivity or synergism. Environmental Health Perspectives, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18‐G‐01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097. 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013. The 2010 European Union Report on Pesticide Residues in Food. EFSA Journal 2013;11(3):3130, 808 pp. 10.2903/j.efsa.2013.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014a. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014b. Use of the EFSA Standard Sample Description for the reporting of data on the control of pesticide residues in food and feed according to Regulation (EC) No 396/2005 (2013 Data Collection). EFSA Journal 2014;12(1):3545, 60 pp. 10.2903/j.efsa.2014.3545 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015a. Scientific report on the pesticide monitoring program: design assessment. EFSA Journal 2015;13(2):4005, 52 pp. 10.2903/j.efsa.2015.4005 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015b. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;EN‐804, 90 pp. 10.2903/sp.efsa.2015.en-804 [DOI]
- EFSA (European Food Safety Authority), 2016a. The 2014 European Union report on pesticide residues in food. EFSA Journal 2016;14(10):4611, 139 pp. 10.2903/j.efsa.2016.4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2016b. National summary reports on pesticide residue analysis performed in 2014. EFSA supporting publication 2016;EN‐1107, 173 pp. 10.2903/sp.efsa.2016.en-1107 [DOI]
- EFSA (European Food Safety Authority), 2017a. The 2015 European Union report on pesticide residues in food. EFSA Journal 2017;15(4):4791, 134 pp. 10.2903/j.efsa.2017.4791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. National summary reports on pesticide residue analysis performed in 2015. EFSA supporting publication 2017;EN‐1211, 165 pp. 10.2903/sp.efsa.2017.en-121 [DOI]
- EFSA (European Food Safety Authority), 2018a. The 2016 European Union report on pesticide residues in food. EFSA Journal 2018;16(7):5348, 139 pp. 10.2903/j.efsa.2018.5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018b. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018c. National summary reports on pesticide residue analysis performed in 2016. EFSA supporting publication 2018;EN‐1454, 175 pp. 10.2903/sp.efsa.2018.en-1454 [DOI]
- EFSA (European Food Safety Authority), Crivellente F, Hart A, Hernandez‐Jerez AF, Hougaard Bennekou S, Pedersen R, Terron A, Wolterink G and Mohimont L, 2019a. Scientific report on the establishment of cumulative assessment groups of pesticides for their effects on the thyroid. EFSA Journal 2019;17(9):5801, 115 pp. 10.2903/j.efsa.2019.5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019b. Scientific Report on the cumulative dietary exposure assessment to pesticides that have chronic effects on the thyroid using SAS® software. EFSA Journal 2019;17(9):5763, 53 pp. 10.2903/j.efsa.2019.5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Hart A, Maxim L, Siegrist M, Von Goetz N, da Cruz C, Merten C, Mosbach‐Schulz O, Lahaniatis M, Smith A and Hardy A, 2019c. Guidance on Communication of Uncertainty in Scientific Assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Dujardin B and Kirwan L, 2019d. Technical report on the raw primary commodity (RPC) model: strengthening EFSA's capacity to assess dietary exposure at different levels of the food chain, from raw primary commodities to foods as consumed. EFSA supporting publication 2019;EN‐1532, 30 pp. 10.2903/sp.efsa.2019.en-1532 [DOI]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2008. Scientific Opinion to evaluate the suitability of existing methodologies and, if appropriate, the identification of new approaches to assess cumulative and synergistic risks from pesticides to human health with a view to set MRLs for those pesticides in the frame of Regulation (EC) 396/2005. EFSA Journal 2008;6(4):705, 61 pp. 10.2903/j.efsa.2008.705 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on the use of probabilistic methodology for modelling dietary exposure to pesticide residues. EFSA Journal 2012;10(10):2839, 95 pp. 10.2903/j.efsa.2012.2839 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013a. Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile (2014 update). EFSA Journal 2013;11(7):3293, 131 pp. 10.2903/j.efsa.2013.3293 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2013b. Scientific Opinion on relevance of dissimilar mode of action and its appropriate application for cumulative risk assessment of pesticides residues in food. EFSA Journal 2013;11(12):3472, 40 pp. 10.2903/j.efsa.2013.3472 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2016. Guidance on the establishment of the residue definition for dietary risk assessment. EFSA Journal 2016;14(12):4549, 129 pp. 10.2903/j.efsa.2016.4549 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More SJ, Hardy A, Bampidis V, Benford D, Bennekou SH, Bragard C, Boesten J, Halldorsson TI, Hernandez‐Jerez AF, Jeger MJ, Knutsen HK, Koutsoumanis KP, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Nielsen SS, Schrenk D, Solecki R, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Laskowski R, Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Testai E, Dujardin B, Kass GEN, Manini P, Zare Jeddi M, Dorne J‐LCM and Hogstrand C, 2019. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), Ockleford C, Adriaanse P, Hougaard Bennekou S, Berny P, Brock T, Duquesne S, Grilli S, Hernandez‐Jerez AF, Klein M, Kuhl T, Laskowski R, Machera K, Pelkonen O, Pieper S, Smith R, Stemmer M, Sundh I, Teodorovic I, Tiktak A, Topping CJ, Gundert‐Remy U, Kersting M, Waalkens‐Berendsen I, Chiusolo A, Court Marques D, Dujardin B, Kass GEN, Mohimont L, Nougadere A, Reich H and Wolterink G, 2018. Scientific opinion on pesticides in foods for infants and young children. EFSA Journal 2018;16(6):5286, 75 pp. 10.2903/j.efsa.2018.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Germini A, Martino L, Merten C, Mosbach‐Schulz O, Smith A and Hardy A, 2018b. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2011. The Rapid Alert System for Food and Feed, 2011 Annual Report. Available online: http://ec.europa.eu/food/food/rapidalert/docs/rasff_annual_report_2011_en.pdf
- European Commission , 2015. Summary Report of the Standing Committee on Plants, Animals, Food and Feed Held in Brussels on 11 June 2015–12 June 2015 (Section Phytopharmaceuticals ‐ Pesticides Residues). Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/sc_phytopharmaceuticals_sum_2015061112_ppr_en.pdf
- European Commission , Directorate General for health and food safety, 2017a. Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. SANTE/11813/2017, 21 22 November 2017 rev.0.
- European Commission , Directorate General for health and food safety, 2017b. Guidance document (Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs). SANCO 7525/VI/95 Rev. 10.3, 13 June 2017.
- European Commission , 2018. (letter). Available online: http://www.efsa.europa.eu/sites/default/files/SANTE_CRA_Mandate.pdf
- FAO/WHO , 2019. Summary report of a FAO/WHO Expert Consultation on Dietary risk assessment of chemical mixtures (Risk assessment of combined exposure to multiple chemicals). WHO, Geneva, 16–18 April 2019. Available online: https://www.who.int/foodsafety/areas_work/chemical-risks/Euromix_Report.pdf)
- Garthwaite D, Sinclair CJ, Glass R, Pote A, Trevisan M, Sacchettini G, Spanoghe P, Ngoc KD, Fevery D, Machera K, Charistou A, Nikolopoulou D, Aarapaki N, Tskirakis A, Gerritsen‐Ebben R, Spaan S, González FE, Stobiecki S, Śliwiński W, Stobiecki T and Hakaite P, 2015. Collection of pesticide application data in view of performing environmental risk assessments for pesticides. EFSA supporting publication 2015;EN‐846, 246 pp.
- Goedhart PW, van der Voet H, Knüppel S, Dekkers ALM, Dodd KW, Boeing H and van Klaveren JD, 2012. A comparison by simulation of different methods to estimate the usual intake distribution for episodically consumed foods. Supporting Publications 2012;EN‐299, 65 pp. Available online: www.efsa.europa.eu
- Keikotlhaile BM, Spanoghe P and Steurbaut W, 2010. Effects of food processing on pesticide residues in fruits and vegetables: a meta‐analysis approach. Food and Chemical Toxicology, 48, 1–6. [DOI] [PubMed] [Google Scholar]
- Oakley JE and O'Hagan A, 2016. SHELF: the Sheffield Elicitation Framework (version 3.0). School of Mathematics and Statistics, University of Sheffield, UK. Available online: http://tonyohagan.co.uk/shelf
- van Klaveren J, 2017. Cumulative exposure assessment to pesticide residues regarding two chronic effects on the thyroid conducted with the MCRA tool 8.2 (unpublished).
- RIVM letter report 2015‐0191 (P.E. Boon, H. van der Voet), 2015. Probabilistic dietary exposure models relevant for acute and chronic exposure assessment of adverse chemicals in food. Available online: https://www.rivm.nl/bibliotheek/rapporten/2015-0191.pdf
- Scholz R, 2018. European database of processing factors for pesticides. EFSA supporting publication 2018;EN‐1510, 50 pp. 10.2903/sp.efsa.2018.en-1510 [DOI]
- US EPA , 1997. Guiding principles for Monte‐Carlo analysis. EPA/630/R‐97/001 March 1997.
- US EPA , 2003. Framework for Cumulative Risk Assessment. 2003. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington, DC 20460. EPA/630/P‐02/001F. May 2003. Available online: https://www.epa.gov/sites/production/files/2014-11/documents/frmwrk_cum_risk_assmnt.pdf
- Van Klaveren JD, Kruisselbrink JW, de Boer WJ, van Donkersgoed G, te Biesebeek JD, Sam M and van der Voet H, 2019. Cumulative dietary exposure assessment of pesticides that have chronic effects on the thyroid using MCRA software. EFSA Supporting Publication 2019;EN‐1707, 85 pp. 10.2903/sp.efsa.2019.en-1707 [DOI]
- Vereecken C, Pedersen TP, Ojala K, Krølner R, Dzielska A, Ahluwalia N, Giacchi M and Kelly C, 2015. Fruit and vegetable consumption trends among adolescents from 2002 to 2010 in 33 countries. European Journal of Public Health, 25, 16–19. [DOI] [PubMed] [Google Scholar]


