Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the competent authority of the initial rapporteur Member State the United Kingdom and the new rapporteur Member State Italy for the pesticide active substance chloropicrin are reported. The context of the peer review was that required by Regulation (EC) No 1107/2009 of the European Parliament and of the Council. The conclusions were reached on the basis of the evaluation of the representative uses of chloropicrin as a soil fumigant on strawberries, tomatoes, peppers, cucurbits (field and greenhouse applications) and tree crops: pome fruit, stone fruit, citrus, olives (field applications). The reliable endpoints, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are identified.
Keywords: chloropicrin, peer review, risk assessment, pesticide, soil fumigant
Summary
Chloropicrin is considered a new active substance for which, in accordance with Article 7 of Regulation (EC) No 1107/2009 of the European Parliament and of the Council, the initial rapporteur Member State (initial RMS), the United Kingdom, received an application from the European Chloropicrin Group (ECG) in December 2013 for approval. Complying with Article 9 of the Regulation, the completeness of the dossier was checked by the RMS and the date of admissibility of the application was recognised as being 18 June 2014.
An initial evaluation of the dossier on chloropicrin was provided by the RMS United Kingdom in the draft assessment report (initial DAR) and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by the European Food Safety Authority (EFSA) in accordance with Article 12 of Regulation (EC) No 1107/2009. During the peer review process, due to the intention of the United Kingdom to withdraw from the European Union (EU) pursuant to Article 50 of the Treaty on the EU, Italy took over the responsibility for this substance as RMS since June 2019.
The conclusions of the peer review process are summarised below.
The uses of chloropicrin as a soil fumigant according to the representative uses proposed at EU level result in a sufficient efficacy against the target organisms.
The assessment of the data package revealed no issues that could not be finalised or that need to be included as critical areas of concern with respect to identity, physical/chemical properties and analytical methods.
In the area of mammalian toxicology, the mutagenic potential of chloropicrin could not be concluded on the basis of the available data; consequently toxicological reference values could not be established and the non‐dietary exposure estimates for operators, workers, residents and bystanders could not be finalised (critical area of concern). Similarly, the toxicological profile of the metabolite dichloronitromethane (DCNM), including genotoxicity, could not be concluded based on the available data.
In the area of residues, the consumer risk assessment could not be finalised pending the lack of reference values (critical area of concern), the confirmation of the residue definition for risk assessment and due to the lack of appropriate information to address the effect of water treatment processes on the nature of residues of chloropicrin and its metabolite DCNM, potentially present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water (issue not finalised).
In the area of fate and behaviour in the environment, reliable soil degradation studies under aerobic conditions and a water/sediment investigation were not available. The available surface water modelling was based on best‐case estimated parameters and has been maintained for illustrative purposes. Even when using best‐case input parameters, the groundwater concentrations of chloropicrin are above the parametric drinking water limit of 0.1 μg/L for all the representative uses simulated and all the FOCUS scenarios/crops simulated, resulting in a critical area of concern being identified for possible contamination of groundwater. Groundwater exposure assessment for the major known breakdown product DCNM is not possible due to the lack of reliable data.
In the area of ecotoxicology, the following assessments could not be finalised: the long‐term risk to birds for both dietary and inhalation exposure (data gap for a suitable endpoint), the risk to aquatic organisms (data gaps for suitable algae endpoint and for a reliable exposure estimate), the chronic risk to honey bees via inhalation (data gap for a suitable endpoint), the off‐field risk to foliar‐dwelling non‐target arthropods (data gap for suitable endpoints), the chronic risk to earthworms (data gap for a suitable endpoint), and the risk to non‐target terrestrial plants (data gap for a suitable endpoint). A critical area of concern has been identified for soil macroorganisms and soil microorganisms.
The available evidence was not considered sufficient to draw a conclusion on the endocrine‐disrupting properties of chloropicrin for non‐target organisms.
Background
Regulation (EC) No 1107/2009 of the European Parliament and of the Council1 (hereinafter referred to as ‘the Regulation’) lays down, inter alia, the detailed rules as regards the procedure and conditions for approval of active substances. This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States and the applicant(s) for comments on the initial evaluation in the draft assessment report (DAR), provided by the rapporteur Member State (RMS), and the organisation of an expert consultation, where appropriate.
In accordance with Article 12 of the Regulation, EFSA is required to adopt a conclusion on whether an active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation (also taking into consideration recital (10) of the Regulation) within 120 days from the end of the period provided for the submission of written comments, subject to an extension of 30 days where an expert consultation is necessary, and a further extension of up to 150 days where additional information is required to be submitted by the applicant(s) in accordance with Article 12(3).
Chloropicrin is considered a new active substance for which, in accordance with Article 7 of the Regulation, the initial RMS, the United Kingdom, received an application from the European Chloropicrin Group (ECG) in December 2013 for approval. Complying with Article 9 of the Regulation, the completeness of the dossier was checked by the initial RMS and the date of admissibility of the application was recognised as being 18 June 2014.
The RMS UK provided its initial evaluation of the dossier on chloropicrin in the DAR, which was received by EFSA on 12 December 2017 (United Kingdom, 2017). Since the dossier was submitted before the new data requirements entered into force on 1 January 2014, the DAR has been prepared by the RMS UK following the old data requirements in accordance with Commission Regulations (EC) No 544/20112 and 545/20113.
During the peer review process, due to the intention of the United Kingdom to withdraw from the European Union (EU) pursuant to Article 50 of the Treaty on the European Union, Italy has been allocated as new RMS for this substance and took over the responsibility for this substance as RMS since June 2019.
The peer review was initiated on 20 February 2018 by dispatching the initial DAR to the Member States and the applicants, the ECG, for consultation and comments. EFSA also provided comments. In addition, EFSA conducted a public consultation on the DAR. The comments received were collated by EFSA and forwarded to the RMS United Kingdom for compilation and evaluation in the format of a reporting table. The applicants were invited to respond to the comments in column 3 of the reporting table. The comments and the applicants’ response were evaluated by the RMS UK in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicants in accordance with Article 12(3) of the Regulation were considered in a telephone conference between EFSA and the RMS United Kingdom on 13 June 2018. On the basis of the comments received, the applicants’ response to the comments and the RMS's evaluation thereof, it was concluded that additional information should be requested from the applicants, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments is reflected in the conclusions set out in column 4 of the reporting table. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation where this took place, were reported in the final column of the evaluation table.
In accordance with Article 12 of the Regulation, EFSA should adopt a conclusion on whether chloropicrin can be expected to meet the approval criteria provided for in Article 4 of the Regulation, taking into consideration recital (10) of the Regulation. A final consultation on the conclusions arising from the peer review of the risk assessment took place with Member States via a written procedure in December 2019–January 2020.
This conclusion report summarises the outcome of the peer review of the risk assessment on the active substance and the representative formulations evaluated on the basis of the representative uses of chloropicrin as a soil fumigant on strawberries, tomatoes, peppers, cucurbits (field and greenhouse applications) and tree crops: pome fruit, stone fruit, citrus, olives (field applications), as proposed by the applicants. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the DAR and considered during the peer review, if any, are presented in the conclusion. Furthermore, this conclusion also addresses the assessment required from EFSA under Article 12 of Regulation (EC) No 396/2005, provided that the active substance will be approved under Regulation (EC) No 1107/2009 without restrictions affecting the residue assessment. In the event of a non‐approval of the active substance or an approval with restrictions that have an impact on the residue assessment, the potential maximum residue level (MRL) proposals, if any, from this conclusion might no longer be relevant and a new assessment under Article 12 of Regulation (EC) No 396/2005 will be required. A list of the relevant end points for the active substance and the formulations is provided in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2020), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views where applicable, can be found:
the comments received on the DAR;
the reporting table (14 June 2018);
the evaluation tables (21 January 2020);
the reports of the scientific consultation with Member State experts (where relevant);
the comments received on the assessment of the additional information (where relevant);
the comments received on the draft EFSA conclusion.
Given the importance of the DAR including its revisions (United Kingdom, 2017; United Kingdom and Italy, 2019) and the peer review report, these documents are considered as background documents to this conclusion.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Chloropicrin is the ISO common name for trichloronitromethane (IUPAC).
The representative formulated products for the evaluation are ‘Chloropicrin 99’, a vapour‐realising product (VP) containing 990 g/kg chloropicrin and ‘Chloropicrin EC’, an emulsifiable concentrate (EC) containing 940 g/kg chloropicrin.
The representative uses evaluated were shank injection and drip application to bare soil (pre‐planting) for the control of various fungal diseases, nematodes and germinating weed seeds in a range of crops such as strawberries, tomatoes, peppers, cucurbits (with and without edible peel) for field and greenhouse uses, and tree crops (pome fruit, stone fruit, citrus, olives) in field. Full details of the Good Agricultural Practices (GAPs) can be found in the list of end points in Appendix A.
Data were submitted to conclude that the use of chloropicrin as a soil fumigant according to the representative uses proposed at EU level results in a sufficient efficacy against the target organisms, following the guidance document SANCO/10054/2013 ‐ rev. 3 (European Commission, 2013).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: European Commission (2000a,b, 2010).
The proposed specification for chloropicrin is based on batch data from industrial plant production. The proposed minimum purity of the technical material is 990 g/kg. It should be noted that the evaluation of the toxicological relevance of one impurity was not concluded (see data gap in Section 2). The batches used in the (eco)toxicological assessment support the proposed specification. There is no FAO specification available for chloropicrin.
The main data regarding the identity of chloropicrin and its physical and chemical properties are given in Appendix A. Data gaps were identified for an accelerated storage stability study for the formulation ‘Chloropicrin 99’ and for additional information on the technical characteristics before and after 2 years storage of the formulation ‘Chloropicrin EC’.
Methods of analysis are available for the determination of the active substance in the technical material and in the representative formulations and for the determination of the respective impurities in the technical material.
Chloropicrin residues in high water and high acid content plant commodities can be monitored by gas chromatography (GC)‐ECD (electron capture detector) with a limit of quantification (LOQ) of 0.005 mg/kg. Analytical methods for determination of the residue definition in dry and high oil content commodities were not provided (data gap). The efficiency of the extraction procedure used in the monitoring methods was not verified since no residues above the LOQ as a result of the representative uses were detected in all matrix groups.
An analytical method for food of animal origin is not required since MRL in animal products was not proposed (see Section 3).
Chloropicrin residues in soil, water and air can be monitored by GC‐ECD with LOQs of 0.05 mg/kg, 0.1 μg/L and 0.83 μg/m3, respectively. The residue definition for monitoring in soil and water was not concluded (see data gap in Section 4); as a consequence, additional monitoring methods might be required should new components be included in the residue definitions. The residue definition for monitoring in air was concluded as chloropicrin and phosgene; as a consequence, a data gap for an analytical method for monitoring of phosgene in air was set.
Gas chromatography–mass spectrometry (GC–MS) and GC‐ECD methods can be used for monitoring of chloropicrin residues in body fluids (urine and blood) with LOQs of 0.05 mg/L. Chloropicrin residues in body tissues can be determined by GC‐ECD with a LOQ of 0.01 mg/kg.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion: European Commission (2003, 2012) and EFSA PPR Panel (2012).
Chloropicrin was discussed at the Pesticide Peer Review Experts’ Meeting 13 in September 2019.
As regards the proposed technical specification, the batches used in the toxicological studies are representative, taking into account the high purity of the active substance. The assessment of the toxicological relevance of one impurity could not be finalised on the basis of the available data (data gap).
After oral administration, chloropicrin is very well absorbed (> 85%) and mainly excreted via expired air, to a lesser extent via urine and faeces. With a limited body distribution (mainly in stomach and liver), chloropicrin did not demonstrate a potential for bioaccumulation. The proposed major metabolic pathway would include a rapid dechlorination, and the minor pathway would involve adduct formation with thiol proteins.
With regard to acute toxicity, chloropicrin has a harmonised classification4 for all routes of exposure (Harmful if swallowed, Fatal if inhaled; skin, eye and respiratory tract irritant). Based on the available data, criteria for more severe classification may be met (Toxic if swallowed and in contact with skin, Fatal if inhaled and Corrosive). Owing to the strong irritating properties of chloropicrin, the need to perform a skin sensitisation study can be waived.
In short‐term toxicity studies, after oral administration, the relevant no observed adverse effect level (NOAEL) for rats was 10 mg/kg body weight (bw) per day based on mortality, decreased body weight and changes in the non‐glandular forestomach; and emesis was observed in dogs with a relevant NOAEL of 0.1 mg/kg bw per day. In toxicity studies by inhalation with rats and mice, toxicity was manifested in the respiratory tract (nasal cavities and lungs) at the low dose level (0.3 ppm) while systemic toxicity was observed at higher dose levels.
For the in vitro genotoxicity, chloropicrin was demonstrated to be mutagenic in bacteria in the presence of exogenous metabolic activation, and clastogenic in cultured mammalian cells in the absence of metabolic activation only at concentrations producing significant toxicity. Based on the limitations of the available in vitro mammalian cell gene mutation assay (i.e. no experiment with 24 h exposure without metabolic activation), the experts agreed that the mutagenic potential of chloropicrin should be further investigated5 (data gap). Based on negative results obtained in in vivo studies (including mouse bone marrow micronucleus test and rat liver unscheduled DNA synthesis (UDS) assay), chloropicrin can be concluded as having no clastogenic potential. Taking into account the data gap for further investigation of its mutagenic potential, the genotoxic potential of chloropicrin cannot be concluded (issue not finalised).
In long‐term toxicity studies by oral and inhalation routes, chloropicrin did not induce any carcinogenic effect. Adverse findings after oral administration were observed in the non‐glandular forestomach, with a NOAEL of 0.1 mg/kg bw per day (rats). After exposure by inhalation of rats and mice, the no observed adverse effect concentration (NOAEC) was 0.1 ppm based on mortality (rats), decreased body weight gain (rats, mice) and histopathological findings in the respiratory tract (mice).
In the rat multigeneration study by inhalation, no adverse effect was observed on the reproductive parameters and offspring development, while pulmonary inflammation was observed in parental females with a NOAEC of 1.0 ppm. In the developmental toxicity studies, no teratogenic effect was observed. In rats, maternal toxicity included decreased body weight gain and food consumption while the developmental toxicity was based on delayed ossification and skeletal variations (in the presence of maternal toxicity). In rabbits, the maternal toxicity included mortalities, clinical signs, necropsy findings (lungs) and reduced body weight (gain), while the developmental toxicity was based on increased foetal mortality and abortions (in the presence of maternal toxicity). All NOAECs were 0.4 ppm.
Based on the available data chloropicrin is unlikely to be neurotoxic.
For the derivation of toxicological reference values, according to Regulation (EC) No 1107/2009, the assessment of an active substance or a plant protection product should not be based on tests or studies involving the deliberate administration of the active substance or plant protection product to humans. Consequently, a human sensory irritation study presented in the dossier was not further considered for the setting of toxicological reference values for chloropicrin.6 However, since the mutagenic potential of chloropicrin could not be concluded based on the available data (data gap), and acknowledging that this can be a non‐threshold effect, no toxicological reference values can be derived to conduct the non‐dietary exposure estimates for operators, workers, residents and bystanders7 (critical area of concern).
For phosgene, air metabolite of chloropicrin, harmonised occupational exposure limits8 have been considered for non‐dietary exposure estimates. For operators and workers, the 8‐h time‐weighted average (TWA) value of 0.4 mg/m3 has been applied as acceptable operator exposure concentration (AOEC) while for residents and bystanders, the 15‐min short‐term exposure limit (STEL value) of 2 mg/m³ has been used to derive an acute acceptable operator exposure concentration (AAOEC) of 0.2 mg/m³, with an additional uncertainty factor of 10 for intraspecies variation. The non‐dietary exposure estimates from valid field studies resulted in measured values at or below the LOQ (0.0023 μg/L) in the inhalation air samplers of operators/workers during the use as shank injection, at or below the LOQ for operators during the use as drip irrigation, and below the AOEC for workers re‐entering greenhouse for ventilation for the use as drip irrigation. For residents and bystanders, measured values are below the LOQ at 50 and 200 m from field edges for the use as shank injection, and the maximum estimated peak exposure is lower than the reference value at 20 m from field edges for the drip irrigation use (no values were measured for residents/bystanders positioned at less than 20 m).
For the metabolite DCNM, only data from public literature were available (with limited reliability due to limited reporting), showing indications of a genotoxic potential in in vitro genotoxicity studies. Consequently, no conclusion can be drawn about the (geno)toxicity profile of DCNM (data gap and issue not finalised).
3. Residues
The assessment in the residue section is based on the following guidance documents: European Commission (1999) and JMPR (2004, 2007).
Chloropicrin was discussed at the Pesticides Peer Review Experts’ Teleconference 08 in September 2019.
Metabolism was studied in a good laboratory practice (GLP)‐compliant study on strawberries, red beet and green beans which covered the conditions of the representative uses. The study shows some shortcomings (e.g. storage times of specimen not covered by storage stability data, no identification of metabolites), which render the study not fully guideline compliant and not suitable to establish the metabolic pathway of chloropicrin in plants. However, given the physical properties of the compound (high volatility and instability) and the high percentage of tissue bound radioactivity, the results do not warrant the request for a new metabolism study. Instead, the applicant is requested to further investigate the metabolism of chloropicrin in plants considering the chemical structure of chloropicrin, the overall data available on the metabolism in plants, residue trials, the degradation pathway of chloropicrin in soil (as the representative uses consist of a soil application), the potential toxicological relevance of the metabolites identified in soil and any relevant literature search on the parent and the soil metabolites. (data gap). The plant residue definition for monitoring is set as chloropicrin. For risk assessment, the residue definition is provisionally set as chloropicrin and should be revisited pending upon the requested additional information to confidently address the metabolic pathway of chloropicrin in plants. The proposed residue definitions are for all categories of crops following soil treatment.
Residue field trials performed in greenhouses for the representative uses on strawberries, tomatoes, pepper and cucurbits were provided with results below the LOQ (0.005 or 0.01 mg/kg) for both chloropicrin and DCNM. These trials did not address all different combinations in the application techniques (shank vs. drip tarped) and the practice of cutting and consequent planting the seedlings (cutting the film and then planting through the virtually impermeable film (VIF) vs. cutting and removing the film one day later) nor the application in the open field (data gap). For the tree crops, no data from residue field trials are needed as the GAPs foresee application only every 15 years.
Given that the residue definition for risk assessment is provisional and the maximum period for which storage stability for chloropicrin can be assumed in high water content commodities needs confirmation by an additional storage stability study (data gap),9 the results of all presented residue field trials which are found valid10 should be regarded as provisional.
The consumer risk assessment could not be finalised pending the lack of reference values for chloropicrin (critical area of concern, see Section 2), the confirmation of the residue definition for risk assessment and due to the lack of appropriate information to address the effect of water treatment processes on the nature of residues of chloropicrin and its metabolite DCNM, potentially present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water (issue not finalised, see Section 4).
4. Environmental fate and behaviour
Chloropicrin was discussed at the Pesticide Peer Review Experts’ Meeting 15 in September 2019.
The laboratory soil degradation studies presented did not allow the mass balance to be closed nor major metabolites to be identified. The soil degradation studies available did not enable reliable degradation parameters to be derived. The studies only allowed the determination of dissipation DissT50. However, it was not possible to determine to what extent the dissipation occurring in the laboratory system is representative of field situations. Therefore, a data gap was identified for reliable soil degradation studies under aerobic conditions with mass balance closed, reliable kinetic analysis of the degradation of chloropicrin and formation and degradation of metabolites (including DCNM) and adequately identifying volatiles. These studies should also be used to confirm if any metabolites are formed, other than DCNM, and to identify them. During the experts’ meeting, it was noted that under the impermeable film it cannot be excluded that the soil environment becomes at least partly anaerobic. Under anaerobic conditions, the available anaerobic soil incubation indicated that the major transformation product was nitromethane. It might be hypothesised that this is also an aerobic metabolite. Though not identified in significant amounts in the available aerobic incubations, this metabolite may eventually be a component of the not recovered radioactivity. Therefore, the experts considered that reliable route of degradation information under aerobic conditions would be needed before the possible consequences for the need of further metabolism data under anaerobic conditions might be concluded on.
Chloropicrin exhibited high to very high mobility in soil. DCNM exhibited very high mobility. There were no indications that the adsorption of chloropicrin or DCNM was pH dependent.
Chloropicrin was stable to hydrolysis at environmentally relevant temperatures and all pH in the range between 5 and 9. In the laboratory aqueous photolysis studies, chloropicrin exhibited very low persistence.
The available incubations in dark aerobic natural water sediment systems did not allow the route of degradation to be characterised. Therefore, a data gap was identified for a water/sediment investigation with a closed mass balance, allowing to characterise the route of degradation of chloropicrin in the aquatic environment. The experts agreed that for the parent chloropicrin whole system DegT50 derived from these incubations might be considered reliable and that transfer to sediment was limited.
The available surface water modelling (FOCUS (2001) predicted environmental concentrations (PECs) for surface water and sediment) have been maintained for illustrative purposes. The initial calculations provided assumed degradation during the soil coverage with a degradation half‐life equivalent to the dissipation rate observed in the laboratory experiments. Since this dissipation rate is mostly attributable to volatilisation, the experts considered it a best‐case situation when representing degradation under a covered soil (where dissipation particularly by volatilisation is minimised). Therefore, the original calculations have been complimented with further calculations assuming no degradation of chloropicrin during the period the soil was covered, to show the relevance of the situations, which, in the absence of degradation, would result in surface water concentrations of chloropicrin above the aquatic ecotoxicological endpoint (see Section 5). The experts in the meeting considered that all these PECSW could also illustrate the potential use in permanent greenhouses, since no separate PECSW/sed were deemed necessary for protected crop uses, provided that the soil is covered in the same way as for the field uses. The lack of reliable information on the formation and degradation of metabolite DCNM (both in soil and sediment water systems) did not allow the surface water and sediment exposure assessments for this breakdown product known to be formed in soil, to be completed. As noted above, the possible formation of other soil metabolites remains open. These might have the potential to move to surface water.
A groundwater exposure assessment for chloropicrin consequent to the representative uses assessed (assuming leaching was not possible and degradation in soil occurred whilst the VIF was in place for 7 days after soil injection), already indicated a high potential for groundwater contamination over a wide range of geoclimatic conditions as represented by annual average recharge concentrations leaving the top 1 m soil layer. This assessment used standard first tier FOCUS (2009) groundwater scenarios and the models PEARL 4.4.4 and PELMO 5.5.3 and the dissipation DissT50 parameter to represent degradation whilst the soil is tarp covered. This assumption was considered as best case by the experts. Due to the lack of reliable degradation parameters, the available groundwater modelling has been maintained for illustrative purposes to show that even when using best‐case input parameters, the groundwater concentrations of chloropicrin are above the parametric drinking water limit of 0.1 μg/L for all the representative uses simulated and all the FOCUS scenarios/crops simulated11 (critical area of concern). Other groundwater assessments (such as field experiments of leaching, that might have been used to refine/inform modelling approaches or pertinent reliable groundwater monitoring) were not included in the applicant's dossier. Groundwater exposure assessment for the major known breakdown product DCNM is not possible due to the lack of reliable data on its fate and behaviour in soil (issue not finalised). Groundwater exposure assessment of the impurity of the active substance for which the toxicological relevance assessment could not be performed (see Section 2), may be needed, depending on the final result of that assessment since this impurity has the potential to be applied to soil in significant amounts.
The applicant did not provide appropriate information to address the effect of water treatment processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water. This has led to the identification of a data gap and results in the consumer risk assessment not being finalised.
According to the available information, it cannot be excluded that the atmospheric half‐life of chloropicrin is greater than 2 days. The photodecomposition products of chloropicrin are phosgene and nitrosyl chloride.
The initial PEC in soil and the illustrative PEC in surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix A of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents: European Commission (2002a,b), EFSA (2009), SETAC (2001) and ECHA/EFSA (2018).
Some specific aspects related to the environmental risk assessment of chloropicrin were discussed at the Pesticide Peer Review Experts’ Meeting 12 in September 2019.
For potential uses in permanent high‐technology greenhouses, limited dietary exposure to wild terrestrial vertebrates is anticipated, and hence the risk was considered as low. Nevertheless, for all other uses, exposure cannot be ruled out and a risk assessment is needed.
Acute oral toxicity data for chloropicrin were available for both birds and wild mammals. A specific scenario for the applications described in the GAP (shank injection, drip irrigation) is not available in EFSA (2009). In order to assess the risk, the ‘bare soil scenario’ for spray applications was used. At the tier 1, a high acute risk was identified to both birds and wild mammals. A more realistic exposure estimate based on residue data on soil arthropods and seeds was used in a refined risk assessment, leading to a conclusion of a low acute risk for both birds and mammals.
A reproductive dietary study was available for birds. However, exposure in this study was only lasting 6 weeks vs the standard (minimum) twenty weeks exposure used in OECD 206. This issue was discussed at the experts’ meeting.12 Due to the inability of the available study to cover all possible reproductive phases, the experts agreed that the endpoint is not suitable for the reproductive risk assessment (data gap). Therefore, the reproductive risk to birds could not be finalised.
Appropriate reproductive dietary endpoints were available for wild mammals. A high reproductive risk was identified at the tier 1. A refined risk assessment was carried out considering refined residue levels and decline data, and a refined endpoint (discussed and agreed at the experts’ meeting13). Based on such refined assessment, a low dietary reproductive risk could be concluded for wild mammals.
No relevant plant metabolites were identified and therefore exposure to plant metabolites has not been considered further.
Due to the characteristics of chloropicrin, no bioaccumulation is expected; therefore, a quantitative risk assessment for exposure via secondary poisoning was not considered necessary. A low risk was concluded for both birds (acute only) and wild mammals (acute and reproductive) for what concerns exposure via consumption of contaminated water.
Due to the volatile nature of chloropicrin and its air metabolites (phosgene), a risk assessment for exposure via inhalation was also considered relevant for all representative uses (also those potentially in permanent high‐technology greenhouses). Short‐term inhalation studies were available for birds and mammals, and for the latter also a long‐term inhalation study. Since reliable exposure estimates were available from the operator exposure studies, a risk assessment was carried out and was discussed at the experts’ meeting.14 The experts agreed that the acute inhalation risk from chloropicrin could be considered low for both birds and wild mammals. Similarly, a low chronic risk was concluded for wild mammals. Only a 5‐day study with chloropicrin was available for birds, where animals were exposed for 4 h/day. This was not considered sufficient to cover for chronic exposure (data gap), particularly for birds nesting in the vicinity of the treated field. Hence, the chronic inhalation risk assessment for birds could not be finalised for chloropicrin.
Exposure via air is in principle possible for the metabolite phosgene gas. Nevertheless, in the available operator exposure studies, phosgene concentrations were below the limit of quantification in the majority of cases (see Section 2). On this basis, a low risk to birds and wild mammals from inhalation of phosgene gas is expected.
Data were available for assessing the toxicity of the active substance chloropicrin to fish, aquatic invertebrates and macrophytes. Valid studies were not available to characterise the toxicity of chloropicrin to algae (data gap). The available information nevertheless indicates that algae are likely to be driving the aquatic risk assessment, which therefore cannot be finalised.
In addition, as already mentioned in Section 4, there were significant uncertainties in the prediction of the exposure level of chloropicrin in surface waters. The environmental fate experts concluded that the available surface water modelling (applicable to uses in open field and in non‐permanent structures) cannot be relied upon and has been maintained for illustrative purposes only. Two sets of illustrative PECsw values (one best‐case and one worst‐case) were produced for the uses in field based on opposite extreme assumptions for the degradation during the soil coverage (see Section 4 for details). PEC calculations were performed for applications in June and September, i.e. the boundaries of the application window according to the GAP.
The risk assessment for aquatic organisms other than algae is driven by the acute risk to fish. The worst‐case illustrative PECs at step 3 were above the regulatory acceptable concentration (RAC) for at least half of the scenarios for all the uses and for both application techniques (shank injection and drip), when merging results from the June and September applications. The assessment is similar for chronic risk to fish. The application of mitigation measures (20 m buffer zones) would not change this outcome. For aquatic invertebrates (acute and chronic) and macrophytes, the number of unresolved scenarios is smaller, but high risk was identified in at least one scenario for each use and for both application techniques (except for macrophytes when the shank injection is performed on pome fruits). The best‐case illustrative risk assessment based on PECsw at step 3 resulted in a rather different picture, with a number of unresolved scenarios mainly for the drip application uses. For those, a high risk was concluded. No reliable step 4 calculations are available for the best case. Due to the different pictures drawn by the two sets of illustrative PECs (best‐case vs. worst‐case) for the shank injection uses, it was not possible to conclude either a low or a high risk. As such, the risk assessment could not be finalised for any of the relevant aquatic taxa.
No reliable exposure estimation was available for potential uses under permanent high‐technology greenhouse; hence this assessment could not be finalised either for any aquatic organism.
In addition to the risk assessment considering the standard entry routes (i.e. drift, drainage, runoff) of the active ingredients in water bodies, an additional risk assessment has been carried out for entries via atmospheric deposition, i.e. volatilisation to the atmosphere and subsequent deposition to surface water. This assessment is not use‐specific, but rather application‐specific. Based on the calculated PECs a high acute risk to fish was identified for ditches (all application techniques) and for streams (only application via drip irrigation in protected crop). A low risk was concluded for the remaining aquatic organisms other than algae and fish. The risk to fish due to exposure via atmospheric deposition could be resolved by using buffer zones of 50 m.
Valid acute toxicity data for fish, Daphnia and macrophytes were available for the metabolite DCNM, but not for algae (data gap). As commented in Section 4, the lack of reliable information on the formation and degradation of this metabolite did not allow to perform a surface water exposure assessment. Hence, the risk assessment cannot be finalised.
Other water‐sediment polar metabolites have been only tentatively identified. No toxicity data are available for those. However, some quantitative structure–activity relationship (QSAR) estimations have been submitted. These are considered rather unreliable and mostly inconsistent, but they indicate that these metabolites are unlikely to be more toxic than chloropicrin. Pending on the data gap identified in Section 4 regarding the route of degradation of chloropicrin in the aquatic environment, this issue may be considered further.
The available studies investigating the toxicity of chloropicrin to honey bees for acute (oral and contact) exposure were not considered reliable. Nevertheless, the experts at the meeting15 agreed that, for the representative GAPs, exposure to honey bees via contact and oral routes is unlikely and therefore a low risk could be concluded.
On the contrary, exposure via inhalation was deemed relevant for all uses (including those potentially in permanent high‐technology greenhouse). A 1 h acute inhalation test was available. The RMS provided a comparison between the measured air concentration of chloropicrin in the field operator studies with the highest tested concentration from the acute inhalation toxicity study (=no observed effect concentration (NOEC)). Such comparison indicated that the maximum measured exposure is by a factor of 279 lower than the estimated NOEC. Even in the lack of a specific risk assessment scheme for exposure via inhalation, the experts agreed that the margin of safety was sufficient for concluding a low acute risk to honey bees.
Chronic inhalation toxicity studies were not available for honey bees. The operator exposure studies confirmed that measurable levels of chloropicrin in air were still present up to 28 days after the treatment (see Section 2, Appendix A). All experts agreed that the chronic risk to honey bees could not be excluded based on the available data. The experts agreed that further data would be needed to either exclude chronic inhalation exposure to honey bees or to quantify the level of chronic toxicity (data gap). Hence, the chronic risk to honey bees via inhalation cannot be finalised.
The efficacy of the mitigation measures proposed by the RMS (i.e. remove hives during application and the VIF plastic sheeting removal) was not considered sufficiently demonstrated.
No data on the standard species of foliar dwelling non‐target arthropods were available (data gap). In view of the application time and technique, exposure in field is not anticipated for foliar dwellers. However, exposure via redeposition to the off‐crop environment cannot be excluded for all uses (including those potentially in permanent high‐technology greenhouse). Owing to the lack of specific toxicity data, the risk assessment for non‐target arthropods cannot be finalised.
Data were available for different species of soil‐dwelling non‐target arthropods. The tier 1 assessment indicated a high in‐field risk. However, aged residue studies were available to confirm that the expected in‐field effects would disappear rather quickly, in line with the expected volatility of chloropicrin. As the off‐field risk for the same soil‐dwelling species was assessed as low, it was considered that there is potential for recolonization within one year.
For the potential uses in permanent high‐technology greenhouse, due to the limited exposure, a low risk can be concluded for all soil organisms.
For the other uses (open field and non‐permanent protected structures) a risk assessment needs to be carried out. Both acute and chronic laboratory studies with chloropicrin were made available for earthworms. Nevertheless, none of them were considered reliable (data gap) during the experts’ meeting.16 As such, no proper tier‐1 risk assessment could be performed for earthworms. However, it was noted that, if the results of the available chronic study were used in the risk assessment, a high risk would be indicated for all the uses of chloropicrin (other than those in permanent high‐technology greenhouse). Other two chronic studies were carried out with aged soils (i.e. organisms were introduced a number of days after the application). These were also discussed at the experts’ meeting,16 where it was noted that the aged residue studies for earthworms are not a standard refinement. These studies were not considered sufficient for demonstrating recovery/recolonisation of earthworms as these organisms are likely to move very slowly from the off‐field. Overall, the risk to earthworms cannot be finalised with the available data.
Regarding other soil macroorganisms, one tier‐1 toxicity study was available for Folsomia candida. This indicated that 100% mortality is expected when chloropicrin is applied at field rate, thus indicating a high risk. Aged residue studies were available for Folsomia and for Hypoaspis aculeifer. It is noted that this option is generally designed to assess the risk for non‐target arthropods and not for soil macroorganisms. However, the two studies have been considered for the risk assessment. Concerns about the exposure were raised for both. Particularly for the second study on H. aculeifer, the results were not considered reliable (data gap), as only marginal mortality was seen even immediately after the application of chloropicrin. In general, the experts agreed16 that the available data were not sufficient for demonstrating potential for recovery/recolonisation within 1 year. Hence, a high risk to soil macroinvertebrates is concluded for all uses in open field and in non‐permanent structures (critical area of concern).
No data are available for assessing the toxicity of the soil metabolite DCNM and any other potential soil metabolites (see data gap in Section 4) to any non‐target soil macroorganism (data gap).
Two tier‐1 studies with soil microorganisms and chloropicrin were available and highlighted effects > 25% at the end of the studies (98 and 142 days, respectively). Based on those, a high risk was indicated for all uses of chloropicrin (other than those in permanent high‐technology greenhouse). A third study was available where soils were treated and aged for 29 days in the field, and then moved to the laboratory for the assessment of nitrogen and carbon transformation. In this study, effects were below 25% after 28 further days. Nevertheless, the study was considered not representative for the GAP, as the tarp was only maintained on the soil for 8 days instead of 21 days, thus likely reducing the length of the exposure. In addition, the soil aged in the field has been strongly manipulated (sieved) before the start of the laboratory phase, with a potential significant reduction of residues in soil. As such, a high risk was concluded for soil microorganisms following the uses of chloropicrin in open field and in non‐permanent structures (critical area of concern).
No data are available for assessing the toxicity of the soil metabolite DCNM and any other potential soil metabolites (see data gap in Section 4) to soil microorganisms (data gap).
Non‐target terrestrial plants in the off‐field environment may be exposed to volatilised chloropicrin (even from permanent high‐technology greenhouse). One study was available for assessing the toxicity of the active substance in form of a vapour. In the test, plants were exposed for 6‐h on two consecutive days. The RMS considered that this exposure length is not sufficient to address the potential effects in the field, where exposure can last longer (data gap). As such, the risk assessment to non‐target terrestrial plants cannot be finalised.
One study was available for assessing the effects of chloropicrin to activated sludge. Considering the application pattern of chloropicrin and the results of the toxicity study, a low risk was concluded.
6. Endocrine disruption properties
The assessment of the endocrine disruption potential of chloropicrin was performed based on the new scientific criteria for the determination of endocrine‐disrupting properties, as laid down in Commission Regulation (EU) 2018/60517 and implemented in the EFSA/ECHA guidance (2018) for the identification of endocrine disruptors.
As regards the assessment of the endocrine disruption potential of chloropicrin for humans, for the T‐modality, the data package is complete, and no T‐mediated adversity was observed. For the oestrogen, androgen and steroidogenesis (EAS) modalities, the data package is not complete. However, given the mode of action of the active substance, i.e. local irritant with minimal systemic effects, no further testing is necessary (acceptable data waiver). According to point 3.6.5 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, it can be concluded that chloropicrin is not an endocrine disruptor in humans.
The outcome of the assessment reported above for humans also applies to wild mammals as non‐target organisms.
For non‐target organisms other than mammals, no relevant data for assessing the endocrine properties of chloropicrin through the T‐modality were available. For assessing the endocrine‐disrupting properties through the EAS modalities, one chronic study with fish (early life‐stage (ELS) test according to OECD 210) and one reproductive toxicity study with birds (OECD TG 206) were available. However, those studies provide little information concerning potential endocrine‐related effects. No information is available on endocrine activity for the EATS modalities. Thus, the available evidence was not considered sufficient to draw a conclusion on the endocrine‐disrupting properties for non‐target organisms (data gap and issue not finalised).
In line with the assessment strategy proposed in the ECHA/EFSA Guidance (2018), level 3 tests would be required to complete the current data package, i.e. a study in line with OECD TG 231 (Amphibian Metamorphosis Assay (AMA))15 and a study in line with the OECD TG 229 (Fish Short‐Term Reproduction Assay (FSTRA)).
Those tests are relevant to investigate potential EATS‐mediated endocrine activity and, if negative, to exclude that chloropicrin has endocrine properties according to the scientific criteria for the determination of endocrine‐disrupting properties as set out in point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605. However, in case of positive result/s based on any of these tests for at least one modality, additional testing (i.e. a test in line with OECD 241 (Larval Amphibian Growth and Development Test) and/or a test in line with OECD 240 (Medaka Extended One‐Generation Reproduction Test)) might be needed in order to further investigate the adversity.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 2, 3, 4, 5)
Table 2.
Groundwater
| Compound (name and/or code) | Mobility in soil | > 0.1 μg/L at 1 m depth for the representative usesa | Pesticidal activity | Toxicological relevance |
|---|---|---|---|---|
| Chloropicrin |
High to very high mobility KFoc = 24.9–122 mL/g |
Yes Illustrative calculations with best‐case degradation estimations: Apple > 0.1 μg/L at 8 out of 8 scenarios assessed 0.3–18 μg/L Citrus > 0.1 μg/L at 4 out of 4 scenarios assessed 0.11–4.9 μg/L Tomatoes > 0.1 μg/L at 4 out of 4 scenarios assessed 3.28–18.08 μg/L Strawberries > 0.1 μg/L at 4 out of 4 scenarios assessed 0.715–49.64 μg/L |
Yes | Yes |
| Dichloronitromethane (DCNM) |
Very high mobility KFoc = 14.1–37.1 mL/g |
Data gap | Open | Open (data gap) |
KFoc: Freundlich organic carbon adsorption coefficient.
At least one FOCUS scenario or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Chloropicrin | Data gap (algae) |
| Dichloronitromethane (DCNM) | Data gap (algae) |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Chloropicrin | Fatal if inhaled – Rat LC50 = 0.044 mg/L (4 h; nose only) |
| Phosgene | Fatal if inhaled |
LC50: lethal dose, median.
Table 5.
Overview of concerns
| Representative use | Chloropicrin 99 (VP) – SEU, shank injection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G/F* (*areas intended for walk in tunnels) | F | ||||||||||||
| Strawberries | Tomatoes | Peppers | Cucurbits (with and without edible peel) | Strawberries (nursery) | Tomatoes (nursery) | Peppers (nursery) | Cucurbits (with and without edible peel) (nursery) | Tree crops: Pome fruit | Tree crops: Stone fruit | Tree crops: Citrus | Tree crops: Olives | ||
| Operator risk | Risk identified | ||||||||||||
| Assessment not finalised | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | |
| Worker risk | Risk identified | ||||||||||||
| Assessment not finalised | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | |
| Resident/bystander risk | Risk identified | ||||||||||||
| Assessment not finalised | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | |
| Consumer risk | Risk identified | ||||||||||||
| Assessment not finalised | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3 * ,12 | X1,3 * ,12 | X1,3 * ,12 | X1,3 * ,12 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | ||||||||||||
| Assessment not finalised | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | |
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 |
| Assessment not finalised | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | |
| Risk to aquatic organisms | Risk identified | ||||||||||||
| Assessment not finalised | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | X6,11 | |
| Groundwater exposure to active substance | Legal parametric value breached | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 |
| Assessment not finalised | |||||||||||||
| Groundwater exposure to metabolites | Legal parametric value breacheda | ||||||||||||
| Parametric value of 10 μg/Lb breached | |||||||||||||
| Assessment not finalised | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | |
| Representative use | Chloropicrin EC (drip application) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G/F* (*areas intended for walk in tunnels) SEU | G (existing protected structure greenhouse/walk‐in tunnel) SEU + CEU | F SEU + CEU | |||||||||||||||
| Strawberries | Tomatoes | Peppers | Cucurbits (with and without edible peel) | Strawberries | Tomatoes | Peppers | Cucurbits (with and without edible peel) | Strawberries | Tomatoes | Peppers | Cucurbits (with and without edible peel) | Tree crops: Pome fruit | Tree crops: Stone fruit | Tree crops: Citrus | Tree crops: Olives | ||
| Operator risk | Risk identified | ||||||||||||||||
| Assessment not finalised | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | |
| Worker risk | Risk identified | ||||||||||||||||
| Assessment not finalised | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | |
| Resident/bystander risk | Risk identified | ||||||||||||||||
| Assessment not finalised | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | X1,12 | |
| Consumer risk | Risk identified | ||||||||||||||||
| Assessment not finalised | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3,12 | X1,3 *** ,12 | X1,3 *** ,12 | X1,3 *** ,12 | X1,3 *** ,12 | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | ||||||||||||||||
| Assessment not finalised | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | X5,11 | |
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | X14,15 | X14,15 | X14,15 | X14,15 | X14 * ,15 * | X14 * ,15 * | X14 * ,15 * | X14 * ,15 * | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 | X14,15 |
| Assessment not finalised | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9 * ,10 | X7,8,9 * ,10 | X7,8,9 * ,10 | X7,8,9 * ,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | X7,8,9,10 | |
| Risk to aquatic organisms | Risk identified | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** | X** |
| Assessment not finalised | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | X11 | |
| Groundwater exposure to active substance | Legal parametric value breached | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 | X13 |
| Assessment not finalised | |||||||||||||||||
| Groundwater exposure to metabolites | Legal parametric value breacheda | ||||||||||||||||
| Parametric value of 10 μg/Lb breached | |||||||||||||||||
| Assessment not finalised | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | X2,4 | |
G: greenhouse; F: field; SEU: southern European Union; CEU: central European Union.
Not applicable for uses in permanent high‐technology greenhouse.
Despite the lack of a suitable endpoint for algae, likely driving the risk assessment for aquatic organisms, for the drip irrigation uses a high risk to aquatic organisms could be concluded even when using the ‘best case’ PECsw and the available endpoints (e.g. acute fish).
No data from residue field trials needed as application only every 15 years.
The superscript numbers relate to the numbered points indicated in Sections 10.1 and 10.2. Where there is no superscript number, see Sections 2–7 for further information.
Based on classification made in the context of this evaluation procedure under Regulation (EC) No 1107/2009. It should be noted that harmonised classification and labelling is formally proposed and decided in accordance with Regulation (EC) No 1272/2008. Or It should be noted that the classification proposed in the context of this evaluation procedure under Regulation (EC) No 1107/2009 concurs with the harmonised classification and labelling in accordance with Regulation (EC) No 1272/2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
8. Data gaps
This is a list of data gaps identified during the peer review process, including those areas in which a study may have been made available during the peer review process but not considered for procedural reasons (without prejudice to the provisions of Article 56 of the Regulation concerning information on potentially harmful effects).
Accelerated storage stability study for the formulation ‘Chloropicrin 99’ (relevant for the shank injection uses; see Section 1).
Additional information on the technical characteristics before and after 2 years storage of the formulation ‘Chloropicrin EC’ (relevant for the drip application uses; see Section 1).
Analytical methods for monitoring of the residue definition in dry and high oil content commodities (relevant for all representative uses evaluated; see Section 1).
An analytical method for monitoring of phosgene in air is required (relevant for all representative uses evaluated; see Section 1).
Further assessment of the toxicological relevance of one impurity included in the technical specification is required (relevant for all representative uses; see Section 2).
Further assessment/data on the gene mutation potential of chloropicrin, as follow up for a positive Ames test, should be provided (e.g. transgenic rodent mutation assay or rodent comet assay, including inhalation exposure and adequate target tissues; relevant for all representative uses; see Section 2).
Further assessment of the toxicological profile (first step: genotoxicity, second step: other toxicological endpoints in view of deriving reference values for consumers) of the metabolite DCNM is required, including full assessment of the relevance and reliability of the published literature (relevant for all representative uses; see Section 2).
A storage stability study on chloropicrin in a crop representative of the high water content commodities (preferably fruiting vegetables) and covering the maximum storage time interval of the residue samples in the trials on fruit crops in order to conclude on the validity of these trials is required (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
Further investigation on the metabolism of chloropicrin in plants is required considering the chemical structure of chloropicrin, the overall data available on the metabolism in plants, residue trials, the degradation pathway of chloropicrin in soil (as the representative uses consist of a soil application), the potential toxicological relevance of the metabolites identified in soil and any relevant literature search on the parent and metabolites (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
Northern European Union (NEU) zone: Three residue trials each on tomato, pepper and strawberry supporting the proposed uses in open field with drip tarped application and removing the VIF 1 day after cutting of the film (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
-
Southern European Union (SEU) zone:
Three residue trials each on tomato, pepper and strawberry supporting the proposed uses in open field with drip tarped application and removing the VIF 1 day after cutting of the film (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
Four residue trials each on tomato, pepper and strawberry supporting the proposed uses in open field with shank tarped application and removing the VIF 1 day after cutting of the film (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
Four residue trials each on tomato, pepper and strawberry supporting the proposed uses in open field with shank tarped application and planting through the VIF (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
One, four and two residue trials each on tomato, pepper and strawberry, respectively, supporting the proposed uses in greenhouse with drip tarped application and planting through the VIF (relevant for the uses in strawberry, tomato, pepper and cucurbits; see Section 3).
Reliable soil degradation studies under aerobic conditions with mass balance closed, reliable kinetic analysis of the degradation of chloropicrin and formation and degradation of metabolites (including DCNM), adequately identifying volatiles are required (relevant for all representative uses evaluated; see Section 4).
Reliable water/sediment investigation with a closed mass balance, allowing to characterise the route of degradation of chloropicrin in the aquatic environment (relevant for all representative uses evaluated; see Section 4).
Appropriate information to address the effect of water treatment processes on the nature of the residues that might be present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water (relevant for all representative uses evaluated; see Section 4).
A suitable avian dietary study with chloropicrin covering all reproductive stages (e.g. OECD 206) (relevant for all representative uses except those in permanent high technology greenhouse, see Section 5).
Toxicity data to address the long‐term risk to birds from inhalation of chloropicrin (relevant for all representative uses, see Section 5).
Reliable toxicity data for algae for chloropicrin and DCNM (relevant for all representative uses, see Section 5).
Chronic inhalation toxicity data for honeybees (relevant for all representative uses, see Section 5).
Toxicity data with chloropicrin on the standard species of foliar dwelling non‐target arthropods (relevant for all representative uses, see Section 5).
Valid studies with chloropicrin for earthworms and H. aculeifer (relevant for all representative uses except those in permanent high technology greenhouse, see Section 5).
Studies to characterise the toxicity of DCNM and any other potential soil metabolites (see data gap in Section 4) to all soil organisms (relevant for all representative uses except those in permanent high technology greenhouse, see Section 5).
A toxicity study able to appropriately characterise the toxicity of chloropicrin to non‐target terrestrial plants over a realistic exposure period (relevant for all representative uses, see Section 5).
A test according to OECD 231 (AMA) and a test according to OECD 229 (FSTRA) would be needed for further investigating the endocrine disruption potential of chloropicrin (assuming that those 2 tests are performed in parallel, the applicant would need to complete the data package to support a conclusion on absence of EATS‐mediated adversity/endocrine activity within an estimated time period of 19 months). However, if one of this test is positive, further tests according to OECD 241 and/or OECD 240 might be needed in order to further investigate the adversity and an additional estimated time period of 28 months would be needed (relevant for all representative uses; see Section 5).
9. Particular conditions proposed to be taken into account to manage the risk(s) identified
No particular conditions are proposed for the representative uses evaluated.
10. Concerns
10.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for the representative uses in line with the uniform principles in accordance with Article 29(6) of the Regulation and as set out in Commission Regulation (EU) No 546/201118 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of the Regulation.
-
1
The mutagenic potential of chloropicrin in vivo cannot be excluded (see Section 2).
-
2
The toxicological relevance of the groundwater metabolite DCNM, pending confirmation that the groundwater level is above 0.1 μg/L (see Sections 2 and 4).
-
3
Consumer risk assessment cannot be finalised pending on the confirmation of the residue definition for risk assessment and due to the lack of appropriate information to address the effect of water treatment processes on the nature of residues of chloropicrin and its metabolite DCNM, potentially present in surface water and groundwater, when surface water or groundwater are abstracted for drinking water (see Sections 3 and 4).
-
4
The potential contamination of groundwater by soil metabolites, including DCNM could not be finalised (see Section 4).
-
5
The long‐term dietary and inhalation risk to birds could not be finalised for all uses of chloropicrin, due to lack of a suitable hazard characterisation (see Section 5).
-
6
The risk assessment for aquatic organisms could not be finalised due to lack of toxicity data on algae for both chloropicrin and DCNM and due to the lack of reliable PECs (see Sections 4 and 5).
-
7
The chronic risk assessment to bees for exposure via inhalation could not be finalised (see Section 5).
-
8
In view of the lack of specific toxicity data, the risk assessment for foliar‐dwelling non‐target arthropods could not be finalised (see Section 5).
-
9
Due to the lack of reliable toxicity data for the parent, metabolite DCNM and any other potential soil metabolites, the risk assessment for earthworms could not be finalised (see Section 5).
-
10
The risk assessment to non‐target terrestrial plants could not be finalised, due to lack of a suitable hazard characterisation (see Section 5).
-
11
The assessment of the endocrine‐disrupting properties of chloropicrin for non‐target organisms could not be finalised due to the lack of suitable data (see Section 5).
10.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of the Regulation and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment performed at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of the Regulation.
-
12
In the absence of derivation of toxicological reference values (due to the inconclusive genotoxic potential of chloropicrin), the dietary and non‐dietary risk assessment for humans cannot be finalised (see Sections 2 and 3).
-
13
Potential groundwater contamination by chloropicrin at levels above 0.1 μg/L (see Section 4).
-
14
A high risk to soil macro‐organisms was identified for all uses of chloropicrin (see Section 5).
-
15
A high risk to soil micro‐organisms was identified for all uses of chloropicrin (see Section 5).
10.3. Overview of the concerns identified for each representative use considered
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
Table 1.
Soil
| Compound (name and/or code) | Persistence | Ecotoxicology |
|---|---|---|
| Chloropicrin | Data gap | Data gap (earthworms)/high risk (other organisms) |
| Dichloronitromethane (DCNM) | Data gap | Data gap |
In addition to the issues indicated below, the assessment of the endocrine‐disrupting properties of chloropicrin for non‐target organisms according to the scientific criteria for the determination of endocrine‐disrupting properties as set out in point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, could not be finalised.
Abbreviations
- AAOEC
acute acceptable operator exposure concentration
- AMA
Amphibian Metamorphosis Assay
- AOEC
acceptable operator exposure concentration
- bw
body weight
- CEU
central European Union
- DAR
draft assessment report
- DCNM
dichloronitromethane
- EAS
oestrogen, androgen and steroidogenesis
- EC
emulsifiable concentrate
- ECG
European Chloropicrin Group
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- ELS
early life‐stage
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- FSTRA
Fish Short‐Term Reproduction Assay
- GAP
Good Agricultural Practice
- GC‐ECD
gas chromatography‐electron capture detector
- GC–MS
gas chromatography–mass spectrometry
- GLP
good laboratory practice
- InChiKey
International Chemical Identifier Key
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- KFoc
Freundlich organic carbon adsorption coefficient
- LC50
lethal concentration, median
- LOQ
limit of quantification
- MRL
maximum residue level
- NEU
northern European Union
- NOAEC
no observed adverse effect concentration
- NOAEL
no observed adverse effect level
- NOEC
no observed effect concentration
- OECD
Organisation for Economic Co‐operation and Development
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- QSAR
quantitative structure–activity relationship
- RAC
regulatory acceptable concentration
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- STEL
short‐term exposure limit
- TWA
time‐weighted average
- UDS
unscheduled DNA synthesis
- VIF
virtually impermeable film
- VP
vapour‐realising product
- WHO
World Health Organization
Appendix A – List of end points for the active substance and the representative formulation
1.
Appendix A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2020.6028
Appendix B – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulab |
|---|---|---|
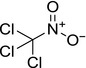
| chloropicrin |
Trichloronitromethane O=[N+](C(Cl)(Cl)Cl)[O‐] LFHISGNCFUNFFM‐UHFFFAOYSA‐N |
|
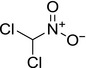
| dichloronitromethane (DCNM) |
dichloro(nitro)methane O=[N+](C(Cl)Cl)[O‐] XUNYLLBGLKGFHO‐UHFFFAOYSA‐N |
|
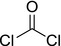
| phosgene |
phosgene ClC(Cl)=O YGYAWVDWMABLBF‐UHFFFAOYSA‐N |
|
| nitrosyl chloride |
nitrosyl chloride O=NCl VPCDQGACGWYTMC‐UHFFFAOYSA‐N |
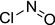
|
| nitromethane |
nitromethane C[N+]([O‐])=O LYGJENNIWJXYER‐UHFFFAOYSA‐N |
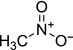
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ChemBioDraw v.13.0.2.3021.
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Arena M, Auteri D, Brancato A, Bura L, Carrasco Cabrera L, Chaideftou E, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lostia A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Pedersen R, Reich H, Santos M, Serafimova R, Sharp R, Stanek A, Sturma J, Szentes C, Terron A, Tiramani M, Vagenende B and Villamar‐Bouza L, 2020. Conclusion on the peer review of the pesticide risk assessment of the active substance chloropicrin. EFSA Journal 2020;18(3):6028, 25 pp. 10.2903/j.efsa.2020.6028
Requestor: European Commission
Question number: EFSA‐Q‐2014‐00456
Acknowledgement: EFSA wishes to thank the initial rapporteur Member State the United Kingdom and the new rapporteur Member State Italy for the preparatory work on this scientific output.
Approved: 30 January 2020
Notes
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 545/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for plant protection products. OJ L 155, 11.6.2011, p. 67–126.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, 1–1355. It is noted that this harmonised classification has been transferred from agreements under previous Directive 67/548/EEC.
See expert consultation point 2.1 from the report of the Pesticide Peer Review Meeting 13 (EFSA, 2020).
See expert consultation point 2.6 from the report of the Pesticide Peer Review Meeting 13 (EFSA, 2020).
See expert consultation point 2.9 from the report of the Pesticide Peer Review Meeting 13 where tentative reference values were derived by the experts (EFSA, 2020).
Recommendations of the Scientific Committee on Occupational Exposure Limits (European Commission ‐ Employment, Social Affairs & Inclusion Health and Safety at work – May 2013).
See also Evaluation table, expert consultation point 3.1 (EFSA, 2020).
See Appendix 1 in the Evaluation Table, expert consultation point 3.3 (EFSA, 2020).
Just application at Thiva and then just for specific application windows (part of the representative uses) were the predictions below the drinking water limit illustrated.
See expert consultation point 5.2 from the report of the Pesticide Peer Review Meeting 12 (EFSA, 2020).
See expert consultation point 5.3 from the report of the Pesticide Peer Review Meeting 12 (EFSA, 2020).
See expert consultation point 5.1 from the report of the Pesticide Peer Review Meeting 12 (EFSA, 2020).
See expert consultation point 5.4 from the report of the Pesticide Peer Review Meeting 12 (EFSA, 2020).
See expert consultation point 5.5 from the report of the Pesticide Peer Review Meeting 12 (EFSA, 2020).
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
See report of the Peer Review experts’ meeting PREV 12 (September 2019) expert consultation point 5.6 (EFSA, 2020). The experts discussed that considering the lack of T‐mediated adversity in the mammalian data package, the XETA might also be a suitable test.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311,135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18‐G‐01‐EN [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance chloropicrin. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- European Commission , 1999. Guidelines for the generation of data concerning residues as provided in Annex II Part A, Section 6 and Annex III, Part A, Section 8 of Directive 91/414/EEC concerning the placing of plant protection products on the market, 1607/VI/97‐rev. 2, 10 June 1999.
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2013. Guidance document on data requirements on efficacy for the dossier to be submitted for the approval of new active substances contained in plant protection products. SANCO/10054/2013‐rev. 3, 11 July 2013.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.1, March 2012.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use), 2009. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 1, 604 pp., as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.0, January 2011.
- JMPR (Joint Meeting on Pesticide Residues), 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues), 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- SETAC (Society of Environmental Toxicology and Chemistry), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2.
- United Kingdom , 2017. Draft Assessment Report (DAR) on the active substance chloropicrin prepared by the rapporteur Member State the United Kingdom in the framework of Regulation (EC) No 1107/2009, December 2017. Available online: www.efsa.europa.eu
- United Kingdom and Italy , 2019. Revised Draft Assessment Report (DAR) on chloropicrin prepared by the initial rapporteur Member State the United Kingdom and the new rapporteur Member State Italy in the framework of Regulation (EC) No 1107/2009, March and October 2019. Available online: www.efsa.europa.eu
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation


