Abstract
In 2019, no lumpy skin disease (LSD) outbreaks were reported in South‐Eastern Europe, the mass vaccination regional campaign with homologous LSD vaccine continued for the fourth year with over 1.8 million bovines vaccinated in the region, preventing further outbreaks since 2016. LSD outbreaks were reported in Turkey, including western Turkey, in Russia and in eastern Asia affecting China, Bangladesh and India for the first time. The use of homologous vaccine should be considered in the countries still affected in order to eliminate the virus. Besides passive surveillance, which is implemented in all the countries, active surveillance for early detection based on clinical examination could be conducted ideally during April–October every 5 weeks in at‐risk areas, based on possible re‐emergence or re‐introduction from affected neighbouring countries. Active surveillance for proving disease freedom could be based on serological testing (enzyme‐linked immunosorbent assay (ELISA)) targeting 3.5% seroprevalence and conducted on a random sample of cattle herds on non‐vaccinated animals. LSD re‐emerged in Israel in 2019, after vaccination became voluntary. This shows that, if the virus is still circulating in the region, the reduced protection might result in re‐emergence of LSD. In case of re‐emergence, a contingency plan and vaccine stockpiling would be needed, in order to react quickly. From a study performed in Israel to test side effects of live‐attenuated homologous LSD vaccine, milk production can be reduced during 7 days after vaccination (around 6–8 kg per cow), without a significant loss in the 30 days after vaccination. Research needs should be focused on the probability of transmission from insect to bovine, the virus inactivation rate in insects, the collection of baseline entomological data, the capacity of vector species in LSDV transmission linked to studies on their abundance and the control of Stomoxys calcitrans being the most important vector in LSD transmission.
Keywords: lumpy skin disease, spread, vaccine, mathematical model, surveillance, diagnostic test
Summary
In 2019, no lumpy skin disease (LSD) outbreaks were reported in South‐Eastern Europe. The mass vaccination regional campaign with homologous LSD vaccine has been continued in 2019 in South‐Eastern Europe, over 1.8 million bovines were vaccinated in the region, preventing further outbreaks since 2016 (since 2017 for Albania and few outbreaks in Greece and North Macedonia). LSD further spread to Eastern Asia with new countries affected for the first time, China, Bangladesh and India, thus substantially increasing the global LSD virus spread.
In 2019, LSD has been reported in Turkey for the seventh year, with an increased number of outbreaks compared to 2017 and 2018, including outbreaks reported in western Turkey close to Thrace and facing the Greek islands, which may represent a risk of further spread to South‐Eastern Europe. To reduce the risk of further spread to South‐Eastern Europe, homologous vaccine should be used in Turkey not only in Thrace as recommended by Global Framework for the progressive control of Transboundary Diseases (GF‐TADs), but also in the western part of the country opposite to Greek islands. Additionally, for virus elimination, this type of vaccine should be used in the entire country.
In Russia, LSD outbreaks have been reported in 2019 to the east of previous outbreaks since 2015. The vaccination based on sheep‐pox strain vaccine has not resulted in virus elimination yet.
In term of surveillance, in South‐Eastern Europe, passive surveillance is implemented nation‐wide in all countries; while active surveillance based on clinical examination and virological test for confirmation is in place in Albania, Bulgaria, Croatia, Greece, Kosovo*, North Macedonia, either as specifically for LSD or as part of other programmes; it allowed to spot seven suspected cases in 2019, that were then tested by polymerase chain reaction (PCR) and turned out negative.
Active surveillance for early detection could be conducted in at‐risk areas, determined either according to the last outbreaks in the country or in the neighbouring country from where possible incursion can be expected. Ideally this type of surveillance should be conducted every 5 weeks in the at‐risk period (April–October) targeting an expected prevalence of 0.042%, corresponding to the prevalence reached by the disease at 35 days after introduction. If this level of surveillance effort is not feasible (too many herds to be visited), the surveillance activities can be strengthened or partially replaced by adding systematic clinical examinations for LSD at live animal markets, before cattle leave their herds for any reasons (e.g. pre‐movement clinical checks) and during ante‐mortem examinations on animals to be slaughtered. These activities could be also combined with other surveillance programmes on the cattle population in place in the country.
Active surveillance for proving disease freedom could be based on serological testing (ELISA (ELISA)) targeting 3.5% seroprevalence conducted on a sample of cattle herds randomly selected from the whole country, testing the animals not vaccinated and where maternal immunity period has waned, to avoid interference with vaccine antibodies.
Based on the Israeli experience about reoccurrence of LSD in 2019, when vaccination becomes voluntary a sharp reduction in vaccination coverage can be expected, and if the virus is still circulating in the region and/or in neighbouring countries, the reduced protection might result in re‐emergence of LSD. Therefore, a contingency plan and vaccine stockpiling, even on a regional basis, would be needed, in order to react quickly with emergency vaccination.
From an observational controlled study performed in Israel to test the extent of side effects of live‐attenuated homologous LSD vaccine, it emerged that milk production is primarily reduced during 7 days after vaccination. The reduction along the whole week is around 6–8 kg per cow (depending on parity). For the rest of the 30 days after vaccination, the total reduction in milk production is not significant. In order to analyse the possible production losses linked to LSD vaccination, a randomised controlled trial with vaccinated and non‐vaccinated cows randomised in the same herds should be used. If this is not possible, then a large number of herds should be included in the analysis to control for other possible factors influencing survival and milk production.
There is experimental evidence that Stomoxys calcitrans and Haematopota spp. can mechanically transmit LSDV, and S. calcitrans being likely the most important vector, among those studied, in LSD transmission in South‐Eastern Europe. There is a need of better understanding the capacity of the other vectors present among countries and their potential role in LSDV transmission. These results should be linked to studies on the abundance of vectors in different regions.
Experimental evidence showed also that the LSD incubation period in bovine hosts can vary between 6 and 26 days. Based on this, the length of quarantine periods for bovines being moved from infected to non‐infected regions should be reconsidered.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
LSD is a viral disease of cattle that reached continental Europe via Turkey in 2015, affecting North Eastern Greece, between August and December. The disease returned again in the spring of 2016, this time affecting seven countries of South‐Eastern (SE) Europe with numerous outbreaks, namely Greece, Bulgaria, (the Republic of) North Macedonia, Serbia, Kosovo*, Albania and Montenegro.
By 2017, thanks to the coordinated vaccination campaigns in all affected countries of SE Europe (annual vaccination of all cattle, with live homologous vaccines, since 2016) the disease was contained. In addition, two non‐affected countries resorted to LSD vaccination too, as a preventive measure, namely Croatia (2016‐2017) and Bosnia and Herzegovina (2017). As a result of this combined effort, no new LSD outbreaks were reported in those countries where sufficient vaccination coverage was achieved.
The regional LSD vaccination strategy continued in 2019, in line with the recommendations of the latest meeting of the Standing Group of Experts on Lumpy Skin Disease in South‐East Europe under the GF‐TADs umbrella (GF TADs SGE LSD5, 19‐20 October 2017, Budva, Montenegro), where EFSA was present too.
Mass vaccination of cattle against LSD using live homologous vaccines figures clearly as the most effective control policy. Nevertheless, there is evidence that LSD virus remains present (e.g. recurrence of LSD in Albania in 2017) and non‐immune cattle remain at risk, even in areas of relatively high vaccine coverage.
In addition, 2019 will be the fourth consecutive year of mass LSD vaccination across South‐East Europe and the countries in the region, affected or at risk for LSD, have already expressed their will to collaborate in drafting an LSD exit strategy.
To this end updated scientific information and analysis is needed to assist the countries of South‐East Europe in drafting a regional roadmap on an LSD exit strategy from 2018 onwards, as described in the GF TADs SGE LSD5 recommendations.
EFSA, at the request of DG SANTE, has been systematically collecting and analysing LSD epidemiological data since 2016 and has already produced two (2) LSD scientific reports.
1.1.1. Terms of references
In view of the above and in the context of Articles 31 of Regulation (EC) No. 178/2002, EFSA is requested to provide scientific and technical assistance to the Commission on the continuation of the collection and analysis of the LSD epidemiological data in the context of article 31 of Regulation (EC) No. 178/2002, as detailed in the reference letter Ares(2016) 3953310, of 27 July 2016, for another two (2) years. In the framework of this exercise two reports should be produced, one in January 2019 and the other in January 2020. Given the extent and duration of LSD vaccination in South‐East Europe, every effort should be taken to include in the analysis as much information as possible, in relation to the performance of the LSD homologous vaccines (effectiveness, safety, etc.), in use since the beginning of the epidemic.
1.2. Introduction and interpretation of the Terms of Reference
2. Data and methodologies
2.1. Epidemiological data and analysis
The LSD outbreak data up to November 2019 were obtained from national authorities and Animal Disease Notification System (ADNS1) for those countries that notify to ADNS and from OIE and EMPRES Global Animal Disease Information System (Empres‐I2) for the other countries.
The population data and vaccination data at farm level related to the vaccination campaign conducted in 2019 were provided by the national authorities of Albania, Bulgaria, Greece, Kosovo*, Montenegro, North Macedonia, Serbia.
The data were described in a spatial and temporal context using GIS software.
2.2. Surveillance data from Croatia
Data of LSD serological surveillance of animals tested by ELISA were obtained from the Croatian veterinary services, 9089 entries of test result (positive or negative and, for the positive the OD values) with indication of animal geographical origin, date of sampling, date of vaccinations (2016, 2017 or both3) and animal characteristics like age, sex, breed, etc. Unfortunately, the data set was not arranged per animal ID, but different ID codes per animal were used by the lab., making the analysis difficult. Moreover, OD values were entered manually only for positive animals; thus, the analysis of OD trend over time since vaccination was not possible. The only outcome that could be tested was the ELISA result expressed as negative or positive.
A subset of the data set could be re‐organised to include unique animal IDs and dates of sampling, resulting in 4543 unique entries belonging to 1718 unique animal IDs and including animals tested multiple times between 14/6/2018 and 21/12/2018. Of those 1112 were tested at least twice, of which 665 gave always negative results and 447 gave positive results at least once. In Croatia, there were two vaccination campaigns, the vaccination dates of the tested animals in 2016 were between August and November 2016 and in 2017 between May and December 2017.
The data allowed to test:
The age profile of the positive animals that were never vaccinated;
If there was any correlation between ELISA result and time lag between vaccination and test and number of vaccinations.
A generalised linear mixed model was used in which a random intercept for each animal was considered to account for potential correlation between results coming from the same animal. The model was fitted using the number of vaccinations that an animal had received as well as the time lag between each of the vaccinations and the date of sampling.
3. Assessment
3.1. Epidemiological situation
3.1.1. Update of the spatial and temporal development of LSD epidemics in Eurasia
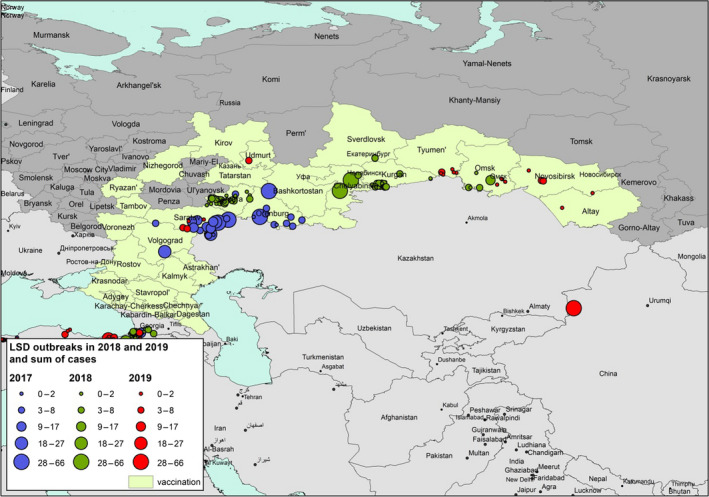
Considering Eurasia, up to November 2019, LSD outbreaks were reported in Russia (26 outbreaks), Turkey (131 outbreaks), Israel, Syria and, for the first time, in China (1), Bangladesh (2) and India (3 outbreaks). Compared to 2018, the LSD epidemic in the Russian Federation expanded northward and eastward along the border with Kazakhstan, while in Turkey, most outbreaks were reported in the Eastern regions. No outbreaks were reported in the Balkan region. Figures 1 shows the geographical distribution of LSD outbreaks in Europe and Asia.
Figure 1.
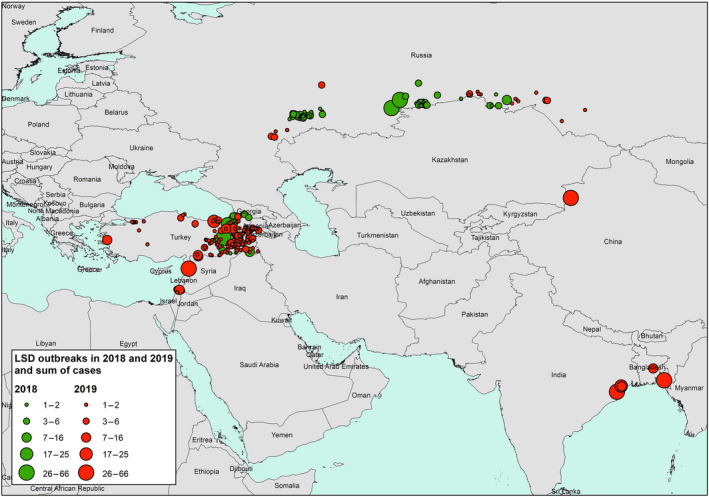
LSD outbreaks notified in Europe and Asia in 2019 compared to 2018 in relation to the sum of cases (amount of affected animals) (Data sources: national authorities and ADNS for those countries that notify to ADNS and Empres‐I/OIE for the other countries)
Figure 1 shows: (i) large number of LSD outbreaks in South Eastern Turkey, bordering Syria, but also few cases in Western Turkey; (ii) LSD outbreaks in the Russian Federation along the border with Kazakhstan expanding eastward in 2019 compared to 2018; (iii) LSD expanding eastward in Asia with outbreaks reported in China, Bangladesh and India for the first time in 2019; (iv) LSD reappeared in Northern Israel, bordering Lebanon and Syria, where LSD had not been reported since 2013.
In Figure 2, the temporal distribution of LSD outbreaks since 2012 is shown for those European and Asian countries that reported LSD in 2019 and/or 2018.
Figure 2.
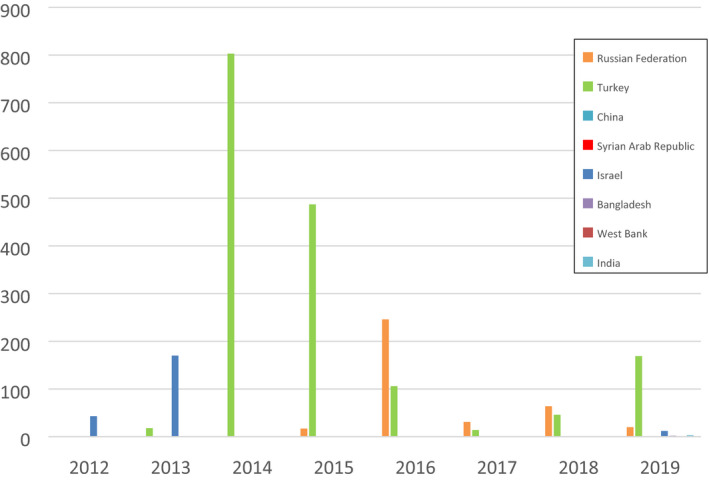
Temporal distribution of LSD outbreaks in European and Asian countries that reported any in 2019 and 2018 since 2012 (Data sources: Empres‐I and ADNS for Turkey)
Apart from the still active epidemics in Turkey and Russia, it is notable that LSD has been reported in China, India and Bangladesh for the first time, and re‐appeared in Israel after 6 years (see Section 3.1.4). Moreover, LSD was reported in 2019 in areas neighbouring Israel like Syria and the West Bank.
In Figure 3, the spatial distribution of outbreaks in 2018 and 2019 is shown by month.
Figure 3.
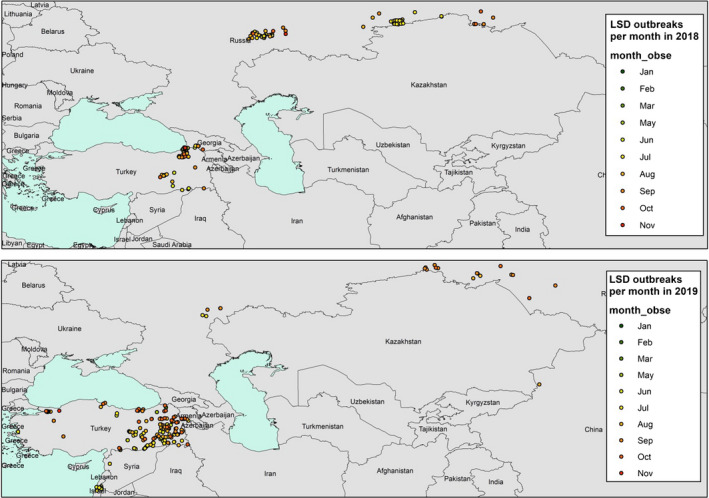
Monthly spatial distribution of the LSD outbreaks (coloured bubbles) in 2018 and 2019 (end of November). (Data source: Empres‐I and ADNS)
In Turkey, the outbreaks were reported between May and November, while in Russia, they occurred between July and October, thus confirming the seasonality of LSD outbreaks in the northern hemisphere. The few outbreaks in China, India and Bangladesh were reported in July and August.
3.1.2. LSD in Turkey in 2019
In 2019, 131 outbreaks were reported in Turkey, mostly in the eastern part of the country and mostly between April and October. Twenty outbreaks in western Turkey close to Thrace region and in Izmir opposite the Greek islands were reported. After a sharp decrease from 803 to 106 outbreaks between 2014 and 2016, when three to five times doses of sheep pox vaccine were used in the whole population, the number of reported outbreaks in Turkey has been slightly increasing from 14 in 2017 to 169 outbreaks in 2019 (Figure 4).
Figure 4.
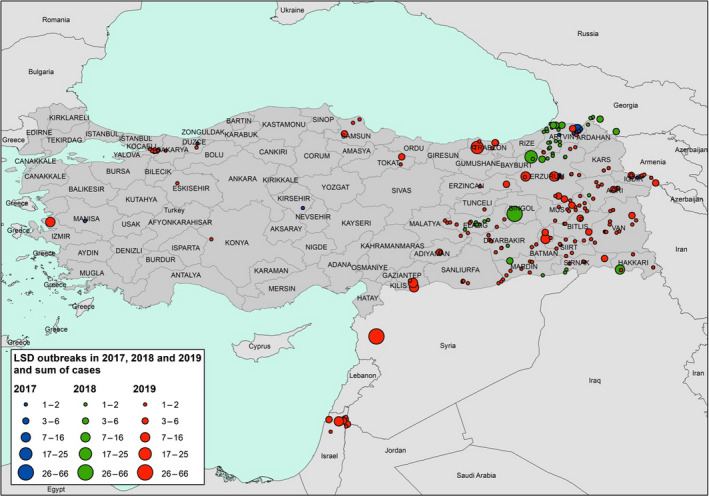
Reported LSD outbreaks in Turkey in 2017–2019 and sum of cases (number of affected animals) (source: ADNS)
Turkey has been combatting LSD intensively since the first occurrence of the disease in the country with a close collaboration with the international organisations such as OIE, FAO, EU. Turkey has a selective culling policy with 100% compensation, mandatory mass vaccination which is free of charge for farmers, and quarantine measures.
The reported outbreaks are the numbers of notifications which may be affected by some reporting bias and give slightly different indication than the real occurrence of the disease. Despite this, the recent increase of reported outbreaks in 2019 is remarkable, and some hypotheses can be advanced to explain it, including the level of protection conferred by the sheep pox vaccine, the timing of vaccination, biosecurity measures etc.
First consideration is about the level of protection conferred by the type of vaccine used, which may not be complete. A country‐wide mass vaccination campaign is in place since 2013, which reached 93% coverage in 2019.4 Vaccination in 2019 was, however, applied using the 5x dose sheep pox Bakırköy strain (heterologous vaccine) on all cattle older than 3 months. It should be remarked that the strain used in Turkey is Bakırköy strain sheep pox strain and not sheep and goat pox as reported by Şevik and Doğan (2017).5 For 2020, the vaccination based on homologous LSD strain is foreseen in the Thrace region, while in Anatolia sheep pox strain will be still used, with a recommendation by GF TADs, that consideration should be given to the use of 10x sheep dose, at least in high risk areas.6 Considering the vaccine effectiveness, a complete data set of field data on vaccination recorded at farm or village level with the date of vaccination and relative location would be needed to estimate the vaccination effectiveness of the sheep pox vaccine, so to compare the relative risk in vaccinated/unvaccinated villages towards the infected/uninfected ones. Vaccination data from two provinces (Aydın/Samsun) were provided to European Food Safety Authority (EFSA), although only from randomly selected villages and not from all the villages in the provinces; therefore, this analysis could not be finalised yet and the results are still pending.
Regarding the timing of vaccination in 2019, it was reported that the vaccine supply in 2019 was late, thus vaccination after June 2019 may have not protected the whole population. Also, incomplete application of biosecurity measures could have played a role in increasing LSD outbreaks. There are intense animal movements in Turkey linked to some religious fests, like Kurban Bayrami which took place on August 11 in 2019.
In the Thrace Region, a risk‐based surveillance programme is in place targeting a between‐herd and within‐herd prevalence of 5% for detecting the clinical signs among susceptible animals of each epidemiological unit with a 95% level of confidence. 65,369 farms with 624,420 animals were clinically examined four times a year in 2019; the suspected outbreaks were confirmed by PCR. Up to October 2019, 131 outbreaks were confirmed out of 214 LSD suspected farms (61%).
The seasonality of outbreaks reported in 2017, 2018 and 2019 in Turkey and the mean temperatures per month in 2017, 2018 and averaged over the last 30 years (at the time of writing mean temperatures of 2019 were not available) is displayed in Figure 5.
Figure 5.
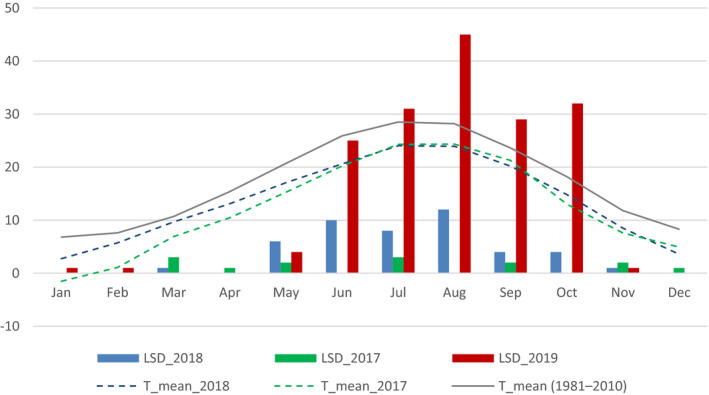
Monthly reported number of LSD outbreaks in Turkey in 2017, 2018 and 2019, and mean temperature in 2017, 2018 and averaged in the last 30 years
The seasonality in 2019 reflects the expected pattern with the vast majority of outbreaks between May and November (with two outbreaks reported in January and February), and 1‐month broader time window in the previous years.
3.1.3. LSD in Russia in 2019
Between March and up to October 2019, 26 LSD outbreaks were reported in six Russian provinces, around one‐third of the outbreaks reported in 2018. LSD outbreaks have been reported in 2019 to the east of previous outbreaks since 2015, along the border with Kazakhstan, across southern Siberia until Novosibirsk and Altai. LSD has been present in Russia since 2015 (Figure 6).
Figure 6.
Reported LSD outbreaks in Russian Federation in 2016–2019 and number of affected animals in each outbreak (source: OIE, GF TADs)
LSD vaccination was initially performed as a ring vaccination around the outbreaks then followed by a regional vaccination campaign of livestock in the regions bordering Caspian Sea and Kazakhstan using a sheep pox strain vaccine and achieving 80–100% coverage in the vaccinated area.7 Kononov et al. (2019) reported the detection of the Russian LSDV field strain as well as a vaccine‐like LSDV strain in 4 out of 13 outbreaks in 2017. The vaccine‐like LSDV strain was found 100–300 km from the Kazakhstan border while the majority of the Russian field LSDV outbreaks largely congregated along the border of neighbouring Kazakhstan. The authors suggest that a recombinant vaccine‐like LSDV strain may have emerged after natural coinfection of a host with a field and a vaccine‐like strain. However, the authors also mention that, in the Volgogradskaya oblast, the field virus might have already been circulating when vaccination was first initiated and that the field virus and the vaccine‐like virus were both found in the oblast Orenburgskaya; therefore, caution should be taken in the interpretation of their current findings, considering also the lack of literature concerning transmission of Neethling‐based vaccine strains. Sprygin et al. (2018) reported the finding of a recombination lumpy skin disease virus in a cow with typical LSD clinical signs. A total of 27 recombination events were identified. This is surprising as recombination in poxviruses is an extremely rare event and the authors mention themselves that published examples of poxvirus recombination clearly arising in the field are still lacking except in some laboratory conditions. The authors further conclude that the impact of their finding remains to be clarified.
It should be stressed that in South‐Eastern Europe, an LSDV vaccine strain was only found in rare cases related to vaccine side effects (Neethling‐response) occurring in less than 0.5% of all vaccinated animals (EFSA, 2017). Furthermore, several LSDV isolates from outbreaks occurring in South‐Eastern Europe were analysed using Full Genome Sequencing and no recombinant strain was found (Agianniotaki et al., 2017; Toplak et al., 2017; Lojkić et al., 2018; Sprygin et al., 2019).
3.1.4. LSD spread towards eastern Asia
In 2019, LSD has further spread to Eastern Asia, with outbreaks reported for the first time in China, Bangladesh and India.
In China, the outbreak was suspected on 03/08/2019 with 65 animals affected in a village in the Ili Kazak Autonomous Prefecture, in the north‐west Xinjiang province at the border with Kazakhstan, and it was confirmed on 10 August by QPCR. The last time LSD was reported in Kazakhstan was in November 2016 but in the western part of the territory at the border with Russia on the Caspian Sea.
In the Xinjiang province, there are around 4 million cattle. At the end of summer in this region, Kazakh nomads migrate with their sheep and cattle to autumn pastures.8
In Bangladesh in 2019, two outbreaks of LSD were reported, one in July and one in September, with 66 animals affected out of 360 exposed, in Chittagong and Dhaka, respectively.
In India, three outbreaks were reported to OIE in Orissa state, in Eastern India, with 79 affected animals out of 932 exposed.
Among the countries in Central Asia south from Russia (Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan), the only country that reported LSD was, as said above, Kazakhstan, in 2016, where vaccination with homologous vaccine (Lumpivax, Kenya Veterinary Vaccine Institute, KEVEVAPI9) has been ongoing since 2017, covering 100% of cattle in the western regions of the country, as reported to GF TADs.10
3.1.5. LSD recrudescence in Israel in 2019
From March 2013 to June 2016, vaccination against LSD in Israel was mandatory in cattle all over the country and the spread of LSD was stopped. Since June 2016, vaccination has been voluntary in Israel. Data were collected from farmers insured by ‘Hachaklait’, which is the biggest veterinary cooperative in Israel. Immediately after the cessation of mandatory vaccination, the vaccination coverage started decreasing and reached 21% of farms prior to the new LSD epidemic in May 2019. The steepest decline in vaccine application was observed in feedlot cattle and mixed herds, followed by beef cattle while the lowest decline was observed in non‐grazing dairy herds (according to data by ‘Hachaklait’).
On May 31, 2019, LSD cases were observed in several calves in a dairy herd in the Golan Heights with 1,300 cows and heifers. Although most of the herd was vaccinated, the disease occurred in a group of 200 young heifers that were not vaccinated. Emergency vaccination was applied in this group as soon as LSD cases were detected. A total of 18 calves eventually developed LSD: seven were euthanised due to welfare consideration and the rest recovered spontaneously.
Up to until October 2019, 18 herds had been detected, with a total of 72 cases out of 7,202 animals exposed in these herds in north Israel. Except for one case, which occurred 30 days after vaccination, all cases occurred either in non‐vaccinated animals or within 2 weeks after vaccination. In these non‐vaccinated animals, morbidity was up to 9%.
The main actions taken by the veterinary authorities were:
Mandatory vaccination performed by private veterinarians. However, vaccination needed to be postponed several days due to the detection of BTV contamination in a vaccine batch.
Zoning, movement control and quarantine of affected herds.
Increased surveillance in outbreak areas and areas at high risk.
Slaughtering only due to welfare considerations and not for the control of the epidemic.
The lessons learned from this recent re‐emergence of LSD are:
The situation in Israel demonstrates that when vaccination is voluntary, a sharp reduction in coverage is expected, even in highly industrialised dairy herds.
If the virus is circulating in the neighbouring regions, low coverage of vaccination might result in the re‐emergence of LSD. In such episodes (similar to the experience in the Balkans), it takes at least a few months until the disease is completely controlled again.
When vaccination becomes voluntary, stockpiling of vaccine is an option in order to act quickly with emergency vaccination in case of re‐emergence;
In areas bordering with countries where LSD outbreaks are ongoing, it is recommended that vaccination is continued, otherwise, in case of re‐introduction, it may take long to stop a recurrent epidemic. Therefore, if vaccination is stopped, a contingency plan for quick reaction should be in place.
Key messages:
In 2019, no LSD outbreaks reported in South‐East Europe.
In 2019, LSD further spread to Eastern Asia with new countries affected for the first time, China, Bangladesh and India, thus substantially increasing the global LSD virus spread;
-
LSD has been reported in Turkey, for the seventh year, with an increased number of outbreaks in 2019 compared to 2017 and 2018; for this reason, and according to OIE, Turkey can be considered as a LSD endemic country (Tuppurainen and Galon, 2016; Şevik and Doğan, 2017)
-
o
Although most of outbreaks reported in 2019 until November are located in the eastern part of Turkey, 20 new outbreaks have been reported also in western Turkey close to Thrace and opposite Greek islands, thus representing a risk of further spread to South‐East Europe (Figure 4);
-
o
The endemic situation in Turkey may be linked to different factors including the ineffectiveness of the vaccination campaign, in place since 2013, in eliminating the virus, and/or the incomplete application of biosecurity measures on animal movements. The reasons of virus circulation can be investigated in further epidemiological studies for which Turkish veterinary authorities can provide a complete data set including individual animal records;
-
o
The high vaccination coverage, as reported by Turkey, may raise doubts about the effectiveness of sheep pox strain‐based vaccine;
-
o
In Russia, similar to Turkey, LSD outbreaks have been reported in 2019 to the east of previous outbreaks since 2015; the vaccination based on SPP strain vaccine has not resulted in virus elimination.
LSD outbreaks mainly occur between May and November.
-
Based on the Israeli experience, when vaccination becomes voluntary, a sharp reduction in vaccination coverage can be expected, and if the virus is still circulating in the region and/or in neighbouring countries, the reduced protection might result in re‐emergence of LSD.
-
o
When vaccination is stopped, in case of LSD re‐emergence, in order to act quickly with emergency vaccination vaccine stockpiling, even on a regional basis, would be needed.
-
o
3.2. Situation about LSD control and prevention in South‐East Europe
In 2019, all countries in South Eastern Europe (Albania, Bosnia and Herzegovina, Bulgaria, Greece, Kosovo*, Montenegro, North Macedonia, Serbia) continued to vaccinate against LSD; over 1.8 million cattle were vaccinated, with the exception of Croatia that stopped in 2018. In all these countries, a live‐attenuated vaccine based on a homologous LSD vaccine strain was used. The vaccines used in South‐Eastern Europe were from different companies, either based on Neethling strain like Lumpy Skin Disease Vaccine for Cattle (Onderstepoort Biological Products; OBP, South Africa11) or Bovivax (MCI Santé Animale, Morocco12), or based on SIS Neethling type (Lumpyvax, MSD Animal Health‐Intervet, South Africa.13)
The level of the vaccination coverage calculated as the proportion of immunised animals, i.e. animals that received a vaccination in the last 12 months14 out of the total animals present) achieved in April and October 2019 (beginning and end of the vector season) in the Balkan region is displayed in Figure 7.
Figure 7.
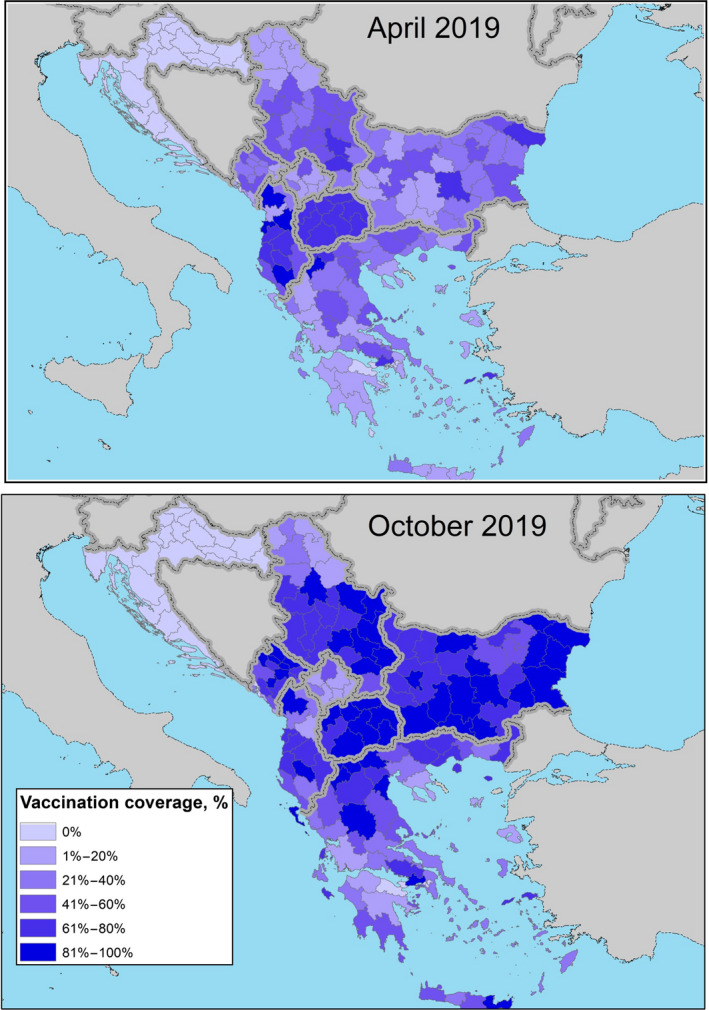
Vaccination coverage achieved in 2019 (proportion of immunised animals out of the total animals present) in the Balkan region at the beginning (April) and at the end (October) of the vector season (for Bosnia and Herzegovina, data were not available).
The situation shown in Figure 7 is up to end October 2019, approximately up to the end of vector season, although in most countries the vaccination campaign went on until end of 2019, reaching higher coverage. For example in Albania, till 31 December 2019, 283,137 animals were vaccinated out of 295,835 present animals in the whole country in that moment (around 96% coverage).
In Table 1, an overview about the past, current and future status of LSD vaccination and surveillance in South‐Eastern Europe is shown.
Table 1.
Overview of LSD vaccination and surveillance activities in South‐East European countries
| Albania | Bosnia and Herzegovina | Bulgaria | Croatia | Greece | Kosovo* | Montenegro | North Macedonia | Serbia | Turkey | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LSD spread |
Years with outbreaks1 (No of outbreaks per year) |
2016 (250) 2017 (50) |
never | 2016 (217) | Never |
2015 (117) 2016 (104) 2017 (2) |
2016 (46/1415) | 2016 (436) |
2016 (1454) 2017 (4) |
2016 (225) |
2013–current 2016 (106) 2017(14) 2018 (46) 2019 (169) |
| Vaccination | Years under Vaccination |
2016 2017 2018 2019 |
2017 2018 |
2016 2017 2018 2019 |
2016 2017 |
2015 2016 2017 2018 2019 |
2016 2017 2018 2019 |
2016 2017 2018 2019 |
2016 2017 2018 2019 |
2016 2017 2018 2019 |
2013–current |
| Type of Vaccines | Live homologous | Live homologous | Live homologous | Live homologous | Live homologous | Live homologous | Live homologous | Live homologous | Live homologous | Live heterologous (sheep pox strain, 3–5 times dose) | |
| Commercial Names |
Lumpy Skin Disease (OBP) Lumpyvax (MSD) |
Lumpy Skin Disease (OBP) Lumpyvax (MSD) |
Lumpy Skin Disease (OBP) Lumpyvax (MSD) BovivaxLSD‐N |
Lumpy Skin Disease (OBP) Lumpyvax (MSD) | Lumpy Skin Disease (OBP) Lumpyvax (MSD) | Lumpy Skin Disease (OBP) | Lumpy Skin Disease (OBP) | Lumpy Skin Disease (OBP) Lumpyvax (MSD) |
Lumpy Skin Disease (OBP) Bovivax LSD‐N |
Penpox‐M (PVCI) Poxdoll (Dollvet) Poxvac (Vetal) |
|
| Area of Vaccination | Whole country | High risk areas (border area with Serbia and Montenegro) | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | |
| Plan for 2020 | To be continued in whole country | No vaccination | To be continued in whole country | No vaccination | To be continued in the whole country | To be stopped | To be stopped | To be stopped | Partially continued | To be continued in the whole country | |
|
Passive Surveillance |
Present or not | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Area of implementation | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | Whole country | |
| Active Risk based Surveillance | Present or not | Yes | No | No | Yes | Yes | No | No | No | No | Yes |
| Area of implementation | Previous affected areas | The whole country | High risk area Konavle, Neretva |
North Greece Islands opposite Turkey |
– | – | – | – |
|
||
| Clinical examination | Yes | Yes, In case of suspicion | Yes | Yes | – | – | – | – | Yes | ||
| Serological tests | No | No | Yes | No | – | – | – | – | No | ||
| Virological tests | No | Yes, In case of suspicion | Yes for the seropositive | Yes for the suspicions | – | – | – | – | No | ||
| Active surveillance in the framework of other programs | Present or not | No | No | Yes | No | Yes | Yes | No | Yes | No | No |
| Context | – |
TB prog. BT prog. |
– |
slaughterhouses Brucellosis prog. TB prog. LSD vacc. prog. |
Brucellosis prog. TB prog. |
– |
LSD vacc. prog. TB prog. Brucellosis prog. |
– | – | ||
| Area of implementation | – |
border area (west‐southern part) The whole country |
– | Whole country | Whole country | – | Whole country | – | – | ||
| Clinical examination | – | Yes | – | Yes | No | – | Yes | – | – | ||
| Serological tests | – | No | – | No | Yes | – | Yes for the suspicions | – | – | ||
| Virological tests | – | Yes for the suspicions | – | Yes for the suspicions | Yes for the suspicions | – | Yes for the suspicions | – | – | ||
| Suspicions | Number of suspicions2 (herds per year) |
0 (2016) 345 (2017) 7 (2018) 0 (2019) |
2 (2016) 0 (2017) 0 (2018) 0 (2019) |
0 (2016) 0 (2017) 0 (2018) 0 (2019) |
5 (2016) 1 (2017) 3 (2018) 1 (2019) |
20 (2016) 3 (2017) 1 (2018) 3 (2019) |
0 (2016) 0 (2017) 0 (2018) 0 (2019) |
No data (2016) 9 (2017) 5 (2018) 1 (2019) |
In 2017, 15 farms suspected, all negative |
No data (2016‐2018) 1 (2019) |
295 (2016) 117 (2017) 166 (2018) 423 (2019) |
| Number of reactions to vaccination3 (herds per year) | No data |
0 (2016) 20 (2017) 0 (2018) 0(2019) |
0 (2016) 0 (2017) 0 (2018) 0 (2019) |
298 (2016) 389 (2017) 0 (2018) 0 (2019) |
2 (2016) 0 (2017) 0 (2018) 1 (2019) |
0 (2016) 0 (2017) 0 (2018) 0 (2019) |
No data | No data | No data |
0 (2016) 0 (2017) 0 (2018) 0 (2019) |
The number of outbreaks for Albania, Kosovo*, Montenegro and North Macedonia as reported in (EFSA, 2017). The number of outbreaks in Albania has been confirmed in January 2020 by the national authority.
The number of herds where there was a suspicion of LSD that turned to be negative after the laboratory investigation.
The number of herds where there was a suspicion of LSD and where the vaccine strain was then detected.
Key messages:
The mass vaccination regional campaign with homologous LSD vaccine has been continued in 2019 in South‐Eastern Europe (Albania, Bulgaria, Greece, Montenegro, North Macedonia, Serbia), the fourth year of vaccination for most of the countries in the region (the fifth year for Greece); over 1.8 million bovines were vaccinated in the region, preventing further outbreaks since 2016 (since 2017 for Albania and few outbreaks in Greece and North Macedonia);
Passive surveillance is implemented in all the countries nationwide;
Active surveillance based on clinical examination and virological test for confirmation is in place in Albania, Bulgaria, Croatia, Greece, Kosovo*, North Macedonia, either as specifically for LSD or as part of other programmes; it allowed to spot seven suspected cases in 2019, that were then tested by PCR and turned out negative.
3.3. Sero‐surveillance in Croatia
The data set of sero‐surveillance results from Croatia as discussed in Section 2.2 was analysed. The map below in Figure 8 represents the spatial distribution of results in the animals tested in Croatia in 2018; it shows how the sampling was concentrated in the areas closest to the last affected countries in 2016, Serbia and Montenegro.
Figure 8.
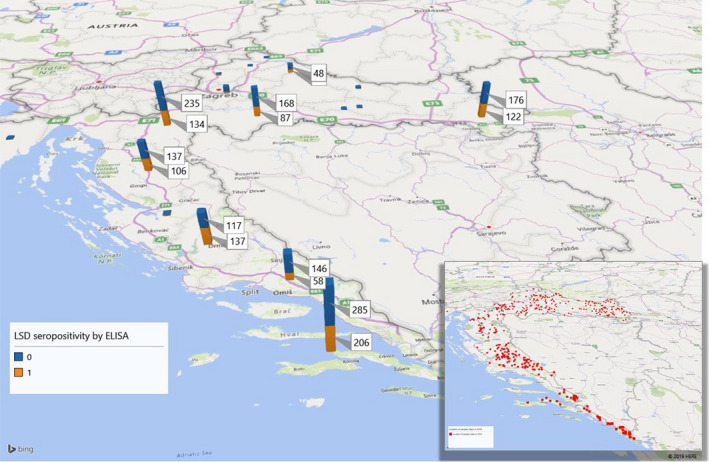
Geographical distribution of the test results of the samples tested for LSD by ELISA in Croatia in 2018 per county (in the small map the distribution of the samples with farm location where XY were available)
The number of animals which resulted negative or positive by ELISA, according to the number of vaccinations they have been subjected to, is shown in Figure 9.
Figure 9.
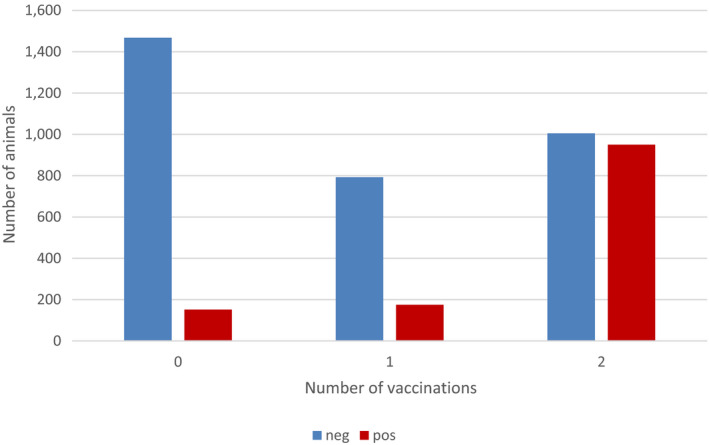
Number of positive and negative animals tested for antibodies according to number of vaccinations received
Figure 9 shows that the proportion of positive animals increases with the number of vaccinations received. This effect was also tested by generalised linear mixed model in the animals above 6 months of age (to avoid bias coming from maternal immunity), and resulted statistically significant (Annex A). This is in line with the study by (Milovanovic et al. (2019) who checked the humoral immune response against LSDV after re‐vaccination and found that re‐vaccination leads to a secondary response in the cattle population and significantly increases the number of animals with detectable antibody titres.
One hundred and fifty‐two animals never vaccinated tested positive. It must be taken into account that animals that are not vaccinated are the younger ones, and maternal immunity may have interfered with the serological test. In fact, when checking the age of the animals according to how many vaccinations they received, the median age of those never vaccinated and tested positive was 247 days (Q1–Q3: 150–355 days). Considering that Croatia is a free country, this may indicate that maternal immunity could last longer than what is normally expected (3–6 months as in other viral diseases), although this should be confirmed in further experimental studies. The distribution of the age of the animals according to the results of ELISA and number of vaccinations received is given in Figure 10, which shows that older animals are the ones that also received more than one vaccination.
Figure 10.
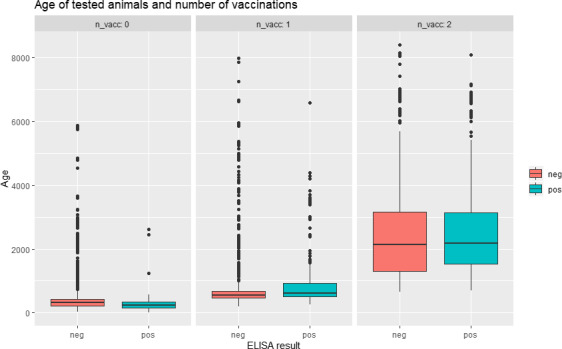
Age (days) of tested animals according to ELISA results and number of vaccinations received
The proportion of positive and negative ELISA results of animals aged more than 180 days (to avoid the possible interference of the maternal immunity) according to time post vaccination (1 and 2 vaccinations) is shown in Figure 11.
Figure 11.
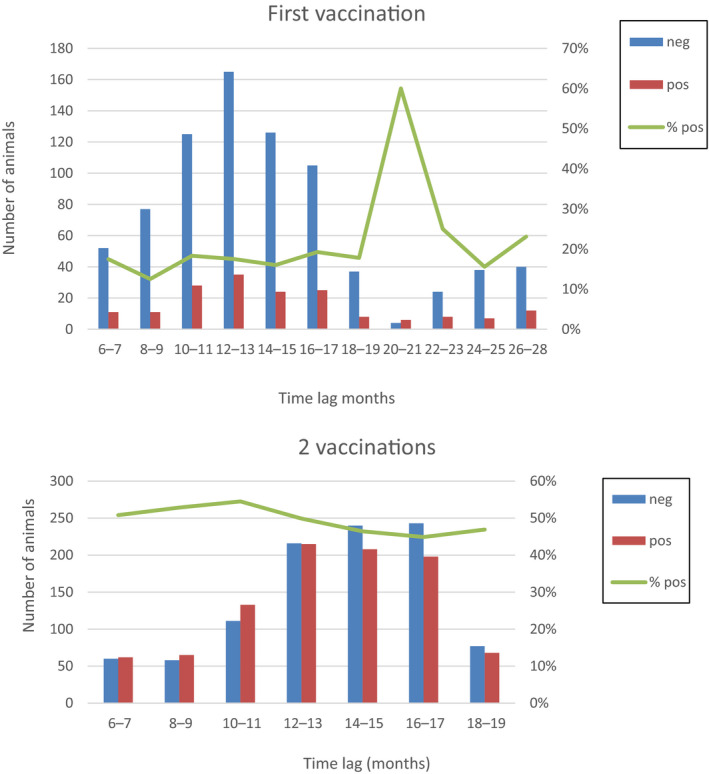
Proportion of seropositive animals compared to time after vaccination (1 and 2 vaccinations)
It can be seen that up to 26 months after the first vaccination and up to 18 months after the second vaccination, around 23% and 47% of the animals, respectively, were still positive, in line with the study by Milovanovic et al. (2019) where 33% of animals were still seropositive at ELISA after several months (11 months).
Nevertheless, while in the study by Milovanovic et al. (2019), the proportion of seropositive animals decreased over time, which could be expected, in Figure 11, it was more or less constant over time post vaccination. This was also observed when applying the generalised linear mixed model; the time lag variable in the model provided the same information as the number of vaccinations, so the more parsimonious model was chosen. This lack of the effect of time post vaccination on the proportion of seropositive should be still confirmed.
Key messages:
From the field data on serological surveillance as provided by Croatian national authorities, the preliminary analysis shows that the proportion of seropositive is influenced by the number of vaccinations received (more seropositive after second vaccination – booster – than after first vaccination).
Seropositive animals were tested positive in ELISA until 26 months post vaccination.
Nevertheless, a better‐shaped data set according to each animal with OD values for both positive and negative animals is needed in order to perform more robust analyses and to confirm this preliminary evidence.
3.4. Side effects of vaccination: survey in Israel
It is widely accepted that good vaccines should keep a balance between effectiveness and safety. There are several publications referring to the side effects after vaccination with Neethling LSD vaccine. This aspect has been reviewed in the EFSA report (EFSA, 2019):
‘Neethling disease’ in 0.4% of the vaccinated cows (Ben‐Gera et al., 2015);
Follow‐up of one vaccinated (Neethling vaccine OBP®) dairy farm (215 cows) showed: (i) local reaction 26/215 (12%), (ii) skin nodules – 19/215 (9%) and of these 9/19 viraemia, (iii) drop in milk production – no accurate estimation due to a small sample size and no use of controls (Katsoulos et al., 2018);
Identification of the vaccine virus in five vaccinated animals (Agianniotaki et al., 2017);
Demonstration of LSD vaccine DNA in blood, skin lesions, milk and saliva from vaccinated cows. (Bedekovic et al., 2017);
Decrease in milk production (RM65 vaccine) (Abutarbush and Tuppurainen, 2018);
Various reports on adverse effects, collected by passive surveillance (EFSA, 2019);
It is known that any vaccine might cause some side effects. However, the challenge is to quantify those losses in order to weigh the pros and cons of vaccination. In order to answer this question, the analysis should fulfil some prerequisites:
the analysis should be controlled (i.e. vaccinated animals should be compared to non‐vaccinated animals),
potential confounders (e.g. lactation, days in milk, season) should be controlled for,
it should be taken into account that usually all the cows in the herd are vaccinated together and therefore if something else occurs in the herd in parallel to the time of vaccination, the perception of the farmer is that this is due to vaccination and
analysis should be performed on a sufficiently large number of herds to enable control for such possible biases.
For this purpose, a study has been designed and is still in progress by Koret School of Veterinary Medicine in the Hebrew University of Jerusalem in Israel to analyse the changes in milk production after vaccination.
The methodology used was as follows:
86 herds were analysed, vaccinated between 60 and 210 days post‐partum;
Individual daily records of milk production were used;
The cows were divided into three lactation groups for which each analysis was performed separately;
The daily milk production in the 30 days before vaccination was compared to the milk production after vaccination;
A mixed effect general linear model was fitted to the data with the week of observation included as a fixed effect (to control for seasonal changes in milk production) and the cow and herd as random variables.
Preliminary conclusions of the study:
Milk production is primarily reduced during the 7 days after vaccination. The reduction for this entire week is around 6–8 kg per cow (depending on parity).
For the rest of the 30 days after vaccination, the total reduction in milk production is not significant.
This is one of the few studies conducted in a controlled way aiming at quantifying the production losses due to LSD vaccination side effects;
One limitation of this study is that it is conducted on re‐vaccinated animals, and not on animals vaccinated for the first time, which might experience different effects.
Recommendations for designing studies for vaccine side effects:
The best method is a randomised controlled trial with vaccinated and non‐vaccinated cows randomised in the same herds;
If this is not possible, then a large number of herds should be included in the analysis to control for other possible factors influencing survival, adverse effects and milk production;
The fact that an event occurs after vaccination does not necessarily imply causation.
3.5. Surveillance planning
In this section, some scenarios for planning the surveillance in the different previously LSD‐affected countries in South‐Eastern Europe is presented, both for early detection and for proving disease freedom, together with the calculation of sample size for both cases. Given the small median herd size in South‐Eastern Europe (two to four animals per farm in most countries), a two‐stage sample size calculation would not be useful; the epidemiological unit to be considered should be the herd. In case of larger herds (e.g. median herd around 100 animals), a two‐stage sampling can be performed. For this purpose, the within‐herd prevalence is needed; this has been estimated based on the mathematical model presented earlier (EFSA, 2017, 2018) and discussed in the next section.
3.5.1. Two‐stage sampling: estimation of within‐herd prevalence
The simulated dynamics of LSDV in a cattle herd when transmission is by the bites of S. calcitrans are shown in Figure 12 and details are given in Annexes B and C. The median (95% prediction interval) for the peak prevalence of infection (which also corresponds with the proportion of animals showing clinical signs) is around 46% (27–76%), which occurs at around 27 (19–44) days post introduction. The prevalence of infection (%) at 7, 14, 21 and 28 days post introduction is: 0 (0, 1.3), 1.0 (1.0, 34.8), 27.5 (1.7, 72.1) and 42.5 (4.1, 71.6), respectively. The seroprevalence (assumed to be equal to the proportion of recovered cattle) is around 46% (27–76%).
Figure 12.
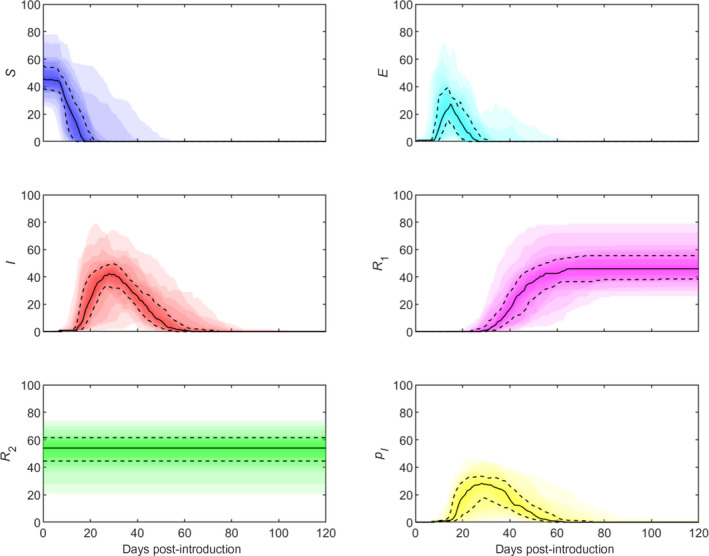
Simulated dynamics of lumpy skin disease virus in a cattle herd when transmission is by the bites of the stable fly, Stomoxys calcitrans
- Panels show the number of susceptible (S; blue), latent (E; cyan), infectious (I; red), recovered (R 1; magenta) and resistant (R 2; green) cattle and the proportion of infected vectors (p I; yellow) over time. Plots show the median (solid black line), 25th and 75th percentiles (dashed lines) and five percentile bands (up to the 5th and 95th percentiles; shading). Simulations assume a herd size of 100 cattle and infection is initiated by a single infected vector. Results are based on 1000 replicates of the model; only results for those replicates in which there was an outbreak (i.e. there was at least one infected bovine) are shown. The proportion of replicates resulting in an outbreak was 4.8%.
3.5.2. Surveillance for early detection in South‐East Europe countries
As described previously (EFSA, 2018), the following elements should be considered for planning the surveillance for early detection:
The period for testing by active surveillance: ideally every 5 weeks in the vector season, between April and October.
The test to be used is clinical examination; sensitivity is 75% (EFSA et al., 2018).
For the definition of the target area and the population at risk, a buffer of 60 km (area where LSD has a 99% probability of not spreading further, EFSA et al., 2018) is set for each country according to either the last outbreaks in the country or from the neighbouring country from where possible incursion can be expected; the related bovine population is calculated based on the geographical coordinates of the farms falling inside the buffer zone (Figure 13 and Table 2).
Figure 13.
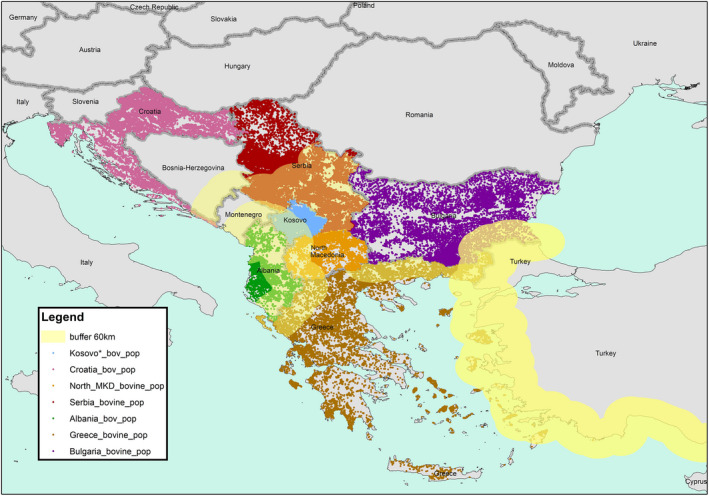
Buffer zone of 60 km for the definition of the surveillance area in each of the countries previously affected by LSD in South‐eastern Europe
Table 2.
Sample size for surveillance for early detection in each country in South‐Eastern Europe
| country | Buffer zone 60 km set according to | Total bovine herds | % herds in the buffer zone 60 km | Number of herds in the buffer zone | Median herd size in the buffer zone | Sample size herds |
|---|---|---|---|---|---|---|
| Albania | Last outbreaks in the country | 102,000 herds | Whole country | 102,000 | 2 | 9,184 |
| Bulgaria | Border with Turkey | 67,700 | 13% | 8,800 | 3 | 1,248 |
| Croatia | Border with closest last affected neighbouring country (MNE) | 25,000 | 0.4% | 179 | 2 | 118 |
| Greece | Border with Turkey and northern Greece (where surveillance is going on) | 18,000 | 33% | 6,000 | 19 | 2,634 |
| Kosovo* | Border with closest last affected neighbouring country (Albania) | 44,000 | 69% | 30,000 | 4 | 6,082 |
| Montenegro | Border with closest last affected neighbouring country (Albania) | 22,900 | 60% | 13,000 | 2 | 4,931 |
| North Macedonia | Border with closest last affected neighbouring country (Albania) | 19,500 | 47% | 9,100 | 4 | 3,778 |
| Serbia | Last outbreaks in the country (2016) | 114,000 | 68% | 77,000 | 3 | 6,297 |
Target population: as said above, given the small herd size (up to 8–10 animals), sampling should be done considering the herds as units, all animals in the herd should be examined. In case of bigger farms (100–1000 animals) and considering the within‐herd prevalence 46% (27 days post introduction, as in Section 3.5.1), then a random sampling of animals within the herd should be done, which gives a sample size of eight animals to be randomly selected within the herd.
design prevalence: between‐herd prevalence set at 0.042%, which corresponds to the expected prevalence at 35 days post introduction after stop vaccination (EFSA, 2018);
confidence level: 95%
The sample size values for each country are given in Table 2.
If this level of surveillance effort is not feasible, given the logistic and organisational difficulties in planning repeated visits to a such high number of cattle herds, the surveillance activities can be strengthened or partially replaced by adding systematic clinical examinations for LSD at live animal markets, before cattle leave their herds for any reasons (pre‐movement clinical checks) and during ante‐mortem examinations on animals to be slaughtered. These activities could be also combined with other surveillance programmes on cattle population in place in the country. Active surveillance can be feasible only in at‐risk areas and during the risk period.
3.5.2.1. Surveillance for testing freedom from disease
As described previously (EFSA, 2018), the following elements should be considered for planning the surveillance for proving disease freedom:
Period for testing: ideally the sampling should be at the end of vector season (e.g. October);
Target area: whole country, randomly selected herds;
Diagnostic test: serological assay (ELISA), test sensitivity is set at 80%. Under experimental conditions, the Sensitivity (Se) was estimated as 83%, while under field conditions in a preliminary study in Bulgaria it was 59% (EFSA, 2018). Nevertheless, in later field trials in Serbia and North Macedonia, the Se values were 75–80%, thus more in line with what observed under experimental conditions. For calculating the sample size, this can justify the ELISA Se value of 80%. The specificity is 99.7% (EFSA, 2018). In case of positivity, positive animals and the herds which they belong to should be followed up.
Target population: total number of herds randomly selected over the country; all eligible animals should be sampled in the farm; serological testing for disease freedom should target the ones not vaccinated and where the maternal immunity period has waned;
Design prevalence (between‐herd prevalence): 3.5%.
Confidence level: 95%
The sample size has been calculated and is equal to 106 herds in all the countries considered.
3.6. Update on research conducted on LSD in 2019
The scientific evidence from the papers on LSD published in 2019 is summarised in this section.
3.6.1. Diagnostics
A brief overview is given of several articles related to diagnostics for lumpy skin disease and published in 2019.
Möller et al. (2019) used specimens from the field and from experimental inoculation to evaluate different diagnostic tools, including a pan‐Capripox real‐time qPCR, a duplex real‐time qPCR assays and several serological methods (ELISA, indirect immunofluorescence test and serum neutralisation test [SNT]). The authors reported a high analytical sensitivity of both tested duplex real‐time qPCR systems for the reliable distinction of LSDV field and vaccine strains. The ID‐Vet antibody ELISA was as specific as the SNT, and therefore, they concluded that this ELISA provides an excellent tool for rapid and simple serological examination of LSDV‐vaccinated or infected cattle.
Milovanovic et al. (2019) described the humoral immune response to repeated lumpy skin disease virus vaccinations and the comparative performance of the ID‐Vet antibody ELISA to the virus neutralisation test (VNT) and the indirect fluorescent antibody test (IFAT). The detection rate of antibody‐positive animals one year after the primo vaccination was 33%, 73% 1 month after re‐vaccination and 59% 5 months post re‐vaccination. These figures were highly comparable to the VNT and IFAT.
Alexander et al. (2019) reported the development of a new real‐time PCR for the detection of LSD virus (LSDV) field, vaccine and recombinant strains as a follow‐up of the finding by Kononov et al. (2019) of vaccine‐like LSDV strains outbreaks in Russia and of a recombined LSDV as reported by Sprygin et al. (2018).
Adedeji et al. (2019) used samples from field outbreaks of Capripox virus infections in Nigeria to evaluate a newly developed partial sequencing protocol. This method allows a first, short molecular classification of strains and further phylogenetical analysis was done.
Erster et al. (2019) reported the use of the LSDV126, containing a duplicated region of 27 bp, to differentiate virulent and vaccine LSD viruses.
Douglass et al. (2019) used full genome sequencing to prove that the LSDV vaccine Herbivac LS is different from the Neethling vaccine strain in the locus encoding a superoxide dismutase (SOD) homolog. The presence of a SOD homolog, be it full length (as in Herbivac LS) or truncated (as in Neethling) may affect vaccine immunogenicity.
Munyanduki et al. (2020) reported how vaccine stocks can be cleared of BVDV by passage through an avian host, non‐permissive to Bovine viral diarrhoea virus (BVDV), but permissive to LSDV. This might be of importance as BVDV is a common contaminant of Madin‐Darby bovine kidney (MDBK) cells as well as fetal calf serum (FCS). MDBK cells are used in the generation of recombinant LSDV and have been used for the growth of LSDV vaccines.
Haegeman et al. (2019) developed an Immuno‐Peroxidase Monolayer Assay (IPMA) for the detection of antibodies against LSDV in an easy and low‐tech setting. The test was shown to be highly sensitive, specific and repeatable. In comparison to the VNT and a commercial ELISA, the LSDV‐IPMA was able to detect the LSDV antibodies earlier in infected, vaccinated and vaccinated/infected animals. The assay is very flexible as it can be easily adapted for the detection of sheep‐pox or goat‐pox antibodies and it can be scaled‐up to handle medium size sample sets by preparing the IPMA plates in advance. These plates are safe and can be handled in low biosafety level labs.
3.6.2. Epidemiology
Two papers have reported on the epidemiology of LSDV.
In the first paper, Manic et al. (2019) described outbreaks of LSDV in five districts in South‐Eastern Serbia in 2016. There were 189 outbreaks in the study area (out of 225 in Serbia as a whole), with the first case detected in June 2016. Overall morbidity and mortality were 13.6% and 0.4%, respectively. The median distance between outbreaks was 4.3 km (interquartile range (IQR): 1.89–8.88 km) and the median time between outbreaks was 9 days (IQR: 5–16 days). Vaccination with Neethling vaccine strain of LSDV commenced 3 weeks after the first reported case. The last case in the study region was in August 2016, and the last case in Serbia was in October 2019, a month and a half after the vaccination campaign ended. Information on post‐vaccination complications in the study area was not collected systematically, but there were no reports of complications from cattle owners.
In the second paper, Gubbins (2019) assessed the risk of transmission of LSDV by calculating the basic reproduction number (R 0) for five species of biting insects (the stable fly, Stomoxys calcitrans, the biting midge, Culicoides nubeculosus, and three mosquito species, Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus). This involved analysing previously published data on the mechanical transmission of LSDV and collating life history parameters from the published literature for each of the five species. Results suggested that S. calcitrans is likely to be the most efficient at transmitting LSDV (median estimate (95% prediction interval): R 0=15.5 (1.4–81.9)), with Ae. aegypti also an efficient vector (R 0 = 7.4 (1.3–17.6)). By contrast, C. nubeculosus (R 0 = 1.8 (0.06–13.5)), Cx. quinquefasciatus (R 0 = 0.8 (0.09–3.5)) and An. stephensi (R 0 = 1.6 (0.2–6.0)) were likely to be less efficient vectors of LSDV. However, there was considerable uncertainty associated with the estimates of R 0. Sensitivity analysis suggested that future work should focus on estimating the probability of transmission from insect to bovine and the virus inactivation rate in insects, as well as collecting baseline entomological data for each vector species.
3.6.2.1. New evidence on the role of vectors in LSD transmission
LSDV transmission is an important factor in understanding the epidemiology of the disease. A limited number of publications have shown several haematophagous arthropod species, like dipterans and ticks, to play a role in the transmission of LSDV. Tentative links have been made for the stable fly S. calcitrans, such as the presence of live LSDV in the flies and the capacity to transmit sheep‐pox, but no direct conclusive data is available on the importance of biting flies and horseflies as potential vectors for LSDV. Therefore, in vivo transmission studies were carried out to investigate possible LSDV transmission by S. calcitrans biting flies and Haematopota spp. horseflies from experimentally infected viraemic donor cows to acceptor cows. LSDV transmission by S. calcitrans was evidenced in three independent experiments (Sohier et al., 2019). Transmission was demonstrated not only by the development of clinical signs (noduli) but also by viraemia and seroconversion. Interestingly, the incubation period was variable and was found to be between 6 and 26 days and needs to be kept in mind when establishing quarantine periods. As in one of the animal trials only a short period between feeding on donor and acceptors was used, it can be stated that S. calcitrans has the capacity to transmit LSDV mechanically. Furthermore, one of the two acceptor cows receiving horseflies, fed on LSDV viraemic donor cows, became clinically ill and developed viraemia, indicating that this vector is also capable of transmitting LSDV.
These data provide for the first‐time direct evidence for the mechanical transmission of LSDV by S. calcitrans and shows that multiple vectors (including Haematopota spp.) can be involved in LSDV epidemiology. This study supports the need of better understanding the capacity of the different vectors present among countries and their potential role in LSDV transmission. These results should be linked to studies on the abundance of vectors in different regions.
3.6.3. Risk assessment
Two studies explored the spatial and temporal risk of LSDV (Allepuz et al., 2019; Machado et al., 2019). These first examined the relationship between the occurrence of reported outbreaks in South‐Eastern Europe, Turkey and Russia and livestock density, land cover and environmental variables. The resulting statistical models were then used to assess the risk of LSDV in Eastern Europe, the Caucasus and central Asia.
Allepuz et al. (2019) used logistic regression modelling to identify factors associated with LSDV occurrence, while Machado et al. (2019) used a combination of ecological niche modelling and Poisson regression. Allepuz et al. (2019) identified increased odds of LSDV associated with land cover (specifically, cropland, grassland or shrubland compared with forest), higher cattle densities, higher annual mean temperature and higher diurnal temperature ranges. By contrast, Machado et al. (2019) did not identify any association between land cover and the risk of LSDV but did find an increased risk associated with higher temperatures, higher precipitation and lower wind speeds. Despite the differences in underlying risk factors, both studies identified similar regions as being at high risk of LSDV transmission.
Two studies examined the risk of introduction of LSDV via imported cattle (Saegerman et al., 2019; Taylor et al., 2019). Saegerman et al. (2019) carried out a quantitative import risk assessment for the introduction of LSDV to France. By contrast, Taylor et al. (2019) developed a generic framework for quantitative risk assessment for disease introduction but illustrated its use by applying it to the case of the introduction of LSDV to unaffected countries in Europe via imported cattle (Taylor et al., 2019). Both approaches considered the probabilities along the risk pathway from importing animals from at‐risk areas to infection being transmitted to local cattle. The model of Saegerman et al. (2019) was parameterised using a combination of expert opinions, field data and published literature, while that of Taylor et al. (2019) used publicly available data and published literature.
Saegerman et al. (2019) estimated that the probability of introducing LSDV through imported cattle, it being transmitted to French cattle was 5.4 × 10−4 (95% prediction interval (PI): 0.4 × 10−4–28.7 × 10−4) in summer months, when vector activity is high, and 1.8 × 10−4 (95% PI: 0.1 × 10−4–15.0 × 10−4) in winter months, when vector activity is low. These probabilities were most sensitive to assumptions about the within‐herd prevalence of LSDV and the probability that an infected animal is infectious. Taylor et al. (2019) found that Croatia had the highest mean annual probability of infection (assuming that vaccination was not carried out), with Italy, Hungary and Spain at the next highest risk of introduction. These results were consistent across spatial scales from countries to regions and to farms. In addition, Taylor et al. (2019) estimated that France had a negligible probability of LSDV being introduced.
Casal et al. (2019) developed a simple model to estimate the number of vaccine doses that would be needed to control an epidemic and applied it to the case of a putative LSDV epidemic in France. The model, which is provided by the authors as an Excel spreadsheet and an R script, is based on the rate of disease spread (i.e. distance travelled by the disease per week), the time taken to achieve effective vaccination (i.e. the time from introduction to detection, the time needed to vaccinate the population and the time from vaccination to protection) and cattle densities in the affected area. Assuming a 7‐week period is needed to vaccinate all animals and a spread rate of 7.3 km/week (i.e. that estimated for the Balkans in 2015–2016), 741,000 vaccine doses would be sufficient to control an epidemic in 90% of the simulations and 609,000 would be sufficient in 75% of the simulations.
4. Conclusions
In 2019, no LSD outbreaks were reported in South‐Eastern Europe.
The mass vaccination regional campaign with homologous LSD vaccine has been continued in 2019 in South‐Eastern Europe, the fourth year of vaccination for most of the countries in the region (the fifth year for Greece), over 1.8 million bovines were vaccinated in the region, preventing further outbreaks since 2016 (since 2017 for Albania and few outbreaks in Greece and North Macedonia);
In South‐Eastern Europe, passive surveillance is implemented nationwide in all countries; while active surveillance based on clinical examination and virological test for confirmation is in place in Albania, Bulgaria, Croatia, Greece, Kosovo*, North Macedonia, either as specifically for LSD or as part of other programmes; it allowed to spot seven suspected cases in 2019, that were then tested by PCR and turned out negative.
Active surveillance for early detection could be conducted in at‐risk areas, determined either according to the last outbreaks in the country or in the neighbouring country from where possible incursion can be expected. Ideally this type of surveillance should be conducted every 5 weeks in the at‐risk period (April–October) targeting an expected prevalence of 0.042%, corresponding to the prevalence reached by the disease at 35 days after introduction. If this level of surveillance effort is not feasible (too many herds to be visited), the surveillance activities can be strengthened or partially replaced by adding systematic clinical examinations for LSD at live animal markets, before cattle leave their herds for any reasons (e.g. pre‐movement clinical checks) and during ante‐mortem examinations on animals to be slaughtered. These activities could also be combined with other surveillance programmes on the cattle population in place in the country.
Active surveillance for proving disease freedom could be based on serological testing (ELISA) targeting 3.5% seroprevalence conducted on a sample of cattle herds randomly selected from the whole country, testing the animals not vaccinated and where maternal immunity period has waned, to avoid interference with vaccine antibodies;
When planning the LSD surveillance, where a two‐stage sampling is needed (in case of larger herds, median herd size around 100 animals) both the within‐herd and between‐herd prevalence is needed. By a mathematical model, the expected within‐herd prevalence of LSD infection has been estimated at 7, 14, 21 and 28 days post introduction as 0% (0, 1.3), 1.0% (1,0, 34.8), 27.5% (1.7, 72.1) and 42.5% (4.1, 71.6), respectively (median and confidence intervals).
In 2019, LSD further spread to Eastern Asia with new countries affected for the first time, China, Bangladesh and India, thus substantially increasing the global LSD virus spread;
LSD has been reported in Turkey for the seventh year, with an increased number of outbreaks in 2019 compared to 2017 and 2018, including outbreaks reported in western Turkey close to Thrace and facing the Greek islands, which may represent a risk of further spread to South‐Eastern Europe;
The endemic situation in Turkey may be linked to different factors including the ineffectiveness of the vaccination campaign in place since 2013 to eliminate the virus, and/or the incomplete application of biosecurity measures on animal movements. The high vaccination coverage, as reported by Turkey, may raise doubts about the effectiveness of sheep‐pox strain‐based vaccine;
In Russia, LSD outbreaks have been reported in 2019 to the east of previous outbreaks since 2015. The vaccination based on sheep‐pox strain vaccine has not resulted in virus elimination yet.
Based on the Israeli experience about reoccurrence of LSD in 2019, when vaccination becomes voluntary a sharp reduction in vaccination coverage can be expected, and if the virus is still circulating in the region and/or in neighbouring countries, the reduced protection might result in re‐emergence of LSD.
From the field data on serological surveillance as provided by Croatian national authorities, the preliminary analysis shows that the proportion of seropositives is influenced by the number of vaccinations received, and vaccinated animals can be tested positive at ELISA until 26 months post vaccination. Nevertheless, better‐shaped data sets are needed in order to perform a more robust analysis and to confirm this preliminary evidence.
From an observational controlled study performed in Israel to test the extent of side effects of live‐attenuated homologous LSD vaccine, it emerged that milk production is primarily reduced during 7 days after vaccination. The reduction along the whole week is around 6–8 kg per cow (depending on parity). For the rest of the 30 days after vaccination, the total reduction in milk production is not significant.
There is experimental evidence that Stomoxys calcitrans and Haematopota spp. can mechanically transmit LSDV, and S. calcitrans being likely the most important vector, among those studied, in LSD transmission in South‐Eastern Europe.
There is experimental evidence that the LSD incubation period in bovine hosts can vary between 6 and 26 days.
5. Recommendations
To reduce the risk of further spread to South‐Eastern Europe, homologous vaccine should be used in Turkey not only in Thrace as recommended by GF TADs but also in the western part of the country opposite to Greek islands. Additionally, for virus elimination, this type of vaccine should be used in the entire country.
When vaccination is stopped or becomes voluntary, in case of LSD re‐emergence, a contingency plan and vaccine stockpiling, even on a regional basis, would be needed, in order to react quickly with emergency vaccination.
Since the evidence suggests that S. calcitrans is the most important vector in LSD transmission, research should be performed about possible control of this species.
Future work should focus on estimating the probability of transmission from insect to bovine and the virus inactivation rate in insects, as well as collecting baseline entomological data for each vector species.
There is a need of better understanding the capacity of the different vectors present among countries and their potential role in LSDV transmission. These results should be linked to studies on the abundance of vectors in different regions.
In order to analyse the possible production losses linked to LSD vaccination, a randomised controlled trial with vaccinated and non‐vaccinated cows randomised in the same herds should be used. If this is not possible, then a large number of herds should be included in the analysis to control for other possible factors influencing survival and milk production.
Based on new evidence about the length of incubation period, the length of quarantine periods for bovines being moved from infected to non‐infected regions should be reconsidered.
Abbreviations
- ADNS
Animal Disease Notification System
- BVDV
Bovine viral diarrhoea virus
- ELISA
enzyme‐linked immunosorbent assay
- FCS
fetal calf serum
- IFAT
indirect fluorescent antibody test
- IPMA
Immuno‐Peroxidase Monolayer Assay
- IQR
interquartile range
- LSD
Lumpy skin disease
- LSDV
Lumpy skin disease virus
- MDBK
Madin‐Darby bovine kidney
- PCR
Polymerase chain reaction
- SNT
Serum neutralisation test
- SOD
Superoxide dismutase
- GF‐TADs
Global Framework for the progressive control of Transboundary Diseases
- VNT
Virus neutralisation test
Annex A – Effect of number of vaccinations and time lag between first or second vaccination
1.
A generalised linear mixed model was used in which a random intercept for each animal was considered to account for potential correlation between results coming from the same animal. The model was fitted using the number of vaccinations that an animal had received as well as the time lag between each of the vaccinations and the date of sampling. A backward selection procedure was followed, identifying the number of vaccinations as the only significant effect. The number of vaccinations was considered as a saturated effect in order to allow other types of relationship than the linear one.
The effect of number of vaccinations and time lag between first or second vaccination and date of sampling on the seropositivity was tested in the animals above 6 months of age (to avoid bias coming from maternal immunity), the results of the generalised linear mixed model are shown below:
| Estimate | Std. Error | z value | Pr(> |z|) | |
|---|---|---|---|---|
| (Intercept) | −7.2964 | 0.4339 | −16.814 | < 2e‐16*** |
| as.factor(n_vacc)1 | 1.6505 | 0.4447 | 3.711 | 0.000206*** |
| as.factor(n_vacc)2 | 6.5178 | 0.5110 | 12.755 | < 2e‐16*** |
Signif. codes: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1 ‘ ’ 1.
As said in Section 2.2, the time lag variables in the model provide the same information as the number of vaccinations, so the more parsimonious model was chosen.
The model results show the effect of number of vaccinations on the ELISA result is significant and the estimated effect obtained is positive when two vaccinations are done, indicating that the proportion of sero‐converted samples increases if the number of vaccinations increases for 1 and 2 vaccinations. The estimated probability of a positive ELISA increases from 0.0007 in non‐vaccinated animals to 0.315 in animals vaccinated twice (Figure A.1). In single vaccinated animals, it was only slightly higher than in non‐vaccinated animals.
Figure A.1.
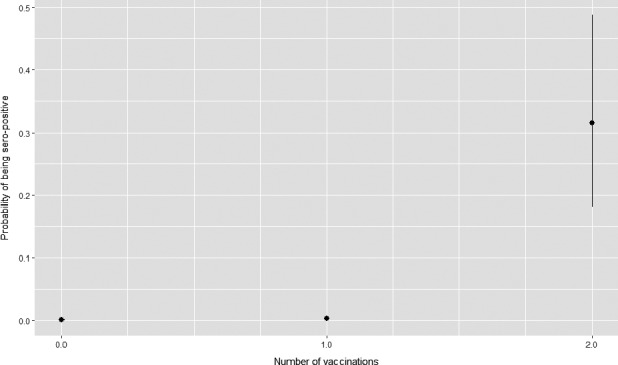
Probability of being seropositive according to the number of vaccinations received
Annex B – The dynamics of lumpy skin disease virus in a cattle herd
B.1. Modelling approach
B.1.1. Transmission model for LSDV
A stochastic compartmental model was developed to describe the transmission of lumpy skin disease virus (LSDV) in a cattle herd via the bites of insect vectors. This was adapted from an earlier deterministic model presented in Gubbins (2019). For simplicity, a single vector species, the stable fly, Stomoxys calcitrans, is assumed to transmit LSDV on a farm.
The cattle population (H) is subdivided into the number of susceptible (i.e. uninfected), latent (i.e. infected, but not yet infectious), infectious (and showing clinical signs, specifically skin lesions), recovered and resistant animals, denoted by S, E, I, R1 and R2, respectively. Resistant cattle do not develop generalised disease (i.e. skin lesions) and are assumed not to be infectious. To allow for more general (gamma) distributions for the latent and infectious periods, the latent and infectious cattle populations, E and I, are subdivided into ni stages each of mean duration 1/niri (so that the latent and infectious periods follow gamma distributions with means 1/ri and variances 1/niri 2 for i = E,I, respectively) (Anderson & Watson, 1980). Disease‐associated mortality is assumed to be negligible, so the cattle population remains constant.
The vector population (N) is subdivided into the number of susceptible (i.e. uninfected) and infected insects, denoted by X and Y, respectively. Because transmission is mechanical and there is no need for the virus to replicate in the insect before transmission can occur, a vector becomes infectious as soon as it takes an infected blood meal (i.e. there is no extrinsic incubation period) and remains infectious until the virus is inactivated (which occurs at rate γ), when it become susceptible again, or the insect dies. Vector mortality occurs at the same rate (μ) in both classes and is balanced by the recruitment of susceptible vectors, so that the total vector population (N) remains constant.
The force of infection for cattle is given by,
| (1) |
where b is the probability of transmission from insect to bovine, m=N/H is the vector‐to‐host ratio, a is the reciprocal of the time interval between blood meals, pR is the proportion of cattle resistant to disease and Y/N is the proportion of bites which are from infected insects. The force of infection for vectors is given by,
| (2) |
where b is the probability of transmission from bovine to insect and the remaining terms are defined above.
B.1.2. Parameter estimation
Parameters related to mechanical transmission of LSDV by S. calcitrans and the duration of latent and infectious periods (using detection of virus or viral DNA in skin lesions as a proxy measure for infectiousness) were estimated using data from challenge experiments (Babiuk et al., 2008; Chihota et al., 2001, 2003; Tuppurainen et al., 2005) analysed with Bayesian methods (Gubbins, 2019). The proportion of cattle resistant to disease was estimated using the numbers of cattle that did not develop generalised disease following challenge in published experiments (Babiuk et al. 2008; Carn & Kitching, 1995; Möller et al., 2019; Tuppurainen et al., 2005) (see appendix for details). Plausible ranges for life history parameters for S. calcitrans were extracted from the published literature (Gubbins, 2019). The parameters are summarised in Table 2.
For each replicate of the model, a set of parameters was obtained by sampling from the joint posterior distributions for the mechanical transmission parameters (b, β and γ), the latent and infectious period parameters (nE, rE, nI and rI) and the proportion of cattle resistant to disease (pR). Life history parameters (m, a and μ) were sampled from their plausible ranges.
B.1.3. Model implementation
Population sizes in the model take integer values, while transitions between compartments are stochastic processes (Table 1). The number of transitions of each type during a small time interval δt was drawn from a binomial distribution with population size n and transition probability q (the appropriate per capita rate multiplied by δt) (Table 1). However, binomial random variables are computationally expensive to simulate and an approximating distribution was used wherever possible. If: (i) nq(1−q) > 25; (ii) nq(1−q) > 5 and 0.1 < q < 0.9; or (iii) min(nq,n(1−q)) > 10, an approximating normal variate with mean nq and variance nq(1−q) was used, while if q < 0.1 and nq < 10, an approximating Poisson variate with mean nq was used (Forbes et al., 2011).
The initial number of cattle in each class is S(0) = H–R2(0), Ej(0) = 0 (j = 1,…,nE), Ij(0) = 0 (j = 1,…,nI) and R1(0) = 0, where R2(0) is drawn from a binomial distribution with probability of success, p R, and number of trials, H. The initial number of susceptible and infected vectors are X(0) = N−1 and Y(0) = 1, respectively.
B.2. Results
The simulated dynamics of LSDV in a cattle herd when transmission is by the bites of S. calcitrans are shown in Figure 1. The median (95% prediction interval) for the peak prevalence of infection (which also corresponds with the proportion of animals showing clinical signs) is around 46% (27–76%), which occurs at around 27 (19–44) days post introduction. The prevalence of infection (%) at 7, 14, 21 and 28 days post introduction is: 0 (0, 1.3), 1.0 (1,0, 34.8), 27.5 (1.7, 72.1) and 42.5 (4.1, 71.6), respectively. The seroprevalence (assumed to be equal to the proportion of recovered cattle) is around 46% (27–76%).
References
Anderson D & Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198.
Babiuk S, Bowden TR, Parkyn G, Dalman B, Manning L, Neufeld J, Embury‐Hyatt C, Copps J & Boyle DB, 2008. Quantification of lumpy skin disease virus following experimental infection in cattle. Transboundary and Emerging Diseases, 55, 299–307.
Carn VM & Kitching RP, 1995. An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiology and Infection, 114, 219–226.
Chihota CM, Rennie LF, Kitching RP & Mellor PS, 2001. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiology and Infection, 126, 317–321.
Chihota CM, Rennie LF, Kitching RP & Mellor PS, 2003. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Medical and Veterinary Entomology, 17, 294–300.
Forbes C, Evans M, Hastings N & Peacock B, 2011. Statistical distributions (4th edition). Hoboken, New Jersey, U.S.A.: John Wiley & Sons.
Gelman A, Carlin JB, Stern HS & Rubin DB, 2004. Bayesian data analysis (2nd edition). Boca Raton, Florida, U.S.A.: Chapman Hall/CRC.
Gubbins S, 2019. Using the basic reproduction number to assess the risk of transmission of lumpy skin disease virus by biting insects. Transboundary and Emerging Diseases, 66, 1873–1883.
Möller J, Moritz T, Schlottau K, Krstevski K, Hoffmann D, Beer M. & Hoffmann B, 2019. Experimental lumpy skin disease virus infection: comparison of a field and a vaccine strain. Archives of Virology, 164, 2931–2941.
Tuppurainen ESM, Venter EH & Coetzer JAW, 2005. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort Journal of Veterinary Research, 72, 153–164.
Annex C – Estimating the proportion of resistant cattle
1.
The proportion of cattle resistant to disease (p R) was estimated based on the numbers of cattle that did not develop generalised disease following challenge in published experiments: 9 out of 14, 4 out of 25 and 8 out of 11 (Carn & Kitching, 1995); 3 out of 6 (Möller et al., 2019); 3 out of 6 (Tuppurainen et al., 2005) and 1 out of 7 (Babiuk et al., 2008).
Parameters were estimated in a Bayesian framework. The likelihood for the data is
where Ri and Ni are the number of resistant cattle and the number of challenged cattle, respectively, and pi is the proportion of resistant cattle in experiment i. To allow the pis to vary among experiments, a hierarchical structure was assumed, such that,
where αp and βp are hierarchical parameters. Exponential priors (with mean 100) were assumed for αp and βp. Samples from the joint posterior distribution were generated by Markov chain–Monte Carlo methods implemented in OpenBUGS (version 3.2.3). Two chains each of 100,000 samples were run, with the preceding 20,000 iterations discarded to allow for burn‐in. The chains were then thinned (selecting every 20th sample) to reduce autocorrelation. The posterior median (95% credible interval (CI)) for αp and βp were 45.8 (3.1, 263.5) and 35.5 (2.9, 198.1), respectively, with a corresponding posterior median for pR of 0.56 (95% CI: 0.32–0.75).
A simpler model in which the proportion of resistant cattle was common to all experiments was also considered. However, this yielded a poorer fit to the data, as judged by the deviance information criterion: 31.6 (p varying) vs. 35.7 (p common).
Table C.1.
Transitions, probabilities and population sizes in the model for the transmission of LSDV in a cattle herd
| Description | transition | probability | population size | |
|---|---|---|---|---|
| Cattle | ||||
| infection |
|
λCδt | S | |
| completion of latent stage j(j = 1,…,nE–1) |
|
nErEδt | Ej | |
| completion of latent period |
|
nErEδt | Eng | |
| completion of infectious stage j(j = 1,…,nI–1) |
|
nIrIδt | Ij | |
| recovery |
|
nIrIδt | InI | |
| Vectors | ||||
| infection |
|
λVδt | X | |
| virus inactivated on infected vector |
|
γδt | Y | |
| mortality of infected vectors(and compensatory recruitment) |
|
μδt | Y |
Table C.2.
Parameters for the transmission of LSDV in a cattle herd by the bites of the stable fly, S. calcitrans (Gubbins, 2019)
| parameter | symbol | estimate (95% CI)a or range |
|---|---|---|
| mechanical transmission | ||
| virus inactivation rate (day−1) | γ | 1.71 (0.80, 3.34) |
| probability of transmission from insect to bovine | b | 0.07 (0.002, 0.64) |
| probability of transmission from bovine to insect | β | 0.46 (0.23, 0.71) |
| vector life history | ||
| biting rate (day−1) | a | 0.33–6.0 |
| vector to host ratio | m | 30–145 |
| vector mortality rate (day−1) | μ | 0.04–0.11 |
| latent and infectious periods in cattle | ||
| number of stages in latent period | nE | 26 (5, 81) |
| mean latent period (days) | 1/rE | 7.3 (5.9, 9.7) |
| number of stages in infectious period | nI | 11 (2, 36) |
| mean infectious period (days) | 1/rI | 23.1 (16.4, 36.2) |
| proportion of cattle resistant to disease | pR | 0.56 (0.32, 0.75) |
posterior median (95% credible interval).
Suggested citation: EFSA (European Food Safety Authority) , Calistri P, De Clercq K, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Marojevic D, Antoniou SE and Broglia A, 2020. Scientific report on the lumpy skin disease epidemiological report IV: data collection and analysis. EFSA Journal 2020;18(2):6010, 36 pp. 10.2903/j.efsa.2020.6010
Requestor: European Commission
Question number: EFSA‐Q‐2018‐00291
Acknowledgements: EFSA wishes to thank the AHAW Panel members Klaus Depner, Bruno Garin‐Bastuji, Liisa Sihvonen, Karl Ståhl, for reviewing the document, and EFSA staff member: Yves Van der Stede for the support provided to this scientific output. A special thanks is due to: Ali Lilo and Blerina Luke from Ministry of Agriculture, Rural Development and Water Administration, Albania; Aleksandra Miteva from Food Safety Agency, Bulgaria; Žaklin Acinger‐Rogić from Ministry of Agriculture, Croatia; Chrysoula Dile of the Animal Health Directorate of the Greek Ministry of Rural Development and Food, Greece; Bafti Murati from the Food and Veterinary Agency, Kosovo*, Vesna Dakovic from the Ministry of Agriculture and Rural Development, Montenegro; Vanja Kondratenko, Food and Veterinary Agency, North Macedonia; Tatjana Labus from the Ministry of Agriculture and Environmental Protection, Republic of Serbia; Anil Demeli from General Directorate of Food and Control Department of Animal Health and Quarantine, Ministry of Agriculture and Forestry, Turkey for the precious collaboration and timely provision of the epidemiological data.
Approved: 30 January 2020
* This designation is without prejudice to positions on status, and is in line with UNSCR 1244 and the ICJ Opinion on the Kosovo Declaration of Independence. This footnote applies all the time Kosovo is mentioned in this document.
Notes
In Croatia vaccination against LSD was done in these two years
http://web.oie.int/RR-Europe/eng/Regprog/docs/docs/LSD9/LSD%20presentations/TR_LSD_PPT_SGE_LSD9.pdf
Live attenuated vaccines against LSD are commercially available. Homologous vaccines are based on a LSDV strain, the most used strain being the Neethling strain from South Africa. Heterologous vaccines are based on other Capripox virus strains, mainly sheep and goat pox virus (SGPV strains such as Kenyan SGP O‐240, Yugoslavian RM‐65 and Romanian SPPV strains), which are supposed to confer a certain level of cross protection in cattle, although this is not satisfactory. An increased effectiveness can be achieved when the vaccine is used at 10‐fold doses than the one used in small ruminants for the control of sheep and goat pox. One new inactivated vaccine has been developed by MCI Santé Animale in Morocco but its efficacy and safety is currently under testing.
http://web.oie.int/RR-Europe/eng/Regprog/docs/docs/LSD9/LSD%20presentations/RU_LSD_PPT_SGE_LSD9.pdf
https://www.sciencedirect.com/science/article/abs/pii/S014362281300249X; http://www.china.org.cn/travel/2017-06/08/content_40987529_3.htm
The indication of vaccine producers is to re‐vaccinate animals every year, so it is assumed that the immunity duration is one year.
References
- Abutarbush SM and Tuppurainen ES, 2018. Serological and clinical evaluation of the Yugoslavian RM 65 sheep pox strain vaccine use in cattle against lumpy skin disease. Transboundary and Emerging Diseases, 65, 1657–1663. [DOI] [PubMed] [Google Scholar]
- Adedeji AJ, Möller J, Meseko CA, Adole JA, Tekki IS, Shamaki D and Hoffmann B, 2019. Molecular characterization of Capripox viruses obtained from field outbreaks in Nigeria between 2000 and 2016. Transboundary and Emerging Diseases. [DOI] [PubMed] [Google Scholar]
- Anderson D and Watson R, 1980. On the spread of a disease with gamma distributed latent and infectious periods. Biometrika, 67, 191–198. [Google Scholar]
- Agianniotaki EI, Mathijs E, Vandenbussche F, Tasioudi KE, Haegeman A, Iliadou P, Chaintoutis SC, Dovas CI, Van Borm S, Chondrokouki ED and De Clercq K, 2017. Complete genome sequence of the lumpy skin disease virus isolated from the first reported case in Greece in 2015. Genome Announcements, 5, e00550–00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Olga B, Svetlana K, Valeriy Z, Yana P, Pavel P and Aleksandr K, 2019. A real‐time PCR screening assay for the universal detection of lumpy skin disease virus DNA. BMC Research Notes, 12, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allepuz A, Casal J and Beltran‐Alcrudo D, 2019. Spatial analysis of lumpy skin disease in Eurasia‐Predicting areas at risk for further spread within the region. Transboundary and Emerging Diseases, 66, 813–822. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Bowden TR, Parkyn G, Dalman B, Manning L, Neufeld J, Embury‐Hyatt C, Copps J and Boyle DB, 2008. Quantification of lumpy skin disease virus following experimental infection in cattle. Transboundary and Emerging Diseases, 55, 299–307. [DOI] [PubMed] [Google Scholar]
- Bedekovic T, Simic I, Kresic N and Lojkic I, 2017. Detection of lumpy skin disease virus in skin lesions, blood, nasal swabs and milk following preventive vaccination. Transbound Emerging Disease, 65, 491–496. [DOI] [PubMed] [Google Scholar]
- Ben‐Gera J, Klement E, Khinich E, Stram Y and Shpigel NY, 2015. Comparison of the efficacy of Neethling lumpy skin disease virus and x10RM65 sheep‐pox live attenuated vaccines for the prevention of lumpy skin disease ‐The results of a randomized controlled field study. Vaccine, 33, 4837–4842. [DOI] [PubMed] [Google Scholar]
- Carn VM and Kitching RP, 1995. An investigation of possible routes of transmission of lumpy skin disease virus (Neethling). Epidemiology and Infection, 114, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Saegerman C, Bertagnoli S, Meyer G, Ganiere JP, Caufour P, De Clercq K, Jacquiet P, Hautefeuille C, Etore F and Napp S, 2019. A simple method to estimate the number of doses to include in a bank of vaccines. The case of Lumpy Skin Disease in France. PLoS ONE, 14, e0210317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota CM, Rennie LF, Kitching RP and Mellor PS, 2001. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiology and Infection, 126, 317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihota CM, Rennie LF, Kitching RP and Mellor PS, 2003. Attempted mechanical transmission of lumpy skin disease virus by biting insects. Medical and Veterinary Entomology, 17, 294–300. [DOI] [PubMed] [Google Scholar]
- Douglass N, Van Der Walt A, Omar R, Munyanduki H and Williamson AL, 2019. The complete genome sequence of the lumpy skin disease virus vaccine Herbivac LS reveals a mutation in the superoxide dismutase gene homolog. Archives of Virology, 164, 3107–3109. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Lumpy skin disease: I. Data collection and analysis. EFSA Journal 2017;15(4):4773, 54 pp. 10.2903/j.efsa.2017.4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Calistri P, DeClercq K, De Vleeschauwer A, Gubbins S, Klement E, Stegeman A, Cortiñas Abrahantes J, Antoniou SE and Broglia A, 2018. Lumpy skin disease: scientific and technical assistance on control and surveillance activities. EFSA Journal 2018;16(10):5452, 46 pp. 10.2903/j.efsa.2018.5452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Calistri P, DeClercq K, Gubbins S, Klement E, Stegeman A, Corti˜nas Abrahantes J, Antoniou S‐E, Broglia A and Gogin A, 2019. Scientificreport on lumpy skin disease: III. Data collection and analysis. EFSA Journal 2019;17(3):5638, 26 pp. 10.2903/j.efsa.2019.5638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster O, Rubinstein MG, Menasherow S, Ivanova E, Venter E, Sekler M, Kolarevic M and Stram Y, 2019. Importance of the lumpy skin disease virus (LSDV) LSDV126 gene in differential diagnosis and epidemiology and its possible involvement in attenuation. Archives of Virology, 164, 2285–2295. [DOI] [PubMed] [Google Scholar]
- Forbes C, Evans M, Hastings N and Peacock B, 2011. Statistical distributions, 4.th edition. John Wiley & Sons, Hoboken, New Jersey, U.S.A. [Google Scholar]
- Gelman A, Carlin JB, Stern HS and Rubin DB, 2004. Bayesian data analysis (2nd edition). Boca Raton, Florida, U.S.A.: Chapman Hall/CRC.
- Gubbins S, 2019. Using the basic reproduction number to assess the risk of transmission of lumpy skin disease virus by biting insects. Transboundary and Emerging Diseases, 66, 1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, De Leeuw I, Mostin L, Van Campe W, Aerts L, Vastag M and De Clercq K, 2019. An Immunoperoxidase Monolayer Assay (IPMA) for the detection of lumpy skin disease antibodies. Journal of Virological Methods, 113800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulos PD, Chaintoutis SC, Dovas CI, Polizopoulou ZS, Brellou GD, Agianniotaki EI, Tasioudi KE, Chondrokouki E, Papadopoulos O, Karatzias H and Boscos C, 2018. Investigation on the incidence of adverse reactions, viraemia and haematological changes following field immunization of cattle using a live attenuated vaccine against lumpy skin disease. Transboundary and Emerging Diseases, 65, 174–185. [DOI] [PubMed] [Google Scholar]
- Kononov A, Byadovskaya O, Kononova S, Yashin R, Zinyakov N, Mischenko V, Perevozchikova N and Sprygin A, 2019. Detection of vaccine‐like strains of lumpy skin disease virus in outbreaks in Russia in 2017. Archives of Virology, 164, 1575–1585. [DOI] [PubMed] [Google Scholar]
- Lojkić I, Šimić I, Krešić N and Bedeković T, 2018. Complete Genome Sequence of a Lumpy Skin Disease Virus Strain Isolated from the Skin of a Vaccinated Animal. Genome Announcements, 6, e00482–00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado G, Korennoy F, Alvarez J, Picasso‐Risso C, Perez A and VanderWaal K, 2019. Mapping changes in the spatiotemporal distribution of lumpy skin disease virus. Transboundary and Emerging Diseases, 66, 2045–2057. [DOI] [PubMed] [Google Scholar]
- Manic M, Stojiljkovic M, Petrovic M, Nisavic J, Bacic D, Petrovic T, Vidanovic D and Obrenovic S, 2019. Epizootic features and control measures for lumpy skin disease in south‐east Serbia in 2016. Transboundary and Emerging Diseases, 66, 2087–2099. [DOI] [PubMed] [Google Scholar]
- M€oller J, Moritz T, Schlottau K, Krstevski K, Hoffmann D, Beer M. and Hoffmann B, 2019. Experimental lumpy skin disease virus infection: comparison of a field and a vaccine strain. Archives of Virology, 164, 2931–2941. [DOI] [PubMed] [Google Scholar]
- Milovanovic M, Dietze K, Milicevic V, Radojicic S, Valcic M, Moritz T and Hoffmann B, 2019. Humoral immune response to repeated lumpy skin disease virus vaccination and performance of serological tests. BMC Veterinary Research, 15, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller J, Moritz T, Schlottau K, Krstevski K, Hoffmann D, Beer M and Hoffmann B, 2019. Experimental lumpy skin disease virus infection of cattle: comparison of a field strain and a vaccine strain. Archives of Virology, 164, 2931–2941. [DOI] [PubMed] [Google Scholar]
- Munyanduki H, Omar R, Douglass N and Williamson A‐L, 2020. Removal of bovine viral diarrhea virus (BVDV) from lumpy skin disease virus (LSDV) vaccine stocks by passage on chorioallantoic membranes of fertilized hens’ eggs. Journal of Virological Methods, 275, 113752. [DOI] [PubMed] [Google Scholar]
- Saegerman C, Bertagnoli S, Meyer G, Ganiere JP, Caufour P, De Clercq K, Jacquiet P, Hautefeuille C, Etore F and Casal J, 2019. Risk of introduction of Lumpy Skin Disease into France through imports of cattle. Transboundary and Emerging Diseases, 66, 957–967. [DOI] [PubMed] [Google Scholar]
- Şevik M and Doğan M, 2017. Epidemiological and molecular studies on lumpy skin disease outbreaks in Turkey during 2014–2015. Transboundary and Emerging Diseases, 64, 1268–1279. [DOI] [PubMed] [Google Scholar]
- Sohier C, Haegeman A, Mostin L, De Leeuw I, Van Campe W, De Vleeschauwer A, Tuppurainen E, van den Berg T, De Regge N and De Clercq K, 2019. Experimental evidence of mechanical lumpy skin disease virus transmission by Stomoxys calcitrans biting flies and Haematopota spp. horseflies. Scientific Reports, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprygin A, Babin Y, Pestova Y, Kononova S, Wallace DB, Van Schalkwyk A, Byadovskaya O, Diev V, Lozovoy D and Kononov A, 2018. Analysis and insights into recombination signals in lumpy skin disease virus recovered in the field. PLoS ONE, 13, e0207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprygin A, Babin Y, Pestova Y, Kononova S, Byadovskaya O and Kononov A, 2019. Complete Genome Sequence of the Lumpy Skin Disease Virus Recovered from the First Outbreak in the Northern Caucasus Region of Russia in 2015. Microbiol Resour Announc, 8, e01733–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RA, Berriman ADC, Gale P, Kelly LA and Snary EL, 2019. A generic framework for spatial quantitative risk assessments of infectious diseases: Lumpy skin disease case study. Transboundary and Emerging Diseases, 66, 131–143. [DOI] [PubMed] [Google Scholar]
- Toplak I, Petrovic T, Vidanovic D, Lazic S, Sekler M, Manic M, Petrovic M and Kuhar U, 2017. Complete Genome Sequence of Lumpy Skin Disease Virus Isolate SERBIA/Bujanovac/2016, Detected during an Outbreak in the Balkan Area. Genome Announc, 5, e00882–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppurainen ESM, Venter EH and Coetzer JAW, 2005. The detection of lumpy skin disease virus in samples of experimentally infected cattle using different diagnostic techniques. Onderstepoort Journal of Veterinary Research, 72, 153–164. [DOI] [PubMed] [Google Scholar]
- Tuppurainen E and Galon N, 2016. TECHNICAL ITEM II LUMPY SKIN DISEASE: CURRENT SITUATION IN EUROPE AND NEIGHBOURING REGIONS AND NECESSARY CONTROL MEASURES TO HALT THE SPREAD IN SOUTH‐EAST EUROPE.


