Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance metaflumizone. To assess the occurrence of metaflumizone residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived in the framework of Commission Regulation (EU) No 188/2011, the MRLs established by the Codex Alimentarius Commission and the European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Some information required by the regulatory framework was missing and a possible acute risk to consumers was identified. Hence, the consumer risk assessment is considered indicative only, some MRL proposals derived by EFSA still require further consideration by risk managers and measures for reduction of the consumer exposure should also be considered.
Keywords: metaflumizone, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, insecticide, E‐ and Z‐isomers, metabolite M320I04
Summary
Metaflumizone was approved on 1 January 2015 by means of Commission Implementing Regulation (EU) 922/2014 in the framework of Regulation (EC) No 1107/2009 as amended by Commission Implementing Regulations (EU) No 540/2011 and 541/2011.
As the active substance was approved after the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(1) of the aforementioned regulation.
As the basis for the MRL review, on 22 November 2018 EFSA initiated the collection of data for this active substance. In a first step, Member States were invited to submit by 21 December 2018 their national Good Agricultural Practices (GAPs) in a standardised way, in the format of specific GAP forms, allowing the designated rapporteur Member State, Sweden, to identify the critical GAPs in the format of a specific GAP overview file. Subsequently, Member States were requested to provide residue data supporting the critical GAPs, within a period of 1 month, by 26 April 2019. On the basis of all the data submitted by Member States and by the European Reference Laboratories for Pesticides Residues (EURLs), EFSA asked the rapporteur Member State (RMS) to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report, together with Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file were provided by the RMS to EFSA on 29 August 2019. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Based on the information provided by the RMS, Member States and the EURLs, and taking into account the conclusions derived by EFSA in the framework of Commission Regulation (EU) No 188/2011 and the MRLs established by the Codex Alimentarius Commission, EFSA prepared in February 2020 a draft reasoned opinion, which was circulated to Member States and EURLs for consultation via a written procedure. Comments received by 11 March 2020 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The metabolism of metaflumizone in plants was investigated in primary and rotational crops. According to the results of the metabolism studies and of the residue trials, a general residue definition for enforcement and risk assessment can be proposed as metaflumizone (sum of E‐ and Z‐isomers). As metabolite M320I04 was above the limit of quantification (LOQ) in lettuce and Chinese cabbage and no conclusion could be drawn on its toxicity (data gap), for leafy crops the proposed residue definition for risk assessment should be considered tentative only. In processed commodities, based on the results from the hydrolysis and the processing studies, the residue definitions are also proposed on a tentative basis as metaflumizone (sum of E‐ and Z‐isomers) and M320I04, expressed as metaflumizone. A specific residue definition for rotational crops is not deemed necessary considering that no significant residues are expected to occur.
Fully validated analytical methods are available for the enforcement of the proposed residue definition (raw commodities) in the four main plant matrices at the combined LOQ of 0.02 mg/kg. According to the EURLs, the combined LOQ of 0.02 mg/kg is achievable in high oil content commodities by using the QuEChERS method in routine analyses, and of 0.01 mg/kg in high water content, high acid content and dry commodities.
Available residue trials data were considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for kale where no data are available and for escarole and broccoli as no safe use could be identified. The MRLs derived for cauliflower are tentative since additional residue trials compliant with the GAPs are still required, as well as for tomato, sweet pepper, gherkin, Chinese cabbage, lettuce and other leafy crops extrapolated from lettuce, since further toxicological data on metabolite M320I04 are required.
Metaflumizone is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. The dietary burdens calculated for cattle, swine and sheep were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues was therefore assessed in these groups of livestock.
The metabolism of metaflumizone residues in livestock was investigated in lactating goats and laying hens at dose rate covering the maximum dietary burdens calculated in this review. According to the results of these studies, the residue definition for enforcement and risk assessment in livestock commodities was proposed as metaflumizone (sum of E‐ and Z‐isomers). An analytical method for the enforcement of the proposed residue definition at the combined LOQ of 0.02 mg/kg in all animal tissues and of 0.01 mg/kg in milk and eggs is available. According to screening data generated by EURLs, the E‐Isomer of metaflumizone can be monitored in milk with a screening detection limit (SDL) of 0.005 mg/kg, in egg with an SDL of 0.01 mg/kg and in muscle and honey with an SDL of 0.02 mg/kg.
A livestock feeding study on lactating cows was used to derive MRL and risk assessment values in milk and tissues of ruminants. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs. No MRLs were required for poultry, as the dietary burden calculation is not triggered and very low exposure to metaflumizone residues is expected.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 3.1 of the EFSA PRIMo. For kale where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation. In a first scenario (EU1), an exceedance of the acute reference dose (ARfD) was identified for lettuces, escaroles and broccoli, representing 208%, 154% and 122% of the ARfD, respectively. An exceedance of the ARfD was also observed for processed commodities of escaroles and broccoli, representing 254% and 231% of the ARfD, respectively. Considering a fall‐back MRL for lettuces (based on the southern data set only) and excluding escaroles and broccoli from the calculation (scenario EU2), the highest chronic exposure represented 15% of the acceptable daily intake (ADI) (GEMS/Food G10 diet) and the highest acute exposure amounted to 92% of the ARfD (unprocessed lettuces). Processing studies on broccoli, for which no processing factors were available, are required to refine the risk assessment (data gap).
Apart from the MRLs evaluated in the framework of this review, internationally recommended Codex residue limits (CXLs) have also been established for metaflumizone. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out (scenario CX1) and an exceedance of the ARfD was identified for the existing CXL in lettuces (146%). Excluding this CXL from the calculation (scenario CX2 equivalent to EU2), the highest chronic exposure represented 15% of the ADI (GEMS/Food G10 diet) and the highest acute exposure amounted to 92% of the ARfD (lettuces).
Considering the limited metabolism of metaflumizone in plants and the toxicological studies previously carried out with both isomers concluding that they share the same toxicity, the potential change of isomer ratios in the final residues is unlikely to be of concern for the authorised use reported in the framework of this review.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(1) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide within 12 months from the date of the inclusion or non‐inclusion of an active substance in Annex I to Directive 91/414/EEC2 a reasoned opinion on the review of the existing MRLs for that active substance.
As metaflumizone was approved on 1 January 2015 by means of Commission Implementing Regulation (EU) 922/20143 in the framework of Regulation (EC) No 1107/20094 as amended by Commission Implementing Regulations (EU) No 540/20115 and 541/20116, EFSA initiated the review of all existing MRLs for that active substance.
By way of background information, in the framework of Commission Regulation (EU) No 188/20117 Metaflumizone was evaluated by United Kingdom, designated as rapporteur Member State (RMS). Subsequently, a peer review on the initial evaluation of the RMS was conducted by EFSA, leading to the conclusions as set out in the EFSA scientific output (EFSA, 2013b).
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC repealed by Regulation (EC) No 1107/2009. It should be noted, however, that, in the framework of Regulation (EC) No 1107/2009, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU), and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Regulation (EC) No 1107/2009 is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
As the basis for the MRL review, on 22 November 2018, EFSA initiated the collection of data for this active substance. In a first step, Member States were invited to submit by 21 December 2018 their Good Agricultural Practices (GAPs) that are authorised nationally, in a standardised way, in the format of specific GAP forms. In the framework of this consultation, 11 Member States provided feedback on their national authorisations of metaflumizone. Based on the GAP data submitted, the reassigned RMS, Sweden, was asked to identify the critical GAPs to be further considered in the assessment, in the format of a specific GAP overview file. Subsequently, in a second step, Member States were requested to provide residue data supporting the critical GAPs by 26 April 2019.
On the basis of all the data submitted by Member States and the EU Reference Laboratories for Pesticides Residues (EURLs), EFSA asked Sweden to complete the PROFile and to prepare a supporting evaluation report. The PROFile and the supporting evaluation report, together with the Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file, were submitted to EFSA on 29 August 2019. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Considering all the available information, and taking into account the MRLs established by the Codex Alimentarius Commission (CAC) (i.e. codex maximum residue limit; CXLs), EFSA prepared in February 2020 a draft reasoned opinion, which was circulated to Member States and EURLs for commenting via a written procedure. All comments received by 11 March 2020 were considered by EFSA during the finalisation of the reasoned opinion.
The evaluation report submitted by the RMS (Sweden, 2019), taking into account also the information provided by Member States during the collection of data, and the EURLs report on analytical methods (EURLs, 2019) are considered as main supporting documents to this reasoned opinion and, thus, made publicly available.
In addition, further supporting documents to this reasoned opinion are the completeness check report (EFSA, 2020a) and the Member States consultation report (EFSA, 2020b). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Furthermore, the exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) and the PROFile as well as the GAP overview file listing all authorised uses are key supporting documents and made publicly available as background documents to this reasoned opinion. A screenshot of the report sheets of the PRIMo is presented in Appendix C.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Metaflumizone is the ISO common name for (EZ)‐2′‐[2‐(4‐cyanophenyl)‐1‐(α,α,α‐trifluoro‐m‐tolyl)ethylidene]‐4‐(trifluoromethoxy)carbanilohydrazide (IUPAC). Metaflumizone consists of a mixture of two stereoisomers: E‐isomer and Z‐isomer, with a minimum E/Z‐ratio of 9/1.
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
The EU MRLs for metaflumizone are established in Annexes IIIA of Regulation (EC) No 396/2005. Codex maximum residue limits (CXLs) for metaflumizone were also established by the Codex Alimentarius Commission (CAC). An overview of the MRL changes that occurred since the entry into force of the Regulation mentioned above is provided below (Table 1).
Table 1.
Overview of the MRL changes since the entry into force of Regulation (EC) No 396/2005
| Procedure | Legal implementation | Remarks |
|---|---|---|
| MRL application | Regulation (EC) No 318/2014a | Various crops (EFSA, 2013a) |
| Implementation of CAC 2010 | Regulation (EC) No 520/2011b | On 9 July 2010, the Codex Alimentarius Commission (CAC) adopted Codex maximum residue limits (CXLs) |
Commission Regulation (EU) No 318/2014 of 27 March 2014 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fenarimol, metaflumizone and teflubenzuron in or on certain products. OJ L 93, 28.3.2014, p. 28–57.
Commission Regulation (EU) No 520/2011 of 25 May 2011 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for benalaxyl, boscalid, buprofezin, carbofuran, carbosulfan, cypermethrin, fluopicolide, hexythiazox, indoxacarb, metaflumizone, methoxyfenozide, paraquat, prochloraz, spirodiclofen, prothioconazole and zoxamide in or on certain products. OJ L 140, 27.5.2011, p. 2–47.
For the purpose of this MRL review, all the uses of metaflumizone currently authorised within the EU as submitted by the Member States during the GAP collection, have been reported by the RMS in the GAP overview file. The critical GAPs identified in the GAP overview file were then summarised in the PROFile and considered in the assessment. The details of the authorised critical GAPs for metaflumizone are given in Appendix A. The RMS did not report any use authorised in third countries that might have a significant impact on international trade.
Assessment
EFSA has based its assessment on the following documents:
the PROFile submitted by the RMS;
the evaluation report accompanying the PROFile (Sweden, 2019);
the draft assessment report (DAR) and its addenda prepared under Council Directive 91/414/EEC (United Kingdom, 2012, 2013);
the conclusion on the peer review of the pesticide risk assessment of the active substance metaflumizone (EFSA, 2013b);
the Joint Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 2009a);
the previous reasoned opinion on metaflumizone (EFSA, 2013a).
The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20118 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a, 1997b, 1997c, 1997d, 1997e, 1997f, 1997g, 2000, 2010a, 2010b, 2017; OECD, 2008, 2011, 2013).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of metaflumizone was investigated after foliar treatment in fruiting vegetables, leafy vegetables and pulses and oilseeds (United Kingdom, 2012) and assessed in the framework of a previous article 10 reasoned opinion and the peer review (EFSA, 2013a,b). In all studies, metaflumizone was radiolabelled in the trifluoromethoxyphenyl or benzonitrile ring of the molecule (T‐label and B‐label, respectively). The trifluoromethyl‐phenyl ring of the molecule was not labelled in any study. However, there is little evidence of cleavage and the cleavage product is not expected to be of significance, thus no additional metabolism studies are needed.
The metabolism of metaflumizone in tomato was investigated both under field and greenhouse conditions. After six foliar applications of 331–378 g a.s./ha at 7 days interval, tomato samples were harvested at 0 and 7 days after treatment (DAT). In field conditions, the major component identified at both preharvest intervals (PHI) was metaflumizone (E‐ and Z‐ isomers), representing up to 62% of the total radioactive residues (TRR) (0.37 mg/kg) in B‐label and up to 81% TRR (0.32 mg/kg) in T‐label. Metabolite M320I04 was also significant, accounting for up to 13% TRR (0.076 mg/kg) in B‐label study only. Other metabolites were identified, however, in proportions below 10% TRR. The same metabolic profile was observed for indoor grown tomatoes, with slightly higher concentrations (up to 84% TRR; 0.57 mg/kg for metaflumizone (E‐ and Z‐isomers) and up to 16% TRR; 0.12 mg/kg for M320I04).
After four foliar applications of 280 g a.s./ha on white cabbage under greenhouse conditions, the major components identified in cabbage leaves were metaflumizone isomers, ranging from 75% TRR (7.3 mg/kg) 3 DAT in B‐label, to 98% TRR (14.4 mg/kg) 7 DAT in T‐label. Metabolite M320I04 was again present in significant proportions and levels (up to 16% TRR; 2.1 mg/kg) in B‐label study (EFSA, 2013a). Minor metabolites were also identified in cabbage leaves but not in significant proportions.
After six foliar applications of 307–350 g a.s./ha on cotton seeds with 7 days interval, metaflumizone E‐isomer and Z‐isomer were the most significant residues, representing up to 26% TRR (5 mg/kg) and 39% TRR (7.5 mg/kg), respectively, in cotton gin by‐products, and up to 21% TRR (0.029 mg/kg) and 26% TRR (0.036 mg/kg), respectively, in cotton seeds. In B‐label study, M320I04 was identified at significant levels in cotton gin by‐products (13% TRR; 3.8 mg/kg) and cotton seeds (16% TRR; 0.06 mg/kg). The same minor metabolites as in tomato were identified (< 10% TRR) (EFSA, 2013a).
The metabolic pathway of metaflumizone was similar in all crops, including an isomerisation of E‐isomer to Z‐isomer, the ring closure to form metabolite M320I23 and the cleavage of the parent molecule to form M320I04 containing the trifluoromethyl‐phenyl ring (EFSA, 2013a,b).
1.1.2. Nature of residues in rotational crops
Metaflumizone is authorised on crops that may be grown in rotation. The field DT90 reported in the soil degradation studies evaluated in the framework of the peer review was 177 days for parent metaflumizone. Therefore, it is required to investigate the nature of metaflumizone in rotational crops. Metabolite M320I23 was found to degrade slowly in soil and to be very highly persistent, with a laboratory DT50 above 1,000 days (EFSA, 2013b) and accumulation in soil would need to be considered (see Section 1.2.2).
Two confined rotational crop studies with T‐labelled and B‐labelled metaflumizone were available for this review (United Kingdom, 2012, EFSA, 2013a,b). In the first study, metaflumizone was applied twice at a rate of 560 g a.s./ha (i.e. total rate of 1.12 kg a.s./ha) onto bare soil. Crops were planted at nominal plant back intervals (PBI) of 30, 60/62, 120 and 365 DAT. Crops planted at each interval consisted of leafy vegetables (lettuce), roots (radish) and cereals (wheat). In the second study, metaflumizone was applied once onto bare soil at a rate of 1.168 kg a.s./ha and the same crops were planted 30 DAT.
In the first study and for both labels, total residues in lettuce and radish root were low (maximum of 0.01 mg/kg 30 to 60/62 DAT and 0.02 mg/kg 30 DAT, respectively) and decreasing over time, particularly in B‐label. Highest levels of TRR were observed in radish tops (0.038 mg/kg 30 DAT) and in wheat matrices (up to 0.05 mg/kg in forage and 0.60 mg/kg in hay 30 DAT, 0.25 mg/kg in straw 60/62 DAT and 0.16 mg/kg in grain 120 DAT).
Parent metaflumizone was not identified in any of the rotational crops and most of the radioactivity was described as polar and medium polar unknowns and was not further characterised (EFSA, 2013a). In the second confined rotational crop study, identification of compounds was greater analysing for parent and additional metabolites.
In this second study, the highest TRR were also observed in wheat matrices ranging from 0.10 mg/kg (forage) to 0.91 mg/kg (straw), while residues were lower in lettuce and radish (up to 0.02 mg/kg and 0.05 mg/kg, respectively). The identified compounds were E‐isomer and Z‐isomer of metaflumizone in both labels and minor metabolites including M320I04 in B‐label (however not individually exceeding 6% TRR). In some matrices, the unknown fractions were major (up to 87% TRR in lettuce, and 69% in wheat straw) (EFSA, 2013a).
As a large part of the extracted TRR could not be identified or characterised, the depiction of the metabolic pathway in rotational crops is not clear enough to conclude whether the metabolic pathway in rotational crops is similar to that observed in primary crops. Nevertheless, in these studies performed at overdosed rates (2.4N the rate of the most cGAP assessed under this review), low overall TRR and individual residue levels were observed in plant matrices. Therefore, no significant residues are expected in rotated crops and a residue definition is not considered necessary (EFSA, 2013b).
1.1.3. Nature of residues in processed commodities
Studies investigating the nature of residues in processed commodities were assessed (United Kingdom, 2012, EFSA, 2013a,b).
These studies were conducted with B‐labelled and T‐labelled metaflumizone, simulating representative hydrolytic conditions for pasteurisation (20 min at 90°C, pH 4), boiling/brewing/baking (60 min at 100°C, pH 5) and sterilisation (20 min at 120°C, pH 6).
No significant degradation products were observed, except in B‐label studies where metabolite M320I04 was the only degradation product exceeding 10% of the applied radioactivity (AR), formed under pasteurisation and boiling conditions (15% and 25% AR, respectively). In these studies, at low pH, residues appeared to convert from E‐isomer to M320I04 (see also Section 1.2.3). Nevertheless, parent metaflumizone was observed in high proportions in B‐label studies (62% AR, 78% AR and 89% AR under boiling conditions, pasteurisation conditions and sterilisation conditions, respectively) and found to be stable in all T‐label studies (77% to 94% AR).
It can be concluded that the metabolic pathway of metaflumizone in processed commodities is similar to the one observed in primary crops (United Kingdom, 2012, EFSA, 2013a,b). However, metabolite M320I04 needs to be considered and included in the residue definitions for processed commodities.
1.1.4. Methods of analysis in plants
During the peer review and in the framework of a previous MRL application (United Kingdom, 2012, EFSA, 2013a,b), a hyphenated analytical method based on liquid chromatography (LC) coupled to MS/MS detection was validated for the determination of E‐isomer and Z‐isomer of metaflumizone separately in all plant matrices (high water content, high acid content, high oil content and dry commodities), with an LOQ of 0.01 mg/kg for each analyte (i.e. a combined LOQ of 0.02 mg/kg). The method covers also the determination of metabolites M320I04 and M320I23 with individual LOQs of 0.01 mg/kg. Two transitions were monitored for each analyte in the primary method, considered to be sufficiently specific. This primary method is supported by an independent laboratory validation (ILV).
The multi‐residue method DFG‐S19 was tested and found to be not applicable to the analysis of metaflumizone (United Kingdom, 2012).
During the completeness check, the EURLs provided a QuEChERS multi‐residue analytical method using LC‐MS/MS, with a combined LOQ of 0.01 mg/kg for the routine analysis of metaflumizone (E‐ and Z‐isomers) in high water content, high acid content and dry commodities and a combined LOQ of 0.02 mg/kg in high oil content commodities (EURLs, 2019).
1.1.5. Stability of residues in plants
The storage stability of parent metaflumizone (E‐ and Z‐isomers) and its metabolites M320I04 and M320I23 was investigated in the framework of the peer review (United Kingdom, 2012, 2013, EFSA, 2013b) and a previous reasoned opinion (EFSA, 2013a).
Three studies were assessed. In the first one, tomato, potato, white cabbage, lettuce and cotton seed were fortified with a mixture of E‐ and Z‐isomers and separately with metabolite M320I04. In the second one, the stability of incurred residues of E‐isomer, Z‐isomer, metabolites M320I04 and M320I23 was investigated only in high water content matrices (white cabbage, lettuce, celery, broccoli, mustard greens and tomato). The third study investigated the stability of incurred residues of E‐isomer, Z‐isomer, and metabolites M320I04 and M320I23 in high water content (cucumber, snap bean and potato), high oil content (sunflower seed) and high acid content commodities (strawberry).
It was concluded that in high water content, high acid content and high oil content matrices, the sum of E‐ and Z‐isomers, as well as the two metabolites M320I04 and M320I23, were stable for 2 years when samples are stored deep frozen (−20°C) (EFSA, 2013b; United Kingdom, 2013).
1.1.6. Proposed residue definitions
The metabolism of metaflumizone was similar in all crops assessed. The metabolism in rotational crops was not sufficiently elucidated to conclude whether the metabolic pathway was the same as in primary crops. However, considering that no significant residues are expected in rotated crops, no specific residue definition is deemed necessary (EFSA, 2013b).
As the parent compound (sum of both isomers) was found to be a sufficient marker in fruiting and leafy vegetables and in pulses and oilseeds, a general residue definition for enforcement is proposed as metaflumizone (sum of E‐ and Z‐ isomers).
Although it was concluded that the processing of metaflumizone is not expected to modify the nature of residues (see Section 1.1.3), significant residues of M320I04 were found in the studies investigating the effect of processing on the magnitude of residues. In particular, parent compound was not found in canned tomato and tomato juice, where the only relevant compound was metabolite M320I04 (see Section 1.2.3). Therefore, the residue definition, both for enforcement and risk assessment, in processed commodities is proposed as metaflumizone (sum of E‐ and Z‐ isomers) and M320I04, expressed as metaflumizone.
An analytical method for the enforcement of the proposed residue definition for plants (raw commodities) at the combined LOQ of 0.02 mg/kg in all four main plant matrices is available (EFSA, 2013a,b). Although not required as MRLs are set considering the enforcement residue definition of raw plant commodities, this analytical method is also validated for the enforcement of the proposed residue definition for processed commodities at the combined LOQ of 0.038 mg/kg. According to the EURLs, a combined LOQ of 0.01 mg/kg is achievable by using the QuEChERS method in routine analyses in high water content, high acid content and dry commodities, and a combined LOQ of 0.02 mg/kg is achievable in high oil content commodities (EURLs, 2019).
Both isomers of metaflumizone are toxicologically relevant and a clear isomerisation was observed. Thus, for risk assessment, E‐ and Z‐isomers should be considered in the consumer exposure. Metabolite M320I04 was encountered in plant commodities in metabolism studies but is minor in the rat metabolism and data were not sufficient to conclude on its toxicological profile (including genotoxicity). However, since this metabolite was always below or at the LOQ in the residue trials supporting the representative uses, it was not proposed for inclusion in the residue definition for plants, and additional data on the toxicity were thus, not required during the peer review (EFSA, 2013a,b, United Kingdom, 2013).
Considering the authorised uses assessed under the current review, it should be noted that residues of metabolite M320I04 in plant commodities were below the LOQ in each of the GAP‐compliant field trials, except in lettuce and Chinese cabbage (see Section 1.2.1. and Appendix B.1.2.1). Although residue levels of M320I04 measured in these two crops are very low compared to the residue levels of the parent and are not expected to have a significant impact on the calculated MRLs, further information on the toxicity of this metabolite is still required in order to confirm that it is not relevant for the risk assessment of leafy crops (data gap). Therefore, the residue definition for risk assessment in plants (raw commodities) derived during the peer review as metaflumizone (sum of E‐ and Z‐ isomers) is still considered appropriate for the uses under assessment, except for leafy crops where it is proposed as tentative only, pending the submission of additional data to conclude on the toxicity of this metabolite. Pending clarification on its toxicological profile, Member States are in any case recommended to ask for residue trials analysing for metabolite M320I04 when granting new uses.
Regarding processed commodities, the proposed residue definitions for enforcement and risk assessment as stated above are also tentative only, considering the lack of information on the toxicity of metabolite M320I04.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of metaflumizone residues resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (Sweden, 2019) as well as the residue trials evaluated in the framework of the peer review (United Kingdom, 2012; EFSA, 2013b) and of a previous MRL application (EFSA, 2013a). The samples were analysed for the parent compound in accordance with the residue definitions for enforcement and risk assessment, and additionally analysed for metabolite M320I04, for which residue levels were usually below the LOQ of 0.01 mg/kg, except in lettuce (ranging from < LOQ to 0.10 mg/kg) and in Chinese cabbage (ranging from < LOQ to 0.07 mg/kg). All residue trial samples considered in this framework were stored in compliance with the conditions for which storage stability of residues was demonstrated. Decline of residues during storage of the trial samples is therefore not expected.
The number of residue trials and extrapolations was evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2017).
Residue trials are not available to support the authorisations on kale. Therefore, MRL and risk assessment values could not be derived for this crop and the following data gap was identified:
Kale: four trials compliant with the southern outdoor GAP are required. For all other crops, available residue trials are sufficient to derive (tentative) MRL and risk assessment values, taking note of the following considerations:
Tomato: although tentative MRL and risk assessment values can be derived from the indoor data, eight trials compliant with the southern GAP are still required.
Aubergine: although MRL and risk assessment values can be derived from the indoor data on tomato, four trials compliant with the southern GAP are still required.
Cauliflower: although tentative MRL and risk assessment values can be derived from the southern limited data set, four additional trials compliant with the southern GAP are still required.
1.2.2. Magnitude of residues in rotational crops
There were no studies investigating the magnitude of residues in rotational crops available for this review.
Nevertheless, based on the confined rotational crop studies and considering the overdosing factor of these studies (2.4N the maximum application rate assessed in this review) and the fact that active substance was applied to a bare soil (interception of active substance by the plants is expected in practice), it can be concluded that significant residue levels in rotational commodities are not expected, provided that metaflumizone is applied in compliance with the GAPs reported in Appendix A (see Section 1.1.2).
The possible accumulation in soil of the very highly persistent metabolite M320I23 was already considered during the peer review (EFSA, 2013b). Additional uses (on brassica and leafy vegetables) were authorised since this peer review and are assessed under this Article 12 review. However, all these authorised uses are covered by the representative uses assessed during the peer review regarding the GAP parameters (application rate, number of treatments, PHI). Therefore, the previous conclusions on rotational crops laid down under the peer review are still valid. Consequently, no further data are deemed necessary. This could be reconsidered if in the future new uses were to be authorised.
1.2.3. Magnitude of residues in processed commodities
The effect of industrial processing and/or household preparation was assessed on studies conducted on tomato, lettuce, cotton seeds and head cabbage (Italy, 2011; United Kingdom, 2012, EFSA, 2013a,b). An overview of all available processing studies is available in Appendix B.1.2.3.
Robust processing factors (fully supported by data) could be derived for tomato (canned, sauce, paste and juice), head cabbage (cooked, fermented and fermented juice) and lettuce (washed inner and outer leaves). Limited processing factors were derived for cotton seeds, due to insufficient number of studies, however, reported for information only since no use is authorised on this crop under the current review.
Results of these studies indicate that residues are significantly reduced in processed head cabbage, washed lettuce and processed tomato (except in tomato paste where residues tend to concentrate).
No processing studies, and thus no processing factors, were available for broccoli. However, considering the outcome of the risk assessment (see Section 3), processing studies would be useful to refine the risk assessment for broccoli (data gap).
In addition, if further robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed for the other processed commodities where a tentative processing factor is derived.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for kale where no data are available and for broccoli and escaroles as a risk to consumers could not be excluded for these crops and no fall‐back GAPs were identified. MRL is tentative for cauliflowers, since additional residue trials compliant with the southern GAP are missing. Considering the need for additional toxicological data on metabolite M320I04, MRLs are also tentative for tomato, sweet pepper, gherkin, Chinese cabbage, lettuce and all the leafy crops for which data are extrapolated from lettuces (see Appendix B.1.2.1).
2. Residues in livestock
Metaflumizone is authorised for use on crops (potato, kale, head cabbage) that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance (OECD, 2013), which has now also been agreed upon at European level. The input values for all relevant commodities are summarised in Appendix D. The dietary burdens calculated were found to exceed the trigger value of 0.1 mg/kg dry matter (DM) for cattle, swine and sheep (see Appendix B.2). Behaviour of residues should therefore be assessed in all groups of livestock, except in poultry.
It is highlighted that for kale no residue data were available. The animal intake of metaflumizone residues via this commodity has therefore not been assessed and may have been underestimated. However, this is not expected to have a major impact on the outcome of the dietary burden considering the high contribution of cabbage (heads and leaves).
2.1. Nature of residues and methods of analysis in livestock
The metabolism of metaflumizone residues in livestock was investigated in lactating goats and laying hens at dose rates covering the maximum dietary burdens calculated in this review. These studies using B‐labelled metaflumizone and T‐labelled metaflumizone were assessed in the framework of the peer review (United Kingdom, 2012; EFSA, 2013b).
In the study performed on lactating goats, highest residue levels were found in liver (1.29–2.85 mg eq./kg), fat (0.73–2.87 mg eq./kg), kidney (0.21–0.38 mg eq./kg) and milk (0.20–0.53 mg eq./kg) while in muscle residue levels were lower (0.07–0.18 mg eq./kg). For each commodity, the highest level reported was measured in B‐label study. Parent compound was predominant in all matrices and for both label studies, with E‐isomer being the major component identified accounting for 30–102% TRR and Z‐isomer present at up to 16% TRR. In T‐label study, other metabolites were identified but they were all present in very small amounts (< 10% TRR). In B‐label study, the only metabolites found in significant proportions were M320I25 and M320I26 present in liver hydrolysates of non‐extractable residues at 20% TRR and 11% TRR, respectively.
In laying hens, the highest TRR were found in fat up to 27 mg eq./kg in B‐label. In the other matrices, total residue levels ranged from 1.1 mg eq./kg in muscle to 4.6 mg eq./kg in liver. As for goats, the main compound identified in all matrices was E‐isomer of metaflumizone (55‐97% TRR). No metabolites were identified in T‐label study, while in B‐label study, minor metabolites were found in liver, however, in proportions below 10% TRR.
EFSA concludes that the metabolism of metaflumizone in livestock is adequately elucidated. Considering the exaggerated dose rate of both studies (57N the maximum dietary burden calculated for ruminants and 440N the one calculated for poultry), no significant metabolites are expected in animal commodities. Parent metaflumizone (E‐ and Z‐isomers) is the most relevant component of the residues expected to be found in all livestock commodities. As the parent compound was found to be a sufficient marker in livestock commodities, the residue definitions for enforcement and risk assessment are proposed as metaflumizone (sum of E‐ and Z‐isomers).
No storage stability study was available for livestock commodities. However, the stability of the residues was investigated during the metabolism studies. Analyses on stored samples indicate that the metabolite profiles were stable in goat samples and extracts (tissues and milk) during frozen storage for at least 17–19 months and for around 24 months for hen samples (tissues and eggs). No concerns were identified from the metabolism studies (United Kingdom, 2012).
An analytical method was fully validated for the determination of metaflumizone (each isomer individually) in all animal tissues with a combined LOQ of 0.02 mg/kg, and in milk and eggs with a combined LOQ of 0.01 mg/kg (United Kingdom, 2012, EFSA, 2013a,b). This method available for the enforcement of the proposed residue definition is supported by an ILV. According to screening data generated by EURLs, the E‐Isomer of metaflumizone can be monitored in milk with a screening detection limit (SDL) of 0.005 mg/kg, in egg with an SDL of 0.01 mg/kg and in muscle and honey with an SDL of 0.02 mg/kg (EURLs, 2019).
2.2. Magnitude of residues in livestock
In the framework of the peer review, feeding studies were performed with dairy cows and laying hens (United Kingdom, 2012), although not required for poultry under the current review.
In these studies, metaflumizone was administered to cows using different dosing levels ranging from 0.2 to 16.5 mg/kg feed (equivalent to 0.0077–0.63 mg/kg body weight (bw) per day), and to hens at dosing levels from 0.1 to 0.9 mg/kg feed (equivalent to 0.0068–0.062 mg/kg bw per day). Samples of tissues, eggs and milk were analysed for metaflumizone, sum of E‐ and Z‐isomers, within 33 days (cow matrices) and 28 days (poultry matrices) of collection and these storage periods are covered by the conclusions raised in the metabolism study performed on goats and laying hens (see Section 2.1). Thus, decline of residues during storage of the trial samples is not expected.
The study performed on dairy cows was used to derive MRL and risk assessment values in milk and tissues of ruminants. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs.
As poultry are not expected to be exposed to significant residues resulting from the uses of metaflumizone currently authorised, further investigation was not required and setting of MRLs for poultry products is not needed.
Based on the study on dairy cows, MRL and risk assessment values were derived for all commodities of ruminants and pigs, in compliance with the latest recommendations on this matter (FAO, 2009b). It is noted that significant levels of metaflumizone are only expected in liver and fat at the highest feeding levels (equivalent to at least 33N the maximum dietary burden calculated for dairy cows), therefore, in all ruminant matrices, the MRLs are proposed at the LOQ.
3. Consumer risk assessment
In the framework of this review, only the uses of metaflumizone reported by the RMS in Appendix A were considered; however, the use of metaflumizone was previously also assessed by the JMPR (FAO, 2009a). The CXLs, resulting from this assessment by JMPR and adopted by the CAC, are now international recommendations that need to be considered by European risk managers when establishing MRLs. To facilitate consideration of these CXLs by risk managers, the consumer exposure was calculated both with and without consideration of the existing CXLs. Following the extra 2019 JMPR, new CXLs for metaflumizone are currently under discussion. As they are not yet implemented, they are not considered under the current assessment.
3.1. Consumer risk assessment without consideration of the existing CXLs
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed using revision 3.1 of the EFSA PRIMo (EFSA, 2018, 2019). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where an (tentative) MRL could be derived by EFSA in the framework of this review, input values were derived according to the internationally agreed methodologies (FAO, 2009b). For kale where data were insufficient to derive an MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. All input values included in the exposure calculations are summarised in Appendix D.
The exposure values calculated were compared with the toxicological reference values for metaflumizone, derived by EFSA (2013b). In a first scenario (EU1), the highest chronic exposure was calculated for Spanish adults, representing 27% of the acceptable daily intake (ADI). Regarding the acute exposure, however, an exceedance of the ARfD was identified for lettuces, escaroles and broccoli, representing 208%, 154% and 122% of the ARfD, respectively. An exceedance of the ARfD was also observed for processed commodities of escaroles (boiled) and broccoli (boiled), representing 254% and 231% of the ARfD, respectively.
A second exposure calculation was therefore performed (scenario EU2), considering a fall‐back MRL for lettuces (derived from the southern data set). For escaroles and indoor lettuces, no fall‐back GAP could be identified by EFSA. For broccoli, a fall‐back GAP was identified, but still leading to an exceedance of the ARfD for processed broccoli (processing factors would be thus required to further refine the risk assessment). Escaroles and broccoli were therefore excluded from the calculation. According to the results of this second calculation, the highest chronic exposure declined to 15% of the ADI for the GEMS/Food G10 diet, and the highest acute exposure is then calculated for unprocessed lettuces, representing 92% of the ARfD.
Based on these calculations, an acute risk to consumers was identified for the most critical GAPs of metaflumizone on broccoli, lettuces and escaroles. However, a fall‐back MRL was calculated for lettuces, for which a second risk assessment did not indicate risk to consumers. No further refinement of the risk assessment was possible for escaroles, indoor lettuces and processed broccoli. For the remaining commodities, although major uncertainties remain due to the data gaps identified in the previous sections, the indicative exposure calculation did not indicate a risk to consumers.
Considering the limited metabolism of metaflumizone in plants and the toxicological studies carried out with both isomers concluding that they share the same toxicity (EFSA, 2013b), the potential change of isomer ratios in the final residues is not expected to be of concern for the authorised uses reported in the framework of this review.
3.2. Consumer risk assessment with consideration of the existing CXLs
To include the CXLs in the calculations of the consumer exposure, CXLs were compared with the EU MRL proposals in compliance with Appendix E and all data relevant to the consumer exposure assessment have been collected from JMPR evaluations. The EU MRLs and the CXLs are established for the same residue definition and are therefore comparable.
The EU MRL proposals are covering all the CXLs established, except for lettuce (where the fall‐back EU MRL proposal is lower than the CXL). Therefore, an additional exposure calculation was performed to include this CXL in the calculation. An overview of the input values used for this exposure calculation is also provided in Appendix D.
Chronic and acute exposure calculations were also performed using revision 3.1 of the EFSA PRIMo and the exposure values calculated were compared with the toxicological reference values derived for metaflumizone. The highest chronic exposure was calculated for the GEMS/Food G10 diet, representing 17% of the ADI. Regarding the acute exposure, however, an exceedance of the ARfD was identified for lettuces representing 146% of the ARfD (scenario CX1).
Excluding the CXL for this crop, the input values and the results of the exposure calculation would be the same as for scenario EU2, meaning that the highest chronic exposure would decline to 15% of the ADI for the GEMS/Food G10 diet, and the highest acute exposure is then calculated for lettuces, representing 92% of the ARfD (scenario CX2 = scenario EU2).
Based on these calculations, EFSA concludes that the CXLs for metaflumizone are not of concern for European consumers, except for the CXL on lettuces where a potential acute risk to consumers was identified and no further refinements of the risk assessment were possible. For the remaining CXLs, the indicative exposure calculation did not indicate a risk to consumers.
Conclusions
The metabolism of metaflumizone in plants was investigated in primary and rotational crops. According to the results of the metabolism studies and of the residue trials, a general residue definition for enforcement and risk assessment can be proposed as metaflumizone (sum of E‐ and Z‐ isomers). As metabolite M320I04 was above the limit of quantification (LOQ) in lettuce and Chinese cabbage and no conclusion could be drawn on its toxicity (data gap), for leafy crops, the proposed residue definition for risk assessment should be considered tentative only. In processed commodities, based on the results from the hydrolysis and the processing studies, the residue definitions are also proposed on a tentative basis as metaflumizone (sum of E‐ and Z‐isomers) and M320I04, expressed as metaflumizone. A specific residue definition for rotational crops is not deemed necessary considering that no significant residues are expected to occur.
Fully validated analytical methods are available for the enforcement of the proposed residue definition (raw commodities) in the four main plant matrices at the combined LOQ of 0.02 mg/kg. According to the EURLs, the combined LOQ of 0.02 mg/kg is achievable in high oil content commodities by using the QuEChERS method in routine analyses, and of 0.01 mg/kg in high water content, high acid content and dry commodities.
Available residue trials data were considered sufficient to derive (tentative) MRL proposals as well as risk assessment values for all commodities under evaluation, except for kale where no data are available and for escarole and broccoli as no safe use could be identified. The MRLs derived for cauliflower are tentative since additional residue trials compliant with the GAPs are still required, as well as for tomato, sweet pepper, gherkin, Chinese cabbage, lettuce and other leafy crops extrapolated from lettuce, since further toxicological data on metabolite M320I04 are required.
Metaflumizone is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. The dietary burdens calculated for cattle, swine and sheep were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues was therefore assessed in these groups of livestock.
The metabolism of metaflumizone residues in livestock was investigated in lactating goats and laying hens at dose rate covering the maximum dietary burdens calculated in this review. According to the results of these studies, the residue definition for enforcement and risk assessment in livestock commodities was proposed as metaflumizone (sum of E‐ and Z‐isomers). An analytical method for the enforcement of the proposed residue definition at the combined LOQ of 0.02 mg/kg in all animal tissues and of 0.01 mg/kg in milk and eggs is available. According to screening data generated by EURLs, the E‐Isomer of metaflumizone can be monitored in milk with a screening detection limit (SDL) of 0.005 mg/kg, in egg with an SDL of 0.01 mg/kg and in muscle and honey with an SDL of 0.02 mg/kg.
A livestock feeding study on lactating cows was used to derive MRL and risk assessment values in milk and tissues of ruminants. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs. No MRLs were required for poultry, as the dietary burden calculation is not triggered and very low exposure to metaflumizone residues is expected.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 3.1 of the EFSA PRIMo. For kale where data were insufficient to derive an MRL, EFSA considered the existing EU MRL for an indicative calculation. In a first scenario (EU1), an exceedance of the acute reference dose (ARfD) was identified for lettuces, escaroles and broccoli, representing 208%, 154% and 122% of the ARfD, respectively. An exceedance of the ARfD was also observed for processed commodities of escaroles and broccoli, representing 254% and 231% of the ARfD, respectively. Considering a fall‐back MRL for lettuces (based on the southern data set only) and excluding escaroles and broccoli from the calculation (scenario EU2), the highest chronic exposure represented 15% of the acceptable daily intake (ADI) (GEMS/Food G10 diet) and the highest acute exposure amounted to 92% of the ARfD (unprocessed lettuces). Processing studies on broccoli, for which no processing factors were available, are required to refine the risk assessment (data gap).
Apart from the MRLs evaluated in the framework of this review, internationally recommended Codex residue limits (CXLs) have also been established for metaflumizone. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out (scenario CX1) and an exceedance of the ARfD was identified for the existing CXL in lettuces (146%). Excluding this CXL from the calculation (scenario CX2 equivalent to EU2), the highest chronic exposure represented 15% of the ADI (GEMS/Food G10 diet) and the highest acute exposure amounted to 92% of the ARfD (lettuces).
Considering the limited metabolism of metaflumizone in plants and the toxicological studies previously carried out with both isomers concluding that they share the same toxicity, the potential change of isomer ratios in the final residues is unlikely to be of concern for the authorised use reported in the framework of this review.
Recommendations
MRL recommendations were derived in compliance with the decision tree reported in Appendix E of the reasoned opinion (see Table 2). All MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 2 footnotes for details).
Table 2.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition (existing): metaflumizone (sum of E‐ and Z‐isomer) f | |||||
| 211000 | Potatoes | 0.05 | 0.02* | 0.02* | Recommendeda |
| 231010 | Tomatoes | 0.6 | 0.6 | 0.7 | Further consideration neededb |
| 231020 | Sweet peppers | 1 | 0.6 | 1.5 | Further consideration neededb |
| 231030 | Aubergines (egg plants) | 0.6 | 0.6 | 0.7 | Recommendeda |
| 232010 | Cucumbers | 0.4 | – | 0.4 | Recommendedc |
| 232020 | Gherkins | 0.4 | – | 0.4 | Further consideration neededd |
| 232030 | Courgettes | 0.4 | – | 0.4 | Recommendedc |
| 241010 | Broccoli | 3 | – | – | Further consideration needede |
| 241020 | Cauliflower | 1.5 | – | 0.5 | Further consideration neededd |
| 242010 | Brussels sprouts | 1 | 0.8 | 1 | Recommendeda |
| 242020 | Head cabbage | 1 | – | 0.15 | Recommendedc |
| 243010 | Chinese cabbage | 7 | 6 | 8 | Further consideration neededb |
| 243020 | Kale | 0.05 | – | 0.05 | Further consideration neededf |
| 251010 | Lamb's lettuce | 10 | – | 20 | Further consideration neededd |
| 251020 | Lettuce | 5 | 7 | 6 | Further consideration neededg |
| 251030 | Escarole (broad‐leaf endive) | 0.05 | – | – | Further consideration needede |
| 251040 | Cress | 10 | – | 20 | Further consideration neededd |
| 251050 | Land cress | 10 | – | 20 | Further consideration neededd |
| 251060 | Rocket, Rucola | 10 | – | 20 | Further consideration neededd |
| 251070 | Red mustard | 10 | – | 20 | Further consideration neededd |
| 251080 | Leaves and sprouts of Brassica spp. | 10 | – | 20 | Further consideration neededd |
| 1011010 | Swine muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1011020 | Swine fat (free of lean meat) | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1011030 | Swine liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1011040 | Swine kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012010 | Bovine muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012020 | Bovine fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012030 | Bovine liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012040 | Bovine kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013010 | Sheep muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013020 | Sheep fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013030 | Sheep liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013040 | Sheep kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014010 | Goat muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014020 | Goat fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014030 | Goat liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014040 | Goat kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015010 | Horse muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015020 | Horse fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015030 | Horse liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015040 | Horse kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1020010 | Cattle milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| 1020020 | Sheep milk | 0.02 | 0.01* | 0.01* | Recommendededa |
| 1020030 | Goat milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| 1020040 | Horse milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| – | Other commodities of plant and/or animal origin | See Reg. 318/2014 | – | – | Further consideration neededh |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
The residue definition is fat soluble.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination H‐III in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); existing CXL is covered by the tentative MRL (combination F‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination H‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination F‐I in Appendix E).
GAP evaluated at EU level is not fully supported by data and a risk to consumers cannot be excluded; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination E‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination D‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); CXL is higher, supported by data but a risk to consumers cannot be excluded (combination F‐VI in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A–I in Appendix E).
In particular, some tentative MRLs and existing EU MRL need to be confirmed and, as no safe MRL could be proposed for broccoli, the following data are required to further refine the risk assessment:
four residue trials supporting the southern outdoor GAP on kale;
four additional residue trials supporting the southern outdoor GAP on cauliflower;
processing studies on broccoli to derive processing factors;
additional studies to conclude on the toxicological profile of metabolite M320I04 (relevant for tomato, sweet pepper, gherkin, Chinese cabbage, lettuce and other leafy crops for which data were extrapolated from lettuce).
It is highlighted that some of the (tentative) MRLs derived result from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
eight additional residue trials supporting the southern outdoor GAP on tomato;
four additional residue trials supporting the southern outdoor GAP on aubergine.
If the above‐reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level.
Furthermore, it is highlighted that a possible risk to consumers was identified for the most critical GAPs that are currently authorised for SEU escaroles (Spain), SEU broccoli (Portugal) and indoor lettuces (Portugal). Member States are therefore in any case recommended to withdraw their national authorisations for escaroles and indoor lettuces where no fall‐back MRLs could be derived by EFSA. For broccoli, where a fall‐back MRL could be derived (based on the GAP reported by Greece), however, for which a risk to consumers from processed broccoli could not be excluded, Member States are also recommended to withdraw their national authorisations, pending the submission of processing studies to refine the risk assessment. For lettuces (SEU, currently authorised in Portugal), EFSA recommends that the national authorisations are modified in order to comply with the fall‐back MRL identified by EFSA, based on the GAP reported by Italy and Croatia.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- cGAP
critical GAP
- CS
capsule suspension
- CV
coefficient of variation (relative standard deviation)
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DM
dry matter
- DS
powder for dry seed treatment
- DT90
period required for 90% dissipation (define method of estimation)
- EDI
estimated daily intake
- EFSA
European Food Safety Authority
- MS
evaluating Member State
- eq.
residue expressed as a.s. equivalent
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- FID
flame ionisation detector
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐FID
gas chromatography with flame ionisation detector
- GC‐MS
gas chromatography with mass spectrometry
- GC‐MS/MS
gas chromatography with tandem mass spectrometry
- GS
growth stage
- HPLC
high performance liquid chromatography
- HPLC‐MS
high performance liquid chromatography with mass spectrometry
- HPLC‐MS/MS
high performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LC
liquid chromatography
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- MW
molecular weight
- NEDI
national estimated daily intake
- NESTI
national estimated short‐term intake
- NEU
northern European Union
- NTMDI
national theoretical maximum daily intake
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
pre‐harvest interval
- Pow
partition coefficient between n‐octanol and water
- ppm
parts per million (10−6)
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- SL
soluble concentrate
- SP
water soluble powder
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- WHO
World Health Organization
- WP
wettable powder
Appendix A – Summary of authorised uses considered for the review of MRLs
A.1. Authorised outdoor uses in northern EU
| Crop and/or situation | MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc | Number (min–max) | Min. interval between applications (days) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||||
| Potatoes | AT | F | SC | 240 g/L | Foliar treatment – spraying | 2 | 7 | – | – | 60 g a.s./ha | 14 | |||
MS: Member State; a.s.: active substance.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
A.2. Authorised outdoor uses in southern EU
| Crop and/or situation | MS or country | F G or Ia | Pests or group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc | Number (min–max) | Min. interval between applications (days) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||||
| Potatoes | ES, EL, IT, PT | F | Leptinotarsa decemlineata | SC | 240 g/L | Foliar treatment – spraying | 15–89 | 3 | – | – | – | 60 g a.s./ha | 14 | – |
| Tomatoes | PT | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 51–89 | 2 | 7 | – | – | 240 g a.s./ha | 1 | – |
| Sweet peppers | IT | F | LEPIDOPTEREN (V) (LEPIDO), SCROBIPALPULA ABSOLUTA (GNORAB) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Aubergines | ES, PT | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 51–89 | 2 | 7 | – | – | 240 g a.s./ha | 1 | – |
| Broccoli | PT | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 12–49 | 2 | 7 | – | – | 240 g a.s./ha | 3 | Exceedance of the ARfD observed for this most critical GAP |
| EL | F | Insect pests | Foliar treatment – general | 12–49 | 2 | 7 | – | – | 180 g a.s./ha | 1 | Fall‐back GAP considered to refine the risk assessment. However, exceedance of the ARfD is still observed for processed broccoli | |||
| Cauliflowers | PT | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 12–49 | 2 | 7 | – | – | 240 g a.s./ha | 3 | – |
| Brussels sprouts | IT, PT, HR | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 40–49 | 2 | 7 | – | – | 240 g a.s./ha | 3 | – |
| Head cabbages | IT, PT, HR | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 40–49 | 2 | 7 | – | – | 240 g a.s./ha | 3 | Authorisation also on other Head brassica: Savoy cabbage |
| Chinese cabbages | HR | F |
HELIOTHIS ARMIGERA PLUSIA SP.; SPODOPTERA SP. |
SC | 240 g/L | Foliar treatment – general | 40–49 | 2 | 7 | – | – | 240 g a.s./ha | 3 | Authorisation also on other leafy Brassica: Indian mustard (Brassica juncea) |
| Kales | HR | F |
HELIOTHIS ARMIGERA PLUSIA SP.; SPODOPTERA SP. |
SC | 240 g/L | Foliar treatment – general | 40–49 | 2 | 7 | – | – | 240 g a.s./ha | 3 | GAP not supported by residue trials |
| Lamb's lettuces | IT | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Lettuces | PT | F | Insect pests | SC | 240 g/L | Foliar treatment – general | 12–73 | 2 | 7 | – | – | 240 g a.s./ha | 1 | Exceedance of the ARfD observed for this most critical GAP |
| IT, HR | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – spraying | 40–49 | 1–2 | 7 | – | – | 240 g a.s./ha | 7 | Fall‐back GAP considered to refine the risk assessment | |
| Escaroles | ES | F | SC | 240 g/L | Foliar treatment – spraying | 1 | – | – | 240 g a.s./ha | 3 |
Exceedance of the ARfD observed No fall‐back GAP identified |
|||
| Cresses | IT, HR | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 40–49 | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast |
| Land cresses | IT | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | ‐ | ‐ | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Roman rocket | IT, HR, ES | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 40–49 | 1–2 | 7 | ‐ | ‐ | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast |
| Red mustards | IT | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | ‐ | ‐ | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Baby leaf crops | IT | F | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | ‐ | ‐ | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
MS: Member State; a.s.: active substance.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
A.3. Authorised indoor uses in EU
| Crop and/or situation | MS or country | F G or Ia | Pests or Group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days)d | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc | Number (min–max) | Min. interval between applications (days) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||||
| Tomatoes | PT | I | Insect pests | SC | 240 g/L | Foliar treatment – general | 15–89 | 2 | 7 | – | – | 240 g a.s./ha | 1 | – |
| Sweet peppers | AT, PT | I | Insect pests | SC | 240 g/L | Foliar treatment – general | 15–89 | 2 | 7 | – | – | 240 g a.s./ha | 1 | – |
| Aubergines | ES, PT | I | Insect pests | SC | 240 g/L | Foliar treatment – general | 15–89 | 2 | 7 | – | – | 240 g a.s./ha | 1 | – |
| Cucumbers | NL | I | Insect pests | SC | 240 g/L | Foliar treatment – spraying | 12–73 | 2 | 7 | – | – | 240 g a.s./ha | 3 | – |
| Gherkins | NL | I | Insect pests | SC | 240 g/L | Foliar treatment – spraying | 12–73 | 2 | 7 | – | – | 240 g a.s./ha | 3 | – |
| Courgettes | NL | I | Insect pests | SC | 240 g/L | Foliar treatment – spraying | 12–73 | 2 | 7 | – | – | 240 g a.s./ha | 3 | – |
| Lamb's lettuces | IT | I | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Lettuces | PT | I | Insect pests | SC | 240 g/L | Foliar treatment – general | 12–73 | 2 | 7 | – | – | 240 g a.s./ha | 3 |
Exceedance of the ARfD observed No fall‐back GAP identified |
| Cresses | IT | I | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Land cresses | IT | I | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Roman rocket | IT | I | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Red mustards | IT | I | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
| Baby leaf crops | IT | I | LEPIDOPTEREN (V) (LEPIDO) | SC | 240 g/L | Foliar treatment – general (see comment field) | 1–2 | 7 | – | – | 240 g a.s./ha | 3 | Method kind: normal volume spraying broadcast | |
MS: Member State; a.s.: active substance.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Tomato | Foliar: 6 × 331–378 g a.s./ha, 7 days interval (from early fruiting) | 0, 7 |
Benzonitrile (B‐label) 14C‐metaflumizone or trifluoromethoxyphenyl (T‐label) 14C‐metaflumizone Studies performed both in field and in glasshouse (United Kingdom, 2012) |
|
| Leafy crops | White cabbage | Foliar: 4 × 280 g a.s./ha, 7 days interval (from 106 days after planting) | 0, 3, 7 | B‐labelled or T‐labelled metaflumizone. Studies performed in glasshouse conditions (United Kingdom, 2012) | |
| Pulses/oilseeds | Cotton seeds | Foliar: 6 × 307–350 g a.s./ha, 7 days interval (from mid‐flowering) | 21 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | Radish | Bare soil: 2 × 560 g a.s./ha, 20 days interval | 30, 60/62, 120, 365 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) | |
| Bare soil: 1 × 1.168 kg a.s./ha | 30 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) | |||
| Leafy crops | Lettuce | Bare soil: 2 × 560 g a.s./ha, 20 days interval | 30, 60/62, 120, 365 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) | |
| Bare soil: 1 × 1.168 kg a.s./ha | 30 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) | |||
| Cereal (small grain) | Wheat | Bare soil: 2 × 560 g a.s./ha, 20 days interval | 30, 60/62, 120, 365 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) | |
| Bare soil: 1 × 1.168 kg a.s./ha | 30 | B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes |
Metaflumizone (sum of E‐ and Z‐isomers) is stable in both studies performed with B‐label or T‐label (77–78% of applied radioactivity; AR) Degradation products observed with B‐label study, mainly M320I04 (15% AR) (United Kingdom, 2012) |
|
| Baking, brewing and boiling (60 min, 100°C, pH 5) | No |
Metaflumizone (sum of E‐ and Z‐isomers) is stable in the study performed with T‐label. However, not with B‐label (62% AR) Degradation products observed with B‐label study, mainly M320I04 (25% AR) (United Kingdom, 2012) |
|
| Sterilisation (20 min, 120°C, pH 6) | Yes |
Metaflumizone (sum of E‐ and Z‐isomers) is stable in both studies performed with B‐label or T‐label (89–94% AR) No degradation products observed (United Kingdom, 2012) |

B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Tomato, white cabbage, lettuce, potato | −20 | 24 | Months | Metaflumizone (E‐ and Z‐isomers) and M320I04 separately | United Kingdom (2012) | |
| Potato | −5 | 24 | Months | Metaflumizone E‐isomer, metaflumizone Z‐isomer, M320I04 and M320I23 | Stability of incurred residues was investigated United Kingdom (2013) | ||
| White cabbage, lettuce, celery, broccoli, tomato, mustard green | −20 | 24 | Months | Metaflumizone (E‐ and Z‐isomers), M320I04 and M320I23 |
Stability of incurred residues was investigated. Decline of more than 30% of incurred residues in tomato M320I04 stable, except in lettuce and white cabbage where a rapid decrease was observed (recoveries < 50%) (United Kingdom, 2012) |
||
|
Cucumber Snap been |
−5 | 24 | Months | Metaflumizone E‐isomer, metaflumizone Z‐isomer, M320I04 and M320I23 | Stability of incurred residues was investigated (United Kingdom, 2013) | ||
| High oil content | Cotton seed | −20 | 24 | Months | Metaflumizone (E‐ and Z‐isomers) and M320I04 separately | United Kingdom (2012) | |
| Sunflower seed | −5 | 24 | Months | Metaflumizone E‐isomer, metaflumizone Z‐isomer, M320I04 and M320I23 | Stability of incurred residues was investigated (United Kingdom, 2013) | ||
| High acid content | Strawberry | −5 | 24 | Months | Metaflumizone E‐isomer, metaflumizone Z‐isomer, M320I04 and M320I23 | Stability of incurred residues was investigated (United Kingdom, 2013) | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials – Primary crops
| Commodity | Region/indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| Potatoes | NEU | 10 × < 0.02 |
5 trials on potato compliant with GAP and 5 overdosed trials on potato performed with 1.7N rate and one more application (United Kingdom, 2012), deemed acceptable since residues < LOQ. Residues of M320I04 < 0.01 mg/kg MRLOECD = 0.02 Demonstrated stability: Yes |
0.02* | 0.02 | 0.02 |
| SEU | 10 × < 0.02 |
5 trials on potato compliant with GAP and 5 overdosed trials on potato performed with 1.7N rate (United Kingdom, 2012), deemed acceptable since residues < LOQ. Residues of M320I04 < 0.01 mg/kgMRLOECD = 0.02 Demonstrated stability: Yes |
0.02* | 0.02 | 0.02 | |
| Tomatoes | SEU | – | No data available | – | – | – |
| EU | 0.03; 0.09; 0.10; 0.11; 0.14; 0.15; 0.19; 0.23; 0.38; 0.41 |
Trials on tomatoes compliant with GAP (United Kingdom, 2012). 4 selected values correspond to higher residue levels observed at a longer PHI. Residues of M320I04 < 0.01 mg/kg Extrapolation from tomatoes to aubergines is applicable MRLOECD = 0.68 Demonstrated stability: Yes |
0.70 (tentative)d | 0.41 | 0.15 | |
| Aubergines/eggplants | SEU | – | No data available | – | – | – |
| EU | 0.03; 0.09; 0.10; 0.11; 0.14; 0.15; 0.19; 0.23; 0.38; 0.41 |
Trials on tomatoes compliant with GAP (United Kingdom, 2012). 4 selected values correspond to higher residue levels observed at a longer PHI. Residues of M320I04 < 0.01 mg/kg Extrapolation from tomatoes to aubergines is applicable MRLOECD = 0.68 Demonstrated stability: Yes |
0.70 | 0.41 | 0.15 | |
| Sweet peppers/bell peppers | SEU | 0.05; 0.14; 0.15; 2 × 0.16; 0.18; 0.23; 0.50 |
Trials on sweet peppers compliant with GAP (Italy, 2011). Residues of M320I04 < 0.01 mg/kg MRLOECD = 0.73 Demonstrated stability: Yes |
0.80 (tentative)d | 0.50 | 0.16 |
| EU | 0.10; 0.18; 0.21; 0.24; 0.28; 0.30; 0.34; 0.35; 0.41; 0.81 |
Trials on sweet peppers compliant with GAP (United Kingdom, 2012). 3 selected values correspond to higher residue levels observed at a longer PHI. Residues of M320I04 < 0.01 mg/kg MRLOECD = 1.1 Demonstrated stability: Yes |
1.50 (tentative)d | 0.81 | 0.29 | |
|
Cucumbers Gherkins Courgettes |
EU | < 0.02; 2 × 0.05; 0.06; 0.1; 0.15; 0.19; 0.2 |
Trials on cucumbers compliant with GAP (Italy, 2011). One selected value corresponds to higher residue at a longer PHI. Residues of M320I04 < 0.01 mg/kg Extrapolation from cucumbers to gherkins and courgettes is acceptable MRLOECD = 0.38 Demonstrated stability: Yes |
0.40 (tentative for gherkins)d | 0.20 | 0.08 |
| Broccoli | SEU | 0.37; 0.54; 0.61; 3.82 |
Trials on broccoli compliant with GAP (Italy, 2011). Residues of M320I04 < 0.01 mg/kg MRLOECD = 7.97 Demonstrated stability: Yes Exceedances of the ARfD is observed for broccoli (unprocessed and processed) |
10 | 3.82 | 0.58 |
| SEU (fall‐back) | 0.28; 0.41; 0.46; 2.87 |
Trial results scaled down with the fall‐back GAP (Italy, 2011; Sweden, 2019) MRLOECD = 5.99 Demonstrated stability: Yes Exceedance of the ARfD is still observed for processed broccoli. No safe fall‐back MRL could be calculated |
8 | 2.87 | 0.44 | |
| Cauliflowers | SEU | < 0.02; 0.05; 0.07; 0.17 |
Trials on cauliflowers compliant with GAP (Italy, 2011). Last value corresponds to higher residue level observed at a longer PHI. Residues of M320I04 < 0.01 mg/kg MRLOECD = 0.34 Demonstrated stability: Yes |
0.50 (tentative)e | 0.17 | 0.06 |
| Brussels sprouts | SEU | 0.1; 0.12; 0.35; 0.39 |
Trials on Brussels sprouts compliant with GAP (Italy, 2011). All selected values correspond to higher residue levels observed at a longer PHI. Residues of M320I04 < 0.01 mg/kg MRLOECD = 0.84 Demonstrated stability: Yes |
1 | 0.39 | 0.24 |
| Head cabbages | SEU | 2 × < 0.02; 0.05; 0.06 |
Trials on head cabbages compliant with GAP (Italy, 2011). Residues of M320I04 < 0.01 mg/kg MRLOECD = 0.12 Demonstrated stability: Yes |
0.15 | 0.06 | 0.04 |
| Chinese cabbages/pe‐tsai | SEU | 0.13; 0.26; 1.33; 2.89 |
Trials on Chinese cabbage compliant with GAP (Italy, 2011). One selected value corresponds to higher residue level observed at a longer PHI. Residues of M320I04 ranged from < 0.01 mg/kg to 0.08 mg/kg MRLOECD = 6.26 Demonstrated stability: Yes |
8 (tentative)f | 2.89 | 0.80 |
| Kales | SEU | – | No data compliant with GAP available | – | – | – |
|
Lamb's lettuces/corn salads Escaroles/broad‐leaved endives Cresses and other sprouts and shoots Land cresses Roman rocket/rucola Red mustards Baby leaf crops (including brassica species) |
SEU | 0.76; 2.68; 2.85; 2.98; 3.61; 4.98 |
Trials on lettuce compliant with GAP (only residue levels from open leaf varieties are reported) (Italy, 2011; Sweden, 2019). Residues of M320I04 ranged from < 0.01 mg/kg to 0.10 mg/kg Extrapolation from open leaf varieties of lettuce to other salad plants is acceptable MRLOECD = 8.93 Demonstrated stability: Yes Exceedances of the ARfD is observed for escaroles/broad‐leaved endives (unprocessed and processed). No fall‐back MRL could be calculated for this crop |
9 (tentative)f | 4.98 | 2.92 |
|
Lamb's lettuces/corn salads Cresses and other sprouts and shoots Land cresses Roman rocket/rucola Red mustards Baby leaf crops (including brassica species) |
EU | 4.7; 5; 5.5; 6.4 |
Trials on lettuce compliant with GAP (only residue levels from open leaf varieties are reported) (Italy, 2011; Sweden, 2019). Residues of M320I04 ranged from 0.01 mg/kg to 0.09 mg/kg Extrapolation from open leaf varieties of lettuce to other salad plants is acceptable MRLOECD = 16.2 Demonstrated stability: Yes |
20 (tentative)f | 6.40 | 5.25 |
| Lettuces | SEU | 2.31; 3.44; 3.58; 3.62; 3.63; 5.34; 6.49; 7.11 |
Trials on lettuce compliant with GAP (Italy, 2011). All selected values correspond to higher values observed at a shorter PHI. Residues of M320I04 ranged from < 0.01 mg/kg to 0.03 mg/kg Extrapolation from lettuce (open leaf varieties) to other salad plants is acceptable MRLOECD = 13.32 Demonstrated stability: Yes Exceedance of the ARfD is observed for lettuces |
15 (tentative)f | 7.11 | 3.63 |
| SEU (fall‐back) | 0.15; 0.94; 1.12; 1.17; 1.52; 1.61; 2.39; 3.14 |
Trials on lettuce compliant with fall‐back GAP (Italy, 2011; Sweden, 2019). Residues of M320I04 ranged from < 0.01 mg/kg to 0.07 mg/kg MRLOECD = 5.17 Demonstrated stability: Yes |
6 (tentative)f | 3.14 | 1.35 | |
| EU | 2; 2.4; 3.1; 4.4; 4.7; 5; 5.5; 6.4 |
Trials on lettuce compliant with GAP (Italy, 2011). One selected value corresponds to higher value observed at a longer PHI. Residues of M320I04 ranged from < 0.01 mg/kg to 0.09 mg/kg Extrapolation from lettuce (open leaf varieties) to other salad plants is acceptable MRLOECD = 12.56 Demonstrated stability: Yes Exceedance of the ARfD is observed for lettuces. No fall‐back MRL could be calculated |
15 (tentative)f | 6.40 | 4.55 |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level; PHI: preharvest interval.
Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
MRL is tentative considering that residue levels of metaflumizone in raw tomato and residue levels of metabolite M320I04 in processed tomato are significant, while the toxicological relevance of this metabolite is not fully elucidated. Considering that processing of tomato, sweet pepper and gherkin is comparable, MRL is tentative also for these commodities.
MRL is tentative because additional trials compliant with GAP are needed.
MRL is tentative considering that residue levels of metabolite M320I04 are measured in the GAP‐compliant field trials on lettuce and Chinese cabbage, while the toxicological relevance of this metabolite is not fully elucidated.
B.1.2.2. Residues in rotational crops
a) Overall summary

B.1.2.3. Processing factors
| Processed commodity | Number of valid studiesa | Processing Factor (PF) | Comment/Source | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Head cabbage, cooked | 4 | 0.02; 0.02; 0.02; 0.08 | 0.02 | Residues of M320I04 below LOQ in cooked cabbage (Italy, 2011) |
| Head cabbage, fermented | 4 | 0.02; 0.02; 0.03; 0.05 | 0.02 | Residues of Z‐isomer below LOQ and residues of M320I04 range from < 0.018 to 0.04 mg eq./kg in fermented cabbage (Italy, 2011) |
| Head cabbage, juice of fermented | 4 | 0.01; 0.02; 0.02; 0.05 | 0.02 | Residues of Z‐isomer and M320I04 below LOQ in juice of fermented cabbage (Italy, 2011) |
| Tomato, canned | 4 | 0.07; 0.32; 0.48; 0.55 | 0.40 | Residues of metaflumizone E‐ and Z‐isomers below LOQ and residues of M320I04 range from < 0.018 to 0.073 mg eq./kg in canned tomato (United Kingdom, 2012) |
| Tomato, sauce | 4 | 0.42; 0.52; 0.92; 0.99 | 0.72 | Residues of Z‐isomer below LOQ and residues of M320I04 range from 0.04 to 0.16 mg eq./kg in tomato sauce (United Kingdom, 2012) |
| Tomato, paste | 4 | 1.44; 2.75; 3.06; 4.56 | 2.90 | Residues of Z‐isomer below LOQ and residues of M320I04 range from 0.24 to 0.51 mg eq./kg in tomato paste (United Kingdom, 2012) |
| Tomato, juice | 4 | 0.16; 0.51; 0.54; 0.85 | 0.52 | Residues of metaflumizone E‐ and Z‐isomers below LOQ and residues of M320I04 range from 0.04 to 0.07 mg eq./kg in tomato juice United Kingdom, 2012) |
| Head lettuce, washed (outer leaves) | 4 | 0.38; 0.54; 0.54; 0.76 | 0.54 | Residues of M320I04 range from 0.12 to 0.61 mg eq./kg in washed lettuce (Italy, 2011) |
| Head lettuce, washed (inner leaves) | 4 | 0.05; 0.05; 0.08; 0.10 | 0.07 | Residues of M320I04 range from < 0.018 to 0.04 mg eq./kg in washed lettuce (Italy, 2011) |
| Cotton seeds, meal | 1 | – | 0.13b | Mean value from 2 samples. Residues of M320I04 below LOQ in processed cotton seeds (Italy, 2011) |
| Cotton seeds, hulls | 1 | – | 1.15b | Mean value from 2 samples. Residues of M320I04 range from 0.04 to 0.05 mg eq./kg in processed cotton seeds (Italy, 2011) |
| Cotton seeds, crude oil | 1 | – | 0.85b | Mean value from 2 samples. Residues of M320I04 below LOQ in processed cotton seeds in processed cotton seeds (Italy, 2011) |
| Cotton seeds, refined oil | 1 | – | 0.84b | Mean value from 2 samples. Residues of M320I04 below LOQ in processed cotton seeds (Italy, 2011) |
PF: Processing factor (=Residue level in processed commodity expressed according to RD‐Mo/Residue level in raw commodity expressed according to RD‐Mo).
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Median of the individual conversion factors for each processing residues trial.
A tentative PF is derived based on a limited data set.
B.2. Residues in livestock
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Yes/No) | Comments | |||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Median | Maximum | Median | Maximum | |||||
| Cattle (all) | 0.005 | 0.006 | 0.14 | 0.18 | Cattle (dairy) | Cabbage, heads, leaves | Yes | – |
| Cattle (dairy only) | 0.005 | 0.006 | 0.13 | 0.16 | Cattle (dairy) | Cabbage, heads, leaves | Yes | – |
| Sheep (all) | 0.004 | 0.005 | 0.12 | 0.14 | Sheep (ram/ewe) | Cabbage, heads, leaves | Yes | – |
| Sheep (ewe only) | 0.004 | 0.005 | 0.12 | 0.14 | Sheep (ram/ewe) | Cabbage, heads, leaves | Yes | – |
| Swine (all) | 0.002 | 0.003 | 0.11 | 0.12 | Swine (breeding) | Cabbage, heads, leaves | Yes | – |
| Poultry (all) | 0.002 | 0.002 | 0.03 | 0.03 | Poultry (layer) | Cabbage, heads, leaves | No | – |
| Poultry (layer only) | 0.002 | 0.002 | 0.03 | 0.03 | Poultry (layer) | Cabbage, heads, leaves | No | – |
bw: body weight; DM: dry matter.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | Comment/Source |
|---|---|---|---|---|
| Laying hens | 0.89–0.96 | 14 |
Study performed with B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) Dose rate recalculated assuming body weight of 1.9 kg and feed intake of 0.13 kg per day |
|
| Lactating ruminants | 0.34 | 14 |
Study performed on goats with B‐labelled or T‐labelled metaflumizone (United Kingdom, 2012) Dose rate recalculated assuming body weight of 70 kg and feed intake of 2 kg per day |

B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| Goats | Muscle, fat, liver, kidney, milk | −18 | 18 | Months | Metaflumizone (E‐ and Z‐isomers) | Storage stability demonstrated in the metabolism study (United Kingdom, 2012) | |
| Poultry | Muscle, fat, liver, eggs | −18 | 24 | Months | Metaflumizone (E‐ and Z‐isomers) | Storage stability demonstrated in the metabolism study (United Kingdom, 2012) | |
B.2.2. Magnitude of residues in livestock
B.2.2.1. Summary of the residue data from livestock feeding studies
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | ||
|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | ||
| Cattle (all) – Closest feeding level (0.0077 mg/kg bw; 1.3 N rate)c | |||||
| Muscle | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Fat | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Liver | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Kidney | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Cattle (dairy only) – Closest feeding level (0.0077 mg/kg bw; 1.3 N rate)c | |||||
| Milkd | < 0.01 | < 0.01 | 0.01 | 0.01 | 0.01* |
| Sheep (all) e − Closest feeding level (0.0077 mg/kg bw; 1.6 N rate)c | |||||
| Muscle | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Fat | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Liver | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Kidney | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Sheep (ewe only) e − Closest feeding level (0.0077 mg/kg bw; 1.6 N rate)c | |||||
| Milkd | < 0.01 | < 0.01 | 0.01 | 0.01 | 0.01* |
| Swine (all) e − Closest feeding level (0.0077 mg/kg bw; 2.5 N rate)c | |||||
| Muscle | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Fat | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Liver | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Kidney | < 0.02 | < 0.02 | 0.02 | 0.02 | 0.02* |
| Poultry (all) – dietary burden not triggered, no MRL needed | |||||
| Muscle | – | – | – | – | – |
| Fat | – | – | – | – | – |
| Liver | – | – | – | – | – |
| Poultry (layer only) – dietary burden not triggered, no MRL needed | |||||
| Eggs | – | – | – | – | – |
Indicates that the MRL is proposed at the limit of quantification.
Median residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the median dietary burden.
Highest residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the maximum dietary burden.
Closest feeding level and N dose rate related to the maximum dietary burden.
For milk, mean was derived from samplings performed from day 1 to day 45 (daily mean of three cows).
Since extrapolation from cattle to other ruminants and swine is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in sheep and swine.
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs
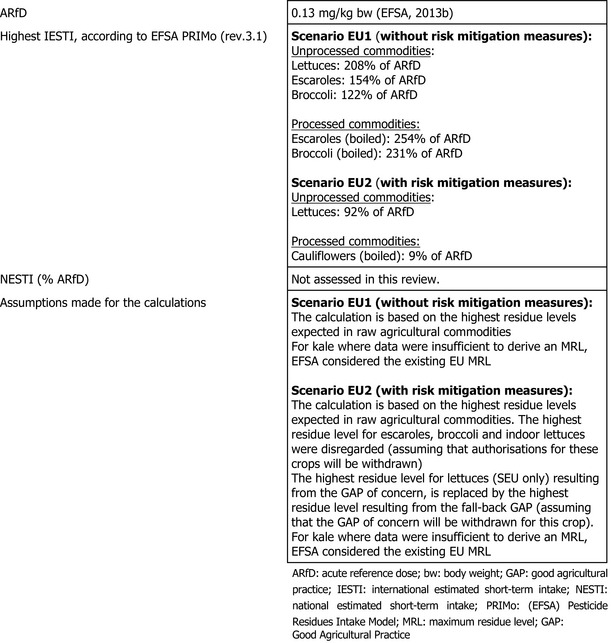


B.3.2. Consumer risk assessment with consideration of the existing CXLs


B.4. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |
|---|---|---|---|---|---|
| MRL (mg/kg) | Comment | ||||
| Enforcement residue definition (existing): metaflumizone (sum of E‐ and Z‐isomer) F | |||||
| 211000 | Potatoes | 0.05 | 0.02* | 0.02* | Recommendeda |
| 231010 | Tomatoes | 0.6 | 0.6 | 0.7 | Further consideration neededb |
| 231020 | Sweet peppers | 1 | 0.6 | 1.5 | Further consideration neededb |
| 231030 | Aubergines (egg plants) | 0.6 | 0.6 | 0.7 | Recommendeda |
| 232010 | Cucumbers | 0.4 | – | 0.4 | Recommendedc |
| 232020 | Gherkins | 0.4 | – | 0.4 | Further consideration neededd |
| 232030 | Courgettes | 0.4 | – | 0.4 | Recommendedc |
| 241010 | Broccoli | 3 | – | – | Further consideration needede |
| 241020 | Cauliflower | 1.5 | – | 0.5 | Further consideration neededd |
| 242010 | Brussels sprouts | 1 | 0.8 | 1 | Recommendeda |
| 242020 | Head cabbage | 1 | – | 0.15 | Recommendedc |
| 243010 | Chinese cabbage | 7 | 6 | 8 | Further consideration neededb |
| 243020 | Kale | 0.05 | – | 0.05 | Further consideration neededf |
| 251010 | Lamb's lettuce | 10 | – | 20 | Further consideration neededd |
| 251020 | Lettuce | 5 | 7 | 6 | Further consideration neededg |
| 251030 | Escarole (broad‐leaf endive) | 0.05 | – | – | Further consideration needede |
| 251040 | Cress | 10 | – | 20 | Further consideration neededd |
| 251050 | Land cress | 10 | – | 20 | Further consideration neededd |
| 251060 | Rocket, Rucola | 10 | – | 20 | Further consideration neededd |
| 251070 | Red mustard | 10 | – | 20 | Further consideration neededd |
| 251080 | Leaves and sprouts of Brassica spp. | 10 | – | 20 | Further consideration neededd |
| 1011010 | Swine muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1011020 | Swine fat (free of lean meat) | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1011030 | Swine liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1011040 | Swine kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012010 | Bovine muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012020 | Bovine fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012030 | Bovine liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1012040 | Bovine kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013010 | Sheep muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013020 | Sheep fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013030 | Sheep liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1013040 | Sheep kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014010 | Goat muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014020 | Goat fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014030 | Goat liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1014040 | Goat kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015010 | Horse muscle | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015020 | Horse fat | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015030 | Horse liver | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1015040 | Horse kidney | 0.02 | 0.02* | 0.02* | Recommendeda |
| 1020010 | Cattle milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| 1020020 | Sheep milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| 1020030 | Goat milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| 1020040 | Horse milk | 0.02 | 0.01* | 0.01* | Recommendeda |
| – | Other commodities of plant and/or animal origin | See Reg. 318/2014 | – | – | Further consideration neededh |
MRL: maximum residue level; CXL: codex maximum residue limit.
Indicates that the MRL is set at the limit of quantification.
The residue definition is fat soluble.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination H‐III in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); existing CXL is covered by the tentative MRL (combination F‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available (combination H‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination F‐I in Appendix E).
GAP evaluated at EU level is not fully supported by data and a risk to consumers cannot be excluded; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination E‐I in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination D‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); CXL is higher, supported by data but a risk to consumers cannot be excluded (combination F‐VI in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo(EU1)

PRIMo(EU2)

PRIMo(CX1)

PRIMo(CX2) = PRIMo(EU2)
Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Potato, culls | 0.02* | STMR | 0.02* | HR |
| Potato, process waste | 0.02* | STMRa | 0.02* | STMRa |
| Potato, dried pulp | 0.02* | STMRa | 0.02* | STMRa |
| Cabbage, heads, leaves | 0.04 | STMR | 0.06 | HR |
STMR: supervised trials median residue; HR: highest residue.
Indicates that the input value is proposed at the limit of quantification.
For processed commodities of potatoes, no default processing factor was applied because residues of metaflumizone are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2. Consumer risk assessment without consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: metaflumizone (sum of E‐ and Z‐isomers) | ||||
| Potatoes | 0.02* | STMR | 0.02* | HR |
| Tomatoes | 0.15 | STMR (tentative) | 0.41 | HR (tentative) |
| Sweet peppers/bell peppers | 0.29 | STMR (tentative) | 0.81 | HR (tentative) |
| Aubergines/egg plants | 0.15 | STMR | 0.41 | HR |
| Cucumbers | 0.08 | STMR | 0.2 | HR |
| Gherkins | 0.08 | STMR (tentative) | 0.2 | HR (tentative) |
| Courgettes | 0.08 | STMR | 0.2 | HR |
| Broccoli | 0.58 | STMR | 3.82 | HR |
| – | No fall‐back STMR | – | No fall‐back HR | |
| Cauliflowers | 0.06 | STMR (tentative) | 0.17 | HR (tentative) |
| Brussels sprouts | 0.24 | STMR | 0.39 | HR |
| Head cabbages | 0.04 | STMR | 0.06 | HR |
| Chinese cabbages/pe‐tsai | 0.80 | STMR (tentative) | 2.89 | HR (tentative) |
| Kales | 0.05 | EU MRL | 0.05 | EU MRL |
| Lamb's lettuce/corn salads | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Lettuces | 4.55 | STMR (tentative) | 7.11 | HR (tentative) |
| 1.35 | STMR (fall‐back, tentative) | 3.14 | HR (fall‐back, tentative) | |
| Escaroles/broad‐leaved endives | 2.92 | STMR (tentative) | 4.98 | HR (tentative) |
| – | No fall‐back STMR | – | No fall‐back HR | |
| Cress and other sprouts and shoots | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Land cress | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Roman rocket/rucola | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Red mustards | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Baby leaf crops (including brassica species) | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Swine: Meat | 0.02* | STMR | 0.02* | HR |
| Swine: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Swine: Liver | 0.02* | STMR | 0.02* | HR |
| Swine: Kidney | 0.02* | STMR | 0.02* | HR |
| Bovine: Meat | 0.02* | STMR | 0.02* | HR |
| Bovine: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Bovine: Liver | 0.02* | STMR | 0.02* | HR |
| Bovine: Kidney | 0.02* | STMR | 0.02* | HR |
| Sheep: Meat | 0.02* | STMR | 0.02* | HR |
| Sheep: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Sheep: Liver | 0.02* | STMR | 0.02* | HR |
| Sheep: Kidney | 0.02* | STMR | 0.02* | HR |
| Goat: Meat | 0.02* | STMR | 0.02* | HR |
| Goat: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Goat: Liver | 0.02* | STMR | 0.02* | HR |
| Goat: Kidney | 0.02* | STMR | 0.02* | HR |
| Equine: Meat | 0.02* | STMR | 0.02* | HR |
| Equine: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Equine: Liver | 0.02* | STMR | 0.02* | HR |
| Equine: Kidney | 0.02* | STMR | 0.02* | HR |
| Milk: Cattle | 0.01* | STMR | 0.01* | STMR |
| Milk: Sheep | 0.01* | STMR | 0.01* | STMR |
| Milk: Goat | 0.01* | STMR | 0.01* | STMR |
| Milk: Horse | 0.01* | STMR | 0.01* | STMR |
STMR: supervised trials median residue; HR: highest residue; MRL: maximum residue level; CXL: Codex MRL.
Indicates that the input value is proposed at the limit of quantification.
D.3. Consumer risk assessment with consideration of the existing CXLs
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: metaflumizone (sum of E‐ and Z‐isomers) | ||||
| Potatoes | 0.02* | STMR | 0.02* | HR |
| Tomatoes | 0.15 | STMR (tentative) | 0.41 | HR (tentative) |
| Sweet peppers/bell peppers | 0.29 | STMR (tentative) | 0.81 | HR (tentative) |
| Aubergines/egg plants | 0.15 | STMR | 0.41 | HR |
| Cucumbers | 0.08 | STMR | 0.2 | HR |
| Gherkins | 0.08 | STMR (tentative) | 0.2 | HR (tentative) |
| Courgettes | 0.08 | STMR | 0.2 | HR |
| Broccoli | – | No fall‐back STMR | – | No fall‐back HR |
| Cauliflowers | 0.06 | STMR (tentative) | 0.17 | HR (tentative) |
| Brussels sprouts | 0.24 | STMR | 0.39 | HR |
| Head cabbages | 0.04 | STMR | 0.06 | HR |
| Chinese cabbages/pe‐tsai | 0.80 | STMR (tentative) | 2.89 | HR (tentative) |
| Kales | 0.05 | EU MRL | 0.05 | EU MRL |
| Lamb's lettuce/corn salads | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Lettuces | 2 | STMR (CXL) | 5 | HR (CXL) |
| 1.35 | STMR (fall‐back, tentative, scenario EU2) | 3.14 | HR (fall‐back, tentative, scenario EU2) | |
| Escaroles/broad‐leaved endives | – | No fall‐back STMR | – | No fall‐back HR |
| Cress and other sprouts and shoots | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Land cress | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Roman rocket/rucola | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Red mustards | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Baby leaf crops (including brassica species) | 5.25 | STMR (tentative) | 6.4 | HR (tentative) |
| Swine: Meat | 0.02* | STMR | 0.02* | HR |
| Swine: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Swine: Liver | 0.02* | STMR | 0.02* | HR |
| Swine: Kidney | 0.02* | STMR | 0.02* | HR |
| Bovine: Meat | 0.02* | STMR | 0.02* | HR |
| Bovine: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Bovine: Liver | 0.02* | STMR | 0.02* | HR |
| Bovine: Kidney | 0.02* | STMR | 0.02* | HR |
| Sheep: Meat | 0.02* | STMR | 0.02* | HR |
| Sheep: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Sheep: Liver | 0.02* | STMR | 0.02* | HR |
| Sheep: Kidney | 0.02* | STMR | 0.02* | HR |
| Goat: Meat | 0.02* | STMR | 0.02* | HR |
| Goat: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Goat: Liver | 0.02* | STMR | 0.02* | HR |
| Goat: Kidney | 0.02* | STMR | 0.02* | HR |
| Equine: Meat | 0.02* | STMR | 0.02* | HR |
| Equine: Fat tissue | 0.02* | STMR | 0.02* | HR |
| Equine: Liver | 0.02* | STMR | 0.02* | HR |
| Equine: Kidney | 0.02* | STMR | 0.02* | HR |
| Milk: Cattle | 0.01* | STMR | 0.01* | STMR |
| Milk: Sheep | 0.01* | STMR | 0.01* | STMR |
| Milk: Goat | 0.01* | STMR | 0.01* | STMR |
| Milk: Horse | 0.01* | STMR | 0.01* | STMR |
STMR: supervised trials median residue; HR: highest residue; MRL: maximum residue level; CXL: Codex MRL.
Indicates that the input value is proposed at the limit of quantification.
Appendix E – Decision tree for deriving MRL recommendations
1.

Appendix F – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| Metaflumizone BAS 320 I ( E ‐isomer) |
(2E)‐2‐{2‐(4‐cyanophenyl)‐1‐[3‐(trifluoromethyl)phenyl]ethylidene}‐N‐[4‐(trifluoromethoxy)phenyl]hydrazinecarboxamide FC(F)(F)Oc1ccc(cc1)NC(=O)N\N=C(/Cc2ccc(C#N)cc2)c3cccc(c3)C(F)(F)F MIFOMMKAVSCNKQ‐QNKGDIEWSA‐N |

|
|
Metaflumizone BAS 320 I (Z‐ isomer) |
(2Z)‐2‐{2‐(4‐cyanophenyl)‐1‐[3‐(trifluoromethyl)phenyl]ethylidene}‐N‐[4‐(trifluoromethoxy)phenyl]hydrazinecarboxamide FC(F)(F)Oc1ccc(cc1)NC(=O)N\N=C(\Cc2ccc(C#N)cc2)c3cccc(c3)C(F)(F)F MIFOMMKAVSCNKQ‐HWIUFGAZSA‐N |

|
| M320I23 |
4‐{(5RS)‐5‐hydroxy‐3‐oxo‐4‐[4‐(trifluoromethoxy)phenyl]‐6‐[3‐(trifluoromethyl)phenyl]‐2,3,4,5‐tetrahydro‐1,2,4‐triazin‐5‐yl}benzonitrile FC(F)(F)Oc1ccc(cc1)N3C(=O)NN=C(c2cc(ccc2)C(F)(F)F)C3(O)c4ccc(C#N)cc4 WVLWMHZHXMIYJC‐UHFFFAOYSA‐N |

|
| M320I26 |
(4RS)‐4‐amino‐5‐{(1RS)‐2‐(4‐cyanophenyl)‐1‐[3‐(trifluoromethyl)phenyl]ethoxy}‐5‐oxopentanoic acid (unstated stereochemistry) FC(F)(F)c1cccc(c1)C(Cc2ccc(C#N)cc2)OC(=O)C(N)CCC(=O)O QUTHADOJIJMNHR‐UHFFFAOYSA‐N |
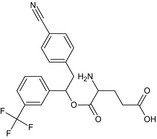
|
| M320I04 |
4‐{2‐oxo‐2‐[3‐(trifluoromethyl)phenyl]ethyl}benzonitrile FC(F)(F)c1cccc(c1)C(=O)Cc2ccc(C#N)cc2 CMHGFDOROXXOOB‐UHFFFAOYSA‐N |

|
| M320I25 |
4‐{(2RS)‐2‐hydroxy‐2‐[3‐(trifluoromethyl)phenyl]ethyl}benzonitrile FC(F)(F)c1cccc(c1)C(O)Cc2ccc(C#N)cc2 SKSGGSZKBBQVKZ‐UHFFFAOYSA‐N |

|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2020. Reasoned Opinion on the review of the existing maximum residue levels for metaflumizone according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2020;18(6):6123, 60 pp. 10.2903/j.efsa.2020.6123
Requestor: European Commission
Question number: EFSA‐Q‐2014‐00596
Acknowledgement: EFSA wishes to thank the following EFSA staff members Chris Anagnostopoulos, Laszlo Bura, Georgios Chatzisotiriou, Viktoria Krivova, Silvia Ruocco and Viktor Toth for the support provided and the rapporteur Member State Sweden for the preparatory work on this scientific output.
Approved: 27 April 2020
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Implementing Regulation (EU) No 922/2014 of 25 August 2014 approving the active substance metaflumizone, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Implementing Regulation (EU) No 540/2011. OJ L 252, 26.8.2014, p. 6–10.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Commission Regulation (EU) No 188/2011 of 25 February 2011 laying down detailed rules for the implementation of Council Directive 91/414/EEC as regards the procedure for the assessment of active substances which were not on the market 2 years after the date of notification of that Directive. OJ No L 53, 26.2.2011, p. 51–55.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food Safety Authority), 2013a. Reasoned opinion on the modification of the existing MRLs for metaflumizone in various commodities. EFSA Journal 2013;11(7):3316, 50 pp. 10.2903/j.efsa.2013.3316 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. Conclusion on the peer review of the pesticide risk assessment of the active substance metaflumizone. EFSA Journal 2013;11(10):3373, 98 pp. 10.2903/j.efsa.2013.3373 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019;EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2020a. Completeness check report on the review of the existing MRLs of metaflumizone prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 03 February 2020. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2020b. Member States consultation report on the review of the existing MRLs of metaflumizone prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 15 April 2020. Available online: www.efsa.europa.eu
- EURLs (European Union Reference Laboratories for Pesticide Residues), 2019. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and capability of official laboratories to be considered for the review of the existing MRLs for metaflumizone 7 May 2019. Available online: www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- FAO (Food and Agriculture Organization of the United Nations), 2009a. Metaflumizone. In: Pesticide residues in food 2009. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues. FAO Plant Production and Protection Paper 196.
- FAO (Food and Agriculture Organization of the United Nations), 2009b. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- Italy , 2011. Evaluation report on the modification of MRLs for metaflumizone in cucumber, courgette, gherkin, melon, head cabbage, lettuce and similar, scarole, herbs, Brussels sprouts, Chinese cabbage, beans and peas with pods, broccoli, cauliflower, artichokes and cotton seed and animal origin products prepared by the evaluating Member State (EMS) Italy under Article 8 of Regulation (EC) No 396/2005. 179 pp. Available online: www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2008. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96. ENV/JM/MONO(2008)23, 29 July 2008.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- Sweden , 2019. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Review of the existing MRLs for metaflumizone, August 2019, revised November 2019. Available online: www.efsa.europa.eu
- United Kingdom , 2012. Draft assessment report on the active substance metaflumizone prepared by the rapporteur Member State United Kingdom in the framework of Council Directive 91/414/EEC, June 2012. Available online: www.efsa.europa.eu
- United Kingdom , 2013. Final addendum to the draft assessment report on the active substance metaflumizone, compiled by EFSA, August 2013. Available online: www.efsa.europa.eu


