Abstract
EFSA received from the European Commission a mandate to deliver a reasoned opinion according to Article 43 of Regulation (EC) No 396/2005 on the safety of a proposed temporary maximum residue level (tMRL) for chlorpropham in potatoes to consumers. Sprout inhibitors based on the active substance chlorpropham have been widely used in commercial storage facilities in Europe over multiple seasons. Following the non‐renewal of approval of chlorpropham, representatives of potato trade organisations and manufacturers of chlorpropham reported an issue of cross‐contamination above the limit of quantification (LOQ) of untreated potatoes stored in facilities with a history of applications of chlorpropham as a post‐harvest treatment. The evaluating Member State (EMS) the Netherlands, in accordance with Articles 6(2) and 16 (1)(a) of Regulation (EC) No 396/2005, submitted an application for the setting of a tMRL for chlorpropham in potatoes in order to address the cross‐contamination in commercial storages. The EMS proposed to set a tMRL for the active substance at a level ranging between 0.3 and 0.5 mg/kg. Based on the assessment of the available data and information with different methodologies, optional tMRL proposals of 0.3, 0.4 and 0.5 mg/kg were derived, and an indicative consumer risk assessment was carried out. The tMRL proposals require further consideration by risk managers, mainly with regard to identified uncertainties, and measures for further reduction of occurrence of chlorpropham residues will also need to be considered. With this prospect, EFSA concluded that the short‐term and long‐term intake of residues of chlorpropham and 3‐chloroaniline resulting from cross‐contamination in potatoes is unlikely to present a risk to consumer health.
Keywords: chlorpropham, 3‐chloroaniline, potatoes, pesticide, temporary MRL, consumer risk assessment
Summary
In accordance with Articles 6(2) and 16 (1) (a) of Regulation (EC) No 396/2005, an application for the setting of a temporary maximum residue level (tMRL) for chlorpropham in potatoes, in order to address the cross‐contamination in storage facilities, was submitted by the competent national authority in the Netherlands (evaluating Member State, EMS) to the European Commission. Subsequently, the European Commission, in the framework of Article 43 of Regulation (EC) No 396/2005, requested the European Food Safety Authority (EFSA) to carry out an assessment of the potential dietary exposure and risk to consumers for the temporary maximum residue level (MRL) recommended for chlorpropham by the Netherlands. The proposed temporary MRL should accommodate for residues of chlorpropham in untreated potatoes exceeding the limit of quantification (LOQ) of 0.01 mg/kg due to cross‐contamination from storage facilities, previously treated with a post‐harvest application.
The Netherlands drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to EFSA on 7 October 2019. The submission of several final study reports was still pending at the time of submission of the first evaluation report by the EMS to EFSA. Successively, EFSA identified points for further clarification that was requested from the EMS. On 10 January 2020, the EMS submitted the requested information in a revised evaluation report, which replaced the previously submitted evaluation report. The EMS proposed to set a tMRL for chlorpropham at a level ranging between 0.3 and 0.5 mg/kg depending on the data and methodology used to derive tMRLs.
Based on the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009, and the additional data provided by the EMS in the framework of this assessment, the following conclusions are derived.
The nature of residues following cross‐contamination of untreated potatoes stored in warehouses with a history of chlorpropham treatments is sufficiently understood.
Chlorpropham was shown susceptible to high temperature hydrolysis in studies simulating industrial and household processing conditions and degraded strongly under formation of 3‐chloroaniline at conditions reflecting oven‐baking of potato.
The residue definition for risk assessment for potatoes and processed products thereof is derived as (1) chlorpropham (free and conjugated), expressed as chlorpropham and (2) 3‐chloroaniline (free and conjugated), expressed as 3‐chloroaniline and is only applicable to assessments conducted for contaminated potatoes. The residue definition differs from the residue definition set by the peer review for crops receiving chlorpropham treatment.
The residue definition for enforcement is proposed as chlorpropham. Sufficiently validated analytical methods are available to quantify residues in contaminated potatoes according to the enforcement residue definition. The methods enable quantification of residues at or above 0.01 mg/kg (LOQ).
Based on the assessment of the available data and information, different tMRL proposals ranging between 0.3 and 0.5 mg/kg were derived using different methodologies that accounted for the different quality of the available data sets. The proposed options for a tMRL require further consideration by risk managers.
Tentative processing factors (PF) for the crops under assessment were derived from a processing study provided for
Chlorpropham – Potato/oven‐baked wedges: PF 2
3‐chloroaniline – Potato/oven‐baked wedges: PF 4
Potatoes and their by‐products are used as feed products. The calculated livestock dietary burden exceeds the trigger value of 0.004 mg/kg body weight (bw) for all relevant animal species in separate assessments for chlorpropham (free and conjugated) and 3‐chloroaniline (free and conjugated) and further data to address specifically the carry‐over of 3‐chloroaniline and occurrence of 3‐chloro 4‐hydroxyaniline in animal commodities would normally be required to complete the consumer risk assessment. EFSA suggests that the uncertainty arising from the non‐consideration of animal commodities in the consumer risk assessment should be taken into account when the risk assessment outcome for 3‐chloroaniline is considered.
The toxicological profile of chlorpropham and of 3‐chloroaniline was assessed in the framework of the EU pesticides peer review under Regulation (EC) No 1107/2009. The metabolite 3‐chloroaniline included in the residue definition for risk assessment is of higher toxicity than chlorpropham; therefore the two compounds should be considered separately in the risk assessment.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo), considering different scenarios according to the available data sets.
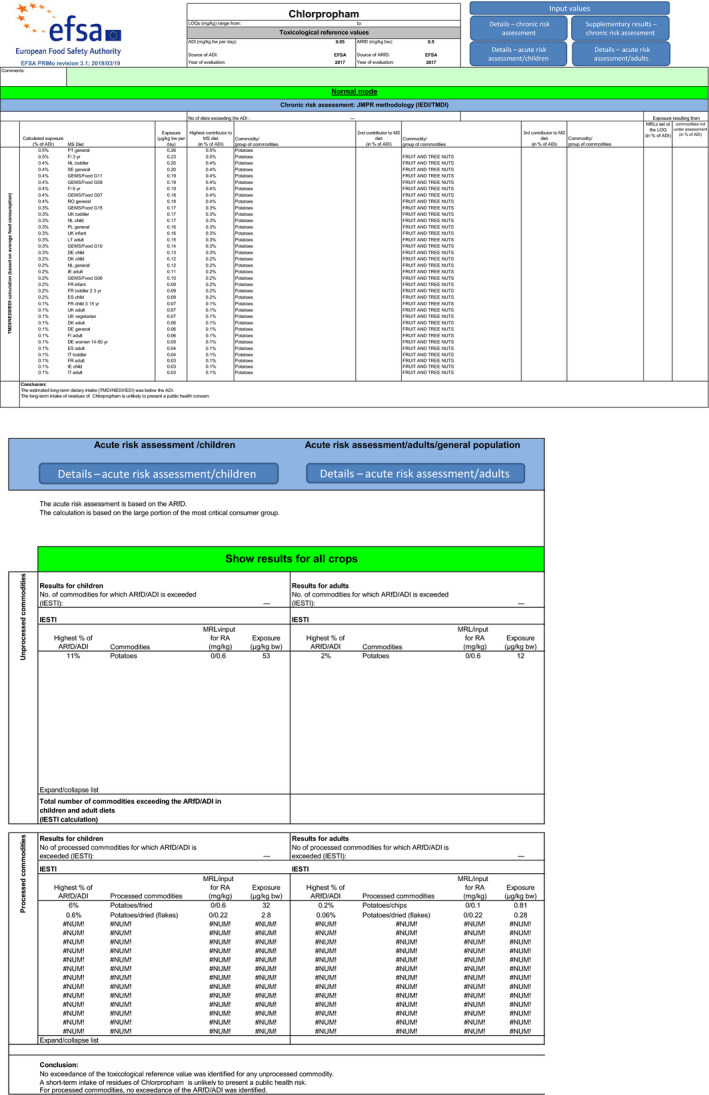
With regard to chlorpropham, the estimated long‐term dietary intake was less than 1% of the acceptable daily intake (ADI). The short‐term exposure did not exceed the acute reference dose (ARfD) for any of the commodities assessed in four calculated scenarios, the maximum calculated exposure corresponding to 18% of the ARfD. Due to lack of data, animal commodities could not be included in the chronic and acute risk assessment.
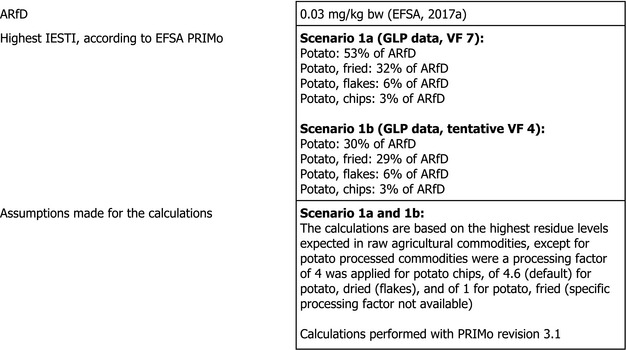
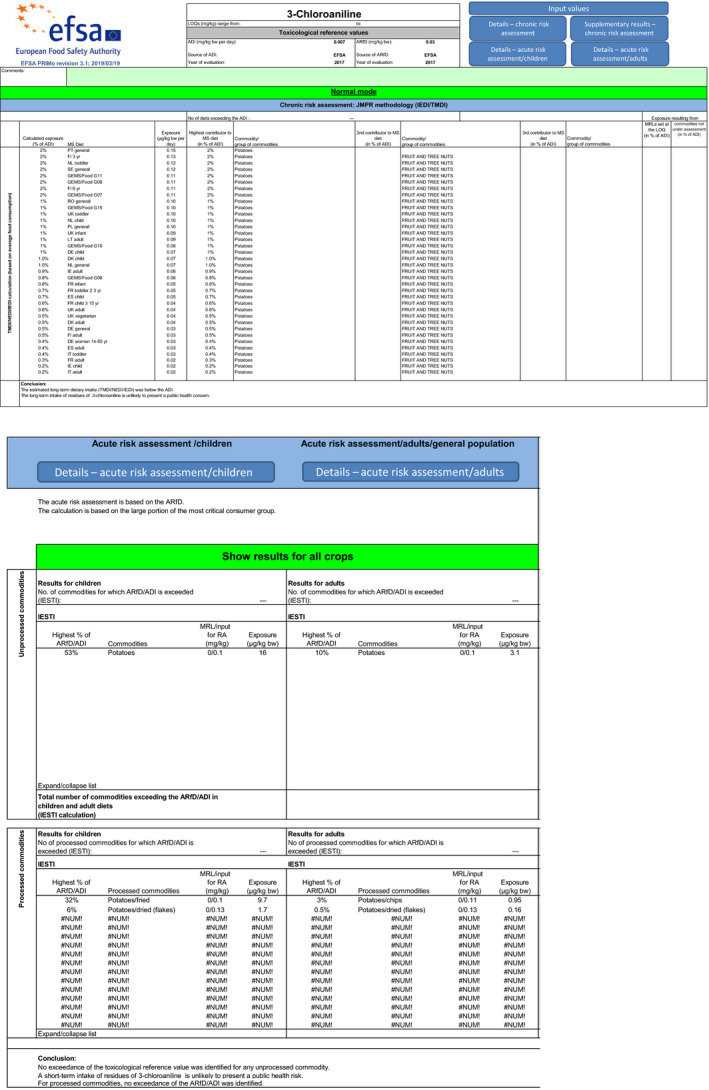
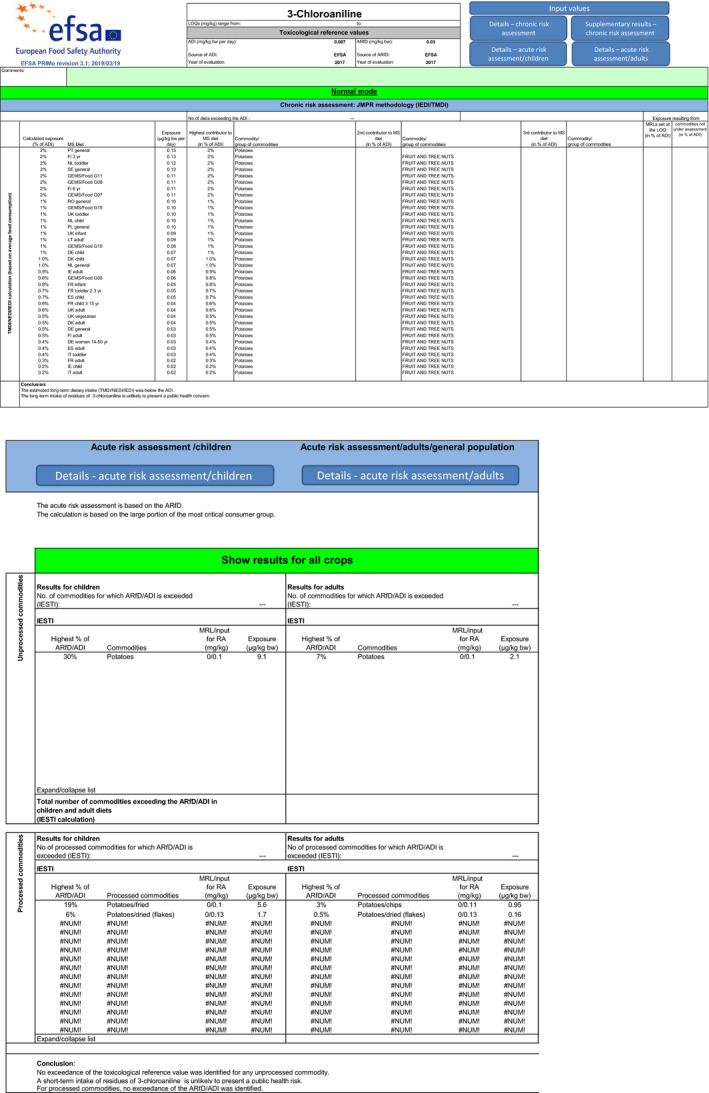
With regard to 3‐chloroaniline, the estimated long‐term dietary intake was 2% of the ADI. The short‐term exposure did not exceed the ARfD for any of the commodities assessed in two calculated scenarios, the maximum calculated exposure corresponding to 53% of the ARfD. Due to lack of data, two additional short‐term exposure scenarios could not be calculated, and animal commodities could not be included in the chronic and acute risk assessment.
With the prospect that carry‐over of residues to stored potatoes will be further reduced by implementation of effective cleaning practices, EFSA concluded that the proposed options for setting an tMRL in potatoes will result in a consumer exposure unlikely exceeding the toxicological reference values for chlorpropham and 3‐chloroaniline.
EFSA proposes to amend the existing MRLs as reported in the summary table below.
Full details of all endpoints, including the input values considered for the exposure calculation and the consumer risk assessment can be found in Appendices Appendix A – List of end points, Appendix B – Pesticide Residue Intake Model (PRIMo)–C.
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU tMRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Chlorpropham | ||||
| 0211000 | Potatoes | 10 | 0.3 or |
Further risk management considerations are required The tMRL was derived from monitoring data with the FAO methodology for setting an extraneous MRL, based on the 95th percentile. Despite unavailability of acceptable data for 3‐chloroaniline, the consumer risk assessment is inherently covered by the assessment for the next highest tMRL proposal Risk for consumers unlikely |
| 0.4 or |
Further risk management considerations are required. The tMRL was derived from GLP trials with OECD MRL calculator; and is supported by monitoring data on chlorpropham with the FAO methodology for setting an extraneous MRL, based on the 97.5th percentile Risk for consumers unlikely with regard to chlorpropham and 3‐chloroaniline |
|||
| 0.5 |
Further risk management considerations are required The tMRL was derived from monitoring data with the FAO methodology for setting an extraneous MRL, based on the 99th percentile. The sample size was not sufficient to reliably estimate this percentile and the derived value is thus surrounded by uncertainty Risk for consumers with regard to 3‐chloroaniline occurrence could not be assessed due to missing data |
|||
tMRL: temporary maximum residue level; FAO: Food and Agriculture Organization of the United Nations; MRL: maximum residue level; GLP: Good Laboratory Practice; OECD: Organisation for Economic Co‐operation and Development.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Background
In the framework of Article 43 of Regulation (EC) No 396/2005, the European Food Safety Authority (EFSA) was requested to carry out an exposure assessment of the potential dietary exposure and risk to consumers for the temporary maximum residue level (tMRL) proposed by the evaluating Member State (EMS) the Netherlands for chlorpropham in potatoes.
Chlorpropham is the ISO common name for isopropyl 3‐chlorocarbanilate (IUPAC). The chemical structures of the active substance and its main metabolites are reported in Appendix D.
Chlorpropham was initially included in Annex I to Council Directive 91/414/EEC1 with the Netherlands designated as rapporteur Member State (RMS) for the representative uses as a post‐harvest plant growth regulator for sprout suppression of potatoes and as herbicide in many edible and non‐edible crops. The process of renewal of the first approval under Regulation (EC) No 1107/20092 has been completed, and the renewal assessment report (RAR) prepared by the RMS the Netherlands (Netherlands, 2016, 2017) was peer reviewed by EFSA (EFSA, 2017a, 2017b). Based on the EFSA conclusion, which was issued in July 2017, a decision of the non‐renewal of approval of the active substance was taken by Commission Implementing Regulation 2019/989.3
The EU MRLs for chlorpropham are established in Annexes II of Regulation (EC) No 396/20054. The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed (EFSA, 2012). Proposed modifications have been implemented in the MRL legislation.5
EFSA based its assessment on the evaluation report submitted by the EMS (Netherlands, 2019), the RAR (Netherlands, 2017) prepared under Regulation (EC) 1107/2009, the Commission review report on chlorpropham (European Commission, 2019), the conclusion on the peer review of the pesticide risk assessment of the active substance (EFSA, 2017a) and the peer review report on chlorpropham (EFSA, 2017b).
For this assessment, the data requirements established in Regulation (EU) No 283/20136 and the guidance documents applicable at the date of submission to the EMS have been used (European Commission, 2000, 2010a,b, 2017; OECD, 2008, 2009, 2011, 2013). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20117.
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application are presented in Appendix A.
The evaluation report submitted by the EMS (Netherlands, 2019) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) rev. 3.1 are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Terms of Reference
On 7 October 2019, EFSA received a request from the European Commission to carry out an assessment of the potential dietary exposure and risk to consumers for the t‐MRL for chlorpropham in potatoes, as recommended in the Evaluation Report prepared by the Netherlands and to deliver a Reasoned Opinion according to Article 43 of Regulation (EC) No 396/2005 on the safety for consumers. The deadline to deliver the reasoned opinion was agreed to be 13 March 2020. EFSA accepted the mandate and included it in the EFSA Register of Questions with the reference number EFSA‐Q‐2019‐00653.
2. Assessment
2.1. Ex ante considerations
As for its long‐established use as sprout suppressant, chlorpropham has contaminated construction material and storage equipment of many potato warehouses and appears to persist even after industrial cleaning. Chlorpropham was found to migrate in quantifiable concentrations to potatoes stored in these facilities. The tMRL is requested to account for potential contamination of potatoes above the default MRL of 0.01 mg/kg when stored in facilities with a history of chlorpropham use. Consequently, a Good Agricultural Practice (GAP) is not proposed and assessed within the remit of this tMRL application.
The tMRL is based on existing data and information previously peer reviewed, as applicable, and new data reported within the remit of the tMRL application and considered relevant to assess the contamination in stored potatoes.
When compared to the agricultural use of chlorpropham as post‐harvest treatment the following differences should be noted for the contamination scenario as these will determine how the available data on treatment and contamination are weighted and used in the presented assessment (Table 1).
Table 1.
Comparison of GAP use and contamination scenario
| Parameter | GAP use | Contamination |
|---|---|---|
| Route | Treatment of potato storages involves, in most cases, application of chlorpropham as a hot fog which is introduced into the storage building under pressure. Best practice requires use of the store ventilation system to help to distribute this fog evenly throughout the crop | Chlorpropham has deposited on or migrated into the construction material of potato storages e.g. concrete, foam insulation and panels, or storage equipment e.g. crates and boxes. Possible routes are direct contact of potatoes with contaminated surfaces as well as volatilisation from contaminated materials and distribution in the storage by the ventilation system |
| Rate and time | Application in potatoes is temporally defined and occurs at a much higher rate than contamination | Potatoes are exposed to comparably low concentrations but steadily during the entire period of their storage |
| Nature | Exposure to the technical a.s. chlorpropham | Little is known about the precise nature of deposits in the construction and equipment materials and whether they consist of chlorpropham alone. Chlorpropham has been found low to moderate persistent in environmental compartments, forming the major metabolite 3‐chloroaniline. Chlorpropham degrades also abiotically into 3‐chloroaniline. A potato store is not a sterile environment and can be moreover subjected to cleaning activities involving water and high temperature (e.g. pressure washing or steam cleaning). In turn, it cannot be excluded that part of the chlorpropham deposits might have altered their nature over time |
2.2. Residues in plants
2.2.1. Metabolism in plants and the occurrence of metabolites
Despite the differences outlined in Table 1, the available studies on the nature of residues in potatoes previously peer reviewed (EFSA, 2017a, 2017b) are considered still applicable to the present assessment. The metabolism of chlorpropham was investigated in stored potatoes upon post‐harvest treatment. In these studies, chlorpropham was applied at higher rates than the concentrations released from construction material of potato storages. The duration of at least one study covers a period representative for the length of commercial potato storage in the EU (approx. from September to July). The metabolism of chlorpropham is expected to be qualitatively comparable, irrespective of whether contact of stored potato tubers with chlorpropham was intentional or unintentional.
In addition, exposure of tubers to 3‐chloroaniline formed from degrading chlorpropham in the warehouse structure and storage equipment cannot be excluded. However, 3‐chloroaniline occurred also as a metabolite in the pathway of plants and its behaviour in plants is therefore covered by the available studies.
In post‐harvest treated potato tubers, chlorpropham and 4′‐hydoxychlorpropham, mainly in its conjugated form were found to be the predominant compounds, and 3‐chloroaniline was identified as a minor metabolite. It is noted that data available during the peer review indicated very limited recoveries of 3‐chloroaniline and 4′‐hydoxychlorpropham in freezer storage experiments and thus uncertainty remained over the reported quantities of these metabolites. Supplemented by additional metabolism studies in lettuce and onions, the peer review concluded that the major metabolic pathway in all investigated crops is similar and consisted of hydroxylation of the phenyl ring of chlorpropham followed by conjugation with amino acids or oligosaccharides, and of the formation of 3‐chloroaniline with further N‐glucosylamine conjugation (EFSA, 2017a, 2017b).
Any new metabolism data were not submitted with this tMRL application. However, new trials in contaminated ware potatoes with analysis of chlorpropham, 4′‐hydoxychlorpropham, 3‐chloroaniline and their conjugates were used as an additional source of information to support the evaluation. (Netherlands, 2019). The newly submitted trials in contaminated potato tubers have demonstrated the absence of 4′‐hydroxychorpropham in either free or conjugated form, while conjugated chlorpropham was present at quantifiable concentrations. This information supports a refinement of the definitions of the relevant residues in contaminated ware potatoes.
2.2.2. Nature of residues in processed commodities
The effect of processing on the nature of chlorpropham was investigated in the framework of the EU pesticides peer review (EFSA, 2017a, 2017b). Chlorpropham was concluded to be stable under standard hydrolysis conditions simulating pasteurisation, boiling and sterilisation. A data gap was set to investigate the behaviour of chlorpropham and 3‐chloroaniline under high temperature processing conditions (such as frying and oven‐baking). Therefore, specific studies were submitted with the current application (Netherlands, 2019). These studies showed that chlorpropham is subject to only very minor degradation to 3‐chloroaniline under conditions simulating frying (6 min, 180°C, pH 7) but almost totally degraded to 3‐chloroaniline under conditions simulating oven baking (20 min, 220°C, pH 7).
2.2.3. Methods of analysis in plants
2.2.3.1. Analytical method for enforcement
A multi‐residue method (QuEChERS) by high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) has been reviewed and approved during the peer review (EFSA, 2017a) for the determination of free chlorpropham and 3‐chloroaniline residues in food or feed of plant origin. An independent laboratory validation (ILV) is available for both residues in potato matrix and the limit of quantification (LOQ) was set at 0.01 mg/kg for both chlorpropham and 3‐chloroaniline.
2.2.3.2. Analytical method for residue data generation
A new analytical method was developed and concluded to be valid for determination of the concentration of free and conjugated chlorpropham, free and conjugated 4‐hydroxychlorpropham, and free and conjugated 3‐chloroaniline in potatoes. The method consists of extraction and analysis of the free residues followed by hydrolytic steps to release conjugated residues (Netherlands, 2019).
2.2.4. Storage stability of residues in plants
The storage stability of chlorpropham in plants stored under frozen conditions was investigated in the framework of the EU pesticides peer review (EFSA, 2017a).
It was demonstrated that in potato, residues were stable for at least 13 months when stored at −18°C.
In newly submitted study with incurred residues of free 3‐chloroaniline and conjugated 3‐chloroaniline it was demonstrated that in crops assessed in the framework of this application, residues were stable for at least 14 days when stored at −18°C (freezer), and for 7 days when stored at 9°C (cooled) (Netherlands, 2019).
2.2.5. Proposed residue definitions
Based on the pattern identified in metabolism studies, the results of hydrolysis studies, the toxicological significance of metabolites and/or degradation products, the capabilities of enforcement analytical methods and the supportive information from the newly submitted Good Laboratory Practice (GLP) trials in contaminated potato tubers, the following residue definitions were proposed for assessment of the contamination scenario in potato.
-
residue for risk assessment:
chlorpropham (free and conjugated), expressed as chlorpropham.
3‐chloroaniline (free and conjugated), expressed as 3‐chloroaniline.
residue definition for enforcement: chlorpropham.
The same residue definitions are applicable to processed products.
The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above‐mentioned enforcement residue definition.
Taking into account the additional information provided with this application and the particular context of the assessment, EFSA concluded that these residue definitions are appropriate and that it is justified to amend the residue definition for risk assessment established by the peer review for root and leafy crops treated with chlorpropham (EFSA, 2017a, 2017b). It is emphasised that the newly proposed residue definition is only applicable to the investigated contamination scenario in potatoes, while the residue definitions derived during the peer review are still considered valid for agricultural uses of chlorpropham.
2.3. Magnitude of residues in plants
Studies investigating the magnitude of residues or their variability in contaminated potatoes cannot be surrogated by data generated on potatoes treated according to agricultural practices due to significant differences expected (see Table 1). Therefore, different data sets were used to derive tMRLs (Section 2.3.1) and to assess the variability of the residues in potatoes (Section 2.3.2).
2.3.1. Magnitude of residues in ware potatoes resulting from contamination
Three data sets were submitted that aimed to present the extent of contamination of potatoes stored in locations with a history of chlorpropham use. According to the quality of the individual data sets submitted, a different approach was applied to estimate the appropriate tMRL and risk assessment input values.
2.3.1.1. GLP trials
Twelve GLP trials investigating residue levels following storage in contaminated facilities were submitted by applicants Certis/UPL. Validated analytical methods were used to determine in potato tubers the concentrations of all components of the residue definition for monitoring and risk assessment derived by the peer review and of the amended residue definition for risk assessment proposed for the contamination scenario. The trials were conducted in storages in Belgium (four trials), the Netherlands (four trials) and Germany (four trials) and are representing two different types of storage, bulk and pallet box storage. All facilities had a multiannual history of chlorpropham use in potatoes (ranging between at least 3 years and more than 20 years), but chlorpropham was not necessarily used in the foregoing season. Chlorpropham was not applied to the potatoes stored in the present season. These new GLP trials are considered representative of commercial storage conditions throughout Europe and their results can be fully relied on.
The GLP trials determined the free and conjugated residues of each chlorpropham, 4′‐hydroxychlorpropham and 3‐chloroaniline. Levels of 4′‐hydroxychlorpropham were consistently below LOQ and were therefore disregarded in the further assessment (see also Section 2.2.1).
The trials provide information on nine bulk storages and two pallet box storages. Although pallet box storages are underrepresented in the data set, the current findings do not point towards a significantly different residue situation in bulk and box storages.
The trials were found appropriate to use the OECD‐MRL calculator for deriving a tMRL proposal for chlorpropham (free) and to derive STMR and HR values according to the residue definition for risk assessment (see Appendix A.1.2.1). Based on the trials, a conversion factor (CF) of 1.2 was derived to convert concentrations of free chlorpropham determined by the enforcement method into chlorpropham (free and conjugated) used in the risk assessment.
Based on these trials, a correlation between the years of chlorpropham usage and the level of contamination in potatoes was not observed. It is noted that in one of the trials, warehouse management practices were unusual, favouring increased contamination of the potatoes in one particular area of the warehouse. Moreover, the samples for analysis were collected specifically from this area, an assumed contamination hotspot, and not in a randomised whole‐store approach (Netherlands, 2019). Therefore, EFSA and the EMS agreed not to consider this trial as representative for deriving the tMRL proposal. For the risk assessment, EFSA considered the use of a variability factor sufficient to account for observed inhomogeneities of residues in a lot.
Although a single observation and not representing the majority of the cases studied, the finding for the said trial should also not be disregarded but considered a realistic worst case that marks residue levels that may occasionally arise in some areas of individual warehouses if contamination hotspots are not mitigated. If such warehouse with hotspots is gradually unloaded in smaller batches, reaching the market, the probability of residues possibly exceeding the tMRL may increase. Therefore, the findings of this trial should be used to identify reasons for the high level of contamination observed and to improve the instructions for appropriate store management and cleaning in order to minimise the extent of contamination of the stored tubers in all areas of the warehouse.
As for 3‐chloroaniline (free and conjugated), the results were ranging from 0.02 to 0.1 mg/kg. For the same reasons explained earlier, the result obtained in the trial highlighted above for the different store management and sampling practices is reported for information only and is not considered for risk assessment purposes.
The EMS attempted to derive a conversion factor for 3‐chloroaniline (free and conjugated) to be applied on the residue definition for enforcement (free chlorpropham) in order to estimate co‐occurring residues of 3‐chloroaniline (free and conjugated). However, EFSA did not find a good correlation between observed concentrations of chlorpropham and 3‐chloroaniline (free and conjugated) in the GLP trials. Conversion factors derived from individual analyses in the residue trials were ranging between 0.11 and 4.33 (Netherlands, 2019). The high variability observed on the individual conversion factors derived from the numerically limited set of GLP trials does not provide reassurance that a meaningful conversion factor can be proposed, specifically since residues of free 3‐chloroaniline below the LOQ observed in the majority of trials are expected to bias the derived conversion factors, resulting in an overestimation of the actual conversion factor.
2.3.1.2. Data collected in industry monitoring and surveillance programmes
For the assessment of a tMRL based on data collected in surveillance programmes (monitoring data) by the Potato Value Chain and the UK FPSA, the FAO methodology for estimation of extraneous MRLs was applied (FAO, 2016). It is noted that EFSA did not verify the calculation of respective percentiles of the monitoring data and the resulting temporary MRLs using the FAO approach for setting EMRLs conducted by the EMS (Netherlands, 2019). It is further noted that for a reliable estimation of the higher percentiles the sample size in both data sets is considered insufficient by EFSA, as a minimum sample size of 119 and 299 would be required to provide valid estimates of the 97.5th and 99th percentile, respectively. Therefore, where indicated in Tables 2 and 3, reported estimates for the respective percentiles should be considered tentative.
Table 2.
Potato Value Chain monitoring data using FAO approach for the setting of EMRLs
| Pooled data of bulk and box stores | ||
|---|---|---|
| Approach 1 Mean value of samples per store and sampling date | Approach 2 Mean value of all samples per store | |
| Number of samples | 124 | 73 |
| Highest residue level (HR) | 0.50 mg/kg | 0.48 mg/kg |
| Median residue level (STMR) | 0.04 mg/kg | 0.05 mg/kg |
| P90 | 0.21 mg/kg | 0.24 mg/kg |
| P95 | 0.31 mg/kg | 0.37 mg/kg |
| P97.5 | 0.41 mg/kg | 0.43 mg/kg a |
| P99 | 0.47 mg/kg a | 0.47 mg/kg a |
| MRL proposal (rounded) P95 | 0.3 mg/kg | 0.4 mg/kg |
| MRL proposal (rounded) P97.5 | 0.4 mg/kg | 0.4 mg/kg a |
| MRL proposal (rounded) P99 | 0.5 mg/kg a | 0.5 mg/kg a |
Sample size not sufficient to reliably estimate this percentile.
Table 3.
UK FPSA monitoring data using FAO approach for the setting of EMRLs
| Pooled data of box stores and of unknown store types | |
|---|---|
| Number of samples | 72 |
| Highest residue level (HR) | 0.48 mg/kg |
| Median residue level (STMR) | 0.01 mg/kg |
| P90 | 0.04 mg/kg |
| P95 | 0.04 mg/kg |
| P97.5 | 0.11 mg/kg a |
| P99 | 0.31 mg/kg a |
| MRL proposal (rounded) P95 | 0.04 mg/kg |
| MRL proposal (rounded) P97.5 | 0.1 mg/kg a |
| MRL proposal (rounded) P99 | 0.3 mg/kg a |
Sample size not sufficient to reliably estimate this percentile.
Potato Value Chain surveillance programme
The Potato Value Chain, i.e. representatives of potato farmers (COPA‐COGECA), potato traders (EUROPATAT), producers of potato crisps (ESA), potato starch (StarchEurope) and potato processors (EUPPA) surveyed potato storage facilities that have previously stored chlorpropham treated potatoes and collected samples for analysis. Their surveillance programme covered 73 different warehouses distributed across France, the United Kingdom, Belgium, the Netherlands and Germany, representing two different types of storage (bulk or pallet box storage) and two types of outlets (fresh or processing). According to the data submitter, the potatoes in this surveillance program were of local origin and not imported. The data are non‐GLP. Free chlorpropham and 3‐chloroaniline were determined. Despite the analysis in accredited laboratories, for several samples some relevant parameters are unknown, such as the conditions and length of sample storage before analysis and the sample preparation. Other parameters varied between laboratories such as the analytical methods used (e.g. multi‐method, common moiety method analysing for total residues). As specifically for 3‐chloroaniline the reliability of results is strongly dependent of appropriate sample storage and application of the correct analytical procedure, the results for 3‐chloroaniline were not taken into account as relied on values and only results reported for chlorpropham were considered.
Of the 73 different European warehouses surveyed by the Potato Value Chain, a total of 358 individual samples were analysed for chlorpropham, the high number arising from replicated sampling in several of the facilities. In some warehouses, samples were taken also at different dates. Sampling was either targeted at predefined areas or done during store loading/unloading (mixed samples), the latter most representative of lots of potatoes entering the market. In these mixed samples, chlorpropham levels ranged from < 0.01 to 0.17 mg/kg. The number of mixed samples is however too limited to derive an MRL proposal (Netherlands, 2019). Therefore, two alternative approaches were used to prepare the data from the replicated sampling for the derivation of the tMRL:
Approach 1: Use of the mean value of replicated samples per store per sampling date, deemed most representative of batches of potatoes unloaded from warehouses at different time points (n = 124).
Approach 2: Use the mean value of all replicated samples per store, disregarding different sampling dates (n = 73).
The tMRL calculations based on both options result largely in the same tMRL proposals (see Table 2).
The monitoring data do not point towards a significantly different residue situation in bulk storages and box storages and were thus pooled for derivation of the tMRL.
UK FPSA monitoring data
The UK provided industry monitoring data collected by the UK Fresh Potato Suppliers Association (FPSA) in the country during the seasons 2014–2017. Samples were analysed for free chlorpropham only. Sampling was mostly done in box stores, which is the primary method of storing potatoes intended for the fresh produce market in the UK. For some of the samples, the storage type could not be specified, and it was not reported whether the storage facilities had a history of chlorpropham use in previous years. Notably, the data set included samples of potatoes imported from third countries and it is thus unknown whether these potatoes may have received a treatment with chlorpropham before being sent to the UK. The data are non‐GLP. Detailed information e.g. on sampling method, method of analysis and reporting limits (such as the LOQ) is not available. Altogether this data set was considered the least robust and most locally restricted data set among the three submissions supporting the setting of a tMRL.
The UK FPSA data (n = 72) show mainly low positive residues up to 0.05 mg/kg chlorpropham with 44 samples (61%) below LOQ (undefined). A couple of samples with higher carryover of around 0.2–0.5 mg/kg were reported. All over, the data appear consistent with the Potato Value Chain survey data. The UK FPSA data set is presented for information only and was mainly introduced for comparison with the other two data sets (Netherlands, 2019).
2.3.2. Variability of residues in potato tubers resulting from contamination
The variability factor (VF) is a key factor in the acute consumer exposure assessment and the highest likely residue at an individual tuber level should be understood. The following data were submitted in the remit of the tMRL application for chlorpropham and have not been previously peer reviewed.
-
a)
Six residues trials (non‐GLP) investigating unit to unit variability in the commercial scale setting of both box and bulk stores following fogging applications of chlorpropham in ware potatoes with analysis of free chlorpropham in all trials and free 3‐chloroaniline in four of the trials.
-
b)
Two GLP trials investigating unit to unit variability of concentrations of free chlorpropham on ware potatoes in commercial bulk stores in Europe in which chlorpropham had been used previously.
Variability studies following GAP use of chlorpropham (a.) are not considered applicable as primary data source for assessment of the unit to unit variability of residues in potatoes following contamination (see Table 1). Yet, these new studies were assessed and included for completeness in the evaluation report by the EMS. Two trials of 2016 were not relied upon due to different shortcomings in the study design. The four trials of 2017 were considered valid by the EMS to derive VF for chlorpropham and free 3‐chloroaniline; however, EFSA does only partly agree with this assessment. EFSA is of the opinion that the sampling was inappropriate to assess variability of residues in at least one of the trials in bulk potatoes and was also not fully satisfactory in the second trial in bulk potatoes as the way the sampling points distribute over the bulk is expected to lead to biased data sets. According to information reported by the applicant in the sampling protocols, in the first trial, samples were collected from the front and top of the bulk (targeted) and not across the entire bulk, while in the second trial, sampling was of inconsistent density across the entire store. A thorough re‐analysis of the variability trials in treated bulk potatoes for the purpose of deriving a VF is recommended if these data were intended to be used for future assessments. The variability factors for chlorpropham (free) derived from the two acceptable experiments in box stores were 3.4 and 5.5. The data for box stores do not provide sufficient confidence that the VF is significantly different from general observations on variability of pesticide residues in crops (EFSA PPR Panel, 2005) and would justify the use of a VF lower than the default factors currently applied in the EU risk assessment. Variability factors were also proposed for residues of free 3‐chloroaniline; however, due to the observed behaviour of 3‐chloroaniline to readily form conjugated residues (that were not analysed), the meaningfulness of these VF for risk assessment is very limited.
Of the two trials investigating the unit to unit variability of residues in potato upon storage in contaminated warehouses (b.) only one trial could be used to derive a tentative VF of 4 for chlorpropham (free). In the second trial, chlorpropham concentrations were below LOQ in most of the samples and a reliable factor could not be derived. The uncertainty arising from a VF based on a single experiment is noted. The study does also not included analysis of conjugated chlorpropham. The precisions of the factor could thus be affected. Since the free chlorpropham is the driver for the total residues of free and conjugated chlorpropham, the expected impact of the imprecision on the assessment is marginal.
Based on all above considerations, the hypothesis of the applicants UPL/Certis that contamination would lead to less variability than a fogging treatment can currently not be confirmed.
Since appropriate data are not available to derive a VF for 3‐chloronaniline (free and conjugated), it is proposed to extrapolate from the result obtained for chlorpropham. The assessment uncertainty caused by using extrapolation in lieu of experimental data on 3‐chloronaniline (free and conjugated) is noted, particularly since the presence of 3‐chloronaniline in the tubers, unlike that of chlorpropham, could be induced in two different ways, namely by direct contamination and as a plant metabolite of chlorpropham (see Table 1).
In view of the picture obtained from the available data, EFSA decided to conduct the exposure assessments with both VF, the tentative VF and the EU default VF.
2.3.3. Magnitude of residues in processed commodities
One processing study in oven‐baked potato wedges demonstrated that baking leads to a concentration of chlorpropham residues (PF = 2) as well as to formation and concentration of 3‐chloroaniline (PF = 4) in the processed product (Netherlands, 2019).
The quality of the processing study is acceptable; however, only tentative processing factors can be derived based on a single study. It is likely that substantial processing data to robustly cover all forms of processing would probably be needed to obtain a more refined understanding that can be harnessed in terms of PFs to apply to risk assessment for potato processed products.
2.3.4. Proposed MRLs
Because of the different quality of the data sets provided, i.e. GLP trials and potato industry monitoring data, the methodologies applied to derive the tMRL proposals differ. Four options resulting into three different proposals for a tMRL for chlorpropham in potatoes are available for risk managers, of which one option is surrounded by additional non‐standard uncertainty.
Table 4.
Proposed MRLs
| Source of data relied on | Size of data set | Methodology | MRL proposal |
|---|---|---|---|
| GLP trials (Certis/UPL) | 11 | OECD MRL‐calculator | 0.4 mg/kg |
| Monitoring data (Potato Value Chain) | 124 | EMRL approach: 95th percentile | 0.3 mg/kg |
| EMRL approach: 97.5th percentile | 0.4 mg/kg | ||
| EMRL approach: 99th percentilea | 0.5 mg/kg |
GLP: Good Laboratory Practice; MRL: maximum residue level; OECD: Organisation for Economic Co‐operation and Development.
Sample size not sufficient to reliably estimate this percentile.
EFSA did not verify the calculation of respective percentiles of the monitoring data and the resulting temporary MRLs using the FAO approach for setting EMRLs.
In Section 4, EFSA assessed whether the carry‐over of residues on potatoes are likely to pose a consumer health risk.
3. Residues in livestock
Potatoes and their by‐products are used as feed products. The calculated livestock dietary burden exceeded the trigger value of 0.004 mg/kg body weight (bw) for all relevant animal species in separate assessments for chlorpropham and 3‐chloroaniline. The nature of chlorpropham residues in livestock has been investigated during the EU pesticides peer review (EFSA, 2017a). The conclusions by the peer review remain unchanged and further data to address specifically the carry‐over of 3‐chloroaniline and occurrence of 3‐chloro 4‐hydroxyaniline in animal commodities would normally be required to complete the consumer risk assessment. EFSA take note that the EMS considers the requirement of vertebrate studies to support a temporary MRL as inappropriate and that the setting of tMRLs for chlorpropham in commodities of animal origin is not intended. Yet, ESFA suggests that the uncertainty arising from the non‐consideration of animal commodities in the consumer risk assessment should be taken into account when the risk assessment outcome for 3‐chloroaniline is considered.
4. Consumer risk assessment
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2018, 2019). This exposure assessment model contains food consumption data for different sub‐groups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016).
The toxicological reference values for chlorpropham and 3‐chloroaniline used in the risk assessment (i.e. ADI and ARfD values) were derived in the framework of the EU pesticides peer review (EFSA, 2017a). The metabolite included in the risk assessment residue definition was considered more toxic than the parent compound and therefore separate risk assessments were conducted for chlorpropham and 3‐chloroaniline.
Two different data sets were used, the GLP trials and the monitoring data generated by the Potato Value Chain. The calculations were based on the HR or STMR, and where appropriate HR‐P and STMR‐P, derived from these datasets and the complete list of input values can be found in Appendix C.2.
In all scenarios presented in the following sections, potential residues in animal commodities could not be taken into account due to lack of data (see Section 3).
4.1. Chlorpropham, free and conjugated
4.1.1. Short‐term (acute) dietary risk assessment
The short‐term exposure assessment was performed for the commodities assessed in this application in accordance with the internationally agreed methodology (FAO, 2016), taking into account the HR values and processing factors, where appropriate, derived in the previous sections.
Scenario 1 – GLP trials
A first exposure scenario (sub 1.a) was calculated with the EU default VF and a second scenario (sub 1. b) with the indicative VF of 4 derived from a single unit‐to‐unit variability trial in contaminated potatoes.
Scenario 2 – Potato Value Chain monitoring data
A CF of 1.2 was applied to account for conjugated residues of chlorpropham which were not determined for the monitoring samples. The CF has been derived from the GLP trial data. Again, a first exposure scenario (sub 2.a) was calculated with the default VF and a second (sub 2.b) with the indicative VF derived from one unit‐to‐unit variability trial in contaminated potatoes.
Although uncertainties remain as reported in the previous sections, the exposure calculation performed with both scenarios did not indicate a risk to consumer health. The short‐term exposure did not exceed the acute reference dose (ARfD) for any of the commodities assessed in this application (see Table 5).
Table 5.
Risk assessment for chlorpropham (free and conjugated)
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| IEDI | Comment | IESTI | Comment | |
| Scenario 1 GLP trials | ||||
| Sub‐scenario 1.a with EU default VF | ||||
| Potato | 0.6% | PT general | 7% | UK infant (Potatoes) |
| Potatoes/fried | – | – | 4% | NL toddler (Potatoes fried) |
| Potatoes/dried (flakes) | – | – | 0.6% | DE child |
| Potatoes/chips | – | – | 0.2% | NL general population |
| Sub‐scenario 1.b with tentative VF = 4 | ||||
| Potato | 0.6% | PT general | 4% | UK infant (Potatoes) |
| Potatoes/fried | – | – | 2% | NL toddler (Potatoes fried) |
| Potatoes/dried (flakes) | – | – | 0.6% | DE child |
| Potatoes/chips | – | – | 0.2% | NL general population |
| Scenario 2 Monitoring data | ||||
| Sub‐scenario 2.a with EU default VF | ||||
| Potato | 0.5% | PT general | 18% | UK infant (Potatoes) |
| Potatoes/fried | – | – | 11% | NL toddler (Potatoes fried) |
| Potatoes/dried (flakes) | – | – | 0.6% | DE child |
| Potatoes/chips | – | – | 0.2% | NL general population |
| Sub‐scenario 2.b with tentative VF = 4 | ||||
| Potato | 0.5% | PT general | 11% | UK infant (Potatoes) |
| Potatoes/fried | – | – | 6% | NL toddler (Potatoes fried) |
| Potatoes/dried (flakes) | – | – | 0.6% | DE child |
| Potatoes/chips | – | – | 0.2% | NL general population |
IEDI: international estimated daily intake; IESTI: international estimated short‐term intake.
4.1.2. Long‐term (chronic) dietary risk assessment
Scenario 1 – GLP trials
The long‐term exposure assessment was performed, taking into account the STMR values derived in the previous sections.
Scenario 2 – Potato Value Chain monitoring data
The long‐term exposure assessment was performed, taking into account the STMR values derived for the commodities assessed in this application. A CF of 1.2 was applied to account for conjugated residues of chlorpropham which were not determined for the monitoring samples. The CF has been derived from the GLP trial data.
In both scenarios, the estimated long‐term dietary intake was less than 1% of the ADI. Although uncertainties remain as indicated in the previous sections, the exposure calculation did not indicate a risk to consumer health (see Table 5). Contributions from additional commodities were not considered as following the non‐renewal of approval of chlorpropham, any crop other than potato should not contain any residues resulting from the use of this active substance.
4.2. 3‐Chloroaniline, free and conjugated
The risk assessment was performed using the data generated in the GLP trials. Scenarios using the monitoring data were not calculated since reliable data for 3‐chloroaniline were not available for the monitoring samples.
4.2.1. Short‐term (acute) dietary risk assessment
The short‐term exposure assessment was performed for the commodities assessed in this application in accordance with the internationally agreed methodology (FAO, 2016), taking into account the HR values and processing factors, where appropriate, derived in the previous sections.
A first exposure scenario (1.a) was calculated with the EU default VF and a second scenario (1.b) with the extrapolated indicative VF of 4 derived from a single unit‐to‐unit variability trial with chlorpropham in contaminated potatoes.
Although uncertainties remain as indicated in the previous sections, the exposure calculation performed with both scenarios did not indicate a risk to consumer health as the short‐term exposure did not exceed the ARfD for any of the commodities assessed in this application (see Table 6).
Table 6.
Risk assessment for 3‐chloroaniline (free and conjugated)
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| IEDI | Comment | IESTI | Comment | |
| GLP trials | ||||
| Scenario 1.a with EU default VF | ||||
| Potato | 2% | PT general | 53% | UK infant |
| Potatoes/fried | – | – | 32% | NL toddler |
| Potatoes/dried (flakes) | – | – | 6% | DE child |
| Potatoes/chips | – | – | 3% | NL general population |
| Scenario 1.b with extrapolated tentative VF=4 | ||||
| Potato | 2% | PT general | 30% | UK infant |
| Potatoes/fried | – | – | 29% | NL toddler |
| Potatoes/dried (flakes) | – | – | 6% | DE child |
| Potatoes/chips | – | – | 3% | NL general population |
IEDI: international estimated daily intake; IESTI: international estimated short‐term intake.
It is noted that exposure to 3‐chloroaniline for fried potato may be underestimated applying a PF of 1. The tentative PF determined for oven‐baked wedges (PF4) was not considered appropriate for fried potatoes, as firstly, the formation of 3‐chloroaniline during high temperature operations is likely time‐dependent, and secondly, dehydration during oven‐baking is an additional factor determining the resulting PF. Uncertainty remains also with regard to the variety of processing procedures for potatoes that were not explicitly studied (see also 2.3.3).
4.2.2. Long‐term (chronic) dietary risk assessment
The long‐term exposure assessment was performed, taking into account the STMR values derived in the previous sections.
The estimated long‐term dietary intake was 2% of the acceptable daily intake (ADI). Although uncertainties remain as indicated in the previous sections, the exposure calculation did not indicate a risk to consumer health (see Table 6). Contributions from additional commodities were not considered as following the non‐renewal of approval of chlorpropham, any crop other than potato should not contain residues resulting from the use of this active substance.
For further details on the exposure calculations, screenshots of the Report sheets of the PRIMo are presented in Appendix B.
5. Conclusion and Recommendations
Based on the data available to support the setting of a temporary MRL for chlorpropham in potatoes in order to address cross‐contamination in storage facilities, options for setting a tMRL in potato could be proposed. The risk assessment contains assumptions that lead to non‐standard uncertainties.
Because of the different quality and quantity of the data provided, i.e. GLP trials and potato industry monitoring data, the methodologies applied to derive MRL proposals differ. Four options resulting into three proposals for a temporary MRL for chlorpropham in potatoes are available for risk managers (see Appendix A.4).
It is noted that the monitoring data were obtained exclusively from storages with chlorpropham use in previous seasons and without implementation of effective cleaning practices as the research in this area is still ongoing. EFSA support the EMS recommendation that manufacturers should propose an efficient and robust regime for cleaning, considering different aspects such as areas of the stores where deposits of chlorpropham might have accumulated, e.g. area on/around fans, ‘fabric of store’ including insulation material, boxes. The EMS has pointed out that some of these accumulation hotspots e.g. around the fans can be areas that are not usually cleaned and that also underground ducts are often not cleaned (Netherlands, 2019). Evidence suggests that, if insufficient cleaning combines with other factors arising from suboptimal store management practices (e.g. store loading schemes that are triggering unbalanced ventilation), the probability for contamination levels over average and possibly leading to an exceedance of the tMRL and for a risk for the consumer specifically with regard to 3‐chloroaniline exposure is significantly increased.
With the prospect that carry‐over of residues to stored potatoes should be further reduced by implementation of effective cleaning practices, EFSA concluded that the short‐term and long‐term intake of residues resulting from the assessed contamination scenario for chlorpropham in ware potatoes is unlikely to result in a consumer exposure exceeding the toxicological reference values.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- bw
body weight
- CF
conversion factor for enforcement to risk assessment residue definition
- DAR
draft assessment report
- DM
dry matter
- EMS
evaluating Member State
- FAO
Food and Agriculture Organization of the United Nations
- FPSA
Fresh Potato Suppliers Association
- GAP
Good Agricultural Practice
- GLP
Good Laboratory Practice
- HPLC‐MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- MRL
maximum residue level
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PF
processing factor
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- STMR
supervised trials median residue
- t‐MRL
temporary maximum residue level
Appendix A – List of end points
A.1. Residues in plants
A.1.1. Nature of residues and methods of analysis in plants
A.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Frying (6 min, 180°C, pH 7) | Yes | ||
| Oven baking (20 min, 220°C, pH 7) | No | Chlorpropham significantly (> 75%) degrades into 3‐chloroaniline |

A.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High starch | Potato | 9 | 7 | Days | 3‐chloraniline free and conjugated | Incurred residues | |
| −18 | 14 | Days | 3‐chloraniline free and conjugated | Incurred residues | |||
A.1.2. Magnitude of residues in plants
A.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) | CFd |
|---|---|---|---|---|---|---|---|
| Potato | Indoor |
Mo: < 0.01; 0.01; 0.01; 0.02; 0.02; 0.04; 0.07; 0.10; 0.10; 0.12; 0.21 RA 1: Chlorpropham (free + conjugated) < 0.02; 0.02; 0.02; 0.03; 0.03; 0.05; 0.08; 0.11; 0.11; 0.14; 0.22 RA 2: 3‐chloroaniline (free + conjugated) 0.02; 0.02; 0.02; 0.03; 0.03; 0.03; 0.03; 0.03; 0.03; 0.04; 0.10 |
Residues values (except store 7) are shown All residue values are based on the mean of 3 replicate samples per store MRL‐OECD unrounded = 0.32 mg/kg |
0.4 |
Mo: 0.21 RA 1: 0.22 RA 2: 0.10 |
Mo: 0.04 RA 1: 0.05 RA 2: 0.03 |
RA1: 1.2 RA 2: Not derived |
| Indoor |
Mo: 0.81 RA 1: Chlorpropham (free + conjugated): 0.96 RA 2: 3‐chloroaniline (free + conjugated): 0.21 |
Assumed Hotspot sample (store 7), excluded from MRL calculation and risk assessment, but included to derive CF for chlorpropham | n/a | n/a | n/a | See above |
MRL: maximum residue level; GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; Mo: monitoring; RA: risk assessment.
N/A: not applicable.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
A.1.2.2. Processing factors
| Processed commodity | Number of valid studiesa | Processing Factor (PF) | Comment/Source | |
|---|---|---|---|---|
| Individual values | Median PF | |||
| Chlorpropham: Potato, wedges baked | 1 | 2 | n/a | Tentativeb |
| 3‐chloroaniline: Potato, wedges baked | 1 | 4 | n/a | Tentativeb |
N/A: not applicable.
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
A tentative PF is derived based on a limited dataset.
A.2. Residues in livestock
Dietary burden calculation according to OECD (2013).
Chlorpropham, free and conjugated
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Y/N) | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Median | Maximum | Median | Maximum | ||||
| Cattle (all) | 0.103 | 0.113 | 3.54c | 3.80c | Cattle, dairy | Potato, process waste | Y |
| Cattle (dairy only) | 0.103 | 0.113 | 2.68 | 2.94 | N/A | Potato, process waste | Y |
| Sheep (all) | 0.118 | 0.127 | 3.54 | 3.80 | Ram/ewe | Potato, process waste | Y |
| Sheep (ewe only) | 0.118 | 0.127 | 3.54 | 3.80 | N/A | Potato, process waste | Y |
| Swine (all) | 0.043 | 0.053 | 1.86 | 2.29 | Swine, breeding | Potato, process waste | Y |
| Poultry (all) | 0.034 | 0.040 | 0.48 | 0.56 | Broiler | Potato, dried pulp | Y |
| Poultry (layer only) | 0.025 | 0.031 | 0.36 | 0.45 | N/A | Potato, dried pulp | Y |
| Fish | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
bw: body weight; DM: dry matter.
N/A: not applicable.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
The highest dietary burden expressed in mg/kg DM result from Beef cattle.
3‐chloroaniline, free and conjugated
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Y/N) | |||
|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | ||||||
| Median | Maximum | Median | Maximum | ||||
| Cattle (all) | 0.056 | 0.060 | 1.91c | 2.02c | Cattle, dairy | Potato, process waste | Y |
| Cattle (dairy only) | 0.056 | 0.060 | 1.44 | 1.56 | N/A | Potato, process waste | Y |
| Sheep (all) | 0.064 | 0.067 | 1.91 | 2.0 | Ram/ewe | Potato, process waste | Y |
| Sheep (ewe only) | 0.064 | 0.067 | 1.91 | 2.0 | N/A | Potato, process waste | Y |
| Swine (all) | 0.023 | 0.028 | 1.00 | 1.19 | Swine, breeding | Potato, process waste | Y |
| Poultry (all) | 0.018 | 0.021 | 0.26 | 0.29 | Broiler | Potato, dried pulp | Y |
| Poultry (layer only) | 0.013 | 0.016 | 0.20 | 0.23 | N/A | Potato, dried pulp | Y |
| Fish | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
bw: body weight; DM: dry matter; N/A: not applicable.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
The highest dietary burden expressed in mg/kg DM result from Beef cattle.
A.3. Consumer risk assessment
Chlorpropham, free and conjugated


3‐chloroaniline, free and conjugated

A.4. Recommended MRLs
| Codea | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Chlorpropham | ||||
| 0.3 or |
Further risk management considerations are required The tMRL was derived from monitoring data with the FAO methodology for setting an extraneous MRL, based on the 95th percentile. Despite unavailability of acceptable data for 3‐chloroaniline, the consumer risk assessment is inherently covered by the assessment for the next highest tMRL proposal Risk for consumers unlikely |
|||
| 0.4 or |
Further risk management considerations are required. The tMRL was derived from GLP trials with OECD MRL calculator; and is supported by monitoring data on chlorpropham with the FAO methodology for setting an extraneous MRL, based on the 97.5th percentile Risk for consumers unlikely with regard to chlorpropham and 3‐chloroaniline |
|||
| 0.5 |
Further risk management considerations are required The tMRL was derived from monitoring data with the FAO methodology for setting an extraneous MRL, based on the 99th percentile. The sample size was not sufficient to reliably estimate this percentile and the derived value is thus surrounded by uncertainty Risk for consumers with regard to 3‐chloroaniline occurrence could not be assessed due to missing data |
|||
tMRL: temporary maximum residue level; FAO: Food and Agriculture Organization of the United Nations; MRL: maximum residue level; GLP: Good Laboratory Practice; OECD: Organisation for Economic Co‐operation and Development.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Fat soluble.
Appendix B – Pesticide Residue Intake Model (PRIMo)
1.
EFSA_PRIMo_rev3.1_ Chlorpropham_Scen.1a

EFSA_PRIMo_rev3.1_ Chlorpropham_Scen.1b

EFSA_PRIMo_rev3.1_ Chlorpropham_Scen.2a

EFSA_PRIMo_rev3.1_ Chlorpropham_Scen.2b
EFSA_PRIMo_rev3.1_3chloroaniline_scen. 1a
EFSA_PRIMo_rev3.1_3chloroaniline_scen. 1b
Appendix C – Input values for the exposure calculations
C.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1 (Chlorpropham, free and conjugated) | ||||
| Potato | 0.052 | STMRa | 0.224 | HR |
| Risk assessment residue definition 2 (3‐chloroaniline, free and conjugated) | ||||
| Potato | 0.028 | STMRa | 0.104 | HR |
STMR: supervised trials median residue; HR: highest residue.
For potato process waste and dried pulp, in the absence of processing factors supported by data, default processing factors of 20 and 38 were respectively included in the calculation to consider the potential concentration of residues in these commodities.
C.2. Consumer risk assessment
Chlorpropham, free and conjugated
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Scenario 1. GLP trials: Mean chlorpropham (free+ conj.) values of samples taken per store | ||||
| Potato | 0.052 | STMRtotal CIPC | 0.224 | HRtotal CIPC |
| Potatoes/chips | – | – | 0.104 (0.052 × 2) | STMRtotal CIPC × PFCIPC |
| Potato, fried | – | – | 0.224 | HRtotal CIPC |
| Potato, dried (flakes) | – | – | 0.2329 (0.052 × 4.6) | STMRtotal CIPC × default PF |
| Scenario 2. Monitoring data: Mean chlorpropham (free) values of samples taken per store per sampling date | ||||
| Potato | 0.048 (0.04 × 1.2) | STMR × CFfree CIPC to total CIPC | 0.60 (0.50 × 1.2) | HR × CFfree CIPC to total CIPC |
| Potatoes/chips | – | – | 0.096 (0.04 × 2.0 × 1.2) | STMR × PFCIPC × CFfree CIPC to total CIPC |
| Potato, fried | – | – | 0.60 (0.50 × 1.2) | HR × CFfree CIPC to total CIPC |
| Potato, dried (flakes) | – | – | 0.2208 (0.04 × 1.2 × 4.6) | STMR × default PF × CFfree CIPC to total CIPC |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; CF: conversion factor for enforcement to risk assessment residue definition; GLP: Good Laboratory Practice.
3‐chloroaniline, free and conjugated
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Scenario 1. GLP trials: Mean 3‐chloroaniline values (free + conj.) of samples taken per store | ||||
| Potato | 0.028 | STMRtotal 3‐CA | 0.104 | HRtotal 3‐CA |
| Potatoes/chips | – | – | 0.112 (0.028 × 4.0) | STMRtotal 3‐CA × PF3‐CA |
| Potato, fried | – | – | 0.104 | HRtotal 3‐CA |
| Potato, dried (flakes) | – | – | 0.1288 (0.028 × 4.6) | STMRtotal 3‐CA × default PF |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor; GLP: Good Laboratory Practice.
Appendix D – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
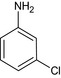
| 3‐chloroaniline |
3‐chloroaniline Nc1cc(Cl)ccc1 PNPCRKVUWYDDST‐UHFFFAOYSA‐N |
|
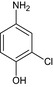
| 3‐chloro‐4‐hydroxyaniline |
4‐amino‐2‐chlorophenol Nc1cc(Cl)c(O)cc1 ZYZQSCWSPFLAFM‐UHFFFAOYSA‐N |
|
|
4‐OH.chlorpropham or 4′‐hydroxychlorpropham |
propan‐2‐yl (3‐chloro‐4‐hydroxyphenyl)carbamate Oc1ccc(NC(=O)OC(C)C)cc1Cl LODWUFQKCTUNBU‐UHFFFAOYSA‐N |

|
| 3‐chloroacetanilide |
N‐(3‐chlorophenyl)acetamide Clc1cc(NC(C)=O)ccc1 MUUQHCOAOLLHIL‐UHFFFAOYSA‐N |

|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019)
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019)
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2020. Reasoned opinion on the setting of temporary maximum residue levels for chlorpropham in potatoes. EFSA Journal 2020;18(6):6061, 39 pp. 10.2903/j.efsa.2020.6061
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00653
Acknowledgments: EFSA wishes to thank the following for the support provided to this scientific output: Silvia Ruocco, Laszlo Bura, Georgios Chatzisotiriou, and Viktor Toth.
Adopted: 13 March 2020
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2019/989 of 17 June 2019 concerning the non‐renewal of approval of the active substance chlorpropham, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 160, 18.6.2019, p. 11–13.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) No 79/2014 of 29 January 2014 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bifenazate, chlorpropham, esfenvalerate, fludioxonil and thiobencarb in or on certain products. OJ L 27, 30.1.2014, p. 9–55.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- EFSA (European Food SSafety Authority), 2012. Review of the existing maximum residue levels (MRLs) for chlorpropham according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2012;10(2):2584. 53 pp. 10.2903/j.efsa.2012.2584 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Arena M, Auteri D, Barmaz S, Bellisai G, Brancato A, Brocca D, Bura L, Byers H, Chiusolo A, Court Marques D, Crivellente F, De Lentdecker C, De Maglie M, Egsmose M, Erdos Z, Fait G, Ferreira L, Goumenou M, Greco L, Ippolito A, Istace F, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Magrans JO, Medina P, Miron I, Molnar T, Nougadere A, Padovani L, Parra Morte JM, Pedersen R, Reich H, Sacchi A, Santos M, Serafimova R, Sharp R, Stanek A, Streissl F, Sturma J, Szentes Cs, Tarazona J, Terron A, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017a. Conclusion on the peer review of the pesticide risk assessment of the active substance chlorpropham. EFSA Journal 2017;15(7):4903, 37 pp. 10.2903/j.efsa.2017.4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2017b. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance chlorpropham. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschne R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1. EFSA supporting publication 2019;16(3): EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant protection products and their Residues), 2005. Opinion of the PPR Panel related to the appropriate variability factor(s) to be used for acute dietary exposure assessment of pesticide residues in fruit and vegetables. EFSA Journal 2005;3(3):177, 61 pp. updated on 2 February 2006. 10.2903/j.efsa.2005.177 [DOI] [Google Scholar]
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.3, 13 June 2017.
- European Commission , 2019. Final Renewal report for the active substance chlorpropham finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 22 February 2019 in view of the non‐renewal of the approval of chlorpropham as active substance in accordance with Regulation (EC) No 1107/20091. SANTE/11966/2017 rev. 5, 22 February 2019.
- FAO (Food and Agriculture Organization of the United Nations), 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 298 pp.
- Netherlands , 2016. Renewal Assessment Report (RAR) on the active substance chlorpropham prepared by the rapporteur Member State the Netherlands, in the framework of Commission Implementing Regulation (EU) No 844/2012, April 2016. Available online: www.efsa.europa.eu
- Netherlands , 2017. Revised Renewal Assessment Report (RAR) on chlorpropham prepared by the rapporteur Member State the Netherlands in the framework of Regulation (EU) No 844/2012, April 2017. Available online: www.efsa.europa.eu
- Netherlands , 2019. Evaluation report Prepared under Article 8 of Regulation (EC) No 396/2005. Application on the setting of a temporary MRL for Chlorpropham in potatoes. October 2019, revised January 2020, 139 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2008. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96. ENV/JM/MONO(2008)23, 29 July 2008.
- OECD (Organisation for Economic Co‐operation and Development), 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.


