Abstract
Data on antimicrobial resistance (AMR) in zoonotic and indicator bacteria from humans, animals and food are collected annually by the EU Member States (MSs), jointly analysed by EFSA and ECDC and reported in a yearly EU Summary Report. The annual monitoring of AMR in animals and food within the EU is targeted at selected animal species corresponding to the reporting year. The 2017 monitoring specifically focussed on pigs and calves under 1 year of age, as well as their derived carcases/meat, while the monitoring performed in 2018 specifically focussed on poultry and their derived carcases/meat. Monitoring and reporting of AMR in 2017/2018 included data regarding Salmonella, Campylobacter and indicator Escherichia coli isolates, as well as data obtained from the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolates. Additionally, some MSs reported voluntary data on the occurrence of meticillin‐resistant Staphylococcus aureus in animals and food, with some countries also providing data on antimicrobial susceptibility. This report provides, for the first time, an overview of the main findings of the 2017/2018 harmonised AMR monitoring in the main food‐producing animal populations monitored, in related carcase/meat samples and in humans. Where available, data monitoring obtained from pigs, calves/cattle, broilers, laying hens and turkeys, as well as from carcase/meat samples and humans were combined and compared at the EU level, with particular emphasis on multiple drug resistance, complete susceptibility and combined resistance patterns to critically important antimicrobials, as well as Salmonella and E. coli isolates exhibiting presumptive ESBL‐/AmpC‐/carbapenemase‐producing phenotypes. The outcome indicators for AMR in food‐producing animals, such as complete susceptibility to the harmonised panel of antimicrobials in E. coli and the prevalence of ESBL‐/AmpC‐producing E. coli have been also specifically analysed over the period 2014–2018.
Keywords: antimicrobial resistance, zoonotic bacteria, indicator bacteria, ESBL, MRSA
Summary
In 2017–2018, data on antimicrobial resistance (AMR) in zoonotic and indicator bacteria, submitted by 28 EU Member States (MSs), were jointly analysed by the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC). Resistance in zoonotic Salmonella and Campylobacter from humans, animals and food, as well as resistance in indicator Escherichia coli and meticillin‐resistant Staphylococcus aureus (MRSA) from animals and food were addressed. ‘Microbiological’ resistance was assessed using epidemiological cut‐off (ECOFF) values; for some countries, qualitative data on human isolates were interpreted in a way which corresponds closely to the ECOFF‐defined ‘microbiological’ resistance.
In Salmonella spp. from human cases in 2018, resistance to ampicillin, sulfonamides and tetracyclines were observed at overall high levels, particularly among serovars commonly found in pigs, while resistance to third‐generation cephalosporins were noted at overall very low/low levels. A decline in resistance to ampicillin and tetracyclines in Salmonella Typhimurium from humans was observed in several countries over the period 2013–2018. In Salmonella spp. and indicator E. coli isolates recovered from animals and food during the 2017/2018 routine monitoring, resistance to ampicillin, tetracyclines and sulfonamides was also frequently detected, and resistance to third‐generation cephalosporins was uncommon; paralleling that observed in Salmonella isolates reported from human cases. Additionally, in 2018, resistance to (fluoro)/quinolones was observed at very high/high levels among Salmonella spp. and indicator E. coli isolates recovered from broilers, fattening turkeys and poultry carcases/meat, and at high to extremely high levels in Salmonella isolates from humans in serovars commonly found in poultry (namely Infantis and Kentucky), with increasing levels of resistance over time.
The monitoring included assessment of the levels of presumptive extended‐spectrum beta‐lactamase (ESBL)‐/AmpC‐/carbapenemase producers among Salmonella spp. from human cases, food‐producing animals and animal carcases; as well as among indicator E. coli isolates from food‐producing animals. At the reporting MS‐group level, the proportion of presumptive ESBL or AmpC producers was low among all indicator E. coli isolates recovered from the animal sector (fattening pigs, calves, broilers and fattening turkeys) and very low to low among Salmonella spp. recovered from animals/carcases (fattening pigs, broilers, laying hens and fattening turkeys) and from human cases, although higher in some Salmonella serovars. Within both the routine and specific monitoring (non‐selective and selective media, respectively), varying occurrence/prevalence rates of presumptive ESBL or AmpC producers were observed in different reporting countries. Carbapenemase‐producing E. coli was detected in a single sample from a fattening pig in one MS in 2017; while no presumptive or confirmed carbapenemase‐producing E. coli was detected from broilers and their derived meat in 2018. Carbapenemase‐producing Salmonella were reported in one domestically‐acquired case and four human cases lacking information on travel status in 2018.
Resistance to colistin was generally uncommon among Salmonella spp. and E. coli isolates recovered from food‐producing animals (fattening pigs, calves/cattle, Gallus gallus and fattening turkeys) and carcases/meat derived from these animals.
In Campylobacter from humans, food‐producing animals and poultry meat, resistance to ciprofloxacin and tetracycline generally ranged from high to extremely high, particularly in Campylobacter coli isolates from humans and from poultry and derived meat. Erythromycin resistance was observed at much lower levels in Campylobacter jejuni but at moderate levels in C. coli isolates from pigs and humans. Ciprofloxacin and tetracycline resistance increased over time in both C. jejuni and C. coli from humans in several countries, while erythromycin resistance was more commonly decreasing in C. jejuni. In five countries, high to very high proportions of C. coli from humans were resistant to both ciprofloxacin and erythromycin, leaving few options for treatment of severe Campylobacter infections.
Combined resistance to critically important antimicrobials in Salmonella, C. jejuni and E. coli from both humans and animals was generally uncommon, although very high to extremely high multiple drug resistance levels to other antimicrobials were observed in certain Salmonella serovars. Notably, S. Infantis accounted for most of the multiple drug resistant Salmonella spp. recovered from broilers and their derived carcases (79% and 75.3%, respectively), and monophasic S. Typhimurium accounted for 52.3% and 56.7% of the multiple drug‐resistant Salmonella spp. recovered from fattening pigs and their derived carcases, respectively. Furthermore, S. Kentucky accounted for most of the Salmonella isolates from both humans and poultry, which exhibited high‐level resistance to ciprofloxacin (140/180 and 180/252 isolates, respectively), in addition to the detection of third‐generation cephalosporin resistance in some isolates.
The voluntary monitoring of MRSA from food, healthy animals and following clinical investigations in 2017/2018 revealed that most reported spa‐types were those associated with LA‐MRSA lineages in both reporting years (94.9% in 2017 and 97.6% in 2018). Spa‐types associated with community‐associated (CA)‐ and healthcare‐associated (HA)‐MRSA were also reported, as well as mecC‐MRSA. The occasional detection of lineages of CA‐ and HA‐MRSA primarily associated with humans is presumably associated with the sporadic interchange of strains between humans and animals.
The outcome indicators for AMR in food‐producing animals, such as complete susceptibility to the harmonised panel of antimicrobials in E. coli and the prevalence of ESBL‐/AmpC‐producing E. coli, have also been specifically analysed over the period 2014–2018. There are marked variations in both outcome indicators among reporting countries. A positive development manifested by statistically significant decreasing trends in the prevalence of ESBL‐/AmpC‐producing E. coli in food‐producing animals is observed in 12 countries (11 MSs and 1 non‐MS), whereas statistically significant increasing trends in complete susceptibility in indicator E. coli from food‐producing animals is registered in 6 MSs. These outcome indicators show that some encouraging progress has been registered in reducing AMR in food‐producing animals in several EU MSs over the last years.
1. Introduction
Legal basis
Monitoring of AMR in bacteria from food‐producing animals and derived meat
Regulation (EC) 178/2002 1 Article 33 establishes that EFSA is responsible for examining data on AMR collected from the Member States (MSs) in accordance with Directive 2003/99/EC and for preparing the EU Summary Report from the results.
Directive 2003/99/EC 2 on the monitoring of zoonoses and zoonotic agents lays down the provisions for monitoring of AMR in zoonotic and indicator bacteria in food‐producing animals and derived meat. The Directive obliges EU MSs to collect relevant and, where applicable, comparable data on zoonoses, zoonotic agents, AMR and food‐borne outbreaks.
Implementing Decision 2013/652/EU 3 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria was adopted as part of the 2011–2016 European Commission action plan. It applies from 2014 to 2020 and sets up priorities for the monitoring of AMR from a public health perspective, drafts a list of combinations of bacterial species, food‐producing animal populations and foodstuffs and lays down detailed requirements on the harmonised monitoring and reporting of AMR in food‐producing animals and food.
Monitoring of AMR in bacteria from humans
Decision 2012/506/EU 4 lays down the case definitions that are to be followed when reporting data on infectious diseases, including AMR, to ECDC. These were replaced by Decision 2018/945/EU 5 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions, which came into force in July 2018. The new decision stipulates mandatory testing and reporting of a representative subset of isolates using methods and criteria specified in the EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates (ECDC, 2016).
The data collection on human diseases from MSs is conducted in accordance with Decision 1082/2013/EU 6 on serious cross‐border threats to health.
Terms of Reference
In accordance with the Zoonoses Directive 2003/99/EC the EU MSs are required to assess trends and sources of zoonoses, zoonotic agents and AMR, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the EC covering the data collected.
In accordance with Article 9 of Directive 2003/99/EC, EFSA shall examine the submitted national reports of the MSs and publish a summary report on the trends and sources of zoonoses, zoonotic agents and AMR in the EU.
ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2007, data on human cases have been reported from The European Surveillance System (TESSy), maintained by ECDC.
The antimicrobial agents used in food‐producing animals in Europe are frequently the same, or belong to the same classes, as those used in human medicine. Antimicrobial resistance (AMR) is the main undesirable side‐effect of antimicrobial use in both humans and animals, and results from the continuous positive selection of resistant bacterial clones, whether these are pathogenic, commensal or even environmental bacteria. This will change the population structure of microbial communities, leading to accelerated evolutionary trends with unpredictable consequences for human and animal health. Both the route of administration and the administered quantities of antimicrobials may differ between humans and food‐producing animals; moreover, there are important variations between and within food‐producing animal populations, as well as between countries.
Antimicrobial resistance
Is defined as the inability or reduced ability of an antimicrobial agent to inhibit the growth of a bacterium, which, in the case of a pathogenic organism, can lead to therapy failure. A bacterial strain can acquire resistance by mutation, by the uptake of exogenous genes by horizontal transfer from other bacterial strains or by the activation/triggering of a genetic cascade, thereby inducing the expression of resistance mechanisms (EMA and EFSA, 2017). Resistance development can be triggered by different factors such as inappropriate use of antimicrobials in human and veterinary medicine, poor hygiene conditions and practices in healthcare settings or in the food chain facilitating the transmission of resistant microorganisms. Over time, this makes antimicrobials less effective and ultimately useless.
Bacterial resistance to antimicrobials occurring in food‐producing animals can spread to humans not only via food‐borne routes, but also by routes such as water or other environmental contamination, as well as through direct animal contact. Campylobacter, Salmonella and some strains of Escherichia coli are examples of zoonotic bacteria that can infect humans by the food‐borne route. Infections with bacteria that are resistant to antimicrobials may result in treatment failures or necessitate the use of second‐line antimicrobials for therapy. The commensal bacterial flora can also form a reservoir of resistance genes, which may be transferred between bacterial species, including organisms capable of causing disease in both humans and animals (EFSA, 2008).
The monitoring of AMR in zoonotic and commensal bacteria in food‐producing animals and their food products is a pre‐requisite for understanding the development and diffusion of resistance, providing relevant risk assessment data, and evaluating targeted interventions. Resistance monitoring entails specific and continuous data collection, analysis and reporting and should enable the following of temporal trends in the occurrence and distribution of resistance to antimicrobials and also allow for the identification of emerging or specific patterns of resistance.
This EU Summary Report includes data related to the occurrence of AMR both in isolates from animals and foodstuffs and in isolates from human cases. The report is a collaboration between the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) with the assistance of EFSA's contractors. The European Union Member States (EU MSs), other reporting countries, the European Commission and the relevant EU Reference Laboratory (EURL‐AR) were consulted, while preparing the report. The efforts made by the MSs and the other reporting countries in the reporting of data on AMR and in the preparation of this report are gratefully acknowledged.
The information and data on AMR collected by the EU MSs and compiled in the EU Summary Report on AMR are used to perform wider analyses, such as the joint report on consumption of antimicrobial agents (AMC) and AMR in animals, food and humans, produced by ECDC, EFSA and the European Medicines Agency (EMA), under a One Health approach on a regular basis (JIACRA I and II reports: ECDC, EFSA and EMA, 2015, 2017). This report provides evidence‐based analysis of the possible association between AMC and AMR in humans and food‐producing animals. The JIACRA III report should be issued by the Agencies in December 2020.
A new EU action plan against antimicrobial resistance
The European Commission adopted a new Action Plan to tackle Antimicrobial Resistance (AMR) on 29 June 2017.7 The Action Plan is underpinned by a One Health approach that addresses resistance in both humans and animals. The key objectives of this new plan are built on three main pillars:
Pillar 1: Making the EU a best practice region: as the evaluation of the 2011 action plan highlighted, this will require better evidence, better coordination and surveillance, and better control measures: EU action will focus on key areas and help Member States in establishing, implementing and monitoring their own One Health action plans on AMR, which they agreed to develop at the 2015 World Health Assembly.
Pillar 2: Boosting research, development and innovation by closing current knowledge gaps, providing novel solutions and tools to prevent and treat infectious diseases, and improving diagnosis in order to control the spread of AMR.
Pillar 3: Intensifying EU effort worldwide to shape the global agenda on AMR and mitigate the related risks in an increasingly interconnected world.
In particular, under the first pillar, EU actions will focus on the areas with the highest added value for MSs, e.g. promoting the prudent use of antimicrobials, enhancing cross‐sectorial work, improving infection prevention and consolidating surveillance of AMR and antimicrobial consumption. Examples of support include providing evidence‐based data with the support of EFSA, EMA and ECDC, updating EU implementing legislation on monitoring and reporting of AMR in zoonotic and commensal bacteria in farm animals and food, to take into account new scientific development and monitoring needs, enabling mutual learning, exchange of innovative ideas and consensus building, and co‐fund activities in MSs to tackle AMR.
The new plan includes more than 75 concrete actions with EU added value that the EU Commission will develop and strengthen as appropriate in the coming years. All these actions are important in themselves, but they are also interdependent and need to be implemented in parallel to achieve the best outcome.
1.1. Monitoring and reporting of antimicrobial resistance at the EU level8
1.1.1. Monitoring of antimicrobial resistance in animals and food
According to Commission Implementing Decision 2013/652/EU, monitoring of AMR is mandatory in Salmonella, Campylobacter jejuni and indicator commensal E. coli in the major food‐producing animal populations – broilers, laying hens, fattening turkeys, fattening pigs, calves – and their derived meat. Monitoring is performed on a rotating basis, targeting fattening pigs and bovine animals under 1 year of age and meat derived thereof in odd years and different poultry populations and their derived meat in even years. MSs are also required to conduct specific monitoring of extended‐spectrum β‐lactamase (ESBL)‐, AmpC‐ and carbapenemase‐producing Salmonella and indicator commensal E. coli. The legislation specifies those types of animals that should be monitored in particular years.
The collection and reporting of data are performed at the isolate level, to enable more in‐depth analyses to be conducted, in particular on the occurrence and traits of multiple drug resistance (MDR). Representative random sampling is performed according to the legislation and to the detailed technical specifications issued by EFSA in 2014. Monitoring of AMR in food‐producing animals is performed in domestically produced animal populations, corresponding to different production types with the aim of collecting data that could be combined with those on exposure to antimicrobials. MSs may also performed complementary monitoring, such as that of MRSA, on a voluntary basis. Commission Implementing Decision 2013/652/EU applied as of 1 January 2014 and until December 2020.
Microdilution methods for testing should be used and results should be interpreted by the application of European Committee on Antimicrobial Susceptibility Testing (EUCAST) epidemiological cut‐off (ECOFF) values9 for the interpretation of ‘microbiological’ resistance. The harmonised panels of antimicrobials used for Salmonella, Campylobacter and indicator E. coli include substances that either are important for human health, such as critically important antimicrobials (CIAs), or can provide clearer insight into the resistance mechanisms involved. The concentration ranges to be used embrace both the ECOFF and the clinical breakpoints (CBPs), as defined by EUCAST (2019a), so that comparability of results with human data is made possible.
A particular feature of the monitoring scheme for Salmonella and E. coli is the use of a supplementary panel of antimicrobials for testing isolates that show resistance to third‐generation cephalosporins or carbapenems in the first panel. The reporting of isolate‐based data allows in‐depth phenotypic characterisation of certain mechanisms of resistance, for example, third‐generation cephalosporin resistance and carbapenem resistance can be further characterised.
External quality assurance is provided by the EURL‐AR, which distributes panels of well characterised organisms to all MSs for susceptibility testing, arranges proficiency tests (PTs) trials for the National Reference Laboratories for Antimicrobial Resistance (NRLs‐AR) of the MSs on a yearly basis, and, together with EFSA and the MSs, performs a reference testing exercise that includes re‐testing the antimicrobial susceptibility and whole genome sequencing analysis of selected isolates (Annex A, Materials and methods). The EURL‐AR also provides a source of reference for MSs in cases in which there are issues or problems with the susceptibility test methodology.
1.1.2. Monitoring of antimicrobial resistance in humans
Together with its Food‐ and Waterborne Diseases and Zoonoses (FWD) network, ECDC has developed an EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2014, 2016). This document is intended for the National Public Health Reference Laboratories to guide the susceptibility testing required for EU surveillance and reporting to ECDC. Consultation was also sought from EFSA, EUCAST and the EURL for antimicrobial resistance to facilitate comparison of data between countries and with results from the AMR monitoring performed in isolates from animals and from food products. The protocol is effective from 2014 and supports the implementation of the Commission Action Plan on AMR. One of the recommendations is that, for the purpose of the joint EFSA‐ECDC report, human data should also be interpreted based on ECOFFs. As this requires quantitative data, ECDC introduced reporting of quantitative antimicrobial susceptibility testing (AST) results in the 2013 data collection and encourages countries to use it. As the EU protocol is not a legal document in itself, it is for each National Public Health Reference Laboratory to decide whether to adapt their practices to the protocol. Since the entry into force of Decision 2018/945/EU in July 2018, however, laboratories are obliged to report their AMR test results to ECDC according to the methods and criteria specified in the EU protocol. In 2017 and 2018, most laboratories had adopted the priority panel of antimicrobials suggested in the protocol with the exception of the last‐line antimicrobials, which were tested by fewer laboratories. The protocol also proposes a testing algorithm for screening and confirmation of ESBL‐producing Salmonella spp., including detection of AmpC. This has been implemented by some laboratories while others use a modification of the algorithm or test suspected isolates directly with PCR or whole genome sequencing. Further testing for ESBL and AmpC was performed in 15 of 20 countries with the third‐generation cephalosporin resistance detected in Salmonella isolated from humans in 2018.
External quality assessment to support laboratories in implementing the recommended test methods and antimicrobials and obtaining high‐quality AST results is provided by ECDC via a contract with Statens Serum Institute in Denmark.
1.2. Further harmonised monitoring of antimicrobial resistance
To facilitate comparability of data the methodology for AMR surveillance should be harmonised across countries as far as possible. The main issues when comparing AMR data originating from different countries are the use of different laboratory methods and different interpretive criteria of resistance. These issues have been addressed by the development of ECDC's protocol for harmonised monitoring and reporting of resistance in humans and by the legislation on harmonised monitoring in food‐producing animals and the food produced.
So as to respond effectively to the constantly evolving threat of AMR, further enhancements and specific adaptations will be regularly required on an ongoing basis. Under the new One Health action plan (2017) the European Commission is committed to review this legislation, to take into account new scientific developments and data collection needs. It is envisaged that the new legislation replacing Commission Implementing Decision 2013/652/EU will apply as of 2021. In view of reviewing this Decision, EFSA received a mandate from the EC to review and update the technical specifications on harmonised monitoring of AMR issued in 2012 and 2014 and, notably, specifically address in these updates the possible use of molecular typing methods. The new technical specifications were published in June 2019 (EFSA, 2019) and provide solid scientific advice to support amendments in the existing legislation (see text box below).
New technical specifications on harmonised monitoring of AMR in food‐producing animals
EFSA issued new technical specifications in June 2019 with proposals for implementing updated guidelines for further harmonised monitoring of AMR in food‐producing animals and derived meat in the EU and for ensuring continuity in following up further trends in AMR (EFSA, 2019).
The combinations of bacterial species, food‐producing animals and meat for mandatory monitoring were reviewed and it is proposed to reinforce the approach of prioritising potential consumers’ exposure by targeting zoonotic Salmonella spp. and Campylobacter jejuni and Campylobacter coli, as well as indicator commensal E. coli from the major domestically produced animal populations. One of the major aims is the collection of AMR data that can be investigated in combination with data on exposure to antimicrobials. Although monitoring performed on a yearly basis would allow earlier detection of trends in AMR, than monitoring at greater intervals, it is proposed to retain and reinforce the current monitoring performed on a rotating basis. Thus, the potential benefits of an increased frequency of monitoring were reviewed considering competing priorities, as well as the need to get a balanced output from each of the most important sectors. In addition to routine monitoring on a biennial basis, the undertaking of complementary baseline cross‐sectional surveys in order to assess specifically the situation on certain AMR issues, such as MRSA, AMR in bacteria from sea food and from the environment is suggested.
Limited revisions and/or additions to the antimicrobial panel have been proposed to both account for recent trends in AMR and continue following up further temporal trends for the sake of continuity. In particular, it is proposed to complement the first harmonised panel of antimicrobials for Salmonella and indicator E. coli with amikacin to improve the detection of 16S rRNA methyltransferase enzymes that confer resistance to all aminoglycosides except streptomycin. Slight alterations to the antimicrobial panel for Campylobacter have also been suggested and, in order to improve the comparability of Campylobacter prevalence and AMR data between MSs, it is proposed that a harmonised protocol should be provided.
The approach and the results of the sample size analyses and calculation in the previous EFSA technical specifications were reviewed. Considering differing sample sizes, numerical simulations have been performed to evaluate the related statistical power available for assessing occurrence and temporal trends in resistance, with a predetermined accuracy, to support the choice of harmonised sample size. Randomised sampling procedures, based on a generic proportionate stratified sampling process, have been reviewed and reinforced. As regards the laboratory methodologies, it is confirmed that broth microdilution is the preferred method and that EUCAST epidemiological cut‐off values should be used as interpretative criteria to define microbiological resistance. The concentration ranges to be used should ensure that both the epidemiological cut‐off values and the clinical breakpoints (CBPs) are included so that comparability of results with human data is made possible.
Considering the advantages inherent in the whole genome sequencing (WGS) technology but also its current limitations, as well as the expected evolution of the present situation, it is proposed to follow a gradual, phased approach to integration of WGS within the harmonised AMR monitoring. The integration process could be initiated by complementing the harmonised phenotypic monitoring with WGS on a voluntary basis in the early phase of the period 2021–2026 and at the end of the period envisage the replacement of the standard routine phenotypic antimicrobial susceptibility testing with the systematic use of WGS. The period 2021–2026 should therefore be seen as a transitory period for the implementation of WGS, expected to be a reasonable transition period for the MSs to gain experience and acquire WGS technology. As part of this flexible approach the voluntary replacement of the phenotypic antimicrobial susceptibility testing method for detection of ESBL‐/AmpC/carbapenemase‐producing E. coli is proposed to begin in 2021.
1.3. The 2017–2018 EU Summary Report on AMR
Most data reported to EFSA by the MSs comprise data collected in accordance with Commission Implementing Decision 2013/652/EU. The antimicrobial susceptibility data reported to EFSA for 2017 and 2018 for Campylobacter, Salmonella and indicator E. coli isolates from animals and food were analysed and all quantitative data were interpreted using ECOFFs. This report also includes results of phenotypic monitoring of resistance to third‐generation cephalosporins and/or carbapenems caused by ESBLs, AmpC β‐lactamases or carbapenemases in Salmonella and indicator E. coli, as well as the investigation at the EU level of the occurrence of complete susceptibility and MDR in data reported at the isolate level. All the information on the methodology applied, list of antimicrobials, criteria, etc. can be found in Annex A ‘Materials and methods’ available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3628719. Additional information on the data reported in 2017 can also be found in EFSA and ECDC (2019).
The report also includes resistance in Salmonella and Campylobacter isolates from human cases of salmonellosis and campylobacteriosis, respectively. These data were reported by MSs to TESSy either as quantitative or categorical/qualitative data. The quantitative data were interpreted using EUCAST ECOFFs, where available. The qualitative data had been interpreted using CBPs to guide medical treatment of the patient. The breakpoints for ‘clinical’ resistance are, in many cases, less sensitive than the ECOFF for a specific bacterium–drug combination resulting in higher levels of ‘microbiological’ resistance than ‘clinical’ resistance. By combining the categories of ‘clinically’ resistant and intermediate resistant into a non‐susceptible category, however, close correspondence with the ECOFF was achieved. CBPs enable clinicians to choose the appropriate treatment based on information relevant to the individual patient. ECOFFs recognise that epidemiologists need to be aware of small changes in bacterial susceptibility, which may indicate emerging resistance and allow for appropriate control measures to be considered. ECOFFs, CBPs and related concepts on antimicrobial resistance/susceptibility are presented in detail in Annex A ‘Materials and methods’.
2. Antimicrobial resistance in Salmonella spp.10
Non‐typhoidal salmonellas (NTS) are the focus of this section, which summarises the occurrence and AMR patterns of isolates recovered from humans and various food‐producing animal populations and their derived carcases. Whereas typhoidal salmonellas are human host‐adapted organisms that cause typhoid fever and paratyphoid fever; non‐typhoidal strains may be host generalists, infecting or colonising a broad range of animals, or tend to be host‐specific to particular animal species (Crump et al., 2015). Typhoidal salmonellas refer to Salmonella enterica subsp. enterica serovars Typhi, Paratyphi A, Paratyphi B (d‐tartrate negative) and Paratyphi C, while all other serovars within the subspecies enterica (including the d‐tartrate positive Paratyphi B variant Java) refer to NTS.
The World Health Organization states that transmission of bacterial infection from non‐human sources to humans, with the ability to cause disease, is more evident in particular bacteria (including non‐typhoidal Salmonella, Campylobacter spp. and E. coli) and comments that the potential for such transmission should be recognised (WHO, 2006). In 2018, salmonellosis was the second most common zoonosis in the European Union, with 91,857 confirmed human cases, as well as the most frequent cause of food‐borne outbreaks accounting for 30.7% of all cases reported in 2018 (EFSA and ECDC, 2019a,b). A recent review inferred that MDR NTS infections may have more serious human health implications compared to those of pan‐susceptible strains (Parisi et al., 2018).
2.1. Data on AMR in Salmonella spp. addressed
Commission Implementing Decision 2013/652/EU stipulates detailed protocols for the harmonised monitoring and reporting of AMR in zoonotic and commensal bacteria. The monitoring of AMR in Salmonella isolates recovered from carcase swabs of fattening pigs and calves (under 1 year of age) at slaughter was mandatory in 2017, in accordance with Regulation (EC) No 2073/2005; similarly, the monitoring of AMR in Salmonella isolates recovered from carcase swabs of broilers and fattening turkeys at slaughter was mandatory in 2018. Additionally, in 2018, the monitoring of AMR in Salmonella isolates recovered from faecal samples and/or environmental samples (boot swabs or dust) of broiler, laying hen and fattening turkey flocks was mandatory, in accordance with Regulation (EC) No 2160/2003, collected as part of National Control Programmes (NCPs) for Salmonella in poultry. In 2017, some MSs also reported Salmonella AMR data from fattening pigs and cattle at slaughter, where in general one representative sample of caecal contents was collected per epidemiological unit (i.e. the holding) to prevent clustering. The reporting of such data was not mandatory but was included for completeness.
The Salmonella spp. data includes results for all serovars reported from the different animal categories, where no more than one isolate per Salmonella serovar from the same epidemiological unit per year was tested for AMR (Decision 2013/652/EU). As the potential for acquiring or occurrence of AMR markedly varies between serovars, the relative contribution of different serovars to the total significantly influences overall resistance levels for Salmonella spp. data. Therefore, results have also been presented for selected serovars because of their importance and/or prevalence. Resistance profiles were also considered when less than ten isolates were recovered from a given animal category in a country, to account for the low prevalence of certain serovars, to prevent exclusion of emerging serovars and to ensure that the analysis included all relevant data. The spread of particular resistant clones and the occurrence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. Other factors, such as foreign travel by humans, international food trade, animal movements, farming systems, animal husbandry and the pyramidal structure of some types of animal primary production, may also influence the spread of resistant Salmonella clones.
It is of note that countries reported Salmonella spp. data from the different animal categories according to their national situation. Notably, some MSs did not obtain any positive Salmonella isolates from the carcase and animal origins and, therefore, data are not presented for these countries. The number of countries reporting results for pig and broiler carcases was considerably higher than those for calf and turkey carcases, because the size of the veal calf and turkey sectors is relatively small in certain EU MSs, with production levels below the threshold at which mandatory monitoring is required. Additionally, the number of isolates reported by countries varied because of varying Salmonella prevalence, and these factors may introduce a source of variation to results when considering all reporting countries.
In both 2017 and 2018, data for Salmonella spp. from human cases were also reported. Section 2.2 presents data for 2018 since 2017 data on humans were published in the EU Summary report for 2017 (EFSA and ECDC, 2019a,b). The analysis of AMR in Salmonella isolates from human cases includes that of prevalent serovars corresponding to those occurring in animal species.
2.2. Antimicrobial resistance in Salmonella from humans
Data reported
For 2018, 23 MSs and 1 non‐MS reported data on AMR in Salmonella isolates from human cases of non‐typhoidal salmonellosis. Fifteen countries provided data as measured values (quantitative data) and nine as data interpreted with CBPs. Not all countries reported results for all antimicrobials in the harmonised panel (ECDC, 2016). The reported data represented 23.0% of the confirmed human cases with non‐typhoidal Salmonella reported in the EU/EEA in 2018.
Resistance to commonly used antimicrobials in human and/or veterinary medicine
In 2018, high proportions of human Salmonella isolates were resistant to sulfonamides (30.5%), tetracyclines (28.8%) and ampicillin (25.9%) – see Figure 1 and Annex B, Table 1. By serovar, resistance to these compounds ranged from low in S. Enteritidis to extremely high in monophasic S. Typhimurium 1,4,[5],12:i:‐ and S. Kentucky. The variation in the proportion of resistance by country was large. For S. Enteritidis, outliers in terms of high proportions of resistance were observed in Belgium and Poland for both ampicillin and tetracycline (see Annex B, Table 2). For S. Infantis, Italy had much higher resistance (75.0%) to ampicillin than the EU average (see Annex B, Table 5). For monophasic S. Typhimurium 1,4,[5],12:i:‐, Malta reported a much lower proportion (53.7%) of ampicillin resistance than other countries (see Annex B, Table 4). Resistance to gentamicin was overall low (2.9%) (see Annex B, Table 1) with the exception of S. Kentucky where it was very high (51.1%) (Annex B, Table 6). Similarly, levels of chloramphenicol were overall low (6.5%) (see Annex B, Table 1) but moderate (17.3%) in S. Typhimurium (Annex B, Table 3).
Figure 1.
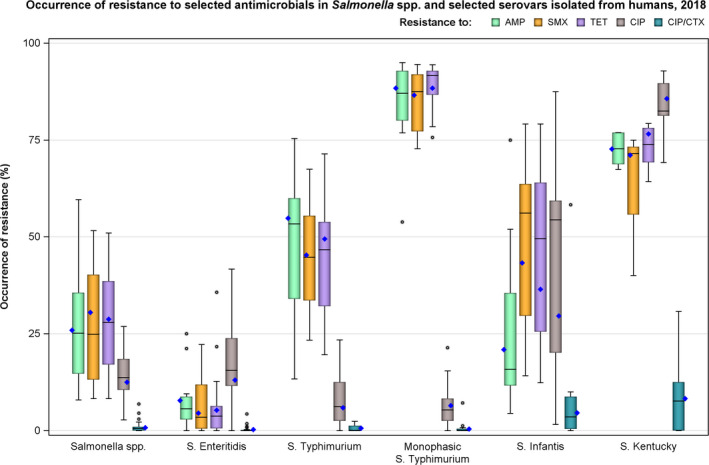
Occurrence of resistance to selected antimicrobials in Salmonella spp. and selected serovars isolated from humans, 2018
- Horizontal line represents median, and blue diamond represents the resistance at the reporting‐MS level.
| EU total | AMP | SMX | TET | CIP | CTX | Combined CIP/CTX | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Salmonella spp. (23 MSs) | 18,103 | 25.9 | 8,377 | 30.5 | 13,571 | 28.8 | 16,996 | 12.5 | 14,983 | 1.5 | 14,335 | 0.8 |
| S. Enteritidis (23 MSs) | 6,543 | 7.8 | 2,518 | 4.5 | 4,304 | 5.3 | 5,670 | 13.1 | 5,034 | 0.6 | 4,596 | 0.3 |
| S. Typhimurium (23 MSs) | 2,731 | 54.8 | 1,089 | 45.3 | 2,178 | 49.5 | 2,678 | 5.9 | 2,325 | 1.3 | 2,272 | 0.6 |
| Monophasic S. Typhimurium (15 MSs) | 1,731 | 88.4 | 1,496 | 86.6 | 1,606 | 88.4 | 1,731 | 6.5 | 1,645 | 0.7 | 1,643 | 0.4 |
| S. Infantis (20 MSs) | 808 | 20.9 | 406 | 43.3 | 694 | 36.5 | 796 | 29.6 | 727 | 8.3 | 713 | 4.6 |
| S. Kentucky (14 MSs) | 322 | 72.7 | 187 | 71.1 | 278 | 76.6 | 322 | 85.7 | 291 | 8.2 | 290 | 8.3 |
AMP: ampicillin; CIP: ciprofloxacin; CTX: cefotaxime; SMX: sulphonamides; TET: tetracyclines.
Occurrence of resistance to the highest priority ‘critically important antimicrobials’
The proportion of Salmonella isolates resistant to the CIA ciprofloxacin was overall 12.5% (see Annex B, Table 1) with extremely high proportions being resistant in S. Kentucky (85.7%) (see Annex B, Table 6), and in S. Infantis ranging from 1.6% in Germany to 87.5% in Italy (EU average 29.6%) – see Figure 1 and Annex B, Table 5. For the two antimicrobials cefotaxime and ceftazidime, representing third‐generation cephalosporins, another class of CIAs for Salmonella, resistance levels were generally low (1.5% and 1.2%, respectively) (see Annex B, Table 1) but with higher levels (6.1–8.2%) in S. Infantis and S. Kentucky (see Annex B, Tables 5 and 6). Outliers for both cephalosporins were observed in Italy regarding S. Infantis (58.3% and 50.0% resistant to cefotaxime and ceftazidime, respectively – see Annex B, Table 5) and in Malta for S. Kentucky (30.8% resistant to both – see Annex B, Table 6). Combined resistance to ciprofloxacin and cefotaxime was overall low in Salmonella spp. (0.8%) but significantly higher in S. Infantis (4.3%) and S. Kentucky (7.9%) with particularly high proportions of combined resistance among S. Infantis isolates from Italy (58.3%) – see Annex B, Table 5 – and among S. Kentucky isolates from Malta (30.8%) – see Figure 2 and Annex B, Table 6.
Figure 2.
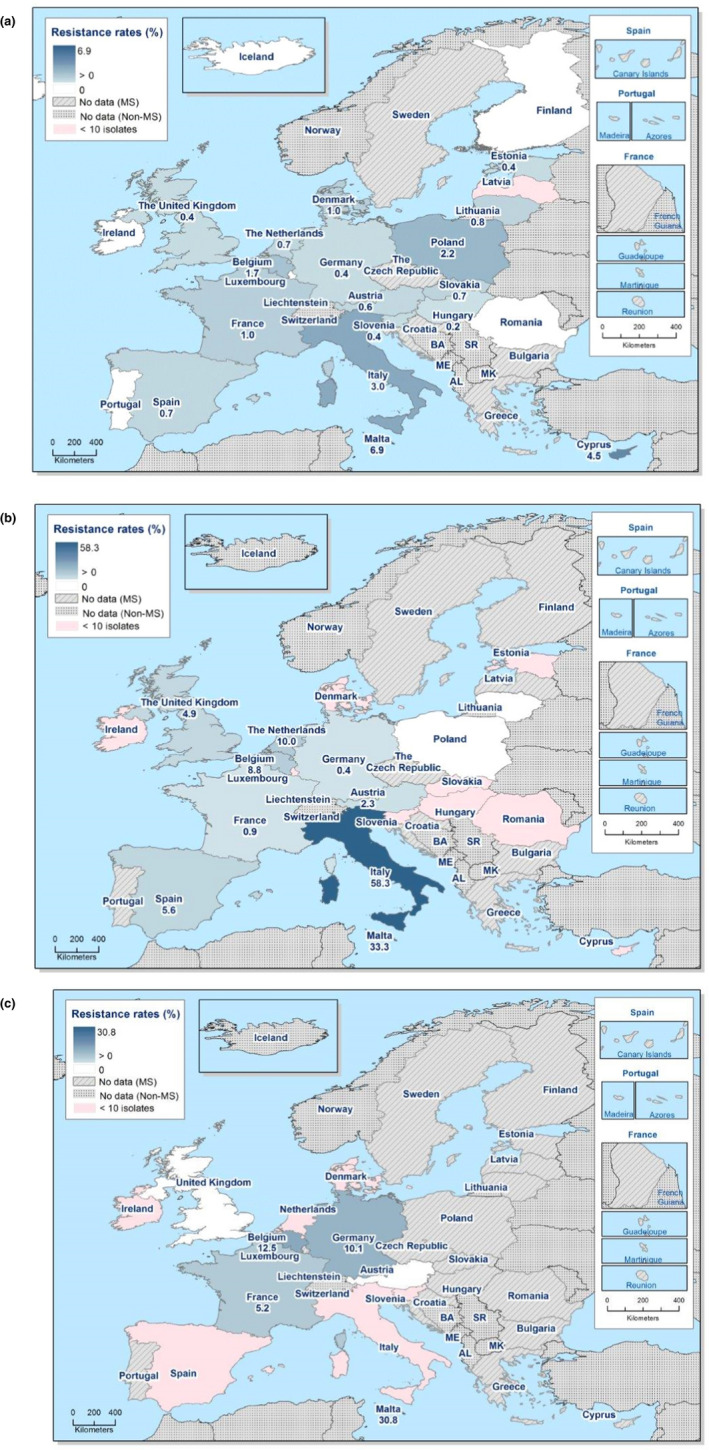
Spatial distribution of combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime among (a) Salmonella spp., (b) S. Infantis and (c) S. Kentucky isolated from human cases, 2018
- Pink indicates less than 10 isolates tested.
Only seven and eight countries tested resistance to the last line antimicrobials azithromycin and tigecycline, respectively. Resistance was overall low (1.6% and 1.7%, respectively) with Belgium reporting the highest proportions (4.7% and 8.5%, respectively) (Annex B). By serovar, a higher proportion of S. Infantis isolates were resistant to tigecycline (4.2%) compared to all Salmonella spp. and a higher proportion of S. Kentucky to azithromycin and tigecycline (6.5% and 5.9%, respectively). Resistance to colistin was detected in 7.8% of isolates, although 83.6% of the resistant isolates were either S. Enteritidis or S. Dublin, which have been reported to have higher natural tolerance to colistin (Agersø et al., 2012).
ESBL‐, AmpC‐ and carbapenemase‐producing Salmonella
In 2018, 15 MSs (of 20 reporting isolates resistant to cephalosporins) further tested all or some of their suspected isolates for the presence of ESBL and/or AmpC. Presumptive ESBL‐producing Salmonella were identified in 0.8% of the tested isolates in the EU MSs with the highest occurrence in Malta (6.9%) and Italy (2.6%) (Annex B). AmpC was less frequent, identified in 0.2% of tested isolates. No isolates were reported to be both AmpC‐ and ESBL‐producing. ESBL was reported in 16 different serovars in 2018, most commonly in S. Corvallis, S. Infantis, S. Give, S. Haifa and S. Kentucky (ranging between 4.5% and 6.1%) (Table 1). ESBL‐production was more frequent in S. Typhimurium (0.8%) than in monophasic S. Typhimurium 1,4,[5],12:i:‐ (0.3%) and S. Enteritidis (0.2%). The proportion of S. Kentucky with ESBL decreased from 20.3% in 2017 to 4.5% in 2018 with only two countries reporting S. Kentucky with CTX‐M‐14b/CTX‐M‐9/14 in 2018. AmpC‐type β‐lactamases were reported in ten different serovars, most commonly in S. Anatum, S. Bredeney and S. Thompson (ranging between 2.4% and 3.7%), although the proportions were higher due to the low frequency of these serovars in human cases.
Table 1.
ESBL, AmpC and carbapenemase phenotypes and genotypes in Salmonella spp. isolates from humans by serovar*, 2018
| Serovar | Tested for CTX and/or CAZ | Res to CTX and/or CAZ | Resistance phenotype | Genotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ESBL | AmpC | AmpC + ESBL | Carbapenemase | ||||||||
| N | N | N | % | N | % | N | % | N | % | ||
| Anatum | 27 | 1 | 1 | 3.7 | |||||||
| Blockley | 4 | 2 | 2 | NA | SHV‐12 (2) | ||||||
| Bovismorbificans | 70 | 2 | 2 | 2.9 | CTX‐M (2) | ||||||
| Bredeney | 36 | 1 | 1 | 2.8 | CMY‐2 | ||||||
| Corvallis | 33 | 3 | 2 | 6.1 | 1 | 3.0 | CTX‐M, CTX‐M‐55, OXA‐48 | ||||
| Derby | 201 | 1 | 1 | 0.5 | CTX‐M‐14 | ||||||
| Dublin | 111 | 1 | 1 | 0.9 | CMY‐2 | ||||||
| Enteritidis | 3,205 | 9 | 8 | 0.2 | 1 | 0.0 | CTX‐M, SHV‐12, CIT | ||||
| Give | 35 | 2 | 2 | 5.7 | CTX‐M, CTX‐M‐55 | ||||||
| Haifa | 18 | 1 | 1 | 5.6 | SHV‐12 | ||||||
| Infantis | 450 | 36 | 26 | 5.8 | 1 | 0.2 | CTX‐M‐65 (5), CTX‐M‐1 group (4), CTX‐M‐9/14 (3), CTX‐M, CTX‐M‐32, CTX‐M‐15, CMY‐2 | ||||
| Kentucky | 200 | 15 | 9 | 4.5 | 3 | 1.5 | 2 | 1.0 | CTX‐M‐14b (4), CTX‐M‐9/14 (3), CMY‐2 (3), OXA‐48 (2), CTX‐M‐15, SHV‐12 | ||
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 1,562 | 16 | 4 | 0.3 | 1 | 0.1 | CTX‐M‐55 (2), CTX‐M (2) | ||||
| Muenchen | 28 | 1 | 1 | 3.6 | CTX‐M‐8 | ||||||
| Napoli | 47 | 1 | 1 | 2.1 | |||||||
| Newport | 289 | 2 | 1 | 0.3 | 1 | 0.3 | CMY‐2 | ||||
| Panama | 29 | 1 | 1 | 3.4 | CTX‐M‐2 | ||||||
| Rissen | 44 | 1 | 1 | 2.3 | KPC | ||||||
| Saintpaul | 53 | 2 | 2 | 3.8 | CTX‐M‐15 | ||||||
| Thompson | 42 | 2 | 1 | 2.4 | CIT | ||||||
| Typhimurium | 1,355 | 27 | 11 | 0.8 | 4 | 0.3 | 1 | 0.1 | CMY‐2 (4), CTX‐M (2), CTX‐M‐3 (2), CTX‐M‐9 (2), CTX‐M‐1, CTX‐M‐15, CTX‐M‐64, SHV, VIM | ||
CTX: cefotaxime; CAZ: ceftazidime; ESBL: extended spectrum beta‐lactamase.
1 S. Oranienburg and 2 isolates of unspecified serotype that were cephalosporin‐resistant but neither ESBL‐, AmpC‐ nor carbapenemase producing were not included in the table.
Five isolates resistant to meropenem were reported by three MS (Italy, France and Spain) in 2018. This is the first report of carbapenem resistance in Salmonella from humans not related to known travel outside the EU/EEA: one case (in Italy) was reported to be domestically acquired. For the other four, information on travel status was missing. Four of five cases were in elderly persons aged 75 years or more, with isolation of the bacteria from urine or other body sites, rather than from stool. Of the five carbapenemase‐producing isolates, two were S. Kentucky (OXA‐48), as well as single isolates of S. Corvallis (OXA‐48), S. Rissen (KPC) and S. Typhimurium (VIM). In 8 of 23 reporting countries, meropenem results were interpreted with CBPs and the CBP is much less sensitive than the ECOFF.
MDR
MDR was high overall (28.5%) in the EU (Figure 3). For the investigated serovars, MDR was most frequently reported among monophasic S. Typhimurium 1,4,[5],12:i:‐ (80.5%), followed by S. Kentucky (77.4%), S. Infantis (41.8%), S. Typhimurium (38.2%) and lastly S. Enteritidis (3.5%). Eleven isolates (seven S. Infantis, two S. Kentucky and single isolates of S. Corvallis and S. Typhimurium) were resistant to eight of the nine tested substances, only susceptible to meropenem.
Figure 3.
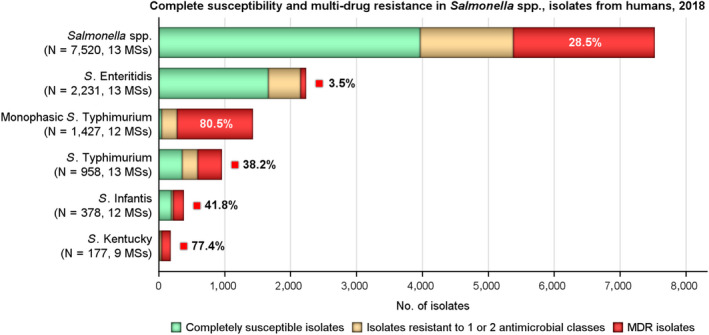
Number of MDR isolates, isolates resistant to 1 and/or 2 antimicrobial classes and completely susceptible Salmonella isolates from humans in 2018
Temporal trends
Trends in resistance over the period 2013–2018 were assessed with logistic regression. Trends varied by country for the different serovars and antimicrobials (Table 2, graphs in Annex B). Increasing trends in resistance were more commonly observed than decreasing trends for ciprofloxacin/quinolones in S. Infantis and S. Enteritidis, and for ampicillin in monophasic S. Typhimurium and S. Infantis. More countries also reported increasing than decreasing trends for tetracyclines in S. Enteritidis. Decreasing trends in resistance were more commonly observed for ampicillin in S. Enteritidis and S. Typhimurium (9 countries with decreasing trend) and also in Salmonella spp. overall, for cefotaxime in S. Enteritidis and for tetracycline in S. Typhimurium (with 11 countries observing a decreasing trend).
Table 2.
Number of countries with statistically significant (p < 0.05) increasing or decreasing trends in resistance to selected antimicrobials for Salmonella spp. and selected serovars in humans in 2013–2018*
| Serovar | Ampicillin | Cefotaxime | Ciprofloxacin/quinolones | Tetracyclines | ||||
|---|---|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | |
| Salmonella spp. (24 MSs + 1 non‐MS) | 2 (BE, EL) | 8 (DE, EE, ES, IT, LT, PT, RO, UK) | 3 (BE, MT, NL) | 1 (FR) | 6 (BE, DE, IE, NL, NO, SK) | 6 (AT, EL, ES, FR, HU, MT) | 5 (BE, EL, NO, SI, UK) | 6 (EE, ES, FR, IE, IT, PT) |
| S. Enteritidis (22 MSs + 1 non‐MS) | 4 (AT, BE, FR, NL) | 6 (ES, IE, LT, LU, MT, RO) | – | 5 (EE, HU, IT, NO, SI) | 5 (AT, BE, NO, RO, SK) | 3 (ES, LT, MT) | 7 (AT, BE, DE, NL, SI, SK, UK) | 3 (EE, LT, RO) |
| S. Typhimurium (22 MSs + 1 non‐MS) | 3 (BE, DK, SK) | 9 (DE, EE, EL, ES, IE, LU, NO, PT, UK) | 1 (BE) | 2 (IT, NO) | 4 (AT, BE, FI, LT) | 3 (EL, SI, SK) | 3 (BE, DK, RO) | 11 (AT, DE, EE, EL, ES, FR, HU, IE, LU, NL, PT) |
| Monophasic S. Typhimurium (13 MSs) | 3 (EL, IT, LU) | – | – | 1 (LU) | 2 (AT, HU) | 2 (EL, IT) | 2 (EL, IT) | 3 (ES, HU, NL) |
| S. Infantis (13 MSs) | 3 (BE, DE, SK) | – | 2 (DE, UK) | 1 (FR) | 5 (BE, DE, LT, NL, SK) | 1 (HU) | 1 (NL) | – |
| S. Kentucky (7 MSs) | – | – | 1 (BE) | – | – | – | – | 1 (AT) |
Only countries reporting data for at least 10 isolates for a specific combination and for at least 3 years in the 6‐year period were included.
High ciprofloxacin resistance
In 2018, 4.6% (180 of 3,953) of Salmonella spp. expressed high‐level resistance to ciprofloxacin (minimum inhibitory concentration (MIC) ≥ 4 mg/L) (Table 3). Such isolates were reported from eight of the ten countries reporting MIC values for ciprofloxacin. Among the fourteen serovars reported with MIC ≥ 4 mg/L, high‐level ciprofloxacin resistance was most frequently observed in S. Kentucky (in 88.6% of tested S. Kentucky) followed by S. Agona (14.3%) and S. Oranienburg (7.7%).
Table 3.
Occurrence of high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) in Salmonella serovars from human cases in 2018, 10 MSs
| Serovar | N | High‐Level resistance to ciprofloxacin (MIC ≥ 4 mg/L) | |
|---|---|---|---|
| n | % | ||
| S. Agona | 28 | 4 | 14.3 |
| S. Chester | 46 | 3 | 6.5 |
| S. Derby | 134 | 1 | 0.7 |
| S. Dublin | 99 | 1 | 1.0 |
| S. Enteritidis | 918 | 7 | 0.8 |
| S. Infantis | 238 | 4 | 1.7 |
| S. Kentucky | 158 | 140 | 88.6 |
| S. Livingstone | 39 | 1 | 2.6 |
| Monophasic S. Typhimurium | 681 | 2 | 0.3 |
| S. Newport | 199 | 2 | 1.0 |
| S. Oranienburg | 26 | 2 | 7.7 |
| S. Stanley | 43 | 2 | 4.7 |
| S. Stourbridge | 2 | 1 | NA |
| S. Typhimurium | 661 | 10 | 1.5 |
| Other | 681 | 0 | 0.0 |
| Total (10 MSs) | 3,953 | 180 | 4.6 |
Additional data on certain resistance traits of Salmonella spp. isolates from humans are provided hereafter and presented in parallel to corresponding data on Salmonella spp. from animals and food.
2.3. Occurrence of antimicrobial resistance in Salmonella from poultry, porcine and bovine populations, and carcases from these species
In 2017, AMR data for Salmonella isolates recovered from carcases of pigs (fatteners) and calves (under 1 year of age), in some cases with additional data obtained from the monitoring of caecal contents of fattening pigs and cattle, were reported by 25 MSs and 2 non‐MS; while in 2018, AMR data for Salmonella isolates recovered from carcases of broilers and fattening turkeys, as well as data obtained from National Control Plan samples (boot swabs or dust) of broiler, laying hen and fattening turkey flocks, were reported by 26 MSs and 2 non‐MSs. Annex B (available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3628719) presents the occurrence of antimicrobial resistance (%) in Salmonella spp. from carcases of pigs, calves, broilers and turkeys, as well as from pigs, cattle, broilers, laying hens and turkeys, at both the MS and MS‐group level.
2.3.1. Resistance in Salmonella spp. from carcases of food‐producing animals
Occurrence of resistance to commonly used antimicrobials in veterinary medicine
Carcases of pigs and calves
Among Salmonella spp. recovered from carcase swabs of pigs and calves in 2017, the highest levels of resistance were noted to ampicillin, sulfamethoxazole and tetracycline considering all reporting MSs. High to extremely high levels of resistance to these antimicrobials were recorded in pig carcases by most of the MSs included in the analysis; while resistance to these compounds generally ranged from moderate to very high among isolates from calf carcases (overall resistance in pig carcases: 53%, 59.5% and 56.8%, respectively; overall resistance in calf carcases: 24.4%, 30.5% and 28%, respectively). Among Salmonella isolates recovered from calf carcases, overall resistance levels were mostly lower than those observed for pig carcases, with the exception of colistin resistance (3.7%) which was slightly higher than the value registered for pig carcases (0.6%); however, the total number of isolates from calf carcases (N = 82) was considerably lower than that from pig carcases (N = 954).
Carcases of poultry
Considering all MSs reporting Salmonella spp. data from carcase swabs of poultry in 2018, overall resistance to ampicillin, sulfamethoxazole and tetracycline ranged from moderate to very high. Ampicillin resistance was observed at overall moderate levels in both broiler and turkey carcases (13.7% and 16.5%, respectively); sulfamethoxazole resistance was noted at an overall high level in broiler carcases and a moderate level in turkey carcases (33.9% and 13.7%, respectively); while tetracycline resistance was noted at an overall high level in broiler carcases and a very high level in turkey carcases (35.5% and 57.3%, respectively). Among Salmonella isolates recovered from turkey carcases, overall resistance levels were generally lower than those observed for broiler carcases with the exception of chloramphenicol, ampicillin and colistin resistance which were slightly higher than the values registered for broiler carcases. Notably, tetracycline resistance was reported at a much higher level among isolates from turkey carcases compared to that from broiler carcases considering all reporting MSs (57.3% and 35.5%, respectively).
Occurrence of resistance to ‘critically important antimicrobials’
Fluoroquinolones and third‐generation cephalosporins are categorised as highest priority, CIA in human medicine (WHO, 2019). Although fluoroquinolones may not be recommended for use in children, these CIAs often constitute first‐line treatment for invasive salmonellosis in humans and as such, the monitoring of resistance to these compounds in zoonotic bacteria, including Salmonella spp., originating from animals is of particular interest. These classes are represented by ciprofloxacin and cefotaxime/ceftazidime, compounds which are specified in the antimicrobial panels for the monitoring and reporting of AMR in Salmonella spp. The WHO also recognises tigecycline and azithromycin as CIAs. Additionally, colistin is considered as a highest priority CIA for the treatment of serious human infection with some Gram‐negative bacteria (WHO, 2019).
Considering Salmonella spp. recovered from broiler carcases in 2018, resistance to the (fluoro)quinolone antimicrobial agents, ciprofloxacin and nalidixic acid, were reported at high to extremely high levels by many of the MSs included in the analysis (with overall resistance at 51.4% and 48.8%, respectively). Resistance levels to ciprofloxacin and nalidixic acid in isolates from turkey carcases ranged from low or not detected to extremely high among reporting MSs (overall, 32.4% and 23.7%, respectively). In certain Salmonella serovars recovered from carcases of pigs and poultry, isolates resistant to ciprofloxacin but not to nalidixic acid were observed; possibly indicating the occurrence of plasmid‐mediated quinolone resistance (PMQR) mechanisms. This was particularly apparent among 13 S. Hadar isolates reported from turkey carcases by Romania in 2018, where all isolates displayed ciprofloxacin resistance, yet none showed resistance to nalidixic acid. Similarly, 16/32 S. Rissen isolates reported from pig carcases by Spain in 2017 displayed ciprofloxacin resistance, yet only 9/32 isolates showed nalidixic acid resistance.
‘Microbiological’ resistance to third‐generation cephalosporins (cefotaxime and ceftazidime) in Salmonella spp. from carcases of these food‐producing animals was either not discerned or detected at low levels in most of the reporting MSs, with the exception of Portugal (N = 6) which reported moderate levels of resistance in 1/6 Salmonella spp. from broiler carcases, as well as Lithuania (N = 2) which reported high levels of resistance in 1/2 isolates from pig carcases. No reporting countries detected third‐generation cephalosporin resistance among Salmonella isolates from carcases of calves or turkeys. Section 2.3.5 provides further information on the phenotypic characterisation of third‐generation cephalosporin resistance among Salmonella isolates from pig and broiler carcases.
Spain was the only country to report combined ‘microbiological’ resistance to both ciprofloxacin and cefotaxime in two Salmonella isolates from pig carcases (of serovars Bredeney and Rissen); while Portugal was the only country to report combined ‘microbiological’ resistance to these antimicrobial agents in an isolate from a broiler carcase (S. Paratyphi B var. Java). Therefore, considering all reporting MSs, ‘microbiological’ combined resistance to these agents among isolates from pig and broiler carcases were observed at overall very low levels (0.2% and 0.1%, respectively) – see Figure 4. Notably, when CBPs were applied, only the single S. Paratyphi B var. Java isolate recovered from a broiler carcase by Portugal exhibited ‘clinical’ resistance to these compounds.
Figure 4.
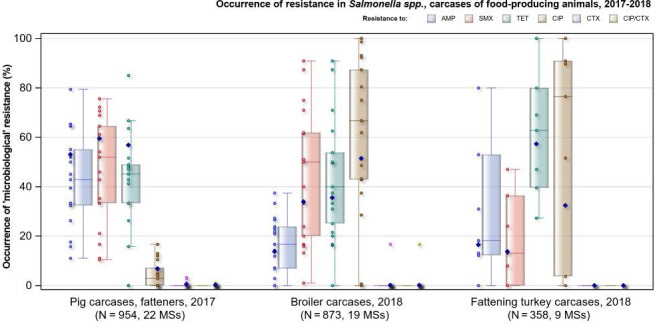
Occurrence of resistance to selected antimicrobials in Salmonella spp. from carcases of pigs, calves (< 1 year of age), broilers and fattening turkeys, reporting EU MSs, 2017/2018
- AMP: ampicillin, SMX: sulfamethoxazole, TET: tetracycline, CIP: ciprofloxacin, CTX: cefotaxime, CIP/CTX: combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime, N: total number of Salmonella spp. reported by MSs. Blue diamond shows resistance at the reporting‐MS group level.
Resistance to azithromycin (a highest priority CIA) in Salmonella spp. from carcases of pigs, calves and poultry was generally low or not detected, although there were a few exceptions: a moderate level of resistance to this compound was detected among isolates from pig carcases by Portugal (11.8%), as well as calf carcases by Denmark (20%) and broiler carcases by Portugal (16.7%), although Denmark and Portugal reported a very low number of isolates from calf and broiler carcases (N = 5 and N = 6, respectively), and Portugal reported a low number of isolates from pig carcases (N = 34). Where azithromycin resistance was detected among isolates from each of the carcase origins, MDR was not a feature.
Tigecycline resistance was not detected in Salmonella isolates from calf carcases and considering all MSs, low/very low levels were noted in isolates from carcases of pigs, broilers and turkeys (1.4%, 1.9% and 0.6%, respectively). Where countries reported resistance to this antimicrobial, generally low/very low levels were observed, with the exception of Portugal which reported a moderate level of resistance to tigecycline (16.7%) in 1/6 isolates recovered from broiler carcases; however, the small sample size should be considered when interpreting this result. Similarly, the Netherlands reported a moderate level of resistance to tigecycline (19%) in 4/21 isolates recovered from broiler carcases. Excluding pig carcases (where 53.8% of tigecycline‐resistant isolates exhibited MDR), all tigecycline‐resistant isolates from broiler and turkey carcases were multiresistant (n = 17 and n = 2, respectively).
Overall, colistin resistance was reported at low levels among isolates from turkey carcases and calf carcases (2.5% and 3.7%, respectively), and at very low levels in isolates from pig carcases and broilers (0.6% and 1%, respectively). Where countries reported resistance to this antimicrobial among isolates from the carcase origins, generally very low or low levels were noted, however, there were a few exceptions. A moderate level of resistance at 12.9% was noted by Germany (N = 31) in pig carcases, as well as a moderate level (16.7%) reported by Portugal (N = 6) in broiler carcases. Additionally, a high level (38.5%) was reported by Romania (N = 13) in turkey carcases, as well as a very high level (60%) noted by Denmark (N = 5) in calf carcases. Notably, some of these countries provided data for a very low number of isolates, therefore results may be subject to variation.
Figure 4 summarises the overall resistance to selected antimicrobials, as well as combined ‘microbiological’ resistance to cefotaxime and ciprofloxacin within the four carcase origins.
Complete susceptibility and MDR
The levels of MDR, defined as resistance to three or more antimicrobial classes, among Salmonella isolates from carcases of these food‐producing animals are shown in Figure 5. Overall, MDR was observed at high levels in Salmonella spp. recovered from carcases of pigs, broilers and calves (47.4%, 32.7% and 22%, respectively), and at a moderate level in Salmonella isolates recovered from turkey carcases (15.1%). Considering only countries where 10 or more isolates were assessed, MDR among isolates recovered from pig carcases ranged from moderate in Slovakia, Hungary and Malta (10.5%, 15.8% and 17.6%, respectively) to extremely high in Spain (75.6%). Although an extremely high level (77.8%) of MDR was noted in isolates from calf carcases by Croatia, only nine isolates were submitted for assessment; moderate levels of 13.6% and 18.8% were reported in isolates from calf carcases by Spain and France, respectively. In poultry carcases and where 10 or more isolates were submitted for analysis, MDR among isolates from broiler carcases ranged from not detected in the UK to extremely high in Austria and Slovenia (87.3% and 90.9%, respectively), and among isolates from turkey carcases between 0% in Romania to 52.9% in Poland.
Figure 5.
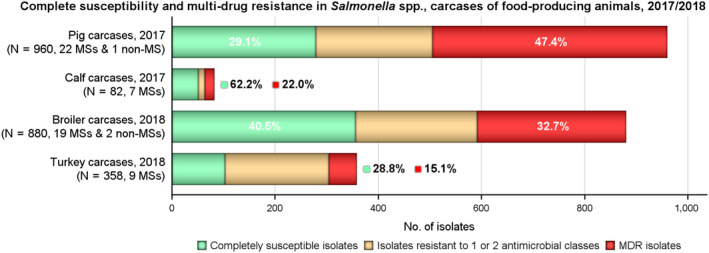
MDR and completely susceptible Salmonella spp. recovered from carcases of pigs (fatteners), calves (under 1 year of age), broilers and fattening turkeys, for all reporting countries (including 1 non‐MS in pig carcases and 2 non‐MSs in broiler carcases) in 2017/2018
- MDR and complete susceptibility levels are also expressed as a percentage; N: total number of Salmonella spp. reported by MSs and non‐MSs.
The levels of complete susceptibility (defined as susceptibility to all of the 14 antimicrobials tested in the harmonised panel) also varied between reporting countries within most of the carcase origins (Figures 6 and 7). Considering countries reporting data for ten or more Salmonella isolates, complete susceptibility among isolates recovered from pig carcases ranged from 7.2% in Spain to 68.4% in Hungary and 78.9% in Slovakia. In calf carcases, only two countries reported data on ten or more Salmonella isolates, with complete susceptibility ranging from high in France (50%) to extremely high in Spain (75%). Considering countries reporting data from poultry carcases and where ten or more isolates were submitted for analysis, the proportion of completely susceptible isolates from broiler carcases ranged from not detected in Greece and Slovenia to extremely high in the Czech Republic and the UK (71.4% and 99%, respectively), and for turkey carcases between 0% in Spain and Romania to 35.6% in France. Differences in the prevalence of particular serovars and phage types of Salmonella in different countries and animal populations, and their associated patterns of resistance are likely to explain some of the differences in the levels of MDR and complete susceptibility. The proportions of isolates which were completely susceptible and MDR among particular Salmonella serovars within the carcases origins are presented in Annex B.
Figure 6.
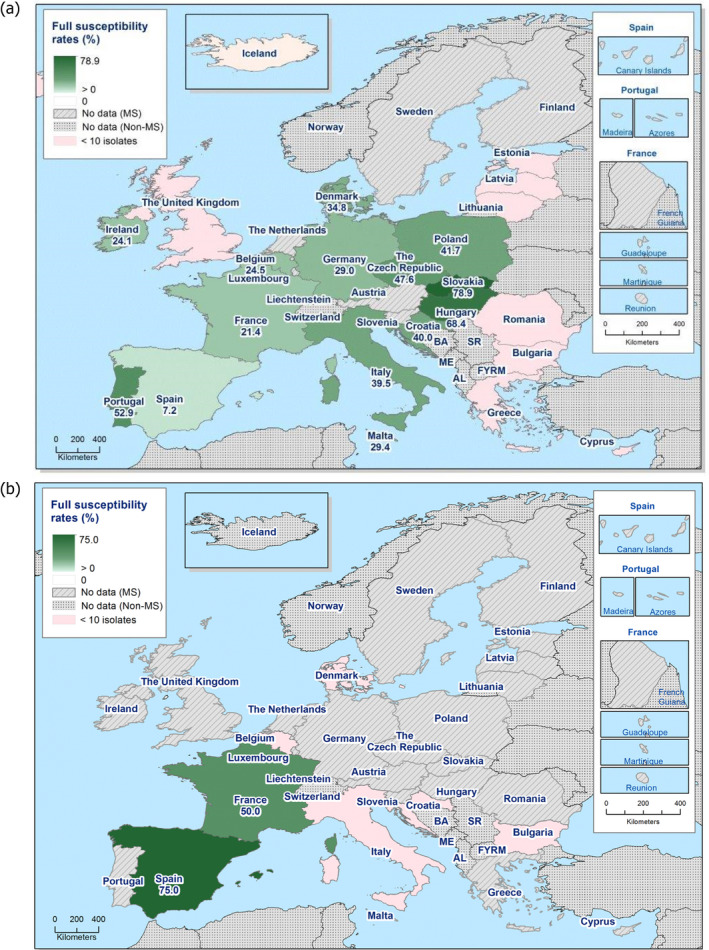
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) fattening pig carcases and (b) calf carcases (less than 1 year of age), using harmonised ECOFFs, 2017
Figure 7.
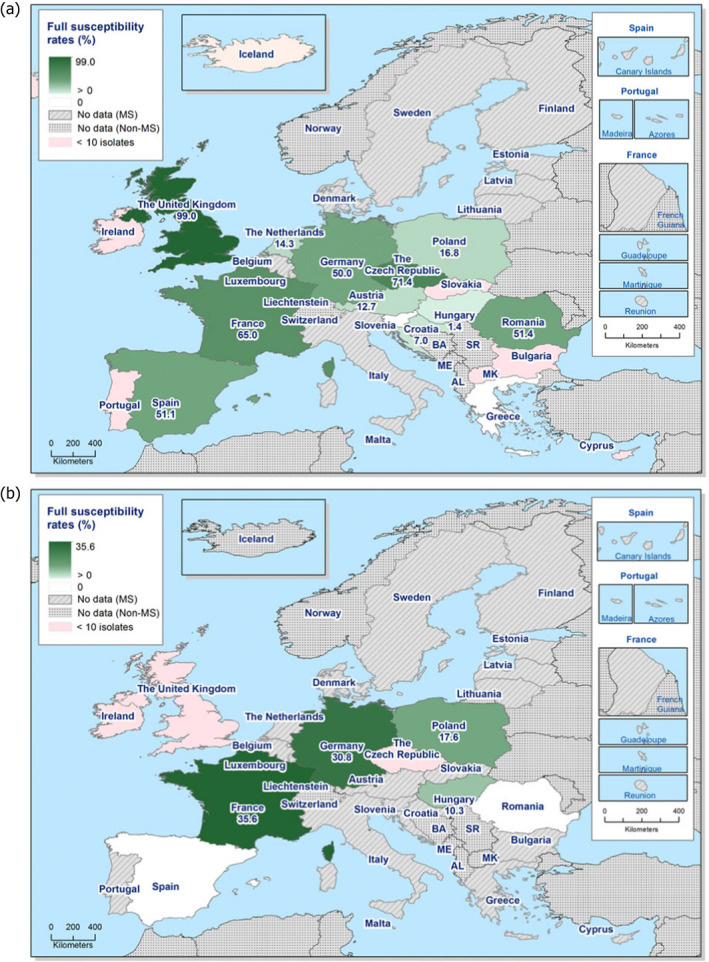
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) broiler carcases and (b) fattening turkey carcases, using harmonised ECOFFs, 2018
2.3.2. Resistance in Salmonella spp. from food‐producing animals
Occurrence of resistance to commonly/formerly used antimicrobials in veterinary medicine
Among Salmonella spp. recovered from fattening pigs in 2017, as well as flocks of broilers and fattening turkeys in 2018, most MSs reported moderate or high to extremely high resistance to tetracyclines and sulfonamides. Among isolates recovered from cattle in 2017, 4/7 and 3/7 MSs recorded no resistance to tetracycline and sulfamethoxazole, respectively. Resistance to these antimicrobials were generally observed at lower levels among laying hen flocks than broiler flocks in 2018, with most MSs registering low to high levels of resistance which did not exceed 37% in flocks of laying hens. Considering reporting MSs, resistance levels to ampicillin were generally observed at similar or slightly lower levels to those of tetracycline and sulfamethoxazole within all food‐producing animal origins; and overall resistance levels to these antimicrobials were highest in isolates from pigs and turkeys (Figure 8). While an overall high level of resistance to chloramphenicol was noted in isolates from cattle (22.7%), a moderate level was noted in isolates from pigs (14.6%) and overall low levels were reported in isolates from broilers, laying hens and turkeys (2.1%, 1.4% and 3.7%, respectively). Overall, resistance to gentamicin was noted at similarly low levels in isolates from pigs, broilers, laying hens and turkeys (5.9%, 2.4%, 1.1% and 7.2%, respectively); while an overall moderate level was observed in isolates from cattle (10.9%).
Figure 8.
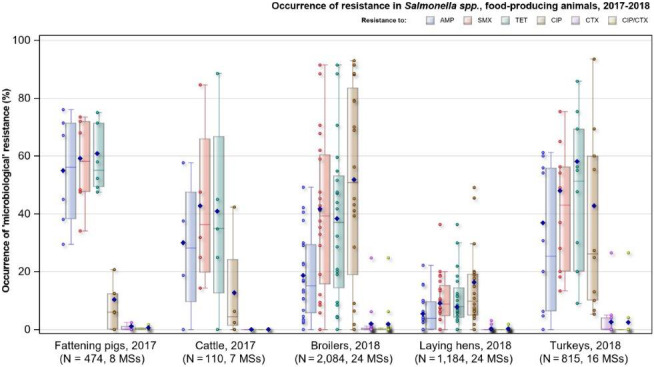
Occurrence of resistance to selected antimicrobials in Salmonella spp. from fattening pigs, cattle, broilers, laying hens and fattening turkeys, reporting EU MSs, 2017/2018
-
AMP: ampicillin, SMX: sulfamethoxazole, TET: tetracycline, CIP: ciprofloxacin, CTX: cefotaxime, CIP/CTX: combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime, N: total number of Salmonella spp. reported by MSs. Blue diamond shows resistance at the reporting‐MS group level.Note: Member States reporting at least 10 isolates are shown in the graph; all isolates are included in the calculation of resistance at the reporting‐MS group level.
Occurrence of resistance to ‘critically important antimicrobials’
Azithromycin resistance among Salmonella isolates from pigs and cattle, as well as flocks of broilers and turkeys was either not detected or observed at very low/low levels by reporting countries, resulting in overall low/very low levels considering all reporting MSs (2.5%, 1.8%, 0.3% and 0.5%, respectively). Resistance to azithromycin was not detected in Salmonella spp. recovered from laying hen flocks, and where resistance was detected among isolates from the other animal origins, MDR was not a feature.
Overall, tigecycline resistance was reported at low levels among isolates from pigs, broilers and turkeys (1.5%, 2.6% and 4.8%, respectively), and at very low levels in isolates from cattle and laying hens (0.9% and 0.3%, respectively). Where countries reported resistance to this antimicrobial among isolates from pigs, cattle and laying hens, very low or low levels were noted. However, among isolates from broilers, moderate levels of resistance at 14.3% and 10.1% were noted by the Netherlands (N = 7) and Slovenia (N = 129), respectively; and a high level of 25.7% was reported by Cyprus (N = 35). Similarly, moderate/high levels of resistance at 20% and 20.6% were reported from turkey isolates by Slovakia (N = 5) and Hungary (N = 170), respectively. Notably, some of these countries provided data for a very low number of isolates from broilers or turkeys, therefore results may be subject to variation. Where tigecycline‐resistant isolates were detected within the animal origins, the majority of isolates exhibited MDR (among tigecycline‐resistant isolates were reported at levels of 66.7% in laying hens, 98.1% in broilers, and 100% in pigs, cattle and turkeys; although the total number of tigecycline‐resistant isolates reported from some origins was very low).
Considering all reporting MSs, colistin resistance was reported at overall low levels among isolates from turkeys, broilers, pigs and laying hens (1.5%, 1.8%, 1.9% and 8.1%, respectively); while an overall moderate level was noted among isolates from cattle (14.5%). Estonia, Sweden and the Netherlands (N = 4, N = 4 and N = 40, respectively) were the only countries to report colistin resistance among cattle isolates at high levels of 25%, 25% and 35%, respectively. Where countries reported resistance to this antimicrobial among isolates from the other animal origins, generally very low or low levels were noted, however, there were a few exceptions. Moderate levels of resistance were noted by Estonia in pigs (14.3%, N = 7), by the Czech Republic in broilers (10.3%, N = 116) and by Austria in turkeys (13.3%, N = 15), as well as moderate levels of 15% and 17.6% noted by Austria (N = 40) and Bulgaria (N = 34), respectively, in laying hens. Additionally, high levels of resistance were reported among isolates from laying hens by Germany (29.6%, N = 108) and the Netherlands (26.7%, N = 15). Notably, some of these countries provided data for a very low number of isolates, therefore results may be subject to variation.
Overall, very high/high levels of resistance to ciprofloxacin and nalidixic acid were observed in Salmonella spp. from broilers (51.8% and 48.8%, respectively) and turkeys (42.7% and 33.7%, respectively), compared with moderate levels recorded in Salmonella isolates from laying hens (16.2% and 14.9%, respectively), and moderate/low levels reported in isolates from pigs (10.3% and 6.3%, respectively) and cattle (12.7% and 10%, respectively) – see Figure 8. Salmonella isolates exhibiting ciprofloxacin resistance and nalidixic acid susceptibility were evident, possibly indicating the occurrence of PMQR mechanisms. This was particularly apparent among 39 S. Newport isolates reported from turkeys by Hungary, where all isolates displayed ciprofloxacin resistance, yet only 23/39 showed resistance to nalidixic acid. Similarly, 14/15 S. Livingstone isolates reported from broilers by Greece displayed ciprofloxacin resistance, yet only 5/15 isolates showed nalidixic acid resistance. The findings were therefore similar for ciprofloxacin and nalidixic acid resistance in Salmonella spp. from turkeys and broilers to those observed in isolates from their derived carcases.
Quinolone/fluoroquinolone (i.e. nalidixic acid/ciprofloxacin) resistance in Salmonella usually arises due to point mutations within the DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) genes, at locations comprising the quinolone resistance‐determining regions (QRDR) of the bacterial chromosome. Additionally, PMQR mechanisms have also been recognised, including the action of efflux pumps (qepA and oqxAB genes), enzymatic modifications (aac(6′)Ib‐cr gene – also conferring resistance to kanamycin), and protection of the DNA gyrase (qnrA, qnrB, qnrC, qnrD, qnrS and qnrVC genes) (Li et al., 2013; Luk‐In et al., 2017).
The CBP for ciprofloxacin in Salmonella has been lowered by EUCAST from > 1 mg/L to > 0.06 mg/L, resulting in the CBP and ECOFF (microbiological breakpoint) for ciprofloxacin applying the same threshold (MIC > 0.064 mg/L). The presence of two‐point mutations in the QRDR will usually confer resistance to ciprofloxacin, with isolates typically exhibiting MICs of > 1 mg/L, as well as conferring resistance to nalidixic acid. In contrast, isolates harbouring only one‐point mutation in the QRDR will usually still display resistance to ciprofloxacin and nalidixic acid, but the degree of resistance to ciprofloxacin is reduced (MIC > 0.064 mg/L). Salmonella isolates causing systemic infections in humans and displaying MICs of > 0.064 mg/L but < 1 mg/L, have shown a poor response to treatment in some studies. This provides the rationale for setting the CBP at > 0.064 mg/L and it follows that monitoring of low‐level resistance to this compound is therefore indicated.
In the absence of other fluoroquinolone resistance mechanisms, the presence of PMQR determinants (i.e. primarily qnr genes) in a bacterium usually confers resistance to ciprofloxacin, with an MIC of > 0.064 mg/L, but the isolate remains susceptible to nalidixic acid. This contrasts with mutation in the QRDR regions of the bacterial chromosome, which confer resistance to both ciprofloxacin and nalidixic acid.
Resistance to cefotaxime and ceftazidime in Salmonella isolates from these animal origins was either not discerned or detected at very low/low levels by reporting MSs (Figure 8), although there were a few exceptions. Among isolates from broilers, high levels of 24.8% were noted by Italy (N = 121), as well as moderate levels of 14.3% noted by the Netherlands which reported data on a low number of isolates (N = 7). Similarly, Italy (N = 49) reported high levels of third‐generation cephalosporin resistance at 26.5% from turkey isolates. The Republic of North Macedonia also reported resistance to third‐generation cephalosporins in 1/9 isolates from laying hens, resulting in moderate levels of resistance at 11.1%. No resistance to third‐generation cephalosporins was detected in cattle, consistent with the result obtained for Salmonella spp. from calf carcases; however, only 110 cattle isolates were obtained by 7 MSs in 2017, which was considerably lower than the total number of isolates reported for the other animal sectors. Sections 2.3.5 and 5 provide further information on the phenotypic characterisation of third‐generation cephalosporin resistance among Salmonella isolates from the animal origins.
Where MSs reported combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime in Salmonella isolates from pigs or broilers, laying hens or turkeys, this was observed at very low or low levels, although the same exceptions as above were noted. Among isolates from broilers, a high level of 24.8% was reported by Italy (30/121 isolates), as well as a moderate level of 14.3% reported by the Netherlands (1/7 isolates). The Republic of North Macedonia also reported a moderate level of combined ‘microbiological’ resistance to these compounds in 1/9 isolates from laying hens (11.1%). Among isolates from turkeys, Italy again reported a high level of combined ‘microbiological’ resistance to these antimicrobials at 26.5% (13/49 isolates). Nevertheless, when ciprofloxacin and cefotaxime resistance was interpreted using CBPs, only five isolates recovered from broilers (four from Malta and one from the Netherlands) and one from laying hens (Hungary) displayed ‘clinical’ resistance; these were all S. Kentucky (5 isolates from broilers and 1 isolate from laying hens). Combined ‘clinical’ resistance to these antimicrobials was not observed in the other isolates from pigs or turkeys.
Complete susceptibility and MDR
The levels of MDR and complete susceptibility among Salmonella isolates recovered from these food‐producing animals are shown in Figure 9. Overall, MDR was observed at a very high level in Salmonella spp. from pigs (51.3%), at high levels in isolates from turkeys, broilers and cattle (38.8%, 38.2% and 29.5%, respectively), and at a low level in isolates from laying hens (6.5%). Considering only countries where ten or more isolates were assessed, MDR among isolates recovered from pigs ranged from 27.3% in Denmark to 69.4% in Germany. Among isolates recovered from cattle, MDR ranged from not detected in Croatia to very high in Italy (61.5%). In poultry and where 10 or more isolates were submitted for analysis, MDR among isolates from broilers ranged from not detected in Ireland to 91.4% in Cyprus, and among isolates from turkeys between 4.7% in the UK to 68.8% in Hungary. Generally, MDR among isolates from laying hens spanned much lower levels; from not detected in Bulgaria, Denmark, Greece and the Netherlands to 36.4% in Slovenia.
Figure 9.
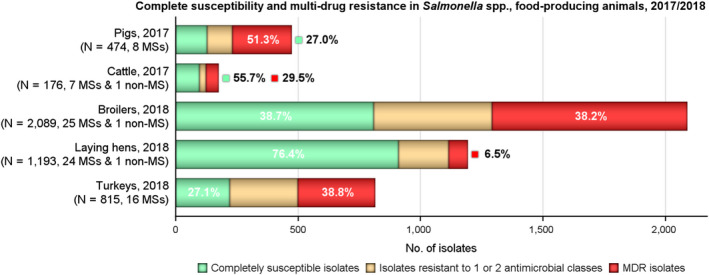
MDR and completely susceptible Salmonella spp. recovered from fattening pigs, cattle, broilers, laying hens and fattening turkeys, all reporting countries, 2017/2018
- MDR and complete susceptibility are expressed as percentages; N: total number of Salmonella spp. reported by MSs and non‐MSs.
Considering the proportions of isolates exhibiting susceptibility to all of the 14 antimicrobials tested in the harmonised panel, there was also a wide variation in the levels of complete susceptibility among the animal origins. Overall, 76.4%, 55.7%, 38.7%, 27.1% and 27% of the isolates reported from laying hens, cattle, broilers, turkeys and pigs, respectively, were completely susceptible (Figure 9). Furthermore, the levels of complete susceptibility varied widely between reporting countries for each of the animal populations monitored (Figures 10 and 11). Considering countries reporting data for ten or more Salmonella isolates, complete susceptibility among isolates recovered from pigs ranged from 11% in Spain to 50% in Croatia. Among those isolates recovered from cattle, complete susceptibility ranged from low in Italy (7.7%) to extremely high in Spain and Croatia (75% and 85.7%, respectively). Considering countries reporting data for poultry and where 10 or more isolates were submitted for analysis, the proportion of completely susceptible isolates from broilers ranged from 6.2% in Slovenia to 90.9% in Ireland, and for turkeys between 4.7% in Hungary to 70% in the Czech Republic. Generally, complete susceptibility spanned higher levels among isolates from laying hens; ranging from 46.4% in Italy to 94.8% in France. However, as mentioned previously, the prevalence of particular serovars in different countries and animal populations, and their associated patterns of resistance, may account for the differences in the levels of MDR and complete susceptibility among Salmonella spp. data. Notably in laying hens, S. Enteritidis predominated (accounting for 30.6% of Salmonella isolates recovered from this poultry origin) with 83.3% of isolates exhibiting complete susceptibility. The proportions of isolates which were completely susceptible and MDR among particular Salmonella serovars within the animal origins are presented in Annex B.
Figure 10.
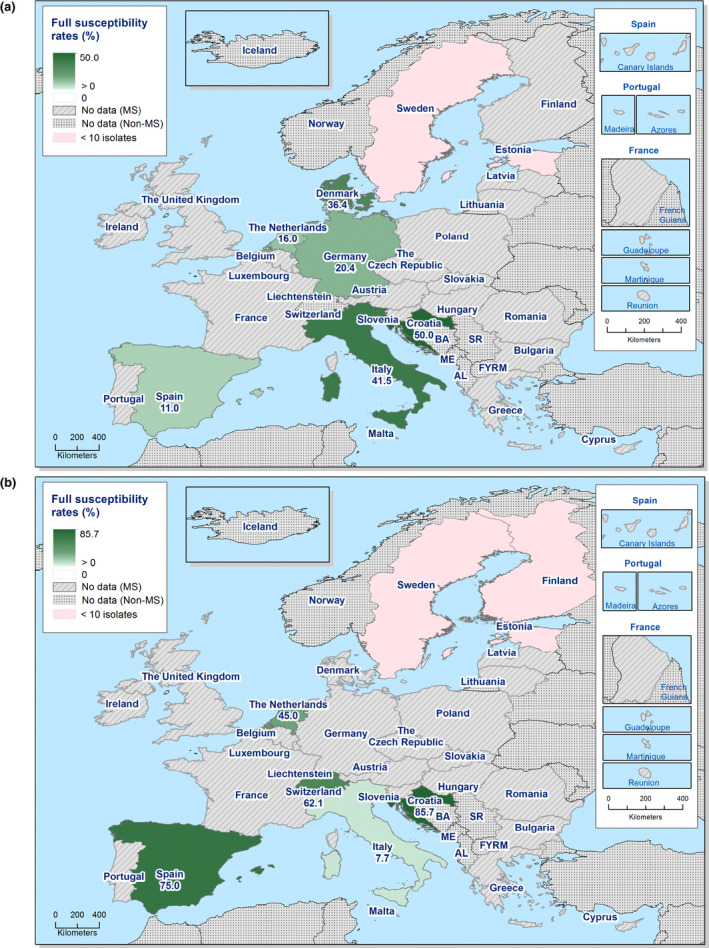
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) fattening pigs and (b) cattle, using harmonised ECOFFs, 2017
Figure 11.
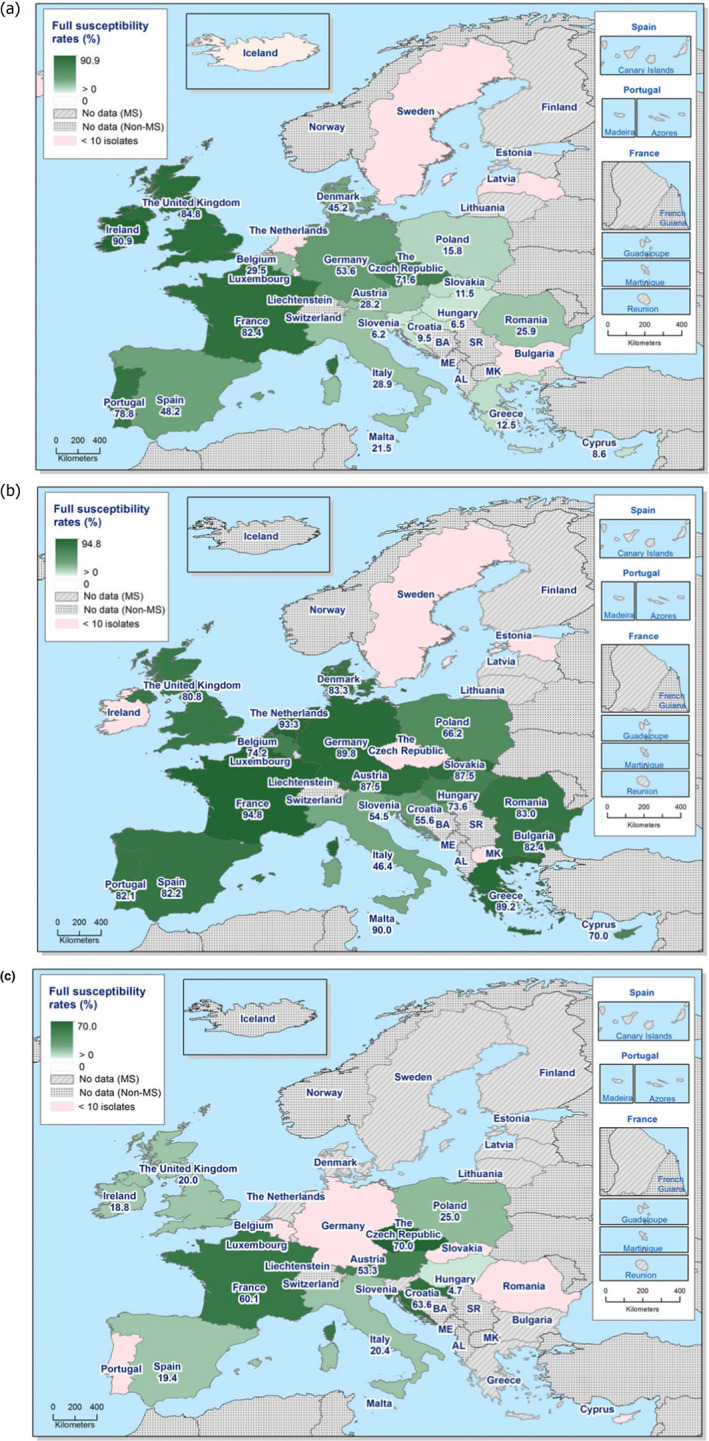
Spatial distributions of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from (a) broilers, (b) laying hens and (c) fattening turkeys, using harmonised ECOFFs, 2018
2.3.3. High‐level resistance to ciprofloxacin (CIP) in Salmonella spp.
High‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) was not observed in Salmonella spp. recovered from pig carcases or calf carcases, or from pigs or cattle. Considering the total number of Salmonella isolates monitored from the different types of poultry by MSs in 2018, the highest proportions of isolates displaying ciprofloxacin MICs of ≥ 4 mg/L were noted in broilers and turkeys, with levels of 1.3% (15/1,184), 2.5% (9/358), 5.6% (49/873), 6% (125/2,084) and 6.6% (54/815) reported from laying hens, turkey carcases, broiler carcases, broilers and turkeys, respectively.
Among Salmonella isolates displaying ciprofloxacin resistance, 49/449 (10.9%) isolates from broiler carcases and 9/116 (7.8%) isolates from turkey carcases exhibited MIC ≥ 4 mg/L. Considering the total number of CIP‐resistant isolates reported by MSs from flocks of broilers (n = 1,080), laying hens (n = 192) and turkeys (n = 348), most Salmonella isolates displaying high‐level ciprofloxacin resistance originated from broilers and turkeys (125 and 54 isolates, corresponding to levels of 11.6% and 15.5%, respectively). A lower proportion of CIP‐resistant isolates displayed MICs of ≥4 mg/L from laying hens (15/192 CIP‐resistant isolates, 7.8%).
The distribution of CIP‐resistant isolates displaying levels of ‘microbiological’ resistance or ‘clinical’ resistance or high‐level resistance to ciprofloxacin within each of the animal/carcase categories is illustrated in Figure 12. Notably, the distribution of MICs is provided only for CIP‐resistant isolates; the total number of Salmonella isolates monitored is provided in the legend.
Figure 12.
Distribution of MIC levels among ciprofloxacin‐resistant Salmonella spp. from carcases of pigs, calves, broilers and turkeys, as well as fattening pigs, cattle, broilers, laying hens and fattening turkeys, for all reporting EU MSs, 2017/2018
- n: Total number of Salmonella spp. exhibiting CIP resistance (MSs only); N: total number of Salmonella spp. reported by MSs.
- 1In accordance with breakpoints stated in Decision 2013/652/EU.
- The proportion of isolates showing high‐level resistance is not included with those exhibiting ‘clinical’ or ‘microbiological’ resistance; similarly, the proportion of isolates showing ‘clinical’ resistance is not included with those displaying ‘microbiological’ resistance. The Figure above excludes one isolate reported from laying hens (by the Republic of North Macedonia), which was ‘microbiologically’ resistant to ciprofloxacin.
The serovars which displayed high‐level resistance to fluoroquinolones are of interest from both epidemiological and public/animal health perspectives. A complementary analysis on the high‐level resistance to ciprofloxacin in S . Kentucky and other Salmonella serovars is presented in Appendix A and Annex B.
2.3.4. Tigecycline and colistin resistance in Salmonella serovars
Tigecycline resistance in Salmonella serovars
The World Health Organization also recognises tigecycline as a CIA (WHO, 2019). Although tigecycline is not recommended for use in pregnant women or children, this CIA may be considered as a last resort for the treatment of serious infection in adults caused by MDR bacteria.
Considering tigecycline resistance among the animal/carcase origins, certain serovars displayed ‘microbiological’ resistance (MIC > 1 mg/L – see Annex A, ‘Materials and methods’), which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars. Figure 13 shows the number of tigecycline‐resistant isolates where detected from the animal/carcase origins by reporting MSs, and the predominant serovars accounting for this resistance. More than half (57.1%) of the tigecycline‐resistant isolates recovered from pigs were S. Typhimurium, while S. Rissen accounted for more than half (53.8%) of those recovered from pig carcases. Serovar Infantis accounted for most of the resistant isolates recovered from broilers and their derived carcases (85.2% and 88.2%, respectively), while S. Bredeney accounted for most/all of the tigecycline‐resistant isolates recovered from turkeys and their derived carcases (71.8% and 100%, respectively). Additionally, S. Infantis accounted for all tigecycline‐resistant isolates from laying hens (100%), although only three resistant isolates were reported in total.
Figure 13.
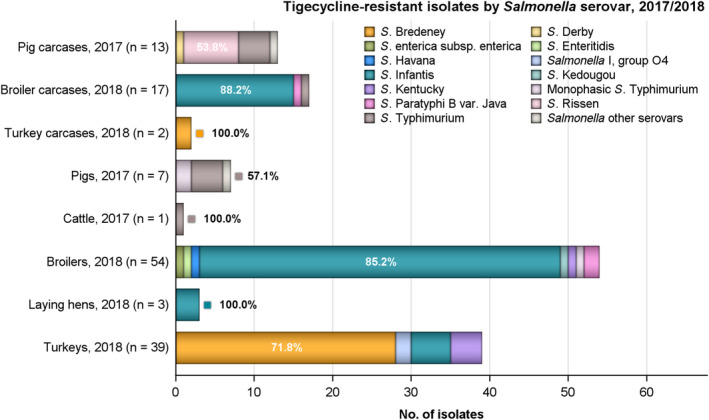
Breakdown of the number of tigecycline‐resistant isolates by serovar, where detected among the animal/carcase origins by reporting MSs in 2017/2018
- n: Total number of tigecycline‐resistant isolates reported by the MSs; predominant serovars are also expressed as a percentage.
- Note: No tigecycline‐resistant isolates were reported among Salmonella spp. from calf carcases (N = 82, 7 MSs).
Where tigecycline resistance was reported among certain serovars within the carcase/animal origins, MDR was often a feature (with the exception of S. Rissen in pig carcases). For instance, among broilers and their derived carcases, all tigecycline‐resistant S. Infantis isolates (n = 46 and n = 15, respectively) were multiresistant, with ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline resistance being a feature of all MDR isolates; a pattern typical of recent MDR broiler clones of S. Infantis (Nógrády et al., 2012). Among turkeys and their derived carcases, all tigecycline‐resistant S. Bredeney isolates (n = 28 and n = 2, respectively) were multiresistant, with ampicillin, nalidixic acid and tetracycline resistance being a feature of all MDR isolates. Similarly, among pigs, all tigecycline‐resistant S. Typhimurium isolates (n = 4) were MDR, of which all showed resistance to ampicillin, sulfamethoxazole, trimethoprim and tetracycline. Conversely, MDR among the tigecycline‐resistant S. Rissen isolates recovered from pig carcases (n = 7) was not a common feature, where only 28.6% exhibited MDR (a single isolate from Spain and 1/6 isolates from France).
Considering individual countries reporting tigecycline resistance, certain features relating to resistance were also evident. For example, Germany reported four of the seven resistant isolates recovered from pigs, while France reported seven of the 13 resistant isolates recovered from pig carcases. Additionally, 35/39 tigecycline‐resistant isolates recovered from turkeys and 14/54 from broilers were reported by Hungary and Belgium, respectively. Notably, where tigecycline‐resistant isolates were detected among the carcase/animal origins, most displayed MICs just above the ECOFF of > 1 mg/L, with only a small proportion of isolates displaying ‘clinical’ resistance (MIC > 2 mg/L).
Tigecycline is structurally related to the tetracycline class of antibiotics and is active against Gram‐positive and Gram‐negative bacteria, as well as tetracycline‐resistant bacteria and some anaerobes (WHO, 2006). In a recent study, two transferable plasmid‐mediated tigecycline resistance genes, tet (X3) and tet (X4), were reported in numerous Enterobacteriaceae and Acinetobacter that were isolated from animals and meat (chicken and pork) in China, as well as from hospital patients from different cities around the country (He et al., 2019). Both genes were reported to confer clinically significant levels of tigecycline resistance, with isolates displaying MICs of ≥ 32 mg/L. Furthermore, in a subsequent investigation carried out by Bai et al. (2019), seven tet(X4) positive E. coli isolates were identified from retail pork samples in China (Bai et al., 2019). These isolates were all MDR and displayed tigecycline MICs ranging from 16 to 32 mg/L. The tet(X4) gene conferring such resistance in these isolates was located on various conjugative plasmids of diverse replicon types, indicating that the gene may be captured by a range of mobile genetic elements circulating among bacterial strains. The authors also comment that the occurrence of tet(X3) and tet(X4) in food‐producing animals could potentially lead to an increased risk of infection by strains harbouring these genes and treatment failure in humans (Bai et al., 2019).
The potential for other bacteria within the Enterobacteriaceae family (such as Salmonella) to acquire such transferable tigecycline resistance genes is therefore highlighted, and the importance of monitoring tigecycline resistance through determination of MICs or by molecular investigation such as WGS is further underlined.
Colistin resistance in Salmonella spp.
Colistin is an antimicrobial compound, belonging to the polymyxin class and considered as a highest priority CIA for the treatment of serious human infection with some Gram‐negative bacteria (WHO, 2019).
Among Salmonella isolates recovered from poultry in 2018, ‘microbiological/clinical’ resistance to colistin (MIC > 2 mg/L) was generally observed in S. Enteritidis isolates; this serovar accounting for 33.3%, 63.2% and 89.6% of the colistin‐resistant isolates recovered from broiler carcases, broilers and laying hens, respectively. A single colistin‐resistant S. Enteritidis isolate was also reported from turkeys. Considering the monitoring performed in 2017, all colistin‐resistant isolates reported from calf carcases (n = 3) and cattle (n = 16) were serotyped as S. Dublin, with the Netherlands reporting 14 of these isolates from cattle. Notably, both S. Enteritidis and S. Dublin are group D salmonellas (serogroup O9). Salmonella belonging to group D tend to show decreased susceptibility to colistin without having any known acquired or mutational colistin resistance mechanisms (Agersø et al., 2012). This is exemplified by the proportion of colistin‐resistant isolates belonging to S. Dublin and S. Enteritidis in 2017 and 2018, respectively. Figure 14 presents the number of colistin‐resistant isolates where detected from the animal/carcase origins by reporting MSs, and the predominant serovars accounting for this resistance. With the exception of S. Eastbourne and S. Napoli, the other serovars listed in Figure 14 do not belong to group D (serogroup O9). Serovars Eastbourne and Napoli are also group D salmonellas; a single colistin‐resistant S. Eastbourne isolate was recovered from a broiler carcase, and single S. Napoli isolates which displayed colistin resistance were recovered from a broiler and laying hen flock.
Figure 14.
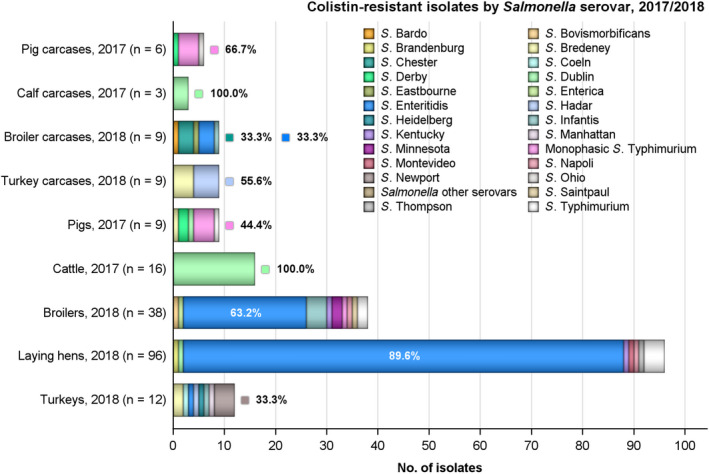
Breakdown of the number of colistin‐resistant isolates by serovar, where detected among the animal/carcase origins by reporting MSs in 2017/2018
- n: Total number of colistin‐resistant isolates reported by the MSs; predominant serovars are expressed as a percentage.
S. Newport and S. Hadar accounted for 33.3% and 55.6% of the colistin‐resistant isolates from turkeys (n = 12) and their derived carcases (n = 9), respectively; while monophasic S. Typhimurium predominated among the colistin‐resistant isolates from pigs (n = 9) and their derived carcases (n = 6), accounting for 44.4% and 66.7% of isolates from these animal/carcase origins, respectively. In addition to these colistin‐resistant monophasic S. Typhimurium isolates (pigs: 4/9; pig carcases: 4/6), two S. Derby and single isolates of S. Typhimurium, S. Bredeney and S. Dublin were reported from pigs; while the remaining colistin‐resistant isolates reported from pig carcases were attributed to single isolates of S. Derby and S. Ohio. In an Italian study, Carnevali et al. (2016) detected mcr‐1 in a number of Salmonella serovars, of which monophasic S. Typhimurium was the most frequent (isolates from pigs, pork and man) and S. Derby was the second most frequently found (isolates from pigs).
While resistance to colistin was reported in a diverse range of serovars from poultry (including serovars Bardo, Bovismorbificans, Brandenburg, Bredeney, Chester, Coeln, Heidelberg, Infantis, Kedougou, Kentucky, Manhattan, Minnesota, Montevideo, Saintpaul, Thompson, Typhimurium and its monophasic variant), no colistin‐resistant isolates reported from any of the carcase/animal origins exhibited MDR.
Considering individual serovars in which the highest colistin MICs were observed, two S. Derby isolates displayed MICs of ≥ 16 mg/L in 2017; one originated from a pig carcase in Germany (MIC > 16 mg/L) and the other from a fattening pig in Estonia (MIC 16 mg/L). From the monitoring of poultry in 2018, a single S. Bredeney isolate exhibiting a colistin MIC of 16 mg/L was reported from turkeys by France.
2.3.5. Phenotypic characterisation of third‐generation cephalosporin in Salmonella spp.11
Further phenotypic characterisation of those Salmonella isolates that exhibited resistance to third‐generation cephalosporins within each of the animal categories and for Salmonella isolates from humans (Appendix B) was performed in 2017/2018 (Table B.1). Notably, no Salmonella isolates recovered from cattle, or carcases of calves and turkeys exhibited resistance to third‐generation cephalosporins.
Table B.1.
Occurrence of resistance to third‐generation cephalosporins and fluoroquinolones in non‐typhoidal Salmonella spp. from food‐producing animals, animal carcases and humans, reported by MSs in 2017/2018
| Human/animal category | No. of MSs | N | Cefotaxime | Ceftazidime | Ciprofloxacin/pefloxacin | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Humans – 2017* | See footnote below | – | 1.9%a | – | 1.1%b | – | 13%c | |
| Humans – 2018* | – | 1.5%d | – | 1.2%e | – | 12.5%f | ||
| Pig carcases – 2017 | 22 MSs | 954 | 5 | 0.5% | 5 | 0.5% | 65 | 6.8% |
| Calf carcases (< 1 year) – 2017 | 7 MSs | 82 | 0 | 0% | 0 | 0% | 2 | 2.4% |
| Broiler carcases – 2018 | 19 MSs | 873 | 1 | 0.1% | 1 | 0.1% | 449 | 51.4% |
| Turkey carcases – 2018 | 9 MSs | 358 | 0 | 0% | 0 | 0% | 116 | 32.4% |
| Fattening pigs – 2017 | 8 MSs | 474 | 5 | 1.1% | 4 | 0.8% | 49 | 10.3% |
| Cattle – 2017 | 7 MSs | 110 | 0 | 0% | 0 | 0% | 14 | 12.7% |
| Broilers – 2018 | 25 MSs | 2,084 | 40 | 1.9% | 40 | 1.9% | 1,080 | 51.8% |
| Laying hens – 2018 | 24 MSs | 1,184 | 3 | 0.3% | 2 | 0.2% | 192 | 16.2% |
| Fattening turkeys – 2018 | 16 MSs | 815 | 21 | 2.6% | 15 | 1.8% | 348 | 42.7% |
N: Total number of isolates tested/reported by MSs; n: Total number of isolates resistant; MSs: Member states.
In several countries, ciprofloxacin has been replaced by pefloxacin for screening for fluoroquinolone resistance with disk diffusion, as recommended by EUCAST.
N = 12,580, 23 MSs.
N = 10,848, 21 MSs.
N = 14,864, 24 MSs.
N = 14,982, 22 MSs.
N = 13,667, 19 MSs.
N = 16,996, 23 MSs.
Salmonella spp. from food‐producing animals and derived carcases
Considering only isolates from the animal sector, a low number (78/6,934, 1.1% of all Salmonella recovered from all animals/carcases in 2017/2018) demonstrated third‐generation cephalosporin resistance and were subjected to the supplementary testing. Within the different animal species and production types (Table 4), the highest to lowest proportion of isolates exhibiting ESBL, AmpC or ESBL+AmpC phenotypes were: turkeys (2.6%), broilers (2.1%), pigs (1.1%), pig carcases (0.5%), laying hens (0.2%) and then broiler carcases (0.1%). Given the total number of Salmonella isolates reported by the MSs within the animal categories, the percentage of presumptive ESBL, AmpC or ESBL+AmpC producers was similar, with the ESBL phenotype more frequently detected than the AmpC phenotype among pigs, broilers and turkeys. Considering the individual MSs reporting cephalosporin‐resistant isolates from pigs and poultry and related matrices, where presumptive ESBL, AmpC or ESBL+AmpC producers were identified, they were observed at very low or low levels, although there were a few exceptions. Italy reported the highest number of isolates from both broilers and turkeys, with 30 isolates from broilers and 13 isolates from turkeys exhibiting the ESBL phenotype (24.8% and 26.5% of all isolates tested by Italy, respectively). Although only single isolates were reported to exhibit an ESBL phenotype in broilers by the Netherlands and an ESBL+AmpC phenotype in broiler carcases by Portugal, moderate levels were observed at the MS level (14.3% and 16.7%, respectively) due to a low number of isolates tested (N = 7 and N = 6, respectively). Similarly, only two Salmonella isolates were reported in total from pig carcases by Lithuania, with one identified as a presumptive AmpC‐producer (50% of all isolates tested by this MS).
Table 4.
Summary of phenotypic characterisation of third‐generation cephalosporin resistance in non‐typhoidal Salmonella spp. from food‐producing animals, animal carcases and humans, reported in 2017/2018
| Matrix | Presumptive ESBL and/or AmpC producersa n (%R) | Presumptive ESBL producersb n (%R) | Presumptive AmpC producersc n (%R) | Presumptive ESBL + AmpC producersd n (%R) |
|---|---|---|---|---|
| Humans ‐ 2017 (N = 8,018, 12 MSs)* | 77 (1.0) | 62 (0.8) | 12 (0.1) | 3 (0.04) |
| Humans ‐ 2018 (N = 9,894, 15 MSs) | 91 (0.9) | 75 (0.8) | 16 (0.2) | 0 (0) |
| Pig carcases (N = 954, 22 MSs) | 5 (0.5) | 2 (0.2) | 3 (0.3) | 0 (0) |
| Broiler carcases (N = 873, 19 MSs) | 1 (0.1) | 1 (0.1) | 1 (0.1) | 1 (0.1) |
| Fattening pigs (N = 474, 8 MSs) | 5 (1.1) | 4 (0.8) | 1 (0.2) | 0 (0) |
| Broilers (N = 2,084, 25 MSs) | 43 (2.1) | 40 (1.9) | 9 (0.4) | 6 (0.3) |
| Laying hens (N = 1,184, 24 MSs) | 2 (0.2) | 1 (0.1) | 1 (0.1) | 0 (0) |
| Fattening turkeys (N = 815, 16 MSs) | 21 (2.6) | 21 (2.6) | 3 (0.4) | 3 (0.4) |
N: Total number of isolates reported by the MSs; n: number of the isolates resistant; %R: percentage of resistant isolates; ESBL: extended‐spectrum β‐lactamase.
For humans (2017), the total number of isolates exhibiting a combined ESBL+AmpC phenotype(d) are not included within the total number of presumptive ESBL producers(b) or the total number of presumptive AmpC producers(c).
Isolates exhibiting only ESBL‐ and/or only AmpC‐ and/or combined ESBL+AmpC phenotype.
Isolates exhibiting an ESBL‐ and/or combined ESBL+AmpC‐phenotype.
Isolates exhibiting an AmpC and/or combined ESBL+AmpC‐phenotype.
Isolates exhibiting a combined ESBL+AmpC phenotype.
Salmonella serovars from food producing animals and carcases
When assessing the 2017 data by serovar, the ESBL or AmpC phenotype was detected in six serovars among porcine isolates, these being: S. Derby, S. Bredeney, S. Rissen, S. Kapemba, S. Typhimurium and its monophasic variant. Among pig carcases, two Salmonella isolates displayed an ESBL phenotype (one S. Derby from Germany and one S. Rissen from Spain), and three displayed an AmpC phenotype (three S. Bredeney from Lithuania, Portugal and Spain). In pigs, four Salmonella isolates displayed an ESBL phenotype (single isolates of S. Kapemba and S. Typhimurium from Italy, and single isolates of S. Rissen and monophasic S. Typhimurium from Spain) and one displayed an AmpC phenotype (a monophasic S. Typhimurium from Italy).
Considering the 2018 data on poultry, the ESBL or AmpC phenotype was associated with certain serovars, suggesting the possible clonal expansion of particular strains: namely, S. Infantis, S. Kentucky, S. Bareilly and S. Bredeney. Among both broilers and turkeys, presumptive ESBL‐producing Salmonella were identified more frequently than presumptive AmpC‐producing Salmonella and encompassed a greater number of serovars. The ESBL phenotype was identified in four different serovars from broilers (Infantis, Kentucky, Livingstone and Rissen) and six different serovars from turkeys (Agona, Bareilly, Bredeney, Derby, Infantis and Typhimurium), while the AmpC phenotype was identified in only two different serovars from these origins (Infantis and Orion in broilers; Infantis and Derby in turkeys). Six of the AmpC‐carrying S. Infantis from broilers and two from turkeys, as well as the AmpC‐carrying S. Derby from turkeys, also expressed an ESBL phenotype. Where presumptive ESBL, AmpC or ESBL+AmpC producers were identified from broilers (43/2,084 isolates), most were attributed to S. Infantis (30 isolates reported by Italy and four by Hungary) and S. Kentucky (four isolates reported by Malta and one by the Netherlands). All 30 S. Infantis isolates reported by Italy displayed an ESBL phenotype, as well as an AmpC phenotype in six of these; while of the four S. Infantis isolates reported by Hungary, two presented with an ESBL phenotype and two with an AmpC phenotype. Conversely, only the ESBL phenotype was expressed in the five S. Kentucky isolates. Where presumptive ESBL, AmpC or ESBL+AmpC producers were identified from turkeys (21/815 isolates), most were attributed to S. Infantis (7 isolates reported by Italy), S. Bareilly (six isolates reported by Italy) and S. Bredeney (4 isolates reported by Spain). All seven S. Infantis isolates reported by Italy displayed an ESBL phenotype, as well as an AmpC phenotype in two of these; while the six S. Bareilly and four S. Bredeney (reported by Italy and Spain, respectively) presented an ESBL phenotype only. Among laying hens, a single S. Infantis isolate reported by Italy was also identified as a presumptive ESBL‐producer, and a single S. Kentucky isolate reported by Hungary was identified as a presumptive AmpC‐producer. Additionally, both the ESBL and AmpC phenotype were detected in a single S. Paratyphi B var. Java isolate reported from a broiler carcase by Portugal.
2.3.6. Carbapenem resistance in Salmonella spp. from food‐producing animals and carcases
Resistance to meropenem was not detected in Salmonella spp. recovered from pigs or cattle, or derived carcases from these species, in 2017. Similarly, none of the Salmonella isolates recovered from any of the poultry origins were ‘microbiologically’ resistant to meropenem in 2018.
2.3.7. Resistance exhibited by dominant Salmonella serovars
The detailed reporting of results at the serovar level clearly demonstrated the major contribution of a few serovars to the observed overall occurrence of resistance when considering aggregated data for Salmonella spp. The patterns of resistance associated with these different serovars have a marked influence on the overall resistance levels in Salmonella spp., as the proportion of completely susceptible and MDR isolates may vary significantly among particular serovars recovered from each of the carcase origins/food‐producing animal populations studied. The analysis of antimicrobial resistance at the serovar level is presented in Appendix C.
2.4. Comparing resistance in Salmonella from humans and food‐producing animals
A further comparison of human Salmonella data by serovar to that in food‐producing animals for the years 2017/2018 was performed and is detailed in Appendix D. Comparable AMR data are presented for serovars S. Typhimurium and its monophasic variant, S. Derby, S. Infantis, S. Enteritidis and S. Kentucky, and are discussed in this corresponding Appendix. The prevalence of particular Salmonella serovars within countries and animal populations, and their associated patterns of resistance, may explain some of the observed differences in the occurrence of antimicrobial resistance and MDR. The spread of resistant clones and the presence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. However, it should be noted that relating the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated because other sources of Salmonella occur; such evaluations should be performed and interpreted taking into account the complex epidemiology of salmonellosis.
2.5. Discussion
In 2018, information on AMR in Salmonella isolates from human clinical cases was reported by 23 MSs and 1 non‐MS. This is two countries less than in 2017 as in 2018, one country focused its resources on implementing sequencing of all Salmonella isolates and the other was lacking resources for performing AST of Salmonella. Fifteen countries provided data as measured values (quantitative data) and nine as data interpreted with CBPs. In July 2018, the Commission Implementing Decision 2018/945/EU ‘on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions,’ came into force. The Decision stipulates mandatory testing and reporting of a representative subset of Salmonella isolates using methods and criteria specified in the EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates (ECDC, 2016). The Decision is expected to result in an increase in the number of reporting countries from 2019 onwards.
In 2017, AMR data for Salmonella isolates recovered from the mandatory carcase swabbing of fattening pigs and calves (less than 1 year of age) at slaughter were reported by 22 MSs and 1 non‐MS for fattening pigs and 7 MSs for calves; while in 2018, AMR data for Salmonella isolates recovered from the mandatory carcase swabbing of broilers and fattening turkeys at slaughter were reported by 19 MSs and 2 non‐MSs for broilers and 9 MSs for turkeys. Additionally in 2018, 26 MSs and 2 non‐MSs reported mandatory AMR data for Salmonella isolates recovered from flocks of broilers, laying hens and fattening turkeys (boot swabs or dust samples), in accordance with Regulation (EC) No 2160/2003 and as part of National Control Programmes (NCPs) of Salmonella in poultry. Notably, some MSs did not obtain any positive Salmonella isolates from these carcase/animal origins and, therefore, data are not presented for these countries in corresponding results. In 2017, nine MSs also reported voluntary data on Salmonella isolates recovered from caecal contents of fattening pigs and cattle at slaughter, where in general one representative sample of caecal contents was collected per epidemiological unit (i.e. the holding) to prevent clustering. The reporting of isolate‐based data enables the analysis of MDR patterns, detection of high‐level ciprofloxacin resistance, and co‐resistance to ciprofloxacin and cefotaxime; first‐line agents critically important for treating human salmonellosis. Resistance levels were also reported by serovar for the different animal/carcase origins (see Appendix C), which allows detailed analysis and, as required by Decision 2013/652/EU, all MSs included information on serovars and production type. In line with this decision, streptomycin is no longer included in the specified test panels for the monitoring and reporting of AMR in Salmonella, which has an impact on how MDR patterns are interpreted. The numbers of MSs reporting data in 2017 and 2018 from carcases and animals in the various sectors represents an increase on the numbers of reporting MSs in 2015 and 2016 for pigs, broilers, laying hens and turkeys, as well as carcases of pigs and turkeys. In 2015 and 2017, an equal number of MSs reported data on Salmonella isolates recovered from calf carcases (7 MSs), and in 2016 and 2018, an equal number of MSs reported data on Salmonella spp. from broiler carcases (19 MSs). Additionally, 3 MSs reported data on Salmonella isolates recovered from calves in 2015, while 7 MSs reported data on Salmonella spp. from cattle in 2017. MSs which have a very low prevalence or zero prevalence of Salmonella in certain sectors may of course only contribute in years when Salmonella is detected in those sectors and this may result in fluctuations to the numbers of contributing MSs.
Antimicrobials such as ampicillin, sulfamethoxazole and tetracycline have been widely used for many years in veterinary medicine to treat infections in production animals. Generally, moderate to high levels of resistance to these antimicrobials were reported by MSs from these animals and overall high levels in isolates from humans. Overall in 2017, the highest levels of resistance to ampicillin, sulfamethoxazole and tetracycline were recorded in Salmonella isolates recovered from pig carcases and fattening pigs; the lowest levels were reported in Salmonella isolates recovered from calf carcases (less than 1 year of age). Among pigs and cattle, as well as derived carcases of these species, resistance levels to ampicillin were generally observed at similar or slightly lower levels to those of tetracycline and sulfamethoxazole. This may be related to the occurrence of underlying genetic structures responsible for resistance and the proportion of Salmonella spp. carrying genetically linked resistance genes to these agents. Considering individual serovars, monophasic S. Typhimurium generally showed the highest resistance to these compounds across most of the animal/carcase origins (including pigs, cattle and derived carcases from these species, as well as flocks of broilers, laying hens and turkeys). The same observation was made in isolates from humans, where overall extremely high levels of resistance to these antimicrobials were found in monophasic S. Typhimurium and also in S. Kentucky.
Fluoroquinolones (a class represented by ciprofloxacin in the panel of tested antimicrobials) are CIAs in human medicine and consequently their use in food‐producing animals is the subject of prudent use initiatives which aim to minimise use. From the monitoring of poultry in 2018, the highest levels of resistance were generally noted to ciprofloxacin/nalidixic acid, sulfamethoxazole and tetracycline, with the exception of sulfamethoxazole resistance among turkey carcases where overall resistance to ampicillin exceeded that of sulfamethoxazole in view of all reporting MSs (16.5% and 13.7%, respectively). Considering individual serovars, Infantis and Kentucky generally showed the highest resistance to ciprofloxacin and nalidixic acid across the poultry origins, both cases reflecting likely spread of resistant clones belonging to these serovars. In humans, S. Infantis and S. Kentucky showed the highest resistance to these substances. Resistance to ciprofloxacin/nalidixic acid, sulfamethoxazole and tetracycline is typical of a clone of S. Infantis which is prevalent in Europe in broilers (Nógrády et al., 2012) and S. Infantis is a serovar commonly reported in the monitoring by some MSs. Excluding colistin resistance, overall AMR levels were much lower among isolates from laying hens compared to those from broilers and turkeys. This observation most likely reflects in part the predominance of S. Enteritidis, which accounted for 30.6% of Salmonella isolates recovered from laying hens and where 83.3% of S. Enteritidis isolates exhibited complete susceptibility. Additionally, only a limited number of antimicrobial compounds are authorised for the treatment of laying hens in many EU countries, and this factor may also be reflected in overall AMR levels in Salmonella isolates from this sector. Considering all reporting MSs, ciprofloxacin resistance was observed at higher levels among isolates from broilers and their derived carcases to those noted in turkeys and their derived carcases. Conversely, overall resistance levels to tetracycline among these origins showed the opposite finding.
Within each of the carcase origins and animal populations, overall resistance to ciprofloxacin and nalidixic acid was generally very similar. However, Salmonella isolates exhibiting ciprofloxacin resistance and nalidixic acid susceptibility were also evident, possibly indicating the occurrence of PMQR mechanisms (qepA, oqxAB, aac(6′)Ib‐cr, qnr genes). This was particularly apparent among 13 S. Hadar isolates reported from turkey carcases by Romania, where all isolates displayed ciprofloxacin resistance, yet none showed resistance to nalidixic acid. Ciprofloxacin resistance was also detected in 39 S. Newport isolates reported from turkeys by Hungary, while nalidixic acid resistance was detected in only 23 of these isolates. Similarly, 16/32 S. Rissen isolates reported from pig carcases by Spain displayed ciprofloxacin resistance, yet only 9/32 isolates showed nalidixic acid resistance.
Although high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) was not detected among Salmonella isolates from pigs or cattle, or derived carcases of these species, this was observed among isolates from poultry and their derived carcases. Considering the total number of Salmonella isolates monitored from the different types of poultry by MSs in 2018, high‐level resistance to this compound ranged from 1.3% in laying hens to 6.6% in turkeys. While many serovars (including Infantis) were noted to exhibit resistance by this definition, S. Kentucky accounted for most of the Salmonella isolates recovered from poultry which exhibited ciprofloxacin MICs of ≥ 4 mg/L (180/252). The same finding was made in isolates from humans where high‐level‐ciprofloxacin resistance was most commonly found in S. Kentucky (representing 140/180 isolates with high‐level resistance and expressed in 140/158 S. Kentucky with MIC results). S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance are likely to belong to the multilocus sequence type (ST) 198 clone, which has shown epidemic spread in North Africa and the Middle East (Le Hello et al., 2011, 2013). Notably in 2018, the occurrence of this serovar exhibiting high‐level resistance was observed by many MSs from most parts of Europe, suggesting further clonal expansion (S. Kentucky ST198‐X1) within poultry populations. Furthermore, a very high proportion of the poultry S. Kentucky isolates displaying ciprofloxacin MICs of ≥ 4 mg/L (n = 180) were also multiresistant (57.2%), primarily showing resistance to ampicillin, gentamicin, nalidixic acid, sulfamethoxazole and tetracycline (AMP‐CIP‐GEN‐NAL‐SMX‐TET). The same observation was noted among S. Kentucky isolates from humans.
‘Microbiological’ resistance to cefotaxime and ceftazidime (third‐generation cephalosporins) in Salmonella isolates recovered from the carcase origins and animal populations monitored was either not discerned, or was generally detected at very low/low levels by most of the reporting MSs. Considering the total number of Salmonella isolates recovered from all carcase/animal origins, a low number (78/6,934, 1.1%) demonstrated third‐generation cephalosporin resistance and were subjected to supplementary testing with a further panel of antimicrobials. Notably, no Salmonella isolates recovered from cattle, or carcases of calves and turkeys exhibited resistance to third‐generation cephalosporins. The supplementary testing revealed the presence of isolates with an ESBL, AmpC or combined ESBL + AmpC phenotype. Particularly among poultry isolates, the ESBL or AmpC phenotype was associated with certain serovars, suggesting the possible clonal expansion of particular strains: namely, S. Infantis, S. Kentucky, S. Bareilly and S. Bredeney. Among both broilers and turkeys, presumptive ESBL‐producing Salmonella were identified more frequently than presumptive AmpC‐producing Salmonella and encompassed a greater number of serovars. With the exception of one MS, where countries reported data on ten or more Salmonella isolates from pigs and poultry, presumptive ESBL, AmpC or ESBL+AmpC producers were identified at very low or low levels. Italy however, reported the ESBL phenotype in 24.8% of Salmonella spp. from broilers and 26.5% of Salmonella spp. from turkeys (N = 121 and N = 49, respectively). All presumptive ESBL producers identified from broilers in Italy (n = 30) were attributed to S. Infantis; six of which also possessed an AmpC phenotype. Similarly, more than half of the presumptive ESBL producers identified from turkeys in Italy (7/13) were attributed to S. Infantis; two of which also exhibited an AmpC phenotype. While Hungary also reported cephalosporin‐resistant S. Infantis among broilers (n = 4) in 2018, the proportion of presumptive ESBL/AmpC producers attributed to this serovar within broilers and turkeys in Italy suggests clonal expansion and spread among these animal populations in this country. The findings in poultry are interesting because there are no authorised products for use in the poultry sector in the EU which include third‐generation cephalosporins and off‐label use of third‐generation cephalosporins in poultry is not permitted (Franco et al., 2015). In humans, presumptive ESBL‐producing Salmonella were identified in 0.8% of the tested isolates in 2018 with the highest occurrence in Malta (6.9%) and Italy (2.6%), with S. Kentucky bla CTX‐M‐14b and S. Infantis (genotype not specified) dominating in Malta and S. Infantis bla CTX‐M‐1 dominating in Italy. AmpC was less frequent and no isolates were reported to be both AmpC‐ and ESBL‐producing. Of the 16 serovars identified with an ESBL phenotype from humans in 2018, this was most commonly reported in S. Corvallis, S. Infantis, S. Give, S. Haifa and S. Kentucky (ranging between 4.5% and 6.1%). The proportion of S. Kentucky with an ESBL phenotype decreased from 20.3% in 2017 to 4.5% in 2018, with only two countries reporting S. Kentucky with CTX‐M‐14b/CTX‐M‐9/14 in 2018. AmpC‐type β‐lactamases were reported in ten different serovars, most commonly in S. Anatum, S. Bredeney and S. Thompson (ranging between 2.4% and 3.7%).
MDR and ESBL‐producing S. Infantis
In 2018, the proportion of all Salmonella isolates showing MDR in broilers and their derived carcases was greatly influenced by the occurrence of multiresistant S. Infantis, which accounted for 79% and 75.3% of the MDR Salmonella isolates from these origins, respectively. Additionally, this serovar accounted for 15.8% and 13% of the MDR isolates in turkeys and their derived carcases, respectively. In humans, 41.8% of S. Infantis were MDR and eight countries reported S. Infantis with an ESBL phenotype. Of the seven countries reporting on genotype, only Italy reported the genotype bla CTX‐M‐1 (Table 1). All ESBL‐carrying S. Infantis were also ciprofloxacin‐resistant.
Over the last decade, multiresistant S. Infantis has increasingly been reported from food‐producing animals and humans in Italy. Subsequently, an S. Infantis clone harbouring a pESI‐like megaplasmid and carrying the ESBL gene bla CTX‐M‐1 (mediating cefotaxime resistance), as well as the resistance genes tet(A), sul1, dfrA1 and dfrA14 (conferring resistance to tetracycline, sulfamethoxazole and trimethoprim, respectively), was identified from food‐producing animals and humans in Italy (Franco et al., 2015). This MDR clone was mainly detected among the Italian broiler chicken industry, where it is thought to have disseminated through the food chain to humans (Franco et al., 2015). A proportion of the Italian isolates of MDR S. Infantis also possessed the streptomycin resistance gene aadA1.
The pESI megaplasmid (pESI=‘plasmid for emerging S. Infantis;’ Tate et al., 2017) was first reported among S. Infantis isolates from Israel; and while these isolates were susceptible to extended‐spectrum cephalosporins, this megaplasmid also conferred resistance to tetracycline, sulfamethoxazole and trimethoprim (Aviv et al., 2014).
Additionally, a S. Infantis clone harbouring the pESI‐like megaplasmid, but carrying the ESBL gene bla CTX‐M‐65, has been reported in the United States (Tate et al., 2017), as well as Switzerland (Hindermann et al., 2017). In the US, this genotype has been associated with travel to South America (Brown et al., 2018). In 2017, Luxembourg and the Netherlands reported one human case each of bla CTX‐M‐65 carrying‐S. Infantis, where travel history was unknown. In 2018, Denmark, the Netherlands and the UK together reported two domestically acquired cases of bla CTX‐M‐65 carrying‐S. Infantis, three cases with unknown travel history and five related to travel. Of the five travel‐related cases, three reported travel to Peru. The majority of the bla CTX‐M‐65 isolates were resistant to ciprofloxacin, chloramphenicol, gentamicin, sulfamethoxazole, tetracycline and trimethoprim, in addition of being ESBL‐producing.
Genotypic screening of the presumptive ESBL‐producing S. Infantis identified from broilers (n = 30) and turkeys (n = 7) in Italy also revealed the presence of CTX‐M enzymes, although type(s) were not specified.
Third‐generation cephalosporins and fluoroquinolones are highest priority CIAs for the treatment of human invasive salmonellosis (WHO, 2019), and therefore, this sets the rationale for monitoring combined resistance to these antimicrobial classes within food‐producing animal populations. Considering all reporting MSs, combined ‘microbiological’ resistance to cefotaxime and ciprofloxacin was detected at overall very low levels among Salmonella isolates recovered from pig carcases, broiler carcases, pigs and laying hens; while overall low levels were reported among isolates from broilers and turkeys. No Salmonella isolates recovered from cattle, or carcases of calves and turkeys displayed combined resistance to these antimicrobials. Notably, where cefotaxime and ciprofloxacin MICs were interpreted using CBPs, only a single isolate recovered from a broiler carcase by Portugal (S. Paratyphi B var. Java) and five isolates recovered from broilers by Malta and the Netherlands (all S. Kentucky), as well as a single isolate recovered from laying hens by Hungary (S. Kentucky) exhibited ‘clinical’ resistance to these compounds. Combined ‘clinical’ resistance to these antimicrobials was not observed among the other resistant isolates recovered from pig carcases, pigs or turkeys.
Colistin is also a highest priority CIA (WHO, 2019), considered as a last resort for the treatment of serious human infections. Although not frequently used in human medicine due to its nephrotoxic effects, colistin has been widely used in veterinary medicine for prophylactic/metaphylactic treatment (Kieffer et al., 2017). Considering the total number of Salmonella isolates reported by MSs from the carcase/animal categories, colistin resistance was detected at a very low level among isolates from pig carcases (0.6%); at low levels among isolates from broiler carcases, turkeys, pigs, broilers, turkey carcases, calf carcases and laying hens (1%, 1.5%, 1.9%, 1.8%, 2.5%, 3.7% and 8.1%, respectively); and a moderate level among isolates from cattle (14.5%). Notably, where colistin resistance was detected among isolates from each of the carcase/animal origins, MDR was not a feature. Among those isolates recovered from poultry in 2018, colistin resistance was generally observed in S. Enteritidis isolates; this serovar accounting for 33.3%, 63.2% and 89.6% of the colistin‐resistant isolates recovered from broiler carcases, broilers and laying hens, respectively. Considering the monitoring performed in 2017, all colistin‐resistant isolates reported from cattle and calf carcases were serotyped as S. Dublin. Both S. Enteritidis and S. Dublin are group D salmonellas (serogroup O9); Salmonella belonging to group D tend to show decreased susceptibility to colistin without having any known acquired or mutational colistin resistance mechanisms and, therefore, show a degree of intrinsic resistance to colistin (Agersø et al., 2012). Considering other serovars, colistin‐resistance was most frequently reported among S. Newport in turkeys (33.5%) and S. Hadar in turkey carcases (55.6%), while monophasic S. Typhimurium predominated among the colistin‐resistant isolates from pigs and their derived carcases (44.4% and 66.7%, respectively). Mechanisms of polymyxin resistance in Gram‐negative bacteria have been described (lipopolysaccharide modifications, efflux pumps, capsule formation and over‐expression of membrane protein – Olaitan et al., 2014); and transferable mobile colistin resistance (mcr) genes have also been detected in Salmonella isolates (Campos et al., 2016; Carnevali et al., 2016; Skov and Monnet, 2016). Further molecular characterisation of colistin‐resistant isolates obtained from the EU AMR monitoring, to determine the underlying genetic mechanisms, would assist in identifying the emergence and dissemination of colistin‐resistant Salmonella clones and also identify colistin resistance plasmids occurring in Salmonella associated with livestock.
Carbapenems are recognised as CIAs (WHO, 2019) and include meropenem, a compound which is specified in the antimicrobial panels for the monitoring and reporting of AMR in Salmonella spp. (as stipulated by Decision 2013/652/EU). This class of antimicrobials are not therapeutically used in food‐producing animals but are reserved for use in humans. In both 2017 and 2018, no Salmonella spp. recovered from any of the carcase/animal origins were ‘microbiologically’ resistant to meropenem. In humans, however, five Salmonella isolates from three MSs were found to carry carbapenemase genes in 2018 (detected via phenotypic screening of meropenem resistance). One case was reported to be domestically acquired in Italy: this is the first confirmed report of carbapenem resistance in Salmonella from humans not related to travel outside the EU/EEA. Information on travel status was missing for the other four cases. Four of the five cases were in elderly persons, aged 75 years or more, with isolation of the bacteria from urine or other body sites rather than stool. This could possibly indicate nosocomial transmission, i.e. transmission occurring in a hospital or healthcare setting. There are several examples from such settings where carbapenemase genes have been shared between different bacterial species within the order Enterobacterales via horizontal gene transfer within and/or between patients (Borgia et al., 2012; Torres‐González et al., 2015; Bosch et al., 2017). Carbapenemase acquisition by a Salmonella from other Enterobacterales in a healthcare context has been suggested by Ktari et al. (2015) as the cause of human infection by OXA‐48 in MDR S. Kentucky in North Africa. Additional information gathered from one of the five EU patients revealed that the individual was immunocompromised, had been treated with antibiotics 2 months prior to the onset of a urinary‐tract infection with Salmonella Rissen and routinely attended a healthcare facility (C. Lucarelli, ISS, Italy, personal communication 19 Dec 2019). Of the five carbapenemase‐producing isolates, two were S. Kentucky (OXA‐48), as well as single isolates of S. Corvallis (OXA‐48), S. Rissen (KPC) and S. Typhimurium (VIM). To note also is that in eight of 23 reporting countries, meropenem results were interpreted using the EUCAST CBP and since the CBP is much less sensitive than the ECOFF, microbiological resistance to meropenem may have existed among some isolates from these countries.
Tigecycline is also considered as a CIA (WHO, 2019), which may be considered as a last resort for the treatment of serious infections caused by MDR bacteria. Where resistance to this antimicrobial was reported among the carcase/animal origins (no tigecycline‐resistant isolates were reported from calf carcases), most isolates displayed MICs just above the ECOFF of > 1 mg/L, with only a small proportion of isolates displaying ‘clinical’ resistance (MIC > 2 mg/L). Certain serovars displayed ‘microbiological’ resistance to this antimicrobial, which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars. Although low numbers of resistant isolates were reported, more than half (57.1%) of the tigecycline‐resistant isolates recovered from pigs were S. Typhimurium, while S. Rissen accounted for more than half (53.8%) of those recovered from pig carcases. Serovar Infantis accounted for most of the resistant isolates recovered from broilers and their derived carcases (85.2% and 88.2%, respectively), as well as all of the tigecycline‐resistant isolates from laying hens (100%), although only three resistant isolates were reported in total from laying hens. Additionally, S. Bredeney accounted for most/all of the tigecycline‐resistant isolates recovered from turkeys and their derived carcases (71.8% and 100%, respectively), although again a low number of resistant isolates were reported in total from turkey carcases (n = 2). With the exception of pig carcases and laying hens (where 53.8% and 66.7% of tigecycline‐resistant isolates exhibited MDR, respectively), most/all tigecycline‐resistant isolates recovered from the other carcase/animal origins were multiresistant. Determining the susceptibility of tigecycline is not straightforward as this compound may be affected (inactivated) by oxidation and exposure to light, which may lead to falsely reported ‘microbiological’ resistance. Several mechanisms of resistance to tigecycline in Salmonella and other members of the family Enterobacteriaceae have previously been described: increased activity of efflux pumps (AcrAB), mutation of the ribosomal protein S10 and modification of the Mla system involved in phospholipid transport in cell membranes (He et al., 2016). The mechanisms of development of microbiological resistance, which may involve upregulation of normal cell pathways or processes, probably also contribute to the occurrence of a ‘tail’ of isolates on the MIC distribution with values just above the ECOFF.
MDR, defined as resistance to three or more antimicrobial classes, varied between reporting countries and among the animal/carcase origins, with overall levels ranging from 6.6% in laying hens to 51.3% in pigs. Considering all reporting MSs, MDR was higher among Salmonella spp. from pigs and pig carcases (51.3% and 47.4%, respectively) to that noted among isolates from cattle and calf carcases (29.5% and 22%, respectively). Similarly, overall levels were higher in isolates from turkeys and broilers (38.8% and 38.2%, respectively) compared to that in isolates from laying hens (6.5%). While an overall high level of MDR was reported among isolates from broiler carcases (32.7%), an overall moderate level was noted among isolates from turkey carcases (15.1%). It should be noted however, that the countries reporting Salmonella spp. data from these origins differed and the number of isolates reported by countries varied because of varying Salmonella prevalence; these factors may introduce a source of variation to results when considering all reporting countries. Furthermore, resistance levels varied among serovars which may exhibit particular MDR patterns, so the relative contribution of individual serovars within the different animal origins and between MSs should be considered when comparing the situation between reporting countries.
In both pig carcases and pigs, the proportion of all Salmonella isolates exhibiting MDR, was greatly influenced by the occurrence of MDR monophasic S . Typhimurium, this serovar accounting for 56.7% and 52.3% of the MDR isolates in pig carcases and pigs in 2017, respectively. In human cases from 2017, monophasic S. Typhimurium was the third most common serovar, showing the highest proportion of MDR (81.4%). This serovar has spread widely among European pig populations. Particular MDR patterns are associated with monophasic S. Typhimurium and because this serovar was prevalent in many countries, these patterns greatly influenced the overall resistance figures. This is exemplified by resistance to ampicillin, sulfamethoxazole and tetracycline which occurred as an MDR pattern without additional resistances in 199/334 (59.6%) monophasic S. Typhimurium isolates from pig carcases and in 91/161 (56.5%) monophasic S. Typhimurium isolates from pigs. This resistance pattern (together with resistance to streptomycin) is typical of the European clone of monophasic S. Typhimurium (Hopkins et al., 2010). The genes conferring resistance to these antimicrobials are commonly found in association together with IS26 mobile genetic elements, responsible for their integration at different chromosomal locations, in recently described European strains of monophasic S. Typhimurium (Sun et al., 2019). It is noteworthy that MDR in the European clone of monophasic S. Typhimurium appears to have originated from integration of MDR plasmids into the chromosome, facilitated by the presence of these IS26 mobile genetic elements (Sun et al., 2019).
In 2017, S . Typhimurium was the most dominant serovar reported in cattle, the second most commonly reported serovar in humans, and the third most commonly reported in pigs and pig carcases. Among S. Typhimurium isolates recovered from humans, cattle, pigs and pig carcases, MDR was also frequently observed (39.7%, 30.8%, 59.3% and 64.2%, respectively). A wide range of different MDR patterns were reported among S. Typhimurium isolates from pig carcases and pigs. The most frequent MDR core pattern among isolates from pigs was resistance to ampicillin, chloramphenicol, sulfamethoxazole and tetracycline. Among MDR isolates from pig carcases, two core resistance patterns predominated: ampicillin, sulfamethoxazole and tetracycline, and the same pattern with the addition of chloramphenicol. This latter core pattern (ampicillin, chloramphenicol, sulfamethoxazole and tetracycline) was also the most frequently noted among MDR isolates from broilers and turkeys; as well as among MDR isolates from cattle but with the addition of ciprofloxacin/nalidixic acid. Although genotypic data were not reported, mobile genetic elements which could account for this resistance pattern in S. Typhimurium isolates have previously been described. Salmonella genomic island 1 (SGI1), known to contain a MDR region located on a complex class 1 integron designated In104, confers pentavalent resistance (the ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, tetracycline resistance phenotype – ACSSuT) and has widely been documented in a range of Salmonella serovars.
Multiresistant S. Rissen isolates were recovered from pigs, broilers and laying hens, as well as carcases of pigs and broilers. Although the proportion of MDR Salmonella isolates in pigs was mostly influenced by the occurrence of multiresistant S. Typhimurium and its monophasic variant (72% in total), S. Rissen accounted for 14% of the MDR Salmonella isolates recovered from this animal population. MDR was frequently observed among S. Rissen isolates from pigs and their derived carcases (66.7% and 46.5%, respectively), with a wide range of different resistance patterns noted. In pigs, the most frequent pattern of resistance was to ampicillin, sulfamethoxazole, trimethoprim and tetracycline (32.4%). Similarly, this combination (ampicillin, sulfamethoxazole, trimethoprim and tetracycline) with the addition of chloramphenicol was the most common resistance pattern noted among pig carcases (24.2%). García‐Fierro et al. (2016) previously identified a dominant S. Rissen clone in pigs, pork and humans in Spain, which was shown to carry genes conferring resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline and trimethoprim at varying frequencies, mostly on integrons. S. Rissen is also a common serovar in pigs, chicken, pork and man in some parts of Asia. Pornsukarom et al. (2015) demonstrated that S. Rissen isolates originating from Thai pig farms were frequently MDR to most of the antimicrobials listed above.
S. Derby was the sixth most common serovar detected in humans in 2018, as well as the most common serovar detected in turkeys, and the second most frequently reported from pigs and pig carcases. While MDR was not as frequently reported among S. Derby isolates from these animal origins (22.9%, 15.3% and 11.8%, respectively), the most common resistance pattern was to sulfamethoxazole, trimethoprim and tetracycline, with the addition of ampicillin in turkeys.
S. Infantis was the fourth most commonly reported serovar in humans in 2018, the most frequently reported serovar from broilers and their derived carcases, (37.6% and 36.3%, respectively), and the second most frequently reported from laying hens and turkeys (9.5% and 8.1%, respectively). Although a wide range of different MDR patterns were reported among S. Infantis isolates from poultry, the most frequent core pattern of resistance was to ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline. Where MDR was detected, this resistance profile (resistance to only ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline) accounted for 46%, 50%, 60.3%, 74.2% and 100% of the multiresistant S. Infantis isolates recovered from turkeys, laying hens, broilers, broiler carcases and turkey carcases, respectively. Multiresistant S. Infantis was also reported from pig carcases (six isolates were MDR out of 31 isolates reported, 19.4%), where all MDR isolates showed resistance to ampicillin, sulfamethoxazole and trimethoprim. The most common pattern of resistance (83.3%) among MDR isolates from pig carcases was to chloramphenicol, ampicillin, sulfamethoxazole, trimethoprim and tetracycline; all isolates exhibiting this resistance pattern were reported by Spain. Although genotypic data were not reported, previous scientific publications in Europe highlight the involvement of plasmids, which appear to be responsible for resistance in many European MDR S. Infantis isolates (Nógrády et al., 2012; Franco et al., 2015). In Australia, an S. Infantis strain harbouring a SGI1 homologue with an integron related to In104 and conferring resistance to streptomycin, sulfamethoxazole and trimethoprim was identified (Levings et al., 2005). For information on ESBL‐carrying S. Infantis, please see the text box above.
S. Kentucky was the seventh most commonly reported serovar in humans in 2018, and the third most commonly reported serovar in laying hens and turkeys, as well as the fourth most frequently reported in broilers. While MDR was observed at an extremely high level in S. Kentucky isolates from humans and turkeys (77.4% and 84.9%, respectively), isolates recovered from broilers and laying hens were less frequently MDR (77.4% and 84.9%, respectively). This variation in MDR was also apparent among S. Kentucky isolates recovered from carcases of turkeys and broilers (75% and 37%, respectively), although the total number of isolates available for analysis from these origins was relatively low (N = 8 and N = 27, respectively). A wide range of different MDR patterns were reported among S. Kentucky isolates from broilers, laying hens and turkeys. Among all poultry origins (including carcases of broilers and turkeys), the most frequent core pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid, gentamicin, sulfamethoxazole and tetracycline. Where MDR was detected, this resistance profile (resistance to only ampicillin, ciprofloxacin, nalidixic acid, gentamicin, sulfamethoxazole and tetracycline) accounted for 27.8%, 64.9%, 75.6%, 80% and 83.3% of the multiresistant S. Kentucky isolates recovered from laying hens, broilers, turkeys, broiler carcases and turkey carcases, respectively. The same resistance pattern was also frequently found in S. Kentucky isolates from humans. Additionally, ESBL and AmpC‐carrying S. Kentucky were identified in human isolates, as well as a few ESBL‐carrying S. Kentucky from broilers and a single AmpC‐carrying S. Kentucky isolate from laying hens (see Table 1 and Section 2.3.5 for further details). Two S. Kentucky isolates from humans were also identified as carbapenemase producers.
In contrast, S. Enteritidis isolates recovered from the monitoring in 2018 exhibited much lower multiresistance. This serovar was the most commonly reported in humans (49.6%) and laying hens (30.6%), the second most commonly reported in broilers (7.8%), and the third most frequently reported in broiler carcases (7.8%). While complete susceptibility to the harmonised panel of antimicrobials was observed at 44.9% in S. Enteritidis isolates from broiler carcases; in isolates recovered from broilers and laying hens, the majority exhibited complete susceptibility (66.7% and 83.8%, respectively). In S. Enteritidis from humans, 18 of 23 MSs found moderate to high levels of resistance to ciprofloxacin. Belgium and Poland also reported high levels of resistance to ampicillin and tetracycline in this serovar from human isolates.
In summary, the prevalence of particular Salmonella serovars within countries and animal populations, and their associated patterns of resistance, are likely to explain many of the observed differences in the overall levels of antimicrobial resistance and MDR. The spread of resistant clones and the occurrence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. Within a given MS, any attempt to relate the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated (see Appendix D), as much of the food consumed in a MS may have originated from other MSs or non‐member countries. Salmonella infections can also be associated with foreign travel, other types of animal contact (such as pets, including reptiles) or the environment. Additionally, some human infections may result from human to human transmission. To improve investigation of these relationships, human isolates from cases notified as having been acquired during travel outside of the reporting country were excluded from the analysis.
3. Antimicrobial resistance in Campylobacter spp.12
3.1. Data on AMR in Campylobacter spp. addressed
The monitoring of AMR in Campylobacter spp. from food‐producing animals and food is focused13 on the species C. jejuni and C. coli. While the biennial monitoring and reporting of AMR in C. jejuni isolates recovered from caecal samples of broilers and fattening turkeys, is mandatory, the annual monitoring of AMR in C. coli isolates recovered from food‐producing animals is performed on a voluntary basis. C. jejuni is the main Campylobacter species responsible for human infections and usually predominant in poultry, whereas C. coli is recognised as the second most common Campylobacter species affecting humans, and likewise is frequently found in poultry, sometimes at higher rates than C. jejuni (Pergola et al., 2017). C. coli also typically displays higher levels of resistance to important antimicrobials in comparison to C. jejuni, thus MSs are encouraged to monitor AMR levels in C. coli.
While food‐producing animals are considered to be a major source of human campylobacteriosis through contamination of food products, other sources – such as wild birds, pets and environmental water – should also be considered as potential modes of transmission (Moré et al., 2017; Szczepanska et al., 2017; EFSA, 2019).
The monitoring of AMR in C. jejuni isolates recovered from caecal samples of broilers and fattening turkeys at slaughter was made mandatory in 2018; while the monitoring of AMR in Campylobacter isolates recovered from caecal samples of fattening pigs and calves (under 1 year of age) at slaughter, was performed on a voluntary basis during 2017. In addition, the voluntary monitoring of AMR in Campylobacter isolates recovered from meat samples (of broilers, turkeys, bovine and pigs) at retail, as well as C. coli isolates recovered from caecal samples of broilers and turkeys was performed by some MSs in 2017 and 2018. However, no country reported information on more than 10 Campylobacter isolates recovered from bovine meat or pig meat samples.
In 2017 and 2018, data for C. jejuni and C. coli from human cases were also reported. Only data for 2018 from humans are presented below as the 2017 data has been presented in the EU Summary report for 2017 (EFSA and ECDC, 2019a,b).
3.2. Occurrence of antimicrobial resistance in humans
3.2.1. Data reported
For 2018, 19 MSs and 1 non‐MS reported data on AMR in Campylobacter isolates from human cases of campylobacteriosis. Twelve countries provided data as measured values (quantitative data) and eight as data interpreted with CBPs. Not all countries reported results for all antimicrobials in the harmonised panel (ECDC, 2016). The reported data represented 20.8% and 21.7% of the confirmed human cases with C. jejuni and C. coli, respectively, reported in the EU/EEA in 2018.
3.2.2. Occurrence of resistance
Occurrence of resistance
In 2018, very high to extremely high resistance levels to ciprofloxacin were reported in human C. jejuni isolates from all reporting countries with the exception of Denmark, Ireland and the UK, where high levels were reported, and Iceland, where a moderate level was reported (Figure 15 and Annex C, Table 1). For C. coli, 13 out of 15 countries reporting more than 10 isolates had levels of ciprofloxacin resistance of > 70–98.1% (Annex C, Table 2). The EU average for ciprofloxacin resistance was 59.3% and 65.2% for C. jejuni and C. coli, respectively. The proportion of human C. jejuni isolates resistant to erythromycin was low overall at 1.8% but markedly higher in C. coli, 14.3%, with high proportions (22.5–31.6%) of C. coli being resistant in 5 MSs and a very high proportion (60.7%) in one MS (Portugal). High (47.2%) and extremely high (71.3%) proportions of resistance to tetracycline were observed in C. jejuni and C. coli, respectively. Low proportions of Campylobacter isolates were resistant to gentamicin and amoxicillin‐clavulanic acid, except in Luxembourg, Malta and Spain where 20–27.3% of C. coli were resistant to clavulanic acid‐amoxicillin (Annex C).
Figure 15.
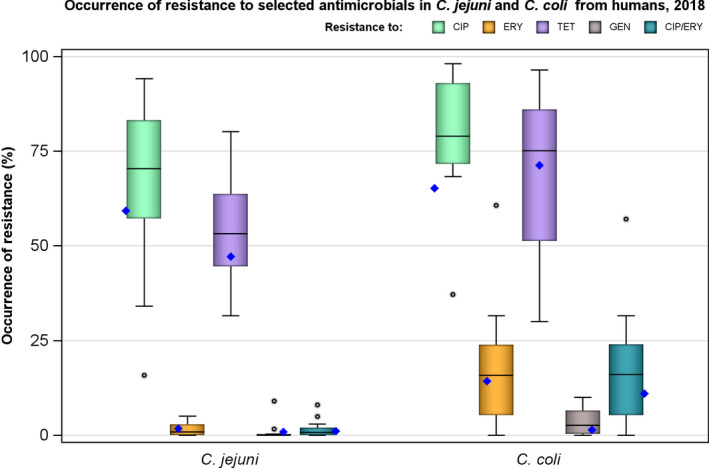
Occurrence of resistance to selected antimicrobials in C. jejuni and C. coli isolates from humans, 2018
- Horizontal line represents median, and blue diamond represents the resistance at the reporting‐MS level.
| EU total | CIP | ERY | TCY | GEN | Combined CIP/ERY | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| C. jejuni (19 MSs) | 23,241 | 59.3 | 21,481 | 1.8 | 16,933 | 47.2 | 7,467 | 0.9 | 21,250 | 1.1 |
| C. coli (18 MSs) | 2,973 | 65.2 | 2,736 | 14.3 | 2,333 | 71.3 | 1,245 | 1.4 | 2,708 | 11.0 |
CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; TCY: tetracyclines.
Combined microbiological, as well as clinical, resistance to both ciprofloxacin and erythromycin, which are considered critically important for treatment of campylobacteriosis, was generally low (microbiological resistance 1.1%, clinical resistance 1.0%) in C. jejuni and moderate (11.0% for both) in C. coli for 2018 (Figure 15). Two countries (Poland and Portugal) reported higher levels of combined resistance in C. jejuni from humans (8.0% and 5.0%, respectively), four countries (Estonia, Finland, Italy and Spain) reported high levels (> 20%) of combined resistance in C. coli and one country (Portugal) reported very high levels (> 50%) (Figure 16 and Annex C, Tables 3 and 4).
Figure 16.
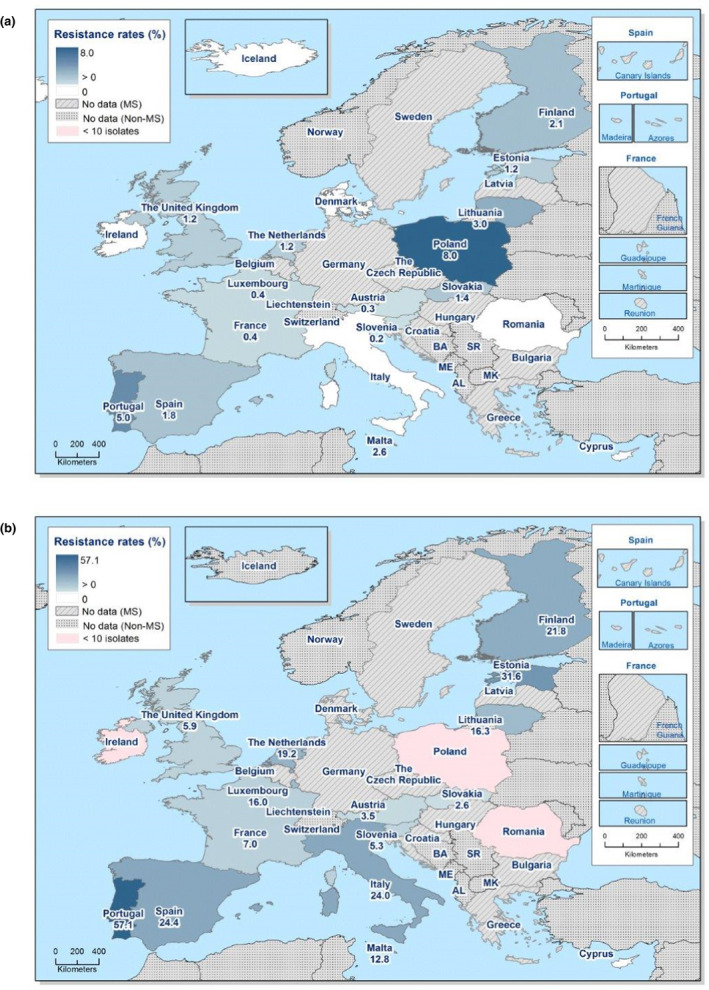
Combined resistance to the critically important antimicrobials ciprofloxacin and erythromycin in (a) C. jejuni and (b) C. coli isolates from humans, 2018
- Note: For Finland, travel information was missing from the AMR data while from other sources, travel‐associated cases were known to account for 80% of Finnish Campylobacter infections in 2018.
MDR in isolates tested for four antimicrobial classes (fluoroquinolones, macrolides, tetracyclines and aminoglycosides) was overall low in C. jejuni but moderate in C. coli (Figure 17 and Annex C, Tables 5 and 6). The most common resistance pattern in both C. jejuni and C. coli was resistance to both ciprofloxacin and tetracycline, observed in 39.4% of C. jejuni isolates and 51.9% of C. coli isolates. The second most common pattern in C. jejuni (in 33.9% of isolates) was complete susceptibility to the four antimicrobial classes in the harmonised panel while in C. coli (14.9%) it was tetracycline resistance alone.
Figure 17.
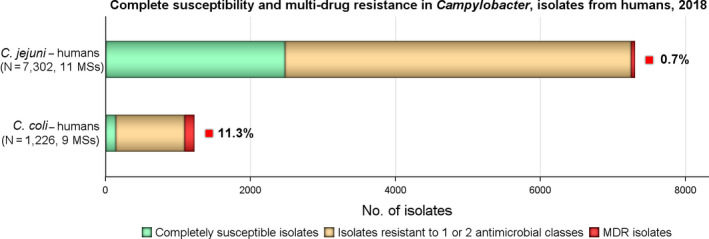
Number of MDR isolates, isolates resistant to 1 and/or 2 antimicrobials and completely susceptible Campylobacter isolates from humans, 2018
Temporal trends were analysed for countries reporting data for at least 3 years over the 6‐year period 2013–2018. Statistically significant (p < 0.05) increasing trends of fluoroquinolone resistance were observed in C. jejuni in seven MSs and in C. coli in three, while a decreasing trend was observed in only one MS in C. coli (Table 5). Similarly, tetracycline resistance increased significantly in seven MS for C. jejuni and in five for C. coli, while only one MS observed a decrease in C. jejuni in the same period. Erythromycin resistance, on the other hand, decreased in five MSs for C. jejuni and increased in one MS and one non‐MS, while for C. coli one MS observed a decreasing trend and one MS reported an increasing trend. For country‐specific trend graphs, please see Annex C, Figures 1 and 2.
Table 5.
Number of countries with significantly increasing or decreasing trends in resistance to selected antimicrobials for Campylobacter jejuni and Campylobacter coli in humans, 2013–2018
| Species | Ciprofloxacin | Erythromycin | Tetracyclines | |||
|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | |
| C. jejuni (18 MSs + 1 non‐MS) | 7 (AT, EE, FI, FR, LT, SI, SK) | – | 2 (NO, SK) | 5 (FR, IT, LU, MT, SI) | 7 (AT, EE, LU, NL, SI, SK, UK) | 1 (FR) |
| C. coli (14 MSs) | 3 (LT, NL, SK) | 1 (UK) | 1 (UK) | 1 (FR) | 5 (FR, LT, NL, SI, SK) | – |
High‐level resistance to erythromycin (MIC > 128 mg/L) was assessed as a possible indication for transferrable erythromycin resistance due to the presence of the erm(B) gene. However, also a point mutation in the 23S rRNA was shown to be sufficient for high level resistance against erythromycin in Campylobacter spp. Further molecular analysis will be of interest to distinguish between both resistance mechanisms (Bohlinger and Kathariou, 2017). In C. jejuni, 1.1% of the isolates (N = 2,209, 6 MSs) had MIC > 128 mg/L while in C. coli this proportion was substantially higher, 16.3% (N = 307, 5 MSs) (Figure 18). Similarly, in 1.4% (N = 3,333, 8 MSs) of C. jejuni and 17.2% (N = 424, 8 MSs) of C. coli tested with disk diffusion no inhibition zone could be observed (6 mm zone equals the disk size), which corresponds to a MIC of ≥ 128 mg/L (EUCAST, 2019a).
Figure 18.
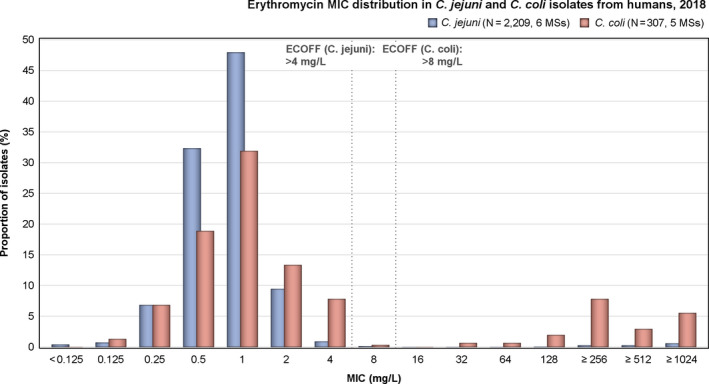
Erythromycin MIC distribution in C. jejuni and C. coli isolates from humans, 2018
- MIC: minimum inhibitory concentration.
3.3. Occurrence of antimicrobial resistance in food‐producing animals, and meat derived from broilers and turkeys
3.3.1. Data reported
In 2017, 7 MSs and 2 non‐MSs voluntary reported data on C. coli isolates recovered from caecal samples of fattening pigs, and 5 MSs voluntary reported data on C. jejuni isolates recovered from caecal samples of calves (Annex C, Tables 8 and 9), while in 2018, 25 MSs and 4 non‐MSs reported mandatory data on C. jejuni isolates recovered from caecal samples of broilers, and 10 MS and 1 non‐MS reported data on C. jejuni isolates recovered from caecal samples of fattening turkeys (Annex C, Tables 10 and 11). Additionally, some countries voluntary reported data on C. coli isolates recovered from caecal samples of broilers and fattening turkeys, and derived meat (Annex C, Table 12).
3.3.2. Campylobacter from meat samples of broilers and turkeys
Considering Campylobacter isolates recovered from meat samples of broilers and turkeys collected in 2018, resistance was generally observed at higher levels in C. coli than in C. jejuni. Among C. jejuni and C. coli isolates recovered from poultry meat, the highest levels of resistance were noted for ciprofloxacin, nalidixic acid and tetracycline (overall percentages: 54–83%) considering all reporting MSs. Generally, most MSs reported high to extremely high levels of resistance to these antimicrobials in Campylobacter isolates. Resistance to gentamicin in C. jejuni and C. coli isolates recovered from poultry meat was not observed in most countries. While resistance to streptomycin was either not detected or observed at very low/low levels in C. jejuni isolates, resistance to this antimicrobial was noted at higher levels in C. coli isolates (15.7%). Similarly, erythromycin resistance was generally higher among C. coli isolates (14.3%) compared to C. jejuni isolates (< 2%).
3.3.3. Campylobacter from poultry, pigs and calves
Occurrence of resistance
Comparison of resistance levels between bacterial and animal species should be interpreted cautiously because of the dispersion of resistance rates between countries and because numbers of isolates and reporting countries vary, particularly for voluntary reporting.
Generally, tetracycline resistance ranged from high to very high within each of the animal origins; overall, the highest levels of resistance were noted in C. coli isolates recovered from broilers (61.4%) and C. jejuni from turkeys (56.1%). The highest levels of resistance to streptomycin were noted in C. coli isolates recovered from fattening pigs (overall, 64.4%), with much lower levels observed in poultry and calves’ isolates. Overall, moderate levels were noted in C. coli recovered from broilers, respectively (15.6%) and C. jejuni from calves (15.6%), whereas low levels were noted in C. jejuni recovered from broilers and fattening turkeys (8.7% and 6.4%, respectively). Resistance to gentamicin in Campylobacter isolates from these animals was detected at very low/low levels by reporting MSs.
Considering Campylobacter isolates recovered from caecal samples of broilers and fattening turkeys, overall resistance to ciprofloxacin and nalidixic acid was very high to extremely high (from 66.0% for nalidixic resistance in C. jejuni from turkeys to 86.7% for nalidixic acid and ciprofloxacin resistance of C. coli from broilers); resistance levels to these antimicrobials were generally lower in C. coli isolates recovered from fattening pigs (52.3% for both antimicrobials) and C. jejuni from calves (52.1% for nalidixic acid). Among C. jejuni from poultry and calves, erythromycin resistance was either not discerned or detected at very low/low to moderate levels by most reporting MSs (overall, 1.1%, 1.3% and 1.2% in turkeys, broilers and calves, respectively). Generally, erythromycin resistance was observed at higher levels in C. coli isolates recovered from fattening pigs (overall, 15.6%), although resistance varied markedly between individual MSs. For instance, Spain reported very high levels (N = 170, 61.8%) for pigs, whereas resistance to this compound was very low for pigs in Sweden (N = 137, 0.7%).
Combined resistance to ciprofloxacin and erythromycin
The occurrence of Campylobacter isolates displaying combined resistance to ciprofloxacin and erythromycin is of great importance to public health, since both compounds are recognised as CIAs for the treatment of Campylobacter infections in humans (WHO, 2019). Considering all reporting countries (including non‐MSs), overall combined resistance to these antimicrobials was detected in 9.7% of C. coli isolates recovered from pigs (136/395), 6.5% of C. coli isolates from broilers (22/339), 1.1% of C. jejuni isolates from broilers (43/3,757 – or 42/3,519 for MSs only), 1.0% of C. jejuni isolates from turkeys (12/1,190 or 2.0% – 12/1,174 for MSs only) and 1.0% in C. jejuni isolates from calves (6/585) (Figure 5).
Figure 19.
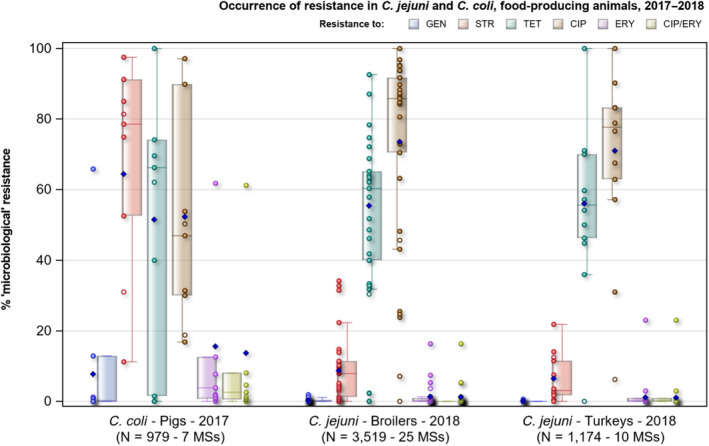
Occurrence of resistance to selected antimicrobials in C. jejuni/C. coli isolates from broilers, fattening turkeys and fattening pigs, reporting EU MSs, 2017/2018
- GEN: gentamicin, STR: streptomycin, TET: tetracycline CIP: ciprofloxacin, ERY: erythromycin, CIP/ERY: combined ‘microbiological’ resistance to ciprofloxacin and erythromycin. N: Total number of isolates reported by all Member States (MSs). Blue diamond: occurrence of resistance at the reporting‐MS group level.
Combined resistance to both ciprofloxacin and erythromycin in C. jejuni from broilers was detected in 7 out of 29 reporting countries in 2018 and assessed at 1.22% (38/3,117) in all reporting EU MSs. Among those countries recording combined resistance to ciprofloxacin and erythromycin in C. jejuni from broilers, two groups can be observed: first, Bulgaria, Italy and Portugal, registering a combined resistance of 5.3%, 5.3% and 16.4% respectively, and second, four countries (the Czech Republic, Germany, Romania and Switzerland), reporting a combined resistance lower than 1.5%. Among the six MSs (Austria, the Czech Republic, Estonia, the Netherlands, Slovenia and Spain), reporting on resistance in C. coli from broilers for 2018 on a voluntary basis (overall 339 isolates), four MSs (Austria, the Czech Republic, the Netherlands, and Spain) reported combined resistance to both ciprofloxacin and erythromycin, at levels starting from 1.2% (Austria) to 14.3% (Spain). Where comparison of the levels of combined resistance is possible between C. jejuni and C. coli, the levels in C. coli are greater than those observed in C. jejuni in the Czech Republic, the Netherlands and Spain.
Out of 10 reporting MSs, combined resistance to both ciprofloxacin and erythromycin in C. jejuni from fattening turkeys was detected in Spain, Italy and Portugal with resistance in 0.9%, 2.9% and 23.1% of the isolates tested, respectively (Figure 20). The overall occurrence of combined resistance to ciprofloxacin and erythromycin in C. jejuni was 1.0%, when considering all reporting MSs.
Figure 20.
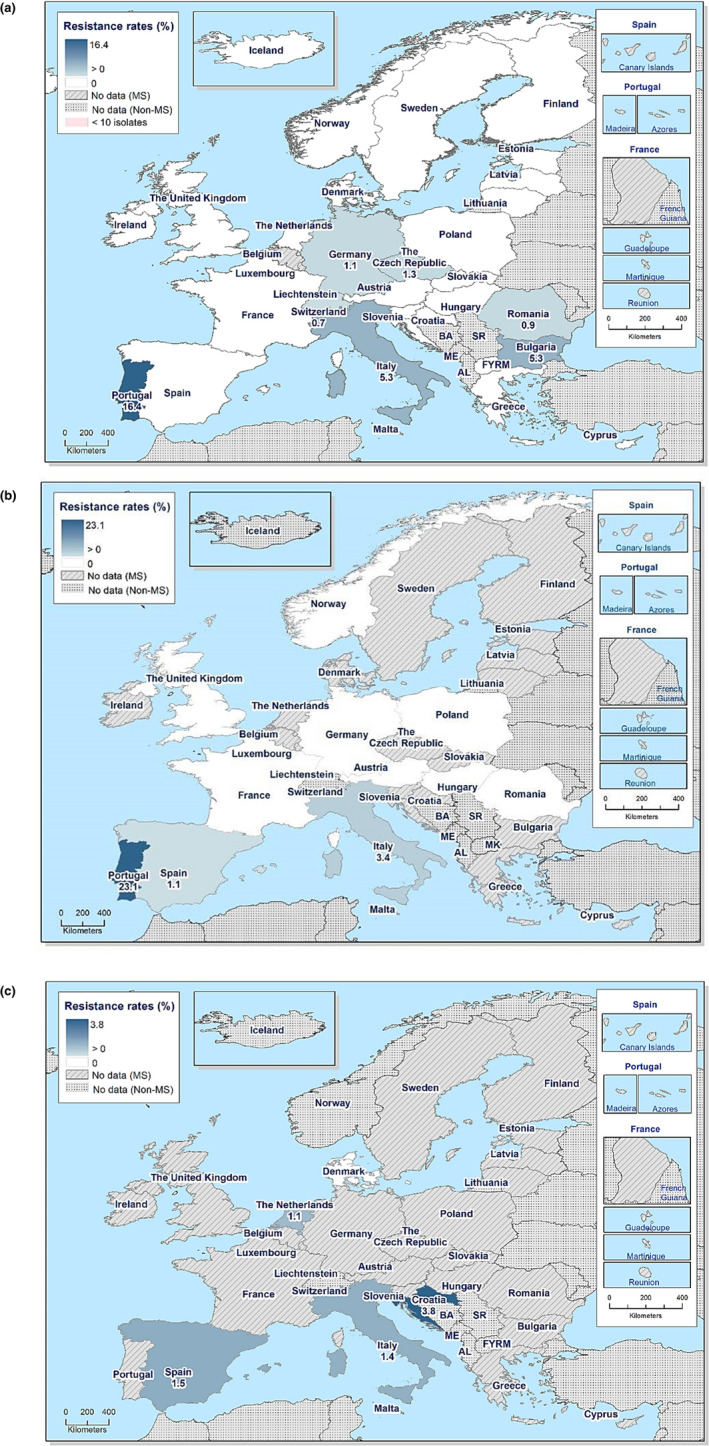
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter jejuni from (a) broilers (29 EU/EEA MSs, 2018), (b) fattening turkeys (11 EU/EEA MSs, 2018) and (c) calves (5 MSs, 2017)
Considering the five MS reporting data for C. jejuni from calves in 2017, no C. jejuni isolate from Denmark was found to be resistant to the two important therapeutic compounds, erythromycin and ciprofloxacin. In the other reporting countries, only one isolate (Italy, the Netherlands) or two isolates (Croatia and Spain) were resistant to ciprofloxacin and erythromycin. The overall level of combined resistance to ciprofloxacin and erythromycin was 1.0%.
Considering the seven MSs reporting data on C. coli from pigs in 2017 (Croatia, the Czech Republic, Estonia, Finland, Germany, Spain and Sweden), the overall combined resistance to both ciprofloxacin and erythromycin was 13.7% (134/979 isolates). The highest proportion was observed in Spain (61.2%). Levels of combined resistance were much lower in Germany (8.1%) and in other countries (less than 5% of reported isolates) in 2017 (Figure 21).
Figure 21.
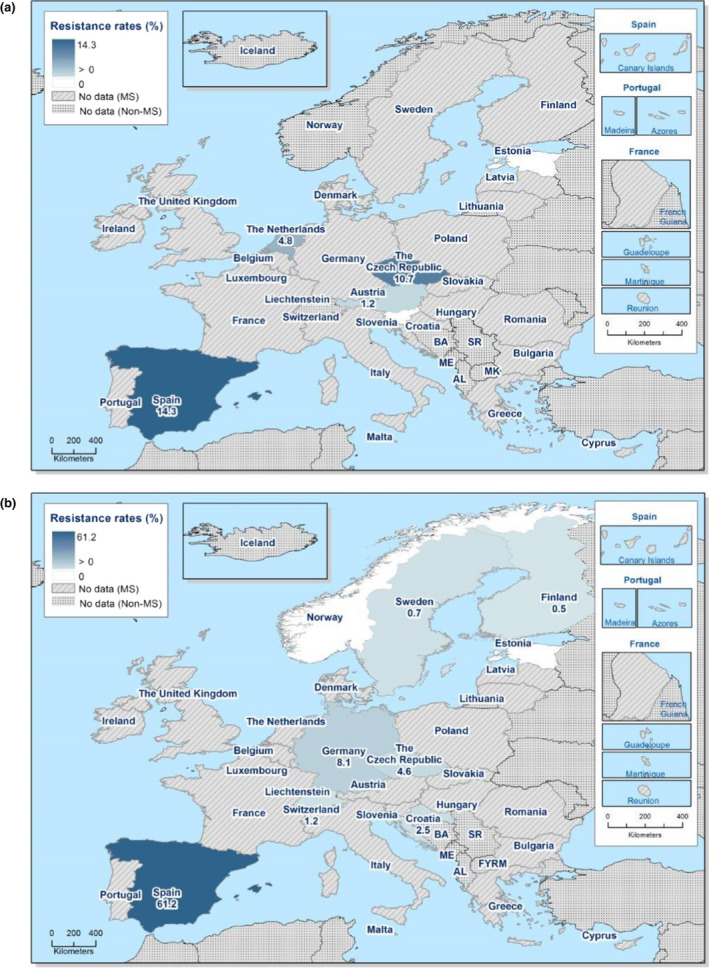
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter coli isolates from a) broilers, 6 EU/EEA MSs, 2018 and b) fattening pigs, 7 EU/EEA MSs, 2017
Complete susceptibility and MDR in reporting countries
The levels of MDR, defined as resistance to three or more antimicrobial classes of the harmonised panel tested, among Campylobacter isolates recovered from these food‐producing animals by MSs and non‐MSs are shown in Figure 22. Overall, MDR was observed at a moderate level in C. coli isolates recovered from fattening pigs (16.8%), and at lower levels in C. coli isolates recovered from broilers (8.0%) and in C. jejuni isolates recovered from calves (4.1%), broilers (1.2%), and fattening turkeys (1.2%).
Figure 22.
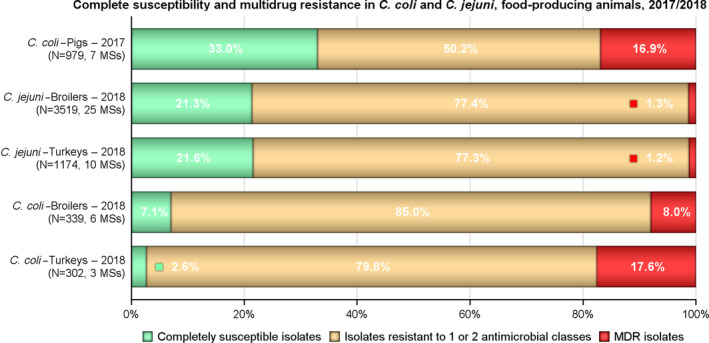
Proportions of isolates completely susceptible and MDR in C. jejuni and C. coli from fattening pigs, broilers and fattening turkeys, reporting EU/EEA MSs, 2017/2018
- N: Total number of isolates reported by the EU MSs.
Considering complete susceptibility to the four antimicrobial classes (ciprofloxacin/nalidixic acid, erythromycin, tetracycline and gentamicin), the highest proportions of isolates displaying complete susceptibility were noted among C. jejuni from calves (39.5%) and C. coli from pigs (33.0%). The proportions were lower for C. jejuni isolates from broilers and turkeys (21.3% and 21.6% respectively) and below 10% for C. coli isolates from broilers (7.1%). Among poultry isolates, complete susceptibility was generally noted at slightly higher levels in C. jejuni isolates compared to those in C. coli isolates. Marked differences could be detected between countries with, for example percentages of complete susceptibility in C. jejuni from broilers ranging from < 5% in Cyprus, Latvia, Portugal and Lithuania to > 70% in Finland, Sweden, Iceland and Norway.
Temporal trends in resistance in C . jejuni and C. coli from broilers
Temporal trends in resistance in C. jejuni from broilers could be studied from data from 16 EU MSs and 2 non‐MSs over the period 2009–2018 and are displayed in Figure 23. Due to the lack of longitudinal data, evaluation of temporal trends in resistance cannot yet be assessed for all countries participating in the monitoring. A significant increase in resistance to ciprofloxacin was recorded in 10 MSs (Austria, Croatia, the Czech Republic, Denmark, Finland, France, Germany, the Netherlands, Romania, and Sweden) and 1 non‐MS (Switzerland) (Table 6). An increase in resistance was also detected for streptomycin in 4 MSs and for tetracycline in 10 MSs and 1 non‐MS. A decrease in resistance was detected in erythromycin, streptomycin and tetracycline in two, four and three MSs, respectively.
Figure 23.
Trends in ciprofloxacin (CIP), erythromycin (ERY), streptomycin (STR) and tetracycline (TET) resistance in C. jejuni from broilers, 2009–2018
Table 6.
Number of countries with significantly increasing or decreasing trends in resistance to selected antimicrobials for C. jejuni and C. coli in broilers, 2009–2018
| Campylobacter from broilers | Ciprofloxacin | Erythromycin | Tetracyclines | |||
|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | |
| C. jejuni (16 MSs + 2 non‐MS) | 10 (AT, HR, CZ, DK, FI, FR, DE, NL, RO, SW, CH) | 2 (RO, SP) | 11 (AT, BE, HR, CZ, DK, FI, FR, DE, SW, CH, UK) | 3 (IT, NL, SI) | ||
| C. coli (6 MSs + 1 non‐MS) | 3 (CZ, DE, NL) | 1 (CZ) | 2 (AT, DE) | 3 (CZ, DE, NL) | ||
Trends in C. coli from broilers could be evaluated in only six MSs and one non‐MS. Increases of resistance were observed for ciprofloxacin (3 MSs), erythromycin (1 MS), streptomycin (1 MS) and tetracycline (3 MSs), whereas decreases were observed only for erythromycin (2 MSs) and streptomycin (2 MSs). Remarkably, the trend was usually the same for C. coli and C. jejuni within a same production, with the exceptions of streptomycin and tetracycline in the Netherlands.
Temporal trends in resistance in C . jejuni from turkeys
The comparison of resistance in C. jejuni isolates from fattening turkeys between 2014 and 2018 showed statistically significant changes in proportions of resistant isolates. Significant increasing trends in resistance to ciprofloxacin between 2014 and 2018 were notably detected in Poland and Portugal, whereas a significant decreasing trend was recorded in Hungary (Figure 24). For resistance to tetracyclines, significant decreasing trends were observed in France, Germany, Italy, Hungary, Spain and the UK. At the overall level (nine MSs), whereas increasing trends in resistance to streptomycin was registered between 2014 and 2018, decreasing trends in resistance to erythromycin and tetracyclines were also registered.
Figure 24.
Trends in ciprofloxacin (CIP), erythromycin (ERY), streptomycin (STR), and tetracycline (TET) resistance in C. jejuni from turkeys, reporting EU MSs, 2014–2018
High‐level resistance to erythromycin
While erythromycin resistance was reported overall at very low, low and moderate levels in Campylobacter spp. recovered from caecal samples of the food‐producing animals, isolates displaying MICs > 128 mg/L were detected (Figure 25). Notably, an erythromycin MIC of > 128 mg/L exceeds the highest concentration tested, in accordance with the harmonised method set out in Decision 2013/652/EU. Figure 26 illustrates the proportion of isolates reported by MSs and non‐MSs solely displaying ‘microbiological/clinical’ resistance (C. jejuni: MIC > 4 mg/L; C. coli: MIC > 8 mg/L) in comparison to those displaying high‐level resistance (MICs > 128 mg/L) to this antimicrobial within each of the animal categories. Interestingly, 87% of C. coli isolates displaying erythromycin resistance from pigs (N = 161) exhibited an MIC of > 128 mg/L in 2017, while 69.2% and 52.9% of erythromycin‐resistant C. jejuni isolates from turkeys (N = 13) and broilers (N = 51), respectively, exhibited an MIC of > 128 mg/L in 2018. In pigs, 88% of the high‐level erythromycin‐resistant C. coli isolates were reported by Germany and Spain. In broilers and turkeys, 70% and 89% of the high‐level erythromycin‐resistant C. jejuni strains reported were isolated in Portugal and Italy.
Figure 25.
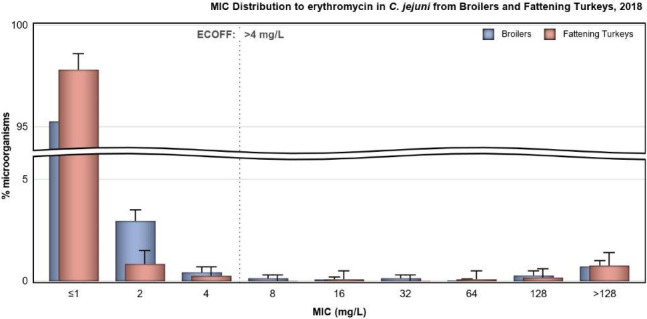
MICs of Campylobacter jejuni isolates exhibiting erythromycin resistance in broilers and turkeys, reporting EU MSs and non‐EU MSs, 2017/2018
- N: Total number of C. jejuni or C. coli isolates exhibiting erythromycin resistance.
- *: Includes data on erythromycin‐resistant isolates reported by non‐EU MSs.
Figure 26.
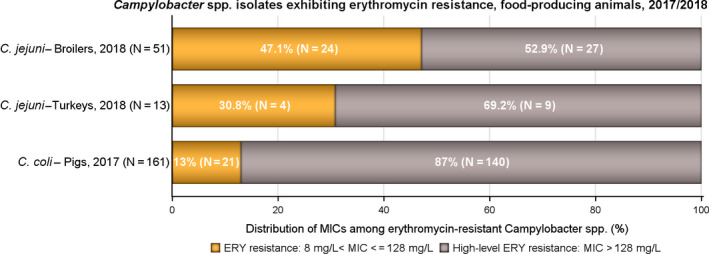
MICs of Campylobacter spp. isolates exhibiting erythromycin resistance in pigs, broilers and turkeys, reporting EU MSs and non‐EU MSs in 2017/2018
- N: Total number of C. jejuni or C. coli isolates exhibiting erythromycin resistance; ERY: erythromycin.
The erm(B) gene encodes an rRNA methylase and confers a high‐level of resistance to macrolides, lincosamides and/or streptogramin B antibiotics. This gene is widely distributed in Gram‐positive and Gram‐negative bacteria (Roberts, 2008) and has been recently recognised to confer high‐level resistance to erythromycin in Campylobacter spp. (Wang et al., 2014). Recent studies in China indicate that this gene was observed more frequently in C. coli than in C. jejuni but it was also found on C. jejuni from poultry resistant to all clinically important antimicrobial agents (Liu et al., 2019). In Europe, erm(B) has been reported in C. coli from broilers and turkeys in Spain and from a broiler isolate in Belgium (Florez‐Cuadrado et al., 2017; Elhadidy et al., 2019). Among Campylobacter sp. the erm(B) gene has been detected on plasmids, or more frequently on multiple drug resistance islands (MDRI); the latter frequently containing additional resistance genes, such as those conferring resistance to tetracycline and aminoglycosides (Florez‐Cuadrado et al., 2017). MDRI carrying erm(B) are transferable by natural transformation between strains of Campylobacter (Wang et al., 2014). The presence of transferable resistance genes, either on plasmids or MDRI in Campylobacter, represents a recent development, because hitherto, resistance to macrolides in Campylobacter was considered to occur mainly as the result of mutations in rRNA or ribosomal proteins and was not transferable. The occurrence of MDR plasmids or MDRI conferring resistance to several important therapeutic options, means that use of any one of these options will result in co‐selection of MDR isolates (EFSA, 2019). Furthermore, as erm(B) genes have been most frequently reported among C. coli rather than C. jejuni in many published studies, and to facilitate early detection, inclusion of C. coli monitoring has benefits in relation to detection of emerging macrolide resistance (EFSA, 2019).
Although transferable erythromycin resistance conferred by erm(B) generally results in a high‐level pf resistance to erythromycin, mutational resistance can also result in high‐level resistance to erythromycin. Mutational resistance may also result in lower MICs (< 128 mg/L), although this is still above the ECOFF, dependent on the particular mutations which have occurred. Where the erm(B) gene has been confirmed, isolates have demonstrated erythromycin MICs of ≥ 512 mg/L. Those isolates exhibiting MICs ≥ 512 mg/L therefore have an erythromycin resistance phenotype consistent with either possession of transferable erm(B) or mutational resistance (Wang et al., 2014), whereas isolates with erythromycin MICs below this figure have a phenotype consistent with mutational resistance. EFSA advise therefore, that increasing the tested concentrations of erythromycin (up to 512 mg/L instead of 128 mg/L) should enable better targeted (phenotypic) screening of isolates which may be carrying this resistance gene or MDRI, and that such isolates are be subsequently analysed by molecular methods, if possible by WGS (EFSA, 2019).
3.4. Comparison of human and animal data on Campylobacter spp.
In 2017/2018, quantitative human data were interpreted using EUCAST ECOFF values, where available, in the same way as for the animal and food data. In the absence of ECOFFs (i.e. gentamicin), CBPs from the French Society for Microbiology (CA‐SFM) were applied. Figure 27 presents the CBPs and ECOFFs used to interpret the MIC data reported for Campylobacter spp. from humans, animals or food. Notably, there is concordance across interpretive categories, with the exception of the EUCAST CBP for tetracycline in C. jejuni which is one dilution above the EUCAST ECOFF.
Figure 27.
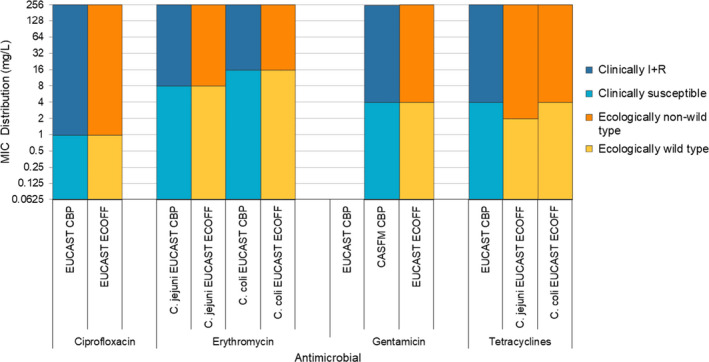
Comparison of clinical breakpoints (CBPs) and epidemiological cut‐off values (ECOFFs) used to interpret MIC data reported for Campylobacter spp. from humans, animals or food
Considering all data submitted from MSs, resistance to ciprofloxacin among C. jejuni isolates reported in 2017/2018 was detected in 57.7% (2017) and 59.3% (2018) of human isolates, 73.5% of isolates from broilers, 70.9% of isolates from fattening turkeys and 52.5% of isolates from calves. Overall resistance to erythromycin was reported at 2.0% (2017) and 1.8% (2018) in isolates from humans, 1.3% in isolates from broilers, 1.1% in isolates from fattening turkeys and 1.2% of isolates from calves. Combined resistance to ciprofloxacin and erythromycin was reported at 1.2% (2017) and 1.1% (2018) in isolates from humans, and 1.2%, 1.0% and 1.0% in isolates from broilers, turkeys, and calves, respectively (excluding non‐MSs). Considering MSs’ reports on the four antimicrobials (ciprofloxacin/nalidixic acid, erythromycin, tetracycline and gentamicin), complete susceptibility was reported at levels of 31.3% (2017) and 33.9% (2018) in isolates from humans, and 21.3%, 21.6% and 39.5% in isolates from broilers (25 MSs), turkeys (10 MSs), and calves (5 MSs), respectively. However, it must be noted that all countries used EUCAST ECOFFs (MIC > 1 mg/L) to determine resistance in C. jejuni isolates from animals, whereas some countries used CBPs (MIC > 2 mg/L) to determine resistance in C. jejuni isolates from humans.
Assessing C. jejuni AMR data at the country level revealed significant differences in ciprofloxacin resistance among isolates from broilers and humans in Finland, with a lower occurrence of ciprofloxacin resistance in broilers than for humans, whereas similar or higher percentages were obtained for isolates from broilers in most MS. This discrepancy is most likely due to the fact that the human resistance data from Finland include both travel‐related infections and domestically acquired infections, without any possibility to discern one from the other. From the national campylobacteriosis data, it is reported that 79.9% of the Finnish Campylobacter infections in 2018 were related to travel outside the country, primarily to countries in southern Europe and Asia (ECDC Surveillance Atlas, TESSy). Resistance to ciprofloxacin was significantly more frequent in human isolates than in turkey isolates in the UK, than in cattle isolates in the Netherlands and Spain. However, in France and Italy, resistance was significantly lower in human isolates than in turkey isolates. For erythromycin, significant differences were noted in Poland (higher percentage of isolates from humans being resistant compared to percentages from broilers and turkeys) and Portugal (higher percentages of isolates from broilers and turkeys compared to human ones). Combined resistance to ciprofloxacin and erythromycin was significantly more frequent in broiler and turkey isolates compared to human isolates in Portugal. More isolates of human origin were found susceptible to the four classes of antimicrobials compared to isolates from broilers and turkeys in France and in Italy, to isolates from broilers in the UK, and isolates from cattle in Denmark.
Considering all reports from MSs, resistance to ciprofloxacin among C. coli isolates was reported in 63.5% (2017) and 65.2% (2018) of isolates from humans, 52.3% of isolates from fattening pigs (7 MSs), and 86.7% of isolates from broilers (6 MSs). Overall, resistance to erythromycin was reported in 12.8% (2017) and 14.3% (2018) in isolates from humans, and 15.6% and 6.5% of isolates from fattening pigs and broilers were resistant. Combined resistance to ciprofloxacin and erythromycin was reported overall at 10.2% (2017) and 11.0% (2018) in isolates from humans and at 13.7% in isolates from fattening pigs (excluding non‐MSs), and at 6.5% in isolates from broilers. In view of the reporting countries (excluding non‐MSs), complete susceptibility to the four antimicrobial classes was reported at levels of 11.9% (2017) and 11.4% (2018) for humans and 33.0%, and 7.1% in isolates from fattening pigs and broilers, respectively.
Considering the countries reporting information on C. coli isolates originating from both fattening pigs (2017), broilers (2018) and humans (2017 or 2018), significant differences in ciprofloxacin resistance were noted in Estonia and Finland with significantly higher percentages of resistance in isolates from humans compared to isolates from pigs. For erythromycin, the percentages of resistance were also significantly higher for human isolates compared to pig isolates in Finland, but the opposite finding was observed in Spain. In the Netherlands, the human isolates were more frequently resistant to erythromycin compared to those from broilers. Combined resistance to ciprofloxacin and erythromycin was also significantly more frequent in isolates from humans compared to pig isolates in Finland but the opposite finding was observed in Spain. In Estonia, isolates from pigs were significantly more often susceptible to the four antimicrobial classes, compared to human ones.
Comparison of trends in resistance to ciprofloxacin, erythromycin and tetracyclines for isolates from humans (2013–2018) and broilers (2009–2018) was possible for 10 MSs and one non‐MS regarding C. jejuni and four MSs regarding C. coli. The results show various situations (Table 7). For example, similar increasing trends for resistance to ciprofloxacin and tetracycline were observed for C. jejuni from humans and from broilers in Austria, or for C. coli from humans and from broilers in the Netherlands. In France, resistance to ciprofloxacin increased in C. jejuni from humans and broilers, but resistance to tetracycline decreased in humans and increased in broilers.
Table 7.
Number of countries with significantly increasing or decreasing trends in resistance to selected antimicrobials for Campylobacter jejuni and Campylobacter coli in humans, 2013–2018, and in broilers, 2009–2018
| Species | Ciprofloxacin | Erythromycin | Tetracyclines | ||||
|---|---|---|---|---|---|---|---|
| Incr. | Decr. | Incr. | Decr. | Incr. | Decr. | ||
| Human | C. jejuni (18 MS + 1 non‐MS) | 7 (AT, EE, FI, FR, LT, SI, SK) | – | 2 (NO, SK) | 5 (FR, IT, LU, MT, SI) | 7 (AT, EE, LU, NL, SI, SK, UK) | 1 (FR) |
| C. coli (14 MSs) | 3 (LT, NL, SK) | 1 (UK) | 1 (UK) | 1 (FR) | 5 (FR, LT, NL, SI, SK) | – | |
| Broilers | C. jejuni (16 MS + 2 non‐MS) | 10 (AT, HR, CZ, DK, FI, FR, DE, NL, RO, SE, CH) | 2 (RO, ES) | 11 (AT, BE, HR, CZ, DK, FI, FR, DE, SW, CH, UK) | 3 (IT, NL, SI) | ||
| C. coli (6 MS + 1 non‐MS) | 3 (CZ, DE, NL) | 1 (CZ) | 2 (AT, DE) | 3 (CZ, DE, NL) | |||
3.5. Discussion
Globally, the data obtained from Campylobacter jejuni and C. coli from human and animal origins in 2017–2018, showed very high to extremely high levels of resistance to fluoroquinolones, which are CIAs for the treatment of Campylobacter infections in humans. An increasing trend in resistance was observed in several countries in both humans and animals. Resistance to quinolones and fluoroquinolones is most usually due to mutations in the gyrase gene, the C257T mutation on gyrA gene being the major mechanism for ciprofloxacin resistance. Modifications in the expression of the efflux pump CmeABC may also result in higher MICs of various antimicrobials including ciprofloxacin, and recently highly resistant isolates bearing a super efflux pump variant of CmeABC (RE‐CmeABC) were described in China (Yao et al., 2016). This RE‐CmeABC coding region could be transferred between Campylobacter isolates by natural transformation and the MICs of ciprofloxacin, and also of florfenicol, chloramphenicol, erythromycin and tetracycline, were increased in the transformants.
Resistance to erythromycin was detected at low levels in C. jejuni from humans and animals, but higher levels in C. coli isolates. Resistance to erythromycin is usually associated with mutations in one or several copies of the ribosomal RNA genes, such as A2074G, A2074C, and A2075G, or in the ribosomal proteins L4 and L22 (Luangtongkum et al., 2009). Additionally, the transferable erm(B) gene encoding an rRNA methylase, usually present on multiple drug resistance genomic islands (MDRGI) or plasmids, may confer a high level of resistance to macrolides, lincosamides and/or streptogramin B antibiotics (Wang et al., 2014). Initially described in Asia, this emerging resistance mechanism has now also been detected in animal isolates in Europe (Florez‐Cuadrado et al., 2017; Elhadidy et al., 2018).
Other new mechanisms of antimicrobial resistance in Campylobacter have emerged or have been evidenced in the last few years, such as gentamicin resistance genes borne on chromosomal genomic island or on self‐transmissible plasmids (Zhao et al., 2015; Yao et al., 2017) or the cfr(C) gene, borne on a conjugative plasmid and conferring resistance to phenicols, lincosamides, pleuromutilins and oxazolidinones (Tang et al., 2017).
As these mechanisms (efflux pumps) and/or their genetic support (plasmids, MDRGI) confer resistance to one or several families of antimicrobials of major importance for therapy (macrolides, fluoroquinolones or aminoglycosides) or could favour co‐selection of resistant clones or plasmids, it is necessary to optimise methods aimed at their early detection. Several modifications of the monitoring protocol have been proposed (EFSA, 2019), such as enlargement of the range of concentrations tested for erythromycin and ciprofloxacin and evaluation of the susceptibilities of additional molecules, such as phenicols. Whole genome sequencing of isolates with MDR, high‐level resistance to erythromycin or ciprofloxacin, or resistance to gentamicin should be implemented to evidence the involved genes, detect resistant clones and for comparison to human isolates.
Differences of occurrence of resistance of isolates from animals were observed between countries. These differences are probably associated to differences in use of antimicrobials. For human isolates, some of the differences observed between countries may result from the origins of reported data, according to local medical and diagnostic practices, which may result in the reporting of various clinical or regional subsets of isolates. Within a given MS, relating the occurrence of AMR in human Campylobacter isolates to that in isolates from food/food‐producing animals is complicated, as parts of the food consumed in a MS have originated from other MSs or third countries. Cases of human infection may also be associated with foreign travel and notably, while 80% of the Finnish Campylobacter infections in 2018 were related to travel, travel‐associated cases could not be excluded from the Finnish AMR data collected from primary laboratories. Human contamination from sources other than food animals – such as wild birds, pets and environmental water – should also be considered as potential modes of transmission (Moré et al., 2017; Szczepanska et al., 2017). Still, recent source attribution studies concluded that ruminants play an important role in human Campylobacter cases (Mossong et al., 2016; Thépault et al., 2018). A better knowledge of the resistance levels of C. jejuni and C. coli, not only in poultry but also in pigs and ruminants, is necessary, and mandatory monitoring of these two Campylobacter species in the different animal productions is suggested (EFSA, 2019).
4. Antimicrobial resistance in indicator E. coli 14
4.1. Data on AMR in indicator E. coli addressed
Throughout 2017 and 2018, data on AMR in indicator E. coli were obtained from caecal samples of food‐producing animals at slaughter according to the requirements laid down in Commission Implementing Decision 2013/652/EU. In 2017, it was mandatory to report data on E. coli isolates from fattening pigs and calves under 1 year of age and in 2018 on isolates from broilers and fattening turkeys. The specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli recovered from caecal samples of broilers, fattening turkeys, fattening pigs and calves under 1 year of age, as well as from fresh meat samples of broilers, pigs and bovines, was also mandatory over these reporting years (see Section 5 ESBL).
Studying phenotypic AMR of commensal ‘indicator’ E. coli from the intestinal flora of healthy food‐producing animals and from food derived from these animals provides information on the reservoirs of resistant bacteria that could potentially be transferred between animal populations and between animals and humans. It also provides indirect information on the reservoirs in animals and food of resistance genes that could be transferred to bacteria that are pathogenic for humans and/or animals. Such monitoring, therefore, has relevance to both public and animal health. The occurrence of resistance to antimicrobials in indicator E. coli is likely to depend on a number of factors including: the selective pressure exerted by the use of antimicrobials in various food‐producing animal populations; clonal spread of resistant organisms; dissemination of particular genetic elements, such as resistance plasmids; and the effects of co‐selection in bacteria exhibiting MDR.
4.2. Antimicrobial resistance in poultry, porcine and bovine populations
In 2017, 28 MSs and 3 non‐MSs reported quantitative AMR data on indicator E. coli isolates from caecal samples of fattening pigs and 10 MSs and 2 non‐MSs data on isolates from calves under 1 year of age. In 2018, 28 MSs and 4 non‐MSs reported data on isolates from caecal samples of broilers and 11 MSs and 1 non‐MS data on isolates from fattening turkeys.
4.2.1. Occurrence of resistance
Resistance to ampicillin, sulfamethoxazole, trimetoprim and tetracycline were the most common traits and most countries reported high or very high levels of resistance to these antimicrobials in all four animal populations (Figure 28 and Annex D). There were however large differences between countries and in broilers, turkeys and pigs some countries reported extremely high levels of resistance to these antimicrobials whereas, in contrast, others reported moderate or low levels in all four animal categories. Ciprofloxacin and nalidixic acid resistance were reported by more than half of the countries at very high or extremely high levels in broilers and turkeys, whereas low or moderate levels were mainly reported for pigs and calves (Figure 28 and Annex D). Generally, nalidixic acid resistance was reported at slightly lower levels than ciprofloxacin resistance.
Figure 28.
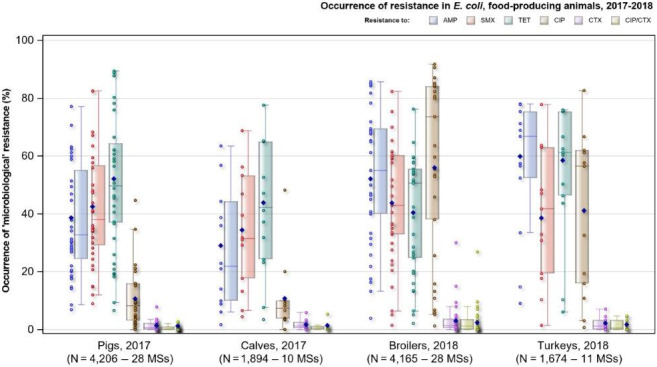
Distribution of occurrence of resistance to selected antimicrobials in indicator E. coli isolates recovered from fattening pigs and calves under 1 year of age, 2017 and from broilers and fattening turkeys, 2018, EU MSs and non‐MSs, 2017/2018
-
AMP: ampicillin, SMX: sulfamethoxazole, TET: tetracycline, CIP: ciprofloxacin, CTX: cefotaxime, CIP/CTX: combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime, N: total number of E. coli reported by MSs. Blue diamond shows resistance at the reporting‐MS group level.Note: Member States reporting at least 10 isolates are shown in the graph; all isolates are included in the calculation of resistance at the reporting‐MS group level.
Chloramphenicol resistance was mainly reported at low or moderate levels in all animal categories, but high, very high and even extremely high levels were reported by some countries (Annex D). In most countries, resistance to gentamicin, cefotaxime, ceftazidime, colistin or azithromycin was rare or reported at very low or low levels in all four animal categories although higher levels were reported by single countries (Annex D). Meropenem resistance was not detected in any isolate of indicator E. coli and tigecycline resistance in only 3 isolates from Belgium, 2 from broilers and 1 from pigs.
Occurrence of resistance to ‘critically important antimicrobials’
Among the antimicrobials tested in the mandatory monitoring, ciprofloxacin (fluoroquinolones), cefotaxime and ceftazidime (third‐generation cephalosporins), meropenem (carbapenems), colistin (polymyxins) and azithromycin (macrolides) have been categorised by the WHO as CIAs and among substances of the highest priority (WHO, 2019).
In 2017 and 2018, meropenem resistance was not observed among indicator E. coli from the four animal categories and colistin and azithromycin resistance mainly at low or very low levels. In contrast, at the EU‐level, very high or extremely high levels of resistance to fluoroquinolones/quinolones were observed in indicator E. coli isolates from broilers (median 73.5% for ciprofloxacin and 64.1% for nalidixic acid), and high levels also in isolates from turkeys (median levels 34.8% for ciprofloxacin and 56.5% for nalidixic acid) (Figure 28). Resistance to ciprofloxacin and nalidixic acid were reported at much lower levels in isolates from pigs (median 7.4% and 6.2%, respectively) and calves (median 8.4% and 4.2%, respectively). There were however large variations between reporting countries for each of the animal categories (Figure 29). In non‐MS, resistance to fluroquinolones/quinolones was either not detected or found at low or very low levels in animal categories reported. An exception was Switzerland who reported high levels of resistance to both nalidixic acid (45.8%) and ciprofloxacin (45.3%) in isolates from broilers.
Figure 29.
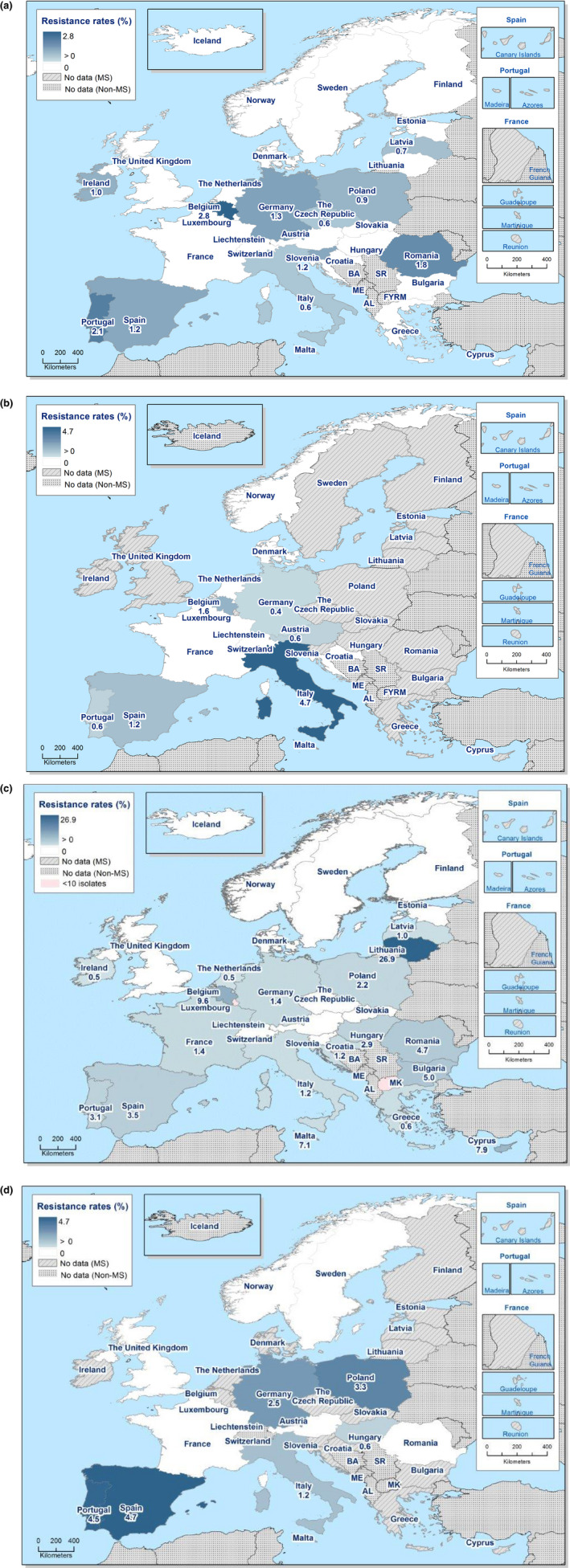
Spatial distribution of combined ‘microbiological’ resistance to cefotaxime and ciprofloxacin in indicator Escherichia coli. (a) fattening pigs, 28 MSs and 3 non‐MSs 2017, (b) calves under 1 year of age, 10 MSs and 2 non‐MSs 2017, (c) broilers, 27 MSs and 3 non‐MSs 2018, (d) fattening turkeys, 11 MSs and 1 non‐MSs 2018
In all animal categories, resistance to third generation cephalosporins (cefotaxime or ceftazidime) was either not observed or in some countries detected at very low or low levels (Figure 28). Exceptions were Lithuania, which in isolates from broilers reported a high level of resistance (30.1%), and Belgium which reported a moderate level (14.8–16.2%). At the EU‐level, median levels of resistance to cefotaxime and ceftazidime were similar in the four animal categories at 0.6% vs. 0.6% in isolates from pigs, 1.2% vs. 0.9% in isolates from calves, 1.4% vs. 1.4% in isolates from broilers, and 1.2% vs. 1.2% in isolates from turkeys. Resistance to third generation cephalosporins was not reported in any animal category by non‐MS, except for Norway who reported 2 isolates (0.7%) from calves.
Combined resistance to ciprofloxacin and cefotaxime
In most reporting countries, ‘microbiological’ combined resistance to ciprofloxacin and cefotaxime was either not observed or detected at very low or low levels in all four animal categories except in Lithuania where the level was high (26.9%) in isolates from broilers (Figure 29). Considering all reporting countries, mean levels of ‘microbiological’ combined resistance, were very low in pigs and calves and low in broilers and turkeys and mean levels of ‘clinical’ combined resistance were very low in all four animal categories (Table 8).
Table 8.
Overall levels of combined resistance to ciprofloxacin and cefotaxime applying ECOFFs and clinical breakpoints issued by EUCAST, EU MSs and non‐MSs
| Food‐producing animal category | ‘Microbiological’ combined resistance to CIP & CTX (using EUCAST ECOFFs) | ‘Clinical’ combined resistance to CIP & CTX (using clinical breakpoints) | ||
|---|---|---|---|---|
| No. of isolates | % Resistance | No. of isolates | % Resistance | |
| Fattening pigs (2017, N = 4,747, 28 MSs, 3 non‐MSs) | 24 | 0.5% | 12 | 0.3% |
| Calves < 1 year (2017, N = 2,383, 10 MSs, 2 non‐MSs) | 16 | 0.7% | 6 | 0.3% |
| Broilers (2018, N = 4,739, 28 MSs, 4 non‐MSs) | 100 | 2.1% | 40 | 0.8% |
| Fattening turkeys (2018, N = 1,810, 11 MSs, 1 non‐MSs) | 28 | 1.5% | 12 | 0.7% |
N: total number of E. coli isolates reported by MSs and non‐MSs; CIP: ciprofloxacin; CTX; cefotaxime.
4.2.2. Temporal trends in resistance among indicator E. coli
Due to the lack of longitudinal data, evaluation of temporal trends in resistance cannot yet be made for all the countries participating in the harmonised monitoring. For countries that have provided data on indicator E. coli from caecal content of fattening pigs, calves under 1 year of age and broilers for 4 years or more in 2009–2018 and for fattening turkeys for 3 years or more in 2014–2018, trends in resistance to ampicillin, ciprofloxacin, cefotaxime and tetracycline are presented below. The statistical significance (p ≤ 0.05) of trends was tested by logistic regression.
Fattening pigs
Eleven countries (10 MSs and 1 non‐MS) have provided data on indicator E. coli from fattening pigs for 4 years or more in the period 2009–2017 (Figure 30). Resistance to ampicillin has decreased in four countries (France, Hungary, the Netherlands and Switzerland) and increased in five countries (Austria, Belgium, Denmark, Poland and Spain). Resistance to cefotaxime has decreased in three countries (France, Hungary and the Netherlands) and increased in one country (Belgium). Ciprofloxacin resistance has decreased in three countries (Belgium, the Netherlands and Poland) and increased in one country (Spain). Tetracycline resistance has decreased in seven countries (Austria, Belgium, Estonia, France, the Netherlands, Poland and Switzerland) and increased in one country (Hungary). Overall, in the 11 countries, there are 17 decreasing and 8 increasing trends over the period. In Estonia, France, the Netherlands and Switzerland, only decreasing trends are detected. Notably in the Netherlands, resistance is decreasing for all four antimicrobials considered, and in France, resistance to three of the substances is decreasing. In contrast, in three countries there are only increasing trends: in Spain for two antimicrobials (ampicillin, ciprofloxacin) and Austria and Denmark to 1 antimicrobial (ampicillin). In Belgium, Hungary and Poland, both decreasing and increasing trends are detected and in Finland resistance is stable at low levels.
Figure 30.
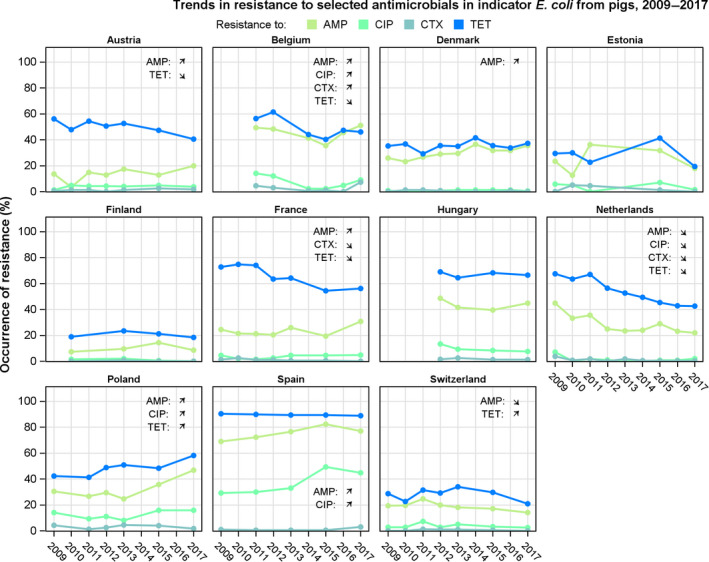
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from pigs, reporting countries, 2009–2017
Calves under 1 year of age
Eight countries (7 MSs and 1 non‐MS) have provided data on indicator E. coli from calves under 1 year of age for 4 years or more in the period 2009–2017 (Figure 31). Resistance to ampicillin has decreased in two countries (Germany and the Netherlands) and increased in two countries (Austria and Switzerland). Resistance to cefotaxime has decreased in three countries (Belgium, Germany and the Netherlands) and increased in one country (Poland). Ciprofloxacin resistance has decreased in three countries (Belgium, Germany and the Netherlands) and increased in two countries (Austria and Switzerland). Tetracycline resistance has decreased in two countries (Germany and the Netherlands) and increased in three countries (Austria, Belgium and Switzerland).
Figure 31.
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from calves under 1 year of age, 2009–2017
Overall, in the 8 countries, there are 10 decreasing and 8 increasing trends over the period. In two countries (Germany and Netherlands), there are only decreasing trends and notably in both countries, levels of resistance are decreasing for all four antimicrobials considered. In contrast in three countries there are only increasing trends, in Austria and Switzerland for three antimicrobials and in Poland for one antimicrobial. For two countries (Denmark and, Spain), there are no statistical trends in resistance and levels are stable at low levels in Denmark and at high levels in Spain.
Broilers
Fifteen countries (13 MSs and 2 non‐MS) have provided data on indicator E. coli from broilers for 4 years or more in the period 2009–2018 (Figure 32). Resistance to ampicillin has decreased in seven countries (Croatia, France, Germany, Ireland, the Netherlands, Norway and Spain) and increased in three countries (Belgium, Finland and Poland). Resistance to cefotaxime has decreased in seven countries (Croatia, France, Germany, the Netherlands, Poland, Spain and Switzerland) and increased in one country (Belgium). Ciprofloxacin resistance has decreased in four countries (Austria, Ireland, the Netherlands and Sweden) and increased in five countries (Finland, Hungary, Norway, Poland and Switzerland). Tetracycline resistance has decreased in seven countries (France, Germany, Ireland, the Netherlands, Norway, Spain and Switzerland) and increased in two countries (Belgium and Poland).
Figure 32.
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracyclines (TET) in indicator E. coli from broilers, 2009–2018
Overall, in the 15 countries, there are 25 decreasing and 11 increasing trends over the period. In eight countries (Austria, Croatia, France, Germany, Ireland, the Netherlands, Spain and Sweden), there are only decreasing trends to one or more of the antimicrobials. In contrast, in three countries, there are only increasing trends, in Belgium for three antimicrobials, in Hungary for one antimicrobial and in Finland for two antimicrobials, although at low levels in the latter country. For three countries (Norway, Poland and Switzerland), there are both increasing and decreasing trends and in one country (Denmark) resistance is stable at low levels.
Fattening turkeys
Eleven MSs have provided data on indicator E. coli from fattening turkeys for 3 years or more in the period 2014–2018 (Figure 33). There are no increasing trends for any of the four antimicrobials evaluated. However, decreasing trends are observed for ampicillin in four countries (Austria, Sweden, Spain and the UK) for ciprofloxacin in four countries (Austria, Romania, Spain and the UK) and for tetracyclines in seven countries (France, Hungary, Poland, Portugal, Spain, Sweden and the UK). Notably, in Spain and the UK resistance to all three antimicrobials has decreased, and at the overall EU level. Over the period evaluated, resistance to cefotaxime has remained stable at low levels in all 11 countries. Overall, there are 15 decreasing trends and no increasing trend in the 11 countries evaluated. Since only data for 3 years were available for evaluation, and only for a limited period, the positive trends should be interpreted with caution and need to be confirmed over a longer period.
Figure 33.
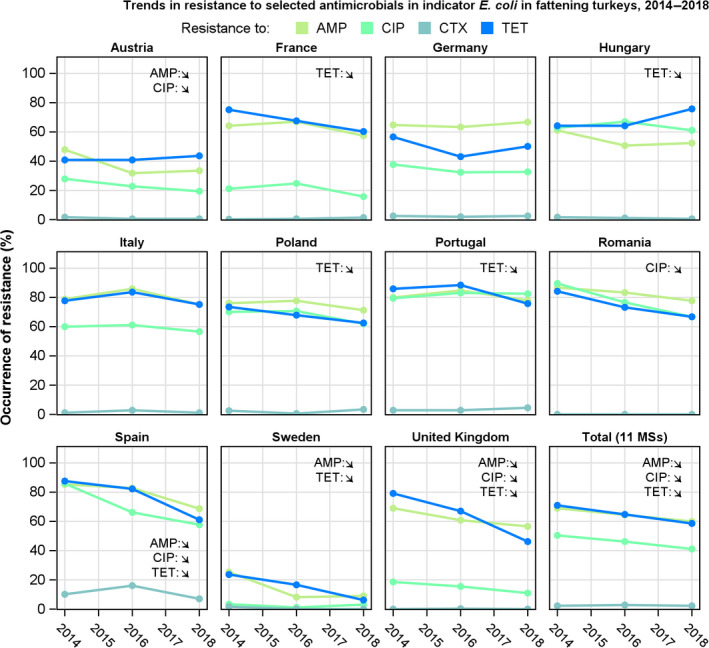
Trends in resistance to ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP) and tetracycline (TET) in indicator E. coli from fattening turkeys, 2014–2018
4.2.3. Phenotypic characterisation of third‐generation cephalosporin and carbapenem resistance in indicator E. coli from caecal samples15
A low number of indicator E. coli isolates from caecal samples from pigs and calves under 1 year of age in 2017 and from broilers and fattening turkeys in 2018 were phenotypically resistant to third‐generation cephalosporins (cefotaxime or ceftazidime) on initial testing on panel 1 (see Annex A, ‘Materials and methods’). Further phenotypic characterisation of these isolates for presumptive production of ESBL‐ and/or AmpC‐enzymes on panel 2 showed that the total number of presumptive ESBL‐ and/or AmpC producers was low in all four animal categories but that occurrence was higher in isolates from broilers and turkeys than in isolates from pigs and calves (Table 9).
Table 9.
Occurrence of resistance to third‐generation cephalosporins in indicator E. coli isolates from fattening pigs, calves under 1 year of age, broilers and fattening turkeys. EU MSs and non‐MSs, 2017/2018
| Animal category | No. of MSs/non‐MSs | N | Cefotaxime | Ceftazidime | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Fattening pigs – 2017 | 28/3 | 4,747 | 58 | 1.2% | 55 | 1.2% |
| Calves, < 1 year – 2017 | 10/2 | 2,383 | 32 | 1.3% | 29 | 1.2% |
| Broilers – 2018 | 28/4 | 4,739 | 125 | 2.6% | 116 | 2.4% |
| Fattening turkeys – 2018 | 11/4 | 1,810 | 36 | 2.0% | 34 | 1.9% |
N: Total number of isolates tested by MSs; n: Total number of isolates resistant; MSs: Member states.
Presumptive ESBL‐ and/or AmpC‐producing isolates were reported from pigs by 14 of 28 MSs, from calves by 5 of 10 MSs, from broilers by 21 of 28 MSs and from turkeys by 8 of 11 MSs. None of the non‐MSs reported presumptive ESBL‐ and/or AmpC‐producing isolates (pigs 3 non‐MSs; calves 2 non‐MSs; broilers 4 non‐MSs; turkeys and 1 non‐MSs).
In countries reporting presumptive ESBL‐ and/or AmpC‐producing isolates occurrence was generally low, ranging from 0.6% to 6.3% in isolates from pigs, from 1.2% to 5.3% in isolates from calves and from 0.6% to 7.1% in isolates from turkeys. Occurrence was generally low also in broilers and in 19 of the MSs ranged from 0.6% to 7.9% but was moderate in Belgium (12.8%) and high in Lithuania (29%). Presumptive ESBL producers were more common than AmpC producers in all animal categories and isolates with a combined phenotype (ESBL+AmpC) were uncommon (Table 10).
Table 10.
Phenotypes of presumptive ESBL‐, AmpC‐ or CP‐ producing indicator E. coli subjected to supplementary testing (panel 2). EU MSs and non‐MSs, 2017/2018
| Animal category | ESBL and/or AmpC n (% R) | ESBLa n (% R) | AmpCb n (% R) | ESBL + AmpCc n (% R) | CPd n (%R) |
|---|---|---|---|---|---|
| Fattening pigs, 2017 | 52 (1.1) | 38 (0.8) | 14 (0.3) | 0 | 0 |
| Calves < 1 year, 2017 | 28 (1.2) | 26 (1.1) | 7 (0.3) | 5 (0.2) | 0 |
| Broilers, 2018 | 115 (2.4) | 82 (1.7) | 38 (0.8) | 5 (0.1) | 0 |
| Fattening turkeys, 2018 | 35 (1.9) | 31 (1.7) | 5 (0.3) | 1 (0.1) | 0 |
ESBL: extended‐spectrum β‐lactamase; CP: carbapenemase; N: Total number of isolates reported by MSs and non‐MSs; n: number of isolates with this phenotype; % R: percentage of isolates from the total tested; ESBL; extended‐spectrum β‐lactamase.
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
No isolate of indicator E. coli recovered from caecal samples by MSs and non‐MSs from the four animal categories in 2017/2018 showed microbiological resistance to carbapenems (meropenem) on initial testing on panel 1.
4.2.4. MDR and complete susceptibility in indicator E. coli
MDR
MDR, defined as ‘microbiological’ resistance to three or more antimicrobial classes, was observed in 34.9% (1,668/4,774) of indicator E. coli isolates from pigs, in 27.7% (659/2,383) from calves, in 42.2% (2,002/4,739) from broilers and in 43.5% (787/1,810) from turkeys. There were large variations between reporting countries and MDR was generally observed at higher levels among isolates from broilers and turkeys than among isolates from pigs and calves.
MDR patterns
A wide variety of resistance patterns were observed in MDR isolates. The antimicrobials most often represented in the patterns of isolates from pigs and calves were tetracycline, ampicillin, sulfamethoxazole and trimethoprim. About half of the MDR isolates from pigs (48.5%, 809/1,668) and calves (54.5%, 359/659) were resistant to all these four antimicrobials and often also to other substances. These antimicrobials, alone or in combination with other substances, were also common in resistance patterns of MDR isolates from broilers (43.4%, 869/2,002) and turkeys (45.7%, 360/787). MDR patterns of isolates from poultry often included quinolones at 78.9% (1,579/2,002) for broilers and 71.7% (560/787) for turkeys. In contrast, quinolones were less often included in the patterns of MDR isolates from pigs (24.8%, 414/1,668) and calves (28.9%, 191/659).
Resistance to colistin was uncommon in the patterns of MDR isolates, at 0.5% (9/1,668) in pigs, 2.3% (15/659) in calves, 1.3% (25/2,002) in broilers 6.5% (51/787) and in turkeys. Also, resistance to third‐generation cephalosporins was uncommon at 3.3% (54/1,668) in pigs, 3.6% (24/659) in calves, 6.2% (124/2,002) in broilers and 4.3% (34/787) in turkeys.
Completely susceptible isolates
Occurrence of resistance can also be addressed by considering the proportion of indicator E. coli isolates exhibiting susceptibility to all the 14 antimicrobials tested, using ECOFF values for interpretation. Overall, 39.2% (1,875/4,774) of isolates from pigs, 56.7% (1,350/2,383) from calves, 27.8% (1,319/4,742) from broilers and 27.8% (504/1,810) from turkeys showed complete susceptibility. However, for all animal categories the levels of complete susceptibility varied widely between individual countries (Figure 34). Thus, complete susceptibility in isolates from pigs ranged from 5.3% in Spain and Cyprus to 84.5% in Norway and among isolates from calves, from 19,41% in Italy to more than 90% in Norway and Denmark. Likewise, the proportion of completely susceptible isolates from broilers ranged from 1.8% in Greece to over 90% in Norway and Finland and in isolates from turkeys from between 7.6% in Portugal to 80.3% in Sweden. Typically, the highest levels of complete susceptibility in all four animal categories were in isolates from the Nordic countries, with levels generally decreasing in a north to south gradient.
Figure 35.
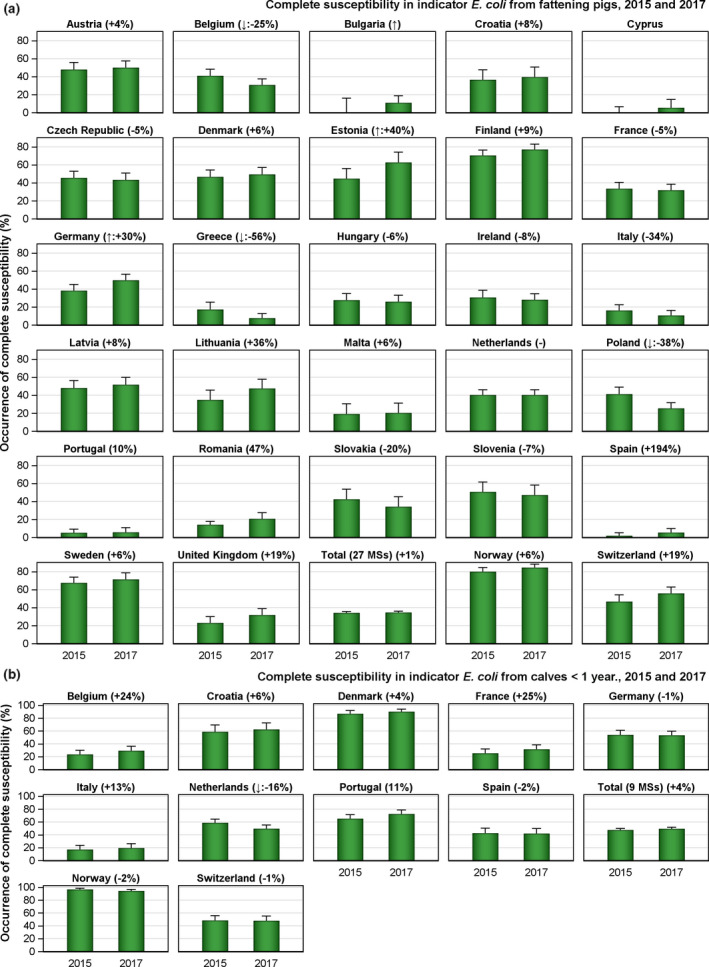
Changes in the occurrence of complete susceptibility to the panel of antimicrobials tested in indicator E. coli isolates from (a) fattening pigs and (b) calves < 1 year of age, between 2015 and 2017
-
(↓)/(↑): indicates statistically significant trends between 2015 and 2017.The upper bounds of the 95% confidence interval of the occurrence of complete susceptibility and the rate of change (in %) are also indicated.
Changes in complete susceptibility
For pigs, there was no significant difference in the level of complete susceptibility between 2015 and 2017 at the overall MSs level (Figure 35). However, in Bulgaria, Estonia and Germany, levels have increased significantly in the period, whereas there are significant decreases in Belgium, Greece and Poland. For calves, complete susceptibility has also remained stable at the MSs level between 2015 and 2017 as in most countries, except in the Netherlands where the level has increased (Figure 35).
For broilers, there was a significant increase in complete susceptibility at the overall MSs level over the years 2014, 2016 and 2018 (Figure 36). Also, the level of full susceptibility has increased in 11 individual countries (Austria, Bulgaria, France, Ireland, Italy, Latvia, Lithuania, the Netherlands, Romania, Slovakia and the UK) whereas it has decreased in 2 countries (Denmark and Germany). The complete susceptibility has increased significantly also for turkeys at the overall level and in seven MSs (France, Hungary, Romania, Spain, Sweden and the UK) (Figure 36).
Figure 36.
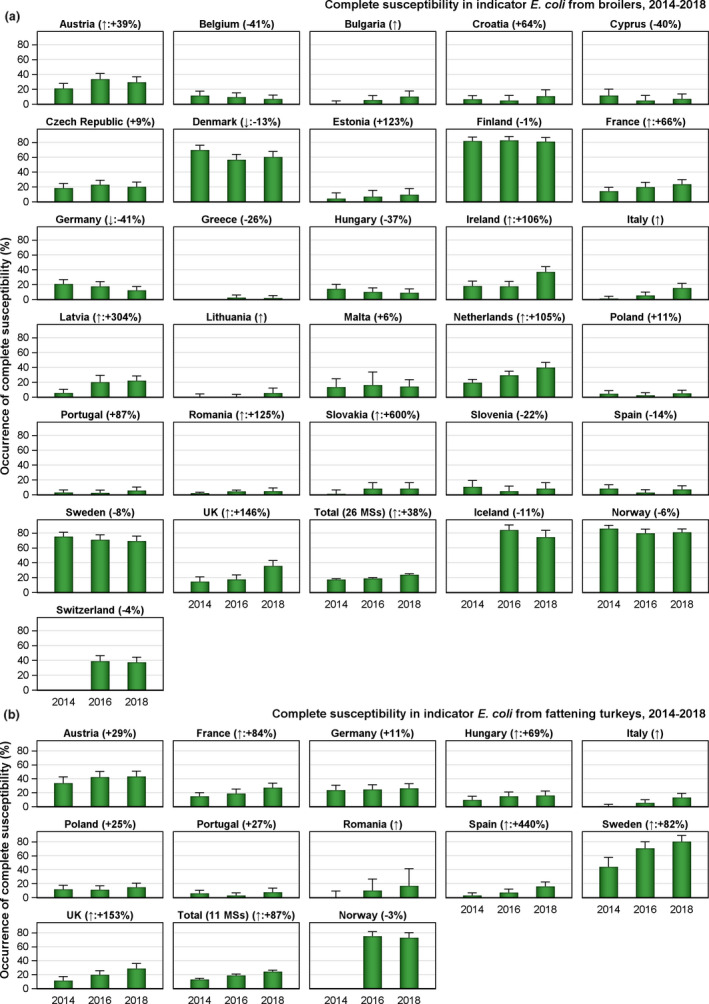
Changes in the occurrence of complete susceptibility to the panel of antimicrobials tested in indicator E. coli isolates from (a) broilers and (b) fattening turkeys, 2014–2018
- (↓)/(↑): indicates statistically significant trends over the 2014–2018 period. The upper bounds of the 95% confidence interval of the occurrence of complete susceptibility and the rate of change (in %) are also indicated.
Figure 34.
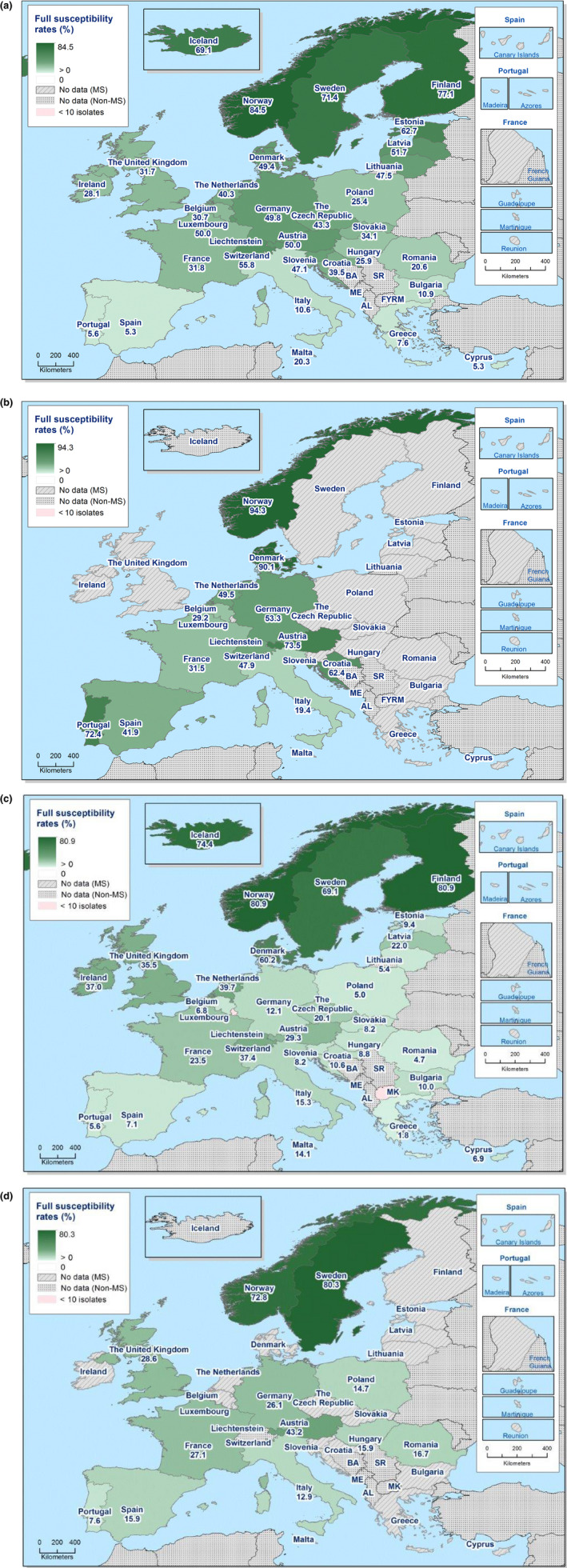
Spatial distribution of complete susceptibility to the antimicrobials tested in indicator E. coli. (a) fattening pigs, 28 MSs, 3 non‐MSs, 2017; (b) calves < 1 year of age, 10 MSs, 2 non‐MSs, 2017; (c) broilers, 28 MSs, 4 non‐MSs, 2018; (d) fattening turkeys, 11 MSs, 1 non‐MSs, 2018, EU MSs and non‐MSs
Key outcome indicator of complete susceptibility
The proportion of indicator E. coli isolates from the most important production animals, i.e. broilers, fattening turkeys, fattening pigs and calves (collected in the framework of Commission Implementing Decision 2013/652/EU), weighted by the size of the four animal populations, that are completely susceptible to the entire panel of antimicrobials defined in the Decision, has been retained as the primary outcome indicator (OICS) in food‐producing animals. The harmonised AMR monitoring in the EU yields data based on use of the same panel of antimicrobials and applying criteria (ECOFF) when interpreting resistance (Moyaert et al., 2014). Adherence to legislation would guarantee this uniformity. Indicator E. coli is selected as the reporting organism instead of zoonotic organisms, since it is expected to better represent the overall AMR situation, including resistance due to plasmid‐mediated AMR genes. Plasmid‐mediated AMR genes are considered to be a more significant part of the total resistance that could be transferred from the agricultural sector to human healthcare than most antimicrobial‐resistant zoonotic pathogens (Hammerum et al., 2014). A general and abundant reporter species representing the overall AMR situation is therefore more relevant than less abundant zoonotic species. The OICS can be used to assess the development of AMR in relation to the total use of antimicrobials in food‐producing animals (Queenan et al., 2016; ECDC, EFSA and EMA, 2017). The assumption underlying the choice of this specific indicator is that only E. coli that is rarely, if ever, exposed to antimicrobials will be fully susceptible (Martinez, 2014). Therefore, it is to be expected that a reduction of the use of antimicrobials in food‐producing animals would result in a noticeable improvement of this indicator.
The populations of food‐producing animals differ in size within and between European countries. The relative size of those varying populations may influence resistance issues related to the overall food animal production at the country level as well as at the European level. This makes it difficult to evaluate overall trends and to assess the overall magnitude of resistance in food‐producing animals within and between countries. To account for differences in the relative size of food animal populations in a country, the OICS was calculated as the weighted mean of the proportions of completely susceptible indicator E. coli isolates in each of the four animal populations monitored (fattening pigs, calves under 1 year of age, broilers, fattening turkeys). For calculation of the OICS, the value for each population was weighted in relation to the relative size of the populations within a country using the ‘population correction unit’ (PCU). Regarding cattle, only calves under 1 year of age were included in calculation of the PCU. PCU is a technical unit of measurement used as an indicator of animal population size and was developed by the EMA, primarily to estimate sales of antimicrobials corrected by the animal population in individual countries. The data sources and methodology for the calculation of PCU are comprehensively described in EMA's report ‘Sales of veterinary antimicrobial agents in 31 European countries in 2017’ (EMA, 2019). For each country, OICS was calculated using data reported for two consecutive years. Thus, values for 2014–2015 were calculated from data for broilers and fattening turkeys reported in 2014 and on data for fattening pigs and calves under 1 year of age reported in 2015. Likewise, the values for 2015–2016 were calculated from data reported for pigs and calves in 2015 and on data for broilers and fattening turkeys reported in 2016, and so on. For each value of OICS calculated for a single country, data for broilers and pigs were included. However, since all countries haven't reported data for calves and turkeys regularly, all calculations did not include data for these categories.
OICS and its rate of change (expressed in %) for the 27 MSs and 3 non‐MSs reporting data on resistance over the period 2014–2018, are presented in Figure 37. Marked variations in OICS were registered between countries: in 8 countries, OICS were noted at levels of < 20%, in 10 countries at 20–40%, in 7 countries at 40–60%, in 4 countries at 60–80% and in one country (Norway) at > 80% for 2017–2018. The lowest OICS were generally observed in countries from Eastern and Southern Europe, while the highest OICS were generally noted in countries from northern parts of Europe. For some countries, OICS have been stable at a high level over the period studied and in others, at a low level. Interestingly, statistically significant increasing trends in OICS were registered by 6 MSs, whereas 3 MSs registered statistically significant decreasing trends. Ten other MSs also recorded non‐significant increasing trends greater than 5% over the study period. The 2014–2018 trends in OIcs need to be confirmed through further follow‐up.
Notably, the relative contribution from the data submitted from the different animal populations studied by the individual reporting countries and the relative size of those animal populations have an impact on the calculation of summary OICS. In 6 of the 9 countries, a positive or negative trend in OICS, is concurrent with a similar trend in the levels of complete susceptibility of isolates from pigs (Figure 35). Similarly, in countries where there is a positive or negative trend in complete susceptibility in pigs, this is reflected in the OICS. Positive or negative trends in one animal category of small relative size within a country may therefore go unnoticed if masked by opposing changes in another category, if the summary OICS is used as the sole indicator. For example, in Germany, a significant negative trend in complete susceptibility in isolates from broilers (Figure 36) is outweighed by a significant positive trend in pigs, resulting in a statistically significant positive trend in OICS. Conversely, in France, the significant increasing trends in complete susceptibility observed in broilers and turkeys is masked overall by the lack of significant changes in complete susceptibility in pigs and calves. Data on resistance/complete susceptibility should therefore also be evaluated at the level of the individual animal populations to fully appreciate the situation within a given country.
Figure 37.
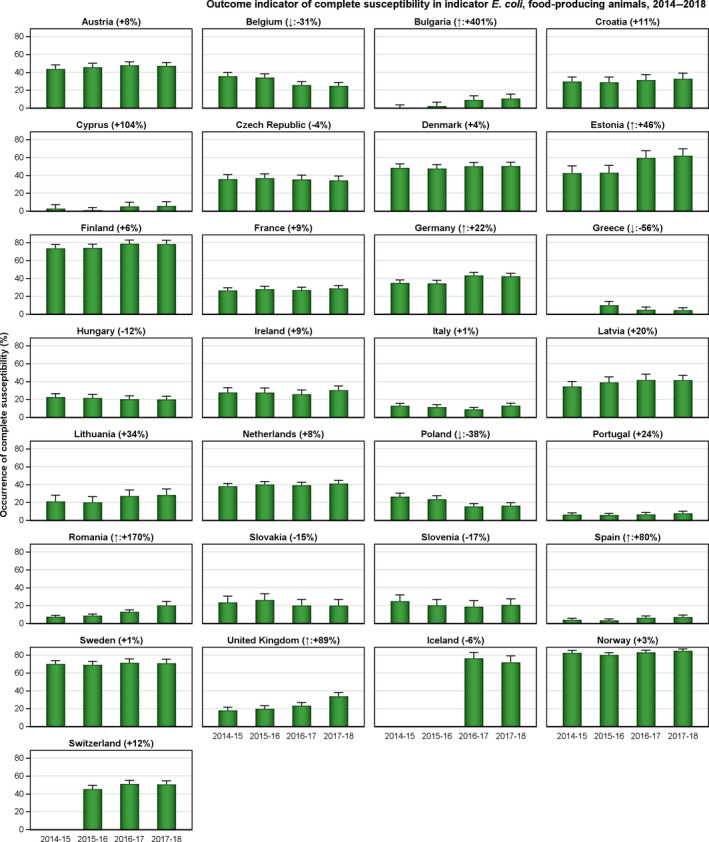
Changes in outcome indicator of complete susceptibility (OICS), 26 MSs and 3 non‐MSs, 2014–2018
- (↓)/(↑): indicates statistically significant decreasing/increasing trends over the 2018–2014 period. The upper bounds of the 95% confidence interval of the OICS and the rate of change (in %) are also indicated.
4.2.5. Colistin resistance in indicator E. coli
Colistin (polymyxin E) is an antimicrobial of the polymyxin group that has been used extensively in farm animals all over the world, including Europe. In human medicine, use of colistin has historically been limited. However, in recent years there has been an increased usage in human medicine due to the need for last resort antimicrobials to treat infections caused by MDR Gram‐negative bacteria. Consequently, polymyxins are now among the five antimicrobials listed by WHO as critically important and of highest priority for human medicine. The discovery of transferable genetic elements (e.g. mcr‐genes) conferring resistance to colistin, further underlines the importance of monitoring such resistance in food animals.
In the EU MSs, colistin resistance in indicator E. coli was observed at very low mean levels in isolates from pigs (mean 0.3%; median 0%), calves under 1 year of age (mean 0.8%; median 0%) and broilers (mean 0.7%; median 0%) and at a low level in isolates from fattening turkeys (mean 3.2%; median 0%) (Annex D).
About one‐third of the countries (9/28 MSs, 0/3 non‐MSs) reported colistin resistance in isolates from pigs at levels ranging between 0.4% and 2.1% in the individual countries. Also, for broilers about one‐third of the countries reported colistin resistance (8/28 MSs, 0/4 non‐MSs) at levels ranging between 0.6% up to 4.7% in Germany and Romania. About one‐third of the countries (4/10 MSs, 0/2 non‐MSs) reported colistin resistance in isolates from calves at levels ranging between 1.0% and 2.9%. In contrast, colistin resistance in isolates from fattening turkeys was reported by about half of the countries (5/11 Mss, 1/1 non‐MSs) at low/moderate levels ranging from 0.7% and up to 9.1% in Germany and 17.4% in Portugal.
The levels of colistin resistance in isolates from pigs and calves reported in 2017 are about the same as those reported in 2015 (pigs 0.4%; calves 0.3%) and also levels in individual countries are about the same in the two years. However, for broilers and fattening turkeys, the levels reported in 2018 are lower than those reported in 2016, (broilers 1.9%; fattening turkeys 6.1%). For isolates from broilers, most individual countries reported about the same levels of resistance in 2016 and 2018 but, in some countries marked reductions were observed from 2016 to 2018, notably in Cyprus from 9.5% to 2.9% and in Portugal from 5.6% to 1.2%. Also, among isolates from fattening turkeys were marked reductions in resistance observed in individual countries, notably in Portugal from 25.1% to 2.2%, in Italy from 14.7% to 1.2% and in Romania from 6.7% to 0%.
One MSs (Italy), voluntarily reported data for indicator E. coli from both meat and caecal content of pigs and bovines in 2017. In pigs, colistin resistance was higher in isolates from meat than in isolates from caecal content (5.3% vs. 0.6%), whereas occurrence was similar in both matrices for bovines (2.1% vs. 2.9%).
The mandatory monitoring according to Decision 2013/652/EU is based on phenotypic susceptibility and does not discriminate between different resistance mechanisms. Therefore, molecular testing would be required to confirm the underlying mechanisms of resistance and inference regarding the presence of mcr‐genes.
4.2.6. Discussion
To study phenotypic AMR of commensal ‘indicator’ E‐coli from caecal content of healthy food‐producing animals provides information on the reservoirs of resistant bacteria that could potentially be transferred between animals and between animals and humans. Monitoring, therefore, has relevance to both public and animal health. AMR in indicator E. coli is likely to depend on several factors, such as the selective pressure from the use of antimicrobials in food‐producing animals, the co‐selection of bacteria with multiple resistance, the clonal spread of resistant bacteria and the dissemination of genetic elements, such as plasmids, between bacteria.
Representative monitoring
The data on AMR in E‐ coli from caecal content of healthy food‐producing animals in the present report was collected in the years 2014–2018 in accordance with the methodology for AMR monitoring laid down in Commission Implementing. Decision 2013/652/EU. The presented data is therefore harmonised with respect to sampling design, laboratory methodology, reporting and interpretation of resistance. Data collected previously may, however, be impacted by differences in methodology.
In the period 2014–2018, data on E. coli from caecal content of fattening pigs and broilers was reported by the majority of EU MSs. Thus, data for pigs was reported by 27 and 28 MSs in 2015 and 2017, respectively, and for broilers by 27, 27 and 28 MSs in 2014, 2016 and 2018, respectively. The data for pigs and broilers can therefore be considered representative at the EU level. In the same period, a minority of MSs reported data for calves under 1 year of age and for fattening turkeys. Thus, 10 MS reported data for calves in 2015 and 2017, and 11 MSs data for turkeys in 2014, 2016 and 2018. The data for calves and turkeys can still be considered representative at the EU level because the main producers of meat derived from these animal categories in EU (Eurostat) are among the reporting MSs.
General observations
At the EU level, resistance to ampicillin, sulfamethoxazole, trimetoprim and tetracycline was common in indicator E. coli from caecal content and reported by most MSs at high or very high levels in pigs and calves in 2017 and in broilers and turkeys in 2018. In poultry, resistance to ciprofloxacin and nalidixic acid was also common and several MSs reported very high or extremely high levels in broilers and fattening turkeys. The high levels of resistance probably reflect a common past, and present use of these antimicrobials in food‐producing animals in several MSs.
There were notable spatial differences in the occurrence of resistance for most antimicrobials as well as in occurrence of MDR and complete susceptibility and as well as for the summary index SICS. Regarding pigs and broilers, the situation was generally more favourable in northern than in southern and eastern Europe. The limited number of countries reporting data for calves and turkeys precludes valid conclusions on spatial differences, but the available data for turkeys indicate a similar spatial distribution as for pigs and broilers. For calves, the picture is more complex and although the most favourable situation was reported by the Nordic countries (Norway, Denmark), countries in southern (Portugal, Croatia) and central Europe (Austria) also reported favourable situations in comparison to neighbouring countries in these regions.
Overall, in several countries, there appears to be a trend towards an improved situation regarding resistance in intestinal E. coli from food‐producing animals, although starting from different levels. It should however be noted that in some countries, levels of resistance to individual antimicrobials, complete susceptibility and SICS have been consistently stable at low levels and major changes cannot be expected. The overall positive trend is possibly to some extent due to the overall decline in sales of antimicrobials for use in animals since 2011, as noted in the recent ESVAC report (EMA, 2019).
Comparison of resistance in fattening pigs, calves under 1 year of age, broilers and fattening turkeys
There were no major differences in occurrence of resistance to gentamicin, cefotaxime, ceftazidime, meropenem, tigecycline, azithromycin and colistin between the four animal categories. At the EU level, median levels of resistance to these antimicrobials was rare, very low or low in all four categories, although levels could be considerably higher in individual countries. Also, for sulfamethoxazole and trimetoprim resistance, there were no major differences, and median levels in MSs were high in all four animal categories.
In contrast, median levels of resistance to some antimicrobials in MSs were higher in poultry than in pigs and calves. Thus, median levels of ampicillin resistance were higher in broilers (55.0%) and turkeys (66.8%) than in pigs (32.5%) and calves (21.9%). Likewise, levels of ciprofloxacin and nalidixic acid resistance were much higher in broilers (73.5%/64.1%) and turkeys (56.5%/34.8%) than in pigs (7.4%/6.2%) and calves (8.4%/4.2%). Additionally, median levels of chloramphenicol resistance were higher in turkeys (23.5%) than in pigs, calves and broilers (12.0–14.1%) and median levels of tetracycline resistance were higher in turkeys (61.2%) than in pigs, broilers and calves (42.2–50.6%).
Indications that resistance is more common in isolates of indicator E. coli from poultry than in isolates from pigs and calves are also found in the data on occurrence of MDR and completely susceptible isolates. Thus, median levels of MDR isolates were higher in broilers (49.4%) and turkeys (52.4%) than in pigs (31.1%) and calves (28.4%). On the contrary, median levels of completely susceptible isolates in MSs were lower in broilers (10.6%) and turkeys (16.7%) than in pigs (34.1%) and calves (51.4%).
The observed differences between animal species could reflect a difference in the quantity of antimicrobials used, but possibly also the mode of administration. In poultry, flock treatment is almost exclusively practised, whereas pigs and calves often are in some countries mainly treated individually.
Trends in resistance
Trends in levels of resistance to ampicillin, ciprofloxacin, cefotaxime and tetracycline as well as in levels of completely susceptible isolates and SICS were assessed by logistic regression for countries that have provided relevant data the different animal categories. Overall, the trend analyses reveal a progress towards lower levels of resistance in several of the reporting countries.
Regarding resistance to the individual antimicrobials, there were 64 decreasing and 24 increasing trends in the period 2009–2018 and for all the four substances evaluated there were more decreasing than increasing trends. Notably, in the Netherlands, resistance to all 4 antimicrobials has decreased in pigs, calves and broilers and for turkeys there were no increasing trend observed in any of the 11 countries evaluated.
For levels of complete susceptibility in indicator E. coli, there were no significant differences at the EU‐ level for pigs and calves between 2015 and 2017. However, levels in isolates from pigs have increased significantly in 3 MSs and decreased in 3 MSs, and in calves the level has increased in one MSs. For both broilers and turkeys there were significant increases at the overall MSs level over the years 2014, 2016 and 2018. Notably, for broilers, the level of full susceptibility has increased in 11 MS and decreased in only 2 MSs and for turkeys the level has increased in 7 MSs.
The summary index, OICS, intended to account for differences in the relative size of food animal populations in a country in evaluation of risks related to resistance, has in most countries been stable at a high or low level. In six countries, there are significant trends towards a higher OICS whereas in 3 countries there are trends towards decreasing values. Trends in complete susceptibility of isolates from pigs are reflected in OICS whereas trends in isolates from broilers and turkeys have smaller impact and are not always mirrored in the summary index.
Complete susceptibility and MDR
Considering all reporting countries, the occurrence of E. coli isolates susceptible to all antimicrobial classes tested was lower in broilers (27.8%) and turkeys (27.8%) than in pigs (39.2%) and calves (56.7%). Conversely, MDR isolates were more common in broilers (42.2%) and turkeys (43.5%) than in pigs (34.9%) and calves (27.7%). However, for all animal categories there were marked differences in levels of complete susceptibility as well as MDR between countries. Generally, completely susceptible isolates from pigs, broilers and turkeys were more common in northern than in southern and eastern Europe, whereas the converse situation was observed for MDR. For calves there was no obvious spatial pattern and a favourable situation was reported from the Nordic countries (Norway, Denmark) as well as in countries in southern and central Europe (Austria, Portugal, Croatia).
Tetracycline, ampicillin, sulfamethoxazole and trimethoprim were the antimicrobials most often represented in the pattern of MDR isolates, often in combination with other substances. About half of the MDR isolates from pigs (48.5%) and calves (54.5%) were resistant to all these antimicrobials and they were common also in MDR isolates from broilers (43.4% and turkeys (45.7%). Additionally, quinolone resistance was common in MDR isolates from broilers (78.9%) and turkeys (71.7%) but less common in isolates from pigs (24.8%) and calves (28.9%). The frequent occurrence of these substances as a core component of MDR patterns presumably reflects an extensive usage in several countries over many years and that genes conferring resistance to these substances often are linked on mobile genetic elements, resulting in co‐selection.
Resistance to critically important antimicrobials
Of the antimicrobials tested in the mandatory monitoring, ciprofloxacin (fluoroquinolones), cefotaxime and ceftazidime (third‐generation cephalosporins), meropenem (carbapenems), colistin (polymyxin E) and azithromycin (macrolides) are categorised by the WHO as CIA and among substances of the highest priority (WHO, 2019). To monitor resistance to these antimicrobials in food‐producing animals is of particular interest because there is a risk that animal reservoirs of bacteria resistant to these substances could spread to humans along the food chain.
Phenotypic resistance to third‐generation cephalosporins (cefotaxime and ceftazidime) at the EU level was overall low in indicator E. coli from caecal content. About half of the countries reported isolates resistant to cefotaxime and/or ceftazidime from pigs and calves in 2017 at levels up to at most 6.3% in pigs. Resistant isolates from poultry were reported by a larger proportion of countries (≈ 75%) at levels up to at most 7.1% in turkeys and 30.1% in broilers. The more common occurrence of resistant isolates in poultry is likely a consequence of spread by breeding animal through the production pyramid documented in several countries in Europe. Within the mandatory monitoring, samples of caecal content are also cultured on selective media to specifically detect the presence of E. coli resistant to third‐generation cephalosporins. The results of these analyses are presented in Section 5 ESBL.
Of the 13,679 isolates of indicator E. coli from caecal content of pigs, calves, broilers and turkeys phenotypically tested in 2017 and 2018, resistance to carbapenems (meropenem) was not detected. This provides a strong indication that carbapenem resistance is infrequent in E. coli from these food‐producing animals in Europe. Further information on carbapenem resistance is found in Section 5 ESBL.
Median levels of both ciprofloxacin and nalidixic acid resistance in E. coli isolates from pigs and calves were low at the EU‐level in 2017. In contrast, median levels of ciprofloxacin resistance were extremely high in broilers and very high in turkeys and levels of nalidixic acid resistance were very high in broilers and high in turkeys. A substantial proportion of isolates from all animal categories were resistant to ciprofloxacin only which indicates presence of transmissible genes mediating quinolone resistance.
Only 168 of the 13,679 isolates of indicator E. coli tested in 2017 and 2018 were showed ‘microbiological’ resistance to both ciprofloxacin and third‐generation cephalosporins and 70 of these isolates also ‘clinical’ resistance to both substances. The level of ‘microbiological’ co‐resistance to these substances was highest in broilers (2.1%) and turkeys (1.5%) and lower in pigs (0.5%) and calves (0.7%).
Median levels of azithromycin resistance in MSs were very low in pigs and calves and low in broilers and turkeys. Most countries reported no azithromycin resistance or single isolates only, but a few countries reported higher levels, up to about 10% for broilers and turkeys and up to 16.2% for pigs. Azithromycin is an azalide antimicrobial which is a subgroup of the macrolides, not used in animals. Possibly, selection pressure exerted by use of other macrolides, e.g. tylosin, in food‐producing animals may have favoured emergence of azithromycin resistance.
Median levels of colistin resistance in MSs were 0% for all animal categories and altogether only 112 of the 13,679 isolates tested in 2017 and 2018 showed phenotypic resistance to this antimicrobial. Higher levels were however reported in individual countries, up to 17.4% in turkeys, 4.7% in broilers, 2.1% in pigs and 2.9% in calves. Colistin resistance is likely due to selection from use of colistin in animal production and the high occurrence in some animal categories in some countries indicates large differences in the usage of colistin in Europe as documented in the ESVAC report (EMA, 2019).
5. Extended‐spectrum β‐lactamase (ESBL)‐, AmpC‐ and/or carbapenemase‐producing Salmonella and Escherichia coli 16
The occurrence of ESBL, AmpC, or carbapenemase‐producing bacteria in the intestinal flora of animals is undesirable, as it might lead to dissemination of resistant bacteria from food and farm animals to healthy humans or patients. Bacteria from animals with such resistance should also be considered as a reservoir of resistance genes which may be transferable to other bacteria including food‐borne zoonoses, such as Salmonella spp., further adding to the potential public health consequences. The epidemiology of ESBL‐, AmpC‐ and carbapenemase‐producing E. coli in animals, food and humans is complex and the performance of a harmonised monitoring to specifically investigate their prevalence provides additional information to the data already available in different countries.
As outlined in Commission Implementing Decision 2013/652/EU, the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli in caecal samples of fattening pigs and cattle (calves under 1 year of age), as well as pig meat and bovine meat gathered at retail was mandatory in 2017, whereas the specific monitoring in caecal samples of broilers, fattening turkeys and fresh broiler meat (at retail) was mandatory in 2018. In 2017, the specific monitoring was carried out by 28 MSs and three non‐MSs for meat from pigs, fattening pigs, and meat from bovine animals and by 10 MSs and 2 non‐MSs for calves under 1 year of age. In 2018, the monitoring was performed by 28 MSs and four non‐MSs for broiler meat, 28 MSs and three non‐MSs for broilers, and 11 MSs and 1 non‐MS for fattening turkeys.
When assessing the data, it should be understood that the classification of isolates as being ESBL‐, AmpC‐ or carbapenemase‐producing is based merely on the phenotype of the isolates (done according to EUCAST guidelines, EUCAST 2017, and criteria described in Materials and Methods, Annex A). This means that most, but not all isolates resistant to extended‐spectrum cephalosporins (ESC) are classified into these categories and that all classified isolates, in particular those with an AmpC phenotype, do not necessarily carry any transferrable genes. In order to know if the isolates carry any transferrable genes encoding resistance to ESC, molecular investigations would be needed. However, such investigations are not mandatory according to the current legislation. Also, as only one isolate per sample is to be further investigated the relative abundance of bacteria with an ESBL and/or AmpC phenotype present in the sample will influence the probability of detecting either phenotype.
5.1. Routine antimicrobial resistance monitoring in food‐producing animals and derived meat: presumptive ESBL/AmpC/CP producers
In 2017 and 2018, third‐generation cephalosporin resistance was identified in Salmonella spp. from broilers, fattening turkeys, and laying hens and from carcases (meat) of broilers, pigs and calves under 1 year of age (bovine) as well as in indicator E. coli isolates from broilers, fattening turkeys, fattening pigs and calves under 1 year of age tested with the harmonised panel of antimicrobial substances (panel 1). All Salmonella and indicator E. coli isolates exhibiting microbiological resistance to cefotaxime, ceftazidime or meropenem were subsequently subjected to further testing using a supplementary panel of substances (Panel 2) to obtain more detailed phenotypic characterisation of any resistance detected to third‐generation cephalosporins and/or the carbapenem compound meropenem (see Annex A, Materials and methods).
ESBL/AmpC phenotypes in indicator E. coli
The proportion of the ESC‐resistant (isolates tested with panel 2 by the MSs) indicator E. coli isolates collected within the routine monitoring was generally low in 2017 and 2018 (between 1.4% and 2.8% of the investigated isolates depending on the animal category, Table 9; Annex B, Tables 7–15; Annex E, Tables 23 and 24). Among the reporting MSs, the occurrence of ESC resistance varied from 0% to 7.9% in fattening pigs; from 0% to 5.9% in calves under 1 year of age; from 0% to 30.1% in broilers, and from 0% to 7.1% in fattening turkeys (see chapter 4, E. coli, for further details).
The variation in this ESC resistance occurrence observed is in accordance with the results from the specific monitoring of ESBL/AmpC‐producing E. coli. At the MS group level, the occurrence of presumptive ESBL, AmpC or ESBL+AmpC‐producing E. coli was 2.8% in broilers, 2.1% in turkeys, 1.2% in fattening pigs and 1.4% in calves under 1 year of age (Table 11). For all matrices, the occurrence of the ESBL phenotype was more prevalent than the AmpC phenotype. Detailed data per matrix and country can be found in Annex E (Tables 2, 3, 11 and 12).
Table 11.
Summary of presumptive ESBL‐/AmpC‐producing Salmonella spp. from animals and meat (carcases) and indicator E. coli from caecal samples collected within the routine monitoring, EU MSs, 2017 and 2018
| Matrix | Presumptive ESBL and/or AmpC producersa n (%R) | Presumptive ESBL producersa , b n (%R) | Presumptive AmpC producersa , c n (%R) | Presumptive ESBL+AmpC producersa , d n (%R) | Presumptive CP producerse n (%R) |
|---|---|---|---|---|---|
| Salmonella | |||||
| Broiler meat (N = 873, 19 MSs) | 1 (0.1) | 1 (0.1) | 1 (0.1) | 1 (0.1) | 0 |
| Broilers (N = 2,084, 24 MSs) | 43 (2.1) | 40 (1.9) | 9 (0.4) | 6 (0.3) | 0 |
| Fattening turkeys (N = 815, 16 MSs) | 21 (2.6) | 21 (2.6) | 3 (0.4) | 3 (0.4) | 0 |
| Laying hens (N = 1,184, 24 MSs) | 2 (0.2) | 1 (0.1) | 1 (0.1) | 0 | 0 |
| Pig meat (N = 954, 22 MSs) | 5 (0.5) | 2 (0.2) | 3 (0.3) | 0 | 0 |
| Bovine meat (N = 82, 7 MSs) | 0 | 0 | 0 | 0 | 0 |
| E. coli | |||||
| Broilers (N = 4,165, 28 MSs) | 115 (2.8) | 82 (2.0) | 38 (0.9) | 5 (0.1) | 0 |
| Fattening turkeys (N = 1,674, 11 MSs) | 35 (2.1) | 31 (1.9) | 5 (0.3) | 1 (0.1) | 0 |
| Fattening pigs (N = 4,205, 28 MSs) | 52 (1.2) | 38 (0.9) | 14 (0.3) | 0 | 0 |
| Calves, < 1 year (N = 1,893, 10 MSs) | 26 (1.4) | 25 (1.3) | 5 (0.3) | 4 (0.2) | 0 |
N: total of isolates reported for this monitoring by the MSs; n: number of the isolates resistant; %R: percentage of resistant isolates; ESBL: extended‐ spectrum b‐lactamase; MSs: EU Member States.
According to EUCAST Guidelines (EUCAST, 2017), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Annex A, Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
ESBL/AmpC phenotypes in Salmonella spp.
The proportion of the ESC resistant (isolates tested with panel 2 by the MSs) Salmonella spp. isolates collected within the routine monitoring was generally low in 2017 and 2018 (between 0% and 2.6% of the investigated isolates, depending on the animal category, see Appendix B, Table B.1; Annex B, Tables 9–15; Annex E, Table 22). Notably, the occurrence of Salmonella isolates resistant to ESC from a specific animal category can be largely affected by a high occurrence in certain countries. As an example, 70% of the Salmonella isolates from broilers derive from one single MS (see chapter 2, Salmonella, for further details).
At the reporting MS group level, the prevalence of presumptive ESBL, AmpC or ESBL+AmpC‐producing Salmonella spp. was 2.1% in broilers, 2.6% in turkeys, 0.2% in laying hens, 0.5% in fattening pigs and 0% in calves under 1 year of age (Table 11). In broilers and turkeys, the occurrence of the ESBL phenotype was much greater than that of the AmpC phenotype. Detailed data per country and matrix can be found in Annex E (Tables 1, 10 and 22).
5.2. Specific monitoring of ESBL/AmpC‐producing E. coli in food‐producing animals and derived meat
5.2.1. Prevalence and occurrence of presumptive ESBL/AmpC/CP producers
The specific monitoring employs culture of samples on selective media (including cefotaxime at 1 mg/L), which can detect very low numbers of resistant isolates present within a sample. The ‘screening’ breakpoint for cefotaxime (> 1 mg/L) applied to look for ESBL and AmpC producers was used as recommended by EUCAST. The method is described in more detail in Materials and Methods (Annex A) and protocols are available in https://www.eurl-ar.eu/protocols.aspx. The occurrence and prevalence of E. coli showing an ESBL, AmpC or ESBL+AmpC phenotype from the food‐producing animal populations and derived meat, assessed at the reporting MS‐group level, are presented in Table 12: The general prevalence of presumptive ESBL or AmpC‐producing E. coli for all matrices tested in both 2017–2018 are shown in Figure 38.
Table 12.
Summary of presumptive ESBL‐/AmpC‐producing E. coli from food‐producing animals and derived meat, specific monitoring, EU MSs, 2017 and 2018
| Matrix | Presumptive ESBL and/or AmpC producersa | Presumptive ESBL producersb | Presumptive AmpC producersc | Presumptive ESBL and AmpC producers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Prev % | N | Occ % | Prev % | n | Occ % | Prev % | n | Occ % | Prev % | |
| Pig meat – 2017 (28 MSs, Ns = 6,803, N = 380) | 378 | 6.0 | 298 | 78.4 | 4.4 | 99 | 26.1 | 1.6 | 19 | 5.0 | 0.3 |
| Bovine meat – 2017 (28 MSs, Ns = 6,621, N = 304) | 298 | 4.8 | 238 | 78.0 | 3.9 | 67 | 22.0 | 1.1 | 7 | 2.3 | 0.1 |
| Broiler meat – 2018 (28 MSs, Ns = 7,424, N = 2,970) | 2,943 | 39.8 | 1,896 | 63.8 | 25.7 | 1,190 | 40.1 | 16.1 | 143 | 4.8 | 1.9 |
| Pigs – 2017 (28 MSs, Ns = 6,836, N = 2,819) | 2,783 | 43.8 | 2,180 | 77.0 | 34.4 | 703 | 24.8 | 11.1 | 100 | 3.5 | 1.6 |
| Calves, < 1 year – 2017 (10 MSs, Ns = 3,113, N = 1,312) | 1,312 | 44.5 | 1,223 | 92.2 | 41.5 | 177 | 13.3 | 6.0 | 88 | 6.6 | 3.0 |
| Broilers – 2018 (28 MSs, Ns = 9,049, N = 4,037) | 3,982 | 48.3 | 2,628 | 65.1 | 31.9 | 1,558 | 38.6 | 18.9 | 204 | 5.1 | 2.5 |
| Turkeys – 2018 (11 MSs, Ns = 2,926, N = 1,082) | 1,072 | 39.3 | 925 | 85.5 | 33.9 | 215 | 19.9 | 7.9 | 68 | 6.3 | 2.5 |
Ns: Number of animal/meat samples; N: Number of isolates tested; n: Number of resistant isolates; % Occ: Percentage of cephalosporin‐resistant isolates presenting a presumptive phenotype; % Prev: Percentage of samples harbouring a presumptive ESBL/AmpC‐producing E. coli; ESBL; extended‐spectrum β‐lactamase.
Isolates exhibiting only ESBL‐ and/or only AmpC‐ and/or ESBL+AmpC phenotype.
Isolates exhibiting an ESBL‐ and/or ESBL+AmpC‐phenotype.
Isolates exhibiting an AmpC and/or ESBL+AmpC‐phenotype.
Figure 38.
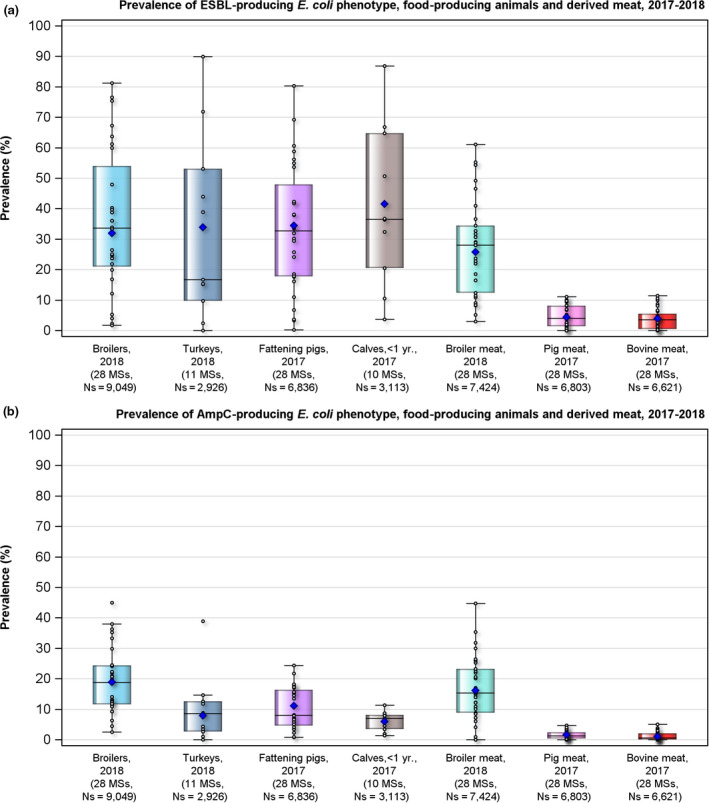
Prevalence of presumptive ESBL‐producing (a) and AmpC‐producing (b) E. coli from the specific monitoring of ESBL/AmpC‐producing E. coli, 2017/2018
Detailed data per country and matrix can be found in Annex E (Tables 4–9, 13–20). Data on the resistance to the different antimicrobials tested in Panel 1 and Panel 2 can be found in Annex E (Tables 25–30 for poultry, 2018) and EFSA–ECDC, 2019 (pigs and cattle, and meat thereof, 2017).
There were however marked variations between MSs and for example, the prevalence of presumptive E. coli ESBL and/or AmpC producers (E. coli showing an ESBL, AmpC or ESBL+AmpC phenotype) ranges from 0.8% (Cyprus) to 87.4% (Italy) in fattening pigs; from 7.1% (Denmark) to 89.0% (Italy) in calves under 1 year of age; from 10.3% (the UK) to 100% (Malta) in broilers; and from 0% (Sweden) to 76.5% (Portugal) in fattening turkeys (for poultry, see Annex E, Tables 6 and 8); for pigs and calves under 1 year of age, see Annex E, Tables 15 and 19, and EFSA and ECDC, 2019a,b).
The differences among reporting countries withstands also when assessing the occurrence of isolates with ESBL or AmpC phenotypes separately (Figures 38, 40 and 42–45 as well as Annex E, Tables 6, 8, 15 and 19). In so that the prevalence of presumptive E. coli ESBL‐producers (E. coli showing an ESBL phenotype) ranges from 0.3% (Finland) to 80.3% (Malta) in fattening pigs; from 3.7% (Denmark) to 86.8% (Italy) in calves under 1 year of age; from 1.7% (Finland) to 81.2% (Malta) in broilers; and from 0% (Sweden) to 89.9% (Portugal) in fattening turkeys. Likewise, the prevalence of presumptive E. coli AmpC‐producers (E. coli showing an AmpC phenotype) ranges from 0.9% (Malta) to 24.4% (Slovenia) in fattening pigs; from 1.4% (Portugal) to 11.3% (Spain) in calves under 1 year of age; from 2.5% (Latvia) to 38.0% (Lithuania) in broilers; and from 0% (Sweden) to 38.9% (Romania) in fattening turkeys.
Figure 40.
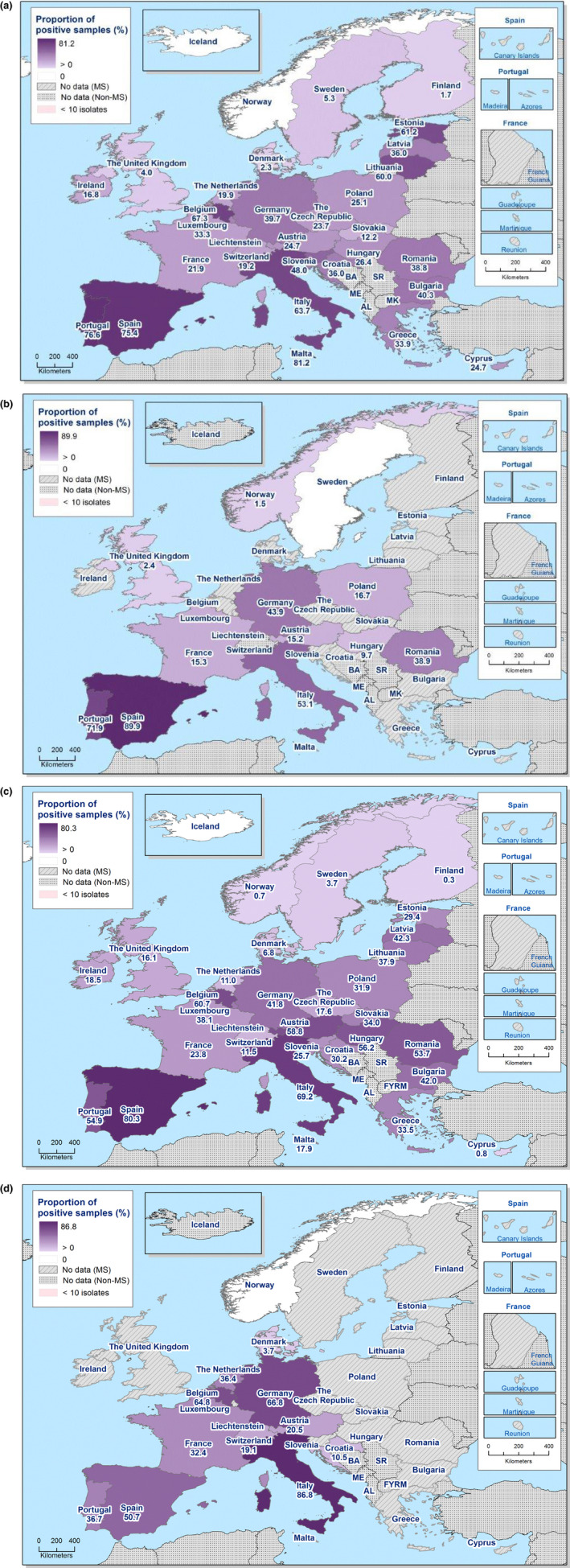
Spatial distribution of the prevalence of presumptive ESBL‐producing E. coli from (a) broilers in 2018, (b) fattening turkeys in 2018, (c) fattening pigs in 2017 and (d) calves under 1 year of age in 2017, EU MSs and non‐MSs, 2017/2018
Figure 42.
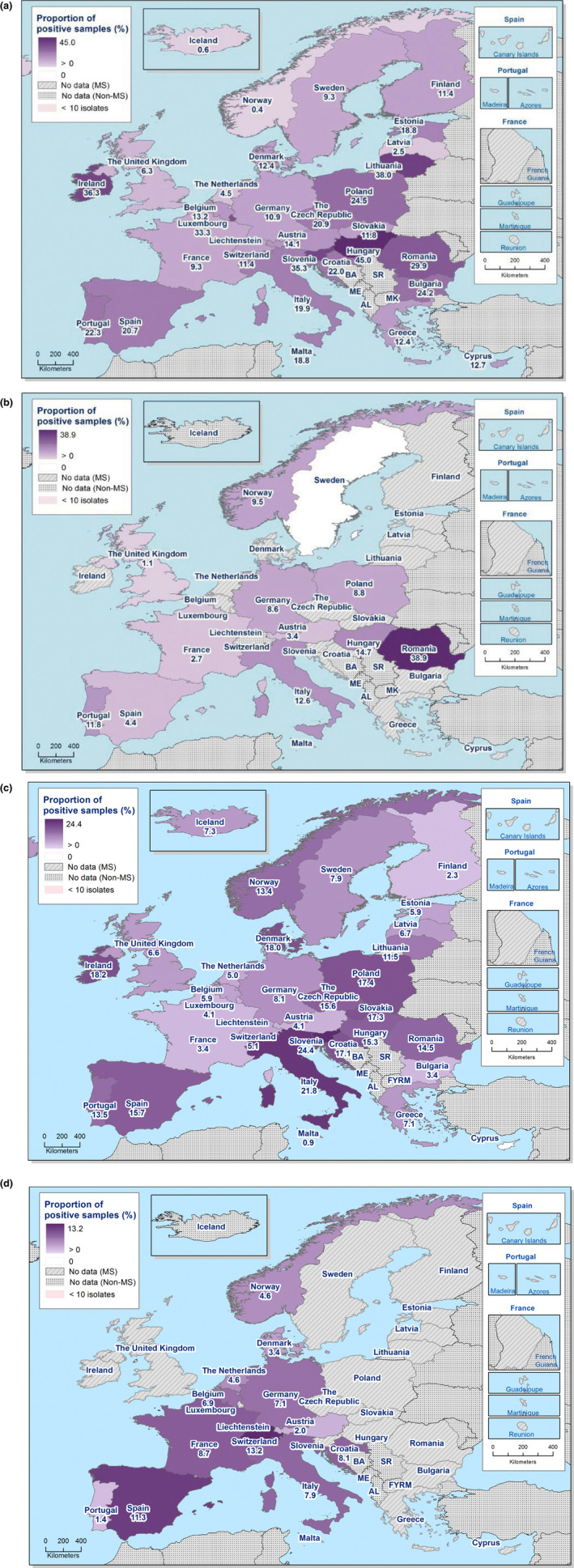
Spatial distribution of prevalence of presumptive AmpC‐producing E. coli from (a) broilers in 2018, (b) fattening turkeys in 2018, (c) fattening pigs in 2017, and (d) calves under 1 year of age in 2017, EU MSs and non‐MSs, 2017/2018
Figure 45.
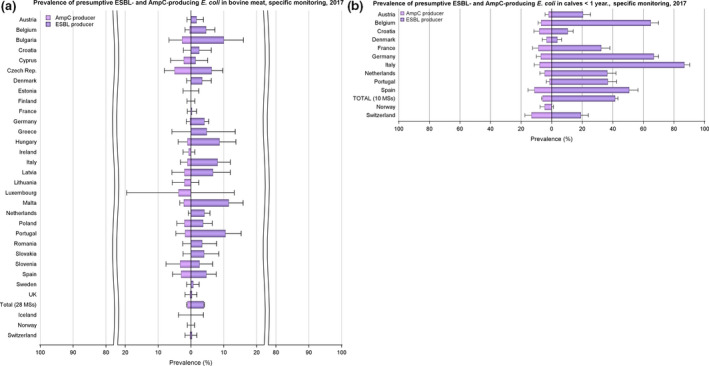
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated.
Likewise, there were large differences among MSs in the prevalence of presumptive E. coli ESBL and/or AmpC producers (E. coli showing an ESBL, AmpC or ESBL+AmpC phenotype) in meat from broilers, ranging from 11.6% (Malta) to 78.0% (Spain), whereas the prevalence in meat from pigs and bovines were less diverse, ranging from 0% (Finland, Luxembourg and Sweden) to 14.4% (Romania) for meat from pigs and 0% (Finland and Estonia) to 13.1% (Malta) for meat from bovine animals (for broiler meat, see Annex E, Table 4; for pig meat and bovine meat, see Annex E, Tables 13 and 17, and EFSA and ECDC, 2019a,b).
The differences among reporting countries withstands also when assessing the occurrence of isolates with ESBL or AmpC phenotypes separately (Figures 38–39, 41 and 43–45 as well as Annex E, Tables 4, 13, and 17). In so that the prevalence of presumptive E. coli ESBL‐producers (E. coli showing an ESBL phenotype) ranges from 0% (Finland, Luxembourg, Sweden and United Kingdom) to 11.1% (Malta) in meat from pigs; from 0% (Estonia, Finland, Ireland, Lithuania and Luxembourg) to 10.5% (Portugal) in meat from bovine animals; from 3% (Finland) to 61.1% (Portugal) in meat from broilers. Likewise, the prevalence of presumptive E. coli AmpC‐producers (E. coli showing an AmpC phenotype) ranges from 0% (Finland, France, Lithuania, Luxembourg and Sweden) to 4.0% (Spain) in meat from pigs; from 0% (Austria, Croatia, Denmark, Estonia, Finland, France, Greece, the Netherlands, Romania, Slovakia, and Sweden) to 5.0% (Czech Republic) in meat from bovine animals; from 0% (Luxembourg) to 44.8% (Hungary) in meat from broilers.
Figure 39.
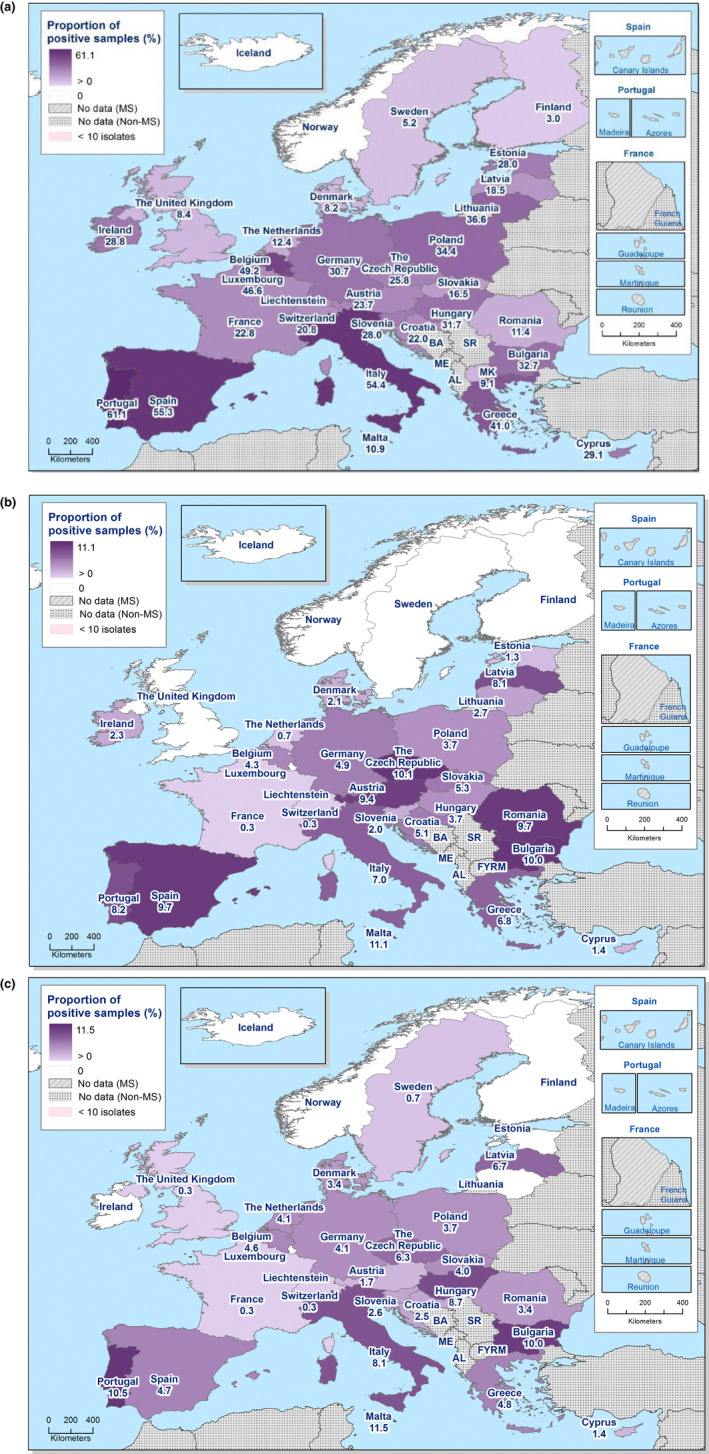
Spatial distribution of the prevalence of presumptive ESBL‐producing E. coli from (a) meat from broilers in 2018, (b) meat from pigs in 2017 and (c) bovine meat in 2017, EU MSs and non‐MSs, 2017/2018
Figure 41.
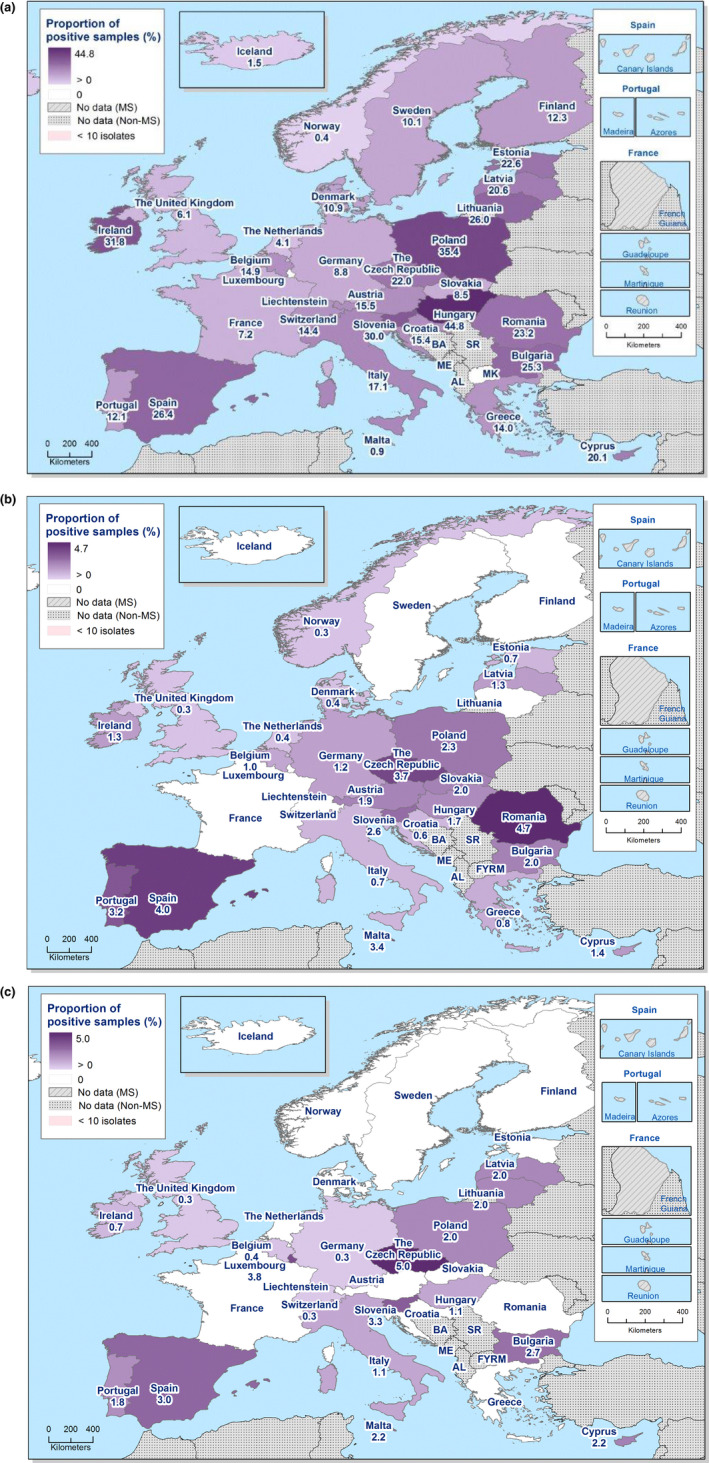
Spatial distribution of prevalence of presumptive AmpC‐producing E. coli from (a) meat from broilers in 2018, (b) meat from pigs in 2017, and (c) bovine meat in 2017, EU MSs and non‐MSs, 2017/2018
Figure 43.
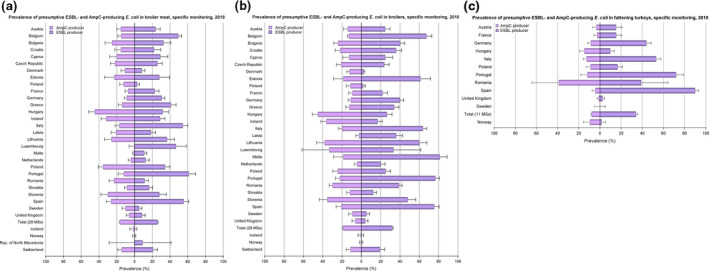
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated.
5.2.2. Relative abundance of presumptive ESBL/AmpC producers
As only one isolate per sample is to be further investigated, the relative abundance of E. coli with an ESBL and/or AmpC phenotype present in the sample will influence the probability of detecting either phenotype. In those animal populations/food matrices monitored, at the reporting MS‐group level and in the majority of the countries, the detection of presumptive ESBL E. coli exceeded that of presumptive AmpC E. coli (Figures 39, 40, 41, 42, 43, 44–45, Annex E). Nevertheless, the occurrence of the different phenotypes varied considerably among the MSs. After excluding MSs with less than 10 isolates tested, the occurrence of the ESBL phenotype ranged from 27.4% (Denmark) to 100% (Malta) in fattening pigs (Annex E, Table 16); from 50% (Denmark) to 99.1% (Portugal) in calves under 1 year of age Annex E, Table 20); from 13.2% (Finland) to 89.7% (Portugal) in broilers (Annex E, Table 7); from 40.8% (Hungary) to 96.9% (Spain) in fattening turkeys (Annex E, Table 9), from 19.6% (Finland) to 96.4% (Luxembourg) in meat from broilers (Annex E, Table 5); from 63.6% (Ireland) to 100% in meat from pigs (Annex E, Table 14); and from 40% (Slovenia) to 100% (Denmark and the Netherlands) in meat from bovine (Annex E, Table 18).
Figure 44.
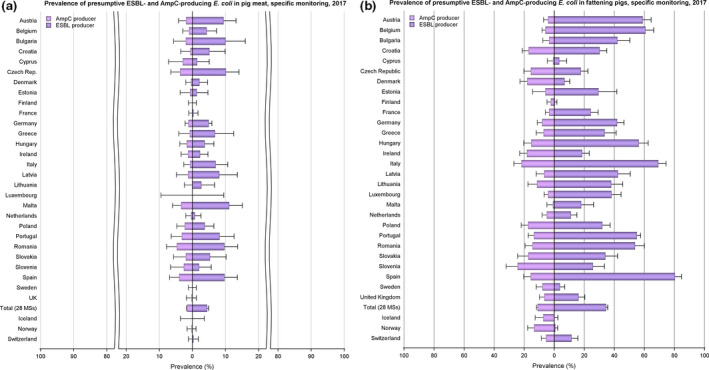
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated.
5.2.3. Evolution of the prevalence of presumptive ESBL/AmpC/CP producers
The evolution of the prevalence of presumptive ESBL and AmpC producing E. coli in each separate animal population and meat category since the starting of the harmonised monitoring is presented at the reporting country and at the MS‐group level in Figures 46 and 47.
Figure 46.
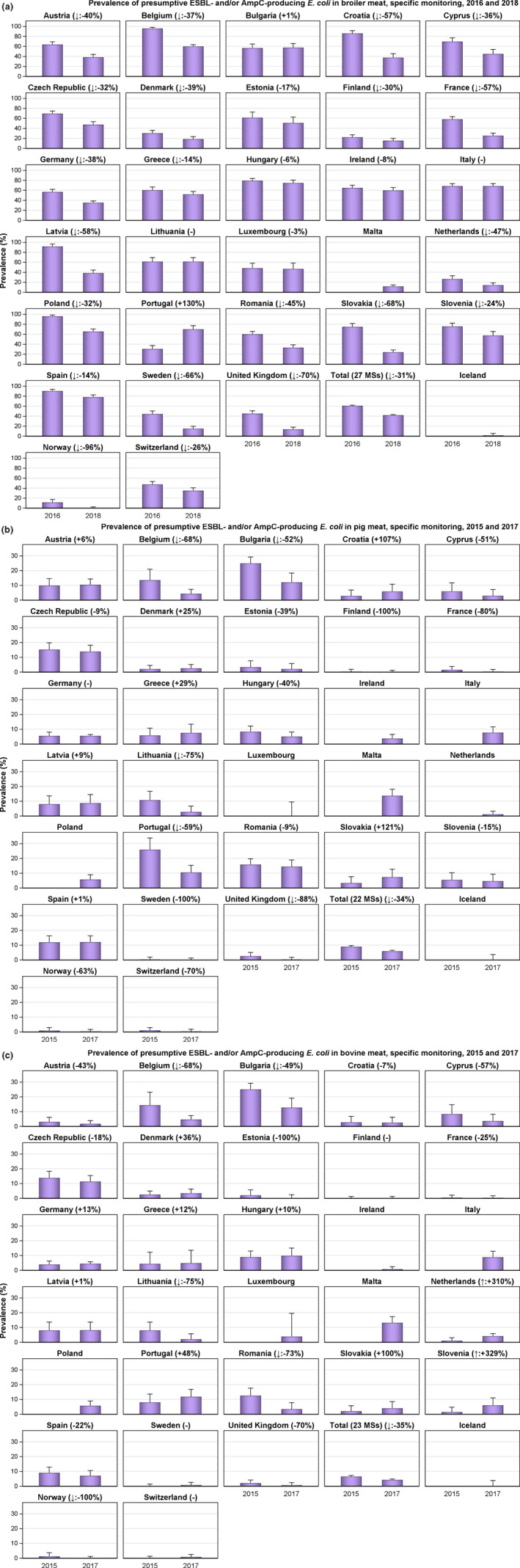
Trends on the prevalence of presumptive ESBL and/or AmpC‐producing E. coli in (a) meat from broilers, (b) meat from pigs, and (c) bovine meat over the period 2015–2017, EU MSs and non‐MSs
- To improve the visibility of the differences, different scales were used for the y‐axis for the different sub‐figures (a, 0–100%; b–c, 0–30%). The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli are also indicated.
Figure 47.
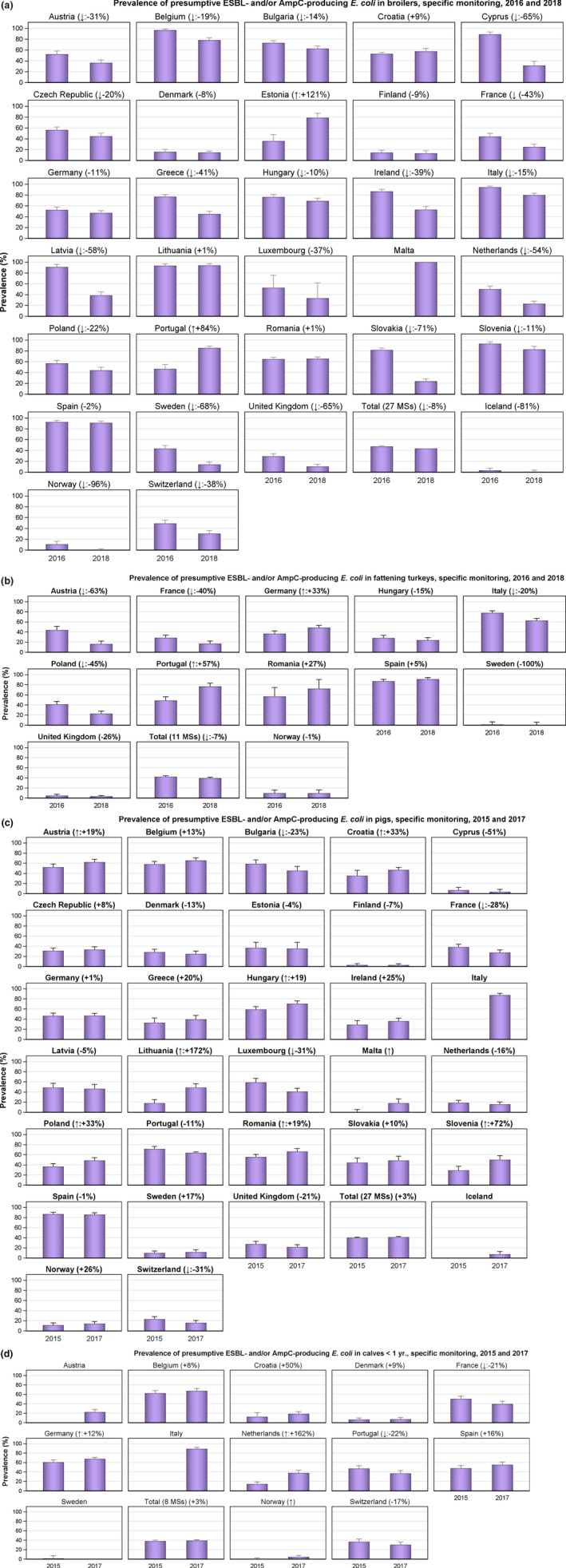
Trends on the prevalence of presumptive ESBL and/or AmpC‐producing E. coli in (a) broilers, (b) fattening turkeys, (c) fattening pigs and (d) calves under 1 year of age, over the period 2015–2017, EU MSs and non‐MSs
- The upper bounds of the 95% confidence interval of the prevalence of ESBL‐ and/or AmpC‐producing E. coli and the rate of change (in %) are also indicated.
The prevalence of presumptive ESBL, AmpC or ESBL + AmpC producing E. coli observed in fattening pigs (43.8%) and calves under 1 year of age (44.6%) in 2017 is comparable with that assessed in the same animal populations in 2015 (40.1% and 39.6%, respectively). Likewise, the prevalence in meat from pigs and meat from bovine animals (6.0% and 4.8%, respectively) observed in 2017 is comparable with that assessed in the same kinds of meat in 2015 (approximately 9% and 6%, respectively). When considering only those MSs having reported consistently for both 2015 and 2017, the point estimates of the prevalence assessed at the MS‐group level in 2015 and 2017 equal, respectively, 40.1% and 39.1% in fattening pigs, 38.0% and 39.1% in calves under 1 year of age, approximately 9% and 5.9% in meat from pigs, and approximately 6% and 4.2% in meat from bovines.
The prevalence of presumptive ESBL, AmpC or ESBL+AmpC‐producing E. coli observed in broilers (48.3%) and fattening turkeys (39.3%) in 2018 is comparable with that assessed in the same animal populations in 2016 (47.4% and 42.2%, respectively). Notably, the prevalence in meat from broilers in 2018 (39.8%) is markedly lower compared to 2016 (57.4%). When considering only those MSs having reported consentingly data for both 2016 and 2018 the point estimates of the prevalence assessed at the MS‐group level in 2016 and 2018 equal, respectively, 47.4% and 43.5% in broilers, 57.4% and 41.9% in meat from broilers. In fattening turkeys, the MSs reporting data in 2018 are the same as in 2016. However, even if, for most matrices, the prevalence of ESBL, AmpC or ESBL+AmpC producing E. coli remains of the same magnitude between the years, when addressing specifically the group of MSs having reported data on both years for each matrix, respectively, statistically significant decreasing trends are demonstrated in all animal populations and meat categories, except in fattening pigs and calves under 1 year of age.
Nevertheless, it is worth noting that those slight decreases at the reporting‐MS group level may mask more important decreases registered in several MSs. A decreased prevalence is observed in many of the reporting countries, and some MSs report a considerable improvement. Concordantly, the difference compared to 2016 withstands even if the data from Malta is not included in the MS‐group level result (41.9% vs. 57.4%; Figure 47a). The improvement is however not uniform and some MSs have reported a high or very high prevalence in both 2018 and 2016, and one MS (Portugal) even registered an increase from high to very high prevalence. In 2018, one additional MS (Malta) compared to 2016 reported data and there the prevalence of presumptive ESBL, AmpC or ESBL+AmpC‐producing E. coli isolates in samples of meat from broilers was moderate.
Detailed information of the prevalence obtained by country and matrix for 2017 and 2018 monitoring can be found in Annex E. For 2015 and 2016, detailed data can be found in EFSA and ECDC, 2017, 2018, respectively.
5.2.4. Key Outcome Indicator of prevalence of ESBL and/or AmpC producers.
The proportion of samples from broilers, fattening turkeys, fattening pigs and calves under 1 year, weighted by PCU, that are identified as positive for presumptive ESBL‐ and/or AmpC‐producing indicator E. coli in the framework of the specific monitoring for ESBL‐/AmpC‐/carbapenemase‐producing indicator E. coli according to Commission Implementing Decision 2013/652/EU has been retained as the key outcome indicator of prevalence of ESBL‐ and/or AmpC‐producing E. coli (OIESC). Resistance to third‐ and fourth‐generation cephalosporins can provide insight on the selection for ESBL‐encoding plasmids due to veterinary antimicrobial usage and on abundance of AmpC‐expressing isolates.
One of the most medically relevant forms of AMR is mediated by plasmid‐encoded ESBL genes (EFSA BIOHAZ Panel, 2011; Maslikowska et al., 2016). In contrast, the AmpC β‐lactamases in E. coli are often chromosomally encoded and upregulated by overexpression of existing AmpC genes (Handel et al., 2014). Genes for AmpC can also be located on plasmids and transferred between strains. Within the broadly defined ESBL/AmpC group, the pathogens resistant to 3rd‐ and 4th‐generation cephalosporins are of particular concern, as these belong to the HCIA list defined by the World Health Organization (WHO, 2019).
There are many different enzymes that can destroy the β‐lactam ring (Pimenta et al., 2014), with a corresponding variety of genes and plasmids (Chong et al., 2011). The observation that ESBL‐carrying isolates from humans are often more related to chicken isolates than are susceptible isolates indicates that a proportion of ESBL‐ and/or AmpC‐encoding isolates from agricultural settings may be of importance in human healthcare situations (Torneke et al., 2015). Plasmids carrying ESBL encoding genes can be transferred rapidly between E. coli strains (Handel et al., 2015) and selection can be driven by the use of many β‐lactam antimicrobials (Cavaco et al., 2008).
To account for differences in the relative size of food animal populations in a country, a weighted Outcome Indicator of the prevalence of ESBL‐ and/or AmpC‐producing E. coli (OIESC) was calculated. The indicator is the weighted mean of the prevalence of ESBL‐ and/or AmpC‐producing E. coli in each of the four animal populations monitored. For the calculation of the mean, the value for each population was weighted in relation to the relative size of the populations within a country using the ‘population correction unit’ (PCU). PCU is a technical unit of measurement used as an indicator of animal population size and was developed by the EMA, primarily to estimate sales of antimicrobials corrected by the animal population in individual countries. The data sources and methodology for the calculation of PCU are comprehensively described in EMA's report ‘Sales of veterinary antimicrobial agents in 31 European countries in 2017’ (EMA, 2019). For each country, OIESC was calculated using data reported for two consecutive years. Thus, values for 2015–2016 were calculated from data reported for fattening pigs and cattle under 1 year of age in 2015 and on data for broilers and fattening turkeys reported in 2016. Likewise, values for 2016–2017 were calculated from data for broilers and fattening turkeys reported in 2016 and on data for fattening pigs and calves under 1 year of age reported in 2017, and so on.
Assessed at the reporting MS‐group level (26 MSs), the OIESC for 2017–2018 was high at 46.4% but has shown a statistically significant decrease of 6% since 2015‐16 (Figure 48). However, considering individual countries and related matrices, the OIESC differs greatly among countries, ranging from 9.4% in Cyprus to 85.7% in Spain. Furthermore, marked variations in OIESc were registered between countries: in 7 countries (5 MSs and 2 non‐MSs), OICS were noted at moderate or lower levels of < 20%, in 6 countries (5 MSs and 1 non‐MS) at 20–40%, in 10 MSs at 40–60%, in 6 MSs at 60–80% and in 2 MSs at > 80%. Interestingly, a positive development is the statistically significant decreasing trends in OIESC observed by 11 MSs (and 1 non‐MS). It is, however, noteworthy that in six of these MSs, some decreases are observed starting from very high or extremely high levels, and that one country (Italy) still records an OIESC at an extremely high level despite a statistically decreasing trend since 2016–2017. Conversely, four MSs showed a statistically increasing trend in OIESC and all of those recorded a high level prevalence. Notably, the relative contribution from the data submitted from the different animal populations studied by the individual reporting countries and the relative size of those animal populations impact the calculation of summary OIESC, and OIESC should be considered in view of the prevalence of ESBL assessed at the level of the animal populations. The 2015–2018 trends in OIESC still need to be confirmed through further follow‐up.
Figure 48.
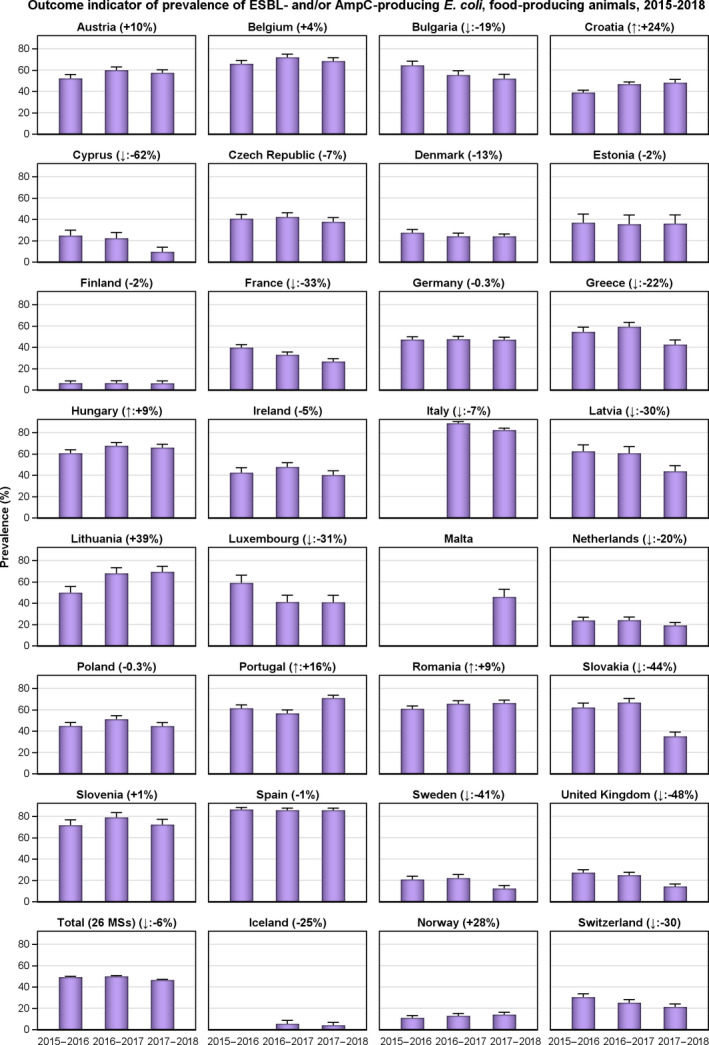
Changes in Outcome Indicator of ESBL‐ and/or AmpC producing E. coli (OIESC), 28 EU MSs and 3 non‐MSs, 2015–2018
- (↓)/(↑): indicates statistically significant decreasing/increasing trends over the 2015–2018 period. The upper bounds of the 95% confidence interval of the OICS and the rate of change (in %) are also indicated. Note: ‘Total’ values from 26 MSs do not include Italy and Malta.
5.2.4. Summary and discussion
Overall, the specific monitoring highlighted that presumptive ESBL, AmpC or ESBL+AmpC‐producing E. coli were frequently detected in caecal samples of all investigated animal categories. The prevalence was slightly lower in turkeys than in the other animal categories; however, only the main producing countries report data on turkeys (according to the Commission Implementing Decision, monitoring in turkeys and calves under 1 year of age is mandatory only in those countries with a production greater than 10,000 tonnes slaughtered per year), and therefore, the difference should be interpreted with caution.
In all monitored animal populations/food matrices, isolates with an ESBL phenotype was more common than isolates with an AmpC phenotype at the reporting MS‐group level and in the majority of the countries. The occurrence of the different phenotypes did however vary considerably among the MSs and in some countries the AmpC phenotype dominated.
The prevalence of presumptive ESBL, AmpC or ESBL+AmpC‐producing E. coli in broiler meat was slightly lower but still comparable to that reported in caecal samples of broilers at slaughter. Conversely, the prevalence of ESBL, AmpC or ESBL+AmpC‐producing E. coli in meat samples of pigs and cattle was much lower than that detected in the caecum of fattening pigs and calves under 1 year of age at slaughter. The range in prevalence of these phenotypes in pig and bovine meat by different MSs also tended to be narrower than that observed in the caecum of these animals at slaughter. The findings suggest that many of these animals are carrying ESBL‐, AmpC‐ or ESBL+AmpC‐producing E. coli in their intestinal content, but that the bacteria do not contaminate the carcases during the slaughter process, alternatively that the bacteria do contaminate the carcases but are somehow removed later in the process.
Furthermore, it is worth noting that there may be several potential sources for bacteria on meat, including the animals from which the meat was derived, other cross‐contaminating products, machinery and the environment, as well as those workers who are handling the meat. Even if the origin of the isolates does not affect the risk for public health, this is of importance when elaborating on effective risk management strategies.
5.3. Monitoring of carbapenemase‐producing E. coli
5.3.1. Mandatory E. coli ESBL/AmpC/carbapenemase producers monitoring
The specific monitoring of ESBL/AmpC‐producing E. coli on selective media (including cefotaxime) also enables the detection of isolates with some mechanisms of carbapenem resistance. In 2017, one isolate with carbapenemase phenotype from a caecal sample collected at slaughter from a pig in Germany was detected. Germany also reported an isolate with carbapenemase phenotype from a pig caecal sample in 2015. Both isolates were confirmed to produce VIM‐1. Those isolates belonged to different genetic types which indicates that the occurrence of the bla‐VIM‐1 gene is not restricted to a specific type of E. coli (EMA and EFSA, 2017). Within the 2018 mandatory ESBL/AmpC monitoring, no isolates of carbapenem‐resistant E. coli were detected. In 2016, isolates of suspected carbapenem‐resistant E. coli were detected in broiler meat samples from Cyprus. However, further analysis of the isolates did not confirm these suspicions.
5.3.2. Voluntary specific carbapenemase producers monitoring
In 2017 and 2018, specific monitoring of carbapenemase‐producing microorganisms using selective media for carbapenemase producers, in accordance with a protocol developed by the EURL on AMR,17 , 18 was performed on a voluntary basis by a number of countries (see Annex A, Materials and methods and Annex E, Table 21). Together during the 2 years, the 20 countries (18 MSs and 2 non‐MSs) investigated 5,208 samples from fattening pigs; 2,827 samples from calves under 1 year of age; 6,168 samples from broilers; 2,419 samples from fattening turkeys, 4,846 samples from meat from pigs, 4,615 samples from meat from cattle and 4,615 samples from meat from broilers. This gives a grand total of 30,698 samples, all of which were negative for carbapenemase‐producing E. coli (Annex E, Table 21). These results are generally in accordance with the results of the voluntary monitoring performed in 2015 and 2016. In these 2 years, a total of 6,751 (2015) and 11,935 (2016) samples, respectively, where investigated and only 5 of these samples generated suspected carbapenemase‐producing E. coli. Three of these, two from broilers and one from meat from broilers, isolated by Romania have been confirmed as bla OXA‐162 (bla oxa‐48‐like) carriers. The other two isolates, one isolate from broiler meat and one from broiler, reported in 2016 by Cyprus, were not confirmed as CP producers.
The Netherlands also reported data on additional specific monitoring of carbapenemase‐producing E. coli (Annex E, Table 21) using a different isolation protocol (EFSA and ECDC, 2019a,b). All the poultry samples tested (n = 301) were negative.
5.3.3. Summary and discussion
Among all samples and isolates investigated within the harmonised monitoring in 2017 and 2018, only one E. coli with elevated MIC to meropenem was detected (from a fattening pig in Germany). Notably, this isolate was detected within the specific monitoring of E. coli with resistance to third‐generation cephalosporins (isolated in plates with cefotaxime) but not within the specific voluntary monitoring of carbapenemase‐producing microorganisms using selective media for carbapenemase producers. The reasons for this remain to be clarified. In conjunction to this, it could be noted that specific isolation methods for faecal samples targeting bacteria with the gene (bla VIM‐1) detected in Germany have recently been developed (Irrgang et al., 2019).
That no more than one isolate was detected in 2017 and 2018, together with the fact that only a few isolates have been detected in the previous years, indicates that carbapenemase‐producing E. coli is still rare among the investigated animal species in Europe (EFSA and ECDC, 2019a,b). Thereby, potential actions to preserve this situation can hopefully still be effective, ensuring that farm animals do not become an important source of such bacteria for humans. Due to the public health importance of carbapenemase‐producing E. coli and/or Salmonella, both as pathogens and as vectors for resistance mechanisms there is a need to follow further developments in this area for farm animals and food derived thereof. Especially as carbapenemase‐producing Enterobacteriaceae has been reported, not only in farm animals and food derived thereof but also from vegetables from many parts of the world including Europe (Zurfluh et al., 2015; Touati et al., 2017; Brouwer et al., 2018; Köck et al., 2018; Liu et al., 2018; Irrgang et al., 2019).
Furthermore, it should be noted that there are several potential sources for bacteria on meat, including the animals from which the meat was derived, other cross‐contaminating products, machinery and the environment, as well as those workers who are handling the meat product. Even if the origin of the isolates does not affect the risk for public health, it is of importance when elaborating on effective risk management strategies.
6. Antimicrobial resistance in meticillin‐resistant Staphylococcus aureus 19
Monitoring of MRSA in food‐producing animals, particularly those intensively reared, carried out periodically in conjunction with systematic surveillance of MRSA in humans, allows trends in the diffusion and evolution of zoonotically acquired MRSA in humans to be identified (EFSA, 2009a,b, 2012). Isolates representative of various animal and food origins should therefore optimally be analysed for determination of lineage, antimicrobial susceptibility and virulence‐associated traits. The monitoring of MRSA in animals and food is currently voluntary and only a limited number of countries reported MRSA data in 2017 and 2018, with some countries additionally reporting data on spa and/or sequence type and antimicrobial susceptibility. Such monitoring may provide an early indication of the occurrence of types of MRSA in animals which have previously not been recognised in animal populations. Furthermore, monitoring of other non‐food animal species, with which certain types of MRSA can be associated, provides additional useful information.
MRSA has been recognised for decades as a serious cause of infections in humans. Strains of MRSA that cause infections in humans can be divided into three broad categories: community‐associated (CA‐), healthcare‐associated (HA‐) and livestock‐associated (LA‐) MRSA. Strains assigned to these different categories of MRSA differ in their epidemiology, although distinctions between types can be blurred. LA‐MRSA has been detected in pigs, poultry and veal calves, as well as in other farm animal species, companion animals and horses in many countries worldwide. LA‐MRSA isolates in Europe predominantly belong to clonal complex (CC) 398, although other livestock‐associated clonal lineages have been reported. HA‐MRSA and CA‐MRSA include strains that predominantly affect humans, and these are much less frequently reported from food‐producing animals. LA‐MRSA may also be carried by humans, especially those persons who have repeated occupational contact with colonised livestock and their derived carcases. The severity of LA‐MRSA infection has been shown to be generally similar to that of other MRSA strains. Indeed, public health surveillance in the Netherlands (2003–2014) and Denmark (1999–2011) detected distinct LA‐MRSA strains disseminating into the community (the Netherlands) or capable of transmission in the community in the absence of livestock contact (Denmark; Kinross et al., 2017).
A variant of the meticillin resistance gene mecA, termed mecC, was identified in 2011 in MRSA from humans and cattle in Europe (García‐Álvarez et al., 2011; Shore et al., 2011), and has subsequently been detected in ruminants, pigs and companion animals, with increasing reports from wild animals (Paterson et al., 2014; Bengtsson et al., 2017). Although first identified in 2011, mecC‐MRSA isolates have now been found dating back to 1975 (Petersen et al., 2013), with the mecC gene sharing 70% identity with mecA at the DNA level (García‐Álvarez et al., 2011). Petersen et al. (2013) demonstrated that mecC‐MRSA infections in humans were primarily community acquired, typically affecting people living in rural areas and older than was typical for CA‐mecA‐MRSA patients. Although our understanding of the epidemiology of mecC‐MRSA is incomplete, studies have indicated that animal contact and zoonotic transmission are likely to be important. Paterson et al. (2014) reported that when tested, mecC‐MRSA strains have been negative for Panton–Valentine leukocidin (PVL) toxin – a virulence feature typically associated with CA‐MRSA – and negative for human immune evasion cluster (IEC) genes, chp (chemotaxis inhibitor protein), sak (staphylokinase) and scn (encoding the staphylococcal complement protein inhibitor). Carriage of these IEC genes is considered an adaptation to enable S. aureus colonisation and infection of humans, and is not usually a feature of animal S. aureus strains (Cuny et al., 2015a).
Antimicrobial susceptibility in European invasive Staphylococcus aureus isolates from humans is reported by the MSs to the European Antimicrobial Resistance Surveillance Network (EARS‐Net) hosted by ECDC. MRSA typing data are not reported and, therefore, when there may be possible links to the animal reservoir of LA‐MRSA, these cannot easily be detected with current monitoring procedures, at least at the European level. The EU/EEA population‐weighted mean proportion of MRSA among invasive S. aureus infections reported to EARS‐Net decreased significantly from 19.0% in 2015 to 16.4% in 2018, with similar decreasing trends reported from more than a quarter of the individual EU/EEA countries. Nevertheless, MRSA remains an important human pathogen in the EU/EEA, as the levels of MRSA were still high in several countries and combined resistance to other antimicrobial groups was common (ECDC, 2019).
6.1. MRSA in food and animals
LA‐MRSA isolates are the main focus of this section, which summarises the occurrence of MRSA and its susceptibility to antimicrobials in various food categories (including meat samples from various species) and food‐producing animals reported by six MSs and two non‐MSs in 2017 and in 2018 (excluding clinical investigations). In 2017, Finland and Switzerland were the only countries to report susceptibility data for MRSA isolates from meat samples (both countries also reported molecular typing data); Belgium and Switzerland were the only countries in 2017 to report such data for MRSA isolates from food‐producing animals (both countries also reported molecular typing data, as did Finland, Norway and Spain). In 2018, Austria and Switzerland were the only countries to report susceptibility data on MRSA isolates from meat samples, with both countries additionally reporting molecular typing data; Belgium was the only country in 2018 to report susceptibility data on isolates from food‐producing animals (and also provided molecular typing data, as did Denmark). This chapter also summarises MRSA occurrence data reported from clinical investigations of food‐producing and companion animals in 2017/2018. Antimicrobial susceptibility and molecular typing data of MRSA isolates recovered from dogs, goats, sheep, horses, a cat and a rabbit were also provided by Sweden in 2017 and are presented in Annex F, Table 7a. The methods for the isolation of MRSA from food and animals are not yet harmonised at the EU level and, therefore, the methods used by individual reporting MSs may differ in sensitivity. Similarly, the sampling strategies used by reporting MSs are not harmonised at the EU level and these may also influence the results obtained.
6.1.1. Monitoring of MRSA in food
In both 2017 and 2018, a low number of countries reported data on the occurrence of MRSA in food (N = 5). Slovakia examined a range of food products (including meat samples from cattle, pigs and poultry in 2017) with no samples testing positive for MRSA (see Annex F, Table 1). In 2017, MRSA was detected in meat from pigs by three countries (Finland, Spain and Switzerland), as well as meat from cattle and rabbits by two countries (Germany and Spain, respectively). In 2018, MRSA was detected in poultry meat by four countries (broiler meat: Austria, Germany, the Netherlands and Switzerland; turkey meat: Austria, Germany and the Netherlands), as well as meat from cattle and pigs by one country (the Netherlands). Over 2017/2018, the reported prevalence of MRSA ranged from very low to low in pig meat (0.7% to 5.9%), low to moderate in meat from cattle (2.1% to 11.3%), low to high in broiler meat (1.3% to 20.2%), high to extremely high in turkey meat (42.7% to 100%) and at a low level in rabbit meat from Spain (4.0%). Notably in 2018, the Netherlands tested a very low number of samples from turkey meat (N = 3), which all proved positive for MRSA resulting in the extremely high prevalence recorded (100%). Similarly, Austria tested a single sample of turkey meat and following the detection of MRSA, the result of which was reported (100% prevalence). The occurrence of MRSA in meat can reflect colonisation of the animals from which the meat was derived with MRSA. However, MRSA is not generally considered to be transmitted by food to humans, and detection often involves selective culture techniques which may detect very low levels of contamination.
In 2017, spa‐typing data were reported for 15 of 80 MRSA isolates recovered from meat (Finland and Switzerland were the only countries to report corresponding spa‐type from pig meat); while in 2018, spa‐typing data were reported for only 8 of 345 MRSA isolates recovered from meat (only Austria and Switzerland provided typing data for isolates recovered from poultry meat). In 2017, all spa‐types reported by Finland in batches of pig meat were those associated with CC398 (spa‐types t011, t034 and t2741), the most common LA‐MRSA lineage occurring in Europe. Of the two positive isolates recovered from Swiss pig meat in 2017, one was reported as spa‐type t011 (associated with CC398) and the other as spa‐type t002. MRSA spa‐type t002 is most commonly associated with ST5 (CC5), a sequence type which includes MRSA isolates considered as either CA or healthcare‐associated (HA) MRSA. Additionally, the t002‐ST5 genotype has also been suggested to represent a livestock‐associated (LA) lineage. Although further molecular typing data (including PVL status) were not available, the isolate was considered most likely to represent a HA‐MRSA lineage. In 2018, Switzerland reported four spa‐types associated with the LA lineages CC398 (spa‐types t034 and t571) and CC9 (t1430 and t13177) from broiler meat. MRSA belonging to CC9 represent a further LA‐MRSA lineage which is disseminated worldwide, although particularly prevalent among various species of livestock in Asia (Cuny et al., 2015b). Austria reported four MRSA isolates associated with CC398 (spa‐types t011 and t034) from the monitoring of broiler and turkey meat in 2018.
In summary, meat from cattle, pigs, broilers, turkeys or rabbits proved positive for MRSA in 2017/2018, although the prevalence varied between meats of different origins (Figure 49).
Figure 49.
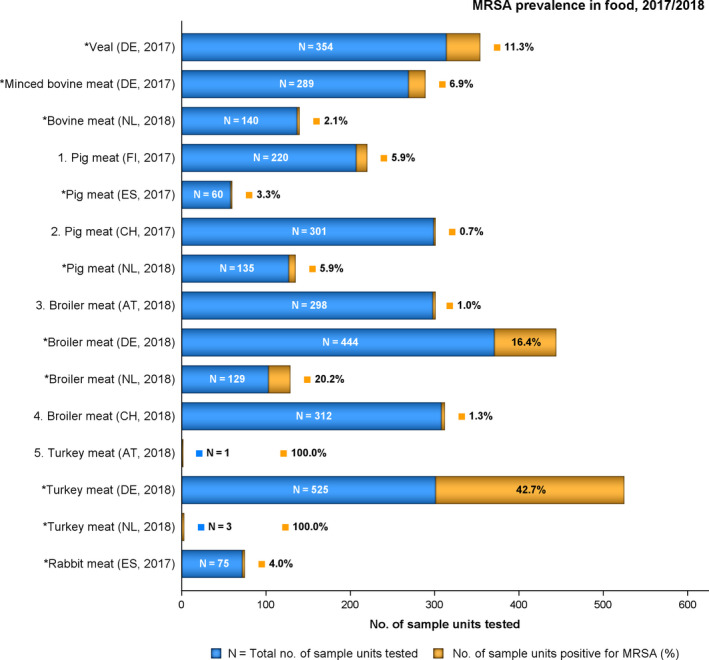
MRSA prevalence in food, 2017/2018 (only food origins where positive isolates were obtained are presented)
-
N: Total number of sample units tested; DE: Germany; NL: the Netherlands; FI: Finland; ES: Spain; CH: Switzerland; AT: Austria.1. spa‐types: t034 (11 isolates), t011 (1), t2741 (1).2. spa‐types: t011 (1 isolate), t002 (1). PVL status of the t002 isolate was not reported.3. spa‐types: t011 (2 isolates), t034 (1).4. spa‐types: t034 CC398 (1 isolate), t1430 (1), t571 CC398 (1), t13177 (1).5. spa‐types : t011 (1).*: spa‐types not reported.
6.1.2. Monitoring of MRSA in animals
Monitoring of MRSA in healthy food‐producing animals
Seven countries reported data on the occurrence of MRSA in healthy food‐producing animals in 2017; while four countries provided MRSA occurrence data on healthy food‐producing animals and horses in 2018. The voluntary monitoring performed over 2017/2018 examined cattle, pigs and chickens of varying production types, as well as fattening turkeys, farmed mink and horses in 2018 – see Annex F, Tables 1a,b, and 3a,b. MRSA was detected in cattle, pigs and chickens by all reporting countries in 2017 (2/2, 1/1 and 6/6 countries, respectively); while in 2018, MRSA was detected in cattle by 2/2 reporting countries, in pigs by 1/2 reporting countries, in laying hens, mink and horses by 1/1 reporting countries, and in fattening turkeys by 1/2 reporting countries. Figure 2 presents MRSA prevalence for the animal origins where positive samples were obtained.
From the monitoring of cattle in 2018, Belgium reported a moderate MRSA prevalence in herds of dairy cows and a low prevalence in herds of meat production animals (14.0% and 8.7%, respectively), while a very high prevalence was reported by Belgium in herds of calves under 1 year of age (54.5%). As part of a national survey in 2018, Denmark reported a low MRSA prevalence in herds of dairy cows (6.1%); and at the animal level, MRSA prevalence in calves at slaughter (under 1 year of age) reported by Germany and Switzerland in 2017 was 39.7% and 8.1%, respectively.
There was also a large degree of variation between reporting countries in the occurrence of MRSA in pigs, with 0.4% to 90.4% of animals/herds/slaughter batches testing positive in 2017 and 0% to 89.2% of pig herds testing positive in 2018. This variation highlights the success of Norwegian eradication programmes (0.4% prevalence in 2017 and no pig herds testing positive in 2018), but also likely reflects the differences in sampling protocols performed in 2017, for example whether testing individual or batches of pigs and whether animals were sampled at slaughter or on farms.
Interestingly in 2018, Denmark sampled herds of fattening pigs, both raised under controlled housing conditions (CHC, N = 130) and not raised under CHC (N = 104), with MRSA herd prevalence reported at levels of 89.2% and 20.2%, respectively. Notably, the pigs raised under CHC represented conventional indoor fattening pig herds, whilst those not raised under these conditions represented free‐range fattening pig herds including organic production herds (DANMAP, 2018).
Considering the monitoring of poultry flocks, a low MRSA prevalence was reported in broiler and laying hen flocks by Belgium in 2017, as well as laying hen flocks by Denmark in 2018 (2.5%, 1.3% and 3.2%, respectively). MRSA prevalence was reported at a moderate level in fattening turkey flocks by Germany in 2018 (17.2%); no flocks of meat production turkeys tested by Denmark in 2018 proved positive for MRSA (N = 19). In 2018, Denmark also tested mink farms and horses at the premises (stable) level, with MRSA farm/stable prevalence reported at high (25.4%) and low levels (8.1%), respectively.
Figure 50.
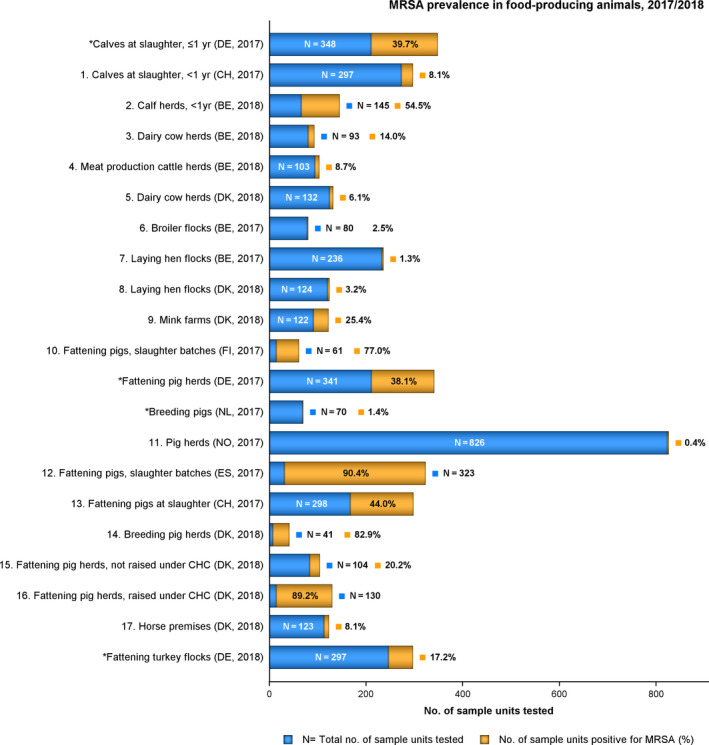
MRSA prevalence in food‐producing animals (excluding clinical investigations), 2017/2018 (only origins where positive isolates were obtained are presented)
-
N: Total number of sample units tested; DE: Germany; CH: Switzerland; BE: Belgium; DK: Denmark; FI: Finland; NL: the Netherlands; NO: Norway; ES: Spain; CHC: controlled housing conditions.1: spa‐types: t011 (14 isolates), t034 (7), t127 (1), t17339 (2). PVL status of the t127 isolate was not reported.2: spa‐types: t011 CC398 (65 isolates), t034 CC398 (8), t1451 CC398 (1), t1580 CC398 (2), t3423 CC398 (1), t3479 CC398 (1), t9433 CC398 (1).3: spa‐types: t011 CC398 (8 isolates), t034 CC398 (1), t223 (3), t1257 (1). The t223 isolates were PVL negative; TSST status was not determined. The PVL status of the t1257 isolate was not reported.4: spa‐types: t011 CC398 (5 isolates), t1451 CC398 (1), t223 (2), t223 ST22 (1). All three t223 isolates were PVL negative. One t223 isolate was subjected to WGS and confirmed to belong to ST22 and harbour the tst gene.5. spa‐types: t034 (7 isolates), t267 CC97 (1).6. spa‐types: t011 CC398 (2 isolates).7. spa‐types: t011 CC398 (2 isolates), t037 ST239 (1). WGS of the t037 isolate confirmed it to belong to ST239 and carry sak and scn genes.8. spa‐types: t011 CC398 (2 isolates), t034 CC398 (2).9. spa‐types: t011 CC398 (6 isolates), t034 CC398 (19), t571 CC398 (1), t588 CC398 (1), t1456 CC398 (1), t1457 CC398 (2), t13790 CC1 (1).10. spa‐types: t034 (32 isolates), t2741 (25), t011 (9), t108 (6), t1250 (1), t1255 (1), t17061 (1). NB. All MRSA isolates were subject to spa‐typing; from one slaughter batch, up to three different spa‐types were detected. Therefore, the total number of individual spa‐types exceeds the number of positive batches.11. spa‐types: t091 CC7 (1 isolate), t843 CC130 (1), t6292 CC425 (1). The t091 isolate was PVL negative, spa‐types t843 and t6292 were confirmed to carry the mecC gene.12. spa‐types: t011 (203 isolates), t034 (32), t108 (14), t109 (1), t899 (2), t1197 (11), t1255 (2), t1451 (13), t1606 (1), t2011 (5), t2346 (1), t2748 (1), t3041 (2), t4208 (2), t17304 (1), t17627 (1).13. spa‐types: t034 (63 isolates), t011 (61), t899 (2), t1451 (3), t2330 (1), t2876 (1).14. spa‐types: t011 CC398 (6 isolates), t034 CC398 (24), t1250 CC398 (2), t1793 CC398 (1), t3171 CC398 (1).15. spa‐types: t011 CC398 (4 isolates), t034 CC398 (15), t588 CC398 (1), t1456 CC398 (1).16. spa‐types: t011 CC398 (22 isolates), t034 CC398 (85), t571 CC398 (3), t898 CC398 (1), t2383 CC398 (1), t2974 CC398 (1), t3423 CC398 (1), t4652 CC398 (1), t9266 CC398 (1).17. spa‐types: t011 CC398 (3 isolates), t034 CC398 (6), t843 CC130 (1). spa‐type t843 was confirmed to carry the mecC gene.*: spa‐types not reported.
In 2017, spa‐typing data were reported for 530 MRSA isolates recovered from pigs, calves and broiler/laying hen flocks; while in 2018, spa‐typing data were reported for 325 MRSA isolates recovered from pigs, cattle, laying hens, mink and horses at the herd/flock/farm/stable level. Additional sequence typing data were available for most of the 325 reported spa‐types in 2018, in comparison to a low number available for the 530 reported spa‐types in 2017.
In 2017, most reported spa‐types were those associated with LA‐MRSA (524/530) – see Figure 51a. These included the novel spa‐types t17061, t17304 and t17627 which were reported from batches of Finnish or Spanish fattening pigs at slaughter, as well as MRSA isolates of spa‐type t899 which were reported from Swiss and Spanish fattening pigs at slaughter.
While spa‐types t17061, t17304 and t17627 appear not to have been previously reported or associated with particular MRSA sequence types, based upon similarities of their spa repeats to other spa‐types associated with CC398, they were inferred to belong to CC398. Additionally, Switzerland reported the novel spa‐type t17339 from two calves at slaughter which was confirmed to belong to CC398.
spa‐type t899 can be associated with different clonal lineages, including CC398 and CC9. LA‐MRSA CC9/CC398 displaying spa‐type t899 is a mosaic strain, consisting of a CC398 chromosomal backbone having acquired the CC9 region containing the staphylococcal protein A gene (Guardabassi et al., 2009; Larsen et al., 2016).
Figure 51.
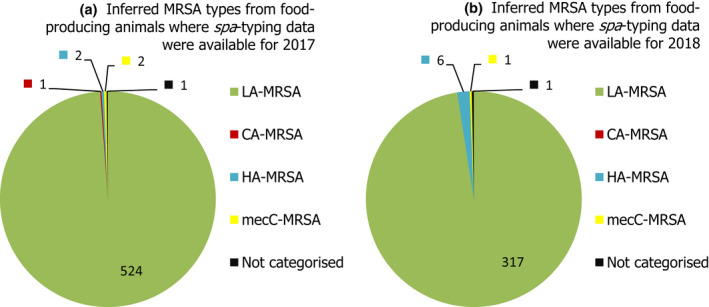
MRSA types reported from food‐producing animals in (a) 2017 and (b) 2018, inferred from spa‐typing data
-
Inferred MRSA types in (a) 2017 were recovered from calves, pigs and broiler/laying hen flocks; inferred MRSA types in (b) 2018 were recovered from pigs, cattle, laying hens, mink and horses at the herd/flock/farm/stable level.NB. All MRSA isolates recovered from Finnish fattening pigs in 2017 were subject to spa‐typing; from a slaughter batch of pigs, up to three different spa‐types were detected.
MRSA spa‐types which were not attributed to LA‐MRSA in 2017 (recovered from pigs, calves and a laying hen flock) are summarised below:
In calves at slaughter (under 1 year of age), Switzerland reported spa‐type t127. This spa‐type has been associated with MRSA belonging to several sequence types within CC1, as well as to types in CC474, but is most frequently associated with ST1 (CC1) and considered a CA‐MRSA regardless of PVL status. The isolate was considered most likely to represent a CA‐MRSA, although the establishment of spa‐type t127 (CC1) within livestock has also been reported.
Spain reported spa‐type t109 from a batch of fattening pigs at slaughter. This spa‐type has been associated with ST5 and ST228 (both members of CC5) but is generally associated with ST228 and was considered a HA‐MRSA lineage.
spa‐type t091 was reported from a multiplier pig herd in Norway; multilocus sequence typing (MLST) confirmed the isolate belonged to CC7. Additionally, Norway reported that the t091 isolate was PVL negative, which could indicate a HA‐MRSA lineage; however, meticillin‐sensitive S. aureus (MSSA) belonging to this spa‐type have also been reported in pigs (Krupa et al., 2015) and therefore a category was not inferred.
mecC‐MRSA was reported in two Norwegian farrow‐to‐finish pig herds; spa‐types t843 and t6292. MLST confirmed them to belong to CC130 and CC425, respectively.
spa‐type t037 was reported from a laying hen flock in Belgium. This spa‐type is generally associated with ST239, a dominant sequence type of HA‐MRSA and mosaic strain which has descended from ST8 and ST30 parents; whole genome sequencing (WGS) confirmed the isolate belonged to ST239 and the isolate was categorised as a HA‐MRSA lineage.
In 2018, again most reported spa‐types were considered to represent LA lineages (317/325) – see Figure 51b. These included spa‐type t267 which was reported from a dairy cow herd in Denmark, as well as spa‐type t13790 which was reported from farmed mink in Denmark.
Although spa‐type t267 has been associated with CC80 and CC97, Denmark confirmed the isolate belonged to CC97. CC97 MRSA has been detected in pigs and cattle (associated with bovine mastitis) in Europe and is considered a LA MRSA lineage; spa‐type t267 was detected in cattle in Italy in 2011 (Feltrin et al., 2016). While MRSA isolates belonging to this clonal lineage have also been reported from humans as community‐associated (CA) clones (Monecke et al., 2011; Spoor et al., 2013; Egea et al., 2014), this isolate was considered a LA‐MRSA.
Denmark reported that spa‐type t13790 recovered from a mink farm belonged to CC1. LA‐MRSA in mink has been considered to originate from contaminated pig by‐products used in the production of mink feed (Hansen et al., 2017; Fertner et al., 2019) and MRSA belonging to CC1 has been detected in breeding pigs and in pork in Denmark (DANMAP, 2016). The occurrence of LA‐MRSA CC1 in pigs and pork in Denmark may therefore account for the detection in mink, and while MRSA isolates belonging to CC1 may be regarded as either a CA‐ or LA‐MRSA, the isolate was considered most likely to represent a LA‐MRSA. Additionally, Denmark reported that the t13790 isolate was negative for the human immune evasion cluster (IEC) gene scn, which may also suggest a link to animals.
MRSA spa‐types which were not attributed to LA‐MRSA in 2018 (recovered from cattle herds and an equine premises) are summarised below:
Belgium reported spa‐type t223 from three dairy cow herds and three meat production cattle herds. All isolates were PVL negative. spa‐type t223 is associated with ST22 (CC22), a dominant sequence type and spa‐type combination of HA‐MRSA; and Belgium confirmed that one isolate from meat production cattle belonged to sequence type (ST) 22 and was SCCmec type IV2B/IVa2B from whole genome sequence (WGS) data. Additionally, this isolate was reported to harbour the tst gene encoding for toxic shock syndrome toxin 1 (TSST‐1) and the human IEC genes (chp, sak and scn). All six bovine t223 isolates were therefore categorised as HA‐MRSA.
spa‐type t1257 was also reported from a dairy cow herd in Belgium. This spa‐type has been associated with sequence types within CC8 (ST239 and ST612) but appears to be more frequently associated with sequence type ST612. While the t1257‐ST612 genotype may be regarded as either a CA‐ or HA‐MRSA, the t1257 isolate from a dairy herd was not categorised to a particular lineage; further typing (including PVL testing) would aid such characterisation.
Denmark reported spa‐type t843 from an equine premise. The isolate was confirmed to belong to CC130 and carry the mecC gene.
Overall, where spa‐typing data were available forMRSA isolates recovered from these food‐producing animals in 2017/18 (excluding clinical investigations), most were considered to represent LA‐MRSA ‐ see Figure 51 (a and b).
Monitoring of MRSA in animals following clinical investigations
Typically, clinical investigations differ from monitoring studies in food‐producing animals; as selective culture methods may not be used, the number of units tested may be low and the sample may involve a biased sample population. Although these data do not allow prevalence to be inferred and cannot be extrapolated at the population level, it is still considered relevant to report the range of animal species/populations which were affected and the lineages of MRSA detected, where reported.
In food‐producing animals
Ireland, the Netherlands and Slovakia reported data following clinical investigations for MRSA in various food‐producing animals in 2017 (cattle, goats or sheep); while Slovakia was the only country to report data in 2018 (cattle, broilers, goats and sheep) – see Annex F, Tables 4a and b. From the 2017/2018 monitoring, the Netherlands were the only country to detect MRSA among dairy cows (N = 1,062) in 2017, with MRSA detected in 0.9% of samples from individual animals. Corresponding spa‐typing data were not available. No other food‐producing animals tested in 2017/2018 proved positive for MRSA; however in most cases, sample sizes were small.
In companion animals
In 2017/2018, the Netherlands and Slovakia reported data on MRSA in companion animals following clinical investigations (Annex F, Tables 5a and b). Slovakia tested cats and dogs, as well as guinea pigs, rabbits and horses in 2018. No animals tested positive for MRSA; however, a small number of cats, guinea pigs, rabbits and horses were tested. In 2017 and 2018, the Netherlands tested more than 250 cats, dogs and horses, with MRSA occurrence reported at levels of 0.9%, 1.3% and 6.3% in 2017, and 1.4%, 0.2% and 9.5% in 2018, respectively. Corresponding spa‐typing data were not reported. In 2017, Sweden also reported data (antimicrobial susceptibility and molecular typing data) on MRSA isolates recovered from dogs, goats, sheep, horses, a cat and a rabbit following clinical investigations; these are discussed in Section 1.3.
6.2. Temporal trends of MRSA prevalence in various types of meat and food‐producing animals (excluding clinical investigations)
Isolation of MRSA from food‐producing animals and the farm environment
In June 2018, the European Union Reference Laboratory‐Antimicrobial Resistance (EURL‐AR) published revised recommendations for the isolation of MRSA from food‐producing animals and the farm environment, which omit the use of a second enrichment step with cefoxitin and aztreonam (EURL‐AR, 2018). Prior to this, the recommended method for the detection of MRSA comprised a pre‐enrichment step and a selective enrichment step (known as the 2‐S method). The revised recommendations followed a study of Danish and Norwegian pig herds which reported a high ratio of false‐negative results using the 2‐S method (Larsen et al., 2017). During this investigation, sensitivity of the 2‐S method was evaluated by comparison with an alternative 1‐S method, whereby the selective enrichment step was bypassed. From 2014 to 2016, LA‐MRSA samples were collected from Danish and Norwegian pigs and their environment and examined by each method. Results confirmed that the 1‐S method resulted in a lower proportion of false‐negative results than the 2‐S method; the 1‐S method and the 2‐S method detecting MRSA in 82% and 74% of the Danish samples, and in 5.6% and 3.8% of the Norwegian samples, respectively. The authors urged caution in extrapolating the results to animals other than pigs and commented that previous studies in Belgium in poultry and cattle did not find significant differences between the performance of the two methods. Notably, changes to the recommended method of isolation may impact longitudinal studies, since direct comparison of the data obtained using the different protocols should be performed with caution.
In 2017, all countries (8/8) reporting data on the occurrence of MRSA in food and food‐producing animals used the 2‐S method; while in 2018, occurrence data was obtained using the 2‐S method by 2/4 reporting countries in food‐producing animals and by 3/5 reporting countries in food (with the remaining countries using the 1‐S method). Considering the monitoring performed in 2018 and for previous years, comparable longitudinal porcine data were available for Denmark and Norway, with both countries using the 1‐S method of isolation in 2018. Where longitudinal data are comparable with the monitoring carried out in 2017/2018, these temporal trends of MRSA prevalence in various types of meat and food‐producing animals are presented in Annex F, Tables 3a,b and 6a,b, respectively.
Temporal trends of MRSA prevalence in various types of meat
In view of the monitoring of food performed in 2017, comparable longitudinal data were available for veal in Germany, pig meat in Finland and Spain, and rabbit meat in Spain; while longitudinal data comparable to the monitoring of food carried out in 2018, included broiler meat in Germany and Switzerland, as well as turkey meat in Germany (see Figure 4). Considering veal, Germany reported annual results on MRSA prevalence in 2012 and 2017, with moderate levels of around 10% detected in both years. In pig meat, Finland reported MRSA prevalence data from batches of fresh pig meat in 2015 and 2017. Although in both years, prevalence was low, interestingly, it nearly doubled from 2015 to 2017 (3.0% in 2015 and 5.9% in 2017), corresponding to a statistically significant increase (Cochran‐Armitage trend test). In both years, sample size examined remained high and common spa‐types associated with CC398 were reported. Spain reported annual results on MRSA prevalence in fresh pig meat in 2011, 2012, 2013, 2014 and 2017, testing a relatively low number of samples each year. With the exception of 2013 (8.3%), similar levels were recorded: 2.4% (2011), 1.7% (2012), 3.2% (2014) and 3.3% (2017). Spain also reported data on the annual prevalence of MRSA in fresh rabbit meat from 2015 to 2017 (testing a relatively low number of samples each year). Prevalence remained at similar levels in 2015 and 2016 (8.3% and 8.0%, respectively), decreasing to 4.0% in 2017.
Regarding poultry meat, Germany reported MRSA prevalence data for fresh broiler meat in 2011, 2013, 2016 and 2018, with sample size remaining similar throughout all years. While prevalence remained at a similar high level in 2011 and 2013 (26.5% and 24.2% respectively), this fell to a moderate level in 2016 (13.0%), increasing slightly in 2018 (16.4%). Switzerland also reported annual results on MRSA prevalence in fresh broiler meat, for years 2014, 2016 and 2018. Although the number of units tested in these years was similar, the sampling differed in 2014, analysing batches of meat in comparison to single meat samples in 2016 and 2018. Throughout all years, prevalence was low and has shown a steady decline (6.9%, 3.0% and 1.3%, respectively). Most isolates were spa‐types associated with LA‐MRSA, with the exception of t032 in 2014 and t153 in 2016. Longitudinal data were also available for fresh turkey meat in Germany, for years 2012, 2014, 2016 and 2018. Although MRSA prevalence increased slightly from 2012 to 2016 and showed a slight decline in 2018, similar high levels were reported throughout (37.7%, 42.5%, 44.5% and 42.7%, respectively).
Considering the monitoring performed in 2017/2018 and where comparable longitudinal data were available, a decline in the occurrence of MRSA in rabbit meat was noted by Spain, as well as broiler meat by Switzerland and turkey meat by Germany. The reasons for these observed declines are unclear, but findings are interesting because generally the occurrence of MRSA in food and animals has shown a progressive increase, where it has been investigated. For example, an increase was observed in Finnish pig meat from 2015 to 2017, with statistical analysis detecting an increasing trend.
Figure 52.
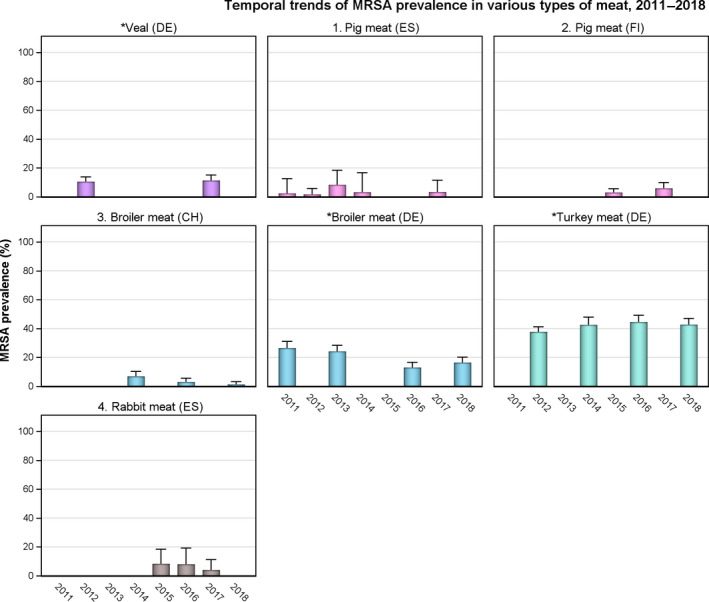
Temporal trends of MRSA prevalence in various types of meat, 2011–2018
-
DE: Germany; ES: Spain; FI: Finland; CH: Switzerland.Where comparable longitudinal data were available, all reporting countries (4/4) used the 2‐S method of isolation (2011–2018).*: spa‐types not reported.1: In 2011, spa‐type: t011 (1 isolate).In 2012, 2013, 2014 and 2017, spa‐types not reported.2: In 2015, spa‐types: t034 (6 isolates), t2741 (3).In 2017, spa‐types: t034 (11 isolates), t011 (1), t2741 (1).3: In 2014, spa‐types: t011 (3 isolates), t032 (3), t034 (14), t571 (1) t899 (1).In 2016, spa‐types: t034 (3 isolates), t153 (1), t1430 (3), t2123 (2). PVL status of the t153 isolate was not reported.In 2018, spa‐types: t034 CC398 (1 isolate), t1430 (1), t571 CC398 (1), t13177 (1).4: In 2015 and 2017, spa‐types not reported.In 2016, spa‐types: t011 (3 isolates), t1190 (1). PVL status of the t1190 isolate was not reported.
Temporal trends of MRSA prevalence in food‐producing animals (excluding clinical investigations)
Considering the monitoring of food‐producing animals in 2017, comparable longitudinal data were available for calves in Germany and Switzerland, batches of pigs in Finland and Spain, herds of pigs in Germany and Norway, as well as pigs in Switzerland. Longitudinal data which was comparable to the monitoring of food‐producing animals in 2018 included cattle herds in Belgium, pig herds in Denmark and Norway, as well as fattening turkey flocks in Germany (see Figures 5 and 6 for comparable trends in cattle and pigs). Germany reported annual results on MRSA prevalence in fattening turkey flocks in 2012, 2014 and 2018, with sample size remaining high throughout. Interestingly, prevalence increased from a moderate level in 2012 to a high level in 2014 (12.8% to 21.9%, respectively), and then declined slightly to a moderate level in 2018 (17.2%). These trends are only presented in Annex F, Table 6b.
Considering the monitoring of cattle herds in 2018 (Figure 5), longitudinal data were available for Belgian calves, dairy cows and meat production animals, with a similar number of herds tested within each production type. In herds of calves (under 1 year of age), MRSA prevalence increased sharply from a high level in 2012 to an extremely high level in 2015 (47.1% to 78.9%, respectively), and then declined to a very high level in 2018 (54.5%). Most isolates were spa‐types associated with CC398 (LA‐MRSA), except for spa‐types t037 and t044 detected in 2015. Among Belgian dairy cow herds, MRSA prevalence remained at similar levels in 2012, 2015 and 2018, although a slight increase was noted over these years (9.9%, 10.4% to 14.0%, respectively). Most isolates were spa‐types associated with CC398 (LA‐MRSA), apart from spa‐types t037 and t388 detected in 2012, as well as spa‐types t223 (HA‐MRSA) and t1257 (not categorised) in 2018. While MRSA prevalence remained at similar levels in Belgian meat production cattle herds, a modest increase was observed from 2012 to 2015 (10.2% to 15.4%, respectively), followed by a modest decline in 2018 (8.7%). Most isolates were spa‐types associated with CC398 (LA‐MRSA), except for spa‐type t121 detected in 2012 and spa‐type t223 in 2018 (categorised as HA‐MRSA). At the animal level, Germany reported annual results on the prevalence of MRSA in calves at the slaughterhouse in 2012 and 2017. In both years, a similar number of calves were tested, and prevalence remained at a high level, although declined slightly from 2012 to 2017 (45% to 39.7%, respectively). In 2015 and 2017, Switzerland also monitored MRSA prevalence in calves at the slaughterhouse, testing a similar number of animals in both years. Although prevalence remained low, this increased slightly from 2015 to 2017 (6.5% to 8.1%, respectively). Most isolates were spa‐types associated with CC398 (LA‐MRSA), except for spa‐type t008 in 2015 and spa‐type t127 in 2017; both categorised as CA‐MRSA.
Figure 53.
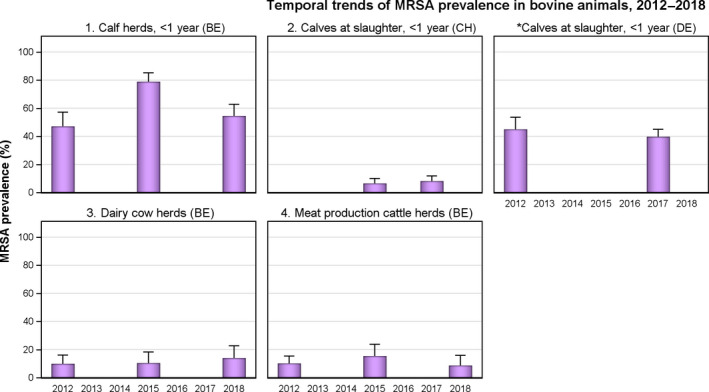
Temporal trends of MRSA prevalence in cattle, 2012–2018
-
BE: Belgium; CH: Switzerland; DE: Germany. Where comparable longitudinal data were available, all reporting countries (3/3) used the 2‐S method of isolation (2009–2018).*: spa‐types not reported.1: In 2012, spa‐types: t011 (40 isolates), t1451 (3), t1456 (1), t1985 (3), t3423 (1), untypable (1).In 2015, spa‐types: t011 (64 isolates), t034 (15), t037 (8), t044 (3), t1451 (3), t1580 (7), t1985 (8), t2287 (2), t3423 (5), untypable (1).. The t044 isolates were PVL negative.In 2018, spa‐types: t011 CC398 (65 isolates), t034 CC398 (8), t1451 CC398 (1), t1580 CC398 (2), t3423 CC398 (1), t3479 CC398 (1), t9433 CC398 (1).2: In 2015, spa‐types: t011 (11 isolates), t034 (6) and t008 (2). The t008 isolates were PVL positive.In 2017, spa‐types: t011 (14 isolates), t034 (7), t127 (1), t17339 (2). PVL status of the t127 isolate was not reported.3: In 2012, spa‐types: t011 (8 isolates), t037 (1), t388 (1), t1456 (1), t6228 (2), untypable (1).In 2015, t011 (4 isolates), t034 (1), t1580 (1), t1985 (2), t2383 (1), untypable (1).In 2018, spa‐types: t011 CC398 (8 isolates), t034 CC398 (1), t223 (3), t1257 (1). The t223 isolates were PVL negative; TSST status was not determined. The PVL status of the t1257 isolate was not reported.4: In 2012, spa‐types: t011 (16 isolates), t121 (1), t1456 (1), t1985 (1).In 2015, spa‐types: t011 (9 isolates), t034 (2), t1451 (1), t1580 (2), t2287 (1), t3423 (1).In 2018, spa‐types: t011 CC398 (5 isolates), t1451 CC398 (1), t223 (2), t223 ST22 (1). All three t223 isolates were PVL negative. One t223 isolate was confirmed to belong to ST22 and harbour the tst gene from WGS data.
Regarding the monitoring of pigs (Figure 6), MRSA prevalence data for Swiss fattening pigs at slaughter were reported from 2009 to 2015 and in 2017. Generally, prevalence has increased annually, rising from 2.2% in 2009 to 44.0% in 2017; and from 2015 to 2017, a marked increase was observed from 25.7% to 44.0%, respectively. Following statistical analyses (Cochran‐Armitage trend test), a significant increasing trend was detected over these years. Notably, spa‐types associated with CC398 exhibited a steady increase in prevalence, and in 2017, all reported isolates were those associated with CC398, with most belonging to spa‐types t011 and t034. In 2017, Finland reported MRSA prevalence at 77% in batches of fattening pigs at slaughter. Although in previous years, comparable data were not submitted to EFSA, Finland state that in 2009–2010 an equivalent study was performed, reporting MRSA prevalence at 22% (FINRES‐Vet, 2016–2017). Following statistical analyses (Cochran‐Armitage trend test), a significant increasing trend was detected over these years. Notably in 2010, the most common spa‐types reported were t108 and t127; while in 2017, spa‐types t034 and t2741 predominated, and all spa‐types in 2017 were associated with CC398. Spain reported data on MRSA prevalence in batches of fattening pigs at slaughter in 2011, 2015 and 2017. Although MRSA prevalence remained extremely high throughout, a slight increase was noted from 2011 to 2015 (84.1% in 2011 to 91.4% in 2015), while a slight decline was noted from 2015 to 2017 (91.4% in 2015 to 90.4% in 2017). Statistical analyses (Cochran‐Armitage trend test) revealed a significant increasing trend over these years. spa‐typing data were available for 123 isolates in 2011 and for all isolates in 2017; all spa‐types were those associated with CC398, with the exception of a single isolate of spa‐type t109 in 2017 (categorised as HA‐MRSA). Germany reported data on MRSA prevalence in fattening pig herds in 2015 and 2017. Although prevalence remained high in both years, this decreased slightly from 2015 to 2017 (41.3% to 38.1%, respectively). As part of a national surveillance programme, Norway has reported annual data on MRSA prevalence among pig herds since 2014. From 2014 to 2017, similar very low levels of prevalence were recorded (0.1%, 0.5%, 0.1% and 0.4%, respectively), and in 2018, no pig herds tested positive for MRSA. Notably in 2018, Norway used the 1‐S method of isolation. These trends highlight the favourable impact of the Norwegian programme in eradicating and maintaining freedom of MRSA from most pig herds. Considering breeding pigs, Denmark reported annual results on the prevalence of MRSA in breeding pig herds in 2016 and 2018. Although in 2016, the 2‐S method of isolation was used in comparison to the 1‐S in 2018, prevalence declined over these years from 100% to 82.9%, respectively. This apparent decline is likely to reflect in part the differences in sample size; notably in 2016, Denmark tested a very low number of breeding herds (N = 6) resulting in the extremely high prevalence (100%), while in 2018 a larger number of breeding herds were tested (N = 41). Corresponding spa‐typing data were not reported in 2016, however, all reported spa‐types in 2018 were those associated with CC398 (LA‐MRSA). In 2016 and 2018, Denmark also reported data on MRSA prevalence among fattening pig herds. In 2016, randomly selected conventional pig herds were sampled (DANMAP, 2016); while in 2018, both herds raised under controlled housing conditions (CHC) and herds not raised under CHC were tested. Interestingly, prevalence was reported at extremely high levels in 2016 (conventional herds) and from herds raised under CHC in 2018 (87.7% and 89.2%, respectively); while a much lower level was reported from herds not raised under CHC in 2018 (20.2%). Notably in 2018, the pigs raised under CHC represented conventional indoor fattening pig herds, whilst those not raised under these conditions represented free‐range fattening pig herds including organic production herds (DANMAP, 2018). In 2016, Denmark used the 2‐S method of isolation in comparison to the 1‐S in 2018. Once again, Denmark did not report corresponding spa‐typing data in 2016; however, all isolates recovered from the conventional and free‐range herds in 2018 were spa‐types associated with CC398 (LA‐MRSA).
Figure 54.
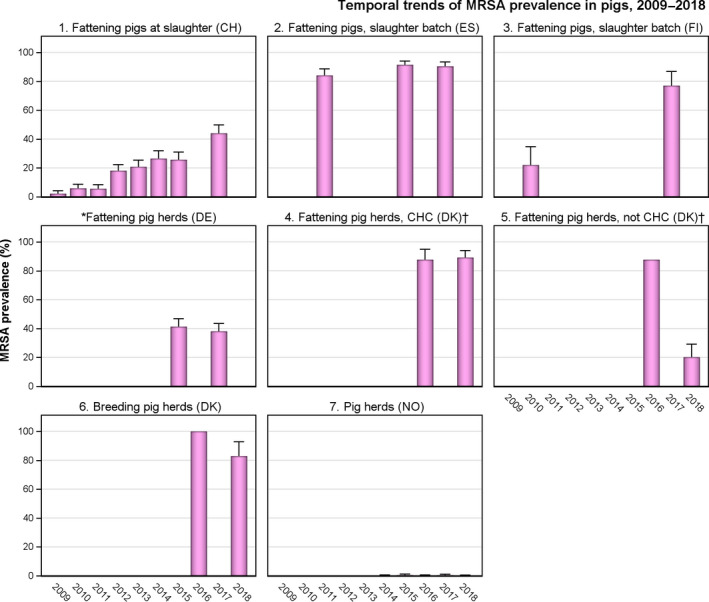
Temporal trends of MRSA prevalence in pigs, 2009–2018
-
CH: Switzerland; ES: Spain; FI: Finland; DE: Germany; DK: Denmark; NO: Norway; CHC: controlled housing conditions. 4/6 reporting countries used the 2‐S method of isolation (2009–2018). NO and DK used the 1‐S method of isolation in 2018.*: spa‐types not reported.†: Prevalence data for 2016 is from conventional fattening pig herds.1: In 2009, spa‐types not reported.In 2010, spa‐types: t034 ST398 (17 isolates), t011 ST398 (1), t208 ST49 (5).In 2011, spa‐types: t034 ST398 (19 isolates), t011 ST398 (1), t208 ST49 (1), t2279 ST1 (1).In 2012, spa‐types: t034 CC398 (61 isolates), t011 CC398 (9), t208 ST49 (2).In 2013, spa‐types: t034 (63 isolates), t011 (10).In 2014, spa‐types: t034 (57 isolates), t011 (19), t208 (1), t899 (1), t2741 (1).In 2015, spa‐types: t034 (48 isolates), t011 (23), t032 (1), t571 (1), t899 (1), t1145 (1), t1250 (1), t4475 (1).In 2017, spa‐types: t034 (63 isolates), t011 (61), t899 (2), t1451 (3), t2330 (1), t2876 (1).2: In 2011, spa‐types: t011 (97 isolates), t034 (8), t108 (3), t1197 (7), t1451 (3), t2346 (3), unspecified (68).In 2015, spa‐types not reported.In 2017, spa‐types: t011 (203 isolates), t034 (32), t108 (14), t109 (1), t899 (2), t1197 (11), t1255 (2), t1451 (13), t1606 (1), t2011 (5), t2346 (1), t2748 (1), t3041 (2), t4208 (2), t17304 (1), t17627 (1).3: In 2010, spa‐types: t108 (6 isolates) and t127 (5) were the most commonly detected.In 2017, spa‐types: t034 (32 isolates), t2741 (25), t011 (9), t108 (6), t1250 (1), t1255 (1), t17061 (1). NB. All MRSA isolates were subject to spa‐typing; from one slaughter batch, up to three different spa‐types were detected.4: In 2016, spa‐types not reported.In 2018, spa‐types: t011 CC398 (22 isolates), t034 CC398 (85), t571 CC398 (3), t898 CC398 (1), t2383 CC398 (1), t2974 CC398 (1), t3423 CC398 (1), t4652 CC398 (1), t9266 CC398 (1).5: In 2016, spa‐types not reported.In 2018, spa‐types: t011 CC398 (4 isolates), t034 CC398 (15), t588 CC398 (1), t1456 CC398 (1).6: In 2016, spa‐types not reported.In 2018, spa‐types: t011 CC398 (6 isolates), t034 CC398 (24), t1250 CC398 (2), t1793 CC398 (1), t3171 CC398 (1).7: In 2014, spa‐type: t011 (1).In 2015, spa‐type: t034 CC398 (2), t177 CC1 (2).In 2016, spa‐type: t034 CC398 (1).In 2017, spa‐types: t091 CC7 (1 isolate), t843 CC130 (1), t6292 CC425 (1). The t091 isolate was PVL negative, spa‐types t843 and t6292 were confirmed to carry the mecC gene.In 2018, no herds tested positive for MRSA.
6.3. Summary data on the occurrence and susceptibility of MRSA
Determination of the susceptibility of MRSA isolates to antimicrobials, including those of particular medical importance, such as linezolid and vancomycin, provides valuable information on the MRSA situation in animals and food. The importance of monitoring AMR patterns among different lineages is underlined by the potential for multiple resistance genes harboured by less virulent strains to spread to other S. aureus strains (Sahibzada et al., 2017).
Data on the antimicrobial susceptibility of MRSA isolates were reported by Belgium, Finland, Switzerland and Sweden in 2017, as well as Austria, Belgium and Switzerland in 2018 (see Annex F, Tables 7a,b). All countries used a broth dilution method and applied EUCAST ECOFFs to determine the susceptibility of isolates. As expected, all MRSA isolates were resistant to penicillin and cefoxitin. Linezolid and vancomycin are antimicrobials of last resort for treating S. aureus infections in humans. All countries reporting susceptibility data in 2017/2018 tested isolates for linezolid susceptibility and all isolates proved susceptible. All isolates in 2018 and those where tested in 2017, were susceptible to vancomycin; which was as expected, since resistance to vancomycin is currently extremely rare in S. aureus. (MRSA isolates reported by Sweden following clinical investigations in 2017 were not tested for vancomycin susceptibility.)
Susceptibility data of MRSA isolates obtained from meat and food‐producing animals (excluding clinical investigations)
In 2017/2018, tetracycline resistance was extremely high (at 100%) in MRSA isolates from Swiss calves, Belgian broiler and laying hen flocks, Swiss fattening pigs and Finnish pig meat, as well as Austrian poultry meat and Belgian calf herds; and all but two of these isolates – spa‐type t127 recovered from a Swiss calf in 2017 and spa‐type t037 recovered from a Belgian laying hen flock in 2017 – were spa‐types associated with CC398. This was expected as LA MRSA isolates belonging to CC398 are usually tetracycline resistant (Crombé et al., 2013).
The extremely high level of MRSA isolates showing resistance to trimethoprim and tiamulin in fresh pig meat from Finland in 2017 (92.3% and 100%, respectively) presumably reflects the relatively common usage of these compounds in pig medicine in many European countries. Resistance levels to these compounds were lower in Swiss fattening pigs at slaughter in 2017 (51.9% and 50.4%, respectively). Considering the MRSA isolates reported from Finnish pig meat in 2017, resistance to quinupristin/dalfopristin was also reported to be extremely high (100%); lincosamide and macrolide resistance were reported to be extremely high (100%) and high (30.8%), respectively. Isolates from Swiss fattening pigs at slaughter in 2017 showed resistance in one or more isolates to all antimicrobials tested (with the exception of vancomycin and linezolid). Again, clindamycin resistance was observed at a higher level when compared to erythromycin. This pattern was also noted among Belgian calf herds in 2018, where lincosamide and macrolide resistance were reported at levels of 88.6% and 84.8%, respectively. Among the MRSA isolates reported from Swiss calves in 2017, lincosamide and macrolide resistance was reported at an equal extremely high level (70.8%). Similarly, clindamycin and erythromycin resistance were observed at an equal very high level (60%) in Belgian broiler and laying hen flocks in 2017; an equal high level of resistance to these antimicrobials (33.3%) was also observed among isolates from Belgian meat production cattle herds in 2018. Figures 7 and 8 present the overall resistance to selected antimicrobials within the meat and food‐producing animal origins.
Figure 55.
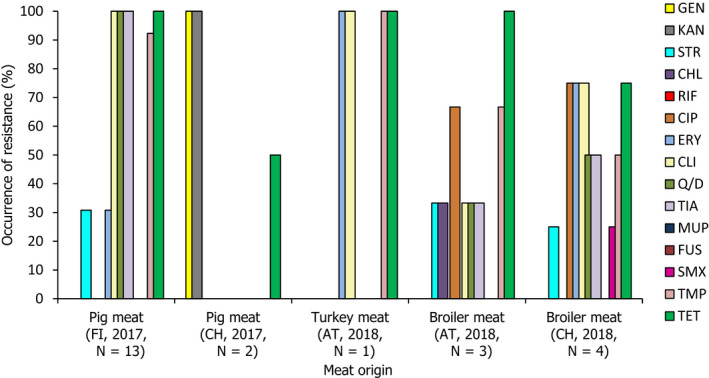
Occurrence of resistance (%) to selected antimicrobials in MRSA isolates from food, 2017/2018
-
N: Number of MRSA isolates reported/tested; FI: Finland; CH: Switzerland; AT: Austria.All isolates were tested against GEN: gentamicin; KAN: kanamycin; STR: streptomycin; CHL: chloramphenicol; RIF: rifampicin; CIP: ciprofloxacin; ERY: erythromycin; CLI: clindamycin; Q/D: quinupristin/dalfopristin; TIA: tiamulin; MUP: mupirocin; FUS: fusidic acid; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline. All MRSA isolates were resistant to penicillin and cefoxitin, as expected. All isolates were susceptible to vancomycin and linezolid.
Figure 56.
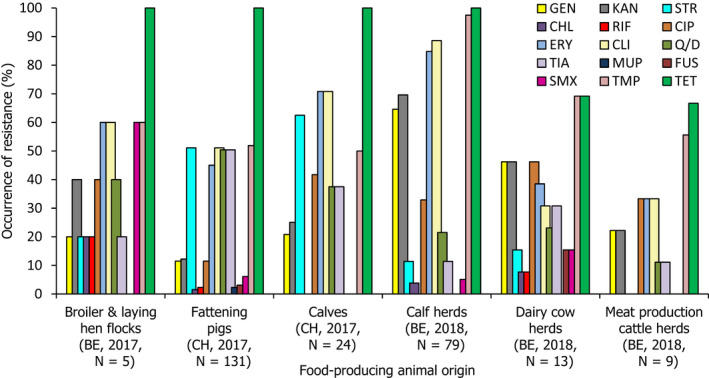
Occurrence of resistance (%) to selected antimicrobials in MRSA isolates from food‐producing animals, 2017/2018
-
N: Number of MRSA isolates reported/tested; BE: Belgium; CH: Switzerland.All isolates were tested against GEN: gentamicin; KAN: kanamycin; STR: streptomycin; CHL: chloramphenicol; RIF: rifampicin; CIP: ciprofloxacin; ERY: erythromycin; CLI: clindamycin; Q/D: quinupristin/dalfopristin; TIA: tiamulin; MUP: mupirocin; FUS: fusidic acid; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline. All MRSA isolates were resistant to penicillin and cefoxitin, as expected. All isolates were susceptible to vancomycin and linezolid.
Susceptibility data of MRSA isolates obtained from clinical investigations (Sweden, 2017)
Antimicrobial susceptibility and molecular typing data of MRSA isolates from dogs, goats, sheep, horses, a cat and a rabbit (following clinical investigations) were also provided by Sweden in 2017:
spa‐type t786 was reported from a pet cat and only showed resistance to trimethoprim and the combination of trimethoprim + sulfonamide (in addition to cefoxitin and penicillin resistance, as expected).
Eight MRSA isolates were reported from pet dogs – spa‐types t008, t022, t032, t034, t127, t891, t2734 and t5634 – with 5/8 displaying ciprofloxacin resistance. Additionally, the canine isolates showed varied resistance patterns to antimicrobials including gentamicin, erythromycin, clindamycin, fusidic acid, trimethoprim, tetracycline and the combination of trimethoprim + sulfonamide; illustrating the diversity of spa‐types reported.
Fusidic acid resistance was reported in a MRSA isolate from a pet rabbit (spa‐type t132); no other resistance was observed (with the exception of cefoxitin and penicillin).
Among the low number (N = 7) of MRSA isolates from horses, all showed resistance to gentamicin, tetracycline and trimethoprim. Resistance to ciprofloxacin, erythromycin, clindamycin and the combination of trimethoprim + sulfonamide ranged from extremely high to high (71.4%, 57.1%, 28.6% and 28.6%, respectively). These isolates were spa‐types t011 and t1257. One t011 isolate was susceptible to oxacillin, with the MIC at the ECOFF.
Ten mecC‐MRSA isolates (spa‐types t373 and t9268) were reported from goats following on‐farm clinical investigations; no resistance was recorded to antimicrobials with the exception as expected of cefoxitin (10/10) and penicillin (10/10), oxacillin was not tested.
mecC‐MRSA was also detected in two sheep at a zoo. The isolates were spa‐type t9268, with a similar resistance pattern, showing resistance to β‐lactams only (cefoxitin and penicillin; oxacillin was not tested).
Figure 9 presents the overall resistance to selected antimicrobials in MRSA isolates obtained from clinical investigations in Sweden in 2017.
Figure 57.
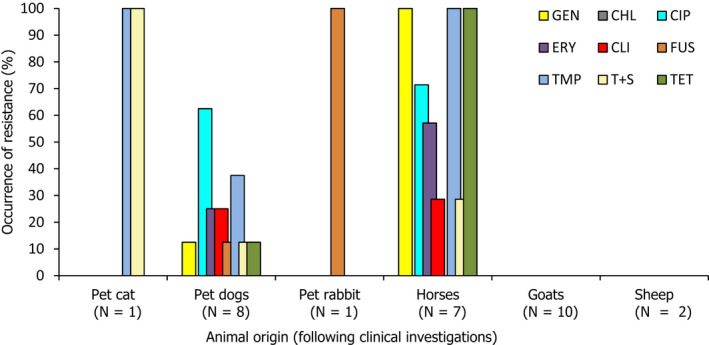
Occurrence of resistance (%) to selected antimicrobials in MRSA isolates obtained from clinical investigations by Sweden in 2017
- N: Number of MRSA isolates reported/tested. All isolates were tested against GEN: gentamicin; CHL: chloramphenicol; CIP: ciprofloxacin; ERY: erythromycin; CLI: clindamycin; FUS: fusidic acid; TMP: trimethoprim; T+S: trimethoprim + sulfonamide; TET: tetracycline. All MRSA isolates were resistant to penicillin and cefoxitin, as expected, and susceptible to linezolid; vancomycin susceptibility was not reported.
6.4. Discussion
The monitoring of MRSA in animals and food was voluntary in 2017/2018 and only a limited number of countries reported data on the occurrence of MRSA, with some countries additionally reporting data on spa‐type and antimicrobial susceptibility. Where typing data were available, most MRSA isolates detected were those associated with LA‐MRSA (94.9% in 2017 and 97.6% in 2018); Figure 10 provides an overview of the types of MRSA detected.
The monitoring of food in 2017/2018 comprised investigations of various food products including meat derived from different animal sources. The monitoring of MRSA in various food products performed by MSs consistently indicates that MRSA can be detected, quite frequently, in different types of food. Such food included meat from cattle and pigs, as well as rabbit meat in 2017 and poultry meat in 2018. It should be underlined that the laboratory techniques used to detect MRSA employ selective bacterial culture and therefore, very low levels of contamination can be detected. Cross‐contamination between carcases on slaughterhouse lines or during production processes may also result in a higher prevalence in meat produced from animals than in the animals themselves. LA‐MRSA is considered a poor coloniser of humans and occurs uncommonly in persons without direct or indirect contact with livestock or their carcases (Graveland et al., 2010). Although a previous report has cautiously suggested that some strains of LA‐MRSA may be adapted to colonise and infect humans and implicate poultry meat as a possible source for humans (Larsen et al., 2016), food is not generally considered to be a significant source of MRSA infection or colonisation of humans (EFSA, 2009b). A recent risk assessment published by the UK Food Standards Agency reached the same conclusion (FSA, 2017).
The spa‐typing and susceptibility data reported in 2017/2018 provided useful information in categorising MRSA isolates; and in 2018, data on spa‐type and sequence type/clonal complex were provided for most isolates (84.8%) recovered from food‐producing animals. However, further typing data would in many cases provide extremely useful additional information to aid classification and help assess the origin and significance of the MRSA isolates. For example, possession of the IEC genes (chp, sak and scn) is considered an adaptation facilitating colonisation and infection of humans and is not usually a feature of animal strains (Cuny et al., 2015a; Larsen et al., 2016). Similarly, the presence of the PVL toxin is a virulence feature typically associated with most CA‐MRSA strains; other genetic factors can be associated with particular strains or may suggest a particular host preference (e.g. lukM has been associated with certain animal strains, particularly those affecting ruminants).
Monitoring of MRSA in food
In 2017 and 2018, spa‐typing data were available for 15/80 and 8/345 MRSA isolates recovered from meat, respectively. Considering the three broad categories of MRSA – CA, HA and LA – most reported spa‐types in 2017 (14/15) and all of those reported in 2018 (8/8) were those associated with LA‐MRSA. In 2017, 14/15 spa‐types were those associated with CC398, the most common LA‐MRSA lineage occurring in Europe; the remaining isolate, spa‐type t002, was recovered from fresh pig meat by Switzerland. spa‐type t002 has been associated with several sequence types within CC5 but is most commonly associated with ST5 (CC5), a sequence type which can be considered as either a CA or HA MRSA. Although further molecular typing data (including PVL status) were not available, the isolate was considered likely to represent a HA‐MRSA lineage. In 2011, Monecke et al. documented that ST5‐MRSA‐II was the most frequently isolated strain from intensive care units in Dresden/Saxony (Monecke et al., 2011). In addition to β‐lactams (cefoxitin and penicillin), the t002 isolate was resistant to the aminoglycosides, gentamicin and kanamycin and susceptible to all other tested antimicrobials including streptomycin. Interestingly, a study carried out in the USA suggests that t002‐ST5 may also represent a LA MRSA lineage, whereby this genotype was most frequently recovered during investigations focused on the short‐term exposures experienced by veterinary students conducting diagnostic enquiries on pig farms. The t002‐ST5 genotype accounted for 75% of MRSA isolates recovered from pigs, 83.8% of MRSA isolates from the farm environment, and 76.9% of MRSA isolates from veterinary students visiting these corresponding farms in the USA (Frana et al., 2013). In 2018, spa‐types associated with the LA lineages CC398 and CC9 were reported from broiler meat in Switzerland, as well as types associated with CC398 from broiler and turkey meat in Austria. CC9 is also an LA‐MRSA clonal lineage. It is disseminated worldwide and is particularly prevalent among various species of livestock in Asia (Cuny et al., 2015b). Kraushaar et al. (2016) reported that MRSA from poultry (chickens and turkeys) collected along the production chains in Germany mainly belonged to ST9, ST398 and ST5, and resistance to clindamycin, erythromycin tetracycline and trimethoprim was most frequently detected. Among the eight LA‐MRSA isolates recovered from poultry meat by Austria and Switzerland, this pattern of resistance was generally reported.
Monitoring of MRSA in healthy food‐producing animals and horses
Considering food‐producing animals, spa‐types associated with each type of MRSA (LA‐, CA‐ and HA‐MRSA) were reported in 2017, while spa‐types associated with LA‐ and HA‐MRSA were reported in 2018; as well as mecC‐MRSA in both 2017 and 2018. In total, spa‐typing data were available for 530 MRSA isolates reported in 2017 and 325 isolates reported in 2018, with most spa‐types considered to represent LA lineages (524/530 isolates in 2017 and 317/325 isolates in 2018). Of the 524 isolates categorised as LA‐MRSA in 2017, four novel spa‐types were reported (see text box below); while of the 317 isolates considered to represent LA‐MRSA in 2018, these included spa‐types t267 and t13790. spa‐type t267 was reported from a Danish dairy cow herd and was confirmed to belong to CC97. Although MRSA isolates belonging to this clonal lineage have been reported from humans as CA clones (Monecke et al., 2011; Spoor et al., 2013; Egea et al., 2014), CC97 MRSA has been detected in pigs and cattle (associated with bovine mastitis) in Europe and is considered a LA lineage. In an earlier European study, an MRSA isolate of spa‐type t267 was reported from dairy cattle in Italy (Feltrin et al., 2016). Certain characteristics of CC97 S. aureus isolates can be associated with human or animal hosts; for example the LukM/F leukotoxin and von Willebrand binding protein have ruminant host‐specific activity and were associated with a proportion of isolates from cattle but were not detected in isolates from humans in a European study (Spoor et al., 2013). EFSA recently proposed that WGS should be used to determine MRSA strains and lineages, as well as to investigate the presence of important virulence and host‐adaptation factors and those specific genetic markers (e.g. phages) associated with certain animal hosts (EFSA, 2019). The CC97 isolate provides a good illustration of the potential benefits of adopting this approach and the type of additional information which could be obtained. The aforementioned spa‐type which was also considered to represent a LA lineage in 2018 (spa‐type t13790) was recovered from a Danish mink farm (sample of mink paw) and reported to belong to CC1. LA‐MRSA in mink has been considered to originate from contaminated pig by‐products used in the production of mink feed and in a 2016 survey of mink feed samples, 19% (20/108) were reported to be positive for LA‐MRSA (Hansen et al., 2017). LA‐MRSA has been detected on the paws and pharynx of mink after exposure to feed contaminated with LA‐MRSA and may persist for more than 26 days (Fertner et al., 2019). MRSA belonging to CC1 has been detected in breeding pigs and in pork in Denmark, although at a much lower frequency than MRSA CC398 (DANMAP, 2016). The occurrence of LA‐MRSA CC1 in pigs and pork in Denmark may therefore account for the detection in mink and while MRSA isolates belonging to CC1 may be regarded as either a CA‐ or LA‐MRSA, the isolate was considered most likely to represent a LA‐MRSA. LA‐MRSA CC1 has also been reported in pigs in other European countries, notably in Italy (Alba et al., 2015). Additionally, Denmark reported that the t13790 isolate was negative for the human IEC gene scn, which may also suggest a link to animals. These findings in mink are interesting because, although LA‐MRSA is not considered a food‐borne disease in humans, either food‐borne spread or contamination of the mink environment through their food and subsequent colonisation of the animals, appears to have occurred in the farmed mink.
Detection of ‘new’ spa ‐types in 2017
Principally, spa‐typing is a sequence‐based technique which analyses variable number tandem repeats (VNTR) in the 3’ coding region of the staphylococcal protein A gene (spa). Base sequences are assigned unique repeat codes, which comprise the repeat succession (spa repeats) for a given strain and determine spa‐type. Therefore, alterations to spa repeats may give rise to ‘new’ spa‐types, as a consequence of slipped strand mispairing during DNA synthesis (van Belkum, 1999). Unlike spa‐typing, multilocus sequence typing (MLST) is a technique which types multiple loci; namely seven S. aureus housekeeping genes. The DNA sequences within each housekeeping gene are assigned as distinct alleles, and a sequence type/clonal lineage is allocated by comparing the set of alleles to other isolate profiles. Although some spa‐types can belong to several sequence types (some rarely possessing mosaic or hybrid genomes), generally most spa‐types are associated with a particular sequence type.
In 2017, spa‐types t17061, t17304 and t17627 were reported; MLST data were not available. Although these spa‐types appear not to have been previously sequenced typed, based upon similarities of spa repeats to other spa‐types associated with CC398, they were inferred to belong to CC398 – see Table 13. Additionally, Switzerland reported the novel spa‐type t17339 from two calves, which was confirmed to belong to CC398; spa repeats of t17339 are also shown in Table 13.
Considering the origins of these novel spa‐types in 2017 – Finnish fattening pigs, Spanish fattening pigs and Swiss calves – their detection illustrates how rapidly S. aureus is able to evolve through repeat deletion, duplication and point mutation. Although the likelihood is that spa‐types t17061, t17304 and t17627 are also associated with CC398, the possibility that they possess mosaic or hybrid genomes cannot be definitively excluded, and EFSA recommend that novel spa‐types be sequence typed to confirm concordance between spa‐typing and assignment of a given isolate to a sequence type or lineage (EFSA, 2012).
Table 13.
VNTR compositions of spa‐types t17061, t17304, t17339 and t17627, and of common spa‐types associated with CC398
| spa‐type | VNTR/repeat successiona | spa repeat similarities |
|---|---|---|
| t2741b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 ‐ 16 | t17061 differs from t2741 by only one repeat |
| t17061 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ ‐ ‐ ‐ 25 ‐ 16 | |
| t1456b | 08 ‐ 16 ‐ 02 ‐ 25 | t17304 differs from t1456 by only one repeat |
| t17304 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 13 | |
| t034b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ ‐ ‐ ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | t17339 differs from t034 by only one repeat |
| t17339 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 51 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | |
| t011b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | t17627 differs from t011 by only one repeat |
| t17627 | 08‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 24 | |
spa repeats as published on Ridom Spa Server (https://spa.ridom.de/spatypes.shtml).
Common spa‐types associated with CC398.
In 2017, the six isolates which were not categorised as LA‐MRSA from food‐producing animals included spa‐types t037, t091, t109, t127, t843 and t6292; the latter two spa‐types were confirmed to carry the mecC gene (see text box below). spa‐type t037 (which was recovered from a Belgian laying hen flock) is generally associated with ST239, a dominant sequence type of HA‐MRSA and mosaic strain which has descended from ST8 and ST30 parents. spa‐type t037 has also been associated with ST110 and ST241 (Fossum and Bukholm, 2006). The occurrence of mosaic strains, which are hybrid strains formed by recombination of the genome of MRSA belonging to different lineages, has the consequence that certain spa‐types may be associated with more than one sequence type. The t037 isolate recovered from a laying hen flock was considered to represent a HA‐MRSA lineage, and Belgium confirmed that the isolate belonged to ST239 and carried both sak and scn genes from WGS data. Concerning porcine isolates, spa‐type t091 was recovered from a Norwegian multiplier pig herd; and although additional molecular data was available (clonal complex 7, PVL‐negative), a MRSA category was not inferred. The PVL status of this isolate would suggest a HA‐MRSA lineage – as possession of the PVL toxin is typical of CA‐MRSA strains – however, MSSA t091 isolates have been frequently reported in pigs/pork meat from south west Poland (Krupa et al., 2015). Therefore, the possibility that this MRSA genotype has emerged, through meticillin‐sensitive S. aureus in pigs acquiring the SCCmec cassette, cannot be discounted. Additionally, spa‐type t109 was recovered from a slaughter batch of Spanish fattening pigs in 2017; this spa‐type has been associated with ST5 and ST228 (both members of CC5) but is generally associated with ST228 and considered as a HA‐MRSA lineage. Concerning bovine isolates reported in 2017, a single t127 isolate was recovered from a Swiss calf at slaughter. Although spa‐type t127 has been associated with MRSA belonging to several sequence types within CC1, as well as to types in CC474, it is most frequently associated with ST1 (CC1); whereby this spa/sequence type combination represents a CA‐MRSA regardless of PVL status. The t127 isolate was therefore categorised as a CA‐MRSA, although the ST1 lineage has also been recognised as a LA MRSA (Feltrin et al., 2016) and the establishment of spa‐type t127 within livestock has also been reported. In the EU baseline survey of breeding pig holdings, the potential clonal spread of spa‐type t127 (ST1) among Italian pig populations was documented (EFSA, 2009c; Franco et al., 2011). Additionally, t127‐ST1 has frequently been detected among ruminants and/or their produce in Italy (Carfora et al., 2016; Luini et al., 2015; Parisi et al., 2016; Macori et al., 2017), and from horses in Austria (Loncaric et al., 2014).
Regarding the monitoring of food‐producing animals in 2018, the eight isolates which were not categorised as LA‐MRSA comprised spa‐types t223, t1257 and t843; the latter confirmed to represent a mecC‐MRSA (see text box below). Belgium reported spa‐type t223 from three dairy cow herds and three meat production cattle herds. spa‐type t223 is associated with ST22 (CC22) – a dominant sequence type and spa‐type combination of HA‐MRSA – and Belgium confirmed that one isolate from meat production cattle belonged to ST22 and SCCmec type IV2B/IVa2B from WGS data. ST22‐MRSA‐IV is the pandemic HA strain known as EMRSA‐15, which was first identified in the UK during the early 1990s and has since been reported in many countries. The classical EMRSA‐15 strain typically lacks certain virulence features such as PVL and toxic shock syndrome toxin 1 (TSST‐1), but possesses the enterotoxin C gene, sec (Wolter et al., 2008; Monecke et al., 2011). Belgium confirmed that all six bovine t223 isolates were PVL negative, and while WGS of the isolate from meat production cattle was found to harbour the tst gene encoding for TSST‐1, many variants of EMRSA‐15 have been described (Moneke et al., 2011). Such variants are not unforeseen since the genes encoding these virulence toxins reside on mobile genetic elements. Wolter et al. (2008) reported an EMRSA‐15 variant from the USA which was negative for sec and the PVL gene but positive for tst. Other variable virulence markers in ST22‐MRSA‐IV strains include the human IEC genes, chp, sak and scn (Monecke et al., 2011), which Belgium additionally reported from WGS data of the isolate from meat production cattle. spa‐type t1257 was also reported from a Belgian dairy cow herd in 2018. This spa‐type has been associated with sequence types within CC8 (ST239 and ST612) but appears to be more frequently associated with sequence type ST612. While the t1257‐ST612 genotype may be regarded as either a CA‐ or HA‐MRSA, the t1257 isolate from a dairy herd was not categorised to a particular lineage; further typing (including PVL testing) would aid such characterisation.
mecC ‐MRSA reported from food‐producing animals and a horse in 2017/2018
In 2017, mecC‐MRSA isolates were recovered from two Norwegian farrow‐to‐finish pig herds; spa‐types t843 and t6292, MLST confirming them to belong to CC130 and CC425, respectively. Additionally, Denmark reported spa‐type t843 from an equine premise in 2018. The isolate was confirmed to belong to CC130 and carry the mecC gene. Antimicrobial resistance patterns of these porcine/equine mecC isolates were not reported. Of note, is the detection of mecC‐MRSA CC130 (spa‐type t528) for the first time from Danish horses in 2015 (DANMAP, 2016; Islam et al., 2017), although mecC‐MRSA isolates have also been recovered from other animal sources in Denmark (Angen et al., 2017; Petersen et al., 2013; Harrison et al., 2013). In another recent study, the occurrence of mecC‐MRSA in wild hedgehogs from three regions of Sweden was investigated (Bengtsson et al., 2017); whereby mecC‐MRSA was isolated from 64% of 55 wild hedgehogs and spa‐type t843 was most commonly found (49%). These two spa‐types (t843 and t6292) have previously been observed in humans (Paterson et al., 2014; Swedres‐Svarm, 2017) and possible transmission between humans and animals is documented (Peterson et al., 2013; Harrison et al., 2013; Angen et al., 2017). Angen et al. (2017) identified the first case of mecC‐MRSA in domesticated pigs and findings strongly indicated transmission between farmers and pigs. Additionally, the study of Bengtsson et al. (2017) supports the hypothesis that wildlife may constitute a reservoir of mecC‐MRSA.
Temporal trends of MRSA prevalence in various types of meat and food‐producing animals
Considering that the temporal prevalence of MRSA in Swiss fattening pigs at slaughter has shown a steady increase from 2009 to 2015, a more marked increase from 2015 to 2017 was observed. This marked increase represents the diffusion of spa‐types t011 and t034 within Swiss fattening pig populations; and in 2017, all reported isolates were those associated with CC398, with most belonging to spa‐types t011 and t034. Moreover, statistical tests (Cochran‐Armitage trend test) performed on the Swiss longitudinal data revealed a statistically significant increasing trend over these years. A longitudinal study carried out by Kraemer et al. (2017) also supports these trends, in which MRSA prevalence of pig farms in Western Switzerland were reported to increase from 7.3% in 2008 to 31% in 2015. The complete epidemiological data should however be considered when evaluating trends apparent in this chapter, because the summary data reported to EFSA may not include full details of any methodological or other changes to monitoring procedures. A detailed longitudinal study illustrated that pigs are intermittently and repeatedly colonised, and that colonisation may also occur during transportation and while in the lairage (Bangerter et al., 2016). The detection of intermittent, repeated colonisation suggests that the number of animals sampled as part of a batch, including whether individual animals are sampled to represent a herd or batch, is likely to influence the batch or herd prevalence obtained. These factors should therefore be taken into consideration with regard to the statistical analyses, as the Swiss annual MRSA monitoring examines a single pig from a herd at slaughter. Regarding longitudinal data available for other countries, a decline in MRSA prevalence was noted in German calves at slaughter from 2012 to 2017, as well as German fattening pig herds from 2015 to 2017. The reasons for these modest declines were not apparent and could possibly reflect sampling variability, with no statistically significant differences detected from the German longitudinal data, however findings are interesting because, generally, MRSA prevalence in animals and food has shown a progressive increase, where it has been investigated. For example, a marked increase was observed in batches of Finnish fattening pigs at slaughter from 2010 to 2017; illustrating the possible dissemination of spa‐types t034 and t2741 within Finnish fattening pig populations. Furthermore, a study conducted in 2015, identified spa‐type t2741 as a new dominant clone among Finnish fattening pigs at slaughter (Heikinheimo et al., 2016). Tests for statistical significance in relation to the changes in MRSA prevalence in Finnish fattening pigs at slaughter confirmed a statistically significant increasing trend from 2010 to 2017 (Cochran‐Armitage trend test). Similarly, MRSA prevalence in Finnish pig meat was reported at a higher level in 2017 compared to that observed in 2015, with statistical analysis also detecting an increasing trend (Cochran‐Armitage trend test). A modest increase in MRSA prevalence was also noted among Belgian dairy cow herds from 2012 to 2018, as well as among conventional fattening pig herds in Denmark from 2016 to 2018. A significant observation from the monitoring of Danish fattening pig herds in 2018 is the considerable difference in MRSA prevalence among differing herd types. Prevalence was reported at a substantially lower level among free‐range production type herds (including organic production) in comparison to conventional indoor production herds (20.2% and 89.2%, respectively); corroborating findings from the DANMAP (2016) report, which concludes that MRSA is less well maintained in free‐range pig herds compared to conventional pig herds.
Susceptibility testing of MRSA isolates obtained from meat and food‐producing animals
Lincosamide resistance and macrolide susceptibility is an unusual phenotype which may be conferred by lnu genes. In a study of Finnish fattening pigs at slaughter, this unusual phenotype was observed among some CC398 isolates, and was associated with isolates lacking ermB, but harbouring lnuB (Heikinheimo et al., 2016). Considering the susceptibility of MRSA isolates to clindamycin and erythromycin, there was an equal occurrence of resistance to both compounds in Swiss calves and Belgian chicken flocks in 2017, as well as Swiss broiler meat and Belgian meat production cattle herds in 2018. Conversely, clindamycin resistance exceeded that of erythromycin in Swiss pigs and Finnish pork in 2017, as well as Belgian calf herds in 2018; this phenotype suggesting the possible presence of lnu genes.
MRSA isolates obtained from clinical investigations (Sweden, 2017)
In 2017, spa‐types associated with each type of MRSA, as well as mecC‐MRSA, were also reported following clinical examinations carried out by Sweden; denominator data were not provided. mecC‐MRSA was reported in ten goats and two sheep, discussed further in the text box below. Following veterinary‐clinic clinical investigations, Sweden reported spa‐type t1257 from two horses. As discussed previously, this spa‐type is generally associated with sequence type ST612 (CC8); and although the t1257‐ST612 genotype may be regarded as either a CA‐ or HA‐MRSA, Sweden confirmed that the isolates were PVL‐negative which is indicative of a probable HA lineage. Susceptibility testing revealed that both isolates were resistant to ciprofloxacin, erythromycin, tetracycline, trimethoprim, gentamicin, and the combination of trimethoprim + sulfonamide (in addition to β‐lactams); which reflects the fact that these horses were in the same animal hospital so transmission between animals is a possibility. Additionally, LA MRSA was reported in five Swedish horses; these were spa‐type t011 (associated with CC398) and all were tetracycline‐resistant. Köck et al. (2017) documented that LA‐MRSA CC398 has recently emerged as a significant cause of primarily nosocomial infections in horses. Considering Swedish companion animals, spa‐type t132 was reported from a pet rabbit. While MLST was not reported, this spa‐type is associated with ST45 (CC45), and the isolate was inferred to represent a HA MRSA due to its PVL‐negative status. With the exception of β‐lactams (penicillin and cefoxitin; oxacillin not tested), the isolate only showed resistance to fusidic acid. Conversely, ST45‐MRSA CA lineages have also been recognised in humans. In particular, a ST45‐MRSA‐N1 clone was found among intravenous drug users and their contacts in Switzerland; whereby this clone was reported to be similar to the epidemic Berlin MRSA clone (Qi et al., 2005). Considering MRSA cases reported in dogs, Sweden reported the isolation of spa‐types t008, t022, t032, t034, t127, t891, t2734 and t5634; representing all three MRSA categories. spa‐types t034 and t2734 were attributed to LA‐MRSA, whereby these types are associated with CC398 and CC97, respectively. spa‐type t2734 (CC97) has however, also been recognised as a CA‐MRSA in Argentina (Egea et al., 2014). spa‐types t891 and t127 were attributed to CA‐MRSA, whereby these types are associated with ST22 (CC22) and most frequently ST1 (CC1), respectively. Both CA‐ and HA‐MRSA have been reported among ST22 isolates, however, spa‐type t891 was reported to be PVL‐positive suggesting a CA lineage as CA‐MRSA frequently possess the PVL toxin, which may confer an increase in virulence, although the exact role of the PVL toxin has been debated (Chadwick et al., 2013). Conversely, spa‐type t127 was reported to be PVL‐negative, yet this isolate was still considered most likely to represent a CA‐MRSA regardless of PVL status. Considering the remaining canine isolates, spa‐types t008, t022, t032 and t5634, all were considered to represent HA‐MRSA. spa‐type t008 has been associated with many sequence types within CC8 (ST8, ST247, ST250 and ST254), but is most commonly associated with ST8; Sweden confirmed that the isolate belonged to ST8 from WGS data. This spa‐type and sequence type combination is seen in isolates of the globally significant CA‐MRSA USA300 strain, which is PVL positive and frequently possesses arginine catabolic mobile element (ACME) genes. The CA‐MRSA USA300 strain can cause severe infections in humans and has a markedly different epidemiology from HA‐MRSA strains (Tenover and Goering, 2009). However, Sweden confirmed that the isolate was PVL‐negative and ACME genes (arcA) were not detected. Therefore, the isolate is likely to represent a HA‐MRSA. spa‐types t022, t032 and t5634 are all associated with ST22 (CC22) and both CA‐ and HA‐MRSA have been reported within this sequence type. All three spa‐types were however reported to be PVL‐negative and were therefore categorised as HA‐MRSA. The final isolate reported by Sweden in 2017 was spa‐type t786 from a pet cat and although this isolate was not sequence typed, spa‐type t786 is associated with CC88 (sequence types ST78 and ST88). While the t786 isolate was reported to be PVL‐negative, the CC88 lineage is predominantly regarded as a CA MRSA and was categorised as such. Detection of CA‐MRSA and HA‐MRSA within these companion animals most likely represents colonisation or infection with human MRSA strains rather than persistent establishment within these species. This is supported by the common occurrence of some of these spa‐types within the Swedish human population.
mecC ‐MRSA reported following clinical investigations by Sweden in 2017
In addition to the three mecC‐MRSA isolates reported from food‐producing animals in 2017/2018, ovine and caprine mecC‐MRSA isolates were also detected following zoo/on‐farm clinical investigations by Sweden in 2017. In total, 12 mecC‐MRSA isolates were reported. spa‐type t9268 was recovered from two sheep at a zoo, and sequence typing of one t9268 isolate confirmed it to belong to CC130. Considering the caprine isolates, spa‐types t373 (nine isolates) and t9268 (one isolate) were reported; both of which are associated with CC130 (Peterson et al., 2013; SWEDRES, 2011). From 2011 to mid‐2017, spa‐type t373 was the second (20/92 cases) most common domestically acquired mecC‐MRSA spa‐type reported from humans in Sweden (Swedres‐Svarm, 2017); spa‐type t9268 has also been reported in man (Swedres‐Svarm, 2017).
Resistance to non‐β‐lactam antibiotics is currently uncommon among mecC ‐MRSA isolates (Paterson et al., 2014) and, typically, the t373 and t9268 isolates from clinical investigations were susceptible to non‐β‐lactams. Although Sweden did not report oxacillin susceptibility for the caprine/ovine isolates, all were resistant to penicillin and cefoxitin; oxacillin has been demonstrated to be a less reliable marker than cefoxitin for detection of mecC‐MRSA (Paterson et al., 2014; Bengtsson et al., 2017).
In summary, the monitoring of MRSA in 2017 and 2018 provided extremely useful information on the occurrence of MRSA in livestock and food. The situation continues to develop and evolve and there is a clear requirement for the continued monitoring and appropriate molecular characterisation of MRSA isolates recovered from livestock and food. Nevertheless, where countries reported spa‐typing data of MRSA isolates from food‐producing animals in 2018, most additionally provided data on multilocus sequence type or clonal complex (319/325), which proved extremely useful to categorise isolates. Molecular characterisation is however, becoming increasingly necessary to fully evaluate the significance of MRSA isolates and there are limitations to the analyses which can be performed when spa‐typing is used as the only technique to characterise isolates. Conversely, the presence of the PVL toxin may not always be indicative of CA‐MRSA, highlighted in 2017 by genotypes t786‐CC88 and t127‐CC1 which are predominantly CA lineages yet lack PVL. Notably, the movement of live animals, as well as human travel, are important contributing factors to the spread of MRSA between countries, and therefore the occurrence data contained in this report may reflect such circumstances. Similarly, the occurrence of MRSA among meat samples reported by certain countries may not reflect the situation in corresponding animal populations for that country, as the summary data reported to EFSA does not include details of whether such meats were imported. Most reporting countries did not report susceptibility data for MRSA isolates recovered in 2017/2018, which also provides useful information for characterising isolates. A significant observation from the 2018 monitoring included the considerable difference in MRSA prevalence reported from free‐range pig herds in comparison to that noted among conventional pig herds in Denmark. Additionally, the monitoring includes some new findings: spa‐types t17061, t17304, t17339 and t17627 were reported from food‐producing animals in 2017, and these spa‐types appear not to have been reported previously. Although the likelihood is that t17061, t17304 and t17627 are associated with CC398, the findings once again illustrate the limitations of spa‐typing as a single method of definitively assigning novel isolates to particular lineages, where MLST has not previously been undertaken. In conclusion, Figure 10 illustrates the genetic diversity of MRSA isolates recovered from food, healthy animals and following clinical investigations in 2017/2018. Most reported spa‐types were those associated with LA‐MRSA lineages in both reporting years (94.9% in 2017 and 97.6% in 2018). However, spa‐types associated with CA‐ and HA‐MRSA were also reported, as well as mecC‐MRSA. The occasional detection of lineages of CA‐ and HA‐MRSA primarily associated with humans is perhaps not surprising, since the sporadic interchange of strains between man and animals may be expected. While the monitoring of MRSA is voluntary and not all countries contribute data, Figure 10 provides a summary of all reported findings in 2017/2018, against which changes in the reported occurrence of different MRSA lineages may be assessed in future.
Figure 58.
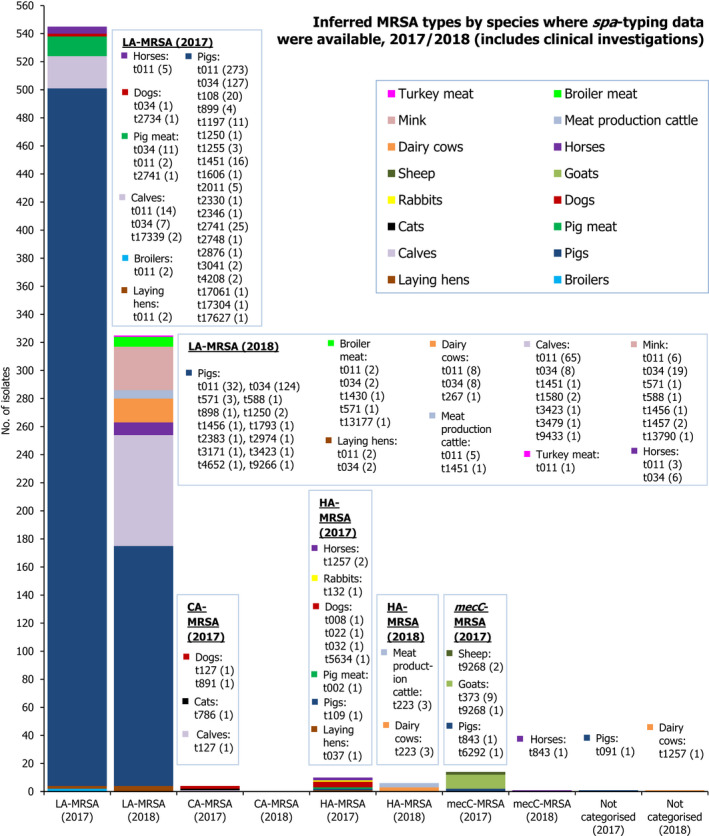
Overview of MRSA types by species reported in 2017 and 2018, including isolates recovered from food, healthy animals, and following clinical investigations
Abbreviations
- %
percentage of resistant isolates per category of susceptibility or multiple resistance
- % f
percentage frequency of isolates tested
- % Res
percentage of resistant isolates
- –
no data reported
- AAC
aminoglycoside acetyltransferases
- ACME
arginine catabolic mobile element
- AMC
consumption of antimicrobials agents
- AMR
antimicrobial resistance
- ARM
At‐retail monitoring
- AST
antimicrobial susceptibility testing
- BIOHAZ
EFSA Panel on Biological Hazards
- CA
community‐associated
- CA‐SFM
French Society for Microbiology
- CBP
clinical breakpoints
- CC
clonal complex
- CLSI
Clinical and Laboratory Standards Institute
- CP
carbapenemase producer
- CTX‐M
cefotaxime
- DD
disc diffusion method
- DIN
Deutsches Institut für Normung
- DL
dilution/dilution method
- EARS‐Net
European Antimicrobial Resistance Surveillance Network
- ECDC
European Centre for Disease Prevention and Control
- ECOFF
epidemiological cut‐off value
- EEA
European Economic Area
- EFSA
European Food Safety Authority
- EMA
European Medicines Agency
- ESBL
extended spectrum beta‐lactamase
- ESC
extended‐spectrum cephalosporins
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- EURL‐AR
EU Reference Laboratory for Antimicrobial Resistance (www.crl-ar.eu)
- FWD
food‐ and waterborne diseases and zoonoses
- HA
healthcare‐associated
- I
intermediate
- IEC
immune evasion cluster
- IR
inverted repeat
- JIACRA
Joint Interagency Antimicrobial Consumption and Resistance Analysis
- LA
livestock‐associated
- LOS
lipo‐oligosaccharide
- MDR
multiple drug resistance
- MDRI
multiple drug resistance islands
- MDRGI
multiple drug resistance genomic island
- MIC
minimum inhibitory concentration
- MRSA
meticillin‐resistant Staphylococcus aureus
- MS
Member State
- MSSA
meticillin‐susceptible Staphylococcus aureus
- NA
not applicable/not available
- NCP
National Control Programme
- NRL
National Reference Laboratory
- NTS
non‐typhoidal salmonellas
- OICS
outcome indicator of complete susceptibility
- PCU
PCU population correction unit
- PMQR
plasmid‐mediated quinolone resistance
- PVL
Panton–Valentine leukocidin
- Q
quantitative
- QRDR
quinolone resistance‐determining regions
- R
resistant
- S
susceptible
- SICS
summary index of complete susceptibility
- SIR
susceptible, intermediate, resistant
- ST
sequence type
- TESSy
The European Surveillance System
- VNTR
variable number tandem repeats
- WGS
whole genome sequencing
- WHO
World Health Organization
Antimicrobial substances
- AMC
amoxicillin/clavulanate
- AMP
ampicillin
- AZM
azithromycin
- CAZ
ceftazidime
- CHL
chloramphenicol
- CIP
ciprofloxacin
- CLA
clavulanate
- CLI
clindamycin
- CST
colistin
- CTX
cefotaxime
- ERY
erythromycin
- FUS
fusidic acid
- GEN
gentamicin
- KAN
kanamycin
- LZD
linezolid
- MEM/MER
meropenem
- MUP
mupirocin
- NAL
nalidixic acid
- QD
quinupristin/dalfopristin
- RIF
rifampicin
- SUL
sulfonamides
- STR
streptomycin
- SMX
sulfamethoxazole
- TGC
tigecycline
- TIA
tiamulin
- TET/TCY
tetracycline
- TMP
trimethoprim
MSs of the EU and other reporting countries in 2015
- Austria
AT
- Belgium
BE
- Bulgaria
BG
- Croatia
HR
- Cyprus
CY
- Czech Republic
CZ
- Denmark
DK
- Estonia
EE
- Finland
FI
- France
FR
- Germany
DE
- Greece
GR
- Hungary
HU
- Ireland
IE
- Italy
IT
- Latvia
LV
- Lithuania
LT
- Luxembourg
LU
- Malta
MT
- Netherlands
NL
- Poland
PL
- Portugal
PT
- Romania
RO
- Slovakia
SK
- Slovenia
SI
- Spain
ES
- Sweden
SE
- United Kingdom
UK
Non‐MSs reporting, 2016
- Iceland
IS
- Norway
NO
- Switzerland
CH
Definitions
- ‘Antimicrobial‐resistant isolate’
-
In the case of quantitative data, an isolate was defined as ‘resistant’ to a selected antimicrobial when its minimum inhibitory concentration (MIC) value (in mg/L) was above the cut‐off value or the disc diffusion diameter (in mm) was below the cut‐off value. The cut‐off values, used to interpret MIC distributions (mg/L) for bacteria from animals and food, are shown in Material and methods, Table 5–7.
In the case of qualitative data, an isolate was regarded as resistant when the country reported it as resistant using its own cut‐off value or break point
- ‘Level of antimicrobial resistance’
The percentage of resistant isolates among the tested isolates
- ‘Reporting MS group’
MSs (MSs) that provided data and were included in the relevant table for antimicrobial resistance data for the bacteria–food/animal category–antimicrobial combination
- Terms used to describe the antimicrobial resistance levels
-
Rare: < 0.1%
Very low: 0.1% to 1.0%
Low: > 1.0% to 10.0%
Moderate: > 10.0% to 20.0%
High: > 20.0% to 50.0%
Very high: > 50.0% to 70.0%
Extremely high: > 70.0%
Appendix A – High‐level resistance to ciprofloxacin among certain Salmonella serovars recovered from poultry
High‐level resistance to ciprofloxacin in S. Kentucky
Considering individual serovars, S. Kentucky accounted for most of the Salmonella isolates recovered from poultry which exhibited MICs to ciprofloxacin of ≥ 4 mg/L (180/252). Within each of the poultry origins, the highest number of Salmonella isolates exhibiting high‐level resistance to this antimicrobial were attributed to S. Kentucky; this serovar accounting for 44.9%, 88.9%, 73.6%, 60% and 90.7% of the total number of isolates displaying MICs of ≥ 4 mg/L from broiler carcases, turkey carcases, broilers, laying hens and turkeys, respectively. S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance are likely to belong to the multilocus sequence type (ST) 198 clone, which has shown epidemic spread across Africa first, then to the Middle East, Asia and Europe (Le Hello et al., 2011, 2013; Hawkey et al., 2019). Notably in 2018, the occurrence of this serovar exhibiting high‐level resistance was observed by many MSs from most parts of Europe, suggesting further clonal expansion (S. Kentucky ST198‐X1) within poultry populations. In view of reported MIC values, most of the S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance displayed MICs of ≥ 8 mg/L (only one S. Kentucky isolate from a broiler flock displayed an MIC of 4 mg/L). Additionally, a very high proportion of the S. Kentucky isolates displaying ciprofloxacin MICs of ≥ 4 mg/L (n = 180) were also multiresistant (57.2%), primarily showing resistance to ampicillin, gentamicin, nalidixic acid, sulfamethoxazole and tetracycline (AMP‐CIP‐GEN‐NAL‐SMX‐TET). Figure A.1 presents the overall AMR levels in S. Kentucky isolates which exhibited high‐level ciprofloxacin resistance in poultry.
Figure A.1.
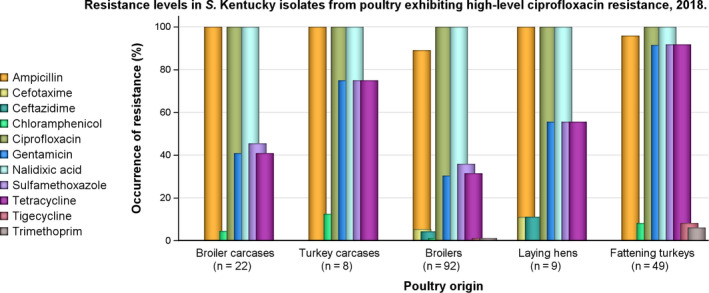
Resistance levels to other selected antimicrobials in S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance from poultry, reported by MSs in 2018
- n: Total number of S. Kentucky isolates exhibiting high‐level ciprofloxacin resistance.
In 2018, S. Kentucky was the seventh most commonly reported serovar in humans, with 663 cases reported by EU/EEA countries. From the monitoring of human cases in 2018, very high to extremely high levels of resistance were noted to gentamicin (51.1%), sulfonamides (71.1%), ampicillin (72.7%), tetracyclines (76.6%), ciprofloxacin (85.7%) and nalidixic acid (87.3%); consistent with the multiresistance patterns observed in isolates from the monitoring of poultry in 2018, and the possible dissemination of the S. Kentucky ST198 strain within Europe. Furthermore, of 3,953 Salmonella isolates from humans where ciprofloxacin MIC data was available, 180 of these (4.6%) exhibited MICs of ≥ 4 mg/L, of which S. Kentucky accounted for 140 (88.6%). Figure A.2 shows the spatial d istributions of ciprofloxacin resistance among S. Kentucky isolates reported from human cases in 2018.
Figure A.2.
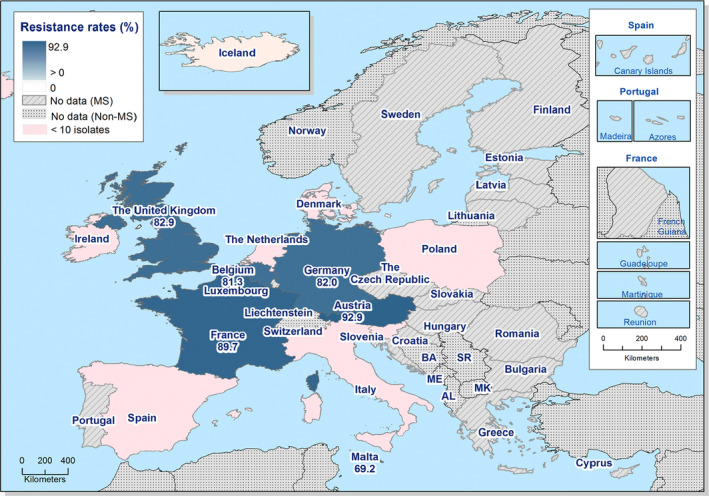
Spatial distribution of ciprofloxacin resistance among S. Kentucky from human cases in reporting countries in 2018
Hawkey et al. (2019) recently documented that MDR S. Kentucky ST198 is a globally disseminated clone, capable of rapid spread and accumulation of last‐line AMR determinants. Acquisition of SGI1 and plasmids, as well as mutations in the QRDR, were the only genetic features found during this study to explain the global epidemiological success of the MDR S. Kentucky ST198 lineage which is highly resistant to ciprofloxacin.
High‐level resistance to ciprofloxacin among other Salmonella serovars
While S. Kentucky generally accounted for most of the Salmonella isolates exhibiting high‐level resistance and there was a significant contribution from S. Infantis in broilers, laying hens and broiler carcases, many other serovars exhibiting resistance by this definition were noted among the poultry origins (namely S. Newport, S. Bardo, S. Enteritidis, S. Bovismorbificans, S. Paratyphi B var. Java, S. Muenster, S. Ohio and S. Saintpaul). Figure A.3 shows the number of isolates exhibiting high‐level resistance to ciprofloxacin by serovar within each of the poultry origins.
Figure A.3.
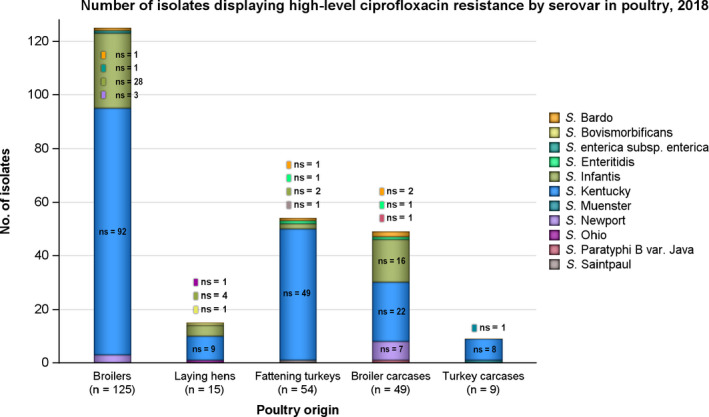
Number of isolates displaying high‐level ciprofloxacin resistance by serovar, reported from the different poultry origins by MSs in 2018
- n: Total number of Salmonella isolates exhibiting high‐level ciprofloxacin resistance; ns: number of isolates by serovar exhibiting high‐level ciprofloxacin resistance.
Considering ciprofloxacin MICs among the serovars presented in Figure A.3 (excluding S. Kentucky), MICs of 4 mg/L were generally reported, although there were a few exceptions. MICs of 8 mg/L were noted in two S. Infantis isolates from broilers, a single S. Bovismorbificans isolate from laying hens and a single S. Enteritidis isolate from turkeys, as well as from single S. Bardo, S. Enteritidis and S. Newport isolates from broiler carcases. Additionally, MICs of > 8 mg/L were noted in an S. Ohio isolate from laying hens, as well as an S. Infantis isolate and an isolate of unspecified serovar from broilers. Single S. Infantis and S. Muenster isolates from broiler and turkey carcases, respectively, also displayed MICs of > 8 mg/L.
Appendix B – Cefotaxime, ceftazidime and ciprofloxacin resistance in Salmonella spp. recovered from each of the animal/carcase origins and humans considering all reporting MSs in 2017/2018
1.
1.1.
Table B.1 summarises cefotaxime, ceftazidime and ciprofloxacin resistance in Salmonella spp. recovered from each of the animal/carcase origins and humans considering all reporting MSs in 2017/2018.
Appendix C – Occurrence of resistance at the Salmonella serovar level
In carcases of food‐producing animals
Breakdown of the most prevalent serovars
The detailed reporting of results at the serovar level clearly demonstrated the major contribution of a few serovars to the observed occurrence of resistance in Salmonella spp. Figure C.1 illustrates the relative contribution of some of the most dominant serovars recovered from each of the carcase origins. In pig carcases, six serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Infantis and London) accounted for 86.6% of Salmonella spp.; while in calf carcases, serovars monophasic Typhimurium, Meleagridis, Mbandaka, Derby, Dublin and Livingstone accounted for 56.1% of the total Salmonella spp. isolated from this origin. Additionally, in broiler carcases, six serovars (Infantis, Indiana, Enteritidis, Chester, Montevideo and Derby) accounted for 67.4% of Salmonella isolates; while in turkey carcases, Bredeney, monophasic Typhimurium, Agona, Newport, Hadar and Indiana accounted for 73.7% of Salmonella spp. isolated from this origin.
Figure C.1.
Commonly reported serovars from carcases of pigs (fatteners), calves (under 1 year of age), broilers and fattening turkeys in 2017/2018
- From calf carcases, S. Livingstone, S. Montevideo and S. Typhimurium were joint sixth most frequently reported.
Complete susceptibility and MDR
Patterns of resistance associated with these different serovars have a marked influence on the overall resistance levels in Salmonella spp., and Figure C.2 summarises the proportion of completely susceptible and MDR isolates among particular serovars recovered from each of these carcase origins. Large contributions of a few resistant serovars to the overall level of MDR among Salmonella spp. were evident within some of the carcase origins; notably S. Infantis in broiler carcases, and S. Typhimurium and its monophasic variant in pig carcases.
Figure C.2.
Proportions of isolates completely susceptible and MDR in Salmonella spp. and particular Salmonella serovars from carcases of pigs (fatteners), calves (under 1 year of age), broilers and fattening turkeys, for all reporting countries in 2017/2018
- N: Total number of Salmonella spp. or total number of particular serovars recovered from the carcase monitoring.
In food‐producing animal populations
Breakdown of the most prevalent serovars
The relative contribution of some of the most dominant serovars recovered from each of the food‐producing animal populations is illustrated in Figure C.3. In pigs, six serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Brandenburg and London) accounted for 86.9% of Salmonella spp.; while in cattle, serovars Typhimurium, monophasic Typhimurium, Dublin, Enteritidis, Derby and Mbandaka accounted for 87.5% of the total Salmonella spp. isolated from this origin. Additionally, in broilers, six serovars (Infantis, Enteritidis, Mbandaka, Kentucky, Livingstone and Senftenberg) accounted for 62.9% of Salmonella isolates, while in laying hens six serovars (Enteritidis, Infantis, Kentucky, Typhimurium, Senftenberg and Mbandaka) accounted for 62.4% of isolates; and in turkeys, serovars Derby, Infantis, Kentucky, Newport, Bredeney and Hadar accounted for 58% of Salmonella isolates.
Figure C.3.
Commonly reported serovars recovered from fattening pigs, cattle, broilers, laying hens and fattening turkeys in 2017/2018
- From cattle, S. Derby and S. Mbandaka were the joint fifth most frequently reported.
Complete susceptibility and MDR
The patterns of resistance associated with these different serovars influenced the overall resistance levels in Salmonella isolates, and Figure C.4 summarises the proportion of completely susceptible and MDR isolates among particular serovars recovered from each of these food‐producing animal populations.
Figure C.4.
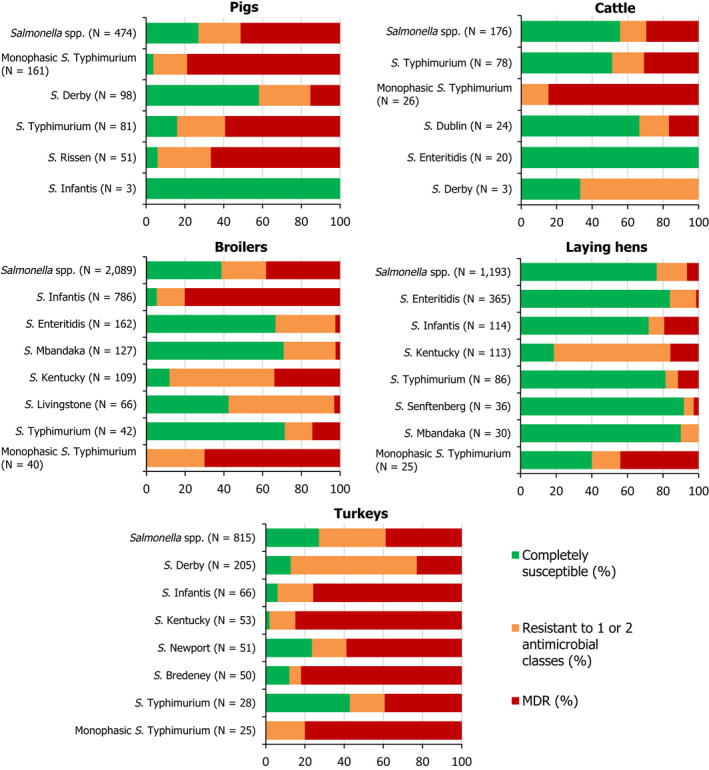
Proportions of isolates completely susceptible and MDR in Salmonella spp. and certain serovars recovered from fattening pigs, cattle, broilers, laying hens and fattening turkeys, for all reporting countries in 2017/2018
- N: Total number of Salmonella spp. or total number of particular serovars recovered from the monitoring of animals.
Resistance exhibited by particular serovars
S. Derby was the most common serovar detected in fattening turkeys, as well as the second most frequently recovered from pig carcases and fattening pigs, accounting for 25.2%, 26.5% and 20.7% of Salmonella isolates recovered from these animal/carcase origins, respectively (see Figures C.1 and C.3). While MDR was not frequently observed among S. Derby isolates from pigs and pig carcases (15.3% and 11.8%, respectively), it was detected at a high level in isolates from turkeys (22.9%); with 87.3% (179/205) of isolates showing resistance to one or more antimicrobials.
In pigs, where 15/98 (15.3%) isolates were MDR, in pig carcases where 30/254 (11.8%) isolates were MDR and in turkeys where 47/205 (22.9%) isolates were MDR, the most common resistance pattern was to sulfamethoxazole, trimethoprim and tetracycline, with the addition of ampicillin in turkeys. Resistance to five antimicrobial classes was observed in three isolates recovered from pig carcases, in two isolates from pigs and in 17 isolates from turkeys. Ciprofloxacin/nalidixic acid resistance among MDR isolates was reported in a single isolate recovered from pigs, two isolates from pig carcases and 24 isolates from turkeys. Tigecycline resistance was only observed in a single MDR isolate recovered from a pig carcase.
Resistance to third‐generation cephalosporins was not detected in S. Derby isolates from pigs, and only single isolates recovered from pig carcases by Germany (N = 7) and turkeys by Poland (N = 1) were determined to be resistant to this antimicrobial class. Both S. Derby isolates exhibited an ESBL phenotype, with the isolate from turkeys also expressing an AmpC phenotype. Combined ‘microbiological’ resistance to two of the highest priority critically important antimicrobials (CIA), ciprofloxacin and cefotaxime, was not detected in any S. Derby isolates recovered from the carcase/animal origins.
Monophasic S . Typhimurium commonly exhibited resistance, and was the most dominant serovar recovered from pig carcases, pigs and calf carcases, as well as the second most dominant serovar recovered from cattle and turkey carcases; accounting for 34.8%, 34%, 14.6%, 14.8% and 12.6% (see Figures C.1 and C.3) of Salmonella isolates recovered from these animal/carcase origins, respectively. Notably, the proportion of all Salmonella isolates showing MDR in calf carcases, pig carcases, pigs and cattle was greatly influenced by the occurrence of multiresistant monophasic S. Typhimurium, which accounted for 61.1% (11/18), 56.7% (258/455), 52.3% (127/243) and 42.3% (22/52) of the MDR Salmonella isolates recovered from these carcase/animal origins, respectively (see Figure C.5). Similarly, this serovar contributed the highest level of multiresistance (13%, 7/54) to overall MDR levels among Salmonella isolates recovered from turkey carcases, as did S. Infantis.
Figure C.5.
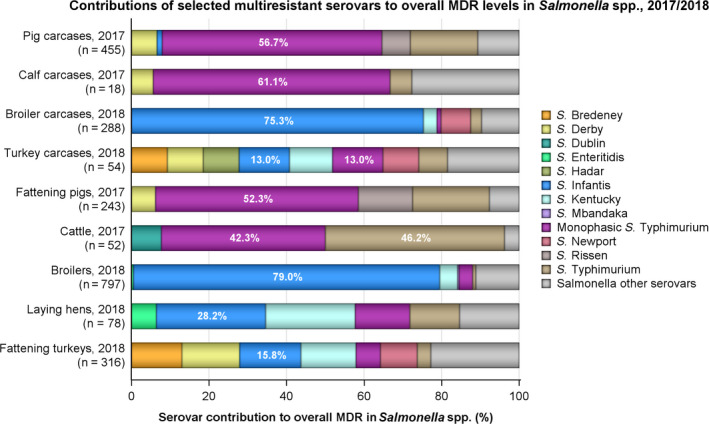
Proportions of certain serovars exhibiting multiresistance to overall MDR levels in Salmonella spp. recovered from each of the food‐producing animal populations and derived carcases, for all reporting countries in 2017/2018
- n: Total number of Salmonella isolates exhibiting MDR; serovars contributing the highest levels of MDR to overall MDR levels in Salmonella spp. are illustrated with a percentage.
Although a greater number of monophasic S. Typhimurium isolates were recovered from pigs and their derived carcases, this serovar exhibited MDR among all carcase/animal origins, with the most frequent pattern of resistance to ampicillin, sulfamethoxazole and tetracycline. This was followed in pigs by the same pattern with the addition of ciprofloxacin/nalidixic acid, and in pig carcases by the pattern ampicillin, gentamicin, sulfamethoxazole and tetracycline. Resistance to ampicillin, sulfamethoxazole and tetracycline (together with streptomycin resistance) is typical of monophasic S. Typhimurium (Hopkins et al., 2010). Notably among the MDR isolates recovered from cattle and pigs, as well as carcases of pigs and calves, sulfamethoxazole resistance was observed at levels of 95.5%, 99.2%, 99.6% and 100% from these origins, respectively. Among the multiresistant monophasic S. Typhimurium isolates recovered from poultry, all isolates displayed resistance to sulfamethoxazole. Resistance to five antimicrobial classes was observed among isolates from pigs and broilers, as well as carcases of pigs and calves; resistance to six antimicrobial classes was noted in six isolates from pig carcases, four isolates from pigs and a single isolate from broilers. Three isolates originating from pigs and a single isolate from cattle also exhibited resistance to seven antimicrobial classes. Ciprofloxacin/nalidixic acid resistance among MDR isolates from cattle, pig carcases, broilers, calf carcases, pigs and turkey carcases were observed at levels of 4.5%, 5.4%, 7.1%, 9.1%, 10.2% and 28.6%, respectively. Tigecycline resistance was reported in two MDR isolates from pigs, as well as a single MDR isolate from broilers.
In 2017, monophasic S. Typhimurium was the third most frequent serovar causing human infection in Europe, with 6,322 cases reported by EU/EEA countries. While extremely high levels of MDR (81.4%) were observed among 1,636 isolates from human cases in 2017 (i.e. those tested against the full panel of nine antimicrobial classes), combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was very low among 1,685 tested isolates from human cases (0.7%). Notably, combined ‘microbiological’ resistance to two of the highest priority CIAs (ciprofloxacin and cefotaxime) was not detected among monophasic S. Typhimurium isolates recovered from the carcase/animal origins. Considering resistance to third‐generation cephalosporins among the carcase/animal origins, only two monophasic S. Typhimurium isolates recovered from pigs displayed resistance to this antimicrobial class; an isolate from Italy was resistant to both cefotaxime and ceftazidime, while an isolate from Spain was resistant to cefotaxime only. An AmpC phenotype was detected in the isolate from Italy, while an ESBL phenotype was identified in the isolate from Spain. No monophasic S. Typhimurium isolates recovered from cattle, broilers, laying hens or turkeys, as well as carcases of pigs, calves, broilers or turkeys displayed resistance to third‐generation cephalosporins. From the monitoring of human monophasic S. Typhimurium cases reported to ECDC in 2017, 8/1,250 isolates for which data were available had an ESBL phenotype and 4/1,250 had an AmpC phenotype, with mainly different types of CTX‐M enzymes detected.
S . Typhimurium was the most dominant serovar reported in cattle, as well as the third most commonly reported serovar in pigs and pig carcases, accounting for 44.3%, 17.1% and 12.8% of Salmonella isolates recovered from these origins, respectively (see Figures C.1 and C.3). Among S. Typhimurium isolates recovered from cattle, pigs and pig carcases, MDR was also frequently observed: 30.8%, 59.3% and 64.2%, respectively. Notably, the proportion of all Salmonella isolates showing MDR in cattle was greatly influenced by the occurrence of multiresistant S. Typhimurium, which accounted for 46.2% (24/52) of the MDR Salmonella isolates recovered from this animal population (see Figure C.5).
Although a greater number of S. Typhimurium isolates were recovered from pigs, cattle, laying hens and pig carcases, this serovar exhibited MDR among all carcase/animal origins. A wide range of different MDR patterns were reported among S. Typhimurium isolates from pig carcases and pigs. The most frequent MDR core pattern among isolates from pigs and calf carcases was resistance to ampicillin, chloramphenicol, sulfamethoxazole and tetracycline; although only one S. Typhimurium isolate exhibited MDR from calf carcases. Among MDR isolates from pig carcases, two core resistance patterns predominated: ampicillin, sulfamethoxazole and tetracycline, and the same pattern with the addition of chloramphenicol. This latter core pattern (ampicillin, chloramphenicol, sulfamethoxazole and tetracycline) was also the most frequently noted among MDR isolates from broilers and turkeys; as well as among MDR isolates from cattle but with the addition of ciprofloxacin/nalidixic acid. In laying hens, the most frequent MDR core pattern was to gentamicin, sulfamethoxazole and tetracycline; while in broiler carcases, the pattern ampicillin, sulfamethoxazole and tetracycline was most commonly reported. Of only four MDR S. Typhimurium isolates recovered from turkey carcases, four different combinations were noted. Notably, all MDR S. Typhimurium isolates from pigs, cattle and turkeys, as well as carcases of pigs, calves and turkeys exhibited resistance to ampicillin (100%); while resistance to this antimicrobial was noted in most of the MDR isolates from broilers and their derived carcases. Resistance to five antimicrobial classes was observed among isolates from pigs, cattle and pig carcases, as well as a couple of isolates from turkeys and their derived carcases. Among a few isolates from cattle, broilers and pig carcases, resistance to six antimicrobial classes was noted. Furthermore, resistance to seven antimicrobial classes was observed in single isolates originating from cattle, turkeys and pig carcases, as well as two isolates originating from pigs; one isolate recovered from a pig carcase also exhibited resistance to eight antimicrobial classes. Ciprofloxacin/nalidixic acid resistance among MDR isolates from pig carcases, laying hens, pigs, turkey carcases, broilers, cattle and turkeys were observed at levels of 8.9%, 10%, 20.8%, 25%, 33.3%, 37.9% and 45.5%, respectively. Tigecycline resistance among multiresistant S. Typhimurium isolates was reported in four MDR isolates from pigs, four MDR isolates from pig carcases and a single MDR isolate from a broiler carcase.
While resistance to third‐generation cephalosporins was not detected among any S. Typhimurium isolates recovered from the carcase origins, or cattle, laying hens or broilers, this was detected in single isolates from pigs and turkeys. Italy reported resistance to this antimicrobial class in 1/5 S. Typhimurium isolates from pigs (ESBL phenotype), while Spain reported resistance to this class in 1/4 isolates from turkeys (ESBL phenotype). Additionally, where third‐generation cephalosporin resistance was reported in these two isolates, ‘microbiological’ resistance to ciprofloxacin was also observed. Considering human cases of S. Typhimurium, this serovar was identified as the second most common in 2017, with 10,675 cases reported by EU/EEA countries. While MDR among human isolates was observed at a lower level (39.7% of 1,266 isolates which were tested against the full panel of nine antimicrobial classes) to that noted among its monophasic variant (81.4%), combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime were observed at similar levels (0.6% of 2,046 tested S. Typhimurium isolates and 0.7% of 1,685 tested monophasic S. Typhimurium isolates). Additionally of 1,250 human S. Typhimurium isolates for which data were available to ECDC in 2017, 17 isolates exhibited an ESBL phenotype and 2 isolates exhibited an AmpC phenotype; different types of CTX‐M enzymes, as well as DHA, OXA‐1 and SHV‐64 were detected.
Interestingly, S. Rissen isolates recovered from pigs displayed similar levels of MDR to those of S. Typhimurium isolates (recovered from pigs and their derived carcases), where 66.7% (34/51) of S. Rissen isolates were multiresistant. While the proportion of MDR Salmonella isolates in pigs was mostly influenced by the occurrence of multiresistant S. Typhimurium and its monophasic variant (72%, 175/243), S. Rissen accounted for 14% (34/243) of the MDR Salmonella isolates recovered from this animal population (see Figure C.5).
Multiresistant S. Rissen isolates were recovered from pigs, broilers and laying hens, as well as carcases of pigs and broilers. Among pigs where 34/51 (66.7%) isolates exhibited MDR and pig carcases where 33/71 (46.5%) isolates exhibited MDR, a wide range of different resistance patterns were evident. In pigs, the most frequent pattern of resistance was to ampicillin, sulfamethoxazole, trimethoprim and tetracycline (32.4%); this core pattern was also reported in the only two MDR S. Rissen isolates recovered from laying hens (N = 12). Similarly, this combination (ampicillin, sulfamethoxazole, trimethoprim and tetracycline) with the addition of chloramphenicol was the most common resistance pattern noted among pig carcases (24.2%); a single S. Rissen isolate recovered from broiler carcases also exhibited resistance to ampicillin, chloramphenicol, sulfamethoxazole, trimethoprim and tetracycline. In broilers, where 5/30 (16.7%) S. Rissen isolates exhibited MDR, four different combinations were noted (the most common being resistance to ampicillin, cefotaxime, chloramphenicol, ciprofloxacin, gentamicin, sulfamethoxazole, trimethoprim and tetracycline). Tigecycline resistance was reported in two MDR isolates from pig carcases, as well as a single MDR isolate from pigs.
Resistance to third‐generation cephalosporins was detected in two S. Rissen isolates reported by Spain; one originating from a pig (ESBL phenotype) and the other from a pig carcase (ESBL phenotype). Additionally, two S. Rissen isolates reported from broilers by Spain displayed resistance to cefotaxime, with ESBL phenotypes. Where third‐generation cephalosporin resistance was reported in these four isolates, ‘microbiological’ resistance to ciprofloxacin was also observed.
Considering S. Infantis, this serovar was most frequently recovered from broilers and their derived carcases, accounting for 37.6% and 36.3% of Salmonella isolates recovered from these origins, respectively (see Figures C.1 and C.3). Additionally, this serovar was the second most frequently reported in laying hens and turkeys (9.6% and 8.1%, respectively), as well as the fifth most common among pig carcases (3.2%). While MDR was common among S. Infantis isolates from broilers and their derived carcases, as well as turkeys and their derived carcases (80.2%, 68%, 75.8% and 87.5%, respectively), isolates recovered from laying hens (N = 114) were less frequently MDR (19.3%). This was also apparent in S. Infantis isolates recovered from pig carcases (MDR: 19.35%), although the total number of isolates available for analysis was relatively low (N = 31). Notably, the proportion of all Salmonella isolates showing MDR in broilers and their derived carcases was greatly influenced by the occurrence of multiresistant S. Infantis, which accounted for 79% (630/797) and 75.3% (217/288) of the MDR Salmonella isolates from these origins, respectively (see Figure C.5). Similarly, this serovar contributed the highest levels of multiresistance to overall MDR among Salmonella isolates recovered from laying hens, turkeys and turkey carcases (as did monophasic S. Typhimurium in turkey carcases).
Although a wide range of different MDR patterns were reported among S. Infantis isolates from poultry, the most frequent core pattern of resistance was to ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline. This resistance pattern is typical of a major European clone of S. Infantis which is prevalent among broilers (Nógrády et al., 2012). Where MDR was detected, this resistance profile (resistance to only ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline) accounted for 46%, 50%, 60.3%, 74.2% and 100% of the multiresistant S. Infantis isolates recovered from turkeys, laying hens, broilers, broiler carcases and turkey carcases, respectively. Resistance to five antimicrobial classes was noted among isolates from all poultry origins, with the exception of turkey carcases where all isolates displayed the core pattern described above. Resistance to six antimicrobial classes was noted among isolates from broilers and turkeys, as well as a single isolate from laying hens; while four isolates from broilers also displayed resistance to seven antimicrobial classes. Additionally, tigecycline resistance was observed among some MDR isolates from poultry, with the exception once more of turkey carcases. Multiresistant S. Infantis was also reported from pig carcases (six isolates were MDR out of 31 isolates reported, 19.4%). Among the MDR isolates, all showed resistance to ampicillin, sulfamethoxazole and trimethoprim. The most common pattern of resistance (83.3%) among MDR isolates was to chloramphenicol, ampicillin, sulfamethoxazole, trimethoprim and tetracycline; all isolates exhibiting this resistance pattern were reported by Spain.
Resistance to third‐generation cephalosporins was detected in 34 S. Infantis isolates recovered from broilers, 30 originating from Italy (all displaying an ESBL phenotype, with 6/30 also exhibiting an AmpC phenotype) and 4 from Hungary (2 exhibiting an ESBL phenotype and 2 exhibiting an AmpC phenotype). Additionally, Italy reported resistance to this antimicrobial class in 7/12 S. Infantis isolates from turkeys and in 1/11 isolates from laying hens. An ESBL phenotype was identified in the isolate from laying hens and seven isolates from turkeys, as well as an AmpC phenotype in two of the seven isolates from turkeys. Where third‐generation cephalosporin resistance was reported, 32/34 isolates from broilers and all seven isolates from turkeys, as well as the single isolate from laying hens, displayed ‘microbiological’ resistance to ciprofloxacin (MIC > 0.064 mg/L). Nevertheless, when ciprofloxacin and cefotaxime resistance were interpreted using clinical breakpoints (CBPs), no isolates displayed combined ‘clinical’ resistance. While high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) was mostly observed among S. Kentucky isolates from poultry, 19.8% (50/252) of isolates displaying resistance by this definition were attributed to S. Infantis. Particular MDR patterns are associated with S. Infantis and because this serovar was prevalent in many countries, these patterns greatly influenced the overall resistance figures. Moreover, resistance to third‐generation cephalosporins, as well as high‐level resistance to ciprofloxacin, further underline the significance of this multiresistant serovar.
In contrast, S. Enteritidis isolates exhibited much lower multiresistance. This serovar was the most frequently reported in laying hens, the second most commonly reported in broilers, and the third most frequently reported in broiler carcases, accounting for 30.6%, 7.8% and 7.8% of Salmonella spp. recovered from these poultry origins, respectively (see Figures C.1 and C.3). While complete susceptibility to the harmonised panel of antimicrobials was observed at 44.9% in S. Enteritidis isolates from broiler carcases; in isolates recovered from broilers and laying hens, the majority of isolates exhibited complete susceptibility (66.7% and 83.8%, respectively). S. Enteritidis belongs to group D Salmonella (serogroup O9) which tend to show elevated colistin MICs, a phenomenon considered to reflect slightly decreased intrinsic susceptibility of wild‐type isolates belonging to Group D (Agersø et al., 2012). This is exemplified by the proportion of colistin‐resistant isolates attributed to S. Enteritidis (from laying hens, broilers and broiler carcases) in comparison to other serovars belonging to different serogroups. Notably, where multiresistant S. Enteritidis isolates were recovered from poultry (four isolates from broilers, five from laying hens and a single isolate from turkeys), colistin resistance was not a feature.
S. Kentucky was the third most commonly reported serovar in laying hens and turkeys, as well as the fourth most frequently reported in broilers, accounting for 9.5%, 6.5% and 5.2% of Salmonella spp. recovered from these poultry origins, respectively (see Figure C.3). While MDR was observed at an extremely high level in S. Kentucky isolates from turkeys (84.9%), isolates recovered from broilers and laying hens were less frequently MDR (33.9% and 15.9%, respectively). This variation in MDR was also apparent among S. Kentucky isolates recovered from carcases of turkeys and broilers (75% and 37%, respectively), although the total number of isolates available for analysis from these carcase origins was relatively low (N = 8 and N = 27, respectively).
A wide range of different MDR patterns were reported among S. Kentucky isolates from broilers, laying hens and turkeys. Among all poultry origins (including carcases of broilers and turkeys), the most frequent core pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid, gentamicin, sulfamethoxazole and tetracycline. Where MDR was detected, this resistance profile (resistance to only ampicillin, ciprofloxacin, nalidixic acid, gentamicin, sulfamethoxazole and tetracycline) accounted for 27.8%, 64.9%, 75.6%, 80% and 83.3% of the multiresistant S. Kentucky isolates recovered from laying hens, broilers, turkeys, broiler carcases and turkey carcases, respectively. Resistance to six antimicrobial classes was noted in three isolates from turkeys, as well as single isolates from broiler carcases and turkey carcases. Additionally, resistance to seven antimicrobial classes was noted in four isolates from turkeys and a single isolate from broilers. The broiler isolate which showed resistance to seven antimicrobial classes was resistant to tigecycline; four MDR isolates from turkeys also showed tigecycline resistance.
Considering isolates exhibiting high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L), S. Kentucky accounted for most of the Salmonella isolates recovered from poultry which exhibited resistance by this definition (180/252). Additionally, resistance to third‐generation cephalosporins was detected in five S. Kentucky isolates recovered from broilers by Malta (4 isolates) and the Netherlands (1 isolate), as well as an isolate recovered from laying hens by Hungary. An ESBL phenotype was reported in the five isolates from broilers, while an AmpC phenotype was reported in the single isolate from laying hens. Where third‐generation cephalosporin resistance was reported in these S. Kentucky isolates, ‘microbiological’ resistance to ciprofloxacin was also observed. The detection of third‐generation cephalosporin resistance and high‐level resistance to ciprofloxacin, underline the significance of this serovar; and notably, when cefotaxime and ciprofloxacin resistance were interpreted using CBPs, the five isolates from broilers as well as the single isolate from laying hens displayed combined ‘clinical’ resistance to these compounds.
S. Newport isolates recovered from turkeys displayed very high levels of MDR, where 58.8% (30/51) of isolates were multiresistant. Notably, the level of MDR among turkeys was greatly influenced by one MS, with Hungary (N = 39) reporting 30 multiresistant isolates. While a relatively low number of S. Newport isolates were available for analysis from broiler and turkey carcases (N = 26 and N = 27, respectively), a greater proportion of isolates from broiler carcases were multiresistant in comparison to those from turkey carcases (84.6% and 18.5%, respectively). Once more however, the level of MDR among broiler carcases was greatly influenced by one MS, with Poland (N = 22) reporting 22 multiresistant isolates.
Among MDR S. Newport isolates recovered from turkeys and their derived carcases, the most frequent pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid and tetracycline; followed by the same pattern but without nalidixic acid resistance. In broiler carcases, the combination ampicillin, ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline predominated. This pattern was also the second most frequently reported in broilers, although the combination chloramphenicol, ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline was most frequently noted.
Interestingly, MDR was observed at an extremely high level among S. Bredeney isolates from turkeys (82%); while a low level was noted among S. Bredeney isolates from turkey carcases (4.7%). Notably, among isolates reported from turkeys (N = 50), the level of MDR was greatly influenced by one MS, with Hungary (N = 31) reporting 31 multiresistant isolates.
Among MDR S. Bredeney isolates recovered from turkeys, the most frequent pattern of resistance was to ampicillin, ciprofloxacin, nalidixic acid, tigecycline and tetracycline (34.1%); followed by the same pattern but with the addition of trimethoprim (24.4%). This second core pattern was also the most commonly noted among turkey carcases (40%). While a wide range of different resistance patterns were noted among S. Bredeney isolates from turkeys, tigecycline resistance among MDR isolates from this animal origin was observed at 68.3% (all MDR isolates displaying tigecycline‐resistance originated from Hungary).
Spain detected third‐generation cephalosporin resistance in four S. Bredeney isolates from turkeys (all four isolates displayed an ESBL phenotype), as well as a single isolate from a pig carcase (displaying an AmpC phenotype). Where third‐generation cephalosporin resistance was reported in these five S. Bredeney isolates, ‘microbiological’ resistance to ciprofloxacin was also observed. Additionally, Lithuania and Portugal reported third‐generation cephalosporin resistance in single S. Bredeney isolates from pig carcases (both identified as presumptive AmpC producers); these, however did not exhibit combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime.
Multiresistant S. Bareilly was recovered from turkeys, where 6/6 isolates reported by Italy exhibited MDR. All six isolates showed resistance to the same core pattern (ampicillin, cefotaxime, ceftazidime, ciprofloxacin, nalidixic acid, sulfamethoxazole, trimethoprim and tetracycline) and all were identified as presumptive ESBL producers. Although combined ‘microbiological’ resistance to cefotaxime and ciprofloxacin was reported in these isolates, when MICs to these antimicrobials were interpreted using CBPs, combined ‘clinical’ resistance was not detected.
Multiresistant serovars
The contributions of particular multiresistant serovars to overall MDR levels in Salmonella spp. from each of the animal/carcase categories are illustrated in Figure C.5.
Appendix D – Comparison of human Salmonella data by serovar to that in food‐producing animals
1.
In 2017/2018, the quantitative human data were interpreted using EUCAST ECOFF values (categorised into wild‐type and non‐wild type), when available, in the same way as for the animal and food data, following Decision 2013/652/EU. Where ECOFFs do not exist, EUCAST or Clinical and Laboratory Standards Institute (CLSI) CBPs were applied. Notably, for sulfamethoxazole/sulfonamides, there is no EUCAST interpretative criterion for Salmonella and therefore a threshold of > 256 mg/L was applied to both the human and animal data. For qualitative data interpreted with clinical breakpoints (S = susceptible, I = susceptible with increased exposure* and R = resistant), I+R results were combined into one category. When aligning susceptible isolates with wild‐type isolates and I+R isolates with non‐wild‐type isolates, there is generally close concordance across categories (Figure D.1). An exception is meropenem where the EUCAST CBP is substantially higher (+4 dilutions) than the ECOFF.
Figure D.1.
Comparison of CBPs and ECOFFs used to interpret MIC data reported for Salmonella spp. from humans, animals or food
- *: EUCAST has changed the definitions of SIR from 2019 (EUCAST, 2019b ‐ http://www.eucast.org/newsiandr/). For I, the new definition ‘susceptible, increased exposure’ is used when there is a high likelihood of therapeutic success because exposure to the agent is increased by adjusting the dosing regimen or by its concentration at the site of infection.
It is of note that the countries reporting data on particular Salmonella serovars from human cases are not always the same as those reporting corresponding serovar data within the animal categories. Additionally, the number of isolates reported from human cases and from the animal origins varied, both at the MS and MS‐group level. These factors may introduce a source of variation to results when comparing overall percentage resistance to particular antimicrobials and MDR levels among human and animal isolates.
Comparison of 2017 human data to that in pig carcases, calf carcases, pigs and cattle
S. Typhimurium was the second most common Salmonella serovar identified in human cases in 2017, with 10,675 cases reported in the EU/EEA. Considering all reporting MSs, the highest levels of resistance in S. Typhimurium from humans were observed for ampicillin (53.3%), sulfonamides (48.1%) and tetracyclines (44.5%); as was the case for isolates from pigs, cattle and carcases of pigs and calves. Figure D.2 presents the resistance levels to these compounds considering all reporting MSs. Notably for human isolates, four MSs assessed tetracycline resistance using the CLSI CBP which is one dilution below the EUCAST ECOFF value.
Figure D.2.
Occurrence of resistance to selected antimicrobials in S. Typhimurium from humans, carcases of pigs and calves, fattening pigs and cattle, reported by MSs in 2017
Considering all reporting countries (including one non‐MS in cattle), MDR levels in S. Typhimurium were reported at 39.7%, 64.2%, 25%, 59.3% and 30.8% in isolates from humans (14 MSs), pig carcases (17 MSs), calf carcases (2 MSs), pigs (7 MSs) and cattle (7 MSs and 1 non‐MS), respectively. While 1,266 isolates were included in the MDR analysis from humans (i.e. those tested against the full panel of nine antimicrobial classes), a much lower number of isolates were available from animals and their derived carcases. Assessment of human and animal S. Typhimurium AMR data at the country level was not performed, as where comparable data were available, few isolates were reported from animals in comparison to humans by given MSs; small sample sizes are subject to high statistical variation. Furthermore, in isolates from human cases, some MSs interpreted antimicrobial susceptibility using clinical breakpoints (i.e. tetracycline).
Monophasic S . Typhimurium was the third most common serovar reported from human cases in 2017, with 6,322 registered cases in the EU/EEA. Considering all reporting MSs, the highest levels of resistance in monophasic S. Typhimurium from humans were observed for ampicillin (86.8%), sulfonamides (86.7%) and tetracyclines (87.9%); as was also the case for isolates from pigs, cattle and carcases of pigs and calves. Notably, this resistance pattern (together with resistance to streptomycin) is typical of monophasic S. Typhimurium (Hopkins et al., 2010). Figure D.3 presents resistance levels to these compounds considering all reporting MSs.
Figure D.3.
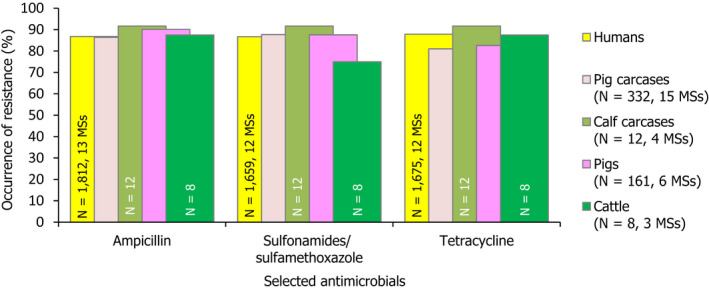
Occurrence of resistance to selected antimicrobials in monophasic S. Typhimurium from humans, carcases of pigs and calves, fattening pigs and cattle, reported by MSs in 2017
Considering all reporting countries (including one non‐MS in pig carcases and cattle), MDR levels in monophasic S. Typhimurium were reported at 81.4%, 77.2%, 91.7%, 78.9% and 84.6% in isolates from humans (12 MSs), pig carcases (15 MSs and 1 non‐MS), calf carcases (4 MSs), pigs (6 MSs) and cattle (3 MSs and 1 non‐MS), respectively. In total, 1,636 isolates were included in the MDR analysis from humans (i.e. those tested against the full panel of nine antimicrobial classes), while a much lower number of isolates were available from animals and their derived carcases, particularly in calf carcases and cattle (N = 12 and N = 26, respectively). Assessment of human and animal monophasic S. Typhimurium AMR data at the country level was not performed, as where comparable data were available, a much lower number of isolates were reported from animals.
S . Derby was the seventh most common serovar reported from human cases in 2017, with 612 cases registered by EU/EEA countries. While MDR was not as frequently observed among human/animal S. Derby isolates in comparison to S. Typhimurium and its monophasic variant, resistance to sulfonamides and tetracycline was relatively common in S. Derby isolates from human cases (30% and 26.2%, respectively). This was also observed among S. Derby isolates from the animal/carcase origins. Figure D.4 presents resistance levels to these compounds considering all reporting MSs. For human isolates, two MSs assessed tetracycline resistance using the CLSI CBP. Additionally for trimethoprim, the EUCAST ECOFF of > 2 mg/L was applied to the animal/carcase data; while in humans, at least one MSs provided interpreted categorical AST (qualitative) data using the EUCAST CBP of > 4 mg/L. Assessment of human and animal S. Derby AMR data at the country level was not performed due to the low number of isolates reported by MSs from human cases and within the animal categories.
Figure D.4.
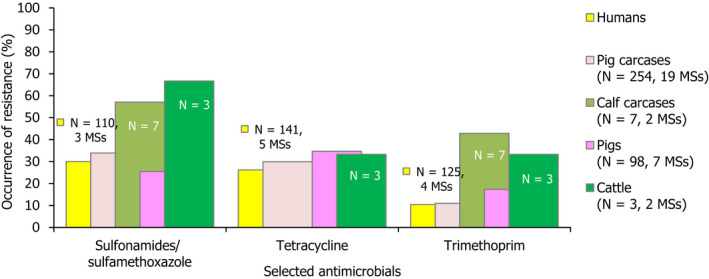
Occurrence of resistance to selected antimicrobials in S. Derby from humans, carcases of pigs and calves, fattening pigs and cattle, reported by MSs in 2017
Comparison of 2018 human data to that in poultry and their derived carcases
S. Infantis was the fourth most common serovar identified in human cases in 2018, with 1,868 cases reported in the EU/EEA. Considering all reporting MSs, the highest levels of resistance in S. Infantis from humans were noted to ciprofloxacin/pefloxacin (29.6%), nalidixic acid (36.4%), sulfonamides (43.3%) and tetracyclines (36.5%), although levels varied markedly between reporting countries. At the reporting MS‐group level for S. Infantis from poultry, generally very high or extremely high resistance to ciprofloxacin, nalidixic acid, sulfamethoxazole and tetracycline was reported, with the exception of laying hens where much lower resistance levels to these antimicrobials were noted. Figure D.5 presents the resistance levels to these four antimicrobials considering all reporting MSs. Notably for human S. Infantis isolates, Germany, Lithuania, Slovakia and the United Kingdom provided interpreted categorical AST data for tetracycline.
Figure D.5.
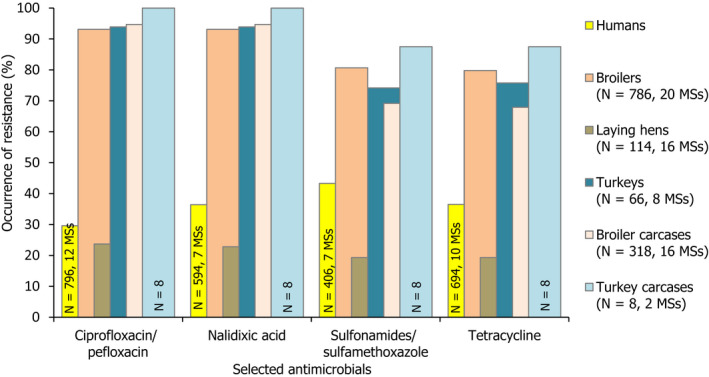
Occurrence of resistance to selected antimicrobials in S. Infantis from humans, poultry and poultry carcases, reported by MSs in 2018
With the exception of laying hens, MDR among S. Infantis was reported at higher levels in isolates from poultry compared to those from humans considering all reporting countries. In human isolates, overall MDR was observed at 41.8% (12 MSs); while 68%, 75.8%, 80.2% and 87.5% of isolates from broiler carcases (16 MSs and 1 non‐MS), turkeys (8 MSs), broilers (20 MSs) and turkey carcases (2 MSs) were MDR, respectively. At the reporting MS‐group level for S. Infantis isolates recovered from laying hens, MDR was noted at a much lower level of 19.3% (16 MSs). Notably, only eight S. Infantis isolates were reported by two MSs in turkey carcases and only 66 isolates were reported by eight MSs in turkeys; comparative assessment of AMR data to that in humans at the country level was therefore not considered for these categories. Comparative data for nalidixic acid resistance among isolates from both broilers and humans was available for six MSs. Considering only countries reporting a similar number of S. Infantis isolates from both broilers and humans and where more than ten isolates were reported (3 MSs), a higher percentage occurrence of nalidixic acid resistance was noted in isolates originating from broilers in comparison to those from humans by Austria, Italy and Spain. Similarly, Austria, Belgium, Italy, Poland, Slovakia and Spain reported a higher percentage occurrence of ciprofloxacin/pefloxacin resistance in isolates from broilers compared to those from human cases. Notably, these 6 MSs were the only countries where a similar number, as well as ten or more S. Infantis isolates were reported from both broilers and humans for comparative assessment of ciprofloxacin/pefloxacin resistance. Concerning sulfamethoxazole resistance, Austria, Belgium, Italy and Spain were the only countries to report on ten or more S. Infantis isolates from both broilers and humans, with a higher percentage occurrence of resistance noted for all four countries in isolates from broilers compared to isolates from humans. For tetracycline, a comparative assessment of resistance (based on the number of isolates available for both broilers and humans) could be made for five MSs, with Austria, Belgium, Italy and Spain again reporting a higher percentage occurrence of resistance in isolates from broilers compared to isolates from humans. Slovakia reported a lower percentage occurrence of tetracycline resistance in isolates from broilers (N = 51, 54.9%) compared to those from humans (N = 19, 78.9%), however, this MS assessed tetracycline resistance among human S. Infantis isolates using the CLSI CBP (which is one dilution below the EUCAST ECOFF). When applying the same considerations to AMR data for S. Infantis from both broiler carcases and humans (i.e. where a similar number and ten or more isolates were reported), apparent differences in the levels of nalidixic acid resistance were noted by Austria and Spain; with a higher percentage occurrence of resistance in isolates from broiler carcases compared to those from humans. This was also the case for ciprofloxacin/pefloxacin resistance, where Austria, Hungary, Poland and Spain reported a higher percentage occurrence of resistance in isolates from broiler carcases compared to isolates from humans. A higher percentage occurrence of resistance to sulfamethoxazole was also noted in isolates from broiler carcases by Austria and Spain, as well as a higher level of tetracycline resistance in isolates from broiler carcases reported by Austria and Spain. Although comparable AMR data for S. Infantis from both laying hens and humans was available, a much lower number of isolates were reported from laying hens, with only 5 MSs reporting data on ten or more isolates from this poultry origin. Belgium, Italy and Spain reported a lower percentage occurrence of resistance to nalidixic acid, ciprofloxacin, sulfamethoxazole and tetracycline among isolates from laying hens in comparison to isolates from humans. Additionally, Poland reported a lower percentage occurrence of resistance to ciprofloxacin among isolates from laying hens in comparison to those from humans.
S. Enteritidis was the most common Salmonella serovar identified in human cases in 2018, with 40,463 cases reported in the EU/EEA. While MDR was uncommon among S. Enteritidis isolates (from both humans and poultry), the highest levels of resistance in S. Enteritidis from humans were noted to ciprofloxacin/pefloxacin (13.1%), nalidixic acid (16.3%) and colistin (19.2%). Colistin resistance among S. Enteritidis is not uncommon, since this serovar belongs to group D salmonellas (serogroup O9) which tend to show decreased intrinsic susceptibility to colistin (Agersø et al., 2012). Figure D.6 presents the resistance levels to these antimicrobials considering all reporting MSs.
Figure D.6.
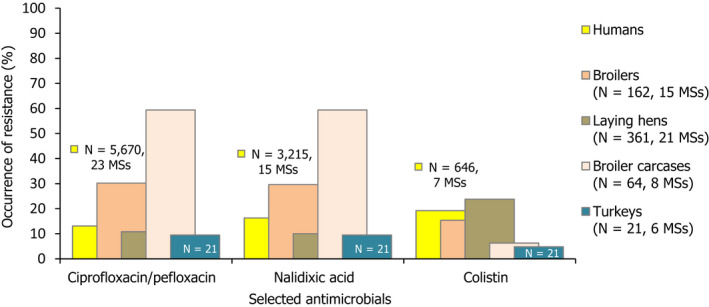
Occurrence of resistance to selected antimicrobials in S. Enteritidis from humans, poultry and broiler carcases, reported by MSs in 2018
- Note: S. Enteritidis was not reported from turkey carcases.
Only 21 S. Enteritidis isolates were reported by six MSs in turkeys; comparative assessment of AMR data to that in humans at the country level was therefore not considered for these categories. Considering data available for broiler carcases, the Czech Republic and Poland were the only countries to report on ten or more S. Enteritidis isolates from this poultry origin. While corresponding human AMR data were not available for the Czech Republic, AMR data from human isolates were reported by Poland. A much higher percentage occurrence of nalidixic acid and ciprofloxacin/pefloxacin resistance was reported among isolates from broiler carcases in comparison to those from humans, however for ciprofloxacin/pefloxacin, a considerably lower number of isolates were available from broiler carcases in comparison to those from humans (N = 39 and N = 345, respectively). Similarly, the Czech Republic, Poland and France were the only countries to report on ten or more S. Enteritidis isolates from broilers. While nalidixic acid and ciprofloxacin/pefloxacin resistance was not detected among broiler isolates from France and moderate/high levels of resistance to these antimicrobials were reported among human isolates (18.6% and 20.9%, respectively), a much lower number of isolates were available from broilers in comparison to humans (N = 10 and N = 86, respectively). Once more, a much higher percentage occurrence of nalidixic acid and ciprofloxacin/pefloxacin resistance was reported among broiler isolates from Poland in comparison to those from humans, however for ciprofloxacin/pefloxacin, a considerably lower number of isolates were available from broilers in comparison to those from humans (N = 39 and N = 345, respectively). While eight MSs reported data on ten or more S. Enteritidis isolates from laying hens, generally a much lower number of isolates were reported by these countries for laying hens in comparison to isolates from human cases; comparative assessment of AMR data to that in humans was therefore not considered. In isolates from human cases, resistance to ciprofloxacin/pefloxacin was reported at 13.1% (N = 5,670) and to nalidixic acid at 16.3% (N = 3,215); while in laying hens (N = 361), ciprofloxacin and nalidixic acid resistance were reported at levels of 10.8% and 10%, respectively.
Considering S. Kentucky, the seventh most commonly reported serovar from human cases in 2018, with 663 cases reported in the EU/EEA, the highest levels of resistance in human isolates were noted to ampicillin (72.7%), ciprofloxacin/pefloxacin (85.7%), gentamicin (51.1%), nalidixic acid (87.3%), sulfonamides (71.1%) and tetracyclines (76.6%). Figure D.7 presents the resistance levels to these antimicrobials in human and poultry isolates considering all reporting MSs. For gentamicin, the clinical breakpoints used for the categorical data from Germany (DIN, Deutsches Institut für Normung) was one dilution higher than the ECOFF while for tetracycline, both Germany and the UK provided interpreted data using CBPs which was one dilution lower than the ECOFF.
Figure D.7.
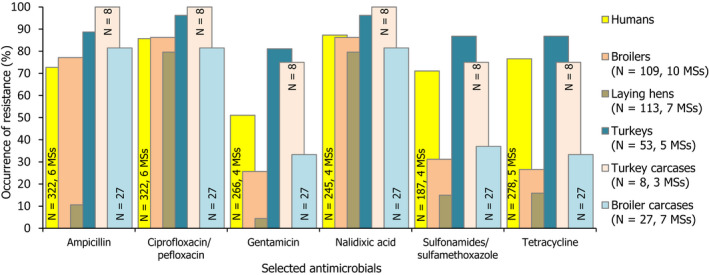
Occurrence of resistance to selected antimicrobials in S. Kentucky from humans, poultry and poultry carcases, reported by MSs in 2018
Considering all reporting countries, extremely high levels of MDR were reported among S. Kentucky isolates from humans, turkeys and turkey carcases (77.4%, 84.9% and 75%, respectively), although the number of isolates recovered from turkeys and their derived carcases was relatively low (N = 53 and N = 8, respectively). While an overall high level of MDR was noted among isolates from broilers and their derived carcases (33.9% and 37%, respectively), an overall moderate level was reported among isolates from laying hens (15.9%). Only 27 S. Kentucky isolates were reported by seven MSs in broiler carcases and only 8 isolates were reported by three MSs in turkey carcases; comparative assessment of AMR data to that in humans at the country level was therefore not considered for these categories. Although comparable AMR data for S. Kentucky from both laying hens and humans was available, only Malta reported data for ten or more isolates from both laying hens and humans (N = 10 and N = 13, respectively). A much higher percentage occurrence of resistance to ampicillin and ciprofloxacin/pefloxacin was noted in human isolates (76.9% and 69.2%, respectively) in comparison to those from laying hens (30%) by Malta, however, results may be subject to imprecision due to the low number of isolates. Overall, fluoroquinolone resistance was noted at a similar level in isolates from humans and laying hens (85.7% and 79.6%?%, respectively); while resistance to ampicillin was noted at a much higher level in isolates from humans compared to those from laying hens (72.7% and 10.6%, respectively). Similarly, Malta was the only country to report AMR data for ten or more isolates from both broilers and humans (N = 23 and N = 13, respectively). A higher percentage occurrence of resistance to ampicillin was noted in isolates from humans (76.9%) compared to those from broilers (60.9%); while a lower percentage occurrence of resistance to ciprofloxacin/pefloxacin was reported in isolates from humans (69.2%) compared to those from broilers (82.6%). No comparable AMR data for S. Kentucky from both turkeys and humans was available.
Within a given MS, any attempt to relate the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated, as much of the food consumed in a MS may have originated from other MSs or non‐member countries. Salmonella infections can also be associated with foreign travel, other types of animal contact (such as pet reptiles) or the environment. Additionally, some human infections may result from human to human transmission and, although known travel‐associated isolates from human cases were excluded from the analysis, a large proportion of cases lacked information on travel status. Such circumstances may influence the human AMR data at the reporting MS level. Furthermore, the local medical and diagnostic practices and policies for referral to clinical laboratories may vary between countries, which may result in reporting of various clinical or regional subsets of isolates from humans.
Appendix E – Additional information and supporting data
List of Annexes
The annexes are available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3628719
The annexes contain the following information:
Annex A – Materials and methods
1.
The annex contains the materials and methods used for producing the EU Summary Report on antimicrobial resistance in zoonotic bacteria from humans, animals and food for the period 2017/2018.
Annex B – Data reported on antimicrobial resistance in Salmonella spp.
1.
The annex contains tables on antimicrobial resistance data:
-
–
Antimicrobial resistance in Salmonella spp. from humans, 2018;
-
–
Occurrence of resistance to selected antimicrobials in Salmonella spp. from animal carcases, 2017 and 2018;
-
–
Occurrence of resistance to selected antimicrobials in Salmonella spp. from animals, 2017 and 2018;
-
–
Occurrence of resistance (%) to selected antimicrobials in specific Salmonella serovars.
Annex C – Data reported on antimicrobial resistance in Campylobacter spp.
1.
The annex contains tables and figures showing antimicrobial resistance data:
-
–
Antimicrobial resistance in Campylobacter spp. from humans, 2018 and trends for 2013‐2017 period;
-
–
Data reported on antimicrobial resistance and occurrence of resistance to selected antimicrobials in Campylobacter spp. from food‐producing animals and derived meat, for 2017 and 2018.
Annex D – Data reported on AMR in indicator Escherichia coli from food‐producing animals and derived meat
1.
The annex contains tables on data reported on AMR in indicator Escherichia coli from food‐producing animals and derived meat.
Annex E – Data on presumptive ESBL‐, AmpC‐ and/or carbapenemase‐producing microorganisms and their resistance occurrence (routine and specific monitorings)
1.
The annex contains the tables (Tables 1–30) with the data reported on presumptive ESBL‐, AmpC‐ and/or carbapenemase‐producing microorganisms for poultry (2018) and pigs and cattle (2018) and meat thereof, and their resistance occurrence (routine and specific monitorings):
-
–
ESBL‐, AmpC‐, carbapenemase‐producers prevalence and occurrence tables – poultry 2018;
-
–
ESBL‐, AmpC‐producers prevalence and occurrence tables – pigs and cattle and meat thereof, 2017;
-
–
Specific carbapenemase‐producing E. coli monitoring 2017‐2018;
-
–
Occurrence of antimicrobial resistance in poultry isolates collected in 2018.
Annex F – Data reported on antimicrobial resistance in MRSA from food‐producing animals and derived meat
1.
The annex contains tables on data reported on the prevalence, genetic diversity and antimicrobial resistance of MRSA from food‐producing animals and derived meat.
Supporting data
All tables produced for the European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2018 are available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.3628719.
The aggregated dataset submitted on the negative results for extended‐spectrum β‐lactamase (ESBL) is also available on the Knowledge Junction at: https://doi.org/10.5281/zenodo.3635794
Country Datasets
All country datasets containing the tables on the occurrence of antimicrobial resistance per each country are available on the EFSA Knowledge Junction community on Zenodo – please see below the list and corresponding link to the datasets.
The countries that submitted datasets on the 2018 monitoring data year are: the 28 EU Member States, the 3 non‐EU Member States, and Republic of North Macedonia as pre‐accession country.
Suggested citation: EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2020. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA Journal 2020;18(3):6007, 166 pp. 10.2903/j.efsa.2020.6007
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00090
Acknowledgements: EFSA and ECDC wish to thank the members of the Scientific Network for Zoonoses Monitoring Data (EFSA) and the Food‐ and Waterborne Diseases and Zoonoses Network (ECDC) who provided the data and reviewed the report and the members of the Scientific Network for Zoonoses Monitoring Data, for their endorsement of this scientific output. Also, the contribution of EFSA staff members: Pierre‐Alexandre Beloeil, Beatriz Guerra, Anca‐Violeta Stoicescu, Kenneth Mulligan, Krisztina Nagy, Gina Cioacata, and Karoline Noerstrud, the contributions of ECDC staff member: Therese Westrell, and the contributions of EFSA's contractors: Björn Bengtsson, Isabelle Kempf, Oskar Nilsson and Catherine Tallentire for the support provided to this scientific output. Also, the support from the EURL‐AR, specifically Inge Marianne Hansen, Jette Sejer Kjeldgaard, Anne Mette Seyfarth, Pimlapas Leekitcharoenphon, Valeria Bortolaia, and Rene S. Hendriksen for the confirmatory testing are gratefully acknowledged.
Approved: 31 January 2020
Notes
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the EFSA and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L325/31, 12.12.2003, p. 31–40.
Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, p. 26–39.
Commission Decision 2012/506/EU amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. OJ L 262, 27.9.2012, p. 1–57.
Commission Implementing Decision 2018/945/EU of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. OJ L 170, 6.7.2018, p. 1–74.
Decision No 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No. 2119/98/EC. OJ L 293, 5.11.2013, p. 1–15.
Links to additional information on Materials and methods (Annex A) and supporting data for this chapter (Annex B) are provided in Appendix E.
The epidemiological cut‐off (ECOFF) values separate the naive, susceptible wild‐type bacterial populations from isolates that have developed reduced susceptibility to a given antimicrobial agent (Kahlmeter et al., 2003). The ECOFFs may differ from breakpoints used for clinical purposes, which are set out against a background of clinically relevant data, including therapeutic indication, clinical response data, dosing schedules, pharmacokinetics and pharmacodynamics. The use of harmonised methods and ECOFFs ensures the comparability of data over time at the country level and also facilitates the comparison of resistance between MSs.
Links to additional information on Materials and methods (Annex A) and supporting data for this chapter (Annex B) are provided in Appendix E.
Additional information on the presumptive ESBL‐, AmpC‐ and/or carbapenemase‐producing Salmonella spp. from different matrices for the different MSs and their beta‐lactams resistance can be found in Section 5 and Annex E (Tables 1, 10 and 22).
Links to additional information on Materials and methods (Annex A) and supporting data for this chapter (Annex C) are provided in Appendix E.
As outlined in the Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, p. 26–39.
Links to additional information on Materials and methods (Annex A) and supporting data for this chapter (Annex D) are provided in Appendix E.
Additional information on the presumptive ESBL‐, AmpC‐, and/or carbapenemase‐producing E. coli from different matrices for the different MSs and their beta‐lactams resistance can be found in Section 5 and Annex E (Tables 2, 3, 11, 12, 23 and 24).
Contributor Information
European Food Safety Authority, Email: zoonoses@efsa.europa.eu.
European Centre for Disease Prevention and Control, Email: FWD@ecdc.europa.eu.
References
- Agersø Y, Torpdahl M, Zachariasen C, Seyfarth A, Hammerum AM and Nielsen EM, 2012. Tentative colistin epidemiological cut‐off value for Salmonella spp. Foodborne Pathogens and Disease, 9, 367–369. 10.1089/fpd.2011.1015 [DOI] [PubMed] [Google Scholar]
- Alba P, Feltrin F, Cordaro G, Porrero MC, Kraushaar B, Argudín MA, Nykäsenoja S, Monaco M, Stegger M, Aarestrup FM, Butaye P, Franco A and Battisti A, 2015. Livestock‐associated methicillin resistant and methicillin susceptible Staphylococcus aureus sequence type (CC)1 in European farmed animals: high genetic relatedness of isolates from Italian cattle herds and humans. PLoS ONE, 10, e0137143 10.1371/journal.pone.0137143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angen Ø, Stegger M, Larsen J, Lilje B, Kaya H, Pedersen KS, Jakobsen A, Petersen A and Larsen AR, 2017. Report of mecC‐carrying MRSA in domestic swine. Journal of Antimicrobial Chemotherapy, 72, 60–63. 10.1093/jac/dkw389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv G, Tsyba K, Steck N, Salmon‐Divon M, Cornelius A, Rahav G, Grassl GA and Gal‐Mor O, 2014. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environmental Microbiology, 16, 977–994. 10.1111/1462-2920.12351 [DOI] [PubMed] [Google Scholar]
- Bai L, Du P, Du Y, Sun H, Zhang P, Wan Y, Lin Q, Fanning S, Cui S and Wu Y, 2019. Detection of plasmid‐mediated tigecycline‐resistant gene tet(X4) in Escherichia coli from pork, Sichuan and Shandong Provinces, China, February 2019. Euro Surveillance, 24, 4 10.2807/1560-7917.es.2019.24.25.1900340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangerter PD, Sidler X, Perreten V and Overesch G, 2016. Longitudinal study on the colonisation and transmission of methicillin‐resistant Staphylococcus aureus in pig farms. Veterinary Microbiology, 183, 125–134. 10.1016/j.vetmic.2015.12.007 [DOI] [PubMed] [Google Scholar]
- van Belkum A, 1999. The role of short sequence repeats in epidemiologic typing. Current Opinion in Microbiology, 2, 306–311. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Persson L, Ekström K, Unnerstad HE, Uhlhorn H and Börjesson S, 2017. High occurrence of mecC‐MRSA in wild hedgehogs (Erinaceus europaeus) in Sweden. Veterinary Microbiology, 207, 103–107. [DOI] [PubMed] [Google Scholar]
- Borgia S, Lastovetska O, Richardson D, Eshaghi A, Xiong J, Chung C, Baqi M, McGeer A, Ricci G, Sawicki R, Pantelidis R, Low DE, Patel SN and Melano RG. 2012. Outbreak of carbapenem‐resistant Enterobacteriaceae containing blaNDM‐1, Ontario, Canada. Clinical Infectious Disease, 55, e109–e117. [DOI] [PubMed] [Google Scholar]
- Bosch T, Lutgens SPM, Hermans MHA, Wever PC, Schneeberger PM, Renders NHM, et al., 2017. Outbreak of NDM‐1‐Producing Klebsiella pneumoniae in a Dutch Hospital, with interspecies transfer of the resistance plasmid and unexpected occurrence in unrelated health care centers. Journal of Clinical Microbiology, 55, 2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer MSM, Rapallini M, Geurts Y, Harders F, Bossers A, Mevius DJ, Wit B and Veldman KT, 2018. Enterobacter cloacae complex isolated from shrimps from Vietnam encoding blaIMI‐1, resistant to carbapenems but not cephalosporins. Antimicrobial Agents and Chemotherapy, 62. 10.1128/AAC.00398-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Chen JC, Francois Watkins LK, Campbell D, Folster JP, Tate H, Wasilenko J, Van Tubbergen C and Friedman CR, 2018. CTX‐M‐65 extended‐spectrum β‐lactamase–producing Salmonella enterica serotype Infantis, United States. Emerging Infectious Diseases, 24, 2284–2291. 10.3201/eid2412.180500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J, Cristino L, Peixe L and Antunes P, 2016. MCR‐1 in multidrug‐resistant and copper‐tolerant clinically relevant Salmonella 1,4[5],12:i:‐ and S. Rissen clones in Portugal, 2011 to 2015. Euro Surveillance, 21, 5 10.2807/1560-7917.ES.2016.21.26.30270 [DOI] [PubMed] [Google Scholar]
- Carfora V, Giacinti G, Sagrafoli D, Marri N, Giangolini G, Alba P, Feltrin F, Sorbara L, Amoruso R and Caprioli A, Amatiste S, Battisti A, 2016. Methicillin‐resistant and methicillin‐susceptible Staphylococcus aureus in dairy sheep and in‐contact humans: an intra‐farm study.. Journal of Dairy Science, 99, 4251–4258. 10.3168/jds.2016-10912 [DOI] [PubMed] [Google Scholar]
- Carnevali C, Morganti M, Scaltriti E, Bolzoni L, Pongolini S and Casadei G, 2016. Occurrence of mcr‐1 in colistin‐resistant Salmonella enterica isolates recovered from humans and animals in Italy, 2012 to 2015. Antimicrobial Agents and Chemotherapy, 60, 7532–7534. 10.1128/AAC.01803-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco LM, Abatih E, Aarestrup FM and Guardabassi L, 2008. Selection and persistence of CTX‐M‐producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrobial Agents and Chemotherapy, 52, 3612–3616. 10.1128/aac.00354-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick SG, Prasad A, Smith WL, Mordechai E, Adelson ME and Gygax SE, 2013. Detection of epidemic USA300 community‐associated methicillin‐resistant Staphylococcus aureus strains by use of a single allele‐specific PCR assay targeting a novel polymorphism of Staphylococcus aureus pbp3. Journal of Clinical Microbiology, 51, 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong Y, Ito Y and Kamimura T, 2011. Genetic evolution and clinical impact in extended‐spectrum beta‐lactamase producing Escherichia coli and Klebsiella pneumoniae. Infection Genetics and Evolution, 11, 1499–1504. 10.1016/j.meegid.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Crombé F, Argudín MA, Vanderhaeghen W, Hermans K, Haesebrouck F and Butaye P, 2013. Transmission dynamics of methicillin‐resistant Staphylococcus aureus in pigs. Frontiers in Microbiology, 4 10.3389/fmicb.2013.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump JA, Sjölund‐Karlsson M, Gordon MA and Parry CM, 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clinical Microbiology Reviews, 28, 901–937. 10.1128/CMR.00002-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Abdelbary M, Layer F, Werner G and Witte W, 2015a. Prevalence of the immune evasion gene cluster in Staphylococcus aureus CC398. Veterinary Microbiology, 177, 219–223. 10.1016/j.vetmic.2015.02.031 [DOI] [PubMed] [Google Scholar]
- Cuny C, Wieler LH and Witte W, 2015b. Livestock‐associated MRSA: the impact on humans. Antibiotics (Basel), 4, 521–543. 10.3390/antibiotics4040521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANMAP , 2016. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600‐2032.
- DANMAP , 2018. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600‐2032.
- ECDC (European Centre for Disease Prevention and Control), 2014. EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates. Stockholm: ECDC; 2014.
- ECDC (European Centre for Disease Prevention and Control), 2016. EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates – June 2016. Stockholm: ECDC; 2016. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/antimicrobial-resistance-Salmonella-Campylobacter-harmonised-monitoring.pdf
- ECDC (European Centre for Disease Prevention and Control), 2019. Surveillance of antimicrobial resistance in Europe 2018. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS‐Net). Stockholm: ECDC; 2019.
- ECDC, EFSA and EMA (European Centre for Disease Prevention and Control, European Food Safety Authority and European Medicines Agency), 2015. ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals. Stockholm/Parma/London: ECDC/EFSA/EMA, 2015. EFSA Journal 2015;13(1):4006, 114 pp. 10.2903/j.efsa.2015.4006 [DOI] [Google Scholar]
- ECDC, EFSA and EMA (European Centre for Disease Prevention and Control, European Food Safety Authority and European Medicines Agency), 2017. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals ‐ Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA Journal 2017;15(7):4872, 135 pp. 10.2903/j.efsa.2017.4872 Available online: https://www.ema.europa.eu/en/documents/report/ecdc/efsa/ema-first-joint-report-integrated-analysis-consumption-antimicrobial-agents-occurrence-antimicrobial_en.pdf and https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2017.4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA Journal 2008;6(4):141, 44 pp. 10.2903/j.efsa.2008.141r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009a. Joint scientific report of ECDC, EFSA and EMEA on meticillin resistant Staphylococcus aureus (MRSA) in livestock, companion animals and foods. EFSA‐Q‐2009‐00612 (EFSA Scientific Report (2009) 301, 1–10) and EMEA/CVMP/SAGAM/62464/2009. EFSA Journal 2009;7(6):301r, 10 pp. 10.2903/j.efsa.2009.301r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Scientific opinion of the Panel on Biological Hazards on a request from the European Commission on Assessment of the public health significance of meticillin resistant Staphylococcus aureus (MRSA) in animals and foods. EFSA Journal 2009;7(3):993, 73 pp. 10.2903/j.efsa.2009.993 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009c. Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part A: MRSA prevalence estimates; on request from the European Commission. EFSA Journal 2009;7(11):1376, 82 pp. 10.2903/j.efsa.2009.1376 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Technical specifications for the harmonised monitoring and reporting of antimicrobial resistance in methicillin‐resistant Staphylococcus aureus in food‐producing animals and foods. EFSA Journal2012; 10(10): 2897, 56 pp. 10.2903/j.efsa.2012.2897 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the public health risks of bacterial strains producing extended‐spectrum b‐lactamases and/or AmpC b‐lactamases in food and food producing animals. EFSA Journal 2011;9(8):2322, 95 pp. 10.2903/j.efsa.2011.2322 Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2011.2322/full [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA Journal 2017;15(2):4694, 212 pp. 10.2903/j.efsa.2017.4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA Journal2018;16(2):5182, 270 pp. 10.2903/j.efsa.2018.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019a. Scientific report on the European Union One Health 2018 Zoonoses Report. EFSA Journal2019;17(12):5926, 276 pp. 10.2903/j.efsa.2019.5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019b. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA Journal2019;17 (2):5598, 278 pp. 10.2903/j.efsa.2019.5598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and EMA (European Food Safety Authority and European Medicines Agency), 2017. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals – Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA Journal 2017;15(7):4872, 135 pp. 10.2903/j.efsa.2017.4872 Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2015/01/WC500181485.pdf and http://onlinelibrary.wiley.com/enhanced/exportCitation/doi/10.2903/j.efsa.2017.4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Aerts M, Battisti A, Hendriksen R, Kempf I, Teale C, Tenhagen B‐A, Veldman K, Wasyl D, Guerra B, Liébana E, Thomas‐López D and Belœil P‐A, 2019. Scientific report on the technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food‐producing animals and food. EFSA Journal 2019;17(6):5709, 122 pp. 10.2903/j.efsa.2019.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea AL, Gagetti P, Lamberghini R, Faccone D, Lucero C, Vindel A, Tosoroni D, Garnero A, Saka HA, Galas M, Bocco JL, Corso A and Sola C, 2014. New patterns of methicillin‐resistant Staphylococcus aureus (MRSA) clones, community‐associated MRSA genotypes behave like healthcare‐associated MRSA genotypes within hospitals, Argentina. International Journal of Medical Microbiology, 304, 1086–1099. Available online: https://pdfs.semanticscholar.org/313e/a7681483ab77327a6a9d3aab2a871e7b1880.pdf [DOI] [PubMed] [Google Scholar]
- Elhadidy M, Miller WG, Arguello H, Álvarez‐Ordóñez A, Dierick K and Botteldoorn N, 2019. Molecular epidemiology and antimicrobial resistance mechanisms of Campylobacter coli from diarrhoeal patients and broiler carcasses in Belgium. Transbound Emerg Dis., 66, 463–475. 10.1111/tbed.13046 [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), European Surveillance of Veterinary Antimicrobial Consumption, 2019. Sales of veterinary antimicrobial agents in 31 European countries in 2017. (EMA/294674/2019).
- EMA and EFSA (European Medicines Agency and European Food Safety Authority), 2017. Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA Journal 2017;15(1):4666, 245 pp. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2017.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCAST (European Committee on Antimicrobial Susceptibility Testing), 2017. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 2.0, 2017. Available online: http://www.eucast.org
- EUCAST (European Committee for Antimicrobial Susceptibility Testing), 2019a. Calibration of zone diameter breakpoints to MIC values ‐ Campylobacter jejuni and coli, January 2019. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_criteria/Validation_2019/Camplyobacter_v_2.4_January_2019.pdf
- EUCAST (European Committee on Antimicrobial Susceptibility Testing), 2019b. New definitions of S, I and R from 2019. Available online: http://www.eucast.org/newsiandr/
- EURL‐AR (European Union Reference Laboratory for Antimicrobial Resistance), 2018. Laboratory Protocol, Isolation of methicillin‐resistant Staphylococcus aureus (MRSA) from food‐producing animals and farm environment. Technical University of Denmark.
- Feltrin F, Alba P, Kraushaar B, Ianzano A, Argudín MA, Di Matteo P, Porrero MC, Aarestrup FM, Butaye P, Franco A and Battisti A, 2016. A livestock‐associated, multidrug‐resistant, methicillin‐resistant Staphylococcus aureus clonal complex 97 lineage spreading in dairy cattle and pigs in Italy. Applied Environmental Microbiology, 82, 816–821. 10.1128/AEM.02854-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertner M, Pedersen K and Chriél M, 2019. Experimental exposure of farmed mink (Neovison vison) to livestock‐associated methicillin‐resistant Staphylococcus aureus contaminated feed. Veterinary Microbiology, 231, 45–47. 10.1016/j.vetmic.2019.02.033 [DOI] [PubMed] [Google Scholar]
- FINRES‐Vet , 2016–2017. Finnish Veterinary Antimicrobial Resistance Monitoring and Consumption of Antimicrobial Agents, Finnish Food Safety Authority Evira, Helsinki, Finland, ISSN 1797‐299X, ISBN 978‐952‐225‐173‐2. Available online: https://www.ruokavirasto.fi/globalassets/viljelijat/elaintenpito/elainten-laakitseminen/evira_publications_5_2018.pdf
- Florez‐Cuadrado D, Ugarte‐Ruiz M, Meric G, Quesada A, Porrero MC, Pascoe B, Saez‐Llorente JL, Orozco GL, Dominguez L and Sheppard SK, 2017. Genome comparison of erythromycin resistant Campylobacter from turkeys identifies hosts and pathways for horizontal spread of erm(B) genes. Frontiers in Microbiology, 8, 2240 10.3389/fmicb.2017.02240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum AE and Bukholm G, 2006. Increased incidence of methicillin‐resistant Staphylococcus aureus ST80, novel ST125 and SCCmecIV in the south‐eastern part of Norway during a 12‐year period. Clinical Microbiology and Infectious Diseases, 12, 627–633. 10.1111/j.1469-0691.2006.01467.x [DOI] [PubMed] [Google Scholar]
- Frana TS, Beahm AR, Hanson BM, Kinyon JM, Layman LL, Karriker LA, Ramirez A and Smith TC, 2013. Isolation and characterization of methicillin‐resistant Staphylococcus aureus from pork farms and visiting veterinary students. PLoS ONE, 8, e53738 10.1371/journal.pone.0053738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Hasman H, Iurescia M, Lorenzetti R, Stegger M, Pantosti A, Feltrin F, Ianzano A, Porrero MC, Liapi M and Battisti A, 2011. Molecular characterization of spa type t127, sequence type 1 methicillin‐resistant Staphylococcus aureus from pigs. Journal of Antimicrobial Chemotherapy, 66, 1231–1235. 10.1093/jac/dkr115 [DOI] [PubMed] [Google Scholar]
- Franco A, Leekitcharoenphon P, Feltrin F, Alba P, Cordaro G, Iurescia M, Tolli R, D'Incau M, Staffolani M, Di Giannatale E, Hendriksen RS and Battisti A, 2015. Emergence of a clonal lineage of multidrug‐resistant ESBL‐producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS ONE, 10. 10.1371/journal.pone.0144802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSA (Food Standards Agency), 2017. Risk assessment on meticillin‐resistant Staphylococcus aureus (MRSA), with a focus on livestock‐associated MRSA in the UK Food Chain. Available online: https://pdfs.semanticscholar.org/4fca/b9277652e3d8cc34a8150b31de865a838e67.pdf
- García‐Álvarez L, Holden MTG, Lindsay H, Webb CR, Brown DFJ, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RLR, Larsen AR, Skov RL, Peacock SJ, Maskell DJ and Holmes MA, 2011. Meticillin‐resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infectious Diseases, 11, 595–603. 10.1016/S1473-3099(11)70126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Fierro R, Montero I, Bances M, González‐Hevia MÁ and Rodicio MR, 2016. Antimicrobial drug resistance and molecular typing of Salmonella enterica serovar rissen from different sources. Microbial Drug Resistance, 22, 211–217. [DOI] [PubMed] [Google Scholar]
- Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E and Heederik D, 2010. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS ONE, 5, e10990 10.1371/journal.pone.0010990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardabassi L, O'Donoghue M, Moodley A, Ho J and Boost M, 2009. Novel lineage of methicillin‐resistant Staphylococcus aureus, Hong Kong. Emerging and Infectious Diseases, 15, 1998–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerum AM, Larsen J, Andersen VD, Lester CH, Skytte TSS, Hansen F, Olsen SS, Mordhorst H, Skov RL, Aarestrup FM and Agerso Y, 2014. Characterization of extended‐spectrum beta‐lactamase (ESBL)‐producing Escherichia coli obtained from Danish pigs, pig farmers and their families from farms with high or no consumption of third‐or fourth‐generation cephalosporins. Journal of Antimicrobial Chemotherapy, 69, 2650–2657. 10.1093/jac/dku180 [DOI] [PubMed] [Google Scholar]
- Handel N, Schuurmans JM, Feng YF, Brul S and ter Kuile BH, 2014. Interaction between mutations and regulation of gene expression during development of De Novo antibiotic resistance. Antimicrobial Agents and Chemotherapy, 58, 4371–4379. 10.1128/aac.02892-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel N, Otte S, Jonker M, Brul S and ter Kuile BH, 2015. Factors that affect transfer of the IncI1 beta‐lactam resistance plasmid pESBL‐283 between E. coli strains. PLoS ONE, 10, e0123039 10.1371/journal.pone.0123039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JE, Larsen AR, Skov RL, Chriél M, Larsen G, Angen Ø, Larsen J, Lassen DCK and Pedersen K, 2017. Livestock‐associated methicillin‐resistant Staphylococcus aureus is widespread in farmed mink (Neovison vison). Veterinary Microbiology, 207, 44–49. 10.1016/j.vetmic.2017.05.027 [DOI] [PubMed] [Google Scholar]
- Harrison EM, Paterson GK, Holden MTG, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Bak Zeuthen A, Heltberg O, Harris SR, Zadoks RN, Parkhill J, Peacock SJ and Holmes MA, 2013. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC. EMBO Molecular Medicine, 5, 509–515. 10.1002/emmm.201202413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey J, Le Hello S, Doublet B, Granier SA, Hendriksen RS, Fricke WF, Ceyssens P‐J, Gomart C, Billman‐Jacobe H, Holt KE and Weill F‐X, 2019. Global phylogenomics of multidrug‐resistant Salmonella enterica serotype Kentucky ST198. Microbial Genomics, 5 10.1099/mgen.0.000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xu J, Wang J, Chen Q, Hua X, Fu Y and Yu Y, 2016. Decreased susceptibility to tigecycline mediated by a mutation in mlaA in Escherichia coli strains. Antimicrobial Agents and Chemotherapy, 60, 7530–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z, Shen Y, Wang G, Sun L, Lei L, Lv Z, Li Y, Pang M, Wang L, Sun Q, Fu Y, Song H, Hao Y, Shen Z, Wang S, Chen G, Wu C, Shen J and Wang Y, 2019. Emergence of plasmid‐mediated high‐level tigecycline resistance genes in animals and humans. Nature Microbiology, 4, 1450–1456. 10.1038/s41564-019-0445-2 [DOI] [PubMed] [Google Scholar]
- Heikinheimo A, Johler S, Karvonen L, Julmi J, Fredriksson‐Ahomaa M and Stephan R, 2016. New dominant spa type t2741 in livestock‐associated MRSA (CC398‐MRSA‐V) in Finnish fattening pigs at slaughter. Antimicrobial Resistance and Infection Control, 5, 6 10.1186/s13756-016-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindermann D, Gopinath G, Chase H, Negrete F, Althaus D, Zurfluh K, Tall BD, Stephan R and Nüesch‐Inderbinen M, 2017. Salmonella enterica serovar Infantis from food and human infections, Switzerland, 2010–2015: poultry‐related multidrug resistant clones and an emerging ESBL producing clonal lineage. Frontiers in Microbiology, 8, 1322 10.3389/fmicb.2017.01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins KL, Kirchner M, Guerra B, Granier SA, Lucarelli C, Porrero MC, Jakubczak A, Threlfall EJ and Mevius DJ, 2010. Multiresistant Salmonella enterica serovar 4,[5],12:i:‐ in Europe: a new pandemic strain?. Eurosurveillance, 15, pii=19580. Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19580 [PubMed] [Google Scholar]
- Irrgang A, Tenhagen B‐A, Pauly N, Schmoger S, Kaesbohrer A and Hammerl JA, 2019. Characterization of VIM‐1‐Producing E. coli isolated from a German fattening pig farm by an improved isolation procedure. Frontiers in Microbiology, 10, 2256 10.3389/fmicb.2019.02256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MZ, Espinosa‐Gongora C, Damborg P, Sieber RN, Munk R, Husted L, Moodley A, Skov R, Larsen J and Guardabassi L, 2017. Horses in Denmark are a reservoir of diverse clones of methicillin‐resistant and ‐susceptible Staphylococcus aureus . Frontiers in Microbiology, 8, 543, 10 pp. 10.3389/fmicb.2017.00543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P and Vatopoulos A, 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. Journal of Antimicrobial Chemotherapy, 52, 145–148. 10.1093/jac/dkg312 [DOI] [PubMed] [Google Scholar]
- Kieffer N, Aires‐de‐Sousa M, Nordmann P and Poirel L, 2017. High Rate of MCR‐1–producing Escherichia coli and Klebsiella pneumoniae among Pigs, Portugal. Emerging Infectious Diseases, 23, 2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross P, Petersen A, Skov R, Van Hauwermeiren E, Pantosti A, Laurent F, Voss A, Kluytmans J, Struelens MJ, Heuer O and Monnet DL and the European human LA‐MRSA study group , 2017. Livestock‐associated meticillin‐resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance, 22, pii=16‐00696. 10.2807/1560-7917.es.2017.22.44.16-00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck R, Kreienbrock L, van Duijkeren E and Schwarz S, 2017. Antimicrobial resistance at the interface of human and veterinary medicine. Veterinary Microbiology, 200, 1–5. [DOI] [PubMed] [Google Scholar]
- Köck R, Daniels‐Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, Schwarz S and Jurke A, 2018. Carbapenem‐resistant Enterobacteriaceae in wildlife, food‐producing, and companion animals: a systematic review. Clinical Microbiology and Infection, 24, 1241–1250. 10.1016/j.cmi.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Kraemer JG, Pires J, Kueffer M, Semaani E, Endimiani A, Hilty M and Oppliger A, 2017. Prevalence of extended‐spectrum β‐lactamase‐producing Enterobacteriaceae and methicillin‐resistant Staphylococcus aureus in pig farms in Switzerland. Science of the Total Environment, 603–604, 401–405. 10.1016/j.scitotenv.2017.06.110 [DOI] [PubMed] [Google Scholar]
- Kraushaar B, Ballhausen B, Leeser D, Tenhagen BA, Käsbohrer A and Fetsch A, 2016. Antimicrobial resistances and virulence markers in Methicillin‐resistant Staphylococcus aureus from broiler and turkey: a molecular view from farm to fork. Veterinary Microbiology, 200, 25–32. 10.1016/j.vetmic.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Krupa P, Bystroń J, Podkowik M, Empel J, Mroczkowska A and Bania J, 2015. Population structure and oxacillin resistance of Staphylococcus aureus from pigs and pork meat in south‐west of Poland. BioMed Research International, 2015, 141475 10.1155/2015/141475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktari S, Le Hello S, Ksibi B, Courdavault L, Mnif B, Maalej S, Fabre L, Hammami A and Weill FX. 2015. Carbapenemase‐producing Salmonella enterica serotype Kentucky ST198, North Africa. The Journal of Antimicrobial Chemotherapy, 70, 3405–3407. [DOI] [PubMed] [Google Scholar]
- Larsen J, Stegger M, Andersen PS, Petersen A, Larsen AR, Westh H, Agersø Y, Fetsch A, Kraushaar B, Käsbohrer A, Feβler AT, Schwarz S, Cuny C, Witte W, Butaye P, Denis O, Haenni M, Madec J, Jouy E, Laurent F, Battisti A, Franco A, Alba P, Mammina C, Pantosti A, Monaco M, Wagenaar JA, de Boer E, van Duijkeren E, Heck M, Domínguez L, Torres C, Zarazaga M, Price LB and Skov RL, 2016. Evidence for human adaptation and foodborne transmission of livestock‐associated methicillin‐resistant Staphylococcus aureus . Clinical Infectious Diseases, 63, 1349–1352. 10.1093/cid/ciw532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Sunde M, Islam MZ, Urdahl AM, Barstad AS, Larsen AR, Grøntvedt CA and Angen Ø, 2017. Evaluation of a widely used culture‐based method for detection of livestock‐associated meticillin‐resistant Staphylococcus aureus (MRSA), Denmark and Norway, 2014 to 2016. Euro Surveillance, 22, pii=30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hello S, Hendriksen RS, Doublet B, Fisher I, Nielsen EM, Whichard JM, Bouchrif B, Fashae K, Granier SA, Jourdan‐Da Silva N, Cloeckaert A, Threlfall EJ, Angulo FJ, Aarestrup FM, Wain J and Weill FX, 2011. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. Journal of Infectious Diseases, 204, 675–684. 10.1093/infdis/jir409 [DOI] [PubMed] [Google Scholar]
- Le Hello S, Bekhit A, Granier SA, Barua H, Beutlich J, Zając M, Münch S, Sintchenko V, Bouchrif B, Fashae K, Pinsard JL, Sontag L, Fabre L, Garnier M, Guibert V, Howard P, Hendriksen RS, Christensen JP, Biswas PK, Cloeckaert A, Rabsch W, Wasyl D, Doublet B and Weill FX, 2013. The global establishment of a highly‐fluoroquinolone resistant Salmonella enterica serotype Kentucky ST198 strain. Frontiers in Microbiology, 4, 395 10.3389/fmicb.2013.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings RS, Lightfoot D, Partridge SR, Hall RM and Djordjevic SP, 2005. The genomic island SGI1, containing the multiple antibiotic resistance region of Salmonella enterica serovar typhimurium DT104 or variants of it, is widely distributed in other S. enterica serovars. Journal of Bacteriology, 187, 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liao X, Yang Y, Sun J, Li L, Liu B, Yang S, Ma J, Li X, Zhang Q and Liu Y, 2013. Spread of oqxAB in Salmonella enterica serotype Typhimurium predominantly by IncHI2 plasmids. Journal of Antimicrobial Chemotherapy, 68, 2263–2268. 10.1093/jac/dkt209 [DOI] [PubMed] [Google Scholar]
- Liu BT, Zhang XY, Wan SW, Hao JJ, Jiang RD and Song FJ, 2018. Characteristics of Carbapenem‐resistant Enterobacteriaceae in ready‐to‐eat vegetables in China. Frontiers in Microbiology, 9, 1147. 10.3389/fmicb.2018.01147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liu W, Lv Z, Xia J, Li X, Hao Y, Zhou Y, Yao H, Liu Z, Wang Y, Shen J, Ke Y and Shen Z, 2019. Emerging erm(B)‐mediated macrolide resistance associated with novel multidrug resistance genomic islands in Campylobacter . Antimicrobial Agents and Chemotherapy, 63 10.1128/AAC.00153-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncaric I, Künzel F, Licka T, Simhofer H, Spergser J and Rosengarten R, 2014. Identification and characterization of methicillin‐resistant Staphylococcus aureus (MRSA) from Austrian companion animals and horses. Veterinary Microbiology, 168, 381–387. 10.1016/j.vetmic.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM and Zhang Q, 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiology, 4, 189–200. 10.2217/17460913.4.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini M, Cremonesi P, Magro G, Bianchini V, Minozzi G, Castiglioni B and Piccinini R, 2015. Methicillin‐resistant Staphylococcus aureus (MRSA) is associated with low within‐herd prevalence of intra‐mammary infections in dairy cows: genotyping of isolates. Veterinary Microbiology, 178, 270–274. 10.1016/j.vetmic.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Luk‐In S, Pulsrikarn C, Bangtrakulnonth A, Chatsuwan T and Kulwichit W, 2017. Occurrence of a novel class 1 integron harboring qnrVC4 in Salmonella Rissen. Diagnostic Microbiology and Infectious Disease, 88, 282–286. [DOI] [PubMed] [Google Scholar]
- Macori G, Giuseppina G, Bellio A, Gallina S, Bianchi DM, Sagrafoli D, Marri N, Giangolini G, Amatiste S and Decastelli L, 2017. Molecular epidemiology of Staphylococcus aureus in the ovine dairy chain and in farm‐related humans. Toxins, 9, 11 10.3390/toxins9050161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL, 2014. General principles of antibiotic resistance in bacteria. Drug Discovery Today. Technologies, 11, 33–39. 10.1016/j.ddtec.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Maslikowska JA, Walker SAN, Elligsen M, Mittmann N, Palmay L, Daneman N and Simor A, 2016. Impact of infection with extended‐spectrum beta‐lactamase‐producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. Journal of Hospital Infection, 92, 33–41. 10.1016/j.jhin.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, Chow H, Ip M, Jatzwauk L, Jonas D, Kadlec K, Kearns A, Laurent F, O'Brien FG, Pearson J, Ruppelt A, Schwarz S, Scicluna E, Slickers P, Tan H‐L, Weber S and Ehricht R, 2011. A field guide to pandemic, epidemic and sporadic clones of methicillin‐resistant Staphylococcus aureus . PLoS ONE, 6, e17936 10.1371/journal.pone.0017936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moré E, Ayats T, Ryan PG, Naicker PR, Keddy KH, Gaglio D, Witteveen M and Cerdà‐Cuéllar M, 2017. Seabirds (Laridae) as a source of Campylobacter spp., Salmonella spp. and antimicrobial resistance in South Africa. Environmental Microbiology, 19, 4164–4176. 10.1111/1462-2920.13874 [DOI] [PubMed] [Google Scholar]
- Mossong J, Mughini‐Gras L, Penny C, Devaux A, Olinger C, Losch S, Cauchie HM, van pelt W and Ragiumbeau C, 2016. Human campylobacteriosis in Luxembourg, 2010‐2013: a case‐control study combined with multilocus sequence typing for source attribution and risk factor analysis. Scientific Reports, 6, 20939 10.1038/srep20939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyaert H, de Jong A, Simjee S and Thomas V, 2014. Antimicrobial resistance monitoring projects for zoonotic and indicator bacteria of animal origin: common aspects and differences between EASSA and EFSA. Veterinary Microbiology, 171, 279–283. 10.1016/j.vetmic.2014.02.038 [DOI] [PubMed] [Google Scholar]
- Nógrády N, Király M, Davies R and Nagy B, 2012. Multidrug resistant clones of Salmonella Infantis of broiler origin in Europe. International Journal of Food Microbiology, 157, 108–112. [DOI] [PubMed] [Google Scholar]
- Olaitan AO, Morand S and Rolain J‐M, 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Frontiers in Microbiology, 5, 643, 18 pp. 10.3389/fmicb.2014.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi A, Caruso M, Normanno G, Latorre L, Sottili R, Miccolupo A, Fraccalvieri R and Santagada G, 2016. Prevalence, antimicrobial susceptibility and molecular typing of methicillin‐resistant Staphylococcus aureus (MRSA) in bulk tank milk from southern Italy. Food Microbiology, 58, 36–42. 10.1016/j.fm.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Parisi A, Crump JA, Glass K, Howden BP, Furuya‐Kanamori L, Vilkins S, Gray DJ and Kirk MD, 2018. Health outcomes from multidrug‐resistant Salmonella infections in high‐income countries: a systematic review and meta‐analysis. Foodborne Pathogens and Disease, 15. 10.1089/fpd.2017.2403 [DOI] [PubMed] [Google Scholar]
- Paterson GK, Harrison EM and Holmes MA, 2014. The emergence of mecC methicillin‐resistant Staphylococcus aureus . Trends in Microbiology, 22, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola S, Franciosini MP, Comitini F, Ciani M, De Luca S, Bellucci S, Menchetti L and Casagrande Proietti P, 2017. Genetic diversity and antimicrobial resistance profiles of Campylobacter coli and Campylobacter jejuni isolated from broiler chicken in farms and at time of slaughter in central Italy. Journal of applied microbiology, 122, 1348–1356. 10.1111/jam.13419 [DOI] [PubMed] [Google Scholar]
- Petersen A, Stegger M, Heltberg O, Christensen J, Zeuthen A, Knudsen LK, Urth T, Sorum M, Schouls L, Larsen J, Skov R and Larsen AR, 2013. Epidemiology of methicillin‐resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clinical Microbiology and Infection, 19, E16–E22. 10.1111/1469-0691.12036 [DOI] [PubMed] [Google Scholar]
- Pimenta AC, Fernandes R and Moreira IS, 2014. Evolution of drug resistance: insight on TEM beta‐lactamases structure and activity and beta‐lactam antibiotics. Mini Reviews in Medicinal Chemistry, 14, 111–122. [DOI] [PubMed] [Google Scholar]
- Pornsukarom S, Patchanee P, Erdman M, Cray PF, Wittum T, Lee J and Gebreyes WA, 2015. Comparative phenotypic and genotypic analyses of Salmonella Rissen that originated from food animals in Thailand and United States. Zoonoses and Public Health, 62, 151–158. [DOI] [PubMed] [Google Scholar]
- Qi W, Ender M, O'Brien F, Imhof A, Ruef C, McCallum N and Berger‐Bächi B, 2005. Molecular epidemiology of methicillin‐resistant Staphylococcus aureus in Zürich, Switzerland (2003): prevalence of type IV SCCmec and a new SCCmec element associated with isolates from intravenous drug users. Journal of Clinical Microbiology, 43, 5164–5170. 10.1128/JCM.43.10.5164-5170.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenan K, Hasler B and Rushton J, 2016. A one Health approach to antimicrobial resistance surveillance: is there a business case for it? International Journal of Antimicrobial Agents, 48, 422–427. 10.1016/j.ijantimicag.2016.06.014 [DOI] [PubMed] [Google Scholar]
- Roberts MC, 2008. Update on macrolide‐lincosamide‐streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiology Letetrs, 282, 147–159. 10.1111/j.1574-6968.2008.01145.x [DOI] [PubMed] [Google Scholar]
- Sahibzada S, Abraham S, Coombs GW, Pang S, Hernández‐Jover M, Jordan D and Heller J, 2017. Transmission of highly virulent community‐associated MRSA ST93 and livestock‐associated MRSA ST398 between humans and pigs in Australia. Scientific Reports, 7, 5273 10.1038/s41598-017-04789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, Ehricht R and Coleman DC, 2011. Detection of staphylococcal cassette chromosome mec Type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin‐resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy., 55, 3765–3773. 10.1128/AAC.00187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov RL and Monnet DL, 2016. Plasmid‐mediated colistin resistance (mcr‐1 gene): three months later, the story unfolds. Eurosurveillance, 21, pii=30155. Available online: https://www.eurosurveillance.org/images/dynamic/EE/V21N09/art21403.pdf [DOI] [PubMed] [Google Scholar]
- Spoor LE, McAdam PR, Weinert LA, Rambaut A, Hasman H, Aarestrup FM, Kearns AM, Larsen AR, Skov RL and Fitzgerald JR, 2013. Livestock origin for a human pandemic clone of community‐associated methicillin‐resistant Staphylococcus aureus . mBio, 4, e00356‐13. 10.1128/mBio.00356-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Wan Y, Du P and Bai L, 2019. The epidemiology of monophasic Salmonella Typhimurium. Foodborne Pathogens and Disease. 10.1089/fpd.2019.2676 [DOI] [PubMed] [Google Scholar]
- SWEDRES , 2011. A report on Swedish antibiotic utilisation and resistance in human medicine. Available online: https://www.folkhalsomyndigheten.se/contentassets/822c49b59bc44d58b18f64c913b6ce59/swedres-svarm-2011.pdf
- Swedres‐Svarm , 2017. Consumption of antibiotics and occurrence of resistance in Sweden. Solna/Uppsala ISSN1650‐6332. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/swedres_svarm2017.pdf
- Szczepanska B, Andrzejewska M, Spica D and Klawe JJ, 2017. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiol, 17, 80 10.1186/s12866-017-0991-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Dai L, Sahin O, Wu Z, Liu M and Zhang Q, 2017. Emergence of a plasmid‐borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. Journal of Antimicrobial Chemotheraphy, 72, 1581–1588. 10.1093/jac/dkx023 [DOI] [PubMed] [Google Scholar]
- Tate H, Folster JP, Hsu C‐H, Chen J, Hoffmann M, Li C, Morales C, Tyson GH, Mukherjee S, Brown AC, Green A, Wilson W, Dessai U, Abbott J, Joseph L, Haro J, Ayers S, McDermott PF and Zhaoa S, 2017. Comparative analysis of extended‐spectrum‐β‐lactamase CTX‐M‐65‐producing Salmonella enterica serovar Infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrobial Agents and Chemotherapy, 61 10.1128/aac.00488-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC and Goering RV, 2009. Methicillin‐resistant Staphylococcus aureus strain USA300: origin and epidemiology. Journal of Antimicrobial Chemotherapy, 64, 441–446. 10.1093/jac/dkp241 [DOI] [PubMed] [Google Scholar]
- Thépault A, Poezevara T, Quesne S, Rose V, Chemaly M and Rivoal K, 2018. Prevalence of Thermophilic Campylobacter in Cattle Production at Slaughterhouse Level in France and Link Between C. jejuni Bovine Strains and Campylobacteriosis. Front Microbiol, 9, 471 10.3389/fmicb.2018.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torneke K, Torren‐Edo J, Grave K and Mackay DK, 2015. The management of risk arising from the use of antimicrobial agents in veterinary medicine in EU/EEA countries ‐ a review. Journal of Veterinary Pharmacology and Therapeutics, 38, 519–528. 10.1111/jvp.12226 [DOI] [PubMed] [Google Scholar]
- Torres‐González P, Bobadilla‐del Valle M, Tovar‐Calderón E, Leal‐Vega F, Hernández‐Cruz A, Martínez‐Gamboa A, Niembro‐Ortega MD, Sifuentes‐Osornio J and Ponce‐de‐León A, 2015. Outbreak caused by Enterobacteriaceae harboring NDM‐1 metallo‐β‐lactamase carried in an IncFII plasmid in a Tertiary Care Hospital in Mexico City. Antimicrobial Agents and Chemotherapy, 59, 7080–7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati A, Mairi A, Baloul Y, Lalaoui R, Bakour S, Thighilt L, Gharout A and Rolain J‐M, 2017. First detection of Klebsiella pneumoniae producing OXA‐48 in fresh vegetables from Béjaïa city, Algeria. Journal of Global Antimicrobial Resistance, 9, 17–18. 10.1016/j.jgar.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q and Shen J, 2014. Emergence of multidrug‐resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrobial Agents and Chemotherapy, 58, 5405–5412. 10.1128/AAC.03039-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization), 2006. WHO Drug Information, 20, 237–280. Available online: https://www.who.int/medicines/publications/druginformation/issues/DrugInfo06vol20_4/en/
- WHO (World Health Organization ‐ Advisory Group on Integrated Surveillance of Antimicrobial Resistance), 2019. Critically important antimicrobials for human medicine, 6th Revision 2018. 45 pp. Available online: https://www.who.int/foodsafety/publications/antimicrobials-sixth/en/
- Wolter DJ, Chatterjee A, Varman M and Goering RV, 2008. Isolation and characterization of an epidemic methicillin‐resistant Staphylococcus aureus 15 variant in the central United States. Journal of Clinical Microbiology, 46, 3548–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su C, Wang B, Wang S, Wu C, Yu EW, Zhang Q and Shen J, 2016. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. MBio, 7, e01543–16. 10.1128/mBio.01543-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Liu D, Wang Y, Zhang Q and Shen Z, 2017. High Prevalence and Predominance of the aph(2′′)‐If GeneConferring Aminoglycoside Resistance in Campylobacter. Antimicrobial Agents and Chemotherapy, 61, e00112–e00117. 10.1128/aac.00112-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Mukherjee S, Chen Y, Li C, Young S, Warren M, Abbott J, Friedman S, Kabera C, Karlsson M and McDermott PF, 2015. Novel gentamicin resistance genes in Campylobacter isolated from humans and retail meats in the USA. Journal of Antimicrobial Chemotheraphy, 70, 1314–1321. 10.1093/jac/dkv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh K, Poirel L, Nordmann P, Klumpp J and Stephan R, 2015. First detection of Klebsiella variicola producing OXA‐181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrobial Resistance and Infection Control, 4, 38 10.1186/s13756-015-0080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


