Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Liriomyza sativae (Diptera: Agromyzidae) for the EU. L. sativae (the cabbage or vegetable leaf miner; EPPO code: LIRISA) is a polyphagous pest native to the Americas which has spread to Africa, Asia and Oceania. L. sativae can have multiple overlapping generations per year. Eggs are inserted in the leaves of host plants. Three larval instars, which feed internally on field vegetables (leaves and stems), follow. Then, the larva jumps into the soil where a fourth larval instar occurs immediately before pupation, which takes place in the soil. L. sativae is regulated in the EU by Commission Implementing Regulation (EU) 2019/2072 (Annex IIA). Within this Regulation, import of soil or growing medium as such or attached to plants for planting from third countries other than Switzerland is regulated. Therefore, entry of L. sativae pupae is prevented. However, immature stages on plants for planting (excluding seeds) and fresh leafy hosts for consumption, cut branches, flowers and fruit with foliage provide potential pathways for entry into the EU. L. sativae has been repeatedly intercepted in the EU, especially in basil (Ocimum spp.). Climatic conditions and the wide availability of host plants provide conditions to support establishment in the EU, both in open fields and greenhouses. Impacts on field vegetables and ornamentals as well as hosts in greenhouses would be possible. Phytosanitary measures are available to reduce the likelihood of entry. L. sativae satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. Although human‐assisted movement of vegetables is considered the main spread way for L. sativae, this agromyzid does not meet the criterion of occurring in the EU for it to be regarded as a potential Union regulated non‐quarantine pest.
Keywords: Agromyzid, European Union, pest risk, plant health, plant pest, quarantine, cabbage leaf miner, vegetable leaf miner
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community established the previous European Union plant health regime. The Directive laid down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union was prohibited, was detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and applied from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorisations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002,3 to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pest categorisations should be delivered by end 2020.
For the above mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocanthus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricidas Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Little cherry pathogen (non‐ EU isolates) |
| Black raspberry latent virus | Naturally spreading psorosis |
| Blight and blight‐like | Palm lethal yellowing mycoplasm |
| Cadang‐Cadang viroid | Satsuma dwarf virus |
| Citrus tristeza virus (non‐EU isolates) | Tatter leaf virus |
| Leprosis | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Mycosphaerella larici‐leptolepis Ito et al. |
| Chrysomyxa arctostaphyli Dietel | Mycosphaerella populorum G. E. Thompson |
| Cronartium spp. (non‐EU) | Phoma andina Turkensteen |
| Endocronartium spp. (non‐EU) | Phyllosticta solitaria Ell. and Ev. |
| Guignardia laricina (Saw.) Yamamoto and Ito | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Gymnosporangium spp. (non‐EU) | Thecaphora solani Barrus |
| Inonotus weirii (Murril) Kotlaba and Pouzar | Trechispora brinkmannii (Bresad.) Rogers |
| Melampsora farlowii (Arthur) Davis | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Liriomyza sativae Blanchard is one of a number of pests listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether it fulfils the criteria of a quarantine pest or those of a regulated non‐quarantine pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
Following the adoption of Regulation (EU) 2016/2031 on 14 December 2019 and the Commission Implementing Regulation (EU) 2019/2072 for the listing of EU regulated pests, the Plant Health Panel interpreted the original request (ToR in Section 1.1.2) as a request to provide pest categorisations for the pests in the Annexes of Commission Implementing Regulation (EU) 2019/2072.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on Liriomyza sativae was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name Liriomyza sativae as a search term. Relevant papers were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, 2019a,b) and relevant publications.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission, and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States (MS) and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for Liriomyza sativae, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018) and in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
This work was initiated following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest (RNQP) in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as an RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as an RNQP that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1 ) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2 ) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be an RNQP. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3 ) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest‐free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential RNQP were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel.
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
3.1.1.1.
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of Liriomyza sativae is well established.
Liriomyza sativae Blanchard 1938 is an insect of the order Diptera, family Agromyzidae. This species, native to the Americas, was originally described from specimens obtained from infested leaves of lucerne (Medicago sativa L.) collected in Argentina (CABI, 2019). However, it was inadvertently re‐described twice (Scheffer and Lewis, 2005). Its common English names include cabbage leaf miner, tomato leaf miner and vegetable leaf miner (EPPO GD, 2019). This species has many junior synonyms (CABI, 2019; EPPO GD, 2019; FAO, 2016): Agromyza subpusilla Frost, 1943); Liriomyza canomarginis Frick, 1952; L. guytona Freeman, 1958; L. lycopersicae Pla & de la Cruz, 1981; L. minutiseta Frick, 1952; L. munda Frick, 1957; L. propepusilla Frost, 1954; L. pullata Frick, 1952; and L. verbenicola Hering, 1951. The EPPO code (Griessinger and Roy, 2015; EPPO, 2019a,b) for this species is LIRISA4 (EPPO GD, 2019).
According to Scheffer and Lewis (2005), there has been a long history of taxonomic confusion regarding L. sativae, which together with numerous misidentifications, make the literature on this species before the 1970s difficult to interpret.
3.1.2. Biology of the pest
Although nearly all Liriomyza species are host‐specific, Liriomyza sativae is one of the few Agromyzidae of economic importance considered to be truly polyphagous (Parrella, 1987; Kang et al., 2009). Indeed, this species is considered a pest of many vegetable and flower crops (Spencer, 1973a,b, 1990). Larvae feed internally on plants, often as leaf and stem miners, thus the common name of leaf miner.
L. sativae is a multivoltine species which cannot survive cold areas except in greenhouses. In warm climates (including glasshouses), this species can breed continuously, with many overlapping generations per year (Capinera, 2017; CABI, 2019). Eggs, which are inserted into plant tissue just beneath the leaf surface (Capinera, 2017), hatch in 2–8 days depending on temperature (Parrella, 1987). Many eggs can be laid on the same leaf. A lower development threshold for this stage was estimated to be 7°C (Webb and Smith, 1970). First instar larvae start feeding immediately after hatching and will continue feeding until they reach the third instar. At this stage, the larva cuts a semi‐circular slit in the mined leaf and usually exits the mine, jumps off the leaf and burrows into the soil to a depth of only a few centimetres to form a puparium (Capinera, 2017). A fourth non‐feeding larval instar occurs between puparium formation and pupation (Parrella, 1987). The lower development threshold of this stage has been estimated to be in the range 4.6–7.9°C (Oatman and Michelbacher, 1959; Webb and Smith, 1970). The pupal stage may take 7–14 days at temperatures between 20 and 30°C (Leibee, 1982). At lower temperatures, emergence is delayed and this stage becomes the overwintering stage (Parrella, 1987). Indeed, pupae can endure some time at freezing temperatures. The LT50 of 4‐day‐old puparia exposed to 0, −5, and −10°C is around 9 days, 2 days, and less than 1 hour, respectively (Zhao and Kang, 2000). Immature development time takes around 25 days at 15°C. At optimal temperatures (30°C), the whole cycle is completed in about 15 days (Capinera, 2017). One day after emergence, adults become sexually active. They can mate several times for up to a month post‐emergence before dying (Capinera, 2017). Adults feed on plant exudates, e.g. caused by oviposition. Females often make feeding punctures without depositing eggs and only about 15% of punctures contain viable eggs (Parrella et al., 1981). Mean fecundity ranges from 200 to 700 eggs per female, with a daily oviposition rate of 30–40 eggs, which decreases as females get older.
Adult agromyzid flies are not considered strong fliers and tend to remain close to their target crops, only moving short distances between host plants. Although they can be passively dispersed over long distances by the wind (Malipatil et al., 2016), dispersal over long distances is attributed to human‐assisted movement of planting material (EPPO GD, 2019).
3.1.3. Intraspecific diversity
The existence of a host race of L. sativae on melons (misidentified as L. pictella) was reported by Parrella (1987). Later, Scheffer and Lewis (2005) found distinct mitochondrial clades in different L. sativae populations from native (the Americas) and invaded areas (Asia), which suggested that L. sativae could be a cryptic species complex. Interestingly, only one clade seemed to be invasive on a worldwide scale. However, this study was not conclusive and further research is needed to clarify the situation.
3.1.4. Detection and identification of the pest
3.1.4.1.
Are detection and identification methods available for the pest?
Yes, there are standard protocols for detection and identification of L. sativae (EPPO, 2005; FAO, 2017). Moreover, taxonomic keys for the identification of L. sativae exist (Spencer and Steyskal, 1986).
There are almost 400 species in the genus Liriomyza (Kang et al., 2009; EPPO GD, 2019), of which around 140 are found naturally in Europe (Seymour, 1994; de Jong et al., 2014). According to EPPO (EPPO GD, 2019), the adult flies of all these minute species (1–3 mm long) look very similar. From above, they are seen to be mostly black, with a bright yellow scutellum in most species. As a result, separating these species can be difficult. Diagnosticians have to distinguish indigenous and naturalised Liriomyza spp. from quarantine agromyzid species.
FAO developed a diagnostic protocol for these species including morphological and molecular tools for both adults and immature stages of this fly (ISPM 27; FAO 2016). EPPO also produced a standard for L. sativae (PM 7/53; EPPO, 2005). A summary of the most remarkable features in these diagnostic protocols follows:
-
Detection
-
−
Symptoms: Feeding punctures and leaf mines are usually the first and most obvious signs of the presence of Liriomyza spp. Mines remain intact and relatively unchanged over a period of weeks. Mine configuration is affected by the host, by the physical and physiological condition of each leaf and by the number of larvae mining the same leaf. Therefore, species identification from mine configuration alone is not advisable, especially for polyphagous Liriomyza spp. like L. sativae.
-
−
Adults: Small free‐flying minute flies (1.3–2.3 mm in body length, 1.3–2.3 mm in wing length; females slightly larger than males), which can be observed on leaf surfaces while producing feeding and oviposition punctures. Species‐specific characteristics of L. sativae include bright‐yellow scutellum, shining black prescutum and scutum and inner vertical setae usually standing on yellow ground. Accurate identification, though, requires dissection of male terminalia (see below).
-
−
Immature stages: Egg: Elliptical, 0.20–0.30 × 0.10–0.15 mm, off‐white and slightly translucent, and inserted into plant tissue. Larva: headless maggots up to 3 mm long when mature. First instar larvae are colourless when hatching but turn yellowish as they grow older. Later larval instars are yellow‐orangish. Third instars abandon the mine and usually burrow into the soil (a few centimetres deep) where a fourth and last non‐feeding larval instar occurs. Petitt (1990) provided characters to distinguish the larval instars of L. sativae. Puparium: Elliptical, 1.5 × 0.75 mm, slightly flattened ventrally, reddish‐brown, located a few centimetres deep into the soil.
-
−
-
Identification
-
−
Morphological identification: Because the morphological characters used to diagnose species are based on male genitalia (particularly the distiphallus, the terminal part of the aedeagus), adult males are needed in order to confirm species identification. There are no adequate keys for the species‐level identification of adult females (which are often identifiable with certainty to genus level only), eggs, larvae or pupae.
-
−
Molecular identification: Various polymerase chain reaction (PCR)‐based molecular tests have been used to identify Liriomyza species, including PCR‐restriction fragment length polymorphism (RFLP), end‐point PCR using species‐specific primers, real‐time PCR and DNA sequence comparison. Considering the specific limitations of molecular tests, a negative molecular test result does not exclude the possibility of positive identification by morphological tests. In fact, it is advisable to combine morphology and molecular‐based identification methods for accurate species identification.
-
−
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
Liriomyza sativae is endemic to the Americas. Although originally limited to this continent, it is now found in many areas of Africa, Asia and Oceania (Figure 1). It is not clear whether it may be present in the European part of Turkey. According to EPPO GD (2019), in Turkey, L. sativae is restricted to the regions of the Aegean and south east Anatolia. However, the original information dates from 2005 (Çıkman and Civelek, 2005).
Figure 1.
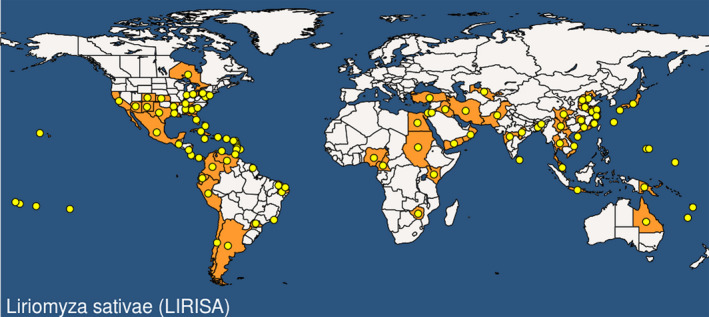
Global distribution map for Liriomyza sativae (extracted from the EPPO Global Database updated 30/01/2020 accessed on 17/2/2020)
Appendix C shows the details about the worldwide pest presence and absence on the base of EPPO Global Database accessed on 17/11/2019.
3.2.2. Pest distribution in the EU
3.2.2.1.
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, L. sativae is not present in the EU territory (EPPO GD, 2019)
3.3. Regulatory status
3.3.1. Regulation 2016/2031
Liriomyza sativae is listed in Annex II of Commission Implementing Regulation (EU) 2019/20725 and of Regulation (EU) 2016/2031 of The European Parliament. Details are presented in Table 2.
Table 2.
Liriomyza sativae in Commission Implementing Regulation (EU) 2019/2072
| Annex II | List of Union quarantine pests and their respective codes |
|---|---|
| Part A: | Pests not known to occur in the Union territory |
| Quarantine Pests and their codes assigned by EPPO | |
| C. Insects and mites | |
| 37. Liriomyza sativae Blanchard [LIRISA] |
3.3.2. Legislation addressing the hosts of Liriomyza sativae
Regulated hosts and commodities that may involve L. sativae in Annexes of Commission Implementing Regulation (EU) 2019/2072 are shown in Table 3.
Table 3.
List of plants, plant products and other objects, originating from third countries and the corresponding special requirements for their introduction into the Union territory in Commission Implementing Regulation (EU) 2019/2072
| Annex VII | List of plants, plant products and other objects, originating from third countries and the corresponding special requirements for their introduction into the Union territory | |||
|---|---|---|---|---|
| Plants, plant products and other objects | CN codes a | Origin | Special requirements | |
| 8 | Plants for planting of herbaceous species, other than bulbs, corms, plants of the family Poaceae, rhizomes, seeds, tubers, and plants in tissue culture |
ex 0602 10 90 0602 90 20 ex 0602 90 30 ex 0602 90 50 ex 0602 90 70 ex 0602 90 91 ex 0602 90 99 ex 0704 10 00 ex 0704 90 10 ex 0704 90 90 ex 0705 11 00 ex 0705 19 00 ex 0705 21 00 ex 0705 29 00 ex 0706 90 10 ex 0709 40 00 ex 0709 99 10 ex 0910 99 31 ex 0910 99 33 |
Third countries where Liriomyza sativae (Blanchard) and (…) are known to occur |
Official statement that the plants have been grown in nurseries and: (a) originate in an area established by the national plant protection organisation in the country of origin as being free from Liriomyza sativae (Blanchard) (…) in accordance with relevant International Standards for Phytosanitary Measures which is mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Additional declaration’, or (b) originate in a place of production, established by the national plant protection organisation of the country of origin as being free from Liriomyza sativae |
|
(Blanchard) (…) in accordance with the relevant International Standards for Phytosanitary Measures, and which is mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031, under the rubric ‘Additional declaration’, and declared free from Liriomyza sativae (Blanchard) (…) on official inspections carried out at least monthly during the three months prior to export, or (c) immediately prior to export, have been subjected to an appropriate treatment against Liriomyza sativae (Blanchard) (…) and have been officially inspected and found free from Liriomyza sativae (Blanchard) (…). Details of the treatment referred in point (c) shall be mentioned on the phytosanitary certificate referred to in Article 71 of Regulation (EU) No 2016/2031 |
||||
| 28 | Cut flowers of Chrysanthemum L., Dianthus L., Gypsophila L. and Solidago L., and leafy vegetables of Apium graveolens L. and Ocimum L. |
0603 12 00 0603 14 00 ex 0603 19 70 0709 40 00 ex 0709 99 90 |
Third countries |
Official statement that the cut flowers and the leafy vegetables: (a) originate in a country free from Liriomyza sativae (Blanchard) (…), or (b) immediately prior to their export, have been officially inspected and found free from Liriomyza sativae (Blanchard) (…)). |
Further details on the CN codes is provided in Annex XI of Commission Implementing Regulation (EC) 2019/2072.
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
Liriomyza sativae is a highly polyphagous species, with more than 60 host plants in 18 different botanical families: Amaranthaceae, Apiaceae, Asteraceae, Brassicaceae, Caryophyllaceae, Chenopodiaceae, Convolvulaceae, Cucurbitaceae, Euphorbiaceae, Fabaceae, Lamiaceae, Liliaceae, Malvaceae, Moringaceae, Poaceae, Polemoniaceae, Solanaceae and Tropaeolaceae (Appendix A). Hosts include cultivated monocots (e.g. maize, sorghum) and dicots (e.g. potatoes, cabbages, sugar beet, melons), and ornamentals (e.g. dahlia, phlox), as well as plants considered as weeds in America (e.g. the nightshade, Solanum americanum and Spanish needles, Bidens alba).
As a Union quarantine pest, its introduction into the EU is banned irrespective of the host plant.
3.4.2. Entry
3.4.2.1.
Is the pest able to enter into the EU territory?
Yes, L. sativae has been repeatedly intercepted in different commodities entering into the EU. The main pathways are fruit and vegetables and cut flowers and branches with foliage. Plants for planting can also constitute a pathway.
Liriomyza sativae is a polyphagous species and its different life stages could use different pathways to enter the EU, as noted in Table 4.
Table 4.
Potential pathways for Liriomyza sativae and existing mitigations
| Pathways | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI) or special requirements (Annex VII)] |
|---|---|---|
| Plants for planting (excluding seeds) | Eggs and larvae | |
| Cut flowers and branches with foliage | Eggs and larvae | Annex VII applies only to Chrysanthemum, Dianthus, Gypsophila and Solidago other ornamental hosts exist such as Phlox and Dahlia |
| Fruits and vegetables | Eggs and larvae | Annex VII applies to Apium graveolens and Ocimum |
| Soil & growing media | Pupae |
Annex VI of Commission Implementing Regulation 2019/2072 bans the introduction of soil and growing medium as such into the Union from third countries other than Switzerland Specific regulations apply to soil/growing medium attached to plants for planting for vitality |
| Hitchhiking adults | Adults |
The soil/growing medium pathway can be considered as closed, as import of soil/growing medium as such from third countries other than Switzerland is banned from entering into the EU (Annex VI). If necessary, for vitality, when attached to plants for planting, specific regulations are in place for import (Annex VII).
With the implementation of the Plant Health Regulation (EC 2016/2031), consignments of almost all fruits and vegetables require a phytosanitary certificate indicating that they have been inspected and are free from harmful organisms before entry into the EU.
3.4.2.2. Interceptions
There are 624 records of L. sativae interceptions in the Europhyt database between 1996 and November 2019 (accessed 17/11/2019). Most of these interceptions refer to basil (Ocimum spp.) (Figure 2) and to commodities imported from Thailand (Figure 3). L. sativae has been intercepted in many EU countries (Europhyt, 2019) because it is transported with plant material (Capinera, 2017).
Figure 2.

Host plants where L. sativae was intercepted between 1996 and 2019 (n = 624). Hosts where the pest was intercepted less than 10 times have been grouped as ‘Others’. This category includes Amaranthus sp., Amaranthus viridis, Artemisia dracunculus, Brassica alboglabra, Brassica sp., Cassia sp., Cestrum sp., Chrysantemum sp., Coriandrum sativum, Dendranthema sp., Dianthus sp., Gypsophila sp., Ipomoea sp., Momordica charantia, Moringa oleifera, Solanum sp., Solidago sp., Spinacia sp., Trigonella sp. and Trigonella foenum‐graecum
Figure 3.

Countries of origin of the commodity where L. sativae was intercepted between 1996 and 2019 (n = 624). Countries from which the pest was intercepted less than 10 times have been grouped as ‘Others’. This category includes Congo, Colombia, Dominican Republic, Ecuador, Egypt, Ethiopia, Ghana, Iran, Jordan, Sri Lanka, Morocco, Mexico, Nigeria, Pakistan, Tanzania and Uganda
56% of interceptions refer to fruit and vegetables (Europhyt classification code 140), 39% to cut flowers and branches with foliage (code 120). The remaining 5% corresponds to other living plants (codes !, 102, and 122). The number of interceptions substantially decreased between 1997 and 2003, and then again starting in 2009 (Figure 4). The average number of interceptions between 2009 and 2018 was 25.2 per year. However, without information on the number of inspections made, it is difficult to interpret interception data.
Figure 4.

Annual number of interceptions of L. sativae between 1996 and 2019 (n = 624)
3.4.3. Establishment
3.4.3.1.
Is the pest able to become established in the EU territory?
Yes, biotic and abiotic conditions are conducive for the establishment of L. sativae in some parts of the EU where potential hosts occur (either cultivated or not).
3.4.3.2. EU distribution of main host plants
Many potential hosts of L. sativae (Appendix A) would be available to this insect in the EU. Because of the high polyphagy of this Dipteran, many crops widely grown in the EU, including those grown in glasshouses, could support the reproduction and immature development of this insect (Table 5).
Table 5.
EU 28 crop production (2014–2018) of the main host plants affected by Liriomyza sativae
| Crop | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| Brassicas | : | 273.77 | 273.01 | 279.90 | : |
| Lettuces | 96.03 | 93.95 | 91.19 | 91.00 | 88.33 |
| Tomatoes | 248.09 | 254.43 | 247.00 | 241.07 | 243.44 |
| Cucumbers | 37.31 | 33.51 | 32.43 | 31.91 | : |
| Gourds and pumpkins | : | : | : | : | : |
| Muskmelons | 76.46 | 73.73 | 73.27 | 72.60 | : |
| Watermelons | 75.56 | 76.39 | 75.29 | 76.47 | : |
‘:’ data not available.
3.4.3.3. Climatic conditions affecting establishment
The distribution of L. sativae in its native range in the Americas, extending from Canada to Argentina and Chile, covers a large area where all climate types also occurring in the EU can be found (Figure 5). Therefore, we assume that climatic conditions in the EU would not limit the ability of L. sativae to establish.
Figure 5.

Köppen–Geiger climate type zones (MacLeod and Korycinska, 2019). In its native range in the Americas, L. sativae is established from Canada to Argentina and Chile (dotted rectangle), a zone including all climate types also occurring in the EU
3.4.4. Spread
3.4.4.1.
Is the pest able to spread within the EU territory following establishment?
Yes, adults can fly. However, L. sativae seems not to be a good flyer. It can be passively dispersed by wind currents.
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
Yes, wide‐scale and international spread of L. sativae seems to be mostly dependent on human‐mediated movement of plants.
As pointed out in Section 3.1.2, agromyzid flies are not considered strong fliers and tend to remain close to their host crops, only moving short distances between host plants. Although they can be passively dispersed over long distances by the wind (Malipatil et al., 2016), dispersal over long distances is attributed to human‐assisted moving of infested host plant material (EPPO GD, 2019).
3.5. Impacts
3.5.1.
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, the introduction of L. sativae would most probably have an economic impact in the EU through qualitative and quantitative effects on agricultural production.
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting? 6
Yes, should L. sativae be present in plants for planting, an economic impact on their intended use would be expected.
According to CABI (2019), L. sativae is the most serious of the agromyzid pests, causing severe damage and loss of yield in many southern states of the US and also in South America. Damage to the plant is caused in several ways: (i) by the stippling that results from punctures made by females with their ovipositor for feeding on sap and laying eggs; (ii) by the internal mining by the larvae; (iii) by allowing microorganisms to enter the leaf through the feeding punctures and (iv) by mechanical transmission of some plant viruses (Malipatil et al., 2016). Young plants are particularly susceptible to damage and consequent reduced efficiency or death, while older plants may also be seriously damaged through leaf loss due to many mines occurring in each leaf (CABI, 2019). Losses of 80% have been reported for celery in Florida and up to 80% in lucerne in Argentina (Spencer, 1973b). 30–60% yield increases were reported by Sharma et al. (1980), who studied the value of controlling this pest in squash in California. L. sativae is difficult to eradicate because of its ability to survive in many weed plants which normally occur in areas adjacent to crop fields (CABI, 2019).
Liriomyza sativae can mechanically transmit the Potyviridae Celery Mosaic Virus and Watermelon Mosaic Virus in experimental conditions (Zitter and Tsai, 1977). However, the same authors say that ‘the likelihood of achieving natural spread of potyviruses by leaf miners is at best remote’. Legislation does not address these viruses which are widespread and not regulated in the EU (EPPO GD, 2019).
3.6. Availability and limits of mitigation measures
3.6.1.
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, the existing measures (see sections 3.3 and 3.4.2) can mitigate the risks of entry, establishment, and spread within the EU. As a pest listed in Annex IIA, its introduction and spread in the EU is banned irrespective of what it may be found on.
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
Yes, sourcing plants and plant parts from PFA would mitigate the risk.
3.6.2. Identification of additional measures
Phytosanitary measures are currently applied to soil. Some host plants are listed in the import prohibitions of Annex VI (e.g. Fragaria and Poaceae from specified third countries) or in specific requirements in Annex VII of Commission Implementing Regulation 2019/2072 (see Sections 3.3 and 3.4.2).
3.6.3. Additional control measures
Potential additional control measures are listed in Table 6.
Table 6.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Control measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| Growing plants in isolation | Description of possible exclusion conditions that could be implemented to isolate the crop from pests and if applicable relevant vectors, e.g. a dedicated structure such as greenhouses | Entry, establishment, spread, impact |
| Crop rotation, associations and density, weed/volunteer control |
Crop rotation, associations and density, weed/volunteer control are used to prevent problems related to pests and are usually applied in various combinations to make the habitat less favourable for pests The measures deal with (1) allocation of crops to field (over time and space) (multi‐crop, diversity cropping) and (2) to control weeds and volunteers as hosts of pests/vectors Nitrogen level and reflective mulches are sometimes said to influence leaf miner populations, but responses have not been consistent (Chalfant et al., 1977; Hanna et al., 1987). Placement of row covers over cantaloupe has been reported to prevent damage by L. sativae (Orozco‐Santos et al., 1995) |
Impact |
| Heat and cold treatments |
Controlled temperature treatments aimed to kill or inactivate pests without causing any unacceptable prejudice to the treated material itself. The measures addressed in this information sheet are: autoclaving; steam; hot water; hot air; cold treatment All stages are killed within a few weeks by cold storage at 0°C. Newly laid eggs are, however, the most resistant stage and it is recommended that cuttings of infested ornamental plants be maintained under normal glasshouse conditions for 3–4 days after lifting to allow eggs to hatch. Subsequent storage of the plants at 0°C for 1–2 weeks should then kill off the larvae of leaf miner species (Webb and Smith, 1970) |
Entry, spread, impact |
| Chemical treatments on crops including reproductive material | Foliar application of insecticides is often frequent in susceptible crops. Insecticide susceptibility varies greatly both spatially and temporally. Many insecticides are no longer effective. Insecticides are disruptive to naturally occurring biological control agents, and leaf miner outbreaks are sometimes reported to follow chemical insecticide treatment for other insects (Capinera, 2017) | Impact |
| Use of resistant and tolerant plant species/varieties |
Resistant plants are used to restrict the growth and development of a specified pest and/or the damage they cause when compared to susceptible plant varieties under similar environmental conditions and pest pressure It is important to distinguish resistant from tolerant species/varieties Some crops vary in susceptibility to leaf mining. This has been noted, e.g. in cultivars of tomato, cucumber, cantaloupe, and beans (Hanna et al., 1987). However, the differences tend to be moderate, and not adequate for reliable protection (Capinera, 2017) |
Impact |
| õBiological control and behavioural manipulation | The parasitoids of L. sativae are not specific (Capinera, 2017) and usually attack other (i.e. Coleoptera, Hymenoptera, Lepidoptera) | Impact |
3.6.3.1. Additional supporting measures
Potential additional supporting measures are listed in Table 7.
Table 7.
Selected supporting measures (a full list is available in EFSA PLH Panel et al., 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Supporting measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| Inspection and trapping | Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5).The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques | Entry |
| Laboratory testing | Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests | Entry |
| Certified and approved premises | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by a National Plant Protection Organization in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries | Entry |
| Sampling | According to ISPM 31, it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing. For inspection, testing and/or surveillance purposes the sample may be taken according to a statistically based or a non‐statistical sampling methodology | Entry |
| Phytosanitary certificate and plant passport | An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5)a) export certificate (import)b) plant passport (EU internal trade)To avoid the introduction of L. sativae EPPO (EPPO, 1990) recommends that propagating material (except seeds) of Capsicum, carnations, celery, chrysanthemums, Cucumis, Gerbera, Gypsophila, lettuces, Senecio hybridus and tomatoes from countries where the pest occurs must have been inspected at least every month during the previous 3 months and found free from the pests. A phytosanitary certificate should be required for cut flowers and for vegetables with leaves. | Entry |
| Certification of reproductive material (voluntary/official) | – | Entry |
| Surveillance | – | Entry |
3.6.3.2. Biological or technical factors limiting the effectiveness of measures to prevent the entry, establishment and spread of the pest
Minute size of all developmental stages of L. sativae
Mobility of adults
Egg and larval stages within and protected by plant tissue
Long pupal stage occurring in the soil
Control with insecticides is usually complicated by the insect's biology, including the ability of Liriomyza spp. to develop resistance to insecticides (Parrella, 1987).
3.6.3.3. Biological or technical factors limiting the ability to prevent the presence of the pest on plants for planting
Fast development time
High reproductive capability
3.7. Uncertainty
There are no uncertainties affecting the conclusions of this pest categorisation.
4. Conclusions
L. sativae satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. L. sativae does not meet the criteria of occurring in the EU for it to be regarded as a potential Union regulated non‐quarantine pest. Pest categorisation's conclusions are presented in the Table 8.
Table 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pests (Section 3.1) | The identity of Liriomyza sativae is well established and there are taxonomic keys available for its identification to species level | The identity of Liriomyza sativae is well established and there are taxonomic keys available for its identification to species level | |
| Absence/presence of the pest in the EU territory (Section 3.2) | L. sativae is not present in the EU | L. sativae is not present in the EU. Therefore, it does not fulfil this criterion to be regulated as a RNQP | |
| Regulatory status (Section 3.3) | The pest is listed in Annex IIA Commission Implementing Regulation (EU) 2019/2072 | There are no grounds to consider its status as a quarantine pest is to be revoked | |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | L. sativae could enter into, become established in, and spread within, the EU territory. The main pathways are: Fresh leafy hosts for consumption, cut branches, fruit and flowers with foliage, leafy plants for planting | Although adults can fly, natural spread is not considered its main dispersal mode but human‐assisted transport (including plants for planting) | |
| Potential for consequences in the EU territory (Section 3.5) | The pests’ introduction would most probably have an economic impact in the EU | Should L. sativae be present on plants for planting, an economic impact on its intended use would be expected | |
| Available measures (Section 3.6) | There are measures available to prevent the entry into, establishment within or spread of the pest within the EU (i.e. sourcing plants from PFA) | There are measures available to prevent pest presence on plants for planting (i.e. sourcing plants from PFA, PFPP) | |
| Conclusion on pest categorisation (Section 4) | All criteria assessed by EFSA above for consideration as a potential quarantine pest are met with no uncertainties | Although the criterion of plants for planting being the main means of spread for consideration as a RNQP is met, the criterion of the pest being present in the EU territory, which is a prerequisite for consideration as a potential RNQP, is not met | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | None | ||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PCR
polymerase chain reaction
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- RFLP
Restriction fragment length polymorphism
- RNQP
Regulated non‐quarantine pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 1995, 2017)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Greenhouse
The term ‘greenhouse’ is used in the current opinion as defined by EPPO (https://gd.eppo.int/taxon/3GREEL) as a walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment. A similar definition is also given in EFSA Guidance Document on protected crops (2014) https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2014.3615
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zones (PZ)
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
Appendix A – Host plants for Liriomyza sativae.
1.
| Host plant | Family | EPPO GD (accessed 17/11/2019) | CABI (accessed 17/11/2019) | Other sources |
|---|---|---|---|---|
| Abelmoschus esculentus (okra) | Malvaceae | Main | ||
| Allium | Liliaceae | Main | ||
| Amaranthaceae | Other | |||
| Amaranthus | Amaranthaceae | Wild/Weed | ||
| Amaranthus viridis | Amaranthaceae | Europhyt (this opinion) | ||
| Apium graveolens (celery) | Apiaceae | Minor | Main | |
| Arachis hypogaea (groundnut) | Fabaceae | Main | ||
| Artemisia dracunculus | Asteraceae | Europhyt (this opinion) | ||
| Aster | Asteraceae | Other | ||
| Beta vulgaris var. saccharifera (sugar beet) | Chenopodiaceae | Main | ||
| Bidens alba | Asteraceae | Weed (Capinera, 2017) | ||
| Brassica alboglabra | Brassicaceae | Europhyt (this opinion) | ||
| Brassica oleracea (cabbages, cauliflowers) | Brassicaceae | Main | ||
| Brassica rapa cultivar group Mizuna | Brassicaceae | Main | ||
| Brassica rapa subsp. rapa (turnip) | Brassicaceae | Main | ||
| Brassicaceae (cruciferous crops) | Main | |||
| Cajanus cajan (pigeon pea) | Fabaceae | Main | ||
| Capsicum (peppers) | Solanaceae | Main | ||
| Capsicum annuum (bell pepper) | Solanaceae | Minor | Main | |
| Cassia sp. | Fabaceae | Europhyt (this opinion) | ||
| Cestrum (jessamine) | Solanaceae | Other | ||
| Chrysanthemum | Asteraceae | Europhyt (this opinion) | ||
| Cicer arietinum (chickpea) | Fabaceae | Other | ||
| Citrullus lanatus (watermelon) | Cucurbitaceae | Main | ||
| Coriandrum sativum | Apiaceae | Europhyt (this opinion) | ||
| Cucumis | Cucurbitaceae | Minor | ||
| Cucumis melo (melon) | Cucurbitaceae | Minor | Main | |
| Cucumis sativus (cucumber) | Cucurbitaceae | Minor | Main | |
| Cucurbita (pumpkin) | Cucurbitaceae | Main | ||
| Cucurbita maxima (giant pumpkin) | Cucurbitaceae | Main | ||
| Cucurbita pepo (marrow) | Cucurbitaceae | Major | Main | |
| Cucurbitaceae (cucurbits) | Main | |||
| Dahlia hybrids | Asteraceae | Minor | ||
| Dahlia pinnata (garden dahlia) | Asteraceae | Other | ||
| Datura (thorn‐apple) | Solanaceae | Other | ||
| Daucus carota (carrot) | Apiaceae | Main | ||
| Dendranthema x grandiflorum | Asteraceae | Minor | ||
| Dendranthema x grandiflorum | Asteraceae | Minor | ||
| Fabaceae (leguminous plants) | Minor | Main | ||
| Dianthus sp. | Caryophyllaceae | Europhyt (this opinion) | ||
| Gypsophila sp. | Caryophyllaceae | Europhyt (this opinion) | ||
| Gossypium (cotton) | Malvaceae | Main | ||
| herbaceous ornamental plants | Minor | |||
| Indigofera (indigo) | Fabaceae | Other | ||
| Ipomoea sp. | Convolvulaceae | Europhyt (this opinion) | ||
| Lactuca sativa (lettuce) | Asteraceae | Main | ||
| Lathyrus | Fabaceae | Minor | Other | |
| Lathyrus odoratus (sweet pea) | Fabaceae | Main | ||
| Medicago sativa (lucerne) | Fabaceae | Minor | Main | |
| Melilotus (melilots) | Fabaceae | Other | ||
| Momordica charantia | Cucurbitaceae | Europhyt (this opinion) | ||
| Moringa oleifera | Moringaceae | Europhyt (this opinion) | ||
| Nicotiana tabacum (tobacco) | Solanaceae | Main | ||
| Ocimum basilicum (basil) | Lamiaceae | Main | ||
| Phaseolus (beans) | Fabaceae | Main | ||
| Phaseolus lunatus | Fabaceae | Minor | ||
| Phaseolus vulgaris (common bean) | Fabaceae | Minor | Main | |
| Phlox | Polemoniaceae | Other | ||
| Physalis (Groundcherry) | Solanaceae | Other | ||
| Pisum (pea) | Fabaceae | Main | ||
| Pisum sativum (pea) | Fabaceae | Minor | Main | |
| Raphanus sativus (radish) | Brassicaceae | Main | ||
| Ricinus communis (castor bean) | Euphorbiaceae | Minor | Other | |
| Solanaceae | Minor | Main | ||
| Solanum americanum | Solanaceae | Weed (Capinera, 2017) | ||
| Solanum lycopersicum (tomato) | Solanaceae | Major | Main | |
| Solanum melongena (aubergine) | Solanaceae | Minor | Main | |
| Solanum tuberosum (potato) | Solanaceae | Major | Main | |
| Solidago sp. | Asteraceae | Europhyt (this opinion) | ||
| Sorghum bicolor | Poaceae | Minor | ||
| Spinacia oleracea (spinach) | Chenopodiaceae | Minor | Main | |
| Symphyotrichum novi‐belgii | Asteraceae | Minor | ||
| Trifolium (clovers) | Fabaceae | Main | ||
| Trigonella foenum‐graecum | Fabaceae | Europhyt (this opinion) | ||
| Trigonella sp. | Fabaceae | Europhyt (this opinion) | ||
| Tropaeolum majus | Tropaeolaceae | Incidental | ||
| Vegetable plants | Minor | |||
| Vicia faba | Fabaceae | Minor | ||
| Vigna (cowpea) | Fabaceae | Minor | Main | |
| Zea mays (maize) | Poaceae | Main |
Appendix B – EU member state production of some L. sativae hosts
1.
EU28 crop production in standard humidity Eurostat (Area (cultivation/harvested/production) (1,000 ha) (accessed 11.11.2019)
Brassicas
| Area\year | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 countries | : | 273.77 | 273.01 | 279.9 | : |
| Austria | 1.76 | 1.64 | 1.57 | 1.53 | 1.44 |
| Belgium | 8.58 | 8.73 | 8.98 | 9.82 | 9.58 |
| Bulgaria | : | 2.11 | 3.03 | 1.85 | 2.13 |
| Croatia | 0.94 | 1.66 | 1.67 | 2.13 | 1.98 |
| Cyprus | 0.13 | 0.12 | 0.14 | 0.15 | 0.16 |
| Czech Republic | 1.68 | 1.71 | 1.77 | 1.64 | 1.47 |
| Denmark | : | 1.65 | 1.87 | 2.07 | 2.18 |
| Estonia | 0.3 | 0.3 | 0.28 | 0.29 | 0.38 |
| Finland | 1.27 | 1.22 | 1.21 | 1.49 | 1.46 |
| France | 26.89 | 26.09 | 26.23 | 26.39 | 26 |
| Germany | 19.53 | 18.7 | 18.8 | 20.09 | 18.84 |
| Greece | 9.73 | 7.15 | 6.32 | 5.89 | 6.22 |
| Hungary | 4.46 | 4.37 | 4.43 | 4.24 | 3.55 |
| Ireland | 1.9 | 1.9 | 1.82 | 1.68 | 1.78 |
| Italy | : | 30.26 | 29.74 | 29.81 | : |
| Latvia | 0.9 | 1 | 0.8 | 0.6 | 0.7 |
| Lithuania | 2.41 | 2.04 | 2.22 | 1.99 | 2.16 |
| Luxembourg | 0 | 0.01 | 0.01 | 0.01 | 0.03 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 10.08 | 9.65 | 10.27 | 11.14 | 10.85 |
| Poland | 43.3 | 44 | 39.98 | 40.69 | 41.58 |
| Portugal | 10.57 | 8.71 | 10.17 | 9.35 | 9.47 |
| Romania | 31.45 | 32.41 | 30.76 | 30.9 | 32.08 |
| Slovakia | 0 | 0.55 | 0.6 | 0.51 | 0.44 |
| Slovenia | : | 0.91 | 0.97 | 0.97 | 0.94 |
| Spain | : | 38.84 | 42.16 | 45.98 | 46.99 |
| Sweden | 1.18 | 1.18 | 1.2 | 1.4 | 1.38 |
| United Kingdom | 27 | 26.88 | 26 | 27.3 | 25.6 |
data not available.
Lettuces
| Area\year | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 | 96.03 | 93.95 | 91.19 | 91 | : |
| Austria | 1.41 | 1.32 | 1.45 | 1.39 | 1.31 |
| Belgium | 1.25 | 1.33 | 1.29 | 1.28 | 1.18 |
| Bulgaria | 0.29 | 0.18 | 0.12 | 0.29 | 0.24 |
| Croatia | 0.1 | 0.2 | 0.28 | 0.2 | 0.25 |
| Cyprus | 0.08 | 0.15 | 0.28 | 0.19 | 0.18 |
| Czech Republic | 0.18 | 0.14 | 0.15 | 0.59 | 0.62 |
| Denmark | 0.67 | 0.61 | 0.42 | 0.56 | 0.53 |
| Estonia | 0 | 0 | 0 | 0 | 0 |
| Finland | 0.65 | 0.65 | 0.7 | 0.59 | 0.67 |
| France | 8.96 | 8.84 | 8.86 | 8.6 | 8.43 |
| Germany | 6.7 | 6.56 | 6.5 | 7.09 | 6.93 |
| Greece | 4.76 | 3.67 | 3.56 | 3.29 | 3.31 |
| Hungary | 0.31 | 0.37 | 0.4 | 0.34 | 0.28 |
| Ireland | 0.3 | 0.3 | 0.31 | 0.26 | 0.26 |
| Italy | 19.78 | 18.58 | 15.67 | 15.66 | : |
| Latvia | 0 | 0 | 0 | 0 | 0 |
| Lithuania | 0.22 | 0.24 | 0.27 | 0.24 | 0.25 |
| Luxembourg | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 3.51 | 3.48 | 3.52 | 3.45 | 3.35 |
| Poland | 1.7 | 1.8 | 2.31 | 2.78 | 2.53 |
| Portugal | 2.42 | 2.15 | 2.18 | 2.28 | 1.93 |
| Romania | 0.15 | 0.16 | 0.15 | 0.14 | 0.15 |
| Slovakia | 0.2 | 0.04 | 0.02 | 0.02 | 0.01 |
| Slovenia | 0.67 | 0.73 | 0.75 | 0.74 | 0.71 |
| Spain | 33.87 | 34.31 | 35.65 | 34.51 | 33.67 |
| Sweden | 1.85 | 1.71 | 1.63 | 1.7 | 1.81 |
| United Kingdom | 6 | 6.43 | 4.7 | 4.8 | 4.8 |
data not available.
Tomatoes
| Area\Year | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 countries | 248.09 | 254.43 | 247 | 241.07 | 243.44 |
| Austria | 0.19 | 0.19 | 0.18 | 0.18 | 0.2 |
| Belgium | 0.51 | 0.51 | 0.51 | 0.52 | 0.55 |
| Bulgaria | 3.59 | 3.28 | 4.2 | 5.01 | 4.52 |
| Croatia | 0.32 | 0.42 | 0.37 | 0.45 | 0.49 |
| Cyprus | 0.21 | 0.27 | 0.22 | 0.26 | 0.26 |
| Czech Republic | 0.28 | 0.2 | 0.34 | 0.24 | 0.3 |
| Denmark | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 |
| Estonia | 0 | 0 | 0.01 | 0 | 0 |
| European Union – 28 countries | 248.09 | 254.43 | 247 | 241.07 | 243.44 |
| Finland | 0.11 | 0.11 | 0.11 | 0.11 | 0.1 |
| France | 5.83 | 5.69 | 5.65 | 5.75 | 5.74 |
| Germany | 0.33 | 0.33 | 0.34 | 0.37 | 0.4 |
| Greece | 17.26 | 15.25 | 14.01 | 13.32 | 16.02 |
| Hungary | 1.88 | 2.26 | 2.08 | 2.19 | 2.5 |
| Ireland | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Italy | 103.11 | 107.18 | 96.78 | 92.67 | 100.9 |
| Latvia | 0 | 0 | 0 | 0 | 0 |
| Lithuania | 0.54 | 0.49 | 0.57 | 0.55 | 0.57 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 1.78 | 1.76 | 1.78 | 1.79 | 1.79 |
| Poland | 13.5 | 13.8 | 12.42 | 12.64 | 13.11 |
| Portugal | 18.46 | 18.66 | 20.85 | 20.87 | 15.83 |
| Romania | 24.43 | 24.84 | 22.71 | 22.21 | 22.97 |
| Slovakia | 0.51 | 0.57 | 0.68 | 0.6 | 0.59 |
| Slovenia | 0.23 | 0.19 | 0.21 | 0.2 | 0.19 |
| Spain | 54.75 | 58.13 | 62.72 | 60.85 | 56.13 |
| Sweden | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| United Kingdom | 0.2 | 0.23 | 0.2 | 0.2 | 0.18 |
data not available.
Cucumbers
| Area\year | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 | 37.31 | 33.51 | 32.43 | 31.91 | : |
| Austria | 0.21 | 0.21 | 0.19 | 0.19 | 0.2 |
| Belgium | 0.04 | 0.04 | 0.06 | 0.06 | 0.06 |
| Bulgaria | 0.73 | 0.71 | 0.73 | 0.67 | 0.93 |
| Croatia | 0.14 | 0.13 | 0.16 | 0.11 | 0.09 |
| Cyprus | 0.22 | 0.2 | 0.2 | 0.19 | 0.19 |
| Czech Republic | 0.05 | 0.03 | 0.05 | 0.04 | 0.05 |
| Denmark | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 |
| Estonia | 0.1 | 0.1 | 0.09 | 0.1 | 0.1 |
| Finland | 0.96 | 0.09 | 0.08 | 0.08 | 0.1 |
| France | 1.56 | 1.56 | 1.64 | 1.71 | 1.68 |
| Germany | 0.33 | 0.34 | 0.37 | 0.37 | 0.39 |
| Greece | 2.34 | 1.85 | 1.85 | 1.88 | 1.89 |
| Hungary | 0.23 | 0.25 | 0.4 | 0.38 | 0.31 |
| Ireland | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Italy | 2.02 | 1.89 | 1.84 | 1.79 | : |
| Latvia | 0.1 | 0.1 | 0 | 0.1 | 0.1 |
| Lithuania | 1.17 | 0.96 | 1.13 | 1.08 | 1.11 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 0.6 | 0.55 | 0.54 | 0.6 | 0.59 |
| Poland | 10.6 | 10.1 | 9.49 | 9.19 | 9.17 |
| Portugal | 0.19 | 0.22 | 0.13 | 0.11 | 0.13 |
| Romania | 6.44 | 5.73 | 5.7 | 5.44 | 6.04 |
| Slovakia | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Slovenia | 0.08 | 0.06 | 0.06 | 0.06 | 0.06 |
| Spain | 8.9 | 8.1 | 7.44 | 7.48 | 7.5 |
| Sweden | 0.08 | 0.09 | 0.09 | 0.08 | 0.09 |
| United Kingdom | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
data not available.
Gourds and pumpkins
| Area\year | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 | : | : | : | : | : |
| Austria | 0.5 | 0.5 | 0.55 | 0.6 | 0.7 |
| Belgium | 0.21 | 0.3 | 0.51 | 0.51 | 0.46 |
| Bulgaria | 0 | 2.44 | 11.76 | 1.87 | 1.57 |
| Croatia | 0.09 | 0.27 | 0.16 | 0.21 | 0.14 |
| Cyprus | 0 | 0 | 0 | 0 | 0 |
| Czech Republic | 0 | 0 | 0 | 0 | 0 |
| Denmark | 0 | 0 | 0 | 0 | 0 |
| Estonia | 0 | 0 | 0 | 0 | 0 |
| Finland | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 |
| France | 3.85 | 3.83 | 4.08 | 4.31 | 4.21 |
| Germany | 3.23 | 3.49 | 3.99 | 4.48 | 4.15 |
| Greece | 0 | 0 | 0 | 0 | 0 |
| Hungary | 0.96 | 0.73 | 1.17 | 1.39 | 1.54 |
| Ireland | 0 | 0 | 0 | 0 | 0 |
| Italy | : | : | 0 | 0 | : |
| Latvia | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| Lithuania | 0.1 | 0.1 | 0.13 | 0.22 | 0.21 |
| Luxembourg | 0 | 0 | 0.01 | 0.02 | 0.01 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 0.29 | 0.82 | 0.82 | 0.93 | 0.76 |
| Poland | 1.1 | 1.3 | 1.34 | 1.66 | 1.69 |
| Portugal | 3.25 | 3.06 | 2.94 | 2.95 | 2.86 |
| Romania | 3.36 | 2.46 | 1.29 | 1.18 | 1.23 |
| Slovakia | 0 | 2.25 | 2.87 | 0.67 | 0.21 |
| Slovenia | : | : | : | : | : |
| Spain | 2 | 2.89 | 3.17 | 3.74 | 4.05 |
| Sweden | 0.12 | 0.12 | 0.12 | 0.19 | 0.2 |
| United Kingdom | 0 | 0 | 0 | 0 | 0 |
data not available.
Muskmelons
| Area\time | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 countries | 76.46 | 73.73 | 73.27 | 72.6 | : |
| Austria | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 |
| Belgium | 0 | 0 | 0 | 0 | 0 |
| Bulgaria | 0.48 | 0.66 | 1.75 | 2.67 | 2.77 |
| Croatia | 0.1 | 0.11 | 0.17 | 0.15 | 0.22 |
| Cyprus | 0.14 | 0.17 | 0.15 | 0.15 | 0.15 |
| Czech Republic | 0 | 0 | 0 | 0 | 0 |
| Denmark | 0 | 0 | 0 | 0 | 0 |
| Estonia | 0 | 0 | 0 | 0 | 0 |
| Finland | 0 | 0 | 0 | 0 | 0 |
| France | 14.1 | 14.02 | 14.17 | 14.16 | 13.41 |
| Germany | 0 | 0 | 0 | 0 | 0 |
| Greece | 4.72 | 4.22 | 3.91 | 4.03 | 3.74 |
| Hungary | 0.59 | 0.8 | 0.83 | 0.64 | 0.57 |
| Ireland | 0 | 0 | 0 | 0 | 0 |
| Italy | 25.03 | 24.8 | 24.72 | 24.17 | : |
| Latvia | 0 | 0 | 0 | 0 | 0 |
| Lithuania | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 0 | 0 | 0 | 0 | 0 |
| Poland | 0 | 0 | 0 | 0 | 0 |
| Portugal | 3.26 | 2.56 | 2.08 | 1.84 | 1.94 |
| Romania | 4.19 | 4.18 | 4.73 | 4.26 | 4.26 |
| Slovakia | 0.04 | 0.04 | 0.04 | 0.03 | 0.01 |
| Slovenia | 0 | 0.01 | 0.02 | 0.01 | 0.01 |
| Spain | 23.79 | 22.14 | 20.69 | 20.47 | 19.03 |
| Sweden | 0 | 0 | 0 | 0 | 0 |
| United Kingdom | 0 | 0 | 0 | 0 | 0 |
data not available.
Watermelons
| Area\Year | 2014 | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| European Union – 28 countries | 75.56 | 76.39 | 75.29 | 76.47 | : |
| Austria | 0 | 0 | 0 | 0 | 0 |
| Belgium | 0 | 0 | 0 | 0 | 0 |
| Bulgaria | 2.86 | 3.21 | 4.74 | 4.82 | 4.32 |
| Croatia | 0.69 | 0.61 | 0.68 | 0.68 | 0.97 |
| Cyprus | 0.6 | 0.53 | 0.47 | 0.44 | 0.43 |
| Czech Republic | 0 | 0 | 0 | 0 | 0 |
| Denmark | 0 | 0 | 0 | 0 | 0 |
| Estonia | 0 | 0 | 0 | 0 | 0 |
| Finland | 0 | 0 | 0 | 0 | 0 |
| France | 0.8 | 0.89 | 0.91 | 0.93 | 0.94 |
| Germany | 0 | 0 | 0 | 0 | 0 |
| Greece | 12.54 | 11.41 | 10.76 | 11.13 | 9.62 |
| Hungary | 6.12 | 6.02 | 5.41 | 5.27 | 5.09 |
| Ireland | 0 | 0 | 0 | 0 | 0 |
| Italy | 11.42 | 11.58 | 12.01 | 12.84 | : |
| Latvia | 0 | 0 | 0 | 0 | 0 |
| Lithuania | 0 | 0 | 0 | 0 | 0 |
| Luxembourg | 0 | 0 | 0 | 0 | 0 |
| Malta | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 0 | 0 | 0 | 0 | 0 |
| Poland | 0 | 0 | 0 | 0 | 0 |
| Portugal | 0.87 | 1.05 | 1.11 | 1.11 | 0.93 |
| Romania | 21.55 | 21.81 | 19.9 | 19.09 | 17.8 |
| Slovakia | 0.15 | 0.12 | 0.14 | 0.12 | 0.06 |
| Slovenia | 0 | 0.03 | 0.02 | 0.01 | 0.01 |
| Spain | 17.95 | 19.15 | 19.16 | 20.03 | 20.4 |
| Sweden | 0 | 0 | 0 | 0 | 0 |
| United Kingdom | 0 | 0 | 0 | 0 | 0 |
data not available.
Appendix C – Detailed global distribution of Liriomyza sativae on the base of EPPO Global Database
1.
| Continent | Country | State | Status |
|---|---|---|---|
| Africa | Cameroon | Present, no details | |
| Congo | Absent, unreliable record | ||
| Egypt | Present, no details | ||
| Ethiopia | Absent, unreliable record | ||
| Kenya | Present, no details | ||
| Morocco | Absent, unreliable record | ||
| Nigeria | Present, no details | ||
| South Africa | Absent, unreliable record | ||
| Sudan | Present, no details | ||
| Tanzania | Absent, unreliable record | ||
| Uganda | Absent, unreliable record | ||
| Zimbabwe | Present, restricted distribution | ||
| Americas | Antigua and Barbuda | Present, no details | |
| Argentina | Present, widespread | ||
| Bahamas | Present, restricted distribution | ||
| Barbados | Present, restricted distribution | ||
| Brazil | Present, restricted distribution | ||
| Ceara | Present, no details | ||
| Parana | Present, no details | ||
| Pernambuco | Present, no details | ||
| Rio de Janeiro | Present, no details | ||
| Rio Grande do Norte | Present, no details | ||
| Canada | Present, restricted distribution | ||
| Ontario | Present, no details | ||
| Chile | Present, restricted distribution | ||
| Colombia | Present, restricted distribution | ||
| Costa Rica | Present, no details | ||
| Cuba | Present, no details | ||
| Dominica | Present, no details | ||
| Dominican Republic | Present, no details | ||
| French Guiana | Present, no details | ||
| Guadeloupe | Present, no details | ||
| Jamaica | Present, restricted distribution | ||
| Martinique | Present, widespread | ||
| Mexico | Present, no details | ||
| Montserrat | Present, no details | ||
| Netherlands Antilles | Present, no details | ||
| Nicaragua | Present, no details | ||
| Panama | Present, no details | ||
| Peru | Present, restricted distribution | ||
| Puerto Rico | Present, no details | ||
| Saint Lucia | Present, no details | ||
| St Kitts‐Nevis | Present, no details | ||
| St Vincent and the Grenadines | Present, widespread | ||
| Suriname | Absent, unreliable record | ||
| Trinidad and Tobago | Present, no details | ||
| United States of America | Present, restricted distribution | ||
| Alabama | Present, no details | ||
| Arizona | Present, no details | ||
| Arkansas | Present, no details | ||
| California | Present, no details | ||
| Florida | Present, no details | ||
| Georgia | Present, no details | ||
| Hawaii | Present, no details | ||
| Indiana | Present, no details | ||
| Louisiana | Present, no details | ||
| Maryland | Present, no details | ||
| New Jersey | Present, no details | ||
| Ohio | Present, no details | ||
| Pennsylvania | Present, no details | ||
| South Carolina | Present, no details | ||
| Tennessee | Present, no details | ||
| Texas | Present, no details | ||
| Venezuela | Present, restricted distribution | ||
| Asia | Bangladesh | Present, widespread | |
| Cambodia | Absent, unreliable record | ||
| China | Present, widespread | ||
| Anhui | Present, no details | ||
| Fujian | Present, no details | ||
| Guangdong | Present, no details | ||
| Hainan | Present, no details | ||
| Hebei | Present, no details | ||
| Henan | Present, no details | ||
| Hunan | Present, no details | ||
| Shanxi | Present, no details | ||
| Sichuan | Present, no details | ||
| Yunnan | Present, no details | ||
| Zhejiang | Present, no details | ||
| India | Present, restricted distribution | ||
| Uttar Pradesh | Present, no details | ||
| Indonesia | Present, no details | ||
| Java | Present, no details | ||
| Iran | Present, widespread | ||
| Israel | Present, no details | ||
| Japan | Present, restricted distribution | ||
| Honshu | Present, restricted distribution | ||
| Kyushu | Present, restricted distribution | ||
| Ryukyu Archipelago | Present, restricted distribution | ||
| Jordan | Present, no details | ||
| Lao | Absent, unreliable record | ||
| Malaysia | Present, no details | ||
| West | Present, no details | ||
| Oman | Present, no details | ||
| Pakistan | Present, no details | ||
| Sri Lanka | Present, no details | ||
| Thailand | Present, restricted distribution | ||
| Uzbekistan | Present, restricted distribution | ||
| Viet Nam | Present, widespread | ||
| Yemen | Present, few occurrences | ||
| Europe | Belgium | Absent, intercepted only | |
| Croatia | Absent, confirmed by survey | ||
| Estonia | Absent, confirmed by survey | ||
| Finland | Absent, intercepted only | ||
| Lithuania | Absent, confirmed by survey | ||
| Netherlands | Absent, confirmed by survey | ||
| Poland | Absent, invalid record | ||
| Slovenia | Absent, no pest record | ||
| Turkey* | Present, restricted distribution | ||
| United Kingdom | Absent, intercepted only | ||
| Oceania | American Samoa | Present, widespread | |
| Australia | Present, restricted distribution | ||
| Queensland | Present, restricted distribution | ||
| Cook Islands | Present, restricted distribution | ||
| French Polynesia | Present, no details | ||
| Guam | Present, restricted distribution | ||
| Micronesia | Present, no details | ||
| New Caledonia | Present, restricted distribution | ||
| Northern Mariana Islands | Present, no details | ||
| Samoa | Present, widespread | ||
| Vanuatu | Present, no details |
Although Turkey is included in Europe, L. sativae has been reported only from Asian locations see Section 3.2.1.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques MA, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Czwienczek E, Streissl F and MacLeod A, 2020. Scientific Opinion on the pest categorisation of Liriomyza sativae . EFSA Journal 2020;18(3):6037, 37 pp. 10.2903/j.efsa.2020.6037
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00579
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe Lucien Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © EPPO
Adopted: 30 January 2020
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonised system to facilitate the management of plant and pest names in computerised databases, as well as data exchange between IT systems (Griessinger and Roy, 2015; EPPO, 2019a,b).
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. OJ L 319, 10.12.2019, p. 1–279.
See Section 2.1 on what falls outside EFSA's remit.
References
- CABI , 2019. Invasive species compendium. Avilable online: https://www.cabi.org/isc/datasheet/30960 [Accessed: 19 Novrmber 2019]
- Capinera JL, 2017. Featured Creatures: Liriomyza sativae Blanchard (Insecta: Dipetra: Agromyzidae). Available online: http://entnemdept.ufl.edu/creatures/veg/leaf/vegetable_leafminer.htm [Accessed: 21 Novemeber 2019].
- Chalfant RB, Jaworski CA, Johnson AW and Summer DR, 1977. Reflective film mulches, millet barriers, and pesticides: effects on watermelon mosaic virus, insects, nematodes, soil‐borne fungi, and yield of yellow summer squash. Journal of the American Society of Horticultural Science, 102, 11–15. [Google Scholar]
- Çıkman E and Civelek HS, 2005. Contributions to the leaf miner fauna from Turkey, with four new records. Phytoparasitica, 33, 391–396. [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO , 1990. Specific quarantine requirements. EPPO Technical Documents No. 1008.
- EPPO , 2005. Data sheets on Quarantine Pests. Liriomyza sativae.
- EPPO , 2019a. EPPO codes. Available online: https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO , 2019b. How to use the EPPO Global Database?. Available online: https://gd.eppo.int/media/files/general_user-guide_2019_09.pdf
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 09 January 2019].
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests.FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2016. ISPM (International standards for phytosanitary measures) No 27. Diagnostic protocols for regulated pests. DP 16: Genus Liriomyza. Available online: https://www.ippc.int/static/media/files/publication/en/2017/01/DP_16_2016_En_2017-01-30.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International standards for phytosanitary measures) No 5. Glossary of phytosanitary terms. Available online: https://www.ippc.int/en/publications/622/
- Griessinger D and Roy AS, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Hanna HY, Story RN and Adams AJ, 1987. Influence of cultivar, nitrogen, and frequency of insecticide application on vegetable leaf miner (Diptera: Agromyzidae) population density and dispersion on snap beans. Journal of Economic Entomology, 80, 107–110. [Google Scholar]
- de Jong Y, Verbeek M, Michelsen V, De Place Bjørn P, Los W, Steeman F, Bailly N, Basire C, Chylarecki P, Stloukal E, Hagedorn G, Wetzel FT, Glöckler F, Kroupa A, Korb G, Hoffmann A, Häuser C, Kohlbecker A, Müller A, Güntsch A, Stoev P and Penev L, 2014. Fauna Europaea ‐ all European animal species on the web. Biodiversity Data Journal, 2, e4034. 10.3897/BDJ.2.e4034. Available at https://fauna-eu.org/ [Accessed: 20 November 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Chen B, Wei JN and Liu TX, 2009. Roles of thermal adaptation and chemical ecology in Liriomyza distribution and control. Annual Review of Entomology, 54, 127–145. [DOI] [PubMed] [Google Scholar]
- Leibee GL, 1982. Development of Liriomyza trifolii on celery In: Schuster DJ. (ed.). Proceedings of IFAS‐Industry Conference on Biology and Control of Liriomyza leaf miners. Lake Buéna Vista, Florida: pp. 35–41. [Google Scholar]
- MacLeod A and Korycinska A, 2019. Detailing Koppen‐Geiger climate zones at a country and regional level: a resource for pest risk analysis. EPPO Bulletin, 49, 73–82. [Google Scholar]
- Malipatil M, Blacket M, Wainer J, Ridland P and Reviewer Jones DC (Subcommittee on Plant Health Diagnostics), 2016. National Diagnostic Protocol for Liriomyza trifolii– NDP27 V1. Available online: http://plantbiosecuritydiagnostics.net.au/resource-hub/priority-pest-diagnostic-resources/ [Accessed: 21 November 2019].
- Oatman ER and Michelbacher AE, 1959. The melon leaf miner Liriomyza pictella (Thomson) (Diptera: Agromyzidae).II. Ecological studies.. Journal of Economic Entomology, 52, 83–89. [Google Scholar]
- Orozco‐Santos M, Perez‐Zamora O and Lopez‐Arriaga O, 1995. Floating row cover and transparent mulch to reduce insect populations, virus diseases and increase yield in cantaloupe. Florida Entomologist, 78, 493–501. [Google Scholar]
- Parrella MP, 1987. Biology of Liriomyza . Annual Review of Entomology, 32, 201–224. [Google Scholar]
- Parrella MP, Robb KL and Bethke JA, 1981. Oviposition and pupation of Liriomyza trifolii (Burgess). See Ref., 87, 5a–55. [Google Scholar]
- Petitt FL, 1990. Distinguishing larval instars of the vegetable leaf miner Liriomyza sativae (Diptera: Agromyzidae). Florida Entomologist, 73, 280–286. [Google Scholar]
- Scheffer SJ and Lewis ML, 2005. Mitochondrial phylogeography of vegetable pest Liriomyza sativae (Diptera: Agromyzidae): Divergent clades and invasive populations. Annals of the Entomological Society of America, 98, 181–186. [Google Scholar]
- Seymour PR, 1994. Taxonomy and morphological identification. In: Final Report on EU Contract No. 90/399005 – Evaluation, development of rapid detection, identification procedures for Liriomyza species: taxonomic differentiation of polyphagous Liriomyza species of economic importance. EU, Brussels (BE).
- Sharma RK, Durazo A and Mayberry KS, 1980. Leaf miner control increases summer squash yields. California Agriculture, 34, 21–22. [Google Scholar]
- Spencer KA, 1973a. Agromyzidae (Diptera) of economic importance. Series Entomologica 9. The Hague, W. Junk. 418 pp.
- Spencer KA, 1973b. The Agromyzidae of Venezuela. Revista Facultad Agronómica, Maracay, 7, 5–107. [Google Scholar]
- Spencer KA, 1990. Host specialization in the world Agromyzidae (Diptera). Series Entomologica 45. Dordrecht, Netherlands, Kluwer Academic Publishers. 444 pp.
- Spencer KA and Steyskal GC, 1986. Manual of the Agromyzidae (Diptera) of the United States. USDA, ARS Agricultural Handbook 638. 478 pp.
- Webb RE and Smith FF, 1970. Survival of eggs of Liriomyza munda in chrysanthemums during cold storage. Journal of Economic Entomology, 63, 1359–1361. [Google Scholar]
- Zhao YX and Kang L, 2000. Cold tolerance of the leaf miner Liriomyza sativae (Dipt., Agromyzidae). Journal of Applied Entomology, 124, 185–189. [Google Scholar]
- Zitter TA and Tsai JH, 1977. Transmission of three potyviruses by the leaf miner Liriomyza sativae (Diptera: Agromyzidae). Plant Disease Reporter, 61, 1025–1029. [Google Scholar]


