Abstract
An alternative method for the production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products was assessed. The method is based on a pre‐cleaning process, acidic esterification/transesterification of tallow using 1.5% methanesulfonic acid w/w; 140°C; 5.5 bar absolute pressure (bara); 4 h, followed by fractional distillation. The application focuses on the capacity of the alternative method to inactivate prions. Given the limitations that biodiesel presents for direct measurement of prion infectivity, the BIOHAZ Panel considered, based on the outcome of previous EFSA Opinions and current expert evaluation, that a reduction of 6 log10 in detectable PrPS c signal would be necessary to consider the process at least equivalent to previously approved methods for Category 1 animal by‐products. This is in addition to the inactivation achieved by the pressure sterilisation method applied before the application of any biodiesel production method. Experimental data were provided via ad hoc studies commissioned to quantify the reduction in detectable PrPS c in material spiked with scrapie hamster strain 263K, as measured by western blot, for the first two steps, with distillation assumed to provide at least an additional 3 log10 reduction, based on published data. Despite the intrinsic methodological caveats of the detection of PrPS c in laboratory studies, the BIOHAZ Panel considers that the alternative method, including the final fractional distillation, is capable of achieving the required 6 log10 reduction of the strain 263K PrPS c signal. Therefore, the method under assessment can be considered at least equivalent to the processing methods previously approved for the production of biodiesel from all categories of animal by‐product raw materials. It is recommended to check the feasibility of the proposed HACCP plan by recording the main processing parameters for a certain time period under real industrial conditions.
Keywords: ABP, biodiesel, prion, TSE, esterification, transesterification
Summary
On 3 July 2019, the European Food Safety Authority (EFSA) received from the Irish Competent Authority (Department of Agriculture, Food and the Marine) the application (mandate and technical dossier) (EFSA‐Q‐2019‐00432) under Regulation (EC) No 1069/2009 and Regulation (EU) No 142/2011 referring to the evaluation of an alternative method for production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products, submitted by College Proteins (hereinafter referred to as the applicant).
The proposed new method, which would be applied after treatment of the feedstock using processing Method 1 (pressure sterilisation), involves three key parts: (i) a pre‐cleaning process for the removal of insoluble impurities in excess of 0.15% w/w. These cleaned liquids will then be pumped to the biodiesel tank farm where they are stored, as biodiesel feedstocks, until processing; (ii) an acidic esterification/transesterification (1.5% methanesulfonic acid (MSA) w/w; 140°C; 5.5 bar absolute pressure (bara); 4 h) for conversion of the cleaned feedstock into biodiesel; and (iii) fractional distillation: the biodiesel is fractionated (≥ 220°C; 10–35 milibara (mbara)) into multiple refined products, each containing carbon chains of a particular length, resulting in batches of biodiesel with differing specifications.
The material to be treated is tallow produced from Category 1, 2 and 3 animal by‐products plus raw material other than animal by‐products. Given the high resistance of prions to destruction and their high thermostability, the BIOHAZ Panel agreed with the approach used by the applicant of focusing on the capacity of the alternative method to inactivate transmissible spongiform encephalopathies (TSE) agents.
The applicant included in the dossier the output of a quantitative risk assessment of the residual bovine spongiform encephalopathy (BSE) risk in the treated material after the application of the proposed alternative method, taking into account both current disease prevalence and the pre‐testing of material contributing to the feedstock. The BIOHAZ Panel considers this to be outside the remit of the assessment of the alternative method as EFSA is only required to assess if the proposed method is at least equivalent (in terms of hazard reduction), for the relevant categories of animal by‐products, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1) of Regulation 1069/2009.
Two independent studies were carried out on behalf of the applicant: one on the reduction of detectable PrPSc due to acid‐catalysed esterification and transesterification only, and the second on the removal of PrPSc due to the pre‐cleaning phase together with the reduction of detectable PrPSc following acid‐catalysed esterification and transesterification.
Inactivation studies based on experimentally derived laboratory TSE strains, such as the hamster‐adapted scrapie strain 263K, and the use of Western blotting (WB) to assess the presence of detectable PrPSc following treatment, have been used in previous inactivation studies and have been considered acceptable in several EFSA Opinions (EFSA BIOHAZ Panel, 2015, EFSA BIOHAZ Panel, 2017) to qualify the safety of renewable fuels. This methodology is increasingly being considered suboptimal for predicting the resistance of natural TSE when they are subjected to such inactivating protocols, due to the limited analytical sensitivity and biological relevance of WB for the detection of prion infectivity. However, the experimental approach followed by the applicant is considered acceptable.
The prion reduction factor claimed by the applicant for the pre‐cleaning/acid esterification and transesterification steps combined was on average ≥ 4.28 log10, with the fractional distillation assumed to result in at least an additional 3 log10 reduction, based on published data. This is considered to be a realistic assessment of the overall achievable reduction factor of the evaluated method at industrial level as it avoids the need for any assumption of the additive impact of the individual pre‐cleaning and acidic esterification and transesterification steps, for which individual data sets were also provided.
Despite the inherent methodological caveats, the BIOHAZ Panel considers that the alternative method, including the final distillation, is capable of achieving the 6 log10 reduction factor as required. Therefore, the method under assessment can be considered at least equivalent to the processing methods previously approved for the production of biodiesel from all categories of animal by‐products (ABP) raw materials.
The applicant provided a detailed theoretical Hazard Analysis and Critical Control Point (HACCP) scheme that has not been verified in a full industrial‐scale plant. The CCPs designated in the HACCP plan are correctly addressed and the corrective actions proposed are appropriate. They cover the main hazardous events that could alter the risk of exposure to any prions that may be present. However, since no full‐scale equipment has been built so far, it is not possible to conclude on the feasibility of the given HACCP plan in a full‐scale plant, where records of the main parameters (e.g. time, pH, temperature) should be assessed for a certain period under real operating conditions.
The applicant provided a detailed description of the risks associated with the interdependent processes and of the procedures that would be implemented for dealing with these risks. The end product of the proposed alternative method is biodiesel. Considering the nature and foreseen uses of this final product, exposure of animals or humans to prions resulting from the interdependent processes and the intended end use of the product is not expected
1. Introduction
1.1. Background
On 3 July 2019, the European Food Safety Authority (EFSA) received from the Irish Competent Authority (Department of Agriculture, Food and the Marine) the application (mandate and technical dossier) (EFSA‐Q‐2019–00432) under Regulation (EC) No 1069/20091 and Commission Regulation (EU) No 142/20112 referring to the evaluation of an alternative method for production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products (ABP), submitted by College Proteins (hereinafter referred to as the applicant).
The applicant submitted an application as suggested in the procedure for authorisation of an alternative method of use or disposal of ABP or derived products, laid down in Article 20 of the Regulation (EC) No 1069/2009.
During the completeness check, performed according to Regulation (EC) No 1069/2009, it was noticed that some information was missing or incomplete. Therefore, the dossier could not be considered complete. On 14 August 2019, EFSA sent a letter to the applicant with three requests:
The applicant provided only one reference. The applicant was requested to send all the cited references as separate pdf format files. References should be provided in a dedicated folder ‘References’.
Confidential information: The applicant was requested to highlight which information should be considered confidential (in the text, in a report) and to fill in a table in attachment. Evidence has to be provided for each piece of information claimed confidential.
According to Article 20 of Regulation (EC) 1069/2009, EFSA shall receive from the Member State Competent Authority an evaluation report and the technical dossier submitted by the applicant. The evaluation report from the Member State was missing and the applicant was requested to submit it. EFSA also requested it from the Member State.
On 17 September 2019, the EFSA received the missing information on the application EFSA‐Q‐2019–00432, following its request dated 14 August 2019. After checking the content of the full dossier, EFSA considered that the application EFSA‐Q‐2019–00432 was valid on 8 October 2019. According to Regulation (EC) No 1069/2009, EFSA shall respect the deadline of 6 months to deliver a scientific opinion. Therefore, the scientific opinion must be delivered by 8 April 2020.
This application presents a new method for biodiesel production. Raw materials include ABP Category 1, 2 and 3 tallow, used cooking oil and fats, oils and greases. Additional feedstocks intended for use and that are not subject to EFSA approval include, but are not limited to, vegetable oils, edible oils and fatty acid distillates produced during the refinement of these oils.
According to Section 2.D, Chapter IV, Annex IV of Commission Regulation (EU) 142/2011, biodiesel production shall be carried out according to the following processing standards:
-
Unless fish oil or rendered fat are used which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No 853/20043, respectively, the fat fraction derived from ABP must be first processed using:
for Category 1 or 2 materials, processing Method 1 (pressure sterilisation) as set out in Chapter III;
for Category 3 materials, any of the processing methods 1–5 or processing method 7 or, for material derived from fish, processing methods 1–7 as set out in Chapter III.
-
The processed fat must then be processed further using one of the following methods:
a process in which the processed fat must be separated from the protein and for fat from ruminant origin, insoluble impurities in excess of 0.15% by weight must be removed, and the processed fat must be subsequently submitted to esterification and transesterification. However, esterification is not required for processed fat derived from Category 3 material. For esterification, the pH must be reduced to less than 1 by adding sulfuric acid (H2SO4) or an equivalent acid and the mixture must be heated to 72°C for at least 2 h during which it must be intensely mixed. Transesterification must be carried out by increasing the pH to about 14 with potassium hydroxide or with an equivalent base at 35–50°C for at least 15 min. Transesterification shall be carried out twice under the conditions described in this point using a new base solution. This process must be followed by refinement of the products, including vacuum distillation at 150°C, leading to biodiesel.
a process using equivalent process parameters authorised by the competent authority.
The proposed new method, which will be applied after treatment of the feedstock using processing Method 1 (pressure sterilisation), involves three key parts:
Pre‐cleaning: A pre‐cleaning process for the removal of insoluble impurities in excess of 0.15% w/w will occur. These cleaned liquids will then be pumped to the biodiesel tank farm where they will be stored, as biodiesel feedstocks, until processing.
Acidic esterification/transesterification: The feedstocks will undergo an acidic esterification and transesterification process (1.5% methanesulfonic acid (MSA) w/w; 140°C; 5.5 bara; 4 h) for conversion into biodiesel.
Fractional distillation: The biodiesel will be fractionated (≥ 220°C; 10–35 mbara) resulting in multiple refined products each containing carbon chains of a particular length. This results in batches of biodiesel with differing specifications.
As set out in Article 20 of Regulation (EC) No 1069/2009, EFSA is required to assess whether the method submitted ensures that any risks to public or animal health are reduced to a degree that is at least equivalent to that achieved by the processing methods that have already been approved for the same category of ABP.
2. Data and methodologies
2.1. Data
The data used in the assessment were provided by the applicant as requested in Annex VII of Commission Regulation (EU) No 142/2011 and its amendment by Commission Regulation (EU) No 749/20114. A process flow diagram and a HACCP plan were included in the application dossier as well as a description of risk reduction studies carried out on behalf of the applicant. The report submitted by the Competent Authority (CA) related to the application was also considered. Relevant scientific papers provided by experts of the Working Group (WG) were also considered during the assessment.
2.2. Methodologies
The EFSA Panel on Biological Hazards (BIOHAZ) evaluated the application for a new alternative biodiesel production process by individually assessing the following steps as set out in the ‘EFSA Scientific Opinion on the format for applications for new alternative methods for ABP’ (EFSA BIOHAZ Panel, 2010). These steps are:
full description of the process;
full description of the material to be treated;
hazard identification;
level of risk reduction;
HACCP plan;
risks associated with interdependent processes;
risks associated with the intended end use of the product.
The applicant is required to document as fully as possible the different aspects of each of these steps and, according to the CA, the application meets the requirements as laid down in the above‐mentioned EFSA Opinion.
As set out in Article 20 of European Union Regulation (EC) No 1069/2009, EFSA is required to assess whether the method submitted ensures that the risks to public or animal health are:
-
a)
‘controlled in a manner which prevents their proliferation before disposal in accordance with this Regulation or the implementing measures thereof’; or
-
b)
‘reduced to a degree which is at least equivalent, for the relevant categories of animal by‐products, to the processing methods laid down pursuant to point (b) of the first subparagraph of Article 15(1)’.
This requirement for applications is described in the Commission Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 and amended by Commission Regulation (EU) No 749/2011. According to point 2(d), Chapter II, Annex VII of Commission Regulation (EU) No 142/2011, any application for the evaluation of alternative methods shall:
‘show that the most resistant biological hazards associated with the category of materials to be processed are reduced in any products generated during the process, including the waste water, at least to the degree achieved by the processing standards laid down in this Regulation for the same category of animal by‐products. The degree of risk reduction must be determined with validated direct measurements, unless modelling or comparisons with other processes are acceptable’.
The validation requirements are further described on in the 2010 EFSA Opinion. According to the Opinion and to Chapter II, Annex VII of Commission Regulation (EU) No 142/2011 (point 3), validated direct measurements as referred to above shall mean:
-
‘measuring the reduction of viability/infectivity of endogenous indicator organisms during the process, where the indicator is:
consistently present in the raw material in high numbers,
not less resistant to the lethal aspects of the treatment process, but also not significantly more resistant, than the pathogens for which it is being used to monitor,
relatively easy to quantify and relatively easy to identify and to confirm;
using a well‐characterised test organism or virus introduced in a suitable test body into the starting material.’
The Opinion states that:
‘results should be accompanied by evidence’. Such evidence ‘includes, for measurements, information on the methodology used, nature of samples that have been analysed and evidence that samples are representative (e.g. number of samples, number of tests performed and selection of measuring points). If several treatment steps are involved, an assessment should be performed on the degree to which individual titre reduction steps are additive, or whether early steps in the process may compromise the efficacy of subsequent steps. In any case it is necessary to provide the sensitivity and specificity of the detection methods applied. Data on the repeatability and statistical variability of the measures obtained during the experiments should also be presented.’
It states also that:
‘Generally, the level of risk reduction for human and animal health which can be achieved by the process should be evaluated on the basis of direct measurements (validation).’
Should:
‘no direct measurements of the risk reduction be available (i.e. no validation as defined above is feasible), modelling or comparison with other processes may be acceptable if:
the factors leading to the risk reduction are well known;
the model of risk reduction is well established; and
continuous direct measurements of the factors leading to the risk reduction are provided for the full‐scale process which demonstrate that these factors are homogeneously applied throughout the treated batch.’
The risk reduction achieved as a result of the standard processing methods of Category 1, 2 and 3 ABP materials, as described in the Regulation, is not specified. So, there are no definitive specific risk reduction standards for proposed alternative methods for biodiesel production using ABP. However, in a previous EFSA Opinion (EFSA BIOHAZ Panel, 2017) dealing with proposed alternative processing methods for Category 1 ABP, the BIOHAZ Panel decided that a reduction of 6 log10 in prion infectivity by the alternative method is required to consider it at least equivalent, for Category 1 ABP, to the processing methods laid down in the legislation. This is in addition to the inactivation achieved by the pressure sterilisation method (Method 1) before the application of the alternative method. The BIOHAZ Panel decided to use the same standard in the evaluation of the current application.
3. Assessment
3.1. The alternative method as proposed by the applicant
The description presented in this chapter has been extracted verbatim from the application, with some editorial changes to summarise the technical procedures.
The alternative method consists of three main steps:
Step One: Raw materials that have already been treated according to Method 1 will undergo a cleaning process for the removal of solids, particulates and phospholipids. The raw materials will then be pumped to the biodiesel tank farm where they will be stored, as biodiesel feedstocks, until processing.
Step Two: Cleaned feedstocks will undergo an acidic esterification and transesterification process for complete conversion into biodiesel.
Step Three: The final step in the biodiesel production process is fractional distillation, which separates the biodiesel product into fractions containing defined length carbon chains. This results in batches of biodiesel with differing specifications.
3.1.1. Mandatory preliminary treatment
According to point D, Section 2, Chapter IV, Annex IV of Regulation 142/2011 as amended, a fat fraction derived from ABP of all categories may be used for the production of biodiesel. Category 1 or Category 2 materials must be first processed using processing Method 1 (pressure sterilisation) as set out in Chapter III of the same Annex.
According to the application, all Category 1, 2 and 3 materials either produced by College Proteins or another rendering company, or collected from the hospitality sector, will be sterilised according to Method 1 (20 min, 133°C, 3 bara).
The raw material (Category 1, 2 and/or 3) is pumped into the steriliser. Pressure is increased to 3 bara. Temperature is increased to 133°C. Temperature and pressure are maintained for a retention time of 22 min without interruption. After that, pressure is reduced to atmospheric pressure. Sterilised raw material is discharged to a designated feedstock storage tank in the biodiesel tank farm.
3.1.2. Step one: pre‐cleaning process
According to the application, the sterilised raw material and bentonite are mixed using an agitator as the first step in the process of precipitating solids so the resulting oil has less than 0.15% w/w solid impurities. The mixture is then pumped to the tricanter feed tank where it is split into three phases: solids (including bentonite), water and oil. The oil fraction is pumped into a separator where the addition of water and phosphoric acid occurs. The separator again separates the material into three components: solids (including phospholipids), water and clean oil. This clean oil is the raw material for the esterification and transesterification stage of the process. A flow chart of the sterilisation and pre‐cleaning process is provided in Figure A.1 of Appendix A.
Figure A.1.
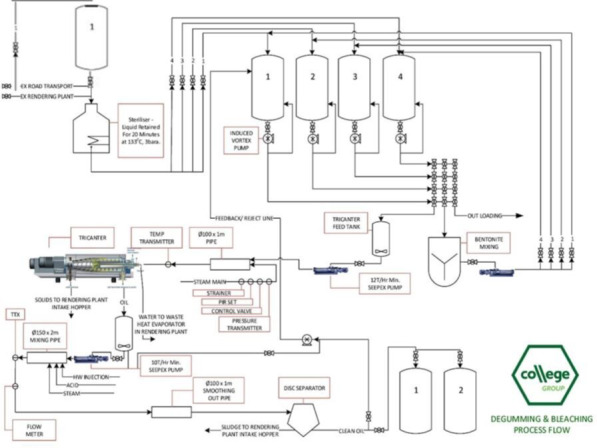
Schematic of the sterilisation and pre‐cleaning process for the removal of solids and impurities from the biodiesel raw materials
3.1.3. Step two: acidic esterification and transesterification
The esterification of fatty acids and the transesterification of triglycerides with methanol are carried out according to the reaction equations listed below. This is an acid‐catalysed equilibrium reaction.
Esterification:
Chemical Formula:
Transesterification:
Chemical Formula:
The following is a reduced summary of the process steps as described in the application: (see Figure 1)
The feedstock is fed into two batch reactor vessels where it is pre‐heated.
The catalyst (MSA 99%; 1.5% w/w) and methanol (≥ 3%) are added to the reactor vessels.
A circulation pump re‐circulates and mixes the contents of the tank.
The esterification/transesterification reaction is carried out at a minimum temperature of 140°C and an operating pressure in the reactor of minimum 5.5 bara with a minimum retention time of 4 h.
Excess methanol (and the water created by the reaction) are continuously removed. The methanol and water are separated using condensation, and the recovered methanol is added back into the reaction.
The crude ester is then pumped out of the reactors into the settlement tanks after a minimum residence time of 4 h.
Biodiesel and the glycerol/water mix are pumped out of the settlement tanks, from where the biodiesel (FAME) is sent for further processing in the fractional distillation system into various C‐fractions (determined by the length of their carbon chains) and the glycerol/water mix is pumped to the tank farm before being transported off‐site as a by‐product.
Figure 1.
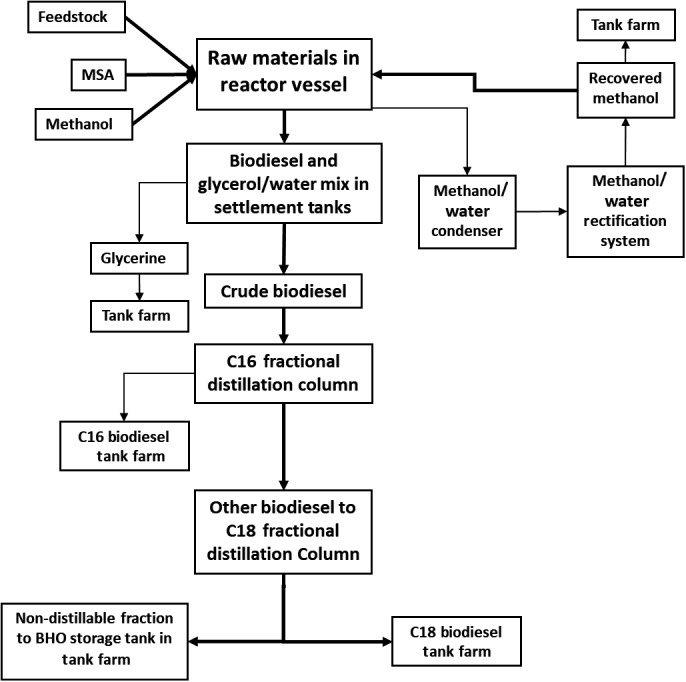
Acid‐only esterification and transesterification process for the production of biodiesel as in the application
3.1.4. Step three: FAME fractional distillation
The following is a reduced summary of the process steps as described in the application (see Figure 1):
The FAME mixture is pumped over a preheater and dryer into the C16 fractionation column to obtain 16C‐FAME molecules.
The system operates at a vacuum of lower than 35 mbara and a temperature in the circulating sump product of ≥ 220°C. Re‐distillation is required to effectively separate the C16 and C18 FAME.
The distillate, which is enriched mainly with C16 FAME and contains a maximum of 10% C18 FAME, is pumped directly to designated C16 biodiesel product tanks in the tank farm.
The FAME, enriched with C18, is discharged from the C16 fractionation column and fed to the C18 distillation column. The system operates at a vacuum of lower than 30 mbara and a temperature in the circulating sump product of ≥ 220°C.
The ascending FAME vapours pass through a structured packing to reach the main condenser. The FAME vapours are condensed in the C18 condenser and collected in a storage tank.
The C18 fractionation column output is transferred to a third column for increasing the yield of the process and pumped directly to C18 biodiesel product tanks.
The non‐distillable components [bio‐heating oil (BHO)] are collected from the tank bottom and sent to the tank farm.
Table 1 shows the comparison of the processing parameters proposed in the alternative method with those included in the legislation.
Table 1.
Comparison of processing parameters of the alternative method and the approved in the legislation
| Regulation (EU)142/2001 – Chapter IV, Section 2 D | Application | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Process | pH | Temperature | Pressure | Time | Final product | Process | pH | Temperature | Pressure | Time | Final product |
| Pressure sterilisation (Method 1) | Not specified | 133°C | 3 bar | 20 min | Pressure sterilisation (Method 1) | Not specified | 133°C | 3 bar | 22 min | ||
| Removal of insoluble impurities | Not specified | Not specified | Not specified | Not specified | Cleaned raw material with solid content < 0.15% w/w | Pre‐cleaning (bentonite adsorption and phosphoric acid precipitation) | Not specified | Min 98°C | Not applicable | 30 min continuously recirculation | Cleaned raw material with solid content < 0.15% w/w |
| Esterification | Less than 1 by adding sulfuric acid or equivalent | 72°C | Not specified | Min 2 h mixed intensely | FAME (biodiesel) | Esterification/transesterification | Not specified | 140°C | 5.5 bara | Min 4 h | FAME (biodiesel) |
| Trans‐esterification | Increasing to 14 by adding potassium hydroxide or equivalent | 35–50°C | Not specified | Min 15 min, performed twice with new base solution | |||||||
| Vacuum distillation | Not specified | 150°C | Not specified | Not specified | Glycerine and FAME (biodiesel) | Fractional distillation | Not specified | ≥ 220°C | 30/35 mbara | Not specified | Glycerine and FAME (biodiesel) |
3.2. Material to be treated
3.2.1. Material to be treated as described by the applicant
Tallow is produced from the heating, filtration and sterilising of Category 1, 2 and 3 ABP in compliance with Regulation (EU) No 1069/2009.
The following is a description of the material to be treated as described in the application.
3.2.1.1. Tallow derived from Category 1 material
Category 1 tallow is produced from animals or ABP presenting a TSE risk, including specified risk material. This material includes ABP presenting a TSE risk or unknown risk, or a risk related to treatment with illegal substances or to environmental contaminants. Intake material can include whole animals or parts of animals. This material is high risk. Please see Category 1 tallow specifications in Table 2 below.
Table 2.
Specifications of tallow
| Parameter | Critical control limit |
|---|---|
| Free fatty acids a | < 25% |
| Insoluble impurities | < 0.15% |
| Polyethylene | < 200 ppm |
| Moisture | < 1% |
| Glycerol triheptanoate (GTH) | 250 ppm |
The free fatty acid (FFA) and insoluble impurity critical control limits listed are specific to Category 1 tallow produced by the applicant. The polyethylene and moisture levels are dictated by the customer and the GTH levels are dictated by the Department of Agriculture, Food and the Marine of Ireland.
3.2.1.2. Tallow derived from Category 3 material
Category 3 tallow includes material which was previously found ‘fit for human consumption’, including catering waste, raw meat and fish, hides and skins; parts of slaughtered animals which are fit for human consumption but which are not intended for human consumption for commercial reasons, or due to problems of manufacturing or packaging defects; ABP derived from the processing of products intended for human consumption (e.g. degreased bones and greaves); and blood from healthy ruminants.
3.2.1.3. Used cooking oil (UCO)
Used cooking oil (UCO) is vegetable/seed/animal oil that has been used to cook foodstuff for human consumption. Article 1 point 22 of Commission Regulation (EC) No. 142/2011 describes UCO as catering waste and classifies it as a Category 3 waste product. Since this raw material is used to cook meat for human consumption, the exposure of humans to prions is not expected.
UCO imported from a non‐member country regulated in accordance with Commission Regulation (EU) No 142/2011 (Annex XIV, Chapter 1) must be sourced from one of the countries on the ‘Approved List’ in Annex II of Commission Regulation (EU) No 206/20105.
3.2.1.4. Raw materials other than animal by‐products
All fatty acid distillates (FAD) produced as by‐products, wastes or residues during the refinement of vegetable and seed oils are suitable for conversion to biodiesel using the acidic esterification and transesterification process. Palm FAD, for example, is a processing residue from the refining of food‐grade palm oil for use in the food industry. Additionally, fresh, virgin cooking oils themselves are viable options as raw materials for the process. Virgin cooking oils include vegetable and seed oils extracted from seeds or plants. The same as animal fats, they are a mixture of triglycerides. Potential candidates include rapeseed oil, sunflower oil, soybean oil and vegetable oil.
3.2.2. BIOHAZ Panel assessment of the material to be treated
The materials intended to be used for the production of biodiesel using the proposed alternative method are described in detail by the applicant. Category 1 ABP tallow, pre‐treated according to Method 1, with a maximum level of insoluble impurities of 0.15%, is the highest risk material used, due to the possible presence of the abnormal prion protein, PrPSc. Other ABP materials used as feedstock are Category 3 ABP tallow and UCO. In addition, other non‐ABP raw materials, such as by‐products from the refinement of vegetable and seed oils, are also used.
3.3. Hazard identification
3.3.1. Hazard identification as provided by the applicant
According to the application, the highest level of risk is associated with use of raw materials derived from Category 1 ABP. The key hazard associated with this category of raw materials is the abnormal prion protein, PrPSc and the associated risk of transmission of TSE. Category 1 material can contain bacterial spores and viruses but as the process is considered suitable for the inactivation of prions, all other microorganisms are believed to be inactivated in line with the reasoning presented in the EFSA Scientific Opinion of 2017 (EFSA BIOHAZ Panel, 2017).
3.3.2. BIOHAZ Panel assessment of the hazard identification
The method proposed by the applicant is suitable for all categories of animal fats to be used as feedstock. Category 1 material can contain various prion strains known to cause classical and atypical forms of both bovine spongiform encephalopathy (BSE) and scrapie. Category 1 material can also contain a wide range of other biological hazards (including some highly heat‐resistant bacterial spores and viruses). A wide range of bacterial, viral, parasitic, protozoan and fungal pathogens can also be found in Category 2 and Category 3 materials. Given their high resistance to destruction, and, in particular, the high thermostability of prions (Somerville et al., 2009), it is assumed that if the alternative method ensures the inactivation of the prions, then all microorganisms, including spore‐forming bacteria and thermo‐resistant viruses, will be completely inactivated. Therefore, the BIOHAZ Panel agrees with the approach used by the applicant of focusing on the capacity of the alternative method to reduce the risk associated with prions.
3.4. Level of risk reduction
3.4.1. Level of risk reduction as provided by the applicant
Two independent studies were carried out on behalf of the applicant: (1) Reduction of PrPSc signal due to acid‐catalysed esterification and transesterification (study 1); and (2) Reduction of PrPSc signal due to the combined effects of pre‐cleaning and acid‐catalysed esterification and transesterification (study 2). The description presented in the current section has been extracted verbatim from the application with minor editorial changes for clarity purposes.
To obtain indicative data on the clearance of prions by the pre‐cleaning process, the applicant commissioned a laboratory‐scale facsimile experiment of the industrial pre‐cleaning process, as well as an evaluation of the acid esterification and transesterification process itself, using hamster 263 K prions and WB of prion protein as a surrogate for infectivity measurements (circumventing animal bioethics objections and toxicity issues).
According to the results of the studies, the acidic esterification and transesterification process alone demonstrated a reduction factor of ≥ 3.58 log10 of the abnormal protein, PrPSc (Table 3). The applicant argued that the results of the two independent studies, in part contribute towards addressing the query on the degree to which individual titre reduction steps are additive. In Table 4, it can be seen that the pre‐cleaning process alone results in a ≥ 2.98 log10 reduction in abnormal prion protein, whereas the acidic esterification and transesterification process alone results in a reduction factor of ≥ 3.58 log10. In Table 4, it is shown that when the entire process is evaluated with a prion spiked into the tallow before pre‐cleaning and a final titre is calculated for the biodiesel post‐acidic esterification and transesterification, an overall reduction factor for the entire process of ≥ 4.28 log10 (average) is achieved. This demonstrates that the addition of the second processing step does result in an additive effect and a further lowering of the abnormal prion protein level.
Table 3.
Reduction factors of the abnormal prion protein PrPSc for study 1
| Reduction factors [log10] | Run 1 | Run 2 |
|---|---|---|
| Reduction factor of combined glycerol and biodiesel after 2 h of transesterification vs. spiked starting material (SSM) | ≥ 3.38 | ≥ 3.38 |
| Reduction factor of glycerol fraction after 4 h of transesterification vs. SSM | ≥ 4.29 | ≥ 4.61 |
| Reduction factor of biodiesel fraction after 4 h of transesterification vs. SSM | ≥ 3.59 | ≥ 3.58 |
Table 4.
Reduction factors of the abnormal prion protein PrPSc for study 2
| Reduction factors [log10] | Run 1 | Run 2 |
|---|---|---|
| Reduction factor of supernatant after phosphoric acid precipitation vs SSM | ≥ 2.98 | ≥ 2.98 |
| Reduction factor of combined glycerol and biodiesel after 4 h of esterification/transesterification vs. SSM | ≥ 3.27 | ≥ 3.20 |
| Reduction factor of biodiesel fraction after 4 h of esterification/transesterification vs. SSM | ≥ 4.32 | ≥ 4.25 |
The applicant concluded that these investigations and conclusions are limited by the dynamic range of the assay with the ‘≥’ symbol indicating that the analysis has reached the limit of quantitation of the assay. Therefore, it is not possible to conclude definitively if the two process steps are entirely additive. It is important to note, however, that the individual titre reduction steps are three independent physicochemical mechanisms: (1) phase separation (adsorption to bentonite and distillation); (2) chemical treatment (acidification); and (3) heat (140°C reflux).
In each study, the experiment (facsimile of the industrial process) was conducted twice on two separate days. The facsimile experiments also provided information in relation to the level of prion clearance observed in the glycerol phase of the end product. In Table 3, according to the applicant, it can be seen that the acidic esterification and transesterification process reduces the risk associated with the glycerol produced during the biodiesel process. An average reduction factor of ≥ 4.45 log10 was achieved for the glycerol sample at 4 h retention time.
The applicant quoted the previous EFSA BIOHAZ Panel (2017), on the reduction factor achieved by fractional distillation, representing the last step of the process:
‘The distillation of biodiesel under vacuum at 150°C is already part of the approved biodiesel production process according to Commission Regulation (EU) No. 142/2011 Chapter IV Section 2. According to the applicant, Mittelbach et al. (2007) showed that such distillation has a reduction factor of at least 103 (3 log10) for TSE infectivity. As proteins are not volatile under the conditions of distillation, it was assumed that they remained in the distillation residue. Mittelbach et al. (2007) found no traces of prion protein in either the distilled samples or in the distillation residue. Therefore, the reported effect on the level of risk reduction was considered realistic’.
On the basis of the conclusion in the 2017 EFSA Opinion that a reduction of 3 log10 for the fractional distillation step of the applicant process is realistic, the applicant has used this reduction factor for the fractional distillation step of the process under review.
3.4.2. BIOHAZ Panel assessment of the level of risk reduction
3.4.2.1. Assessment of the residual BSE quantitative risk assessment (QRA)
Apart from the experimental studies described above, the applicant included in the dossier the output of a quantitative risk assessment (QRA) of the residual BSE risk in the treated material after applying the proposed alternative method.
When dealing with the factors relevant to the QRA of the hazard in the process feedstock, a direct reference has been made to the recent update of the EFSA QRA of the BSE risk posed by processed animal proteins (EFSA BIOHAZ Panel, 2018). Based on that, the applicant presented an estimate of worst‐case residual risk in biodiesel after its production through the acid‐only esterification and transesterification process. In the application, the following input factors are considered in the QRA: estimates of the prevalence of BSE in the source herds, the amount of prion protein in each animal, the number of animals in each batch of tallow feedstock and the level of inactivation of bovine prions achieved by the processing.
In the context of this assessment, the reference to the prevalence of BSE within the total amount of rendered material is not relevant. As explained by the applicant, in the worst‐case scenario, the probability of a clinical case of BSE in a batch (obtained by applying the hypothetical detection limit of the passive surveillance to the size of the batch per rendering run) is not used as an input for the subsequent steps. The assessment starts with the main assumption of the inclusion of at least one infected cow in a batch size of 400 cattle, and the population prevalence is disregarded. The same initial assumption is also made when a ‘more likely’ scenario was compared with the ‘worst‐case’ scenario. So, the shift from the ‘worst’ to ‘more likely’ case scenario is not based on a different prevalence scenario but on a different and more realistic partition (100 times less) of the infectivity in tallow.
As the required focus of this assessment is to evaluate whether the public and animal health risks are reduced to a degree which is at least equivalent, for the relevant categories of ABP, to the approved methods, it is not relevant to consider the initial probability of a clinical case in a batch and, therefore, the QRA outputs provided by the applicant are not considered in the assessment of this application. Instead, the assessment focuses on whether the proposed alternative process is able to achieve the 6 log10 reduction in prion infectivity or, as a proxy, detectable PrPSc, required to consider it at least equivalent, for Category 1 ABP, to the biodiesel production method laid down in the legislation.
3.4.2.2. BIOHAZ Panel assessment of the PrPsc signal reduction
Inactivation studies based on experimentally derived laboratory strains, such as the hamster‐adapted scrapie strain 263K, and with the use of WB to assess the presence of detectable PrPSc following treatment are increasingly being considered suboptimal as predictors of the resistance of natural TSEs when they are subjected to such inactivating protocols (see Appendix B) due to the inherent limited analytical sensitivity and biological relevance of WB for the detection of prion infectivity. However, similar methods have been used in previous inactivation studies and official guidelines (EMA, 2019). The latter acknowledges that most studies, aimed at following the partition/removal of PrPSc and/or infectivity during plasma fractionation processes, also use:
‘rodent‐adapted TSE agent (263K hamster strain) brain homogenate and microsomal brain fractions as a spike and rely on direct [PrPSc] immunodetection tools (western blot or conformation‐dependent immunoassay) to demonstrate a drop in the TSE agent content in processed fractions and on bioassay infectivity measurements to confirm the results’.
This approach has been considered acceptable in previous EFSA Opinions (EFSA BIOHAZ Panel, 2015, 2017) to assess the safety of renewable fuels.
The prion reduction factor claimed by the application for the pre‐cleaning/acid esterification and transesterification steps combined is on average ≥ 4.28 log10. This is considered to be a realistic assessment of the overall achievable reduction factor of the evaluated method at industrial level since it avoids the need for any assumption of the additive impact of the individual pre‐cleaning and acidic esterification and transesterification steps.
Based on previous studies, the final process step (fractional distillation) has been assumed to have an additional 3 log10 reduction (Mittelbach et al., 2007). In the process described in this application, the conditions of the distillation (≥ 220°C at 30–35 mbara) are higher than those required by the legislation (vacuum distillation at 150°C). It cannot be assumed that a more aggressive treatment would necessarily achieve an increased reduction in infectivity. As stated in a previous EFSA opinion (EFSA BIOHAZ Panel, 2015), there is no consistent linear relationship between increasing temperature and decreasing PrPSc infectivity. Nevertheless, it can be expected that the proposed fractional distillation will achieve at least the same level of reduction as reported elsewhere.
The applicant did not provide any data about the level of inactivation achieved by the manufacturing process on the naturally occurring cattle prion strains that might be present in the raw material entering the industrial process (i.e. C‐BSE, L‐BSE and H‐BSE).
The methodological caveats described in Appendix B may cause an under‐/overestimation of the actual level of reduction. Despite this, the BIOHAZ Panel considers that the reduction factor demonstrated for the pre‐cleaning, and acid esterification and transesterification steps of this process, added to the reduction expected from the final distillation step, reaches the required minimum of 6 log10 estimated to be achieved by the existing methods when assessed using similar models, although direct extrapolation of this outcome to naturally occurring TSE agents may not be appropriate. It is reassuring, nevertheless, that new data relating specifically to BSE strains (Chapman et al., 2020) indicate that the reduction in infectivity achievable by Method 1 (pressure sterilisation) is greater than the previously reported 3 log10 by at least one log10 cycle. This provides a safety margin when considering the overall inactivation achieved in alternative ABP processing methods where Method 1 is requested, such as in the biodiesel production process.
3.5. HACCP plan
3.5.1. HACCP plan and CCPs, as provided by the applicant
The applicant provided a theoretical HACCP plan. The plan was in accordance with the Codex Alimentarius General Principles of Food Hygiene and it identified the main hazards and the risks associated with each (considering the likelihood of occurrence and the severity of the possible impact). Specific control measures were also included which contained the interventions/steps considered to be CCPs according to the applicant. Finally, a list of proposed verification methods and corrective actions to be taken when monitoring indicates that CCPs are not under control were included. A summary of the critical control points, critical limit parameters and corrective actions included in the HACCP plan submitted by the applicant are displayed in Table 5. A flow chart showing the critical control points identified in the HACCP plan by the applicant is displayed in Figure A.2 of Appendix A.
Table 5.
Summary of the critical control points, critical limit parameters and corrective actions included in the HACCP plan submitted by the applicant
| CCP no. | Critical point | Control parameters | Critical limits for parameters | Corrective action (summary) | Records | Verification |
|---|---|---|---|---|---|---|
| 1 | Raw material reception | Identification, weight, documentation | Absolute | Hold material until all correct | Daily intake sheet, Weighbridge management system | Sign off |
| 2 | Raw material steriliser | Temperature, pressure, time | ≥ 133°C, 3 bar, 20 min | Repair and repeat cycle | Programmable Logic Controller (PLC) Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 3 | Bentonite treatment area | Mass, treatment time, mixing speed, temperature | ≥ 6% w/w, ≥ 30 min, ≥ 300 rpm, ≥ 98°C | Repair and repeat cycle | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 4 | Tricanter | Speed and retention time | ≥ 3,000 g, ≥ 2 min | Repair and repeat if target not met | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 5 | Phosphoric acid treatment area | Volume, temperature, time | ≥ 0.5% v/v, ≥ 98°C, ≥ 2 min | Repair and repeat process | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 6 | Separator | Speed | 10,000 g | Suspension of process and repair | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 7 | Tallow testing area | Residual solids | ≤ 0.15% w/w | Repeat process | Laboratory results | Laboratory quality manager |
| 8 | FAME conversion reactor | Mass of tallow, MSA, methanol and pressure, temperature, time | kg, ≥ 1.5% w/w, ≥ 3% w/w and ≥ 5.5 bara, ≥ 140°C, ≥ 240 min | Process suspended, restarted or batch might be rejected | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 9 | Fractionation system C16 | Pressure, temperature | < 35 mbar, ≥ 220°C | Repair and repeat cycle | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
| 10 | Fractionation system C18 | Pressure, temperature | < 30 mbar, ≥ 220°C | Repair and Repeat Cycle | PLC Visual, PLC contemporaneous, Historical, Data Logger | Production manager |
Figure A.2.

Critical control points identified in the HACCP by the applicant
According to the application, all critical parameters associated with the process are measured and controlled by purpose‐specific probes and technological devices that are calibrated and serviced according to a strict preventative maintenance schedule as dictated by the manufacturers and described in the technical data sheets for the equipment. All steps of the production process and all key process parameters are continuously monitored and recorded by a Programmable Logic Controller (PLC) system. One‐off measurements such as batch weight are also measured and recorded by the PLC system. The PLC system will issue an alert if any deviation from the specified parameters is detected and will enter stand‐by mode. If specific parameters are not achieved, the batch will be rejected and assessed either for reprocessing or disposal by incineration. The following critical control points are included in the HACCP plan proposed by the applicant:
3.5.1.1. CCP 1: Raw Material Intake
Before the material is discharged, it undergoes a documentation check and is weighed. If it is not possible to correctly identify a load of raw material or correctly complete the accompanying commercial documentation, the load will be processed as Category 1 raw material.
3.5.1.2. CCP 2: Raw material steriliser
After discharge, the raw material is pumped into a holding tank. From there, it is fed to the raw material steriliser. The temperature is increased to 133°C and the pressure to 3 bar. The temperature and pressure must be maintained at this level for at least 22 consecutive uninterrupted minutes. The raw material is circulated inside the vessel during sterilisation to ensure proper exposure to the sterilisation conditions. If there is failure in the process for any reason, the steriliser will then automatically recommence the batch from time zero.
3.5.1.3. CCP 3/4/5/6: Bentonite treatment, tricanter, phosphoric acid treatment and separator (pre‐cleaning)
The raw materials undergo a pre‐cleaning process following sterilisation to convert them to feedstocks for the biodiesel production process. All steps in the process are monitored by an automated PLC recording and control system that will keep the process under control allowing the parameters to be consistently achieved.
CCP 3: In the event of a failure in the bentonite dosing system, the system is stopped, inspected, repaired and reset to repeat the entire dosing process. In the event of agitator failure, heating failure or a power cut, the timer will restart at 0 min.
CCP 4: If the tricanter speed drops below 3,000 g, a warning will be issued. An investigation will be undertaken. The reaction will proceed and if CCP7 (solids < 0.15%) is achieved, the batch will be accepted. If the tricanter stops, the feed pump is automatically turned off and the process is suspended until the tricanter is repaired.
CCP 5: In the event of a failure in the phosphoric acid dosing system, the system is stopped, inspected, repaired and reset to repeat the entire dosing process. If the temperature in the phosphoric acid dosing system is not achieved, the system is stopped, inspected, repaired and reset to repeat the entire phosphoric acid dosing process. If the mixing time is not achieved, the reaction will proceed and if CCP7 (solids < 0.15%) is achieved, the batch will be accepted. If not, the batch will be reprocessed. In the event of a failure of the hot water flow meter, the system is stopped, inspected, repaired and reset to repeat the entire phosphoric acid dosing process.
CCP 6: If the separator speed drops below 10,000 g, a warning will be issued. An investigation will be undertaken. The reaction will proceed and if CCP7 (solids ≤ 0.15% w/w) is achieved, the batch will be accepted. If the separator stops, the feed pump is automatically turned off and the process is suspended until the separator is repaired.
3.5.1.4. CCP 7: Tallow testing area
This is a standard method based on ISO 663:2017 (E). Cleaned oil is sampled and tested after the pre‐cleaning process for solids content before pumping to the biodiesel tank farm for storage as a biodiesel feedstock. No raw material will be accepted as a feedstock for biodiesel production unless solids are ≤ 0.15% w/w. If the solids are high at the storage tanks, the material must be pumped back to the raw material storage tanks for reprocessing.
3.5.1.5. CCP 8: FAME conversion reactor
Acidic esterification and transesterification of feedstocks at the minimum parameters of 140°C for 4 h at 5.5 bara is controlled by a PLC. Crude ester is not discharged from the reactor vessel until all the parameters have been achieved for 4 h continuously and a satisfactory record of all parameters has been recorded. If the reaction stops due to a power cut or critical equipment failure, or any other reason, maintenance will be performed, and the batch will be reprocessed from zero time. If for any other reason the batch must be rejected at this time, it will be treated as Category 1 and incinerated in accordance with the Category 1 disposal guidelines in Chapter II, Section 2, Article 12 of Regulation (EU) No 1069/2009.
3.5.1.6. CCP: 9 and 10 Fractionation
Crude ester is distilled through a series of three distillation columns allowing it to be purified as well as separated into batches of biodiesel of particular carbon chain lengths and characteristics. This occurs at high temperature under vacuum. All steps and parameters in the process are monitored by operators on an automated PLC recording and control system. If the vacuum or temperature is lost in the fractional distillation column, the distillation system will automatically shut down. Maintenance will be performed, and the process will be resumed. If for any reason it is necessary to dispose of this batch, it will be burned in a boiler in accordance with the Category 1 disposal guidelines in Chapter II, Section 2, Article 12 of Regulation (EU) No 1069/2009.
3.5.2. BIOHAZ Panel assessment of the HACCP plan
The applicant provided a detailed theoretical HACCP plan which has not been verified under real industrial‐scale conditions. It includes a description of the steps, and two figures presenting a schematic representation of the process. There are some differences in terminology between the text and figures, these are:
In the pre‐cleaning process description, process step 2, it is stated that: ‘The oil will be collected in a designated tank and will be pumped through a flow smoothing pipe to the vertical bowl separator.’ In the figure, instead of vertical bowl separator, it is indicated as ‘disc separator’; the same terminology should be used. Additionally, the water phase separated in this step is not mentioned in Figure A.1 of Appendix A.
In the hazard identification section, for biological hazards, bovine prions are the only relevant biological hazard mentioned. Although it is justified, other hazards that may be present should be mentioned, as well as the latest information about differences in resistance among TSE strains. In this section, control of the different hazards according to literature data and the production process itself are included.
The CCPs designated in the HACCP plan are correctly addressed and the corrective actions proposed are appropriate. They cover the main hazardous events that could alter the risk of exposure to prions.
The proposed HACCP plan can be considered satisfactory if the whole scheme is verified under full‐scale conditions.
3.6. Risk associated with interdependent processes
3.6.1. Risk associated with interdependent processes as provided by the applicant
Point 5(a), Chapter II, Annex VII of Regulation (EU) 142/2011 requires that the applicant provides information on the risks associated with interdependent processes. In particular, the applicant is required to provide information on the outcome of an evaluation of possible indirect impacts, which may: (i) influence the level of risk reduction of a particular process; or (ii) arise from transport or storage of any products generated during the process and from the safe disposal of such products, including waste water. The description presented in the current section has been extracted verbatim from the application, with minor editorial changes for clarity purposes.
3.6.1.1. Storage
All end products (tallow) and by‐products (wastewater, solids) of the pre‐cleaning process are stored in bunded, designated tanks or processed. All end products (biodiesel and glycerine) and by‐products (wastewater, methanol water) from the acid esterification and transesterification and distillation processes are stored in specifically designated tanks in the bunded and monitored tank farm. Any environmental risk or risk to human or animal health due to spills or leaks during storage or filling of tankers is mitigated by the use of bunded spaces and sumps that can be pumped back to designated storage.
3.6.1.2. Transport
All end products or by‐products that are transported off‐site will be hauled in designated tankers, constructed from the appropriate corrosion‐resistant materials, by licensed hauliers. Tankers will be labelled with the appropriate signage and all loads will be accompanied by the correct legal and environmental paperwork. If a tanker containing either biodiesel or glycerine crashed and due to some extreme event the specialised, heavy‐duty tanker was breached and biodiesel or glycerine was discharged to the road and verge, exposure to prions from that glycerine or biodiesel would not be expected.
3.6.1.3. Safe disposal of by‐products
Solids from the pre‐cleaning process
All solids removed from raw materials during the pre‐cleaning process, either by the tricanter or separator, will be transported back by a one‐directional screw conveyor system into the intake pit of the Category 1 rendering plant. There, the material will be processed in accordance with Commission Regulation (EU) No 142/2011 and in accordance with Article 12 of Regulation (EC) No 1069/2009 under Department of Agriculture Approval No. R911.
Wastewater from the pre‐cleaning process
Wastewater from the pre‐cleaning process that is removed from the raw materials either by the tricanter or separator will enter the waste heat evaporator in the Category 1 rendering plant and be processed in accordance with Section II of Chapter 1 of Annex IV of Commission Regulation (EU) 142/2011.
Glycerine
Glycerine that is produced as a by‐product from the acidic esterification and transesterification process may be used as a fuel source for power generation or will be disposed as a Category 1 by‐product by incineration at an appropriately licensed facility in accordance with Article 12 of Regulation (EC) No 1069/2009.
Bio‐heating oil
Bio‐heating oil (BHO) collected from the tank bottoms of the fractional distillation columns will be burned in a boiler in accordance with Article 12 of Regulation (EC) No 1069/2009.
Wastewater from the biodiesel production and methanol rectification processes
Wastewater that is produced as a by‐product from the acidic esterification and transesterification process will be processed in the wastewater treatment plant (WWTP) in accordance with Section II of Chapter 1 of Annex IV of Commission Regulation (EU) 142/2011. All wastewater will have been evaporated and condensed during either the acidic esterification and transesterification or methanol rectification process and will be free of prions since proteins are not known to evaporate. Additionally, the methanol rectification process is dosed with NaOH to maintain a neutral range of pH 6–7.
3.6.2. BIOHAZ Panel assessment of the risk associated with interdependent processes
The applicant provided a detailed description of the risks associated with the interdependent processes and of the procedures that would be implemented for dealing with these risks.
The procedures on the storage and transport of ABP and derived products are in compliance with the requirements set out in Article 21 of Regulation (EC) No 1069/2009, Article 17 and Annex VIII of Commission Regulation (EU) No 142/2011 and various other parts of these Regulations.
The procedure for dealing with wastewater from the pre‐cleaning process and from the acid‐only esterification and transesterification process is in compliance with the requirements set out for wastewater treatment in ABP processing plants in Section 2, Chapter 1, Annex IV of Commission Regulation (EU) No 142/2011. The procedures for dealing with other by‐products of the alternative process, including solids from the pre‐cleaning process, methanol, glycerine and BHO from the acid‐only esterification and transesterification process are in compliance with Article 12 of Commission Regulation (EC) No 1069/2009 and with Section 3, Chapter IV of Annex IV of Commission Regulation (EU) No 142/2011. Measures to mitigate any risks that may arise from interdependent processes are also set out in the HACCP plan accompanying the application. Considering the nature of these by‐products and the procedures for dealing with them, exposure of animals or humans to prions resulting from/related to interdependent processes would not be expected.
3.6.3. Risk associated with the intended end use of the product
3.6.3.1. Risk associated with the intended end use of the products from the process as provided by the applicant
The description presented in the current section has been extracted verbatim from the application with minor editorial changes for clarity purposes.
Biodiesel will be blended with fossil diesel for use in domestic and commercial vehicles and will be dispensed at forecourts of retail filling stations. As discussed in Mittelbach et al. (2007), a limited number of potential routes of infection of humans with BSE exist because of the residual risk associated with biodiesel. There are two main routes of potential infection that exist: oral and subcutaneous. It is highly unlikely that biodiesel would be intentionally ingested and equally, prions will not be inhaled ‘since proteins are not known to evaporate’ (Mittelbach et al., 2007). The subcutaneous route represents a more viable potential route of exposure if the barrier created by the skin in some way suffered a loss of integrity such as an open cut or wound. If a wound or trauma had compromised the integrity of the skin, the volume of biodiesel that could penetrate the underlying cells would be in the range of less than a millilitre. So, the exposure to prions via filling a vehicle with biodiesel is not expected. If because of an explosion, leak or accidental submersion, a person became entirely immersed in biodiesel, the exposure prions via biodiesel would be equally not expected.
The possibility of exposure of an animal either by ingesting or being exposed by the subcutaneous route to prions in fossil diesel containing biodiesel is not expected. For this to occur, it would be necessary for either a spillage or other major incident to happen. All areas of the biodiesel facility where a spill or leak could occur are bunded to an appropriate capacity. The entire bottom floor of the production facility is bunded to a level of minus 0.5 m. The loading station will be bunded and have a sump that can be pumped back into a storage tank. The tank farm is constructed in a bund that can hold 110% the capacity of the largest tank or 25% the capacity of the tank farm. As a result of these bunds and sumps, it is extremely unlikely that any animal would come into contact with 100% biodiesel. All wholesale fuel facilities would have similar environmental protection strategies and on‐site security. Additionally, due to the hazardous nature of fuel, retail filling stations are extremely unlikely to have present exposed fuel. Therefore, the exposure of an animal to prions via contact with sufficient biodiesel is not expected.
3.6.4. BIOHAZ Panel assessment of the risk associated with the intended end use of the products
The end product of the proposed alternative method is biodiesel. Considering the nature and foreseen uses of this final product, exposure of animals or humans to prions resulting from the intended end use is not expected.
4. Conclusions
Since the starting material includes Category 1 tallow, the applicant considered that, of any biological hazards that may be present, prions would be the most resistant. The BIOHAZ Panel agrees with the approach used by the applicant of focusing on the capacity of the alternative method to inactivate prions.
The dossier contains a detailed theoretical HACCP plan and information about the risks of the interdependent processes and those associated with the intended end use. The BIOHAZ Panel considers that the measures proposed in the dossier to deal with these risks are compliant with the relevant legislation.
The dossier contains studies commissioned by the applicant to specifically evaluate the detectable PrPSc reduction factor achievable by the proposed alternative method, using the same substrate and processing parameters. Despite its inherent limitations, the experimental approach followed is considered acceptable.
In addition to estimates of prion reduction factors associated with the individual steps of the processing method, the overall achievable reduction factor of the evaluated method has been provided. This avoids the need to assume an additive impact for individual steps and offers a more realistic assessment.
Previous EFSA Opinions established that a reduction in prion infectivity, or detectable PrPSc, of at least 6 log10 should be achieved to consider a method at least equivalent, for the relevant category of ABP (Category 1), to the processing methods previously approved.
The prion reduction factor claimed in the application for the pre‐cleaning and acid esterification and transesterification processes combined was at least 4.3 log10, as established by the estimated titre of a 263K hamster strain spike, using WB detection of the residual PrPSc signal. The final step (fractional distillation) is assumed to achieve at least an additional 3 log10 reduction, based on published data.
Inactivation studies based on experimentally derived laboratory TSE strains, such as the hamster‐adapted scrapie strain 263K, and with the use of WB to assess the presence of detectable PrPSc following treatment, are increasingly being considered suboptimal as predictors of the resistance of natural TSE when they are subjected to such inactivating protocols, due to the inherent limited analytical sensitivity and biological relevance of WB for the detection of prion infectivity. Despite these methodological caveats, the BIOHAZ Panel considers that the alternative method, including the final fractional distillation, is capable of achieving the 6 log10 reduction factor that enables it to be considered at least equivalent to the processing methods previously approved for the production of biodiesel from all categories of ABP raw materials.
5. Recommendations
To check the feasibility of the proposed HACCP plan, the records of the main processing parameters (e.g. time, pH, temperature) should be assessed for a certain time period under real industrial conditions.
6. Documentation as provided to EFSA
Application for evaluation of an alternative process for production of biodiesel from rendered fat of all categories of animal by‐products. First submission. July 2019. Submitted by: College Proteins Unlimited Company. College, Nobber, Co. Meath, A82 XT61, Ireland.
Application for evaluation of an alternative process for production of biodiesel from rendered fat of all categories of animal by‐products. Second submission. September 2019. Submitted by: College Proteins Unlimited Company. College, Nobber, Co. Meath, A82 XT61, Ireland.
Glossary
- Absolute pressure
Pressure zero – referenced against a perfect vacuum. The standard absolute atmosphere pressure is 101,325 Pa or 1.01325 bar (gauge pressure plus atmospheric pressure) It can be indicated by an ‘a’ after the pressure unit (e.g. ‘bara’) https://www.esi-tec.com/blog-pressure-sensors-transmitter-transducer/2013/06/difference-between-gauge-and-absolute-pressure-measurement
- Bar
Non‐international system of units (SI unit) for pressure, accepted for use but not encouraged. One hundred thousand pascals are called a bar (100,000 Pa = 1 bar). Commonly used for technical applications. https://www.nist.gov/pml/special-publication-811/nist-guide-si-chapter-5-units-outside-si
- Bara
Absolute pressure measured in bar
- Barg
Gauge pressure measured in bar
- Biodiesel
Renewable fuel comprised of mono‐alkyl esters of long chain fatty acids derived from vegetable oils or animal fats https://www.biodiesel.org/what-is-biodiesel/biodiesel-basics
- C16
Obtained biodiesel fraction after distillation with fatty acid methyl esters derived from fatty acids consisting of a chain of 16 carbon atoms
- C18
Obtained biodiesel fraction after distillation with fatty acid methyl esters derived from fatty acids consisting of a chain of 18 carbon atoms
- Distillation
Separation of different components in a liquid by evaporation and condensation using various boiling points of the substances to be separated https://www.britannica.com/science/distillation
- Esterification
The reaction between an alcohol (R‐COH) and a carboxylic acid (R’‐COOH) forming in the presence of a catalyst an ester (R‐COO‐R’) and water (H2O). Typical alcohols used in esterification are methanol and ethanol. A reaction with free fatty acids results in fatty acid alkyl esters and water https://www.britannica.com/science/alcohol/Esterification#ref998542
- FAME
Fatty acid methyl ester. An ester obtained by reactions of fatty acids with methanol https://www.ebi.ac.uk/chebi/searchId.do?chebiId=4986
- Gauge pressure
Pressure zero – referenced against the environment (ambient air pressure). The standard gauge atmosphere pressure is 0 Pa or 0 bar (absolute pressure minus atmospheric pressure) Can be indicated by an ‘g’ after the pressure unit (e.g. ‘barg’) https://physics.stackexchange.com/questions/20460/gauge-pressure-vs-absolute-pressure
- Methyl ester
An ester obtained by reactions with the alcohol methanol (R‐COO‐CH3) https://www.darpro-bioenergy.com/about-dar-pro-bioenergy/contact/methyl-ester-faqs
- Pascal
Standard unit for pressure as defined by the International System of Units (SI unit). It indicates the ratio of force applied per area covered (kg m−1 s−2) https://www.nist.gov/pml/special-publication-811/nist-guide-si-chapter-4-two-classes-si-units-and-si-prefixes
- Tallow
Animal fat obtained after rendering of animal by‐products https://www.daera-ni.gov.uk/articles/animal-by-products-specific-guidance
- Transesterification
The reaction between an alcohol (R’’‐OH) and an ester (R‐COO‐R’) forming in the presence of a catalyst a different ester (R‐COO‐R’’) and a different alcohol (R’‐OH) with exchanged R groups A reaction with triglycerides results in fatty acid alkyl esters and glycerol http://www.etipbioenergy.eu/value-chains/conversion-technologies/conventional-technologies/transesterification-to-biodiesel
Abbreviations
- ABP
animal by‐products
- BHO
bio‐heating oil
- BIOHAZ
EFSA Panel on Biological Hazards
- BSE
bovine spongiform encephalopathy
- CA
Competent Authority
- C‐BSE
classical bovine spongiform encephalopathy
- CCP
critical control point
- FAD
fatty acid distillates
- FAME
fatty acid methyl ester
- GTH
glycerol triheptanoate
- H2SO4
sulfuric acid
- HACCP
hazard analysis and critical control point
- H‐BSE
H‐type bovine spongiform encephalopathy
- L‐BSE
L‐type bovine spongiform encephalopathy
- MSA
methanesulfonic acid
- PLC
programmable logic controller
- PMCA
protein misfolding cyclic amplification
- PrPSc
abnormal protease‐resistant isoform of prion protein
- QRA
quantitative risk assessment
- RT‐QuIC
real‐time quaking‐induced conversion
- SSM
spiked starting material
- TSE
transmissible spongiform encephalopathies
- UCO
used cooking oil
- WB
western blotting
- WG
Working Group
- WWTP
wastewater treatment plant
Appendix A – Flow charts
1.
Appendix B – Issues relating to methods for prion quantification
1.
For TSE, the most reliable approach to determining the potential infectivity of a sample is to bioassay it in a rodent model that is appropriate for the strain and/or host species under consideration. However, this process requires specialist facilities and resources, and may take years to complete. In addition, the toxic nature of some substrates, such as those involved with biodiesel production, render this methodology inappropriate for the validation or quantification of the TSE inactivation achieved by this process.
In the context of disease confirmation, the use of abnormal PrP immunochemical detection methods such as immunohistochemistry and WB has become the mainstay of statutory diagnostic procedures. So, the presence of detectable PrPSc has become widely used as a proxy for infectivity, although it is accepted that WB is less sensitive than bioassay (EFSA BIOHAZ Panel, 2015). Additionally, studies have demonstrated that there is no consistent or linear relationship between the measurable amount of PrPSc and the amount of infectivity (Gonzalez et al., 2012; Marín‐Moreno et al., 2019). In a range of contexts, infectivity has been demonstrated in the absence of detectable PrPSc (Lasmézas et al., 1997; Wells et al., 2005; Bruederle et al., 2008; Andréoletti et al., 2011; Simmons et al., 2011; Marín‐Moreno et al., 2016; Ackermann et al., 2017), and PrPSc has also been reported in the absence of infectivity (Piccardo et al., 2013; Serra et al., 2017, 2018).
Seminal studies on prion decontamination/inactivation relied on the use of laboratory‐adapted rodent prion strains derived from naturally occurring scrapie (such as ME7, or 263K), or classical BSE (301V) (Brown et al., 1990; Taylor, 1991, 1999). Over the last 30 years, however, it has become clear that the strains involved in naturally occurring prion diseases (scrapie, BSE, CWD or CJD) display very different resistance/sensitivity to decontaminating treatments (autoclaving or chemical inactivation) (Taylor, 1986, 2000; Taylor et al., 1994).
It has also been established that even different prion strains from naturally occurring TSE in the same host species can behave very differently. In cattle, C‐BSE, H‐BSE and L‐BSE all have different responses to inactivation by the ABP sterilisation ‘Method 1’ (Chapman et al., 2020); similarly, in sheep, classical and atypical scrapie behave differently when they are subjected to Method 1 conditions (Spiropoulos et al., 2019). So, the resistance/sensitivity of different prion strain/host combinations to decontamination or inactivation treatments may vary substantially, and should, ideally, be experimentally characterised for each given situation.
Over the last 20 years, in vitro amplification of prions by either protein misfolding cyclic amplification (PMCA) (Saborio et al., 2001; Castilla et al., 2005; Kurt et al., 2007; Lacroux et al., 2012) or real‐time quaking‐induced conversion (RT‐QuiC) (Atarashi et al., 2011; Orrú et al., 2011, 2012) have emerged as sensitive and efficient methodologies for the detection and quantification of prions. Both rely on the detection of PrP structural conversion and polymerisation upon addition of PrPSc ‘seeds’ contained within infected samples (for a review, see Krauss and Vorberg, 2013). The sensitivity of detection achieved by PMCA and RT‐QuiC exceeds the sensitivity of the reference bioassay models. A relationship between seeding activity (as measured by RT‐QuiC or PMCA) and infectivity (measured by bioassay in a reference model) can be established (Makarava et al., 2012; Boerner et al., 2013; Douet et al., 2017).
In the context of the quantification of prion reduction achieved during processes such as the manufacturing of biodiesel, in vitro amplification methodologies such as PMCA or RT‐QuiC now offer a better experimental approach than the measurement of the PrPSc WB signal, and would enable the testing of additional prion strains, such as BSE from cattle, that would better reflect the field situation.
Suggested citation: EFSA Panel on Biological Hazards (BIOHAZ) , Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Andréoletti O, Fernández Escamez P, Griffin J, Spiropoulos J, Ashe S, Ortiz‐Peláez A and Alvarez‐Ordóñez A, 2020. Scientific Opinion on the evaluation of an alternative method for production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products (submitted by College Proteins). EFSA Journal 2020;18(4):6089, 29 pp. 10.2903/j.efsa.2020.6089
Requestor: European Commission
Question number: EFSA‐Q‐2019–00432
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Amendment: The paragraph ‘Waiver’, not included in error at the time of publication, has been inserted to account for a waiver granted to John Griffin, Working Group member. To avoid confusion, the original version of the scientific opinion has been removed from the EFSA Journal, but is available on request as is a version showing all the changes made.
Waiver: In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management a waiver was granted to John Griffin, Working Group member. Pursuant to Article 21(6) of the aforementioned Decision, the concerned expert was allowed to take part in the discussions and in the drafting of the scientific output but was not allowed to be or act as chair, vice‐chair or rapporteur of the Working Group. Any competing interests are recorded in the respective minutes of the meetings of the Working Group, available at: https://www.efsa.europa.eu/sites/default/files/wgs/biological-hazards/wg-biohaz-ABP-biodisel-fats.pdf
Acknowledgements: The Panel wishes to thank Katrin Bote and Citlali Pintado for their contributions to the drafting of this scientific output.
Adopted: 18 March 2020
Amended: 22 Jun 2020
Notes
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002. OJ L 300, 14.11.2009, p. 1.
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 54, 26.2.2011, p. 1.
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, p. 55.
Commission Regulation (EU) No 749/2011 of 29 July 2011 amending Regulation (EU) No 142/2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive Text with EEA relevance.
Commission Regulation (EU) No 206/2010 of 12 March 2010 laying down lists of third countries, territories or parts thereof authorised for the introduction into the European Union of certain animals and fresh meat and the veterinary certification requirements.
References
- Ackermann I, Balkema‐Buschmann A, Ulrich R, Tauscher K, Shawulu JC, Keller M, Fatola OI, Brown P and Groschup MH, 2017. Detection of PrP BSE and prion infectivity in the ileal Peyer's patch of young calves as early as 2 months after oral challenge with classical bovine spongiform encephalopathy. Veterinary Research, 48, 88 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andréoletti O, et al., 2011. PLoS Pathogen, 7, e1001285 10.1371/journal.ppat.1001285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S and Yamada M, 2011. Ultrasensitive human prion detection in cerebrospinal fluid by real‐time quaking‐induced conversion. Nature Medicine, 17, 175–178. [DOI] [PubMed] [Google Scholar]
- Boerner S, Wagenführ K, Daus ML, Thomzig A and Beekes M, 2013. Towards further reduction and replacement of animal bioassays in prion research by cell and protein misfolding cyclic amplification assays. Laboratory Animals, 47, 106–115. [DOI] [PubMed] [Google Scholar]
- Brown P, Liberski PR, Wolff A and Gajdusek DC, 1990. Resistance of scrapie infectivity to steam autoclaving after formaldehyde fixation and limited survival after washing at 360°C: practical and theoretical implications. Journal of Infectious Diseases, 161, 467–472. [DOI] [PubMed] [Google Scholar]
- Bruederle CE, Hnasko RM, Kraemer T, Garcia RA, Haas MJ, Marmer WN and Carter JM, 2008. Prion infected meat‐and‐bone meal is still infectious after biodiesel production. PLoS ONE, 3, e2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla J, Saa P, Hetz C and Soto C, 2005. In vitro generation of infectious scrapie prions. Cell, 121, 195–206. [DOI] [PubMed] [Google Scholar]
- Chapman GE, Lockey R, Beck KE, Vickery C, Arnold M, Thorne L, Thorne JK, Walker SR, van Keulen L, Casalone C, Griffiths PC, Simmons MM, Terry LA and Spiropoulos J, 2020. Inactivation of H‐type and L‐type BSE following recommended autoclave decontamination procedures. Transboundary and Emerging Diseases, 10.1111/tbed.13513. [DOI] [PubMed] [Google Scholar]
- Douet JY, Lacroux C, Aron N, Head MW, Lugan S, Tillier C, Huor A, Cassard H, Arnold M, Beringue V and Ironside JW, 2017. Distribution and quantitative estimates of variant Creutzfeldt‐Jakob disease prions in tissues of clinical and asymptomatic patients. Emerging Infectious Diseases, 23, 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Statement on technical assistance on the format for applications for new alternative methods for animal by‐products. EFSA Journal 2010;8(7):1680, 12 pp. 10.2903/j.efsa.2010.1680 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on a continuous multiple‐step catalytic hydro‐treatment for the processing of rendered animal fat (Category 1). EFSA Journal 2015;13(11):4307, 23 pp. 10.2903/j.efsa.2015.4307 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Alvarez Ordoñez A, Griffin J, Spiropoulos J, Vanopdenbosch E, Correia S and Fernandez Escamez PS, 2017. Scientific Opinion on the evaluation of the Application for new alternative biodiesel production process for rendered fat of Cat 1 (BDI‐RepCat process, AT). EFSA Journal 2017;15(11):5053, 22 pp. 10.2903/j.efsa.2017.5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Gironés R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Skandamis P, Snary E, Speybroeck N, Kuile BT, Threlfall J, Wahlström H, Adkin A, Greiner M, Marchis D, Prado M, Da Silva Felicio T, Ortiz‐Pelaez A and Simmons M, 2018. Scientific opinion on an updated quantitative risk assessment (QRA) of the BSE risk posed by processed animal protein (PAP). EFSA Journal 2018;16(7):5314, 111 pp. 10.2903/j.efsa [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA , 2019. CHMP position statement on Creutzfeldt–Jakob disease and plasma‐derived and urine‐derived medicinal products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/chmp-position-statement-creutzfeldt-jakob-disease-plasma-derived-urine-derived-medicinal-products_en-0.pdf
- Gonzalez L, Thorne L, Jeffrey M, Martin S, Spiropoulos J, Beck KE, Lockey RW, Vickery CM, Holder T and Terry L, 2012. Infectious titres of sheep scrapie and bovine spongiform encephalopathy agents cannot be accurately predicted from quantitative laboratory test results. Journal of General Virology, 93, 2518–2527. [DOI] [PubMed] [Google Scholar]
- Krauss S and Vorberg I, 2013. Prions ex vivo: what cell culture models tell us about infectious proteins. International Journal of Cell Biology, 2013, 704546 10.1155/2013/704546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt TD, Perrott MR, Wilusz CJ, Wilusz J, Supattapone S, Telling GC, Zabel MD and Hoover EA, 2007. Efficient in vitro amplification of chronic wasting disease PrPRES . Journal of Virology, 81, 9605–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroux C, Comoy E, Moudjou M, Perret‐Liaudet A, Lugan S, Litaise C, Simmons H, Jas‐Duval C, Lantier I, Beringue V and Groschup M, 2012. Preclinical detection of variant CJD and BSE prions in blood. PLoS Pathogens, 10, e1004202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasmézas CI, Deslys JP, Robain O, Jaegly A, Beringue V, Peyrin JM, Fournier JG, Hauw JJ, Rossier J and Dormont D, 1997. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science, 275, 402–404. [DOI] [PubMed] [Google Scholar]
- Makarava N, Kovacs GG, Savtchenko R, Alexeeva I, Ostapchenko VG, Budka H, Rohwer RG and Baskakov IV, 2012. A new mechanism for transmissible prion diseases. Journal of Neuroscience, 32, 7345–7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín‐Moreno A et al., 2016. An assessment of the long‐term persistence of prion infectivity in aquatic environments. Environmental Research, 151, 587–594. 10.1016/j.envres.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Marín‐Moreno A, Aguilar‐Calvo P, Moudjou M, Espinosa JC, Béringue V and Torres JM, 2019. Thermostability as a highly dependent prion strain feature. Science Reports, 9, 11396 10.1038/s41598-019-47781-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbach M, Pokits B, Müller H, Müller M and Riesner D, 2007. Risk assessment for prion protein reduction under the conditions of the biodiesel production process. European Journal of Lipid Science and Technology, 109, 79–90. [Google Scholar]
- Orrú CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ and Caughey B, 2011. Prion disease blood test using immunoprecipitation and improved quaking‐induced conversion. MBio, 2, e00078‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrú CD, Wilham JM, Vascellari S, Hughson AG and Caughey B, 2012. New generation QuIC assays for prion seeding activity. Prion, 6, 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccardo P, King D, Telling G, Manson JC and Barron RM, 2013. Dissociation of prion protein amyloid seeding from transmission of a spongiform encephalopathy. Journal of Virology, 87, 12349–12356. 10.1128/JVI.00673-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio GP, Permanne B and Soto C, 2001. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature, 411, 810–813. [DOI] [PubMed] [Google Scholar]
- Serra F, Müller J, Gray J, Lüthi R, Dudas S, Czub S and Seuberlich T, 2017. PrP‐C1 fragment in cattle brains reveals features of the transmissible spongiform encephalopathy associated PrPSc . Brain Research, 1659, 19–28. 10.1016/j.brainres.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Serra F, Dudas S, Torres JM, Anderson R, Oevermann A, Espinosa JC, Czub S and Seuberlich T, 2018. Presumptive BSE cases with an aberrant prion protein phenotype in Switzerland, 2011: lack of prion disease in experimentally inoculated cattle and bovine prion protein transgenic mice. Transboundary and Emerging Diseases, 65, 1348–1356. 10.1111/tbed.12884 [DOI] [PubMed] [Google Scholar]
- Simmons MM, Moore SJ, Konold T, Thurston L, Terry LA, Thorne L, Lockey R, Vickery C, Hawkins SA, Chaplin MJ and Spiropoulos J, 2011. Experimental oral transmission of atypical scrapie to sheep. Emerging Infectious Diseases, 17, 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RA, Fernie K, Smith A, Andrews R, Schmidt E and Taylor DM, 2009. Inactivation of a TSE agent by a novel biorefinement system. Process Biochemistry, 44, 1060–1062. [Google Scholar]
- Spiropoulos J, Lockey R, Beck KE, Vickery C, Holder TM, Thorne L, Arnold M, Andreoletti O, Simmons MM and Terry LA, 2019. Incomplete inactivation of atypical scrapie following recommended autoclave decontamination procedures. Transboundary and Emerging Diseases, 66, 1993–2001. 10.1111/tbed.13247 [DOI] [PubMed] [Google Scholar]
- Taylor DM, 1986. Decontamination of Creutzfeldt‐Jakob disease agent. Annals of Neurology, 20, 749–750. [DOI] [PubMed] [Google Scholar]
- Taylor DM, 1991. Resistance of the ME7 scrapie agent to peracetic acid. Veterinary Microbiology, 27, 19–24. [DOI] [PubMed] [Google Scholar]
- Taylor DM, 1999. Inactivation of prions by physical and chemical means. Journal of Hospital Infection, 43, S69–S76. [DOI] [PubMed] [Google Scholar]
- Taylor DM, 2000. Inactivation of transmissible degenerative encephalopathy agents: a review. The Veterinary Journal, 159, 10–17. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Fraser H, McConnell I, Brown DA, Brown KL, Lamza KA and Smith GR, 1994. Decontamination studies with the agents of bovine spongiform encephalopathy and scrapie. Archives of Virology, 139, 313–326. [DOI] [PubMed] [Google Scholar]
- Wells GA, Spiropoulos J, Hawkins SA and Ryder SJ, 2005. Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Veterinary Record, 156, 401–407. [DOI] [PubMed] [Google Scholar]


