Abstract
Following a request from the European Commission, the Panel on Additives and Products or substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of monosodium l‐glutamate monohydrate produced by fermentation using Corynebacterium glutamicum KCCM 80188 when used as a sensory additive (flavouring compound) in feed and water for drinking for all animal species. The production strain is not genetically modified. Viable cells of the production strain were not detected in the final additive. The additive does not give rise to any safety concern regarding the production strain. Monosodium l‐glutamate monohydrate produced using C. glutamicum KCCM 80188 is considered safe for the target species, for the consumer and for the environment. Monosodium l‐glutamate monohydrated produced by C. glutamicum KCCM 80188 is considered not toxic by inhalation, not irritant to skin or eyes and not a dermal sensitiser. The FEEDAP Panel expressed reservations on the use of the additive in water for drinking due to concerns on its impact on the hygienic conditions of the water. The Panel concluded that the additive is efficacious to contribute to the flavour of feed.
Keywords: sensory, flavouring compounds, monosodium glutamate, safety, efficacy
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7.
The European Commission received a request from CJ Europe GmbH2 for authorisation of the product monosodium l‐glutamate, when used as a feed additive for all animal species (category: sensory; functional group: flavouring compounds).
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 1 March 2019.
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the product monosodium l‐glutamate, when used under the proposed conditions of use (see Section 3.1.5).
1.2. Additional information
The product under assessment, which is not authorised as a feed additive in the European Union, is based on monosodium l‐glutamate and is produced by fermentation with a non‐genetically modified strain of Corynebacterium glutamicum (KCCM 80188).
The FEEDAP Panel issued a scientific opinion on the safety and efficacy of the use of amino acids (chemical group 34), when used as flavourings for all animal species, which included monosodium l‐glutamate (EFSA FEEDAP Panel, 2014). Monosodium l‐glutamate (purity minimum 99%) is currently authorised for use in feed for all animal species as a sensory additive (functional group: flavouring compounds) only when produced by chemical synthesis or protein hydrolysis.3
Glutamic acid was assessed by JECFA (WHO, 1987, as flavour enhancer, seasoner) and the Scientific Committee for Food (European Commission, 1991, technological functions) as food additive. Monosodium glutamate has been assessed by the EFSA's Scientific Panel on food Contact Material, Enzymes, flavourings and Processing Aids (CEF) for use in food contact materials (EFSA CEF Panel, 2013) and the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) assessed glutamic acid and its salts as food additive (EFSA ANS Panel, 2017). Glutamic acid (E 620) and its salts (E 621 to E 625) are included in the Union list of food additives as ‘additives other than colours and sweeteners’, ‘group I (with a maximum of 10 g/kg), ‘other additives that may be regulated combined’, category 12.1.2 salt substitutes and category 12.2.2. seasoning and condiment.4
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier5 in support of the authorisation request for the use of sodium l‐glutamate produced by C. glutamicum KCCM 80188 as a feed additive.
The FEEDAP Panel used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies to deliver the present output.
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the monosodium l‐glutamate in animal feed. The Executive Summary of the EURL report can be found in Annex A.6
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of monosodium l‐glutamate produced by C. glutamicum KCCM 80188 is in line with the principles laid down in Regulation (EC) No 429/20087 and the relevant guidance documents: Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a,b,c), Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a,b), Guidance on the assessment of the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017a,b,c, Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017a,b,c), Guidance on the assessment of the efficacy of feed additives (EFSA FEEDAP Panel, 2018a,b) and Guidance on the assessment of the safety of feed additives for the environment (EFSA FEEDAP Panel, 2019).
3. Assessment
The additive under assessment contains monosodium l‐glutamate produced by Corynebacterium glutamicum KCCM 80188 and it is intended to be used as a sensory additive (functional group: flavouring compounds) in feed and water for drinking for all animal species.
3.1. Characterisation
3.1.1. Characterisation of the production organism
The production strain is a non‐genetically modified strain of Corynebacterium glutamicum which is deposited at the Korean Culture Centre of Microorganisms (KCCM) with the accession number KCCM 80188.8 ■■■■■■■■■■
The taxonomic identification of the production strain was confirmed ■■■■■■■■■■■■■■■■■■■■
The applicant tested the susceptibility of the strain ■■■■■ against the list of antibiotics proposed for ‘Corynebacterium and other Gram‐positive’ in the Guidance on the characterisation of microorganisms used as feed additives or as production organisms (EFSA FEEDAP Panel, 2018a,b).■■■■■ All the MICs were equal or below the cut‐off values established in this guidance.
The whole genome sequence data (WGS) of the production strain was interrogated for the presence of antimicrobial resistance genes (AMR) ■■■■■ No genes of concern were identified.■■■■■
3.1.2. Manufacturing process
■■■■■
■■■■■■■■■■
3.1.3. Characterisation of the additive
Monosodium l‐glutamate monohydrate (MNG; International Union of Pure and Applied Chemistry (IUPAC) name: sodium (2S)‐2‐amino‐4‐carboxybutanoate hydrate (synonyms: l‐2‐aminopentandioic acid, l‐glutamic acid monosodium salt monohydrate), a compound identified with the Chemical Abstracts Service (CAS) No 6106‐04‐3, the European Inventory of Existing Commercial chemical Substances (EINECS) No 205‐538‐1) is the active substance of the additive and has a molecular mass of 187.13 g/mol. The molecular formula of monosodium l‐glutamate is C5H8O4NNa ·H2O and the structural formula is given in Figure 1.
Figure 1.
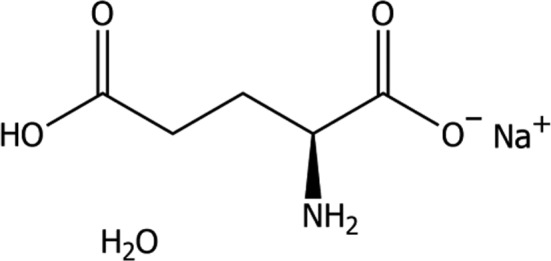
Structural formula of monosodium l‐glutamate monohydrate
The additive contains by specification ≥ 99% monosodium l‐glutamate monohydrate on ‘as is’ basis and ≤ 0.5% water.14 The analysis of five batches showed an average value of monosodium l‐glutamate 99.9% on ‘dry matter basis’ (range 99.3–100.2%) with water loss on drying of 0.1% (range 0.07–0.12%).15
In another analytical report,16 the applicant analysed five additional batches for monosodium l‐glutamate monohydrate (higher than 99% ‘as is’ basis), water (0.2%), nitrogen containing components (ammonium, nitrates, nitrites and betaine, not detected, limit of detection (LOD) 0.01 mg/kg), free amino acids (LOD, 0.5 mg/kg), organic acids (formic, acetic, citric, malic, succinic, lactic, not detected, LOD 1 mg/kg) and other elements (sodium 12.7% (12.6–12.8%)17 ‘as is’ basis, potassium, magnesium, calcium, fluoride, bromide, chloride, phosphate, sulfate, not detected, LOD 0.01 mg/kg).
The specific optical rotation was measured in three batches of the final product and the average was +25.0° (range +24.9° to +25.1°),18 which is within the range for monosodium l‐glutamate monohydrate (+24.2 to +25.5, pubChem)19 and demonstrates the identity of the l‐enantiomer and is in accordance with the specifications set for monosodium glutamate (E 621) as a food additive.20
Three batches of the additive were analysed for other chemical impurities. Heavy metals (lead, cadmium and mercury) and arsenic were below the LODs.21 In the same batches, the analysis of polychlorinated dibenzodioxins (PCDDs, 0.036 WHO‐TEQ ng/kg), polychlorinated dibenzofurans (PCDFs, 0.034 WHO‐TEQ ng/kg) and the sum of PCDD/PCDF and dioxin‐like polychlorinated biphenyls (PCB) (0.12 WHO‐TEQ ng/kg) was conducted.22 Aflatoxins (B1, B2, G1, G2), ochratoxin A, zearalenone, deoxynivalenol, fumonisins B1 and B2 were below the corresponding LOD.23 In the same batches, nitrofurans (furazolidone, furaltadone, nitrofurazone and nitrofurantoin) and nitrofuran metabolites (amino oxazolidinone, amino morpholino oxazolidinone, semi carbazide, amino hydantoin) were below the corresponding LODs.24 A multiresidue pesticide analysis showed that none of the 358 pesticides analysed was detected in the three batches.25 The applicant set specifications for microbial contamination as total bacterial count < 103 colony‐forming unit (CFU)/g, yeasts and filamentous fungi < 5 × 101 CFU/g, while Salmonella spp., Escherichia coli and coliforms absent in 25 g. The analysis of three batches showed compliance with these specifications.26
The presence of viable cells of the production strain in the final product was investigated in three batches of the product.27 ■■■■■ No colonies were detected.
The additive is a white powder with a solubility in water of 740 g/L. The dusting potential of the additive measured in three batches following the Stauber–Heubach method gave results ranging from 3 to 11 mg/m3.28 The particle size was measured by sieving method, particles below 100 μm amounted > 85% (w/w), particles below 44 μm amounted up to 24.5%.29
3.1.4. Stability and homogeneity
A shelf‐life of 36 months at 25°C was demonstrated in three batches of the additive when stored in bags corresponding to the commercial packaging.30
The stability of the additive (three batches) in a vitamin and mineral premixture for chickens for fattening (without choline chloride) was studied when added at 5% and stored at 25°C for 6 months (in closed bags). No losses were observed in the content of the active substance in the premixture up to 6 months.31
The stability of the additive (three batches) was evaluated when added at 0.4% to a mash32 and pelleted33 feed for chickens for fattening after storage at 25°C for 3 months (in closed bags). In the mash feed the mean loss the active substance after 3 months was 10% (ranging 3–16%), no losses were observed in the pelleted feed. The data do not allow to assess the effect of pelleting.
Analytical data on the stability in water of three batches of monosodium l‐glutamate were provided in two different studies.34 The test was done for each batch at two different concentrations 1 g or 12.5 mg/L of drinking water. The substance was dissolved at 30°C (in ultrasonic bath) and stored at a 25°C or 40°C for up to 48 h. No losses in the content of the active substance were observed. The content of monosodium glutamate was measured in 10 subsamples (concentration 1 g/L) to measure the capacity to homogeneously distribute, the coefficient of variation was of 1.3% (mean content of monosodium glutamate of 1 g/L).
3.1.5. Conditions of use
Monosodium l‐glutamate monohydrate is intended to be used in feedingstuffs/complementary feedingstuffs or water for drinking in all animal species as a flavouring compound. The applicant proposes a maximum use level of 25 mg monosodium l‐glutamate monohydrate/kg feed. For its use in water, the applicant recommends 12.5 mg/L water for drinking for rabbits, poultry species and pigs, for the rest of the species, the applicant recommends that the use in water should not exceed the daily amount that would be consumed via feed.35
3.2. Safety
3.2.1. Safety for the target species, consumer and environment
Safety concerns from the additive may derive either from the active substance or from the residues of the fermentation process/production strain remaining in the final product. The product under assessment is highly purified (less than 1% unidentified material is present in the additive). The production strain, KCCM 80188, belongs to a species, C. glutamicum, that qualifies for the qualified presumption of safety (QPS) approach to safety assessment (EFSA, 2007) when used for production purposes (EFSA BIOHAZ Panel, 2020). The production strain was unambiguously identified as C. glutamicum, was shown to be susceptible to the relevant antibiotics and not to contain antimicrobial resistance genes, and there were also no viable cells of the production strain in the final product. It can be concluded that no safety concerns for target animals, consumers and the environment would arise from the use of C. glutamicum KCCM 80188 as the production strain.
The recommended levels of use of monosodium l‐glutamate monohydrate in feed are well below the ones that may be present in the diets when using feedstuffs like soya bean meal, which contains 9.2% of glutamic acid (FAO, 2014).36 Therefore, no concerns for the target animals would arise for the supplementation of the diets with monosodium l‐glutamate monohydrate at 25 mg/kg feed. The applicant established conditions of use in water that would mirror the intakes resulting from the supplementation in feed; however, the FEEDAP Panel has reservations on the use of the additive via water due to hygienic reasons (EFSA FEEDAP Panel, 2010).
Regarding the safety for consumers, the vast majority of monosodium l‐glutamate monohydrate is metabolised in the gastrointestinal tract of the target animals and only a very small proportion enters either the systemic or the portal blood supply. It is not expected that the composition of tissues and products of animal origin will be affected by the use of monosodium l‐glutamate monohydrate as a feed additive. The FEEDAP Panel also notes that the same substance is authorised as additive in food at levels up to 10,000 mg/kg or L.
The use of monosodium l‐glutamate monohydrate as a feed additive at the levels proposed is not expected to increase its concentration in the environment, and therefore, it is of no safety concern for the environment.
Overall, the FEEDAP Panel concludes that monosodium l‐glutamate monohydrate produced by C. glutamicum KCCM 80188 is safe under the proposed conditions of use for the target species, for the consumer and for the environment. However, the Panel has reservations on the use of the additive in water for drinking of the target animals due to concerns on its impact on the hygienic conditions of the water.
3.2.2. Safety for user
3.2.2.1. Effects on the respiratory system
The additive has a very low dusting potential (up to 11 mg/m3).
In an acute inhalation toxicity study, conducted following OECD Guideline 403,37 the rats exposed to a concentration of 5.13 mg of the test item/L exhibited irregular respiration after exposure. All animals recovered by day 2 after exposure and no other signs were found during the 14‐day observation period. Further, no findings were evidenced in the gross pathology examination at the conclusion of the study. Therefore, the LC50 is greater than 5.13 mg/L.
3.2.2.2. Effects on skin and eyes
The skin irritation potential of monosodium l‐glutamate was tested in a valid study performed according to OECD guideline 439,38 which showed that it is not a skin irritant.
The eye irritation potential of monosodium l‐glutamate was tested in a valid study performed according to OECD guideline 437,39 which showed that it is not an eye irritant.
In a valid local lymph‐node assay (LLNA) following OECD guideline 429,40 monosodium l‐glutamate did not show any skin sensitisation potential.
3.2.2.3. Conclusions on safety for the user
The additive is not toxic by inhalation, not irritant to skin or eyes and is not a dermal sensitiser.
3.3. Efficacy
Monosodium glutamate is mentioned in Fenaroli's Handbook of Flavour Ingredients (Burdock, 2010), by the Flavour and Extract Manufactures Association (FEMA) as a flavour enhancer, i.e. a substance with no specific taste on its own but which has an ability to enhance existing flavours. l‐Glutamic acid is listed in Fenaroli's Handbook of Flavour Ingredients with the reference number 3285. Further, Monosodium glutamate is authorised under Commission Regulation (EU) No 1129/2011 on food additives as flavour enhancer in many processed food.
The Panel considers that the effect of monosodium l‐glutamate monohydrate to increase the taste of food is well documented, and therefore, no further demonstration of efficacy is necessary.
4. Conclusions
The additive is produced by a non‐genetically modified strain of C. glutamicum, KCCM 80188, and no viable cells of the production strain were detected in the final additive. The additive does not give rise to any safety concern regarding the production strain.
Monosodium l‐glutamate monohydrate produced by C. glutamicum KCCM 80188 is considered to be safe for the target species, for the consumer and for the environment. However, the use of the additive in water for drinking raises concerns for the target species due to its likely impact on the hygienic conditions of the water.
Monosodium l‐glutamate monohydrate produced by C. glutamicum KCCM 80188 is considered not toxic by inhalation, not irritant to skin or eyes and not a dermal sensitiser.
The FEEDAP Panel concludes that the additive is efficacious to contribute to the flavour of feed.
5. Documentation as provided to EFSA/Chronology
| Date | Event |
|---|---|
| 18/12/2018 | Dossier received by EFSA. Monosodium l‐glutamate for all animal species. CJ Europe GmbH |
| 18/01/2019 | Reception mandate from the European Commission |
| 01/03/2019 | Application validated by EFSA – Start of the scientific assessment |
| 29/03/209 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation |
| 19/08/2019 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 01/06/2019 | Comments received from Member States |
| 28/06/2019 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives |
| 22/10/2019 and 20/12/2019 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation |
| 17/01/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 02/03/2020 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: Characterisation |
| 16/03/2020 | Reception of supplementary information from the applicant ‐ Scientific assessment re‐started |
| 19/03/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AMR
Antimicrobial resistance genes
- ARDB
Antibiotic Resistance Genes Database
- CFU
Colony‐forming unit
- EURL
European Union Reference Laboratory
- FEEDAP
Panel on Additives and Products or substances used in Animal Feed
- FEMA
Flavour and Extract Manufactures Association
- HPLC‐UV
High‐performance liquid chromatography coupled with ultraviolet detection
- LLNA
Local lymph‐node assay
- LOD
Limit of detection
- LOQ
Limit of quantification
- PCB
polychlorinated biphenyls
- PCDDs
Polychlorinated dibenzodioxins
- PCDFs
Polychlorinated dibenzofurans
- QPS
Qualified presumption of safety
- WGS
Whole genome sequence data
Annex A – Executive Summary of the Evaluation Report of the European Union Reference Laboratory for Feed Additives on the Method(s) of Analysis for Monosodium l‐glutamate produced by fermentation with Corynebacterium glutamicum KCCM 80188
1.
In the current application, authorisation is sought under Article 4(1) for monosodium l‐ glutamate (MSG) produced by fermentation with Corynebacterium glutamicum KCCM80188, under the category/functional group 2(c) ‘sensory additives’/‘flavouring compound’, according to Annex I of Regulation (EC) No 1831/2003. Authorisation is sought for all animal species.
According to the Applicant, MSG has a minimum purity (mass fraction) of 99%. The feed additive is intended to be added directly into feedingstuffs (or through premixtures) and water for drinking. The Applicant proposed a maximum content of MSG in feedingstuffs of 25 mg/kg.
For the quantification of MSG in the feed additive, the Applicant submitted an in‐house validated analytical method based on reversed phase high‐performance liquid chromatography coupled with ultraviolet detection (HPLC‐UV). While in the frame of the validation study satisfactory performance characteristics were derived, the Applicant did not present a verification study or any additional test performed by a second independent laboratory applying the above‐mentioned method.
For the quantification of MSG in premixtures and feedingstuffs, the Applicant submitted the ring‐trial validated European Union method (Commission Regulation (EC) No 152/2009) based on ion exchange chromatography coupled to photometric detection (IEC‐VIS). This method, designed only for the analysis of amino acids in premixtures and feedingstuffs, does not distinguish between the salts (MSG) and the amino acid enantiomers. The method was further ring‐trial validated resulting in the EN ISO 13903:2005 method. The following performance characteristics were reported for the quantification of glutamic acid: RSDr ranging from 0.9% to 2.7% and RSDR ranging from 6.2% to 9.1%. However, while the lowest limit of quantification (LOQ) of 30 mg/kg has been reported for the analysis of certain amino acids, a specific LOQ for glutamic acid has not been indicated. Therefore, the method does not ensure the accurate determination of MSG when added into feed at the proposed maximum content (i.e. 25 mg/kg feedingstuffs). Hence, the EURL recommends for official control the European Union method based on IEC‐VIS for the quantification of MSG in premixtures only.
The Applicant did not provide any experimental data to determine MSG in water. Nevertheless, as concluded in previous EURL reports on amino acids, the EURL recommends for official control the procedure based on IEC‐VIS and described in the ring‐trial validated European Union method (or in equivalent ring‐trial validated methods e.g. VDLUFA Method 4.11.6.) to quantify MSG in the feed additive and water.
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Dusemund B, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Herman L, Glandorf B, Saarela M, Galobart J, Holczknecht O, Manini P, Pettenati E, Call JT, Pizzo F and Anguita M, 2020. Scientific opinion on the safety and efficacy of monosodium l‐glutamate monohydrate produced by Corynebacterium glutamicum KCCM 80188 as a feed additive for all animal species. EFSA Journal 2020;18(4):6085, 12 pp. 10.2903/j.efsa.2020.6085
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00037
Panel members: Giovanna Azimonti, Vasileios Bampidis Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Mojca Kos Durjava, Maryline Kouba, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Edoardo Villa and Ruud Woutersen.
Legal notice: Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Commission. The full output has been shared with the European Commission, EU Member States and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Commission.
Adopted: 19 March 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
CJ Europe GmbH, Ober der Roeth 4, 65824 Schwalbach/Taunus, Germany.
Commission implementing Regulation (EU) 2018/249 of 15 February 2018 concerning the authorisation of taurine, beta‐alanine, l‐alanine, l‐arginine, l‐aspartic acid, l‐histidine, d,l‐isoleucine, l‐leucine, l‐phenylalanine, l‐proline, d,l‐serine, l‐tyrosine, l‐methionine, l‐valine, l‐cysteine, glycine, monosodium glutamate and l‐glutamic acid as feed additives for all animal species and l‐cysteine hydrochloride monohydrate for all species except cats and dogs. OJ L 53, 23.2.2018, p. 134.
Commission Regulation (EU) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives Text with EEA relevance OJ L 295, 12.11.2011, p. 1.
FEED dossier reference: FAD‐2018‐0090.
The full report is available on the EURL website: https://ec.europa.eu/jrc/sites/jrcsh/files/finrep_fad-2018-0090-msglutamate.pdf
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
■■■■■
Technical dossier/Section II/Annex II.1.01 and supplementary information December 2019. The measurements are done with an HPLC method using glutamic acid as standard, validated method. The transformation from the standard glutamic acid to the active substance was done assuming the molecule as a monosodium and monohydrate.
Technical dossier/Section II/Annex II.1.03 and supplementary information December 2019.
Technical dossier/Supplementary information/March 2020/Annex 1.
This content of sodium would be in line with the stechiometry of the molecule assumed.
Technical dossier/Section II/Annex II.1.02.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council (Text with EEA relevance). OJ L 83, 22.3.2012, p. 1.
Technical dossier/Section II/Annex II.1.05. LOD (in mg/kg) were 1 for arsenic, lead and cadmium, 5 for mercury.
Technical dossier/Supplementary information/January 2020/Annex 1.
Technical dossier/Section II/Annex II.1.05. LOD (in µg/kg) was 0.1 for aflatoxins (B1, B2, G1, G2) and ochratoxin A; 1.5 for zearalenone; 0.5 for deoxynivalenol and 5 for fumonisin B1 and B2.
Technical dossier/Section II/Annex II.1.05. LOD (in µg/kg) was 0.09–0.15 for nitrofurans and 0.11–0.16 for nitrofuran metabolites.
Technical dossier/Section II/Annex II.1.06. LOD (in µg/kg) was 0.5–8.
Technical dossier/Section II/Annex II.1.05.
Technical dossier/Supplementary information August 2019/Subfolder 19082019/Annex 3.
Technical dossier/Section II/Annex II.1.08.
Technical dossier/Section II/Annex II.1.07.
Technical dossier/Section II/Annex_ II_4_01 and Supplementary information December 2019.
Technical dossier/Section II/Annex_ II_4_04 and Supplementary information December 2019.
Technical dossier/Section II/Annex_ II_4_02 and Supplementary information December 2019.
Technical dossier/Section II/Annex_ II_4_03.
Technical dossier/Section II/Annex_ II_4_05/Supplementary information December 2019/Annex SI.
Technical dossier/Supplementary information December 2019/annex SIN_03, values would equate (in mg/day) to 50 for veal calf, 227 for cattle for fattening, 568 for dairy cow, 34 for sheep/goat, 227 for horses, 7.1 for dog and 1.70 for cats.
A complete feed with a 20% of soya bean meal would present 1.8 g of glutamic acid per kg feed.
Technical dossier/Section III/Annex III.3.04 and Supplementary information August 2019/subfolder 190604.
Technical dossier/Section III/Annex III.3.06.
Technical dossier/Section III/Annex III.3.05.
Technical dossier/Section III/Annex III.3.07.
References
- Burdock GA, 2010. Fenaroli's Handbook of Flavor Ingredients. 6th Edition CRC press. Taylor & Francis Group; Boca Raton, FL. 143 pp. [Google Scholar]
- EFSA (European Food Safety Authority), 2007. Opinion of the Scientific Committee on a request from EFSA on the introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA. EFSA Journal 2007;5(11):587. 16 pp. 10.2903/j.efsa.2007.587 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen A, Aguilar F, Crebelli R, Di Domenico A, Dusemund B, Frutos MJ, Galtier P, Gott D, Gundert‐Remy U, Leblanc J‐C, Lindtner O, Moldeus P, Mosesso P, Parent‐Massin D, Oskarsson A, Stankovic I, Waalkens‐Berendsen I, Woutersen RA, Wright M, Younes M, Boon P, Chrysafidis D, Gurtler R, Tobback P, Altieri A, Rincon AM and Lambr € e C, 2017. Scientific Opinion on the re‐evaluation of glutamic acid (E 620), sodium glutamate (E 621), potassium glutamate (E 622), calcium glutamate (E 623), ammonium glutamate (E 624) and magnesium glutamate (E 625) as food additives. EFSA Journal 2017;15(7):4910, 90 pp. 10.2903/j.efsa.2017.4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Cocconcelli PS, Fernández Escámez PS, Maradona MP, Querol A, Suarez JE, Sundh I, Vlak J, Barizzone F, Correia S and Herman L, 2020. Scientific Opinion on the update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA Journal 2020;18(2):5966, 56 pp. 10.2903/j.efsa.2020.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2013. Scientific Opinion on the safety evaluation of the active substances iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite for use in food contact materials. EFSA Journal 2013;11(4):3155, 12 pp. 10.2903/j.efsa.2013.3155 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances Used in Animal Feed), 2010. Scientific Opinion on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2014. Scientific Opinion on the safety and efficacy of the use of amino acids (chemical group 34) when used as flavourings for all animal species. EFSA Journal 2014;12(5):3670, 19 pp. 10.2903/j.efsa.2014.3670 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Glandorf B, Herman L, Kärenlampi S, Aguilera J, Anguita M, Brozzi R and Galobart J, 2018a. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2018b. Guidance on the assessment of the efficacy of feed additives. EFSA Journal 2018;16(5):5274, 25 pp. 10.2903/j.efsa.2018.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Bampidis V, Bastos ML, Christensen H, Dusemund B, Kouba M, Kos Durjava M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brock T, Knecht J, Kolar B, Beelen P, Padovani L, Tarrés‐Call J, Vettori MV and Azimonti G, 2019. Guidance on the assessment of the safety of feed additives for the environment. EFSA Journal 2019;17(4):5648, 78 pp. 10.2903/j.efsa.2019.5648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 1991. Report of the Scientific Committee for Food (twenty‐fifth series). First series of food additives of various technological functions. Commission of the European Communities. Directorate General Internal Market and Industrial Affairs. Report EUR 13416. Luxembourg. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_reports_25.pdfvale
- FAO (Food and Agriculture Organization), 2014. FAO's Animal Feed Resources Information System (1991–2002) and from Bo Göhl's Tropical Feeds (1976–1982). Available online: http://www.feedipedia.org/node/222
- WHO (World Health Organization), 1987. Evaluation of certain food additives and contaminants. Thirty‐first report of the Joint FAO/WHO Experts Committee on Food Additives. Geneva, 16–25 February 1987. WHO Technical Report Series, No 759. Geneva, Switzerland. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_759.pdf [PubMed]


