Abstract
On‐land transport/storage of fresh fishery products (FFP) for up to 3 days in ‘tubs’ of three‐layered poly‐ethylene filled with freshwater and ice was compared to the currently authorised practice (fish boxes of high‐density poly‐ethylene filled with ice). The impact on the survival and growth of biological hazards in fish and the histamine production in fish species associated with a high amount of histidine was assessed. In different modelling scenarios, the FFP are stored on‐board in freshwater or seawater/ice (in tubs) and once on‐land they are ‘handled’ (i.e. sorted or gutted and/or filleted) and transferred to either tubs or boxes. The temperature of the FFP was assumed to be the most influential factor affecting relevant hazards. Under reasonably foreseeable ‘abusive’ scenarios and using a conservative modelling approach, the growth of the relevant hazards (i.e. Listeria monocytogenes, Aeromonas spp. and non‐proteolytic Clostridium botulinum), is expected to be < 0.2 log10 units higher in tubs than in boxes after 3 days when the initial temperature of the fish is 0°C (‘keeping’ process). Starting at 7°C (‘cooling‐keeping’ process), the expected difference in the growth potential is higher (< 1 log10 for A. hydrophila and < 0.5 log10 for the other two hazards) due to the poorer cooling capacity of water and ice (tub) compared with ice (box). The survival of relevant hazards is not or is negligibly impacted. Histamine formation due to growth of Morganella psychrotolerans under the ‘keeping’ or ‘cooling‐keeping’ process can be up to 0.4 ppm and 1.5 ppm higher, respectively, in tubs as compared to boxes after 3 days, without reaching the legal limit of 100 ppm. The water uptake associated with the storage of the FFP in tubs (which may be up to 6%) does not make a relevant contribution to the differences in microbial growth potential compared to boxes.
Keywords: Fish tubs, fish boxes, transport, storage, fresh fishery products, biological hazards, histamine
Summary
Following a request from the European Commission, the EFSA Scientific Panel on Biological Hazards (BIOHAZ) was asked to provide a scientific opinion on the use of fish tubs compared to fish boxes for transporting and storing fresh fishery products (FFP) upon their arrival at the first on‐land establishment.
In Term of Reference 1 (ToR1), EFSA was requested to compare the impact that transport and storage of FFPs in on‐land establishments in tubs, when compared to currently authorised practices (fish boxes), could have on the survival and growth of biological hazards and the production of histamine in fish species associated with a high amount of histidine. The ToR2 was to estimate the impact that transport and storage in tubs have on the water content of the fish meat compared to boxes and on its consequences on the survival and growth of biological hazards.
It was clarified that the tubs are composed of three‐layered poly‐ethylene (PE) and filled with freshwater and ice while the boxes are composed of high density poly‐ethylene (HDPE) and are filled with layers of fish and ice. Fish are expected to be kept in tubs for a maximum of 3 days, with an exceptional maximum duration of 5 days.
The FFP should have been transported on‐board using tubs filled with fresh or seawater and ice. Upon the arrival at the first on‐land establishment, the FFP may be gutted and/or filleted before on‐land transport/storage as whole, gutted or filleted fish. The assessment compared the following conditions:
Baseline or current condition: the FFP are unloaded and transferred to boxes with ice, where they are kept during transport and storage on‐land until dispatched and/or marketed;
Alternative condition: the FFP are unloaded and transferred to tubs with freshwater and ice, where they are kept during transport and storage on‐land until dispatched and/or marketed.
Good practice with handling fish catches as well as the use of enough ice and re‐icing was assumed. It was also assumed that the initial status of the fish (e.g. intrinsic characteristics, conditions on‐board, hygienic status) was common for the two conditions, and thus, the relative impact during the on‐land transport and storage in boxes or tubs would be equivalent.
The relevant biological hazards were identified from the literature, considering their ability to cause human illness associated with FFP and to grow and/or survive on FFP within the temperature range of −3°C to 7°C. The hazards of interest may be present on the fish surface, fish meat or intestines. The relevant biological hazards for growth on FFP at refrigeration temperatures < 7°C are Aeromonas spp., non‐proteolytic Clostridium botulinum and Listeria monocytogenes, while those relevant for survival (no change or reduction) are pathogenic Escherichia coli, Salmonella, Staphylococcus aureus, Vibrio spp. and Nematodes (Anisakis spp.). The relevant histamine‐producing bacteria in FFPs are Enterobacter spp., Morganella spp. and Photobacterium spp.
Due to the scarcity of data on fish temperatures comparing both conditions, heat transfer modelling was used. The temperature of the fish surface was considered the main impacting factor. Two processes were considered:
the capacity of maintaining (i.e. ‘keeping’) the temperature when fish from tubs on‐board are transferred to either boxes or tubs for transport and storage on‐land, assuming no change of the fish temperature during such transfer, and
the ‘cooling’ capacity when the fish is handled (e.g. gutting and/or filleting) after landing, which causes an increase of the fish temperature to 7°C, and are then transferred to either boxes or tubs for transport and storage on‐land (i.e. ‘cooling‐keeping’).
In an ‘ideal’ scenario with proper practices and assuming that the initial fish temperature is 0°C and the fish is in perfect contact with ice in boxes and with a perfect mixing of water and ice in tubs, the temperature of the fish surface would be equal for both types of containers and equivalent to the temperature of melting ice (i.e. 0°C) throughout the transport/storage period.
Only two experiments using lean small fish (plaice) provided ‘observed’ time–Temperature (t/T) profiles of fish upon storage/transport in both type of containers. The fish temperature fluctuated and relevant differences were observed depending on the location of the fish within the containers, which can be related to the distance from the ice layer as well as to the container walls. Overall, the median fish temperature was about 1.0°C higher when transported/stored in tubs compared to boxes, but after the short initial cooling stage, fish temperature never exceeded 3.1°C during the subsequent storage/transport.
The heat transfer model predicted fish surface temperatures under reasonable foreseeable ‘abusive’ scenarios of the outside temperature where temperature is mostly at 2°C but including some abusive peaks up to 6°C, assuming that in boxes, fish are surrounded by two layers of ice, and in tubs, fish are in water below an ice layer without mixing. The model provided satisfactory outputs when compared with observed data, for both types of containers. The model was then applied to generate the fish surface t/T profiles for both processes using both type of containers under the same conditions of transport/storage, considering the size of the fish (e.g. small flat fish such as plaice vs. bigger fish with a broad oval cross section, such as salmon) and their fat content (1–4% vs. 10–20%). The fat content and the dimensions of the fish have only limited impact on the t/T profiles as compared to the impact of the initial fish temperature and outside temperature of the chilling room where tubs and boxes are stored, or transported. The fish surface temperatures depend on the location of the fish within the containers, with fish located in positions more distant from the ice (the centre between ice layers in a box and the bottom of the tub) and closer to the walls of the container being the worst‐case scenario (warmest). It is important to note that, at earlier stages of storage, fish cools down faster in boxes than in tubs. Later, as the ice melts, the capacity to keep the temperature low is less in boxes than in tubs, which can be related to the insulating properties of the tubs.
The impact of the transport and storage of FFP in tubs and boxes on the water content of the fish meat was assessed through review of the available published data in the scientific literature as was the impact on the physico‐chemical characteristics of the FFP relevant for microbial behaviour. From available data on water and salt content of fish, the water phase salt (WPS) content was calculated. FFP stored/transported in tubs with fresh water and ice may increase the water content from 0% to 6%, causing a reduction of the WPS concentration (%) ranging from 0 to 0.019 units in comparison with the FFP transferred to boxes.
The impact of the fish temperature on the growth or survival of the identified hazards during the transport and storage of FFP in tubs in comparison with boxes was assessed applying available predictive models for specific pathogens and histamine accumulation. t/T profiles from observed data and predicted through heat transfer modelling were used as input. The impact of the WPS change due to potential water uptake upon storage of FFP in tubs was assessed by changing this input factor in the predictive models. The impact of other factors (e.g. pH, initial concentration of histamine producing bacteria, lag phase, oxygen availability, internalisation) was considered in the uncertainty analysis. When no predictive models were available for a particular hazard, its behaviour was assessed using the evidence obtained through the literature review.
Under an ‘ideal’ scenario described above, there is no difference in the growth potential of Aeromonas spp., non‐proteolytic Cl. botulinum and L. monocytogenes in FFP when transported/stored in tubs compared to boxes as FFP temperature is maintained at 0°C throughout the storage/transport. Under ‘reasonably foreseeable abusive’ scenarios (referred to ‘abusive’ scenarios), the initial fish temperature when transferring the FFP to the tub or box needs to be considered. If the initial fish temperature equals 0°C (referred to as ‘keeping’ process), the growth potential (log10 increase) of the relevant hazards (i.e. A. hydrophila, L. monocytogenes or non‐proteolytic Cl. botulinum) is up to 0.12 log10 units, 0.17 log10 units, and 0.27 log10 units higher in tubs than in boxes after 2 days, 3 days, and 5 days, respectively. Instead, if the initial fish temperature is equal to 7°C (referred to as ‘cooling‐keeping’ process), the difference in the growth potential (log10 increase) of the relevant hazards is of a higher magnitude compared to the ‘keeping’ process mainly as a result of the poorer cooling capacity of water with ice (in tubs) compared to ice (in boxes). More specifically, for A. hydrophila, L. monocytogenes, and non‐proteolytic Cl. botulinum growth, the log10 increase is up to 1 log10 units, 0.5 log10 units and 0.5 log10 units higher in tubs than in boxes, respectively, after 3 days of storage/transport. An exceptional duration of the storage/transport of 5 days would result in a limited additional increase (≤ 0.1 log10) of the differences in the growth potential of the relevant hazards between boxes and tubs. Under the conditions of the assessment based on the fish t/T profiles that may occur under ‘abusive’ conditions of transport/storage of FFP, no substantial differences in the magnitude of reduction of pathogens between boxes and tubs are expected.
As for growth of the histamine‐producing hazards, under the ‘ideal’ scenario, there is no difference in the growth potential of M. psychrotolerans on FFP and on histamine accumulation when transported/stored in tubs compared to boxes. Under ‘abusive’ scenarios, when the initial fish temperature at transfer to the tub or box equals either 0°C or 7°C (referred to as ‘keeping process’ or ‘cooling‐keeping’ process), the histamine formation due to the growth of M. psychrotolerans can be up to 0.4 and 1.5 ppm higher, respectively, in tubs compared to boxes after 3 days. After the exceptional maximum duration of 5 days, the maximum difference can be up to 16 ppm and in any case the limit of 100 ppm histamine as defined in Commission Regulation (EC) 2073/200510 is not reached. Though it was not possible to quantify the temperature‐dependent growth and histamine production by Enterobacter spp. and Photobacterium spp, for the present assessment, these two histamine‐forming bacteria are considered less relevant than M. psychrotolerans because they have a lower histamine‐producing potential.
As the t/T profile is only slightly affected by the size and fat content of the fish, so is the associated growth potential of the relevant hazards. The location of the fish within the container impacted the t/T profile and thus the associated microbial growth.
The foreseeable decrease in WPS due to the uptake of water when fish is stored/transported in tub (in water and ice) has a negligible impact on the growth rate of all identified biological hazards and, consequently, on the growth potential of relevant pathogens, and on the histamine formation in FFP stored/transported in tubs compared to boxes.
In principle, any condition leading to more growth (log10 increase) of the relevant hazards would increase the public health risk. A higher histamine accumulation in tubs compared to boxes would be increase the public health risk, only if the threshold benchmark dose is exceeded. Quantification of the risk to public health associated with the storage/transport in tubs compared to boxes would require a quantitative microbial risk assessment (QMRA), including an exposure assessment taking into account subsequent steps of the FFP supply chain including the consumer handling and consumption habits as well as the dose–response relationship for each relevant hazard. Such a model is not available, and its development was beyond the scope of the present mandate.
Several recommendations for the sector for limiting the growth of pathogens when using tubs filled with water and ice are included, such as using precooled tubs made of insulating material and with a lid. To fill the tubs with sufficient water at a temperature as close to 0°C as possible and with enough ice on top to cover the whole surface of the tubs, making sure that all fish are below the ice layer, as well as to keep the temperature of the chilling room close to the melting ice temperature throughout the whole period of storage and transport. During transport and storage, with an absolute maximum duration of 5 days, it is recommended to re‐ice if needed and to circulate water inside the tubs to achieve uniform temperatures within the container. At last, tubs have to be transport and stored (as for boxes) in a cool environment.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
In accordance with Annex III, Section VIII, Chapter III point A of Regulation (EC) No 853/20041, when chilled, unpackaged [fishery] products are not distributed, dispatched, prepared or processed immediately after reaching an establishment on‐land, they must be stored under ice in appropriate facilities. Re‐icing must be carried out as often as necessary.
The same point A states that containers (fish boxes) used for the dispatch or storage of unpackaged prepared fresh fishery products stored under ice must ensure that melt water does not remain in contact with the products. However, whole and gutted fresh fishery products may be transported and stored in cooled water on board vessels. They may also continue to be transported in cooled water after landing, and be transported from aquaculture establishments, until they arrive at the first establishment on‐land carrying out any activity other than transport or sorting.
The industry claims that fish boxes are keeping the upper fish cool, while the bottom ones are pressed together and the required temperature is difficult to reach in those conditions, as the ice prevents full surface contact. In addition, the use of ice boxes causes mechanical damage to the fish and enhances the formation of bacterial ‘slime’.
According with a study published by ILVO (Research Institute for Agriculture, Fisheries and Food) an internationally recognized scientific institute part of the Government of Flanders,2 commissioned by the Flemish Fish Auctions, the preservation of fish in tubs (large plastic containers measuring 1 × 1 × 1 meters (or 1 × 1 × 0.5 meters), filled with ice and water) was excellent and even slightly better than in fish boxes (polystyrene boxes3 where the fish is placed under ice, normally used by the industry), but storage in tubs also affected the water content of the fish. The temperature differences and fluctuations proved limited in tubs. Based on this research, ILVO formulated recommendations for the use of tubs.
The study demonstrated that, during a shelf life experiment, a temperature difference within the same tub of up to 2.8°C was recorded. The threshold level of 4°C for the fish temperature was never exceeded despite some fluctuations in the ambient temperature. Although the fish in tubs reached slightly higher temperatures than the fish in the boxes, this had no effect on the quality of the fish. The sensory and chemical analyses showed that plaice in tubs even had a longer shelf life than plaice in boxes. Although the microbiological parameters were also better in the tubs than in the boxes, these differences were not significant. Transport did not affect any parameter. The storage in tubs did have an effect on the water content of the fish meat. This effect was evident already after 2 days of storage: fish in tubs clearly had higher water content than in boxes.
EFSA is asked to provide a scientific opinion on the use of “tubs” compared to fish boxes for transporting and storing fresh fishery products in on‐land establishments. In particular, EFSA is requested:
Terms of Reference (ToR) 1: To compare the impact that transport and storage of fresh fishery products in on‐land establishments in “tubs”, when compared to currently authorised practices (fish boxes), could have on:
-
a)
the survival and growth of biological hazards.
-
b)
the production of histamine in fish species associated with a high amount of histidine.4
ToR 2: To estimate the impact that transport and storage in tubs have on the water content of the fish meat compared to currently authorised practices (fish boxes) and its consequences on the survival and growth of biological hazards.
1.2. Interpretation of the Terms of Reference
The term ‘fishery products’ as defined by Regulation (EC) No 853/20041, Annex I, comprises all seawater and freshwater animals (except for live bivalve molluscs, live echinoderms, live tunicates and live marine gastropods, and all mammals, reptiles and frogs) whether wild or farmed and including all edible forms, parts and products of such animals. In Annex I, ‘fresh fishery products’ (FFP) are defined as unprocessed fishery products, whether whole or prepared, including products packaged under vacuum or in a modified atmosphere, that have not undergone any treatment to ensure preservation other than chilling. However, as the current mandate is restricted to unpackaged FFP, with packaged FFP being beyond the scope of this assessment. It was clarified that the specific FFP to which the ToR apply also include ‘prepared fishery products’, in particular unprocessed fishery products that have undergone an operation affecting their anatomical wholeness, such as gutting, heading and filleting. The FFP considered in the assessment are derived from the marine and land‐based environment.
The assessment focuses on the EU/EEA regarding the transport and storage of unpackaged FFP (referred to as ‘FFP’ throughout the rest of the document) from the first on‐land establishments onwards using ‘boxes’ (as authorised practice) in comparison with ‘tubs’ (as alternative practice) until they are marketed or processed. The terms ‘box’ and ‘tub’ encompass plastic containers (Figure 1) made of different materials and with different dimensions depending on its use on‐board or on‐land. Tubs are large containers which are usually filled with ice and seawater (only on‐board) or ice and freshwater (on‐board or on‐land). Boxes are filled with layers of fish and ice.
Figure 1.

Example of a fish box (white) and a tub (red) (Source of picture: Visfederatie, 2020)
The tubs to be considered for on‐land use are composed of three‐layered poly‐ethylene (PE) and are filled with ice and freshwater. The boxes are composed of high‐density poly‐ethylene (HDPE) and are filled with layers of fish and ice. Polystyrene boxes are not to be evaluated, as the focus of interest is mainly on business to business transport. Fish were expected to be kept in tubs for a maximum of 3 days. However, the absolute and exceptional maximum duration considered was 5 days.
It was clarified with the requestor that FFP transported on‐board using tubs (filled with fresh or seawater and ice) should be considered for the assessment, so the use on‐board of boxes (with ice) is beyond the scope of this opinion.
It was agreed with the requestor to assume that the freshwater and seawater as well as the ice (made either from fresh or seawater) used to fill the tubs and/or boxes comply with the legal standard. Thus, when either potable water or clean seawater are used, according to their definitions in Regulation (EC) 852/20045 , 6, they should not be a source of microbiological contamination of the fish. The growth and survival of spoilage microorganisms as well as quality traits (e.g. sensory, commercial value etc.) are also beyond the scope of this mandate.
The term ‘impact’ of a given condition on the growth or survival of a biological hazard (including accumulation of histamine) was interpreted as the change of the concentration of the hazard, e.g. microorganism log10 concentration change or histamine concentration change. Subsequently, the requestor clarified that in case of any evidence of a different survival or growth of biological hazards in FFP submitted to the alternative practice in comparison with the baseline, to indicate if this could represent a potential risk for public health.
ToR 2 is considered implicitly included in ToR 1 as the output of ToR 2 is needed to answer ToR 1. Therefore, as the two ToRs are interlinked, it was agreed to combine the conclusions for both ToRs.
Based on the interpretations described above, the following assessment questions (AQs) were formulated in order to address the ToR.
AQ 1: What is the reduction potential (i.e. log10 decrease) or growth potential (i.e. log10 increase) of relevant biological hazards when FFP, initially stored in freshwater or seawater/ice (in tubs) on board, are subsequently ‘handled’ (i.e. sorted or gutted and/or filleted) at the first on‐land establishment and then transferred to freshwater/ice (in three‐layered PE tubs) compared to being transferred to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days? Is there a potential increased risk for public health as a result of using tubs compared to boxes?
AQ 2: What is the magnitude of histamine accumulation in fish species associated with a high amount of histidine when FFP, initially stored in freshwater or seawater/ice (in tubs) on board, are then ‘handled’ (i.e. sorted or gutted and/or filleted) at the first on‐land establishment before being transferred to freshwater/ice (in three‐layered PE tubs) compared to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days? Is there a potential increased risk for public health as a result of using tubs compared to boxes?
AQ 3: What is the contribution of the water content change of the fish meat on previous AQs outcomes when FFP, first stored in freshwater or seawater/ice (in tubs) on board, are ‘handled’ at the first on‐land establishment and then transferred to freshwater/ice (in three‐layered PE tubs) compared to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days?
1.3. Additional information
1.3.1. Additional background information
1.3.1.1. Previous EFSA scientific opinions and reports
In 2015, EFSA published a report on the assessment of the temperature conditions, including a possible tolerance, to be applied for storage and transport of packaged FFP, gutted or entire, including some parts of them, at retail level where icing is not possible. The main temperature‐dependent hazards identified were histamine as well as the psychrotrophic Listeria monocytogenes, Clostridium botulinum and Yersinia enterocolitica. It was concluded that it is possible to store packaged FFP at refrigeration temperatures above 0°C (e.g. 3–5°C) and still be compliant with the current EU and international regulated microbiological criteria. For this, the storage time and the concentration of CO2 in the packaging headspace need to be adjusted accordingly. The report provides several scenarios equivalent to storage at 0°C, consisting of combinations of storage temperature, shelf‐life and CO2 concentration in the package (EFSA, 2015).
Also in 2015, EFSA published a scientific opinion of the Panel on Biological Hazards (BIOHAZ) and the Panel on Contaminants in the Food Chain (CONTAM) on the minimum hygiene criteria to be applied to clean seawater and on the public health risks and hygiene criteria for bottled seawater intended for domestic use. It was concluded that the comprehensiveness of the sanitary survey, the stringency of microbiological criteria and the need for treatment depend on the relative exposures associated with the different uses of seawater. For uses with low exposure to microbiological hazards, a basic sanitary survey and microbiological criteria based on the Directive 2006/7/EC7 are considered appropriate. For uses with a higher exposure, a more comprehensive sanitary survey, mandatory water treatment and microbiological criteria based on Council Directive 98/83/EC8 with an additional criterion for Vibrio spp. are considered appropriate (EFSA BIOHAZ and CONTAM Panel, 2015). Low exposure could be considered when using seawater to fill the tubs on‐board, while high exposure could be considered when seawater is in contact with prepared, processed and/or ready‐to‐eat (RTE) fishery products.
In 2017, EFSA assessed the incidents of histamine intoxication in some EU countries that were linked to consumption of tuna and were notified through the Rapid Alert System for Food and Feed (RASFF). All incidents of histamine intoxication were evaluated to highlight common factors in the food distribution chain that potentially contributed to the human cases, and to verify the possible correlation upstream in the food supply chain through the food business operators (FBO) involved. Due to the nature of histamine and the conditions that favour its production, it was concluded that it is likely that several concurrent factors have occurred in several stages along the food chain. It was recommended to maintain adequate chilling rates, carefully manage the cold chain and ensure hygienic conditions at each step of the supply chain of this product (EFSA, 2017).
1.3.1.2. Legal background
According to food safety requirements of the Regulation (EC) 178/20029, food shall not be placed on the marked if it is unsafe, i.e. if it is considered to be injurious to health and/or unfit for consumption (e.g. due to spoilage) taking into account the normal conditions of use at each stage of production, processing distribution and the consumer.
Regulation (EC) No 853/20041 lays down specific rules on the hygiene of food of animal origin for FBOs and supplements Regulation (EC) No 852/20045 on the hygiene of foodstuffs. Section VIII of Annex III of Regulation (EC) No 853/20041 deals with fishery products. It states that clean water may be used for the handling and washing of fishery products and the production of ice used to chill fishery products. It supplements the requirements of Annex II, Chapter VII to that Regulation stating that clean water may be used with whole fishery products. When clean water is used, adequate facilities and procedures are to be available for its supply to ensure that such use is not a source of contamination for the foodstuff.
Regulation (EC) 852/20045 defines ‘clean seawater’ as natural, artificial or purified seawater or brackish water that does not contain microorganisms, harmful substances or toxic marine plankton in quantities capable of directly or indirectly affecting the health quality of food. ‘Potable water’ means water meeting the minimum requirements laid down in Council Directive 98/83/EC of 3 November 19988 on the quality of water intended for human consumption; ‘clean water’ includes clean seawater and freshwater of a similar quality.
Relevant information from the Chapters in Annex III, Section VIII of the Regulation (EC) No 853/20041 is summarised here. Chapter I states that vessels designed and equipped to preserve fishery products for more than 24 h must be equipped with holds, tanks or containers for the storage of fishery products at the temperatures laid down in Chapter VII (see below). Holds and containers must ensure that melt water does not remain in contact with the products. In vessels equipped for chilling fishery products in cooled clean seawater, tanks must incorporate devices for achieving a uniform temperature throughout the tanks, reaching, after loading, not more than 3°C and not more than 0°C within 6 h and 16 h, respectively, and must allow monitoring/recording of temperatures.
Chapter II defines the requirements during and after landing. It specifies that, when it is not possible to refrigerate them on board vessels, FFPs shall be refrigerated as soon as possible after their landing and stored at a temperature close to that of melting ice. It also states that FBOs displaying FFPs for sale must ensure their refrigerated storage.
Chapter III defines the requirements for establishments, including vessels, which handle fishery products. It states that:
Where chilled, unpackaged products are not distributed, dispatched, prepared or processed immediately after reaching an establishment on land, they must be stored under ice in appropriate facilities. Re‐icing must be carried out as often as necessary. Packaged FFPs must be chilled to a temperature approaching that of melting ice.
Operations such as heading and gutting must be carried out hygienically and as quickly as possible after the products have been caught or landed. The products must be washed thoroughly immediately after these operations.
Operations such as filleting and cutting must be carried out so as to avoid contamination or spoilage of fillets and slices. Fillets and slices must not remain on the worktables beyond the time necessary for their preparation and must be wrapped and, where necessary, packaged and must be chilled as quickly as possible after their preparation.
Containers used for the dispatch or storage of unpackaged prepared FFPs stored under ice must ensure that water from melted ice does not remain in contact with the fish.
Whole and gutted FFPs may be transported and stored in cooled water on‐board vessels. They may also continue to be transported in cooled water after landing, and be transported from aquaculture establishments, until they arrive at the first establishment on land carrying out any activity other than transport or sorting.
Chapter V states that FBOs must ensure that the limits with regard to histamine are not exceeded. These limits are defined in Commission Regulation (EC) 2073/200510 as food safety criteria (FSC) in two different types of fishery products placed on the market during their shelf‐life:
In fishery products from fish species associated with a high amount of histamine (particularly fish species of the families Scombridae, Clupeidae, Engraulidae, Coryfenidae, Pomatomidae and Scombresosidae), out of n = 9 units comprising the sample, c = 2 units may have a histamine concentration between m = 100 and M = 200 mg/kg, none may be > M, and the mean value observed should be ≤ m.
The second type comprises fishery products from the same fish species that have undergone enzyme maturation treatment in brine. Out of the n = 9 units comprising the sample, c = 2 units may have a histamine concentration between m = 200 and M = 400 mg/kg, while none may be > M.
Chapter VI states that containers in which FFPs are kept on ice must be water resistant. Chapter VII states that FFPs must be maintained at a temperature approaching that of melting ice during their storage while Chapter VIII states that this also applies during their transport and reiterates that melt water must not remain in contact the FFPs, when kept under ice.
1.3.1.3. Fresh fishery products, production and supply chains
Fish can originate from aquaculture or be caught in the wild either from the sea or from freshwater environments. Fish exist at the ambient temperature of their environment, so their initial temperature is generally above 0°C. Seawater and freshwater temperatures vary significantly depending on many factors (e.g. season, latitude, depth, freshwater sources or oceanographic currents) and EU waters range from ~ 4°C (north) to 25°C or higher (south) (EFSA, 2015).
The handling of fishery products on‐board depends on the fish species and aims to assure the quality of the catch fish to be marketed (Huss, 1995; Mai et al., 2010; Valtýsdóttir et al., 2010; Matis, 2017). Small fish, such as herring or mackerel, are stored without bleeding or gutting, while bigger fish such as cod or salmon are bled after slaughtering and gutted as soon as possible thereafter (Borderías and Sánchez‐Alonso, 2011; Matis, 2017). However, a delay of up to 24 h for gutting can be applied for bigger fish provided that they are rapidly chilled. As stated by the current regulation (see Section 1.3.1.2), fish, which are bled or gutted, are washed before being stored on‐board.
Cooling and careful handling at all stages of the fish supply chain are key factors to assure good quality, maximum shelf‐life and high value of fishery products. Additional factors which can affect the quality of the fishery products are fishing season, area, fishing and handling equipment, nutritional status and age, rigor mortis as well as handling before and after processing. Once the fish are caught, they are chilled to achieve a temperature close to that of melting ice. The chilling methods used include immersion in ice slurry, refrigerated seawater (RSW) or flake ice. For instance, flake ice is widely used to reduce the temperature of fresh fish down to final levels slightly above 0°C; slurry ice (a mixture of small ice crystals suspended in water) is often used for superchilled products because it allows the product temperature to be reduced to just below the initial freezing temperature (−0.5°C to −2.8°C) (Stonehouse and Evans, 2015; Laguerre et al., 2018). The resultant temperature gradient brings the fish surfaces in contact with ice close to 0°C relatively rapidly, while the core temperature drops more slowly. Factors which may affect the rate of cooling include the ratio of fish to ice, contact of fish with ice, the size and the initial temperature of the fish, the type and temperature of the ice, the type of containers (including their insulating properties), the temperature outside the container and, for longer fishing trips, the frequency of re‐icing (Huss, 1995; Shawyer and Pizzal, 2003). On‐board, the stowage can be in fish boxes or tubs with ice, in fish tubs with a mixture of seawater or freshwater and ice and also by other means such as on shelves in the ship hold covered with ice or in RSW/chilled sea water (CSW) tanks (Stonehouse and Evans, 2015; Laguerre et al., 2018).
As a consequence of the heat transfer from the fish to the ice, the ice melts. Whilst the temperature of slush‐ice mixture arguably approaches that of melting ice, the efficacy of icing to maintain the cold chain is optimised by replacing melted ice (re‐icing) and removing water (melted ice) in contact with fish, making use of the drainage holes on the containers. Melted ice replacement also helps to remove organic matter and some of the microbial contamination carried by the water (Huss, 1995; Adams and Moss, 2006).
FFP going directly to the fish processing plant are kept in the same container from the catching ground to the processing plant. Otherwise, after landing fish is usually sorted and transferred to new containers (boxes) in fish auction centres. Throughout the subsequent distribution chain, transport and storage of FFP on‐land are carried out in ice and away from water (from melted ice) whilst in boxes. Tubs made with insulating material are also being used to keep fish in ice (Seafish Fact Sheet, 2008; Margeirsson et al., 2010; Margeirsson, 2012).
1.3.2. Approach to answer the ToR
A conceptual map of the conditions to be addressed in the current assessment is depicted in Figure 2. Upon arrival at the first on‐land establishment, the conditions to be compared in the assessment include:
Baseline or current condition: the FFP are unloaded and transferred to boxes with ice, where they are kept during transport and storage on‐land until dispatched and/or marketed; and
Alternative condition: the FFP are unloaded and transferred to tubs with freshwater and ice, where they are kept during transport and storage on‐land until dispatched and/or marketed.
Figure 2.
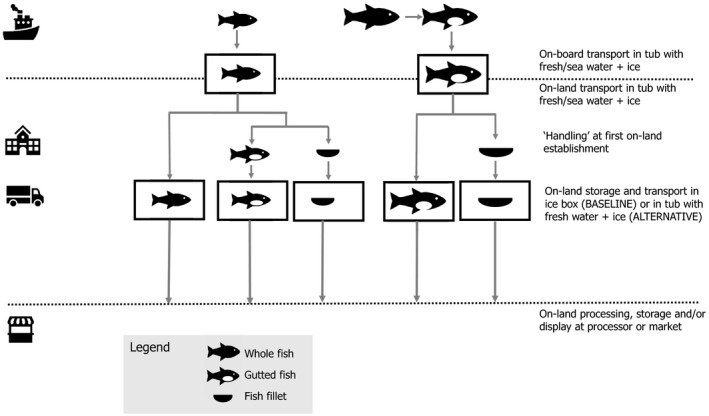
General flow diagram of the transport and storage of fresh fishery products
For the present assessment, either whole or gutted FFP are transported on‐board using tubs filled with freshwater or seawater and ice. As fish filleted on‐board are always frozen, fish filleted on‐board is out of the scope of this assessment. Once on‐land, the FFP may be gutted and/or filleted at the first establishment before the on‐land transport/storage as whole, gutted or filleted fish in boxes with ice (baseline) or in tubs with freshwater and ice (alternative condition). Good practice when handling fish catches as well as the use of sufficient ice and re‐icing was assumed. It was also assumed that the initial status of the fish (e.g. intrinsic characteristics, conditions on‐board, hygienic status) was common for the two conditions, and thus, the relative impact during the on‐land transport and storage in boxes or tubs would be equivalent.
To address the AQs to answer the ToRs, the following steps were undertaken:
The relevant biological hazards (including histidine‐decarboxylase bacteria and the consequent histamine accumulation) were identified from the literature, considering their ability to cause human illness associated with FFP and to grow and/or survive (either no change or reduction) on FFP considering the temperatures encountered during transport and storage in boxes and tubs. The hazards of interest are those that may be present on the fish surface or within fish meat or intestines.
-
The temperature of the fish surface was considered to be the main factor impacting the growth and/or survival of the identified hazards during the storage and transport of FFP in tubs as compared to boxes. Due to scarcity of data for fish temperatures in both conditions, heat transfer modelling was used to assess:
the capacity for maintaining (i.e. ‘keeping’) the temperature when fish from tubs on‐board are transferred to either boxes or tubs for transport and storage on‐land, assuming no change of the fish temperature during such transfer, and
the ‘cooling’ capacity when the fish handling (e.g. gutting and/or filleting) after landing causes an increase of the fish temperature which are then transferred to either boxes or tubs for transport and storage on‐land.
The impact of the transport and storage of FFP in tubs and boxes on the water content of the fish meat was assessed through literature and subsequently, its impact on the physico‐chemical characteristics of the FFP (relevant for microbial behaviour) was assessed. From available data on water and salt content of fish, the water phase salt (WPS) content was derived.
The impact of the fish temperature on the behaviour (growth or survival) of the identified hazards during the transport and storage of FFP in tubs compared to boxes was assessed applying available predictive models for specific pathogens and histamine accumulation using as input temperature profiles from observed data and predicted through heat transfer modelling. The impact of the WPS change due to potential water uptake upon storage of FFP in tubs was assessed by changing this factor in the predictive models. The impact of other factors (e.g. pH, initial concentration of histamine producing bacteria, lag phase) was considered in the uncertainty analysis. When no predictive models were available for a particular hazard, its behaviour was assessed through literature review.
For the quantification of the potential risk to public health, a quantitative microbial risk assessment (QMRA) model would be needed, integrating the level of exposure of the relevant hazards due to the consumption of FFP and a dose–response (DR) relationship for each considered hazard. Such a QMRA was not available and its development would require the collection of a large data set and information which was out of the scope of the present mandate. Consequently, the potential risk to public health was not quantified, but factors that would need to be included in such assessment were described.
2. Data and methodologies
2.1. Hazard identification
The listing of the biological hazards (including histidine‐decarboxylase bacteria and the consequent histamine accumulation) associated with FFP was based on the scientific opinion (EFSA BIOHAZ and CONTAM Panel, 2015) and report (EFSA, 2015) summarised in Section 1.3.1.1 and considered hazards potentially present in FFP, including fishes, crustacea, cephalopods and sea urchins. Bivalve shellfish were not considered as they are sold either live or frozen. For the selection of hazards relevant for FFP, the compiled list was screened considering first the evidence of these hazards to cause human illness (i.e. report of human cases or outbreaks) associated with fishery products that had not been further processed (e.g. salted, marinated, smoked etc.). Cl. botulinum was included in the screening for its potential for growth under anaerobic conditions that may occur in specific niches within fishery products. For Cl. botulinum, evidence of human illness associated with products other than canned fish was considered.
The hazards complying with this first criteria were then evaluated for their ability to grow and/or to survive in raw fishery products, considering a temperature range of the fish (from −3°C to 7°C11) that encompassed the reasonably foreseeable temperatures in boxes or tubs during storage and transport, including temperature increases associated with ‘handling’, such as gutting and/or filleting. In literature screening of the ability of the hazards to grow and/or to survive in raw fishery products, growth and reduction were considered relevant when an increase or decrease ≥ 0.5 log10 units was reported. This criterion was defined in agreement with the reliability (circa 0.5 log10) generally attributed to quantitative enumeration methods on the basis of early studies (Jarvis et al., 1977; Kramer and Gilbert, 1978), and confirmed by the analysis of measurement uncertainty (median: 0.6 log10 CFU/g) associated with microbiological methods for specific organisms count (Jarvis et al., 2007). Furthermore, the threshold of ≥ 0.5 log10 units was considered conservative compared to other values (i.e. ≥ 1.0 log10 units) in some instances adopted as a measure of significant variation in food microbiology.
Other physio‐chemical parameters (such as pH and WPS) relevant for growth and/or survival were not considered at screening level. Compared to EFSA (2015), additional analysis was performed to consider the survival (as no change or reduction in the concentration) of the hazards. The strategy for conducting the literature searches and screening is provided in Appendix A.
2.2. Description of the conditions of the assessment
The baseline condition (i.e. box filled with fish in ice) and the alternative condition (i.e. tub filled with fish in freshwater and ice) for transport/storage were compared by assessing (i) the temperature‐related processes regarding the initial fish temperature; (ii) the type of fish and (iii) different potential scenarios as described below and depicted in Figure 3.
Figure 3.
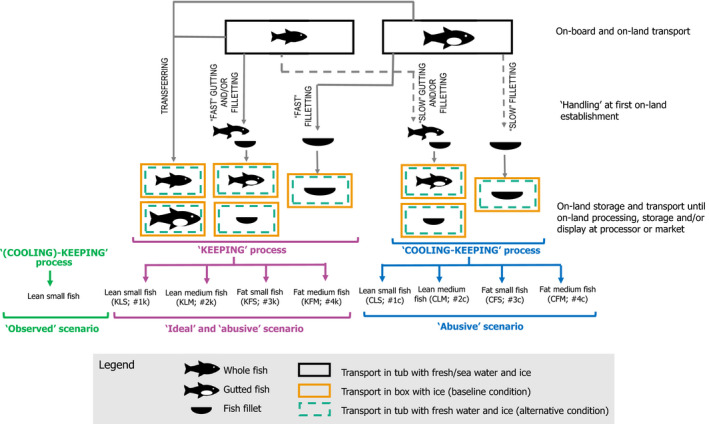
Conceptual map showing the scenarios and case studies to be assessed for transport/storage of fresh fishery products comparing the baseline condition (i.e. box filled with fish in ice) and the alternative condition (i.e. tub filled with fish in freshwater and ice)
Temperature‐related processes:
Keeping the chill temperature of the fish (referred to as ‘keeping’ process), which applies to whole fish (either ungutted or gutted on‐board), not ‘handled’ after landing other than transferring to a new container, being either a tub or a box. This process also includes fish that is gutted and/or filleted on‐land fast enough to not cause a temperature increase of the fish before its transfer to a tub or a box. The initial fish temperature is assumed equal to the temperature inside the tubs (i.e. 0°C) upon arrival at the first on‐land establishment.
Cooling and then keeping the chill temperature of the fish (referred to as ‘cooling‐keeping’ process), which apply when fish gutting and/or filleting at the first on‐land establishment causes an initial increase of the fish temperature up to 7°C. This initial temperature is considered, assuming that the fish temperature upon arrival at the first on‐land establishment is in the range from 0°C to 3°C, increases 3–4°C for every half an hour of ‘handling’, the time for ‘handling’ is maximum 30 min (Margeirsson et al., 2010; Valtýsdóttir et al., 2010), and then, the handled fish is transferred to a tub or a box.
Two major criteria were considered to define the type of fish for the assessment:
The fat content of the fish was considered by selecting fish with a low (such as plaice having a fat and water content of 1–4% and 79–81%, respectively) and high (such as Atlantic salmon having a fat and water content of 10–20% and 60–70%, respectively) fat content, referred to as ‘lean’ fish and ‘fat’ fish.
Two different dimensions and geometries were considered, i.e. small flat fish vs. bigger fish with a broad oval cross section. These were categorised as follows; ‘small’ fish (e.g. a plaice of a size class 4 having a weight of 150–300 g) and ‘medium‐sized’ fish (e.g. salmon with a length of 50 cm). The size of the latter was restricted by the size of the fish box. Whole or gutted fish was considered to have the same geometric dimension, while fillets would be at least half of the dimension in one of the three axes. The inclusion of two different dimensions accounts for the variety of sizes of whole (including gutted) or filleted fish within the assessed range.
For each process and type of fish, three potential scenarios were assessed based on the initial fish temperature and outside temperature (i.e. the temperature of the room/chamber/truck in which the fish containers (boxes/tubs) will be transported/stored) and the configuration inside the container:
the ‘ideal’ scenario: it assumes that the initial fish temperature equals the temperature inside the tubs (i.e. 0°C) upon arrival at the first on‐land establishment and the fish temperature does not increase when transferred to the on‐land container because in case of a box, the fish is surrounded by ice with perfect contact with fish surface; while in case of a tub, the fish is surrounded by water in equilibrium with ice and there is perfect mixing by aeration. Under this scenario, the gutting and/or filleting of the fish is considered fast enough so as not to cause a temperature increase in the fish. The outside temperature is low enough to avoid a temperature increase inside the container (box or tub). Therefore, the fish temperature is constant and equal to 0°C during the whole ‘keeping’ process, irrespectively of the type of fish and type of container. This scenario is not applicable to the ‘cooling‐keeping’ process.
the ‘observed’ scenario: based on experimental data of the fish temperature records of the experiments of the ‘Qualitubfish’ project (Section 2.3.1) dealing with small lean fish, which included a short cooling period followed by keeping the chill temperature. It is referred to as a ‘(cooling)‐keeping’ process.
-
the last scenario is applicable to the ‘keeping’ and ‘cooling‐keeping’ process and takes into account reasonably foreseeable abuse of the following factors (‘abusive’ scenarios):
-
(a)
for the ‘keeping’ process, the initial fish temperature equals the temperature inside the tubs (i.e. 0°C) upon arrival at the first on‐land establishment. For the cooling‐keeping’ process, the fish temperature rises to 7°C during its handling.
-
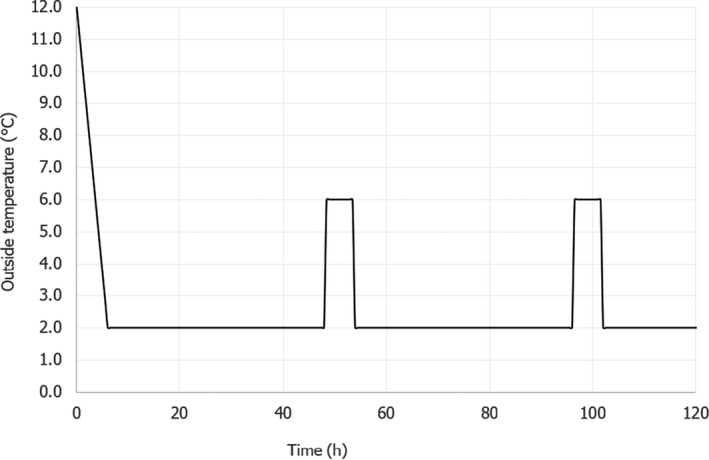
(b)
the outside temperature (i.e. temperature in the chamber or truck where the container is stored/transported) follows the profile shown in Figure 4, starting from 12°C, decreasing to 2°C in 6 h, then following 48‐h time/Temperature (t/T) cycles, including abusive temperatures, consisting of: 42 h at 2°C, increase from 2°C to 6°C in 30 min, 5 h at 6°C, decrease from 6°C to 2°C in 30 min. This was based on expert opinion considering the t/T profiles derived from the ‘Qualitubfish’ project (Bekaert et al., 2016a) and as described in Thordarson et al. (2017).
-
(c)
for boxes, fish is surrounded by air and two layers of ice (bottom and top); while for tubs, fish is in water below an ice layer on the top of the tub without mixing.
-
(a)
For these scenarios, the fish t/T profiles were obtained from heat transfer modelling (Section 2.3.2) for the combinations shown in Figure 3. Among the entire set of modelled temperatures, the temperature of fish located in the ‘warmest spot’ within the container was considered to assess microbial growth. For boxes, the temperature of two fish located in the middle of the box was used, i.e. one fish in the centre and one in the corner close to the box wall. For tubs, the temperature was extracted for two fish located in the bottom of the tub, i.e. one in the centre and the other in the corner.
Figure 4.
For all cases, the duration of the transport and storage in the tub/box on‐land would be constrained by the time of on‐board transport and in the first on‐land establishment. For modelling purposes, it was considered that the total duration of the transport/storage on‐land would be maximum 5 days in accordance with the exceptional absolute maximum duration assessed. However, fish is expected to be kept in a tub for a maximum up to 3 days (see Section 1.2 and Table 3 in Section 2.5.2).
Table 3.
Input values used for assessing the growth of hazards through the application of selected predictive models
| Variables and parameters affecting microbial growth, accounted for by the models | Background information | Final selection of input values for modelling | Data sources and/or reasoning for selected input values |
|---|---|---|---|
| Temperature of fish surface | Refer to assumptions about t/T profiles that may occur during ‘cooling‐keeping’ and/or ‘keeping’ processes (Sections 1.3.2 and 2.3) | Data shown in Section 3.2.1 | ‘Observed’ scenarios: data from the ‘Qualitubfish’ experiments 1 and 2 |
| Data shown in Section 3.2.3 | ‘Abusive’ scenarios: data from the heat transfer model (for boxes and tubs), which was validated against the observed ‘Qualitubfish’ data and considered reliable for simulating the keeping and cooling profiles | ||
| WPS (%) |
Median = 0.3711 Mean = 0.513 SD = 0.474 Min = 0.143 Max = 1.739 n = 10 |
0.37 (A. hydrophila, Cl. botulinum and M. psychrotolerans) 0.6% (L. monocytogenes, as this is the minimum level accepted by FSSP tool) |
Median of n = 10 values for fresh fish of different species was extracted from scientific papersa |
| pH |
Median = 6.5 Mean = 6.4 SD = 0.27 Min = 5.78 Max = 6.89 n = 31 |
6.5 | Median of n = 31 values for fresh fish of different species was extracted from scientific papersb |
| Oxygen availability or aerobic/anaerobic conditions | – |
Aerobic (A. hydrophila, L. monocytogenes, M. psychrotolerans) Anaerobic (Cl. botulinum) |
Applies to obligatory anaerobic hazards that may multiply under the anaerobic gut environment, where these organisms reside |
| Lag time | Relative lag time (RLT; ho) for FSSP; physiological state (ao) for ComBase | No lag time | For the uncertainty analysis, comparisons were carried out between (sub‐) selected growth simulations without and with lag time, associated with different (assumed) initial physiological states. The subselected simulations were those of t/T scenarios that showed the maximum log10 increases without lag |
| Lactic acid (endogenous origin) | Up to 1% (10,350 ppm) Yellowfin tuna Emborg et al. (2005) | 0.7% (7,000 ppm) lactate in water phase | Mejlholm et al. (2010) |
| Initial concentration of histidine |
Median = 5,000 Mean = 5,915 SD = 3,917 Min = 1,187 Max = 13,970 n = 15c |
10,750 ppm | High enough so it is not the limiting factor for histamine formation (e.g. from 10,750 ppm of histidine, up to 7,700 ppm of histamine could be formed) (EFSA, 2015) |
| Initial concentration of M. psychrotolerans | 10; 100; 10,000 CFU/gd | 1,000 CFU/g | The level of 1,000 CFU/g was chosen as a rather conservative initial level, i.e. not very high as 10,000 CFU/g, but not such low that would probably mask the differences in histamine accumulation between boxes and tubs for the t/T conditions of the current assessment. The same levels was also tested in the previous EFSA report (EFSA, 2015) |
| Initial concentration of histamine | 0 ppm | Assuming fresh fish with no relevant histidine‐decarboxylase activity before fish lands | |
| Time points of interest for the assessment e | 1 and 2 days | Possible durations as below the realistic maximum duration according to the clarification with the requestor and in agreement with the ‘Qualitubfish’ data (in 82% of the 61 surveyed fish transport/storage the duration was ≤ 2 days). The duration of 2 days also approximately corresponds with the end of cooling | |
| 3 days | The realistic maximum duration according to the clarification with the requestor and in agreement with the ‘Qualitubfish’ data (duration of ≤ 3 days for 97% of transported/stored fish surveyed) | ||
| 5 days | The exceptional absolute maximum duration according to the clarification with the requestor and in agreement with the ‘Qualitubfish’ data (duration below 5 days for all 61 surveyed transported/stored fish) |
WPS: Water phase salt; t/T: time/Temperature.
Based on values reported in (Emborg et al., 2005; Magnússon et al., 2010; Valtýsdóttir et al., 2010; Digre et al., 2011; Thordarson et al., 2017).
Based on values reported in (Ruiz‐Capillas and Moral, 2001; Gimenez et al., 2002; Baixas‐Nogueras et al., 2003; Pons‐Sanchez‐Cascado et al., 2006; Erkan, 2007; Kilinc et al., 2007; Olsson et al., 2007; Sallam, 2007; Emborg and Dalgaard, 2008; Hernandez et al., 2009; Magnússon et al., 2010; Khalafalla and El‐Sayed, 2015).
Based on (Abe, 1983; Fletcher et al., 1995; Antoine et al., 1999; Shirai et al., 2002; Kanki et al., 2004; Ruiz‐Capillas and Moral, 2004; Emborg et al., 2005).
Low initial concentrations (≤ 10 CFU/g) can be expected in the skin and gills of FFP. Higher concentrations can be found in the intestines and contaminate the FFP when handheld either on‐board and on‐land (Emborg et al., 2005; Emborg and Dalgaard, 2008). An exceptional worst‐case scenario of initial concentration of M. psychrotolerans was assumed to be 10,000 CFU/g.
For the ‘observed’ scenario, the objective of the ‘Qualitubfish’ experiments was to evaluate the shelf‐life of fish in a tub, so the maximum duration monitored (i.e. 12 days and 7 days) is not considered a realistic practice. Therefore, the actual assessed time for these t/T profiles was cut to 5 days in accordance with the exceptional maximum duration that fish could be kept in a tub.
2.3. Fish temperature in boxes and tubs
A literature search was conducted to retrieve data comparing fish temperature profiles when transporting or storing (either keeping or cooling) fish in tubs (with freshwater and ice) or boxes (with ice), keeping the other conditions fixed such as the outside temperature, type of fish and conditions before transport or storage. Two studies compared the t/T profile of fish when kept in such boxes and tubs. Both studies were conducted in the frame of the ‘Qualitubfish’ project and consisted of storing/transporting lean small fish (plaice) in boxes and tubs under the same external temperature conditions mimicking the foreseeable conditions of on‐land transport and storage. The studies have been described in two reports. In the first experiment described in Bekaert et al. (2016a), the fish were stored for 3 days on ice in boxes on‐board a fishing vessel before the start of the experiment, while in the second experiment (Bekaert et al., 2016b), storage was for 8 days on ice before the start.
Otherwise, studies are limited to the currently authorised practices, both for use of tubs and boxes. That is the transport of fish in boxes after the first on‐land establishment, and the on‐board transport of fish in tubs. For example, studies are available on the use of tubs with ice, dry ice, slurry ice or gel/ice packs but without water, or with seawater but not freshwater, thus not covering the conditions to be assessed in the present mandate.
Due to the lack of experimental data, thermodynamic models were developed enabling the comparison of the impact on the fish temperature of current authorised practice (baseline condition, using boxes) and alternative condition (using tubs) for on‐land transport or storage under the same conditions of initial fish temperature and outside temperature.
The temperature dynamics in boxes and tubs were modelled considering the processes, type of fish and scenarios described in Figure 3. When data were available (as in the ‘(cooling)‐keeping’ process for lean small fish), the heat transfer model was validated (Section 2.3.2.2).
2.3.1. Recorded time/temperature profiles during storage/transport
Temperature data gathered in the first experiment of the ‘Qualitubfish’ project (Bekaert et al., 2016a) were used (i) to assess the dynamics of the fish temperature in boxes with ice and in tubs with freshwater and ice (Section 3.2.1), (ii) to validate the thermodynamic models (Section 3.2.2) and (iii) to assess the behaviour of selected relevant hazards through the application of predictive models (Section 3.4). In this experiment, plaice (Pleuronectes platessa) of size class 4 (having a weight of 150–300 g) were stored for 3 days on ice in boxes on‐board a fishing vessel before being transferred on‐land to tubs or boxes. The PE tubs were filled with 400 kg of fish, 110 L of freshwater at 2.7°C and 20 kg of flake ice. The HDPE boxes were filled with 40 kg of fish and 15 kg of flake ice on the bottom, in the middle and on top of the boxes. The temperature of fish was recorded by inserting temperature loggers into the core of the fish through the gutting cut. The temperature monitored fish were marked with a strap and placed in different zones of two tubs, namely on the bottom, in the middle and on top. Logged fish were also placed in the middle of four different boxes. One tub and three boxes were immediately stored for 12 days in a cold storage room (non‐transported). One tub and three boxes were also transported by truck to the Netherlands and back the next day to be further stored in the same cold storage. After 7 days of storage, boxes and tubs were re‐iced. The temperature of the cold storage (outside temperature) was also monitored.
In the second experiment of the ‘Qualitubfish’ project (Bekaert et al., 2016b), plaice of the same size class were stored for 8 days on ice in boxes on‐board and transported with trucks to the auction before being transferred on‐land to tubs or boxes. One PE tub was filled with 400 kg of fish, water was added to cover all fish and a layer of ice was put on top. HDPE boxes were filled with ~ 40 kg of fish and 15 kg of flake ice (layer of ice on top, in the middle and on the bottom). Both the tub and boxes were further stored for 7 days in a cold storage room. The temperature of the fish inside tubs and boxes was registered as in the previous experiment. The outside temperature was not available and therefore not allowing validation of the thermodynamic model, but allowing to assess the dynamics of the fish temperatures and behaviour of selected relevant hazards as the first experiment.
For the assessment of the behaviour of selected biological hazards, the actual t/T profiles from both experiments were cut to 5 days in accordance with the maximum duration to be assessed (see Section 1.2).
As part of the study described in the report (Bekaert et al., 2016a), a total of 61 tubs were followed from the auction in Belgium to the filleting company in the Netherlands. In most cases, the fish was processed on the same day (n = 16 tubs; 26.2%) or on the next day (n = 29 tubs; 47.5%). For the rest of the tubs, the duration before processing was 2 days, 3 days and 4 days for n = 5 (8.2%), n = 9 (14.8%) and n = 2 (3.3%) tubs, respectively.
2.3.2. Modelling of fish temperature dynamics in boxes and tubs
The heat transfer modelling was applied to estimate the fish surface temperature under the process of cooling and/or keeping fish in ice (in boxes) vs. in water and ice (in tubs) under the same conditions of transport/storage. Figure 3 shows the considered conditions regarding the fat content of the fish, its dimensions and initial temperature. The outside temperature depends on the scenario of ‘ideal’ or ‘abusive’.
As mentioned in Section 2.2, for the ‘ideal’ scenarios, temperatures inside containers can be predicted without the use of mathematical models assuming ideal conditions, like perfect contact of ice with fish (in boxes) and perfect mixing of water and ice (in tubs).
For ‘abusive’ scenarios, the t/T profiles inside the containers were modelled using mathematical heat transfer models. For each container, the model consists of a partial differential equation simulating the temperature dynamics (along the time) and distribution (on space) with different thermodynamic parameters for each considered material (e.g. air, water, ice, lean/fat fish, PE container material). Therefore, the model required to define geometries and positions for each of the materials (referred to as ‘domains’), mesh these domains (spatial discretisation of the partial differential equation) and evaluate the resulting equations in each of the elements of the mesh. The partial differential equation was solved using the software COMSOL® (COMSOL Multiphysics Reference Manual, version 5.4”, COMSOL, Inc, www.comsol.com). More details about numerical methods, discretisation in time and space and further details about the simplifications and assumptions can be found in Appendix B. In short, the model, named the Temperature Simulator of Fish Stored in Tubs and Boxes (FishT‐TaB Simulator), relies on the following simplifications and assumptions:
-
1
Heat transfer by conduction is simulated, disregarding any other type of heat transfer phenomena;
-
2
The phase change from ice to water is simulated using the ‘apparent heat capacity method’ in which the latent heat is included as an additional term in the heat capacity;
-
3
Only two fish, assuming an ellipsoid geometry, were explicitly modelled in each container. For the contribution of the other fish in the same container, a matrix of water/fish in tubs and air/fish in boxes is simulated using the standard approximations for modelling porous materials;
-
4
Among all the modelled fish surface temperatures distributed in space, three t/T profiles were retrieved for each container. The highest temperatures were selected as follows:
-
For boxes, the maximum temperatures (Tmax) are expected in the centre (in the vertical and horizontal axis), i.e. the furthest location from both ice layers on the top and bottom. Therefore, the selection was (see Figure 5 for illustration):
Tmax on the surface of a fish located in the centre of the box;
Tmax on the surface of a fish located in the centre, but close to the box wall; and
the maximum overall temperature in the food/air matrix.
-
For tubs, from the thermodynamic principles, Tmax is expected on the bottom (i.e. the furthest vertical location from the top ice layer); selected temperatures were:
Tmax on the surface of a fish located in the bottom centre of the tub;
Tmax on the surface of a fish located in the bottom corner of the tub; and
Tmax obtained for each time within the whole food/water matrix.
-
For the validation of the heat transfer model, the temperature locations are selected based on the position of the hardware sensors in the ‘Qualitubfish’ project experiments (Bekaert et al., 2016a) with some adjustments due to modelling only two ice layers, whereas the experiments were carried out with three layers (see Section 2.3.2.2 for details). The fish temperatures modelled for the validation consisted of the average temperature of the whole fish, as data loggers were inserted into the fish through the gutting cut.
-
5
Convective heat flux was considered with the usual heat transfer coefficient without air flow;
-
6
Initial conditions were assumed homogenous in space, i.e. with the same temperatures for all the points in the same domain; and
-
7
Whenever a parameter, initial condition or boundary condition may be case‐dependent, the usual practice was considered. Assumed values, such as the container‐specific characteristics of the tubs and boxes, are described in Table 1. The fish‐specific characteristics are provided in Table 2.
Figure 5.
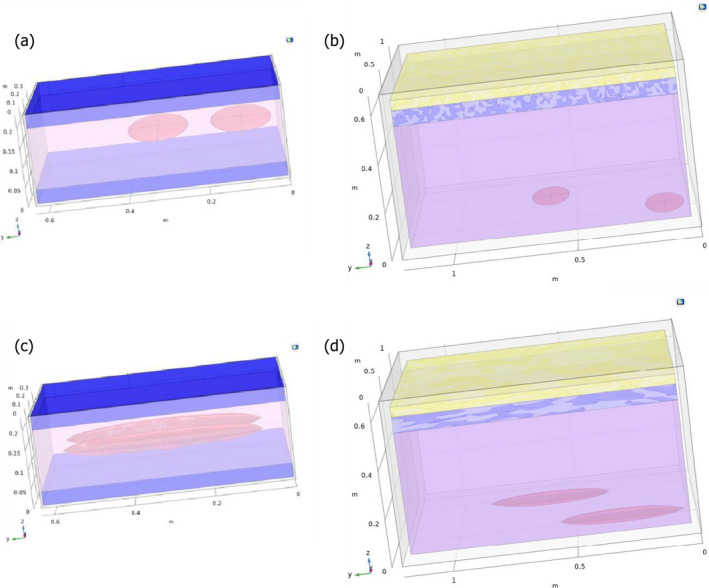
Geometries and configuration of the container (box: a and c; tub: b and d) considered for modelling the surface temperature of (a) lean small fish in boxes with ice, (b) lean small fish in tubs with ice and water, (c) lean medium fish in boxes with ice and (d) lean medium fish in tubs with ice and water
- Blue, yellow and grey domains represent ice, air and container material, respectively. Domains are scaled to represent cooling conditions (63 and 15 kg of ice in tubs and boxes, respectively). The pink domain is the air/fish matrix in boxes and the water/fish matrix in tubs. The red ellipsoids represent the fish where the temperature was captured and used to simulate the hazard behaviour. The box is open on the top without any lid, while the tub is closed with the same foam. Note that the orientation of small and medium fish is different to accommodate the medium fish inside the container
Table 1.
Container‐specific characteristics of the boxes and tubs
| Parameter | Boxes | Tubs |
|---|---|---|
| Container outer dimensions (L × W × H) | 80 × 45 × 27 cma | 120 × 100 × 79 cmb |
| Container inner dimensions (L × W × H) | 62 × 37 × 23 cma | 111–115 × 91–95 × 60.5–61.5 cmc |
| Container thickness | 0.5 cm | 4.6 cm |
| Container material | HDPEc | Triple‐walled with a PE structure of the outer walls and PE core of the container (3.6 cm PE foam core in between 0.5 cm HDPE skin)c , d |
| Container weight | 4.18 kga | 55 kgb |
| Container volume | 60 La | 630 Lb |
| Fish added in the container | 30 kge | 440 kgf |
| Ice added in the container | 15 kg (for ‘keeping’ and ‘cooling‐keeping’ processes)e | 38 kg (‘keeping’ process) and 63 kg (‘cooling‐keeping’ process)f |
| Water added in tubs | NA | 100 kg (‘keeping’ process) and 100 kg (‘cooling‐keeping’ process)f |
| Type of water used | NA | Freshwater without salt added |
| Type of ice and arrangement | Top and bottom layers of ice | Water with ice on top |
HDPE: high‐density polyethylene; NA: not applicable; PE: polyethylene.
Based on the ‘180° fish box ‐ 800 × 450 × 270 mm ‐ solid base and walls ‐ 2 open handholes’.10
Based on the ‘Seaplast 630 Insulated Storage Bulk Container’.11
Considered having following properties (as of PE skin): thermal conductivity of 0.44 W/(m·°C), density of 930 kg/m3 and heat capacity of 1.64 kJ/(kg·°C) at constant pressure.
Considered having following properties (as of PE foam of the core of the container): thermal conductivity of 0.05 W/(m·°C), density of 70 kg/m3 and heat capacity of 2.3 kJ/(kg·°C) at constant pressure.
Considered having following properties: thermal conductivity of 0.035 W/(m·°C), density of 31 kg/m3 and heat capacity of 1.28 kJ/(kg·°C) at constant pressure.
For boxes, the amount of fish and ice considered the practice applied in the ‘Qualitubfish’ project (Bekaert et al., 2016a,b) and relied on the ice/fish ratio of 1/2 according to (Graham et al., 1992). Smaller quantities of ice with respect to fish have been also reported (Thordarson et al., 2017; Laguerre et al., 2019), which may lead to an earlier need of re‐icing to ensure the presence of ice in the container.
For tubs, the amount of fish and water was based on the practice applied in the ‘Qualitubfish’ project (Bekaert et al., 2016a,b), while the amount of ice was estimated through the ice calculator for tubs that can be retrieved at https://isreiknir.matisprojects.com/ (Ragnarsson, 2017). Convection on both sides of the wall was not considered and it assumed that the container is closed and is similar to 660 L‐PE. The calculations were made to ensure ice was not completely melted when the tub would be stored for 7 days at 2°C (with safety margin of 2 days). It assumed an initial fish and water temperature of 0°C and 3°C, respectively, for the ‘keeping’ process. It assumed an initial fish and water temperature of 7°C and 0.5°C, respectively, for the ‘cooling‐keeping’ process.
Table 2.
Fish‐specific characteristics
| Fish temperature | ||
|---|---|---|
| Initial temperature | 0°C or 7°C depending on the scenario (see Section 2.2) | |
| Small fish (e.g. plaice)a | Medium fish (e.g. salmon)b | |
| Fish dimensions: length | 29 cm | 50 cm |
| Fish dimensions: height | 15.5 cm | 10.7 cm |
| Fish dimensions: thickness | 0.85 cm | 5 cm |
| Lean fish (e.g. haddock/cod) c | Fat fish (e.g. salmon) d | |
| Thermal conductivity (k) | 0.43 W/m°C | 0.41 W/m°C |
| Specific heat capacity (Cp) | 3.73 kJ/kg°C | 3.50 kJ/kg°C |
| Density (ρ) | 1,054 kg/m3 | 1,025 kg/m3 |
Average length for Atlantic salmon is 70–75 cm and its average weight is 3.5–5.5 kg. The maximum reported length for Atlantic salmon is 150 cm and a maximum reported weight of 46.8 kg (https://www.fws.gov/fisheries/freshwater-fish-of-america/atlantic_salmon.html). The salmon with a length of 50 cm would correspond to a weight of around 1.4 kg.
Representative of a plaice of a size class 4 having a weight of 150–300 g (https://lv.vlaanderen.be/nl/visserij/cijfers-marktoverzichten/prijsnoteringen).
Based on Margeirsson et al. (2012).
2.3.2.1. ‘Cooling’ and ‘cooling‐keeping’ processes of fish in boxes and tubs
Figure 5 illustrates the geometries applied when modelling the temperature of the fish surface for the cooling and ‘cooling‐keeping’ processes of lean small or medium fish in boxes and tubs. Each domain is represented with a different colour. The red ellipsoids represent the fish that has been considered for recording its surface temperature to assess the hazard behaviour (Sections 2.5.2 and 2.5.3).
For fat fish, domains were recalculated to maintain the same ratios of fish/water/ice in tubs and fish/air/ice in boxes (Table 1) considering that the fat fish density is slightly lower than that of the lean fish (see Table 2). For the ‘keeping’ process, the domains were scaled to have 38 kg instead of 63 kg of ice in tubs. For boxes, no changes were required as the same amount of ice was used for ‘keeping’ and ‘cooling‐keeping’ process.
The water or air used inside the containers was considered precooled at 0.5°C for the ‘cooling‐keeping’ process, but not for the ‘keeping’ process, in which the value measured in the ‘Qualitubfish’ experiment (i.e. 2.7°C) was used. For both ‘keeping’ and ‘cooling‐keeping’ process, 1.5°C was used for the material of the container and −0.5°C for ice (see modelling assumption 2 for justification).
2.3.2.2. Validation of the heat transfer model
To validate the predictive performance of the heat transfer model, predicted and observed data from the first experiment of the ‘Qualitubfish’ project were compared. Some parameters and initial conditions were different from those in the case studies described for the ‘abusive’ scenarios. In these cases, the parameter value used was based on the actual measurements carried out in the project experiments. Details are shown in Appendix B.
The first experiment evaluated temperatures of one fish in a box and three fish on the bottom, middle and top of a tub. The top fish was subjected to higher temperatures than expected as it was not fully covered with ice and this location (fish between ice and air domains) could not be simulated with the model; therefore, this record was excluded for the validation.
For the other temperatures, the following locations were assumed:
Fish in the box was modelled by placing the fish close to the box wall and just below the ice layer. The position close to the ice was selected because in the experiments, boxes were filled with three layers of ice (top, middle and bottom), whereas only two layers were assumed (top and bottom) for modelling (as a worst case). Total amounts of ice were the same in the experiments and modelling;
Fish in the tub bottom was simulated by placing the fish on the bottom close to the corner; and
Fish in the tub middle was simulated by placing the fish in the centre of the horizontal plane (x–y axis) but just behind the ice layer for the vertical (z axis). Results for a fish located on the centre in the vertical (z axis) showed higher temperatures than in the experiments. It should be noted that the exact location of the fish in the experiment is unknown and the fish with the temperature logger might move up at some point.
To make the temperatures reported by the model and the loggers placed inside the fish more comparable, average temperatures inside each of the fish were calculated. It should be noted that using the same approach as for the case studies, i.e. Tmax in each of the containers or Tmax on the fish surface, would have significantly overestimated the observed temperatures.
2.4. Water absorption and consequences on fish WPS
A literature search was performed to gather scientific publications, reports and official documents relevant to the water absorption in fish stored under different conditions. Focusing on publications between 1965 and 2019 (inclusive) and relying on expertise in the WG (e.g. relevant peer reviewed papers, scientific reports, book chapters), this was further developed using ‘footnote chasing’ until sufficient coverage of the subject area was achieved.
According to water sorption isotherms, at the high aw values of fresh fish, the increase of water content has little impact on aw. Furthermore, the inaccuracy of the water sorption isotherms at the upper extreme (aw > 0.90) is high as the studies usually focus on the desorption isotherm (built for drying or dehydrating products with aw < 0.96) and do not represent the sorption isotherm representing the intake of water by fresh fish that has not been dried before. It was decided to use instead the WPS as a parameter for assessing the impact of fish water uptake on the behaviour of relevant hazards. The WPS can be derived from the salt and water content of foods. For foods without added salt, the percentage of endogenous salt in the WPS is calculated by dividing the salt content by the water content multiplied by 100, to express it as percentage.
The potential impact of the storage conditions of FFP in water and ice (in tubs) on parameters relevant for bacterial growth (e.g. oxygen availability, internalisation) was assessed from literature review.
2.5. Behaviour of relevant hazards
The impact of the fish temperature on the behaviour (survival or growth) of the identified hazards during the transport and storage of FFP in tubs compared to boxes was assessed applying predictive models, when available, for specific pathogens and histamine accumulation using as input the observed t/T profiles and those derived through heat transfer modelling. For the latter, the following was used: (i) the ‘observed’ scenarios through the fish t/T recorded in the ‘Qualitubfish’ experiment as detailed in Section 2.3.1 and/or (ii) the ‘abusive’ scenarios from predicted surface t/T profiles from the heat transfer model in boxes and tubs considering different processes (‘cooling‐keeping’ and ‘keeping’), fat content and dimensions of fish (Section 2.3.2, Figure 3).
When no predictive models were available for a particular hazard, its behaviour was assessed using the relevant studies selected from the literature review (Section 2.1) reporting data on the levels of the hazards during the storage of FFP at different temperatures. Survival was considered either as no change or as a reduction of the concentration of relevant selected hazards. The potential impact of factors other than temperature associated with the storage/transport of FFP in tubs with water and ice was taken into account in the uncertainty analysis.
To report the predicted log10 changes (decrease or increase) of relevant hazards for each t/T profile, different ‘sampling times’ along the storage/transport were selected according to the clarification with the requestor (Section 1.2) and in agreement with the ‘Qualitubfish’ data ((Bekaert et al., 2016a), Section 2.3.1) regarding the expected duration of the on‐land transport/storage of the fish in tubs. These were 1 and 2 days (as the most probable expected duration), 3 days (as the realistic maximum) and 5 days (as the exceptional absolute maximum duration) as specified in Table 3.
2.5.1. Survival of relevant hazards
A predictive model was available describing the reduction of Vibrio parahaemolyticus in salmon meat as a function of the storage temperature within the range from 0 to 12°C (Yang et al., 2009). The inactivation model describes the log10 reduction (log10R; i.e. inactivation or decrease) of the V. parahaemolyticus concentration as a function of the storage time (t; in h) and the temperature‐dependent primary inactivation kinetic parameters of the Weibull model, i.e. the shape (n) and scale factors (b):
| (1) |
with b and n depending on the storage temperature (T) according to the empirical polynomial equations:
| (2) |
| (3) |
The predictive performance of the model was confirmed with additional experiments performed in salmon and tuna fish, and the predictions agreed well with observed viable counts irrespectively of the fish matrix (Yang et al., 2009).
The log10 decrease (as the log10 concentration at a given ‘sampling time’ minus the initial log10 concentration) represents reduction potential and was used to assess the impact of the t/T profile predicted for FFP storage/transport in boxes (in ice) compared to tubs (in water and ice). In particular, the t/T profiles predicted through the heat transfer modelling for the ‘abusive’ scenario (Section 2.3.2) were used for this purpose.
2.5.2. Growth of relevant hazards
When available, predictive microbial growth models were used to simulate the growth of the selected hazards. The change in the concentration (log10 increase, as log10 concentration at a given ‘sampling time’ minus the initial log10 concentration) was based on the estimated growth rate provided by the predictive tool, without considering a lag phase (conservative assumption). The log10 change represents the growth potential and was used to compare between box and tub storage/transport. For simulation purposes, the initial concentration of the relevant hazard was set at 1 CFU/g (i.e. 0 log10 CFU/g). This factor has no impact on the magnitude of the log10 change. Different ‘sampling times’ were selected to report the results of the log10 increase, as described in Section 2.5 and shown in Table 3, together with the values of the other input parameters.
The growth of L. monocytogenes was simulated using the predictive model available in Food Spoilage and Safety Predictor (FSSP for Windows, v. 4.0),12 which was successfully validated for a variety of seafood (Mejlholm et al., 2010).
For the simulation of A. hydrophila growth the following information was considered: its minimum growth temperature (ICMSF, 1996) is between 0°C and 4°C, most commonly 2°C. The ComBase13 models are based on data from laboratory broth, known to support higher (mainly unconstrained by indigenous competitors) and/or faster growth than food matrices. The ComBase model predicts some (yet limited) growth at 2°C when no lag is considered, and no simulation can be done below 2°C. To overcome the ComBase limitations and overestimation in the growth, the following gamma model was used that described the effect of temperature on maximum growth rate (μmax) of A. hydrophila, calibrated for fish using the maximum growth rate of the bacterium on fish at the reference temperature of 4°C, μref (h−1) (Leung et al., 1992; Fernandes et al., 1998). To perform the simulations, the highest value reported was considered, i.e. μref = 0.039 h−1. With this structure and mathematical assumptions, the model encompasses the impact of intrinsic fish factors underpinning the behaviour of the bacterium on fish.
| (4) |
No systematic validation study has been carried out and published specifically for FFP. However, the growth rate predictions provided by the Combase growth model of A. hydrophila were on average 3.3‐fold higher than those observed in the experiments using catfish and rainbow trout stored at 4°C (Leung et al., 1992; Fernandes et al., 1998). Therefore, the growth of A. hydrophila foreseen by the polynomial models of Combase at 2°C may lead to a conservative (i.e. fail‐safe) but unrealistic overestimation of growth. As such, the correction factor μref coupled with the above gamma model was used to approximate the predicted growth rate to that observed in actual fish matrices (Leung et al., 1992; Fernandes et al., 1998). Based on the above, all growth simulations were carried out with the growth rate gamma model, in which the Tmin and the variation of true μref are the major uncertainty sources and without considering a lag phase (Figure C.1; Appendix C).
Figure C.1.
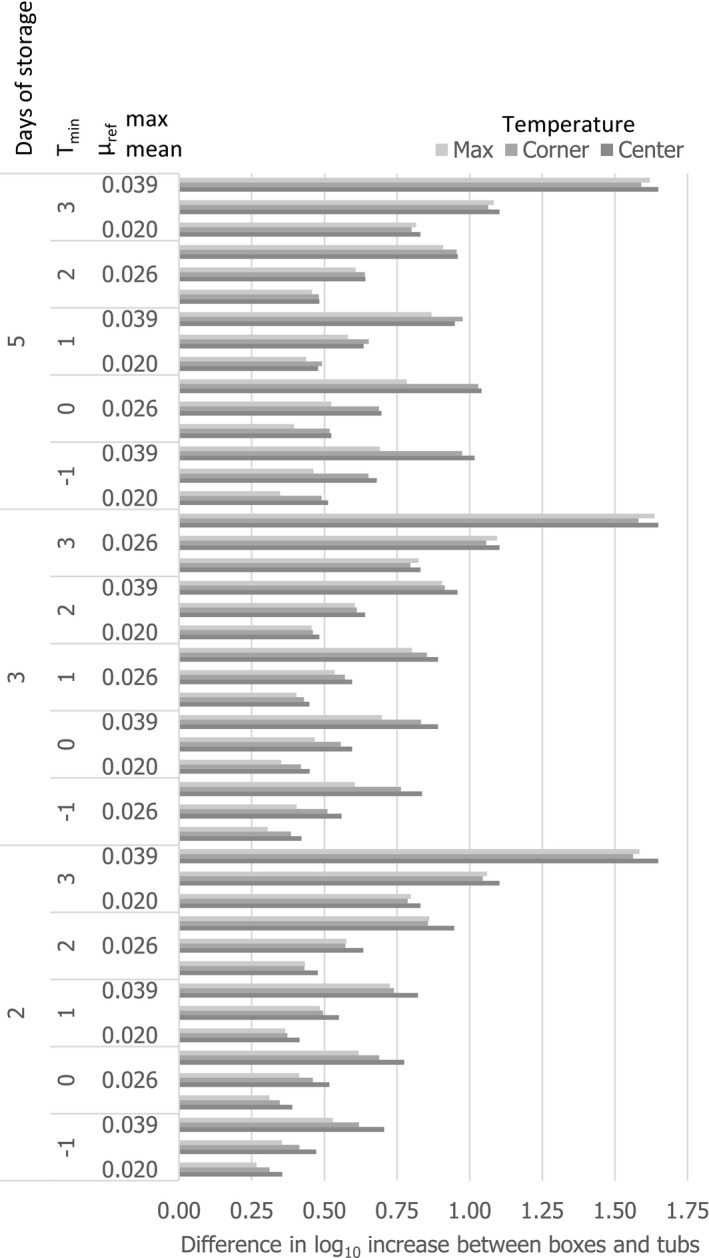
Difference in log10 increase of Aeromonas hydrophila between boxes and tubs for a Tref = 4°C and different values of Tmin as well as maximum growth rate at 4°C for different durations of storage
- Different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) are considered and as calculated from the time/Temperature profile of the case study ‘cooling‐keeping’ process of lean medium fish (CLM; #2c) under the ‘abusive’ scenarios.
The growth of non‐proteolytic (i.e. group II, psychrotrophic) Cl. botulinum was simulated using the predictive growth models available in Combase, assuming no lag. The latter information was accounted for by the simulations, by setting the physiological state parameter (a0) equal to 1, i.e. 100% of cells grow without lag. The growth of Cl. botulinum was included in the assessment assuming that growth may take place in the anaerobic gut environment, where this organism resides, during the on‐land transport/storage of ungutted fish in boxes or tubs. The minimum growth temperature of Cl. botulinum is 3.3°C (ICMSF, 1996), but Combase does not perform simulations < 4°C, at which growth is supported, albeit limited. Thus, there is a temperature interval from 3.3°C to 4°C, where growth is expected but cannot be simulated by Combase. Therefore, Combase simulations were performed considering the growth rate equal to that at 4°C for the temperature records between 3.3°C and 4°C. Although this introduces a slight overestimation of the actual growth, this is not expected to have major impact on the assessment as the growth of Cl. botulinum is rather limited (Section 3.4.2), besides the overestimation associated with the model developed from broth data.
The approach used to account for the lag time was as follows. The lag time is highly dependent on the combination of the physiological state of cells at the time they experience a shift in their environment and the magnitude and direction (e.g. temperature up/down‐shift) of the encountered shift. The targeted microorganisms are to have the same history when whole or gutted fish arrive on‐land, and while residing on fish, they encounter the outside environments of boxes or tubs, each inducing uncertain changes (if any) in the micro‐environmental conditions affecting the sites of microbial growth on fish. As such, to quantitatively address the impact of this uncertainty on lag time, the latter may be expressed as different initial physiological states of cells on fish arriving for the first time on‐land. These will represent the scenario of no lag, i.e. all cells continue growing exponentially in boxes or upon transfer in tubes, and a physiological state representing increased stress history, i.e. cells having experienced increasing level of stress or injury that increases their need to adapt prior to (re‐) growth. Particularly, the physiological state in growth models is expressed via the following parameters (Baranyi et al., 1995; Mellefont et al., 2003): (i) the parameter ao, as in Combase, that describes the % of cells that continue to grow (undisturbed) exponentially without lag, upon the shift in the environment and ranges from 0 (no growth) to 1 (no lag) and (ii) ho, also termed ‘relative lag time’ (Mellefont et al., 2003) (equal to the lag time divided by the generation time) that practically represents the ‘work to be done’ by the cells so that they enter the exponential phase of growth.
2.5.3. Histamine accumulation
The predictive model available at the FSSP about the growth of histidine‐decarboxylase Morganella psychrotolerans and the consequent histamine accumulation in fish was used. The assumptions regarding the values of the input parameters are described in Table 3. The predictive performance of this model was validated by the model developed for FFP, and on average, it provides slightly conservative (fail‐safe) predictions according to the FSSP developer (Emborg and Dalgaard, 2008).
2.6. Uncertainty analysis
Based on the EFSA guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a) and scientific opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018b), special attention was given to: (i) the interpretation of the ToRs, i.e. framing of the mandate and the AQs, (ii) identifying sources of uncertainty and (iii) their impact on the outcome of the assessment. The identified assumptions and other sources of uncertainty were listed.
3. Assessment
3.1. Hazard identification
3.1.1. Selection of hazards to be included in the assessment
An overview of the hazards potentially present in seawater and/or freshwater FFPs is given in Tables 4 and 5 along with the evidence of an association of these hazards with human illness and their ability for survival and/or growth encompassing temperatures within the range of conditions for box and tub transport. More details are provided in Tables A.3 and A.4 in Appendix A.
Table 4.
Overview of bacteria, viruses and parasites as relevant biological hazards based on the selection against two criteria: (1) evidence of causing human illness and being associated with fresh fishery products and (2) evidence for survival or growth on fresh fishery products within the range –3°C to 7°C
| Group of hazards | Hazards included in the assessment | Hazards excluded from the assessment | |||
|---|---|---|---|---|---|
| Hazard | Evidence for survival (S) or growth (G) | Assessment approach | Hazard | Reason for exclusion | |
| BACTERIA | Aeromonas spp. | G | Simulation applying predictive models for A. hydrophila | B. cereus | Evidence of human illness but no evidence of S or G |
| Cl. botulinum (non‐proteolytic) | G (evidence of toxin production) | Simulation applying predictive models for Cl. botulinum | Cl. perfringens | Evidence of human illness but no evidence of S or G | |
| L. monocytogenes | G | Simulation applying predictive models for L. monocytogenes | Plesiomonas shigelloides | Evidence of human illness but no evidence of S or G | |
| Human pathogenic E. coli | S | Appraisal of bibliographic data | Shigella spp. | Weak evidence of human illness but no evidence of S or G | |
| Salmonella spp. | S | Appraisal of bibliographic data | Thermophilic Campylobacter spp. | No evidence of human illness | |
| St. aureus | S | Appraisal of bibliographic data | Y. enterocolitica a | No evidence of human illness | |
| Vibrio spp. | S | Appraisal of bibliographic data | |||
| VIRUSES | Norovirus | Evidence of human illness but no evidence of S or G | |||
| Hepatitis A virus | Evidence of human illness but no evidence of S or G | ||||
| Hepatitis E virus | Weak evidence of human illness but no evidence of S or G | ||||
| PARASITES | Nematodes (Anisakis spp.) | S | Appraisal of bibliographic data | Cestodes | Evidence of human illness but no evidence of S or G |
| Trematodes | Evidence of human illness but no evidence of S or G | ||||
| Giardia | Evidence of human illness but no evidence of S or G | ||||
| Cryptosporidium | No evidence of human illness | ||||
| Toxoplasma | No evidence of human illness | ||||
G: growth; S: survival.
Y. enterocolitica was included as hazard in the assessment dealing with pre‐packed fishery products at retail (EFSA, 2015) based on its occurrence in fishery products and the growth capability of the hazard under refrigerated conditions. However, there was no scientific evidence of Y. enterocolitica causing human illness associated with fishery products.
Table 5.
Overview of histamine‐producing bacteria as relevant biological hazards based on the selection against two criteria: (1) evidence of causing human illness (histamine intoxication) associated with fresh fishery products and (2) evidence of biogenic amines production in fresh fishery products within the range −3°C to 7°C
| Hazards included in the assessment | Hazards excluded from the assessment | ||
|---|---|---|---|
| Hazard | Assessment approach | Hazard | Reason for exclusion |
| Enterobacter spp.a | Appraisal of bibliographic data | Aeromonas spp. | No evidence of human illness |
| Morganella spp. | Simulation applying predictive models for M. psychrotolerans | Citrobacter spp. | No evidence of human illness |
| Photobacterium spp. | Appraisal of bibliographic data | Cl. perfringens | No evidence of human illness |
| Hafnia alvei | Weak evidence of human illness but no evidence of biogenic amine production | ||
| Klebsiella spp. | Evidence of human illness but no evidence of biogenic amine production | ||
| Proteus spp. | No evidence of human illness | ||
| Providencia spp. | No evidence of human illness | ||
| Pseudomonas spp. | No evidence of human illness | ||
| Raoultella spp. | Evidence of human illness but no evidence of biogenic amine production | ||
| Serratia spp. | Weak evidence of human illness but no evidence of biogenic amine production | ||
| Staphylococcus spp. | Weak evidence of human illness but no evidence of biogenic amine production | ||
| Vibrio spp. | No evidence of human illness | ||
For consistency among information sources, Enterobacter spp. were considered according to the standing classification at the time of publishing of the screened articles.
Table A.3.
Long list of bacteria, viruses and parasites as biological hazards and the result of the selection based on the two criteria: (1) evidence of causing human illness and being associated with fresh fishery products and (2) evidence of survival and/or growth within the range of −3°C to 7°C. Those included in the assessment are shown in bold
| Group of hazards | Hazard | Evidence of human illnessa | Reference | Evidence of G or S | Reference |
|---|---|---|---|---|---|
| BACTERIA | Aeromonas spp. | Y | Morinaga et al. (2011) | G | Davies and Slade (1995), Carrascosa et al. (2014, 2016), Zhang et al. (2015c), Hoel et al. (2018) |
| B. cereus | Y | Hernando et al. (2007), Iwamoto et al. (2010), Domenech‐Sanchez et al. (2011) | N | ||
| Cl. botulinum (non proteolytic) | Y | Badhey et al. (1986), Telzak et al. (1990), Weber et al. (1993), Sobel et al. 2007), Horowitz (2010), Leclair et al. (2013), Walton et al. (2014) | G (evidence of toxin production) | Riedo et al. (1994), Betts and Gaze (1995), Farber et al. (2000), Miya et al. (2010), Lopez‐Valladares et al. (2014), Nakari et al. (2014), Lassen et al. (2016), Liu et al. (2016), Costa et al. (2019) | |
| Cl. perfringens | Y | Hewitt et al. (1986), Ciarrone et al. (1997) | N | ||
| L. monocytogenes | Y | Riedo et al. (1994), Farber et al. (2000), Tham et al. (2000), Nakari et al. (2014) | G | Eom et al. (2009), Lorentzen et al. (2010), Liu et al. (2016), Boulares et al. (2017) | |
| Pathogenic E. coli | Y | EFSA BIOHAZ Panel, (2013) | S | Masniyom et al. (2006), Jalali et al. (2016) | |
| Plesiomonas shigelloides | Y | Reilly and Kaferstein (1997), Stock (2004), Janda et al. (2016) | N | ||
| Salmonella spp. | Y | Iwamoto et al. (2010), Barrett et al. (2017), Hassan et al. (2018), Venkat et al. (2018) | S | Tassou et al. (2004), Norhana et al. (2010), Provincial et al. (2013b), Liu et al. (2016), Li et al. (2018), Pattanayaiying et al. (2019) | |
| Shigella spp | Y (weak) | Kumar et al. (2019) | N | ||
| St. aureus | Y | Wieneke et al. (1993), Gallina et al. (2013) | S | Poli et al. (2006), Binsi et al. (2015), Du et al. (2017) | |
| Thermophilic Campylobacter spp. | N | – | |||
| Vibrio spp. | Y | Finelli et al. (1992), Vandy et al. (2012), Martinez‐Urtaza et al. (2016), Jung (2018), Kim et al. (2018) | S b | Reily and Hackney (1985), Corrales et al. (1994), Wong et al. (1995), Magalhaes et al. (2000), Johnston and Brown (2002), Vasudevan et al. (2002), Januario and Dykes (2005), Vasudevan and Venkitanarayanan (2006), Provincial et al. (2013a), Gui et al. (2014), Wang et al. (2014), Zhang et al. (2015d), Pattanayaiying et al. (2019), Telli and Dogruer (2019) | |
| Y. enterocolitica | N | – | |||
| VIRUSES | NoV | Y | Barrett et al. (2017), Elbashir et al. (2018), Hardstaff et al. (2018) | N | |
| HAV | Y | Elbashir et al. (2018) | N | ||
| HEV | Y (weak) | Sridhar et al. (2017) | N | ||
| PARASITES | Cestodes | Y | Iwamoto et al. (2010) | N | |
| Nematodes | Y | Iwamoto et al. (2010) | S c | Pascual et al. (2010) | |
| Trematodes | Y | Iwamoto et al. (2010) | N | ||
| Giardia | Y | Iwamoto et al. (2010) | N | ||
| Cryptosporidium | N | – | |||
| Toxoplasma | N | – |
G: growth; S: survival.
The note ‘weak’ (evidence) was included for cases in which: a) the hazard was detected in outbreaks/cases‐related food together with other microorganisms, or b) mixed food that included seafood components where reported as a possible outbreak/case source, or c) contamination was reported as probably associated with food manipulation or cross‐contamination from other foods.
Predominant condition according to literature review.
Data related to Anisakis spp.
Table A.4.
Long list of histamine‐producing bacteria as biological hazards and the result of the assessment against the two criteria: (1) evidence of causing human illness associated with fresh fishery products and (2) evidence of biogenic amines production within the range of −3°C to 7°C. Those included in the assessment are shown in bold
| Hazard | Evidence of human illnessa | Reference | Evidence of biogenic amine production | Reference |
|---|---|---|---|---|
| Aeromonas spp. | N | – | ||
| Citrobacter spp. | N | – | ||
| Cl. perfringens | N | – | ||
| Enterobacter spp. a | Y | Lee et al. (2013) | Y | Tsai et al. (2005) |
| Hafnia alvei | Y (weak) | Lee et al. (2016) | N | |
| Klebsiella spp | Y | Taylor et al. (1979), Chen et al. (2008), Velut et al. (2019) | N | |
| Morganella spp. | Y | Dalgaard et al. (2008), Lee et al. (2013) | Y | Aytac et al. (2000), Emborg and Dalgaard (2008) |
| Photobacterium spp. | Y | Kanki et al. (2004), Dalgaard et al. (2008) | Y | Torido et al. (2012) |
| Proteus spp. | N | – | ||
| Providencia spp. | N | – | ||
| Pseudomonas spp. | N | – | ||
| Raoultella spp. | Y | Tsai et al. (2007), Lee et al. (2013, 2016), Lam and Salit (2014) | N | |
| Serratia spp. | Y (weak) | Tsai et al. (2007), Chen et al. (2008), Lee et al. (2016) | N | |
| Staphylococcus spp. | Y (weak) | Chang et al. (2008) | N | |
| Vibrio spp. | N | – |
The note ‘weak’ (evidence) was included for cases in which: a) the hazard was detected in outbreaks/cases‐related food together with other microorganisms, or b) mixed food that included seafood components where reported as a possible outbreak/case source, or c) contamination was reported as probably associated with food manipulation or cross‐contamination from other foods.
For consistency among information sources, Enterobacter spp. where considered according to the standing classification at the time of publishing of the screened articles.
Based on this analysis, Aeromonas spp., non‐proteolytic Cl. botulinum, L. monocytogenes, human pathogenic Escherichia coli, Staphylococcus aureus, Vibrio spp. for bacteria, nematodes (Anisakis spp.) for parasites, and Enterobacter spp.14 Morganella spp. and Photobacterium spp. for histamine‐producing bacteria were considered for inclusion in the present assessment.
With regard to the hazards for which either evidence of human illness or evidence of growth or survival (reduction) in FFP was not retrieved from the scientific literature and that were therefore excluded from the assessment, the following additional considerations were made based on available information on their ecology, minimum growth temperature, potential for pathogenicity and histamine production, as well as their life cycle:
-
•
Psychrotrophic strains of Bacillus cereus ‘sensu latu’ belong mainly to B. weihenstephanensis and to B. mycoides. Bacillus weihenstephanensis and B. mycoides have not been described as food poisoning agents (EFSA BIOHAZ Panel, 2016). Although these species can carry the enterotoxin genes, their toxigenic potential remains uncertain (Stenfors et al., 2002) and B. weihenstephanensis has been reported to produce emetic toxins only at temperatures ≥ 8°C (Thorsen et al., 2009; Guerin et al., 2017). Available data on the temperature dependence of the growth rate of the fastest growing B. cereus ‘sensu latu’ strains, show that their recorded maximum growth rate at 7°C (i.e. at the minimum temperature for which growth rate is available) in broth culture, ranges from 0.04 to 0.09 h−1 (Carlin et al., 2013). As such, they are expected to result in almost no growth in FFP at the even lower temperatures of the current assessment, according to the predictions of the heat transfer model.
-
•
Clostridium perfringens, Plesiomonas shigelloides, Shigella spp. and thermophilic Campylobacter spp. are not expected to grow within the range −3°C to 7°C based on their minimum growth temperatures (ICMSF, 1996);
-
•
Norovirus (NoV), hepatitis A virus (HAV), hepatitis E virus (HEV), Cestodes, Trematodes, Giardia, Cryptosporidium and Toxoplasma are not expected to replicate outside their respective living hosts;
-
•
It was considered unlikely that the absence of evidence of human illness for the Y. enterocolitica‐FFP hazard–food combination was due to continuous and systematic non‐reporting of cases or outbreaks and that, therefore, the results of the systematic literature review reflected the absence of epidemiological relevance of yersiniosis associated with FFP consumption.
-
•
Aeromonas spp., Citrobacter spp., Cl. perfringens, Hafnia alvei, Klebsiella spp., Pseudomonas spp., Serratia spp. and Vibrio spp. are considered weak histamine producers (Chang et al., 2008; Bjornsdottir et al., 2009; Visciano et al., 2012; Bjornsdottir‐Butler et al., 2013) and are prevalently mesophilic, while strong histamine‐producing bacteria as Proteus spp., Providencia spp., Raoultella spp. and Staphylococcus spp. are all mesophilic with negligible growth and histamine production at temperatures below 7–10°C (Dalgaard et al., 2008; Bjornsdottir‐Butler, 2018).
3.1.2. Uncertainties associated with hazard identification
The uncertainties related to the hazard identification are described in Table D.1 in Appendix D. The uncertainties relate to the literature search and screening, leading to a potential underestimation of the relevance of a hazard, and to data extraction, leading to a potential over‐ or underestimation of the relevance of a hazard.
3.1.3. Concluding remarks
-
•
The relevant biological hazards in FFP, considering both their association with human illnesses associated with FFP and their potential for growth on FFP within the temperature range of −3°C to 7°C, are: Aeromonas spp., non‐proteolytic Cl. botulinum and L. monocytogenes as regards bacteria. Those relevant for survival are: pathogenic E. coli, Salmonella, St. aureus and Vibrio spp., and Nematodes (Anisakis spp.) for parasites.
-
•
The relevant histamine‐producing bacteria in FFPs considering both their association with human illnesses (histamine intoxication) associated with FFP and their potential for histamine production, within the temperature range −3°C to 7°C, are: Enterobacter spp., Morganella spp. and Photobacterium spp.
-
•
The exclusion of hazards based on the absence of evidence of human illness associated with FFPs or of evidence for survival/growth on FFPs within the considered temperature range was further substantiated through information on their ecology, minimum growth temperature, potential for pathogenicity and histamine production, and life cycle. No further consideration could be made on the potential for survival of the excluded hazards.
3.2. Fish surface temperature dynamics in boxes and tubs
3.2.1. Recorded time/Temperature profiles of fish in boxes and tubs
The temperatures of plaice recorded in the first 5 days of the experiments during the ‘Qualitubfish’ project are shown in Figure 6 (first experiment, (Bekaert et al., 2016a)) and Figure 7 (second experiment, (Bekaert et al., 2016b)). The study consisted mainly of a ‘keeping’ process. Nevertheless, a short ‘cooling’ process took place at the beginning of the experiment, since the fish were exposed to the outside temperature before being introduced in the box/tub. The initial temperature of the manipulated fish with temperature loggers ranged between 2.5°C and 5.5°C.
Figure 6.
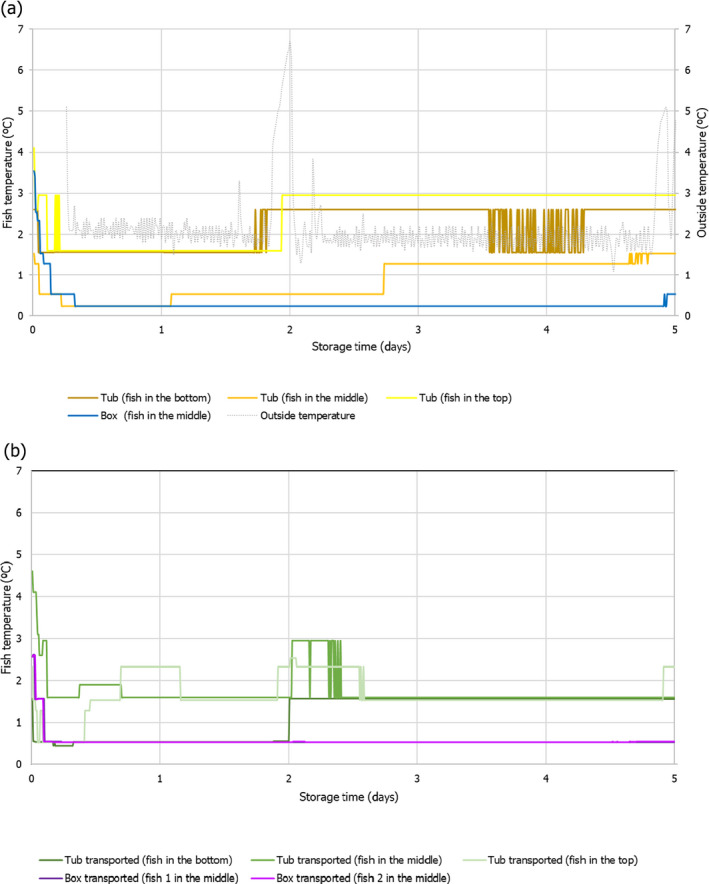
Time/Temperature records of plaice during the first 5 days of the first experiment of the ‘Qualitubfish’ project for (a) non‐transported and (b) transported boxes and tubs (Bekaert et al., 2016a). For the calculation of the boxplots (Figure 8), the first 3 days of storage were considered
Figure 7.
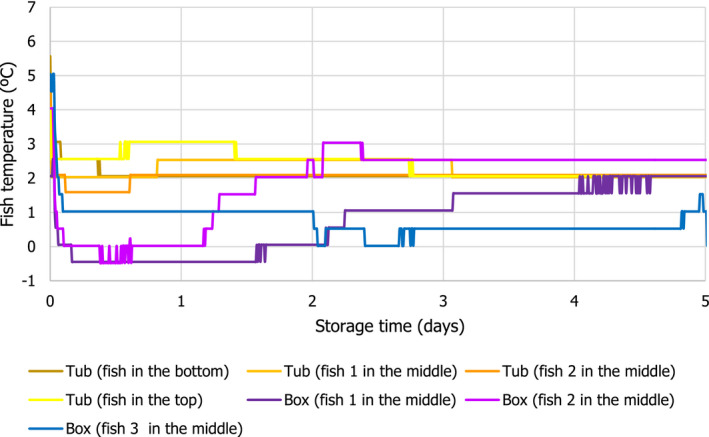
Time/Temperature records of plaice during the first 5 days of the second experiment of the ‘Qualitubfish’ project for non‐transported boxes and tubs (Bekaert et al., 2016b)
In the first experiment, the outside temperature in the cool cell was mostly 2°C but cyclically fluctuated between 1.1 and 6.7°C due to activities for the auction. After the initial short cooling process (1 h), the fish temperature in the transported tub varied between 0.5°C and 3.1°C and overall the mean temperature in the three locations was 1.7°C (top), 1.8°C (middle) and 0.9°C (bottom) during the first 3 days of storage. The maximum temperature difference depending on the position of the fish in the tub was 2.6°C (initial short cooling period not considered). In the non‐transported tub, the mean temperature in the three locations was higher for the first 3 days of storage except for the fish in the middle: 2.1°C (top), 0.5°C (middle) and 2.0°C (bottom). The maximum temperature difference, depending on the position of the fish in the tub, was 2.7°C. The fish temperature in the boxes was more stable than in the tubs and averaged 0.6°C (transported boxes) and 0.3°C (non‐transported box). The highest measured temperature was 1.6°C during the first 3 days of storage (initial short cooling not considered).
In the second experiment, the fish temperature in the tub varied between 1.6°C and 2.6°C during the first 3 days of storage after the first cooling period of 2 h. The temperature fluctuated more when fish were stored in boxes, specifically between −0.5 and 3.0°C. The highest temperature difference at any given time in the tubs was 1.0°C (for fish at the upper and lower position) and in boxes was 3.5°C. The mean temperature in the three locations in the box were 0.2°C, 0.9°C and 1.4°C (middle) and in the tub were 2.0°C and 2.4°C (middle), 2.1°C (bottom) and 2.6°C (top).
Overall, fish transport/storage during 3 days in boxes resulted in lower average fish temperatures in comparison with the tubs, as shown in Figure 8. The figure is similar for 5 days of transport/storage (data not shown). Regardless, a considerable variability was recorded in both boxes and tubs. In experiment 1, the fish temperature in the boxes was more stable than in the tubs, while this was reversed in experiment 2. In the latter, the variation recorded for different fish located in the middle layer of the box was considerable.
Figure 8.
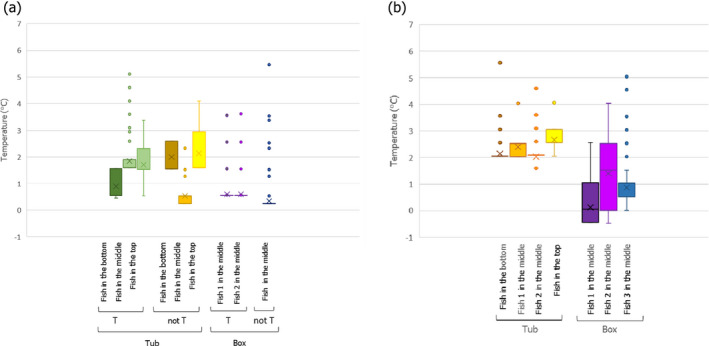
Boxplot distribution of the temperatures recorded in fish located in different positions of the box (with ice) and tubs (with freshwater and ice) during the experiments 1 (a) for transported (T) and not transported (not T) (Bekaert et al., 2016a) and experiment 2 (b) (Bekaert et al., 2016b) of the ‘Qualitubfish’ project for the first 3 days of transport/storage
The location of the fish in the container also influenced the fish temperature, which was related to the distance from the ice layer as well as to the distance from the walls of the container. Also, the position of the container in the storage room could play a role (e.g. stacking, temperature distribution within the storage room), though this aspect was not recorded in the experiments. Within the tub, there were differences between the bottom, middle and top position of the fish; the fish located in the top being at a higher temperature. This finding does align with the thermodynamic theory, according to which the bottom fish would be at the highest temperature as shown later (in Section 3.2.3), while the fish in the top would be at the lowest temperature as long as they are just below and in close contact with the ice layer on the top. The reason for this finding was that the top layer of fish/water was directly in contact with the higher temperatures of the storage room once the ice melted and before re‐icing of the fish was carried out (e.g. experiment 1 for non‐transported tubs).
3.2.2. Validation of the heat transfer model
The heat transfer model was validated by comparing the model predictions against some of the measurements in boxes and tubs extracted from experiment 1 of the ‘Qualitubfish’ project (see Section 2.3.1). As stated before, the study consisted mainly of a keeping process with an initial short cooling. The initial temperature of the manipulated fish with temperature loggers ranged between 2.5°C and 5.5°C. Non‐manipulated fish were exposed to the outside temperature for a shorter time, and therefore, expecting a lower, though unknown, initial temperature. For the validation, manipulated fish initial temperatures were considered as measured while for non‐manipulated fish 1.5°C was assumed.
The positions of the fish inside the containers and the simulation of the reported temperatures (i.e. average temperatures in the fish instead of surface maximum fish temperatures) were selected to realistically mimic the experiments, as described in Section 2.3.2.2. Therefore, temperatures of the validation model tend to reproduce the experiments, but not to capture Tmax within the containers.
The measured fish temperatures for a non‐transported box correspond well with the model predictions (see Figure 9), although the model overestimated the initial cooling of the fish for boxes. One reason may be the uncertainty of the initial temperature of the non‐manipulated (i.e. without a temperature probe) fish, but it may be also because of the approximation used to model the heat transfer inside the container (porous material of fish/air for box and fish/water for tub). The approximation is expected to perform better for water than for air, and therefore, the predictions were better for tubs. At time 168 h the box was re‐iced, and after this time point higher the discrepancy between model predictions and the measurements was observed (data not shown) because the model did not include the re‐icing. However, this discrepancy has little impact on the actual assessment because shorter duration (maximum 5 days) and the presence of ice throughout the duration of the assessment (i.e. no need for re‐icing) was assumed.
Figure 9.
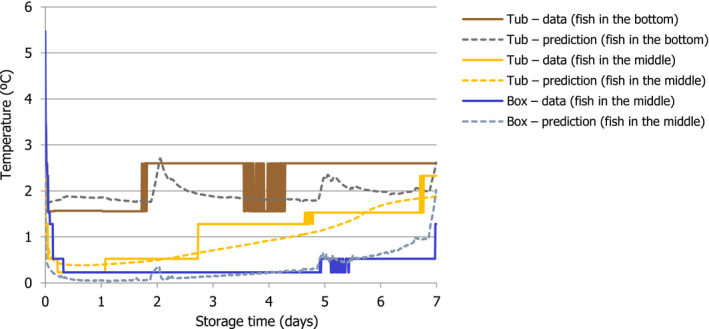
- The observed data were obtained in the first experiment of the ‘Qualitubfish’ project (Bekaert et al., 2016a).
Fish t/T profiles for non‐transported tubs used for the validation considered a fish located in the middle or bottom of the container. Measurements demonstrated great variability with fish on the bottom having higher temperatures compared to the middle (see Figure 9). The measured temperature of the fish in the middle of a tub was compared with the model predictions of fish at 0.01 m below the top ice, instead of in the geometric middle. Registered data showed abrupt changes from 1°C to 0.5°C, while the model predictions provided a smooth temperature profile. The model predictions are satisfactory overall, with a slight underestimation of the temperature being more evident in the tub (particularly for the fish located in the bottom) than in the box (Figure 9).
Note that the measured temperature of the fish in the bottom location remains at 2.6°C, when the mean outside temperature is 2°C with very short abusive temperature peaks (Figure 9). The model cannot predict such high temperatures over long periods of time, even if the fish are located in the warmest spot, i.e. bottom and close to the wall of the tub. A possible reason for the discrepancy could be that the model does not include natural convection (water movement due to density differences which is maximal at 4°C). Therefore, for fish located on the bottom, the model may underestimate the actual temperature.
The heat transfer model facilitates simulation of the temperature at different locations in the space (temperature distributions), for different materials (fish, water, container wall‐foam) and at different times (temperature dynamics). For example, after 1 day, the model predicts the temperature distributions shown in Figure 10. Usually, the fish temperature is at its maximum for the most distant position in relation to the ice and when closest to the container wall. Therefore, for boxes, the least cold points are on the corners and on the vertical middle (after 1 day, there is still ice on top and bottom layers), while for tubs, these are usually located on the bottom corners.
Figure 10.
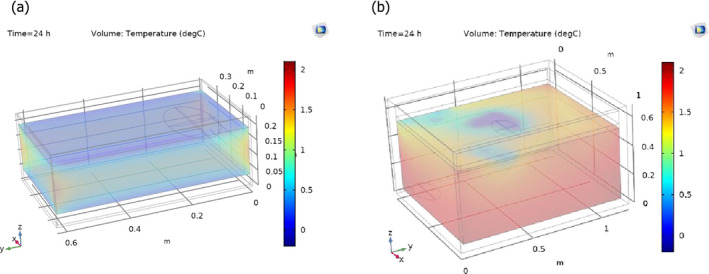
Modelled temperature distribution in the boxes (a) and tubs (b) after 1 day based on data obtained in the first experiment of the ‘Qualitubfish’ project (Bekaert et al., 2016a)
3.2.3. Predictions of fish temperatures using the heat transfer model
3.2.3.1. Temperature distribution inside containers
Fish surface temperatures depend on the location of the fish inside the containers and the configuration arrangement of the containers. This effect can be analysed with the heat transfer model providing for each time the temperatures at different points of the containers (i.e. one temperature for each spatial discretisation point).
Figure 11 shows the temperature distributions inside the container of the ‘abusive’ scenario for the ‘cooling‐keeping’ process of lean small (CLS) fish (i.e. case study #1c) (see Figure 3). The discretisation resulted in 44,782 points for boxes and 14,067 points for tubs following the discretisation options detailed in Appendix B. Probabilities are calculated for temperature intervals (histogram bins) of 0.025°C width, between 0 and 4°C. The results are shown after 1 day of cooling (a, b), after 2 days including the first cycle of abusive temperature (c, d), after 3 days and after 5 days.
Figure 11.
Temperature distribution at different times inside the container for the simulation of the ‘abusive’ scenario for the ‘cooling‐keeping’ process of lean small (CLS) fish (i.e. case study #1c)
Results show that after 1 day, fish was cooled down faster in boxes (a) than in tubs (b). This was most likely because more fish have to be cooled in the tub and this was not compensated for by the increase in ice weight. Figure 11a shows a two‐peak‐like distribution for boxes. One peak is at 0.14°C and corresponds with temperatures close to the ice, and the other peak at 0.63°C corresponds to temperatures close to the wall. For tubs, the distribution is more spread at cooler temperature (between 2.75°C and 3.18°C), with a peak at the warmer temperatures (at 3.49°C), corresponding with the bottom of the tub (Figure 11b).
Before the first abusive temperature after 2 days of cooling, the temperature distribution for boxes is similar to the profile after 1 day of cooling, while a decrease in temperatures is observed for tubs, i.e. tubs require more time for cooling (Figure 11c and d). However, the temperature distribution is wider for boxes. Two factors determine this finding: the container configuration consisting of two layers of ice on the top and bottom for boxes, but only one layer of ice on top for tubs, as well as the better transmission of the temperature effect due to water in the tub compared with the air of the box.
After 3 days (Figure 11e and f), temperatures in boxes are between 0 and 1.5°C and in tubs around 2°C. After 5 days (Figure 11g and h), temperatures increase for boxes, but not for tubs, resulting in temperatures showing similar distribution ranges.
3.2.3.2. Temperature dynamics
Three t/T profiles at specific locations in the containers were used to analyse the effect of the outside temperature: the surface temperature of two fish located at the warmest spots within the container and Tmax in the matrix of fish/water or fish/air (Section 2.3.2). It should be noted that Tmax in the matrix has a very low frequency of occurrence (tail of the distributions shown in Figure 11) and may not necessarily correspond with the same location inside the container throughout the storage time.
Figure 12 shows the t/T profiles captured for all the case studies for the ‘abusive’ scenarios described in Figure 3.
Figure 12.
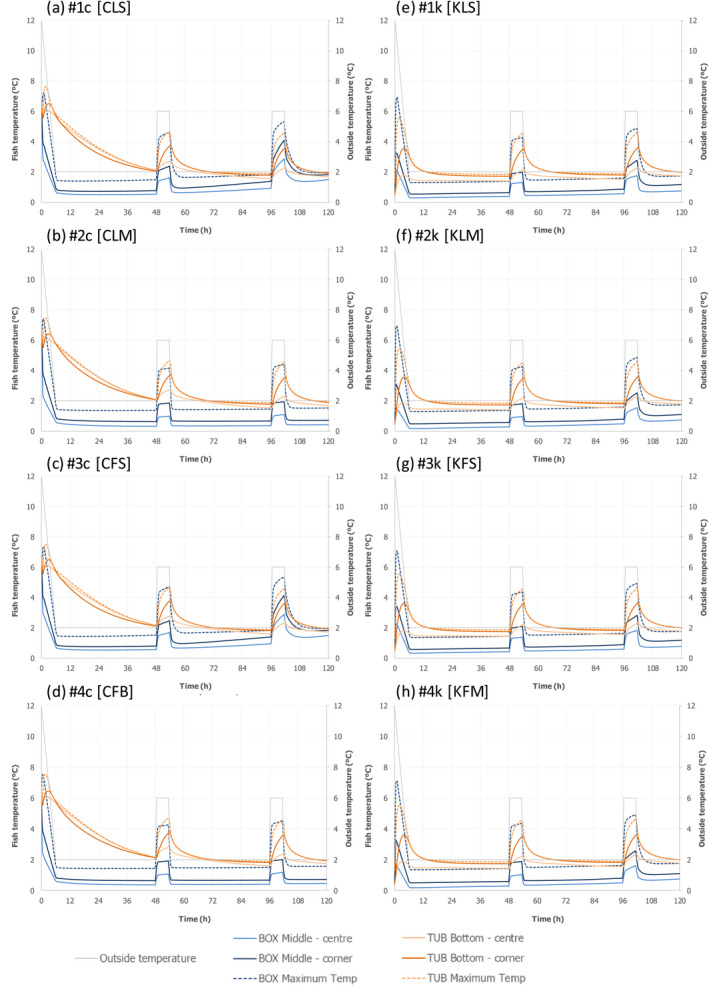
Time/Temperature profiles of fish located at the warmest spots within the container (middle centre and middle corner for boxes and bottom centre and bottom corner for tubs) and the maximum temperature in the matrix of fish/water or fish/air during transport/storage of fish as predicted by the heat transfer model for all the case studies assessed for ‘abusive’ scenarios (see Figure 3)
- C: ‘Cooling‐keeping’ process; K: ‘Keeping’ process; L: Lean fish; F: Fat fish; S: Small fish; M: Medium‐sized fish.
Boxes appear to provide a more efficient means of cooling fish, and therefore perform better during the earlier stages of storage. However, as the ice melts, the capacity to maintain the temperature is worse in boxes than in tubs, which can be explained by the non‐insulating material of the boxes compared to the insulating material of tubs. Figure 12 also demonstrates that the dimensions of the fish influence the t/T profiles to a limited extent. More specifically, small differences were observed for fish in boxes at the end of storage in the ‘cooling‐keeping’ process as medium‐sized fish seem to maintain the temperature better than small fish. The t/T profiles are not affected significantly by the fat content of the fish (lean or fat).
Abusive outside temperatures have a larger impact on Tmax detected in each container at each sampling for tubs than boxes. This effect is, however, not so relevant when focusing on the temperatures of the surface of a fish in a fixed position.
3.2.4. Uncertainty associated with the fish temperature
The uncertainties mainly relate to the scarcity of (real) observed data as well as to the modelling of the fish surface temperature are described in Table D.2 in Appendix D. The assessment assumed the application of good practices. The impact of improper practices (e.g. no re‐icing) or not fully covering the FFP will lead to higher temperatures than expected and modelled and has not been covered by the heat transfer modelling.
Table D.2.
Potential sources of uncertainty identified in the assessment of the fish temperature in boxes or tubs and assessment of the impact that these uncertainties could have on the conclusion (i.e. over/underestimation of the fish surface temperature in boxes or tubs and the extent of the over/underestimation)
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| t/T profiles of fish under ‘observed’ conditions | Only three sets of data from two experiments and on one fish type (i.e. plaice, representative of lean small fish) are available comparing transport/storage of FFP in water and ice (tub) vs. in ice (boxes) (‘Qualitubfish’ project). Temperatures were recorded inside the fish but considering the thickness of the fish this would not be so different compared to fish surface temperatures |
Reasonably foreseeable temperature conditions for storage/transport of FFP are expected to be within the ‘ideal’ and ‘abusive’ scenario included in the assessment, which addressed the fish surface temperature However, the real t/T profiles may deviate from this one set of experimental conditions |
| Heat transfer model assumption 1: Model focuses on conduction |
The model focuses on conduction and disregards other heat transfer phenomena, such as:
The model disregards any other liquid or solid movement:
|
Assuming that fish are covered with ice (proper practice as considered in the assessment), and, the model tends to overestimate temperatures in tubs (mainly due to disregarding forced convection) and in boxes (mainly due to disregarding the effect of the movement of water). Radiation has lower impact compared with conduction. It is unknown whether the extent of these overestimations is the same for boxes and tubs, and it depends on the location of the fish in the container |
| Heat transfer model assumption 2: ice melting using the ‘Apparent Heat Capacity method’ | The model includes the latent heat in the heat capacity and does not respond to a proper phase change with moving boundaries. For the considered parameters, the so‐called ice domain is a perfect mixture of 50% water with 50% ice at the transition temperature (Tt = 0°C), while all is ice at temperatures below −0.5°C and water above 0.5°C | The fish surface temperatures held in tubs and boxes may be either underestimated or overestimated the temperature. The impact would mostly affect temperatures in the range of –0.5 to 0.5°C in the ice‐water layer, but in any case towards the same direction and to the same extent in both containers, making the impact in the difference irrelevant |
| Heat transfer model assumption 3: Approximation to simplification of fish modelling and avoiding explicitly modelling the tens of fish in the containers |
Fish of interest (where temperatures are reported) are considered ellipsoids of two different dimensions and the fish surface temperature was used as input for modelling survival and growth of hazards in the worst‐case ‘abusive’ scenarios Around the fish of interest, the model assumes a matrix mixture consisting of fish/water for tubs and fish/air for boxes. It uses the standard mathematics for porous materials, where the volume fraction of fish is calculated based on ratios fish/water in tubs |
The fish surface temperatures held in tubs and boxes may be either underestimated or overestimated, being unknown the impact on the difference between containers |
| Heat transfer model assumption 4: location of the fish where temperatures are reported | Three locations are considered for each case study and container: the overall maximum temperature in the interior of the container at each time and the fish surface temperatures located in the warmest zone within the containers (except for validation of the heat transfer modelling, for which locations similar to the experimental ones were selected) | The fish surface temperatures FFP held in tubs and boxes are expected to be overestimated as worst‐case locations are considered, or directly the maximum temperature in the container. However, as this maximum temperature does not constantly occur on the same spot of either container, it does not represent a complete t/T profile on the surface of a fish. Instead, it is an evidence of the high likelihood of markedly higher temperatures occurring overall in tubs than in boxes regardless of the modelled process. Therefore, this value is an overestimated t/T profile |
| Heat transfer model assumption 5: Boundary conditions | Boundary conditions determine the heat flux between the container and the environment. The model assumes the standard parameter without air flow (no wind). However, this may change depending on the air movement around the container, for instance being limited when containers are in direct contact with the floor, walls or other containers |
Over/underestimation of the fish surface temperatures with low impact for containers made of insulating materials (such as tubs). Heat flux increases when the heat transfer coefficient increases, particularly in non‐insulating materials (such as boxes) Depending on the difference of temperatures between the container and the outside, the flux goes in one or other direction and to the same or different extent in boxes and tubs |
| Heat transfer model assumption 6: homogeneous initial conditions | Initial conditions are kept constant for each domain. As such, the initial temperature distributions of, for example, the fish, the water and the container material were not considered. Some of the initial temperatures, such as the initial temperature of the water in tubs, had to be assumed |
Overestimation of fish temperatures during cooling, as 7°C is assumed for the whole fish matrix Over/underestimation with uncertain impact for the other temperatures |
| Heat transfer model assumption 7: variability and uncertainty in model parameters and geometry |
|
The model parameters were carefully selected to represent reasonably foreseeable conditions, tending to be conservative. When the real conditions involve parameter values outside the range considered in the model, the simulation could both underestimate or overestimate the fish surface temperature From the multiple tests conducted with the model, the results are especially sensitive to the ratios of water/fish/ice for tubs and fish/ice for boxes, particularly affecting the time when re‐icing is needed to replace melted ice However, according to the current regulation, re‐icing is a practice that must be carried out to ensure that enough ice remains in the container. If properly done, the impact of the initial amount of ice would be minimised Bad practices in this respect were out of the scope of the present assessment |
| Numerical errors | Models of partial differential equations may not be sufficiently precise for coarse discretisation in space and stiff dynamics. The model was discretised sufficiently to see no effect on the discretisation and right integrator methods were selected | The numerics were tested to reduce this effect, expecting to be minimal. |
| Predictive performance of the heat transfer model | The model was validated by the comparison of observed and predicted t/T profiles associated with one experiment. Some of the assumptions were adjusted to make a more realistic comparison of the model and data. For example, fish location is not in the warmest zone but in a location similar to that of the fish bearing the logger in the experiment and instead of calculating the maximum temperature on the fish surface, the comparisons are carried out with the mean temperature in the fish (as the logger was inserted into the fish through the gutting cut) | Small discrepancies (slight underestimation) were recorded, its impact on the microbial behaviour was minimal |
| Fish temperature: surface and internal parts | The distribution of initial fish temperatures was not available and various options have been considered in the assessment. First, it was assumed to be 0°C (‘ideal’ scenario) without differences between the type of fish, as it equals the temperature inside the tubs upon arrival at the first on‐land establishment. A better scenario could be that temperature of lean fish is −1 to −0.5°C and the temperature of fat fish is −2°C to −1°C. Second, it was assumed to be 7°C (‘abusive’ scenario). The ‘observed’ scenario showed that fish temperature ranges from 0 to 2°C but in this case the fish were not handled (e.g. gutted) at an on‐land establishment | The ‘abusive’ scenario overestimates the initial temperature, and thus, the temperatures of FFP held in tubs and boxes, but in any case towards the same direction and to the same extent in both containers, making the impact in the difference minimal |
FFP: fresh fishery products; PE: poly‐ethylene.
Limited number of studies are available providing comparative data on t/T profiles of fish when stored/transported in ice (box) and in mixed ice and water (tub), which introduces a considerable uncertainty relating to the FFP temperatures during their storage/transport in tubs compared to boxes.
The FFP t/T profiles has been estimated from ‘ideal’ to ‘reasonably foreseeable abusive conditions’, in both cases based on simplification of the reality. A heat transfer modelling approach was used to analyse the ‘abusive’ conditions. Both the ‘ideal’ and the ‘abusive’ scenarios have inherent limitations and uncertainties associated with the applied assumptions.
Among the uncertainties when modelling fish temperature, the ones with the highest impact are due to the following assumptions:
-
•
disregarding any type of water or air movement in the containers due to mainly convection (forced or natural) in tubs and dripping of melting ice in boxes; and
-
•
assuming a matrix mixture of water/fish and air/fish to account for the contribution of the fish where temperatures are not reported.
These uncertainties are relevant and tend to overestimate the FFP temperature in both types of containers. However, the magnitude of the impact may be different in tubs compared to boxes and also may depend on the location of the fish within the container.
Other important sources of uncertainty are due to the values used for the parameters involved in the model that were considered to be representative of the conditions in boxes and tubs, such as the size of the container, the total mass of fish (and type of fish) in each container, the initial temperatures of each component (fish, water, air, container) or the amount of ice used to cool and/or keep the temperature of the FFP. Through the ‘reasonable foreseeable conditions’ a range of scenarios have been assessed, but these may have underestimated or overestimated the impact on FFP temperature associated with other possible combinations of conditions.
Overall, the approach implemented tends to overestimate the FFP temperature, mainly due to measuring the temperature on the fish located in the warmest positions inside both containers, as not considering the dripping melted water in boxes or forced convection around tubs. The uncertainty tending to underestimate the FFP temperature that could have the greatest impact on the fish at the bottom of tubs, due to not including natural convection in the modelling approach.
3.2.5. Concluding remarks
In an ‘ideal’ scenario15 with proper practices and assuming that the initial fish temperature is 0°C and the fish is in perfect contact with ice in boxes and with a perfect mixing of water and ice in tubs, the temperature of the fish surface would be equal for both types of containers (i.e. three‐layered poly‐ethylene PE tubs filled with freshwater/ice and HDPE boxes filled with ice) and equivalent to the temperature of melting ice (i.e. 0°C) throughout the storage/transport period of 5 days.
Only two experiments using lean small fish (i.e. plaice) provided ‘observed’ t/T profiles of fish during storage/transport in both type of containers. The fish temperature luctuated during the first 5 days of storage and after the initial short cooling. In the first ‘Qualitubfish’ experiment, the temperature fluctuated more in tubs, while in the second experiment, this was reversed. Relevant differences were observed depending on the location of the fish within the containers, which can be related to the distance from the ice layer as well as to the distance from the container walls. Overall, the median fish temperature was about 1.0°C higher when transported/stored in ice and water (tubs) compared to ice (boxes), but after the short initial cooling, a temperature of 3.1°C was never exceeded.
A heat transfer model was developed to predict fish surface temperatures under ‘reasonable foreseeable abusive’ scenarios,16 providing satisfactory outputs when compared with the observed data, for both type of containers.
-
The model was applied to generate the fish surface temperature profiles under the ‘cooling‐keeping’ and ‘keeping’ process for FFP in both type of containers under the same conditions of transport/storage, considering different types of fish (regarding fat content and dimensions).17 According to the model predictions, for the considered parameters and configuration arrangement of the containers:
-
○
Fish surface temperatures depend on the location of the fish inside the containers with the warmest spots most distant from the ice layer; that is the centre between the two ice layers in boxes (ice on top and bottom) and in the bottom of tubs filled with water and ice (ice on the top). The fat content and the dimensions of the fish have only limited impact on the t/T profiles as compared to the impact of the initial fish temperature and temperature of the chilling room where tubs and boxes are stored or transported (i.e. outside temperature).
-
○
In earlier stages of the storage, fish cools down faster in boxes than in tubs (‘cooling‐keeping’ process). Later, as the ice melts, the capacity to keep the temperature low is less in boxes (made of non‐insulating material) than in tubs (made of insulating material) (both ‘cooling‐keeping’ and ‘keeping’ processes).
-
○
3.3. Impact of the storage conditions of fish on parameters relevant for bacterial growth
Storage of fish in tubs or boxes may have an impact on the water content of the fish flesh depending on many factors including the type of ice or liquid used. This change in the water content may further influence parameters of relevance for bacterial growth such as aw or WPS. Storage in water also raises the question of oxygen availability and internalisation of microorganisms. These topics are further discussed below.
3.3.1. Changes in water content
The proximate composition including the water content of live fish is very variable. Water content will vary between species, but also from one individual fish to another. Other parameters such as fishing area, fish age, sex, season and fish size will also play a role in the variability (Lemon and Regier, 1977; Huss, 1995).
The storage methods are known to also impact on the water content of the stored fish. Table 6 summarises the available studies on the change either in weight (presumably due to water uptake) or water content of fish during its storage under different conditions, including ice (unspecified type), flake ice, slurry ice, RFW or RSW for a variable time period.
Table 6.
Overview of changes in weight or water content during storage of fresh fishery products. The studies shown in bold type represent the specific conditions of interests of the present opinion
| Fish species | Storage typea | Duration of storageb | Weight or water content changec | Reference |
|---|---|---|---|---|
| Weight change | ||||
| Cod (Gadus morhua), farmed | Flake ice | 2 days | − 1% | Digre et al. (2011) |
| 14 days | − 1% | |||
| Slurry ice | 2 days | + 5% | ||
| 13 days | + 17% | |||
| Cod (Gadus morhua) | Flake ice | 3 days | No change | Joensen et al. (2001) |
| 11 days | + 1% | |||
| Ice and freshwater mixture | 3 days | + 6% | ||
| 1 day | + 13% | |||
| Slurry ice | 3 days | + 5% | ||
| 11 days | + 10% | |||
| Ice and seawater | 3 days | + 3% | ||
| 11 days | + 7% | |||
| Herring (Clupea harengus) | RSW (5 L container) | 6 days | + 6.4 to 17.7% | Hjelm et al. (2006) |
| Atlantic salmon, gutted (Salmo salar) | Flake ice | 11 days | − 2% | Erikson et al. (2011) |
| Slurry ice | 11 days | + 2.5 to 6% | ||
| Dressed chinook salmon ( Oncorhynchus tshawytscha ) | Flake ice | 2 days | − 0.2% | Bronstein et al. (1985) |
| 4 days | + 0.4% | |||
| 7 days | − 0.2% | |||
| RFW | 2 days | + 3% | ||
| 4 days | + 5% | |||
| 7 days | + 5.45% | |||
| RSW | 2 days | + 0.6% | ||
| 4 days | + 2.6% | |||
| 7 days | + 3.8% | |||
| Sockeye salmon (Oncorhynchus nerka) | RSW | 14 days | + 3.5% | Tomlinson et al. (1965) |
| Sprat (Sprattus sprattus) | RSW (5 L container) | 6 days | + 4.1 to 16.5% | Hjelm et al. (2006) |
| Steelhead trout (Oncorhynchus mykiss) | RSW | 14 days | + 3.7% | Tomlinson et al. (1965) |
| Water content change | ||||
| Mackerel (Scomber scombrus) | Flake ice | 8 days | No change | Lemon and Regier (1977) |
| RSW | 8 days | No change | ||
| Plaice, gutted (Pleuronectes platessa) | Flake ice (after 3 days on board in ice) | 2 days | + 0.7% | Bekaert et al. (2016a) |
| 5 days | − 0.2% | |||
| 9 days | + 3.6% | |||
| Ice and freshwater mixture (after 3 days on board in ice) | 2 days | + 2.0% | ||
| 5 days | + 1.6% | |||
| 9 days | + 6.0% | |||
| Plaice, gutted (Pleuronectes platessa) | Flake ice (after 8 days on board in ice) | 2 days | + 1.1% | Bekaert et al. (2016b) |
| 5 days | − 1.4% | |||
| 7 days | − 0.3% | |||
| Ice and freshwater mixture (after 8 days on board in ice) | 2 days | + 3.3% | ||
| 5 days | + 1.9% | |||
| 7 days | + 2.9% | |||
| Gilthead seabream (Sparus aurata) | Flake ice | 20 days | + 1% | Huidobro et al. (2001) |
RFW: refrigerated freshwater; RSW: refrigerated seawater.
RSW and slurry ice are included as a storage method on‐board the fishing vessels, which can also be responsible for water uptake.
Storage time selected from the information available in the article as the closest to 5 days (maximum duration considered in the present assessment).
Weight change is considered to be due to water uptake. Increases or decreases are calculated as percentages with respect of the water content of fish at time 0 (at the beginning of the storage).
Storage of fish in flake ice generally results in smaller increases in fish weight or water content compared to storage in water. No change or even a decrease has often been recorded during storage in flake ice, depending on the storage period and the fish species. For example, in the first experiment of the ‘Qualitubfish’ project (see Figure 13a) (fish was stored for 3 days on board in flake ice before being transferred to tubs filled with water and ice) plaice stored for 2 and 5 days in flake ice after landing, showed a water content increase of 0.7% and a slight decrease of 0.2%, respectively, as compared to an increase of 2.0% and 1.6% in refrigerated freshwater (Bekaert et al., 2016a). During the second experiment (see Figure 13b) (fish for 8 days on board in flake ice), the water content increased with 1.1% after 2 days but decreased with 1.3% after 5 days of on land storage in flake ice, while there was an increase of 3.2% and 1.9% during storage in freshwater (2 and 5 days respectively). Huidobro et al. (2001) recorded a small water content increase of 1% in gilthead seabream stored for 20 days in flake ice. In contrast, dressed chinook salmon (Gallart‐Jornet et al., 2007), gutted Atlantic salmon (Erikson et al., 2011) and farmed cod (Digre et al., 2011) decreased in weight after 7–14 days of storage in flake ice. Lemon and Regier (1977) found no changes in the water content of mackerel when stored for 8 days in flake ice or RSW. This was also the case for cod in the study of Joensen et al. (2001) after 3 days of storage.
Figure 13.
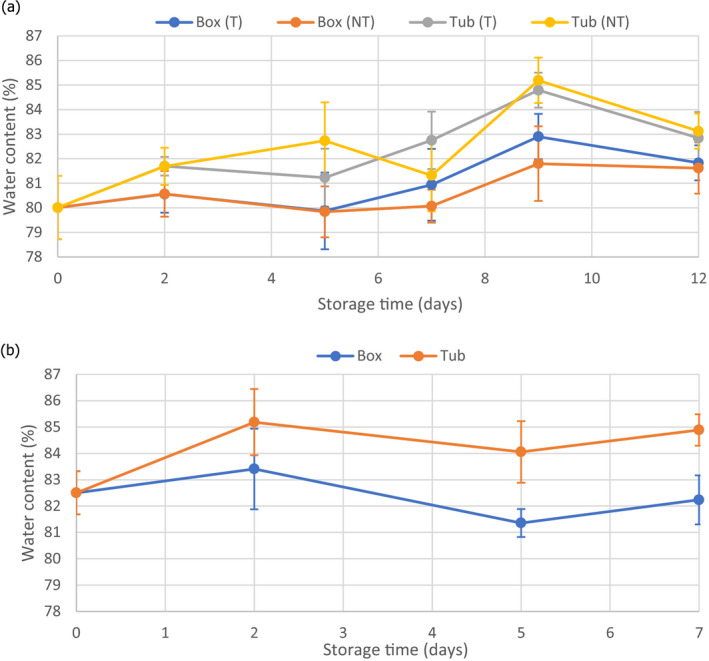
- In the first experiment, fish was stored for 3 days on board in ice before starting the storage experiment, while in the second experiment, fish was stored for 8 days on board in ice before starting the storage experiment.
Storage in water systems promotes water uptake by fish. For herring and sprat stored for 6 days in RSW, the water uptake varied between 6.4% and 17.7% and 4.1–16.5%, respectively (Hjelm et al., 2006). Tomlinson et al. (1965) found an increase for sockeye salmon and steelhead trout of 3.7% and 3.5%, respectively, after 11 days of storage in RSW. Studies comparing storage in RSW and RFW for the same species showed that the water uptake is higher in RFW than in RSW. For example, Bronstein et al. (1985) held dressed chinook salmon in RFW and RSW and found a significant weight gain of the fish after 2 and 4 days storage of 3 and 5%, respectively, in RFW and 0.6% and 2.6%, respectively, in RSW. The observed fish water uptake was much faster in RFW than the RSW. According to Joensen et al. (2001), the weight gain of cod was 6% when kept in an ice and freshwater mixture for 3 days and 3% when kept in an ice and seawater mixture. Storage in slurry ice, a mixture of small ice crystals and seawater, also favoured water uptake up to 5% (Joensen et al., 2001). Other studies with slurry ice confirmed the weight increase: up to 2.5–6% in Atlantic salmon after 11 days of storage (Erikson et al., 2011) and up to 5% for farmed cod after 2 days of storage (Digre et al., 2011).
When looking at the water uptake dynamics, Hjelm et al. (2006) found that the mean water uptake (by weight) was highest during the first 24 h of storage of herring and sprats in salt water, irrespectively of their size class and catching area. Although the general trend was a steady increase in weight, in some cases, the weight was stable or decreased. The salinity of the water used in the experiment (i.e. from 8 to 32 practical salinity units (PSU)) had little or no impact on the total water uptake. Water uptake was affected by catching area and salinity for herring, while for sprats, it was affected by area and size. The difference between the two species was attributed to their fat content. The experiments in the lab started 3–9 h after landing because of varying transportation times to the lab, but this did not seem to impact the water uptake for the different batches. The water uptake was similar in whole and gutted fishes, confirming that the water is absorbed through the body. In the first experiment of the ‘Qualitubfish’ project (fish stored 3 days on‐board in flake ice before transferring to tubs), water uptake was 2% after the first 2 days of storage in tubs, but steadily increased to reach a maximum of 6% after 9 days in tubs (Bekaert et al., 2016a). In the second experiment (fish stored 8 days on‐board in flake ice before transferring to tubs), water uptake was 3% after 2 days in tubs, but did not increase any more thereafter (Bekaert et al., 2016b). The study of Bronstein et al. (1985) also showed an immediate important weight gain of chinook salmon the first days of storage. The experiment started 18 h after catch. The water uptake rate was much higher if the fish was stored in freshwater as compared to seawater. However, from day 7 of storage when fish were transferred back to ice, all fish lost weight as the water taken up during storage was quickly lost. In contrast to the previous study, Tomlinson et al. (1965) found that trout and sockeye salmon stored in RSW lost weight during the first 2 days of storage and only gained weight thereafter. This was attributed to the continuation of slime secretion post‐mortem. For skinned fish muscle, however, the uptake of water was delayed for 2 days in rested fish (i.e. fish removed from the tank and killed immediately), but was immediate in exhausted fish (i.e. fish forced to swim vigorously during 30 min and then to struggle 15 min in air before being killed). This study of Tomlinson et al. (1965) was performed with aquacultured fish with no delay between killing the fish and the start of the experiment, while in experiments with wild fish, there is often a 1‐ or 2‐day delay due to landing and transportation of fish from the ship to the lab before a controlled experiment can start. This complicates the evaluation of the first days of storage and might explain the difference in water uptake dynamics during the first days. Indeed, as fish undergoes important changes (rigor mortis) during the first hours or day after catch, this might influence the water uptake and cause variability. The water uptake of the steelhead trout in the study of Tomlinson et al. (1965) only started after resolution of rigor mortis. The time to go into and pass through rigor mortis will depend on the species, the physical condition, the degree of exhaustion, the size, the amount of handling and the storage temperature of the fish (Stroudt, 2001).
3.3.2. Changes of relevance for microbial growth
3.3.2.1. Water phase salt content
Calculated values of the WPS content of fish from the literature can be found in Table 7. The WPS of farmed cod derived from one study was 0.88% and 1.77% after 14 days of storage in ice and slurry ice, respectively (Digre et al., 2011). Salt content was not reported at the start of storage period, but the salt in the slurry ice must have contributed to the increased salt content, and hence WPS, of the fish fillet. This high uptake of salt during storage in slurry ice was not confirmed in other studies (Magnússon et al., 2010; Thordarson et al., 2017) in which the salt content hardly varied during storage. The study of Magnússon et al. (2010) provided a WPS between 0.37% and 0.38% (as a maximum value as the salt content was often below the analytical detection limit). Thordarson et al. (2017) reported no change in water nor salt content in traditionally stored (ice) and sub‐chilled (liquid ice) cod, having a 0.24% WPS. The WPS of cod varied between 0.24% − wild cod when stored for 16 days in ice or slurry ice (Thordarson et al., 2017)−, and 1.77% − for farmed cod stored for 14 days in slurry ice (Digre et al., 2011). The fact that farmed cod was used in the study of Digre et al. (2011) could have impacted on the uptake of salt and water as even though farmed and wild cod are the same species, it appears there are differences between them (Olsson et al., 2007). Moreover, the salinity of the slurry ice, the physiological state or size of the fish could be different between the studies leading to different salt uptake levels.
Table 7.
Overview of the water phase salt (WPS) content during storage of fresh fishery products
| Fish species | Time and storage type | Water content (%) | Salt content (%) | WPS (%) | Reference |
|---|---|---|---|---|---|
| Cod, farmed | 14 days, ice | 79.8 | 0.7 | 0.88 | Digre et al. (2011) |
| Cod, farmed | 14 days, slurry | 79.1 | 1.4 | 1.77 | Digre et al. (2011) |
| Cod | Cooled with crushed plate ice after 1 day (min) | 80.0 | 0.3 | 0.38 | Magnússon et al. (2010) |
| Cod | Whole, bled gutted cod cooled with liquid ice after 8 days (max) | 81.5 | 0.3 | 0.37 | Magnússon et al. (2010) |
| Cod | 0 and 16 days, ice, no variation in salt and water throughout storage time | 82.0 | 0.2 | 0.24 | Thordarson et al. (2017) |
| Cod | 0 and 16 days, slurry, no variation in salt and water throughout storage time | 82.0 | 0.2 | 0.24 | Thordarson et al. (2017) |
| Salmon | 7 days, ice | 70.0 | 0.1 | 0.14 | Thordarson et al. (2017) |
| Salmon | 7 days, slurry ice | 70.0 | 0.2 | 0.29 | Thordarson et al. (2017) |
| Chinook salmon | 7 days, ice | 70.0a | 0.096 | 0.14 | Bronstein et al. (1985) |
| Chinook salmon | 7 days, RFW | 73.8b | 0.084 | 0.11 | Bronstein et al. (1985) |
| Chinook salmon | 7 days, RSW | 72.7b | 1.137 | 1.56 | Bronstein et al. (1985) |
| Cod, fillet | Storage time unknown, before liquid cooling | 82.4 | 0.4 | 0.48 | Valtýsdóttir et al. (2010) |
| Tuna, loin | Storage time unknown, has been frozen | 71.0 | 0.27 | 0.60 | Emborg et al. (2005) |
RFF: refrigerated freshwater; RSW: refrigerated seawater.
Assumed value, not available in the publication.
Calculated value based on reported weight increase % in the publication.
The WPS content derived from literature review for salmon varied between 0.11% in ice and 1.56% in RSW due to a significant increase in the salt content during the 7 days of storage. Bronstein et al. (1985) reported salt levels around 0.1% in Chinook salmon during storage in ice and RFW, but > 1% salt in RSW, leading to a high WPS of 1.56%. However, the reported salt levels could be above the average as the belly flap of the fish, known to contain higher salt levels, was included in the sample. WPS values of salmon derived from the study of Thordarson et al. (2017) were 0.14% (in ice) and 0.29% (in slurry ice) indicating a slight increase in salt content during storage in slurry ice.
From the literature above, the salt content increases markedly in RSW during storage leading to a clear increase in WPS during storage. In slurry ice, both situations of high and low salt uptake are possible. The WPS will only increase during storage in tubs when high uptake of salt occurs. In ice and RFW, the salt content does not seem to vary much during storage. Therefore, the WPS will usually remain stable during storage in ice (not much absorption of water) and decrease slightly in RFW or in water with ice due to uptake of water.
RSW, slurry ice and flake ice are often used for storage on board fishing vessels. Hence, WPS of fish can already differ at landing depending on the storage method on board.
In summary, the impact of the water uptake on the WPS will be rather limited during 2–5 days of storage of fish in freshwater with ice (in tubs) and practically irrelevant when fish is stored in ice (in boxes). Based on the literature review, the storage/transport of fish in fresh water with ice (as in tubs) can increase the water content of fish from 1.6% to 6%. The impact of this water uptake on the median WPS of fish (0.37%, see Table 3) is a decrease of the WPS value of fish from 0.364% to 0.349%, respectively.
3.3.2.2. Internalisation of microorganisms
In live fish, microorganisms are present in the intestines and on the outer surface of the fish in contact with the environment such as the skin and the gills. Once the fish dies and the immune system of the fish collapses, bacteria colonise the scale pockets on the skin and may invade the fish flesh by moving between the muscle fibres during storage (Gram, 1995). The invasion of bacteria is considered to start at the gills and the kidneys, to continue through the vascular system or directly through the peritoneal lining (Tomiyasu and Zenitani, 1957). However, the extent of this invasion (internalisation) is actually limited in chilled fish as mentioned by Murray and Shewan (1979). Bacteria could only be detected in the fish flesh once the number of microorganisms on the skin reached 106 CFU/cm2 (Ruskol and Bendsen, 1992). There was no difference in detection threshold between iced and ambient temperatures nor difference in invasive pattern between specific spoilage bacteria and non‐spoilage bacteria. As spoilage seems to occur mostly at the surface of the flesh, it is considered mainly to be a consequence of the diffusion of bacterial enzymes into the flesh (Gram, 1995). No evidence could be found that the bacterial internalisation patterns or rates of spoilage microorganisms differ between storage on ice (in box) or in water and ice (in tubs).
The above‐mentioned studies did not deal with pathogenic bacteria, but the potential for internalisation could be similar to that of spoilage bacteria. In fact, internalisation of pathogenic bacteria has been reported in terrestrial livestock animal meat (Shirai et al., 2017; Tozzo et al., 2018). Bacteria can migrate through the gaps between the endomysia and the muscle fibres that shrink during the development of rigour (Gill et al., 1984). The extend of internalisation has been reported to depend on the type of bacteria (e.g. aerobic vs. anaerobic, motile vs. non‐motile) and is the result of a balance among different factors including motility, chemotaxis and proteolysis (Shirai et al., 2017). However, neither the proteolysis nor the motility seems crucial to allow bacterial pathogens to penetrate into the meat, and other intrinsic and extrinsic factors (e.g. osmotic pressure, temperature, tissue moisture) and the presence of background microbiota may also play a role (Thomas et al., 1987; Tozzo et al., 2018).
Although no scientific article has been found reporting bacterial pathogen internalisation in fish muscle, fish muscle flesh has a structure similar to that of livestock muscle meat. A gradual breakdown of the endomysium and detachment of the fibres due to the rupture of attachments between the endomysium and the myoseptum occurs during tenderisation of fish flesh (Listrat et al., 2016), which could also enable the internalisation of bacteria. However, different to meat, fish is stored in ice (in boxes) or in water and ice (in tubs), which introduces other factors (associated with temperature, water uptake, rinsing/dilution effects etc.) that may modulate bacterial internalisation into fish flesh to a potentially different extent when fish is stored in water (in tubs with water and ice) compared to being stored in ice (in boxes).
Compared to bacteria on the fish surface, bacteria internalised in the fish matrix may be exposed to slightly different environmental conditions, for instance regarding temperature (less fluctuating) and oxygen availability (lower) with consequent impact on the bacterial growth.
3.3.2.3. Anaerobic conditions during storage
No significant differences were observed during the ‘Qualitubfish’ project (Bekaert et al., 2016a) in the total psychrotrophic bacteria or specific spoilage bacteria between fish stored in tubs (with freshwater and ice) or in boxes (in ice), although microbiological counts were slightly lower in fish in tubs. This was confirmed in different studies. Chilled water systems retarded bacterial growth as compared to ice in Bronstein et al. (1985). Also in the study of Digre et al. (2011), cod stored in slurry ice had significantly lower bacterial loads (total viable count (1.3 log10 difference) and sulfide‐producing bacteria (0.6 log10 difference) than ice‐stored cod after 14 days and in the study by Tomlinson et al. (1974), RSW was much more effective than ice in controlling bacterial growth in different fish species.
Though nutrient dilution or contamination rinsing effects of water ingress cannot be discounted, the slower bacterial growth in water systems has been attributed to the reduced oxygen availability, reducing the growth rate of the aerobic spoilage microbiota compared to the growth under aerobic conditions occurring during storage in ice (Bronstein et al., 1985). The low level of oxygen, leading to growth of anaerobic bacteria is also mentioned by Graham et al. (1992). In water systems, oxygen will decrease and give rise to more anaerobic conditions with the formation of hydrogen sulfide by certain bacteria. In these studies (Chinivasagam et al., 1996; Chinivasagam et al., 1998), it was shown that the bacterial spoilage microbiota in tropical prawns were predominantly H2S‐producers (mainly Shewanella putrefaciens) during storage in slurry ice while this was not the case during storage in flake ice (mainly Pseudomonas fragi). The presence of two different dominant spoilage bacteria was explained by the inhibitory effect that Pseudomonas strains can have on S. putrefaciens during storage in ice (Gram, 1993). As selection of microbiota during spoilage is not only determined by growth rate but also by microbial interaction, storage in slurry ice could have altered the inhibitory effect. Also, S. putrefaciens, which is capable of anaerobic respiration, could benefit from the more anaerobic conditions in water systems, similar to reports relating to vacuum packed fish (Huss, 1995).
Although no scientific paper was found dealing with the differential growth of pathogenic microorganisms in fish when stored in tubs compared to boxes, the different oxygen availability of the environment surrounding the fish when stored in water systems, e.g. in tubs, compared to aerobic storage in ice, could influence the growth behaviour.
3.3.3. Uncertainties associated with water uptake and WPS
The uncertainties associated with the water uptake and WPS are described in Table D.3 in Appendix D.
Table D.3.
Potential sources of uncertainty identified in relation to the impact of storage conditions and the water uptake and its influence on critical factors for microbial growth
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Factors affecting water uptake | There are many factors affecting the water uptake of fish such as salinity of water used to fill the tub, storage temperature, season, exhaustion state of the fish, fish size, fish species, storage time, integrity of the skin. It is impossible to consider all these combinations. Evidence from experiments that changed the relevant factor (e.g. storage in ice vs. water) was used, keeping the other factors unchanged | Over/underestimation of the water uptake and the quantitative effects of the influencing factors |
| Water uptake before landing | Fish stored in tubs with water (freshwater or seawater) and ice on‐board will uptake water to a variable extend depending on exhaustion state of fish, fish size, fish species as well as the time on board | Overestimation of the water uptake and its impact on the physico‐chemical characteristics determining microbial growth and survival as the assessment assumed that all water uptake occur during the transport and storage on‐land |
| Determination of median WPS of fish | Scarce availability in literature on WPS values of fresh fish and uptake dynamics of salt during storage in ice or water systems to calculate WPS | Over/underestimation of the median WPS because values could be missed in literature |
| Internalisation of microorganisms | The mechanisms of internalisation of microorganisms and its extent is unknown. Internalisation would lead to slightly different environmental conditions for the hazards | Underestimation of internalisation of microorganisms, which will be associated with an overestimation of the growth of aerobic hazards due to the lower oxygen availability in the internal parts of fish compared to the aerobic surface |
| Anaerobic conditions in water storage | The anaerobic conditions found in water systems seem to limit growth of spoilage bacteria. Though no evidence has been found for pathogenic bacteria, the more anaerobic environment may reduce the growth potential of aerobic pathogens and increase the growth potential of anaerobic pathogens |
Overestimation of the growth of aerobic/facultative anaerobic pathogenic growth in water system (in tubs) as the growth has been assessed assuming aerobic environment The growth of anaerobic pathogens (such as non‐proteolytic Cl. botulinum) may have also been overestimated if the water system (in tubs) is not strictly anaerobic |
WPS: water phase salt.
Both under‐ and overestimation of the water uptake and/or WPS is possible both in tubs and in boxes as linked to following uncertainties:
-
•
There are numerous factors affecting the water uptake during on‐land storage such as species, on‐board storage conditions, season and exhaustion state. It is impossible to consider all combinations; and
-
•
Literature could have been missed for the determination of the median WPS.
Though the extent of the internalisation of microorganisms in FFP when stored in boxes or in tubs is unknown, this uncertainty would lead to an overestimation of the growth of aerobic bacteria, as internalised microorganisms may encounter lower oxygen availability compared to aerobic storage in ice.
On the other hand, in tubs, overestimation of pathogenic growth could have taken place in the assessments, as in the case of aerobic bacteria because more anaerobic conditions occur in water storage as compared to ice. For anaerobic bacteria, an overestimation can also be expected because the water in tubs is not strictly anaerobic.
3.3.4. Concluding remarks
-
•
The water content of fish generally increases more during storage when fish are in tubs (with water and ice) compared to boxes (with ice). The amount of water uptake by fish depends on the water salinity, fishing area, fish species and size as well as the duration of storage.
-
•
The storage of fish in tubs filled with water and ice on‐board may result in a variable amount of water being absorbed by the fish. On‐land, further water uptake by fish during storage in tubs may continue until saturation. Absorbed water can also be lost after transferring fish from tubs on‐board to boxes on‐land.
-
•
The water uptake of fish associated with a 2–5 days period of storage in freshwater may range from 1.6% to 6%. As a consequence, the WPS content (0.37%) reduction may range from 0.006 (i.e. to 0.364%) to 0.019 (i.e. to 0.349%). It is reasonable to assume that the fish WPS decrease will be even lower on land, or not occur at all, because the fish may already be saturated during the on‐board storage in tubs filled with seawater.
-
•
The storage of fish in water such as in tubs (with water and ice) can have an impact on other factors relevant for microbial behaviour. For instance, water systems can reduce the oxygen availability in the environment. Consequently, compared to the aerobic environment of the fish stored in ice (in boxes), the growth of strictly aerobic pathogenic bacteria may be reduced, while the growth of facultative anaerobic pathogens could be less affected.
3.4. Behaviour of relevant biological hazards and histamine accumulation during transport boxes and tubs
3.4.1. Survival of relevant hazards
The available evidence suggests inactivation of the mesophilic pathogens St. aureus, Salmonella spp. and pathogenic E. coli is not likely under the conditions of the assessment (i.e. pH 6.5, aw > 0.98 corresponding to a WPS up to 2%, and temperatures from −3°C to 7°C) (Alford and Palumbo, 1969; Skandamis et al., 2002; Ross et al., 2008; Pin et al., 2011). Even though the data refer to studies in broth, or studies with ground meat and cheese, the bacterial inactivation is associated with the lethality effects of the intrinsic factors (such as pH and aw) and not the substrate itself. The pH and aw become bactericidal at values that prevent growth; e.g. pH < 4.5 and aw < 0.94 (Presser et al., 1998; Koutsoumanis et al., 2004; Valero et al., 2009). Under these conditions, the inactivation rate increases with temperature, suggesting a dominant role of storage temperature in non‐thermal inactivation associated with a metabolic exhaustion mechanism (Leistner, 2000). Despite the evidence that prolonged storage in Brain–Heart Infusion (BHI) broth under the pH and aw conditions of the current assessment may cause some inactivation of St. aureus, the corresponding D‐value (i.e. the time needed for a 1 log10 reduction) is more than 2,000 h at 5°C, which is far beyond the maximum storage duration considered in the present assessment. Likewise, Salmonella Derby, Thomson and Enterititis decreased by < 1 to > 2 log10 units in ground pork within 14 days of aerobic storage at 4°C, but the reduction started only after the indigenous microbiota reached the maximum growth levels of 8 log10 CFU/g at stationary phase. The latter suggests that Salmonella inactivation was the result of microbial interaction and/or the concomitant changes in the intrinsic properties of meat, e.g. reduction of pH, increase of metabolites (Alford and Palumbo, 1969). In another study, however, less than 0.5 log10 reduction of Salmonella Typhimurium was observed during storage of beef fillets for 15 days under aerobic conditions, modified atmospheres or vacuum at 5°C, regardless of the level of spoilage microbiota (Skandamis et al., 2002). These observations are further supported by existing inactivation models based on data collection from various studies. In particular, the Arrhenius type model of Pin et al. (2011) that was fitted to 190 inactivation rates of various Salmonella serovars at pH 3.2–7.3, aw 0.781–0.999 and temperatures 0–45°C. In pork products (data extracted from Combase), the model predicts no inactivation at pH 6.5 and aw of 0.98 up to 8°C. Similar results are reported for E. coli in the meta‐analysis study of Ross et al. (2008), who illustrated the aforementioned prominent role of temperature in the inactivation under lethal conditions of pH and aw, i.e. below the growth boundaries (Presser et al., 1998). Under the current assessment conditions, some bacterial death may occur due to mechanical damage of cells caused by the formation of crystals if freezing conditions occur in specific locations of the containers; however, such a decay is not attributable to temperature and thus, is not affected by the small temperature fluctuations above 0°C that may occur in boxes and tubs. Overall, the above suggests that under the conditions of the current assessment (including storage duration), inactivation of St. aureus, Salmonella spp. and pathogenic E. coli is either not likely, or not temperature dependent and thus, comparison between inactivation in boxes and tubs is not applicable.
A summary of the information retrieved through the systematic literature review on survival of relevant hazards is reported in Table 8. In the following lines, an in‐depth analysis of survival data per pathogen shown in the table is provided.
Table 8.
Systematic review of the survival of the microbial hazards selected for the assessment in fish and fishery products stored at chilling temperatures(a)
| Hazard | Temperature | Matrix | Hazard considered (if not already defined at species level) | First time point in which survival is reported | Last time point in which survival is reported | Characterisation of the survival (no change or reduction)a | Reference |
|---|---|---|---|---|---|---|---|
| Pathogenic E. coli | 4°C | Seabass | E. coli O157 | 3 days | 21 days |
Survival: no change No change at 3 days until 6 days; no change at 21 days |
Masniyom et al. (2006) |
| Salmonella spp. | 0 ± 1°C | Sea bream | S. Enteriditis | 4 days | 16 days |
Survival: reduction strain CECT4300: ~ 1 log10 reduction at 4 days until 8 days; 2.78 log10 reduction at 16 days strain CECT4145: ~ 1 log10 reduction at 4 days until 8 days; 1.70 log10 reduction at 16 days |
Provincial et al. (2013b) |
| 0 ± 1°C | Red mullet | S. Enteriditis | 2 days | 14 days |
Survival: no change no change at 2 days until 4 days; 0.6 log10 increase at 6 days until 14 days |
Tassou et al. (2004) | |
| 0 ± 1°C | Carp | S. Enteriditis | 1 day | 15 days |
Survival: reduction 1.0–1.4 log10 reduction at 1 day until 7 days; no change at 15 days |
Tassou et al. (2004) | |
| 4°C | Shrimp | S. Senftenberg; S. Typhimurium | 7 days |
Survival: reduction S. Senftenberg: 0.80 reduction at 7 days S. Typhimurium: 0.91 log10 reduction at 7 days |
Norhana et al. (2010) | ||
| 4 ± 1°C | Sea bream | S. Enteriditis | 4 days | 16 days |
Survival: no change strain CECT4300: no change at 4 days until 16 days; strain CECT4145: ~ 0.5 log10 increase at 4 days; no change at 8 days until 16 days |
Provincial et al. (2013b) | |
| 4°C | Salmon | S. Enteriditis | – | 8 h | Survival: no change | Li et al. (2018) | |
| 4°C | Indian mackerel | S. Weltevreden; S. Typhi | 1 day | 7 days |
Survival: reduction S. Weltevreden: ~ 0.5 log10 reduction at 1 day; ~ 1 log10 reduction at 3 days until 5 days; ~ 2.0 log10 reduction at 7 days; S. Typhi: ~ 0.5 log10 reduction at 1 day; ~ 1.5 log10 reduction at 3 days; ~ 2 log10 reduction at 5 days until 7 days |
Kumar et al. (2015) | |
| 5–7°C | Yellowfin tuna | S. Weltevreden; S. Newport | 2 days | 14 days |
Survival: reductionb ~ 0.5/1 log10 reduction at 2 days; ~ 0.5/1 log10 reduction at 4 days; ~ 0.5/1 log10 reduction at 6 days; S. Weltevreden: 1.63 log10 reduction at 14 days S. Newport: 0.8 log10 reduction at 14 days |
Liu et al. (2016) | |
| St. aureus | Ice | White prawn | – | 1 day | 10 days |
Survival: no change no change at 1 day until 5 days; ~ 2 log10 reduction at 10 days |
Du et al. (2017) |
| 4 ± 2°C | Catfish | – | 5 days | 20 days |
Survival: no change no change at 5 days; no change at 10 days |
Binsi et al. (2015) | |
| Vibrio spp. | 0 ± 1°C | Sea bream | V. parahaemolyticus | 4 days | 16 days |
Survival: reduction no change at 4 days ~ 0.5 log10 reduction at 8 days; ~ 1 log10 reduction at 16 days |
Provincial et al. (2013a) |
| 1.5 ± 0.5°C | Prawns | V. cholerae | 1 day | 14 days |
Survival: reduction no change at 1 day; ~ 1 log10 reduction at 2 days until 6days; ~ 3.5 log10 reduction at 14 days |
Januario and Dykes (2005) | |
| 4°C | Sheep head porgy | V. parahaemolyticus | 1 day | 9 days |
Survival: reduction no change at 1 day until 5 days; ~ 1 log10 reduction at 9 days |
Vasudevan et al. (2002) | |
| 4°C | Sheepshead porgy homogenate | V. parahaemolyticus | 1 day | 12 days |
Survival: reduction ~ 1 log10 reduction at 1 day until 5 days; ~ 1.5 log10 reduction at 12 days |
Vasudevan and Venkitanarayanan (2006) | |
| 4°C | Shrimps | V. cholerae | 2 days | 6 days |
Survival: reduction ~ 0.5 log10 reduction at 2 days; ~ 1 log10 reduction at 4 days until 6 days |
Wong et al. (1995) | |
| 4°C | Shrimp | V. parahaemolyticus | 8 h | 106 h |
Survival: reduction ~ 1 log10 reduction at 1 day; ~ 1.5 log10 reduction at 2 days; ~ 2 log10 reduction at 3.5 days; ~ 2 log10 reduction at 106 h |
Wang et al. (2014) | |
| 4°C | Bigeye snapper | V. parahaemolyticus | 1 day | 28 days |
Survival: no change no change at 1 day; ~ 0.5 log10 increase at 3 days; ~ 0.5 log10 reduction at 7 days; no change at 28 days |
Pattanayaiying et al. (2019) | |
| 4°C | Tiger prawn | V. parahaemolyticus | 1 day | 28 days |
Survival: reduction no change at 1 day; ~ 0.5 log10 reduction at 3 days; ~ 2 log10 reduction at 7 days; ~ 3 log10 reduction at 28 days |
Pattanayaiying et al. (2019) | |
| 4°C | Shrimps | V. parahaemolyticus | 1 day | 9 days |
Survival: reduction ~ 0.5 log10 reduction at 1 day; ~ 1 log10 reduction at 2 days; ~ 1.5 log10 reduction at 3 days; ~ 2 log10 reduction at 4 days until 8 days; ~ 2.5 log10 reduction at 9 days |
Zhang et al. (2015d) | |
| 4°C | Sea bass | V. parahaemolyticus | 3 days | 14 days |
Survival: reduction 1.55 log10 reduction at 3 days; > 4.5 log10 reduction (no detection) at 7 days until 14 days |
Telli and Dogruer (2019) | |
| 6°C | Lobster | V. parahaemolyticus | 3 days | 60 days |
Survival: reduction ~ 1 log10 reduction at 3 days; ~ 1.5 log10 reduction at 5 days; ~ 3 log10 reduction at 60 days |
Magalhaes et al. (2000) | |
| 7°C | Crab homogenate | V. cholerae | 3 days | 21 days |
Survival: reduction ~ 3.5 log10 reduction at 3 days; ~ 4 log10 reduction at 7 days; ~ 5.5 log10 reduction at 21 days |
Reily and Hackney (1985) | |
| 7°C | Shrimp homogenate | V. cholerae | 3 days | 21 days |
Survival: reduction ~ 4 log10 reduction at 3 days; ~ 4.5 log10 reduction at 7 days; ~ 6 log10 reduction at 21 days |
Reily and Hackney (1985) | |
| 7°C | Spotted surubim | V. cholerae | 18 days | Survival: reduction Detection up to 18 days with initial inoculum of 106 CFU/g | Corrales et al. (1994) | ||
| 7°C | Shrimp | V. parahaemolyticus | 8 h | 106 h |
Survival: reduction ~ 0.5 log10 reduction at 1 day; ~ 1 log10 reduction at 2 days; ~ 2 log10 reduction at 3 days; ~ 2 log10 reduction at 106 h |
Wang et al. (2014) | |
| Nematodes | 3°C | Hake | Anisakis | 3 days | 15 days |
Survival: no change Motility retained by all larvae |
Pascual et al. (2010) |
Growth or reduction was considered relevant when an increase or decrease ≥ 0.5 log10 units was reported (see Section 2.1).
Ice = storage on ice, temperature not provided.
Considering a temperature range from −3°C to 7°C, wide enough to also capture the temperature range for the mandate on the use of the so‐called ‘superchilling’ technique for the transport of fresh fishery products11.
The observations are presented at the first time point in which growth/survival is reported, at the time points relevant for the assessment, and the last observation time point included in the study. Numbers followed by ‘day’ indicate the day of observation (i.e. 2 days = 2nd day of observation; 2 days until 6 days = from the 2nd day to the 6th day of observation). ‘~’ is used for data not provided as punctual numbers (e.g. pictures, graphs); for these data numbers are expressed by increments of 0.5 log10.
Data from one of the two available experiments (102–3 CFU/g inoculum).
3.4.1.1. Human pathogenic E. coli
Very limited data are available for pathogenic E. coli behaviour in FFPs stored between 0°C and 7°C as only one study investigated the fate of E. coli O157 in sea bass kept at 4°C (Masniyom et al., 2006), reporting no changes of the inoculated strain counts over a period of 21 days. Based on the available evidence, it is not possible to quantify the impact of on‐land transport of FFPs in tubs compared to boxes, on the survival of pathogenic E. coli. It is considered that, in the absence of other factors affecting microorganism viability (i.e. low pH and/or low aw), inactivation of human pathogenic E. coli is not temperature dependent within the temperature range considered in this assessment.
3.4.1.2. Salmonella spp.
According to experimental data, Salmonella present on FFPs stored between 0 and 7°C either undergo a reduction of their counts (Tassou et al., 2004; Norhana et al., 2010; Provincial et al., 2013b; Kumar et al., 2015; Liu et al., 2016) or remain stable during the time of observation (Tassou et al., 2004; Provincial et al., 2013b; Pattanayaiying et al., 2019). An approximate reduction of 1 log10 within 3–5 days is reported for S. Enteriditis in sea bream and carp stored at 0°C (Tassou et al., 2004; Provincial et al., 2013b). In the same time range (3–5 days), the reduction was between 1.5 log10 and 2 log10 for S. Weltevreden and S. Typhi in mackerel stored at 4°C (Kumar et al., 2015), while a lower reduction (< 1 log10) was reported for S. Weltevreden and S. Newport in yellowfish tuna at 5–7°C (Liu et al., 2016). Based on the available evidence, it is expected that Salmonella on FFPs will remain stable or will decrease in the range of temperature conditions applied in this assessment. Considering current data and methodology limitations, it is not possible to quantify the impact of on‐land transport and storage of FFPs in tubs compared to boxes on the survival of Salmonella. It is considered that, in the absence of other factors affecting microorganism viability (i.e. low pH and/or low aw), inactivation of Salmonella is not temperature dependent within the temperature range considered in the assessment.
3.4.1.3. Staphylococcus aureus
Two studies report data on St. aureus behaviour during storage of FFPs. According to Du et al. (2017), St. aureus decreased slightly (between 0 and 0.5 log10) in white prawn during 5 days of incubation on ice, and further decreased (up to 2.0 log10) in the subsequent 5 days. A 0.5 log10 reduction was also recorded in catfish stored at 4°C (Binsi et al., 2015). Based on the limited available evidence and on the minimum temperature for growth of St. aureus (7°C; (ICMSF, 1996)), the growth of St. aureus will not be sustained in the temperature conditions applied in this assessment. Considering the available data, it is not possible to quantify the impact of on‐land transport and storage of FFPs in tubs compared to boxes on the survival of St. aureus. It is concluded that inactivation of St. aureus is not temperature dependent within the temperature range considered in the assessment.
3.4.1.4. Vibrio spp.
Several authors reported on Vibrio spp. (particularly V. parahaemolyticus and V. cholerae) behaviour on FFPs held at temperatures between 0 and 7°C. In the majority of studies, a reduction of Vibrio counts (from 0.5 to 4 log10) within the first 3 days was reported. V. cholerae showed a 1 log10 decrease after 4 days in prawns stored at 1.5°C and in shrimps at 4°C (Wong et al., 1995; Januario and Dykes, 2005) and between 3 and 4 log10 decrease after 3 days in crab and shrimp homogenates stored at 7°C. As regard to V. parahaemolyticus, two studies report no substantial variations of counts in sheep head porgy and bigeye snapper stored at 4°C over 5 and 28 days, respectively (Vasudevan and Venkitanarayanan, 2006; Pattanayaiying et al., 2019) and one described growth (~ 2.5 log10 in 4 days) in sea bass at 4°C (Provincial et al., 2013a). Overall, however, reductions variable from 0.5 log10 to 2 log10 within 3 days are displayed by V. parahaemolyticus in different studies in tiger prawns, shrimps, sea bass and sheep head porgy held at 4°C (Vasudevan and Venkitanarayanan, 2006; Wang et al., 2014; Zhang et al., 2015d; Pattanayaiying et al., 2019; Telli and Dogruer, 2019), as well as at higher storage temperatures (7°C; (Wang et al., 2014)). Under stress conditions, including unfavourable temperatures, Vibrio spp. are known to enter the viable but nonculturable (VBNC) state (Oliver et al., 1995; Wong and Wang, 2004; Wu et al., 2016). While the VBNC state may account for the progressive decrease of Vibrio spp. counts in FFP held at refrigeration temperatures, studies conducted with the use of viability assays (i.e. propidium monoazide (PMA)PCR, propidium monoazide (PMA)LAMP, epifluorescence staining, etc.) confirmed a reduction of viable V. parahaemolyticus of ~ 0.5 log10 during 3 to 4 days in shrimp and seabass held at 4°C and in lobster homogenate stored at 6°C (Magalhaes et al., 2000; Zhang et al., 2015d; Telli and Dogruer, 2019).
The predictive model developed by (Yang et al., 2009) was used to simulate the log10 reduction of V. parahaemolyticus as a function of the fish surface t/T profile predicted for the case studies of ‘abusive’ scenarios, the survival of the pathogen is practically not affected, as < 0.03 and 0.05 log10 reduction after 3 and 5 days of storage, respectively, is recorded (Table C.1 in Appendix C). Consequently, the differences in the log10 reduction between the storage in boxes and in tubs are negligible.
Table C.1.
Predicted log10 decrease of Vibrio parahaemolyticus by the predictive model developed by Yang et al. (2009) in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies of the ‘cooling‐keeping’ and ‘keeping’ processes of fresh fishery products with different fat content and size
| Process | Fat content | Fish dimension | Case study # | Time (day) | Location of fish in box or tub | Difference between boxes and tubsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||||
| ‘Cooling‐keeping’ | Lean | Small | 1c | 2 | −0.018 | −0.014 | −0.018 | −0.015 | −0.017 | −0.014 | 0.004 | 0.003 | 0.003 |
| 3 | −0.027 | −0.022 | −0.027 | −0.022 | −0.025 | −0.022 | 0.005 | 0.004 | 0.003 | ||||
| 5 | −0.044 | −0.039 | −0.043 | −0.039 | −0.041 | −0.038 | 0.005 | 0.005 | 0.003 | ||||
| Medium | 2c | 2 | −0.018 | −0.014 | −0.018 | −0.014 | −0.017 | −0.014 | 0.004 | 0.003 | 0.003 | ||
| 3 | −0.028 | −0.022 | −0.027 | −0.022 | −0.025 | −0.022 | 0.005 | 0.005 | 0.003 | ||||
| 5 | −0.046 | −0.039 | −0.045 | −0.039 | −0.042 | −0.038 | 0.007 | 0.006 | 0.004 | ||||
| Fat | Small | 3c | 2 | −0.018 | −0.014 | −0.018 | −0.014 | −0.017 | −0.014 | 0.004 | 0.003 | 0.003 | |
| 3 | −0.027 | −0.022 | −0.026 | −0.022 | −0.025 | −0.022 | 0.005 | 0.004 | 0.003 | ||||
| 5 | −0.044 | −0.039 | −0.043 | −0.038 | −0.041 | −0.038 | 0.005 | 0.005 | 0.003 | ||||
| Medium | 4c | 2 | −0.018 | −0.014 | −0.018 | −0.014 | −0.017 | −0.014 | 0.004 | 0.004 | 0.003 | ||
| 3 | −0.027 | −0.022 | −0.027 | −0.022 | −0.025 | −0.022 | 0.005 | 0.005 | 0.003 | ||||
| 5 | −0.046 | −0.039 | −0.045 | −0.038 | −0.041 | −0.038 | 0.007 | 0.006 | 0.004 | ||||
| ‘Keeping’ | Lean | Small | 1k | 2 | −0.019 | −0.017 | −0.018 | −0.017 | −0.017 | −0.016 | 0.001 | 0.002 | 0.001 |
| 3 | −0.028 | −0.026 | −0.027 | −0.025 | −0.025 | −0.024 | 0.002 | 0.002 | 0.001 | ||||
| 5 | −0.046 | −0.042 | −0.044 | −0.041 | −0.041 | −0.040 | 0.003 | 0.004 | 0.001 | ||||
| Medium | 2k | 2 | −0.019 | −0.017 | −0.018 | −0.017 | −0.017 | −0.016 | 0.001 | 0.002 | 0.001 | ||
| 3 | −0.028 | −0.026 | −0.027 | −0.025 | −0.025 | −0.024 | 0.002 | 0.003 | 0.001 | ||||
| 5 | −0.046 | −0.042 | −0.045 | −0.041 | −0.041 | −0.040 | 0.004 | 0.004 | 0.001 | ||||
| Fat | Small | 3k | 2 | −0.018 | −0.017 | −0.018 | −0.017 | −0.017 | −0.016 | 0.001 | 0.002 | 0.001 | |
| 3 | −0.027 | −0.026 | −0.027 | −0.024 | −0.025 | −0.024 | 0.002 | 0.002 | 0.001 | ||||
| 5 | −0.045 | −0.042 | −0.044 | −0.041 | −0.041 | −0.040 | 0.003 | 0.004 | 0.001 | ||||
| Medium | 4k | 2 | −0.019 | −0.017 | −0.018 | −0.017 | −0.017 | −0.016 | 0.001 | 0.002 | 0.001 | ||
| 3 | −0.028 | −0.026 | −0.027 | −0.025 | −0.025 | −0.024 | 0.002 | 0.003 | 0.001 | ||||
| 5 | −0.046 | −0.042 | −0.045 | −0.041 | −0.041 | −0.040 | 0.004 | 0.004 | 0.001 | ||||
Maximum temperature on the surface of a fish located in the middle of the box, close to the wall.
Maximum temperature on the surface of a fish located at the bottom corner of the tub.
Calculated by subtracting the log10 decrease in tubs of the specified column number from the corresponding log10 decrease in the box, also indicated by the appropriate column number. A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values.
Considering the available data and methodology limitations, it is not possible to provide such assessment on the survival of Vibrio ssp. other than V. parahaemolyticus. Based on the available evidence and the minimum growth temperatures, human pathogenic Vibrio spp. can decrease to a variable extent within the temperature interval considered in the assessment. Based on the predictive model developed for V. parahaemolyticus inactivation in fish, the impact of the assessed t/T profiles in the decrease of other pathogenic Vibrio spp. may be negligible.
3.4.1.5. Nematodes
Only one study addressing Anisakis spp. viability during fish storage at refrigerated temperatures was retrieved. In this study by Pascual et al. (2010), third larval stage (L3) of Anisakis spp., retrieved from infected fish, was inoculated in fresh European hake, stored at 3°C for up to 15 days and visually checked for signs of vitality. All inoculated larvae retained vitality, as assessed by motility, throughout the experiment time. Based on this limited evidence and on the prolonged survival of Anisakis at temperatures below the freezing point (144 h at −5°C; (ICMSF, 1996)), it was concluded that the temperature conditions applied in this assessment will only minimally affect the viability of these parasites.
3.4.2. Growth potential of relevant hazards
The experiments described in the scientific publications gathered in Table 9 monitored the pathogens at single isothermal chilling conditions (< 7°C) and illustrated that FFP support the growth of the several pathogenic bacteria. However, none of the studies specifically dealt with the comparison of the storage of FFP in ice (box) and in ice with water (tubs). Therefore, the design of the experiment and the reported results do not allow the AQ to be addressed; i.e. to estimate the contribution of the water content change of the fish meat on the reduction or growth potential of relevant biological hazards or on the magnitude of the histamine accumulation in fish species associated with a high amount of histidine when FFP, initially stored in freshwater or seawater/ice (in tubs) on board, are subsequently ‘handled’ (i.e. sorted or gutted and/or filleted) at the first on‐land establishment and then transferred to freshwater/ice (in three‐layered PE tubs) compared to being transferred to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days.
Table 9.
Systematic review of the growth of the microbial hazards selected for the assessment in fish and fishery products stored at chilling temperatures(a)
| Hazard | Temperature | Matrix | Hazard considered (if not already defined at species level) | Maximum time in which growth is not observed | First time point in which growth is reported | Last time point in which growth is reported | Characterisation of the growtha | Reference |
|---|---|---|---|---|---|---|---|---|
| Aeromonas spp. | Ice | Sea bream | Aeromonas spp. | – | 2 days | 18 days |
1.23 log10* increase at 2 days; 3.04 log10 increase at 4 days; 4.24 log10 increase at 7 days; 7.49 log10 increase at 18 days |
Carrascosa et al. (2016) |
| Ice | Sea bass | Aeromonas spp. | – | 2 days | 18 days |
1.97 log10* increase at 2 days; 3.76 log10 increase at 4 days; 4.87 log10 increase at 7 days; 7.95 log10 increase at 18 days |
Carrascosa et al. (2014) | |
| 0 ± 1°C | Carp | Aeromonas spp. | 3 days | 6 days | 18 days |
2.68 log10 increase at 6 days; 6.54 log10 increase at 18 days |
Zhang et al. (2015c) | |
| 0 ± 1°C | Sea bream | A. hydrophila | 4 days | 16 days | No change at 4 days to 16 days | Provincial et al. (2013a) | ||
| 0°Cb | Cod | A. hydrophila and other Aeromonas spp. | 10 days | 14 days | 21 days |
~ 1 log10 increase at 14 days; ~ 1 log10 increase at 21 days |
Davies and Slade (1995) | |
| Rainbow trout | A. hydrophila and other Aeromonas spp. | 3 days | 7 days | 21 days |
~ 1.5 log10 increase at 7 days; ~ 4 log10 increase at 21 days |
|||
| 4°C | Salmon | A. salmonicida | 1 day | 2 days | 5 days | ~ 0.5 log10 increase at 2 days;~ 1 log10 increase at 3 days;~ 2 log10 increase at 5 days | Hoel et al., (2018) | |
| 4 ± 1°C | Sea bream | A. hydrophila | 4 days | 16 days |
~ 2 log10 increase at 4 days; ~ 2 to 2.5 log10 increase at 8 days **; ~ 2 to 3 log10 increase at 16 days ** |
Provincial et al. (2013a) | ||
| 5°Cb | Cod | A. hydrophila and other Aeromonas spp. | 3 days | 7 days | 21 days |
~ 1 log10 increase at 7 days; ~ 1.5 log10 increase at 21 days |
Davies and Slade (1995) | |
| Rainbow trout | A. hydrophila and other Aeromonas spp. | 3 days | 21 days |
~ 0.5 log10 increase at 3 days; ~ 3.5 log10 increase at 7 days; ~ 4 log10 increase at 21 days |
||||
| Clostridium botulinum (non proteolytic) | 4.4°C | Crab | – | – | 55 days *** | Increase with 106 spores/sample; longer time required for lower concentrations | Betts and Gaze (1995) | |
| Listeria monocytogenes | 4°C | Cod muscle juice | – | – | – | 2.8 days | 2 log10 increase with both 1% and 3% NaCl | Lorentzen et al. (2010) |
| 4°C | Sea bass | – | – | 7 days | 21 days |
1.86 log10 increase at 7 days; 3.32 log10 increase at 21 days |
Boulares et al. (2017) | |
| 4°C | Imitation crab meat | – | – | – | – | growth rate: 0.014 log10/h | Eom et al. (2009) | |
| 5–7°C | Yellowfin tuna | – | – | 2 days | 14 days |
~ 0.5 log10 increase at 2 days; ~ 1 log10 increase at 4 days; ~ 1.5 log10 increase at 6 days; ~ 3 log10 increase at 14 days |
Liu et al. (2016) | |
| 7°C | Cod muscle juice | – | – | – | 0.8/0.9 days | 2 log10 increase with 1% and 3% NaCl, respectively | Lorentzen et al. (2010) | |
| Vibrio spp. | 4 ± 1°Cc | Sea bream | V. parahaemolyticus | 4 days | 16 days |
~ 2.0 log10 increase at 4 days; ~ 2.5 to 3 log10 increase at 8 days to 16 days |
Provincial et al. (2013a) |
Growth or reduction was considered microbiologically relevant when an increase or decrease ≥ 0.5 log10 units was recorded (see Section 2.1).
Ice = storage on ice, temperature not provided.
Considering a temperature range between −3°C and 7°C, wide enough to also capture the temperature range for the ‘superchilling’ mandate11.
The observations are presented at the first time point in which growth/survival is reported, at the time points relevant for the assessment, and the last observation time point included in the study. Numbers followed by ‘day’ indicate the day of observation (i.e. 2 days = 2nd day of observation; 2 days until 6 days = from the 2nd day to the 6th day of observation). ‘~’ is used for data not provided as punctual numbers (i.e. pictures, graphs); for these data numbers are expressed by increments of 0.5 log10; * values in which the analytical limit of detection was subtracted; ** data from experiments with two different strains; *** evidence of toxin production.
Experimental design including an inoculum with a mixture of A. hydrophila and other Aeromonas species.
The reported increase here should be considered with caution, as it is not consistent with other relevant reports that suggest no growth of V. vulnificus and V. parahaemolyticus below 5°C or even 10°C, as well as available predictive growth models that estimate minimum growth temperature for the above species between 5°C and 7°C in fish and seafood.
Under the assumption that temperature is the most important factor determining the microbial growth during the storage of FFP, secondary models describing the relationship between the storage temperature and the growth rate can be used to quantify the impact of transport/storage temperature on the growth behaviour of relevant hazards. Figure 14 depicts the maximum growth rates (μmax) of the selected relevant hazards as a function of temperature. A. hydrophila shows the highest values and is also one of the more sensitive to temperature changes as it occurs with non‐proteolytic Cl. botulinum. Therefore, in principle, these hazards would be more affected by differences in fish temperatures when transported/stored in tubs vs. boxes. The histamine‐producing M. psychrotolerans shows the highest growth rates and the most psychotrophic character than the other hazards considered, suggesting that it may grow to higher levels than the other pathogens under the temperature conditions of the current assessment. The risk associated with M. psychrotolerans growth is linked to histamine accumulation and is discussed in Section 3.4.3. Overall, L. monocytogenes shows slower growth rates than the other hazards, but is able to grow at lower temperatures (Tmin = −1.5°C) than A. hydrophila (Tmin = 0–2°C) and non‐proteolytic Cl. botulinum (Tmin = 3.3°C), and makes it particularly relevant at the target temperature (i.e. 0°C) for storing and transporting FFP along the supply chain.
Figure 14.
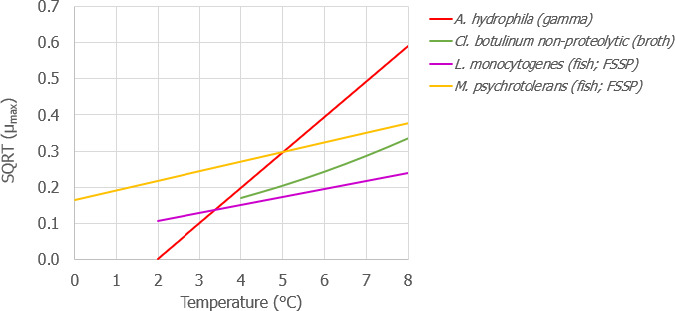
Square root of the maximum growth rate (μmax) as a function of temperature predicted for selected relevant hazards according to FSSP (Listeria monocytogenes and histamine‐producing M. psychrotolerans in fish) and ComBase (non‐proteolytic Clostridium botulinum broth) models
- Input values: pH 6.5; WPS 0.37% and lactic acid WP: 7,000 ppm) as well as the application of the gamma model approach (for A. hydrophila, with μ ref = 0.039 and Tmin = 2°C). FSSP: Food Spoilage and Safety Predictor.
3.4.2.1. Growth potential of the relevant hazards under the ‘ideal’ scenario
Under the ‘ideal’ scenario described in Section 2.2, the fish surface temperature would be 0°C throughout the transport/storage time (isothermal conditions) irrespective of the use of boxes or tubs. Therefore, the growth potential of the biological hazards will be equivalent in both type of containers. Hence, the potential difference in log10 increase during transport or storage would be zero.
Based on the minimum temperature for growth of the identified hazards, both non‐proteolytic Cl. botulinum and A. hydrophila will not be able to grow at 0°C, while growth of L. monocytogenes and M. psychrotolerans can occur but will also be very limited. Unfortunately, the predictive models available do not allow simulation the growth of L. monocytogenes at temperature below 1°C (ComBase) and 2°C (FSSP). The maximum growth rate (μmax) at 0°C extrapolated from the cardinal parameter‐based model available in FSSP is 0.0035 h−1 and the associated log10 increase at 2, 3 and 5 days is 0.07, 0.11 and 0.18 log10 units, respectively.
3.4.2.2. Growth potential of the relevant hazards under the ‘observed’ scenario
Tables 10 and 11 present the values of the growth potential (log10 increase) associated with the t/T profiles recorded in the ‘Qualitubfish’ project, comparing the storage/transport of FFP (i.e. plaice) in box (with ice) and in tubs (with water and ice). The results consist of the log10 increase and the range of differences in log10 increases between boxes and tubs.
Table 10.
Predicted log10 increase and log10 difference of Listeria monocytogenes and Aeromonas hydrophila on fresh fishery products when transported/stored in tubs or boxes based on the observed time/Temperature profile in experiment 1 of the ‘Qualitubfish’ project (Bekaert et al., 2016a)
| Pathogen | Time (day) | Box | Tub | Differencea | |||
|---|---|---|---|---|---|---|---|
| Middle fish | Middle fish | Bottom fish | Middle fish | Top fish | Range (min–max) | ||
| L. monocytogenes | Not transported | ||||||
| 2 | 0.10 | – | 0.21 | 0.10 | 0.21 | 0 to 0.11 | |
| 3 | 0.15 | – | 0.35 | 0.17 | 0.37 | 0.02 to 0.22 | |
| 5 | 0.23 | – | 0.62 | 0.34 | 0.71 | 0.11 to 0.48 | |
| Transported | |||||||
| 2 | 0.12 | 0.12 | 0.11 | 0.21 | 0.20 | −0.01 to 0.09 | |
| 3 | 0.17 | 0.17 | 0.21 | 0.33 | 0.31 | 0.04 to 0.16 | |
| 5 | 0.28 | 0.28 | 0.40 | 0.53 | 0.51 | 0.12 to 0.25 | |
| A. hydrophila | Not transported | ||||||
| 2 | 0.047 | – | 0.0098 | 0.0006 | 0.02 | −0.046 to −0.027 | |
| 3 | 0.047 | – | 0.046 | 0.0006 | 0.11 | −0.046 to 0.065 | |
| 5 | 0.047 | – | 0.102 | 0.0006 | 0.30 | −0.046 to 0.248 | |
| Transported | |||||||
| 2 | 0.006 | 0.0059 | 0.00066 | 0.053 | 0.0312 | −0.005 to 0.047 | |
| 3 | 0.006 | 0.0059 | 0.00066 | 0.082 | 0.0385 | −0.005 to 0.076 | |
| 5 | 0.006 | 0.0059 | 0.00066 | 0.082 | 0.0392 | −0.005 to 0.076 | |
A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values. The range represents the minimum and maximum difference considering any combination between the log10 increase in tubs and boxes.
Table 11.
Predicted log10 increase and log10 difference of Listeria monocytogenes and Aeromonas hydrophila on fresh fishery products when transported/stored in tubs or boxes based on the observed time/Temperature profile in experiment 2 of the ‘Qualitubfish’ project (Bekaert et al., 2016b)
| Pathogen | Time (day) | Box | Tub | Differencea | |||||
|---|---|---|---|---|---|---|---|---|---|
| Middle fish | Middle fish | Middle fish | Bottom fish | Middle fish | Middle fish | Top fish | Range (min–max) | ||
| L. monocytogenes | 2 | 0.06 | 0.13 | 0.16 | 0.25 | 0.27 | 0.23 | 0.31 | 0.07 to 0.25 |
| 3 | 0.13 | 0.28 | 0.21 | 0.37 | 0.41 | 0.35 | 0.45 | 0.07 to 0.32 | |
| 5 | 0.33 | 0.57 | 0.32 | 0.61 | 0.65 | 0.59 | 0.69 | 0.02 to 0.37 | |
| A. hydrophila | 2 | 0.00066 | 0.010 | 0.022 | 0.020 | 0.035 | 0.0028 | 0.13 | −0.019 to 0.13 |
| 3 | 0.00066 | 0.061 | 0.022 | 0.021 | 0.064 | 0.0038 | 0.16 | −0.057 to 0.16 | |
| 5 | 0.00095 | 0.12 | 0.022 | 0.022 | 0.066 | 0.0056 | 0.16 | −0.11 to 0.16 | |
A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values. The range represents the minimum and maximum difference considering any combination between the log increase in tubs and boxes.
According to the simulations carried out, among the relevant hazards included in the assessment, L. monocytogenes has the highest growth potential under the t/T profiles observed in the experiments, though in all cases, the log10 increase was below 0.5 log10 units at 3 days of transport/storage, which is in agreement with the overall higher temperatures recorded in tubs compared to boxes. The maximum log10 increase was associated with the temperature record of the fish located at the top of the tubs and reached 0.71 log10 units only at 5 days. Although the top of the tub would not be, a priori, the warmest position of this container, the reason for this finding was thought to be related with the fact that the top layer of fish/water was directly in contact with the higher temperatures of the storage room once the ice on the top was melted (Section 3.2.1).
The growth of A. hydrophila was limited during the 3 days of transport/storage irrespectively of the type of container and the consequent difference in the log10 increase between tubs and boxes was practically irrelevant for most of the comparisons (i.e. below 0.2 log10 units). The results, and particularly the lower growth of A. hydrophila than L. monocytogenes, are further supported by the temperature dependence of growth rates of relevant hazards (Figure 14). From this Figure, it is evident that A. hydrophila grows faster than L. monocytogenes above 5°C and the opposite happens below this temperature, with growth of A. hydrophila ceasing at 2°C. As such, given that the temperature records from ‘Qualitubfish’ project were dominated by temperatures below 4°C (including records with slightly below 0°C), it is expected that L. monocytogenes grows faster, and thus at higher levels, than A. hydrophila within the period of the assessment.
As the t/T profiles observed in the fish did not record temperatures above 3.3°C, no growth of non‐proteolytic Cl. botulinum is expected, irrespectively of the type of container.
3.4.2.3. Growth potential of the relevant hazards under the ‘abusive’ scenarios
Tables C.2, C.3–C.4 in Appendix C show the results of the growth simulation of L. monocytogenes, A. hydrophila and Cl. botulinum associated with the t/T profiles generated through the heat transfer model for each case study defined in Figure 3. The results consist of the log10 increase and the difference of the log10 increase between boxes and tubs.
Table C.2.
Predicted log10 increase of Listeria monocytogenes in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies of the ‘cooling‐keeping’ and ‘keeping’ processes of fresh fishery products with different fat content and size
| Process | Fat content | Fish Dimension | Case study # | Time (day) | Location of fish in box or tub | Difference between boxes and tubsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||||
| ‘Cooling‐keeping’ | Lean | Small | 1c | 2 | 0.13 | 0.45 | 0.16 | 0.43 | 0.25 | 0.48 | 0.32 | 0.27 | 0.24 |
| 3 | 0.20 | 0.58 | 0.25 | 0.58 | 0.39 | 0.65 | 0.38 | 0.33 | 0.26 | ||||
| 5 | 0.37 | 0.79 | 0.46 | 0.83 | 0.66 | 0.91 | 0.42 | 0.37 | 0.25 | ||||
| Medium | 2c | 2 | 0.12 | 0.46 | 0.15 | 0.44 | 0.24 | 0.48 | 0.33 | 0.29 | 0.24 | ||
| 3 | 0.18 | 0.58 | 0.22 | 0.58 | 0.37 | 0.64 | 0.41 | 0.36 | 0.27 | ||||
| 5 | 0.28 | 0.80 | 0.35 | 0.83 | 0.59 | 0.91 | 0.52 | 0.47 | 0.32 | ||||
| Fat | Small | 3c | 2 | 0.14 | 0.46 | 0.16 | 0.44 | 0.25 | 0.49 | 0.32 | 0.28 | 0.24 | |
| 3 | 0.21 | 0.59 | 0.25 | 0.59 | 0.39 | 0.65 | 0.38 | 0.34 | 0.26 | ||||
| 5 | 0.37 | 0.80 | 0.46 | 0.84 | 0.67 | 0.92 | 0.43 | 0.38 | 0.25 | ||||
| Medium | 4c | 2 | 0.12 | 0.47 | 0.16 | 0.45 | 0.25 | 0.49 | 0.34 | 0.29 | 0.24 | ||
| 3 | 0.18 | 0.60 | 0.23 | 0.59 | 0.37 | 0.65 | 0.42 | 0.37 | 0.28 | ||||
| 5 | 0.29 | 0.81 | 0.36 | 0.84 | 0.60 | 0.92 | 0.53 | 0.48 | 0.32 | ||||
| ‘Keeping’ | Lean | Small | 1k | 2 | 0.11 | 0.18 | 0.14 | 0.23 | 0.23 | 0.28 | 0.08 | 0.10 | 0.05 |
| 3 | 0.16 | 0.29 | 0.21 | 0.37 | 0.36 | 0.43 | 0.12 | 0.16 | 0.07 | ||||
| 5 | 0.29 | 0.49 | 0.37 | 0.62 | 0.60 | 0.70 | 0.20 | 0.25 | 0.10 | ||||
| Medium | 2k | 2 | 0.10 | 0.19 | 0.13 | 0.24 | 0.23 | 0.27 | 0.09 | 0.11 | 0.05 | ||
| 3 | 0.15 | 0.29 | 0.20 | 0.37 | 0.35 | 0.42 | 0.14 | 0.17 | 0.07 | ||||
| 5 | 0.27 | 0.50 | 0.35 | 0.62 | 0.60 | 0.69 | 0.23 | 0.27 | 0.09 | ||||
| Fat | Small | 3k | 2 | 0.11 | 0.19 | 0.14 | 0.24 | 0.23 | 0.27 | 0.07 | 0.10 | 0.04 | |
| 3 | 0.17 | 0.29 | 0.21 | 0.38 | 0.36 | 0.43 | 0.12 | 0.16 | 0.07 | ||||
| 5 | 0.30 | 0.50 | 0.37 | 0.63 | 0.61 | 0.70 | 0.20 | 0.25 | 0.09 | ||||
| Medium | 4k | 2 | 0.10 | 0.19 | 0.13 | 0.24 | 0.23 | 0.27 | 0.09 | 0.11 | 0.03 | ||
| 3 | 0.15 | 0.30 | 0.20 | 0.38 | 0.36 | 0.43 | 0.14 | 0.17 | 0.07 | ||||
| 5 | 0.27 | 0.51 | 0.35 | 0.63 | 0.61 | 0.70 | 0.28 | 0.06 | 0.09 | ||||
Maximum temperature on the surface of a fish located in the middle of the box, close to the wall.
Maximum temperature on the surface of a fish located at the bottom corner of the tub.
Calculated by subtracting the log10 increase in tubs of the specified column number from the corresponding log10 increase in the box, also indicated by the appropriate column number. A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values.
Table C.3.
Predicted log10 increase of Aeromonas hydrophila in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies of the ‘cooling‐keeping’ and ‘keeping’ processes of fresh fishery products with different fat content and size
| Process | Fat content | Fish Dimension | Case study # | Time (days) | Location of fish in box or tub | Difference between boxes and tubsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||||
| ‘Cooling‐keeping’ | Lean | Small | 1c | 2 | 0.00 | 0.91 | 0.02 | 0.87 | 0.27 | 1.17 | 0.91 | 0.85 | 0.89 |
| 3 | 0.00 | 0.92 | 0.03 | 0.94 | 0.41 | 1.31 | 0.92 | 0.91 | 0.90 | ||||
| 5 | 0.01 | 0.92 | 0.09 | 0.98 | 0.65 | 1.44 | 0.91 | 0.89 | 0.79 | ||||
| Medium | 2c | 2 | 0.00 | 0.95 | 0.02 | 0.88 | 0.28 | 1.14 | 0.95 | 0.86 | 0.86 | ||
| 3 | 0.00 | 0.96 | 0.02 | 0.93 | 0.37 | 1.28 | 0.96 | 0.91 | 0.90 | ||||
| 5 | 0.00 | 0.96 | 0.02 | 0.97 | 0.49 | 1.40 | 0.96 | 0.96 | 0.91 | ||||
| Fat | Small | 3c | 2 | 0.00 | 0.95 | 0.03 | 0.88 | 0.29 | 1.19 | 0.94 | 0.86 | 0.90 | |
| 3 | 0.00 | 0.96 | 0.03 | 0.95 | 0.43 | 1.34 | 0.96 | 0.92 | 0.91 | ||||
| 5 | 0.01 | 0.96 | 0.09 | 1.00 | 0.68 | 1.47 | 0.95 | 0.91 | 0.79 | ||||
| Medium | 4c | 2 | 0.00 | 0.99 | 0.02 | 0.90 | 0.29 | 1.19 | 0.99 | 0.88 | 0.90 | ||
| 3 | 0.00 | 1.00 | 0.02 | 0.97 | 0.40 | 1.34 | 1.00 | 0.94 | 0.94 | ||||
| 5 | 0.00 | 1.00 | 0.02 | 1.01 | 0.52 | 1.47 | 1.00 | 0.99 | 0.95 | ||||
| ‘Keeping’ | Lean | Small | 1k | 2 | 0.00 | 0.00 | 0.01 | 0.04 | 0.24 | 0.20 | 0.00 | 0.03 | −0.04 |
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.34 | 0.32 | 0.00 | 0.07 | −0.02 | ||||
| 5 | 0.00 | 0.00 | 0.02 | 0.13 | 0.51 | 0.45 | 0.00 | 0.11 | −0.06 | ||||
| Medium | 2k | 2 | 0.00 | 0.00 | 0.01 | 0.04 | 0.24 | 0.19 | 0.00 | 0.03 | −0.05 | ||
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.34 | 0.31 | 0.00 | 0.07 | −0.03 | ||||
| 5 | 0.00 | 0.00 | 0.01 | 0.12 | 0.51 | 0.43 | 0.00 | 0.12 | −0.07 | ||||
| Fat | Small | 3k | 2 | 0.00 | 0.00 | 0.01 | 0.04 | 0.26 | 0.20 | 0.00 | 0.03 | −0.06 | |
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.37 | 0.32 | 0.00 | 0.08 | −0.05 | ||||
| 5 | 0.00 | 0.00 | 0.01 | 0.13 | 0.54 | 0.45 | 0.00 | 0.12 | −0.09 | ||||
| Medium | 4k | 2 | 0.00 | 0.00 | 0.01 | 0.04 | 0.26 | 0.20 | 0.00 | 0.03 | −0.06 | ||
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.37 | 0.32 | 0.00 | 0.08 | −0.05 | ||||
| 5 | 0.00 | 0.00 | 0.01 | 0.13 | 0.54 | 0.45 | 0.00 | 0.12 | −0.09 | ||||
Maximum temperature on the surface of a fish located in the middle of the box, close to the wall.
Maximum temperature on the surface of a fish located at the bottom corner of the tub.
Calculated by subtracting the log10 increase in tubs of the specified column number from the corresponding log10 increase in the box, also indicated by the appropriate column number. A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values.
Table C.4.
Predicted log10 increase of Clostridium botulinum in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies of the ‘cooling‐keeping’ and ‘keeping’ processes of fresh fishery products with different fat content and size
| Process | Fat content | Fish Dimension | Case study # | Time (days) | Location of fish in box or tub | Difference between boxes and tubs c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||||
| ‘Cooling‐keeping’ | Lean | Small | 1c | 2 | 0.01 | 0.46 | 0.02 | 0.41 | 0.11 | 0.53 | 0.45 | 0.39 | 0.42 |
| 3 | 0.01 | 0.46 | 0.02 | 0.46 | 0.19 | 0.62 | 0.45 | 0.44 | 0.43 | ||||
| 5 | 0.01 | 0.46 | 0.07 | 0.48 | 0.32 | 0.70 | 0.45 | 0.41 | 0.38 | ||||
| Medium | 2c | 2 | 0.01 | 0.47 | 0.02 | 0.42 | 0.11 | 0.53 | 0.46 | 0.40 | 0.42 | ||
| 3 | 0.01 | 0.47 | 0.02 | 0.45 | 0.18 | 0.62 | 0.46 | 0.43 | 0.44 | ||||
| 5 | 0.01 | 0.47 | 0.02 | 0.48 | 0.26 | 0.69 | 0.46 | 0.46 | 0.43 | ||||
| Fat | Small | 3c | 2 | 0.01 | 0.46 | 0.02 | 0.42 | 0.11 | 0.55 | 0.45 | 0.40 | 0.44 | |
| 3 | 0.01 | 0.46 | 0.02 | 0.47 | 0.20 | 0.64 | 0.45 | 0.45 | 0.44 | ||||
| 5 | 0.01 | 0.46 | 0.07 | 0.49 | 0.33 | 0.71 | 0.45 | 0.42 | 0.38 | ||||
| Medium | 4c | 2 | 0.01 | 0.49 | 0.02 | 0.42 | 0.22 | 0.53 | 0.48 | 0.40 | 0.31 | ||
| 3 | 0.01 | 0.49 | 0.02 | 0.47 | 0.30 | 0.61 | 0.48 | 0.45 | 0.31 | ||||
| 5 | 0.01 | 0.49 | 0.02 | 0.49 | 0.37 | 0.69 | 0.48 | 0.47 | 0.32 | ||||
| ‘Keeping’ | Lean | Small | 1k | 2 | 0.00 | 0.00 | 0.00 | 0.03 | 0.09 | 0.09 | 0.00 | 0.03 | 0.00 |
| 3 | 0.00 | 0.00 | 0.00 | 0.06 | 0.16 | 0.17 | 0.00 | 0.06 | 0.01 | ||||
| 5 | 0.00 | 0.00 | 0.00 | 0.09 | 0.25 | 0.26 | 0.00 | 0.09 | 0.01 | ||||
| Medium | 2k | 2 | 0.00 | 0.00 | 0.00 | 0.04 | 0.09 | 0.10 | 0.00 | 0.04 | 0.01 | ||
| 3 | 0.00 | 0.00 | 0.00 | 0.06 | 0.16 | 0.17 | 0.00 | 0.06 | 0.01 | ||||
| 5 | 0.00 | 0.00 | 0.00 | 0.09 | 0.25 | 0.24 | 0.00 | 0.09 | −0.01 | ||||
| Fat | Small | 3k | 2 | 0.00 | 0.00 | 0.01 | 0.05 | 0.10 | 0.10 | 0.00 | 0.04 | 0.00 | |
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.18 | 0.18 | 0.00 | 0.07 | 0.00 | ||||
| 5 | 0.00 | 0.00 | 0.01 | 0.11 | 0.28 | 0.25 | 0.00 | 0.10 | −0.03 | ||||
| Medium | 4k | 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.21 | 0.02 | 0.00 | 0.06 | −0.19 | ||
| 3 | 0.00 | 0.00 | 0.00 | 0.09 | 0.29 | 0.03 | 0.00 | 0.09 | −0.26 | ||||
| 5 | 0.00 | 0.00 | 0.00 | 0.12 | 0.39 | 0.04 | 0.00 | 0.12 | −0.35 | ||||
Maximum temperature on the surface of a fish located in the middle of the box, close to the wall.
Maximum temperature on the surface of a fish located at the bottom corner of the tub.
Calculated by subtracting the log10 increase in tubs of the specified column number from the corresponding log10 increase in the box, also indicated by the appropriate column number. A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values.
Tables 12 and 13 provide a summary overview for these three hazards considering the case studies dealing with lean small fish and fat medium fish for both ‘cooling‐keeping’ and ‘keeping’ processes. The plots of the growth simulation for each t/T profile are in Figures 15 and 16.
Table 12.
Predicted log10 increase of Listeria monocytogenes, Aeromonas hydrophila and Clostridium botulinum in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies ‘cooling‐keeping’ process of lean small fish (CLS; #1c) and ‘keeping’ process of lean small fish (KLS; #1k) under the ‘abusive’ scenarios
| Process | Hazard | Time (day) | Location of fish in box or tub | Difference between boxes and tubsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||
| ‘Cooling‐keeping’ | L. monocytogenes | 2 | 0.13 | 0.45 | 0.16 | 0.43 | 0.25 | 0.48 | 0.32 | 0.27 | 0.24 |
| 3 | 0.20 | 0.58 | 0.25 | 0.58 | 0.39 | 0.65 | 0.38 | 0.33 | 0.26 | ||
| 5 | 0.37 | 0.79 | 0.46 | 0.83 | 0.66 | 0.91 | 0.42 | 0.37 | 0.25 | ||
| A. hydrophila | 2 | 0.00 | 0.91 | 0.02 | 0.87 | 0.27 | 1.17 | 0.91 | 0.85 | 0.89 | |
| 3 | 0.00 | 0.92 | 0.03 | 0.94 | 0.41 | 1.31 | 0.92 | 0.91 | 0.90 | ||
| 5 | 0.01 | 0.92 | 0.09 | 0.98 | 0.65 | 1.44 | 0.91 | 0.89 | 0.79 | ||
| Cl. botulinum | 2 | 0.01 | 0.46 | 0.02 | 0.41 | 0.11 | 0.53 | 0.45 | 0.39 | 0.42 | |
| 3 | 0.01 | 0.46 | 0.02 | 0.46 | 0.19 | 0.62 | 0.45 | 0.44 | 0.43 | ||
| 5 | 0.01 | 0.46 | 0.07 | 0.48 | 0.32 | 0.70 | 0.45 | 0.41 | 0.38 | ||
| ‘Keeping’ | L. monocytogenes | 2 | 0.11 | 0.18 | 0.14 | 0.23 | 0.23 | 0.28 | 0.08 | 0.10 | 0.05 |
| 3 | 0.16 | 0.29 | 0.21 | 0.37 | 0.36 | 0.43 | 0.12 | 0.16 | 0.07 | ||
| 5 | 0.29 | 0.49 | 0.37 | 0.62 | 0.60 | 0.70 | 0.20 | 0.25 | 0.10 | ||
| A. hydrophila | 2 | 0.00 | 0.00 | 0.01 | 0.04 | 0.24 | 0.20 | 0.00 | 0.03 | −0.04 | |
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.34 | 0.32 | 0.00 | 0.07 | −0.02 | ||
| 5 | 0.00 | 0.00 | 0.02 | 0.13 | 0.51 | 0.45 | 0.00 | 0.11 | −0.06 | ||
| Cl. botulinum | 2 | 0.00 | 0.00 | 0.00 | 0.03 | 0.09 | 0.09 | 0.00 | 0.03 | 0.00 | |
| 3 | 0.00 | 0.00 | 0.00 | 0.06 | 0.16 | 0.17 | 0.00 | 0.06 | 0.01 | ||
| 5 | 0.00 | 0.00 | 0.00 | 0.09 | 0.25 | 0.26 | 0.00 | 0.09 | 0.01 | ||
Based on the maximum overall temperature in the food/air matrix.
Based on the maximum temperature obtained for each time within the whole food/water matrix.
Calculated by subtracting the log10 increase in tubs of the specified column number from the corresponding log10 increase in the box, also indicated by the appropriate column number. A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values.
Table 13.
Predicted log10 increase of Listeria monocytogenes, Aeromonas hydrophila and Clostridium botulinum in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies ‘cooling‐keeping’ process of fat medium fish (CFM; #4c) and ‘keeping’ process of fat medium fish (KFM; #4k) under the ‘abusive’ scenarios
| Process | Hazard | Time (day) | Location of fish in box or tub | Difference between boxes and tubsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||
| ‘Cooling‐keeping’ | L. monocytogenes | 2 | 0.12 | 0.47 | 0.16 | 0.45 | 0.25 | 0.49 | 0.34 | 0.29 | 0.24 |
| 3 | 0.18 | 0.60 | 0.23 | 0.59 | 0.37 | 0.65 | 0.42 | 0.37 | 0.28 | ||
| 5 | 0.29 | 0.81 | 0.36 | 0.84 | 0.60 | 0.92 | 0.53 | 0.48 | 0.32 | ||
| A. hydrophila | 2 | 0.00 | 0.99 | 0.02 | 0.90 | 0.29 | 1.19 | 0.99 | 0.88 | 0.90 | |
| 3 | 0.00 | 1.00 | 0.02 | 0.97 | 0.40 | 1.34 | 1.00 | 0.94 | 0.94 | ||
| 5 | 0.00 | 1.00 | 0.02 | 1.01 | 0.52 | 1.47 | 1.00 | 0.99 | 0.95 | ||
| Cl. botulinum | 2 | 0.01 | 0.49 | 0.02 | 0.42 | 0.22 | 0.53 | 0.48 | 0.40 | 0.31 | |
| 3 | 0.01 | 0.49 | 0.02 | 0.47 | 0.30 | 0.61 | 0.48 | 0.45 | 0.31 | ||
| 5 | 0.01 | 0.49 | 0.02 | 0.49 | 0.37 | 0.69 | 0.48 | 0.47 | 0.32 | ||
| ‘Keeping’ | L. monocytogenes | 2 | 0.10 | 0.19 | 0.13 | 0.24 | 0.23 | 0.27 | 0.09 | 0.11 | 0.04 |
| 3 | 0.15 | 0.30 | 0.20 | 0.38 | 0.36 | 0.43 | 0.14 | 0.17 | 0.07 | ||
| 5 | 0.27 | 0.51 | 0.35 | 0.63 | 0.61 | 0.70 | 0.28 | 0.06 | 0.09 | ||
| A. hydrophila | 2 | 0.00 | 0.00 | 0.01 | 0.04 | 0.26 | 0.20 | 0.00 | 0.03 | −0.06 | |
| 3 | 0.00 | 0.00 | 0.01 | 0.08 | 0.37 | 0.32 | 0.00 | 0.08 | −0.05 | ||
| 5 | 0.00 | 0.00 | 0.01 | 0.13 | 0.54 | 0.45 | 0.00 | 0.12 | −0.09 | ||
| Cl. botulinum | 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.21 | 0.02 | 0.00 | 0.06 | −0.19 | |
| 3 | 0.00 | 0.00 | 0.00 | 0.09 | 0.29 | 0.03 | 0.00 | 0.09 | −0.26 | ||
| 5 | 0.00 | 0.00 | 0.00 | 0.12 | 0.39 | 0.04 | 0.00 | 0.12 | −0.35 | ||
Based on the maximum overall temperature in the food/air matrix.
Based on the maximum temperature obtained for each time within the whole food/water matrix.
Calculated by subtracting the log10 increase in tubs of the specified column number from the corresponding log10 increase in the box, also indicated by the appropriate column number. A positive value indicates a greater growth potential (log10 increase) in tubs compared to boxes, while the opposite is true for the negative values.
Figure 15.
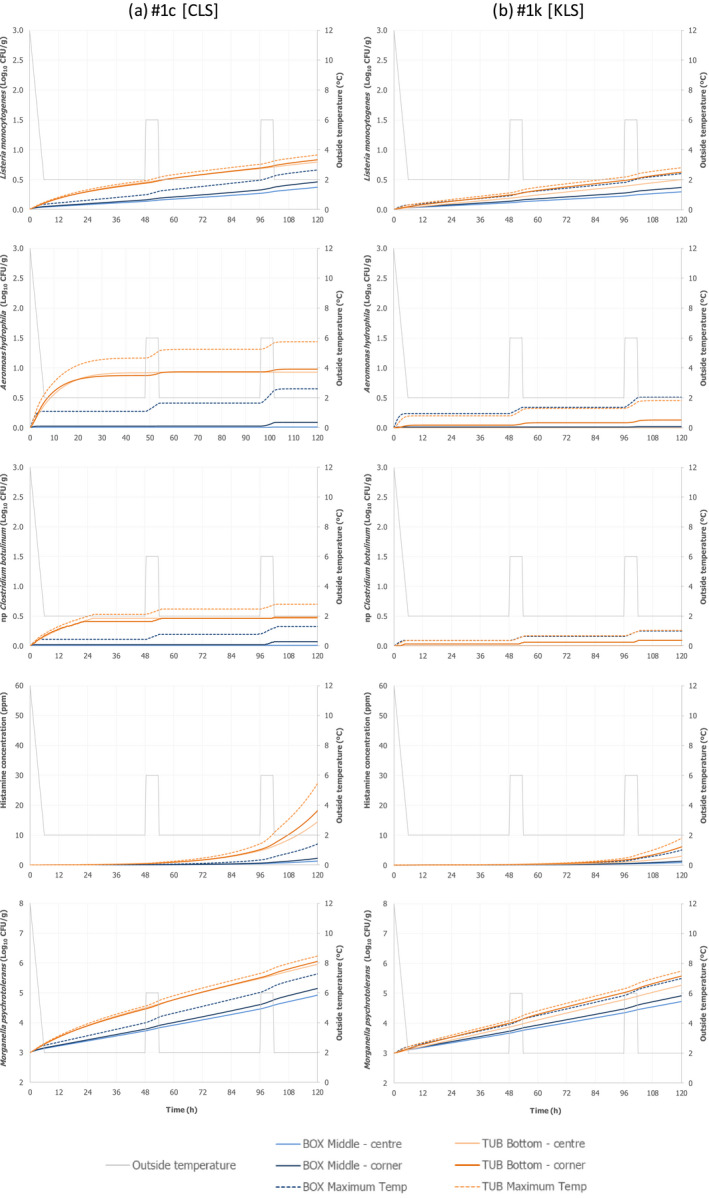
Growth of relevant hazards and histamine accumulation on the fish surface and the maximum temperature in the matrix of fish/water or fish/air during transport/storage of fish based on the predicted time/Temperature profiles of selected case studies of the ‘abusive’ scenario: (a) ‘cooling‐keeping’ process of lean small fish (CLS; #1c) and (b) ‘keeping’ process of lean small fish (KLS; #1k)
Figure 16.
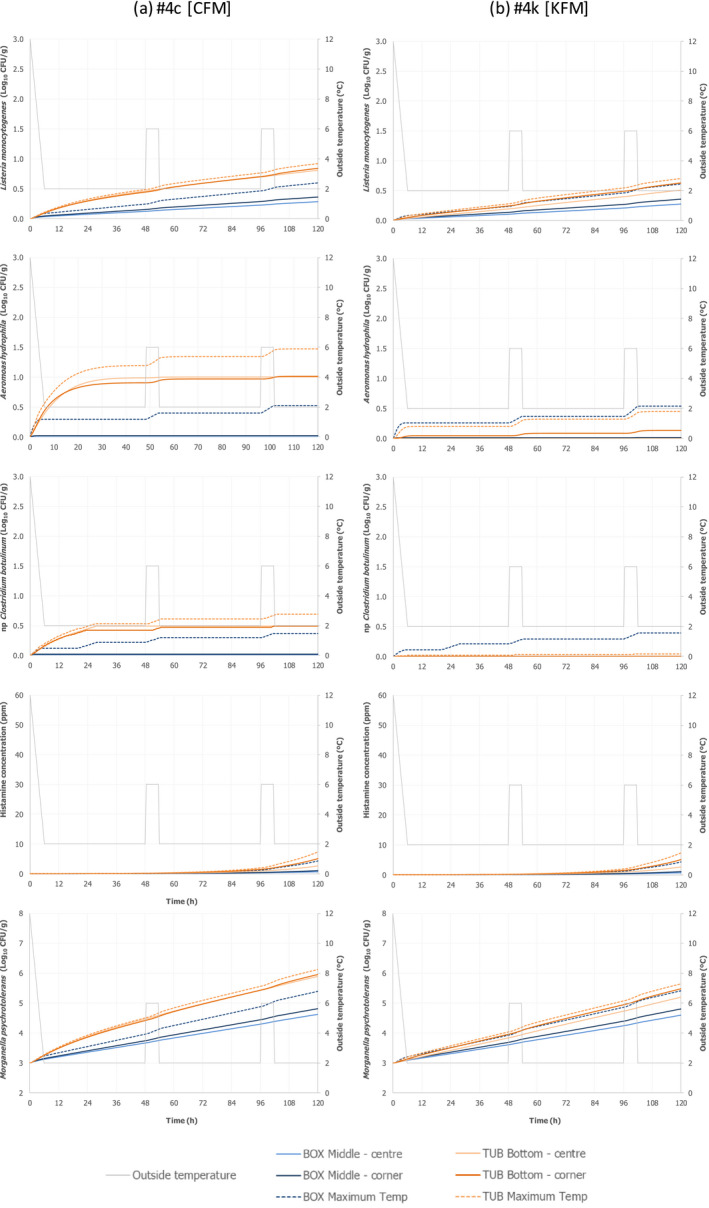
Growth of relevant hazards and histamine accumulation on the fish surface and the maximum temperature in the matrix of fish/water or fish/air during transport/storage of fish based on the predicted time/Temperature profiles of selected case studies of the ‘abusive’ scenario: (a) ‘cooling‐keeping’ process of fat medium fish (CFM; #4c) and (b) ‘keeping’ process of fat medium fish (KFM; #4k)
For the ‘abusive’ scenarios, A. hydrophila showed higher growth potential than L. monocytogenes, which is the opposite in the ‘observed’ scenarios. This can be explained by the higher temperature in the t/T profiles predicted for the case studies within the ‘abusive’ scenario compared to those observed in the ‘Qualitubfish’ experiments. The higher predicted log10 increases of A. hydrophila compared to L. monocytogenes, especially during the initial cooling, can be explained by the response of A. hydrophila to temperature increases (as shown in Figure 14; the growth rate of A. hydrophila is higher than that of L. monocytogenes at temperatures above 5°C) and also due to the assumption that the minimum temperature allowing growth (Tmin) of this pathogen is 2°C (see Section 2.5).
The differences in the simulated increase of L. monocytogenes, A. hydrophila and Cl. botulinum on the fish surface between boxes and tubs for a period of 2–5 days ranged from 0.27 to 0.53, 0.85 to 1.00 and 0.39 to 0.48 log10, respectively, for the ‘cooling‐keeping’ process considering the various case studies and the two positions of the fish at the warmest position within the container. The comparison of the growth potential associated with the maximum predicted temperatures reached within the containers provided similar differences to those recorded for the temperatures predicted for the surface of the fish located at the warmest zone of the container.
In comparison, for the ‘keeping’ process differences are smaller corresponding to −0.12 to 0.27, 0 to 0.12 and 0 to 0.12 log10, respectively. The negative values indicate a minority of the cases where growth potential (log10 increase) was greater in boxes than tubs and the opposite is true for the positive values. Only when the comparison was calculated using the maximum predicted temperatures reached within the containers, the differences in the log10 increase indicate that slightly higher growth occurred in boxes compared to tubs, which can be associated with the better capacity of the tubs (filled with water and ice) to keep the temperature compared with the boxes (with ice only) under the specific abusive conditions assessed.
The smaller log10 increase differences for the ‘keeping’ process indicate a more similar capacity of boxes and tubs to keep the fish temperatures during storage. The higher positive predicted log10 increases for the ‘cooling‐keeping’ process suggests that temperatures occurring at the initial cooling are responsible for the higher microbial growth. In other words, in this situation, the poorer cooling capacity of water with ice (in tubs) compared to ice (in boxes) leads to a higher log10 increase difference of the three hazards. For L. monocytogenes and non‐proteolytic Cl. botulinum, the maximum log10 increase difference between tubs and boxes is around 0.5 log10 at day 5, which may be considered of similar magnitude to the error of microbiological analysis and thus, of low impact from a microbiological point of view. For A. hydrophila, the magnitude of the difference in log10 increases is higher, with values in the range of 0.90–1.00 log10 units, especially in ‘cooling‐keeping’ processes, where, as stated above, higher temperatures occur on fish surfaces as compared to the ‘keeping’ process, till they (asymptotically) reach the level of 0°C, when the ‘keeping’ process practically applies.
Slightly higher growth of the hazards in boxes than tubs was predicted only in the ‘keeping’ process for fat medium fish; namely, only after 2 days for L. monocytogenes when fish are located close to the corner of either container type (i.e. at the warmest spots) (−0.12 log10 increase difference), after 2 and 5 days at the maximum predicted temperatures reached within the containers for L. monocytogenes (−0.33 and −0.18 log10 increase difference and after 2, 3 and 5 days at the maximum predicted temperatures reached within the containers for Cl. botulinum (−0.19, −0.26 and −0.35 log10 increase difference, respectively).
3.4.2.4. Impact of the lag time and pH on the growth potential of the relevant hazards
The predicted growth potential (log10 increases) of all three pathogens selected for the growth simulations seem to be less than 1 log10. Only the growth of A. hydrophila in tubs was estimated to be higher than 1 log10 when the maximum predicted temperatures within the containers at each time is considered. However, this t/T profile is an extreme worst case, focussing on the maximum temperature (warmest spots) occurring within the tub along the time (Tables 12 and 13). In all cases, including lag time in these simulations is expected to reduce the predicted log10 increases. Regarding the comparative growth potential in tubs and boxes, the following outcome is expected based on the options to consider or not lag time: since microbial growth in boxes is already limited, i.e. close to no growth (and lower than in tubs) without lag, inclusion of lag is expected to limit the magnitude of microbial changes in tubs more than in boxes. Thus, differences in log10 increases between tubs and boxes are expected to decrease if the hazards growth with lag time as compared to growth without lag.
The predicted growth of A. hydrophila with and without lag in tubs is presented in Table 14 for the case study of the ‘abusive’ scenario for the ‘cooling‐keeping’ process of fat medium fish (CFM; #4c), as these conditions showed the maximum predicted increases in log10 units of the organism without lag. The dynamic growth model is updated with lag time information through the value of the parameter (ao), i.e. the percentage of cells growing without lag. This value may range from 0 (= no growth) to 1 (= no lag). The corresponding comparisons in boxes were not tested because the growth simulations in boxes without lag, showed markedly lower growth than in tubs (Tables 12 and 13).
Table 14.
Predicted log10 increases of Aeromonas hydrophila on the surface of fish in the ‘abusive’ scenario for fat medium fish, located in different spots inside tubs during the ‘cooling‐keeping’ process (CFM; #4c), assuming different initial physiological states (and thus, potential lag time) of cells at the beginning of simulations
| Assumption of lag | Time (days) | TUB Bottom – centre | TUB Bottom – corner | TUB Maxa |
|---|---|---|---|---|
| No lag (a 0 = 1) | 2 | 0.99 | 0.90 | 1.19 |
| 3 | 1.00 | 0.97 | 1.34 | |
| 5 | 1.00 | 1.01 | 1.47 | |
| Lag defined by a 0 = 0.75 | 2 | 0.832 | 0.757 | 1.020 |
| 3 | 0.845 | 0.814 | 1.157 | |
| 5 | 0.846 | 0.854 | 1.276 | |
| Lag defined by a 0 = 0.25 | 2 | 0.368 | 0.328 | 0.476 |
| 3 | 0.374 | 0.358 | 0.559 | |
| 5 | 0.375 | 0.380 | 0.635 |
a0 is the percentage of cells growing without lag. This value may range from 0 (= no growth) to 1 (= no lag).
Based on the maximum temperature obtained for each time within the whole food/water matrix.
The foreseeable distribution of pH of FFP based on scientific reports is between 5.78 and 6.68 (i.e. minimum to maximum values recorded in the literature, Table 3). The predictive models available for L. monocytogenes and non‐proteolytic Cl. botulinum allow the estimation of the growth rate of these organisms at different pH values within the above range, keeping the other input parameters constant and then the calculation of the associated growth potential (log10 increase) at a given temperature. As shown in Table 15, in FFP with the minimum reported pH value of 5.78, the growth potential of the hazards is reduced, compared with the simulations at the reference pH value of 6.5 that is used in all simulations of the assessment (Table 3). As a consequence, in FFP with lower pH than 6.5, the difference in the log10 increase between boxes and tubs will also be lower.
Table 15.
Predicted log10 increases of Listeria monocytogenes and non‐proteolytic Clostridium botulinum on the surface of fish after 3 and 5 days at 4°C (isothermal conditions) assuming different pH values of the fresh fishery products
| Pathogen | After 3 days of storage | After 5 days of storage | ||||
|---|---|---|---|---|---|---|
| pH = 5.78 | pH = 6.5 | pH = 6.89 | pH = 5.78 | pH = 6.5 | pH = 6.89 | |
| L. monocytogenes | 0.32 | 0.71 | 0.74 | 0.53 | 1.18 | 1.24 |
| Non‐proteolytic Cl. botulinum | 0.25 | 0.38 | 0.41 | 0.42 | 0.63 | 0.68 |
When a higher pH than 6.5 is assumed in FFP, such as the maximum reported pH value (6.89), a negligible increase in the growth potential (0.03–0.06 log10 units) is predicted for both hazards after 3 and 5 days, as compared to the reference pH value of 6.5. This can be explained by the fact that both pH values (6.5 and 6.68) are close to the optimum pH near 7.0. Moreover, the calculated log10 increases shown in Table 15, albeit limited, they are already overestimated, as they have been calculated for an isothermal temperature of 4°C, which is substantially higher than the majority of temperature records predicted or observed (i.e. 0–2°C) during storage of FFP in tubs and boxes. Therefore, the impact of the uncertainty around the pH of the FFP on growth of hazards and histamine accumulation is considered negligible.
3.4.2.5. Impact of the WPS change due to water uptake on the growth potential of the relevant hazards
The magnitude of the WPS decrease due to the potential water uptake of fish during 5 days of transport/storage in tubs was estimated to be between 0.006 and 0.021 (associated with an increase of 1.6% to 6% of the water content, see Section 3.3.2). This decrease in the WPS has no effect on the predicted growth rate of A. hydrophila and non‐proteolytic Cl. botulinum, while for L. monocytogenes, a negligible increase of the maximum growth rate is expected according to the predictive models considered in the present assessment.
Consequently, the predicted growth potential of the relevant hazards on FFP during their transport/storage in tubs and the subsequent difference compared with boxes will not be affected by the small decrease of the WPS value.
3.4.3. Histamine formation by relevant histamine‐producing bacteria
A summary of the information retrieved through the systematic literature review on histamine production by the relevant hazards is reported in Table 16.
Table 16.
Systematic review of the histamine (HI) production of the microbial hazards selected for the assessment, in fish and fishery products stored within the temperature range −3°C to 7°C
| Hazard | Temperature | Matrix | Hazard considered (if not already defined at species level) | Maximum time in which histamine production is not observed | First/last time point in which histamine production is reported | Characterisation of the growth or of survival (no change or reduction) a | Reference |
|---|---|---|---|---|---|---|---|
| Enterobacter spp. a | 4°C | Sailfish | E. aerogenes a [Klebsiella aerogenes] | – | 1 day/4 days |
No change at 1 day; ~ 0.5 log10 increase at 4 days 63 ppm at 4 days |
Tsai et al. (2005) |
| 4°C | Milkfish | E. aerogenes a [Klebsiella aerogenes] | – | 1 day/4 days |
No change at 1 day; ~ 0.5 log10 increase at 4 days 96 ppm at 4 days |
Tsai et al. (2005) | |
| Morganella spp. | 4 ± 1°C | Mackerel | M. morganii | – | 1 day/8 days |
~ 1 log10 increase at 1 day; ~ 2 log10 increase at 2 days until 6 days ~ 900 ppm increase HI at 1 day; ~ 1,200 ppm increase HI at 2 days; ~ 500 ppm increase HI at 8 days |
Aytac et al. ( 2000 ) |
| 2.1°C | Tuna | M. psychrotolerans | – |
Time to 100 ppm HI: 6.3 days; time to 500 ppm HI: 8.4 days; time to 1000 ppm HI: 10.8 days |
Emborg and Dalgaard ( 2008 ) | ||
| 5°C | Tuna juice | M. psychrotolerans | – |
Time to 100 ppm HI: 9.1 day; Time to 500 ppm HI: 9.5 days; time to 1,000 ppm HI: 11.2 days |
Emborg and Dalgaard ( 2008 ) | ||
| Photobacterium spp. | 0°C | Garfish | P. phosphoreum | 12 days | 18 days/20 days |
No change at 3 days; ~ 2 log10 increase at 6 days; ~ 7 log10 increase at 20 days ~ 20 ppm increase HI at 18 days; ~ 25 ppm increase HI at 20 days |
Dalgaard et al. ( 2006 ) |
| 4°C | Swordfish | P. iliopiscarum | 3 days | 5 days/7 days | ~ 2 log10 increase at 3 days;~ 4 log10 increase at 5 days until 7 days~ 350 ppm increase HI at 5 days;~ 1750 ppm increase HI at 7 days | Torido et al. ( 2012 ) | |
| 4°C | Swordfish | P. phosphoreum | 3 days | 5 days/7 days |
~ 3 log10 increase at 3 days; ~ 4 log10 increase at 5 days until 7 days ~ 400 ppm increase HI at 5 days; ~ 870 ppm increase HI at 7 days |
Torido et al. ( 2012 ) | |
| 5°C | Garfish | P. phosphoreum | – | 7 days/13 days |
~ 2 log10 increase at 3 days; ~ 7 log10 increase at 5 days until 13 days ~ 200 ppm increase HI at 7 days; ~ 1,200 ppm increase HI at 13 days |
Dalgaard et al. ( 2006 ) |
HI: histamine.
In the column are reported the observations at the first time point in which growth/survival is reported, at the time points relevant for the assessment, and the last observation time point included in the study. Numbers followed by ‘day’ indicate the day of observation (i.e. 2 days = 2nd day of observation; 2 days until 6 days = from the 2nd day to the 6th day of observation). ‘~’ is used for data not provided as punctual numbers (e.g. pictures, graphs); for these data, numbers are expressed by increments of 0.5 log10.
3.4.3.1. Histamine‐producing bacteria: Enterobacter spp.
Limited data are available on growth and histamine production by Enterobacter spp. in FFPs. In Tsai et al. (2005), a limited growth (~ 0.5 log10) was obtained after 4 days of storage (4°C) of experimentally inoculated sailfish and milkfish. In the same experiment, histamine levels did not exceed 100 ppm at the end of the incubation (Tsai et al., 2005). In other relevant studies, no histamine accumulation was recorded at 4°C for 36–96 h, but only above 15°C without exceeding 100 ppm up to 96 h of maximum experimental storage period (Lee, 2012; Zou and Hou, 2017).
3.4.3.2. Histamine‐producing bacteria: Photobacterium spp.
Growth and histamine production by Ph. phosphoreum and Ph. iliopiscarum within the temperature range of 0–5°C has been addressed in two studies, with initial inoculation levels of a mixture of Photobacterium species at 104 CFU/g (Dalgaard et al., 2006; Torido et al., 2012). In the experiments performed, on swordfish stored at 4°C, a 2 log10 growth was observed for Ph. iliopiscarum and 3 log10 for Ph. phosphoreum within 3 days, and the two species resulted in histamine accumulation of 350 ppm and 400 ppm of histamine, respectively, up to 5 days of storage (Torido et al., 2012). A slightly lower growth and histamine production was reported in another study on stored garfish at 5°C, in which Ph. phosphoreum increase (7 log10) and histamine production (200 ppm) were registered after 7 days. Significantly slower growth and minimum histamine production was instead observed on garfish stored at 0°C, in which Ph. phosphoreum increased by 2 log10 within 6 days but no histamine was detected during this time (Dalgaard et al., 2006). According to the above studies, histamine accumulation was detected after Photobacterium population exceeded the level of 106 CFU/g. Overall, the available data indicate that growth of Photobacterium spp. and the associated histamine production may occur under certain temperature conditions applied in this assessment, but histamine accumulation is negligible at temperatures close to 0°C, e.g. as in the case of ‘keeping’ profiles. Even though temperatures occurring at the beginning of the abusive ‘cooling’ profiles (i.e. till fish cools to a target temperature close to 0°C) could exceed 4°C, shown to support growth and histamine production by Photobacterium after at least 3–4 days (and 4 log10 CFU/g initial level of Photobacterium), the actual duration of fish exposure to such temperatures is much shorter (i.e. maximum 16–20 h in tubs and only 2–3 h in boxes) to eventually enable histamine production.
Combining the information discussed in the above two paragraphs (Sections 3.4.3.1 and 3.4.3.2), with the relevant numerical details shown in Table 16, it may be suggested that the expected histamine accumulation levels by the two organisms under the conditions of the assessment is limited especially for Enterobacter spp., regardless of the container type. This is explained by the short duration of the assessment (i.e. up to 5 days) and the low temperatures occurring on the surface of the FFP during the assessed cooling and keeping durations, in comparison to the t/T conditions reported to favour histamine accumulation by the reviewed evidence. Given the lack of available predictive models of temperature‐dependent histamine accumulation on aerobically stored FFP, associated with the growth of the above organisms, it is not possible to quantitatively assess the impact of on‐land transport and storage of FFPs in tubs compared to boxes on histamine production by Enterobacter and Photobacterium species, based on the reasonably foreseeable abusive t/T profiles predicted by the heat‐transfer model. However, the production of histamine by these two bacteria under chilling conditions (< 7°C) is expected to be lower than that produced by M. psychrotolerans (see Section 3.4.3.3).
3.4.3.3. Histamine‐producing bacteria: Morganella psychrotolerans
The histamine accumulation due to M. psychrotolerans initiates above the level of 105 CFU/g in fish. This, in combination with an initial Morganella level of 103 CFU/g is expected to result in detectable levels of histamine at in boxes and tubs at the different temperature conditions assessed as described below.
Histamine formation under the ideal scenario
Under the ideal scenario described in Section 2.2, the fish surface temperature would be 0°C throughout the transport/storage time (isothermal conditions) irrespectively of the use of boxes (with ice) or tubs (with ice and water). Therefore, the amount of histamine formed will be equivalent in both type of containers and equal to a maximum of 0.4 ppm after 5 days according to the predictions provided by the FSSP tool using the conservative input values defined in Table 3.
Histamine formation under the ‘observed’ scenarios
Tables 17 and 18 gather the results of the accumulation of histamine, due to the growth of M. psychrotolerans in fish predicted for the t/T profiles observed in the experiments of the ‘Qualitubfish’ project. Very small amounts of histamine were recorded even after 5 days of storage/transport irrespectively of the container (box or tub). The recorded histamine accumulation was below the maximum tolerable limits (100 ppm) set by the Commission Regulation (EC) 2073/200510. In fact, the time to reach 100 ppm, taken the whole available t/T profiles was 6.7 days in the worst case (e.g. due to the temperature recorded for fish located in the top of the tub of the non‐transported fish in the experiment 1).
Table 17.
Predicted levels of histamine (ppm) accumulation due to growth of Morganella psychrotolerans on FFP when transported/stored in tubs or boxes based on the observed time/Temperature profile in experiment 1 of the ‘Qualitubfish’ project (Bekaert et al., 2016a)
| Time (day) | Box | Tub | Differencea | |||
|---|---|---|---|---|---|---|
| Middle fish | Middle fish | Bottom fish | Middle fish | Top fish | Range (min–max) | |
| Not transported | ||||||
| 2 | 0.06 | 0.12 | 0.05 | 0.12 | −0.01 to 0.06 | |
| 3 | 0.13 | 0.49 | 0.11 | 0.56 | −0.02 to 0.43 | |
| 5 | 0.58 | 6.17 | 0.49 | 9.86 | −0.09 to 9.28 | |
| Time to 100 ppm | 11.0 | 7.2 | 11.6 | 6.7 | ||
| Transported | ||||||
| 2 | 0.06 | 0.06 | 0.06 | 0.13 | 0.11 | 0 to 0.07 |
| 3 | 0.15 | 0.16 | 0.20 | 0.43 | 0.39 | 0.04 to 0.28 |
| 5 | 0.79 | 0.79 | 1.65 | 3.54 | 3.13 | 0.86 to 2.75 |
| Time to 100 ppm | 11.3 | 10.5 | 8.6 | 8.1 | 9.1 | |
A positive value indicates a greater histamine accumulation (ppm increase) in tubs compared to boxes, while the opposite is true for the negative values.
Table 18.
Predicted levels (ppm) of histamine (ppm) accumulation due to growth of Morganella psychrotolerans on FFP when transported/stored in tubs or boxes based on the observed time/Temperature profile in experiment 2 of the ‘Qualitubfish’ project (Bekaert et al., 2016b)
| Time (day) | Box | Tub | Differencea | |||||
|---|---|---|---|---|---|---|---|---|
| Middle fish | Middle fish | Middle fish | Bottom fish | Middle fish | Middle fish | Top fish | Range (min–max) | |
| 2 | 0.04 | 0.07 | 0.09 | 0.16 | 0.18 | 0.14 | 0.24 | 0.05 to 0.2 |
| 3 | 0.11 | 0.31 | 0.20 | 0.55 | 0.69 | 0.50 | 0.88 | 0.19 to 0.77 |
| 5 | 1.07 | 4.43 | 1.00 | 5.79 | 7.18 | 5.34 | 9.12 | 0.91 to 8.12 |
| Time to 100 ppm | NR | NR | NR | NR | NR | NR | 7.1 | |
NR: not reached within the whole temperature record available.
A positive value indicates a greater histamine accumulation (ppm increase) in tubs compared to boxes, while the opposite is true for the negative values.
The difference of the increase of the histamine levels between box and tubs was practically irrelevant, i.e. less than 1 ppm at 3 days, and below 10 ppm at 5 days of transport/storage of FFP.
Histamine formation under the ‘abusive’ scenarios
Table C.5 in Appendix C shows the results of the histamine accumulation due to growth of M. psychrotolerans on the surface of fish associated with the t/T profiles generated through the heat transfer model for each case study defined in Figure 3. The predicted differences in histamine accumulation due to growth of M. psychrotolerans between boxes and tubs for a period of 2, 3 and 5 days were 0, less than 1.5 ppm and less than 15.9 ppm, respectively (Table 19). Consistently, with the predicted log10 increase of pathogens, the highest values in histamine accumulation levels were observed in tubs as compared to boxes and on the surface of fish located close to the wall of either container type, as compared to the fish located in the centre of the containers.
Table C.5.
Predicted levels (ppm) of histamine accumulation due to growth of Morganella psychrotolerans in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies of the ‘cooling‐keeping’ and ‘keeping’ processes of fresh fishery products with different fat content and size
| Process | Fat content | Fish Dimension | Case study # | Time (days) | Location of fish in box or tub | Difference between boxes and tubsc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Maxa | TUB Maxb | Centre | Corner | Max | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||||
| ‘Cooling‐keeping’ | Lean | Small | 1c | 2 | 0.1 | 0.5 | 0.1 | 0.4 | 0.1 | 0.6 | 0.4 | 0.4 | 0.4 |
| 3 | 0.2 | 1.7 | 0.2 | 1.7 | 0.6 | 2.3 | 1.5 | 1.5 | 1.8 | ||||
| 5 | 1.3 | 14.4 | 2.2 | 18.1 | 7.0 | 27.2 | 13.1 | 15.9 | 20.2 | ||||
| Medium | 2c | 2 | 0.1 | 0.4 | 0.1 | 0.4 | 0.1 | 0.5 | 0.4 | 0.3 | 0.4 | ||
| 3 | 0.1 | 1.4 | 0.2 | 1.5 | 0.4 | 1.9 | 1.3 | 1.3 | 1.5 | ||||
| 5 | 0.7 | 11.8 | 1.0 | 14.2 | 3.8 | 20.6 | 11.1 | 13.2 | 16.7 | ||||
| Fat | Small | 3c | 2 | 0.1 | 0.4 | 0.1 | 0.4 | 0.1 | 0.5 | 0.4 | 0.3 | 0.4 | |
| 3 | 0.2 | 1.5 | 0.2 | 1.5 | 0.5 | 2.0 | 1.3 | 1.3 | 1.5 | ||||
| 5 | 1.2 | 12.4 | 2.0 | 15.0 | 5.9 | 22.3 | 11.2 | 13.0 | 16.4 | ||||
| Medium | 4c | 2 | 0.1 | 0.5 | 0.1 | 0.4 | 0.1 | 0.5 | 0.4 | 0.3 | 0.4 | ||
| 3 | 0.1 | 1.5 | 0.2 | 1.5 | 0.5 | 2.0 | 1.4 | 1.3 | 1.6 | ||||
| 5 | 0.7 | 12.8 | 1.1 | 15.1 | 4.0 | 22.2 | 12.1 | 14.0 | 18.2 | ||||
| ‘Keeping’ | Lean | Small | 1k | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 |
| 3 | 0.1 | 0.3 | 0.2 | 0.6 | 0.5 | 0.7 | 0.2 | 0.4 | 0.3 | ||||
| 5 | 0.8 | 2.9 | 1.3 | 6.1 | 5.0 | 9.0 | 2.1 | 4.8 | 3.9 | ||||
| Medium | 2k | 2 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 | ||
| 3 | 0.1 | 0.3 | 0.2 | 0.5 | 0.4 | 0.6 | 0.2 | 0.3 | 0.2 | ||||
| 5 | 0.6 | 2.5 | 1.0 | 4.9 | 4.1 | 7.1 | 1.9 | 3.9 | 3.0 | ||||
| Fat | Small | 3k | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 | |
| 3 | 0.1 | 0.3 | 0.2 | 0.5 | 0.4 | 0.6 | 0.2 | 0.3 | 0.2 | ||||
| 5 | 0.8 | 2.6 | 1.2 | 5.1 | 4.3 | 7.3 | 1.8 | 4.0 | 3.0 | ||||
| Medium | 4k | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 | ||
| 3 | 0.1 | 0.3 | 0.2 | 0.5 | 0.4 | 0.6 | 0.2 | 0.3 | 0.2 | ||||
| 5 | 0.6 | 2.6 | 1.0 | 5.0 | 4.3 | 7.3 | 2.0 | 4.0 | 3.0 | ||||
Maximum temperature on the surface of a fish located in the middle of the box, close to the wall.
Maximum temperature on the surface of a fish located at the bottom corner of the tub.
Calculated by subtracting the histamine levels (ppm) in tubs of the specified column number from the corresponding histamine levels (ppm) in the box, also indicated by the appropriate column number. A positive value indicates a greater histamine accumulation (ppm increase) in tubs compared to boxes, while the opposite is true for the negative values.
Table 19.
Predicted levels (ppm) of histamine accumulation due to the growth of Morganella psychrotolerans in different spots inside tubs and boxes (on the fish surface and the maximum temperature in the matrix of fish/water or fish/air) of the case studies ‘cooling‐keeping’ process of lean small fish (CLM; #1c), ‘keeping’ process of lean medium fish (KLM; #1k), ‘cooling‐keeping’ process of fat medium fish (CFM; #4c) and ‘keeping’ process of fat medium fish (KFM; #4k) under the ‘abusive’ scenarios
| Process | Fat content and dimension of the fish | Time (day) | Location of fish in box or tub | Difference between boxes and tubs(c) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BOX Middle – centre | TUB Bottom – centre | BOX Middle – corner | TUB Bottom – corner | BOX Max(a) | TUB Max(b) | Centre | Corner | Max | |||
| 1 | 2 | 3 | 4 | 5 | 6 | (2) – (1) | (4) – (3) | (6) – (5) | |||
| ‘Cooling‐keeping’ | Lean small fish | 2 | 0.1 | 0.5 | 0.1 | 0.4 | 0.1 | 0.6 | 0.4 | 0.4 | 0.4 |
| 3 | 0.2 | 1.7 | 0.2 | 1.7 | 0.6 | 2.3 | 1.5 | 1.5 | 1.8 | ||
| 5 | 1.3 | 14.4 | 2.2 | 18.1 | 7.0 | 27.2 | 13.1 | 15.9 | 20.2 | ||
| ‘Keeping’ | Lean small fish | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 |
| 3 | 0.1 | 0.3 | 0.2 | 0.6 | 0.5 | 0.7 | 0.2 | 0.4 | 0.3 | ||
| 5 | 0.8 | 2.9 | 1.3 | 6.1 | 5.0 | 9.0 | 2.1 | 4.8 | 3.9 | ||
| ‘Cooling‐keeping’ | Fat medium fish | 2 | 0.1 | 0.5 | 0.1 | 0.4 | 0.1 | 0.5 | 0.4 | 0.3 | 0.4 |
| 3 | 0.1 | 1.5 | 0.2 | 1.5 | 0.5 | 2.0 | 1.4 | 1.3 | 1.6 | ||
| 5 | 0.7 | 12.8 | 1.1 | 15.1 | 4.0 | 22.2 | 12.1 | 14.0 | 18.2 | ||
| ‘Keeping’ | Fat medium fish | 2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 |
| 3 | 0.1 | 0.3 | 0.2 | 0.5 | 0.4 | 0.6 | 0.2 | 0.3 | 0.2 | ||
| 5 | 0.6 | 2.6 | 1.0 | 5.0 | 4.3 | 7.3 | 2.0 | 4.0 | 3.0 | ||
(a): Based on the maximum overall temperature in the food/air matrix.
Based on the maximum temperature obtained for each time within the whole food/water matrix.
Calculated by subtracting the histamine levels (ppm) in tubs of the specified column number from the corresponding histamine levels (ppm) in the box, also indicated by the appropriate column number. A positive value indicates a greater histamine accumulation (ppm increase) in tubs compared to boxes, while the opposite is true for the negative values.
In any case, the maximum limit of 100 ppm was not achieved in any of the assessed scenarios.
3.4.3.4. Impact of the lag time and initial concentration of M. psychrotolerans on the histamine formation
Little information is available about the actual prevalence and concentration of M. psychrotolerans in FFP. The input value of 1,000 CFU/g used to assess the histamine accumulation (Section 3.4.3.3) can be considered conservative, as the contamination in the fish skin and gills has been assumed to be low, from 5 to 10 CFU/g (Emborg, 2007; Emborg and Dalgaard, 2008). When this low value of initial contamination is used to simulate the histamine accumulation for the worst‐case ‘abusive’ scenario t/T profile shown in Table 19, i.e. the ‘cooling‐keeping’ process of lean small fish located in the bottom corner of the tub, the level of histamine accumulated is decreased to 0.02 and 0.18 ppm at day 3 and 5, respectively, assuming no lag for M. psychrotolerans growth (Table 20). As the level of histamine in boxes were markedly lower, it is expected that by lowering the initial concentration of M. psychrotolerans, the difference in the histamine accumulation between tubs and boxes will be reduced.
Table 20.
Predicted levels (ppm) of histamine accumulation due to the growth of Morganella psychrotolerans on the fish surface assuming different initial concentration of M. psychrotolerans and with or without lag time.a A fish located in bottom corner inside tubs for the case study ‘cooling‐keeping’ process of lean small fish (CLM; #1c) under the ‘abusive’ scenarios is considered
| Time (day) | 10 CFU/g | 1,000 CFU/g | 10,000 CFU/g | |||
|---|---|---|---|---|---|---|
| Laga | No lag | Lag | No lag | Lag | No lag | |
| 2 | 0.00 | 0.00 | 0.06 | 0.45 | 0.63 | 4.43 |
| 3 | 0.00 | 0.02 | 0.28 | 1.70 | 2.77 | 16.72 |
| 5 | 0.03 | 0.18 | 3.14 | 18.14 | 30.67 | 167.69 |
FSSP: food spoilage and safety predictor.
FSSP predictive model uses a relative lag time (RLT) of 2.55 for M. psychrotolerans (Emborg and Dalgaard, 2008).
However, higher concentrations of the histamine‐producing bacteria can be found in the intestines of fish and the FFP could be contaminated when handled either on‐board or on‐land (Emborg, 2007). Under the exceptional event of an extremely high initial concentration of M. psychrotolerans of 10,000 CFU/g, the amount of histamine accumulated would be less than 20 ppm after 3 days of storage/transport (Table 20). In this case, the critical limit of 100 ppm would be reached after 4.5 days of storage/transport and up to 167 ppm could be accumulated after 5 days. When this simulation is performed using the t/T profile corresponding to the fish located in the middle corner of the boxes, the maximum concentration of histamine accumulated after 5 days is 22 ppm. Therefore, under such an extremely rare worst‐case scenario, the difference in histamine accumulation between tubs and boxes are considerable (i.e. 145 ppm).
The lag time of M. psychrotolerans in naturally contaminated FFP is uncertain. With respect of the conservative approach of not considering lag time applied to assess the impact of ‘abusive’ scenarios, the inclusion of lag time would probably provide more realistic simulations for naturally contaminated products. According to the simulations, the inclusion of lag time reduces the amount of histamine accumulation compared with the simulations without lag. The extent of reduction was five‐ to sevenfold when using the t/T profile of the worst‐case ‘cooling‐keeping’ process of lean small fish, Table 20). As a consequence, it is expected that the differences in the histamine accumulation between boxes and tubs will also be considerably reduced, not reaching the critical limit of 100 ppm in any of the initial concentrations of M. psychrotolerans assessed.
3.4.3.5. Impact of WPS change due to water uptake on the histamine formation of M. psychrotolerans
The magnitude of the WPS decrease due to the potential water uptake of fish during the exceptional duration of 5 days of storage/transport in tubs was estimated to be from 0.006 (associated with an increase of 1.6% of the water content) to 0.021 (associated with an increase of 6% of the water content, see Section 3.3.2). This WPS reduction has negligible impact on the growth rate of M. psychrotolerans and thus on the formation of histamine and the difference between boxes and tubs.
3.4.4. Potential public health risks
The potential risk to public health associated with the transport/storage of FFP is dependent on many factors and its quantification requires the development of a QMRA including the exposure assessment and hazard characterisation (dose‐response). For the exposure assessment, the initial concentration of the hazards at the moment of arrival at the first on‐land establishment would be required and the behaviour of each hazard during the transport/storage on‐land (which was assessed in this opinion) complemented by the frequency of occurrence of the various t/T profiles. Next, all the subsequent steps of the supply chain would need to be accounted for, including the FFP transformation, the consumer habits and behaviour during storage, the handling and mode of consumption (e.g. consumed as raw having a higher risk compared to consumed cooked) and the size and the frequency of consumption of each type of FFP or the products thereof. Finally, the susceptibility of the consumer group is also a key factor in the hazard characterisation to be able to estimate the probability of illness.
No QMRA is available addressing the relevant factors and its development, including the collection of the required data is out of the scope of the present mandate.
In principle, risk would potentially increase with the use of tubs in comparison to boxes as the temperature of the fish in tubs would lead to substantially more growth (log10 increase) of the identified hazards in tubs than in boxes. This is provided that the remaining steps of the food supply chain up to the consumption step would have equal impact on the levels of the hazards until the consumption.
For a higher histamine accumulation in tubs than in boxes, public health risk would potentially increase depending on the predetermined level of extra risk selected as the threshold of the benchmark dose used. A FAO risk assessment estimated, using the benchmark dose methodology, that a hazard dose of 50 mg corresponded to an increased risk level of 10% (lower confidence interval) for healthy individuals, which under a assumed serving size of 250 g corresponded to a threshold of 200 ppm histamine in fish (FAO and WHO, 2013). These figures are difficult to combine with the results obtained in the present assessment, as the impact of subsequent steps of the FFP supply chain on the histamine concentration, the serving size as well as the susceptibility variation within the population, including more vulnerable groups are not taken into consideration.
Within the food safety management systems (FSMS), the purpose of maintaining the cold chain is to control the biological hazards. Chilling conditions are not applied with the intention of reducing the survival of hazards. Although better survival of the identified hazards in one condition compared to the other (e.g. tubs in comparison with in boxes) would lead to a higher potential risk to public health, the impact of higher survival was considered of lower impact in comparison with that of a higher growth of pathogens and histamine accumulation. However, the quantification of the increase of the risk due to the a higher growth or survival of the hazards and/or higher histamine accumulation in tubs compared to boxes and the assessment of its actual relevance for public health was not carried out.
3.4.5. Uncertainties associated with the behaviour of relevant biological hazards
The uncertainties associated with the behaviour of relevant biological hazards are described in Table D.4 in Appendix D.
Table D.4.
Potential sources of uncertainty identified in the assessment of the behaviour of relevant biological hazards and estimation of the impact that these uncertainties could have on the conclusion (i.e. over/underestimation extent of the survival, growth or histamine accumulation)
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Temperature as the critical factor distinguishing boxes and tubs |
Temperature was considered the only quantitative factor affecting the behaviour of the hazards on the fish surface. Thus, the assessment was based on differences on the t/T profiles observed or predicted in boxes (in ice) compared to tubs (in ice and water). The predicted t/T profiles correspond to ‘reasonably foreseeable abusive’ case studies applying an overall conservative approach to get the results Other factors associated with the presence of water in tub and not in boxes may have an impact on the behaviour of the hazards (see next uncertainty sources) |
Overestimation of the growth potential of the hazards, including histamine accumulation due to the overestimation of the FFP temperatures is expected in both tubs and boxes. Thus, the difference in the log10 increase between tubs and boxes will also be overestimated |
| Presence of water in tubs |
In tubs with water and ice, a more planktonic‐like mode of growth could occur, which in theory is known to be faster than colonial/sessile type of growth occurring in the surface of fish in a box. However, the results of the ‘Qualitubfish’ experiment do not confirm this hypothesis. On the contrary, the growth of the different microbial groups monitored were similar between fish stored in tubs and in boxes, despite the temperatures recorded in tubs were overall higher than in boxes Possible eluting/disorbing effect of the water present in tubs but not in boxes (e.g. rinsing, dilution, of nutrients and metabolites, or dilution and planktonic dispersion of background microbiota and hazards) were not considered in the assessment Dispersion/dissemination of nutrients, also due to tissue injury by ice crystals, was not considered either Oxygen availability is expected be lower when fish is stored in water and ice (in tubs) than in ice without water (in box). However, the growth of A. hydrophila, L. monocytogenes and M. psychrotolerans were simulated in aerobic (non limiting oxygen availability) conditions. On the other hand, non‐proteolytic Cl. botulinum growth was simulated in totally anaerobic conditions, despite dissolved oxygen will be present in the water (in tubs) |
Overestimation for the assessed (simulated) pathogens, the effect of water in tubs (e.g. dilution, elution, lowering oxygen availability) could reduce the growth of the hazards in tub and as a consequence also reduce the differences in the log10 increase between tubs and boxes as compared with the growth potential quantified with the predictive models as a result of the different t/T profiles |
| t/T profiles predicted by heat transfer modelling |
The temperatures used as input parameter for the predictive microbial models have been derived from heat transfer modelling and represent the output of a modelling exercise, subjected to error and uncertainty (Table D.2) The impact of the heat transfer model performance results were assessed by comparing the growth potential of L. monocytogenes and the histamine accumulation provided by predictive models in response to the predicted t/T profiles vs. the observed temperature records. A slightly lower log10 increase of L. monocytogenes (0.12 or lower) and histamine accumulation (40 ppm) were estimated from the heat transfer modelling t/T profile (for a total of 168 h, Figure 9) in tubs compared with the observed t/T profile. Smaller discrepancies between observations and predictions of the heat transfer model were detected in boxes |
Underestimation (slight) of the growth of the hazards and histamine accumulation when t/T profiles come from the heat transfer modelling compared to the actual observations. Limited extent of underestimation in tubs and even lower in boxes. As a consequence, the difference in log10 increase of the hazards between boxes and tubs is expected to be slightly underestimated |
| Predictive performance of predictive models used for assessing the behaviour of pathogens and histamine formation |
The predictive models used for the assessment of the behaviour of the relevant pathogens are, as any model, dependent on the validity of its inputs, based on several important assumptions and subject to error and uncertainty associated with model fitting, experimental (e.g. plating) error of data used to fit the models and possible overestimation of microbial responses in laboratory (especially liquid, i.e. broth) media, used for collection of modelling data, as compared to the actual microbial growth on FFP The performance of the predictive models used were assessed (validated) by the model developers as discussed in Sections 2.5.1 (for survival) and 2.5.2 (for growth) Even though the approach undertaken for simulating A. hydrophila growth is not validated against independent experimental data, the gamma (growth) model used is taking into account (through the parameter μref) the impact of the factors affecting the growth of the pathogen on fish surface. Uncertainty around this model is associated with the above correction factor μref and the selected value for the minimum temperature allowing growth of A. hydrophila. These model parameters are also strain dependent (see next uncertainty source) |
Over/underestimation (slight) of the temperature‐dependent survival or growth behaviour of V. parahaemolyticus and L. monocytogenes, associated with the accuracy of the predictive model. Consequently, it is expected that the difference between tubs and boxes regarding the log10 decrease (V. parahaemolyticus) and log10 increase (L. monocytogenes) can be under/overestimated to a limited extend The slight fail‐safe predictions associated with the histamine formation model is expected to cause a slight overestimation of the temperature‐dependent histamine accumulation both in boxes and tubs. Consequently, an overestimation of the difference of the growth potential between boxes and tubs can be expected A considerable overestimation of the non‐proteolytic Cl. botulinum growth is expected as the model was built from laboratory broth data and also due to the fact that growth rate within the temperature interval from 3.3 to 4°C, was assumed to be equal to the growth rate at 4°C. However, the predicted absolute growth is very low in both boxes and tup and thus, the impact of this uncertainty source on the actual log10 differences between tubs and boxes is very limited Over/underestimation (slight) of the temperature‐dependent A. hydrophila growth depending on the actual value of the model parameters (T min and μ ref). As a consequence, the difference in the log10 increase between boxes and tubs is expected to be slightly over/underestimated |
| Lag time duration |
Pathogens in fish coming from on‐board were assumed to be adapted to cold temperatures and fish characteristics upon arrival at the first establishment on‐land. Therefore, simulations were performed without lag More details on how lag time was to be addressed are provided in Section 2.5.2 |
When no lag time is included, an overestimation of the growth potential and histamine formation may occur and consequently causing an overestimation of the differences in the log10 increase. Including lag time, in terms of different potential physiological states of cells on fish (but common in the two types of containers), reduces the differences in log10 increase between tubs and boxes |
| Strain variability |
Different strains of each type of hazard could contaminate the FFP, the behaviour of other strains could be different from the ones with available scientific data and predictive models Strain variability is reflected on variability in the minimum temperature allowing growth and in the reference growth rate (μref) or the optimal growth rate (μopt, also used in similar approaches in the same context) of the hazards on the fish. As such, it may impact the overall temperature dependence of the growth of hazards |
Over/underestimation. The impact of strain variability may be high on absolute log10 increase, but it will be rather similar on both types of containers. The difference in the log10 increase between boxes and tubs will also be over/underestimated to an unknown extend |
| Susceptibility of fish to microbial growth |
Variability in the texture, roughness and softness of the fish surface and underneath tissues Intestinal leakage is expected to deliver more nutrients thus, favouring growth of hazards, and reduce the growth of anaerobes, but both situations can be counteracted by the simultaneous release of high populations of commensal gut microbiota on fish surface (where hazards reside) that may outcompete the hazards. Differences between whole fish with skin vs. gutted fish vs. filleted fish (flesh totally exposed) were not considered Fish structure damage due to ice (in boxes) could be lower when stored in water with ice (in tubs) |
Over/underestimation The impact of mechanical (texture) properties of the fish surface may be high on absolute log10 increase, but it will be rather similar on both types of containers. The difference in the log10 increase between boxes and tubs will also be over/underestimated to an unknown extend |
| Internalisation/intestine habitat (oxygen availability, temperature, pH…) |
Oxygen availability decreases to some extent, in the direction from the surface to the interior. More microaerophilic or anaerobic conditions are expected in the intestines) Intestines provide a rather different environment (physico‐chemical characteristics, microbial competition, etc.) than the one described by the input values used for the simulation of the growth behaviour. The growth of strict anaerobes such as Cl. botulinum was simulated assuming no oxygen dissolved in the water or in the internal parts of the fish |
Overestimation of the growth of aerobic biological hazards, as the growth was simulated under aerobic conditions. The difference in the log10 increase between boxes and tubs will also be overestimated to an unknown extend |
| Background microbiota |
The assessment assumed a high microbiological quality of the fish to minimise the impact of background microbiota due to microbial interaction mechanisms Some spoilage (mainly aerobic) organisms, such as Pseudomonas spp. have also been reported to favour growth of some pathogens, such as L. monocytogenes when reaching counts of > 5 log10 CFU/g (Marshall et al., 1992; Zhang et al., 2015b) Initial levels of histamine‐producing bacteria could be lower (as discussed in Section 3.4.3) A variable (in quality and quantity) composition of background microbiota can compete with pathogens and limit pathogen growth, to an uncertain variable extend Specific spoilage organism may cause product spoilage before the hazardous concentration of pathogen could be reached |
Overestimation of the pathogen growth potential equally in tubs and boxes (though to a variable and perhaps different extend). Underestimation of the L. monocytogenes growth. The underestimation would equally affect both boxes and tubs, with limited impact as the scenario is not foreseeable, due to the short time period of the assessment and the low temperatures, that (when considered in combination) do not allow pseudomonads to reach high levels required to stimulate L. monocytogenes. Thus, the underestimation of the final output (difference in the log10 decrease between tubs and boxes) is expected to be very low Overestimation of the predicted histamine accumulation, and thus the differences between tubs and boxes Overestimation of the impact of the growth of pathogen This is not reasonably foreseen, due to the short time period of the assessment |
| Storage time (assessment time) | The duration of the storage and transport in the tub/box on‐land would be constrained by the time of on‐board transport and in the first on‐land establishment. It was considered that the total duration of the storage/transport on‐land would be exceptionally maximum 5 days, though 3, 2 and 1 day were considered reasonable (e.g. 97% of the cases from 61 surveyed fish transport in Belgium, 3% remaining consist of 4 days of storage) | Overestimation of the impact of the growth of pathogen and the difference in the growth potential between the storage in boxes or tubs associated with the longest storage times, rarely occurring |
The sources of uncertainty identified in the assessment of the behaviour of relevant biological hazards are mainly associated with the assumption that temperature is the only quantitative factor determining the survival or growth of biological hazards (including histamine accumulation).
In this context, the occurrence of water in tubs, which is not present in boxes, introduces a source of uncertainty on the bacterial behaviour, which could not quantified and is expected to overestimate the growth of the hazards in tubs but not in boxes. Therefore, this uncertainty causes an overestimation of the difference in the log10 increase of the relevant hazards.
As the temperature of ‘reasonable foreseeable abusive’ conditions tend to overestimate the fish surface temperature both in boxes and tubs, the growth potential of the hazards is also expected to be overestimated. Any factor favouring faster, or more, growth of the biological hazards occurring in boxes and in tubs will maximise the differences in the growth potential between boxes and tubs associated with the impact of the temperature. Therefore, the uncertainties expected to cause an overestimation of the temperature‐dependent growth of the biological hazards (including histamine accumulation) both in tubs and boxes are also expected to cause an overestimation of the difference of the growth potential between the two types of containers.
The use of predictive microbiology models is subjected to errors and uncertainties. They are applied using a limited number of input factors, the value of which have been set from a conservative point of view, i.e. leading to a faster, or more, growth or histamine formation (no lag time, high initial concentration of histamine forming bacteria, no microbial interactions, etc.). These assumptions cause an overestimation of the growth potential of the hazards.
3.4.6. Concluding remarks
Concluding remarks related to survival of relevant hazards
The extensive literature review provided evidence that the viability of pathogenic E. coli, Salmonella spp., St. aureus and Vibrio spp. will not change or be reduced during the storage of FFP at chill temperatures (< 7°C). Under the conditions of the current assessment (including pH and WPS of FFP as well as storage temperature and time), reduction of mesophilic pathogens such as pathogenic E. coli, Salmonella spp. and St. aureus is either not likely or not temperature dependent. As the comparison of the survival of hazards on FFP when transported/stored in boxes or tubs was based on the effect of temperature only, this comparison is not applicable for the mesophilic pathogens.
The survival of V. parahaemolyticus on FFP is temperature dependent. Based on the available predictive model and the specific assumptions applied, the t/T profiles associated with the transport/storage of FFP in boxes and tubs caused minimal differences in the reduction of V. parahaemolyticus between the two containers, being < 0.007 log10 in all the scenarios assessed. It is not possible to provide such assessment on the survival of pathogenic Vibrio ssp. other than V. parahaemolyticus, though a reduction of other pathogenic Vibrio spp. at a variable extent is expected at the temperature conditions of the current assessment.
Transport/storage of FFP in either type of container will have a minimal effect on the viability of Anisakis spp. as freezing temperatures are needed to cause a relevant inactivation of this parasite.
Concluding remarks related to growth of relevant hazards and histamine formation
The extensive literature review provided evidence that FFP under chilling conditions supports the growth of Aeromonas spp., non‐proteolytic Cl. botulinum, L. monocytogenes and histamine‐forming bacteria, such as Enterobacter spp. Photobacterium spp. and M. psychrotolerans.
Under the ‘ideal’ scenario,16 there will be no difference in the growth potential of A. hydrophila, non‐proteolytic Cl. botulinum and L. monocytogenes, or in the histamine accumulation of FFP when transported/stored in tubs as compared to boxes as the fish temperature will be equal to 0°C in any case.
The growth potential estimated from the ‘observed’ scenario t/T profiles (consisting of a short initial cooling of a small lean fish) confirmed that, in general, higher temperatures were recorded in tubs compared to boxes, thus faster growth could be expected in tubs, the difference being up to from −0.11 to 0.25 log10 units for A. hydrophila and from −0.01 to 0.48 log10 for L. monocytogenes, while no growth of non‐proteolytic Cl. botulinum could be possible as the temperatures were below the Tmin.
-
Under reasonable foreseeable ‘abusive’ scenarios17 of fish surface temperature predicted through heat transfer modelling, and considering the two processes, different types of fish based on the fat content (lean vs. fat) and size (small vs. medium)18 and location of the fish within the container, the following conclusions apply:
-
o
Greater differences, comparing both containers, in the growth potential of the relevant hazards, including histamine accumulation, between containers, were estimated in the ‘cooling‐keeping’ process compared to the ‘keeping’ process, which can be explained by higher microbial growth at the higher temperatures throughout the cooling combined with the poorer cooling capacity of water with ice (in tubs) compared to ice (in boxes).
-
o
The predicted growth potential of A. hydrophila for ‘cooling‐keeping’ of FFP for 3 days was up to 1 log10 units higher in tubs compared to boxes. Similar growth potential differences were predicted for 5 days. By contrast, the growth of A. hydrophila in FFP ‘kept’ in tubs was up to 0.08 log10 units (3 days) and 0.12 log10 units (5 days) higher than in boxes.
-
o
The predicted growth potential of L. monocytogenes and non‐proteolytic Cl. botulinum in FFP in the ‘cooling‐keeping’ process for 3 days was up to 0.5 log10 units higher in tubs compared to boxes. Similar growth potential differences were predicted for 5 days. In contrast, the growth of L. monocytogenes in FFP ‘kept’ in tubs was up to 0.2 log10 units (3 days) and 0.3 log10 units (5 days) higher than in boxes, and for non‐proteolytic Cl. botulinum, it was up to 0.09 log10 units (3 days) and 0.12 log10 units (5 days) higher than in boxes.
-
o
The predicted histamine formation due to the growth of M. psychrotolerans when FFP are ‘cooled‐kept’ or ‘kept’ for 3 days can be up to 1.5 and 0.4 ppm higher, respectively, in tubs compared to boxes. After the exceptional maximum duration of 5 days, the maximum difference on the fish surface is 16 ppm, and thus, the limit of 100 ppm histamine as defined in Commission Regulation (EC) 2073/200510 was not reached.
-
o
It is not possible to make such conclusions regarding the temperature‐dependent growth and histamine production by Enterobacter spp. and Photobacterium spp. However, these two histamine‐forming bacteria have a lower histamine‐producing potential in comparison with the above‐mentioned M. psychrotolerans, thus they are less relevant.
-
o
The fish t/T profiles were negligibly affected by the size and fat content of the fish; consequently, the associated growth potential was only impacted to a limited extent.
-
o
The impact of WPS change on the growth potential of the identified relevant hazards due to the water uptake during the storage of FFP in tubs with water and ice is negligible.
-
o
The inclusion of lag time for the growth of the relevant hazards, including histamine‐producing M. psychrotolerans, reduces their simulated growth potential and amount of histamine accumulated compared with the simulations without lag. As a consequence, it is expected that the differences in the growth potential and histamine accumulation between boxes and tubs will also be reduced.
-
o
All simulation results are dependent on the validity of the input data and on several assumptions subjected to uncertainty. Based on a considerable number of uncertainties associated with the assessment methodology as well as the overall conservative approach applied when assessing fish temperature and the associated growth of the hazards through predictive models, the differences in the log10 increase obtained can be considered an overestimate. Underestimation is possible, but it is less likely than overestimation.
-
o
Concluding remarks related to public health impact
In principle, any condition leading to more growth (log10 increase) would potentially increase the public health risk. Nevertheless, to determine the actual risk to public health of FFP stored/transported in ice (in boxes) compared to being stored/transported in water with ice (in tubs), an exposure assessment at the consumer phase is needed starting from the first on‐land establishment until the consumption step. In addition, a DR relationship is needed for each hazard.
4. Conclusions
AQ 1: What is the reduction potential (i.e. log10 decrease) or growth potential (i.e. log10 increase) of relevant biological hazards when FFP, initially stored in freshwater or seawater/ice (in tubs) on board, are subsequently ‘handled’ (i.e. sorted or gutted and/or filleted) at the first on‐land establishment and then transferred to freshwater/ice (in three‐layered PE tubs) compared to being transferred to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days? Is there a potential increased risk for public health as a result of using tubs compared to boxes?
The relevant biological hazards in FFP, considering both their association with human illnesses linked to FFP and their potential for growth on FFP at refrigeration temperatures < 7°C are: Aeromonas spp., non‐proteolytic Cl. botulinum and L. monocytogenes.
Under an ‘ideal’ scenario considering proper practices, assuming that the initial fish temperature is 0°C and the fish is in perfect contact with ice in boxes and with a perfect mixing of water and ice in tubs, there is no difference in the growth potential of Aeromonas spp., non‐proteolytic Cl. botulinum and L. monocytogenes in FFP when transported/stored in tubs compared to boxes as FFP temperature is maintained at 0°C throughout the storage/transport.
-
Under reasonably foreseeable ‘abusive’ scenarios of the outside temperature, where temperature is mostly at 2°C but including some abusive peaks of up to 6°C assuming that in boxes, fish is surrounded by two layers of ice, and in tubs, fish is in water below an ice layer without mixing, the following are concluded:
-
o
If the initial fish temperature equals 0°C when transferring the FFP to the tub or box (referred to as ‘keeping’ process), the growth potential (log10 increase) of the relevant hazards (i.e. A. hydrophila, L. monocytogenes or non‐proteolytic Cl. botulinum) is up to 0.12 log10 units, 0.17 log10 units and 0.27 log10 units higher in tubs than in boxes after 2 days, 3 days and 5 days, respectively.
-
o
If the initial fish temperature equals 7°C when transferring the FFP to a tub or box (referred to as ‘cooling‐keeping’ process), the difference in the growth potential (log10 increase) of the relevant hazards between tubs and boxes is of higher magnitude as compared to the ‘keeping’ process as follows:
-
■
Growth of A. hydrophila, L. monocytogenes and non‐proteolytic Cl. botulinum is up to 1 log10 units, 0.5 log10 units and 0.5 log10 units higher in tubs than in boxes, respectively, after 3 days of storage/transport.
-
■
an exceptional duration of the storage/transport of 5 days would result in a limited additional increase (≤ 0.1 log10) of the differences in the growth potential of the relevant hazards between boxes and tubs.
-
■
the higher impact of temperature conditions on hazard relative growth in tubs than in boxes is mainly a result of the poorer cooling capacity of water with ice (in tubs) compared to ice (in boxes) under the modelled conditions.
-
■
-
o
The t/T profile and the associated growth potential are only slightly affected by the size (e.g. small flat fish such as plaice vs. bigger with a broad oval cross section fish such as salmon) and fat content (1–4% vs. 10–20%) of the fish.
-
o
The location of the fish within the container impacted the t/T profile and the associated microbial growth, with fish located in positions more distant from the ice (the centre between ice layers in a box and the bottom of the tub) and closer to the walls of the container being exposed to the highest temperatures and thus representing the warmest spots (worst case).
-
o
Due to the overall conservative modelling approaches and assumptions applied, the obtained differences in log10 increase between boxes and tubs would be most likely an overestimate of the differences that may occur in reality. For instance, the t/T profiles of the ‘observed’ scenarios and the estimated difference of the growth potential of the relevant hazards between boxes and tubs are below the log10 increases under the ‘abusive’ scenario. Underestimation is possible, but it is less likely than overestimation.
-
•
The relevant biological hazards in FFP, considering both their association with human illnesses linked to FFP and their potential for survival (no change or reduction of their concentration) on FFP at refrigeration temperatures (< 7°C) are: pathogenic E. coli, Salmonella, St. aureus, Vibrio spp. and Nematodes (Anisakis spp.).
-
•
Under the conditions of the assessment based on the fish t/T profiles that may occur under reasonably foreseeable ‘abusive’ conditions of transport/storage of FFP, no substantial differences in the magnitude of reduction of pathogens between boxes and tubs are expected.
-
•
In principle, any condition leading to more growth (log10 increase) would potentially increase the public health risk. Nevertheless, to determine the actual risk to public health when using tubs compared to boxes, a QMRA would be needed, including an exposure assessment that would take into account the cumulative impact of subsequent steps of the FFP supply chain, including the consumer handling and consumption habits as well as the DR relationship for each relevant hazard on the consumed dose.
-
o
AQ 2: What is the magnitude of histamine accumulation in fish species associated with a high amount of histidine when FFP, initially stored in freshwater or seawater/ice (in tubs) on board, are then ‘handled’ (i.e. sorted or gutted and/or filleted) at the first on‐land establishment before being transferred to freshwater/ice (in three‐layered PE tubs) compared to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days? Is there a potential increased risk for public health as a result of using tubs compared to boxes?
The relevant biological hazards in FFP for histamine production, considering both their association with human illnesses (histamine intoxication) and potential for histamine production at refrigeration temperatures (< 7°C), are: Enterobacter spp., Morganella spp. and Photobacterium spp. with M. psychrotolerans being one of the most prolific producers of histamine‐producing bacteria under the chill conditions of the current assessment.
Under the ‘ideal’ scenario, there is no difference in the growth potential of M. psychrotolerans on FFP and in histamine accumulation when FFP is transported/stored in tubs compared to boxes.
Under reasonably foreseeable ‘abusive’ scenarios, when the initial fish temperature at transfer to the tub or box is equal to either 0°C (referred to as ‘keeping process’) or 7°C (‘cooling‐keeping’ process), the histamine formation due to the growth of M. psychrotolerans can be up to 0.4 and 1.5 ppm higher, respectively, in tubs as compared to boxes after 3 days. After the exceptional maximum duration of 5 days, the maximum difference of histamine accumulation between tubs and boxes is 16 ppm. The limit of 100 ppm histamine as defined in Commission Regulation (EC) 2073/200510 is never reached.
Due to the overall conservative modelling approaches and assumptions applied, the obtained differences in the histamine accumulation between boxes and tubs would be an overestimate of the differences that may occur in reality. For instance, the t/T profiles of the ‘observed’ scenarios and the estimated difference of histamine accumulation by M. psychrotolerans between boxes and tubs are below what is reported for the ‘abusive’ scenario. Underestimation is possible, but it is less likely than overestimation.
In principle, any condition leading to more accumulation of histamine would potentially increase the public health risk depending on the predetermined level of extra risk selected as the threshold of the benchmark dose used. Nevertheless, to determine the actual risk to public health of FFP stored/transported in ice (in boxes) compared to being stored/transported in water with ice (in tubs), a full QMRA would be needed, including an assessment of the exposure to histamine due to the consumption of FFP or products thereof taking into account subsequent steps in the FFP supply chain, including the consumer practices and consumption habits as well as a dose–response relationship for histamine.
AQ 3: What is the contribution of the change of the water content of the fish meat on previous AQs outcomes when FFP, first stored in freshwater or seawater/ice (in tubs) on board, are ‘handled’ at the first on‐land establishment and then transferred to freshwater/ice (in three‐layered PE tubs) compared to ice (in HDPE boxes) for further transport and storage on‐land for a maximum duration of 3 days with an exceptional maximum duration of 5 days?
The water content of FFP stored/transported in tubs with fresh water and ice may increase from 0% to 6%, causing a reduction of the water phase salt concentration (WPS, %) ranging from 0 to 0.019 units in comparison with the FFP transferred to boxes.
The foreseeable decrease in WPS has a negligible impact on the growth rate of all identified biological hazards. Consequently, there is a negligible difference in the growth potential of relevant pathogens, and on the histamine formation in FFP stored/transported in tubs compared to boxes. Consequently, the water uptake associated with the storage of the fish in tubs does not make a relevant contribution to the outcome of the previous AQs.
5. Recommendations
Recommendations for the sector for limiting the growth of pathogens when using tubs filled with water and ice for the transport/storage of FFP include:
The use of clean and undamaged tubs made of insulating material and with a lid, and precooled before filling with fish/water/ice.
The use of sufficient water having a temperature as close to 0°C as possible to cover all the fish.
The use of enough ice on top to cover the whole surface of the water within tubs, making sure that all fish is below the ice layer.
The use of the right proportion of fish/water/ice to ensure proper cooling (if initial fish temperature is higher than 0°C), and to ensure that enough ice is present during the whole storage/transport duration (or re‐ice properly) and reduce pressure damage to the fish.
The circulation of water inside the tubs to achieve uniform temperatures within the container.
The transport and storage of the tubs (as for boxes) in a cool environment and for less than 5 days (absolute maximum).
Abbreviations
- AIEC
Adherent Invasive E. coli
- BHI
Brain Heart Infusion
- BoNTs
botulinum neurotoxins
- CSW
chilled sea water
- DAEC
Diffusely Adherent E. coli
- DR
dose‐response
- EAEC
Enteroaggregative E. coli
- EHEC
Enterohaemorrhagic E. coli
- EIEC
Enteroinvasive E. coli
- EPEC
Enteropathogenic E. coli
- ETEC
Enterotoxigenic E. coli
- FBO
food business operators
- FFP
fresh fishery products
- FSC
food safety criteria
- FSMS
food safety management systems
- FSSP
Food Spoilage and Safety Predictor
- HDPE
high density poly‐ethylene
- HAV
hepatitis A virus
- HEV
hepatitis E virus
- NoV
Norovirus
- PE
poly‐ethylene
- PSU
practical salinity units
- QMRA
quantitative microbial risk assessment
- RASFF
Rapid Alert System for Food and Feed
- RSW
refrigerated seawater
- RTE
ready‐to‐eat
- STEC
Shiga toxin‐producing E. coli
- t/T
time‐Temperature
- VBNC
viable but nonculturable
- WPS
water phase salt
Appendix A – Search strategies and outcome of the literature searches supporting the hazard identification
1.
1.1.
1.1.1.
1.1.1.1. Literature search on human illness associated with the hazards in fishery products
Two literature searches in the Web of Science™ Core Collection (1975–present). The search strategies are reported in Tables A.1 and A.2. The first search was conducted on 16 and 22 July 2019 and on 27 September 2019 (for Yersinia). The second search was conducted between 7 and 21 August 2019.
The first literature search collected information on the evidence of hazards potentially present in seawater and/or freshwater fishery products (including fishes, crustacea, cephalopods and urchins) to cause human illness (i.e. report of human cases or outbreaks) associated with fishery products. Only hazards for which evidence of association to human cases or outbreaks was not already available for previous scientific report (EFSA, 2015) were included in the search. For these hazards, further evidence was retrieved through non‐systematic literature review.
The second literature search, performed on the hazards remaining after screening for evidence of association with human cases/outbreaks, aimed to collect information on their ability for growth and/or survival/persistence/inactivation considering a temperature range (−3°C to 7°C) encompassing temperatures of ice and tub transport. Other physiochemical parameters (as aw or pH) were not considered at screening level.
Table A.1.
Details of search strings used for literature searches on human illness associated with the hazards in fishery products using Web of Science™ Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TOPIC: (fish OR fishes OR “fishery product” OR “fishery products” OR seafood OR seafoods OR albacore OR amberjack OR anchovy OR angler OR argentine OR bacha OR barbel OR barracuda OR basa OR bass OR “sea bass” OR beluga OR bib OR bigeye OR blackfish OR bleak OR blenny OR bluefish OR “blue runner” OR “blue shark” OR bonito OR branzino OR bream OR seabream OR “sea bream” OR brill OR burbot OR butterfish OR carp OR catfish OR catshark OR chub OR cod OR comber OR conger OR corb OR cutlassfish OR dab OR “danubian wels” OR dentex OR dogfish OR eel OR emperor OR flathead OR flounder OR “flying fish” OR forkbeard OR garfish OR garrick OR goby OR goldline OR grouper OR guitarfish OR gunard OR haddock OR hake OR halibut OR hammerhead OR herring OR hoki OR huss OR icefish OR “John dory” OR lamprey OR lanternfish OR leerfish OR ling OR “little tunny” OR lythe OR mackerel OR “mahi mahi” OR marlin OR megrim OR melva OR monkfish OR moonfish OR mullet OR needlefish OR oreo OR pacu OR pandoras OR panga OR pangasius OR parrotfish OR “parrot fish” OR perch OR picarel OR pike OR pilchard OR pilotfish OR “pilot fish” OR plaice OR pollan OR Pollack OR Pollock OR ponyfish OR porbeagle OR pout OR ray OR ribbonfish OR rigg OR rockfish OR rosefish OR sablefish OR sailfish OR salmon OR sandeel OR sardine OR sardinella OR scabbardfish OR scorpionfish OR sheatfish OR “shi drum” OR sild OR sillago OR skipjack OR smelt OR smooth hound OR “smooth‐hound” OR snapper OR snook OR sole OR sparling OR spearfish OR “St Peter's fish” OR stargazer OR stingray OR sturgeon OR “surgeon fish” OR swordfish OR tailor OR tench OR tilapia OR threadfin OR triggerfish OR trout OR tubefish OR tuna OR turbot OR tusk OR walleye OR weever OR whitebait OR whiting OR wrasse OR yellowtail OR octopus OR squid OR crab OR lobster OR prawn OR shrimp OR cuttlefish OR crayfish OR langoustine OR scampi OR urchin) | 2,472,592 a |
| #2 | TOPIC: (Outbreak OR Outbreaks OR ((Human OR Humans) NEAR/2 (Case OR Cases OR Disease OR Diseases OR Illness OR Illnesses OR Health OR Risk OR Risks)) OR (Public NEAR/2 Health) OR (Histamin* NEAR/2 (Intoxication* OR Poison*)) OR ((Scombroid OR Scombrotoxin*) NEAR/2 (Poison* OR Intoxication*)) OR “Risk Profile” OR “Risk Ranking”) | 544,273 a |
| #3 | TOPIC: (Outbreak OR Outbreaks OR ((Human OR Humans) NEAR/2 (Case OR Cases OR Disease OR Diseases OR Illness OR Illnesses OR Health OR Risk OR Risks)) OR (Public NEAR/2 Health) OR “Risk Profile” OR “Risk Ranking”) | 544,009 a |
| TOPIC: (Aeromonas) AND #1 AND #2 | 439 b | |
| TOPIC: (Citrobacter) AND #1 AND #2 | 18 b | |
| TOPIC: (Hafnia) AND #1 AND #2 | 12 b | |
| TOPIC: (Klebsiella) AND #1 AND #2 | 75 b | |
| TOPIC: (Proteus) AND #1 AND #2 | 23 b | |
| TOPIC: (Providencia) AND #1 AND #2 | 4 b | |
| TOPIC: (Pseudomonas) AND #1 AND #2 | 257 b | |
| TOPIC: (Raoultella) AND #1 AND #2 | 16 b | |
| TOPIC: (Serratia) AND #1 AND #2 | 25 b | |
| TOPIC: (Staphylococcus) AND #1 AND #2 | 318 b | |
| TOPIC: (Vibrio) AND #1 AND #2 | 927 b | |
| TOPIC: (Yersinia) AND #1 AND #2 | 132 b) | |
| TOPIC: (Campylobacter) AND #1 AND #3 | 75 b | |
| TOPIC: (Clostridium) AND #1 AND #3 | 154 b | |
| TOPIC: (Plesiomonas) AND #1 AND #3 | 27 b | |
| TOPIC: (Shigella) AND #1 AND #3 | 46 b | |
| TOPIC: (Norovirus OR (hepatitis NEAR/1 virus) OR HAV OR (enteric NEAR/1 virus) AND #1 AND #3 | 198 b | |
| TOPIC: (cryptosporidium) AND #1 AND #3 | 79 b | |
| TOPIC: (toxoplasma) AND #1 AND #3 | 36 b |
DocType = All document types; Language = All languages; Timespan = All years.
DocType = All document types; Language = English; Timespan = All years.
Screening at title level:
Question: does the record contains info about human illness related to the consumption of fish, fish fillets or other fishery products with the exception of bivalve shellfish for the selected hazard?
Reply:
-
•
Yes: go to abstract screening
-
•
No: exclude
-
•
Unclear: go to abstract screening
Screening at abstract level:
Question 1: does the record report on the selected hazard?
Reply to question 1:
-
•
Yes: go to next question
-
•
No: exclude
-
•
Unclear: go to next question
Question 2:
(all but Cl. botulinum) does the record report on human illnesses related to consumption of fish, fish fillets or other fishery products with the exception of bivalve shellfish that has not been transformed (so excluded: smoked, marinated, canned)
(Cl. botulinum) does the record report on human illnesses related to consumption of fish, fish fillets or other fishery products with the exception of bivalve shellfish apart from those canned
Reply to question 2:
-
•
Yes: go to full text screening
-
•
No: exclude
-
•
Unclear: go to full text screening
Screening at full text level:
Question 1: does the record report on the selected hazard?
Reply to question 1:
-
•
Yes: go to next question
-
•
No: exclude
Question 2:
(all but Cl. botulinum) does the record report on human illness related to consumption of fish, fish fillets or other fishery products with the exception of bivalve shellfish that has not been transformed (so excluded: smoked, marinated, canned)
(Cl. botulinum) does the record report on human illness related to consumption of fish, fish fillets or other fishery products with the exception of bivalve shellfish apart from those canned
Reply to question 2:
-
•
Yes: include
-
•
No: exclude
If another reference seems relevant in the obtained records, it will be retrieved and screened.
1.1.1.2. Literature search on growth and/or survival/persistence of hazards under low temperatures
Table A.2.
Details of search strings used for literature searches on growth and/or survival/persistence of hazards under low temperatures (considered as −3°C to 7°C) using Web of Science™ Core Collection (1975–present)
| Set number | Search | No of records |
|---|---|---|
| #1 | TOPIC: (fish OR fishes OR “fishery product” OR “fishery products” OR seafood OR seafoods OR albacore OR amberjack OR anchovy OR angler OR argentine OR bacha OR barbel OR barracuda OR basa OR bass OR “sea bass” OR beluga OR bib OR bigeye OR blackfish OR bleak OR blenny OR bluefish OR “blue runner” OR “blue shark” OR bonito OR branzino OR bream OR seabream OR “sea bream” OR brill OR burbot OR butterfish OR carp OR catfish OR catshark OR chub OR cod OR comber OR conger OR corb OR cutlassfish OR dab OR “danubian wels” OR dentex OR dogfish OR eel OR emperor OR flathead OR flounder OR “flying fish” OR forkbeard OR garfish OR garrick OR goby OR goldline OR grouper OR guitarfish OR gunard OR haddock OR hake OR halibut OR hammerhead OR herring OR hoki OR huss OR icefish OR “John dory” OR lamprey OR lanternfish OR leerfish OR ling OR “little tunny” OR lythe OR mackerel OR “mahi mahi” OR marlin OR megrim OR melva OR monkfish OR moonfish OR mullet OR needlefish OR oreo OR pacu OR pandoras OR panga OR pangasius OR parrotfish OR “parrot fish” OR perch OR picarel OR pike OR pilchard OR pilotfish OR “pilot fish” OR plaice OR pollan OR Pollack OR Pollock OR ponyfish OR porbeagle OR pout OR ray OR ribbonfish OR rigg OR rockfish OR rosefish OR sablefish OR sailfish OR salmon OR sandeel OR sardine OR sardinella OR scabbardfish OR scorpionfish OR sheatfish OR “shi drum” OR sild OR sillago OR skipjack OR smelt OR smooth hound OR “smooth‐hound” OR snapper OR snook OR sole OR sparling OR spearfish OR “St Peter's fish” OR stargazer OR stingray OR sturgeon OR “surgeon fish” OR swordfish OR tailor OR tench OR tilapia OR threadfin OR triggerfish OR trout OR tubefish OR tuna OR turbot OR tusk OR walleye OR weever OR whitebait OR whiting OR wrasse OR yellowtail OR octopus OR squid OR crab OR lobster OR prawn OR shrimp OR cuttlefish OR crayfish OR langoustine OR scampi OR urchin) | 2,395,527 a |
| #2 | TOPIC: (Surviv* OR Persist* OR viability OR viable or “bacterial growth” OR “bacterial increase” OR “Growth Rate” or “kinetic model*” OR “growth model*” OR “bacterial decrease” OR “Inactivation rate” OR “inactivation model*” OR “nonthermal inactivation” OR “nonthermal reduction” OR “non‐thermal inactivation” OR “non‐thermal reduction” or “bacterial injury” or “bacterial injuries”) | 2,429,599 a |
| #3 | TOPIC: (Refrigerat* OR (Cold NEAR/3 (Storage OR adaptation)) OR Cooling OR ((low OR lower) NEAR/3 temperature*) OR ice OR chill* OR psychrotroph* OR fridge NOT “ice cream”) | 1,033,160 a |
| #4 | TOPIC: (superchill* OR super‐chill* OR “super chill*” OR “partial* freez*” OR “partial* frozen” OR deepchill* OR deep‐chill* OR “deep chill*” OR subchill* OR sub‐chill* OR “sub chill*” OR supercool* OR super‐cool* OR “super cool*”) | 24,202 a |
| #5 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Aeromonas | 27 |
| #6 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Bacillus | 10 |
| #7 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Clostridium | 11 |
| #8 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Enterobacter | 7 |
| #9 | TOPIC: #1 AND #2 AND (#3 OR #4) AND (Escherichia coli OR “E. coli” OR “E coli” OR STEC OR EHEC) | 17 |
| #10 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Hafnia | 2 |
| #11 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Klebsiella | 0 |
| #12 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Listeria | 93 |
| #13 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Morganella | 5 |
| #14 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Photobacterium | 19 |
| #15 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Plesiomonas | 1 |
| #16 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Raoultella | 1 |
| #17 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Shigella | 0 |
| #18 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Serratia | 3 |
| #19 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Staphylococcus | 21 |
| #20 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Vibrio | 35 |
| #21 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Salmonella | 77 |
| #22 | TOPIC: #1 AND #2 AND (#3 OR #4) AND (Norovirus OR (hepatitis NEAR/1 virus) OR HAV OR (enteric NEAR/1 virus) | 0 |
| #23 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Giardia | 0 |
| #24 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Cryptosporidium | 0 |
| #25 | TOPIC: #1 AND #2 AND (#3 OR #4) AND Toxoplasma | 1 |
| #26 | TOPIC: #1 AND #2 AND (#3 OR #4) AND (trematode* OR opistorchis OR chlonorchis OR paragonimus) | 0 |
| #27 | TOPIC: #1 AND #2 AND (#3 OR #4) AND (Cestodes OR Diphyllobothrium) | 0 |
| #28 | TOPIC: #1 AND #2 AND (#3 OR #4) AND (Anisaki* OR Pseudoterranova) | 6 |
DocType = All document types; Language = All languages; Timespan = All years.
DocType = All document types; Language = English; Timespan = All years.
Screening at title level:
Question: does the record report on growth or survival of the selected hazard in (sea)food products at low temperatures (e.g. below 10°C)
Reply:
-
•
Yes: go to abstract screening
-
•
No: exclude
-
•
Unclear: go to abstract screening
Screening at abstract level:
Question 1: does the record report on the selected hazard?
Reply to question 1:
-
•
Yes: go to next question
-
•
No (*): exclude
-
•
Unclear: go to next question
(*) Includes records reporting ‘no detection’ of the hazard.
Question 2: does the record report (either in the test samples or in the experimental controls) on growth or survival of the hazard in a temperature range encompassing −3°C to 7°C
Reply to question 2:
-
•
Yes: go to full text screening
-
•
No: exclude
-
•
Unclear: go to full text screening
Screening at full text level:
Question 1: does the record report on the selected hazard?
Reply to question 1:
-
•
Yes: go to next question
-
•
No: exclude
Question 2: does the record report on growth or survival of the hazard in raw (fresh or defrosted) fish, fish fillets or other fishery products (i.e. not smoked, salted, marinated, cooked, etc.)
Reply to question 2:
-
•
Yes: go to next question
-
•
No: exclude
Question 3: does the record report on growth or survival of the hazard under aerobic conditions?
Reply to question 3:
-
•
Yes: go to question 5
-
•
No: go to question 4
Question 4: does the record report on growth or survival of the hazard under anaerobic conditions (e.g. vacuum packaging)?
Reply to question 4:
-
•
Yes: go to question 5
-
•
No: exclude
Question 5: does the record report on growth or survival of the hazard in a temperature range encompassing −3°C to 7°C?
Reply to question 5:
-
•
Yes: retain
-
•
No: exclude
1.1.1.3. Background information on selected hazards
Aeromonas
Aeromonas are Gram‐negative, facultative anaerobic bacteria that are ubiquitous and widely occurring in aquatic environments (Janda and Abbott, 2010), whose first report of association with gastroenteritis dates back to the 1960s (Rosner, 1964). While the role of Aeromonas spp. as food‐borne pathogens has been controversial for a long time, four species are clearly associated with human disease: A. hydrophila, A. cavie, A. veronii and A. dhakensis, the latter a reclassification of A. hydrophila subsp. Dhakensis and A. aquariorum (Beaz‐Hidalgo et al., 2013). Clinical manifestations of Aeromonas infection include mainly gastroenteritis, but wound and soft tissues infections, bacteraemia, septicaemia, respiratory and urinary tract infections have also been documented (reviewed in Janda and Abbott (2010)). The Aeromonas genus includes both mesophilic and psychrotrophic species, with optimum growth temperatures of 35–37°C and 22–28°C, respectively (Isonhood and Drake, 2002), and an extended temperature range for certain strains of 0–45°C (ICMSF, 1996). Growth at refrigeration temperature, indeed, has been reported for several strains, including A. hydrophila (Palumbo et al., 1985; Davies and Slade, 1995; De Silvestri et al., 2018), making aeromonads significant microorganisms in refrigerated foods. Detection of Aeromonas species in seafood is a common occurrence, its presence being reported in 71% of retail sushi boxes from Norwegian supermarkets (Hoel et al., 2015), 65%, 77%, 25% and 25% of fresh fish from commercial outlets in France, UK, Portugal and Greece (Davies et al., 2001), and 40%, 93%, 20% and 72% of seafood in studies at retail conducted in Spain, Finland, Germany and Belgium, respectively (Pin et al., 1994; Hänninen et al., 1997; Neyts et al., 2000; Ullmann et al., 2005; Carrascosa et al., 2014).
Clostridium botulinum
Botulism is a paralytic disease derived by the action of botulinum neurotoxins (BoNTs), either as a result of the growth of Cl. botulinum in food (food‐borne botulism, involving preformed toxins) or from bacterial germination within the human organism (wound and infant botulism). Symptoms typically include descending flaccid paralysis, aphasia, diplopia and difficulties in swallowing. Botulism occurs rarely in the EU, with ~ 100 cases reported to ECDC annually, largely from Italy, Romania and Poland (ECDC website18). Seven BoNTs serotypes are recognised (A, B, C1, D, E, F and G), with type A, B, E and F involved in human botulism, and type C and D mostly affecting animals. BoNTs are recognised as the most acutely toxic substances known for humans and vertebrate animals (Gill, 1982). The spores of Cl. botulinum are ubiquitous in the environment, though types A and B are generally found on land, while type E is the most common type in water environments, including marine waters, and is often isolated from fishery products and aquatic animals. Three surveys on fish from farms in Nordic countries, for instance, detected type E Cl. botulinum in 0%, 15% and 10–40% of samples, respectively (Hielm et al., 1996, 1998; Hyytiä‐Trees et al., 1999), while the prevalence was 30% in a Bavarian study (Hyytiä‐Trees et al., 1999) and 0% in a EU study conducted in France, UK, Greece and Portugal (Davies et al., 2001). Further to toxin type, Cl. botulinum is divided according to its phenotypic and metabolic properties in four groups. Group I comprises the proteolytic strains of types A, B and F, that use amino acids as carbon and energy sources during growth, and group II includes non‐proteolytic strains of type B and F, and type E strains, whose growth is supported through carbohydrates. Proteolytic and non‐proteolytic Cl. botulinum differ substantially in their growth properties; proteolytic strains being more heat resistant and showing growth in the temperature range between 10°C and 48°C, and non‐proteolytic strains having higher cold tolerance, with a minimum growth temperature of 3.3°C (ICMSF, 1996). At this temperature growth and BoNTs formation take place slowly and several weeks may be required before reaching concentrations that are lethal in mouse bioassay (Betts and Gaze, 1995); however, due to this potential for growth, a combination of growth barriers should be applied together with refrigeration to ensure safety. Several botulism episodes are reported following seafood consumption, particularly in association with traditional dishes, including Norwegian ‘rakfish’ (Skåra et al., 2015), the ‘stinky heads’ and ‘stinky eggs’ of the Canadian Arctic region (Horowitz, 2010; Leclair et al., 2013) or recipes using uneviscerated fish as ‘kapchunka’ (Badhey et al., 1986; Telzak et al., 1990) and ‘fasheik’ (Weber et al., 1993; Sobel et al., 2007; Walton et al., 2014), in which the incorrect preparation may lead to the development of anaerobic conditions supporting Cl. botulinum growth.
Listeria monocytogenes
Listeria monocytogens are Gram‐positive, facultative anaerobic bacteria, that are geographically widespread and ubiquitous in the environment. Soil and water are the primary sources for transmission of L. monocytogenes to raw materials from primary production, animals and to the food chain (Linke et al., 2014). Human listeriosis cases prevalently occur in specific high‐risk groups (elderly, children, pregnant women and immunocompromised) and include clinical manifestations such as bacteriaemia, septicaemia and meningitis and, in pregnancy, severe consequence for the fetus (granulomatous lesions, stillbirth and abortion). In 2018, a total of 2,549 confirmed cases of listeriosis were reported in EU, with a case fatality of 15.6% (EFSA and ECDC, 2019). RTE foods play an important role in human listeriosis, given the ability of some of these products to support the growth of L. monocytogenes and the potential for growth of this microorganism also under refrigerated conditions (minimum growth temperature: −0.4°C; (ICMSF, 1996)). As regard to seafood products, the occurrence of L. monocytogenes is well documented in both seawater (Bou‐m'handi and Marrakchi, 2003; El‐Shenawy and El‐Shenawy, 2006; Rodas‐Suárez et al., 2006; Beleneva, 2011) and fresh water (Stea et al., 2015; Linke et al., 2014; Wilkes et al., 2009; Lyautey et al., 2007; Arvanitidou et al., 1997), including aquatic environments for fish farming (Hansen et al., 2006). As a consequence, the presence of L. monocytogenes in FFP has been reported, with large variations, in several studies worldwide. Occurrence in finfish, for instance, ranged from null to 43.3% (reviewed in Jami et al. (2014)) being, in earlier as in more recent studies, predominantly in the interval between 0% and 10% (Yan et al., 2010; Das et al., 2013; Jami et al., 2014; Chen et al., 2015, 2018; Jamali et al., 2015; İKİZ et al., 2016; Tao et al., 2017; Pyz‐Łukasik and Paszkiewicz, 2018; Rezai et al., 2018; Li et al., 2019). Similar occurrence of L. monocytogenes was reported also in crustacean and cephalopods, in which prevalence varies between zero and 28.8%, with values often below 5% (Wang et al., 2011; Pagadala et al., 2012; Momtaz and Yadollahi, 2013; Jami et al., 2014).
Pathogenic E. coli
Escherichia coli is a facultative anaerobic, Gram‐negative, non‐spore forming bacterium of the Enterobacteriaceae family. It is part of the normal gastrointestinal flora of humans and of many warm‐blooded animals, often as a harmless commensal. Pathogenic E. coli, on the other hand, include variants causing enteric or extra‐intestinal infections. Among the first group, different pathogenicity mechanisms are recognised, on the basis of which seven E. coli pathotypes are defined: Shiga toxin‐producing E. coli (STEC), Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Enteroinvasive E. coli (EIEC), Enteroaggregative E. coli (EAEC), Diffusely Adherent E. coli (DAEC) and Adherent Invasive E. coli (AIEC). A description of STEC is provided in EFSA BIOHAZ Panel (2020). Pathogenic E. coli may be present in aquatic environments following the release in water bodies of faecal material from the natural hosts. Survival of pathogenic E. coli in the open environment depends on several factors, including temperature, pH, osmotic stress, solar radiation, nutrients availability, predation by other microorganisms, etc.; however, survival of E. coli O157:H7 after 5 days at 10°C and 15 days at 27°C was reported in seawater (Miyagi et al., 2001; Williams et al., 2007) and detection after 30 days at 10°C was described in lake and river waters (Avery et al., 2008). Fishery and fishery products contamination may arise from faecal contamination of the farming or catching areas and pathogenic E. coli (mainly STEC, but also ETEC and EPEC) have been detected in fresh fish at landing or at market in Brazil, India, South Korea, Algeria and Egypt (Sanath Kumar et al., 2001; Teophilo et al., 2002; Cardozo et al., 2012; Koo et al., 2012; Murugadas, 2016; Dib et al., 2018; Hussein et al., 2018) as well as in fish in aquaculture settings (Alagarsamy et al., 2010; Siddhnath et al., 2018), fishing ponds (Ribeiro et al., 2016) and fish droppings in aquaponic systems (Wang et al., 2020). Refrigeration temperatures are non‐permissive for pathogenic E. coli, the minimum growth temperature being 7–8°C (ICMSF, 1996); however, outbreaks associated with fish or crustacean consumption are occasionally reported (EFSA BIOHAZ Panel, 2013, 2020).
Salmonella
Salmonella are Gram‐negative bacteria responsible for acute gastroenteritis in humans. Salmonella are the most frequently reported agents of food‐borne outbreaks in EU and fish and fishery products are involved in Salmonella transmission in a minority of cases (26 strong evidence outbreaks out of 2,306 reported between 2010 and 2018 (EFSA and ECDC, 2019)). Salmonella are shed in the faeces of infected humans or in those of the wide range of animals that may host this microorganism, and may therefore reach aquatic environments through sewage discharges and land run‐offs. Survival of Salmonella in water environments largely depends on solar irradiation, temperature and salinity fluctuations, as the decimal reduction time increases at lower temperatures (i.e. 16.7 days at 6°C vs. 3.8 days at 20°C; (Wait and Sobsey, 2001)) and at lower salinity (i.e. 80 h in brackish waters vs. 54 in marine ones; (Gabutti et al., 2000)). In relation to Salmonella presence in their production environments or as a consequence of contamination during handling and processing, Salmonella detection in fish and fishery products (excluding bivalve shellfish) is not uncommon. In surveys performed in the United States, contamination was absent in crabs and fillets, but was 1.3% in fish, 1.6% in shrimps and 3.9% in crustacea (Reinhard et al., 1996; Heinitz et al., 2000; Koonse et al., 2005; Pao et al., 2008). In South‐East Asian countries and in China, Salmonella prevalence in marketed products ranges from 4.6% to 75% in shrimps, and from 3.5% to 36.6% for fish (Kamalika et al., 2008; Kumar et al., 2009; Minami et al., 2010; Banerjee et al., 2012; Woodring et al., 2012; Budiati et al., 2013; Yang et al., 2015; Zhang et al., 2015a; Nguyen et al., 2016; Sing et al., 2016; Li et al., 2019; Yen et al., 2020), though a higher prevalence can be reached depending on aquaculture systems (Budiati et al., 2013). In EU countries, the prevalence of Salmonella in raw fish and fishery products is presumably very low, this pathogen having been detected in fish and crustacea only in a large survey from Italy (10/2,965 samples, 0.3%; (Busani et al., 2005)), while prevalence has been zero in other studies conducted in France, UK, Portugal, Spain, Greece, Croatia, Poland and Latvia (Davies et al., 2001; Herrera et al., 2006; Popovic et al., 2010; Terentjeva et al., 2015; Pyz‐Łukasik and Paszkiewicz, 2018). One serovar, S. enterica Weltevreden, seems to display a stronger association with fish and fishery products, particularly in South‐East Asia and North America, while in Europe, S. Typhimurium and S. Senftenberg are more often reported in association with this food category (Ferrari et al., 2019). In the event of Salmonella contamination, temperature control is crucial to avoid their proliferation. Salmonella are mesophilic microorganisms and their growth rate is substantially reduced below 15°C. Further to this, the majority of isolates are not able to grow at temperatures below 7°C (ICMSF, 1996) and, in seafood matrices, either no change or a slight reduction of viable microorganisms has been reported in the temperature range between 0°C and 7°C (Tassou et al., 2004; Norhana et al., 2010; Provincial et al., 2013b; Kumar et al., 2015; Liu et al., 2016; Li et al., 2018; Pattanayaiying et al., 2019).
Staphylococcus aureus
Staphylococcus is a genus including small, spherical Gram‐positive bacteria that are commensal and opportunistic pathogens for humans, which colonise skin, mucosae and the nasal cavity. Beside humans, a wide range of warm‐blooded animals, including both wild animals and those of zootechnical interest, may host St. aureus. Staphylococcus aureus is environmentally resistant, surviving drying and freezing, and is salt‐tolerant, growing at an aw as low as 0.85 (ICMSF, 1996). Given its ecological distribution, seafood contamination mostly occurs as a result of cross‐contamination from carriers, particularly food‐handlers (Vazquez‐Sanchez et al., 2012), but association with quality of the rearing waters cannot be ruled out (Huss, 1994; Ismail et al., 2016). Occurrence of St. aureus in fresh fish and fishery products has been reported with variable prevalence, ranging from zero in two studies on samples taken in Croatian markets and Spanish restaurants (Popovic et al., 2010; Sospedra et al., 2015), to 1.0% in jacopevers and plaice in Korean restaurants (Yoon et al., 2016), and to 2.2% and 5% in seafood marketed in India and Iran, respectively (Singh and Kulshreshtha, 1994; Zarei et al., 2012). Detection frequency, on the other hand, reached 17–25% in other seafood types, as frozen fishery products or farmed shrimp (Simon and Sanjeev, 2007; Zarei et al., 2012; Arfatahery et al., 2015), and was 43% in FFP from Galician markets (Vazquez‐Sanchez et al., 2012). St. aureus exhibits a minimum growth temperature of 7°C (ICMSF, 1996), but higher temperatures (> 10°C) are required for the production of its pathogenicity determinants, the staphylococcal enterotoxins.
Vibrio
Vibrios are microorganisms autochthonous of marine and estuarine waters and in their evolutive history have adapted to the fluctuating conditions of these environments, developing strategies to respond to temperature, osmotic and pH shifts and to starvation (Conner et al., 2016). While several Vibrio species have been reported as pathogenic, three of them, V. cholerae, V. parahaemolyticus and V. vulnificus, play a major role in human infections. In industrialised countries, Vibrio infections are either food‐borne or consequence of recreational or professional exposure to marine waters. In the case of food‐borne transmission, the implicated vehicle is almost exclusively seafood, with a predominant involvement of bivalve shellfish. Vibrios account for an estimated 80,000 illnesses in the United States every year,19 but vibriosis are seldom detected in the EU, where less than 170 food‐borne cases were reported between 2016 and 2018 (EFSA and ECDC, 2017, 2018, 2019). Within each Vibrio species, pathogenicity is restricted to strains expressing specific virulence traits that are present in a minority of the environmental isolates, as cholera toxin (associated with serogroups O1 and O139 of V. cholerae) or thermostable and thermostable‐related haemolysins (TDH and TRH) of V. parahaemolyticus. In aquatic environments, Vibrios prefer temperate waters, and their concentration remains low when temperatures drop below 16°C. As temperature represents a primary driver in the ecology of pathogenic Vibrio, climate change deeply affects the distribution of this genus, as indicated by the rise of vibriosis cases in Northern Europe (Baker‐Austin et al., 2013, 2016). Overall, V. cholerae and V. vulnificus growth is not reported at temperatures below 10°C and 8°C, respectively (ICMSF, 1996), while growth of V. parahaemolyticus at refrigeration temperature has been occasionally reported (Provincial et al., 2013a). With regard to water salinity, pathogenic species display different behaviours. V. cholerae prefers the low salinity of freshwaters, brackish and estuarine waters and grows in the salinity range 0.1–4.0%. V. vulnificus and V. parahaemolyticus instead require a minimum concentration of sodium of 0.5% and grow in the presence of NaCl concentrations up to 5.0% and 10.0%, respectively (ICMSF, 1996), therefore spreading predominantly in coastal waters and off‐shore. Vibrio presence in farming or catching waters is reflected in fishery products. Excluding bivalve shellfish, V. cholerae is reported at low but significant prevalence in many countries: in Europe has been detected in 0.6% of seafood samples in France and 2.9% in Switzerland (Scharer et al., 2011; Robert‐Pillot et al., 2014), but was not present in two studies conducted in Spain and Italy on fish and crustacean, respectively (Herrera et al., 2006; Caburlotto et al., 2016). A prevalence ranging from 0% to 6% was also reported in surveys carried on fresh fish, shrimps or prawns in South Africa, Iran, Brazil, Senegal, China, Turkey, Morocco, Egypt and Burkina Faso (Hosseini et al., 2004; Da Silva et al., 2010; Coly et al., 2013; Mus et al., 2014; Traore et al., 2014; Kriem et al., 2015; Fri et al., 2017; Ahmed et al., 2018; Li et al., 2019), while higher values, 36% and 81%, were reported in fish sampled in Mexico and in Bangladesh, respectively (Torres‐Vitela et al., 1997; Hossain et al., 2018). In all these studies, however, isolates carrying genes associated with cholera toxin production represented a minority. Similar to V. cholerae, V. vulnificus has been detected in surveys on fishery products worldwide. V. vulnificus presence was not detected for instance in crustacea and seafood in Switzerland and Italy (Scharer et al., 2011; Caburlotto et al., 2016), but prevalence was 6.8% in fish from Croatian coasts and 12.6% in seafood distributed in France (Jakšić et al., 2002; Robert‐Pillot et al., 2014). Other studies from Iran, South‐East Asian countries and China reported prevalence values between 0% and 58.6%, with a slightly lower occurrence in fish than in crustacean (e.g. shrimps and prawns) (Elhadi et al., 2004; Yano et al., 2004; Gopal et al., 2005; Ji et al., 2011; Koralage et al., 2012; Raissy et al., 2012; Pan et al., 2013; Paydar and Thong, 2013; Tey et al., 2015; Wong et al., 2015; Tra et al., 2016; Amalina et al., 2019; Yan et al., 2019), while high detection rates were reported for fish from Mexico Gulf (37–51%) and for crabs from the Atlantic coast of USA (Tao et al., 2012; Baumeister et al., 2014; Rodgers et al., 2014). As for V. parahaemolyticus, according to a recent meta‐analysis of available studies, the prevalence of this species in seafood amounts to 48.3% (95% CI: 0.454–0.512) in crustaceans and to 51.0% (95% CI: 0.476–0.544) in fish and cephalopod (Odeyemi, 2016).
Nematodes
Fish‐borne Nematode diseases are most commonly associated with the Anisakidae family (EFSA BIOHAZ Panel, 2010), which includes Anisakis spp., Pseudoterranova, Phocascaris and Contracaecum. The species more frequently responsible of human infection are A. simplex, A. pegreffii and Pseudoterranova decipiens. Anisakids typical definitive hosts are marine mammals and birds, while fish and cephalopod molluscs (squids) act as transport hosts for third‐stage larvae and as food vehicles for human infection. Worldwide over 200 fish and 25 cephalopods species have been reported to host the larval stage of A. simplex (Abollo et al., 2001), with species as herring, anchovy, sardine and cod frequently involved in anisakiasis in European countries (Audicana et al., 1995; Guardone et al., 2018). Overall, a few thousands anisakiasis cases are diagnosed every year, 90% of which from Japan (2,000–3,000 cases reported annually), the remaining from countries (Spain, the Netherlands, Korea, Germany, Italy) where consumption of raw or marinated fish is common (Bao et al., 2017). Salting and marinating are effective for Anisakis larvae inactivation only under specific conditions (ICMSF, 1996; EFSA BIOHAZ Panel, 2010), therefore European legislation requires freezing (at −20°C for not less than 24 h or −35°C for not less than 15 h) of products intended to be consumed raw or marinated/salted if the treatment is insufficient to kill the viable parasites (Regulation (EC) No 853/20041). Refrigerated temperatures display no evident activity on Anisakis larvae viability (Pascual et al., 2010).
Histamine‐producing bacteria
Histamine is a heat‐stable biogenic amine naturally occurring in the human body, where it is involved in neurotransmission and in immune response, including allergic reactions. Beside its endogenous production, histamine may also be introduced in the human organism from food sources containing high concentrations of this compound, overcoming the physiological detoxification processes. This may lead to an allergy type of food poisoning with a rapid onset (usually within 1 h, (Dalgaard et al., 2006)) characterised by skin flushing, rash, headache, gastrointestinal symptoms, itchiness and tingling fingers. Each year several hundred cases of histamine intoxication are reported in the EU (EFSA and ECDC, 2018, 2019), with outbreaks usually associated with fish and fishery products (Huss et al., 2000). The species most often involved in outbreaks include fishes of the Scombridae family (i.e. tuna, mackerel, bonito), whose muscle tissue is particularly rich in histidine, but also other species which have a high content of naturally occurring histidine as sardine, anchovy, swordfish, herring, amberjack, mahi mahi, bluefish, marlin. Histamine production in fish and fishery products results from decarboxylation of free histidine by the bacterial enzyme histidine decarboxylase, which is produced by a wide range of bacteria including Morganella morganii and psychrotolerans, Photobacterium phosphoreum, psychrotolerans and damselae, Hafnia alvei, Citrobacter koseri, Cl. perfringens, Aeromonas hydrophila, as well as some species of the Enterobacter, Klebsiella, Proteus, Pseudomonas, Raoultella, Serratia, Staphylococcus and Vibrio genuses (Kanki et al., 2004; Emborg et al., 2006; Ozogul and Ozogul, 2006; Tsai et al., 2007; Chang et al., 2008; Dalgaard et al., 2008; Lin et al., 2016). Most of these species are mesophilic; however, growth in fish at refrigeration temperatures and histamine production was reported, at various levels, for psychrotolerant or psychrotrophic M. morganii (Aytac et al., 2000), M. psychrotolerans (Emborg and Dalgaard, 2008), Photobacterium phosphoreum and iliopiscarium (Torido et al., 2012) and Enterobacter (currently Klebsiella) aerogens (Tsai et al., 2005). Further to this, while the production of histidine decarboxylase is affected by bacterial growth and therefore by storage temperature, the enzyme activity is retained at low temperatures (Fuji et al., 1994) and histidine decarboxylase formed in the event of temperature abuse remains active even after correct storage conditions are restored.
Enterobacter
Different species of the Enterobacter genus20 have been reported to produce histamine, including Enterobacter aerogenes (Yoshinaga and Frank, 1982), agglomerans (Garcia‐Tapia et al., 2013), cloacae (Allen et al., 2005), intermedium (López‐Sabater et al., 1996), pyrinus (McCarthy et al., 2015) and kobei (Ohshima et al., 2019). In a survey on 235 scombrotoxin‐forming fish from the Gulf of Mexico, Enterobacter (Klebsiella) aerogenes represented 4% of histamine‐producing bacteria, being the third most common species after Photobacterium damselae and Morganella morganii (Bjornsdottir‐Butler et al., 2015), while the Enterobacter cloacae complex was the most frequently isolated histamine‐producing species in a study on mackerels and sardines in Egypt (Sabry et al., 2019). Further to this, Enterobacter (Klebsiella) aerogenes, together with other histamine‐producing species, was also isolated from suspected foods during investigations following three histamine intoxication outbreaks in Taiwan in 2011 (67 cases) and in 2014 (37 and 7 cases, respectively) (Lee et al., 2013, 2016; Hwang et al., 2019).
Morganella
The genus Morganella was established in 1978 through reassignment of the species previously known as Proteus morganii (Brenner et al., 1978) and its role in histamine production in fish and fish products was already clearly defined in the early 1990s (Lopez‐Sabater et al., 1994; Lopez‐Sabater et al., 1996; Rodriguez‐Jerez et al., 1994). Subsequent characterisation studies showed that, in tuna fish infusion at 25°C, histamine production by M. morganii could reach up to 5000 ppm in 48 h (Kim et al., 2000). Between 2005 and 2006, the isolation of psychrotolerant M. morganii‐like bacteria from fish products implicated in histamine poisoning events led to the identification of a second species within the genus, M. psychrotolerans (Emborg et al., 2006). M. morganii and M. psychrotolerans are considered, together with Photobacterium spp., the most relevant histamine‐producing species in fish. While both Morganella species are reported to grow at refrigeration temperatures, significant differences were reported with regard to their optimal and maximum growth temperatures, as well as the for minimum growth temperature, that ranges from −8.3 to −5.9°C and from 0.3 to 2.8°C for M. psychrotolerans and for M. morganii, respectively (Emborg and Dalgaard, 2008).
Photobacterium
Similar to Morganella, histamine production by Photobacterium spp. was reported three decades ago, in studies conducted on P. phosphoreum and P. damselae subsp. damselae strains isolated from the marine environment and from fish sources (Fuji et al., 1994). More recent studies provided evidence that histamine production is a feature common to several Photobacterium species, including P. iliopiscarium (Torido et al., 2012), P. kishitanii, P. angustum (Bjornsdottir‐Butler et al., 2016bb), and P. aquimaris (Bjornsdottir‐Butler et al., 2016aa), therefore being present in at least three of the Photobacterium clades: Phosphoreum, Damselae and the recently proposed Leiognathi clade (Labella et al., 2018). Significant histamine production (> 200 ppm) is reported for P. angustum, P. aquimaris, P. kishitanii, P. damselae and P. phosphoreum (Bjornsdottir‐Butler, 2018), with isolates of the latter species showing a histamine production variable from 2,080 to 4,490 ppm at 5°C (Dalgaard et al., 2006). Investigations of histamine intoxication events also pointed out P. phosphoreum involvement in outbreaks following tuna and sardine consumption (Kanki et al., 2004; Emborg and Dalgaard, 2006).
Appendix B – Supporting information for modelling temperatures inside tubs and boxes
1.
The heat transfer model, named the Temperature Simulator of Fish Stored in Tubs and Boxes (FishT‐TaB Simulator), has been made available through the Knowledge Junction under the https://doi.org/10.5281/zenodo.3725616
2.
2.1.
2.1.1.
2.1.1.1. Simulation of temperature dynamics in tubs and iced boxes
The general heat transfer in fluids follows the following standard convection–diffusion equation:
where T is the temperature, ρ the density, Cp the specific heat capacity and k the conductivity. Certain terms are zero in the model due to the approximations of no fluid movement (velocity vector u) and heat sources (Q, Qp and Qvd). Therefore, the equation of interest reads:
When using the porous medium approximation and the ‘apparent heat capacity method’, the thermal parameters are functions of the different materials parameters (see Comsol help for details). For water and air, thermodynamic parameters are also function of the temperature, for the rest are considered constant.
This heat transfer by conduction is completed with initial conditions (as described in the main text) and the following boundary conditions:
where h is the heat transfer coefficient and Text the outside temperature.
The model is implemented and solved using Comsol software. Comsol is based on the Finite Element Method to solve the previous Partial Derivative Equation. The procedure for all the case studies and validation is similar. To add a context, in the following, the procedure is described in general but with references, when necessary for clarification, to the model validated with the ‘Qualitubfish’ data. For the case studies, only minor changes, such as the size and type of the fish or the initial and outside temperatures, are changed.
The steps are as following
-
•
Define the critical parameters (see Table B.1 for the model used for the validation) and the profile of the outside temperature. Other parameters are functions of these critical parameters, such as for example the volume of air in tubs, that is the space left on top of the container without fish, ice or water.
-
•
Define the different spatial domains, i.e. the geometry, its position and material for each domain. For example, for tubs, there are the following domains: the container wall made of Polyethylene foam, two ellipsoids made of lean fish, a matrix (rectangle inside the container) made of a mixture of fish and liquid water, a rectangle on the top of this matrix made of ice that can change phase to water and another rectangle on the top of the ice with air. See Figure 5 for illustration and Table B.2 for the value of the thermodynamic parameters of each material used.
-
•
Mesh the domains. The discretisation used by default was the ‘normal’ discretisation in Comsol, selecting ‘finer’ discretisation for the ice domain and the fish ellipsoids. Ice domain needed more discretisation due to the approximation used for the change of phase. Fish ellipsoids are discretised with finer meshes as are the domains where temperature is reported and are too thin compared with other geometries when simulating small fish. It was checked that results do not change with further discretisations in space.
-
•
Select the options of the time integrator. The default option is used with a few exceptions. For example, the integrator maximum step is lowed to force considering all the fluctuations with low frequency on the outside temperature profile obtained from loggers in the ‘Qualitubfish’ project.
-
•
After testing different time integrator and space discretisations (see previous points), the model is simulated for final results. Different so‐called ‘probes’ in Comsol are used to extract the interesting temperatures, such as the maximum temperature on the fish surface or the maximum temperature in the fish matrix for the ‘abusive’ scenarios. For the validation, the probe used for comparison with the experimental data was set to the mean temperature in (inside and surface) the fish ellipsoids.
-
•
Results were compared with data from the ‘Qualitubfish’ project. Parameters that were not known with precision (such as the temperature of the non‐manipulated fish) were set to a reasonable value determined by expert knowledge. We could not use the data to estimate those parameters due to identifiability problems common of these type of systems.
Table B.1.
Parameters in the simple box/tub model based on the experiment of the ‘Qualitubfish’ project
| Name | Value | Value in S.I. | Description |
|---|---|---|---|
| fish_w | (29/2) cm | 0.145 m | Width fish radius of ellipsoid |
| fish_d | (15.5/2) cm | 0.0775 m | Depth fish radius of ellipsoid |
| fish_h | (0.85/2) cm | 0.00425 m | Height fish radius of ellipsoid |
| dT | 1 K | 1 K | Transition Interval (ΔT) |
| Ttrans | 0°C | 273.15 K | Transition temperature (Tt) |
| lm | 333.5 kJ/kg | 3.335E5 J/kg | Latent heat of fusion (λ) |
| hh | 5 W/(m2 K) | 5 W/(m2·K) | Heat transfer coefficient |
| theta | (Vfish)/(Vfish+Vwater) | 0.80675 | Volume fraction |
| Vfish | 440 kg/1,054 kg/m3 | 0.41746 m3 | Fish volume (function of fish density) |
| Vwater | 100 L | 0.1 m3 | Tub‐water volume |
| fish_T0 | 1.5°C | 274.65 K | Initial temperature fish |
| foam_T0 | 1.5°C | 274.65 K | Initial temperature foam |
| air_T0 | 2.7°C | 275.85 K | Initial temperature air inside box/tub |
| T_fishsmall_T0 | 5.5°C | 278.65 K | Tub – Initial temperature manipulated fish |
| B_fishsmall_T0 | 2.5°C | 275.65 K | Box – Initial temperature manipulated fish |
| T_water_T0 | 2.7°C | 275.85 K | Tub – Initial water temperature |
| ice_T0 | −0.5°C | 273.85 K | Initial ice temperature |
Table B.2.
Table of basic thermodynamic parameters for different insulators and fish, water, ice and air
| Thermal conductivity (k; in W/m °C) | Specific heat capacity (Cp, in kJ/kg°C) | Density (ρ, in kg/m3) | Reference | ||||
|---|---|---|---|---|---|---|---|
| Fat fish | 0.41 | 3.50 | 1,025 | Rahman (2009), Tolstorebrov et al. (2019) and Radhakrishnan (1997) | |||
| Lean fish | 0.43 | 3.73 | 1,054 | Margeirsson et al. (2012) | |||
| Tubs a | 0.05 | 2.3 | 70 | ||||
| Non‐insulated boxes b | 0.44 | 1.64 | 930 | ||||
| Water |
|
|
|
Comsol default function of temperature (T) | |||
| Air | pA × 0.02897/Rcons[K × mol/J]/T with Rconst[K × mol/J] = 8.3145 and |
|
|
Comsol default function of temperature (T) | |||
| Ice | 2.31 | 2.052 | 918 | Comsol (example of ‘Phase change’ in library) |
Polyethylene foam of the core of the container.
High‐density polyethylene (HDPE).
2.1.1.2. Simplifications and assumptions of the heat transfer model
The model relies on the following simplifications and assumptions as shown in Table B.3.
Table B.3.
Simplifications and assumptions used in the heat transfer modelling
| Simplifications | Assumptions |
|---|---|
| The model simulates heat transfer by conduction, disregarding any other type of heat transfer phenomena | Forced convection due to aeration (forced water mixing) was not considered as it helps to homogenise the temperature distributions, avoiding the emergence of hot/warm spots (especially in tubs). Natural convection and radiation have a minor contribution compared with conduction and forced convection and were not modelled. In boxes, movement of melting water due to gravity was neither considered |
| The model simulates the change of phase from ice to water using the ‘Apparent Heat Capacity method’ where the latent heat is included as an additional term in the heat capacity (Bonacina et al., 1973) | The following parameters were used: transition temperature (Tt) 0°C, transition interval (ΔT) 1°C and latent heat of fusion (λ) 333.5 kJ/kg. Therefore, the so‐called ‘ice domain’ is a perfect mixture of 50% water with 50% ice at the transition temperature (Tt = 0°C), where all is ice at temperatures below −0.5°C (Tt–(1/2) ΔT) and all is water above 0.5°C (Tt + (1/2) ΔT) |
| Only two fish, assuming an ellipsoid geometry, were explicitly modelled in each container. Instead, for the contribution of the other fish in the same container, the model considered a matrix inspired by the approximation used for porous materials | Certain approximations were required to model the temperature of the surface of the tens of fish in both types of containers. Only two fish, assuming an ellipsoid geometry, were explicitly modelled in each container. The contribution of the other fish could not be modelled using the same approach as tens of ellipsoids requires fine meshes, and therefore, many equations that could not be dealt with by standard computing in terms of memory required and computational times. Instead, for the contribution of the other fish in the same container, the model considered a matrix inspired by the approximation used for porous materials made of a mixture of
|
| Among all the modelled fish surface temperatures distributed in space (which changed from one location to another), three t/T profiles were retrieved and reported for each container. Thus, from the large number of temperatures simulated at each time in each mesh point), the following highest temperatures were selected |
|
| Convective heat flux was considered without air flow | As boundary condition (heat transfer between the outside surface of the container and the air of the storage/transport chamber) convective heat flux was considered with the usual heat transfer coefficient (5 W/(m2 °C)) without air flow |
| Initial conditions were assumed homogenous in space | This means that the initial conditions were assumed homogenous in space, i.e. same temperatures for all the points in the same domain. Therefore, the initial spatial distribution of temperatures of, for example, fish, water or container material was not considered |
| Whenever a parameter, initial condition or boundary condition may be case dependent, the usual practice was considered | Assumed values, such as the container‐specific characteristics of the tubs and boxes, are described in Table 1. The fish‐specific characteristics are provided in Table 2 |
Appendix C – Behaviour of relevant hazards
1.
Figure C.2.
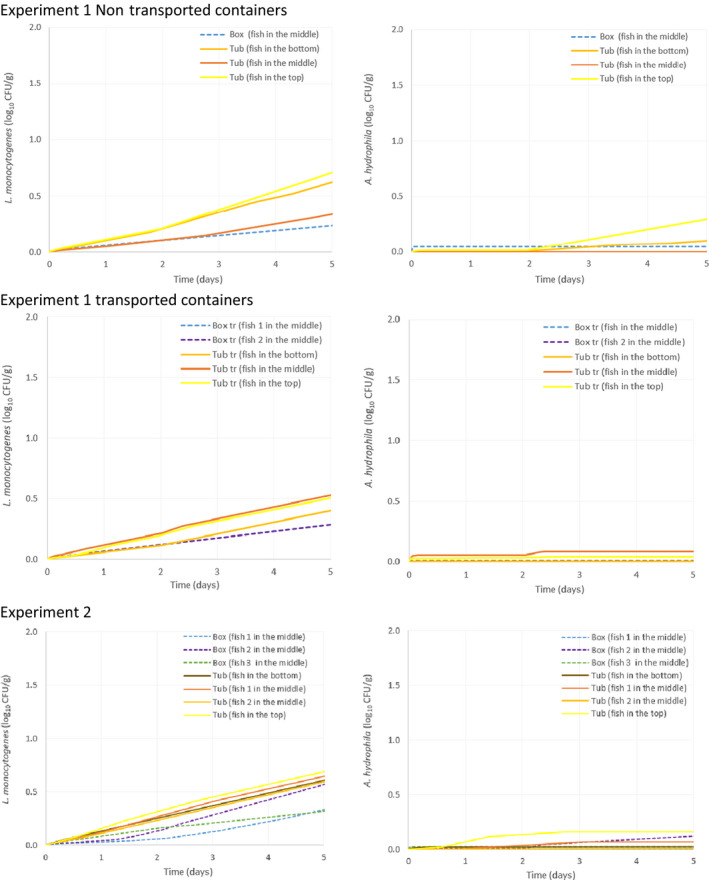
Predicted growth of Listeria monocytogenes (plots in the left) and Aeromonas hydrophila (plots in the right) based on the ‘observed’ scenarios based on the time/Temperature profile recorded in experiment 1 (Bekaert et al., 2016a) and experiment 2 (Bekaert et al., 2016b) of the ‘Qualitubfish’ project
Figure C.3.
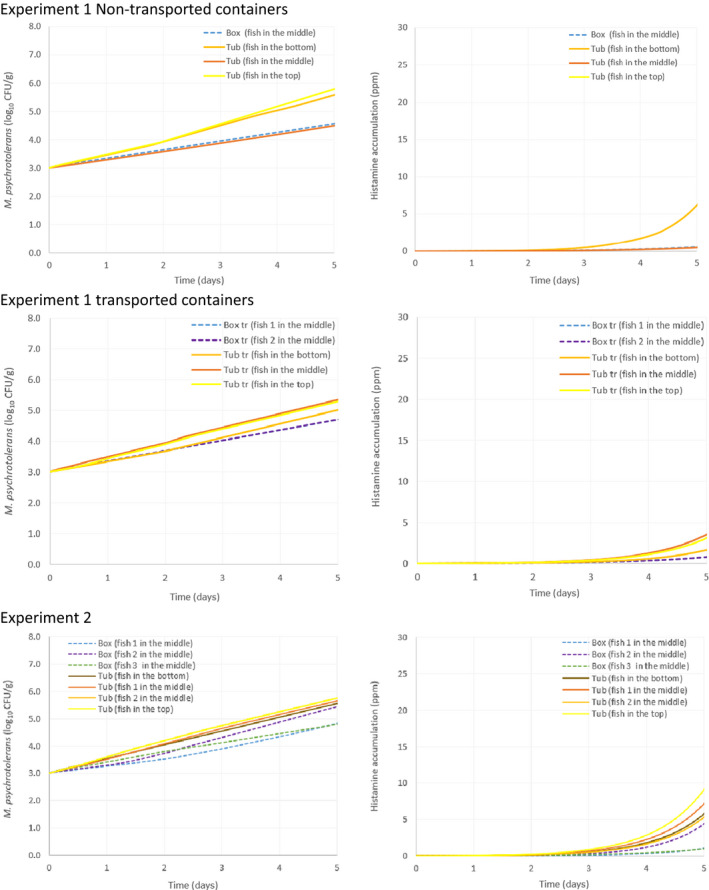
Growth of Morganella psychrotolerans (plots on the right) and the consequent histamine formation (ppm, plots on the left) predicted by food spoilage and safety predictor based on the time/Temperature profile recorded in experiment 1 (Bekaert et al., 2016a) and experiment 2 (Bekaert et al., 2016b) of the ‘Qualitubfish’ project
Appendix D – Tables of uncertainty assessment
1.
Table D.1.
Sources of uncertainty identified in the hazard identification and qualitative assessment of the impact that these uncertainties could have on the conclusions
| Source or location of the uncertainty | Nature or cause of the uncertainty | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Hazard identification (literature search and screening) | Evidence on (1) hazards relevance for human illness associated with FFP or on (2) survival (no change or reduction) or growth of the hazard in FFP within the range −3°C to 7°C may have been missed or may be absent due to:
|
Potential underestimation of the relevance of a hazard. However, given the use of extensive literature reviews, uncertainty associated with missing evidence in this step has been reduced as much as possible In particular, in consideration of the search extent, exclusion of a relevant hazard due to protracted non‐reporting of human disease was considered unlikely For hazards excluded from the assessment due to absence of evidence of growth or histamine production within the range −3°C to 7°C, other available information based on microbial ecology principles was considered as a scientific support for exclusion |
| Hazard identification (data extraction) |
Uncertainty in the description of the growth/survival behaviour of the hazards due to inter‐strain variability, heterogeneous distribution of the microorganisms on the FFP and variability of the performance of the enumeration methods A systematic appraisal analysis for quality of the studies, particularly with regard to survival or growth of the selected hazards at temperatures relevant for the assessment, was not performed |
Potential over‐ or underestimation of the relevance of a hazard The adoption of a criterion for the assessment of relevant growth/survival (threshold of 0.5 log10 in the increase/decrease of microorganisms) limited the uncertainty in the interpretation of the hazards’ behaviour To avoid overestimation of growth/survival, particularly of mesophilic microorganisms, critical appraisal and exclusion of results was applied for publications in which inconsistencies in the experimental design or the analytical methods were detected |
FFP: fresh fishery products.
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Arason S, Bekaert K, García MR, Georgiadis M, Messens W, Mosbach‐Schulz O and Bover‐Cid S, 2020. Scientific Opinion on the use of the so‐called ‘tubs’ for transporting and storing fresh fishery products. EFSA Journal 2020;18(4):6091, 123 pp. 10.2903/j.efsa.2020.6091
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00053
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: EFSA wishes to acknowledge the contribution of Maria Francesca Iulietto to this opinion for the support in the literature searches related to the hazard identification.
Adopted: 19 March 2020
Notes
Laying down specific hygiene rules for food of animal origin. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. O.J. L. 139, 30.4.2004, p. 1–55.
https://pure.ilvo.be/portal/files/4784279/ILVO_mededeling_221_Qualitubfish.pdf (as assessed on 19.12.2018).
The study has used high‐density polyethylene (HDPE) boxes instead.
Family Scombridae, Clupeidae, Engraulidae, Coryfenidae, Pomatomidae, Scombresosidae.
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJ L 139, 30.4.2004, p. 1–54.
Potable water is defined as water meeting the minimum requirements laid down in Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, while clean seawater is defined as natural, artificial or purified seawater or brackish water that does not contain microorganisms, harmful substances or toxic marine plankton in quantities capable of directly or indirectly affecting the health quality of food.
Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. OJ L 64, 4.3.2006, p. 1–37.
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.98, p. 1–32.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, p. 1–26, as amended by Commission Regulation (EC) No 2019/229 of 7 February 2019.
Note that the temperature range was widened to also capture the temperature range for the mandate on the use of the so‐called ‘superchilling’ technique for the transport of fresh fishery products (http://registerofquestions.efsa.europa.eu/roqFrontend/questionLoader?question=EFSA-Q-2019-00437).
For consistency among information sources, Enterobacter spp. where considered according to the standing classification at the time of publishing of the screened articles. Over time, the classification of certain histamine‐producing Enterobacter spp. such as E. aerogenes, E. agglomerans, E. cloacae, E. intermedium, E. pyrinus has been revised [Klebsiella aerogenes (Tindall et al., 2017) (Hoffmann and Roggenkamp, 2003), Kluyvera intermedia (Pavan et al., 2005), Pluralibacter pyrinus] (Brady et al., 2013), with the former names considered as homotypic synonyms.
Considering proper practices and assuming that the initial fish temperature equals 0°C and the fish is in perfect contact with ice in boxes and with a perfect mixing of water and ice in tubs. This scenario is not applicable to the ‘cooling‐keeping’ process.
Assuming that the initial fish temperature equals 0°C (‘keeping’ process) or 7°C (‘cooling‐keeping’ process) and the outside temperature is mostly at 2°C but includes some abusive temperature peaks up to 6°C. For boxes, the fish is surrounded by air and two layers of ice (bottom and top) while for tubs, the fish is in water below an ice layer on the top of the tub without mixing.
Plaice was considered as a lean small fish having a fat and water content 1–4% and 79–81%, respectively, and weight of 150–300 g; and Atlantic salmon as a fat medium‐sized fish having a fat and water content 10–20% and 60–70%, respectively. Fish fillets were assumed to be covered by dimensions of the flat fish.
For consistency among information sources, Enterobacter spp. where considered according to the standing classification at the time of publishing of the screened articles. Over time, the classification of certain histamine‐producing Enterobacter spp. such as E. aerogenes, E. agglomerans, E. cloacae, E. intermedium, E. pyrinus has been revised [Klebsiella aerogenes (Tindall et al., 2017), Pantoea agglomerans (Gavini et al., 1989), E. cloacae complex (Hoffmann and Roggenkamp, 2003), Kluyvera intermedia (Pavan et al., 2005), Pluralibacter pyrinus (Brady et al., 2013)], with the former names considered as homotypic synonyms.
References
- Abe H, 1983. Distribution of free L‐histidine and its related compounds in marine fishes. Bulletin of the Japanese Society of Scientific Fisheries, 49, 1683–1687. [Google Scholar]
- Abollo E, Gestal C and Pascual S, 2001. Anisakis infestation in marine fish and cephalopods from Galician waters: an updated perspective. Parasitology Research, 87, 492–499. [DOI] [PubMed] [Google Scholar]
- Adams MR and Moss MO, 2006. Food Microbiology, 2nd Edition Published by The Royal Society of Chemistry. [Google Scholar]
- Ahmed HA, Abdelazim RS, Gharieb RMA, Abou Elez RMM and Awadallah MAI, 2018. Prevalence of antibiotic resistant V. parahaemolyticus and V. cholerae in fish and humans with special reference to virulotyping and genotyping of V. parahaemolyticus. Slovenian Veterinary Research, 55, 251–262. [Google Scholar]
- Alagarsamy S, Thampuran N and Joseph TC, 2010. Virulence genes, serobiotypes and antibiotic resistance profile of Escherichia coli strains isolated from aquaculture and other sources. Aquaculture Research, 41, 1003–1014. [Google Scholar]
- Alford JA and Palumbo SA, 1969. Interaction of salt, pH, and temperature on the growth and survival of salmonellae in ground pork. Applied Microbiology, 17, 528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Green DP, Bolton GE, Jaykus LA and Cope WG, 2005. Detection and identification of histamine‐producing bacteria associated with harvesting and processing mahimahi and yellowfin tuna. Journal of Food Protection, 68, 1676–1682. [DOI] [PubMed] [Google Scholar]
- Amalina NZ, Santha S, Zulperi D, Amal MNA, Yusof MT, Zamri‐Saad M and Ina‐Salwany Y, 2019. Prevalence, antimicrobial susceptibility and plasmid profiling of Vibrio spp. isolated from cultured groupers in Peninsular Malaysia. Bmc Microbiology, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine FR, Wei CI, Littell RC and Marshall MR, 1999. HPLC method for analysis of free amino acids in fish using o‐phthaldialdehyde precolumn derivatization. Journal of Agricultural and Food Chemistry, 47, 5100–5107. [DOI] [PubMed] [Google Scholar]
- Arfatahery N, Mirshafiey A, Abedimohtasab TP and Zeinolabedinizamani M, 2015. Study of the prevalence of Staphylococcus aureus in marine and farmed shrimps in Iran aiming the future development of a prophylactic vaccine. Procedia of the 8th Vaccine & Isv Congress. Ed DeSousa, CBP, 44–49. [Google Scholar]
- Audicana MT, Fernández de Corres L, Muñoz D, Fernández E, Navarro JA and del Pozo MD, 1995. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. Journal of Allergy and Clinical Immunology, 96, 558–560. [DOI] [PubMed] [Google Scholar]
- Avery L, Williams AP, Killham K and Jones D, 2008. Survival of Escherichia coli O157:H7 in waters from lakes, rivers, puddles and animal‐drinking troughs. The Science of the Total Environment, 389, 378–385. [DOI] [PubMed] [Google Scholar]
- Aytac SA, Ozbas ZY and Vural H, 2000. Effects of irradiation, antimicrobial agents and modified‐atmosphere packaging on histamine production by Morganella morganii in mackerel fillets. Archiv Fur Lebensmittelhygiene, 51, 28–30. [Google Scholar]
- Badhey H, Cleri DJ, Damato RF, Vernaleo JR, Veinni V, Tessler J, Wallman AA, Mastellone AJ, Giuliani M and Hochstein L, 1986. 2 fatal cases of type‐e adult foodborne botulism with early symptoms and terminal neurologic signs. Journal of Clinical Microbiology, 23, 616–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baixas‐Nogueras S, Bover‐Cid S, Veciana‐Nogues T, Nunes ML and Vidal‐Carou MC, 2003. Development of a Quality Index Method to evaluate freshness in Mediterranean hake (Merluccius merluccius). Journal of Food Science, 68, 1067–1071. [Google Scholar]
- Baker‐Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A and Martinez‐Urtaza J, 2013. Emerging Vibrio risk at high latitudes in response to ocean warming. Nature Climate Change, 3, 73–77. [Google Scholar]
- Baker‐Austin C, Triñanes J, Salmenlinna S, Löfdahl M, Siitonen A, Taylor N and Martinez‐Urtaza J, 2016. Heat Wave–Associated Vibriosis, Sweden and Finland, 2014. Emerging Infectious Diseases, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Ooi M, Shariff M and Khatoon H, 2012. Antibiotic resistant salmonella and vibrio associated with farmed litopenaeus vannamei. The Scientific World Journal, 2012, 130136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Pierce GJ, Pascual S, Gonzalez‐Munoz M, Mattiucci S, Mladineo I, Cipriani P, Buselic I and Strachan NJC, 2017. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Scientific Reports, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranyi J, Robinson TP, Kaloti A and Mackey BM, 1995. Predicting growth of brochothrix thermosphacta at changing temperature. International Journal of Food Microbiology, 27, 61–75. [DOI] [PubMed] [Google Scholar]
- Barrett KA, Nakao JH, Taylor EV, Eggers C and Gould LH, 2017. Fish‐Associated Foodborne Disease Outbreaks: United States, 1998‐2015. Foodborne Pathogens and Disease, 14, 537–543. [DOI] [PubMed] [Google Scholar]
- Baumeister L, Hochman ME, Schwarz JR and Brinkmeyer R, 2014. Occurrence of vibrio vulnificus and toxigenic vibrio parahaemolyticus on sea catfishes from galveston bay, Texas. Journal of Food Protection, 77, 1784–1786. [DOI] [PubMed] [Google Scholar]
- Beaz‐Hidalgo R, Martinez‐Murcia A and Figueras MJ, 2013. Reclassification of Aeromonas hydrophila subsp dhakensis Huys et al. 2002 and Aeromonas aquariorum Martinez‐Murcia et al. 2008 as Aeromonas dhakensis sp nov comb nov and emendation of the species Aeromonas hydrophila. Systematic and Applied Microbiology, 36, 171–176. [DOI] [PubMed] [Google Scholar]
- Bekaert K, Deloof D, Vandermeersch G, De Witte B, Vlaemynck G and De Reu K (Institute for Agricultural and Fisheries Research (ILVO)), 2016a. Project Qualitubfish. Opvolging van de kwaliteitsveranderingen van pladijs gedurende de opslag in tubs. 35 pp.
- Bekaert K, Deloof D, Vandermeersch G, Devriese L, De Reu K, Vlaemynck G and Polet H (Institute for Agricultural and Fisheries Research (ILVO)), 2016b. Verslag Project Qualitubfish. Opvolging van de kwaliteitsveranderingen van pladijs gedurende de opslag in tubs ‐ Tweede houdbaarheidsexperiment. 21 pp.
- Beleneva IA, 2011. Incidence and characteristics of Staphylococcus aureus and Listeria monocytogenes from the Japan and South China seas. Marine Pollution Bulletin, 62, 382–387. [DOI] [PubMed] [Google Scholar]
- Betts GD and Gaze JE, 1995. Growth and heat‐resistance of psychrotrophic clostridium‐botulinum in relation to sous vide products. Food Control, 6, 57–63. [Google Scholar]
- Binsi PK, Viji P, Visnuvinayagam S, Ninan G, Sangeeta G, Triveni A and Ravishankar CN, 2015. Microbiological and shelf life characteristics of eviscerated and vacuum packed freshwater catfish (Ompok pabda) during chill storage. Journal of Food Science and Technology‐Mysore, 52, 1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdottir K, Bolton GE, McClellan‐Green PD, Jaykus LA and Green DP, 2009. Detection of gram‐negative histamine‐producing bacteria in fish: A comparative study. Journal of Food Protection, 72, 1987–1991. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir‐Butler K, McCarthy S, Burkhardt W and Benner RA, 2013. Importance of histamine‐producing clostridium perfringens in scombrotoxin‐forming fish. Journal of Food Protection, 76, 1283–1287. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir‐Butler K, 2018. Scombrotoxin (chapter 99) In: Liu D (ed.). Handbook of Foodborne Diseases. CRC Press. [Google Scholar]
- Bjornsdottir‐Butler K, Bowers JC and Benner RA, 2015. Prevalence and characterization of high histamine‐producing bacteria in gulf of Mexico fish species. Journal of Food Protection, 78, 1335–1342. [DOI] [PubMed] [Google Scholar]
- Bjornsdottir‐Butler K, Leon MS, Dunlap PV and Benner RA, 2016a. Draft genome sequences of histamine‐ and non‐histamine‐producing photobacterium strains. Microbiology Resource Announcements, 4, 10.1128/genomeA.01008-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdottir‐Butler K, McCarthy SA, Dunlap PV and Benner RA, 2016b. Photobacterium angustum and photobacterium Kishitanii, psychrotrophic high‐level histamine‐producing bacteria indigenous to tuna. Applied and Environmental Microbiology, 82, 2167–2176. 10.1128/aem.02833-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacina C, Comini G, Fasano A and Primicerio M, 1973. Numerical solution of phase‐change problems. International Journal of Heat and Mass Transfer, 16, 1825–1832. [Google Scholar]
- Borderías AJ and Sánchez‐Alonso I, 2011. First processing steps and the quality of wild and farmed fish. Journal of Food Science, 76, R1–R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulares M, Mankai M, Sadok S and Hassouna M, 2017. Anti‐Listerial inhibitory lactic acid bacteria in fresh farmed sea bass (Dicentrarchus labrax) fillets during storage at 4 degrees C under vacuum‐packed conditions. Journal of Food Safety, 37. [Google Scholar]
- Bou‐m'handi N and Marrakchi A, 2003. Incidence de Listeria spp. en milieu marin au Maroc. Sciences Des Aliments, 23, 513–523. [Google Scholar]
- Brady C, Cleenwerck I, Venter S, Coutinho T and De Vos P, 2013. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Systematic and Applied Microbiology, 36, 309–319. [DOI] [PubMed] [Google Scholar]
- Bronstein MN, Price RJ, Strange EM, Melvin EF, Dewees CM and Wyatt BB, 1985. Storage of dressed chinook salmon, Oncorhynchus‐Tshawytscha, in refrigerated fresh‐water, diluted seawater, seawater, and in ice. Marine Fisheries Review, 47, 68–72. [Google Scholar]
- Brenner DJ, Farmer JJ, Fanning GR, Steigerwalt AG, Klykken P, Wathen HG, Hickman FW and Ewing WH, 1978. Deoxyribonucleic‐acid relatedness of Proteus and Providencia species. International Journal of Systematic Bacteriology, 28, 269–282. [Google Scholar]
- Budiati T, Rusul G, Wan‐Abdullah WN, Arip YM, Ahmad R and Thong KL, 2013. Prevalence, antibiotic resistance and plasmid profiling of Salmonella in catfish (Clarias gariepinus) and tilapia (Tilapia mossambica) obtained from wet markets and ponds in Malaysia. Aquaculture, 372–375, 127–132. [Google Scholar]
- Busani L, Cigliano A, Taioli E, Caligiuri V, Chiavacci L, Di Bella C, Battisti A, Duranti A, Gianfranceschi M, Nardella Mc, Ricci A, Rolesu S, Tamba M, Marabelli R and Caprioli A and Epidemiology Obotigov , 2005. Prevalence of Salmonella enterica and Listeria monocytogenes Contamination in Foods of Animal Origin in Italy. Journal of Food Protection, 68, 1729–1733. [DOI] [PubMed] [Google Scholar]
- Caburlotto G, Suffredini E, Toson M, Fasolato L, Antonetti P, Zambon M and Manfrin A, 2016. Occurrence and molecular characterisation of Vibrio parahaemolyticus in crustaceans commercialised in Venice area, Italy. International Journal of Food Microbiology, 220, 39–49. [DOI] [PubMed] [Google Scholar]
- Cardozo MV, Borges CA, Beraldo LG, de Oliveira FE and de Avila FA, 2012. Prevalence and characterization of shigatoxigenic (STEC) and enteropathogenic (EPEC) Escherichia coli strains from fishes for human consumption isolated by Polymerase Chain Reaction (PCR). The FEBS Journal, 279, 530. [Google Scholar]
- Carlin F, Albagnac C, Rida A, Guinebretiere MH, Couvert O and Nguyen‐The C, 2013. Variation of cardinal growth parameters and growth limits according to phylogenetic affiliation in the Bacillus cereus Group. Consequences for risk assessment. Food Microbiology, 33, 69–76. [DOI] [PubMed] [Google Scholar]
- Carrascosa C, Millan R, Saavedra P, Jaber JR, Montenegro T, Raposo A, Perez E and Sanjuan E, 2014. Predictive models for bacterial growth in sea bass (Dicentrarchus labrax) stored in ice. International Journal of Food Science and Technology, 49, 354–363. [Google Scholar]
- Carrascosa C, Saavedra P, Millan R, Jaber JR, Montenegro T, Raposo A and Sanjuan E, 2016. Microbial growth models in gilthead sea bream (Sparus aurata) stored in ice. Journal of Aquatic Food Product Technology, 25, 307–322. [Google Scholar]
- Chang SC, Kung HF, Chen HC, Lin CS and Tsai YH, 2008. Determination of histamine and bacterial isolation in swordfish fillets (Xiphias gladius) implicated in a food borne poisoning. Food Control, 19, 16–21. [Google Scholar]
- Chen HC, Kung HF, Chen WC, Lin WF, Hwang DF, Lee YC and Tsai YH, 2008. Determination of histamine and histamine‐forming bacteria in tuna dumpling implicated in a food‐borne poisoning. Food Chemistry, 106, 612–618. [Google Scholar]
- Chen M, Wu Q, Zhang J, Wu S and Guo W, 2015. Prevalence, enumeration, and pheno‐ and genotypic characteristics of Listeria monocytogenes isolated from raw foods in South China. Frontiers in Microbiology, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Cheng J, Wu Q, Zhang J, Chen Y, Xue L, Lei T, Zeng H, Wu S, Ye Q, Bai J and Wang J, 2018. Occurrence, Antibiotic Resistance, and Population Diversity of Listeria monocytogenes Isolated From Fresh Aquatic Products in China. Frontiers in Microbiology, 9, 2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinivasagam HN, Bremner HA, Thrower SJ and Nottingham SM, 1996. Spoilage pattern of five species of Australian Prawns. Journal of Aquatic Food Product Technology, 5, 25–50. [Google Scholar]
- Chinivasagam HN, Bremner HA, Wood AF and Nottingham SM, 1998. Volatile components associated with bacterial spoilage of tropical prawns. International Journal of Food Microbiology, 42, 45–55. [DOI] [PubMed] [Google Scholar]
- Ciarrone R, Maini P, Simoncello I, Stevanin A, Gottardello L and Renzulli G, 1997. An outbreak of Clostridium perfringens associated with consumption of “baccala alla vicentina” a stock‐fish Italian speciality. Igiene Moderna, 107, 1–9. [Google Scholar]
- Coly I, Sow AG, Seydi M and Martinez‐Urtaza J, 2013. Vibrio cholerae and Vibrio parahaemolyticus Detected in Seafood Products from Senegal. Foodborne Pathogens and Disease, 10, 1050–1058. [DOI] [PubMed] [Google Scholar]
- Conner JG, Teschler JK, Jones CJ and Yildiz FH, 2016. Staying Alive: Vibrio cholerae's Cycle of Environmental Survival, Transmission, and Dissemination. Microbiology Spectrum, 4. [Google Scholar]
- Corrales MT, Bainotti AE and Simonetta AC, 1994. Survival of vibrio‐cholerae‐01 in common foodstuffs during storage at different temperatures. Letters in Applied Microbiology, 18, 277–280. [Google Scholar]
- Costa J, Bover‐Cid S, Bolivar A, Zurera G and Perez‐Rodriguez F, 2019. Modelling the interaction of the sakacin‐producing Lactobacillus sakei CTC494 and Listeria monocytogenes in filleted gilthead sea bream (Sparus aurata) under modified atmosphere packaging at isothermal and non‐isothermal conditions. International Journal of Food Microbiology, 297, 72–84. [DOI] [PubMed] [Google Scholar]
- Da Silva ML, Matte GR, Germano PML and Matte MH, 2010. Occurrence of pathogenic microorganisms in fish sold in Sao Paulo, Brazil. Journal of Food Safety, 30, 94–110. [Google Scholar]
- Dalgaard P, Madsen HL, Samieian N and Emborg J, 2006. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone) – effect of modified atmosphere packaging and previous frozen storage. Journal of Applied Microbiology, 101, 80–95. [DOI] [PubMed] [Google Scholar]
- Dalgaard P, Emborg J, Kjolby A, Sorensen ND and Ballin NZ, 2008. Histamine and biogenic amines: formation and importance in seafood In: Borresen T. (ed.). Improving Seafood Products for the Consumer. British Welding Research Association, Cambridge, pp. 292–324. [Google Scholar]
- Das S, Lalitha KV, Thampuran N and Surendran PK, 2013. Isolation and characterization of Listeria monocytogenes from tropical seafood of Kerala, India. Annals of Microbiology, 63, 1093–1098. [Google Scholar]
- Davies AR and Slade A, 1995. Fate of Aeromonas and Yersinia on modified‐atmosphere‐packaged (MAP) cod and trout. Letters in Applied Microbiology, 21, 354–358. [DOI] [PubMed] [Google Scholar]
- Davies AR, Capell C, Jehanno D, Nychas GJE and Kirby RM, 2001. Incidence of foodborne pathogens on European fish. Food Control, 12, 67–71. [Google Scholar]
- De Silvestri A, Ferrari E, Gozzi S, Marchi F and Foschino R, 2018. Determination of Temperature Dependent Growth Parameters in Psychrotrophic Pathogen Bacteria and Tentative Use of Mean Kinetic Temperature for the Microbiological Control of Food. Frontiers in Microbiology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib AL, Agabou A, Chahed A, Kurekci C, Moreno E, Espigares M and Espigares E, 2018. Isolation, molecular characterization and antimicrobial resistance of enterobacteriaceae isolated from fish and seafood. Food Control, 88, 54–60. [Google Scholar]
- Digre H, Erikson U, Aursand IG, Gallart‐Jornet L, Misimi E and Rustad T, 2011. Rested and stressed farmed atlantic cod (Gadus morhua) chilled in ice or slurry and effects on quality. Journal of Food Science, 76, S89–S100. [DOI] [PubMed] [Google Scholar]
- Domenech‐Sanchez A, Laso E, Perez MJ and Berrocal CI, 2011. Emetic disease caused by bacillus cereus after consumption of tuna fish in a beach Club. Foodborne Pathogens and Disease, 8, 835–837. [DOI] [PubMed] [Google Scholar]
- Du LH, Fan XR, Liu F, Zhou Q, Yuan J and Ju XR, 2017. Changes of Dominant Spoilage Bacteria and Biogenic Amines of Taihu White Prawn (Exopalaemon modestus) during Ice Storage. Journal of Food Protection, 80, 2099–2104. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific and technical assistance on the evaluation of the temperature to be applied to pre‐packed fishery products at retail level. EFSA Journal 2015;13(7):4162, 48 pp. 10.2903/j.efsa.2015.4162 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2017. Assessment of the incidents of histamine intoxication in some EU countries. EFSA supporting publication 2017;EN‐1301. 37 pp. 10.2903/sp.efsa.2017.en-1301 [DOI]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2016. EFSA Journal, 15(1831–4732), e05077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2017. EFSA Journal, 16(1831–4732), e05500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2019. The European Union One Health 2018 Zoonoses Report. EFSA Journal, 17(1831–4732), e05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ and CONTAM Panel (EFSA Panel on Biological Hazards and EFSA Panel on Contaminants in the Food Chain), 2015. Scientific Opinion on the minimum hygiene criteria to be applied to clean seawater and on the public health risks and hygiene criteria for bottled seawater intended for domestic use. EFSA Journal 2012;10(3):2613, 85 pp. 10.2903/j.efsa.2012.2613 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Scientific Opinion on risk assessment of parasites in fishery products. EFSA Journal 2010;8(4):1543, 91 pp. 10.2903/j.efsa.2010.1543 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on VTEC‐seropathotype and scientific criteria regarding pathogenicity assessment. EFSA Journal 2013;11(4):3138, 106 pp. 10.2903/j.efsa.2013.3138 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2016. Scientific opinion on the risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA Journal 2016;14(7):4524, 93 pp. 10.2903/j.efsa.2016.4524 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2020. Pathogenicity assessment of Shiga toxin‐producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA Journal 2020;18(1831–4732), e05967. [Google Scholar]
- EFSA Scientific Committee , 2018a. The principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S, Parveen S, Schwarz J, Rippen T, Jahncke M and DePaola A, 2018. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiology, 70, 85–93. [DOI] [PubMed] [Google Scholar]
- Elhadi N, Radu S, Chen CHS and Nishibuchi M, 2004. Prevalence of potentially pathogenic Vibrio species in the seafood marketed in Malaysia. Journal of Food Protection, 67, 1469–1475. [DOI] [PubMed] [Google Scholar]
- El‐Shenawy MA and El‐Shenawy MA, 2006. Listeria spp. in the coastal environment of the Aqaba Gulf, Suez Gulf and the Red Sea. Epidemiology and Infection, 134, 752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg J, Laursen BG and Dalgaard P, 2005. Significant histamine formation in tuna (Thunnus albacares) at 2 degrees C ‐ effect of vacuum‐ and modified atmosphere‐packaging on psychrotolerant bacteria. International Journal of Food Microbiology, 101, 263–279. [DOI] [PubMed] [Google Scholar]
- Emborg J and Dalgaard P, 2006. Formation of histamine and biogenic amines in cold‐smoked tuna: an investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. Journal of Food Protection, 69, 897–906. [DOI] [PubMed] [Google Scholar]
- Emborg J and Dalgaard P, 2008. Growth, inactivation and histamine formation of Morganella psychrotolerans and Morganella morganii ‐ development and evaluation of predictive models. International Journal of Food Microbiology, 128, 234–243. [DOI] [PubMed] [Google Scholar]
- Emborg J, Dalgaard P and Ahrens P, 2006. Morganella psychrotolerans sp nov., a histamine‐producing bacterium isolated from various seafoods. International Journal of Systematic and Evolutionary Microbiology, 56, 2473–2479. [DOI] [PubMed] [Google Scholar]
- Emborg J, 2007. Morganella psychrotolerans ‐ Identification, histamine formation and importance for histamine fish poisoning (PhD thesis). Available online: https://core.ac.uk/download/pdf/13732311.pdf
- Eom S, Jung Y and Yoon K, 2009. Effect of sanitizer stress response on the growth kinetics of listeria monocytogenes on imitation crabmeat and in broth as a function of temperature. Journal of Food Safety, 29, 564–574. [Google Scholar]
- Erikson U, Misimi E and Gallart‐Jornet L, 2011. Superchilling of rested Atlantic salmon: different chilling strategies and effects on fish and fillet quality. Food Chemistry, 127, 1427–1437. [Google Scholar]
- Erkan N, 2007. Sensory, chemical, and microbiological attributes of sea bream (Sparus aurata): Effect of washing and ice storage. International Journal of Food Properties, 10, 421–434. [Google Scholar]
- FAO and WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2013. Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Meeting report.
- Farber JM, Daley EM, Mackie MT and Limerick B, 2000. A small outbreak of listeriosis potentially linked to the consumption of imitation crab meat. Letters in Applied Microbiology, 31, 100–104. [DOI] [PubMed] [Google Scholar]
- Fernandes CF, Flick GJ and Thomas TB, 1998. Growth of inoculated psychrotrophic pathogens on refrigerated fillets of aquacultured rainbow trout and channel catfish. Journal of Food Protection, 61, 313–317. [DOI] [PubMed] [Google Scholar]
- Ferrari RG, Rosario DKA, Cunha‐Neto A, Mano SB, Figueiredo EES and Conte‐Junior CA, 2019. Worldwide Epidemiology of <em>Salmonella</em> Serovars in Animal‐Based Foods: a Meta‐analysis. Applied and Environmental Microbiology, 85, e00591‐00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finelli L, Swerdlow D, Mertz K, Ragazzoni H and Spitalny K, 1992. Outbreak of cholera associated with crab brought from an area with epidemic disease. Journal of Infectious Diseases, 166, 1433–1435. [DOI] [PubMed] [Google Scholar]
- Fletcher GC, Summers G, Winchester RV and Wong RJ, 1995. Histamine and Histidine in New Zealand Marine Fish and Shellfish Species, Particularly Kahawai (Arripis trutta). Journal of Aquatic Food Product Technology, 4, 53–74. [Google Scholar]
- Fri J, Ndip RN, Njom HA and Clarke AM, 2017. Occurrence of Virulence Genes Associated with Human Pathogenic Vibrios Isolated from Two Commercial Dusky Kob (Argyrosmus japonicus) Farms and Kareiga Estuary in the Eastern Cape Province, South Africa. International Journal of Environmental Research and Public Health, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji T, Kurihara K and Okuzumi M, 1994. Viability and histidine‐decarboxylase activity of halophilic histamine‐forming bacteria during frozen storage. Journal of Food Protection, 57, 611–613. [DOI] [PubMed] [Google Scholar]
- Gabutti G, De Donno A, Bagordo F and Montagna MT, 2000. Comparative survival of faecal and human contaminants and use of staphylococcus aureus as an effective indicator of human pollution. Marine Pollution Bulletin, 40, 697–700. [Google Scholar]
- Gallart‐Jornet L, Barat JM, Rustad T, Erikson U, Escriche I and Fito P, 2007. Influence of brine concentration on Atlantic salmon fillet salting. Journal of Food Engineering, 80, 267–275. [Google Scholar]
- Gallina S, Bianchi DM, Bellio A, Nogarol C, Macori G, Zaccaria T, Biorci F, Carraro E and Decastelli L, 2013. Staphylococcal poisoning foodborne outbreak: epidemiological investigation and strain genotyping. Journal of Food Protection, 76, 2093–2098. [DOI] [PubMed] [Google Scholar]
- Garcia‐Tapia G, Barba‐Quintero G, Gallegos‐Infante JA, Pacheco Aguilar R, Ruiz‐Cortes JA and Ramirez JA, 2013. Influence of physical damage and freezing on histamine concentration and microbiological quality of yellowfin tuna during processing. Food Science and Technology, 33, 463–467. [Google Scholar]
- Gavini F, Mergaert J, Beji A, Mielcarek C, Izard D, Kersters K and De Ley J, 1989. Transfer of Enterobacter agglomerans (Beijerinck 1888) Ewing and Fife 1972 to Pantoea gen. nov. as Pantoea agglomerans comb. nov. and Description of Pantoea dispersa sp. nov. International Journal of Systematic and Evolutionary Microbiology, 39, 337–345. [Google Scholar]
- Gill DM, 1982. Bacterial toxins: a table of lethal amounts. Microbiological Reviews, 46, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill CO, Leet NG and Penney N, 1984. Structural‐changes developing with rigor that facilitate bacterial invasion of muscle‐tissue. Meat Science, 10, 265–274. [DOI] [PubMed] [Google Scholar]
- Gimenez B, Roncales P and Beltran JA, 2002. Modified atmosphere packaging of filleted rainbow trout. Journal of the Science of Food and Agriculture, 82, 1154–1159. [Google Scholar]
- Gopal S, Otta SK, Kumar S, Karunasagar I, Nishibuchi M and Karunasagar I, 2005. The occurrence of Vibrio species in tropical shrimp culture environments; implications for food safety. International Journal of Food Microbiology, 102, 151–159. [DOI] [PubMed] [Google Scholar]
- Graham J, Johnston WA and Nicholson FJ, 1992. Ice in fisheries. [Google Scholar]
- Gram L, 1993. Inhibitory effect against pathogenic and spoilage bacteria of Pseudomonas strains isolated from spoiled and fresh fish. Applied and Environmental Microbiology, 59, 2197–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram L, 1995. Bacteriological changes In: HH H (ed.). Quality and quality changes in fresh fish. Rome: FAO Tech Paper No. 348. pp. 51–63. [Google Scholar]
- Guardone L, Armani A, Nucera D, Costanzo F, Mattiucci S and Bruschi F, 2018. Human anisakiasis in Italy: a retrospective epidemiological study over two decades., Parasite, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin A, Ronning HT, Dargaignaratz C, Clavel T, Broussolle V, Mahillon J, Granum PE and Nguyen‐The C, 2017. Cereulide production by Bacillus weihenstephanensis strains during growth at different pH values and temperatures. Food Microbiology, 65, 130–135. [DOI] [PubMed] [Google Scholar]
- Gui M, Binzhao Song JY, Zhang ZC, Hui PZ and Li PL, 2014. Biogenic amines formation, nucleotide degradation and TVB‐N accumulation of vacuum‐packedminced sturgeon (Acipenser schrencki) stored at 4 degrees C and their relation to microbiological attributes. Journal of the Science of Food and Agriculture, 94, 2057–2063. [DOI] [PubMed] [Google Scholar]
- Hänninen M‐l, Oivanen P and Hirvelä‐koski V, 1997. Aeromonas species in fish, fish‐eggs, shrimp and freshwater. International Journal of Food Microbiology, 34, 17–26. [DOI] [PubMed] [Google Scholar]
- Hansen CH, Vogel BF and Gram L, 2006. Prevalence and survival of Listeria monocytogenes in Danish aquatic and fish‐processing environments. Journal of Food Protection, 69, 2113–2122. [DOI] [PubMed] [Google Scholar]
- Hardstaff JL, Clough HE, Lutje V, McIntyre KM, Harris JP, Garner P and O'Brien SJ, 2018. Foodborne and food‐handler norovirus outbreaks: a systematic review. Foodborne Pathogens and Disease, 15, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R, Tecle S, Adcock B, Kellis M, Weiss J, Saupe A, Sorenson A, Klos R, Blankenship J, Blessington T, Whitlock L, Carleton HA, Concepcion‐Acevedo J, Tolar B, Wise M and Neil KP, 2018. Multistate outbreak of Salmonella Paratyphi B variant L(+) tartrate(+) and Salmonella Weltevreden infections linked to imported frozen raw tuna: USA, March‐July 2015. Epidemiology and Infection, 146, 1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinitz ML, Ruble RD, Wagner DE and Tatini SR, 2000. Incidence of Salmonella in Fish and Seafood†. Journal of Food Protection, 63, 579–592. [DOI] [PubMed] [Google Scholar]
- Hernandez MD, Lopez MB, Alvarez A, Ferrandini E, Garcia BG and Garrido MD, 2009. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chemistry, 114, 237–245. [Google Scholar]
- Hernando V, Arranz LN, Catalan S, Gomez P, Hidalgo C, Barrasa A and Herrera D, 2007. Investigation of a foodborne intoxication in a high‐density penitentiary center. Gaceta Sanitaria, 21, 452–457. [DOI] [PubMed] [Google Scholar]
- Herrera FC, Santos JA, Otero A and García‐López ML, 2006. Occurrence of foodborne pathogenic bacteria in retail prepackaged portions of marine fish in Spain. Journal of Applied Microbiology, 100, 527–536. [DOI] [PubMed] [Google Scholar]
- Hewitt JH, Begg N, Hewish J, Rawaf S, Stringer M and Theodoregandi B, 1986. Large outbreaks of clostridium‐perfringens food poisoning associated with the consumption of boiled salmon. Journal of Hygiene, 97, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hielm S, Hyytiä E, Ridell J and Korkeala H, 1996. Detection of Clostridium botulinum in fish and environmental samples using polymerase chain reaction. International Journal of Food Microbiology, 31, 357–365. [DOI] [PubMed] [Google Scholar]
- Hielm S, Bjorkroth J, Hyytia E and Korkeala H, 1998. Prevalence of Clostridium botulinum in Finnish trout farms: pulsed‐field gel electrophoresis typing reveals extensive genetic diversity among type E isolates. Applied and Environmental Microbiology, 64, 4161–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm J, Hultgren M and Cardinale M, 2006. Water uptake in herring (Clupea harengus) and sprat (Sprattus sprattus) as a function of area, salinity and fat content. Fisheries Research, 81, 94–99. [Google Scholar]
- Hoel S, Mehli L, Bruheim T, Vadstein O and Jakobsen AN, 2015. Assessment of microbiological quality of retail fresh sushi from selected sources in Norway. Journal of Food Protocology, 78, 977–982. [DOI] [PubMed] [Google Scholar]
- Hoel S, Vadstein O and Jakobsen AN, 2018. Growth of mesophilic Aeromonas salmonicida in an experimental model of nigiri sushi during cold storage. International Journal of Food Microbiology, 285, 1–6. [DOI] [PubMed] [Google Scholar]
- Hoffmann H and Roggenkamp A, 2003. Population Genetics of the Nomenspecies Enterobacter cloacae. Applied and Environmental Microbiology, 69, 5306–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormaeche E and Edwards PR, 1960. A proposed genus enterobacter. International Journal of Systematic and Evolutionary Microbiology, 10, 71–74. [Google Scholar]
- Horowitz BZ, 2010. Type E botulism. Clinical Toxicology, 48, 880–895. [DOI] [PubMed] [Google Scholar]
- Hossain ZZ, Farhana I, Tulsiani SM, Beguml A and Jensen PKM, 2018. Transmission and Toxigenic Potential of Vibrio cholerae in Hilsha Fish (Tenualosa ilisha) for Human Consumption in Bangladesh. Frontiers in Microbiology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini H, Cheraghali AM, Yalfani R and Razavilar V, 2004. Incidence of Vibrio spp. in shrimp caught off the south coast of Iran. Food Control, 15, 187–190. [Google Scholar]
- Huidobro A, Mendes R and Nunes ML, 2001. Slaughtering of gilthead seabream (Sparus aurata) in liquid ice: influence on fish quality. European Food Research and Technology, 213, 267–272. [Google Scholar]
- Huss HH, 1994. Assurance of seafood quality. FAO (Food and Agriculture Organization of the United Nations) Fisheries Technical Paper, No. 334, ix‐169.
- Huss H, 1995. Quality and quality changes in fresh fish. FAO (Food and Agriculture Organization of the United Nations) Fisheries Technical Paper, No. 348, 1–195.
- Huss HH, Reilly A and Karim Ben Embarek P, 2000. Prevention and control of hazards in seafood. Food Control, 11, 149–156. [Google Scholar]
- Hussein MA, Merwad AMA, Elabbasy MT, Suelam IIA, Abdelwahab AM and Taha MA, 2018. Prevalence of Enterotoxigenic Staphylococcus aureus and Shiga Toxin Producing Escherichia coli in Fish in Egypt: Quality Parameters and Public Health Hazard. Vector‐Borne and Zoonotic Diseases, 19, 255–264. [DOI] [PubMed] [Google Scholar]
- Hwang CC, Tseng PH, Lee YC, Kung HF, Huang CY, Chen HC and Tsai YH, 2019. Determination of Histamine in Japanese Spanish Mackerel (Scomberomorus niphonius) Meat Implicated in a Foodborne Poisoning. Journal of Food Protection, 82, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Hyytiä‐Trees E, Lindström M, Schalch B, Stolle A and Korkeala H, 1999. Clostridium botulinum type E in Bavarian fish Archiv Fur Lebensmittelhygiene, 50, 79–82. [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods), 1996. Microorganisms in Foods 5‐Microbiological specifications of food pathogens.
- İKİZ S, DÜMen E, Başaran Kahraman B, Bayrakal G, Kahraman T and ErgİN S, 2016. Deniz Ürünlerinde Salmonella spp. ve Listeria monocytogenes Varlığının Kültürel Metotlar ve PCR İle Araştırılması. Kafkas Universitesi Veteriner Fakultesi Dergisi, 22.
- Ismail NIA, Amal MNA, Shohaimi S, Saad MZ and Abdullah SZ, 2016. Associations of water quality and bacteria presence in cage cultured red hybrid tilapia, Oreochromis niloticus x O. mossambicus. Aquaculture Reports, 4, 57–65. [Google Scholar]
- Isonhood JH and Drake M, 2002. Aeromonas species in foods. Journal of Food Protection, 65, 575–582. [DOI] [PubMed] [Google Scholar]
- Iwamoto M, Ayers T, Mahon BE and Swerdlow DL, 2010. Epidemiology of Seafood‐Associated Infections in the United States. Clinical Microbiology Reviews, 23, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ben‐Gigirey B, Barros‐Velazquez J, Price RJ and An HJ, 2000. Histamine and biogenic amine production by Morganella morganii isolated from temperature‐abused albacore. Journal of Food Protection, 63, 244–251. [DOI] [PubMed] [Google Scholar]
- Jakšić S, Uhitil S, Petrak T, Bažulić D and Gumhalter Karolyi L, 2002. Occurrence of Vibrio spp. in sea fish, shrimps and bivalve molluscs harvested from Adriatic sea. Food Control, 13, 491–493. [Google Scholar]
- Jalali N, Ariiai P and Fattahi E, 2016. Effect of alginate/carboxyl methyl cellulose composite coating incorporated with clove essential oil on the quality of silver carp fillet and Escherichia coli O157:H7 inhibition during refrigerated storage. Journal of Food Science and Technology‐Mysore, 53, 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali H, Paydar M, Ismail S, Looi CY, Wong WF, Radmehr B and Abedini A, 2015. Prevalence, antimicrobial susceptibility and virulotyping of Listeria species and Listeria monocytogenes isolated from open‐air fish markets. Bmc Microbiology, 15, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami M, Ghanbari M, Zunabovic M, Domig KJ and Kneifel W, 2014. Listeria monocytogenes in aquatic food products—a review. Comprehensive Reviews in Food Science and Food Safety, 13, 798–813. [Google Scholar]
- Janda JM and Abbott SL, 2010. The genus aeromonas: taxonomy, pathogenicity, and infection. Clinical Microbiology Reviews, 23, 35–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda JM, Abbott SL and McIver CJ, 2016. Plesiomonas shigelloides Revisited. Clinical Microbiology Reviews, 29, 349–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januario FES and Dykes GA, 2005. Effect of sodium metabisulphite and storage temperature on the survival of Vibrio cholerae on prawns (Penaeus monodon). World Journal of Microbiology & Biotechnology, 21, 1017–1020. [Google Scholar]
- Jarvis B, Lach VH and Wood JM, 1977. Evaluation of the spiral plate maker for the enumeration of micro‐organisms in foods. Journal of Applied Bacteriology, 43, 149–157. [Google Scholar]
- Jarvis B, Corry JEL and Hedges AJ, 2007. Estimates of measurement uncertainty from proficiency testing schemes, internal laboratory quality monitoring and during routine enforcement examination of foods. Journal of Applied Microbiology, 103, 462–267. [DOI] [PubMed] [Google Scholar]
- Ji H, Chen Y, Guo YC, Liu XM, Wen J and Liu H, 2011. Occurrence and characteristics of Vibrio vulnificus in retail marine shrimp in China. Food Control, 22, 1935–1940. [Google Scholar]
- Joensen S, Sorensen N and Akse L, 2001. Cod: storage in ice water increases weight. Fiskeriforskning Info. February No 1, 2 pp.
- Johnston MD and Brown MH, 2002. An investigation into the changed physiological state of Vibrio bacteria as a survival mechanism in response to cold temperatures and studies on their sensitivity to heating and freezing. Journal of Applied Microbiology, 92, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Jung SW, 2018. A foodborne outbreak of gastroenteritis caused by Vibrio parahaemolyticus associated with cross‐contamination from squid in Korea. Epidemiology and Health, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalika J, Ubeyratne H, Kleer J, Hildebrandt G, Fries R, Khattiya R, Padungtod P, Baumann M and Zessin K‐H, 2008. Prevalence of Salmonella in marketed Penaeus monodon shrimps in North Western Province, Sri Lanka. Berliner und Münchener tierärztliche Wochenschrift, 121, 418–421. [PubMed] [Google Scholar]
- Kanki M, Yoda T, Ishibashi M and Tsukamoto T, 2004. Photobacterium phosphoreum caused a histamine fish poisoning incident. International Journal of Food Microbiology, 92, 79–87. [DOI] [PubMed] [Google Scholar]
- Khalafalla MM and El‐Sayed B, 2015. Biological treatment of broad bean hulls and its evaluation through tilapia fingerlings (Oreochromis niloticus) feeding. Journal of Aquaculture Research and Development, 6, 353. [Google Scholar]
- Kilinc B, Cakli S, Cadun A, Dincer T and Tolasa S, 2007. Comparison of effects of slurry ice and flake ice pretreatments on the quality of aquacultured sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) stored at 4 degrees C. Food Chemistry, 104, 1611–1617. [Google Scholar]
- Kim JH, Lee J, Hong S, Lee S, Na HY, Jeong YI, Choi EJ, Kim J, Kawk HS and Cho E, 2018. Cholera Outbreak due to Raw Seafood Consumption in South Korea, 2016. American Journal of Tropical Medicine and Hygiene, 99, 168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H‐J, Kwak H‐S, Yoon S‐H and Woo G‐J, 2012. Phylogenetic group distribution and prevalence of virulence genes in Escherichia coli isolates from food samples in South Korea. World Journal of Microbiology and Biotechnology, 28, 1813–1816. [DOI] [PubMed] [Google Scholar]
- Koonse B, Burkhardt III W, Chirtel S and Hoskin Gp, 2005. Salmonella and the Sanitary Quality of Aquacultured Shrimp. Journal of Food Protection, 68, 2527–2532. [DOI] [PubMed] [Google Scholar]
- Koralage MSG, Alter T, Pichpol D, Strauch E, Zessin KH and Huehn S, 2012. Prevalence and Molecular Characteristics of Vibrio spp. Isolated from Preharvest Shrimp of the North Western Province of Sri Lanka. Journal of Food Protection, 75, 1846–1850. [DOI] [PubMed] [Google Scholar]
- Koutsoumanis KP, Ashton LV, Geornaras I, Belk KE, Scanga JA, Kendall PA, Smith GC and Sofos JN, 2004. Effect of single or sequential hot water and lactic acid decontamination treatments on the survival and growth of Listeria monocytogenes and spoilage microflora during aerobic storage of fresh beef at 4, 10, and 25 degrees C. Journal of Food Protection, 67, 2703–2711. [DOI] [PubMed] [Google Scholar]
- Kramer JM and Gilbert RJ, 1978. Enumeration of micro‐organisms in food: a comparative study of five methods. Journal of Hygiene, 81, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriem MR, Banni B, El Bouchtaoui H, Hamama A, El Marrakchi A, Chaouqy N, Robert‐Pillot A and Quilici ML, 2015. Prevalence of Vibrio spp. in raw shrimps (Parapenaeus longirostris) and performance of a chromogenic medium for the isolation of Vibrio strains. Letters in Applied Microbiology, 61, 224–230. [DOI] [PubMed] [Google Scholar]
- Kumar R, Surendran PK and Thampuran N, 2009. Distribution and genotypic characterization of Salmonella serovars isolated from tropical seafood of Cochin, India. Journal of Applied Microbiology, 106, 515–524. [DOI] [PubMed] [Google Scholar]
- Kumar R, Datta TK and Lalitha KV, 2015. Salmonella grows vigorously on seafood and expresses its virulence and stress genes at different temperature exposure. Bmc Microbiology, 15, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NR, Murugadas V, Joseph TC, Lalitha KV, Basha AK, Muthulakshmi T and Prasad MM, 2019. Need for an Optimized Protocol for Screening Seafood and Aquatic Environment for Shigella sp. Fishery Technology, 56, 164–167. [Google Scholar]
- Labella AM, Castro MD, Manchado M and Borrego JJ, 2018. Description of new and amended clades of the genus photobacterium. Microorganisms, 6 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguerre O, Derens E and Flick D, 2018. Modelling of fish refrigeration using flake ice. International Journal of Refrigeration‐Revue Internationale Du Froid, 85, 97–108. [Google Scholar]
- Laguerre O, Chaomuang N, Derens E and Flick D, 2019. How to predict product temperature changes during transport in an insulated box equipped with an ice pack: Experimental versus 1‐D and 3‐D modelling approaches. International Journal of Refrigeration‐Revue Internationale Du Froid, 100, 196–207. [Google Scholar]
- Lam PW and Salit IE, 2014. Raoultella planticola bacteremia following consumption of seafood. Canadian Journal of Infectious Diseases & Medical Microbiology, 25, E83–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen SG, Ethelberg S, Bjorkman JT, Jensen T, Sorensen G, Jensen AK, Muller L, Nielsen EM and Molbak K, 2016. Two listeria outbreaks caused by smoked fish consumption‐using whole‐genome sequencing for outbreak investigations. Clinical Microbiology and Infection, 22, 620–624. [DOI] [PubMed] [Google Scholar]
- Leclair D, Fung J, Isaac‐Renton JL, Proulx JF, May‐Hadford J, Ellis A, Ashton E, Bekal S, Farber JM, Blanchfield B and Austin JW, 2013. Foodborne Botulism in Canada, 1985–2005. Emerging Infectious Diseases, 19, 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y‐C, 2012. Histamine production by Enterobacter aerogenes in tuna dumpling stuffing at various storage temperatures. Food Chemistry, 131, 405–412. [Google Scholar]
- Lee YC, Lin CM, Huang CY, Huang YL, Chen HC, Huang TC and Tsai YH, 2013. Determination and frying loss of histamine in striped marlin fillets implicated in a foodborne poisoning. Journal of Food Protection, 76, 860–866. [DOI] [PubMed] [Google Scholar]
- Lee YC, Kung HF, Wu CH, Hsu HM, Chen HC, Huang TC and Tsai YH, 2016. Determination of histamine in milkfish stick implicated in food‐borne poisoning. Journal of Food and Drug Analysis, 24, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistner L, 2000. Basic aspects of food preservation by hurdle technology. International Journal of Food Microbiology, 55, 181–186. [DOI] [PubMed] [Google Scholar]
- Lemon DW and Regier LW, 1977. Holding of Atlantic mackerel (scomber‐scombrus) in refrigerated sea‐water. Journal of the Fisheries Research Board of Canada, 34, 439–443. [Google Scholar]
- Leung CK, Huang YW and Harrison MA, 1992. Fate of listeria‐monocytogenes and aeromonas hydrophila on packaged channel catfish fillets stored at 4‐degrees‐C. Journal of Food Protection, 55, 728–730. [DOI] [PubMed] [Google Scholar]
- Li X, Kim M‐J and Yuk H‐G, 2018. Influence of 405 nm light‐emitting diode illumination on the inactivation of Listeria monocytogenes and Salmonella spp. on ready‐to‐eat fresh salmon surface at chilling storage for 8 h and their susceptibility to simulated gastric fluid. Food Control, 88, 61–68. [Google Scholar]
- Li Y, Pei X, Yan J, Liu D, Zhang H, Yu B, Li N and Yang D, 2019. Prevalence of foodborne pathogens isolated from retail freshwater fish and shellfish in China. Food Control, 99, 131–136. [Google Scholar]
- Lin CS, Kung HF, Lin CM, Tsai HC and Tsai YH, 2016. Histamine production by Raoultella ornithinolytica in mahi‐mahi meat at various storage temperatures. Journal of Food and Drug Analysis, 24, 305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke K, Rückerl I, Brugger K, Karpiskova R, Walland J, Muri‐Klinger S, Tichy A, Wagner M and Stessl B, 2014. Reservoirs of Listeria Species in Three Environmental Ecosystems. Applied and Environmental Microbiology, 80, 5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, Picard B and Bugeon J, 2016. How muscle structure and composition influence meat and flesh quality. The Scientific World Journal, 2016, 3182746‐Article ID 3182746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Mou J and Su YC, 2016. Behavior of Salmonella and Listeria monocytogenes in Raw Yellowfin Tuna during Cold Storage. Food, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Sabater EI, Rodríguez‐Jerez J, Hernández‐Herrero M and Mora‐Ventura MT, 1996. Incidence of histamine‐forming bacteria and histamine content in scombroid fish species from retail markets in the Barcelona area. International Journal of Food Microbiology, 28, 411–418. [DOI] [PubMed] [Google Scholar]
- Lopez‐Sabater EI, Rodriguez‐Jerez JJ, Roig‐Sagues AX and Mora‐Ventura MAT, 1994. Bacteriological quality of tuna fish (Thunnus‐Thynnus) destined for canning ‐ Effect of tuna handling on presence of histidine‐decarboxylase bacteria and histamine level. Journal of Food Protection, 57, 318–323. [DOI] [PubMed] [Google Scholar]
- Lopez‐Valladares G, Tham W, Parihar VS, Helmersson S, Andersson B, Ivarsson S, Johansson C, Ringberg H, Tjernberg I, Henriques‐Normark B and Danielsson‐Tham ML, 2014. Human isolates of Listeria monocytogenes in Sweden during half a century (1958‐2010). Epidemiology and Infection, 142, 2251–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentzen G, Olsen RL, Bjorkevoll I, Mikkelsen H and Skjerdal T, 2010. Survival of Listeria innocua and Listeria monocytogenes in muscle of cod (Gadus morhua L.) during salt‐curing and growth during chilled storage of rehydrated product. Food Control, 21, 292–297. [Google Scholar]
- Magalhaes TF, Vieira R, Facanha SHF, Hofer E and Martin AM, 2000. Note. Growth of Vibrio parahaemolyticus in lobster homogenates at different temperatures. Food Science and Technology International, 6, 145–150. [Google Scholar]
- Magnússon H, Sveinsdóttir K, Thorvaldsson L, Guðjónsdóttir M, Lauzon H, Reynisson E, Rúnarsson Á, Magnússon S, Viðarsson J, Arason S and Martinsdóttir E (MATIS), 2010. The effect of different cooling techniques on the quality changes and shelf life of whole cod (Gadus morhua). 28 pp.
- Mai N, Gretar Bogason S, Arason S, Víkingur Árnason S and Geir Matthíasson T, 2010. Benefits of traceability in fish supply chains – case studies. British Food Journal, 112, 976–1002. [Google Scholar]
- Margeirsson B, Magnússon H, Sveinsdóttir K, Valtýsdóttir K, Reynisson E and Arason S, 2010. The effect of different precooling media during processing and cooling techniques during packaging of cod (Gadus morhua) fillets. Matis report 15–10. Available at: http://www.matis.is/media/matis/utgafa/15-10-The-effect-of-different-cooling-media.pdf, 0140‐7007, 30 pp.
- Margeirsson B, 2012. Modelling of temperature changes during transport of fresh fish products. Available online: http://www.matis.is/media/utgafa/krokur/BMPhDThesis.pdf
- Margeirsson B, Lauzon HL, Palsson H, Popov V, Gospavic R, Jonsson MP, Sigurgisladottir S and Arason S, 2012. Temperature fluctuations and quality deterioration of chilled cod (Gadus morhua) fillets packaged in different boxes stored on pallets under dynamic temperature conditions. International Journal of Refrigeration‐Revue Internationale Du Froid, 35, 187–201. [Google Scholar]
- Marshall DL, Andrews LS, Wells JH and Farr AJ, 1992. Influence of modified atmosphere packaging on the competitive growth of Listeria monocytogenes and Pseudomonas fluorescens on precooked chicken. Food Microbiology, 9, 303–309. [Google Scholar]
- Martinez‐Urtaza J, Powell A, Jansa J, Rey JLC, Montero OP, Campello MG, Lopez MJZ, Pousa A, Valles MJF, Trinanes J, Hervio‐Heath D, Keay W, Bayley A, Hartnell R and Baker‐Austin C, 2016. Epidemiological investigation of a foodborne outbreak in Spain associated with US West Coast genotypes of Vibrio parahaemolyticus. Springerplus, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masniyom P, Benjakul S and Visessanguan W, 2006. Synergistic antimicrobial effect of pyrophosphate on Listeria monocytogenes and Escherichia coli O157 in modified atmosphere packaged and refrigerated seabass slices. Lwt‐Food Science and Technology, 39, 302–307. [Google Scholar]
- Matis , 2017. Instructions leaflet. The importance of good onboard handling of fish. Available online: http://www.matis.is/media/baeklingar/the-importance-of-good-onboard-handling-of-fish_en.pdf
- McCarthy S, Bjornsdottir‐Butler K and Benner R, 2015. Storage time and temperature effects on histamine production in Tuna salad preparations. Journal of Food Protection, 78, 1343–1349. [DOI] [PubMed] [Google Scholar]
- Mejlholm O, Gunvig A, Borggaard C, Blom‐Hanssen J, Mellefont L, Ross T, Leroi F, Else T, Visser D and Dalgaard P, 2010. Predicting growth rates and growth boundary of Listeria monocytogenes ‐ An international validation study with focus on processed and ready‐to‐eat meat and seafood. International Journal of Food Microbiology, 141, 137–150. [DOI] [PubMed] [Google Scholar]
- Mellefont LA, McMeekin TA and Ross T, 2003. The effect of abrupt osmotic shifts on the lag phase duration of foodborne bacteria. International Journal of Food Microbiology, 83, 281–293. [DOI] [PubMed] [Google Scholar]
- Minami A, Chaicumpa W, Chongsa‐Nguan M, Samosornsuk S, Monden S, Takeshi K, S‐i Makino and Kawamoto K, 2010. Prevalence of foodborne pathogens in open markets and supermarkets in Thailand. Food Control, 21, 221–226. [Google Scholar]
- Miya S, Takahashi H, Ishikawa T, Fujii T and Kimura B, 2010. Risk of listeria monocytogenes contamination of raw ready‐to‐eat seafood products available at retail outlets in Japan. Applied and Environmental Microbiology, 76, 3383–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi K, Omura K, Ogawa A, Hanafusa M, Nakano Y, Morimatsu S and Sano K, 2001. Survival of Shiga toxin‐producing Escherichia coli O157 in marine water and frequent detection of the Shiga toxin gene in marine water samples from an estuary port. Epidemiology and Infection, 126, 129–133. [PMC free article] [PubMed] [Google Scholar]
- Momtaz H and Yadollahi S, 2013. Molecular characterization of Listeria monocytogenes isolated from fresh seafood samples in Iran. Diagnostic Pathology, 8, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga Y, Yanagihara K, Araki N, Harada Y, Yamada K, Akamatsu N, Matsuda J, Nishino T, Hasegawa H, Izumikawa K, Kakeya H, Yamamoto Y, Yasuoka A, Kohno S and Kamihira S, 2011. Clinical characteristics of seven patients with aeromonas Septicemia in a Japanese Hospital. Tohoku Journal of Experimental Medicine, 225, 81–84. [DOI] [PubMed] [Google Scholar]
- Murray CK and Shewan JM, 1979. The microbial spoilage of fish with special reference to the role of psychrotrophs In: Russell AD, Fuller R. (eds.). Cold tolerant microbes in spoilage and the environment. Academic Press, pp. 117–136. [Google Scholar]
- Murugadas V, 2016. Distribution of Pathotypes of E.coli in seafood from retail markets of Kerala (India). Indian Journal of Fisheries, 63. [Google Scholar]
- Mus TE, Cetinkaya F and Celik U, 2014. Occurrence of Vibrio, Salmonella and Staphylococcus aureus in retail fresh fish, mussel and shrimp. Acta Veterinaria Brno, 83, 75–78. [Google Scholar]
- Nakari UM, Rantala L, Pihlajasaari A, Toikkanen S, Johansson T, Hellsten C, Raulo SM, Kuusi M, Siitonen A and Rimhanen‐Finne R, 2014. Investigation of increased listeriosis revealed two fishery production plants with persistent Listeria contamination in Finland in 2010. Epidemiology and Infection, 142, 2261–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts K, Huys G, Uyttendaele M, Swings J and Debevere J, 2000. Incidence and identification of mesophilic Aeromonas spp. from retail foods. Letters in Applied Microbiology, 31, 359–363. [DOI] [PubMed] [Google Scholar]
- Nguyen DT, Kanki M, Nguyen PD, Le HT, Ngo PT, Tran DN, Le NH, Dang CV, Kawai T, Kawahara R, Yonogi S, Hirai Y, Jinnai M, Yamasaki S, Kumeda Y and Yamamoto Y, 2016. Prevalence, antibiotic resistance, and extended‐spectrum and AmpC beta‐lactamase productivity of Salmonella isolates from raw meat and seafood samples in Ho Chi Minh City. Vietnam. Int J Food Microbiol, 236, 115–122. [DOI] [PubMed] [Google Scholar]
- Norhana MNW, Poole SE, Deeth HC and Dykes GA, 2010. The effects of temperature, chlorine and acids on the survival of Listeria and Salmonella strains associated with uncooked shrimp carapace and cooked shrimp flesh. Food Microbiology, 27, 250–256. [DOI] [PubMed] [Google Scholar]
- Odeyemi OA, 2016. Incidence and prevalence of Vibrio parahaemolyticus in seafood: a systematic review and meta‐analysis. Springerplus, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima C, Sato F, Takahashi H, Kuda T, Kimura B and Tao ZH, 2019. Draft Genome Sequence of the Histamine‐Producing Bacterium Enterobacter kobei Strain 42‐12. Microbiology Resource Announcements, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J, Hite F, McDougald D, Andon N and Simpson L, 1995. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Applied and Environmental Microbiology, 61, 2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson GB, Seppola MA and Olsen RL, 2007. Water‐holding capacity of wild and farmed cod (Gadus morhua) and haddock (Melanogrammus aeglefinus) muscle during ice storage. Lwt‐Food Science and Technology, 40, 793–799. [Google Scholar]
- Ozogul F and Ozogul Y, 2006. Biogenic amine content and biogenic amine quality indices of sardines (Sardina pilchardus) stored in modified atmosphere packaging and vacuum packaging. Food Chemistry, 99, 574–578. [Google Scholar]
- Pagadala S, Parveen S, Rippen T, Luchansky JB, Call JE, Tamplin ML and Porto‐Fett ACS, 2012. Prevalence, characterization and sources of Listeria monocytogenes in blue crab (Callinectus sapidus) meat and blue crab processing plants. Food Microbiology, 31, 263–270. [DOI] [PubMed] [Google Scholar]
- Palumbo SA, Morgan DR and Buchanan RL, 1985. Influence of temperature, NaCI, and pH on the growth of aeromonas hydrophila. Journal of Food Science, 50, 1417–1421. [Google Scholar]
- Pan JH, Zhang YJ, Jin DZ, Ding GQ, Luo Y, Zhang JY, Mei LL and Zhu MY, 2013. Molecular characterization and antibiotic susceptibility of vibrio vulnificus in retail shrimps in Hangzhou, People's Republic of China. Journal of Food Protection, 76, 2063–2068. [DOI] [PubMed] [Google Scholar]
- Pao S, Ettinger MR, Khalid MF, Reid AO and Nerrie BI, 2008. Microbial quality of raw aquacultured fish fillets procured from internet and local retail markets†. Journal of Food Protection, 71, 1544–1549. [DOI] [PubMed] [Google Scholar]
- Pascual S, Antonio J, Cabo ML and Pineiro C, 2010. Anisakis survival in refrigerated fish products under CO2 modified‐atmosphere. Food Control, 21, 1254–1256. [Google Scholar]
- Pattanayaiying R, Sane A, Photjanataree P and Cutter CN, 2019. Thermoplastic starch/polybutylene adipate terephthalate film coated with gelatin containing nisin Z and lauric arginate for control of foodborne pathogens associated with chilled and frozen seafood. International Journal of Food Microbiology, 290, 59–67. [DOI] [PubMed] [Google Scholar]
- Pavan ME, Franco RJ, Rodriguez JM, Gadaleta P, Abbott SL, Janda JM and Zorzópulos J, et al. 2005. Phylogenetic relationships of the genus Kluyvera: transfer of Enterobacter intermedius Izard et al., 1980 to the genus Kluyvera as Kluyvera intermedia comb. nov. and reclassification of Kluyvera cochleae as a later synonym of K. intermedia. International Journal of Systematic and Evolutionary Microbiology, 55, 437–442. [DOI] [PubMed] [Google Scholar]
- Paydar M and Thong KL, 2013. Prevalence and genetic characterization of vibrio vulnificus in raw seafood and seawater in Malaysia. Journal of Food Protection, 76, 1797–1800. [DOI] [PubMed] [Google Scholar]
- Pin C, Marin ML, Garcia ML, Tormo J, Selgas MD and Casas C, 1994. Incidence of motile Aeromonas spp. in foods. Microbiologia, 10, 257–262. [PubMed] [Google Scholar]
- Pin C, Avendano‐Perez G, Cosciani‐Cunico E, Gomez N, Gounadakic A, Nychas GJ, Skandamis P and Barker G, 2011. Modelling Salmonella concentration throughout the pork supply chain by considering growth and survival in fluctuating conditions of temperature, pH and a(w). International Journal of Food Microbiology, 145, S96–S102. [DOI] [PubMed] [Google Scholar]
- Poli BM, Messini A, Parisi G, Scappini F, Vigiani V, Giorgi G and Vincenzini M, 2006. Sensory, physical, chemical and microbiological changes in European sea bass (Dicentrarchus labrax) fillets packed under modified atmosphere/air or prepared from whole fish stored in ice. International Journal of Food Science and Technology, 41, 444–454. [Google Scholar]
- Pons‐Sanchez‐Cascado S, Veciana‐Nogues MT, Bover‐Cid S, Marine‐Font A and Vidal‐Carou MC, 2006. Use of volatile and non‐volatile amines to evaluate the freshness of anchovies stored in ice. Journal of the Science of Food and Agriculture, 86, 699–705. [Google Scholar]
- Popovic NT, Skukan AB, Dzidara P, Coz‐Rakovac R, Strunjak‐Perovic I, Kozacinski L, Jadan M and Brlek‐Gorski D, 2010. Microbiological quality of marketed fresh and frozen seafood caught off the Adriatic coast of Croatia. Veterinární medicína, 55, 233–241. [Google Scholar]
- Presser KA, Ross T and Ratkowsky DA, 1998. Modelling the growth limits (growth no growth interface) of Escherichia coli as a function of temperature, pH, lactic acid concentration, and water activity. Applied and Environmental Microbiology, 64, 1773–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provincial L, Guillen E, Alonso V, Gil M, Roncales P and Beltran JA, 2013a. Survival of Vibrio parahaemolyticus and Aeromonas hydrophila in sea bream (Sparus aurata) fillets packaged under enriched CO2 modified atmospheres. International Journal of Food Microbiology, 166, 141–147. [DOI] [PubMed] [Google Scholar]
- Provincial L, Guillen E, Gil M, Alonso V, Roncales P and Beltran JA, 2013b. Survival of Listeria monocytogenes and Salmonella Enteritidis in sea bream (Sparus aurata) fillets packaged under enriched CO2 modified atmospheres. International Journal of Food Microbiology, 162, 213–219. [DOI] [PubMed] [Google Scholar]
- Pyz‐Łukasik R and Paszkiewicz W, 2018. Microbiological quality of farmed grass carp, bighead carp, Siberian sturgeon, and wels catfish from Eastern Poland. Journal of Veterinary Research, 62, 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S, 1997. Measurement of the thermal properties of seafood. Masters of Science in Biological Systems Engineering, Faculty of the Virginia Polytechnic Institute and. State University, Blacksburg, Virginia: 92. [Google Scholar]
- Ragnarsson SÖ, 2017. Dynamic Thermal Models for the Atlantic Cod and Evaluation of Thermal Resistance of Fish Containers. M.Sc. thesis, Faculty of Industrial Engineering, Mechanical Engineering and Computer Science, University of Iceland. [Google Scholar]
- Rahman S, 2009. Food properties handbook, 2nd Edition CRC Press, Publisher, Boca Raton, FL: 859 pp. [Google Scholar]
- Raissy M, Moumeni M, Ansari M and Rahimi E, 2012. Occurrence of vibrio spp. in lobster and crab from the persian gulf. Journal of Food Safety, 32, 198–203. [Google Scholar]
- Reilly A and Kaferstein F, 1997. Food safety hazards and the application of the principles of the hazard analysis and critical control point (HACCP) system for their control in aquaculture production. Aquaculture Research, 28, 735–752. [Google Scholar]
- Reily LA and Hackney CR, 1985. Survival of vibrio‐cholerae during cold‐storage in artificially contaminated seafoods. Journal of Food Science, 50, 838–839. [Google Scholar]
- Reinhard RG, Mc AT, Flick GJ, Croonenberghs RE, Wittman RF, Diallo AA and Fernandes C, 1996. Analysis of Campylobacter jejuni, Campylobacter coli, Salmonella, Klebsiella pneumoniae, and Escherichia coli O157:H7 in Fresh Hand‐Picked Blue Crab (Callinectes sapidus) Meat (dagger). Journal of Food Protection, 59, 803–807. [DOI] [PubMed] [Google Scholar]
- Rezai R, Ahmadi E and Salimi B, 2018. Prevalence and Antimicrobial Resistance Profile of Listeria Species Isolated from Farmed and On‐Sale Rainbow Trout (Oncorhynchus mykiss) in Western Iran. Journal of Food Protection, 81, 886–891. [DOI] [PubMed] [Google Scholar]
- Ribeiro LF, Barbosa MMC, de Rezende Pinto F, Guariz CSL, Maluta RP, Rossi JR, Rossi GAM, Lemos MVF and do Amaral LA, 2016. Shiga toxigenic and enteropathogenic Escherichia coli in water and fish from pay‐to‐fish ponds. Letters in Applied Microbiology, 62, 216–220. [DOI] [PubMed] [Google Scholar]
- Riedo FX, Pinner RW, Tosca MD, Cartter ML, Graves LM, Reeves MW, Weaver RE, Plikaytis BD and Broome CV, 1994. A point‐source foodborne listeriosis outbreak ‐ documented incubation period and possible mild illness. Journal of Infectious Diseases, 170, 693–696. [DOI] [PubMed] [Google Scholar]
- Robert‐Pillot A, Copin S, Himber C, Gay M and Quilici ML, 2014. Occurrence of the three major Vibrio species pathogenic for human in seafood products consumed in France using real‐time PCR. International Journal of Food Microbiology, 189, 75–81. [DOI] [PubMed] [Google Scholar]
- Rodas‐Suárez OR, Flores‐Pedroche JF, Betancourt‐Rule JM, Quiñones‐Ramírez EI and Vázquez‐Salinas C, 2006. Occurrence and Antibiotic Sensitivity of Listeria monocytogenes Strains Isolated from Oysters, Fish, and Estuarine Water. Applied and Environmental Microbiology, 72, 7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers C, Parveen S, Chigbu P, Jacobs J, Rhodes M and Harter‐Dennis J, 2014. Prevalence of Vibrio parahaemolyticus, and Vibrio vulnificus in blue crabs (Callinectes sapidus), seawater and sediments of the Maryland Coastal Bays. Journal of Applied Microbiology, 117, 1198–1209. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Jerez JJ, Mora‐Ventura MT, Lopez‐Sabater EI and Hernandez‐Herrero M, 1994. Histidine, lysine and ornithine decarboxylase bacteria in Spanish salted semi‐preserved anchovies. Journal of Food Protection, 57, 784–791. [DOI] [PubMed] [Google Scholar]
- Rosner R, 1964. Aeromonas hydrophila as the etiologic agent in a case of severe gastroenteritis. American Journal of Clinical Pathology, 42, 402–404. [DOI] [PubMed] [Google Scholar]
- Ross T, Zhang D and McQuestin OJ, 2008. Temperature governs the inactivation rate of vegetative bacteria under growth‐preventing conditions. International Journal of Food Microbiology, 128, 129–135. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Capillas C and Moral A, 2001. Correlation between biochemical and sensory quality indices in hake stored in ice. Food Research International, 34, 441–447. [Google Scholar]
- Ruiz‐Capillas C and Moral A, 2004. Free amino acids and biogenic amines in red and white muscle of tuna stored in controlled atmospheres. Amino Acids, 26, 125–132. [DOI] [PubMed] [Google Scholar]
- Ruskol D and Bendsen P, 1992. Invasion of S. putrefaciens during spoilage of fish M.Sc. Thesis, Technological Laboratory and the Technical University, Denmark. [Google Scholar]
- Sabry MA, Mansour HA, Ashour RM and Hamza E, 2019. Histamine‐Producing Bacteria and Histamine Induction in Retail Sardine and Mackerel from Fish Markets in Egypt. Foodborne Pathogens and Disease, 16, 597–603. [DOI] [PubMed] [Google Scholar]
- Sallam KI, 2007. Chemical, sensory and shelf life evaluation of sliced salmon treated with salts of organic acids. Food Chemistry, 101, 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanath Kumar H, Otta SK, Karunasagar I and Karunasagar I, 2001. Detection of Shiga‐toxigenic Escherichia coli (STEC) in fresh seafood and meat marketed in Mangalore, India by PCR. Letters in Applied Microbiology, 33, 334–338. [DOI] [PubMed] [Google Scholar]
- Scharer K, Savioz S, Cernela N, Saegesser G and Stephan R, 2011. Occurrence of vibrio spp. in fish and shellfish collected from the Swiss market. Journal of Food Protection, 74, 1345–1347. [DOI] [PubMed] [Google Scholar]
- Seafish Fact Sheet , 2008. Options to improve catch quality on inshore vessels. FS7‐11.08 Nov 2008. Available online: https://www.seafish.org/media/Publications/FS7_11_08_ImproveCatchquality.pdf
- Shawyer M and Pizzal AFM, 2003. The use of ice on small fishing vessels. FAO fisheries technical paper 436.
- Shirai N, Terayama M and Takeda H, 2002. Effect of season on the fatty acid composition and free amino acid content of the sardine Sardinops melanostictus. Comparative Biochemistry Physiology Part B: Biochemistry & Molecular Biology, 131, 387–393. [DOI] [PubMed] [Google Scholar]
- Shirai H, Datta AK and Oshita S, 2017. Penetration of aerobic bacteria into meat: A mechanistic understanding. Journal of Food Engineering, 196, 193–207. [Google Scholar]
- Siddhnath K, Majumdar RK, Parhi J, Sharma S, Mehta NK and Laishram M, 2018. Detection and characterization of Shiga toxin‐producing Escherichia coli from carps from integrated aquaculture system. Aquaculture, 487, 97–101. [Google Scholar]
- Simon SS and Sanjeev S, 2007. Prevalence of enterotoxigenic Staphylococcus aureus in fishery products and fish processing factory workers. Food Control, 18, 1565–1568. [Google Scholar]
- Sing CK, Khan Z, Daud HH and Aziz AR, 2016. Prevalence of salmonella sp. in African Catfish (Clarias gariepinus) Obtained from Farms and Wet Markets in Kelantan. Malaysia and Their Antibiotic Resistance, 45, 1597–1602. [Google Scholar]
- Singh B and Kulshreshtha SB, 1994. A study on prevalence of Staphylococcus aureus in fish and fish products and their public health significance. Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases, 14, 25–28. [Google Scholar]
- Skandamis P, Tsigarida E and Nychas GJE, 2002. The effect of oregano essential oil on survival/death of Salmonella typhimurium in meat stored at 5 degrees C under aerobic, VP/MAP conditions. Food Microbiology, 19, 97–103. [Google Scholar]
- Skåra T, Axelsson L, Stefánsson G, Ekstrand B and Hagen H, 2015. Fermented and ripened fish products in the northern European countries. Journal of Ethnic Foods, 2, 18–24. [Google Scholar]
- Sobel J, Malavet M and John S, 2007. Outbreak of clinically mild botulism type E illness from home‐salted fish in patients presenting with predominantly gastrointestinal symptoms. Clinical Infectious Diseases, 45, E14–E16. [DOI] [PubMed] [Google Scholar]
- Sospedra I, Rubert J, Soriano JM, Manes J and Fuentes MV, 2015. Prevalence of bacteria and absence of anisakid parasites in raw and prepared fish and seafood dishes in Spanish restaurants. Journal of Food Protection, 78, 615–618. [DOI] [PubMed] [Google Scholar]
- Sridhar S, Lo SKF, Xing FF, Yang J, Ye HY, Chan JFW, Teng JLL, Huang C, Yip CCY, Lau SKP and Woo PCY, 2017. Clinical characteristics and molecular epidemiology of hepatitis E in Shenzhen, China: a shift toward foodborne transmission of hepatitis E virus infection. Emerging Microbes and Infections, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenfors LP, Mayr R, Scherer S and Granum PE, 2002. Pathogenic potential of fifty Bacillus weihenstephanensis strains. Fems Microbiology Letters, 215, 47–51. [DOI] [PubMed] [Google Scholar]
- Stock I, 2004. Plesiomonas shigelloides: an emerging pathogen with unusual properties. Reviews in Medical Microbiology, 15, 129–139. [Google Scholar]
- Stonehouse GG and Evans JA, 2015. The use of supercooling for fresh foods: a review. Journal of Food Engineering, 148, 74–79. [Google Scholar]
- Stroudt G, 2001. Rigor in Fish ‐ The Effect on Quality. Torry advisory note 36.
- Tao Z, Larsen AM, Bullard SA, Wright AC and Arias CR, 2012. Prevalence and Population Structure of Vibrio vulnificus on Fishes from the Northern Gulf of Mexico. Applied and Environmental Microbiology, 78, 7611–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Chen Q, Bie X, Lu F and Lu Z, 2017. Investigation on prevalence of Listeria spp. and Listeria monocytogenes in animal‐derived foods by multiplex PCR assay targeting novel genes. Food Control, 73, 704–711. [Google Scholar]
- Tassou CC, Lambropoulou K and Nychas GJE, 2004. Effect of prestorage treatments and storage conditions on the survival of Salmonella enteritidis PT4 and Listeria monocytogenes on fresh marine and freshwater aquaculture fish. Journal of Food Protection, 67, 193–198. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Guthertz LS, Leatherwood M and Lieber ER, 1979. Histamine production By Klebsiella‐Pneumoniae and an incident of scombroid fish poisoning. Applied and Environmental Microbiology, 37, 274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telli AE and Dogruer Y, 2019. Discrimination of viable and dead Vibrio parahaemolyticus subjected to low temperatures using Propidium Monoazide ‐ Quantitative loop mediated isothermal amplification (PMA‐qLAMP) and PMA‐qPCR. Microbial Pathogenesis, 132, 109–116. [DOI] [PubMed] [Google Scholar]
- Telzak EE, Bell EP, Kautter DA, Crowell L, Budnick LD, Morse DL and Schultz S, 1990. An international outbreak of type‐e botulism due to uneviscerated fish. Journal of Infectious Diseases, 161, 340–342. [DOI] [PubMed] [Google Scholar]
- Teophilo G, dos Fernandes Vieira R, dos Prazeres Rodrigues D and Menezes FR, 2002. Escherichia coli isolated from seafood: toxicity and plasmid profiles. International Microbiology, 5, 11–14. [DOI] [PubMed] [Google Scholar]
- Terentjeva M, Eizenberga I, Valciņa O, Novoslavskij A, Strazdiņa V and BĒRZIŅŠ A, 2015. Prevalence of foodborne pathogens in freshwater fish in Latvia. Journal of Food Protection, 78, 2093–2098. [DOI] [PubMed] [Google Scholar]
- Tey YH, Jong KJ, Fen SY and Wong HC, 2015. Occurrence of vibrio parahaemolyticus, vibrio cholerae, and vibrio vulnificus in the aquacultural environments of Taiwan. Journal of Food Protection, 78, 969–976. [DOI] [PubMed] [Google Scholar]
- Tham W, Ericsson H, Loncarevic S, Unnerstad H and Danielsson‐Tham ML, 2000. Lessons from an outbreak of listeriosis related to vacuum‐packed gravad and cold‐smoked fish. International Journal of Food Microbiology, 62, 173–175. [DOI] [PubMed] [Google Scholar]
- Thomas CJ, O'Rourke RD and McMeekin TA, 1987. Bacterial penetration of chicken breast muscle. Food Microbiology (London), 4, 87–96. [Google Scholar]
- Thordarson G, Arason S and Karlsdottir M (MATIS), 2017. Subchilling of fish. 65 pp.
- Thorsen L, Budde BB, Henrichsen L, Martinussen T and Jakobsen M, 2009. Cereulide formation by Bacillus weihenstephanensis and mesophilic emetic Bacillus cereus at temperature abuse depends on pre‐incubation conditions. International Journal of Food Microbiology, 134, 133–139. [DOI] [PubMed] [Google Scholar]
- Tindall BJ, Sutton G and Garrity GM, 2017. Enterobacter aerogenes Hormaeche and Edwards 1960 (Approved Lists 1980) and Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980) share the same nomenclatural type (ATCC 13048) on the Approved Lists and are homotypic synonyms, with consequences for the name Klebsiella mobilis Bascomb et al. 1971 (Approved Lists 1980). International Journal of Systematic and Evolutionary Microbiology, 67, 502–504. [DOI] [PubMed] [Google Scholar]
- Tolstorebrov I, Eikevik T, Widell K and Nordtvedt T, 2019. Study of convective heat transfer and chilling kinetics of Atlantic salmon in refrigerated sea water: experiments and modelling., Montreal, Canada: 8 pp. [Google Scholar]
- Tomiyasu Y and Zenitani B, 1957. Spoilage of fish and its preservation by chemical agents In: Mrak EM. and Stewart GF. (eds.). Advances in Food Research. Academic Press, Massachusetts, USA: pp. 41–82. [Google Scholar]
- Tomlinson N, Geiger SE and Kay WW, 1965. Sodium, potassium, and magnesium concentration and weight changes in fish stored in refrigerated sea‐water in relation to biochemical changes associated with rigor mortis. Journal of Food Science, 30, 126–134. [Google Scholar]
- Tomlinson N, Geiger SE, Boyd JW, Southcotl BA, Gibbard GA and Roach SW, 1974. Comparsion between refrigerated sea water (with or without added carbon dioxide) and ice as storage media for fish to be subsequently frozen. Bulletin de l'Institut International du Froid Annex, 1974–1, 163–168. [Google Scholar]
- Torido Y, Takahashi H, Kuda T and Kimura B, 2012. Analysis of the growth of histamine‐producing bacteria and histamine accumulation in fish during storage at low temperatures. Food Control, 26, 174–177. [Google Scholar]
- Torres‐Vitela MR, Castillo A, Finne G, Rodriguez‐Garcia MO, Martinez‐Gonzales NE and Navarro‐Hidalgo V, 1997. Incidence of vibrio cholerae in fresh fish and Ceviche in Guadalajara, Mexico. Journal of Food Protection, 60, 237–241. [DOI] [PubMed] [Google Scholar]
- Tozzo K, Neto AFG, Spercoski KM, Ronnau M, Soares VM and Bersot LS, 2018. Migration of Salmonella serotypes Heidelberg and Enteritidis in previously frozen chicken breast meat. Food Microbiology, 69, 204–211. [DOI] [PubMed] [Google Scholar]
- Tra VTT, Meng L, Pichpol D, Pham NH, Baumann M, Alter T and Huehn S, 2016. Prevalence and antimicrobial resistance of Vibrio spp. in retail shrimps in Vietnam. Berliner und Münchener tierärztliche Wochenschrift, 129, 48–51. [PubMed] [Google Scholar]
- Traore O, Martikainen O, Siitonen A, Traore AS, Barro N and Haukka K, 2014. Occurrence of Vibrio cholerae in fish and water from a reservoir and a neighboring channel in Ouagadougou, Burkina Faso. Journal of Infection in Developing Countries, 8, 1334–1338. [DOI] [PubMed] [Google Scholar]
- Tsai YH, Chang SC, Kung HF, Wei CI and Hwang DF, 2005. Histamine production by Enterobacter aerogenes in sailfish and milkfish at various storage temperatures. Journal of Food Protection, 68, 1690–1695. [DOI] [PubMed] [Google Scholar]
- Tsai YH, Kung HF, Chen HC, Chang SC, Hsu HH and Wei CI, 2007. Determination of histamine and histamine‐forming bacteria in dried milkfish (Chanos chanos) implicated in a food‐borne poisoning. Food Chemistry, 105, 1289–1296. [Google Scholar]
- Ullmann D, Krause G, Knabner D, Weber H and Beutin L, 2005. Isolation and characterization of potentially human pathogenic, cytotoxin‐producing aeromonas strains from retailed seafood in Berlin, Germany. Journal of Veterinary Medicine, Series B, 52, 82–87. [DOI] [PubMed] [Google Scholar]
- Valero A, Perez‐Rodriguez F, Carrasco E, Fuentes‐Alventosa JM, Garcia‐Gimeno RM and Zurera G, 2009. Modelling the growth boundaries of Staphylococcus aureus: effect of temperature, pH and water activity. International Journal of Food Microbiology, 133, 186–194. [DOI] [PubMed] [Google Scholar]
- Valtýsdóttir K, Margeirsson B, Arason S, Lauzon H and Martinsdóttir E (MATIS), 2010. Guidelines for precooling of fresh fish during processing and choice of packaging with respect to temperature control in cold chains. 43 pp.
- Vandy S, Leakhann S, Phalmony H, Denny J and Roces MC, 2012. Vibrio parahaemolyticus enteritis outbreak following a wedding banquet in a rural village ‐ Kampong Speu, Cambodia, April 2012. Western Pacific Surveillance and Response, 3, 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan P and Venkitanarayanan K, 2006. Role of the rpoS gene in the survival of Vibrio parahaemolyticus in artificial seawater and fish homogenate. Journal of Food Protection, 69, 1438–1442. [DOI] [PubMed] [Google Scholar]
- Vasudevan P, Marek P, Daigle S, Hoagland T and Venkitanarayanan KS, 2002. Effect of chilling and freezing on survival of Vibrio parahaemolyticus on fish fillets. Journal of Food Safety, 22, 209–217. [Google Scholar]
- Vazquez‐Sanchez D, Lopez‐Cabo M, Saa‐Ibusquiza P and Rodriguez‐Herrera JJ, 2012. Incidence and characterization of Staphylococcus aureus in fishery products marketed in Galicia (Northwest Spain). International Journal of Food Microbiology, 157, 286–296. [DOI] [PubMed] [Google Scholar]
- Velut G, Delon F, Merigaud JP, Tong C, Duflos G, Boissan F, Watier‐Grillot S, Boni M, Derkenne C, Dia A, Texier G, Vest P, Meynard JB, Fournier PE, Chesnay A and de Santi VP, 2019. Histamine food poisoning: a sudden, large outbreak linked to fresh yellowfin tuna from Reunion Island, France, April 2017. Eurosurveillance, 24, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkat H, Matthews J, Lumadao P, Caballero B, Collins J, Fowle N, Kellis M, Tewell M, White S, Hassan R, Classon A, Joung Y, Komatsu K, Weiss J, Zusy S and Sunenshine R, 2018. Salmonella enterica Serotype Javiana Infections Linked to a Seafood Restaurant in Maricopa County, Arizona, 2016. Journal of Food Protection, 81, 1283–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visciano P, Schirone M, Tofalo R and Suzzi G, 2012. Biogenic amines in raw and processed seafood. Frontiers in Microbiology, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wait DA and Sobsey MD, 2001. Comparative survival of enteric viruses and bacteria in Atlantic Ocean seawater. Water and Science Technology, 43, 139–142. [PubMed] [Google Scholar]
- Walton RN, Clemens A, Chung J, Moore S, Wharton D, Haydu L, de Villa E, Sanders G, Bussey J, Richardson D and Austin JW, 2014. Outbreak of type E foodborne botulism linked to traditionally prepared salted Fish in Ontario, Canada. Foodborne Pathogens and Disease, 11, 830–834. [DOI] [PubMed] [Google Scholar]
- Wang F, Jiang L, Yang Q, Han F, Chen S, Pu S, Vance A and Ge B, 2011. Prevalence and antimicrobial susceptibility of major foodborne pathogens in imported seafood. Journal of Food Protection, 74, 1451–1461. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Sun WS, Jin MT, Liu HQ, Zhang WJ, Sun XH, Pan YJ and Zhao Y, 2014. Fate of Vibrio parahaemolyticus on shrimp after acidic electrolyzed water treatment. International Journal of Food Microbiology, 179, 50–56. [DOI] [PubMed] [Google Scholar]
- Wang Y‐J, Deering AJ and Kim H‐J, 2020. The occurrence of shiga toxin‐producing E. coli in aquaponic and hydroponic systems. Horticulturae, 6, 1. [Google Scholar]
- Weber JT, Hibbs RG, Darwish A, Mishu B, Corwin AL, Rakha M, Hatheway CL, Elsharkawy S, Elrahim SA, Alhamd MFS, Sarn JE, Blake PA and Tauxe RV, 1993. A massive outbreak of type‐E Botulism associated with traditional salted fish in Cairo. Journal of Infectious Diseases, 167, 451–454. [DOI] [PubMed] [Google Scholar]
- Wieneke AA, Roberts D and Gilbert RJ, 1993. Staphylococcal food poisoning in the United‐Kingdom, 1969–90. Epidemiology and Infection, 110, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AP, Avery LM, Killham K and Jones DL, 2007. Persistence, dissipation, and activity of Escherichia coli O157:H7 within sand and seawater environments. FEMS Microbiology Ecology, 60, 24–32. [DOI] [PubMed] [Google Scholar]
- Wong HC, Chen LL and Yu CM, 1995. Occurrence of vibrios in frozen seafoods and survival of psychrotrophic vibrio‐cholerae in broth and shrimp homogenate at low‐temperatures. Journal of Food Protection, 58, 263–267. [DOI] [PubMed] [Google Scholar]
- Wong HC and Wang P, 2004. Induction of viable but nonculturable state in Vibrio parahaemolyticus and its susceptibility to environmental stresses. Journal of Applied Microbiology, 96, 359–366. [DOI] [PubMed] [Google Scholar]
- Wong HC, Jiang HY, Lin HY and Wang YT, 2015. Microbiological quality of seafood marketed in Taiwan. Journal of Food Protection, 78, 1973–1979. [DOI] [PubMed] [Google Scholar]
- Woodring J, Srijan A, Puripunyakom P, Oransathid W, Wongstitwilairoong B and Manson C, 2012. Prevalence and antimicrobial susceptibilities of vibrio, salmonella, and aeromonas isolates from various uncooked seafoods in Thailand. Journal of Food Protection, 75, 41–47. [DOI] [PubMed] [Google Scholar]
- Wu B, Liang W and Kan B, 2016. Growth Phase, Oxygen, Temperature, and Starvation Affect the Development of Viable but Non‐culturable State of Vibrio cholerae. Frontiers in Microbiology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Neogi SB, Mo Z, Guan W, Shen Z, Zhang S, Li L, Yamasaki S, Shi L and Zhong N, 2010. Prevalence and characterization of antimicrobial resistance of foodborne Listeria monocytogenes isolates in Hebei province of Northern China, 2005‐2007. International Journal of Food Microbiology, 144, 310–316. [DOI] [PubMed] [Google Scholar]
- Yan L, Pei XY, Zhang XL, Guan WY, Chui HX, Jia HY, Ma GZ, Yang SR, Li Y, Li N and Yang D, 2019. Occurrence of four pathogenic Vibrios in Chinese freshwater fish farms in 2016. Food Control, 95, 85–89. [Google Scholar]
- Yang ZQ, Jiao XA, Li P, Pan ZM, Huang JL, Gu RX, Fang WM and Chao GX, 2009. Predictive model of Vibrio parahaemolyticus growth and survival on salmon meat as a function of temperature. Food Microbiology, 26, 606–614. [DOI] [PubMed] [Google Scholar]
- Yang X, Wu Q, Zhang J, Huang J, Chen L, Liu S, Yu S and Cai S, 2015. Prevalence, enumeration, and characterization of Salmonella isolated from aquatic food products from retail markets in China. Food Control, 57, 308–313. [Google Scholar]
- Yano Y, Yokoyama M, Satomi M, Oikawa H and Chen SS, 2004. Occurrence of Vibrio vulnificus in fish and shellfish available from markets in China. Journal of Food Protection, 67, 1617–1623. [DOI] [PubMed] [Google Scholar]
- Yen NTP, Nhung NT, Van NTB, Cuong NV, Tien Chau LT, Trinh HN, Tuat CV, Tu ND, Phu Huong Lan N, Campbell J, Thwaites G, Baker S and Carrique‐Mas J, 2020. Antimicrobial residues, non‐typhoidal Salmonella, Vibrio spp. and associated microbiological hazards in retail shrimps purchased in Ho Chi Minh city (Vietnam). Food Control, 107, 106756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Hwang JH, Han A, Choi YS, Hyun JE and Lee SY, 2016. Evaluation of the microbiological quality of jacopevers and plaices in Korea, 2015‐2016. Food Science and Biotechnology, 25, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga DH and Frank HA, 1982. Histamine‐producing bacteria in decomposing skipjack tuna (Katsuwonus‐Pelamis). Applied and Environmental Microbiology, 44, 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei M, Maktabi S and Ghorbanpour M, 2012. Prevalence of Listeria monocytogenes, Vibrio parahaemolyticus, Staphylococcus aureus, and Salmonella spp. in seafood products using multiplex polymerase chain reaction. Foodborne Pathogens and Disease, 9, 108–112. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang X, Kuang D, Shi X, Xiao W, Zhang J, Gu Z, Xu X and Meng J, 2015a. Prevalence of antimicrobial resistance of non‐typhoidal Salmonella serovars in retail aquaculture products. International Journal of Food Microbiology, 210, 47–52. [DOI] [PubMed] [Google Scholar]
- Zhang P, Baranyi J and Tamplin M, 2015b. Interstrain interactions between bacteria isolated from vacuum‐packaged refrigerated beef. Applied and Environmental Microbiology, 81, 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Qin N, Luo YK and Shen HX, 2015c. Changes in Biogenic Amines and ATP‐Related Compounds and Their Relation to Other Quality Changes in Common Carp (Cyprinus carpio var. Jian) Stored at 20 and 0 degrees C. Journal of Food Protection, 78, 1699–1707. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Liu HQ, Lou Y, Xiao LL, Liao C, Malakar PK, Pan YJ and Zhao Y, 2015d. Quantifying viable Vibrio parahaemolyticus and Listeria monocytogenes simultaneously in raw shrimp. Applied Microbiology and Biotechnology, 99, 6451–6462. [DOI] [PubMed] [Google Scholar]
- Zou Y and Hou X, 2017. Histamine production by Enterobacter aerogenes in chub mackerel (Scomber japonicus) at various storage temperatures. Food Science and Technology, 37, 76–79. [Google Scholar]


