Abstract
The EFSA Panel on Food Additives and Flavourings (FAF) provides a scientific opinion on the safety of the proposed amendment of the specifications for steviol glycosides (E 960) as a food additive, in particular to expand the list of steviol glycosides to 60 steviol glycosides identified in the leaves of Stevia Rebaudiana Bertoni. With the existing specifications, the food additive must be comprised of not less than 95% of the 11 named steviol glycosides. The proposed change is to include all 60 steviol glycosides in the same limit value of 95% and this would allow the presence of up to 5% of impurities. FAF Panel considered that all steviol glycosides share the same metabolic fate, and therefore, the safety of 60 identified steviol glycosides can be based on read‐across from toxicological data previously evaluated by EFSA and the acceptable daily intake (ADI) of 4 mg/kg body weight (bw) per day will apply to all those steviol glycosides. However, according to the proposed change in specifications, there remains a small but not insignificant fraction of the additive that would be undefined and therefore cannot be evaluated by the Panel. The Panel concluded that the inclusion of the 60 steviol glycosides in the proposed specifications for steviol glycoside (E960) would not be of safety concern. However, the Panel cannot conclude on the safety of the proposed amendment to the specifications of steviol glycosides (E 960) as food additive if the purity assay value of not less than 95% for the total content of steviol glycosides is maintained.
Keywords: Steviol glycosides, E960, food additive
Summary
Following a request from the European Commission to the European Food Safety Authority (EFSA), the Panel on Food Additives and Flavourings (FAF) was asked to provide a scientific opinion on the safety of a proposed amendment of the specifications of the food additive steviol glycosides (E 960), in accordance with Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
The present evaluation is based on the data on steviol glycosides in a newly submitted dossier by the applicant and additional information submitted by the applicant during the assessment process in response to a request by EFSA.
The safety of steviol glycosides as a food additive was evaluated by EFSA in 2010 and an acceptable daily intake (ADI) of 4 mg/kg body weight (bw) per day, expressed as steviol equivalents. Following a subsequent EFSA assessment in 2015 (EFSA ANS Panel, 2015a), rebaudioside D and M were included in the specifications for steviol glycosides (E 960).
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) established an ADI for steviol glycosides of 4 mg/kg bw per day, expressed as steviol (JECFA, 2009). At the JECFA meeting in 2019, a framework was adopted for developing specifications for steviol glycosides by four different methods of production (JECFA, 2020). Specifications for steviol glycosides from Stevia Rebaudiana Bertoni obtained by water extraction were prepared (JECFA, 2020).
The applicant asked to amend the existing EU specifications for steviol glycosides to allow for the inclusion of 60 steviol glycosides identified in S. rebaudiana Bertoni leaves, including both ‘major’ and ‘minor’ steviol glycosides, that may comprise the assay value of not less than 95% total steviol glycosides (Appendix A). With the existing specifications, the food additive must be comprised of not less than 95% of the 11 named steviol glycosides. The proposed change is to include all 60 steviol glycosides in the same limit value of 95% and this would allow the presence of up to 5% of impurities. Panel noted that this proposal would allow to have commercial products of lower purity than is currently the case.
Data on ADME of some of steviol glycosides currently listed in the EU specifications have been considered and summarised in previous EFSA opinions (EFSA ANS Panel, 2010, 2015a). Based on the available data set, stevioside and Rebaudioside A are not hydrolysed by digestive enzymes of the upper gastrointestinal tract due to the presence of β‐glycosidic bonds, but subject to microbial metabolism in the colon resulting in the release of the aglycone steviol which is then absorbed. In rats and humans, steviol is further glucuronidated; steviol glucuronide is excreted with urine and faeces.
Two unpublished studies testing major and minor steviol glycosides in human faecal homogenate have been provided by the applicant. Based on the results from unpublished and published studies, the different rebaudiosides analysed share the same metabolic degradation (i.e. deglycosylation) by the gut microbiome in the colon leading to the formation of steviol. Considering the data set, it can be predicted that all 60 steviol glycosides considered in Appendix A follow the same metabolic fate in the colon with an hydrolysation to steviol.
The Panel agreed that the overall metabolic fate of the steviol glycosides listed in Appendix A is the same; therefore, it would be acceptable to use a read across approach taking the toxicological data previously evaluated by EFSA, for the safety assessment of the 60 steviol glycosides and the ADI of 4 mg/kg bw per day will apply to all those steviol glycosides.
Regarding the genotoxicity, none of the submitted studies are considered adequate to assess the genotoxic potential of the steviol glycosides preparations to be used as a food additive.
As part of the application dossier, the applicant has provided two published studies investigating the effect of dietary administration of rebaudioside A and S. rebaudiana extract on reproductive organs. Overall, in a study on ovarian function in rats with rebaudioside A, no effects were observed relevant for hazard assessment. In addition, the study on sexual function in diabetic rats with a S. rebaudiana extract cannot be used for hazard assessment.
The findings from the submitted genotoxicity studies and studies on reproductive organs and related effects do not change the conclusions reached by the EFSA ANS Panel on genotoxicity and reproductive toxicity when steviol glycosides were evaluated for their authorisation as a food additive (EFSA ANS Panel, 2010). However, it is not possible to conclude if the mixture tested in all the various genotoxicity studies previously evaluated by EFSA, can be considered to be sufficiently representative of other kinds of mixtures that the proposed changes in the specifications would allow.
Overall, the Panel considered that read‐across is justified and the list of 60 steviol glycosides listed in Appendix A could provide a basis for an amendment of the specifications for steviol glycosides. However, the proposed change from 11 to 60 specified steviol glycosides, whilst maintaining the assay value of not less than 95%, would allow less pure preparations of the food additive onto the market. According to the proposed change in specifications, there remains a small but not insignificant fraction of the additive that would be undefined and therefore cannot be evaluated by the Panel.
The Panel concluded that the inclusion of the 60 steviol glycosides in the proposed specifications for steviol glycoside (E960) would not be of safety concern. However, the Panel cannot conclude on the safety of the proposed amendment to the specifications of steviol glycosides (E 960) as food additive if the purity assay value of not less than 95% for the total content of steviol glycosides is maintained.
1. Introduction
The present scientific opinion deals with the safety evaluation of a proposed modification of the EU specifications of the already authorised food additive, steviol glycosides (E 960) to expand the list of steviol glycosides to 60 steviol glycosides identified in the leaves of Stevia Rebaudiana Bertoni.
1.1. Background and Terms of Reference as provided by the European Commission
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives.1 Only food additives that are included in the Union list, in particular in Annex II to that regulation, may be placed on the market and used as in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in Commission Regulation (EU) No 231/2012.2
Steviol glycosides (E 960) is an authorised food additive in the European Union for use in several food categories and specifications have been adopted for it. Presently, those specifications stipulate steviol glycosides (E 960) as a final product containing not less than 95% of 11 identified steviol glycosides ‐ stevioside, rebaudiosides A, B, C, D, E, F, and M, steviolbioside, rubusoside and dulcoside in any combination and ratio.
In 2017, the European Commission received a request vis‐a‐vis and amendment of the specifications of steviol glycosides (E 960) to include all steviol glycosides identified in Stevia rebaudiana Bertoni leaf extracts, including both “major” and “minor” glycosides, that may contribute to the assay value of not less than 95% on a dried weight basis total steviol glycosides. The request was assessed by EFSA (EFSA ANS Panel, 2018) which concluded that the submitted data were insufficient to assess the safety of the proposed amendment of specifications.
In January 2019, the European Commission received an updated application containing additional information, which according to the applicant, should address the concerns and questions raised in the EFSA's scientific opinion (EFSA ANS Panel, 2018).
1.1.2. Terms of Reference
The Commission requests the European Food Safety Authority to provide a scientific opinion on the safety of the proposed amendment of the specifications of that food additive in accordance with the Regulation (EC) No 1331/2008 establishing a common authorisation procedure for food additives, food enzymes and food flavourings.
1.2. Information on existing authorisations and evaluations
Steviol glycosides (E 960) from water extraction of the leaves of the Stevia rebaudiana Bertoni plant and described as ‘not less than 95% steviolbioside, rubusoside, dulcoside A, stevioside, rebaudiosides A, B, C, D, E, F and M on the dried basis, in any combination and ratio’ is an authorised food additive in the EU according to Regulation (EC) No 1333/2008 on food additives and specifications have been defined in Commission Regulation (EU) No 231/2012.
The safety of steviol glycosides as a food additive was evaluated by EFSA in 2010 and an acceptable daily intake (ADI) of 4 mg/kg body weight (bw) per day, expressed as steviol equivalents, based on application of a 100‐fold uncertainty factor to the no observed adverse effect level (NOAEL) from a 2‐year carcinogenicity study in the rat was established (EFSA ANS Panel, 2010). Following a subsequent EFSA assessment in 2015 (EFSA ANS Panel, 2015a), rebaudioside D and M were included in the specifications for steviol glycosides (E 960).
In 2019, the EFSA FAF Panel performed a safety evaluation for a modification of the specifications following a new production process of steviol glycosides (E 960) (EFSA FAF Panel, 2019). The FAF Panel concluded that there is no safety concern for Rebaudioside M produced via enzymatic bioconversion, however recommended that the European Commission considers establishing separate specifications for Rebaudioside M produced via enzymatic bioconversion of purified stevia leaf extract in Commission Regulation (EU) No 231/2012.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) established an ADI for steviol glycosides of 4 mg/kg bw per day, expressed as steviol (JECFA, 2009).
In 2016, JECFA confirmed that rebaudioside A from multiple gene donors expressed in Yarrowia lipolytica is included in the ADI of 4 mg/kg bw, expressed as steviol (JECFA, 2016). JECFA has prepared new specifications for Rebaudioside A from Multiple Gene Donors Expressed in Yarrowia lipolytica) for the yeast‐derived product, recognising that it was manufactured by a distinctly different, biosynthetic process compared with stevia leaf‐derived products (JECFA, 2017).
JECFA recently issued new specifications for ‘Steviol Glycosides from Stevia rebaudiana Bertoni’ that consist of a mixture of compounds containing a steviol backbone conjugated to any number or combination of the principal sugar moieties (glucose, rhamnose, xylose, fructose and deoxyglucose) in any of the orientations occurring in the leaves of S. rebaudiana Bertoni, provided that the total percentage of steviol glycosides is not less than 95% (JECFA, 2017).
At the JECFA meeting in 2019, a framework was adopted for developing specifications for steviol glycosides by four different methods of production (JECFA, 2020). Specifications for steviol glycosides from Stevia Rebaudiana Bertoni obtained by water extraction were prepared (JECFA, 2020).
2. Data and methodologies
Data
The present evaluation is based on the data submitted in the application dossier (‘Documentation provided to EFSA’ No 1) and additional information submitted by the applicant during the assessment process following a request by EFSA (‘Documentation provided to EFSA’ No 2 and Documentation provided to EFSA’ No 3).
Following the request for additional data sent by EFSA on 25 November 2019, the applicant requested a clarification teleconference on 06 December 2020, after which the applicant provided additional data on 03 January 2020.
Methodologies
This opinion was formulated following the principles described in the EFSA Guidance of the Scientific Committee on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing Guidances from the EFSA Scientific Committee.
The Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012) was followed for the assessment.
3. Assessment
3.1. Technical data
3.1.1. Proposed identity of the food additive by the applicant
Specifications for steviol glycosides (E 960) have been defined in the Commission Regulation (EU) No 231/2012. The current EU specifications for steviol glycosides (E 960) stipulate that a steviol glycoside preparation must contain not less than 95% of 11 named steviol glycosides: stevioside, rebaudiosides A, B, C, D, E, F and M, steviolbioside, rubusoside and dulcoside A.
According to the applicant, the stevia leaf extracts contain three groups of steviol glycosides which they have described as: ‘major’ steviol glycosides for which commercial quantitative reference standards are available; ‘minor’ steviol glycosides which are characterised compounds but for which commercial quantitative reference standards are not available: and ‘related’ steviol glycosides which contain the steviol backbone but for which complete structural elucidation has not been determined (Documentation provided to EFSA No 2). The method of analysis including quantification is described at Section 3.1.2 below. The ‘major’ and ‘minor’ steviol glycosides referred by the applicant are listed in Appendix A.
The applicant asked to amend the existing EU specifications for steviol glycosides to allow for the inclusion of 60 steviol glycosides identified in S. rebaudiana Bertoni leaves, including both ‘major’ and ‘minor’ steviol glycosides, that may comprise the assay value of not less than 95% total steviol glycosides (Appendix A).
The applicant stated that slight modifications can be applied at certain stages of the steviol glycoside production processes (e.g. extraction, purification), while still adhering to the same general manufacturing principles (hot water extraction of Stevia leaves followed by isolation and step‐wise purification using ion exchange resins and alcohol solvents). Therefore, it is possible to obtain steviol glycoside preparations with higher levels of ‘minor’ steviol glycosides than the levels present in currently available steviol glycoside products (Documentation provided to EFSA No 1). Steviol glycoside mixtures with different ratios of steviol glycosides may be produced; the steviol glycoside distribution is specific to each individual preparation and could contain any of the identified steviol glycosides (see Appendix A) (Documentation provided to EFSA No 2).
According to the applicant, all steviol glycoside constituents are glycosylated derivatives of the aglycone steviol and as such, all share the same backbone structure, differing only with respect to the type and number of glycoside units (glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose and galactose) at positions R1 and R2 (Figure 1). Sixty steviol glycosides have been identified in S. rebaudiana ranging from the lowest molecular weight (steviolmonoside, 481 g/mol) to the highest one (rebaudioside IX, 1,778 g/mol) (See Appendix A). Steviol glycosides were isolated from different steviol glycoside extracts after several extraction and crystallisation steps; not every stevia leaf extract may contain all of the steviol glycosides in measurable quantities.
Figure 1.

Structure of the steviol backbone
3.1.2. Specifications
According to the applicant (Documentation provided to EFSA n. 1), steviol glycosides are completely hydrolysed to steviol in the intestine at generally similar rates and, therefore, the necessity for steviol glycoside preparations from S rebaudiana Bertoni to be comprised of specific individual steviol glycoside is no longer relevant. The following three changes to the EU specifications for E 960 steviol glycosides are proposed by the applicant (see Appendix B):
The definition of steviol glycosides be expanded to include 60 steviol glycosides identified in S. rebaudiana Bertoni leaves (conjugated with glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose and galactose) that may comprise the assay value of not less than 95%;
The listing of the chemical names, molecular formulas, molecular weights and Chemical Abstracts Service (CAS) numbers for individual steviol glycosides be replaced with 1 structural formula depicting the steviol backbone with R1 and R2 groups that ‘can be 1 or more sugar moieties, including glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose, and galactose’, as listed in Appendix A in this opinion;
Microbiological parameters be added to the specification as follows: Total (aerobic) plate count – not more than 1,000 CFU/g; Yeasts and moulds – not more than 200 CFU/g; E. coli – negative in 1 g; Salmonella – negative in 25 g.
According to the applicant (Documentation provided to EFSA n. 3), the current EU specification of steviol glycosides specifies that the purity of the leaf extract should be no less than 95% of the 11 named glycosides. The specifications also lists total ash < 1%, loss of drying < 6% and residual solvents, methanol < 0.2% and ethanol < 0.5%. Adding the ash and residual solvents together with the major and minor steviol glycosides, the total composition gets close to 100%. The difference between the estimated composition and 100% is mainly contributed by related glycosides. Therefore, according to the applicant, the assay purity should be maintained at not less than 95%.
According to the applicant, the additive may contain residues of processing aid used in the manufacturing process. Several other related steviol glycosides that may be present in the Stevia rebaudiana plant or generated as a result of the production process, have been identified in small amounts (0.1–2.0% w/w) (Appendix B).
The Panel noted that even allowing for the presence of ash, residuals solvents and residues of processing aids and these other related glycosides, the proposed specification value of 95% still gives room for an appreciable unknown fraction.
Regarding toxic elements, in addition to the maximum limits for lead and arsenic already included in the current EU specifications for E 960, results for the analysis of cadmium and mercury were also reported (< 0.005 mg/kg for cadmium and mercury). Based on the analytical data provided, the Panel noted that maximum limits for cadmium and mercury should be added to the proposed specifications. Additionally, lower levels of the maximum limits for lead and arsenic could be considered in the proposed specifications since the results obtained in the five batches analysed were < 0.011 mg/kg for arsenic and < 0.42 mg/kg for lead.
In view of the botanical origin of the stevia, limits for the presence of pesticides should be considered. The same samples as were tested for toxic elements were subjected to a multiresidue pesticide screen that covered a range of commonly used pesticides. No pesticide residues were detected in any of the finished samples. The Panel noted that MRLs for pesticides set under Regulation (EC) 396/20053 apply to stevia (Stevia rebaudiana) as listed in Part B of Annex I. For processed products derived from stevia, the provisions of Article 20 are applicable, meaning that the changes in the levels of pesticide residues caused by processing need to be taken into account.
No information was provided by the applicant on particle size of the product in powder form.
With regard to a method of analysis, the applicant described an HPLC/UV assay in tandem with MS detection. By this method, steviol glycoside extract preparations can be assayed for ‘major’ and ‘minor’ steviol glycosides, including compounds for which commercial quantitative reference standards are not presently available, to determine the total steviol glycoside content. The principle of the methods is the same as those described in the JECFA monograph (JECFA, 2017), whereby the 13 major steviol glycosides can be separated and quantified by a validated HPLC/UV method against the available glycoside standards. The remaining minor steviol glycosides are identified by HPLC/UV/MS, and the concentration of each of them is calculated using their HPLC‐UV peak area, corrected for the molecular mass of the minor glycoside ratioed to the molecular mass of rebaudioside A (967 amu), and the rebaudioside A UV standard curve.
Information on the composition of two representative steviol glycosides preparations that do not meet the current EU specifications was provided. The content of the 11 steviol glycosides recognised in the EU specifications is below 95%, however, if the content of rebaudioside N, rebaudioside O and other ‘minor’ steviol glycosides are included in the assay value, then the total steviol glycoside content is above the 95% limit value.
3.1.3. Manufacturing process
The crushed Stevia leaves are extracted with hot water and the resulting extract is subjected to isolation and purification steps (by use of ion exchange resins and alcohol solvents (methanol and/or ethanol)). It is recognised that slight modifications can be applied at certain stages of the production process (e.g. extraction, purification), while still adhering to the same general principles, to obtain steviol glycoside preparations with specific distributions of individual steviol glycosides (‘major’ and ‘minor’). In particular, the adsorption column system contains different sections that adsorb different proportions of the various steviol glycosides from a mixture. Because these different sections are desorbed separately with solvent(s), steviol glycoside mixtures with different ratios of individual steviol glycosides may be produced simultaneously. This initial stage is followed by additional purification steps, including further and repeated recrystallisation and separation steps. The production process involves the use of other processing aids, calcium hydroxide as a flocculant and activated carbon as decolourising agent. This process results in a preparation that contains 95% total steviol glycosides (Documentation provided to EFSA No.1).
3.1.4. Methods of analysis in food
No method of analysis of steviol glycosides in foods was provided.
3.1.5. Stability of the substance, and reaction and fate in food
A pH stability test with two steviol glycosides mixtures (one with > 50% Reb A and another with Reb D (55–68%) and Reb M (17–27%)) at different temperatures (5–56°C) was provided. The degradation was observed at pH 2 at temperature above 25°C and at pH 3–8 at 56°C. Steviol glycosides mixture (> 50% Reb A) in powder was found to be stable during 122 weeks storage in glass amber bottles at 40°C.
According to the applicant: ‘stability data for rebaudioside A and stevioside in food matrices can be extrapolated to steviol glycosides in general, and the conclusions regarding the stability of steviol glycosides made by EFSA and other scientific bodies (that steviol glycosides are thermally and hydrolytically stable for use in foods and acidic beverages under normal processing and storage conditions) can be extended to include the “major” and “minor” steviol glycosides present in the steviol glycoside mixtures that are the subject of this safety assessment’. Based on the structural similarities between steviol glycosides, the Panel agreed that the stability findings previously evaluated (EFSA, 2010) can be extrapolated to include the major and the minor steviol glycosides as suggested by the applicant.
3.2. Proposed uses and use levels
Maximum level of steviol glycosides (E 960) expressed as steviol equivalents are defined in Annex II to Regulation (EC) No 1333/2008.1
The proposed amendment to expand the definition of steviol glycosides to include all those identified in the extracts from the Stevia Rebaudiana plant is proposed for use as high‐intensity sweetener in food and beverages under the same conditions as those already approved for steviol glycosides (E 960) in the EU (Regulation (EC) No 1333/2008) (Documentation provided to EFSA n. 1).
3.3. Exposure data
Because the proposed uses and use levels for the proposed amendment to expand the definition of steviol glycosides to include all those identified in the extracts from the Stevia Rebaudiana plant are the same as the already authorised food additive steviol glycosides (E 960), the applicant did not provide an exposure estimate but made reference to the latest estimated exposure to E 960 (e.g. EFSA ANS Panel, 2015b).
The Panel considers that if steviol glycosides would be replaced by steviol glycosides mixture containing both ‘major’ and ‘minor’ steviol glycosides, exposure to steviol glycosides mixture (expressed as steviol equivalent) will not be higher than the last EFSA estimate of exposure to steviol glycosides (E 960) (EFSA ANS Panel, 2015b).
3.4. Biological and Toxicological data
Within the application dossier, scientific publications considered by the applicant relevant to the safety assessment of steviol glycosides were submitted.
The present evaluation will focus on existing and new data provided by the applicant on the rates of in vitro hydrolysis of ‘major’ and ‘minor’ steviol glycosides by pooled faecal homogenates from human volunteers.
Only toxicological studies with steviol glycosides not previously evaluated by EFSA have been considered in the current assessment by the Panel.
3.4.1. Absorption, distribution, metabolism and excretion
Data on ADME of some of steviol glycosides currently listed in the EU specifications have been considered and summarised in previous EFSA opinions (EFSA ANS Panel, 2010, 2015a). According to these opinions, stevioside and Rebaudioside A are not hydrolysed by digestive enzymes of the upper gastrointestinal tract due to the presence of β‐glycosidic bonds. After entering the colon intact, these two steviol glycosides are subject to microbial degradation by the gut microbiome, resulting in the release of the aglycone steviol which is then absorbed. In rats and humans, absorbed steviol is glucuronidated; steviol glucuronide is then excreted in the urine and faeces.
The microbial hydrolysis of different steviol glycosides, in particular rebaudioside A, B, C, D, E, F, M, steviolbioside and stevioside (with different purity levels or purity not specified) has been investigated in vitro with human faecal incubations.
Summary of published studies submitted by the applicant
Published ADME studies testing different steviol glycosides have been considered. A tabulated summary of these studies is available in Appendix C. These are in vivo studies in both rats and humans with oral administration of rebaudioside A or stevioside.
In vivo rat studies analysed the levels of steviol, rebaudioside A and stevioside in blood, urine, faeces and organs. These studies showed that rebaudioside A and stevioside are metabolised in the lower gut by gut microbioma and do not accumulate in the organs (Wingard et al., 1980; Nakayama et al., 1986; Roberts and Renwick, 2008; Roberts et al., 2016).
Human in vivo studies with stevioside and rebaudioside A confirmed that both products are degraded to steviol by gut microbioma (Simonetti et al., 2004; Geuns et al., 2006; Wheeler et al., 2008). One in vivo study investigated the kinetic of stevioside in human and showed that the final metabolite steviol reaches the Cmax between 19 and 20 h post‐administration and is not detectable in blood at 48 h post‐dose (Roberts et al., 2016). The results of these studies showed clear similarities in metabolic disposition between rat and human.
In vitro studies with human faecal homogenates showed that different rebaudiosides shared the same metabolic fate with a complete hydrolysis to steviol (Koyama et al., 2003; Purkayastha et al., 2014, 2015, 2016). The rate of degradation varies to some extent with the number and type of sugars bound to the steviol aglycone structure. Rebaudioside A has the fastest hydrolysation rate and rebaudioside F has the slowest rate (Purkayastha et al., 2016). One early in vitro study with rat caecal bacterial cells with rebaudioside A confirmed the degradation of rebaudioside A to steviol (Wingard et al., 1980). One study investigated the metabolism of stevia mixture (rebaudioside A, stevioside, rebaudioside C and Dulcoside A) and a mixture of enzymatically modified stevia (α‐monoglucosylrebaudioside A‐1, α‐monoglucosylrebaudioside A‐2, α‐monoglucosylrebaudioside A‐3, α‐monoglucosylstevioside‐1, α‐monoglucosylstevioside‐2 and α‐diglucosylstevioside) in faecal homogenate. This study showed that rebaudioside A, stevioside, α‐monoglucosylrebaudioside A and α‐monoglucosylstevioside are deglycosylated first and then hydrolysed to steviol (Koyama et al., 2003).
The Panel noted that the steviol glycosides tested (A, B, C, D, E, F, M, steviobioside and stevioside) are already authorised and were previously assessed by EFSA (EFSA ANS Panel, 2010, 2015a,b).
Additional unpublished in vitro studies submitted by the applicant
A mixture of steviol glycosides (RAF) composed by 7% w/w rebaudioside A and 93% w/w fructosylated rebaudioside A has been tested in human faecal homogenate samples (BRI, 2015 Documentation provided to EFSA N.1). RAF (0.2 mg/mL) were incubated with male and female faecal homogenate samples under anaerobic conditions at 37°C for 4–48 h. Liquid chromatography‐mass spectrometry (LC/MS) was used to measure the rate of formation and amount of steviol generated from the microbial hydrolysis. Rebaudioside A and fructosylated rebaudioside A were completely metabolised to steviol after both 24‐ and 48‐hours incubation.
A subsequent in vitro metabolic study in human faecal homogenate samples has been performed with a mixture of steviol glycoside A95 or a mixture of minor steviol glycosides (rebaudioside AM, W2, Y, U2, V N and O) over an incubation period of 72 h (BRI, 2018 Documentation provided to EFSA N.1). Steviol glycoside A95 is a mixture of 13 different steviol glycosides with the main components being rebaudioside D and M (58 and 21%, respectively). Other steviol glycosides such as rebaudioside A, B, C, E, F, O, N and stevioside are present in low percentage. Steviol glycoside A95 was incubated in faecal homogenates of adult male, adult female and paediatric samples at a concentration of 0.2 mg/mL under anaerobic conditions at 37°C for between 4 and 72 h. The steviol glycoside mixture prepared with minor steviol glycosides was incubated with adult male and adult female pooled faecal homogenate samples at concentrations of 0.2 and 0.4 mg/mL under anaerobic conditions at 37°C for 4–48 h. Liquid chromatography‐mass spectrometry (LC/MS) analysis was used to provide metabolic mass balance on the molar equivalent formation of the steviol metabolite over the time course. Rebaudioside M and D present in steviol glycoside A95 deglucosylated to steviol over the first 12 hours in both adult and paediatric samples. Individual minor steviol glycosides (Reb AM, W2, Y, U2, V, N and O) showed also a deglucosylation to steviol within 12 hours of incubation in adult samples. Differences in the rate of degradation were observed among major steviol glycosides A95 with the rebaudioside M and D showing an almost complete deglucosylation at the 12‐hour time point. Among the minor steviol glycosides rebaudiosides AM and O had the quickest rate of degradation with a detected concentration of approximately 50% detected at the 4‐hour time point and almost complete deglucosylation at the 12 h. Samples from female adults showed a more rapid degradation compared to male adult samples for rebaudioside AM, N and O.
In vitro metabolism studies demonstrated that major and minor steviol glycosides are extensively metabolised to steviol within 12 h. The data also confirm similar degradation rates between gender and between adults and children, except for rebaudioside AM, N and O for which a more rapid degradation has been measured in the female adult samples. The Panel noted that some ‘minor’ glycosides have been tested only in one in vitro metabolic study (BRI, 2018 Documentation provided to EFSA N.2).
Based on available knowledge, the Panel noted steviol glycosides with β‐glycosidic bonds are not hydrolysed by digestive enzymes of the upper gastrointestinal tract but subject to microbial metabolism in the colon. Considering the data set, it can be presumed that all 60 steviol glycosides considered in Appendix A follow the same metabolic fate in the colon with an hydrolysation to steviol. Some minor differences in degradation rate can be anticipated depending on the number and type of sugar moieties bound to the steviol (aglycone).
3.4.2. Acute toxicity
No new acute toxicity studies were provided.
3.4.3. Short‐term and subchronic toxicity
No new short‐term or subchronic toxicity studies were provided.
3.4.4. Genotoxicity
Sharif and co‐workers investigated the genotoxic potential of stevioside in CCD18Co myofibroblasts and human HCT116 cells using the in vitro alkaline comet assay (Sharif et al., 2017). A single concentration (200 μM) was tested and no information on the composition and the purity of the test item was provided. The authors concluded that the test item did not exhibit genotoxic effects. Considering that the testing method used is not validated for regulatory purposes and the nature of the test item is not clear, this study could not be used for the assessment of genotoxicity.
The genotoxic potential of stevia was evaluated using in vitro micronucleus and chromosomal aberration tests in human lymphocytes (Uçar et al., 2018). In both the assays, the test item (stevia extract, no further information on steviol glycoside composition) was added up to a concentration of 16 μg/mL, 24 and 48 h after the culture initiation and cells were harvested after 72 h. The range of the concentration used was chosen on the basis of the ADI, with the maximum concentration corresponding to 4× ADI. No significant increases in the number of chromosomal aberrations or micronuclei were reported at any stevia test concentration when compared to the negative control. The Panel noted that the experimental conditions used in this study do not meet the recommendations of the OECD test guidelines 473 and 487. In particular: (i) the tests were conducted only in the absence of metabolic activation; (ii) the treatment timings used for both the assays were different from the indications of the OECD guidelines; (iii) the tested concentrations were chosen on the basis of the ADI rather than considering the cytotoxicity of the treatment; (iv) the cytotoxicity of the treatment was not reported. Moreover, the information provided on the test item are not sufficient to clarify if it is representative of the additive under evaluation. Overall, also these tests cannot be considered suitable to assess the genotoxic potential of the stevioside.
A third experimental study (Zhang et al., 2017) submitted by the applicant included three different assays conducted with an ethanolic extract of S. rebaudiana Bertoni leaves: a bacterial reverse mutation assay, an in vivo micronucleus and a mouse sperm malformation assay.
The bacterial reverse mutation assay was conducted in S. typhimurium TA97, TA98, TA100, and TA102, exposed to concentrations of up to 5,000 μg/plate of stevia extract in the presence or absence of metabolic activation. No increase in the number of revertants was observed in any experimental condition. This test was conducted in line with the OECD test guideline 471, except that the strain TA1535 was not included in the strain battery.
In the in vivo micronucleus assay, the animals (Kunming mice, 5/sex per group) were administered the test item by oral gavage at doses up to 10,000 mg/kg bw twice at a 24‐h interval. For each animal, 200 red blood cells and 1000 polychromatic erythrocytes (PCE) were observed to determine the proportion of PCE and incidence of micronuclei. No mutagenic effect was reported at any dose evaluated. However, no evidence of target cells exposure (alteration of NCE/PCE ratio) was provided; therefore, the study should be considered inconclusive.
The mouse sperm malformation assay is based on a morphological evaluation of the treatment effect on spermatozoa: it is not a genotoxicity test and it is not validated for any endpoint relevant to regulatory toxicology.
Also, in these tests, the information provided on the test item are not sufficient to clarify if it is representative of the additive under evaluation.
3.4.5. Chronic toxicity and carcinogenicity
No new chronic toxicity or carcinogenicity studies were available.
3.4.6. Studies on reproductive organs and related effects
As part of the application dossier, the applicant has provided the published studies Jiang et al. (2018) and Ghaheri et al. (2018) investigating the effect of dietary administration of rebaudioside A and S. rebaudiana extract on reproductive organs.
The potential effect of rebaudioside A on ovary function in rats was investigated by Jiang et al. (2018). Rebaudioside A was provided in drinking water to weanling Sprague‐Dawley rats (n = 6 females/group) for a total of 48 days at dose levels of 0, 0.5 or 2.5 mM (equal to 0, 210 or 1,430 mg/kg bw per day). Food and water were provided ad libitum. In the high‐dose group, body weight was significantly decreased from day 18 to 30 of the study; however, at the end of the study, no significant difference in body weight between the high‐dose rebaudioside A and control groups was observed. Water consumption was significantly increased in the high‐dose group during the entire study period, while the low‐dose group was reported to be notably different from the control group after 21 days of treatment. Furthermore, the high‐dose group water consumption was reported to be remarkable higher than the low‐dose group in the last 3 weeks of treatment. Conversely, rebaudioside A was not reported to influence food intake in either group. No significant differences between the control and test article treated groups were reported for puberty onset (vaginal opening) or body weights at puberty, nor were differences in oestrous cycles observed. No morphological changes in the ovaries of the rebaudioside A treated groups were reported. Serum levels of progesterone in the rebaudioside A groups were significantly decreased compared to the control group and decreased expression of 3β‐hydroxysteroid dehydrogenase, an enzyme involved in progesterone synthesis, was measured in the ovaries via Western blotting. Several other steroidogenesis‐related factors were also reported to decrease based on the Western blot results; however, the significance of these findings with respect to safety is limited as no effects on ovarian morphology or oestrous cyclicity were reported following rebaudioside A exposure for 48 days. The Panel concluded that no clearly adverse effects were observed in the study.
Ghaheri et al. (2018) investigated the effects of an aqueous S. rebaudiana extract on reproduction function in diabetes‐induced healthy adult male albino rats of Wistar strain. Diabetes mellitus was induced in healthy rats via intraperitoneal injection of 50 mg streptozotocin/kg bw. The rats that reached fasting glucose levels greater than 250 mg/dL after 72 hours were selected for the study. Animals (n = 7/group) were administered stevia extract at doses of 5, 50 or 100 mg/kg bw per day by gavage for 28 days. A diabetic and non‐diabetic control group received 2 mL distilled water only. Sexual behaviours of the rats were recorded for 30 minutes every 2 weeks for 1 month, including mount latency, intromission latency, mount frequency, intromission frequency, ejaculation latency, the mount latency post ejaculation and ejaculation frequency. Following the study period, animals were killed, and serum concentration of testosterone was measured. Histological examination was carried out on the right testis and epididymis. In diabetic rats, a significant increase in the frequency of intromission was observed in the low‐dose group, compared to diabetic control rats. In addition, diabetic rats of the low‐dose group showed a significant increase in the frequency of ejaculation, compared to the diabetic control and high‐dose animals. However, a significant decrease in the latency of ejaculation was observed in the low‐dose group when compared to the high‐dose animals, although the effect was not significant between the treated animals and the controls. Significant differences in other sexual behaviour parameters measured were not observed in the animals. Furthermore, a significant reduction in the number of Leydig cells in high‐dose animals was noted, compared to the non‐diabetic control group; however, this effect was not significantly different compared to the diabetic control rats. Relative testis and epididymides weights and serum testosterone levels showed no significant differences among the study animals. Based on the results of the study, the authors concluded that there is no risk related to reproductive parameters following consumption of stevia. The Panel noted that this study did not report results relevant for hazard assessment.
Overall, in a study on ovarian function in rats with rebaudioside A, no effects were observed relevant for hazard assessment. In addition, the study on sexual function in diabetic rats with a S. rebaudiana extract cannot be used for hazard assessment.
3.4.7. Hypersensitivity, allergenicity and food intolerance
In vivo and in vitro effects of stevioside (>95% purity) on the release of cytokines tumour necrosis factor (TNF‐α) and interleukin (IL‐1β) from isolated rat peripheral blood mononuclear cells (PBMCs) and presence in the plasma was investigated in rats (Noosud et al., 2017). Stevioside was administered via oral gavage to male Wistar rats (n = 6/group) at doses of 0, 500 and 1000 mg/kg bw per day over a period of 6 weeks. PBMCs were stimulated with and without lipopolysaccharide (LPS) in vitro for 24 hours to induce cytokine production. Oral administration of stevioside did not show any toxicity in PBMCs, and at the end of treatment period, the plasma levels of TNF‐α and IL‐1β were not detectable in plasma. In stevioside‐fed animals, the levels of TNF‐α in LPS‐stimulated PBMCs were significantly decreased compared to the control group. Results of this study demonstrated that oral ingestion of stevioside inhibits the formation of inflammatory cytokines TNF‐α and IL‐1β in rats at high doses.
The Panel noted that no conclusion on hypersensitivity and allergenicity can be drawn from this study.
3.4.8. Human studies
Human studies performed with commercially available products containing Stevia or stevia extract were submitted within the application dossier (Kassi et al., 2016; Ritu and Nandini, 2016; Shin et al., 2016; Al‐Dujaili et al., 2017; Ahmad et al., 2018a; Rizwan et al., 2018). The Panel noted that the purpose of these studies was to investigate the efficacy of product containing stevia on the glucose handling and insulin levels. The Panel noted that substances tested in these studies were not fully characterised and represent samples of commercially available stevia derivatives. None of the studies provided information that was considered relevant for the current assessment.
3.4.9. Other studies
The applicant has provided studies investigating potential mechanisms of action of steviol glycosides (Akbarzadeh et al., 2015; Holvoet et al., 2015; Abo Elnaga et al., 2016; Aranda‐González et al., 2016; Assaei et al., 2016; Perumal et al., 2016; Elzinga et al., 2017; Philippaert et al., 2017; Potočnjak et al., 2017; Reynolds IV et al., 2017; Ilić et al., 2017; Ahmad and Ahmad, 2018; Ahmad et al., 2018a; Barrios‐Correa et al., 2018). The Panel noted that these studies have been performed with extract prepared in the laboratory and for which the composition is unknown or based on rebaudioside A or stevioside in isolation. None of the studies provided information that was considered relevant for the current assessment.
3.5. Discussion
According to the applicant, steviol glycosides are completely hydrolysed to steviol in the intestine at generally similar rates and, therefore, the necessity for steviol glycosides preparations from S. rebaudiana Bertoni to be comprised of specific individual steviol glycoside is no longer relevant. Therefore, to reiterate, the following three changes to the EU specifications for E 960 steviol glycosides were proposed by the applicant:
The definition of steviol glycosides be expanded to include 60 steviol glycosides identified in S. rebaudiana Bertoni leaves (conjugated with glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose and galactose) as listed in Appendix A that may comprise the assay value of not less than 95%;
The listing of the chemical names, molecular formulas, molecular weights and Chemical Abstracts Service (CAS) numbers for individual steviol glycosides be replaced with one structural formula depicting the steviol backbone with R1 and R2 groups that ‘can be 1 or more sugar moieties, including glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose, and galactose’, as listed in Appendix A in this opinion;
Microbiological parameters be added to the specifications.
Regarding the proposal from the applicant to expand the definition of food additive E960 to include 60 steviol glycosides identified in S. rebaudiana Bertoni leaves in the assay value of not less than 95%, the Panel noted that this proposal would allow to have commercial products of lower purity than is currently the case. With the existing specifications, the food additive must be comprised of not less than 95% of the 11 named steviol glycosides. These specifications have an inbuilt degree of conservatism concerning the possible presence of impurities. In current commercial products, the remaining 5% of non‐defined material will in fact be made up (in‐part or even largely) of other minor and related steviol glycosides. The proposed change is to include all 60 steviol glycosides (major and minor steviol glycosides) in the same limit value of 95%, when in the current EU specifications, only major steviol glycosides (11 named steviol glycosides) are included in the 95%, and this would permit the presence of up to 5% of impurities. The Panel noted that even taking into account the presence of ash, residuals solvents and residues of processing aids and the other related steviol glycosides, the proposed specification value of 95% still gives room for an appreciable unknown fraction. Taking into account the data submitted in the application dossier, the Panel noted that it is technically possible to manufacture steviol glycosides (E960) with a purity higher than 95% for the total steviol glycosides. In the absence of a higher purity assay value, the Panel considered that identity and quantity of the impurities in the proposed specifications of steviol glycosides (E 960) should be better defined to ensure that an assay value of not less than 95% for the total content of steviol glycosides would not be of safety concern.
The Panel noted and supported the proposal made by the applicant for amending the specifications with the inclusion of a generic backbone structure and a reference to the 60 steviol glycosides listed in Appendix A.
The Panel agreed with the proposal from the applicant to include microbiological criteria in the EU specifications.
Regarding toxic elements, in addition to the maximum limits for lead and arsenic already included in the current EU specifications for E 960, results for the analysis of cadmium and mercury were also reported by the applicant (< 0.005 mg/kg for cadmium and mercury). Based on the analytical data provided, the Panel noted that maximum limits for cadmium and mercury should be added to the proposed specifications. Additionally, lower levels of the maximum limits for lead and arsenic could be considered in the proposed specifications since the results obtained in the five batches analysed were < 0.011 mg/kg for arsenic and < 0.42 mg/kg for lead.
Data on ADME of some of steviol glycosides currently listed in the EU specifications have been considered and summarised in previous EFSA opinions (EFSA ANS Panel, 2010, 2015a). Based on the available data set, stevioside and Rebaudioside A are not hydrolysed by digestive enzymes of the upper gastrointestinal tract due to the presence of β‐glycosidic bonds, but subject to microbial metabolism in the colon resulting in the release of the aglycone steviol which is then absorbed. In rats and humans, steviol is further glucuronidated; steviol glucuronide is excreted with urine and faeces.
Two unpublished studies testing major and minor steviol glycosides in human faecal homogenate have been provided by the applicant. Based on the results from unpublished and published studies, the different rebaudiosides analysed share the same metabolic degradation (i.e. deglycosylation) by the gut microbiome in the colon leading to the formation of steviol. Some minor differences in degradation rate can be anticipated depending on the number and type of sugar moieties bound to the steviol (aglycone). Yet, given the variations of metabolic rates in the reported studies and considering anticipated human exposure levels, the observed differences are not large enough to rebut the concept of similar metabolic rates of various steviol glycosides. Considering the data set, it can be presumed that all 60 steviol glycosides considered in Appendix A follow the same metabolic fate in the colon with an hydrolysation to steviol.
The Panel agreed that the overall metabolic fate of the steviol glycosides listed in Appendix A is the same; therefore, it would be acceptable to use a read‐across approach taking the toxicological data previously evaluated by EFSA, for the safety assessment of the 60 steviol glycosides and the ADI of 4 mg/kg bw per day will apply to all those steviol glycosides.
In none of the submitted genotoxicity studies, it was possible to ascertain if the test item is representative of the additive under evaluation. Moreover, in the study by Sharif et al., 2017, a testing method not validated for risk assessment was used; the study by Uçar et al., 2018 was not conducted in line with the current scientific standard and in compliance with the OECD test guidelines; in the study by Zhang et al., 2017, the Ames test was not conducted on all the bacterial strains that are recommended by the relevant OECD guideline and the in vivo micronucleus was inconclusive (negative, but without evidence of target exposure). Overall, none of the submitted studies are considered adequate to assess the genotoxic potential of the steviol glycosides preparations to be used as a food additive.
The findings from the submitted genotoxicity studies (Sharif et al., 2017; Zhang et al., 2017) and studies on reproductive organs and related effects (Ghaheri et al., 2018; Jiang et al., 2018) do not change the conclusions reached by the EFSA ANS Panel on genotoxicity and reproductive toxicity when steviol glycosides were evaluated for their authorisation as a food additive (EFSA ANS Panel, 2010). However, it is not possible to conclude if the mixture tested in all the various genotoxicity studies previously evaluated by EFSA, can be considered to be sufficiently representative of other kinds of mixtures that the proposed changes in the specifications would allow. For the particular mixtures that the applicant characterised and provided assay data on, this would not necessarily be a concern because the sum of all steviol glycosides (major, minor and related) can approach 100%. However, in a generic authorisation procedure used by European Commission, other products and other producers have to be anticipated as well. The proposed amendment of the specifications could result in a higher fraction of impurities; therefore, characterisation of the nature and amount of the various impurities would be required.
Overall, the Panel considered that read‐across is justified and the list of 60 steviol glycosides listed in Appendix A could provide a basis for an amendment of the specifications for steviol glycosides. However, the proposed change from 11 to 60 specified SGs, whilst maintaining the assay value of not less than 95%, would allow less pure preparations of the food additive onto the market. According to the proposed change in specifications, there remains a small but not insignificant fraction of the additive that would be undefined and therefore cannot be evaluated by the Panel. For these reasons, the Panel cannot conclude on the safety of the proposed amendment of the specifications for steviol glycosides (E 960).
4. Conclusions
The Panel concluded that the inclusion of the 60 steviol glycosides in the proposed specifications for steviol glycoside (E960) would not be of safety concern. However, the Panel cannot conclude on the safety of the proposed amendment to the specifications of steviol glycosides (E 960) as food additive if the purity assay value of not less than 95% for the total content of steviol glycosides is maintained.
Taking into account the data submitted in the application dossier, the Panel noted that it is technically possible to manufacture steviol glycosides (E960) with a purity higher than 95% for the total steviol glycosides.
Documentation provided to EFSA
Dossier “Application for a change in the steviol glycoside specification to expand the list of steviol glycosides in the European Union to all those identified in the leaves of Stevia rebaudiana Bertoni”. December 2018. Submitted by PureCircle Limited.
Additional information on 27 December 2019. Submitted by PureCircle Limited in response to a request from EFSA.
Additional information on 10 February 2020. Submitted by PureCircle Limited in response to a request from EFSA.
BRI Report no. RPT‐PUR‐2015‐001, 2015. Pilot In Vitro Metabolism of RAF in Male and Female Pooled Human Intestinal Fecal Homogenates Under Physiological Anaerobic Conditions.Unpublished report. Submitted within the application dossier. Submitted within the application dossier.
BRI Report no. RPT‐PUR‐2018‐001, 2018. In Vitro Anaerobic Metabolism of Steviol Glycosides A95‐27A in Pooled Human Intestinal Fecal Homogenates From Healthy Male and Female Adult and Pediatric Subjects. Unpublished report. Submitted within the application dossier.
Abbreviations
- ADI
acceptable daily intake
- ANS
EFSA Panel on Food Additives and Nutrient Sources added to Food
- BIOHAZ
EFSA Panel on Biological Hazards
- bw
body weight
- CAS
Chemical Abstracts Service
- CFU
colony forming units
- FAF
EFSA Panel on Food Additives and Flavourings
- FAO
Food and Agriculture Organisation
- FEEDAP
EFSA Panel on Additives and Products or Substances used in Animal Feed
- HPLC
high‐performance liquid chromatography
- IL‐1β
interleukin
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC/MS
liquid chromatography–mass spectrometry
- NOAEL
no observed adverse effect level
- PBMCs
peripheral blood mononuclear cells
- PCE
polychromatic erythrocytes
- TNF‐α
tumour necrosis factor
- WHO
World Health Organization
Appendix A – Summary of trivial formula, CAS Number, molecular weight, steviol equivalent and R‐group (from Figure 1) of identified steviol glycosides from the leaves of Stevia rebaudiana Bertoni (Documentation provided to EFSA No 2)
1.

Appendix B – Proposed specifications for Steviol Glycosides (E960) (Documentation provided to EFSA n. 5)
1.
|
Steviol glycosides (E 960) Commission Regulation 231/2012 |
Proposed specifications for E 960 Steviol (Documentation provided to EFSA n. 1) | |||||
|---|---|---|---|---|---|---|
| Definition | The manufacturing process comprises two main phases: the first involving water extraction of the leaves of the Stevia rebaudiana Bertoni plant and preliminary purification of the extract by employing ion exchange chromatography to yield a steviol glycoside primary extract, and the second involving recrystallisation of the steviol glycosides from methanol or aqueous ethanol resulting in a final product containing not less than 95% of the below identified 11 related steviol glycosides, in any combination and ratio. The additive may contain residues of ion‐exchange resins used in the manufacturing process. Several other related steviol glycosides that may be generated as a result of the production process, but do not occur naturally in the Stevia rebaudiana plant have been identified in small amounts (0.10 to 0.37% w/w). |
Steviol glycosides consists of a mixture of compounds containing a steviol backbone conjugated to any number or combination of the principal sugar moieties (glucose, rhamnose, xylose, fructose, arabinose, galactose and deoxyglucose) in any of the orientations occurring in the leaves of Stevia rebaudiana Bertoni as listed in Appendix A. The product is obtained from the leaves of Stevia rebaudiana Bertoni. The leaves are extracted with hot water and the aqueous extract is passed through an adsorption resin to trap and concentrate the component steviol glycosides. The resin is washed with a solvent alcohol to release the glycosides and product is recrystallised from methanol or aqueous ethanol. Ion exchange resins may be used in the purification process. The final product may be spray‐dried. The additive may contain residues of processing aid used in the manufacturing process. Several other related steviol glycosides that may be present in the Stevia rebaudiana plant or generated as a result of the production process, have been identified in small amounts (0.1 to 2.0% w/w). |
||||
| Chemical name |
Steviolbioside: 13‐[(2‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid Rubusoside: 13‐β‐D‐glucopyranosyloxykaur‐16‐en‐18‐oic acid, β‐D‐glucopyranosyl ester Dulcoside A: 13‐[(2‐O‐α–L‐rhamnopyranosyl‐β–D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, β‐D‐glucopyranosyl ester Stevioside: 13‐[(2‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, β‐D‐glucopyranosyl ester Rebaudioside A: 13‐[(2‐O‐β‐D‐glucopyranosyl‐3‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, β‐D‐ glucopyranosyl ester Rebaudioside B: 13‐[(2‐O‐β–D‐glucopyranosyl‐3‐O‐β–D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid Rebaudioside C: 13‐[(2‐O‐α–L‐rhamnopyranosyl‐3‐O‐β–D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, β‐D‐glucopyranosyl ester Rebaudioside D: 13‐[(2‐O‐β‐D‐glucopyranosyl‐3‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, 2‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl ester Rebaudioside E: 13‐[(2‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, 2‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl ester Rebaudioside F: 13[(2‐O‐β‐D‐xylofurananosyl‐3‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, β‐D‐glucopyranosyl ester Rebaudioside M: 13‐[(2‐O‐β‐D‐glucopyranosyl‐3‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl)oxy]kaur‐16‐en‐18‐oic acid, 2‐O‐β‐D‐glucopyranosyl‐3‐O‐β‐D‐glucopyranosyl‐β‐D‐glucopyranosyl ester |
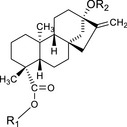 Where R1 and R2 can be one or more sugar moieties, including, but not limited to glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose and galactose as listed in Appendix A Where R1 and R2 can be one or more sugar moieties, including, but not limited to glucose, rhamnose, xylose, fructose, deoxyglucose, arabinose and galactose as listed in Appendix A
|
||||
| Molecular formula | Trivial name | Formula | Conversion factor | |||
|
Steviol Steviolbioside Rubusoside Dulcoside A Stevioside Rebaudioside A Rebaudioside B Rebaudioside C Rebaudioside D Rebaudioside E Rebaudioside F Rebaudioside M |
C20H30O3 C32H50O13 C32H50O13 C38H60O17 C38H60O18 C44H70O23 C38H60O18 C44H70O20 C50H80O28 C44H70O23 C43H68O22 C56H90O33 |
1,00 0,50 0,50 0,40 0,40 0,33 0,40 0,34 0,29 0,33 0,34 0,25 |
||||
| Molecular weight and CAS No | Trivial name | CAS Number | Molecular weight | |||
|
Steviol Steviolbioside Rubusoside Dulcoside A Stevioside Rebaudioside A Rebaudioside B Rebaudioside C Rebaudioside D Rebaudioside E Rebaudioside F Rebaudioside M |
41093‐60‐1 64849‐39‐4 64432‐06‐0 57817‐89‐7 58543‐16‐1 58543‐17‐2 63550‐99‐2 63279‐13‐0 63279‐14‐1 438045‐89‐7 1220616‐44‐3 |
318,46 642,73 642,73 788,87 804,88 967,01 804,88 951,02 1 129,15 967,01 936,99 1 291,30 |
||||
| Assay | Not less than 95% steviolbioside, rubusoside, dulcoside A, stevioside, rebaudiosides A, B, C, D, E, F and M, on the dried basis, in any combination and ratio | Not less than 95% of total steviol glycosides on the dried basis | ||||
| Description | White to light yellow powder, approximately between 200 and 350 times sweeter than sucrose (at 5% sucrose equivalency). | White to light yellow powder, approximately between 200 and 350 times sweeter than sucrose (at 5% sucrose equivalency) | ||||
| Identification | ||||||
| Solubility | Freely soluble to slightly soluble in water | Freely soluble to slightly soluble in water | ||||
| pH | Between 4,5 and 7,0 (1 in 100 solution) | Between 4,5 and 7,0 (1 in 100 solution) | ||||
| Purity | ||||||
| Total ash | Not more than 1% | Not more than 1% | ||||
| Loss on drying | Not more than 6% (105 °C, 2 h) | Not more than 6% (105 °C, 2 h) | ||||
| Residual solvent | Not more than 200 mg/kg methanol | Not more than 200 mg/kg methanol | ||||
| Not more than 5000 mg/kg ethanol | Not more than 5,000 mg/kg ethanol | |||||
| Arsenic | Not more than 1 mg/kg | Not more than 1 mg/kg | ||||
| Lead | Not more than 1 mg/kg | Not more than 1 mg/kg | ||||
| Microbiological criteria | ||||||
| Total (aerobic) plate count | Not more than 1,000 CFU/g | |||||
| Yeast and moulds | Not more than 200 CFU/g | |||||
| E. coli | Negative in 1 g | |||||
| Salmonella | Negative in 25 g | |||||
Appendix C – Tabulated summary of studies on ADME
1.
| Reference |
Study type |
Species |
Substance/ purity |
Dosing | Samples analysed | Analysis | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Wingard et al. (1980) | In vitro | Sprague Dawley Rats |
Stevioside (2.5 mg/ml); Rebaudioside A (3.0 mg/ml); Steviol (0.2 mg/ml) Purity not specified |
6 days incubation | Anaerobial caecal bacterial cells | HPLC |
Stevioside was completely transformed into steviol within 2 days Rebaudioside A was transformed into steviol after 6 days |
Both stevioside and rebaudioside A can be degraded to steviol by microbiota in the mammalian lower bowel with a degradation rate ranging between 0.4 and 0.8 mg converted/h per g caecal contents |
| In vivo | Steviol‐17‐[14C] |
Oral in suspension 1 ml (1.7 uCi, 0.7 mg) of Steviol‐17‐[14C] |
Urine and faeces | Radioactive level | Steviol‐17‐[14C] was completely absorbed from the bowel after oral administration. The radioactivity was largely excreted in the urine | After oral administration, Steviol‐17‐[14C] is readily absorbed by mammalian lower bowel | ||
| Nakayama et al. (1986) | In vivo | Wistar Rats |
[3H] Stevioside Purity not specified |
Stomach tube in suspension [3H] Stevioside 125 ml/5 ml/kg (10.4–120 uCi/kg) |
Blood (0.5, 1, 2 and every 2 hours up to 120 hours) urine and faeces (at 24 hours interval for 120 hours) Tissues distribution |
Liquid scintillation counter Thin‐layer radiochromatogram of metabolites |
In blood: [3H] Stevioside reached Cmax at 8 hours and then decreased slowly with an elimination half‐life of 24 hours In urine and faeces: Faecal excretion was 68% and urinary excretion was 2.3% at 120 hours, respectively. Tissues distribution: in the stomach and small intestine, the highest levels were reached at 1 hour and in the caecum, the highest concentration was reached at 4 hours. Less than 1% of [3H] Stevioside was found in other tissues after 1 hour. Radiochromatogram of metabolites showed that in the stomach and faeces steviol was the main metabolites. Other metabolites were detected but not identified |
[3H] Stevioside concentration reached higher concentration at 1 hour and 4 hours after the administration in the GI tract and in the blood, respectively. Most of the compound stays in the GI tract with low distribution in other organs. Stevioside hydrolysed into steviol in the intestine and mainly excreted via faeces |
| Koyama et al. (2003) | In vitro | Human |
Stevia mixture and enzymatically modified stevia Highest purity commercially available or of HPLC grade |
0.2 or 10 mg/ml stevia mixture, rebaudioside A, stevioside, α‐monoglucosylrebaudioside A or α‐monoglucosylstevioside or 0.08 or 0.2 mg/ml steviol 24 hours incubation |
Faecal homogenate samples | LC/ESI/MS and LC/MS/MS |
Stevioside is hydrolysed into steviol and similarly monoglucosylstevioside is first α‐deglucosilate and then hydrolysed to steviol. Rebaudioside A and α‐monoglucosylrebaudioside A are eventually hydrolysed to steviol mainly via stevioside. No degradation of steviol was found after 24 hours incubation period and no other peaks were found for steviol of stevia mixture and enzymatically modified stevia |
Steviol is confirmed as the final metabolite from stevia mixture and enzymatically modified stevia after human intestinal metabolism |
| Gardana et al. (2003) | In vitro | Human | Stevia extract containing either 85% w/w stevioside or 90% w/w rebaudioside A and pure steviolbioside |
stevioside or rebaudioside A 40 mg 24 hours incubation |
Faecal homogenate samples | HPLC |
Stevioside was completely degraded into steviol in approximately 10 hours. Rebaudioside A was completely degraded into steviol in approximately 24 hours. Steviol remained unchanged for 72 hours incubation period |
Human faecal microbiota completely hydrolysed stevioside and rebaudioside A to the common aglycon steviol in 10 and 24 hours, respectively. Steviol did not further degraded |
| Simonetti et al. (2004) | In vivo | Human | Stevia extract (Stevioside 85% w/w) | Stevioside 375 mg (5.16 mg/kg/bw) single dose | Plasma samples, urine and faeces | LC/MS | Stevioside was detected in 7 out of 9 subjects at 1 hour after dosing | Stevioside is absorbed after oral administration and steviol‐glucoronide was the only metabolite found in plasma and in urine. Steviol was present only in the faeces suggesting its formation after intestinal microflora metabolism |
| Geuns et al. (2006) | In vivo | Human | Stevioside (97% purity) | Stevioside 250 mg capsules × 3 time a day for 3 days | Urine | IR/NMR | Steviol‐glucoronide was the only metabolite detected in the urine. No free steviol was found | As only steviol‐glucoronide was the detected in the urine, it is suggested that stevioside is degraded into steviol by colon microbioma and then transport in the liver where steviol‐glucuronide is formed |
| Wheeler et al. (2008) | In vivo | Human | Rebaudioside A (98.7% purity) and Stevioside (96.6% purity) | 5 mg/kg rebaudioside A and 4.2 mg/kg stevioside single dose | Plasma, urine and faeces | LC/MS/MS |
Steviol was detected only in one subject whether steviol‐glucuronide was found in plasma samples. Steviol‐glucuronide derived from rebaudioside A showed a peak at 12 hours, Steviol‐glucuronide derived from stevioside showed a peak at 8 hours. Steviol‐glucuronide was primary excreted in the urine and was not found in the faeces |
Rebaudioside A and stevioside are hydrolysed to steviol in the GI tract. The main circulating metabolite is Steviol‐glucuronide which is then excreted in the urine. Rebaudioside A has one additional glucose moiety that must be removed before hydrolysation and this can explain the lower Cmax and longer Tmax than stevioside |
| Roberts and Renwick (2008) | In vivo | Sprague Dawley Rats | Rebaudioside A (> 97% purity) and stevioside (> 97% purity) |
Rebaudioside A 5 mg/kg bw Stevioside 4.2 mg/kg bw Single dose by gavage |
Plasma, urine and faeces | LSC/HPLC/TLC |
Main radioactive metabolite was steviol and in lower amount steviol‐glucoronide. Steviol was most component in faeces |
Rebaudioside A and stevioside are metabolised to steviol by gut microbioma and excreted in the faeces. Steviol‐glucoronide was present in the urine |
| Purkayastha et al. (2014) | In vitro | Human | Rebaudioside B (96.5% purity), rebaudioside D (96.46% purity), rebaudioside M (97.33% purity), steviolbioside (96.81% purity) and stevioside (97.37% purity) | 0.2 or 2 mg/ml of each rebaudioside for 24 or 48 h incubation period | Human faecal homogenate | LC/MS |
In vitro metabolism of rebaudioside A, B and D are comparable. At 0.2 mg/ml rebaudioside A degraded to steviol (> 88%) within 8 h and was completely metabolised by 24 h. Similarly, rebaudioside D was degraded into steviol by 8 hours and rebaudioside B had lower level on degradation to 83% into steviol after 8 hours incubation. Rebaudioside A, B and D at 2 mg/ml showed slower metabolism to steviol compared to 0.2 mg/ml but still showing full conversion to steviol at 24 h. Rebaudioside M was tested at 0.2 mg/ml and showed a slower rate of metabolism after 8 h incubation. Rebaudioside M was fully degraded at 16 h |
Rebaudioside A, B, D and M also defined as parent steviol glycosides, were degraded by gut microbioma to steviol within 24 hours but with the majority metabolised at 8 hours. No significant differences have been identified between rebaudioside A and other rebaudiosides except for the slower rate of degradation of rebaudioside D and M compared to rebaudioside A, which could be explained by the additional glucose moiety present in rebaudioside D and M |
| Purkayastha et al. (2015) | In vitro | Human | Rebaudioside E and Rebaudioside A purity not specified | Rebaudioside E and Rebaudioside A at 0.2 and 2 mg/mL | Human faecal homogenate | LC/MS/MS |
Rebaudioside E at 0.2 mg/ml was metabolised to steviolbioside and steviol within 24 h incubation period. The intermediate metabolites steviolbioside was found at 4 h and 8 h samples but then was not found at 24 h. Incubation of rebaudioside E at 2 mg/mL showed slower metabolism compared to 0.2 mg/mL |
No significant differences were identified in the metabolism between rebaudioside A and E, which are both degraded to steviol within 24 h. A metabolic intermediate of rebaudioside E, steviolbioside was identified. Steviolbioside is then hydrolysed into steviol |
| Purkayastha et al. (2016) | In vitro | Human | Rebaudioside A,B,C,D,E,F,M, steviolbioside and dulcoside A | Rebaudioside A,B,C,D,E,F,M, steviolbioside and dulcoside A at 0.2 and 2 mg/ml | Human faecal homogenate | LC/MS |
At 2 mg/mL, rebaudioside A, B and D were metabolised up to 17% at 8 h and at up to 102% of hydrolysis at 24 h. The rats of metabolism at 0.2 mg/mL were quicker. At 2 mg/mL, Rebaudioside C has slower rate of hydrolysis compared to Rebaudioside A. At 2 mg/mL, Rebaudioside M achieved complete hydrolysis at 16 h. At 2 mg/mL, Rebaudioside E has similar rate of hydrolysis than rebaudioside A. Rebaudioside F showed solubility issue at 2 mg/mL. At 0.2 and 2 mg/mL rate of hydrolysis was slower than rebaudioside A |
At 2 mg/ml rebaudioside A, B, C and D were completely hydrolysed within 48 hours, whether at lower concentration of 0.2 mg/mL the hydrolysis rate was more rapid. For rebaudioside A, B and E at 24 h approximately 50–80% of hydrolysis takes place, whether for rebaudioside C and D the level of hydrolysis ranges from 20–46% and 60–100%, respectively, at 24 h. Rebaudioside F has the lowest rate of hydrolysis with a range of 2.9–6.6% at 24 h |
| Roberts et al. (2016) | In vivo | Sprague Dawley Rats | Stevioside (> 95% purity) | Stevioside at 40 and 1,000 mg/kg bw single oral gavage | Blood, tissues | LC/MS/MS | After stevioside administration at 40 mg/kg bw, steviol was detectable from 2 h post‐dose and reached Cmax between 2 and 6 hours post‐dose. Steviol was not detectable at 36 h. At 1,000 mg/kg bw, steviol was detected from 1 h post‐dose reaching th Cmax between 6 and 12 h post‐dose. Steviol was not detected at 72 h post‐dose | In humans, Cmax for steviol occurred slightly later than in rats. AUC to steviol in humans were approximately 2.8‐fold higher than in rats |
| Human | Stevioside at 40 mg/kg bw | Blood | Cmax of steviol was reached between 19 and 20 h post‐dose after administration of 40 mg/kg bw stevioside. Steviol was below limit of detection at 48 h post‐dose |
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes M, Aquilina G, Engel K‐H, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert‐Remy U, Husøy T, Manco M, Mennes W, Moldeus P, Passamonti S, Shah R, Waalkens‐Berendsen I, Wölfle D, Wright M, Degen G, Giarola A, Rincon AM and Castle L, 2020. Scientific Opinion on the safety of a proposed amendment of the specifications for steviol glycosides (E 960) as a food additive: to expand the list of steviol glycosides to all those identified in the leaves of Stevia Rebaudiana Bertoni . EFSA Journal 2020;18(4):6106, 32 pp. 10.2903/j.efsa.2020.6106
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00063
Panel members: Gabriele Aquilina, Laurence Castle, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Rainer Gürtler, Ursula Gundert‐Remy, Trine Husøy, Melania Manco, Wim Mennes, Peter Moldeus, Sabina Passamonti, Romina Shah, Ine Waalkens‐Berendsen, Detlef Wölfle, Matthew Wright and Maged Younes.
Acknowledgements: The FAF Panel wishes to acknowledge all European competent institutions, Member State bodies and other organisations that provided data for this scientific output.
Adopted: 24 March 2020
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © Stockphoto
Notes
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) no 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005.
References
- Abo Elnaga NIE, Massoud MI, Yousef MI and Mohamed HHA, 2016. Effect of stevia sweetener consumption as non‐caloric sweetening on body weight gain and biochemical's parameters in overweight female rats. Annals of Agricultural Science, 61, 155–163. [Google Scholar]
- Ahmad U and Ahmad RS, 2018. Anti diabetic property of aqueous extract of Stevia rebaudiana Bertoni leaves in Streptozotocin‐induced diabetes in albino rats. BMC Complementary and Alternative Medicine, 18, 179[11 pp]. 10.1186/s12906-018-2245-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad J, Khan I, Johnson SK, Alam I and Din ZU, 2018a. Effect of incorporating stevia and moringa in cookies on postprandial glycemia, appetite, palatability, and gastrointestinal well‐being. Journal of the American College of Nutrition, 37, 133–139. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh S, Eskandari F, Tangestani H, Bagherinejad ST, Bargahi A, Bazzi P, Daneshi A, Sahrapoor A, O'Connor WJ and Rahbar AR, 2015. The effect of Stevia rebaudiana on serum omentin and visfatin level in STZ‐induced diabetic rats. Journal of Dietary Supplements, 12, 11–22. [DOI] [PubMed] [Google Scholar]
- Al‐Dujaili EAS, Twaij H, Bataineh YA, Arshad U and Amjid F, 2017. Effect of stevia consumption on blood pressure, stress hormone levels and anthropometrical parameters in healthy persons. American Journal of Pharmacology and Toxicology, 12, 7–17. [Google Scholar]
- Aranda‐González I, Moguel‐Ordóñez Y, Chel‐Guerrero L, Segura‐Campos M and Betancur‐Ancona D, 2016. Evaluation of the antihyperglycemic effect of minor steviol glycosides in normoglycemic and induced‐diabetic Wistar rats. Journal of Medicinal Food, 19, 844–852. [DOI] [PubMed] [Google Scholar]
- Assaei R, Mokarram P, Dastghaib S, Darbandi S, Darbandi M, Zal F, Akmali M and Ranjbar Omrani GH, 2016. Hypoglycemic effect of aquatic extract of stevia in pancreas of diabetic rats: PPARγ‐dependent regulation or antioxidant potential. Avicenna Journal of Medical Biotechnology, 8, 65–74. [PMC free article] [PubMed] [Google Scholar]
- Barrios‐Correa AA, Estrada JA, Martel C, Olivier M, López‐Santiago R and Contreras I, 2018. Chronic intake of commercial sweeteners induces changes in feeding behavior and signaling pathways related to the control of appetite in BALB/c mice. BioMed Research International, 2018, Article No 3628121 [15 pp, plus supplementary figures]. 10.1155/2018/3628121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2010. Scientific Opinion on safety of steviol glycosides for the proposed uses as a food additive. EFSA Journal 2010;8(4):1537, 85 pp. 10.2903/j.efsa.2010.1537 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources), 2015a. Scientific opinion on the safety of the proposed amendment of the specifications for steviol glycosides (E 960) as a food additive. EFSA Journal 2015;13(12):4316, 29 pp. 10.2903/j.efsa.2015.4316 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2015b. Scientific Opinion on the extension of use of steviol glycosides (E 960) as a food additive. EFSA Journal 2015;13(6):4146, 20 pp. 10.2903/j.efsa.2015.4146 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2018. Safety of the proposed amendment of the specifications of the food additive steviol glycosides (E 960). EFSA Journal 2018;16(3):5236, 11 pp. 10.2903/j.efsa.2018.5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2019. Safety of the proposed amendment of the specifications for steviol glycosides (E 960) as a food additive: Rebaudioside M produced via enzyme‐catalysed bioconversion of purified stevia leaf extract. EFSA Journal 2019;17(10):5867, 12 pp. 10.2903/j.efsa.2019.5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on Transparency in the Scientific Aspects of Risk Assessments carried out by EFSA. Part 2: General Principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- Elzinga SE, Rohleder B, Schanbacher B, McQuerry K, Barker VD and Adams AA, 2017. Metabolic and inflammatory responses to the common sweetener stevioside and a glycemic challenge in horses with equine metabolic syndrome. Domestic Animal Endocrinology, 60, 1–8. [DOI] [PubMed] [Google Scholar]
- Gardana C, Simonetti P, Canzi E, Zanchi R and Pietta P, 2003. Metabolism of stevioside and rebaudioside A from Stevia rebaudiana extracts by human microflora. J Agric Food Chem., 51, 6618–6622. [DOI] [PubMed] [Google Scholar]
- Geuns JMC, Buyse J, Vankeirsbilck A, Temme EHM, Compernolle F and Toppet S, 2006. Identification of steviol glucuronide in human urine. Journal of Agricultural and Food Chemistry, 54, 2794–2798. [DOI] [PubMed] [Google Scholar]
- Ghaheri M, Miraghaee S, Babaei A, Mohammadi B, Kahrizi D, Saivosh Haghighi ZM and Bahrami G, 2018. Effect of Stevia rebaudiana Bertoni extract on sexual dysfunction in Streptozotocin‐induced diabetic male rats. Cellular and Molecular Biology (Noisy‐le‐Grand, France), 64, 6–10. [DOI] [PubMed] [Google Scholar]
- Holvoet P, Rull A, García‐Heredia A, López‐Sanromà S, Geeraert B, Joven J and Camps J, 2015. Stevia‐derived compounds attenuate the toxic effects of ectopic lipid accumulation in the liver of obese mice: a transcriptomic and metabolomic study. Food and Chemical Toxicology, 77, 22–33[plus supplementary tables]. [DOI] [PubMed] [Google Scholar]
- Ilić V, Vukmirovic S, Stilinovic N, Capo I, Arsenovic M and Milijaševic B, 2017. Insight into anti‐diabetic effect of low dose of stevioside. Biomedicine & Pharmacotherapy = Biomedecine et Pharmacotherapie, 90, 216–221. [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2009. Steviol glycosides (addendum). In: Toxicological evaluation of certain food additives. Sixty‐ninth report of JECFA, June 17‐26‐29, 2008, Rome. WHO Food Additives series, No. 60, 183–219.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2016. Rebaudioside A from multiple gene donors expressed in Yarrowia lipolytica [Prepared at the 82nd JECFA, 2016). In: Combined compendium of food additive specifications. 82nd Meeting, June 7‐16, Geneva, Switz. Food and Agriculture Organization of the United Nations (FAO), Rome, Italy/World Health Organization (WHO), Geneva, Switz. FAO JECFA Monographs 19, 91–96.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2017. Monograph 20. Combined compendium of food additive specifications. Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017. Steviol Glycosides from Stevia rebaudiana Bertoni. Available online: http://www.fao.org/ag/agn/jecfa-additives/search.html
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2020. Joint FAO/WHO Expert Committee on Food Additives, Eighty‐seventh meeting, 4‐13 June 2019. Summary and conclusions. Issued 26 June 2019. Available online: http://www.fao.org/3/ca5270en/ca5270en.pdf
- Jiang J, Qi L, Wei Q and Shi F, 2018. Effects of daily exposure to saccharin sodium and rebaudioside A on the ovarian cycle and steroidogenesis in rats. Reproductive Toxicology, 76, 35–45. [DOI] [PubMed] [Google Scholar]
- Kassi E, Landis G, Pavlaki A, Lambrou G, Mantzou E, Androulakis I, Giannakou A, Papanikolaou E and Chrousos GP, 2016. Acute effects of Stevia rebaudiana extract on postprandial glucose metabolism in patients with metabolic syndrome. Endocrine Reviews, 37(Suppl. 1), abstract SUN‐691. [Google Scholar]
- Koyama E, Kitazawa K, Ohori Y, Izawa O, Kakegawa K, Fujino A and Ui M, 2003. In vitro metabolism of the glycosidic sweeteners, stevia mixture and enzymatically modified stevia in human intestinal microflora. Food and Chemical Toxicology, 41, 359–374. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kasahara D and Yamamoto F, 1986. Absorption, distribution, metabolism and excretion of stevioside in rats. Shokuhin Eiseigaku Zasshi. Journal of the Food Hygienic Society of Japan, 27, 1–8. [Google Scholar]
- Noosud J, Lailerd N, Kayan A and Boonkaewwan C, 2017. In vitro and in vivo assessment of inhibitory effect of stevioside on pro‐inflammatory cytokines. Avicenna Journal of Phytomedicine, 7, 101–106. [PMC free article] [PubMed] [Google Scholar]
- Perumal V, Manickam T, Bang K‐S, Velmurugan P and Oh B‐T, 2016. Antidiabetic potential of bioactive molecules coated chitosan nanoparticles in experimental rats. International Journal of Biological Macromolecules, 92, 63–69 [plus supplementary figures]. [DOI] [PubMed] [Google Scholar]
- Philippaert K, Pironet A, Mesuere M, Sones W, Vermeiren L, Kerselaers S, Pinto S, Segal A, Antoine N, Gysemans C, Laureys J, Lemaire K, Gilon P, Cuypers E, Tytgat J, Mathieu C, Schuit F, Rorsman P, Talavera K, Voets T and Vennekens R, 2017. Steviol glycosides enhance pancreatic beta‐cell function and taste sensation by potentiation of TRPM5 channel activity. Nature Communications, 8, 1–16. 10.1038/ncomms14733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potočnjak I, Broznić D, Kindl M, Kropek M, Vladimir‐Knežević S and Domitrović R, 2017. Stevia and stevioside protect against cisplatin nephrotoxicity through inhibition of ERK1/2, STAT3, and NF‐κB activation. Food and Chemical Toxicology, 107, 215–225. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Pugh G Jr, Lynch B, Roberts A, Kwok D and Tarka SM Jr, 2014. In vitro metabolism of rebaudioside B, D, and M under anaerobic conditions: comparison with rebaudioside A. Regulatory Toxicology and Pharmacology: RTP, 68, 259–268. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Bhusari S, Pugh G Jr, Teng X, Kwok D and Tarka SM Jr, 2015. In vitro metabolism of rebaudioside E under anaerobic conditions: comparison with rebaudioside A. Regulatory Toxicology and Pharmacology: RTP, 72, 646–657. [DOI] [PubMed] [Google Scholar]
- Purkayastha S, Markosyan A, Prakash I, Bhusari S, Pugh G Jr, Lynch B and Roberts A, 2016. Steviol glycosides in purified stevia leaf extract sharing the same metabolic fate. Regulatory Toxicology and Pharmacology: RTP, 77, 125–133. [DOI] [PubMed] [Google Scholar]
- Reynolds TH IV, Soriano RA, Obadi OA, Murkland S and Possidente B, 2017Long term rebaudioside A treatment does not alter circadian activity rhythms, adiposity, or insulin action in male mice. PLoS ONE, 12, e0177138 11 pp. 10.1371/journal.pone.0177138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritu M and Nandini J, 2016. Nutritional composition of Stevia rebaudiana a sweet herb and its hypoglycaemic and hypolipidaemic effect on patients with non‐insulin dependent diabetes mellitus. Journal of the Science of Food and Agriculture, 96, 4231–4234. [DOI] [PubMed] [Google Scholar]
- Rizwan F, Rashid HU, Yesmine S, Monjur F and Chatterjee TK, 2018. Preliminary analysis of the effect of Stevia (Stevia rebaudiana) in patients with chronic kidney disease (stage I to stage III). Contemporary Clinical Trials Communications, 12, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A and Renwick AG, 2008. Comparative toxicokinetics and metabolism of rebaudioside A, stevioside and steviol in rats. Food and Chemical Toxicology, 46(7, Suppl.), S31–S39. [DOI] [PubMed] [Google Scholar]
- Roberts A, Lynch B, Rogerson R, Renwick A, Kern H, Coffee M, Cuellar‐Kingston N, Eapen A, Crincoli C, Pugh G, Bhusari S, Purkayashtha S and Carakostas M, 2016. Chemical‐specific adjustment factors (inter‐species toxicokinetics) to establish the ADI for steviol glycosides. Regulatory Toxicology and Pharmacology: RTP, 79, 91–102. [DOI] [PubMed] [Google Scholar]
- Sharif R, Chan KM, Ooi TC and Mohammad NF, 2017. Cytotoxicity and genotoxicity evaluation of stevioside on CCD18Co and HCT 116 cell lines. International Food Research Journal, 24, 341–345. [Google Scholar]
- Shin DH, Lee JH, Kang MS, Kim TH, Jeong SJ, Kim CH, Kim SS and Kim IJ, 2016. Glycemic effects of rebaudioside A and erythritol in people with glucose intolerance. Diabetes & Metabolism Journal, 40, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti P, Gardana C, Bramati L and Pietta PG, 2004. Bioavailability of stevioside from Stevia rebaudiana in humans: preliminary report. In: Geuns JMC, Buyse J, editors. Safety of stevioside: proceedings of the first symposium sponsored by KULeuven, Apr. 16, 2004, Leuven, Belgium. Euprint ed., Heverlee, Belgium, 51‐62.
- Uçar A, Yılmaz S, Yılmaz Ş and Kılıç MS, 2018. A research on the genotoxicity of stevia in human lymphocytes. Drug and Chemical Toxicology, 41, 221–224. 10.1080/01480545.2017.1349135 [DOI] [PubMed] [Google Scholar]
- Wheeler A, Boileau AC, Winkler PC, Compton JC, Prakash I, Jiang X and Mandarino DA, 2008. Pharmacokinetics of rebaudioside A and stevioside after single oral doses in healthy men. Food and Chemical Toxicology, 46(7, Suppl.), S54–S60. [DOI] [PubMed] [Google Scholar]
- Wingard RE, Brown JP, Enderlin FE, Dale JA, Hale RL and Seitz CT, 1980. Intestinal degradation and absorption of the glycosidic sweeteners stevioside and rebaudioside A. Experientia, 36, 519–520. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang H, Li Y, Liu H and Jia X, 2017. Toxicological evaluation of ethanolic extract from Stevia rebaudiana Bertoni leaves: Genotoxicity and subchronic oral toxicity. Regulatory Toxicology and Pharmacology, 86, 253–259. 10.1016/j.yrtph.2017.03.021 [DOI] [PubMed] [Google Scholar]


