Abstract
A multi‐country outbreak of Listeria monocytogenes ST6 linked to blanched frozen vegetables (bfV) took place in the EU (2015–2018). Evidence of food‐borne outbreaks shows that L. monocytogenes is the most relevant pathogen associated with bfV. The probability of illness per serving of uncooked bfV, for the elderly (65–74 years old) population, is up to 3,600 times greater than cooked bfV and very likely lower than any of the evaluated ready‐to‐eat food categories. The main factors affecting contamination and growth of L. monocytogenes in bfV during processing are the hygiene of the raw materials and process water; the hygienic conditions of the food processing environment (FPE); and the time/Temperature (t/T) combinations used for storage and processing (e.g. blanching, cooling). Relevant factors after processing are the intrinsic characteristics of the bfV, the t/T combinations used for thawing and storage and subsequent cooking conditions, unless eaten uncooked. Analysis of the possible control options suggests that application of a complete HACCP plan is either not possible or would not further enhance food safety. Instead, specific prerequisite programmes (PRP) and operational PRP activities should be applied such as cleaning and disinfection of the FPE, water control, t/T control and product information and consumer awareness. The occurrence of low levels of L. monocytogenes at the end of the production process (e.g. < 10 CFU/g) would be compatible with the limit of 100 CFU/g at the moment of consumption if any labelling recommendations are strictly followed (i.e. 24 h at 5°C). Under reasonably foreseeable conditions of use (i.e. 48 h at 12°C), L. monocytogenes levels need to be considerably lower (not detected in 25 g). Routine monitoring programmes for L. monocytogenes should be designed following a risk‐based approach and regularly revised based on trend analysis, being FPE monitoring a key activity in the frozen vegetable industry.
Keywords: Listeria monocytogenes, growth, blanched frozen vegetables, public health risk, risk factors, control options, food safety management systems
Summary
Following a request from the European Commission, the Scientific Panel on Biological Hazards (BIOHAZ) was asked to provide a scientific opinion on the public health risk posed by Listeria monocytogenes and, if considered relevant by EFSA, other pathogens that may contaminate fruit, vegetables and herbs (FVH) which are processed (e.g. blanched) prior to being placed on the market frozen. It was clarified that fruit and herbs are out of scope of the assessment, as these are typically not blanched while some or all groups of vegetables may be blanched. These will be referred to as blanched frozen vegetables (bfV).
In particular, EFSA was asked to provide an estimation of the public health impact of L. monocytogenes contamination, and if considered relevant also of other pathogens in bfV, compared with other known pathogen–food combinations (Term of Reference 1; ToR1). Based on the number of human cases involved in the food‐borne outbreaks (FBOs) in the European Union (EU) (2005–2018), L. monocytogenes is the most relevant pathogen in bfV for public health. Therefore, the public health impact of L. monocytogenes contamination of bfV was compared with the better‐known risk of food‐borne illness associated with ready‐to‐eat (RTE) foods such as RTE meat products, dairy products and/or fishery products. Comparison was done using data on strong FBOs at EU/EEA level from 2008 to 2018 related to L. monocytogenes for the whole population. The ‘dairy’ food category was responsible for five outbreaks by L. monocytogenes involving 47 cases, while ‘fish and seafood’ and ‘meat and meat products’ caused nine and 16 outbreaks involving 63 and 190 cases, respectively. Cases linked to bfV were reported only in 2018 and involved 46 persons (all hospitalised) and five deaths. Also, a quantitative microbiological risk assessment (QMRA) model was used to estimate the listeriosis risk associated with the consumption of bfV by the elderly population of the age group 65–74 years old. The bfV were separated into two subcategories to encompass the range of consumer habits in relation to the mode of use/consumption; namely those consumed uncooked or cooked (i.e. boiled, fried or microwave heated). The estimated individual risk, i.e. the probability of illness per serving, is lower for bfV than for any of the RTE food subcategories evaluated, i.e. cold‐smoked fish, hot‐smoked fish, gravad fish, cooked meat, sausage, pâté and soft and semi‐soft cheese. It is up to 3,600 times greater for bfV consumed uncooked rather than cooked and judged to be very unlikely (5–10%) to be higher for bfV consumed uncooked than for soft and semi‐soft cheese. The estimated public health impact, i.e. the annual number of cases for elderly females in the EU, taken as an example, is less than two cases per year, which is, considering also the uncertainty, lower than any of the evaluated RTE food categories. The public health impact of bfV is dominated by the proportion of total servings consumed uncooked.
The main risk factors of contamination and growth of L. monocytogenes in bfV from processing until consumption were assessed in ToR 2. The production steps considered started from the receipt of the raw material at the processing plant, while the consumption steps included storage after thawing, food preparation and consumption habits. The main factors that may increase the contamination and/or growth of L. monocytogenes in bfV during processing are: (i) the hygiene status of the incoming raw materials; (ii) the hygienic conditions of the food processing environment (FPE), including food contact surfaces (FCSs) and non‐FCSs; (iii) the microbiological quality of the process water; and (iv) the time/Temperature (t/T) combinations used for storage, washing, blanching, cooling and freezing. Blanching (depending on t/T applied in the process) and water disinfection (to maintain the microbiological quality of process water) can reduce the contamination of L. monocytogenes in bfV at processing level. The main factors affecting contamination and/or growth of L. monocytogenes in bfV after processing are: (i) the intrinsic characteristics of the bfV (e.g. pH, aw, nutrients, presence of antimicrobial compounds and natural microbiota); (ii) the t/T profiles during thawing and storage; and (iii) the cooking conditions applied, including the cooking method and equipment.
In ToR 3, recommendations were requested on possible control options that may be implemented by food business operators (FBOp) during the production process of bfV. Control options are based on prerequisite programmes (PRPs), including good hygiene practises (GHP), good manufacturing practices (GMP) and operational PRPs (oPRPs), as well as procedures based on the hazard analysis and critical control points (HACCP) principles. Analysis of the hazards and activities of the target FBOp suggest that PRPs are sufficient to reduce contamination and the application of a complete HACCP plan is either not possible or would not further enhance food safety. In total, 11 PRP categories were identified, which, if implemented together, are very likely (95–99%) to reduce the probability of contamination of bfV by L. monocytogenes. Hygienic design of equipment, cleaning and disinfection of the processing environment and water control are of utmost importance to reduce the probability of introduction, survival and growth of L. monocytogenes. Additionally, t/T combinations applied during washing, blanching, cooling and freezing must be controlled to prevent the potential for any surviving L. monocytogenes to grow. Four different oPRPs are suggested as control measures and linked to seven different processing stages including: (i) equipment and processing environment (oPRP1: cleaning and disinfection); (ii) processing steps where water is used (oPRP2: water control); (iii) washing (oPRP3: t/T control); (iv) blanching (also oPRP3: t/T control); (v) cooling (also oPRP3: t/T control); (vi) freezing (also oPRP3: t/T control); and (vii) consumer practices (oPRP4: product information and consumer awareness). Additional control measures which the aim to reduce or eliminate L. monocytogenes in the product or on food process surfaces have been identified. However, not all of these measures are commercially available. In addition, their efficacy is not yet fully validated in industrial settings.
In ToR 3, recommendations were requested on routine monitoring for L. monocytogenes in the bfV processing environment and final product. This was carried out by critically appraising available guidelines for the industry. It was clarified that sampling and monitoring recommendations have the purpose to verify that the food safety management system (FSMS) implemented by the FBOp is well designed and has the appropriate control measures. Environmental monitoring (EM) can be used to validate or verify specific PRPs or as a strategy to monitor the environment for unhygienic conditions. An EM program should establish the sampling strategies and microbiological methods for L. monocytogenes detection most appropriate for maximising the identification of sources and routes of L. monocytogenes contamination in the FPE. Well‐established routine EM programmes should be designed on a risk‐based approach, considering the nature and size of the food operation and reflecting aspects related to the raw materials, the production processes and the final product application, but they also need to be regularly revised based on trend analysis. To establish a routine monitoring program, the FBOp should consider the following criteria: (i) the identification of the sampling points; (ii) the target microorganisms; (iii) the sample size; (iv) the frequency of testing; and (v) the selection of sampling, detection and quantification methods. It is not possible to give specific advice regarding the sampling sites that should be selected or the number of samples and frequency of sampling because these must be chosen on a case‐by‐case basis and established on a risk‐based approach and trend analysis. Sampling, detection and enumeration methods should follow validated methods. Subtyping of L. monocytogenes isolates by molecular methods (such as whole genome sequencing; WGS) is necessary to establish whether the isolates belong to a persistent clone.
It was clarified that, if the FBO decides to establish an intermediate level of L. monocytogenes concentrations in bfV at the end of the production process (i.e. performance objective; PO) compatible with the food safety objective (FSO) of 100 CFU/g at the moment of consumption without cooking, the PO would need to be estimated considering reasonably foreseen storage conditions (e.g. 48 h 12°C). This was mainly inferred from a study that considered the potential growth of L. monocytogenes in different bfV during storage for a maximum of 120 h at 5 to 12°C. It was concluded that the occurrence of relatively low levels of L. monocytogenes at the end of the production process, e.g. < 10 CFU/g (detection limit of the quantification method), would be compatible with that limit of 100 CFU/g as long as any labelling recommendations given are strictly followed (i.e. 24 h at 5°C). However, considering reasonably foreseeable conditions of use by the consumers beyond the labelling instructions (i.e. 48 h at 12°C), levels need to be considerably lower, even below the detection sensitivity of the current available standard analytical procedure/methods (not detected in 25 g) for those vegetables that best support pathogen growth. Microbiological criteria, set by the risk manager, can be used as a tool to verify that the threshold of the L. monocytogenes concentration in bfV at the end of production (compatible with the limit) is not exceeded. Sampling plans should be designed to take into consideration the expected heterogeneity of the contamination, the specificity and the sensitivity of the analytical methodology as well as the statistical confidence required for acceptance or rejection of non‐compliant lots. The impact of possible food safety criteria (FSC) on public health and/or on product compliance would be useful information to support risks managers’ decisions in this respect.
Consumer education and standardised label information are recommended to promote better understanding by consumers. It is also recommended to raise the awareness of the public health risks associated with the consumption of uncooked bfV, particularly by susceptible population groups. It is also recommended to perform subtyping of L. monocytogenes isolates detected during the routine monitoring program in FPE by molecular methods (e.g. WGS) and to improve collection and reporting of data on human listeriosis, including underlying conditions.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
A multi‐country outbreak of Listeria monocytogenes ST6 that caused 53 cases and 10 deaths over the period 2015–2018, was linked in 2018 to frozen vegetables. The processing of such vegetables included a blanching step before their freezing. The outbreak was detected using very specific molecular analytical methods, facilitating the linkage between human and food isolates. Environmental contamination of a freezing plant was indicated as the source of the persistence of the strain causing the outbreak from 2015 until 2018. At the request of the Commission EFSA published, on 3 July 2018, recommendations on the sampling strategies and established microbiological methods for maximising the sensitivity of detection of L. monocytogenes in processing water and the environment of premises producing frozen fruit, vegetables or herbs (FVH) as well as on the final food produced. Recommendations were also provided on the identification of critical sampling sites for environmental monitoring (EM) of L. monocytogenes.
Outbreak investigations conducted showed that some frozen FVH can be defrosted and used as such in salads by consumers or as ingredients in other ready‐to‐eat (RTE) products subsequently sold to consumers without undergoing any process to eliminate or reduce the level of pathogens. If such defrosted FVH are stored for a prolonged period at refrigeration temperature, the potential growth of L. monocytogenes could represent a serious public health risk.
Commission Regulation (EC) No 2073/20051 lays down the microbiological criteria for certain micro‐organisms, their toxins or metabolites and the implementing rules to be complied with by food business operators (FBOp). In particular, different L. monocytogenes food safety criteria have been laid down for RTE foods, depending on their ability to support the growth of this pathogen. While L. monocytogenes does not grow in frozen foods and therefore, a threshold of 100 CFU/g has been accepted for such products, such criteria might not sufficiently protect the consumer if defrosted FVH are stored for a certain period, even at refrigeration temperatures, before consumption.
Following a major outbreak of Shiga toxin‐producing Escherichia coli (STEC) O104:H4 in Germany in 2011, EFSA issued seven scientific opinions on pathogens in food of non‐animal origin, including an opinion providing outbreak data analysis and risk ranking of food/pathogen combinations, adopted on 6 December 2012. No combination of L. monocytogenes with food of non‐animal origin were included in that ranking, due to both the absence of reported L. monocytogenes outbreaks implicating food of non‐animal origin and the criteria considered in the ranking tool.
Prevention of contamination of FVH with pathogens is based on hygiene requirements and implementation of the HACCP principles, to be respected by FBOp at all stages of the food production chain and laid down in general in Regulation (EC) No 852/2004 on the hygiene of foodstuffs.2 Recommendations on the specific application of these requirements in the production and storage of frozen FVH may further reduce the risk from the potential contamination of such food with foodborne pathogens.
In view of the above, there is a need to evaluate and recommend specific control measures to reduce public health risks arising from the consumption of frozen FVH.
EFSA is asked to issue a scientific opinion on the public health risk posed by L. monocytogenes and, if considered relevant by EFSA, by other pathogens that may contaminate fruit, vegetables and herbs which are processed (e.g. blanched) prior to be placed on the market frozen. More specifically, EFSA is asked to:
-
provide an estimation of the public health impact of L. monocytogenes contamination, and if considered relevant of other pathogens of frozen fruit, vegetables and herbs blanched before freezing. For this purpose, EFSA should make a semi‐quantitative estimation of the risk posed by the “Listeria/pathogen – frozen fruit, vegetables and herbs” combination by comparing such risk with better known risks, for example:
-
–
with the risk of L. monocytogenes from other food such as meat products, dairy products and/or fishery products, or
-
–
with the risk of food of non‐animal origin/pathogen combination, as done in the previous EFSA scientific opinion on the risk posed by pathogens in food of non‐animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations), adopted on 6 December 2012.
-
–
assess the main risk factors of contamination and growth of pathogens in frozen FVH during all stages from processing (excluding at primary production) until consumption (including e.g. storage after thawing, food preparation and consumption habits).
-
provide recommendations:
-
a)
on possible control options that may be implemented by FBOp during the production process of frozen FVH and assess their efficacy to reduce public health risks, and
-
b)
on routine monitoring for L. monocytogenes in frozen FVH taking into account good hygiene practises (GHP) and procedures based on the HACCP principles.
-
a)
1.2. Interpretation of the Terms of Reference
It was clarified that, for the three ToRs, the assessment was exclusively for the commercial production of blanched frozen FVH. Based on the information kindly provided by the European Association of Fruit and Vegetable Processors (PROFEL; Appendix A), no fruit groups and typically no herbs are blanched while some or all groups of vegetables may be blanched. Therefore, fruit and herbs are out of scope of the assessment. Frozen meals (e.g. vegetables with meat) were also beyond the scope of the assessment but mixtures of frozen vegetables were to be considered.
For some products, the blanching process depends on the customer requirements, factory or process. Mostly, hot water blanching and steam blanching are applied in freezing plants in the EU and these technologies will be addressed in the assessment.
In a previous scientific opinion (EFSA BIOHAZ Panel, 2013), a categorisation of foods of non‐animal origin (FoNAO) was made to permit a risk ranking among different commodities with respect to the main biological hazards covered in the assessment. This categorisation of FoNAO is compatible with the definition of the food commodities used in EU food‐borne outbreaks (FBOs) and food consumption databases and followed the conventional categories of botanical groups (e.g. citrus fruit, stone fruit, apples and related fruit, berries, melon, tomatoes, leafy vegetables, roots and tubers, etc). In this opinion, the same categorisation is followed, and fruiting vegetables/vegetable fruits are considered vegetables.
The frozen products may have undergone a size reduction (e.g. cut, sliced and diced) but shall not be smashed or juiced (e.g. into smoothies and purees) before freezing. However, the frozen vegetables can be thawed and used for example in smoothies. New specialities include frozen vegetables that have been grilled or coated, but these have not been considered within this opinion.
It was agreed that after the identification of the relevant pathogens for frozen vegetables blanched before freezing in the ToR1, answers to ToR2 and ToR3 would be elaborated considering the selected pathogens in ToR1.
Based on the above, ToR 1 aims to provide an estimation of the public health impact of L. monocytogenes contamination of blanched frozen vegetables (bfV). If considered relevant, other pathogens associated with bfV could be considered.
After discussion with the European Commission, several points were clarified for ToR 1 as follows:
-
–
The criteria to be used for considering another pathogen as relevant are to be defined by EFSA.
-
–
Also, the bfV as one group or as subgroups are to be defined by EFSA depending on their importance and available data to distinguish such subgroups.
-
–
The methodology to be used is to be defined by EFSA. It could be qualitative, semi‐quantitative, or quantitative depending on the available data.
ToR 1 was reformulated as assessment questions (AQs):
Which are the pathogen(s) (in addition to L. monocytogenes) in bfV of relevance for public health/illness in the EU?
What is the public health risk posed by L. monocytogenes/pathogen – bfV (subgroups) in comparison with other known pathogen–food combinations using the methodology best suited based on the available data?
For ToR 2, it was clarified that:
-
–
Both bfV and non‐bfV are processed in the same company using the same processing lines and steps. Therefore, if the processing of non‐blanched products is a risk factor for blanched products, non‐blanched products will also be considered.
-
–
Although frozen vegetables are traditionally considered as non‐RTE products, these products may be consumed uncooked. According to the British Frozen Food Federation (BFFF, 2018), historically frozen vegetables have not been represented as RTE and ready‐to‐defrost‐and‐eat (RTDE) foods. However, changing consumer behaviour towards ‘healthy’, ‘on‐the go’, ‘convenience’ meals has changed consumer perception meaning that frozen vegetables can be treated by consumers as safe to eat without cooking. Also a recent internal review of products in the frozen market made by that Federation (BFFF, 2018) identified variation in the labelling information, with some products clearly stating that they are RTE, others implying the need to cook, and others being unclear. Additionally, in several informative leaflets and websites of companies manufacturing frozen vegetables, the FBOp explicitly suggests eating them directly after thawing, without cooking.
-
–
The risk factors for contamination are interpreted as sources of contamination (increasing the prevalence and/or the level of contamination) and those affecting growth (increasing growth and thus the level of contamination). Cross‐contamination at the consumer level is not considered.
The ToR 2 has been reformulated as (AQs):
What are the main factors affecting contamination and/or growth of the pathogens defined in ToR 1 in bfV during processing (i.e. until the product is released to retail/packaged)?
What are the main factors affecting contamination of the product and/or growth of pathogens defined in ToR 1 in bfV after processing and until consumption (including e.g. storage after thawing, food preparation and consumption habits)?
For ToR 3, it was clarified that:
-
–
Food businesses are obliged to develop and implement food safety management systems (FSMS) including prerequisite program (PRP) activities and hazard analysis and critical control point (HACCP) principles. For this reason, control options will be based on prerequisite programmes (PRPs; e.g. cleaning and disinfection), and operational prerequisite programmes (oPRPs) and, if possible, control points (CPs) and critical control points (CCPs; i.e. the steps at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level). PRPs are preventive practices and conditions needed prior to and during the implementation of HACCP and which are essential for food safety. However, some prerequisites, typically linked to the production process, may be identified as essential to control the likelihood of the introduction, survival and/or proliferation of food safety hazards in the product(s) or in the processing environment. These are referred to as oPRPs.
-
–
The control options are to be compared based on their efficacy (i.e. reducing the L. monocytogenes occurrence or levels on the final product), but the impact of this reduction cannot necessarily be translated to public health.
-
–
The starting point will be the receipt of the raw material at the processing plant.
-
–
Recommendations on routine EM program for L. monocytogenes in bfV processing environment and final product included in this scientific opinion have the purpose to verify that the FSMS implemented by the FBOp is well designed and has the appropriate control measures. The aim of the EM program is to establish the sampling strategies and microbiological methods for L. monocytogenes detection most appropriate for maximising the identification of sources and routes of L. monocytogenes contamination in the FPE.
-
–
L. monocytogenes concentrations in bfV at the end of the production process compatible with 100 CFU/g would need to be estimated based on the potential growth of L. monocytogenes at reasonably foreseen storage conditions. This would help if the FBOp decides to establish an intermediate level to guarantee that the limit of 100 CFU/g is not exceeded at the moment of consumption without cooking.
ToR 3 has been reformulated as (AQs):
Which possible control options can be recommended to be implemented by the FBOp during the production process of bfV, along with their efficacy to reduce contamination (prevalence and/or levels) of L. monocytogenes, within the frame of procedures based on the HACCP principles and GHP (as part of the PRPs)?
What is the L. monocytogenes concentration in bfV at the end of the production process as performance objective (PO)3 that would be compatible with the food safety objective (FSO)4 of 100 CFU/g at the moment of consumption without cooking, for different times and temperatures of storage once the frozen vegetable is removed from the freezer.
What routine monitoring for L. monocytogenes in bfV processing environment and final product can be recommended to verify the correct application of GHP and/or within the frame of procedures based on the HACCP principles?
1.3. Additional information
1.3.1. Additional background information
1.3.1.1. Previous EFSA scientific opinions and reports
After the major FBO of STEC O104:H4 in Germany in 2011, EFSA was asked to identify and rank specific food/pathogen combinations most often linked to human cases, including frozen fruits and vegetables. Based on a semi‐quantitative model, the top‐ranking food/pathogen combination was Salmonella spp. and leafy greens eaten raw followed by (in equal rank) Salmonella spp. and tomatoes, Salmonella spp. and melons, Salmonella spp. and bulb and stem vegetables, and pathogenic Escherichia coli and fresh pods, legumes or grains. Among the limitations of the model, it was highlighted that more efforts should be given to collect additional data, even in the absence of reported FBOs, as well as to enhance the quality of the EU‐specific data. In addition, to assist future microbiological risk assessments (MRA), consideration should be given to the collection of additional information on how food has been processed, stored and prepared as part of the above data collection exercises (EFSA BIOHAZ Panel, 2013).
In the scientific opinion on L. monocytogenes contamination of RTE foods and the risk for human health in the EU, time series analysis for the 2008–2015 period in the EU/EEA indicated an increasing trend of the monthly notified incidence rate of confirmed human invasive listeriosis for the over 75 age groups and female age group between 25 and 44 years old (probably related to pregnancies). A conceptual model was used to identify factors in the food chain as potential drivers for L. monocytogenes contamination of RTE foods and listeriosis. Factors considered likely to be responsible for the increasing trend in cases are the increased population size of the elderly and susceptible population except for the 25–44 female age group. For the increased incidence rates and cases, the likely factor is the increased proportion of susceptible persons in the age groups over 45 years old for both genders. Quantitative modelling suggests that more than 90% of invasive listeriosis is caused by ingestion of RTE food containing > 2,000 CFU/g, and that one‐third of cases are due to growth of the organism in the consumer phase (EFSA BIOHAZ Panel, 2018). In this opinion, three EFSA outsourcing activities under ‘Closing gaps for performing a risk assessment on L. monocytogenes in RTE foods’ are summarised, including: an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE foods (Pérez‐Rodríguez et al., 2017); a quantitative risk characterisation on L. monocytogenes in RTE foods, starting from the retail stage (Pérez‐Rodríguez et al., 2017); and the comparison of isolates from different compartments along the food chain, and in humans using whole genome sequencing (WGS) analysis (Møller Nielsen et al., 2017).
In 2018, EFSA and ECDC published the multi‐country outbreak report on L. monocytogenes serogroup 4b, multi‐locus sequence type (MLST) 6, infections linked to frozen corn and possibly to other frozen vegetables (EFSA and ECDC, 2018). This FBO of invasive L. monocytogenes infections confirmed by WGS had been ongoing in Austria, Denmark, Finland, Sweden and the United Kingdom since 2015. As of 15 June 2018, 47 cases were reported, and nine patients died.5 Cases were detected in Finland (23 cases), United Kingdom (11 cases), Sweden (7 cases), Denmark (4 cases) and Austria (2 cases). The median age of cases was 72 years (interquartile range 56–85), 26 (55%) cases were females. Information on hospitalisation was available for 16 patients, who were all hospitalised. WGS analysis of 29 non‐human L. monocytogenes isolates found them to be closely related to the multi‐country human cluster of L. monocytogenes serogroup 4b, MLST 6. The non‐human isolates were obtained from frozen corn, frozen vegetable mixes including corn, frozen spinach products, frozen green beans and two environmental samples. Traceability information for the contaminated products pointed to the source of contamination in a Hungarian freezing plant. It is possible that frozen vegetables other than corn processed in this plant could also be a vehicle of human infection. The maximum concentration found was 1,400 CFU/g in one frozen corn batch. However, the strain was not related to the FBO. L. monocytogenes 4b, ST6, but matches the outbreak strain in frozen corn and other frozen vegetables produced in the 2016, 2017 and 2018 production seasons at the plant of the Hungarian company A. This suggests, according to the report, that the strain was persisting in the processing environment after standard cleaning and disinfection procedures carried out in conjunction with periods of inactivity in the plant, as well as the rotation of the processed products. Production sites were also sampled in the Hungarian company A. The first positive sample was taken after the grinding stage which occurs after blanching (96°C for 110 s) and cooling of spinach.
Based on the consumption information included in the multi‐country outbreak report, no information on consumption as RTE (without cooking) of the implicated foods was provided. However, consumers may have eaten these thawed products without having cooked them properly or at all. For example, foods cooked in the microwave may still have cold spots where the bacteria could survive. Moreover, it was stated that ‘the consumption of thawed corn and thawed vegetables without cooking them is not an unusual practice (e.g. in salads, smoothies, etc.).’ and ‘In order to reduce the risk of L. monocytogenes infection due to the consumption of contaminated non ready‐to‐eat frozen vegetables, consumers should thoroughly cook these products before consumption, as it is not unusual for them to be consumed without being cooked (e.g. in salads, smoothies)’.
In 2018, EFSA provided scientific and technical assistance for the design of sampling and testing strategies for the detection of L. monocytogenes in the processing plants of frozen FVH. This was expected to support the competent authorities (CA) and FBOp in the, at that moment ongoing, multi‐country outbreak investigation mentioned above. Recommendations to the European Commission were provided on the sampling strategies and established microbiological methods most appropriate for maximising the sensitivity of detection of L. monocytogenes for microbial source tracking (MST) to identify the point of origin of microbial contamination in a food processing environment (FPE) including raw vegetables, processing water and the environment of premises producing frozen FVH as well as on the final food produced. Seven steps were defined for a fit‐for‐purpose sampling strategy, including the identification of critical sampling sites (CSSs) for EM of L. monocytogenes, which is expected to support food‐borne outbreak investigations where frozen FVH are implicated. The relevant CSSs can be defined based upon critical inspection inside a freezing plant and the background information described in the report. The international standards EN ISO 11290‐1:20176 and EN ISO 11290‐2:20177 were recommended for L. monocytogenes detection and enumeration. It was concluded that characterisation of L. monocytogenes isolates using well‐established molecular techniques is needed to establish links between isolates from humans and from implicated FVH (EFSA, 2018).
This FBO triggered PROFEL to take steps as a sector association together with DG SANTE. Two actions were agreed: (a) Investigate the risks associated with frozen vegetables – the growth rate of L. monocytogenes in five products (challenge tests) and the subsequent recommendation for on‐pack instructions and guidance to consumers; (b) Develop an industry guide to good hygiene practices, which will be used to raise awareness and improve standards across the industry. In collaboration with the Commission and EU MS, the guidelines aim to meet the requirements of Regulation (EC) No 852/20042 in order to obtain recognition as EU community guides for the production and food safety management of quick‐frozen FVH, starting from the receipt of raw materials and ending with the packed end products ready for next step in the food supply chain, business to business (B2B), business to consumer (B2C). The target is that FBOp active in the commercial production and/or trade of quick‐frozen FVH may use the guidelines as a starting point for their own FSMS, helping on the elaboration of good practices, PRPs and HACCP principles. The scope for the challenge tests is frozen FVHs, categorised to identify the most relevant products based on pH, sugar content, anti‐bacterial compounds, nutrient level and structure/texture of the product. From each category, a model product was chosen on which challenge tests were performed to identify the risk of possible L. monocytogenes growth. The selected products were white cabbage, sweetcorn kernels, sweet potatoes, peas and parsnips. The draft hygienic guidelines were submitted to DG SANTE by the end of October 2019 and have simultaneously been shared for consultation with broader interested stakeholder organisations representing suppliers or users of frozen FVH and other relevant interest groups (farmers, HORECA/catering, food and drink industry, consumer organisations, retailers, etc.) and with EFSA (see documentation provided to EFSA). These will be referred to as draft PROFEL guidelines in this document.
1.3.1.2. Production of bfV
Information kindly provided by PROFEL indicates that around 3.8 million tonnes of frozen vegetables were produced in the EU in 2017, which corresponds to data from 16 Member States (MS). Table 1 shows the production values for the different MS. In the draft PROFEL guidelines (PROFEL, 2019), the EU annual production of quick‐frozen vegetables8 is estimated at 4 million tonnes, including all MS.
Table 1.
Production of frozen vegetables in the EU (tonnes) as provided by Mrs. Nele Cattoor (General Secretary at PROFEL) by e‐mail on 28 June 2019 (Cattoor, 2019b)
| Country | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| Belgium, the Netherlands, Germany, UK | 1,481,105 | 1,415,079 | 1,442,825 | 1,511,392 |
| Portugal, Spain | 603,100 | 666,700 | 703,600 | 843,400 |
| Austria, Czechia, Denmark, Finland, Italy, Sweden | 394,384 | 351,211 | 317,132 | 377,328 |
| Poland | 404,000 | 449,800 | 585,600 | 707,000 |
| France | 391,324 | 399,535 | 381,000 | 321,015 |
| Hungary | 70,629 | 70,150 | 62,587 | 36,950 |
| Greece | 30,000 | 30,000 | 30,000 | 30,000 |
| Total | 3,374,542 | 3,382,475 | 3,522,744 | 3,827,085 |
In the previous EFSA report (EFSA, 2018), the production of frozen FVH has been described in detail. As this assessment is restricted to bfV, the flow charts have been revised and are presented in Figures 1a and b. They provide a general description of the process to produce bfV from the moment of receipt of the fresh vegetables/ingredients at the freezing plant and/or handling facilities. It should be noted that the details of this flow chart might vary between companies.
Figure 1.
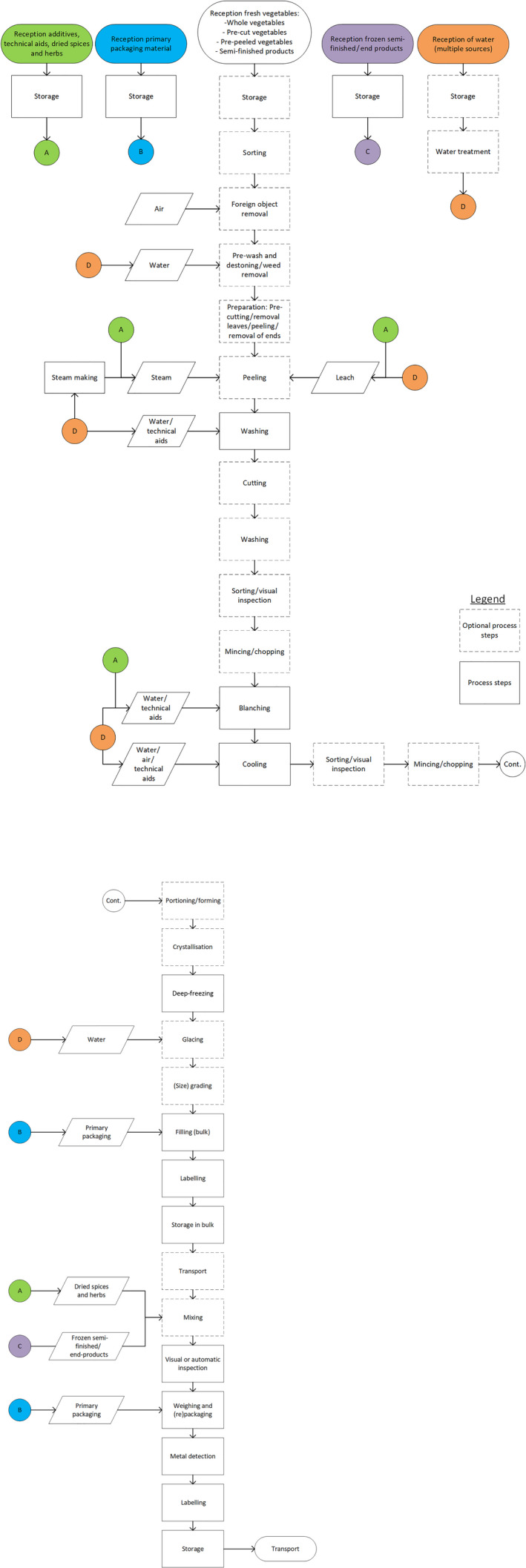
General flow chart for the production of blanched frozen vegetables from the moment of receipt of the fresh vegetables at the freezing plant and/or handling facilities
- This flow chart might vary between companies and only represents a general description of the process.
1.3.1.3. Legal background
Food safety criteria (FSC) for L. monocytogenes in RTE foods have been applied from 2006 onwards (Commission Regulation (EC) 2073/20051). This Regulation requires the following:
-
–
In RTE foods intended for infants and RTE foods for special medical purposes, L. monocytogenes must not be detected in 25 g of sample (n = 10 sample units);
-
–
In RTE foods that are unable to support the growth of the bacterium, other than those intended for infants and for special medical purposes, L. monocytogenes must not exceed 100 CFU/g during the shelf‐life (n = 5 sample units); and
-
–
In RTE foods that are able to support the growth of the bacterium, other than those intended for infants and for special medical purposes:
-
○
L. monocytogenes must not be detected in 25 g of sample before they have left the immediate control of the producing FBOp (n = 5 sample units), when the FBOp is not able to demonstrate, to the satisfaction of the CA, that the product will not exceed the limit of 100 CFU/g throughout the shelf‐life; and
-
○
L. monocytogenes must not exceed 100 CFU/g during the shelf‐life (n = 5 sample units) when the FBOp is able to demonstrate, to the satisfaction of the CA, that the product will not exceed the limit of 100 CFU/g throughout the shelf‐life.
-
○
In this Regulation, RTE food is defined, as Food intended by the producer or the manufacturer for direct human consumption without the need for cooking or other processing effective to eliminate or reduce to acceptable level microorganisms of concern.
1.3.2. Approach to answer the ToR
Term of reference 1
To answer to ToR 1, the pathogen(s), in addition to L. monocytogenes, in bfV of relevance for public health in the EU were identified and defined based on data of FBOs and of the Rapid Alert System for Food and Feed (RASFF). The information kindly provided by PROFEL (Appendix A) was used to distinguish the vegetables that are usually blanched before the freezing process. Then, the blanched vegetable subgroup(s) of interest were chosen considering the pathogens selected and the available data. Finally, the public health impact of L. monocytogenes contamination of bfV was compared with the better‐known risk of food‐borne illness associated with RTE foods such as RTE meat products, dairy products and/or fishery products. This was done using data on FBOs at EU/EEA level from 2008 to 2018 related to L. monocytogenes for the whole population and using a QMRA model to estimate the listeriosis risk with the consumption of bfV by the elderly population of the age group 65–74 years old. This population category was selected because it represents a higher risk than the younger population, but it does not represent the worst‐case scenario (population over 75 years old). For the modelling approach, bfV were separated into two different food subcategories to encompass the range of consumer habits in relation to the mode of use/consumption. Namely those consumed uncooked (i.e. consumed without any cooking step able to eliminate L. monocytogenes) and those consumed cooked (i.e. boiled, fried or microwave heated, as recommended by most of the producers). Since full cooking of vegetables would result in negligible risk, the latter subcategory also includes heat treatments that would not result in the elimination of L. monocytogenes.
Term of Reference 2
The approach taken to answer to ToR 2 was to identify the main factors affecting contamination and growth of the pathogen(s) in bfV during the production and consumption stages. The production steps started from the receipt of the raw material at the processing plant (excluding primary production), while the consumption steps included e.g. storage after thawing, food preparation and consumption habits.
Term of Reference 3
The approach taken to answer to ToR 3 was to identify the food safety management options during the production process, based on the outcome of ToR 2 regarding factors affecting contamination and growth. These control options include PRPs, including GHP, good manufacturing practices (GMP) and oPRPs, as well as procedures based on the HACCP principles. Also, alternative methods reported by the scientific literature as potential intervention options for the inactivation of L. monocytogenes and, in most cases, not implemented by the industry were considered. Considering that control options are based on preventive measures, the efficacy of the establishment of specific PRPs on the reduction of contamination in the product cannot be assessed. Recommendations on routine monitoring for L. monocytogenes in the bfV processing environment and final product were carried out by critically appraising available guidelines for the industry taking into account that sampling and monitoring recommendations have the purpose to verify that the FSMS implemented by the FBOp is well designed and has the appropriate control measures. The growth of L. monocytogenes in different bfV after different holding periods under reasonably foreseeable conditions of use by the consumers was calculated, using data on growth kinetic parameters and growth potential, to estimate the L. monocytogenes concentration at the end of the production process that would be compatible with the FSO of 100 CFU/g.
2. Data and methodologies
2.1. Data
2.1.1. Data request through EFSA's Microbiological Risk Assessment network
The Microbiological Risk Assessment (MRA) network was requested to provide information about:
the presence and concentration of pathogens (incl. L. monocytogenes) in frozen vegetables and, if available, their link to human cases (e.g. FBOs);
the risk factors and possible control options (and efficacy) from processing until consumption;
the growth of L. monocytogenes in thawed vegetables; and
the consumer behaviour for handling frozen vegetables (e.g. temperature/time conditions of thawing; % consuming uncooked; % thawed beforehand) and consumption (e.g. importance of industrially frozen vs. fresh vegetables).
2.1.2. Data on pathogens of public health relevance in bfV
2.1.2.1. Food‐borne outbreaks
Within the framework of the EU Zoonoses Directive 2003/99/EC,9 the EU MS are required to submit data on the occurrence of zoonoses, zoonotic agents, antimicrobial resistance and FBOs. EFSA, in collaboration with ECDC, coordinates the collation and analysis of these data to produce the annual EU One Health Zoonoses Report10 which include data on FBOs. The latter represents the most comprehensive set of data available at an EU level for assessing the burden of FBOs in the EU/EEA and the related contributing risk factors. The technical specifications for harmonised reporting of FBOs through the EU reporting system, in accordance with the aforementioned EU Zoonoses Directive can be found in EFSA (2014).
For the identification of pathogens of public health relevance in bfV, data on ‘strong and weak evidence’ FBOs from the years 2005–2018 were extracted from the EFSA zoonoses database on 29 July 2019.
To compare the vehicles involved in the outbreaks related to L. monocytogenes, only data on ‘strong’ FBOs at EU/EEA level from 2008 to 2018 were considered and were extracted from the EFSA zoonoses database on 29 July 2019.
2.1.2.2. EU Rapid Alert System for Food and Feed data
Commission Regulation (EU) No 16/201111 lays down the implementing measures for the requirements of Regulation (EC) No 178/200212 around the RASFF.13 This is established as a system facilitating the notification of food and feed safety alerts among the CAs of MS. Although, the RASFF system is primarily a communication facility and cannot be considered to be an epidemiological surveillance system as explained previously (EFSA BIOHAZ Panel, 2018), RASFF data were used in this assessment to provide information about the hazards present in frozen vegetables. A search was conducted on 1 August 2019 of the RASFF database using as product category ‘food’, as the hazard category ‘pathogenic microorganisms’, as the product categories ‘fruits and vegetables’ or ‘herbs and spices’ and as subject ‘frozen’ or ‘freezing’. No time restriction was applied. Manual screening was performed to gather information about the product being a frozen vegetable not being smashed or juiced before freezing.
2.1.3. Data on L. monocytogenes in frozen vegetables
2.1.3.1. EFSA monitoring data
The monitoring data collected by EFSA on L. monocytogenes in RTE food are mainly food chain control data (official monitoring) and are collected by the CA conducting investigations to verify whether the FBOp implement correctly the FSC (see Section 1.3.1.3). As stated by Boelaert et al. (2016) and EFSA (EFSA BIOHAZ Panel, 2018), L. monocytogenes belongs to a second category of monitoring data that are less harmonised compared to a first category of fully harmonised and comparable data. Although the matrices sampled are harmonised and the sampling and analytical methods for L. monocytogenes are harmonised to a certain extent, the sampling objectives, the place of sampling and the sampling frequency vary or are interpreted differently between MS and according to food types. Data on the occurrence of pathogens on frozen vegetables were extracted (matrix level 3) for the years 2008–2018. Only the data retrieved using detection methods (presence/absence) were used as these are considered to have a higher sensitivity compared to the L. monocytogenes enumeration method. Data were summarised considering the sampling units (single units and batches), sampling stages (processing and retail stages), sampling context (e.g. surveillance, monitoring) and sampling strategy (e.g. ‘census sampling’, ‘convenience sampling’ and ‘objective sampling’ vs. ‘suspect sampling’, ‘selective sampling’).
2.1.3.2. Data from scientific literature and outsourcing activities
The strategy for conducting the literature searches is provided in Appendix B. Two searches were conducted in Web of Science™ Core Collection (1975–present), CABI: CAB Abstracts® (1910–present) and PubMed on 8 April 2019. The search aimed to retrieve information on hazards in frozen FVH (first search) and related to L. monocytogenes in frozen FVH (second search).
The records were complemented with relevant records from the report by Jofré et al. (2016). In that report, the results of an extensive literature search considering the time span 1990–2015, is described with the aim to gather information on the occurrence and levels of contamination of L. monocytogenes in different foods (i.e. RTE foods, leafy greens and melons and traditional meat products) and risk factors for L. monocytogenes contamination of various foods. Information was extracted about the study, type of product (population) and analytical methodology, risk factors (exposure and comparators) and results (outcomes) about prevalence and concentration of L. monocytogenes. Also, data obtained through the MRA network and extracted from the monitoring data were assessed (see Section 2.1.1).
Prevalence data were included considering random sampling taking place after 2010 from Europe and North America, covering retail or processing plants (data obtained from one plant being repeatedly sampled was avoided), independent of analytical method, and for ‘frozen vegetables’ or ‘mixed vegetables’ which according to Appendix A are always, or may be, blanched. Concentration data, in most cases left‐censored, were extracted from the subset of prevalence studies reporting this and were fitted to a beta‐general distribution.
2.1.3.3. Multi‐country outbreak report
To complement the limited concentration data, quantitative results from sampling and analysis of frozen vegetables were extracted from the previously mentioned multi‐country outbreak report (EFSA and ECDC, 2018) where possible.
2.1.4. Consumption data
To estimate the serving size of bfV, the acute food consumption data (considering the consuming days only) were extracted from the EFSA Comprehensive European Food Consumption Database14 for the elderly (65–74 years old) age group for a representative set of vegetables that are commonly blanched and used as frozen products. The vegetables were: carrots, peas, beans, broccoli, corn and asparagus. Data were available from 19 MS and 23 surveys. The most recent survey per MS was considered to estimate summary statistics. Thus, data were considered from 19 surveys. The survey starting dates ranged from 2000 to 2015.
To estimate the consumption of bfV and consequently the number of servings, data were extracted by considering those vegetables in the FoodEx2 list that are (i) always blanched, (ii) always or sometimes blanched and (iii) always or sometimes blanched but without potatoes. Potential blanching was based on Appendix A. For the estimations, it was assumed that about 8% of the total consumption events would have been derived from industrially frozen vegetables. This figure was derived from a recent Belgian survey on home consumption in 2018, in which the total vegetable consumption per capita was 45 kg, of which the frozen vegetables accounted for 3.6 kg.15 This proportion is in agreement with the figure of 8.2% on average (interquartile range (IQR) = 5.4–11.2%) derived from data available in the EU Data Food Networking (DAFNE16) databank, the Eurostat report about the fruit and vegetable sector (201917) and the PROFEL production data.18 The surveys available from 28 countries indicate that, on average 16% (IQR = 11–22%) of the total vegetable consumption comes from the category ‘processed vegetables’, including frozen, tinned and others (such as pickled, dried, ready meals, etc). Assuming that frozen and canned vegetables represent approximately 75% of the processed vegetable production, while the group of others representing 25% (Eurostat). Overall, the production of frozen vegetables is double that of canned, but there are exceptions: France, where it is the opposite and Poland that produces about eightfold more frozen than canned vegetables (PROFEL data).
2.2. Methodologies
2.2.1. ToR 1
2.2.1.1. Identification of pathogens of public health relevance in bfV
The identification of pathogens, in addition to L. monocytogenes, in bfV of relevance for public health/illness in the EU was performed using data on ‘strong and weak evidence’ FBOs from the years 2005–2018 as explained in Section 2.1.2.1. The vehicle category ‘Vegetables and juices and other products thereof’ was selected. Manual screening of the ‘vehicle info fields’ and ‘rescom’ fields was performed to gather information about the vehicle being a frozen vegetable.
2.2.1.2. Public health impact based on food‐borne outbreak data
The data on ‘strong’ FBOs at EU/EEA level from 2008 to 2018 related to L. monocytogenes were used to compare the number of outbreaks, number of human cases, number of hospitalised cases and number of fatal cases for the food vehicles involved in the outbreaks.
2.2.1.3. Public health impact based on a QMRA
The estimation of the public health risk posed by L. monocytogenes and bfV in comparison with other known pathogen–food combinations was performed using a model that was developed based on the generic QMRA (gQMRA) as described in a previous scientific opinion (EFSA BIOHAZ Panel, 2018). This model is called generic because users can add food categories and their own data. A revision of the model was needed since that model aimed to estimate the risk associated with consumption of different RTE food categories (e.g. cold‐smoked fish, hot‐smoked fish, gravad fish, cooked meat, sausage, pâté and soft and semi‐soft cheese) for all age and gender groups. The food categories were subcategorised by the type of atmosphere packaging (i.e. normal atmosphere packaging (NAP) or reduced oxygen packaging (ROP)), except for soft and semi‐soft cheese, resulting in 13 RTE food subcategories. Furthermore, the model does not distinguish risk associated with the different foods, only total risk and did not include the bfV as food category. Thus, a revised model, named the modified generic Quantitative Microbiological Risk Assessment (mgQMRA), was developed using blocks from the gQMRA as a starting point (Figure 2).
Figure 2.
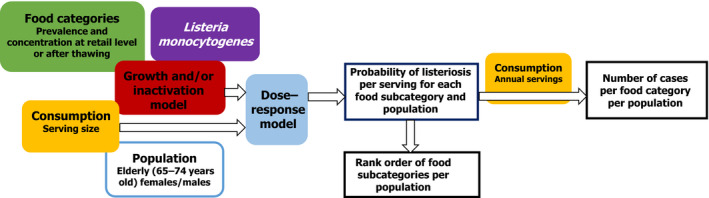
Overview of the mgQMRA developed to assess the risk associated with blanched frozen vegetables (two subcategories, with or without cooking) and 13 ready‐to‐eat food subcategories (based on type of atmosphere packaging)
The objective of the mgQMRA is to estimate and rank the mean probability of listeriosis per serving of the different food categories for the elderly population. The 13 RTE food subcategories defined above were supplemented with two subcategories of bfV considering the mode of use/consumption, being either cooked (i.e. consumed after cooking at variable extent) or uncooked (i.e. consumed as a RTE product). Public health impact was also estimated by comparing the predicted annual number of listeriosis cases. The model scripts were implemented in R version 3.5.1 (R Core Team, 2018, Appendix C). The input data for bfV are shown in Table 2 whereas for the 13 RTE food subcategories, it can be found in EFSA BIOHAZ Panel (2018), which sourced data from Pérez‐Rodríguez et al. (2017).
Table 2.
Summary of the generic Quantitative Microbiological Risk Assessment (mgQMRA) input data for blanched frozen vegetables (bfV) consumed with or without cooking for the baseline scenario and best‐case and worst‐case uncertainty scenarios
| Parameter | Distributiona | Input parameters | Baseline scenario | Input parameters for best‐case uncertainty scenario | Input parameters for worst‐case uncertainty scenario | Comments or percentiles used for best‐case and worse‐case |
|---|---|---|---|---|---|---|
| Prevalence of L. monocytogenes in bfV | Beta |
alpha = (147 + 1) beta = (1,288‐147 + 1) |
p = 0.114 | p = 0.098 | p = 0.133 | 2.5th and 97.5th percentiles |
| Initial concentration of L. monocytogenes in bfV (log10 CFU/g) – surveys/studies + FBO data | Beta‐general |
Min = −1.69 Max = 5 Shape 1 = 0.094 Shape 2 = 2.839 |
Used as baseline |
Shape 1 = 0.121 Shape 2 = 3.819 |
Shape 1 = 0.073 Shape 2 = 2.217 |
2.5th and 97.5th percentiles |
| Exponential growth rate (expressed as log10 /h) of L. monocytogenes in heat‐treated vegetables at a reference temperature of 5°C (EGR5°C) | Lognormal |
Mean = 0.0117 SD = 0.00816 Max = 0.0319 |
All scenarios | All scenarios | All scenarios | Not evaluated in the uncertainty analysis |
| Serving size of vegetables (g) | Constant | Mean = 49 | Used as baseline | 31 g | 106 g | 20th and 80th percentiles, respectively |
| Maximum population density (log10 CFU/g) of L. monocytogenes in vegetables | Constant | Mean = 7.83 | Used as baseline | 5.43 | 9.78 | Minimum and maximum Nmax |
|
Consumer storage time outside the freezer = (remaining shelf‐life × proportion being used) Remaining shelf‐life (h) Proportion being used |
Exponential with rate = 1/mean shelf‐life Beta‐Pert |
Mean shelf‐life = 12 h (‘cooked’ vegetables) Mean shelf‐life = 24 h (‘RTE’ vegetables) Max = 96 h Minimum = 0 Most likely = 0.3 Maximum = 1.1 |
All scenarios | All scenarios | All scenarios | Not evaluated |
| Storage temperature at consumer fridge (°C) | Truncated Normal |
Mean = 5.9°C SD = 2.9°C Lower = −2°C Upper = 15°C |
All scenarios | All scenarios | All scenarios | Not evaluated – same for all foods |
| Reduction of L. monocytogenes in the event of cooking (log10 units) | Beta‐Pert |
Min = 1 log10 Most likely = 5 log10 Maximum = 9 log10 |
Used as baseline | Same as baseline | Most likely = 3 | Reasonable assumptions |
| Dose response of L. monocytogenes for the elderly population | Log‐normal exponential model |
Females: Mean = −13.7020 SD = 1.6154 Males: Mean = −13.5598 SD = 1.6154 |
All scenarios | All scenarios | All scenarios | Not evaluated‐ The same uncertainty over all food categories so less important for the uncertainty related to the comparison |
| Annual number of servings of bfV for the elderly population | Constant | 2.65 × 109 (females); 2.28 × 109 (males) | Baseline | 1.63 × 109 (females); 1.38 × 109 (males) | 3.15 × 109 (females); 2.74 × 109 (males) | Public health impact evaluated in scenario analysis |
| Proportion of servings of bfV consumed cooked or uncooked by the elderly population | Constant | Cooked = 50, 60, 70, 80, 90, 100 and uncooked = 50, 40, 30, 20, 10, 0 | Proportions based on label information Willis et al. (2019): cooked: 96%; uncooked = 4% | Proportions based on label information Willis et al. (2019): cooked: 77%, uncooked = 23% | Different proportions were evaluated in separate scenario analyses |
The same distributions as used in the gQMRA in EFSA BIOHAZ Panel (2018), except for the log10 reduction for cooking which was not included in that model.
The exposure was estimated based on the prevalence and initial contamination of L. monocytogenes level at retail, taking potential growth during storage and inactivation during cooking, and serving sizes for the population of interest into consideration (Figure 2). The exposure was then combined with an exponential dose response (DR) model to estimate the mean probability of listeriosis per serving using the model parameters for the elderly population developed in EFSA BIOHAZ Panel (2018). Distributions were used to describe the variability of model parameters and to estimate the mean probability of illness following consumption of a contaminated serving. This estimate was multiplied by the mean prevalence of L. monocytogenes in the food subcategories to obtain the risk per any serving. To achieve a sufficient number of iterations and consistent ranking of food subcategories, multiple simulations were run (250 simulations) each involving 2 × 105 iterations (Figure C.1; Appendix C). Ranking of risk per serving was based on the mean probability of illness per serving (Pérez‐Rodríguez et al. (2017)).
Figure C.1.
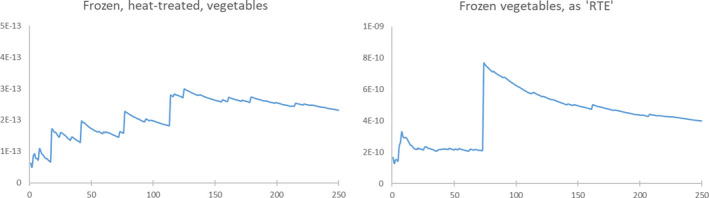
The estimated mean probability of illness per serving as a function of the number of simulations for the two scenarios; thawed frozen vegetables consumed after cooking or without cooking (i.e. as RTE food)
- The impact of random high‐risk iterations resulting in higher risk simulations is illustrated. The impact is decreasing with the number of simulations and 250 simulations were selected as a balance between the required precision and the execution time.
The public health impact of bfV in terms of the number of cases per year in the female/male elderly age group was evaluated by combining the predicted mean probability of illness per serving with the number of servings per year in the EU/EEA. The estimation of the total number of bfV servings in the EU/EEA is described in Section 2.1.4, and scenarios of different proportions of servings consumed uncooked or cooked were evaluated. The predicted number of cases for bfV was compared with the predicted number of cases associated with the seven RTE food categories mentioned above.
As mentioned above, the L. monocytogenes contamination of the RTE food categories was characterised by the prevalence and concentration at retail. The uncertainty in the prevalence estimation was described by a beta‐distribution as in the gQMRA model (EFSA BIOHAZ Panel, 2018). Very sparse concentration data were available representing bfV and these data were mostly reported as below the limit of detection of the plate count procedure (e.g. < 10 CFU/g to < 100 CFU/g depending on the analytical methodology used). The concentration data were fitted to a beta‐general distribution using the fitdistrplus package in R (Delignette‐Muller and Dutang, 2015). A non‐parametric bootstrap resampling method was applied to estimate uncertainty in the parameters of the distribution fitted to the data. The 95% confidence interval (CI; 2.5th and 97.5th percentiles) for the parameters is reported in Table 2 and were used in the evaluation of uncertainty. Two distributions were developed, one based on the literature data and the other one supplementing these data with concentration data from the multi‐country outbreak report (EFSA and ECDC, 2018).
A weighted mean serving size of bfV was estimated from the mean serving sizes derived as reported in Section 2.1.4. As the number of consuming days in the various surveys varied, the reported mean serving sizes were weighted by the number of consuming days of the respective survey in order to obtain a weighted mean serving size. The three estimates of the total number of servings of bfV described in Section 2.1.4, representing uncertainty, were assumed to represent a baseline (always or sometimes blanched but without potatoes), best‐case (always blanched) and worst‐case scenario (always or sometimes blanched with potatoes). The potential public health impact was evaluated by assuming different proportions of the total number of servings being consumed uncooked (as RTE) and cooked, respectively.
Changes in L. monocytogenes levels were described by estimating growth using the same approach as in EFSA BIOHAZ Panel (2018). The exponential growth rate (EGR) of one specific strain or a pool of L. monocytogenes strains (up to a 12‐strain mixture) was obtained from scientific literature for different vegetables including corn, green peas, carrots, broccoli, beans, asparagus (Beuchat and Brackett, 1990; Kataoka et al., 2017) and records available in ComBase.19 Experiments using vegetables previously heated (e.g. blanched or an equivalent heat treatment) were also considered as the pathogen seems able to grow faster in heat‐treated vegetables (see Section 3.3.2). EGR values collected were either reported in the scientific article or estimated by primary growth model fit using the DMFit tool from ComBase. The EGR values at specific storage temperatures were standardised to 5°C (EGR5°C) using the square‐root‐based secondary model for bacterial growth using Tmin = −1.18°C as in FDA and FSIS (2003) and EFSA BIOHAZ Panel (2018). The variability of EGR5°C of L. monocytogenes was assumed to be log‐normally distributed and the mean, standard deviation and truncated maximum rate (mean + 2 standard deviations) considered (Table 2). No lag time was included in the growth simulation; this assumption may overestimate growth and the associated risk. The mean and standard deviation of the maximum population density of L. monocytogenes as result of growth (MPD) was extracted from the data in the same studies used to extract the EGR. The minimum and maximum MPD (MPDmin and MPDmax) was used in the best‐ and worst‐case scenario, respectively (Table 2).
Different heat treatment intensities (t/T combinations) have been considered for cooking, varying between very strong heat treatments (i.e. fully cooking) to lesser heat treatments (i.e. slight microwaving). The impact of cooking on the exposure to L. monocytogenes, in terms of log‐reduction, was modelled as a beta‐pert distribution, assuming a reduction between 1 and 9 log10 units, with 5 log10 as the most likely value (equivalent to the minimum required for a fully cooking treatment of food (NACMCF, 2006). The parameters of this distribution were based on considerations of potential heat treatments, as well as the variability of heat resistance of L. monocytogenes reported by the meta‐analysis carried out by van Asselt and Zwietering (2006). That study reported a mean decimal reduction time (D‐value) at 70°C (D70°C) of 0.09 min and an upper 95% prediction interval, used as a conservative estimation D70°C = 0.52 min, and the thermal resistance constant z = 4–11°C (EURL‐L. monocytogenes and ANSES, 2019b). To address the uncertainty with this assumption, a most likely value of 3 log10 units was evaluated representing a higher risk scenario associated with less effective heating methods than cooking in boiling water.
The remaining shelf‐life in the consumer refrigerator was modelled as an exponential distribution with a rate described by 1/mean shelf‐life. The mean shelf‐life was assumed 0.5 day for cooked vegetables and 1 day for vegetables consumed uncooked. The maximum remaining shelf‐life resulting from the exponential distribution was assumed 4 day. The actual consumer storage time is calculated as the remaining shelf‐life multiplied by a proportion of remaining shelf‐life at which time consumption takes place. The proportion is described by a beta‐pert distribution, with a minimum, mode and maximum proportion of 0, 0.3 and 1.1 as in the previous scientific opinion (EFSA BIOHAZ Panel, 2018). For bfV, this time represents the delay between vegetables being thawed and consumed (uncooked subcategory) or between being thawed and cooked (cooked subcategory). Storage temperature was assumed to follow a normal distribution among consumers but constant during the storage period for a given consumer (EFSA BIOHAZ Panel, 2018).
In the previous scientific opinion (EFSA BIOHAZ Panel, 2018), the number of cases of the seven RTE food categories were calculated based on the probability of illness per type of packaging (i.e. NAP or ROP), using data on the proportions of food with each packaging produced and consumed. For bfV, the proportion of consumption as cooked and uncooked (as RTE) was needed. Since there are limited data on this, different proportions were assumed and used in scenarios to evaluate total number of cases based on estimates of 1) the probability of illness per serving and 2) the total number of servings of bfV. In addition, the number of cases was also predicted based on the reported proportions of bfV with information on the label to cook or not before consumption (Willis et al., 2019).
The same DR model and parameters developed in the previous scientific opinion were used for the elderly female and male age groups (EFSA BIOHAZ Panel, 2018). The r‐value is the key parameter in this DR model and represents the probability that one single cell will survive the different barriers in the human intestinal tract and multiply in the human host. This value depends on the susceptibility of the host and the virulence of the L. monocytogenes strain.
2.2.2. ToR 2
First, the stages from processing (excluding primary production in the field) until consumption (including e.g. storage after thawing and consumption habits) of bfV were identified based on the EFSA technical report (EFSA, 2018) and combined with expert knowledge on the current industrial practices thanks to the exchange of information between the WG and PROFEL regarding the type of products subjected to blanching (Appendix A) as well as the processing steps involved in the production chain (draft PROFEL guidelines). Secondly, scientific papers retrieved by the literature search described in Section 2.1.3.2 (including ComBase) as well as the knowledge and expertise of the WG members were considered to identify the main factors affecting contamination and/or growth of L. monocytogenes in bfV during the production and consumption stages.
2.2.3. ToR 3
Scientific papers as well as the knowledge and expertise of the WG members were considered to identify possible control options that may be implemented by FBOp during the production process of bfV. The proposal for control options highlights the most critical steps to be considered by the industry taking into account prerequisite programmes (PRPs) and procedures based on the HACCP principles. The identification of PRPs, oPRP or CCP was performed based on the Commission Notice on the implementation of FSMS covering PRPs and procedures based on the HACCP principles, including the facilitation/flexibility of the implementation in certain food businesses (Commission Notice (EC) No 2016/C 278/0120).
The control measures considered are based on preventive measures, and for this reason, the efficacy of the establishment of specific preventive measures on the reduction of contamination in the product cannot be assessed. Reviewed research papers, focussing on additional technologies (which in most cases are not fully implemented by the industry), have been retrieved by the literature search. Recommendations on routine monitoring for L. monocytogenes in bfV processing environments and final product were carried out by critically appraising available guidelines for the industry taking into account that sampling and monitoring recommendations have the purpose to verify that the FSMS implemented by the FBOp is well designed and has the appropriate control measures. Key documents were selected based on WG discussion. These included the EURL Lm‐ANSES ‘Guidelines on Sampling the Food Processing Area and Equipment for the Detection of Listeria monocytogenes’ (EURL‐L. monocytogenes and ANSES, 2019a), the international standard EN ISO 18593:2018,21 the document from the Food and Drug Administration ‘Testing Methodology for Listeria species or L. monocytogenes in Environmental Samples’ (FDA, 2015), and the ‘Environmental Monitoring Handbook for the Food and Beverage Industries’ published by the University of Cornell in collaboration with 3M (3M and Cornell, 2019). For frozen vegetables produced as RTE, the draft guidance from the Food and Drug Administration/Center for Food Safety and Applied Nutrition ‘Control of Listeria monocytogenes in Ready‐To‐Eat Foods: Guidance for Industry’ (FDA‐CFSAN, 2017), among others, were considered for the assessment. Information provided by the draft PROFEL guidelines (PROFEL, 2019) has been included.
To estimate L. monocytogenes concentrations at the end of production process compatible with the FSO of 100 CFU/g, L. monocytogenes growth in different bfV was calculated considering the period between the removal of the product from the freezer and the subsequent storage for a certain period of time under reasonably foreseeable conditions of use by the consumers (8–12°C for a maximum of 120 h). For this, the growth kinetic parameters of a 12‐strain cocktail of L. monocytogenes in frozen–thawed corn and green peas were obtained from the predictive models developed specifically for these vegetables by Kataoka et al. (2017), which included a rapid thawing process of the 25 g portions used in the experiment. In addition, the growth potential during thawing and storage at 9°C for 24 and 48 h obtained in the challenge tests reported in the draft PROFEL guidance of 200 g portions of different types of frozen vegetables, were taken into consideration.
2.2.4. Uncertainty
Based on the EFSA guidance on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018b), and the scientific opinion on the principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018a), special attention was given to: (i) the interpretation of the ToRs, i.e. framing of the mandate and the AQs, (ii) identifying sources of uncertainty and (iii) their impact on the outcome of the assessment. The identified assumptions and other sources of uncertainty were listed.
For ToR1, the uncertainty of the rankings based on comparisons to other known risks using the mgQMRA model was evaluated by running a baseline and two uncertainty scenarios, termed a best‐ and worst‐case scenario. This resulted in 19 different outcomes for the food subcategories and scenarios being evaluated and ranked; 13 RTE food subcategories and 2 subcategories of bfV each with three scenarios. For the predicted number of cases, scenarios with different estimates of the total number of servings of bfV and proportions of consumer behaviour in terms of consuming uncooked or cooked were evaluated. The identified sources of quantified and non‐quantified uncertainties and the outcomes of the best and worst‐case scenarios were used to estimate the overall uncertainty in the response to ToR1. In support of this analysis, the mgQMRA model was implemented in the @Risk Analysis Add‐in (version 7.5.0, Palisade Corporation) for Excel. First, sensitivity and scenario analyses were carried out for the effect of variability in input parameters on the variability of the output mean risk per serving for the separate scenarios. Second, a sensitivity analysis of the uncertain parameters serving size, prevalence, initial concentration and MPD was evaluated by changing one parameter at the time (in Table 2) using the Simtable function in @Risk. A total of 200,000 iterations of the model per simulation were used in the sensitivity analyses.
For ToR2 and 3, uncertainties may result in the incomplete identification or misclassification of (1) factors of contamination or growth of L. monocytogenes in bfV; (2) control options; and (3) recommendations on routine monitoring. The following were considered:
Incompleteness: some factors/control options/recommendations may be missed in the identification process and so would be considered non‐existent or not relevant.
Misclassified: some factors of contamination/control options/recommendations may be wrongly included in the list of an outcome table without being relevant factor.
The uncertainty analysis was limited to the quantification of the probability of incompleteness or misclassified factors/control options/recommendations. For the incompleteness of factors/control options/recommendations, the expert knowledge was elicited on the probability that at least one factor/control option/recommendation was missed in the outcome table. For the misclassification, the experts elicited the probability that each factor/control option/recommendation included in the outcome table was correctly included.
3. Assessment
3.1. Pathogens of public health relevance in bfV
3.1.1. Evidence from food‐borne outbreaks
An overview of the FBOs in the EU/EEA where frozen vegetables were implicated as reported in EFSA's zoonoses database (2005–2018) can be found in Table D.1 in Appendix D. Before 2018, two outbreaks were reported with 17 cases, no hospitalisations or deaths, implicating frozen vegetables. The Staphylococcus aureus outbreak22 in frozen beans took place at a school or kindergarten in Belgium in 2009 and caused 14 cases. It was reported that storage time/Temperature (t/T) abuse was a contributory factor (i.e. a fault or circumstance that singly or in combination led to the FBO). Based on Appendix A, the beans were likely to have been blanched.
Table TableD.1.
Summary of food‐borne outbreaks (FBOs) in the EU/EEA where frozen vegetables were implicated as reported in EFSA's zoonoses database (2005–2018)
| Year | Causative agent | Countrya | Number of human cases | Number of hospitalised cases | Number of deaths | Food vehicleb | Blanchedc | Place of exposured | Contributory factorh | Nature of evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | Staphylococcus aureus | BE | 14 | 0 | 0 | Frozen beans | Probably | School or kindergartene | Storage t/T abuse | Laboratory detection in implicated food |
| 2015 | Clostridium perfringens | DE | 3 | 0 | 0 | Frozen onions | Likely not | Canteen or workplace cateringf | Inadequate chilling | Detection of causative agent in food vehicle or its component – Symptoms and onset of illness pathognomonic to causative agent |
| 2018 | Listeria monocytogenes | DK | 4 | 4 | 0 | Frozen corn and other frozen vegetables | Yes | Multiple places of exposure in more than one countryg | Unprocessed contaminated ingredient | Detection of causative agent in food vehicle or its component – Detection of indistinguishable causative agent in humans; Detection of causative agent in food chain or its environment – Detection of indistinguishable causative agent in humans; Descriptive epidemiological evidence |
| 2018 | Listeria monocytogenes | FI | 30 | 30 | 3 | Frozen corn | Yes | Multiple places of exposure in more than one countryf | Inadequate heat treatment before consumption | Product‐tracing investigations; Descriptive environmental evidence; Detection of causative agent in food vehicle or its component – Detection of indistinguishable causative agent in humans; Descriptive epidemiological evidence |
| 2018 | Listeria monocytogenes | UK | 12 | 12 | 2 | Frozen sweetcorn | Yes | Multiple places of exposure in more than one countryf | Inadequate heat treatment before consumption | Detection of causative agent in food vehicle or its component – Detection of indistinguishable causative agent in humans; Descriptive epidemiological evidence |
FBOs: food‐borne outbreaks; t/T: time/Temperature.
BE: Belgium; DE: Germany; DK: Denmark; FI: Finland; UK: the United Kingdom.
Food (or foodstuff) that is suspected of causing human cases.
This is the location (‘setting’) where the food was consumed or where the final stages of preparation of the food vehicle took place.
This is also the place of origin of the problem.
The place of origin of the problem is not reported.
The place of origin of the problem is a processing plant.
Fault or circumstance that singly or in combination led to the FBO.
The Clostridium perfringens outbreak23 relating to frozen onions took place at a canteen or workplace catering facility in Germany in 2015 and caused three cases. It was reported that inadequate chilling was a contributory factor. Based on Appendix A, the onions may have been blanched before the freezing process. The draft PROFEL guidelines (PROFEL, 2019) state, however, that ‘Some products cannot be blanched because of detrimental effects on the product quality (e.g. onions or leafy herbs as basil)’. Therefore, it is likely these onions were not blanched.
Given the minimum temperature for toxin production for S. aureus (> 12°C) and for growth of Cl. perfringens (> 10°C) (ICMSF, 1996), it was assumed that these outbreaks were caused by keeping the vegetables above the reasonable foreseeable refrigeration temperatures applied by consumers to store frozen vegetables after removing from the freezer (i.e. 9°C based on the draft PROFEL guidelines). Data reported in the zoonoses database only indicates that the contributory factor was storage t/T abuse in the case of S. aureus and inadequate chilling in the case of Cl. perfringens. Therefore, these hazards were not considered relevant for the present assessment.
In 2018, the multi‐country outbreak of L. monocytogenes ST6 was linked to frozen vegetables (EFSA and ECDC, 2018), but apart from this outbreak, no other outbreaks with L. monocytogenes in frozen vegetables have been reported in the EU/EEA. As stated in the background, that outbreak caused 53 cases and 10 deaths over the period 2015–2018. The processing of these vegetables included a blanching step before freezing. In 2018, three countries (Denmark, Finland and UK) reported cases linked to this outbreak with in total 46 cases of which all hospitalised and five died.
In the US, only one outbreak implicating frozen vegetables was reported during 2009–2019. This outbreak was associated with L. monocytogenes during 2013–2016 causing nine cases and three deaths.24 The contamination source was the FPE and the vegetables were probably blanched.
3.1.2. Evidence from RASFF notifications
A total of 14 notifications were reported in the RASFF database related to pathogenic microorganisms in frozen vegetables since the start of the reporting system. These are summarised in Table D.2 in Appendix D.
Table TableD.2.
Number of Rapid Alert System for Food and Feed (RASFF) notifications in the EU with frozen vegetables (2006–2018)
| Year | Hazard | Product | Blancheda | Notification type | Subject of notification (country notifying)b |
|---|---|---|---|---|---|
| 2016 | L. monocytogenes | Vegetable mix | Maybe | Alert | FBO suspected (Listeria monocytogenes) to be caused by frozen organic vegetable mix from the United States (UK)(b) |
| 2018 | L. monocytogenes | Corn and mixes | Yes | Alert | Consumer recall of frozen vegetables from BE in relation to a multi‐country FBO (DE) |
| 2018 | L. monocytogenes | Corn | Yes | Alert | Consumer recall of frozen corn from HU in relation to a multi‐country FBO (DK) |
| 2018 | L. monocytogenes | Spinach | Yes | Alert | Consumer recall of frozen spinach from HU in relation to a multi‐country FBO (HR) |
| 2018 | L. monocytogenes | Corn | Yes | Alert | Consumer recall of frozen corn from FR in relation to a multi‐country FBO (NL) |
| 2018 | L. monocytogenes | Corn | Yes | Alert | Consumer recall of frozen corn from HU in relation to a multi‐country FBO (NL) |
| 2018 | L. monocytogenes | Corn | Yes | Alert | Consumer recall of frozen vegetables from UK in relation to a multi‐country FBO (UK) |
| 2007 | Salmonella Lexington | Lime leaves | Unknown | Information | Salmonella Lexington (presence) in frozen lime leaves from Thailand (FI) |
| 2010 | Salmonella Montevideo | Tomatoes | Maybe | Alert | Salmonella Montevideo (presence /25 g) in frozen tomatoes from ES (FR) |
| 2011 | Salmonella Aberdeen | Vegetable mix | Maybe | Information for follow‐up | Salmonella Aberdeen (presence /25 g) in frozen vegetable mix from SE (FR) |
| 2013 | Salmonella enterica | Tomatoes | Maybe | Alert | Salmonella enterica (presence /25 g) in frozen diced tomatoes from ES (FR) |
| 2015 | Salmonella Stanley | Okra | Yes | Border rejection | Salmonella Stanley and unauthorised substance acephate (0.035 mg/kg ‐ ppm) in frozen okra from Vietnam (FI) |
| 2015 | Salmonella | Tomatoes | Maybe | Alert | Salmonella (presence /25 g) in frozen cube tomatoes from ES (FR) |
FBO: food‐borne outbreak.
Based on Appendix A.
Is linked to the vegetable outbreak in the US (link included: http://www.cdc.gov/listeria/outbreaks/frozen-vegetables-05-16/index.html).
BE: Belgium; DE: Germany; DK: Denmark; ES: Spain; FI: Finland; FR: France; HR: Croatia; HU: Hungary; NL: the Netherlands; SE: Sweden; UK: the United Kingdom.
The six notifications of L. monocytogenes in frozen corn, spinach or mixes in 2018 were in relation to the multi‐country outbreak described above. The other notification of L. monocytogenes in frozen vegetable mix was linked to a vegetable‐associated outbreak in the US, also described above.25
The six notifications involving Salmonella included three alerts for its presence in frozen tomatoes and one information for follow‐up in a frozen vegetable mix. The other notifications were a border rejection for Salmonella in okra and an information report of Salmonella in lime leaves. None of these notifications were associated with a reported FBO.
3.1.3. Concluding remarks
The FBOs in the EU/EEA where frozen vegetables were implicated as reported in EFSA's zoonoses database (2005–2018) were reviewed.
In addition to the L. monocytogenes outbreak linked to frozen vegetables in 2018, which affected 46 people and caused 10 deaths, two outbreaks were reported involving frozen vegetables. The S. aureus outbreak linked to frozen beans, which were likely to have been blanched, caused 14 cases. The Cl. perfringens outbreak related to frozen onions, which were probably not blanched before the freezing process, caused three cases. Data reported in the zoonoses database only indicates that the contributory factor was storage time/temperature abuse in the case of S. aureus and inadequate chilling in the case of Cl. perfringens.
Based on the evidence from FBOs, particularly the number of human cases involved in these FBOs and the main identified contributory factors, S. aureus and Cl. perfringens in bfV are not considered relevant for public health/illness.
Therefore, only the risk posed by the combination of L. monocytogenes in bfV will be compared with better‐known risks such as the risks of L. monocytogenes from other food.
3.2. Relative public health risk posed by L. monocytogenes in bfV
3.2.1. Occurrence of L. monocytogenes in bfV
The retrieved studies on the prevalence and enumeration of L. monocytogenes in bfV are summarised in Table 3. In some literature studies, sampling took place in one processing plant only. These studies illustrate that the occurrence of positive samples can vary considerably between processing plants; from 5.5% (Skowron et al., 2019) to 46.8% (Pappelbaum et al., 2008).
Table 3.
Overview of the available studies related to the prevalence and concentration of Listeria monocytogenes in blancheda frozen vegetables, with studies in bold type included in the prevalence estimationb
| Reference | Product sampled | Available information on sampling and analytical methodologies | Year of sampling | No of samples tested | No of positive samples | Prevalence (%) | Concentration |
|---|---|---|---|---|---|---|---|
| Literature data | |||||||
| Aguado et al. (2004) | Frozen vegetables (artichoke, green beans, broccoli, carrot, cauliflower, peas, spinach, tomato) | Samples were obtained as final product from a processing plant in ES | 1997–2000 | 906 | 11 | 1.2 | NR |
| Lee et al. (2007b) | Frozen vegetables (Brussels sprouts, onions, peas, peppers, tomatoes) | Samples were taken to reveal the incidence of L. monocytogenes in vegetables processed in Bursa (TR) | NR < 2007 | 44 | 3 | 6.8 | NR |
| Lee et al. (2007a) | Frozen peppers | Samples were taken during four separate visits at monthly intervals over a processing season in a factory (Bursa, TR) that processes and exports frozen strip and cube pepper | NR < 2007 | 12 | 0 | 0.0 | NR |
| Majczyna and Bialasiewicz (2006) | Frozen vegetables | Not provided | 2001–2005 | 62 | 8 | 12.9 | NR |
| Mena et al. (2004) | Frozen vegetables (aubergine, broccoli, courgette, peas, green and red peppers) | Commercial food products were obtained from producers and retailers in PT | 2000–2001 | 271 | 35 | 12.9 | NR |
| Moravkova et al. ( 2017 ) | Frozen vegetables (e.g., peas, carrot, maize; mixed, packed or not packed) | Samples from 32 manufacturers originating from 10 MS were collected in nine supermarkets in CZ | 2014 | 43 | 9 | 20.9 | < 50 CFU/g |
| Moreno et al. ( 2012 ) | Frozen vegetables (spinach, broccoli, stew, stir‐fry) | Samples were collected in supermarkets in Valencia (ES) | NR < 2012 | 33 | 4 | 12.1 | < 100 CFU/g |
| Pappelbaum et al. (2008) | Frozen vegetables (cauliflower, mushrooms, mixed vegetables, broccoli, string bean, green pea, onion, carrot, zucchini, spinach, tomatoes, celery, parsley, paprika, Brussels sprouts) | Samples were collected once a week from a Polish produce processor | 2001–2005 | 1,691 | 791 | 46.8 | NR |
| Skowron et al. (2019) | Frozen vegetable mix (broccoli, carrot, corn, green beans, green pea, pepper, red beans, onion, red and potato) | Samples produced in a freezing plant in PO were yearly tested | 2003–2007 | 9,100 | 504 | 5.5 | NR |
| Vitas et al. (2004) | Frozen vegetables (potatoes, carrot, spinach, broccoli, string‐beans, peas, artichoke, cauliflower) | Samples were obtained in retail outlets of Navarra (ES) | 1997–1999 | 1,750 | 31 | 1.8 | NR |
| Vojkovska et al. ( 2017 ) | Frozen vegetables (broccoli, Brussels sprout, carrot, corn, green beans, green peas, mix, spinach) | Samples were randomly collected from different supermarkets, local stores and green markets in nine cities of CZ | 2014 | 66 | 0 | 0.0 | NR |
| Survey data | |||||||
| Personal communication from Jim McLauchlin from Public Health England (McLauchlin, 2019 ) and poster (Willis et al., 2019 ) | Frozen vegetables (e.g. vegetable mix, peas, sweetcorn, beans, carrot, spinach, peppers, broccoli, sprouts, okra, cauliflower, cabbage, mushrooms, cassava, bitter gourd, onions, squash, asparagus, fenugreek, swede, artichoke, kale, molokhia, yam) |
Sampling focused on catering and retail premises and included any frozen fruit or vegetables Samples (25 g each) were tested for the presence of Listeria species using ISO 11290‐1:2017.6 Identification of Listeria isolates was performed as outlined in the standard methods above |
2019 | 631 | 71 | 11.3 | 2 samples with 20–100 CFU/g, rest < 20 CFU/g |
| Personal communication from Paul Ellis from Public Health Wales (Ellis, 2019 ) | Frozen fruit and vegetables (e.g. sweetcorn, mixed vegetables; broccoli, mushrooms; cauliflower, mixed peppers; carrot, cauliflower, green beans mix, spinach, carrot, peas, sweetcorn mix, butternut squash, melon, peas, cherries and berry smoothie mix) | Enrichment method using 25 g. Direct enumeration was performed in which Listeria species (not monocytogenes) were found in two samples. Sweet corn having 10 CFU/g and chopped onions having 10 CFU/g | 2019 | 265 | 27 | 10.2 | |
| Monitoring data | |||||||
| EU monitoring data (AT, BG, DE, ES, HU) | Pre‐cut frozen vegetables | Monitoring and surveillance at retail level through official sampling using objective sampling (single samples) | 2015–2018 | 118 | 15 | 12.7 | |
| EU monitoring data (BG, CY, FR) | Pre‐cut frozen vegetables | Surveillance and survey at retail level through official sampling using suspect or selective sampling (single or batch samples)c | 2017–2018 | 51 | 11 | 21.6 | |
| EU monitoring data (DE, ES) | Pre‐cut frozen vegetables | Surveillance and survey at packing centre and processing level through official sampling using objective sampling (single samples) | 2015, 2018 | 9 | 3 | 33.3 | |
| EU monitoring data (BG) | Pre‐cut frozen vegetables | Surveillance and monitoring at processing plant through HACCP and own check using objective and census sampling (single and batch samples) | 2015–2017 | 23 | 0 | 0 | |
| Data received through MRA network request | |||||||
| CZ | Frozen vegetables (e.g. spinach, mixed vegetable such as carrot, corn, peas, beans) | Samples were collected from retail; majority were of Czech origin | 2015–2018 | ˜ 100 d | 18 | 18 | < 100 CFU/g |
| CYf | Frozen vegetables (e.g. sweetcorn, mixed vegetables) | Samples were collected from the market (non‐official single samples) | 20 | 6 | 30 | < 10 CFU/g | |
| CYf | Frozen vegetables (e.g. sweetcorn) | Samples were collected from the market (official samples in batches of 5) | 20 | 4 | 20 | < 10 CFU/g | |
| LT | Frozen vegetables (e.g. beans, broccoli, corn, peas, pumpkin cubes, vegetable mix) | Samples were collected at retail (28 samples) and producer (two samples) level in relation to Listeria outbreak from frozen corn in the EU; 24 products were produced in POc | 2018 | 30 | 24 | 80f | < 10 CFU/g or < 40 CFU/ge |
| DEf | Frozen vegetables (broccoli, Brussels sprouts, carrots, cauliflower, mixed vegetables, onions, peas and carrots, spinach, yellow boletus) | Mainly 2018, few 2011 and 2016 | 68 | 12 | 17.6 | ||
MRA: microbiological risk assessment; NR: not reported; RTE: ready‐to‐eat.
Countries: AT: Austria; BG: Bulgaria; CY: Cyprus; CZ: Czechia; DE: Germany; FR: France; HU: Hungary; LT: Lithuania; ES: Spain; PO: Poland; PT: Portugal; TR: Turkey.
Some samples collected in the studies were excluded from the table when these were either not considered vegetables (e.g. parsley in the study by Moreno et al. (2012) or are not blanched according to Appendix A (e.g. leek in the study by Pappelbaum et al. (2008)).
Some studies were excluded to estimate the prevalence because they were only sampled at one processing plant and/or sampling took place before 2010.
These data were excluded to estimate the prevalence because there was evidence that the samples were taken using suspect or selective sampling.
Considered as 100 in the calculation of the prevalence. 17 isolates were of serotype 1/2a and one serotype 1/2b. Data from WGS are available, none of them matched with those of human origin (in 1‐year period).
Each sample consists of five number of units (as ‘n’ in Regulation (EC) 2073/20051). For all the 5 units, L. monocytogenes detection analysis was performed. When Listeria was detected, additionally L. monocytogenes enumeration analysis was done. A sample was considered positive when at least one unit was found positive by detection. In all but two samples, all enumeration results were below the detection limit of 10 CFU/g. In two samples, one unit was < 40 CFU/g.
These data were excluded to estimate the prevalence because they were already retrieved in the monitoring data.
The prevalence estimate was derived from the following data. Three studies from the literature data considered random sampling that took place after 2010; these yielded a prevalence of 9.2% (13 positive samples out of 142). The surveys carried out in the UK and Wales resulted in prevalence estimates of 11.3% and 10.2%, respectively. The data retrieved through the monitoring and MRA request resulted in a prevalence estimate of 14.4% (36 positive samples out of 250). The combined estimated prevalence of L. monocytogenes in bfV was 11.4% (147 of 1,288 samples).
There were very few studies reporting enumeration of L. monocytogenes in frozen vegetables and in most of the cases, the numbers were below the limit of enumeration of the plate count procedure applied in each of the studies (e.g. < 10, < 20, < 50 and < 100 CFU/g) (Table D.3 in Appendix D).
Table TableD.3.
Concentration data extracted from the multi‐country outbreak report (EFSA and ECDC, 2018) used in developing the distribution of the concentration of L. monocytogenes in blanched frozen vegetables
| Country | Batches/samples | Concentration (CFU/g) | Product sampled |
|---|---|---|---|
| Poland | 1 batch/3 samples | < 10 | Frozen corn batch G |
| 1/1 | 10 | Frozen corn used for batch G | |
| 1/1 | < 10 | Frozen corn used for batch G | |
| 3/3 | < 150 | Frozen corn used for batches A, B, C | |
| 1/1 | < 70 | Frozen corn used for batch D | |
| Hungary | 10/10 | < 10 | Frozen corn batches X1, X2, X12, X15, X16, X21, X23, X25, X26, X27 |
| 1/1 | 1.4 × 103 | Frozen corn batch X28 | |
| 3/3 | 60 | Frozen classical vegetable mix (pea, baby carrot, corn) batches H, I, L | |
| 1/1 | 50 | Frozen classical vegetable mix batch M | |
| 1/1 | 30 | Frozen classical vegetable mix batch N | |
| 1/1 | < 30 | Frozen spinach puree batch W | |
| 1/1 | < 10 | Frozen spinach puree batch Y | |
| Belgium | 1/2 (sample 1) | < 10 | Frozen corn batch Z1 |
| 1/2 (sample 2) | 80 | Frozen corn batch Z2 | |
| 1/4 (samples 1–4) | < 100 | Frozen corn used for batches Q, R, S | |
| France | 1/1 | < 10 | Frozen corn batch F |
| Austria | 1/1 | < 10 | Frozen Mexican vegetable mix (corn, red kidney beans, onion, red and yellow paprika, spices, etc) batch S |
3.2.2. Public health impact based on food‐borne outbreak data
A summary of the ‘strong‐evidence’ FBO caused by L. monocytogenes (2008–2018) at EU/EEA level can be found in Table 4. A total of 53 strong evidence FBO were reported with 679 human cases, 283 hospitalisations and 54 deaths. The ‘dairy’ food category was responsible for five of these outbreaks causing 47 cases, while ‘fish and seafood’ and ‘meat and meat products’ food categories were responsible for 9 and 16 of these outbreaks causing 63 and 190 cases, respectively. Vegetables and juices and other products thereof caused 8 outbreaks and 87 cases. Reports from three countries (Denmark, Finland, and UK) in 2018 were linked to the multi‐country frozen vegetable outbreak with in total 46 cases of which all were hospitalised and five died (see Section 3.1.1; (EFSA and ECDC, 2018)).
Table 4.
Summary of reported strong evidence food‐borne outbreaks (FBOs) caused by Listeria monocytogenes in the EU/EEA as reported in EFSA's zoonoses database (2008–2018)
| Food vehicle | No of FBOs | No of cases | No of hospitalised cases | No of deaths | No of reporting countries | Distribution of FBOs per country (year of FBOs)n |
|---|---|---|---|---|---|---|
| Dairy products | 5 | 47 | 42 | 11 | ||
| Cheesea | 5 | 47 | 42 | 11 | 4 | AT (2009), BE (2011, 2013), DE (2009), SE (2017) |
| Fish and seafood products | 9 | 63 | 22 | 4 | ||
| Crustaceans, shellfish, molluscs and products thereofb | 3 | 10 | 8 | 2 | 2 | FR (2013), UK (2013, 2013) |
| Fish and fish productsc | 6 | 53 | 14 | 2 | 3 | DE (2010), DK (2010, 2014, 2017), NO (2013, 2018) |
| Meat and meat products | 16 | 190 | 119 | 20 | ||
| Bovine meat and products thereofd | 2 | 12 | 12 | 2 | 3 | DK (2009), UK (2012), RO (2018) |
| Meat and meat productse | 4 | 58 | 20 | 2 | 4 | AT (2017), CH (2016), DE (2016), SE (2013) |
| Other or mixed red meat and products thereoff | 4 | 41 | 30 | 5 | 4 | DK (2016), FI (2012), SE (2014), UK (2010) |
| Pig meat and products thereofg | 6 | 79 | 57 | 11 | 6 | AT (2008), BE (2013), CH (2011), CZ (2009), DE (2018), IT (2015) |
| Other | 22 | 375 | 96 | 19 | ||
| Vegetables and juices and other products thereofh | 8 | 87 | 53 | 11 | 6 | AT (2017), CH (2014, 2017), DE (2013), DK (2018), ES (2015), FI (2018), UK (2018) |
| Bakery productsi | 2 | 16 | 16 | 1 | 2 | FI (2011), UK (2012) |
| Cereal products including rice and seeds/pulses (nuts, almonds)j | 1 | 2 | 0 | 0 | 1 | SE (2018) |
| Buffet mealsk | 3 | 47 | 5 | 0 | 3 | FI (2015), RO (2018), UK (2014) |
| Mixed foodsl | 7 | 193 | 21 | 6 | 4 | DE (2014, 2015), PT (2015), SE (2015, 2018), UK (2011, 2012) |
| Other foodsm | 2 | 34 | 5 | 1 | 2 | AT (2018), UK (2010) |
| All | 53 | 679 | 283 | 54 |
Acid curd cheese; more information about food vehicle not reported; more information about food vehicle not reported; cheese (acid curd) made from pasteurised milk; washed rind cheeses.
More information about food vehicle not reported; crab meat; crab meat.
Herring casserole in vegetable oil; gravad salmon; smoked salmon; cold smoked salmon; half‐fermented trout; more information about food vehicle not reported.
Beef stew (sous vide); pressed beef also called potted beef and beef stew.
More information about food vehicle not reported; meat pâté; cured pork belly with juniper or similar products; more information about food vehicle not reported.
Different cold cuts; meat jelly; sausage; tongue, beef, pork, ham, chicken, turkey.
Sliced jelly pork; more information about food vehicle not reported; more information about food vehicle not reported; more information about food vehicle not reported; blood sausage; more information about food vehicle not reported.
More information about food vehicle not reported; pre‐cut salad; leaf lettuce; mixed salad; the food sources identified for this international outbreak were frozen corn and other frozen vegetables from a distinct production site in Hungary. The actual source of illness in Denmark was not found, however products from the Hungarian production site was traded in Denmark; more information about food vehicle not reported (this FBO was reported as ‘weak evidence’ but has been considered as ‘strong evidence’ for this opinion; frozen corn; frozen sweetcorn.
Sponge cake; pork pies.
More information about food vehicle not reported.
More information about food vehicle not reported; food dishes (fresh chicken meat, pressed ham, meat chicken products ready to eat, cheeses made from cows’ milk); sandwiches.
Iceberg lettuce with yoghurt dressing; rice pudding; more information about food vehicle not reported; likely dill which then contaminated crustaceans and cheese; more information about food vehicle not reported; sandwiches various and prepared salad dishes; sandwiches.
More information about food vehicle not reported; salmon and cress sandwiches, egg mayonnaise sandwiches.
AT: Austria; BE: Belgium; CH: Switzerland; CZ: Czechia; DE: Germany; DK: Denmark; ES: Spain; FI: Finland; FR: France; IT: Italy; NO: Norway; PT: Portugal; RO: Romania; SE: Sweden; UK: the United Kingdom. Data from Spain before 2015 have not been included in this table because it was provided outside the EFSA zoonoses database and in a different format of aggregation.
3.2.3. Public health impact using the mgQMRA model
The individual public health risk (probability of illness per serving) posed by L. monocytogenes in two bfV subcategories (i.e. uncooked and cooked after thawing) was compared with the 13 RTE food subcategories for elderly males and females using the mgQMRA model. Blanched frozen vegetables were separated into two food subcategories to represent different end‐points in the spectrum of consumer behaviour in relation to the mode of use/consumption to encompass the minimum to maximum risk associated with bfV.
To evaluate the potential public health impact of bfV, the predicted number of cases for elderly females was estimated as an example based on scenarios for the annual number of servings and the proportions of servings consumed with or without cooking. The number of cases in the scenario analysis was compared with the predicted number of cases associated with the 13 RTE food subcategories.
3.2.3.1. Input data developed for the mgQMRA model
The distribution of the mean prevalence of L. monocytogenes in bfV is shown in Figure 3a. The mean value was 0.114. Data from nine studies in Table 3 representing a range of years, geographic areas and products were used. Uncertainty was considered using the mean, 2.5th and 97.5th percentiles in the baseline, best‐ and worst‐case uncertainty scenarios, respectively (Table 2).
Figure 3.
Distributions related to the occurrence of L. monocytogenes in blanched frozen vegetables
- (a) Uncertainty of the prevalence based on nine studies in Table 3. The dotted lines indicate the 2.5th and 97.5th percentiles used in the uncertainty scenarios; (b) Cumulative density plot (CDF = cumulative density frequency) of the beta‐general distribution fitted to the censored concentration data in Table 3 (surveys/studies data) or the same data complemented with data from the multi‐country outbreak report (EFSA and ECDC, 2018) (surveys/studies data + outbreak), compared to the fitted concentration distribution for soft and semi‐soft cheese developed in EFSA BIOHAZ Panel (2018).
Two distributions describing the concentration were developed, one based on surveys/studies from the literature and the other supplementing those with data from the multi‐country outbreak report (EFSA and ECDC, 2018). As seen in Figure 3b, both distributions are quite similar and concentrations are lower than those estimated for soft and semi‐soft cheese in EFSA BIOHAZ Panel (2018) being the subcategory distribution located furthest to the left on the log10 concentration axis of the RTE subcategories. It was decided to use the distribution based on the combined data (surveys/studies + FBOs) to make use of the scarce available data, although this may potentially represent an overestimation. The 95% CI (2.5th and 97.5th percentiles) for the parameters was used in the evaluation of uncertainty (Table 2).
The consumer storage time of uncooked and cooked bfV outside the freezer is presented in Figure 4. This time represents the delay between vegetables being thawed and consumed (uncooked subcategory) or between being thawed and cooked (cooked subcategory).
Figure 4.
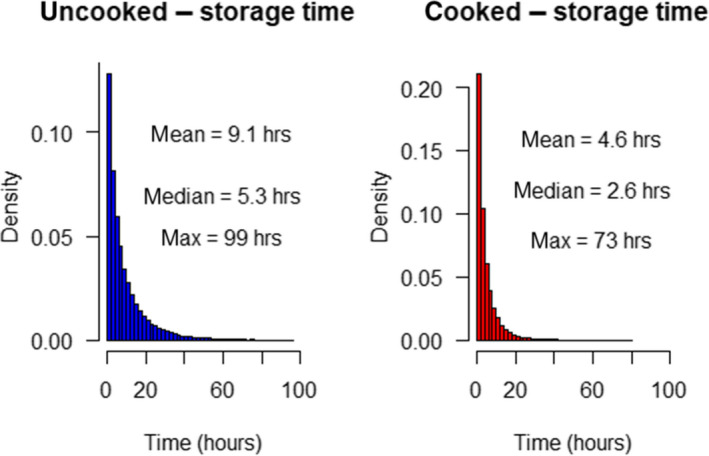
Density plots of the consumer storage time outside the freezer of uncooked and cooked blanched frozen vegetables considering the remaining shelf‐life and proportion being used (description in Table 2)
Figure 5 shows the distribution of the exponential growth rate at 5°C (EGR 5°C, log10/h) using data collected from scientific literature and Combase records reporting L. monocytogenes growth at different temperatures in heat‐treated (blanched) vegetables, which has been converted to EGR 5°C using the square‐root‐based secondary model for bacterial growth. The intensity of the heat treatment ranged from 50°C for 60 s up to 90°C for 10 min. The mean, standard deviation and truncated maximum (mean + 2 standard deviations) of the growth rate was used for the mgQMRA (Table 2). The mean value was estimated to be 0.0117 log10/h, meaning that, on average, after 10 h at 5°C the population increases by 0.117 log10 units
Figure 5.
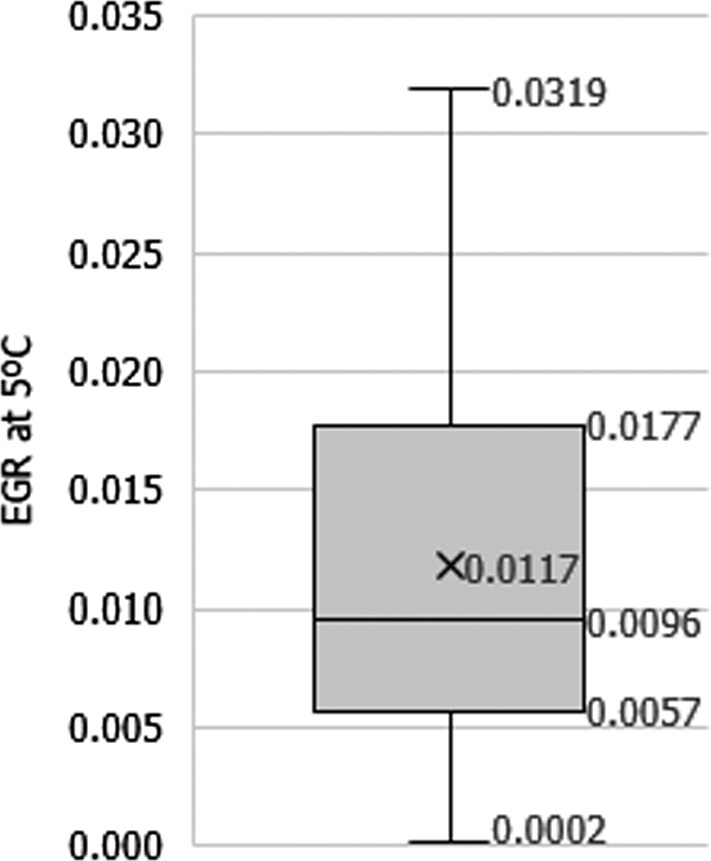
Boxplot showing the distribution of the exponential growth rate at 5°C (EGR 5°C) (log10/h) of L. monocytogenes in heat‐treated (blanched) vegetables including corn, green peas, carrots, broccoli, beans and asparagus stored in air
- Data extracted from the literature and ComBase were transformed to EGR 5°C, using Tmin = −1.18°C (FDA and FSIS, 2003).
The estimated total number of servings of bfV consumed by elderly females and males are shown in Table 5. Estimations are provided for three groups (A–C) based on different vegetables.
Table 5.
The estimated total number of blanched frozen vegetables (bfV) servings yearly consumed by females and males, 65–74 years of age in the EU
| Gender | Population size | Group Aa | Group Bb | Group Cc |
|---|---|---|---|---|
| Female | 2.61 × 107 | 1.63 × 109 | 3.15 × 109 | 2.65 × 109 |
| Male | 2.29 × 107 | 1.38 × 109 | 2.74 × 109 | 2.28 × 109 |
| Total | 4.89 × 10 7 | 3.02 × 10 9 | 5.88 × 10 9 | 4.93 × 10 9 |
Group A is based on vegetables that are always blanched and this estimate has been used a best‐case scenario.
Group B is based on vegetables that are always blanched or sometimes blanched (i.e. also including potatoes) and this estimate has been used a worst‐case scenario.
Group C is the same as group B but without potatoes and this estimate has been used as baseline scenario.
3.2.3.2. Ranking based on probability of illness per serving (individual risk)
The ranking of the mean probability of illness per serving as estimated for elderly females and males in the EU is shown in Table 6. The mean probability of illness per serving was slightly but consistently higher for elderly males compared to elderly females both for bfV consumed uncooked or cooked in the different scenarios. This reflects the higher r‐value in the DR model since all other input parameters were the same. The sensitivity analysis of the variable inputs indicate the importance of the susceptibility of the consumer and the virulence of the L. monocytogenes strains as reflected in iterations with high r‐values, low log10 reductions during cooking and to a lesser degree a high initial concentration on the predicted mean probability of illness per serving (Appendix C). This probability is largely determined by the upper percentile ranges of these parameters, i.e. by iterations when conditions with sensitive consumers (high r‐vales), low log10 reduction, and high initial concentrations are at hand. In these iterations, the other variable parameters, related to storage and growth, affect the final predicted magnitude of risk to a similar extent, as reflected in their effect on the mean probability per serving.
Table 6.
Summary and ranking of the estimated mean probability of illness per serving blanched frozen vegetables (bfV) compared to 13 RTE food subcategories for elderly females and males (65–74 years old) in the EU using the mgQMRA model and the baseline, best‐case and worst‐case uncertainty scenarios for bfV (see input parameters in Table 2)
| Females | Males | ||||||
|---|---|---|---|---|---|---|---|
| Rank | Food subcategory | Mean probability of illness per serving | Number of cases per 1012 servings | Rank | Food subcategory | Mean probability of illness per serving | Number of cases per 1012 servings |
| 1 | Gravad fish, NAP | 6.1 × 10−7 | 610,000 | 1 | Gravad fish, NAP | 7.5 × 10−7 | 750,000 |
| 2 | Hot‐smoked fish, ROP | 7.2 × 10−8 | 72,000 | 2 | Hot‐smoked fish, ROP | 9.6 × 10−8 | 96,000 |
| 3–4 | Cold‐smoked fish, ROP | 6.4 × 10−8 | 64,000 | 3–4 | Cold‐smoked fish, ROP | 8.4 × 10−8 | 84,000 |
| 3–4 | Hot‐smoked fish, NAP | 6.0 × 10−8 | 60,000 | 3–4 | Hot‐smoked fish, NAP | 8.0 × 10−8 | 80,000 |
| 5 | Paté, NAP | 3.9 × 10−8 | 39,000 | 5 | Paté, NAP | 4.8 × 10−8 | 48,000 |
| 6–8 | Paté, ROP | 2.7 × 10−8 | 27,000 | 6–8 | Cold‐smoked fish, NAP | 3.6 × 10−8 | 36,000 |
| 6–8 | Cold‐smoked fish, NAP | 2.6 × 10−8 | 26,000 | 6–8 | Paté, ROP | 3.2 × 10−8 | 32,000 |
| 6–8 | Gravad fish, ROP | 2.3 × 10−8 | 23,000 | 6–8 | Gravad fish, ROP | 2.9 × 10−8 | 29,000 |
| 9–12 | Cooked meat, ROP | 1.1 × 10−8 | 11,000 | 9–12 | Cooked meat, NAP | 1.5 × 10−8 | 15,000 |
| 9–12 | Cooked meat, NAP | 1.1 × 10−8 | 11,000 | 9–12 | Cooked meat, ROP | 1.5 × 10−8 | 15,000 |
| 9–12 | Sausage, NAP | 1.0 × 10−8 | 10,000 | 9–12 | Sausage, NAP | 1.3 × 10−8 | 13,000 |
| 9–12 | Sausage, ROP | 9.7 × 10−9 | 9,700 | 9–12 | Sausage, ROP | 1.3 × 10−8 | 13,000 |
| 13 | bfV, uncooked (worst‐case) | 2.6 × 10−9 | 2,600 | 13 | bfV, uncooked (worst‐case) | 3.8 × 10−9 | 3,800 |
| 14 | Soft and semi‐soft cheese | 2.1 × 10−9 | 2,100 | 14 | Soft and semi‐soft cheese | 2.6 × 10−9 | 2,600 |
| 15 | bfV, uncooked (baseline) | 4.0 × 10−10 | 400 | 15 | bfV, uncooked (baseline) | 1.9 × 10−9 | 1,900 |
| 16 | bfV, uncooked (best case) | 2.8 × 10−10 | 280 | 16 | bfV, uncooked (best case) | 4.0 × 10−10 | 400 |
| 17 | bfV, cooked (worst case) | 1.0 × 10−11 | 10 | 17 | bfV, cooked (worst‐case) | 1.5 × 10−11 | 15 |
| 18 | bfV, cooked (baseline) | 2.3 × 10−13 | 0.23 | 18 | bfV, cooked (baseline) | 5.3 × 10−13 | 0.53 |
| 19 | bfV, cooked (best case) | 7.9 × 10−14 | 0.079 | 19 | bfV, cooked (best case) | 1.2 × 10−13 | 0.12 |
bfV: Blanched frozen vegetables; NAP: normal atmosphere packaging; ROP: reduced oxygen packaging.
Considering the 13 RTE food subcategories and the three uncertainty scenarios for the two bfV subcategories, the mean probability of illness per serving ranged from 1 in every 12.7 trillion servings to 1 in every 1.6 million servings for elderly females and from 1 in every 8.3 trillion servings to 1 in every 1.3 million servings for elderly males (depending on the food sub‐category). The order of the food subcategories between genders based on risk per serving is similar but not identical. This is due to differences in consumption, i.e. serving sizes, but also the effect of randomness between simulations. To acknowledge the latter, no distinction is made in rank between RTE foods with similar predicted risk (Table 6).
The important observation is that all uncertainty scenarios for cooked bfV are associated with a lower predicted mean probability of illness per serving, a factor of 250–3,600 between uncertainty scenarios, than those for uncooked. Further, all bfV scenarios except for the uncooked worst‐case scenario ranked below the RTE food subcategories. In this worst‐case scenario, the bfV ranked just above soft and semi‐soft cheese, which is the lowest ranked of the RTE food subcategories.
3.2.3.3. Ranking based on predicted number of cases (population risk)
Since the focus of the assessment is on comparing the listeriosis risk associated with bfV with other known foods and the rank order was similar between genders, the public health impact in terms of the annual number of cases is illustrated using only the female group as an example. Additional information is needed about the proportion of bfV consumed with or without cooking and, unfortunately, there is very little information about this.
The impact of different assumed proportions of consumption of bfV either cooked or uncooked on the predicted number of cases is illustrated for the baseline and worst‐case uncertainty scenarios using different estimates of the total number of servings (Figure 6). In the baseline estimate, i.e. considering the baseline estimates for the total number of servings (i.e. 2.65 × 109) and mgQMRA uncertainty scenario (with a probability of illness per serving of 4.0 × 10−10), less than one annual case for the elderly female group is predicted, except if all servings are consumed uncooked (Figure 6a). With the same total number of servings, and in the mgQMRA worst‐case uncertainty scenario (with a probability of illness per serving of 2.6 × 10−9), there are up to seven annual cases (Figure 6b, considering 100% uncooked). With a total consumption of 3.15 × 109 servings, likely (66–90%) to be an overestimation, up to eight annual cases are predicted (Figure 6c, considering 100% uncooked), and, basically all cases are due to consumption of uncooked bfV.
Figure 6.
Illustration of the effect of the proportion of total servings of blanched frozen vegetables consumed with or without cooking on the number of annual cases in the elderly female (65–74 year age) group of the EU population considering following estimates of total consumption and uncertainty scenarios
- (a) baseline estimate of total number of servings (i.e. 2.65 × 109) and baseline mgQMRA uncertainty scenario (with a probability of illness per serving of 4.0 × 10−10), (b) baseline estimate of total number of servings (i.e. 2.65 × 109) and worst‐case mgQMRA uncertainty scenario (with a probability of illness per serving of 2.6 × 10−9), (c) worst‐case estimate of total number of servings (i.e. 3.15 × 109) and worst‐case mgQMRA uncertainty scenario (with a probability of illness per serving of 2.6 × 10−9).
Information from Willis et al. (2019) on the intended handling practices of frozen vegetables was used. This information was gathered through a survey to assess the microbiological quality of frozen fruit and vegetables with respect to the presence of L. monocytogenes. Survey results are included in Table 3. Samples were collected at catering businesses and from retail sale in England. Data were collected for 673 packages of frozen vegetables. The first estimate assumes that only 4% of servings are consumed uncooked (i.e. the proportion of vegetables with a label stating ‘RTE’ or indicating to consume directly without cooking). The second estimate assumes that 23% could be consumed uncooked (i.e. 4% vegetables with a label stating ‘RTE’, 12% with no information on the intended use and 7% with a label that could not clearly inform on the intended use). The predicted number of cases based on these two proportions and the mgQMRA worst‐case or baseline uncertainty scenario for probability of illness per serving, and the baseline estimate of total number of servings (2.65 × 109 servings per year) were ranked based on a comparison with the number of cases predicted for the seven RTE food categories.
Irrespective of the proportion of consumption of uncooked bfV, the predicted total number of cases in the female elderly group is lower than that of the seven RTE food categories (Table 7). The yearly number of cases for bfV ranged from 0.04 (baseline estimate of probability of illness per serving) to 1.6 (worst‐case estimate of probability of illness per serving).
Table 7.
Ranking of the predicted number of listeriosis cases per year in the female 65–74 year age group in the EU by consumption of blanched frozen vegetables (bfV) compared to seven RTE food categories
| Rank | Food category/subcategory | Predicted number of cases per year | ||||
|---|---|---|---|---|---|---|
| Total | Type of packaging | Mode of use/consumption | ||||
| ROP | NAP | Uncookeda | Cookedb | |||
| 1 | Cold‐smoked fish | 41.6 | 41 | 0.6 | NA | NA |
| 2 | Cooked meat | 37 | 32 | 5 | NA | NA |
| 3 | Gravad fish | 32 | 4 | 28 | NA | NA |
| 4 | Paté | 22 | 15 | 7 | NA | NA |
| 5 | Hot‐smoked fish | 20 | 15 | 5 | NA | NA |
| 6 | Sausage | 17.6 | 14 | 3.6 | NA | NA |
| 7 | Soft and semi‐soft cheese | 1.9 | NA | NA | NA | NA |
| 8 | bfV, 23% uncooked, probability of illness per serving for worst‐case scenarioc | 1.62 | NA | NA | 1.6 | 0.02 |
| 11e | bfV, 4% uncooked, probability of illness per serving for baseline scenariod | 0.0406 | NA | NA | 0.04 | 0.0006 |
Note: For the bfV category, a baseline or a worst‐case mgQMRA uncertainty scenario was assumed based on the different proportions of total number of servings being uncooked.
bfV: blanched frozen vegetables; NA: not applicable; NAP: normal atmosphere packaging; ROP: reduced oxygen packaging.
For vegetables consumed uncooked (i.e. as ready‐to‐eat, RTE).
For vegetables consumed cooked (i.e. as recommended by most of the producers).
Considering a baseline estimate of total number of servings (2.65 × 109 servings per year) and a worst‐case scenario of the probability of illness per serving of 2.6 × 10−9.
Considering a baseline estimate of total number of servings (2.65 × 109 servings per year) and a baseline scenario of the probability of illness per serving of 4.0 × 10−10.
Positions 9 and 10 of the ranking have not been included in this table for clarity.
3.2.3.4. Uncertainty related to mgQMRA model results
There are multiple sources of uncertainties affecting the data and methodology of the assessment. Some of these are general or related to the hazard and thus, affecting all food subcategories, whereas others are restricted to bfV. A list of sources of uncertainty is shown in Table C.1. Most of these, i.e. prevalence, initial concentration, log10 reduction, MPD, serving size, number of servings, proportion of servings being cooked were evaluated within the model by running uncertainty scenarios as described above, where best‐ and worst‐case parameter values were defined based on estimated uncertainties in the distributions used or assumptions based on other information (Appendix C). Of the sources of uncertainty not quantitatively evaluated within the modelling scenarios, the impact of the DR relation would be the same for all foods. For the additional sources of uncertainty, i.e. the description of storage temperature and time, and the assumption of no lag phase, this was the same approach as used for the RTE foods, and the qualitative uncertainty evaluation indicates that this may have contributed to some overestimation of risk. The remaining uncertainty sources were related to estimation of growth, and inactivation during freezing and thawing of bfV, and the qualitative evaluation indicates that this uncertainty may also have contributed to some overestimation of risk (Table C.1). Taken together, it is believed that the uncertainties not evaluated in the modelling scenarios would have contributed to an overestimation of risk.
Table TableC.1.
Potential sources of uncertainty identified in the mgQMRA model and a qualitative assessment of the impact that these uncertainties could have on the outcome, i.e. the probability of illness per serving of uncooked or cooked frozen vegetables and the number of cases due to blanched frozen vegetables (bfV) for elderly females and males (65–74 years old) in the EU
| Factor considered | Source of uncertainty/considerations | Impact on the factor (e.g. prevalence)a | Impact of the uncertainty on the probability of illness per serving of bfVb | Impact of the uncertainty on the number of cases of bfVb | ||
|---|---|---|---|---|---|---|
| As non‐cooked | As cooked | As non‐cooked | As cooked | |||
| Data: L. monocytogenes prevalence in bfV | As most laboratory methods are using a standardised method for detection (ISO), not much impact is expected on the estimated prevalence. | – | – | – | – | – |
| As most studies were carried out recently (since 2010) and especially the most impacting, largest, studies have been carried out very recently, not much impact is expected on the estimated prevalence. | +/− | +/− | +/− | +/− | +/− | |
| As studies are representing a few geographical locations and have mostly been taken from retail, these may not be representative for the EU market prevalence. | +/− | +/− | +/− | +/− | +/− | |
| The blanching of some vegetables is in some cases unknown and some un‐blanched frozen vegetables may have been considered in the prevalence distribution. As blanching can reduce contamination, but subsequent processing steps may introduce contamination and because not many unknown bfV have been included, not much impact is expected on the estimated prevalence. | +/− | +/− | +/− | +/− | +/− | |
| Data: L. monocytogenes concentration in bfV | FBO samples were included in the estimation of the concentration. These are suspect samples and could have had a higher concentration but on the other hand ‘the highest count sample’ was not related to the outbreak. | + or none | + or none | + or none | + or none | + or none |
| As most laboratory methods are using a standardised method for enumeration (ISO), not much impact expected on the concentration. | – | – | – | – | – | |
| As most studies were carried out recently (since 2012 and for most samples later), not much impact expected on the concentration. | +/− | +/− | +/− | +/− | +/− | |
| As studies are representing a few geographical locations only (few studies/few countries), this contributes uncertainty to the estimation of EU market L. monocytogenes concentration. | +/− | +/− | +/− | +/− | +/− | |
| The blanching of some vegetables is in some cases unknown. However, most samples were from FBOs using samples that have been blanched. | +/− | +/− | +/− | +/− | +/− | |
| Data: serving size | Data were available from 19 MS and is therefore considered representative of EU serving sizes. | +/− | ++/−− | ++/−− | ++/−− | ++/−− |
| Most surveys were carried out after 2000 (2000–2015) and based on expert opinion and survey data it is believed that serving size has not changed. | +/− | ++/−− | ++/−− | ++/−− | ++/−− | |
| The vegetables considered (carrots, peas, beans, broccoli, corn and asparagus) are representative of bfV. | +/− | +/− | +/− | +/− | +/− | |
| Data: number of servings | Group A is based on vegetables that are always blanched and this group is probably representing an underestimation of consumption with 62.5 servings per female per year. These figures have been validated against Belgian data being 3.6 kg of frozen vegetables per year per person. Considering a serving size of 50 g this gives 72 servings per year. | – | none | none | −− | −− |
| Group B is based on vegetables that are always blanched and those that are sometimes blanched (i.e. also including potatoes that are seldom a part of frozen vegetables). This group is believed to represent an overestimation of total consumption with 120.7 servings per female per year. | ++ | none | none | ++ | ++ | |
| Group C is the same as group B but without potatoes. This is believed to be to be the best estimate with 101.5 servings per female per year. | + | none | none | ++ | ++ | |
| Data were available from 19 MS and is therefore considered representative. | +/− | none | none | ++/−− | ++/−− | |
| Most surveys were carried out after 2000 (2000–2015) and, based on expert opinion and survey data, it is believed that the number of servings has not changed. | +/− | none | none | ++/−− | ++/−− | |
| Proportion of 8% considered being frozen: based on two estimates (from Belgium and from 28 MS) as described in Section 2.1.4. | +/− | none | none | ++/−− | ++/−− | |
| Data: L. monocytogenes growth rate | A high proportion of data on L. monocytogenes growth is associated with vegetables as the most favourable for L. monocytogenes growth | + | ++ | ++ | ++ | ++ |
| The frozen vegetables were artificially contaminated using selected strains (also FBO strains) and different methodologies, but the conditions are chosen to mimic realistic conditions (strains adapted). Depending on conditions, this could give a slight over‐ or underestimation of the growth rate and of L. monocytogenes counts. | +/− | +/− | +/− | +/− | +/− | |
| Not all data come from blanched frozen and thawed vegetables. Some studies involved heated treated (blanched) products that were not frozen–thawed. This could lead to some underestimation of the growth rate and of L. monocytogenes counts. | – | – | – | – | – | |
| Data: MPD | Maximum population density estimates are based on the same data as used for estimation of growth rate. The limited data could contribute either over‐ or underestimation of growth. | +/− | ++/−− | ++/−− | ++/−− | ++/−− |
| Methodology: L. monocytogenes growth rate | EGR at the experimental temperature was estimated by Baranyi model fitting, sometimes to very few data (n = 5 or 3), making the EGR estimates very uncertain. This could lead to moderate underestimation of the growth rate. | −− | −− | −− | −− | −− |
| The EGR at 5°C was calculated using Tmin = –1.18°C. More values have been reported that would lead to a lower growth rate. This may lead to an overestimation of growth rate. | + | + | + | + | + | |
| Dose response | The same approach has been applied as in previous scientific opinion (EFSA BIOHAZ Panel, 2018), hence any uncertainties will be inherent and affect all foods the same. | +/− | +++/−−− | +++/−−− | +++/−−− | +++/−−− |
| Assumptions: lag phase | Assumption of a lack of lag phase. Many experiments in the laboratory show that short or no lag occurs, the physiological state and the adaptation capacity of ‘natural’ L. monocytogenes contaminants (usually at low level) may differ and be more variable from L. monocytogenes strains used in the challenge testing. This may lead to some overestimation of growth. | + | + | + | + | + |
| Assumptions: log reduction by cooking process | Assumption in the absence of data on time and temperature conditions of the cooking process. Inactivation extent during cooking (consumer practices include fully cooking, e.g. through boiling, but also other treatments that may not be so efficient, e.g. in the microwave). It is considered that underestimation of the log reduction may be at hand in the upper part of the distribution, but assumption is reasonable for the lower part of the distribution. | −− | NA | (+) to none | NA | (+) to none |
| Assumption: No inactivation during thawing | No inactivation assumed during thawing although this may be carried out with heat. May lead to overestimation of L. monocytogenes counts when thawing is with heat. | + | (+) | (+) | (+) | (+) |
| Assumptions: storage time | The same approach as for other RTE foods but for bfV based on assumption, not data. The time distribution may be less relevant for frozen vegetables than for RTE foods. May lead to overestimation of the L. monocytogenes counts. | + | ++ | ++ | ++ | ++ |
| Assumptions: storage temperature | Assumption based on the literature review carried out in the previous scientific opinion (EFSA BIOHAZ Panel, 2018), but is the same for all foods and scenarios. The temperature distribution may be less relevant for frozen vegetables than for the RTE foods as the storage time is usually shorter. | +/− | ++/−− | ++/−− | ++/−− | ++/−− |
| A constant temperature per consumer is considered, which is a simplification of a dynamic process and at higher temperatures would lead to substantial growth potential. This scenario can be considered to reflect also storage at higher temperatures, e.g. room temperatures, during shorter times. Overall, may lead to some overestimation of growth. | + | ++ | ++ | ++ | ++ | |
| Assumptions: freezing inactivation | No inactivation effect of freezing during frozen storage. No effect or a slight reduction could be expected on the levels of L. monocytogenes (100 d of freezing would lead to 1 log10 reduction in cauliflower/Brussels sprouts) which would be associated with some overestimation or no effect. | (+) | (+) to none | (+) to none | (+) to none | (+) to none |
| Scenarios: uncooked and cooked (i.e. boiled, fried or microwave heated) | Uncertain parameter that was solved by taking into account the whole range of scenarios (until 100% consumed as RTE). Made two assumptions based on data. | + (for the worst‐case (23%) | NA | NA | +++ | +++ |
| –/+ (for the best case, 4%) | NA | NA | −−/++ | −−/++ | ||
bfV: blanched frozen vegetables; DR: dose response; EGR: exponential growth rate; NA: not applicable; RTE: ready‐to‐eat.
+ or – signs indicate whether the uncertainty associated with the factor would lead to an increased (+) or a decreased (–) estimated risk.
+ or – signs indicate the direction and the impact the uncertainty would have on the estimated risk.
The only bfV scenario having a higher ranking (based on the mean risk per serving) than any RTE food subcategory was the worst‐case uncertainty scenario for the uncooked bfV subcategory. The mean probability of illness per serving in this scenario was similar though slightly greater (2.6 × 10−9 compared to 2.1 × 10−9) than the RTE food category soft‐ and semi‐soft cheese. Compared to the baseline scenario, the probability of illness per serving in the worst‐case scenario was about six times greater (2.6 × 10−9/4.0 × 10−10, see Table 6) due to assumptions of higher values for the initial concentration, mean serving size, MPD and prevalence. Since the worst‐case scenario assumes the high percentiles of all these uncertain parameters, it is considered that it may represent an overestimation of risk. The most influential uncertain parameters according to the sensitivity analysis are the MPD and serving size, followed by the initial concentration and prevalence (Table C.2). On the one hand, the worst‐case mean serving size of 106 g is believed to be a serious overestimation, which alone, in comparison with the more reasonable baseline serving size of 49 g, would explain a higher predicted probability of illness per serving than for the soft‐ and semi‐soft cheese. On the other hand, there were quite limited data on all these parameters and considering the variety of types of bfV and the hygienic conditions under which they are produced, uncertainty is associated with the estimated probability of illness per serving and consequently on the ranking of bfV. In conclusion, the probability of illness per serving of bfV is lower than the RTE food categories evaluated but the estimated uncertainty interval (Table 6), for vegetables consumed without cooking, using the model and the best and worst‐case scenarios, includes also the estimated mean value for the soft and semi‐soft cheese subcategory. This means that even though the probability of illness associated with consumption of uncooked bfV is lower in the baseline scenario than that for soft or semi‐soft cheese, the possibility that it is higher (in worst‐case scenario) cannot be excluded. However, considering the additional sources of uncertainties mentioned above this is very unlikely (5–10%). Importantly, the probability of illness is always lower than for the next RTE category (sausage).
Table TableC.2.
The impact and ranking of the importance of uncertain parameters in the mgQMRA model on the probability of illness per serving for uncooked frozen vegetables
| Additional scenarios (using same seed) | Probability of illness per servinga | Rscenario/Rbaseline | Rank |
|---|---|---|---|
| Baseline | 6.24 × 10−10 | 1 | NR |
| Baseline + worst‐case for prevalence of L. monocytogenes in bfV | 7.26 × 10−10 | 1.2 | 4 |
| Baseline + worst‐case for initial concentration of L. monocytogenes in bfV (C0) | 8.96 × 10−10 | 1.4 | 3 |
| Baseline + worst‐case for serving size of vegetables | 1.35 × 10−9 | 2.2 | 2 |
| Baseline + worst‐case for MPD of L. monocytogenes in vegetables | 1.57 × 10−9 | 2.5 | 1 |
The impact was evaluated by replacing each parameter in the baseline scenario, one at the time, with the parameter value of the worst‐case scenario and running a simulation in @Risk.
bfV: blanched frozen vegetables; MPD: maximum population density; NR: not relevant.
Based on fewer iterations; thus, there is no total correspondence with Table 7.
Based on the estimated number of annual cases and the evaluated uncertainty range, it was concluded that the public health impact of bfV consumption is ranked lower than for the consumption of the seven RTE food categories included in the analysis (Table 7). This conclusion is based on the probability of illness per serving, and in addition, the total number of servings and the proportion consumed as cooked or uncooked. The latter data reflect current consumer preferences and habits, both of which have a direct influence on the predicted number of cases and may be subject to change.
3.2.4. Concluding remarks
During 2008–2018 and at EU/EEA level, 53 strong evidence FBOs by L. monocytogenes were reported with 679 human cases, 283 hospitalisations and 54 deaths. The ‘dairy’ food category was responsible for five outbreaks involving 47 cases, while ‘fish and seafood’ and ‘meat and meat products’ food categories were responsible for nine and 16 of these outbreaks causing 63 and 190 cases. In comparison, cases linked to bfV were reported only in 2018, and involved 46 persons, of which all were hospitalised and five deaths. However, it should be considered that most surveillance and notification systems are affected by a degree of underestimation, and therefore, this represents a source of uncertainty.
The probability of illness per serving of bfV consumed either uncooked or cooked was evaluated by a risk assessment model, the mgQMRA, to encompass the range of consumer habits of females and males in the elderly group (65–74 years) of the EU population. The probability of illness per serving is up to 3,600 times greater for bfV consumed uncooked rather than cooked. The variable parameters having the largest impact on this outcome is the r‐value of the DR model and the L. monocytogenes concentration in bfV before thawing, and, for cooked vegetables, also log reduction during cooking. Sources of uncertainties were identified and their impact on the assessment was quantified by modelling of uncertainty scenarios or by a qualitative uncertainty assessment. The main sources of uncertainties are the concentration and prevalence of L. monocytogenes in these foods, serving sizes, log reduction and MPD. The qualitative uncertainty assessment related to bfV indicated that the additional sources of uncertainty not evaluated in the modelling scenarios contributed an overestimation of risk. The mean probability of illness per serving of bfV is lower than the evaluated RTE food subcategories, i.e. cold‐smoked fish, hot‐smoked fish, gravad fish, cooked meat, sausage, pâté and soft and semi‐soft cheese. The probability of illness per serving for bfV consumed uncooked may be higher than for the lowest ranked RTE food category of soft and semi‐soft cheese (uncooked bfV uncertainty interval: 2.8 × 10−10–2.6 × 10−9, cheese 2.1 × 10−9). The possibility that the risk of bfV is higher (in worst‐case scenarios) than that of cheese cannot be excluded but considering the additional sources of uncertainty, this is very unlikely (5–10%).
The public health impact in terms of the annual number of cases in the EU was evaluated for the female, 65–74 age group. Under assumptions of the total number of servings and the proportion consumed uncooked, the yearly number of cases for bfV ranged from 0.04 (baseline estimate of probability of illness per serving) to 1.6 (worst‐case estimate of probability of illness per serving). Based on the estimated number of annual cases and the evaluated uncertainty interval, it is concluded that the public health impact of bfV consumed with or without cooking is ranked lower than the evaluated RTE foods. This conclusion is based on the probability of illness per serving, and additional data on current consumer preferences and habits, both of which would have a direct impact on the predicted number of cases and may be subject to change.
3.3. Factors of contamination and growth of L. monocytogenes in bfV during processing until consumption
The following section summarises information on factors that may increase or decrease contamination with L. monocytogenes in bfV during processing and before consumption. Such an approach may be jeopardised by the fact that L. monocytogenes contamination is often a consequence of unforeseeable or poorly managed situations such as technical breakdowns, unplanned change of personnel, and hygienic circumstances due to seasonal work peaks (e.g. before Christmas). A recent publication has shown a large shift of the L. monocytogenes population structure to disease‐associated clones during a period of reconstruction in a meat processing environment (Stessl et al., 2020). Such situations could amplify the impact of the listed main factors affecting contamination and growth of L. monocytogenes in bfV during all stages of processing (excluding primary production) (see Section 3.3.1) and consumption (including storage after thawing, food preparation and consumer habits) (see Section 3.3.2).
3.3.1. Main factors affecting contamination and/or growth of L. monocytogenes in bfV during processing
3.3.1.1. Factors thought to increase the contamination of L. monocytogenes at processing level
In the processing facilities, both the occurrence of L. monocytogenes in the FPE and in the frozen product is to be considered when evaluating potential risk factors. Table 8 summarises the main factors at processing level that affect the contamination of L. monocytogenes in bfV. It is recognised that the environment of a food processing facilities can be an important source of contamination that may negatively affect food safety and quality (3M and Cornell, 2019).
Table 8.
Main factors identified at processing level that affect the contamination of blanched frozen vegetables with L. monocytogenes
| Stage | Factor | Reasoning/justification | Source of information |
|---|---|---|---|
| All | Lack of implementation of PRPs included in the FSMS | FBOp are obliged to develop and implement FSMS including PRP activities and HACCP principles. PRP cleaning and disinfection includes SSOPs. When daily SSOP intervention strategies were used, L. monocytogenes load (log10 CFU per sample) remained unchanged but persistent strains were eliminated in some FCS | Expert knowledge |
| All | Humidity control/condensation | Condensation from overhead pipes and cooling systems that can drop onto the exposed food has been identified as a possible source of L. monocytogenes. Condensation drippings have been directly linked to food safety recalls. This phenomenon is of concern in frozen processing plants due to the temperature fluctuation and production traffic patterns. Insulated panels represent a big problem once waterlogged | FDA‐CFSAN (2017), 3M and Cornell (2019) |
| All | t/T combinations | If present in the vegetables, L. monocytogenes can grow during storage or during different processing steps such as washing, blanching, cooling and freezing if the correct t/T combinations are not well maintained | Kataoka et al. (2017) |
| Receipt of fresh vegetables | Hygiene of the incoming raw material | Raw material coming from the field contains dust, soila and debris that can be contaminated with L. monocytogenes. Raw material can be stored before processing at a t/T combination that may allow L. monocytogenes growth | Beuchat (1996), Pappelbaum et al. (2008), Jorgensen et al. (2020) |
| Equipment and processing environment | Environmental contamination | FPE can be important sources of L. monocytogenes contamination. Several studies have evidenced that contamination of Listeria spp. and L. monocytogenes from the environment is associated with FCS but also non‐FCS | Cox et al. (1986), Spurlock and Zottola (1991), Pappelbaum et al. (2008), Kovacevic et al. (2012), Simmons et al. (2014), Inuwa et al. (2017), Jorgensen et al. (2020) |
| Transport from and within the processing facility | Conveyor equipmentb | Rollers, belts and conveyor systems from frozen vegetable processing plants have been found to be contaminated with L. monocytogenes even when cleaned and sanitised every day. Conveyor equipment can be of poor design and made of material that is very difficult to clean and may facilitate biofilm formation. L. monocytogenes has also been isolated from conveyor belts in food processing facilities from other sectors (i.e. dairy) | Midelet and Carpentier (2002), Tolvanen et al. (2007), Pappelbaum et al. (2008), Chaitiemwong et al. (2010), Morey et al. (2010), Moretro et al. (2019) |
| Processing steps, where water is being used (e.g. washing, cleaning, cooling) | Microbiological quality of process water | Process water can be a source of cross‐contamination between different product batches if contaminated with L. monocytogenes. The microbiological quality of the water must be maintained to avoid cross‐contamination | Pappelbaum et al. (2008), Tadepalli et al. (2018), Smith et al. (2019) |
| Deep freezing | Environmental contamination of freezing tunnel | Freezing tunnels are wet environments that may enable survival of L. monocytogenes. L. monocytogenes has been isolated from freezing tunnels. ‘New generation’ freezer tunnels do not allow for a full/complete defrost, so once contaminated, it is almost impossible to eradicate bacteria | Inuwa et al. (2017), EFSA (2018) |
| Filling (bulk) | Packaging (either bulk or package) | GHPs are essential to have a clean environment and prevent cross‐contamination at (re)packaging | FDA‐CFSAN (2017) |
| Cutting and repackaging at a different facility | Opening packages, cutting and repackaging should be done under strict hygiene conditions to avoid contamination | EFSA and ECDC (2018) |
Note: Expert knowledge and references from other processing plants were also considered in the absence of data on frozen vegetables processing plants.
FCS: food contact surface; FPE: food processing environment; FSMS: food safety management systems; GHP: good hygiene practice; HACCP: hazard analysis and critical control points; PRP: prerequisite program; SSOP: sanitation standard operating procedure; t/T: time/Temperature.
Native L. monocytogenes survives in soils and depends on moisture (Falardeau et al., 2018).
Conveyor equipment is known to transfer microbes to the food. Since vegetable processing uses transportation of raw materials over long distances and given the fact that some of those are non‐removable, conveyor belts will pose a risk for transmission. Using chemical sanitisers was found to be very much debris‐dependent and in general quite ineffective. Ultrasonic cleaning experiments have shown that stainless steel is easier to decontaminate than plastic materials (polypropylene and acetal). A strong reduction effect was achieved in experiments employing UV light on conveyor belts where numbers of L. monocytogenes (7 log10 CFU/g) were decreased below the quantification level of the method in three out of four cases. Plastic surfaces were also described more resistant to decontamination in a study using hydrogen peroxide mist and whole room disinfection.
L. monocytogenes may be introduced into a processing facility via soil debris remaining on produce. This bacterium was also shown to be an efficient endophytic coloniser of different raw produce species (Kljujev et al., 2018). L. monocytogenes contamination of food products within processing plants evidences that L. monocytogenes strains are often isolated from FPEs (e.g. drains and equipment), including sites close to FCSs (e.g. dicing machines), rather than from raw materials (Ferreira et al., 2014; Zoellner et al., 2018). For example, L. monocytogenes in dried ice cream mixes on a stainless‐steel surface could still be detected for up to 6 weeks at room temperature and for 9 weeks at 4°C (Inuwa et al., 2017).
Pappelbaum et al. (2008) investigated the L. monocytogenes prevalence in different types of frozen vegetables and in freezer environments over a 4‐year period. The processing equipment in direct contact with the food (food contact surfaces or FCSs) such as slicing machines, feeders, vibratory transporters, freezing tunnels and conveyor belts, as well as non‐FCSs such as the floor were found to be heavily contaminated with L. monocytogenes. This illustrates extensive contamination of the FPE. The contaminated raw produce as well as the gloves of the operators were identified as sources of the contamination cycle in the processing facility. The authors concluded that, because the isolates recovered from gloves were indistinguishable by pulsed‐field gel electrophoresis (PFGE) from those isolated from the processed produce, the lack in personnel hygiene, as well as the environment contamination, was supporting the contamination cycle in that processing plant. Spurlock and Zottola (1991) highlighted that Listeria presence in a floor drain could possibly lead to contamination of food products through airborne contamination. They reported aerosol generation, that was shown to prevail for up to 210 min when the initial inoculum was very high. Condensate dripping from overhead pipes and cooling systems that can drop onto the exposed food has been identified as a possible source of L. monocytogenes in several food processing operations (FDA‐CFSAN, 2017; 3M and Cornell, 2019). The contamination problems associated with the freezing process are less well covered and usually not monitored for L. monocytogenes at processing facility level.
According to the system of contamination scenarios as set up by Muhterem‐Uyar et al. (2015), contamination of FPEs is often widely spread involving several processing steps. The EFSA technical report (EFSA, 2018) includes a comprehensive list of potential sources of L. monocytogenes contamination of frozen FVH considering the different activities performed in the freezing plants and handling facilities.
In most cases, cross‐contamination of food from the FPE will not result in high numbers as the L. monocytogenes numbers on surfaces are in general low (Zoellner et al., 2018). An exception could be a sort of ‘sloughing effect’ that could result in higher numbers of L. monocytogenes in samples, usually from a single lot. Sloughing effect means a release of an accumulation of L. monocytogenes, whether organised in biofilms or not, that is residing on FCSs for a while before dispatch onto the food. Repeated investigation usually does not confirm the prior positive result for L. monocytogenes. That a sort of sloughing effect exists was exemplified by a study in which some particular processing lines were highly positive whereas other lines were negative for L. monocytogenes (Lee et al., 2007a).
Biofilms are complex structures mainly composed of extracellular DNA, exopolymers and microbial cells and can grow on polypropylene, steel, rubber or glass surfaces throughout the industry (Blackman and Frank, 1996; Colagiorgi et al., 2016; Galie et al., 2018; Skowron et al., 2018; Dygico et al., 2020). Biofilms establish preferentially in surface irregularities of conveyor belts, e.g. potentially constituting harbourage sites for persistent contamination (Fagerlund et al., 2017). Biofilms grown in zones subjected to shear and at the wetting front are significantly more resistant to mechanical stress and sanitisers. Therefore, poor design features resulting in difficult to clean cavities, dead ends, dirty flanges and welds (such as washing tanks) appear critical in terms of hygiene. Although L. monocytogenes is capable of rapid attachment to various food processing surfaces such as stainless steel, the debate continues about whether L. monocytogenes can form biofilms. However, mixed‐species biofilms are the predominant form of biofilms found in FPEs (Colagiorgi et al., 2017).
3.3.1.2. Factors thought to decrease the contamination of L. monocytogenes at processing level
Both blanching (at specific t/T combinations) and the use of water disinfection treatments to maintain the microbiological quality of process water, may decrease the L. monocytogenes contamination of bfV at processing level.
Water disinfection treatments
Water supply plays an important role in frozen vegetable processing and different purposes in its use are interconnected: (i) water for cleaning of raw materials containing soil and dust, (ii) water for rinsing and cooling blanched product and (iii) water (ice) accumulated during the freezing step, among others. The water supply can be configured in a circular (water is re‐used) or non‐circular system. In some processing facilities, washing of raw material is done by recirculated water whereas post‐blanching steps are supplied with potable water. However, whenever a washing tank is used, large amounts of produce are exposed to the same volume of water, representing a risk for cross‐contamination. While the safety of a circulated water supply is often ensured by physical treatments (e.g. heat treatment, ultraviolet‐C), the use of a water disinfection treatment (e.g. by the use of chemical sanitisers such as sodium hypochlorite and peroxyacetic acid) is recommended to maintain the microbiological quality of the water. The presence of residual concentrations of sanitisers in the process water to avoid cross‐contamination during washing may reduce the microbial load present on the surface of the vegetables; however, the complete elimination of pathogens from the plant tissue is not feasible and should not be the aim of the washing step (Gil et al., 2009). Nevertheless, studies showed either a large reduction of L. monocytogenes in peppers after ozonisation/hydrogen peroxide washing or even a decrease to non‐detectable levels in a combined antimicrobial washing/freezing model for blueberries (Alexandre et al., 2011; Tadepalli et al., 2018). However, these studies were performed under laboratory conditions, which cannot be directly extrapolated to the situation in industrial settings.
Blanching
Blanching is a heat treatment applied to the vegetables after the initial washing and, if applicable cutting and mincing/chopping (see Figure 1). The process conditions (t/T combinations) applied during blanching vary greatly, depending on the food company and the type of vegetable. Blanching is a technological heat treatment applied for enzymatic inactivation, but it can reduce L. monocytogenes levels by more than 5 log10 units (Mazzotta, 2001; Ceylan et al., 2017).
Inoculation studies carried out by Mazzotta (2001) using a variety of vegetables (broccoli florets, sweet green peppers, onions, mushrooms and peas) showed that blanching could be used as a 5‐log inactivation process of L. monocytogenes provided that the cold spots of vegetables reached 75°C for at least 10 s or 82°C instantaneously. Moreno et al. (2012) reported that blanching for 60 s at 98°C eliminated approximately 6 log10 of inoculated L. monocytogenes. Other researchers compared steam and hot water blanching. Steam blanching at 85°C reduced L. monocytogenes by more than 5 log10 on carrots and spinach within 2 min and on broccoli and peas within 3.5 min. L. monocytogenes was reduced more than 5 log10 within 1 min on carrot, spinach, peas and broccoli by steam blanching at 96.7°C. The study by Ceylan et al. (2017) revealed that shorter t/T treatment is applicable when using hot water blanching. However, either hot water or steam blanching can achieve the desired 5 log10 reduction in L. monocytogenes when adequate t/T combinations are applied. However, this study was performed at laboratory scale, and therefore, care should be taken to conclude that a similar reduction is achieved in industrial settings.
Besides its thermal inactivation, in the EFSA report (EFSA, 2018), blanching was highlighted as a possible risk factor for several reasons: (i) blanching followed by cooling can increase L. monocytogenes prevalence mainly due to cross‐contamination of post‐blanched products; (ii) thermal treatment modifies the structure and/or characteristics of the matrix and the resulting product may facilitate growth if storage temperature allows it (see Section 3.3.2); (iii) thermal treatment reduces endogenous/background microbiota, which could facilitate establishment and growth of L. monocytogenes, due to a loss of antagonistic effects. However, the impact of bacterial competition between endogenous microbiota and pathogens on vegetable safety is still unclear. If the Total Bacterial Count (TBC) of the vegetables consists of vegetative bacteria, the reduction in numbers may be considerable, while it may be negligible if the TBC consists of spore‐forming bacteria.
3.3.2. Main factors affecting contamination and/or growth of L. monocytogenes in bfV after processing
The behaviour of L. monocytogenes in bfV after processing (at the consumer stage) will be determined by the consumer practices. Unfortunately, no (or limited) data is published on consumer practices in relation to handling and consumption habits, but the foreseeable steps are illustrated in Figure 7. The main factors affecting growth of L. monocytogenes associated with these practices are summarised in Table 9.
Figure 7.
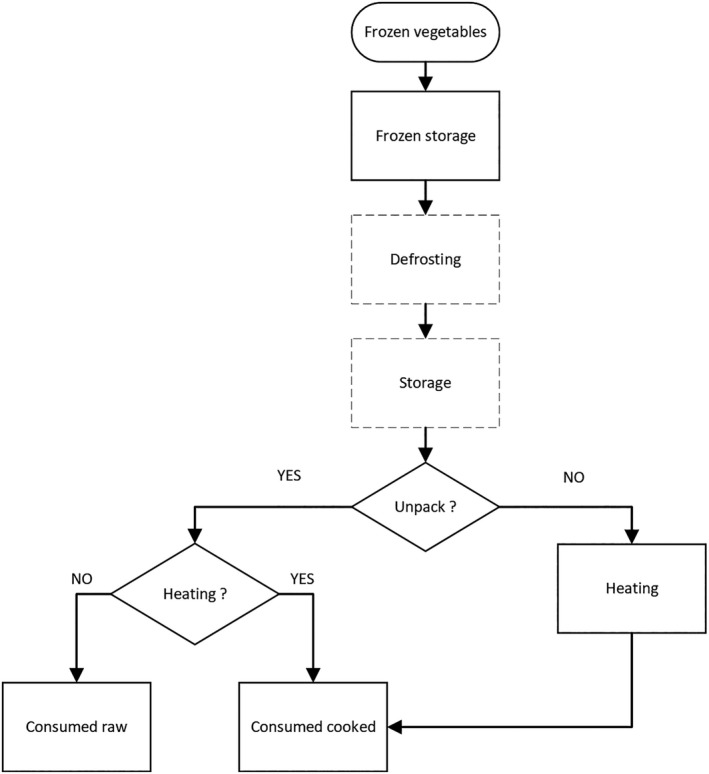
Flow chart for the preparation and consumption of (blanched) frozen vegetables
Table 9.
Main factors at consumer level affecting the concentration of L. monocytogenes in blanched frozen vegetables (bfV)
| Stage | Factor | Reasoning/justification | Source of information |
|---|---|---|---|
| Handling/storage | Intrinsic characteristics of the bfV | In the presence of L. monocytogenes, the intrinsic characteristics (e.g. pH, aw, nutrients, antimicrobial compounds) of the bfV will determine whether the products support growth or not, and the pathogen growth rate in case it is supported | Beuchat and Brackett (1990), Palumbo and Williams (1991), Flessa et al. (2005), Pappelbaum et al. (2008), Uyttendaele et al. (2009), Bardsley et al. (2019), PROFEL (2019) |
| Thawing/storage | t/T profile during thawing and storage | If the vegetables are kept long enough at temperatures supporting growth during thawing and subsequent storage, growth of L. monocytogenes on the vegetables is possible. This is only relevant if the product is thawed and not sufficiently cooked before consumption to guarantee decreasing L. monocytogenes to ‘safe’ levels | Sant'Ana et al. (2012), Kataoka et al. (2017), Huang et al. (2019), PROFEL (2019) |
| Cooking/consumption | Product consumed without sufficient cooking; instructions not properly validated | When the vegetables are not sufficiently cooked before consumption to guarantee decreasing L. monocytogenes to ‘safe’ levels or the cooking instructions are not properly validated or no proper label instructions exist indicating it has to be cooked and how. This is also linked to the type of equipment used for cooking | Benlloch‐Tinoco et al. (2014), Renna et al. (2017), Gonzalez‐Tejedor et al. (2018), Kim and Pao (2019), Willis et al. (2019) |
| Consumption without cooking (uncooked) | Product consumed as RTE | Some vegetables might be used for food preparations without cooking at all or without any other transformation to reduce or eliminate L. monocytogenes (e.g. smoothies, salads). Some vegetables are specifically intended to be RTE by the FBOp or the consumer may use as such despite the information provide in the label | EFSA and ECDC (2018), Willis et al. (2019) |
aw: water activity; bfV: blanched frozen vegetables; FBOp: food business operator; RTE: ready‐to‐eat; t/T: time/Temperature.
Frozen foods do not support L. monocytogenes growth while kept at freezing temperatures. However, L. monocytogenes can survive for extended periods at freezing temperatures (e.g. for 120 days at −18°C on whole and cut cucumbers (Bardsley et al., 2019) or at least 28 days at −20°C on whole and cut strawberries (Flessa et al., 2005)) particularly in low acid food, including vegetables (Palumbo and Williams, 1991; Pappelbaum et al., 2008; Bardsley et al., 2019). Even if some cell injury may occur, the reduction of the pathogen during the frozen storage of low acid vegetables is very limited (ca. 1 log10 in 100 days based on (Pappelbaum et al., 2008)).
Technological factors such as slicing, grinding or juicing of raw materials will have an impact on the subsequent growth (Francis and O'Beirne, 2001; Gleeson and O'Beirne, 2005).
For many frozen vegetables, cooking before consumption is advised on the label of the consumer packages of bfV. For instance, in a recent survey at retail conducted in England (Willis et al., 2019), 77% of those packages recommended cooking before consumption, yet 4% specified RTE and on 19% of the packages, there was no indication of cooking instructions or the cooking instructions were unknown.
Preparation instructions often include ‘cook from frozen’ and the time for cooking varies considerably depending on the product, producer and the heating method. Cooking may be done in‐package or unpacked. The cooking method for unpacked products may include frying, pressure cooking in water, steaming or microwaving. The latter two may also be used for in‐package heating mostly with the aim of cooking but also to defrost the product.
In principle, by following the cooking instructions, inactivation of L. monocytogenes (e.g. at least 5 log10 and up to 9 log10 reduction) can be expected. The microwave power can considerably influence the inactivation rate. Although microwave‐mediated microbial inactivation seems to be faster than conventional heating (Benlloch‐Tinoco et al., 2014; Renna et al., 2017), ‘cold spots’ may occur in domestic microwaving due to non‐uniform heat distribution. The consequently lower lethality in some parts of the product may increase the probability of L. monocytogenes remaining in the final product, making the conventional cooking (e.g. in water, steam, frying and oven) more efficient at decreasing overall microbial levels (Heddleson and Doores, 1994; Anantheswaran and Ramaswamy, 2001).
Consumers may thaw frozen vegetables at room or chilled temperatures or by microwave before cooking it or before consuming it as a RTE product. In general, labelling instructions indicate that storage should be under refrigeration conditions for a limited time (e.g. 24 h), but storage at room temperature may occasionally occur, although this is an inappropriate practice and could be considered as a worst‐case scenario.
The hygiene of the storage place (e.g. freezer and refrigerator) is also relevant. The presence of L. monocytogenes in the freezer/fridge can be associated with storage of raw vegetables and/or poor hygiene. To avoid cross‐contamination, it is important to keep bfV and thawed product away from dirty environments and to frequently clean the freezer and refrigerator.
Besides some exceptions (e.g. raw carrots as shown by Beuchat and Brackett (1990)), vegetables usually support L. monocytogenes growth. Thus, L. monocytogenes levels may increase if frozen vegetables are thawed and kept at conditions enabling pathogen growth. For instance, smoothies consisting of cucumber (34%), sugar beet (12%), broccoli (8%), purple cabbage (3%) and purple seedless grapes (43%) allowed a growth to 7.5–8 log10 CFU/ml at 25°C, but also at storage at 15°C. At 10°C, growth was only observed at the highest inoculum level of 6.6 log10 CFU/mL (Gonzalez‐Tejedor et al., 2018).
The extent of growth of L. monocytogenes in thawed blanched vegetables will depend on several factors, including the type of product (e.g. due to its intrinsic factors such as pH, aw, sugar/starch content, presence of antimicrobial compounds resistant to the blanching process and occurrence of cut surfaces or packaging conditions in cases where modified atmosphere is applied), the presence, type and level of competitive background microbiota as well as the t/T profile of the product during its storage.
Data describing the growth of L. monocytogenes inoculated in bfV during thawing and/or storage are scarce. Moreover, the scope and design of experiments in terms of vegetable type, L. monocytogenes strains (single or cocktail) and method of inoculation, temperature (either dynamic or constant), time, analytical methods and outputs vary greatly in the limited number of experiments undertaken (Kataoka et al. (2017); Beuchat and Brackett (1990); ComBase). The methodology used to assess L. monocytogenes growth on vegetables can be influenced by many factors, including, but not limited to, the vegetable species, size of the inoculum used, the storage temperature, the strains used for inoculation and how they were prepared and the method to determine growth (McManamon et al., 2017; Ziegler et al., 2019). Predictive microbiology models (growth/no growth probability models (boundaries)) can be used to assess if L. monocytogenes growth on a vegetable is probable, and if so, growth kinetic models can quantify the extent of growth. Many predictive microbiology models only consider some major factors such as temperature, pH and aw, and, when developed from data obtained in laboratory media, in most of the cases, they do not consider intrinsic and implicit factors in the vegetables that may influence growth. When the predictive model is developed from the experiments made with heat‐treated (i.e. blanched) frozen vegetables, using a 12‐strain cocktail (Kataoka et al., 2017), the results may be much more representative, though still product specific.
As previously mentioned, the heat treatment associated with blanching of vegetables may result in a product that facilitates L. monocytogenes growth during subsequent storage after thawing due to various factors (see Section 3.3.1.2). ComBase contains a set of comparable records regarding the growth behaviour of a cocktail of L. monocytogenes in beans and broccoli either raw (not heat‐treated) and heated at different t/T combinations (including 50–52°C for 60–90 s up to 55–90°C for 2–10 min) and then stored at 7°C and 10°C. According to the comparison, the standardised EGR5°C tends to be higher in heat‐treated (e.g. blanched) vegetables than in raw vegetables (Figure 8). The limited data for beans did not allow statistical differences to be determined, but for broccoli as well as for the overall data set, both the mean and median values of EGR5°C were significantly different (p < 0.05) between the two groups (not‐heated and heated), with a higher EGR5°C in the heated product.
Figure 8.
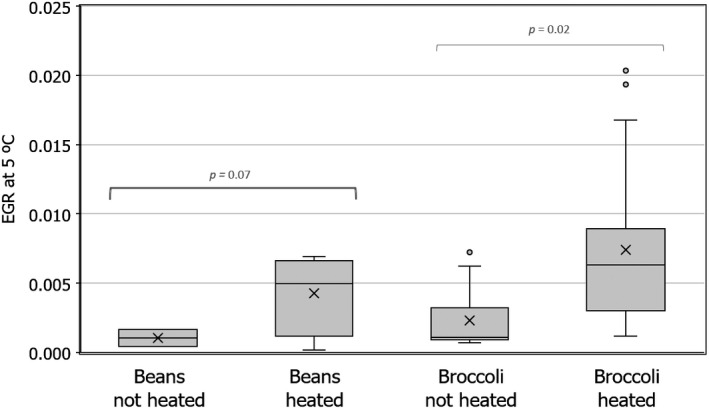
Distribution (boxplot) of the estimated exponential growth rate (EGR, log10/h) at 5°C of L. monocytogenes in raw beans (n = 3) and broccoli (n = 10) in comparison with heat‐treated beans (n = 4) and broccoli (n = 18)
In ComBase, the growth of L. monocytogenes in heat‐treated (e.g. blanched) vegetables was also available for corn, green peas, carrots and asparagus at different temperatures. In most of the records, L. monocytogenes was able to grow without a lag time (in 73% of the records) or after a (very) short lag time (i.e. with a lag time shorter than 4 h in 9% of the records), indicating that it is well adapted to the vegetable characteristics and can initiate its growth almost immediately when the product temperature allows it.
The EGR values retrieved from ComBase26 and the literature are shown in Figure 9. The EGR was variable between and within vegetable types. Despite the limited data, it seems that some vegetables favour a faster growth of L. monocytogenes (e.g. asparagus, green peas, corn and carrots) than others (e.g. broccoli and beans). However, the data can hardly be compared as they come from different experiments, using specific strains and experimental conditions.
Figure 9.
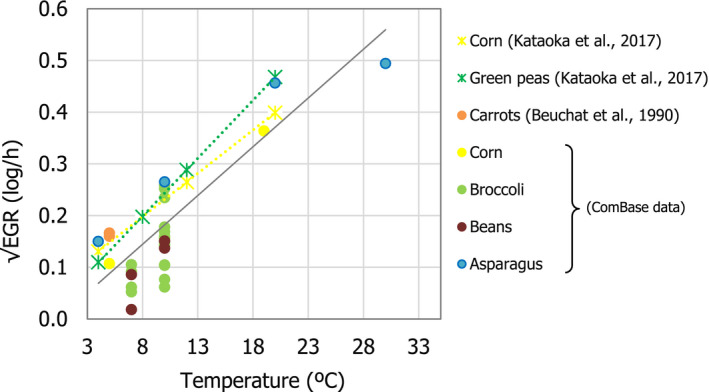
Exponential growth rate (EGR, in log10/h) for L. monocytogenes in heat‐treated vegetables as a function of the storage temperature
- Dots are the observed data obtained from the literature and Combase and lines show the fit of the square root model to the whole data set (grey), green peas (green) and corn (yellow) reported by Kataoka et al. (2017).
Figure 9 also presents the relationship between the EGR (log10/h) of L. monocytogenes in heat‐treated vegetables and the storage temperature. The secondary square root model was used to fit the whole set of data and the estimated parameters are shown in Equation 1 (residual sum of square, RSS = 0.150 and adjusted coefficient of correlation, = 0.70).
| (1) |
Only the study by Kataoka et al. (2017) dealt with a cocktail of cold‐adapted L. monocytogenes being inoculated onto frozen vegetables (blanched individually quick frozen corn with a pH 7.2 and individually quick frozen green peas with a pH 6.8), then frozen for 1 week at −18°C and thawed and stored at 4, 8, 12 and 20°C. L. monocytogenes grew well in corn and green peas, with the EGR in the upper range i.e. worse case, in comparison with those observed in other studies for other vegetables. Consequently, the line describing the square root model fit to the EGR of corn and green peas from this study is above the one fitting the whole set of data recorded (Figure 9).
The experimental design of the study by Kataoka et al. (2017) enabled the quantification of the impact of the storage temperature on the growth kinetic parameters (i.e. lag phase and EGR) of a 12‐strain cocktail of L. monocytogenes. The available mathematical models enable estimating the time to achieve a given log10 increase (Table 10). This can either be the time needed for L. monocytogenes to reach 100 CFU/g assuming an initial contamination level of 1 CFU/g (i.e. time to 2 log10 increase) or the time needed for an increase of 0.5 log10, as the threshold above which food is considered to support growth (FAO, 2007; EURL‐L. monocytogenes and ANSES, 2019b). A relatively short lag time was recorded, indicating that there was no relevant effect of freezing and thawing. Moreover, it is uncertain whether the short lag time was due to the adaptation of the pathogen (i.e. an actual lag phase) or due to the thawing process (from −18°C to the target temperature), since inoculated frozen vegetables (25 g portions) were transferred to a cabinet set at the target temperature and L. monocytogenes was monitored from the start. The EGR increased with temperature; the increase observed for green peas being higher than for corn. At low temperatures, L. monocytogenes grew slightly faster in corn than in green peas, e.g. at 4°C the time to 2 log10 increase was 118 and 167 h, respectively. By contrast, at higher temperatures, growth was faster in green peas than in corn, e.g. at 12°C the time to 2 log10 increase was 24 and 29 h, respectively. An increase of 0.5 log10 of L. monocytogenes levels would be reached in less than 48 h in all cases. Considering 24 h as the maximum time to store bfV under refrigeration according to some labelling instructions, the maximum temperature of storage to avoid a 0.5 log10 increase would be 4.9°C (for corn) and 5.6°C (for green peas). In that study, a cocktail of 12 strains of L. monocytogenes was used to account for the strain variability in the growth behaviour. Therefore, the estimates for the growth rate will be highly dependent on the growth rate of the fastest growing strain.
Table 10.
Growth kinetic parameters of L. monocytogenes inoculated in vegetables (corn and green peas), frozena and thawedb and stored at different temperatures
| Parameter | Frozen–thawed corn | Frozen–thawed green peas | ||||||
|---|---|---|---|---|---|---|---|---|
| 4°C | 8°C | 12°C | 20°C | 4°C | 8°C | 12°C | 20°C | |
| Lag time (h) | 13.2 | 7.0 | 2.9 | 0.5 | 12.2 | 6.8 | 3.5 | 3.4 |
| EGR (log10/h) | 0.017 | 0.039 | 0.070 | 0.16 | 0.012 | 0.039 | 0.083 | 0.219 |
| Growth potential after 24 h (c),(d) (log10 units) | 0.4 | 0.9 | 1.7 | 3.8 | 0.3 | 0.9 | 2.0 | 5.3 |
| Growth potential after 48 h (c),(d) (log10 units) | 0.8 | 1.9 | 3.3 | 7.6 | 0.6 | 1.9 | 4.0 | 10.5 |
| Time to 0.5 log 10 increase c (h) | 30 | 13 | 7 | 3 | 43 | 13 | 6 | 2 |
| Time to 2 log 10 increase c (h) | 119 | 52 | 29 | 13 | 172 | 51 | 24 | 9 |
Note: Predicted lag time and exponential growth rate (EGR) according to the models developed by Kataoka et al. (2017) for a 12‐strain cocktail.
Individually quick frozen, kept at −18°C before and during inoculation and during 7 days before thawing to monitor L. monocytogenes growth.
Inoculated frozen vegetables (25 g portion) were transferred to the target temperatures and L. monocytogenes was monitored during thawing and storage (not at the time the vegetable reached the target temperature).
Log10 increase of concentration after 24 h and at 48 h of storage at the corresponding temperature.
The lag time was not considered for the calculation.
However, unsafe practices related to t/T combinations of food storage are quite common (EFSA BIOHAZ Panel, 2018). At consumer level, the storage temperature can be > 5°C in a notable proportion of household refrigerators. According to the probability distribution of storage temperatures in the refrigerators in the EU (EFSA BIOHAZ Panel, 2018), in 23% of the cases, the storage temperature would be at 8°C or higher, during which L. monocytogenes could increase 0.5 log10 in 13 h or less (Table 10).
Three experiments of challenge tests are described in the draft PROFEL guidance. The growth potential was derived of a cocktail of four strains of L. monocytogenes (which included the ST6 multi‐country outbreak strain), inoculated on five types of frozen vegetables (green peas, parsnips, sweet corn, sweet potatoes and white cabbage) using an inoculum level of 100 CFU/g. After inoculation, packs of 200 g samples were frozen for a maximum of 2 w and then transferred to a refrigerator at 9°C during defrosting and storage for 24–48 h. The selected vegetables green peas, parsnips, sweet corn and sweet potatoes were identified a priori as the most favourable for L. monocytogenes growth according to score criteria related to their pH, sugar and starch content, and presence of anti‐listeria components. White cabbage was part of the second highest risk group and was added to include also a vegetable belonging to the leafy green group. The storage temperature of 9°C was selected to mimic reasonably foreseen temperature abuse in household refrigerators. The actual t/T profiles of the products during thawing and storage varied depending on the product and the load of the refrigerator; the more loaded the refrigerator, the slower the thawing process. For example, the defrosting time (to reach 0°C from −18°C) took either up to 20 h (higher load) or less than 5 h (lower load: each food type stored separately). Consequently, the growth potential of L. monocytogenes observed in each experiment was notably affected by a defrosting rate factor. Among the five types of vegetables studied, green peas, sweet corn and sweet potatoes showed the highest growth potential, though the results varied between experiments. With the highly loaded refrigerator, between 0.6 and 0.9 log10 increase was observed in 24 h, and 1.2–1.8 log10 after 48 h for the three above‐mentioned vegetables. With the lower loaded refrigerator, a 0.2–1.3 log10 increase was observed after 24 h and 0.8–2.4 log10 increase was observed after 48 h. The highest values were recorded for sweet corn (2.4 log10) and green peas (2.2 log10) after 48 h at 9°C. These values are in agreement with the simulations provided by the Kataoka model at 9°C (Kataoka et al., 2017), being 2.2 and 2.3 log10 for sweet corn and green peas, respectively.
3.3.3. Uncertainty related to factors of contamination and growth
Potential sources of uncertainty identified for the main factors affecting the contamination and growth of L. monocytogenes in bfV during processing and until consumption are included in Table 11. Uncertainties refer to whether, in addition to the identified factors during the production process of frozen vegetables and after processing of bfV, there are other factors that have been omitted. The impact of this uncertainty on the conclusions is expected to be low as the WG members believe that they identified the main factors affecting contamination and/or growth of L. monocytogenes in bfV during processing and until consumption in the answer to this ToR. Additionally, the impact of the uncertainty linked to the misclassification of some factors has been identified as low, because it is believed that there is a low probability that there are other factors apart from those already considered.
Table 11.
Potential sources of uncertainty identified for the main factors affecting the contamination and growth of L. monocytogenes pathogens in blanched frozen vegetables during processing (excluding primary production) and consumption (including storage after thawing, food preparation and consumer habits)
| Source or location of the uncertainty | Nature or cause of the uncertainty as described by the experts | Impact of the uncertainty on the conclusions (e.g. over/underestimation) |
|---|---|---|
| Scarcity of data | Overreliance on a limited number of publications | This factor can lead to both, underestimation or overestimation of specific risk factors due to data gaps |
| Nature of product | bfV encompass a huge variety of different product categories. The consumed parts of a vegetable (either sprouts, roots, tubers etc.) might have a divergent contamination status; risk assessment over the whole of frozen vegetables is difficult | This factor can lead to both, underestimation or overestimation of contamination |
| Use of data of fresh or processed vegetables (such as fresh‐cut salads, heat‐treated vegetables) to determine growth potential of L. monocytogenes | It is possible that blanched and/or frozen vegetables may be more vulnerable for growth than fresh. Results for the respective products may be higher or lower in relation to the products focused on in this report | This factor can lead to both, underestimation or overestimation of the potential growth |
| Type of experiment | Naturally contaminated vegetables vs. inoculated vegetables and laboratory‐scale experiments vs. pilot‐scale or industrial‐scale experiments. Laboratory tested microorganisms may be more vulnerable or more resistant compared to natural contaminants and in an industrial setting; growth predictions are widely hampered | This factor can lead to both, underestimation or overestimation of the potential growth |
| Methodology | Little standardisation with regard to practical approaches, especially with regard to sampling concepts. Comparison of data is difficult, extrapolating conclusions from single plants to a general situation is difficult | This factor can lead to both underestimation or overestimation of the potential growth |
| Technological impacts – harvest | Vegetable harvest and distribution results in management of huge numbers of bulky material; storage of raw material is often uncontained. Data may fluctuate over locations and categories of raw materials | This factor can lead to both, underestimation or overestimation of the potential growth |
| Technological impacts – processing | Vegetable processing is a non‐continuous process and depends very much on campaigns; processing in general leads to a food that is minimally processed. Data may fluctuate over seasons and categories of raw materials under processing; little risk reduction over the entire process | This factor can lead to both, underestimation or overestimation of the potential growth |
| The relative impact of raw materials and processing environment as contamination source |
Raw material is the primary source of contamination, which can be spread all along the processing plant (e.g. by workers and equipment flows, gloves). This would apply for sporadically occurring L. monocytogenes strains The relative importance may be processing plant dependent Some studies point out that post‐processing (e.g. post‐blanching) is an important source of contamination. This might be particularly true for persistent L. monocytogenes strains |
This factor can lead to both, underestimation or overestimation of contamination |
| Consumer behaviour | Very scarce information is available concerning consumer behaviour (e.g. handling practices) for bfV. Exact data on the percentage of consumers not cooking or improper cooking practices are not described | This factor can lead to both, underestimation or overestimation of specific risk factors |
bfV: blanched frozen vegetables.
3.3.4. Concluding remarks
-
•
The main factors that are very likely (90–95%) to impact the contamination and/or growth of L. monocytogenes in bfV during processing are:
-
○
The hygiene status of the incoming raw materials;
-
○
The hygienic conditions of the FPE, including FCSs and non‐FCSs;
-
○
The microbiological quality of the process water;
-
○
t/T combinations used for storage, washing, blanching, cooling and freezing.
-
○
-
•
The identified factors that could increase contamination with L. monocytogenes include the inappropriate hygienic conditions of the post‐blanching processing environment particularly linked to the conveyor equipment and freezing tunnels.
-
•
Factors that may decrease the contamination of L. monocytogenes in bfV during processing are:
-
○
Blanching: L. monocytogenes can be reduced up to 5 log10 units when the t/T combinations applied are equivalent to a heat treatment where the cold spots of the product reach 75°C for at least 10 s or 82°C instantaneously;
-
○
Water disinfection treatments applied to maintain the microbiological quality of the process water usually reduce the microbial load present on the surface of the vegetables but complete elimination of microorganisms is not feasible.
-
○
-
•
The heat treatment associated with blanching of vegetables may result in a product in which L. monocytogenes can grow better and/or faster during subsequent storage after thawing.
-
•
There is no growth of L. monocytogenes in products kept frozen, but contamination during processing may lead to growth if bfV are thawed and stored at a temperature and for times that allow L. monocytogenes growth.
-
•
Based on the available data, the intrinsic characteristics of the bfV (e.g. pH, aw, nutrients, antimicrobial compounds, natural microbiota) and t/T profile during thawing and storage affect the growth of L. monocytogenes in bfV once thawed.
-
•
Cooking conditions, including the method and the equipment (set parameters vs. real values e.g. in a microwave) determine the reduction of L. monocytogenes, if present, and the remaining levels of the pathogen in the product before consumption.
-
•
Based on predictive microbiology models in different bfV at different temperatures, it was observed that L. monocytogenes was able to grow without a lag time or after a (very) short lag time, indicating that it is well adapted to the vegetable characteristics.
-
•
The EGR of L. monocytogenes increased with temperature and it was dependent on the type of vegetable studied, and would probably be affected by strain variation. Green peas, sweet corn and asparagus support the highest growth rates (as well as carrots at the reported temperature). Beans and broccoli support lower growth rates.
-
•
The presence of L. monocytogenes within cold storage equipment can be associated with storage of raw vegetables and/or poor hygiene. Contamination can be avoided by keeping bfV and thawed product away from dirty environments and by frequently cleaning freezers and refrigerators.
3.4. Control options during the production process of bfV
Analysis of the hazards and activities of the target processing plants as performed in the EFSA technical report (EFSA, 2018) suggests that PRP activities are sufficient and the application of HACCP, including CCPs, is either not possible or would not further enhance food safety. This is why potential control options described in this section are based on PRPs (focusing on the hygiene and organization of the production environment) and HACCP‐plan (focusing on the process control) conform to the Commission Notice (EC) No 2016/C 278/0120. PRPs are the basic conditions and activities necessary to maintain a hygienic environment. Prerequisites include GHP and GMP among other good practices and, although food business specific, may be divided into 13 categories (based on the Commission Notice and a recent scientific opinion on hazard analysis approaches for certain small retail establishments in view of the application of their FSMSs (EFSA BIOHAZ Panel, 2017b)): infrastructure; cleaning and disinfection; pest control; technical maintenance and calibration; physical and chemical contamination from production environment (not applicable here); allergens (not applicable here); waste management; water and air control; personnel; raw materials; temperature control; working methodology; product information and consumer awareness.
3.4.1. Prerequisite program
Article 17 of Regulation (EC) No 178/200212 states that: ‘Food and feed business operators must ensure that food and feed at all stages of production, processing and distribution in farms under their management comply with the requirements of food law applicable for their activity and check whether these regulations are indeed being complied with’.
Annex 1 of Regulation (EC) No 852/20042 stipulates, among other things, that procedures, practices and methods to ensure that food is processed, handled, packaged, stored and transported under appropriate hygienic conditions, including effective cleaning and control of hazards, should be in place’. Annex 2 of that regulation stipulates, among other things, that ‘the organisation, design, construction, location and dimensions of spaces for foodstuffs must be such that: (a) maintenance, cleaning and disinfection can be adequately carried out, air pollution is prevented as far as possible and sufficient working space is available to carry out all operations satisfactorily.
EN 1672‐2:2005+A1:200927 part 2 proposes, among other things, ‘the implementation of a risk analysis for food processing machines, based on hygienic aspects, provides requirements for hygienic materials and design, gives examples of hygienic or unsanitary design and proposes the verification of compliance with requirements’.
Based on this legal context, the European Hygienic Engineering and Design Group (EHEDG) provides a practical interpretation of how this is possible for, among other things: closed equipment, open equipment, buildings and their utilities to exclude or control all possible contamination based on HACCP principles.
3.4.1.1. PRP raw material
The documentation with incoming fresh raw produce should be checked to ensure that it meets the plant‐established purchase specifications. Personnel in charge of the receipt of the raw material should audit the certifications of suppliers demonstrating that they comply with GAP.
3.4.1.2. PRP infrastructure
Design and organisation of infrastructure, equipment and devices are important in the prevention of L. monocytogenes in the FPE. As described by Zoellner et al. (2019), areas of a facility are often prioritised according to levels or required hygienic care (hygienic areas) and surfaces within each area may be designated into zones (also known as zoning) according to different levels of L. monocytogenes control and their proximity to food products. The intensity of the verification of PRP will be different based on the potential for product contamination. Table 12 shows the established zone characterisation with some examples of locations linked to these zones.
Table 12.
The classification of surfaces into four different zones with examples of locations (FDA‐CFSAN, 2017)
| Zones | Description | Examples of locations |
|---|---|---|
| Zone 1 | FCSs | Utensils, table surfaces, slicers, pipe interiors, tank interiors, filler bowls, packaging and conveyors, hoppers |
| Zone 2 | Non‐FCSs in close proximity to food and FCSs | Equipment housing or framework, and some walls, floors or drains in the immediate vicinity of FCSs carts |
| Zone 3 | More remote non‐FCSs that are in or near the processing areas and could lead to contamination of zones 1 and 2 | Forklifts, hand trucks and carts that move within the plant and some walls, floors or drains not in the immediate vicinity of FCSs |
| Zone 4 | Non‐FCSs, remote areas outside of the processing area, from which environmental pathogens can be introduced into the FPE | Locker rooms, cafeterias and hallways outside the production area or outside areas where raw materials or finished foods are stored or transported |
FCSs: food‐contact surfaces; FPE: food processing environment.
The establishment of hygienic areas is used by FBOp to divide the FPE in different areas in an attempt to differentiate dirty (e.g. outside reception area of raw materials) from clean (e.g. post blanching area) areas. While this approach is used in different processing plants, it is usually not easy to segregate different areas within the plant mostly because of the lack of physical barriers (e.g. walls) between one area and another. One possible solution is to use a colour‐coding system, which allows clear identification of equipment allocated in particular areas.
Next to the zoning, the hygienic design of equipment plays an important role in controlling microbiological safety. There are several aspects that should be considered by the food processor before reengineering or introducing process equipment into the plant. Effective hygienic design (Directive 2006/42/EC,28 CEN EN 1672‐2,29 the international standard ISO 14159:200230) describes some selected criteria and basic requirements, related to:
materials used for the construction: e.g. must be inert under operating conditions as well as the surface finish being undamaged; the materials must be corrosion‐resistant and mechanically stable;
surface roughness (or smoothness): FCSs should be smooth enough to be easily cleanable. Porous surfaces are usually unacceptable. The surfaces must be free from crevices, sharp corners, protrusions and shadow zones and this during their entire functional lifetime;
accessibility of all parts of the equipment: for inspection, maintenance, cleaning, preferably without the use of tools;
no liquid collection: equipment should be self‐draining, by having e.g. the framework round or inclined at 45 degrees; no horizontal surfaces, hollow bodies, crevices, dead spaces (as these may lead to accumulation of water, dust, product); and
no niches: e.g. welds should be flush, and free of pits, occlusions and corrosion.
A few examples are given to illustrate this, and which are of importance in processing facilities:
The distance between the conveyor systems and the floors and drains must be sufficient to prevent contamination from the latter to the conveyor belts. Also, the conveyor belt between the blancher and the freezer needs to be kept as short as possible.
Drains must be designed to function adequately; be accessible for cleaning; there should be no trench drains in areas where foods are processed; flow should not be from areas where raw foods are processed or exposed towards processed foods; drains of restrooms should not be connected to the drains from areas where foods are processed; accumulation of standing water in and around drains should be prevented; floors should be properly sloped to the drain(s) in a way that ensures that floors drain freely and water does not accumulate (FDA‐CFSAN, 2017).
Freezers (spiral, tunnel and other types of design) have been identified as relevant for contaminating the areas surrounding freezers and finished products with L. monocytogenes (AFFI, no date). The following points are important:
to ensure that there is no damage in the walls and ceiling of the freezer as these openings serve as entry points for moisture in the insulation of the structure;
to consider the lights inside freezers as condensation commonly forms inside the lights and can serve as a source of contamination;
to consider the roof of the freezer as excessive condensation and accumulation of moisture on the roof of the freezer, where refrigeration piping is often located, can result in water dripping, puddles and pooling. Appropriate design and inspection frequencies are the best way to minimise this risk; and
to consider that ice is usually shovelled out from freezers and often stacked on the ground of the surrounding area. If L. monocytogenes is present in the ice, floor contamination may occur.
Humidity control is important, as lower humidity environments are not a favourable environment to support retention of viable L. monocytogenes on a stainless steel surface (Redfern and Verran, 2017). Condensate dripping from cooling systems has been identified as a possible source of L. monocytogenes in a number of food processing operations. Therefore, it is essential that all drainage ducting from air‐handlers and condensers is piped directly into the drains and not on the floor or ground. Condensation on walls and ceilings must be avoided as foods, FCSs or food packaging material may become contaminated.
3.4.1.3. PRP cleaning and disinfection
Establishment of a general sanitation program
Cleaning and sanitising are critical operations to ensure control of L. monocytogenes and to minimise conditions that promote the survival or growth of L. monocytogenes in the FPE. The general sanitation program aims to reduce contamination of the product, the FCSs but also of the non‐FCSs with L. monocytogenes (see also Section 3.4.1.2). A compliant, effective sanitation program must be well structured and have a detailed description of what is to be done, when it is to be done, how it is to be done, and by whom, captured in Sanitation Standard Operating Procedures (SSOPs) (Gordon, 2017). The SSOPs are generally documented steps that must be followed to ensure adequate cleaning of FCSs and non‐FCSs. The specific steps used to clean and sanitise equipment and the FPE are unique to each processor but in most of the cases, the same sanitisers are applied for different processing practices. Special focus should be given to the prevention of contamination of the product from FCSs and non‐FCSs. The efficacy of the sanitation program should be verified to avoid harbourage sites of L. monocytogenes. The verification program should follow a risk‐based approach for EM, which should include sampling sites, frequency of the sampling, test procedures and corrective actions based on the characteristics of the final product and the processing methods used to produce those products (FDA‐CFSAN, 2017). See also Section 3.5.
A schedule with the frequency of routine cleaning and sanitation should be based on the condition of the production plant, sanitary design, production schedule and product characteristics. Rooms should be kept as dry as possible as moisture fosters growth and transfer of L. monocytogenes (FDA‐CFSAN, 2017). In principle, the locations (defined in Table 12) in direct contact with foods are more frequently cleaned and disinfected compared to the ones without direct contact with the foods. The sanitation plan should also report if the equipment must be disassembled, the method of cleaning and disinfection (e.g. foam cleaning, cleaning‐in place (CIP)), types and concentration of cleaning compounds and disinfectants t/T/pressures to be used (PROFEL, 2019). Example of a template to define a sampling plan aiming to identify potential sources of L. monocytogenes in freezing plants/handling facilities for frozen FVH has been already suggested. The schedule with the frequency of cleaning and sanitation may be revised in time based on the outcome of the sampling plan. Regular control of the efficiency of the cleaning and disinfection needs to be done by microbiological sampling of contact surfaces (with different targeted groups of bacteria, including L. monocytogenes) and by ATP measurement (presence of organic material) (see also Section 3.5).
A critical aspect of cleaning freezers includes manual cleaning activities. Automated cleaning systems are strongly recommended for freezers. Ensuring that CIP systems are properly designed and installed, maintenance is critical. Portions of the conveyor system that extend outside the blancher and the freezer enclosure require special consideration. Biofilms established preferentially in surface irregularities of conveyor belts, potentially constituting harbourage sites for persistent contamination. The transfer of L. monocytogenes from FCSs such as conveyor belts to processed food products has been documented. Although the underside of a conveyor belt is not intended to be in direct contact with food, it may confer harbourage sites from which bacteria can shelter and contaminate FCSs during processing (Fagerlund et al., 2017). In all cases, the equipment manufacturer, the chemical supplier and a multidisciplinary team of the FBOp must agree upon decisions regarding the best cleaning methods and cleaning products for the tasks (AFFI, no date).
Actions must be taken to avoid splashing already completely cleaned and disinfected equipment and areas. To prevent aerosols from contacting food, FCSs, and food packaging materials, personnel should not use high‐pressure water hoses during production in areas where foods are exposed or after equipment has been cleaned and sanitised (FDA‐CFSAN, 2017). Therefore, drains should be cleaned and disinfected in a manner that prevents contamination of other surfaces in the room. Utensils for cleaning drains should be easily distinguishable and be dedicated to that purpose to minimise the potential for contamination. Floor drains should not be cleaned during production. High‐pressure hoses should not be used to remove visual dirt or clean a drain, as aerosols will be created that may spread contamination throughout the room. If a drain backup occurs in finished product areas, production should stop until the water has been removed and the areas have been cleaned and disinfected. Employees who have been cleaning drains should not contact or clean FCSs without changing clothes, and washing and disinfecting hands (FAO, 2007).
After production, the equipment must be cleaned and disinfected. But the production of these bfV is seasonal, which leads to machines that will not be used for a certain time. It is essential to have a cleaning and disinfection after this period, before starting up the production, i.e. cleaning and disinfection needs to be followed immediately by production or a new cleaning and disinfection needs to be planned.
Special attention must be paid to the status of cleanliness of mobile equipment (PROFEL, 2019) as well as the flows within the hygienic areas or zones of the production plant (when moving from dirty to clean areas) to avoid contamination among different areas.
3.4.1.4. PRP pest control
Pests may also carry L. monocytogenes; therefore, a pest control program which includes proofing of the facility as well as control practices will minimise potential ingress of Listeria‐carrying animals and birds (Khan et al., 2016; Grinyer, 2018).
3.4.1.5. PRP technical maintenance
A preventive maintenance plan must be drawn with appropriate inspection frequencies to minimise the risks of contamination.
Tools intended for maintenance of equipment used in areas where there is direct contact with the food should be dedicated to these areas or need to be cleaned and disinfected after maintenance and prior to use (FDA‐CFSAN, 2017).
Special attention must be paid to the freezing tunnel and blancher, ventilation and air condition systems, but also to other equipment. Inspection for damage is critical and should be conducted during pre‐operational checks and during preventive maintenance activities. Conduits in freezers need to be inspected for junction separation, corrosion and water encroachment. These conditions provide a source of contamination inside the freezer, as well as raising safety concerns when water is introduced to electrical systems.
3.4.1.6. PRP waste management
Waste areas need to be clearly separated from the areas where foods are processed, exposed or stored (FDA‐CFSAN, 2017) and must be indicated in the specific flow diagram of the FPE. Waste management control activities are described in PRP 7 (waste management) of Commission Notice 2016/C 278/0120 (EFSA BIOHAZ Panel, 2017b).
3.4.1.7. PRP water and air control
Water (both its availability/usage and its quality) is coming under increasing pressure so care has to be taken by the companies that the internal re‐use of water is not a source of contamination for food products with L. monocytogenes.
The water source (e.g. tap water, rain water, ground water, treated recycled water) and supply systems are of importance and need to be free of L. monocytogenes.
The water quality needs to be controlled (microbiologically as well as chemically). Large volumes of water are commonly used in the frozen food industry for washing, cooling and transport of food, among other uses. If the water quality is not well maintained, this can cause cross‐contamination with harmful microorganisms or chemicals between different lots of product (Gil et al., 2015). The existing literature indicates that the disinfection of the wash water is a necessary intervention strategy to reduce microbiological risks where water is used (Danyluk and Schaffner, 2011; Gombas et al., 2017; Maffei et al., 2017). Depending on the final use of the water and the type of product being in contact with the water, different types of water might be required (e.g. potable and clean water). For example, the water used for glazing must be of potable water quality.
When recycled or re‐used water is used, the company must be aware of the possible accumulation of microorganisms, including pathogens such as L. monocytogenes and avoid cross‐contamination and dispersion of the pathogens to the batches of product in contact with that water. The use of optimised residual concentrations of disinfectant are needed to eliminate L. monocytogenes in the process water and to avoid cross‐contamination between different batches (Banach et al., 2015), in this case we speak about an oPRP (see Section 3.4.2). Based on the current Regulation (EC) No 852/20042, the recycled water that is used in the production process must be of drinking water quality, unless the CA has determined that the quality of water cannot affect the healthiness of food products in their final form.
For water reconditioning, next to disinfectants (such as chlorine compounds, peracetic acid) also physical methods such as ultraviolet‐C and reverse osmosis may be used, but one must keep in mind that cross‐contamination may not be prevented when used for water disinfection in situ (Banach et al., 2015). All disinfection techniques need to be controlled (e.g. continuously monitoring and keeping a residue in the water) and validated.
A water management plan needs to be elaborated in function of the source and quality of the water and the water disinfection technologies (PROFEL, 2019). The water management plan should include the sampling and analytical procedures for the verification of the quality of the water.
Avoid contamination of the water in the production area with drain water/effluent water (PROFEL, 2019).
Air flow systems could be of benefit. If installed, the design should be as follows: higher air pressures where products are being processed and lower air pressures in areas where unprocessed (‘raw’) foods are handled (FDA‐CFSAN, 2017). If incoming air is filtered to remove contaminating particles, there should be a documented filter maintenance program in place, with a record kept of all filter maintenance carried out. Filters will need to be checked, cleaned and replaced at frequent intervals. Intakes should be upwind from the prevailing wind, exhaust vents, inwards goods and rubbish disposal sites. The location of the air intake should not be adjacent to the location of the air exhaust or other sources of airborne contamination such as waste disposal areas.
3.4.1.8. PRP personnel
Training programmes are essential to raise awareness of L. monocytogenes amongst the operators and the personnel involved in sanitation or maintenance activities. Programs designed for training employees in proper handling, cleaning or engineering practices raise awareness of L. monocytogenes, minimise its introduction into the facility, and reduce opportunities for contamination (FDA‐CFSAN, 2017; Grinyer, 2018).
The movements of the personnel between different areas (from dirty to clean or from under construction to production) or zones is also very important, as the personnel can transfer L. monocytogenes from the FPE to the final product. The risk of contamination via the movement of personnel between areas or zones should be minimised. For example, employees who handle trash, floor sweepings, drains, packaging waste or scrap product, should not touch the food, FCSs or food packaging material, unless they change their smock or outer clothing, wash and disinfect hands, and wear clean new gloves for tasks requiring gloves. Adequate training and supervision should be provided to assure hygienic practices are accomplished (FAO, 2007).
3.4.1.9. PRP time/Temperature (t/T) control
Minimise the amount of time that ingredients and other raw materials, in‐process materials, and finished products are stored under conditions that allow growth of L. monocytogenes, apply first‐in, first‐out or use the ones expiring first (FDA‐CFSAN, 2017).
The time and temperature need to be measured on an ongoing basis. Freezing is a particularly effective temperature control option to prevent growth during storage, but will not eliminate L. monocytogenes (FDA‐CFSAN, 2017; Bardsley et al., 2019). Blanching may reduce the microbial load (e.g. of L. monocytogenes) and if the t/T of the process has been validated, it would be an oPRP (see Section 3.4.2). However, as previously pointed out in Section 3.3.1.2, blanching is a technological heat treatment intended to inactivate enzymes that cause product spoilage.
3.4.1.10. PRP working methodology
Personnel follow work descriptions, and standard operating procedures (SOP) need to be controlled frequently (EFSA BIOHAZ Panel, 2017b). There is a need for the establishment of SOPs for among others cleaning and disinfections procedures (SSOPs), sanistations audits, maintenance. A SOP is a set of written instructions that documents a routine or repetitive activity followed by an organisation (EPA, 2007). Based on the guidance for preparing SOPs elaborated by the US Environmental Protection Agency (EPA, 2007), the development and use of SOPs are an integral part of a successful quality system as it provides individuals with the information to perform a job properly, and facilitates consistency in the quality and integrity of a product or end results. Traffic flow patterns for personnel, food products, food packaging materials and equipment need to be controlled.
3.4.1.11. PRP product information and consumer awareness
The FBOp decides if the product is RTE or not. In case it is not RTE, the cooking instructions need to be validated as well as the storage/handling conditions (either freezing, thawing, t/T conditions after thawing etc.). Where appropriate, product labels should include information on safe handling practices and/or advice on the time frames in which the product should be eaten (FAO, 2007). In this case, the labelling is an oPRP (see Section 3.4.2).
3.4.2. Operational Prerequisite Programs
The oPRPs are prerequisites that may be identified as critical to control a specific hazard. Both CCPs and oPRPs are designated if there are any safety measures necessary to control a specific hazard.
The CCPs are designated at the stage where control can be applied to prevent or eliminate the hazard or reduce it to an acceptable level. The oPRPs are designed with reference to a product or FPE as the basic component of managing the likelihood of the introduction of food safety hazards (Jackowska‐Tracz et al., 2018). As previously mentioned, no CCPs are determined in the process for bfV and potential control options are based on PRPs. Most PRPs are general in nature and their purpose is to ensure the general hygiene conditions of the food business. The draft PROFEL guidelines (PROFEL, 2019) identified four different oPRPs: oPRP 1: water contamination in washing tanks in case of unblanched products; oPRP 2: blanching process, t/T; oPRP 3: temperature follow‐up of cooling water and oPRP 4: freezing t/T. All of these (except oPRP1) refer to different stages of the process where the impact that t/T conditions might have on the L. monocytogenes growth.
The factors contributing to increased/decreased occurrence of L. monocytogenes and the control activities linked to oPRPs identified in the HACCP plan of this scientific opinion can be found in Table 13.
Table 13.
Factors contributing to increased/decreased occurrence of L. monocytogenes and the control activities linked to oPRPs identified in the food safety management systems (FSMS)
| Processing stage | Factors contributing to increased/decreased occurrence of L. monocytogenes | Control activitya |
|---|---|---|
| Equipment and processing environment | Food processing environments can be important sources of L. monocytogenes contamination. Contamination from the environment is associated with FCS but also non‐FCS | oPRP1: Cleaning and disinfection |
| Processing steps where water is used (e.g. washing, cleaning, cooling etc.) | Process water can be a source of cross‐contamination between different product batches if contaminated with L. monocytogenes. The microbiological quality of the water must be maintained to avoid cross‐contamination | oPRP2: Water control |
| Washing (t/T) | The t/T combination applied during washing allows growth or survival of L. monocytogenes in the water | oPRP3: Control the temperature over time |
| Blanching (t/T) | The t/T combination applied during blanching allows growth or survival of L. monocytogenes in the water and/or product | oPRP3: Control the temperature over time |
| Cooling (t/T) | The t/T combination applied during cooling allows growth of L. monocytogenes | oPRP3: Control the temperature over time |
| Freezing (t/T) | Control of the freezing process and breakdown in the freezing process may result in a t/T combination applied during freezing that may/ may not allow growth of L. monocytogenes | oPRP3: Control the temperature over time |
| Setting information for the consumer | Relevant information to assure food safety during storage, handling and preparation of the product is not included in the label or it has not been previously proved (validated) to ensure the control of the hazard, regarding:
|
oPRP4: Product information and consumer awareness |
FCS: food contact surface; RTE: ready‐to‐eat; t/T: time/Temperature.
Prerequisite programmes (PRPs) that are considered operational PRPs (oPRPs).
The cleaning and disinfection of equipment and the processing environment are considered as an oPRP. The processing environment has been identified as a source of L. monocytogenes, which makes cleaning and disinfection a relevant control measure to minimise contamination of bfV. The efficacy of cleaning and disinfection can be validated and verified based on EM. The presence of positive samples provides evidence of conditions that are unhygienic or otherwise increase the risk of food safety issues (3M and Cornell, 2019). SOPs should be implemented to ensure adequate cleaning and disinfection of FCSs and non‐FCSs.
The use of water disinfection treatments is needed to maintain the microbiological quality of the process wash or cooling water, which is critical to avoid cross‐contamination between contaminated and non‐contaminated products (EFSA BIOHAZ Panel, 2014). Thus, water control including maintenance of the water quality by means of a water disinfection treatment should be considered as an oPRP. Efficient antimicrobial treatment of process water is critical to avoid microbial risks of the processed product (Gil et al., 2009). SOPs should be implemented to maintain optimum residual disinfectant concentrations that avoid contamination.
Time/Temperature control is also considered an oPRP. The time that raw materials and other ingredients are stored under conditions that allow growth of L. monocytogenes before processing must be avoided. This is also applicable for thawed bfV, as the t/T combinations of storage after thawing will affect the growth potential of L. monocytogenes.
Although outside current food safety legislation, consumers should ensure the food is stored, handled and prepared in a manner that ensures it is safe for consumption. This stage in the food chain is especially important as it includes interventions (such as cooking) capable of eliminating pathogenic bacteria that inevitably contaminates a small percentage of food batches and survives the processing and retail stages (EFSA BIOHAZ Panel, 2017b). Producers should provide the consumer with information to assure food safety during storage, handling and preparation of the product. Relevant information may originate from national food safety authorities and include optimum storage temperature, shelf‐life, cooking instructions, etc. (EFSA BIOHAZ Panel, 2017b). This is why in addition to the oPRP1 cleaning and disinfection, oPRP2 water control and the oPRP3 control the temperature over time, also the PRP product information and consumer awareness has been identified as oPRP4.
Relevant information that producers should provide to the consumer to assure food safety during storage, handling and preparation of the product include: (1) time of storage under various freezing conditions at home; (2) thawing instructions that should indicate the time period needed to thaw the product in the refrigerator, microwave or in boiling water as well as instructions about the appropriate t/T conditions to store the thawed product before cooking/consumption or indicate immediate use/consumption; and (3) cooking instructions, which should be included on the label if the product is intended to be consumed as non‐RTE. In this case, relevant information should be included on the labels referring to regular cooking practices used by the consumer in order to achieve minimum treatments equivalent to the standard treatment, which guarantees that the inner part (cold spot) of the product reaches at least 70°C for 2 min (Gaze et al., 1989). Specific cooking instructions such as, time in boiling water, time in the microwave at a given power in the microwave, time in a frying pan or in the oven depending on the amount of vegetable to be cooked and the initial temperature (e.g. direct from frozen state), need to be previously validated by the FBOp as part of the FSMS.
Several control options have been described as suitable strategies to reduce contamination of bfV, although it should be taken into account that the production steps in place in the freezing plants may not be able to eliminate L. monocytogenes in frozen vegetables as no full mitigation strategy is applied in the production process (EFSA, 2018). Consequently, no CCPs are determined in the process for bfV. This is in agreement with the draft PROFEL guidance (PROFEL, 2019), where nine different PRPs have been identified. In the guidance, the PRP pest control and PRP product information and consumer awareness are not incorporated compared to this opinion. Most of the reported options to reduce L. monocytogenes in foods, including frozen vegetables, focus on the establishment of good hygienic and sanitary production and processing practices mainly to reduce the contamination of foods and the environment with L. monocytogenes (Adzitey and Huda, 2010).
3.4.3. Additional methods for control of L. monocytogenes
Several additional methods (technologies and antimicrobial solutions) have been tested with the aim to reduce or eliminate L. monocytogenes not only in the environment, mainly on surfaces, but also on the product. Most of these studies are laboratory‐based experiments. Industrial‐scale trials are still needed to demonstrate practical efficacy, especially these need to be tested on frozen vegetables or the FPE. The technologies are aimed at reducing L. monocytogenes in the product, removing them on food process surfaces or eliminating or preventing biofilms. A few examples are given in Appendix E.
It should be noted that these methods are not yet commercially available (with some exceptions) or their efficacy is not yet fully validated in an industrial setting. For some alternatives, it must also be investigated whether they can represent a potential health hazard or whether they make sensory changes to the products. For some alternatives such as gamma radiation, one must consider the consumer acceptance.
3.4.4. Uncertainty related to control options
Uncertainties linked to the possible control options that may be implemented by FBOp during the production process of bfV refer to whether, in addition to the recommended control options to be applied during the production process of bfV, there are other control options that have been omitted. The impact of this uncertainty in the conclusions is expected to be low as it is believed that the control options addressing the identified main factors affecting contamination and/or growth of pathogens in bfV have been included in the answer of this ToR.
Regarding additional methods to control L. monocytogenes, most of the suggested treatments have only been tested at laboratory scale conditions using inoculated foods. In most of the cases, very promising results are reported in the scientific papers, but the obtained efficacy under these conditions is difficult to extrapolate to industrial settings.
3.4.5. Concluding remarks
-
•
Previous analysis of the hazards and activities of the processing/freezing plants suggests that PRP activities can be applied as control measures because the application of HACCP, including CCPs, is either not possible or would not further enhance food safety.
-
•
In total, 11 PRP categories, if implemented together, are very likely (90–95%) to reduce the probability of L. monocytogenes contamination of bfV, namely infrastructure; cleaning and disinfection; pest control; technical maintenance; waste management; water and air control; personnel; raw materials; t/T control; working methodology; and product information and consumer awareness. The main important categories are those that maintain the hygiene level in the FPE high (e.g. the hygienic design of equipment, technical maintenance and cleaning and disinfection) as well as t/T control.
-
•
Four different oPRPs have been suggested as control measures and they have been linked to seven different processing stages including:
-
o
equipment and processing environment (oPRP1: cleaning and disinfection);
-
o
processing steps where water is used (e.g. washing, cleaning, cooling etc.) (oPRP2: water control);
-
o
washing (oPRP3: t/T control);
-
o
blanching (oPRP3: t/T control);
-
o
cooling (oPRP3: t/T control);
-
o
freezing (oPRP3: t/T control); and
-
o
consumer practices (oPRP4: Product information and consumer awareness).
-
o
-
•
Several control options have been described as suitable strategies to reduce L. monocytogenes contamination of bfV, although it should be taken into account that the production steps in place in the freezing plants may not be able to eliminate L. monocytogenes in bfV as no full mitigation strategy is applied in the production process.
-
•
Most of the reported options to reduce L. monocytogenes in bfV focus on the establishment of good hygienic and sanitary production and processing practices mainly to reduce the contamination of foods and the FPE with L. monocytogenes.
-
•
There is a need for food worker education and training, and consumer awareness and responsibility. To improve consumer compliance, it is also important to provide targeted risk communication (education) and proper labelling instructions, which clearly states the conditions of storage, thawing, preparation and cooking.
-
•
Additional technologies and antimicrobial solutions have been identified with the aim of reducing or eliminating L. monocytogenes in the product (e.g. Gamma irradiation, bacteriophages and phage‐derived proteins, bacteriocins, essential oils) or on food process surfaces or eliminating or preventing biofilm (e.g. phages and phage‐derived proteins, bacteriocins, nanotechnology agents, essential oils (or components), photocatalysis, biosurfactants). Not all of these technologies/solutions are yet commercially available, or their efficacy is not yet fully validated under an industrial setting.
3.5. Recommendations on routine monitoring for L. monocytogenes in bfV
L. monocytogenes contamination from environmental sources in food facilities has been shown to play an important role in the finished product contamination (Norton et al., 2001; Simmons and Wiedmann, 2018). Routine processing EM programmes for L. monocytogenes aim to reduce the probability of contamination of frozen vegetables and has been identified as the most sensitive tool to assess control of the FPE and risk of product contamination (CFIA, 2011).
Many guidance documents have been published in the past years focusing on recommendations on effective routine monitoring procedures for L. monocytogenes in food processing areas. Examples include, among other: (1) ‘guidelines on sampling the food processing area and equipment for the detection of L. monocytogenes’ (EURL‐L. monocytogenes and ANSES, 2019a); (2) the international standard EN ISO 18593:201821; (3) the ‘Testing Methodology for Listeria spp. or L. monocytogenes in Environmental Samples’ (FDA, 2015); and (4) the ‘Guidance on environmental monitoring and control of listeria for the fresh produce industry’ (UFPA, 2018). Most of the recommendations included in these guidelines are fully applicable to the frozen vegetable industry. However, frozen vegetables pose specific challenges that must be taken into account, as described below.
Monitoring L. monocytogenes in a freezing plant or handling facility for frozen vegetables can be implemented for different reasons, for example:
Batch sampling (product sampling) to judge on acceptance or non‐acceptance of a batch of fresh vegetables and/or finished products;
Surveillance sampling (product sampling) to seek for the prevalence of pathogens (e.g. official monitoring of CAs, food products on the market);
Sampling of areas after cleaning to verify that a cleaning and sanitising program works;
Monitoring the efficacy of L. monocytogenes control programmes and strategies including PRPs, HACCP system or other FSMS to determine, among other, if the facility design and infrastructure support the safe production of frozen vegetables. If this is the aim, sampling should be done during processing, contaminated sites identified and targeted for cleaning and sanitisation; and
Identification of specific problem sites in facilities (Simmons and Wiedmann, 2018).
Maintenance of a L. monocytogenes‐free FPE is relatively difficult to achieve as many factors can affect the occurrence of L. monocytogenes. These can include, for example, contaminated incoming raw materials, staff members as carriers, insufficient cleaning strategies and sampling programmes the facility design to minimise the likelihood of contamination, the location of the facility near a farm etc. Another major factor in the occurrence is the awareness of the potential risk of the processing facility management and staff regarding the risk of L. monocytogenes. Thus, awareness, sampling and analysis are key trend factors in the successful control of L. monocytogenes in the FPE. If L. monocytogenes is detected, it can be eliminated through targeted intervention measures, thus minimising the likelihood of final product re‐contamination.
Although frozen vegetables are traditionally consumed cooked, consumer trends are moving towards fresher products, which has increased the proportion of frozen vegetables consumed raw. Additionally, the packaging labelling is not always clear regarding cooking instructions, leading to confusion for the consumers. In some cases, the product is specified as RTE, so there are no cooking instructions needed. The relative importance of verifying control of the FPE should be reflective of the risk to consumers if the food becomes contaminated. Therefore, frozen vegetables considered as RTE should be of concern.
The monitoring and control programmes implemented in a processing plant are to be designed in consideration of the production volume (e.g. regarding sampling selection, frequency of sampling, numbers of samples, method of sampling) to reduce the risk of contamination with L. monocytogenes.
The objective of this section is to identify the most critical steps that should be considered when establishing a routine monitoring program for L. monocytogenes in frozen vegetables and their FPE, considering available guidance documents. Recording of data obtained is critical for the processor. As well as recording the results of a process environment monitoring program, it is also important to record data on ingredients and raw materials so that any correlations can be identified, e.g. processing environment contamination coinciding with a batch of raw material or a change in supplier. In this way, as specified in the current legislation (Commission Regulation (EC) 2073/20051) trends in test results should be analysed, as they can reveal unwanted developments in the manufacturing process, enabling the FBOp to take corrective actions before the process is out of control. The routine monitoring program should include both the routine monitoring of the FPE and product testing. The section is divided in two subsections to cover the specific characteristics of both types of monitoring.
3.5.1. Recommendations on processing environment monitoring for L. monocytogenes in bfV processing plants
The establishment of effective EM programmes for L. monocytogenes in food processing facilities according to a sampling scheme is a critical component of any FSMS. The main objectives of a routine EM program are 1) to prevent transient contamination from becoming entrenched, forming biofilms and spreading within the facility, 2) verifying that existing control measures are effective, 3) detecting L. monocytogenes that has become entrenched in the produce handling environment before it can spread to the point of contaminating product and 4) determining when and what corrective action is appropriate, which can be used as an early warning system to identify and eliminate problematic sources of contamination (UFPA, 2018). A well‐designed process environment program is one that finds L. monocytogenes. In that way, the data can be used in trend analysis to track trends towards the increase or decrease of the organism in the FPE.
Well‐established routine EM programmes should be designed on a risk‐based approach, considering the nature and size of the food operation and reflecting aspects related to the raw materials, the production processes and the final product. But they also need to be regularly revised based on trend analysis. As described by Zoellner et al. (2019), the current approach to designing an EM program relies heavily on zoning (see Table 12) and sanitary design (whether a surface is cleanable or not, or how well it could be cleaned).
However, performance of effective EM programmes has started to be validated through mathematical modelling, such as agent‐based modelling (ABM). Zoellner et al. (2019) have recently developed an ABM that allows an in silico approach to map Listeria spp. persistence and dispersal and to evaluate interventions using a data‐driven methodology. The model is entitled EnABLe (‘Environmental monitoring with an Agent‐Based Model of Listeria’). In general, there is a consensus regarding the use of a risk‐based approach when establishing the strategies for processing EM, particularly when defining the sampling sites and frequency, based on the nature and size of the food operation and should reflect aspects related to the raw materials, the production processes and the final product application (BFFF, 2015; FDA‐CFSAN, 2017).
It is well known that L. monocytogenes does not grow in frozen products and will not be substantially reduced in number. Therefore, and based on the recommendations from the BFFF, the EM program should also be focused on processes prior to freezing. The complexity of FPE and diversity of equipment present major barriers to systematic monitoring (Zoellner et al., 2018).
There are several important decisions to be made within a routine EM program (Figure 10). Recommendations specified that FBOp should have a technically competent individual with the responsibility and authority to manage L. monocytogenes EM and control (BFFF, 2015).
Figure 10.
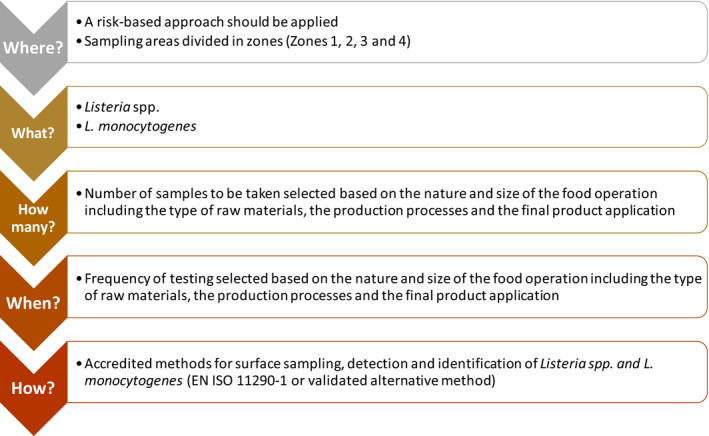
Critical decisions that should be included in a routine processing environmental sampling program.
3.5.1.1. Where to sample
FBOp have to identify the locations and number of sites from which samples will be collected during routine EM for the identification and eradication of L. monocytogenes. The wide variety of surfaces to be monitored in food processing facilities makes the selection and description of sampling sites a critical component of a well‐designed program. In the case of a routine monitoring program, sampling should be targeted at sites that are good indicators of control and should reflect prior knowledge of facility risks (Tompkin, 2002; Zoellner et al., 2018). Based on the literature, L. monocytogenes is mostly found in wet or soiled places where it can survive and it can be found in all types of equipment and surfaces (UFPA, 2018). Therefore, site selection is not necessarily based on random or systematic random sampling methods, and it can be focused on the wet zones of the FPE, e.g. the cleaning station, water condensates and drains if any (for review, see (Carpentier and Cerf, 2011; Valderrama and Cutter, 2013)) or allocated by the proximity of the site to food products (Zoellner et al., 2018).
For the purpose of collecting and testing EM samples for the presence of L. monocytogenes, most of the guidelines on strategy recommend that the FBOp characterises areas according to the potential for product contamination. Thus, the selection of specific sampling locations should be done according to historical data linked to each factory and after step‐by‐step examination of the process because harbourage risks within the FPE are individual to each operation (BFFF, 2015; EURL‐L. monocytogenes and ANSES, 2019a). As already mentioned in Section 3.4.1.2, the EM design relies on zoning. However, the designation of sampling sites to zones is not an easy task. For instance, surfaces that are located above foods, which might have condensation that can drop onto the exposed food, are usually considered zone 1, but may be classified into zone 2 if zone classification is performed during a time of low humidity when no visible condensation is present (3M and Cornell, 2019). Another example of variable zoning is drains; they are typically classified into zone 3, but if they are located immediately under food contact surfaces may be considered zone 2 sites (3M and Cornell, 2019).
In general, the number of samples should be higher in zones 1 and 2 due to the increased risk for product contamination and if a zone‐based system is not established or used. Sampling sites should be otherwise characterised in a way that distinguishes between FCS and non‐FCS (FDA‐CFSAN, 2017). When an FPE is being sampled for the first time, a broad sampling approach should be taken to identify contamination routes and potential harbourage sites. If a history of monitoring is available, or the contamination status is already known, the sampling sites can be restricted. To select the most critical sampling points, FBOp should go into the areas where produce is moved, particularly where it is exposed to the environment, and observe employee and product movement and employee practices and add sites to the list based on handling and risk, or stop practices if not appropriate (UFPA, 2018). It is recommended to divide larger sites (e.g. conveyors), into parts such as beginning, middle and/or end of belt. Sampling plans should incorporate sites that are always sampled (‘static’), sites that are frequently sampled (‘rotating’) and sites that are chosen at the time of sampling (‘random’) (Zoellner et al., 2019).
Results obtained using mathematical modelling, such as EnABLe (Zoellner et al., 2019), demonstrated that when designing EM there are several other factors to consider. These include:
The location connectivity (how many contacts are made with a surface per unit time and with which other surfaces contacts are made); and
Ranking with respect to the expected length and level of contamination of a surface if Listeria spp. is in the facility based on the facility design. This characteristic is a composite function of several factors, including connectivity, sanitary design and likelihood and vehicles of Listeria spp. introduction into the environment.
In the future, this approach may help in designing a company‐specific EM program.
3.5.1.2. What to sample for
The two alternatives when determining the focus of testing are to test for Listeria spp. or L. monocytogenes. Most of the available guidelines recommend testing for Listeria spp. because it will allow the detection of a potential contamination in a sampling point. The EURL Lm‐ANSES guideline (EURL‐L. monocytogenes and ANSES, 2019a) states that according to Commission Regulation (EC) 2073/20051 only sampling for L. monocytogenes is required. However, they indicate that according to the Codex Alimentarius (CAC, 2007), effective monitoring programmes may also involve testing for Listeria spp. as their presence is a good indicator of conditions supporting the potential presence of L. monocytogenes. Tests for Listeria spp. typically include an enrichment step followed by agar‐based detection methods (using selective and differential media) and confirmation of Listeria spp. in which a positive can be identified but not confirmed to the species level. As the relationship between Listeria spp. and L. monocytogenes will depend on the unique ecology of each food processing facility, it is important to consider that a rapid response to a positive Listeria spp. sample to address a problem or contamination risk will depend on the type of information and the time at which it is received (Zoellner et al., 2018). However, it is recommended that the freezing plant focuses on L. monocytogenes.
3.5.1.3. How many samples and when to sample
Most guidance documents do not give advice on the number of sampling points as these have to be chosen on a case‐by‐case basis (BFFF, 2015; FDA‐CFSAN, 2017; EURL‐L. monocytogenes and ANSES, 2019a). In the UFPA guidance (UFPA, 2018), general recommendations are included providing examples of specific situations which cannot be directly translated from one industry to another. Trend analysis is critical to determine the number and frequency of sampling of each type of zone because it provides evidence of the harbourage places for L. monocytogenes.
The timing of sampling is often referred to the production time where sampling is conducted (e.g. before production, during production, after production or after cleaning and sanitation). It is recommended that routine sampling is performed weekly, monthly or quarterly depending on the amount of product produced, risk and facility history.
Based on the sampling objective, the results obtained will inform on different aspects. In the case of a routine monitoring processing environment program, regulatory documents suggest that some FCS should be sampled before production, but also a few hours into production or just prior to clean‐up because this allows time for L. monocytogenes (if present) to work its way out of harbourage sites and contaminate the environment, the processing line (including FCS sites), and, potentially, final products (FDA‐CFSAN, 2017).
The recommendation is to take samples at different sampling zones and times starting with a standard protocol such as: 1) 25% after sanitation samples for all zones 1–4; 2) 65% zones 2–3 mid‐shift samples, and 3) no more than 10% zone 4 mid‐shift, with some zone 1 samples taken after equipment has been running. This will provide samples after disinfection to verify the efficacy of the disinfection procedure but also to identify potential ‘hot spots’ during processing. However, the sampling protocol should be dynamic and always based on trend analysis.
3.5.1.4. How to sample
Sampling, detection and enumeration methods should follow validated methods, which describe surface sampling methods to detect or enumerate viable microorganisms, as well as the detection and enumeration methods that should be used for L. monocytogenes.
However, the L. monocytogenes enumeration method in the analysis of processing environment samples cannot be done, as swabbing does not detach all bacterial cells, or all cells may not be detached from the swab material and the proportion of detached cells is unknown and variable. Quantitative data from swabbing could only be applied to an internal comparison of the processing facility over time. In addition, L. monocytogenes cells are not evenly distributed on a surface and comparisons of results from large and small areas would thus be invalid (EURL‐L. monocytogenes and ANSES, 2019a).
The international standard EN ISO 18593:201821 and the European Guidelines (EURL‐L. monocytogenes and ANSES, 2019a) specify horizontal methods for sampling techniques using contact plates, stick swabs, sponges and gauze pads on surfaces in the food chain environment to detect L. monocytogenes (FDA‐CFSAN, 2017; EURL‐L. monocytogenes and ANSES, 2019a). However, no large surveys have been performed with the aim of comparing different sampling practices in food industries. In general, it is recommended to sample most surfaces using surface sponging, except for small or hard‐to‐access surfaces where swabbing works better. The sample size depends on the methodology being used, but specific recommendations are included in the international standard EN ISO 18593:2018.21
Different tools can be used for different purposes and depending on the type of surface. These include sponge swabs, stick swabs or a gauze pad, with or without hydration or a neutralising agent. Swabs may be composed of different materials that influence both the adsorption and the release of bacteria from the swab (e.g. organic materials like cotton, inorganic material like polyurethane). Stick swabs can be used for small areas (e.g. 10 cm2) or difficult to access sites while sponge swabs can be used for larger sampling areas. It is recommended by the international standard EN ISO 18593:201821 that, where possible, a surface area of 900 cm2 should be swabbed. Larger areas for swabbing have also been suggested (e.g., between 1,000 and 3,000 cm2 when possible, i.e. when the areas are open and flat) (EURL‐L. monocytogenes and ANSES, 2019a). If the swabbing is conducted on wet surfaces, dry swabs should be used; conversely the hydration of swabs with sterile diluent (e.g. phosphate‐buffered saline or buffered peptone water) is needed to facilitate microbial recovery from dry surfaces. If the swabbing is conducted after disinfection, or if the presence of residual disinfectant is expected, swabs should be pre‐moistened with a neutralising diluent. Sodium thiosulfate is generally used to neutralise chlorine or chlorine‐releasing compounds (EN ISO 18593:201821). No ‘universal’ neutraliser is available, therefore, if a different disinfectant is used, a different neutralising buffer may be needed. The appropriate diluent (sterile diluent or neutralising agent) should be chosen on a case‐by‐case basis, depending on the swabbing being undertaken because sometimes, adding neutralisers to the swabs can reduce recovery by stimulating overgrowth of competing bacteria. Additionally, it is recommended to consult with the manufacturers on the most appropriate swab to use. Before collecting the swab samples, the operator should make sure to remove excess liquid from the area (i.e. pools of water/standing water on the floor). The swab should be vigorously and forcefully rubbed back and forth in two perpendicular directions for 30 s using its entire absorbing surface (rotating the swab or using both sides of the sponge) (Nicolau and Bolocan, 2014).
A brief summary on the performance of a routine EM plan can be also found in the scientific report (EFSA, 2018). The technical report (EFSA, 2018) includes relevant information that should be collected during the performance of a routine sampling program to identify potential sources of L. monocytogenes contamination in freezing plants/handling facilities for frozen vegetables.
3.5.2. Characterisation of isolates
After processing environment and end‐product sampling and sample analysis, all samples positive by L. monocytogenes detection or enumeration can be further analysed to characterise the L. monocytogenes isolates.
Subtype characterisation of L. monocytogenes isolates should be done to identify the point of contamination with L. monocytogenes in the freezing plants/handling facilities for bfV. This can be done using well‐established molecular techniques (EFSA, 2018). Subtyping L. monocytogenes isolates by a genotyping method (such as whole genome sequencing (WGS), pulsed‐field gel electrophoresis (PFGE), multilocus variable‐number tandem‐repeat analysis (MLVA) or fluorescent amplified fragment length polymorphism (AFLP)) will be necessary to establish whether the isolates belong to a persistent clone (EURL‐L. monocytogenes and ANSES, 2019a). WGS technology has introduced a new way of subtyping isolates (EFSA BIOHAZ Panel, 2019). Using WGS‐based subtyping schemes, the resolution of isolate typing is higher than that obtained using traditional phenotyping (serotyping) or older genotyping methods (listed above). Thereafter, the approach chosen to analyse WGS data (e.g. based on allele or nucleotide differences) may also result in different levels of discrimination (Franz et al., 2016).
3.5.3. Recommendations on monitoring for L. monocytogenes in bfV final product
End‐product sampling is currently a primary mechanism for analysis of processed foods before release onto the market. It is a relevant part of the verification of a FSMS (Zwietering et al., 2016). In fact, product analysis is usually done to verify the efficacy of L. monocytogenes control programmes and strategies including PRPs, HACCP system or other FSMS.
End‐product testing is not effective for controlling food safety because it provides little information on the source of the product contamination and how to mitigate it, and lacks sensitivity if contamination is non‐uniform. A more beneficial strategy, therefore, is to have in place a validated and effective processing environment monitoring program. Basically, if L. monocytogenes is not in the FPE, then the likelihood of cross‐contamination to the final product is reduced.
Therefore, it is generally agreed that validated process or preventive control will always be more reliable to ensure final product safety than testing the product itself because the absence of evidence is not evidence of absence (FDA‐CFSAN, 2017). This is why some organisations recommend testing finished product on a periodic basis, while others recommend product testing in limited circumstances, i.e. when there is reason to suspect contamination with the microorganism or when there is evidence that a prerequisite program or food safety process has failed or is out of control (FDA‐CFSAN, 2017; UFPA, 2018).
The BFFF guidance (BFFF, 2015) recommends the implementation of positive‐release procedures (i.e. when negative for the presence). They also recommend that sampling plans should include samples of final products at the end of shelf‐life post‐thawing. In this case, if the product is stored in chilled conditions, after thawing, then the storage temperature conditions for the test should reflect those used by the consumer. However, it is more the totality of information on the FPE and product that provides the confidence in safety. The company resources should focus on the implementation of good FSMS rather than on positive release testing to guarantee the safety of the product.
3.5.4. Performance objective for L. monocytogenes at the end of the production process
FSC for end‐products are implemented to verify the acceptability of the production lots. FSC for L. monocytogenes in RTE foods have been regulated as described in Section 1.3.1.3. When the RTE food supports growth of the pathogen, the FBOp may fix intermediate limits (at the end of the production process), that should be low enough to guarantee that the limit of 100 CFU/g is not exceeded at the moment of consumption without cooking (i.e. as RTE food) (Commission Regulation (EC) 2073/20051). Furthermore, these intermediate limits should be established taking into account the potential growth of the pathogen in the product once it is removed from the freeze and keeping it for a certain period of time under temperature conditions allowing its growth, as shown in Section 3.3.2. The reasonably foreseeable conditions of use by the consumers need to be considered beyond the recommendations provided by the FBOp through the product labelling. In this respect, it has been recommended to consider temperatures from 8 to 12°C as reasonably foreseeable temperature abuse in household refrigerators (EURL‐L. monocytogenes and ANSES, 2019b; PROFEL, 2019). Occasionally, consumers may maintain bfV at room temperature for a limited time (e.g. 24 h) for thawing. However, this is an inappropriate practice and cannot be considered as reasonably foreseeable temperature abuse but as a worst‐case scenario. It has also been reported that consumers may consume the product after the expiry date indicated on the label (Van Boxstael et al., 2014; Bover‐Cid et al., 2015); for instance, up to 48 h outside the freezer (in a refrigerator) has been considered in the challenge tests reported in the draft PROFEL guidelines as reasonably foreseeable condition and up to 4 day has been considered as the maximum in the mgQMRA used in Section 3.2.3.
Table 14 shows possible L. monocytogenes concentrations (CFU/g) that could be considered as a PO at the end of the production process, immediately before releasing the frozen vegetables on the market, compatible with the FSO of 100 CFU/g. The levels were calculated taking into account the growth a 12‐strain cocktail of L. monocytogenes, in frozen–thawed corn and green peas, as reported by Kataoka et al. (2017). Levels including thawing/lag time or not, for different storage times and temperatures were calculated in order to assure that the FSO of 100 CFU/g is not exceeded when the consumer thaws and stores the product [and does not cook it before consumption]. Additionally, calculations considering the growth potential recorded in the aforementioned challenge tests were performed. More information on both studies can be found in Section 3.3.2.
Table 14.
Calculated L. monocytogenes concentrations (CFU/g) on corn and green peas at the end of the production process (i.e. performance objective) that would be compatible with the food safety objective (FSO) of 100 CFU/g at the moment of consumption without cooking, for different times and temperatures of storage
| Vegetable | Storage temperature (°C)a | Storage time (h) | |||||
|---|---|---|---|---|---|---|---|
| 12 | 24 | 48 | 72 | 96 | 120 | ||
| Corn | 5 | 97b (55)c | 54 (31) | 17 (9) | 5 (3) | 2 (0.9) | 0.5 (0.3) |
| 8 | 64 (34) | 22 (12) | 3 (1) | 0.3 (0.2) | 0.04 (0.02) | 0.004 (0.002) | |
| 9 | 52 (28) | 15 (8) [5–24]d | 1 (0.6) [0.4–5] | 0.1 (0.05) | 0.008 (0.004) | 0.0006 (0.0003) | |
| 12 | 23 (15) | 3 (2) | 0.07 (0.05) | 0.002 (0.001) | 0.00003 (0.00002) | 0.0000007 (0.0000004) | |
| Green peas | 5 | 95b (63)c | 59 (39) | 23 (15) | 9 (6) | 4 (2) | 1 (0.9) |
| 8 | 62 (34) | 21 (12) | 2 (1) | 0.3 (0.2) | 0.03 (0.02) | 0.004 (0.002) | |
| 9 | 49 (26) | 13 (7) [19–24]d | 0.9 (0.5) [0.7–14] | 0.06 (0.03) | 0.004 (0.002) | 0.0003 (0.0002) | |
| 12 | 20 (10) | 2 (1) | 0.02 (0.01) | 0.0002 (0.0001) | 0.000002 (0.000001) | 0.00000002 (0.00000001) | |
5°C corresponds to the storage temperature recommended on the product labelling (PROFEL, 2019); 8°C is the 75th percentile of the refrigerator temperature in the survey by Roccato et al. (2017); 9°C is the temperature considered in the challenge tests reported in the draft PROFEL guidelines to mimic reasonably foreseeable temperature abuse in household refrigerators; 12°C is the temperature at the consumer phase considered in the EURL‐Lm guidelines to conduct shelf‐life studies (EURL‐L. monocytogenes and ANSES, 2019b) when the percentile 95% of the actual distribution of the temperatures at consumer level is not known.
Based on Kataoka et al. (2017) including the thawing/lag time.
Based on Kataoka et al. (2017), without including the thawing/lag time.
Based on the challenge tests described in the draft PROFEL guidelines, considering following situations: (1) batch 1 with high‐volume loading of the refrigerator simulating defrosting in catering or business to business refrigerator scenario (331 litre refrigerator holding in total 35–55 of 200 g packs of frozen vegetables) and using a four‐strain cocktail of L. monocytogenes (i.e. including the L. monocytogenes MLST 6 multi‐country outbreak strain); (2) batches 2 and 3 with low volume loading of the refrigerator simulating defrosting in household refrigerator scenario (331 litre refrigerator holding in total 7–11 of 200 g packs of frozen vegetables) and using the same four‐strain cocktail of L. monocytogenes, (3) as batch 3 but using a three‐strain cocktail of L. monocytogenes (i.e. excluding the L. monocytogenes MLST 6 multi‐country outbreak strain).
The occurrence of relatively low levels of L. monocytogenes at the end of the production process, from 31 to 59 CFU/g in frozen vegetables, would be compatible with the limit of 100 CFU/g at the moment of consumption without cooking as long as any labelling recommendations given are strictly followed (i.e. 24 h at 5°C). However, considering reasonably foreseeable conditions of use by the consumers beyond the labelling instructions (i.e. 48 h at 12°C), L. monocytogenes levels need to be considerably lower at the end of processing (e.g. absence in 25 g) (Table 14). The examples given are based on the growth of L. monocytogenes in corn and green peas, which have been recognised as among the vegetables most favourable for pathogen growth. In the challenge tests described in the draft PROFEL guidelines, the growth potential of L. monocytogenes was assessed also for parsnip, sweet potatoes and white cabbage (similarly as done for corn and green peas in Table 14). After 24 h of storage at 9°C, and depending on the batch and loading of the refrigerator, end‐product L. monocytogenes concentrations (CFU/g) compatible with 100 CFU/g at the moment of consumption without cooking, ranged from 14 to 74 CFU/g, 13 to 59 CFU/g and 26 to 36 CFU/g for parsnip, sweet potatoes and white cabbage, respectively. The corresponding concentrations when storage was prolonged to 48 h were 2–11 CFU/g, 2–8 CFU/g and 2–1631 CFU/g.
The calculated L. monocytogenes concentrations constitute the basis to establish targets to be met by the FBOp. In this context, to verify that the targets are met, FSC need to be defined in terms of sampling plans, taking into account aspects such as the expected heterogeneity of the contamination (standard deviation of L. monocytogenes counts in samples from a lot), the specificity and the sensitivity of the analytical methodology used to quantify the hazard (e.g. concentrations < 10 CFU/g need to be detected), and the statistical confidence required for acceptance or rejection of non‐compliant lots (Van Schothorst et al., 2009). The establishment of such FSC as intermediate levels applicable at the end of production process is a risk management decision. The impact of possible FSC on public health and/or on product compliance would be useful information to support risks managers decisions in this respect (EFSA BIOHAZ Panel, 2017a). However, this assessment was out of the scope of the present mandate.
3.5.5. Uncertainty related to recommendations on routine monitoring
Potential sources of uncertainty identified refer to whether, in addition to the recommended routine monitoring practices to be applied during the production process of bfV, there are other recommendations that have been omitted. The impact of this uncertainty in the conclusions is expected to be low (0–5%) as it is believed that the most relevant recommendations when establishing a routine EM program have been identified. Additionally, the impact of the uncertainty linked to the misclassification of some recommendations has been identified as low (0–5%), because it is believed that there is a low (0–5%) probability that there are other relevant recommendations apart from those already considered.
The uncertainties associated with the L. monocytogenes concentration at the end of the production process that would be compatible with 100 CFU/g at the moment of consumption without cooking, for different times and temperatures of storage once the frozen vegetable is removed from the freezer rely on predictive models and the growth potential data used to made the calculations. Despite evidence being available only for vegetables identified a priori as the most favourable for L. monocytogenes growth, and cocktails of L. monocytogenes strains were used for spiking in the growth experiments, it is uncertain whether other products or other L. monocytogenes strains could lead to a faster growth rate. An additional uncertainty refers to the thawing conditions, i.e. actual product t/T profile, of the frozen vegetables, which is highly dependent on the consumer practices in relation to for instance the size and portion of the vegetables being thawed and the external temperature.
3.5.6. Concluding remarks
-
•
Monitoring for L. monocytogenes includes both monitoring the FPE and the final product. End‐product sampling is currently being used as a mechanism for assessing the safety of processed foods before they are released onto the market. It provides information limited to the product tested and although it is not informative for the safety of the whole batch produced it provides relevant information for trend analysis. A validated and effective process EM program is the most beneficial strategy for minimising the likelihood of final product contamination.
-
•
EM is a core activity in the frozen vegetable industry and should be carefully planned. Sources of cross‐contamination should be traced and carefully sanitised.
-
•
Well‐established routine EM programmes should be designed using a risk‐based approach, considering the nature and size of the food operation and reflecting aspects related to the raw materials, the production processes and the final product application but they also need to be regularly revised based on trend analysis.
-
•
The current approach to designing an EM relies mostly on zoning (standard division of surfaces in a facility with respect to the proximity and contact with foods) and sanitary design (whether a surface is cleanable or not, or how well it could be cleaned). Mathematical models have started to be used to evaluate the performance of effective EM programmes.
-
•
To establish a routine monitoring program, the FBOp should consider the following criteria:
-
o
Identification of the sampling points;
-
o
The target microorganisms;
-
o
Sample size;
-
o
Frequency of testing; and
-
o
Selection of sampling, detection and quantification methods.
-
o
-
•
It is not possible to give specific advice regarding the sampling sites that should be selected as well as the number of samples and frequency of sampling because these must be chosen on a case‐by‐case basis. Trend analysis is critical to determine the number of samples of each type of zone, how often and when because it provides evidence of the harbourage places for L. monocytogenes.
-
•
Sampling, detection and enumeration methods should follow validated methods (e.g. the international standard EN ISO 18593:201821; EN ISO 11290‐1:20176; EN ISO 11290‐2‐20177).
-
•
Subtyping L. monocytogenes isolates by a molecular method is necessary to establish whether the isolates belong to a persistent clone.
-
•
The occurrence of relatively low levels of L. monocytogenes at the end of the production process, e.g. < 10 CFU/g (detection limit of the quantification method), would be compatible with the limit of 100 CFU/g at the moment of consumption as long as any labelling recommendations given are strictly followed (i.e. 24 h at 5°C). However, considering reasonably foreseeable conditions of use by the consumers beyond the labelling instructions (i.e. 48 h at 12°C), L. monocytogenes levels need to be considerably lower, even below the detection sensitivity of the current available standard analytical procedure/methods (not detected in 25 g) for those vegetables that best support pathogen growth.
-
•
Microbiological criteria, set by the risk manager, can be used as a tool to verify that the threshold of the L. monocytogenes concentration in bfV at the end of production (compatible with the limit) is not exceeded. Sampling plans should be designed to take into consideration the expected heterogeneity of the contamination, the specificity and the sensitivity of the analytical methodology as well as the statistical confidence required for acceptance or rejection of non‐compliant lots.
-
•
The impact of possible FSC on public health and/or on product compliance would be useful information to support risks managers’ decisions in this respect.
4. Conclusions
ToR 1: to provide an estimation of the public health impact of L. monocytogenes contamination, and if considered relevant of other pathogens of frozen fruit, vegetables and herbs blanched before freezing. For this purpose, EFSA should make a semi‐quantitative estimation of the risk posed by the ‘ Listeria /pathogen – frozen fruit, vegetables and herbs’ combination by comparing such risk with better known risks
AQ 1: Which are the pathogen(s) (in addition to L. monocytogenes) in bfV of relevance for public health/illness in the EU?
Based on the number of human cases involved in the FBOs in the EU (2005–2018) and the main identified contributory factors, L. monocytogenes is the most relevant pathogen in bfV for public health/illness in the EU.
AQ 2: What is the public health risk posed by L. monocytogenes/pathogen – bfV (subgroups) in comparison with other known pathogen–food combinations using the methodology best suited based on the available data?
During 2008–2018 and at EU/EEA level, the ‘dairy’ food category was responsible for five outbreaks by L. monocytogenes involving 47 cases, while ‘fish and seafood’ and ‘meat and meat products’ caused 9 and 16 outbreaks, respectively, involving 63 and 190 cases. Cases linked to bfV were reported only in 2018 and involved 46 persons (all hospitalized) and 5 deaths.
The estimated individual risk, i.e. the probability of illness per serving for the elderly EU population of the age group 65–74 years old, is lower for bfV than for any of the RTE food subcategories evaluated, i.e. cold‐smoked fish, hot‐smoked fish, gravad fish, cooked meat, sausage, pâté and soft and semi‐soft cheese.
The probability of illness per serving is up to 3,600 times greater for bfV consumed uncooked rather than cooked. It is judged to be very unlikely (5–10%) that the risk per serving for bfV consumed uncooked is higher than for soft and semi‐soft cheese.
The estimated public health impact, i.e. the annual number of cases for elderly females in the EU, is less than two cases per year, which is, considering also the uncertainty, lower than any of the evaluated RTE food categories. The public health impact of bfV is dominated by the proportion of total servings consumed uncooked.
ToR 2: to assess the main risk factors of contamination and growth of pathogens in frozen FVH during all stages from processing (excluding at primary production) until consumption (including e.g. storage after thawing, food preparation and consumption habits)
AQ 3: What are the main factors affecting contamination and/or growth of the pathogens defined in ToR 1 in bfV during processing (i.e. until the product is released to retail/packaged)?
-
•
The main factors that may impact the contamination and/or growth of the L. monocytogenes in bfV during processing are:
-
o
the hygiene status of the incoming raw materials;
-
o
the hygienic conditions of the FPE, including FCSs and non‐FCSs;
-
o
the microbiological quality of the process water;
-
o
The t/T combinations used for storage, washing, blanching, cooling and freezing.
-
o
-
•
Blanching (if enough time and temperature are applied in the process) and water disinfection applied to maintain the microbiological quality of process water are factors that can reduce the contamination by L. monocytogenes of bfV at processing level.
-
•
Blanching of vegetables may result in a product in which L. monocytogenes can grow faster during subsequent storage after thawing.
AQ 4: What are the main factors affecting contamination of the product and/or growth of the pathogens defined in ToR 1 in bfV after processing and until consumption (including e.g. storage after thawing, food preparation and consumption habits)?
-
•
The main factors affecting contamination and/or growth of L. monocytogenes in bfV after processing and until consumption are:
-
o
the intrinsic characteristics of the bfV (e.g. pH, aw, nutrients, presence of antimicrobial compounds, natural microbiota);
-
o
the t/T profiles during thawing and storage; and
-
o
the cooking conditions applied, including the cooking method and equipment.
-
o
-
•
L. monocytogenes is able to grow without a lag time or after a (very) short lag time, indicating that it is well adapted to the vegetable characteristics. The EGR of L. monocytogenes increases with temperature and it was notably affected by the type of vegetable studied.
ToR 3: to provide recommendations (a) on possible control options that may be implemented by FBOp during the production process of frozen FVH and assess their efficacy to reduce public health risks, and (b) on routine monitoring for L. monocytogenes in frozen FVH taking into account good hygiene practises (GHP) and procedures based on the HACCP principles
AQ 5: Which possible control options can be recommended to be implemented by FBOp during the production process of bfV, along with their efficacy to reduce contamination (prevalence and/or levels) of L. monocytogenes, within the frame of procedures based on the HACCP principles and GHP (as part of the PRPs)?
-
•
Analysis of the hazards and activities of freezing plants suggests that PRP activities are sufficient to reduce contamination and the application of a complete HACCP plan approach, including CCPs, is either not possible or would not further enhance food safety.
-
•
Most of the reported options to reduce L. monocytogenes in bfV focus on the establishment of good hygienic and sanitary production and processing practices mainly to reduce the contamination of foods and the FPE with L. monocytogenes.
-
•
The 11 PRP categories, if implemented together, are very likely (90–95%) to reduce the probability of contamination of bfV by L. monocytogenes.
-
o
PRPs which focus on the hygiene of the FPE, including cleaning and disinfection of equipment and facilities, hygienic design and equipment maintenance, are of utmost importance to reduce the probability of introduction, survival and growth of L. monocytogenes.
-
o
t/T combinations applied during washing, blanching, cooling and freezing must be controlled to prevent the potential for any surviving L. monocytogenes to grow.
-
o
-
•
Four different oPRPs are suggested as control measures and linked to seven different processing stages including:
-
o
equipment and processing environment (oPRP1: cleaning and disinfection)
-
o
processing steps where water is used (e.g. washing, cleaning, cooling etc.) (oPRP2: water control);
-
o
washing (oPRP3: t/T control);
-
o
blanching (oPRP3: t/T control);
-
o
cooling (oPRP3: t/T control);
-
o
freezing (oPRP3: t/T control); and
-
o
consumer practices (oPRP4: Product information and consumer awareness).
-
o
-
•
Additional control measures are identified with the aim of reducing or eliminating L. monocytogenes in the product or on food process surfaces (e.g. bacteriophages and phage‐derived proteins, bacteriocins, essential oils, nanotechnology agents etc.). Not all of these measures are commercially available. In addition, their efficacy is not yet fully validated in industrial settings.
AQ 6: What is the L. monocytogenes concentration in bfV at the end of the production process as PO that would be compatible with the FSO of 100 CFU/g at the moment of consumption without cooking, for different times and temperatures of storage once the frozen vegetable is removed from the freezer?
The occurrence of relatively low levels of L. monocytogenes at the end of the production process, e.g. < 10 CFU/g (detection limit of the quantification method), would be compatible with the limit of 100 CFU/g at the moment of consumption as long as any labelling recommendations given are strictly followed (i.e. 24 h at 5°C). However, considering reasonably foreseeable conditions of use by the consumers beyond the labelling instructions (i.e. 48 h at 12°C), L. monocytogenes levels need to be considerably lower, even below the detection sensitivity of the current available standard analytical procedure/methods (not detected in 25 g) for those vegetables that best support pathogen growth.
Microbiological criteria, set by the risk manager, can be used as a tool to verify that the threshold of the L. monocytogenes concentration in bfV at the end of production (compatible with the limit) is not exceeded. Sampling plans should be designed to take into consideration the expected heterogeneity of the contamination, the specificity and the sensitivity of the analytical methodology as well as the statistical confidence required for acceptance or rejection of non‐compliant lots.
The impact of possible FSC on public health and/or on product compliance would be useful information to support risks managers’ decisions in this respect.
AQ 7: What routine monitoring for L. monocytogenes in bfV processing environment and final product can be recommended to verify the correct application of GHP and/or within the frame of procedures based on the HACCP principles?
-
•
A validated and effective process EM program is the most beneficial strategy for minimising the likelihood of final product contamination. EM should be a key activity in frozen vegetable industries and should be carefully planned.
-
•
Well‐established routine EM programmes should be designed on a risk‐based approach, considering the nature and size of the food operation and reflecting aspects related to the raw materials, the production processes and the final product application, but they also need to be regularly revised based on trend analysis. To establish a routine monitoring program for FBOp, the following criteria should be considered:
-
o
the identification of the sampling points;
-
o
the target microorganisms;
-
o
the sample size;
-
o
the frequency of testing; and
-
o
the selection of sampling, detection and quantification methods.
-
o
-
•
It is not possible to give specific advice regarding the sampling sites that should be selected or the number of samples and frequency of sampling because these must be chosen on a case‐by‐case basis.
-
•
Sampling, detection and enumeration methods should follow validated methods (e.g. the international standard EN ISO 18593:201821; EN ISO 11290‐1:20176; EN ISO 11290‐2‐20177).
-
•
Subtyping of L. monocytogenes isolates by molecular methods (such as WGS) is necessary to establish whether the isolates belong to a persistent clone.
5. Recommendations
To educate consumers on hygienic practices such as the storage of frozen or thawed vegetables in a clean freezer or refrigerator (at the appropriate temperature) and on how to prepare (thawing and cooking) the purchased food in a proper and safe manner.
To standardise the labelling used by the industry to promote better understanding by consumers.
To raise the awareness of the public health risks associated with the consumption of uncooked bfV, particularly to susceptible population groups.
To apply harmonised terminology in the categorisation of foods, even though collected for different reasons, e.g. monitoring, surveillance, outbreak investigation and consumption. In addition, to assist future MRAs, consideration should be given to the collection of additional information on how food has been prepared, processed and stored as part of this data collection.
To perform subtyping of L. monocytogenes isolates detected during the routine monitoring program in FPE by molecular methods (e.g. WGS).
To improve collection and reporting of data on human listeriosis, including underlying conditions (e.g. pregnancy, different types of cancer, immunodeficiency, renal or liver failure).
Documentation provided to EFSA
Hygiene Guidelines For The Production Of Quick‐Frozen Fruits, Vegetables And Herbs In Control Of Listeria monocytogenes. Draft as shared for consultation. 24 October 2019. Submitted by the European Association of Fruit and Vegetable Processors (PROFEL).
Glossary
- Fruit, berries and juices and other products thereof
Fruit is defined as all fruit. Tomatoes are not regarded as fruit (Council Directive 2001/112/EC). Fruit purée is defined as the fermentable but unfermented product obtained by sieving the edible part of whole or peeled fruit without removing the juice (Council Directive 2001/112/EC). Fruit juice is defined as: The fermentable but unfermented product obtained from fruit which is sound and ripe, fresh or preserved by chilling, of one or more kinds mixed together, having the characteristic colour, flavour and taste typical of the juice of the fruit from which it comes. Flavour, pulp and cells from the juice which are separated during processing may be restored to the same juice. In the case of citrus fruits, the fruit juice must come from the endocarp. Lime juice, however, may be obtained from the whole fruit, by suitable production processes whereby the proportion of constituents of the outer part of the fruit is reduced to a minimum. The product obtained from concentrated fruit juice by: – replacing, in the concentrated fruit juice, water extracted from that juice during concentration, restoring the flavours and, if appropriate, pulp and cells lost from the juice but recovered during the process of producing the fruit juice in question or of fruit juice of the same kind. The water added must display appropriate characteristics, particularly from the chemical, microbiological and organoleptic viewpoints, in such a way as to guarantee the essential qualities of the juice. The product thus obtained must display organoleptic and analytical characteristics at least equivalent to those of an average type of juice obtained from fruits of the same kind within the meaning of (a) (Council Directive 2001/112/EC). Concentrated fruit juice is the product obtained from fruit juice of one or more kinds by the physical removal of a specific proportion of the water content. Where the product is intended for direct consumption that removal will be of at least 50% (Council Directive 2001/112/EC). Fruit nectar is the fermentable but unfermented product obtained by adding water and sugars and/or honey to the products to fruit purée or to a mixture of those products, that, moreover, meet the requirements of Annex IV (Council Directive 2001/112/EC). (outbreaks data model of EFSA's zoonoses database)
- Herbs and spices
Herbs are the aromatic leaves of plants without woody stems that grow in temperate zones. Spices are seasonings obtained from the bark, buds, fruit or flower parts, roots, seeds or stems of various aromatic plants and trees. Herbs and spices are usually derived from botanical sources, which may be dehydrated and are either ground or whole. Examples of herbs include basil, oregano and thyme. Examples of spices include cumin and caraway seeds. Spices may also be found as blends in powder or paste form. Examples of spice blends include chilli seasoning, chilli paste, curry paste, curry roux and dry cures or rubs that are applied to external surfaces of meat or fish (outbreaks data model of EFSA's zoonoses database)
- Vegetables and juices and other products thereof
Vegetables are plants or parts of plants cultivated for food. Some foods that are botanically fruits, such as tomatoes and cucumbers, and seeds, such as peas and beans, are included with the vegetables; some plants, such as rhubarb, are classed as fruit, although they are not botanically fruits. The distinction in popular usage depends on whether they are eaten as savoury (vegetables) or sweet (fruit) dishes. Examples of vegetables include cauliflower, broccoli, pea, cucumber, lentil, avocado and garlic. ‘Sea vegetables’ like sea lettuce and seaweed are also part of this group. Vegetable juice is the juice obtained from vegetables and usually made from carrots, beets, pumpkin or tomatoes. Please specify the plant species or cultivar group as well as the treatment (e.g. raw, cooked juice) in the free text data element (for example: ‘raw iceberg lettuce’) (outbreaks data model of EFSA's zoonoses database)
- Food Safety Management (or control) system (FSMS)
The combination of PRPs as preventive control measures; traceability, recall and communication as preparedness and HACCP plan defining CCPs and/or oPRPs as control measures linked to the production process. The FSMS is also the combination of control measures and assurance activities. The latter aims at providing evidence that control measures are working properly such as validation and verification, documentation and record keeping (definition proposed by the Commission Notice 2016/C27820)
- PRPs or prerequisite programmes
preventive practices and conditions needed prior to and during the implementation of HACCP and which are essential for food safety. The PRPs needed depend on the segment of the food chain in which the sector operates and the type of sector (definition proposed by the Commission Notice 2016/C27820)
- CCP or critical control point
a step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level (e.g. pasteurisation) (definition proposed by the Commission Notice 2016/C27820)
- oPRPs or operational prerequisite programmes (= CPs or control points)
PRPs that are typically linked to the production process and are identified by the hazard analysis as essential, in order to control the likelihood of the introduction, survival and/or proliferation of food safety hazards in the product(s) or in the processing environment (e.g. more intensive cleaning and disinfection in high care areas) (definition proposed by the Commission Notice 2016/C27820)
- Glazing
is the application of a protective layer of ice formed at the surface of a frozen product by spraying it with, or dipping it into, clean seawater, potable water, or potable water with approved additives, as appropriate. The method of glazing preserves the structure of the vegetable during processing and subsequent handling and reduces fines loss
- Crystallisation
is the transformation of existing water into ice crystals. Crystallization is a spontaneous and stochastic process. Crystallization is a pre‐cooling of e.g. the spinach: on a belt it goes through the freezer tunnel for the first time before portions are formed after which the portions of spinach go through the freezer tunnel again to freeze to −18°C.
Abbreviations
- ABM
agent‐based modelling
- AFLP
amplified fragment length polymorphism
- AQ
assessment question
- bfV
blanched frozen vegetables
- BIOHAZ Panel
EFSA Panel on Biological Hazards
- B2B
business to business
- B2C
business to consumer
- BFFF
British Frozen Food Federation
- CA
competent authority
- CCP
critical control point
- CFU
colony forming unit(s)
- CI
confidence interval
- CP
control point
- CSS
critical sampling site
- D‐value
decimal reduction time or the time required at a given temperature to kill 90% of the exposed microorganisms
- DR
dose response
- ECDC
European Centre for Disease Prevention and Control
- EGR
exponential growth rate
- EM
environmental monitoring
- EnABLe
Environmental monitoring with an Agent‐Based Model of Listeria
- FBO
food‐borne outbreaks
- FBOp
food business operator
- FCS
food contact surface
- FoNAO
foods of non‐animal origin
- FPE
food processing environment
- FSC
food safety criteria
- FSMS
food safety management system
- FSO
food safety objective
- FVH
fruit, vegetables or herbs
- GHP
good hygiene practices
- GMP
good manufacturing practices
- HACCP
hazard analysis and critical control points
- IQR
interquartile range
- mgQMRA
modified generic QMRA model
- MLVA
multi‐locus variable number tandem repeat analysis
- MLST
multi‐locus sequence type
- MPD
maximum population density
- (MRA) network
Microbiological Risk Assessment network
- MRA
microbiological risk assessment
- MS
Member State
- MST
Microbial Source Tracking
- NAP
normal atmosphere packaging
- oPRP
operational prerequisite program
- PFGE
pulsed‐field gel electrophoresis
- PO
performance objective
- PROFEL
European Association of Fruit and Vegetable Processors
- PRP
prerequisite program
- QMRA
Quantitative Microbiological Risk Assessment
- RASFF
Rapid Alert System for Food and Feed
- ROP
reduced oxygen packaging
- RTDE
ready‐to‐defrost‐and‐eat
- RTE
ready‐to‐eat
- SOP
standard operating procedure
- SSOP
sanitation standard operating procedures
- STEC
Shiga toxin‐producing Escherichia coli
- TBC
Total Bacterial Count
- ToR
Terms of Reference
- WGS
whole genome sequencing
- z‐value
thermal resistance constant or the temperature required for one log10 increase or decrease in the D‐value
Appendix A – List of blanched and unblanched frozen FVH
1.
Table A.1 provides an overview of the use of the blanching process for the commercial production of frozen FVH as provided by Mrs. Nele Cattoor (General Secretary at PROFEL) by e‐mail on 23 April 2019 (Cattoor, 2019a). PROFEL consulted its members to draft this list. Some products are blanched and unblanched, depending on the customer requirements, factory or process.
Table TableA.1.
Overview of the use of the blanching process for the commercial production of frozen fruit, vegetables and herbs as provided by PROFEL
| Blanched | Unblanched | Blanched and unblanched | Not in scope | ||
|---|---|---|---|---|---|
| FRUITS, FRESH or FROZEN; TREE NUTS | |||||
| Citrus fruits | Grapefruits | X | |||
| Oranges | X | ||||
| Lemons | X | ||||
| Limes | X | ||||
| Mandarins | X | ||||
| Others (2) | |||||
| Pome fruits | Apples | X | |||
| Pears | X | ||||
| Quinces | X | ||||
| Medlars | X | ||||
| Loquats/Japanese medlars | X | ||||
| Others (2) | |||||
| Stone fruits | Apricots | X | |||
| Cherries (sweet) | X | ||||
| Peaches | X | ||||
| Plums | X | ||||
| Others (2) | |||||
| Berries and small fruits | (a) grapes | X | |||
| Table grapes | X | ||||
| Wine grapes | X | ||||
| (b) strawberries | X | ||||
| (c) cane fruits | X | ||||
| Blackberries | X | ||||
| Dewberries | X | ||||
| Raspberries (red and yellow) | X | ||||
| Others (2) | |||||
| (d) other small fruits and berries | X | ||||
| Blueberries | X | ||||
| Cranberries | X | ||||
| Currants (black, red and white) | X | ||||
| Gooseberries (green, red and yellow) | X | ||||
| Rose hips | X | ||||
| Mulberries (black and white) | X | ||||
| Azaroles/Mediterranean medlars | X | ||||
| Elderberries | X | ||||
| Others (2) | |||||
| Miscellaneous fruits with | (a) edible peel | ||||
| Dates | X | ||||
| Figs | X | ||||
| Table olives | X | ||||
| Kumquats | X | ||||
| Carambolas | X | ||||
| Kaki/Japanese persimmons | X | ||||
| Jambuls/jambolans | X | ||||
| Others (2) | |||||
| (b) inedible peel, small | X | ||||
| Kiwi fruits (green, red, yellow) | X | ||||
| Litchis/lychees | X | ||||
| Passion fruits/maracujas | X | ||||
| Prickly pears/cactus fruits | X | ||||
| Star apples/cainitos | X | ||||
| American persimmons/Virginia kaki | X | ||||
| Others (2) | |||||
| (c) inedible peel, large | X | ||||
| Avocados | X | ||||
| Bananas | X | ||||
| Mangoes | X | ||||
| Papayas | X | ||||
| Granate apples/pomegranates | X | ||||
| Cherimoyas | X | ||||
| Guavas | X | ||||
| Pineapples | X | ||||
| Breadfruits | X | ||||
| Durians | X | ||||
| Soursops/guanabanas | X | ||||
| Others (2) | |||||
| VEGETABLES, FRESH or FROZEN | |||||
| Root and tuber vegetables | (a) potatoes | Xa | |||
| (b) tropical root and tuber vegetables | |||||
| Cassava roots/manioc | ? | ||||
| Sweet potatoes | X | ||||
| Yams | X | ||||
| Arrowroots | ? | ||||
| Others (2) | ? | ||||
| (c) other root and tuber vegetables except sugar beets | |||||
| Beetroots | X | ||||
| Carrots | X | ||||
| Celeriacs/turnip rooted celeries | X | ||||
| Horse radishes | X | ||||
| Jerusalem artichokes | X | ||||
| Parsnips | X | ||||
| Parsley roots/Hamburg roots parsley | X | ||||
| Radishes | X | ||||
| Salsifies | X | ||||
| Swedes/rutabagas | X | ||||
| Turnips | X | ||||
| Others (2) | |||||
| Bulb vegetables | Garlic | X | |||
| Onions | X | ||||
| Shallots | X | ||||
| Spring onions/green onions and Welsh onions | X | ||||
| Others (2) | |||||
| Fruiting vegetables | (a) Solanaceae and Malvaceae | ||||
| Tomatoes | X | ||||
| Sweet peppers/bell peppers | X | ||||
| Chili | X | ||||
| Aubergines/eggplants | X | ||||
| Okra/lady's fingers | X | ||||
| Others (2) | |||||
| (b) cucurbits with edible peel | |||||
| Cucumbers | X | ||||
| Gherkins | X | ||||
| Courgettes | X | ||||
| Others (2) | |||||
| (c) cucurbits with inedible peel | |||||
| Melons | X | ||||
| Pumpkins | X | ||||
| Watermelons | X | ||||
| Others (2) | |||||
| (d) sweet corn | X | ||||
| (e) other fruiting vegetables | |||||
| Brassica vegetables (excluding brassica roots and brassica baby leaf crops) | (a) flowering brassica | ||||
| Broccoli | X | ||||
| Cauliflowers | X | ||||
| Others (2) | |||||
| (b) head brassica | |||||
| Brussels sprouts | X | ||||
| Head cabbages | X | ||||
| Others (2) | |||||
| (c) leafy brassica | |||||
| Chinese cabbages/pe‐tsai | Xb | ||||
| Kales | Xb | ||||
| Others (2) | |||||
| (d) kohlrabies | X | ||||
| Leaf vegetables, herbs and edible flowers | (a) lettuces and salad plants | ||||
| Lamb's lettuces/corn salads | X | ||||
| Lettuces | X | ||||
| Escaroles/broad‐leaved endives | ? | ||||
| Endives | |||||
| Cresses and other sprouts and shoots | X | X | |||
| Land cresses | X | ||||
| Roman rocket/rucola | X | ||||
| Red mustards | X | ||||
| Baby leaf crops (including brassica species) | X | ||||
| Others (2) | |||||
| (b) spinaches and similar leaves | |||||
| Spinaches | X | ||||
| Purslanes | X | ||||
| Chards/beet leaves | X | ||||
| Others (2) | |||||
| (c) grape leaves and similar species | X | ||||
| (d) watercresses | X | ||||
| (e) witloofs/Belgian endives | X | ||||
| (f) herbs and edible flowers | |||||
| Chervil | X | ||||
| Chives | X | ||||
| Celery leaves | X | ||||
| Parsley | X | ||||
| Sage | X | ||||
| Rosemary | X | ||||
| Thyme | X | ||||
| Basil and edible flowers | X | ||||
| Laurel/bay leaves | X | ||||
| Dill | X | ||||
| Tarragon | X | ||||
| Others (2) | |||||
| Legume vegetables | Beans (with pods) | X | |||
| Beans (without pods) | X | ||||
| Peas (with pods) | X | ||||
| Peas (without pods) | X | ||||
| Lentils | X | ||||
| Others (2) | |||||
| Stem vegetables | Asparagus | X | |||
| Cardoons | X | ||||
| Celeries | X | ||||
| Florence fennels | X | ||||
| Globe artichokes | X | ||||
| Leeks | X | ||||
| Rhubarbs | X | ||||
| Bamboo shoots | X | ||||
| Palm hearts | X | ||||
| Others (2) | |||||
| Fungi, mosses and lichens | Cultivated fungi | X | |||
| Wild fungi | X | ||||
| Mosses and lichens | X | ||||
| Algae and prokaryotes organisms | |||||
| PULSES | |||||
| Beans | X | ||||
| Lentils | X | ||||
| Peas | X | ||||
| Lupins/lupini beans | X | ||||
| Others (2) | |||||
| SPICES | |||||
| Seed spices | Anise/aniseed | X | |||
| Black caraway/black cumin | X | ||||
| Celery | X | ||||
| Coriander | X | ||||
| Cumin | X | ||||
| Dill | X | ||||
| Fennel | X | ||||
| Fenugreek | X | ||||
| Nutmeg | X | ||||
| Others (2) | |||||
| Fruit spices | Allspice/pimento | X | |||
| Sichuan pepper | X | ||||
| Caraway | X | ||||
| Cardamom | X | ||||
| Juniper berry | X | ||||
| Peppercorn (black, green and white) | X | ||||
| Vanilla | X | ||||
| Tamarind | X | ||||
| Others (2) | |||||
| Bark spices | Cinnamon | X | |||
| Others (2) | |||||
| Root and rhizome spices | Liquorice | X | |||
| Ginger | X | ||||
| Turmeric/curcuma | X | ||||
| Horseradish | X | ||||
| Others (2) | |||||
Sulfitised in case of unblanched.
Blanched/flash blanched.
Appendix B – Search strategies for literature searches
1.
Literature searches were conducted on 8 April 2019 in the Web of Science™ Core Collection (1975–present), CABI: CAB Abstracts® (1910–present) and PubMed. The search strategies are reported in Tables B.1 and B.2. Searches retrieved 494 (Web of Science), 330 (CABI) and 39 (PubMed) hits.
Table TableB.1.
Details of search strings used for literature searches – search strings for Web of Science and CABI
| Set number | Search |
|---|---|
| 4 | #4 AND (#3 OR #2) AND #1 |
| 4 | TOPIC: (frozen OR freezing OR thawed OR thawing OR frost* OR defrost* OR iced OR chilled OR melt*) |
| 3 | TITLE: (meal OR food OR produce OR Fruit OR Acerola OR Amla OR Apple OR Apricots OR Avocado OR Banana OR *Berry OR *Berries OR Blackcurrant OR Blackberries OR Blueberries OR Breadfruit OR Cantaloupe OR Carambola OR Cherimoya OR Cherries OR Clementine OR Coconut OR Cranberries OR Custard‐Apple OR Date Fruit OR Durian OR Elderberries OR Feijoa OR Figs OR Gooseberries OR Grapefruit OR Grapes OR Guava OR Herb OR Honeydew Melon OR Jackfruit OR Java‐Plum OR Jujube Fruit OR Kiwifruit OR Kiwi OR Kumquat OR Lemon OR Lime OR Longan OR Loquat OR Lychee OR Mandarin OR Mango OR Mangosteen OR Mulberries OR Nectarine OR Olives OR Orange* OR Papaya OR Passion Fruit OR Peaches OR Pear OR Persimmon OR Caki* OR Pineapple OR Pitanga OR Plantain OR Plums OR Pomegranate OR Prickly Pear OR Prunes OR Pummelo OR Pomelo OR Quince OR Raspberries OR Rhubarb OR Rose‐Apple OR Sapodilla OR Sapote Mamey OR Soursop OR Strawberries OR Sugar‐Apple OR Tamarind OR Tangerine OR Watermelon OR Vegetable OR Amaranth Leave* OR Arrowroot OR Artichoke OR Arugula OR Asparagus OR Bamboo Shoots OR Green Beans OR Beets OR Belgian Endive OR Bitter Melon* OR Bok Choy OR Broad Beans OR Fava Beans OR Broccoli OR Broccoli Rabe OR Brussel Sprouts OR Sprout* Cabbage Red OR Carrot OR Cassava OR Cauliflower OR Celeriac OR Celery OR Chayote* OR Chicory OR Collards OR Corn OR Crookneck OR Cucumber OR Daikon OR Dandelion Greens OR Edamame OR Soybeans OR Eggplant OR Fennel OR Fiddleheads OR Ginger Root OR Horseradish OR Jicama OR Kale OR Kohlrabi OR Leeks OR Legume OR Romaine Lettuce OR Mushrooms OR Mustard Green* OR Okra OR Onion* OR Parsnip OR Green Peas OR Pepper OR Potato OR Pumpkin OR Radicchio OR Radishes OR Rutabaga OR Salsify OR Oysterplant OR Shallots OR Snow Peas OR Sorrel OR Dock OR Spaghetti Squash OR Spinach OR Spinacia OR Butternut OR Sweet Potato OR Swiss Chard OR Tomatillo OR Tomato* OR Turnip OR Watercress OR Yam Root OR Zucchini OR Herbs OR Basil OR Bay Laurel OR Borage OR Caraway OR Catnip OR Chervil OR Chives OR Cilantro OR Dill OR Epazote OR Fennel OR Garlic OR Lavender OR Lemon Grass OR Lemon Balm OR Lemon Verbena OR Lovage OR Marjoram OR Mints OR Nasturtium OR Parsley OR Oregano OR Rosemary OR Sage OR Salad Burnet OR Savory OR Scented Geranium OR Sorrel OR Spice OR Tarragon OR Thyme)DocType=All document typesLanguage=All languagesTimespan=All years |
| 2 | TOPIC: (Fruit OR Acerola OR Amla OR Apple OR Apricots OR Avocado OR Banana OR *Berry OR *Berries OR Blackcurrant OR Blackberries OR Blueberries OR Breadfruit OR Cantaloupe OR Carambola OR Cherimoya OR Cherries OR Clementine OR Coconut OR Cranberries OR Custard‐Apple OR Date Fruit OR Durian OR Elderberries OR Feijoa OR Figs OR Gooseberries OR Grapefruit OR Grapes OR Guava OR Herb OR Honeydew Melon OR Jackfruit OR Java‐Plum OR Jujube Fruit OR Kiwifruit OR Kiwi OR Kumquat OR Lemon OR Lime OR Longan OR Loquat OR Lychee OR Mandarin OR Mango OR Mangosteen OR Mulberries OR Nectarine OR Olives OR Orange* OR Papaya OR Passion Fruit OR Peaches OR Pear OR Persimmon OR Caki* OR Pineapple OR Pitanga OR Plantain OR Plums OR Pomegranate OR Prickly Pear OR Prunes OR Pummelo OR Pomelo OR Quince OR Raspberries OR Rhubarb OR Rose‐Apple OR Sapodilla OR Sapote Mamey OR Soursop OR Strawberries OR Sugar‐Apple OR Tamarind OR Tangerine OR Watermelon OR Vegetable OR Amaranth Leave* OR Arrowroot OR Artichoke OR Arugula OR Asparagus OR Bamboo Shoots OR Green Beans OR Beets OR Belgian Endive OR Bitter Melon* OR Bok Choy OR Broad Beans OR Fava Beans OR Broccoli OR Broccoli Rabe OR Brussel Sprouts OR Sprout* Cabbage Red OR Carrot OR Cassava OR Cauliflower OR Celeriac OR Celery OR Chayote* OR Chicory OR Collards OR Corn OR Crookneck OR Cucumber OR Daikon OR Dandelion Greens OR Edamame OR Soybeans OR Eggplant OR Fennel OR Fiddleheads OR Ginger Root OR Horseradish OR Jicama OR Kale OR Kohlrabi OR Leeks OR Legume OR Romaine Lettuce OR Mushrooms OR Mustard Green* OR Okra OR Onion* OR Parsnip OR Green Peas OR Pepper OR Potato OR Pumpkin OR Radicchio OR Radishes OR Rutabaga OR Salsify OR Oysterplant OR Shallots OR Snow Peas OR Sorrel OR Dock OR Spaghetti Squash OR Spinach OR Spinacia OR Butternut OR Sweet Potato OR Swiss Chard OR Tomatillo OR Tomato* OR Turnip OR Watercress OR Yam Root OR Zucchini OR Herbs OR Basil OR Bay Laurel OR Borage OR Caraway OR Catnip OR Chervil OR Chives OR Cilantro OR Dill OR Epazote OR Fennel OR Garlic OR Lavender OR Lemon Grass OR Lemon Balm OR Lemon Verbena OR Lovage OR Marjoram OR Mints OR Nasturtium OR Parsley OR Oregano OR Rosemary OR Sage OR Salad Burnet OR Savory OR Scented Geranium OR Sorrel OR Spice OR Tarragon OR Thyme)DocType=All document typesLanguage=All languagesTimespan=All years |
| 1 | TOPIC: (Listeria OR L.monocytogenes OR monocytogenes)DocType=All document typesLanguage=All languagesTimespan=All years |
Table TableB.2.
Details of search strings used for literature searches – search strings for PubMed
| Set number | Search |
|---|---|
| 4 | #3 AND #2 AND #1 |
| 3 | (((((frozen[Title/Abstract] OR freezing[Title/Abstract] OR thawed[Title/Abstract] OR thawing[Title/Abstract] OR frost*[Title/Abstract] OR defrost*[Title/Abstract] OR iced[Title/Abstract] OR chilled[Title/Abstract] OR melt*[Title/Abstract])) OR food, frozen[MeSH Terms]) OR foods, frozen[MeSH Terms]) OR frozen food[MeSH Terms]) OR frozen foods[MeSH Terms]DocType=All document typesLanguage=All languagesTimespan=All years |
| 2 | (((((Fruit[Title/Abstract] OR Acerola[Title/Abstract] OR Amla[Title/Abstract] OR Apple[Title/Abstract] OR Apricots[Title/Abstract] OR Avocado[Title/Abstract] OR Banana[Title/Abstract] OR *Berry[Title/Abstract] OR *Berries[Title/Abstract] OR Blackcurrant[Title/Abstract] OR Blackberries[Title/Abstract] OR Blueberries[Title/Abstract] OR Breadfruit[Title/Abstract] OR Cantaloupe[Title/Abstract] OR Carambola[Title/Abstract] OR Cherimoya[Title/Abstract] OR Cherries[Title/Abstract] OR Clementine[Title/Abstract] OR Coconut[Title/Abstract] OR Cranberries[Title/Abstract] OR Custard‐Apple[Title/Abstract] OR Date Fruit[Title/Abstract] OR Durian[Title/Abstract] OR Elderberries[Title/Abstract] OR Feijoa[Title/Abstract] OR Figs[Title/Abstract] OR Gooseberries[Title/Abstract] OR Grapefruit[Title/Abstract] OR Grapes[Title/Abstract] OR Guava[Title/Abstract] OR Herb[Title/Abstract] OR Honeydew Melon[Title/Abstract] OR Jackfruit[Title/Abstract] OR Java‐Plum[Title/Abstract] OR Jujube Fruit[Title/Abstract] OR Kiwifruit[Title/Abstract] OR Kiwi[Title/Abstract] OR Kumquat[Title/Abstract] OR Lemon[Title/Abstract] OR Lime[Title/Abstract] OR Longan[Title/Abstract] OR Loquat[Title/Abstract] OR Lychee[Title/Abstract] OR Mandarin[Title/Abstract] OR Mango[Title/Abstract] OR Mangosteen[Title/Abstract] OR Mulberries[Title/Abstract] OR Nectarine[Title/Abstract] OR Olives[Title/Abstract] OR Orange*[Title/Abstract] OR Papaya[Title/Abstract] OR Passion Fruit[Title/Abstract] OR Peaches[Title/Abstract] OR Pear[Title/Abstract] OR Persimmon[Title/Abstract] OR Caki*[Title/Abstract] OR Pineapple[Title/Abstract] OR Pitanga[Title/Abstract] OR Plantain[Title/Abstract] OR Plums[Title/Abstract] OR Pomegranate[Title/Abstract] OR Prickly Pear[Title/Abstract] OR Prunes[Title/Abstract] OR Pummelo[Title/Abstract] OR Pomelo[Title/Abstract] OR Quince[Title/Abstract] OR Raspberries[Title/Abstract] OR Rhubarb[Title/Abstract] OR Rose‐Apple[Title/Abstract] OR Sapodilla[Title/Abstract] OR Sapote Mamey[Title/Abstract] OR Soursop[Title/Abstract] OR Strawberries[Title/Abstract] OR Sugar‐Apple[Title/Abstract] OR Tamarind[Title/Abstract] OR Tangerine[Title/Abstract] OR Watermelon[Title/Abstract] OR Vegetable[Title/Abstract] OR Amaranth Leave*[Title/Abstract] OR Arrowroot[Title/Abstract] OR Artichoke[Title/Abstract] OR Arugula[Title/Abstract] OR Asparagus[Title/Abstract] OR Bamboo Shoots[Title/Abstract] OR Green Beans[Title/Abstract] OR Beets[Title/Abstract] OR Belgian Endive[Title/Abstract] OR Bitter Melon*[Title/Abstract] OR Bok Choy[Title/Abstract] OR Broad Beans[Title/Abstract] OR Fava Beans[Title/Abstract] OR Broccoli[Title/Abstract] OR Broccoli Rabe[Title/Abstract] OR Brussel Sprouts[Title/Abstract] OR Sprout* Cabbage Red[Title/Abstract] OR Carrot[Title/Abstract] OR Cassava[Title/Abstract] OR Cauliflower[Title/Abstract] OR Celeriac[Title/Abstract] OR Celery[Title/Abstract] OR Chayote*[Title/Abstract] OR Chicory[Title/Abstract] OR Collards[Title/Abstract] OR Corn[Title/Abstract] OR Crookneck[Title/Abstract] OR Cucumber[Title/Abstract] OR Daikon[Title/Abstract] OR Dandelion Greens[Title/Abstract] OR Edamame[Title/Abstract] OR Soybeans[Title/Abstract] OR Eggplant[Title/Abstract] OR Fennel[Title/Abstract] OR Fiddleheads[Title/Abstract] OR Ginger Root[Title/Abstract] OR Horseradish[Title/Abstract] OR Jicama[Title/Abstract] OR Kale[Title/Abstract] OR Kohlrabi[Title/Abstract] OR Leeks[Title/Abstract] OR Legume[Title/Abstract] OR Romaine Lettuce[Title/Abstract] OR Mushrooms[Title/Abstract] OR Mustard Green*[Title/Abstract] OR Okra[Title/Abstract] OR Onion*[Title/Abstract] OR Parsnip[Title/Abstract] OR Green Peas[Title/Abstract] OR Pepper[Title/Abstract] OR Potato[Title/Abstract] OR Pumpkin[Title/Abstract] OR Radicchio[Title/Abstract] OR Radishes[Title/Abstract] OR Rutabaga[Title/Abstract] OR Salsify[Title/Abstract] OR Oysterplant[Title/Abstract] OR Shallots[Title/Abstract] OR Snow Peas[Title/Abstract] OR Sorrel[Title/Abstract] OR Dock[Title/Abstract] OR Spaghetti Squash[Title/Abstract] OR Spinach[Title/Abstract] OR Spinacia[Title/Abstract] OR Butternut[Title/Abstract] OR Sweet Potato[Title/Abstract] OR Swiss Chard[Title/Abstract] OR Tomatillo[Title/Abstract] OR Tomato*[Title/Abstract] OR Turnip[Title/Abstract] OR Watercress[Title/Abstract] OR Yam Root[Title/Abstract] OR Zucchini[Title/Abstract] OR Herbs[Title/Abstract] OR Basil[Title/Abstract] OR Bay Laurel[Title/Abstract] OR Borage[Title/Abstract] OR Caraway[Title/Abstract] OR Catnip[Title/Abstract] OR Chervil[Title/Abstract] OR Chives[Title/Abstract] OR Cilantro[Title/Abstract] OR Dill[Title/Abstract] OR Epazote[Title/Abstract] OR Fennel[Title/Abstract] OR Garlic[Title/Abstract] OR Lavender[Title/Abstract] OR Lemon Grass[Title/Abstract] OR Lemon Balm[Title/Abstract] OR Lemon Verbena[Title/Abstract] OR Lovage[Title/Abstract] OR Marjoram[Title/Abstract] OR Mints[Title/Abstract] OR Nasturtium[Title/Abstract] OR Parsley[Title/Abstract] OR Oregano[Title/Abstract] OR Rosemary[Title/Abstract] OR Sage[Title/Abstract] OR Salad Burnet[Title/Abstract] OR Savory[Title/Abstract] OR Scented Geranium[Title/Abstract] OR Sorrel[Title/Abstract] OR Spice[Title/Abstract] OR Tarragon[Title/Abstract] OR Thyme)[Title/Abstract])) OR (meal[Title] OR food[Title] OR Produce[Title])) OR vegetables[MeSH Terms]) OR vegetable[MeSH Terms]DocType=All document typesLanguage=All languagesTimespan=All years |
| 1 | (((Listeria[Title/Abstract] OR L.monocytogenes[Title/Abstract] OR monocytogenes)[Title/Abstract])) OR listeria[MeSH Terms]DocType=All document typesLanguage=All languagesTimespan=All years |
Three EndNote X7 files were created, containing the outputs from the three searches, including all indexed fields per hit (e.g. title, authors, abstract). Files were combined and duplicate records removed yielding 624 records after de‐duplication. Records in Indonesia, Japanese, Korean and Thai were removed and these < 1970 leaving 594 records.
Appendix C – Additional information related to the modified Listeria monocytogenes generic QMRA (mgQMRA) model
1. Model code and number of simulations and iterations
The R‐code of the mgQMRA model and the inputs (in Excel files containing tables) have been made available through the Knowledge Junction under https://doi.org/10.5281/zenodo.3725608.
The predicted mean probability of illness per serving was slow to converge during simulations since the level of predicted risk is very low making the outcomes very sensitive to iterations reflecting rare events, i.e. random sampling from low probability tails (high‐end) of the distributions. Thus, it was important to run a sufficient number of iterations. As limitations of the software restricted the number of iterations and to obtain consistent ranking of food categories, multiple simulations were run (250 simulations) each involving 2 × 105 iterations (Figure C.1). Ranking of probability of illness per serving was based on the mean probability of illness per serving (cf. Pérez‐Rodríguez et al. (2017)).
2. Uncertainty assessment
The identified assumptions and other sources of uncertainty of the mgQMRA output are listed in Table C.1.
3. Sensitivity and scenario analyses of the uncertainty scenarios – variable parameters
Sensitivity analyses of the variable parameters in the separate uncertainty scenarios show that the r‐values and initial concentration, and for cooked vegetables, also log reduction are the inputs that are most correlated (Spearman rank correlation) to the output mean probability of illness per serving (Figures C.2 and C.3 lower part). The correlation with the other parameters, i.e. those affecting growth is weak.
Figure C.2.
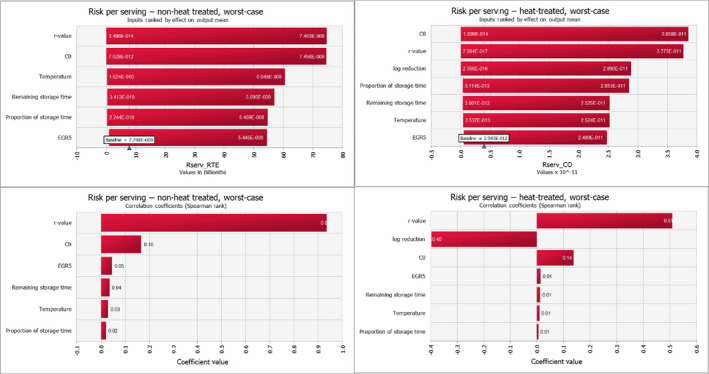
Sensitivity analysis of worst‐case output probability of illness per serving for non‐cooked (left) and cooked blanched frozen vegetables (right) in the worst‐case scenario
- The variable inputs are ranked based on the effect on the mean probability of illness per serving (upper) and correlation (lower). The baseline indicated by the blue arrow in the upper graphs is the mean probability of illness per serving and the numbers in the bars are the extreme estimates of the mean for the minimum and maximum bins of that input data.
Figure C.3.
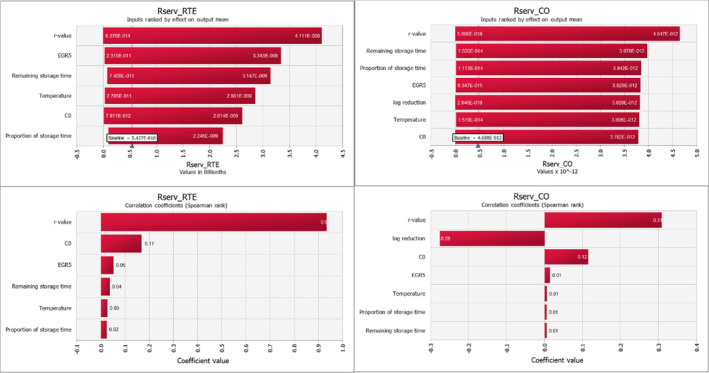
Sensitivity analysis of baseline scenario output probability of illness per serving for non‐cooked (upper) and cooked blanched frozen vegetables (lower) in the worst‐case scenario
- The variable inputs are ranked based on the effect on the mean probability of illness per serving (upper) and correlation (lower). The baseline indicated by the blue arrow in the upper graphs is the mean probability of illness per serving and the numbers in the bars are the extreme estimates of the mean for the minimum and maximum bins of that input data.
Inputs were also ranked based on their impact on simulated mean probability of illness per serving when the range, from minimum to maximum, of input values were divided into 10 equal sized bins (graphs in upper part of the figures). In the figure, the baseline indicated by the blue triangle is the mean probability of illness per serving and the numbers in the bars are the estimates of the mean for the minimum and maximum bins of that input data. In comparison with ranking based on correlation, the effect of the variable input parameters on the output mean probability of illness per serving are more variable. The parameter having the greatest effect on the mean probability of serving is the r‐value in the DR model (Figures C.2 and C.3). The relative effect of the remaining parameters related to initial concentration, log reduction during cooking (cooked vegetables) and growth through the time for growth (storage time and proportion of that used), temperature and growth rate (EGR5°C) vary between the vegetables subcategories and uncertainty scenarios (Figures C.2 and C.3, upper part).
The interpretation of these results is that the effects of growth on mean probability of illness per serving are only important in cases when a high r‐value, inefficient cooking (low log reduction), and to a lesser extent a high initial concentration, are at hand. Thus, the sensitivity to the inputs indicate the importance of the susceptibility of the consumer and the virulence of the L. monocytogenes strains as reflected in the variability of the r‐value, of log reduction during cooking and to a lesser degree to the initial concentration on the mean probability per serving. The risk per serving is largely determined by the upper percentile range when these conditions are at hand, and then the magnitude of risk is more equally affected by the other variable parameters as reflected in the effect on the mean probability per serving. In comparison, Zoellner et al. (2019) concluded that the initial concentration of L. monocytogenes in the lot, the temperature at which the product is thawed/stored and whether a serving is cooked are main predictors for illness from a lot.
A scenario analysis of the worst‐case scenario for risk per serving > 99 percentiles indicates that the only significant inputs are the initial concentration and the r‐value for non‐cooked frozen vegetable (C0 > 1.4 log10 CFU/g, log r > –11.2) and in addition for cooked vegetables log reduction (C0 > 0.5 log10 CFU/g, log10 r > −10.8, log10 reduction < 2.1). The corresponding targets for the baseline scenario are for non‐cooked vegetables (C0 > 0.6 log10 CFU/g, log r > −10.8) and for cooked (C0 > −0.7 log10 CFU/g, log10 r > −10.7, log10 reduction < 2.8).
4. Sensitivity analysis of uncertain parameters
The sensitivity analysis of the uncertain parameters initial concentration (C0), MPD, serving size, log reduction and prevalence associated with the baseline and worst‐case scenarios as described in Table 2 was run in the @risk model (Table C.2). The impact of the uncertain parameters serving size and prevalence is as expected direct and of the same magnitude as the ratio between parameters in the baseline and worst‐case scenario. The impact of the MPD and C0 was a factor of 2.5 and 1.4, respectively, according to simulations. Thus, the sensitivity analysis indicates that the uncertainty associated with the MPD has the largest impact on the predicted probability of illness per serving, followed by serving size, C0 and prevalence.
Appendix D – Supporting information on the food‐borne outbreaks in the EU/EEA where frozen vegetables were implicated
1.
Appendix E – Additional information related to additional methods for control of L. monocytogenes
1.
For L. monocytogenes reduction on the product (not limiting list):
Gamma irradiation has attracted attention as a potential non‐thermal decontamination strategy to ensure the safety of fresh fruits and vegetables. Several studies have demonstrated the high efficiency of this technology applied on different vegetables to control pathogens, but vegetables tolerance to radiation can vary, and irradiation temperature may have an impact, so process validation is required. At −20°C, radiation doses sufficient to achieve a 5‐log10 reduction (3.9–4.6 kGy) caused significant softening of peas and broccoli stems but not of corn or lima beans (Niemira et al., 2002; Finten et al., 2017). The consumer perception of foods treated with irradiation may be negative. Frozen vegetables are not on the list of authorisation of food and food ingredients granted by MS that can be treated with ionising radiation, vegetables are on the list in the UK (2001/C 241/03/EC).32
The use of bacteriophages, bacteriocin‐producing cultures or bacteriocins as ‘biopreservatives’ against L. monocytogenes to replace chemical preservatives has received much attention (Oliveira et al., 2014; Skariyachan and Govindarajan, 2019; Truchado et al., 2020). Bacteriophages are bacterial viruses that infect and kill bacteria cells with high specificity (Brovko et al., 2012). However, targeted bacterial populations may not be eradicated by phages or bacteriocin‐producing cultures, and viable L. monocytogenes cells could still be recovered, albeit in much lower numbers (EFSA BIOHAZ Panel, 2016). There could be several possible explanations for this: (i) L. monocytogenes cells could be exhibiting resistance to phage infection or bacteriocins, (ii) phages resistant mutants may have developed, (iii) the phages did not come into direct contact with some L. monocytogenes cells after the phages or the cultures were sprayed onto the foods, which resulted in those bacterial cells not being lysed. In this latter scenario, using larger spray volumes, fine (mist‐like) sprays, rotating/tumbling foods during phage application and otherwise ensuring thorough surface coverage with phages or cultures may help enhance the effectiveness of phage biocontrol (Moye et al., 2018). Perera et al. (2015) found approximatively 2.2 log10 reduction of L. monocytogenes in frozen entrées, which contained broccoli when using a bacteriophage cocktail.
In situ results showed that an antibacterial edible coating essential oils (components) on RTE frozen pre‐cut green peppers presented a large spectrum activity against Gram‐positive and Gram‐negative bacteria (against among others Listeria innocua) (Maherani et al., 2019).
For biofilm removal on the food process surfaces (not limiting list):
Phages and phage‐derived proteins are highly effective for biofilm removal at laboratory scale on foods and FPE, though some commercial examples exist. However, more research is needed before their complete implementation as part of the standard cleaning processes in the food industry (Galie et al., 2018). Some commercially available phage‐based products were effective in removing 72 h‐old biofilms formed on stainless steel surfaces by most of the assayed strains after a 4 h treatment at 12°C. For some strains, moreover, one product showed complete removal of adhered bacteria from 48‐h‐old biofilms formed on polystyrene surfaces after 4 h of treatment at 32°C (Gutierrez et al., 2017).
Resistance of some L. monocytogenes to bacteriocins, and subsequent growth of these resistant strains, is a challenge in the use of bacteriocins. The most promising results are produced by a combination of novel biotechnologies, including a mixture of natural antagonistic bacteria and their bacteriocins, or a combination of antagonistic bacteria, bacteriocins and bacteriophages, or the utilisation of bacteriocins and bacteriophages (Jordan et al., 2018).
The difficulty in removing biofilms with conventional control methods highlights the urgent need for alternative antibacterial and antibiofilm agents. One promising approach focuses on nanotechnology agents, such as carbon‐based materials (fullerenes and carbon nanotubes), dendrimers that provide cavities for other molecules, nanocomposites, natural nanoparticles and metal‐based nanoparticles, including silver, gold, metal oxides (such as ZnO and CuO) (Galie et al., 2018).
Essential oils (or components) (e.g. oregano and thyme oils, the component carvacrol) have an important antibiofilm activity on L. monocytogenes biofilms (Galie et al., 2018). On stainless steel coupons, a 0.5% concentration of these compounds was adequate to eliminate 4‐day‐old biofilms at 7 log10 CFU per coupon (Desai et al., 2012).
Photocatalysis may help in the elimination of L. monocytogenes biofilms on stainless steel or glass surfaces (Galie et al., 2018). The use of titanium dioxide (TiO2) nanostructured photocatalysts may result in 3 log10 CFU/cm2 after 90 min irradiation by UVA (Chorianopoulos et al., 2011).
Biosurfactants (natural compounds, usually of microbial origin, such as lichenysin) can modify the hydrophobic characteristics of the bacterial surface. This alters the adhesion properties and binding capacities to any given surface (Coronel‐Leon et al., 2016; Galie et al., 2018).
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Jordan K, Sampers I, Wagner M, Da Silva Felicio MT, Georgiadis M, Messens W, Mosbach‐Schulz O and Allende A, 2020. Scientific Opinion on the public health risk posed by Listeria monocytogenes in frozen fruit and vegetables including herbs, blanched during processing. EFSA Journal 2020;18(4):6092, 102 pp. 10.2903/j.efsa.2020.6092
Requestor: European Commission
Question number: EFSA‐Q‐2018‐01006
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgements: The Panel wishes to thank Jim McLauchlin from Public Health England, Paul Ellis from Public Health Wales, the representatives of the Microbiological Risk Assessment (MRA) Network and PROFEL for providing data for this scientific output. The Panel wishes to acknowledge the hearing experts Liesbeth Jacxsens, Nigel Thorgrimsson and Mieke Uyttendaele (representing PROFEL) and Martin Wiedmann. The Panel also wishes to thank Davide Arcella to extract food consumption data.
Adopted: 19 March 2020
Amendment: On page 53 the reference and corresponding footnote to the legislation on machinery has been corrected, as Directive 98/37/EC has been repealed and replaced by Directive 2006/42/EC. To avoid confusion, the original version of the scientific opinion has been removed from the EFSA Journal, but is available on request, as is a version showing all the changes made.
Amendment: 28 Jul 2020
Notes
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, p. 1–26 as amended by Commission Regulation (EC) No 2019/229 of 7 February 2019.
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs OJ L 139, 30.4.2004, p. 1–54.
PO is defined as ‘the maximum frequency and/or concentration of a hazard in a food at a specified step in the food chain before consumption that provides or contributes to an FSO or ALOP, as applicable’.
FSO is defined as ‘the maximum frequency and/or concentration of a hazard in a food at the time of consumption that provides or contributes to the appropriate level of (health) protection (ALOP)’ (van Schothorst, 2009 Food Control 20 (2009) 967–979 https://doi.org/10.1016/j.foodcont.2008.11.005).
As stated in the background provide by the European Commission, updated figures indicate that the multi‐country outbreak caused 53 cases and 10 deaths over the period 2015–2018.
EN ISO 11290‐1:2017. Microbiology of the food chain – Horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp. – Part 1: Detection method.
EN ISO 11290‐2:2017. Microbiology of the food chain – Horizontal method for the detection and enumeration of Listeria monocytogenes and of Listeria spp. – Part 2: Enumeration method.
Excluding potatoes and tomatoes, but including quick‐frozen sweet corn.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40.
For example the last report published in 2019: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2019.5926
Commission Regulation (EU) No 16/2011 of 10 January 2011 laying down implementing measures for the Rapid alert system for food and feed. OJ L 6, 11.1.2011, p. 7–10.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
https://www.vlam.be/public/uploads/files/feiten_en_cijfers/groenten_en_fruit/Thuisverbruik_van_groenten_en_fruit_in_Belgie_in_2018_DEF.pdf and https://www.vlam.be/public/uploads/files/feiten_en_cijfers/verwerkte_groenten/thuisverbruik_verwerkte_groenten_2018.pdf
http://www.hhf-greece.gr/DafnesoftWebV2 and https://link.springer.com/article/10.1007/s10389-004-0094-6
https://profel-europe.eu/_library/_files/Final_-_Frozen_vegetables_statistics_2016_-_per_country.pdf and https://profel-europe.eu/_library/_files/Final_-_canned_vegetables_statistics_2016_-_per_country(2).pdf
Commission Notice (EC) No 2016/C 278/01 of 30 July 2016 on the implementation of food safety management systems covering prerequisite programmes (PRPs) and procedures based on the HACCP principles, including the facilitation/flexibility of the implementation in certain food businesses. OJ C 278, 30.7.2016, p. 1–56.
ISO 18593:2018. Microbiology of the food chain — Horizontal methods for surface sampling.
Information retrieved from http://www.efsa.europa.eu/sites/default/files/assets/zoocountryreport09be.pdf
Information retrieved from https://www.bvl.bund.de/EN/01_Food/_01_tasks/09_FBO_Zoonoses/FBO_zoonoses_node.html
ID codes: ELC0343; J007_14; J007_15; J007_18; J007_23; J007_32; J007_37; J007_02; J007_03; J007_04; J007_05; J007_07; J007_08; J007_09; J007_11; J007_12; J007_19; J007_25; J007_27; J007_28; J007_29; J007_33; J007_39; LisAsp_1; LisAsp_2; LisAsp_3; LisAsp_4; ELC0347; GMW_0484; GMW_0485.
EN 1672‐2:2005+A1:2009 Food processing machinery ‐ Basic concepts ‐ Part 2: Hygiene requirements 44 pp.
Directive 2006/42/EC of the European Parliament and of the Council of 17 May 2006 on machinery, and amending Directive 95/16/EC. OJ L 157, 9.6.2006, p. 24–86.
CEN – EN 1672‐2. Food processing machinery ‐ Basic concepts ‐ Part 2: Hygiene requirements.
EN ISO 14159:2002. Safety of machinery — Hygiene requirements for the design of machinery.
For batch 1 of white cabbage after 48 h of storage, an inconsistent result was found which was not considered in the range.
Information and Notices (EC) No 2001/C 241/03 on the communication from the Commission on foods and food ingredients authorised for treatment with ionising radiation. OJ C 241, 29.8.2001, p. 1–17.
References
- Adzitey F and Huda N, 2010. Listeria monocytogenes in foods: incidences and possible control measures. African Journal of Microbiology Research, 4, 2848–2855. [Google Scholar]
- AFFI (American Frozen Food Institut), no date. Freezer Management Guidelines for Listeria Control. Available online: https://drive.google.com/file/d/1Z6tsFjeqklEcq4h2h70sgplOIdBgSVqy/view (Accessed: 19 September 2019). 7 pp.
- Aguado V, Vitas AI and Garcia‐Jalon I, 2004. Characterization of Listeria monocytogenes and Listeria innocua from a vegetable processing plant by RAPD and REA. International Journal of Food Microbiology, 90, 341–347. [DOI] [PubMed] [Google Scholar]
- Alexandre EMC, Santos‐Pedro DM, Brandao TRS and Silva CLM, 2011. Influence of aqueous ozone, blanching and combined treatments on microbial load of red bell peppers, strawberries and watercress. Journal of Food Engineering, 105, 277–282. [Google Scholar]
- Anantheswaran RC and Ramaswamy HS, 2001. Bacterial destruction and enzyme inactivation during microwave heating. Handbook of Microwave Technology for Food Applications, 109, 191–213. [Google Scholar]
- van Asselt ED and Zwietering MH, 2006. A systematic approach to determine global thermal inactivation parameters for various food pathogens. International Journal of Food Microbiology, 107, 73–82. [DOI] [PubMed] [Google Scholar]
- Banach JL, Sampers I, Van Haute S and van der Fels‐Klerx HJ, 2015. Effect of disinfectants on preventing the cross‐contamination of pathogens in fresh produce washing water. International Journal of Environmental Research and Public Health, 12, 8658–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley CA, Truitt LN, Pfuntner RC, Danyluk MD, Rideout SL and Strawn LK, 2019. Growth and survival of Listeria monocytogenes and Salmonella on whole and sliced cucumbers. Journal of Food Protection, 82, 301–309. [DOI] [PubMed] [Google Scholar]
- Benlloch‐Tinoco M, Pina‐Perez MC, Martinez‐Navarrete N and Rodrigo D, 2014. Listeria monocytogenes inactivation kinetics under microwave and conventional thermal processing in a kiwifruit puree. Innovative Food Science & Emerging Technologies, 22, 131–136. [Google Scholar]
- Beuchat LR, 1996. Listeria monocytogenes: incidence on vegetables. Food Control, 7, 223–228. [Google Scholar]
- Beuchat LR and Brackett RE, 1990. inhibitory effects of raw carrots on listeria‐monocytogenes. Applied and Environmental Microbiology, 56, 1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BFFF (British Frozen Food Federation), 2015. Guide to the management of Listeria in food processing.
- BFFF (British Frozen Food Federation), 2018. Guide to the Management of Risk of Listeriosis from Frozen Vegetables. 7 pp.
- Blackman IC and Frank JF, 1996. Growth of Listeria monocytogenes as a biofilm on various food‐processing surfaces. Journal of Food Protection, 59, 827–831. [DOI] [PubMed] [Google Scholar]
- Boelaert F, Amore G, Van der Stede Y and Hugas M, 2016. EU‐wide monitoring of biological hazards along the food chain: achievements, challenges and EFSA vision for the future. Current Opinion in Food Science, 12, 52–62. [Google Scholar]
- Bover‐Cid S, Jofré A, Dolors Guardia M, Latorre‐Moratalla ML and Garriga M, 2015. Consumption Habits and Storage Attitudes towards RTE Cooked Meat Products – Useful Information for Reliable Risk Assessments. Proceedings of the IAFP European Symposium on Food Safety, Cardiff, Wales, 20–22 April 2015.
- Brovko LY, Anany H and Griffiths MW, 2012. Bacteriophages for Detection and Control of Bacterial Pathogens in Food and Food‐Processing Environment. In: Advances in Food and Nutrition Research, Vol 67. Ed Henry J, 241–288. [DOI] [PubMed] [Google Scholar]
- CAC (Codex Alimentarius Commission), 2007. Guidelines on the application of general principles of food hygiene to the control of Listeria monocytogenes in foods. CAC/GL 61‐2007. Available online: http://www.codexalimentarius.net/web/more_info.jsp?id_sta=10740 accessed September 2019.
- Carpentier B and Cerf O, 2011. Review ‐ persistence of Listeria monocytogenes in food industry equipment and premises. International Journal of Food Microbiology, 145, 1–8. [DOI] [PubMed] [Google Scholar]
- Cattoor N, 2019a. RE: EFSA Scientific Opinion on Listeria monocytogenes in frozen fruit and vegetables including herbs. Message to Winy Messens. 23 April 2019. E‐mail.
- Cattoor N, 2019b. RE: EFSA Scientific Opinion on Listeria monocytogenes in frozen fruit and vegetables including herbs. Message to Winy Messens. 28 June 2019. E‐mail.
- Ceylan E, McMahon W and Garren DM, 2017. Thermal inactivation of Listeria monocytogenes and Salmonella during water and steam blanching of vegetables. Journal of Food Protection, 80, 1550–1556. [DOI] [PubMed] [Google Scholar]
- CFIA (Canadian Food Inspection Agency), 2011. Policy on the control of Listeria monocytogenes in ready‐to‐eat (RTE) foods. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/legislation/pol/policy_listeria_monocytogenes_2011-eng.pdf [Accessed 11 November 2019]. [Google Scholar]
- Chaitiemwong N, Hazeleger WC and Beumer RR, 2010. Survival of Listeria monocytogenes on a conveyor belt material with or without antimicrobial additives. International Journal of Food Microbiology, 142, 260–263. [DOI] [PubMed] [Google Scholar]
- Chorianopoulos NG, Tsoukleris DS, Panagou EZ, Falaras P and Nychas GJE, 2011. Use of titanium dioxide (TiO2) photocatalysts as alternative means for Listeria monocytogenes biofilm disinfection in food processing. Food Microbiology, 28, 164–170. [DOI] [PubMed] [Google Scholar]
- Colagiorgi A, Di Ciccio P, Zanardi E, Ghidini S and Ianieri A, 2016. A Look inside the Listeria monocytogenes Biofilms Extracellular Matrix. Microorganisms, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colagiorgi A, Bruini I, Di Ciccio PA, Zanardi E, Ghidini S and Ianieri A, 2017. Listeria monocytogenes biofilms in the wonderland of food industry. Pathogens (Basel, Switzerland), 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronel‐Leon J, Marques AM, Bastida J and Manresa A, 2016. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. Journal of Applied Microbiology, 120, 99–111. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Kleiss T, Cordier JL, Cordellana C, Konkel P, Pedrazzini C, Beumer R and Siebenga A, 1986. Listeria spp. in food processing, non‐food and domestic environments. Food Microbiology, 6, 49–61. [Google Scholar]
- Danyluk MD and Schaffner DW, 2011. Quantitative assessment of the microbial risk of leafy greens from farm to consumption: preliminary framework, data, and risk estimates. Journal of Food Protection, 74, 700–708. [DOI] [PubMed] [Google Scholar]
- Delignette‐Muller M and Dutang C, 2015. fitdistrplus: an R package for fitting distributions. Journal of Statistical Software, 64, 1–34. Available online: http://www.jstatsoft.org/v64/i04/ [Google Scholar]
- Desai MA, Soni KA, Nannapaneni R, Schilling MW and Silva JL, 2012. Reduction of listeria monocytogenes biofilms on stainless steel and polystyrene surfaces by essential oils. Journal of Food Protection, 75, 1332–1337. [DOI] [PubMed] [Google Scholar]
- Dygico LK, Gahan C, Grogan H and Burgess C, 2020. The ability of Listeria monocytogenes to form biofilm on surfaces relevant to the mushroom production environment. International Journal of Food Microbiology, 317. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Update of the technical specifications for harmonised reporting of food‐borne outbreaks through the European Union reporting system in accordance with Directive 2003/99/EC. EFSA Journal 2014;12(3):3598, 25 pp. 10.2903/j.efsa.2014.3598 [DOI] [Google Scholar]
- EFSA (Eurpean Food Safety Authority), 2018. Urgent scientific and technical assistance to provide recommendations for sampling and testing in the processing plants of frozen vegetables aiming at detecting Listeria monocytogenes . EFSA supporting publication 2018:EN‐1445. 41 pp. 10.2903/sp.efsa.2018.EN-1445, 1831‐4732. [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 1 (outbreak data analysis and risk ranking of food/pathogen combinations). EFSA Journal 2013;11(1):3025, 138 pp. 10.2903/j.efsa.2013.3025 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014. Scientific Opinion on the risk posed by pathogens in food of non‐animal origin. Part 2 (Salmonella and Norovirus in leafy greens eaten raw as salads). Current Opinion in Food Science. EFSA Journal 2014;12(3):3600, 118 pp. 10.2903/j.efsa.2014.3600 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2016. Scientific Opinion on the evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready‐to‐eat (RTE) food products. EFSA Journal 2016;14(8):4565, 94 pp. 10.2903/j.efsa.2016.4565 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2017a. Scientific Opinion on the guidance on the requirements for the development of microbiological criteria. EFSA Journal 2017;15(11):5052, 60 pp. 10.2903/j.efsa.2017.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2017b. Scientific opinion on hazard analysis approaches for certain small retail establishmentsin view of the application of their food safety management systems. EFSA Journal 2017;15(3):4697, 52 pp. 10.2903/j.efsa.2017.4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2018. Scientific Opinion on the Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Journal 2018;16(1):5134, 173 pp. 10.2903/j.efsa.2018.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2019. Scientific Opinion on the whole genome sequencing and metagenomics for outbreak investigation, source attribution and risk assessment of food‐borne microorganisms. EFSA Journal 2019;17(12):5898, 78 pp. 10.2903/j.efsa.2019.5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018. Multi‐country outbreak of Listeria monocytogenes serogroup IVb, multi‐locus sequence type 6, infections linked to frozen corn and possibly to other frozen vegetables – first update. EFSA supporting publication 2018:EN‐1448. 22 pp. 10.2903/sp.efsa.2018.en-1448 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2018a. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis P, 2019. RE: Presentation ‘Listeria in frozen foods and imported foods’ provided at the 13th workshop of theLm NRLs > Surrey results. Message to Winy Messens. 23 September 2019. E‐mail.
- EPA (Environmental Protection Agency), 2007. Guidance for Preparing Standard Operating Procedures (SOPs). 55 pp.
- EURL‐L monocytogenes and ANSES (European Union Reference Laboratory for Listeria monocytogenes and French Agency for Food, Environmental and Occupational Health Safety), 2019a. Guidelines on sampling the food processing area and equipment for the detection of Listeria monocytogenes. Version 3 of 6 June 2014 – Amendment 1 of 21 February 2019. 47 pp.
- EURL‐L monocytogenes and ANSES (European Union Reference Laboratory for Listeria monocytogenes and French Agency for Food, Environmental and Occupational Health Safety), 2019b. EURL Lm TECHNICAL GUIDANCE DOCUMENT for conducting shelf‐life studies on Listeria monocytogenes in ready‐to‐eat foods ‐ Version 3 of 6 June 2014 – Amendment 1 of 21 February 2019. 47 pp.
- Fagerlund A, Moretro T, Heir E, Briandet R and Langsrud S, 2017. Cleaning and disinfection of biofilms composed of listeria monocytogenes and background microbiota from meat processing surfaces. Applied and Environmental Microbiology, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falardeau J, Walji K, Haure M, Fong K, Taylor G, Ma Y, Smukler S and Wang SY, 2018. Native bacterial communities and Listeria monocytogenes survival in soils collected from the Lower Mainland of British Columbia, Canada. Canadian Journal of Microbiology, 64, 695–705. [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization), 2007. Guidelines on the Application of General Principles of Food Hygiene to the Control of Listeria Monocytogenes in Foods. 28 pp.
- FDA (U.S. Food and Drug Administration), 2015. Testing Methodology for Listeria species or L. monocytogenes in Environmental Samples. 11 pp.
- FDA and FSIS (Food and Drug Administration of the US Department of Health and Human Services and Food Safety and Inspection Service of the US Department of Agriculture), 2003. Quantitative assessment of relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready‐to‐eat foods. FDA, 572 pp. Available online: https://www.fda.gov/food/foodscienceresearch/risksafetyassessment/ucm183966.
- FDA‐CFSAN (Office of Food Safety at the U.S. Food and Drug Administration ‐ Center for Food Safety and Applied Nutrition), 2017. Control of Listeria monocytogenes in Ready‐To‐Eat Foods: Guidance for Industry ‐ Draft Guidance. 79 pp.
- Ferreira V, Wiedmann M, Teixeira P and Stasiewicz MJ, 2014. Listeria monocytogenes persistence in food‐associated environments: epidemiology, strain characteristics, and implications for public health. Journal of Food Protection, 77, 150–170. [DOI] [PubMed] [Google Scholar]
- Finten G, Garrido JI, Cova MC, Narvaiz P, Jagus RJ and Aguero MV, 2017. Safety improvement and quality retention of gamma irradiated spinach leaves. Journal of Food, Safety, 37. [Google Scholar]
- Flessa S, Lusk DM and Harris LJ, 2005. Survival of Listeria monocytogenes on fresh and frozen strawberries. International Journal of Food Microbiology, 101, 255–262. [DOI] [PubMed] [Google Scholar]
- Francis GA and O'Beirne D, 2001. Effects of vegetable type, package atmosphere and storage temperature on growth and survival of Escherichia coli O157:H7 and Listeria monocytogenes. Journal of Industrial Microbiology Biotechnology, 27, 111–116. [DOI] [PubMed] [Google Scholar]
- Franz E, Gras LM and Dallman T, 2016. Significance of whole genome sequencing for surveillance, source attribution and microbial risk assessment of foodborne pathogens. Current Opinion in Food Science, 8, 74–79. [Google Scholar]
- Galie S, Garcia‐Gutierrez C, Miguelez EM, Villar CJ and Lombo F, 2018. Biofilms in the food industry: health aspects and control methods. Frontiers in Microbiology, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze JE, Brown GD, Gaskell DE and Banks JG, 1989. Heat resistance of Listeria monocytogenes in homogenates of chicken, beef steak and carrot. Food Microbiology, 6, 251–259. [Google Scholar]
- Gil MI, Selma MV, Lopez‐Galvez F and Allende A, 2009. Fresh‐cut product sanitation and wash water disinfection: Problems and solutions. International Journal of Food Microbiology, 134, 37–45. [DOI] [PubMed] [Google Scholar]
- Gil MI, Selma MV, Suslow T, Jacxsens L, Uyttendaele M and Allende A, 2015. Pre‐ and postharvest preventive measures and intervention strategies to control microbial food safety hazards of fresh leafy vegetables. Critical Reviews in Food Science and Nutrition, 55, 453–468. [DOI] [PubMed] [Google Scholar]
- Gleeson E and O'Beirne D, 2005. Effects of process severity on survival and growth of Escherichia coli and Listeria innocua on minimally processed vegetables. Food Control, 16, 677–685. [Google Scholar]
- Gombas D, Luo Y, Brennan J, Shergill G, Petran R, Walsh R, Hau H, Khurana K, Zomorodi B, Rosen J, Varley R and Deng K, 2017. Guidelines to validate control of cross‐contamination during washing of fresh‐cut leafy vegetables. Journal of Food Protection, 80, 312–330. [DOI] [PubMed] [Google Scholar]
- Gonzalez‐Tejedor GA, Garre A, Esnoz A, Artes‐Hernandez F and Fernandez PS, 2018. Effect of storage conditions in the response of Listeria monocytogenes in a fresh purple vegetable smoothie compared with an acidified TSB medium. Food Microbiology, 72, 98–105. [DOI] [PubMed] [Google Scholar]
- Gordon A, 2017. In: Gordon A. (ed.). Introduction: effective implementation of food safety and quality systems: prerequisites and other considerations.
- Grinyer L, 2018. Six steps to control Listeria. Available online: https://www.leatherheadfood.com/files/2018/08/Six-steps-to-control-Listeria-in-foods_3.pdf (Accessed: 19 September 2019). [Google Scholar]
- Gutierrez D, Rodriguez‐Rubio L, Fernandez L, Martinez B, Rodriguez A and Garcia P, 2017. Applicability of commercial phage‐based products against Listeria monocytogenes for improvement of food safety in Spanish dry‐cured ham and food contact surfaces. Food Control, 73, 1474–1482. [Google Scholar]
- Heddleson RA and Doores S, 1994. Factors affecting microwave‐heating of foods and microwave‐induced destruction of foodborne pathogens ‐ a review. Journal of Food Protection, 57, 1025–1037. [DOI] [PubMed] [Google Scholar]
- Huang JW, Luo YG, Zhou B, Zheng J and Nou XW, 2019. Growth and survival of Salmonella enterica and Listeria monocytogenes on fresh‐cut produce and their juice extracts: impacts and interactions of food matrices and temperature abuse conditions. Food Control, 100, 300–304. [Google Scholar]
- ICMSF (International Commission on Microbiological Specifications for Foods), 1996. Microorganisms in Foods 5: Characteristics of Microbial Pathogens. 514 pp.
- Inuwa A, Lunt A, Czuprynski C, Miller G and Rankin SA, 2017. Hygienic shortcomings of frozen dessert freezing equipment and fate of listeria monocytogenes on ice cream‐soiled stainless steel. Journal of Food Protection, 80, 1897–1902. [DOI] [PubMed] [Google Scholar]
- Jackowska‐Tracz A, Tracz M and Anusz K, 2018. Integrated approach across prerequisite programmes and procedures based on HACCP principles. Medycyna Weterynaryjna‐Veterinary Medicine‐Science and Practice, 74, 219–223. [Google Scholar]
- Jofré A, Garriga M, Aymerich T, Pérez‐Rodríguez F, Valero A, Carrasco E and Bover‐Cid S, 2016. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 1, an extensive literature search and study selection with data extraction on L. monocytogenes in a wide range of RTE food. EFSA Supporting Publication 2016:13(12):EN‐1141, 184 pp. 10.2903/sp.efsa.2016.EN-1141 [DOI] [Google Scholar]
- Jordan K, Hunt K, Lourenco A and Pennone V, 2018. Listeria monocytogenes in the Food Processing Environment. Current Clinical Microbiology Reports, 5, 106–119. [Google Scholar]
- Jorgensen J, Joy W‐C and Kovacevic J, 2020. Prevalence of Listeria spp. in produce handling and processing facilities in the Pacific Northwest. Food Microbiology, 90, 103468. [DOI] [PubMed] [Google Scholar]
- Kataoka A, Wang H, Elliott PH, Whiting RC and Hayman MM, 2017. Growth of Listeria monocytogenes in thawed frozen foods. Journal of Food Protection, 80, 447–453. [DOI] [PubMed] [Google Scholar]
- Khan I, Khan J, Miskeen S, Tango CN, Park YS and Oh DH, 2016. Prevalence and control of Listeria monocytogenes in the food industry ‐ a review. Czech Journal of Food Sciences, 34, 469–487. [Google Scholar]
- Kim C and Pao S, 2019. Utilizing kitchen steamers to inactivate Listeria monocytogenes and Salmonella enterica on whole cantaloupe melons. Journal of Food Safety, 39. [Google Scholar]
- Kljujev I, Raicevic V, Jovicic‐Petrovic J, Vujovic B, Mirkovic M and Rothballer M, 2018. Listeria monocytogenes ‐ Danger for health safety vegetable production. Microbial Pathogenesis, 120, 23–31. [DOI] [PubMed] [Google Scholar]
- Kovacevic J, McIntyre LF, Henderson SB and Kosatsky T, 2012. Occurrence and distribution of listeria species in facilities producing ready‐to‐eat foods in British Columbia, Canada. Journal of Food Protection, 75, 216–224. [DOI] [PubMed] [Google Scholar]
- Lee S, Cetinkaya F and Soyutemiz GE, 2007a. Occurrence of Listeria species in the processing stages of frozen pepper. Journal of Food Safety, 27, 134–147. [Google Scholar]
- Lee S, Cetinkaya F and Soyutemiz GE, 2007b. Investigation on the presence of Listeria monocytogenes in some foods produced for exporting [Ihracata yonelik uretilen bazi gidalarda Listeria monocytogenes varliginin arastirilmasi]. Veteriner Fakultesi Dergisi (Istanbul), 33, 1–11. [Google Scholar]
- 3M and Cornell , 2019. Environmental Monitoring Handbook for the food and beverage industries. ST. PAUL, Minn. and ITHACA, N.Y. – June 13, 2019 – 3M Food Safety and Cornell University's College of Agriculture and Life Sciences (CALS). Available online: www.3M.com/EnvironmentalMonitoringProgram. Accessed February 2020. 122 pp.
- Maffei DF, Sant'Ana AS, Franco B and Schaffner DW, 2017. Quantitative assessment of the impact of cross‐contamination during the washing step of ready‐to‐eat leafy greens on the risk of illness caused by Salmonella. Food Research International, 92, 106–112. [DOI] [PubMed] [Google Scholar]
- Maherani B, Harich M, Salmieri S and Lacroix M, 2019. Antibacterial properties of combined non‐thermal treatments based on bioactive edible coating, ozonation, and gamma irradiation on ready‐to‐eat frozen green peppers: evaluation of their freshness and sensory qualities. European Food Research and Technology, 245, 1095–1111. [Google Scholar]
- Majczyna D and Bialasiewicz D, 2006. Characteristic of Listeria spp. bacteria isolated from food products (Charakterystyka bakterii z rodzaju Listeria wyizolowanych z zywnosci). Medycyna Doswiadczalna i Mikrobiologia, 58, 119–126. [PubMed] [Google Scholar]
- Mazzotta AS, 2001. Heat resistance of Listeria monocytogenes in vegetables: Evaluation of blanching processes. Journal of Food Protection, 64, 385–387. [DOI] [PubMed] [Google Scholar]
- McLauchlin J, 2019. RE: Presentation ‘Listeria in frozen foods and imported foods’ provided at the 13th workshop of the Lm NRLs. Message to Winy Messens. 31 May 2019. E‐mail.
- McManamon O, Scollard J and Schmalenberger A, 2017. Inoculation density is affecting growth conditions of Listeria monocytogenes on fresh cut lettuce. World Journal of Microbiology & Biotechnology, 33. [DOI] [PubMed] [Google Scholar]
- Mena C, Almeida G, Carneiro L, Teixeira P, Hogg T and Gibbs PA, 2004. Incidence of Listeria monocytogenes in different food products commercialized in Portugal. Food Microbiology, 21, 213–216. [Google Scholar]
- Midelet G and Carpentier B, 2002. Transfer of microorganisms, including Listeria monocytogenes, from various materials to beef. Applied and Environmental Microbiology, 68, 4015–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller Nielsen E, Björkman JT, Kiil K, Grant K, Dallman T, Painset A, Amar C, Roussel S, Guillier L, Félix B, Rotariu O, Perez‐Reche F, Forbes K and Strachan N, 2017. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 3, the comparison of isolates from different compartments along the food chain, and in humans using whole genome sequencing (WGS) analysis. EFSA Supporting Publication 2017:EN‐1151. 170 pp. 10.2903/sp.efsa.2017.EN-1151 [DOI] [Google Scholar]
- Moravkova M, Verbikova V, Michna V, Babak V, Cahlikova H, Karpiskova R and Kralik P, 2017. Detection and quantification of listeria monocytogenes in ready‐to‐eat vegetables, frozen vegetables and sprouts examined by culture methods and real‐time PCR. Journal of Food and Nutrition Research, 5, 832–837. [Google Scholar]
- Moreno Y, Sanchez‐Contreras J, Montes RM, Garcia‐Hernandez J, Ballesteros L and Ferrus MA, 2012. Detection and enumeration of viable Listeria monocytogenes cells from ready‐to‐eat and processed vegetable foods by culture and DVC‐FISH. Food Control, 27, 374–379. [Google Scholar]
- Moretro T, Fanebust H, Fagerlund A and Langsrud S, 2019. Whole room disinfection with hydrogen peroxide mist to control Listeria monocytogenes in food industry related environments. International Journal of Food Microbiology, 292, 118–125. [DOI] [PubMed] [Google Scholar]
- Morey A, McKee SR, Dickson JS and Singh M, 2010. Efficacy of ultraviolet light exposure against survival of listeria monocytogenes on conveyor belts. Foodborne Pathogens and Disease, 7, 737–740. [DOI] [PubMed] [Google Scholar]
- Moye ZD, Woolston J and Sulakvelidze A, 2018. Bacteriophage Applications for Food Production and Processing. Viruses‐Basel, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhterem‐Uyar M, Dalmasso M, Bolocan AS, Hernandez M, Kapetanakou AE, Kuchta T, Manios SG, Melero B, Minarovicovaa J, Nicolau AI, Rovira J, Skandamis PN, Jordan K, Rodriguez‐Lazaro D, Stessl B and Wagner M, 2015. Environmental sampling for Listeria monocytogenes control in food processing facilities reveals three contamination scenarios. Food Control, 51, 94–107. [Google Scholar]
- NACMCF (National Advisory Committee On Microbiological Criteria For Foods), 2006. Requisite scientific parameters for establishing the equivalence of alternative methods of pasteurization ‐ National Advisory Committee on Microbiological Criteria for Foods. Journal of Food Protection, 69, 0362‐028X, 1190–1216. [DOI] [PubMed] [Google Scholar]
- Nicolau AI and Bolocan AS, 2014. Sampling the processing environment for Listeria Listeria monocytogenes. Humana Press, New York, NY: pp. 3–14. [DOI] [PubMed] [Google Scholar]
- Niemira BA, Fan XT and Sommers CH, 2002. Irradiation temperature influences product quality factors of frozen vegetables and radiation sensitivity of inoculated Listeria monocytogenes. Journal of Food Protection, 65, 1406–1410. [DOI] [PubMed] [Google Scholar]
- Norton DM, McCamey MA, Gall KL, Scarlett JM, Boor KJ and Wiedmann M, 2001. Molecular studies on the ecology of Listeria monocytogenes in the smoked fish processing industry. Applied and Environmental Microbiology, 67, 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M, Vinas I, Colas P, Anguera M, Usall J and Abadias M, 2014. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh‐cut fruits and fruit juices. Food Microbiology, 38, 137–142. [DOI] [PubMed] [Google Scholar]
- Palumbo SA and Williams AC, 1991. Resistance of Listeria monocytogenes to freezing in foods. Food Microbiology, 8, 63–68. [Google Scholar]
- Pappelbaum K, Grif K, Heller I, Wurzner R, Hein I, Ellerbroek L and Wagner M, 2008. Monitoring hygiene on‐ and at‐line is critical for controlling Listeria monocytogenes during produce processing. Journal of Food Protection, 71, 735–741. [DOI] [PubMed] [Google Scholar]
- Perera MN, Abuladze T, Li MR, Woolston J and Sulakvelidze A, 2015. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods. Food Microbiology, 52, 42–48. [DOI] [PubMed] [Google Scholar]
- Pérez‐Rodríguez F, Carrasco E, Bover‐Cid S, Jofré A and Valero A, 2017. Closing gaps for performing a risk assessment on Listeria monocytogenes in ready‐to‐eat (RTE) foods: activity 2, a quantitative risk characterization on L. monocytogenes in RTE foods; starting from the retail stage. EFSA Supporting Publication 2017:EN‐1252, 211 pp. 10.2903/sp.efsa.2017.EN-1252 [DOI] [Google Scholar]
- PROFEL (European Association of Fruit and Vegetable Processors), 2019. Draft Hygiene guidelines for the production of quick‐frozen fruits, vegetables and herbs in control of Listeria monocytogenes. 45 pp.
- R Core Team 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/
- Redfern J and Verran J, 2017. Effect of humidity and temperature on the survival of Listeria monocytogenes on surfaces. Letters in Applied Microbiology, 64, 276–282. [DOI] [PubMed] [Google Scholar]
- Renna M, Gonnella M, de Candia S, Serio F and Baruzzi F, 2017. Efficacy of combined sous vide‐microwave cooking for foodborne pathogen inactivation in ready‐to‐eat chicory stems. Journal of Food Science, 82, 1664–1671. [DOI] [PubMed] [Google Scholar]
- Roccato A, Uyttendaele M and Membre JM, 2017. Analysis of domestic refrigerator temperatures and home storage time distributions for shelf‐life studies and food safety risk assessment. Food Research International, 96, 171–181. [DOI] [PubMed] [Google Scholar]
- Sant'Ana AS, Barbosa MS, Destro MT, Landgraf M and Franco B, 2012. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready‐to‐eat vegetables stored at variable temperature conditions during shelf‐life. International Journal of Food Microbiology, 157, 52–58. [DOI] [PubMed] [Google Scholar]
- Simmons CK and Wiedmann M, 2018. Identification and classification of sampling sites for pathogen environmental monitoring programs for Listeria monocytogenes: results from an expert elicitation. Food Microbiology, 75, 2–17. [DOI] [PubMed] [Google Scholar]
- Simmons C, Stasiewicz MJ, Wright E, Warchocki S, Roof S, Kause JR, Bauer N, Ibrahim S, Wiedmann M and Oliver HF, 2014. Listeria monocytogenes and Listeria spp. contamination patterns in retail delicatessen establishments in three US States. Journal of Food Protection, 77, 1929–1939. [DOI] [PubMed] [Google Scholar]
- Skariyachan S and Govindarajan S, 2019. Biopreservation potential of antimicrobial protein producing Pediococcus spp. towards selected food samples in comparison with chemical preservatives. International Journal of Food Microbiology, 291, 189–196. [DOI] [PubMed] [Google Scholar]
- Skowron K, Hulisz K, Gryn G, Olszewska H, Wiktorczyk N and Paluszak Z, 2018. Comparison of selected disinfectants efficiency against Listeria monocytogenes biofilm formed on various surfaces. International Microbiology, 21, 23–33. [DOI] [PubMed] [Google Scholar]
- Skowron K, Grudlewska K, Lewandowski D, Gajewski P, Reslinski A and Gospodarek‐Komkowska E, 2019. Antibiotic susceptibility and ability to form biofilm of listeria monocytogenes strains isolated from frozen vegetables. Acta Alimentaria, 48, 65–75. [Google Scholar]
- Smith A, Hearn J, Taylor C, Wheelhouse N, Kaczmarek M, Moorhouse E and Singleton I, 2019. Listeria monocytogenes isolates from ready to eat plant produce are diverse and have virulence potential. International Journal of Food Microbiology, 299, 23–32. [DOI] [PubMed] [Google Scholar]
- Spurlock AT and Zottola EA, 1991. The survival of listeria‐monocytogenes in aerosols. Journal of Food Protection, 54, 910–912. [DOI] [PubMed] [Google Scholar]
- Stessl B, Szakmary‐Brandle K, Vorberg U, Schoder D and Wagner M, 2020. Temporal analysis of the Listeria monocytogenes population structure in floor drains during reconstruction and expansion of a meat processing plant. International Journal of Food Microbiology, 314. [DOI] [PubMed] [Google Scholar]
- Tadepalli S, Bridges DF, Driver R and Wu VCH, 2018. Effectiveness of different antimicrobial washes combined with freezing against Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes inoculated on blueberries. Food Microbiology, 74, 34–39. [DOI] [PubMed] [Google Scholar]
- Tolvanen R, Lunden J, Korkeala H and Wirtanen G, 2007. Ultrasonic cleaning of conveyor belt materials using Listeria monocytogenes as a model organism. Journal of Food Protection, 70, 758–761. [DOI] [PubMed] [Google Scholar]
- Tompkin RB, 2002. Control of Listeria monocytogenes in the food‐processing environment. Journal of Food Protection, 65, 709–725. [DOI] [PubMed] [Google Scholar]
- Truchado P, Elsser‐Gravesen A, Gil MI and Allende A, 2020. Post‐process treatments are effective strategies to reduce Listeria monocytogenes on the surface of leafy greens: a pilot study. International Journal of Food Microbiology, 313, 108390. [DOI] [PubMed] [Google Scholar]
- UFPA (United Fresh Produce Association), 2018. Guidance on Environmental Monitoring and Control of Listeria for the Fresh Produce Industry, 2th Edition. 36 pp. [Google Scholar]
- Uyttendaele M, Busschaert P, Valero A, Geeraerd AH, Vermeulen A, Jacxsens L, Goh KK, De Loy A, Van Impe JF and Devlieghere F, 2009. Prevalence and challenge tests of Listeria monocytogenes in Belgian produced and retailed mayonnaise‐based deli‐salads, cooked meat products and smoked fish between 2005 and 2007. International Journal of Food Microbiology, 133, 94–104. [DOI] [PubMed] [Google Scholar]
- Valderrama WB and Cutter CN, 2013. An ecological perspective of listeria monocytogenes biofilms in food processing facilities. Critical Reviews in Food Science and Nutrition, 53, 801–817. [DOI] [PubMed] [Google Scholar]
- Van Boxstael S, Devlieghere F, Berkvens D, Vermeulen A and Uyttendaele M, 2014. Understanding and attitude regarding the shelf life labels and dates on pre‐packed food products by Belgian consumers. Food Control, 37, 85–92. [Google Scholar]
- Van Schothorst M, Zwietering MH, Ross T, Buchanan RL and Cole MB, 2009. Relating microbiological criteria to food safety objectives and performance objectives. Food Control, 20, 967–979. [Google Scholar]
- Vitas AI, Aguado V and Garcia‐Jalon I, 2004. Occurrence of Listeria monocytogenes in fresh and processed foods in Navarra (Spain). International Journal of Food Microbiology, 90, 349–356. [DOI] [PubMed] [Google Scholar]
- Vojkovska H, Myskova P, Gelbicova T, Skockova A, Kolackova I and Karpiskova R, 2017. Occurrence and characterization of food‐borne pathogens isolated from fruit, vegetables and sprouts retailed in the Czech Republic. Food Microbiology, 63, 147–152. [DOI] [PubMed] [Google Scholar]
- Willis C, Amar C, Aird H, Jørgensen F, Lai S and McLauchlin J, 2019. Survey on the presence of Listeria monocytogenes in frozen fruit and vegetables collected in England in 2018–19., Toronto, Canada. [Google Scholar]
- Ziegler M, Kent D, Stephan R and Guldimann C, 2019. Growth potential of Listeria monocytogenes in twelve different types of RTE salads: impact of food matrix, storage temperature and storage time. International Journal of Food Microbiology, 296, 83–92. [DOI] [PubMed] [Google Scholar]
- Zoellner C, Ceres K, Ghezzi‐Kopel K, Wiedmann M and Ivanek R, 2018. Design elements of listeria environmental monitoring programs in food processing facilities: a scoping review of research and guidance materials. Comprehensive Reviews in Food Science and Food Safety, 17, 1156–1171. [DOI] [PubMed] [Google Scholar]
- Zoellner C, Jennings R, Wiedmann M and Ivanek R, 2019. EnABLe: an agent‐based model to understand Listeria dynamics in food processing facilities. Scientific Reports, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwietering MH, Jacxsens L, Membre J‐M, Nauta M and Peterz M, 2016. Relevance of microbial finished product testing in food safety management. Food Control, 60, 31–43. [Google Scholar]


