Abstract
Following a request from the European Commission, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) was asked to deliver a scientific opinion on the safety and efficacy of turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L. rhizome when used as sensory additives in feed and in water for drinking for all animal species. The FEEDAP Panel concludes that the additives under consideration are safe at the maximum proposed use levels: (i) turmeric extract at 15 mg/kg complete feed (or in water for drinking at comparable exposure) for all animal species; (ii) turmeric essential oil at 80 mg/kg feed for veal calves (milk replacer) and 20 mg/kg complete feed (or 20 mg/L) for all other species; (iii) turmeric oleoresin at 30 mg/kg complete feed (or 30 mg/L) for chickens for fattening and laying hens and 5 mg/kg complete feed (or 5 mg/L) for pigs, veal calves, cattle for fattening and dairy cows, sheep, goats, horses, rabbits and fish; (iv) turmeric tincture at 0.8 mL/L water for drinking for poultry, 6 mL per head and day for horses and 0.05 mL tincture/kg complete feed for dogs. No concerns for consumers were identified following the use of the additives at the proposed use level in animal nutrition. Turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture should be considered as irritants to skin and eyes and the respiratory tract and as skin sensitisers. The use of the additives in feed is not expected to pose a risk for the environment. Since turmeric and its preparations are recognised to flavour food and their function in feed would be essentially the same as that in food, no further demonstration of efficacy is considered necessary.
Keywords: sensory additives, flavouring compounds, turmeric, Curcuma longa L., safety
1. Introduction
1.1. Background and Terms of Reference
Regulation (EC) No 1831/20031 establishes the rules governing the Community authorisation of additives for use in animal nutrition. In particular, Article 4(1) of that Regulation lays down that any person seeking authorisation for a feed additive or for a new use of a feed additive shall submit an application in accordance with Article 7 and in addition, Article 10(2) of that Regulation specifies that for existing products within the meaning of Article 10(1), an application shall be submitted in accordance with Article 7, within a maximum of 7 years after the entry into force of this Regulation.
The European Commission received a request from the Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG)2 for authorisation/re‐evaluation of nine preparations (namely turmeric oil, turmeric oleoresin, turmeric extract (sb) and turmeric tincture from Curcuma longa L., cardamom oil from Elettaria cardamomum (L.) Maton, ginger oil, oleoresin, tincture and extract from Zingiber officinale Roscoe) belonging to botanically defined group (BDG) 9 ‐ Zingiberales, when used as feed additives for all animal species (category: sensory additives; functional group: flavourings). During the assessment, the applicant withdrew the application for ginger extract.3 During the course of the assessment, this application was split and the present opinion covers only four out of the nine preparations under application: turmeric oil, turmeric oleoresin, turmeric extract and turmeric tincture from C. longa for all animal species.
According to Article 7(1) of Regulation (EC) No 1831/2003, the Commission forwarded the application to the European Food Safety Authority (EFSA) as an application under Article 4(1) (authorisation of a feed additive or new use of a feed additive) and under Article 10(2) (re‐evaluation of an authorised feed additive). EFSA received directly from the applicant the technical dossier in support of this application. The particulars and documents in support of the application were considered valid by EFSA as of 3 January 2011.4
According to Article 8 of Regulation (EC) No 1831/2003, EFSA, after verifying the particulars and documents submitted by the applicant, shall undertake an assessment in order to determine whether the feed additive complies with the conditions laid down in Article 5. EFSA shall deliver an opinion on the safety for the target animals, consumer, user and the environment and on the efficacy of the products turmeric oil, turmeric oleoresin, turmeric extract and turmeric tincture from C. longa, when used under the proposed conditions of use (see Sections 3.2.1.3, 3.3.1.3, 3.4.1.3 and 3.5.1.3).
The remaining five preparations belonging to botanically defined group (BDG) 9 ‐ Zingiberales under application are assessed in separate opinions.
1.2. Additional information
The four preparations under assessment, namely turmeric oil, turmeric oleoresin, turmeric extract and turmeric tincture from Curcuma longa L., are currently authorised as feed additives according to the entry in the European Union Register of Feed Additives pursuant to Regulation (EC) No 1831/2003 (2b natural products – botanically defined). They have not been assessed as feed additives in the European Union (EU).
There is no specific EU authorisation for any C. longa preparation when used to provide flavour in food. However, according to Regulation (EC) No 1334/20085 flavourings preparations produced from food or food ingredients with flavouring properties, may be used without an evaluation and approval as long as ‘they do not, on the basis of the scientific evidence available, pose a safety risk to the health of the consumer, and their use does not mislead the consumer’.
A turmeric rhizome extract is authorised as food additive (colour) under the name curcumin (E 100) in the EU (Commission Regulation (EU) No 1129/20116). According to Commission Regulation (EU) No 231/20127, the following definition is allocated to this food additive: ‘Curcumin is obtained by solvent extraction of turmeric i.e. the ground rhizomes of strains of C. longa L. In order to obtain a concentrated curcumin powder, the extract is purified by crystallization. The product consists essentially of curcumins; i.e. the colouring principle (1,7‐bis(4‐hydroxy‐3‐methoxyphenyl)hepta‐1,6‐dien‐3,5‐dione8) and its two desmethoxy derivatives9 in varying proportions. Minor amounts of oils and resins naturally occurring in turmeric may be present. … Only the following solvents may be used in the extraction: ethyl acetate, acetone, carbon dioxide, dichloromethane, n‐butanol, methanol, ethanol, hexane, propan‐2‐ol’.
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) assessed the food additive curcumin (turmeric rhizome extract) in 2003 and established an acceptable daily intake (ADI) of 0–3 mg/kg body weight (bw) (WHO, 2004a,b). In 2010, the EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) adopted a scientific opinion on the re‐evaluation of the food additive colour curcumin (E 100) (turmeric rhizome extract) and concluded that the available data set supports the ADI allocated by JECFA based on the NOAEL of 250–320 mg/kg bw per day from the reproductive toxicity study in rats for a decreased body weight gain in the F2 generation observed at the highest dose level, and an uncertainty factor of 100 (EFSA ANS Panel, 2010). In 2014, the European Food Safety Authority (EFSA) took into account additional information on the use of curcumin (E 100) in foods and carried out a refined exposure assessment (EFSA, 2014).
The European Medicines Agency (EMA, 2018a,b) assessed C. longa L., rhizoma, as herbal medicinal product in the form of powdered herbal substance, comminuted herbal substance, dry extract (13–25:1, extraction solvent: ethanol 96% (v/v)), dry extract (5.5–6.5:1, extraction solvent: ethanol 50% (v/v)) and tinctures (1:5 or 1:10, extraction solvent: ethanol 70% (v/v)).
The preparations from C. longa are listed in the report on botanical flavourings of the Council of Europe (CoE) with the number 163 (CoE, 2000).
2. Data and methodologies
2.1. Data
The present assessment is based on data submitted by the applicant in the form of a technical dossier10 in support of the authorisation request for the use of turmeric oil, turmeric oleoresin, turmeric extract and turmeric tincture from C. longa as feed additives.
The Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) used the data provided by the applicant together with data from other sources, such as previous risk assessments by EFSA or other expert bodies, peer‐reviewed scientific papers, other scientific reports and experts’ knowledge, to deliver the present output.
Many of the components of the essential oil under assessment have been already evaluated by the FEEDAP Panel as chemically defined flavourings. The applicant submitted a written agreement to use the data submitted for the assessment of chemically defined flavourings (dossiers, publications and unpublished reports) for the risk assessment of preparations from C. longa.11
EFSA has verified the European Union Reference Laboratory (EURL) report as it relates to the methods used for the control of the phytochemical markers in the feed additives from botanically defined flavourings group 09 (BDG 09) – Zingiberales. The EURL delivered in 2018 an evaluation report related to the Botanically Defined Flavourings Group BDG 09 ‐ Zingiberales.12 In this report, only analytical methods for cardamom oil were evaluated. On 25 February 2020, the EURL delivered an addendum to the above‐mentioned report, in which the remaining feed additives included in this group were evaluated. In particular, regarding the feed additives subject of the present scientific opinion, the method of analysis for ar‐turmerone and beta‐turmerone in turmeric oil, and for total curcuminoids in turmeric oleoresin, turmeric extract and turmeric tincture were evaluated. The full report including the addendum is available on the EURL website: https://ec.europa.eu/jrc/en/eurl/feed-additives/evaluation-reports/fad-2010-0335.
2.2. Methodologies
The approach followed by the FEEDAP Panel to assess the safety and the efficacy of turmeric oil, turmeric oleoresin, turmeric extract and turmeric tincture from C. longa is in line with the principles laid down in Regulation (EC) No 429/200813 and the relevant guidance documents: Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements (EFSA Scientific Committee, 2009), Compendium of botanicals that have been reported to contain toxic, addictive, psychotropic or other substances of concern (EFSA, 2012), Guidance for the preparation of dossiers for sensory additives (EFSA FEEDAP Panel, 2012a), Guidance on the identity, characterisation and conditions of use of feed additives (EFSA FEEDAP Panel, 2017a), Technical Guidance for assessing the safety of feed additives for the environment (EFSA, 2008), Guidance for the preparation of dossiers for additives already authorised for use in food (EFSA FEEDAP Panel, 2012b), Guidance on studies concerning the safety of use of the additive for users/workers (EFSA FEEDAP Panel, 2012c), Guidance on the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), Guidance on the assessment of the safety of feed additives for the consumer (EFSA FEEDAP Panel, 2017c), Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017), Guidance document on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals (EFSA Scientific Committee, 2019a), Statement on the genotoxicity assessment of chemical mixtures (EFSA Scientific Committee, 2019b).
3. Assessment
The additives under assessment are turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L. and are intended for use as sensory additives (flavourings) in feed and in water for drinking.
The FEEDAP Panel noted that the term ‘curcumin’ is used to describe both the chemical compound 1,7‐bis(4‐hydroxy‐3‐methoxyphenyl) hepta‐1,6‐dien‐3,5‐dione and the food additive E 100, a turmeric rhizome extract containing curcumin and its two desmethoxy‐derivatives.
In this opinion, the term ‘curcumin’ is only used to describe the chemical compound 1,7‐bis(4‐hydroxy‐3‐methoxyphenyl) hepta‐1,6‐dien‐3,5‐dione. In case of reference to a turmeric rhizome extract, which meets the specifications of the food additive E 100, containing a mixture of curcumin and its two desmethoxy‐derivatives, the term ‘curcumin (E 100, turmeric rhizome extract)’ is used.
3.1. Origin and extraction
Turmeric (C. longa L.; synonym: C. domestica Valeton) is a rhizomatous herbaceous perennial flowering plant which belongs to the Zingiberaceae family.
The plant is native to tropical Asia, especially India, and has been widely introduced and naturalised to many tropical and subtropical countries. The parts of C. longa used for production of the preparations for feed flavouring under evaluation are dried rhizomes.14 Rhizomes from C. longa, which are designated by the name ‘turmeric’ as the plant itself, have a long traditional use as a spice to flavour and colour food, especially in Indian curries, and as medicinal products including traditional Ayurvedic medicine (e.g. Teuscher, 2003; FAO, 2004; Ziegler, 2007). Preparations from other parts than rhizomes of C. longa (e.g. turmeric leaf oil) are described and available on the market (e.g. Raina et al., 2005; Singh et al., 2010) but not addressed here.
The plant components present in the different preparations depend on the selectivity of the extraction process. The different extraction processes used for the additives which are the subject of this opinion, namely turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture, are described under their respective headings.
3.2. Turmeric rhizome extract (referred to as turmeric extract)
This application concerns turmeric extract produced by extraction of dried rhizomes, using organic solvents in a process as described by JECFA (FAO, 2004; WHO 2006) and the ANS Panel (EFSA ANS Panel, 2010) for the manufacturing of the food additive curcumin (E 100, turmeric rhizome extract). Primary extraction by ethyl acetate and/or acetone or by hexane and ethyl acetate is followed by purification using isopropanol or ethyl acetate or mixtures of solvents (isopropanol/ethanol or ethyl acetate/hexane). As a final step, solvents are removed under vacuum.
The plant components present in turmeric extract are curcuminoids, mainly curcumin (I), desmethoxycurcumin (II) and bis‐desmethoxycurcumin (III). The molecular structure of curcuminoids is shown in Figure 1.
Figure 1.
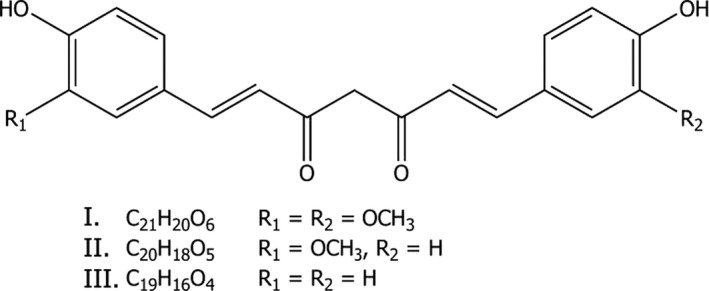
Structural and molecular formula of curcuminoids, the main components of turmeric extract: curcumin (I), desmethoxycurcumin (II) and bis‐desmethoxycurcumin (III)
3.2.1. Characterisation of turmeric extract
Turmeric extract is described as a yellow orange hygroscopic powder with characteristic odour and taste15 and poorly soluble in water (WHO, 2006). It is identified with the Chemical Abstracts Service (CAS) number 8024‐37‐1, the European Inventory of Existing Chemical Substances (EINECS) number 283‐882‐1 and Flavour Extract Manufacturers Association (FEMA) number 3086. However, these identifiers are applied indiscriminately to different kinds of extracts and derivatives from C. longa, none of which accurately describes the additive under application
The feed additive contains by specification at least 90% curcuminoids (the active substances, as the sum of curcumin (I), desmethoxycurcumin (II) and bis‐desmethoxycurcumin (III)). The product is in compliance with the specifications of the food additive colour curcumin (E 100, turmeric rhizome extract) according to Commission Regulation (EU) No 231/201216 and according to JECFA (FAO 2004, WHO 2006). Analysis of nine batches from three different companies showed compliance with these specifications (Table 1).17 Individual curcuminoids were determined by high‐performance liquid chromatography (HPLC) with spectrophotometric detection.18
Table 1.
Composition of turmeric extract based on the analysis of nine batches from three different companies. The results are expressed as % (w/w)
| Constituent | Chemical formula | Molecular weight | CAS No | Meana | Range |
|---|---|---|---|---|---|
| % (w/w) | % (w/w) | ||||
| Curcumin (I) | C21H20O6 | 368.39 | 458‐37‐7 | 77.02 | 74.84–78.76 |
| Desmethoxycurcumin (II) | C20H18O5 | 338.39 | 33171‐16‐3 | 16.34 | 15.3–18.46 |
| Bis‐desmethoxycurcumin (III) | C19H16O4 | 308.39 | 33171‐05‐0 | 3.62 | 2.18–4.58 |
| Total curcuminoids | 96.68 | 94.88–97.68 |
CAS No: Chemical Abstracts Service number.
Mean calculated on nine batches.
The results of the proximate analysis of the same batches are summarised in Table 2.
Table 2.
Results of the proximate analysis of nine batches of turmeric extract from three different companies. The results are expressed as % (w/w)
| Constituent | Meana | Range |
|---|---|---|
| % (w/w) | % (w/w) | |
| Protein | 0.71 | < 0.1–1.1 |
| Lipids | 0.82 | < 0.6–1.2 |
| Fibre | 0.42 | n.d.–1.2 |
| Other organic compounds | 0.27 | n.d.–1.2 |
| Ash | 0.09 | n.d.–0.25 |
| Water | 0.71 | 0.33–1.59 |
| Total | 3.02 | 2.45–3.81 |
Mean calculated on nine batches.
3.2.1.1. Impurities
The purity criteria for the turmeric extract under assessment fully comply with those in the specifications of the food additive colour E 100.19
Data on residual solvents in five batches of the feed additive indicated that residual solvents (acetone, ethyl acetate, isopropanol and ethanol) fully comply with purity criteria of the food colour.20
Data on impurities were provided for three batches of turmeric extract. The concentrations of lead and mercury were ≤ 0.04 mg/kg and ≤ 0.09 mg/kg, respectively. Cadmium and arsenic were below the respective limit of quantification (LOQ).21 All comply with the specifications of the food additive (arsenic < 3 mg/kg, lead < 10 mg/kg, mercury and cadmium < 1 mg/kg). Pesticides (multiresidue analysis)22 and aflatoxins (B1, B2, G1 and G2)23 were below the LOQ in three additional batches.
Dioxin‐like polychlorinated biphenyls (PCBs) ranged between 0.01 and 0.04 ng WHO‐PCB‐TEQ (World Health Organisation polychlorinated biphenyl (PCB) toxic equivalents)/kg, the sum of dioxins was in the range 0.21–0.26 ng WHO PCDD/F‐TEQ (World Health Organisation polychlorinated dibenzo‐p‐dioxin (PCDD) and polychlorinated dibenzofuran (PCDF) toxic equivalents)/kg and the sum of dioxins and dioxin‐like PCBs was 0.22–0.27 ng WHO‐PCDD/F‐PCB‐TEQ (World Health Organisation PCDD, PCDF and PCB toxic equivalents)/kg.24 None of the data on chemical impurities raised concerns.
Analysis of microbial contamination of three batches of turmeric extract indicated that Salmonella spp. was absent in 25 g, Escherichia coli and Staphylococcus aureus were absent in 1 g. The total bacterial count was between < 102 and < 104 colony‐forming unit (CFU)/g, yeasts and moulds were < 102 CFU/g.
3.2.1.2. Shelf‐life
According to the JECFA report (FAO, 2004), curcuminoids from turmeric extract are stable under dry conditions (in dry food).
In water, they are relatively stable at acidic pH, but rapidly decompose at pHs above neutral. Initial degradation products at pH 7–10 are ferulic acid and feruloylmethane. The latter rapidly forms coloured condensation products (FAO, 2004).
Depending on the physical conditions, curcumin may be affected by chemical and or photochemical oxidative degradation and autoxidation (Heger et al., 2014; Gordon et al., 2015; Nelson et al., 2017).25 Nelson et al. (2017) also reported that in consequence of photodegradation, reaction with organic solvents like isopropanol as a substrate may occur.
3.2.1.3. Conditions of use
Turmeric extract is intended to be added to feed and water for drinking for all animal species without withdrawal period.26 The maximum proposed use level is 15 mg/kg complete feed. No specific use level has been proposed by the applicant for the use in water for drinking.
3.2.2. Safety
The assessment of safety is based on the use level proposed by the applicant.
3.2.2.1. Absorption, distribution, metabolism and excretion
In the course of the safety assessment of the food additive colour curcumin (E 100, turmeric rhizome extract), studies on the absorption, distribution, metabolism and excretion (ADME) with curcumin or purified turmeric extracts in laboratory animals and humans were evaluated by JECFA (WHO, 2004a,b) and the ANS Panel (EFSA ANS Panel, 2010). In the opinion of the ANS Panel, the ADME studies of curcumin and curcuminoids in experimental animals and humans were reviewed and data generally showed very poor absorption of the compounds at the intestinal level with excretion being mainly in the faeces. One paper that was identified in the ANS assessment indicated that curcumin does not distribute to any specific organs in appreciable levels (Wahlstrom and Blennow, 1978 as referenced by EFSA ANS Panel, 2010).
The applicant submitted an updated literature search on ADME of curcumin and its desmethoxy‐derivatives, including some reviews (Heger et al., 2014; Shen et al., 2016; Nelson et al., 2017). Overall, all reviewed studies on ADME point to a very low bioavailability of curcumin via oral route, both in experimental animals and humans. Several factors can contribute to this low bioavailability, namely i) its chemical instability ii) its reactivity with proteins and glutathione in the intestinal mucus and retention therein, iii) efflux of the compounds taken up by the intestinal cells by the efflux pumps. Metabolism of the absorbed curcumin and of its reduced compounds in the enterocytes occurs mainly by further reduction and conjugation reactions. Hepatic metabolism of the absorbed curcumin and of its metabolites occurs mainly by reductive and/or conjugation reactions and elimination through the bile into the intestine. Excretion occurs mainly in faeces, and to a minor extent via urine.
Some experimental studies and clinical trials in human volunteers performed with standardised curcumin and curcuminoids or formulations containing these compounds are briefly described.
In suspensions of freshly isolated rat and human hepatocytes, the metabolism of curcumin is qualitatively similar, with the formation of hexahydrocurcumin and hexahydrocurcuminol in the cells of both species, being more rapid and extensive in the rat hepatocytes. Sulfate and glucuronide derivatives were also characterised as minor metabolites in these in vitro models (Ireson et al., 2001). In the rat, after a single oral dose of a solution of curcumin in dimethylsulfoxide (DMSO) at 500 mg/kg bw, conjugate derivatives of curcumin determined by liquid chromatography‐mass spectrometry (LC‐MS) were present in plasma (glucuronide: 930 ng/mL at 30 min and 820 ng/mL at 6 h; sulfate: 90 ng/mL at 30 min and 160 ng/mL at 2 h; curcumin glucuronide sulfate was also identified). Hexahydrocurcumin and hexahydrocurcuminol were present at very low levels and curcumin levels were lower than the limit of detection (LOD: 1.84 ng/mL) of the LC‐MS method (Ireson et al., 2001).
Also 42 min after a single oral administration to rats of 500 mg curcumin/kg bw plasma free curcumin Cmax determined by liquid chromatography tandem mass spectrometry (LC‐MS/MS) was 60 ng/mL (LOD: 1 ng/mL). The bioavailability was calculated to be about 1% and the t1/2β was 45 min (Yang et al., 2007).
Marczylo et al. (2009) administered to rats a single oral dose of a formulation delivering 340 mg/kg bw of curcumin. Animals were killed 20 min after dosing and blood, liver, kidneys, heart and gastrointestinal tract were removed for LC‐MS/MS analysis (LOD: 2.5 ng/mL). Free curcumin was present in plasma (16 ng/mL), in intestinal mucosa (1.4 mg/g), liver, kidney and heart (3.7, 0.21 and 0.81 μg/g, respectively). In plasma, desmethoxycurcumin was present at 13.6 ng/mL, curcumin glucuronide at 61 ng/mL and desmethoxycurcumin glucuronide at 96 ng/mL. In urine, curcumin levels were below LOD, and glucuronides of curcumin and of desmethoxycurcumin were 426 and 532 ng/mL, respectively. In liver and kidney, hexahydrocurcumin and its sulfoconjugate were identified and in the liver also dihydrocurcumin, tetrahydrocurcumin and its sulfoconjugates. All three parent curcuminoids were detected in intestinal mucosa, liver and kidney. No metabolites were detected in heart and intestinal mucosa. Curcumin levels in tissues were higher than in plasma: intestinal mucosa > liver > heart > kidney. The major metabolites of curcuminoids were the glucuronide conjugates.
Free curcumininoids were below the LOD in the plasma of rats receiving 100 mg curcumin/kg bw (LC‐MS, LOD: 5 ng/mL), whereas after enzymatic hydrolysis of the conjugates, the highest concentration of curcumin was 209 ng/mL determined 1 h after administration (Asai and Miyazawa, 2000). Similar results were reported by Liu et al. (2006). When an oral dose of 100 mg of curcumin was given to rats, the Cmax for the sum of curcumin and tetrahydrocurcumin in plasma was 267 ng/mL (Tmax 1.62 h) when determined after enzymatic hydrolysis of the conjugates by LC‐MS/MS (LOQ: 0.5 ng/mL).
In a study carried out to compare the bioavailability of curcumin in different formulations, the oral administration to rats of a water suspension of standard curcumin at 500 mg/kg bw led to a plasma free curcumin concentration of 3.2 ng/mL at 30 min (LC‐MS; LOD: 1.8 ng/L) (Teixeira et al., 2016).
Sharma et al., 2004, carried out in 15 human patients a dose escalation study giving daily 0.45–3.6 g of curcumin in capsules up to 4 months. Each capsule contained 500 mg curcuminoids (450 mg curcumin + 40 mg desmethoxycurcumin + 10 mg bisdesmethoxycurcumin). Mean plasma levels of curcumin at 1 h post dosing of 3.6 g on day 1, 2, 8 or 29 days were similar: 4.1 ng/mL curcumin, 8.6 ng/mL glucuronide and 4 ng/mL sulfate (LC‐MS; LOD: 1.84 ng/mL curcumin). For the other doses and blood collection time points, curcumin was not detected. Conjugates of desmethoxycurcumin were also detected in plasma of all six patients treated with 3.6 g curcumin. In urine, the sulfate and glucuronide derivatives of curcumin were 8.5–20 ng/mL and 114–278 ng/mL, respectively, and curcumin was up to 479 ng/mL. Curcumin was excreted in faeces after all the doses (in day 8 after 3.6 g ingestion: 9.2–42.7 μg/g dry matter).
Jäger et al., 2014, performed a randomised, double‐blind, crossover study in 12 healthy volunteers by oral administration of a single dose of 1.8 g of curcuminoids (from a standardised curcumin mixture) in gelatine capsules or of a formulation with volatile oils of turmeric rhizome containing 0.376 g of total curcuminoids. Blood was collected at 1 h up to 12 h post dose and analysis of curcumin, desmethoxycurcumin, bis‐desmethoxycurcumin and tetrahydrocurcumin was performed in plasma after enzymatic hydrolysis (LC‐MS/MS; LOD not given). The concentration of total curcuminoids in blood was 1.3 times higher after administration of the oil formulation as compared with a standardised curcumin mixture. The respective Tmax values were reached after 1.8 h and 9.5 h. The study demonstrated that the presence of volatile oil shortened the time of absorption and increased the maximum blood levels of curcuminoids and their metabolites.
Another comparative randomised, double‐blind, crossover study was recently made in 12 healthy human volunteers, orally given a single dose in hard gel capsules of standardised unformulated curcumin extract (SC, 1.95 g total curcuminoids: 1.774 g curcumin, 0.162 g desmethoxycurcumin and 0.09 g bis‐desmethoxycurcumin) or a commercially available formulation of curcumin with essential oils turmeric extracted from the rhizome (CEO, 0.392 g total curcuminoids: 0.355 g curcumin, 0.035 g desmethoxycurcumin and 0.0018 g bis‐desmethoxycurcumin) (Purpura et al., 2018). Blood was collected at 1 h up to 12 h after dosing and curcuminoids and the metabolite tetrahydrocurcumin were analysed in plasma after enzymatic hydrolysis (LC‐MS/MS; LOD not given). Curcumin and bis‐desmethoxycurcumin were more efficiently absorbed from the oil formulation compared to the standardised curcuminoids extract (1.7 and 1.4 times, respectively). Plasma Cmax of curcumin was 0.5 ng/mL and 0.9 ng/mL, attained at 12 h and 6 h, respectively, for SC and CEO and Cmax of bis‐desmethoxycurcumin was 0.2 ng/mL and 0.3 ng/mL. The corresponding values for the area under the curve (AUC0‐12h) of curcumin were 3.9 and 6.7 ng/mLh for SC and CEO, respectively, and AUC0‐12h for total curcuminoids 10.4 and 12.1 ng/mLh for SC and CEO, respectively. In terms of total curcuminoids, their absorption was slightly higher in the oil formulation (1.1 relative absorption units).
From the studies described, it can be concluded that after the administration of high doses of curcumin to rats (300–500 mg/kg bw) and to humans (up to 3.6 g/day), very low plasma levels of free curcumin were consistently found, in the range of ng/mL (3.2–60 ng/mL). The concentration of conjugated curcumin in plasma was in the range of 200–700 ng/mL (mainly as glucuronide) following the administration of a single oral dose to rats (100–500 mg/kg bw per day).
Studies on ADME of curcumin and curcuminoids in target animals are not available. The phase I and phase II metabolic pathways involved in the biotransformation of curcumin and curcuminoids in experimental animals and humans were generally identified in several target species (EFSA FEEDAP Panel, 2015, 2016). Thus, a similar ADME profile is expected in target animals given feed containing turmeric extract.
There are a number of publications which describe the metabolism of curcumin/curcuminoids by individual intestinal isolates or under simulated gut conditions (reviewed by Shen and Ji, 2019). A curcumin specific reductase (CurA) has been identified in strains of E. coli which leads, via dihydrocurcumin, to the production of tetrahydrocurcumin (Hassaninasab et al., 2011, as referenced in Shen and Ji, 2019). Demethylation reactions produced by other isolated intestinal bacteria (e.g. Blautia sp. and Bacillus megaterium) also have been described with the resulting formation of demethylcurcumin, bisdemethylcurcumin and demethyldesmethoxycurcumin (Burapan et al., 2017; An et al., 2017, as referenced in Shen and Ji, 2019).
Consortia of intestinal bacteria appear more effective than individual isolates. In a human faecal fermentation model, only 24%, 61% and 87% of curcumin, desmethoxycurcumin and bis‐desmethoxycurcumin initially present could be recovered after 24 h incubation. The three main metabolites formed were tetrahydrocurcumin, dihydroferulic acid and 1‐(4‐hydroxy‐3‐methoxyphenyl)‐2‐propanol (Tan et al., 2015, as referenced in Shen and Ji, 2019). In a similar study, 23 metabolites were identified by LC‐MS, resulting from reduction, demethoxylation, hydroxylation, methylation and acetylation reactions (Lou et al., 2015, as referenced in Shen and Ji, 2019). Although in vitro or in vivo studies describing the degradation of curcuminoids by the gut microbiota of target animals could not be identified, it is reasonable to assume that a similar metabolic capacity exists.
From the above data, it can be concluded that curcumin presents a very low bioavailability, remaining in a great extension in the intestine, as such or as metabolites. However, considering the instability of curcumin at pHs above neutral, its extensive metabolism in intestine and liver and the ability of some microorganisms to degrade curcumin in the gut, the concentrations of orally administered curcumin cannot be maintained for several hours in the gastrointestinal tract.
3.2.2.2. Genotoxicity and carcinogenicity
Antioxidant and pro‐oxidant properties of curcumin, its desmethoxy‐derivatives and its metabolites
Curcuminoids have antioxidant properties scavenging free radicals and becoming weak free radicals themselves said to be short‐lived products (FAO, 2004).
Curcumin is a potent antioxidant that interacts with different types of radicals, including hydroxyl radical (OH), nitric oxide (NO), oxidised glutathione and oxidants such as hydrogen peroxide. Curcumin was also shown to be able to react with non‐physiological radicals and peroxides, like tert‐butyl peroxide (Heger et al., 2014). The chemical structure of curcumin, which includes a wide conjugated system of double bonds, can easily accept single electrons from reactive oxygen species (ROS) by formation of semiquinone radical structure, or lead to the formation of OH‐radicals and H2O2, depending on the concentration and the chemical environment (e.g. presence of transition metals). These features are also shared by curcumin reductive metabolites (tetrahydrocurcumin, hexahydrocurcumin and octahydrocurcumin) and methoxy‐analogues (desmethoxycurcumin and bis‐desmethoxycurcumin, representing on average 16% and 3.6% of the additive under assessment).
The antioxidant properties of curcumin are not affected by reduction, as tetrahydrocurcumin is a stronger antioxidant than curcumin. This suggests that the enolic hydroxy group, present in the enol tautomer, is responsible for the antioxidant properties. Concerning the desmethoxy‐ and bis‐desmethoxy derivatives, their antioxidant properties have been reported to be considerably lower than that of curcumin. The order of the antioxidative capacity of curcuminoids towards 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH, a stable nitrogen‐centred radical) is tetrahydrocurcumin > hexahydrocurcumin > octahydrocurcumin > curcumin > > desmethoxycurcumin > >> bisdemethoxycurcumin. With respect to galvinoxyl radicals, the order is curcumin > > octahydrocurcumin > tetrahydrocurcumin (Somparn et al., 2007; Feng and Liu, 2009, as reported by Heger et al., 2014). When the scavenging properties were investigated in two in vitro 1O2‐generating systems, the extent of protection was in the order curcumin > desmethoxycurcumin > bisdesmethoxycurcumin, suggesting that the methoxy groups play a role in 1O2 scavenging (Subramanian et al., 1994, as reported by Heger et al., 2014).
Curcumin metabolites, including vanillin, ferulic acid and 4‐vinylguaiacol, are also antioxidants (Heger et al., 2014).
On the other hand, experimental studies have demonstrated that, although low concentrations of curcumin induce antioxidant effects, higher concentrations of this compound increase the cellular levels of ROS, such as superoxide anion, hydroxyl radical and hydrogen peroxide (reviewed in Burgos‐Morón et al., 2010). For example, the two α,β‐unsaturated ketones in the structure of curcumin can react covalently with thiol groups of cysteine (Michael addition), resulting in the generation of ROS. In the presence of transition metals, curcumin can generate reactive oxygen species and behave as a prooxidant in cells (Yoshino et al., 2004) or act as a chelating agent (reviewed in Burgos‐Morón et al., 2010).
Genotoxicity
The potential of turmeric rhizome extracts and curcumin to induce genotoxicity was repeatedly assessed in the past. JECFA concluded in 1996, that in limited studies with curcumin preparations of up to 85% purity, or of unknown purity, no mutagenic activity was reported in bacteria and equivocal activity was observed for the induction of chromosomal aberrations. Therefore, there was no evidence of curcumin genotoxicity. No new studies were evaluated in the assessment by JECFA (WHO, 2004a).
In 2010, the ANS Panel considered in addition new studies and concluded ‘that the indications provided by the positive results for curcumin in several in vitro and in vivo tests for genotoxicity, especially those detecting chromosomal aberrations and DNA adducts should not be disregarded, and that the available in vivo genotoxicity studies were insufficient to eliminate the concerns regarding genotoxicity’.
The applicant submitted copies of the studies already assessed by JECFA and EFSA between 1974 and 2010 for the evaluation of curcumin and turmeric extract with respect to their genotoxicity, mutagenicity and carcinogenicity and performed a structured literature search covering the period 2010–2019. The search included the databases Livivo, Toxnet, OVID, Pubmed/Medline and Web of Science and the search terms ‘Curcuma longa’, ‘turmeric’, ‘curcumin’, ‘8024‐37‐1’, ‘617‐027‐4’, ‘283‐882‐1’, ‘458‐37‐3’, ‘207‐280‐5’ ‘genotox*’, ‘carcinogen*’. The search identified 683 hits and 82 publications were identified as relevant for the present assessment.27
The studies were evaluated considering the relevance of the test item (in comparison with the additive under assessment, turmeric extract containing > 90% curcuminoids), the reliability of the results (evaluated as the degree of compliance with the corresponding technical guidance) and the relevance of the results for the current assessment. The outcome of the evaluation of the individual studies is available in Appendix A. A short summary of the evaluation is presented below.
It should be noted that in several studies, the test item was not free curcumin but nanoparticles loaded with curcumin produced for pharmaceutical purposes to increase its bioavailability (reviewed by Her et al., 2018). A number of studies were designed with the aim to demonstrate the alleged protective properties of curcumin against the effects of some genotoxic substances (doxorubicin, cis‐platin, cyclophosphamide perfluorooctane sulfonate and β‐cyfluthrin).
The FEEDAP Panel notes that in the genotoxicity and carcinogenicity studies performed within the National Toxicology Program (NTP, 1993), a turmeric extract containing 79–85% curcumin, 11.3–16.9% desmethoxycurcumin and 1.3–3.1% bis‐desmethoxycurcumin was tested, which is very similar in its composition to the turmeric extract under evaluation.
In vitro studies
Bacterial reverse mutation test
Thirteen studies, either pre‐Organisation for Economic Co‐operation and Development (OECD) or conducted according to OECD TG 471, consistently indicate that different test items, including pure curcumin (tested in two studies), are not mutagenic in bacteria (Salmonella Typhimurium). In particular, the NTP study conducted with turmeric oleoresin (major component 79–85% curcumin compound I, CAS No 458‐37‐7), a test item comparable in its composition to the turmeric extract under evaluation, concluded that turmeric oleoresin was not mutagenic in Salmonella Typhimurium strains TA100, TA1535, TA1537 and TA98 with or without exogenous metabolic activation (S9) (NTP, 1993).
In vitro chromosomal aberrations
Synthetic curcumin (purity ranging between 90% and 99.4%) was tested in five out of the seven studies.
In Chinese Hamster ovary (CHO) cells, a turmeric extract composed of 79–85% curcumin, 11.3–16.9% desmethoxycurcumin and 1.3–3.1% bis‐desmethoxycurcumin was tested at 5, 10 and 16 μg/mL and induced chromosomal aberrations at the highest dose tested (16 μg/mL) in the absence of S9 (NTP, 1993). In the same cell type in the absence of metabolic activation, curcumin with a purity > 94% induced DNA damage at 5 and 10 μg/mL and potentiated the effect of doxorubicin (Antunes et al., 1999), a potent generator of semiquinone radicals which cooperated with curcumin sharing the same ability. In another study performed in CHO cells, negative results were obtained with a formulation containing 25% curcuminoids when tested up to 15 μg/mL (corresponding to 3.75 μg curcumin/mL) (Ravikumar et al., 2018).
A dose‐dependent increase of chromosomal aberration was reported in human lymphocytes exposed to curcumin solutions in ethanol at concentrations of 5 and 10 μg/mL, in the absence of S9 (Sebastià et al., 2012), while peripheral blood lymphocytes treated with curcumin (purity > 94%) at 6.3, 12.5 and 25 μg/mL showed an increase in the frequency of aberrant cells at the highest concentration tested only in the presence of S9 (Damarla et al., 2018).
In vitro micronucleus test
Two out of the three in vitro micronucleus tests performed with synthetic curcumin (purity > 94%) showed positive results.
In metabolically competent human hepatoma G2 (HepG2) cells exposed to pure curcumin at 0, 2, 4, 8 and 16 μg/mL, a significant increase of the frequency of micronuclei was observed at the highest concentrations tested (8 and 16 μg/mL). In the same study, pretreatment of the cells with curcumin (2 μg/mL) reduced the frequency of micronuclei induced by cyclophosphamide (Cao et al., 2007). Similarly, a significant increase in the frequency of micronuclei was observed in rat pheochromocytoma (PC12) cells at the highest concentration tested (10 μg/mL), whereas pre‐incubation with curcumin (purity > 94%) at lower concentrations (1, 2.5 or 5 μg/mL) reduced the frequency of micronuclei induced by cis‐platin (Mendonça et al., 2009).
In vitro Comet assay
Six out of nine studies conducted with pure curcumin (purity > 94%) consistently indicated that curcumin induced a dose‐related increase of DNA strand breaks at concentrations above 2 μg/mL.
Blasiak et al. (1999a,b) showed that curcumin induced DNA strand breaks in human lymphocytes and gastric mucosa cells in vitro when tested at concentrations ranging from 3.7 to 18.4 μg/mL. The authors also reported an additive effect with hexavalent chromium, confirming that transition metals may enhance the formation of radicals by polyphenols and the induction of oxidative stress (Sakihama et al., 2002). It is well known that reduction of hexavalent chromium generates reactive oxygen species (ROS), leading to oxidative DNA damage (De Flora and Wetterhahn, 1989 as referenced in Blasiak).
In this respect, Cao et al. (2006) reported that in metabolically competent HepG2 cells curcumin induced oxidative DNA damage in mitochondrial and nuclear DNA. Moreover, it was observed that curcumin at concentrations ≥ 5 μg/mL increased the production of ROS in a dose‐dependent manner, while curcumin trapped ROS at concentrations of 2 μg/mL and lower, suggesting an antioxidant effect of curcumin at low doses and a pro‐oxidant activity at high doses (Cao et al., 2006; Kocyigit and Guler, 2017).
The FEEDAP Panel notes that although the in vitro Comet assay is not implemented into an official regulatory test guideline, the results obtained with this assay could be relevant to providing mechanistic information.
In vivo studies
In vivo chromosomal aberrations
Three out of 10 studies were conducted with curcumin (of unknown purity in two cases) and seven with different test materials (nanoparticles, turmeric spice, turmeric powder, complexes with phosphatidylcholine or essential oils). All the studies gave negative results, and were conducted following OECD TGs with some limitation. Although none of the studies showed clear evidence of bone marrow exposure, in one study curcumin was tested up to the highest recommended dose (2,000 mg/kg bw) by the OECD guideline (Aggarwal et al., 2016).
Oral administration of 0.2% curcumin in the diet (purity 97%) decreased by 70% the frequency of chromosomal aberrations induced by β‐cyfluthrin, a pyrethroid inducing oxidative stress and genotoxicity. The Panel notes that the reduction in the frequency of chromosomal aberrations could be considered as indirect evidence of systemic bioavailability of curcumin, since the protective effect was observed in bone marrow cells. However, the results of the study were considered of limited relevance as curcumin (one dose) was only tested in combination with β‐cyfluthrin (Verma et al., 2016).
In vivo micronucleus test
Four out of the 14 in vivo micronucleus test studies were performed with appropriate test material (curcumin, purity > 94%) and the remaining 10 studies with different test materials (nanoparticles, turmeric spice, turmeric powder, complexes with phosphatidylcholine or essential oils). All the studies were negative but showed some limitation mainly related to the level of the dose tested and the lack of evidence of bone marrow exposure. However, in two out of the four studies conducted with curcumin, the compound was tested up to the highest recommended dose by OECD TG 474 (2,000 mg/kg bw) (Aggarwal et al., 2016; Damarla et al., 2018).
In addition, one study performed with pure curcumin showed some indication of systemic exposure and indirect evidence of bone marrow exposure, when curcumin administered by gavage showed 50% reduction of micronuclei induced by cisplatin administered intraperitoneal (Mendonça et al., 2015).
In vivo Comet assay
Four out of seven in vivo Comet assays were conducted with curcumin (purity > 94%) and the remaining three with nanoparticles coated with curcumin. Four studies were designed to demonstrate the protective properties of curcumin against the effects of some genotoxic substances (e.g. cis‐platin, β‐cyfluthrin and cyclophosphamide). All studies were negative regarding the genotoxicity of curcumin, but were conducted following OECD TG with some limitation. In all the studies, the highest dose tested was much below the OECD recommendations, while in three studies, only one dose level was tested. A limited number of targets were analysed: only one tissue (blood or bone marrow) in three studies, blood and kidney in one study. None of the studies investigated genotoxic effects in the liver or at the site of contact (gastro‐intestinal tract).
Other in vivo studies
In Long‐Evans Cinnamon rats, exposure to 0.5% curcumin (95% purity) in the diet enhanced the formation of etheno‐DNA adducts in liver 9‐ to 25‐fold in nuclear DNA and three‐ to fourfold in mitochondrial DNA (Nair et al., 2005). The rat strain has a genetic abnormality which leads to the accumulation of copper in the liver. It is used as a model for the human Wilson's disease, which is characterised by a massive accumulation of copper in various tissues. This leads to an abnormal degree of oxidative stress and tissue damage, but not to cancer. The enhanced formation of etheno‐DNA adducts after treatment of the rats with curcumin is due to the concurrent effect of copper and curcumin in the formation of ROS and cannot be used as a model for healthy subjects.
Carcinogenicity
Based on dose finding in the 13‐week studies (NTP, 1993) described above, NTP (1993) conducted 2‐year studies in mice and rats.
Rats
F344/N rats (60 animals/sex per dose) were fed ad libitum diets containing 0, 2,000, 10,000 or 50,000 mg/kg turmeric extract (composition: 79–85% curcumin, 11.3–16.9% desmethoxycurcumin, 1.3–3.1% bis‐desmethoxycurcumin) for 103 weeks, which were estimated to have finally delivered average doses of 80/90, 460/440 or 2,000/2,400 turmeric extract/kg bw per day in males and females, respectively (NTP, 1993).
Pathology findings included nonneoplastic and neoplastic changes. Nonneoplastic lesions occurred in the gastrointestinal tract of animals of the highest dose group: increased incidences of ulcers, hyperplasia and hyperkeratosis of the forestomach in males; ulcers, chronic inflammation and hyperplasia of the caecum in males and females; similar lesions in the colon of males. Furthermore, male and female rats that received 50,000 mg/kg and male rats that received 10,000 mg/kg had significantly increased incidences of sinus ectasia of the mesenteric lymph node.
With respect to tumour development, no neoplasms were found in male rats. The incidences of clitoral gland adenoma were significantly increased in all exposed groups of females. Clitoral gland carcinomas occurred in one control female and in four low dose females, but not in females that received higher doses. The incidences of clitoral gland adenoma and carcinoma (combined) in all exposed groups were higher than in the controls (6/50) but not dose related since all treated groups showed comparable values (16/48, 15/47, 16/49).
The conclusion in the NTP report was that there was no evidence of carcinogenic activity of the turmeric extract in male F344/N rats administered 2,000, 10,000 or 50,000 ppm, but that there was equivocal evidence of carcinogenic activity of the turmeric extract in female F344/N rats based on increased incidences of clitoral gland adenomas in all exposed groups (NTP, 1993).
Mice
B6C3F1 mice (60 animals/sex per dose) were fed ad libitum the same diets as in the rat experiment for 103 weeks, which were estimated to deliver average doses of 0, 220/320, 1,520/1,620 or 6,000/8,400 mg turmeric extract/kg bw per day in males and females, respectively.
In contrast to the rat study, no nonneoplastic lesions of the gastrointestinal tract were observed. With respect to tumour development, the incidences of hepatocellular adenoma in male and female mice of the mid‐dose group, but not of the low‐ and high‐dose groups, were significantly increased (male: 25/50 (control), 28/50, 35/50, 30/50; female: 7/50 (control), 8/50, 19/51, 14/50). Three males which received 2,000 ppm and three males which received 10,000 ppm had carcinomas of the small intestine; neoplasms of the small intestine were not observed in control males or in males that received 50,000 ppm.
The conclusion in the NTP report was that there was equivocal evidence of carcinogenic activity of the turmeric extract in male B6C3F1 mice based on a marginally increased incidence of hepatocellular adenoma at the 10,000 ppm level, and the occurrence of carcinomas of the small intestine at 2,000 and 10,000 ppm. There was equivocal evidence of carcinogenic activity of the turmeric extract in female B6C3F1 mice based on an increased incidence of hepatocellular adenomas at 10,000 ppm.
Overall JECFA (WHO, 1982, 1996) draw the following conclusions on the results of the 2‐year NTP studies in mice and rats (NTP, 1993): ‘On the basis of the results of these studies, the Committee concluded that the effects were not dose‐related, and that curcumin was not a carcinogen’.
The ANS Panel noted ‘that all statistically significant effects noted by the NTP refer to benign neoplastic lesions (adenomas) and that the incidences for malignant neoplastic lesions (carcinomas), including the small intestine carcinomas of male mice, did not reach statistical significance. The Panel also noted that the effects observed were not dose‐dependent, were in line with historical control values and were not consistent across sexes and/or species. The Panel noted moreover that hepatocellular tumors occurring in untreated and treated B6C3F1 mice are not relevant for humans’. ‘The Panel also noted that the absence of dose‐related effects in the NTP study is not due to saturating absorption kinetics because the data demonstrated that blood plasma concentrations increased linearly in a dose related manner over the dietary concentration range of 0.1–2.5%, and that plasma levels of curcumin tended to plateau only at the higher dietary level of 5.0%. The (ANS) Panel agrees with JECFA that curcumin is not carcinogenic’ (EFSA ANS Panel, 2010).
The ANS Panel also noted that ‘in the NTP (1993) studies, gastrointestinal irritation (ulcers, hyperplasia and inflammation) was common in male and female rats in the high‐dose group but this was not observed in mice. The NOAEL for gastrointestinal effects in rats was 10,000 mg/kg in the diet, equal to 440 mg/kg bw per day’. The adverse effects are likely due to oxidative stress, induced by high doses of curcuminoids and are in agreement with the findings detected in the in vitro Comet assay, which showed that high doses of curcuminoids induce oxidative stress, leading to cell damage.
Discussion on genotoxicity and carcinogenicity
In order to draw conclusions on the potential genotoxicity of curcumin (E 100, turmeric rhizome extract), the FEEDAP Panel considered a weight of evidence assessment (EFSA Scientific Committee, 2017). Information from different lines of evidence was integrated including (i) the structure of curcumin and its ability to act both as antioxidant and prooxidant, (ii) the outcome of in vitro genotoxicity studies and (iii) that of in vivo genotoxicity studies, (iv) the outcome of carcinogenicity studies in rat and mice and (v) the limited absorption of curcumin by oral route.
The chemical structure of curcumin, which includes a wide conjugated system of double bonds, can easily accept single electrons from ROS by formation of semiquinone radical structure, or lead to the formation of hydroxyl radicals and H2O2 depending on the concentration and the chemical environment (e.g. in the presence of transition metals). As many other polyphenols, curcumin is able to interact with ROS and can behave as antioxidant (FAO, 2004) or as prooxidant depending on its concentration (Banerjee et al., 2008). Because of its ability to trap or generate ROS, curcumin can induce DNA damage or inhibit DNA damage caused by other compounds, as long as an oxidative mechanism is involved.
The results of in vitro studies in mammalian cells showed genotoxic effects at concentrations above 5 μg curcumin/mL. The FEEDAP Panel notes that these effects could be attributable to indirect mechanisms of genotoxicity since curcumin at concentrations above 5 μg/mL may increase the production of ROS (Sakihama et al., 2002; Yoshino et al., 2004), the level of 8‐oxo‐guanine (Lewinska et al., 2015), inhibit topoisomerase II (Saleh et al., 2012; Ketron et al., 2013; Gordon et al., 2015), and inhibit histone deacetylase (Hassan et al., 2019).
In vivo, no genotoxicity was observed in the bone marrow after oral administration up to 2,000 mg curcumin/kg bw, indicating that DNA damage induced in vitro is not expressed in vivo, most probably due to the low absorption of curcumin.
The FEEDAP Panel notes that none of the studies showed direct evidence of bone marrow exposure even when tested up to the top recommended dose. According to OECD TG 474 (2016), target tissue exposure (e.g. systemic toxicity) needs to be addressed. In this respect, indirect evidence of bone marrow exposure could be deduced from two in vivo studies where co‐administration of curcumin reduced the DNA damage induced by β‐cyfluthrin and cis‐platin in bone marrow (Mendonça et al., 2015; Verma et al., 2016). In addition, evidence that curcumin could be systemically available at 2,000 mg/kg bw was deduced from ADME data in laboratory animals (see Section 3.2.2.1) showing that the concentration of free curcumin in plasma after a single dose administration of 300–500 mg curcumin/kg bw per day was in the order of ng/mL (3.2–60 ng/mL), whereas the concentration of conjugated curcumin in plasma was one or two orders of magnitude higher (200–700 ng/mL, mainly as glucuronide, after a single oral dose of 100–500 mg/kg bw per day).
In respect to the genotoxic effects at other potentially relevant targets, such as the site of first contact, the in vivo micronucleus test, applied as follow‐up of in vitro positive results, has a limited value when there is no direct evidence of bone marrow exposure. In fact, the negative results associated with the systemic exposure do not allow to rule out concern for genotoxicity at the site of contact, where the concentrations of the test item or its metabolites may be higher than the concentrations reached in the bone marrow. In this case, the genotoxic effects in the liver or GI tract should be evaluated (EFSA Scientific Committee, 2011). However, none of the in vivo studies investigated genotoxic effects at the first site of contact (i.e. the mucosa of the GI tract). In the absence of this information, data on genotoxicity were integrated with the outcome of the 2‐year carcinogenicity study (NTP, 1993) in order to conclude on the genotoxic potential of curcumin at the site of contact. The NTP study showed an increase in tumours in liver and the gastrointestinal tract of mice, which were considered not biologically relevant and not dose‐related, respectively. Non‐neoplastic lesions (ulcers, hyperplasia and inflammation) observed in the GI tract in male and female rats in the high‐dose group, but not in mice (NTP, 1993), were considered to be thresholded effects compatible with oxidative stress, inflammation and apoptosis induced by high doses of curcuminoids. Based on the results of the 2‐year carcinogenicity studies in rat and mice, curcumin was considered not carcinogenic and concern for genotoxic effects at the first site of contact was ruled out.
Conclusions on genotoxicity and carcinogenicity
No induction of gene mutations was observed for curcuminoids and turmeric extract in vitro and in vivo. Clastogenic effects (chromosomal damage and micronucleus) and DNA strand breaks observed in in vitro assays are likely to be due to oxidative stress at concentrations > 2 μg/mL, which are two to three orders of magnitude higher than the maximum serum concentration reached in vivo after treatment up to 500 mg/kg bw. In view of the lack of genotoxicity in vivo and the negative results obtained in carcinogenicity studies in rats and mice, the FEEDAP Panel concludes that the use of the additive in feed does not pose concern for genotoxicity and carcinogenicity.
3.2.2.3. Other repeated dose toxicity studies
In the course of the safety assessment of the food additive curcumin (E 100, turmeric rhizome extract), toxicity studies and human studies with curcumin or purified turmeric extracts were evaluated by JECFA (WHO, 2004a,b) and the ANS Panel (EFSA ANS Panel, 2010).
Short‐term and subchronic toxicity
According to the ANS Panel assessment, the short‐term toxicity of curcumin appears to be low. A gastric erosion was observed in rats following curcumin administration of a daily oral dose of 100 mg/kg bw for 6 days (Gupta et al., 1980 as referenced by EFSA ANS Panel, 2010). However, this effect was not seen in 13‐week studies with mice and rats fed a turmeric extract at dietary concentrations of 0, 0.1, 0.5, 1.0, 2.5 or 5.0% (NTP, 1993). For the study in mice, the ANS Panel identified 5% turmeric extract in the diet as the NOAEL, corresponding to 9,280 and 7,700 mg turmeric extract/kg bw per day in females and males, respectively, the highest doses tested. For the rat study, the ANS Panel identified an NOAEL of 2.5% turmeric extract in the diet, equivalent to 1,300 mg/kg bw per day for males and 1,450 mg/kg bw per day for females, based on hyperplasia of the mucosal epithelium in the caecum and colon of males and females at the highest dose level. The Panel noted that in these 13‐week studies, a turmeric extract containing 79–85% curcumin, 11.3–16.9% desmethoxycurcumin and 1.3–3.1% bis‐desmethoxycurcumin was tested (NTP, 1993), which is very similar in its composition to the turmeric extract under evaluation.
Metabolites of curcumin
A subchronic oral toxicity test in rats performed with a mixture of tetra‐, hexa‐ and octahydrocurcumin at doses of 200, 400 and 800 mg/kg bw for 90 days gave no evidence of treatment‐related toxicity with respect to mortality, body weight gain, feed consumption, clinical observations, haematology, organ weights and histopathological findings (Gopi et al., 2016).
Reproductive and developmental toxicity
JECFA evaluated at its 61st meeting in 2003 (WHO, 2004a,b) an unpublished study on reproductive toxicity with curcumin (E 100, turmeric rhizome extract) (Ganiger, 2002, as referenced by the EFSA ANS Panel, 2010) referred by the ANS Panel in its 2010 assessment (EFSA ANS Panel, 2010).
According to JECFA (WHO, 2004b), a multigeneration study was conducted by Ganiger (2002) in Wistar rats in compliance with the OECD guideline 416 (1983). The test material contained 80% curcumin, with a total curcuminoids content of 99%, and met the specifications of the food additive curcumin (E 100, turmeric rhizome extract), established by JECFA in 2003. The test preparation was fed to groups of 30 males and 30 females in the diet at concentrations of 0, 1,500, 3,000 or 10,000 mg/kg (corresponding to 0, 130–140, 250–290 or 850–960 mg/kg bw per day in males and 0, 160, 310–320 or 1,000–1,100 mg/kg bw per day in females) from 10 weeks before mating and then throughout mating. Females were treated throughout pregnancy and weaning of the offspring. The medium dose, equal to 250–320 mg/kg bw per day for the F1 generation, was identified by JECFA to represent the NOEL of this study based on body weight reduction of the offspring at the highest dose. Based on this NOEL, JECFA allocated for the food additive curcumin (E 100, turmeric rhizome extract) an ADI of 0–3 mg/kg bw per day by applying an uncertainty factor of 100 (WHO, 2004b).
As noted in the ANS opinion (EFSA ANS Panel, 2010), the original work of Ganiger had been published in the open literature (Ganiger et al., 2002, 2007 as referenced by EFSA ANS Panel, 2010). In this report, the test material is described as curcumin (1,7‐bis‐(4‐hydroxy‐3‐methoxy‐phenyl)‐1,6‐heptadiene‐3,5‐dione) with a minimum purity of 95%. The study authors conclude that the NOAEL for the reproductive toxicity of the test material is 10,000 mg/kg, which is equivalent to 847 and 959 mg/kg bw per day for male rats and 1,043 and 1,076 mg/kg bw per day for females for the F0 and F1 generations, respectively.
In agreement with JECFA, the ANS Panel concluded in 2010 ‘that the present database supports an ADI of 3 mg/kg bw per day based on the NOAEL of 250‐320 mg/kg bw/day from the reproductive toxicity study for a decreased body weight gain in the F2 generation observed at the highest dose level, and an uncertainty factor of 100’ (EFSA ANS Panel, 2010).
Human clinical studies
No toxic effects were observed in a phase I study in 25 patients with high‐risk cancerous conditions, receiving up to 8 g of curcumin (99.3% of purity) per day for 3 months for anticancer treatment (Cheng et al., 2001, EFSA ANS Panel, 2010). No side effects were reported in 18 patients with rheumatoid arthritis treated with daily doses of 1.2 g curcumin for 2 weeks (Deodhar et al., 1980 as referenced by EFSA ANS Panel, 2010). In a clinical study in 207 patients with irritable bowel syndrome, receiving daily oral doses of 72 mg or 144 mg of standardised turmeric extract (curcumin content not specified) for 8 weeks, no major side effects were observed. Dry mouth and flatulence were reported by approximately 25% of these patients (Bundy et al., 2004 as referenced by EFSA ANS Panel, 2010).
Conclusions on toxicology
A low oral toxicity of curcuminoids was observed in subchronic toxicity studies and a developmental toxicity study in rats. For curcumin with a minimum purity of 95%, an NOAEL of 250–320 mg/kg bw per day was derived from a reproduction toxicity study and selected as lowest NOAEL. This value can be used as point of departure for the estimation of a maximum safe dose for target species, fed with curcumin as a flavouring in the diet.
3.2.2.4. Safety for the target species
The maximum feed concentration which can be considered safe for the target animals can be derived from the lowest no observed effect level (NOAEL) of 250 mg curcumin/kg bw per day identified by the JECFA (WHO, 2004a,b) and confirmed by the EFSA ANS Panel (2010) in a reproductive toxicity study in rat.28
Applying an uncertainty factor (UF) of 100 to the NOAEL, the safe daily dose for the target species was derived following the EFSA Guidance on the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), and thus, the maximum safe feed concentration was calculated (Table 3).
Table 3.
Maximum safe concentration in feed for different target animals for turmeric extract
| Body weight (kg) | Feed intake (g DM/day) | Daily feed intake (g DM/kg bw) | Maximum safe concentration (mg/kg feed)1 | |
|---|---|---|---|---|
| Chickens for fattening | 2 | 158 | 79 | 28 |
| Laying hens | 2 | 106 | 53 | 42 |
| Turkeys for fattening | 3 | 176 | 59 | 38 |
| Piglets | 20 | 880 | 44 | 50 |
| Pigs for fattening | 60 | 2,200 | 37 | 60 |
| Sow lactating | 175 | 5,280 | 30 | 78 |
| Veal calves (milk replacer) | 100 | 1,890 | 19 | 125 |
| Cattle for fattening | 400 | 8,000 | 20 | 110 |
| Dairy cows | 650 | 20,000 | 31 | 72 |
| Sheep/goat | 60 | 1,200 | 20 | 110 |
| Horse | 400 | 8,000 | 20 | 110 |
| Rabbit | 2 | 100 | 50 | 44 |
| Salmon | 0.12 | 2.1 | 18 | 126 |
| Dogs | 15 | 250 | 17 | 132 |
| Cats2 | 3 | 60 | 20 | 22 |
| Ornamental fish | 0.012 | 0.054 | 5 | 489 |
Complete feed containing 88% DM, milk replacer 94.5% DM.
The uncertainty factor for cats is increased by an additional factor of 5 because of the reduced capacity of glucuronidation.
Because glucuronidation of the metabolites of curcuminoids is an important metabolic reaction to facilitate the excretion of these compounds (see Section 3.2.2.1), the calculation of safe concentrations in cat feed needs an additional UF of 5. This factor is due to the unusually low capacity for glucuronidation in cats (Court and Greenblatter, 1997).
The FEEDAP Panel concludes that turmeric extract added to the feed of all animal species is safe at the maximum proposed use level of 15 mg/kg feed.
No specific proposals have been made by the applicant for the use level in water for drinking. Therefore, the FEEDAP Panel considered the same use level in water for drinking (15 mg/L) as proposed for feed (15 mg/kg). When used at 15 mg/L water for drinking, the intake of the additive via water would be two to three times higher than the intake via feed for poultry, pigs and rabbits (EFSA FEEDAP Panel, 2010). Considering the magnitude of the margin of safety, a concentration of 15 mg/L water for drinking is considered safe for all animal species, except chickens for fattening, laying hens, turkeys for fattening, piglets, rabbits and cats. For these species, the use of turmeric extract in water for drinking should be reduced to ensure that the exposure is comparable to that from feed at the maximum proposed use level (15 mg/kg).
3.2.2.5. Safety for the consumer
In agreement with the human clinical data presented in the assessments of JECFA and the ANS Panel (WHO, 2004b; EFSA ANS Panel, 2010), EMA concluded that no major side effects have been reported in clinical studies after oral intake of turmeric extracts and curcumin in doses up to 8 g curcumin per day for 3 months (EMA, 2018b) (see Section 3.2.2.2).
The use of curcumin (E 100, turmeric rhizome extract) is authorised as a food additive for colouring purposes with combined maximum limits.6
Owing to the low bioavailability of curcumin, its rapid metabolism and efficient excretion in rodents and humans (Section 3.2.2.1), it is not expected that curcuminoids or their metabolites accumulate in edible tissues and products of the target species. Curcumin was not detected in yolk and albumen of eggs collected on days 14 and 21 from layers supplemented with 30 and 50 mg curcumin/kg feed (Galli et al., 2018).
Considering the reported human exposure due to the direct use of curcumin (E 100, turmeric rhizome extract) (EFSA ANS Panel, 2010), the FEEDAP Panel considers it as unlikely that consumption of products from animals given turmeric extract at the proposed maximum dose (15 mg/kg) would significantly increase human background exposure to curcuminoids from food.
Consequently, no safety concern would be expected for the consumer from the use of turmeric extract up to the highest safe use level in feed.
3.2.2.6. Safety for the user
No specific data were provided by the applicant regarding the safety of the feed additive for users.
Isolated cases of contact dermatitis and contact urticaria caused by curcumin and/or tetrahydrocurcumin have been reported (EFSA ANS Panel, 2010). Curcumin (synthetic) has been notified to the European Chemical Agency (ECHA) for classification and labelling according to Classification Labelling and Packaging (CLP)29 criteria as skin irritant (H315) and eye irritant (H319). Curcuma longa extracts have been notified to ECHA for classification according to CLP as aspiratory toxic (H304), skin irritant (H315), skin sensitiser (H317), eye irritant (H319) and respiratory irritant (H335).
3.2.2.7. Safety for the environment
The addition of naturally occurring substances that will not result in a substantial increase of the concentration in the environment is exempt from further assessment (EFSA, 2008). This exemption applies to botanical preparations from plants native to Europe. However, C. Longa is not native to Europe. Therefore, the safety for the environment is assessed based on the individual components of the extract.
Curcumin, desmethoxycurcumin and bis‐desmethoxycurcumin have not been evaluated by EFSA with respect to its safety for the environment. As described in Section 3.2.2.2, curcumin has a very low bioavailability and remains to a high extent in the intestine, as such or as metabolites. However, considering the instability of curcumin at pHs above neutral (see Section 3.2.1.2), its extensive metabolism in the intestine and liver, and the ability of some microorganisms to degrade curcumin in the gut, orally administered curcumin is not expected to survive for several hours in the gastrointestinal tract and reach the environment as intact molecule. Therefore, the environmental exposure from faecal material predominately will not be due to the parental compound but to a variety of metabolites, all of them are naturally occurring compounds, e.g. ferulic acid, ferulic aldehyde, vanillin, vanillic acid, 4‐vinyl guaiacol, feruloyl methane. Therefore, no risk for the environment is foreseen.
3.3. Turmeric rhizome essential oil (referred to as turmeric oil)
This application concerns the essential oil derived by steam distillation from the dried rhizomes of C. longa of Indian origin. After steam distillation and water separation, the oil is filtered, dried with anhydrous sodium sulfate and filtered a second time.
The amount of oil present in the dried rhizomes ranges between 3 and 6%. The oil mainly contains ar‐turmerone (about 60%).30 Other components are β‐turmerone, α‐turmerone, (E)‐atlantone, ar‐curcumene, β‐sesquiphellandrene, α‐zingiberene and β‐bisabolene. The molecular structures of the main components of the essential oil are shown in Figure 2.
Figure 2.

Molecular formula of the main components of turmeric essential oil
3.3.1. Characterisation of turmeric oil
The essential oil under assessment is a pale yellow to reddish‐brown oily liquid with characteristic spicy turmeric odour. In three batches of the additive, the optical rotation at 25°C ranged between +0.30° and +2.17° (specification: +3.0 to –32.0°). The refractive index ranged between 1.5131 and 1.5288 (specification: 1.4990–1.5210) and the specific gravity (25°C) between 0.942 and 0.981 g/mL (specification: 0.9010–0.9710 g/mL) in five batches.31 Turmeric oil is identified with the single CAS number 8024‐37‐1, EINECS number 283‐882‐1 and FEMA number 3085.
The product specifications as proposed by the applicant are based on the main components of the essential oil namely ar‐turmerone (40–60%), β‐turmerone (5–15%), ar‐curcumene (3–6%), β‐sesquiphellandrene (3–6%), α‐zingiberene (1–5%), (E)‐atlantone (2‐4%), other constituents < 3%. Analysis of five batches of the additive32 showed compliance with these specifications (Table 4). These six compounds account for about 73.1% on average (range 72.0–75.1%) of the product, expressed as area per cent (%) of the gas chromatographic (GC) profile.
Table 4.
Major constituents of the essential oil from the rhizomes of Curcuma longa L. as defined by specifications (based on the analysis of five batches). The content of each constituent is expressed as the area per cent of the corresponding chromatographic peak (% GC area), assuming the sum of chromatographic areas of all detected peaks as 100%
| Constituent | CAS no | Specification GC Area % | Percentage of oil | |
|---|---|---|---|---|
| EU register name | Meana | Range | ||
| ar‐Turmeroneb | 532‐65‐0 | 40–60 | 47.45 | 43.34–58.75 |
| ß‐Turmerone (curlone) | 82508‐14‐3 | 5–15 | 11.01 | 6.61–12.97 |
| ar‐Curcumene | 644‐30‐4 | 3–6 | 4.49 | 3.97–4.84 |
| ß‐Sesquiphellandrene | 20307‐83‐9 | 3–6 | 3.88 | 1.20–5.20 |
| α‐Zingiberene | 495‐60‐3 | 1–5 | 3.21 | 1.71–3.85 |
| (E)‐Atlantone | 108645‐54‐1 | 2–4 | 3.04 | 2.79–3.22 |
| Total | 73.1 | 72.0–75.1 | ||
EU: European Union; CAS no. Chemical Abstracts Service number
Mean calculated on five batches.
Sum of five isomers identified under different retentions times.
The applicant provided the full characterisation of the five batches obtained by gas chromatography coupled with mass spectrometry (GC‐MS). In total, 105 constituents were detected, 95 of which were identified, accounting for 98.56% of the GC chromatogram peaks area. The unidentified compounds accounted for 1.44% (0.87–1.67%), with the single highest chromatogram peak area < 0.4%. The 13 compounds not included in the specifications but occurring at concentrations > 1% are listed in Table 5. The remaining 76 compounds (ranging between 0.005% and 1%) are listed in the footnote.33
Table 5.
Constituents of the essential oil from the rhizomes of Curcuma longa L. accounting for > 1% of the composition in at least one batch (based on the analysis of five batches) not included in the specification. The content of each constituent is expressed as the area per cent of the corresponding chromatographic peak (% GC area), assuming the sum of chromatographic areas of all detected peaks as 100%
| Constituent | CAS no | Percentage of oil | |
|---|---|---|---|
| EU register name | Meana | Range | |
| (E)‐γ‐Atlantone | 108549‐47‐9 | 1.692 | 1.32–1.90 |
| Benzene, 1‐methyl‐4‐(1‐methylpropyl)‐ | 1595‐16‐0 | 1.482 | 0–2.24 |
| (6R,7R)‐Bisabolone | 72441‐71‐5 | 1.272 | 1.21–1.48 |
| β‐Bisabolene | 495‐61‐4 | 1.11 | 1.08–1.13 |
| m‐Camphorene | 20016‐73‐3 | 0.988 | 0.82–1.24 |
| β‐Bisabolol | 15352‐77‐9 | 0.966 | 0.24–1.39 |
| 4‐tert‐Butylcyclohexane‐1‐carboxylic acid (γ‐curcumene) | 451‐55‐8 | 0.948 | 0.30–3.36 |
| 2‐Methyl‐6‐(p‐tolyl)hept‐2‐en‐4‐ol (ar‐turmerol) |
38142‐57‐3 120710‐98‐7 |
1.58 | 0.12–2.18 |
| Naphthalene, 1,2,3,4,4a,7‐hexahydro‐1,6‐dimethyl‐4‐(1‐methylethyl)‐ | 16728‐99‐7 | 0.846 | 0.38–1.15 |
| 1,8‐Cineole | 470‐82‐6 | 0.672 | 0–1.13 |
| 6‐(3‐Hydroxy‐4‐methylphenyl)‐2‐methylhept‐2‐en‐4‐one | 139085‐16‐8 | 0.622 | 0–2.38 |
| Benzene, (1,1,4,6,6‐pentamethylheptyl)‐ | 55134‐07‐1 | 0.548 | 0.16–1.27 |
| Curcuphenol | 69301‐27‐5 | 0.42 | 0.17–1.11 |
| Total | 13.14 | 11.51–14.16 | |
EU: European Union; CAS no. Chemical Abstracts Service number.
Mean calculated on five batches.
The applicant performed a literature search regarding substances of concern and chemical composition of the plant species C. longa and its preparations.34 The occurrence of methyleugenol in leaf oil (up to 3%) but not in rhizomes oil has been reported for C. longa L. (Awasthi and Dixit, 2009). Methyleugenol in rhizome oil was not detected by the analysis of five batches of the feed additive under assessment (LOD of 0.05%).32
3.3.1.1. Impurities
No information on the concentrations of undesirable compounds in the essential oil is given. The applicant makes reference to the ‘periodic testing’ of some representative flavourings premixtures for heavy metals (mercury, cadmium and lead), arsenic, fluoride, dioxins and dioxin‐like polychlorinated biphenyls (PCBs), organo‐chloride pesticides, organo‐phosphorous pesticides, aflatoxin B1, B2, G1, G2 and ochratoxin A. Since turmeric oil is produced by steam distillation, the likelihood of any measurable carry‐over of heavy metals is low except for mercury (Tascone et al., 2014).
3.3.1.2. Shelf‐life
The typical shelf‐life of turmeric oil is stated to be at least 18 months, when stored in tightly closed containers under standard conditions (in a cool, dry place protected from light).35 No data were provided to support this statement.
3.3.1.3. Conditions of use
Turmeric oil is intended to be added to feed and water for drinking for all animal species without withdrawal period.26 The maximum recommended use level is 20 mg/kg complete feed for all species, except veal calves (milk replacer) for which the recommended maximum use level is 80 mg/kg (on dry matter basis). No specific use level has been proposed by the applicant for the use in water for drinking.
3.3.2. Safety
The assessment of safety is based on the maximum use level proposed by the applicant.
3.3.2.1. Absorption, distribution, metabolism and excretion
Turmerones, the major components of the essential oil, and atlantones are ketones, which are expected to be absorbed, reduced to the corresponding secondary alcohol and then excreted after conjugation. This is supported by preliminary data from Mehrotra et al. (2009) who reported for several turmerones a rapid clearance in rabbits after intravenous administration of turmeric oil36 with elimination half‐lives between 2.0 and 3.5 h. The enzymes involved in the biotransformation pathways of these compounds are present in all the target species (food‐producing and non‐food producing), with the exception of cats which have a low capacity of glucuronidation (reviewed in EFSA FEEDAP Panel, 2016).
Other minor constituents are aliphatic mono‐ or sesquiterpenes, which are expected to be extensively metabolised and excreted as innocuous metabolites and carbon dioxide in the target animals (EFSA FEEDAP Panel, 2016).
3.3.2.2. Toxicological studies
The applicant submitted a subchronic 90‐day oral toxicity rat study performed with of an essential oil from turmeric (C. longa) rhizomes (Liju et al., 2013). Analysis shows that the essential oil tested is similar in composition and content to the essential oil under assessment (Table 6). The differences in the composition in the characterised fraction are mainly due to the variability of the major component ar‐turmerone, for which the difference lies in the range of 3–18%.
Table 6.
Comparison of the test item used in the genotoxicity and subchronic oral toxicity study by Liju et al. (2013) (A) and the turmeric oil under application (B)
| Constituent | Essential oil A (%) | Essential oil B (%) |
|---|---|---|
| ar‐Turmerone | 61.79 | 47.45 (43.34–58.75) |
| ß‐Turmerone | 12.48 | 11.01 (6.61–12.97) |
| ar‐Curcumene | 6.11 | 4.49 (3.97–4.84) |
| ß‐Sesquiphellandrene | 2.81 | 3.88 (1.20–5.20) |
| α‐Zingiberene | 2.98 | 3.21 (1.71–3.85) |
| β‐Bisabolene | 1.48 | 1.11 (1.08–1.13) |
| Total | 86.17 | 71.15 |
A total of 50 male and female Wistar rats (5 males and 5 females per group) were given 0 (control), 0 (vehicle control), 100, 250 or 500 mg of essential oil/kg bw per day via the diet for 90 days. The study was carried out following the OECD Guideline 408. No deaths and no significant differences in growth were observed among the groups. The results on haematology, blood chemistry, gross pathology and histology showed no evidence of any treatment‐related adverse effects. From this study, the highest dose tested (500 mg/kg bw per day) was identified by the authors as the no observed adverse effect level (NOAEL). The FEEDAP Panel agrees with the conclusions of the authors of the study. The NOAEL of 500 mg essential oil/kg bw per day would correspond to an NOAEL of 310 mg/kg bw per day for the major component of the oil, ar‐turmerone.
The genotoxicity of turmeric essential oil (A) was also investigated by Liju et al. (2013). The essential oil did not produce any mutagenicity to Salmonella Typhimurium TA98, TA100, TA102 and TA1535 with or without metabolic activation at concentrations of 0.1, 1 and 3 mg/plate. Administration of turmeric oil to rats (1 g/kg bw) by oral gavage for 14 days did not produce any chromosomal aberration or micronuclei in rat bone marrow cells. No evidence of bone marrow exposure was observed; however, the study was performed testing the top recommended dose by OECD TG 474 for an administration period of 14 days. The FEEDAP Panel concludes that the use of the additive in feed is unlikely to pose a concern for genotoxicity.
Human clinical studies
In a human clinical study, daily intake of high doses of a turmeric oil, containing turmerones and zingiberene as major components, over 3 months did not result in major side effects apart from the allergic reaction in one subject and a change in serum lipids in another. Nine volunteers were administered a daily dose of 0.6 mL of turmeric (C. longa) oil (plant part of origin not specified; given composition: 59% turmerone and ar‐turmerone, 25% zingiberene, 1% cineole, 1% α‐phellandrene, 0.6% d‐sabinene, 0.5% borneol, further components: α and β–allatone, sesquiterpene alcohol) in three aliquots for 1 month and a daily dose of 1 mL in three aliquots for 2 months. Blood pressure, pulse, haematological parameters, liver and kidney functions and side effects were recorded regularly. One of the subjects interrupted the treatment on the 3rd day for allergic skin rashes, another discontinued on the 7th day for intercurrent fever requiring antibiotic treatment. In the remaining seven volunteers, no side effects were observed except for a reversible change in serum lipids in one subject (Joshi et al., 2003, as referenced by the EFSA ANS Panel, 2010).
3.3.2.3. Safety for the target species
Tolerance studies and/or toxicological studies made with the essential oil under application were not submitted. In the absence of these data, the approach to the safety assessment of the whole mixture can be based on read‐across from a sufficiently similar mixture (EFSA Scientific Committee, 2019a). The FEEDAP Panel considers the composition of the turmeric rhizome oil tested in the 90‐day study (Liju et al., 2013) sufficiently similar to that of the oil under assessment. In addition, the turmeric oil under assessment is well characterised (up to 98.56%) and does not contain substances of concern in the characterised fraction. Therefore, the FEEDAP Panel identifies the NOAEL of 500 mg/kg bw per day from this 90‐day study as a suitable reference point to assess the safety of the turmeric oil under assessment.
Applying an UF of 100 to the NOAEL the safe daily dose for the target species was derived following the EFSA Guidance on the safety of feed additives for the target species (EFSA FEEDAP Panel, 2017b), and thus, the maximum safe feed concentration was calculated (Table 7).
Table 7.
Maximum safe concentration in feed for different target animals for turmeric oil
| Body weight (kg) | Feed intake (g DM/day) | Daily feed intake (g DM/kg bw) | Maximum safe concentration (mg/kg feed)a | |
|---|---|---|---|---|
| Chickens for fattening | 2 | 158 | 79 | 56 |
| Laying hens | 2 | 106 | 53 | 83 |
| Turkeys for fattening | 3 | 176 | 59 | 75 |
| Piglets | 20 | 880 | 44 | 100 |
| Pigs for fattening | 60 | 2,200 | 37 | 120 |
| Sow lactating | 175 | 5,280 | 30 | 146 |
| Veal calves (milk replacer) | 100 | 1,890 | 19 | 233 |
| Cattle for fattening | 400 | 8,000 | 20 | 220 |
| Dairy cows | 650 | 20,000 | 31 | 143 |
| Sheep/goat | 60 | 1,200 | 20 | 220 |
| Horse | 400 | 8,000 | 20 | 220 |
| Rabbit | 2 | 100 | 50 | 88 |
| Salmon | 0.12 | 2.1 | 18 | 251 |
| Dogs | 15 | 250 | 17 | 264 |
| Catsb | 3 | 60 | 20 | 55 |
| Ornamental fish | 0.012 | 0.054 | 5 | 978 |
Complete feed containing 88% DM, milk replacer 94.5% DM.
The uncertainty factor for cats is increased by an additional factor of 5 because of the reduced capacity of glucuronidation.
Because glucuronidation of Phase I metabolites of turmerones and structurally related compounds is an important metabolic reaction to facilitate the excretion of these compounds (see Section 3.3.2.1), the calculation of safe concentrations in cat feed needs an additional UF of 5. This factor was due to the unusually low capacity for glucuronidation in cats (Court and Greenblatter, 1997).
The FEEDAP Panel concludes that turmeric oil added to the feed of all animal species is safe at the maximum proposed use level of 20 mg/kg feed. The higher maximum use level of 80 mg/kg for veal calves is also considered safe for this species category.
No specific proposals have been made by the applicant for the use level in water for drinking. Therefore, the FEEDAP Panel considered the same use level in water for drinking (20 mg/L) as proposed for feed (20 mg/kg). When used at 150 mg/L water for drinking, the intake of the additive via water would be two to three times higher than the intake via feed for poultry, pigs and rabbits (EFSA FEEDAP Panel, 2010). Considering the magnitude of the margin of safety, a concentration of 20 mg essential oil/L water for drinking is considered safe for all animal species.
3.3.2.4. Safety for the consumer
Rhizomes of C. longa and their preparations including the essential oil are added to a wide range of food categories for flavouring purposes. Consumption figures for the EU are not available. The Fenaroli's handbook of flavour ingredients (Burdock, 2010) cites values of 2.175 mg/kg bw per day for turmeric rhizomes.
No data on residues formation in products of animal origin were made available for any of the constituents of the essential oil. However, the Panel recognises that the constituents of turmeric oil are expected to be absorbed, extensively absorbed, distributed, metabolised and excreted in laboratory animals and in the target species (Section 3.3.2.1). Therefore, a relevant increase of the uptake of the individual constituents by humans consuming products of animal origin is not expected.
Considering the reported human exposure due to direct use of turmeric rhizomes and its preparations in food (Burdock, 2010), it is unlikely that consumption of products from animals given turmeric oil at the proposed maximums use level would significantly increase human background exposure.
Consequently, no safety concern would arise for the consumer from the use of turmeric oil up to the highest safe level in feed.
3.3.2.5. Safety for the user
No specific data were provided by the applicant regarding the safety of the feed additive for users.
Curcuma longa extracts (which include the essential oil) have been notified to ECHA for classification according to CLP29 as aspiratory toxic (H304), skin irritant (H315), skin sensitiser (H317), eye irritant (H319) and respiratory irritant (H335).
3.3.2.6. Safety for the environment
The additions of naturally occurring substances that will not result in a substantial increase of the concentration in the environment are exempt from further assessment (EFSA, 2008). This exemption applies to botanical preparations from plants native to Europe. However, C. longa is not native to Europe. Therefore, the safety for the environment is assessed based on the individual components of the essential oil.
The major components of turmeric oil, ar‐turmerone, β‐turmerone, ar‐curcumene, β‐sesquiphellandrene, a‐zingiberene and (E)‐atlantone, as well as the minor constituents present in turmeric essential oil, have not been evaluated by the EFSA FEEDAP Panel with respect to its safety for the environment. These compounds are aliphatic or aromatic sesquiterpenes, some of them (turmerones and atlantones) are characterised by the presence of a ketone functional group and are expected to be metabolised by the target species (see Section 3.3.2.1). These hydrocarbon derivatives are chemically related to the substances evaluated by EFSA as chemical group (CG) 31 for use in animal feed (EFSA FEEDAP Panel, 2015, 2016) for which EFSA concluded that they were ‘extensively metabolised by the target species and excreted as innocuous metabolites or carbon dioxide’. Average feed levels of constituents of turmeric essential oil in animal feed are much lower than the authorised use levels for CG 31 substances. Therefore, no risk for the safety of the environment is foreseen. The same conclusion applies to the substances chemically related to those evaluated in CG 31.
The use of turmeric essential oil up to the highest safe level in feed is not expected to pose a risk for the environment.
3.4. Turmeric rhizome oleoresin (referred to as turmeric oleoresin)
Turmeric oleoresin is obtained by solvent extraction of dried rhizomes of C. longa of Indian origin. The dried rhizomes are ground and the resulting turmeric powder is extracted with solvent (ethyl acetate). The solvent is removed by distillation.
Besides fibre, lipids and proteins, the main components of turmeric oleoresin are curcuminoids (curcumin, desmethoxycurcumin and bis‐desmethoxycurcumin) and volatile components from the essential oil.
3.4.1. Characterisation of turmeric oleoresin
The additive is a reddish‐brown viscous liquid with characteristic odour.37 It contains by specification 20–35% total curcuminoids (w/w) and 30–35% essential oil (v/w).27
Table 8 summarises the results of proximate analysis38 and Table 9 the characterisation of the fraction of secondary metabolites in four batches of the additive from one producer (from Indian origin). The content of essential oil was determined by distillation. Individual curcuminoids were determined by HPLC with UV detection,38 the essential oil fraction was characterised by GC‐MS.39 The relative concentration of the components of the essential oil (as g/100 mL) was converted in g/100 g considering the average density of the essential oil (936 kg/m3).
Table 8.
Proximate analysis of turmeric oleoresin (Curcuma longa L.) based on the analysis of four batches (mean and range). The results are expressed as % (w/w)
| Constituent | Meana | Range |
|---|---|---|
| % (w/w) | % (w/w) | |
| Humidity | 19.1 | 12.7–24.9 |
| Ash | 0.12 | < 0.1–0.18 |
| Lipids (acid hydrolysis) | 1.47 | 0.39–3.37 |
| Proteins | 1.78 | 1.59–2.12 |
| Fibre | 2.13 | 0.30–3.58 |
| Total | 24.6 | 19.7–28.6 |
Mean calculated on four batches.
Table 9.
Characterisation of the fraction of secondary metabolites of turmeric oleoresin (Curcuma longa L.) based on the analysis of four batches (mean and range). The results are expressed as % (w/w) of turmeric oleoresin
| Constituent | CAS no | Meana | Range |
|---|---|---|---|
| % (w/w) | % (w/w) | ||
| Essential oila | – | 31.5 | 30–33 |
| Total curcuminoids | 27.5 | 26.0–29.3 | |
| Curcumin (I) | 458‐37‐7 | 18.14 | 16.63–20.06 |
| Desmethoxycurcumin (II) | 33171‐16‐3 | 5.14 | 4.78–5.61 |
| Bis‐desmethoxycurcumin (III) | 33171‐05‐0 | 4.21 | 3.92–4.89 |
| Total identified (essential oil + curcuminoids) | 59.0 | 57.0–62.3 |
The essential oil fraction was shown to have essentially the same composition40 as the turmeric essential oil described in Section 3.3.1. The fraction of total curcuminoids in the oleoresin contains a higher percentage of desmethoxycurcumin and bis‐desmethoxycurcumin compared to turmeric extract (see Section 3.2.1).
Overall, it is estimated that results of the proximate analysis taken together with the values obtained for the essential oil and the curcuminoids account for approximately 83% of the composition of oleoresin.
3.4.1.1. Impurities
Residual solvent (ethyl acetate) determined in five batches ranged between 17 and 18 mg/kg.37 Data on chemical and microbial impurities were provided in three batches of turmeric oleoresin.41 The concentrations of heavy metals were below the corresponding limit of quantification (LOQ), with the exception of lead (0.06 mg/kg) in one batch. Mycotoxins (aflatoxins B1, B2, G1 and G2) were below the LOQ except aflatoxin B1 in two batches (1.0 and 4.5 μg/kg). Pesticides were not detected in a multiresidue analysis, with the exception of cypermethrin (0.79 mg/kg) and malathion (0.25 mg/kg) in one batch, phorate (0.89–22.9 mg/kg) and phorate‐sulfoxide in two batches, chlorpyrifos ethyl (0.32–0.79 mg/kg) in three batches. In the same batches, the sum of polychlorinated dibenzo‐p‐dioxin (PCDD), polychlorinated dibenzofuran (PCDF) and dioxin‐like polychlorinated biphenyls (PCBs) ranged between 0.331 and 0.535 ng WHO (2005) PCDD/F+PCB TEQ (toxic equivalents)/kg wet weight (upper bond). None of the data on chemical impurities raised concerns.
Analysis of microbial contamination of three batches of turmeric oleoresin indicated that Salmonella spp. was absent in 25 g, Enterobacteriaceae, total viable count, yeasts and moulds were < 10 colony‐forming unit (CFU)/g, except Enterobacteriaceae in one batch (40 CFU/g).
3.4.1.2. Shelf‐life
The applicant states that the typical shelf‐life of flavourings is at least 12 months, when stored in tightly closed containers under standard conditions. No stability studies were performed for the oleoresin.
3.4.1.3. Conditions of use
Turmeric oleoresin is intended to be added to feed and water for drinking for all animal species without withdrawal period.26 The applicant proposed a use level of 30 mg/kg complete feed for chickens for fattening and laying hens and 5 mg/kg for pigs, veal calves, cattle for fattening and dairy cows, sheep, goats, horses, rabbit and fish. No specific use level has been proposed by the applicant for non‐food producing animals and for the use in water for drinking.
3.4.2. Safety
The assessment of safety is based on the maximum use levels proposed by the applicant.
The ADME of the individual components of turmeric oleoresin has been already described in Section 3.2.2.1 (curcuminoids) and in Section 3.3.2.1 (components of turmeric essential oil). The FEEDAP Panel notes that concomitant application of curcumin with the essential oil present in turmeric oleoresin enhances the bioavailability of curcuminoids by a factor 1.1–1.3 (Jäger et al., 2014; Purpura et al., 2018).
Toxicological studies with the turmeric oleoresin under assessment are not available to the FEEDAP Panel. The studies relevant to the assessment of the known individual components of turmeric oleoresin have been already described in Sections 3.2.2.2 and 3.3.2.2.
3.4.2.1. Safety for the target species
Tolerance studies and/or toxicological studies made with the oleoresin under application were not submitted.
In the absence of these data, the approach to the safety assessment of a mixture whose individual components are known is based on the safety assessment of each individual component (component‐based approach). This approach requires that the mixture is sufficiently characterised. The individual components can be grouped into assessment groups, based on structural and metabolic similarity. The combined toxicity can be predicted using the dose addition assumption within an assessment group, taking into account the relative toxic potency of each component (EFSA Scientific Committee, 2019a).
Based on considerations related to structural and metabolic similarities, the identified components of the oleoresins were allocated to two assessment groups: curcuminoids and volatile components of the essential oil (Table 10).
Table 10.
Compositional data, intake values, reference points and margin of exposure (MOE) for the individual components of turmeric oleoresin classified according to assessment groups(a)
| Oleoresin composition | Exposure | Hazard characterisation | Risk characterisation | |||
|---|---|---|---|---|---|---|
| Assessment group | Max conc. in the oleoresin | Max Feed conc. | Intake | Cramer class | NOAEL | MOE |
| % (w/w) | mg/kg | mg/kg bw | mg/kg bw | |||
| Curcuminoids | 29.34 | 8.80 | 0.6954 | 250 | 360 | |
| Essential oil | 33.0 | 9.90 | 0.7821 | – | 500 | 639 |
NOAEL: no observed adverse effect level; MOE: margin of exposure.
Intake calculations for the individual components are based on the use level of 30 mg/kg in feed for chickens for fattening, the species with the highest ratio of feed intake/body weight. The MOE for each component is calculated as the ratio of the reference point (NOAEL) to the intake.
For each assessment group, exposure in target animals was estimated considering the use levels in feed, the percentage of the fraction in the oleoresin and the default values for feed intake according to the guidance on the safety of feed additives for target species (EFSA FEEDAP Panel, 2017b). Default values on body weight are used to express exposure in terms of mg/kg bw. The intake levels of the individual components calculated for chickens for fattening, the species with the highest ratio of feed intake/body weight, are shown in Table 10.
For hazard characterisation, toxicological data from which NOAEL values could be derived were available for curcuminoids (see section 3.2.2.3) and for turmeric essential oil (see section 3.3.2.2). From these studies a NOAEL of 250 mg/kg bw per day was derived for curcuminoids and a NOAEL of 500 mg/kg bw per day for the essential oil.
For risk characterisation, the margin of exposure (MOE) was calculated for each assessment group (EFSA Scientific Committee, 2019a). An MOE > 100 allowed for interspecies‐ and intra‐individual variability (as in the default 10 × 10 uncertainty factor).
The approach to the safety assessment of turmeric oleoresin for the target species is summarised in Table 10. As the calculations were done for chickens for fattening, the species with the highest ratio of feed intake/body weight and represent the worst‐case scenario at the use level of 30 mg/kg, the same conclusion can be extended to all animal species for which the additive is intended.
As shown in Table 6, the MOE was > 100 for all the assessment groups, indicating that no concern would arise from the identified components when the additive is used up to the highest proposed use level in feed for the target species. The magnitude of the MOE for curcuminoids is wide enough to account for the enhanced bioavailability (1.1–1.3 fold) when administered together with essential oil.
The identified compounds together with ash, protein, lipids, fibre and moisture account for approximately 83% w/w of the oleoresin. Carbohydrate would be expected to account for much of the unidentified fraction, since carbohydrates comprise up to 70% of the dried rhizome and, in part at least, would be extractable with a semi‐polar solvent such as ethyl acetate. While analysis of various rhizome samples shows that the diarylheptanoids and terpenoids are the dominant groups of secondary metabolites present, small amounts of other compounds including simple phenolics (ferulic and vanillic acids), saponins and steroids (β‐sitosterol) may be present to varying extents (Li et al., 2011). Given the capacity of the ethyl acetate to extract both polar and non‐polar constituents, such minor constituents when present in the rhizome would be expected to be extracted into the oleoresin. However, none of these minor components detected in rhizome samples give rise to safety concerns and, consequently, the unidentified fraction of the oleoresin is considered unlikely to present a hazard. This view is supported by feeding studies in rats, which showed no adverse effects when 1% turmeric rhizome powder (equivalent to 2,000 mg/kg bw per day) or up to 0.25% turmeric ethanolic extract (equivalent to 500 mg/kg bw per day) was administered in the diet for 90 days (Deshpande et al., 1998 as cited by EMA, 2018b).
The FEEDAP Panel concludes that turmeric oleoresin added to the feed of all animal species is safe at the maximum proposed use level of 30 mg/kg complete feed for chickens for fattening and laying hens and 5 mg/kg complete feed for the other species.
No specific proposals have been made by the applicant for the use level in water for drinking. Therefore, the FEEDAP Panel considered the same use level in water for drinking (30 mg/L for poultry and 5 for the other species) as proposed for feed (30 mg/kg for poultry and 5 mg/kg for the other species). When used at 30 mg/L water for drinking, the intake of the additive via water would be two to three times higher than the intake via feed for poultry, pigs and rabbits (EFSA FEEDAP Panel, 2010). Considering the magnitude of the margin of exposure, a concentration of 30 mg/L water for drinking for poultry and of 5 mg/L for the other species is considered safe for all animal species.
3.4.2.2. Safety for the consumer
Rhizomes of C. longa and their preparations including the oleoresin are added to a wide range of food categories for flavouring purposes. Consumption figures for the EU are not available. The Fenaroli's handbook of flavour ingredients (Burdock, 2010) cites values of 2.187 mg/kg bw per day for turmeric rhizomes and 0.2033 mg/kg bw per day for the oleoresin.
No data on residues formation in products of animal origin were made available for any of the constituents of the oleoresin. When considering the ADME of the individual components, curcuminoids (see Section 3.3.2.1) as well as the volatile components of the essential oil, which show a rapid conjugation and elimination (see Section 3.3.2.1), a relevant increase of the uptake of these compounds by humans consuming products of animal origin is not expected.
Considering the reported human exposure due to direct use of turmeric rhizomes and its preparations in food (Burdock, 2010), it is unlikely that consumption of products from animals given turmeric oleoresin at the proposed maximum use level would significantly increase human background exposure.
Consequently, no safety concern would arise for the consumer from the use of turmeric oleoresin up to the highest safe level in feed.
3.4.2.3. Safety for the user
No specific data were provided by the applicant regarding the safety of the feed additive for users.
Isolated cases of contact dermatitis and contact urticaria caused by curcumin and/or tetrahydrocurcumin have been reported (EFSA ANS Panel, 2010). Curcuma longa extracts (which includes the oleoresin) have been notified to ECHA for classification according to CLP29 as aspiratory toxic (H304), skin irritant (H315), skin sensitiser (H317), eye irritant (H319) and respiratory irritant (H335).
3.4.2.4. Safety for the environment
The additions of naturally occurring substances that will not result in a substantial increase of the concentration in the environment are exempt from further assessment (EFSA, 2008). This exemption applies to botanical preparations from plants native to Europe. However, C. longa is not native to Europe. Therefore, the safety for the environment is assessed based on the individual components of the oleoresin.
The use of the oleoresin at the maximum proposed use levels in feed (30 mg oleoresin/kg feed) would result in concentrations of curcuminoids and of the major components of the essential oil (ar‐turmerone, β‐turmerone, ar‐curcumene and β‐sesquiphellandrene) lower than those resulting, respectively, from the use of turmeric extract at the proposed use level of 15 mg/kg and of the essential oil at 20 mg/kg.
Therefore, the same considerations on the safety for the environment of curcuminoids (see Section 3.2.2.7) and of the components of the essential oil (see Section 3.3.2.6) applies to the oleoresin.
The use of turmeric essential oil up to the highest safe level in feed is not expected to pose a risk for the environment.
3.5. Turmeric rhizome tincture (referred to as turmeric tincture)
The tincture is obtained by extraction of ground‐dried rhizomes using a water/ethanol mixture (55/45% v/v). After pressing to remove the solid material and filtration, the tincture is obtained. Besides sugars, lipids and proteins, the dry matter (DM) fraction of the tincture contains curcuminoids (see Figure 1), volatile components from the essential oil (see Figure 2) and other non‐volatile phenols.
3.5.1. Characterisation of turmeric tincture
The tincture is a colourless to pale yellow‐brownish liquid with characteristic odour and taste. It has a density of 927–968 kg/m3 (951 kg/m3 on average). The product is a water/ethanol (55/45% v/v) solution, which contains by specification 400–900 μg/mL of total curcuminoids (expressed as curcumin and determined by spectrophotometry as dicinnamoyl methane derivatives42).
Table 11 summarises the results of proximate analysis of six batches of turmeric tincture (origin not specified) expressed as % (w/w).43 The solvent represents up to 97% of the feed additive, the DM content of the tincture ranged between 2.43 and 3.08 g/100 mL (average: 2.65 g/100 mL).44
Table 11.
Proximate analysis of turmeric tincture (Curcuma longa L.) based on the analysis of six batches (mean and range). The results are expressed as % (w/w)
| Constituent | Meana | Range |
|---|---|---|
| % (w/w) | % (w/w) | |
| Dry matterb | 2.78 | 2.62–3.18 |
| Ash | 0.72 | 0.4–0.8 |
| Total sugars | 0.6 | < 0.5–0.8 |
| Lipids | 0.1 | 0.1 |
| Protein | 0.1 | < 0.1–0.1 |
| Fibre | < 0.5 | < 0.5 |
| Solvent (water/ethanol, 55/45) | 97.22 | 96.82–97.38 |
Mean calculated on five batches.
Values converted in g/100 g considering the density of the tincture.
The fraction of secondary metabolites was characterised in the same batches of the additive and the results are summarised in Table 12. Individual curcuminoids were determined by HPLC‐UV/VIS,45 the essential oil fraction was characterised by gas chromatography coupled with a flame ionisation detector (GC‐FID) and mass spectrometry (GC‐MS).46 Phenols determined by spectrophotometry are expressed as gallic acid equivalents.47 Analytical results are expressed as μg/mL (w/v). With respect to the secondary metabolites, the tincture contains on average 1,295 μg/mL volatile compounds (corresponding to 0.136% (w/w), when considering the average density of the tincture 951 kg/m3) and 1,278 μg/mL phenols (0.134% (w/w)), including 597 μg/mL curcuminoids (0.063% (w/w)). The corresponding figures for the maximum concentrations are 1,537 μg/mL (0.166%, (w/w)) volatile compounds, 1,489 μg/mL phenols (0.156% (w/w)) and 798 μg/mL (0.086%, (w/w)). The fraction of secondary metabolites including volatile compounds accounts on average for 10% of the dry matter fraction of the tincture (range: 9–12%) and the other plant constituents for about 90%.48
Table 12.
Characterisation of the fraction of secondary metabolites (including volatiles) of turmeric tincture (Curcuma longa L.) based on the analysis of six batches (mean and range). The results are expressed as μg/mL of turmeric tincture
| Constituent | CAS No | FLAVIS No | Meana | Range |
|---|---|---|---|---|
| μg/mL | μg/mL | |||
| Phenols (as gallic acid equivalent) | – | – | 1,278 | 1,060–1,489 |
| Total curcuminoidsb (as curcumin) | – | – | 597 | 464–798 |
| Curcuminoidsc | – | – | 417 | 304–581 |
| Curcumin (I) | 458‐37‐7 | – | 114 | 83–182 |
| Desmethoxycurcumin (II) | 33171‐16‐3 | – | 127 | 80–175 |
| Bis‐desmethoxycurcumin (III) | 33171‐05‐0 | – | 177 | 139–224 |
| Essential oil | – | – | 1,295 | 1,176–1,537 |
| ar‐Turmerone | – | 419 | 349–507 | |
| Turmerone | – | 202 | 77–298 | |
| β‐Turmerone (curlone) | – | 166 | 119–222 | |
| 1,8 Cineole | 03.001 | 27.2 | 8–111 | |
| α‐Terpineol | 02.014 | 4.2 | 2–9 | |
| α‐Farnesene | 01.040 | 2.4 | 2–3 | |
| Total unidentified | – | – | 475 | 435–524 |
Mean calculated on five batches.
Determined by spectrophotometry as dicinnamoyl methane derivatives.
Determined by high‐performance liquid chromatography (HPLC).
3.5.1.1. Impurities
Data on impurities were provided for three batches of turmeric tincture. The concentrations of heavy metals were below the corresponding LOQ. In the same batches, pesticides were not detected in a multiresidue analysis and mycotoxins (aflatoxins B1, B2, G1 and G2) were below the LOQ.49 The sum of PCDD and PCDF was in the range 0.15–0.27 pg WHO (2005) PCDD/F‐TEQ/L.44 None of the data on chemical impurities raised concerns.
Analysis of microbial contamination of six batches of turmeric tincture indicated that Salmonella spp. was absent in 25 g, E. coli and Enterobacteriaceae were < 10 colony‐forming unit (CFU)/g.43
3.5.1.2. Shelf‐life
The applicant states that the typical shelf‐life of flavourings is at least 12 months, when stored in tightly closed containers under standard conditions. No stability studies were performed for the tincture.
3.5.1.3. Conditions of use
Turmeric tincture is intended to be added to water for drinking for poultry, and to feed for horses and dogs without withdrawal period. The maximum proposed use level is 0.8 mL/L water for drinking (corresponding to 1.6 mL/kg complete feed or to 1.52 g/kg complete feed, considering the average density of the feed additive of 951 kg/m3) for poultry, 6 mL per head and day (corresponding to 0.75 mL/kg or 0.71 g/kg complete feed) for horses and 0.05 mL tincture/kg (or 0.048 g/kg) complete feed for dogs.
No specific proposal was made for the remaining target species/categories.
3.5.2. Safety
The assessment of safety is based on the use level proposed by the applicant.
The ADME of the individual components of turmeric tincture has been already described in Section 3.2.2.1 (curcuminoids) and in Section 3.3.2.1 (components of turmeric essential oil).
Toxicological studies with the turmeric tincture under assessment are not available to the FEEDAP Panel. The studies relevant to the assessment of the known individual components of turmeric oleoresin have been already described in Sections 3.2.2.2 and 3.3.2.2.
3.5.2.1. Safety for the target species
Tolerance studies and/or toxicological studies made with the tincture under application were not submitted.
In the absence of these data, the approach to the safety assessment of a mixture whose individual components are known is based on the safety assessment of each individual component (component‐based approach, EFSA Scientific Committee, 2019a).
The tincture consists of 97.22% of a water/ethanol mixture. The concentration of plant‐derived compounds is about 2.78% of the tincture, of which 1.52% was identified as ash, protein, lipids, sugars and fibre. These components identified by the proximate analysis are not of concern and are not further considered. Among the identified secondary metabolites, 0.136% on average (maximum: 0.166%) is volatile, 0.134% (0.156%) is phenolic in nature and 0.063% (0.086%) is constituted by curcuminoids (which are also phenolic compounds). The concentration of unidentified compounds in the tincture is < 1%.
The approach to the safety assessment of turmeric tincture for the target species follows the principles described in Section 3.4.2.1 for the oleoresin and is summarised in Table 13. Based on considerations related to structural and metabolic similarities, the identified components were allocated to two assessment groups: curcuminoids and volatile components from the essential oil.
Table 13.
Compositional data, intake values, reference points and margin of exposure (MOE) for the individual components of turmeric tincture classified according to assessment groupsa
| Tincture composition | Exposure | Hazard characterisation | Risk characterisation | |||||
|---|---|---|---|---|---|---|---|---|
| Assessment group | FLAVIS‐No | Max conc. in the tincture | Max Feed conc. | Intake | Cramer class | NOAELb | MOE | MOET |
| Constituent | – | % (w/w) | mg/kg | mg/kg bw | – | mg/kg bw | – | – |
| Curcuminoids | 0.0861 | 1.309 | 0.103 | 250 | 2,427 | |||
| Essential oil | 0.1658 | 2.5202 | 0.1991 | |||||
| 1,8‐Cineole | 03.001 | 0.0117 | 0.1772 | 0.0140 | II | 562.5 | 40,176 | |
| α‐Terpineol | 02.014 | 0.00095 | 0.0144 | 0.0011 | I | 250 | 220,224 | |
| α‐Farnesene | 01.040 | 0.00032 | 0.0048 | 0.0004 | I | 44 | 116,278 | |
| ar‐turmerone | – | 0.0547 | 0.8313 | 0.0657 | II | 310 c | 4,721 | |
| Turmerone | – | 0.0315 | 0.4886 | 0.0386 | II | 310 | 8,031 | |
| β‐Turmerone | – | 0.0239 | 0.3640 | 0.0288 | I | 310 | 10,780 | |
| 2,230 | ||||||||
FLAVIS No: EU Flavour Information System number; NOAEL: no observed adverse effect level; MOE: margin of exposure; MOET: combined margin of exposure.
Intake calculations for the individual components are based on the use level of 1.52 g/kg in feed for chickens for fattening, the species with the highest ratio of feed intake/body weight. The MOE for each component is calculated as the ratio of the reference point (NOAEL) to the intake. The combined margin of exposure (MOET) is calculated for each assessment group as the reciprocal sum of the reciprocals of the MOE of the individual substances.
values in bold refer to those components for which the NOAEL value was available, other values (plain text) are NOAELs extrapolated by using read‐across.
NOAEL derived from the 90‐day study with the essential oil.
Toxicological data, from which NOAEL values could be derived, were available for curcuminoids (see Section 3.2.2.3), ar‐turmerone (see Section 3.3.2.2), α‐terpineol [02.014] (EFSA FEEDAP Panel, 2012d) and 1,8 cineole [03.001] (EFSA FEEDAP Panel, 2012e).
Read‐across was applied using the NOAEL of 310 mg/kg bw per day for ar‐turmerone to turmerone and β‐turmerone.
For each assessment group, the combined (total) margin of exposure (MOET) was calculated. As the calculations were done for chickens for fattening, the species with the highest ratio of feed intake/body weight and represent the worst‐case scenario at the use level of 1.52 g/kg, the same conclusion can be extended to all animal species for which the additive is intended.
As shown in Table 13, the MOE(T) was > 100 for all the assessment groups, indicating that no concern would arise from the identified components when the additive is used up to the highest proposed use level in feed for the target species.
More than 50 components detected in the volatile fraction remained unidentified. Taken together, they represent 0.05% of the tincture and would lead to no more than 0.8 mg/kg feed. The largest unidentified compound of the volatile fraction (0.005% of the tincture) would lead to 0.08 mg/kg feed, which would be below the threshold for Cramer Class I and II compounds but above the threshold value for Cramer III compounds. However, a literature survey did not identify compounds of concern in extracts from dried rhizomes of C. longa and the FEEDAP Panel considers it unlikely that this compound and the other unidentified compounds in the volatile fraction would be of concern.
Similarly, the concentration of phenols (0.134% of the tincture on average and maximum 0.156%) at the maximum proposed use level in feed of 1.6 mL tincture/kg feed would be on average 2.0 mg/kg feed (maximum 2.37 mg/kg). Nearly half of this fraction consists of curcuminoids (maximum 1.31 mg/kg, see Table 12). The remaining fraction contains different compounds of phenolic nature. Phenols are used by plants as antioxidants and appear in almost all plants. They are usually of low toxicity for animals and can easily be conjugated with glucuronic acid or sulfate and excreted via urine or bile. The concentration of phenols other than curcuminoids in feed would be around 1 mg/kg, which is related to the maximum safe concentration of Cramer class I compounds. The occurrence of Cramer class II or III‐compounds in the phenolic fraction is very unlikely. Therefore, the occurrence of phenols other than curcuminoids in the tincture is considered safe for all animal species at the maximum use level.
The FEEDAP Panel concludes that turmeric tincture added to the feed of all animal species is safe at the maximum proposed use level of 0.8 mL/L water for drinking (corresponding to 1.6 mL/kg complete feed) for poultry, 6 mL per head and day (corresponding to 0.75 mL/kg complete feed) for horses and 0.05 mL tincture/kg complete feed for dogs.
3.5.2.2. Safety for the consumer
Rhizomes of C. longa and their preparations including the tincture are added to a wide range of food categories for flavouring purposes. Consumption figures for the EU are not available. The Fenaroli's handbook of flavour ingredients (Burdock, 2010) cites values of 2.187 mg/kg bw per day for turmeric rhizomes.
No data on residues formation in products of animal origin were made available for any of the constituents of the tincture. When considering the ADME of the individual components, curcuminoids (see Section 3.3.2.1) as well as the volatile components of the essential oil, which show a rapid conjugation and elimination (see Section 3.3.2.1), a relevant increase of the uptake of these compounds by humans consuming products of animal origin is not expected.
Considering the reported human exposure due to direct use of turmeric rhizomes and its preparations in food (Burdock, 2010), it is unlikely that consumption of products from animals given turmeric tincture at the proposed maximum use level would significantly increase human background exposure.
Consequently, no safety concern would arise for the consumer from the use of turmeric tincture up to the highest safe level in feed.
3.5.2.3. Safety for the user
No specific data were provided by the applicant regarding the safety of the feed additive for users.
The additive contains 55% ethanol which is the main hazard present. Curcuma longa extracts (which includes the tincture) have been notified to ECHA for classification according to CLP29 as aspiratory toxic (H304), skin irritant (H315), skin sensitiser (H317), eye irritant (H319) and respiratory irritant (H335). Ar‐turmerone is classified by ECHA CLP as skin sensitiser (H317) and eye irritant (H319). As curcuminoids and turmerones are present at low concentrations in the tincture, these effects will be secondary to the ones possibly caused by ethanol.
3.5.2.4. Safety for the environment
No data were provided by the applicant regarding the safety of turmeric tincture for the environment.
Curcuminoids, the most abundant constituents in tincture, are expected to be metabolised and degraded in the target animal (see Section 3.2.2.1). The volatile components of the essential oil will be present in the water for drinking of horses and poultry at low concentrations (< 1 mg/kg feed) and are unlikely to present any environmental concern. The use of turmeric tincture at the proposed use levels in poultry and horses is not expected to pose a risk for the environment.
3.6. Efficacy
Turmeric and its extracts are listed in Fenaroli's Handbook of Flavour Ingredients (Burdock, 2010) and by FEMA with the reference number 3085 (turmeric), 3086 (turmeric extract), turmeric oleoresin (3087).
Since turmeric and its extracts are recognised to flavour food and their function in feed would be essentially the same as that in food, no further demonstration of efficacy is considered necessary.
4. Conclusions
The FEEDAP Panel concludes that the four preparations under consideration, turmeric extract, turmeric essential oil, turmeric oleoresin and turmeric tincture from Curcuma Longa L, are safe for the target species at the following use levels:
turmeric extract is safe for all animal species at the maximum proposed use level of 15 mg/kg feed (or in water for drinking at comparable exposure)
turmeric essential oil is safe for all animal species up to the maximum proposed use level of 20 mg/kg feed (or 20 mg/L water for drinking). The higher maximum use level of 80 mg/kg for veal calves is also considered safe for this species category
turmeric oleoresin is safe at the maximum proposed concentration of 30 mg/kg complete feed (or 30 mg/L water for drinking) for chickens for fattening and laying hens and 5 mg/kg (or 5 mg/L water for drinking) for pigs, veal calves, cattle for fattening and dairy cows, sheep, goats, horses, rabbit and fish
turmeric tincture is safe at the maximum proposed concentrations of 0.8 mL/L water for drinking (corresponding to 1.6 mL/kg complete feed) for poultry, 6 mL per head and day (corresponding to 0.75 mL/kg complete feed) for horses and 0.05 mL tincture/kg complete feed for dogs
The use of turmeric extract, turmeric essential oil, turmeric oleoresin and turmeric tincture up to the highest proposed use level in feed is considered safe for the consumer.
Turmeric extract, turmeric essential oil, turmeric oleoresin and turmeric tincture should be considered as irritants to skin and eyes and the respiratory tract and as skin sensitisers.
The use of turmeric extract, turmeric essential oil, turmeric oleoresin and turmeric tincture in feed is not expected to pose a risk for the environment.
Since turmeric and its preparations are recognised to flavour food and their function in feed would be essentially the same as that in food, no further demonstration of efficacy is considered necessary.
5. Recommendation
The FEEDAP Panel recommends that the authorisation should apply only to the preparations obtained from rhizomes of Curcuma longa L.
If turmeric extract is used simultaneously in feed and water for drinking, overdosage should be avoided.
If the essential oil is used simultaneously in feed and water for drinking, overdosage should be avoided.
Documentation provided to EFSA/Chronology
| Date | Event |
|---|---|
| 05/11/2010 | Dossier received by EFSA. Botanically defined flavourings from Botanical Group 09 ‐ Zingiberales for all animal species and categories. Submitted by Feed Flavourings Authorisation Consortium European Economic Interest Grouping (FFAC EEIG) |
| 11/11/2010 | Reception mandate from the European Commission |
| 03/01/2011 | Application validated by EFSA – Start of the scientific assessment |
| 01/04/2011 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended. Issues: analytical methods |
| 05/04/2011 | Comments received from Member States |
| 17/10/2012 | Reception of supplementary information from the applicant |
| 26/02/2013 | EFSA informed the applicant (EFSA ref. 7150727) that, in view of the workload, the evaluation of applications on feed flavourings would be re‐organised by giving priority to the assessment of the chemically defined feed flavourings, as agreed with the European Commission |
| 24/06/2015 | Technical hearing during risk assessment with the applicant according to the “EFSA's Catalogue of support initiatives during the life‐cycle of applications for regulated products”: data requirement for the risk assessment of botanicals |
| 12/05/2016 | Technical hearing during risk assessment with the applicant according to the “EFSA's Catalogue of support initiatives during the life‐cycle of applications for regulated products”. Discussion on the ongoing work regarding the pilot dossiers BDG08 and BDG 09 |
| 17/06/2016 | Spontaneous submission of information by the applicant. Issues: characterisation |
| 27/04/2017 | Trilateral meeting organised by the European Commission with EFSA and the applicant FEFANA on the assessment of botanical flavourings: characterisation, substances of toxicological concern present in the botanical extracts, feedback on the pilot dossiers |
| 24/07/2017 | EFSA informed the applicant that the evaluation process restarted. |
| 12/10/2017 | Request of supplementary information to the applicant in line with Article 8(1)(2) of Regulation (EC) No 1831/2003 – Scientific assessment suspended Issues: characterisation, safety for target species, safety for the consumer, safety for the user and environment |
| 29/05/2018 | Reception of supplementary information from the applicant (partial submission) |
| 10/08/2018 | Reception of supplementary information from the applicant (partial submission) |
| 19/03/2019 | Reception of supplementary information from the applicant |
| 17/02/2020 | Reception of the Evaluation report of the European Union Reference Laboratory for Feed Additives ‐ Scientific assessment re‐started |
| 02/04/2020 | Spontaneous submission of information by the applicant. Issues: safety for the consumer |
| 27/04/2020 | Spontaneous submission of information by the applicant. Issues: safety for the environment |
| 07/05/2020 | Opinion adopted by the FEEDAP Panel. End of the Scientific assessment |
Abbreviations
- AFC
EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- B[a]p
benzo[a]pyrene
- BDG
Botanically defined group
- β‐cyf
β‐cyfluthrin
- BM
bone marrow
- bw
body weight
- C‐
negative control
- C+
positive control
- CAS
Chemical Abstracts Service
- CA
Chromosomal aberration
- CBPI
Cytokinesis Block Proliferation Index
- CD
Commission Decision
- cDDP
cisplatin
- CEC
curcuminoids‐essential oil complex
- CG
chemical group
- CHO
Chinese Hamster ovary cells
- CMN
curcumin
- CNP
chitosan nanoparticle
- CP/CPA
cyclophosphamide
- CU
curcumin
- CU‐HSPC
Curcumin‐hydrogenated soya phosphatidylcholine
- CU‐PC
Curcumin‐ phosphatidylcholine
- DM
dry matter
- DMSO
dimethylsulfoxide
- DXR
doxorubicin
- EEIG
European economic interest grouping
- EINECS
European Inventory of Existing Chemical Substances
- EMA
European Medicines Agencies
- EURL
European Union Reference Laboratory
- FAS
Food Additives Series
- FEEDAP
EFSA Scientific Panel on Additives and Products or Substances used in Animal Feed
- FEMA
Flavor Extract Manufacturers Association
- FFAC
Feed Flavourings authorisation Consortium of (FEFANA) the EU Association of Specialty Feed Ingredients and their Mixtures
- FLAVIS
the EU Flavour Information System
- FL‐No
FLAVIS number
- GC
gas chromatography
- GC‐FID
gas chromatography with flame ionisation detector
- GC–MS
gas chromatography–mass spectrometry
- GLP
Good laboratory practices
- HepG2
human hepatoma G2 cells
- HPBL
human peripheral blood lymphocytes
- ISO
International standard organisation
- LOD
limit of detection
- JECFA
The Joint FAO/WHO Expert Committee on Food Additives
- MI
Mitotic index
- MMC
mitomycin C
- MN
micronucleus/micronuclei
- MOE
margin of exposure
- MOET
combined margin of exposure (total)
- NB
nuclear buds
- NCE
normochromatic erythrocytes
- NOAEL
no observed adverse effect level
- NP
nanoparticle
- OECD
Organization for Economic Co‐operation and Development
- PCBs
polychlorobiphenyls
- PCDD/F
polychlorinated dibenzo‐p‐dioxins and dibenzofurans
- PCE
polychromatic erythrocytes
- PFOS
perfluorooctane sulfonate
- RI
replication index
- RPI
relative proliferation index
- ROS
reactive oxygen species
- SCE
sister chromatid exchange
- SSB
Single strand break
- TE
Total erythrocytes
- TI
Tail intensity
- TL
tail length
- TM
tail moment
- TMO
tail movement olive
- TG
technical guidance
- UF
uncertainty factor
- WHO
World Health Organization
- WMA
whole mixture approach
Appendix A – Summary of the available in vitro and in vivo genotoxicity studies
1.
Explanatory notes to the table:
The table is organised in tables, by type of genotoxicity test, in vitro and in vivo. When a publication includes more than one test, the study is cited in all the corresponding tables.
The reference to the study is given in the column ‘Ref.’
The cell line or the animal strain is described in the column ‘Experimental test system’. When the study is not only aimed at investigating mutagenicity or genotoxicity, it is specified as aim of the study.
In the column ‘Test substance/Relevance’ information on the test item (purity, manufacturer, formulation) is given in order to evaluate the relevance of the test item for the substance under assessment curcumin (E100), total curcuminoids > 90% (average of five batches: 96.7%, Curcumin 77.0%, desmethoxycurcumin 16.3% and bis‐desmethoxycurcumin 3.%). The relevance of the item is scored using verbal expressions, i.e. high/high to limited/limited/low/very low/none.
Experimental detail are given in the column ‘Exposure conditions’.
The main results and the outcome of the study are given in the column ‘Results’. The outcome is reported as Positive/Negative/Equivocal.
In the column ‘Comments/Reliability’, the compliance with GLP and OECD GD is reported together with the limitations identified, resulting in an evaluation of the study as Reliable/Reliable with minor restrictions/ Reliable with restrictions/Not reliable.
In the column ‘Relevance of the results’, the overall evaluation of the study is scored using verbal expressions, i.e. high/high to limited/limited/low/very low/none, taking into account the relevance of the test item and the reliability of the study.
Bacterial reverse mutation studies
| Ref. | Experimental test system (aim of the study) | Test substance/relevance | Exposure conditions | Results | Comments/reliability | Relevance of the results |
|---|---|---|---|---|---|---|
| Aggarwal et al. (2016) | S. Typhimurium TA98, TA100, TA102, TA1535 TA1537 |
Curcuminoids‐essential oil complex (CEC, with 95% curcuminoid complex), provided by Arjuna Natural Extracts Ltd. (Aluva, India), highly bioavailable (sevenfold higher than normal curcumin) Relevance: high vehicle DMSO |
Plate incorporation assay (+/− rat liver S9) five doses tested, from 1,000 to 5,000 μg/plate |
No treatment‐related increase in revertant colonies Adequate response of positive controls Negative |
Reliable with restriction (According to OECD TG 471 (1997), however, not all results reported in detail, unclear if triplicate plates, enzyme induction not reported, only one experiment) |
Limited |
| Damarla et al. (2018) | S. Typhimurium TA98, TA100, TA102, TA1535, TA1537 |
Synthetic curcumin (99.4% purity) manufactured by Laurus Labs (Visakhapatnam, India), batch 25027‐1VSP10410915 (August 2015) Relevance: High vehicle DMSO |
Plate incorporation assay, triplicate (+/− rat liver S9) Preliminary assay (TA98 and TA100): eight doses tested (from 1.6–5,000 μg/plate), precipitation observed at 5,000 μg/plate five doses tested, from 5.0 to 1,600 μg/plate |
No treatment‐related increase in revertant colonies Adequate response of positive controls Negative |
GLP compliant Reliable with minor restriction (According to OECD TG 471 (1997), however, not reported if liver enzymes were induced, only one experiment) |
High to limited |
| Jensen (1982) | S. Typhimurium TA98, TA100, TA1535 |
Curcumin (powder with 90 ± 2.5% curcumin) Turmeric oleoresin (curcumin content about 20%) Relevance: high (powder), low (oleoresin) vehicle DMSO |
Preliminary assay (TA100): six doses tested (1.28–20,000 μg/plate), toxicity observed at 160 μg/plate for both test items five doses tested, from 1.28 to 160 μg/plate, five parallel plates (+/− rat liver S9), tests were performed twice |
No treatment‐related increase in revertant colonies for both test items Adequate response of positive controls Negative |
According to Ames et al. (1975) as referenced by Jensen (1982) pre‐OECD Reliable with restriction (only three strains used, not reported if liver enzymes were induced) Evaluated by JECFA, FAS 17 as negative |
Limited |
| Liju et al. (2015) | S. Typhimurium TA98, TA100, TA102 |
Curcumagalactomannosides (CGM), a water soluble formulation containing 40.2% curcumin Manufactured by Akay Flavours & Aromatics Pvt Ltd (Kerala, India) from commercial curcumin (95%) Relevance: limited vehicle DMSO |
Plate incorporation assay, triplicate (+/− rat liver S9) five doses tested, from 100 to 5,000 μg/plate |
No treatment‐related increase in revertant colonies Adequate response of positive controls Negative |
According to Ames et al. (1975) as referenced in Liju et al. (2015) OECD TG 471 not mentioned Reliable with restriction (only three strains used, not reported if liver enzymes were induced, only one experiment) |
Limited |
| Nagabhushan and Bhide (1986) |
S. Typhimurium TA98, TA100, TA1535, TA1538 Aim of the study: mutagenicity and antimutagenicity of different test items |
Alcoholic extract of fresh or dried turmeric extract (10 g in 100 mL alcohol) Individual components (I‐III) separated Pyrolysed products (10 mg each of turmeric powder and curcumin suspended in 5 mL water and kept at 160°C for 1 h) Relevance: limited (individual components, identity unclear) |
Pre‐incubation assay: one dose tested of each test item, 50 μL corresponding to 360 μg/plate (fresh turmeric extract), 250 μg/plate (dried turmeric extract), 200 μg/plate (pyrolysed products) Individual components (I‐III) tested in TA100 and TA98 at four doses (60.2–500 μg/plate) (+/− rat liver S9) Antimutagenicity tested (interaction with chili extract and capsaicin) in TA98 with S9 |
No increase in revertant colonies for fresh and dried turmeric extracts, pyrolysed turmeric and curcumin at the dose tested No treatment‐related increase in revertant colonies for the three individual components Adequate response of positive controls Negative With S9 in TA98, dose‐dependent decrease of mutagenicity of chili extract and capsaicin |
According to Ames et al. (1975), as referenced in Shah and Netrawali (1988), OECD TG 471 not mentioned Reliable with restriction (only four strains used, only one concentration tested, only two plates per concentration, identity of the test substances unclear) Evaluated by JECFA, FAS 35 as negative for mutagenicity |
Limited |
| Nagabhushan et al. (1987) |
S. Typhimurium TA98 and TA100 Aim of the study: antimutagenicity of curcumin |
Curcumin (Sigma Chemical Co., purity > 94%) Relevance: high vehicle DMSO |
Antimutagenicity of curcumin tested against bidi smoke condensate, cigarette smoke condensate, tobacco and masheri extracts, B[a]P and B[a]anthracene: 50 μL curcumin + 50 μL mutagen (+/− rat liver S9) Five curcumin doses tested (0‐250 μg/plate) |
Curcumin inhibited mutagenicity in a dose‐dependent manner, only in the presence of S9 mix |
According to Ames et al. (1975), OECD TG 471 not mentioned Antimutagenicity only Limited number of strain tested Evaluated by JECFA, FAS 35 |
Low |
| Nagabhushan and Bhide (1987) |
S. Typhimurium TA98 Aim of the study: antimutagenicity of turmeric extract |
Alcoholic extract of turmeric (10 g powder in 100 mL) The extract dried under vacuum contains 40% curcumin Relevance: limited vehicle DMSO |
Antimutagenicity of turmeric extract against benzo(a)pyrene and dimethylbenzanthracene (B[a]p and DMBA): 50 μL curcumin + 50 μL mutagen (+/− rat liver S9) | The alcoholic extract of turmeric inhibited mutagenicity of B[a]P and DMBA in strain TA98 and with S9 mix, in a dose‐dependent manner (50% inhibition at 125 μg/plate, 75% at 500 μg/plate) |
According to Ames et al. (1975), OECD TG 471 not mentioned Antimutagenicity only Only one strain and only one dose tested Evaluated by JECFA, FAS 35 |
Low |
| NTP (1993) | S. Typhimurium TA98, TA100, TA1535, TA1538 |
Turmeric oleoresin with a high curcumin content (79‐85%) CAS No. 8024‐37‐1 Relevance: high vehicle DMSO |
Pre‐incubation assay (+/− rat liver S9) Five doses tested, up to 333 μg/plate, selection of the highest dose based on toxicity observed at higher doses |
No treatment‐related increase in revertant colonies Adequate response of positive controls Negative |
According to Mortelmans et al. (1986) as referenced by NTP (1993) OECD TG 471 not mentioned Reliable with minor restriction (only four strains tested) Evaluated by JECFA, FAS 35 as negative |
High to limited |
| Ravikumar et al. (2018) | S. Typhimurium TA98, TA100, TA1535, TA1537, E.coli WP2uvrA |
CuroWhite (Aurea Biolabs, Ltd., Kerala, India), defined as 25–27% standardised hydrogenated curcumin powder. Obtained from turmeric rhizome powder by extraction, hydrogenation, encapsulation with beta‐cyclodextrin and spray drying Relevance: limited vehicle DMSO |
Preliminary solubility test (1,000–5,000 μg/plate): test item soluble in DMSO only Preliminary cytotoxicity test (50–5,000 μg/plate) (+/‐ rat liver S9) Plate incorporation and pre‐incubation assay, each in triplicate Seven doses tested (62–5,000 μg/plate) (+/− rat liver S9) |
No treatment‐related increase in revertant colonies Adequate response of positive controls Historical control range given Negative |
GLP compliant According to OECD TG 471 (1997) Reliable without restriction |
Limited |
| Shah and Netrawali (1988) | S. Typhimurium TA98, TA100 and TA97a |
Ethanol soluble extract obtained from turmeric powder of dry rhizhome (100 g in 400 mL ethanol/water 70/30, v/v), curcumin content: 33–35% Relevance: limited Vehicle ethanol |
Plate incorporation assay (+/− rat liver S9) Three doses tested 50, 100 and 200 μg/plate corresponding to 17.5, 35, 50 μg curcumin/plate), five replicates of four separate experiments (each dose) |
No treatment‐related increase in revertant colonies Adequate response of positive controls Negative |
According to Ames et al. (1975), Shah and Netrawali (1988), OECD TG 471 not mentioned Reliable with restriction (only three strains tested, only three concentrations) Evaluated by JECFA, FAS 35 as negative |
Limited |
| Sivaswamy et al. (1991) | S. Typhimurium TA1535, TA1537, TA1538 |
Food item (turmeric, source, characterisation not available) Relevance: very low vehicle DMSO |
Two doses tested (50 and 100 μg/plate) |
Turmeric was not mutagenic Negative |
Not reliable (limited information, only summary report, only three strains, only two concentrations, negative controls resulted in unusually high revertant frequencies) Evaluated by JECFA, FAS 35 as negative (weak positive at 50 μg/plate) |
Low |
| Spalding (1983) unpublished report (NIH) | S. Typhimurium TA98, TA100,TA1535, TA1537 |
Turmeric oleoresin Relevance: low |
+/− rat liver S9 |
Not mutagenic Negative |
Not reliable (paper could not be retrieved, limited information) Evaluated by JECFA, FAS 21 as negative |
Low |
| Srividya et al. (2013) | S. Typhimurium TA98 and TA100 |
Curcumin (Sami labs, India, purity not specified) and 50% hydro alcoholic extracts from Curcuma aromatica and Curcuma zedoaria Relevance: limited |
One dose tested (50 μg/mL), triplicate experiments (+/− rat liver S9) |
Slight increase in revertant colonies compared to control (not significant) Adequate response of positive controls Negative |
Not reliable (test carried out with modifications (Meshram et al., 1992, as referenced in Srividya et al., 2013), only two strains, only one concentration, negative controls resulted in low revertant frequencies, test items poorly described) | Low |
In vitro chromosomal aberrations and sister chromatid exchange
| Ref | Experimental test system (aim of the study) | Test substance/relevance | Exposure conditions | Result | Comments/Reliability | Relevance of the results |
|---|---|---|---|---|---|---|
| Antunes et al. (1999) |
Chinese Hamster Ovary cells (CHO‐9 line) Aim of the study: modulation of damage induced by the antitumoral doxorubicin (DXR) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high C‐: untreated and solvent DMSO C+: doxorubicin (DXR) S9: No |
Cells pre‐treated with 0 (untreated), 2.5, 5 and 10 μg curcumin/mL Treatment with DXR (1 μg/mL) for 30’ during different phases of cell cycle: harvesting 3, 8 and 12 after DXR treatment Triplicate cultures 300 metaphases scored (100 per experiment) MI scored on 3,000 cells per treatment |
Cytotoxicity at > 10 μg/mL Curcumin induces chromosomal damage at 5 and 10 μg/mL In combination with DXR: Increase in the frequency of chromosomal damage at 5 and 10 μg/mL in comparison to the effect of DXR alone Positive |
OECD not mentioned, but compliant Reliable Evaluated by EFSA, 2010 as positive |
High |
| Au and Hsu (1979) |
CHO cells Aim of thestudy: Several dyes (48) were tested |
Curcumin (Eastman, purity 90%) Relevance: medium to high C−: solvent alcohol C+: several dyes, known to increase chromosomal damage were tested S9 not tested |
Cells treated with 20 μM curcumin and incubated for 5 h Only−S9 50 metaphases scored for each experimental period |
Curcumin did not induce chromosomal damage Negative |
Not reliable (only summary information S9 not tested) Evaluated by JECFA, FAS 17 as negative |
Low |
| Damarla et al. (2018) |
Human lymphocytes (HPBL) (pooled blood from healthy young male non‐smoking donors) |
Synthetic curcumin (99.4% purity) manufactured by Laurus Labs (Visakhapatnam, India), batch 25027‐1VSP10410915 (August 2015) Relevance: high C−: solvent DMSO C+: −S9: MMC, + S9: CP S9: rat liver |
Preliminary toxicity test: max. dose tested: 250 μg/mL, −/+ S9. −S9: short expo (4 h + 18 h recovery); continuous expo (22 h); + S9: short expos (4 h + 20 h recovery) CA test: 1st exp. (short term): − S9: 4 h expo., 0 (DMSO), 10, 20 and 40 μg/mL; + S9: 4 h expo., 0 (DMSO), 6.3, 12.5 and 25 μg/mL 2d. exp.: −S9: 20 h expo., harvesting at end of treatment, 0 (DMSO), 6.3, 12.5 and 25 μg/mL; Duplicate cultures 300 metaphases scored (150 from each duplicate) MI scored on 1,000 cells per treatment |
Mitotic Index (MI): ‐S9: 41% of negative controls at high‐dose level; +S9: 47% of negative controls at high‐dose level −S9: negative +S9: 1st. exp: statistically significant increase in the frequency of aberrant cells at 25 μg/mL 2d. exp: decrease of MI at 6.3, 12.5 and 25 μg/mL (MI: 25%, 33% and 51% of negative control, respectively). No increase in the frequency of aberrant cells No changes in the frequency of numerical chromosome aberrations Positive +S9 |
GLP compliant According to OECD TG 473 (2014) Reliable |
High |
| Haverić et al. (2018) |
Human lymphocytes (pooled blood from four healthy male non‐smoking donors) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high C−: ddH2O C+: no S9: no |
Cells treated with curcumin in water at 1, 2, 4 and 8 mM (7–56 ug/mL) (100 μL) 4 replicates/treatment 200 metaphases per replicate |
No significant increase in the frequencies of chromosome aberrations Negative |
OECD not mentioned Not reliable Limited information on experimental details (solubility, cytotoxicity, dose selection, exposure time) |
Low |
| Ravikumar et al. (2018) | CHO cells |
CuroWhite (Aurea Biolabs, Ltd., Kerala, India), defined as 25–27% standardised hydrogenated curcumin powder. Obtained from turmeric rhizome powder by extraction, hydrogenation, encapsulation with beta‐cyclodextrin and spray drying Composition given Relevance: limited C−: solvent DMSO (0.1%) C+: ‐S9: mitomycin C (MMC), + S9: CP S9: rat liver |
Preliminary cytotoxicity test: range finding up to 20 μg/mL, ‐/+S9: expo. 3 h CA main test: 1st exp.: −/+ S9: 3 h expo., harvesting 18 h, 0 (DMSO), 5, 10 and 15 μg/mL; 2d. exp.: ‐S9: 18 h expo., harvesting 18 h, 0 (DMSO), 5, 10 and 15 μg/mL; Duplicate cultures 200 metaphases per experimental group |
MI: −S9: 56% of C− at HD, +S9: 56% of C‐ at HD Solubilised in DMSO at 10 mg/mL, at 0.1 mg/mL no precipitation or change of pH Cytotoxicity observed at 20 μg/mL 1st. exp: No differences compared to negative controls in % of CAs without gaps at any concentration, −/+S9 2d. exp: same as above (−S9 only). No polyploidy or endoreduplicated chromosomes observed, no precipitation Negative |
GLP compliant According to OECD TG 473 (1997) Reliable |
Limited |
| Sebastià et al. (2012) |
Human lymphocytes (HPBL) (no further info) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high C−: HPBL only and solvent control (95% ethanol) C+: none S9: No |
Cells treated with curcumin at 0 (ethanol), 0.5, 1, 2, 5, 10 and 50 μg/mL, incubated for 48 or 72 h CA analysed in 100 metaphases per experimental group SCE analysed in 25 second division metaphase per concentrations |
MI higher at all dose tested compared to C− CAs: No increase of the frequencies of chromatid‐type aberrations; significant increase of chromosome‐type aberrations (acentric fragments) in a dose‐dependent manner (1–50 μg/mL) SCEs rate in treated cells was not different from the control group Positive CAs Negative SCE |
MI and RPI calculated according to Rojas (1993) Reliable with minor restrictions OECD not mentioned Limited information (HPBL, no S9, no C+) 100 metaphases analysed/dose |
High (CAS) Limited (SCE) |
| NTP (1993) | CHO |
Turmeric oleoresin with a high curcumin content (79–85%) CAS No. 8024‐37‐1 Relevance: high C−: solvent DMSO C+: −S9: MMC, + S9: CP S9: rat liver |
CA test 1st exp: − S9: 10 h treatment., harvesting 2 h later, 0 (DMSO), 5, 10 and 16 μg/mL; + S9: 2 h treatment., harvesting 11 h later, 0 (DMSO), 3, 5 and 10 μg/mL 2nd exp: ‐S9, repeated SCE test 1st exp: − S9: 26 h incubation, 0 (DMSO), 0.16, 0.50, 1.60 and 5 μg/mL, BrdU added 2 h after start; medium removed;+2 h with fresh medium, BrdU and colcemid; + S9, 2 h incubation, 0 (DMSO), 0.16, 0.50, 1.60 and 5 μg/mL, medium removed, +26 h with serum and BrdU (colcemid last 2 h) 2nd exp.: −S9 repeated |
CA test −S9: statistically significant increase in the frequency of aberrant cells at 16 μg/mL +S9: No increase SCE test −S9: exp 1 statistically significant increase of SCE at the highest dose not confirmed in exp. 2 +S9: exp 1: negative results; in exp. 2, significant increases in SCEs observed at the two highest doses (1.60 and 5.00 pg/mL) CAS: Positive −S9/ Negative +S9 SCE: Equivocal |
Test as reported by Galloway et al. (1987), as referenced by NTP (1993) OECD not mentioned Tests with positive or equivocal outcome were repeated Reliable Evaluated by JECFA, FAS 35 as positive for CAS at 15 μg/mL and equivocal for SCE |
High (CAS) Low (SCE) |
In vitro Micronucleus
| Ref. | Experimental test system (aim of the study) | Test substance/relevance | Exposure conditions | Result | Comments/reliability | Relevance of the results |
|---|---|---|---|---|---|---|
| Cao et al. (2007) |
Human hepatoma G2 (HepG2) cells Aim of the study: genotoxicity and antigenotoxicity of curcumin, tested in combination with cyclophosphamide (CPA) |
Curcumin (purity > 95.6%) from Xi'an Chongxin Natural Additives Co. Ltd. (Xi'an, China) Relevance: high C−: solvent DMSO (0.1%) C+: CPA (800 μM) S9: HepG2 cells, no need of metabolic activation |
Experiment 1: Cells treated with curcumin at 0 (DMSO), 2, 4, 8 and 16 μg/mL (corresponding to 0, 5.2, 10.4, 20.8 and 41.5 μM) for 24 h Experiment 2: Cells pretreated with 2 μg/mL curcumin for 2 h; curcumin washed out and incubation with CPA (800 μM) for 22 h Cytochalasin B (4.5 μg/mL) for 20 h Three independent experiments, each run in triplicate 1,000 binucleated cells/experiment scored |
MN frequency increased at 8 and 16 μg/mL (p < 0.05) Statistically significant reduction of MN induced by CPA when cells were pretreated with curcumin (2 μg/mL) Positive |
OECD not mentioned Reliable with minor restrictions Cytotoxicity (CBPI or RI) not given Evaluated by EFSA 2010 as positive (curcumin induced a small but significant increase of MN in HepG2 cells at concentrations of 8 and 16 μg/mL). |
High |
| Haverić et al. (2018) |
Human lymphocytes (pooled blood from four healthy male non‐smoking donors) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high C−: ddH2O C+: none S9: no |
CBMN‐cyt assay Cultures treated with curcumin in water at 1, 2, 4, 8 mM (100 μL) Cytochalasin B (4.5 μg/mL) 4 replicates/treatment MN, nuclear buds (NB) and nucleoplasmic bridges (NPBs) in 2000 binucleated cells NDI (nuclear division index) calculated in 500 cells/replicate |
No increase of MN Statistically significant increase in the frequency of nuclear buds at 4 and 8 mM No increase in NDI Negative |
OECD not mentioned Not reliable Limited information on experimental details (solubility, cytotoxicity, dose selection, exposure time), no S9, no C+ |
Low |
| Mendonça et al. (2009) |
PC12 cells (rat pheochromocytoma) Aim: genotoxicity and antigenotoxicity of curcumin. Associated treatment with Cisplatin (cDDP) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high C−: culture medium solvent DMSO (0.4%) C+: cDDP (0.1 μg/mL) S9: not used |
Experiment 1: Cells incubated for 72 h at 37 °C, with curcumin (1, 2.5, 5 and 10 μg/mL) for the last 48 h Experiment 2: Cells preincubated for 2 h with curcumin (1, 2.5, and 5 μg/mL), cDDP (0.1 μg/mL) added and harvested 48 h later Cytochalasin B (6 μg/mL) Triplicate experiments 2,000 binucleated cells/replicate CBI calculated in 500 cells/replicate |
Cytotoxicity test (MTT): 16% at 8 μg/mL; statistically significant decrease at 16 ug/mLmL and above. Curcumin statistically significant increase of MN at 10 μg/mL Curcumin (1, 2.5 and 5 μg/mL) significantly reduced the frequency of MN induced by cDDP (0.1 μg/mL) Positive |
According to Fenech, 2000 with slight modifications OECD not mentioned Reliable Historical control data not available Evaluated by EFSA 2010 as positive (curcumin increased MN frequency at highest concentrations of 10 μg/mL and reduced the frequency of MN induced by cisplatin) |
High |
In vitro alkaline comet assay
It is to be noted that this in vitro test method is not implemented into an official regulatory test guideline, and therefore, the reliability of the study was not assessed and the relevance of the results was considered ‘limited’ at maximum.
| Ref. | Experimental test system (aim of the study) | Test substance/Relevance | Exposure conditions | Result | Comments | Relevance of the results |
|---|---|---|---|---|---|---|
| Ayoub et al. (2019) | Lymphocytes |
Nanoemulsion formulations of curcuminoids, prepared using limonene and oleic acid as oil phases. Microsuspension solutions, prepared by suspending curcuminoid particles in isotonic solution (saline solution) of 0.02% Tween 80 (surfactant) Relevance: limited |
Nanoemulsion tested at three curcumin concentrations (100, 250 and 500 μg/mL) Incubation 30 min at 37 °C Computerised image system to measure Comet parameters, % olive tail moment used for statistics |
The DNA damage observed for six nanoemulsion formulations was lower than that of the negative control indicating some protective effect of the curcumin nanoemulsions Negative |
Test as reported by Ostling and Johanson, 1984, Singh et al. 1988 and Olive et al., 1990 |
Low (as in vitro comet) |
| Blasiak et al. (1999a,b) |
Human lymphocytes (healthy, non‐smoking donors) and gastric mucosa cells (healthy tissues) Aim of the study: modulation of damage induced by chromium |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high Positive control: H2O2 Curcumin tested alone and also in combination with potassium dichromate (Blasiak et al., 1999a) |
Comet assay Three concentrations tested (10, 25 and 50 μM) Incubation 1 h at 37°C Curcumin (50 μM) tested with potassium dichromate (500 μM) Fifty images for each sample, comet tail moment measured |
Curcumin induced DNA damage in both cell lines in a dose‐dependent manner Curcumin significantly increased DNA damage induced by chromium (effect addition) Positive |
Test as described by Singh et al. (1988) with slight modification Evaluated in EFSA, 2010 as positive (Curcumin induced DNA damage in human lymphocytes and gastric mucosa in the low micromolar range (10–50 μM). Curcumin works in an additive fashion with hexavalent chromium) |
Limited (as in vitro comet) |
| Bojko et al. (2015) |
LN229 human brain cancer cells Aim of the study: modulation effects of curcumin and EGFR kinase inhibitors. |
Curcumin (Fluka, purity > 94%) Relevance: high Solvent: ethanol Negative control: culture medium (DMEM/F12 1:1) Positive control: paclitaxel Also tested in combination with EGFR kinase inhibitors: tyrphostins AG494 and AG1478 |
Curcumin tested at 1x IC 50 (7.1 μM) and 2x IC50, 48 h incubation, alone and in combination with AG494 and AG1478 (1x and 2xIC50) Three independent experiments Two replicate wells/concentration for each experiment |
Curcumin induced DNA damage in a dose‐dependent manner (max 23% at 2xIC50) alone) Positive |
Low (as in vitro comet) |
|
| Cao et al. (2006) | Human Hepatoma G2 (HepG2) cells |
Curcumin (China, purity > 95.6%) Relevance: high Positive control: H2O2 |
Cells incubated with 0, 2.5, 5, 10, 20 and 40 μg/mL at 37°C for 1 h Software to analyse Comet parameters Other parameters: cell viability, QPCR, immunocytochemistry (8OHdG), ROS, lipid peroxidation (TBARs) |
Comet: dose‐dependent increase of tail moment, more extensive damage (SSB) at 40 μg/mL Positive at conc ≥ 2.5 μg/mL (comet tails) Curcumin induced damage (detected by qPCR) to both the mitochondrial and nuclear genomes, in a dose dependent manner, particularly in mtDNA Oxidative damage indicated by increase 8‐OHdG content Strong cytotoxicity at concentrations > 20 μg/mL Positive (ox damage) |
Test as described by Singh and Stephen (1997) Evaluated in EFSA, 2010 as positive (Curcumin induced DNA damage measured in the Comet assay) |
Limited (as in vitro comet) |
| Kocyigit and Guler (2017) |
B16‐F10 cells (from mouse melanoma cells) L‐929 cells (from mouse fibroblast cells, normal cells) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high Negative control: DMSO (0.1%) |
Cells incubated with curcumin (2.5, 5, 10, 30, 40 and 50 μM in 1% DMSO) at 37 °C for 24 h Computerised image system. DNA % in tail (tail intensity %) as measure of DNA damage Other parameters: cell viability, ROS generation, apoptosis |
Curcumin increased DNA damage, in both cell lines in a dose‐dependent manner, significant at all doses except at 2.5 μM. Curcumin also decreased cell viability, increased apoptosis and reactive oxygen species (ROS) levels in both cell lines, particularly in melanoma cells There were positive strong relationships between DNA damage, apoptosis, cytotoxicity and ROS generation in both cell lines Positive (ox damage) |
According to Singh et al. (1988) with slight modifications |
Low (as in vitro comet) |
| Mendonça et al. (2010) |
PC12 cells (rat pheochromocytoma, model for oxidative damage induction in neurons) Aim of the study: modulation of damage induced by Cisplatin (cDDP) |
Curcumin (94% purity, Sigma Aldrich) Relevance: high Also tested in combination with cDDP |
Alkaline comet assay cells pre‐treated with curcumin (1 or 5 μg/mL) for 2 h and exposed to cisplatin (0.1 μg/mL), harvested after 3 h Triplicate experiments Computerised image system. DNA % in tail (tail intensity %), tail moment and olive moment. Comet classes 1–4 Cytotoxicity at concentrations > 16 μg/mL |
Curcumin alone did not induce DNA damage, olive and tail moment. The effect was a decrease in %DNA, olive moment and tail moment (also for cDDP) Significant reduction of DNA migration in cells pre‐treated with 5 μg/mL curcumin before cisplatin treatment Negative |
Test performed according to Singh et al., 1988 and Tice et al. 2000 Not reliable |
Low (as in vitro comet) |
| Papiez (2013) |
LT12 cells (rat myeloid leukemia cell line) Aim of the study: modulation of damage induced by etoposide |
Curcumin (Sigma Aldrich, > purity 94%) Relevance: high Solvent: DMSO Negative control: 1% ethanol In combination with etoposide (0.25–2.0 μM) |
Curcumin (1–20 μM) Exposure: 1 h |
Curcumin (1–10 μM) did not induce DNA damage Significant increase DNA damage after exposure to 20 μM (1 h exposure) Significant increase of DNA damage induced by etoposide (2 μM) in combination with 10 and 20 μM curcumin Positive |
Test performed according to Tice et al. (1991) |
Low (as in vitro comet) |
| Srividya et al. (2013) | Human lymphocytes |
Curcumin (Sami labs, India, purity not specified) and 50% hydro alcoholic extracts from Curcuma aromatica and Curcuma zedoaria Relevance: low |
Cells incubated with curcumin (50 μg/mL) at 37 °C for 30 min −/+S9 DNA damage (ratio of tail to head length) scored into four classes |
Curcumin slightly increased DNA damage (SSB), with or without S9, particularly with S9 Positive |
According to Singh et al. (1988) with slight modifications |
Low (as in vitro comet) |
| Urbina‐Cano et al. (2006) |
Balb‐C mouse lymphocytes (three healthy animals) Aim of the study: modulation of damage induced by copper |
Curcumin (source not specified, CAS number: 458‐37‐7) Relevance: low (source not provided) Negative control: untreated Positive control: H2O2 In combination with copper |
Curcumin (50 μM) tested alone and in combination with copper (10, 100, 200 μM) and H2O2 (50 μM) Exposure: 1 h at 37°C |
Curcumin increased DNA damage (SSB), alone and in the presence of copper Positive |
According to Singh et al., 1988 Evaluated in EFSA, 2010 as positive (50 μM curcumin alone or in the presence of 100–200 μM copper induced DNA damage in mouse lymphocytes) |
Low (as in vitro comet) |
In vivo chromosomal aberrations
| Ref. | Experimental test system (aim of the study) | Test substance/Relevance | Exposure conditions | Result | Comments/Reliability | Relevance of the results |
|---|---|---|---|---|---|---|
| Aggarwal et al. (2016) |
BM cells of Swiss albino rats, 8–12 weeks old Males+Females (30M+30F, 3 groups, 10M/10F per group treated, C+ and C‐) Parallel 90‐day toxicity study in males and female rats (OECD no. 480). Maximal Tolerates Dose, Minimal Lethal Dose and LD50: > 5,000 mg/kg bw |
Curcuminoids‐essential oil complex (with 95% curcuminoid complex, India) Relevance: high Negative control: corn oil (oral) Positive control: CP (i.p., 50 mg/kg bw, sampling: 24 h) |
Curcumin (oral admin): 2,000 mg/kg bw (max tolerated dose), single dose Sampling time: 18 and 42 h after administration MI calculated based on 1,000 cells Two slides/animal for scoring 100 metaphases analysed/slide |
Positive controls: stat. signif. increase in % aberrant cells and decrease in MI No evidence of numerical or structural aberrations were observed at the maximum tolerated dose at any time point of bone marrow harvest No decrease in MI Negative |
OECD 475 not mentioned, but compliant Reliable with minor restrictions Only one dose tested Historical control data not provided No indication if BM was exposed The compound was tested up to the highest recommended dose by OECG TG 475 |
Limited |
| Dandekar et al., 2010a; |
BM cells of Holtzman rats, 6–8 weeks old Males+Females (n = 10, 5M/5F per group, six groups) Parallel acute (2,000 mg/kg bw per kg and sub‐acute (28 d, up to 200 mg/kg) toxicity studies in males and female rats (OECD, 1996, 1995, as referenced by Dandekar et al. (2010a) no. 425 and 407): no signs of toxicity |
Curcumin nanoparticles (NP) of Eudragit® S100 Relevance: limited Vehicle: distilled water Positive control: CP (40 mg/kg bw) |
Curcumin NP: vehicle control, blank NP, 100, 200 and 300 mg/kg bw (gavage), administered once daily on 2 consecutive days 100 metaphases/animal |
No (statistical) increase in % aberrant cells by curcumin NP Positive controls: stat. signif. increase in % aberrant cells Negative |
OECD not mentioned Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided MI not reported No indication if BM was exposed |
Low |
| Dandekar et al., 2010b; |
BM cells of Holtzman rats, adult Males+Females (n = 10, 5M/5F per group, six groups) Parallel acute (2,000 mg/kg bw per kg and sub‐acute (28 d, up to 200 mg/kg) toxicity studies in males and female rats (see above) |
Hydrogel nanoparticles (NP) of curcumin (95%) based on HPMC and PVP Relevance: limited Vehicle: distilled water Positive control: CP (40 mg/kg bw) |
Curcumin NP: vehicle control, blank NP, 100, 200 and 300 mg/kg bw (gavage), administered once daily on 2 consecutive days 100 metaphases analysed/animal |
No (statistical) increase in % aberrant cells by curcumin Positive controls: stat. signif. increase in % aberrant cells Negative |
OECD not mentioned Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided MI not reported No indication if BM was exposed |
Low |
| El‐Makawy and Sharaf (2006) |
BM cells of Wistar rats Males (10/group, males only) |
Curcumin spice (not characterised) Relevance: very low Vehicle: distilled water Positive control: CP (25 mg/kg bw, i.p.) |
Curcumin spice: 0.5, 5, 10, 25 and 50 mg/kg bw, daily oral administration for 4 weeks |
Curcumin caused a significant dose‐dependent increase of total chromosomal aberrations at doses ≥ 5 mg/kg bw Positive controls: stat. signif. increase in % aberrant cells Positive |
OECD not mentioned Not reliable Study protocol not appropriate for CA Historical data not provided Evaluated by EFSA 2010 as positive, but the Panel noted that the curcumin tested was not adequately specified. |
Very low |
| Giri et al., 1990; |
BM cells of rat (unknown strain), 10–12 weeks old Males (5 animals/group, 3 exposure times) |
Curcumin (Gurr, UK, purity not given) Positive control: MMC (2.5 mg/kg) Relevance: limited |
Curcumin: 100, 200, 500 and 1,000 ppm, daily oral for 3, 6 and 9 months (5 animals/exposure time) 100 metaphases analysed/animal |
No (statistical) increase in % aberrant cells by curcumin after 3 and 6 months, but increase after 9 months at 500 and 1000 ppm Positive controls: stat. signif. increase in % aberrant cells Negative up to 6 months/Positive after 9 months |
According to WHO, 1985 Not reliable Protocol not appropriate for CA Evaluated by JECFA FAS35 as negative after 3 and 6 months, and positive after 9 months at 500 and 1,000 ppm |
Very low |
| Jain et al. (1987) |
BM cells of mice Males (4 per group) |
Turmeric powder, dried methanolic extract Relevance: limited Vehicle: DMSO Positive control: MMC (2 mg/kg bw, i.p., single dose) |
Doses tested: 100, 250 and 500 mg turmeric powder/kg bw, by single i.p. injection |
Positive controls: stat. signif. increase in % aberrant cells No significant increase in % aberrant cells at any dose of turmeric powder Negative |
OECD not mentioned Not reliable Limited description Inadequate study protocol and statistical evaluation of results Evaluated by JECFA FAS 35 (frequency of aberrant cells including gaps was 2.00, 1.73 and 6.22% at 100, 200 and 500 turmeric powder; negative and positive controls 0.5 and 12.8%) |
Very low |
| Khatik et al. (2016) |
BM cells of Balb/c mice, 6–8 weeks old 4 groups, 4 animals per group, sex not specified |
Complexes (1:1) between curcumin (CU) phosphatidylcholine (PC) and hydrogenated soya PC (HSPC) Relevance: limited Vehicle: distilled water Positive control: CP (40 mg/kg bw) |
One dose tested: 100 mg/kg bw of CU (as CU‐PC and CU‐HSPC), administered by gavage once daily over a period of 2 days 100 metaphases analysed/animal |
No (statistical) increase in % aberrant cells by curcumin with both complexes Negative |
OECD not mentioned Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided MI not reported No indication if BM was exposed |
Low |
| Verma et al. (2016) |
BM cells of Swiss albino mice, adult Male (6 animals/dose group, 6 groups) Aim of the study: modulation on effects of beta‐cyfluthrin (β‐CYF) |
Curcumin (purity 97%) Relevance: high Control: corn oil Positive control: CP (25 mg/kg bw) |
One dose of curcumin (0.2%), oral, administered alone (feed pellets) and in combination with low (13 mg/kg bw) and high (26 mg/kg bw) dose of β‐CYF for 21 days 100 metaphases analysed/ animal |
Positive controls: stat. signif. increase in % aberrant cells and decrease in MI Curcumin decreased the % aberrant cells induced by β‐CYF (70%) Inconclusive because curcumin was only tested in combination with cyfluthrin (or tested alone but data not reported) |
Preparation (Adler et al., 1984), analysis (Verma et al., 2013) OECD not mentioned Reliable with limitations Only one dose tested Missing data on CA for curcumin when tested alone No indication if BM was exposed but indirect evidence The study is not relevant regarding for the genotoxicity of curcumin (data not reported) but provides some evidence of bioavailability of curcumin in relation to modulation of genotoxic effects of β‐CYF |
Low |
| Vijayalaxmi (1980) |
BM cells of albino mice and Wistar rats 1st experiment Swiss albino mice Males and females (30, 15/15), 3 groups, 5M and 5F per treatment 2nd experiment: Wistar rats Male and females (5M/5F per group), 4 groups |
Turmeric and curcumin added to diet (1st experiment) or turmeric in cooked diet (2nd experiment) Relevance: high (curcumin), limited (turmeric) Control: normal diet Positive control: none |
1st experiment Added to diet: control (0), turmeric (0.5%) and curcumin (0.015%,) for 12 weeks 50 metaphases analysed/animal Polyploidy scored in 1000 cells per animal 2nd experiment Turmeric added to diet: 0, 0.05%, 0.5% (steamed), 0.5% (uncooked) for 12 weeks |
No (statistical) increase in % aberrant cells and polyploidy induced by turmeric and curcumin compared to control Negative but only low doses tested |
OECD not mentioned Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided, no C+ No indication if BM was exposed Evaluated by JECFA FAS 17 as negative |
Low |
| Zheng et al. (2016) |
BM cells of Balb/c mice, 6–8 weeks old 5 groups, 4 animals per group |
Two types of chitosan nanoparticles (CNP) loaded with curcumin (chitosan nanoparticles CNPs‐CU and phosphatidylserine‐coated chitosan nanoparticles PS‐CNPs‐CU) (release investigated) Relevance: limited Vehicle control C‐: Distilled water Positive control: CP (40 mg/kg bw) |
One dose tested (40 mg/kg bw) of the two formulations, corresponding to 100 mg curcumin/kg (gavage?), administered once daily on 2 consecutive days 100 metaphases analysed/animal |
Positive controls: stat. signif. increase in % aberrant cells No (statistical) increase in % aberrant cells by curcumin in both forms Negative (but limited reliability for test item and protocol) |
OECD not mentioned Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
In vivo micronucleus
| Type of test | Experimental test system (aim of the study) | Test substance/relevance | Exposure conditions | Result | Comments/Reliability | Relevance of the results |
|---|---|---|---|---|---|---|
| Aggarwal et al. (2016) |
BM cells of Swiss albino mice, 8–12 weeks old Males+Females (10M + 10F per group, 3 groups, one treated group, negative and positive controls Parallel 90‐day toxicity study in males and female rats (OECD no. 480). Maximal Tolerates Dose, Minimal Lethal Dose and LD50: > 5,000 mg/kg bw |
Curcuminoids‐essential oil complex (CEC, with 95% curcuminoid complex, India), with increased bioavailability Relevance: high Negative control: corn oil (oral) Positive control: CP (i.p., 40 mg/kg bw, sampling: 24 h) |
2,000 mg CEC/kg bw (max tolerated dose), oral administration Sampling time: 24 and 48 h after administration (5M+5F per time point) 200 erythrocytes/slide counted for % PCE; 2,000 PCE/animal scored for MN |
No mortality, no signs of toxicity Positive controls: stat. signif. increase in the number of MNPCE No statistical increase in frequency of MN PCE No effect on PCE/NCE (no cytotoxicity) Acute toxicity: no clinical signs of toxicity at the dose of 5,000 mg/kg bw Negative |
OECD 474 not mentioned, but compliant Reliable with minor restrictions Only one dose tested Historical data not provided No indication if BM was exposed The compound was tested up to the highest recommended dose by OECD TG 474 |
Limited |
| Çelik et al. (2013) |
BM cells of Swiss albino rats (Wistar rats), 6–8 weeks old Females (9 groups of 6 rats) curcumin treated group; 3 PFOS treated groups, 3 PFOS + Curcumin treated groups, negative and positive controls Aim of the study: modulation on effects of perfluorooctane sulfonate (PFOS) |
Curcumin (Sigma Aldrich purity > 99%) Relevance: high Negative control: Vehicle: saline Positive control: MMC (i.p., single dose 2 mg/kg at the 16th week) |
80 mg/kg bw curcumin by gavage for 30 days at 48 h intervals, sacrificed 30 h after last treatment 200 erythrocytes/animal for % PCE; 2,000 PCE/animal scored for MN |
Positive controls: stat. signif. increase in the number of MNPCE No stat. signif. increase in the MN frequency by curcumin Dose‐related increase of MN frequency by PFOS alone and also in combination with curcumin No decrease in PCE/200 TE by curcumin alone Negative |
Procedure described by Schmidt (1993) and Agarwal (1994) Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| Damarla et al. (2018) |
BM cells of Swiss albino mice, 7–9 weeks old Males+Females (5M+5F per group, 5 groups, 3 treated, negative and positive controls |
Synthetic curcumin (99.4% purity) Relevance: high Negative control: Vehicle: 0.5% w/v CMC Positive control: CP (30 mg/kg bw, single dose, gavage, sampling 24 h) |
0, 500, 1,000 and 2,000 mg/kg bw, by gavage, for 2 consecutive days Sampling time: 24 after second administration 500 erythrocytes/ animal scored; 4,000 PCE/animals scored for MN |
No mortality, no signs of toxicity Positive controls: stat. signif. increase in the number of MNPCE Negative controls: within historical control laboratory values No statistical signif. increase in frequency of MN PCE No effect on PCE/TE Negative |
GLP compliant According to OECD 474 (2014) Reliable with minor restrictions No indication if BM was exposed The compound was tested up to the highest recommended dose by OECD TG 474 |
Limited |
| Dandekar et al., 2010a; |
BM cells of Swiss albino mice, 6–8 weeks old Males+Females (n = 10, M/F 5/5 each group) Six groups:3 treated groups, Negative and positive controls Parallel acute (2,000 mg/kg bw per kg and sub‐acute (28 d, up to 200 mg/kg) toxicity studies in males and female rats (OECD, 1996, 1995, as referenced by Dandekar et al. (2010a) no. 425 and 407): no signs of toxicity |
Curcumin nanoparticles (NP) of Eudragit® S100 Relevance: limited Negative control: Vehicle: distilled water Blank nanoparticles Positive control: CP (40 mg/kg bw) |
Four curcumin NP doses tested, corresponding to 0 (blank NP), 100, 200 and 300 mg/kg bw (gavage), administration once daily on 2 consecutive days; sampling at the end of treatment 2,000 erythrocytes per animal; PCE and NCE scored |
Positive controls: stat. signif. increase in the number of MNPCE and decrease in PCE/NCE No stat. signif increase in frequency of MN PCE No effect on PCE/NCE (no cytotoxicity) Negative |
OECD not mentioned Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| Dandekar et al. (2010b) |
BM cells of Swiss albino mice Males+Females (5M/5F per group, six groups) Parallel acute (2,000 mg/kg bw per kg and sub‐acute (28 d, up to 200 mg/kg) toxicity studies in males and female rats (see above) |
Hydrogel nanoparticles (NP) of curcumin (95%) based on HPMC and PVP Relevance: limited Negative control: Vehicle: distilled water Positive control: CP (40 mg/kg bw) |
Four curcumin NP doses tested, corresponding to o (blank NP), 100, 200 and 300 mg/kg bw (gavage), administration once daily on 2 consecutive days; sampling at the end of treatment 2,000 erythrocytes per animal; PCE and NCE scored |
Positive controls: stat. signif. increase in the number of MNPCE and decrease in PCE/NCE No stat. signif increase in frequency of MN PCE No effect on PCE/NCE (no cytotoxicity) Negative |
OECD not mentioned Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| El‐Makawy and Sharaf (2006) |
BM cells of Wistar rats Males (10/group) 7 groups: 5 treated groups, negative and positive controls |
Curcumin spice (not characterised) Relevance: very low Negative control: Vehicle: distilled water Positive control: CP (25 mg/kg bw, i.p., single dose) |
Five doses tested, 0.5, 5, 10, 25 and 50 mg/kg bw, daily oral administration for 4 weeks Sampling 24 h after last administration 2,000 PCE/animal scored for MN |
Positive controls: stat. signif. increase in the number of MNPCE Curcumin caused a significant dose‐dependent increase of MNPCE, significant at doses ≥ 5 mg/kg bw No data on PCE/NCE Positive |
OECD not mentioned Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided No bone marrow exposure Evaluated by EFSA 2010 as positive, but the Panel noted that the curcumin tested was not adequately specified |
Very low |
| Farag et al., 2014; |
Whole blood of chickens 6 groups: 2 turmeric groups, endosulfan group, 2 endosulfan+ turmeric groups, negative controls, Also tested in combination with endosulfan (protective effects of turmeric) |
Turmeric (Curcuma longa) Relevance: limited Negative control:normal diet Positive control: endosulfan (30 mg/kg) |
Doses tested: 5 and 10 mg turmeric/kg diet, for 5 weeks 1,000 erythrocytes/group scored for MN |
No stat. signif increase in frequency of MN induced by turmeric Endosulfan increased MN, decreased by co‐exposure to turmeric Negative |
Historical data not provided Not reliable in vivo MN not validated in chicken, experimental protocol not standardised |
None |
| Farhadi et al. (2018) |
Human blood samples 21 patients with differentiated thyroid carcinoma, 11 (6F) patients receiving curcumin, 10 (5F) receiving placebo Aim of thestudy: Radioprotective effects against genotoxicity induced by Iodine‐131 |
Nano‐curcumin (nano micellar soft gel capsules) Relevance: limited |
Curcumin 160 mg/day, orally given from 3 days before to 7 days after 131I therapy blood sampling before treatment and 1 week after Phytohemagglutinin incubated at 37°C for 44 h Cytochalasin B Two paired cultures per sample At least 1,000 binucleated cells/per patient, before and after therapy |
Baseline MN same in patients receiving curcumin and placebo Treatment with 131I sign. increased MN After 1 week treatment with 131I, the frequency of MN decreased in patients receiving curcumin by 32% Negative |
Not relevant for the purpose of the assessment (biomonitoring study in a group of patients) |
None |
| Jain et al. (1987) |
BM cells of mice (unspecified) Males (4 per group) |
Turmeric powder, dried methanolic extract (containing about 3% curcumin) Relevance: limited Negative control: Vehicle: DMSO Positive control: MMC (2 mg/kg bw, i.p., single dose) |
Doses tested: 100, 250 and 500 mg/kg bw, i.p., single dose, after 22 h, colchicine injected (0.2 mg/kg bw), animals sacrificed after 2 h At least 1,000 PCE/animal scored for MN |
Positive controls: stat. signif. increase in the number of MNPCE Statistical signif. increase in MN frequency at the dose 250 mg/kg bw No dose‐response No data on PCE/NCE Negative |
Not reliable Historical data not provided No indication if BM was exposed (No data on PCE/NCE) Evaluated by JECFA FAS 35 as negative |
Low |
| Khatik et al. (2016 |
BM cells of Balb/c mice, 6‐8 week old 4 groups, 4 animals per group (two treated groups, Negative and positive controls |
Complexes (1:1) between curcumin phosphatidylcholine (CU‐PC) and hydrogenated soya PC (CU‐HSPC) Negative control: Vehicle: distilled water Positive control: CP (40 mg/kg bw) Relevance: limited |
One dose tested: 100 mg/kg bw of CU (as CU‐PC and CU‐HSPC), administration once daily on 2 consecutive days, gavage Sampling at the end of the treatment At least 1,000 PCE scored for the presence of MN |
Positive controls: stat. signif. increase in the number of MNPCE No statistical signif. increase in frequency of MN PCE and no effect on PCE/NCE by curcumin with both complexes Negative |
OECD not mentioned Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| Mendonça et al. (2015) |
BM cells of Wistar albino Rats, 5–6 weeks old Males, 12 groups, 6 animals per treatment Aim of the study: modulation on effects induced by cisplatin (cDDP) |
Curcumin (CMN Sigma Aldrich purity > 99%) and curcumin solid dispersion (CMN SD) Relevance: high (CMN) Negative control: Saline solution and GLA (components used for SD) Positive control: Cisplatin (cDDP), 6 mg/kg bw CMN and CMN SD in combination with cDDP |
CMN 50 mg/kg bw and CMN SD 5, 25 and 50 mg/kg bw, by gavage at 72, 48, 24 h and 30 min before i.p. administration of saline or cDDP Three slides/animal PCE and NCE scored in 500 erythrocytes; 2,000 PCE per animal scored for MN Oxidative stress parameters also measured in kidney (TBARS, GSH, Tp53 gene expression levels) |
Positive controls: stat. signif. increase in the number of MN PCE No stat. signif. increase in frequency of MN PCE by curcumin in both forms CMN and CMN SD significantly decreased the formation of MN by cDDP Negative |
OECD not mentioned but probably in line Reliable with restrictions Only one CMN dose tested Highest dose tested below OECD recommendations Historical data not provided No direct indication if BM was exposed, but indirect evidence The study is of low relevance regarding for the genotoxicity of curcumin but provide some evidence of bioavailability of curcumin in relation to modulation of genotoxic effects of cisplatin |
Limited |
| Ravikumar et al. (2018) |
BM cells of Wistar rats, 6–8 weeks old Males and females (5+5 per group) |
Curo White (Aurea Biolabs, Ltd., Kerala, India) 25–27% standardised hydrogenated curcumin powder (from turmeric rhizome powder by extraction, hydrogenation, encapsulation with beta‐cyclodextrin and spray drying) Relevance: limited Negative control: Vehicle: DMSO Positive control: CP (50 mg/kg bw, gavage) |
Three curcumin doses tested, 200, 400 and 800 mg/kg bw, administration once daily on 2 consecutive days, gavage Sampling time: 24 after second administration PCE and NCE scored in 200 erythrocytes; 2,000 PCE/animal scored for MN |
No mortality, no clinical signs of toxicity Positive controls: stat. signif. increase in the number of MNPCE Negative controls: within historical controls No stat. signif. increase in frequency of MN PCE No effect on PCE/NCE (no cytotoxicity) Negative |
GLP compliant According to OECD 474 (1997) Reliable with minor restrictions Highest dose tested below OECD recommendations No indication if BM was exposed |
Low |
| Vijayalaxmi (1980) |
BM cells of Swiss albino mice Females (24), 3 groups, 8 animals per treatment 2 treated group (turmeric and curcumin) negative control group |
Turmeric and curcumin Relevance: limited Control: normal diet Positive control: none |
0.5% turmeric and 0.015% curcumin added to diet, for 12 weeks Sampling at the end of the study 2,000 PCE per animal scored for MN |
No stat. signif. increase in frequency of MN induced by turmeric and curcumin compared to control Negative |
OECD not mentioned Reliable with restrictions No positive controls Only one dose tested No indication if BM was exposed Evaluated by JECFA FAS 17 as negative |
Low |
| Zheng et al. (2016) |
BM cells of Balb/c mice, 6–8 weeks old 5 groups, 4 animals per group |
Two types of chitosan nanoparticles (CNP) loaded with curcumin (release investigated) Relevance: limited Negative control: vehicle: distilled water Positive control: CP (40 mg/kg bw) |
One dose tested (40 mg/kg bw) of the two formulations, corresponding to 100 mg curcumin/kg (gavage), administration once daily on 2 consecutive days; sampling at the end of the study 1,000 PCE per animal scored for MN |
Positive controls: stat. signif. increase in the number of MN No stat. signif. increase in frequency of MN by curcumin in both forms Negative |
OECD not mentioned Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
In vivo comet assay
| Type of test | Experimental test system | Test substance/relevance | Exposure conditions | Result | Comments/Reliability | Relevance of the results |
|---|---|---|---|---|---|---|
| Avci et al., 2016; |
Lymphocytes of Wistar albino Rats, 3 months old Females (6 animals/group, 6 groups) Aim of the study: modulation on effects induced by cyclophosphamide (CP) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high Vehicle: corn oil Negative control: nothing was administered Positive control: CP (30 mg/kg bw), ipi for 7 days |
Curcumin (100 mg/kg bw, gavage for 14 days) Curcumin (as above) + CP (30 mg/kg bw, ip, for 7 days starting from day 8) Parameters: %Tail DNA, tail moment (by software) |
C+: stat. signif. increase of DNA damage Curcumin does not induce DNA damage and reduced damage by CP Negative DNA fragmentation in liver and kidney cells (ELISA): not increased by curcumin |
According to Singh (1988), as referenced by Avci et al. (2016) and Collins (2004) as referenced by Avci et al. (2016) No OECD Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided |
Low |
| Çelik et al., 2013 |
BM cells of Swiss albino rats (Wistar rats), 6–8 week old Females (9 groups of 6 rats) curcumin treated group; 3 PFOS treated groups, 3 PFOS + Curcumin treated groups Negative and positive controls Aim of the study: modulation on effects induced by perfluorooctane sulfonate (PFOS) |
Curcumin (Sigma Aldrich, purity > 94%) Relevance: high Vehicle: saline Positive control: MMC (2 mg/kg i.p.) |
Curcumin 80 mg/kg bw by gavage for 30 days at 48 h intervals, sacrificed 30 h after last treatment Co‐administered with PFOS (0.6, 1.25 and 2.5 mg/kg bw) 100 comet images scored per treatment; visually by two scorers, intensity for 0 (undamaged) to 4 (high damage) |
C+: stat. signif. increase of DNA damage Curcumin does not induce DNA damage PFOS induce DNA damage in a dose‐dependent manner, reduced by curcumin (by 40%) Negative |
Procedure described by Singh et al. (1988) as referenced by Çelik et al. (2013). Scoring according to Collins et al. (1995) as referenced by Çelik et al. (2013) No OECD Reliable with restrictions Only one dose tested Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| Dandekar et al. (2010a) |
BM cells of Holtzman rats, 6–8 weeks old Males + Females (n = 10, M/F 5/5 each per group), six groups Parallel acute (2,000 mg/kg bw/kg and sub‐acute (28 days, up to 200 mg/kg) toxicity studies in males and female rats (OECD, 1996, 1995 as referenced by Dandekar et al. (2010a), no. 425 and 407): no signs of toxicity |
Curcumin nanoparticles (NP) of Eudragit® S100 Relevance: limited Vehicle: distilled water Positive control: CP (40 mg/kg bw) |
Four curcumin NP doses tested, corresponding to o (blank NP), 100, 200 and 300 mg/kg bw (gavage), administered once daily on 2 consecutive days 100 cells/animal evaluated Parameters: TL, TM, TMO, % DNA damage (by software) |
C+: stat. signif. increase in all parameters and formation of distinct comets No (statistical) increase in any parameter comet formation, similarly to controls Negative |
Pre‐OECD Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided No indicationif BM was exposed |
Low |
| Dandekar et al., 2010b; |
BM cells of Holtzman rats, 6–8 week old Males+Females (n = 10, M/F 5/5 each per group), six groups Parallel acute (2,000 mg/kg bw/kg and sub‐acute (28 d, up to 200 mg/kg) toxicity studies in males and female rats (see above) |
Hydrogel nanoparticles (NP) of curcumin (95%) based on HPMC and PVP Relevance: limited Vehicle: distilled water Positive control: CP (40 mg/kg bw) |
Four curcumin NP doses tested, corresponding to o (blank NP), 100, 200 and 300 mg/kg bw (gavage), administered once daily on 2 consecutive days 100 cells/animal evaluated Parameters: TL, TM, TMO, % DNA damage (by software) |
C+: stat. signif. increase in all parameters and formation of distinct comets No (statistical) increase in any parameter comet formation, similar to controls Negative |
Pre‐OECD Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| Mendonça et al. (2015) |
Kidney and peripheral blood cells of Wistar albino Rats, 5–6 weeks old Males, 12 groups, 6 animals per treatment Aim of the study: modulation on effects induced by cisplatin (cDDP) |
Curcumin (CMN) and curcumin solid dispersion (CMN SD) Relevance: high (CMN) Negative control: Saline solution and GLA (components used for SD) Positive control: Cisplatin (cDDP), 6 mg/kg bw CMN and CMN SD in combination with cDDP |
CMN 50 mg/kg bw and CMN SD 5, 25 and 50 mg/kg bw, by gavage at 72, 48, 24 h and 30 min before cDDP (i.p.) 100 nucleoids (2 slides of 50 each) per animal analysed Parameters: %Tail DNA (by software) |
C+: stat. signif. increase in %tail by cDDP in renal tissue No (statistical) increase in %tail by curcumin in both forms, alone and in combination with cDDP Negative |
Protocol according to Singh et al. (1988) and Tice et al. (2000) as referenced by Mendonça et al. (2015) OECD not mentioned Reliable with restrictions Only one dose tested (CMN) Highest dose tested below OECD recommendations Historical data not provided No direct indication if BM was exposed |
Low |
| Sherin et al. (2017) |
Sprague dawley rats, 2–3 months old Males (number of animals/group not reported, 8 groups) |
Curcumin (Sigma Aldrich) loaded on TiO2 nanoparticles (CTNP) Relevance: limited Negative control: unspecified Positive control: silver NP (180 mg/kg bw) Biodistribution studies (longer half‐life curcumin in CTNP) |
Curcumin (1 and 20 mg/kg bw), TNPs (1 and 5 mg/kg bw), CNTPs (5 and 10 mg/kg bw) 100 cells (2 slides of 50 each) per exp group analysed Parameters: TL, %Tail DNA, TI and TM, etc. (by software) |
C+: stat. signif. Increase in all comet parameters No (statistical) increase in comet parameters by curcumin compared to negative control Negative |
According to Singh et al. (1988) OECD not mentioned Reliable with restrictions Highest dose tested below OECD recommendations Historical data not provided No indication if BM was exposed |
Low |
| Verma et al. (2016) |
BM cells of Swiss albino mice Male (6 animals/dose group, 6 groups) Aim of the study: modulation on effects of beta‐cyfluthrin (β‐CYF) |
Curcumin (purity 97%) Relevance: high Control: corn oil Positive control: CP (25 mg/kg bw) |
One dose of curcumin (0.2%), oral, administered alone (feed pellets) and in combination with low (13 mg/kg bw) and high (26 mg/kg bw) dose of β‐CYF for 21 days 100 cells (2 slides of 50 each) per exp group analysed. Parameters: TL, %Tail DNA, TI and TM (by software) |
C+: stat. signif. Increase in all comet parameters No (statistical) increase in TL, TI and TM by curcumin compared to control Curcumin decreased TL, TI and TM induced by β‐CYF Inconclusive because curcumin was only tested in combination with cyfluthrin (or tested alone but data not reported) |
According to Singh et al. (1988) OECD not mentioned Reliable with restrictions Historical data not provided Only one dose tested Missing data for curcumin when tested alone No indication if BM was exposed but indirect evidence The study is not relevant regarding for the genotoxicity of curcumin (data not reported) but provides some evidence of bioavailability of curcumin in relation to modulation of genotoxic effects of β‐CYF |
Low |
Other in vivo studies
| Ref. | Experimental test system (aim of the study) | Test substance | Exposure conditions | Result | Comments | Relevance of the results |
|---|---|---|---|---|---|---|
| Nair et al. (2005) |
Lipid peroxidation (LPO)‐induced‐ethano‐DNA adducts Long‐Evans Cinnamom (LEC) rats, model for Wilson's disease, 4 week old Males (52 animals, 2 groups, treated and control) Aim of the study: investigation of synergistic role of copper and curcumin in LEC rats, a model for human Wilson's disease |
Curcumin (95%, Schuschardt, Germany) Negative control: standard diet Relevance: high |
One doses tested: 0.5% curcumin added to standard diet, animals (n) killed after 6 (4), 8(4), 12 (6), 16 (4) and 32 (8) weeks Parameters: etheno‐DNA adducts (to adenine and cytidine) in nuclear and mitochondrial DNA, apoptosis, CD95L RNA expression, GSH and GSSG, liver enzymes (ASAT, ALAT), Cu and Fe |
The levels of adducts is higher in mitochondrial DNA compared to nuclear DNA Curcumin treatment increased the levels of adducts 10–20 times in nuclear DNA and 3–4 times in mitochondrial DNA Positive |
The enhanced formation of etheno‐DNA adducts after treatment of the rats with curcumin is due to the concurrent effect of copper and curcumin in the formation of ROS Evaluated by EFSA, 2010 as positive (exposure to 0.5% curcumin (95% purity) in the diet enhanced etheno‐DNA adduct formation 9‐ to 25‐fold in nuclear DNA and three‐ to fourfold in mitochondrial DNA. LEC rats are a model for human Wilson's disease and develop chronic hepatitis and liver tumours owing to accumulation of copper and induced oxidative stress) |
Limited |
| Polasa et al. (1991) |
Urinary mutagens Wistar rats, 8–10 weeks old Males, 6–8 rats per group (Exp. 1: 8 groups, Exp. 2: 5 groups, Exp. 3: 6 groups) Aim of the study: In vivo model to test antimutagenicity |
Turmeric sticks (from the local market), powdered and incorporated into standard diet Relevance: low Vehicle: groundnut oil Carcinogens: benzo[a]pyrene (Ba[a]P) and 3‐methyl cholanthrenene (3‐MC) |
Several doses tested: Exp.1 (0, 1, 5 and 10% turmeric), Exp. 2 and 3 (0, 0.1 and 0.5% turmeric) curcumin added to standard diet for 1, 2 or 3 months, then i.p. administration of Ba[a]P (1 or 5 mg) or 3‐MC (1 or 5 mg) Urine collected for 24 h Mutagenicity assay on urine (TA100 and TA98), +/− rat liver S9 |
Turmeric fed at 0.5% and above inhibited B[a]P and 3 MC‐mediated mutagenicity |
Antimutagenicity only Evaluated by JECFA FAS 35 |
Low |
Suggested citation: EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , Bampidis V, Azimonti G, Bastos ML, Christensen H, Kos Durjava M, Kouba M, López‐Alonso M, López Puente S, Marcon F, Mayo B, Pechová A, Petkova M, Ramos F, Sanz Y, Villa RE, Woutersen R, Brantom P, Chesson A, Westendorf J, Gregoretti L, Manini P and Dusemund B, 2020. Scientific Opinion on the safety and efficacy of turmeric extract, turmeric oil, turmeric oleoresin and turmeric tincture from Curcuma longa L. rhizome when used as sensory additives in feed for all animal species. EFSA Journal 2020;18(6):6146, 74 pp. 10.2903/j.efsa.2020.6146
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00532
Panel members: Giovanna Azimonti, Vasileios Bampidis, Maria de Lourdes Bastos, Henrik Christensen, Birgit Dusemund, Maryline Kouba, Mojca Kos Durjava, Marta López‐Alonso, Secundino López Puente, Francesca Marcon, Baltasar Mayo, Alena Pechová, Mariana Petkova, Fernando Ramos, Yolanda Sanz, Roberto Villa and Ruud Woutersen.
Acknowledgements: The EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) wishes to thank the following for the support provided to this scientific output: the experts of the Scientific Committee Working group on genotoxicity: Gabriele Aquilina, Diane Benford, Margherita Bignami, Claudia Bolognesi, Riccardo Crebelli, Rainer Gürtler, Elsa Nielsen, Josef Schlatter and Christiane Vleminckx and EFSA staff: Maria Carfí, Jaume Galobart, Orsolya Holczknecht, Daniela Maurici and Fabiola Pizzo.
Adopted: 7 May 2020
Notes
Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. OJ L 268, 18.10.2003, p. 29.
On 13/3/2013, EFSA was informed by the applicant that the applicant company changed to FEFANA asbl, Avenue Louise 130 A, Box 1, 1050 Brussels, Belgium.
On 27 February 2019, EFSA was informed about the withdrawal of the application on ginger extract.
On 26 February 2013, EFSA duly informed the applicant (EFSA ref. 7150727) that, in view of the workload, the evaluation of applications on feed flavourings would be re‐organised by giving priority to the assessment of the chemically defined feed flavourings, as agreed with the European Commission. On 24 July 2017, EFSA informed the applicant that the evaluation process restarted.
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Regulation (EC) No 1601/91 of the Council, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34.
Commission Regulation (EC) No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. OJ L 295, 12.11.2011, p. 1.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Synonym: curcumin (CAS No 458‐37‐7).
Desmethoxycurcumin (CAS No33171‐16‐3) and Bis‐desmethoxycurcumin (CAS No 33171‐05‐09).
FEED dossier reference: FAD‐2010‐0419.
Technical dossier/Supplementary information February 2018/ 2018‐01‐30_SInReply_cardamom.
Reference: EURL evaluation report related to FAD‐2010‐0335 ‐ Botanically Defined Flavourings Group BDG 09 ‐ Zingiberales (JRC F.5/CvH/ZE/AS/Ares (2018)5225574) issued on 11/10/2018.
Commission Regulation (EC) No 429/2008 of 25 April 2008 on detailed rules for the implementation of Regulation (EC) No 1831/2003 of the European Parliament and of the Council as regards the preparation and the presentation of applications and the assessment and the authorisation of feed additives. OJ L 133, 22.5.2008, p. 1.
Technical dossier/Section II/Annex_II_4.
Technical dossier/Section II, Table II. 3, p. 8.
Commission Regulation (EC) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1.
Technical dossier/Supplementary information May 2018/Annex_II_Turm_extr_Batch_to_batch.
Technical dossier/Supplementary information May 2018/Turmeric extract_ZIN002c_SIn_Reply_final.
Technical dossier/Supplementary information May 2018/Turm_extr_ZIN002c_SIn_Reply_final.
Technical dossier/Supplementary information May 2018/Annex_II_Turm_extr_Batch_to_batch and June 2016/Summary Additional_Data_Curcuma longa extracts.
Technical dossier/Supplementary information May 2018/Annex_III_Turm_extr_Heavy_metals, Limit of quantification (LOQ) for lead, cadmium, mercury and arsenic: 0.01 mg/kg.
Technical dossier/Supplementary information May 2018/Annex_IV_Turm_extr_Pesticides, LOQ for individual pesticides: 0.01 mg/kg.
Technical dossier/Supplementary information May 2018/Annex_V_Turm_extr_Mycotoxins. LOQ (two batches): 0.5 µg/kg for aflatoxins B1, B2, G1 and G2; LOQ (one batch): 3 µg/kg for aflatoxin B1, 9 µg/kg for the sum of aflatoxins B1, B2, G1 and G2.
Technical dossier/Supplementary information May 2018/Annex_VI_Turm_extr_Dioxins.
Technical dossier/Supplementary information May 2018/Turm_Extr/References.
During the assessment the applicant has clarified that insoluble additives are properly formulated to allow homogeneous distribution in water for drinking.
Technical dossier/Supplementary information/August 2019.
The NOAEL refers to curcumin with a minimum purity of 95%; however, it is considered relevant for the turmeric extract under evaluation which is considered to be of slightly lower biological activity containing only 74.84–78.6% curcumin, accompanied by 15.3–18.46% desmethoxycurcumin and 2.18–4.58% bis‐desmethoxycurcumin.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353 of 31.12.2008, p. 1.
Technical dossier/Section II/Annex 4.
Technical dossier/Supplementary information August 2018/Annex_II_Turm_Oil_CoA and Annex IV_Turm_Oil_Specifications.
Technical dossier/Supplementary information August 2018/Annex_I_Turm_Oil_Batch‐to‐batch.
Technical dossier/Supplementary information August 2018/Annex_III_Turm_Oil_Constituents_consolidated. Additional constituents: α‐phellandrene, β‐pinene, d‐limonene, o‐cymene, (+)‐4‐carene, 7‐epi‐sesquithujene, (‐)‐α‐santalene, (E)‐α‐bergamotene, (3R,4aS,8aS)‐8a‐methyl‐5‐methylene‐3‐(prop‐1‐en‐2‐yl)‐1,2,3,4,4a,5,6,8aoctahydronaphthalene; caryophyllene, tricyclo[3.3.0.0(2,8)]octan‐3‐one, 8‐methyl‐; epi‐(β)‐santalene, γ‐elemene, muurola‐4,11‐diene, β‐farnesene, sesquisabinene, α‐terpineol, zonarene, (R)‐1‐methyl‐4‐(6‐methylhept‐5‐en‐2‐yl)cyclohexa‐1,4‐diene, 4,5‐dehydroisolongifolene, (E)‐γ‐bisabolene; cis‐4,7,10,13,16,19‐docosahexaenoic acid, picolinyl ester; 2‐methylacetophenone, megastigma‐3,7(E),9‐triene, (Z)‐sabinol, 2‐methoxyphenol, 2,3‐dibromo‐8‐phenyl‐menthane, humulene oxide II; 1‐(1,2,3‐trimethylcyclopent‐2‐enyl)‐ethanone; 4,2,8‐ethanylylidene‐2H‐1‐benzopyran, octahydro‐2‐methyl‐; 1,4‐methanoazulen‐7(1H)‐one, octahydro‐1,5,5,8a‐tetramethyl‐; 4‐methyl‐4‐phenylpentan‐2‐one; isolongifolene, 4,5,9,10‐dehydro‐; beta‐caryophyllene oxide; bicyclo[3.2.2]non‐8‐en‐6‐ol, (1R,5‐cis,6‐cis)‐; 2‐methyl‐1‐(4‐methylphenyl)‐3‐buten‐1‐ol; α‐santalol, 2,4‐dimethylphenethyl alcohol; nerolidol, cinnamyl‐2‐methylcrotonate, dihydro‐ar‐turmerone; β‐elemenone, zingiberenol, 5‐(2,3‐dimethyltricyclo[2.2.1.02,6]heptan‐3‐yl)pentan‐2‐one; 2‐methoxy‐4‐vinylphenol; 3,6‐dimethyl‐6‐(1‐methylethyl)‐2‐cyclohexen‐1‐one; pregna‐3,5‐dien‐9‐ol‐20‐one; carotol, bisacurol, 6,10‐dodecadien‐1‐yn‐3‐ol, 3,7,11‐trimethyl‐; geranyl‐α‐terpinene, spiro[4.4]nona‐3,8‐diene; longiverbenone, 2‐nonadecanone, 3‐methyl‐but‐2‐enoic acid, 1,7,7‐trimethylbicyclo[2.2.1]hept‐2‐yl ester; benzenebutanal, γ‐4‐dimethyl‐; 4,2,8‐ethanylylidene‐2H‐1‐benzopyran, octahydro‐2‐methyl‐; 1,4‐cyclohexadiene, 3,3,6,6‐tetramethyl‐; oleic acid, piperityl tiglate, trans‐, vanillin, vanillydene acetone, 6‐(3‐hydroxy‐4‐methylphenyl)‐2‐methylhept‐2‐en‐4‐one; isobornyl acrylate, (R)‐curcumin, 3‐hexene, 2,5‐dimethyl‐3,4‐bis(1‐methylethyl)‐; 3‐decen‐5‐one, 2,10‐dodecadien‐1‐ol, 3,7,11‐trimethyl‐, (Z)‐; (S)‐3‐methyl‐6‐((S)‐6‐methyl‐4‐oxohept‐5‐en‐2‐yl)cyclohex‐2‐enone; bornyl tiglate, 4‐hydroxycinnamoylmethane; 3‐phenylpropyl cyclohexanecarboxylate; hexadecenoic acid.
Technical dossier/Supplementary information May 2018/Literature search_Curcuma longa L_final. Details of the search (databases searched, search terms) were provided, together with the reference list in RIS format and the relevant references (n = 39) cited in the review.
Technical dossier/Supplementary information August 2018/Annex IV_Turm_Oil_Specifications.
no indication from which plant parts (e.g. rhizomes or leaves) the oil is originating.
Technical dossier/Supplementary information/August 2019/Annex_I_Turm_Oleo_Certificates of analysis.
Technical dossier/Supplementary information August 2019/Annex_II_Turm_Oleo_Composition.
Technical dossier/Supplementary information August 2019/Annex_III_Turm_Oleo_Essential_oils_constituents.
Technical dossier/Supplementary information/August 2019/SIn_reply_Turmeric_Oleoresin.
Technical dossier/Supplementary information August 2019/Annex VI. LOQ for heavy metals and arsenic: < 0.1 mg/kg for lead, < 0.005 mg/kg for mercury, < 0.01 mg/kg for cadmium and < 0.1 mg/kg for arsenic; LOQ for individual pesticides: 0.01–0.4 mg/kg; LOQ for mycotoxins: < 0.1 µg/kg for aflatoxins B1, B2, G1 and G2.
Technical dossier/Supplementary information May 2018/Annex_Ia_Turm_Tinct_Photometer_Dicinnamoyl_methane_deriv.
Technical dossier/Supplementary information May 2018/Annex_III_Turm_Tinct_Nutr_Anal_Microbiol.
Technical dossier/Supplementary information May 2018/Annex_II_Turm_Tinct_Gravim_Anal_PCCD‐PCDF.
Technical dossier/Supplementary information May 2018/Annex_Ib_Turm_Tinct_Curcuminoids_HPLC.
Technical dossier/Supplementary information May 2018/Annex_IV_Turm_Tinct_GC‐MS_GC‐FID.
Technical dossier/Supplementary information May 2018/Annex_V_Turm_Tinct_Total_Phenols.
Average of 10% calculated by dividing the sum of the fractions (expressed as %, w/w) of phenols (0.13%) and volatiles (0.13%) and by the average DM (2.78%, w/w). The range (9–12%) is obtained by dividing the sum of phenols and volatiles calculated for each individual batch by the corresponding value of the DM in the same batch.
Technical dossier/Supplementary information May 2018/Annex_XIII_Turm_Tinct_Other_impurities. LOQ for heavy metals and arsenic: < 0.01 mg/kg for lead and arsenic, < 0.002 mg/kg for mercury and cadmium; LOQ for individual pesticides: 0.001–0.005 mg/L; LOQ for mycotoxins: < 0.1 µg/kg for aflatoxins B1, B2, G1 and G2 and ochratoxin A, < 2 µg/kg for zearalenon, HT2‐toxin and T2‐toxin, < 5 µg/kg for nivalenol and < 10 for deoxynivalenol.
References
- Aggarwal ML, Chacko KM and Kuruvilla BT, 2016. Systematic and comprehensive investigation of the toxicity of curcuminoid essential oil complex: a bioavailable turmeric formulation. Molecular Medicine Reports, 13, 592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes LM, Araujo MC, Dias FL and Takahashi CS, 1999. Modulatory effects of curcumin on the chromosomal damage induced by doxorubicin in Chinese hamster ovary cells. Teratogenesis, Carcinogenesis, and Mutagenesis, 19, 1–8. [DOI] [PubMed] [Google Scholar]
- Asai A and Miyazawa T, 2000. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sciences, 2785–2793. [DOI] [PubMed] [Google Scholar]
- Au W and Hsu TC, 1979. Studies on the clastogenic effects of biologic stains and dyes. Environmental Mutagenesis, 1, 27–35. [DOI] [PubMed] [Google Scholar]
- Avci H, Sekkin S, Boyacioğlu M, Akşit H, Tunca R, Epikmen ET and Birincioğlu SS, 2016. Ratlarda deneysel siklofosfamid toksikasyonunda silimarin ve curcuminin koruyucu ve antigenotoksik etkileri. Kafkas Univ Vet Fak Derg, 10.9775/kvfd.2016.15145 [DOI] [Google Scholar]
- Awasthi PK and Dixit SC, 2009. Chemical composition of Curcuma longa leaves and Rhizomes Oil form the Plains of Northern India. Journal of Young Pharmacists, 1, 312–316. [Google Scholar]
- Ayoub Y, Gopalan RC, Najafzadeh M, Mohammad MA, Anderson D, Paradkar A and Assi KH, 2019. Development and evaluation of nanoemulsion and microsuspension formulations of curcuminoids for lung delivery with a novel approach to understanding the aerosol performance of nanoparticles. International Journal of Pharmaceutics, 557, 254–263. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Kunwar A, Mishra B and Priyadarsini KI, 2008. Concentration dependent antioxidant/pro‐oxidant activity of curcumin Studies from AAPH induced hemolysis of RBCs. Chemico‐Biological Interactions, 174, 134–139. [DOI] [PubMed] [Google Scholar]
- Blasiak J, Trzeciak A and Kowalik J, 1999a. Curcumin damages DNA in human gastric mucosa cells and lymphocytes. Journal of Environmental Pathology, Toxicology and Oncology, 18, 271–276. [PubMed] [Google Scholar]
- Błasiak J, Trzeciak A, Małecka‐Panas E, Drzewoski J, Iwanienko T, Szumiel I and Wojewódzka M, 1999b. DNA damage and repair in human lymphocytes and gastric mucosa cells exposed to chromium and curcumin. Teratogenesis, Carcinogenesis, and Mutagenesis, 19, 19–31. [DOI] [PubMed] [Google Scholar]
- Bojko A, Cierniak A, Adamczyk A and Ligeza J, 2015. Modulatory effects of curcumin and tyrphostins (AG494 and AG1478) on growth regulation and viability of LN229 human brain cancer cells. Nutrition and Cancer, 67, 1170–1182. [DOI] [PubMed] [Google Scholar]
- Burdock GA, 2010. Fenaroli's handbook of flavor ingredients, 6th Edition CRC Press. Taylor & Francis Group, Boca Raton, FL: pp. 276–277. [Google Scholar]
- Burgos‐Morón E, Calderón‐Montaño JM, Salvador J, Robles A and López‐Lázaro M, 2010. The dark side of curcumin. International Journal of Cancer, 126, 1771–1775. [DOI] [PubMed] [Google Scholar]
- Cao J, Jia L, Zhou HM, Liu Y and Zhong LF, 2006. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicological Sciences, 91, 476–483. [DOI] [PubMed] [Google Scholar]
- Cao J, Jiang LP, Liu Y, Yang G, Yao XF and Zhong LF, 2007. Curcumin‐induced genotoxicity and antigenotoxicity in HepG2 cells. Toxicon, 49, 1219–1222. [DOI] [PubMed] [Google Scholar]
- Çelik A, Eke D, Ekinci SY and Yıldırım S, 2013. The protective role of curcumin on perfluorooctane sulfonate‐induced genotoxicity: single cell gel electrophoresis and micronucleus test. Food and Chemical Toxicology, 53, 249–255. [DOI] [PubMed] [Google Scholar]
- CoE (Council of Europe), 2000. Natural sources of flavourings. Report No. 1. Curcuma longa L., Council of. Europe Publishing; pp. 161–162. [Google Scholar]
- Court MH and Greenblatter DJ, 1997. Molecular basis for deficient acetaminophen glucuronidation in cats. Biochemical Pharmacology, 53, 1041–1047. [DOI] [PubMed] [Google Scholar]
- Damarla SR, Komma R, Bhatnagar U, Rajesh N and Sadik MAM, 2018. An Evaluation of the Genotoxicity and Subchronic Oral Toxicity of Synthetic Curcumin. Hindawi, Journal of Toxicology, 2018, Article ID 6872753, 27 p. 10.1155/2018/6872753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar P, Dhumal R, Jain R, Tiwari D, Vanage G and Patravale V, 2010a. Toxicological evaluation of pH‐sensitive nanoparticles of curcumin: acute, sub‐acute and genotoxicity studies. Food and Chemical Toxicology, 48, 2073–2089. [DOI] [PubMed] [Google Scholar]
- Dandekar PP, Jain R, Patil S, Dhumal R, Tiwari D, Sharma S, Vanage G and Patravale V, 2010b. Curcumin‐loaded hydrogel nanoparticles: application in anti‐malarial therapy and toxicological evaluation. Journal of Pharmaceutical Sciences, 99, 4992–5010. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Technical Guidance of the Scientific Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) for assessing the safety of feed additives for the environment. EFSA Journal 2008;6(10):842, 28 pp. 10.2903/j.efsa.2008.842 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Compendium of botanicals reported to contain naturally occurring substances of possible concern for human health when used in food and food supplements. EFSA Journal 2012;10(5):2663, 60 pp. 10.2903/j.efsa.2012.2663 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Refined exposure assessment for curcumin (E 100). EFSA Journal 2014;12(10):3876, 43 pp. 10.2903/j.efsa.2014.3876 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2010. Scientific Opinion on the re‐evaluation of curcumin (E 100) as a food additive. EFSA Journal 2010;8(9):1679, 46 pp. 10.2903/j.efsa.2010.1679 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2010. Statement on the use of feed additives authorised/applied for use in feed when supplied via water. EFSA Journal 2010;8(12):1956, 9 pp. 10.2903/j.efsa.2010.1956 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012a. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012b. Guidance for the preparation of dossiers for additives already authorised for use in food. EFSA Journal 2012;10(1):2538, 4 pp. 10.2903/j.efsa.2012.2538 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012c. Guidance on studies concerning the safety of use of the additive for users/workers. EFSA Journal 2012;10(1):2539, 5 pp. 10.2903/j.efsa.2012.2539 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012d. Scientific opinion on the safety and efficacy of aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols and esters with esters containing tertiary alcohols ethers (chemical group 6) when used as flavourings for all animal species. EFSA Journal 2012;10(11):2966, 25 pp. 10.2903/j.efsa.2012.2966 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012e. Scientific Opinion on the safety and efficacy of aliphatic and alicyclic ethers (chemical group 16) when used as flavourings for all animal species. EFSA Journal 2012;10(11):2967, 17 pp. 10.2903/j.efsa.2012.2967 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2015. Scientific Opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species. EFSA Journal 2015;13(3):4053, 22 pp. 10.2903/j.efsa.2015.4053 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2016. Scientific opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical Group 31) when used as flavourings for all animal species and categories. EFSA Journal 2016;14(1):4339, 17 pp. 10.2903/j.efsa.2016.4339 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on additives and products or substances used in animal feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J and Innocenti ML, 2017a. Guidance on the identity, characterisation and conditions of use of feed additives. EFSA Journal 2017;15(10):5023, 12 pp. 10.2903/j.efsa.2017.5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Galobart J, Innocenti ML and Martino L, 2017b. Guidance on the assessment of the safety of feed additives for the target species. EFSA Journal 2017;15(10):5021, 19 pp. 10.2903/j.efsa.2017.5021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), Rychen G, Aquilina G, Azimonti G, Bampidis V, Bastos ML, Bories G, Chesson A, Cocconcelli PS, Flachowsky G, Gropp J, Kolar B, Kouba M, López‐Alonso M, López Puente S, Mantovani A, Mayo B, Ramos F, Saarela M, Villa RE, Wallace RJ, Wester P, Anguita M, Dujardin B, Galobart J and Innocenti ML, 2017c. Guidance on the assessment of the safety of feed additives for the consumer. EFSA Journal 2017;15(10):5022, 17 pp. 10.2903/j.efsa.2017.5022 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance on safety assessment of botanicals and botanical preparations intended for use as ingredients in food supplements, on request of EFSA. EFSA Journal 2009;7(9):1249, 19 pp. 10.2093/j.efsa.2009.1249 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2011. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA Journal 2011;9(9):2379, 69 pp. 10.2903/j.efsa.2011.2379 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtuena Martínez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More SJ, Hardy A, Bampidis V, Benford D, Bennekou SH, Bragard C, Boesten J, Halldorsson TI, Hernandez‐Jerez AF, Jeger MJ, Knutsen HK, Koutsoumanis KP, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Nielsen SS, Schrenk D, Solecki R, Turck D, Younes M, Benfenati E, Castle L, Cedergreen N, Laskowski R, Leblanc JC, Kortenkamp A, Ragas A, Posthuma L, Svendsen C, Testai E, Dujardin B, Kass GEN, Manini P, Zare Jeddi M, Dorne J‐LCM and Hogstrand C, 2019a. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA Journal 2019;17(3):5634, 77 pp. 10.2903/j.efsa.2019.5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , More S, Bampidis V, Benford D, Boesten J, Bragard C, Halldorsson T, Hernandez‐Jerez A, Hougaard‐Bennekou S, Koutsoumanis K, Naegeli H, Nielsen SS, Schrenk D, Silano V, Turck D, Younes M, Aquilina G, Crebelli R, Gürtler R, Hirsch‐Ernst KI, Mosesso P, Nielsen E, Solecki R, Carfì M, Martino C, Maurici D, Parra Morte J and Schlatter J, 2019b. Statement on the genotoxicity assessment of chemical mixtures. EFSA Journal 2019;17(1):5519, 11 pp. 10.2903/j.efsa.2019.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Makawy A and Sharaf HA, 2006. Cytogenetical and histochemical studies on curcumin in male rats. WIT Transactions on Biomedicine and Health, 10, 169–180. [Google Scholar]
- EMA (European Medicines Agency), 2018a. Committee on Herbal Medicinal Products. Herbal Monograph on Curcuma longa L. rhizoma. EMA/507445/2018. Available online: https://www.ema.europa.eu/en/documents/herbal-opinion/opinion-hmpc-european-union-herbal-monograph-curcuma-longa-l-rhizoma-revision-1_en.pdf
- EMA (European Medicines Agency), 2018b. Committee on Herbal Medicinal Products. Assessment report on Curcuma longa L. rhizoma. EMA/HMPC/749518/2016. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-curcuma-longa-l-rhizoma-revision-1_en.pdf
- FAO (World Health Organization), 2004. Curcumin, Chemical and Technical Assessment (CTA). Available online: http://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/61/Curcumin.pdf
- Farag MR, Alagawany MM and Dhama K, 2014. Antidotal effect of turmeric (Curcuma longa) against endosulfan‐induced cytogenotoxicity and immunotoxicity in broiler chicks. International Journal of Pharmacology, 10, 429–439. [Google Scholar]
- Farhadi M, Bakhshandeh M, Shafiei B, Mahmoudzadeh A and Hosseinimehr SJ, 2018. The radioprotective effects of nano‐curcumin against genotoxicity induced by iodine‐131 in patients with differentiated thyroid carcinoma (DTC) by micronucleus assay. International Journal of Cancer Management, 11, e14193. [Google Scholar]
- Galli GM, Da Silva AS, Biazus AH, Reis JH, Boiago MM, Topazio JP, Migliorini MJ, Guarda NS, Moresco RN, Ourique AF, Santos CG, Lopes LS, Baldissera MD and Stefani LM, 2018. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Research in Veterinary Sciences, 118, 101–106. [DOI] [PubMed] [Google Scholar]
- Giri AK, Das SK, Talukder G and Sharma A, 1990. Sister chromatid exchange and chromosome aberrations induced by curcumin and tartrazine on mammalian cells in vivo. Cytobios, 62, 111–117. [PubMed] [Google Scholar]
- Gopi S, Jacob J and Mathur KY, 2016. Acute and subchronic oral toxicity studies of hydrogenated curcuminoid formulation ‘CuroWhite’ in rats toxicology. Reports, 3, 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon ON, Luis PB, Ashley RE, Osheroff N and Schneider C, 2015. Oxidative transformation of demethoxy‐ and bisdemethoxycurcumin: products, mechanism of formation, and poisoning of human topoisomerase Iiα. Chemical Research in Toxicology, 28, 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F, Rehman S, Khan MS, Ali MA, Nawaz A and Yang C, 2019. Curcumin as an alternative epigenetic modulator: mechanism of action and potential effects. Frontiers in Genetics, 10, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverić A, Haverić S, Hadžić M, Lojo‐Kadrić N and Ibrulj S, 2018. Genotoxicity and cytotoxicity analysis of curcumin and sunset yellow in human lymphocyte culture. Cellular and Molecular Biology, 64, 87–91. [DOI] [PubMed] [Google Scholar]
- Heger M, van Golen RF, Broekgaarden M and Michel MC, 2014. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacological Reviews, 66, 222–307. [DOI] [PubMed] [Google Scholar]
- Her C, Venier‐Julienne MC and Roger E, 2018. Improvement of curcumin bioavailability for medical applications. Medicinal and Aromatic Plants, 7, 6. [Google Scholar]
- Ireson C, Orr S, Jones DJL, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, Steward WP and Gescher A, 2001. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester‐induced Prostaglandin E2 production. Cancer Research, 61, 1058–1064. [PubMed] [Google Scholar]
- Jäger R, Lowery RP, Calvanese AV, Joy JM, Purpura M and Wilson JM, 2014. Comparative absorption of curcumin formulations. Nutrition Journal, 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain AK, Tezuka H, Kada T and Tomita I, 1987. Evaluation of genotoxic effects of turmeric in mice. Current Science. Bangalore, 56, 1005–1006. [Google Scholar]
- Jensen NJ, 1982. Lack of mutagenic effect of turmeric oleoresin and curcumin in the Salmonella/mammalian microsome test. Mutation Research, 105, 393–396. [DOI] [PubMed] [Google Scholar]
- Ketron AC, Gordon ON, Schneider C and Osheroff N, 2013. Oxidative metabolites of curcumin poison human type II topoisomerases. Biochemistry, 52, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatik R, Dwivedi P, Shukla A, Srivastava P, Rath SK, Paliwal SK and Dwivedi AK, 2016. Development, characterization and toxicological evaluations of phospholipids complexes of curcumin for effective drug delivery in cancer chemotherapy. Drug Delivery, 23, 1067–1078. [DOI] [PubMed] [Google Scholar]
- Kocyigit A and Guler EM, 2017. Curcumin induce DNA damage and apoptosis through generation of reactive oxygen species and reducing mitochondrial membrane potential in melanoma cancer cells. Cellular and Molecular Biology, 63, 97–105. [DOI] [PubMed] [Google Scholar]
- Lewinska A, Wnuk M, Grabowska W, Zabek T, Semik E, Sikora E and Bielak‐Zmijewska A, 2015. Curcumin induces oxidation‐dependent cell cycle arrest mediated by SIRT7 inhibition of rDNA transcription in human aortic smooth muscle cells. Toxicology Letters, 233, 227–238. [DOI] [PubMed] [Google Scholar]
- Li S, Yuan W, Deng G, Wang P, Yang P and Aggarwal BB, 2011. Chemical composition and product quality control of turmeric (Curcuma longa L,). Pharmaceutical Crops, 2, 28–54. [Google Scholar]
- Liju VB, Jeena K and Kutta R, 2013. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L). Food and Chemical Toxicology, 53, 5261. [DOI] [PubMed] [Google Scholar]
- Liju VB, Jeena K, Kumar D, Maliakel B, Kuttan R and Krishnakumar IM, 2015. Enhanced bioavailability and safety of curcumagalactomannosides as a dietary ingredient. Food & Function, 6, 276–286. [DOI] [PubMed] [Google Scholar]
- Liu A, Lou H, Zhao L and Fan P, 2006. Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. Journal of Pharmaceutical and Biomedical Analysis, 40, 720–727. [DOI] [PubMed] [Google Scholar]
- Marczylo T, Steward WP and Gescher AJ, 2009. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. Journal of agricultural and Food Chemistry, 57, 797–803. [DOI] [PubMed] [Google Scholar]
- Mehrotra N, Sabarinath S, Suryawanshi S, Raj K and Gupta RC, 2009. LC–UV assay for simultaneous estimation of aromatic turmerone, α/β‐turmerone and curlone: major bisabolane sesquiterpenes of turmeric oil in rabbit plasma for application to pharmacokinetic studies. Chromatographia, 69, 1077–1082. [Google Scholar]
- Mendonça LM, Dos Santos GC, Antonucci GA, Dos Santos AC, Bianchi MLP and Antunes LMG, 2009. Evaluation of the cytotoxicity and genotoxicity of curcumin in PC12 cells. Mutation Research, 675, 29–34. [DOI] [PubMed] [Google Scholar]
- Mendonça LM, Dos Santos GC, dos Santos RA, Takahashi CS, Bianchi MLP and Antunes LMG, 2010. Evaluation of curcumin and cisplatin‐induced DNA damage in PC12 cells by the alkaline comet assay. Human & Experimental Toxicology, 29, 635–643. [DOI] [PubMed] [Google Scholar]
- Mendonça LM, Machado CD, Teixeira CCC, Freitas LAP, Bianchi MLP and Antunes LMG, 2015. Comparative study of curcumin and curcumin formulated in a solid dispersion: evaluation of their antigenotoxic effects. Genetics and Molecular Biology, 38, 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushan M and Bhide SV, 1986. Nonmutagenicity of curcumin and its antimutagenic action versus chili and capsaicin. Nutrition and Cancer, 8, 201–210. [DOI] [PubMed] [Google Scholar]
- Nagabhushan M and Bhide SV, 1987. Antimutagenicity and anticarcinogenicity of turmeric (Curcuma longa). Journal of Nutrition Growth and Cancer, 4, 83–89. [Google Scholar]
- Nagabhushan M, Amonkar AJ and Bhide SV, 1987. In vitro antimutagenicity of curcumin against environmental mutagens. Food and Chemical Toxicology, 25, 545–547. [DOI] [PubMed] [Google Scholar]
- Nair J, Strand S, Frank N, Knauft J, Wesch H, Galle PR and Bartsch H, 2005. Apoptosis and age‐dependant induction of nuclear and mitochondrial etheno‐DNA adducts in Long‐Evans Cinnamon (LEC) rats: enhanced DNA damage by dietary curcumin upon copper accumulation. Carcinogenesis, 26, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF and Walters MA, 2017. The essential medicinal chemistry of curcumin. Journal of Medicinal Chemistry, 60, 1620–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), , 1993. Toxicology and carcinogenesis studies of turmeric oleoresin (CAS No. 8024‐37‐1) (major components 79%‐85% curcumin CAS No. 458‐37‐7) in F344/N Rats and B6C3F1 Mice (feed studies). Technical Report Series No. 427, 1–275. [PubMed] [Google Scholar]
- Papiez MA, 2013. The influence of curcumin on the action of etoposide in a rat acute myeloid leukemia cell line. Folia Medica Cracoviensia, 53, 61–72. [PubMed] [Google Scholar]
- Polasa K, Sesikaran B, Krishna TP and Krishnaswamy K, 1991. Turmeric (Curcuma longa)‐induced reduction in urinary mutagens. Food and Chemical Toxicology, 29, 699–706. [DOI] [PubMed] [Google Scholar]
- Purpura M, Lowery RP, Wilson JM, Mannan H, Münch G and Razmovski‐Naumovski V, 2018. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. European Journal of Nutrition, 57, 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina VK, Srivastava SK and Syamsundar KV, 2005. Rhizome and leaf oil composition of Curcuma longa from the lower Himalayan Region of Northern India. Journal of Essential Oil Research, 17, 4 pp. [Google Scholar]
- Ravikumar AN, Jacob J, Gopi S and Jagannath TS, 2018. A Toxicological Evaluation of a Standardized Hydrogenated Extract of Curcumin (CuroWhite™). Hindawi, Journal of Toxicology, Article ID 5243617, 16 p, 10.1155/2018/5243617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakihama Y, Cohen MF, Grace SC and Yamasaki H, 2002. Plant phenolic antioxidant and prooxidant activities: phenolics‐induced oxidative damage mediated by metals in plants. Toxicology, 177, 67–80. [DOI] [PubMed] [Google Scholar]
- Saleh EM, El‐awady R, Eissa NA and Abdel‐Rahman WM, 2012. Antagonism between curcumin and the topoisomerase II inhibitor etoposide. A study of DNA damage, cell cycle regulation and death pathways. Cancer Biology & Therapy, 13, 1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastià N, Soriano JM, Barquinero JF, Villaescusa JI, Almonacid M, Cervera J, Such E, Silla MA and Montorso A, 2012. In vitro cytogenetic and genotoxic effects of curcumin on human peripheral blood lymphocytes. Food and Chemical Toxicology, 50, 3229–3233. [DOI] [PubMed] [Google Scholar]
- Shah RH and Netrawali MS, 1988. Evaluation of mutagenic activity of turmeric extract containing curcumin, before and after activation with mammalian cecal microbial extract of liver microsomal fraction, in the Ames Salmonella test. Bulletin of Environmental Contamination and Toxicology, 40, 350–357. [DOI] [PubMed] [Google Scholar]
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, Plummer SM, Pirmohamed M, Gescher AJ and Steward WP, 2004. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clinical Cancer Research, 10, 6847–6854. [DOI] [PubMed] [Google Scholar]
- Shen LL and Ji HF, 2019. Bidirectional interactions between dietary curcumin and gut microbiota. Critical Reviews in Food Science and Nutrition, 59(19). [DOI] [PubMed] [Google Scholar]
- Shen L, Liu C, An C and Ji H, 2016. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Nature. Scientific Reports, 6, 20872 10.1038/srep20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin S, Sheeja S, Sudha Devi R, Balachandran S, Soumya RS and Abraham A, 2017. In vitro and in vivo pharmacokinetics and toxicity evaluation of curcumin incorporated titanium dioxide nanoparticles for biomedical applications. Chemico‐Biological Interactions, 275, 35–46. [DOI] [PubMed] [Google Scholar]
- Singh S, Panda MK, Subudhi E and Nayak S, 2010. Chemical composition of leaf and rhizome oil of an elite genotype Curcuma longa L. from South Eastern Ghats of Orissa. Journal of Pharmacy Research, 3, 1630–1633. [Google Scholar]
- Sivaswamy SN, Balachandran B, Balanehru S and Sivaramakrishnan VM, 1991. Mutagenic activity of south Indian food items. Indian Journal of Experimental Biology, 29, 730–737. [PubMed] [Google Scholar]
- Spalding JW, 1983. Genetic toxicity report on turmeric oleoresin (8024–37‐1). Unpublished report submitted to WHO by the National Institutes of Health, Research Triangle Park, NC, USA. [Google Scholar]
- Srividya AR, Dhanbal SP, Sathish Kumar MN and Vishnuvarthan VJ, 2013. Comparison of genotoxicity produced by hydro alcoholic extract of Curcuma aromatica salisb, Curcuma zedoaria with curcumin by Ames test, comet assay and micronucleus test. International Research Journal Pharmacology, 4, 113–119. [Google Scholar]
- Tascone O, Roy C, Filippi J‐J and Meierhenrich UJ, 2014. Use, analysis, and regulation of pesticides in natural extracts, essential oils, concretes, and absolutes. Analytical and Bioanalytical Chemistry, 406, 971–980. [DOI] [PubMed] [Google Scholar]
- Teixeira CCC, Mendonça LM, Bergamaschi MM, Queiroz RHC, Souza GEP, Antunes LMG and Freitas LAP, 2016. Microparticles containing curcumin solid dispersion: stability, bioavailability and anti‐inflammatory activity. An Official Journal of the American Association of Pharmaceutical Scientists, 17, 252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher E, 2003. Gewürzdrogen. Wissenschaftliche Verlagsgesellschaft mbH Stuttgart, Ein Handbuch der Gewürze, Gewürzkrauter, Gewürzmischungen und ihrer ätherischen Öle. ISBN 3‐8047‐1867‐1. [Google Scholar]
- Urbina‐Cano P, Bobadilla‐Morales L, Ramírez‐Herrera MA, Corona‐Rivera JR, Mendoza‐Magaña ML, Troyo‐Sanromán R and Corona‐Rivera A, 2006. DNA damage in mouse lymphocytes exposed to curcumin and copper. Journal of applied genetics, 47, 377–382. [DOI] [PubMed] [Google Scholar]
- Verma R, Awasthi KK, Rajawat NK, Soni I and John PJ, 2016. Curcumin modulates oxidative stress and genotoxicity induced by a type II fluorinated pyrethroid, beta‐cyfluthrin. Food and Chemical Toxicology, 97, 168–176. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi , 1980. Genetic effects of turmeric and curcumin in mice and rats. Mutation Research, 79, 125–132. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization), 1982. Curcumin. WHO Food additives Series (FAS), 17 Available online: http://www.inchem.org/documents/jecfa/jecmono/v17je30.htm [Google Scholar]
- WHO (World Health Organization), 1996. Curcumin. WHO Food additives Series, (FAS), 35 Available online:http://www.inchem.org/documents/jecfa/jecmono/v35je09.htm [Google Scholar]
- WHO (World Health Organization), 2004a. Evaluation of certain food additives and contaminants. Sixty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 922. Geneva. Available online: http://whqlibdoc.who.int/trs/WHO_TRS_922.pdf
- WHO (World Health Organization), 2004b. WHO Food additives Series (FAS): 52. Available online: http://www.inchem.org/documents/jecfa/jecmono/v52je04.htm
- WHO (World Health Organization), 2006. Combined compendium of food additive specifications ‐ all specifications monographs from the 1st to the 65th meeting (1956‐2005). FAO JECFA Monographs Series, No. 1 Volume 1–3. Available online: http://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/detail/en/c/442/
- Yang KY, Lin LC, Tseng TY, Wang SC and Tsai TH, 2007. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. Journal of Chromatography B, 853, 183–189. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Haneda M, Naruse M, Htay HH, Tsubouchi R, Qiao SL, Li WH, Murakami K and Yokochi T, 2004. Prooxidant activity of curcumin: copper‐dependent formation of 8‐hydroxy‐2’‐deoxyguanosine in DNA and induction of apoptotic cell death. Toxicology in Vitro, 18, 783–789. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chen Y, Jin LW, Ye HY and Liu G, 2016. Cytotoxicity and genotoxicity in human embryonic kidney cells exposed to surface modify chitosan nanoparticles loaded with curcumin. An Official Journal of the American Association of Pharmaceutical Scientists, 17, 1347–1352. [DOI] [PubMed] [Google Scholar]
- Ziegler H (ed), 2007. Turmeric, Curcuma In: Flavourings. Production, Composition, Applications, Regulations. Second completely revised version. Wiley‐VCH Verlag GmbH & Co. KGA, Weinheim, p. 246. [Google Scholar]


