Abstract
According to Article 12 of Regulation (EC) No 396/2005, EFSA has reviewed the maximum residue levels (MRLs) currently established at European level for the pesticide active substance fluopyram. To assess the occurrence of fluopyram residues in plants, processed commodities, rotational crops and livestock, EFSA considered the conclusions derived in the framework of Commission Regulation (EU) No 188/2011, the MRLs established by the Codex Alimentarius Commission as well as the import tolerances and European authorisations reported by Member States (including the supporting residues data). Based on the assessment of the available data, MRL proposals were derived and a consumer risk assessment was carried out. Some information required by the regulatory framework was missing and a possible chronic risk to consumers was identified. Hence, the consumer risk assessment is considered indicative only, some MRL proposals derived by EFSA still require further consideration by risk managers and measures for reduction of the consumer exposure should also be considered. Hence, the consumer risk assessment is considered indicative only and some MRL proposals derived by EFSA still require further consideration by risk managers.
Keywords: fluopyram, MRL review, Regulation (EC) No 396/2005, consumer risk assessment, fungicide
Summary
Fluopyram was approved on 1 February 2014 by means of Commission Implementing Regulation (EU) No 802/2013 under Regulation (EC) No 1107/2009 as amended by Commission Implementing Regulations (EU) No 540/2011 and 541/2011.
As the active substance was approved after the entry into force of Regulation (EC) No 396/2005 on 2 September 2008, the European Food Safety Authority (EFSA) is required to provide a reasoned opinion on the review of the existing maximum residue levels (MRLs) for that active substance in compliance with Article 12(1) of the aforementioned regulation.
As the basis for the MRL review, on 13 October 2017 EFSA initiated the collection of data for this active substance. In a first step, Member States were invited to submit by 13 November 2017 their national Good Agricultural Practices (GAPs) in a standardised way, in the format of specific GAP forms, allowing the designated rapporteur Member State (RMS) Germany to identify the critical GAPs in the format of a specific GAP overview file. Subsequently, Member States were requested to provide residue data supporting the critical GAPs, within a period of 1 month, by 9 May 2018. On the basis of all the data submitted by Member States and by the EU Reference Laboratories for Pesticides Residues (EURL), EFSA asked the RMS to complete the Pesticide Residues Overview File (PROFile) and to prepare a supporting evaluation report. The PROFile and evaluation report, together with Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file, were provided by the RMS to EFSA on 27 September 2018. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Based on the information provided by the RMS, Member States and the EURL, and taking into account the conclusions derived by EFSA in the framework of Commission Regulation (EU) No 188/2011 and the MRLs established by the Codex Alimentarius Commission, EFSA prepared in July 2019 a draft reasoned opinion, which was circulated to Member States for consultation via a written procedure. Comments received by 3 September 2019 were considered during the finalisation of this reasoned opinion. The following conclusions are derived.
The metabolism of fluopyram in plant was investigated in primary and rotational crops. According to the results of the metabolism studies, the plant residue definition for enforcement can be proposed as ‘fluopyram’ and for risk assessment as ‘sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram’. These residue definitions are also applicable to processed commodities. Fully validated analytical methods are available for the enforcement of the proposed residue definition in all major matrices at the limit of quantification (LOQ) of 0.01 mg/kg. According to the EURLs, the LOQ of 0.01 mg/kg is achievable by using the QuEChERS method in routine analyses.
Fluopyram is a persistent substance which may accumulate in soil following multiannual uses. To account for the potential uptake of such residues accumulated in soil in rotational crops two options were considered. Both options assumed that the most critical indoor GAP on tomatoes is restricted to growing on artificial substrates or other means to prevent carry‐over of residues from treated soil to succeeding crops. In addition to this restriction:
Option 1: assumed that adequate risk mitigation measures are in place to avoid significant residues in crops grown in rotation with crops treated with fluopyram. These measures included a plant‐back interval (PBI) of 1 year for root and tuber vegetables, and leafy vegetables; and a PBI of 120 days for cereals.
Option 2: assumed that no risk mitigation is implemented other than the above restriction on the most critical indoor GAP on tomatoes.
For Option 1, the available data are considered sufficient to derive MRL proposals as well as risk assessment values for all commodities under evaluation, except for lemons, mandarins, cherries, banana, spring onions, tomatoes, melons, watermelon, Chinese cabbage, escaroles, land cresses, red mustards, spinaches, chards/beet leaves, globe artichokes and leeks, where tentative MRLs are derived, and for lime, cherimoya and chicory roots where the available data were insufficient to derive tentative MRLs.
For Option 2, specific MRLs, considering that residues uptake in succeeding crops are not avoided, were also derived for cassava roots/manioc, sweet potatoes, yams, arrowroots, root vegetables, broccoli, cauliflower, Brussels sprouts, head cabbage, kales, kohlrabies, watercress, herbal infusions (roots), sugar beets, sweet corn, maize grain, buckwheat and millet grain, as well as tentative MRLs for chicory roots. It is underlined that MRLs values derived from rotational crop field data are subject to a high degree of uncertainty.
Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items.
The effect of industrial processing and/or household preparation was assessed and robust processing factors could be derived for processed commodities from wine grapes, strawberries, tomatoes, melons, apples, bananas and rapeseeds. Tentative processing factors are also proposed for citrus, sugar beet, potato and peanuts.
Fluopyram is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. Residues from primary uses without (Option 1) or with (Option 2) residues in rotational crops were considered. For both scenarios, the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM) in both cases. Behaviour of residues was therefore assessed in all commodities of animal origin.
The metabolism of fluopyram residues in livestock was investigated in lactating goats and laying hens at dose rates covering the maximum dietary burdens calculated in this review. According to the results of these studies, the residue definition for enforcement in all livestock commodities was proposed as the ‘sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram’ and for risk assessment as the ‘sum of fluopyram, fluopyram‐benzamide (M25), and fluopyram‐ E / Z ‐olefine (M02/M03), expressed as fluopyram’. An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.02 mg/kg in all matrices is available. According to the EURLs, a combined LOQ of 0.02 mg/kg is achievable for commodities of animal origin.
Livestock feeding studies on cows and laying hens were used to derive two sets of MRL and risk assessment values in milk, eggs, and tissues of ruminants and poultry in view of the two dietary burdens (with or without rotational crops), each set corresponding to one of the 2 options described above. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 3.1 of the EFSA PRIMo. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation.
In the light of the possible contribution of residues from rotational uses to consumer exposure pending the implementation of risk mitigation measures, the two options described above were considered.
The highest chronic exposure was calculated for the Dutch toddler, representing 86% (Option 1) and 100% (Option 2) of the acceptable daily intake (ADI). The highest acute exposure was calculated for lettuce, representing 76% of the acute reference dose (ARfD) for both options.
Apart from the MRLs evaluated in the framework of this review, internationally recommended codex maximum residue limits (CXLs) have also been established for fluopyram. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out.
The highest chronic exposure was calculated for Dutch toddler, representing 110% (Option 1) and 128% (Option 2) of the ADI. The highest acute exposure was calculated for lettuce, accounting 76% of the ARfD for both options.
For Option 1, as a potential risk management option, the risk assessment was re‐calculated by considering the European animal diet for cattle and swine and thus disregarding the CXLs for these animal commodities. According to this scenario, the chronic exposure represents 92% of the ADI. Nonetheless, it is highlighted that this scenario was only provided as a potential option for risk managers to consider and does not exclude or suggest alternative options may not be available for risk managers.
For Option 2, given that the chronic exposure based on the authorised EU uses, import tolerances and the uptake of fluopyram accumulated in soil following multiannual use already accounted for 100% of the ADI (NL toddlers), and as there may be several alternative options at the discretion of risk managers to exclude a potential chronic risk, the only safe scenario assessed was that disregarding from the calculation all CXLs higher than the derived EU MRL.
Altogether, the calculations indicate a potential chronic risk to consumers if all the existing CXLs are incorporated in the assessment. For Option 1, a safe scenario could be identified, excluding the CXLs for cattle and swine tissues from the calculation. For Option 2, a safe scenario could be identified disregarding from the calculation all CXLs higher than the derived EU MRL.
Background
Regulation (EC) No 396/20051 (hereinafter referred to as ‘the Regulation’) establishes the rules governing the setting and the review of pesticide maximum residue levels (MRLs) at European level. Article 12(1) of that Regulation stipulates that the European Food Safety Authority (EFSA) shall provide within 12 months from the date of the inclusion or non‐inclusion of an active substance in Annex I to Directive 91/414/EEC2 a reasoned opinion on the review of the existing MRLs for that active substance.
As fluopyram was approved on 1 February 2014 by means of Commission Implementing Regulation (EU) No 802/20133 in accordance with Regulation (EC) No 1107/20094 as amended by Commission Implementing Regulations (EU) No 540/20115 and 541/20116,EFSA initiated the review of all existing MRLs for that active substance.
By way of background information, in the framework of Commission Regulation (EU) No 188/20117 Fluopyram was evaluated by Germany, designated as rapporteur Member State (RMS). Subsequently, a peer review on the initial evaluation of the RMS was conducted by EFSA, leading to the conclusions as set out in the EFSA scientific report (EFSA, 2013a).
According to the legal provisions, EFSA shall base its reasoned opinion in particular on the relevant assessment report prepared under Directive 91/414/EEC repealed by Regulation (EC) No 1107/2009. It should be noted, however, that, in the framework of Regulation (EC) No 1107/2009, only a few representative uses are evaluated, whereas MRLs set out in Regulation (EC) No 396/2005 should accommodate all uses authorised within the European Union (EU), and uses authorised in third countries that have a significant impact on international trade. The information included in the assessment report prepared under Regulation (EC) No 1107/2009 is therefore insufficient for the assessment of all existing MRLs for a given active substance.
To gain an overview of the pesticide residues data that have been considered for the setting of the existing MRLs, EFSA developed the Pesticide Residues Overview File (PROFile). The PROFile is an inventory of all pesticide residues data relevant to the risk assessment and MRL setting for a given active substance. This includes data on:
the nature and magnitude of residues in primary crops;
the nature and magnitude of residues in processed commodities;
the nature and magnitude of residues in rotational crops;
the nature and magnitude of residues in livestock commodities;
the analytical methods for enforcement of the proposed MRLs.
As the basis for the MRL review, on 13 October 2017, EFSA initiated the collection of data for this active substance. In a first step, Member States were invited to submit by 13 November 2017 their Good Agricultural Practices (GAPs), in a standardised way, in the format of specific GAP forms. In the framework of this consultation 17 Member States provided feedback on their national authorisations of fluopyram. Based on the GAP data submitted, the designated RMS Germany was asked to identify the critical GAPs to be further considered in the assessment, in the format of a specific GAP overview file. Subsequently, in a second step, Member States were requested to provide residue data supporting the critical GAPs by 9 May 2018.
On the basis of all the data submitted by Member States and the EU Reference Laboratories for Pesticides Residues (EURL), EFSA asked Germany to complete the PROFile and to prepare a supporting evaluation report. The PROFile and the supporting evaluation report, together with the Pesticide Residues Intake Model (PRIMo) calculations and an updated GAP overview file, were submitted to EFSA on 27 September 2018. Subsequently, EFSA performed the completeness check of these documents with the RMS. The outcome of this exercise including the clarifications provided by the RMS, if any, was compiled in the completeness check report.
Considering all the available information, and taking into account the MRLs established by the Codex Alimentarius Commission (CAC) (i.e. codex maximum residue limit (CXLs)), EFSA prepared in July 2019 a draft reasoned opinion, which was circulated to Member States for commenting via a written procedure. All comments received by 3 September 2019 including additional GAPs submitted (Netherlands, 2019) were considered by EFSA during the finalisation of the reasoned opinion.
The evaluation report submitted by the RMS (Germany, 2018), taking into account also the information provided by Member States during the collection of data, the EURL report on analytical methods (EURL, 2018) and the evaluation reports received during the Member State consultation (Belgium, 2019; Netherlands, 2019) are considered as main supporting documents to this reasoned opinion and, thus, made publicly available.
In addition, further supporting documents to this reasoned opinion are the completeness check report (EFSA, 2019c) and the Member States consultation report (EFSA, 2019e). These reports are developed to address all issues raised in the course of the review, from the initial completeness check to the reasoned opinion. Furthermore, the exposure calculations for all crops reported in the framework of this review performed using the EFSA Pesticide Residues Intake Model (PRIMo) and the PROFile as well as the GAP overview file listing all authorised uses and import tolerances are key supporting documents and made publicly available as background documents to this reasoned opinion. A screenshot of the report sheet of the PRIMo is presented in Appendix C.
Terms of Reference
According to Article 12 of Regulation (EC) No 396/2005, EFSA shall provide a reasoned opinion on:
the inclusion of the active substance in Annex IV to the Regulation, when appropriate;
the necessity of setting new MRLs for the active substance or deleting/modifying existing MRLs set out in Annex II or III of the Regulation;
the inclusion of the recommended MRLs in Annex II or III to the Regulation;
the setting of specific processing factors as referred to in Article 20(2) of the Regulation.
The active substance and its use pattern
Fluopyram is the ISO common name for N‐{2‐[3‐chloro‐5‐(trifluoromethyl)‐2‐pyridyl]ethyl}‐α,α,α‐trifluoro‐o‐toluamide (IUPAC).
The chemical structure of the active substance and its main metabolites are reported in Appendix F.
The EU MRLs for fluopyram are established in Annex IIIA of Regulation (EC) No 396/2005. CXLs for fluopyram were also established by the CAC. An overview of the MRL changes that occurred since the entry into force of the Regulation mentioned above is provided below (Table 1).
Table 1.
Overview of the MRL changes since the entry into force of Regulation (EC) No 396/2005
| Procedure | Legal implementation | Remarks |
|---|---|---|
| MRL application | Not yet implemented | Modification of the existing maximum residue level for fluopyram in herbal infusions from leaves, herbs and flowers (EFSA, 2019d) |
| MRL application | Commission Regulation (EU) 2019/1791a | Modification of the existing maximum residue level for fluopyram in broccoli (EFSA, 2019a) |
| Implementation of CAC 2018 | Commission Regulation (EU) 2019/552b | On 6 July 2018, the Codex Alimentarius Commission (CAC) adopted Codex limits (CXLs) for fluopyram. These CXLs have been included in Regulation (EC) No 396/2005 as MRLs |
| MRL application | Commission Regulation (EU) 2018/685c | Modification of the existing maximum residue level for fluopyram in purslanes (EFSA, 2017) |
| MRL application | Commission Regulation (EU) 2017/978d | Modification of the existing maximum residue levels for fluopyram in solanacea, other fruiting vegetables, cardoons, celeries, Florence fennels, other stem vegetables, cotton seeds, other oilseeds, common millet/proso millet, other cereals, herbal infusions from any other parts of the plant, seed spices, carawayand other sugar plants (EFSA, 2016) |
| Implementation of CAC 2015 | Commission Regulation (EU) 2017/626e | On 11 July 2015, the Codex Alimentarius Commission (CAC) adopted Codex limits (CXLs) for fluopyram. These CXLs have been included in Regulation (EC) No 396/2005 as MRLs |
| MRL application | Commission Regulation (EU) 2017/171f | Modification of the existing maximum residue levels for apricots, peppers, ‘spinaches and similar leaves’, witloof, ‘herbs and edible flowers’, peas (with pods), lentils, other legume vegetables of code 0260990, sesame seeds, sunflower seeds, pumpkin seeds, safflower seeds, borage seeds, hemp seeds, castor beans, barley, buckwheat, oats and sugar beet (EFSA, 2016) |
| Implementation of CAC 2014 | Commission Regulation (EU) 2016/567a | On 18 July 2014, Codex Alimentarius Commission (CAC) adopted Codex maximum residue limits (CXLs) for fluopyram. These CXLs have been included in Regulation (EC) No 396/2005 as MRLs |
| MRL application | Commission Regulation (EU) 2015/1101g | Modification of the existing MRLs in various crops: apricots, peaches, plums, cane fruit, small fruits and berries, root and tuber vegetables, aubergines, escaroles, spinaches, witloof, beans (without pods), peas (with pods), linseed, poppy seed, mustard seed, gold of pleasure, herbal infusions (dried roots), hops, spices (roots or rhizome), chicory roots (EFSA, 2014) |
| Implementation of CAC 2013 | Commission Regulation (EU) No 491/2014h | On 5 July 2013, Codex Alimentarius Commission (CAC) adopted Codex maximum residue limits (CXLs) for fluopyram. These CXLs have been included in Regulation (EC) No 396/2005 as MRLs |
| MRL application | Commission Regulation (EU) No 270/2012i | Setting new MRLs and import tolerances in various commodities (EFSA, 2011) |
MRL: maximum residue level.
Commission Regulation (EU) 2019/1791 of 17 October 2019 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 1‐decanol, 2,4‐D, ABE‐IT 56, cyprodinil, dimethenamid, fatty alcohols, florpyrauxifen‐benzyl, fludioxonil, fluopyram, mepiquat, pendimethalin, picolinafen, pyraflufen‐ethyl, pyridaben, S‐abscisic acid and trifloxystrobin in or on certain products.OJ L 277, 29.10.2019, p. 1–65.
Commission Regulation (EU) 2019/552 of 4 April 2019 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for azoxystrobin, bicyclopyrone, chlormequat, cyprodinil, difenoconazole, fenpropimorph, fenpyroximate, fluopyram, fosetyl, isoprothiolane, isopyrazam, oxamyl, prothioconazole, spinetoram, trifloxystrobin and triflumezopyrim in or on certain products. OJ L 96, 5.4.2019, p. 6–49.
Commission Regulation (EU) 2018/685 of 3 May 2018 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for abamectin, beer, fluopyram, fluxapyroxad, maleic hydrazide, mustard seeds powder and tefluthrin in or on certain products. OJ L 121, 16.5.2018, p. 1–29.
Commission Regulation (EU) 2017/978 of 9 June 2017 amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for fluopyram; hexachlorocyclohexane (HCH), alpha‐isomer; hexachlorocyclohexane (HCH), beta‐isomer; hexachlorocyclohexane (HCH), sum of isomers, except the gamma isomer; lindane (hexachlorocyclohexane (HCH), gamma‐isomer); nicotine and profenofos in or on certain products. OJ L 151, 14.6.2017, p. 1–37.
Commission Regulation (EU) 2017/626 of 31 March 2017 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for acetamiprid, cyantraniliprole, cypermethrin, cyprodinil, difenoconazole, ethephon, fluopyram, flutriafol, fluxapyroxad, imazapic, imazapyr, lambda‐cyhalothrin, mesotrione, profenofos, propiconazole, pyrimethanil, spirotetramat, tebuconazole, triazophos and trifloxystrobin in or on certain products. OJ L 96, 7.4.2017, p. 1–43.
Commission Regulation (EU) 2017/171 of 30 January 2017 amending Annexes II, III and IV to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for aminopyralid, azoxystrobin, cyantraniliprole, cyflufenamid, cyproconazole, diethofencarb, dithiocarbamates, fluazifop‐P, fluopyram, haloxyfop, isofetamid, metalaxyl, prohexadione, propaquizafop, pyrimethanil, Trichoderma atroviride strain SC1 and zoxamide in or on certain products. OJ L 30, 3.2.2017, p. 45–111.
Commission Regulation (EU) 2016/567 of 6 April 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for chlorantraniliprole, cyflumetofen, cyprodinil, dimethomorph, dithiocarbamates, fenamidone, fluopyram, flutolanil, imazamox, metrafenone, myclobutanil, propiconazole, sedaxane and spirodiclofen in or on certain products. OJ L 100, 15.4.2016, p. 1–60.
Commission Regulation (EU) 2015/1101 of 8 July 2015 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for difenoconazole, fluopicolide, fluopyram, isopyrazam and pendimethalin in or on certain products. OJ L 181, 9.7.2015, p. 27–53.
Commission Regulation (EU) No 491/2014 of 5 May 2014 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for ametoctradin, azoxystrobin, cycloxydim, cyfluthrin, dinotefuran, fenbuconazole, fenvalerate, fluopyram, fluopyram, flutriafol, fluxapyroxad, glufosinate‐ammonium, imidacloprid, indoxacarb, MCPA, methoxyfenozide, penthiopyrad, spinetoram and trifloxystrobin in or on certain products. OJ L 146, 16.5.2014, p. 1–91.
Commission Regulation (EU) No 270/2012 of 26 March 2012 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for amidosulfuron, azoxystrobin, bentazone, bixafen, cyproconazole, fluopyram, imazapic, malathion, propiconazole and spinosad in or on certain products. OJ L 89, 27.3.2012, p. 5–63.
For the purpose of this MRL review, all the uses of fluopyram currently authorised within the EU and in third countries as submitted by the Member States during the GAP collection, have been reported by the RMS in the GAP overview file. The critical GAPs identified in the GAP overview file were then summarised in the PROFile and considered in the assessment. The details of the authorised critical GAPs for fluopyram are given in Appendix A.
Assessment
EFSA has based its assessment on the following documents:
the PROFile submitted by the RMS;
the evaluation report accompanying the PROFile (Germany, 2018);
the draft assessment report (DAR) and its addenda prepared under in the framework of Commission Regulation (EU) No 188/2011 (Germany, 2011, 2012);
the conclusion on the peer review of the pesticide risk assessment of the active substance fluopyram (EFSA, 2013a);
the Joint Meeting on Pesticide residues (JMPR) Evaluation report (FAO, 2010, 2012, 2014, 2015, 2017);
the previous reasoned opinions on fluopyram (EFSA, 2011 2014 2016 2019c 2017 d).
The assessment is performed in accordance with the legal provisions of the uniform principles for evaluation and authorisation of plant protection products as set out in Commission Regulation (EU) No 546/20118 and the currently applicable guidance documents relevant for the consumer risk assessment of pesticide residues (European Commission, 1997a, 1997b, 1997c, 1997d, 1997e, 1997f, 1997g, 2000, 2010a, 2010b, 2017; OECD, 2011, 2013, 2018).
More detailed information on the available data and on the conclusions derived by EFSA can be retrieved from the list of end points reported in Appendix B.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of fluopyram was investigated in the framework of the peer review (Germany, 2011; EFSA, 2013a). Studies are available after foliar treatment in three crop groups: fruits and fruiting vegetables (grapes), root and tuber (potato) and pulses (beans) and following drip irrigation in fruiting vegetables (pepper). In addition, as supporting information to assist the identification of the metabolic pathway, a study using cell culture is also available.
In grapes, metabolism was limited after 100 g a.s./ha foliar spray application followed bsy 2 × 200 g a.s./ha. Fluopyram represented over 95% of the total radioactive residues (TRR) 18 days after the last treatment.
Another study investigated metabolism following drip irrigation in mature pepper fruits 55–97 days following treatment. Plants treated with 5 mg [phenyl‐UL‐14C]fluopyram/plant, resulted in low TRR (0.038 mg eq/kg), with parent accounting for 49% of TRR and metabolite fluopyram‐benzamide (M25) for 16% of TRR. When [pyridyl‐2,6‐14C]fluopyram was applied at a rate of 5 or 20 mg/plant, the TRR was 0.06 or 0.149 mg eq/kg, with fluopyram present at 16% or 33% TRR, and metabolites fluopyram‐pyridyl‐acetic‐acid‐glycoside (M42) accounting for 32% or 38%, while fluopyram‐pyridyl‐carboxylic acid (M43) for 20% or 44% of the TRR, respectively.
In potatoes, after three foliar applications of 167 g a.s./ha using two different radiolabels, the overall TRR was low (0.008 and 0.012 mg eq/kg in potato tubers). In the study using the phenyl label, fluopyram accounted for 69% and metabolite M25 for 7% of the TRR. In the case of the pyridyl‐2,6 label, metabolite fluopyram‐pyridyl‐carboxylic acid (M43) was identified up to 50% of the TRR, whereas fluopyram was present at 23% of the TRR.
In beans, after two foliar applications of 250 g a.s./ha, while initially metabolism was not observed, later it became more extensive. In green beans, 4 days following treatment, fluopyram accounted for 94‐99% of the TRR (1.3 and 3.9 mg e.g./kg). By 29 days after treatment, the overall TRR dropped substantially in succulent (0.07–0.17 mg eq/kg) and dry beans (0.12–0.31 mg eq/kg). While fluopyram represented only up to 13% of the TRR, metabolite M25 represented up to 64% of the TRR, whereas metabolites M43 and fluopyram‐pyridyl‐acetic acid (M40) contributed up to 30 and 32 of the TRR% in succulent and dry beans, respectively.
In all foliar applications (grapes, potato and bean), very limited metabolism was observed in the leaves/foliage with parent contributing in the range of 87–98% of the TRR. Even following drip irrigation parent remained above 70% of the TRR in the whole plant.
In addition, a cell suspension study derived from apples was submitted and was considered as supporting information to help identification of metabolites in plant and animal metabolism studies.
It can be concluded that the metabolic pathway of fluopyram was qualitatively similar throughout all crop groups and treatments. Nonetheless, quantitative differences were observed. Fluopyram remained unchanged after foliar application in fruit crops. Meanwhile, in pulses and after soil irrigation in fruits at longer periods after treatment, metabolism entailing cleavage between the phenyl and the pyridyl rings occurred, resulting in the formation of metabolites M25 (phenyl) and M40, M42, M43 (pyridyl moiety).
1.1.2. Nature of residues in rotational crops
Fluopyram is authorised on crops that may be grown in rotation. It is a highly persistent substance; the field DT90 reported in the soil degradation studies evaluated in the framework of the peer review was above 1,000 days (EFSA, 2013a). In soil, the primary metabolic pathway following microbial degradation was suggested to be via hydroxylation of fluopyram to fluopyram‐7‐hydroxy (M08) followed by cleavage to M25 and M43, with M43 further metabolised to methyl‐sulfoxide (M45) (Germany, 2011). None of the soil metabolites were highlighted as persistent during the peer review (EFSA, 2013a).
Two confined rotational crop studies with fluopyram radiolabelled on either the phenyl or the pyridyl moiety were assessed during the peer review (Germany, 2011; EFSA, 2013a). Fluopyram was applied at a rate of 534 or 514 g a.s./ha onto bare soil, which covers the accumulated multiannual soil plateau concentration calculated for the second most critical GAPs (outdoor strawberry, 1.2N) but not that of the indoor tomato GAP submitted during the Member State Consultation (0.12 N) (see Section 1.2.2). Crops were planted at nominal plant‐back intervals (PBIs) of 30, 139 and 280 days after treatment (DAT). Crops planted at each interval consisted of leafy vegetables (Swiss chard), roots (turnips) and cereals (spring wheat).
Residues in wheat straw, grain, Swiss chard, and turnips declined over time, while residues in hay and forage remained at similar levels. However, significant residues were observed even at 280 DAT in all crops (up to 1.97 mg eq/kg in straw).
Parent fluopyram was the major component of the TRR (50–95% TRR; up to 4.9 mg eq/kg in straw at 30 DAT) in all crops. However, in grains metabolites M43 and M45 and in chards metabolite M08 were more prominent (up to 56%, 49% and 38.6% of the TRR, respectively). M08 and its conjugate were also observed in straw and hay at significant levels (up to 12.6% TRR). M08 and its conjugates were also observed in primary crops at low levels. Similarly to primary crops, M25 was also identified in all crops at low levels, in the range of 2.8–11.7% TRR.
Overall, the metabolism and distribution of fluopyram in rotational crops is similar to the metabolic pathway observed in primary crops, involving hydroxylation followed by cleavage between the two rings. Nonetheless, some metabolites may be specific to one metabolic pathway (M45 for rotational crops) and/or the relative proportions may vary, for example hydroxylated parent compounds (M08) and their conjugates occur at much higher levels in rotational crops, whereas M25 is observed at higher levels in primary crops.
1.1.3. Nature of residues in processed commodities
Studies investigating the nature of residues in processed commodities were assessed (Germany, 2011; EFSA, 2013a). Studies were conducted with radiolabelled fluopyram and metabolites M08, M25, M40 and M43 on either their phenyl or their pyridyl moiety simulating representative hydrolytic conditions for pasteurisation (20 min at 90°C, pH 4), boiling/brewing/baking (60 min at 100°C, pH 5) and sterilisation (20 min at 120°C, pH 6). Fluopyram, M08, M25 and M43 were stable to hydrolysis under standard conditions of pasteurisation, baking/brewing/boiling and sterilisation (Germany, 2011; EFSA, 2013a) whereas M40 ([3‐chloro‐5‐(trifluoromethyl)pyridin‐2‐yl]acetic acid) degraded to fluopyram‐picoline (3‐chloro‐2‐methyl‐5‐(trifluoromethyl)pyridine). Nonetheless, based on the peer review, M40 is not expected to be present in significant levels in raw agricultural commodities (EFSA, 2013a). Overall, it can be concluded that processing will not impact the nature of residues in processed commodities and is similar to that in primary crops.
1.1.4. Methods of analysis in plants
During the peer review a hyphenated analytical method based on gas chromatography (GC) coupled to mass spectrometry (MS) detection was fully validated for the enforcement of fluopyram in high water content (lettuce), high oil content (oilseed rape), high acid content (orange) and dry matrices (wheat grain, peas seed), with a LOQ of 0.01 mg/kg. This primary method is supported by independent laboratory validation (ILV) (EFSA, 2013a). During the completeness check, the EURLs concluded that fluopyram can be monitored by using the QuEChERS method in high water content and high acid content commodities with a LOQ of 0.002 mg/kg and in high oil content and dry commodities with a LOQ of 0.01 mg/kg (EURL, 2018).
EFSA notes that specific analytical methods for dill seeds (seed spice) were not provided, whereas for hops, the ILV of the method is missing. Nonetheless, considering that for all four main groups fully validated analytical methods were provided with an LOQ of 0.01 mg/kg and the MRLs proposed based on CXLs for these commodities are high (≥ 60 mg/kg), this is considered a minor deficiency and therefore submission of the fully validated analytical methods specific for these matrices is only desirable.
1.1.5. Stability of residues in plants
The storage stability of parent and its metabolite M25 was investigated in the framework of the peer review (EFSA, 2013a) and in a subsequent reasoned opinion on fluopyram (EFSA, 2014). Storage stability for both fluopyram and its metabolite M25 was demonstrated in high water content (lettuce, cabbage), high acid content (orange), high oil content (rapeseed) matrices and dry/high starch content (dry pea, wheat grain) commodities for a period of 36 months when stored at –18°C.
It is noted that no specific study is available for the storage stability in hops, seed spices and straw. However, as storage stability was investigated and demonstrated in the four main plant matrices for at least 36 months, and considering that samples from these crops were stored for a maximum of 18 months, a significant decline of residues in these samples is not excepted to have occurred. Therefore, no additional storage stability studies are required.
During the peer review, storage stability was proven for M40 and M43 for at least 2 years in water‐, starch‐, protein‐ and oil‐containing matrices and at least 6 months in acidic matrices, and for M08 and M45 for at least 2 years in water and starch containing matrices when stored at or below −18°C (EFSA, 2013a).
1.1.6. Proposed residue definitions
A wide range of growing conditions and crop groups was investigated (spraying in fruits, pulses, and tuber crops; drip irrigation in fruits; as well as cereals, root crops and leafy crops grown in rotation). Fluopyram is also authorised as primary seed treatment on oil seeds and as a local treatment (pre‐forcing) on chicory roots (witloofs). As the metabolite pattern is essentially the same in all crop categories even under different application systems, the above studies are considered to cover also the latter uses. Overall, the studies experimental designs were representative of the authorised uses and no further study is required.
As the parent compound was found to be a sufficient marker in all crops investigated, the residue definition for enforcement is proposed as ‘fluopyram’ only.
An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.01 mg/kg in all four main plant matrices is available (EFSA, 2013a). According to the EURLs, the LOQ of 0.002 mg/kg in high water content and high acid content commodities and the LOQ of 0.01 mg/kg in high oil content and dry commodities is achievable by using the QuEChERS method in routine analyses (EURL, 2018).
The metabolic pathway of fluopyram in plants can be regarded as essentially the same in all crops investigated, with the parent compound being one of the major constituents of the residues. The metabolic pathway primarily consists of the hydroxylation of parent compound (M08), followed by cleavage of the hydroxylated parent compound leading to metabolite M25 (fluopyram‐benzamide) from the phenyl moiety and metabolites M40 (primary crops only, including its hexose‐conjugate M42), M45 (rotational crop only) and M43 from the pyridyl moiety of the active substance.
In the supervised field trials assessed in the current review M25 was detected only in a few commodities (up to a level of 0.16 mg/kg in rape seed) (see Section 1.2.1). In rotational crop field trials, solely M25 and M08 were found in significant amounts, and only in straw (see Section 1.2.2). However, as the relative contribution of M08 is little and would have very limited impact on the animal burden, if at all, its inclusion in the residue definition for risk assessment that would be specific to rotational cereals (straw) is not proposed. The peer review concluded that metabolite M40 does not need to be included in the residue definition as is of no toxicological concern at the levels detected in supervised field trials and it may be covered by the concurrently detected phenyl specific M25, included in the residue definition (Germany, 2011).
M08, M25, M40 and its conjugate M42 were considered covered by the toxicological profile of the parent compound (EFSA, 2013a). M43 and M45, are common metabolites with active substance fluopicolide. In the light of their levels in food and feed items, and the conclusion for fluopicolide, the peer review considered these metabolites as toxicologically not relevant (Germany, 2011).
Altogether, the residue definition for risk assessment is proposed to remain ‘sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram’ as set by the peer review (EFSA, 2013a).
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
To assess the magnitude of fluopyram residues resulting from the reported GAPs, EFSA considered all residue trials reported by the RMS in its evaluation report (Germany, 2018) and the evaluation reports received during the Member State Consultation (Belgium, 2019; Netherlands, 2019) as well as the residue trials evaluated in the framework of previous MRL applications (EFSA, 2011 2014 2016 2019c 2017 d). Based on the information received during the Member States Consultation, EFSA disregarded the uses initially mistakenly considered as existing uses (EFSA, 2019e). All residue trial samples considered in this framework were stored in compliance with the conditions for which storage stability of residues was demonstrated. Decline of residues during storage of the trial samples is therefore not expected.
The number of residue trials and extrapolations were evaluated in accordance with the European guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs (European Commission, 2017).
Residue trials are not available to support the authorisations on lime, cherimoyas, and chicory roots. Therefore, MRL and risk assessment values could not be derived for these crops and the following data gaps were identified:
Lime: Four trials compliant with the import tolerance GAP are required.
Cherimoyas: Four trials compliant with the indoor GAP are required.
Chicory roots: Four trials compliant with the northern outdoor GAP are required. The available trials are not GAP compliant, as they include an additional treatment of the roots by dipping and therefore cannot be used to support the outdoor use.
For all other crops, available residue trials are sufficient to derive (tentative) MRL and risk assessment values, taking note of the following considerations:
Lemons, mandarins: Although tentative MRL and risk assessment values can be derived from the import tolerance limited data set, two additional trials compliant with the import tolerance GAP are still required.
Rose hips: Trials on currants were scaled to the northern outdoor GAP (scaling factor of 0.75). Further residue trials are not required.
Banana: Although tentative MRL and risk assessment values can be derived from the southern outdoor data, four trials compliant with the southern outdoor GAP are required.
Apricots: Although MRL and risk assessment values can be derived from the southern outdoor data, eight trials compliant with the import tolerance GAP are still required.
Cherries: Although tentative MRL and risk assessment values can be derived from the northern limited data set, one additional trial compliant with the northern GAP and two additional trials compliant with the import tolerance are still required.
Plums: Although MRL and risk assessment values can be derived from the northern data, two additional trials compliant with the import tolerance GAP are still required.
Hazelnuts: MRL and risk assessment values can be derived from the import tolerance data. As the northern GAP is clearly less critical, further residue trials compliant with the northern outdoor GAP are not required.
Carrots: Trials on carrots and radishes were combined in the import tolerance application, since residue levels in carrot and radish roots are expected to be comparable as the foliar application is done close to the harvest. Further residue trials are therefore not required.
Radishes: Trials on carrots and radishes were combined in the import tolerance application, since residue levels in carrot and radish roots are expected to be comparable as the foliar application is done close to the harvest. Although MRL and risk assessment values can be derived from the import tolerance GAP, four trials compliant with the northern outdoor GAP are still required.
Tomatoes: Although tentative MRL and risk assessment values can be derived from the indoor limited data set, one additional trial compliant with the indoor GAP is still required.
Aubergines: Six trials were compliant with the indoor GAP and eight indoor residue trials were conducted with two applications instead of three. The trials on tomatoes performed with two instead of three applications were deemed acceptable since residues are in the same range or higher compared to residues of the GAP compliant trials. Further residue trials are therefore not required.
Spring onions: Residue trials supporting the northern and the southern outdoor GAPs were conducted with two applications instead of one. Although tentative MRL and risk assessment values can be derived from the southern overdosed residue trials, four trials compliant with the southern GAP are still required. As the northern GAP is clearly less critical, further residue trials compliant with the northern outdoor GAP are not required.
Sweet peppers: As MRL and risk assessment values can be derived from the import tolerance data and the reduced number of residue trials supporting the southern outdoor GAP confirms that this use is less critical, additional trials compliant with the southern outdoor GAP are not required.
Cucumbers, courgettes: As MRL and risk assessment values can be derived from the indoor data and the reduced number of residue trials (at least 4) supporting the outdoor GAPs confirms that the outdoor uses are less critical, additional trials compliant with the outdoor GAPs are not required.
Melons: Although tentative MRL and risk assessment values can be derived from the import tolerance limited data set, two additional trials compliant with the import tolerance GAP and four additional trials compliant with the indoor GAP are still required. EFSA highlights, in case the MRL is to be lowered in the future, risk managers should consider that another GAP leading to a lower MRL (0.3 mg/kg) fully supported by data is authorised in France.
Watermelons: Although tentative MRL and risk assessment values can be derived from the indoor limited data set, four additional trials compliant with the indoor GAP are still required. EFSA highlights that in case the MRL is to be lowered in the future, risk managers should consider that another GAP leading to a lower MRL (0.3 mg/kg) fully supported by data is authorised in France.
Sweet corn: The number of residue trials supporting the import tolerance GAP is not compliant with the data requirements for this crop. However, the reduced number of residue trials for this minor crop is considered acceptable in this case because all results were below the LOQ and no residue is expected. Further residue trials are therefore not required.
Chinese cabbage: Residue trials were conducted with two applications instead of one. Although tentative MRL and risk assessment values can be derived from these northern overdosed trials, four trials compliant with the northern GAP are still required.
Lettuces: The southern outdoor residue trials were conducted with two applications instead of one. Nevertheless, as the indoor GAP is clearly more critical, further residue trials compliant with the outdoor GAP are not required.
Lamb's lettuces, cresses and other sprouts and shoots, Roman rocket, purslanes (sea lavender), baby leaf crops: Northern outdoor residue trials were conducted with two applications instead of one. Nevertheless, as the indoor GAP is clearly more critical, further residue trials compliant with the northern outdoor GAPs are not required.
Escaroles, land cresses, red mustards, spinaches, chards/beet leaves: Residue trials were conducted with two applications instead of one. Although tentative MRL and risk assessment values can be derived from these northern overdosed residue trials, four trials compliant with the northern GAP are still required.
Herbs and edible flowers: Residue trials were conducted in parsley, chervil, sage and savoury. As the highest residue was measured in savoury, all trials in fresh herbs were included in the MRL derivation for the whole group of fresh herbs. It is noted that according to the current EU guidance on extrapolation (European Commission, 2017), extrapolation from sage and savoury to the whole group is not supported, and if these trials are disregarded a lower MRL may be derived.
Globe artichokes: Although tentative MRL and risk assessment values can be derived from the import tolerance limited data set, one additional trial compliant with the import tolerance GAP is still required.
Leeks: Residue trials were conducted with two applications instead of one. Although tentative MRL and risk assessment values can be derived from these southern overdosed residue trials, four trials compliant with the southern GAP are still required.
Beans, peas (dry): Although MRL and risk assessment values can be derived from the import tolerance data, eight additional trials compliant with the northern outdoor GAPs are still required.
The available residue trials also allow to derive conversion factors (CFs) from enforcement to risk assessment. In order to avoid excessive overestimation of the risk assessment the following considerations were applied when calculating the CFs. A CF of 1 was applied for all commodities where the metabolite M25 was found at or below the LOQ in all trials. The results from trials performed in different geographical zones were combined, if mode of application allowed. For the import tolerances (with the exception of cotton) the metabolite M25 was not measured. In these cases, and where applicable, the CFs for the same commodities or group of commodities were used. For all import tolerances on fruit crops, the CF of 1 as derived from the available trials on other fruits crops with foliar treatment was applied; for pulses, a CF of 1.3 based on beans/peas without pods; whereas for oilseeds the CF of 1.2 was based on rapeseed. An overview of the derived CFs is reported in Appendix B.1.2.1. Considering the overall data available, although the metabolite was not always analysed, additional trials are not required to confirm the proposed conversion factors.
1.2.2. Magnitude of residues in rotational crops
The confined rotational crop studies suggest that residues of fluopyram cannot be excluded in rotational crops. Therefore, rotational field studies were required to assess potential residues uptake in rotational crops following multiannual use of fluopyram.
1.2.2.1. Plateau concentration in soil
As the DT90 value exceeds one year, fluopyram is likely to accumulate in soils treated for several consecutive years. Therefore particular attention has to be paid to the plateau concentration expected in soil after several years of applications. The total soil concentration of fluopyram (PECsoil total) resulting from the multiannual use of fluopyram at the critical GAP (plateau background 0.08 mg/kg soil at 20 cm depth) plus from the maximal seasonal application rate is calculated as 0.146 mg/kg after 10 years based on the most critical authorised use of strawberries (foliar, 2 × 250 g/ha). During the Member States consultation, data to support a recently authorised more critical GAP was submitted (Netherlands, 2019). Based on this indoor GAP on tomatoes (4 × 500 g/ha; soil drip application, preharvest interval (PHI) of 1 day), the calculated total plateau concentration over 20 cm is 1.42 mg/kg after 11 years.
Several rotational crop field trials conducted in Europe, the USA or Canada provided in the framework of the peer review (Germany, 2011; EFSA, 2013a) or submitted in the framework of an MRL application (EFSA, 2014) were considered in the present MRL review. In all these trials, fluopyram was applied on bare soil, or early post‐emergence applications of a primary crop at the dose rate of 500 g a.s./ha equivalent to a PECsoil total of 0.17 mg a.s./kg soil (20 cm soil of a density of 1.5 g/cm3; no plant‐soil interception). Therefore, the dose rate of the rotational field studies represents roughly 1.2N and 0.12N compared to the PECsoil estimated at 0.146 and 1.42 mg/kg for the northern European Union (NEU) GAP on strawberries and the indoor GAP on tomato, respectively.
Since the rotational crop field studies were underdosed compared to the plateau concentration for the indoor soil application on tomato, the possible occurrence of residues of fluopyram following multiannual applications according to this GAP could not be assessed for this use. Therefore, Member States granting an authorisation for this indoor tomato GAP should request additional rotational crop field studies conducted with application rates that cover the plateau background concentrations for this use. Pending the submission of these studies, Member States are recommended to implement mitigation measures (e.g. restriction on the use to growing substrate) in order to avoid uptake of residues from soil following the above use, not covered by the present assessment.
The current assessment covers residue uptake from previously treated soils following multiannual applications for all other authorised uses.
1.2.2.2. Rotational crop field trials: residues in succeeding crops
In the above rotational field trials, residues in succeeding crops with PBIs of around 30 days (28–49 days), 90–240 days or 286–320 days were evaluated (EFSA, 2013a). Samples from rotational crops (turnips/carrot, head lettuce and wheat) were taken 100–425 days following last treatment and residues were analysed for parent, and metabolites M08, M25, M43 and M45. Fluopyram was found at up to 0.05 and 0.03 mg/kg in carrots, 0.03 and 0.01 mg/kg in lettuce (PBI: 30–36 days and 90–240 days, respectively); and in one sample at 0.01 mg/kg (PBI 30 days) in wheat grains. At 286/320 PBIs, in the edible part of crops, residues of fluopyram and its metabolites were all below the LOQ of 0.01 mg/kg. With regard to feed items, in straw and green material (considered as surrogate for forage) fluopyram was detected at all PBIs, with highest residues observed at the PBI of ~ 30 days (up to 0.28 mg/kg and 0.12 mg/kg, respectively).
Regarding the metabolites, none were detected in lettuce or root crops at any of the PBIs. In straw, M08 was detected up to 0.11 mg/kg and M25 at up to 0.14 mg/kg at the PBI of ~ 30 days. M45 was also detected in all wheat parts, with highest residue detected at the PBI of 30 days.
In the rotational crop field trials submitted under a previous MRL application, residues at the PBI of 30 days were investigated in potato and spinach (EFSA, 2014). Fluopyram was detected between 0.02 to 0.09 mg/kg in spinach, whereas in potato tubers it was detected at 0.02 mg/kg. Compared to the previous trials on lettuce (EFSA, 2013a), residue data on spinach leaves show higher residues levels.
Additional field rotational crop trials (mustard green, alfalfa and cotton) conducted in the US and Canada using 2 applications of 250 g/ha fluopyram sprayed on bare soil or early post‐emergence applications (primary crops) with a target PBI of 14 days or 240 DAT, considered less representative than the European trials, completed the data set (Germany, 2011). These trials indicate that fluopyram is present in mustard green grown in rotation (up to 0.035 mg/kg at the PBI of 240 days), whereas in succeeding cotton its presence is unlikely. Cotton was grown following 14 days of bare soil treatment and fluopyram was not detected in cotton seeds, only in cotton gin by‐products in 2 out of 11 trials (0.02 mg/kg) (Germany, 2011).
Results of the rotational field studies are reported in Appendix B.1.2.2(b).
There are no rotational field trials available on fruits and fruiting vegetables. Therefore, the available authorised soil treatment uses on tomatoes (excluding the new indoor use), sweet peppers and cucurbits (see Appendix B.1.2.1) were considered as surrogate for fruits and fruiting vegetables grown in rotation. These trials suggests that significant residue uptake from rotated uses might not be expected in this group of crops. As the application rate of the new indoor use on tomato is much higher compared to the plateau expected in soil (see above), this use was not considered.
Based on the available rotational crop trials, residues may only be expected to be below 0.01 mg/kg in the edible parts of succeeding crops if appropriate risk mitigation measures are implemented, such as limiting the use of the new indoor GAP on tomato to substrate growth, setting a PBI of 120 days for cereals, and a PBI of 1 year for root and tuber vegetables and leafy crops, and provided that fluopyram is applied in compliance with the GAPs reported in Appendix A. EFSA investigated two options to account for the possible carry‐over of residues to crops grown in rotation following multiannual use. In both options, the most critical indoor GAP on tomatoes is considered to be restricted to substrate growth.
Option 1: assumed that adequate risk mitigation measures are in place to avoid residues above 0.01 mg/kg in the edible part of crops grown in rotation with crops treated with fluopyram. As described above, these measures included a PBI of 1 year for root and tuber vegetables, and leafy vegetables; and a PBI of 120 days for cereals. It is highlighted that at national level alternative risk mitigations measures may be also be available.
Option 2: assumed that no additional risk mitigation is implemented.
1.2.2.3. Calculation of MRLs in rotational crops (Option 2)
On the basis of the above reported studies the peer review proposed default MRLs of 0.1 mg/kg for root/tuber and leafy crops and of 0.01 mg/kg for cereals and oilseed (EFSA, 2013a), and EFSA recommended 0.2 mg/kg for spinaches and similar leaves, except purslanes and 0.05 mg/kg for potatoes (EFSA, 2014).
In the framework of this MRL review, EFSA further considered the available data to estimate the impact of residue uptake from soil following multiannual use on the MRLs and risk assessment values, in case appropriate risk mitigation measures are not in place to prevent carry‐over (Option 2).
The MRL review should be performed according to the old data requirements applicable at the time of the peer review. Nevertheless, as the European Commission guidance document on rotational crops (European Commission, 1997c) provides only limited guidance on how to derive MRLs for rotational crops, EFSA considered the methodology described by the recent OECD guidance on rotational crops (OECD, 2018) which is in principle fully applicable only with the new data requirements.
For annual crops, EFSA performed a rough estimate whether or not uptake of fluopyram residues from the soil could contribute significantly to the overall fluopyram residue levels.
Based on the rotational field studies, considering the worst case scenario of crop failure (PBI of 30 days) highest fluopyram residues were 0.05, 0.09 and 0.28 mg/kg in root and tuber vegetables, leafy vegetables and straw, respectively. Residues were below the LOQ of 0.01 mg/kg in cotton seeds (pulses and oil seeds) (see Section 1.2.2.2 and Appendix B.1.2.2). Residues from soil uptake in succeeding crops were extrapolated from spinaches/lettuces to all leafy vegetables, brassicas; from potatoes to all tuber vegetables; from carrots/turnips to root; and from wheat to cereals. Residues resulting from the primary crop use were compared to the residue levels observed through soil uptake in the rotational field trials. If the additional contribution by rotational crop residues (highest residue (HR) values) is < 25% of the residues arising after primary treatment (HR values), the primary use was considered as representative of the residues from the combined sources.
For root and tuber vegetables, and brassica vegetables, the uptake of residues from rotational crops exceeded 25% of the residue from primary uses. Therefore, the HR and supervised trials median residue (STMR) values from the two uses were summed and the MRL was rounded up to account for the combined uses. It is noted, when residues from soil uptake were combined with does originating from the primary use, the existing GAPs from third countries (import tolerances) were disregarded. For all crops that may be grown in rotation but for which no primary crop use is authorised, the STMR, HR and MRL values were derived from extrapolation from the relevant rotational crops data.
As in succeeding crops M25 was above the LOQ only in cereal green material and straw, for which residues following primary use were significantly higher, the CF from enforcement to risk assessment derived for primary crops are considered applicable for the combined uses.
In the absence of data on the primary crop use of chicory roots the MRL proposal and risk assessment values were also derived directly from the rotational field trials. It is stressed that setting MRLs for rotational crops based on the available limited data set is associated with large uncertainties.
An overview of the derived MRLs is reported Appendix B.1.2.2(c).
In fruit crops, the available data suggest that potential uptake in succeeding crops is likely covered by the MRLs derived from the authorised uses. The following data are therefore considered desirable but not essential:
additional rotational field trials on fruits and fruiting vegetables.
Moreover, for bulb and stem vegetables specific data for rotational crops are not available, EFSA was not able to assess the potential uptake in succeeding crops. Although it is not expected to modify the outcome of the risk assessment, the following data should be generated if risk managers intend to set MRLs in these crops:
four additional rotational field trials on bulb and stem vegetables.
1.2.3. Magnitude of residues in processed commodities
The effect of industrial processing and/or household preparation was assessed on studies conducted on oranges, grapes, strawberries, tomato, melon, apple, banana, oilseed, potato, sugar beet, peanuts (EFSA, 2011; Germany, 2011). An overview of all available processing studies is available in Appendix B.1.2.3. Robust processing factors (fully supported by data) could be derived for grapes (washed; juice, dry and wet pomace, must, wine, and dried raisins) strawberries (jam), tomatoes (peeled and canned; juice), melons (peeled), apples (washed; juice, dry and wet pomace, and sauce), bananas (peeled) and rapeseeds (crude oil, refined oil and meal/press cake). Tentative processing factors are available for citrus (pulp, dried pulp and juice), potato tuber (peeled), sugar beet (refined sugar, molasses, dried pulp) and for peanut (meal/pressed cake and refined oil) based on only one study.
Further processing studies are not required as they are not expected to affect the outcome of the risk assessment. However, if more robust processing factors were to be required by risk managers, in particular for enforcement purposes, additional processing studies would be needed.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive MRL proposals as well as risk assessment values for all commodities under evaluation, except for lemons, mandarins, cherries, banana, spring onions, tomatoes, melons, watermelon, Chinese cabbage, escaroles, land cresses, red mustards, spinaches, chards/beet leaves, globe artichokes and leeks, where tentative MRLs are derived, and for lime, cherimoya, and chicory roots, where the available data were insufficient to derive tentative MRLs. As fluopyram is a very persistent substance, these MRL proposals assume that appropriate risk mitigation measure are implemented to avoid carry‐over from treated soil (such as limiting the use of the new indoor GAP on tomato to substrate growth, setting a PBI of 120 days for cereals, and a PBI of one year for root and tuber vegetables and leafy crops; Option 1).
In addition, specific MRLs from rotational crops considering a worst case scenario (PBI of 30 days, no risk mitigation measure other than restricting the new tomato indoor use is in place; Option 2) were also derived for: cassava roots/manioc, sweet potatoes, yams, arrowroots, root vegetables, broccoli, cauliflower, Brussels sprouts, head cabbage, kales, kohlrabies, watercress, herbal infusions (roots), spice roots, sugar beets, chicory roots (tentative), sweet corn, maize, rice, buckwheat and millet grain.
It is noted that following multiannual applications according to the new indoor soil treatment use on tomato, if carry‐over of treated soil is not mitigated, the possible occurrence of residues of fluopyram at levels higher than the derived MRL reported in this review cannot be excluded for this use.
Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items.
2. Residues in livestock
Fluopyram is authorised for use on several crops that might be fed to livestock, in addition residues in feed items from crops grown in rotation cannot be ruled out. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance (OECD, 2013).
In a first scenario only the residues from primary uses were considered. In order to cover the possible contribution from rotational crops, a second calculation was carried out considering the STMR and HR values as derived in Appendix B.1.2.2.(c) based on the combined residues from primary and rotational crops. The input values for all relevant commodities, corresponding to each option, have been selected according to the recommendations of JMPR (FAO, 2009) and are summarised in Appendix D.
The calculated dietary burdens for all groups of livestock are summarised in Appendix B.2.(a) considering primary uses only (Option 1) and in Appendix B.2.(b) considering also residues from additional soil uptake (Option 2). The calculated dietary burden for ruminants nearly doubled in Option 2, driven by residues in processed potato waste, whereas it had only a minor impact the dietary burden for poultry (most critical commodity swede roots).
It is highlighted that for turnip tops, no residue data were available for primary crops. Nonetheless, residues extrapolated from rotated carrot leaves were considered in the second calculation. The animal intake of fluopyram residues via the primary use of turnip leaves has therefore not been assessed and may have been underestimated. However, this is not expected to have a major impact on the outcome of the dietary burden considering the overwhelming contribution of other feed items (e.g. cereals and potato).
The calculated dietary burdens for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg dry matter (DM). Behaviour of residues was therefore assessed in all commodities of animal origin.
2.1. Nature of residues and methods of analysis in livestock
The metabolism of fluopyram residues in livestock was investigated in lactating goats and laying hens at dose rates covering the maximum dietary burdens calculated in this review (Germany, 2011). These studies were assessed in the framework of the peer review (EFSA, 2013a).
In all studies fluopyram was radiolabelled in the phenyl or pyridyl ring of the molecule and administered at nominal rates of 2 mg/kg body weight (bw) per day to lactating goats or laying hens. The studies indicate a significant transfer of residues to all animal matrices. Substantial label dependent differences were observed in the uptake and distribution of radioactivity in both hens and goats. The rate of uptake of pyridyl labelled compounds was faster, in contrast, the accumulation of the phenyl labelled compounds in milk, eggs and tissues were several fold higher. In lactating goats, highest residue levels (phenyl label) were found in liver and kidney (8.7 and 2.3 mg eq/kg, respectively) whilst lower transfer is expected in milk (up to 0.3 mg eq/kg), muscle (0.7 mg eq/kg) and fat (0.4 mg eq/kg). In hens, the highest residue transfer was observed in liver (9.4 mg eq/kg), followed by eggs, muscle and fat (3.6, 3.3 and 1.6 mg eq/kg, respectively).
Fluopyram was extensively metabolised in all animals and was only detected at very low levels in poultry and goat matrices in the metabolism studies. Radioactive residues were composed of several metabolites. The phenyl specific metabolite fluopyram‐benzamide (M25) was the most predominant compound in all matrices identified; in hen between 67% and 99% TRR and in goat in the range of 49% to 98% TRR. Other main metabolites were fluopyram‐E/Z‐olefine (M02, M03), observed at significant levels in fat of poultry (up to 0.425 mg/kg; 26% TRR) and ruminant (up to 0.125 mg/kg; 34% TRR). In addition, in goat other metabolites found above 10% TRR included fluopyram‐7‐hydroxy (M08), its conjugates. Their relative contribution to the consumer exposure compared to other metabolites is expected to be low. All other identified metabolites were present at lower levels (< 10% TRR).
As fluopyram and fluopyram‐benzamide were found to be sufficient markers in all livestock commodities, the residue definition for enforcement is proposed as the ‘sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram’.
An analytical method using high‐performance liquid chromatography with tandem mass spectrometry (HPLC–MS/MS) was fully validated for the determination of fluopyram and fluopyram‐benzamide in all animal tissues, milk and eggs, with a combined LOQ of 0.02 mg/kg (EFSA, 2013a). According to the EURLs, a combined LOQ of 0.02 mg/kg is achievable for the proposed residue definition for commodities of animal origin (sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram) (EFSA, 2019e).
Based on the metabolism and feeding studies fluopyram residues are not fat soluble, as preferential concentration in fat tissues and/or milk is not observed.
For risk assessment, fluopyram and fluopyram‐benzamide (M25), fluopyram‐E/Z‐olefine (M02/M03) are considered toxicologically relevant. Fluopyram‐benzamide (M25) and fluopyram‐E/Z‐olefine (M02/M03) are encountered in the rat metabolism (EFSA, 2013a). Therefore, the residue for risk assessment was defined as the ‘sum of fluopyram, fluopyram‐benzamide (M25), and fluopyram‐ E / Z ‐olefine (M02/M03), expressed as fluopyram’.
It is noted that a study was provided on the metabolism of fish in the framework of the peer review (Germany, 2011). In case MRLs will need to be set for fish commodities in the future, this study could be considered.
2.2. Magnitude of residues in livestock
In the framework of the peer review, feeding studies were performed with dairy cows and laying hens (Germany, 2011). In the ruminant feeding study, fluopyram was administered using different dosing levels ranging from 0.04 to 4.05 mg/kg bw per day and. The study also included a separate group to investigate depuration of fluopyram residues, that was fed at a dose rate of 4.38 mg/kg bw per day during the feeding phase. In the poultry feeding study, fluopyram was administered at dosing levels ranging from 0.035 to 0.32 mg/kg bw per day.
The studies performed on cows and hens were used to derive MRL and risk assessment values in milk, eggs, and tissues of ruminants and poultry. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs. In these studies, samples of tissues, milk and eggs were analysed for fluopyram, and its metabolites fluopyram‐benzamide (M25), fluopyram‐E/Z‐olefine (M02/M03), and are expressed as fluopyram. All tissue, milk and eggs samples were analysed within 30 days of collection and stored ≤ −18°C thus decline of residues during storage of the trial samples is not expected.
Based on these studies, MRL and risk assessment values were derived for all commodities of ruminants, pigs and poultry in compliance with the latest recommendations on this matter considering the dietary burdens with or without risk mitigation measures preventing residue uptake from rotational uses (FAO, 2009).
Based on the livestock feeding studies and the calculated livestock dietary burden, EFSA also derived a conversion factor for risk assessment of 1.3 and 1.4 in fat for poultry and ruminants, respectively and a CF of 1 for all other tissues (see Appendix B.2.2).
3. Consumer risk assessment
In the framework of this review, only the uses of fluopyram reported by the RMS in Appendix A were considered; however, the use of fluopyram was previously also assessed by the JMPR (FAO, 2010, 2012, 2014, 2015, 2017). The CXLs, resulting from these assessments by JMPR and adopted by the CAC, are now international recommendations that need to be considered by European risk managers when establishing MRLs. To facilitate consideration of these CXLs by risk managers, the consumer exposure was calculated both with and without consideration of the existing CXLs.
In the light of the possible contribution of residues from rotational uses to consumer exposure pending the implementation of risk mitigation measures, two options were considered.
In both options, it is assumed that the most critical indoor GAP on tomatoes is restricted to growing on artificial substrates or other means to prevent carry‐over of residues from treated soil to succeeding crops. In addition to this restriction:
Option 1: assumed that adequate risk mitigation measures are in place to avoid significant residues in crops grown in rotation with crops treated with fluopyram. These measures included a PBI of 1 year for root and tuber vegetables, and leafy vegetables; and a PBI of 120 days for cereals.
Option 2: assumed that no risk mitigation is implemented other than the restriction on the most critical indoor GAP on tomatoes.
Finally, it is highlighted that fluopyram‐benzamide (M25) was recently identified to be a common metabolite with flutolanil in the on‐going renewal of the latter (Netherlands, 2018). Although the MRL review for flutolanil did not assess the presence of M25 (EFSA, 2013b), as only a limited number of GAPs are authorised with relatively low existing MRLs, it can be reasonably expected that exposure to M25 from the use of flutolanil is not significant compared to the uses on fluopyram and will not impact the risk assessment performed under the present MRL review.
3.1. Consumer risk assessment without consideration of the existing CXLs
Chronic and acute exposure calculations for all crops reported in the framework of this review were performed with revision 3.1 (EFSA, 2019e, 2018). Input values for the exposure calculations were derived in compliance with the decision tree reported in Appendix E. Hence, for those commodities where a (tentative) MRL could be derived by EFSA in the framework of this review, risk assessment values were derived according to the internationally agreed methodologies (FAO, 2009). For those commodities where data were insufficient to derive an MRL in Section 1, EFSA considered the existing EU MRL for an indicative calculation. Further to the crops reported in the framework of this review, these calculations also include the potential uptake of fluopyram residues in crops that may be grown in rotation. All input values included in the exposure calculations are summarised in Appendix D.
The exposure values calculated were compared with the toxicological reference values for fluopyram, derived by EFSA (2013a).
The highest chronic exposure was calculated for the Dutch toddler, representing 86% (Option 1) and 100% (Option 2) of the acceptable daily intake (ADI). The highest acute exposure was calculated for lettuce, representing 76% of the acute reference dose (ARfD) for both options. As the exposure calculated did not exceed the toxicological reference values, no further refinement of the risk assessment was performed but it is noted that for Option 2 the major contributors to the chronic exposure are milk (20%), apples (18%) and banana (9%).
Although uncertainties remain due to the data gaps identified in the previous sections, it is highlighted that chronic intake is 100% of the ADI if risk mitigation measures are not in place to avoid carry‐over of residues from previously treated soils (Option 2).
3.2. Consumer risk assessment with consideration of the existing CXLs
To include the CXLs in the calculations of the consumer exposure, CXLs were compared with the EU MRL proposals in compliance with Appendix E and all data relevant to the consumer exposure assessment have been collected from JMPR evaluations. It is highlighted that the existing EU MRL established by Reg. (EU) 2017/626 for milk of 0.6 mg/kg was based on a CXL adopted by CAC in 2016. However, in 2018, CAC adopted a higher CXL for milk and revoked the associated CXL. The increased CXL was not taken over in Reg. (EU) 2019/552 due to intake concerns. As the original CXL was revoked, there were no longer basis to consider the former CXL of 0.6 mg/kg for milk. An overview of the input values used for this exposure calculation is also provided in Appendix D.
It is noted that for plant commodities the residue definition established by the JMPR for both enforcement and risk assessment of the CXLs is ‘fluopyram’. For risk assessment the definition proposed by EFSA is wider compared to the one for CXLs. Therefore, the relevant conversion factors derived in Sections 1.2 and 2,2 were applied for the same commodities or group of commodities.
Chronic and acute exposure calculations were also performed using revision 3.1 of the EFSA PRIMo and the exposure values calculated were compared with the toxicological reference values derived for fluopyram.
The highest chronic exposure was calculated for Dutch toddler, representing 110% (Option 1) and 128% (Option 2) of the ADI. The highest acute exposure was calculated for lettuce, accounting for 76% of the ARfD for both options.
For Option 1, as a possible option for risk managers consideration, the risk assessment was recalculated by disregarding the CXLs for cattle and swine tissues and considering for these commodities the STMR values derived from the European animal diet. According to this calculation, the chronic exposure represents 92% of the ADI.
For Option 2, given that the chronic exposure considering the authorised EU uses and import tolerances and the uptake from rotational crops already accounted for 100% of the ADI (NL toddlers), and as there may be several alternative options to exclude a potential chronic risk, the only safe scenario assessed was disregarding from the calculation all CXLs higher than the derived EU MRL. Overall, for Option 1, a safe scenario could be identified, excluding the CXLs for cattle and swine tissues from the calculation. For Option 2, a safe scenario could be identified disregarding from the calculation all CXLs higher than the derived EU MRL.
Conclusions
The metabolism of fluopyram in plant was investigated in primary and rotational crops. According to the results of the metabolism studies, the plant residue definition for enforcement can be proposed as ‘fluopyram’ and for risk assessment as ‘sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram’. These residue definitions are also applicable to processed commodities. Fully validated analytical methods are available for the enforcement of the proposed residue definition in all major matrices at the LOQ of 0.01 mg/kg. According to the EURLs the LOQ of 0.01 mg/kg is achievable by using the QuEChERS method in routine analyses.
Fluopyram is a persistent substance which may accumulate in soil following multiannual uses. To account for the potential uptake of such residues accumulated in soil in rotational crops two options were considered. Both options assumed that the most critical indoor GAP on tomatoes is restricted to growing on artificial substrates or other means to prevent carry‐over of residues from treated soil to succeeding crops. In addition to this restriction:
Option 1: assumed that adequate risk mitigation measures are in place to avoid significant residues in crops grown in rotation with crops treated with fluopyram. These measures included a plant back interval (PBI) of 1 year for root and tuber vegetables, and leafy vegetables; and a PBI of 120 days for cereals.
Option 2: assumed that no risk mitigation is implemented other than the above restriction on the most critical indoor GAP on tomatoes.
For Option 1, the available data are considered sufficient to derive MRL proposals as well as risk assessment values for all commodities under evaluation, except for lemons, mandarins, cherries, banana, spring onions, tomatoes, melons, watermelon, Chinese cabbage, escaroles, land cresses, red mustards, spinaches, chards/beet leaves, globe artichokes and leeks, where tentative MRLs are derived, and for lime, cherimoya and chicory roots where the available data were insufficient to derive tentative MRLs.
For Option 2, specific MRLs, considering that residues uptake in succeeding crops are not avoided, were also derived for cassava roots/manioc, sweet potatoes, yams, arrowroots, root vegetables, broccoli, cauliflower, Brussels sprouts, head cabbage, kales, kohlrabies, watercress, herbal infusions (roots), sugar beets, sweet corn, maize grain, buckwheat and millet grain, as well as tentative MRLs for chicory roots. It is underlined that MRLs values derived from rotational crop field data are subject to a high degree of uncertainty.
Tentative MRLs were also derived for cereal straw in view of the future need to set MRLs in feed items.
The effect of industrial processing and/or household preparation was assessed and robust processing factors could be derived for processed commodities from wine grapes, strawberries, tomatoes, melons, apples, bananas and rapeseeds. Tentative processing factors are also proposed for citrus, sugar beet, potato and peanuts.
Fluopyram is authorised for use on crops that might be fed to livestock. Livestock dietary burden calculations were therefore performed for different groups of livestock according to OECD guidance. Residues from primary uses without (Option 1) or with (Option 2) residues in rotational crops were considered. For both scenarios, the dietary burdens calculated for all groups of livestock were found to exceed the trigger value of 0.1 mg/kg DM in both cases. Behaviour of residues was therefore assessed in all commodities of animal origin.
The metabolism of fluopyram residues in livestock was investigated in lactating goats and laying hens at dose rates covering the maximum dietary burdens calculated in this review. According to the results of these studies, the residue definition for enforcement in all livestock commodities was proposed as the ‘sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram’ and for risk assessment as the ‘sum of fluopyram, fluopyram‐benzamide (M25), and fluopyram‐ E / Z ‐olefine (M02/M03), expressed as fluopyram’. An analytical method for the enforcement of the proposed residue definition at the LOQ of 0.02 mg/kg in all matrices is available. According to the EURLs a combined LOQ of 0.02 mg/kg is achievable for commodities of animal origin.
Livestock feeding studies on cows and laying hens were used to derive two sets of MRL and risk assessment values in milk, eggs, and tissues of ruminants and poultry in view of the two dietary burdens (with or without rotational crops), each set corresponding to one of the 2 options described above. Since extrapolation from ruminants to pigs is acceptable, results of the livestock feeding study on ruminants were relied upon to derive the MRL and risk assessment values in pigs.
Chronic and acute consumer exposure resulting from the authorised uses reported in the framework of this review was calculated using revision 3.1 of the EFSA PRIMo. For those commodities where data were insufficient to derive a MRL, EFSA considered the existing EU MRL for an indicative calculation.
In the light of the possible contribution of residues from rotational uses to consumer exposure pending the implementation of risk mitigation measures, the two options described above were considered.
The highest chronic exposure was calculated for the Dutch toddler, representing 86% (Option 1) and 100% (Option 2) of the ADI. The highest acute exposure was calculated for lettuce, representing 76% of the ARfD for both options.
Apart from the MRLs evaluated in the framework of this review, internationally recommended CXLs have also been established for fluopyram. Additional calculations of the consumer exposure, considering these CXLs, were therefore carried out.
The highest chronic exposure was calculated for Dutch toddler, representing 110% (Option 1) and 128% (Option 2) of the ADI. The highest acute exposure was calculated for lettuce, accounting 76% of the ARfD for both options.
For Option 1, as a potential risk management option, the risk assessment was re‐calculated by considering the European animal diet for cattle and swine and thus disregarding the CXLs for these animal commodities. According to this scenario, the chronic exposure represents 92% of the ADI. Nonetheless, it is highlighted that this scenario was only provided as a potential option for risk managers to consider and does not exclude or suggest alternative options may not be available for risk managers.
For Option 2, given that the chronic exposure based on the authorised EU uses, import tolerances and the uptake of fluopyram accumulated in soil following multiannual use already accounted for 100% of the ADI (NL toddlers), and as there may be several alternative options at the discretion of risk managers to exclude a potential chronic risk, the only safe scenario assessed was that disregarding from the calculation all CXLs higher than the derived EU MRL.
Altogether, the calculations indicate a potential chronic risk to consumers if all the existing CXLs are incorporated in the assessment. For Option 1, a safe scenario could be identified, excluding the CXLs for cattle and swine tissues from the calculation. For Option 2, a safe scenario could be identified disregarding from the calculation all CXLs higher than the derived EU MRL.
Recommendations
MRL recommendations were derived in compliance with the decision tree reported in Appendix E of the reasoned opinion.
Since fluopyram is highly persistent in the soil, for root, tuber and brassica vegetables as well as certain crops that may be grown in rotation but for which no primary crop use is authorised (kales, kohlrabies, watercresses, buckwheat and millet grain), it cannot be excluded that residues above the derived MRLs occur in succeeding crops, unless appropriate risk mitigation measures are in place.
Therefore, two different options were derived. In both options, it is assumed that the most critical indoor GAP on tomatoes is restricted to growing on artificial substrates or other means to prevent carry‐over of residues from treated soil to succeeding crops. In addition to this restriction:
Option 1: assumed that adequate risk mitigation measures are in place to avoid significant residues in crops grown in rotation with crops treated with fluopyram. These measures included a PBI of 1 year for root and tuber vegetables, and leafy vegetables; and a PBI of 120 days for cereals.
Option 2: assumed that no risk mitigation is implemented other than the restriction on the most critical indoor GAP on tomatoes.
For Option 1, all MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 2 footnotes for details). In particular, some tentative MRLs and/or existing EU MRLs need to be confirmed by the following data:
additional residue trials on lime, mandarins, bananas, cherimoya, tomatoes, melons, watermelons, Chinese cabbage, escaroles, land cresses, red mustards, spinaches, chards/beet leaves, globe artichokes, leeks and chicory roots.
Table 2.
Summary table
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |||
|---|---|---|---|---|---|---|---|
| Option 1 (PBIs, restriction on indoor tomato use) | Option 2 (Restriction on indoor tomato use) | ||||||
| MRL (mg/kg) | Comment | MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: Fluopyram | |||||||
| 110010 | Grapefruit | 0.4 | 0.4 | 0.5 | Recommendeda | 0.5 | Recommendeda |
| 110020 | Oranges | 0.6 | 0.6 | 0.6 | Recommendedb | 0.5 | Further consideration neededc |
| 110030 | Lemons | 1 | 1 | 1 | Recommendedd | 0.9 | Further consideration needede |
| 110040 | Limes | 1 | 1 | 1 | Further consideration neededf | 1 | Further consideration neededf |
| 110050 | Mandarins | 0.6 | 0.6 | 0.9 | Further consideration neededg | 0.9 | Further consideration neededg |
| 120010 | Almonds | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120020 | Brazil nuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120030 | Cashew nuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120040 | Chestnuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120050 | Coconuts | 0.04 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120060 | Hazelnuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120070 | Macadamia | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120080 | Pecans | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120090 | Pine nuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120100 | Pistachios | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120110 | Walnuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 130010 | Apples | 0.6 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130020 | Pears | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130030 | Quinces | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130040 | Medlar | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130050 | Loquat | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 140010 | Apricots | 1.5 | 1 | 1.5 | Recommendeda | 1.5 | Recommendeda |
| 140020 | Cherries | 2 | 2 | 2 | Recommendedd | 2 | Recommendedd |
| 140030 | Peaches | 1.5 | 1 | 1.5 | Recommendeda | 1.5 | Recommendeda |
| 140040 | Plums | 0.5 | 0.5 | 0.6 | Recommendeda | 0.6 | Recommendeda |
| 151010 | Table grapes | 1.5 | 2 | 2 | Recommendeda | 2 | Recommendeda |
| 151020 | Wine grapes | 1.5 | 2 | 2 | Recommendedb | 1.5 | Further consideration neededc |
| 152000 | Strawberries | 2 | 0.4 | 2 | Recommendeda | 2 | Recommendeda |
| 153010 | Blackberries | 5 | 5 | 5 | Recommendeda | 5 | Recommendeda |
| 153020 | Dewberries | 5 | 5 | 5 | Recommendeda | 5 | Recommendeda |
| 153030 | Raspberries | 5 | 5 | 5 | Recommendeda | 5 | Recommendeda |
| 154010 | Blueberries | 7 | 7 | 7 | Recommendeda | 7 | Recommendeda |
| 154020 | Cranberries | 3 | – | 4 | Recommendedh | 4 | Recommendedh |
| 154030 | Currants (red, black and white) | 7 | 7 | 7 | Recommendedb | 4 | Further consideration neededc |
| 154040 | Gooseberries | 7 | 7 | 7 | Recommendedb | 4 | Further consideration neededc |
| 154050 | Rose hips | 7 | 7 | 7 | Recommendedb | 3 | Further consideration neededc |
| 154060 | Mulberries | 7 | – | 4 | Recommendedh | 4 | Recommendedh |
| 154080 | Elderberries | 7 | – | 4 | Recommendedh | 4 | Recommendedh |
| 163020 | Bananas | 0.8 | 0.8 | 0.8 | Further consideration neededg | 0.8 | Further consideration neededg |
| 163030 | Mangoes | 1 | 1 | 1 | Recommendedi | – | Further consideration neededj |
| 163060 | Cherimoyas | 0.01* | – | – | Further consideration neededk | – | Further consideration neededk |
| 211000 | Potatoes | 0.15 | 0.15 | 0.15 | Recommendedb | 0.08 | Further consideration neededc |
| 212010 | Cassava | 0.1 | – | – | Further consideration neededl | 0.06 | Recommendedh |
| 212020 | Sweet potatoes | 0.1 | – | 0.06 | Recommendedh | 0.15 | Recommendedh |
| 212030 | Yams | 0.1 | – | 0.06 | Recommendedh | 0.15 | Recommendedh |
| 212040 | Arrowroot | 0.1 | – | – | Further consideration neededl | 0.06 | Recommendedh |
| 213010 | Beetroot | 0.3 | – | 0.06 | Recommendedh | 0.2 | Recommendedh |
| 213020 | Carrots | 0.4 | 0.4 | 0.4 | Recommendedb | 0.4 | Recommendeda |
| 213030 | Celeriac | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213040 | Horseradish | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213050 | Jerusalem artichokes | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213060 | Parsnips | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213070 | Parsley root | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213080 | Radishes | 0.3 | – | 0.3 | Recommendedh | 0.4 | Recommendedh |
| 213090 | Salsify | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213100 | Swedes | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213110 | Turnips | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 220010 | Garlic | 0.1 | 0.07 | 0.07 | Recommendeda | 0.07 | Recommendeda |
| 220020 | Onions | 0.1 | 0.07 | 0.07 | Recommendeda | 0.07 | Recommendeda |
| 220030 | Shallots | 0.1 | – | 0.07 | Recommendedh | 0.07 | Recommendedh |
| 220040 | Spring onions | 15 | 15 | 15 | Recommendedb | 3 | Further consideration needede |
| 231010 | Tomatoes | 0.9 | 0.5 | 0.5 | Further consideration neededg | 0.5 | Further consideration neededg |
| 231020 | Peppers | 3 | 3 | 3 | Recommendedb | 2 | Further consideration neededc |
| 231030 | Aubergines (egg plants) | 0.9 | 0.5 | 0.5 | Recommendedb | 0.4 | Further consideration neededc |
| 232010 | Cucumbers | 0.5 | 0.5 | 0.6 | Recommendeda | 0.6 | Recommendeda |
| 232020 | Gherkins | 0.5 | – | 0.6 | Recommendedh | 0.6 | Recommendedh |
| 232030 | Courgettes | 0.5 | – | 0.6 | Recommendedh | 0.6 | Recommendedh |
| 233010 | Melons | 0.4 | – | 0.9 | Further consideration neededm | 0.9 | Further consideration neededm |
| 233020 | Pumpkins | 0.4 | – | 0.4 | Recommendedh | 0.4 | Recommendedh |
| 233030 | Watermelons | 0.4 | – | 0.4 | Further consideration neededm | 0.4 | Further consideration neededm |
| 234000 | Sweet corn | 0.01* | 0.01* | 0.01* | Recommendeda | 0.02 | Recommendeda |
| 241010 | Broccoli | 0.4 | 0.3 | 0.4 | Recommendeda | 0.5 | Recommendeda |
| 241020 | Cauliflower | 0.2 | 0.09 | 0.1 | Recommendeda | 0.3 | Recommendeda |
| 242010 | Brussels sprouts | 0.3 | 0.3 | 0.3 | Recommendeda | 0.4 | Recommendeda |
| 242020 | Head cabbage | 0.3 | 0.15 | 0.15 | Recommendeda | 0.3 | Recommendeda |
| 243010 | Chinese cabbage | 0.7 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 243020 | Kale | 0.1 | – | – | Further consideration neededl | 0.15 | Recommendedh |
| 244000 | Kohlrabi | 0.1 | – | – | Further consideration neededl | 0.15 | Recommendedh |
| 251010 | Lamb's lettuce | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 251020 | Lettuce | 15 | 15 | 15 | Recommendeda | 15 | Recommendeda |
| 251030 | Escarole (broad‐leaf endive) | 1.5 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 251040 | Cress | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 251050 | Land cress | 15 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 251060 | Rocket, Rucola | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 251070 | Red mustard | 15 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 251080 | Baby leaf crops | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 252010 | Spinach | 0.2 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 252020 | Purslane | 20 | – | 20 | Recommendedh | 20 | Recommendedh |
| 252030 | Beet leaves (chard) | 0.2 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 254000 | Watercress | 0.1 | – | – | Further consideration neededl | 0.15 | Recommendedh |
| 255000 | Witloof | 0.3 | 0.15 | 0.3 | Recommendeda | 0.3 | Recommendeda |
| 256010 | Chervil | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256020 | Chives | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256030 | Celery leaves | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256040 | Parsley | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256050 | Sage | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256060 | Rosemary | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256070 | Thyme | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256080 | Basil | 70 | 70 | 70 | Recommendedb | 60 | Further consideration neededc |
| 256090 | Bay leaves (laurel) | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256100 | Tarragon | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 260010 | Beans (fresh, with pods) | 1 | 1 | 3 | Recommendeda | 3 | Recommendeda |
| 260020 | Beans (fresh, without pods) | 0.2 | 0.2 | 0.2 | Recommendedb | 0.15 | Further consideration neededc |
| 260030 | Peas (fresh, with pods) | 1.5 | – | 3 | Recommendedh | 3 | Recommendedh |
| 260040 | Peas (fresh, without pods) | 0.2 | 0.2 | 0.2 | Recommendedb | 0.15 | Further consideration neededc |
| 260050 | Lentils (fresh) | 0.2 | – | 0.15 | Recommendedh | 0.15 | Recommendedh |
| 270010 | Asparagus | 0.01* | 0.01* | 0.01* | Recommendeda | 0.01* | Recommendeda |
| 270030 | Celery | 0.01* | – | 20 | Recommendedh | 20 | Recommendedh |
| 270050 | Globe artichokes | 0.5 | 0.4 | 4 | Further consideration neededg | 4 | Further consideration neededg |
| 270060 | Leek | 0.7 | 0.15 | 0.8 | Further consideration neededg | 0.8 | Further consideration neededg |
| 300010 | Beans (dry) | 0.4 | 0.15 | 0.5 | Recommendeda | 0.5 | Recommendeda |
| 300020 | Lentils (dry) | 0.4 | 0.7 | 0.7 | Recommendedb | 0.5 | Further consideration neededc |
| 300030 | Peas (dry) | 0.4 | 0.7 | 0.7 | Recommendedb | 0.5 | Further consideration neededc |
| 300040 | Lupins (dry) | 0.4 | 0.15 | 0.5 | Recommendeda | 0.5 | Recommendeda |
| 401020 | Peanuts | 0.2 | 0.2 | 0.2 | Recommendedb | 0.02 | Further consideration neededc |
| 401030 | Poppy seed | 0.3 | – | 0.4 | Recommendedh | 0.4 | Recommendedh |
| 401050 | Sunflower seed | 0.7 | 0.7 | 0.7 | Recommendeda | 0.7 | Recommendeda |
| 401060 | Rape seed | 1 | 1 | 1 | Recommendeda | 1 | Recommendeddeda |
| 401070 | Soya bean | 0.3 | 0.3 | 0.3 | Recommendedb | 0.08 | Further consideration neededc |
| 401080 | Mustard seed | 0.3 | – | 0.4 | Recommendedh | 0.4 | Recommendedh |
| 401090 | Cotton seed | 0.8 | 0.8 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 500010 | Barley grain | 0.2 | 0.2 | 0.2 | Recommendeda | 0.2 | Recommendeda |
| 500020 | Buckwheat grain | 0.2 | – | – | Further consideration neededl | 0.02 | Recommendedh |
| 500030 | Maize grain | 0.02 | 0.02 | 0.02 | Recommendedb | 0.02 | Recommendeda |
| 500040 | Millet grain | 0.01* | – | – | Further consideration neededl | 0.02 | Recommendedh |
| 500050 | Oats grain | 0.2 | 0.2 | 0.2 | Recommendeda | 0.2 | Recommendeda |
| 500060 | Rice | 0.01* | 1.5 | – | Further consideration neededl | 0.02 | Recommendedh |
| 500070 | Rye grain | 0.9 | 0.9 | 0.9 | Recommendedb | 0.07 | Further consideration neededc |
| 500080 | Sorghum grain | 1.5 | – | 4 | Recommendedh | 4 | Recommendedh |
| 500090 | Wheat grain | 0.9 | 0.9 | 0.9 | Recommendeda | 0.9 | Recommendeda |
| 631000 | Herbal infusions (dried, flowers) | 0.1 | – | 40 | Recommendedh | 40 | Recommendedh |
| 632000 | Herbal infusions (dried, leaves) | 0.1 | – | 40 | Recommendedh | 40 | Recommendedh |
| 633000 | Herbal infusions (dried, roots) | 2.5 | – | – | Further consideration neededl | 1 | Recommendedh |
| 700000 | Hops (dried) | 50 | 50 | 60 | Recommendeda | 60 | Recommendeda |
| 810060 | Dill seeds | 70 | 70 | 70 | Recommendeda | 70 | Recommendeda |
| 840000 | Spices (roots and rhizome) | – | – | – | Further consideration neededdedl | 1 | Recommendedh |
| 900010 | Sugar beet (root) | 0.1 | 0.04 | 0.04 | Recommendedi | 0.1 | Further consideration neededc |
| 900030 | Chicory roots | 0.1 | – | – | Further consideration neededk | 0.1 | Further consideration neededk |
| Enforcement residue definition 2: Sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram | |||||||
| 1011010 | Swine muscle | 0.8 | 1.5 | 0.09 | Further consideration neededc | 0.1 | Further consideration neededc |
| 1011020 | Swine fat tissue | 0.5 | 1.5 | 0.08 | Further consideration neededc | 0.09 | Further consideration neededc |
| 1011030 | Swine liver | 5 | 8 | 0.50 | Further consideration neededc | 0.5 | Further consideration neededc |
| 1011040 | Swine kidney | 0.8 | 8 | 0.08 | Further consideration neededc | 0.08 | Further consideration neededc |
| 1012010 | Bovine muscle | 0.8 | 1.5 | 0.10 | Further consideration neededc | 0.15 | Further consideration neededc |
| 1012020 | Bovine fat tissue | 0.5 | 1.5 | 0.09 | Further consideration neededc | 0.15 | Further consideration neededc |
| 1012030 | Bovine liver | 5 | 8 | 0.50 | Further consideration neededc | 0.8 | Further consideration neededc |
| 1012040 | Bovine kidney | 0.8 | 8 | 0.08 | Further consideration neededc | 0.15 | Further consideration neededc |
| 1013010 | Sheep muscle | 0.8 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1013020 | Sheep fat tissue | 0.5 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1013030 | Sheep liver | 5 | 8 | 8 | Recommendedb | 0.8 | Further consideration neededc |
| 1013040 | Sheep kidney | 0.8 | 8 | 8 | Recommendedb | 0.15 | Further consideration neededc |
| 1014010 | Goat muscle | 0.8 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1014020 | Goat fat tissue | 0.5 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1014030 | Goat liver | 5 | 8 | 8 | Recommendedb | 0.8 | Further consideration neededc |
| 1014040 | Goat kidney | 0.8 | 8 | 8 | Recommendedb | 0.15 | Further consideration neededc |
| 1015010 | Equine muscle | 0.8 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1015020 | Equine fat tissue | 0.5 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1015030 | Equine liver | 0.7 | 8 | 8 | Recommendedb | 0.8 | Further consideration neededc |
| 1015040 | Equine kidney | 0.7 | 8 | 8 | Recommendedb | 0.15 | Further consideration neededc |
| 1016010 | Poultry muscle | 0.5 | 1.5 | 1.5 | Recommendedb | 0.07 | Further consideration neededc |
| 1016020 | Poultry fat tissue | 0.2 | 1 | 1 | Recommendedb | 0.07 | Further consideration neededc |
| 1016030 | Poultry liver | 2 | 5 | 5 | Recommendedb | 0.3 | Further consideration neededc |
| 1020010 | Cattle milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.07 | Further consideration neededh |
| 1020020 | Sheep milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.06 | Further consideration neededh |
| 1020030 | Goat milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.06 | Further consideration neededh |
| 1020040 | Horse milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.07 | Further consideration neededh |
| 1030000 | Birds eggs | 1 | 2 | 2 | Recommendedb | 0.15 | Further consideration neededc |
| – | Other commodities of plant and/or animal origin | See Reg. 2019/1791 | – | – | Further consideration neededl | ||
MRL: maximum residue level; CXL: codex maximum residue limit; PBI: plant‐back interval.
* Indicates that the input value is proposed at the limit of quantification.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination H‐III in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is also fully supported by data, leads to a lower MRL (combination H‐VII in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; CXL is higher, supported by data but a chronic risk to consumers cannot be excluded considering some (Option 1)/or all additional CXLs (Option 2) (combination H‐VI/VII in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower or same tentative MRL (combination F‐VII in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition; CXL is higher, supported by data but a chronic risk to consumers cannot be excluded considering some (Option 1)/or all additional CXLs (Option 2) (combination F‐VI/VII in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); existing CXL is covered by the existing EU MRL (combination D‐III in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); existing CXL is covered by the tentative MRL (combination F‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available or CXL was not considered further due to reservations raised by the EU delegation. (combination H‐I in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; there are no relevant authorisations or import tolerances reported at EU level (combination A‐VII in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; CXL is supported by data but a chronic risk to consumers cannot be excluded considering all additional CXLs (Option 2). Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐VI in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination D‐I in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available or CXL was not considered further due to reservations raised by the EU delegation. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination F‐I in Appendix E).
It is highlighted, however, that some of the MRLs derived result from a CXL or from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
additional residue trials on lemons, cherries, apricots, plums, spring onions, radishes, dry beans and peas.
If the above reported data gaps are not addressed in the future, Member States are recommended to withdraw or modify the relevant authorisations at national level.
Option 2 is presented in order to facilitate decision by risk managers but, it is underlined that all MRLs values derived from rotational crop field data are subject to a higher degree of uncertainty. They are based on a limited data set, with conservative assumptions and a very high degree of uncertainty with regards to actual concentrations of fluopyram in soil, and is also pending on the actual use pattern of fluopyram. EFSA recommends that residues uptake in succeeding crops should be avoided as much as possible. Furthermore, given that the chronic exposure based on the European authorised uses, the import tolerances and the uptake from soil accounted already for 100% of the ADI (NL toddlers), according to this option, it was not possible to consider the current CXLs higher than the derived EU MRL. As there may be several alternative options, at the discretion of risk managers, to exclude the potential chronic risk, MRLs not covering the existing CXLs require further considerations by risk managers. In particular, a chronic risk was identified if also all CXLs are considered, but it does not mean that all CXLs contribute significantly to the chronic intake and would lead to a potential intake concern. For Option 2, all MRL values listed as ‘Recommended’ in the table are sufficiently supported by data and are therefore proposed for inclusion in Annex II to the Regulation. The remaining MRL values listed in the table are not recommended for inclusion in Annex II because they require further consideration by risk managers (see Table 2 footnotes for details). In particular, some tentative MRLs and/or existing EU MRLs need to be confirmed by the following data:
additional residue trials on lime, lemons, mandarins, bananas, cherimoyas, spring onions, tomatoes, melons, watermelons, Chinese cabbage, escaroles, land cresses, red mustards, spinaches, chards/beet leaves, globe artichokes, leeks and chicory roots.
It is highlighted, however, that some of the MRLs derived result from a GAP in one climatic zone only, whereas other GAPs reported by the RMS were not fully supported by data. EFSA therefore identified the following data gaps which are not expected to impact on the validity of the MRLs derived but which might have an impact on national authorisations:
additional residue trials on cherries, apricots, plums, radishes, dry beans and peas.
additional rotational field trials on fruit and fruiting vegetables.
Moreover, for bulb and stem vegetables specific data for rotational crops are not available, EFSA was not able to assess the potential uptake in succeeding crops. Although it is not expected to modify the outcome of the risk assessment, the following data should be generated if risk managers intend to set MRLs in these crops:
4 additional rotational field trials on bulb and stem vegetables.
Minor deficiencies were also identified in the assessment but they are not expected to impact either on the validity of the MRLs derived or on the national authorisations. The following data are therefore considered desirable but not essential:
a fully validated analytical method for the determination of fluopyram in seed spices and an ILV of the method in hops.
Abbreviations
- a.i.
active ingredient
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CF
conversion factor for enforcement residue definition to risk assessment residue definition
- CXL
codex maximum residue limit
- DAR
draft assessment report
- DAT
days after treatment
- DB
dietary burden
- DF
default drying factor
- DM
dry matter
- DT90
period required for 90% dissipation (define method of estimation)
- EC
emulsifiable concentrate
- eq
residue expressed as a.s. equivalent
- EURLs
European Union Reference Laboratories for Pesticide Residues (former CRLs)
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC–MS
gas chromatography with mass spectrometry
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- InChiKey
International Chemical Identifier Key
- ISO
International Organisation for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- LOQ
limit of quantification
- Mo
monitoring
- MRL
maximum residue level
- MS
Member States
- NEDI
national estimated daily intake
- NESTI
national estimated short‐term intake
- NEU
northern European Union
- NTMDI
national theoretical maximum daily intake
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant‐back interval
- PECsoil
predicted environmental concentration in soil
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- PROFile
(EFSA) Pesticide Residues Overview File
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SE
suspoemulsion
- SEU
southern European Union
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- WHO
World Health Organization
Appendix A – Summary of critical authorised uses considered for the review of MRLs
A.1. Authorised outdoor uses in northern EU
| Crop and/or situation | MS or country | F G or Ia | Preparation | Application | Application rate per treatment | PHI (days)d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||
| Hazelnuts | PL | F | SC | 250 g/L | Foliar treatment – spraying | 1 | – | – | 120 g a.i./ha | 21 | ||
| Apples | HU | F | SC | 200 g/L | Foliar treatment – spraying | 57–84 | 3 | 7 | – | – | 150 g a.i./ha | 14 |
| Pears | NL | F | SC | 200 g/L | Foliar treatment – spraying | 72–87 | 3 | 21 | – | – | 150 g a.i./ha | 14 |
| Quinces | NL | F | SC | 200 g/L | Foliar treatment – spraying | 72–87 | 3 | 21 | – | – | 150 g a.i./ha | 14 |
| Medlars | NL | F | SC | 200 g/L | Foliar treatment – spraying | 72–87 | 3 | 21 | – | – | 150 g a.i./ha | 14 |
| Loquats | NL | F | SC | 200 g/L | Foliar treatment – spraying | 72–87 | 3 | 21 | – | – | 150 g a.i./ha | 14 |
| Apricots | CZ | F | SC | 200 g/L | Foliar treatment – spraying | 59–87 | 2 | 21 | – | – | 150 g a.i./ha | 3 |
| Cherries | HU | F | SC | 500 g/L | Foliar treatment – spraying | 61–85 | 2 | 7 | – | – | 250 g a.i./ha | 7 |
| Peaches | FR | F | SC | 200 g/L | Foliar treatment – spraying | 77–89 | 2 | – | – | 100 g a.i./ha | 3 | |
| Plums | FR | F | SC | 200 g/L | Foliar treatment – spraying | 77–89 | 2 | 7 | – | – | 100 g a.i./ha | 3 |
| Table grapes | RO | F | SC | 500 g/L | Foliar treatment – spraying | 69–89 | 2 | 12 | – | – | 250 g a.i./ha | 21 |
| Wine grapes | RO | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 69–89 | 2 | 12 | – | – | 250 g a.i./ha | 21 |
| Strawberries | CZ | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 15–87 | 2 | 7 | – | – | 250 g a.i./ha | 1 |
| Blackberries | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Dewberries | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Raspberries | AT, DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | – | – | 200 g a.i./ha | 3 | |
| Blueberries | AT, DE, PL | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Cranberries | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Currants | AT, DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Gooseberries | AT, DE | F | SC | 250 g/L | Foliar treatment – general | 15–89 | 2 | – | – | 200 g a.i./ha | 7 | |
| Rose hips | NL | F | SC | 250 g/L | Foliar treatment – broadcast spraying | – | 2 | 14 | – | – | 150 g a.i./ha | 7 |
| Mulberries | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Elderberries | AT, DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Sweet potatoes | NL | F | SC | 400 g/L | Soil treatment – spraying | BBCH 00 | 1 | – | – | – | 250 g a.i./ha | n.a. |
| Yams | NL | F | SC | 400 g/L | Soil treatment – spraying | BBCH 00 | 1 | – | – | – | 250 g a.i./ha | n.a. |
| Carrots | SI | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 1–2 | 14 | – | – | 150 g a.i./ha | 14 |
| Beetroots | NL | F | SC | 400 g/L | Soil treatment – spraying | BBCH 00 | 1 | – | – | – | 250 g a.i./ha | n.a. |
| Celeriacs | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 150 g a.i./ha | 14 |
| Horseradishes | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 150 g a.i./ha | 14 |
| Jerusalem artichokes | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 150 g a.i./ha | 14 |
| Parsnips | DE, PL | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 150 g a.i./ha | 14 |
| Parsley roots | DE, PL | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 150 g a.i./ha | 14 |
| Radishes | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 1 | – | – | 200 g a.i./ha | 7 | |
| Salsifies | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 150 g a.i./ha | 14 |
| Swedes | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 2 | – | – | 150 g a.i./ha | 14 | |
| Turnips | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 2 | – | – | 150 g a.i./ha | 14 | |
| Garlic | AT | F | SC | 200 g/L | Foliar treatment – general | 41–49 | 2 | – | – | 100 g a.i./ha | 7 | |
| Onions | AT | F | SC | 200 g/L | Foliar treatment – general | 41–49 | 2 | – | – | 100 g a.i./ha | 7 | |
| Shallots | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 100 g a.i./ha | 7 |
| Spring onions | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 1 | – | – | 200 g a.i./ha | 21 | |
| Cucumbers | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 61–79 | 2 | 14 | – | – | 200 g a.i./ha | 3 |
| Gherkins | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 61–79 | 2 | 14 | – | – | 200 g a.i./ha | 3 |
| Sweet corn | HU | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 30–69 | 2 | 14 | – | – | 125 g a.i./ha | 14 |
| Broccoli | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 2 | – | – | 180 g a.i./ha | 14 | |
| Cauliflowers | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 2 | – | – | 180 g a.i./ha | 14 | |
| Brussels sprouts | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 180 g a.i./ha | 14 |
| Head cabbages | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 2 | 14 | – | – | 180 g a.i./ha | 14 |
| Chinese cabbages | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 1 | – | – | 200 g a.i./ha | 7 | |
| Lamb's lettuces | AT, DE | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Lettuces | PL | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 41–49 | 1–2 | 7 | – | – | 200 g a.i./ha | 7 |
| Escaroles | AT, DE | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Cresses | AT | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Land cresses | AT | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Roman rocket | AT, DE | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Red mustards | AT | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Baby leaf crops | AT, DE | F | SC | 250 g/L | Foliar treatment – general | 13–49 | 1 | – | – | 200 g a.i./ha | 7 | |
| Spinaches | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 1 | – | – | 200 g a.i./ha | 7 | |
| Purslanes | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 1 | – | – | 200 g a.i./ha | 7 | |
| Chards | DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13 | 1 | – | – | 200 g a.i./ha | 7 | |
| Chervil | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Chives | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Celery leaves | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Parsley | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Sage | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Rosemary | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Thyme | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Basil | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Laurel | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Tarragon | DE | F | SC | 200 g/L | Foliar treatment – broadcast spraying | n.a. | 1 | – | – | 200 g a.i./ha | 14 | |
| Beans (with pods) | BE, CZ, NL | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 51–79 | 2 | 7 | – | – | 250 g a.i./ha | 7 |
| Beans (without pods) | BE, CZ, NL | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 60–79 | 2 | 7 | – | – | 250 g a.i./ha | 7 |
| Peas (with pods) | CZ | F | SC | 502 g/L | Foliar treatment – general | 60–79 | 1–2 | 7 | – | – | 250 g a.i./ha | 7 |
| Peas (without pods) | CZ, NL | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 51–79 | 2 | 7 | – | – | 250 g a.i./ha | 7 |
| Asparagus | AT, DE | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 49–95 | 1–2 | 10 | – | – | 200 g a.i./ha | > 200 |
| Leeks | SI | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–49 | 1–2 | 14 | – | – | 200 g a.i./ha | 21 |
| Beans (dry) | BE, CZ | F | SC | 501 g/L | Foliar treatment – general | 60–79 | 1–2 | 7 | – | – | 250 g a.i./ha | 7 |
| Peas (dry) | CZ | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 60–79 | 2 | 7 | – | – | 250 g a.i./ha | 7 |
| Poppy seeds | CZ, HU | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 14–65 | 2 | 21 | – | – | 125 g a.i./ha | 56 |
| Sunflower seeds | CZ, HU | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 16–69 | 2 | 14 | – | – | 125 g a.i./ha | 28 |
| Rapeseeds | HU | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 14–73 | 2 | 14 | – | – | 125 g a.i./ha | 28 |
| Mustard seeds | CZ, HU | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 57–69 | 2 | 14 | – | – | 125 g a.i./ha | 56 |
| Barley | DK | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 1–2 | 14 | – | – | 125 g a.i./ha | 35 |
| Maize | CZ, DE, DK, EE, HU, LT | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 30–69 | 2 | 14 | – | – | 125 g a.i./ha | n.a. |
| Oat | DK | F | Foliar treatment – broadcast spraying | 30–61 | 1 | – | – | 125 g a.i./ha | n.a. | |||
| Rye | DK | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 1–2 | 14 | – | – | 125 g a.i./ha | 35 |
| Wheat | DK | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 30–61 | 1–2 | 14 | – | – | 125 g a.i./ha | 35 |
| Hops | PL | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 37–79 | 1–2 | 14 | – | – | 150 g a.i./ha | 21 |
| Chicory roots | BE | F | SC | 250 g/L | Foliar treatment – general | 41–49 | 1 | – | – | – | 150 g a.i./ha | 7 |
MS: Member State; a.s.: active substance; a.i.: active ingredient; n.a.: not applicable; SC: suspension concentrate; SE: suspoemulsion.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
A.2. Authorised outdoor uses in southern EU
| Crop and/or situation | MS or country | F G or Ia | Preparation | Application | Application rate per treatment | PHI (days)d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||
| Apples | EL | F | SC | 200 g/L | Foliar treatment – spraying | 57–87 | 2 | 7 | – | – | 150 g a.i./ha | 14 |
| Pears | EL, IT | F | SC | 200 g/L | Foliar treatment – spraying | 57–87 | 2 | 7 | – | – | 150 g a.i./ha | 14 |
| Quinces | FR | F | SC | 200 g/L | Foliar treatment – spraying | 57–89 | 1 | – | – | 150 g a.i./ha | 14 | |
| Medlars | FR | F | SC | 200 g/L | Foliar treatment – spraying | 57–89 | 1 | – | – | 150 g a.i./ha | 14 | |
| Loquats | FR | F | SC | 200 g/L | Foliar treatment – spraying | 57–89 | 1 | – | – | 150 g a.i./ha | 14 | |
| Apricots | IT | F | SC | 500 g/L | Foliar treatment – spraying | 61–87 | 2 | – | – | 250 g a.i./ha | 3 | |
| Cherries | IT | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 61–87 | 1–2 | 7 | – | – | 250 g a.i./ha | 3 |
| Peaches | IT | F | SC | 500 g/L | Foliar treatment – spraying | 61–87 | 2 | – | – | 250 g a.i./ha | 3 | |
| Plums | IT | F | SC | 500 g/L | Foliar treatment – spraying | 61–87 | 2 | – | – | 250 g a.i./ha | 3 | |
| Table grapes | HR | F | SC | 500 g/L | Foliar treatment – spraying | 71–83 | 2 | 12 | – | – | 250 g a.i./ha | 3 |
| Wine grapes | HR | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 71–83 | 2 | 12 | – | – | 250 g a.i./ha | 21 |
| Strawberries | FR | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 40–89 | 1 | – | – | 200 g a.i./ha | 3 | |
| Blackberries | FR | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13–89 | 1 | – | – | 200 g a.i./ha | 3 | |
| Dewberries | FR | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13–89 | 1 | – | – | 200 g a.i./ha | 3 | |
| Raspberries | FR | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13–89 | 1 | – | – | 200 g a.i./ha | 3 | |
| Blueberries | FR | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13–89 | 1 | – | – | 200 g a.i./ha | 7 | |
| Gooseberries | FR | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 13–89 | 1 | – | – | 200 g a.i./ha | 7 | |
| Bananas | FR | F | SC | 500 g/L | Foliar treatment – broadcast spraying | 13–81 | 1–3 | 7 | – | – | 300 g a.i./ha | 1 |
| Potatoes | IT | F | SC | 400 g/L | Soil treatment – general | BBCH 00 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Carrots | IT | F | SC | 400 g/L | Soil treatment – general | BBCH 00 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Garlic | EL; ES, PT | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–91 | 1–1 | – | – | 200 g a.i./ha | 7 | |
| Onions | EL; ES, PT | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–91 | 1–1 | – | – | 200 g a.i./ha | 7 | |
| Shallots | EL | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–91 | 1–1 | – | – | 0.2 kg a.i./ha | 7 | |
| Spring onions | EL | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–91 | 1–1 | – | – | 200 g a.i./ha | 7 | |
| Tomatoes | IT | F | SC | 400 g/L | Soil treatment – general | 0–9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Sweet peppers | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Aubergines | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Cucumbers | IT | F | SC | 400 g/l | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Gherkins | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Courgettes | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Melons | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Pumpkins | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Watermelons | IT | F | SC | 400 g/L | Soil treatment – general | n.a. to 9 | 1–1 | – | – | 250 g a.i./ha | n.a. | |
| Lettuces | ES, IT | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 41–49 | 1–1 | – | – | 200 g a.i./ha | 7 | |
| Beans (with pods) | IT | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 59–75 | 1–2 | 14 | – | – | 200 g a.i./ha | 14 |
| Asparagus | IT | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 43–91 | 1–2 | 10 | – | – | 150 g a.i./ha | > 200 |
| Globe artichokes | EL, IT | F | SC | 250 g/L | Foliar treatment – broadcast spraying | 14–87 | 1–3 | 7 | – | – | 75 g a.i./ha | 7 |
| Leeks | EL | F | SC | 200 g/L | Foliar treatment – broadcast spraying | 41–91 | 1–1 | – | – | 200 g a.i./ha | 14 | |
| Rapeseeds | FR, HR, IT, PT | F | SE | 125 g/L | Foliar treatment – broadcast spraying | 14–73 | 1 | – | – | 125 g a.i./ha | 56 | |
| Barley | FR | F | EC | 65 g/L | Foliar treatment – broadcast spraying | 30–61 | 1 | – | – | 78 g a.i./ha | n.a. | |
| Oat | FR | F | EC | 65 g/L | Foliar treatment – broadcast spraying | 30–61 | 1 | – | – | 78 g a.i./ha | n.a. | |
| Rye | FR | F | EC | 65 g/L | Foliar treatment – broadcast spraying | 30–61 | 1 | – | – | 97.5 g a.i./ha | n.a. | |
| Wheat | FR | F | EC | 65 g/L | Foliar treatment – broadcast spraying | 30–61 | 1 | – | – | 97.5 g a.i./ha | n.a. | |
MS: Member State; a.s.: active substance; a.i.: active ingredient; n.a.: not applicable; SC: suspension concentrate; SE: suspoemulsion; EC: emulsifiable concentrate.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
A.3. Authorised indoor uses in EU
| Crop and/or situation | MS or country | F G or Ia | Preparation | Application | Application rate per treatment | PHI (days)d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||
| Strawberries | NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 250 g a.i./ha | 1 | |
| Blackberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 51–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Dewberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 51–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Raspberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 51–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Blueberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Cranberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Currants | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Gooseberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Rose hips | NL | G/I | SC | 250 g/L | Foliar treatment – broadcast spraying | – | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Mulberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 51–89 | 2 | 7 | – | – | 200 g a.i./ha | 3 |
| Elderberries | DE | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 15–89 | 2 | 7 | – | – | 200 g a.i./ha | 7 |
| Cherimoyas | PT | I | SC | 150 g/L | Foliar treatment – general | 15–89 | 2 | 7 | – | – | 120 g a.i./ha | 3 |
| Tomatoes | NL | G/I | SC | 500 g/L | Soil treatment – drip irrigation | – | 4 | 7 | – | – | 500 g a.i./ha | 1 |
| Sweet peppers | CZ | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 61–83 | 2 | 7 | – | – | 300 g a.i./ha | 3 |
| Aubergines | EL | I | SC | 250 g/L | Foliar treatment – broadcast spraying | 14–89 | 1–3 | 14 | – | – | 150 g a.i./ha | 3 |
| Cucumbers | NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 300 g a.i./ha | 1 | |
| Gherkins | NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 300 g a.i./ha | 1 | |
| Courgettes | NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 300 g a.i./ha | 1 | |
| Melons | ES | I | SC | 250 g/L | Foliar treatment – general | 3 | 14 | – | – | 100 g a.i./ha | 3 | |
| Pumpkins | ES | I | SC | 250 g/L | Foliar treatment – general | 3 | 14 | – | – | 100 g a.i./ha | 3 | |
| Watermelons | ES | I | SC | 250 g/L | Foliar treatment – general | 3 | 14 | – | – | 100 g a.i./ha | 3 | |
| Lamb's lettuces | BE, NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 250 g a.i./ha | 7 | |
| Lettuces | BE, CZ, NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 250 g a.i./ha | 7 | |
| Cresses | BE, NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 250 g a.i./ha | 7 | |
| Roman rocket | BE, NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 250 g a.i./ha | 7 | |
| Baby leaf crops | BE, NL | I | SC | 500 g/L | Foliar treatment – broadcast spraying | 2 | 7 | – | – | 250 g a.i./ha | 7 | |
| Purslanes (sea lavender) | NL | G | SC | 250 g/L | Foliar spraying | 12–49 | 2 | 7 | – | 200–1,000 | 200 g a.i./ha | 7 |
| Witloofs | BE | I | SC | 500 g/L | Local treatment – general | 1 | – | – | 0.05 kg a.i./ton | 21 | ||
| Beans (with pods) | BE, NL | I | SC | 501 g/L | Foliar treatment – general | 60–79 | 2 | 7 | – | – | 250 g a.i./ha | 7 |
MS: Member State; a.s.: active substance; a.i.: active ingredient; n.a.: not applicable; SC: suspension concentrate.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
A.4. Import tolerance
| Crop and/or situation | MS or country | F G or Ia | Preparation | Application | Application rate per treatment | PHI (days)d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb | Conc. a.s. | Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
a.s./hL min–max |
Water L/ha min–max |
Rate and unit | ||||
| Grapefruits | US | F | SC | 500 g/L | Foliar treatment – general | 81–89 | 2 | 5 | – | – | 250 g a.i./ha | 7 |
| Oranges | US | F | SC | 500 g/L | Foliar treatment – general | 81–89 | 2 | 5 | – | – | 250 g a.i./ha | 7 |
| Lemons | US | F | SC | 500 g/L | Foliar treatment – general | 81–89 | 2 | 5 | – | – | 250 g a.i./ha | 7 |
| Limes | US | F | SC | 500 g/L | Soil treatment – general | 81–89 | 2 | 2 | – | – | 250 g a.i./ha | 7 |
| Mandarins | US | F | SC | 500 g/L | Foliar treatment – general | 81–89 | 2 | 5 | – | – | 250 g a.i./ha | 7 |
| Almonds | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Brazil nuts | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Cashew nuts | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Chestnuts | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Coconuts | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Hazelnuts | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Macadamias | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Pecans | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Pine nut kernels | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Pistachios | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Walnuts | US | F | SC | 500 g/L | Foliar treatment – general | 79–89 | 2 | 6 | – | – | 250 g a.i./ha | 14 |
| Apples | US/CAN | F | SC | 500 g/L | Foliar treatment – spraying | 81–87 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Pears | US/CAN | F | SC | 500 g/L | Foliar treatment – spraying | 81–87 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Quinces | US/CAN | F | SC | 500 g/L | Foliar treatment – spraying | 81–87 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Medlars | US/CAN | F | SC | 500 g/L | Foliar treatment – spraying | 81–87 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Loquats | US/CAN | F | SC | 500 g/L | Foliar treatment – spraying | 81–87 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Apricots | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 30 | – | – | 250 g a.i./ha | 0 |
| Cherries | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 30 | – | – | 250 g a.i./ha | 0 |
| Peaches | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 30 | – | – | 250 g a.i./ha | 0 |
| Plums | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 30 | – | – | 250 g a.i./ha | 0 |
| Table grapes | US | F | SC | 500 g/L | Foliar treatment – spraying | 85–89 | 2 | 12 | – | – | 250 g a.i./ha | 7 |
| Wine grapes | US | F | SC | 500 g/L | Foliar treatment – spraying | 85–89 | 2 | 12 | – | – | 250 g a.i./ha | 7 |
| Strawberries | US | F | SC | 500 g/L | Foliar treatment – spraying | 85–89 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Blackberries | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Dewberries | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Raspberries | US | F | SC | 500 g/L | Foliar treatment – spraying | 87–89 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Blueberries | US | F | SC | 500 g/L | Foliar treatment – spraying | 85–89 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Potatoes | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2 | 3 | – | – | 250 g a.i./ha | 7 |
| Carrots | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Radishes | US | F | SC | 500 g/L | Foliar treatment – general (see also comment field) | 250 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Tomatoes | US | F | SC | 500 g/L | Foliar treatment – general (see also comment field) | 250 | 2 | 6 | – | – | 250 g a.i./ha | 0 |
| Sweet peppers | US | F | SC | 500 g/L | Foliar treatment – general (see also comment field) | 250 | 2 | 7 | – | – | 250 g a.i./ha | 0 |
| Melons | US | F | SC | 500 g/L | Foliar treatment – general (see also comment field) | 250 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Basil | US | F | SC | 500 g/L | Foliar treatment – general (see also comment field) | 2 | 7 | – | – | 250 g a.i./ha | 0 | |
| Beans (with pods) | US | F | SC | 500 g/L | Foliar treatment – general (see also comment field) | 250 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Beans (without pods) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Peas (with pods) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2 | 5 | – | – | 250 g a.i./ha | 0 |
| Peas (without pods) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2–2 | 5 | – | – | 250 g a.i./ha | 0 |
| Lentils (fresh) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2–2 | 5 | – | – | 250 g a.i./ha | 0 |
| Celeries | US | F | Foliar treatment – general | 250 | 2 | – | – | 250 g a.i./ha | 0 | |||
| Globe artichokes | US | F | Foliar treatment – general | 250 | 2 | – | – | 250 g a.i./ha | 0 | |||
| Beans (dry) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2–2 | 5 | – | – | 250 g a.i./ha | 14 |
| Lentils (dry) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2–2 | 5 | – | – | 250 g a.i./ha | 14 |
| Peas (dry) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2–2 | 5 | – | – | 250 g a.i./ha | 14 |
| Lupins (dry) | US | F | SC | 500 g/L | Foliar treatment – general | 250 | 2–2 | 5 | – | – | 250 g a.i./ha | 14 |
| Peanuts | US | F | SC | 500 g/L | Foliar treatment – general | 2 | 12 | – | – | 250 g a.i./ha | 7 | |
| Sunflower seeds | US | F | SC | 500 g/L | Foliar treatment – general | 2–2 | 12 | – | – | 250 g a.i./ha | 14 | |
| Soya beans | US | F | SC | 500 g/L | Seed treatment – general | 1–2 | 7 | – | – | 250 g a.i./ha | 14 | |
| Cotton seeds | US | F | SC | 500 g/L | Foliar treatment – general | 1–2 | – | – | 250 g a.i./ha | 30 | ||
| Maize | US | F | SC | 500 g/L | Foliar treatment – general | 85–89 | 2–2 | 5 | – | – | 250 g a.i./ha | 14 |
| Sorghum | US | F | SC | 500 g/L | Foliar treatment – general | 83–89 | 2–2 | 12 | – | – | 250 g a.i./ha | 14 |
| Wheat | US | F | SC | 500 g/L | Foliar treatment – general | 75–87 | 2–2 | 12 | – | – | 250 g a.i./ha | 14 |
| Hops | US | F | SC | 500 g/L | Foliar treatment – general | 88 | 2 | – | – | 250 g a.i./ha | 7 | |
| Seed spices (Dill) | US | F | SC | 500 g/L | Foliar treatment – general | 85 | 2 | 7 | – | – | 250 g a.i./ha | 14 |
MS: Member State; a.s.: active substance; n.a.: not applicable; SC: suspension concentrate.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Grape | Foliar, 1 × 100 + 2 × 200 g a.s./ha | 18–19 | Radiolabelled active substance: phenyl‐UL‐14C and Pyridyl‐2,6‐14C (Germany, 2011; EFSA, 2013a) | |
| Pepper | Drip irrigation, 5 and 20 mg/plant | 55–97 | |||
| Root/tuber crops | Potato | Foliar, 3 × 167 g a.s./ha | 51 | ||
| Pulses/oilseeds | Bean | Foliar, 2 × 250 g a.s./ha | 4–29 | ||
| Cell culture | n.a. | n.a. | n.a. | Supplemental information (Germany, 2011) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | Turnips | Bare soil, 534 or 514 g a.s./ha | 30, 139, 280 |
Phenyl‐UL‐14C and Pyridyl‐2,6‐14C (Germany, 2011; EFSA, 2013a) Rotational crop study on cereals surrogate for primary seed treatment |
|
| Leafy crops | Swiss chard | Bare soil, 534 or 514 g a.s./ha | 30, 139, 280 | ||
| Cereal (small grain) | Spring wheat | Bare soil, 534 or 514 g a.s./ha | 30, 139, 280 |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Fluopyram, M08, M25 and M43 are stable. M40 is not stable, but not expected in the RAC in significant levels (Germany, 2011; EFSA, 2013a) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||
| Sterilisation (20 min, 120°C, pH 6) | Yes |

B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Lettuce, cabbage | –18°C | 36 | Months | Fluopyram, M25 | EFSA (2014) | |
| Lettuce | –18°C | 24 | Months | M40, M43, M08, M45 | EFSA (2013a) | ||
| High oil content | Rapeseed | –18°C | 36 | Months | Fluopyram, M25 | EFSA (2014) | |
| Rapeseed | –18°C | 24 | Months | M40, M43 | EFSA (2013a) | ||
| High protein/starch content | Dry pea, wheat grain | –18°C | 36 | Months | Fluopyram, M25 | EFSA (2014) | |
| Wheat grain, dry pea | –18°C | 24 | Months | M40, M43, M08, M45 | EFSA (2013a) | ||
| High acid content | Orange | –18°C | 36 | Months | Fluopyram, M25 | EFSA (2014) | |
| Orange, grapes | –18°C | 6 | Months | M40, 43 | EFSA (2013a) | ||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials – Primary crops
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) | CFd |
|---|---|---|---|---|---|---|---|
| Grapefruits, oranges | Import (US) |
Mo: Oranges: 0.04; 0.06; 0.11; 0.12; 0.12; 0.13; 0.14; 0.15; 0.25; 0.32; 0.25 Grapefruits: 0.06; 0.08; 0.14; 0.04; 0.11; 0.12 RA: – |
Trials on oranges and grapefruits compliant with GAP (Germany, 2018). Extrapolation to oranges and grapefruits is possible MRLOECD = 0.44 |
0.5 | 0.32 | 0.12 | 1.00e |
| Lemons, mandarins | Import (US) |
Mo: Lemons: 0.3; 0.29; 0.3; 0.32; 0.27 Mandarins: 0.2 RA: – |
Trials on lemons and mandarins compliant with GAP. Extrapolation to lemons and mandarins is possible (Germany, 2018) MRLOECD = 0.84 |
0.9 (tentative)f | 0.32 | 0.29 | 1.00e |
| Limes | Import (US) | – | No trials available for soil treatment | – | – | – | – |
| Hazelnuts | NEU | – | No trials available. However, the import tolerance GAP is clearly more critical (both application rate and PHI), therefore no further trials are required | – | – | – | – |
| Tree nuts | Import (US) |
Mo: Almonds: 6× < 0.01; 0.01; 0.017 Pecans: 4× < 0.01; 0.024 RA: – |
Trials on almonds and pecans compliant with GAP (Germany, 2018). Extrapolation to tree nuts is possible MRLOECD = 0.03 |
0.03 | 0.02 | 0.01 | 1.00e |
| Pome fruits | NEU |
Mo: Apples: 0.08; 0.09; 0.10; 0.11; 2× 0.12; 0.13; 0.18; 0.21 Pears: 0.10; 0.11; 0.12; 0.13; 0.19; 0.26 RA: Apples: 0.09; 0.10; 0.11; 0.12; 2× 0.13; 0.14; 0.19; 0.22 Pears: 0.11; 0.12; 0.13; 0.14; 0.20; 0.27 |
Combined data set on apples and pears compliant with GAP (Germany, 2018). Extrapolation to pome fruits is possible MRLOECD = 0.41 |
0.5 | 0.26 | 0.12 | 1.00 |
| SEU |
Mo: Apples: 0.03; 0.04; 0.06; 2× 0.08; 0.15 Pears: 0.20; 0.27 RA: Apples: 0.04; 0.05; 0.07; 2× 0.09; 0.16 Pears: 0.21; 0.28 |
Trials on apples and pears with application rate within 25% deviation. Extrapolation to pome fruits is proposed MRLOECD = 0.45 |
0.5 | 0.27 | 0.08 | 1.00 | |
| Import (US/CAN) |
Mo: Apples: 0.08; 0.11; 0.11; 2× 0.15; 0.16; 0.17; 0.18; 0.19; 2× 0.21; 0.22; 0.23; 0.24; 0.24; 0.39; 0.6 Pears: 0.18; 2× 0.2; 0.29; 0.41; 0.51 RA: – |
Combined data set on apples and pears compliant with GAP (Germany, 2018). Extrapolation to pome fruits is possible MRLOECD = 0.75 |
0.8 | 0.60 | 0.20 | 1.00e | |
| Apricots | NEU |
Mo: 0.12; 0.20; 0.30; 0.45 RA: 0.13; 0.21; 0.31; 0.46 |
Trials on apricots compliant with GAP (Germany, 2018) MRLOECD = 0.84 |
1 | 0.45 | 0.25 | 1.00 |
| SEU |
Mo: Apricots 0.27; 0.28; 0.33; 0.37; 0.43; 0.55; 0.58; 0.95 Peaches: 0.20; 0.26; 2× 0.28; 0.31; 0.36; 0.63; 0.73 RA: 0.28; 0.29; 0.34; 0.38; 0.44; 0.56; 0.59; 0.96; 0.21; 0.27; 0.29; 0.29; 0.32; 0.37; 0.64; 0.74 |
Combined data set on apricots and peaches compliant with GAP (Germany, 2018) Extrapolation to apricots and peaches is possible MRLOECD = 1.28 |
1.5 | 0.95 | 0.33 | 1.00 | |
| Import (US) | – | No data available | – | – | – | – | |
| Cherries (sweet) | NEU |
Mo: 0.20; 0.26; 0.27; 0.56; 0.56; 0.59; 1.1 RA: 0.21; 0.27; 0.28; 0.57; 0.57; 0.60; 1.11 |
Trials on cherries compliant with GAP (Germany, 2018) MRLOECD = 1.74 |
2 (tentative)f |
1.10 | 0.56 | 1.00 |
| SEU |
Mo: 0.12; 0.41; 0.47; 0.49 RA: 0.13; 0.42; 0.48; 0.50 |
Trials on cherries compliant with GAP (Germany, 2018) MRLOECD = 1.12 |
1.5 | 0.49 | 0.44 | 1.00 | |
| Import (US) |
Mo: 0.11; 0.25; 0.41; 0.58; 0.6; 0.89 RA: – |
Trials on cherries compliant with GAP (Germany, 2018) MRLOECD = 1.58 |
2 (tentative)f |
0.89 | 0.49 | 1.00e | |
| Peaches | NEU |
Mo: 0.05; 0.16; 0.17; 0.47 RA: 0.06; 0.18; 0.20; 0.48 |
Overdosed trials on peaches performed with 3 × 125 g/ha, PHI 3 days (Germany, 2018). As the SEU GAP is clearly more critical no additional trials are required MRLOECD = 0.93 |
1.5 | 0.47 | 0.16 | 1.00 |
| SEU |
Mo: Apricots 0.27; 0.28; 0.33; 0.37; 0.43; 0.55; 0.58; 0.95 Peaches: 0.20; 0.26; 2× 0.28; 0.31; 0.36; 0.63; 0.73 RA: Apricots 0.28; 0.29; 0.34; 0.38; 0.44; 0.56; 0.59; 0.96 Peaches: 0.21; 0.27; 0.29; 0.29; 0.32; 0.37; 0.64; 0.74 |
Combined data set on apricots and peaches compliant with GAP (Germany, 2018). Extrapolation to apricots and peaches is possible MRLOECD =1.28 |
1.5 | 0.95 | 0.33 | 1.00 | |
| Import (US) |
Mo: 0.17; 0.20; 0.22; 0.31; 0.34; 0.37; 0.4; 2× 0.42 RA: – |
Trials on peaches compliant with GAP (Germany, 2018) MRLOECD = 0.95 |
1 | 0.42 | 0.34 | 1.00e | |
| Plums | NEU |
Mo: 0.10; 0.13; 0.14; 0.18; 0.19; 0.20; 0.22; 0.24; 0.27 RA: 0.11; 0.14; 0.15; 0.19; 0.20; 0.21; 0.23; 0.25; 0.28 |
Trials on plums performed with application rate within 25% deviation (Germany, 2018) MRLOECD = 0.56 |
0.6 | 0.27 | 0.19 | 1.00 |
| SEU |
Mo: 0.08; 0.09; 0.12; 0.15; 0.19; 0.05; 0.07; 0.09 RA: 0.09; 0.10; 0.13; 0.16; 0.20; 0.06; 0.08; 0.10 |
Trials on plums compliant with GAP (Germany, 2018) MRLOECD = 0.32 |
0.4 | 0.19 | 0.09 | 1.00 | |
| Import (US) |
Mo: 0.02; 0.04; 0.05; 0.06; 0.06; 0.27 RA: – |
Trials on plums compliant with GAP (Germany, 2018) MRLOECD = 0.46 |
0.5 (tentative)f |
0.27 | 0.05 | 1.00e | |
| Table grapes | NEU |
Mo: 0.18; 0.29; 0.36; 0.44; 0.46; 0.56; 0.63; 0.65; 0.66 RA: 0.19; 0.30; 0.37; 0.45; 0.47; 0.57; 0.64; 0.66; 0.67 |
Trials on grapes compliant with GAP (Germany, 2018). Extrapolation to table grapes is applicable MRLOECD = 1.41 |
1.5 | 0.66 | 0.46 | 1.00 |
| SEU |
Mo: 0.30; 0.34; 0.36; 0.55; 0.58; 0.60; 0.63; 0.66; 0.96; 1.0 RA: 0.31; 0.35; 0.37; 0.58; 0.60; 0.61; 0.64; 0.68; 0.97; 1.02 |
Trials on grapes compliant with GAP for table grapes (Germany, 2018) MRLOECD = 1.79 |
2 | 1.00 | 0.59 | 1.00 | |
| Import (US) |
Mo: 0.1; 2× 0.15; 0.19; 0.21; 0.27; 0.32; 0.37; 0.43; 0.47; 0.49; 0.52; 0.58; 0.62; 0.63; 0.95 RA: – |
Trials on table grapes compliant with GAP (Germany, 2018) MRLOECD = 1.32 |
1.5 | 0.95 | 0.40 | 1.00e | |
| Wine grapes | NEU |
Mo: 0.18; 0.29; 0.36; 0.44; 0.46; 0.56; 0.63; 0.65; 0.66 RA: 0.19; 0.30; 0.37; 0.45; 0.47; 0.57; 0.64; 0.66; 0.67 |
Trials on grapes compliant with GAP (Germany, 2018). Extrapolation to table grapes and wine grapes is applicable MRLOECD = 1.41 |
1.5 | 0.66 | 0.46 | 1.00 |
| SEU |
Mo: 0.13; 0.22; 0.26; 0.28; 0.34; 0.35; 0.41; 0.44; 0.61; 0.63 RA: 0.15; 0.23; 0.28; 0.29; 0.36; 0.36; 0.43; 0.45; 0.65; 0.65 |
Trials on wine grapes compliant with GAP (Germany, 2018) MRLOECD = 1.1 |
1.5 | 0.63 | 0.35 | 1.00 | |
| Import (US) |
Mo: 0.1; 2× 0.15; 0.19; 0.21; 0.27; 0.32; 0.37; 0.43; 0.47; 0.49; 0.52; 0.58; 0.62; 0.63; 0.95 RA: – |
Trials on table grapes compliant with GAP (Germany, 2018). Extrapolation to wine grapes is applicable MRLOECD = 1.32 |
1.5 | 0.95 | 0.40 | 1.00e | |
| Strawberries | NEU |
Mo: 0.15; 2× 0.17; 0.19; 0.24; 0.35; 0.36; 0.43; 0.69 RA: 0.16; 2× 0.18; 0.20; 0.25; 0.36; 0.37; 0.44; 0.70 |
Trials on strawberries compliant with GAP (Germany, 2018) MRLOECD = 1.01 |
1 | 0.69 | 0.24 | 1.00 |
| SEU |
Mo: 0.07; 0.14; 0.18; 0.23; 0.28; 0.33; 0.50; 0.56 RA: 0.08; 0.15; 0.19; 0.24; 0.29; 0.34; 0.51; 0.57 |
Trials on strawberries performed with 2 applications instead 1 application (Germany, 2018). As the NEU GAP is clearly more critical no additional trials are required MRLOECD = 0.97 |
1 | 0.56 | 0.26 | 1.00 | |
| EU |
Mo: 0.12; 0.13; 0.20; 0.25; 0.28; 0.33; 0.71; 0.79 RA: 0.13; 0.24; 0.21; 0.26; 0.29; 0.34; 0.72; 0.8 |
Trials on strawberries compliant with GAP (Germany, 2018) MRLOECD = 1.38 |
1.5 | 0.79 | 0.27 | 1.00 | |
| Import (US) |
Mo: 0.20; 0.24; 0.29; 0.3; 0.36; 0.5; 0.68; 0.7; 0.83; 1.01 RA: – |
Trials on strawberries compliant with GAP (Germany, 2018) MRLOECD = 1.63 |
2 | 1.01 | 0.43 | 1.00e | |
| Cane fruits | NEU |
Mo: 0.51; 0.70; 0.88; 1.2 RA: 0.52; 0.71; –; – |
Trials on raspberries compliant with GAP (Germany, 2018). Extrapolation to cane fruits is applicable. Only 2 samples were analysed for fluopyram benzamide (M25) MRLOECD = 2.47 |
3 | 1.20 | 0.79 | 1.00 |
| SEU |
Mo: 0.69; 0.87; 0.99; 0.84; 0.29; 0.42 RA: –; –; 1.0; 0.85; 0.30; 0.43 |
Trials on raspberries performed with 2 instead 1 application (Germany, 2018). As the NEU GAP is clearly more critical no additional trials are required. Extrapolation to blackberries and dewberries is applicable MRLOECD = 2.05 |
2 | 0.99 | 0.77 | 1.00 | |
| EU |
Mo: 0.19; 0.35; 0.42; 0.98 RA: 0.20; 0.36; 0.43; 0.99 |
Trials on raspberries compliant with GAP (Germany, 2018). Extrapolation cane fruits is applicable MRLOECD = 1.86 |
3 | 0.98 | 0.39 | 1.00 | |
| Import (US) |
Mo: Raspberries 0.43; 0.71; 1.12 Blackberries 1.53; 2.39 RA: – |
Combined data set on raspberries and blackberries compliant with GAP (Germany, 2018). Extrapolation to cane fruits is applicable MRLOECD = 4.31 |
5 | 2.39 | 1.12 | 1.00e | |
| Blueberries | Import (US) |
Mo: 0.51; 0.58; 0.88; 1.14; 1.14; 1.32; 1.49; 4.33 RA: – |
Trails on blueberries compliant with GAP (Germany, 2018) MRLOECD = 6.32 |
7 | 4.33 | 1.14 | 1.00e |
| Blueberries, cranberries, currants, gooseberries and elderberries | NEU |
Mo: 0.24; 0.26; 0.35; 0.40; 0.44; 0.64; 0.92; 0.96; 1.01; 1.63; 1.69; 2.1 RA: 0.25; 0.27; 0.36; 0.41; 0.45; 0.65; 0.93; 0.97; 1.02; 1.64; 1.70; 2.11 |
Trials on currants compliant with GAP (Germany, 2018). Extrapolation to blueberries, cranberries, gooseberries and elderberries is applicable MRLOECD = 3.38 |
4 | 2.10 | 0.78 | 1.00 |
| SEU |
Mo: 0.12; 0.40; 0.47; 1.20 RA: 0.13; 0.41; 0.48; 1.21 |
Trials on currants performed with 2 instead 1 application (Germany, 2018). As the NEU GAP is clearly more critical no additional trials are required. Extrapolation to blueberries and gooseberries is applicable. No GAP is authorised for currants, cranberries, or elderberries MRLOECD = 2.39 |
3 | 1.20 | 0.44 | 1.00 | |
| EU |
Mo: 0.15; 0.25; 0.36; 0.38; 0.42; 0.42 RA: 0.16; 0.26; 0.37; 0.39; 0.43; 0.43 |
Trials on currants compliant with GAP (Germany, 2018). Extrapolation to other small fruits and berries is applicable MRLOECD = 1.15 |
1.5 | 0.42 | 0.37 | 1.00 | |
| Rose hips | NEU |
Unscaled: Mo: 0.24; 0.26; 0.35; 0.40; 0.44; 0.64; 0.92; 0.96; 1.01; 1.63; 1.69; 2.1 RA: 0.25; 0.27; 0.36; 0.41; 0.45; 0.65; 0.93; 0.97; 1.02; 1.64; 1.70; 2.11 Scaled: Mo: 0.18; 0.2; 0.26; 0.3; 0.33; 0.48; 0.69; 0.72; 0.76; 1.22; 1.22; 1.27; 1.58 RA: 0.19; 0.2, 0.27; 0.31; 0.34; 0.49; 0.7; 0.73; 0.77; 1.23; 1.28; 1.58 |
Trials on currants scaled to GAP (scaling factor 0.75) (Netherlands, 2019). Extrapolation to rose hips is applicable MRLOECD = 2.61 |
3 | 1.58 | 0.69 | 1.00 |
| EU |
Mo: 0.15; 0.25; 0.36; 0.38; 0.42; 0.42 RA: 0.16; 0.26; 0.37; 0.39; 0.43; 0.43 |
Trials on currants compliant with GAP (Germany, 2018). Extrapolation to rose hips is applicable MRLOECD = 1.15 |
1.5 | 0.42 | 0.37 | 1.00 | |
| Mulberries (black and white) | NEU |
Mo: 0.41; 0.44; 0.57; 1.0; 1.4; 2.1 RA: 0.42; 0.45; 0.58; 1.01; 1.41; 2.11 |
Trials on currents compliant with GAP (Germany, 2018). Extrapolation to mulberries is applicable MRLOECD = 3.65 |
4 | 2.10 | 0.79 | 1.00 |
| EU |
Mo: 0.15; 0.36; 0.37; 0.42; 0.42; 0.47 RA: 0.16; 0.37; 0.38; 0.43; 0.43; 0.48 |
Trials on currants compliant with GAP (Germany, 2018). Extrapolation to mulberries is applicable MRLOECD = 1.1 |
1.5 | 0.47 | 0.40 | 1.00 | |
| Bananas | SEU |
Mo: 0.02; 2× 0.04; 0.05; 0.06; 0.07; 0.19; 0.20; 0.25; 2× 0.26; 0.28; 0.37; 0.53 RA: 0.03; 2× 0.05; 0.06; 0.07; 0.08; 0.20; 0.21; 0.26; 2× 0.27; 0.29; 0.38; 0.54 |
Trials on banana performed with 6 instead of 3 applications with a PHI of 0 day used on a tentative basis (EFSA, 2011) MRLOECD = 0.79 |
0.8 (tentative)g |
0.53 | 0.2 | 1.00 |
| Cherimoyas | EU | Mo/RA: – | No trials available. Applicant not aware of such use (EFSA, 2019e) | – | – | – | – |
| Potatoes | SEU |
Mo: 7× < 0.01; 0.02 RA: 7× < 0.02; 0.03 |
Trails on potatoes compliant with GAP (Germany, 2018) MRLOECD = 0.02 |
0.02 | 0.02 | 0.01 | 1.00 |
| Import (US) |
Mo: 21× < 0.01; 6× 0.01; 5x 0.02; 2× 0.03; 0.04; 0.05; 0.06; 0.07 RA: – |
Trials on potatoes compliant with GAP (Germany, 2018) MRLOECD = 0.07 |
0.08 | 0.07 | 0.01 | 1.00e | |
| Sweet potatoes and yams | NEU |
Mo: < 0.01; 5× 0.02; 2× 0.03 RA: < 0.02; 5× 0.03; 2× 0.04 |
Trials on potatoes compliant with GAP (Netherlands, 2018) MRLOECD = 0.06 |
0.06 | 0.03 | 0.02 | 1.00 |
| Other root and tuber vegetables except beetroots, carrots, radishes and sugar beets | NEU |
Mo: 0.02; 0.03; 2× 0.04; 3× 0.05; 0.08; 0.13 RA: 0.03; 0.04; 2× 0.05; 3× 0.06; 0.09; 0.14 |
Trials on carrots compliant with GAP (Germany, 2018). Extrapolation to other root and tuber vegetables possible MRLOECD = 0.18 |
0.2 | 0.13 | 0.05 | 1.00 |
| Carrots | NEU |
Mo: 0.02; 0.03; 2× 0.04; 3× 0.05; 0.08; 0.13 RA: 0.03; 0.04; 2× 0.05; 3× 0.06; 0.09; 0.14 |
Trials on carrots compliant with GAP (Germany, 2018) MRLOECD = 0.18 |
0.2 | 0.13 | 0.05 | 1.00 |
| SEU |
Mo: < 0.01; 2× 0.01; 3× 0.02; 0.03; 0.06 RA: < 0.02; 2× 0.03; 3× 0.03; 0.04; 0.07 |
Trials on carrots compliant with GAP (Germany, 2018) MRLOECD = 0.09 |
0.09 | 0.06 | 0.02 | 1.00 | |
| Import (US) |
Carrots: Mo: 0.02; 2× 0.04; 2× 0.06; 0.09 Radishes: 0.05; 0.07; 0.1; 0.12; 0.13 RA: – |
Trials on carrots and radishes compliant with GAP (Germany, 2018). Extrapolation to carrots accepted MRLOECD = 0.21 |
0.3 | 0.13 | 0.06 | 1.00e | |
| Radishes | NEU |
Mo: – RA: – |
No trials compliant with the GAP is available | – | – | – | – |
| Import (US) |
Carrots: Mo: 0.02; 2× 0.04; 2× 0.06; 0.09 Radishes: Mo: 0.05; 0.07; 0.1; 0.12; 0.13 RA: – |
Trials on carrots and radishes compliant with GAP (Germany, 2018). Extrapolation to radishes accepted MRLOECD = 0.21 |
0.3 | 0.13 | 0.06 | 1.00e | |
| Beetroots | NEU |
Mo: 2× < 0.01; 0.01; 3× 0.02; 0.03; 0.04 RA: 2× < 0.02; 0.02; 3× 0.03; 0.04; 0.05 |
Trials on carrots compliant with the GAP (Netherlands, 2019). Extrapolation to beetroots possible MRLOECD = 0.06 |
0.06 | 0.04 | 0.02 | 1.00 |
| Onions, garlic, shallots | NEU |
Mo: 5× < 0.01; 0.02; 0.03; 0.04 RA: 5× < 0.02; 0.03; 0.04; 0.05 |
Trials on onions compliant with GAP (Germany, 2018). Extrapolation to shallots and garlic is applicable MRLOECD = 0.06 |
0.07 | 0.04 | 0.01 | 1.00 |
| SEU |
Mo: 6× < 0.01; 0.03; 0.04 RA: 6× < 0.02; 0.04; 0.05 |
Trials on onions compliant with GAP (Germany, 2018). Extrapolation to shallots and garlic is applicable MRLOECD = 0.06 |
0.07 | 0.04 | 0.01 | 1.00 | |
| Spring onions/green onions and Welsh onions | NEU |
Mo: 0.07; 0.11; 0.12; 0.29 RA: 0.16; 0.18; 0.13; 0.30 |
Overdosed trials on spring onions performed with 2 instead 1 application (Germany, 2018). As the SEU GAP is clearly more critical no additional trials are required MRLOECD = 0.54 |
0.7 | 0.29 | 0.12 | 1.10 |
| SEU |
Mo: 0.27; 0.41; 0.61; 1.2 RA: 0.32; 0.42; 0.63; 1.22 |
Overdosed trials on spring onions performed with 2 instead 1 application (Germany, 2018) used on tentative basis MRLOECD = 2.26 |
3 (tentative)g |
1.20 | 0.51 | 1.10 | |
| Tomatoes | SEU |
Mo: 4× < 0.01 RA: 4× < 0.02 |
Trials on tomato compliant with soil application GAP (Germany, 2018) MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 | 1.00 |
| EU |
Mo: 0.01; 0.04; 0.08; 0.11; 0.14; 0.18; 0.23 RA: 0.03; 0.05; 0.1; 0.15; 0.5; 0.23; 0.24 |
Trials compliant with the GAP using drip irrigation submitted during Member States Consultation (Netherlands, 2019) MRLOECD = 0.42 |
0.5 (tentative)f |
0.23 | 0.11 | 1.3 | |
| Import (US) |
Mo: 0.02; 0.06; 0.07; 2× 0.08; 0.09; 0.10; 0.11; 0.16; 0.17; 0.18; 2× 0.19; RA: – |
Trials on tomatoes compliant with GAP (Germany, 2018) MRLOECD = 0.35 |
0.4 | 0.19 | 0.11 | 1.00e | |
| Aubergines | SEU |
Mo: 4× < 0.01 RA: 4× < 0.02 |
Trials on tomato compliant with soil application GAP (Germany, 2018). Extrapolation to aubergines is possible MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 | 1.00 |
| EU |
Mo: 0.04; 0.04h; 0.05h; 0.07; 2× 0.08; 0.11h; 0.12h; 0.12; 0.13h; 0.13; 0.15h; 0.21h; 0.23h; RA: 0.05; 0.05h; 0.06h; 0.08; 2× 0.09; 0.12h; 0.13h; 0.13; 0.14h; 0.14; 0.16h; 0.22h; 0.24h |
Trials on tomatoes GAP compliant, or performed with 2 instead of 3 foliar applications deemed acceptable, since residues are in the same range or higher compared to residues of the GAP compliant trials (Germany, 2018). Extrapolation to aubergines is possible MRLOECD = 0.34 |
0.4 | 0.23 | 0.12 | 1.00 | |
| Sweet peppers/bell peppers | SEU |
Mo: < 0.01; 0.01; 2× 0.02 RA: < 0.02; 0.02; 2× 0.03 |
Four trials compliant with soil application GAP (Germany, 2018). As the indoor GAP is clearly more critical no additional trials are required MRLOECD = 0.03 |
0.04 | 0.02 | 0.01 | 1.00 |
| EU |
Mo: 0.16; 2× 0.25; 2× 0.29; 2× 0.31; 0.42; 0.58 RA: 0.17; 0.26; 0.26; 0.30; 0.30; 0.32; 0.32; 0.43; 0.59 |
Trials on peppers compliant with GAP (Germany, 2018) MRLOECD = 0.95 |
1 | 0.58 | 0.29 | 1.00 | |
| Import (US) |
Mo: sweet peppers: 0.04; 0.09; 0.13; 0.14; 0.17; 0.36; Chilli peppers: 0.12; 1.23 RA: – |
Trials on peppers and chilli peppers compliant with GAP (Germany, 2018) MRLOECD = 1.86 |
2 | 1.23 | 0.14 | 1.00e | |
| Cucumbers, gherkins, courgettes | NEU |
Mo: 0.02; 0.03; 0.04; 0.05; 0.06 RA: 0.03; 0.04; 0.05; 0.06; 0.07 |
Trials on cucumbers compliant with the GAP. (Germany, 2018) Extrapolation to gherkins is applicable. No authorised NEU GAP for courgettes reported. As the indoor GAP is clearly more critical no additional trials are required MRLOECD = 0.12 |
0.15 | 0.06 | 0.04 | 1.00 |
| SEU |
Mo: 2× < 0.01; 0.01; 0.02 RA: 2× < 0.02; 0.02; 0.03 |
Trials on cucumbers compliant with the soil application GAP (Germany, 2018). Extrapolation to courgettes and gherkins is applicable. As the indoor GAP is clearly more critical no additional trials are required MRLOECD = 0.02 |
0.03 | 0.02 | 0.01 | 1.00 | |
| EU |
Mo: 0.08; 0.10; 2× 0.13; 0.14; 0.22; 0.26; 0.30 RA: 0.09; 0.11; 0.14; 0.14; 0.15; 0.23; 0.27; 0.31 |
Trails on cucumbers compliant with GAP (Germany, 2018). Extrapolation to courgettes and gherkins is applicable MRLOECD = 0.51 |
0.6 | 0.30 | 0.14 | 1.00 | |
| Melons, watermelons, pumpkins | SEU |
Mo: 6× < 0.01; 0.02; 0.04; 2× 0.06 RA: 6× < 0.02; 0.04; 0.06; 2× 0.07 |
Trials on melons compliant with soil application GAP (Germany, 2018). Extrapolation to pumpkins and watermelons is applicable MRLOECD = 0.11 |
0.15 | 0.06 | 0.01 | 1.00 |
| EU |
Mo: < 0.01; 0.02; 0.07; 0.12 RA: < 0.02; 0.03; 0.08; 0.13 |
Trials on melons compliant with GAP used (Germany, 2018). Extrapolation to pumpkins and watermelons is applicable MRLOECD = 0.26 |
0.4 (tentative for watermelons and melons)f |
0.12 | 0.05 | 1.00 | |
| Import (US) |
Mo: 0.07; 0.08; 0.14; 0.23; 0.38; 0.44 RA: – |
Trials on melons compliant with GAP (Germany, 2018). Authorised GAP only for melons MRLOECD = 0.85 |
0.9 (tentative)f |
0.44 | 0.19 | 1.00e | |
| Sweet corn | NEU |
Mo: 3× < 0.01 RA: 3× < 0.02 |
Trials on sweet corn compliant with the GAP (Germany, 2018). The reduced number of residue trials is considered acceptable as all results were below the LOQ and no residue is expected MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 | 1.00 |
| Broccoli | NEU |
Mo: < 0.01; 0.02; 0.05; 0.14 RA: < 0.02; 0.03; 0.06; 0.15 |
Trials on broccoli compliant with the GAP (Germany, 2018) MRLOECD = 0.29 |
0.4 | 0.14 | 0.04 | 1.00 |
| Cauliflowers | NEU |
Mo: 2× < 0.01; 3× 0.01; 0.02; 2× 0.05 RA: 2× < 0.02; 3× 0.02; 0.03; 2× 0.06 |
Trials on cauliflower with 25% deviation in application rate (Germany, 2018) MRLOECD = 0.18 |
0.1 | 0.05 | 0.01 | 1.00 |
| Brussels sprouts | NEU |
Mo: 0.01; 4× 0.04; 2× 0.07; 0.14 RA: 0.02; 4× 0.05; 2× 0.08; 0.15 |
Trials on brussels sprouts with dose rates within 25% deviation (Germany, 2018) MRLOECD = 0.21 |
0.3 | 0.14 | 0.04 | 1.00 |
| Head cabbages | NEU |
Mo: 3× < 0.01; 3× 0.01; 0.02; 0.04; 0.08 RA: 3× < 0.02; 3× 0.02; 0.03; 0.05; 0.09 |
Trials on head cabbage with dose rates within 25% deviation (Germany, 2018) MRLOECD = 0.12 |
0.15 | 0.08 | 0.01 | 1.00 |
| Chinese cabbages/pe‐tsai | NEU |
Mo: 0.22; 0.29; 0.42; 0.84 RA: 0.23; 0.3; 0.43; 0.85 |
Trials with 2 applications instead of one on Chinese cabbage used on a tentative basis (Germany, 2018) MRLOECD = 1.55 |
2 (tentative)g |
0.84 | 0.36 | 1.00 |
| Escaroles, land cresses, red mustards | NEU |
Mo: 0.05; 0.11; 0.21; 0.26; 0.37; 0.58; 0.62; 0.84; 0.98 RA: 0.06; 0.12; 0.22; 0.27; 0.38; 0.59; 0.63; 0.85; 0.99 |
Trials on open leaf lettuce with 2 application instead of 1 (Germany, 2018). Extrapolation to escaroles, land cresses, and red mustards is possible MRLOECD = 1.75 |
2 (tentative)g |
0.98 | 0.37 | 1.00 |
| Lamb's lettuces, cresses and other sprouts shoots, Roman rocket and purslanes (sea lavender) and baby leaf crops | NEU |
Mo: 0.05; 0.11; 0.21; 0.26; 0.37; 0.58; 0.62; 0.84; 0.98 RA: 0.06; 0.12; 0.22; 0.27; 0.38; 0.59; 0.63; 0.85; 0.99 |
Trials on open leaf lettuce with 2 application instead of 1 (Germany, 2018). As the indoor GAP is clearly more critical no additional trials are required for. Extrapolation to subgroup of lettuces and salad plants is possible MRLOECD = 1.75 |
2 | 0.98 | 0.37 | 1.00 |
| EU |
Mo: 0.83; 0.92; 0.94; 1.6; 3.6; 3.9; 10 RA: 0.84; 0.94; 0.95; 1.61; 3.63; 3.91; 10.01 |
Trials on open leaf variety lettuce with dose rates within 25% deviation (Germany, 2018). Extrapolation to subgroup of lettuces and salad plants is possible MRLOECD = 16.31 |
20 | 10.00 | 1.60 | 1.00 | |
| Lettuces | NEU |
Mo: 0.12; 0.13; 2× 0.18; 0.26; 0.53; 0.57; 0.61; 0.62; 0.63; 0.93; 0.05; 0.11; 0.21; 0.26; 0.37; 0.58; 0.62; 0.84; 0.98 RA: 0.13; 0.14; 0.19; 0.19; 0.27; 0.55; 0.59; 0.62; 0.64; 0.65; 0.96; 0.06; 0.12; 0.22; 0.27; 0.38; 0.59; 0.63; 0.85; 0.99 |
Trials on open and closed variety lettuces within 25% application rate (Germany, 2018). As the indoor GAP is clearly more critical no additional trials are required MRLOECD = 1.59 |
2 | 0.98 | 0.45 | 1.00 |
| SEU |
Mo: 0.74; 1.5; 0.6; 0.55; 0.71; 0.14; 1.2; 2.2; 0.57 RA: 0.75; 1.52; 0.62; 0.56; 0.72; 0.15; 1.21; 2.21; 0.58 |
Trials on open leaf lettuce varieties with 2 applications instead of 1 (Germany, 2018). As the indoor GAP is clearly more critical no additional trials are required MRLOECD = 3.4 |
4 | 2.20 | 0.71 | 1.00 | |
| EU |
Mo: 0.23; 0.83; 0.92; 0.94; 1.4; 1.6; 2.1; 3.6; 3.9; 10 RA: 0.24; 0.84; 0.94; 0.95; 1.42; 1.61; 2.11; 3.63; 3.91; 10.01 |
Trials on open and closed leaf variety lettuce within 25% deviation of application rate (Germany, 2018) MRLOECD = 14.06 |
15 | 10.00 | 1.50 | 1.00 | |
| Spinaches, chards/beet leaves | NEU |
Mo: 0.05; 0.11; 0.21; 0.26; 0.37; 0.58; 0.62; 0.84; 0.98 RA: 0.06; 0.12; 0.22; 0.27; 0.38; 0.59; 0.63; 0.85; 0.99 |
Trials on open leaf lettuce with 2 application instead of 1 used on a tentative basis (Germany, 2018). Extrapolation to spinaches and similar leaves possible MRLOECD = 1.75 |
2 (tentative)g |
0.98 | 0.37 | 1.00 |
| Witloofs/Belgian endives | EU |
Mo: 0.04; 0.07; 2× 0.12 RA: 0.06; 0.08; 0.13; 0.14 |
Trials on witloof compliant with GAP (EFSA, 2016) MRLOECD = 0.26 |
0.3 | 0.12 | 0.10 | 1.20 |
| Herbs, and edible flowers | NEU |
Mo: Parsley: 0.31; 0.39; 0.54; 0.64; Chervil: 0.08; 0.38 Sage: 0.31 Savoury: 0.11; 3.64 RA: Parsley: 0.32; 0.4; 0.55; 0.65; Chervil: 0.09; 0.39 Sage: 0.32 Savoury: 0.12; 3.65 |
Combined data set of residue trials on parsley, chervil, sage and savoury (Germany, 2018). Extrapolation to fresh herbs possible MRLOECD = 5.16 (1.16) (without savoury) |
6 (1.5)i |
3.64 (0.67)i |
0.38 (0.38)i |
1.00 |
| Basil | Import (US) |
Mo: Chives: 6.05; 7.83; 19.8; Basil 18.78; 19.36; 30.0 RA: – |
Combined data set on chives and basil compliant with the GAP. Only parent analysed (Germany, 2018) MRLOECD = 52.28 |
60 | 30.00 | 19.07 | 1.00e |
| Beans, peas (with pods) | NEU |
Mo: Beans: 0.05; 0.06; 0.10; 0.18 Peas: 0.03; 0.04; 0.05; 0.06; 0.13; 0.14; 0.19; 0.53 RA: Beans: 0.08; 0.10; 0.11; 0.19; Peas: 0.04; 0.05; 0.06; 0.08; 0.14; 0.15; 0.20; 0.54 |
Trials in pea/bean (200 g/ha) scaled to the nominal rate of 250 g/ha (EFSA, 2016). Extrapolation to beans and peas with pods possible MRLOECD = 1.13 |
0.7 | 0.53 | 0.08 | 1.10 |
| SEU |
Mo: < 0.01; 0.01; 0.02; 2× 0.03; 2× 0.04; 3× 0.05; 0.06; 0.07; 2× 0.08; 0.10; 0.14; 0.16; 0.32; 0.82 RA: < 0.02; 0.02; 0.03; 0.09; 0.06; 0.04; 0.05; 0.07; 0.08; 2× 0.07; 0.07; 0.11; 0.09; 0.14; 0.20; 0.22; 0.39; 0.96 |
Trials on beans with pods, application rate within 25% deviation (Germany, 2018). No authorised use for peas (with pods) MRLOECD = 0.86 |
0.9 | 0.82 | 0.05 | 1.10 | |
| EU |
Mo: 0.07; 0.12; 2× 0.16; 0.20; 2× 0.22; 0.23; 0.26; 2× 0.40; 0.43; 0.69; 0.78; 0.95; 1.5; RA: 0.08; 0.13; 2× 0.17; 0.21; 2× 0.23; 0.24; 0.28; 0.41; 0.69; 0.44; 0.70; 0.8; 1.05; 1.51; |
Trials on beans with pods with application rate within 25% deviation (Germany, 2018). No authorised use for peas (with pods) MRLOECD = 1.95 |
2 | 1.50 | 0.23 | 1.10 | |
| Import (US) |
Mo: 0.13; 0.15; 0.17; 0.25; 0.41; 0.7; 0.78; 1.14; 1.24 RA: – |
Combined data set on beans and peas with pods (Germany, 2018). Extrapolation to beans and peas with pods is possible MRLOECD = 2.28 |
3 | 1.24 | 0.41 | 1.10e | |
| Beans, peas (without pods), lentils (fresh) | NEU |
Mo: 3× < 0.01; 2× 0.01; 4× 0.02; 3× 0.03; 2× 0.05 RA: 3× < 0.02; 2× 0.02; 4× 0.03; 3× 0.04; 2× 0.06 |
Trials on peas without pods (Germany, 2018). Extrapolation to beans without pods possible. No authorised use for lentils in NEU MRLOECD = 0.08 |
0.08 | 0.05 | 0.02 | 1.30 |
| Import (US) |
Mo: < 0.01; 2× 0.01; 0.02; 0.03; 0.04; 0.05; 2× 0.06; 2× 0.07 RA: – |
Combined data sets from beans w/o pods and peas w/o pods compliant with GAP (Germany, 2018). Extrapolation to legume vegetables possible MRLOECD = 0.14 |
0.15 | 0.07 | 0.04 | 1.30e | |
| Asparagus | NEU |
Mo: 4× < 0.01 RA: 4× < 0.02 |
Trials on asparagus compliant with the GAP (Germany, 2018) MRLOECD = 0.01 |
0.01 | 0.01 | 0.01 | 1.00 |
| SEU |
Mo: 4× < 0.01 RA: 4× < 0.02 |
Trials on asparagus with three applications instead of two (Germany, 2018). No further data needed as even overdosed trials are below the LOQ MRLOECD = 0.01 |
0.01 | 0.01 | 0.01 | 1.00 | |
| Celeries | Import (US) |
Mo: 0.20; 1.58; 2.24; 3.82; 5.44; 9.74 RA: – |
Trials on celery compliant with the GAP. M‐25 not analysed (Germany, 2018) MRLOECD = 17.49 |
20 | 9.74 | 3.03 | 1.00 |
| Globe artichokes | SEU |
Mo: 0.05; 0.09; 0.1; 0.14; 0.16; 0.18; 0.21; 0.29 RA: 0.11; 0.15; 0.06; 0.1; 0.17; 0.19; 0.22; 0.30 |
Trials on globe artichokes with some applications slightly overdosed, outside the 25% range (3 × 100 g/ha instead of 3 × 75 g/ha) (Germany, 2018) MRLOECD = 0.46 |
0.5 | 0.29 | 0.15 | 1.00 |
| Import (US) |
Mo: 1.02; 1.27; 1.37 RA: – |
Trials on artichoke compliant with the GAP (Germany, 2018). Only parent analysed MRLOECD = 3.66 |
4 (tentative)f |
1.37 | 1.27 | 1.00e | |
| Leeks | NEU |
Mo: 0.01; 2× 0.02; 0.03; 0.07; 0.11; 0.17; 0.32 RA: 0.02; 0.03; 0.03; 0.04; 0.08; 0.12; 0.18; 0.33; |
Trials on leek compliant with the GAP (Germany, 2018) MRLOECD = 0.52 |
0.6 | 0.32 | 0.05 | 1.10 |
| SEU |
Mo: 0.07; 0.16; 0.28; 0.31 RA: 0.08; 0.18; 0.3; 0.32 |
Overdosed trials on leek with two applications instead of one (Germany, 2018) MRLOECD = 0.65 |
0.8 (tentative)g |
0.31 | 0.22 | 1.10 | |
| Beans, Peas, Lentils, Lupins/lupini beans (dry) | NEU |
Mo: – RA: – |
No GAP compliant trials available. Only authorised use reported for beans and peas (dry) | – | – | – | – |
| Import (US) |
Mo: Beans 3× < 0.01; 0.01; 0.02; 0.03; 0.05; 0.07; Peas: 0.03; 0.04; 0.06; 0.16; 0.35 RA: – |
Combined data set on dry beans and peas compliant with GAP (Germany, 2018). Extrapolation to pulses is possible. Only parent analysed MRLOECD = 0.44 |
0.5 | 0.35 | 0.03 | 1.30e | |
| Peanuts/groundnuts | Import (US) |
Mo: 10× < 0.01; 0.01; 0.0175 RA: – |
Trials on peanuts compliant with the GAP. Only parent analysed (Germany, 2018) MRLOECD = 0.02 |
0.02 | 0.02 | 0.01 | 1.20 |
| Poppy seeds, mustard seeds | NEU |
Mo: 0.02; 0.04; 0.08; 0.09; 0.1; 2× 0.11; 0.19; 0.26 RA: 0.03; 0.05; 0.09; 0.12; 0.13; 0.14; 0.13; 0.22; 0.30 |
Trials on rapeseed compliant with NEU GAP (Germany, 2018). Extrapolation to minor oilseeds possible MRLOECD = 0.41 |
0.4 | 0.26 | 0.10 | 1.20 |
| Sunflower seeds | NEU |
Mo: 5× < 0.01; 0.01; 0.02; 0.17 RA: 5×3 < 0.02; 0.02; 0.03; 0.18 |
Trials on sunflower seeds compliant with the GAP (EFSA, 2016) MRLOECD = 0.26 |
0.3 | 0.17 | 0.01 | 1.00 |
| Import (US) |
Mo: 0.02; 0.05; 0.06; 2× 0.08; 0.22; 0.25; 0.38 RA: – |
Trials on sunflower seed compliant with the GAP (Germany, 2018) MRLOECD = 0.65 |
0.7 | 0.38 | 0.08 | 1.00e | |
| Rapeseeds/canola seeds | NEU, SEU |
NEU: Mo: 0.10; 0.26; 0.27; 0.29; 0.34; 0.35; 0.47; 0.61; RA: 0.13; 0.3; 0.34; 0.33; 0.42; 0.38; 0.51; 0.65 SEU: Mo: 0.14; 0.25; 0.27; 0.33; 2× 0.38; 2× 0.46 RA: 0.19; 0.27; 0.32; 0.42; 0.44; 0.51; 0.54; 0.62 |
Combined data set on rapeseed compliant with NEU and SEU GAP (EFSA, 2016) MRLOECD = 1.01 |
1 | 0.61 | 0.34 | 1.20 |
| Soya beans | Import (US) |
Mo: 12× < 0.01; 3× 0.01; 2× 0.02; 2× 0.04; 0.06 RA: – |
Trials on soya beans compliant with GAP. Only parent analysed (Germany, 2018) MRLOECD = 0.07 |
0.08 | 0.06 | 0.01 | 1.20 |
| Cotton seeds | Import (US) |
Mo: 2× < 0.01; 2× 0.02; 0.04; 0.08; 0.14; 0.16; 0.29; 0.47 RA: – |
Trials on cotton seeds not fully GAP compliant: seed treatment + 2 × foliar altogether 500 g a.s./ha. Only parent analysed (Germany, 2018) MRLOECD = 0.72 |
0.8 | 0.47 | 0.06 | 1.20 |
| Barley grains, oat grains | NEU |
Mo: 0.01; 3× 0.02; 4x 0.03; RA: 0.02; 3× 0.03; 3× 0.04; 0.05; |
Trials on barley compliant with the GAP (Germany, 2018). Extrapolation to oat possible MRLOECD = 0.19 |
0.07 | 0.03 | 0.02 | 1.00 |
| SEU |
Mo: 2× < 0.01; 0.01; 0.02; 2× 0.03; 0.08; 0.11 RA: 2× < 0.02; 0.02; 0.03; 2× 0.04; 0.09; 0.12 |
Trials on barley compliant with the GAP (Germany, 2018). Extrapolation to oat possible MRLOECD = 0.19 |
0.2 | 0.11 | 0.02 | 1.00 | |
| Maize/corn grains | NEU |
Mo: 8× < 0.01 RA: 8× < 0.02 |
Trials on maize compliant with the GAP (Germany, 2018) MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 | 1.00 |
| Import (US) |
Mo: 15× < 0.01 RA: – |
Trials on maize (ear without husk) compliant with the GAP. Only parent analysed (Germany, 2018) MRLOECD = 0.01 |
0.01* | 0.01 | 0.01 | 1.00e | |
| Sorghum grains | Import (US) |
Mo: 0.23; 0.24; 2× 0.25; 2× 0.26; 0.45; 0.50; 0.64; 0.69; 0.71; 3.03 RA: – |
Trials on sorghum compliant with the GAP. Only parent analysed (Germany, 2018) MRLOECD = 3.75 |
4 | 3.03 | 0.36 | 1.00 |
| Wheat, rye grains | NEU |
Mo: 4× < 0.01; 4× 0.01; 0.02 RA: 4× < 0.02; 4× 0.02; 0.03 |
Trials on wheat with application rate within 25% deviation (Germany, 2018). Extrapolation to rye possible MRLOECD = 0.03 |
0.03 | 0.02 | 0.01 | 1.00 |
| SEU |
Mo: 5× < 0.01; 0.01; 0.02; 0.05 RA: 5× < 0.02; 0.02; 0.03; 0.06 |
Trials on wheat compliant with the GAP (Germany, 2018). Extrapolation to rye possible MRLOECD = 0.07 |
0.07 | 0.05 | 0.01 | 1.00 | |
| Import (US) |
Mo: 0.04; 2× 0.13; 0.15; 0.16; 0.17; 2× 0.19; 0.2; 0.21; 2× 0.23; 0.25; 0.30; 0.72 RA: – |
Trials on wheat compliant with the GAP (Germany, 2018). Only parent analysed. No GAP on rye grain authorised MRLOECD = 0.82 |
0.9 | 0.72 | 0.19 | 1.00e | |
| Hops | NEU |
Mo: 0.27; 0.40; 0.45; 0.92; 0.93; 1.1; 1.3; 1.0; 0.93 RA: 0.29; 0.45; 0.57; 0.96; 1.27; 1.46; 1.55; 1.05; 1.08 |
Trials in hops compliant with the GAP (Germany, 2018) MRLOECD = 2.43 |
3 | 1.30 | 0.93 | 1.20 |
| Import (US) |
Mo: 5.80; 6.71; 13.5; 25.4 RA: –; –; 13.77; – |
Trials on hops compliant with the GAP (Germany, 2018) MRLOECD = 49.02 |
60 | 25.40 | 10.11 | 1.20e | |
| Dill (Seed spices) | Import (US) |
Mo: 9.16; 25.9; 29.6; 19.1 RA: –; –; –; 19.17 |
Trials on dill seeds compliant with the GAP. M25 analysed in 1 trial only (Germany, 2018) MRLOECD = 62.82 |
70 | 29.60 | 22.50 | 1.00 |
| Chicory roots | NEU |
Mo: – RA: – |
No trials compliant with the GAP available | – | – | – | – |
| Barley, oat straw | NEU |
Mo: 0.03; 0.06; 0.07; 0.08; 0.11; 0.13; 2× 0.14 RA: 0.04; 0.07; 0.08; 0.09; 0.14; 0.17; 0.15; 0.16; |
Trials on barley compliant with the GAP (Germany, 2018). Extrapolation to oat possible MRLOECD = 0.19 |
0.3 (tentative)(j) |
0.14 | 0.10 | 1.10 |
| SEU |
Mo: 0.03; 0.08; 2× 0.1; 0.18; 0.4; 0.77; 1.1 RA: 0.04; 0.12; 2× 0.12; 0.2; 0.42; 0.8; 1.16 |
Trials on barley compliant with GAP (Germany, 2018). Extrapolation to oat possible MRLOECD = 1.91 |
2 (tentative)(j) |
1.10 | 0.14 | 1.10 | |
| Maize/corn stover | NEU |
Mo: 0.13; 0.34; 0.37; 0.38; 0.46; 0.8; 0.99; 1.7 RA: 0.14; 0.35; 0.38; 0.39; 0.47; 0.81; 1; 1.75 |
Trials on maize compliant with the GAP (Germany, 2018) MRLOECD = 2.67 |
3 (tentative)(j) |
1.70 | 0.42 | 1.00 |
| Wheat, rye straw | NEU |
Mo: 0.06; 0.09; 3× 0.11; 0.13; 0.16; 0.20; 0.21; 0.26; 0.28; 0.35 RA: 0.07; 0.12; 0.13; 2× 0.15; 0.16; 0.17; 2× 0.26; 0.29; 0.35; 0.38 |
Trials on wheat with application rate within 25% deviation (Germany, 2018). Extrapolation to rye possible MRLOECD = 0.53 |
0.6 (tentative)(j) |
0.35 | 0.15 | 1.10 |
| SEU |
Mo: 0.11; 2× 0.13; 0.17; 0.63; 0.09; 0.67; 1.1 RA: 0.12; 0.14; 0.15; 0.19; 0.67; 0.28; 0.71; 1.13 |
Trials on wheat compliant with the GAP (Germany, 2018). Extrapolation to rye possible MRLOECD = 1.88 |
2 (tentative)(j) |
1.10 | 0.15 | 1.10 | |
| Turnip tops | NEU |
Mo: – RA: – |
No GAP compliant trials available | – | – | – | – |
GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level; PHI: preharvest interval.
* Indicates that the input value is proposed at the limit of quantification.
Mo: residue levels expressed according to the monitoring residue definition; RA: residue levels expressed according to risk assessment residue definition.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
In the absence of residue data for metabolite M25 included in the RD‐RA, the CF was derived from the same commodities or group of commodities. For pulses, a CF of 1.3 was based on beans/peas without pods, whereas for peanuts and soya beans the CF of 1.2 was based on rapeseed.
MRL proposal is tentative because additional trials are required.
MRL proposal is tentative as supporting trials were overdosed.
Value from trials performed with 2 applications instead 3.
Based on trials on parsley and chervil only. Trials on sage and savoury disregarded.
Tentative MRLs are derived for feed commodities in view of the future need to set MRLs in these commodities.
B.1.2.2. Residues in rotational crops
(a) Overall summary

(b) Summary of residues data from the rotational crops residue trials
| Commodity (Relevant crop group/extrapolation) | Region/Indoora | PBI (days)b | Residue levels observed in the supervised residue trials | Comments/Source | Calculated MRLc (mg/kg) | HRd (mg/kg) | STMRe (mg/kg) | CFf |
|---|---|---|---|---|---|---|---|---|
| Carrot and turnip root (root and tuber) | NEU | 30 |
Mo: < 0.01; 0.01 RA: < 0.02; 0.02 |
Rotational crops field trials conducted at a dose rate of application covering the max PECsoil for parent (~ 1.2N) (Germany, 2011) | 0.1 | 0.05 | 0.02 | 1 |
| SEU | 30 |
Mo: 0.02; 0.05 RA: 0.03; 0.06 |
||||||
| NEU | 90/216 |
Mo: < 0.01; 0.02 RA: < 0.02; 0.03 |
– | 0.01 | 0.01 | |||
| SEU | 120 |
Mo: 0.03 RA: 0.04 |
||||||
| NEU | 320 |
Mo: < 0.01 RA: < 0.02 |
0.01* | 0.01 | 0.01 | |||
| SEU | 365 |
Mo: < 0.01 RA: < 0.02 |
||||||
| USA | 228–245 |
Mo: 3× < 0.01 RA: 3× < 0.02 |
||||||
|
Carrot and turnip top (leaves and tops) |
NEU | 30 |
Mo: 0.02; 0.04 RA: 0.03; 0.05 |
0.09 | 0.04 | 0.03 | 1 | |
| SEU | 30 |
Mo: 0.04; 0.01 RA: 0.05; 0.02 |
||||||
| NEU | 90/216 |
Mo: < 0.01; 0.04 RA: < 0.02; 0.05 |
0.04 | 0.02 | 0.01 | |||
| SEU | 154/240 |
Mo: 0.02; 0.01 RA: 0.03; 0.02 |
||||||
|
Potato (tuber vegetables) |
NEU | 30 |
Mo: 2× 0.02 RA: 2× 0.03 |
Rotational crops field trials conducted at a dose rate of application covering the max PECsoil for parent (~ 1.2N) (EFSA, 2014) | 0.06 | 0.02 | 0.02 | 1 |
| SEU | 30 |
Mo: 2× 0.02 RA: 2× 0.03 |
||||||
|
Spinach and lettuce (leafy vegetables) |
NEU | 30 |
Mo: 0.01; 0.02; 2× 0.03 RA: 0.02; 0.03; 2× 0.04 |
Rotational crops field trials conducted at a dose rate of application covering the max PECsoil for parent (Germany, 2011; EFSA, 2014) | 0.15 | 0.09 | 0.03 | 1 |
| SEU | 30 |
Mo: < 0.01; 0.02; 0.03; 0.09 RA: < 0.02; 0.03; 0.04; 0.1 |
||||||
| NEU | 90/230 |
Mo: 2× 0.01 RA: 2× 0.02 |
0.02 | 0.01 | 0.01 | |||
| SEU |
155/240/ 320 |
Mo: 3× < 0.01 RA: 3× < 0.02 |
||||||
|
Mustard green (Brassica vegetables) |
USA | 228–245 |
Mo: < 0.01; 0.01; 0.035 RA: – |
Indicative rotational crop field studies performed at 1.2N PECsoil, but considered less representative of European uses (Germany, 2011) | – | 0.035 | 0.01 | – |
|
Cotton seeds (Oil seeds) |
USA/CAN | 12–14 |
Mo: 11× < 0.01 RA:– |
0.01* | 0.01 | 0.01 | – | |
|
Cotton gin by‐product (feed by‐product) |
USA/CAN | 12–14 |
Mo: 9× < 0.01; 2× 0.02 RA:– |
0.03 | 0.02 | 0.01 | – | |
|
Wheat grain (cereals) |
NEU | 28–30 |
Mo: 2× < 0.01 RA: 2× < 0.02 |
Rotational crop field studies performed at ~ 1.2N PECsoil (Germany, 2011) | 0.02 | 0.01 | 0.01 | 1 |
| SEU | 30 |
Mo: < 0.01; 0.01 RA: < 0.02; 0.02 |
||||||
| NEU | 100–286 |
Mo: 3× < 0.01 RA: 3× < 0.02 |
0.01* | 0.01 | 0.01 | |||
| SEU | 120–154 |
Mo: 2× < 0.01 RA: 2× < 0.02 |
||||||
|
Wheat green material (forage) |
NEU | 28–30 |
Mo: 0.07; 0.12 RA: 0.08; 0.13 |
0.3 | 0.12 | 0.11 | 1 | |
| SEU | 30–49 |
Mo: 2× 0.11 RA: 2× 0.12 |
||||||
| NEU | 100–146 |
Mo: 0.08; 0.05 RA: 0.09; 0.06 |
0.15 | 0.05 | 0.04 | |||
| SEU | 120–154 |
Mo: 0.07; 0.05 RA: 0.08; 0.06 |
||||||
| NEU | 286 |
Mo: 0.1 RA: 0.11 |
||||||
|
Wheat straw (straw and fodder) |
NEU | 28–30 |
Mo: 0.07; 0.28 RA: 0.12; 0.33 |
0.7 | 0.28 | 0.11 | 1.5 | |
| SEU | 30–49 |
Mo: 0.15; 0.05 RA: 0.2; 0.19 |
||||||
| NEU | 100–146 |
Mo: 0.09; 0.17 RA: 0.14; 0.22 |
0.4 | 0.19 | 0.09 | 1.5 | ||
| SEU | 120–154 |
Mo: < 0.05; 0.19 RA: < 0.1; 0.24 |
||||||
| NEU | 286 |
Mo: 0.06 RA: 0.11 |
PBI: plant‐back interval; GAP: Good Agricultural Practice; OECD: Organisation for Economic Co‐operation and Development; MRL: maximum residue level; PECsoil: predicted environmental concentration in soil.
* Indicates that the input value is proposed at the limit of quantification.
Mo: residue levels expressed according to the monitoring residue definition; RA: residue levels expressed according to risk assessment residue definition.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Country code: if non‐EU trials.
Plant‐back interval (PBI): The interval (days, months, years) between the final application of a pesticide product to a primary crop and the planting of a rotational crop.
Based on the shortest nominal PBI of 30 days representing crop failure.
Highest residue. The highest residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment (RA) refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment. When M25 was below the LOQ in all of the samples, a CF of 1 was derived.
(c) Summary of residues data from the combined primary and rotational crops uses
| Commodity | Primary crops (GAP used to derive MRL, including import tolerance (IT) uses) | Rotational crops | HRrotation > 25% HRprimary (Y/N) | Combined assessment, based on European GAPs (excluding IT) | CFa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median residue (mg/kg) | Highest residue (mg/kg) | MRL proposal (mg/kg) | Relevant crop group | Median residue (mg/kg) | Highest residue (mg/kg) | Median residue (mg/kg) | Highest residue (mg/kg) | MRL proposal (mg/kg) | |||
| Potatoes | 0.01 | 0.07 | 0.08 | Tuber | 0.02 | 0.02 | Y | 0.03b | 0.04b | 0.06b | 1 |
| Sweet potatoes, yams | 0.02 | 0.03 | 0.06 | Tuber | 0.02 | 0.02 | Y | 0.04 | 0.05 | 0.15 | 1 |
| Arrowroot, Cassava | n.r. | n.r. | n.r. | Tuber | 0.02 | 0.02 | n.r. | 0.02 | 0.02 | 0.06 | 1 |
| Beetroot | 0.03 | 0.05 | 0.06 | Root | 0.02 | 0.05 | Y | 0.05 | 0.1 | 0.2 | 1 |
| Carrots, radishes | 0.06 | 0.13 | 0.3 | Root | 0.02 | 0.05 | Y | 0.07b | 0.18 | 0.4 | 1 |
| Celeriac, horseradish, Jerusalem artichokes, parsnips, parsley root, salsify, swedes, turnips | 0.05 | 0.13 | 0.2 | Root | 0.02 | 0.05 | Y | 0.07 | 0.18 | 0.4 | 1 |
| Broccoli | 0.04 | 0.14 | 0.4 | Leafy and brassicas | 0.03 | 0.09 | Y | 0.07 | 0.23 | 0.5 | 1 |
| Cauliflowers | 0.01 | 0.05 | 0.1 | Leafy and brassicas | 0.03 | 0.09 | Y | 0.04 | 0.14 | 0.3 | 1 |
| Brussels sprouts | 0.04 | 0.14 | 0.3 | Leafy and brassicas | 0.03 | 0.09 | Y | 0.07 | 0.23 | 0.4 | 1 |
| Head cabbages | 0.01 | 0.08 | 0.15 | Leafy and brassicas | 0.03 | 0.09 | Y | 0.04 | 0.17 | 0.3 | 1 |
| Chinese cabbages/pe‐tsai | 0.36 | 0.84 | 2 (tentative)c | Leafy and brassicas | 0.03 | 0.09 | N | 0.36 | 0.84 | 2 (tentative)c | 1 |
| Kales, kohlrabies, watercresses | n.r. | n.r. | n.r. | Leafy and brassicas | 0.03 | 0.09 | n.r. | 0.03 | 0.09 | 0.15 | 1 |
| Lamb's lettuces, cresses and other sprouts and shoots, Roman rocket and purslanes (sea lavender), baby leaf crops | 1.60 | 10.00 | 20 | Leafy and brassicas | 0.03 | 0.09 | N | 1.60 | 10.00 | 20 | 1 |
| Lettuces | 1.50 | 10.00 | 15 | Leafy and brassicas | 0.03 | 0.09 | N | 1.50 | 10.00 | 15 | 1 |
| Escaroles, land cresses, red mustards, spinaches, chards/beet leaves | 0.37 | 0.98 | 2 (tentative)c | Leafy and brassicas | 0.03 | 0.09 | N | 0.37 | 0.98 | 2 (tentative)c | 1 |
| Herbs, and edible flowers | 0.38 | 3.64 | 6 | Leafy and brassicas | 0.03 | 0.09 | N | 0.38 | 3.64 | 6 | 1 |
| Basil and edible flowers | 19.07 | 30.00 | 60 | Leafy and brassicas | 0.03 | 0.09 | N | 19.07 | 30.00 | 60 | 1 |
| Barley, oat (grain) | 0.02 | 0.11 | 0.2 | Cereals | < 0.01 | 0.01 | N | 0.02 | 0.11 | 0.2 | 1 |
| Buckwheat, millet, rice (grain) | n.r. | n.r. | n.r. | Cereals | < 0.01 | 0.01 | n.r. | < 0.01 | 0.01 | 0.02 | 1 |
| Maize (grain), sweet corn | 0.01* | 0.01* | 0.01* | Cereals | 0.01* | 0.01 | Y | 0.01* | 0.01 | 0.02d | 1 |
| Rye, wheat (grain) | 0.72 | 0.19 | 0.9 | Cereals | 0.02 | 0.02 | N | 0.72 | 0.19 | 0.9 | 1 |
| Sorghum (grain) | 3.03 | 0.36 | 4 | Cereals | 0.02 | 0.02 | N | 3.03 | 0.36 | 4 | 1 |
| Herbal infusions from flowers, leaves and herbs | 2.24a | 25.2a | 40a | Leafy and Brassicas | 0.03 × 10(DF) | 0.09 × 10 (DF) | N | 0.3 | 0.9 | 40 | 1 |
| Herbal infusions (dried, roots) | n.r. | n.r. | n.r. | Root | 0.02 × 10 (DF) | 0.05 × 10 (DF) | n.r. | 0.2 | 0.5 | 1 | 1 |
| Spices (other than roots) | 22.50 | 29.60 | 70 | Leafy and Brassicas | 0.02 | 0.05 | N | 22.50 | 29.60 | 70 | 1 |
| Spices, roots | n.r. | n.r. | n.r. | Root | 0.02 × 10 (DF) | 0.05 × 10 (DF) | n.r. | 0.2 | 0.5 | 1 | 1 |
| Sugar beet (roots) | n.r. | n.r. | n.r. | Root | 0.02 | 0.05 | n.r. | 0.02 | 0.05 | 0.1 | 1 |
| Chicory roots | n.c. | n.c. | n.c. | Root | 0.02 | 0.05 | n.c. | 0.02 | 0.05 | 0.1 tentativec | 1 |
| Barley, oat straw | 0.14 | 1.1 | 2 (tentative)a | Cereal (straw) | 0.11 | 0.28 | N | 0.14 | 1.1 | 2 (tentative)a | 1.1 |
| Maize/corn stover | 0.42 | 1.70 | 3 (tentative)a | Cereal (straw) | 0.06 | 0.14 | N | 0.42 | 1.70 | 3 (tentative)a | 1 |
| Wheat, rye straw | 0.15 | 1.1 | 2 (tentative)a | Cereal (straw) | 0.11 | 0.28 | N | 0.15 | 1.1 | 2 (tentative)a | 1.1 |
| Buckwheat, millet, rice (straw) | n.r. | n.r. | n.r. | Cereal (straw) | 0.06 | 0.14 | n.r. | 0.11 | 0.28 | (tentative)a | 1.1 |
| Turnip (top) | n.c. | n.c. | n.c. | Root (top) | 0.03 | 0.04 | n.c. | 0.03 | 0.04 | 0.09 (tentative)(c,f) | 1 |
| Barley, common millet, grass, maize, oat, rye, wheat (forage) | n.r. | n.r. | n.r. | Cereal (forage) | 0.22 | 0.24 | n.r. | 0.22 | 0.24 | 0.3 (tentative)a | 1.1 |
| Fodder beet (root) | n.r. | n.r. | n.r. | Root | 0.02 | 0.05 | n.c. | 0.04 | 0.1 | 0.15 (tentative)a | 1 |
| Fodder beet (top) | n.r. | n.r. | n.r. | Root (top) | 0.03 | 0.04 | n.c. | 0.06 | 0.06 | 0.09 (tentative)a | 1 |
GAP: Good Agricultural Practice; MRL: maximum residue level; HR: highest residue; n.c.: not conclusive as residues trials on primary crops are not available; n.r.: not registered for use on primary crops; DF: default drying factor.
* Indicates that the input value is proposed at the limit of quantification.
Conversion factor to recalculate residues according to the residue definition for monitoring and to the residue definition for risk assessment. As in succeeding crops M25 was above the LOQ only in cereal green material and straw, for which commodities residues following primary use were significantly higher, the CF was derived for primary crops are considered applicable for the combined uses.
Although residues derived from the import tolerance are higher compared to other European uses, for combining residues from rotation use with primary uses the most critical European use (NEU/SEU/EU) is considered.
MRL proposal is tentative, as additional trials are required to support the primary crop use.
Based on the rotational use, as a no residues are expected from primary use.
Based on recently adopted opinion on modification of MRL (EFSA, 2019d).
Tentative MRLs are derived for feed commodities in view of the future need to set MRLs in these commodities.
B.1.2.3. Processing factors
| Processed commodity | Number of valid studiesa | Processing Factor (PF) | CFP b | Comment/Source | |
|---|---|---|---|---|---|
| Individual values | Median PF | ||||
| Orange, pulp | 1 | 0.16 | 0.16 | – | Tentativec; EFSA (2011) |
| Orange, juice | 1 | 0.01 | 0.01 | – | Tentativec; EFSA (2011) |
| Orange, dried pulp | 1 | 0.93 | 0.93 | – | Tentativec; EFSA (2011) |
| Grape, washed berries | 4 | 0.5; 0.59; 0.66; 0.74 | 0.62 | 1.05 | EFSA (2011) |
| Wine grapes, juice | 4 | 0.10; 0.12; 0.14; 0.16; 0.54 | 0.14 | 1.2 | EFSA (2011); Germany (2011) |
| Wine grapes, dry pomace | 4 | 4.83; 5.88; 7.24; 7.50 | 6.56 | 1 | Germany (2011) |
| Wine grapes, wet pomace | 4 | 2.24; 3.14; 3.62; 3.89 | 3.38 | 1 | EFSA (2011); Germany (2011) |
| Wine grapes, must | 6 | 0.21; 2× 0.22; 0.31; 0.68, 1.08 | 0.26 | 1.1 | Germany (2011) |
| Wine grapes, red wine (unheated) | 4 | 0.14; 0.17; 0.19; 0.20 | 0.18 | 1.2 | Germany (2011) |
| Wine grapes, white wine | 2 | 0.64; 0.74 | 0.69 | 1 | Germany (2011) |
| Table grapes, dried (raisins) | 4 | 2.00; 2.44; 2.88; 3.2; 6.56 | 3.04 | 1 | Germany (2011) |
| Strawberries, jam | 4 | 0.28; 0.58; 0.63; 0.64 | 0.61 | 1.1 | Germany (2011) |
| Tomatoes, peeled and canned | 5 | 0.07; 0.18; 0.21; 0.25; 0.33 | 0.21 | 1.3 | Germany (2011) |
| Tomatoes, juice | 5 | 0.09; 0.27; 0.42; 0.44; 0.56 | 0.42 | 1.15 | Germany (2011) |
| Melons, peeled | 18 | 0.03; 0.05; 2× 0.06; 0.08, 0.09; 2× 0.11; 4× 0.13; 0.17; 0.20; 2× 0.25; 0.50 | 0.13 | 1 | EFSA (2011) |
| Apples, washed | 5 | 0.36; 0.43; 0.55; 0.7; 1.38 | 0.55 | 1 | EFSA (2011) |
| Apples, juice | 5 | 0.05; 2× 0.09; 0.13; 0.44 | 0.09 | 1.00 | EFSA (2011) |
| Apples, dry pomace | 4 | 5.45; 5.71; 7.64; 11.88 | 6.68 | 1.01 | EFSA (2011) |
| Apples, wet pomace | 5 | 1.73; 1.24; 2.26; 4.13; 2.45; | 2.26 | 1.05 | EFSA (2011) |
| Apples, sauce | 5 | 0.01; 0.24; 2× 0.36; 0.63 | 0.36 | 1.30 | EFSA (2011) |
| Bananas, peeled | 4 | 0.82; 1.47; 0.44; 1.15 | 0.98 | 1.2 | EFSA (2011) |
| Rapeseeds, crude oil | 4 | 1.00; 1.25; 1.27; 2.14 | 1.26 | 1.12 | EFSA (2011) |
| Rapeseeds, refined oil | 4 | 0.64; 0.83; 1.00; 1.71 | 0.92 | 1.17 | EFSA (2011) |
| Rapeseeds, meal/press cake | 4 | 0.67; 0.71; 0.75; 1.27 | 0.73 | 1.29 | EFSA (2011) |
| Potato tuber, peeled | 1 | 0.67 | 0.67 | – | Tentativec; EFSA (2011) |
| Sugar beet/Refined sugar | 1 | 1.27 | 1.27 | – | Tentativec; EFSA (2011) |
| Sugar beet/Molasses | 1 | 0.92 | 0.92 | – | Tentativec; EFSA (2011) |
| Sugar beet/Pulp (dried) | 1 | 1.27 | 1.27 | – | Tentativec; EFSA (2011) |
| Peanut, meal/press cake | 1 | 0.19 | 0.19 | – | Tentativec; EFSA (2011) |
| Peanut, refined oil | 1 | 0.24 | 0.24 | – | Tentativec; EFSA (2011) |
PF: Processing factor (= Residue level in processed commodity expressed according to RD‐Mo/Residue level in raw commodity expressed according to RD‐Mo).
CFp: Conversion factor for risk assessment in processed commodity (= Residue level in processed commodity expressed according to RD‐RA/Residue level in processed commodity expressed according to RD‐Mo).
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Median of the individual conversion factors for each processing residues trial.
A tentative PF is derived based on a limited data set.
B.2. Residues in livestock
(a) Dietary Burden based on residues from primary uses (Option 1)
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Y/N) | ||||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Median | Maximum | Median | Maximum | |||||
| Cattle (all diets) | 0.032 | 0.050 | 0.97 | 1.63 | Dairy cattle | Corn, field | Stover | Yes |
| Cattle (dairy only) | 0.032 | 0.050 | 0.83 | 1.29 | Dairy cattle | Corn, field | Stover | Yes |
| Sheep (all diets) | 0.042 | 0.061 | 0.99 | 1.48 | Lamb | Wheat | Milled by‐pdts | Yes |
| Sheep (ewe only) | 0.031 | 0.049 | 0.94 | 1.48 | Ram/Ewe | Potato | Process waste | Yes |
| Swine (all diets) | 0.030 | 0.040 | 1.01 | 1.56 | Swine (finishing) | Wheat | Milled by‐pdts | Yes |
| Poultry (all diets) | 0.045 | 0.056 | 0.64 | 0.82 | Poultry layer | Wheat | Milled by‐pdts | Yes |
| Poultry (layer only) | 0.044 | 0.056 | 0.64 | 0.82 | Poultry layer | Wheat | Milled by‐pdts | Yes |
bw: body weight; DM: dry matter.
When several diets are relevant (e.g. cattle, sheep and poultry ‘all diets’), the most critical diet is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘g/kg bw per day’.
(b) Dietary Burden based on residues from combined primary and rotational uses (Option 2)
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroupa | Most critical commodityb | Trigger exceeded (Y/N) | ||||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Median | Maximum | Median | Maximum | |||||
| Cattle (all diets) | 0.074 | 0.092 | 2.48 | 3.13 | Dairy cattle | Potato | Process waste | Yes |
| Cattle (dairy only) | 0.074 | 0.092 | 1.93 | 2.39 | Dairy cattle | Potato | Process waste | Yes |
| Sheep (all diets) | 0.083 | 0.101 | 2.50 | 3.04 | Ram/Ewe | Potato | Process waste | Yes |
| Sheep (ewe only) | 0.083 | 0.101 | 2.50 | 3.04 | Ram/Ewe | Potato | Process waste | Yes |
| Swine (all diets) | 0.037 | 0.051 | 1.61 | 2.21 | Swine (breeding) | Potato | Process waste | Yes |
| Poultry (all diets) | 0.049 | 0.061 | 0.72 | 0.90 | Poultry layer | Swede | Roots | Yes |
| Poultry (layer only) | 0.049 | 0.061 | 0.72 | 0.90 | Poultry layer | Swede | Roots | Yes |
bw: body weight; DM: dry matter.
When several diets are relevant (e.g. cattle, sheep and poultry ‘all diets’), the most critical diet is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.2.1. Nature of residues and methods of analysis in livestock
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | Comment/Source |
|---|---|---|---|---|
| Laying hens | 2.03 or 2.02 | 14 | Radiolabelled active substance: phenyl‐UL‐14C and Pyridyl‐2,6‐14C (Germany, 2011) | |
| Lactating ruminants | 1.91 | 5 | Goat, radiolabelled active substance: phenyl‐UL‐14C and Pyridyl‐2,6‐14C (Germany, 2011) | |
| Fish | 60 μg as/L water | 7 or 14 | Radiolabelled active substance: Pyridyl‐2,6‐14C (Germany, 2011) |
bw: body weight.

B.2.1.2. Stability of residues in livestock
Storage stability studies are not available. As all samples of the feeding studies were stored ≤ −18°C and analysed within 30 days of collection further storage stability studies are not required (Germany, 2011).
B.2.2. Magnitude of residues in livestock
(a) Summary of the residue data from livestock feeding studies based on residues from primary uses (Option 1)
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CFc | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | |||
| Cattle (all) – Closest feeding level (0.04 mg/kg bw; 0.8 N rate)d | ||||||
| Muscle | 0.02 | 0.02 | 0.02 | 0.09 | 0.1 | 1.0 |
| Fat | 0.02 | 0.02 | 0.02 | 0.08 | 0.09 | 1.4 |
| Liver | 0.34 | 0.36 | 0.27 | 0.49 | 0.5 | 1.0 |
| Kidney | 0.03 | 0.03 | 0.02 | 0.08 | 0.08 | 1.0 |
| Cattle (dairy only) – Closest feeding level (0.04 mg/kg bw; 0.8 N rate)d | ||||||
| Milke | 0.03 | 0.04 | 0.02 | 0.05 | 0.05 | 1.0 |
| Sheep (all) – Closest feeding level (0.04 mg/kg bw; 0.7 N rate)d | ||||||
| Muscle | 0.02 | 0.02 | 0.05 | 0.10 | 0.1 | 1.0 |
| Fat | 0.02 | 0.02 | 0.04 | 0.09 | 0.09 | 1.4 |
| Liver | 0.34 | 0.36 | 0.37 | 0.53 | 0.6 | 1.0 |
| Kidney | 0.03 | 0.03 | 0.04 | 0.09 | 0.09 | 1.0 |
| Sheep (ewe only) – Closest feeding level (0.04 mg/kg bw; 0.8 N rate)d | ||||||
| Milke | 0.03 | 0.04 | 0.02 | 0.05 | 0.05 | 1.0 |
| Swine (all) – Closest feeding level (0.04 mg/kg bw; 1.0 N rate)d | ||||||
| Muscle | 0.02 | 0.02 | 0.02 | 0.09 | 0.09 | 1.0 |
| Fat | 0.02 | 0.02 | 0.02 | 0.08 | 0.08 | 1.4 |
| Liver | 0.34 | 0.36 | 0.26 | 0.44 | 0.5 | 1.0 |
| Kidney | 0.03 | 0.03 | 0.02 | 0.07 | 0.08 | 1.0 |
| Poultry (all) – Closest feeding level (0.035 mg/kg bw; 0.6 N rate)d | ||||||
| Muscle | 0.04 | 0.04 | 0.04 | 0.06 | 0.06 | 1.0 |
| Fat | 0.04 | 0.04 | 0.05 | 0.06 | 0.06 | 1.3 |
| Liver | 0.16 | 0.16 | 0.20 | 0.24 | 0.3 | 1.0 |
| Poultry (layer only) – Closest feeding level (0.035 mg/kg bw; 0.6 N rate)d | ||||||
| Eggsf | 0.08 | 0.09 | 0.1 | 0.13 | 0.15 | 1.0 |
Median residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the median dietary burden.
Highest residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the maximum dietary burden.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
Closest feeding level and N dose rate related to the maximum dietary burden.
Highest residue level from day 21 to day 29 (daily mean of 3 cows).
Highest residue level from day 21 to day 28 (daily mean of 3 laying hens).
(b) Summary of the residue data from livestock feeding studies based on residues from combined primary and rotational uses (Option 2)
| Animal commodity | Residues at the closest feeding level (mg/kg) | Estimated value at 1N | MRL proposal (mg/kg) | CFc | ||
|---|---|---|---|---|---|---|
| Mean | Highest | STMRMo a (mg/kg) | HRMo b (mg/kg) | |||
| Cattle (all) – Closest feeding level (0.04 mg/kg bw; 0.4 N rate)d | ||||||
| Muscle | 0.02 | 0.02 | 0.07 | 0.13 | 0.15 | 1.0 |
| Fat | 0.02 | 0.02 | 0.05 | 0.11 | 0.15 | 1.4 |
| Liver | 0.34 | 0.36 | 0.51 | 0.71 | 0.8 | 1.0 |
| Kidney | 0.03 | 0.03 | 0.06 | 0.11 | 0.15 | 1.0 |
| Cattle (dairy only) – Closest feeding level (0.04 mg/kg bw; 0.4 N rate)d | ||||||
| Milke | 0.02 | 0.02 | 0.04 | 0.06 | 0.07 | 1.0 |
| Sheep (all) – Closest feeding level (0.04 mg/kg bw; 0.4 N rate)d | ||||||
| Muscle | 0.02 | 0.02 | 0.07 | 0.13 | 0.15 | 1.0 |
| Fat | 0.02 | 0.02 | 0.05 | 0.11 | 0.15 | 1.4 |
| Liver | 0.34 | 0.36 | 0.53 | 0.70 | 0.8 | 1.0 |
| Kidney | 0.03 | 0.03 | 0.06 | 0.11 | 0.15 | 1.0 |
| Sheep (ewe only) – Closest feeding level (0.04 mg/kg bw; 0.4 N rate)d | ||||||
| Milke | 0.02 | 0.02 | 0.05 | 0.06 | 0.06 | 1.0 |
| Swine (all) – Closest feeding level (0.04 mg/kg bw; 0.8 N rate)d | ||||||
| Muscle | 0.02 | 0.02 | 0.02 | 0.09 | 0.1 | 1.0 |
| Fat | 0.02 | 0.02 | 0.02 | 0.08 | 0.09 | 1.4 |
| Liver | 0.34 | 0.36 | 0.32 | 0.49 | 0.5 | 1.0 |
| Kidney | 0.03 | 0.03 | 0.03 | 0.08 | 0.08 | 1.0 |
| Poultry (all) – Closest feeding level (0.035 mg/kg bw; 0.6 N rate)d | ||||||
| Muscle | 0.04 | 0.04 | 0.05 | 0.06 | 0.07 | 1.0 |
| Fat | 0.04 | 0.04 | 0.05 | 0.07 | 0.07 | 1.3 |
| Liver | 0.16 | 0.16 | 0.21 | 0.26 | 0.3 | 1.0 |
| Poultry (layer only) – Closest feeding level (0.035 mg/kg bw; 0.6 N rate)d | ||||||
| Eggsf | 0.07 | 0.08 | 0.10 | 0.13 | 0.15 | 1.0 |
Median residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the median dietary burden.
Highest residues expressed according to the residue definition for monitoring, recalculated at the 1N rate for the maximum dietary burden.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
Closest feeding level and N dose rate related to the maximum dietary burden.
Highest residue level from day 21 to day 29 (daily mean of 3 cows).
Highest residue level from day 21 to day 28 (daily mean of 3 laying hens).
B.3. Consumer risk assessment
B.3.1. Consumer risk assessment without consideration of the existing CXLs



B.3.2. Consumer risk assessment with consideration of the existing CXLs


B.4. Proposed MRLs
| Code number | Commodity | Existing EU MRL (mg/kg) | Existing CXL (mg/kg) | Outcome of the review | |||
|---|---|---|---|---|---|---|---|
| Option 1 (PBIs, restriction on indoor tomato use) | Option 2 (Restriction on indoor tomato use) | ||||||
| MRL (mg/kg) | Comment | MRL (mg/kg) | Comment | ||||
| Enforcement residue definition: Fluopyram | |||||||
| 110010 | Grapefruit | 0.4 | 0.4 | 0.5 | Recommendeda | 0.5 | Recommendeda |
| 110020 | Oranges | 0.6 | 0.6 | 0.6 | Recommendedb | 0.5 | Further consideration neededc |
| 110030 | Lemons | 1 | 1 | 1 | Recommendedd | 0.9 | Further consideration needede |
| 110040 | Limes | 1 | 1 | 1 | Further consideration neededf | 1 | Further consideration neededf |
| 110050 | Mandarins | 0.6 | 0.6 | 0.9 | Further consideration neededg | 0.9 | Further consideration neededg |
| 120010 | Almonds | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120020 | Brazil nuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120030 | Cashew nuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120040 | Chestnuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120050 | Coconuts | 0.04 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120060 | Hazelnuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120070 | Macadamia | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120080 | Pecans | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120090 | Pine nuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120100 | Pistachios | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 120110 | Walnuts | 0.05 | 0.04 | 0.04 | Recommendedb | 0.03 | Further consideration neededc |
| 130010 | Apples | 0.6 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130020 | Pears | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130030 | Quinces | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130040 | Medlar | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 130050 | Loquat | 0.5 | 0.5 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 140010 | Apricots | 1.5 | 1 | 1.5 | Recommendeda | 1.5 | Recommendeda |
| 140020 | Cherries | 2 | 2 | 2 | Recommendedd | 2 | Recommendedd |
| 140030 | Peaches | 1.5 | 1 | 1.5 | Recommendeda | 1.5 | Recommendeda |
| 140040 | Plums | 0.5 | 0.5 | 0.6 | Recommendeda | 0.6 | Recommendeda |
| 151010 | Table grapes | 1.5 | 2 | 2 | Recommendeda | 2 | Recommendeda |
| 151020 | Wine grapes | 1.5 | 2 | 2 | Recommendedb | 1.5 | Further consideration neededc |
| 152000 | Strawberries | 2 | 0.4 | 2 | Recommendeda | 2 | Recommendeda |
| 153010 | Blackberries | 5 | 5 | 5 | Recommendeda | 5 | Recommendeda |
| 153020 | Dewberries | 5 | 5 | 5 | Recommendeda | 5 | Recommendeda |
| 153030 | Raspberries | 5 | 5 | 5 | Recommendeda | 5 | Recommendeda |
| 154010 | Blueberries | 7 | 7 | 7 | Recommendeda | 7 | Recommendeda |
| 154020 | Cranberries | 3 | – | 4 | Recommendedh | 4 | Recommendedh |
| 154030 | Currants (red, black and white) | 7 | 7 | 7 | Recommendedb | 4 | Further consideration neededc |
| 154040 | Gooseberries | 7 | 7 | 7 | Recommendedb | 4 | Further consideration neededc |
| 154050 | Rose hips | 7 | 7 | 7 | Recommendedb | 3 | Further consideration neededc |
| 154060 | Mulberries | 7 | – | 4 | Recommendedh | 4 | Recommendedh |
| 154080 | Elderberries | 7 | – | 4 | Recommendedh | 4 | Recommendedh |
| 163020 | Bananas | 0.8 | 0.8 | 0.8 | Further consideration neededg | 0.8 | Further consideration neededg |
| 163030 | Mangoes | 1 | 1 | 1 | Recommendedi | – | Further consideration neededj |
| 163060 | Cherimoyas | 0.01* | – | – | Further consideration neededk | – | Further consideration neededk |
| 211000 | Potatoes | 0.15 | 0.15 | 0.15 | Recommendedb | 0.08 | Further consideration neededc |
| 212010 | Cassava | 0.1 | – | – | Further consideration neededl | 0.06 | Recommendedh |
| 212020 | Sweet potatoes | 0.1 | – | 0.06 | Recommendedh | 0.15 | Recommendedh |
| 212030 | Yams | 0.1 | – | 0.06 | Recommendedh | 0.15 | Recommendedh |
| 212040 | Arrowroot | 0.1 | – | – | Further consideration neededl | 0.06 | Recommendedh |
| 213010 | Beetroot | 0.3 | – | 0.06 | Recommendedh | 0.2 | Recommendedh |
| 213020 | Carrots | 0.4 | 0.4 | 0.4 | Recommendedb | 0.4 | Recommendeda |
| 213030 | Celeriac | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213040 | Horseradish | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213050 | Jerusalem artichokes | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213060 | Parsnips | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213070 | Parsley root | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213080 | Radishes | 0.3 | – | 0.3 | Recommendedh | 0.4 | Recommendedh |
| 213090 | Salsify | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213100 | Swedes | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 213110 | Turnips | 0.3 | – | 0.2 | Recommendedh | 0.4 | Recommendedh |
| 220010 | Garlic | 0.1 | 0.07 | 0.07 | Recommendeda | 0.07 | Recommendeda |
| 220020 | Onions | 0.1 | 0.07 | 0.07 | Recommendeda | 0.07 | Recommendeda |
| 220030 | Shallots | 0.1 | – | 0.07 | Recommendedh | 0.07 | Recommendedh |
| 220040 | Spring onions | 15 | 15 | 15 | Recommendedb | 3 | Further consideration needede |
| 231010 | Tomatoes | 0.9 | 0.5 | 0.5 | Further consideration neededg | 0.5 | Further consideration neededg |
| 231020 | Peppers | 3 | 3 | 3 | Recommendedb | 2 | Further consideration neededc |
| 231030 | Aubergines (egg plants) | 0.9 | 0.5 | 0.5 | Recommendedb | 0.4 | Further consideration neededc |
| 232010 | Cucumbers | 0.5 | 0.5 | 0.6 | Recommendeda | 0.6 | Recommendeda |
| 232020 | Gherkins | 0.5 | – | 0.6 | Recommendedh | 0.6 | Recommendededh |
| 232030 | Courgettes | 0.5 | – | 0.6 | Recommendedh | 0.6 | Recommendedh |
| 233010 | Melons | 0.4 | – | 0.9 | Further consideration neededm | 0.9 | Further consideration neededm |
| 233020 | Pumpkins | 0.4 | – | 0.4 | Recommendedh | 0.4 | Recommendedh |
| 233030 | Watermelons | 0.4 | – | 0.4 | Further consideration neededm | 0.4 | Further consideration neededm |
| 234000 | Sweet corn | 0.01* | 0.01* | 0.01* | Recommendeda | 0.02 | Recommendeda |
| 241010 | Broccoli | 0.4 | 0.3 | 0.4 | Recommendeda | 0.5 | Recommendeda |
| 241020 | Cauliflower | 0.2 | 0.09 | 0.1 | Recommendeda | 0.3 | Recommendeda |
| 242010 | Brussels sprouts | 0.3 | 0.3 | 0.3 | Recommendeda | 0.4 | Recommendeda |
| 242020 | Head cabbage | 0.3 | 0.15 | 0.15 | Recommendeda | 0.3 | Recommendeda |
| 243010 | Chinese cabbage | 0.7 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 243020 | Kale | 0.1 | – | – | Further consideration neededl | 0.15 | Recommendedh |
| 244000 | Kohlrabi | 0.1 | – | – | Further consideration neededl | 0.15 | Recommendedh |
| 251010 | Lamb's lettuce | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 251020 | Lettuce | 15 | 15 | 15 | Recommendeda | 15 | Recommendeda |
| 251030 | Escarole (broad‐leaf endive) | 1.5 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 251040 | Cress | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 251050 | Land cress | 15 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 251060 | Rocket, Rucola | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 251070 | Redmustard | 15 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 251080 | Baby leaf crops | 15 | – | 20 | Recommendedh | 20 | Recommendedh |
| 252010 | Spinach | 0.2 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 252020 | Purslane | 20 | – | 20 | Recommendedh | 20 | Recommendedh |
| 252030 | Beet leaves (chard) | 0.2 | – | 2 | Further consideration neededm | 2 | Further consideration neededm |
| 254000 | Watercress | 0.1 | – | – | Further consideration neededl | 0.15 | Recommendedh |
| 255000 | Witloof | 0.3 | 0.15 | 0.3 | Recommendeda | 0.3 | Recommendeda |
| 256010 | Chervil | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256020 | Chives | 8 | – | 6 | Recommendedh | 6 | Recommendededh |
| 256030 | Celery leaves | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256040 | Parsley | 8 | – | 6 | Recommendedh | 6 | Recommendededh |
| 256050 | Sage | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256060 | Rosemary | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256070 | Thyme | 8 | – | 6 | Recommendedh | 6 | Recommendededh |
| 256080 | Basil | 70 | 70 | 70 | Recommendedb | 60 | Further consideration neededc |
| 256090 | Bay leaves (laurel) | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 256100 | Tarragon | 8 | – | 6 | Recommendedh | 6 | Recommendedh |
| 260010 | Beans (fresh, with pods) | 1 | 1 | 3 | Recommendeda | 3 | Recommendeda |
| 260020 | Beans (fresh, without pods) | 0.2 | 0.2 | 0.2 | Recommendedb | 0.15 | Further consideration neededc |
| 260030 | Peas (fresh, with pods) | 1.5 | – | 3 | Recommendedh | 3 | Recommendedh |
| 260040 | Peas (fresh, without pods) | 0.2 | 0.2 | 0.2 | Recommendedb | 0.15 | Further consideration neededc |
| 260050 | Lentils (fresh) | 0.2 | – | 0.15 | Recommendedh | 0.15 | Recommendedh |
| 270010 | Asparagus | 0.01* | 0.01* | 0.01* | Recommendeda | 0.01* | Recommendeda |
| 270030 | Celery | 0.01* | – | 20 | Recommendedh | 20 | Recommendedh |
| 270050 | Globe artichokes | 0.5 | 0.4 | 4 | Further consideration neededg | 4 | Further consideration neededg |
| 270060 | Leek | 0.7 | 0.15 | 0.8 | Further consideration neededg | 0.8 | Further consideration neededg |
| 300010 | Beans (dry) | 0.4 | 0.15 | 0.5 | Recommendeda | 0.5 | Recommendeda |
| 300020 | Lentils (dry) | 0.4 | 0.7 | 0.7 | Recommendedb | 0.5 | Further consideration neededc |
| 300030 | Peas (dry) | 0.4 | 0.7 | 0.7 | Recommendedb | 0.5 | Further consideration neededc |
| 300040 | Lupins (dry) | 0.4 | 0.15 | 0.5 | Recommendeda | 0.5 | Recommendeda |
| 401020 | Peanuts | 0.2 | 0.2 | 0.2 | Recommendedb | 0.02 | Further consideration neededc |
| 401030 | Poppy seed | 0.3 | – | 0.4 | Recommendedh | 0.4 | Recommendedh |
| 401050 | Sunflower seed | 0.7 | 0.7 | 0.7 | Recommendeda | 0.7 | Recommendeda |
| 401060 | Rape seed | 1 | 1 | 1 | Recommendeda | 1 | Recommendeda |
| 401070 | Soya bean | 0.3 | 0.3 | 0.3 | Recommendedb | 0.08 | Further consideration neededc |
| 401080 | Mustard seed | 0.3 | – | 0.4 | Recommendedh | 0.4 | Recommendedh |
| 401090 | Cotton seed | 0.8 | 0.8 | 0.8 | Recommendeda | 0.8 | Recommendeda |
| 500010 | Barley grain | 0.2 | 0.2 | 0.2 | Recommendeda | 0.2 | Recommendeda |
| 500020 | Buckwheat grain | 0.2 | – | – | Further consideration neededl | 0.02 | Recommendedh |
| 500030 | Maize grain | 0.02 | 0.02 | 0.02 | Recommendedb | 0.02 | Recommendeda |
| 500040 | Millet grain | 0.01* | – | – | Further consideration neededl | 0.02 | Recommendedh |
| 500050 | Oats grain | 0.2 | 0.2 | 0.2 | Recommendeda | 0.2 | Recommendeda |
| 500060 | Rice | 0.01* | 1.5 | – | Further consideration neededl | 0.02 | Recommendedh |
| 500070 | Rye grain | 0.9 | 0.9 | 0.9 | Recommendedb | 0.07 | Further consideration neededc |
| 500080 | Sorghum grain | 1.5 | – | 4 | Recommendedh | 4 | Recommendedh |
| 500090 | Wheat grain | 0.9 | 0.9 | 0.9 | Recommendeda | 0.9 | Recommendeda |
| 631000 | Herbal infusions (dried, flowers) | 0.1 | – | 40 | Recommendedh | 40 | Recommendedh |
| 632000 | Herbal infusions (dried, leaves) | 0.1 | – | 40 | Recommendedh | 40 | Recommendedh |
| 633000 | Herbal infusions (dried, roots) | 2.5 | – | – | Further consideration neededl | 1 | Recommendedh |
| 700000 | Hops (dried) | 50 | 50 | 60 | Recommendeda | 60 | Recommendeda |
| 810060 | Dill seeds | 70 | 70 | 70 | Recommendeda | 70 | Recommendeda |
| 840000 | Spices (roots and rhizome) | – | – | – | Further consideration neededl | 1 | Recommendedh |
| 900010 | Sugar beet (root) | 0.1 | 0.04 | 0.04 | Recommendedi | 0.1 | Further consideration neededc |
| 900030 | Chicory roots | 0.1 | – | – | Further consideration neededk | 0.1 | Further consideration neededk |
| Enforcement residue definition 2: Sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram | |||||||
| 1011010 | Swine muscle | 0.8 | 1.5 | 0.09 | Further consideration neededc | 0.1 | Further consideration neededc |
| 1011020 | Swine fat tissue | 0.5 | 1.5 | 0.08 | Further consideration neededc | 0.09 | Further consideration neededc |
| 1011030 | Swine liver | 5 | 8 | 0.50 | Further consideration neededc | 0.5 | Further consideration neededc |
| 1011040 | Swine kidney | 0.8 | 8 | 0.08 | Further consideration neededc | 0.08 | Further consideration neededc |
| 1012010 | Bovine muscle | 0.8 | 1.5 | 0.10 | Further consideration neededc | 0.15 | Further consideration neededc |
| 1012020 | Bovine fat tissue | 0.5 | 1.5 | 0.09 | Further consideration neededc | 0.15 | Further consideration neededc |
| 1012030 | Bovine liver | 5 | 8 | 0.50 | Further consideration neededc | 0.8 | Further consideration neededc |
| 1012040 | Bovine kidney | 0.8 | 8 | 0.08 | Further consideration neededc | 0.15 | Further consideration neededc |
| 1013010 | Sheep muscle | 0.8 | 1.5 | 1.5 | Recommendededb | 0.15 | Further consideration neededc |
| 1013020 | Sheep fat tissue | 0.5 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1013030 | Sheep liver | 5 | 8 | 8 | Recommendedb | 0.8 | Further consideration neededc |
| 1013040 | Sheep kidney | 0.8 | 8 | 8 | Recommendedb | 0.15 | Further consideration neededc |
| 1014010 | Goat muscle | 0.8 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1014020 | Goat fat tissue | 0.5 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1014030 | Goat liver | 5 | 8 | 8 | Recommendedb | 0.8 | Further consideration neededc |
| 1014040 | Goat kidney | 0.8 | 8 | 8 | Recommendedb | 0.15 | Further consideration neededc |
| 1015010 | Equine muscle | 0.8 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1015020 | Equine fat tissue | 0.5 | 1.5 | 1.5 | Recommendedb | 0.15 | Further consideration neededc |
| 1015030 | Equine liver | 0.7 | 8 | 8 | Recommendedb | 0.8 | Further consideration neededc |
| 1015040 | Equine kidney | 0.7 | 8 | 8 | Recommendedb | 0.15 | Further consideration neededc |
| 1016010 | Poultry muscle | 0.5 | 1.5 | 1.5 | Recommendedb | 0.07 | Further consideration neededc |
| 1016020 | Poultry fat tissue | 0.2 | 1 | 1 | Recommendedb | 0.07 | Further consideration neededc |
| 1016030 | Poultry liver | 2 | 5 | 5 | Recommendedb | 0.3 | Further consideration neededc |
| 1020010 | Cattle milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.07 | Further consideration neededh |
| 1020020 | Sheep milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.06 | Further consideration neededh |
| 1020030 | Goat milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.06 | Further consideration neededh |
| 1020040 | Horse milk | 0.6 | 0.8 | 0.05 | Recommendedh | 0.07 | Further consideration neededh |
| 1030000 | Birds eggs | 1 | 2 | 2 | Recommendedb | 0.15 | Further consideration neededc |
| – | Other commodities of plant and/or animal origin | See Reg. 2019/1791 | – | – | Further consideration neededl | ||
MRL: maximum residue level; CXL: codex maximum residue limit.
* Indicates that the input value is proposed at the limit of quantification.
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; existing CXL is covered by the recommended MRL (combination H‐III in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is also fully supported by data, leads to a lower MRL (combination H‐VII in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; CXL is higher, supported by data but a chronic risk to consumers cannot be excluded considering some (Option 1)/or all additional CXLs (Option 2) (combination H‐VI/VII in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; GAP evaluated at EU level, which is not fully supported by data, leads to a lower or same tentative MRL (combination F‐VII in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition; CXL is higher, supported by data but a chronic risk to consumers cannot be excluded considering some (Option 1)/or all additional CXLs (Option 2) (combination F‐VI/VII in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); existing CXL is covered by the existing EU MRL (combination D‐III in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); existing CXL is covered by the tentative MRL (combination F‐III in Appendix E).
MRL is derived from a GAP evaluated at EU level, which is fully supported by data and for which no risk to consumers is identified; no CXL is available or CXL was not considered further due to reservations raised by the EU delegation. (combination H‐I in Appendix E).
MRL is derived from the existing CXL, which is supported by data and for which no risk to consumers is identified; there are no relevant authorisations or import tolerances reported at EU level (combination A‐VII in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; CXL is supported by data but a chronic risk to consumers cannot be excluded considering all additional CXLs (Option 2). Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐VI in Appendix E).
GAP evaluated at EU level is not supported by data but no risk to consumers was identified for the existing EU MRL (also assuming the existing residue definition); no CXL is available (combination D‐I in Appendix E).
There are no relevant authorisations or import tolerances reported at EU level; no CXL is available or CXL was not considered further due to reservations raised by the EU delegation. Either a specific LOQ or the default MRL of 0.01 mg/kg may be considered (combination A‐I in Appendix E).
Tentative MRL is derived from a GAP evaluated at EU level, which is not fully supported by data but for which no risk to consumers was identified (assuming the existing residue definition); no CXL is available (combination F‐I in Appendix E).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
PRIMo(EU Option 1)
PRIMo(EU Option 2 and CXL2 Option)
PRIMo(CXL1 Option 1)
PRIMo(CXL1 Option 2)
PRIMo(CXL2 Option 1)



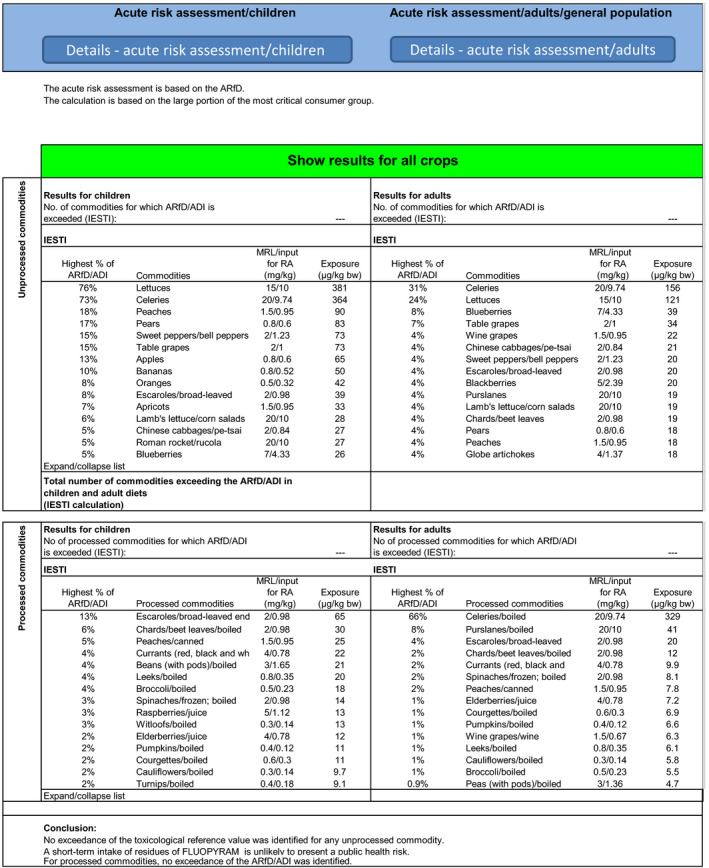



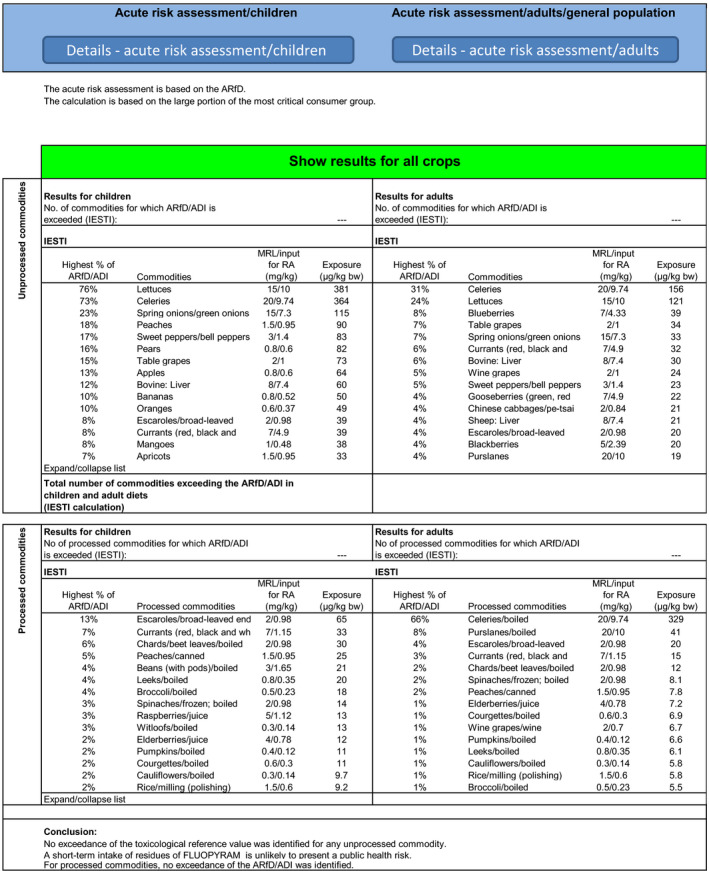
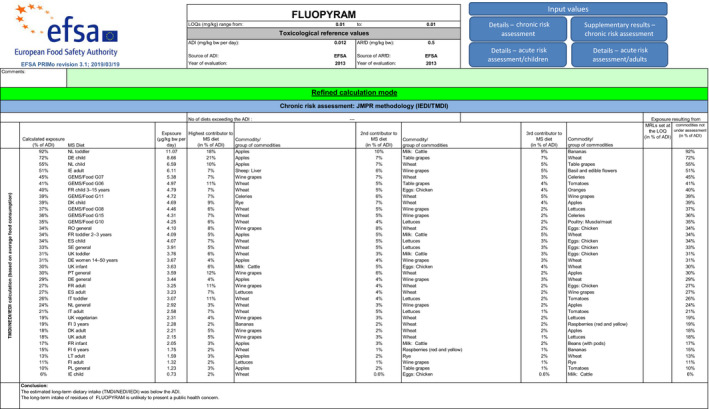

Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Residues from primary crops only (Option 1) | Residues from primary uses and rotational crops (Option 2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Median dietary burden | Maximum dietary burden | Median dietary burden | Maximum dietary burden | |||||
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram | ||||||||
| Grapefruits, dried pulp | 0.12 | STMR × PF (0.93) × CF (1.1)a | 0.12 | STMR × PF (0.93) × CF (1.1)a | 0.12 | STMR × PF (0.93) × CF (1.1)a | 0.12 | STMR × PF (0.93) × CF (1.1)a |
| Oranges, dried pulp | 0.12 | STMR × PF (0.93) × CF (1.1)a | 0.12 | STMR × PF (0.93) × CF (1.1)a | 0.12 | STMR × PF (0.93) × CF (1.1)a | 0.12 | STMR × PF (0.93) × CF (1.1)a |
| Lemons, dried pulp | 0.30 | STMR × PF (0.93) × CF (1.1)a | 0.30 | STMR × PF (0.93) × CF (1.1)a | 0.30 | STMR × PF (0.93) × CF (1.1)a | 0.30 | STMR × PF (0.93) × CF (1.1)a |
| Mandarins, dried pulp | 0.30 | STMR × PF (0.93) × CF (1.1)a | 0.30 | STMR × PF (0.93) × CF (1.1)a | 0.30 | STMR × PF (0.93) × CF (1.1)a | 0.30 | STMR × PF (0.93) × CF (1.1)a |
| Apple, pomace, wet | 0.48 | STMR × PF (2.26) × CF (1.05) | 0.48 | STMR × PF (2.26) × CF (1.05) | 0.48 | STMR × PF (2.26) × CF (1.05) | 0.48 | STMR × PF (2.26) × CF (1.05) |
| Potato, culls | 0.01 | STMR | 0.07 | HR | 0.03 | STMRb | 0.07 | HRb |
| Potato, process waste | 0.20 | STMR × default PF (20) | 0.20 | STMR × default PF (20) | 0.60 | STMRb × default PF (20) | 0.60 | STMRb × default PF (20) |
| Potato, dried pulp | 0.38 | STMR × default PF (38) | 0.38 | STMR × default PF (38) | 1.14 | STMRb × default PF (38) | 1.14 | STMRb × default PF (38) |
| Cassava/tapioca, roots | – | – | – | – | 0.02 | STMRb | 0.02 | HRb |
| Carrot, culls | 0.06 | STMR | 0.13 | HR | 0.10 | STMRb | 0.18 | HRb |
| Swede, roots | 0.05 | STMR | 0.13 | HR | 0.10 | STMRb | 0.18 | HR b |
| Turnip, roots | 0.05 | STMR | 0.13 | HR | 0.10 | STMRb | 0.18 | HRb |
| Cabbage, heads, leaves | 0.01 | STMR | 0.08 | HR | 0.01 | STMRb | 0.08 | HRb |
| Kale, leaves (forage) | – | – | – | – | 0.03 | STMRb | 0.09 | HRb |
| Bean, seed (dry) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) |
| Cowpea, seed | 0.04 | STMR × CF (1.3) | 0.04 | STMR × × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) |
| Pea (Field pea), seed (dry) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) |
| Lupin, seed | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) | 0.04 | STMR × CF (1.3) |
| Lupin seed, meal | 0.05 | STMR × default PF (1.1) × CF (1.3) | 0.05 | STMR × default PF (1.1) × CF (1.3) | 0.05 | STMR × default PF (1.1) × CF (1.3) | 0.05 | STMR × default PF (1.1) × CF (1.3) |
| Peanut, meal | 0.02 | STMR × default PF (2) × CF (1.2) | 0.02 | STMR × default PF (2) CF (1.2) | 0.02 | STMR × default PF (2) × CF (1.2) | 0.02 | STMR × default PF (2) × CF (1.2) |
| Sunflower, meal | 0.15 | STMR × default PF (2) | 0.15 | STMR × default PF (2) | 0.15 | STMR × default PF (2) | 0.15 | STMR × default PF (2) |
| Canola (Rape seed), meal | 0.32 | STMR × PF (0.73) × CF (1.29) | 0.32 | STMR × PF (0.73) × CF (1.29) | 0.32 | STMR × PF (0.73) × CF (1.29) | 0.32 | STMR × PF (0.73) × CF (1.29) |
| Rape, meal | 0.32 | STMR × PF (0.73) × CF (1.29) | 0.32 | STMR × PF (0.73) × CF (1.29) | 0.32 | STMR × PF (0.73) × CF (1.29) | 0.32 | STMR × PF (0.73) × CF (1.29) |
| Soybean, seed | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) |
| Soybean, meal | 0.00 | STMR × PF (0.047) × CF (1.2) | 0.00 | STMR × PF (0.047) × CF (1.2) | 0.00 | STMR × PF (0.047) × CF (1.2) | 0.00 | STMR × PF (0.047) × CF (1.2) |
| Soybean, hulls | 0.02 | STMR × PF (1.31) × CF (1.2) | 0.02 | STMR × PF (1.31) × CF (1.2) | 0.02 | STMR × PF (1.31) × CF (1.2) | 0.02 | STMR × PF (1.31) × CF (1.2) |
| Cotton, undelinted seed | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) |
| Cotton, meal | 0.09 | STMR × default PF (1.25) × CF (1.2) | 0.09 | STMR × default PF (1.25) × CF (1.2) | 0.09 | STMR × default PF (1.25) × CF (1.2) | 0.09 | STMR × default PF (1.25) × CF (1.2) |
| Barley, grain | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR |
| Brewer's grain, dried | 0.07 | STMR × default PF (3.3) | 0.07 | STMR × default PF (3.3) | 0.07 | STMR × default PF (3.3) | 0.07 | STMR × default PF (3.3) |
| Corn, field (Maize), grain | 0.01* | STMR | 0.01* | STMR | 0.01* | STMR | 0.01* | STMR |
| Corn, pop, grain | 0.01* | STMR | 0.01* | STMR | 0.01* | STMR | 0.01* | STMR |
| Corn, field, milled by‐pdts | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc |
| Corn, field, hominy meal | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc |
| Corn, field, distiller's grain (dry) | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc |
| Corn, field, gluten feed | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc |
| Corn, field, gluten, meal | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc | 0.01* | STMRc |
| Millet, grain | – | – | – | – | 0.01 | STMR | 0.01 | STMR |
| Oat, grain | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR |
| Rye, grain | 0.01 | STMR | 0.01 | STMR | 0.01 | STMR | 0.01 | STMR |
| Sorghum, grain | 0.36 | STMR | 0.36 | STMR | 0.36 | STMR | 0.36 | STMR |
| Triticale, grain | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR |
| Wheat, grain | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR |
| Wheat, distiller's grain (dry) | 0.63 | STMR × default PF (3.3) | 0.63 | STMR × default PF (3.3) | 0.63 | STMR × default PF (3.3) | 0.63 | STMR × default PF (3.3) |
| Wheat gluten, meal | 0.34 | STMR × default PF (1.8) | 0.34 | STMR × default PF (1.8) | 0.34 | STMR × default PF (1.8) | 0.34 | STMR × default PF (1.8) |
| Wheat, milled by‐pdts | 1.33 | STMR × default PF (7) | 1.33 | STMR × default PF (7) | 1.33 | STMR × default PF (7) | 1.33 | STMR × default PF (7) |
| Beet, sugar, dried pulp | – | – | – | – | 0.18 | STMRb × default PF (18) | 0.18 | STMRb × default PF (18) |
| Beet, sugar, ensiled pulp | – | – | – | – | 0.03 | STMRb × default PF (3) | 0.03 | STMRb × default PF (3) |
| Beet, sugar, molasses | – | – | – | – | 0.28 | STMRb × default PF (28) | 0.28 | STMRb × default PF (28) |
| Barley, forage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Barley, silage | – | – | – | – | 0.21 | STMRb × default PF (1.3) × CF (1.5) | 0.55 | HRb × default PF (1.3) × CF (1.5) |
| Millet, forage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Corn, field, forage/silage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Oat, forage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Oat, hay | – | – | – | – | 0.50 | STMRb × default PF (3) × CF (1.5) | 1.26 | HRb × default PF (3) × CF (1.5) |
| Rye, forage (greens) | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Sorghum, grain, forage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Sorghum, grain, silage | – | – | – | – | 0.10 | STMRb × default PF (0.6) × CF (1.5) | 0.25 | HRb × default PF (0.6) × CF (1.5) |
| Triticale, forage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Triticale, hay | – | – | – | – | 0.48 | STMRb × default PF (2.9) × CF (1.5) | 1.22 | HRb × default PF (2.9) × CF (1.5) |
| Wheat, forage | – | – | – | – | 0.17 | STMRb × CF (1.5) | 0.42 | HRb × CF (1.5) |
| Wheat, hay (fodder dry) | – | – | – | – | 0.58 | STMRb × default PF (3.5) × CF (1.5) | 1.47 | HRb × default PF (3.5) × CF (1.5) |
| Barley, straw | 0.15 | STMR × CF (1.1) | 1.21 | HR × CF (1.1) | 0.15 | STMRb × CF (1.1) | 1.21 | HRb × CF (1.1) |
| Corn, field, stover (fodder) | 0.42 | STMR | 1.70 | HR | 0.42 | STMR | 1.70 | HR |
| Corn, pop, stover | 0.42 | STMR | 1.70 | HR | 0.42 | STMR | 1.70 | HR |
| Oat, straw | 0.15 | STMR × CF (1.1) | 1.21 | HR × CF (1.1) | 0.15 | STMRb × CF (1.1) | 1.21 | HRb × CF (1.1) |
| Rye, straw | 0.17 | STMR × CF (1.1) | 1.21 | HR × CF (1.1) | 0.17 | STMRb × CF (1.1) | 1.21 | HRb × CF (1.1) |
| Triticale, straw | 0.17 | STMR × CF (1.1) | 1.21 | HR × CF (1.1) | 0.17 | STMRb × CF (1.1) | 1.21 | HRb × CF (1.1) |
| Wheat, straw | 0.17 | STMR × CF (1.1) | 1.21 | HR × CF (1.1) | 0.17 | STMRb × CF (1.1) | 1.21 | HRb × CF (1.1) |
| Beet, mangel, roots | – | – | – | – | 0.01 | STMRb | 0.01 | HRb |
| Beet, mangel, tops | – | – | – | – | 0.01 | STMRb | 0.01 | HRb |
| Beet, sugar, tops | – | – | – | – | 0.01 | STMRb | 0.01 | HRb |
STMR: supervised trials median residue; HR: highest residue; PF: processing factor. In the absence of processing factors supported by data, default processing factors were included in the calculation to consider the potential concentration of residues in these commodities.
* Indicates that the input value is proposed at the limit of quantification.
Tentative PF, based on only 1 value.
The STMR and HR values reflect the combined residues from both primary and rotational crops (sum of the HR/STMR values).
For corn, field by‐products no default processing factor was applied because residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
D.2. Consumer risk assessment without consideration of the existing CXLs, and no risk mitigation implemented to avoid residues from rotational use
| Commodity | Option 1 | Option 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Chronic risk assessment | Acute risk assessment | Chronic risk assessment | Acute risk assessment | |||||
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition 1: sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram | ||||||||
| Grapefruits, oranges | 0.12 | STMR | 0.32 | HR | 0.12 | STMR | 0.32 | HR |
| Lemons, mandarins | 0.29 | STMR (tentative) | 0.32 | HR (tentative) | 0.29 | STMR (tentative) | 0.32 | HR (tentative) |
| Limes | 0.01 | EU MRL | 0.01 | EU MRL | 0.01 | EU MRL | 0.01 | EU MRL |
| Treenuts | 0.01 | STMR | 0.03 | HR | 0.01 | STMR | 0.03 | HR |
| Pome fruits | 0.20 | STMR | 0.60 | HR | 0.20 | STMR | 0.60 | HR |
| Apricots | 0.33 | STMR | 0.95 | HR | 0.33 | STMR | 0.95 | HR |
| Cherries (sweet) | 0.56 | STMR (tentative) | 1.10 | HR (tentative) | 0.56 | STMR (tentative) | 1.10 | HR (tentative) |
| Peaches | 0.34 | STMR | 0.95 | HR | 0.34 | STMR | 0.95 | HR |
| Plums | 0.19 | STMR | 0.27 | HR | 0.19 | STMR | 0.27 | HR |
| Table grapes | 0.59 | STMR | 1.00 | HR | 0.59 | STMR | 1.00 | HR |
| Wine grapes | 0.46 | STMR | 0.95 | HR | 0.46 | STMR | 0.95 | HR |
| Strawberries | 0.43 | STMR | 1.01 | HR | 0.43 | STMR | 1.01 | HR |
| Cane fruits | 1.12 | STMR | 2.39 | HR | 1.12 | STMR | 2.39 | HR |
| Blueberries | 1.14 | STMR | 4.33 | HR | 1.14 | STMR | 4.33 | HR |
| Other small fruits and berries, except rose hips and azaroles | 0.78 | STMR | 2.10 | HR | 0.78 | STMR | 2.10 | HR |
| Rose hips | 0.69 | STMR | 1.58 | HR | 0.69 | STMR | 1.58 | HR |
| Bananas | 0.19 | STMR (tentative) | 0.52 | HR (tentative) | 0.19 | STMR (tentative) | 0.52 | HR (tentative) |
| Cherimoyas | 0.01 | EU MRL | 0.01 | EU MRL | 0.01 | EU MRL | 0.01 | EU MRL |
| Potatoes | 0.01 | STMR | 0.07 | HR | 0.03a | STMR | 0.07 | HR |
| Cassava roots/manioc Arrowroots | n.r. | n.r. | n.r. | n.r. | 0.02b | STMR | 0.02b | HR |
|
Sweet potatoes Yams |
0.02 | STMR | 0.03 | HR | 0.04a | STMR | 0.05a | HR |
| Beetroot | 0.02 | STMR | 0.04 | HR | 0.07a | STMR | 0.1a | HR |
| Carrots, radishes | 0.06 | STMR | 0.13 | HR | 0.07a | STMR | 0.18a | HR |
| other root and tuber vegetables except radishes, carrots, beetroots and sugar beets | 0.05 | STMR | 0.13 | HR | 0.07a | STMR | 0.18a | HR |
| Onions, garlic, shallots | 0.01 | STMR | 0.04 | HR | 0.01 | STMR | 0.04 | HR |
| Spring onions/green onions and Welsh onions | 0.56 | STMR (tentative) × CF (1.1) | 1.32 | HR (tentative) × CF (1.1) | 0.56 | STMR (tentative) × CF (1.1) | 1.32 | HR (tentative) × CF (1.1) |
| Tomatoes | 0.14 | STMR × CF (1.3) | 0.29 | HR × CF (1.3) | 0.14 | STMR × CF (1.3) | 0.29 | HR × CF (1.3) |
| Aubergines | 0.12 | STMR | 0.23 | HR | 0.12 | STMR | 0.23 | HR |
| Sweet peppers/bell peppers | 0.29 | STMR | 1.23 | HR | 0.29 | STMR | 1.23 | HR |
| Cucumbers, gherkins, courgettes | 0.14 | STMR | 0.30 | HR | 0.14 | STMR | 0.30 | HR |
| Melons | 0.19 | STMR (tentative) | 0.44 | HR (tentative) | 0.19 | STMR (tentative) | 0.44 | HR (tentative) |
| Watermelons | 0.05 | STMR (tentative) | 0.12 | HR (tentative) | 0.05 | STMR (tentative) | 0.12 | HR (tentative) |
| Pumpkins, watermelons | 0.05 | STMR | 0.12 | HR | 0.05 | STMR | 0.12 | HR |
| Sweet corn | 0.01* | STMR | 0.01* | HR | 0.01 | STMRa | 0.01 | HRa |
| Broccoli | 0.04 | STMR | 0.14 | HR | 0.06 | STMRa | 0.19 | HRa |
| Cauliflowers | 0.01 | STMR | 0.05 | HR | 0.03 | STMRa | 0.10 | HRa |
| Brussels sprouts | 0.04 | STMR | 0.14 | HR | 0.06 | STMRa | 0.19 | HRa |
| Head cabbages | 0.01 | STMR | 0.08 | HR | 0.04 | STMRa | 0.17 | HRa |
| Chinese cabbages/pe‐tsai | 0.36 | STMR (tentative) | 0.84 | HR (tentative) | 0.36 | STMR (tentative) | 0.84 | HR (tentative) |
| Kales, kohlrabies, watercresses | n.r. | n.r. | n.r. | n.r. | 0.03b | STMR | 0.09b | HR |
| Lamb's lettuces, Cresses, Roman rocket, purslanes, baby leaf crops | 1.60 | STMR | 10.00 | HR | 1.60 | STMR | 10.00 | HR |
| Lettuces | 1.50 | STMR | 10.00 | HR | 1.50 | STMR | 10.00 | HR |
| Escaroles, land cresses, red mustard, spinaches, chards/beet leaves | 0.37 | STMR (tentative) | 0.98 | HR (tentative) | 0.37 | STMR (tentative) | 0.98 | HR (tentative) |
| Witloofs/Belgian endives | 0.12 | STMR × CF (1.2) | 0.14 | HR × CF (1.2) | 0.12 | STMR × CF (1.2) | 0.14 | HR × CF (1.2) |
| Herbs, and edible flowers, except basil | 0.38 | STMR | 3.65 | HR | 0.38 | STMR | 3.65 | HR |
| Basil | 19.12 | STMR | 30.08 | HR | 19.12 | STMR | 30.08 | HR |
| Beans/peas (with pods) | 0.45 | STMR (tentative) × CF (1.1) | 1.65 | HR (tentative) × CF (1.1) | 0.45 | STMR (tentative) × CF (1.1) | 1.65 | HR (tentative) × CF (1.1) |
| Beans/peas (without pods), lentils (fresh) | 0.05 | STMR × CF (1.3) | 0.09 | HR × CF (1.3) | 0.05 | STMR × CF (1.3) | 0.09 | HR × CF (1.3) |
| Asparagus | 0.01* | STMR | 0.01* | HR | 0.01* | STMR | 0.01* | HR |
| Celeries | 3.03 | STMR | 9.74 | HR | 3.03 | STMR | 9.74 | HR |
| Globe artichokes | 1.27 | STMR (tentative) | 1.37 | HR (tentative) | 1.27 | STMR (tentative) | 1.37 | HR (tentative) |
| Leeks | 0.24 | STMR (tentative) × CF (1.1) | 0.35 | HR (tentative) × CF (1.1) | 0.24 | STMR (tentative) × CF (1.1) | 0.35 | HR (tentative) × CF (1.1) |
| Pulses (dry) | 0.05 | STMR × CF (1.4) | 0.05 | STMR × CF (1.4) | 0.05 | STMR × CF (1.4) | 0.05 | STMR × CF (1.4) |
| Peanuts/groundnuts | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) |
| Poppy seeds, mustard seeds | 0.12 | STMR × CF (1.2) | 0.12 | STMR × CF (1.2) | 0.12 | STMR × CF (1.2) | 0.12 | STMR × CF (1.2) |
| Sunflower seeds | 0.08 | STMR | 0.08 | STMR | 0.08 | STMR | 0.08 | STMR |
| Rapeseeds/canola seeds | 0.40 | STMR × CF (1.2) | 0.40 | STMR × CF (1.2) | 0.40 | STMR × CF (1.2) | 0.40 | STMR × CF (1.2) |
| Soya beans | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) | 0.01 | STMR × CF (1.2) |
| Cotton seeds | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) |
| Barley, oat grains | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR |
| Buckwheat, millet, rice grains | n.r. | n.r. | n.r. | n.r. | 0.01b | STMR | 0.01b | STMR |
| Maize/corn grains, rye grains | 0.01 | STMR | 0.01 | STMR | 0.01 | STMR | 0.01 | STMR |
| Sorghum grains | 0.36 | STMR | 0.36 | STMR | 0.36 | STMR | 0.36 | STMR |
| Wheat grains | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR |
| Herbal infusions (dried flowers and leaves) | 2.31 | STMR (EFSA, 2019d) | 25.9 | HR (EFSA, 2019d) | 2.31 | STMR (EFSA, 2019d) | 25.9 | HR (EFSA, 2019d) |
| Herbal infusions (dried roots) | n.r. | n.r. | n.r. | n.r. | 0. 1b | STMR × default DF(10) | 0. 1b | HR × default DF(10) |
| Hops | 12.13 | STMR × CF (1.2) | 30.48 | HR × CF (1.2) | 12.13 | STMR × CF (1.2) | 30.48 | HR × CF (1.2) |
| Seed spices | 22.50 | STMR | 29.60 | HR | 22.50 | STMR | 29.60 | HR |
| Spices (roots or rhizome) | n.r. | n.r. | n.r. | n.r. | 0.2b | STMR × default DF(10) | 0.5b | HR |
| Sugar beet (root) | n.r. | n.r. | n.r. | n.r. | 0.02b | STMR | 0.05b | HR |
| Chicory roots | 0.1 | EU MRL | 0.1 | EU MRL | 0.1 | EU MRL | 0.1 | EU MRL |
| Risk assessment residue definition 2: sum of fluopyram, fluopyram‐benzamide (M25), and fluopyram‐E/Z‐olefine (M02/M03), expressed as fluopyram | ||||||||
| Swine muscle | 0.02 | STMR | 0.09 | HR | 0.02 | STMR | 0.09 | HR |
| Swine fat tissue | 0.03 | STMR × CF (1.4) | 0.11 | HR × CF (1.4) | 0.03 | STMR × CF (1.4) | 0.11 | HR × CF (1.4) |
| Swine liver | 0.26 | STMR | 0.44 | HR | 0.32a | STMR | 0.49a | HR |
| Swine kidney | 0.02 | STMR | 0.07 | HR | 0.03a | STMR | 0.08a | HR |
| Bovine, equine muscle | 0.02 | STMR | 0.09 | HR | 0.07a | STMR | 0.13a | HR |
| Bovine, equine fat tissue | 0.03 | STMR × CF (1.4) | 0.12 | HR × CF (1.4) | 0.07a | STMR × CF (1.4) | 0.16a | HR × CF (1.4) |
| Bovine, equine liver | 0.27 | STMR | 0.48 | HR | 0.51a/0.27 | STMR | 0.71a | HR |
| Bovine, equine kidney | 0.02 | STMR | 0.08 | HR | 0.06a/0.02 | STMR | 0.11a | HR |
| Sheep, goat muscle | 0.05 | STMR | 0.1 | HR | 0.07a/0.05 | STMR | 0.13a | HR |
| Sheep, goat fat tissue | 0.05 | STMR × CF (1.4) | 0.12 | HR × CF (1.4) | 0.07a | STMR × CF (1.4) | 0.15a | HR × CF (1.4) |
| Sheep, goat liver | 0.37 | STMR | 0.53 | HR | 0.53a | STMR | 0.7a | HR |
| Sheep, goat kidney | 0.04 | STMR | 0.09 | HR | 0.06a | STMR | 0.11a | HR |
| Poultry fat tissue | 0.04 | STMR × CF (1.25) | 0.08 | HR × CF (1.25) | 0.07a | STMR × CF (1.25) | 0.08a | HR × CF (1.25) |
| Poultry liver | 0.20 | STMR | 0.24 | HR | 0.21a | STMR | 0.26a | HR |
| Cattle, horse milk | 0.02 | STMR | 0.02 | STMR | 0.04a | STMR | 0.04a | STMR |
| Sheep, goat milk | 0.02 | STMR | 0.02 | STMR | 0.05a | STMR | 0.05a | STMR |
| Birds eggs | 0.10 | STMR | 0.13 | HR | 0.10a | STMR | 0.13a | HR |
STMR: supervised trials median residue; HR: highest residue; CF: Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
n.r.: not registered for use on primary crops.
DF: drying factor.
* Indicates that the input value is proposed at the limit of quantification.
The STMR and HR values reflect the combined residues from both primary and rotational crops (sum of the HR/STMR values).
The STMR and HR values reflect the residues from rotational crops.
D.3. Consumer risk assessment with consideration of the existing CXLs
| Commodity | Option 1 | Option 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Chronic risk assessment | Acute risk assessment | Chronic risk assessment | Acute risk assessment | |||||
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: sum of fluopyram and fluopyram‐benzamide (M25), expressed as fluopyram | ||||||||
| Grapefruits | 0.12 | STMR | 0.32 | HR | 0.12 | STMR | 0.32 | HR |
| Oranges | 0.15 | STMR (CXL) | 0.37 | HR (CXL) | 0.15/0.12 | STMR (CXL/EU) | 0.37/0.32 | HR (CXL/EU) |
| Mandarins | 0.29 | STMR (tentative) | 0.32 | HR (tentative) | 0.29 | STMR (tentative) | 0.32 | HR (tentative) |
| Lemons | 0.33 | STMR (CXL) | 0.51 | HR (CXL) | 0.33/0.29 | STMR (CXL)/STMR (tentative) | 0.51/0.32 | HR (CXL)/HR (tentative) |
| Limes | 0.33 | STMR (CXL) | 0.51 | HR (CXL) | 0.33/0.01 | STMR (CXL)/EU MRL | 0.51/0.01 | HR (CXL)/EU MRL |
| Treenuts | 0.01 | STMR (CXL) | 0.04 | HR (CXL) | 0.01/0.01 | STMR (CXL/EU) | 0.04/0.03 | HR (CXL/EU) |
| Pome fruits | 0.20 | STMR | 0.60 | HR | 0.20 | STMR | 0.60 | HR |
| Apricots | 0.33 | STMR | 0.95 | HR | 0.33 | STMR | 0.95 | HR |
| Cherries (sweet) | 0.56 | STMR (tentative) | 1.10 | HR (tentative) | 0.56 | STMR (tentative) | 1.10 | HR (tentative) |
| Peaches | 0.34 | STMR | 0.95 | HR | 0.34 | STMR | 0.95 | HR |
| Plums | 0.19 | STMR | 0.27 | HR | 0.19 | STMR | 0.27 | HR |
| Table grapes | 0.59 | STMR | 1.00 | HR | 0.59 | STMR | 1.00 | HR |
| Wine grapes | 0.58 | STMR (CXL) | 1.00 | HR (CXL) | 0.58/0.46 | STMR (CXL)/ | 1.00/0.95 | HR (CXL/EU) |
| Strawberries | 0.43 | STMR | 1.01 | HR | 0.43 | STMR | 1.01 | HR |
| Cane fruits | 1.12 | STMR | 2.39 | HR | 1.12 | STMR | 2.39 | HR |
| Blueberries | 1.14 | STMR | 4.33 | HR | 1.14 | STMR | 4.33 | HR |
| Cranberries, elderberries | 0.78 | STMR | 2.10 | HR | 0.78 | STMR | 2.10 | HR |
| Currants, gooseberries | 1.15 | STMR (CXL) | 4.90 | HR (CXL) | 1.15/0.79 | STMR (CXL/EU) | 4.90/2.10 | HR (CXL/EU) |
| Rose hips | 1.15 | STMR (CXL) | 4.90 | HR (CXL) | 1.15/0.69 | STMR (CXL/EU) | 4.90/1.58 | HR (CXL) |
| Mulberries (black and white) | 0.79 | STMR | 2.10 | HR | 0.79 | STMR | 2.10 | HR |
| Bananas | 0.19 | STMR (tentative) | 0.52 | HR (tentative) | 0.19 | STMR (tentative) | 0.52 | HR (tentative) |
| Mangoes | 0.02 | STMR (CXL) × PF(0.11) | 0.05 | HR (CXL) × PF (0.11) | 0.02/n.r. | STMR (CXL) × PF(0.11)/n.r. | 0.05 | HR (CXL) × PF (0.11)/n.r. |
| Cherimoyas | 0.01 | EU MRL | 0.01 | EU MRL | 0.01 | EU MRL | 0.01 | EU MRL |
| Potatoes | 0.02 | STMR (CXL) | 0.08 | HR (CXL) | 0.02/0.03a | STMR (CXL/EU) | 0.08/0.07 | HR (CXL/EU) |
| Cassava roots/Arrowroots | n.r. | n.r. | n.r. | n.r. | 0.02b | STMR | 0.02b | HR |
| Sweet potatoes, yams | 0.02 | STMR | 0.03 | HR | 0.04a | STMR | 0.05a | HR |
| Beetroot | 0.02 | STMR | 0.04 | HR | 0.07a | STMR | 0.1a | HR |
| Carrots | 0.09 | STMR (CXL) | 0.19 | HR (CXL) | 0.07a | STMR | 0.18a | HR |
| other root and tuber vegetables except carrots, beetroots and sugar beets | 0.05 | STMR | 0.13 | HR | 0.07a | STMR | 0.1a | HR |
| Onions, garlic, shallots | 0.01 | STMR | 0.04 | HR | 0.01 | STMR | 0.04 | HR |
| Spring onions/green onions and Welsh onions | 5.6 | STMR (CXL/EU) × CF (1.1) | 8 | HR (CXL/EU) × CF (1.1) | 5.6/0.56 | STMR(CXL/EU) × CF (1.1) | 8/1.32 | HR (CXL/EU) × CF (1.1)) |
| Tomatoes | 0.14 | STMR × CF (1.3) | 0.29 | HR × CF (1.3) | 0.12 | STMR | 0.23 | HR |
| Aubergines | 0.11 | STMR (CXL) | 0.37 | HR (CXL) | 0.11/0.12 | STMR (CXL/EU) | 0.37/0.23 | HR (CXL/EU) |
| Sweet peppers/bell peppers | 0.14 | STMR (CXL) | 1.4 | HR (CXL) | 0.14/0.29 | STMR (CXL/EU) | 1.4/1.23 | HR(CXL/EU) |
| Cucumbers, gherkins, courgettes | 0.14 | STMR | 0.30 | HR | 0.14 | STMR | 0.30 | HR |
| Melons | 0.19 | STMR (tentative) | 0.44 | HR (tentative) | 0.19 | STMR (tentative) | 0.44 | HR (tentative) |
| Watermelons | 0.05 | STMR (tentative) | 0.12 | HR (tentative) | 0.05 | STMR (tentative) | 0.12 | HR (tentative) |
| Pumpkins, watermelons | 0.05 | STMR | 0.12 | HR | 0.05 | STMR | 0.12 | HR |
| Sweet corn | 0.01 | STMR | 0.01 | HR | 0.01 | STMR | 0.01 | HR |
| Broccoli | 0.04 | STMR | 0.14 | HR | 0.06 | STMRa | 0.19 | HRa |
| Cauliflowers | 0.01 | STMR | 0.05 | HR | 0.03 | STMRa | 0.10 | HRa |
| Brussels sprouts | 0.04 | STMR | 0.14 | HR | 0.06 | STMRa | 0.19 | HRa |
| Head cabbages | 0.01 | STMR | 0.08 | HR | 0.04 | STMRa | 0.17 | HRa |
| Chinese cabbages/pe‐tsai | 0.36 | STMR (tentative) | 0.84 | HR (tentative) | 0.36 | STMR (tentative) | 0.84 | HR (tentative) |
| Kales, kohlrabies, watercress | 0.1 | EU MRL | 0.1 | EU MRL | 0.03b | STMR | 0.09b | HR |
| Lamb's lettuces; Cresses; Roman rocket, purslanes, baby leaf crops | 1.60 | STMR | 10.00 | HR | 1.60 | STMR | 10.00 | HR |
| Lettuces | 1.50 | STMR | 10.00 | HR | 1.50 | STMR | 10.00 | HR |
| Escaroles, land cresses, red mustard, spinaches, chards/beet leaves | 0.37 | STMR (tentative) | 0.98 | HR (tentative) | 0.37 | STMR (tentative) | 0.98 | HR (tentative) |
| Witloofs/Belgian endives | 0.12 | STMR × CF (1.2) | 0.14 | HR × CF (1.2) | 0.12 | STMR × CF (1.2) | 0.14 | HR × CF (1.2) |
| Herbs, and edible flowers, except basil | 0.38 | STMR | 3.65 | HR | 0.38 | STMR | 3.65 | HR |
| Basil | 19 | STMR (CXL) | 32 | HR (CXL) | 19/19.12 | STMR (CXL/EU) | 32/30.08 | HR (CXL/EU) |
| Beans/peas (with pods) | 0.45 | STMR (tentative) × CF (1.1) | 1.65 | HR (tentative) × CF (1.1) | 0.45 | STMR (tentative) × CF (1.1) | 1.65 | HR (tentative) × CF (1.1) |
| Beans/peas (without pods) | 0.04 | STMR (CXL) × CF (1.3) | 0.16 | HR (CXL) × CF (1.3) | 0.04/0.05 | STMR (CXL/EU) × CF (1.3) | 0.16/0.09 | HR (CXL/EU) × CF (1.3) |
| Lentils (fresh) | 0.05 | STMR × CF (1.3) | 0.09 | HR × CF (1.3) | 0.05 | STMR × CF (1.3) | 0.09 | HR × CF (1.3) |
| Asparagus | 0.01* | STMR | 0.01* | HR | 0.01* | STMR | 0.01* | HRa |
| Celeries | 3.03 | STMR | 9.74 | HR | 3.03 | STMR | 9.74 | HR |
| Globe artichokes | 1.27 | STMR (tentative) | 1.37 | HR (tentative) | 1.27 | STMR (tentative) | 1.37 | HR (tentative) |
| Leeks | 0.24 | STMR (tentative) × CF (1.1) | 0.35 | HR (tentative) × CF (1.1) | 0.24 | STMR (tentative) × CF (1.1) | 0.35 | HR (tentative) × CF (1.1) |
| Lentils, peas (dry) | 0.08 | STMR (CXL) × CF (1.4) | 0.49 | HR (CXL) × CF (1.4) | 0.08/0.05 | STMR (CXL/EU) × CF (1.4) | 0.49/0.05 | HR (CXL/EU) × CF (1.4) |
| Lupins (dry) | 0.05 | STMR × CF (1.4) | 0.49 | HR × CF (1.4) | 0.05 | STMR × CF (1.4) | 0.49 | HR × CF (1.4) |
| Peanuts/groundnuts | 0.04 | STMR (CXL) × CF (1.2) | 0.16 | STMR × CF(CXL) (1.2) | 0.04/0.01 | STMR (CXL/EU) × CF (1.2) | 0.16/0.01 | STMR × CF(CXL/EU) (1.2) |
| Poppy seeds, mustard seeds | 0.12 | STMR × CF (1.2) | 0.31 | HR × CF (1.2) | 0.12 | STMR × CF (1.2) | 0.12 | STMR × CF (1.2) |
| Sunflower seeds | 0.08 | STMR | 0.38 | HR | 0.08 | STMR | 0.08 | STMR |
| Rapeseeds/canola seeds | 0.40 | STMR × CF (1.2) | 0.73 | HR × CF (1.2) | 0.40 | STMR × CF (1.2) | 0.40 | STMR × CF (1.2) |
| Soya beans | 0.02 | STMR (CXL) × CF (1.2) | 0.25 | HR (CXL) × CF (1.2) | 0.02/0.01 | STMR × CF(CXL/EU) (1.2) | 0.25/0.01 | HR × CF(CXL/EU) (1.2) |
| Cotton seeds | 0.07 | STMR × CF (1.2) | 0.56 | HR × CF (1.2) | 0.07 | STMR × CF (1.2) | 0.07 | STMR × CF (1.2) |
| Barley, oat grains | 0.02 | STMR | 0.02 | STMR | 0.02 | STMR | 0.11 | STMR |
| Buckwheat, millet, rice grains | n.r. | n.r. | n.r. | n.r. | n.r./0.01b | n.r./STMR | n.r./0.01b | n.r./STMR |
| Maize/corn grains | 0.01 | STMR (CXL) | 0.02 | STMR (CXL) | 0.01/0.01 | STMR (CXL/EU) | 0.01/0.01 | STMR (CXL/EU) |
| Rye grains | 0.62 | STMR (CXL) | 2.7 | HR (CXL) | 0.62/0.01 | STMR (CXL/EU) | 0.62/0.01 | STMR (CXL/EU) |
| Sorghum grains | 0.36 | STMR | 0.36 | STMR | 0.36 | STMR | 0.36 | STMR |
| Wheat grains | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR | 0.19 | STMR |
| Herbal infusions (dried flowers and leaves) | 2.31 | STMR | 25.9 | HR | 2.31 | STMR | 25.9 | HR |
| Herbal infusions (dried roots) | n.r. | n.r. | n.r. | n.r. | 0. 1b | STMRxdefault DF(10) | 0. 1b | HR xdefault DF(10) |
| Hops | 12.13 | STMR × CF (1.2) | 30.48 | HR × CF (1.2) | 12.13 | STMR × CF (1.2) | 30.48 | HR × CF (1.2) |
| Seed spices | 22.50 | STMR | 29.60 | HR | 22.50 | STMR | 29.60 | HR |
| Spices (roots or rhizome) | n.r. | n.r. | n.r. | n.r. | 0.2b | STMRxdefault DF(10) | 0.5b | HR |
| Sugar beet (root) | 0.01 | STMR (CXL) | 0.01 | HR (CXL) | 0.02b | STMR | 0.05b | HR |
| Chicory roots | 0.1 | EU MRL | 0.1 | EU MRL | 0.1 | EU MRL | 0.03 | EU MRL |
| Risk assessment residue definition 2: sum of fluopyram, fluopyram‐benzamide (M25), and fluopyram‐E/Z‐olefine (M02/M03), expressed as fluopyram | ||||||||
| Swine muscle | 0.51/0.02 | STMR (CXL/EU) | 1.0/0.09 | HR (CXL/EU) | 0.51/0.02 | STMR (CXL/EU) | 1.0/0.09 | HR (CXL/EU) |
| Swine fat tissue | 0.67/0.03 | STMR (CXL/EU) × CF (1.4) | 1.5/0.08 | HR (CXL) × CF (1.4) | 0.67/0.03 | STMR (CXL/EU) × CF (1.4) | 1.5/0.11 | HR (CXL/EU) × CF (1.4) |
| Swine liver | 3.8/0.26 | STMR (CXL/EU) | 7.4/0.44 | HR (CXL/EU) | 3.8/0.32a | STMR (CXL/EU) | 7.4/0.49a | HR (CXL/EU) |
| Swine kidney | 3.8/0.02 | STMR (CXL/EU) | 7.4/0.07 | HR (CXL/EU) | 3.8/0.03a | STMR (CXL/EU) | 7.4/0.08a | HR (CXL/EU) |
| Bovine muscle | 0.51/0.02 | STMR (CXL/EU) | 1.0/0.09 | HR (CXL/EU) | 0.51/0.07a | STMR (CXL/EU) | 1.0/0.13a | HR (CXL/EU) |
| Bovine fat tissue | 0.67/0.03 | STMR (CXL/EU) × CF (1.4) | 1.5/0.08 | HR (CXL/EU) × CF (1.4) | 0.67/0.07a | STMR (CXL/EU) × CF (1.4) | 1.5/0.16a | HR (CXL/EU) × CF (1.4) |
| Bovine liver | 3.80/0.27 | STMR (CXL/EU) | 7.4/0.49 | HR (CXL/EU) | 0.51a/0.27 | STMR (CXL/EU) | 7.4/0.71a | HR (CXL/EU) |
| Bovine kidney | 3.80/0.02 | STMR (CXL/EU) | 7.4/0.08 | HR (CXL/EU) | 0.06a/0.02 | STMR (CXL/EU) | 7.4/0.11a | HR (CXL/EU) |
| Sheep, goat muscle | 0.51/0.05 | STMR (CXL/EU) | 1.0 | HR (CXL/EU) | 0.51/0.07a | STMR (CXL/EU) | 1.0/0.13a | HR (CXL/EU) |
| Sheep, goat fat tissue | 0.67/0.05 | STMR (CXL/EU) × CF (1.4) | 1.5 | HR (CXL) × CF (1.4) | 0.67/0.07a | STMR (CXL/EU) × CF (1.4) | 1.5/0.15a | HR (CXL/EU) × CF (1.4) |
| Sheep, goat liver | 3.80/0.37 | STMR (CXL/EU) | 7.4 | HR (CXL) | 3.8/0.53a | STMR (CXL/EU) | 7.4/0.7a | HR (CXL/EU) |
| Sheep, goat kidney | 3.80/0.04 | STMR (CXL/EU) | 7.4 | HR (CXL) | 3.8/0.06a | STMR (CXL/EU) | 7.4/0.11a | HR (CXL/EU) |
| Equine muscle | 0.51 | STMR (CXL) | 1.0 | HR (CXL) | 0.51/0.07a | (CXL/EU)STMR | 1.0/0.08a | HR (CXL/EU) |
| Equine fat tissue | 0.67 | STMR (CXL) × CF (1.4) | 1.5 | HR (CXL) × CF (1.4) | 0.67/0.21a | STMR (CXL/EU) × CF (1.4) | 1.5/0.26a | HR (CXL/EU) × CF (1.4) |
| Equine liver | 3.80 | STMR (CXL) | 7.4 | HR (CXL) | 3.8/0.04a | STMR (CXL/EU) | 7.4/0.04a | HR (CXL/EU) |
| Equine kidney | 3.80 | STMR (CXL) | 7.4 | HR (CXL) | 3.8/0.05a | STMR (CXL/EU) | 7.4/0.05a | HR (CXL/EU) |
| Poultry muscle | 0.19 | STMR (CXL) | 1.0 | HR (CXL) | 0.19/0.10a | STMR (CXL/EU) | 1.0/0.13a | HR (CXL/EU) |
| Poultry fat tissue | 0.28 | STMR (CXL) × CF (1.25) | 0.9 | HR (CXL) × CF (1.25) | 0.28/0.02a | STMR (CXL/EU) × CF (1.3) | 0.9/0.09a | HR (CXL/EU) × CF (1.3) |
| Poultry liver | 0.88 | STMR (CXL) | 3.0 | HR | 0.88/0.03a | STMR (CXL/EU) | 3/0.11a | HR (CXL/EU) |
| Cattle, horse milk | 0.02 | STMR | 0.02 | STMR | 0.04a | STMR (EU) | 0.04a | STMR (EU) |
| Sheep, goat milk | 0.02 | STMR | 0.02 | STMR | 0.05a | STMR (EU) | 0.05a | STMR (EU) |
| Birds eggs | 0.46 | STMR (CXL) | 1.4 | HR (CXL) | 0.46/0.1a | STMR (CXL/EU) | 1.4/0.13a | HR (CXL/EU) |
STMR: supervised trials median residue; HR: highest residue; CF: Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment; PF: peeling factor.
n.r.: not registered for use on primary crops.
*: Indicates that the input value is proposed at the limit of quantification.
The STMR and HR values reflect the residues from rotational crops.
The STMR and HR values reflect the combined residues from both primary and rotational crops (sum of the HR/STMR values).
Tentative as no data on NEU authorised use, and therefore it is not known whether the combined primary and rotational crops use is higher than the STMR and HR values derived from the import tolerance.
Appendix E – Decision tree for deriving MRL recommendations
1.


Appendix F – Used compound codes
1.
| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulac |
|---|---|---|
| fluopyram |
N‐{2‐[3‐chloro‐5‐(trifluoromethyl)‐2‐pyridyl]ethyl}‐α,α,α‐trifluoro‐o‐toluamide FC(F)(F)c1ccccc1C(=O)NCCc2ncc(cc2Cl)C(F)(F)F KVDJTXBXMWJJEF‐UHFFFAOYSA‐N |

|
|
M02 fluopyram‐E‐olefine |
N‐{(E)‐2‐[3‐chloro‐5‐(trifluoromethyl)pyridin‐2‐yl]vinyl}‐2‐(trifluoromethyl)benzamide FC(F)(F)c1ccccc1C(=O)N\C=C\c2ncc(cc2Cl)C(F)(F)F ZBXOWVYWCBPUPM‐AATRIKPKSA‐N |

|
|
M03 fluopyram‐Z‐olefine |
N‐{(Z)‐2‐[3‐chloro‐5‐(trifluoromethyl)pyridin‐2‐yl]vinyl}‐2‐(trifluoromethyl)benzamide FC(F)(F)c1ccccc1C(=O)N\C=C/c2ncc(cc2Cl)C(F)(F)F ZBXOWVYWCBPUPM‐WAYWQWQTSA‐N |

|
|
M08 fluopyram‐7‐hydroxy |
N‐{2‐[3‐chloro‐5‐(trifluoromethyl)pyridin‐2‐yl]‐2‐hydroxyethyl}‐2‐(trifluoromethyl)benzamide Clc1cc(cnc1C(O)CNC(=O)c1ccccc1C(F)(F)F)C(F)(F)F LZWQFTDQXOXRHG‐UHFFFAOYSA‐N |
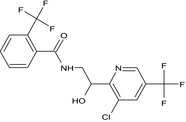
|
|
M25 fluopyram‐benzamide |
2‐(trifluoromethyl)benzamide FC(F)(F)c1ccccc1C(N)=O QBAYIBZITZBSFO‐UHFFFAOYSA‐N |

|
|
M40 fluopyram‐pyridyl‐acetic acid fluopyram‐PAA |
[3‐chloro‐5‐(trifluoromethyl)pyridin2‐yl]acetic acid OC(=O)Cc1ncc(cc1Cl)C(F)(F)F ZCMWOZJSLGQSQV‐UHFFFAOYSA‐N |
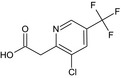
|
|
M42 fluopyram pyridyl‐acetic‐acid‐glycoside |
1‐O‐{[3‐chloro‐5‐(trifluoromethyl)pyridin‐2‐yl]acetyl}‐α‐D‐glucopyranose O=C(O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)Cc1ncc(cc1Cl)C(F)(F)F WLNHNRBMWFDQSH‐KABOQKQYSA‐N |
|
|
M43 fluopyram pyridylcarboxylic acid fluopyram‐PCA (AE C657188) |
3‐chloro‐5‐(trifluoromethyl)pyridine‐2‐carboxylic acid Clc1cc(cnc1C(O)=O)C(F)(F)F HXRMCZBDTDCCOP‐UHFFFAOYSA‐N |

|
|
M45 methyl‐sulfoxide |
3‐(methylsulfinyl)‐5‐(trifluoromethyl)‐2‐pyridinecarboxylic acid OC(=O)c1ncc(cc1S(C)=O)C(F)(F)F RQFCURAIFZONFT‐UHFFFAOYSA‐N |

|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2019.1.1 ACD/Labs 2019 Release (File version N05E41, Build 110555, 18 July 2019).
ACD/ChemSketch 2019.1.1 ACD/Labs 2019 Release (File version C05H41, Build 110712, 24 July 2019).
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2020. Reasoned opinion on the review of the existing maximum residue levels for fluopyram according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2020;18(4):6059, 125 pp. 10.2903/j.efsa.2020.6059
Requestor: European Commission
Question number: EFSA‐Q‐2013‐00775
Acknowledgement: EFSA wishes to thank the rapporteur Member State Germany and Silvia Ruocco, Laszlo Bura, Georgios Chatzisotiriou and Viktor Toth for the preparatory work on this scientific output.
Approved: 6 March 2020
Notes
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32. Repealed by Regulation (EC) No 1107/2009.
Commission Implementing Regulation (EU) No 802/2013 of 22 August 2013 approving the active substance fluopyram, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 225, 23.8.2013, p. 13–16.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 1–186.
Commission Implementing Regulation (EU) No 541/2011 of 1 June 2011 amending Implementing Regulation (EU) No 540/2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the list of approved active substances. OJ L 153, 11.6.2011, p. 187–188.
Commission Regulation (EU) No 188/2011 of 25 February 2011 laying down detailed rules for the implementation of Council Directive 91/414/EEC as regards the procedure for the assessment of active substances which were not on the market 2 years after the date of notification of that Directive. OJ L 53, 26.2.2011, p. 51–55.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
References
- Belgium , 2019. Additional data to be considered for the review of the existing MRLs for fluopyram December 2019. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), 2011. Setting of new MRLs and import tolerances for fluopyram in various crops. EFSA Journal 2011;9(9):2388, 68 pp. 10.2903/j.efsa.2011.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2013a. Conclusion on the peer review of the pesticide risk assessment of the active substance fluopyram. EFSA Journal 2013;11(4):3052, 76 pp. 10.2903/j.efsa.2013.3052 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for flutolanil according to Article 12 of Regulation (EC) No 396/20051. EFSA Journal 2013;11(9):3360, 44 pp. 10.2903/j.efsa.2013.3360 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Reasoned opinion on the modification of the existing MRLs for fluopyram in various crops. EFSA Journal 2014;12(12):3947, 33 pp. 10.2903/j.efsa.2014.3947 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. Reasoned opinion on the modification of the existing maximum residue levels for fluopyram in various crops. EFSA Journal 2016;14(6):4520, 27 pp. 10.2903/j.efsa.2016.4520 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Janossy J, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the modification of the existing maximum residue level for fluopyram in purslanes. EFSA Journal 2017;15(9):4984, 22 pp. 10.2903/j.efsa.2017.4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Brocca D, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Lostia A, Magrans JO, Medina P, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019a. Reasoned opinion on the modification of the existing maximum residue level for fluopyram in broccoli. EFSA Journal 2019;17(3):5624, 23 pp. 10.2903/j.efsa.2019.5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019b. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019:EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI]
- EFSA (European Food Safety Authority), 2019c. Completeness check report on the review of the existing MRLs of fluopyram prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 23 July 2019. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority), Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2019d. Reasoned Opinion on the modification of the existing maximum residue levels for fluopyram in herbal infusions from leaves, herbs and flowers. EFSA Journal 2019;17(12):5942, 25 pp. 10.2903/j.efsa.2019.5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019e. Member States consultation report on the review of the existing MRLs of fluopyram prepared by EFSA in the framework of Article 12 of Regulation (EC) No 396/2005, 12 December 2019. Available online: www.efsa.europa.eu
- EURL (European Union Reference Laboratories for Pesticide Residues), 2018. Evaluation report prepared under Article 12 of Regulation (EC) No 396/2005. Analytical methods validated by the EURLs and overall capability of official laboratories to be considered for the review of the existing MRLs for fluopyram. May 2018. Available online: www.efsa.europa.eu
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/IV/95‐rev., 22 July 1996.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals.7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5) of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev.10.3, June 2017.
- FAO (Food and Agriculture Organization of the United Nations), 2009. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 2nd Edition. FAO Plant Production and Protection Paper 197, 264 pp.
- FAO (Food and Agriculture Organization of the United Nations), 2010. Fluopyram. In: Pesticide residues in food – 2010. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 200.
- FAO (Food and Agriculture Organization of the United Nations), 2012. Fluopyram. In: Pesticide residues in food – 2012. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 215.
- FAO (Food and Agriculture Organization of the United Nations), 2014. Fluopyram. In: Pesticide residues in food – 2014. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues. FAO Plant Production and Protection Paper 221.
- FAO (Food and Agriculture Organization of the United Nations), 2015. Fluopyram. In: Pesticide residues in food – 2015. Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 226.
- FAO (Food and Agriculture Organization of the United Nations), 2017. Fluopyram. In: Pesticide residues in food – 2017. Evaluations, Part I, Residues. FAO Plant Production and Protection Paper 233.
- Germany , 2011. Draft assessment report on the active substance fluopyram prepared by the rapporteur Member State Germany in the framework of Commission Regulation (EU) No 188/2011, August 2011. Available online: www.efsa.europa.eu
- Germany , 2012. Final addendum to the draft assessment report on the active substance fluopyram, compiled by EFSA, September 2012. Available online: www.efsa.europa.eu
- Germany , 2018. Evaluation report prepared under Article 12.1 of Regulation (EC) No 396/2005. Review of the existing MRLs for fluopyram, September 2018. Available online: www.efsa.europa.eu
- Netherlands , 2018. Draft Assessment Report and Proposed decision of the Netherlands prepared in the context of the possible approval of flutolanil under Regulation (EC) 1107/2009. Vol.3.Annex B.7 (AS) B.7, June 2018. Available online: www.efsa.europa.eu
- Netherlands , 2019. Additional data to be considered for the review of the existing MRLs for fluopyram, August 2019. Available online: www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 4 September 2013.
- OECD (Organisation for Economic Co‐operation and Development), 2018. Guidance document on Residues in Rotational Crops. In: Series on Pesticides No 97. ENV/JM/MONO(2018)9, 22 May 2018.


