Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Du Pont (UK) submitted a request to the competent national authority in the United Kingdom to set an import tolerance for the active substance oxathiapiprolin in various crops in support of authorised uses in the United States. The data submitted in support of the request were found to be sufficient to derive maximum residue level (MRL) proposals for citrus fruits, blackberries, raspberries, Chinese cabbage, basil and edible flowers and asparagus. For dewberries, potatoes and sweet potatoes, data gaps were identified which precluded the derivation of MRL proposals. Adequate analytical methods for enforcement are available to control the residues of oxathiapiprolin in plant matrices at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that the long‐term intake of residues resulting from the use of oxathiapiprolin according to the reported agricultural practices is unlikely to present a risk to consumer health.
Keywords: Oxathiapiprolin, various crops, import tolerance, pesticide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, Du Pont (UK) submitted an application to the competent national authority in the United Kingdom (evaluating Member State, EMS) to set import tolerances for the active substance oxathiapiprolin in various crops. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 10 February 2020. The EMS proposed to raise maximum residue levels (MRLs) for all crops under consideration, except for potatoes and sweet potatoes.
EFSA assessed the application and the evaluation report as required by Article 10 of the MRL regulation.
Based on the conclusions derived by EFSA in the framework of Regulation (EC) No 1107/2009, the data evaluated under previous MRL assessment and the additional data provided by the EMS in the framework of this application, the following conclusions are derived.
The metabolism of oxathiapiprolin following foliar treatment of primary crops belonging to fruit, leafy and root crop groups has been investigated in the European Union (EU) pesticides peer review and following soil treatment in the framework of a previous EFSA MRL assessment.
The main residue in most primary crops following foliar treatment was parent oxathiapiprolin, with exception of mature grapes, where metabolites containing the pyrazole moiety (IN‐E8S72 and IN‐WR791) were major residues. Following soil treatment, the main components of the total radioactive residue (TRR) in primary crops were metabolites IN‐E8S72, IN‐WR791, IN‐RZB20 and IN‐RZB21/IN‐RZD74. The actual amounts, however, were low, except for metabolite IN‐WR791 in courgettes.
Studies investigating the effect of processing on the nature of oxathiapiprolin (hydrolysis studies) demonstrated that the active substance is stable. As the authorised use of oxathiapiprolin is on imported crops, investigations of residues in rotational crops are not required.
Based on the metabolic pattern identified in the metabolism studies, hydrolysis studies and the toxicological significance of metabolites, the residue definitions for plant products were proposed by the peer review as ‘oxathiapiprolin’ for enforcement and risk assessment. The same residue definition is implemented in the Regulation (EC) No 396/2005.
EFSA concluded that for the crops assessed in this application, metabolism of oxathiapiprolin in primary and in rotational crops, and the possible degradation in processed products has been sufficiently addressed and that the previously derived residue definitions are applicable.
Sufficiently validated analytical methods based on LC‐MS/MS are available to quantify residues in the crops assessed in this application according to the enforcement residue definition at or above the validated limit of quantification (LOQ) of 0.01 mg/kg.
The available residue trials are sufficient to derive MRL proposals of 0.05 mg/kg for citrus fruits for the authorised foliar use, of 0.5 mg/kg for blackberries and raspberries, of 9 mg/kg for Chinese cabbage, of 10 mg/kg for fresh basil and edible flowers and of 2 mg/kg for asparagus. The authorised soil uses on citrus fruits are not supported by residue data. The submitted residue data were insufficient to derive MRL proposals for dewberries and incompliant to derive MRL proposals for potatoes and sweet potatoes.
Processing factors (PF) for the crops under assessment were derived from processing studies as well as from the supervised residue trials and are recommended to be included in Annex VI of Regulation (EC) No 396/2005 as follows:
-
–
Orange/oil: 47
-
–
Basil/dried basil: 8.8
-
–
Citrus fruit, peeled: < 0.56
As the crops under consideration and their by‐products (dried citrus pulp) can enter EU livestock feed chain, a potential carry‐over of residues into food of animal origin was assessed. The calculated EU livestock dietary burden did not exceed the trigger value of 0.004 mg/kg body weight (bw) per day for any animal species. Furthermore, the contribution of oxathiapiprolin residues in citrus dried pulp to the total livestock exposure was insignificant, and therefore, a modification of the existing MRLs for commodities of animal origin was considered unnecessary.
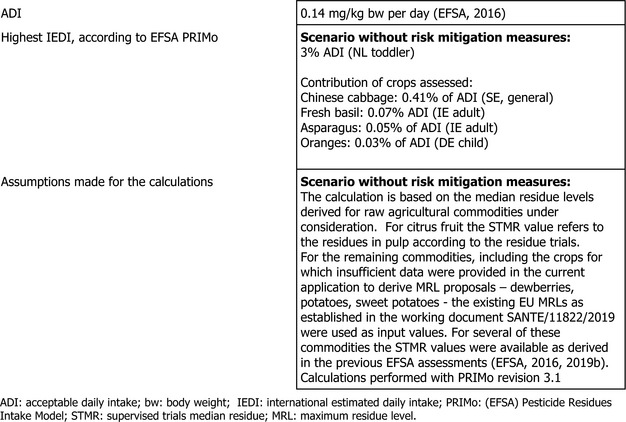
The toxicological profile of oxathiapiprolin was assessed in the framework of the EU pesticides peer review under Regulation (EC) No 1107/2009 and the data suffice to derive an acceptable daily intake (ADI) of 0.14 mg/kg bw per day. An acute reference dose (ARfD) was not considered necessary and thus was not derived.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo). The estimated long‐term dietary intake accounted for a maximum of 3% of the ADI for NL toddler diet.
EFSA concluded that the authorised use of oxathiapiprolin on the crops under consideration and the existing uses of oxathiapiprolin will not result in a consumer exposure exceeding the toxicological reference value and therefore is unlikely to pose a risk to consumers’ health.
EFSA proposes to amend the existing MRLs as reported in the summary table below. Full details of all endpoints and the consumer risk assessment can be found in Appendices B–D.
| Codea | Commodity |
Existing EU MRL (mg/kg) |
Proposed EU MRL (mg/kg) |
Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin | ||||
| 011000 | Citrus fruits | 0.01* | 0.05 |
The submitted data are sufficient to derive an MRL proposal for the authorised foliar use in the USA. Risk for consumers unlikely The submitted data are not sufficient to support the authorised soil use |
| 153010 | Blackberries | 0.01* | 0.5 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 153020 | Dewberries | 0.01* | No MRL proposal | The submitted data are not sufficient to derive an MRL proposal for the import tolerance |
| 153030 | Raspberries (red and yellow) | 0.01* | 0.5 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 211000 | Potatoes | 0.01* | No MRL proposal | The submitted data are incompliant to derive an MRL proposal for the import tolerances |
| 212020 | Sweet potatoes | 0.01* | No MRL proposal | The submitted data are incompliant to derive an MRL proposal for the import tolerances |
| 243010 | Chinese cabbage/pe‐tsai | 0.01* | 9 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 256080 | Basil and edible flowers | 0.01* | 10 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 270010 | Asparagus | 0.01* | 2 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Assessment
The European Food Safety Authority (EFSA) received an application from Du Pont to modify the existing maximum residue level (MRL) for oxathiapiprolin in various crops. The detailed description of the authorised uses of oxathiapiprolin in the United States (USA) on various crops, which are the basis for the current MRL application, is reported in Appendix A.
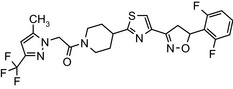
Oxathiapiprolin is the ISO common name for 1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}‐1‐piperidyl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone (IUPAC). The chemical structures of the active substance and its main metabolites are reported in Appendix E.
Oxathiapiprolin was evaluated in the framework of Regulation (EC) No 1107/20091 with Ireland designated as rapporteur Member State (RMS) for the representative uses as a foliar treatment on grapes, potatoes, tomatoes and aubergines. The draft assessment report (DAR) prepared by the RMS has been peer reviewed by EFSA (Ireland, 2015; EFSA, 2016). Oxathiapiprolin was approved2 for the use as fungicide on 3 March 2017.
The EU MRLs for oxathiapiprolin are established in Annex II of Regulation (EC) No 396/20053. The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) is not foreseen as MRLs were assessed in the framework of the first approval of the active substance. So far EFSA has issued one reasoned opinion on the modification of MRLs for oxathiapiprolin (EFSA, 2019b) and provided a scientific support for preparing an EU position in the 51st Session of the Codex Committee on Pesticide Residues (CCPR) (EFSA, 2019c). The proposals of EFSA reasoned opinion are voted in the SCoPAFF meeting in September 2019 and are implemented in the draft Regulation SANTE/11822/20194.
In accordance with Article 6 of Regulation (EC) No 396/2005, Du Pont (UK) submitted an application to the competent national authority in the United Kingdom (evaluating Member State, EMS) to set import tolerances for the active substance oxathiapiprolin in various crops. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the EFSA on 10 February 2020. The EMS proposed to raise MRLs from the LOQ of 0.01 mg/kg for the various crops imported from the US, except for potatoes and sweet potatoes.
EFSA based its assessment on the evaluation report submitted by the EMS (United Kingdom, 2020), the draft assessment report (DAR) (and its addendum/addenda) (Ireland, 2015, 2016) prepared under Regulation (EC) 1107/2009, the Commission review report on oxathiapiprolin (European Commission, 2016), the conclusion on the peer review of the pesticide risk assessment of the active substance oxathiapiprolin (EFSA, 2016), as well as the conclusions from a previous EFSA opinion on oxathiapiprolin (EFSA, 2019b).
For this application, the data requirements established in Regulation (EU) No 283/20135 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 2000, 2010a,b, 2013, 2017; OECD, 2007a, b, c, d, e, f, g h, 2008a, b, 2009a, b, 2011, 2013, 2016, 2018). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20116 .
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application including the end points of relevant studies assessed previously is presented in Appendix B.
The evaluation report submitted by the EMS (United Kingdom, 2020) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
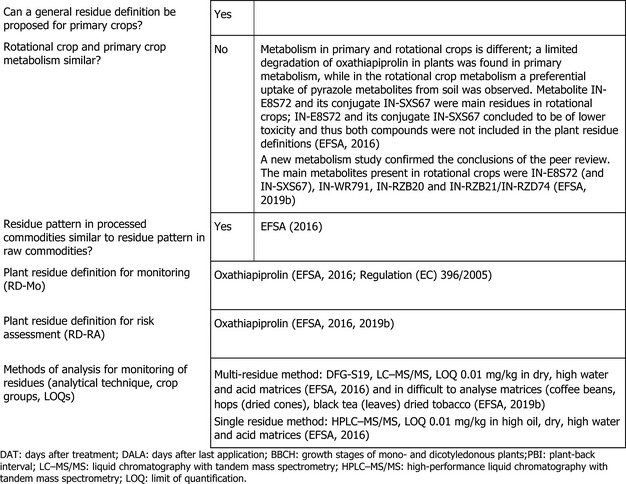
1.1.1. Nature of residues in primary crops
In the framework of the EU pesticides peer review, the metabolism of oxathiapiprolin in primary crops belonging to fruit (grape), leaf (lettuce) and root (potato) crops has been investigated following foliar application (EFSA, 2016). Due to the low total radioactive residue (TRR) at harvest, identification of the residues was not attempted in potato tubers. In grape, lettuce and potato leaves, oxathiapiprolin was observed as the major component of the TRR, accounting for 25–85%. In contrast, in mature grapes, 2 months after the last application, the main components were identified as metabolites IN‐E8S72 and IN‐WR791, representing 14.4% and 18.6% TRR (0.06 mg/kg), respectively.
Additional studies were evaluated in the previous EFSA assessment where the nature of oxathiapiprolin was investigated after soil application in root (potatoes), leafy (lettuce) and fruit (courgettes) crops (EFSA, 2019b).
The main components of the TRR in immature and mature edible matrices (potatoes, lettuce and courgettes) exceeding the trigger value of 10% were metabolites IN‐E8S72, IN‐WR791, IN‐RZB20 and IN‐RZB21/IN‐RZD74. The actual amounts, however, were low, being above 0.01 mg/kg only for metabolite IN‐WR791 in courgettes (0.016 mg/kg). All metabolites identified have also been observed in rotational crops and, to a lesser extent, in primary crops following foliar application (EFSA, 2016, 2019b).
For the authorised uses under consideration, the metabolic behaviour in primary crops is sufficiently addressed.
1.1.2. Nature of residues in rotational crops
Investigations of residues in rotational crops are not required for imported crops.
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of oxathiapiprolin was investigated in the framework of the EU pesticides peer review (EFSA, 2016). These studies showed that oxathiapiprolin is hydrolytically stable under standard processing conditions.
1.1.4. Methods of analysis in plants
Analytical methods for the determination of oxathiapiprolin residues in high oil, high starch, high water and high acid content commodities of plant origin were assessed during the EU pesticides peer review (EFSA, 2016).
The method using LC‐MS/MS is sufficiently validated for quantifying residues of oxathiapiprolin in the crops under consideration at or above the LOQ of 0.01 mg/kg.
1.1.5. Storage stability of residues in plants
The storage stability of oxathiapiprolin in plants stored under frozen conditions was investigated in the framework of the EU pesticides peer review (EFSA, 2016) (See Appendix B.1.1.2). It is concluded that in the relevant crop matrices under consideration, the freezer storage stability of oxathiapiprolin has been addressed for 18 months when stored at −20°C.
1.1.6. Proposed residue definitions
Based on the metabolic pattern identified in metabolism studies, the results of hydrolysis studies, the toxicological significance of metabolites and the capabilities of enforcement analytical methods, the following residue definitions were proposed:
residue definition for risk assessment: oxathiapiprolin
residue definition for enforcement: oxathiapiprolin
The same residue definitions are applicable to rotational crops and processed products. The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical.
Taking in account the authorised uses assessed in this application, EFSA concluded that these residue definitions are appropriate and no modification or further information is required.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In support of the authorised uses in the United States, the applicant submitted residue trials on various crops. The samples were analysed for the parent compound according to the residue definitions for enforcement and risk assessment. According to the assessment of the EMS, the methods used were sufficiently validated and fit for purpose (United Kingdom, 2020).
The samples of these residue trials were stored under conditions for which integrity of the samples has been demonstrated.
Citrus fruits
In support of the authorised foliar and soil treatment‐related outdoor good agricultural practices (GAPs) of oxathiapiprolin in the United States, the applicant submitted 23 outdoor residue trials on various citrus fruits (12 on oranges, 6 on grapefruits and 5 on lemons) performed in the USA from 2013 to 2014.
The trials were not strictly performed according to the registered label since both soil drench and foliar applications were combined and not applied separately, whereby on the registered label, it is stated that ‘foliar and soil applications must not be combined’. All 23 trials demonstrate that residues of oxathiapiprolin following soil application were below the LOQ of 0.01 mg/kg. In addition, all trials exhibit a PHI of 30 days following soil drench treatment and consequently do not reflect the GAP for soil drench treatment. It has therefore to be noted that the MRL proposal is based on the foliar application only.
The applicant proposed to extrapolate with a merged residue data set on oranges, grapefruits and lemons to the whole group of citrus fruits which is not in line with the EU guidance document (European Commission, 2017), since at least three more GAP compliant trials on lemons would be required. However, since it was demonstrated that the orange, grapefruit and lemon data sets were not statistically different, EFSA supports the EMS proposal to derive an MRL for the whole group of citrus fruits.
It is concluded that an MRL of 0.05 mg/kg would suffice to support the authorised foliar outdoor uses of oxathiapiprolin on citrus fruits. For the soil drench treatment, trials are not compliant to support the import tolerance. The tolerance established in the USA7 for oxathiapiprolin in citrus fruit is 0.06 mg/kg.
Cane fruits: raspberries, blackberries and dewberries
In support of the authorised outdoor soil treatment GAP of oxathiapiprolin on cane fruit in Canada, five outdoor trials were provided on raspberries (four trials) and blackberries (one trial) in the 2012 growing season. The trials were independent and in compliance with the authorised GAP.
The applicant proposes to extrapolate the merged residue data set on raspberries and blackberries and to the whole subgroup cane fruits (includes dewberries). According to EU guidance document (European Commission, 2017), such an extrapolation could be supported, provided that one more GAP compliant residue trial on raspberries or blackberries is available. The current residue data set is therefore sufficient to derive an MRL proposal of 0.5 mg/kg only for raspberries and blackberries in support of the authorised GAP of oxathiapiprolin on these crops in the USA. For dewberries, no MRL proposal is derived. The tolerance established in the USA7 for oxathiapiprolin in cane fruit is 0.5 mg/kg.
Potatoes and sweet potatoes
In support of the authorised outdoor GAPs based on either foliar or soil treatments, the applicant submitted 16 residue trials on potatoes performed with a combination of the authorised uses. Trials were performed in the United States over the 2014–2015 growing season.
The residue trials submitted for the authorised soil treatment were not compliant with the GAP, since the second application was performed at BBCH 01 to 60 which corresponds to the growth stages of ‘beginning of sprouting’ and ‘first open flowers’ and not at the time of planting as indicated in the authorised GAP. In addition, in most of the trials, the interval between applications was not according to GAP. Therefore, the trials were deviating from the authorised GAP for more than one parameter and beyond 25% tolerance and trials were thus considered incompliant.
The residue trials submitted for the authorised foliar treatment were also not compliant with the GAP, since of six applications, the first was made in‐furrow at planting, the second by spraying at hilling both at a rate of 140 g a.s./ha, whereby the four subsequent broadcast foliar sprays were performed at a rate of 50 g a.s./ha. However, according to the registered label, foliar and soil applications shall not be combined. It is evident from some trials that residues above LOQ following soil applications occurred. Therefore, both application types contribute to the final residues which consequently cannot be attributed solely to the foliar treatment. Regarding the two initial soil treatments of the 16 trials, the same shortcomings as discussed in the paragraph above apply.
None of the available trials was performed in accordance with the authorised GAPs, and as a result, the trials cannot be used to derive MRL proposals for oxathiapiprolin in potatoes and sweet potatoes. The tolerance established in the USA7 for oxathiapiprolin in potato and sweet potato is 0.04 mg/kg.
Chinese cabbage
In support of the authorised outdoor foliar treatment GAP on Chinese cabbage in the United States, 10 trials were performed on mustard greens in the United States and Canada during the 2013 growing season. In five of the 10 trials, the maximum storage period of trial samples exceeds the acceptable storage period of 18 months at −20°C. Noting, however, that no residue decline was observed within 18 months of storage, the EMS considered a storage interval extension of 10% acceptable, and therefore, two trials within 19.1 and 18.6 months of storage were considered valid (United Kingdom, 2020). EFSA agrees with the proposal of the EMS. Thus, in total, seven GAP compliant residue trials on mustard greens are available. The applicant proposes to extrapolate residue data in mustard greens to Chinese cabbage.
It is noted that according to the EU guidance document (European Commission, 2017), four trials on Chinese cabbage or kale would be required to support an MRL proposal for Chinese cabbage. Nevertheless, the EMS proposed to support an extrapolation from mustard greens to Chinese cabbage in this specific case because both commodities are considered agronomically similar and the seven trials on mustard greens were harvested for their leaves at a suitable stage noting that Chinese cabbage is also grown for its leaves. Since mustard greens, according to Part B of Annex I of Regulation (EU) 2018/628 are classified as a subgroup of Chinese cabbage, EFSA accepts the proposal of the applicant and the EMS to use mustard green residue data for deriving an MRL proposal of 9 mg/kg in Chinese cabbage. The tolerance established in the USA7 for oxathiapiprolin in Chinese cabbage is 10 mg/kg.
Basil and edible flowers
In support of the authorised outdoor foliar treatment GAP on basil in the United States, six trials were performed on basil in the United States and Canada during the 2012 growing season. All trials were performed according to the authorised GAP.
EFSA concludes that the available trials are sufficient to derive an MRL proposal of 10.0 mg/kg on fresh basil and edible flowers in support of the authorised GAP. The tolerance established in the USA7 for oxathiapiprolin in basil and edible flowers is 10.0 mg/kg.
Asparagus
In support of the authorised outdoor soil treatment GAP on asparagus in the United States, eight GAP compliant trials were performed on asparagus in the United States during the 2012 growing season. It is noted that on the USA label two application methods, namely soil‐directed banded spray and drip irrigation (chemigation), are stated. Six residue trials were performed with direct spraying and two trials were performed by drip irrigation. Both trials which were performed with drip irrigation had residues below the LOQ of 0.01 mg/kg. On the other hand, detectable residues were observed by direct spraying. Therefore, to propose an MRL, it was relied upon the six residue trials performed by direct spraying.
EFSA concludes that the available trials are sufficient to derive an MRL proposal of 2.0 mg/kg on asparagus in support of the authorised GAP using direct spraying. The tolerance established in the USA7 for oxathiapiprolin in asparagus is 2.0 mg/kg.
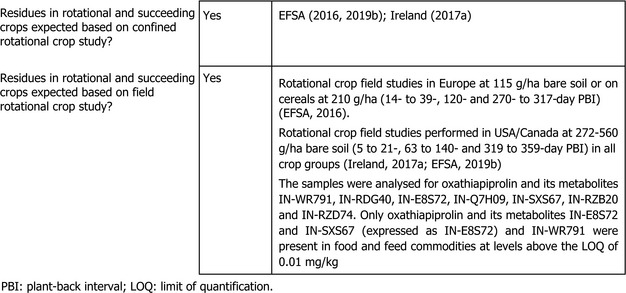
1.2.2. Magnitude of residues in rotational crops
The investigation of rotational crops is of no relevance for the import tolerance requests considered under the assessment. However, it is noted that the maximum application rate of 281 g a.s./ha soil of this assessment is less critical than the rate of 600 g a.s./ha which was assessed by EFSA previously (EFSA, 2016, 2019b), and therefore, the magnitude of rotational crops does not need to be considered any further.
1.2.3. Magnitude of residues in processed commodities
New studies investigating the effect of processing on the magnitude of residues in dried basil, orange juice, orange dried pulp and orange oil have been submitted (United Kingdom, 2020). All these studies, except the study with orange dried pulp, suffice to derive robust processing factors which are recommended to be included in Annex VI of Regulation (EC) No 396/2005.
An overview of derived processing factors is presented in Appendix B.1.2.3.
For orange dried pulp, the processing factors of two available studies differ more than 50% and in principle a third processing study would be needed according to OECD guidance document (OECD, 2008b). For the animal dietary burden, to account for worst‐case situation, the highest processing factor of 3.7 was used.
The applicant proposes to extrapolate available processing factors from studies on canned tomato fruits to canning of small berries (relevant for raspberries and blackberries under consideration) and from grape juice to juice from small berries (United Kingdom, 2020). These processing factors have been previously derived in the framework of the EU pesticides peer review (EFSA, 2016). Considering low overall consumer exposure to oxathiapiprolin residues (see Section 3), these processing factors were not considered by EFSA in the consumer exposure assessment.
In addition, for citrus fruits, a median peeling factor of 0.56 (0.445 for oranges, 0.56 for lemons and 0.83 grapefruits) was derived from the supervised residue trials. It is noted that in pulp measured residues were always below LOQ of 0.01 mg/kg. A concentration of residues was evident in orange dried pulp and dried basil.
However, since the exposure to residues from the intake of citrus fruits, blackberries, raspberries, Chinese cabbage, basil and edible flowers and asparagus to the overall dietary intake is very low (ca 3% of the ADI for NL toddlers), processing studies are not expected to significantly affect the outcome of the exposure assessment.
1.2.4. Proposed MRLs
The available data are sufficient to derive MRL proposals as well as risk assessment values for all commodities under evaluation, except for dewberries, potatoes and sweet potatoes (see Appendix B.1.2.1). In Section 3, EFSA assessed whether residues on these crops resulting from the uses authorised for import tolerance requests are likely to pose a consumer health risk.
2. Residues in livestock
Since some of the imported crops or their by‐products (citrus dried pulp) can enter EU livestock feed chain, EFSA calculated the EU livestock dietary burden, considering residues in citrus dried pulp. The calculated dietary burden did not exceed the trigger value of 0.004 mg/kg bw per day (see Appendices B.2 and D.1). The contribution of residues in citrus dried pulp to the current EU livestock dietary exposure as calculated in the previous EFSA assessment (EFSA, 2019b) was found to be insignificant. Thus, the nature and magnitude of oxathiapiprolin residues in livestock were not investigated further.
3. Consumer risk assessment
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2019a). This exposure assessment model contains food consumption data for different subgroups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (EFSA, 2018, 2019a).
The toxicological reference value for oxathiapiprolin used in the risk assessment (i.e. ADI value of 0.14 mg/kg bw per day) was derived in the framework of the EU pesticides peer review (EFSA, 2016). Considering the toxicological profile of the active substance, a short‐term dietary risk assessment was not required.
The long‐term exposure assessment was performed, taking into account the supervised trials median residue (STMR) values derived for the commodities assessed in this application. For the remaining commodities, including the crops for which the data submitted in the framework of the current assessment were insufficient to derive MRL proposals – dewberries, potatoes, sweet potatoes – the existing EU MRLs as established in the working document SANTE/11822/2019 were used as input values. For several of these commodities, the STMR values were available as derived in the previous EFSA assessments (EFSA, 2016, 2019b). The complete list of input values is presented in Appendix D.2.
The estimated long‐term dietary intake accounted for a maximum of 3% of the ADI for NL toddler diet. The contribution of residues expected in the commodities assessed in this application to the overall long‐term exposure is presented in more detail in Appendix B.3.
EFSA concluded that the long‐term intake of residues of oxathiapiprolin resulting from the existing and the authorised uses is unlikely to present a risk to consumer health. For further details on the exposure calculations, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
4. Conclusion and Recommendations
The data submitted in support of this MRL application were found to suffice to derive MRL proposals for all crops under consideration except for dewberries, potatoes and sweet potatoes. EFSA concluded that the authorised use of oxathiapiprolin on the crops under consideration will not result in a consumer exposure exceeding the toxicological reference value and therefore is unlikely to pose a risk to consumers’ health.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CCPR
Codex Committee on Pesticide Residues
- CF
conversion factor for enforcement to risk assessment residue definition
- cGAP
critical GAP
- CIRCA
(EU) Communication & Information Resource Centre Administrator
- CS
capsule suspension
- CV
coefficient of variation (relative standard deviation)
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DP
dustable powder
- DS
powder for dry seed treatment
- dw
dry weight
- EC
emulsifiable concentrate
- EDI
estimated daily intake
- EMS
evaluating Member State
- eq
residue expressed as a.s. equivalent
- FID
flame ionisation detector
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐MS/MS
gas chromatography with tandem mass spectrometry
- GC‐MS
gas chromatography with mass spectrometry
- GS
growth stage
- HPLC
high‐performance liquid chromatography
- HPLC‐MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HPLC‐MS
high‐performance liquid chromatography with mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LOQ
limit of quantification
- MRL
maximum residue level
- MS/MS
tandem mass spectrometry detector
- MS
mass spectrometry detector
- MS
Member States
- MW
molecular weight
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
pre‐harvest interval
- Pow
partition coefficient between n‐octanol and water
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RAC
raw agricultural commodity
- RA
risk assessment
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SEU
southern Europe
- SL
soluble concentrate
- SP
water‐soluble powder
- STMR
supervised trials median residue
- TAR
total applied radioactivity
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- WHO
World Health Organization
- WP
wettable powder
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
1.
| Crop |
NEU, SEU, MS or country |
F G or Ia |
Pests or Group of pests controlled |
Preparation | Application | Application rate per treatment |
PHI (days)d |
Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typeb |
Conc. a.s. |
Method kind | Range of growth stages & seasonc |
Number min–max |
Interval between application (min) |
g a.s./hL min–max |
Water L/ha min–max |
Rate | Unit | ||||||
| Grapefruits | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Grapefruits | USA | F | Fungi | SC | 200.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Grapefruits | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Grapefruits | USA | F | Fungi | OD | 99.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Oranges | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Oranges | USA | F | Fungi | SC | 200.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Oranges | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Oranges | USA | F | Fungi | OD | 99.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Lemons | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Lemons | USA | F | Fungi | SC | 200.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Lemons | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Lemons | USA | F | Fungi | OD | 99.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Limes | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Limes | USA | F | Fungi | SC | 200.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Limes | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Limes | USA | F | Fungi | OD | 99.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Mandarins | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Mandarins | USA | F | Fungi | SC | 200.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Mandarins | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment* – broadcast spraying | n/a | 1 | n/a | 35 g a.i./ha | 0 | ||||
| Mandarins | USA | F | Fungi | OD | 99.0 g/L | Soil treatment* – general | n/a | 2 | 30 | 140 g a.i./ha | 0 | ||||
| Blackberries | USA | F | Fungi | SC | 200.0 g/L | Soil treatment – general | n/a | 2 | 7 | 281 g a.i./ha | 1 | ||||
| Blackberries | USA | F | Fungi | OD | 99.0 g/L | Soil treatment – general | n/a | 2 | 7 | 281 g a.i./ha | 1 | ||||
| Dewberries | USA | F | Fungi | SC | 200.0 g/L | Soil treatment – general (see also comment field) | n/a | 2 | 7 | 281 g a.i./ha | 1 | ||||
| Dewberries | USA | F | Fungi | OD | 99.0 g/L | Soil treatment – general | n/a | 2 | 7 | 281 g a.i./ha | 1 | ||||
| Raspberries (red and yellow) | USA | F | Fungi | SC | 200.0 g/L | Soil treatment – general | n/a | 2 | 7 | 281 g a.i./ha | 1 | ||||
| Raspberries (red and yellow) | USA | F | Fungi | OD | 99.0 g/L | Soil treatment – general | n/a | 2 | 7 | 281 g a.i./ha | 1 | ||||
| Potatoes | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 5 | ||||
| Potatoes | USA | F | Fungi | SC | 200.0 g/L | Soil treatment – general | planting | 2 | 10–14 | 140 g a.i./ha | n/a | ||||
| Potatoes | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 5 | ||||
| Potatoes | USA | F | Fungi | OD | 99.0 g/L | Soil treatment – general | planting | 2 | 10–14 | 140 g a.i./ha | n/a | ||||
| Sweet potatoes | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 5 | ||||
| Sweet potatoes | USA | F | Fungi | SC | 200.0 g/L | Soil treatment – general | planting | 2 | 10–14 | 140 g a.i./ha | n/a** | ||||
| Sweet potatoes | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 5 | ||||
| Sweet potatoes | USA | F | Fungi | OD | 99.0 g/L | Soil treatment – general | planting | 2 | 10–14 | 140 g a.i./ha | n/a** | ||||
| Chinese cabbages/pe‐tsai | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 0 | ||||
| Chinese cabbages/pe‐tsai | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 0 | ||||
| Basil and edible flowers | USA | F | Fungi | SC | 200.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 0 | ||||
| Basil and edible flowers | USA | F | Fungi | OD | 99.0 g/L | Foliar treatment – broadcast spraying | n/a | 4 | 5 | 35 g a.i./ha | 0 | ||||
| Asparagus | USA | F | Fungi | SC | 200.0 g/L | Soil treatment – general | n/a | 2 | 14 | 281 g a.i./ha | 0 | ||||
| Asparagus | USA | F | Fungi | OD | 99.0 g/L | Soil treatment – general | n/a | 2 | 14 | 281 g a.i./ha | 0 | ||||
NEU: northern European Union; SEU: southern European Union; MS: Member State; n/a: not applicable.
*: According to the registered labels, foliar and soil applications must not be combined.
**: According to the registered labels, oxathiapiprolin should be applied as an in‐furrow application at planting. Therefore, a PHI is not relevant to the soil application use/GAP.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/source |
|---|---|---|---|---|---|
| Fruit crops | Grapes | Foliar: 3 × 70 g/ha (BBCH 63‐65; BBCH 73 and 77; 14 d interval) |
Foliage: 0 DAT1,2,3, 14 DAT2,3, 76 DALA Berries: 14 DAT2,3, 0 DAT3, 76 DALA |
Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |
| Courgette | Soil: 1 × 600 g/ha (pre‐planting) | 44 DAT, 79 DAT (maturity) | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (EFSA, 2019b) | ||
| Root crops | Potatoes | Soil: 1 × 600 g/ha (pre‐planting) | Foliage, tubers: 37 DAT, 72 DAT (maturity) | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (EFSA, 2019b) | |
| Foliar: 3 × 70 g/ha (BBCH 53; BBCH 59 and 69; 14 day interval |
Foliage, tubers: 0 DAT2 (foliage only), 14 DAT1,2,3, 28 DAT3 |
Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |||
| Leafy crops | Lettuce | Foliar: 3 × 70 g/ha (BBCH 15; BBCH 17 and 19; 10 d interval) | 0 DAT1,2,3, 10 DAT1,2, 0 DAT3, 3, 7, 14 DALA | Radiolabelled active substance: pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |
| Soil: 1 × 600 g/ha (pre‐planting) | 30, 44, 57 DAT | Radiolabelled active substance: pyrazole‐14C‐ and isoxazoline‐14C‐oxathiapiprolin (EFSA, 2019b) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/source |
|---|---|---|---|---|---|
| Root/tuber crops | Turnip | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin (EFSA, 2016) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (EFSA, 2019b) | ||||
| Leafy crops | Lettuce | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin. (EFSA, 2016) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (EFSA, 2019b) | ||||
| Cereal (small grain) | Wheat | Soil: 1 × 210 g/ha | 30, 120 and 365 DAT | Radiolabelled active substance: pyrazole‐14C‐, thiazole‐14C‐ and isoxazoline‐14C oxathiapiprolin (EFSA, 2016) | |
| Soil: 1 × 600 g/ha | Radiolabelled active substance: pyrazole‐14C and isoxazoline‐14C oxathiapiprolin (EFSA, 2019b) |
|
Processed commodities (hydrolysis study) |
Conditions | Stable? | Comment/source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Studies performed with pyrazole‐14C‐ and thiazole‐14C‐oxathiapiprolin (EFSA, 2016) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||
| Sterilisation (20 min, 120°C, pH 6) | Yes | ||
| Other processing conditions | – | – |
B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Tomatoes | –20 | 18 | months | Oxathiapiprolin, IN‐Q7H09,IN‐RDG40,IN‐E8S72,IN‐RZB20,IN‐RZD74,IN‐SXS67 and IN‐WR791 | EFSA, 2016 | |
| High oil content | Soybean seed | ||||||
| High protein content | Dried bean seed | ||||||
| Dry/High starch | Potatoes, wheat | ||||||
| High acid content | Grapes | ||||||
| Others | Wheat forage | ||||||
| Rape dry pomace | |||||||
| Wheat straw | |||||||
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/Indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) |
HRb (mg/kg) |
STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin Risk assessment residue definition: Oxathiapiprolin | ||||||
| Citrus fruits (grapefruits, oranges, lemons, limes, mandarins) | USA/outdoor, foliar | Whole fruit:Oranges: 4× < 0.01, 0.01, 0.016, 0.020, 2× 0.022, 0.023, 0.023d, 0.024Grapefruit: 3× < 0.01, 0.011, 0.012, 0.018Lemon: 2× < 0.01, 0.015, 0.022, 0.033Pulp:Oranges: 9× < 0.01Grapefruit: 5× < 0.01Lemons: 5× < 0.01 | Residue trials on citrus fruits with combined soil and foliar application compliant with the GAP. The MRL proposal is based on the data from foliar application, noting that after soil treatment, a no‐residue situation is confirmed.Since residue data on oranges, grapefruits and lemons are not statistically different, it was accepted to combine the residue data and to extrapolate to the whole group of citrus fruits | 0.05 | 0.033 Pulp: < 0.01 | 0.012Pulp: < 0.01 |
| Citrus fruits (grapefruits, oranges, lemons, limes, mandarins) | USA/outdoor, soil treatment | – | No GAP compliant residue trials available | – | – | – |
| Cane fruits (raspberries, blackberries, dewberries) | Canada/outdoor | Raspberries: < 0.01, < 0.01, 0.022d, 0.22Blackberries: < 0.01 | Sufficient number of GAP compliant trials on raspberries and blackberries submitted to derive an MRL proposal for raspberries and blackberriesThe residue data are not sufficient to derive an MRL for dewberries | 0.5 | 0.22 | 0.01 |
| Potatoes, Sweet potatoes | USA/outdoor, foliar treatment | – | Provided 16 trials on potato incompliant with the authorised GAP.An amendment of the current EU MRL of 0.01* mg/kg is not supported | – | – | – |
| Potatoes, Sweet potatoes | USA/outdoor, soil treatment | – | Provided 16 trials on potato incompliant with the authorised GAPAn amendment of the current EU MRL of 0.01* mg/kg is not supported | – | – | – |
| Chinese cabbage | USA, Canada/outdoor | 1.5, 1.7, 2.8, 2.9, 3.0, 4.2, 4.3 | Sufficient number of GAP compliant trials on mustard greens submitted. Extrapolation to Chinese cabbage acceptable | 9.0 | 4.3 | 2.9 |
| Basil and edible flowers | USA, Canada/outdoor | Fresh basil: 2.4, 2.6, 2.9, 3.2, 3.8, 5.4 | Sufficient number of GAP compliant trials on (fresh) basil submitted | 10.0 | 5.4 | 3.05 |
| Asparagus | USA/outdoor | 0.28, 0.35, 0.53, 0.58, 0.71, 0.75 | Sufficient number of GAP compliant residue trials on asparagus submitted. MRL proposal is based on the direct spray application on soil (not chemigation where residues were below LOQ) | 2.0 | 0.75 | 0.56 |
Values in bold are the MRL proposals derived for the cGAP.
Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe; Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Higher residues at a longer PHI interval of 6 days.
B.1.2.2. Residues in rotational crops
B.1.2.3. Processing factors
| Processed commodity | Number of valid studiesa | Processing factor (PF) | CFP b | Comment/source | |
|---|---|---|---|---|---|
| Individual values | Median PF | ||||
| Hops, beer | 3 | < 0.01; < 0.01; < 0.03 | 0.01 | 1 | Ireland (2017b) |
| Grape, Juice | 4 | 0.13; 0.14; 0.18; 0.22 | 0.16 | 1 | EFSA (2016) |
| Grape, Raisins | 4 | 0.9; 1.3; 1.6; 4.1 | 1.45 | 1 | EFSA (2016) |
| Grape, Red wine | 2 | 0.10; 0.18 | No proposal | 1 | EFSA (2016) |
| Grape, White wine | 2 | 0.08; 0.17 | No proposal | 1 | EFSA, 2016 |
| Grape, Overall wine (white and red) | 4 | 0.08; 0.10; 0.17; 0.18 | 0.14 | 1 | EFSA (2016) |
| Tomato, Washed | 3 | 0.4; 2× 0.5 | 0.5 | 1 | EFSA (2016) |
| Tomato, Sun‐dried | 3 | 2.9; 6.9; 7.2 | 6.9 | 1 | EFSA (2016) |
| Tomato, Peeled | 3 | < 0.01; 0.01; 0.05 | 0.01 | 1 | EFSA (2016) |
| Tomato, Canned | 3 | < 0.01; 2× 0.01 | 0.01 | 1 | EFSA (2016) |
| Tomato, juice | 3 | 2× 0.2; 0.3 | 0.2 | 1 | EFSA (2016) |
| Tomato, Wet tomato juice | 3 | 11; 13; 14 | 13 | 1 | EFSA (2016) |
| Tomato, paste | 3 | 0.7; 2× 1.1 | 1.1 | 1 | EFSA (2016) |
| Tomato, puree | 3 | 0.3; 2× 0.6 | 0.6 | 1 | EFSA (2016) |
| Potato, washed tubers | 3 | 0.03; 0.05; 0.7 | 0.05 | 1 | EFSA (2016) |
| Potato, culls | 3 | 2× 0.1; 0.7 | 0.1 | 1 | EFSA (2016) |
| Potato, Steam‐peeled tubers | 3 | < 0.005; < 0.03; < 0.08 | < 0.03 | 1 | EFSA (2016) |
| Potato, steam waste | 3 | 0.9; 1.2; 2.7 | 1.2 | 1 | EFSA (2016) |
| Potato, abrasion‐peeled tubers | 3 | < 0.005; < 0.03; < 0.08 | < 0.03 | 1 | EFSA (2016) |
| Potato, Abrasive waste | 3 | 0.3; 0.4; 3.6 | 0.4 | 1 | EFSA (2016) |
| Potato, dried flakes | 3 | < 0.005; < 0.03; < 0.08 | < 0.03 | 1 | EFSA (2016) |
| Potato, chips | 3 | < 0.005; < 0.03; < 0.08 | < 0.03 | 1 | EFSA (2016) |
| Potato, peeled French fries | 3 | < 0.005; < 0.03; <0.08 | < 0.03 | 1 | EFSA (2016) |
| Potato, unpeeled French fries | 3 | 0.04; 0.05; 0.2 | 0.05 | 1 | EFSA (2016) |
| Potato, unpeeled potatoes | 3 | 0.03; 0.04; 0.2 | 0.04 | 1 | EFSA (2016) |
| Potato, boiled peeled potatoes | 3 | < 0.005; < 0.03; < 0.08 | < 0.03 | 1 | EFSA (2016) |
| Potato, Microwaved unpeeled (baked) | 3 | 0.04; 0.05; 0.4 | 0.05 | 1 | EFSA (2016) |
| Orange, peeled (whole fruit to pulpd | 8 | < 0.063; < 0.42; < 0.43; < 0.44; < 0.45; < 0.45; < 0.5; < 1.0 | < 0.445 | 1 | United Kingdom (2020) |
| Orange, orange juice | 2 | < 0.14; < 0.26 | < 0.02 | 1 | United Kingdom (2020) |
| Orange, dried pulp | 2 | 1.7; 3.7 | 3.7c | 1 | United Kingdom (2020) |
| Orange, orange oil | 2 | 43; 50 | 47 | 1 | United Kingdom, 2020 |
| Grapefruit, peeled (whole fruit to pulpd | 3 | < 0.56; < 0.83; < 0.91 | < 0.83 | 1 | United Kingdom (2020) |
| Lemon, peeled (whole fruit to pulpd | 3 | < 0.45; < 0.67 | < 0.56 | 1 | United Kingdom (2020) |
| Basil, dried basil | 4 | 5.3; 7.6; 10; 11 | 8.8 | 1 | United Kingdom (2020) |
Studies with residues in the RAC at or close to the LOQ were disregarded (unless concentration may occur).
Conversion factor for risk assessment in the processed commodity; median of the individual conversion factors for each processing residues trial.
Tentative, since the two processing studies have more than 50% divergence. A third study required (OECD, 2008b).
The residues in pulp were always < LOQ of 0.01 mg/kg.
B.2. Residues in livestock
Dietary burden calculation according to OECD, 2013.
| Relevant groups (sub groups) | Dietary burden expressed in | Most critical sub groupa | Most critical commodityb | Trigger exceeded (Y/N) | Previous assessment (EFSA, 2019b) | |||
|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | |||||||
| Median | Maximum | Median | Maximum |
Max burden mg/kg bw per day |
||||
| Cattle (all) | 0.002 | 0.002 | 0.05 | 0.05 | potato | process waste | N | 0.002 |
| Cattle (dairy only) | 0.002 | 0.002 | 0.04 | 0.04 | potato | process waste | N | 0.002 |
| Sheep (all) | 0.002 | 0.002 | 0.05 | 0.05 | potato | process waste | N | 0.002 |
| Sheep (ewe only) | 0.002 | 0.002 | 0.05 | 0.05 | potato | process waste | N | 0.002 |
| Swine (all) | 0.001 | 0.001 | 0.04 | 0.04 | potato | process waste | N | 0.001 |
| Poultry (all) | 0.001 | 0.001 | 0.01 | 0.01 | potato | culls | N | 0.001 |
| Poultry (layer only) | 0.000 | 0.000 | 0.01 | 0.01 | potato | culls | N | 0.000 |
| Fish | Not investigated however since only potato protein represents a feed commodity for fish residues are not expected. | n/a | ||||||
bw: body weight; DM: dry matter.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.3. Consumer risk assessment
Not relevant since no ARfD has been considered necessary.
B.4. Recommended MRLs
| Codea | Commodity |
Existing EU MRL (mg/kg) |
Proposed EU MRL (mg/kg) |
Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Oxathiapiprolin | ||||
| 011000 | Citrus fruits | 0.01* | 0.05 | The submitted data are sufficient to derive an MRL proposal for the authorised foliar use in the USA. Risk for consumers unlikelyThe submitted data are not sufficient to support the authorised soil use |
| 153010 | Blackberries | 0.01* | 0.5 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 153020 | Dewberries | 0.01* | No MRL proposal | The submitted data are not sufficient to derive an MRL proposal for the import tolerance |
| 153030 | Raspberries (red and yellow) | 0.01* | 0.5 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 211000 | Potatoes | 0.01* | No MRL proposal | The submitted data are incompliant to derive an MRL proposal for the import tolerances |
| 212020 | Sweet potatoes | 0.01* | No MRL proposal | The submitted data are incompliant to derive an MRL proposal for the import tolerances |
| 243010 | Chinese cabbage/pe‐tsai | 0.01* | 9 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 256080 | Basil and edible flowers | 0.01* | 10 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
| 270010 | Asparagus | 0.01* | 2 | The submitted data are sufficient to derive an MRL proposal for the import tolerance. Risk for consumers unlikely |
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.

Appendix D – Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Risk assessment residue definition: oxathiapiprolin | ||||
| Sunflower seeds meal | 0.01 | STMRa (EFSA, 2019b) | 0.01 | STMRa (EFSA, 2019b) |
| Potato culls | 0.01 | STMR (EFSA, 2016) | 0.01 | STMR (EFSA, 2016) |
| Potato process waste | 0.01 | STMRb (EFSA, 2019b) | 0.01 | STMRb (EFSA, 2019b) |
| Potato dried pulp | 0.01 | STMRb (EFSA, 2019b) | 0.01 | STMRb (EFSA, 2019b) |
| Citrus group, dried pulp | 0.012 | STMR × PFc | 0.012 | STMR × PFc |
STMR: supervised trials median residue; PF: processing factor.
For sunflower seeds meal no default processing factor was applied because oxathiapiprolin is applied early in the growing season and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected.
For potato process waste and potato dried pulp, the default processing factors were not applied as residues in RAC were below the LOQ and residue concentration in processed fractions are not expected.
For citrus, dried pulp, in the absence of a robust processing factor supported by data, the highest processing factor of 3.7 was included in the calculation to consider the potential concentration of residues in these commodities.
D.2. Consumer risk assessment
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Grapefruits | < 0.01 | STMR‐Pulp | Not performed since no ARfD was established and it was not considered necessary | |
| Oranges | < 0.01 | STMR‐Pulp | ||
| Lemons | < 0.01 | STMR‐Pulp | ||
| Limes | < 0.01 | STMR‐Pulp | ||
| Mandarins | < 0.01 | STMR‐Pulp | ||
| Table grapes | 0.12 | STMR (EFSA, 2019b) | ||
| Wine grapes | 0.12 | STMR (EFSA, 2019b) | ||
| Blackberries | 0.01 | STMR | ||
| Raspberries (red and yellow) | 0.01 | STMR | ||
| Potatoes | 0.01 | STMR (EFSA, 2016) | ||
| Garlic | 0.01 | STMR (EFSA, 2019b) | ||
| Onions | 0.01 | STMR (EFSA, 2019b) | ||
| Shallots | 0.01 | STMR (EFSA, 2019b) | ||
| Spring onions/green onions and Welsh onions | 0.57 | STMR (EFSA, 2019b) | ||
| Tomatoes | 0.04 | STMR (EFSA, 2019b) | ||
| Sweet peppers/bell peppers | 0.04 | STMR (EFSA, 2019b) | ||
| Aubergines/egg plants | 0.04 | STMR (EFSA, 2019b) | ||
| Okra/lady's fingers | 0.04 | STMR (EFSA, 2019b) | ||
| Cucumbers | 0.03 | STMR (EFSA, 2019b) | ||
| Gherkins | 0.03 | STMR (EFSA, 2019b) | ||
| Courgettes | 0.03 | STMR (EFSA, 2019b) | ||
| Melons | 0.05 | STMR (EFSA, 2019b) | ||
| Pumpkins | 0.05 | STMR (EFSA, 2019b) | ||
| Watermelons | 0.05 | STMR (EFSA, 2019b) | ||
| Broccoli | 0.12 | STMR (EFSA, 2019b) | ||
| Cauliflowers | 0.12 | STMR (EFSA, 2019b) | ||
| Head cabbages | 0.14 | STMR (EFSA, 2019b) | ||
| Chinese cabbages/pe‐tsai | 2.9 | STMR | ||
| Lamb's lettuce/corn salads | 1.3 | STMR (EFSA, 2019b) | ||
| Lettuces | 1.3 | STMR (EFSA, 2019b) | ||
| Escaroles/broad‐leaved endives | 1.3 | STMR (EFSA, 2019b) | ||
| Cress and other sprouts and shoots | 1.3 | STMR (EFSA, 2019b) | ||
| Land cress | 1.3 | STMR (EFSA, 2019b) | ||
| Roman rocket/rucola | 1.3 | STMR (EFSA, 2019b) | ||
| Red mustards | 1.3 | STMR (EFSA, 2019b) | ||
| Baby leaf crops (including brassica species) | 1.3 | STMR (EFSA, 2019b) | ||
| Spinaches | 3.35 | STMR (EFSA, 2019b) | ||
| Purslanes | 3.35 | STMR (EFSA, 2019b) | ||
| Chards/beet leaves | 3.35 | STMR (EFSA, 2019b) | ||
| Potatoes | 0.01 | STMR (EFSA, 2016) | ||
| Grape leaves and similar species | 8.8 | STMR (EFSA, 2016) | ||
| Basil and edible flowers | 3.05 | STMR | ||
| Peas (with pods) | 0.29 | STMR (EFSA, 2019b) | ||
| Asparagus | 0.56 | STMR | ||
| Leeks | 0.57 | STMR (EFSA, 2019b) | ||
| Sunflower seeds | 0.01 | STMR (EFSA, 2019b) | ||
| Ginseng root | 0.05 | STMR (EFSA, 2019b) | ||
| Hops(dried) | 1.6 | STMR (EFSA, 2019b) | ||
| Other crops/commodities | MRL | SANTE/11822/2019 | ||
STMR: supervised trials median residue.
Appendix E – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notation/InChiKeya | Structural formulab |
|---|---|---|
| Oxathiapiprolin |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluorophenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}‐1‐piperidyl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethenone FC(F)(F)c1cc(C)n(n1)CC(=O)N1CCC(CC1)c1nc(cs1)C=1CC(ON=1)c1c(F)cccc1F IAQLCKZJGNTRDO‐UHFFFAOYSA‐N |
|
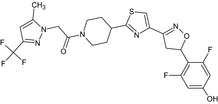
| IN‐Q7H09 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluoro‐4‐hydroxyphenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)cc(O)cc5F XYJWPIOIQYWLNP‐UHFFFAOYSA‐N |
|
| IN‐RDG40 |
1‐(4‐{4‐[(5RS)‐5‐(2,6‐difluoro‐3‐hydroxyphenyl)‐4,5‐dihydro‐1,2‐oxazol‐3‐yl]‐1,3‐thiazol‐2‐yl}piperidin‐1‐yl)‐2‐[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]ethanone FC(F)(F)c1cc(C)n(n1)CC(=O)N2CCC(CC2)c3nc(cs3)C=4CC(ON=4)c5c(F)ccc(O)c5F MCUWVCQCPFWXQQ‐UHFFFAOYSA‐N |
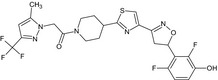
|
| IN‐E8S72 |
3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid FC(F)(F)c1cc(nn1)C(O)=O CIVNBJPTGRMGRS‐UHFFFAOYSA‐N |
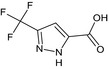
|
| IN‐SXS67 |
1‐β‐D‐glucopyranosyl‐3‐(trifluoromethyl)‐1H‐pyrazole‐5‐carboxylic acid O=C(O)c2cc(nn2[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C(F)(F)F IYVPJWXJEGAHCP‐DDIGBBAMSA‐N |
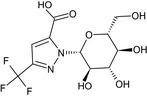
|
| IN‐WR791 |
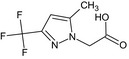
[5‐methyl‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetic acid OC(=O)Cn1nc(cc1C)C(F)(F)F RBHQAIFXLJIFFM‐UHFFFAOYSA‐N |
|
| IN‐RZB20 |
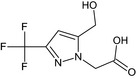
[5‐(hydroxymethyl)‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetic acid OC(=O)Cn1nc(cc1CO)C(F)(F)F LGHWWTCDTBCQQI‐UHFFFAOYSA‐N |
|
| IN‐RZB21 |
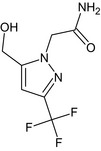
2‐[5‐(hydroxymethyl)‐3‐(trifluoromethyl)‐1H‐pyrazol‐1‐yl]acetamide O=C(N)Cn1nc(cc1CO)C(F)(F)F LDXIZNIPWOQNPY‐UHFFFAOYSA‐N |
|
| IN‐RZD74 |
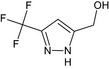
[3‐(trifluoromethyl)‐1H‐pyrazol‐5‐yl]methanol FC(F)(F)c1cc(CO)nn1 KUVPCLYQVMRTPU‐UHFFFAOYSA‐N |
|
ACD/Name 2018.2.2 ACD/Labs 2018 Release (File version N50E41, Build 103230, 21 July 2018).
ACD/ChemSketch 2018.2.2 ACD/Labs 2018 Release (File version C60H41, Build 106041, 07 December 2018).
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Bernasconi G, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2020. Reasoned Opinion on the setting of import tolerances for oxathiapiprolin in various crops. EFSA Journal 2020;18(6):6155, 34 pp. 10.2903/j.efsa.2020.6155
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00135
Acknowledgements: EFSA wishes to thank the following for the support provided to this scientific output: Chris Anagnostopoulos, Laszlo Bura, Georgios Chatzisotiriou, Viktoria Krivova, Silvia Ruocco and Viktor Toth.
Adopted: 18 May 2020
Notes
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2017/239 of 10 February 2017 approving the active substance oxathiapiprolin in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011 C/2017/0694 OJ L 36, 11.2.2017, p. 39.–42
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.03.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.selection&language=EN
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Federal Register/ Volume 81, Number 233, Monday/December 5, 2016/Rules and Regulations.
Commission Regulation (EU) 2018/62 of 17 January 2018 replacing Annex I to Regulation (EC) No 396/2005 of the European Parliament and of the Council, OJ L 18, 23.1.2018, p. 1–73.
References
- EFSA (European Food Safety Authority), 2016. Conclusion on the peer review of the pesticide risk assessment of the active substance oxathiapiprolin. EFSA Journal 2016;14(7):4504, 19 pp. 10.2903/j.efsa.2016.4504 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2019c. Scientific Report on scientific support for preparing an EU position in the 51st Session of the Codex Committee on Pesticide Residues (CCPR). EFSA Journal 2019;17(7):5797, 243 pp. 10.2903/j.efsa.2019.5797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019a. Pesticide Residue Intake Model – EFSA PRIMo revision 3.1. EFSA Supporting Publication 2019:16(3):EN‐1605, 15 pp. doi: 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Anastassiadou M, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2019b. Reasoned opinion on the modification of the existing maximum residue levels and setting of import tolerances for oxathiapiprolin in various commodities. EFSA Journal 2019;17(7):5759, 49 pp. 10.2903/j.efsa.2019.5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirement for Annex II (part A, section 4) and Annex III (part A, section 5 of Directive 91/414. SANCO/3029/99‐rev. 4.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2013. Working document on the nature of pesticide residues in fish. SANCO/11187/2013‐rev. 3, 31 January 2013.
- European Commission , 2016. Final review report for the active substance oxathiapiprolin. Finalised in the Standing Committee on Plants, Animal, Food and Feed at its meeting on 7 December 2016 in view of the approval of oxathiapiprolin as active substance in accordance with Regulation (EC) 1107/2009. SANTE/11169/2016 rev 1, 7 December 2016.
- European Commission , 2017. Appendix D. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. 7525/VI/95‐rev. 10.3, 13 June 2017.
- Ireland , 2015. Draft Assessment Report (DAR) on the active substance oxathiapiprolin prepared by the rapporteur Member State Ireland in the framework of Regulation (EC) No 1107/2009, February 2015. Available online: www.efsa.europa.eu
- Ireland , 2016. Revised Draft Assessment Report (DAR) on oxathiapiprolin prepared by the rapporteur Member State Ireland in the framework of Regulation (EC) No 1107/2009, March 2016. Available online: www.efsa.europa.eu
- Ireland , 2017a. Evaluation report on the setting of an Import Tolerance for oxathiapiprolin in multiple crop commodities. June 2017, updated on April 2018 and February 2019, 253 pp.
- Ireland , 2017b. Evaluation report on the modification of MRLs for oxathiapiprolin in wine grapes, bulb onions, peppers, sunflowers and hops. June 2017, updated on April 2018 and February 2019, 253 pp.
- OECD (Organisation for Economic Co‐operation and Development), 2007a. Test No. 501: Metabolism in Crops, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 25 January 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007b. Test No. 502: Metabolism in Rotational Crops, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 25 January 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007c. Test No. 503: Metabolism in Livestock, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 25 January 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007d. Test No. 504: Residues in Rotational Crops (Limited Field Studies), OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 25 January 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007e. Test No. 505: Residues in Livestock, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 25 January 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007f. Guidance Document on Pesticide Residue Analytical Methods. In: Series on Pesticides No 39 / Series on Testing and Assessment No 72. ENV/JM/MONO(2007)17, 13 August 2007.
- OECD (Organisation for Economic Co‐operation and Development), 2007g. Test No 506: Stability of Pesticide Residues in Stored Commodities, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 15 October 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2007h. Test No. 507: Nature of the Pesticide Residues in Processed Commodities ‐ High Temperature Hydrolysis, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 15 October 2007. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2008a. Guidance document on the magnitude of pesticide residues in processed commodities. In: Series of Testing and Assessment No 96. ENV/JM/MONO(2008)23, 29 July 2008.
- OECD (Organisation for Economic Co‐operation and Development), 2008b. Test No. 508: Magnitude of the Pesticide Residues in Processed Commodities, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 16 October 2008. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2009a. Definition of Residue. In: Series on Pesticides, No 31; Series on Testing and Assessment, No. 63. ENV/JM/MONO(2009)30, revision, published 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development), 2009b, Test No. 509: Crop Field Trial, OECD Guidelines for the Testing of Chemicals, Section 5. OECD Publishing, Paris, 07 Sep 2009. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues.
- OECD (Organisation for Economic Co‐operation and Development), 2013. Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 04 September 2013.
- OECD (Organisation for Economic Co‐operation and Development), 2016. Guidance Document on Crop Field Trials. In: Series on Pesticides No 66 / Series on Testing and Assessment No 164. 2nd Edition. ENV/JM/MONO(2011)50/REV1, ENV/JM/MONO(2011)50/REV1/ANN, 7 September 2016.
- OECD (Organisation for Economic Co‐operation and Development), 2018. Guidance Document on Residues in Rotational Crops. In: Series on Pesticides No 97. ENV/JM/MONO(2018)9, 22 May 2018.
- United Kingdom , 2020. Evaluation report on the MRL application on the setting of Import Tolerances for oxathiapiprolin in citrus fruits, cane fruit, potato, mustard green, basil and asparagus. January 2020, 104 pp.


