Abstract
The EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP) was requested by the European Commission to review the substances for which a Specific Migration Limit (SML) is not assigned in Regulation (EU) No 10/2011. These substances had been covered by the Generic SML of 60 mg/kg food, but with Regulation (EU) 2016/1416 it was removed, necessitating their re‐examination. EFSA was requested to identify those substances requiring an SML to ensure the authorisation is sufficiently protective to health, grouping them in high, medium and low priority to serve as the basis for future re‐evaluations of individual substances. The CEP Panel established a stepwise procedure. This took into account existing hazard assessments for each substance on carcinogenicity/mutagenicity/reprotoxicity (CMR), bioaccumulation and endocrine disruptor (ED) properties along with the use of in silico generated predictions on genotoxicity. Molecular weights and boiling points were considered with regard to their effect on potential consumer exposure. This prioritisation procedure was applied to a total of 451 substances, from which 78 substances were eliminated at the outset, as they had previously been evaluated by EFSA as food contact substances. For 89 substances, the Panel concluded that a migration limit should not be needed. These are in the lists 0 and 1 of the Scientific Committee for Food (SCF), defined as substances for which an Acceptable Daily Intake (ADI) does not need to be established, along with substances that are controlled by existing restrictions and/or generic limits. Of the remaining 284 substances, 179 were placed into the low priority group, 102 were placed into the medium priority group and 3 were placed into the high priority group, i.e. salicylic acid (FCM No 121), styrene (FCM No 193) and lauric acid, vinyl ester (FCM No 436).
Keywords: food contact materials, prioritisation, safety assessment
1. Introduction
1.1. Background and Terms of Reference as provided by the European Commission
Following our own review and discussions with the Member States and the industry, it became apparent that Regulation (EU) No 10/20111 on plastic food contact materials (‘the Regulation’) does not assign a Specific Migration Limit (SML) to approximately 460 substances which are listed in Table 1 of Annex 1. Certain substances may nonetheless require the specification of a limit to ensure their authorisation is sufficiently protective to health. EFSA should assess for which substances a limit would need to be determined.
Commission Regulation (EU) 2016/14162 of 24 August 2016 which amended the Regulation deleted Article 11(2), thereby removing the so‐called Generic Specific Migration Limit (GML). The GML assigned a migration limit of 60 mg/kg food to all substances without an SML. Historically this generic limit complemented overall migration testing only in case of technical problems, but the Regulation made it generally applicable without apparent justification. We deleted the GML because it could cause a significant and often unnecessary testing burden while only for some of these substances an analytical method is available.
For many substances the absence of a limit is correct because their migration may not be of a health concern, or is accounted for otherwise, such as in the overall migration test. However, for certain substances, including volatile substances, this may not be the case. The review of all substances authorised under the Regulation without a specified migration limit is therefore necessary, with the exception of substances for which EFSA published opinions in the context of an application. This review should identify those substances for which EFSA considers that a specific migration limit at or below 60 mg/kg may be required to prevent the transfer of these substances to the food in an amount that could cause adverse health effects.
We therefore ask EFSA to review all substances without an SML and identify those substances for which a specific migration limit would be necessary. Given the large number of the substances concerned, we propose to proceed in two stages.
In the first stage, EFSA should prioritise the need for re‐evaluation of these substances in groups of high, medium, and low priority, setting apart substances for which there is no apparent need for a SML. The enclosed synoptic list may facilitate this work for the older substances.
The prioritisation should be done on the basis of criteria EFSA deems appropriate, such as based on theoretical knowledge about the chemistry and toxicology of the substances, the absence of such information, known migration limiting factors, volatility and other appropriate information available to EFSA. A call for data should not be conducted at this stage.
In the second stage, based on the list of the priorities established in the first stage, the Commission will provide EFSA with separate mandates for re‐evaluation of the individual substances taking into account the assigned priorities.
Terms of Reference
In accordance with Article 12(3) of Regulation (EC) No 1935/20043, the European Commission asks EFSA to review the authorisations of substances listed in Annex I of Regulation (EU) No 10/2011 without a specified Specific Migration Limit (SML). The purpose is to identify those substances requiring a specific migration limit to ensure the authorisation is sufficiently protective to health.
In doing so, EFSA should on the basis of criteria it deems appropriate (e.g. expected toxicity, known migration limiting factors, detection of the substance in overall migration testing, etc.), group these substances in terms of high, medium, and low priority for re‐evaluation, as well as those which do not need a SML. The characterisation thus obtained should allow the risk manager to provide EFSA with individual mandates for the subsequent re‐evaluation of these substances in order of priority and taking into account other legitimate factors.
In carrying out this review, EFSA should not launch a call for data but apply its theoretical knowledge and expertise about the chemistry and toxicology of the substances and about known migration limiting or enhancing factors, (e.g. volatility, polarity, etc.) for the substances and any relevant information available to EFSA.
1.2. Interpretation of the Terms of Reference
Impurities, reaction and transformation products
Substances used to make plastic Food Contact Material (FCM) may contain impurities due to the method of synthesis and production. Food contact substances may also form reaction and transformation products including oligomers when the plastic FCM is made (e.g. polymerisation) and processed (e.g. at high temperature). This review and prioritisation will consider only the food contact substances themselves and not the possible impurities, reaction and transformation products including oligomers, since they are expected to be variable depending on the exact processes used by different business operators. Whereas such information is requested and used in the evaluation of applications for new substances (EFSA, 2008), such information for the substances under consideration here is not available to EFSA and in the absence of a call for data will not become available to EFSA for this prioritisation exercise.
Inorganic substances including minerals and elemental powders
Due to the physical and chemical nature of these types of substances as they are described in the Union list (e.g. metal oxides or halides, aluminium flakes), their migration potential is very limited if not absent. Therefore, establishing SMLs for these substances as listed appears not to be appropriate. Since Regulation (EU) No 10/2011 sets in Table 1 of Annex II general migration limits for metals to be respected – according to Article 10 – as general restrictions related to plastic materials and articles and – according to Article 6(3) – for salts of authorised acids, these substances are under generic control by the migration limits listed in Annex II. As a consequence, substance (as listed) related SMLs are not needed and may not even be possible to be measured due to the dissolution/dissociation effects in the migration test.
Substances possibly in nanoform
Regulation (EU) No 10/2011 makes specific reference to nanosubstances in plastic FCM and established in Article 9(2) that substances in nanoform shall only be used if explicitly authorised. Authorisation of a substance in conventional size/form does not cover the same substance in nanoform. It is stipulated in that Regulation that EFSA will assess substances in nanoform case‐by‐case before authorisation. This being so, and in the absence of any specific information for the substances under review here, it shall be assumed that the substances are not in nanoform. If any substance under review here would be available and used in nanoform, it is understood that the interested business operator(s) would need to come forward with an application for its evaluation by EFSA and subsequent consideration for authorisation by the Commission.
Database searches based on CAS numbers and other identifiers
Due to the lack of a common unique identifier among the plastic FCM Union list and publicly available lists, such substance searches are inherently incomplete. The CAS identifiers from the Union list were used in database searches. It is also noted that the opinion (especially the tables) uses information from various sources and the way the substance is described has been maintained, although it is not always consistent between the different sources. However, care has been taken to remove any ambiguity, even if some inconsistent numbering and nomenclature persists.
Exposure
The tiered approach for risk evaluation described in EFSA (2008) requires the assessment of exposure prior to hazard, in order to set the toxicity requirements per tier. It was acknowledged that this approach cannot be systematically followed in this prioritisation exercise due to the significant lack of exposure data. The issue is partly addressed by placing some substances which would not migrate (based on their structure and physicochemical properties) into the low priority group. Concerning volatile substances, it is clear that exposure will depend on the volatility, assessed from the boiling point, under the conditions of the particular migration test or food contact application. However, due to a lack of clear‐cut criteria on the volatilisation of such substances, quantitative consideration on the exposure cannot be given. Substances in the gas phase at room temperature may no longer be present in the FCM plastics in amounts significant for potential exposure. Such substances are to be examined on a case‐by‐case basis and placed in the appropriate priority group where applicable.
2. Data and methodologies
The mandate requests that the prioritisation is based on appropriate criteria, using theoretical knowledge about the chemistry and toxicology of the substances, the absence of such information, known migration‐limiting factors, volatility and other appropriate information. Sources of such information included the Synoptic document, the EFSA OpenFoodTox Database, non‐confidential information retrieved from ECHA and IARC. Publicly available predictive modelling software, which uses quantitative structure–activity relationship ((Q)SAR) tools, was also applied.
2.1. Data
The initial data set was prepared using the Union list of FCM substances in the latest consolidated version of the Regulation available at the time of receipt of the European Commission mandate. This being Table 1, Annex I of Regulation (EU) No 10/2011, as amended by Regulation (EU) No 2019/374 of 10 January 2019. That Union list contained 451 entries (substances) for which an SML or a total specific migration limit (SML(T)) was not assigned.
Following the exclusion of substances for which EFSA has produced a risk evaluation (as a food contact substance) and other substances which could be set apart for other reasons (Section 2.2.1), the remaining substances were checked against the following lists, databases and other types of information:
Synoptic Document (European Commission, 2005)
EFSA's OpenFoodTox5 (Dorne et al., 2017; Ceriani et al., 2018) and the EFSA Register of Questions6
EFSA Emerging Risks list (Oltmanns et al., 2019)
ECHA substance information7 under the Registration, Evaluation, Authorisation and Restriction of Chemicals8 (REACH) Regulation and the Classification, Labelling and Packaging (CLP) Regulation9
IARC classifications11
SINLIST12
Feedback from the EU Member States on FCM risk assessment conducted at national level.
2.2. Methodologies
A stepwise approach was followed in order to define the applicable group of substances, excluding substances not falling under the remit of this European Commission mandate and to apply the prioritisation strategy, which consisted of four sequential steps: setting apart substances for which an SML should not be needed, and then assigning the remaining substances to low, medium and high priority groups (Figure 1).
Figure 1.
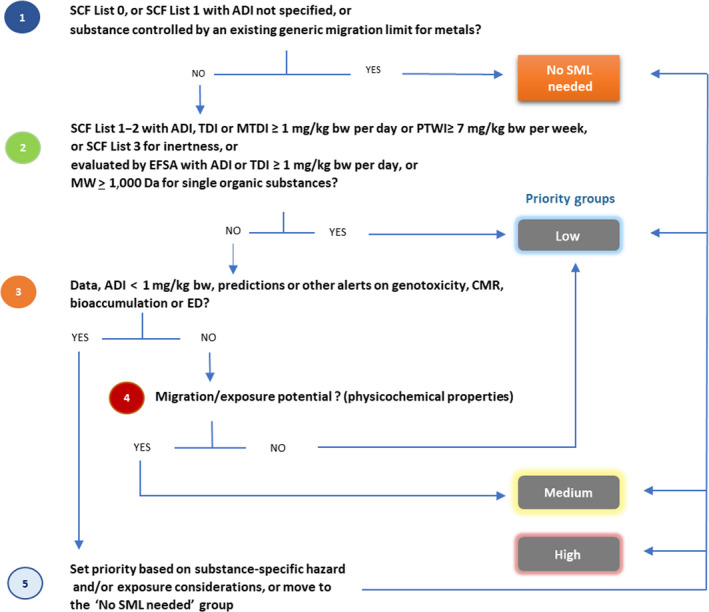
Prioritisation flowchart
2.2.1. Exclusions from the prioritisation exercise
2.2.1.1. EFSA opinions on applications
The European Commission mandate requests the exception of substances for which EFSA has published opinions in the context of an application (i.e. as substances used to make FCM). These were identified following name and CAS number searches from the EFSA Registry of Questions and the EFSA OpenFoodTox Database.
2.2.1.2. Other Union list entries
According to the existing restriction for 2,2‐bis(4‐hydroxyphenyl)propane bis(2,3‐epoxypropyl)ether (also known as bisphenol A diglycidyl ether, BADGE) in Regulation (EU) No 10/2011 referring to Commission Regulation (EC) No 1895/200513, the SML(T) of 9 mg/kg food or food simulant applies. Therefore, BADGE can be excluded from the current prioritisation process.
2.2.2. Prioritisation strategy
In order to define the group of substances for which an SML should not be needed and to produce groups of low, medium and high priority substances, a set of criteria was established, which were then applied in a stepwise approach (Figure 1).
2.2.2.1. Substances for which an SML should not be needed
SCF classification of FCM substances
The Synoptic document contains a list of all FCM substances which had been risk assessed by the Scientific Committee on Food (SCF) and includes chemical names, identification numbers, SCF classification numbers and risk assessment summary information. Where available, FCM substances without an SML were each assigned to an SCF classification taken from the Synoptic Document. Substances falling under an SCF classification of 0 were placed into the group of substances for which an SML should not be needed. List 0 was defined by the SCF (European Commission, 2005) as follows:
List 0: Substances, e.g. foods, which may be used in the production of plastic materials and articles, e.g. food ingredients and certain substances known from the intermediate metabolism in man and for which an ADI need not be established for this purpose.
Substances with the characterisation ‘ADI: not specified’ or ‘Group ADI: not specified’ and classified as SCF List 1 in the Synoptic document were also placed into the group of substances for which an SML should not be needed. The relevant SCF definition (SCF, 1990) is as follows:
‘ADI not specified’ is a term used when, on the basis of the available toxicological, biochemical and clinical data, the total daily intake of the substance, arising from its natural occurrence and/or its present use or uses in food at the levels necessary to achieve the desired technological effect, will not represent a hazard to health. For this reason, the establishment of a numerical limit for the ADI is not considered necessary for these substances.
The Panel did not revisit this classification of substances by the SCF into ‘SCF List 0’ or ‘ADI not specified’ but concluded that, with the definitions as described above, they are a reasonable basis for prioritisation and for identifying substances that should not need an SML for safety reasons. As a check, the EFSA OpenFoodTox database was interrogated to determine if any of these substances have been evaluated more recently by EFSA, i.e. since the SCF classification was made.
FCM substances controlled by existing general restrictions and generic limits
For various forms of elemental metals (such as flakes and powders) or metal compounds (such as metal oxides, hydroxides or salts that dissociate in water or dissolve under acidic conditions), the corresponding general metal‐related SMLs provided in Table 1 of Annex II of Regulation (EU) No. 10/2011 (Al, Ba, Co, Cu, Fe, Li, Mn, Ni or Zn) can be applied. This waives the need of a substance‐as‐listed migration control and allows to place these substances into the group of substances for which an SML should not be needed. This approach is supported by the fact that these types of substances also have a very limited migration potential, if any, in their physico‐chemical form as listed.
It is noted that the following substances of the positive list (Annex I of Regulation (EU) No 10/2011) may also fall under this category; however, they have already been excluded from priority setting due to existing EFSA evaluations on applications: iron phosphide (FCM No 607) (EFSA, 2004) and copper hydroxide phosphate (FCM No 972) (EFSA CEF Panel, 2010).
2.2.2.2. Low priority group
SCF classification of FCM substances
Substances falling under an SCF classification of 1 or 2 with an ADI/Tolerable Daily Intake (TDI)/Maximum Tolerable Daily Intake (MTDI) ≥ 1 mg/kg body weight (bw) per day or a Provisional Tolerable Weekly Intake (PTWI) ≥ 7 mg/kg bw per week were placed in the low priority group. Substances characterised as SCF List 3 due to ‘substance inertness’ were also placed in the same group. The following criteria were used by SCF to classify FCM substances into lists 1, 2 and 3:
List 1: Substances, e.g. food additives, for which an ADI, a t‐ADI (=temporary ADI), an MTDI, a PMTDI (=Provisional Maximum Tolerable Daily Intake), a (P)TWI (=(Provisional) Tolerable Weekly Intake) or the classification ‘acceptable’ has been established by SCF or by JECFA.
List 2: Substances for which a TDI or a t‐TDI has been established by SCF.
List 3: Substances for which an ADI or a TDI could not be established, but where the present use could be accepted. Some of these substances are self‐limiting because of their organoleptic properties or are volatile and therefore unlikely to be present in the finished product. For other substances with very low migration, a TDI has not been set, but the maximum level to be used in any packaging material or a specific limit of migration is stated. This is because the available toxicological data would give a TDI which allows that a specific limit of migration or a composition limit could be fixed at levels very much higher than the maximum likely intakes arising from present uses of the additive.
The CEP Panel noted that for some of the substances falling under the SCF classification of 1 or 2, a health‐based guidance value was not available. However, although implicitly with an ADI/TDI/MTDI ≥ 1 mg/kg bw per day or (P)TWI > 7 mg/kg bw per week, in line with the set criteria, the CEP Panel considered that those substances were deemed to proceed with the next steps of the prioritisation strategy.
FCM substances evaluated by EFSA with an ADI/TDI ≥ 1 mg/kg bw per day
Certain substances used as FCMs are also used in food and feed for other purposes (e.g. food and feed additives, food flavouring substances, pesticide active substances) and, similar to FCM, they have been evaluated by EFSA and/or are authorised under the respective Commission Regulations (e.g. Regulations 1333/200814, 1334/200815 and their implementing measures). Concerning FCM substances that are also food additives and/or flavouring substances, a non‐exhaustive list of such dual‐use additives has been reported in the Commission's Guidelines to Regulation (EU) No 10/2011 (European Commission, 2014).
For the identification of those substances without an SML, which have been previously evaluated by EFSA, the OpenFoodTox Database was used as a source and it was searched by CAS number and substance name. The version updated until 20/01/2020 was used.
An ADI or TDI equal to or above 1 mg/kg bw per day which had been established by EFSA or SCF was defined as one of the criteria for prioritisation. This threshold would correspond to an SML of 60 mg/kg of food or higher, assuming a default body weight of 60 kg and a maximum intake of 1 kg of food per person set in the SCF guidelines (SCF, 2001).
The Panel noted that this approach is protective for adults but not necessarily for infants, toddlers or young children due to their higher relative food consumption on a body weight basis compared to that of adults (EFSA CEF Panel, 2016).
FCM substances of high molecular mass
Single organic substances of a molecular mass equal to or higher than 1,000 Da are unlikely to pass biological membranes (Loewenstein, 1981). They are not expected to be absorbed by the gastrointestinal tract and do not present a systemic toxicological hazard, unless they cause local effects to the gastrointestinal tract or hydrolyse to smaller molecules. If this can be excluded, a molecular mass of 1,000 Da can be considered to be a conclusive cut‐off value. For poly‐ and per‐fluoro substances, a cut‐off value of 1,500 Da could be appropriate, because the molecular volume of C‐F is smaller than that of C‐H molecules of the same molecular mass (EFSA CEF Panel, 2016). Molecular weight information was retrieved from Scifinder(n), Pubchem, eChemPortal, ChemIDPlus (Toxnet) and specific JECFA monographs. FCM substances meeting the molecular weight cut‐off criterion were placed in the low priority group.
2.2.2.3. High priority group
Substances not falling under the low priority group were screened for potential toxicity.
The considered endpoints were genotoxicity, carcinogenicity, mutagenicity, reprotoxicity, bioaccumulation and ED properties. Information was sought from the following sources:
OpenFoodTox
ECHA substance information under the REACH and CLP Regulations, providing information on substance evaluations and harmonised classification and labelling (CLH)
IARC classifications
Risk evaluations conducted at national level by EU Member States
Existing priority lists (Potential Emerging Risks list, EFSA; SIN List, ChemSec) and the overview of Plastic Additives (ECHA)
Genotoxicity alerts triggered by the use of endpoint‐specific profilers from (Q)SAR models (VEGA and OECD QSAR Toolbox)
OpenFoodTox
The OpenFoodTox Database (Dorne et al., 2017; Ceriani et al., 2018) was searched for experimental data and assessments on toxicity of substances without an SML, which have been previously evaluated by EFSA. The database provides, among other information, references to the EFSA opinion and the EFSA Panel, the study category (human or animal health, ecotoxicity), the type and details of the study and the guidelines, the type of toxicity (systemic, developmental, reproductive, etc.), its conclusions on mutagenicity/genotoxicity/carcinogenicity and the health‐based guidance values derived. Substances with an ADI, group ADI, TDI or group TDI value below 1 mg/kg bw per day were candidates for the high priority group, pending case‐by‐case considerations.
ECHA information
EFSA received substance information present in the ECHA public database from the ECHA's Prioritisation Unit. Tonnage information and hyperlinks to each Substance Information Page and Factsheet URL were also kindly provided by ECHA. The Authorisation List (Annex XIV to REACH), the Restriction List (Annex XVII to REACH) and the Classification and Labelling (C&L) Inventory (Annex VI to CLP) were searched for FCM substances which have been evaluated for carcinogenic, mutagenic or toxic for reproduction (CMR), persistent, bioaccumulative and toxic (PBT)/very persistent and very bioaccumulative (vPvB) or ED properties by ECHA. Substances that have passed the screening phase of ECHA and Member State Competent Authorities and are currently included under any of the ongoing processes ‘Substance Evaluation (SEV)’, ‘PBT Assessment’, ‘ED Assessment’, ‘Harmonised Classification and Labelling (CLH)’, ‘Regulatory Management Option Analysis (RMOA)’ and ‘Candidate List of Substances of Very High Concern (SVHC)’, were also identified. Before inclusion of these substances into the high priority group, the relevance to FCM of information retrieved from CLH and RMOA evaluations was confirmed.
IARC classifications
The IARC classifications on carcinogenicity (Agents Classified by the IARC Monographs, Volumes 1–124, update of 2019‐07‐16) were searched for matches with the working list of FCM substances with no SML.
Substances classified by IARC into groups: 1 (carcinogenic to humans), 2A (probably carcinogenic to humans) and 2B (possibly carcinogenic to humans) were assigned to the high priority group. A listing in the IARC group 3 (not classifiable as to its carcinogenicity to humans) was not used for prioritisation.
EU national risk evaluations
EFSA Focal Points, which comprise members from all EU Member States, Iceland and Norway, as well as observers from Switzerland and EU candidate countries, were contacted. They were asked to provide, if available, any additional food safety evaluations conducted at national level regarding the working list of FCM substances with no SML. The information provided was collected and screened based on its relevance to the FCM area and the prioritisation exercise.
Priority lists and overviews
EFSA's list of potential emerging risks.
Oltmanns et al. (2019) described a procedure for the identification of potential emerging risks for food safety using substances registered under REACH. The prioritisation was based on the environmental exposure (tonnage and use pattern), biodegradation and bioaccumulation in food/feed (based on modelling) and toxicity (based on classification for CMR and repeated dose toxicity). The list of 212 substances of ‘potential emerging risks’ identified under this project was searched against the working list of FCM substances without an SML. No matches between the two lists were identified.
SIN List
The SIN List, developed by the non‐profit organisation ChemSec and based on criteria defined within REACH, is a publicly available list of hazardous substances to human health and the environment. The version updated up to 29 May 2019 was used to search against the working list of FCM substances without an SML.
ECHA's Plastic Additives Initiative.
ECHA in collaboration with industry sector organisations characterised the uses of various plastic additives and estimated their potential for release from articles by modelling (ECHA, 2019). Substance properties determining/driving the release from the polymer matrix and information on the additive function, typical concentrations and the types of polymers and articles was collected. This information was obtained from ECHA. However, the presence of a substance in the Plastic Additives inventory was not used as a tool to assign priorities here, due to differences in the selection criteria (e.g. focus on exposure, tonnage information not specific to FCM applications).
In silico methods: (Q)SAR predictions
As regards (Q)SAR tools, two independent and freely available software programmes were used: VEGA16 (version 1.1.5) (Benfenati et al., 2019) and the OECD QSAR ToolBox17 (version 4.3). The VEGA software was selected because it provides information on the applicability domain and makes available the data set on which the models are built (experimental data available) as well as the full report of the predictions performed. The OECD QSAR ToolBox was selected because it contains many databases with updated experimental data. Moreover, the two platforms are generally used and accepted for regulatory purposes (EFSA Scientific Committee, 2017; ECHA, 2016a).
OECD QSAR Toolbox and VEGA were used for predictions of genotoxicity, in particular for the gene mutation endpoint. As regards chromosomal aberration, it was noted that the alerts on this endpoint (e.g. ISS alert for in vivo micronucleus test) are not considered reliable and can be dismissed. Therefore, only gene mutation was taken into account (Benigni et al., 2019; Honma et al., 2019).
In silico positive predictions regarding the gene mutation endpoint were compared, when available, with the conclusion on genotoxicity based on experimental data of substances previously evaluated by EFSA Panels. Substances indicated as positive by the (Q)SAR tools based on experimental data and/or positive alerts were candidates for the high priority group. In cases where predictions from (Q)SAR models contradict available experimental data, the experimental evidence prevails.
For those FCM substances which are also authorised as food flavouring substances, the EFSA evaluations in accordance with Article 9(a) of Regulation (EC) No 1334/2008 (on flavourings and certain food ingredients with flavouring properties for use in and on foods) were taken into account. Specifically, if a substance had experimental in vitro data and/or (Q)SAR predictions indicating a potential for genotoxicity, but for which, based on experimental data or other considerations, the concern for genotoxicity had been ruled out as part of the EFSA evaluation of flavourings, then the substance was assigned not to the high but to the medium priority group.
2.2.2.4. Medium priority group
Substances not assigned to the ‘no SML needed’, low or high priority groups were considered on the basis of their physico‐chemical properties. When these properties suggested a lack of potential for migration into food (e.g. minerals and substances in the gas phase at room temperature), the substance was placed into the low priority group. If not, it was assigned to the medium priority group.
3. Assessment
3.1. Substances without an SML in the Union List of food contact materials and exclusions
3.1.1. FCM substances without an SML
Four hundred and fifty‐one substances without an SML or SML(T) were identified in the Union list of 893 authorised substances of Annex I of Regulation (EU) No 10/2011. The numbers of substances with an SML, SML(T) or an ND characterisation and those without, are shown in Table 1.
Table 1.
Numbers of substances with specific migration limits (SML), group restrictions (SML(T)), not to be detected (ND) or without a specific migration limit or restriction
| Type of limit or restriction | Number of substances in the Union List |
|---|---|
| SML | 263 |
| SML (T) | 139 |
| SML + SML(T) | 9 |
| ND | 31 |
| No SML or SML(T) | 451 |
The full list of substances without an SML or SML(T) is provided in Appendix A, Table A.1.
Table A.1.
Substances without an SML or SML(T)
| FCM substance no. | Ref. no. | CAS no. | Substance name |
|---|---|---|---|
| 1 | 12310 | 266309‐43‐7 | Albumin |
| 2 | 12340 | Albumin, coagulated by formaldehyde | |
| 3 | 12375 | Alcohols, aliphatic, monohydric, saturated, linear, primary (C4‐C22) | |
| 7 | 30370 | Acetylacetic acid, salts | |
| 9 | 30610 | Acids, C2‐C24, aliphatic, linear, monocarboxylic from natural oils and fats, and their mono‐, di‐ and triglycerol esters (branched fatty acids at naturally occurring levels are included) | |
| 10 | 30612 | Acids, C2‐C24, aliphatic, linear, monocarboxylic, synthetic and their mono‐, di‐ and triglycerol esters | |
| 11 | 30960 | Acids, aliphatic, monocarboxylic (C6‐C22), esters with polyglycerol | |
| 12 | 31328 | Acids, fatty, from animal or vegetable food fats and oils | |
| 13 | 33120 | Alcohols, aliphatic, monohydric, saturated, linear, primary (C4‐C24) | |
| 17 | 34281 | Alkyl(C8‐C22)sulphuric acids, linear, primary with an even number of carbon atoms | |
| 18 | 34475 | Aluminium calcium hydroxide phosphite, hydrate | |
| 21 | 42500 | Carbonic acid, salts | |
| 22 | 43200 | Castor oil, mono‐ and diglycerides | |
| 24 | 45280 | Cotton fibers | |
| 34 | 54270 | Ethylhydroxymethylcellulose | |
| 35 | 54280 | Ethylhydroxypropylcellulose | |
| 36 | 54450 | Fats and oils, from animal or vegetable food sources | |
| 37 | 54480 | Fats and oils, hydrogenated, from animal or vegetable food sources | |
| 38 | 55520 | Glass fibers | |
| 39 | 55600 | Glass microballs | |
| 40 | 56360 | Glycerol, esters with acetic acid | |
| 41 | 56486 | Glycerol, esters with acids, aliphatic, saturated, linear, with an even number of carbon atoms (C14‐C18) and with acids, aliphatic, unsaturated, linear, with an even number of carbon atoms (C16‐C18) | |
| 42 | 56487 | Glycerol, esters with butyric acid | |
| 43 | 56490 | Glycerol, esters with erucic acid | |
| 44 | 56495 | Glycerol, esters with 12‐hydroxystearic acid | |
| 45 | 56500 | Glycerol, esters with lauric acid | |
| 46 | 56510 | Glycerol, esters with linoleic acid | |
| 47 | 56520 | Glycerol, esters with myristic acid | |
| 48 | 56535 | Glycerol, esters with nonanoic acid | |
| 49 | 56540 | Glycerol, esters with oleic acid | |
| 50 | 56550 | Glycerol, esters with palmitic acid | |
| 51 | 56570 | Glycerol, esters with propionic acid | |
| 52 | 56580 | Glycerol, esters with ricinoleic acid | |
| 53 | 56585 | Glycerol, esters with stearic acid | |
| 54 | 57040 | Glycerol monooleate, ester with ascorbic acid | |
| 55 | 57120 | Glycerol monooleate, ester with citric acid | |
| 56 | 57200 | Glycerol monopalmitate, ester with ascorbic acid | |
| 57 | 57280 | Glycerol monopalmitate, ester with citric acid | |
| 58 | 57600 | Glycerol monostearate, ester with ascorbic acid | |
| 59 | 57680 | Glycerol monostearate, ester with citric acid | |
| 60 | 58300 | Glycine, salts | |
| 62 | 64500 | Lysine, salts | |
| 63 | 65440 | Manganese pyrophosphite | |
| 64 | 66695 | Methylhydroxymethylcellulose | |
| 65 | 67155 | Mixture of 4‐(2‐benzoxazolyl)‐4′‐(5‐methyl‐2‐benzoxazolyl)stilbene, 4,4′‐bis(2‐benzoxazolyl) stilbene and 4,4′‐bis(5‐methyl‐2‐benzoxazolyl)stilbene | |
| 67 | 67840 | Montanic acids and/or their esters with ethyleneglycol and/or with 1,3‐butanediol and/or with glycerol | |
| 75 | 77702 | Polyethyleneglycol esters of aliph. monocarb. acids (c6‐c22) and their ammonium and sodium sulphates | |
| 79 | 80640 | Polyoxyalkyl (c2‐c4) dimethylpolysiloxane | |
| 80 | 81760 | Powders, flakes and fibers of brass, bronze, copper, stainless steel, tin, iron and alloys of copper, tin and iron | |
| 81 | 83320 | Propylhydroxyethylcellulose | |
| 82 | 83325 | Propylhydroxymethylcellulose | |
| 83 | 83330 | Propylhydroxypropylcellulose | |
| 84 | 85601 | Silicates, natural (with the exception of asbestos) | |
| 85 | 85610 | Silicates, natural, silanated (with the exception of asbestos) | |
| 86 | 86000 | Silicic acid, silylated | |
| 87 | 86285 | Silicon dioxide, silanated | |
| 90 | 92195 | Taurine, salts | |
| 94 | 95859 | Waxes, refined, derived from petroleum based or synthetic hydrocarbon feedstocks, high viscosity | |
| 95 | 95883 | White mineral oils, paraffinic, derived from petroleum based hydrocarbon feedstocks | |
| 96 | 95920 | Wood flour and fibers, untreated | |
| 97 | 720810 | Petroleum hydrocarbon resins (hydrogenated) | |
| 99 | 19460 ‐ 62960 | 50‐21‐5 | Lactic acid |
| 100 | 24490 ‐ 88320 | 50‐70‐4 | Sorbitol |
| 101 | 36000 | 50‐81‐7 | Ascorbic acid |
| 102 | 17530 | 50‐99‐7 | Glucose |
| 103 | 18100 ‐ 55920 | 56‐81‐5 | Glycerol |
| 105 | 22780 ‐ 70400 | 57‐10‐3 | Palmitic acid |
| 106 | 24550 ‐ 89040 | 57‐11‐4 | Stearic acid |
| 107 | 25960 | 57‐13‐6 | Urea |
| 108 | 24880 | 57‐50‐1 | Sucrose |
| 109 | 23740 ‐ 81840 | 57‐55‐6 | 1,2‐Propanediol |
| 110 | 93520 |
59‐02‐9 10191‐41‐0 |
α‐Tocopherol |
| 111 | 53600 | 60‐00‐4 | Ethylenediaminetetraacetic acid |
| 112 | 64015 | 60‐33‐3 | Linoleic acid |
| 113 | 16780 ‐ 52800 | 64‐17‐5 | Ethanol |
| 114 | 55040 | 64‐18‐6 | Formic acid |
| 115 | 10090 ‐ 30000 | 64‐19‐7 | Acetic acid |
| 116 | 13090 ‐ 37600 | 65‐85‐0 | Benzoic acid |
| 117 | 21550 | 67‐56‐1 | Methanol |
| 118 | 23830 ‐ 81882 | 67‐63‐0 | 2‐Propanol |
| 119 | 30295 | 67‐64‐1 | Acetone |
| 120 | 49540 | 67‐68‐5 | Dimethyl sulphoxide |
| 121 | 24270 ‐ 84640 | 69‐72‐7 | Salicylic acid |
| 122 | 23800 | 71‐23‐8 | 1‐Propanol |
| 123 | 13840 | 71‐36‐3 | 1‐Butanol |
| 124 | 22870 | 71‐41‐0 | 1‐Pentanol |
| 125 | 16950 | 74‐85‐1 | Ethylene |
| 126 | 10210 | 74‐86‐2 | Acetylene |
| 131 | 48460 | 75‐37‐6 | 1,1‐Difluoroethane |
| 136 | 41680 | 76‐22‐2 | Camphor |
| 139 | 14680 ‐ 44160 | 77‐92‐9 | Citric acid |
| 143 | 62450 | 78‐78‐4 | Isopentane |
| 146 | 23890 ‐ 82000 | 79‐09‐4 | Propionic acid |
| 155 | 23470 | 80‐56‐8 | α‐Pinene |
| 158 | 23380 ‐ 76320 | 85‐44‐9 | Phthalic anhydride |
| 161 | 92160 | 87‐69‐4 | L‐(+)‐Tartaric acid |
| 162 | 65520 | 87‐78‐5 | Mannitol |
| 165 | 23200 ‐74480 | 88‐99‐3 | o‐Phthalic acid |
| 171 | 38080 | 93‐58‐3 | Benzoic acid, methyl ester |
| 172 | 37840 | 93‐89‐0 | Benzoic acid, ethyl ester |
| 173 | 60240 | 94‐13‐3 | 4‐Hydroxybenzoic acid, propyl ester |
| 174 | 14740 | 95‐48‐7 | o‐Cresol |
| 182 | 19270 | 97‐65‐4 | Itaconic acid |
| 189 | 60200 | 99‐76‐3 | 4‐Hydroxybenzoic acid, methyl ester |
| 190 | 18880 | 99‐96‐7 | p‐Hydroxybenzoic acid |
| 193 | 24610 | 100‐42‐5 | Styrene |
| 194 | 13150 | 100‐51‐6 | Benzyl alcohol |
| 195 | 37360 | 100‐52‐7 | Benzaldehyde |
| 204 | 25180 ‐ 92640 | 102‐60‐3 | N,N,N′,N′‐tetrakis(2‐hydroxypropyl)ethylenediamine |
| 205 | 25385 | 102‐70‐5 | Triallylamine |
| 210 | 13390 ‐ 14880 | 105‐08‐8 | 1,4‐Bis(hydroxymethyl)cyclohexane |
| 213 | 82400 | 105‐62‐4 | 1,2‐Propyleneglycol dioleate |
| 214 | 61840 | 106‐14‐9 | 12‐Hydroxystearic acid |
| 215 | 14170 | 106‐31‐0 | Butyric anhydride |
| 216 | 14770 | 106‐44‐5 | p‐Cresol |
| 221 | 40570 | 106‐97‐8 | Butane |
| 222 | 13870 | 106‐98‐9 | 1‐Butene |
| 224 | 13900 | 107‐01‐7 | 2‐Butene |
| 228 | 13690 | 107‐88‐0 | 1,3‐Butanediol |
| 229 | 14140 | 107‐92‐6 | Butyric acid |
| 232 | 10150 ‐ 30280 | 108‐24‐7 | Acetic anhydride |
| 233 | 24850 | 108‐30‐5 | Succinic anhydride |
| 235 | 14710 | 108‐39‐4 | m‐Cresol |
| 238 | 18070 | 108‐55‐4 | Glutaric anhydride |
| 240 | 45760 | 108‐91‐8 | Cyclohexylamine |
| 244 | 71720 | 109‐66‐0 | Pentane |
| 247 | 24820 ‐ 90960 | 110‐15‐6 | Succinic acid |
| 249 | 17290 ‐ 55120 | 110‐17‐8 | Fumaric acid |
| 250 | 53520 | 110‐30‐5 | N,N′‐Ethylenebisstearamide |
| 251 | 53360 | 110‐31‐6 | N,N′‐Ethylenebisoleamide |
| 252 | 87200 | 110‐44‐1 | Sorbic acid |
| 253 | 15250 | 110‐60‐1 | 1,4‐Diaminobutane |
| 256 | 18010 ‐ 55680 | 110‐94‐1 | Glutaric acid |
| 257 | 13550 ‐ 16660 ‐ 51760 | 110‐98‐5 | Dipropyleneglycol |
| 258 | 70480 | 111‐06‐8 | Palmitic acid, butyl ester |
| 259 | 58720 | 111‐14‐8 | Heptanoic acid |
| 260 | 24280 | 111‐20‐6 | Sebacic acid |
| 265 | 22600 | 111‐87‐5 | 1‐octanol |
| 266 | 25510 ‐ 94320 | 112‐27‐6 | Triethyleneglycol |
| 267 | 15100 | 112‐30‐1 | 1‐decanol |
| 269 | 25090 ‐ 92350 | 112‐60‐7 | Tetraethyleneglycol |
| 270 | 22763 ‐ 69040 | 112‐80‐1 | Oleic acid |
| 271 | 52720 | 112‐84‐5 | Erucamide |
| 272 | 37040 | 112‐85‐6 | Behenic acid |
| 273 | 52730 | 112‐86‐7 | Erucic acid |
| 275 | 23980 | 115‐07‐1 | Propylene |
| 276 | 19000 | 115‐11‐7 | Isobutene |
| 279 | 22840 ‐ 71600 | 115‐77‐5 | Pentaerythritol |
| 287 | 60160 | 120‐47‐8 | 4‐Hydroxybenzoic acid, ethyl ester |
| 288 | 24970 | 120‐61‐6 | Terephthalic acid, dimethyl ester |
| 296 | 23860 | 123‐38‐6 | Propionaldehyde |
| 297 | 23950 | 123‐62‐6 | Propionic anhydride |
| 298 | 14110 | 123‐72‐8 | Butyraldehyde |
| 299 | 63840 | 123‐76‐2 | Levulinic acid |
| 300 | 30045 | 123‐86‐4 | Acetic acid, butyl ester |
| 301 | 89120 | 123‐95‐5 | Stearic acid, butyl ester |
| 302 | 12820 | 123‐99‐9 | Azelaic acid |
| 303 | 12130 ‐ 31730 | 124‐04‐9 | Adipic acid |
| 304 | 14320 ‐ 41960 | 124‐07‐2 | Caprylic acid |
| 306 | 88960 | 124‐26‐5 | Stearamide |
| 307 | 42160 | 124‐38‐9 | Carbon dioxide |
| 308 | 91200 | 126‐13‐6 | Sucrose acetate isobutyrate |
| 309 | 91360 | 126‐14‐7 | Sucrose octaacetate |
| 311 | 16480 ‐ 51200 | 126‐58‐9 | Dipentaerythritol |
| 314 | 23500 | 127‐91‐3 | β‐Pinene |
| 320 | 37680 | 136‐60‐7 | Benzoic acid, butyl ester |
| 321 | 36080 | 137‐66‐6 | Ascorbyl palmitate |
| 322 | 63040 | 138‐22‐7 | Lactic acid, butyl ester |
| 327 | 30140 | 141‐78‐6 | Acetic acid, ethyl ester |
| 328 | 65040 | 141‐82‐2 | Malonic acid |
| 329 | 59360 | 142‐62‐1 | Hexanoic acid |
| 330 | 19470 ‐ 63280 | 143‐07‐7 | Lauric acid |
| 331 | 22480 | 143‐08‐8 | 1‐Nonanol |
| 332 | 69760 | 143‐28‐2 | Oleyl alcohol |
| 335 | 68960 | 301‐02‐0 | Oleamide |
| 336 | 15095 ‐ 45940 | 334‐48‐5 | n‐Decanoic acid |
| 338 | 71020 | 373‐49‐9 | Palmitoleic acid |
| 339 | 86160 | 409‐21‐2 | Silicon carbide |
| 345 | 35840 | 506‐30‐9 | Arachidic acid |
| 346 | 10030 | 514‐10‐3 | Abietic acid |
| 348 | 22350 ‐ 67891 | 544‐63‐8 | Myristic acid |
| 350 | 63920 | 557‐59‐5 | Lignoceric acid |
| 360 | 57920 | 620‐67‐7 | Glycerol triheptanoate |
| 362 | 14350 | 630‐08‐0 | Carbon monoxide |
| 367 | 16697 | 693‐23‐2 | n‐Dodecanedioic acid |
| 393 | 37280 | 1302‐78‐9 | Bentonite |
| 394 | 41280 | 1305‐62‐0 | Calcium hydroxide |
| 395 | 41520 | 1305‐78‐8 | Calcium oxide |
| 396 | 64640 | 1309‐42‐8 | Magnesium hydroxide |
| 397 | 64720 | 1309‐48‐4 | Magnesium oxide |
| 399 | 81600 | 1310‐58‐3 | Potassium hydroxide |
| 400 | 86720 | 1310‐73‐2 | Sodium hydroxide |
| 401 | 24475 | 1313‐82‐2 | Sodium sulphide |
| 402 | 96240 | 1314‐13‐2 | Zinc oxide |
| 403 | 96320 | 1314‐98‐3 | Zinc sulphide |
| 404 | 67200 | 1317‐33‐5 | Molybdenum disulphide |
| 406 | 83300 | 1323‐39‐3 | 1,2‐Propyleneglycol monostearate |
| 408 | 82960 | 1330‐80‐9 | 1,2‐Propyleneglycol monooleate |
| 409 | 62240 | 1332‐37‐2 | Iron oxide |
| 410 | 62720 | 1332‐58‐7 | Kaolin |
| 411 | 42080 | 1333‐86‐4 | Carbon black |
| 413 | 35600 | 1336‐21‐6 | Ammonium hydroxide |
| 414 | 87600 | 1338‐39‐2 | Sorbitan monolaurate |
| 415 | 87840 | 1338‐41‐6 | Sorbitan monostearate |
| 416 | 87680 | 1338‐43‐8 | Sorbitan monooleate |
| 417 | 85680 | 1343‐98‐2 | Silicic acid |
| 418 | 34720 | 1344‐28‐1 | Aluminium oxide |
| 419 | 92150 | 1401‐55‐4 | Tannic acids |
| 421 | 13000 | 1477‐55‐0 | 1,3‐Benzenedimethanamine |
| 426 | 13510 ‐ 13610 | 1675‐54‐3 | 2,2‐Bis(4‐hydroxyphenyl)propane bis(2,3‐epoxypropyl) ether |
| 428 | 95200 | 1709‐70‐2 | 1,3,5‐Trimethyl‐2,4,6‐tris(3,5‐di‐tert‐butyl‐4‐hydroxybenzyl)benzene |
| 432 | 12280 | 2035‐75‐8 | Adipic anhydride |
| 436 | 19480 | 2146‐71‐6 | Lauric acid, vinyl ester |
| 441 | 38160 | 2315‐68‐6 | benzoic acid, propyl ester |
| 445 | 83440 | 2466‐09‐3 | Pyrophosphoric acid |
| 450 | 24430 | 2561‐88‐8 | Sebacic anhydride |
| 458 | 36960 | 3061‐75‐4 | Behenamide |
| 459 | 46870 | 3135‐18‐0 | 3,5‐di‐tert‐butyl‐4‐hydroxybenzylphosphonic acid, dioctadecyl ester |
| 465 | 68040 | 3333‐62‐8 | 7‐[2h‐naphtho‐(1,2‐d)triazol‐2‐yl]‐3‐phenylcoumarin |
| 468 | 71960 | 3825‐26‐1 | Perfluorooctanoic acid, ammonium salt |
| 478 | 60180 | 4191‐73‐5 | 4‐Hydroxybenzoic acid, isopropyl ester |
| 479 | 12970 | 4196‐95‐6 | Azelaic anhydride |
| 480 | 46790 | 4221‐80‐1 | 3,5‐Di‐tert‐butyl‐4‐hydroxybenzoic acid, 2,4‐di‐tert‐butylphenyl ester |
| 486 | 54005 | 5136‐44‐7 | Ethylene‐n‐palmitamide‐n′‐stearamide |
| 488 | 53440 | 5518‐18‐3 | n,n′‐Ethylenebispalmitamide |
| 489 | 41040 | 5743‐36‐2 | Calcium butyrate |
| 491 | 82720 | 6182‐11‐2 | 1,2‐Propyleneglycol distearate |
| 494 | 62140 | 6303‐21‐5 | Hypophosphorous acid |
| 496 | 71680 | 6683‐19‐8 | Pentaerythritol tetrakis[3‐(3,5‐di‐tert‐butyl‐4‐hydroxyphenyl)‐propionate] |
| 499 | 19965 ‐ 65020 | 6915‐15‐7 | Malic acid |
| 501 | 34480 | Aluminium fibers, flakes and powders | |
| 503 | 46080 | 7585‐39‐9 | β‐Dextrin |
| 504 | 86240 | 7631‐86‐9 | Silicon dioxide |
| 507 | 59990 | 7647‐01‐0 | Hydrochloric acid |
| 508 | 86560 | 7647‐15‐6 | Sodium bromide |
| 509 | 23170 ‐ 72640 | 7664‐38‐2 | Phosphoric acid |
| 510 | 12789 ‐ 35320 | 7664‐41‐7 | Ammonia |
| 511 | 91920 | 7664‐93‐9 | Sulphuric acid |
| 514 | 91840 | 7704‐34‐9 | Sulphur |
| 515 | 26360 ‐ 95855 | 7732‐18‐5 | Water |
| 517 | 81520 | 7758‐02‐3 | Potassium bromide |
| 518 | 35845 | 7771‐44‐0 | Arachidonic acid |
| 520 | 65120 | 7773‐01‐5 | Manganese chloride |
| 521 | 58320 | 7782‐42‐5 | Graphite |
| 522 | 14530 | 7782‐50‐5 | Chlorine |
| 523 | 45195 | 7787‐70‐4 | Copper bromide |
| 524 | 24520 | 8001‐22‐7 | Soybean oil |
| 525 | 62640 | 8001‐39‐6 | Japan wax |
| 526 | 43440 | 8001‐75‐0 | Ceresin |
| 527 | 14411 ‐ 42880 | 8001‐79‐4 | Castor oil |
| 528 | 63760 | 8002‐43‐5 | Lecithin |
| 529 | 67850 | 8002‐53‐7 | Montan wax |
| 530 | 41760 | 8006‐44‐8 | Candelilla wax |
| 531 | 36880 | 8012‐89‐3 | Beeswax |
| 533 | 42720 | 8015‐86‐9 | Carnauba wax |
| 534 | 80720 | 8017‐16‐1 | Polyphosphoric acids |
| 535 | 24100 ‐ 24130 ‐ 24190 ‐ 83840 | 8050‐09‐7 | Rosin |
| 536 | 84320 | 8050‐15‐5 | Rosin, hydrogenated, ester with methanol |
| 537 | 84080 | 8050‐26‐8 | Rosin, ester with pentaerythritol |
| 538 | 84000 | 8050‐31‐5 | Rosin, ester with glycerol |
| 539 | 24160 | 8052‐10‐6 | Rosin tall oil |
| 541 | 58480 | 9000‐01‐5 | Gum arabic |
| 542 | 42640 | 9000‐11‐7 | Carboxymethylcellulose |
| 543 | 45920 | 9000‐16‐2 | Dammar |
| 544 | 58400 | 9000‐30‐0 | Guar gum |
| 545 | 93680 | 9000‐65‐1 | Tragacanth gum |
| 546 | 71440 | 9000‐69‐5 | Pectin |
| 547 | 55440 | 9000‐70‐8 | Gelatin |
| 548 | 42800 | 9000‐71‐9 | Casein |
| 549 | 80000 | 9002‐88‐4 | Polyethylene wax |
| 550 | 81060 | 9003‐07‐0 | Polypropylene wax |
| 551 | 79920 |
9003‐11‐6 106392‐12‐5 |
Poly(ethylene propylene) glycol |
| 552 | 81500 | 9003‐39‐8 | Polyvinylpyrrolidone |
| 553 | 14500 ‐ 43280 | 9004‐34‐6 | Cellulose |
| 554 | 43300 | 9004‐36‐8 | Cellulose acetate butyrate |
| 555 | 53280 | 9004‐57‐3 | Ethylcellulose |
| 556 | 54260 | 9004‐58‐4 | Ethylhydroxyethylcellulose |
| 557 | 66640 | 9004‐59‐5 | Methylethylcellulose |
| 558 | 60560 | 9004‐62‐0 | Hydroxyethylcellulose |
| 559 | 61680 | 9004‐64‐2 | Hydroxypropylcellulose |
| 560 | 66700 | 9004‐65‐3 | Methylhydroxypropylcellulose |
| 561 | 66240 | 9004‐67‐5 | Methylcellulose |
| 562 | 22450 | 9004‐70‐0 | Nitrocellulose |
| 564 | 24540 ‐ 88800 | 9005‐25‐8 | Starch, edible |
| 565 | 61120 | 9005‐27‐0 | Hydroxyethyl starch |
| 566 | 33350 | 9005‐32‐7 | Alginic acid |
| 567 | 82080 | 9005‐37‐2 | 1,2‐Propyleneglycol alginate |
| 568 | 79040 | 9005‐64‐5 | Polyethyleneglycol sorbitan monolaurate |
| 569 | 79120 | 9005‐65‐6 | Polyethyleneglycol sorbitan monooleate |
| 570 | 79200 | 9005‐66‐7 | Polyethyleneglycol sorbitan monopalmitate |
| 571 | 79280 | 9005‐67‐8 | Polyethyleneglycol sorbitan monostearate |
| 572 | 79360 | 9005‐70‐3 | Polyethyleneglycol sorbitan trioleate |
| 573 | 79440 | 9005‐71‐4 | Polyethyleneglycol sorbitan tristearate |
| 574 | 24250 ‐ 84560 | 9006‐04‐6 | Rubber, natural |
| 575 | 76721 | 63148‐62‐9 | Polydimethylsiloxane (mw > 6,800 da) |
| 576 | 60880 | 9032‐42‐2 | Hydroxyethylmethylcellulose |
| 577 | 62280 | 9044‐17‐1 | Isobutylene‐butene copolymer |
| 579 | 61800 | 9049‐76‐7 | Hydroxypropyl starch |
| 580 | 46070 | 10016‐20‐3 | α‐Dextrin |
| 581 | 36800 | 10022‐31‐8 | Barium nitrate |
| 585 | 41120 | 10043‐52‐4 | Calcium chloride |
| 586 | 65280 | 10043‐84‐2 | Manganese hypophosphite |
| 589 | 52645 | 10436‐08‐5 | Cis‐11‐eicosenamide |
| 591 | 36160 | 10605‐09‐1 | Ascorbyl stearate |
| 592 | 34690 | 11097‐59‐9 | Aluminium magnesium carbonate hydroxide |
| 593 | 44960 | 11104‐61‐3 | Cobalt oxide |
| 594 | 65360 | 11129‐60‐5 | Manganese oxide |
| 595 | 19510 | 11132‐73‐3 | Lignocellulose |
| 596 | 95935 | 11138‐66‐2 | Xanthan gum |
| 597 | 67120 | 12001‐26‐2 | Mica |
| 598 | 41600 | 12004‐14‐7 | Calcium sulphoaluminate |
| 600 | 60030 | 12072‐90‐1 | Hydromagnesite |
| 601 | 35440 | 12124‐97‐9 | Ammonium bromide |
| 602 | 70240 | 12198‐93‐5 | Ozokerite |
| 603 | 83460 | 12269‐78‐2 | Pyrophyllite |
| 604 | 60080 | 12304‐65‐3 | Hydrotalcite |
| 606 | 65200 | 12626‐88‐9 | Manganese hydroxide |
| 607 | 62245 | 12751‐22‐3 | Iron phosphide |
| 609 | 83455 | 13445‐56‐2 | Pyrophosphorous acid |
| 610 | 93440 | 13463‐67‐7 | Titanium dioxide |
| 611 | 35120 | 13560‐49‐1 | 3‐Aminocrotonic acid, diester with thiobis (2‐hydroxyethyl) ether |
| 613 | 95905 | 13983‐17‐0 | Wollastonite |
| 614 | 45560 | 14464‐46‐1 | Cristobalite |
| 615 | 92080 | 14807‐96‐6 | Talc |
| 616 | 83470 | 14808‐60‐7 | Quartz |
| 623 | 52640 | 16389‐88‐1 | Dolomite |
| 625 | 36720 | 17194‐00‐2 | Barium hydroxide |
| 626 | 57800 | 18641‐57‐1 | Glycerol tribehenate |
| 627 | 59760 | 19569‐21‐2 | Huntite |
| 628 | 96190 | 20427‐58‐1 | Zinc hydroxide |
| 629 | 34560 | 21645‐51‐2 | Aluminium hydroxide |
| 630 | 82240 | 22788‐19‐8 | 1,2‐Propyleneglycol dilaurate |
| 634 | 25910 | 24800‐44‐0 | Tripropyleneglycol |
| 638 | 23590 ‐ 76960 | 25322‐68‐3 | Polyethyleneglycol |
| 639 | 23651 ‐ 80800 | 25322‐69‐4 | Polypropyleneglycol |
| 642 | 64990 | 25736‐61‐2 | Maleic anhydride‐styrene, copolymer, sodium salt |
| 643 | 87760 | 26266‐57‐9 | Sorbitan monopalmitate |
| 644 | 88080 | 26266‐58‐0 | Sorbitan trioleate |
| 647 | 56720 | 26402‐23‐3 | Glycerol monohexanoate |
| 648 | 56880 | 26402‐26‐6 | Glycerol monooctanoate |
| 649 | 47210 | 26427‐07‐6 | Dibutylthiostannoic acid polymer |
| 651 | 88240 | 26658‐19‐5 | Sorbitan tristearate |
| 654 | 88600 | 26836‐47‐5 | Sorbitol monostearate |
| 659 | 82800 | 27194‐74‐7 | 1,2‐Propyleneglycol monolaurate |
| 663 | 64150 | 28290‐79‐1 | Linolenic acid |
| 664 | 95000 | 28931‐67‐1 | Trimethylolpropane trimethacrylate‐methyl methacrylate copolymer |
| 665 | 83120 | 29013‐28‐3 | 1,2‐Propyleneglycol monopalmitate |
| 666 | 87280 | 29116‐98‐1 | Sorbitan dioleate |
| 667 | 55190 | 29204‐02‐2 | Gadoleic acid |
| 668 | 80240 | 29894‐35‐7 | Polyglycerol ricinoleate |
| 669 | 56610 | 30233‐64‐8 | Glycerol monobehenate |
| 671 | 74240 | 31570‐04‐4 | Phosphorous acid, tris(2,4‐di‐tert‐butylphenyl)ester |
| 674 | 46480 | 32647‐67‐9 | Dibenzylidene sorbitol |
| 677 | 82560 | 33587‐20‐1 | 1,2‐Propyleneglycol dipalmitate |
| 681 | 18310 | 36653‐82‐4 | 1‐Hexadecanol |
| 682 | 53270 | 37205‐99‐5 | Ethylcarboxymethylcellulose |
| 683 | 66200 | 37206‐01‐2 | Methylcarboxymethylcellulose |
| 684 | 68125 | 37244‐96‐5 | Nepheline syenite |
| 686 | 61390 | 37353‐59‐6 | Hydroxymethylcellulose |
| 693 | 88160 | 54140‐20‐4 | Sorbitan tripalmitate |
| 696 | 92205 | 57569‐40‐1 | Terephthalic acid, diester with 2,2′‐methylenebis(4‐methyl‐6‐tert‐butylphenol) |
| 699 | 90720 | 58446‐52‐9 | Stearoylbenzoylmethane |
| 702 | 87920 | 61752‐68‐9 | Sorbitan tetrastearate |
| 703 | 17170 | 61788‐47‐4 | Fatty acids, coco |
| 704 | 77600 | 61788‐85‐0 | Polyethyleneglycol ester of hydrogenated castor oil |
| 706 | 17230 | 61790‐12‐3 | Fatty acids, tall oil |
| 707 | 46375 | 61790‐53‐2 | Diatomaceous earth |
| 709 | 87520 | 62568‐11‐0 | Sorbitan monobehenate |
| 712 | 42960 | 64147‐40‐6 | Castor oil, dehydrated |
| 713 | 43480 | 64365‐11‐3 | Charcoal, activated |
| 714 | 84400 | 64365‐17‐9 | Rosin, hydrogenated, ester with pentaerythritol |
| 717 | 84210 | 65997‐06‐0 | Rosin, hydrogenated |
| 718 | 84240 | 65997‐13‐9 | Rosin, hydrogenated, ester with glycerol |
| 719 | 65920 | 66822‐60‐4 | n‐Methacryloyloxyethyl‐n,n‐dimethyl‐n‐carboxymethylammonium chloride, sodium salt ‐octadecyl methacrylate‐ethyl methacrylate‐cyclohexyl methacrylate‐n‐vinyl‐2‐pyrrolidone, copolymers |
| 721 | 46800 | 67845‐93‐6 | 3,5‐Di‐tert‐butyl‐4‐hydroxybenzoic acid, hexadecyl ester |
| 722 | 17200 | 68308‐53‐2 | Fatty acids, soya |
| 723 | 88880 | 68412‐29‐3 | Starch, hydrolysed |
| 724 | 24903 | 68425‐17‐2 | Syrups, hydrolysed starch, hydrogenated |
| 727 | 43360 | 68442‐85‐3 | Cellulose, regenerated |
| 730 | 66930 | 68554‐70‐1 | Methylsilsesquioxane |
| 734 | 46380 | 68855‐54‐9 | Diatomaceous earth, soda ash flux‐calcined |
| 737 | 77370 | 70142‐34‐6 | Polyethyleneglycol‐30 dipolyhydroxystearate |
| 739 | 70000 | 70331‐94‐1 | 2,2′‐Oxamidobis[ethyl‐3‐(3,5‐di‐tert‐butyl‐4‐hydroxyphenyl)‐propionate] |
| 741 | 24070 ‐ 83610 | 73138‐82‐6 | Resin acids and rosin acids |
| 743 | 38950 | 79072‐96‐1 | Bis(4‐ethylbenzylidene)sorbitol |
| 751 | 81515 | 87189‐25‐1 | Poly(zinc glycerolate) |
| 752 | 39890 | 87826‐41‐3 | Bis(methylbenzylidene)sorbitol |
| 753 | 62800 | 92704‐41‐1 | Kaolin, calcined |
| 754 | 56020 | 99880‐64‐5 | Glycerol dibehenate |
| 757 | 95725 | 110638‐71‐6 | Vermiculite, reaction product with citric acid, lithium salt |
| 766 | 38879 | 135861‐56‐2 | Bis(3,4‐dimethylbenzylidene)sorbitol |
| 768 | 34850 | 143925‐92‐2 | Amines, bis(hydrogenated tallow alkyl) oxidised |
| 776 | 76723 | 167883‐16‐1 | Polydimethylsiloxane, 3‐aminopropyl terminated, polymer with dicyclohexylmethane‐4,4′‐diisocyanate |
| 777 | 31542 | 174254‐23‐0 | Acrylic acid, methyl ester, telomer with 1‐dodecanethiol, c16‐c18 alkyl esters |
| 782 | 76725 | 661476‐41‐1 | Polydimethylsiloxane, 3‐aminopropyl terminated, polymer with 1‐isocyanato‐3‐isocyanatomethyl‐3,5,5‐trimethylcyclohexane |
| 789 | 60027 | Hydrogenated homopolymers and/or copolymers made of 1‐hexene and/or 1‐octene and/or 1‐decene and/or 1‐dodecene and/or 1‐tetradecene (mw: 440–12 000) | |
| 794 | 18117 | 79‐14‐1 | Glycolic acid |
| 800 | 94425 | 867‐13‐0 | Triethyl phosphonoacetate |
| 801 | 30607 | Acids, c2‐c24, aliphatic, linear, monocarboxylic, from natural oils and fats, lithium salt | |
| 803 | 33535 | 152261‐33‐1 | α‐Alkenes(c20‐c24) copolymer with maleic anhydride, reaction product with 4‐amino‐2,2,6,6‐tetramethylpiperidine |
| 804 | 80510 | 1010121‐89‐7 | Poly(3‐nonyl‐1,1‐dioxo‐1‐thiopropane‐1,3‐diyl)‐block‐poly(x‐oleyl‐7‐hydroxy‐1,5‐diiminooctane‐1,8‐diyl), process mixture with x = 1 and/or 5, neutralised with dodecylbenzenesulfonic acid |
| 805 | 93450 | Titanium dioxide, coated with a copolymer of n‐octyltrichlorosilane and [aminotris(methylenephosphonic acid), penta sodium salt] | |
| 807 | 93485 | Titanium nitride, nanoparticles | |
| 812 | 80350 | 124578‐12‐7 | Poly(12‐hydroxystearic acid)‐polyethyleneimine copolymer |
| 820 | 76420 | Pimelic acid, salts | |
| 821 | 90810 | Stearoyl‐2‐lactylic acid, salts | |
| 854 | 71943 | 329238‐24‐6 | Perfluoro acetic acid, α‐substituted with the copolymer of perfluoro‐1,2‐propylene glycol and perfluoro‐1,1‐ethylene glycol, terminated with chlorohexafluoropropyloxy groups |
| 855 | 40560 | (Butadiene, styrene, methyl methacrylate) copolymer cross‐linked with 1,3‐butanediol dimethacrylate | |
| 856 | 40563 | 25101‐28‐4 | (Butadiene, styrene, methyl methacrylate, butyl acrylate) copolymer cross‐linked with divinylbenzene or 1,3‐butanediol dimethacrylate |
| 857 | 66765 | 37953‐21‐2 | (Methyl methacrylate, butyl acrylate, styrene, glycidyl methacrylate) copolymer |
| 859 | (Butadiene, ethyl acrylate, methyl methacrylate, styrene) Copolymer crosslinked with divinylbenzene, in nanoform | ||
| 860 | 71980 | 51798‐33‐5 | Perfluoro[2‐(poly(n‐propoxy))propanoic acid] |
| 861 | 71990 | 13252‐13‐6 | Perfluoro[2‐(n‐propoxy)propanoic acid] |
| 865 | 40619 | 25322‐99‐0 | (Butyl acrylate, methyl methacrylate, butyl methacrylate) copolymer |
| 866 | 40620 | (Butyl acrylate, methyl methacrylate) copolymer, cross‐linked with allyl methacrylate | |
| 867 | 40815 | 40471‐03‐2 | (Butyl methacrylate, ethyl acrylate, methyl methacrylate) copolymer |
| 868 | 53245 | 9010‐88‐2 | (Ethyl acrylate, methyl methacrylate) copolymer |
| 869 | 66763 | 27136‐15‐8 | (Butyl acrylate, methyl methacrylate, styrene) copolymer |
| 871 | 287916‐86‐3 | Dodecanoic acid, 12‐amino‐, polymer with ethene, 2,5‐furandione, α‐hydro‐ω‐hydroxypoly (oxy‐1,2‐ethanediyl) and 1‐propene | |
| 873 | 93460 | Titanium dioxide reacted with octyltriethoxysilane | |
| 878 | 31335 | Acids, fatty (c8‐c22) from animal or vegetable fats and oils, esters with branched alcohols, aliphatic, monohydric, saturated, primary (c3‐c22) | |
| 879 | 31336 | Acids, fatty (c8‐c22) from animal or vegetable fats and oils, esters with alcohols, linear, aliphatic, monohydric, saturated, primary (c1‐c22) | |
| 880 | 31348 | Acids, fatty (c8‐c22), esters with pentaerythritol’ | |
| 885 | 45676 | 263244‐54‐8 | Cyclic oligomers of (butylene terephthalate) |
| 896 | 71958 | 958445‐44‐8 | 3h‐perfluoro‐3‐[(3‐methoxy‐propoxy)propanoic acid], ammonium salt |
| 902 | 128‐44‐9 | 1,2‐Benzisothiazol‐3(2h)‐one 1,1‐dioxide, sodium salt | |
| 903 | 37486‐69‐4 | 2h‐Perfluoro‐[(5,8,11,14‐tetramethyl)‐tetraethyleneglycol ethyl propyl ether] | |
| 926 | 71955 | 908020‐52‐0 | Perfluoro[(2‐ethyloxy‐ethoxy)acetic acid], ammonium salt |
| 969 | 24937‐78‐8 | Ethylene‐vinyl acetate copolymer wax | |
| 971 | 25885 | 2459‐10‐1 | Trimethyl trimellitate |
| 972 | 45197 | 12158‐74‐6 | Copper hydroxide phosphate |
| 973 | 22931 | 19430‐93‐4 | (Perfluorobutyl)ethylene |
| 979 | 79987 | (Polyethylene terephthalate, hydroxylated polybutadiene, pyromellitic anhydride) copolymer | |
| 998 | (Butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer not cross‐linked, in nanoform | ||
| 1007 | 976‐56‐7 | Diethyl[[3,5‐bis(1,1‐dimethylethyl)‐4‐hydroxyphenyl]methyl]phosphonate | |
| 1016 | (Methacrylic acid, ethyl acrylate, n‐butyl acrylate, methyl methacrylate and butadiene) copolymer in nanoform | ||
| 1017 | 25618‐55‐7 | Polyglycerol | |
| 1030 | Montmorillonite clay modified by dimethyldialkyl(c16‐c18)ammonium chloride | ||
| 1043 | (Butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer crosslinked with 1,3‐butanediol dimethacrylate, in nanoform | ||
| 1045 | 1190931‐27‐1 | Perfluoro{acetic acid, 2‐[(5‐methoxy‐1,3‐dioxolan‐4‐yl)oxy]}, ammonium salt | |
| 1046 | Zinc oxide, nanoparticles, coated with [3‐(methacryloxy)propyl] trimethoxysilane (fcm no 788) | ||
| 1050 | Zinc oxide, nanoparticles, uncoated | ||
| 1053 | Fatty acids, c16–18 saturated, esters with dipentaerythritol | ||
| 1055 |
7695‐91‐2 58‐95‐7 |
α‐Tocopherol acetate | |
| 1060 | Ground sunflower seed hulls | ||
| 1061 | 80512‐44‐3 | 2,4,4′‐trifluorobenzophenone | |
| 1062 | Mixture composed of 97% tetraethyl orthosilicate (teos) with cas no 78‐10‐4 and 3% hexamethyldisilazane (hmds) with cas no 999‐97‐3 | ||
| 1063 | 1547‐26‐8 | 2,3,3,4,4,5,5‐heptafluoro‐1‐pentene | |
| 1067 | 616‐38‐6 | Dimethyl carbonate | |
| 1068 | 2530‐83‐8 | [3‐(2,3‐epoxypropoxy)propyl]trimethoxy silane | |
| 1069 | 75‐28‐5 | Isobutane |
3.1.2. Exclusions from the prioritisation exercise
Food contact material substances that have been evaluated by EFSA, following mandates on FCM applications by the Member States or mandates of the Commission were excluded from the prioritisation exercise. In total, 78 substances without an SML have been evaluated by EFSA, of which 77 were evaluated on the basis of an application and one substance (BADGE) on the basis described under paragraph 2.2.1.2 and were excluded. The list of substances and corresponding EFSA opinions is provided in Appendix A, Table A.2.
Table A.2.
EFSA opinions on applications of FCM substances without an SML and EFSA opinion on BADGE
| FCM substance no. | Ref. no. | CAS no. | Substance name | EFSA panel | EFSA opinion title | EFSA opinion link |
|---|---|---|---|---|---|---|
| 87 | 86285 | Silicon dioxide, silanated | EFSA CEF | Statement on the safety assessment of the substance silicon dioxide, silanated, FCM Substance No 87 for use in food contact materials | https://doi.org/10.2903/j.efsa.2014.3712 | |
| 97 | 720810 | Petroleum hydrocarbon resins (hydrogenated) | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to a 13th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2006.418 | |
| 258 | 70480 | 111‐06‐8 | Palmitic acid, butyl ester | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 14th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2007.452 |
| 270 | 22763 ‐ 69040 | 112‐80‐1 | Oleic acid | EFSA AFC | Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to a 1st list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2003.3 |
| 301 | 89120 | 123‐95‐5 | Stearic acid, butyl ester | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 14th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2007.452 |
| 411 | 42080 | 1333‐86‐4 | Carbon black | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 9th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2005.248a |
| 426 | 13510 ‐ 13610 | 1675‐54‐3 | 2,2‐Bis(4‐hydroxyphenyl)propane bis(2,3‐epoxypropyl) ether | EFSA CEF | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2‐bis(4‐hydroxyphenyl)propane bis(2,3‐epoxypropyl)ether (Bisphenol A diglycidyl ether, BADGE). REF. No 13510 and 39700 | https://doi.org/10.2903/j.efsa.2004.86 |
| 468 | 71960 | 3825‐26‐1 | Perfluorooctanoic acid, ammonium salt | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 9th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2005.248a |
| 499 | 19965 ‐ 65020 | 6915‐15‐7 | Malic acid | EFSA CEF | 22nd list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2009.961 |
| 549 | 80000 | 9002‐88‐4 | Polyethylene wax | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 3rd list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.37 |
| 550 | 81060 | 9003‐07‐0 | Polypropylene wax | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 3rd list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.37 |
| 551 | 79920 | 9003‐11‐6106392‐12‐5 | Poly(ethylene propylene) glycol | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to an 11th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2006.316 |
| 552 | 81500 | 9003‐39‐8 | Polyvinylpyrrolidone | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 12th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2006.395 |
| 577 | 62280 | 9044‐17‐1 | Isobutylene‐butene copolymer | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 15th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2007.516 |
| 586 | 65280 | 10043‐84‐2 | Manganese hypophosphite | EFSA CEF | Scientific Report of EFSA on the risk assessment of salts of authorised acids, phenols or alcohols for use in food contact materials | https://doi.org/10.2903/j.efsa.2009.1364 |
| 607 | 62245 | 12751‐22‐3 | Iron phosphide | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 6th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.161 |
| 642 | 64990 | 25736‐61‐2 | Maleic anhydride‐styrene, copolymer, sodium salt | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 6th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.161 |
| 671 | 74240 | 31570‐04‐4 | Phosphorous acid, tris(2,4‐di‐tert‐butylphenyl)ester | EFSA CEF | Safety assessment of the substance phosphorous acid, mixed 2,4‐bis(1,1‐dimethylpropyl)phenyl and 4‐(1,1‐dimethylpropyl)phenyl triesters for use in food contact materials | https://doi.org/10.2903/j.efsa.2017.4841 |
| 713 | 43480 | 64365‐11‐3 | Charcoal, activated | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 5th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.109 |
| 724 | 24903 | 68425‐17‐2 | Syrups, hydrolysed starch, hydrogenated | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 5th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.109 |
| 730 | 66930 | 68554‐70‐1 | Methylsilsesquioxane | EFSA AFC | Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to a 4th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.65a |
| 737 | 77370 | 70142‐34‐6 | Polyethyleneglycol‐30 dipolyhydroxystearate | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 5th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2004.109 |
| 776 | 76723 | 167883‐16‐1 | Polydimethylsiloxane, 3‐aminopropyl terminated, polymer with dicyclohexylmethane‐4,4′‐diisocyanate | EFSA AFC | Scientific Opinion of the Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 16th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2007.555 |
| 777 | 31542 | 174254‐23‐0 | Acrylic acid, methyl ester, telomer with 1‐dodecanethiol, C16‐C18 alkyl esters | EFSA AFC | Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to a 1st list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2003.3 |
| 782 | 76725 | 661476‐41‐1 | Polydimethylsiloxane, 3‐aminopropyl terminated, polymer with 1‐isocyanato‐3‐isocyanatomethyl‐3,5,5‐trimethylcyclohexane | EFSA AFC | Scientific Opinion of the Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 16th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2007.555 |
| 789 | 60027 | Hydrogenated homopolymers and/or copolymers made of 1‐hexene and/or 1‐octene and/or 1‐decene and/or 1‐dodecene and/or 1‐tetradecene (Mw: 440–12 000) | EFSA CEF | Scientific Opinion on the safety evaluation of the substance hydrogenated homopolymers and/or copolymers made of 1‐hexene and/or 1‐octene and/or 1‐decene and/or 1‐dodecene and/or 1‐tetradecene (Mw: 440‐12000) for use in food contact materials | https://doi.org/10.2903/j.efsa.2010.1521 | |
| 794 | 18117 | 79‐14‐1 | Glycolic acid | EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 18th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2008.628 |
| 800 | 94425 | 867‐13‐0 | Triethyl phosphonoacetate | EFSA AFC | 19th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2008.699 |
| 801 | 30607 | Acids, C2‐C24, aliphatic, linear, monocarboxylic, from natural oils and fats, lithium salt | EFSA CEF | 20th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2008.816 | |
| 803 | 33535 | 152261‐33‐1 | α‐Alkenes(C20‐C24) copolymer with maleic anhydride, reaction product with 4‐amino‐2,2,6,6‐tetramethylpiperidine | EFSA CEF | 20th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2008.816 |
| 804 | 80510 | 1010121‐89‐7 | Poly(3‐nonyl‐1,1‐dioxo‐1‐thiopropane‐1,3‐diyl)‐block‐poly(x‐oleyl‐7‐hydroxy‐1,5‐diiminooctane‐1,8‐diyl), process mixture with x = 1 and/or 5, neutralised with dodecylbenzenesulfonic acid | EFSA CEF | 20th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2008.816 |
| 805 | 93450 | Titanium dioxide, coated with a copolymer of n‐octyltrichlorosilane and [aminotris(methylenephosphonic acid), penta sodium salt] | EFSA CEF | 20th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2008.816 | |
| 807 | 93485 | Titanium nitride, nanoparticles | EFSA CEF | 20th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2008.816 | |
| 812 | 80350 | 124578‐12‐7 | Poly(12‐hydroxystearic acid)‐polyethyleneimine copolymer | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, poly(12‐hydroxystearic acid)‐polyethyleneimine copolymer, CAS No. 124578‐12‐7, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2125 |
| 854 | 71943 | 329238‐24‐6 | Perfluoro acetic acid, α‐substituted with the copolymer of perfluoro‐1,2‐propylene glycol and perfluoro‐1,1‐ethylene glycol, terminated with chlorohexafluoropropyloxy groups | EFSA CEF | Scientific Opinion on the safety evaluation of the substance perfluoro acetic acid, alpha‐substituted with the copolymer of perfluoro‐1,2‐propylene glycol and perfluoro‐1,1‐ethylene glycol, terminated with chlorohexafluoropropyloxy groups, CAS No. 329238‐24‐6 for use in food contact materials ‐ EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2010.1519 |
| 855 | 40560 | (Butadiene, styrene, methyl methacrylate) copolymer cross‐linked with 1,3‐butanediol dimethacrylate | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (butadiene, styrene, methyl methacrylate) copolymer cross‐linked with 1,3‐butanediol dimethacrylate, for use in food contact materials | https://doi.org/10.2903/j.efsa.2011.2122 | |
| 856 | 40563 | 25101‐28‐4 | (Butadiene, styrene, methyl methacrylate, butyl acrylate) copolymer cross‐linked with divinylbenzene or 1,3‐butanediol dimethacrylate | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (butadiene, styrene, methyl methacrylate, butyl acrylate) copolymer cross‐linked with divinylbenzene or 1,3‐butanediol dimethacrylate for use in food contact materials | https://doi.org/10.2903/j.efsa.2011.2123 |
| 857 | 66765 | 37953‐21‐2 | (Methyl methacrylate, butyl acrylate, styrene, glycidyl methacrylate) copolymer | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (methyl methacrylate, butyl acrylate, styrene, glycidyl methacrylate) copolymer, CAS No. 37953‐21‐2, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2124 |
| 859 | (Butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer crosslinked with divinylbenzene, in nanoform | EFSA CEF | Scientific Opinion on the safety assessment of the substances (butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer either not crosslinked or crosslinked with divinylbenzene or 1,3‐butanediol dimethacrylate, in nanoform, for use in food contact materials | https://doi.org/10.2903/j.efsa.2014.3635 | ||
| 860 | 71980 | 51798‐33‐5 | Perfluoro[2‐(poly(n‐propoxy))propanoic acid] | EFSA CEF | 24th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2009.1157 |
| 861 | 71990 | 13252‐13‐6 | Perfluoro[2‐(n‐propoxy)propanoic acid] | EFSA CEF | 24th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2009.1157 |
| 865 | 40619 | 25322‐99‐0 | (Butyl acrylate, methyl methacrylate, butyl methacrylate) copolymer | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (butyl acrylate, butyl methacrylate, methyl methacrylate) copolymer, CAS No. 25322‐99‐0, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2463 |
| 866 | 40620 | (Butyl acrylate, methyl methacrylate) copolymer, cross‐linked with allyl methacrylate | EFSA CEF | 25th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2009.1196 | |
| 867 | 40815 | 40471‐03‐2 | (Butyl methacrylate, ethyl acrylate, methyl methacrylate) copolymer | EFSA CEF | 25th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2009.1196 |
| 868 | 53245 | 9010‐88‐2 | (Ethyl acrylate, methyl methacrylate) copolymer | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (ethyl acrylate, methyl methacrylate) copolymer, CAS No. 9010‐88‐2, for use in food contact materials ‐ EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2464 |
| 869 | 66763 | 27136‐15‐8 | (Butyl acrylate, methyl methacrylate, styrene) copolymer | EFSA CEF | 25th list of substances for food contact materials – Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2009.1196 |
| 871 | 287916‐86‐3 | Dodecanoic acid, 12‐amino‐, polymer with ethene, 2,5‐furandione, α‐hydro‐ω‐hydroxypoly (oxy‐1,2‐ethanediyl) and 1‐propene | EFSA CEF | Scientific Opinion on the safety assessment of the substance, dodecanoic acid, 12‐amino‐, polymer with ethene, 2,5‐furandione, alpha‐hydro‐omega‐hydroxypoly (oxy‐1,2‐ethanediyl) and 1‐propene, CAS No 287916‐86‐3, for use in food contact materials | https://doi.org/10.2903/j.efsa.2014.3909 | |
| 873 | 93460 | Titanium dioxide reacted with octyltriethoxysilane | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, titanium dioxide reacted with octyltriethoxysilane, CAS no. not assigned, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2003 | |
| 878 | 31335 | Acids, fatty (C8‐C22) from animal or vegetable fats and oils, esters with branched alcohols, aliphatic, monohydric, saturated, primary (C3‐C22) | EFSA CEF | 24th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2009.1157 | |
| 879 | 31336 | Acids, fatty (C8‐C22) from animal or vegetable fats and oils, esters with alcohols, linear, aliphatic, monohydric, saturated, primary (C1‐C22) | EFSA CEF | 24th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2009.1157 | |
| 880 | 31348 | Acids, fatty (C8‐C22), esters with pentaerythritol’ | EFSA CEF | 24th list of substances for food contact materials | https://doi.org/10.2903/j.efsa.2009.1157 | |
| 885 | 45676 | 263244‐54‐8 | Cyclic oligomers of (butylene terephthalate) | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, cyclic oligomers of (butylene terephthalate), CAS No. 263244‐54‐8, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2009.1399 |
| 896 | 71958 | 958445‐44‐8 | 3H‐Perfluoro‐3‐[(3‐methoxy‐propoxy)propanoic acid], ammonium salt | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, 3H‐perfluoro‐3‐[(3‐methoxy‐propoxy)propanoic acid], ammonium salt, CAS No. 958445‐44‐8, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2182 |
| 902 | 128‐44‐9 | 1,2‐Benzisothiazol‐3(2H)‐one 1,1‐dioxide, sodium salt | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, 1,2‐benzisothiazol‐3(2H)‐one 1,1‐dioxide, sodium salt, CAS No. 128‐44‐9, for use in food contact materials ‐ EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2012.2640 | |
| 903 | 37486‐69‐4 | 2H‐perfluoro‐[(5,8,11,14‐tetramethyl)‐tetraethyleneglycol ethyl propyl ether] | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, 2H‐perfluoro‐[(5,8,11,14‐tetramethyl)‐tetraethyleneglycol ethyl propyl ether] CAS No 37486‐69‐4 for use in food contact materials. | https://doi.org/10.2903/j.efsa.2012.2978 | |
| 926 | 71955 | 908020‐52‐0 | Perfluoro[(2‐ethyloxy‐ethoxy)acetic acid], ammonium salt | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, Perfluoro[(2‐ethyloxy‐ethoxy)acetic acid], ammonium salt, CAS No. 908020‐52‐0, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2183 |
| 969 | 24937‐78‐8 | Ethylene‐vinyl acetate copolymer wax | EFSA CEF | Scientific Opinion on the safety assessment of the substance ethylene‐vinyl acetate copolymer wax, CAS No 24937‐78‐8 for use in food contact materials. | https://doi.org/10.2903/j.efsa.2014.3555 | |
| 971 | 25885 | 2459‐10‐1 | Trimethyl trimellitate | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, trimethyl trimellitate, CAS No. 2459‐10‐1, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.1997 |
| 972 | 45197 | 12158‐74‐6 | copper hydroxide phosphate | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, copper hydroxide phosphate, CAS No. 12158‐74‐6, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2010.1838 |
| 973 | 22931 | 19430‐93‐4 | (Perfluorobutyl)ethylene | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (perfluorobutyl)ethylene, CAS No. 19430‐93‐4, for use in food contact materials – EFSA Panel on food contact materials, enzymes, flavourings and processing aids (CEF) | https://doi.org/10.2903/j.efsa.2011.2000 |
| 979 | 79987 | (Polyethylene terephthalate, hydroxylated polybutadiene, pyromellitic anhydride) copolymer | EFSA CEF | Scientific Opinion on the safety evaluation of the substance, (polyethylene terephthalate, hydroxylated polybutadiene, pyromellitic anhydride) copolymer, for use in food contact materials | https://doi.org/10.2903/j.efsa.2011.2462 | |
| 998 | (Butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer not cross‐linked, in nanoform | EFSA CEF | Scientific Opinion on the safety assessment of the substances (butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer either not crosslinked or crosslinked with divinylbenzene or 1,3‐butanediol dimethacrylate, in nanoform, for use in food contact materials | https://doi.org/10.2903/j.efsa.2014.3635 | ||
| 1007 | 976‐56‐7 | Diethyl[[3,5‐bis(1,1‐dimethylethyl)‐4‐hydroxyphenyl]methyl]phosphonate | EFSA CEF | Safety assessment of the substance diethyl[[3,5‐bis(1,1‐dimethylethyl)‐4‐hydroxyphenyl]methyl]phosphonate, for use in food contact materials | https://doi.org/10.2903/j.efsa.2016.4536 | |
| 1016 | (Methacrylic acid, ethyl acrylate, n‐butyl acrylate, methyl methacrylate and butadiene) copolymer in nanoform | EFSA CEF | Scientific Opinion on the safety assessment of the substance (methacrylic acid, ethyl acrylate, n‐butyl acrylate, methyl methacrylate and butadiene) copolymer in nanoform for use in food contact materials | https://doi.org/10.2903/j.efsa.2015.4008 | ||
| 1017 | 25618‐55‐7 | Polyglycerol | EFSA CEF | Scientific Opinion on the safety assessment of the substance, polyglycerol, CAS No 25618‐55‐7, for use in food contact materials. | https://doi.org/10.2903/j.efsa.2013.3389 | |
| 1030 | Montmorillonite clay modified by dimethyldialkyl(C16‐C18)ammonium chloride | Safety assessment of the substance montmorillonite clay modified by dimethyldialkyl(C16‐C18)ammonium chloride for use in food contact materials | https://doi.org/10.2903/j.efsa.2015.4285 | |||
| 1043 | (Bbutadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer crosslinked with 1,3‐butanediol dimethacrylate, in nanoform | EFSA CEF | Scientific Opinion on the safety assessment of the substances (butadiene, ethyl acrylate, methyl methacrylate, styrene) copolymer either not crosslinked or crosslinked with divinylbenzene or 1,3‐butanediol dimethacrylate, in nanoform, for use in food contact materials | https://doi.org/10.2903/j.efsa.2014.3635 | ||
| 1045 | 1190931‐27‐1 | Perfluoro{acetic acid, 2‐[(5‐methoxy‐1,3‐dioxolan‐4‐yl)oxy]}, ammonium salt | EFSA CEF | Scientific Opinion on the safety assessment of the substance, Perfluoro{acetic acid, 2‐[(5‐methoxy‐1,3‐dioxolan‐4‐yl)oxy]}, ammonium salt, CAS No 1190931‐27‐1, for use in food contact materials | https://doi.org/10.2903/j.efsa.2014.3718 | |
| 1046 | Zinc oxide, nanoparticles, coated with [3‐(methacryloxy)propyl] trimethoxysilane (FCM No 788) | EFSA CEF | Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3‐(methacryloxy)propyl] trimethoxysilane, for use in food contact materials | https://doi.org/10.2903/j.efsa.2015.4063 | ||
| 1050 | Zinc oxide, nanoparticles, uncoated | EFSA CEF | Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3‐(methacryloxy)propyl] trimethoxysilane, for use in food contact materials | https://doi.org/10.2903/j.efsa.2015.4063 | ||
| 1053 | Fatty acids, C16–18 saturated, esters with dipentaerythritol | EFSA CEF | Scientific Opinion on the safety assessment of the substance fatty acids, C16–18 saturated, hexaesters with dipentaerythritol for use in food contact materials | https://doi.org/10.2903/j.efsa.2015.4021 | ||
| 1055 | 7695‐91‐258‐95‐7 | α‐tocopherol acetate | EFSA CEF | Safety assessment of the substance α‐tocopherol acetate for use in food contact materials | https://doi.org/10.2903/j.efsa.2016.4412 | |
| 1060 | Ground sunflower seed hulls | EFSA CEF | Safety assessment of the substance ground sunflower seed hulls, for use in food contact materials | https://doi.org/10.2903/j.efsa.2016.4534 | ||
| 1061 | 80512‐44‐3 | 2,4,4′‐Trifluorobenzophenone | EFSA CEF | Safety assessment of the substance 2,4,4’‐trifluorobenzophenone, for use in food contact materials | https://doi.org/10.2903/j.efsa.2016.4532 | |
| 1062 | Mixture composed of 97% tetraethyl orthosilicate (TEOS) with CAS No 78‐10‐4 and 3% hexamethyldisilazane (HMDS) with CAS No 999‐97‐3 | EFSA CEF | Scientific Opinion on the safety assessment of the substances tetraethyl orthosilicate, CAS No. 78‐10‐4, and hexamethyldisilazane, CAS No. 999 97 3, for use in food contact materials | https://doi.org/10.2903/j.efsa.2016.4337 | ||
| 1063 | 1547‐26‐8 | 2,3,3,4,4,5,5‐Heptafluoro‐1‐pentene | EFSA CEF | Safety assessment of the substance 2,3,3,4,4,5,5‐heptafluoro‐1‐pentene, for use in food contact materials | https://doi.org/10.2903/j.efsa.2016.4582 | |
| 1067 | 616‐38‐6 | Dimethyl carbonate | EFSA CEF | Safety assessment of the substance dimethyl carbonate for use in food contact materials | https://doi.org/10.2903/j.efsa.2017.4901 | |
| 1068 | 2530‐83‐8 | [3‐(2,3‐Epoxypropoxy)propyl]trimethoxy silane | EFSA CEF | Safety assessment of the substance [3‐(2,3‐epoxypropoxy) propyl]trimethoxy silane, for use in food contact materials | https://doi.org/10.2903/j.efsa.2017.5014 | |
| 1069 | 75‐28‐5 | Isobutane | EFSA CEF | Safety assessment of the substance isobutane, for use in food contact materials | https://doi.org/10.2903/j.efsa.2018.5116 |
3.2. Prioritisation
3.2.1. Substances for which an SML should not be needed
Thirty‐three of the remaining 373 substances have been classified by the SCF as List 0. They were searched by CAS number and name in the EFSA OpenFoodTox database and, for 19 of these 33 SCF List 0 substances, evaluations by one or more EFSA Panels were identified (Appendix A, Table A.3). In agreement with the earlier SCF classifications, these EFSA evaluations do not raise any safety concern.
Table A.3.
Substances from the SCF List 0 group, for which EFSA opinions have subsequently been produced
| FCM no. | Substance name | Panel | Opinion title | Year | Conclusions on: | Remarks | ||
|---|---|---|---|---|---|---|---|---|
| Mutagenicity | Genotoxicity | Carcinogenicity | ||||||
| 580 | α‐Dextrin | EFSA NDA | Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the safety of alpha-cyclodextrin | 2007 | Negative | Negative | Negative | |
| 553 | Cellulose | EFSA ANS | Safety of the proposed amendment of the specifications for microcrystalline cellulose (E 460(i)) as a food additive | 2017 | No data | No data | No data | The Panel concluded that the amendment to the specifications as regards the solubility of microcrystalline cellulose (E 46(i)) proposed by the applicant would not give rise to a safety concern. However, the Panel recommended that the concentration of sodium hydroxide solution to be used in the solubility test should be indicated in the EU specifications |
| EFSA ANS | Re-evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives | 2018 | Negative | Negative | Negative | |||
| EFSA CEF | Scientific Opinion on the safety evaluation of the active substances iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite for use in food contact materials | 2013 | No data | No data | No data | The CEF Panel concluded that the substances: iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite, do not raise a safety concern when used in oxygen absorbers in sachets, patches or cards, placed in the headspace of the packaging or when used in direct contact with food, excluding liquid food or foods that have an external aqueous liquid phase on the surface such as sliced fruits and fresh meat. Activated carbon should in addition comply with the same purity requirements as for Vegetable Carbon (E 153) set out by Commission Directive 95/45/EC with exception of ash content which can be up to 1% (w/w) | ||
| EFSA CEF | Scientific Opinion on the safety assessment of the active substances, sodium erythorbate, sodium carbonate, sodium bicarbonate, iron sulphate, activated carbon, cellulose, calcium hydroxide, calcium chloride and water, for use as active system in food contact materials | 2014 | Negative | Negative | No data | |||
| 531 | Beeswax | EFSA AFC | Beeswax (E 901) as a glazing agent and as carrier for flavours | 2007 | Negative | Negative | Negative | |
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils - Part II of III | 2012 | Negative | Negative | Negative | Although specific information on beeswax is very limited, there is sufficient information from its human uses, its poor absorption and on its main component groups of chemicals for the CONTAM Panel to conclude that it will not pose any toxicological concern when used as a previous cargo. There is no evidence that it is genotoxic and there is no allergenic potential of concern. It will not give rise to any reaction products with fats and oils of toxicological concern. No impurities of toxicological concern are known or anticipated. The CONTAM Panel therefore concludes that beeswax meets the criteria for acceptability as a previous cargo for edible fats and oils | ||
| 515 | Water | EFSA CONTAM | Scientific Opinion on the abiotic risks for public and animal health of glycerine as co-product from the biodiesel production from Category 1 animal by-products (ABP) and vegetable oils | 2010 | No data | No data | No data | |
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | |||
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 503 (FGE.503): grill flavour ‘Grillin’ CB-200SF’ | 2017 | Not determined | Not determined | Not determined | |||
| EFSA NDA | Scientific Opinion on the safety of ‘Lentinus edodes extract’ (Lentinex) as a Novel Food ingredient | 2010 | No data | No data | No data | |||
| EFSA CEF | Scientific Opinion on the safety evaluation of the active substances iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite for use in food contact materials | 2013 | No data | No data | No data | The CEF Panel concluded that the substances: iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite, do not raise a safety concern when used in oxygen absorbers in sachets, patches or cards, placed in the headspace of the packaging or when used in direct contact with food, excluding liquid food or foods that have an external aqueous liquid phase on the surface such as sliced fruits and fresh meat. Activated carbon should in addition comply with the same purity requirements as for Vegetable Carbon (E 153) set out by Commission Directive 95/45/EC with exception of ash content which can be up to 1% (w/w) | ||
| EFSA CEF | Scientific Opinion on the safety assessment of the active substances, sodium erythorbate, sodium carbonate, sodium bicarbonate, iron sulfate, activated carbon, cellulose, calcium hydroxide, calcium chloride and water, for use as active system in food contact materials. | 2014 | Negative | Negative | Negative | |||
| EFSA CEF | Scientific Opinion on the safety evaluation of the active substances, activated carbon, water, iron powder, kaolin calcined, sulphur and sodium chloride for use as active component in food contact materials | 2012 | No data | No data | No data | |||
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | Negative | |||
| 503 | β‐Dextrin | EFSA ANS | Re-evaluation of Beta-cyclodextrin (E 459) as a food additive | 2016 | Negative | Negative | Negative | The Scientific Committee on Food (SCF) allocated an acceptable daily intake (ADI) of ‐5 mg/kg body weight (bw) per day to beta‐cyclodextrin (E 459) in 1996. The Panel concluded that, based on the available toxicological database, there is no reason to revise the curresnt ADI of 5 mg/kg bw per day for beta‐cyclodextrin. Based on the available reported use and use levels, the Panel also concluded that the ADI was exceeded in the refined brand‐loyal scenario (considered the most relevant scenario) in all population groups except for infants at the mean and in all population groups at the 95th percentile |
| 112 | Linoleic acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel, therefore, concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| 108 | Sucrose | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Ambiguous | Ambiguous | No data | Given its long history of use as a food, and the available information on its components, there are no toxicological concerns regarding the use of molasses obtained from sugar cane, sugar beet, citrus or sorghum, as a previous cargo for edible fats and oils. The amount of sulfite present in some molasses would not be of concern following dilution in edible fats and oils as subsequent cargo. No other impurities or reaction products of concern are known or anticipated. The CONTAM Panel, therefore, concludes that molasses, which has been produced from the conventional sugar processing industry using sugar cane, sugar beet, citrus or sorghum, meets the criteria for acceptability as a previous cargo for edible fats and oils |
| 107 | Urea | EFSA FEEDAP | Scientific Opinion on the safety and efficacy of Urea for ruminants | 2012 | No data | No data | No data | |
| EFSA | Conclusion on the peer review of the pesticide risk assessment of the active substance urea | 2012 | No data | No data | No data | With regard to consumer exposure, it is not necessary to derive an ADI or an ARfD in view of the representative uses (Urea can be used as a fungicide to be applied on fresh‐cut stumps of conifers in forests. It can also be used as an insect attractant for the control and the suppression of the olive fruit fly and the Mediterranean fruit fly in olive trees as a spot bait spray treatment in combination with an insecticide) | ||
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | Not applicable | The CONTAM Panel has previously evaluated calcium ammonium nitrate solution and calcium nitrate (CN‐9) solution and concluded that they meet the criteria for acceptability as previous cargoes. Based on the evaluations for urea, ammonium hydroxide and nitrate, the Panel considers that there are no toxicological concerns regarding urea ammonium nitrate when it is used as a previous cargo. There are no reactions of concern with edible fats and oils, nor are any anticipated impurities likely to be present at levels of toxicological relevance. Therefore, the CONTAM Panel concludes that urea ammonia nitrate solution meets the criteria for acceptability as a previous cargo for edible fats and oils | ||
| 102 | Glucose | EFSA NDA | Scientific Opinion on the safety of ‘Lentinus edodes extract’ (Lentinex) as a Novel Food ingredient | 2010 | No data | No data | No data | |
| 345 | Arachidic acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils - Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| 338 | Palmitoleic acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils - Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| 336 | n‐Decanoic acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils - Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | |||
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 503 (FGE.503): grill flavour ‘Grillin’ CB-200SF’ | 2017 | Not determined | Not determined | Not determined | |||
| 330 | Lauric acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils - Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 503 (FGE.503): grill flavour ‘Grillin’ CB-200SF’ | 2017 | Not determined | Not determined | Not determined | |||
| 329 | Hexanoic acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | |||
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 503 (FGE.503): grill flavour ‘Grillin’ CB-200SF’ | 2017 | Not determined | Not determined | Not determined | |||
| 304 | Caprylic acid | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation |
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | |||
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 503 (FGE.503): grill flavour ‘Grillin’ CB-200SF’ | 2017 | Not determined | Not determined | Not determined | |||
| EFSA AFC | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Treatment of poultry carcasses with chlorine dioxide, acidified sodium chlorite, trisodium phosphate and peroxyacids | 2006 | No data | No data | No data | |||
| 299 | Levulinic acid | EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | |
| 272 | Behenic acid | EFSA NDA | Scientific Opinion related to a notification from DuPont Nutrition Biosciences Aps on behenic acid from mustard seeds to be used in the manufacturing of certain emulsifiers pursuant to Article 21(2) of Regulation (EU) No 1169/2011 – for permanent exemption from labelling | 2016 | No data | No data | No data | |
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Not applicable | Not applicable | Not applicable | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation | ||
| 256 | Glutaric acid | EFSA CEF | Scientific Opinion on Flavouring Group Evaluation 10, Revision 3 (FGE.10Rev3): Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30 | 2012 | Negative | Negative | Not determined | METABOLISM: It can be anticipated that, at the estimated levels of intake as flavouring substance, the candidate substance is metabolised to innocuous products, many of which are endogenous in humans. GENOTOXICITY: For the candidate substance, the genotoxic potential cannot be assessed adequately, however, from the limited data available there were no indications that genotoxicity for these substances should give rise to safety concern. The Panel therefore decided that the substance could be taken through the Procedure. OUTCOME ON THE NAMED COMPOUND: No safety concern based on intake calculated by the MSDI approach of the named compound. OUTCOME ON THE MATERIAL OF COMMERCE: No safety concern at estimated level of intake of the material of commerce meeting the specification (based on intake calculated by the MSDI approach) |
| 229 | Butyric acid | EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | |
During the screening of the SCF List 0 substances, it was noted that the Union list entry β‐dextrin, with FCM No 503, Reference number 46080 and CAS number 7585‐39‐9 has been evaluated in 2016 by EFSA as a food additive (E 459, β‐cyclodextrin) with the same CAS number and with the information that β‐dextrin is a synonym for β‐cyclodextrin. The ADI for E 459 (β‐cyclodextrin) and hence for ‘β‐dextrin’ is 5 mg/kg bw per day (EFSA ANS Panel, 2016a). Consequently, this ADI takes priority over the SCF List 0 classification, and therefore, the ADI value for this substance is used according to the prioritisation scheme, leaving 32 substances not considered further, since they are on SCF List 0.
For 38 substances, an SCF List 1 classification and an ‘ADI: not specified’, or ‘Group ADI: not specified’ characterisation was assigned by the SCF. For 26 of these 38 entries, information could be retrieved from EFSA opinions via the EFSA OpenFoodTox database (Appendix A, Table A.4). No safety concerns were identified in these EFSA evaluations.
Table A.4.
Substances with a List 1 classification and an ‘ADI: not specified’ characterisation by SCF, for which EFSA has produced an opinion
| FCM no. | Substance name | Synoptic SCF Opinion | Panel | Opinion title | Year | Conclusion on: | Remarks | ||
|---|---|---|---|---|---|---|---|---|---|
| Mutagenicity | Genotoxicity | Carcinogenicity | |||||||
| 103 | Glycerol | Group ADI: not specified for glycerol, glycerol diacetate, glycerol triacetate and glycerol monoacetate (SCF, 11th Series, 1981) | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part III of III | 2012 | Negative | Negative | Negative | Both JECFA and the SCF have established an ADI not specified for glycerol, which the CONTAM Panel considers appropriate. |
| EFSA ANS | Re-evaluation of glycerol (E 422) as a food additive | 2017 | Negative | Negative | Negative | ||||
| EFSA CEF | Scientific Opinion on the safety evaluation of the active substances iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite for use in food contact materials | 2013 | No data | No data | No data | The CEF Panel concluded that the substances: iron, sodium chloride, water, silica gel, activated carbon, monosodium glutamate, potassium acid tartrate, powdered cellulose, malic acid, chabazite, hydroxypropyl cellulose, potassium carbonate, sodium thiosulfate, propylene glycol, glycerin, polyethyleneglycol sorbitan monooleate, sodium propionate and clinoptilolite, do not raise a safety concern when used in oxygen absorbers in sachets, patches or cards, placed in the headspace of the packaging or when used in direct contact with food, excluding liquid food or foods that have an external aqueous liquid phase on the surface such as sliced fruits and fresh meat. Activated carbon should in addition comply with the same purity requirements as for Vegetable Carbon (E 153) set out by Commission Directive 95/45/EC with exception of ash content which can be up to 10% (w/w). | |||
| 105 | Palmitic acid | ADI: not specified (SCF, 25th Series, 1990) | EFSA NDA | Scientific Opinion on the safety of ‘coriander seed oil’ as a Novel Food ingredient. | 2013 | Negative | Negative | No data | |
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation. | ||||||
| 106 | Stearic acid | ADI: not specified (SCF, 25th Series, 1990) | EFSA NDA | Scientific Opinion on the safety of ‘coriander seed oil’ as a Novel Food ingredient | 2013 | Negative | Negative | No data | |
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | The fatty acids listed are of no toxicological concern when used as previous cargoes. The CONTAM Panel therefore concludes that the fatty acids specified meet the criteria for acceptability as previous cargoes for edible fats and oils, provided the dioxin and PCB levels in the fatty acids are such that the final concentration in the fats and oils as subsequent cargoes complies with the European legislation. | ||||||
| EFSA ANS | Scientific Opinion on the re-evaluation of sodium stearoyl-2-lactylate (E 481) and calcium stearoyl-2-lactylate (E 482) as food additives. | 2013 | |||||||
| 115 | Acetic acid | Group ADI: not specified (SCF, 25th Series, 1990). | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | Negative | On the basis of its low toxicity and its natural occurrence in food and in the body, the CONTAM Panel does not consider it necessary to establish an ADI for acetic acid. It causes adverse effects only when it is present at sufficient concentration to change the H+ concentration. Studies in experimental animals and humans have shown that the maximum potential levels of acetic acid arising in fats or oils following its transport as a previous cargo would be of no concern. The CONTAM Panel therefore concludes that acetic acid meets the criteria for acceptability as a previous cargo for edible fats and oils. |
| EFSA | Conclusion on the peer review of the pesticide risk assessment of the active substance acetic acid | 2013 | Negative | Negative | Negative | The establishment of an ADI and ARfD for the oral intake of acetic acid is not considered necessary based on the widespread presence of acetic acid in human food and the fact that the substance is a normal metabolite in humans and animals. | |||
| EFSA CEP | Evaluation of the safety and efficacy of the organic acids lactic and acetic acids to reduce microbiological surface contamination on pork carcasses and pork cuts | 2018 | No data | No data | No data | ||||
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | Not determined | ||||
| EFSA CEF | Scientific opinion of Flavouring Group Evaluation 503 (FGE.503): grill flavour ‘Grillin’ CB-200SF’ | 2017 | Not determined | Not determined | Not determined | ||||
| EFSA FEEDAP | Scientific Opinion on the safety and efficacy of acetic acid, sodium diacetate and calcium acetate as preservatives for feed for all animal species | 2012 | Not determined | Not determined | No data | JECFA allocated an ADI of 0 to 15 mg/kg body weight for sodium diacetate. However, the basis for this ADI is not known. | |||
| 139 | Citric acid | Group ADI: not specified for citric acid and its salts (SCF, 25th Series, 1990). | EFSA ANS | Safety of trimagnesium dicitrate anhydrous (TMDC) to be used as a food additive in food supplements in solid and chewable forms | 2016 | No data | No data | No data | |
| EFSA CEF | Safety assessment of the active substances citric acid and sodium hydrogen carbonate for use in active food contact materials | 2016 | |||||||
| 146 | Propionic acid | Group ADI: not specified (SCF, 1st Series, 1974). | EFSA CEF | Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’ | 2017 | Not determined | Not determined | ||
| EFSA ANS | Scientific Opinion on the re-evaluation of propionic acid (E 280), sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) as food additives | 2014 | |||||||
| 247 | Succinic acid | ADI: not specified (SCF, 25th Series, 1990). | EFSA FEEDAP | Scientific Opinion on the safety and efficacy of primary aliphatic saturated or unsaturated alcohols/aldehydes/acids/acetals/esters with a second primary, secondary or tertiary oxygenated functional group including aliphatic lactones (chemical group 9) when used as flavourings for all animal species | 2012 | No data | No data | No data | |
| 327 | Acetic acid, ethyl ester | ADI: not specified. (SCF, 11th Series, 1981). | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part I of III | 2011 | Negative | Negative | No data | JECFA established an ADI of 0–25 mg/kg bw for ethyl acetate, which the CONTAM Panel endorses. |
| 348 | Myristic acid | ADI: Not specified (SCF, 25th Series, 1989). | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Not applicable | Not applicable | ||
| 394 | Calcium hydroxide | ADI: not specified (SCF, 25th Series, 1991) | EFSA CEF | Scientific Opinion on the safety assessment of the active substances iron, iron oxides, sodium chloride and calcium hydroxide for use in food contact materials. | 2013 | No data | No data | No data | |
| EFSA CEF | Scientific Opinion on the safety assessment of the active substances, sodium erythorbate, sodium carbonate, sodium bicarbonate, iron sulphate, activated carbon, cellulose, calcium hydroxide, calcium chloride and water, for use as active system in food contact materials. | 2014 | Negative | Negative | No data | ||||
| 399 | Potassium hydroxide | ADI: not specified (SCF, 25th Series, 1991) | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | Negative | |
| 400 | Sodium hydroxide | ADI: not specified (SCF, 25th Series, 1991) | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | Negative | |
| 410 | Kaolin | ADI: not specified (SCF, 25th Series, 1990). | EFSA CONTAM | Scientific Opinion on the evaluation of substances as acceptable previous cargoes for edible fats and oils | 2009 | No data | No data | No data | Kaolin has been evaluated by JECFA (ADI not specified) and is a permitted anti‐caking food additive (up to 2.5%). |
| 413 | Ammonium hydroxide | ADI: not specified (SCF, 25th Series, 1991) | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | ||
| 504 | Silicon dioxide | ADI: not specified (SCF, 25th Series, 1991) | EFSA NDA | Scientific Opinion on the safety of ‘Cetyl Myristoleate Complex’ as a food ingredient | 2010 | No data | No data | No data | |
| EFSA NDA | Statement on the safety of ‘Cetyl Myristoleate Complex’ as an ingredient in food supplements. | 2010 | No data | No data | No data | ||||
| EFSA NDA | Statement on the safety of ‘Cetyl Myristoleate Complex’ as an ingredient in food supplements | 2014 | No data | No data | No data | ||||
| EFSA NDA | Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Silicon Calcium silicate and silicon dioxide/silicic acid gel added for nutritional purposes to food supplements | 2004 | Negative | Negative | Negative | ||||
| EFSA ANS | Calcium silicate and silicon dioxide/silicic acid gel added for nutritional purposes to food supplements | 2009 | Negative | Negative | No data | ||||
| EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | Negative | ||||
| EFSA ANS | Re-evaluation of silicon dioxide (E 551) as a food additive | 2018 | Negative | Negative | Negative | ||||
| 510 | Ammonia | ADI: not specified (SCF, 25th Series, 1991). | EFSA CEF | Scientific Opinion on Flavouring Group Evaluation 46, Revision 1 (FGE.46Rev1): Ammonia and three ammonium salts from chemical group 30 | 2011 | Not determined | Not determined | Not determined | METABOLISM: The candidate substance is expected to be metabolised to innocuous substances at the anticipated levels of intake as flavouring substance. GENOTOXICITY: Although the genotoxicity data for the flavouring substances in this group are limited, the available data on genotoxicity do not preclude an evaluation of the candidate substances through the Procedure. OUTCOME ON THE NAMED COMPOUND: No safety concern based on intake calculated by the MSDI approach of the named compound. OUTCOME ON THE MATERIAL OF COMMERCE: Tentatively regarded as presenting no safety concern (based on intake calculated by the MSDI approach) pending further information on the purity of the material of commerce and/or information on stereoisomerism |
| EFSA AFC | Opinion Flavouring Group Evaluation 46 (FGE.46): Ammonia and two ammonium salts from chemical group 30 Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) | 2009 | Not determined | Not determined | Not determined | METABOLISM: The substance is accordingly expected to be metabolised to innocuous substances at the anticipated levels of intake as flavouring substance. OUTCOME ON THE NAMED COMPOUND: No safety concern based on intake calculated by the MSDI approach of the named compound. OUTCOME ON THE MATERIAL OF COMMERCE: Tentatively regarded as presenting no safety concern (based on intake calculated by the MSDI approach) pending further information on the purity of the material of commerce and/or information on stereoisomerism | |||
| 511 | Sulphuric acid | ADI: not specified (SCF, 25th Series, 1991) | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils - Part II of III | 2012 | Negative | Negative | No data | No ADI has been established for sulphuric acid. Sulphuric acid is toxic only when it is present at a sufficient concentration to change the H+ concentration. It will be diluted and buffered by the contents of the GI tract so that the levels that would occur following oral ingestion of fats or oils transported subsequent to sulphuric acid do not give rise to any toxicological concern. Studies in experimental animals and humans have shown that the maximum potential levels of sulphuric acid arising in fats or oils following its transport as a previous cargo would be of no concern. No impurities of toxicological concern are known or anticipated. The CONTAM Panel therefore concludes that sulphuric acid meets the criteria for acceptability as a previous cargo for edible fats and oils |
| 528 | Lecithin | ADI: not specified (JECFA 17 M., 1973). | EFSA ANS | Re-evaluation of lecithins (E 322) as a food additive | 2017 | Negative | Negative | Negative | No adverse effects were reported in chronic and carcinogenicity study in rats at the highest dose tested of 3,750 mg lecithins/kg bw per day |
| 541 | Gum arabic | ADI: not specified (JECFA, 35 M., 1989). | EFSA ANS | Re-evaluation of acacia gum (E 414) as a food additive | 2017 | Negative | Negative | Negative | |
| 544 | Guar gum | ADI: not specified (SCF, 7th Series, 1978). | EFSA ANS | Re-evaluation of guar gum (E 412) as a food additive | 2017 | Negative | Negative | Negative | The Panel concluded that there is no need for a numerical ADI for guar gum (E 412), and that there is no safety concern for the general population at the refined exposure assessment for the reported uses of guar gum (E 412) as a food additive |
| 545 | Tragacanth gum | ADI: not specified (SCF, 21th Series, 1989). | EFSA ANS | Re-evaluation of tragacanth (E 413) as a food additive | 2017 | Negative | Negative | Negative | Tragacanth had no observed effects on clinical chemistry, haematological indices, urinalysis parameters, glucose and insulin levels, serum cholesterol, triglycerides and phospholipids, breath hydrogen and methane concentrations |
| 546 | Pectin | ADI: not specified (SCF, 7th Series, 1978). | EFSA ANS | Re-evaluation of pectin (E 440i) and amidated pectin (E 440ii) as food additives | 2017 | Negative | Negative | Negative | |
| 566 | Alginic acid | ADI: not specified (JECFA, 39M., 1992). | EFSA ANS | Re-evaluation of alginic acid and its sodium, potassium, ammonium and calcium salts (E 400-E 404) as food additives | 2017 | Negative | Negative | Negative | |
| EFSA ANS | Re-evaluation of propane-1,2-diol alginate (E 405) as a food additive | 2018 | Negative | Negative | Negative | ||||
| 585 | Calcium chloride | ADI: not specified (SCF, 25th Series, 1991) | EFSA CONTAM | Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils – Part II of III | 2012 | Negative | Negative | No data | |
| 596 | Xanthan gum | ADI: not specified (JECFA, 30M., 1986). | EFSA NDA | Safety of alginate-konjac-xanthan polysaccharide complex (PGX) as a novel food pursuant to Regulation (EC) No 258/97 | 2017 | Negative | Negative | No data | |
| EFSA ANS | Re-evaluation of xanthan gum (E 415) as a food additive | 2017 | Negative | Negative | Negative | ||||
| 615 | Talc | ADI: not specified (SCF, 25th Series, 1991) | EFSA ANS | Re-evaluation of calcium silicate (E 552), magnesium silicate (E 553a(i)), magnesium trisilicate (E 553a(ii)) and talc (E 553b) as food additives | 2018 | Negative | Negative | No data | |
Eighteen substances that are regulated under articles 6(3) and 10 [limits on metals, Annex II of the Commission Regulation (EU) No 10/2011] were also identified.
An SML may not be needed for the above 88 substances and they were not further considered. The 285 remaining substances were subsequently assigned to the low, medium and high priority groups in the next steps.
For one additional substance, FCM No 768 Amines, bis(hydrogenated tallow alkyl) oxidised, which did not fall under any of the above criteria, it was concluded at a later step (Section 3.2.4.4, Figures 1 and 5) that an SML should not be needed, due to existing restrictions of use of this substance under Regulation (EU) No 10/2011. Therefore, the final group of substances for which an SML should not be needed consisted of 89 substances, while 284 substances were finally placed in the low, medium and high priority.
Figure 5.
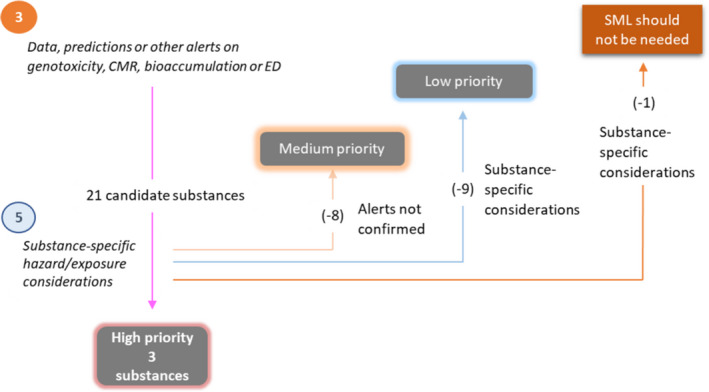
Number of substances placed in the high priority group
The 89 substances for which an SML should not be needed are listed in Appendix A, Table A.5 and are shown schematically in Figure 2.
Table A.5.
Substances for which an SML should not be needed
| FCM substance no. | Ref. no. | CAS no. | Substance name | Metal or metal compounda | SCF lista | ADI: not specifieda |
|---|---|---|---|---|---|---|
| 1 | 12310 | 266309‐43‐7 | Albumin | 0 | ||
| 7 | 30370 | Acetylacetic acid, salts | 0 | |||
| 18 | 34475 | Aluminium calcium hydroxide phosphite, hydrate | Yes | 2–3 | ||
| 21 | 42500 | Carbonic acid, salts | 1 | ADI: not specified | ||
| 55 | 57120 | Glycerol monooleate, ester with citric acid | 1 | ADI: not specified | ||
| 57 | 57280 | Glycerol monopalmitate, ester with citric acid | 1 | ADI: not specified | ||
| 59 | 57680 | Glycerol monostearate, ester with citric acid | 1 | ADI: not specified | ||
| 62 | 64500 | Lysine, salts | 0 | |||
| 63 | 65440 | Manganese pyrophosphite | Yes | 2‐3 | ||
| 80 | 81760 | Powders, flakes and fibers of brass, bronze, copper, stainless steel, tin, iron and alloys of copper, tin and iron | Yes | 2 | ||
| 90 | 92195 | Taurine, salts | 0 | |||
| 99 | 19460 ‐ 62960 | 50‐21‐5 | Lactic acid | 1 | ADI: not specified | |
| 102 | 17530 | 50‐99‐7 | Glucose | 0 | ||
| 103 | 18100 ‐ 55920 | 56‐81‐5 | Glycerol | 1 | ADI: not specified | |
| 105 | 22780 ‐ 70400 | 57‐10‐3 | Palmitic acid | 1 | ADI: not specified | |
| 106 | 24550 ‐ 89040 | 57‐11‐4 | Stearic acid | 1 | ADI: not specified | |
| 107 | 25960 | 57‐13‐6 | Urea | 0 | ||
| 108 | 24880 | 57‐50‐1 | Sucrose | 0 | ||
| 112 | 64015 | 60‐33‐3 | Linoleic acid | 0 | ||
| 115 | 10090 ‐ 30000 | 64‐19‐7 | Acetic acid | 1 | ADI: not specified | |
| 139 | 14680 ‐ 44160 | 77‐92‐9 | Citric acid | 1 | ADI: not specified | |
| 146 | 23890 ‐ 82000 | 79‐09‐4 | Propionic acid | 1 | ADI: not specified | |
| 182 | 19270 | 97‐65‐4 | Itaconic acid | 0 | ||
| 229 | 14140 | 107‐92‐6 | Butyric acid | 0 | ||
| 247 | 24820 ‐ 90960 | 110‐15‐6 | Succinic acid | 1 | ADI: not specified | |
| 256 | 18010 ‐ 55680 | 110‐94‐1 | Glutaric acid | 0 | ||
| 272 | 37040 | 112‐85‐6 | Behenic acid | 0 | ||
| 297 | 23950 | 123‐62‐6 | Propionic anhydride | 1 | ADI: not specified | |
| 299 | 63840 | 123‐76‐2 | Levulinic acid | 0 | ||
| 304 | 14320 ‐ 41960 | 124‐07‐2 | Caprylic acid | 0 | ||
| 307 | 42160 | 124‐38‐9 | Carbon dioxide | 1 | ADI: not specified | |
| 327 | 30140 | 141‐78‐6 | Acetic acid, ethyl ester | 1 | ADI: not specified | |
| 329 | 59360 | 142‐62‐1 | Hexanoic acid | 0 | ||
| 330 | 19470 ‐ 63280 | 143‐07‐7 | Lauric acid | 0 | ||
| 336 | 15095 ‐ 45940 | 334‐48‐5 | n‐Decanoic acid | 0 | ||
| 338 | 71020 | 373‐49‐9 | Palmitoleic acid | 0 | ||
| 345 | 35840 | 506‐30‐9 | Arachidic acid | 0 | ||
| 348 | 22350 ‐ 67891 | 544‐63‐8 | Myristic acid | 1 | ADI: not specified | |
| 350 | 63920 | 557‐59‐5 | Lignoceric acid | 0 | ||
| 394 | 41280 | 1305‐62‐0 | Calcium hydroxide | 1 | ADI: not specified | |
| 395 | 41520 | 1305‐78‐8 | Calcium oxide | 1 | ADI: not specified | |
| 396 | 64640 | 1309‐42‐8 | Magnesium hydroxide | 1 | ADI: not specified | |
| 397 | 64720 | 1309‐48‐4 | Magnesium oxide | 1 | ADI: not specified | |
| 399 | 81600 | 1310‐58‐3 | Potassium hydroxide | 1 | ADI: not specified | |
| 400 | 86720 | 1310‐73‐2 | Sodium hydroxide | 1 | ADI: not specified | |
| 402 | 96240 | 1314‐13‐2 | Zinc oxide | Yes | 2 | |
| 403 | 96320 | 1314‐98‐3 | Zinc sulphide | Yes | 2 | |
| 409 | 62240 | 1332‐37‐2 | Iron oxide | Yes | 2 | ADI: not specified |
| 410 | 62720 | 1332‐58‐7 | Kaolin | 1 | ADI: not specified | |
| 413 | 35600 | 1336‐21‐6 | Ammonium hydroxide | 1 | ADI: not specified | |
| 418 | 34720 | 1344‐28‐1 | Aluminium oxide | Yes | 2 | |
| 489 | 41040 | 5743‐36‐2 | Calcium butyrate | 0 | ||
| 501 | 34480 | Aluminium fibers, fibers and powders | Yes | 2 | ||
| 504 | 86240 | 7631‐86‐9 | Silicon dioxide | 1 | ADI: not specified | |
| 507 | 59990 | 7647‐01‐0 | Hydrochloric acid | 1 | ADI: not specified | |
| 510 | 12789 ‐ 35320 | 7664‐41‐7 | Ammonia | 1 | ADI: not specified | |
| 511 | 91920 | 7664‐93‐9 | Sulphuric acid | 1 | ADI: not specified | |
| 515 | 26360 ‐ 95855 | 7732‐18‐5 | Water | 0 | ||
| 518 | 35845 | 7771‐44‐0 | Arachidonic acid | 0 | ||
| 520 | 65120 | 7773‐01‐5 | Manganese chloride | Yes | 2 | |
| 523 | 45195 | 7787‐70‐4 | Copper bromide | Yes | 2 | |
| 528 | 63760 | 8002‐43‐5 | Lecithin | 1 | ADI: not specified | |
| 531 | 36880 | 8012‐89‐3 | Beeswax | 0 | ||
| 541 | 58480 | 9000‐01‐5 | Gum arabic | 1 | ADI: not specified | |
| 544 | 58400 | 9000‐30‐0 | Guar gum | 1 | ADI: not specified | |
| 545 | 93680 | 9000‐65‐1 | Tragacanth gum | 1 | ADI: not specified | |
| 546 | 71440 | 9000‐69‐5 | Pectin | 1 | ADI: not specified | |
| 547 | 55440 | 9000‐70‐8 | Gelatin | 0 | ||
| 548 | 42800 | 9000‐71‐9 | Casein | 0 | ||
| 553 | 14500 ‐ 43280 | 9004‐34‐6 | Cellulose | 0 | ||
| 564 | 24540 ‐ 88800 | 9005‐25‐8 | Starch, edible | 0 | ||
| 566 | 33350 | 9005‐32‐7 | Alginic acid | 1 | ADI: not specified | |
| 579 | 61800 | 9049‐76‐7 | Hydroxypropyl starch | 1 | ADI: not specified | |
| 580 | 46070 | 10016‐20‐3 | α‐Dextrin | 0 | ||
| 581 | 36800 | 10022‐31‐8 | Barium nitrate | Yes | 2–3 | |
| 585 | 41120 | 10043‐52‐4 | Calcium chloride | 1 | ADI: not specified | |
| 586 | 65280 | 10043‐84‐2 | Manganese hypophosphite | Yes | 2–3 | |
| 592 | 34690 | 11097‐59‐9 | Aluminium magnesium carbonate hydroxide | Yes | 3 | |
| 594 | 65360 | 11129‐60‐5 | Manganese oxide | Yes | 2 | |
| 596 | 95935 | 11138‐66‐2 | Xanthan gum | 1 | ADI: not specified | |
| 606 | 65200 | 12626‐88‐9 | Manganese hydroxide | Yes | 2 | |
| 615 | 92080 | 14807‐96‐6 | Talc | 1 | ADI: not specified | |
| 625 | 36720 | 17194‐00‐2 | Barium hydroxide | Yes | 3 | |
| 628 | 96190 | 20427‐58‐1 | Zinc hydroxide | Yes | 2 | |
| 629 | 34560 | 21645‐51‐2 | Aluminium hydroxide | Yes | 2 | |
| 663 | 64150 | 28290‐79‐1 | Linolenic acid | 0 | ||
| 667 | 55190 | 29204‐02‐2 | Gadoleic acid | 0 | ||
| 723 | 88880 | 68412‐29‐3 | Starch, hydrolysed | 0 | ||
| 768 | 34850 | 143925‐92‐2 | Amines, bis(hydrogenated tallow alkyl) oxidised | 3 | ||
Data complying with the relevant criteria for inclusion in the group of substances for which an SML should not be needed (Section 2.2.2.1) are shown in bold.
Figure 2.
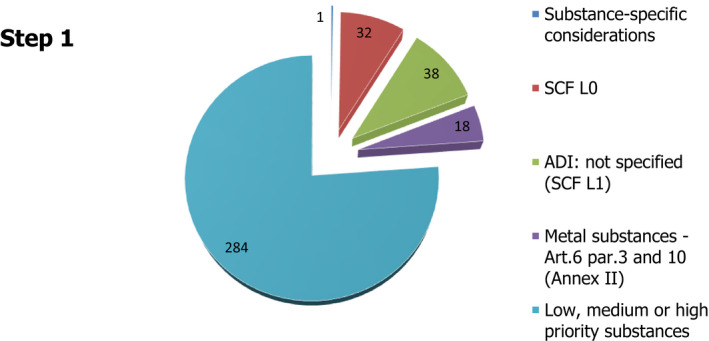
Substances for which no SML should be needed, based on SCF classifications, regulatory provisions on metals, substance‐specific considerations and remaining substances for prioritisation
3.2.2. Low priority group
Following the removal of substances for which an SML should not be needed, 160 substances which:
Have been classified by the SCF Committee as List 1 or List 2, with ADI/group ADI, TDI/group TDI or MTDI ≥ 1 mg/kg bw per day or (P)TWI ≥ 7 mg/kg bw per week, or they have been classified as List 3 (for inertness), or/and
Have an ADI or TDI ≥ 1 mg/kg bw per day established by EFSA, or/and
Have a molecular weight higher or equal to 1,000/1,500 Da for single organic substances,
were placed in the low priority group. Twenty‐nine of these substances matched more than one of the above criteria.
One hundred and twenty‐eight substances fell under criterion (a), with 102 substances being SCF‐classified as List 1 or List 2 and 26 classified as SCF List 3 for inertness.
The OpenFoodTox database was searched for substances for which an ADI or TDI equal to, or above 1 mg/kg bw per day has been assigned, by any of the EFSA Panels, according to criterion (b). Twenty‐two such substances were identified, mainly from flavouring and food additive EFSA evaluations (AFC, CEF, ANS and FAF Panels).
Thirty‐nine substances filled the criterion (c) of high molecular mass. These were either polymeric substances (5), polysaccharides (23) or other substances with a molecular weight higher than 1,000 Da (11). Union list entries for substances with a range of molecular weights, a fraction of which lay below 1,000 Da, were not included in this group.
In addition, 19 substances, which did not fulfil the above criteria, were placed in the low priority group after exclusion from the medium and high priority groups: Ten substances, described under Section 3.2.3, were considered of low potential for migration/exposure (minerals and highly volatile substances) and were moved to the low priority group, as described in the methodology (Section 2.2.2.4). Nine substances which were candidates for the high priority group were also finally placed in the low priority group, following case‐by‐case considerations (Section 3.2.4.4).
The steps taken to construct the low priority group are shown schematically in Figure 3:
Figure 3.
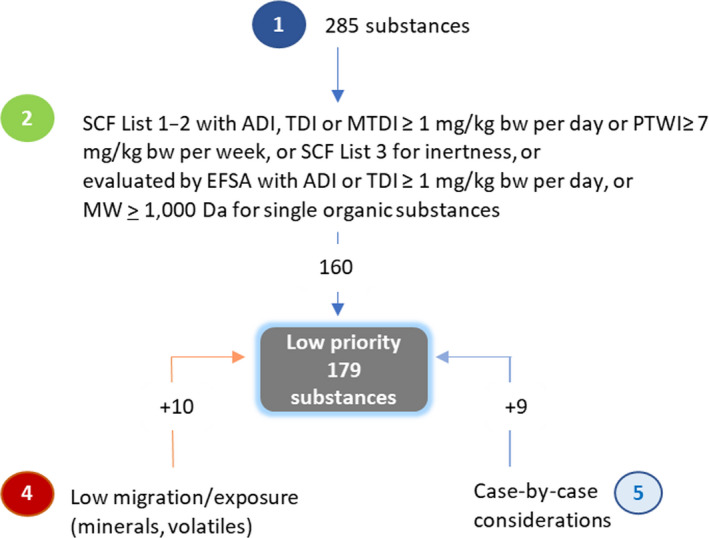
Number of substances placed in the low priority group
The full list of 179 low priority substances is shown in Appendix A, Table A.6. Ten substances that do not fully fall under criterion (a), because of lack of an available or retrievable health‐based guidance value were carried forward to the next steps of the prioritisation and are shown in Table A.7.
Table A.6.
Low Priority Group of substances
| FCM substance no. | Ref. no. | CAS no. | Substance name | SCF Lista | Molecular mass, chemical categorya | ADI or TDI (mg/kg bw per day) | ||
|---|---|---|---|---|---|---|---|---|
| Value established or endorseda | EFSA Panel | Link to the opinion | ||||||
| 2 | 12340 | Albumin, coagulated by formaldehyde | 3 | MW ≥ 1,000 | ||||
| 11 | 30960 | Acids, aliphatic, monocarboxylic (C6‐C22), esters with polyglycerol | 1 | Range, low fraction MW < 1,000 | ||||
| 24 | 45280 | Cotton fibers | 3 ‐inert | Polysaccharide | ||||
| 34 | 54270 | Ethylhydroxymethylcellulose | 2 | Polysaccharide | ||||
| 35 | 54280 | Ethylhydroxypropylcellulose | 2 | Polysaccharide | ||||
| 38 | 55520 | Glass fibers | 3 ‐inert | Inorganic | ||||
| 39 | 55600 | Glass microballs | 3 ‐inert | Inorganic | ||||
| 64 | 66695 | Methylhydroxymethylcellulose | 2 | Polysaccharide | ||||
| 75 | 77702 | Polyethyleneglycol esters of aliph. monocarb. acids (C6‐C22) and their ammonium and sodium sulphates | 2 | Range, low fraction MW < 1,000 | ||||
| 79 | 80640 | Polyoxyalkyl (C2‐C4) dimethylpolysiloxane | 3 | Polymeric | ||||
| 81 | 83320 | Propylhydroxyethylcellulose | 2 | Polysaccharide | ||||
| 82 | 83325 | Propylhydroxymethylcellulose | 2 | Polysaccharide | ||||
| 83 | 83330 | Propylhydroxypropylcellulose | 2 | Polysaccharide | ||||
| 84 | 85601 | Silicates, natural (with the exception of asbestos) | 3 | Mineral | ||||
| 85 | 85610 | Silicates, natural, silanated (with the exception of asbestos) | 3 ‐inert | Mineral | ||||
| 86 | 86000 | Silicic acid, silylated | 3 ‐inert | |||||
| 94 | 95859 | Waxes, refined, derived from petroleum based or synthetic hydrocarbon feedstocks, high viscosity | 2 | Wax | ||||
| 95 | 95883 | White mineral oils, paraffinic, derived from petroleum based hydrocarbon feedstocks | 2 | Wax | ||||
| 96 | 95920 | Wood flour and fibers, untreated | 3 ‐inert | |||||
| 109 | 23740 ‐ 81840 | 57‐55‐6 | 1,2‐Propanediol | 1 | 76.09 | 25 |
CONTAM CEF ANS ANS |
https://doi.org/10.2903/j.efsa.2011.2482 https://doi.org/10.2903/j.efsa.2010.1453 https://doi.org/10.2903/j.efsa.2018.5235 https://doi.org/10.2903/j.efsa.2018.5371 |
| 111 | 53600 | 60‐00‐4 | Ethylenediaminetetraacetic acid | 2 | 292.24 | |||
| 113 | 16780 ‐ 52800 | 64‐17‐5 | Ethanol | 1 | 46.07 | |||
| 114 | 55040 | 64‐18‐6 | Formic acid | 1 | 46.03 | 0–3 | CONTAM | https://doi.org/10.2903/j.efsa.2012.2703 |
| 116 | 13090 ‐ 37600 | 65‐85‐0 | Benzoic acid | 1 | 122.12 | 5 | ANS | https://doi.org/10.2903/j.efsa.2016.4433 |
| 117 | 21550 | 67‐56‐1 | Methanol | 3 | 32.04 | |||
| 118 | 23830 ‐ 81882 | 67‐63‐0 | 2‐Propanol | 1 | 60.10 | 2.4 |
AFC CONTAM |
https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2005.202 https://doi.org/10.2903/j.efsa.2012.2703 |
| 119 | 30295 | 67‐64‐1 | Acetone | 3 | 58.08 | 0.9 | CONTAM | https://doi.org/10.2903/j.efsa.2012.2703 |
| 125 | 16950 | 74‐85‐1 | Ethylene | 3 | 28.05 | |||
| 126 | 10210 | 74‐86‐2 | Acetylene | 3 | 26.04 | |||
| 131 | 48460 | 75‐37‐6 | 1,1‐Difluoroethane | 3 | 66.05 | |||
| 143 | 62450 | 78‐78‐4 | Isopentane | 3 | 72.15 | |||
| 158 | 23380 ‐ 76320 | 85‐44‐9 | Phthalic anhydride | 2 | 148.12 | |||
| 161 | 92160 | 87‐69‐4 | L‐(+)‐Tartaric acid | 1 | 150.09 | 30 | AFC | https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2003.19 |
| 165 | 23200 ‐74480 | 88‐99‐3 | o‐Phthalic acid | 2 | 166.13 | |||
| 171 | 38080 | 93‐58‐3 | Benzoic acid, methyl ester | 2 | 136.15 | |||
| 172 | 37840 | 93‐89‐0 | Benzoic acid, ethyl ester | 2 | 150.17 | |||
| 173 | 60240 | 94‐13‐3 | 4‐Hydroxybenzoic acid, propyl ester | 1 | 180.20 | |||
| 189 | 60200 | 99‐76‐3 | 4‐Hydroxybenzoic acid, methyl ester | 1 | 152.15 | 10 | AFC | https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2004.83 |
| 190 | 18880 | 99‐96‐7 | p‐Hydroxybenzoic acid | 2 | 138.12 | |||
| 194 | 13150 | 100‐51‐6 | Benzyl alcohol | 1 | 108.14 |
4 5 |
FAF CONTAM |
https://doi.org/10.2903/j.efsa.2019.5876 https://doi.org/10.2903/j.efsa.2011.2482 |
| 195 | 37360 | 100‐52‐7 | Benzaldehyde | 1 | 106.12 | |||
| 204 | 25180 ‐ 92640 | 102‐60‐3 | N,N,N′,N′‐Tetrakis(2‐hydroxypropyl)ethylenediamine | 2 | 292.41 | |||
| 213 | 82400 | 105‐62‐4 | 1,2‐Propyleneglycol dioleate | 1 | 604.99 | |||
| 221 | 40570 | 106‐97‐8 | Butane | 3 | 58.12 | |||
| 222 | 13870 | 106‐98‐9 | 1‐Butene | 3 | 56.11 | |||
| 224 | 13900 | 107‐01‐7 | 2‐Butene | 3 | 56.11 | |||
| 228 | 13690 | 107‐88‐0 | 1,3‐Butanediol | 1 | 90.12 | 4 |
CONTAM CEF |
https://doi.org/10.2903/j.efsa.2011.2482 https://doi.org/10.2903/j.efsa.2011.2164 |
| 240 | 45760 | 108‐91‐8 | Cyclohexylamine | 2 | 99.17 | |||
| 244 | 71720 | 109‐66‐0 | Pentane | 3 | 72.15 | |||
| 249 | 17290 ‐ 55120 | 110‐17‐8 | Fumaric acid | 1 | 116.07 | |||
| 252 | 87200 | 110‐44‐1 | Sorbic acid | 1 | 112.13 | 3 | CEF CEF ANS | https://doi.org/10.2903/j.efsa.2009.1205 https://doi.org/10.2903/j.efsa.2011.1924 https://doi.org/10.2903/j.efsa.2015.4144 |
| 257 | 13550 ‐ 16660 ‐ 51760 | 110‐98‐5 | Dipropyleneglycol | 2 | 134.17 | |||
| 260 | 24280 | 111‐20‐6 | Sebacic acid | 2 | 202.25 | |||
| 266 | 25510 ‐ 94320 | 112‐27‐6 | Triethyleneglycol | 2 | 150.17 | |||
| 269 | 25090 ‐ 92350 | 112‐60‐7 | Tetraethyleneglycol | 1 | 194.23 | |||
| 273 | 52730 | 112‐86‐7 | Erucic acid | 3 | 338.57 | 7 | CONTAM | https://doi.org/10.2903/j.efsa.2016.4593 |
| 275 | 23980 | 115‐07‐1 | Propylene | 3 | 42.08 | |||
| 276 | 19000 | 115‐11‐7 | Isobutene | 3 | 56.11 | |||
| 279 | 22840 ‐ 71600 | 115‐77‐5 | Pentaerythritol | 2 | 136.15 | |||
| 287 | 60160 | 120‐47‐8 | 4‐Hydroxybenzoic acid, ethyl ester | 1 | 166.17 | 10 |
AFC CEF AFC |
https://doi.org/10.2903/j.efsa.2006.296 |
| 288 | 24970 | 120‐61‐6 | Terephthalic acid, dimethyl ester | 2 | 194.18 | |||
| 300 | 30045 | 123‐86‐4 | Acetic acid, butyl ester | 1 | 116.16 | 6 | CONTAM | https://doi.org/10.2903/j.efsa.2012.2984 |
| 302 | 12820 | 123‐99‐9 | Azelaic acid | 2 | 188.22 | |||
| 303 | 12130 ‐ 31730 | 124‐04‐9 | Adipic acid | 1 | 146.14 | |||
| 308 | 91200 | 126‐13‐6 | Sucrose acetate isobutyrate | 1 | 846.91 | 20 | ANS | https://doi.org/10.2903/j.efsa.2016.4489 |
| 311 | 16480 ‐ 51200 | 126‐58‐9 | Dipentaerythritol | 2 | 254.28 | |||
| 320 | 37680 | 136‐60‐7 | Benzoic acid, butyl ester | 2 | 178.23 | |||
| 339 | 86160 | 409‐21‐2 | Silicon carbide | 3 ‐inert | 40.10 | |||
| 346 | 10030 | 514‐10‐3 | Abietic acid | 2 | 302.45 | |||
| 362 | 14350 | 630‐08‐0 | Carbon monoxide | 3 | 28.01 | |||
| 393 | 37280 | 1302‐78‐9 | Bentonite | 3 ‐inert | 180.06 | |||
| 404 | 67200 | 1317‐33‐5 | Molybdenum disulphide | 3 ‐inert | 160.07 | |||
| 406 | 83300 | 1323‐39‐3 | 1,2‐Propyleneglycol monostearate | 1 | 342.56 | |||
| 408 | 82960 | 1330‐80‐9 | 1,2‐Propyleneglycol monooleate | 1 | 340.54 | |||
| 414 | 87600 | 1338‐39‐2 | Sorbitan monolaurate | 1 | 346.46 | |||
| 415 | 87840 | 1338‐41‐6 | Sorbitan monostearate | 1 | 430.62 | |||
| 416 | 87680 | 1338‐43‐8 | Sorbitan monooleate | 1 | 428.61 | |||
| 419 | 92150 | 1401‐55‐4 | Tannic acids | 3 | 1,701.20 | |||
| 432 | 12280 | 2035‐75‐8 | Adipic anhydride | 2 | 128.13 | |||
| 441 | 38160 | 2315‐68‐6 | Benzoic acid, propyl ester | 2 | 164.20 | |||
| 445 | 83440 | 2466‐09‐3 | Pyrophosphoric acid | 1 | 177.98 | |||
| 450 | 24430 | 2561‐88‐8 | Sebacic anhydride | 2 | 184.23 | |||
| 459 | 46870 | 3135‐18‐0 | 3,5‐Di‐tert‐butyl‐4‐hydroxybenzylphosphonic acid, dioctadecyl ester | 2 | 805.29 | |||
| 465 | 68040 | 3333‐62‐8 | 7‐[2H‐naphtho‐(1,2‐D)triazol‐2‐yl]‐3‐phenylcoumarin | 2 | 389.41 | |||
| 478 | 60180 | 4191‐73‐5 | 4‐Hydroxybenzoic acid, isopropyl ester | 2 | 180.20 | |||
| 479 | 12970 | 4196‐95‐6 | Azelaic anhydride | 2 | 170.21 | |||
| 480 | 46790 | 4221‐80‐1 | 3,5‐Di‐tert‐butyl‐4‐hydroxybenzoic acid, 2,4‐di‐tert‐butylphenyl ester | 2 | 438.64 | |||
| 491 | 82720 | 6182‐11‐2 | 1,2‐Propyleneglycol distearate | 1 | 609.02 | |||
| 496 | 71680 | 6683‐19‐8 | Pentaerythritol tetrakis[3‐(3,5‐di‐tert‐butyl‐4‐hydroxyphenyl)‐propionate] | 2 | 1,177.63 | |||
| 503 | 46080 | 7585‐39‐9 | β‐Dextrin | 0 | 1,134.98 | 5 | ANS | https://doi.org/10.2903/j.efsa.2016.4628 |
| 508 | 86560 | 7647‐15‐6 | Sodium bromide | 1 | 102.89 | |||
| 509 | 23170 ‐ 72640 | 7664‐38‐2 | Phosphoric acid | 1 | 98.00 | 70 | CONTAM | https://doi.org/10.2903/j.efsa.2011.2482 |
| 514 | 91840 | 7704‐34‐9 | Sulphur | 3 ‐inert | 32.07 | |||
| 517 | 81520 | 7758‐02‐3 | Potassium bromide | 1 | 119.00 | |||
| 521 | 58320 | 7782‐42‐5 | Graphite | 3 ‐inert | 12.01 | |||
| 522 | 7782‐50‐5 | 7782‐50‐5 | Chlorine | 3 | 70.91 | 0.15 | AFC | https://doi.org/10.2903/j.efsa.2006.297 |
| 529 | 67850 | 8002‐53‐7 | Montan wax | 3 ‐inert | Wax | |||
| 534 | 80720 | 8017‐16‐1 | Polyphosphoric acids | 1 | MW < 1,000 | |||
| 535 | 24100 ‐ 24130 ‐ 24190 – 83840 | 8050‐09‐7 | Rosin | 2 | ||||
| 536 | 84320 | 8050‐15‐5 | Rosin, hydrogenated, ester with methanol | 2 | ||||
| 537 | 84080 | 8050‐26‐8 | Rosin, ester with pentaerythritol | 2 | ||||
| 538 | 84000 | 8050‐31‐5 | Rosin, ester with glycerol | 1 | ||||
| 542 | 42640 | 9000‐11‐7 | Carboxymethylcellulose | 2 | Polysaccharide | |||
| 554 | 43300 | 9004‐36‐8 | Cellulose acetate butyrate | 3 ‐inert | Polysaccharide | |||
| 555 | 53280 | 9004‐57‐3 | Ethylcellulose | 2 | Polysaccharide | |||
| 556 | 54260 | 9004‐58‐4 | Ethylhydroxyethylcellulose | 2 | Polysaccharide | |||
| 557 | 66640 | 9004‐59‐5 | Methylethylcellulose | 2 | Polysaccharide | |||
| 558 | 60560 | 9004‐62‐0 | Hydroxyethylcellulose | 2 | Polysaccharide | |||
| 559 | 61680 | 9004‐64‐2 | Hydroxypropylcellulose | 2 | Polysaccharide | |||
| 560 | 66700 | 9004‐65‐3 | Methylhydroxypropylcellulose | 2 | Polysaccharide | |||
| 561 | 66240 | 9004‐67‐5 | Methylcellulose | 2 | Polysaccharide | |||
| 562 | 22450 | 9004‐70‐0 | Nitrocellulose | 3 | MW ≥ 1,000 | |||
| 565 | 61120 | 9005‐27‐0 | Hydroxyethyl starch | 2 | Polysaccharide | |||
| 567 | 82080 | 9005‐37‐2 | 1,2‐Propyleneglycol alginate | 1 | MW ≥ 1,000 | 55 | ANS | https://doi.org/10.2903/j.efsa.2018.5371 |
| 568 | 79040 | 9005‐64‐5 | Polyethyleneglycol sorbitan monolaurate | 1 | 520.66 | 25 | ANS | https://doi.org/10.2903/j.efsa.2015.4152 |
| 569 | 79120 | 9005‐65‐6 | Polyethyleneglycol sorbitan monooleate | 2 | 604.82 | |||
| 570 | 79200 | 9005‐66‐7 | Polyethyleneglycol sorbitan monopalmitate | 1 | 522.671 | 25 | ANS | https://doi.org/10.2903/j.efsa.2015.4152 |
| 571 | 79280 | 9005‐67‐8 | Polyethyleneglycol sorbitan monostearate | 1 | 606.83 | 25 | ANS | https://doi.org/10.2903/j.efsa.2015.4152 |
| 572 | 79360 | 9005‐70‐3 | Polyethyleneglycol sorbitan trioleate | 2 | 1,132.71 | |||
| 573 | 79440 | 9005‐71‐4 | Polyethyleneglycol sorbitan tristearate | 1 | 1,138.76 | 25 | ANS | https://doi.org/10.2903/j.efsa.2015.4152 |
| 574 | 24250 ‐ 84560 | 9006‐04‐6 | Rubber, natural | 3 | Polymeric | |||
| 575 | 76721 | 63148‐62‐9 | Polydimethylsiloxane (Mw > 6,800 Da) | 2 | MW ≥ 1,000 | |||
| 576 | 60880 | 9032‐42‐2 | Hydroxyethylmethylcellulose | 2 | Polysaccharide | |||
| 591 | 36160 | 10605‐09‐1 | Ascorbyl stearate | 1 | 442.59 | |||
| 595 | 19510 | 11132‐73‐3 | Lignocellulose | 3 | Polysaccharide | |||
| 597 | 67120 | 12001‐26‐2 | Mica | 3 ‐inert | Polysaccharide | |||
| 598 | 41600 | 12004‐14‐7 | Calcium sulphoaluminate | 2 | 678.61 | |||
| 600 | 60030 | 12072‐90‐1 | Hydromagnesite | 3 ‐inert | 467.64 | |||
| 601 | 35440 | 12124‐97‐9 | Ammonium bromide | 1 | 97.94 | |||
| 603 | 83460 | 12269‐78‐2 | Pyrophyllite | 3 ‐inert | 181.16 | |||
| 604 | 60080 | 12304‐65‐3 | Hydrotalcite | 3 ‐inert | 603.97 | |||
| 610 | 93440 | 13463‐67‐7 | Titanium dioxide | 1 | 79.87 | |||
| 611 | 35120 | 13560‐49‐1 | 3‐Aminocrotonic acid, diester with thiobis (2‐hydroxyethyl) ether | 2 | 288.36 | |||
| 613 | 95905 | 13983‐17‐0 | Wollastonite | 3 ‐inert | 116.16 | |||
| 614 | 45560 | 14464‐46‐1 | Cristobalite | 3 ‐inert | 60.08 | |||
| 616 | 83470 | 14808‐60‐7 | Quartz | 3 ‐inert | 60.08 | |||
| 623 | 52640 | 16389‐88‐1 | Dolomite | 3 ‐inert | 184.40 | |||
| 626 | 57800 | 18641‐57‐1 | Glycerol tribehenate | 3 | 1,059.80 | |||
| 627 | 59760 | 19569‐21‐2 | Huntite | 3 ‐inert | Mineral | |||
| 630 | 82240 | 22788‐19‐8 | 1,2‐Propyleneglycol dilaurate | 1 | 440.70 | |||
| 634 | 25910 | 24800‐44‐0 | Tripropyleneglycol | 2 | 192.25 | |||
| 638 | 23590 ‐ 76960 | 25322‐68‐3 | Polyethyleneglycol | 2 | Range, low fraction MW < 1,000 | |||
| 639 | 23651 ‐ 80800 | 25322‐69‐4 | Polypropyleneglycol | 3 | Range, low fraction MW < 1,000 | 1.5 | CONTAM | https://doi.org/10.2903/j.efsa.2011.2482 |
| 643 | 87760 | 26266‐57‐9 | Sorbitan monopalmitate | 1 | 402.57 | 10 | ANS | https://doi.org/10.2903/j.efsa.2017.4788 |
| 644 | 88080 | 26266‐58‐0 | Sorbitan trioleate | 2 | 957.51 | |||
| 649 | 47210 | 26427‐07‐6 | Dibutylthiostannoic acid polymer | 2 | Polymeric – UL restriction n = 1.5–2, i.e. MW < 1 kDa | |||
| 651 | 88240 | 26658‐19‐5 | Sorbitan tristearate | 1 | 963.55 | 10 | ANS | https://doi.org/10.2903/j.efsa.2017.4788 |
| 659 | 82800 | 27194‐74‐7 | 1,2‐Propyleneglycol monolaurate | 1 | 258.40 | |||
| 664 | 95000 | 28931‐67‐1 | Trimethylolpropane trimethacrylate‐methyl methacrylate copolymer | 3 | Polymeric | |||
| 665 | 83120 | 29013‐28‐3 | 1,2‐Propyleneglycol monopalmitate | 1 | 314.51 | |||
| 666 | 87280 | 29116‐98‐1 | Sorbitan dioleate | 2 | 693.06 | |||
| 668 | 80240 | 29894‐35‐7 | Polyglycerol ricinoleate | 1 | 520.70 | |||
| 674 | 46480 | 32647‐67‐9 | Dibenzylidene sorbitol | 2 | 358.39 | |||
| 677 | 82560 | 33587‐20‐1 | 1,2‐Propyleneglycol dipalmitate | 1 | 552.91 | |||
| 682 | 53270 | 37205‐99‐5 | Ethylcarboxymethylcellulose | 2 | Polysaccharide | |||
| 683 | 66200 | 37206‐01‐2 | Methylcarboxymethylcellulose | 2 | Polysaccharide | |||
| 684 | 68125 | 37244‐96‐5 | Nepheline syenite | 3 ‐inert | Mineral | |||
| 686 | 61390 | 37353‐59‐6 | Hydroxymethylcellulose | 2 | Polysaccharide | |||
| 693 | 88160 | 54140‐20‐4 | Sorbitan tripalmitate | 2 | 879.39 | |||
| 696 | 92205 | 57569‐40‐1 | Terephthalic acid, diester with 2,2′‐methylenebis(4‐methyl‐6‐tert‐butylphenol) | 2 | 811.10 | |||
| 699 | 90720 | 58446‐52‐9 | Stearoylbenzoylmethane | 2 | 386.61 | |||
| 702 | 87920 | 61752‐68‐9 | Sorbitan tetrastearate | 2 | 1,230.02 | |||
| 707 | 46375 | 61790‐53‐2 | Diatomaceous earth | 3 ‐inert | Mineral | |||
| 709 | 87520 | 62568‐11‐0 | Sorbitan monobehenate | 2 | 486.73 | |||
| 714 | 84400 | 64365‐17‐9 | Rosin, hydrogenated, ester with pentaerythritol | 2 | 420.59 | |||
| 717 | 84210 | 65997‐06‐0 | Rosin, hydrogenated | 2 | ||||
| 719 | 65920 | 66822‐60‐4 | N‐Methacryloyloxyethyl‐N,N‐dimethyl‐N‐carboxymethylammonium chloride, sodium salt ‐octadecyl methacrylate‐ethyl methacrylate‐cyclohexyl methacrylate‐N‐vinyl‐2‐pyrrolidone, copolymers | 2 | Polymeric | |||
| 721 | 46800 | 67845‐93‐6 | 3,5‐di‐tert‐butyl‐4‐hydroxybenzoic acid, hexadecyl ester | 2 | 474.76 | |||
| 727 | 43360 | 68442‐85‐3 | Cellulose, regenerated | 2 | Polysaccharide | |||
| 734 | 46380 | 68855‐54‐9 | Diatomaceous earth, soda ash flux‐calcined | 3 ‐inert | Mineral | |||
| 739 | 70000 | 70331‐94‐1 | 2,2′‐Oxamidobis[ethyl‐3‐(3,5‐di‐tert‐butyl‐4‐hydroxyphenyl)‐propionate] | 2 | 696.91 | |||
| 741 | 24070 ‐ 83610 | 73138‐82‐6 | Resin acids and rosin acids | 2 | ||||
| 743 | 38950 | 79072‐96‐1 | Bis(4‐ethylbenzylidene)sorbitol | 2 | 414.49 | |||
| 751 | 81515 | 87189‐25‐1 | Poly(zinc glycerolate) | 2‐3 | Polymeric | |||
| 752 | 39890 | 87826‐41‐3 | Bis(methylbenzylidene)sorbitol | 2 | 386.44 | |||
| 753 | 62800 | 92704‐41‐1 | Kaolin, calcined | 3 ‐inert | Mineral | |||
| 757 | 95725 | 110638‐71‐6 | Vermiculite, reaction product with citric acid, lithium salt | 2 | Mineral | |||
| 766 | 38879 | 135861‐56‐2 | Bis(3,4‐dimethylbenzylidene)sorbitol | 2 | 414.49 | |||
| 821 | 90810 | Stearoyl‐2‐lactylic acid, salts | 1 | range, MW < 1,000 | ||||
Information in bold indicates the reason for categorising the substance in the low priority group.
Table A.7.
Substances falling under an SCF classification in list 1 or 2 for which the health‐based guidance value was not available
| FCM substance no. | Ref. no. | CAS no. | Substance name | SCF list | Synoptic SCF Opinion |
|---|---|---|---|---|---|
| 60 | 58300 | Glycine, salts | 1 | ADI: acceptable (SCF, 25th Series, 1991) | |
| 162 | 65520 | 87‐78‐5 | Mannitol | 1 | ADI: acceptable (SCF, 16th Series, 1985) |
| 113 | 16780 ‐ 52800 | 64‐17‐5 | Ethanol | 1 | Acceptable (SCF, 11th Series, 1981) |
| 110 | 93520 |
59‐02‐9 10191‐41‐0 |
α‐Tocopherol | 1 | Acceptable (SCF, 22th Series, 1989) |
| 101 | 36000 | 50‐81‐7 | Ascorbic acid | 1 | Acceptable (SCF, 22th Series, 1989) |
| 100 | 24490 ‐ 88320 | 50‐70‐4 | Sorbitol | 1 | Acceptable (SCF, 16th Series, 1985). |
| 428 | 95200 | 1709‐70‐2 | 1,3,5‐trimethyl‐2,4,6‐tris(3,5‐di‐tert‐butyl‐4‐hydroxybenzyl)benzene | 2 |
t‐TDI: 1 mg/kg bw pending check of the reports 2‐year oral studies in rats and dogs and oral carcinogenicity studies in mice and rats. (Shell reports n. TLGR 0023.68. March 1969, TLGR. 0024.68, Sept. 1968, TLGR. 0019.69, March 1969) |
| 321 | 36080 | 137‐66‐6 | Ascorbyl palmitate | 1 | Acceptable (SCF, 22th Series, 1989) |
| 610 | 93440 | 13463‐67‐7 | Titanium dioxide | 1 | Acceptable (SCF, 1st Series, 1975) |
| 591 | 36160 | 10605‐09‐1 | Ascorbyl stearate | 1 | Acceptable. Covered by the assessment for ascorbyl palmitate |
3.2.3. Medium Priority Group
From the pool of 125 substances remaining after application of the low priority criteria, substances which did not meet the criteria for qualification to high priority were placed in the medium priority group.
Following this rationale, 104 substances were initially placed in the medium priority group. To this group, eight substances were added, because the reasons for initial concern for these substances were considered as not of relevance to the risk assessment of FCMs, as described in Section 3.2.4.4 and in Table A.11.
Table A.11.
Substances not included in the high priority group due to rejection criteria
| FCM no. | CAS | Name | ECHA stage | Substance information card | ECHA concerns, not of relevance to FCM risk evaluation |
|---|---|---|---|---|---|
| 235 | 108‐39‐4 | m‐Cresol | CLH | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.253 |
Harmonised classification and labelling: Acute Tox. 3 (H301): is toxic if swallowed Acute Tox. 3 (H311): is toxic in contact with skin Skin Corr. 1B (H314): causes severe skin burns and eye damage |
| 267 | 112‐30‐1 | 1‐Decanol | Substance evaluation | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.597 | Based on information available, the evaluating Member State Competent Authority (eMSCA) of Italy supports the human health self‐classification as Eye Irrit. 2 H319 and the notified classification as Skin Irrit. 2 H315, STOT SE 3 H335 (Respiratory tract) and STOT SE 2 H371 (Central Nervous System) as further explained in the SEV (Substance Evaluation Conclusion and Evaluation Report). Therefore, a harmonised classification of the substance is envisaged as a follow‐up at EU level for these human health endpoints |
| 123 | 71‐36‐3 | 1‐Butanol | Substance evaluation | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.683 |
Conclusion by the eMSCA of Hungary on RMOA concerns for reproductive toxicity and developmental neurotoxicity: no need for regulatory follow‐up action at EU level (CoRAP document and Substance Evaluation Conclusion and Evaluation Report). Harmonised classification and labelling: Acute Tox. 4 (H302): harmful if swallowed Skin Irrit. 2 (H315): causes skin irritation Eye Dam. 1 (H318): causes serious eye damage STOT SE 3 (H335): may cause respiratory irritation STOT SE 3 (H336): may cause drowsiness or dizziness |
| 124 | 71‐41‐0 | 1‐Pentanol | Substance evaluation | https://echa.europa.eu/substance-information/-/substanceinfo/100.000.684 |
According to the evaluation of the eMSCA of Lithuania, the classification as eye damage category 1 with H318 (Causes serious eye damage) is supported. The harmonised classification and labelling to be updated to include eye damage/irritation (Substance Evaluation Conclusion and Evaluation Report). Harmonised classification and labelling: Flam. Liq. 3 (H226): is a flammable liquid and vapour Skin Irrit. 2 (H315): causes skin irritation Acute Tox. 4 (H332): harmful if inhaled STOT SE 3 (H335): may cause respiratory irritation |
| 174 | 95‐48‐7 | o‐Cresol | CLH | https://echa.europa.eu/substance-information/-/substanceinfo/100.002.204 |
Harmonised classification and labelling: Acute Tox. 3 (H301): is toxic if swallowed Acute Tox. 3 (H311): is toxic in contact with skin Skin Corr. 1B (H314): causes severe skin burns and eye damage |
| 233 | 108‐30‐5 | Succinic anhydride | Substance evaluation, CLH | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.246 |
Initial grounds of concern: CMR, Sensitiser, High (aggregated) tonnage, High RCR. According to the eMSCA of Austria, data available demonstrate that succinic anhydride requires further harmonised classification for its sensitising properties (Skin Sens. 1; H317: may cause an allergic skin reaction; Resp. Sens. 1; H334: May cause allergy or asthma symptoms or breathing difficulties if inhaled). Furthermore, data demonstrate that succinic anhydride has corrosive properties and needs to be classified as Skin Corr. 1B (H314: Causes severe skin burns and eye damage) and Eye Dam. 1 (H318: Causes serious eye damage) (Substance Evaluation Conclusion and Evaluation Report) Harmonised classification and labelling: Skin Corr. 1 (H314): causes severe skin burns and eye damage. Acute Tox. 4 (H302): harmful if swallowed. Eye Dam. 1 (H318): causes serious eye damage. Skin Sens. 1 (H317): may cause an allergic skin reaction. Resp. Sens. 1 (H334): may cause allergy or asthma symptoms or breathing difficulties if inhaled |
| 253 | 110‐60‐1 | 1,4‐Diaminobutane | CLH | https://echa.europa.eu/substance-information/-/substanceinfo/100.003.440 |
No Harmonised Classification and Labelling CLH proposal withdrawn |
| 718 | 65997‐13‐9 | Rosin, hydrogenated, ester with glycerol | Substance evaluation, PBT | https://echa.europa.eu/substance-information/-/substanceinfo/100.060.020 |
No Harmonised Classification and Labelling Initial grounds of concern: Suspected PBT/vPvB, exposure to the environment (CoRAP document and Substance Evaluation Decision) PBT under development (no document available) |
Ten substances were subsequently excluded from the medium priority group and placed in the low priority group due to very low potential for migration/exposure, as described below (Section 3.2.3.1).
The medium priority group therefore consisted of 102 substances, schematically shown in Figure 3 and listed in Table A.8 of Appendix A.
Table A.8.
Medium priority group of substances
| FCM substance no. | Ref. no. | CAS no. | Substance name |
|---|---|---|---|
| 3 | 12375 | Alcohols, aliphatic, monohydric, saturated, linear, primary (C4‐C22) | |
| 9 | 30610 | Acids, C2‐C24, aliphatic, linear, monocarboxylic from natural oils and fats and their mono‐, di‐ and triglycerol esters (branched fatty acids at naturally occurring levels are included) | |
| 10 | 30612 | Acids, C2‐C24, aliphatic, linear, monocarboxylic, synthetic and their mono‐, di‐ and triglycerol esters | |
| 12 | 31328 | Acids, fatty, from animal or vegetable food fats and oils | |
| 13 | 33120 | Alcohols, aliphatic, monohydric, saturated, linear, primary (C4‐C24) | |
| 17 | 34281 | Alkyl(C8‐C22) sulphuric acids, linear, primary with an even number of carbon atoms | |
| 22 | 43200 | Castor oil, mono‐ and diglycerides | |
| 36 | 54450 | Fats and oils, from animal or vegetable food sources | |
| 37 | 54480 | Fats and oils, hydrogenated, from animal or vegetable food sources | |
| 40 | 56360 | Glycerol, esters with acetic acid | |
| 41 | 56486 | Glycerol, esters with acids, aliphatic, saturated, linear, with an even number of carbon atoms (C14‐C18) and with acids, aliphatic, unsaturated, linear, with an even number of carbon atoms (C16‐C18) | |
| 42 | 56487 | Glycerol, esters with butyric acid | |
| 43 | 56490 | Glycerol, esters with erucic acid | |
| 44 | 56495 | Glycerol, esters with 12‐hydroxystearic acid | |
| 45 | 56500 | Glycerol, esters with lauric acid | |
| 46 | 56510 | Glycerol, esters with linoleic acid | |
| 47 | 56520 | Glycerol, esters with myristic acid | |
| 48 | 56535 | Glycerol, esters with nonanoic acid | |
| 49 | 56540 | Glycerol, esters with oleic acid | |
| 50 | 56550 | Glycerol, esters with palmitic acid | |
| 51 | 56570 | Glycerol, esters with propionic acid | |
| 52 | 56580 | Glycerol, esters with ricinoleic acid | |
| 53 | 56585 | Glycerol, esters with stearic acid | |
| 54 | 57040 | Glycerol monooleate, ester with ascorbic acid | |
| 56 | 57200 | Glycerol monopalmitate, ester with ascorbic acid | |
| 58 | 57600 | Glycerol monostearate, ester with ascorbic acid | |
| 60 | 58300 | Glycine, salts | |
| 65 | 67155 | Mixture of 4‐(2‐benzoxazolyl)‐4′‐(5‐methyl‐2‐benzoxazolyl)stilbene, 4,4′‐bis(2‐benzoxazolyl) stilbene and 4,4′‐bis(5‐methyl‐2‐benzoxazolyl)stilbene | |
| 67 | 67840 | Montanic acids and/or their esters with ethyleneglycol and/or with 1,3‐butanediol and/or with glycerol | |
| 100 | 24490 ‐ 88320 | 50‐70‐4 | Sorbitol |
| 101 | 36000 | 50‐81‐7 | Ascorbic acid |
| 110 | 93520 |
59‐02‐9 10191‐41‐0 |
α‐Tocopherol |
| 120 | 49540 | 67‐68‐5 | Dimethyl sulphoxide |
| 122 | 23800 | 71‐23‐8 | 1‐Propanol |
| 123 | 13840 | 71‐36‐3 | 1‐Butanol |
| 124 | 22870 | 71‐41‐0 | 1‐Pentanol |
| 136 | 41680 | 76‐22‐2 | Camphor |
| 155 | 23470 | 80‐56‐8 | α‐Pinene |
| 162 | 65520 | 87‐78‐5 | Mannitol |
| 174 | 14740 | 95‐48‐7 | o‐Cresol |
| 205 | 25385 | 102‐70‐5 | Triallylamine |
| 210 | 13390 ‐ 14880 | 105‐08‐8 | 1,4‐Bis(hydroxymethyl)cyclohexane |
| 214 | 61840 | 106‐14‐9 | 12‐Hydroxystearic acid |
| 215 | 14170 | 106‐31‐0 | Butyric anhydride |
| 216 | 14770 | 106‐44‐5 | p‐Cresol |
| 232 | 10150 ‐ 30280 | 108‐24‐7 | Acetic anhydride |
| 233 | 24850 | 108‐30‐5 | Succinic anhydride |
| 235 | 14710 | 108‐39‐4 | m‐Cresol |
| 238 | 18070 | 108‐55‐4 | Glutaric anhydride |
| 250 | 53520 | 110‐30‐5 | N,N′‐Ethylenebisstearamide |
| 251 | 53360 | 110‐31‐6 | N,N′‐Ethylenebisoleamide |
| 253 | 15250 | 110‐60‐1 | 1,4‐Diaminobutane |
| 259 | 58720 | 111‐14‐8 | Heptanoic acid |
| 265 | 22600 | 111‐87‐5 | 1‐Octanol |
| 267 | 15100 | 112‐30‐1 | 1‐Decanol |
| 271 | 52720 | 112‐84‐5 | Erucamide |
| 296 | 23860 | 123‐38‐6 | Propionaldehyde |
| 298 | 14110 | 123‐72‐8 | Butyraldehyde |
| 306 | 88960 | 124‐26‐5 | Stearamide |
| 309 | 91360 | 126‐14‐7 | Sucrose octaacetate |
| 314 | 23500 | 127‐91‐3 | β‐Pinene |
| 321 | 36080 | 137‐66‐6 | Ascorbyl palmitate |
| 322 | 63040 | 138‐22‐7 | Lactic acid, butyl ester |
| 328 | 65040 | 141‐82‐2 | Malonic acid |
| 331 | 22480 | 143‐08‐8 | 1‐Nonanol |
| 332 | 69760 | 143‐28‐2 | Oleyl alcohol |
| 335 | 68960 | 301‐02‐0 | Oleamide |
| 360 | 57920 | 620‐67‐7 | Glycerol triheptanoate |
| 367 | 16697 | 693‐23‐2 | n‐Dodecanedioic acid |
| 401 | 24475 | 1313‐82‐2 | Sodium sulphide |
| 417 | 85680 | 1343‐98‐2 | Silicic acid |
| 421 | 13000 | 1477‐55‐0 | 1,3‐Benzenedimethanamine |
| 428 | 95200 | 1709‐70‐2 | 1,3,5‐Trimethyl‐2,4,6‐tris(3,5‐di‐tert‐butyl‐4‐hydroxybenzyl)benzene |
| 458 | 36960 | 3061‐75‐4 | Behenamide |
| 486 | 54005 | 5136‐44‐7 | Ethylene‐N‐palmitamide‐N′‐stearamide |
| 488 | 53440 | 5518‐18‐3 | N,N′‐Ethylenebispalmitamide |
| 494 | 62140 | 6303‐21‐5 | Hypophosphorous acid |
| 524 | 24520 | 8001‐22‐7 | Soybean oil |
| 525 | 62640 | 8001‐39‐6 | Japan wax |
| 526 | 43440 | 8001‐75‐0 | Ceresin |
| 527 | 14411 ‐ 42880 | 8001‐79‐4 | Castor oil |
| 530 | 41760 | 8006‐44‐8 | Candelilla wax |
| 533 | 42720 | 8015‐86‐9 | Carnauba wax |
| 539 | 24160 | 8052‐10‐6 | Rosin tall oil |
| 543 | 45920 | 9000‐16‐2 | Dammar |
| 589 | 52645 | 10436‐08‐5 | Cis‐11‐eicosenamide |
| 593 | 44960 | 11104‐61‐3 | Cobalt oxide |
| 602 | 70240 | 12198‐93‐5 | Ozokerite |
| 609 | 83455 | 13445‐56‐2 | Pyrophosphorous acid |
| 647 | 56720 | 26402‐23‐3 | Glycerol monohexanoate |
| 648 | 56880 | 26402‐26‐6 | Glycerol monooctanoate |
| 654 | 88600 | 26836‐47‐5 | Sorbitol monostearate |
| 669 | 56610 | 30233‐64‐8 | Glycerol monobehenate |
| 681 | 18310 | 36653‐82‐4 | 1‐Hexadecanol |
| 703 | 17170 | 61788‐47‐4 | Fatty acids, coco |
| 704 | 77600 | 61788‐85‐0 | Polyethyleneglycol ester of hydrogenated castor oil |
| 706 | 17230 | 61790‐12‐3 | Fatty acids, tall oil |
| 712 | 42960 | 64147‐40‐6 | Castor oil, dehydrated |
| 718 | 84240 | 65997‐13‐9 | Rosin, hydrogenated, ester with glycerol |
| 722 | 17200 | 68308‐53‐2 | Fatty acids, soya |
| 754 | 56020 | 99880‐64‐5 | Glycerol dibehenate |
| 820 | 76420 | Pimelic acid, salts |
Figure 4.
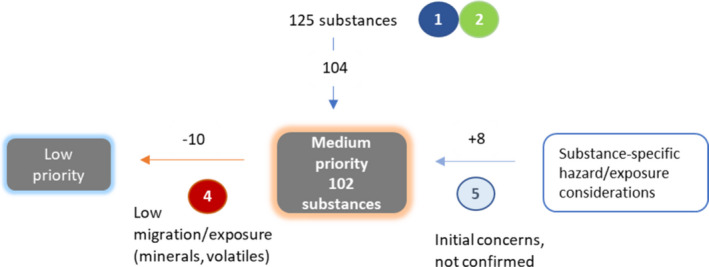
Number of substances placed in the medium priority group
3.2.3.1. Substances with no or very limited potential for migration/exposure
The Union List entries silicates, natural (with the exception of asbestos) (FCM No 84) and vermiculite, reaction product with citric acid, lithium salt (FCM No 757), are minerals and were considered of a low potential for migration. Therefore, these two substances were moved to the low priority group, as mentioned in the previous section.
The following eight volatile substances were also moved from the medium to the low priority group, after considering the likelihood of exposure and relevant hazard information:
FCM No 126: Acetylene and FCM No 125: Ethylene.
Toxicological data on acetylene are limited. According to the reported toxicological studies from ECHA registrations,18 acetylene was negative in three in vitro mutagenicity tests and gave no indications for genotoxicity.
According to the available toxicological studies on ethylene, a low toxicity can be assumed19 (OECD, 2002). However, inhaled ethylene can be metabolically converted to ethylene oxide. Ethylene oxide is a potent alkylating agent and classified as a carcinogen (IARC, 1994). DNA and haemoglobin‐adducts have also been detected after exposure to ethylene in animals, although in vitro and in vivo genotoxicity studies gave no indications for genotoxicity (OECD, 2002). Furthermore, a long‐term inhalation study generated no convincing evidence of carcinogenicity in rats. This absence of toxicity may be due to saturation of ethylene metabolism (Segerbäck, 1983). Indeed, the metabolic conversion of ethylene to ethylene oxide seems to be a rate‐limiting step whereby the produced amount of ethylene oxide via inhalation is insignificant (Bolt and Filser, 1987, Csanády et al., 2000, as reported from ECHA registrations20). No information regarding absorption, distribution, metabolism and excretion (ADME) and carcinogenicity are available for the oral route.
The two substances are gases with very low boiling points of −100°C for ethylene and −84°C for acetylene. Due to the very high volatility of these monomers, it can be expected that they will be effectively volatilised after the polymerisation process and during manufacture of the food contact polymers. On this basis, exposure to ethylene and acetylene due to migration from food contact materials can be excluded. Consequently, these two substances are moved to the low priority group.
FCM No 131: 1,1‐Difluoroethane
According to the OECD Existing Chemicals Database (OECD, 2006), 1,1‐difluoroethane was tested negative in an in vitro bacterial reverse mutation test (Ames test) but showed a weak clastogenic response in an in vitro human lymphocyte chromosome aberration test in the absence of metabolic activation. The clastogenic potential was further tested in an in vivo micronucleus assay which gave negative results. No treatment‐related tumours were observed in male and female rats in a 2‐year inhalation study. In addition, the 2‐year inhalation study revealed no clear evidence of toxicity from repeated exposure to 1,1‐difluoroethane at concentrations of up to 67,500 mg/m3.
1,1‐Difluoroethane is a gas with a boiling point of −25°C and may be used as a polymer production aid for manufacture of fluorinated polymers. Due to the high volatility of this substance and high temperatures applied in the manufacture of such polymers, it is expected that the substance will be volatilised during manufacture of the food contact polymers and not be any longer present in significant amounts in the final food contact material or article. It is concluded that exposure from food consumption, if any, will be very low and, consequently, this substance is moved to the low priority group.
FCM No 222: 1‐Butene, FCM No 224: 2‐Butene and FCM No 276: iso‐Butene
1‐Butene, 2‐butene and iso‐butene (also known as isobutylene) were assessed within a category approach by the OECD (OECD, 2004). Based on the results presented in the assessment, the substances seem to have a low subchronic toxicity and gave no indications for developmental or reproductive toxicity. In a combined repeated toxicity study with reproduction/development toxicity screening by inhalation exposure, the No Observed Adverse Effect Concentration (NOAEC) was higher than 18,400 mg/m3 for 1‐butene and higher or equal to 11,500 mg/m3 for 2‐butene. Isobutylene was not toxic to rats or mice exposed to concentrations up to 4,600 mg/m3 for 105 weeks. For all three substances, no mutagenic responses were observed either in vitro or in vivo.
These three substances are monomers and are gaseous at room temperature, with boiling points of −7°C for 1‐butene, ranging from +0.9°C to +3.7°C for 2‐butene (cis/trans mixture) and −7°C for iso‐butene. Due to their high volatility, it can be expected that these substances will be volatilised during manufacture of the food contact polymers and may be present there only in very low residual amounts. Therefore, exposure to these butenes due to migration from food contact materials, if any, will be very low and, consequently, the three substances were moved to the low priority group.
FCM No 143: iso‐Pentane and FCM No 244: Pentane
According to publicly available data, studies regarding the general toxicity of iso‐pentane are scarce and are restricted to a 4‐week oral toxicity screening study (as cited by ECHA20). For n‐pentane, toxicity studies on the oral route are restricted to a reproductive study in rats (McKee et al., 1998) and a 4‐week oral nephrotoxicity screening study in rats (API, 1985). Nevertheless, taking into account general ADME and systemic toxicity of n‐ and iso‐alkanes, it is considered that both substances are of low toxicity. According to the available information by ECHA18, in the 4‐week oral toxicity screening study in rats with iso‐pentane, mortality was reported to be 10% and 90% in the low‐ and the high‐dose groups (500 and 2,000 mg/kg bw per day), respectively. In the 4‐week oral nephrotoxicity screening study on n‐pentane, a higher mortality (40%) and narcotic effects (20%) relative to controls were reported in the high‐dose group (2,000 mg/kg bw per day). In this study, reported data on histopathology are scarce. Kidneys were the only organs examined microscopically, which were not remarkably changed by the substance. Gross examination of tissue and organs only revealed stomach lesions that could be observed in some animals at the highest dose tested. In a 90‐day repeated dose inhalation study in rats with n‐pentane, no systemic toxicity was observed up to a dose of at least ≥ 20,000 mg/m3, equivalent to 1,766 mg/kg bw per day (US‐EPA, 2009; ECB, 2003).
These two pentane isomers are substances with boiling points of +28°C for iso‐pentane and +36°C for n‐pentane, and hence characterised by high intrinsic volatility. These substances when used as polymer production aids are expected to be volatilised during the thermal conditions in the manufacture of food contact polymers and may be present there only in low residual amounts. Therefore, it is concluded that exposure to these pentanes due to migration from food contact materials will be low and, consequently, these two substances are moved to the low priority group.
In total, 10 substances with a low potential for migration or high volatility were moved to the low priority group.
3.2.4. High Priority Group
Substances complying with any of the criteria described in the methodology section (par. 2.2.2.3) were candidates for the high priority group.
3.2.4.1. Information on genotoxicity, carcinogenicity, mutagenicity, reprotoxicity, bioaccumulation and ED properties
The EFSA OpenFoodTox database was searched for FCM substances not in the low priority group with safety intake levels (ADI, group ADI, TDI and group TDI) below 1 mg/kg bw per day. Two substances were identified: acetone (FCM No 119) and chlorine (FCM No 522).
Three substances were present in the IARC list of classifications: ethanol (FCM No 113), classified as Group 1, styrene (FCM No 193), classified as Group 2A and titanium dioxide (FCM No 610), classified as Group 2B.
No substances were present in the ECHA Candidate list of SVHCs. The list includes substances which meet the criteria for classification as CMR category 1A or 1B in accordance with the CLP Regulation, substances which are persistent, bioaccumulative and toxic (PBT) or very persistent and very bioaccumulative (vPvB) according to REACH Annex XIII, or substances of an equivalent level of concern as CMR or PBT/vPvB substances.
The FCM substances were also checked against the formal regulatory provisions of CLP and REACH, i.e. the C&L Inventory (Annex VI to CLP) for CLH, the Authorisation List of identified SVHCs (Annex XIV to REACH) and the Restriction List (Annex XVII to REACH). One substance with a restriction under REACH (methanol, FCM No 117) and six substances with a CLH were identified (methanol, FCM No 117; salicylic acid. FCM No 121; styrene, FCM No 193; butane, FCM No 221; carbon monoxide, FCM No 362; and titanium dioxide, FCM No 610). No substances were identified in the Authorisation list of REACH.
FCM substances not in the low priority group were also checked against the RMOA tool of ECHA, used to identify appropriate regulatory actions or the most appropriate measures to address concerns. Two substances (methanol, FCM No 117 and styrene, FCM No 193) were found under this category. For methanol, it was concluded (in the RMOA) that there is no need to initiate further regulatory risk management action.
The EU Member States were also requested to provide information on risk assessments of FCM substances conducted at national level. The feedback received, which was mainly based on EFSA hazard assessments, was used as additional information for specific substances, however, it was not used to guide the prioritisation to low, medium and high priority groups. A number of SCF evaluation dossiers were also retrieved through this communication channel.
3.2.4.2. Genotoxicity – in silico predictions
One hundred and twenty‐five substances passed the criteria as candidates for the medium or high priority groups and reached the (Q)SAR prediction step. For 96 substances, the CAS numbers and chemical names were available. The SMILES codes, needed to run the (Q)SAR models, were retrieved by manually checking on PubChem,21 ChemIDPlus22 or ChemSpider,23 using the CAS numbers as input. The SMILES codes were not found for 13 substances, nine substances were classified as inorganic and one as salt or mixture. Since (Q)SAR models are not able to read inorganic compounds or salts/mixtures, these substances were deleted from the data set. The final data set comprised 73 substances.
VEGA was run using the consensus model for genotoxicity. This model provides a qualitative prediction of mutagenicity (Ames test) based on the outcome of the single VEGA mutagenicity models (CAESAR, SarPy, ISS and KNN). Within the VEGA consensus model for genotoxicity, some elements are statistical‐based models and other are built with rules/structural alerts defined by human experts.
The OECD QSAR Toolbox was used applying the following profilers: ‘In vitro mutagenicity (Ames test) alerts by ISS’ and ‘DNA alerts for AMES by OASIS’. Only the models predicting genotoxicity based on the Ames test were selected as models proving most reliable predictions (Benigni et al., 2019; Honma et al., 2019). Indeed, (Q)SAR models providing predictions based on Ames test experimental data give satisfactory results that are comparable with the experimental variability of the test. The reliability of the (Q)SAR models for endpoints other than the Ames test are far less good, giving results close to the random predictions. This can be due to the higher availability and better quality of experimental data for the Ames test used to build the models, compared to the data available for other genotoxicity endpoints.
Results for positive (Mutagen) predictions are reported in Table 2. Negative predictions (Non‐mutagen) are reported in Appendix A, in Tables A.9 and A.10.
Table 2.
Results for positive predictions from VEGA or OECD QSAR ToolBox
| OECD QSAR ToolBox | VEGA | |||||||
|---|---|---|---|---|---|---|---|---|
| FCM no | CAS no | Chemical name | In vitro mutagenicity (Ames test) alerts by ISS | DNA alerts for AMES by OASIS | Consensus Model | Mutagenic score | Non‐mutagenic score | Experimental data available |
| 275 | 115‐07‐1 | Propylene | No alert found | No alert found | Mutagenic | 1 | 0 | YES |
| 436 | 2146‐71‐6 | Lauric acid, vinyl ester | Alpha,beta‐unsaturated aliphatic alkoxy group | No alert found | Non‐mutagenic | 0 | 0.825 | NO |
| 591 | 10605‐09‐1 | Ascorbyl stearate | No alert found | AN2 >> Schiff base formation >> Dicarbonyl compounds | Non‐mutagenic | 0 | 0.45 | NO |
| 768 | 143925‐92‐2 | Amines, bis(hydrogenated tallow alkyl) oxidised | No alert found |
‘SN2 >> Acylation >> N‐Hydroxylamines; SN1 >> Nucleophilic attack after nitrenium ion formation >> N‐Hydroxylamines; Radical >> Radical mechanism via ROS formation (indirect) >> N‐Hydroxylamines; AN2 >> Carbamoylation after isocyanate formation >> N‐Hydroxylamines’ |
Non‐mutagenic | 0 | 0.4 | NO |
Table A.9.
Results for negative predictions considering a score for VEGA ≥ 0.5
| OECD QSAR ToolBox | VEGA | ||||||
|---|---|---|---|---|---|---|---|
| CAS number | Chemical name | In vitro mutagenicity (Ames test) alerts by ISS | DNA alerts for AMES by OASIS | Consensus model | Mutagenic score | Non‐mutagenic score | Experimental data available |
| 99880‐64‐5 | Glycerol dibehenate | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 61788‐47‐4 | Fatty acids, coco | No alert found | No alert found | NON‐mutagenic | 0 | 0.825 | No |
| 67‐56‐1 | Methanol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 67‐64‐1 | Acetone | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 67‐68‐5 | Dimethyl sulphoxide | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 69‐72‐7 | Salicylic acid | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 71‐23‐8 | 1‐Propanol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 71‐36‐3 | 1‐Butanol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 71‐41‐0 | 1‐Pentanol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 74‐85‐1 | Ethylene | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 26402‐26‐6 | Glycerol monooctanoate | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 78‐78‐4 | Isopentane | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 80‐56‐8 | α‐Pinene | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 95‐48‐7 | o‐Cresol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 10436‐08‐5 | Cis‐11‐Eicosenamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 100‐42‐5 | Styrene | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 102‐70‐5 | Triallylamine | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 106‐14‐9 | 12‐Hydroxystearic acid | No alert found | No alert found | NON‐mutagenic | 0 | 0.9 | No |
| 106‐31‐0 | Butyric anhydride | No alert found | No alert found | NON‐mutagenic | 0 | 0.9 | No |
| 106‐44‐5 | p‐Cresol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 106‐97‐8 | Butane | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 106‐98‐9 | 1‐Butene | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 107‐01‐7 | 2‐Butene | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 108‐39‐4 | m‐Cresol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 108‐55‐4 | Glutaric anhydride | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 109‐66‐0 | Pentane | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 110‐30‐5 | N,N′‐ethylenebisstearamide | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 111‐14‐8 | Heptanoic acid | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 111‐87‐5 | 1‐octanol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 112‐30‐1 | 1‐decanol | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 112‐84‐5 | Erucamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 115‐11‐7 | Isobutene | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 8001‐75‐0 | Ceresin | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 123‐38‐6 | Propionaldehyde | Simple aldehyde | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 123‐72‐8 | Butyraldehyde | Simple aldehyde | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 124‐26‐5 | Stearamide | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 127‐91‐3 | β‐pinene | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 141‐82‐2 | Malonic acid | No alert found | No alert found | NON‐mutagenic | 0 | 0.825 | No |
| 143‐28‐2 | Oleyl alcohol | No alert found | No alert found | NON‐mutagenic | 0 | 0.825 | No |
| 301‐02‐0 | Oleamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 3061‐75‐4 | Behenamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.825 | No |
| 2146‐71‐6 | Lauric acid, vinyl ester | alpha,beta‐unsaturated aliphatic alkoxy group | No alert found | NON‐mutagenic | 0 | 0.825 | No |
| 1477‐55‐0 | 1,3‐Benzenedimethanamine | No alert found | No alert found | NON‐mutagenic | 0 | 1 | YES |
| 620‐67‐7 | Glycerol triheptanoate | No alert found | No alert found | NON‐mutagenic | 0 | 0.75 | No |
| 693‐23‐2 | n‐Dodecanedioic acid | No alert found | No alert found | NON‐mutagenic | 0 | 0.9 | No |
| 36653‐82‐4 | 1‐Hexadecanol | No alert found | No alert found | NON‐mutagenic | 0 | 0.675 | No |
| 30233‐64‐8 | Glycerol monobehenate | No alert found | No alert found | NON‐mutagenic | 0 | 0.675 | No |
| 76‐22‐2 | Camphor | No alert found | No alert found | NON‐mutagenic | 0 | 0.575 | No |
| 110‐31‐6 | N,N′‐Ethylenebisoleamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.5 | No |
| 8006‐44‐8 | Candelilla wax | No alert found | No alert found | NON‐mutagenic | 0 | 0.5 | No |
| 8001‐79‐4 | Castor oil | No alert found | No alert found | NON‐mutagenic | 0 | 0.5 | No |
| 126‐14‐7 | Sucrose octaacetate | No alert found | No alert found | NON‐mutagenic | 0 | 0.5 | No |
| 5518‐18‐3 | N,N′‐ethylenebispalmitamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.675 | No |
| 5136‐44‐7 | Ethylene‐N‐palmitamide‐N′‐stearamide | No alert found | No alert found | NON‐mutagenic | 0 | 0.6 | No |
| 143‐08‐8 | 1‐Nonanol | No alert found | No alert found | NON‐mutagenic | 0 | 0.675 | No |
| 105‐08‐8 | 1,4‐Bis(hydroxymethyl)cyclohexane | No alert found | No alert found | NON‐mutagenic | 0.05 | 0.525 | No |
| 108‐24‐7 | Acetic oxide | No alert found | No alert found | NON‐mutagenic | 0 | 1 | Yes |
| 108‐30‐5 | Succinic anhydride | No alert found | No alert found | NON‐mutagenic | 0 | 1 | Yes |
| 110‐60‐1 | Putrescine (1,4‐diaminobutane) | No alert found | No alert found | NON‐mutagenic (Consensus score: 0.825) | 0 | 0.85 | No |
| 137‐66‐6 | Ascorbyl palmitate | No alert found | AN2 >> Schiff base formation >> Dicarbonyl compounds | NON‐mutagenic | 0 | 1 | Yes |
| 138‐22‐7 | Butyl lactate | No alert found | No alert found | NON‐mutagenic | 0 | 0.9 | No |
| 1709‐70‐2 | 1,3,5‐Trimethyl‐2,4,6‐tris(3,5‐di‐tert‐butyl‐4‐hydroxybenzyl)benzene | No alert found | No alert found | NON‐mutagenic | 0 | 0.5 | No |
| 26836‐47‐5 | D‐glucitol monostearate | No alert found | No alert found | NON‐mutagenic (Consensus score: 0.525) | 0 | 0.525 | No |
| 50‐70‐4 | Sorbitol | No alert found | No alert found | NON‐mutagenic (Consensus score: 1) | 0 | 1 | Yes |
| 50‐81‐7 | VITAMIN C (ascorbic acid) | No alert found | AN2 >> Schiff base formation >> Dicarbonyl compounds | NON‐mutagenic (Consensus score: 1) | 0 | 1 | Yes |
| 59‐02‐9 | Alpha‐tocopherol | No alert found | No alert found | NON‐mutagenic (Consensus score: 1) | 0 | 1 | Yes |
| 64‐17‐5 | Ethanol | No alert found | No alert found | NON‐mutagenic (Consensus score: 1) | 0 | 1 | Yes |
| 87‐78‐5 | Mannitol | No alert found | No alert found | NON‐mutagenic (Consensus score: 1) | 0 | 1 | Yes |
Table A.10.
Results for negative predictions considering a consensus score for VEGA < 0.5
| OECD QSAR ToolBox | VEGA | ||||||
|---|---|---|---|---|---|---|---|
| CAS number | Chemical name | In vitro mutagenicity (Ames test) alerts by ISS | DNA alerts for AMES by OASIS | Consensus model | Mutagenic score | NON‐mutagenic score | Experimental data available |
| 143925‐92‐2 | Amines, bis(hydrogenated tallow alkyl) oxidised | No alert found |
SN2 >> Acylation >> N‐Hydroxylamines; SN1 >> Nucleophilic attack after nitrenium ion formation >> N‐Hydroxylamines; Radical >> Radical mechanism via ROS formation (indirect) >> N‐Hydroxylamines; AN2 >> Carbamoylation after isocyanate formation >> N‐Hydroxylamines |
NON‐mutagenic | 0 | 0.4 | No |
| 74‐86‐2 | Acetylene | No alert found | No alert found | NON‐mutagenic | 0.067 | 0.133 | No |
| 75‐37‐6 | 1,1‐Difluoroethane | No alert found | No alert found | NON‐mutagenic | 0.05 | 0.35 | No |
| 10605‐09‐1 | Ascorbyl stearate | No alert found | AN2 >> Schiff base formation >> Dicarbonyl compounds | NON‐mutagenic | 0 | 0.45 | No |
In the VEGA platform, the level of reliability for the predictions for non‐mutagenic (or mutagenic) substances is measured by a score which goes from 0 to 1. If this score is close to zero, the level of uncertainty in the prediction is high. If the score is greater than 0.5, then the level of reliability is acceptable. When the score is 1, it means that experimental data for the specific substance are available in the training/test set of the models.
Out of 73 substances, only one, propylene (FCM No 275), was predicted by VEGA software as ‘Mutagen’ (Table 2), this substance was predicted with a high score since an experimental value is available. This result was not confirmed by the other two predictions obtained with the OECD QSAR Toolbox that did not find any structural alert. However, considering the availability of experimental data for this substance, propylene should be considered as a mutagen.
Two substances, propionaldehyde (FCM No 296) and butyraldehyde (FCM No 298), were predicted as positive by the module In vitro mutagenicity (Ames test) alerts by ISS within the OECD QSAR ToolBox (Appendix A, Table A.9). The structural alert found is ‘simple aldehyde’ which has a very low predictivity (33%) (Benigni et al., 2008). These predictions were not confirmed by the other models used. Moreover, for these two substances, the VEGA software has experimental values in support of negative genotoxicity (Appendix A, Table A.9). Considering the availability of experimental data, propionaldehyde and butyraldehyde should be considered as non‐mutagenic. In addition, the EFSA CEF Panel (EFSA CEF Panel, 2017) ruled out a safety concern regarding genotoxicity and the two substances are authorised food flavourings.
For the substance lauric acid, vinyl ester (FCM No 436), a structural alert (alpha, beta‐unsaturated aliphatic alkoxy group) was found for the module In vitro mutagenicity (Ames test) alerts by ISS within the OECD QSAR ToolBox (Table 2). The predictivity of the structural alert found (50%) is not very high and the prediction is not confirmed by the other models. However, experimental values are not available. Following a conservative approach, this substance should be considered as mutagenic.
The two substances amines, bis(hydrogenated tallow alkyl) oxidised (FCM No 768) and ascorbyl stearate (FCM No 591) were predicted as positive based on the module ‘DNA alerts for AMES by OASIS’ present in the OECD QSAR ToolBox (Table 2). The predictions were not confirmed by the other models. However, the negative predictions obtained with VEGA for these substances have a low predictivity score (Table 2) that may indicate low reliability of the result. Based on this, to be conservative, these substances should be considered as mutagenic before further analyses.
A total of 72 substances were predicted as negative by VEGA, 68 of which had a consensus score ≥ 0.5 (Appendix A, Table A.9). Out of 68 substances, 36 had experimental data confirming the non‐mutagenicity activity. For the other substances, the negative predictions were supported by the results obtained with the other two profilers available in the OECD QSAR ToolBox, except for one substance (lauric acid, vinyl ester, FCM No 436) for which a structural alert was found with the other two models available in the OECD QSAR ToolBox (α,β‐unsaturated aliphatic alkoxy group) with the module of in vitro mutagenicity (Ames test) alerts by ISS. This substance was already considered above. Four substances were predicted as negative (non‐mutagenic) by VEGA with a score < 0.5 (Appendix A, Table A.10). Experimental data for these substances were not available. The negative predictions in VEGA were supported by the results obtained with the other two profilers available in the OECD QSAR ToolBox, except for two substances (FCM No 591 and FCM No 768) for which structural alerts were found; these substances have already been discussed above (Table 2).
3.2.4.3. Alerts from existing priority lists and screening
Two FCM substances were found in the SIN List: styrene (FCM No 193) and carbon monoxide (FCM No 362).
The ECHA database of chemicals registered under REACH and CLP was searched for ongoing risk evaluations, i.e. for substance registrations that have passed the initial screening and compliance check phases and are in the substance evaluation phase. Two substances are currently under assessment by ECHA: Styrene (FCM No 193) under RMOA for concerns on endocrine disruption, mutagenicity and reprotoxicity, and Rosin, hydrogenated, ester with glycerol (FCM No 718): under evaluation as PBT.
No FCM substances of interest were found in the EFSA priority list of potential emerging risks.
3.2.4.4. Case‐by‐case considerations of candidate substances for the high priority group
Eight substances originally identified as being evaluated by ECHA were excluded from the high priority group due to lack of relevance to FCM risk assessment. These substances remain in the medium priority group and are listed in Appendix A, Table A.11.
The 10 substances: ethanol (FCM No 113), methanol (FCM No 117), acetone (FCM No 119), butane (FCM No 221), propylene (FCM No 275), carbon monoxide (FCM No 362), chlorine (FCM No 552), ascorbyl stearate (FCM No 591), titanium dioxide (FCM No 610) and amines, bis(hydrogenated tallow alkyl) oxidised (FCM No 768), were also excluded, for the reasons described below. The initial alerts for these substances are listed in Appendix A, Table A.12.
Table A.12.
Substances assigned to the low priority or ‘no SML needed’ groups, following expert judgement
| FCM substance no. | CAS no. | Substance name | SCF list | Synoptic SCF Opinion | Boiling point (oC) | ADI or TDI (mg/kg bw)a | EFSA Panel | QSAR Mutagenicity (Ames test)a | Evaluation Stage under REACH or CLPa | IARCa | SIN lista |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 113 | 64‐17‐5 | Ethanol | 1 | Acceptable (SCF, 11th Series, 1981) | 78.2 | Dossier evaluation | 1 | ||||
| 117 | 67‐56‐1 | Methanol | 3 | The toxicity profile well known also from intoxication of man. The potential migration into food will not be of toxicological significance (SCF, 6th Series, 1978) | 64.7 | Substance evaluation, CLH, RMOA, Restriction | |||||
| 119 | 67‐64‐1 | Acetone | 3 | Residue in food less than 5 mg/kg. (SCF, 11th Series, 1981) | 56.0 | 0.9 | CONTAM | Screening, Dossier evaluation | |||
| 221 | 106‐97‐8 | Butane | 3 | Volatile compound | −0.5 | CLH | |||||
| 275 | 115‐07‐1 | Propylene | 3 | Residues of this gas in plastics are very small. The gas has a low toxic potential. Migration into food will be toxicologically negligible.(Patty's Industrial Hygiene and Toxicology, 3rd edition, 1981) | −47.6 | Positive | Dossier evaluation | 3 | |||
| 362 | 630‐08‐0 | Carbon monoxide | 3 | Low migration | −191.5 | Dossier evaluation, CLH | ‘Classified CMR according to Annex VI of Regulation 1272/2008’ | ||||
| 522 | 7782‐50‐5 | Chlorine | 3 | Residues of this gas in plastics will be very small. Migration into food would be self‐limiting because of odour | −34.0 | 0.15 | AFC | ||||
| 591 | 10605‐09‐1 | Ascorbyl stearate | 1 |
Acceptable Covered by the assessment for ascorbyl palmitate |
536.0 | Positive | |||||
| 610 | 13463‐67‐7 | Titanium dioxide | 1 | Acceptable (SCF, 1st Series, 1975) | 2,900.0 (approximate value) | Substance evaluation, CLH | 2B | ||||
| 768 | 143925‐92‐2 | Amines, bis (hydrogenated tallow alkyl) oxidised | 3 |
SCF_List: 3 Restriction: Only to be used: ·in polyolefins at 0.1% (w/w) and not for fatty food with a simulant D Reduction Factor less than 3 ·in PET at 0.25% (w/w) and only for food for which simulant D is not required. Remark for Commission: To make sure that also for the migration of Bis (hydrogenated tallow, C16‐18, alkyl) nitrones and (Hydrogenated tallow, C16‐18, alkyl) oximes into fatty foods a sufficient safety margin to the NOEL in the subchronic oral rat study exists the following restriction is proposed: Qm 0,1% in polyolefins, not for fatty foods with a Reduction Factor < 3. See SCF evaluation in the website: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out180_en.pdf |
Positive |
Information in bold indicates the reason for categorising the substance as a possible candidate for the high priority group.
FCM No 113: Ethanol
Ethanol was classified by IARC with carcinogenic hazard to humans in Group 1 based on the evidence of cancers observed in heavy drinkers of alcoholic beverages (IARC, 2012). Potential exposure due to migration of ethanol from plastics when used as a substance for the manufacture of polymers is expected to be several orders of magnitude lower than that from alcoholic beverages. Ethanol is authorised as an extraction solvent to be used in compliance with good manufacturing practice for all uses, during the processing of raw materials, of foodstuffs, of food components or of food ingredients.24 It is concluded that the toxicological and epidemiological basis for the IARC classification is not relevant (in terms of dose) for the potential migration of ethanol from FCMs, and hence, ethanol is placed into the low priority group.
FCM No 117: Methanol
EFSA evaluated the available toxicological information on methanol in the frame of its safety evaluation of the food additive aspartame (E 951) since metabolism of aspartame releases a corresponding 10% by weight of methanol (EFSA ANS Panel, 2013). The ANS Panel concluded that there would be no risk from methanol derived from aspartame, at the ADI for aspartame of 40 mg/kg bw per day.
The ANS Panel also considered the metabolite of methanol, formaldehyde, in its risk assessment. It concluded that based on measurements of basal levels of formaldehyde in blood and on the modelling of its biological turnover and steady‐state concentration in cells, formaldehyde formed from aspartame‐derived methanol would not be of safety concern at the ADI for aspartame of 40 mg/kg bw per day (EFSA, 2014).
These conclusions of the ANS Panel on methanol and on formaldehyde derived from methanol can be used to support that an exposure to methanol of 4 mg/kg bw per day (i.e. 10% of the ADI value for aspartame) is of no safety concern. Since this value is above 1 mg/kg bw per day and according to the prioritisation scheme (Figure 1 and Section 2.2.2.2), methanol was assigned to the low priority group.
FCM No 119: Acetone
Acetone is authorised as an extraction solvent to be used in compliance with good manufacturing practice for all uses during the processing of raw materials, of foodstuffs, of food components or of food ingredients.25 The EFSA CONTAM Panel established for acetone a health‐based guidance value of 0.9 mg/kg bw per day (EFSA CONTAM Panel, 2012), similar to the reference dose for oral exposure previously established by U.S. EPA (US‐EPA, 2003). This value was derived from a No Observed Adverse Effect Level (NOAEL) of 900 mg/kg bw per day, identified in a 90‐day oral study in rats. In the absence of data from chronic exposure, the CONTAM Panel and the US EPA used an additional safety factor of 10, beyond the standard safety factor of 100, to allow for possible chronic exposure. However, according to the Scientific Committee Guideline on default values to be used in the absence of measured data (EFSA Scientific Committee, 2012), an additional factor of 2, instead of 10, should be used to extrapolate from subchronic to chronic exposure. This would result in an ADI higher than 1 mg/kg bw per day, with consequent allocation of acetone to the low priority group. Consequently, acetone is moved to the low priority group.
FCM No 221: Butane
Butane is classified as carcinogenic and mutagenic according to Regulation (EU) No 1272/2008 when containing more than 0.1% 1,3‐butadiene as an impurity. Butane itself is not classified as mutagenic and carcinogenic. 1,3‐Butadiene is regulated under Regulation (EU) No 10/2011. Additionally, it is noted that the SCF did not have a toxicological concern about the use of water‐based emulsion sprays and oil‐based aerosol sprays for baking and frying purposes, which contain propane, butane or isobutane (SCF, 1999). The food additive butane (E 943a) is authorised quantum satis in vegetable oil pan spray and water‐based emulsion spray, according to Regulation (EC) No 1333/2008. Butane is authorised as an extraction solvent to be used in compliance with good manufacturing practice for all uses during the processing of raw materials, of foodstuffs, of food components or of food ingredients25. Butane is a gas with a boiling point of −1°C, thus, any exposure from FCM is expected to be low. Consequently, for these reasons, butane is moved to the low priority group.
FCM No 275: Propylene
IARC (1994) did not classify propylene as a carcinogen to humans but indicated that: ‘Alkylation products of the metabolite, propylene oxide, were found in haemoglobin and in DNA from mice exposed to propylene by inhalation’. The mutagenicity potential is considered to be associated with the formation of propylene oxide (possibly carcinogenic to humans (IARC, 1994)). Propylene was tested positive in one bacteria strain with metabolic activation (Ames test). In vivo tests of propylene were reported as negative (OECD, 2003).
Propylene is a gas with a boiling point of −48°C. Due to the high volatility of this substance and the relatively high intrinsic diffusion properties of polypropylene polymers it can be expected that the substance will be effectively volatilised during manufacture of the food contact polymers. It is concluded that significant amounts of propylene in food contact materials are unlikely and, hence, exposure from food consumption is considered to be low, if any. Consequently, propylene is moved to the low priority group.
FCM No 362: Carbon monoxide
Carbon monoxide is classified as toxic for reproduction according to Annex VI of Regulation (EC) No 1272/2008. However, based on the boiling point of carbon monoxide (−191°C), it is concluded that its presence and subsequent migration in/from food contact materials are highly unlikely and hence exposure from food consumption can be excluded. Consequently, carbon monoxide is moved to the low priority group.
FCM No 552: Chlorine
The AFC Panel in 2006 endorsed the ADI from WHO set at 0.15 mg/kg bw per day for chlorine. Migration to food could lead to what have become known as chlorinated byproducts. However, it is considered that migration of chlorine into food would be negligible, if any, due to its high reactivity, high volatility (boiling point of −34°C) and self‐limiting odor. Chlorine may also be used for the chlorination of polyolefins and the low molecular weight fraction of such polymers may contain chlorinated species of potential toxicological concern. Reaction and transformation products including oligomers are outside the scope of this prioritisation exercise (see section 1.2). Based on these considerations, the CEP Panel moved chlorine to the low priority group.
FCM No 591: Ascorbyl stearate
Ascorbyl stearate (FCM No 591) is very similar in structure to ascorbyl palmitate (FCM No 321) but whereas the palmitate had a positive SCF Opinion placing it into SCF List 1, the stearate seems to have been assigned to SCF list 1 in the Synoptic document by an assumed read‐across from the palmitate but with no traceable SCF Opinion. Ascorbyl stearate and ascorbyl palmitate are authorised for use as food additives (E 304(i) and E 304(ii)) in the EU and the EFSA ANS Panel re‐evaluated these additives in 2015 (EFSA ANS Panel, 2015). The CAS numbers for the food additives are the same as those for the food contact substances and refer specifically to the enantiomers L‐ascorbyl palmitate and L‐ascorbyl stearate (CAS Nos. 137‐66‐6 and 10605‐09‐1, respectively). Although the available toxicological data were too limited to establish an ADI, the ANS Panel concluded that there is no safety concern for the use of ascorbyl palmitate (E 304(i)) and ascorbyl stearate (E 304(ii)) as food additives at the reported uses and use levels, which could give exposure (individually or in combination) of up to 10.8 mg/kg bw per day. Based on this information, both ascorbyl stearate and ascorbyl palmitate are placed into the low priority group.
FCM No 610: Titanium dioxide
Titanium dioxide is used by the food industry in the EU in different applications. In the food contact material sector TiO2 is authorised, but not in a nanoform (Regulation (EU) No 10/2011). Titanium dioxide is also authorised for use as a food additive (E 171) in many food categories, but its specifications do not characterise its particle size distribution (Regulation (EU) No 231/201225, current consolidated version of 23/10/2019). EFSA has been requested to review new and emerging evidence on several occasions (EFSA ANS Panel, 2016b; EFSA ANS Panel, 2018; EFSA, 2019; EFSA FAF Panel, 2019). In 2016, the EFSA ANS Panel completed the re‐evaluation of titanium dioxide E 171 (EFSA ANS Panel, 2016b). Due to limitations of the toxicity database, the ANS Panel did not establish an ADI but used a Margin of Safety (MoS) approach based on an NOAEL of 2,250 mg/kg bw per day from a long‐term chronic study in mice and rats. The calculated MoS was 400 at the mean and 150 at the 95th percentile. The ANS Panel concluded that at the estimated levels of exposure the food additive E 171, which, according to the data reported by the food industry, contains a small component of unavoidable but unintended nanoparticles, was not of safety concern. The ANS Panel recommended the characterisation of the particle size distribution of the food additive E 171. In order to address the uncertainties in the characterisation of the material and in the limitations of the database, the European Commission launched a public call for data on the particle size distribution, and for the performance of a new extended one‐generation reproductive toxicity study (EOGRTS) in rodents. In 2019, the FAF Panel proposed in its published opinion an amendment of the EU specifications for the food additive titanium dioxide (E 171) (EFSA FAF Panel, 2019). A new scientific opinion on the safety of titanium dioxide (E 171) considering all the new relevant data including those falling under the scope of the 2018 Scientific Committee Guidance (EFSA Scientific Committee, 2018) on nanotechnologies is expected by the end of 2020.26
The EFSA CEP Panel recently evaluated the food contact substance ‘titanium dioxide surface treated with fluoride‐modified alumina’ which is a defined mixture of particles of which a fraction of particles have a diameter in the range of 1–100 nm (EFSA CEP Panel, 2019). This is intended to be used as filler and colourant up to 25% w/w in potentially all polymer types. The data evaluated demonstrated that the plastic additive particles stay embedded even in swollen polar polymers such as polyamide, and do not migrate, neither by diffusion nor as a result of abrasion. From these data along with theoretical considerations it can be concluded that for the substance titanium dioxide, modified or not, used as a plastic additive, no migration can be anticipated.
Therefore, based on the above considerations, in the specific context of food contact materials, titanium dioxide is placed into the low priority group.
FCM No 768: Amines, bis(hydrogenated tallow alkyl) oxidised
The substance ‘Amines, bis(hydrogenated tallow alkyl) oxidised’ has been evaluated by the SCF (SCF, 2003) for its use as a processing stabiliser in polyolefins at up to 0.1% (w/w) and in PET up to 0.25% (w/w). It includes: (i) the main substance, bis(hydrogenated tallow, C16–C18, alkyl) hydroxylamine (63–80%) along with lower amounts of; (ii) bis(hydrogenated tallow, C16–C18, alkyl) amine (12–20%); (iii) bis(hydrogenated tallow, C16–C18, alkyl) nitrones (2–10%); (iv) hydrogenated tallow, C16–C18, alkyl) oximes (2–10%); (v) fatty acids (C16–C18) (0–5%); and (vi) (C16–C18) secondary amides (0–7%).
According to the SCF opinion, Amines, bis(hydrogenated tallow alkyl)oxidised, as well as the three migrating substances (ii, iii and iv above) did not show genotoxic properties in the available in vitro studies (gene mutations assays in bacteria and cultured mammalian cells and in vitro chromosomal aberration studies). Therefore, the in silico alerts obtained for this substance, which triggered its consideration for the high priority group, are over‐ruled by the experimental data available.
According to the SCF 2003 evaluation, the main component, bis(hydrogenated tallow, C16–C18, alkyl) hydroxylamine, was not found to migrate and the other components and/or reaction products do not follow a uniform pattern of migration from different types of polyolefins under different time/temperature conditions. For these reasons, an SML was not set by the SCF, which suggested instead, restrictions of use in polyolefins and PET. These were incorporated into regulatory provisions, currently described in Regulation (EU) No 10/2011 as ‘Not to be used for articles in contact with fatty foods for which simulant D1 and/or D2 is laid down. Only to be used in: (a) polyolefins at 0.1% (w/w) and in (b) PET at 0.25% (w/w)’. Considering that the substance is already regulated by restriction of use in polymers and with the exclusion of fatty food contact, the substance Amines, bis(hydrogenated tallow alkyl) oxidised is placed into the group ‘no SML should be needed’.
3.2.4.5. The high priority group of substances
Following the considerations described in the previous section and schematically shown in the flowchart of Figure 5, three substances comprise the final high priority group, listed in Table 3. The evidence from risk evaluations and predictions is summarised in Appendix A, Table A.13.
Table 3.
Substances of the high priority group
| FCM substance no. | CAS No. | Substance name |
|---|---|---|
| 121 | 69‐72‐7 | Salicylic acid |
| 193 | 100‐42‐5 | Styrene |
| 436 | 2146‐71‐6 | Lauric acid, vinyl ester |
Table A.13.
High Priority Group of substances
| FCM substance no. | Ref. no. | CAS no. | Substance name | SCF list | Genotoxicity prediction (Ames test)a | ECHA evaluation stage under REACH or CLPa | IARC classification groupa | SIN list (reasons for inclusion)a |
|---|---|---|---|---|---|---|---|---|
| 121 | 24270 ‐ 84640 | 69‐72‐7 | Salicylic acid | 3 | CLH, Screening under REACH | |||
| 193 | 24610 | 100‐42‐5 | Styrene | 4B | CLH, RMOA: under development | 2A | ‘Styrene is an endocrine disruptor (ED) (cat 1). Reprotoxic as well as carcinogenic and mutagenic effects have been reported. It is highly toxic to aquatic species.’ | |
| 436 | 19480 | 2146‐71‐6 | Lauric acid, vinyl ester | 3 | Positive (OECD Toolbox alerts by ISS) | Dossier evaluation under REACH |
Information in bold indicates the reason for categorising the substance in the high priority group.
FCM No 121: Salicylic acid
Classification of salicylic acid is based on teratogenic properties shown in animals (ECHA, 2016b). Based on the lowest NOAEL of 75 mg/kg bw per day from a developmental toxicity study in rats (Tanaka et al., 1973a,b), the most sensitive species, a tolerable daily intake below 1 mg/kg bw per day could be derived by applying a default uncertainty factor of 100 for inter‐ and intra‐species differences. Therefore, this substance was placed in the high priority group.
FCM No 193: Styrene
As a consequence of a Commission request to assess the IARC reclassification of its carcinogenicity, styrene is currently under risk evaluation by the EFSA CEP Panel.27 According to the European Commission mandate, EFSA should assess whether the evidence examined by IARC would have an impact on the safety of styrene in FCMs and if needed, should determine under what conditions the substance could be safely used in FCM. Therefore, styrene was placed in the high priority group and its re‐evaluation by EFSA is taking place in the context of another mandate.
FCM no. 436: Lauric acid, vinyl ester
According to the Synoptic Document (European Commission, 2005), complete hydrolysis occurs in simulated intestinal fluid into lauric acid (FCM No 330, no SML) and acetaldehyde (FCM No 128, SML(T) of 6 mg/kg food). Gene mutations and chromosomal aberrations observed in in vitro studies are most likely due to the formation of acetaldehyde. According to Table 2 of Annex I of Regulation (EU) No 10/2011, the group restriction no. (1) with an SML(T) of 6 mg/kg food (expressed as acetaldehyde), covers also propionic acid, vinylester (FCM No 211), which like lauric acid, vinyl ester, is a chemical precursor of acetaldehyde in vivo. Therefore, lauric acid, vinyl ester, was placed in the high priority group and it is proposed to include it on a temporary basis into the group restriction no. (1) with an SML(T) of 6 mg/kg food, expressed as acetaldehyde, pending a full evaluation.
4. Discussion
As requested by the Terms of Reference of the mandate received from the European Commission, the CEP Panel has undertaken this review and prioritisation exercise for substances that are listed without a specific migration limit in Table 1 of Annex 1 of Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Certain substances may require an SML (or some other form of limitation) to ensure that their authorisation is sufficiently protective to health.
The Panel considers that prioritisation is a ranking process and it is not a (final) evaluation of any of the substances involved. For this reason, this Opinion has identified substances for which an SML should not be needed along with substances for which an SML may be needed, placed into low, medium and high priority groups. The high, medium and low priority groups should not be interpreted as meaning that an SML is necessary. Rather, the absence of an SML merits further consideration which should be guided by the prioritisation provided.
This review and prioritisation were conducted using existing information that could be retrieved for each substance. As mandated by the Terms of Reference, there was not a call for data from interested parties (other than Member States) nor from business operators that may use the substances. The number of substances actually used nowadays by the industry is not known. Similarly, the technical function and therefore the extent and conditions of use of many substances are not obvious.
Regarding the absence of a call for data, the Panel takes note of the provisions in the Regulation (EC) No 1935/2004, the so‐called ‘Framework Regulation’ on plastic materials and articles. That Regulation provides for the listing of substances. Those substances listed and thus ‘authorised’ can be used by everyone, subject to the restrictions set out in the authorisation. Importantly, at Article 10 (‘Opinion of the Authority’) it is a requirement that ‘The applicant or any business operator using the authorised substance or materials or articles containing the authorised substance shall immediately inform the Commission of any new scientific or technical information, which might affect the safety assessment of the authorised substance in relation to human health. If necessary, the Authority shall then review the assessment.’ This being the case, even in the absence of any call for data for this prioritisation exercise, business operators have the responsibility to inform the Commission of any relevant information that may have developed since the authorisation was made.
The main criteria used for prioritisation were hazard‐based. This took into account existing hazard assessments that could be retrieved for each substance on CMR, bioaccumulation and ED properties and from the qualified structure–activity relationship ((Q)SAR) profiles regarding alerts for genotoxicity. Use of (Q)SAR profilers other than predictions on mutagenicity (based on the Ames test) proved not to be reliable (e.g. alerts for in vivo micronucleus test, for the chromosomal aberration endpoint).
A modelling approach to understand relative levels of exposure was not carried out. It was precluded by the high number and the nature of the uncertainties, mostly stemming from the absence of information on the types of use, use levels and migration levels, of the substances in/from plastic materials in contact with food. The exposure considerations were limited to the default assumption of a person of body weight of 60 kg consuming a maximum of 1 kg of food per day and that food containing the substance having migrated from a plastic used in contact with the food (SCF, 2001). This default exposure scenario is conservative, especially for substances that are used in only niche applications. On the other hand, exposure from other dietary sources, including from non‐plastic FCM, is not known. Also, as already noted, this scenario is generally protective for adults but not necessarily for infants, toddlers or young children due to their higher relative food consumption on a body weight basis compared to that of adults (EFSA CEF Panel, 2016).
The potential for consumer exposure considered the physico‐chemical properties of the substances, notably the molecular weight and boiling point. According to a general understanding of the diffusion process by which chemical migration occurs, the molecular weight (more exactly, the size) of a substance is a major determinant in the extent of migration. The boiling point of a substance indicates its volatility and thus informs on the likelihood or not of the persistence of a substance in a manufactured FCM – which in turn will influence the possible migration levels. These properties of molecular weight (–> size) and boiling point (–> volatility) are used and discussed in a narrative manner in the cases where no clear cut‐off values can be derived and used as such.
5. Conclusions
A total of 451 substances for which an SML or SML(T) was not assigned were put through the prioritisation procedure. These substances were taken from Table 1, Annex I of Regulation (EU) No 10/2011, as amended by Regulation (EU) 2019/37 of 10 January 2019. The full list of substances without an SML or SML(T) is provided in Appendix A, Table A.1.
Seventy‐eight substances have been evaluated previously by EFSA as food contact substances, and therefore, they were eliminated at the outset, according to the mandate received. The list of substances and the corresponding EFSA opinions is provided in Appendix A, Table A.2.
For 89 substances, it was concluded by the Panel that an SML should not be needed. The 89 substances for which an SML should not be needed are listed in Appendix A, Table A.5. The main criteria that defined this group were:
an SCF classification as List 0 or List 1, and/or
an ‘ADI not specified’ by the SCF, and/or
substances that are controlled by existing restrictions and/or generic limits (e.g. salts where the anion is controlled by the general metal‐related SMLs on the corresponding cation) and
no conflict with subsequent EFSA evaluations in domains other than food contact plastics.
One hundred and seventy‐nine substances were placed in the low priority group. The list of 179 low priority substances is shown in Appendix A, Table A.6. The main criteria that defined this group were substances:
for which other EFSA Panels have assigned or endorsed an ADI of ≥ 1 mg/kg bw per day (22 substances, mainly food additives and flavouring substances), and/or
having an ADI set by the SCF of ≥ 1 mg/kg bw per day (102 substances), and/or
with a molecular weight above 1,000 Da (39 substances), and/or
classified by the SCF as List 3 ‘inert’ (26 substances), and/or
with high volatility (8 substances)
One hundred and two substances were placed in the medium priority group. The list of 102 medium priority substances is shown in Appendix A, Table A.8. This group comprises substances for which the information available was not adequate to place them into the ‘low’, ‘high’ or ‘no SML needed’ groups.
Three substances were placed into the high priority group. These three substances that are judged to merit the highest priority for reassessment are salicylic acid (FCM No 121), styrene (FCM No 193) and lauric acid, vinyl ester (FCM No 436). The evidence from risk evaluations and predictions for these high priority substances is summarised in Appendix A, Table A.13. The high prioritisation was due to:
hazard alert(s) from one or more of evaluations by IARC, ECHA or EFSA and/or from (Q)SAR predictions, and
where the hazard alert was not over‐ruled by considerations of low migration/exposure, and/or absence of relevance of the alert to FCM.
6. Recommendations
For lauric acid, vinyl ester, the Panel recommends to include this substance on a temporary basis into the group restriction no. (1) with an SML(T) of 6 mg/kg food, expressed as acetaldehyde, pending a full evaluation.
Abbreviations
- AFC Panel
EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food
- ADI
Acceptable Daily Intake
- ADME
Absorption, Distribution, Metabolism, and Excretion Absorption, Distribution, Metabolism, Excretion
- ANS Panel
EFSA Panel on Food Additives and Nutrient Sources Added to Food
- CAS
Chemical Abstracts Service
- CEF Panel
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CEP Panel
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids
- C&L
Classification and Labelling
- CLH
Harmonised Classification and Labelling
- CLP
Classification, Labelling and Packaging
- CMR
Carcinogenicity, Mutagenicity, Reproductive toxicity
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- ECB
European Chemicals Bureau
- ECHA
European Chemicals Agency
- ED
Endocrine Disruptor
- eMSCA
evaluating Member State Competent Authority
- FAF Panel
EFSA Panel on Food Additives and Flavourings
- FCM
Food contact materials
- GML
Generic Specific Migration Limit
- IARC
International Agency for Research on Cancer
- JECFA
Joint Expert Committee on Food Additives
- MTDI
Maximum Tolerable Daily Intake
- MW
Molecular Weight
- ND
Not Detectable
- NOAEC
No Observed Adverse Effect Concentration
- NOAEL
No Observed Adverse Effect Level
- NOEL
No Observed Effect Level
- OECD
Organisation for Economic Co‐operation and Development
- PBT
Persistent, Bioaccumulative and Toxic
- PET
Polyethylene terephthalate
- PMTDI
Provisional Maximum Tolerable Daily Intake
- (P)TWI
Provisional Tolerable Weekly Intake
- (Q)SAR
Quantitative Structure‐Activity Relationships
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RMOA
Regulatory Management Option Analysis
- SC
Scientific Committee
- SCF
Scientific Committee for Food
- SML
Specific Migration Limit
- SML(T)
Total Specific Migration Limit
- SVHC
Substances of Very High Concern
- TDI
Tolerable Daily Intake
- t‐TDI
temporary Tolerable Daily Intake
- ToR
Terms of Reference
- vPvB
very Persistent, very Bioaccumulative
- WG
Working Group
Appendix A – Tables of substances
1.
Suggested citation: EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) , Silano V, Barat Baviera JM, Bolognesi C, Chesson A, Cocconcelli PS, Crebelli R, Gott DM, Grob K, Lambré C, Lampi E, Mengelers M, Mortensen A, Steffensen I‐L, Tlustos C, Van Loveren H, Vernis L, Zorn H, Benfenati E, Castle L, Di Consiglio E, Franz R, Hellwig N, Milana MR, Pfaff K, Civitella C, Lioupis A, Pizzo F and Rivière G, 2020. Scientific Opinion on the review and priority setting for substances that are listed without a specific migration limit in Table 1 of Annex 1 of Regulation 10/2011 on plastic materials and articles intended to come into contact with food. EFSA Journal 2020;18(6):6124, 104 pp. 10.2903/j.efsa.2020.6124
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00150
Panel members: Jose Manuel Barat Baviera, Claudia Bolognesi, Andrew Chesson, Pier Sandro Cocconcelli, Riccardo Crebelli, David Michael Gott, Konrad Grob, Claude Lambré, Evgenia Lampi, Marcel Mengelers, Alicja Mortensen, Gilles Rivière, Vittorio Silano, Inger‐Lise Steffensen, Christina Tlustos, Henk Van Loveren, Laurence Vernis and Holger Zorn.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: Beat Johannes Brüschweiler (WG and Panel member until June 2019), Giovanni Bernasconi (EFSA), Anastasia Livaniou (EFSA), Elina Karhu and the Prioritisation Unit B3 (ECHA) and the European Competent Authorities for food contact materials.
Adopted: 29 April 2020
Notes
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food Text with EEA relevance OJ L 12, 15.1.2011, p. 1–89.
Commission Regulation (EU) 2016/1416 of 24 August 2016 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food (Text with EEA relevance) OJ L 230, 25.8.2016, p. 22–42.
Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC OJ L 338, 13.11.2004, p. 4–17.
Commission Regulation (EU) 2019/37 of 10 January 2019 amending and correcting Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food OJ L 9, 11.1.2019, p. 88–93.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration,Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000 21 EC OJ L 396, 30.12.2006, p. 1–849.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006 OJ L 353, 31.12.2008, p. 1–1355.
Commission Regulation (EC) No 1895/2005 of 18 November 2005 on the restriction of use of certain epoxy derivatives in materials and articles intended to come into contact with food. OJ L 302, 19.11.2005, p. 28–32.
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. OJ L 354, 31.12.2008, p. 16–33.
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Available online: https://pubchem.ncbi.nlm.nih.gov/
Available online: https://chem.nlm.nih.gov/chemidplus/
Available online: http://www.chemspider.com/
Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients (Recast) OJ L 141, 6.6.2009, p. 3–11.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012, p. 1–295.
References
- API (American Petroleum Institute), 1985. Four‐week oral nephrotoxicity screening study in male F344 rats. TSCATS Section FYI. Fiche #OTS0000280‐2 Unpublished report. Cited in US‐EPA, 2009. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0128tr.pdf
- Benfenati E, Roncaglioni A, Lombardo A and Manganaro A, 2019. Integrating QSAR, Read‐Across and Screening Tools: the VEGAHUB Platform as an Example In: Huixiao H. (ed.). Advances in Computational Toxicology: methodologies and Applications in Regulatory Science. Springer Nature, pp. 365–382. 10.1007/978-3-030-16443-0_18 [DOI] [Google Scholar]
- Benigni R, Bossa C, Jeliazkovab N, Netzevac T and Worthc A, 2008. The Benigni/Bossa rulebase for mutagenicity and carcinogenicity – a module of Toxtree. EUR 23241 EN – 2008. Available online: https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/benigni-bossa-rulebase-mutagenicity-and-carcinogenicity-module-toxtree
- Benigni R, Battistelli CL, Bossa C, Giuliani A, Fioravanzo E, Bassan A, Gatnik MF, Rathman J, Yang C and Tcheremenskaia O, 2019. Evaluation of the applicability of existing (Q)SAR models for predicting the genotoxicity of pesticides and similarity analysis related with genotoxicity of pesticides for facilitating of grouping and read across. EFSA supporting publication 2019:EN‐1598, 221 pp. 10.2903/sp.efsa.2019.en-1598 [DOI] [PubMed] [Google Scholar]
- Bolt HM and Filser JG, 1987. Kinetics and disposition in toxicology. Archives of Toxicology, 60, 73–76. 10.1007/BF00296951 [DOI] [PubMed] [Google Scholar]
- Ceriani L, Ciacci A, Baldin R, Kovarich S, Pavan M, Fioravanzo E and Bassan A, 2018. Final report on the update and maintenance of OpenFoodTox: EFSA's Chemical Hazards Database. EFSA supporting publication 2018:EN‐1438, 59 pp. 10.2903/sp.efsa.2018.en-1438 [DOI] [Google Scholar]
- Csanády GA, Denka B, Pütz C, Kreuzer PE, Kessler W, Bau C, Gargas ML and Filser JC, 2000. A Physiological Toxicokinetic Model for Exogenous and Endogenous Ethylene and Ethylene Oxide in Rat, Mouse, and Human: formation of 2‐Hydroxyethyl Adducts with Hemoglobin and DNA. Toxicology and Applied Pharmacology, 165, 1–26. 10.1006/taap.2000.8918 [DOI] [PubMed] [Google Scholar]
- Dorne JL, Richardson J, Kass G, Georgiadis N, Monguidi M, Pasinato L, Cappe S, Verhagen H and Robinson T, 2017. Editorial: openFoodTox: EFSA's open source toxicological database on chemical hazards in food and feed. EFSA Journal, 15, e15011, 3pp. 10.2903/j.efsa.2017.e15011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECB (European Chemicals Bureau), 2003. European Union Risk Assessment Report. Pentane (CAS No: 109‐66‐0. EINECS No: 203‐692‐4). Joint Research Center ‐ Institute for Health and Consumer Protection (JRC‐IHCP). Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC26088/EUR%2020845%20EN.pdf
- ECHA (European Chemical Agency), 2016a. Preparation of an inventory of substances suspected to meet REACH Annex III criteria. Technical documentation. Available online: https://echa.europa.eu/documents/10162/22332820/annex_iii_preparation_inventory_en.pdf/e42ea5b1-28f0-4390-8d34-6d09e2875ebd
- ECHA (European Chemical Agency), 2016b. Committee for Risk Assessment (RAC), Opinion proposing harmonised classification and labelling at EU level of Salicylic acid, EC Number: 200‐712‐3, CAS Number: 69‐72‐7, CLH‐O‐0000001412‐86‐110/F, Adopted 10 March 2016. Available online: https://echa.europa.eu/documents/10162/13794bcd-8882-b609-46b4-a4bc1263e6e3
- ECHA (European Chemicals Agency), 2019. Plastic additives initiative. Supplementary Information on Scope and Methods. Available online: https://echa.europa.eu/documents/10162/13630/plastic_additives_supplementary_en.pdf
- EFSA (European Food Safety Authority), 2004. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to a 6th list of substances for food contact materials. EFSA Journal 2004;161, 13 pp. 10.2903/j.efsa.2004.161 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Note for Guidance For the Preparation of an Application for the Safety Assessment of a Substance to be used in Plastic Food Contact Materials. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2008.21r
- EFSA (European Food Safety Authority), 2014. Endogenous formaldehyde turnover in humans compared with exogenous contribution from food sources. EFSA Journal 2014;12(2):3550, 11 pp. 10.2903/j.efsa.2014.3550 [DOI] [Google Scholar]
- EFSA (European Food Safety), 2019. EFSA statement on the review of the risks related to the exposure to the food additive titanium dioxide (E 171) performed by the French Agency for Food, Environmental and Occupational Health and Safety (ANSES). EFSA Journal 2019;17(6):5714, 11 pp. 10.2903/j.efsa.2019.5714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2013. Scientific Opinion on the re‐evaluation of aspartame (E 951) as a food additive. EFSA Journal 2013;11(12):3496, 263 pp. 10.2903/j.efsa.2013.3496 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2015. Scientific Opinion on the re‐evaluation of ascorbyl palmitate (E 304(i)) and ascorbyl stearate (E 304(ii)) as food additives. EFSA Journal 2015;13(11):4289, 57 pp. 10.2903/j.efsa.2015.4289 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2016a. Scientific opinion on the re‐evaluation of β‐cyclodextrin (E 459) as a food additive. EFSA Journal 2016; 14(12):4628, 44 pp. 10.2903/j.efsa.2016.4628 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2016b. Scientific Opinion on the re‐evaluation of titanium dioxide (E 171) as a food additive. EFSA Journal 2016;14(9):4545, 83 pp. 10.2903/j.efsa.2016.4545 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2018. Scientific Opinion on the evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171). EFSA Journal 2018;16(7):5366, 27 pp. 10.2903/j.efsa.2018.5366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2010. Scientific Opinion on the safety evaluation of the substance, copper hydroxide phosphate, CAS No. 12158‐74‐6, for use in food contact materials. EFSA Journal 2010;8(10):1838, 11pp. 10.2903/j.efsa.2010.1838. [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2016. Scientific opinion on recent developments in the risk assessment of chemicals in food and their potential impact on the safety assessment of substances used in food contact materials. EFSA Journal 2016; 14(1):4357, 28 pp. 10.2903/j.efsa.2016.4357 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2017. Scientific opinion of Flavouring Group Evaluation 502 (FGE.502): grill flavour ‘Grillin’ 5078’. EFSA Journal 2017;15(9):4973, 33p. 10.2903/j.efsa.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids), 2019. Scientific Opinion on the safety assessment of the substance, titanium dioxide surface treated with fluoride‐modified alumina, for use in food contact materials. EFSA Journal 2019;17(6):5737, 11 pp. 10.2903/j.efsa.2019.5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012. Scientific Opinion on the evaluation of the substances currently on the list in the annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils ‐ Part II of III. EFSA Journal 2012;10(5):2703, 151pp. 10.2903/j.efsa.2012.2703 [DOI] [Google Scholar]
- EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings), 2019. Scientific opinion on the proposed amendment of the EU specifications for titanium dioxide (E 171) with respect to the inclusion of additional parameters related to its particle size distribution. EFSA Journal 2019;17(7):5760, 23 pp. 10.2903/j.efsa.2019.5760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Mart ınez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Chaudhry Q, Cubadda F, Gott D, Oomen A, Weigel S, Karamitrou M, Schoonjans R and Mortensen A, 2018. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: part 1, human and animal health. EFSA Journal 2018;16(7):5327, 95 pp. 10.2903/j.efsa.2018.5327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2005. ‘Synoptic Document’. Provisional list of monomers and additives notified to European Commission as substances which may be used in the manufacture of plastics and coatings intended to come into contact with foodstuffs (updated to June 2005). SANCO D3/AS D(2005).
- European Commission , 2014. Union Guidelines on Regulation (EU) No 10/2011 on plastic materials and articles intended to come into contact with food. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_fcm_plastic-guidance_201110_en.pdf
- Honma M, Kitazawa A, Cayley A, Williams RV, Barber C, Hanser T, Saiakhov R, Chakravarti S, Myatt GJ, Cross KP, Benfenati E, Raitano G, Mekenyan O, Petkov P, Bossa C, Benigni R, Battistelli CL, Giuliani A, Tcheremenskaia O, DeMeo C, Norinder U, Koga H, Jose C, Jeliazkova N, Kochev N, Paskaleva V, Yang C, Daga PR, Clark RD and Rathman J, 2019. Improvement of quantitative structure‐activity relationship (QSAR) tools for predicting Ames mutagenicity: outcomes of the Ames/QSAR International Challenge Project. Mutagenesis, 34, 3–16. 10.1093/mutage/gey031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1994. Some industrial chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 60, 1–560. Available online: http://publications.iarc.fr/78 [PMC free article] [PubMed]
- IARC (International Agency for Research on Cancer), 2012. Personal Habits and Indoor Combustions. Consumption on alcoholic beverages. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 100E, 373‐499. Available online: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100E-11.pdf [PMC free article] [PubMed]
- Loewenstein WR, 1981. Junctional intercellular communication: the cell‐to‐cell membrane channel. Physiological Reviews, 61, 829–913, 84 pp. 10.1152/physrev.1981.61.4.829 [DOI] [PubMed] [Google Scholar]
- McKee R, Frank E, Heath J, Owen D, Przygoda R, Trimmer G and Whitman F, 1998. Toxicology of n‐pentane (CAS No. 109‐66‐0). Journal of Applied Toxicology, 18, 431−442 [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development), 2002. SIDS Final Assessment Report. Ethylene. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=e5d777a4-516f-4fdb-a76d-4ffec89e3aa3 (accessed 16/04/2020).
- OECD (Organisation for Economic Co‐operation and Development), 2003. SIDS Final Assessment Report. Propylene. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=05bca44c-4720-4926-843d-6539599b3de4 (accessed 16/04/2020).
- OECD (Organisation for Economic Co‐operation and Development), 2004. SIDS Final Assessment Report. Butenes. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=11f46903-9226-4233-a9db-36a4c2a81e28 (accessed 16/04/2020).
- OECD (Organisation for Economic Co‐operation and Development), 2006. SIDS Final Assessment Report. 1,1‐Difluoroethane. Available online: https://hpvchemicals.oecd.org/UI/handler.axd?id=6415a8cf-4a7b-4c8e-b943-f61ed5304b0d (accessed 16/04/2020).
- Oltmanns J, Bohlen M‐L, Escher S, Schwarz M and Licht O, 2019. Final report: applying a tested procedure for the identification of potential emerging chemical risks in the food chain to the substances registered under REACH – REACH 2. External Scientific Report. OC/EFSA/SCER/2016/01‐CT1. EFSA supporting publication 2019:EN‐1597. 263 pp. 10.2903/sp.efsa.2019.en-1597 [DOI] [Google Scholar]
- SCF (Scientific Committee for Food), 1990. First series of food additives of various technological functions (Opinion expressed on 18 May 1990). Reports of the Scientific Committee for Food (Twenty fifth series). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_reports_25.pdf
- SCF (Scientific Committee fo Food), 1999. Opinion on propane, butane and iso‐butane as propellant gases for vegetable oil‐based aerosol cooking sprays and water‐based emulsion cooking sprays. SCF/CS/ADD/MsAd/178 Final. 24 March 1999. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out26_en.pdf
- SCF (Scientific Committee for Food), 2001. Guidelines of the Scientific Committee on Food (SCF) for the presentation of an application for safety assessment of a substance to be used in food contact materials prior to its authorisation. SCF/CS/PLEN/GEN/100 Final. 19 December 2001. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out82_en.pdf
- SCF (Scientific Committee for Food), 2003. Opinion of the Scientific Committee on Food on the 22nd additional list of monomers and additives for food contact materials (Opinion expressed on 4 April 2003). SCF/CS/PM/GEN/M94 Final. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/sci-com_scf_out180_en.pdf
- Segerbäck D, 1983. Alkylation of DNA and haemoglobin in the mouse following exposure to ethene and ethene oxide. Chemico‐Biological Interactions, 45, 139–151. 10.1016/0009-2797(83)90064-9 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kawashima K, Nakaura S, Nagao S, Kuwamura T, Takanaka A and Omori Y, 1973a. Studies on teratogenic effects of salicylic acid and aspirin in rats as related to fetal distribution. Department of Pharmacology, National Institute of Hygienic Sciences, Tokyo, Japan, 13, 73–84. [Google Scholar]
- Tanaka S, Kawashima K, Nakaura S, Nagao S, Kuwamura T, Takanaka A and Omori Y, 1973b. Studies on the teratogenicity of food additives (3): teratogenic effect of dietary salicylic acid in rats. Journal of the Food Hygienic Society of Japan, 14, 549–557. Available online: https://www.jstage.jst.go.jp/article/shokueishi1960/14/6/14_6_549/_pdf [Google Scholar]
- US‐EPA (United States ‐ Environmental Protection Agency), 2003. Toxicological review of acetone. (CAS No. 67‐64‐1). In Support of Summary Information on the Integrated Risk Information System (IRIS). EPA/635/R‐03/004. Available online: https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0128tr.pdf
- US‐EPA (United States ‐ Environmental Protection Agency), 2009. Provisional Peer‐Reviewed Toxicity Values for n‐Pentane. (CASRN 109‐66‐0). EPA/690/R‐09/044F. Available online: https://cfpub.epa.gov/ncea/pprtv/documents/Pentanen.pdf


