Abstract
The purpose of this study was to evaluate feasibility of initiating continuous glucose monitoring (CGM) through telehealth as a means of expanding access. Adults with type 1 diabetes (N = 27) or type 2 diabetes using insulin (N = 7) and interest in starting CGM selected a CGM system (Dexcom G6 or Abbott FreeStyle Libre), which they received by mail. CGM was initiated with a certified diabetes care and education specialist providing instruction via videoconference or phone. The primary outcome was days per week of CGM use during the last 4 weeks. Hemoglobin A1c (HbA1c) was measured at baseline and 12 weeks. Participant self-reported outcome measures were also evaluated. All 34 participants (mean age, 46 ± 18 years; 53% female, 85% white) were using CGM at 12 weeks, with 94% using CGM at least 6 days per week during weeks 9 to 12. Mean HbA1c decreased from 8.3 ± 1.6 at baseline to 7.2 ± 1.3 at 12 weeks (P < .001) and mean time in range (70-180 mg/dL, 3.9-10.0 mmol/L) increased from an estimated 48% ± 18% to 59% ± 20% (P < .001), an increase of approximately 2.7 hours/day. Substantial benefits of CGM to quality of life were observed, with reduced diabetes distress, increased satisfaction with glucose monitoring, and fewer perceived technology barriers to management. Remote CGM initiation was successful in achieving sustained use and improving glycemic control after 12 weeks as well as improving quality-of-life indicators. If widely implemented, this telehealth approach could substantially increase the adoption of CGM and potentially improve glycemic control for people with diabetes using insulin.
Keywords: telehealth, continuous glucose monitoring, T1D, T2D
Telehealth provides an opportunity to increase access to continuous glucose monitoring (CGM) and empower patients living with diabetes by providing more data for self-management. Despite the compelling evidence of the benefits of CGM [1-15], many individuals with type 1 diabetes (T1D) or type 2 diabetes (T2D) using insulin have not adopted the use of CGM [6, 16]. Many adults with T1D and most adults with T2D do not regularly see an endocrinologist and receive their diabetes care from their primary care provider [17-19]. Limited CGM awareness among primary care providers may be a factor responsible for the low rate of CGM adoption, particularly in areas where geography affects access to endocrinologists [19].
Training for the initiation of CGM has become easier with advances in CGM technology, including easily inserted, factory-calibrated sensors, making it potentially feasible to initiate CGM through a telemedicine approach. The ability for participants to view their reports on an ongoing basis provides them the opportunity to identify trends and make adjustments, while the ability to share CGM data with providers presents the opportunity to review data not only during but also between visits. With the goal of expanding access to CGM, we conducted a feasibility study to assess whether adults with T1D or T2D using insulin could be trained virtually, outside the routine practice where the individual receives diabetes care, to initiate and use CGM as part of their diabetes self-management. This work predates the current COVID-19 pandemic, but it is even more pertinent now when a telehealth approach is needed to afford access and continuity of care.
1. Materials and Methods
The study was conducted virtually, outside the clinical care office setting. The protocol and informed consent forms were approved by an institutional review board. Electronic informed consent was obtained from each participant. The protocol and study data set are available at www.jaeb.org.
A. Participants
Eligible participants were US residents age 18 years or older with T1D or T2D, using either an insulin pump or basal-bolus insulin injection therapy, and who had not used real-time CGM during the 2 years prior to enrollment. Participants were required to have a smartphone and access to a computer with internet access that could be used to upload the CGM receiver. Exclusions included pregnancy and renal dialysis. Participants were recruited primarily through the Wisconsin Research and Education Network (WREN), a practice-based research network of primary care practices in Wisconsin. Potentially eligible participants received a letter with information about the study that included a link to the electronic consent form and a postcard that they could return to opt out of further information about the study. Interested individuals were able to have a live chat or arrange for a phone call to ask questions about the study as part of the informed consent process. For those who did not consent or opt out, WREN staff followed up by phone to share additional information about the study.
B. Study Procedures
Enrolled participants were assigned a Certified Diabetes Care and Education Specialist (CDCES) who served as the participant’s primary contact during the 12-week study. During the study, the CDCES provided CGM education and training and was a resource for basic diabetes management education. CDCES interactions with participants occurred through video training sessions, texts, emails, and/or phone calls. During the initial contact with the CDCES, general information about CGM was provided to the participant, along with information about the 2 CGM systems used in the study (Dexcom G6 CGM and Abbott FreeStyle Libre). These systems were selected for study inclusion because both have sensors that are factory calibrated and are Food and Drug Administration–approved for dosing insulin without blood glucose monitoring testing. A brief questionnaire was administered to help guide recommendation of a specific CGM system for the participant, but participants were able to choose the CGM system that they preferred. After CGM system selection, the CGM system and other study supplies were shipped to the participant, who used the selected CGM system throughout the study.
Participants were scheduled to complete 3 remote training sessions with the CDCES. The initial training session content covered CGM initiation, including sensor insertion, alerts and alarms (if applicable), uploading data, and visualizing data. After 2 weeks, the second training session was provided. This session included training on how to use data visualization tools and how to use the CGM data to make self-management changes in insulin dosing, meals, and exercise. The third training session, conducted at 4 weeks, included additional CGM instruction to assist participants with individualizing CGM use and provided another opportunity to troubleshoot any concerns or issues. The training approach was individualized to accommodate comfort with technology use. Interim weekly contacts with the CDCES were scheduled to encourage ongoing review of CGM data; interim contacts were initiated by the CDCES or by the participant if assistance was needed. The CDCES reviewed the downloaded glucose data at each contact to assist with self-management. If the CDCES identified a potential need for a change in insulin type or dosing, the recommendation was relayed to the participant’s primary diabetes care provider(s). At the end of 3 months of follow-up, participants received a summary report of their CGM and hemoglobin A1c (HbA1c) results, which they could share with their primary diabetes care providers.
C. Outcome Measures
The primary outcome was days per week of CGM use during the last 4 weeks of the study. To obtain HbA1c values, a kit was sent to participants at baseline and at 12 weeks to collect a fingerstick blood sample, which was returned by mail to the Advanced Research and Diagnostic Laboratory at the University of Minnesota for HbA1c measurement using the Tosoh Automated Glycohemoglobin Analyzer HLC-723G8.
Participants self-reported outcome measures, which included hypoglycemia confidence, hypoglycemia awareness, perceptions regarding diabetes technology and glucose monitoring, and diabetes distress, were completed at enrollment and at 12 weeks [20-24]. The CGM self-efficacy questionnaire was completed at 4 and 12 weeks [25].
Participants were asked monthly during study follow-up to report whether they experienced any adverse events, including severe hypoglycemia (defined as an event that required assistance from another person because of altered consciousness), diabetic ketoacidosis, and hospitalizations. Participants were also asked to report CGM device issues.
2. Statistical Methods
In the calculation of CGM usage, a participant was considered to have used the CGM on a given calendar day if he or she had at least 12 hours of CGM data available that day. All analyses were restricted to participants who initiated CGM use at the start of the study. Summary statistics were reported for CGM metrics calculated on a participant level and a month level in addition to HbA1c at baseline and at the end of the study. Baseline percentage time in range and mean glucose were estimated from HbA1c [26]. For normally distributed variables, mean and SD are reported; otherwise, median and interquartile range are reported. Summary statistics are also reported for questionnaire scores at baseline and at each month. Paired t tests were used to test for significance of the mean change in HbA1c between baseline and postintervention measurement, and Wilcoxon signed rank tests were used to test whether the mean change in questionnaire scores differed from baseline to 12 weeks. Analysis of variance was performed to compare glycemic differences between insulin modality subgroups and type of diabetes subgroups; for skewed outcomes, a Wilcoxon rank sum test was performed. All statistical tests were 2-tailed.
3. Results
Of 35 enrolled participants, 1 withdrew from the study shortly after enrollment prior to starting CGM. All 34 participants completed the 3 planned training sessions; the majority of these key trainings were conducted through video call. The initial training took the most time and subsequent trainings less time. Participants averaged 8 interim contacts during follow-up; interim contacts were conducted through phone call, text, or email depending on participant preference. The other 34 participants were a mean age of 46 ± 18 years; 18 (53%) were female and 29 (85%) were white. Twenty-seven (79%) had T1D, 7 (21%) had T2D, median duration of diabetes was 22 years (interquartile range, 11-30 years), and mean baseline HbA1c was 8.3 ± 1.6 (Table 1). Only one-third of those enrolled listed an endocrinologist as their health care provider.
Table 1.
Participant characteristics at enrollmenta
| Overall | T1D | T2D | Pump | Injections | |
|---|---|---|---|---|---|
| (N = 34) | (N = 27) | (N = 7) | (N = 7) | (N = 27) | |
| Age,y, mean ± SD | 46 ± 18 | 42 ± 18 | 60 ± 7 | 41 ± 19 | 47 ± 17 |
| Sex—female, n (%) | 18 (53%) | 14 (52%) | 4 (57%) | 5 (71%) | 13 (48%) |
| Race/Ethnicity n (%) | |||||
| White | 29 (85%) | 25 (93%) | 4 (57%) | 6 (86%) | 23 (85%) |
| Black/African American | 3 (9%) | 0 (0%) | 3 (43%) | 0 (0%) | 3 (11%) |
| Unknown/not reported | 2 (6%) | 2 (7%) | 0 (0%) | 1 (14%) | 1 (4%) |
| BMI median (quartiles) | 28.9 (25.2, 36.1) | 27.0 (24.4, 33.5) | 37.8 (32.2, 44.0) | 27.8 (21.2, 35.0) | 29.3 (25.2, 37.8) |
| Education level, n (%) | |||||
| < Bachelor degree | 17 (50%) | 12 (44%) | 5 (71%) | 5 (71%) | 12 (44%) |
| Bachelor degree | 11 (32%) | 11 (41%) | 0 (0%) | 2 (29%) | 9 (33%) |
| > Bachelor degree | 6 (18%) | 4 (15%) | 2 (29%) | 0 (0%) | 6 (22%) |
| Annual household income, $, n (%) | |||||
| < 50 000 | 14 (41%) | 11 (41%) | 3 (43%) | 5 (71%) | 9 (33%) |
| 50 000-< 100 000 | 11 (32%) | 9 (33%) | 2 (29%) | 1 (14%) | 10 (37%) |
| ≥ 100 000 | 9 (26%) | 7 (26%) | 2 (29%) | 1 (14%) | 8 (30%) |
| Diabetes duration, y, median (quartiles)b | 22 (11, 30) | 23 (11, 30) | 18 (5, 24) | 26 (14, 47) | 20 (10, 29) |
| HbA1c mean ± SDc | 8.3 ± 1.6 | 8.4 ± 1.7 | 8.1 ± 1.3 | 8.1 ± 2.1 | 8.4 ± 1.5 |
| Estimated % time in range (70-180 mg/dL)d | 48% ± 18% | 48% ± 19% | 51% ± 14% | 51% ± 24% | 48% ± 17% |
| Estimated mean glucose, mg/dLd,e | 196 ± 46 | 197 ± 49 | 189 ± 36 | 190 ± 60 | 197 ± 43 |
Abbreviations: HbA1c, hemoglobin A1c; T1D, type 1 diabetes; T2D, type 2 diabetes.
aExcludes one individual who withdrew from the study shortly after enrollment and did not provide any data.
bMissing for 2 participants with T2D.
cSI conversions: To convert HbA1c to SI units of mmol/mol, multiply HbA1c percentage value × 10.93 and subtract 23.5 from the product.
dEstimated from Beck et al [26].
eSI conversion: To convert glucose to mmol/L, multiply values × 0.0555.
A. Continuous Glucose Monitoring System Use and Changes in Diabetes Management
The Dexcom G6 sensor was used by 31 of the 34 participants and the Abbott FreeStyle Libre by 3. CGM system use was high in the final 4 weeks of the study, with 94% of participants using the CGM at least 6 days per week (Table 2). CGM use was consistent throughout the study; all 34 participants averaged at least 6 days per week of CGM use over the 12 weeks (Table 2).
Table 2.
Continuous glucose monitoring use during the studya
| Overall (N = 34) |
Baseline HbA1c | |||||||
|---|---|---|---|---|---|---|---|---|
|
< 7.5%
(N = 11) |
7.5%-< 9.0%
(N = 11) |
≥ 9.0%
(N = 12) |
T1D
(N = 27) |
T2D
(N = 7) |
Pump
(n = 7) |
Injections
(N = 27) |
||
| Last 4 weeks of study | ||||||||
| Quantity of data available median (quartiles) | ||||||||
| % of possible data available | 96% (93%, 98%) |
96% (93%, 98%) |
97% (97%, 98%) |
94% (85%, 96%) |
97% (95%, 98%) |
93% (86%, 94%) |
97% (96%, 99%) |
96% (93%, 98%) |
| H of data available | 647 (623, 659) |
648 (623, 660) |
653 (649, 662) |
628 (574, 646) |
650 (635, 662) |
622 (579, 629) |
653 (647, 662) |
643 (622, 659) |
| D/wk of CGM use median (quartiles), n (%) | 7.0 (6.7, 7.0) | 7.0 (7.0, 7.0) | 7.0 (6.7, 7.0) | 6.7 (6.5, 7.0) | 7.0 (6.7, 7.0) | 6.7 (6.5, 7.0) | 7.0 (7.0, 7.0) | 7.0 (6.7, 7.0) |
| 7 d/wk | 29 (85%) | 10 (91%) | 10 (91%) | 9 (75%) | 25 (93%) | 4 (57%) | 7 (100%) | 22 (81%) |
| 6 d/wk | 3 (9%) | 1 (9%) | 1 (9%) | 1 (8%) | 0 (0%) | 3 (43%) | 0 (0%) | 3 (11%) |
| ≤ 5 d/wk | 2 (6%) | 0 (0%) | 0 (0%) | 2 (17%) | 2 (7%) | 0 (0%) | 0 (0%) | 2 (7%) |
| Entire study | ||||||||
| Quantity of data available median (quartiles) | ||||||||
| % of possible data available | 95% (93%, 97%) |
97% (95%, 98%) |
97% (93%, 98%) |
92% (89%, 94%) |
97% (94%, 98%) |
90% (89%, 96%) |
97% (95%, 98%) |
95% (90%, 97%) |
| H of data available | 1933 (1874, 1972) |
1957 (1928, 1972) |
1963 (1885, 1974) |
1853 (1790, 1912) |
1957 (1895, 1973) |
1818 (1798, 1943) |
1962 (1913, 1985) |
1928 (1818, 1972) |
| D/wk of CGM use median (quartiles), n (%) | 6.9 (6.7, 7.0) | 7.0 (6.9, 7.0) | 6.9 (6.7, 7.0) | 6.7 (6.3, 6.8) | 6.9 (6.7, 7.0) | 6.7 (6.2, 6.9) | 7.0 (6.9, 7.0) | 6.8 (6.7, 7.0) |
| 7 d/wk | 29 (85%) | 11 (100%) | 10 (91%) | 8 (67%) | 24 (89%) | 5 (71%) | 7 (100%) | 22 (81%) |
| 6 d/wk | 5 (15%) | 0 (0%) | 1 (9%) | 4 (33%) | 3 (11%) | 2 (29%) | 0 (0%) | 5 (19%) |
| ≤ 5 d/wk | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Abbreviations: CGM, continuous glucose monitoring; HbA1c, hemoglobin A1c; T1D, type 1 diabetes; T2D, type 2 diabetes.
aIn all analyses of CGM use, the warm-up period was ignored. This leads to a slight underestimate of use.
During the course of the study, recommended changes in insulin type or dosing were made by the CDCES and relayed to the participant’s primary diabetes care provider for 13 (38%) of the 34 participants (N = 6 for 1 time, N = 4 for 2 times, N = 3 for 3 times).
B. Glycemic Outcomes
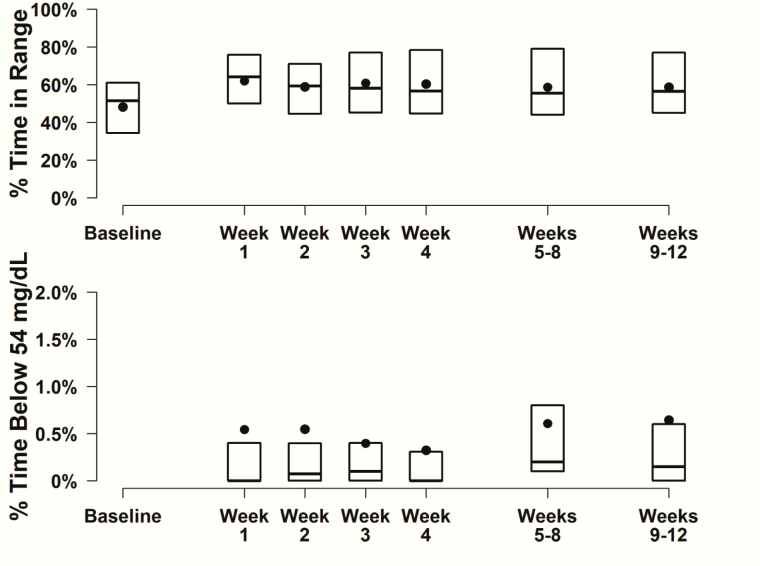
Mean HbA1c decreased from 8.3 ± 1.6 at baseline to 7.2 ± 1.3 at 12 weeks (P < .001). CGM metrics were consistent with this finding, with mean time in range (70-180 mg/dL, 3.9-10.0 mmol/L) increasing from an estimated 48% ± 18% at baseline to CGM-measured 59% ± 20% (P < .001) and mean glucose concentration decreasing from an estimated 196 ± 46 mg/dL (10.9 ± 2.6 mmol/L) at baseline to CGM-measured 170 ± 36 mg/dL (9.4 ± 2.0 mmol/L) (P < .001) over the 12 weeks of the study. Other CGM metrics are shown in Table 3. The increase in time in range was seen in the first week and was sustained through the rest of the 12 weeks of follow-up (Fig. 1).
Table 3.
Continuous glucose monitoring and glycemic control metrics during the study
| Overall (N = 34) |
Baseline HbA1c | |||
|---|---|---|---|---|
|
<7.5%
(N = 11) |
7.5% to < 9.0%
(N = 11) |
≥9.0%
(N = 12) |
||
| HbA1ca,b (%), mean ± SD | ||||
| Baseline | 8.3 ± 1.6 | 6.7 ± 0.7 | 8.1 ± 0.5 | 10.2 ± 1.0 |
| 12 wk | 7.2 ± 1.3 | 6.2 ± 0.7 | 7.1 ± 1.1 | 8.4 ± 1.1 |
| Change from baselinec | –1.1 ± 1.2 | –0.4 ± 0.9 | –1.0 ± 1.1 | –1.8 ± 1.1 |
| Time in range 70-180 mg/dL (%), mean ± SD | ||||
| Baselined | 48% ± 18% | 67% ± 8% | 51% ± 5% | 28% ± 10% |
| Over 12 wk | 59% ± 20% | 74% ± 15% | 65% ± 16% | 41% ± 14% |
| Change from baselinec | 11% ± 16% | 7% ± 16% | 13% ± 18% | 13% ± 16% |
| Mean glucosee (mg/dL) mean ± SD | ||||
| Baselined | 196 ± 46 | 148 ± 20 | 188 ± 13 | 246 ± 27 |
| Over 12 wk | 170 ± 36 | 145 ± 27 | 159 ± 24 | 203 ± 28 |
| Change from baselinec | –26 ± 34 | –3 ± 29 | –29 ± 29 | –44 ± 33 |
| Coefficient of variation over 12 wk, mean ± SD | 35% ± 6% | 33% ± 5% | 35% ± 6% | 37% ± 6% |
| Hypoglycemia median (quartiles) | ||||
| % time < 70 mg/dL over 12 wk (%) | 1.4% (0.8%, 3.1%) | 2.9% (1.2%, 3.7%) | 1.4% (0.5%, 3.1%) | 1.1% (0.7%, 2.2%) |
| % time < 54 mg/dL over 12 wk (%) | 0.2% (0.1%, 0.6%) | 0.5% (0.1%, 0.7%) | 0.1% (0.0%, 0.3%) | 0.2% (0.1%, 0.6%) |
| Hyperglycemia median (quartiles) | ||||
| % time > 180 mg/dL over 12 wk (%) | 41% (20%, 52%) | 16% (10%, 44%) | 34% (22%, 50%) | 55% (47%, 63%) |
| % time > 250 mg/dL over 12 wk (%) | 11% (3%, 16%) | 3% (1%, 15%) | 8% (6%, 12%) | 22% (15%, 36%) |
Abbreviations: CGM, continuous glucose monitoring; HbA1c, hemoglobin A1c; T1D, type 1 diabetes; T2D, type 2 diabetes.
aExcludes one participant in the ≥ 9.0% subgroup who did not have an available measurement at both baseline and 12 weeks.
bSI conversions: To convert HbA1c to SI units of mmol/mol, multiply HbA1c percentage value × 10.93 and subtract 23.5 from the product.
c P value for overall change from baseline is less than .001.
dEstimated from Beck et al [26].
eSI conversion: To convert glucose to mmol/L, multiply values × 0.0555.
Figure 1.
Continuous glucose monitoring metrics over time (N = 34). Box plots displaying the distribution of percentage time in range and percentage time below 54 mg/dL at baseline and through week 12. Black circles represent mean values. Baseline time in range was estimated from Beck et al [26].
The magnitude of change in HbA1c from baseline to 12 weeks was associated with baseline HbA1c, with a greater decrease observed with higher baseline HbA1c (P = .02); mean HbA1c decreased by 1.8% in the 12 participants with baseline HbA1c greater than or equal to 9.0%, by 1.0% in the 11 participants with baseline HbA1c 7.5% to 8.9%, and by 0.4% in the 11 participants with baseline HbA1c less than 7.5%. Mean HbA1c decreased from baseline by 1.3% for the 27 participants with T1D, by 0.3% for the 7 participants with T2D, by 1.6% for the 7 T1D pump users, and by 1.0% for the 27 participants using injections.
CGM-measured hypoglycemia was low throughout the 12 weeks (Fig. 1), but there were no comparator baseline data. There was a suggestion of improved hypoglycemia awareness at 12 weeks compared with baseline (71% with awareness [Clarke Hypoglycemia Awareness Survey score ≤ 2] at baseline vs 82% at 12 weeks, P = .02).
C. Participant-Reported Outcomes
Participants indicated a high degree of satisfaction with the use of CGM. Each participant-reported outcome questionnaire completed at baseline and 12 weeks showed a significant positive change. These included increases in glucose monitoring satisfaction, trust, hypoglycemia confidence, and diabetes technology attitudes; and decreases in diabetes management distress, emotional burden, and behavioral burden (Table 4).
Table 4.
Participant-reported outcomesa
| Baseline (N = 34) |
Month 1 (N = 34) | Month 2b (N = 33) |
Month 3 (N = 34) | P for change from baseline to month 3d | |
|---|---|---|---|---|---|
| CGM Self Efficacy Scale Mean Score | NA | 5.3 (4.7, 5.8) | NA | 5.5 (5.3, 6.0) | NA |
| Median (quartiles) (range, 0-6) | |||||
| Diabetes Distress Scale Management | 1.8 (0.8, 2.5) | 0.6 (0.3, 1.3) | 0.5 (0.3, 1.0) | 0.5 (0.3, 1.3) | < .001 |
| Distress Subscale Mean Score | |||||
| Median (quartiles) (range, 0-5) | |||||
| Glucose Monitoring Satisfaction Survey | 3.2 ± 0.6 | 3.7 ± 0.8 | 3.9 ± 0.6 | 3.9 ± 0.7 | < .001 |
| Mean score | |||||
| Mean ± SD (range, 1-5) | |||||
| Openness Subscale Mean score | 2.6 ± 0.7 | 3.4 ± 0.9 | 3.6 ± 0.6 | 3.7 ± 0.9 | < .001 |
| Mean ± SD (range, 1-5) | |||||
| Emotional Burden Subscale Mean Score | 2.6 ± 0.8 | 2.3 ± 0.9 | 2.1 ± 0.8 | 2.2 ± 0.8 | .003 |
| Mean ± SD (range, 1-5) | |||||
| Behavioral Burden Subscale Mean Score | 2.9 (2.0, 3.3) | 1.5 (1.0, 2.8) | 1.8 (1.3, 2.0) | 1.9 (1.0, 2.5) | < .001 |
| Median (quartiles) (range, 1-5) | |||||
| Trust Subscale Mean Score | 3.7 (3.0, 4.0) | 4.0 (3.0, 4.3) | 4.0 (3.0, 4.7) | 4.0 (3.3, 5.0) | .02 |
| Median (quartiles) (range, 1-5) | |||||
| Hypoglycemia Confidence Survey Mean | 2.1 (1.4, 2.6) | 2.4 (2.2, 2.8) | NA | 2.3 (2.0, 2.9) c | < .001 |
| Score | |||||
| Median (quartiles) (range, 0-3) | |||||
| Diabetes Technology Attitudes Survey | 4.0 (3.6, 4.4) | 4.4 (4.0, 5.0) | NA | 4.8 (4.0, 5.0) | < .001 |
| Mean score | |||||
| Median (quartiles) (range, 1-5) | |||||
| Benefits and Barriers of CGM Survey | 2.2 (1.8, 2.7) | 1.7 (1.4, 2.0) | NA | 1.5 (1.2, 2.2) | < .001 |
| Mean score | |||||
| Median (quartiles) (range, 1-5) |
Abbreviations: CGM, continuous glucose monitoring; HbA1c, hemoglobin A1c; NA, not available.
aShaded rows denote questionnaires for which a higher score is better.
bOne participant did not complete questionnaires at month 2.
cOne participant who completed the questionnaires at month 3 did not complete the Hypoglycemia Confidence Survey.
dBased on a Wilcoxon signed rank test. For the Clarke Hypoglycemia Unawareness Survey; the Fisher exact test was used instead.
D. Safety Outcomes
There was one episode of diabetic ketoacidosis unrelated to CGM use. There were no severe hypoglycemia events.
4. Discussion
Telehealth has the potential to replace clinic visits for the management of diabetes. The COVID-19 pandemic has accelerated the adoption of telemedicine visits for people with diabetes and reimbursement for health care providers. Glucose profiles obtained with CGM can greatly facilitate the ability of health care providers to assess glycemic control and recommend adjustments in diabetes management. In this study, we demonstrated that a virtual approach outside the clinic can be used for successful CGM initiation and incorporation into diabetes self-management for adults with T1D or T2D using insulin. CGM system use was extremely high after 3 months, and participants reported a very positive impact on quality-of-life measures. Additionally, there was a substantial reduction in HbA1c with a low frequency of hypoglycemia in most participants, with an increase of about 2.7 hours per day in the amount of time in range spent with glucose levels between 70 and 180 mg/dL (range, 3.9-10.0 mmol/L), which is the generally accepted target range for most adults with T1D or T2D (excluding pregnancy) [27]. It is noteworthy that the improvement in time in range was seen in the first week and then on average remained reasonably stable, suggesting there was an immediate benefit of starting CGM that was not necessarily enhanced with further training.
The amount of improvement in HbA1c and CGM-measured glycemic metrics was similar to that observed in clinic-based randomized trials [1, 8, 9, 11, 12, 28], but these results should be viewed in the context that this was a small feasibility study with no control group. The study included only adults with T1D or T2D using insulin; therefore, the results should not be applied to youth with diabetes or to patients with T2D who are not using insulin. Additionally, the number of participants with T2D was too small to draw separate conclusions related to the effect of CGM in T2D.
CGM use has been endorsed for patients with T1D by the American Diabetes Association, the American Association of Clinical Endocrinologists, and the International Society for Pediatric and Adolescent Diabetes. Despite recommendations, only a minority of patients with T1D or insulin-using T2D have incorporated CGM into their diabetes management. Reports suggest that a majority of adults with T1D or T2D do not regularly see an endocrinologist, and their diabetes management is prescribed by a primary care provider [17-19]. This undoubtedly has affected the CGM adoption rate because many primary care providers likely have limited knowledge of CGM and do not have the resources to introduce and incorporate CGM into patient diabetes self-management. This study has attempted to address this gap by demonstrating that CGM can be successfully initiated outside the practice setting. We plan to expand this approach in a larger study across the United States to assess the feasibility and efficacy of establishing a virtual diabetes clinic with a focus on introduction of and ongoing use of CGM technology.
Acknowledgments
This work was accepted for oral presentation at Advanced Technologies and Treatments for Diabetes, Madrid, Spain, February 2020.
Financial Support: This work was supported by The Leona M. and Harry B. Helmsley Charitable Trust.
Glossary
Abbreviations
- CDCES
Certified Diabetes Care and Education Specialist
- CGM
continuous glucose monitoring
- HbA1c
hemoglobin A1c
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- WREN
Wisconsin Research and Education Network
Additional Information
Disclosure Summary: R.L.G., N.J.C., P.C., B.B., and H.S. report grants from the Leona M. and Harry B. Helmsley Charitable Trust. D.K. reports grants and personal fees from Abbott Diabetes Care and Dexcom, Inc. R.W.B. reports grants from the Leona M. and Harry B. Helmsley Charitable Trust and grants and nonfinancial support from Bigfoot Biomedical, Tandem Diabetes Care, Dexcom, Inc, and Ascenia Diabetes Care. R.M.B. reports grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Abbott Diabetes Care, Eli Lilly, Hygieia, Johnson and Johnson, Medtronic, Novo Nordisk, Roche Diabetes Care, Sanofi, Dexcom, Inc, Onduo, and United Healthcare. A.H. has nothing to disclose. K.H. reports grants from Dexcom, Inc and personal fees from Lifescan Diabetes Institute and Med-IQ, LLC. M.L.J. reports grants from Abbott Diabetes Care and Dexcom, Inc. T.M. reports personal fees from JCHR. B.A.O. reports stock ownership in Abbott Laboratories. R.S.W. reports grants and personal fees from JCHR, Medtronic, Eli Lilly, Tolerion Inc, Boehringer Ingelheim, Diasome Pharmaceuticals, and Mylan GmbH. S.M.O. reports grants, personal fees, and nonfinancial support from the Leona M. and Harry B. Helmsley Charitable Trust, Children with Diabetes, Juvenile Diabetes Research Fund (JDRF), Med-IQ, LLC, Bayer Pharmaceuticals, Xeris Pharmaceuticals, Highmark, NIDDK, and the Beryl Institute. T.K.O. reports grants, personal fees, and nonfinancial support from the Leona M. and Harry B. Helmsley Charitable Trust, Children with Diabetes, JDRF, Xeris Pharmaceuticals, MannKind Corporation, the Beryl Institute, and NIDDK. G.A. reports grants and personal fees from JCHR, Dexcom, Inc, Eli Lilly, Medtronic, Insulet Corporation, and Novo Nordisk.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Beck RW, Riddlesworth T, Ruedy K, et al. ; DIAMOND Study Group Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. [DOI] [PubMed] [Google Scholar]
- 2. Beck RW, Riddlesworth TD, Ruedy K, et al. ; DIAMOND Study Group Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365-374. [DOI] [PubMed] [Google Scholar]
- 3. Bergenstal RM, Tamborlane WV, Ahmann A, et al. ; STAR 3 Study Group Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320. [DOI] [PubMed] [Google Scholar]
- 4. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. [DOI] [PubMed] [Google Scholar]
- 5. Cox DJ, Taylor AG, Moncrief M, et al. Continuous glucose monitoring in the self-management of type 2 diabetes: a paradigm shift. Diabetes Care. 2016;39(5):e71-e73. [DOI] [PubMed] [Google Scholar]
- 6. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367-1377. [DOI] [PubMed] [Google Scholar]
- 9. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Hirsch I, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane W, Beck RW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464-1476. [DOI] [PubMed] [Google Scholar]
- 11. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. [DOI] [PubMed] [Google Scholar]
- 12. Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37(8):2114-2122. [DOI] [PubMed] [Google Scholar]
- 13. Polonsky WH, Hessler D. What are the quality of life-related benefits and losses associated with real-time continuous glucose monitoring? A survey of current users. Diabetes Technol Ther. 2013;15(4):295-301. [DOI] [PubMed] [Google Scholar]
- 14. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017;31(1):280-287. [DOI] [PubMed] [Google Scholar]
- 15. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixon RF, Zisser H, Layne JE, et al. A virtual type 2 diabetes clinic using continuous glucose monitoring and endocrinology visits. [Published online ahead of print November 25, 2019.] J Diabetes Sci Technol. Doi: 10.1177/1932296819888662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Healy AM, Shubrook JH, Schwartz FL, Cummings DM, Drake AJ, Tanenberg RJ. III. Endocrinologists’ opinions of diabetology as a primary care subspecialty. Clin Diabetes. 2018;36(2):168-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care. 2019;42(12):2220-2227. [DOI] [PubMed] [Google Scholar]
- 19. Vigersky RA, Fish L, Hogan P, et al. The clinical endocrinology workforce: current status and future projections of supply and demand. J Clin Endocrinol Metab. 2014;99(9):3112-3121. [DOI] [PubMed] [Google Scholar]
- 20. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18(4):517-522. [DOI] [PubMed] [Google Scholar]
- 21. Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes technology: uptake, outcomes, barriers, and the intersection with distress. J Diabetes Sci Technol. 2016;10(4):852-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polonsky W, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28(3):626-631. [DOI] [PubMed] [Google Scholar]
- 23. Polonsky WH, Fisher L, Hessler D, Edelman SV. Investigating hypoglycemic confidence in type 1 and type 2 diabetes. Diabetes Technol Ther. 2017;19(2):131-136. [DOI] [PubMed] [Google Scholar]
- 24. Tansey M, Laffel L, Cheng J, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Satisfaction with continuous glucose monitoring in adults and youths with type 1 diabetes. Diabet Med. 2011;28(9):1118-1122. [DOI] [PubMed] [Google Scholar]
- 25. Rasbach LE, Volkening LK, Markowitz JT, Butler DA, Katz ML, Laffel LM. Youth and parent measures of self-efficacy for continuous glucose monitoring: survey psychometric properties. Diabetes Technol Ther. 2015;17(5):327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019;13(4):614-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]