Abstract
Effective suppression of container-inhabiting Asian Tiger [Aedes albopictus (Skuse)] (Diptera: Culicidae) and yellow fever [Aedes aegypti (L.)] (Diptera: Culicidae) mosquitoes presents one of the most intractable problems for modern mosquito control. Traditional tools often fail to control populations of these mosquito species, and are prohibitively expensive or have negative environmental impacts. Novel approaches and tools are urgently needed for integrated container-inhabiting mosquito management programs. One of the most promising techniques is autodissemination. We present the results of a long-term large-scale study conducted in a temperate urbanized environment representing typical Ae. albopictus habitats. Three treatment sites with autodissemination stations and three nearby reference sites were monitored for eggs, immature, and adult mosquitoes over a period of 3 yr from 2014 to 2016. Elevated larval and pupal mortality of 12–19% on average was the most notable outcome in sentinel cups of the treatment sites. The number of eggs in the treatment sites was significantly reduced in 2014, but not in 2015 or 2016. Adult populations remained similar in treatment and reference sites throughout the study. The impact of autodissemination on mosquito populations was lower than reported by previous investigations. Technical and logistical problems associated with wider coverage and working in multiple urban neighborhoods contributed to reduced efficacy. Incorporating autodissemination with routine mosquito control operations and commercializing this methodology for general public use will require further research on combining this tool with other novel or conventional technologies.
Keywords: medical entomology, mosquito control, pest management
The most notable characteristic of the Asian Tiger mosquito [Aedes albopictus (Skuse)] (Diptera: Culicidae) is the overwhelming numbers of biting adults emerging from artificial containers near human habitation (Niebylski and Craig Jr 1994, Faraji et al. 2014). The dispersed nature of this species’ larval habitat poses a challenge for public and private mosquito control professionals (Richards et al. 2008; Rochlin et al. 2013; Unlu et al. 2013, 2014a). This challenge is especially acute in urban areas, where effective Ae. albopictus management is heavily dependent on community involvement (Kay and Vu 2005, Dowling et al. 2013, Jordan et al. 2017, Johnson et al. 2018). Intense public education and involvement can lead to temporary reduction in Asian Tiger mosquito infestations (Jordan et al. 2017, Johnson et al. 2018); however, most studies demonstrate little to no sustained effects in the absence of community-wide active suppression by mosquito control professionals (Schreiber and Morris 1995, Bartlett-Healy et al. 2011, Fonseca et al. 2013, Healy et al. 2014, Faraji and Unlu 2016).
A key operational problem with artificial container remediation on private properties is locating immature mosquito habitats within a reasonable period (Dowling et al 2013). Many habitats are cryptic or inaccessible, e.g., corrugated extension spouts in New Jersey’s residential areas (Unlu et al. 2014a). The cryptic nature of Ae. albopictus larval habitats severely limits the effectiveness of efforts to reduce adult biting populations (Unlu et al. 2016). Targeted delivery of control agents into artificial containers has become a crucial priority for the development of new approaches to this difficult problem. Direct larvicide and pupacide applications usually achieved over 90% control against invasive Aedes species (Pérez et al. 2007, Nelder et al. 2010, Ritchie et al. 2010, Farajollahi et al. 2013); however, these results are only applicable to the rare situation when all individual containers are exposed and can be easily targeted. The direct method is ineffective for dispersed or cryptic habitats (Fonseca et al. 2013).
Most operational mosquito control involves larvicide delivery over large areas within a short duration (Unlu et al. 2018). To achieve this extensive coverage, autodissemination of insect growth regulators (IGRs), such as pyriproxyfen is a promising method (Devine et al. 2009, Mains et al. 2015, Chandel et al. 2016, Unlu et al. 2018). Autodissemination uses adult mosquitoes as a vehicle to treat containers that are inaccessible to direct treatments (Itoh 1995, Gaugler et al. 2012, Geden and Devine 2012). A proof of concept study by Itoh et al. (1995) used female Aedes aegypti (L.) (Diptera: Culicidae) treated with pyriproxyfen to inhibit subsequent adult emergence under laboratory conditions. Laboratory experiments also demonstrated significant adult emergence inhibition for Ae. albopictus mosquitoes (Gaugler et al. 2012). Small-scale field autodissemination studies showed significant reductions of Ae. albopictus and Ae. aegypti (Devine et al. 2009, Caputo et al. 2012, Suman et al. 2014, Abad-Franch et al. 2015, Mains et al. 2015). These field investigations generally focused on measuring juvenile stages in the containers within one small area of mosquito activity. The results were encouraging with approximately 20−70% pupal mortality reported. The adult mosquito populations were monitored in only two studies that produced mixed results (Ponlawat et al. 2013, Suman et al. 2014). Multi-year large-scale trials using autodissemination approach with pyriproxyfen focused on operational use under field conditions have not been reported.
Our field study was conducted in a temperate urban environment supporting high populations of the Asian Tiger mosquito. The study was integrated with operational mosquito control activities by a local mosquito control district from 2014 to 2016. The 2014 analysis evaluating modified autodissemination stations on Ae. albopictus populations was published (Unlu et al. 2018) and the full report is presented here.
Materials and Methods
Study Area
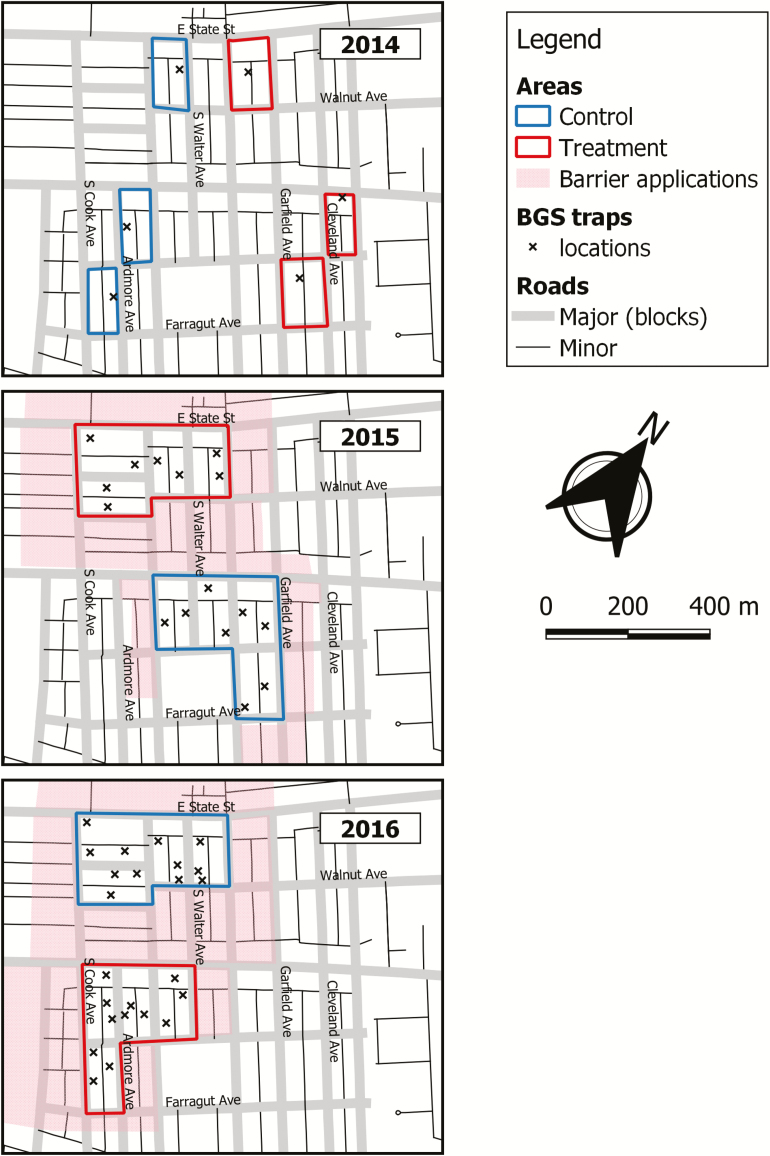
The study area in the City of Trenton, Mercer County, NJ (40°12′N, 74º44′W) has served for long-term monitoring of Ae. albopictus since 2008 (Fonseca et al. 2013; Unlu et al. 2013, 2014b). The 48.6 ha experimental area consisted of 1,251 properties; primarily single family homes and row houses with backyards, as well as commercial and public areas arranged in city blocks (average size ≈ 0.6 ha, range = 0.55–1.5 ha) surrounded by streets and intersected by alleyway between parallel parcels (Unlu et al. 2011, Farajollahi et al. 2012) (Fig. 1).
Fig. 1.
Map of the study area with treatment and reference blocks. BGS traps per block (2014: n = 1, 2015: n = 2, 2016: n = 3, where n is number of traps per each site), autodissemination stations, and oviposition cup locations are shown. Barrier treatments were conducted around treatment and reference blocks inside and outside of the study area.
Identification of Ae. albopictus Hot Spots for Treatment and Reference Sites
A city block was the spatial unit of analysis monitored with Biogents Sentinel (BGS) traps (Biogents AG, Regensburg, Germany) sited near the block’s center (Unlu and Farajollahi 2014). If five or more Ae. albopictus male and female adults were collected, the site was classified as a hot spot for operational mosquito control (Unlu et al. 2011, 2014c). Approximately, 8–10 out of 16 trapping sites were identified as hot spots each year. For the present study, the area was divided into treatment and reference sites using roads as natural borders (Fig. 1) (Unlu et al. 2016). Treatment and reference sites each contained three hot spot city blocks in 2014 and four hot spot city blocks each in 2015 and 2016 (Fig. 1).
Autodissemination Stations
Autodissemination Station Design
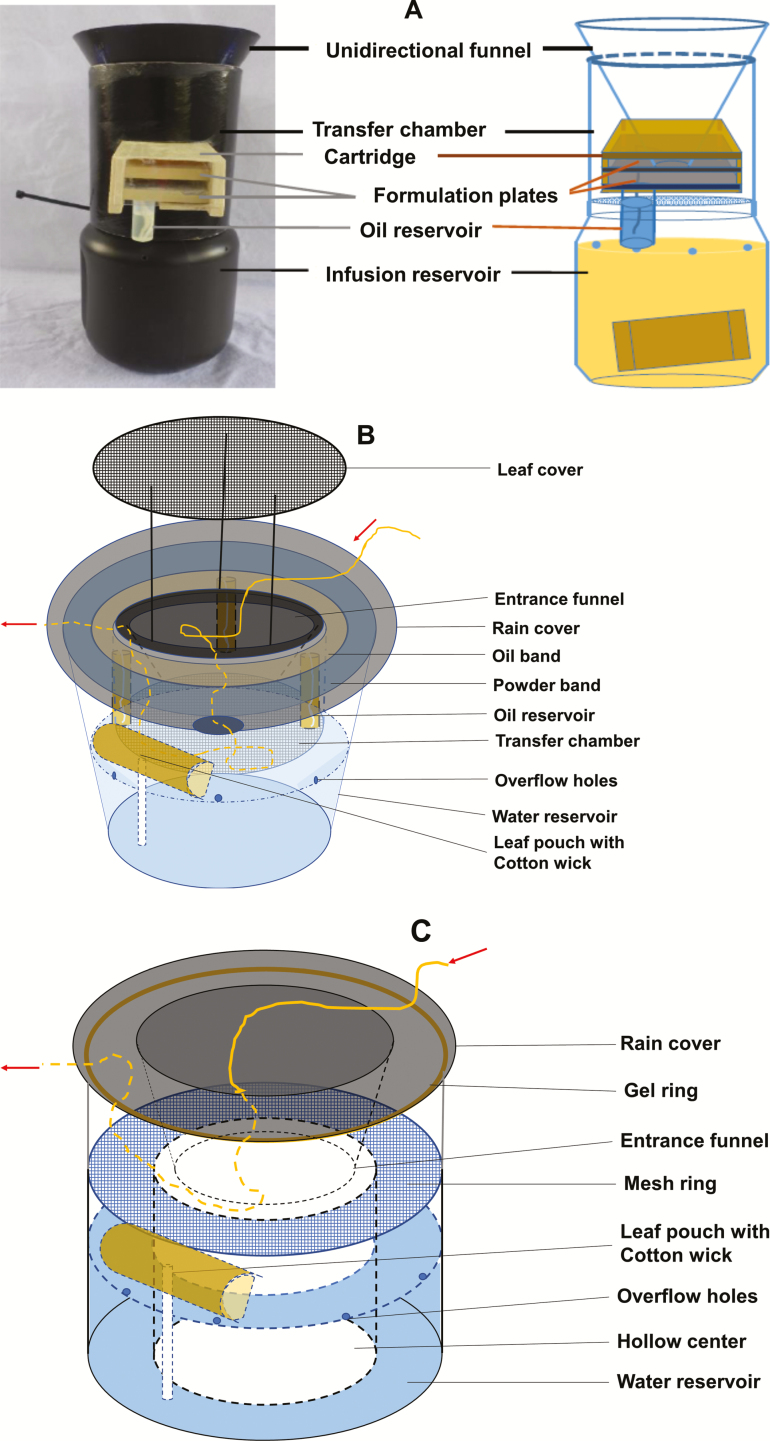
In 2014, autodissemination stations were modified from the earlier design (Gaugler et al. 2012) by incorporating a dual treatment mechanism (Wang et al. 2014) (Fig. 2A). A cartridge inserted into the middle of the chamber body contained two formulation plates (top and bottom, 55 × 50 mm) with oil and powder bands. The oil bands were coated with a formulation consisting of 20% pyriproxyfen active ingredient at 0.62 g of a.i. per station. The powder bands were coated with a formulation containing 60% pyriproxyfen at 0.42 g of a.i. per station (Unlu et al. 2017).
Fig. 2.
Autodissemination stations (ADS) design. ADS consisted of an infusion reservoir, transfer chamber, and a unidirectional funnel with different formulations and delivery systems. (A) 2014 design using a dual treatment mechanism. A cartridge inserted into the middle of the chamber body contained two formulation plates (top and bottom, 55 × 50 mm) each consisting of an oil and a powder bands. The oil bands were coated with 20% pyriproxyfen formulation a.i. (0.62 g/station) and the powder bands were coated with a powder formulation containing 60% pyriproxyfen a.i. (0.42 g/station). (B) 2015 design using a transfer plate consisting of oil and powder formulation bands on top of the reservoir. (C) 2016 design using a gel formulation containing 19% of pyriproxyfen a.i. coated into a narrow grove (5.6 mm width and 6 mm depth) in the gel ring.mm depth in the gel ring.
Further design improvements were implemented in 2015 (Fig. 2B). The reservoir was filled with water to the level of the overflow holes. An oak leaf pouch containing 15 g of shredded oak leaf and 30 g of wood chips was mounted right above the overflow holes. The leaf and wood materials were wrapped into a pouch using a paper towel (Procter & Gamble, Cincinnati, OH) and the pouch was restrained within a porous nylon bag. Chandel et al. (2016) used similar moisture pouch to attract gravid Ae. albopictus over 10 wk (Chandel et al. 2016). A cotton dental wick (15.24 × 0.8 cm, Richmond Premium Dental and Medical Products, Charlotte, NC) connected the pouch and water reservoir (Fig. 2B). The transfer chamber was modified into a mosquito-proof mesh chamber below the entrance funnel (Fig. 2B), which allowed the lure to penetrate and emit into the air. A plate containing oil and powder formulation bands was mounted on top of the reservoir. An oil band (2 cm wide) was created with polyester fabric (White Crepe Fabric Polyester, New York City) that wicked the oil formulation containing 20% pyriproxyfen. To prevent the oil formulation from drying out during a multi-week deployment, three vials containing 6 ml of oil formulation were fixed underneath the plate. A candle wick (EricX Light #24PLY/FT Braided Wick, Chancheng District, Foshan, Guangdong, China) ran down from the fabric polyester to the bottom of the oil reservoir to deliver the formulation into the fabric and promote season-long efficacy (Fig. 2B). The powder band (2 cm wide) was also made of polyester fabric (Low Loft Batting, Hobby Lobby, North Brunswick, NJ) coated with powder formulation with 60% a.i. On top of the funnel entrance, a leaf cover was supported with three 15 cm wires (Fig. 2B).
The stations were further modified for 2016 to address problems encountered in 2015, such as blockage by fallen leaves, powder formulation melting due to high temperatures, and oil formulation leakage onto the powder band. To prevent leaf blockage the center of the autodissemination station was hollowed out to allow leaves and debris to fall through and onto the ground (Fig. 2C). A mesh covered reservoir holding 1.5 liters of water and an infusion pouch as described for the 2015 design was mounted above the water level and below the mesh ring. Instead of using an oil-powder dual treatment platform, a new gel formulation containing 19% a.i. was coated into a narrow grove (5.6 mm width and 6 mm depth) in the gel ring (Fig. 2C). The exit gap between the gel and the rain cover was predetermined at 5 mm to restrict mosquitoes exit to walking instead of flying. Gravid females searching for oviposition sites enter from the top opening following the infusion lure. After failing to reach the water because of the mesh ring, the female mosquitoes exit from gap between the gel ring and the rain cover where they make contact with and become contaminated with the pyriproxyfen gel formulation.
Autodissemination Station Deployment
In treatment sites, 26–28 autodissemination stations per city block were deployed between July and November (Fig. 1). No stations were placed at the reference sites. All residents remained in the program and allowed technical personnel to service the stations throughout the study. The stations were fully serviced once (4 wk postdeployment) to check water and formulation status. At that time, new stations were deployed to replace missing or damaged stations, and water level was checked and the stations refilled as needed. Thereafter, autodissemination stations were serviced weekly by field crews to ensure proper function and to unblock the opening of clogged stations caused by leaves or spider webs in 2014 and 2015. In 2016, the gel formulation was checked and refreshed with a layer of oil formulation containing 20% of a.i. for the ones with dried gel surface every 4 wk. Because of the modifications made in 2016, clogged openings were no longer a problem.
Mosquito Field and Laboratory Surveillance
Eggs
For egg surveillance, previously published sampling protocol was used (Unlu et al. 2017). Briefly, black 360 ml cups (SpringStar, Inc., Woodinville, WA) were secured to a fence at the ground level. Five oviposition cups per each city block were deployed in treatment and reference sites (Fig. 1). Oviposition cups were checked for larvae to calculate the total number of eggs. Eggs were counted and then hatched in the laboratory at 26 ± 1°C and 16:8 (L:D) h. Third instars were identified to species using identification keys (Ward 2005).
Larvae and Pupae
Larval monitoring used sentinel cups (Uline, Pleasant Prairie, WI and ‘naturally’ occurring containers such as buckets, bird baths plant pot saucers, and tires (Unlu et al. 2013). In 2014, 10 sentinel cups with 250 ml of dechlorinated tap water were placed on each city block (Fig. 1A); the number was reduced to five in 2015–2016 (Fig. 1B and C). The sentinel cups were sampled and re-deployed at weekly (2014) or bi-weekly (2015–2016) intervals. Water was filtered to remove debris, organic materials, and immature mosquitoes. The filtrate was then used to conduct laboratory bioassays. Five ‘naturally’ occurring containers per each city block were also sampled using the same protocol. Water collected from sentinel cups and naturally occurring containers was used in laboratory larval bioassays as described by Wang et al. (2014). Briefly, 20 laboratory-raised third instar larvae were added to water removed from sentinel cups and ‘naturally’ occurring containers. Larval and pupal mortality and adult emergence were recorded to estimate efficacy. For laboratory controls, three cups of 20 larvae each were set up using distilled water.
Adults
In 2014, adult surveillance was initiated on 14 May and ended on 12 November when no mosquitoes were collected for over two previous weeks. To reduce adult Ae. albopictus influx into treatment and reference sites from the outside residential properties and alleyways, city blocks adjacent to the study area (Fig. 1) were treated with a water-dispersible granular (WDG) formulation of Bacillus thuringienis var. israelensis (Bti; VectoBac WDG, Valent BioSciences Corp., Libertyville, IL) prior to autodissemination station deployment. We used a CSM2 Mist Sprayer (Buffalo Turbine, Springville, NY) (Williams et al. 2014), for the Bti applications. The first application was on 27 July. Following the initial larviciding, ultra-low volume (ULV) adulticide applications using DUET Dual-action adulticide (Clarke Mosquito Control, Roselle, IL) were performed weekly around the treatment and reference sites until 26 September.
In 2015, adult surveillance was initiated 6 May and ended 13 November after no mosquitoes were collected for two consecutive weeks. In 2016, adult surveillance was initiated in 20 May and ended in 27 October. Our results showed area-wide larviciding and adulticiding conducted in 2014 did not prevent migration of Ae. albopictus into the study sites (Unlu et al. 2017). Therefore, to reduce the adult migration from neighboring areas, we conducted barrier treatment with Suspend Polyzone (deltamethrin, 4.75% a.i, Bayer Environmental Science, Research Triangle Park, NC) in 2015 and 2016. Barrier treatments were initiated a week prior to autodissemination station deployment and trapping, and repeated in 3 wk to all accessible properties, and alleyways. A backpack mist blower (model SR-450, Stihl Corp, Virginia Beach, VA) was used to treat vegetation below 3 m, leaf litter, and other resting habitats (e.g., under the porches and alcoves) (Unlu et al. 2017). For thick foliage, a hand tank with mist blower tip was inserted into the foliage to cover inner areas of dense vegetation (Trout et al. 2007, Unlu et al. 2017).
Data Analysis
To evaluate the effectiveness of the autodissemination stations on the field population of Ae. albopictus, a before-after-control-impact (BACI) design was employed (Stewart-Oaten et al. 1986). BACI design involves comparison between the treatment and reference sites with data collected multiple times before the treatment as well as afterward (Smith et al. 1993). A BACI analysis compares differences between treatment and reference sites, but not the absolute values, before and after an impact or a treatment. The BACI approach was specifically proposed to address naturally occurring changes and fluctuations; therefore, it is superbly suitable to analyze mosquito populations experiencing variability from year to year. So, if the changes in the mosquito populations are similar between treatment and reference sites regardless of their magnitude, there is no impact. However, if these changes differ, then they can be ascribed to the treatment effect (Smith et al. 1993). Since this project was initiated as a multi-year repeated experiment, it was analyzed accordingly to include all 3 yr of experimental data.
The full generalized linear mixed model contained treatment/reference, before/after time periods, and their interactions as fixed effects. Random effects included time (i.e., weeks within years) nested within location (i.e., blocks) to account for potential autocorrelation. The traps within each block were considered pseudoreplicates due to their proximity to each other.
The full model contained random intercept and random slope to account for differences among mosquito populations in different locations. The overall treatment effect was considered significant if the interaction term treatment*before/after application was significant (P < 0.05) in the full model. For egg data, the number of eggs per day was used as response variable in a model with a Gaussian distribution. For the adult data, the total number of mosquitoes collected was used as response variable in the model with negative binomial distribution because of overdispersion.
Laboratory bioassay mortality data were analyzed only post application. The full mixed effects model included the interaction term of treatment and year as the fixed effects, and samples nested within time points (i.e., year and week) as repeated random effects to account for potential autocorrelation and the differences in response among samples at different time points. Since the initial number of introduced larvae in each sample was constant (n = 20), the number of dead pupae instead of proportion was used as response variable in the model with negative binomial distribution because of overdispersion.
All statistical analyses used R v. 3.2.3 (R Development Core Team 2015) and the package lme4 v. 1.1–10 (Pinheiro et al. 2015) for mixed effects models (Bates et al. 2013). P-values were obtained by likelihood ratio tests comparing the full model with and without the effect in question (Crawley 2012). Posthoc tests were performed by planned contrasts with adjusted P-values, or by Tukey HSD test using the package multcomp v. 1.4–8. To check the model’s assumptions, residual plots were visually inspected for obvious deviations from homoscedasticity or normality.
Results
Egg and Adult Field Surveillance
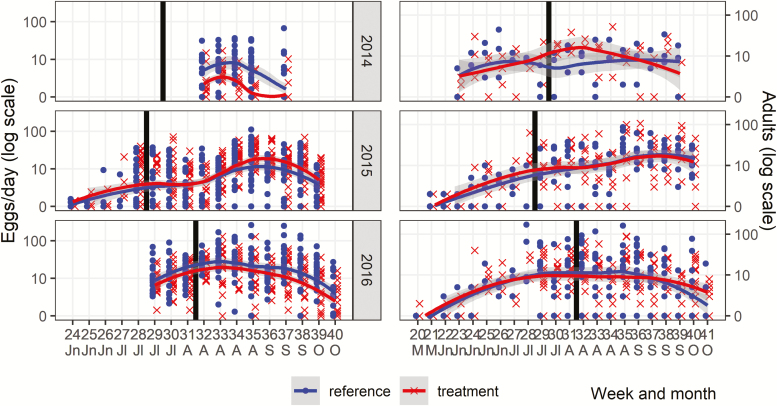
Aedes albopictus egg and adult collections were compared using the before-after-control-impact (BACI) design with the exception of the egg collections in 2014 since no before-treatment data were collected (Fig. 3, Table 1). As previously reported, in 2014, fewer eggs were collected from oviposition cups in the treatment sites (n = 906) compared to those in the reference sites (n = 3350; χ 2 = 42.56; df = 1, P < 0.0001) in the post treatment period. In 2015, a total of 45,342 eggs were collected in treatment sites before (208)/after (24,856) and reference sites before (116)/after (20,162) (refer to Table 1 for averages). In 2016, a total of 25,757 eggs were collected in treatment sites before (2,773)/after (22,984) and reference sites before (4,593)/after (40,205). For egg collections in 2015–2016, the interaction between treatment and before and after treatment time period was not significant (χ2 = 0.17, df = 1, P = 0.679), whereas the main treatment effect was significant (χ 2 = 15.3, df = 1, P < 0.001) suggesting sustained differences between the treatment and reference sites during the entire 2015 and 2016 mosquito seasons (Fig. 3, Table 1).
Fig. 3.
Egg and adult collections at reference and autodissemination treatment sites. Week number and month (M-May, Jn-June, Jl-July, A-August, S-September, O-October) are indicated. The y-axis is on the log scale. Each row corresponds to a year, i.e., 2014, 2015, and 2016. The thick black line corresponds to the autodissemination station deployment week and separates before-treatment from the after-treatment periods. The black (reference) and the gray (treatment) lines are loess regression curves with gray areas indicating 95% confidence intervals.
Table 1.
Summary of pupal mortality, egg, and adult collections
| Year | 2014 | 2015 | 2016 | ||||
|---|---|---|---|---|---|---|---|
| Type | Treatment | Before | After | Before | After | Before | After |
| Pupal mortality (%) | |||||||
| Laboratory | Baseline | nd | 0 | nd | 0.3 ± 0.2 | 0 | 0 |
| Field (deployed) | Reference | nd | 0.6 ± 0.2a | nd | 6.0 ± 0.5a | 0 | 0.2 ± 0.1a |
| Field (deployed) | Treatment | nd | 13.7 ± 2.1b | nd | 18.6 ± 1.3b | 0 | 16.0 ± 1.9b |
| Container (natural) | Reference | nd | nd | nd | 2.2 ± 0.5c | 0 | 0.2 ± 0.2a |
| Container (natural) | Treatment | nd | nd | nd | 14.8 ± 2.5b | 0 | 11.8 ± 3.3b |
| Eggs (average number per day) | |||||||
| Field (ovitrap) | Reference | 31.9 ± 4.5a | 7.3 ± 4.3 | 73.1 ± 5.7 | 114.8 ± 21.1 | 206.2 ± 18.9 | |
| Field (ovitrap) | Treatment | 8.7 ± 1.9b | 13.0 ± 8.6 | 88.8 ± 5.8 | 71.1 ± 10.2 | 121.0 ± 9.9 | |
| Adults (total collected per trap) | |||||||
| Field (BGS trap) | Reference | 8.3 ± 2.8 | 9.0 ± 1.4 | 2.1 ± 0.5 | 18.4 ± 1.7 | 13.8 ± 3.1 | 15.9 ± 2.3 |
| Field (BGS trap) | Treatment | 5.0 ± 1.5 | 11.5 ± 1.7 | 5.6 ± 2.3 | 20.8 ± 2.4 | 10.7 ± 1.5 | 12.5 ± 1.5 |
Average values ± standard errors are shown. Before and after refer to weekly measurements made either before or after autodissemination station deployment (see text for more details). Letters indicate statistically significant difference within each year and measured response. For pupal mortality, laboratory reference samples, containers deployed in the field (Field), and naturally occurring containers (Container, 2015–2016 only) in both the reference (reference) and autodissemination treatment blocks (treatment) are shown. nd designates no data were collected.
In total, 623 adult mosquitoes were collected in 2014 compared to 3,876 and 5,023 in 2015 and 2016, respectively (refer to Table 1 for averages). For adult mosquitoes (combined males and females), no significant differences in abundance were observed between the treatment and reference sites. Neither the interaction between treatment and before/after time period (χ 2 = 0.002, df = 1, P = 0.964), nor the main treatment effect (χ 2 = 0.42, df = 1, P = 0.519) was statistically significant.
Aedes albopictus Pupal Mortality Bioassays
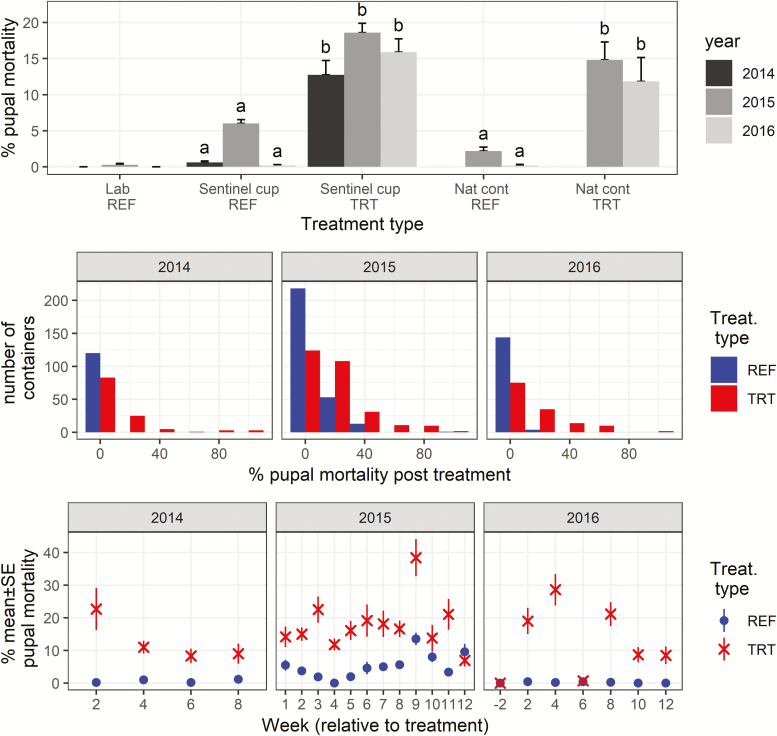
Following the autodissemination station deployment, treatment sites experienced elevated Ae. albopictus pupal mortality sustained over the treatment period during each study year (Fig. 4, Table 1). The average pupal mortality in field experiments using sentinel containers was similar across the 3 yr averaging between 14 and 19% (pairwise comparisons, 2015/14: Z = 0.85, P = 0.691; 2016/14: Z = −0.3, P = 0.951; 2016/15: Z = −1.3, P = 0.387, all df = 1). Comparable pupal mortality of 12–15% was observed for ‘naturally’ occurring containers collected in treatment sites in 2015–2016. Pupal mortalities in containers deployed in the field and naturally occurring containers were similar (pairwise comparisons, 2015: Z = −0.9, P = 0.592 and 2016: Z = −1.1, P = 0.497, all df = 1). Reference sample mortalities in both sentinel cups and ‘naturally’ occurring containers were generally below 1%, but slightly higher in 2015, when sentinel cups had elevated mortality compared to ‘naturally’ occurring containers (Tukey HSD posthoc test, Z = −3.8, df = 1, P < 0.0.001). The laboratory control mortality was always at or close to zero and thus no adjustments were made to account for it. Overall, treatment pupal mortalities were significantly higher than those in the reference samples (Tukey HSD posthoc test, all comparisons, P < 0.001).
Fig. 4.
Pupal mortality in bioassay experiments. The upper panel summarizes data over the study period (mean and SE) for laboratory reference samples (Lab REF), containers deployed in the field (Sentinel cups), and naturally occurring containers (Nat cont, 2015–2016 only) in both the reference (REF) and autodissemination treatment blocks (TRT). Different letters indicate statistical significance at P < 0.001 between categories within each year. The middle panel shows the distribution of mortalities in containers deployed in reference (REF) and treatment (TRT) sites. The data were aggregated at 20% intervals from 0 to 100%. For both treatment types, sentinel cups and naturally occurring containers were combined. The lower panel shows average percent pupal mortality with standard error by week following autodissemination station deployment at week = 0. In 2015–2016, Field and Container categories were combined for reference and treatment blocks. In 2016, the pupal mortality was also measured 2 wk prior to the deployment, i.e., week = −2.
To compare treatment effect over time, field and natural container categories were combined and all designated as either autodissemination treatment or reference samples (Fig. 4). Pupal mortalities in the treatment samples were similar across 3 yr (Tukey HSD posthoc test all P > 0.6) and significantly higher than those in the reference samples (Tukey HSD posthoc test all P < 0.001). However, high pupal mortalities (≥80%) were only observed in a small number of containers deployed in treatment areas (Fig. 4). Out of the total 21 wk of observations over the 3-yr period, the pupal mortalities in the treatment samples were higher than those in the reference samples for 19 wk or approximately 90% of the time.
Autodissemination Stations: Technical Challenges and Solutions
In 2014, 78.9% of the stations experienced exit gap blockage due to falling leaves and spider webs (Fig. 2A). In 2015, the exit gap size was increased, and a cover was added to reduce blockage. Despite the modifications, exit gap blockage remained a problem due to leaf and spider webs, observed in 86.8 and 13.4% of the stations, respectively (Fig. 2B). In addition, failures such as caking or leaking of formulations were corrected during weekly station servicing. In the 2016 final design, over 90% of the blockage and formulation problems were successfully corrected (Fig. 2C).
Discussion
Autodissemination of insecticides selectively targeting container Aedes larval habitats is a promising novel technology to manage mosquito species of high medical importance, namely Ae. albopictus and Ae. aegypti (Devine et al. 2009, Caputo et al. 2012, Snetselaar et al. 2014, Mains et al. 2015, Unlu et al. 2017). This method offers several attractive advantages. Autodissemination precision targets larval habitats using gravid mosquitoes for product delivery of minute amounts of insecticide (Itoh 1995, Devine et al. 2009, Abad-Franch et al. 2015). This limits environmental impact as well as reducing product and labor costs (Sihuincha et al. 2005, Chandel et al. 2016, Unlu et al. 2017). This technique is scalable from a single residential property to the level of neighborhood. It is also compatible with other novel approaches such as sterilized male release (Mains et al. 2015, Pleydell and Bouyer 2019) as well as with more conventional mosquito control measures (Focks et al. 2000, Farajollahi et al. 2012).
Putting aside the favorable environmental and economic profiles, the principal remaining question is whether autodissemination is an effective mosquito control tool. This question has been answered in the affirmative under laboratory conditions, and in semi- or small-scale field experiments (Dash and Ranjit 1992, Dell Chism and Apperson 2003, Devine et al. 2009, Caputo et al. 2012, Abad-Franch et al. 2015, Mains et al. 2015). However, the most important test for any new mosquito control technique is the performance under operational field conditions, which was the focus of our study. Unlike previous autodissemination field testing conducted in tropical or subtropical locales (Devine et al. 2009, Caputo et al. 2012, Ponlawat et al. 2013, Abad-Franch et al. 2015), our study area was in a temperate region representing typical Ae. albopictus habitat in the United States (Rochlin et al. 2013, Faraji et al. 2014).
Only Abad-Franch et al. (2015) had previously conducted a field test within a residential neighborhood, whereas all others experiments were restricted to small areas with clearly delineated physical confines (i.e., a cemetery, a garden, or a small village) (Abad-Franch et al. 2015). Among these studies, only Ponlawat et al (2013) conducted adult surveillance (Ponlawat et al. 2013). All trials were of short duration ranging from a few weeks to a single season. Importantly, some investigations lacked reference sites making it impossible to attribute the observed effects solely to pyriproxyfen autodissemination.
We have addressed some of the shortcomings mentioned above by measuring the impacts of pyriproxyfen autodissemination on egg, immature, and adult mosquito stages. Before-after-control-impact design allowed tracking mosquito populations simultaneously in treatment and reference sites to better separate natural variability from the treatment effect. We further attempted to minimize the migration of adult mosquitoes into the treated or reference sites by barrier treatments around the periphery of the study units. Although we switched from ULV adulticiding to barrier treatment to mitigate the movement of adult mosquitoes into the study sites, we lacked access to every parcel which allowed for some migration. This was a significant limitation likely to be an issue for any large-scale autodissemination study. Based on the analysis performed for 2014 field experiments, the number of BGS traps was increased to improve the statistical estimates. Lastly, to increase statistical power and confidence in the observed results, the treatments were replicated in space (three in each treatment and reference group) and time (three seasons in 2014–2016). The study was designed to reflect an operational environment typically experienced by local mosquito control districts in the United States. We focused on known problematic areas with high Asian Tiger mosquito populations and biting activity, i.e., the hot spots that have been previously characterized and monitored (Unlu et al. 2016).
Similar to the previous studies, our results demonstrated that autodissemination stations can successfully deliver the product into containers. The overall suppression approximated 12–19% on average and was relatively consistent in time (within and across seasons) as well as in place. The significant variability in weekly mortalities was consistent with other field and laboratory experiments (Dell Chism and Apperson 2003, Caputo et al. 2012).
While all autodissemination studies reported increased mortalities for Ae. albopictus and Ae. aegypti immatures, the magnitude differed. Under ideal laboratory conditions, 100% adult mosquito emergence suppression was sometimes achieved, albeit with a high degree of variability (Dell Chism and Apperson 2003, Wang et al. 2014). An average estimate of 70% is probably more realistic (Dell Chism and Apperson 2003, Sihuincha et al. 2005). Generally, Ae. aegypti was found more susceptible to pyriproxyfen than Ae. albopictus (Itoh 1995, Dell Chism and Apperson 2003). This may explain lower pupal mortality of Ae. albopictus compared to Ae. aegypti in the field and greenhouse experiments achieving ≈50–60% adult emergence suppression (Ohba et al. 2013, Wang et al. 2014, Abad-Franch et al. 2015). Abad-Franch et al. (2015) was the only field study reporting autodissemination effects on both container-inhabiting Aedes species. Their pupal mortality results were consistent with others: 50–80% for Ae. aegypti (Devine et al. 2009) and 50–70% for Ae. albopictus (Caputo et al. 2012). These levels of mortality were higher compared to our study (12–19%) and a small-scale trial in the same area (10–20%) (Suman et al. 2014).
Several factors make direct comparisons of our study with other field trials problematic. Caputo et al (2012) was likely the closest study climatically and socioeconomically, but their field sites, a stone cemetery and a garden, were small, nonresidential, and physically confined. These uniform environments lacked the diversity of larval habitats present in our residential community. Abad-Franch et al. 2015 conducted their experiments within an urban neighborhood bearing similarities to our study area in terms of population density, housing and the road network. However, the study site was located in a tropical Amazon basin and was more urbanized with little natural vegetation. The majority of mosquito container habitat was concentrated inside and around the houses. In contrast, in our study, all containers were found in backyards that often had considerable shrub and tree cover. Abad-Franch et al. 2015 study design was also different—all stations were deployed in one core area and no comparable reference site was established making a direct comparison.
Even less data are available on the impact of pyriproxyfen autodissemination on adult mosquito populations. This dearth of information is partially due to difficulties in preventing adult mosquitoes from the surrounding areas to migrate into the treatment sites (Abad-Franch et al. 2015). One way of dealing with this problem is establishing a small core treatment area surrounded by a much larger buffer area. Using this approach, (Ponlawat et al. 2013) detected a reduction in Ae. aegypti BGS trap collections in the autodissemination treatment site. This core and buffer approach would likely work less well for Ae. albopictus because of this species’ greater flight range, of 200–500 m (Niebylski and Craig Jr 1994, Bellini et al. 2010) versus 50–100 m for Ae. aegypti (Reiter et al. 1995). Instead, we treated the buffer zone with a barrier pesticide to reduce adult influx. While the barrier applications worked to some degree (about 40% reduction based on BGS trap counts, data not shown), it did not prevent migration. No differences in adult counts between treatment and reference sites were detected during our study. This was in agreement with the preliminary small-scale trial (Suman et al. 2014) and semifield greenhouse experiments that achieved higher pupal mortalities of ≈50% compared to our study (Ohba et al. 2013). Existing guidelines suggest 80% pupal mortality as the desirable range for IGR products (WHO 2005). Unless this threshold is attained under field condition, any impact due to adult emergence inhibition would be difficult to detect due to considerable natural fluctuations and movements of adult mosquito populations.
An aspect of pyriproxyfen autodissemination largely unexplored by field studies is the IGR’s effect on mosquito eggs. Reduction in female fecundity and egg hatching rate were determined for both Ae. aegypti (Itoh 1995, Sihuincha et al. 2005, Ponlawat et al. 2013) and Ae. albopictus (Dell Chism and Apperson 2003) mosquitoes under laboratory conditions. In greenhouse semifield experiments with Ae. albopictus, egg hatch declined 75–100% and fecundity was also significantly reduced (Ohba et al. 2013). The first year (2014) results from our study seemed to confirm these observations with a ≈73% reduction in egg collections in treatment versus reference sites (Unlu et al. 2017). However, no such reductions were observed during the subsequent 2 yr of the study (2015–2016) leaving this question open to further research.
One possible limitation of an autodissemination approach is the temporary decline in pyriproxyfen effectiveness in the field after heavy rains (Mbare et al. 2013, Suman et al. 2014). Also, the use of plastic containers is mentioned frequently as a concern because pyriproxyfen adhesion to plastic (Suman et al. 2013). However, pyriproxyfen is a juvenile hormone analogue which operates at extremely low concentrations (Lee 2001, Al-Sarar et al. 2011). In 2014, we observed 70% pupal mortality at 0.004 ppb while the highest concentration of 0.0113 ppb achieved 95% pupal mortality (Unlu et al. 2017).
As with any pesticide, pyriproxyfen should be applied using integrated pest management and rotated with other pesticides to prevent resistance. One of the advantages of pyriproxyfen is that exposure to sublethal doses of this IGR also affects reproduction. Mbare et al. (2013) reported 90% reduction in Anopheles gambiae and Anopheles arabiensis fecundity and after exposure to sublethal doses. The delayed sublethal effects of pyriproxyfen and other IGRs also altered the sex ratio and reduced the blood-feeding rates (Loh and Yap 1989, Vasuki 1999). Therefore, even if the mosquitoes are subjected to sublethal doses of the pesticide, the effects might be beneficial for the disease risk reduction. Resistance to pyriproxyfen is a concern, yet widespread use in agriculture resistance has only been reported in a whitefly Bemisia tabaci (Gennadius) population in Israel (Horowitz et al. 2002, 2005).
We conclude that the autodissemination stations successfully delivered the IGR into Ae. albopictus larval habitats, but the impact was insufficient to reduce adult populations. Only 1–4% of the containers in the field treatment sites exhibited high, i.e., ≥80% (WHO 2005) immature stage mortality (Fig. 4). In each year, approximately three-fourth of the containers in the treatment sites exhibited mortalities of <20%. Several problems encountered during this large and long-term experiment likely influenced the outcome. One concern was the stations themselves. The initial design was unsatisfactory because of blockage of the autodissemination stations and exit gap size. This was modified twice through the study. The latest design showed no blockage problem and increased durability of the gel formulation versus oil and powder formulations.
Working in a highly urbanized and densely populated environment was a fundamental challenge of this study. Limited accessibility to individual properties constrained optimal placement of autodissemination stations, a crucial component of effective levels of mosquito control (Caputo et al. 2012). Large-scale barrier treatments to prevent an influx of adult mosquitoes were less effective because many property owners did not provide permission to treat. As a result, our barrier applications lead only to about 40% reduction in adult mosquitoes (data not shown), about one-half of the levels achievable with more uniform coverage (about 72%) (Unlu et al. 2017).
These experimental challenges undoubtedly contributed to reduced efficacy of autodissemination stations resulting in smaller impacts on the mosquito populations compared to the laboratory, semifield, or small-scale field trials. While the stations clearly performed as expected by spreading the IGR into sentinel and ‘natural’ containers leading to higher pupal mortality, the impact was not sufficient to realize a reduction in adult populations.
Operational research requires enhanced support by incorporating different tools such as conventional treatments, autodissemination stations, sterilized male release, and bait stations into a single IPM tactic that has low environmental impact while providing excellent container Aedes control. Such integrated tactic should also appeal to the general public, without whose involvement and support it would be impossible to reduce container inhabiting mosquito habitat. Commercialization of effective techniques adaptable to public use such as autodissemination stations should be also pursued to assist with the efforts of mosquito abatement districts. Our current reliance on conventional pesticide and source reduction may appear logical and simple; however, it is clearly inferior and not sustainable given limited budget and shrinking personnel in many mosquito control districts.
Acknowledgments
We thank Nicholas Indelicato, Walt Voorhees, William Cook, Madison Rittley, Michael Scozzaro, Dan Scozzoro, Ryan Dajczak, Geena Bentz, Dominick Cifelli, and Michael Milewski for technical assistance. Special thanks to Michael Banfield from SpringStar Inc. for his contribution to provide autodissemination stations. This work was supported by the USDA National Institute of Food and Agriculture Hatch project accession number 1020755 through the New Jersey Agricultural Experiment Station, Hatch project NJ08530. This work was also funded in part by the Small Business Innovative Research (SBIR) program collaborative Agreement between the National Institutes of Health, Rutgers University, and SpringStar Inc. All studies were conducted within the jurisdictions of the authors’ respective governance domain by professional mosquito control personnel. All entomological surveys and collections made on private lands or in private residences were conducted after acquisition of oral or written consent from residents. No specific permits were required for the mosquito collections. These studies did not involve endangered or protected species. The authors declare that they have no competing interests. Use of trade names is for identification only and does not imply endorsement by Rutgers University. The views of the authors do not necessarily reflect the position of the Center for Vector Biology, Rutgers University.
References Cited
- Abad-Franch F., Zamora-Perea E., Ferraz G., Padilla-Torres S. D., and Luz S. L... 2015. Mosquito-disseminated pyriproxyfen yields high breeding-site coverage and boosts juvenile mosquito mortality at the neighborhood scale. PLoS Negl. Trop. Dis. 9: e0003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sarar A. S., Al-Shahrani D., Bayoumi A. E., Abobakr Y., and Hussein H. I... 2011. Laboratory and field evaluation of some chemical and biological larvicides against Culex spp. (Diptera: Culicidae) immature stages. Int. J. Agric. Biol. 13: 115–119. [Google Scholar]
- Bartlett-Healy K., Hamilton G., Healy S., Crepeau T., Unlu I., Farajollahi A., Fonseca D., Gaugler R., Clark G. G., and Strickman D... 2011. Source reduction behavior as an independent measurement of the impact of a public health education campaign in an integrated vector management program for the Asian tiger mosquito. Int. J. Environ. Res. Public Health. 8: 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker M. B., and Walker S... 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0-5.
- Bellini R., Albieri A., Balestrino F., Carrieri M., Porretta D., Urbanelli S., Calvitti M., Moretti R., and Maini S... 2010. Dispersal and survival of Aedes albopictus (Diptera: Culicidae) males in Italian urban areas and significance for sterile insect technique application. J. Med. Entomol. 47: 1082–1091. [DOI] [PubMed] [Google Scholar]
- Caputo B., Ienco A., Cianci D., Pombi M., Petrarca V., Baseggio A., Devine G. J., and della Torre A... 2012. The “auto-dissemination” approach: a novel concept to fight Aedes albopictus in urban areas. PLoS Negl. Trop. Dis. 6: e1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel K., Suman D. S., Wang Y., Unlu I., Williges E., Williams G. M., and Gaugler R... 2016. Targeting a hidden enemy: pyriproxyfen autodissemination strategy for the control of the container mosquito Aedes albopictus in cryptic habitats. PLoS Negl. Trop. Dis. 10: e0005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley M. J. 2012. The R book. John Wiley & Sons, Chichester, West Sussex, UK. [Google Scholar]
- Dash A. P., and Ranjit M. R... 1992. Comparative efficacy of aphid extracts and some juvenoids against the development of mosquitoes. J. Am. Mosq. Control Assoc. 8: 247–251. [PubMed] [Google Scholar]
- Dell Chism B., and Apperson C. S... 2003. Horizontal transfer of the insect growth regulator pyriproxyfen to larval microcosms by gravid Aedes albopictus and Ochlerotatus triseriatus mosquitoes in the laboratory. Med. Vet. Entomol. 17: 211–220. [DOI] [PubMed] [Google Scholar]
- Devine G. J., Perea E. Z., Killeen G. F., Stancil J. D., Clark S. J., and Morrison A. C... 2009. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc. Natl Acad. Sci. USA. 106: 11530–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling Z., Armbruster P., LaDeau S. L., DeCotiis M., Mottley J., and Leisnham P. T... 2013. Linking mosquito infestation to resident socioeconomic status, knowledge, and source reduction practices in suburban Washington, DC. Ecohealth. 10: 36–47. [DOI] [PubMed] [Google Scholar]
- Faraji A., and Unlu I... 2016. The eye of the tiger, the thrill of the fight: effective larval and adult control measures against the Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae), in North America. J. Med. Entomol. 53: 1029–1047. [DOI] [PubMed] [Google Scholar]
- Faraji A., Egizi A., Fonseca D. M., Unlu I., Crepeau T., Healy S. P., and Gaugler R... 2014. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban Northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis. 8: e3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A., Healy S. P., Unlu I., Gaugler R., and Fonseca D. M... 2012. Effectiveness of ultra-low volume nighttime applications of an adulticide against diurnal Aedes albopictus, a critical vector of dengue and chikungunya viruses. PLoS One. 7: e49181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajollahi A., Williams G. M., Condon G. C., Kesavaraju B., Unlu I., and Gaugler R... 2013. Assessment of a direct application of two Bacillus thuringiensis israelensis formulations for immediate and residual control of Aedes albopictus. J. Am. Mosq. Control Assoc. 29: 385–388. [DOI] [PubMed] [Google Scholar]
- Focks D. A., Brenner R. J., Hayes J., and Daniels E... 2000. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 62: 11–18. [PubMed] [Google Scholar]
- Fonseca D. M., Unlu I., Crepeau T., Farajollahi A., Healy S. P., Bartlett‐Healy K., Strickman D., Gaugler R., Hamilton G., and Kline D... 2013. Area‐wide management of Aedes albopictus. Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manage. Sci. 69: 1351–1361. [DOI] [PubMed] [Google Scholar]
- Gaugler R., Suman D., and Wang Y... 2012. An autodissemination station for the transfer of an insect growth regulator to mosquito oviposition sites. Med. Vet. Entomol. 26: 37–45. [DOI] [PubMed] [Google Scholar]
- Geden C. J., and Devine G. J... 2012. Pyriproxyfen and house flies (Diptera: Muscidae): effects of direct exposure and autodissemination to larval habitats. J. Med. Entomol. 49: 606–613. [DOI] [PubMed] [Google Scholar]
- Healy K., Hamilton G., Crepeau T., Healy S., Unlu I., Farajollahi A., and Fonseca D. M... 2014. Integrating the public in mosquito management: active education by community peers can lead to significant reduction in peridomestic container mosquito habitats. PLoS One. 9: e108504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A. R., Kontsedalov S., Denholm I., and Ishaaya I... 2002. Dynamics of insecticide resistance in Bemisia tabaci: a case study with the insect growth regulator pyriproxyfen. Pest Manag. Sci. 58: 1096–1100. [DOI] [PubMed] [Google Scholar]
- Horowitz A. R., Kontsedalov S., Khasdan V., and Ishaaya I... 2005. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 58: 216–225. [DOI] [PubMed] [Google Scholar]
- Itoh T. 1995. Utilization of blood fed females of Aedes aegypti as a vehicle for the transfer of the insect growth regulator, pyriproxyfen, to larval habitats. 熱帯医学 Trop. Med. 36: 243–248. [PubMed] [Google Scholar]
- Johnson B. J., Brosch D., Christiansen A., Wells E., Wells M., Bhandoola A. F., Milne A., Garrison S., and Fonseca D. M... 2018. Neighbors help neighbors control urban mosquitoes. Sci. Rep. 8: 15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R. C., Sorensen A. E., and Ladeau S... 2017. Citizen science as a tool for mosquito control. J. Am. Mosq. Control Assoc. 33: 241–245. [DOI] [PubMed] [Google Scholar]
- Kay B., and Vu S. N... 2005. New strategy against Aedes aegypti in Vietnam. Lancet. 365: 613–617. [DOI] [PubMed] [Google Scholar]
- Lee D. K. 2001. Field evaluation of an insect growth regulator, pyriproxyfen, against Aedes togoi larvae in brackish water in South Korea. J. Vector Ecol. 26: 39–42. [PubMed] [Google Scholar]
- Loh P. Y., Yap H. H. 1989. Laboratory studies on the efficacy and sublethal effects of an insect growth regulator, pyriproxyfen (S31183) against Aedes aegypti (Linnaeus). Trop. Biomedicine. 6: 7–12. [Google Scholar]
- Mains J. W., Brelsfoard C. L., and Dobson S. L... 2015. Male mosquitoes as vehicles for insecticide. PLoS Negl. Trop. Dis. 9: e0003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbare O., Lindsay S. W., and Fillinger U... 2013. Dose-response tests and semi-field evaluation of lethal and sub-lethal effects of slow release pyriproxyfen granules (Sumilarv®0.5G) for the control of the malaria vectors Anopheles gambiae sensu lato. Malar. J. 12: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelder M., Kesavaraju B., Farajollahi A., Healy S., Unlu I., Crepeau T., Ragavendran A., Fonseca D., and Gaugler R... 2010. Suppressing Aedes albopictus, an emerging vector of dengue and chikungunya viruses, by a novel combination of a monomolecular film and an insect-growth regulator. Am. J. Trop. Med. Hyg. 82: 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebylski M. L., and Craig G. B. Jr. 1994. Dispersal and survival of Aedes albopictus at a scrap tire yard in Missouri. J. Am. Mosq. Control Assoc. 10: 339–343. [PubMed] [Google Scholar]
- Ohba S. Y., Ohashi K., Pujiyati E., Higa Y., Kawada H., Mito N., and Takagi M... 2013. The effect of pyriproxyfen as a “population growth regulator” against Aedes albopictus under semi-field conditions. PLoS One. 8: e67045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C. M., Marina C. F., Bond J. G., Rojas J. C., Valle J., and Williams T... 2007. Spinosad, a naturally derived insecticide, for control of Aedes aegypti (Diptera: Culicidae): efficacy, persistence, and elicited oviposition response. J. Med. Entomol. 44: 631–638. [DOI] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., DebRoy S., and Sarkar D... 2015. R Core Team. nlme: Linear and Nonlinear mixed effects models. R package version 3.1–117; 2014.
- Pleydell D. R. J., and Bouyer J... 2019. Biopesticides improve efficiency of the sterile insect technique for controlling mosquito-driven dengue epidemics. Commun. Biol. 2: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponlawat A., Fansiri T., Kurusarttra S., Pongsiri A., McCardle P. W., Evans B. P., Evans B. P., and Richardson J. H... 2013. Development and evaluation of a pyriproxyfen-treated device to control the dengue vector, Aedes aegypti (L.) (Diptera:Culicidae). Southeast Asian J. Trop. Med. Public Health. 44: 167–178. [PubMed] [Google Scholar]
- Reiter P., Amador M. A., Anderson R. A., and Clark G. G... 1995. Short report: dispersal of Aedes aegypti in an urban area after blood feeding as demonstrated by rubidium-marked eggs. Am. J. Trop. Med. Hyg. 52: 177–179. [DOI] [PubMed] [Google Scholar]
- Richards S. L., Ghosh S. K., Zeichner B. C., and Apperson C. S... 2008. Impact of source reduction on the spatial distribution of larvae and pupae of Aedes albopictus (Diptera: Culicidae) in suburban neighborhoods of a Piedmont community in North Carolina. J. Med. Entomol. 45: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S. A., Rapley L. P., and Benjamin S... 2010. Bacillus thuringiensis var. israelensis (Bti) provides residual control of Aedes aegypti in small containers. Am. J. Trop. Med. Hyg. 82: 1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin I., Ninivaggi D. V., Hutchinson M. L., and Farajollahi A... 2013. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in Northeastern USA: implications for public health practitioners. PLoS One. 8: e60874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E. T., and Morris C. D... 1995. Evaluation of public information packets for mosquito source reduction in two Florida cities. J. Am. Mosq. Control Assoc. 11: 186–190. [PubMed] [Google Scholar]
- Sihuincha M., Zamora-Perea E., Orellana-Rios W., Stancil J. D., López-Sifuentes V., Vidal-Oré C., and Devine G. J... 2005. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Perú. J. Med. Entomol. 42: 620–630. [DOI] [PubMed] [Google Scholar]
- Smith E. P., Orvos D. R., and Cairns J. Jr.. 1993. Impact assessment using the before-after-control-impact (BACI) model: concerns and comments. Can. J. Fish. Aquat. Sci. 50: 627–637. [Google Scholar]
- Snetselaar J., Andriessen R., Suer R. A., Osinga A. J., Knols B. G., and Farenhorst M... 2014. Development and evaluation of a novel contamination device that targets multiple life-stages of Aedes aegypti. Parasit. Vectors. 7: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Oaten A., Murdoch W. W., and Parker K. R... 1986. Environmental impact assessment:” Pseudoreplication” in time? Ecology 67: 929–940. [Google Scholar]
- Suman D. S., Wang Y., Dong L., and Gaugler R... 2013. Effects of larval habitat substrate on pyriproxyfen efficacy against Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 50: 1261–1266. [DOI] [PubMed] [Google Scholar]
- Suman D. S., Farajollahi A., Healy S., Williams G. M., Wang Y., Schoeler G., and Gaugler R... 2014. Point-source and area-wide field studies of pyriproxyfen autodissemination against urban container-inhabiting mosquitoes. Acta Trop. 135: 96–103. [DOI] [PubMed] [Google Scholar]
- Trout R. T., Brown G. C., Potter M. F., and Hubbard J. L... 2007. Efficacy of two pyrethroid insecticides applied as barrier treatments for managing mosquito (Diptera: Culicidae) populations in suburban residential properties. J. Med. Entomol. 44: 470–477. [DOI] [PubMed] [Google Scholar]
- Unlu I., and Farajollahi A... 2014. A multiyear surveillance for Aedes albopictus with Biogents Sentinel trap counts for males and species composition of other mosquito species. J. Am. Mosq. Control Assoc. 30: 122–125. [DOI] [PubMed] [Google Scholar]
- Unlu I., Farajollahi A., Healy S. P., Crepeau T., Bartlett‐Healy K., Williges E., Strickman D., Clark G. G., Gaugler R., and Fonseca D. M... 2011. Area‐wide management of Aedes albopictus: choice of study sites based on geospatial characteristics, socioeconomic factors and mosquito populations. Pest Manage. Sci. 67: 965–974. [DOI] [PubMed] [Google Scholar]
- Unlu I., Farajollahi A., Strickman D., and Fonseca D. M... 2013. Crouching tiger, hidden trouble: urban sources of Aedes albopictus (Diptera: Culicidae) refractory to source-reduction. PLoS One. 8: e77999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu I., Faraji A., Indelicato N., and Fonseca D. M... 2014a. The hidden world of Asian tiger mosquitoes: immature Aedes albopictus (Skuse) dominate in rainwater corrugated extension spouts. Trans. R. Soc. Trop. Med. Hyg. 108: 699–705. doi: 10.1093/trstmh/tru139. [DOI] [PubMed] [Google Scholar]
- Unlu I., Farajollahi A., Ilia R., Crepeau T. N., Daniel S., and Gaugler R... 2014b. Differences in male-female ratios of Aedes albopictus (Diptera: Culicidae) following ultra-low volume adulticide applications. Acta Trop. 201–205. [DOI] [PubMed] [Google Scholar]
- Unlu I., Farajollahi A., Rochlin I., Crepeau T. N., Strickman D., and Gaugler R... 2014c. Differences in male-female ratios of Aedes albopictus (Diptera: Culicidae) following ultra-low volume adulticide applications. Acta Trop. 137: 201–205. [DOI] [PubMed] [Google Scholar]
- Unlu I., Klingler K., Indelicato N., Faraji A., and Strickman D... 2016. Suppression of Aedes albopictus, the Asian tiger mosquito, using a ‘hot spot’ approach. Pest Manage. Sci. 72: 1427–1432. [DOI] [PubMed] [Google Scholar]
- Unlu I., Suman D. S., Wang Y., Klingler K., Faraji A., and Gaugler R... 2017. Effectiveness of autodissemination stations containing pyriproxyfen in reducing immature Aedes albopictus populations. Parasit. Vectors. 10: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unlu I., Williams G. M., Rochlin I., Suman D., Wang Y., Chandel K., Gaugler R. 2018. Evaluation of lambda-cyhalothrin and pyriproxyfen barrier treatments for Aedes albopictus (Diptera: Culicidae) management in urbanized areas of New Jersey. J. Med. Entomol. 55: 472–6. [DOI] [PubMed] [Google Scholar]
- Vasuki V. 1999. Influence of IGR treatment on oviposition of three species of vector mosquitos at sublethal concentrations. Southeast Asian J. Trop. Med. Public Health 30: 200–203. [PubMed] [Google Scholar]
- Wang Y., Suman D. S., Bertrand J., Dong L., and Gaugler R... 2014. Dual-treatment autodissemination station with enhanced transfer of an insect growth regulator to mosquito oviposition sites. Pest Manag. Sci. 70: 1299–1304. [DOI] [PubMed] [Google Scholar]
- Ward R. D. 2005. Identification and geographical distribution of the mosquitoes of North America, North of Mexico, By Darsie R. F. Jr. and Ward R. A., pp. 416 University Press of Florida, 2005. ISBN0 8130 2784 5. US $75.00. Parasitology 131: 580–580. [Google Scholar]
- World Health Organization (WHO) 2005. Guidelines for laboratory and field testing of mosquito larvicides. Document WHO/CDS/WHOPES/GCDPP/13. Geneva: World Health Organization. [Google Scholar]
- Williams G. M., Faraji A., Unlu I., Healy S. P., Farooq M., Gaugler R., Hamilton G., and Fonseca D. M... 2014. Area-wide ground applications of Bacillus thuringiensis var. israelensis for the control of Aedes albopictus in residential neighborhoods: from optimization to operation. Plos One. 9: e110035. [DOI] [PMC free article] [PubMed] [Google Scholar]