Abstract
Eupatorin is a polymethoxy flavone extracted from Orthosiphon stamineus and was reported to exhibit cytotoxic effects on several cancer cell lines. However, its effect as an anti–breast cancer agent in vivo has yet to be determined. This study aims to elucidate the potential of eupatorin as an anti–breast cancer agent in vivo using 4T1 challenged BALB/c mice model. In this article, BALB/c mice (20-22 g) challenged with 4T1 cells were treated with 5 mg/kg or 20 mg/kg eupatorin, while the untreated and healthy mice were fed with olive oil (vehicle) via oral gavage. After 28 days of experiment, the mice were sacrificed and blood was collected for serum cytokine assay, while tumors were harvested to extract RNA and protein for gene expression assay and hematoxylin-eosin staining. Organs such as spleen and lung were harvested for immune suppression and clonogenic assay, respectively. Eupatorin (20 mg/kg) was effective in delaying the tumor development and reducing metastasis to the lung compared with the untreated mice. Eupatorin (20 mg/kg) also enhanced the immunity as the population of NK1.1+ and CD8+ in the splenocytes and the serum interferon-γ were increased. Concurrently, eupatorin treatment also has downregulated the expression of pro-inflammatory and metastatic related genes (IL-1β. MMP9, TNF-α, and NF-κB). Thus, this study demonstrated that eupatorin at the highest dosage of 20 mg/kg body weight was effective in delaying the 4T1-induced breast tumor growth in the animal model.
Keywords: 4T1, clonogenic, eupatorin, MMP-9, NF-κB, NK1.1, CD8+
Introduction
Globally breast cancer rates are increasing among women, and this cancer is the leading cause of cancer death in women among all types of cancers.1 Among the subtypes of breast cancer, the biologically aggressive triple-negative breast cancer is associated with a high rate of metastasis and poor prognosis. Thus, research into finding the cure for triple-negative breast cancer, including the use of natural-derived molecules as potential antitumor agents, are ongoing.2 Phytochemical compounds have been reported as promising antitumoral agents in breast cancer.3 Among the phytochemical compounds, eupatorin (3′,5-dihydroxy-4′,6,7-trimethoxyflavone), which is one of the essential polymethoxy flavones in Orthosiphon stamineus,4 has been proposed as a potent candidate for anti–breast cancer agents.5,6 Previously, in vitro study on breast cancer cell lines suggested that eupatorin could inhibit cancer cell proliferation through apoptosis induction. Eupatorin regulated the activity of proapoptotic and antisurvival genes, which led to the inhibition of the cells’ metastasis and angiogenesis. Furthermore, cytotoxicity of eupatorin was selective to cancer cells and thus did not harm the normal human breast cells. Although the previous study also found the potential of eupatorin as the antiproliferative and antiangiogenic agent on several cancer cells such as HeLa cervical adenocarcinoma6 and breast cancer cells (MDA-MB-246, MDA-MB-231, and MCF-7),5,7 the in vivo study has not been established yet. Hence, in this study, the potential of eupatorin in treating breast cancer was further investigated in vivo. BALB/c mice challenged with mouse-derived mammary carcinoma cell line 4T1 are an excellent model for invasive breast cancer study. 4T1 is a transplantable tumor cell line that closely resembles metastatic breast cancer in human patients.8 Furthermore, cancer metastasis in this mouse model resembles an advanced stage IV breast cancer.9 Thus, the objective of this study was to evaluate the antitumor effect of eupatorin in 4T1-challenged mice.
Materials and Methods
Materials
Eupatorin (Cat No. E4660) and doxorubicin (Cat No. D1515) were purchased from Sigma-Aldrich, dissolved in phosphate-buffered saline (PBS; Sigma) to prepare a master stock solution at a concentration of 1 mg/mL, and stored at −20 °C before use. The working solution was freshly prepared in the complete culture medium for cytotoxicity assay.
Cell Lines and Culture Conditions
Mouse breast cancer cell line 4T1 was purchased from American Type Culture Collection (ATCC) and cultured in RPMI-1640 (Sigma) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS; PAA), penicillin (100 IU/mL), and streptomycin (100 ng/mL; PAA). The cells were cultured at 37 °C in a 90% humidified incubator with 5% CO2.
Cytotoxicity Assay
The eupatorin effect on 4T1 cells was measured by MTT assay. Cells (0.8 × 105 cells/mL) were seeded in a 96-well plate and incubated with a 2-fold serial dilution of eupatorin starting with the highest concentration at 20 µg/mL for 24, 48, and 72 hours. Doxorubicin was used as a positive control in this experiment. Then, 20 µL of MTT solution (5 mg/mL PBS) was added into each well and incubated for 4 hours. The supernatant was discarded and replaced with 100 µL/well of dimethyl sulfoxide to dissolve the precipitate. The cell viability was estimated by measuring absorbance at 570 nm using a Quant ELISA plate reader (Bio-Tek Instruments). Cytotoxicity was expressed using the IC50 value defined as the concentration of eupatorin inhibiting cell proliferation by 50% and calculated based on the absorbance ratio between cell culture treated with eupatorin and the untreated control multiplied by 100, representing cell viability (percentage of control, %).
In Vivo Study of Eupatorin Compounds as an Antitumor Agent
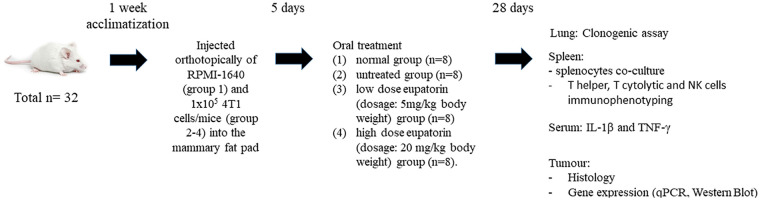
In vivo study using female Balb/c mice was approved by the Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (UPM), Serdang, Selangor (Ethics Approval No. R009/2015). The mice (total N = 32) aged between 4 and 5 weeks were obtained from A-Sapphire Enterprise and acclimatized in plastic cages (4 mice/cage) for 1 week (22 ± 1 °C; 12-hour dark/light cycle). All mice were given distilled water and standard pellet diet ad libitum until the ideal body weight (BW; 20-22 g) was achieved. Then, mice were randomly divided into 4 groups: (1) healthy group (n = 8); (2) untreated group (n = 8); (3) low-dose eupatorin (dosage: 5 mg/kg BW) group (n = 8); and (4) high-dose eupatorin (dosage: 20 mg/kg BW) group (n = 8). Each mouse from groups 2, 3, and 4 was challenged with 4T1 cells by injecting orthotopically 1 × 105 cells/mice into the mammary fat pad. After 5 days of injection, tumor volume was measured by electronic caliper and calculated using the following formula:
All mice were recorded with tumor sized ~0.10 mm3. The 4T1-induced mice in groups 3 and 4 were treated with 100 µL of eupatorin dissolved in olive oil at respective dosage via force-feeding once daily. In addition, untreated and healthy mice were fed with 100 µL of olive oil (vehicle) via force-feeding once daily. After 28 days of treatment, all mice were anaesthetized with 2% isoflurane (Merck) and sacrificed by cervical dislocation. Prior to treatment, all the mice were numbered and weighed and the initial tumor volumes were recorded. Overview of the experimental design is summarized in Figure 1.
Figure 1.
Overview of the in vivo experimental design.
Histology of Tissue Sections
The harvested tumors were fixed in 10% buffered formalin and embedded in paraffin to preserve tissue morphology and retain the antigenicity of the targeted molecules. Then, 5-µm-thick tissue sections were stained with hematoxylin and eosin (H&E) staining according to the standard procedure. The stained tissue sections were viewed under a light microscope (Nikon). For the fluorescent TUNEL apoptosis analysis, the tissue sections were stained with DeadEnd Fluorometric TUNEL kit following the manufacturer’s instruction (Promega). The samples were viewed under a fluorescent microscope using the green fluorescent filter at 520 nm (Nikon).
4T1 Metastasis Evaluation
Metastasis of 4T1 cells into the lung was quantified as described by Yang et al.10 Harvested lung was finely minced and enriched with 300 µL of collagenase (10 µg/mL) at 37 °C for 30 minutes. Then, the cells were filtered through 70-µm nylon cell strainers and washed twice with PBS. The resulting cells were suspended and serially diluted in RPMI medium containing 60 µM 6-thioguanine for clonogenic growth. Because 4T1 cells are resistant to 6-thioguanine, metastasized tumor cells would form foci in 10 days. On the 10th day, the plates were fixed with methanol and stained with 0.5% crystal violet for 2 hours before counting the colonies.
Splenocytes Coculture With 4T1 Cells
4T1 cells (1 × 105 cells/mL) were seeded in 96-well plate overnight. Harvested spleens were dissociated in sterilized PBS. Then, the cells pellet was lysed in 2 mL red blood cell lysis buffer at 4 °C for 10 minutes. The supernatant was discarded, and the cells pellet was resuspended in complete growth medium RPMI-1640 (10% FBS). One hundred microliters of the splenic cells at concentrations of 2 × 105 cells/mL (2 vs 1) and 1 × 106 cells/mL (10 vs 1) were added to the cultured 4T1 cells. Cells were incubated for 24 hours at 37 °C. Then, cells viability was determined using the MTT assay.11,12
Immunophenotyping Assay
Harvested spleens were meshed in sterilized PBS (pH 7.4). Then, the tissues were spun down to collect the pellet and resuspended in 2-mL lysis buffer prior to incubation at 4 °C for 10 minutes. Then, the pellet was resuspended in 200-µL PBS. The cell suspension was divided into 2 portions, and 2 µL of antibody mixtures containing CD3-FITC/NK1.1-APC and CD3-FITC/CD4-PE/CD8-APC were added into each tube, incubated on ice for 1 hour. Then, the pellet was collected by centrifugation at 150 000 rpm for 15 minutes at 4 °C. The pellet was resuspended in 1% paraformaldehyde overnight at 4 °C. Before analysis, 1% paraformaldehyde was removed, washed with PBS twice, and resuspended in 500-µL PBS. Flow cytometry was performed using the FACS Calibur (Becton Dickinson), and all experiments were performed in triplicate.
Serum Cytokine Assay
Blood was collected in BD Vacutainer Serum Separator Tubes (SST, #367988) and spun 10 minutes at 150 000 rpm to obtain the serum. Cytokine in blood serum was determined using kit DuoSet for mouse interleukin (IL)-1β and interferon (IFN)-γ, purchased from R&D Systems and used according to the manufacturer’s instructions. The optical density was measured using a microplate reader set to 450 nm and 540 nm.
RNA Extraction and Real-Time PCR (qPCR) Analysis
Harvested tumors were cut into small size (~1 mm) and submerged in RNAlater solution (Thermo Fisher Scientific) overnight at 4 °C. Then, tumors were collected and meshed in liquid nitrogen using mortar and pestle. Thirty milligrams of the meshed tumor was used for RNA extraction using RNeasy Mini plus kit (Qiagen) according to the manufacturer’s instructions. The quality control of extracted RNA was checked using ultraviolet spectrophotometer (A260/280 and A260/230 ratios ranged from 1.8 to 2.0) and converted to cDNA according to the manufacturer’s instruction of the QuantiTect Reverse Transcription kit (Qiagen) prior to quantitative polymerase chain reaction (qPCR) analysis. The targeted genes of NF-κB, matrix metalloproteinase 9 (MMP9), TNF-α, and IL-1β were evaluated using real-time PCR (qPCR) analysis. All selected genes were obtained from Integrated DNA Technologies (IDT). The primers for targeted genes were designed according to the accession number obtained from the National Centre of Biotechnology Information (NCBI) database (National Library of Medicine). Expression of targeted genes was determined using KAPA SYBR FAST qPCR Kit Master Mix (2×) Universal (KAPA Biosystems) according to the manufacturer’s instruction. The reaction was carried out using CFX 96 Real-time Detection System (Bio-Rad Laboratories, Inc). Thermal cycling was initiated at 95 °C for 3 minutes, followed by 40 cycles consisting of denaturation at 95 °C for 3 seconds and combined annealing/extension steps at 60 °C for 30 seconds. A melting curve analysis was performed by gradually heating the samples from 55 °C to 95 °C with 0.2 °C increment per second, while the fluorescence was measured continuously. Primers with the standard calibration curve that possessed efficiency (E) ≥90% with linear regression (R2) ≥0.980 was accepted for genes quantification. For sample analysis, optimum efficiencies between 94% and 110% for each of the respective primer pairs were obtained. Each sample was run in triplicate, and data were normalized by geometric averaging of the 2 reference genes, namely, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and ACTB. Table 1 presents the PCR primers set that were used during the experiment.
Table 1.
Primers for Real-Time PCR (Quantitative PCR).
| Primers | Accession number | Primer sequence | |
|---|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | ||
| MMP9 | NM_013599 | GCCGACTTTTGTGGTCTTCC | GGTACAAGTATGCCTCTGCCA |
| NF-κB | NM_0086892 | CCTGCTTCTGGAGGGTGAGT | GCCGCTATATGCAGAGGTGT |
| TNF-α | NM_013693 | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGTCTACACG |
| IL-1β | NM_008361 | GCCACCTTTTGACAGTGATGAG | GACAGCCCAGGTCAAAGGTT |
| GAPDH | NM_008084 | GAAGGTGGTGAAGCAGGCATC | GAAGGTGGAAGAGTGGGAGTT |
| ACTB | NM_007393 | TTCCAAGCCTTCCTTCTTG | GGAGCCAGAGCAGTAATC |
Abbreviations: PCR, polymerase chain reaction; MMP9, matrix metalloproteinase 9; NF-κB, nuclear factor κB; TNF-α, tumor necrosis factor; IL-1β, interleukin 1β; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ACTB, β-actin.
Western Blot Analysis of Protein From Tumor
The anti-MMP9 and anti-β-actin antibodies used in this study were purchased from Abcam. Briefly, 100 mg of the meshed tumor was homogenized in 300 µL of ice-cold lysis buffer containing the protease inhibitor and transferred to a QiaShredder (Qiagen). The cells were spun down at 150 000 rpm, 4 °C for 15 minutes. Protein lysate was collected, and total protein was determined using the Bradford method. Then, 12.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) membrane (1 mm thick) was prepared prior to western blot analysis. Separating gel was prepared by mixing 2.19 mL distilled water, 1.56 mL of 40% acrylamide, 1.25 mL of 1.5 M Tris (pH 8.8), 50 mL 10% ammonium persulfate, and 5 mL TEMED, swirled gently, transferred to a plate, and allowed to polymerize. Then, 1.2 mL of stacking gel (3.13 mL distilled water, 0.62 mL of 40% acrylamide/bis stock, 1.25 mL of 1.5 M Tris [pH 6.8], 50 µL of 10% ammonium persulfate, and 5 µL TEMED) was overlaid onto the separating gel and a 10-well of 1.0 mm comb was placed on to the gel. Next, 10 µg of protein lysate samples was loaded into the well of SDS-PAGE gels and separated using the Mini-PROTEAN (Bio-Rad), along with the molecular weight markers. The gel was run at 120 V (±30 mA) for 1 hour or until dye front almost runs off the bottom of the gel. Then, the membrane was cut to 10 cm × 7 cm together with 2 filter papers of the same dimensions and was soaked in Transfer/Towbin buffer for 15 minutes. After separation, proteins were electrotransferred by employing a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad) onto a nitrocellulose membrane at 100 V for 1 hour. Membranes were blocked with 5% bovine serum albumin in a Tris-buffered saline (TBS)/Tween (0.14 M NaCl, 0.05 M HCl, 0.1% Tween 20) for 1 hour and then probed overnight at 4 °C with primary antibodies. After blocking, the membrane was washed 3 times for 5 minutes each with 15 mL of TBST and continued the incubation of the membrane with the species-appropriate horseradish peroxidase (HRP)-conjugated secondary antibody and anti-biotin, HRP-linked antibody (at 1:1000-1:3000) to detect biotinylated protein markers in 10 mL of blocking buffer with gentle agitation for 1 hour at room temperature. Then, the membrane was rewashed with TBST for 5 minutes. For the protein detection and development, the membrane was incubated in 1× Red Alert for 5 minutes and rinsed with water to obtain a bright protein band.
Statistical Analysis
All data are representative of 3 experiments and expressed as mean ± standard deviation (SD). Statistical evaluation was performed by t test using GraphPad Prism 6.0 Software. A P value of less than .05 (P < .05) was considered to be statistically significant.
Results
Eupatorin Caused a Cytotoxic Effect in 4T1 Cells Proliferation
Eupatorin caused a time (24, 48, and 72 hours) and dosage (0.16-20 µg/mL) dependent inhibition of cell proliferation toward 4T1 cells (Table 2; Figure 2). At 24 hours, the IC50 value of eupatorin was higher than 20 µg/mL. When the incubation time was extended for 48 hours, the 4T1 cells exhibited an IC50 value of 6.00 µg/mL. At 72 hours, the IC50 of 4T1 cells was 5 µg/mL. Cytotoxicity of eupatorin was lower than the positive control doxorubicin with the IC50—1.50, 0.90, and 0.60 µg/mL at 24, 48, and 72 hours, respectively (Figure 2).
Table 2.
Percentage of 4T1 Cells Killed After 24 Hours Cocultured With Dissociated Spleen Harvested From Group of Healthy Mice, Untreated Breast Tumor Mice and Group of Mice That Had Received Daily Treatment of Eupatorin at the Dosage of 5 mg/kg and 20 mg/kg Eupatorin Daily After 4T1 Cells Inductiona.
| NK vs 4T1 ratio | Healthy (%) | Untreated (%) | Eupatorin 5 mg/kg (%) | Eupatorin 20 mg/kg (%) |
|---|---|---|---|---|
| 2:1 | 20.11 ± 5.97 | 7.40 ± 1.73 | 24.29 ± 2.87a | 26.65 ± 1.99a |
| 10:1 | 25.92 ± 4.26 | 10.33 ± 2.99 | 27.17 ±2.94b | 32.57 ± 3.19b |
Hundred microliters of viable spleen cells at concentrations 2 × 105 cells/mL (2:1) and 1 × 106 cells/mL (10:1) was plated in a 96-well plate containing 4T1 cells for 24 hours, followed by MTT assessment. (aStatistical significance [P < .05] between untreated mice and treated mice for E:T ratio 2:1; bStatistical significance [P < .05] between untreated mice and treated mice for E:T ratio 10:1).
Figure 2.
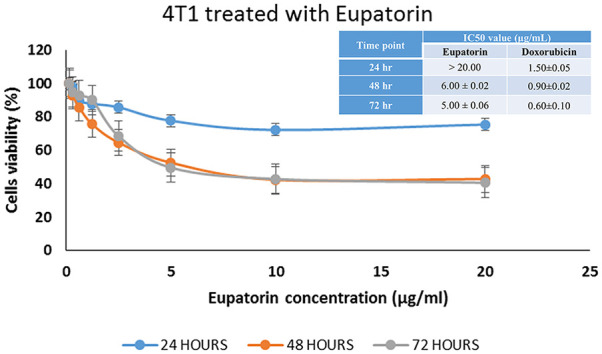
Effect of eupatorin on 4T1 cell cytotoxicity for 24, 48, and 72 hours using MTT assay. The IC50 value of eupatorin after 24-, 48-, and 72-hour incubation time on 4T1 cells. Values are expressed as mean ± SD for 3 independent observations.
Behavior Changes, Physical Assessment, Tumor Growth, and Tumor Weight in Murine Breast Cancer Balb/c Mice for 28 Days of the Experiment
The tumor growth in female Balb/c mice was developed as early as 9 days after the injection of 4T1 cells into the mammary fat pad. During the experiment, all mice survived throughout the 28 days of study.
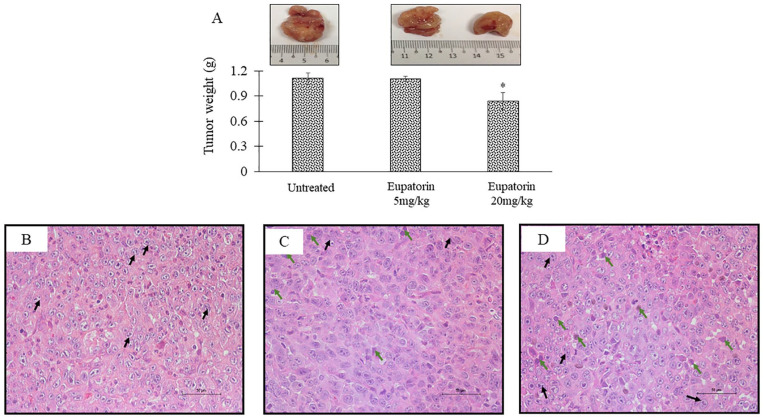
Figure 3A shows the size of tumor that was harvested after 28 days of the experiment. The 4T1-induced mice treated with the high dosage of eupatorin at 20 mg/kg BW had the smallest tumor compared with the mice in the untreated group and low-dosage group. In addition, the group of mice fed with 5 mg/kg BW eupatorin did not show any significant reduction in the tumor weight (TW; 1.102 ± 0.033 g), while tumor mice treated with 20 g/kg BW had significantly lower (P < .05) TW (0.839 ± 0.104 g) as compared with the untreated, which demonstrated the TW of 1.110 ± 0.067 g (Figure 3A). This suggested that eupatorin at the dosage of 20 mg/kg BW is sufficient to reduce the tumor size in mice.
Figure 3.
(A) Tumor weight after 28 days of the experiment. The images of harvested tumor represent the untreated tumor, tumor mice treated with 5 mg/kg eupatorin, and tumor mice treated with 20 mg/kg eupatorin. Excised tissue sections were stained with hematoxylin and eosin (H&E) staining and viewed under the microscope (Nikon) at 20× magnification. The representative images of tumor tissue section stained with H&E in (B) untreated tumor, (C) tumor mice treated with 5 mg/kg eupatorin, and (D) tumor mice treated with 20 mg/kg eupatorin. Statistical analysis was performed using unpaired t test. Data are presented as mean values ± SD of n = 5 independent experiments (*Statistical significance [P < .05] between untreated and treated groups).
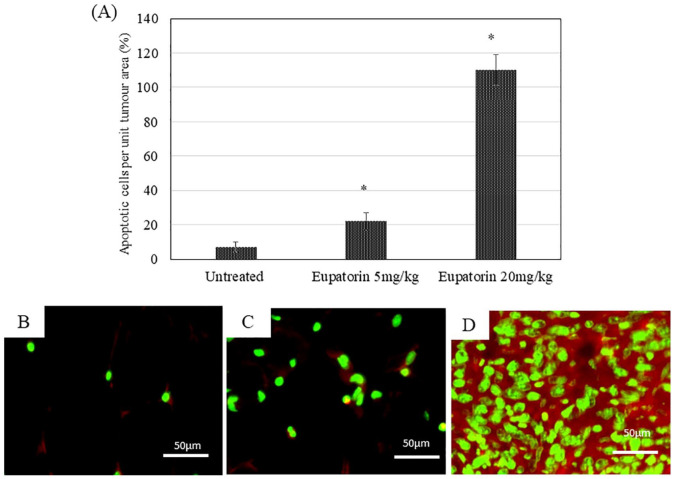
Hematoxylin and eosin staining was performed to evaluate the histology of tumor morphology (Figure 3B). Signs of tissue necrosis were observed in the tumor for mice treated with eupatorin (Figure 3C and D) where apoptotic cells (green arrow) were seen and where nuclei become pyknotic (smaller) or missing. Furthermore, the apoptotic cell appears as a round or oval mass with dark eosinophilic cytoplasm and dense purple nuclear chromatin fragments. Moreover, the cells have shrunk and are smaller in size, the cytoplasm is dense, and the organelles are more tightly packed. In contrast, tumor tissue from mice treated with olive oil revealed the nucleus of the cancer cell was large and oval-shaped, where more chromatin was visible (Figure 3C). Figure 4 revealed that eupatorin has significantly enhanced the number of apoptotic cells, as indicated by the presence of green fluorescent staining cells in the tumor. The number of apoptotic cells present in the tumor treated with eupatorin at 20 mg/kg BW was 15-fold higher, while tumor treated with 5 mg/kg BW eupatorin exhibited 5-fold higher apoptotic cells when compared with the untreated. These results indicate that eupatorin may, directly and indirectly, induce apoptosis of tumor cells.
Figure 4.
Apoptotic cells quantification of the harvested tumor section stained with TUNEL apoptosis detection kit (FITC-labelled) and viewed under the microscope (Nikon) at 40× magnification. The number of apoptotic cells in the tumor section from 5 hematoxylin and eosin (H&E)-stained slides in each group is represented in (A). The representative images of TUNEL labeled tumor tissue from (B) untreated mice, (C) tumor mice treated with 5 mg/kg eupatorin, and (D) tumor mice treated with 20 mg/kg eupatorin. Statistical analysis was performed using unpaired t test. Data are presented as mean values ± SD of n = 5 independent experiments (*statistical significance [P < .05] between untreated and treated groups).
Eupatorin Reduced the Aggressiveness of Metastatic 4T1 Cells Invaded into the Lung
Eupatorin treatment reduced the number of the invaded 4T1 cells in the lung as shown by clonogenic assay. In the clonogenic assay, the number of clonogenic metastatic cells was enumerated because 4T1 cells are resistant to 6-thioguanine, whereas the nonresistant normal cells are killed. The results in Figure 5 showed that the blue spots of clonogenic tumor cells were higher in the lung of the untreated mice compared with those treated mice fed with 5 mg/kg BW and 20 mg/kg BW eupatorin daily. The blue colony of clonogenic tumor cells in mice treated with 20 mg/kg BW showed a remarkably low number of cancer cells that survived compared with mice treated with 5 mg/kg BW eupatorin. Figure 5 shows that the number of blue spots in the clonogenic assay for mice treated with 5 mg/kg BW and 20 mg/kg BW eupatorin was 292.500 × 103 ± 67.175 and 30.700 × 103 ± 10.889 colonies, respectively, while the number of cancer cells surviving in the lung of an untreated mouse was presented by 400.000 × 103 ± 28.284 blue colonies.
Figure 5.
Lung metastases were investigated on day 28 via clonogenic assay from untreated mouse (fed with olive oil), 5 mg/kg and 20 mg/kg body weight (BW) eupatorin-treated mouse. Blue colony represented the breast cancer cells that invaded into the lung and survived. The number of cancer cell colonies that survived in lung harvested from breast cancer mice was enumerated. Statistical analysis was performed using the unpaired t test. Data are presented as mean values ± SD of n = 3 independent experiments (*statistical significance [P < .05] between untreated and treated group).
Eupatorin Enhanced the Expression of Natural Killer (NK) and T-Cells in Breast Cancer Mice
Figure 6 reveals the striking difference in respective spleen size and weight between a healthy spleen and a spleen from a mouse with the tumor for 28 days. As shown in Figure 6A, the spleen harvested from female Balb/c mouse bearing with tumor either treated or untreated was extraordinarily bigger than spleen of control healthy mouse. The spleen size was connected to its weight. Based on Figure 6A, the average weight of spleen removed from the group of untreated tumor mice were 7 times higher than healthy spleen, which demonstrated 0.778 ± 0.047 g, while the average weight of spleen of healthy mice was 0.105 ± 0.014 g. Besides, the spleen weight of tumor mice treated with 5 mg/kg and 20 mg/kg eupatorin exhibited 0.617 ± 0.123 g and 0.590 ± 0.148 g, respectively.
Figure 6.
The weight of harvested spleen was presented in (A) where representative images of harvested spleen presented healthy mice (fed with olive oil), untreated tumor mice (fed with olive oil), mice fed with 5 mg/kg eupatorin in olive oil, and mice fed with 20 mg/kg eupatorin in olive oil. (B-E) Representative images of the spleen tissue section harvested from (B) healthy mice, (C) untreated 4T1 tumor mice (fed with olive oil), (D) 4T1 tumor mice fed with 5 mg/kg body weight (BW) eupatorin in olive oil, and (E) breast cancer mice fed with 20 mg/kg BW eupatorin in olive oil. Excised tissue sections were stained with hematoxylin and eosin staining and viewed under the microscope (Nikon) at 400× magnification. Scale bar: 50 µm. (B) Normal distribution of white pulp (WP) and red pulp (RP) in the healthy spleen. (C) Diffuse WP and RP distribution with the presence of giant cells (black arrow) in the untreated mouse. Statistical analysis was performed using the unpaired t test. Data are presented as mean values ± SD of n = 3 independent experiments (*statistical significance [P < .05] between healthy mice and tumor mice).
Histologic examination (Figure 6B) of the spleen showed a normal distribution of red pulp and white pulp area in the healthy spleen. In contrast, the distribution of white pulp and red pulp in untreated (Figure 6C) spleen was dispersed severely. Besides, multiple giant cells of pale eosinophils, which indicate inflammation, were observed in the untreated mouse spleen. For spleen treated with eupatorin, the number of the giant cells present in the treated mouse spleen with 5 mg/kg eupatorin (Figure 6D) was less than the untreated mouse spleen, while in mouse spleen treated with 20 mg/kg eupatorin (Figure 6E), there was no sign of the existence of giant cells. However, the area of white pulp in mouse spleen for both dosages was expanded, and this was consistent with the size expansion of tumor spleen compared with the healthy spleen.
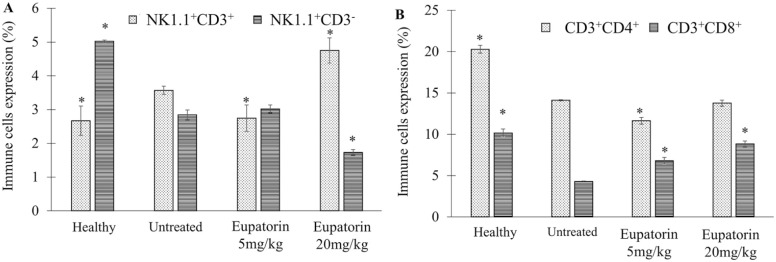
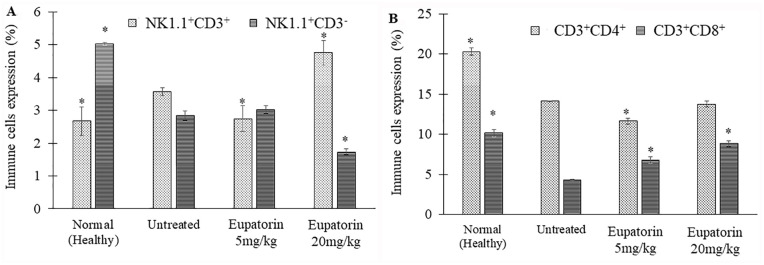
Based on Figure 7A, flow cytometric analysis of immunophenotyping assay revealed that the untreated 4T1 tumor mice exhibited the NK1.1 + CD3 cells (3.57 ± 0.125%), slightly higher than healthy mice (2.67 ± 0.433%; Figure 7A). When the 4T1 tumor mice were treated with eupatorin (20 mg/kg BW), the NK1.1 + CD3 cells production was significantly (P < .05) enhanced to 4.75 ± 0.370%, which was higher than the untreated mice. The population of NK1.1 − CD3 cells in healthy mice were significantly (P < .05) higher than the untreated 4T1 tumor mice; the level of expression was 5.023 ± 0.035% and 2.84 ± 0.150%, respectively. Eventually, the depletion of NK1.1 − CD3 cells was also observed in the eupatorin high-dose group. Eupatorin (20 mg/kg) significantly (P < .05) reduced the expression of the NK1.1 − CD3 to 1.73 ± 0.085%, lower than the untreated mice (2.84 ± 0.150%).
Figure 7.
Immune cells expression by the percentage of (A) NK1.1+CD3+ and NK1.1+CD3− expression and (B) CD3+CD4+ and CD3+CD8+ expression as assessed by flow cytometry analysis. Data are presented as mean values ± SD of n = 3 independent experiments. *Statistical significance (P < .05) compared with the mice in the untreated group.
Figure 7B showed that healthy mice had the highest expression of CD4+ and CD8+ with 20.287 ± 0.623% and 10.16 ± 0.474%, respectively. In contrast, CD8+ depletion was observed in mice induced with 4T1 cells for all groups, including untreated and treated. However, eupatorin treatment at a dosage of 20 mg/kg managed to overcome the reduction of the CD8+ cells occurring in treated mice and raised the CD8+ expression from 4.297 ± 0.067% (untreated) to 8.83 ± 0.358%. For CD4+ expression, the level of expression of treated mice (20 mg/kg eupatorin) was slightly lower (13.76 ± 0.729%) than the untreated (14.107 ± 0.344%) mice.
Suppressor Activity of Splenocytes of Tumor-Bearing Mice Against 4T1 Cells
The 4T1 cells were used as unmodified targets for the detection of cytolytic activity of freshly isolated immune or control splenocytes. Table 2 showed that the percentage of cytotoxicity on the 4T1 cells by the effector cells isolated from untreated mice was the lowest for respective Effector:Target (E:T) ratio 2:1 (7.40 ± 1.73%) and 10:1 (10.33 ± 2.99%). In contrast, splenocytes from mice treated with high-dose eupatorin (20 mg/kg BW) induced cytotoxicity of 32.57 ± 3.19% on 4T1 target cells at E:T ratio 10:1, and significant (P < .05) cytotoxicity was also observed even at E:T ratio 2:1 with 26.65 ± 1.99% when compared with the untreated splenocytes. The previous study reported that this cytotoxicity could be associated with CD8+ T and NK cell activity. Therefore, these data show that eupatorin (20 mg/kg) significantly (P < .05) increased NK cells and T-cell responses to 4T1 tumor cells.
Cytokine Assay of Blood Serum
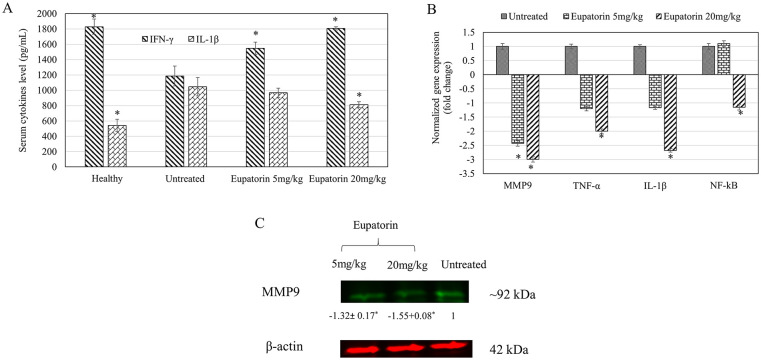
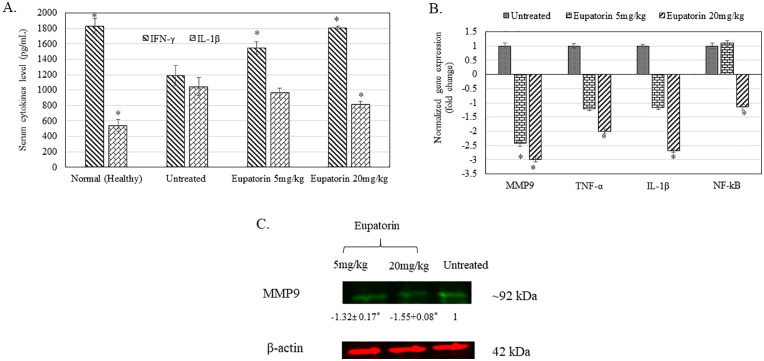
As shown in Figure 8A, the concentration of detected IL-1β and IFN-γ in blood serum harvested from mice (untreated and eupatorin-treated) and healthy mice was revealed. Generally, high-dosage eupatorin (20 mg/kg) has significantly (P < .05) suppressed the level of IL-1β concentration in treated mice to 812.000 ± 57.504 pg/mL, while there is no significant change shown for eupatorin in the low-dosage (5 mg/kg; 968.000 ± 69.282 pg/mL) group when compared with the untreated group (1045.333 ± 257.976 pg/mL). In contrast, significant (P < .05) reduction of the serum IFN-γ cytokine in the untreated tumor mice group (1185.185 ± 187.862 pg/mL) was observed when compared with the healthy mice (1829.630 ± 46.259 pg/mL). By treating the tumor mice with eupatorin, the concentration of IFN-γ cytokine was significantly (P < .05) enhanced. IFN-γ secretion in the blood serum from 4T1-induced mice treated with 5 mg/kg eupatorin and 20 mg/kg eupatorin exhibited 1548.148 ± 114.036 pg/mL and 1407.407 ± 25.660 pg/mL, respectively.
Figure 8.
(A) Cytokine value of IL-1β and IFN-γ in blood serum collected from healthy mice, untreated 4T1-induced mice, and treated group of 4T1-induced mice fed with 5 mg/kg eupatorin and 20 mg/kg eupatorin daily for 28 days. (B) Expression of targeted genes MMP9, TNF-α, IL-1β, and NF-κB in tumor harvested from untreated mice and treated mice with 5 mg/kg and 20 mg/kg eupatorin. Data were normalized with the reference gene of GAPDH and transformed in fold change number. (C) The representative of stained-free gel. Immunofluorescence imaging of protein lysate of the putative housekeeping proteins of β-actin and MMP9, extracted from the tumor. The sample sequences are as follows: untreated tumor mice (right), treated tumor mice fed with 5 mg/kg eupatorin (left), and treated tumor mice fed with 20 mg/kg eupatorin (center) daily for 28 days. Statistical analysis was performed using the unpaired t test. Data are presented as mean values ± SD of n = 3 independent experiments. *Statistical significance (P < .05) compared with the mice in the untreated group.
RT-qPCR Analysis
Figure 8B reveals that expression of MMP9, TNF-α, IL-1β, and NF-κB genes were significantly downregulated (normalized fold change >2) after mice bearing tumors were treated with 20 mg/kg of eupatorin as detected in real-time PCR. For mice treated with 5 mg/kg eupatorin, only MMP9 was significantly downregulated. Overall, these data showed that eupatorin altered the expression of targeted genes in a dosage-dependent manner.
Western Blot Analysis
Figure 8C showed the protein band of MMP9 in tumor mice. Eupatorin at the dosage of 20 mg/kg reduced the level of MMP9 expression in the tumor by −1.55 fold than the untreated. Therefore, this result supported the qPCR gene expression analysis, where eupatorin treatment suppressed the expression of MMP9 in the tumor.
Discussion
A 4T1-based mouse model was developed to mimic the multi-organ metastasis of human breast cancer.9 This animal model was a basal type, triple-negative ductal carcinoma that represented a distinct subtype of invasive carcinoma that has poor prognosis.9,13 Therefore, it is highly reproducible and suitable for testing the success of various chemotherapy drugs used in the treatment of advanced/metastatic breast cancer due to the similarity between the formation of various organ metastasis from its cell line to human breast cancer.13
The in vivo experiments showed no sign of toxic effect caused by eupatorin, and all the mice survived throughout the 28 days of treatment. Eupatorin effectively caused in vitro cytotoxicity to the 4T1 cells by inhibiting 50% of cell viability (IC50) at 6 µg/mL after 48 hours of incubation. In terms of in vivo antitumor effect, eupatorin at 20 mg/kg BW dosage was able to inhibit the tumor growth in Balb/c mice where the harvested tumors of this group were ~27% smaller than the untreated mice. In addition, TUNEL labeling of apoptotic cells of the tumor tissue section revealed that the number of apoptotic cells in eupatorin-treated tumor (20 mg/kg BW dosage) was higher compared with the untreated. This result agrees with the previous in vitro analysis, where eupatorin induced the apoptosis on MDA-MB-231 cells after 24 hours treatment.14 However, the in vitro cytotoxicity of eupatorin to 4T1 cells were ~6 fold and ~8 fold lower compared with the positive control doxorubicin for 48 and 72 hours of treatment (Figure 2). In addition, ~27% reduction of TW by 20 mg/kg of eupatorin was lower when compared with the 37% reduction by 5 mg/kg of doxorubicin, which was reported by Franco et al.15
Besides apoptosis, eupatorin was able to suppress 4T1 metastasis to lung. Usually, metastases in this mouse model are predominately found in the lung than other organs such as brain and lymph nodes, and metastatic tumor cells was reported to destroy the structure of pulmonary alveoli in untreated tumor mouse.12 Eupatorin (5 and 20 mg/kg) prevented the aggressive invasion and migration of the primary tumors and thus reduced ~26.8% and ~92.5% of tumor incidence, respectively, as shown by the clonogenic assay. The anti–lung metastasis effect of 20 mg/kg of eupatorin was better than 86% reduction by 5 mg/kg of doxorubicin, which was reported by Franco et al.15
In the spleen of the untreated mice, the distribution of white pulp and red pulp was dispersed and the presence of giant cells was detected.16 Tumor breast cancer cells spontaneously caused inflammation in the spleen, while the primary tumor was still growing in situ.17 Inflammatory breast cancer is the aggressive form of advanced breast cancer associated with a high probability of metastasis. Pro-inflammatory cytokines, including TNF-α and IL-1β, were the major factors contributing to breast cancer metastasis through induction of epithelial-to-mesenchymal transition (EMT).18 Overexpression of TNF-α and IL-1β activates the NF-κB signaling pathway that subsequently regulates the expression of MMP9, which degrades the extracellular matrix to promote the invasion of cancer cells. In contrast, gene knockdown of MMP9 and TNF-α expression suppressed the regulation of NF-κB genes that are involved in the migration of cancer cells and thus inhibited cells metastases.19-21 Eupatorin (20 mg/kg BW) reduced the inflammatory effect as no giant cells were observed even though the area of white pulp and red pulp was expanded relative to the healthy spleen. Previous study has reported that eupatorin in Orthosiphon stamineus contributed to the anti-inflammatory effect of this herb through inhibition of TNF-α production.22 Similarly, mice treated with 20 mg/kg eupatorin were observed with downregulation of pro-inflammatory cytokines including TNF-α and IL-1β associated with lower level of NF-κB expression. Downregulation of NF-κB expression in the tumor by eupatorin may contribute to the higher apoptosis rate in the tumor as previous study has shown that flavonoid-rich white mulberry fraction induced apoptosis of cancer cells via suppression of NF-κB signaling.23 Moreover, downregulation of NF-κB was also associated with the suppression of MMP9 production in the tumor of the 20 mg/kg eupatorin-treated mice. Overall, this finding postulates that the anti-inflammatory effect of eupatorin suppressed the factors contributing to EMT and thus reduced the intensity of 4T1 metastasis to the lung as observed in Figure 5.
On the other hand, eupatorin enhanced the immunity of tumor mice, which may also contribute to the delay of tumor progression of 4T1 challenged mice. The levels of NK1.1+CD3 and CD8+ cells in tumor mice treated with eupatorin (20 mg/kg BW) were significantly enhanced. This was associated with a higher level of serum IFN-γ and greater splenocyte cytotoxicity against 4T1 cells. IFNs are multifunctional cytokines that regulate cellular and immune responses as well as antiviral and antitumor activity.24 IFNs are pleiotropic cytokines with diverse biological effects, including antiviral, antiangiogenic, immunomodulatory, cell cycle inhibitory effects, and apoptotic functions.24 NK cells are an important source of IFN and play an essential role in early innate response.25 The previous study reported that depletion of CD3−NK1.1+ resulted in low levels of IFNs such as IFN-γ.26 The CD3−NK1.1+ cells produced IFN-γ on stimulation with NK1.1 cross-linking in the presence of IL.26 Numerous studies revealed that IFN-γ is one of the IFNs that play an important role in tumor immunity27,28 through direct suppression of cancer cell proliferation and activation of apoptotic signals.24 From previous reports, IFN-γ has a multiplicity of actions and had been shown to be a critical factor in primary tumor growth and metastasis of breast cancer. Furthermore, IFN-γ has a profound influence on the growth and metastasis of solid tumors including in mice transplanted with the murine mammary carcinoma 4T1 cells.29 Therefore, slowed development of primary tumor in the eupatorin-treated group could be due to the stimulation of IFN-γ cytokine. Besides NK cells, CD8+T cells are the major effector cells mediating tumor cytotoxicity.10 When the antitumor immunity was activated, CD8+ T cells are predominantly recruited and activated according to the higher production of IFN-γ.10 In this study, IFN-γ cytokine production and populations of NK cells and CD8+ T cells were enhanced in tumor mice treated with 20 g/kg BW eupatorin. This immune stimulation by eupatorin may contribute to the antitumor and antimetastatic effects that were observed in the treated mice as previous study has reported that activation of NK and T-cells enhanced the spleen cytotoxicity toward 4T1 cells.12 In addition, DuPre et al29 also suggested that CD8 T cells and NK cells are the best candidates to initiate antitumor immunity via direct cytotoxicity and through secreting intratumoral IFN-γ.
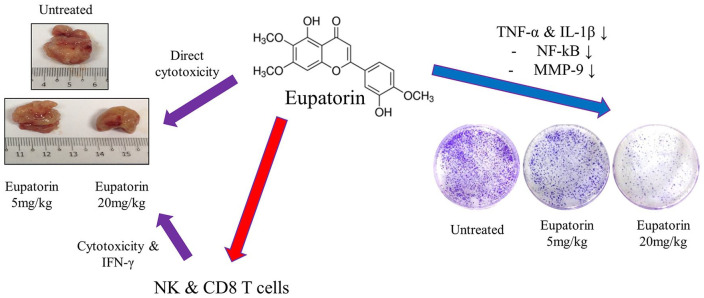
Based on the above-mentioned results, 20 mg/kg of eupatorin was able to kill 4T1 cells via direct cytotoxicity and indirect apoptosis induction by activating the cytotoxicity and IFN-γ cytokine production of NK cells and CD8+ T cells. Eupatorin also suppressed the 4T1 metastasis to the lung by downregulating the expression of TNF-α and IL-1β, which are the pro-inflammatory cytokines that regulate the NF-κB signaling pathway. Suppression of the NF-κB subsequently inhibited the production of MMP9 gelatinase, which is responsible for the degradation of the extracellular matrix during cancer cells metastasis (Figure 9).
Figure 9.
Mode of action for the eupatorin in vivo antitumor and antimetastatic effects.
Conclusion
The present study has demonstrated that the eupatorin at the highest dosage of 20 mg/kg BW is effective for delaying the 4T1-induced breast tumor growth in the animal model. This study signifies the in vivo efficacy and the potential of eupatorin for breast cancer therapeutic purposes.
Footnotes
Author Contributions: SKY, NBA, and KL designed the experiments. NAR, WYH, CYY, and SKY performed the experiments. NAR and WST performed the qPCR experiment and analysis. NAR, WYH, WST, NBA, KL, and SKY performed data analysis. SKY, NBA, WYH, and KL supported the research items. NAR, NBA, and SKY wrote the article. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Universiti Putra Malaysia and MARDI Internal Grant (Vote No. 6300300). The funder has no role/influence in this study.
ORCID iD: Noorjahan Banu Alitheen  https://orcid.org/0000-0003-1966-8580
https://orcid.org/0000-0003-1966-8580
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonofiglio D, Giordano C, De Amicis F, Lanzino M, Ando S. Natural products as promising antitumor agents in breast cancer: mechanisms of action and molecular targets. Mini Rev Med Chem. 2016;16:596-604. [DOI] [PubMed] [Google Scholar]
- 4. Ahamed MBK, Aisha AFA, Nassar ZD, et al. Cat’s whiskers tea (Orthosiphon stamineus) extract inhibits growth of colon tumor in nude mice and angiogenesis in endothelial cells via suppressing VEGFR phosphorylation. Nutr Cancer. 2012;64:89-99. [DOI] [PubMed] [Google Scholar]
- 5. Androutsopoulos V, Arroo RRJ, Hall JF, Surichan S, Potter GA. Antiproliferative and cytostatic effects of the natural product eupatorin on MDA-MB-468 human breast cancer cells due to CYP1-mediated metabolism. Breast Cancer Res. 2008;10:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salmela AL, Pouwels J, Kukkonen-Macchi A, et al. The flavonoid eupatorin inactivates the mitotic checkpoint leading to polyploidy and apoptosis. Exp Cell Res. 2012;318:578-592. [DOI] [PubMed] [Google Scholar]
- 7. Androutsopoulos VP, Ruparelia K, Arroo RRJ, Tsatsakis AM, Spandidos DA. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology. 2009;264:162-170. [DOI] [PubMed] [Google Scholar]
- 8. Taheri A, Dinarvand R, Ahadi F, Khorramizadeh MR, Atyabi F. The in vivo antitumor activity of LHRH targeted methotrexate-human serum albumin nanoparticles in 4T1 tumor-bearing Balb/c mice. Int J Pharm. 2012;431:183-189. [DOI] [PubMed] [Google Scholar]
- 9. McCarthy M, Auda G, Agrawal S, et al. In vivo anticancer synergy mechanism of doxorubicin and verapamil combination treatment is impaired in BALB/c mice with metastatic breast cancer. Exp Mol Pathol. 2014;97:6-15. [DOI] [PubMed] [Google Scholar]
- 10. Yang X, Chu Y, Wang Y, Guo Q, Xiong S. Vaccination with IFN-inducible T cell alpha chemoattractant (ITAC) gene-modified tumor cell attenuates disseminated metastases of circulating tumor cells. Vaccine. 2006;24:2966-2974. [DOI] [PubMed] [Google Scholar]
- 11. Lee BC, Jung MY, Cho D, O-Sullivan I, Cohen EP, Sung T. Immunity to Trop-1, a newly identified breast cancer antigen, inhibits the growth of breast cancer in mice. Vaccine. 2010;28:7757-7763. [DOI] [PubMed] [Google Scholar]
- 12. Mkrtichyan M, Ghochikyan A, Davtyan H, et al. Cancer-testis antigen, BORIS based vaccine delivered by dendritic cells is extremely effective against a very aggressive and highly metastatic mouse mammary carcinoma. Cell Immunol. 2011;270:188-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bao L, Haque A, Jackson K, et al. Increased expression of p-glycoprotein is associated with doxorubicin chemoresistance in the metastatic 4T1 breast cancer model. Am J Pathol. 2011;178:838-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Razak NA, Abu N, Ho WY, et al. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep. 2019;9:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franco MS, Roque MC, Barros ALB, Silva JO, Cassali GD, Oliveira MC. Investigation of the antitumor activity and toxicity of long-circulating and fusogenic liposomes co-encapsulating paclitaxel and doxorubicin in a murine breast cancer animal model. Biomed Pharmacother. 2019;109:1728-1739. [DOI] [PubMed] [Google Scholar]
- 16. Sally AD, Hunter KW. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;2:12-24. [DOI] [PubMed] [Google Scholar]
- 17. Gao ZG, Tian L, Hu J, Park IS, Bae YH. Prevention of metastasis in a 4T1 murine breast cancer model by doxorubicin carried by folate conjugated pH sensitive polymeric micelles. J Control Release. 2011;152:84-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen EN, Gao H, Anfossi S, Mego M, Reddy NG, Debeb B. Inflammation mediated metastasis: immune induced epithelial-to-mesenchymal transition in inflammatory breast cancer cells. PLoS One. 2015;10:e0132710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee SJ, Lim JH, Choi YH, Kim WJ, Moon SK. Interleukin-28A triggers wound healing migration of bladder cancer cells via NF-κB–mediated MMP-9 expression inducing the MAPK pathway. Cell Signal. 2012;24:1734-1742. [DOI] [PubMed] [Google Scholar]
- 20. Lin KL, Chien CM, Hsieh CY, Tsai PC, Chang LS, Lin SR. Antimetastatic potential of cardiotoxin III involves inactivation of PI3K/Akt and p38 MAPK signaling pathways in human breast cancer MDA-MB-231 cells. Life Sci. 2012;90:54-65. [DOI] [PubMed] [Google Scholar]
- 21. Palmieri D, Astigiano S, Barbieri O, et al. Procollagen I COOH-terminal fragment induces VEGF-A and CXCR4 expression in breast carcinoma cells. Exp Cell Res. 2008;314:2289-2298. [DOI] [PubMed] [Google Scholar]
- 22. Laavola M, Nieminen R, Yam MF, et al. Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation. Planta Med. 2012;78:779-786. [DOI] [PubMed] [Google Scholar]
- 23. Alam AK, Hossain AS, Khan MA, et al. The antioxidative fraction of white mulberry induces apoptosis through regulation of p53 and NFκB in EAC cells. PLoS One. 2016;11:e0167536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ryu H, Oh JE, Rhee KJ, et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-β and suppress the growth of MCF-7 human breast cancer cells. Cancer Lett. 2014;352:220-227. [DOI] [PubMed] [Google Scholar]
- 25. Reid-Yu SA, Small CLN, Coombes BK. CD3-NK1.1+ cells aid in the early induction of a Th1 response to an attaching and effacing enteric pathogen. Eur J Immunol. 2013;43:2638-2649. [DOI] [PubMed] [Google Scholar]
- 26. Iwabuchi K, Iwabuchi C, Tone S, et al. Defective development of NK1.1+ T-cell antigen receptor αβ+ cells in zeta-associated protein 70 null mice with an accumulation of NK1.1+ CD3-NK-like cells in the thymus. Blood. 2001;97:1765-1775. [DOI] [PubMed] [Google Scholar]
- 27. Mamidi S, Cinci M, Hasmann M, Fehring V, Kirschfink M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol. 2013;7:580-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Younos I, Donkor M, Hoke T, et al. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11:814-824. [DOI] [PubMed] [Google Scholar]
- 29. DuPre SA, Redelman D, Hunter KW. Microenvironment of the murine mammary carcinoma 4T1: endogenous IFN-γ affects tumor phenotype, growth, and metastasis. Exp Mol Pathol. 2008;85:174-188. [DOI] [PubMed] [Google Scholar]