Abstract
Objective
Our aim was to conduct a pilot randomized controlled trial of a novel cognitive behaviorally based intervention for pediatric PNES called Retraining and Control Therapy (ReACT).
Methods
Participants were randomized to receive either eight sessions of ReACT or supportive therapy, and participants completed follow‐up visits at 7‐ and 60‐days posttreatment. The primary outcome measure was PNES frequency at 7‐days posttreatment. Eligibility criteria included children with video‐EEG confirmed PNES and participant/parent or guardian willingness to participate in treatment. Exclusion criteria included substance use, psychosis, and severe intellectual disability. Forty‐two patients were assessed for eligibility and 32 were randomized. ReACT aimed to retrain classically conditioned, involuntary PNES by targeting catastrophic symptom expectations and a low sense of control over symptoms using principles of habit reversal. Supportive therapy was based on the assumption that relief from stress or problems can be achieved by discussion with a therapist.
Results
Twenty‐nine participants (M age = 15.1 years, SDage = 2.5; 72.2% female; 57.1% Caucasian, 28.6% African American) completed 7‐days postprocedures. For PNES frequency, the Wilcoxon Rank Sum test statistic was 273.5 yielding a normal approximation of Z = 4.725 (P < 0.0001), indicating a significant improvement in PNES frequency for ReACT at 7‐days posttreatment compared to supportive therapy. Participants with PNES in the 7‐days posttreatment were removed from the study for additional treatment, resulting in no 60‐day follow‐up data for supportive therapy.
Interpretation
ReACT resulted in significantly greater PNES reduction than supportive therapy, with 100% of patients experiencing no PNES in 7 days after ReACT. Additionally, 82% remained PNES‐free for 60 days after ReACT.
Introduction
Psychogenic non‐epileptic seizures (PNES) are a functional neurological disorder (FND; also known as conversion disorder) characterized by seizure‐like symptoms without EEG correlates. 1 About 20% of patients in seizure clinics are diagnosed with PNES 2 with typical onset in adolescence or early adulthood. 3 PNES are severely debilitating to children and families, affecting academics, physical functioning, peer relationships, and finances. 4 , 5 , 6 , 7 , 8
The etiological understanding of PNES has evolved over time. Providers often explain PNES as a physical manifestation of psychological distress from disturbances in personal relationships, stress, childhood abuse, or trauma. 9 , 10 However, some patients with FND do not have a comorbid psychiatric diagnosis or trauma history, and for the ones who do, it is unclear if (and if yes, how) it is related to the etiology of their FND. 11 , 12 This suggests trauma and/or psychopathology may not be the most effective targets for PNES intervention.
Recent research has provided evidence for two additional risk factors associated with PNES: catastrophic symptom expectations and perceived control over symptoms. Children with PNES have greater catastrophic symptoms expectations such as, “When my head is pounding, I worry I could have a stroke,” 13 , 14 which can result in the occurrence of the expected symptoms. 15 Additionally, children report no control over their PNES, 16 indicating that children perceive PNES to be involuntary. Experimental research in adults has confirmed the finding of impaired perceived control over actions in patients with FNDs. 17 , 18 , 19 This supports the development of a novel PNES intervention targeting these factors.
Despite the significant prevalence, there are no published randomized clinical trials (RCTs) assessing the treatment of pediatric PNES. However, there have been a few RCTs published assessing treatment of adults with PNES. Based on these studies, psychotherapy appears to be a promising treatment in pilot studies. However, only between 25% and 56% of patients have achieved complete PNES remission in these studies, 20 , 21 , 22 suggesting that further refinement of psychotherapy‐based interventions is needed. These treatments use the psychoanalytic explanation for FND and target fear avoidance or work to attribute patients’ symptoms to psychosocial issues. 22 , 23 In the largest RCT assessing CBT for PNES in adults by targeting fear avoidance, there was no difference in PNES between CBT with standard medical care and standard medical care alone at 12 months after treatment. 24 These negative results suggest mood is not the most effective PNES treatment target and support the investigation of novel treatment targets.
The aim of this pilot study was to determine the effectiveness of Retraining and Control Therapy (ReACT), a novel cognitive behaviorally based therapy for treating pediatric PNES which targets catastrophic symptoms expectations and perceived control over symptoms.
Methods
Design overview
A 1:1 parallel‐group design with participants randomized to one of the two arms: Retraining and Control Therapy (ReACT) versus supportive therapy control. After screening, participants were randomized and follow‐up visits were conducted 7 (primary outcome measure) and 60 days after treatment. Seven days pre and posttreatment were chosen as the comparison times in order to include only prospectively recorded PNES frequency data since the baseline visit and first intervention visit were scheduled about one week apart. In order to prevent patients from being discharged from treatment after the 8‐week intervention, the protocol specified that if participants in either intervention continued to have PNES in the 7 days after the final treatment session they would have the option to be removed from the study and receive the other intervention.
Settings and participants
Participants were enrolled from November 2016 to April 2019 at the University of Alabama at Birmingham Department of Psychiatry. The study is registered at ClinicalTrials.gov (#NCT02801136). Institutional review board approval and written informed consent and assent were obtained. Eligibility criteria included children and adolescents with video‐EEG confirmed PNES and a parent or guardian willing to participate in the treatment. Comorbid diagnosis of epilepsy was acceptable as long as the patient’s neurologist confirmed epileptic seizures were controlled (seizure‐free for at least 6 months). Exclusion criteria included substance use, psychosis or severe intellectual disability.
Randomization and interventions
Randomization was prepared via statistician in closed envelopes in blocks of 6 with a unique randomization number generated for each participant. Research staff were blinded to group assignment during baseline and follow‐up visits, and the statistician was blinded to group assignment during analyses.
Trained Clinical Psychology PhD students conducted the therapy, and the first author (ADF), a clinical psychologist who developed ReACT, supervised each ReACT and supportive therapy session in person for at least 15 min to ensure treatment fidelity. Students who conducted supportive therapy had not received ReACT training in order to prevent treatment contamination. Since studies of these two treatment approaches have not been performed in children and adolescents previously, the study was presented to the PhD student therapists, parents, and children as an assessment of two equal interventions used to treat PNES, and there was no preference or bias assigned to either treatment.
ReACT
This manualized intervention is based on the Integrated Etiological Summary Model in which PNES are described as the result of catastrophic symptom expectations and/or classically conditioned responses. 25 It uses cognitive‐behavioral principles aimed at retraining classically conditioned, involuntary PNES by targeting catastrophic symptom expectations and a low sense of control over symptoms as opposed to adult treatments that target anxiety, depression and/or trauma. 23
The intervention includes four steps (Table 1): (1) a clear etiological description based on the Integrated Etiological Summary Model, 25 (2) an individually tailored patient plan to retrain physical symptoms which challenges catastrophic symptom expectations and teaches patients to engage in behaviors incompatible with PNES similarly to habit reversal, an evidence‐based behavioral treatment for retraining tics, 26 (3) a family plan to react to PNES in which they monitor the patient for safety but otherwise allow the patient to follow their plan to independently control the episode, and (4) a plan to return to school and social activities.
Table 1.
Detailed outline of Retraining and Control Therapy (ReACT) treatment sessions.
| Parent/guardian | Child | |
|---|---|---|
| Session 1 |
|
|
| Sessions 2–3 |
|
|
| Sessions 4–5 |
|
|
| Sessions 6–7 |
|
|
| Session 8 |
|
|
The treatment plan was developed in the initial session separately with the child and parent/guardian, and the child and parent/guardian meet together at the end of the session to review the plan. Topics covered in subsequent sessions were based on the patient’s progress throughout the week. Once PNES subsided, the therapist began generalizing the cognitive‐behavioral strategies to address general stressors. The final session was focused on relapse prevention, and a plan to control potential recurrent symptoms was developed.
Supportive therapy control intervention
Participants randomly assigned to the control group received eight sessions of non‐directive supportive therapy of the same duration and intensity as ReACT sessions. The etiological explanation given was that PNES are the result of stressors and anxiety. The provision of supportive therapy as a standard of care is based on the assumption that relief from stress or problems can be achieved by discussion with a therapist. The purpose is not to acquire new skills or find solutions to problems. 27 The sessions included discussion about life stressors, and therapists offered empathy. This approach is often used for general mood concerns. 27
Outcomes and follow‐up
The primary outcome was PNES frequency during the 7 days posttreatment. Other outcomes included mood, somatization, coping skills, and Health‐Related Quality Of Life (HRQOL). Participants and their parents prospectively recorded the number of PNES that occurred in the 7 days before treatment, 7 days posttreatment, and 60 days after their final visit using a daily PNES log. Discrepancies in PNES reported between the participant and parent were discussed in person, and a consensus was reached. Participants also completed the Adolescent Coping Orientation for Problem Experiences (ACOPE), Behavior Assessment System for Children, Second Edition (BASC‐2), Children's Somatic Symptoms Inventory (CSSI) and the Pediatric Quality of Life Inventory (PedsQL) Generic Core Scales at baseline and 7‐day follow‐up visits and the Childhood Trauma Questionnaire (CTQ) at baseline. The ACOPE identified the behaviors adolescents used in managing problems or difficult situations, 28 the BASC‐2 measured anxiety and depression, 29 the CSSI assessed a variety of somatic symptoms, 30 the PedsQL measured HRQOL, and the CTQ measured childhood history of abuse and neglect. 31 All data were double‐entered by independent researchers and compared for differences. Statistical differences between data entries were <3%, and all differences were assessed and corrected. Participants who discontinued the treatments early were encouraged to complete all follow‐up data assessments.
Statistical analyses
Power analysis
Based on the between‐groups effect size of 0.75 observed in a previous adult RCT for PNES treatment, 21 a sample size of 58 was planned to provide >80% power at the two‐tailed 0.05 α‐level to reject the null hypothesis of no difference in ReACT and control. However, after the first author (ADF) observed during the therapy sessions that all participants in ReACT had obtained PNES cessation compared to only one person in supportive therapy, the statistician (DL) performed the primary analysis and observed a test statistic of 273.5 which yielded a normal approximation of Z = 4.725 and P < 0.0001, resulting in early conclusion of the trial. As this was an unplanned interim analysis, we compared the observed test statistic and P‐value to the appropriate O’Brien‐Fleming boundaries we would have needed if this analysis was prespecified. Using the O’Brien‐Fleming alpha spending function, 32 29/58 = 50% of the data accrued, an overall two‐tailed Type 1 error rate of 0.05, the boundary indicates that any test with P‐value < 0.0056 would indicate a necessary stop. Thus, had we planned for this interim analysis, the trial would have been stopped.
Outcome analyses
Intention‐to‐treat analysis was conducted and included data from participants who discontinued therapy before eight sessions but remained in the study and continued data assessments. Based on published guidelines, 33 data from participants lost to all follow‐up (n = 3) were not imputed because all missing were in the supportive therapy arm which suggests the complete case is biased toward the null and imputing would strengthen the significance of the outcomes. The primary outcome of PNES frequency postintervention was compared using the Wilcoxon Rank Sum test. We initially planned to perform parametric analyses, but the lack of PNES in the ReACT group indicated that there was no variability in that group. Thus, most analyses that could have been considered would not be valid or reach statistical convergence. Therefore, the Wilcoxon Rank Sum test was employed since it tests for distribution differences between groups and not merely mean differences. Average postintervention questionnaire responses were compared between groups using linear models containing fixed effects for group and adjusting for baseline response. All analyses were generated using SAS software, Version 9.4 of the SAS System for Windows. Copyright © 2016 SAS Institute Inc. Cary, NC, USA.
Results
Baseline characteristics
Twenty‐nine participants were analyzed (Mage = 15.1 years, SDage = 2.5; 72.2% female; 57.1% Caucasian, 28.6% African American). Demographic data by the group are shown in Table 2, and epilepsy history and medication use are shown in Table 3. Baseline variables were not different between groups. Family history of epilepsy was present in 41.4%, and 10.3% had comorbid epilepsy. Some participants reported physical and emotional abuse, but no participants reported a history of sexual abuse on the CTQ. Twenty‐eight percent of participants had clinically significant scores for anxiety on the BASC‐2, 10% had clinically significant scores for depression and 21% had clinically significant scores for both anxiety and depression. Forty‐eight percent had no clinically significant elevations for anxiety or depression.
Table 2.
Demographics by group (only completers included).
| ReACT (n = 17) | Supportive (n = 12) | |
|---|---|---|
| Characteristics | % | % |
| Gender | ||
| Female | 82.4 | 58.3 |
| Race | ||
| Caucasian | 58.8 | 54.5 |
| African American | 29.4 | 27.3 |
| Other | 11.8 | 18.2 |
| # With history of physical abuse | 5 | 4 |
| # With history of sexual abuse | 0.0 | 0.0 |
| # With history of emotional abuse | 3 | 4 |
| Mean ± SD | Mean ± SD | |
| Age (y) | 14.9 ± 2.2 | 15.4 ± 2.9 |
| Months since PNES onset | 7.89 ± 7.15 | 7.04 ± 7.45 |
There are no significant differences between ReACT and supportive therapy for any baseline characteristic.
Table 3.
Medications and epilepsy history.
| ReACT (n = 17) | Supportive (n = 12) | |
|---|---|---|
| # | # | |
| Comorbid epilepsy | 2 | 1 |
| Family history of epilepsy | 8 | 4 |
| SSRI | 3 | 3 |
| SNRI | 2 | 0 |
| Buspirone | 1 | 0 |
| Antipsychotic | 3 | 1 |
| Antiepileptic medication | 3 | 2 |
| Benzodiazepine | 0 | 1 |
| Alpha‐2‐agonist | 0 | 1 |
| No medications | 9 | 7 |
There are no significant differences between ReACT and supportive therapy.
Subject disposition
As shown in Figure 1, 71% of the ReACT group and 67% of the supportive therapy group completed all eight therapy sessions. Participants’ reasons for ending treatment early differed by therapy assignment, with those in ReACT discontinuing because of PNES cessation and those in supportive therapy discontinuing because of worsening of or no improvement in symptoms or new cancer diagnosis. Ninety‐one percent of participants enrolled in the study completed at least one follow‐up visit, and all participants lost to all follow‐up visits (9%) had been assigned to the supportive therapy group.
Figure 1.
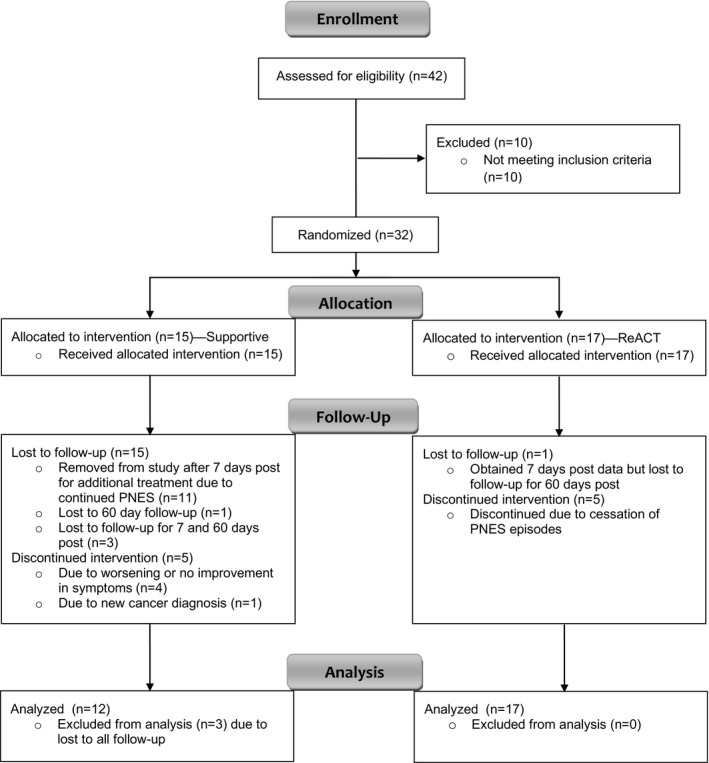
CONSORT flow diagram of the randomized clinical trial for pediatric psychogenic non‐epileptic seizures comparing Retraining and Control Therapy (ReACT) and supportive therapy.
Primary analysis of treatment effect on PNES frequency
The primary outcome measure was PNES frequency in the 7 days after treatment. For the number of PNES, the Wilcoxon Rank Sum test statistic was 273.5 which yielded a normal approximation of Z = 4.725 (P < 0.0001). This indicates that the distribution of PNES frequency at 7‐days post was significantly lower for ReACT than supportive therapy, with all participants in the ReACT group having no PNES and the supportive therapy group having an average of 3.0 (±2.5) PNES in the 7 days after treatment (Table 4). One participant in the supportive therapy group had no PNES 7 days after treatment, but they were lost to follow‐up at 60 days post. Because participants in supportive therapy who had PNES in the 7 days after treatment were removed from the study to receive additional treatment, there is no 60‐day PNES average for the supportive therapy group. It took an average of 4.6 sessions for participants in ReACT to have zero PNES.
Table 4.
PNES outcomes.
| ReACT | Supportive | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| PNES frequency 7 days before therapy | 9.9 ± 12.5 | 3.6 ± 2.8 1 |
| PNES frequency 7 days after therapy | 0.0 ± 0 | 3.0 ± 2.5 |
| PNES frequency 60 days after therapy | 0.56 ± 1.37 | NA 2 |
| Therapy sessions until 0 PNES reached | 4.6 ± 1.9 | NA |
Difference between ReACT and supportive therapy in PNES frequency 7 days before therapy is not significant.
Supportive participants were removed from the study for additional treatment if they experienced PNES within 7 days after completion of therapy, and therefore, there are no 60‐day outcomes data for the supportive group.
Treatment effect on secondary outcomes
On the ACOPE, participants had significantly decreased scores for the Self‐Reliance/Optimism (F(1,26) = −2.34, P = 0.03), Solving Family Problems (F(1,26) = −2.06, P = 0.049), Avoiding Problems (F(1,26) = −2.42, P = 0.02), Seeking Spiritual Support (F(1,26) = −2.55, P = 0.01), Being Humorous (F(1,26) = −2.25, P = 0.03), Relaxing (F(1,26) = −3.05, P = 0.01), and Total Coping (F(1,26) = −2.22, P = 0.04) scales after ReACT. This indicates participants in ReACT engaged in these skills less frequently after treatment compared to supportive therapy. Differences in BASC‐2 anxiety and depression, CSSI somatization and PedsQL scores were not significant (Table 5).
Table 5.
Average of secondary measures and test statistics for linear models containing fixed effects comparing ReACT and supportive therapy at 7 days post and adjusting for 7 days pretreatment results. Higher scores signify greater endorsement of the characteristic.
| Characteristic | ReACT 7 days pre M (SD) | ReACT 7 days post M (SD) | Supportive 7 days pre M (SD) | Supportive 7 days post M (SD) | F | P |
|---|---|---|---|---|---|---|
| ACOPE | ||||||
| Ventilating feelings | 19.2 (2.7) | 12.9 (11.3) | 14.8 (10.3) | 16.9 (9.3) | −1.74 | 0.09 |
| Seeking diversions | 24.5 (5.3) | 14.2 (12.8) | 19.0 (13.0) | 20.7 (11.0) | −1.71 | 0.10 |
| Self‐reliance\optimism | 19.6 (4.7) | 11.8 (10.9) | 15.6 (10.4) | 17.6 (9.3) | −2.34 | 0.03* |
| Developing social support | 22.1 (3.9) | 12.9 (11.5) | 16.2 (10.6) | 17.4 (9.5) | −1.56 | 0.13 |
| Solving family problems | 19.6 (4.1) | 12.2 (11.4) | 13.3 (10.0) | 15.8 (9.1) | −2.06 | 0.049* |
| Avoiding problems | 22.2 (1.6) | 12.8 (11.1) | 15.2 (9.7) | 17.8 (8.6) | −2.42 | 0.02* |
| Seeking spiritual support | 9.2 (4.0) | 4.9 (4.9) | 7.3 (5.9) | 7.4 (5.1) | −2.55 | 0.01* |
| Investing in close friends | 7.2 (1.8) | 4.2 (3.9) | 4.4 (3.6) | 5.5 (3.2) | −1.89 | 0.07 |
| Engaging in demanding activity | 13.9 (3.8) | 7.6 (7.0) | 10.0 (7.7) | 11.6 (6.8) | −1.98 | 0.06 |
| Being humorous | 7.6 (1.5) | 4.9 (4.5) | 5.8 (4.2) | 6.8 (3.8) | −2.25 | 0.03* |
| Relaxing | 13.5 (2.0) | 7.2 (6.4) | 9.6 (5.9) | 12.3 (6.0) | −3.05 | 0.01** |
| Total coping | 183.8 (18.9) | 108.1 (94.1) | 135.0 (87.9) | 153.2 (78.3) | −2.22 | 0.04* |
| BASC‐2 self report | ||||||
| Anxiety T‐score | 54.6 (13.3) | 52.0 (18.4) | 64.0 (14.1) | 61.1 (9.9) | 1.92 | 0.07 |
| Depression T‐score | 53.5 (10.5) | 52.6 (16.5) | 56.7 (15.1) | 51.4 (10.3) | 0.59 | 0.56 |
| CSSI self‐report | ||||||
| Teen total | 38.8 (15.0) | 19.4 (13.4) | 38.7 (16.0) | 28.6 (19.2) | −1.10 | 0.29 |
| Peds‐QL self report | ||||||
| Psychosocial health | 55.9 (13.8) | 69.1 (17.1) | 58.3 (19.5) | 63.2 (17.0) | 0.84 | 0.41 |
| Physical health | 57.9 (28.0) | 73.8 (21.3) | 65.6 (27.7) | 63.1 (27.0) | 0.87 | 0.39 |
| Total score | 56.7 (16.1) | 70.7 (18.1) | 60.9 (20.9) | 63.2 (19.2) | −0.47 | 0.65 |
P ≤ 0.05.
P ≤ 0.01.
Discussion
Three salient points emerge from this pilot RCT. First, ReACT resulted in significantly greater PNES reduction than supportive therapy, with 100% of patients having no PNES in the 7 days after ReACT. Additionally, in the 60 days after ReACT, 82% remained PNES‐free, suggesting that ReACT is effective in treating pediatric PNES. ReACT also works quickly to reduce PNES, with participants reaching PNES cessation after fewer than five sessions on average. The success rate of ReACT is higher than studies using CBT for PNES in adults. 20 , 21 , 23 , 24 However, it is unclear if this is due to a difference in PNES between pediatric and adult populations or due to the treatment targets of the CBT treatments whereas adult PNES CBT treatments target fear avoidance 21 , 22 and work to reattribute patients’ symptoms to psychosocial issues, 20 , 23 , 34 while ReACT aims to decrease catastrophic symptom expectations and directly retrain physical PNES symptoms in order to increase patients’ control over their symptoms. 35
Second, it is noteworthy that significant reductions in PNES were demonstrated after ReACT, while anxiety and depression were not significantly changed. This is consistent with research in adults with PNES in which patients had significantly decreased anxiety and depression through the use of a selective serotonin reuptake inhibitor but no significant reduction of PNES frequency compared to placebo. 34 PNES are often explained as the result of anxiety or depression, and PNES treatments often attempt to treat mood to address PNES. 20 , 21 , 23 Based on the Integrated Etiological Summary Model 25 used in ReACT, tying PNES symptoms to patients’ mood symptoms could result in expectations that PNES will occur in response to feelings of anxiety and depression, resulting in PNES occurring in those situations more often. Our findings suggest that PNES may be successfully treated without targeting mood symptoms. Because anxiety and depression are often chronic issues with high rates of recurrence, 36 , 37 treating PNES directly as opposed to tying it to comorbid mood issues may allow for the prompt treatment of PNES and decrease recurrence rates that might occur with relapses in mood disorders. Further, some patients with PNES do not have comorbid mood disorders, 12 , 38 and targeting other mechanisms of PNES may be more beneficial for them. Additional research is needed to further investigate this finding.
Third, participants used significantly fewer coping strategies after ReACT compared to supportive therapy. Specifically, participants engaged in fewer avoidant or distraction coping strategies, such as using humor, using substances, avoiding situations that cause problems, listening to music, and daydreaming. These results are consistent with ReACT’s focus on directly addressing PNES rather than using distraction or avoidance. Additionally, the reduction in other coping skills such as attempts to solve family problems, engaging in religious behaviors, being organized, and thinking positively about the situation may be the result of an overall reduction in the need for engagement in coping behaviors due to the resolution of PNES after ReACT.
Other secondary measures were not significantly different between ReACT and supportive therapy. However, somatization was reduced and HRQOL was increased after ReACT as compared to supportive therapy. This study was not powered to assess the secondary measures, and a larger sample size may result in significant differences in these outcomes.
These results have several important clinical implications. First, ReACT is the first RCT for the treatment for pediatric PNES. Although the sample size is modest, the robust results suggest ReACT is an efficacious treatment and validates the need for additional research on the treatment. Second, ReACT appears to work in less than five sessions on average as opposed to other CBT treatments which typically require 12 sessions. Psychotherapy wait times are often long and can be expensive for patients without mental health coverage. Therefore, ReACT offers a cost‐effective intervention that may decrease wait times for treatment. Finally, ReACT directly targets and retrains PNES symptoms as opposed to targeting mood or other factors thought to trigger PNES. This allows clinicians to be able to treat PNES without needing the skills to treat mood. This may provide an opportunity for providers without formal training in the treatment of mood disorders to treat PNES, allowing for easier access to treatment.
This study offers several new directions for future research. A trial with a larger sample size is needed to confirm the efficacy of ReACT and to measure the effect of ReACT on secondary outcomes such as pediatric HRQOL, coping skills, and overall somatization. Long‐term outcomes and recurrence rate after ReACT should also be assessed. Further research is needed to assess the mechanisms of action in which ReACT results in the successful treatment of pediatric PNES. Specifically, studies can evaluate if ReACT increases patients’ sense of control over their symptoms and decreases catastrophic symptom expectations or if its success is due to another mechanism. The evaluation of methods to successfully train other providers to complete this treatment is also needed. Finally, future studies can assess if ReACT is successful in treating adult PNES and other pediatric and adult FNDs.
This study is the first RCT for pediatric PNES. The use of a CBT‐based intervention with novel treatment targets of catastrophic symptom expectations and perceived control over symptoms is a strength. Additionally, inclusion and exclusion criteria were not stringent, allowing participants with comorbid epilepsy and psychopathology other than psychosis. Another significant strength is the utilization of an active therapy control as opposed to treatment as usual used by other PNES treatment studies. 20 , 21 This suggests that ReACT’s success is not completely due to the placebo effect or other common factors in therapy, such as empathy, attention, and the therapeutic alliance which have been found to be responsible for as much as 40% of therapy outcomes. 39 However, ReACT is a more intensive treatment than supportive therapy, which could have contributed to improved outcomes. Limitations include small sample size, dropout in the supportive therapy group, less than 75% of either group completing full treatment, and inability to blind patients to treatment condition. Additionally, the primary outcome was limited to a 7‐day follow‐up, which could result in underreporting of PNES. The study only provides the efficacy of ReACT for 60 days postintervention as longer‐term data were not collected; the absence of 60‐day follow‐up data in the supportive therapy group could have prevented a delayed effect of treatment from being observed. However, it is noteworthy that ReACT works quickly, within the eight treatment sessions, even if there is an eventual effect of supportive therapy.
Conclusions
In this pilot RCT of treatments for pediatric PNES, ReACT produced significant PNES reductions compared to supportive therapy. ReACT works quickly, as the cessation of PNES occurred after only 4.6 sessions on average. Notably, ReACT successfully treats PNES without relying on reductions in anxiety or depression. ReACT is the first evidence‐based therapy for pediatric PNES and provides a timely, cost‐effective treatment for this debilitating condition.
Conflicts of Interest
The authors report the following conflicts of interest:
ADF: Funding form NIH (K23 DK106570).
DL: None.
JPS: (last 24 months) Funding: NIH, NSF, Shor Foundation for Epilepsy Research, DoD, UCB Pharma Inc., NeuroPace Inc., Greenwich Biosciences Inc., Biogen Inc., Xenon Pharmaceuticals, Serina Therapeutics Inc., and Eisai, Inc. Consulting/Advisory Boards: SAGE Therapeutics Inc., Greenwich Biosciences Inc., NeuroPace, Inc., Upsher‐Smith Laboratories, Inc., MASA, Serina Therapeutics Inc., LivaNova Inc., UCB Pharma Inc., Lundbeck, SK LifeSciences Inc., and Elite Medical Experts LLC. Editorial board member: Epilepsy & Behavior, Journal of Epileptology, Epilepsy & Behavior Reports (Associate Editor), Journal of Medical Science, Epilepsy Currents, and Folia Medica Copernicana.
Acknowledgments
Thanks to Lindsey Elliott, Christina Mueller, Lauren Bolden, Kathryn Thompson, Doris Pu, Heather Dark, Terence Penn, and Lynnette Short for leadership in initiating data collection and conducting therapy.
Funding Information
National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK106570).
Funding Statement
This work was funded by National Institute of Diabetes and Digestive and Kidney Diseases grant K23 DK106570.
References
- 1. American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5 ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 2. Angus‐Leppan H. Diagnosing epilepsy in neurology clinics: a prospective study. Seizure 2008;17:431–436. [DOI] [PubMed] [Google Scholar]
- 3. Reuber M, Brown RJ. Understanding psychogenic nonepileptic seizures—Phenomenology, semiology and the Integrative Cognitive Model. Seizure. 2017;44:199–205. [DOI] [PubMed] [Google Scholar]
- 4. Gusmão CMD, Guerriero RM, Bernson‐Leung ME, et al. Functional neurological symptom disorders in a pediatric emergency room: diagnostic accuracy, features, and outcome. Pediatr Neurol 2014;51:233–238. [DOI] [PubMed] [Google Scholar]
- 5. Bolger A, Collins A, Michels M, Pruitt D. Characteristics and outcomes of children with conversion disorder admitted to a single inpatient rehabilitation unit, a retrospective study. PM&R 2018;10:910–916. [DOI] [PubMed] [Google Scholar]
- 6. Testa SM, Schefft BK, Szaflarski JP, et al. Mood, personality, and health‐related quality of life in epileptic and psychogenic seizure disorders. Epilepsia 2007;48:973–982. [DOI] [PubMed] [Google Scholar]
- 7. Szaflarski JP, Hughes C, Szaflarski M, et al. Quality of life in psychogenic nonepileptic seizures. Epilepsia 2003;44:236–242. [DOI] [PubMed] [Google Scholar]
- 8. Doss JL, Plioplys S. Pediatric psychogenic nonepileptic seizures. Child Adolesc Psychiatr Clin N Am 2018;27:53–61. [DOI] [PubMed] [Google Scholar]
- 9. Howlett S, Grünewald RA, Khan A, Reuber M. Engagement in psychological treatment for functional neurological symptoms–Barriers and solutions. Psychotherapy 2007;44:354–360. [DOI] [PubMed] [Google Scholar]
- 10. Baslet G. Psychogenic non‐epileptic seizures: a model of their pathogenic mechanism. Seizure 2011;20:1–13. [DOI] [PubMed] [Google Scholar]
- 11. Stone J, LaFrance WC, Levenson JL, Sharpe M. Issues for DSM‐5: conversion disorder. Am J Psychiatry 2010;167:626–627. [DOI] [PubMed] [Google Scholar]
- 12. Crimlisk HL, Bhatia K, Cope H, et al. Slater revisited: 6 year follow up study of patients with medically unexplained motor symptoms. BMJ 1998;316:582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plioplys S, Doss J, Siddarth P, et al. A multisite controlled study of risk factors in pediatric psychogenic nonepileptic seizures. Epilepsia 2014;55:1739–1747. [DOI] [PubMed] [Google Scholar]
- 14. Salpekar JA, Plioplys S, Siddarth P, et al. Pediatric psychogenic nonepileptic seizures: a study of assessment tools. Epilepsy Behav 2010;17:50–55. [DOI] [PubMed] [Google Scholar]
- 15. Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target‐directed expectations of analgesia. J Neurosci 1999;19:3639–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kozlowska K. The developmental origins of conversion disorders. Clin Child Psychol Psychiatry 2007;12:487–510. [DOI] [PubMed] [Google Scholar]
- 17. Baek K, Doñamayor N, Morris LS, et al. Impaired awareness of motor intention in functional neurological disorder: implications for voluntary and functional movement. Psychol Med 2017;47:1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edwards MJ, Moretto G, Schwingenschuh P, et al. Abnormal sense of intention preceding voluntary movement in patients with psychogenic tremor. Neuropsychologia 2011;49:2791–2793. [DOI] [PubMed] [Google Scholar]
- 19. Kranick SM, Moore JW, Yusuf N, et al. Action‐effect binding is decreased in motor conversion disorder: implications for sense of agency. Mov Disord 2013;28:1110–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. LaFrance WC, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures. JAMA Psychiatry 2014;71:997. [DOI] [PubMed] [Google Scholar]
- 21. Goldstein LH, Chalder T, Chigwedere C, et al. Cognitive‐behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology 2010;74:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldstein LH, Deale AC, O’Malley SJM, et al. An evaluation of cognitive behavioral therapy as a treatment for dissociative seizures. Cogn Behav Neurol 2004;17:41–49. [DOI] [PubMed] [Google Scholar]
- 23. LaFrance WC, Miller IW, Ryan CE, et al. Cognitive behavioral therapy for psychogenic nonepileptic seizures. Epilepsy Behav 2009;14:591–596. [DOI] [PubMed] [Google Scholar]
- 24. Goldstein L, Robinson EJ, Mellers J, et al. Cognitive behavioural therapy for adults with dissociative seizures (CODES): a pragmatic, multicentre, randomised controlled trial. Lancet Psych 2020;7:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fobian AD, Elliott L. A review of functional neurological symptom disorder etiology and the integrated etiological summary model. J Psychiatry Neurosci 2019;44:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azrin N, Nunn R. Habit‐reversal: a method of eliminating nervous habits and tics. Behav Res Ther 1973;11:619–628. [DOI] [PubMed] [Google Scholar]
- 27. Cuijpers P, Driessen E, Hollon SD, et al. The efficacy of non‐directive supportive therapy for adult depression: a meta‐analysis. Clin Psychol Rev 2012;32:280–291. [DOI] [PubMed] [Google Scholar]
- 28. Patterson JM, Mccubbin HI. Adolescent coping style and behaviors: conceptualization and measurement. J Adolesc 1987;10:163–186. [DOI] [PubMed] [Google Scholar]
- 29. Reynolds CR, Kramphaus RW. BASC‐2 Behavior assessment system for children, 2nd ed Circle Pines, MN: American Guidance Service, Inc, 2004. [Google Scholar]
- 30. Walker LS, Beck JE, Garber J, Lambert W. Childrens somatization inventory: psychometric properties of the revised Form (CSI‐24). J Pediatr Psychol. 2008;34:430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 1994;151:1132–1136. [DOI] [PubMed] [Google Scholar]
- 32. Cook TD, DeMets DL, eds. Introduction to statistical methods for clinical trials, 1st ed Boca Raton, FL: Chapman and Hall/CRC, 2007. [Google Scholar]
- 33. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials – a practical guide with flowcharts. BMC Med Res Methodol 2017;17:162 https://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874‐017‐0442‐1#citeas [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LaFrance WC, Keitner GI, Papandonatos GD, et al. Pilot pharmacologic randomized controlled trial for psychogenic nonepileptic seizures. Neurology 2010;75:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dutta N, Cavanna AE. The effectiveness of habit reversal therapy in the treatment of Tourette syndrome and other chronic tic disorders: a systematic review. Funct Neurol 2013;28:7–12. [PMC free article] [PubMed] [Google Scholar]
- 36. Keller MB, Lavori PW, Wunder J, et al. Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 1992;31:595–599. [DOI] [PubMed] [Google Scholar]
- 37. Moos RH, Cronkite RC. Symptom‐based predictors of a 10‐year chronic course of treated depression. J Nerv Ment Dis 1999;187:360–368. [DOI] [PubMed] [Google Scholar]
- 38. Wilshire CE, Ward T. Psychogenic explanations of physical illness. Perspect Psychol Sci 2016;11:606–631. [DOI] [PubMed] [Google Scholar]
- 39. Lambert MJ, Barley DE. Research summary on the therapeutic relationship and psychotherapy outcome. Psychotherapy 2001;38:357–361. [Google Scholar]


