Abstract
Objective
We have conducted a study to clarify the association between amphetamine‐related disorders (ARD) and the risk of developing dementia.
Methods
This study used a retrospective cohort design by using Taiwan’s National Health Research Institute Database. A random sample of 68,300 subjects between January 1, 2000, and December 31, 2015, was obtained, comprising of 17,075 patients with ARD, and 51,225 controls without ARD (1:3), matched for gender and age group. After adjusting for covariates, a Fine and Gray’s survival analysis (competing with mortality) was used to compare the risk of dementia during a 15‐year follow‐up period.
Results
In the present study, 1,751 of 17,075 patients with ARD and 2,147 of 51,225 in the control group without ARD (883.10 vs 342.83 per 100,000 person‐years) developed dementia. ARD cohort was more likely to develop dementia (hazard ratio = 4.936 [95% CI: 4.609–5.285, P < 0.001). After adjusting for gender, age groups, education, monthly insured premiums, urbanization level, geographic region, comorbidities, the hazard ratio for ARD patients was 5.034 (95% CI: 4.701–5.391, P < 0.001). ARD has been associated with overall dementia, Alzheimer dementia, vascular dementia, and other dementia. Both the amphetamine use disorder and amphetamine‐induced psychotic disorders were associated with the risk of overall dementia, Alzheimer dementia, vascular dementia, and other dementia.
Interpretation
This study shows that patients with ARD, both the amphetamine use disorder and the amphetamine‐induced psychotic disorder, may have a nearly fivefold risk of developing dementia, including Alzheimer dementia and other types of dementia.
Introduction
Between 2011 and 2012, 130,000 people, or 4.97% of those aged 65 years and over in Taiwan had dementia, 1 which is a heavy burden for dementia patients and their caregivers, community, and society. 2 , 3 , 4 , 5 , 6 Injuries on the brain such as traumatic brain injury, 7 stroke, 8 and attention deficit hyperactivity disorder that is related traumatic brain injury, 9 , 10 contribute to the development of dementia. Several studies have also found that substances, such as alcohol, tobacco, and benzodiazepines, were associated with cognitive impairment, but there were no confirmed association between these substances and dementia. 11 Other exposure to substance intoxication, such as carbon monoxide 12 , 13 , 14 , 15 or nitrogen dioxide, 12 was also associated with the risk of dementia. The usage of central stimulants is a serious problem internationally. Amphetamine exposure has been previously reported to increase the risk of developing Parkinson’s disease in the elderly. 16 However, the amphetamine‐related disorders (ARD) and the risk of dementia has not been investigated.
In Taiwan, most of the abused amphetamines is methamphetamine, which accounts for 40% of the illicit substance use and ranks second only to heroin. 17 In addition, amphetamine medications, such as dextroamphetamine, are not licensed nor reimbursed by the National Health Insurance (NHI) Program in Taiwan. Furthermore, the individuals with childhood poor impulse control might tend to abuse the methamphetamine for its faster‐onset and longer‐lasting effects than the effects of amphetamine medications. 18 , 19 Previous studies have reported that methamphetamine has been investigated and could promote the formation of amyloid‐β42, one of the key Alzheimer’s disease‐like pathological proteins, 20 increase the expression of the tau protein, 21 and produce greater dementia‐related oxidative stress markers including catalase and methane dicarboxylic aldehyde. 22 In addition, methamphetamine may modulate the functions of the immune cells and change the cytokine balance, which leads to neurotoxicity with a compromise of the blood‐brain barrier, and alterations to the brain plasticity, and eventually contributes to age‐related dysfunctions, including dementia. 23
We hypothesize that an early exposure of amphetamine, particularly in those of amphetamine dependency, may enhance the risk of developing dementia afterwards. By employing a nationwide cohort in Taiwan, we conducted this study to investigate the association between amphetamine usage and dementia.
Methods
Study design and sampled participants
In this study, we used data from the National Health Insurance Research Database (NHIRD) to investigate the association between the risk of dementia in subjects with ARD cohort and the non‐ARD cohort, over a 15‐year period, from the two million Longitudinal Health Insurance Database (LHID) in Taiwan, (2000‐2015). The details of the program have been documented in previous studies. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33
This study was a retrospective matched‐cohort design. Patients with ARD were selected from January 1 to December 31, 2000, according to the ICD‐9‐CM codes (Table S1) as: amphetamine dependence, amphetamine abuse, and drug psychosis between January 1, 2000 and December 31, 2000, without other substance use disorders within one year before or after the diagnosis of drug psychosis, with reference from one previous study. 13 In this study, 17,075 patients with ARD, and 51,225 in the age‐, gender‐, and index‐ year, (1:3) matched control group without ARD were enrolled, and the patients aged < 20, diagnoses of ARD and dementia before the tracking in this study were excluded (Fig. 1).
Figure 1.
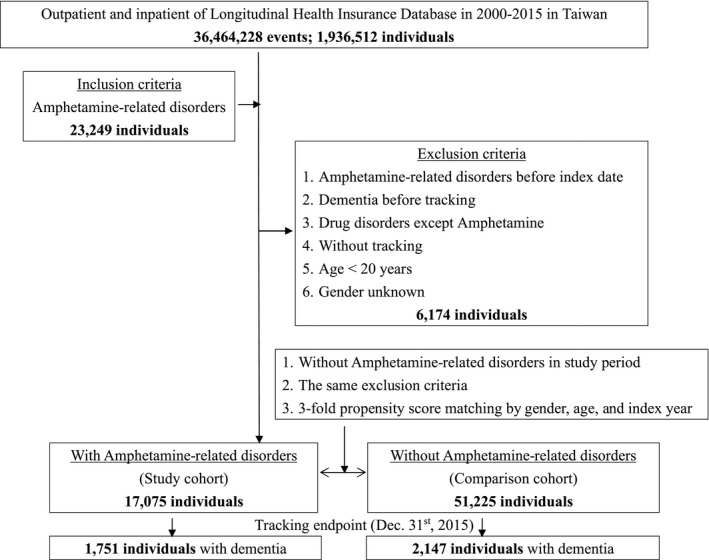
The flowchart of study sample selection.
Ethics approval and consent to participate
The Institutional Review Board of the Tri‐Service General Hospital approved this study (IRB No. 2‐107‐05‐026). The requirement for informed consent was waived by the IRB because all NHIRD data had been de‐identified.
Material and Methods
The NHI Program was launched in Taiwan in 1995, and as of June 2009, included contracts with 97% of the medical providers with approximately 23 million beneficiaries, or more than 99% of the entire population. 34 The NHIRD uses the International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) codes to record diagnoses. 35 All diagnoses of ARD were confirmed by psychiatrists according to the clinical findings, and the dementia diagnosis was made by board‐certified psychiatrists or neurologists, according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition and its Text‐revised edition. 36 , 37 Licensed medical records technicians also reviewed and verified the diagnostic coding before claiming the reimbursements in Taiwan’s hospitals. 38 The NHI Administration randomly reviews the records of ambulatory care visits and inpatient claims periodically to verify the accuracy of the diagnose. 39 Therefore, it is suitable using the NHIRD to study the association between ARD and dementia.
The covariates included gender, age (20–49, 50–64, ≧65), education (<12 years, ≧12 years), geographical area of residence (north, center, south, and east of Taiwan), urbanization level of residence (levels 1 to 4), levels of hospitals as medical centers, regional and local hospitals, and insurance premium (in New Taiwan Dollars [NT$]; <18,000, 18,000–34,999, ≥35,000). The urbanization level of residence was defined according to the population and various indicators of the level of development. Level 1 was defined as a population of >1,250,000, and a specific designation as political, economic, cultural, and metropolitan development. Level 2 was defined as a population between 500,000 and 1,249,999, and as playing an important role in the political system, economy, and culture. Urbanization levels 3 and 4 were defined as a population between 149,999 and 499,999, and <149,999 respectively. 12 The Charlson Comorbidity Index (CCI) was also used to categorize the comorbidities using the ICD‐9‐CM codes, scores each comorbidity category, 40 , 41 , 42 and combines all the scores to calculate a single comorbidity score. A score of zero indicates that no comorbidities were found, and higher scores indicate higher comorbidity burdens. 43
All the study participants were followed from the index date until the onset of dementia including Alzheimer dementia, vascular dementia (VaD), and other degenerative dementia, according to the ICD‐9‐CM codes (Table S1), withdrawal from the NHI program, or the end of 2015.
Statistical analysis
All analyses were performed using the SPSS software version 22 (SPSS Inc., Chicago, Illinois, USA). χ2 and t tests were used to evaluate the distributions of the categorical and continuous variables respectively. In addition, the Fisher exact test for categorical variables was used to statistically examine the differences between the two cohorts, while the sample size was < 5. Fine and Gray’s survival analysis was used to determine the risk of dementia, and the results were presented as a hazard ratio (HR) with a 95% confidence interval (CI). The Fine and Gray’s model benefits from the use of the inclusion of the actual mortality data to study the usage of methamphetamine as a risk factor for dementia. 44 , 45 The difference in the risk of dementia, between the study and control groups, was estimated using the Kaplan–Meier method with the log‐rank test. A 2‐tailed P value < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
Table 1 depicts that the ARD cohort tended to have a lower education level, insured premiums < 18,000 and ≧35,000, CCI score of zero, living in northern and southern, residence of urbanization levels 1 and 2, and seeking medical help in the regional hospitals. There were no significant differences between ARD and non‐ARD cohorts in other covariates.
Table 1.
Characteristics of study subjects at the baseline
| Amphetamine‐related disorders | With | Without | P | ||
|---|---|---|---|---|---|
| Variables | n | % | n | % | |
| Total | 17,075 | 25.00 | 51,225 | 75.00 | |
| Gender | 0.999 | ||||
| Male | 11,992 | 70.23 | 35,976 | 70.23 | |
| Female | 5,083 | 29.77 | 15,249 | 29.77 | |
| Age (years) | 43.85 ± 18.40 | 44.12 ± 16.98 | 0.078 | ||
| Age group (years) | 0.999 | ||||
| 20–49 | 12,185 | 71.36 | 36,555 | 71.36 | |
| 50–64 | 1,750 | 10.25 | 5,250 | 10.25 | |
| ≧65 | 3,140 | 18.39 | 9,420 | 18.39 | |
| Education (years) | <0.001 | ||||
| 0–11 | 11,840 | 69.34 | 17,771 | 34.69 | |
| ≧12 | 5,235 | 30.66 | 33,454 | 65.31 | |
| Insured premium (NT$) | <0.001 | ||||
| <18,000 | 16,280 | 95.34 | 46,787 | 91.34 | |
| 18,000‐34,999 | 234 | 1.37 | 3,312 | 6.47 | |
| ≧35,000 | 561 | 3.29 | 1,126 | 2.20 | |
| CCI_R group | <0.001 | ||||
| 0 | 13,137 | 76.94 | 37,950 | 74.08 | |
| 1 | 2,185 | 12.80 | 6,890 | 13.45 | |
| 2 | 781 | 4.57 | 2,508 | 4.90 | |
| 3 | 443 | 2.59 | 1,958 | 3.82 | |
| ≧4 | 529 | 3.10 | 1,919 | 3.75 | |
| Location | <0.001 | ||||
| Northern Taiwan | 7,379 | 43.22 | 20,708 | 40.43 | |
| Middle Taiwan | 4,075 | 23.87 | 14,135 | 27.59 | |
| Southern Taiwan | 4,612 | 27.01 | 13,126 | 25.62 | |
| Eastern Taiwan | 974 | 5.70 | 3,034 | 5.92 | |
| Outlets islands | 35 | 0.20 | 222 | 0.43 | |
| Urbanization level | <0.001 | ||||
| 1 (The highest) | 5,999 | 35.13 | 17,617 | 34.39 | |
| 2 | 7,317 | 42.85 | 21,063 | 41.12 | |
| 3 | 1,318 | 7.72 | 4,585 | 8.95 | |
| 4 (The lowest) | 2,441 | 14.30 | 7,960 | 15.54 | |
| Level of care | <0.001 | ||||
| Medical center | 5,495 | 32.18 | 15,064 | 29.41 | |
| Regional hospital | 9,081 | 53.18 | 15,638 | 30.53 | |
| Local hospital | 2,499 | 14.64 | 20,523 | 40.06 | |
P, Chi‐square/ Fisher exact test on category variables and t‐test on continue variables; NT$, New Taiwan Dollars; CCI_R, Charlson Comorbidity Index, dementia removed.
Kaplan–Meier model for the cumulative risk of dementia
In the present study, 1,751 of 17,075 patients with ARD (10.25%), and 2,147 of 51,225 (4.19%) in the control group without ARD (883.10 vs. 342.83 per 100,000 person‐years) developed dementia. The Kaplan–Meier analysis revealed that the ARD cohort had a significantly higher 15‐year dementia cumulative incidence rate than the controls. (log‐rank, P < 0.001, Fig. 2). Figure S1 has shown the survival data as the dementia‐free survival for patients with and without amphetamine‐related disorders during the 15‐year follow‐up period in Taiwan (log‐rank, P < 0.001).
Figure 2.
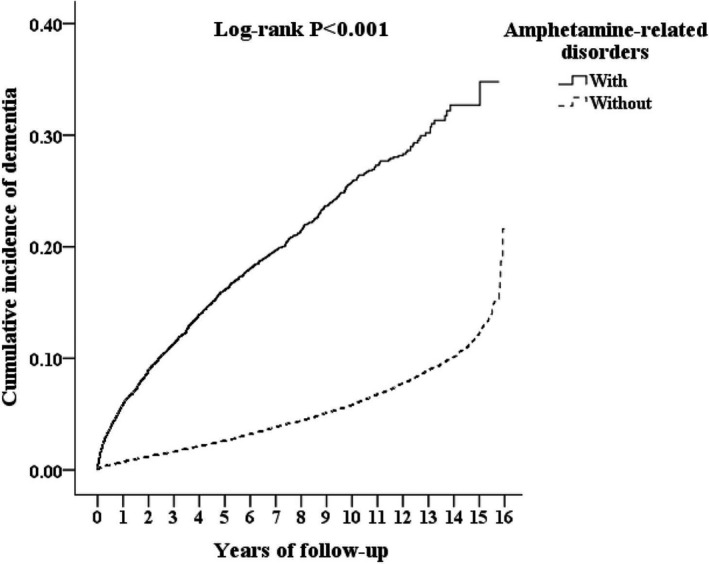
Kaplan–Meier for cumulative incidence of dementia aged 20 and over stratified by Amphetamine‐related disorders with log‐rank test.
Hazard ratios analysis of dementia in the ARD cohort
Table 2 shows that the ARD cohort was more likely to develop dementia (hazard ratio = 4.936 [95% CI:4.609–5.285, P < 0.001). After adjusting for gender, age groups, education, monthly insured premiums, urbanization level, geographic region, comorbidities, the hazard ratio for ARD patients was 5.034 (95% CI: 4.701–5.391, P < 0.001), when compared to the non‐ARD control cohort. In other words, the ARD cohort has a fivefold risk of dementia, when compared to the non‐ARD cohort. Furthermore, male patients with ARD were associated with the risk of dementia (adjusted HR = 1.315, 95% CI: 1.227–1.410, P < 0.001), in comparison to the female patients. The ARD cohort with those aged ≧65, educational years of 0‐11, scores of CCI as 1, 3, and 4, and urbanization levels of 1, 2, and 3, was associated with the increased risk of dementia. On the other hand, the ARD, with those aged 50‐64, insurance premiums of NT$ 18,000‐34,999, and seeking medical care from the medical centers and regional hospitals, was associated with the decreased risk of dementia.
Table 2.
Factors of dementia by using Fine and Gray’s competing risk model
| No competing risk in the model | Competing risk in the model | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Adjusted HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
| Amphetamine‐related disorders | ||||||||
| Without | Reference | Reference | ||||||
| With | 4.936 | 4.609 | 5.285 | <0.001 | 5.034 | 4.701 | 5.391 | <0.001 |
| Gender | ||||||||
| Male | 1.275 | 1.189 | 1.367 | <0.001 | 1.315 | 1.227 | 1.410 | <0.001 |
| Female | Reference | Reference | ||||||
| Age group (years) | ||||||||
| 20–49 | Reference | Reference | ||||||
| 50–64 | 0.753 | 0.675 | 0.840 | <0.001 | 0.775 | 0.695 | 0.865 | <0.001 |
| ≧65 | 3.327 | 3.081 | 3.593 | <0.001 | 3.836 | 3.551 | 4.145 | <0.001 |
| Education (years) | ||||||||
| 0–11 | 1.407 | 1.057 | 1.972 | 0.011 | 1.451 | 1.071 | 2.005 | 0.001 |
| ≧12 | Reference | Reference | ||||||
| Insured premium (NT$) | ||||||||
| <18,000 | Reference | Reference | ||||||
| 18,000–34,999 | 0.640 | 0.465 | 0.881 | 0.006 | 0.641 | 0.466 | 0.883 | 0.006 |
| ≧35,000 | 0.425 | 0.160 | 1.135 | 0.088 | 0.433 | 0.162 | 1.155 | 0.095 |
| CCI_R group | ||||||||
| 0 | Reference | Reference | ||||||
| 1 | 1.051 | 0.971 | 1.137 | 0.218 | 1.084 | 1.002 | 1.173 | 0.045 |
| 2 | 0.973 | 0.872 | 1.086 | 0.628 | 1.053 | 0.943 | 1.175 | 0.359 |
| 3 | 0.632 | 0.540 | 0.738 | <0.001 | 0.729 | 0.623 | 0.852 | <0.001 |
| ≧4 | 0.359 | 0.303 | 0.426 | <0.001 | 0.487 | 0.410 | 0.578 | <0.001 |
| Urbanization level | ||||||||
| 1 (The highest) | 0.779 | 0.705 | 0.860 | <0.001 | 0.818 | 0.740 | 0.904 | <0.001 |
| 2 | 0.797 | 0.731 | 0.868 | <0.001 | 0.816 | 0.749 | 0.889 | <0.001 |
| 3 | 0.697 | 0.612 | 0.794 | <0.001 | 0.692 | 0.608 | 0.788 | <0.001 |
| 4 (The lowest) | Reference | Reference | ||||||
| Level of care | ||||||||
| Medical center | 0.784 | 0.712 | 0.864 | <0.001 | 0.756 | 0.686 | 0.833 | <0.001 |
| Regional hospital | 0.803 | 0.743 | 0.867 | <0.001 | 0.780 | 0.722 | 0.842 | <0.001 |
| Local hospital | Reference | Reference | ||||||
HR, hazard ratio; CI, confidence interval; Adjusted HR, Adjusted variables listed in the Table 1; NT$, New Taiwan Dollars; CCI_R, Charlson Comorbidity Index, dementia removed.
Subgroup analysis in the association between ARD and dementia
The ARD cohort was associated with the increased risk of dementia, in each demographic factor, covariates, scores of CCI, urbanization levels, and the care from the either medical centers, regional hospitals, and local hospitals (Table 3).
Table 3.
Factors of dementia stratified by variables listed in the table by using Fine & Gray's competing risk model
| Amphetamine‐related disorders | With | Without | Competing risk in the model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | PYs | Rate (per 105 PYs) | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | P | |
| Overall | 1,751 | 198,278.28 | 883.10 | 2,147 | 626,249.70 | 342.83 | 5.034 | 4.701 | 5.391 | <0.001 |
| Gender | ||||||||||
| Male | 1,254 | 138,316.11 | 906.62 | 1,453 | 441,082.28 | 329.42 | 5.379 | 5.023 | 5.760 | <0.001 |
| Female | 497 | 59,962.17 | 828.86 | 694 | 185,167.42 | 374.80 | 4.322 | 4.036 | 4.628 | <0.001 |
| Age group (years) | ||||||||||
| 20–49 | 931 | 126,751.31 | 734.51 | 432 | 241,275.04 | 179.05 | 8.017 | 7.487 | 8.586 | <0.001 |
| 50–64 | 162 | 28,046.02 | 577.62 | 303 | 201,048.23 | 150.71 | 7.490 | 6.995 | 8.021 | <0.001 |
| ≧65 | 658 | 43,480.95 | 1,513.31 | 1,412 | 183,926.43 | 767.70 | 3.852 | 3.597 | 4.126 | <0.001 |
| Education (years) | ||||||||||
| 0–11 | 865 | 75,012.46 | 1,153.14 | 1,011 | 232,121.02 | 435.55 | 5.174 | 4.832 | 5.541 | <0.001 |
| ≧12 | 886 | 79,784.87 | 1,110.49 | 1,136 | 210,202.25 | 540.43 | 4.016 | 3.750 | 4.300 | <0.001 |
| Insured premium (NT$) | ||||||||||
| <18,000 | 1,732 | 195,339.81 | 886.66 | 2,124 | 612,609.23 | 346.71 | 4.998 | 4.667 | 5.352 | <0.001 |
| 18,000–34,999 | 18 | 2,780.93 | 647.27 | 20 | 10,324.80 | 193.71 | 6.530 | 6.098 | 6.993 | <0.001 |
| ≧35,000 | 1 | 157.54 | 634.77 | 3 | 3,315.67 | 90.48 | 13.710 | 12.803 | 14.683 | <0.001 |
| CCI_R group | ||||||||||
| 0 | 1,221 | 132,500.97 | 921.50 | 916 | 342,837.75 | 267.18 | 6.740 | 6.294 | 7.218 | <0.001 |
| 1 | 339 | 34,307.78 | 988.11 | 687 | 128,549.44 | 534.42 | 3.613 | 3.374 | 3.870 | <0.001 |
| 2 | 112 | 12,093.61 | 926.11 | 300 | 52,902.59 | 567.08 | 3.192 | 2.980 | 3.418 | <0.001 |
| 3 | 42 | 7,563.04 | 555.33 | 137 | 39,535.27 | 346.53 | 3.132 | 2.925 | 3.354 | <0.001 |
| ≧4 | 37 | 11,812.89 | 313.22 | 107 | 62,424.66 | 171.41 | 3.571 | 3.335 | 3.824 | <0.001 |
| Urbanization level | ||||||||||
| 1 (The highest) | 438 | 57,312.28 | 764.23 | 581 | 187,974.99 | 309.08 | 4.832 | 4.512 | 5.175 | <0.001 |
| 2 | 730 | 86,591.99 | 843.03 | 926 | 272,868.75 | 339.36 | 4.855 | 4.534 | 5.199 | <0.001 |
| 3 | 152 | 18,694.55 | 813.07 | 152 | 55,027.04 | 276.23 | 5.752 | 5.372 | 6.160 | <0.001 |
| 4 (The lowest) | 431 | 35,679.46 | 1,207.98 | 488 | 110,378.91 | 442.11 | 5.340 | 4.986 | 5.718 | <0.001 |
| Level of care | ||||||||||
| Hospital center | 351 | 52,553.35 | 667.89 | 606 | 204,099.71 | 296.91 | 4.396 | 4.105 | 4.708 | <0.001 |
| Regional hospital | 817 | 98,589.28 | 828.69 | 926 | 283,123.01 | 327.07 | 4.952 | 4.624 | 5.303 | <0.001 |
| Local hospital | 583 | 47,135.65 | 1,236.86 | 615 | 139,026.98 | 442.36 | 5.464 | 5.103 | 5.852 | <0.001 |
PYs, Person‐years; Adjusted HR, Adjusted Hazard ratio: Adjusted for the variables listed in Table 1.; CI, confidence interval; NT$, New Taiwan Dollars.
Risk of the types of dementia in the ARD cohort and the sensitivity analysis
Table 4 depicts that the ARD cohort was associated with overall dementia, AD, VaD, and other dementia in the overall follow‐up period. In addition, the ARD cohort was associated with overall dementia, AD, VaD, and other dementia, after the exclusion of dementia diagnosis of the first two years after the diagnosis of ARD. The ARD cohort was associated with overall dementia, AD, and other dementia, but not VaD, after the exclusion of the dementia diagnosis of the first five years after the diagnosis of ARD.
Table 4.
Factors of dementia subgroup and sensitivity test by using Fine and Gray’s competing risk model
| Amphetamine‐related disorders | No competing risk in the model | Competing risk in the model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity test | Dementia subgroup | Adjusted HR | 95% CI | 95% CI | P | Adjusted HR | 95% CI | 95% CI | P |
| Overall | Overall dementia | 4.936 | 4.609 | 5.285 | <0.001 | 5.034 | 4.701 | 5.391 | <0.001 |
| AD | 3.806 | 3.554 | 4.075 | <0.001 | 3.882 | 3.621 | 4.157 | <0.001 | |
| VaD | 2.460 | 2.297 | 2.634 | <0.001 | 2.509 | 2.340 | 2.687 | <0.001 | |
| Other dementia | 5.196 | 4.852 | 5.564 | <0.001 | 5.301 | 4.949 | 5.678 | <0.001 | |
| First 2 years excluded | Overall dementia | 2.862 | 2.671 | 3.065 | <0.001 | 2.918 | 2.725 | 3.128 | <0.001 |
| AD | 2.227 | 2.080 | 2.389 | <0.001 | 2.271 | 2.122 | 2.439 | <0.001 | |
| VaD | 1.580 | 1.476 | 1.692 | <0.001 | 1.613 | 1.502 | 1.729 | <0.001 | |
| Other dementia | 2.999 | 2.798 | 3.213 | <0.001 | 3.055 | 2.851 | 3.270 | <0.001 | |
| First 5 years excluded | Overall dementia | 1.922 | 1.725 | 2.085 | <0.001 | 1.960 | 1.833 | 2.099 | <0.001 |
| AD | 1.313 | 1.021 | 1.534 | 0.022 | 1.339 | 1.101 | 1.443 | 0.004 | |
| VaD | 1.100 | 0.862 | 1.259 | 0.267 | 1.128 | 0.953 | 1.080 | 0.136 | |
| Other dementia | 2.205 | 1.890 | 2.169 | <0.001 | 2.065 | 1.928 | 1.212 | <0.001 | |
PYs, Person‐years; Adjusted HR, Adjusted Hazard ratio, Adjusted for the variables listed in Table 3.; CI, confidence interval; AD, Alzheimer dementia; VaD, vascular dementia.
In addition, both the amphetamine use disorder and amphetamine‐induced psychotic disorder were associated with the risk of overall dementia, AD, VaD, and other dementia (Table 5).
Table 5.
Factors of dementia subgroup stratified by amphetamine‐related disorders by using Fine and Gray’s competing risk model
| Amphetamine‐related disorders | Dementia types | Events | Adjusted HR | 95% CI | 95% CI | P |
|---|---|---|---|---|---|---|
| Amphetamine use disorder (reference: without) | Overall | 200 | 3.189 | 2.971 | 3.419 | <0.001 |
| Amphetamine use disorder (reference: without) | AD | 9 | 3.172 | 2.955 | 3.401 | <0.001 |
| Amphetamine use disorder (reference: without) | VaD | 10 | 2.207 | 2.061 | 2.375 | <0.001 |
| Amphetamine use disorder (reference: without) | Other dementia | 181 | 3.259 | 3.029 | 3.514 | <0.001 |
| Amphetamine‐induced psychotic disorder (reference: without) | Overall | 1,551 | 5.440 | 5.072 | 5.821 | <0.001 |
| Amphetamine‐induced psychotic disorder (reference: without) | AD | 52 | 4.036 | 3.765 | 4.333 | <0.001 |
| Amphetamine‐induced psychotic disorder (reference: without) | VaD | 53 | 2.574 | 2.404 | 2.779 | <0.001 |
| Amphetamine‐induced psychotic disorder (reference: without) | Other dementia | 1,446 | 5.740 | 5.352 | 6.180 | <0.001 |
PYs, Person‐years; Adjusted HR, Adjusted Hazard ratio, Adjusted for the variables listed in Table 1.; CI, confidence interval.
Discussion
Association between ARD cohort and the risk of dementia
In this study, we found that in the 15‐year follow‐up, the ARD cohort was associated with a higher risk of developing dementia. The log rank of the Fine and Gray’s competing risks regression model was significant (P < 0.001). The crude HR of the subject group was 4.936 (95% CI: 4.609–5.285, P < 0.001), and the adjusted HR was 5.034 (95% CI: 4.701–5.391, P < 0.001). The ARD cohort has a fivefold risk of dementia, when compared to the non‐ARD cohort. A subgroup analysis also found that the patients with ARD were associated with the risk of dementia, in all the demographic factors, covariates, scores of CCI, urbanization levels, and the care from the either medical centers, regional and local hospitals, in comparison to the controls without ARDs. We have also conducted two sensitivity analyses to evaluate the influences from protopathic bias. Even though the patients with a diagnosis of dementia within the first two years and five years were excluded, the ARD cohort were still associated with an increased risk of overall dementia and each types of dementia, except for the association between ARD and VaD after the exclusion of the first five years of dementia. To the best of our knowledge, this is the first nationwide, population‐based cohort study that has focused on the association between the ARD cohort and the risk of dementia.
In the present study, the cumulative incidence of dementia within 15 years was 5.9% in all the enrolled subjects, and 4.2% in the controls without ARD, which is compatible to the prevalence of dementia as 2–5%. 46 , 47 , 48
In addition, the 1‐year (between January 1, and December 31, 2000) prevalence rate of ARD was 0.88%, which was just slightly lower than, but close to, that in an epidemiological study for the male patients with methamphetamine use disorder in one county (Taoyuan), as 1.24%, in 2002. 49 However, given the fact that it is difficult to estimate the actual prevalence of illicit drug use disorders, a population‐based study would be a reasonable estimation of the ARD in Taiwan.
Comparison to previous literature
Several previous studies have depicted the association between the medical usage of several drugs and the risk of dementia, such as high doses of opioids, 50 proton pump inhibitors, 51 , 52 , 53 benzodiazepines, 54 disease‐modifying antirheumatic drugs, anticholinergics, 55 and even some types of antidepressants. 56 However, few reports have denoted the association of substance use disorders and the risk of dementia, with the exception of the alcohol use disorder. 5 , 57 , 58 , 59 In addition, the abuse of amphetamines was associated with the risk of Parkinson’s disease, another neurodegenerative disease, in previous studies, including amphetamine and methamphetamine. 60 , 61 , 62 Nonetheless, this is the first on the issues of the association between ARD and risk of dementia. In this study, both the amphetamine use disorders and amphetamine‐induced psychotic disorders were associated with the risk of overall dementia, AD, VaD, and other dementia.
Potential mechanisms of the association between ARD and risk of dementia
The cardinal finding of this study is that we confirm an earlier exposure of amphetamine in Taiwan which led to a higher risk of developing dementia. The potential mechanisms can be twofold. First, as we mentioned earlier, through its central impacts, amphetamine may influence those neurosubstrates associated to the underlying mechanism of dementia, namely, beta‐amyloid cascade, tau protein, oxidative stress, and neural inflammation, 63 , 64 and of which are a time‐consuming process. For example, microglial activation may well be associated to the chronic inflammatory process, 65 and the chronic inflammation condition may contribute to the pathophysiology of AD, 66 and vascular dementia. 67 Second, the image positron emission tomography (PET) study revealed that excessive usage of hedonic substances results in a down‐regulation of the D2 receptors, thus, the addicts need to take “more” and the abstinent users have difficulty experiencing pleasure with the natural reinforcers of life. 68 This appears a possibility of a downward change of brain function following the usage of a cognitive enhancer, which may be interpreted by the opponent process theory, 69 , 70 which was first introduced by Solomon and Corbit (1974, 1980), and is usually employed in the interpretation of drug dependence of central stimulants, which suggests that following a positive hedonic response (upward change above the baseline, a‐process), homeostatic changes in the brain circuits may function to dampen this positive response (downward change below the baseline, b‐process). 69 in terms of drug addiction of the central stimulants, and the compulsive drug consumption can be viewed as a negative reinforcing course to compensate the b‐process. Usually, the a‐process occurs rapidly after the usage of the drug. As to the b‐process, it goes with a slow onset, and in its process, the body exerts itself to achieve homeostasis through change, in which the accumulating brain damage resulting from this chronic aberration, as time goes by, is referred to as allostatic load. 71 , 72
Therefore, if we widen the time scale to a life‐long period and consider the earlier usage of amphetamines as an exposure of the cognitive enhancer which causes upward change of cognition in the beginning, 73 it is possible that the b‐process, on the other hand, reflects its serious allostatic load on a downward change of cognition from the baseline in a quite procrastination manner. In point of fact, for those experiencing earlier amphetamine exposure the cognitive deterioration can be highly regarded as a specific form of compensatory change of the brain function following the exposure of the cognition enhancer, such as amphetamine, thus rendering a high risk of development dementia.
It is interesting to note that in the present study, the ARD cohort was associated with overall dementia, AD, and other dementia, but not VaD. The underlying mechanism could be complicated, however, as it is possibly relevant to the observation that the amphetamine‐associated stroke often occurs when closely following the drug exposure rather than a postponement until elderly, 16 yet dementia diagnosis of the first five years after the diagnosis of ARD, however, was excluded in this study.
Additionally, in the present study, the ARD cohort with monthly insured premiums of ≧ 35,000, in the residence of higher urbanization, and those that received their medical care from the medical centers and regional hospitals, tended to have a lower risk of dementia. Furthermore, the ARD cohort with a lower educational level was associated with a higher risk of dementia. The socioeconomic levels might also play an important role in the development of dementia. The underlying mechanisms between the association of ARD and the risk of dementia need more studies.
Age and gender effects in the risk of dementia
Several previous studies have reported that aging itself is a risk factor of dementia development. 46 , 47 , 48 , 74 ARD patients aged 50–64 were associated with a higher risk of dementia, and the ARD cohort aged ≧65 was associated with a lower risk of dementia in this study, in comparison to those aged 20–49. The reason for this discrepancy might be related to the previous findings that most of the users of amphetamine were middle aged, 49 and a 15‐year period of follow‐up might be enough for these patients to develop the progressive neurodegenerative process to develop dementia. We hypothesize that the effects of amphetamine exposure are life‐stage dependent. And the earlier exposure of amphetamine is the more liable to develop a dementia in later life. When the ARD cohort is aged ≧65, the risk reduces. The interpretation is also along with our hypothesis of the opponent process theory that an earlier exposure to amphetamine, the more readiness for the body to develop a homeostatic change in brain circuits to dampen the positive response.
In addition, male ARD patients were associated with the increased risk of dementia, in comparison to the female patients. The gender effects varied in the risks for different types of dementia as follows. 75 , 76 Further studies may well be needed to examine the association among male patients, anticholinergic usage, and the risk of dementia.
Childhood self‐control, cognitive ability, ARD, and the risk of dementia
In the present study, the cases with other addictions were excluded in both ARD and control cohorts. In addition, when excluding cases developed dementia within first two and first five years, the risks of dementia were significantly decreased, in the sensitivity analysis. Thus, it is possible that drug addiction itself, not just ARD, was associated with dementia. Therefore, a common earlier life event or factor may have contributed to both ARD and dementia. One previous study, in Dunedin, New Zealand, found that the childhood self‐control could predict the physical health, substance dependence, personal finances, and criminal offending outcomes. Lower self‐control had poorer outcomes, including increased risk of substance use disorder. 77 In addition, there might be an indirect connection between the childhood self‐control and dementia, since several studies have found the association between ADHD and risk of dementia 9 , 10 and the poor childhood self‐control is one of the core problems of ADHD. 78 Besides, the individuals with childhood poor impulse control might tend to abuse the methamphetamine for its fast‐onset and long‐lasting effects. 18 , 19 Therefore, further studies are needed to investigate the association among ARD, childhood self‐control, and the risk of dementia.
One case–control study in Scotland, the United Kingdom, has found that lower premorbid cognitive intelligence quotient (IQ) in childhood increases VaD development in later life. 79 This finding also indicates that VaD could occur much earlier than other dementia. The earlier occurrence of VaD might agree with the finding that the ARD cohort was associated with overall dementia, AD, and other dementia, but not VaD, after the exclusion of the dementia diagnosis of the first five years after the diagnosis of ARD, in the sensitivity analysis. Besides, the reports about the association between childhood IQ and risk of adult illicit substance abuse varied. Two civilian cohort studies found that high childhood IQ may increase the risk of illegal drug use in adolescence, adulthood, and middle age. 80 , 81 However, one study in the United States Army found that male veterans with high childhood IQ was associated with less likely to be habitual users of cannabis, cocaine, heroin, amphetamines, barbiturates, and lysergic acid diethylamide (LSD). 82 Therefore, further studies are needed to clarify the link among childhood IQ, VaD, and illicit substance use disorders, including ARD.
Limitations
There were several limitations in the present study. First, our study is retrospective using the ICD‐9‐CM codes, therefore, the lack of detailed records of genetic, nutritional, habitual factors, smoking, and body mass index that were not included in such a claims database study. Second, this national review insurance database cannot provide detailed information, including the severity, stage, and the care‐giver burden of the patients with dementia. Furthermore, Alzheimer dementia is the most common cause of dementia (40–60% in all dementias), followed by vascular dementia (20–30% in all dementias), and mixed or other dementias (7–15%) from previous studies in Taiwan, 46 , 47 but most of the dementia in our study were other types of dementia. The possibility is that clinicians might put these types of dementia with a nature of progressive and gradual decline and no evidence of previous cerebrovascular events, into this category instead of AD.
Conclusion
This study shows that patients with ARD, both amphetamine use disorder and amphetamine‐induced psychotic disorder, may have a nearly fivefold risk of developing dementia in comparison to the non‐ARD cohort. This result could serve as a reminder for clinicians who are in charge of the care of patients with ARD.
Conflict of Interest
None.
Supporting information
Figure S1. Dementia‐free survival for patients with and without amphetamine‐related disorders during the 15‐year follow‐up period in Taiwan.
Table S1. ICD‐9‐CM codes.
Acknowledgments
The authors thank Ms. Hui‐Wen Yeh, who participated in the design of the research and data interpretation, and they appreciate Ms. Wei‐Shan Chiang for her help in paper collection and proof reading. This work was supported by Tri‐Service General Hospital Research Foundation (TSGH‐C108‐003, TSGH‐C108‐151, and TSGH‐B‐109‐010), Medical Affairs Bureau, Ministry of Defense of Taiwan (MAB‐107‐084), and Cheng Hsin General Hospital and National Defense Medical Center (CH‐NDMC‐108‐9, CH‐NDMC‐109‐7) of Taiwan, ROC. The requirement for informed consent was waived by the IRB because all NHIRD data had been de‐identified. We also appreciate Taiwan’s Health and Welfare Data Science Center and Ministry of Health and Welfare (HWDC, MOHW) for providing the National Health Research Database.
Funding Information
This work was supported by Tri‐Service General Hospital Research Foundation (TSGH‐C‐108‐003, TSGH‐C‐108‐151, and TSGH‐B‐109‐010), Medical Affairs Bureau, Ministry of Defense of Taiwan (MAB‐107‐084), and Cheng Hsin General Hospital and National Defense Medical Center (CH‐NDMC‐108‐9, CH‐NDMC‐109‐7) of Taiwan, ROC.
Funding Statement
This work was funded by Cheng Hsin General Hospital Foundation grants CH‐NDMC‐108‐9 and CH‐NDMC‐109‐7; Tri‐Service General Hospital Research Foundation grants TSGH‐C108‐003, TSGH‐C108‐151, and TSGH‐B‐109‐010; Medical Affairs Bureau, Ministry of Defense of Taiwan grant MAB‐107‐084.
References
- 1. Taiwan Alzheimer`s Disease Association . 2015–2056 Expected Dementia popultion Report in Taiwan. Taiwan Alzheimer`s Disease Association. 2013. [cited 2014 September, 10]; Available from: http://www.tada2002.org.tw/tada_know_02.html#01.
- 2. Tzeng NS, Chang CW, Hsu JY, et al. Caregiver burden for patients with dementia with or without hiring foreign health aides: a cross‐sectional study in a Northern Taiwan memory clinic. J Med Sci 2015;35:239–247. [Google Scholar]
- 3. Tzeng NS, Chiang WS, Chen SY, et al. The impact of pharmacological treatments on cognitive function and severity of behavioral symptoms in geriatric elder patients with dementia. Taiwanese J Psychiatry 2017;31:69–79. [Google Scholar]
- 4. Wang HY, Chen JH, Huang SY, et al. Forensic evaluations for offenders with dementia in Taiwan's criminal courts. J Am Acad Psychiatry Law 2018;46:45–51. [PubMed] [Google Scholar]
- 5. Kao LC, Chien WC, Chung CH, et al. The newly diagnosed amnestic disorders and dementia: a nationwide. Cohort Study in Taiwan Taiwanese J Psychiatry 2018;32:18–28. [Google Scholar]
- 6. Yeh TC, Chou YC, Weng JP, et al. Detection of malingering in the memory of patients with dementia: a pilot study on coin‐in‐the‐hand test in a Northern Taiwan memory clinic. J Med Sci 2019;39:81. [Google Scholar]
- 7. Lee YK, Hou SW, Lee CC, et al. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 2013;8:e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang CW, Tzeng NS, Yin YJ, et al. Angiotensin receptor blockers decrease the risk of major adverse cardiovascular events in patients with end‐stage renal disease on maintenance dialysis: a nationwide matched‐cohort study. PLoS One 2015;10:e0140633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tzeng NS, Chung CH, Lin FH, et al. Risk of dementia in adults with ADHD: a nationwide, population‐based cohort study in Taiwan. J Atten Disord 2017;1087054717714057. [DOI] [PubMed] [Google Scholar]
- 10. Golimstok A, Rojas JI, Romano M, et al. Previous adult attention‐deficit and hyperactivity disorder symptoms and risk of dementia with Lewy bodies: a case‐control study. Eur J Neurol 2011;18:78–84. [DOI] [PubMed] [Google Scholar]
- 11. Hulse GK, Lautenschlager NT, Tait RJ, Almeida OP. Dementia associated with alcohol and other drug use. Int Psychogeriatr 2005;17(Suppl 1):S109–S127. [DOI] [PubMed] [Google Scholar]
- 12. Chang KH, Chang MY, Muo CH, et al. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population‐based retrospective cohort study. PLoS One 2014;9:e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai CY, Huang YW, Tseng CH, et al. Patients with carbon monoxide poisoning and subsequent dementia: a population‐based cohort study. Med 2016;95:e2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong CS, Lin YC, Hong LY, et al. Increased long‐term risk of dementia in patients with carbon monoxide poisoning: a population‐based study. Med 2016;95:e2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang SY, Chien WC, Chung CH, et al. Risk of dementia after charcoal‐burning suicide attempts: a nationwide cohort study in Taiwan. J Investig Med 2018;66:1070–1082. [DOI] [PubMed] [Google Scholar]
- 16. Lappin JM, Darke S, Farrell M. Stroke and methamphetamine use in young adults: a review. J Neurol Neurosurg Psychiatry 2017;88:1079–1091. [DOI] [PubMed] [Google Scholar]
- 17. Ministry of Health and Welfare . The Annual of Statistical Data of Cases and Laboratory Examinations for the Drug Abuse 2018: Taiwan Food and Drug Administration, Ministry of Health and Welfare, 2018.
- 18. Fowler JS, Volkow ND, Logan J, et al. Fast uptake and long‐lasting binding of methamphetamine in the human brain: comparison with cocaine. NeuroImage 2008;43:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barr AM, Panenka WJ, MacEwan GW, et al. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Yu P, Zhang L, et al. Methamphetamine exposure induces neuropathic protein beta‐Amyloid expression. Toxicol in vitro 2019;54:304–309. [DOI] [PubMed] [Google Scholar]
- 21. Xu H, Chen X, Wang J, et al. Involvement of insulin signalling pathway in methamphetamine‐induced hyperphosphorylation of Tau. Toxicol 2018;408:88–94. [DOI] [PubMed] [Google Scholar]
- 22. Zhang K, Zhang Q, Jiang H, et al. Impact of aerobic exercise on cognitive impairment and oxidative stress markers in methamphetamine‐dependent patients. Psychiatry Res 2018;266:328–333. [DOI] [PubMed] [Google Scholar]
- 23. Papageorgiou M, Raza A, Fraser S, et al. Methamphetamine and its immune‐modulating effects. Maturitas 2019;121:13–21. [DOI] [PubMed] [Google Scholar]
- 24. Chao PC, Chien WC, Chung CH, et al. Pinworm infections associated with risk of psychiatric disorders‐A nationwide cohort study in Taiwan: pinworm infections and psychiatric disorders. Compr Psychiatry 2019;93:14–19. [DOI] [PubMed] [Google Scholar]
- 25. Chen TY, Huang CH, Chung CH, et al. Sex and age differences in the association between anxiety disorders and narcolepsy: a nationwide population‐based case control study. J Affect Disord 2020;264:130–137. [DOI] [PubMed] [Google Scholar]
- 26. Lin YC, Chen TY, Chien WC, et al. Stimulants associated with reduced risk of hospitalization for motor vehicle accident injury in patients with obstructive sleep apnea‐a nationwide cohort study. BMC Pulm Med 2020;20(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu YP, Chien WC, Chung CH, et al. Are anticholinergic medications associated with increased risk of dementia and behavioral and psychological symptoms of dementia? A nationwide 15‐year follow‐up cohort study in Taiwan. Front Pharmacol 2020;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tseng WS, Chien WC, Chung CH, et al. Risk of sleep disorders in patients with decompression sickness: a nationwide, population‐based study in Taiwan. Psychiatria Danub 2019;31:172–181. [DOI] [PubMed] [Google Scholar]
- 29. Tzeng NS, Chung CH, Chang SY, et al. Risk of psychiatric disorders in pulmonary embolism: a nationwide cohort study. J Investig Med 2019;67:977–986. [DOI] [PubMed] [Google Scholar]
- 30. Wan FJ, Chien WC, Chung CH, et al. Association between traumatic spinal cord injury and affective and other psychiatric disorders‐A nationwide cohort study and effects of rehabilitation therapies. J Affect Disord 2020;265:381–388. [DOI] [PubMed] [Google Scholar]
- 31. Wang DS, Chung CH, Chang HA, et al. Association between child abuse exposure and the risk of psychiatric disorders: a nationwide cohort study in Taiwan. Child Abuse Negl 2020;101:104362. [DOI] [PubMed] [Google Scholar]
- 32. Yang CC, Chien WC, Chung CH, et al. No association between human immunodeficiency virus infections and dementia: a nationwide cohort study in Taiwan. Neuropsychiatr Dis Treat 2019;15:3155–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeh TC, Chien WC, Chung CH, et al. Psychiatric disorders after traumatic brain injury: a nationwide population‐based cohort study and the effects of rehabilitation therapies. Arch Phys Med Rehabil 2020;101(5):822–831. [DOI] [PubMed] [Google Scholar]
- 34. Ho Chan WS. Taiwan’s healthcare report 2010. EPMA J 2010;1:563–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chinese Hospital Association . ICD‐9‐CM English‐Chinese Dictionary. Taipei, Taiwan: Chinese Hospital Association Press, 2000. [Google Scholar]
- 36. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV). American Psychiatric Association; 1994.
- 37. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM‐IV‐TR). American Psychiatric Association; 2000.
- 38. Chen HL, Lea YP, Young SC, Wu CY. A summary report of concurrent peer review of inpatient medical records in a medical center. Taiwan J Public Health 1995;14:103–110. [Google Scholar]
- 39. National Health Insurance Reimbursement Regulations [database on the Internet] . 2014. Available from: http://law.moj.gov.tw/LawClass/LawAllIf.aspx?PCode=L0060006
- 40. McGrogan A, Madle GC, Seaman HE, de Vries CS. The Epidemiology of Guillain‐Barré syndrome worldwide. Neuroepidemiology. 2009;32:150–163. [DOI] [PubMed] [Google Scholar]
- 41. van den Berg B, Walgaard C, Drenthen J, et al. Guillain‐Barre syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol 2014;10:469–482. [DOI] [PubMed] [Google Scholar]
- 42. Sandoglobulin Guillain‐Barre Syndrome Trial Group . Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain‐Barre syndrome. Plasma Exchange/Sandoglobulin Guillain‐Barre Syndrome Trial Group. Lancet 1997;349:225–230. [PubMed] [Google Scholar]
- 43. Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care 2005;20:12–19. [DOI] [PubMed] [Google Scholar]
- 44. Santabarbara J, Villagrasa B, Lopez‐Anton R, et al. Clinically relevant anxiety and risk of Alzheimer's disease in an elderly community sample: 4.5 years of follow‐up. J Affect Disord 2019;250:16–20. [DOI] [PubMed] [Google Scholar]
- 45. Santabarbara J, Lopez‐Anton R, de la Camara C, et al. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr Scand 2019;139:6–14. [DOI] [PubMed] [Google Scholar]
- 46. Liu HC, Lin KN, Teng EL, et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. J Am Geriatr Soc 1995;43:144–149. [DOI] [PubMed] [Google Scholar]
- 47. Lin RT, Lai CL, Tai CT, et al. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Neurol Sci 1998;160:67–75. [DOI] [PubMed] [Google Scholar]
- 48. Sun Y, Lee HJ, Yang SC, et al. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS One 2014;9:e100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chiang SC, Chen CY, Chang YY, et al. Prevalence of heroin and methamphetamine male users in the northern Taiwan, 1999–2002: capture‐recapture estimates. BMC Public Health 2007;7:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dublin S, Walker RL, Gray SL, et al. Prescription opioids and risk of dementia or cognitive decline: a prospective cohort study. J Am Geriatr Soc 2015;63:1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419–428. [DOI] [PubMed] [Google Scholar]
- 52. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol 2016;73:410–416. [DOI] [PubMed] [Google Scholar]
- 53. Eusebi LH, Rabitti S, Artesiani ML, et al. Proton pump inhibitors: risks of long‐term use. J Gastroenterol Hepatol 2017;32:1295–1302. [DOI] [PubMed] [Google Scholar]
- 54. Islam MM, Iqbal U, Walther B, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta‐analysis. Neuroepidemiol 2016;47:181–191. [DOI] [PubMed] [Google Scholar]
- 55. Chou MH, Wang JY, Lin CL, Chung WS. DMARD use is associated with a higher risk of dementia in patients with rheumatoid arthritis: a propensity score‐matched case‐control study. Toxicol Appl Pharmacol 2017;334:217–222. [DOI] [PubMed] [Google Scholar]
- 56. Heser K, Luck T, Rohr S, et al. Potentially inappropriate medication: Association between the use of antidepressant drugs and the subsequent risk for dementia. J Affect Disord 2018;226:28–35. [DOI] [PubMed] [Google Scholar]
- 57. Gutwinski S, Schreiter S, Priller J, et al. Drink and think: impact of alcohol on cognitive functions and dementia ‐ evidence of dose‐related effects. Pharmacopsychiatry 2018;51:136–143. [DOI] [PubMed] [Google Scholar]
- 58. Schwarzinger M, Pollock BG, Hasan OSM, et al. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health 2018;3:e124–e132. [DOI] [PubMed] [Google Scholar]
- 59. Xu W, Wang H, Wan Y, et al. Alcohol consumption and dementia risk: a dose‐response meta‐analysis of prospective studies. Eur J Epidemiol 2017;32:31–42. [DOI] [PubMed] [Google Scholar]
- 60. Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson's disease among hospital patients with methamphetamine‐use disorders. Mov Disord 2010;25:2333–2339. [DOI] [PubMed] [Google Scholar]
- 61. Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine‐type drugs. Drug Alcohol Depend 2012;120:35–40. [DOI] [PubMed] [Google Scholar]
- 62. Curtin K, Fleckenstein AE, Robison RJ, et al. Methamphetamine/amphetamine abuse and risk of Parkinson's disease in Utah: a population‐based assessment. Drug Alcohol Depend 2015;146:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Du X, Wang X, Geng M. Alzheimer's disease hypothesis and related therapies. Transl Neurodegener 2018;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haque MM, Murale DP, Kim YK, Lee JS. Crosstalk between Oxidative Stress and Tauopathy. Int J Mol Sci 2019;20:1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Surendranathan A, Rowe JB, O'Brien JT. Neuroinflammation in Lewy body dementia. Parkinsonism Relat Disord 2015;21:1398–1406. [DOI] [PubMed] [Google Scholar]
- 66. Teixeira FB, Saito MT, Matheus FC, et al. Periodontitis and Alzheimer's Disease: a possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front Aging Neurosci 2017;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Back DB, Kwon KJ, Choi DH, et al. Chronic cerebral hypoperfusion induces post‐stroke dementia following acute ischemic stroke in rats. J Neuroinflammation 2017;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci 2004;5:963–970. [DOI] [PubMed] [Google Scholar]
- 69. Solomon RL, Corbit JD. An opponent‐process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 1974;81:119–145. [DOI] [PubMed] [Google Scholar]
- 70. Solomon RL. The opponent‐process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychol 1980;35:691–712. [DOI] [PubMed] [Google Scholar]
- 71. Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B, Biol Sci 2008;363:3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tzeng NS, Liu YP. Amphetamine exposure and dementia ‐ a hypothesis of the long term sequelae of cognitive enhancers based on opponent process theory. Med Hypotheses 2019;132:109327. [DOI] [PubMed] [Google Scholar]
- 73. Urban KR, Gao WJ. Psychostimulants as cognitive enhancers in adolescents: more risk than reward? Front Public Health 2017;5:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liu HC, Fuh JL, Wang SJ, et al. Prevalence and subtypes of dementia in a rural Chinese population. Alzheimer Dis Assoc Disord 1998;12:127–134. [DOI] [PubMed] [Google Scholar]
- 75. Rocca WA, Mielke MM, Vemuri P, Miller VM. Sex and gender differences in the causes of dementia: a narrative review. Maturitas 2014;79:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cholerton B, Johnson CO, Fish B, et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson's disease. Parkinsonism Relat Disord 2018;50:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moffitt TE, Arseneault L, Belsky D, et al. A gradient of childhood self‐control predicts health, wealth, and public safety. Proc Natl Acad Sci USA 2011;108:2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Neef NA, Bicard DF, Endo S. Assessment of impulsivity and the development of self‐control in students with attention deficit hyperactivity disorder. J Appl Behav Anal 2001;34:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGurn B, Deary IJ, Starr JM. Childhood cognitive ability and risk of late‐onset Alzheimer and vascular dementia. Neurol 2008;71:1051–1056. [DOI] [PubMed] [Google Scholar]
- 80. White J, Batty GD. Intelligence across childhood in relation to illegal drug use in adulthood: 1970 British Cohort Study. J Epidemiol Community Health 2012;66:767–774. [DOI] [PubMed] [Google Scholar]
- 81. White JW, Gale CR, Batty GD. Intelligence quotient in childhood and the risk of illegal drug use in middle‐age: the 1958 National Child Development Survey. Ann Epidemiol 2012;22:654–657. [DOI] [PubMed] [Google Scholar]
- 82. White J, Mortensen LH, Batty GD. Cognitive ability in early adulthood as a predictor of habitual drug use during later military service and civilian life: the Vietnam experience study. Drug Alcohol Depend 2012;125(1–2):164–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dementia‐free survival for patients with and without amphetamine‐related disorders during the 15‐year follow‐up period in Taiwan.
Table S1. ICD‐9‐CM codes.


