Abstract
Objectives
Based on multi‐domain classification of Parkinson disease (PD) subtypes, we sought to determine the key features that best differentiate subtypes and the utility of PD subtypes to predict clinical milestones.
Methods
Prospective cohort of 162 PD participants with ongoing, longitudinal follow‐up. Latent class analysis (LCA) delineated subtypes based on score patterns across baseline motor, cognitive, and psychiatric measures. Discriminant analyses identified key features that distinguish subtypes at baseline. Cox regression models tested PD subtype differences in longitudinal conversion to clinical milestones, including deep brain stimulation (DBS), dementia, and mortality.
Results
LCA identified distinct subtypes: “motor only” (N = 63) characterized by primary motor deficits; “psychiatric & motor” (N = 17) characterized by prominent psychiatric symptoms and moderate motor deficits; “cognitive & motor” (N = 82) characterized by impaired cognition and moderate motor deficits. Depression, executive function, and apathy best discriminated subtypes. Since enrollment, 22 had DBS, 48 developed dementia, and 46 have died. Although there were no subtype differences in rate of DBS, dementia occurred at a higher rate in the “cognitive & motor” subtype. Surprisingly, mortality risk was similarly elevated for both “cognitive & motor” and “psychiatric & motor” subtypes compared to the “motor only” subtype (relative risk = 3.15, 2.60).
Interpretation
Psychiatric and cognitive features, rather than motor deficits, distinguish clinical PD subtypes and predict greater risk of subsequent dementia and mortality. These results emphasize the value of multi‐domain assessments to better characterize clinical variability in PD. Further, differences in dementia and mortality rates demonstrate the prognostic utility of PD subtypes.
Introduction
Parkinson disease (PD) causes motor, cognitive, and psychiatric dysfunction that impair quality of life, health, and lifespan. These clinical features do not occur in isolation, but rather in combination. Specific patterns of motor, cognitive, and psychiatric dysfunction may yield distinct PD subtypes, potentially reflecting differences in neuropathology, 1 , 2 , 3 , 4 , 5 that predict prognosis and treatment response.
The majority of clinically derived subtyping studies focus on motor deficits, dichotomizing PD into tremor dominant or nontremor dominant (akinetic‐rigid or postural instability with gait difficulty [PIGD]). 6 , 7 However, cognitive and psychiatric features also contribute to quality of life and may differentiate subtypes. 8 , 9 , 10 Thus, multi–domain approaches could have greater clinical utility than single‐domain (e.g., motor) classifications.
Multi‐domain classifications, including motor, cognitive, and psychiatric measures, permit broader subtypes capturing the full spectrum of clinical manifestations. However, most multi‐domain studies utilized cognitive screening measures (e.g., Montreal Cognitive Assessment), 11 , 12 , 13 categorized participants based on cognitive status (e.g., mild cognitive impairment [MCI]), 14 included only a single psychiatric rating, 11 , 15 , 16 or combined cognitive and psychiatric function into “non‐motor” symptoms. 17 Even with comprehensive assessments, few studies considered which features best discriminate subtypes. Further, most studies conducted behavioral evaluations while participants were medicated 12 , 14 , 15 , 16 (introducing variability and potential medication effects), focused on early‐stage PD with limited clinical manifestations, 2 , 11 , 12 , 18 or included participants with dementia, 13 , 14 , 15 , 16 precluding the ability to predict dementia. In fact, few multi‐domain studies report longitudinal follow‐up 2 , 14 , 19 thus limiting insight regarding the utility of PD subtypes to predict clinically meaningful outcomes 20 such as dementia or mortality. Predicting progression across PD subtypes could guide prognosis and improve clinical care.
Therefore, this study aims to (1) classify PD subtypes based on comprehensive, multi‐domain clinical evaluations at baseline; (2) determine the specific features that best discriminate subtypes at baseline; and (3) predict longitudinal rates for clinical outcomes, including deep brain stimulation (DBS), dementia, and mortality across subtypes. We applied latent class analysis (LCA) to baseline motor, cognitive, and psychiatric measures in a large, prospective longitudinal sample of nondemented PD participants. DBS, dementia, and mortality rates were compared across baseline PD subtypes to determine clinical utility.
Methods
Standard protocol approvals, registrations, and consents
The Washington University in St. Louis (WUSTL) Human Research Protection Office approved this study. All participants provided written informed consent.
Study overview
As part of a larger, on‐going study, a prospective cohort of 210 PD participants was recruited through the WUSTL Movement Disorders Center and community between January 2006 and September 2015. At study enrollment, participants completed a comprehensive motor, cognitive and clinical evaluation as described below, which is repeated at follow‐up visits. Longitudinal follow‐up visits occur every 1–3 years (average length of follow‐up = 4.8 years [SD = 2.4], range: 0–12 years) depending on date of enrollment, for as long as the participant is willing and able. If a participant is no longer able to attend in‐person testing sessions (e.g., severe cognitive or motor deficits), clinical evaluations are completed over the phone. Longitudinal follow‐up is intended to continue until death and all participants agree to brain donation upon death. To date, 157 participants have completed at least one follow‐up visit, 12 have withdrawn or are lost to follow‐up, and 46 have died (see Fig. S1 for study flowchart diagram). Here, we focus on the baseline evaluation of 162 PD participants to identify clinical subtypes and determine the key features that best distinguish groups. Longitudinal clinical evaluations provide information regarding the clinical milestones of DBS, conversion to dementia, and mortality.
Participants
Parkinson disease diagnosis was based on modified United Kingdom PD Society Brain Bank clinical diagnostic criteria with clear motor response to levodopa, 21 to be confirmed at autopsy. Two participants were drug naive and excluded; two participants were found to not be PD at autopsy, prior to these data analyses, and were excluded. Dementia, defined as clinically significant cognitive decline with functional impairment, 22 was assessed with the Clinical Dementia Rating evaluation (CDR) 23 ; 33 PD participants met dementia criteria (CDR ≥ 1) at baseline and were excluded from analyses. Additional exclusion criteria were: other neurologic diagnosis, head injury with loss of consciousness >5 min or neurologic sequelae (N = 2), brain surgery (DBS exclusion only at enrollment), schizophrenia, bipolar disorder, or incomplete baseline evaluations (N = 9). In total, baseline behavioral evaluations from 162 nondemented PD were included (Fig. S1; see Table 1 for baseline characteristics).
Table 1.
Participant demographics and clinical characteristics at baseline.
| Clinical characteristics | Mean (SD) |
|---|---|
| N | 162 |
| Sex (% male) | 61.7% |
| Age (years) | 66.1 (7.7) |
| Years of education | 16.0 (2.5) |
| Duration of PD symptoms (years) | 6.2 (3.8) |
| Age onset of PD | 60.1 (8.0) |
| UPDRS‐3 OFF total | 24.2 (8.9) |
| LEDD | 764 (493) |
| MMSE | 28.3 (1.5) |
UPDRS‐3, Unified Parkinson Disease Rating Scale, motor subscale 3; LEDD, levodopa equivalent daily dose; MMSE, Mini Mental Status Exam.
Clinical evaluation
Clinical evaluations, with a collateral source and participants on medication, included the CDR (CDR ≥ 1 signifies dementia, 0.5 indicates cognitive decline/impairment, 0 represents intact cognition), MMSE, 24 Brief Smell Identification Test (BSIT), 25 One Day Fluctuations, 26 and Epworth Sleepiness scale. 27 Clinical assessments also include review of medical history and general systems review, including questions about current constipation, orthostasis, or hallucinations, as well as surgical history (e.g., DBS). Symptom duration was computed in years from motor symptom onset to baseline visit.
Motor assessments
After overnight withdrawal of PD medications, in the practically defined “OFF” state, movement disorder specialists completed the Unified Parkinson Disease Rating Scale motor subscale 3 (UPDRS‐3). Tremor, bradykinesia, rigidity, and PIGD subscores and levodopa equivalent daily dose (LEDD) were computed. 28 , 29 At the baseline visit, 37 (23%) participants took dopamine agonists.
Cognitive assessments
Neuropsychological evaluations included tests of attention (Digit Span; 30 Digit Symbol 30 ), memory (California Verbal Learning Test‐II, short form; 31 Logical Memory 32 ), language (Boston Naming Test 33 ), visuospatial (Judgment of Line Orientation; 34 Spatial Relations Test 35 ) and executive function (Trail Making Test; 36 Verbal Fluency‐Switching; 37 Color‐Word Interference 37 ) while OFF PD medications to avoid medication confounds. 38 Age, sex, and education‐adjusted scaled scores, based on test manuals and published normative data, were converted to z‐scores and averaged within each domain. 39 MCI was determined using Movement Disorder Society Level II criteria of at least two tests (>1.5 SD cutoff) within a single domain or across multiple domains. 40
Psychiatric assessments
Psychiatric function, assessed ON medication, was determined by self‐rated Geriatric Depression Scale short form (GDS) 41 and Frontal Systems Behavior Scale – Apathy subscale (FrSBe‐A). 42 Participants and a collateral source completed the brief 12 item Neuropsychiatric Inventory Questionnaire (NPIQ) 43 to assess overall psychiatric function.
Statistical analyses
Identifying clinical subtypes
Latent class analysis (LCA) was used to identify PD subtypes from baseline assessments. Analysis proceeds by modeling 1, 2, 3, and up to k number of classes. Model fit indices include: Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) where lower relative scores indicate better model fit; 44 , 45 Lo‐Mendell‐Rubin Likelihood Ratio Test (LMR LRT) 46 indicates model fit with k classes compared to k−1 classes where P < 0.05 favors the k model; and relative entropy – a measure of classification uncertainty (range: 0–1) with higher values indicating greater classification certainty. Class membership is assigned based on posterior probability values, with ≥0.7 indicating reliable individual class assignment. 47 Higher posterior probabilities for the assigned class yields high overall model entropy. Model selection also is based on meaningful class distinctions and sufficient number of individuals per class to allow further statistical analysis. 48 Thus, the advantages of LCA over more traditional cluster‐based approaches are that (1) LCA is a person‐centered analysis that classifies individuals based on response pattern similarities to measured indicator variables, 49 whereas other clustering methods are variable‐centered and identify relationships among variables 50 , as well as (2) LCA provides indices of model fit and individual‐level class membership probabilities for greater classification certainty.
Indicator variables included the following baseline scores: UPDRS‐3 tremor, bradykinesia, rigidity, and PIGD subscores; attention, memory, language, visuospatial, and executive function cognitive domain scores; and depression and apathy ratings, with age, sex, and education covariates to avoid demographic‐driven classifications. Indicator variables were normalized to z‐scores, based on sample distribution (motor subscores, GDS) or published normative data (cognitive scores, FrSBe‐Apathy subscale).
Determining key features
To determine the key distinguishing features, discriminant analyses, using indicator variables as well as other clinical and demographic variables from the baseline assessments, were conducted. One‐way ANOVAs, nonparametric Kruskal–Wallis, and chi‐square tests compared PD subtypes on indicator variables and additional clinical and demographic information at baseline. Main effects of PD subtype were followed by Tukey’s HSD post hoc pairwise comparisons.
Conversion to clinical milestones
To determine PD subtype differences in clinical milestones (DBS, dementia, mortality), multivariate Cox proportional hazards regression models using the longitudinal follow‐up data were conducted, with censoring based on last date of contact. Survival and events were calculated as such for each milestone: for DBS, events were defined as date of DBS and survival time was calculated as time since baseline visit to most recent contact; for dementia, events were defined as the date when a participant received a CDR score ≥1, and survival time was calculated as time since baseline visit to most recent CDR; and for mortality, events were defined as date of death, and survival time was calculated as time since baseline to most recent contact. As follow‐up visits may only occur every three years, date of last contact (for the DBS and mortality analyses) was based on most recent contact from either a study visit, study contact, or clinical visit.
LCA was conducted using MPlus (Muthen & Muthen, Los Angeles CA). The longitudinal survival analyses (Cox proportional hazards regression) were conducted in R Version 3.5.2, SURVIVAL and SURVMINER packages (R Foundation, Vienna Austria). Additional analyses were conducted with PASW Version 25 (IBM, Chicago, IL). All tests were 2‐tailed and P < 0.05 defined statistical significance.
Results
Baseline subtype classification
The 3‐class LCA model provided the best overall fit (Table S1) and high average posterior probabilities (0.91–0.95), indicating high probability for assigned class membership. Classes captured three PD subtypes: (1) “motor only” – mild motor deficits with intact cognition and healthy psychological state (N = 63); (2) “psychiatric & motor” – prominent depression and apathy, moderate motor deficits, and intact cognition (N = 17); and (3) “cognitive & motor” – impaired cognition, moderate motor deficits, and relatively healthy psychiatric function (N = 82).
The indicator variables discriminated PD subtypes, with 98.3% correct classification, showing excellent subtype separation (Fig. 1A). Stepwise discriminant analysis revealed that depression, executive function, apathy, bradykinesia, visuospatial, attention, and PIGD best discriminated PD subtypes with 95.1% correct classification. Even with just depression, executive function, and apathy, classification accuracy was 89.5%. Notably, tremor scores did not distinguish subtypes despite their frequent use in clinical subtyping. For comparison, we classified participants according to the clinically derived tremor dominant/PIGD motor subtypes, 6 which yielded 19.8% tremor dominant, 59.9% PIGD, and 20.4% unclassified/indeterminate. Thus, psychiatric and cognitive features not only were the defining features for certain subtypes but also substantially increased classification accuracy.
Figure 1.
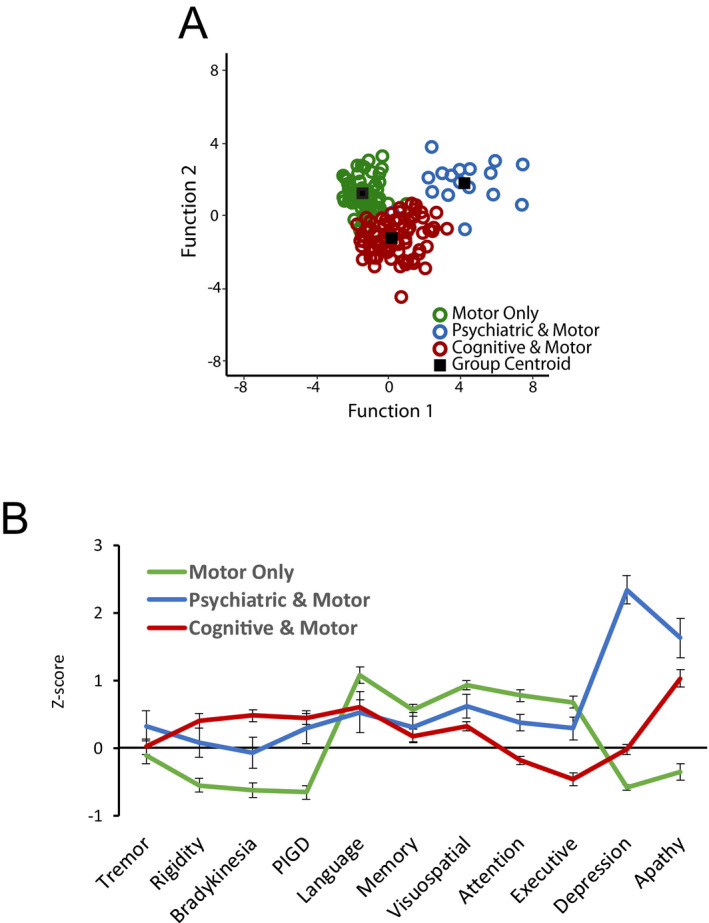
PD Clinical Subtypes. (A) LCA analysis identified three distinct PD subtypes based on the pattern of scores across motor, cognitive and psychiatric domains. Discriminant analyses achieved significant subtype separation and 98.3% classification accuracy based on discriminant functions 1 and 2, which accounted for 60.5% and 39.5% of the variance, respectively. Group centroid represents the standardized mean scores for that subtype on function 1 and 2. (B) Subtypes differed across motor, cognitive, and psychiatric measures. Values represent z‐scores for each measure (indicator). Higher scores represent worse function for motor and psychiatric measures; lower scores represent worse function for cognitive domains. PIGD = postural instability and gait difficulty. Significant subtype differences for all measures, except tremor (P = 0.40) and memory (P = 0.11).
Baseline subtype differences
PD subtypes differed across all indicator variables (ANCOVAs with age, sex, education covariates), except tremor and memory (Fig. 1B; Table S2) at baseline. Post hoc analyses (Table S2) revealed that the “motor only” subtype had the lowest motor ratings, with no significant differences between “psychiatric & motor” and “cognitive & motor” subtypes. The “cognitive & motor” subtype performed the worst across cognitive domains, while the “psychiatric & motor” subtype had the highest depression and apathy ratings.
PD subtypes also differed on select demographic and clinical variables at baseline (Table 2). Although the “motor only” subtype was younger at disease onset and study enrollment, and had shorter symptom duration than the “cognitive & motor” subtype, the “psychiatric & motor” and “cognitive & motor” subtypes did not differ in age (onset or enrollment), or symptom duration.
Table 2.
Comparison of PD subtypes on baseline clinical and demographic characteristics.
| Variable | Class 1: | Class 2: | Class 3: | Omnibus P‐value |
|---|---|---|---|---|
| “Motor Only” | “Psychiatric & Motor” | “Cognitive & Motor” | ||
| N = 63 | N = 17 | N = 82 | ||
| Sex (% male) | 48%bc | 77%a | 70%a | P = 0.01 |
| Age (years) | 63.2 (6.3)c | 64.5 (8.8) | 68.6 (7.6)a | P < 0.001 |
| Years of education | 16 (2.5) | 15 (2.4) | 16 (2.5) | P = 0.15 |
| Age onset of PD | 58.3 (6.6)c | 57.4 (8.7) | 61.9 (8.4)a | P < 0.01 |
| Duration of PD symptoms (years) | 5.1 (3.0)c | 7.2 (3.9) | 6.9 (4.1)a | P < 0.01 |
| LEDD | 613 (380)b | 1004 (664)a | 783 (438) | P = 0.03 |
| DA agonists (no/yes, % using) | 14/49, 22% | 3/14, 18% | 20/62, 24% | P = 0.82 |
| NPIQ total score | 1.8 (2.4)bc | 4.1 (3.7)a | 3.1 (2.9)a | P = 0.01 |
| MMSE | 29.1 (1.1)c | 28.3 (1.4) | 27.6 (1.6)a | P < 0.001 |
| MCI | 3 (4.8%) | 2 (11.8%) | 21 (25.6%) | P = 0.003 |
| CDR (0/.5) | 55/8bc | 9/8ac | 27/55ab | P < 0.01 |
| BSIT | 7.3 (2.6) | 6.4 (2.3) | 5.5 (2.3) | P = 0.24 |
| Epworth sleepiness | 8.9 (4.5) | 10.2 (4.7) | 9.0 (3.6) | P = 0.56 |
| One day fluctuations | 0.5 (1.1) | 0.8 (1.3) | 1.0 (1.7) | P = 0.12 |
| Constipation (no/yes, % yes) 1 | 22/35, 56% | 3/13, 76% | 32/47, 57% | P = 0.26 |
| Orthostasis (no/yes, % yes) 1 | 43/14, 22% | 10/7, 41% | 55/24, 29% | P = 0.40 |
| Hallucinations (no/yes, % yes) | 63/0, 0% | 16/1, 6% | 75/7, 9% | P = 0.06 |
Values represent mean (SD), except sex, MCI, CDR, Constipation, and Orthostasis reported as percentage or total number. LEDD, levodopa equivalent daily dose; DA Agonists, Dopamine Agonists; NPIQ, Neuropsychiatric Inventory Questionnaire; MMSE, Mini Mental Status Exam; MCI, Mild Cognitive Impairment; CDR, Clinical Dementia Rating scale. Superscripts indicate significant differences from (a) motor only; (b) psychiatric & motor; (c) cognitive & motor (i.e., “ab” indicates significant difference from both “motor only” and “psychiatric & motor” subtypes). Significant differences (P < 0.05) are marked in bold.
Total counts reflect only those participants who provided responses to these questions.
The “motor only” subtype had the highest proportion of females, and the “psychiatric & motor” subtype had the highest LEDD; however, there were no differences between groups for number of participants on dopamine agonists. The “cognitive & motor” subtype contained the highest proportion with cognitive impairment and MCI, whereas the “psychiatric & motor” subtype reported the most psychiatric symptoms (NPIQ; Table 2).
Education, presence of anxiety, hallucinations, and constipation were similar across subtypes at baseline (Table 2). PD subtypes also did not significantly differ on the BSIT, Epworth, One Day Fluctuations, or orthostasis (Table 2), although the “cognitive & motor” subtype demonstrated worse sense of smell and more of the “psychiatric & motor” subtype reported orthostasis. Including these variables in the discriminant analysis did not improve group separation or subtype classification (97.1%, compared to 98.3% with just indicator variables) and none were selected as significant variables in stepwise discriminant analyses.
DBS
Since enrollment, 22 participants underwent DBS surgery. Subtypes did not differ in proportion (“motor only”: 9/63, 14.3%; “psychiatric & motor”: 3/17, 17.6%; “cognitive & motor”: 10/82, 12.2%; χ 2 = 0.40, P = 0.82) or rate of DBS surgery (P > 0.26; Table 3, Fig. S2).
Table 3.
PD subtypes predict longitudinal conversion to clinical outcomes.
| Model | Variables | Coefficients | Wald Z | P‐value | Multivariate relative risk (95% CI) |
|---|---|---|---|---|---|
| DBS surgery 1 | |||||
| Covariates | Age | −0.08 | −2.57 | 0.01 | 0.92 (0.87, 0.98) |
| Symptom duration | −0.02 | −0.29 | 0.77 | 0.98 (0.86, 1.11) | |
| Subtype comparisons | "Motor Only" vs. "Psychiatric & Motor" | 0.77 | 1.11 | 0.27 | 2.17 (0.55, 8.55) |
| "Motor Only" vs. "Cognitive & Motor" | 0.23 | 0.47 | 0.64 | 1.26 (0.49, 3.24) | |
| "Cognitive & Motor" vs. "Psychiatric & Motor" | −0.55 | −0.78 | 0.43 | 0.58 (0.15, 2.27) | |
| Dementia conversion 2 | |||||
| Covariates | Education | 0.07 | 1.02 | 0.31 | 1.07 (0.94–1.23) |
| Age | 0.05 | 2.30 | 0.02 | 1.05 (1.01–1.09) | |
| Sex | −0.60 | −1.68 | 0.09 | 0.55 (0.27–1.11) | |
| Symptom Duration | 0.02 | 0.36 | 0.72 | 1.02 (0.93–1.11) | |
| Subtype comparisons | "Motor Only" vs. "Psychiatric & Motor" | 0.22 | 0.27 | 0.79 | 1.25 (0.25–6.28) |
| "Motor Only" vs. "Cognitive & Motor" | 1.44 | 2.51 | 0.01 | 4.20 (1.37–12.88) | |
| "Psychiatric & Motor" vs. "Cognitive & Motor" | 1.22 | 1.97 | <0.05 | 3.37 (1.01–11.30) | |
| Mortality 3 | |||||
| Covariates | Age | 0.06 | 2.72 | 0.007 | 1.06 (1.02–1.11) |
| Sex | −0.41 | −1.19 | 0.23 | 0.66 (0.34–1.30) | |
| Symptom duration | −0.00008 | −0.002 | 0.99 | 1.00 (0.92–1.09) | |
| Subtype comparisons | "Motor Only" vs. "Psychiatric & Motor" | 0.95 | 1.32 | 0.19 | 2.60 (0.63–10.76) |
| "Motor Only" vs. "Cognitive & Motor" | 1.15 | 2.60 | 0.009 | 3.15 (1.33–7.49) | |
| "Psychiatric & Motor" vs. "Cognitive & Motor" | 0.19 | 0.31 | 0.75 | 1.21 (0.36–4.09) | |
Table presents results of longitudinal multivariate Cox proportional hazards regression models comparing baseline PD subtypes in conversion to clinical milestones of DBS, dementia, and mortality.
DBS surgery adjusted for age and symptom duration.
Dementia conversion rate, stratified by baseline CDR, and adjusted for education, age, sex, and symptom duration at baseline.
Mortality rate adjusted for age, sex and symptom duration at baseline. The first subtype listed refers to the reference group in that analysis (e.g., “Motor Only” (reference group) vs. “Psychiatric & Motor”). Significant differences (P < 0.05) are marked in bold.
Dementia
Thus far, forty‐eight participants developed dementia (CDR ≥ 1). The “cognitive & motor” subtype had the highest conversion rate (41/82, 50%), followed by “psychiatric & motor” (3/17, 17.6%) and “motor only” subtypes (4/63, 6.3%) (χ 2 = 33.9, P < 0.001). Multivariate Cox proportional hazards regression, controlling for baseline education, age, sex, symptom duration, and stratified by baseline CDR score, revealed subtype differences (χ 2 = 28.18, P < 0.001; Fig. 2A, Table 3). The “cognitive & motor” subtype demonstrated a faster dementia conversion rate compared to the “motor only” (relative risk [RR] = 4.20; Table 3) and “psychiatric & motor” subtypes (RR = 3.37). Dementia rates did not differ between “psychiatric & motor” (RR = 1.25) and “motor only” subtypes (Fig. 2A).
Figure 2.
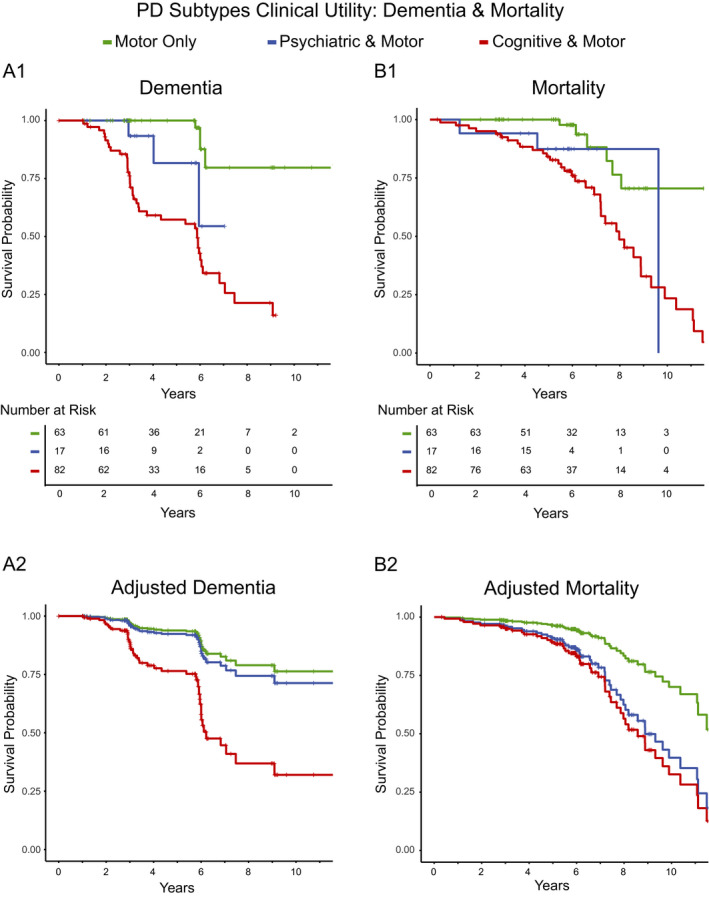
PD subtypes predict conversion to dementia and mortality. Graphs represent Kaplan‐Meier and Cox regression survival plots where “+” indicates censoring and a vertical drop indicates occurrence of an event, either conversion to dementia (panel A) or mortality (panel B). (A1) Top graph represents the Kaplan‐Meier curve for rate of dementia conversion for each subtype without covariates. Table indicates number of participants for each subtype at risk of conversion across time. (A2) Bottom graph represents the Cox regression curves predicting dementia conversion rates for each subtype, accounting for education, sex, age, symptom duration, and CDR score at initial visit, showing a clear difference in conversion rates between the “cognitive & motor” subtypes and both “motor only” and “psychiatric & motor” subtypes. (B1) Kaplan–Meier curves for mortality of each subtype without covariates. Table indicates the number of participants for each subtype at risk of mortality across time. (B2) Bottom graph represents Cox regression curves predicting mortality rates for each subtype, accounting for age, sex, and symptom duration at initial visit, showing similar mortality rates between “cognitive & motor” and “psychiatric & motor” subtypes that are greater than the mortality rate of the “motor only” subtype
Mortality
Forty‐six participants died since study enrollment (Table S3), with a higher proportion in the “cognitive & motor” subtype (N = 36) than the “motor only” (N = 7) and “psychiatric & motor” (N = 3) subtypes (χ 2 = 19.92, P < 0.001). Cause of death (Table S3), “PD‐related” (N = 31) versus “non PD‐related” (N = 15), did not differ between subtypes (χ 2 = 2.30, P = 0.32).
Multivariate Cox proportional hazards regression, controlling for baseline age, sex, and symptom duration, revealed different mortality rates across subtypes (χ 2 = 24.22, P < 0.001; Fig. 2B). Both the “psychiatric & motor” and “cognitive & motor” subtypes showed similarly increased risks of mortality compared to the “motor only” subtype (RR = 2.60 and 3.15; Table 3; Fig. 2B).
Twenty‐four autopsies confirmed PD diagnosis (12 pending neuropathology results; six without brain donation; four found not to have idiopathic PD). Excluding the four non‐PD participants (originally classified as “cognitive & motor”) revealed similar differences in mortality rates across subtypes (Table S4, Fig. S3). The “cognitive & motor” and “psychiatric & motor” subtypes remained at higher mortality risk (RR = 2.73 and 2.38) than the “motor only” subtype.
Discussion
Multi‐domain LCA identified distinct PD clinical subtypes: “motor only,” “psychiatric & motor,” and “cognitive & motor”. The remarkably high classification accuracy (98%) and membership certainty (>90%) demonstrate the robustness of these subtypes. Utilizing a multi‐domain assessment permitted identification of the key features that best distinguish subtypes– depression, executive function, and apathy. Finally, these PD subtypes yield strong prognostic utility for determining conversion to dementia and mortality. Interestingly, both the “cognitive & motor” and “psychiatric & motor” subtypes demonstrated increased mortality risk. These results highlight the need for clinical evaluations that include adequate cognitive and psychiatric assessment to guide prognosis. Ultimately, PD clinical subtypes may provide insight regarding neuropathology and improve patient selection for clinical trials.
Cognitive and psychiatric features distinguish subtypes
Psychiatric and cognitive function distinguish the “psychiatric & motor” and “cognitive & motor” subtypes. In fact, depression, executive function, and apathy best discriminated subtypes. Interestingly, the “psychiatric & motor” and “cognitive & motor” subtypes did not differ in the presence of anxiety or hallucinations, further reinforcing depression and apathy as key psychiatric features. Despite the high rate of psychiatric comorbidities with PD and important treatment implications, few multi‐domain subtyping studies included psychiatric measures. 14 , 15 , 19 Tremor did not discriminate between subtypes and bradykinesia contributed more to subtype distinctions than PIGD, suggesting the classic tremor/PIGD dichotomy is insufficient. In fact, we achieved 98% classification accuracy whereas approximately 20% remained unclassified or indeterminate using the tremor/PIGD classification. These results emphasize the independent and important contributions of psychiatric and cognitive attributes to the clinical presentations of PD.
Our distinct PD subtypes identify a clinically relevant psychiatric subtype with increased mortality risk. Recent subtype classifications emphasize differences in symptom severity and prognosis (e.g., mild/motor, intermediate, diffuse/malignant 14 ), suggesting possible disease stages. Our multi‐domain evaluation specifically highlights the cognitive and psychiatric aspects as the key features that distinguish subtypes. General screening measures, a single “non‐motor” composite score, or reliance on motor scores alone may not provide adequate sensitivity and specificity to classify PD subtypes.
The higher proportion of women in the “motor only” subtype raises the possibility that female PD patients present with different clinical features and progression than males. Previous studies report sex differences across subtypes, typically with more females in the most mildly affected subtype, 12 , 13 , 15 consistent with our current findings. Additional research on sex differences in PD is needed, especially given sex differences in healthy brain aging 51 and tau pathology in preclinical Alzheimer disease. 52
PD subtypes predict clinical milestones
Prospective, longitudinal follow‐up establishes the prognostic utility of these PD subtypes for clinical milestones. The “cognitive & motor” subtype exhibited faster dementia conversion as expected, even after accounting for age, symptom duration, and baseline cognitive status. Cognitive impairment is well established to precede and predict dementia. 53 , 54 Surprisingly, both the “cognitive & motor” and “psychiatric & motor” subtypes had increased mortality rates, regardless of symptom duration. However, there were no subtype differences in DBS rates. Increased dementia and mortality risk for the “cognitive & motor” subtype may be due, in part, to older age at onset and worse cognition. Previous studies also report subtype differences in progression, 14 , 19 , 55 with cognitive and nonmotor symptoms, rather than motor, as the strongest predictors of prognosis. 14 However, prognosis was primarily based on a global composite score 14 and not specific to any clinically relevant milestone. One other prospective study reports mortality rates, 56 with increased mortality associated with more severely affected subtypes suggesting possible disease stage effects. Conversely, our data suggest that presence of cognitive or psychiatric problems increases mortality risk, regardless of age or symptom duration. Previous research indicates that psychosis increases mortality risk, 57 , 58 but our novel results suggest that depression and apathy also increase mortality risk in Parkinson disease.
Distinct subtypes or different disease stages?
Symptom duration and motor severity did not differ between the “cognitive & motor” and “psychiatric & motor” subtypes, suggesting that these subtypes do not merely reflect disease stages. 7 Further, the “cognitive & motor” and “psychiatric & motor” subtypes displayed similarly high mortality risks, but different rates of dementia. Similarity in DBS rates across subtypes also argues against a disease stage model. While it remains possible that the “motor only” subtype represents an early disease stage, the “cognitive & motor” and “psychiatric & motor” do not appear to be sequential PD stages with cumulative symptomatology. Additional longitudinal analyses are required to determine subtype stability and progression. Information regarding neurobiological changes associated with each subtype also may offer insight into subtype differences and progression. However, in the final stages of disease (e.g., near death), PD subtypes may converge in clinical manifestations and neuropathology. 5 In vivo neurobiological measures will be crucial for characterizing the temporal progression of disease pathology in relation to clinical progression.
Strengths & limitations
Using multi‐domain LCA, which avoids the limitations of traditional clustering‐based approaches, we identified distinct PD subtypes and the key distinguishing features, emphasizing the role of cognitive and psychiatric manifestations. The comprehensive assessment conducted OFF medication and broad spectrum of nondemented PD prevents potential medication confounds and classification based on dementia, and permits examination of the predictive utility of baseline subtypes. Subtype classification with drug‐naive PD patients also would eliminate medication confounds. 2 , 11 , 18 However, this would limit analyses to newly diagnosed PD with fewer clinical features. Interestingly, the “psychiatric & motor” subtype reported the highest LEDD, suggesting potential medication effects on clinical presentation or subtype differences in response to medication. 12 , 19 Finally, the prospective, longitudinal follow‐up provides critical information regarding the prognostic utility of PD subtypes for important clinical milestones, here demonstrating subtype differences in dementia and mortality.
The lack of a specific anxiety questionnaire and limited psychiatric assessments represents a potential limitation; however, PD subtypes did not differ on presence of anxiety, suggesting that inclusion of anxiety would not substantially change the PD subtypes. This study, however, did include apathy, which is rarely assessed in other classifications. 12 , 14 Although we did not include other nonmotor symptoms (e.g., fatigue, pain, orthostasis) in our classification, which also affect quality of life 59 , 60 and could contribute to PD subtypes, 14 subtypes did not significantly differ on sense of smell, daytime sleepiness, constipation, or orthostasis nor did these features distinguish subtypes. The relatively low number of DBS and autopsy cases should be interpreted as preliminary evidence.
Future directions
Identification and validation of PD subtypes could aid in prognosis, improve patient selection for clinical trials, and perhaps even suggest new treatment approaches. The current findings provide important information for this endeavor and reinforce the potential clinical utility of PD subtypes. Of course, several additional steps would be required before clinical implementation. First, replication with an independent cohort is necessary to validate these PD clinical subtypes and to determine the sensitivity and specificity to classify at the individual level. Second, the longitudinal progression and stability of PD subtypes remains to be determined. Additional areas of future research should also include examination of potential subtype differences in neuropathology and treatment response.
Conclusion
Psychiatric and cognitive features drive PD subtypes, rather than motor deficits alone. These results demonstrate the value of multi‐domain classification including cognitive and psychiatric measures to better characterize clinical variability in PD. Further, the differences in dementia and mortality rates across subtypes demonstrate the prognostic utility of these PD subtypes.
Conflict of Interest
Dr. Campbell receives salary and research support from the NIH, American Parkinson Disease Association (APDA) Advanced Research Center for PD at WUSTL; Greater St. Louis Chapter of the APDA; McDonnell Center for Systems Neuroscience; Department of Radiology at WUSTL. She also received honoraria from the Department of Veterans Affairs and the Parkinson Foundation. Dr. Myers receives salary and research support from NIH. Ms. Weigand receives salary and research support from NSF. Dr. Foster receives salary and research support from the NIH and American Parkinson Disease Association (APDA) Advanced Research Center for PD at WUSTL; Greater St. Louis Chapter of the APDA. Dr. Cairns receives salary and research support from NIH and the University of Exeter. Dr. Jackson receives salary and research support from NIH and Washington University in St. Louis. Dr. Lessov‐Schlaggar receives salary and research support from NIH. Dr. Perlmutter receives salary and research support from NIH, Washington University in St. Louis, American Parkinson Disease Association (APDA), Greater St. Louis Chapter of the APDA, McDonnell Center for Higher Brain Function, Barnes‐Jewish Hospital Foundation, Huntington’s Disease Society of America, CHDI, MJ Fox Foundation, Fixel Foundation, Oertli Foundation, Riney Foundation and Washington University CTSA/ICTS. He also received honoraria from the American Academy of Neurology, University of Rochester, Parkinson Disease Foundation, St Louis Univ., Harvard, Univ. Michigan, Stanford, CHDI, Huntington Study Group.
Author Contributions
Research project: A. Conception, B. Organization, and C. Execution
Statistical analysis: A. Design, B. Execution, C. Review, and Critique
Manuscript: A. Writing of the first draft, B. Review, and Critique
Campbell: 1. A,B,C; 2. A,B,C; 3. A,B. Myers: 2.A,B; 3B. Weigand: 1C,2B,3B. Foster: 1C; 2C; 3B. Cairns: 1C; 3B. Jackson: 2.A,B,C; 3. B. Lessov‐Schlaggar: 2A,B,C; 3B. Perlmutter: 1A; 3B.
Supporting information
Table S1. Provides the model fit statistics for the latent class analysis, showing how the model fits the data and how the model compares to the previous model.
Table S2. Provides means and standard deviations for motor, psychiatric, and cognitive measures for each subtype.
Table S3. Shows the breakdown of PD‐related and non‐PD‐related deaths for each subtype.
Table S4. Provides the results of the multivariate Cox proportional hazards regression model after the exclusion of participants whose autopsies indicated that they did not have PD.
Figure S1. Depicts a flowchart diagram for the enrollment sample, exclusions, and longitudinal follow‐up.
Figure S2. Depicts the Kaplan–Meier and Cox regression survival plots for the DBS surgery survival analysis.
Figure S3. Depicts the Kaplan–Meier and Cox regression survival plots for the mortality analysis after excluding participants whose autopsies indicated that they did not have PD.
Acknowledgments
We thank the study participants for their time and effort to aid our understanding of Parkinson disease. We also thank the study coordinators and research nurse coordinators, including Phil Lintzenich, Thomas Belcher, Jenny Zhen‐Duan, Anja Pogarcic, My Vu, Barb Merz, Jenny Petros, Katharine Cummings, Selma Avdagic, Kelly McVey, Jacob Wolf, Chris Waller, and especially Johanna Hartlein, for assistance with data collection.
Funding Information
Support for this work was provided by grants from NINDS (NS097437, NS075321, NS41509, NS058714, NS48924, P30 NS048056) and NIH NCRR (UL1RR024992); American Parkinson Disease Association (APDA) Advanced Research Center for PD at WUSTL; Greater St. Louis Chapter of the APDA; Oertli Fund; Barnes‐Jewish Hospital Foundation (BJHF) (Elliot Stein Family Fund & PD Research Fund), and Paula C and Rodger O Riney Foundation.
Funding Statement
This work was funded by National Institute of Neurological Disorders and Stroke grants NS058714, NS075321, NS097437, NS41509, NS48924, and P30 NS048056; NIH NCRR grant UL1RR024992; American Parkinson Disease Association Advanced Research Center grant ; Oertli Fund grant ; Barnes‐Jewish Hospital Foundation: Elliot Stein Family Fund and PD Research Fund grant ; Paula C and Rodger O Riney Foundation grant .
References
- 1. Sauerbier A, Jenner P, Todorova A, Chaudhuri KR. Non motor subtypes and Parkinson's disease. Parkinson Relat Disord 2016;22(Suppl 1):S41–S46. [DOI] [PubMed] [Google Scholar]
- 2. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson's disease: biomarkers and longitudinal progression. Brain 2017;140:1959–1976. [DOI] [PubMed] [Google Scholar]
- 3. Pagano G, Ferrara N, Brooks DJ, Pavese N. Age at onset and Parkinson disease phenotype. Neurology 2016;86:1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marras C, Chaudhuri KR. Nonmotor features of Parkinson's disease subtypes. Mov Disord 2016;31:1095–1102. [DOI] [PubMed] [Google Scholar]
- 5. De Pablo‐Fernandez E, Lees AJ, Holton JL, Warner TT. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 2019;76:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base‐line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990;40:1529–1534. [DOI] [PubMed] [Google Scholar]
- 7. Nutt JG. Motor subtype in Parkinson's disease: different disorders or different stages of disease? Mov Disord 2016;31:957–961. [DOI] [PubMed] [Google Scholar]
- 8. Brennan L, Devlin KM, Xie SX, et al. Neuropsychological subgroups in non‐demented Parkinson's disease: a latent class analysis. J Parkinson's Dis 2017;7:385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landau S, Harris V, Burn DJ, et al. Anxiety and anxious‐depression in Parkinson's disease over a 4‐year period: a latent transition analysis. Psychol Med 2016;46:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldman JG, Weis H, Stebbins G, et al. Clinical differences among mild cognitive impairment subtypes in Parkinson's disease. Mov Disord 2012;27:1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erro R, Vitale C, Amboni M, et al. The heterogeneity of early Parkinson's disease: a cluster analysis on newly diagnosed untreated patients. PLoS One 2013;8:e70244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawton M, Baig F, Rolinski M, et al. Parkinson's disease subtypes in the Oxford Parkinson Disease Centre (OPDC) discovery cohort. J Parkinson's Dis 2015;5:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Rooden SM, Colas F, Martinez‐Martin P, et al. Clinical subtypes of Parkinson's disease. Mov Disord 2011;26:51–58. [DOI] [PubMed] [Google Scholar]
- 14. Fereshtehnejad SM, Romenets SR, Anang JB, et al. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 2015;72:863–873. [DOI] [PubMed] [Google Scholar]
- 15. van Balkom TD, Vriend C, Berendse HW, et al. Profiling cognitive and neuropsychiatric heterogeneity in Parkinson's disease. Parkinsonism Relat Disord 2016;28:130–136. [DOI] [PubMed] [Google Scholar]
- 16. Graham JM, Sagar HJ. A data‐driven approach to the study of heterogeneity in idiopathic Parkinson's disease: identification of three distinct subtypes. Mov Disord 1999;14:10–20. [DOI] [PubMed] [Google Scholar]
- 17. Mu J, Chaudhuri KR, Bielza C, et al. Parkinson's disease subtypes identified from cluster analysis of motor and non‐motor symptoms. Front Aging Neurosci 2017;9:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erro R, Picillo M, Vitale C, et al. Clinical clusters and dopaminergic dysfunction in de‐novo Parkinson disease. Parkinsonism Relat Disord 2016;28:137–140. [DOI] [PubMed] [Google Scholar]
- 19. Lawton M, Ben‐Shlomo Y, May MT, et al. Developing and validating Parkinson's disease subtypes and their motor and cognitive progression. J Neurol Neurosurg Psychiatry 2018;89:1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fereshtehnejad SM, Postuma RB. Subtypes of Parkinson's disease: what do they tell us about disease progression? Curr Neurol Neurosci Rep 2017;17:34. [DOI] [PubMed] [Google Scholar]
- 21. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 23. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 25. Doty RL. The brief smell identification test administration manual. Haddon Heights, New Jersey: Sensonics Inc, 2001. [Google Scholar]
- 26. Walker MP, Ayre GA, Cummings JL, et al. The clinician assessment of fluctuation and the one day fluctuation assessment scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 2000;177:252–256. [DOI] [PubMed] [Google Scholar]
- 27. Johns MH. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 28. Campbell MC, Koller JM, Snyder AZ, et al. CSF proteins and resting‐state functional connectivity in Parkinson disease. Neurology 2015;84:2413–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foster ER, Campbell MC, Burack MA, et al. Amyloid imaging of Lewy body‐associated disorders. Mov Disord 2010;15:2516–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wechsler WD. Wechsler adult intelligence scale – III. San Antonio: The Psychological Corporation, 1997. [Google Scholar]
- 31. Delis D, Kaplan E, Kramer J, Ober B. California verbal learning test‐II. San Antonio, TX: The Psychological Corporation, 2000. [Google Scholar]
- 32. Wechsler D. Wechsler memory scale III. San Antonio, TX: Psychological Corporation, 1997. [Google Scholar]
- 33. Kaplan E, Goodglass H, Weintraub S. Boston naming test. 2nd ed. Austin, TX: Pro‐ED, 2001. [Google Scholar]
- 34. Lezak MD. Neuropsychological assessment, 2004;4th:xiv, 1016.
- 35. Woodcock RW, McGrew KS, Mather N. Woodcock‐Johnson tests of achievement. Itasca, IL: Riverside Publishing Company, 2001. [Google Scholar]
- 36. Lezak MD. Neuropsychological assessment, 3rd ed. Oxford: Oxford University Press, 1995. [Google Scholar]
- 37. Delis DC, Kaplan E, Kramer JH. Delis Kaplan executive function system. San Antonio: The Psychological Corporation, 2001. [Google Scholar]
- 38. Cools R. Dopaminergic modulation of cognitive function‐implications for L‐DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 2006;30:1–23. [DOI] [PubMed] [Google Scholar]
- 39. Buddhala C, Campbell MC, Perlmutter JS, Kotzbauer PT. Correlation between decreased CSF alpha‐synuclein and Abeta(1)(‐)(4)(2) in Parkinson disease. Neurobiol Aging 2015;36:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982;17:37–49. [DOI] [PubMed] [Google Scholar]
- 42. Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the Frontal Lobe Personality Scale. Assessment 1999;6:269–284. [DOI] [PubMed] [Google Scholar]
- 43. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 44. Sclove SL. Application of model‐selection criteria to some problems in multivariate‐analysis. Psychometrika 1987;52:333–343. [Google Scholar]
- 45. Yang CC. Evaluating latent class analysis models in qualitative phenotype identification. Comput Stat Data Anal 2006;50:1090–1104. [Google Scholar]
- 46. Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika 2001;88:767–778. [Google Scholar]
- 47. Nagin DS, Odgers CL. Group‐based trajectory modeling in clinical research. Annu Rev Clin Psychol 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 48. Muthen B. Statistical and substantive checking in growth mixture modeling: comment on Bauer and Curran (2003). Psychol Methods 2003;8:369–377. [DOI] [PubMed] [Google Scholar]
- 49. McCutcheon AC. Latent class analysis. Beverly Hills, CA: Sage, 1987. [Google Scholar]
- 50. Collins LM, Lanza ST. Latent class and latent transition analysis with application in the social, behavioral, and health sciences. Hoboken, New Jersey: John Wiley & Sons Inc, 2010. [Google Scholar]
- 51. Goyal MS, Blazey TM, Su Y, et al. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci USA 2019;116:3251–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buckley RF, Mormino EC, Rabin JS, et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol 2019;76:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aarsland D, Creese B, Politis M, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol 2017;13:217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 2013;70:580–586. [DOI] [PubMed] [Google Scholar]
- 55. Erro R, Picillo M, Vitale C, et al. Non‐motor symptoms in early Parkinson's disease: a 2‐year follow‐up study on previously untreated patients. J Neurol Neurosurg Psychiatry 2013;84:14–17. [DOI] [PubMed] [Google Scholar]
- 56. de Lau LM, Verbaan D, van Rooden SM, et al. Relation of clinical subtypes in Parkinson's disease with survival. Mov Disord 2014;29:150–151. [DOI] [PubMed] [Google Scholar]
- 57. de Lau LM, Verbaan D, Marinus J., van Hilten Jacobus J. Survival in Parkinson's disease. Relation with motor and non‐motor features. Parkinsonism Relat Disord 2014;20:613–616. [DOI] [PubMed] [Google Scholar]
- 58. Forsaa EB, Larsen JP, Wentzel‐Larsen T, Alves G. What predicts mortality in Parkinson disease?: a prospective population‐based long‐term study. Neurology 2010;75:1270–1276. [DOI] [PubMed] [Google Scholar]
- 59. Politis M, Wu K, Molloy S, et al. Parkinson's disease symptoms: the patient's perspective. Mov Disord 2010;25:1646–1651. [DOI] [PubMed] [Google Scholar]
- 60. Martinez‐Martin P, Rodriguez‐Blazquez C, Kurtis MM, Chaudhuri KR. The impact of non‐motor symptoms on health‐related quality of life of patients with Parkinson's disease. Mov Disord 2011;26:399–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Provides the model fit statistics for the latent class analysis, showing how the model fits the data and how the model compares to the previous model.
Table S2. Provides means and standard deviations for motor, psychiatric, and cognitive measures for each subtype.
Table S3. Shows the breakdown of PD‐related and non‐PD‐related deaths for each subtype.
Table S4. Provides the results of the multivariate Cox proportional hazards regression model after the exclusion of participants whose autopsies indicated that they did not have PD.
Figure S1. Depicts a flowchart diagram for the enrollment sample, exclusions, and longitudinal follow‐up.
Figure S2. Depicts the Kaplan–Meier and Cox regression survival plots for the DBS surgery survival analysis.
Figure S3. Depicts the Kaplan–Meier and Cox regression survival plots for the mortality analysis after excluding participants whose autopsies indicated that they did not have PD.


