Abstract
This article elucidates the utilization of a novel platelet concentrate-concentrated growth factor (CGF) for rapid and successful healing outcome in regenerative endodontics. This case report focusses on two cases: 23-year-old and 21-year-old patients with incomplete root formation and periapical lesion. Case 1 and case 2 are classified as stage IV and stage II, respectively, in accordance with Cvek's classification of open apex and had varied outcomes. The extent of open apex, root dentin thickness, and lesion were assessed using periapical radiograph and CBCT. Revascularization procedure was carried out after obtaining patient consent. Following bleeding induction, CGF was prepared, placed, and condensed using pluggers in the root canal space, followed by the placement of mineral trioxide aggregate (MTA) up to the level of CEJ. At 1-year follow-up, apical closure with increased root dentin thickness and reduced periapical radiolucency was evident.
1. Introduction
The developing dentition is perpetually subjected to various mechanical or biological insults pertaining to trauma, caries, and diverse developmental anomalies [1]. There has been tremendous research aimed at the management of nonvital immature anterior permanent teeth to reestablish the pulp-dentin complex. Since the nonvital tooth is devoid of blood supply, the root fails to develop and is subjected to fracture in the long run. Cvek in 1992 classified five stages of root development according to the width of the apical foramen and length of the root where he described teeth with wide divergent apical opening estimated to be less than half, half, two-thirds, nearly completed, and finally teeth with closed apical foramen and completed root development [2].
Revascularization or revitalization is widely described as the restoration of vascularity to a tissue or organ with the sole purpose of reestablishing the pulp-dentin complex, thereby contributing to repair and continued development of the necrotic immature permanent anterior teeth with respect to periapical healing, increase in root dimensions, and closure of the apical foramen [3].The current concept in regenerative endodontics primarily stems from the pioneering work done by Dr. B.W. Hermann wherein the meticulous application of calcium hydroxide for vital pulp therapy was described [4]. The judicious use of calcium hydroxide had gained acceptance worldwide and was also enlisted as the material of choice in apexification procedures before the advent of MTA [5]. However, the conventional endodontic approaches described above failed to offer any benefits as far as continued root development and thickening of root dentinal walls were concerned. As a result, a high incidence of root fracture could be elicited in such cases as reported by Andreasen et al. [6].
Reynolds et al. evaluated a revascularization protocol for rejuvenating the pulp-dentin complex in necrotic immature permanent teeth and reported a case of connective tissue formation in root canals following routine pulpectomy procedure [7]. Rule and Winter further strengthened the concept when pulpal bleeding was induced to revitalize a nonvital immature mandibular premolar [8]. This frank attempt tasted remarkable success when continued root formation and apical closure were detected. The concept of this “induced bleeding” technique has fast gained popularity over the years and catapulted regenerative endodontics into the limelight as an alternative to conventional root canal therapy when all else failed.
In recent years, various attempts have been made to regenerate the lost pulp which involved incorporation of popular platelet concentrates such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). While the former suffers from few shortcomings pertaining to cost, handling, tedious centrifugation, and purification procedures, the latter scores over it in terms of simplicity and cost; the use of bovine thrombin and anticoagulants are also avoided [9]. Apart from the conventional platelet concentrates, CGF has been suggested as an ideal biomaterial as it releases growth factors that induces cell migration and proliferation and chemotactic activity on inflammatory cells and stimulates angiogenesis and tissue remodelling [10]. However, a comprehensive search of the literature revealed the absence of any publication regarding the use of CGF in pulp revitalization, thereby making it a novel platelet concentrate for application in regenerative endodontics. For describing the case reports, we have followed the checklist given in CARE guidelines (http://www.care-statement.org/) [11].
2. Case Presentation
2.1. Case Report 1
A 23-year-old male patient reported to the Department of Conservative Dentistry and Endodontics with chief complaint of broken teeth and pain in the upper front tooth region. There was no contributing medical history. Past dental history revealed traumatic injury 14 years back associated with sharp pain on biting. Intraoral clinical examination revealed fracture of 11 and 12 (Figure 1(a)). Both the teeth were sensitive to percussion and palpation. Cold test was carried out using refrigerant spray (Coltene/Whaledent, Switzerland), and electric pulp testing (Gentle Pulse™ Pulp Vitality Tester, Parkell, USA) showed no response when compared with the control teeth. Periapical radiograph revealed loss of crown structure in 11, immature roots in 12, and radiolucency in the periapical area of 11 and 12 (Figure 1(b)). CBCT (Dentsply Sirona, Orthophos XG 3D) was taken at standardized settings (90 kV, 6 mA, 5∗5.5 cm, 160 μm, 14 s). The lesion extent measured 4.84∗5.59∗9.35 mm (Figure 1(g)). Apical diameter measured 1.96 mm (Figure 1(k)). The root dentin thickness at coronal third was 1.62∗1.80 mm, middle third was 1.59∗1.46 mm, and apical third was 1.23∗1.03 mm (Figure 1(i)).
Figure 1.
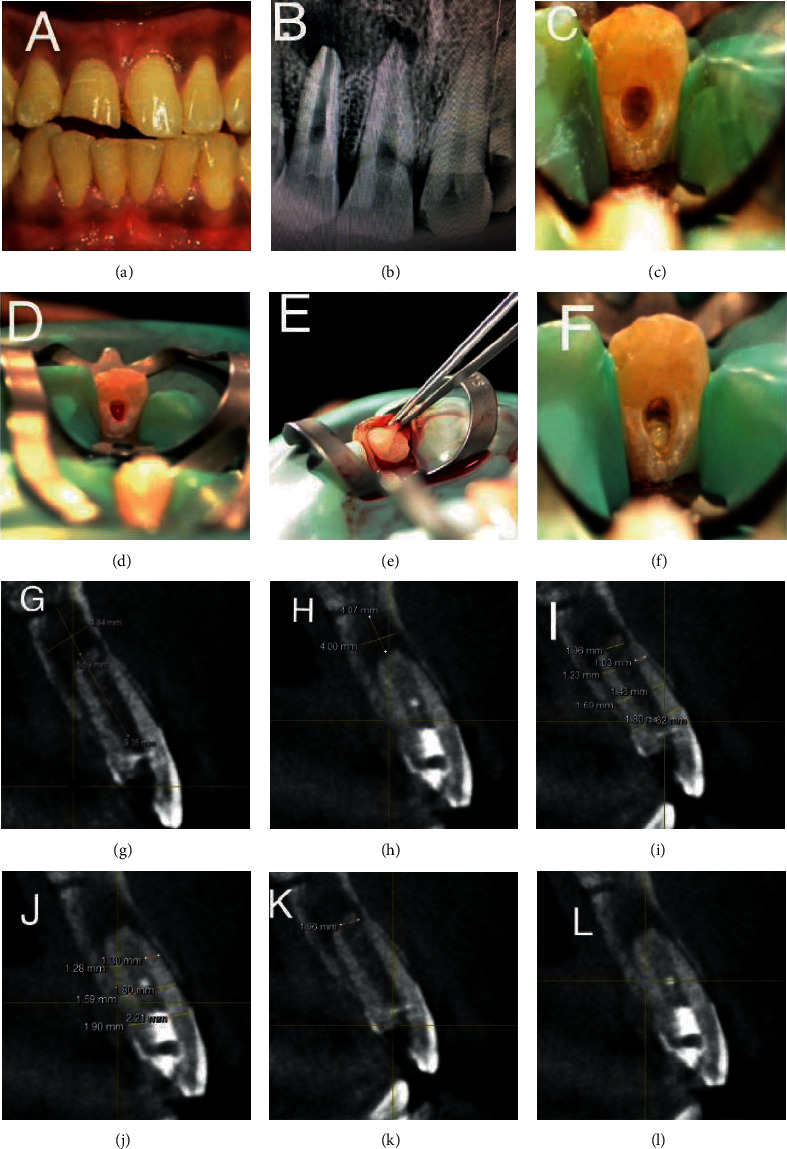
Preoperative clinical, revascularization procedure and radiographic images—IOPA and CBCT images (case report 1). (a) Pre-op clinical photograph showing fractured of 11 and 12. (b) Preoperative IOPA showing the periapical lesion involving the apices of 11 and 12 and immature roots of 12. (c) Access opening done in 12. (d) Bleeding induced in 12 for revascularization. (e) Placement of CGF in the root canal space. (f) MTA plug of 3 mm placed at the level of CEJ in 12. (g) Pre-op CBCT image showing the extent of lesion in sagittal slice. (h) Post-op CBCT image showing the extent of lesion in sagittal slice. (i) Pre-op CBCT showing the root dentin thickness measurement in sagittal slice. (j) Post-op CBCT showing the root dentin thickness measurement in sagittal slice. (k) Pre-op CBCT showing the apical diameter measurement in 12. (l) Post-op CBCT showing the apical diameter in 12.
Based on these findings, the diagnosis made was pulpal necrosis with symptomatic apical periodontitis in 11 and 12. Revascularization protocol was applied for 12, and endodontic therapy was carried out in 11 with placement of intracanal medicament for three weeks (Figures 1(c)–1(f)). No adverse events were reported during the follow-up periods. Intraoral periapical radiograph and CBCT at 12-month follow-up revealed marked closure of the apex in 12 and decreased extent of periapical lesion in 11 and 12. The lesion extent measured 4.00∗4.07∗8.96 mm (Figure 1(h)). The root dentin thickness at coronal third was 1.90∗2.21 mm, middle third was 1.59∗1.80 mm, and apical third was 1.28∗1.30 mm (Figures 1(j)–1(l)).
Livewire segmentation using OSIRIX version 9.5 (PIXMEO, Geneva, Switzerland) was done to delineate the lesion from healthy bone. Preoperative and postoperative volume calculations were 0.1012cm3 and 0.0824 cm3, respectively (Figures 2(a) and 2(b)). The lesion reduction size was found to be 18.57%.
Figure 2.
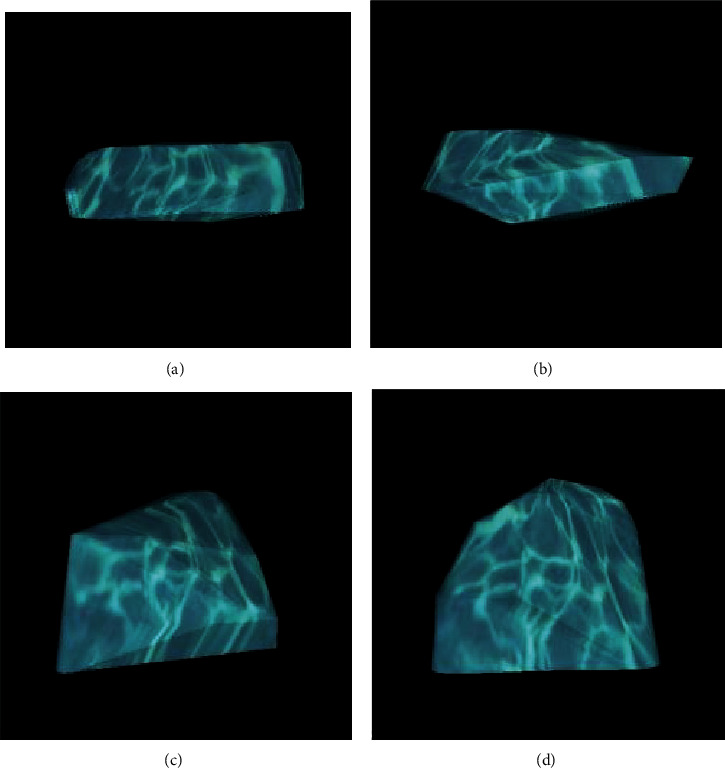
Preoperative and postoperative volume calculations to delineate the lesion from healthy bone. Case report 1: (a, b) preoperative and postoperative volume calculations, respectively. Case report 2: (c, d) preoperative and postoperative volume calculations, respectively.
2.2. Case Report 2
A 21-year-old male patient reported to the Department of Conservative Dentistry and Endodontics with chief complaint of discoloured and broken teeth in the upper front tooth region. Patient revealed a history of trauma 9 years back. Clinical examination revealed discoloured and fractured 21 with sinus opening and pus discharge (Figure 3(a)). On radiographic examination, incomplete root formation was evident in 21 with radiolucency in the periapical region (Figure 3(b)). The lesion extent in CBCT was measured to be 5.41∗4.29∗9.92 mm (Figure 3(g)). The root dentin thickness at coronal third was 2.70∗2.20 mm, middle third was 1.77∗1.83 mm, and apical third was 1.19∗1.26 mm (Figure 3(i)). Apical diameter measured 2.66 mm (Figure 3(k)). Based on these findings, a diagnosis of chronic apical abscess in 21 was made. Revascularization protocol was followed in 21 (Figures 3(c)–3(f)). No adverse events were reported by the patient during the follow-up periods. Intraoral periapical radiograph and CBCT at 12-month follow-up revealed increased root dentin thickness in 21. The lesion extent in CBCT was measured to be 4.00∗3.89∗8.98 mm (Figure 3(h)). The root dentin thickness at coronal third was 2.90∗2.23 mm, middle third was 2.26∗1.84 mm, and apical third was 1.84∗1.47 mm (Figure 3(j)). Apical diameter measured 1.96 mm (Figure 3(l)).
Figure 3.
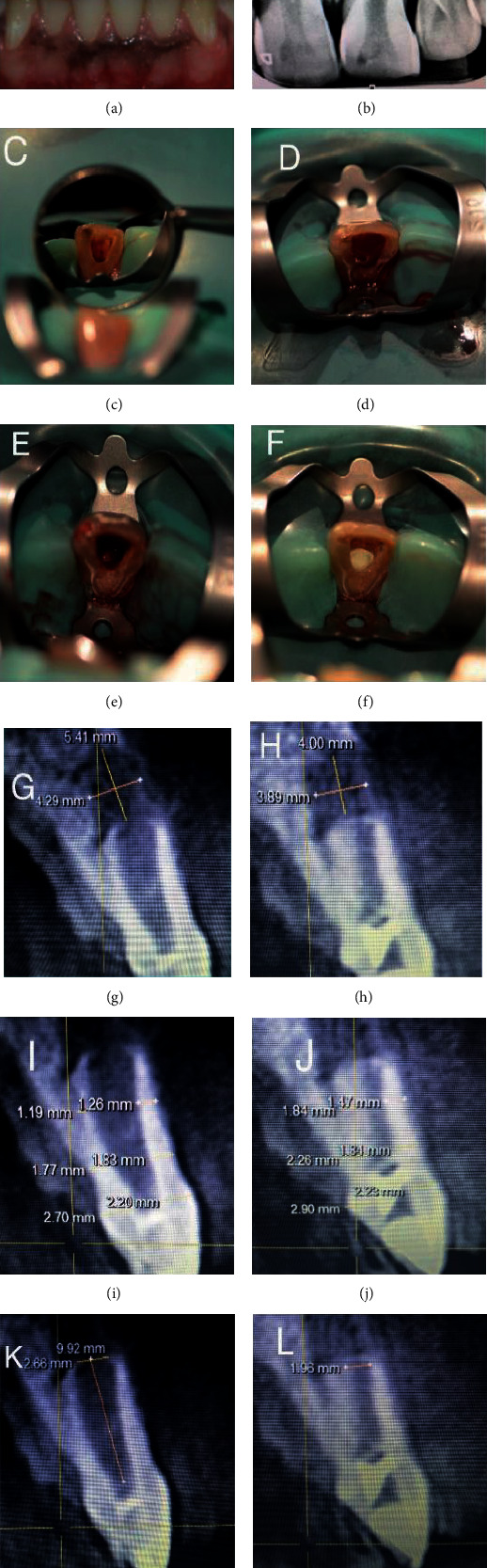
Preoperative clinical, revascularization procedure and radiographic images—IOPA and CBCT images (case report 2). (a) Pre-op clinical photograph showing discoloured and fractured 21 with sinus opening and pus discharge. (b) Preoperative IOPA showing periapical lesion involving the apices of 21 and open apex. (c) Access opening done in 21. (d) Bleeding induced in 21 for revascularization. (e) Placement of CGF in root canal space. (f) MTA plug of 3 mm placed at the level of CEJ in 21. (g) Pre-op CBCT image showing the extent of lesion in sagittal slice. (h) Post-op CBCT image showing the extent of lesion in sagittal slice. (i) Pre-op CBCT showing the root dentin thickness measurement in sagittal slice. (j) Post-op CBCT showing the root dentin thickness measurement in sagittal slice. (k) Pre-op CBCT showing the apical diameter measurement in 21. (l) Post-op CBCT showing the apical diameter in 21.
Preoperative and postoperative volume calculations were 0.0675cm3 and 0.0541 cm3, respectively (Figures 2(c) and 2(d)). The lesion reduction size was found to be 19.85%.
2.3. Common Treatment Protocol
Prior to the revascularization procedure, informed consent was obtained from the patient. On the first visit, teeth were isolated with a rubber dam, followed by access cavity preparation with endo access bur (Dentsply Maillefer, Switzerland) and working length determination using a #80 K-file (Mani, Inc., Japan) radiographically. The canals were irrigated using a 30-gauge side-vented needle with 20 ml of 1.5% sodium hypochlorite (VIP, Vensons, India) followed by saline rinse prior to the placement of calcium hydroxide (RC Cal, Prime dental products, India) as intracanal medicament for three weeks. For temporary sealing of the access cavity, zinc oxide eugenol was used. On the second visit, the patients were asymptomatic. 2% lignocaine (Lignox, India) without vasoconstrictor was administered. The medicament was flushed out of the root canal with copious amount of 0.9% saline (AcuLIFE, India). The root canal was then irrigated with 10 ml of 17% EDTA (Canalarge, Ammdent, India). Following this, the canals were dried with paper points, bleeding was induced using #80 K-file to a length of 2 mm beyond the working length, and the bleeding was made to fill the root canal. A cotton pellet was placed in the pulp chamber to allow clot formation. The protocol thus followed was in accordance with the guidelines proposed by the American Association of Endodontists (AAE).
2.4. Preparation of Concentrated Growth Factor
Two disposable 10 ml nonanticoagulant tubes and a matching centrifuge device (MEDIFUGE, Silfradent SRL, S. Sofia, Italy) were used. 10 ml of intravenous blood sample from the patient was obtained and placed in centrifuge tubes without anticoagulants; accelerated for 30 seconds; centrifuged at 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 4 min, and 3000 rpm for 3 min; and decelerated for 36 secs to stop. At the end of the automated preprogrammed cycle, the centrifuge tube encompassed a concoction of four different layers; the uppermost or the 1st layer contained serum, the 2nd layer was composed of a fibrin buffy coat, the 3rd layer constituted the much needed growth factors, and lastly, the lowermost or the 4th layer was occupied by the red blood cells. The fibrin gel containing the concentrated growth factor was separated from the red blood cells. The CGF obtained was packed into the canals to the level of cementoenamel junction. 3 mm MTA (MTA Angelus Brazil) plug was placed at the level of CEJ, and the coronal cavity was sealed using composite.
3. Discussion
The present case report most notably focused upon achieving revitalization at a flourishing rate, made possible by incorporating the novel autologous platelet concentrate (CGF), well within the guidelines proposed by the AAE. CGF was introduced by Sacco in 2006. As “concentrated” as it seems to be, CGF does stand out from its predecessors by the way it is concocted. This is ascribed to its unique centrifugation technique which depends upon varied time intervals and centrifugation speeds, finally churning out a much concentrated, thicker, and elongated fibrin matrix [12]. In a comparative study, it was claimed that CGF indeed had an upper hand over PRP and PRF in terms of cell proliferation and osteoblastic differentiation as well as a richer growth factor content [13].
In recent years, CGF has carved a niche for itself in terms of sinus ridge augmentation procedures, implant surgeries, and surgical endodontics as reported by Sohn et al., Qiao et al., Ying et al., and Sureshbabu et al., eliciting predictable and positive clinical impact in ridge augmentation and accelerated healing of intrabony defects, lateral cysts, and periapical pathologies, respectively [14–18]. In our recently published case report, we focused on the conspicuous coalescence of CGF and bone graft (sticky bone) to intercept a large periapical bony lesion, for which we garnered resounding success in stemming the repair and regenerative process [14]. Additionally, in our previously published clinical case presentation of two cases, we had implemented CGF as a scaffold in the surgical endodontic treatment procedures with great effect and were able to elicit successful healing outcomes [18]. Furthermore, Hong et al. reviewed the potential application of CGF and concluded that the autologous platelet concentrate could be a promising biomaterial in regenerative endodontics [19].
In case 1, routine IOPAR revealed a slight anatomical variation at the apex of 12 with periapical lesion which was followed by two parallax radiographs to confirm the same. Since periapical radiographs being a two-dimensional imaging modality, we could not ascertain as to whether the said tooth had a closed apical foramen and also could not assess the extent of the lesion in 11 and 12. In case 2, a single periapical radiograph confirmed the presence of blunderbuss canal with periapical lesion in 21. In both cases, the extent of lesion and apical diameter along with root dentin thickness had to be verified and measured with the aid of CBCT. According to AAE guidelines, CBCT scan with a small field of view is recommended in clinical research trials for diagnostic accuracy [20].
The use of anaesthetic solution without a vasoconstrictor was advocated in both our cases which was well in accordance with a study conducted by Petrino et al. [21]. It was reported that several attempts were made to induce bleeding into the canal space when anaesthetic solution containing a vasoconstrictor was employed. Jung et al. suggested that the induction of bleeding into the root canal provides stem cells that can induce dentin formation [22]. Similarly, in both our cases, bleeding was induced using a #80 K-file instrumented 2 mm beyond the working length. According to Wang et al., the outcome of revascularization procedure improved with the inclusion of blood clot in the root canal space which resulted in a process of repair rather than revitalization [23]. To that end, the autologous platelet concentrate has been effectively deployed in these cases as scaffold materials to compensate for the lack of formation of high-quality blood clots.
Rodella et al. demonstrated that growth factors TGF-ß1 and VEGF were found in abundance which according to the literature are highly essential for stimulating cell proliferation, matrix rehabilitation, and angiogenesis [24]. Based on an immunohistochemical analysis, the aforesaid study also confirmed the existence of circulating CD34+ cells in CGF and RBC layers which again is crucial for the revascularization process [25].
Disinfection is of prime importance in enhancing the predictability of regenerative endodontic procedures. According to Ruparel et al., Galler, and Segura-Egea et al., the use of antibiotics in regenerative endodontics should be avoided [26–28]. Althumairy et al. stated that disinfection with calcium hydroxide leads to a greater survival of SCAP cells as opposed to antibiotic pastes [29]. Irrigants are selected based on both their bacteriostatic or bactericidal properties and the ability to sustain the survival of stem cells. The chelating effect of 17% EDTA promoted the release of dentin-derived growth factors which in turn regulates the survival and differentiation of stem cells, whereas 2% chlorhexidine proved to be cytotoxic with no viable cells [30].
The biological mechanism of regeneration depends on the “self-renewal” [31] capacity of the stem cells of apical papilla (SCAP), capable of differentiating into odontoblast-like cells ultimately resulting in the formation of dentin-like tissue [32]. While treating nonvital immature permanent teeth, the preservation of SCAP promotes continuous formation of the root to its completion [33] Induced bleeding into the root canal space triggers higher concentration of mesenchymal stem cells (MSCs) from the apical papilla [34] that may not be sufficient for the formation of pulp-dentin complex; hence, autologous platelet concentrate as scaffold serves as a reservoir of growth factors, thus aiding in the regenerative process [35].
Histologic confirmation of dental pulp with an intact odontoblastic layer and restoration of a functional pulp is a pinnacle in regenerative treatment goals; therefore, further objective should be targeted at histological evaluation of viable pulp tissue [36]. The limitation of these case presentations is that it is not proved that true pulp-dentin complex regeneration has occurred for which histological examination is required. The effect of CGF in regenerative endodontics could be well established by randomized clinical trials and longer follow-up periods.
4. Conclusion
Although evidence concerning the implementation of CGF in revascularization procedures is scarce, however, based on available and limited data, it does deliver phenomenal success in diverse oral surgical applications (described earlier). In this case presentation, CGF was utilized with great effect as a fibrin-rich block, progressively releasing the much noteworthy growth factors to bring about a rapid and successful healing outcome. At one-year follow-up, CBCT assessment confirmed marked apical closure in case 1, increased root dentin thickness in case 2, and complete reduction in size of the periapical lesion in both cases. All things considered, CGF holds substantial promise for use in revascularization techniques to enhance the repair and regenerative process in a manner most beneficial in terms of all-round satisfactory healing of the immature permanent tooth.
Acknowledgments
The authors would like to acknowledge Dr. Sri Prakash for the skillful support rendered for CGF preparation.
Conflicts of Interest
The authors deny any conflicts of interest.
References
- 1.Cvek M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Dental Traumatology. 1992;8(2):45–55. doi: 10.1111/j.1600-9657.1992.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathew B. P., Hegde M. N. Management of non vital immature teeth–case reports and review. Endodontology. 2010;18:18–22. [Google Scholar]
- 3.Murray P. E., Garcia-Godoy F., Hargreaves K. M. Regenerative endodontics: a review of current status and a call for action. Journal of Endodontia. 2007;33(4):377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Chandki R., Kala M., Banthia P., Banthia R. From stem to roots: tissue engineering in endodontics. Journal of Clinical and Experimental Dentistry. 2012;4(1):e66–e71. doi: 10.4317/jced.50678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeeruphan T., Jantarat J., Yanpiset K., Suwannapan L., Khewsawai P., Hargreaves K. M. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. Journal of Endodontia. 2012;38(10):1330–1336. doi: 10.1016/j.joen.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen J. O., Farik B., Munksgaard E. C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dental Traumatology. 2002;18(3):134–137. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds K., Johnson J. D., Cohenca N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: a case report. International Endodontic Journal. 2009;42(1):84–92. doi: 10.1111/j.1365-2591.2008.01467.x. [DOI] [PubMed] [Google Scholar]
- 8.Rule D. C., Winter G. B. Root growth and apical repair subsequent to pulpal necrosis in children. British Dental Journal. 1966;120(12):586–590. [PubMed] [Google Scholar]
- 9.Mirkovic S., Djurdjevic-Mirkovic T., Puskar T. Application of concentrated growth factors in reconstruction of bone defects after removal of large jaw cysts: the two cases report. Vojnosanitetski Pregled. 2015;72(4):368–371. doi: 10.2298/VSP1504368M. [DOI] [PubMed] [Google Scholar]
- 10.Borsani E., Bonazza V., Buffoli B., et al. Biological characterization and in vitro effects of human concentrated growth factor preparation: an innovative approach to tissue regeneration. Biologie et Médecine. 2015;7(5):p. 1. doi: 10.4172/0974-8369.1000256. [DOI] [Google Scholar]
- 11.Gagnier J. J., the CARE Group, Kienle G., et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Journal of Medical Case Reports. 2013;7(1) doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robey P. G., Young M. F., Flanders K. C., et al. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. The Journal of Cell Biology. 1987;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohan Ehrenfest D. M., Pinto N. R., Pereda A., et al. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte-and platelet-rich fibrin (L-PRF) clot and membrane. Platelets. 2018;29(2):171–184. doi: 10.1080/09537104.2017.1293812. [DOI] [PubMed] [Google Scholar]
- 14.Sureshbabu N. M., Ranganath A., Jacob B. Concentrated growth factor – surgical management of large periapical lesion using a novel platelet concentrate in combination with bone graft. Annals of Maxillofacial Surgery. 2020;10(1):246–250. doi: 10.4103/ams.ams_80_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn D. S., Moon J. W., Moon Y. S., Park J. S., Jung H. S. The use of concentrated growth factors (CGF) for sinus augmentation. Journal of Oral Implantology. 2009;38(1):25–38. [Google Scholar]
- 16.Qiao J., Duan J., Zhang Y., Chu Y., Sun C. The effect of concentrated growth factors in the treatment of periodontal intrabony defects. Future Science OA. 2016;2(4, article FS136) doi: 10.4155/fsoa-2016-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying X., Chen Y., Luo N., Shi L., Tong Y., Zhang J. The use of concentrated growth factors and autogenous bone for periodontal soft tissue augmentation and bone regeneration: a case report. Electronic Journal of Biology. 2017;13(3):269–273. [Google Scholar]
- 18.Malli Sureshbabu N., Selvarasu K., Nandakumar M., Selvam D. Concentrated growth factors as an ingenious biomaterial in regeneration of bony defects after periapical surgery: a report of two cases. Case Reports in Dentistry. 2019;2019:6. doi: 10.1155/2019/7046203.7046203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong S., Li L., Cai W., Jiang B. The potential application of concentrated growth factor in regenerative endodontics. International Endodontic Journal. 2019;52(5):646–655. doi: 10.1111/iej.13045. [DOI] [PubMed] [Google Scholar]
- 20.Fayad M. I., Nair M., Levin M. D., et al. AAE and AAOMR joint position statement: use of cone beam computed tomography in endodontics 2015 update. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology. 2015;120(4):508–512. doi: 10.1016/j.oooo.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Petrino J. A., Boda K. K., Shambarger S., Bowles W. R., McClanahan S. B. Challenges in regenerative endodontics: a case series. Journal of Endodontia. 2010;36(3):536–541. doi: 10.1016/j.joen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Jung I., Lee S., Hargreaves K. Biologically based treatment of immature permanent teeth with pulpal necrosis: a case series. Journal of Endodontia. 2008;34(7):876–887. doi: 10.1016/j.joen.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Thibodeau B., Trope M., Lin L. M., Huang G. T.-J. Histologic characterization of regenerated tissues in canal space after the revitalization/revascularization procedure of immature dog teeth with apical periodontitis. Journal of Endodontia. 2010;36(1):56–63. doi: 10.1016/j.joen.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Rodella L. F., Favero G., Boninsegna R., Borgonovo A., Rezzani R., Santoro F. TGF-beta1 and VEGF after fresh frozen bone allograft insertion in oral-maxillo-facial surgery. Histology and Histopathology. 2010;25(4):463–471. doi: 10.14670/HH-25.463. [DOI] [PubMed] [Google Scholar]
- 25.Rodella L. F., Favero G., Boninsegna R., et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microscopy Research and Technique. 2011;74(8):772–777. doi: 10.1002/jemt.20968. [DOI] [PubMed] [Google Scholar]
- 26.Ruparel N. B., Teixeira F. B., Ferraz C. C., Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. Journal of Endodontia. 2012;38(10):1372–1375. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Galler K. M. Clinical procedures for revitalization: current knowledge and considerations. International Endodontic Journal. 2016;49(10):926–936. doi: 10.1111/iej.12606. [DOI] [PubMed] [Google Scholar]
- 28.Segura-Egea J. J., Gould K., Şen B. H., et al. European Society of Endodontology position statement: the use of antibiotics in endodontics. International Endodontic Journal. 2018;51(1):20–25. doi: 10.1111/iej.12781. [DOI] [PubMed] [Google Scholar]
- 29.Althumairy R. I., Teixeira F. B., Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. Journal of Endodontia. 2014;40(4):521–525. doi: 10.1016/j.joen.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Trevino E. G., Patwardhan A. N., Henry M. A., et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. Journal of Endodontia. 2011;37(8):1109–1115. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Egusa H., Sonoyama W., Nishimura M., Atsuta I., Akiyama K. Stem cells in dentistry–part I: stem cell sources. Journal of Prosthodontic Research. 2012;56(3):151–165. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Sonoyama W., Liu Y., Fang D., et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1(1, article e79) doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang G. T.-J., Sonoyama W., Liu Y., Liu H., Wang S., Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. Journal of Endodontia. 2008;34(6):645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovelace T. W., Henry M. A., Hargreaves K. M., Diogenes A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. Journal of Endodontia. 2011;37(2):133–138. doi: 10.1016/j.joen.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Hargreaves K. M., Diogenes A., Teixeira F. B. Treatment options: biological basis of regenerative endodontic procedures. Pediatric Dentistry. 2013;35(2):129–140. [PubMed] [Google Scholar]
- 36.Torabinejad M., Faras H. A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. Journal of Endodontia. 2012;38(6):864–868. doi: 10.1016/j.joen.2012.03.006. [DOI] [PubMed] [Google Scholar]


