Coculture interactions between lasR loss-of-function and LasR+ Pseudomonas aeruginosa strains may explain the worse outcomes associated with the presence of LasR− strains. More broadly, this report illustrates how interactions within a genotypically diverse population, similar to those that frequently develop in natural settings, can promote unpredictably high virulence factor production.
KEYWORDS: Pseudomonas aeruginosa, RhlR, citrate, intraspecies interactions, lasR, pyochelin, quorum sensing
ABSTRACT
The opportunistic pathogen Pseudomonas aeruginosa damages hosts through the production of diverse secreted products, many of which are regulated by quorum sensing (QS). The lasR gene, which encodes a central QS regulator, is frequently mutated in clinical isolates from chronic infections, and loss of LasR function (LasR−) generally impairs the activity of downstream QS regulators RhlR and PqsR. We found that in cocultures containing LasR+ and LasR− strains, LasR− strains hyperproduce the RhlR/RhlI-regulated antagonistic factors pyocyanin and rhamnolipids in diverse models and media and in different strain backgrounds. Diffusible QS autoinducers produced by the wild type were not required for this effect. Using transcriptomics, genetics, and biochemical approaches, we uncovered a reciprocal interaction between wild-type and lasR mutant pairs wherein the iron-scavenging siderophore pyochelin produced by the lasR mutant induced citrate release and cross-feeding from the wild type. Citrate, a metabolite often secreted in low iron environments, stimulated RhlR signaling and RhlI levels in LasR−but not in LasR+ strains. These studies reveal the potential for complex interactions between recently diverged, genetically distinct isolates within populations from single chronic infections.
INTRODUCTION
In chronic infections and healthy microbiomes, genetic diversity frequently arises and persists within clonally derived microbial populations, and recent data highlight that heterogeneity within a population can pose challenges to clearance and treatment (1–4). Genotypic and phenotypic complexity is particularly well documented in the chronic lung infections associated with the genetic disease cystic fibrosis (CF), and studies have convincingly demonstrated that within a species, a common set of genes is under selection across strains and hosts (5–11).
Loss-of-function mutations in Pseudomonas aeruginosa lasR (LasR−) are commonly found in CF isolates and strains from acute infections and from environmental sources (12–16). LasR participates in the regulation of quorum sensing (QS) in conjunction with other transcription factors, including RhlR and PqsR (MvfR). These regulators have one or more autoinducer ligands: 3-oxo-C12-homoserine lactone (3OC12HSL) for LasR, C4-homoserine lactone (C4HSL) for RhlR, and hydroxy-alkyl-quinolones (pseudomonas quinolone signal [PQS] and hydroxy-heptyl quinolone [HHQ]) for PqsR (17). In the regulatory networks described in widely used P. aeruginosa model strains, LasR is an upstream regulator of RhlR and PqsR signaling, and together these regulators control the expression of a suite of genes associated with virulence, including redox-active small-molecule phenazines (18–20), cyanide (21), and rhamnolipid surfactants important for surface motility, biofilm dispersal, and host cell damage (22–24).
Although LasR positively regulates virulence factors, and lasR loss-of-function mutants have reduced virulence in infection models, LasR− strain culture positivity is correlated with worse disease outcomes in acute and chronic infections (12, 13). There are several possible explanations for this apparent contradiction. LasR− clinical isolates (CIs) are frequently found among strains with functional LasR (LasR+) where exoproducts can be shared or signal cross-feeding can occur (14), and some LasR− clinical isolates exhibit rewired QS regulation (25). Loss of LasR function also confers some fitness advantages, including altered catabolic profiles (26) and enhanced growth in low oxygen (27, 28), which may contribute to bacterial burden. Further, LasR− strains can activate QS in response to specific fungal products (29) or culture conditions (30, 31).
In addition to LasR status, iron acquisition strategies are often heterogeneous across P. aeruginosa isolates. P. aeruginosa procures iron through the use of siderophores, including pyochelin (32–34) and pyoverdine (35), from heme, or through a direct iron uptake system (36–38). Although it is common to encounter P. aeruginosa strains with loss-of-function mutations in genes required for biosynthesis of the high-affinity siderophore pyoverdine, genes associated with use of pyochelin, heme utilization, and ferrous iron import are generally intact (39–41). Iron limitation can deprioritize pathways that require abundant iron including the tricarboxylic acid (TCA) cycle (42), and consequently Pseudomonas spp. and other organisms release metabolic intermediates, such as citrate, that accumulate at iron-requiring steps (e.g., aconitase) (43, 44).
Here, we show that mixtures of P. aeruginosa LasR− and LasR+ strains had enhanced production of QS-controlled factors across media, culture conditions, and strain backgrounds. The unpredictably high levels of exoproducts in coculture were produced by LasR− strains due to activation of RhlR, likely through increased C4HSL synthase (RhlI) stability in LasR− strains. Our genetic, transcriptomic, and biochemical studies led us to uncover a set of interactions in which production of the siderophore pyochelin by ∆lasR cells induced citrate release by wild type (WT) but not by ∆lasR cells. We found that citrate led to increased RhlI protein levels and RhlR activity in ∆lasR cells but not in the WT. Together, these intraspecies interactions increased production of exoproducts known to cause host damage.
RESULTS
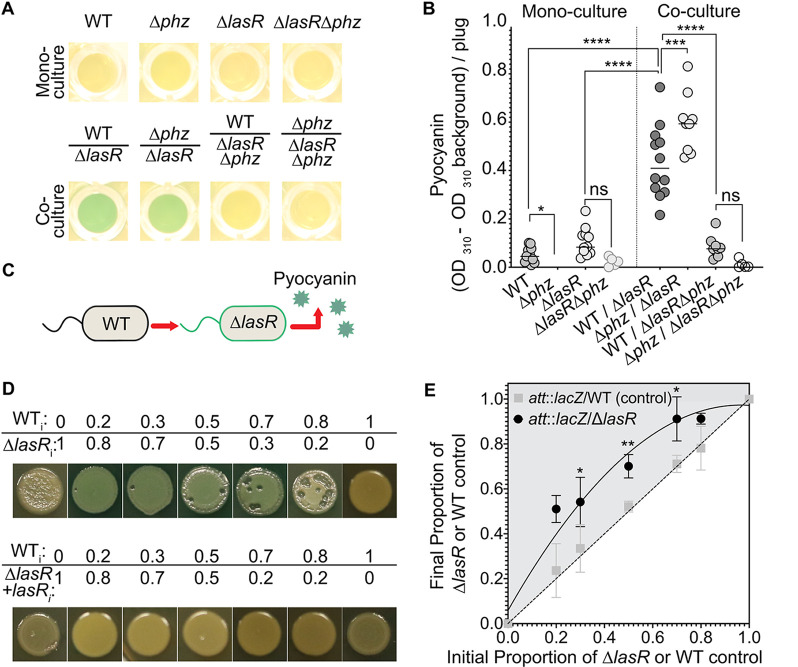
P. aeruginosa ∆lasR overproduces pyocyanin in coculture with the wild type.
We observed that mixtures of P. aeruginosa LasR+ and LasR− strains had high levels of total pyocyanin, a secreted, blue-pigmented phenazine. As shown in spot colonies of the PA14 wild-type (WT), ∆lasR strain, and WT and ∆lasR strain cocultures (here, WT/∆lasR cocultures), the strain mixture showed increased blue pigmentation (Fig. 1A) and a significant 4-fold induction of pyocyanin above the background relative to the level with either strain alone (Fig. 1B). Phenazine-deficient derivatives, ∆phz (∆phzA1 ∆phzB1 ∆phzC1 ∆phzD1 ∆phzE1 ∆phzF1 ∆phzG1 ∆phzA2 ∆phzB2 ∆phzC2 ∆phzD2 ∆phzE2 ∆phzF2 ∆phzG2) and ∆lasR ∆phz (∆lasR ∆phzC1 ∆phzC2) strains, were also included, and as expected, ∆phz and ∆lasR ∆phz cells showed no blue colony pigmentation (Fig. 1A). The higher levels of pyocyanin in WT/∆lasR cocultures relative to levels in single-strain cultures were also observed on artificial sputum medium (ASM) and on phosphate-buffered medium with or without amino acids, indicating that the phenomenon occurred under diverse conditions (see Fig. S1A in the supplemental material). Cocultures of clonally derived LasR+ and LasR− clinical isolates collected from single respiratory sputum samples from chronically infected individuals with cystic fibrosis (14) also had increased production of pyocyanin relative to monoculture levels when LasR+ and LasR− strains were grown together (Fig. S1B and C).
FIG 1.
The ∆lasR strain produces pyocyanin in wild-type/∆lasR cocultures beyond monoculture concentrations. (A) Representative images of the wild-type (WT) and ∆lasR strains and their phenazine-deficient derivatives (∆phz strains) visualized from the bottom of a 96-well LB agar plate after 16 h growth as mono- and cocultures with 70:30 WT-to-∆lasR cell initial ratio. (B) Pyocyanin levels above background, defined as the average signal for the ∆lasR ∆phz strain, quantified for cultures described in panel A. ns, not significant; *, P < 0.05; ***, P < 0.0005; ****, P < 0.0001, as determined by ordinary one-way analysis of variance with Tukey’s multiple-comparison test for n ≥ 8 replicates on three different days. (C) Model of pyocyanin production by the ∆lasR strain in coculture with the WT. (D) Representative pyocyanin production of the wild type cocultured with the ∆lasR strain or the ∆lasR strain complemented with the lasR gene at the native locus (∆lasR + lasR strain) across several initial (designated by the subscript i) proportions on LB medium for 20 h. Three biological replicates were included in at least 3 independent experiments. (E) Average final proportion of 3 replicate colony biofilms quantified after 16 h growth for the WT strain and the ∆lasR strain cocultured with a WT strain tagged with lacZ in 3 independent experiments. Experimental setup was as described in panel D. *, P < 0.05; **, P < 0.005, as determined by two-tailed t tests of paired ratios between att::lacZ/WT (control) and att::lacZ/∆lasR cocultures at each initial ratio. All results that reach significance are marked.
Increased pyocyanin production of LasR+ and LasR− strain cocultures relative to either strain alone is stable across media and strains. (A) Representative images from PA14 wild type (WT) and ∆lasR strain mono- and coculture pyocyanin production on rich medium (LB and artificial sputum medium [ASM]), and minimal (M63 medium, pH 6.8, 0.2% glucose; no Casamino Acids [CAA]) and defined phosphate buffered medium (M63 medium, pH 6.8, 0.2% glucose + 0.2% CAA). (B) Pyocyanin production of mono- and cocultures of strain PA14 WT and the ∆lasR strain and clonally derived clinical isolates DH2417 (LasR+) and DH2415 (LasR−). (C) Pyocyanin quantification of LasR+ and LasR− strains grown in mono- and coculture on 96-well LB agar plugs for 16 h. Strain PA14 WT and the ∆lasR strain and previously characterized clinical isolate pairs DH2417 (LasR+) and DH2415 (LasR−) and DH1133 (LasR+) and DH1132 (LasR−), from two distinct CF patients, are also shown. Data are from two independent experiments with four biological replicates each. (D) Number of CFU in 16-h colony biofilms grown on LB medium. Each strain set is presented relative to the level of the WT or the LasR+ strain. PA14 WT and ∆lasR strain mono- and coculture data are the average from four independent experiments with at least three biological replicates. ns, not significant; **, P < 0.005; ***, P < 0.0005, as determined by analysis of variance with Tukey multiple hypotheses correction for clinical isolate CFU counts. Download FIG S1, PDF file, 0.3 MB (280.8KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To assess individual strain contributions to increased pyocyanin in WT/∆lasR cocultures, we replaced each strain with its phenazine-deficient derivative and measured pyocyanin in coculture. When the ∆lasR strain was cultured with the phenazine biosynthesis mutant ∆phz strain (∆phz/∆lasR coculture), we still observed increased blue pigmentation and total pyocyanin at a level above that of either monoculture (Fig. 1A and B). Surprisingly, pyocyanin production by ∆phz/∆lasR cocultures was statistically higher than that of WT/∆lasR coculture (Fig. 1B). In contrast, WT/∆lasR ∆phz cocultures did not display the high-pyocyanin phenotype (Fig. 1A and B) and resembled ∆phz/∆lasR ∆phz cocultures, where no pyocyanin was produced. Collectively, these data suggested that WT/∆lasR cocultures produced more pyocyanin than either monoculture alone and that the ∆lasR strain contributed the pyocyanin in coculture (Fig. 1C).
That the levels of pyocyanin in WT/∆lasR cocultures were higher than the level in each strain alone was not dependent on the initial ratios of WT to ∆lasR cells (Fig. 1D). We saw increased coculture colony pigmentation when the initial proportions of WT cells were at 0.2, 0.3, 0.5, 0.7, and 0.8 of the initial inoculums, with the balance comprised of ∆lasR cells (Fig. 1D).
No increase in pyocyanin was observed at any ratio when the WT was cocultured with the ∆lasR complemented derivative strain (∆lasR + lasR), indicating that the phenomenon was dependent on the lasR mutation (Fig. 1D). To assess the relative abundances of WT and ∆lasR cells in coculture, we competed each strain against a neutrally tagged WT strain (PA14 att::lacZ). We found that ∆lasR cells increased in proportion after 16 h of growth in colony biofilms regardless of the starting proportion whereas the proportions of untagged WT cells remained stable (Fig. 1E). We have previously shown that Anr activity is higher in ∆lasR strains and contributes to the competitive fitness of the ∆lasR strain against WT P. aeruginosa in colony biofilms (27, 45), but Anr was not required for coculture pyocyanin production (Fig. S2).
∆lasR strain shows Anr-independent increases in pyocyanin in coculture with LasR+ P. aeruginosa. Representative images of WT, ∆anr, and ∆lasR ∆anr strains in mono- and coculture colony biofilms on LB medium after 24 h. Download FIG S2, PDF file, 0.1 MB (152.3KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Pyocyanin is a product regulated by quorum sensing (QS) through the transcription factors LasR, RhlR, and PqsR (46–48), and because QS regulation is cell density dependent, it was important to assess the coculture population size relative to that of the monoculture. Total CFU counts did not increase in WT/∆lasR mixed cultures relative to the level for either strain alone (Fig. S1D). Instead, we found that WT/∆lasR cocultures had fewer CFU than WT monocultures on lysogeny broth (LB) medium (Fig. S1D). Taken together, these data suggested that altered behavior, rather than cell number, contributed to the increased phenazine profile of LasR− strains.
Independent of its ability to produce autoinducers, the WT promotes RhlR/I-dependent signaling in a ∆lasR strain.
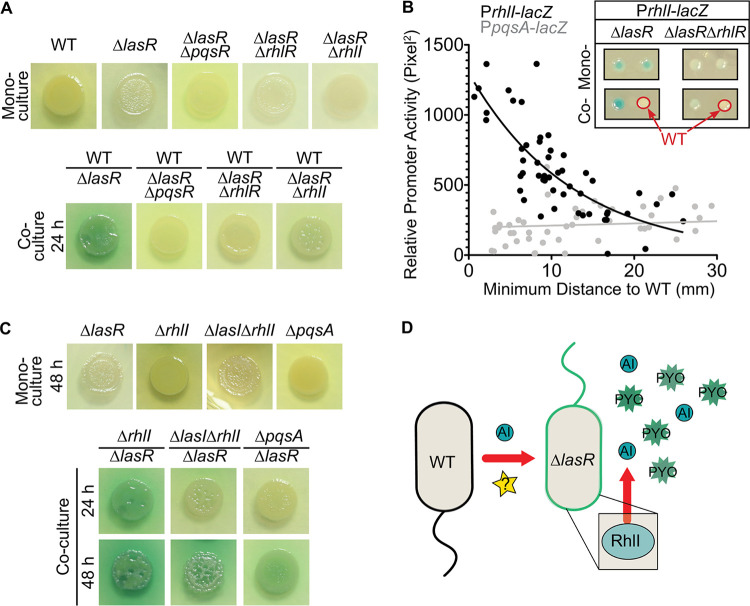
In the canonical QS pathway, LasR regulates both PqsR and RhlR, and mutants lacking either regulator in a WT background have impaired pyocyanin production (49, 50). Both pqsR and rhlR were required in ∆lasR cells for pyocyanin production in coculture with the WT (Fig. 2A). To determine if coculture increased RhlR- or PqsR-dependent signaling in ∆lasR strains, we fused lacZ to the promoters of rhlI and pqsA (PrhlI and PpqsA, respectively) which provide activity readouts of each respective regulator (17). We examined the interactions between WT and the ∆lasR strain in single-cell-derived colonies by spreading suspensions containing ∼50 cells of WT with ∼50 cells of either a ∆lasR PrhlI-lacZ or ∆lasR PpqsA-lacZ strain on LB agar containing the colorimetric β-galactosidase substrate 5-bromo-4-chloro-3-indolyl-d-galactopyranoside (X-Gal). Intercolony distances and β-galactosidase activity in ∆lasR strains were measured. We found that the rise in PrhlI-lacZ activity was inversely correlated with the distance to a WT colony (Fig. 2B). Pearson correlation analyses showed that 54% of the variability in ∆lasR PrhlI- lacZ strain activity could be explained by changes in the distance to a WT colony (P value of ≤ 0.0001). The increased PrhlI-lacZ activity in the ∆lasR strain was not observed in the ∆lasR ∆rhlR strain, and close proximity to another ∆lasR PrhlI-lacZ colony did not affect promoter activity (Fig. 2B, inset). Because C4HSL (which is synthesized by RhlI) activates RhlR and because proximity to the WT stimulated ∆lasR PrhlI-lacZ strain activity, we examined the role of RhlI in the ∆lasR strain response. We observed that a ∆lasR ∆rhlI strain was greatly impaired in the induction of pyocyanin upon coculture with the WT (Fig. 2A), which suggests that WT production of C4HSL was insufficient to complement the ∆lasR ∆rhlI strain and further posits activation of RhlR and C4HSL synthesis in ∆lasR strains. Although PqsR was required in ∆lasR cells for coculture pyocyanin production, there was no significant correlation with proximity to the WT for ∆lasR PpqsA-lacZ strain activity (Fig. 2B). Collectively, these data indicated that a diffusible factor produced by the WT stimulated RhlR-dependent signaling in the ∆lasR strain to induce downstream production of RhlR- and PqsR-dependent factors.
FIG 2.
P. aeruginosa WT induces RhlR/I-dependent pyocyanin production in ∆lasR cells even in the absence of WT autoinducers. (A) Representative pyocyanin production by monocultures and WT cocultures (70% WT at time 0) of ∆lasR and ∆lasR strain derivatives that are deficient in PQS or RhlR/I-dependent quorum sensing on LB medium after 24 h growth. (B) Promoter activity of the ∆lasR PpqsA-lacZ (gray) and ∆lasR PrhlI-lacZ (black) strains, quantified by relative pixel intensity of single-cell-derived ∆lasR colonies grown near unmodified WT colonies. Solid best-fit nonlinear lines are for visualization. The inset shows representative colonies for RhlR-dependent ∆lasR PrhlI-lacZ strain activity in monoculture and coculture with the WT (red circles). The experiment was repeated with 2 replicates on at least 3 independent days. (C) Representative monoculture and ∆lasR coculture images for the ∆pqsA, ∆rhlI, and ∆lasI ∆rhlI mutants on LB medium after 24 h and 48 h. (30% ∆lasR cells at time 0). (D) Model of AHL-dependent and -independent induction of RhlR/I-dependent activity and phenazine production in ∆lasR cells grown in coculture with the WT.
Given differences in colony pigmentation between WT/∆lasR ∆rhlR and WT/∆lasR ∆rhlI (Fig. 2A) cocultures, C4HSL cross-feeding between the WT and ∆lasR strain likely occurred. Because C4HSL is diffusible and produced by WT cells, we tested the hypothesis that C4HSL or other acyl-homoserine lactones (AHLs) produced by the WT were necessary to induce RhlR-dependent activity in ∆lasR cells cocultured with the WT. To test this hypothesis, we cocultured the ∆lasR strain with ∆rhlI cells or ∆lasI ∆rhlI cells which lack both acyl-homoserine lactone synthases. Surprisingly, we found that like WT/∆lasR cocultures, ∆rhlI/∆lasR cocultures had higher levels of pyocyanin than monocultures (Fig. 2C). Similarly, ∆lasI ∆rhlI/∆lasR cocultures had higher levels of pyocyanin production than monocultures though the interaction was delayed by ∼24 h relative to the interaction of the WT/∆lasR cocultures (Fig. 2C). Consistent with the activity of the ∆lasR PpqsA-lacZ strain, which was not induced in coculture with WT, the PQS-deficient ∆pqsA strain supported high pyocyanin colony pigmentation in coculture with ∆lasR cells after 24 h of extended incubation (Fig. 2C). The AHL-independent activation observed in ∆lasI ∆rhlI/∆lasR cocultures and the striking differences in pyocyanin production observed between the strongly stimulating ∆rhlI/∆lasR cocultures and the weakly stimulating WT/∆lasR ∆rhlI cocultures suggested that the ∆lasR strain may rely more heavily on production of its own autoinducer for activation in coculture. Consistent with this model, we found that the ∆lasR strain produces RhlR/RhlI (RhlR/I)-dependent AHLs in coculture with an AHL-sensing reporter strain (i.e., ∆lasI ∆rhlI strain with a lacZ promoter fusion to an AHL-responsive gene) (Fig. S3A and B). The dispensable contribution of WT-produced autoinducers implicated a novel signaling interaction in coculture-dependent activation of RhlR/I activity in the ∆lasR strain (Fig. 2D).
The ∆lasR strain produced RhlR/I-dependent acyl-homoserine lactone (AHL) autoinducers in coculture with the ∆lasI ∆rhlI strain, and this was repressed by iron supplementation. (A) Schematic of experimental setup and quantification of AHL activity for colony biofilms grown on an AHL-sensing reporter strain (i.e., ∆lasI ∆rhlI strain with a lacZ promoter fusion to an AHL-responsive gene). (B) AHL activity for the WT, AHL-deficient ∆lasI ∆rhlI control, ∆lasR, ∆lasR ∆rhlR, and ∆lasR ∆rhlI strains grown on an AHL-sensing reporter strain in X-Gal-containing medium. (C) Representative image of RhlI-dependent activity (i.e., C4HSL) for the ∆lasR strain grown on an AHL-sensing reporter strain across an iron gradient under stable X-Gal-containing conditions. (D) Setup and quantification as described for panel a for the ∆lasR strain on LB medium (−) and on LB medium plus 10 μM FeSO4 (+) including a C4HSL-deficient derivative ∆lasR ∆rhlI strain. Data points are from ≥ 3 different days. *, P < 0.05; ****, P < 0.0001, as determined by ordinary one-way analysis of variance with Dunnett’s multiple-comparison test to the control ∆lasR strain. Download FIG S3, PDF file, 2.4 MB (2.5MB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To assess whether WT-induced RhlR activity in the ∆lasR strain was sufficient to elicit other RhlR/I-controlled phenotypes in addition to pyocyanin production, we tested whether coculture with LasR+ strains enhanced swarming, a surface-associated motility which requires the production of RhlR-regulated rhamnolipid surfactants (51). While the rhamnolipid-defective mutant ∆rhlA, ∆lasR, and ∆lasR ∆rhlR strains were unable to swarm, cocultures of the ∆lasR strain with the ∆rhlA strain swarmed considerably. The phenomenon was dependent on RhlR as the ∆rhlA/∆lasR ∆rhlR cocultures did not swarm (Fig. S4). Altogether, these data implicated broad activation of RhlR-mediated QS in LasR− strains cocultured with LasR+ P. aeruginosa.
The ∆lasR strain showed enhanced production of RhlR-regulated rhamnolipid surfactant in coculture with the LasR+ strain. RhlR-regulated swarming motility on soft agar of the WT, rhamnolipid surfactant biosynthesis mutant (∆rhlA strain), ∆lasR, and ∆lasR ∆rhlR strains in mono- and coculture after 36 h. Download FIG S4, PDF file, 0.3 MB (295KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Pyochelin production by ∆lasR cells is required for coculture interactions.
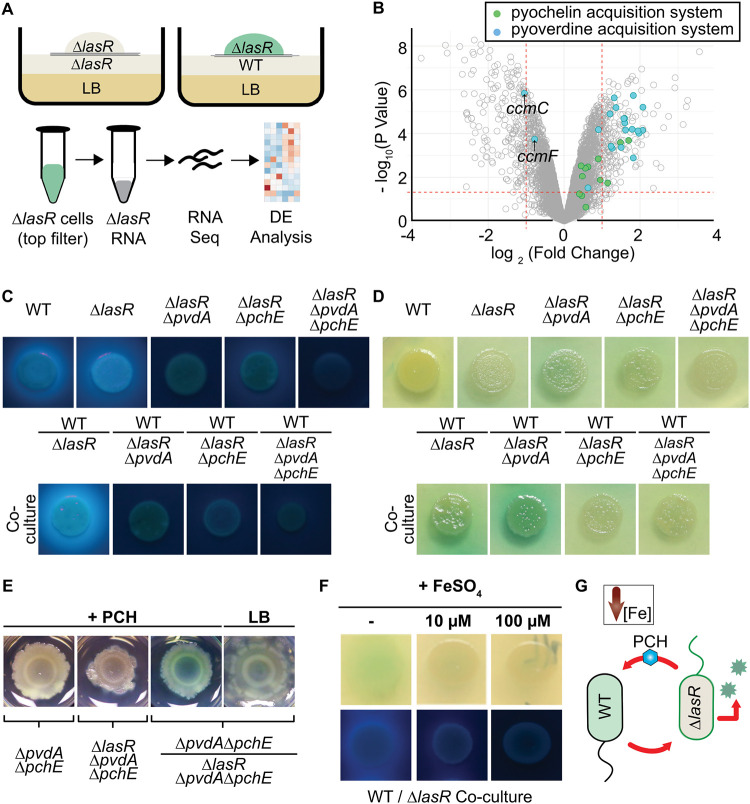
With evidence indicating that induction of RhlR activity in ∆lasR cells can occur in both mixed-strain spot colonies and adjacent colonies independent of autoinducer cross-feeding, we sought to gain further insight into the mechanisms that underlie the WT-∆lasR cell interactions. We investigated the transcriptomes of the lasR mutant in coculture with either the WT or itself via RNA sequencing (RNA-seq). We grew ∆lasR colony biofilms on LB medium physically separated from a lawn of either the ∆lasR or WT strain by two filters with 0.22-μm pores to prevent mixing of genotypes while allowing for the passage of small molecules. In order to examine ∆lasR strain transcriptional profiles, RNA was extracted from cells within the ∆lasR colony biofilms grown on the topmost filter for 16 h (Fig. 3A). As expected, no lasR reads were detected in our sequencing data to suggest that the wild type was sufficiently excluded by filter separation. Expression levels of a total of 199 genes in the ∆lasR strain were higher, and those of 198 genes were lower by a |log2(fold change)| of ≥1 with a P value of <0.05, in coculture with the WT than levels in the ∆lasR strain alone (Table S1). Gene Ontology (GO) term analyses through PantherDB (52) indicated that the upregulated gene set was significantly overrepresented in two pathways related to siderophore biosynthesis: the pyoverdine biosynthetic process and salicylic acid biosynthetic process (an upstream precursor of pyochelin) with ∼44- and ∼77-fold enrichment, respectively (P values of <0.005). Twenty-eight out of the 33 genes in the pyochelin and pyoverdine siderophore biosynthesis- and acquisition-related GO families were significantly upregulated in ∆lasR cells upon coculture with the WT (Fig. 3B) (i.e.,|log2(fold change)| of ≥0 with a P value of <0.05). Other low-iron-responsive genes were differentially expressed, including the has genes involved in heme uptake and antABC genes (Table S1). While we observed stimulation of rhlI promoter activity and increased production of RhlR-regulated products, we did not see a broad transcriptional pattern indicative of RhlR activation at this early time point (Table S1), and this point is discussed below.
FIG 3.
Biosynthesis of the coculture-induced iron scavenging siderophore pyochelin is required in the ∆lasR strain for pyocyanin production when it was cultured with the wild type (WT). (A) Scheme for the collection of RNA from ∆lasR colony biofilms grown above a lawn of ∆lasR or WT cells. DE, differential expression. (B) Volcano plot showing differential expression (log2) for ∆lasR cells grown over WT relative to ∆lasR cells grown over ∆lasR on the x axis; the y axis shows the −log10 P value for the difference between sample types. Genes involved in pyoverdine (blue) and pyochelin (green) iron acquisition systems are indicated. ccmC and ccmF (indicated with arrows) of the pyoverdine GO term are involved in c-type cytochrome biosynthesis, and strains with knockouts of these genes are reported to produce more pyochelin. (C) Monocultures and WT cocultured with ∆lasR strains deficient in pyoverdine (∆pvdA) and/or pyochelin (∆pchE) biosynthesis. Colonies were visualized under UV light in order to see fluorescent siderophores. Images are representative of at least 3 independent experiments. (D) Pyocyanin production visualized for the colonies shown in panel C. (E) Representative pyocyanin production by siderophore-deficient strains grown in mono- and coculture on LB medium or on LB medium with pyochelin-containing extract (+PCH). Colonies were grown in a 12-well plate with a 2-ml total volume and imaged after 24 h. (F) Mixed colony biofilms of wild-type and ∆lasR strains grown on LB medium (−) or LB medium supplemented with either 10 or 100 μM FeSO4 visualized under ambient (top) and UV (bottom) light. (G) Model showing that pyochelin (PCH) production by the ∆lasR strain is required for WT/∆lasR coculture phenazines.
Differentially expressed genes in the ∆lasR strain grown with the wild type versus itself in filter-separated cocultures. PA14 number or gene name, if available, is listed in descending order by log2(∆lasR cells grown on WT/∆lasR cells grown on ∆lasR cells). Differential expression is defined as |log2(∆lasR cells grown on WT/∆lasR cells grown on ∆lasR cells)| >1 with a P value of <0.05. Download Table S1, XLSX file, 0.02 MB (21.7KB, xlsx) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Given that siderophore biosynthesis genes were upregulated in ∆lasR cells cocultured with WT cells, we qualitatively examined production of fluorescent pyoverdine and pyochelin siderophores in monocultures and cocultures. To determine the contribution of both pyoverdine and pyochelin by the ∆lasR strain to fluorescence, genes required for pyoverdine biosynthesis (∆lasR ∆pvdA strain), pyochelin biosynthesis (∆lasR ∆pchE strain), or both pathways (∆lasR ∆pvdA ∆pchE strain) were disrupted (Fig. 3C). Increased fluorescence attributable to both pyoverdine and pyochelin in coculture was due to siderophore production by ∆lasR strains, consistent with the RNA-seq data, as the increased fluorescence in WT/∆lasR cocultures was lost in coculture when the ∆lasR strain was replaced with a ∆lasR ∆pvdA, ∆lasR ∆pchE, or ∆lasR ∆pvdA ∆pchE mutant. While cocultures of the WT and the pyoverdine-deficient derivative ∆lasR ∆pvdA strain (i.e., WT/∆lasR ∆pvdA coculture) showed increased pyocyanin production relative to that of either monoculture, the ∆lasR ∆pchE and ∆lasR ∆pvdA ∆pchE strains did not promote pyocyanin production in coculture with the WT, as observed by colony pigmentation (Fig. 3D). The decrease in ∆lasR strain-derived pyocyanin was not due to decreased fitness as disruption of pvdA and pchE individually in ∆lasR cells had no effect on the final proportions; in contrast, the ∆lasR ∆pvdA ∆pchE strain had a significant defect in competitive fitness compared to the fitness of the ∆lasR parental strain (Fig. S5). These data suggested that pyochelin played a role in the coculture interaction. To test this model, we complemented the pyocyanin defect in the siderophore-deficient ∆pvdA ∆pchE/∆lasR ∆pchE coculture with pyochelin-containing extracts from cultures of ∆pvdA cells which cannot produce pyoverdine or control extracts from siderophore-deficient ∆pvdA ∆pchE cultures (Fig. S6A gives supernatant absorption spectra). The two extracts were analyzed using a chrome azurol S (CAS) assay (53) to confirm that chelator activity was present in the ∆pvdA cell supernatant extracts but not in extracts from ∆pvdA ∆pchE cultures (Fig. S6B). Medium supplemented with pyochelin-containing extracts, but not siderophore-free extracts, restored pyocyanin production in ∆pvdA ∆pchE/∆lasR ∆pchE cocultures (Fig. 3E), lending further support to the idea that pyochelin was required for coculture interactions. Consistent with this requirement, iron supplementation suppressed siderophore production, as expected, and diminished coculture pyocyanin in WT/∆lasR cocultures (Fig. 3F) alongside a decrease in ∆lasR strain RhlR/I-dependent AHL activity in coculture with the AHL-sensing reporter strain (Fig. S3C and D). Collectively, these data support a model in which pyochelin production by the ∆lasR strain is induced and required for pyocyanin-promoting interactions with the WT through initiation of a low-iron response (Fig. 3G).
Fitness of the ∆lasR strain lacking one or both major siderophore biosynthesis pathways. Final proportion of untagged colony forming units was quantified after a 16-h competition with the WT att::lacZ strain. The dotted line indicates the 0.5 initial proportion (Pi). Final proportions (Pf) for the ∆lasR wild-type (WT) strains and the ∆lasR strain complemented with the lasR gene at the native locus (∆lasR strain) on LB medium (white background), for the ∆lasR strain on LB medium supplemented with 10 μM FeSO4 (grey background), and for siderophore-deficient ∆lasR mutant derivatives. a versus b, P < 0.0006; a versus c, P < 0.0001, as determined by one-way analysis of variance for comparison to the ∆lasR strain on LB medium with a Sidak multiple-hypotheses correction for n ≥ 3 experiments. Download FIG S5, PDF file, 0.1 MB (78.8KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Pyochelin-containing extracts are biologically active. (A) Absorbance spectra (230 to 600 nm) of extracts from supernatants of the pyochelin-producing ∆pvdA strain (solid line) and the pyochelin-deficient ∆pvdA ∆pchE strain (dotted line) in a 50/50 MeOH/distilled H2O solution. Grey vertical lines indicate reported peaks for purified, iron-free pyochelin at 248 and 313 nm. (B) Indicated extracts spotted on chrome azurol S (CAS) agar with EDTA metal chelator as a positive control wherein a change from blue to yellow indicates iron-chelating capacity. CAS activity for the extracts from ∆pvdA (+PCH) and ∆pvdA ∆pchE (NEG) strains are shown. Download FIG S6, PDF file, 0.1 MB (106.1KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In coculture with the WT, the ∆lasR strain responds to citrate, a pyochelin-inducible metabolite.
Many of the upregulated genes in the ∆lasR strain upon coculture with the WT have annotations related to organic acids, such as anthranilate and citrate (Table S1 and Fig. S7A). Several lines of evidence suggest that anthranilate was not the factor that stimulated RhlR activity and pyocyanin production in coculture. First, anthranilate supplementation (up to ∼15 mM) did not alter ∆lasR strain phenazine production (Fig. S7B). Further, cocultures of the ∆lasR mutant with the anthranilate synthase mutant ∆phnAB strain, with reduced extracellular anthranilate (Fig. S7C), or with the ∆pqsA mutant (Fig. 2C), which accumulates it (54), did not alter coculture phenazine production. Anthranilate is also generated from tryptophan catabolism through the kynurenine pathway (55). Given that coculture pyocyanin production could occur in the absence of exogenous amino acids (Fig. S1A), we concluded that the kynurenine pathway was likely not involved. Together, these data suggested that anthranilate was not a stimulating metabolite.
Anthranilate (AA) supplementation and loss of potential di- and tricarboxylic acid transporters dctA and PA14_51300 did not alter pyocyanin production of the ∆lasR strain. (A) Volcano plot indicating anthranilate metabolism gene expression (orange) in the ∆lasR strain grown in coculture with the WT. (B) Colony morphology and pyocyanin production are not different upon anthranilate supplementation across a gradient of concentrations. (C) RhlR-dependent pyocyanin production of the ∆lasR mutant and the anthranilate synthase mutant (∆phnAB strain) in mono- and cocultures on LB medium after 18 h. (D) The major succinate, fumarate, and malate transporter dctA was dispensable in the ∆lasR strain background for RhlR-dependent pyocyanin production in coculture with the WT. (E) The broad TCA cycle intermediate transporter PA14_51300 was not required in the ∆lasR strain for pyocyanin production in coculture with the WT. Download FIG S7, PDF file, 0.5 MB (542.7KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
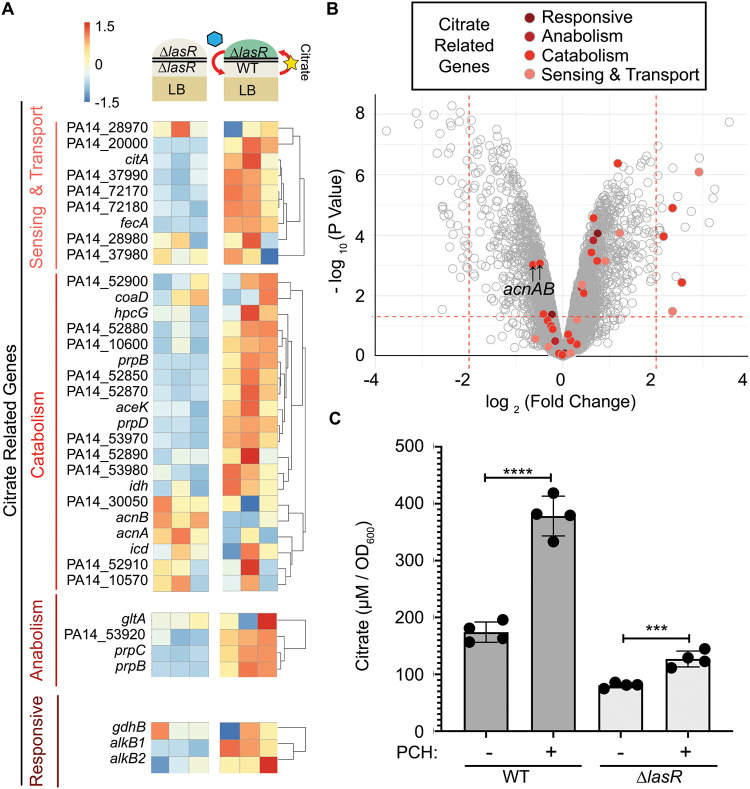
In light of the observation that 20% of the most strongly differentially expressed genes [|log2 (fold change)| of ≥2 with a P value of <0.05] were implicated in citrate sensing, transport, catabolism, and anabolism, as annotated by UniProt (56) and www.pseudomonas.com (57), we looked at all genes with annotations related to citrate to identify broad expression patterns (Fig. 4A). Among the genes induced in ∆lasR/WT cocultures [|log 2 (fold change)| of ≥0 with a P value of <0.05] were genes annotated as citrate responsive or playing roles in citrate sensing and transport or metabolism, with the most strongly upregulated citrate genes involved in sensing, transport, and catabolism specifically (Fig. 4B).
FIG 4.
Citrate release by WT is induced by pyochelin exposure. (A) Subset of expression data in ∆lasR cocultures (Fig. 3A shows the setup) for genes annotated as being involved in citrate sensing, transport, catabolism, anabolism, and those shown to be responsive to citrate. (B) Volcano plot of expression data of ∆lasR cocultures with each point representing the log2(∆lasR cells grown on WT/∆lasR cells grown on ∆lasR cells) expression and –log10(P value) of a single gene. Genes shown in panel A are highlighted in color. (C) Citrate concentrations in supernatants from wild-type and ∆lasR stationary-phase cultures after growth in LB medium supplemented with extracts containing 50 μM pyochelin (PCH+) or an equal volume of control extracts (PCH−). A representative experiment with four biological replicates repeated 3 independent days is shown. ***, P < 0.0005; ****, P ≤ 0.0001, as determined by two-tailed t tests of paired ratios.
We measured citrate in the supernatants of WT and ∆lasR LB cultures based on the following observations: (i) ∆lasR strains induced low-iron-responsive genes when grown near the WT but not itself; (ii) ∆lasR strain pyochelin production was necessary for coculture interactions that led to increased pyocyanin; (iii) citrate sensing and catabolism genes were induced in ∆lasR cells by the presence of WT cells; and (iv) numerous microbes, including Pseudomonas putida, were shown to secrete citrate and other organic acids when iron limited (44, 58–60). Citrate was detected in both WT and ∆lasR strain LB culture supernatants (Fig. 4C), and amendment with extracts containing 50 μM pyochelin increased extracellular citrate concentrations by ∼2-fold in WT cultures compared to levels in cultures supplemented with extracts lacking pyoverdine and pyochelin, with a much smaller stimulation in ∆lasR cultures under the same conditions (Fig. 4C). This suggested that WT-produced citrate may be involved in WT/∆lasR coculture interactions and that citrate release was enhanced by pyochelin produced by the ∆lasR strain.
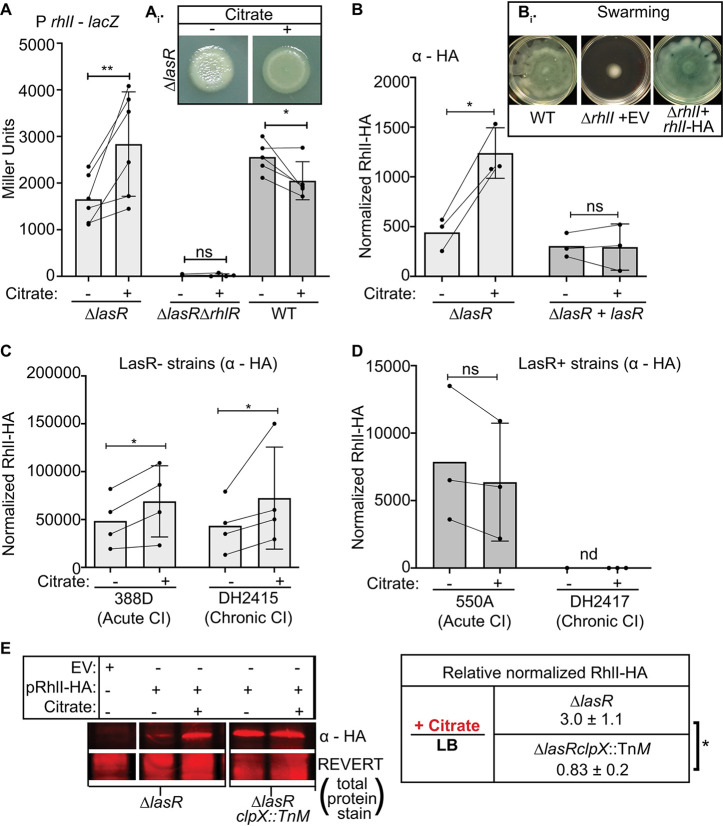
Citrate induces RhlR-dependent activity and RhlI levels in a ClpX protease-dependent manner in ∆lasR cells.
To determine if citrate was sufficient to stimulate RhlR activity in ∆lasR cells, we analyzed its effects on rhlI promoter fusion activity, colony morphology, and RhlI protein levels. We found that citrate increased rhlI promoter activity (PrhlI) in ∆lasR cells and that its effects were dependent on the presence of RhlR (Fig. 5A). Citrate was sufficient to promote increases in colony pigmentation and colony smoothness, previously characterized to be RhlR-mediated in ∆lasR cells (29) (Fig. 5A, inset). In contrast, citrate caused a small but significant reduction in WT PrhlI activity compared to that the LB control (Fig. 5A).
FIG 5.
Citrate induced RhlR-dependent rhlI promoter activity and stabilized RhlI protein in LasR− strains in a ClpX protease-dependent manner. (A) β-Galactosidase activity for ∆lasR, ∆lasR ∆rhlR, and wild-type strains harboring att::PrhlI-lacZ on LB medium with or without 20 mM citrate at 24 h. Each point is the average of three biological replicates from 3 to 4 independent experiments. ns, not significant; *, P < 0.05; **, P < 0.005, as determined by one-way analysis of variance with Dunnett’s multiple hypotheses correction of the indicated comparisons. The inset (Ai) shows a representative image of ∆lasR colony morphology on LB medium with and without 20 mM citrate at 24 h. (B) RhlI-HA protein signal normalized to Revert total protein stain (LiCor) on LB medium with and without 20 mM citrate for the ∆lasR strain and lasR complemented at the native locus (∆lasR strain). ns, not significant; *, P < 0.05; **, P < 0.005, as determined by analysis of variance with Dunnett’s multiple hypothesis correction for n = 3 biological replicates performed on three independent days. The inset (Bi) illustrates that plasmid-borne RhlI-HA, but not the empty vector (EV), complemented an ∆rhlI mutant for swarming. (C) RhlI-HA protein levels on LB medium with and without 20 mM citrate of LasR loss-of-function (LasR−) clinical isolates (CIs) from acute corneal (388D) and chronic CF (DH2415) Infections. *, P < 0.05, as determined by two-tailed t tests of paired ratios for n = 3 experiments for each isolate. (D) RhlI-HA protein levels on LB medium with and without 20 mM citrate of an LasR+ acute corneal CI (550A) of the same multilocus sequence type as 388D and LasR+ chronic CF CI (DH2417) from which DH2415 evolved. ns, not significant as determined by two-tailed t tests of paired ratios for n = 3 experiments for each isolate. nd, not detected. (E) Representative image and quantification of replicates for the anti-HA antibody analysis of ∆lasR and ∆lasR clpX Tn::M strains carrying a plasmid expressing RhlI-HA or an empty vector (pRhlI-HA or pEV, respectively) grown in LB medium with or without 20 mM citrate. *, P < 0.05. as determined by two-tailed t tests of paired ratios for n = 3 replicates from 3 independent days.
To determine if RhlI protein levels were influenced by citrate, we utilized an arabinose-inducible rhlI-hemagglutinin (HA) construct to assess RhlI protein levels and stability in the absence and presence of citrate independent of RhlR transcriptional control. RhlI-HA was functional as swarming defects of the ∆rhlI mutant were complemented upon expression of RhlI-HA but not by the empty vector (Fig. 5B, inset). RhlI-HA protein levels were 3-fold higher in the ∆lasR strain upon citrate supplementation than in the controls (Fig. 5B). Consistent with the absence of an increase in rhlI promoter activity in WT strains (Fig. 5A), RhlI-HA protein levels were not higher with citrate in the ∆lasR complemented strain (∆lasR + lasR strain) (Fig. 5B). The differential responses to citrate were also observed in LasR− and LasR+ pairs of clinical isolates (CIs). LasR− CIs from acute (strain 388D) and chronic (strain DH2415) infections had RhlI-HA levels 1.4- and 1.7-fold higher, respectively, in the presence of citrate (Fig. 5C), whereas alterations in RhlI-HA protein levels in LasR+ CIs from acute (strain 550A) or chronic (strain DH2417) infections were not observed (Fig. 5D). Through this work, we successfully identified citrate as a molecule in coculture that specifically promoted RhlI protein levels in LasR− strains, but not in LasR+ strains, by posttranscriptional control. In an attempt to identify transporters involved in the ∆lasR strain response to citrate and/or other coculture metabolites, we deleted two organic acid transporters: dctA (61) and PA14_51300 (62) in the ∆lasR strain background. We found that the ∆lasR ∆dctA strain showed induction of pyocyanin when it was cocultured with the WT and that induction was dependent on RhlR (Fig. S7D). Similar results were obtained with the ∆lasR ∆PA14_51300 strain (Fig. S7E), suggesting that these transporters were not required for the interaction, perhaps due to redundant functions of other proteins or the involvement of other import mechanisms.
The temporal pattern of activation and the stimulation of RhlI-HA in the absence of RhlR control suggested that the RhlI protein may precede signal amplification via the QS transcriptional network. This would be consistent with a primary effect on posttranscriptional modulation of RhlI-mediated RhlR activity, as has been reported previously to occur through an RhlS small RNA (sRNA)-dependent mechanism (63); however, we found no apparent difference in RhlS expression levels in our RNA-seq reads in coculture. To begin to unravel the mechanisms by which citrate promoted RhlR/I-dependent signaling and RhlI stability in ∆lasR cells, we analyzed the role of two proteases previously found to target and degrade RhlI (i.e., Lon and ClpXP) (64). Given that knockouts of Lon protease have a less substantial rise in RhlR/I expression in ∆lasR strains than in the WT (65), we focused on the role of ClpXP in ∆lasR cells. We found that citrate induction of RhlI-HA protein levels in the ∆lasR strain relative to that in the LB control was dependent on functional ClpX protease (Fig. 5E). More specifically, when ClpX, a protease shown to degrade RhlI, is nonfunctional (i.e., ∆lasR clpX::TnM strain), RhlI-HA levels did not increase on LB plus citrate relative to that of the LB control, unlike the level in the ∆lasR strain comparator (Fig. 5E). Under LB culture conditions, RhlI-HA levels were 3.20- 2.1-fold higher in the ∆lasR clpX::TnM strain than in the ∆lasR strain, which mirrors the 3-fold induction observed for the ∆lasR strain on LB medium with or without citrate. Under citrate-supplemented conditions, no significant difference in RhlI-HA levels was observed for the ∆lasR clpX::TnM relative to that in the ∆lasR strain (fold change of 1.01 0.53). In other words, as previously noted for WT strains, ClpX may degrade RhlI in ∆lasR cells and play a role in the ∆lasR cell response to citrate. The distinct responses and mechanisms identified between LasR+ and LasR− strains under iron limitation and exposure to the low-iron-associated molecules, citrate and pyochelin, enabled increases in antagonistic factors beyond monoculture levels as an emergent property of P. aeruginosa intraspecies interactions.
DISCUSSION
In this study, we described an emergent outcome of coculturing LasR− and LasR+ strains of P. aeruginosa in which their interactions promoted the toxic exoproducts pyocyanin and rhamnolipids (Fig. 6 provides a model). We determined that, in coculture, the ∆lasR strain produces the siderophore pyochelin and that exogenous pyochelin induced citrate release more strongly in the WT than in ∆lasR strain. Citrate increased RhlI protein levels and induced RhlR-dependent activity only in ∆lasR cells and not WT cells (Fig. 6). Western blot analyses of RhlI-HA expressed from a regulated promoter led us to propose that the increase in RhlR-dependent signaling is due to decreased degradation of RhlI by ClpXP, a known negative regulator of RhlI (65, 66), or through other mechanisms of posttranscriptional regulation. The differences in siderophore production, citrate release, and RhlR/I-dependent activation between P. aeruginosa LasR+ and LasR− strains in coculture reflect the pronounced differences between strains that drive QS reactivation. Previous studies have shown that LasR− strain colony morphology and phenazine production change in the presence of other species such as Candida albicans (29) and Staphylococcus aureus (see Fig. 3B in reference 67), and future work will determine if pyochelin and citrate also participate in these interspecies interactions as many microbial interactions have been shown to be influenced by iron availability (68–71). Furthermore, the induction of RhlR activity that can occur in late-stationary-phase ∆lasR cultures (30, 72) may relate to changes in iron or TCA cycle intermediates. While we found that WT-produced autoinducers, including 3OC12HSL, C4HSL, and PQS, were not required for coculture stimulation, they clearly contributed to the enhanced RhlR-dependent activity, which is consistent with intercolony QS interactions demonstrated previously (73).
FIG 6.
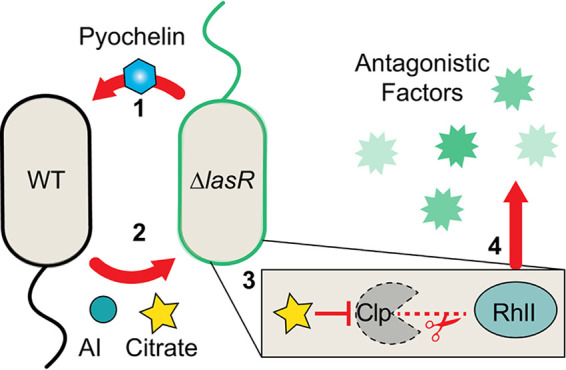
Model for wild-type and ∆lasR coculture interactions. ∆lasR strain-produced pyochelin promotes citrate release in the wild type (1). Citrate (and diffusible autoinducer) released by the wild type in coculture stimulates RhlR/I-dependent activity in a ∆lasR strain (2). Citrate stabilizes RhlI protein in ∆lasR cells potentially through a ClpXP protease-dependent mechanism (3), which ultimately promotes the production of antagonistic factors like pyocyanin toxin and rhamnolipid surfactant above monoculture levels (4).
The stimulatory relationship between LasR+ and LasR− strains was remarkably stable as it was observed when strains were mixed within single spot colonies (Fig. 1A) and when strains were separated by either filters (Fig. 3) or millimeter distances on an agar plate (Fig. 2B). The LasR−/LasR+ interactions occurred across distinct media (see Fig. S1A in the supplemental material), among genetically diverse LasR+ and LasR− clinical isolates (Fig. S1B), and over a wide range of relative proportions of each type (Fig. 1C). Of note, colonies inoculated at a 80:20 WT-to-∆lasR cell ratio had more zones with the lasR mutant-associated phenotypes described as sheen and lysis than colonies with a 20:80 WT-to-∆lasR cell ratio (Fig. 1C). At both ratios, ∆lasR cell numbers increased slightly relative to level of the wild type (Fig. 1D). We propose that the reduced appearance of sheen and lysis in mixed colonies inoculated with more ∆lasR cells reflects a requirement for a sufficient proportion of ∆lasR cells to initiate the WT-∆lasR cell interactions that activate RhlR and restore a more WT-like phenotype to LasR− cells. Furthermore, if RhlR signaling is not fully activated in ∆lasR cells, there may be regions of increased ∆lasR cell killing via WT-produced factors such as cyanide (74).
The consequences of this intraspecies interaction may explain the worse outcomes exhibited by patients in which LasR− strains are detected (13), but future studies that include genotypes, monoculture and coculture phenotypes, and longitudinal outcome data will be required. RhlR plays other important roles in host interactions (75) which may benefit P. aeruginosa LasR− strains. The observation that rhlR mutants are rare in natural isolates and that LasR− strains with active RhlR are virulent (25, 76) underscores the relevance of this mechanism and highlights the importance of understanding how microbial interactions influence RhlR activity.
As studies of inter- and intraspecies interactions progress, it is becoming increasingly clear that the environment can dictate the outcome of microbial interactions (77). In fact, even the importance of QS regulation for fitness depends on nutrient sources and conditions (78, 79). As ∆lasR cell-produced pyochelin was a key component of the interaction and as pyochelin production is repressed under conditions of excess iron, it was not surprising that iron supplementation suppressed the interaction (Fig. 3F and Fig. S3C and D) without significantly altering the final colony CFU count or strain ratios relative to those of the LB control (Fig. S4). Siderophore-mediated iron uptake is often required in vivo (34, 80, 81) due to iron sequestration by host proteins (82–85); thus, in vivo settings may support these interactions.
Interestingly, pyoverdine, the higher-affinity siderophore, was not required for the coculture response, mirroring findings that genes for biosynthesis of pyoverdine, but not pyochelin, are commonly disrupted in isolates from chronic CF patients (39–41). In the absence of pyoverdine (i.e., the ∆lasR ∆pvdA strain), we observed more pyocyanin in coculture with the WT than with the ∆lasR strain (Fig. 3D), and we speculate that this is due to increased pyochelin production by ∆pvdA cells, but future studies will be required to test this model. It was interesting to find that in WT/∆lasR coculture, heme-related proteins, hasAP, hasS, and hasD, were among the top eight most-upregulated genes by the ∆lasR strain because the presence of lasR mutants and heme content are both reported biomarkers of disease progression in CF patients (13, 86). Coculture-induced lasR mutant phenotypes may link these two correlative observations.
Citrate, a TCA intermediate released under iron limitation as a result of overflow metabolism (43, 44, 60, 87), can be used by P. aeruginosa and other microbes for iron acquisition due to its iron chelating properties (88). The increased siderophore production by ∆lasR cells in coculture likely reflects different metabolic strategies between genotypes. Ongoing work will investigate the mechanisms that drive differences in metabolism and iron requirements in order to determine how these differences shape microbial and host interactions. It is likely that Crc-mediated catabolite repression is involved in the response to citrate and the control of RhlI levels (64, 66). That a mechanism exists for the induction of RhlR-mediated QS in response to citrate secreted when iron is limiting dovetails with reports of increased expression of the P. aeruginosa QS regulon in low iron in LasR+ cells (89–91). This coordinate regulation may aid in iron acquisition as QS-controlled phenazines, such as pyocyanin, reduce poorly soluble Fe3+ to Fe2+ and facilitate its uptake via the Feo system (92). Additionally, rhamnolipids have been employed for iron remediation (93, 94), which suggests that their surfactant activity may increase P. aeruginosa substrate iron uptake in part through hydroxy-alkyl-quinolone-dependent mechanisms (95).
Given that anthranilate did not alter ∆lasR colony morphology or phenazine production, we did not further investigate anthranilate as a cross-fed metabolite involved in WT-∆lasR cell interactions. We speculate that the increased expression of anthranilate catabolism genes in coculture may be more reflective of increases in RhlR activity than increased exposure to anthranilate given reports highlighting RhlR activation of antABC and catABC anthranilate catabolism genes (59).
As the presence of heterogeneous genotypes within single-species populations becomes increasingly appreciated, it is important to understand how commonly encountered genotypes interact to influence population-level behavior. Other work shows that cocultures can influence the survival of other genotypes (96, 97). Here, we show that intergenotype interactions lead to increased RhlR-dependent signaling in LasR− strains. It is likely that a wide array of such interactions has yet to be uncovered.
MATERIALS AND METHODS
Strains and growth conditions.
Strains used in this study are listed in Table S2 in the supplemental material. Bacteria were maintained on LB (lysogeny broth) medium with 1.5% agar. Saccharomyces cerevisiae strains for cloning were maintained on yeast-peptone-dextrose (YPD) medium with 2% agar. With the exception of pyochelin complementation experiments, which were performed in 12-well dishes with a 2-ml total volume containing 50 μM pyochelin (or an equal-volume negative-control extract), colony biofilm assays were performed in 100-mm petri dishes with a 25-ml total volume. Where stated, a 20 mM concentration of the indicated metabolite was added to the medium (liquid or molten agar). Planktonic cultures were grown on roller drums at 37°C. Artificial sputum medium (ASM) was made as described previously (27).
Strains and plasmids used in this study. Download Table S2, DOCX file, 0.1 MB (59.2KB, docx) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Competition assays.
Competition assays were performed to determine the relative fitness of P. aeruginosa mutants. Strains to be competed were grown overnight and adjusted to an optical density at 600 nm (OD600) of 1. Competing strains were combined with the PA14 att::lacZ strain in a 1:1 ratio unless otherwise stated. Following a 15-s vortex, 5 μl of the combined suspension was spotted on LB agar. After 16 h, colony biofilms (and agar) were cored, placed in 1.5-ml tubes with 500 μl of LB, and agitated vigorously for 5 min using a Genie Disruptor (Zymo). This suspension was diluted, spread on LB plates supplemented with 150 μg/ml 5-bromo-4-chloro-3-indolyl-d-galactopyranoside (X-Gal) using glass beads, and incubated at 37°C until blue colonies were visible (∼24 h). The numbers of blue and white colonies per plate were counted, and the final proportions were recorded. Each competition was run in triplicate on 3 separate days.
Additional methods.
See Text S1 in the supplemental material for methods describing plasmid construction, pyocyanin quantification, colony proximity image analysis, swarming, RNA collection and processing, pyochelin extraction and quantification, citrate quantification, β-galactosidase quantification, Western blotting, and acyl-homoserine lactone activity assays.
Methods describing plasmid construction, pyocyanin quantification, colony proximity image analysis, swarming, RNA collection and processing, pyochelin extraction and quantification, citrate quantification, β-galactosidase quantification, Western blotting, and acyl-homoserine lactone activity assays. Download Text S1, DOCX file, 0.03 MB (27.7KB, docx) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data availability.
Data for RNA-seq analysis of P. aeruginosa ∆lasR grown on the ∆lasR or WT strain in coculture has been uploaded to the Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE149385.
ACKNOWLEDGMENTS
Research reported in this publication was supported by grants from the Cystic Fibrosis Foundation HOGAN19G0 and NIH/NIAID T32AI007519 (D.L.M.). Additional support came from NIGMS P20GM113132 through the Molecular Interactions and Imaging Core (MIIC), STANTO19R0 from the Cystic Fibrosis Foundation, and NIDDK P30-DK117469 (Dartmouth Cystic Fibrosis Research Center). RNA-seq was carried out at Dartmouth Medical School in the Genomics Shared Resource, which was established by equipment grants from the NIH and NSF and is supported in part by a Cancer Center Core Grant (P30CA023108) from the National Cancer Institute.
We also thank Georgia Doing for preliminary RNA-seq analysis, Pat Occhipinti for swapping the antibiotic marker on the rhlI-HA expression vector, and Carla Cugini for the ∆lasRclpX::TnM mutant.
Footnotes
This article is a direct contribution from Deborah A. Hogan, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Amanda G. Oglesby-Sherrouse, University of Maryland School of Pharmacy, and Ajai A. Dandekar, University of Washington.
Citation Mould DL, Botelho NJ, Hogan DA. 2020. Intraspecies signaling between common variants of Pseudomonas aeruginosa increases production of quorum-sensing-controlled virulence factors. mBio 11:e01865-20. https://doi.org/10.1128/mBio.01865-20.
REFERENCES
- 1.Demers EG, Biermann AR, Masonjones S, Crocker AW, Ashare A, Stajich JE, Hogan DA. 2018. Evolution of drug resistance in an antifungal-naive chronic Candida lusitaniae infection. Proc Natl Acad Sci U S A 115:12040–12045. doi: 10.1073/pnas.1807698115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boles BR, Thoendel M, Singh PK. 2004. Self-generated diversity produces "insurance effects" in biofilm communities. Proc Natl Acad Sci U S A 101:16630–16635. doi: 10.1073/pnas.0407460101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao S, Lieberman TD, Poyet M, Kauffman KM, Gibbons SM, Groussin M, Xavier RJ, Alm EJ. 2019. Adaptive evolution within gut microbiomes of healthy people. Cell Host Microbe 25:656–667.e8. doi: 10.1016/j.chom.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azimi S, Roberts AEL, Peng S, Weitz JS, McNally A, Brown SP, Diggle SP. 2020. Allelic polymorphism shapes community function in evolving Pseudomonas aeruginosa populations. ISME J 14:1929–1942. doi: 10.1038/s41396-020-0652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos LD, Meinardi S, Blake D, Whiteson K. 2016. Bacteria in the airways of patients with cystic fibrosis are genetically capable of producing VOCs in breath. J Breath Res 10:047103. doi: 10.1088/1752-7163/10/4/047103. [DOI] [PubMed] [Google Scholar]
- 6.Jorgensen KM, Wassermann T, Johansen HK, Christiansen LE, Molin S, Hoiby N, Ciofu O. 2015. Diversity of metabolic profiles of cystic fibrosis Pseudomonas aeruginosa during the early stages of lung infection. Microbiology 161:1447–1462. doi: 10.1099/mic.0.000093. [DOI] [PubMed] [Google Scholar]
- 7.Markussen T, Marvig RL, Gómez-Lozano M, Aanæs K, Burleigh AE, Høiby N, Johansen HK, Molin S, Jelsbak L. 2014. Environmental heterogeneity drives within-host diversification and evolution of Pseudomonas aeruginosa. mBio 5:e01592-14. doi: 10.1128/mBio.01592-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilder CN, Allada G, Schuster M. 2009. Instantaneous within-patient diversity of Pseudomonas aeruginosa quorum sensing populations from cystic fibrosis lung infections. Infect Immun 77:5631–5639. doi: 10.1128/IAI.00755-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winstanley C, O'Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo TE, Duong J, Jervis NM, Rabin HR, Parkins MD, Storey DG. 2016. Virulence adaptations of Pseudomonas aeruginosa isolated from patients with non-cystic fibrosis bronchiectasis. Microbiology 162:2126–2135. doi: 10.1099/mic.0.000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workentine ML, Sibley CD, Glezerson B, Purighalla S, Norgaard-Gron JC, Parkins MD, Rabin HR, Surette MG. 2013. Phenotypic heterogeneity of Pseudomonas aeruginosa populations in a cystic fibrosis patient. PLoS One 8:e60225. doi: 10.1371/journal.pone.0060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond JH, Hebert WP, Naimie A, Ray K, Van Gelder RD, DiGiandomenico A, Lalitha P, Srinivasan M, Acharya NR, Lietman T, Hogan DA, Zegans ME. 2016. Environmentally endemic Pseudomonas aeruginosa strains with mutations in lasR are associated with increased disease severity in corneal ulcers. mSphere 1:e00140-16. doi: 10.1128/mSphere.00140-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman LR, Kulasekara HD, Emerson J, Houston LS, Burns JL, Ramsey BW, Miller SI. 2009. Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros 8:66–70. doi: 10.1016/j.jcf.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrol S, Olliver A, Pier GB, Andremont A, Ruimy R. 2003. Transcription of quorum-sensing system genes in clinical and environmental isolates of Pseudomonas aeruginosa. J Bacteriol 185:7222–7230. doi: 10.1128/JB.185.24.7222-7230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denervaud V, TuQuoc P, Blanc D, Favre-Bonte S, Krishnapillai V, Reimmann C, Haas D, van Delden C. 2004. Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J Clin Microbiol 42:554–562. doi: 10.1128/jcm.42.2.554-562.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Zhang L. 2015. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6:26–41. doi: 10.1007/s13238-014-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanthakumar K, Taylor G, Tsang KW, Cundell DR, Rutman A, Smith S, Jeffery PK, Cole PJ, Wilson R. 1993. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect Immun 61:2848–2853. doi: 10.1128/IAI.61.7.2848-2853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, Roberts D, Cole P. 1987. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest 79:221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson R, Roberts D, Cole P. 1985. Effect of bacterial products on human ciliary function in vitro. Thorax 40:125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blumer C, Haas D. 2000. Iron regulation of the hcnABC genes encoding hydrogen cyanide synthase depends on the anaerobic regulator ANR rather than on the global activator GacA in Pseudomonas fluorescens CHA0. Microbiology 146:2417–2424. doi: 10.1099/00221287-146-10-2417. [DOI] [PubMed] [Google Scholar]
- 22.Howe J, Bauer J, Andrä J, Schromm AB, Ernst M, Rössle M, Zähringer U, Rademann J, Brandenburg K. 2006. Biophysical characterization of synthetic rhamnolipids. FEBS J 273:5101–5112. doi: 10.1111/j.1742-4658.2006.05507.x. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz A, Teruel JA, Espuny MJ, Marqués A, Manresa Á, Aranda FJ. 2006. Effects of dirhamnolipid on the structural properties of phosphatidylcholine membranes. Int J Pharm 325:99–107. doi: 10.1016/j.ijpharm.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Moussa Z, Chebl M, Patra D. 2017. Interaction of curcumin with 1,2-dioctadecanoyl-sn-glycero-3-phosphocholine liposomes: intercalation of rhamnolipids enhances membrane fluidity, permeability and stability of drug molecule. Colloids Surf B Biointerfaces 149:30–37. doi: 10.1016/j.colsurfb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Feltner JB, Wolter DJ, Pope CE, Groleau MC, Smalley NE, Greenberg EP, Mayer-Hamblett N, Burns J, Deziel E, Hoffman LR, Dandekar AA. 2016. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa. mBio 7:e01513-16. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Argenio D, Wu M, Hoffman L, Kulasekara H, Déziel E, Smith E, Nguyen H, Ernst R, Larson Freeman T, Spencer D, Brittnacher M, Hayden H, Selgrade S, Klausen M, Goodlett D, Burns J, Ramsey B, Miller S. 2007. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol 64:512–533. doi: 10.1111/j.1365-2958.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clay ME, Hammond JH, Zhong F, Chen X, Kowalski CH, Lee AJ, Porter MS, Hampton TH, Greene CS, Pletneva EV, Hogan DA. 2020. Pseudomonas aeruginosa lasR mutant fitness in microoxia is supported by an Anr-regulated oxygen-binding hemerythrin. Proc Natl Acad Sci U S A 117:3167–3173. doi: 10.1073/pnas.1917576117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basta DW, Bergkessel M, Newman DK. 2017. Identification of fitness determinants during energy-limited growth arrest in Pseudomonas aeruginosa. mBio 8:e01170-17. doi: 10.1128/mBio.01170-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cugini C, Morales DK, Hogan DA. 2010. Candida albicans-produced farnesol stimulates Pseudomonas quinolone signal production in LasR-defective Pseudomonas aeruginosa strains. Microbiology 156:3096–3107. doi: 10.1099/mic.0.037911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabeen MT. 2014. Stationary phase-specific virulence factor overproduction by a lasR mutant of Pseudomonas aeruginosa. PLoS One 9:e88743. doi: 10.1371/journal.pone.0088743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Delden C, Pesci EC, Pearson JP, Iglewski BH. 1998. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect Immun 66:4499–4502. doi: 10.1128/.66.9.4499-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ankenbauer RG, Toyokuni T, Staley A, Rinehart KL, Cox CD. 1988. Synthesis and biological activity of pyochelin, a siderophore of Pseudomonas aeruginosa. J Bacteriol 170:5344–5351. doi: 10.1128/jb.170.11.5344-5351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandel J, Humbert N, Elhabiri M, Schalk IJ, Mislin GL, Albrecht-Gary AM. 2012. Pyochelin, a siderophore of Pseudomonas aeruginosa: physicochemical characterization of the iron(III), copper(II) and zinc(II) complexes. Dalton Trans 41:2820–2834. doi: 10.1039/c1dt11804h. [DOI] [PubMed] [Google Scholar]
- 34.Gi M, Lee KM, Kim SC, Yoon JH, Yoon SS, Choi JY. 2015. A novel siderophore system is essential for the growth of Pseudomonas aeruginosa in airway mucus. Sci Rep 5:14644. doi: 10.1038/srep14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cox CD, Adams P. 1985. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun 48:130–138. doi: 10.1128/IAI.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhakta MN, Wilks A. 2006. The mechanism of heme transfer from the cytoplasmic heme binding protein PhuS to the δ-regioselective heme oxygenase of Pseudomonas aeruginosa. Biochemistry 45:11642–11649. doi: 10.1021/bi060980l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wegele R, Tasler R, Zeng Y, Rivera M, Frankenberg-Dinkel N. 2004. The heme oxygenase(s)-phytochrome system of Pseudomonas aeruginosa. J Biol Chem 279:45791–45802. doi: 10.1074/jbc.M408303200. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Lu F, Latham C, Zander DS, Visner GA. 2004. Heme oxygenase-1 expression in human lungs with cystic fibrosis and cytoprotective effects against Pseudomonas aeruginosa in vitro. Am J Respir Crit Care Med 170:633–640. doi: 10.1164/rccm.200311-1607OC. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen AT, O'Neill MJ, Watts AM, Robson CL, Lamont IL, Wilks A, Oglesby-Sherrouse AG. 2014. Adaptation of iron homeostasis pathways by a Pseudomonas aeruginosa pyoverdine mutant in the cystic fibrosis lung. J Bacteriol 196:2265–2276. doi: 10.1128/JB.01491-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marvig RL, Damkiær S, Khademi SMH, Markussen TM, Molin S, Jelsbak L. 2014. Within-host evolution of Pseudomonas aeruginosa reveals adaptation toward iron acquisition from hemoglobin. mBio 5:e00966-14. doi: 10.1128/mBio.00966-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen SB, Marvig RL, Molin S, Krogh Johansen H, Griffin AS. 2015. Long-term social dynamics drive loss of function in pathogenic bacteria. Proc Natl Acad Sci U S A 112:10756–10761. doi: 10.1073/pnas.1508324112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oexle H, Gnaiger E, Weiss G. 1999. Iron-dependent changes in cellular energy metabolism: influence on citric acid cycle and oxidative phosphorylation. Biochim Biophys Acta 1413:99–107. doi: 10.1016/s0005-2728(99)00088-2. [DOI] [PubMed] [Google Scholar]
- 43.Carlson RP, Beck AE, Phalak P, Fields MW, Gedeon T, Hanley L, Harcombe WR, Henson MA, Heys JJ. 2018. Competitive resource allocation to metabolic pathways contributes to overflow metabolisms and emergent properties in cross-feeding microbial consortia. Biochem Soc Trans 46:269–284. doi: 10.1042/BST20170242. [DOI] [PubMed] [Google Scholar]
- 44.Sasnow SS, Wei H, Aristilde L. 2016. Bypasses in intracellular glucose metabolism in iron-limited Pseudomonas putida. Microbiologyopen 5:3–20. doi: 10.1002/mbo3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammond JH, Dolben EF, Smith TJ, Bhuju S, Hogan DA. 2015. Links between Anr and quorum sensing in Pseudomonas aeruginosa biofilms. J Bacteriol 197:2810–2820. doi: 10.1128/JB.00182-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. 2013. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A 110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lau GW, Hassett DJ, Ran H, Kong F. 2004. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol Med 10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Whiteley M, Lee KM, Greenberg EP. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 96:13904–13909. doi: 10.1073/pnas.96.24.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brint JM, Ohman DE. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol 177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahme LG, Tan MW, Le L, Wong SM, Tompkins RG, Calderwood SB, Ausubel FM. 1997. Use of model plant hosts to identify Pseudomonas aeruginosa virulence factors. Proc Natl Acad Sci U S A 94:13245–13250. doi: 10.1073/pnas.94.24.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Köhler T, Curty LK, Barja F, van Delden C, Pechère JC. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. 2019. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47:D419–D426. doi: 10.1093/nar/gky1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Louden BC, Haarmann D, Lynne AM. 2011. Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12:51–53. doi: 10.1128/jmbe.v12i1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calfee MW, Coleman JP, Pesci EC. 2001. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 98:11633–11637. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrow JM 3rd, Pesci EC. 2007. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J Bacteriol 189:3425–3433. doi: 10.1128/JB.00209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.The UniProt Consortium. 2018. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FS. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res 44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesuisse E, Simon M, Klein R, Labbe P. 1992. Excretion of anthranilate and 3-hydroxyanthranilate by Saccharomyces cerevisiae: relationship to iron metabolism. J Gen Microbiol 138:85–89. doi: 10.1099/00221287-138-1-85. [DOI] [PubMed] [Google Scholar]
- 59.Choi Y, Park HY, Park SJ, Park SJ, Kim SK, Ha C, Im SJ, Lee JH. 2011. Growth phase-differential quorum sensing regulation of anthranilate metabolism in Pseudomonas aeruginosa. Mol Cells 32:57–65. doi: 10.1007/s10059-011-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odoni DI, van Gaal MP, Schonewille T, Tamayo-Ramos JA, Martins dos Santos VAP, Suarez-Diez M, Schaap PJ. 2017. Aspergillus niger secretes citrate to increase iron Bioavailability. Front Microbiol 8:1424. doi: 10.3389/fmicb.2017.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valentini M, Storelli N, Lapouge K. 2011. Identification of C(4)-dicarboxylate transport systems in Pseudomonas aeruginosa PAO1. J Bacteriol 193:4307–4316. doi: 10.1128/JB.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filiatrault MJ, Tombline G, Wagner VE, Van Alst N, Rumbaugh K, Sokol P, Schwingel J, Iglewski BH. 2013. Pseudomonas aeruginosa PA1006, which plays a role in molybdenum homeostasis, is required for nitrate utilization, biofilm formation, and virulence. PLoS One 8:e55594. doi: 10.1371/journal.pone.0055594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomason MK, Voichek M, Dar D, Addis V, Fitzgerald D, Gottesman S, Sorek R, Greenberg EP. 2019. A rhlI 5′ UTR-derived sRNA regulates RhlR-dependent quorum sensing in Pseudomonas aeruginosa. mBio 10:e02253-19. doi: 10.1128/mBio.02253-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang N, Ding S, Chen F, Zhang X, Xia Y, Di H, Cao Q, Deng X, Wu M, Wong CC, Tian XX, Yang CG, Zhao J, Lan L. 2015. The Crc protein participates in down-regulation of the Lon gene to promote rhamnolipid production and Rhl quorum sensing in Pseudomonas aeruginosa. Mol Microbiol 96:526–547. doi: 10.1111/mmi.12954. [DOI] [PubMed] [Google Scholar]
- 65.Takaya A, Tabuchi F, Tsuchiya H, Isogai E, Yamamoto T. 2008. Negative regulation of quorum-sensing systems in Pseudomonas aeruginosa by ATP-dependent Lon protease. J Bacteriol 190:4181–4188. doi: 10.1128/JB.01873-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang N, Lan L. 2016. Pseudomonas aeruginosa Lon and ClpXP proteases: roles in linking carbon catabolite repression system with quorum-sensing system. Curr Genet 62:1–6. doi: 10.1007/s00294-015-0499-5. [DOI] [PubMed] [Google Scholar]
- 67.Hoffman LR, Richardson AR, Houston LS, Kulasekara HD, Martens-Habbena W, Klausen M, Burns JL, Stahl DA, Hassett DJ, Fang FC, Miller SI. 2010. Nutrient availability as a mechanism for selection of antibiotic tolerant Pseudomonas aeruginosa within the CF airway. PLoS Pathog 6:e1000712. doi: 10.1371/journal.ppat.1000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison F, Paul J, Massey RC, Buckling A. 2008. Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa. ISME J 2:49–55. doi: 10.1038/ismej.2007.96. [DOI] [PubMed] [Google Scholar]
- 69.Lopez-Medina E, Fan D, Coughlin LA, Ho EX, Lamont IL, Reimmann C, Hooper LV, Koh AY. 2015. Candida albicans inhibits Pseudomonas aeruginosa virulence through suppression of pyochelin and pyoverdine biosynthesis. PLoS Pathog 11:e1005129. doi: 10.1371/journal.ppat.1005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver VB, Kolter R. 2004. Burkholderia spp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol 186:2376–2384. doi: 10.1128/jb.186.8.2376-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott JE, Li K, Filkins LM, Zhu B, Kuchma SL, Schwartzman JD, O’Toole GA. 2019. Pseudomonas aeruginosa can inhibit growth of Streptococcal species via siderophore production. J Bacteriol 201:e00014-19. doi: 10.1128/JB.00014-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dekimpe V, Deziel E. 2009. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155:712–723. doi: 10.1099/mic.0.022764-0. [DOI] [PubMed] [Google Scholar]
- 73.Darch SE, Simoska O, Fitzpatrick M, Barraza JP, Stevenson KJ, Bonnecaze RT, Shear JB, Whiteley M. 2018. Spatial determinants of quorum signaling in a Pseudomonas aeruginosa infection model. Proc Natl Acad Sci U S A 115:4779–4784. doi: 10.1073/pnas.1719317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M, Schaefer AL, Dandekar AA, Greenberg EP. 2015. Quorum sensing and policing of Pseudomonas aeruginosa social cheaters. Proc Natl Acad Sci U S A 112:2187–2191. doi: 10.1073/pnas.1500704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haller S, Franchet A, Hakkim A, Chen J, Drenkard E, Yu S, Schirmeier S, Li Z, Martins N, Ausubel FM, Liegeois S, Ferrandon D. 2018. Quorum-sensing regulator RhlR but not its autoinducer RhlI enables Pseudomonas to evade opsonization. EMBO Rep 19:e44880. doi: 10.15252/embr.201744880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cruz RL, Asfahl KL, Van den Bossche S, Coenye T, Crabbé A, Dandekar AA. 2020. RhlR-regulated acyl-homoserine lactone quorum sensing in a cystic fibrosis isolate of Pseudomonas aeruginosa. mBio 11:e00532-20. doi: 10.1128/mBio.00532-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doing G, Koeppen K, Occipinti P, Hogan D. 2020. Conditional antagonism in co-cultures of Pseudomonas aeruginosa and Candida albicans: an intersection of ethanol and phosphate signaling distilled from dual-seq transcriptomics. bioRxiv doi: 10.1101/2020.04.20.050765v2. [DOI] [PMC free article] [PubMed]
- 78.Dandekar AA, Chugani S, Greenberg EP. 2012. Bacterial quorum sensing and metabolic incentives to cooperate. Science 338:264–266. doi: 10.1126/science.1227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mund A, Diggle SP, Harrison F. 2017. The fitness of Pseudomonas aeruginosa quorum sensing signal cheats is influenced by the diffusivity of the environment. mBio 8:e00816-17. doi: 10.1128/mBio.00816-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reid DW, Anderson GJ, Lamont IL. 2009. Role of lung iron in determining the bacterial and host struggle in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 297:L795–L802. doi: 10.1152/ajplung.00132.2009. [DOI] [PubMed] [Google Scholar]
- 81.Lamont IL, Konings AF, Reid DW. 2009. Iron acquisition by Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Biometals 22:53–60. doi: 10.1007/s10534-008-9197-9. [DOI] [PubMed] [Google Scholar]
- 82.Britigan BE, Rasmussen GT, Olakanmi O, Cox CD. 2000. Iron acquisition from Pseudomonas aeruginosa siderophores by human phagocytes: an additional mechanism of host defense through iron sequestration? Infect Immun 68:1271–1275. doi: 10.1128/iai.68.3.1271-1275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakashige TG, Zhang B, Krebs C, Nolan EM. 2015. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol 11:765–771. doi: 10.1038/nchembio.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ward PP, Conneely OM. 2004. Lactoferrin: role in iron homeostasis and host defense against microbial infection. Biometals 17:203–208. doi: 10.1023/b:biom.0000027693.60932.26. [DOI] [PubMed] [Google Scholar]
- 85.Michels KR, Zhang Z, Bettina AM, Cagnina RE, Stefanova D, Burdick MD, Vaulont S, Nemeth E, Ganz T, Mehrad B. 2017. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2:e92002. doi: 10.1172/jci.insight.92002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glasser NR, Hunter RC, Liou TG, Newman DK, Mountain West CF Consortium Investigators. 2019. Refinement of metabolite detection in cystic fibrosis sputum reveals heme correlates with lung function decline. PLoS One 14:e0226578. doi: 10.1371/journal.pone.0226578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Folsom JP, Parker AE, Carlson RP. 2014. Physiological and proteomic analysis of Escherichia coli iron-limited chemostat growth. J Bacteriol 196:2748–2761. doi: 10.1128/JB.01606-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marshall B, Stintzi A, Gilmour C, Meyer JM, Poole K. 2009. Citrate-mediated iron uptake in Pseudomonas aeruginosa: involvement of the citrate-inducible FecA receptor and the FeoB ferrous iron transporter. Microbiology 155:305–315. doi: 10.1099/mic.0.023531-0. [DOI] [PubMed] [Google Scholar]
- 89.Oglesby AG, Farrow JM 3rd, Lee JH, Tomaras AP, Greenberg EP, Pesci EC, Vasil ML. 2008. The influence of iron on Pseudomonas aeruginosa physiology: a regulatory link between iron and quorum sensing. J Biol Chem 283:15558–15567. doi: 10.1074/jbc.M707840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim EJ, Sabra W, Zeng AP. 2003. Iron deficiency leads to inhibition of oxygen transfer and enhanced formation of virulence factors in cultures of Pseudomonas aeruginosa PAO1. Microbiology 149:2627–2634. doi: 10.1099/mic.0.26276-0. [DOI] [PubMed] [Google Scholar]
- 91.Kim EJ, Wang W, Deckwer WD, Zeng AP. 2005. Expression of the quorum-sensing regulatory protein LasR is strongly affected by iron and oxygen concentrations in cultures of Pseudomonas aeruginosa irrespective of cell density. Microbiology 151:1127–1138. doi: 10.1099/mic.0.27566-0. [DOI] [PubMed] [Google Scholar]
- 92.Cox CD. 1986. Role of pyocyanin in the acquisition of iron from transferrin. Infect Immun 52:263–270. doi: 10.1128/IAI.52.1.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Akintunde TA, Abioye P, Oyeleke SB, Boboye BE, Ijah UJ. 2015. Remediation of iron using rhamnolipid-surfactant produced by Pseudomonas aeruginosa. Res J Environ Sci 9:169–177. doi: 10.3923/rjes.2015.169.177. [DOI] [Google Scholar]
- 94.Wang S, Mulligan CN. 2009. Rhamnolipid biosurfactant-enhanced soil flushing for the removal of arsenic and heavy metals from mine tailings. Process Biochem 44:296–301. doi: 10.1016/j.procbio.2008.11.006. [DOI] [Google Scholar]
- 95.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, Lamont IL, Kong X, Hider RC, Cornelis P, Camara M, Williams P. 2007. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol 14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 96.Malhotra S, Limoli DH, English AE, Parsek MR, Wozniak DJ. 2018. Mixed communities of mucoid and nonmucoid Pseudomonas aeruginosa exhibit enhanced resistance to host antimicrobials. mBio 9:e00275-18. doi: 10.1128/mBio.00275-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oluyombo O, Penfold CN, Diggle SP. 2019. Competition in biofilms between Cystic Fibrosis isolates of Pseudomonas aeruginosa is shaped by R-Pyocins. mBio 10:e01828-18. doi: 10.1128/mBio.01828-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased pyocyanin production of LasR+ and LasR− strain cocultures relative to either strain alone is stable across media and strains. (A) Representative images from PA14 wild type (WT) and ∆lasR strain mono- and coculture pyocyanin production on rich medium (LB and artificial sputum medium [ASM]), and minimal (M63 medium, pH 6.8, 0.2% glucose; no Casamino Acids [CAA]) and defined phosphate buffered medium (M63 medium, pH 6.8, 0.2% glucose + 0.2% CAA). (B) Pyocyanin production of mono- and cocultures of strain PA14 WT and the ∆lasR strain and clonally derived clinical isolates DH2417 (LasR+) and DH2415 (LasR−). (C) Pyocyanin quantification of LasR+ and LasR− strains grown in mono- and coculture on 96-well LB agar plugs for 16 h. Strain PA14 WT and the ∆lasR strain and previously characterized clinical isolate pairs DH2417 (LasR+) and DH2415 (LasR−) and DH1133 (LasR+) and DH1132 (LasR−), from two distinct CF patients, are also shown. Data are from two independent experiments with four biological replicates each. (D) Number of CFU in 16-h colony biofilms grown on LB medium. Each strain set is presented relative to the level of the WT or the LasR+ strain. PA14 WT and ∆lasR strain mono- and coculture data are the average from four independent experiments with at least three biological replicates. ns, not significant; **, P < 0.005; ***, P < 0.0005, as determined by analysis of variance with Tukey multiple hypotheses correction for clinical isolate CFU counts. Download FIG S1, PDF file, 0.3 MB (280.8KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
∆lasR strain shows Anr-independent increases in pyocyanin in coculture with LasR+ P. aeruginosa. Representative images of WT, ∆anr, and ∆lasR ∆anr strains in mono- and coculture colony biofilms on LB medium after 24 h. Download FIG S2, PDF file, 0.1 MB (152.3KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The ∆lasR strain produced RhlR/I-dependent acyl-homoserine lactone (AHL) autoinducers in coculture with the ∆lasI ∆rhlI strain, and this was repressed by iron supplementation. (A) Schematic of experimental setup and quantification of AHL activity for colony biofilms grown on an AHL-sensing reporter strain (i.e., ∆lasI ∆rhlI strain with a lacZ promoter fusion to an AHL-responsive gene). (B) AHL activity for the WT, AHL-deficient ∆lasI ∆rhlI control, ∆lasR, ∆lasR ∆rhlR, and ∆lasR ∆rhlI strains grown on an AHL-sensing reporter strain in X-Gal-containing medium. (C) Representative image of RhlI-dependent activity (i.e., C4HSL) for the ∆lasR strain grown on an AHL-sensing reporter strain across an iron gradient under stable X-Gal-containing conditions. (D) Setup and quantification as described for panel a for the ∆lasR strain on LB medium (−) and on LB medium plus 10 μM FeSO4 (+) including a C4HSL-deficient derivative ∆lasR ∆rhlI strain. Data points are from ≥ 3 different days. *, P < 0.05; ****, P < 0.0001, as determined by ordinary one-way analysis of variance with Dunnett’s multiple-comparison test to the control ∆lasR strain. Download FIG S3, PDF file, 2.4 MB (2.5MB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The ∆lasR strain showed enhanced production of RhlR-regulated rhamnolipid surfactant in coculture with the LasR+ strain. RhlR-regulated swarming motility on soft agar of the WT, rhamnolipid surfactant biosynthesis mutant (∆rhlA strain), ∆lasR, and ∆lasR ∆rhlR strains in mono- and coculture after 36 h. Download FIG S4, PDF file, 0.3 MB (295KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Differentially expressed genes in the ∆lasR strain grown with the wild type versus itself in filter-separated cocultures. PA14 number or gene name, if available, is listed in descending order by log2(∆lasR cells grown on WT/∆lasR cells grown on ∆lasR cells). Differential expression is defined as |log2(∆lasR cells grown on WT/∆lasR cells grown on ∆lasR cells)| >1 with a P value of <0.05. Download Table S1, XLSX file, 0.02 MB (21.7KB, xlsx) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Fitness of the ∆lasR strain lacking one or both major siderophore biosynthesis pathways. Final proportion of untagged colony forming units was quantified after a 16-h competition with the WT att::lacZ strain. The dotted line indicates the 0.5 initial proportion (Pi). Final proportions (Pf) for the ∆lasR wild-type (WT) strains and the ∆lasR strain complemented with the lasR gene at the native locus (∆lasR strain) on LB medium (white background), for the ∆lasR strain on LB medium supplemented with 10 μM FeSO4 (grey background), and for siderophore-deficient ∆lasR mutant derivatives. a versus b, P < 0.0006; a versus c, P < 0.0001, as determined by one-way analysis of variance for comparison to the ∆lasR strain on LB medium with a Sidak multiple-hypotheses correction for n ≥ 3 experiments. Download FIG S5, PDF file, 0.1 MB (78.8KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Pyochelin-containing extracts are biologically active. (A) Absorbance spectra (230 to 600 nm) of extracts from supernatants of the pyochelin-producing ∆pvdA strain (solid line) and the pyochelin-deficient ∆pvdA ∆pchE strain (dotted line) in a 50/50 MeOH/distilled H2O solution. Grey vertical lines indicate reported peaks for purified, iron-free pyochelin at 248 and 313 nm. (B) Indicated extracts spotted on chrome azurol S (CAS) agar with EDTA metal chelator as a positive control wherein a change from blue to yellow indicates iron-chelating capacity. CAS activity for the extracts from ∆pvdA (+PCH) and ∆pvdA ∆pchE (NEG) strains are shown. Download FIG S6, PDF file, 0.1 MB (106.1KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Anthranilate (AA) supplementation and loss of potential di- and tricarboxylic acid transporters dctA and PA14_51300 did not alter pyocyanin production of the ∆lasR strain. (A) Volcano plot indicating anthranilate metabolism gene expression (orange) in the ∆lasR strain grown in coculture with the WT. (B) Colony morphology and pyocyanin production are not different upon anthranilate supplementation across a gradient of concentrations. (C) RhlR-dependent pyocyanin production of the ∆lasR mutant and the anthranilate synthase mutant (∆phnAB strain) in mono- and cocultures on LB medium after 18 h. (D) The major succinate, fumarate, and malate transporter dctA was dispensable in the ∆lasR strain background for RhlR-dependent pyocyanin production in coculture with the WT. (E) The broad TCA cycle intermediate transporter PA14_51300 was not required in the ∆lasR strain for pyocyanin production in coculture with the WT. Download FIG S7, PDF file, 0.5 MB (542.7KB, pdf) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download Table S2, DOCX file, 0.1 MB (59.2KB, docx) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Methods describing plasmid construction, pyocyanin quantification, colony proximity image analysis, swarming, RNA collection and processing, pyochelin extraction and quantification, citrate quantification, β-galactosidase quantification, Western blotting, and acyl-homoserine lactone activity assays. Download Text S1, DOCX file, 0.03 MB (27.7KB, docx) .
Copyright © 2020 Mould et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Data for RNA-seq analysis of P. aeruginosa ∆lasR grown on the ∆lasR or WT strain in coculture has been uploaded to the Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/) under accession number GSE149385.