Abstract
Inflammatory breast cancer (IBC) appears to have clinical manifestations and a biological behavior different from those of locally advanced breast cancer (LABC), which may be important in studies on pathogenesis and treatment. The laboratory characteristics of IBC identified in this study indicate that the current case definition of IBC is too restrictive.
Background:
Inflammatory breast cancer (IBC) is an aggressive form of breast cancer that on presentation resembles locally advanced breast cancer (LABC). This study identified molecular features of IBC and LABC to investigate pathogenesis.
Materials and Methods:
This study involved 100 IBC cases identified in a national IBC registry and 107 non-IBC LABC cases from the National Cancer Institute’s Cooperative Breast Cancer Tissue Resource (CBCTR). Vascular endothelial growth factor D (VEGF-D) and E-cadherin levels and lymphatic vessel density (LVD) measured by podoplanin staining were examined by immunohistochemistry on paraffin-embedded tumor specimens. Intralymphatic tumor emboli (ILTE) were assessed in IBC and non-IBC tumors. IBC cases diagnosed by clinicians but not meeting the case definitions of the American Joint Committee on Cancer (AJCC) or the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (NCI)(designated atypical IBC) were compared with AJCC- and/or SEER-defined cases (designated classic IBC).
Results:
E-cadherin levels were significantly higher in classic IBC cases compared with non-IBC cases (P = .031), whereas compared with classic IBC, patients with non-IBC LABC had significantly higher LVD (P = .0017) and VEGF-D levels (P < .0001). ILTE was marginally greater in classic IBC than in non-IBC (P = .046). The profile of laboratory values in atypical IBC cases more closely resembled those fitting classic IBC than LABC.
Conclusion:
E-cadherin levels, LVD, VEGF-D expression, and to a lesser extent, ILTE differed between classic IBC and non-IBC LABC. The similarity of laboratory results between atypical IBC and classic IBC vs. LABC suggests the need for broadening both the AJCC and SEER case definitions for this disease.
Keywords: Biomarkers, E-cadherin, Intralymphatic tumor emboli, Lymphovascular density, VEGF-D
Introduction
Inflammatory breast cancer (IBC), considered by some clinicians to be a subset of locally advanced breast cancer (LABC), is the most aggressive type of breast cancer. It has a rapid disease onset with inflammatory breast signs and is rapidly fatal in the absence of treatment.1-4 Histopathologically, it is characterized by tumor emboli in the dermal lymphatic system.5
The diagnosis of IBC is based on clinical presentation and histopathologic features. The American Joint Committee on Cancer (AJCC) defines IBC as breast cancer with a rapid onset of pain, erythema, and a peau d’orange appearance involving the majority of thebreast.6 Before 2007, the Surveillance, Epidemiology, andEnd Results (SEER) database coded a breast cancer as IBC ifthere was a pathologic confirmation of dermal lymphatic invasion (DLI) with or without the typical clinical presentations (SEER program Coding and Staging Manual 2007; http://seer.cancer.gov/tools/codingmanuals). More recently, SEER has accepted the diagnosis of IBC if more than three quarters of the breast is involved in the absence of DLI.3 Survival of patients with IBC is poor compared with patients with non-IBC LABC.3 A review of data from SEER indicates that survival in IBC is improving concomitant with the introduction of newer chemotherapeutic options such as anthracyclines, taxanes, and trastuzumab. A deeper understanding of the biological features of IBC is required for identification of therapeutic targets to improve patient survival.3
Increased angiogenesis and lymphangiogenesis have been found in IBC. Higher intratumor CD31+ microvessel density, endothelial proliferation, and mRNA levels of angiogenic genes were reported in IBC compared with non-IBC LABC.7-11 Agents targeting the angiogenesis pathway, such as bevacizumab and sunitinib, caused reduced tumor blood flow and increased tumor apoptosis in the IBC tumors.12,13 Evaluation of lymphangiogenesis in IBC revealed increased mRNA levels of lymphangiogenic genes and higher lymphatic endothelial proliferation compared with non-IBC.10,14,15 However these studies were limited because of the small number of cases. It is not clear if increased lymphangiogenesis is unique to IBC and if lymphangiogenesis is associated with dermal lymphatic involvement in IBC.
One intriguing observation in IBC is the paradoxical increase of E-cadherin expression by IBC tumor cells.11,16 In a human IBC xenograft model, overexpression of the E-cadherin/α,β-catenin axis and underexpression of sialyl Lewis carbohydrate– binding epitopes were found in the IBC spheroids.17 It has been postulated that increased E-cadherin on the tumor cell surface aids the formation of tumor emboli. The potential link of E-cadherin expression with lymphatic tumor emboli has also been observed in non-IBC tumors.18
To further delineate the differences between IBC and non-IBC LABC, we examined the expression levels of E-cadherin using immunohistochemical staining of archived IBC and non-IBC tumors, specifically focusing on the lymphatic vascular structures.
We also addressed the issue of case definition of IBC, which has not been uniform between major institutions, including AJCC6 and NCI1,3 and has been the subject of a number of publications.19-23 We used these same laboratory tests to assess whether IBC as identified by community physicians and not meeting AJCC or SEER criteria (designated atypical IBC) had the same pattern as IBC meeting AJCC and SEER criteria (designated classic IBC).
Materials and Methods
Patients and Tumor Samples
This study examined IBC cases from a national IBC registry and the biospecimen repository at the George Washington University Medical Center. The IBC registry collected clinical information and tumor specimens from patients diagnosed with IBC from 1991 to 2005 in the United States and Canada. The project was approved by the institutional review boards (IRB) at George Washington University Medical Center and the NCI. All patients who participated in the IBC registry signed an informed consent document. Patient information was handled according to the guidelines of the Health Insurance Portability and Accountability Act. Medical records of the patients were reviewed for clinical history and pathology records. The archival tumor specimens were collected and used for immunohistochemical studies. Pretreatment core biopsies were available for approximately half of the patients, and posttreatment mastectomy samples were used for the others. In those patients in whom both core biopsies and mastectomy samples were tested, results were similar.
A case was considered classic IBC if there was documentation of classic history and physical findings of erythema and peau d’orange appearing acutely (by definition <6 months but usually a few weeks) and involving the majority of the breast or if there was histopathologic confirmation of DLI that met the criteria for IBC diagnosis by the AJCC or SEER. A group of atypical IBC cases with the clinical signs of IBC, also appearing acutely but differing from classic IBC because of involvement of less than half the breast, were compared with the classic IBC cases to investigate the validity of current case definitions. The non-IBC cases included stage III LABC; the tumor specimens and the clinical data were obtained from the National Cancer Institute (NCI) Cooperative Breast Cancer Tissue Resource (CBCTR).24 Stage III LABC included stage IIIA and stage IIIB (per AJCC criteria) based on the clinical information provided by the CBCTR. The size of the tumors in this group was large (median 6 cm).
Grading of tumors was performed by 1 of us (SH) and both estrogen receptor (ER) and progesterone receptor (PR) were assayed by another of us (SY). Human endothelial growth factor receptor 2 (HER-2)/neu results were obtained from IBC registry patient records and were not available for patients with LABC.
Immunohistochemical Evaluation of Tumor Samples
Immunohistochemical staining of ER, podoplanin, and E-cadherin was performed on the IBC and non-IBC paraffin-embedded tumor tissues using previously described methods.10,15 The antibodies used for these studies included mouse monoclonal anti-ER (clone 1D5, Dako North America, Carpinteria, CA), mouse monoclonal antipodoplanin (clone AB03, AngioBio Co, Del Mar, CA), and mouse monoclonal anti–E-cadherin (clone 36B5, Chemicon, Millipore, Billerica, MA).
Analysis of E-Cadherin Expression
E-cadherin expression was quantitatively analyzed using an automated cellular imaging system (ACIS) as previously described.15,25 Six areas of tumors were measured with a ×40 scoring tool in ACIS. Staining index (SI) was determined by staining intensity × % staining per 100. SI results were blinded to the patient diagnosis. To validate the automated image analysis of E-cadherin, every fifth slide was read blindly by a pathologist and compared with the ACIS score. The scores were shown to be correlated, with a correlation coefficient of 0.501.
Lymphatic Vascular Density and Intralymphatic Tumor Emboli
We used podoplanin, a putative marker for lymphatic endothelial cells, to identify the lymphatic vasculature in IBC and non-IBC tumors.26 LVD was determined by manually counting positive podoplanin-staining cells using a “hot spot” approach of quantification in the region of greatest staining density within and adjacent to the edge of a tumor under a ×400 microscopic field.
ILTE evaluation was performed concurrently by identification of tumor emboli within the lymphatic vessels and scored based on a system of no emboli = no intralymphatic tumor cells on a whole slide; few emboli = small clusters of tumor cells in 1 or a few lymphatic vessels on a whole slide; and many emboli = clusters of tumor cells in many vessels with some lumens filled with tumor cells on a whole slide. The ILTE evaluation was internally validated by reevaluation of a subset of cases, which were reviewed by consensus by both SMH and MT. LVD and ILTE evaluations were also blinded to the patient diagnosis.
Statistical Analysis
Means, standard deviations, and 5-number summaries (minimum, first quartile, median, third quartile, maximum) were generated for continuous data, and frequency distributions were generated for categorical data. Histograms and Q-Q plots were used to visually assess the normality of the data, along with the Shapiro-Wilk test for normality. Age at diagnosis was normally distributed within the IBC groups, so analysis of variance was used to examine differences in age at diagnosis between the IBC categories. The Tukey-Kramer method was used for P value adjustment in performing multiple comparisons. Because E-cadherin and LVD were highly right skewed and VEGF-D was highly left skewed, nonparametric tests were applied.
Wilcoxon rank sum tests were used to compare E-cadherin, LVD, and VEGF-D levels between the classic IBC cases and the non-IBC LABC cases. Rank analysis of covariance was used to evaluate differences in E-cadherin, LVD, and VEGF-D between classic IBC and non-IBC LABC while controlling for age at diagnosis (years). Simple ordinal logistic regression was used to test for a significant difference in levels of ILTE (none, few, and many) between classic IBC and non-IBC LABC. A score test indicated that the assumption of proportional odds was satisfied (P = .88). Because DLI is a frequent histopathologic feature of IBC, lymph vascular features (ILTE and LVD) and E-cadherin expression were evaluated in classic IBC tumors with and without DLI. Wilcoxon rank sum tests were used to test for significant differences in LVD and E-cadherin between tumors with and without DLI, and an ordinal logistic regression was used to test for significant differences in ILTE between tumors with and without DLI. The level of statistical significance was set at 0.05. A Dunn posttest was used to perform pairwise comparisons between (1) atypical IBC and classic IBC and (2) atypical IBC and non-IBC LABC at an adjusted significance level of 0.025 to maintain a family-wise error rate of 0.05.
Ordinal logistic regression was used to determine whether ILTE in atypical IBC differed significantly from ILTE in classic IBC and non-IBC LABC.
All statistical data analyses for this article were generated using SAS/STAT software, version 9.1 of the SAS System for Windows (SAS Institute, Cary, NC).
Results
Patient and Tumor Characteristics
Patient characteristics are summarized in Table 1. Patients with classic IBC were significantly younger at diagnosis than were non-IBC LABC controls (P = .0076), but patients with atypical IBC did not differ significantly in age from either of the other 2 groups (classic IBC, P = .95 and LABC, P = 0.24). Patients with classic IBC and those with atypical IBC had respective mean ages of 48.7 and 49.6 years, whereas patients with non-IBC LABC had a mean age of 54.5 years. Among the atypical or classic IBC tumors, 76 (76%) were infiltrating ductal, 62 (62%) were ER−, and 66 (66%) were of high grade. IBC tumors were usually diffuse with no definable borders. The LABC tumors were large, with a median size of 6 cm, and 59 (55%) were ER− and 75 (70%) were of high grade (Table 1). Of the 70 classic IBC tumors, 27 (39%) had evidence of DLI; none was reported in the LABC patients.
Table 1.
Patient Characteristics
| Variable | Classic IBC N (%) |
Atypical IBC N (%) |
Non-IBC LABC N (%) |
|---|---|---|---|
| Patients | 70 (100) | 20 (100) | 107 (100) |
| Age at Diagnosis (years) | |||
| Mean (SD) | 48.7 (9.4) | 49.6 (10.8) | 54.5 (14.0) |
| Median (Q1, Q3) | 48.0 (43.0, 55.0) | 49.5 (44.0, 58.5) | 52.5 (45.0, 65.0) |
| Hormonal Status | |||
| ER+ | 27 (38.6) | 9 (45.0) | 39 (36.5) |
| ER− | 42 (60.0) | 10 (50.0) | 59 (55.1) |
| Unknown | 1 (1.4) | 1 (5.0) | 9 (8.4) |
| HER2 Status | |||
| Positive | 29 (41.4) | 7 (35.0) | NA |
| Negative | 34 (48.6) | 12 (60.0) | NA |
| Unknown | 7 (10.0) | 1 (5.0) | NA |
| Triple-Negative Status | |||
| Yes | 18 (25.7) | 4 (20.0) | NA |
| No | 46 (65.7) | 14 (70.0) | NA |
| Unknown | 6 (8.6) | 2 (10.0) | NA |
| Grade | |||
| Poorly differentiated | 47 (67.1) | 11 (55.0) | 75 (70.1) |
| Moderately differentiated | 16 (22.9) | 7 (35.0) | 30 (28.0) |
| Well differentiated | 1 (1.4) | 1 (5.0) | 0 (0.0) |
| Unknown | 6 (8.6) | 1 (5.0) | 2 (1.9) |
| Dermal Lymphatic Invasion | |||
| Yes | 27 (38.6) | NA | NA |
| No | 42 (60.0) | NA | NA |
| NA | 1 (1.4) | NA | NA |
Abbreviations: ER = estrogen receptor; IBC = inflammatory breast cancer; LABC = locally advanced breast cancer; NA = not applicable; Q1 = first quartile; Q3 = third quartile; SD = standard deviation.
E-Cadherin Levels in Classic IBC and Non-IBC
The IHC staining images of E-cadherin are shown in Figure 1. E-cadherin levels were significantly higher in classic IBC than in non-IBC tumors (P = .040). The median value for E-cadherin was 2.03 for classic IBC cases compared with 1.03 for non-IBC controls (Table 2). This difference became insignificant after controlling for age at diagnosis (P = .077). E-cadherin was higher in younger patients with IBC and LABC and there was a higher proportion of younger patients with IBC.
Figure 1.

Immunohistochemistry Images of Vascular Endothelial Growth Factor D (VEGF-D) and E-Cadherin Expression in Inflammatory Breast Cancer (IBC) (A, C) and Non-IBC (B, D) (×200 Magnification)
Table 2.
LVD and Expression of VEGF-D and E-Cadherin in Classic IBC, Atypical IBC, and Non-IBC LABC
| Variable | Classic IBC | Atypical IBC | Non-IBC LABC | P Valuea | P Valueb | P Valuec |
|---|---|---|---|---|---|---|
| LVD | ||||||
| Mean (SD) | 9.88 (11.63) | 10.10 (9.07) | 15.04 (11.76) | |||
| Median (Q1, Q3) | 7.00 (0.00, 16.00) | 9.50 (1.50, 17.00) | 13.00 (5.00, 25.00) | .0017 | .64 | .10 |
| VEGF-D | ||||||
| Mean (SD) | 144.36 (21.71) | 157.46 (20.03) | 170.33 (12.14) | |||
| Median (Q1, Q3) | 144.60 (128.42, 161.61) | 61.23 (148.58, 172.69) | 172.94 (165.27, 178.50) | < .0001 | .027 | .0079 |
| E-Cadherin | ||||||
| Mean (SD) | 10.45 (15.20) | 13.86 (18.45) | 4.12 (6.76) | |||
| Median (Q1, Q3) | 2.03 (0.13, 16.57) | 2.14 (0.30, 33.15) | 1.03 (0.11,5.14) | .040 | .42 | .029 |
Abbreviations: IBC = inflammatory breast cancer; IQR = interquartile ratio; LABC = locally advanced breast cancer; LVD = lymphatic vessel density; Q1 = first quartile; Q3 = third quartile; VEGF-D = vascular endothelial growth factor D.
Wilcoxon rank sum tests comparing classic IBC to non-IBC LABC.
Dunn tests comparing atypical IBC with classic IBC (adjusted significance level to 0.025).
Dunn tests comparing atypical IBC with non-IBC LABC (adjusted significance level to 0.025).
VEGF-D in Classic IBC and Non-IBC
VEGF-D level was significantly lower in classic IBC than in non-IBC tumors (P < .0001). The median value for VEGF-D was 144.60 among patients with classic IBC compared with 172.94 for non-IBC controls (Table 2). This difference remained significant after controlling for age at diagnosis (P < .0001).
LVD in Classic IBC and Non-IBC
LVD was significantly lower in classic IBC than in non-IBC tumors (P = .0017). The median value for LVD was 7.00 among classic IBC cases compared with 13.00 for non-IBC controls (Table 2). This difference remained significant after controlling for age at diagnosis (P = .0007). Figure 2 shows tumor emboli in podoplanin-positive lymphatic vessels of IBC and non-IBC.
Figure 2.
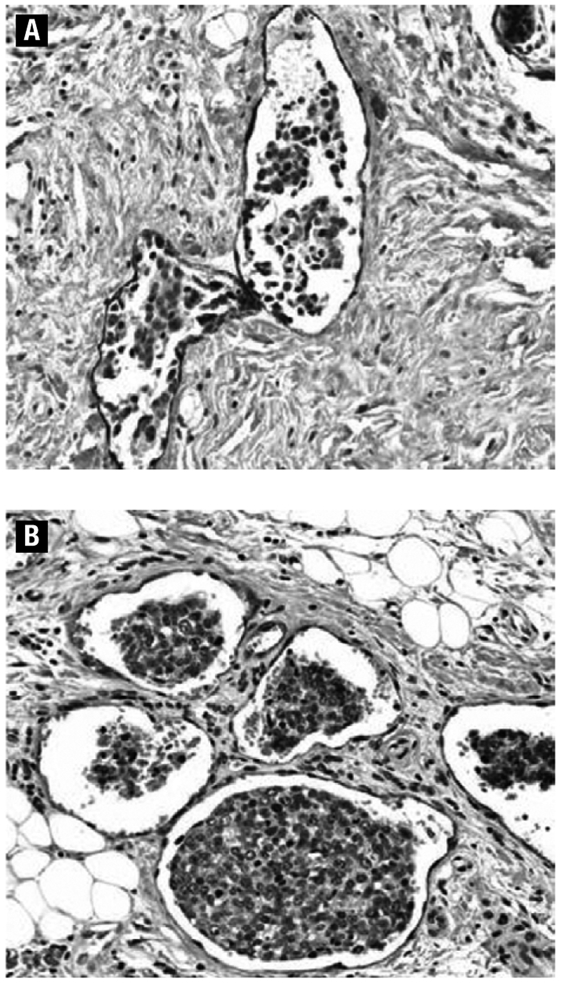
Podoplanin-Expressing Lymphatic Endothelial Cells and Tumor Cells Within the Lymphatic Vessels (Intralymphatic Tumor Emboli) in IBC (A) and Non-IBC (B) (×200 Magnification)
ILTE in Classic IBC and Non-IBC
ILTE was only marginally more prominent in classic IBC than in LABC (P = .047) (Table 3). IBC was not significantly related to ILTE after controlling for age at diagnosis and dichotomized into ≤ 50 and > 50 years (P = .30). Age at diagnosis, however, was statistically significantly associated with ILTE in both patients with classic IBC and those with LABC (P = .0082). The odds of a patient <50 years having many vs. few or no ILTE were significantly higher than the odds of a patient >50 years having many vs. few or no ILTE (odds ratio [OR], 2.27; 95% confidence interval [CI]: 1.24–4.15).
Table 3.
Frequency Distribution of ILTE in Classic IBC, Atypical IBC, and Non-IBC LABC
| Variable ILTE |
Classic IBC N (%) |
Atypical IBC N (%) |
Non-IBC LABC N (%) |
P Valuea |
|---|---|---|---|---|
| Many | 34 (48.6) | 4 (20.0) | 35 (32.7) | .046 |
| Few | 11 (15.7) | 3 (15.0) | 17 (15.9) | |
| None | 24 (34.3) | 13 (65.0) | 49 (45.8) | |
| Unknown | 1 (1.43) | 0 (0.0) | 6 (5.6) |
Abbreviations: IBC = inflammatory breast cancer; LABC = locally advanced breast cancer; ILTE = intralymphatic tumor emboli.
χ2 test from ordinal logistic regression model for classic IBC vs. non-IBC LABC.
DLI vs. ILTE in Classic IBC
Among the classic IBC tumors, 27 (39%) had documented DLI. Of these 27 tumors with DLI, 14 (52%) had many, 7 (26%) had few, and 6 (22%) had no ILTE. Of the 42 tumors without DLI, 20 (48%) had many, 4 (10%) had few, and 17 (41%) had no ILTE. Classic IBC cases with DLI did not differ from classic IBC cases without DLI with respect to the extent of ILTE (P = .38).
Evaluating Differences Between Classic IBC vs. Atypical IBC
For the 3 quantitative tests (E-cadherin, VEGF-D, and LVD), the atypical IBC cases resembled the classic IBC cases rather than the LABC cases (Table 2). The Dunn posttest was applied using an adjusted .025 significance level to determine whether LVD, VEGF-D, and E-cadherin differed significantly between (1) atypical IBC and classic IBC or (2) atypical IBC and non-IBC LABC. For E-cadherin, which was higher in classic IBC than in LABC, no significant difference was found between atypical and classic IBC (P = .42), but atypical IBC was marginally significantly different from LABC (P = .029). For VEGF-D, which was lower in classic IBC than in LABC, atypical IBC was different from both classic IBC and LABC, but the difference between atypical IBC and LABC was greater (P = .0079) than between atypical and classic IBC (P = .027). For LVD, which was lower in classic IBC than in LABC, no significant difference was found between atypical IBC and classic IBC (P = .64) or between atypical IBC and LABC (P = .10).
The evaluation of ILTE, a nonquantitative test, indicated a pattern different from the other 3 tests, with a greater similarity between atypical IBC and LABC than atypical IBC and classic IBC.
The odds of a patient with classic IBC having many vs. few or no ILTE was significantly higher than the odds of a patient with atypical IBC having many vs. few or no ILTE (OR = 3.61;95% CI: 1.32–9.91). There was no significant difference between atypical IBC and LABC in regard to the odds of having many ILTE (OR = 2.00; 95% CI: 0.76–5.31).
Age at diagnosis, however, was statistically significantly associated with ILTE for classic IBC, atypical IBC, and LABC (P = .0087). The odds of a patient <50 years having many vs. few or no ILTE was significantly higher than the odds of a patient > 50 years having many vs. few or no ILTE (OR = 2.16; 95% CI: 1.22–3.84).
Discussion
In this study, we found that all 4 markers evaluated showed significant differences between classic IBC and LABC. The increased levels of E-cadherin in IBC were in concordance with earlier observations.16,17 E-cadherin is 1 of the transmembrane cadherins that mediate cell-cell adhesion. E-cadherin knockout mice exhibit embryonic lethality, indicating its crucial role for cell survival.27 E-cadherin levels are elevated in IBC, and this elevation had been postulated to be crucial for the formation of the lymphatic tumor emboli.16,17,28 Strong E-cadherin expression is also observed in lymphatic tumor emboli of non-IBC tumors.18 In an oral squamous carcinoma cell system, E-cadherin protected cell aggregates from cell death through upregulation of Bcl-2.29 In Ewing tumor cells, E-cadherin suppressed anoikis through ERBB4 activation of the phosphatidylinositol 3-kinase-Akt pathway.30 These results suggest that E-cadherin is a survival factor for anchorage-independent growth.
The other 2 markers that showed the greatest difference between classic IBC and LABC, LVD and VEGF-D, unexpectedly showed higher levels in the patients with LABC than in the patients with classic IBC. It is possible that this could be due in part to the large size of the tumors in LABC and the longer delay in treatment compared with IBC, but the propensity of the tumors to enter the lymphatic system may also be independent of density. Because these data are unexpected, replication would be beneficial. The evaluation of LVD in breast cancer yielded mixed results in the published literature. Van der Auwera et al evaluated multiple parameters of lymphatic vasculature of normal, IBC, and non-IBC tissues and found that lymphatic endothelial cell proliferation was the only parameter that was significantly associated with IBC.10 Two separate studies using the hot spot approach showed that higher LVD was significantly associated with positive lymph node metastasis and shorter survival for patients with mostly stage I and II non-IBC breast tumors.31,32 We found that LVD was higher in non-IBC LABC than in classic IBC but did not evaluate survival. At the present time, it is not clear what the optimal parameters are for evaluating lymphangiogenesis. The differences in methodology for evaluating LVD and in the study populations could also contribute to the differences in results.
VEGF-D, VEGF-C and vascular endothelial growth factor receptor 3 have been shown to play an important role in lymphangiogenesis in many tumor types, including breast cancer.33 In an animal model system, de novo VEGF-D expression was shown to promote tumor lymphangiogenesis and lymphatic invasion.34 In human breast cancer, the difference in expression level of lymphangiogenic factors VEGF-C, and VEGF-D when comparing IBC with non-IBC yielded mixed results. Shirakawa et al using a semiquantitative reverse transcriptase-polymerase chain reaction (PCR) method to measure levels of mRNA in IBC xenograft model WIBC-9 did not find an overexpression of VEGF-C or VEGF-D.35 In contrast, 2 studies with small numbers of tumor samples showed that the mRNA levels of VEGF-D were higher in IBC than in non-IBC using RT-PCR.10,14 None of these studies assessed VEGF-D expression quantitatively. We found that VEGF-D expression levels as measured by immunohistochemistry were quantitatively higher for non-IBC compared with IBC.
Since the clinical picture of an inflammatory appearance in IBC is related to the blockage of dermal lymphatic vessels by IBC tumor emboli rather than inflammation, studies have focused on investigating the nature of DLI in IBC. DLI can be histologically confirmed in less than two thirds of IBC cases and can also be identified in non-IBC. One study reported dermal lymphatic tumor emboli (DLTE) in 7% of non-IBC and 45% of diffuse IBC (involvement of more than two thirds of the breast), and the presence of DLTE was prognostic only in patients with diffuse IBC.36 A high probability of lymph node-positive disease was found in patients with IBC with histologic evidence of DLTE.37 In our study, 63% of the classic IBC tumors had documented DLI, which may be an underestimation because not all the IBC patients had documented histopathologic evaluation of the skin. We did not find any associations of DLTE with LVD, ILTE, and VEGF-D expression. The factors associated with DLTE in IBC are yet to be identified.
LVD and VEGF-D expression were significantly higher in non-IBC LABC compared with classic IBC. There were significant differences in ILTE between classic IBC and atypical IBC and the trend was marginally significant in comparing classic IBC with non-IBC. We showed that E-cadherin expression was higher in classic IBC compared with non-IBC. Our results indicated that LVD and E-cadherin expression did not differ between classic IBC and atypical IBC, and elevated expression of VEGF-D was found in atypical IBC compared with classic IBC. Our data showed that ILTE was associated with pathologic lymphatic invasion in IBC and non-IBC tumors. This result suggests that IBC and non-IBC may share the same mechanisms for formation of tumor emboli in the lymphatic vessels and that the similarities are largely due to the invasive nature of the tumor cells. This is consistent with gene expression array studies in which some genes associated with a biological phenotype identified in IBC have the same molecular expression subgroup signatures seen in non-IBC.38
Another important observation in this study is that the tumor markers investigated in this study document the similarity between IBC diagnosed by clinicians and AJCC- and SEER-defined IBC, which are far more different from LABC. Our earlier pilot study on patients in our IBC registry19 showed that clinical features and survival were similar in the groups that we defined here as classic IBC and atypical IBC. In a review by an international group of investigators with experience in IBC,23 the defining features of IBC were rapid (within 6 months) onset of erythema often associated with an early peau d’orange appearance. This group agreed that the area of erythema could be much more limited than that designated by AJCC, which is reflective of the disease now being recognized at an earlier point than in the past. Our laboratory assays in general suggest that the AJCC and SEER IBC case definitions may be too restrictive.
In atypical IBC, LVD, VEGF-D, and E-cadherin results were all closer to those of the classic IBC cases than the LABC cases. Only ILTE, the most subjective assay, differed from this pattern.
Conclusion
The differential expression of E-cadherin between groups of patients with IBC and LABC may have potential use in separating the 2 in situations in which traditional clinical and pathologic findings are inconclusive. We observed lymphangiogenesis in both IBC and non-IBC tumors, and therefore assays involving this characteristic are unlikely to be of diagnostic importance. Because this study is exploratory, future studies that use standardized methodology are required to permit improvement in expanding and solidifying the case definition ofIBC as well as in investigating molecular prognostic markers in diverse types of breast cancer. The unusual and characteristic behavior of IBC in contrast to non-IBC suggests that the case definition may be improved by adding molecular characterization rather than clinical or pathologic features.
Clinical Practice Points.
Inflammatory breast cancer (IBC) and locally advanced breast cancer (LABC) are believed to have a different pathogenesis because of the difference in clinical presentation and prognosis.
The diagnosis of IBC is usually made based on inflammatory appearance involving more than half the breast and/or the typical pathological feature of dermal lymphatic invasion of the affected breast, the definition of IBC by the American Joint Committee on Cancer (AJCC) and the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program.
In addition, IBC behaves in such a manner to suggest that micrometastases are present at the time of diagnosis and therefore requires neoadjuvant chemotherapy as the initial form of therapy.
The current study provides further supportive evidence of the difference between IBC and LABC.
This study also evaluated the so-called “atypical” IBC cases, where the typical clinical features of IBC are restricted to less than half of the breast and dermal lymphatic invasion is not documented, thus not fitting the definition of classic IBC by AJCC or SEER criteria. The similar molecular marker expression of “atypical” IBC and classic IBC as compared to LABC demonstrates the overly restrictive definition of the disease utilized by AJCC and SEER.
As the standard of care for IBC for initial treatment is neoadjuvant chemotherapy, making the right diagnosis is critically important. When a young woman presents with a small painful red lesion of the breast, IBC needs to be suspected so the appropriate diagnosis can be made.
Acknowledgments
We thank Dat Nguyen for her technical support on immunochemistry for E-cadherin, ER, and podoplanin.
This work was supported in part by Grant No. BC009014 with the Department of Defense and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Levine PH, Steinhorn SC, Ries LG, et al. Inflammatory breast cancer: the experience of the surveillance, epidemiology, and end results (SEER) program. J Natl Cancer Inst 1985; 74:291–7. [PubMed] [Google Scholar]
- 2.Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and noninflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol 2003; 21:2254–9. [DOI] [PubMed] [Google Scholar]
- 3.Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst 2005; 97:966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozzetti F, Saccozzi R, De Lena M, et al. Inflammatory cancer of the breast: analysis of 114 cases. J Surg Oncol 1981; 18:355–61. [DOI] [PubMed] [Google Scholar]
- 5.Taylor GW, Melzer A. Inflammatory carcinoma of the breast. Am J Cancer 1938; 33:33–49. [Google Scholar]
- 6.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. et al. 6th ed. New York, NY: Springer; 2002. [Google Scholar]
- 7.McCarthy NJ, Yang X, Linnoila IR, et al. Microvessel density, expression of estrogen receptor alpha, MIB-1, p53, and c-erbB-2 in inflammatory breast cancer. Clin Cancer Res 2002; 8:3857–62. [PubMed] [Google Scholar]
- 8.Colpaert CG, Vermeulen PB, Dirix LY, et al. Commentary re: NJ McCarthy et al., microvessel density, expression of estrogen receptor alpha, MIB-1, p53 and c-erbB-2 in inflammatory breast cancer. Clin Cancer Res 2002; 8:3857–62. Clin Cancer Res 2003; 9(10 Pt 1):3815-6; author reply 3816. [PubMed] [Google Scholar]
- 9.Bièche I, Lerebours F, Tozlu S, et al. Molecular profiling of inflammatory breast cancer: identification of a poor-prognosis gene expression signature. Clin Cancer Res 2004; 10:6789–95. [DOI] [PubMed] [Google Scholar]
- 10.Van der Auwera I,Van Laere SJ,Van den Eynden GG, et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res 2004; 10:7965–71. [DOI] [PubMed] [Google Scholar]
- 11.Colpaert CG, Vermeulen PB, Benoy I, et al. Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br J Cancer 2003; 88:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol 2006; 24:769–77. [DOI] [PubMed] [Google Scholar]
- 13.Overmoyer B,Fu P, Hoppel C, et al. Inflammatory breast cancer as a model disease to study tumor angiogenesis: results of a phase IB trial of combination SU5416 and doxorubicin. Clin Cancer Res 2007; 13:5862–8. [DOI] [PubMed] [Google Scholar]
- 14.Kurebayashi J, Otsuki T, Kunisue H, et al. Expression of vascular endothelial growth factor (VEGF) family members in breast cancer. Jpn J Cancer Res 1999; 90:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Auwera I, Van den Eynden GG, Colpaert CG, et al. Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res 2005; 11:7637–42. [DOI] [PubMed] [Google Scholar]
- 16.Kleer CG, van Golen KL, Braun T, et al. Persistent E-cadherin expression in inflammatory breast cancer. Mod Pathol 2001; 14:458–64. [DOI] [PubMed] [Google Scholar]
- 17.Alpaugh ML, Tomlinson JS, Ye Y, et al. Relationship of sialyl-Lewis(x/a) underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma. Am J Pathol 2002; 161:619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Deshpande CG, Badve S. Role of E-cadherins in development of lymphatic tumor emboli. Cancer 2003; 97:2341–7. [DOI] [PubMed] [Google Scholar]
- 19.Levine PH, Zolfaghari L, Young H, et al. What is inflammatory breast cancer? Revisiting the case definition. Cancers 2010; 2:143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine PH, Veneroso C. The epidemiology of inflammatory breast cancer. Semin Oncol 2008; 35:11–6. [DOI] [PubMed] [Google Scholar]
- 21.Kim T, Lau J, Erban J. Lack of uniform diagnostic criteria for inflammatory breast cancer limits interpretation of treatment outcomes: a systematic review. Clin Breast Cancer 2006; 7:386–95. [DOI] [PubMed] [Google Scholar]
- 22.Amparo RS,Angel CD, Ana LH, et al. Inflammatory breast carcinoma: pathological or clinical entity? Breast Cancer Res Treat 2000; 64:269–73. [DOI] [PubMed] [Google Scholar]
- 23.Dawood S, Merajver S, Viens P, et al. International expert panel on inflammatory breast cancer (IBC): consensus statement for standardized diagnosis and treatment. Ann Oncol 2011; 22:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass AG, Donis-Keller H, Mies C, et al. The cooperative breast cancer tissue resource: archival tissue for the investigation of tumor markers. Clin Cancer Res 2001; 7:1843–9. [PubMed] [Google Scholar]
- 25.Tan AR, Yang X, Hewitt SM, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol 2004; 22:3080–90. [DOI] [PubMed] [Google Scholar]
- 26.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 1999; 154:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohsugi M, Larue L, Schwarz H, et al. Cell-junctional and cytoskeletal organization in mouse blastocysts lacking E-cadherin. Dev Biol 1997; 185:261–71. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson JS, Alpaugh ML, Barsky SH. An intact overexpressed E-cadherin/alpha-,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res 2001; 61:5231–41. [PubMed] [Google Scholar]
- 29.Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem 1998; 273: 16953–61. [DOI] [PubMed] [Google Scholar]
- 30.Kang HG, Jenabi JM, Zhang J, et al. E-cadherin cell-cell adhesion in Ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res 2007; 67:3094–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura Y, Yasuoka H, Tsujimoto M, et al. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat 2005; 91:125–32. [DOI] [PubMed] [Google Scholar]
- 32.Mohammed RA, Ellis IO, Elsheikh S, et al. Lymphatic and angiogenic characteristics in breast cancer: morphometric analysis and prognostic implications. Breast Cancer Res Treat 2009; 113:261–73. [DOI] [PubMed] [Google Scholar]
- 33.Mylona E, Alexandrou P, Mpakali A, et al. Clinicopathological and prognostic significance of vascular endothelial growth factors (VEGF)-C and -D and VEGF receptor 3 in invasive breast carcinoma. Eur J Surg Oncol 2007; 33:294–300. [DOI] [PubMed] [Google Scholar]
- 34.Stacker SA, Caesar C, Baldwin ME, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7:186–91. [DOI] [PubMed] [Google Scholar]
- 35.Shirakawa K, Shibuya M, Heike Y, et al. Tumor-infiltrating endothelial cells and endothelial precursor cells in inflammatory breast cancer. Int J Cancer 2002; 99: 344–51. [DOI] [PubMed] [Google Scholar]
- 36.Le MG, Arriagada R, Contesso G, et al. Dermal lymphatic emboli in inflammatory and noninflammatory breast cancer: a French-Tunisian joint study in 337 patients. Clin Breast Cancer 2005; 6:439–45. [DOI] [PubMed] [Google Scholar]
- 37.Gruber G, Ciriolo M, Altermatt HJ, et al. Prognosis of dermal lymphatic invasion with or without clinical signs of inflammatory breast cancer. Int J Cancer 2004; 109:144–8. [DOI] [PubMed] [Google Scholar]
- 38.Bertucci F, Finetti P, Rougemont J, et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res 2005;65:2170–8. [DOI] [PubMed] [Google Scholar]


