SUMMARY
Background
Combination antiviral therapy holds the promise of increasing response rates while decreasing antiviral resistance, but has yet to be shown to be beneficial or necessary in chronic hepatitis B.
Aim
To evaluate the benefit of combination therapy with adefovir and lamivudine versus adefovir alone in maintaining virological, biochemical and histological responses.
Methods
Patients with chronic hepatitis B with and without previous lamivudine therapy were randomised to receive adefovir alone (10 mg/daily) or adefovir and lamivudine (100 mg/daily) for up to 192 weeks. Study endpoints were (i) maintained virological (HBV DNA <500 copies/mL), biochemical and histological response, (ii) loss of HBeAg and (iii) loss of HBsAg.
Results
A total of 41 patients were enrolled, including 31 HBeAg -positive and 31 treatment-naïve subjects. 30 patients remained on assigned therapy at 192 weeks. The percentage of patients achieving a combined maintained response was higher in the combination than the monotherapy arm, both at week 48 (59% vs. 26%, P = 0.06) and 192 (68% vs. 31%, P = 0.03). At week 192, 76% of the combination vs. 36% of the monotherapy group had loss of HBeAg (P = 0.03). One patient receiving adefovir cleared HBsAg. Adefovir resistance developed in 6 of 19 (32%) monotherapy but none of 22 combination treated patients (P = 0.03).
Conclusions
Extended combination therapy with lamivudine and adefovir is associated with a high rate of long-term virological and biochemical response. Adefovir monotherapy appears to be less effective mainly because of poor initial response and the ultimate development of antiviral resistance (Clinical.Trials.gov NCT00023309).
INTRODUCTION
Management of chronic hepatitis B improved greatly with the development of orally available nucleoside and nucleotide analogues with potent activity against hepatitis B virus (HBV). These agents can lead to marked improvements in biochemical, virological, serological and histological features of disease.1 Unfortunately, responses are often not sustained once therapy is stopped, and treatment must be continued long-term, if not indefinitely. In addition, long-term therapy is often associated with the development of antiviral drug resistance marked by appearance of mutations in the HBV polymerase gene and rises in serum HBV DNA levels, despite continued treatment.2, 3 The rise in HBV DNA levels is usually followed by a loss of the biochemical and histological responses and absence of a long-term benefit.4 Switching from one nucleoside analogue to another which does not share the same pattern of antiviral resistance can result in reestablishment of clinical benefit, but sequential monotherapy predisposes to multidrug-resistance.5, 6 For these reasons, recent efforts have focused on strategies to prevent antiviral drug resistance.
Borrowing on the paradigm that a drug combination is more effective than monotherapy for treatment of human immunodeficiency virus (HIV), the same approach may be appropriate for chronic hepatitis B. Combination therapy may have heightened antiviral effects, and combining agents that do not share cross resistance has the potential to prevent resistance. The current study was designed to assess whether the combination of adefovir and lamivudine was more efficacious than adefovir alone in providing long-term viral suppression and clinical improvement in chronic hepatitis B. This study was initiated, before the availability of the more potent, recent nucleoside analogues, tenofovir and entecavir.
METHODS
Patients
Adult patients with chronic hepatitis B with HBV DNA levels above 106 copies/mL and raised serum aminotransferase levels were eligible for enrollment, regardless of hepatitis B e antigen (HBeAg) status or previous therapy with lamivudine. A liver biopsy performed within 2 years of enrollment was required to demonstrate chronic hepatitis. Exclusion criteria included, previous or current therapy with adefovir dipivoxil or tenofovir disoproxil fumarate and any antiviral treatment within the previous 6 months. After a patient with lamivudine resistance developed a clinically significant flare of hepatitis after discontinuing therapy in preparation for entry into this study, the protocol was amended to remove the exclusion of antiviral therapy within 6 months. Other exclusion criteria included, co-infection with hepatitis C, hepatitis D and HIV, decompensated liver disease, organ transplantation, immunosuppressive therapy, pregnancy or inability to practise adequate contraception, creatinine clearance <50 mL/min, active alcohol or drug abuse or major medical or psychiatric illness that in the opinion of the investigator would interfere with participation.
Adefovir dipivoxil was provided under a Clinical Trial Agreement between Gilead Sciences (Foster City, CA, USA) and the NIH, and the study was conducted under an Investigation New Drug Application (IND # 66,221) held by the senior author. Lamivudine was provided by the NIH Clinical Center Pharmacy. The study was registered in Clinical.Trials.gov (NCT00023309). The study was approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases, and all patients provided written informed consent.
Study design
Patients were randomly assigned to receive either the combination of lamivudine (100 mg daily) and adefovir (10 mg daily) or adefovir alone (10 mg daily). Enrollment was stratified by HBeAg-status and prior lamivudine therapy. Patients underwent a history and physical examination, before starting therapy. Patients were seen at 2–4 week intervals during the first year and at 8–12 week intervals thereafter for monitoring of safety and efficacy. On each occasion, blood was obtained for routine serum chemistries, complete blood counts, HBV serology and HBV DNA levels. A liver biopsy, having been done before enrollment, was repeated at 48 and 192 weeks of therapy.
Initially, HBV DNA was quantified using the Roche COBAS Amplicor HBV monitor assay which has a lower limit of detection (LLD) of 500 copies per mL (Roche Molecular Diagnostics, Branchburg, NJ, USA). This assay was later replaced by the more sensitive Roche COBAS TaqMan HBV Test with a LLD of 50 copies/mL (Roche Molecular Diagnostics). Stored serum samples from baseline and weeks 48 and 192 initially tested with the Amplicor assay were re-tested using the Taqman assay. Liver biopsy was interpreted by a hepatopathologist who was blinded to the clinical data using the HAI scale for inflammation and necrosis (which has a range 0–18) and the Ishak scoring system for fibrosis (range 0–6, where 0 = no fibrosis and 6 = cirrhosis).
After 48 weeks, patients were admitted for repeat medical evaluation and liver biopsy. Patients who demonstrated a virological response (HBV DNA <105 copies/mL at 48 weeks) and absence of drug toxicity were allowed to continue therapy for up to 4 years (192 weeks). Patients whose HBV DNA was >105 copies/mL but still decreasing and who had a biochemical and histological response were also permitted to continue therapy. All other patients were considered treatment failures and switched to combination therapy if receiving adefovir monotherapy or another agent (tenofovir, entecavir) if already on combination therapy.
Definitions of response
A partial virological response was defined as a decrease in serum HBV DNA level by at least 3 log10 copies/mL from baseline and to below 105 copies/mL by week 48. A full virological response was defined as a decrease in HBV DNA levels to undetectable by the COBAS Amplicor assay (<500 copies/mL). A biochemical response was defined as a decrease in serum ALT levels into the normal range (≤41 U/L). A histological response was defined as a decrease in the HAI score by at least three points with no worsening of the Ishak fibrosis score. Finally, a combined response was defined as a combination of a virological, biochemical and histological responses at weeks 48 and 192. A response was considered maintained if it was present on therapy when last tested (~every 8–12 weeks for ALT and HBV DNA levels, at 1 and 4 years for histological responses). Therapy was stopped only for toxicity or if there was loss of HBsAg as measured on two specimens taken at least 6 months apart. A response was considered sustained if it was present at least 6 months after stopping therapy and when last tested.
End points
The primary endpoint of therapy was a maintained combined response at week 192. Secondary endpoints were loss of HBeAg, loss of hepatitis B surface antigen (HBsAg), the individual types of maintained and sustained responses (virological, biochemical and histological) and the development of lamivudine or adefovir resistance at 48 and 192 weeks. Failure of therapy could be due to drug toxicity or to virological nonresponse or breakthrough. Virological nonresponse was defined as lack of decrease in HBV DNA levels by at least 3 log10 and to below 105 copies/mL at week 48. A virological breakthrough was defined as a confirmed rise in HBV DNA level by ≥1 log10 copies/mL above the nadir value in a patient with an initial virological response who was considered compliant.
Resistance testing
Detection of lamivudine and/or adefovir resistance mutations was performed using the INNO-LiPA HBV genotyping kit (Innogenetics, Ghent, Belgium) on stored serum samples from baseline, week 48 and 192 in patients with detectable serum HBV DNA and in any patient at the time of virological breakthrough.
Statistical analysis
Sample size estimate.
In the phase 3 trial of adefovir monotherapy for chronic hepatitis B, histological improvement was observed in 64% of subjects, 51% achieved undetectable HBV DNA and 72% had normalisation of serum ALT level after 48 weeks of therapy. In this study, we used a combined endpoint that relied upon histological improvement in addition to maintained virological suppression and a normal ALT level. Thus, an optimistic estimate of the expected maintained combined response with adefovir monotherapy was 45%. With combination therapy, we expected an 80% maintained combined response rate at 48 weeks and a 90% rate at 192 weeks. The number of patients needed to show these differences in maintained combined response rate with a significance of 5% and power of 80% would be 30 per group (45% vs. 80%) at 48 weeks and 18 per group at 192 weeks (45% vs. 90%).
The maintained combined response rate between the two groups at 48 and 192 weeks was the primary endpoint for the study and was analysed by Chi-squared test and by life-table analysis. The actual means of secondary endpoints (e.g. HBV DNA level, HAI) and proportion who achieved an endpoint at week 48 and 192 were compared by Student’s t-test and Chi-squared test. McNemar’s test was used to compare the degree of HBsAg and HBcAg staining between weeks 48 and 192 with baseline. For comparison of mean semi-quantitative HBsAg and HBcAg scores, we used paired t-test. A P-value of 0.05 was used to define significance.
RESULTS
Demographic and clinical characteristics
Between January 2002 and September 2006, 41 patients were enrolled; 22 were randomised to receive lamivudine and adefovir and 19 to receive adefovir alone. The two groups were well balanced for baseline demographic and clinical characteristics (Table 1). The mean age was 46 years, the majority of patients were men and either Caucasian (41%) or Asian (46%). Approximately three quarters were HBeAg- positive and a similar proportion were treatment-naive. All patients with previous lamivudine therapy were documented to have lamivudine resistant mutations (rtM204V/I) at the time of virological breakthrough. Genotype A was most common among Caucasian patients (76%) and genotypes B and C among Asian American patients (37% and 47% respectively). The average ALT levels in patients randomised to receive combination therapy (mean = 183 U/L) was higher than that in those randomised to receive monotherapy (mean = 87 U/L), but the differences were not statistically significant. Mean HBV DNA levels in the two groups were similar (8.1 vs. 7.8 log10 IU/mL). The overall mean HAI score was 8.1, and 17% of patients had cirrhosis at baseline.
Table 1 ∣.
Baseline demographic and clinical characteristics
| Feature | Lamivudine & adefovir (n = 22) |
Adefovir (n = 19) |
|---|---|---|
| Age (years)* | 46 (14) | 45 (13) |
| Gender, male | 16 (73%) | 18 (95%) |
| Race | ||
| White | 9 (41%) | 8 (42%) |
| Asian | 9 (41%) | 10 (53%) |
| Black | 4 (18%) | 1 (5%) |
| Treatment-naïve | 17 (77%) | 14 (74%) |
| Genotype | ||
| A | 11 (50%) | 7 (37%) |
| B | 5 (23%) | 3 (16%) |
| C | 2 (9%) | 7 (37%) |
| D | 2 (9%) | 2 (10%) |
| A/G | 2 (9%) | 0 (0%) |
| ALT (U/L)* | 183 (250) | 87 (56) |
| HBeAg-positive | 17 (77%) | 14 (74%) |
| HBV DNA (Log10 Copies/mL)* | 8.1 (1.3) | 7.8 (1.6) |
| Liver histology | ||
| Total HAI* | 8.1 (2.7) | 7.9 (2.4) |
| Ishak Fibrosis Score* | 3.3 (1.7) | 2.4 (1.5) |
| Cirrhosis (%) | 5 (23) | 2 (11) |
HAI, histology activity index.
Mean (s.d.).
Biochemical responses at weeks 48 and 192
All 41 patients completed 48 weeks of therapy. Significantly, more patients receiving combination therapy had normal ALT levels (86%) at week 48 than those receiving adefovir monotherapy (53%), (P = 0.037, Table 2). The difference widened at 192 weeks (95% vs. 63%, P = 0.016). The single patient randomised to combination therapy with elevated ALT levels at week 192 was an overweight Asian male patient with intermittently but minimally elevated ALT values (31–60 U/L), despite lack of HBV DNA in serum; liver biopsies showed steatosis and early cirrhosis.
Table 2 ∣.
Endpoints of efficacy and failure at weeks 48 and 192
| Week 48 |
Week 192 |
|||||
|---|---|---|---|---|---|---|
| Lam & Adv (n = 22) |
Adv (n = 19) |
P-value | Lam & Adv (n = 22) |
Adv (n = 19) |
P-value* | |
| Normal ALT | 86% | 53% | 0.037 | 95% | 63% | 0.016 |
| HBV DNA Mean ± s.d. change (log10 copies/mL) | −6.8 ± 2.1 | −4.5 ± 2.7 | 0.0048‡ | −5.6 ± 1.6 | −3.0 ± 2.8 | 0.0006† |
| HBV DNA <105 copies/mL | 91% | 53% | 0.007 | 91% | 53% | 0.007 |
| HBV DNA <500 copies/mL | 68% | 53% | 0.35 | 77% | 32% | 0.005 |
| HBeAg loss | 41% | 21% | 0.28 | 76% | 36% | 0.03 |
| HBsAg loss | 0% | 5% | 0.46 | 0% | 5% | 0.46 |
| Histological response‡ | 67% (14/21) | 53% (10/19) | 0.52 | 83% (15/18) | 56% (5/9) | 0.18 |
| Genotypic resistance | 0% | 0% | 0% | 32% | 0.03 | |
| Treatment failure | 9% | 37% | 0.06 | 18% | 68% | 0.002 |
| Combined response§ | 59% | 26% | 0.058 | 68% | 31% | 0.03 |
Fisher exact test.
Student’s t-test.
Liver histology was not available on all enroled patients at 48 and 192 weeks; actual numbers given in parentheses. The last result before censoring was used for the week 192 results for subjects who were censored (two in the combination group and nine in the monotherapy group).
Primary endpoint.
Virological responses at weeks 48 and 192
The degree of viral suppression from baseline to week 48 was higher in the combination than monotherapy arm (−6.8 vs. −4.5 log10 copies/mL, P = 0.005, Table 2), and a higher proportion of patients achieved HBV DNA below 105 copies/mL (91% vs. 53%, P = 0.007). However, the proportion of patients with HBV DNA levels below <500 copies/mL (and thus, HBV DNA negative by the assay used at the time) was not significantly different (68% vs. 53%).
Eleven patients (27%) continued to have HBV DNA levels ≥105 copies/mL at week 48, nine receiving monotherapy and two combination therapy (one with pre-existing lamivudine resistance). Thus, 11 patients had a virological failure; the remaining 30 continued on assigned therapy beyond 48 weeks.
At week 192, patients randomised to combination therapy had significantly higher rates of a virological suppression compared with those on monotherapy (−5.6 vs. −3.0 log10 copies/mL, P = 0.0006; Table 2), and a significantly higher proportion on combination therapy was HBV DNA negative (77% vs. 32%, P = 0.005). Of the 22 patients on combination therapy, 17 became HBV DNA negative (15 by week 48, 2 thereafter) and none experienced virological breakthrough. In contrast, of the 19 patients on monotherapy, only 10 became HBV DNA negative (all by week 48), whereas 4 had virological breakthrough and 5 had stable, but persistently detectable HBV DNA levels above 500 copies/mL.
Serological responses at weeks 48 and 192
The rate of HBeAg loss was higher in the combination than the monotherapy group, although the differences did not reach statistical significance until week 192 (76% vs. 36%; P = 0.03, Table 2). The rate of HBeAg loss was somewhat faster in the combination group (Figure 1, P = 0.11). Only one patient became HBsAg negative while on assigned therapy (adefovir monotherapy at week 37).
Figure 1 ∣.
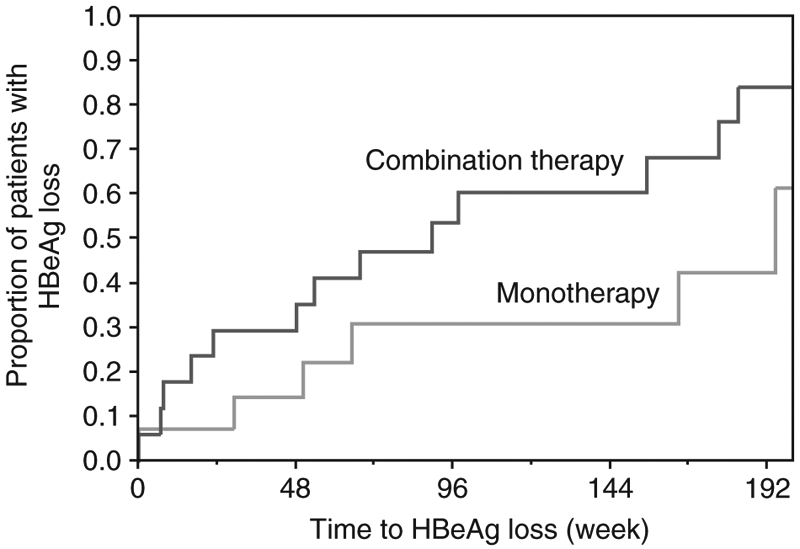
Time to loss of HBeAg by treatment group using life-table analysis.
Histological response at weeks 48 and 192
All patients underwent liver biopsy at baseline, 98% (40/41) had a repeat liver biopsy at week 48 and 68% (28/41) at week 192. Liver biopsy could not be performed in one patient at weeks 48 and 192 because of anatomical deformity of the liver as a result of a partial hepatic resection after a motor vehicular accident. Liver biopsies were not performed in 12 other patients at week 192 because of virological failure and use of other agents in eight, loss of HBsAg in 1 and withdrawal from the study in three others.
Histological responses were more frequent among patients on combination compared with monotherapy, but the differences were not statistically significant either at week 48 (67% vs. 53%, P = 0.52) or 192 (83% vs. 50%, P = 0.09). Similarly, mean HAI scores improved in both treatment arms, but the degree of change was not statistically different (Figure 2a).
Figure 2 ∣.
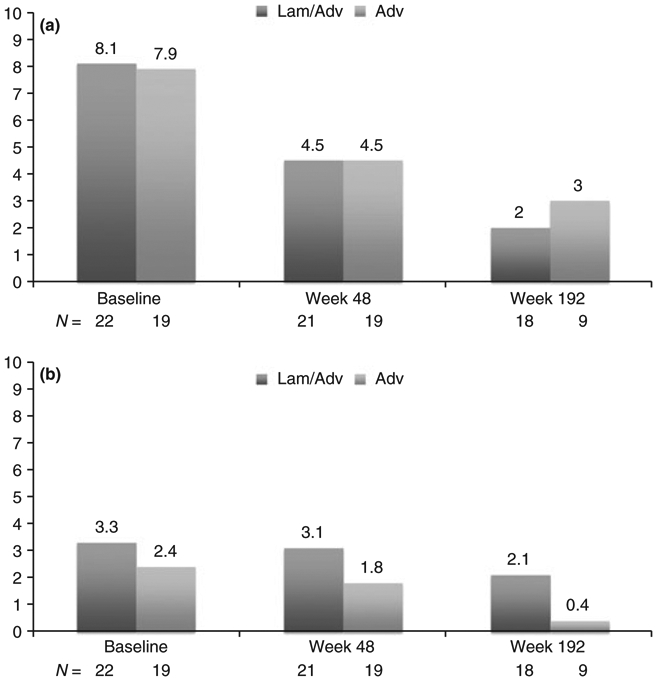
(a) Mean histology activity index scores among patients on combination therapy with lamivudine and adefovir (Lam & Adv) vs. adefovir monotherapy (Adv) at baseline, 48 weeks and 192 weeks. (b) Mean Ishak fibrosis scores among patients on combination therapy with lamivudine and adefovir (Lam & Adv) vs. adefovir monotherapy (Adv) at baseline, 48 weeks and 192 weeks.
Ishak fibrosis scores improved in both groups at week 48 and week 192 compared with baseline (Figure 2b), decreasing from 3.3 to 3.1 at week 48 and 2.1 at week 192 in the combination group, and from 2.4 to 1.8 at week 48 and 0.5 at week 192 in the monotherapy group.
Mean scores and proportions of patients with HBsAg immunostaining did not change between baseline and weeks 48 or 192 in either group (Table 3). In contrast, mean scores of HBcAg immunostaining decreased at both time points in both groups, but the degree of decrease was greater in the combination therapy arm. At week 192, 17% of combination treated compared with 56% of adefovir monotherapy treated subjects still had detectable HBcAg in liver by immunostaining (P = 0.0478).
Table 3 ∣.
Mean scores for HBsAg and HBcAg immunostaining at baseline, week 48 and week 192
| Feature | Baseline (Pre)a |
Week 48b |
Week 192c |
P-values |
P-values |
|||
|---|---|---|---|---|---|---|---|---|
| Lam & Adv (n = 22) |
Adv (n = 19) |
Lam & Adv (n = 21) |
Adv (n = 19) |
Lam & Adv (n = 18) |
Adv (n = 9) |
a vs. b LA/A | a vs. c LA/A | |
| HBsAg Pos | 77% | 100% | 86% | 95% | 78% | 89% | .625/NA | 1.0/NA |
| Mean HBsAg score | 1.18 (0.91) | 1.53 (0.7) | 1.24 (0.77) | 1.47 (0.77) | 1.05 (0.72) | 1.36 (0.88) | .771/.667 | .631/.681* |
| HBcAg Pos | 73% | 74% | 57% | 58% | 17% | 56% | .219/.508 | .006/.25 |
| Mean HBcAg score | 1.50(1.26) | 1.47 (1.17) | 0.71 (0.78) | 1.05 (1.08) | 0.17 (0.38) | 0.82 (1.01) | .006/.177 | .001/.043† |
A, adefovir; LA, lamivudine and adefovir; NA, not applicable.
No significant difference between week 192 and baseline by repeated measures.
Mean week 192 HBcAg results significantly different from baseline by repeated measures.
Combined maintained response
The primary endpoint of the study was a combined biochemical, virological and histological response that was maintained to the end of the study. The proportion of patients with a maintained response by life-table analysis is shown in Figure 3a. Patients on combination therapy were more likely to achieve a combined response, although it was not statistically significant at 48 weeks (59% vs. 26%, P = 0.058), and more likely to sustain it during the study (68% vs. 31%, P = 0.029) (Table 2).
Figure 3 ∣.
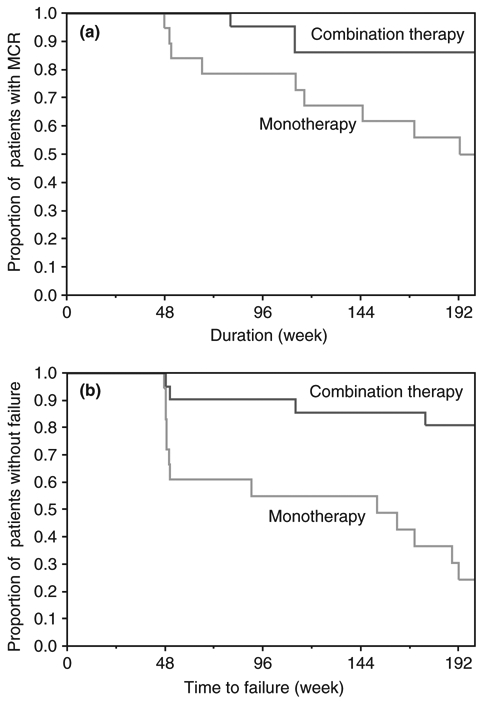
(a) Combined maintain response after 48 weeks by treatment group using life-table analysis. (b) Absence of treatment failure after 48 weeks by treatment group using life-table analysis.
Differences between the two groups were more revealing using the negative endpoint of ‘treatment failure’, defined as persistence of HBV DNA levels >105 copies/mL, virological breakthrough or drug toxicity. Treatment failure was more common in the monotherapy group both at week 48 (47% vs. 9%, P = 0.06) and 192 (68% vs. 18%, P = 0.002). The time to failure is shown in Figure 3b and the difference between the two groups was highly significant (P = 0.0111).
Resistance
Genotypic resistance was assessed at baseline, weeks 48 and 192 and at the time of virological breakthrough. At baseline, no patient had adefovir resistance and 70% of patients with previous exposure to lamivudine had lamivudine resistance. At week 48, no patient had adefovir resistance, despite the fact that 9 patients on adefovir monotherapy failed to achieve even a partial virological response. By 192 weeks, however, adefovir resistance was detected in 6 of 19 (32%) (N236T, n = 4 and A181V, n = 2) patients randomised to adefovir monotherapy compared with no patient on combination therapy (P = 0.027). Three of six patients who developed adefovir resistance had pre-existing lamivudine resistance. At virological breakthrough lamivudine resistance was identified only in patients with prior lamivudine treatment and did not develop in any treatment-naïve patient given the combination of adefovir and lamivudine.
Safety
Both monotherapy and combination therapy were well tolerated. Two patients receiving combination therapy had a persistent increase in serum creatinine >0.5 mg/dL above baseline and were switched to entecavir therapy, whereupon creatinine values returned to baseline levels. No patient in the adefovir monotherapy arm had serum creatinine elevations greater than 0.5 above baseline. Two patients developed a serious adverse event (vocal cord carcinoma and acute cholecystitis), neither of which was considered drug-related.
DISCUSSION
Combination therapy in chronic hepatitis B has two potential advantages: first, the possibility of an additive or synergistic antiviral response and second, prevention of antiviral resistance.7 Few studies have assessed combination therapy in chronic hepatitis B.8-12 Combinations that have been evaluated include lamivudine and peginterferon,10, 11 lamivudine and adefovir,12 lamivudine and telbivudine9 and adefovir and emtricitabine.8 Collectively, these studies have failed to demonstrate a clear advantage of combination over monotherapy in terms of achieving important endpoints, such as loss of HBeAg or HBsAg. However, none of the reported studies monitored patients beyond 1 to 2 years, and none reported long-term histological results. The short duration of follow-up may have been insufficient to demonstrate a benefit of combination therapy over monotherapy, especially in the prevention of antiviral resistance. This study provides the longest follow-up of combination therapy studies including 4 year histological data.
The results showed that a greater proportion of patients receiving combination therapy had a combined response at 1 year compared with those receiving monotherapy, and this difference was significant at 4 years. In this study, the mean reduction in HBV DNA at week 48 was significantly higher with combination therapy compared with adefovir monotherapy, and was incrementally better when compared with previous reports using lamivudine-monotherapy.13, 14 Thus, this study demonstrated a small additive antiviral effect when two nucleos(t)ide analogues are combined as has been reported with other combinations.8, 9, 12 More importantly, in the current study combination therapy lowered the rate of virological breakthrough and antiviral resistance. No patient on both adefovir and lamivudine developed genotypic resistance compared with 32% of those receiving adefovir only – which is similar to previously published rates.15 Other studies have reported a reduction but not prevention of antiviral resistance with combination therapy compared with monotherapy.8, 9, 12 The absence of virological breakthrough and antiviral resistance in this study may have been due to switching patients on combination therapy to other therapies when viral levels remained elevated. The advantages of preventing antiviral resistance were evident at the end of 192 weeks, which revealed a significantly higher rate of complete viral suppression and HBeAg loss in the combination group compared with the monotherapy group. Despite achieving these beneficial endpoints, the rate of HBsAg loss was low occurring in only one patient.
Previous studies of combination therapy did not report on histological benefit. This study with three scheduled liver biopsies demonstrated a substantial histological benefit to maintaining an undetectable HBV DNA level for 192 weeks. Significant improvements in histological activity and fibrosis, including reversal of cirrhosis were evident at 192 weeks compared with baseline in patients who were persistently HBV DNA negative regardless of treatment assignment. At week 192, onethird of patients had complete resolution of fibrosis and one quarter had a normal liver biopsy. Results were similar in the combination and monotherapy groups, probably because analyses were limited to patients who had a continued virological response and, thus eligible for repeat liver biopsy at 192 weeks (in these analyses data were not carried forward).
The results of this and other studies, strongly suggest that neither lamivudine nor adefovir should be used alone because of the high rate of resistance in the former and the low antiviral potency of the latter.15-19 Maintaining viral suppression resulted in better treatment outcomes regardless of how it was achieved, but was more common with combination therapy.
An important question is whether combination therapy is still necessary, now that more potent agents are available that have low rates of antiviral resistance. For example, the rate of resistance in treatment-naïve subjects was reported to be 1% to entecavir at 6 years4 and 0% to tenofovir at 5 years.20 Given these results, it is difficult to justify the expense and the potential for increased toxicity with combining lamivudine and adefovir over monotherapy with entecavir or tenofovir. The combination approach may be considered in certain circumstances, such as in patients with decompensated disease, those who are immunosuppressed and for management of established antiviral resistance. An unanswered question by this and other studies is what are the best agents to combine? Combining two agents that have a higher potency and barrier to resistance would seem logical, but given the superior efficacy of these agents, it would require a large study of long duration to show a treatment benefit of combination therapy over monotherapy.
This study had several limitations. A comparison group receiving lamivudine alone was not included. Use of lamivudine-monotherapy was considered inappropriate because of the number of historical studies, including our own that demonstrated resistance rates of 50% to 80% by 4 years.18, 21-23 In addition, the number of failures at week 192 may have been overestimated because patients who developed resistance or had a poor response after 48 weeks were switched to other therapies and considered long-term failures (based upon intention-to-treat analysis). However, it is possible that some of these patients may have ultimately had spontaneous improvement despite lack of full virological response at an earlier point.
In conclusion, this study revealed that the combination of lamivudine and adefovir was associated with a more durable antiviral response than adefovir alone. This resulted in higher rates of HBeAg loss and lower rates of antiviral resistance. The accumulating evidence indicates that these two agents should no longer be used as monotherapy. However, whether combination therapy is necessary in the era of potent agents with high barrier to resistance and what would be the best combination to use remains to be determined. Clearly other approaches that can lead to higher rates of HBsAg loss are needed.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank the many physicians who cared for the patients described in this report, including Drs. Alejandro Soza, Brian Borg, Rohit Loomba, Apurva Modi, Mazen Nourredin, Adil Abdulla, Naveen Gara and Souvik Sarkar; and the nursing staff of the Liver Diseases Branch Outpatient Clinic. The authors also gratefully acknowledge Gilead Sciences (Foster City, CA) who provided adefovir dipivoxil under a Clinical Trial Agreement. Declaration of personal interests: None. Declaration of funding interests: This research was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute, National Institutes of Health.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
Table S1. Endpoints of efficacy and failure at weeks 48 and 192 analysed by treatment-naïve and –experienced status.
REFERENCES
- 1.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45: 507–39. [DOI] [PubMed] [Google Scholar]
- 2.Dienstag JL, Goldin RD, Heathcote EJ, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology 2003; 124: 105–17. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125: 1714–22. [DOI] [PubMed] [Google Scholar]
- 4.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology 2009; 49: 1503–14. [DOI] [PubMed] [Google Scholar]
- 5.Villet S, Pichoud C, Villeneuve JP, et al. Selection of a multiple drug-resistant hepatitis B virus strain in a livertransplanted patient. Gastroenterology 2006; 131: 1253–61. [DOI] [PubMed] [Google Scholar]
- 6.Yim HJ, Hussain M, Liu Y, et al. Evolution of multi-drug resistant hepatitis B virus during sequential therapy. Hepatology 2006; 44: 703–12. [DOI] [PubMed] [Google Scholar]
- 7.Ghany MG, Doo EC. Antiviral resistance and hepatitis B therapy. Hepatology 2009; 49: S174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hui CK, Zhang HY, Bowden S, et al. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol 2008; 48: 714–20. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Leung N, Teo EK, et al. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 2005; 129: 528–36. [DOI] [PubMed] [Google Scholar]
- 10.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005; 352: 2682–95. [DOI] [PubMed] [Google Scholar]
- 11.Marcellin P, Lau GK, Bonino F, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2004; 351: 1206–17. [DOI] [PubMed] [Google Scholar]
- 12.Sung JJ, Lai JY, Zeuzem S, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol 2008; 48: 728–35. [DOI] [PubMed] [Google Scholar]
- 13.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006; 354: 1001–10. [DOI] [PubMed] [Google Scholar]
- 14.Lai CL, Shouval D, Lok AS, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med 2006; 354: 1011–20. [DOI] [PubMed] [Google Scholar]
- 15.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 2006; 131: 1743–51. [DOI] [PubMed] [Google Scholar]
- 16.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med 1998; 339: 61–8. [DOI] [PubMed] [Google Scholar]
- 17.Dienstag JL, Schiff ER, Wright TL, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med 1999; 341: 1256–63. [DOI] [PubMed] [Google Scholar]
- 18.Chang TT, Lai CL, Chien RN, et al. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol 2004; 19: 1276–82. [DOI] [PubMed] [Google Scholar]
- 19.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 2003; 348: 800–7. [DOI] [PubMed] [Google Scholar]
- 20.Snow-Lampart A, Chappell B, Curtis M, et al. No resistance to tenofovir disoproxil fumarate detected after up to 144 weeks of therapy in patients monoinfected with chronic hepatitis B virus. Hepatology 2011; 53: 763–73. [DOI] [PubMed] [Google Scholar]
- 21.Yao GB, Zhu M, Cui ZY, et al. A 7-year study of lamivudine therapy for hepatitis B virus e antigen-positive chronic hepatitis B patients in China. J Dig Dis 2009; 10: 131–7. [DOI] [PubMed] [Google Scholar]
- 22.Lok AS, Zoulim F, Locarnini S, et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology 2007; 46: 254–65. [DOI] [PubMed] [Google Scholar]
- 23.Lau DT, Khokhar MF, Doo E, et al. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 2000; 32: 828–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


