The BCR-ABL negative myeloproliferative neoplasms (MPNs) include essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF). These MPNs are generally characterized by clonal proliferation of mature hematopoietic cells.1 ET and PV may progress to MF, and all 3 disease entities may progress to acute myeloid leukemia (AML). Amongst the chronic-phase MPNs, ET is generally associated with the lowest rates of transformation to MF and AML.Transformation to AML has a poor prognosis, and the clinical risk factors for transformation as well as biology of this process remains to be understood.2
The hallmark of MPN pathogenesis is activating mutations in the JAK-STAT pathway (JAK2, MPL, CALR). Non JAK-STAT pathway mutations, such as ASXL1, SRSF2, EZH2, IDH1/2, and RAS pathway mutations are all associated with increased risk of transformation of MF to AML.3,4 Of note, mutations in TP53 are observed frequently at the time of leukemic transformation, and appear to play a role in the biology of disease transformation.5
A 57-year-old woman with beta-thalassemia minor presented in March 2014 with an incidental finding of thrombocytosis to 994K/mcl and white blood count (WBC) count of 12.4K/mcl. Molecular genetic analysis identified a JAK2V617F mutation and cytogenetics demonstrated a normal female karyotype. Bone marrow examination was consistent with ET. Given her low-risk IPSET score the patient was started on aspirin. She subsequently developed both a deep venous thrombosis and pulmonary emboli in August 2014 and was started on hydroxyurea and rivaroxaban, which she was maintained on for the next 3.5 years.
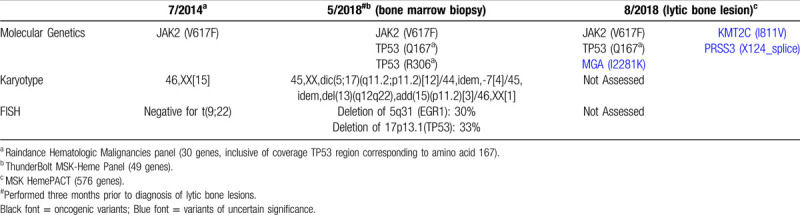
In March 2018, the patient's platelet count began to decrease, and shortly thereafter new leukoerythroblastosis was noted on peripheral blood smear. A bone marrow examination identified dysplastic megakaryocytes and reticulin fibrosis (MF-2) consistent with post-essential thrombocythemia myelofibrosis (pET-MF). No increase in blasts was noted. Cytogenetic analysis detected a complex karyotype in 19 of 20 metaphase cells with a dicentric chromosome, dic(5q;17p), in the stem line. Loss of chromosome 7, and a deletion of the long arm of chromosome 13 and an addition of the short arm of chromosome 15 in 2 subclones, was noted as well. The dic(5q;17p) results in loss of the long arm of chromosome 5 and the short arm of chromosome 17, including TP53 (Fig. 1A), which was confirmed by FISH tests (Table 1). DNA sequencing studies from bone marrow, peripheral blood, and lytic bone lesion (using the MSK-IMPACT platform and Raindance and Thunderbolt platforms as previously described4,6) was performed. These studies demonstrated a JAK2V617F mutation as well as TP53Q167∗ and TP53R306∗ mutations (Table 1).
Figure 1.
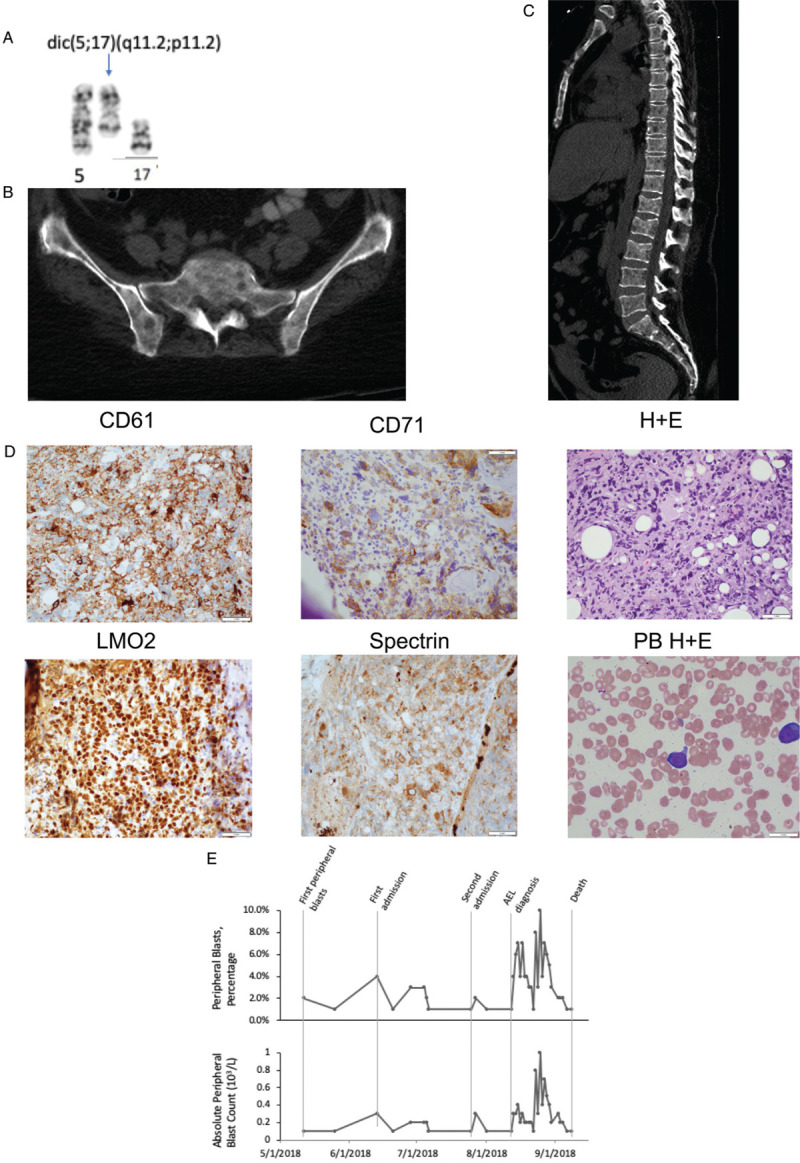
Radiologic, cytogenetic and hematologic changes associated with disease transformation. (A) A partial karyogram with dicentric chromosome dic(5q;17p) which was detected in 19 of 20 metaphase cells (B) Sacral and iliac necrosis secondary to leukemic transformation demonstrating appendicular skeletal involvement (C) Extensive lytic lesions of the axial skeleton (D) Staining of neoplastic cells identifying erythroblastic origin without evidence of significant peripheral blasts. Bone biopsy staining noted positivity for CD61, CD71, LMO2 and Spectrin; peripheral blood however noted few peripheral blasts on blood smear (E) Peripheral blast count trend (absolute count and percentage): over the course of 2018 the patient's peripheral blast count remained low and changed little during the patient's progression from ET to MF and finally transformation to acute erythroid leukemia (AEL). No blasts were noted in the peripheral blood until May 2018, after diagnosis of myelofibrosis.
Table 1.
Molecular and cytogenetic evolution over disease course
Three months later, the patient developed progressive diffuse pain in her shoulders, chest, lower back, and hips associated with generalized weakness, culminating in hospital admission. Physical exam revealed tenderness to palpation of the lumbosacral spine and hips; however, radiographs and a bone scan noted no fractures or focal osseous lesions. An MRI of the left femur noted periostitis for which she received methylprednisolone with a transient response. She was discharged with an opiate regimen and a trial of ruxolitinib for presumed myelofibrosis-related bone pain.
Several weeks later the patient was again admitted for intractable bone pain. Initial labs were notable for hypercalcemia. A CT scan of the abdomen and pelvis noted new lytic lesions of the axial and appendicular skeleton (Fig. 1B-C). Workup for a plasma cell dyscrasia was negative. A bone biopsy of a lytic lesion identified erythroid/megakaryocytic leukemic transformation of disease. Immunohistochemistry of neoplastic cells demonstrated staining for CD61, CD71, CD117, Spectrin, LMO2, and ERG (Fig. 1D). Staining was negative for CD34, MPO, E-Cadherin, CD138 and BCMA. Cytogenetics studies could not be performed. Mutational profiling from the bone lesion demonstrated the presence of a heterozygous JAK2V617F mutation, and TP53Q167∗, as well as several other variants of unknown significance (Table 1).
The patient rapidly declined and developed multi-organ failure. She subsequently expired (within 4 weeks of diagnosis of leukemic transformation).
This case highlights several important and unusual features that are of significance to clinicians treating MPN patients. First, the patient had an unusually rapid progression from ET to post-ET MF to AML (4 years), whereas historical data demonstrates a 10-year incidence of progression from ET to post-ET MF and of leukemic transformation of 0.8% and 0.7%, respectively.7 As well, application of prognostic modeling at the time of progression to MF, utilizing tools such as the MYSEC-PM model risk calculator8 or MIPSS-70,9 predicted for a far longer survival; the patient expired within 6 months of progression to MF as compared to a predicted median survival of 4.5 years.
Second, the presentation of leukemic transformation in this case appears to be extremely rare, as this patient presented only with severe diffuse skeletal pain. Initial imaging found no evidence of osteolytic disease; however, 1 month later her CT scan noted diffuse lytic lesions due to biopsy-proven erythroid/megakaryocytic leukemia. A key cautionary observation in this case was the lack of a markedly elevated or rising peripheral blood blast count. Indeed, the peripheral blood blast count was largely unchanged from the time of MF diagnosis to that of AML diagnosis (Fig. 1E). Our review of the literature identified only three similar cases of myeloproliferative neoplasms associated with osteolytic bone lesions.10–12
Finally, serial genomic and cytogenetic studies clearly indicate a complex clonal evolution. At the time of progression to MF the patient had developed two truncation mutations of TP53 (only one of which was retained at the time of leukemic transformation) and a complex karyotype with dicentric chromosome dic(5q;17p), along with loss of chromosome 7 and deletion of 13q as clonal evolution. Dic(5;17) is a recurring chromosome abnormality in myeloid neoplasia, particularly in therapy-related and secondary leukemia, which result in loss of the TP53 locus on 17p and deletion of 5q, and is frequently associated with TP53 mutations.13 This observation is consistent with prior human genomic and murine functional studies indicating cooperativity between JAK2V617F and TP53 loss in post-MPN AML.5 Notably, acute leukemia with megakaryocytic and erythroid differentiation was recently described in two previously healthy patients without a prior diagnosis of MPN, both of whom harbored JAK2 and TP53 mutations, one of which presented similarly with diffuse lytic lesions of the bone.14 The TP53 pathway can also be altered by changes in MDM2 and MDM4 (which regulate TP53 levels) expression in post-MPN AML.15 Thus, disregulation of the TP53 pathway appears to be a common event in leukemic transformation of MPNs.
Importantly, current prognostic scoring systems in MF do not adequately capture the impact of TP53 mutations, particularly cases with both TP53 mutations and concomitant loss of the TP53 locus owing to chromosomal alterations. The bulk of the evidence from clinical and genomic studies, including this case, strongly suggest that MF/MPN patients with such a genotype should be considered high-risk for leukemic transformation.
This case serves to remind clinicians taking care of MPN patients that while many patients will have favorable outcomes, disease progression may be rapid, present atypically, and that the risk of progression may not always be adequately captured by current risk-scoring systems. Further understanding and refinement of genomic and other biomarkers of disease progression are needed in this patient population.
Sources of Funding
This study was supported by Cancer Center Support Grant/Core Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748); R.K.R is supported by NCI 1K08CA188529-01.
Disclosures
RKR has received consulting fees from: Constellation, Incyte, Celgene, Promedior, CTI, Jazz Pharmaceuticals, Blueprint, Stemline, and research funding from Incyte, Constellation, Stemline.
Footnotes
Citation: Chernak BJ, Sen F, Farnoud N, Ayache JB, Zhang Y, DeWolf S, Rampal RK. Atypical Presentation of Erythroid/Megakaryocytic Leukemic Transformation of a Myeloproliferative Neoplasm Associated with Mutation and Loss of TP53. HemaSphere, 2020;4:4:(e411). http://dx.doi.org/10.1097/HS9.0000000000000411
References
- 1.Saeidi K. Myeloproliferative neoplasms: current molecular biology and genetics. Crit Rev Oncol Hematol. 2016;98:375–389. [DOI] [PubMed] [Google Scholar]
- 2.Abdulkarim K, Girodon F, Johansson P, et al. AML transformation in 56 patients with Ph- MPD in two well defined populations. Eur J Haematol. 2009;82:106–111. [DOI] [PubMed] [Google Scholar]
- 3.Vannucchi AM, Lasho TL, Guglielmelli P, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27:1861–1869. [DOI] [PubMed] [Google Scholar]
- 4.Santos FBS, Getta B, Masarova L, et al. Prognostic impact of RAS-pathway mutations in patients with myelofibrosis. Leukemia. 2020;34:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rampal R, Ahn J, Abdel-Wahab O, et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc Natl Acad Sci U S A. 2014;111:E5401–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng DT, Mitchell TN, Zehir A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbui T, Thiele J, Passamonti F, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29:3179–3184. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Thiele J, Girodon F, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012;120:1197–1201. [DOI] [PubMed] [Google Scholar]
- 9.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-Enhanced International Prognostic Score System for Transplantation-Age Patients With Primary Myelofibrosis. J Clin Oncol. 2018;36:310–318. [DOI] [PubMed] [Google Scholar]
- 10.Chambers I, Truong P, Kallail KJ, et al. Extensive bone marrow necrosis and osteolytic lesions in a case of acute myeloid leukemia transformed from polycythemia vera. Cureus. 2016;8:e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro R, Rizkalla K, Lam S. Extensive bone marrow necrosis in a case of acute myeloid leukemia transformed from a myeloproliferative neoplasm. Case Rep Oncol. 2015;8:345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurisic V, Terzic T, Pavlovic S, et al. Elevated TNF-alpha and LDH without parathormone disturbance is associated with diffuse osteolytic lesions in leukemic transformation of myelofibrosis. Pathol Res Pract. 2008;204:129–132. [DOI] [PubMed] [Google Scholar]
- 13.Wang P, Spielberger RT, Thangavelu M, et al. dic(5;17): a recurring abnormality in malignant myeloid disorders associated with mutations of TP53. Genes Chromosomes Cancer. 1997;20:282–291. [DOI] [PubMed] [Google Scholar]
- 14.Xiao W, Rampal R, Zhang Y, et al. JAK/MAP kinase pathway activation and TP53 mutations in acute leukemia with megakaryocytic and erythroid differentiation. Leukemia. 2018;32:1842–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcellino BK, Hoffman R, Tripodi J, et al. Advanced forms of MPNs are accompanied by chromosomal abnormalities that lead to dysregulation of TP53. Blood Adv. 2018;2:3581–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]


