Abstract
Gastric cancer (GC) is a common gastrointestinal malignancy, and cisplatin (DDP) is an important component of chemotherapeutic regimens for GC. However, the application of DDP is limited by its dose-dependent systemic toxicity. Resveratrol (RES) is a natural polyphenol compound that has chemopreventive and therapeutic effects against various cancers, including GC. However, whether RES can sensitize GC cells to DDP remains unknown. Following RES/DDP combination treatment, cell viability was determined by Cell Counting Kit-8 and colony-forming assays, and cell apoptosis and the cell cycle were detected by FITC-Annexin V/PI staining assay and PI staining assay, respectively, followed by flow cytometry. Moreover, western blotting was performed to evaluate the protein expression levels, and the intracellular free Ca2+ concentration was determined by a Fluo-4 AM probe after cell cotreatment with RES and DDP. The present results demonstrated that RES/DDP combination treatment significantly inhibited cell viability, promoted cell apoptosis and induced G2/M phase arrest in AGS cells. In addition, it was determined that RES combined with DDP significantly increased the levels of Bax, cleaved poly-ADP-ribose polymerase (PARP), glucose-regulated protein 78 (GRP78), PRKR-like ER kinase (PERK), p-eukaryotic translation initiation factor 2α (p-eIF2α), CCAAT/enhancer binding protein homologous protein (CHOP) and cleaved caspase-12, whereas Bcl-2 expression was downregulated following RES/DDP cotreatment. Moreover, RES/DDP cotreatment significantly upregulated phosphorylated cyclin-dependent kinase 1 (p-CDK1, Tyr15), p21Waf1/Cip1 and p27Kip1 protein levels and downregulated Cdc25C protein levels. In conclusion, RES and DDP synergistically inhibited the growth of the gastric adenocarcinoma cell line AGS by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest via activation of the PERK/eIF2α/activating transcription factor 4 (ATF4)/CHOP signaling pathway and caspase-12 and by inactivating the CDK1-cyclin B1 complex. These results indicated that RES is a promising adjuvant for DDP during GC chemotherapy.
Keywords: resveratrol, gastric cancer, cisplatin, apoptosis, endoplasmic reticulum stress, G2/M cell cycle arrest
Introduction
Gastric cancer (GC) is one of the most common gastrointestinal malignancies and remains the leading cause of cancer-related deaths worldwide, with over half of the cases occurring in East Asia (1,2). Adequate surgical resection is the only curative treatment strategy for localized GC, however a high risk of recurrence and metastasis remains after surgical resection (3). Furthermore, most patients are diagnosed at an advanced stage, and chemotherapy is the first-line treatment (4). Cisplatin [cis-diamminedichloroplatinum(II); DDP] is a widely used platinum-based antineoplastic agent for GC treatment, especially for patients at an advanced stage (5). However, its application is limited by drug resistance and its systemic toxicity, such as nephrotoxicity, ototoxicity, neurotoxicity and hepatotoxicity (6). Thus, it is essential to develop novel chemical sensitizing agents for DDP to enhance its efficacy and attenuate side effects in GC patients.
Resveratrol (3,4′,5-trihydroxystilbene; RES) is a nonflavonoid polyphenolic compound notably present in grapes and red wine (7). It plays a protective role against a wide variety of diseases, including cardiovascular diseases (8), neurodegenerative diseases (9), inflammatory diseases (10) and diabetes (11). Moreover, recent preclinical studies have identified RES as a chemopreventive and therapeutic agent as well as a chemical sensitizer for various malignant tumors (12), which displays lower cytotoxicity to normal cells than traditional therapy. Although previous studies have demonstrated that RES exerts its anticancer effects against GC cells by promoting apoptosis and inducing cell cycle arrest or suppressing migration and invasion (13–15), whether it can sensitize GC cells to DDP and the underlying mechanisms remain to be determined.
The endoplasmic reticulum (ER) is the principle organelle responsible for biogenesis, folding and trafficking of over one-third of the proteins in eukaryotic cells (16). Extrinsic and intrinsic perturbations disturb ER homeostasis, trigger ER stress and activate the unfolded protein response (UPR), which promotes cell survival, or induce apoptosis if ER stress is severe or chronic (17). In fact, the UPR promotes cancer cell survival by enhancing cancer cell adaptation to hostile environmental conditions, and regulating the UPR is a promising anticancer strategy (18). Recent studies have demonstrated that RES promoted ER stress-mediated apoptosis in colon cancer cells (19), ovarian cancer cells (20), and malignant melanoma cells (21). However, the role of RES in ER stress-induced apoptosis in GC cells has not been studied to date.
In the present study, it was revealed that RES synergized with DDP in antineoplastic effects against the human GC cell line AGS by inducing ER stress-mediated apoptosis and G2/M cell cycle arrest. Mechanistic experiments revealed that RES/DDP combination treatment activated the PRKR-like ER kinase (PERK)/eukaryotic translation initiation factor 2α (eIF2α)/activating transcription factor 4 (ATF4)-CCAAT/enhancer binding protein homologous protein (CHOP) signaling pathway and upregulated cleaved caspase-12. Furthermore, RES/DDP combination treatment arrested AGS cells in G2/M phase by inactivating the cyclin-dependent kinase 1 (CDK1)-cyclin B1 complex and upregulating p21Waf1/Cip1 and p27Kip protein levels. The present results provided evidence that may lead to the future application of RES as an adjuvant for GC chemotherapy.
Materials and methods
Cell culture
The human GC cell lines AGS, KATO III, MKN-45 and NCI-N87 were obtained from the Type Culture Collection of the Chinese Academy of Sciences. The AGS cells were cultured in Hams F-12K (Kaighns) medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator containing 5% CO2. The KATO III cell line was cultured in Iscoves Modified Dulbeccos Medium (IMDM; Gibco; Thermo Fisher Scientific, Inc.), and the MKN-45 and NCI-N87 cell lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) as described above.
Cell Counting Kit-8 (CCK-8) assay
Cell viability was detected by a CCK-8 assay (Dojindo Molecular Technologies, Inc.). Approximately 5×103 cells/well were seeded into 96-well plates and incubated overnight at 37°C. Next, various doses of RES (0, 5, 10, 20, 30, 40, 50, 60, 70, and 80 µM) and/or DDP (0, 0.5, 1, and 2 µg/ml) (both from Selleck Chemicals) were added. After culturing for another 24, 48, 72 or 96 h, each well was incubated with 10 µl CCK-8 solution for 1–3 h before measuring the absorbance at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Evaluation of the combination effect of RES and DDP
The combination index (CI) was applied to evaluate the combination effect of RES and DDP in AGS cells according to the Chou-Talalay method (22). CI was calculated by CompuSyn software (ComboSyn, Inc.), and the combination effect was defined as follows: CI <1, synergistic effect; CI >1, antagonistic effect; and CI=1, additive effect.
Morphological observation
AGS cells were seeded in 6-well plates (1×105 cells/well), incubated at 37°C overnight, and exposed to RES (20 µM) and DDP (1 µg/ml) alone or in combination for 48 h. Cell morphology was observed using an inverted light microscope (magnification, ×100; Olympus Corporation).
Colony-forming assay
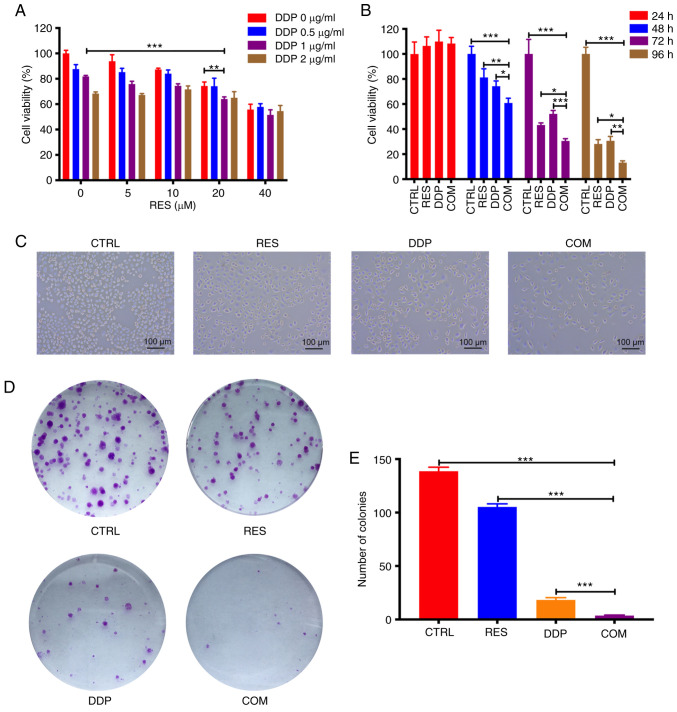
AGS cells were treated with RES (20 µM) and DDP (1 µg/ml) alone or in combination for 48 h. Thereafter, the cells were trypsinized by 0.25% trypsin (Gibco; Thermo Fisher Scientific, Inc.) and dispensed into individual wells of 6-well plates at a density of 1×103 cells/well. Following another 14 days of drug-free culture and changing of the medium every other day, the cells were fixed with 4% paraformaldehyde at 25°C for 15 min and stained with 0.1% crystal violet at 25°C for another 15 min. Finally, colonies (≥10 cells) were counted using an inverted light microscope (magnification, ×100; Olympus Corporation).
Cell apoptosis assay
Apoptotic cells were detected by an FITC-Annexin V Apoptosis Detection Kit (BD Biosciences) according to the manufacturers instructions. The cells (1×105/well) were seeded in 6-well plates and incubated overnight at 37°C. Then, the cells were treated with RES (0, 20, and 40 µM) for 72 h or exposed to RES (20 µM) and DDP (1 µg/ml) alone or in combination for 48 and 72 h. Subsequently, the cells were harvested with EDTA-free trypsin and resuspended in 500 µl of 1X binding buffer followed by incubation with FITC-Annexin V (5 µl) and PI (5 µl) at 25°C in the dark for 15 min. Finally, the apoptotic cells were analyzed by a BD FACSCanto II flow cytometer (BD Biosciences) and FlowJo software (version 10.4; BD Biosciences).
Cell cycle assay
The cell cycle assays were performed according to the manufacturers instructions using a cell cycle staining kit [MultiSciences (Lianke) Biotech Co., Ltd.] as previously described (23). After administration of RES (20 µM) and DDP (1 µg/ml) alone or in combination, the cells were harvested and fixed with 75% ethanol at −20°C overnight. Thereafter, the cells were hydrated with cold phosphate-buffered saline (PBS) for 15 min and incubated with DNA staining solution at 25°C for 30 min in the dark before evaluation by a BD FACSCanto II flow cytometer and ModFit LT software (version 5.0.9; Verity Software House Co., Ltd.).
Detection of intracellular free calcium ions (Ca2+)
The intracellular free Ca2+ concentration was determined by the calcium probe Fluo-4 AM (Beyotime Institute of Biotechnology) according to the manufacturers instructions. After exposure to RES (20 µM) and DDP (1 µg/ml) alone or in combination for 48 h, the cells were collected, washed twice with PBS and incubated with 2 µM Fluo-4 AM probe for 30 min at 37°C. Subsequently, the cells were washed with PBS, and cell fluorescence was determined using a BD FACSCanto II flow cytometer (excitation wavelength, 488 nm; emission wavelength, 525 nm).
Western blot analysis
Following treatment with RES (10, 20, and 40 µM) and DDP (1 µg/ml) alone or combination treatment of RES (20 µM) and DDP (1 µg/ml) for 48 h, total protein from AGS cells was extracted using cell lysis buffer (Cell Signaling Technology, Inc.) according to the manufacturers instructions. Western blot analysis was performed as previously described (24). Briefly, 20 µg of protein was loaded onto each lane and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with primary antibodies overnight at 4°C, followed by goat anti-rabbit secondary antibody (1:5,000, cat. no. HA1001; HuaBio Co., Ltd.) or goat anti-mouse secondary antibody (1:10,000, cat. no. BL001A, BioSharp Co., Ltd.) for 1 h at 25°C. The primary antibodies used were rabbit antibodies against CHOP (also named DDIT3; 1:1,000; product code ab179823; Abcam), poly-ADP-ribose polymerase (PARP; 1:1,000; cat. no. 9532; Cell Signaling Technology, Inc.), Bax (1:1,000, cat. no. 2774; Cell Signaling Technology, Inc.), Bcl-2 (1:1,000, cat. no. 2872, Cell Signaling Technology, Inc.), glucose-regulated protein 78 (GRP78; 1:1,000; product code ab108615; Abcam), PERK (1:1,000; cat. no. 5683; Cell Signaling Technology, Inc.), eIF2α (1:1,000; cat. no. 5324; Cell Signaling Technology, Inc.), p-eIF2α (1:1,000; cat. no. 3398; Cell Signaling Technology, Inc.), caspase-12 (1:1,000; product code ab62484; Abcam), cyclin B1 (1:1,000; cat. no. 4138; Cell Signaling Technology, Inc.), p-CDK1 (Tyr15; 1:1,000; cat. no. 4539; Cell Signaling Technology, Inc.), Cdc25C (1:1,000; cat. no. 4688; Cell Signaling Technology, Inc.), p21Waf1/Cip1 (1:1,000; cat. no. 2947; Cell Signaling Technology, Inc.), p27Kip1 (1:1,000; cat. no. 3686; Cell Signaling Technology, Inc.) and GAPDH (1:5,000; cat. no. 2118; Cell Signaling Technology, Inc.), and mouse monoclonal antibody against CDK1 (1:1,000; cat. no. 9116; Cell Signaling Technology, Inc.). The protein bands were quantified using ImageJ software (version 1.52a; National Institutes of Health). The phosphorylated protein bands were normalized to their total protein levels, and the other bands were normalized to GAPDH.
Statistical analysis
The results are presented as the mean ± standard deviation (SD). All statistical analyses were performed by Prism 7.0 (GraphPad Software, Inc.) using one-way ANOVA followed by Tukeys multiple comparisons test. Differences were considered to be statistically significant at P<0.05. All experiments were performed in triplicate.
Results
RES inhibits viability and promotes apoptosis of AGS cells
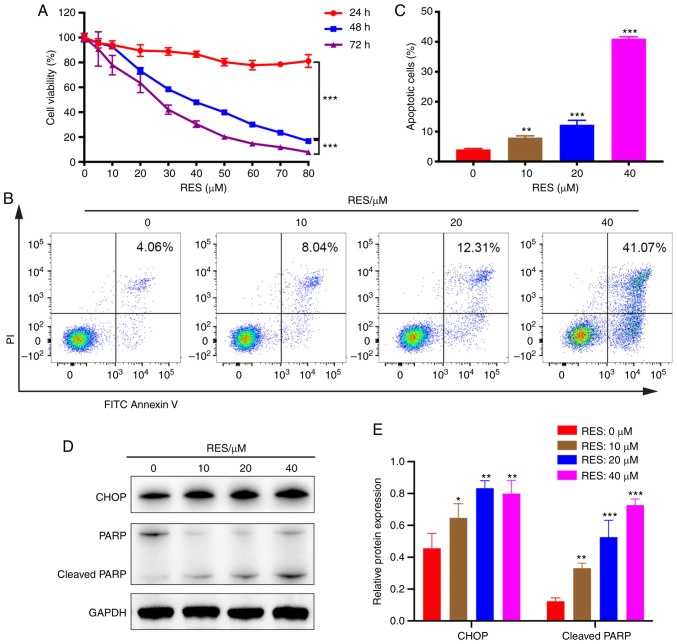
First, cell viability assays in 4 GC cell lines (AGS, KATO III, MKN-45 and NCI-N87) were performed and cell apoptosis was detected in 3 GC cell lines (AGS, KATO III and MKN-45) following RES/DDP cotreatment (Figs. S1, 2B and 3A-C). It was determined that the most significant results were obtained in AGS cells. Therefore, the human gastric adenocarcinoma cell line AGS was selected for the present study. After exposure to RES, the cell viability of AGS was explored by CCK-8 assay. The RES concentration gradient ranged from 5 to 80 µM. Our results demonstrated that RES displayed a dose-and time-dependent inhibitory effect on AGS cellular viability (P<0.001; Fig. 1A). Flow cytometric analysis indicated that RES treatment (10, 20 and 40 µM) consistently upregulated AGS cell apoptosis in a dose-dependent manner, with the percentage of cells undergoing apoptosis increasing from 4.06 to 41.07% with increasing RES concentration (P<0.001; Fig. 1B and C). Furthermore, the protein expression of CHOP, an early protein in ER stress, was detected after administration of RES and DDP alone or in combination for 24, 36, and 48 h. The most notable results were obtained for the 48-h time-point (data not shown). Thus, this time-point was selected for subsequent experiments. Western blot analysis revealed that RES administration (10, 20 and 40 µM) significantly increased the protein levels of CHOP and cleaved PARP in a dose-dependent manner (P<0.05; Fig. 1D and E). Collectively, the present results indicated that RES exerts anticancer activity against AGS cells by inhibiting cell viability and promoting cell apoptosis.
Figure 2.
RES and DDP synergistically inhibit AGS cell viability. (A) Cell viability was determined by CCK-8 assay after cotreatment with different doses of RES and DDP for 48 h. (B) Following RES (20 µM) and DDP (1 µg/ml) combination treatment for 24, 48, 72 and 96 h, cell viability was detected by CCK-8 assay. (C) The morphological changes in AGS cells were determined after RES (20 µM) and DDP (1 µg/ml) cotreatment for 48 h (magnification, ×100). (D) Following RES (20 µM)/DDP (1 µg/ml) combination treatment for 48 h, colony-forming assays were performed. (E) Colonies in each well were counted. The results are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001. RES, resveratrol; DDP, cisplatin; CCK-8, Cell Counting Kit-8; CTRL, control; COM, combination treatment.
Figure 3.
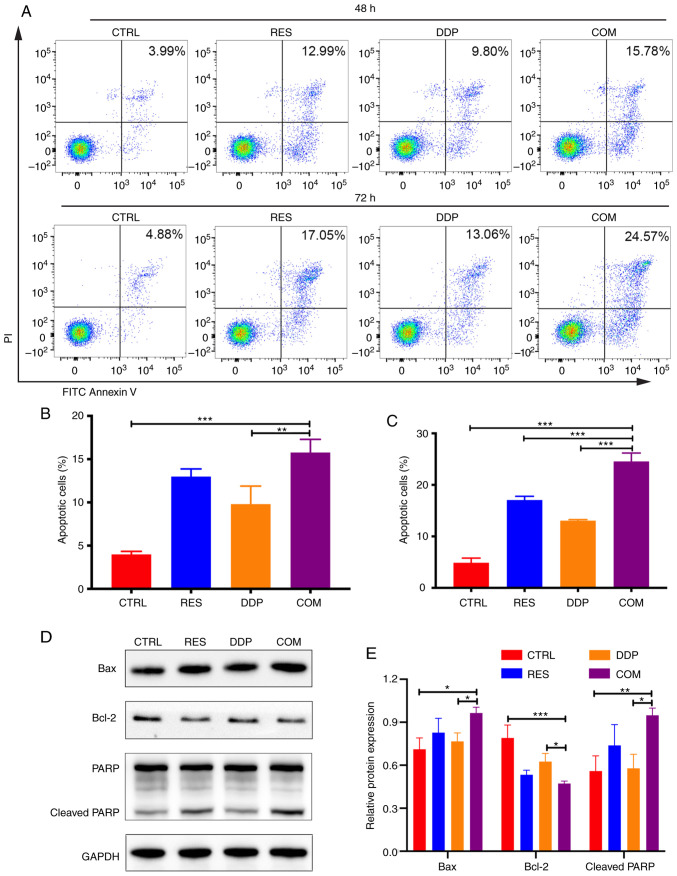
RES sensitizes AGS cells to DDP by inducing cell apoptosis. (A) Cell apoptosis was detected after RES (20 µM) and DDP (1 µg/ml) cotreatment for 48 and 72 h through FITC-Annexin V/PI assays and flow cytometry. (B and C) Histograms revealing the percentage of apoptotic cells. (D and E) The expression levels of Bax, Bcl-2 and PARP were detected by western blotting. The results are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001. RES, resveratrol; DDP, cisplatin; PARP, poly-ADP-ribose polymerase; CTRL, control; COM, combination treatment.
Figure 1.
RES inhibits viability and promotes apoptosis of AGS cells. (A) Cell viability was detected by CCK-8 assay after RES treatment (5–80 µM) for 24, 48 and 72 h. (B) Following exposure to RES (10, 20 and 40 µM) for 48 h, FITC-Annexin V/PI assays and flow cytometry were performed to detect cell apoptosis. (C) The percentage of apoptotic cells (Annexin V-FITC+/PI+ quadrant and Annexin V-FITC+/PI− quadrant) was determined. (D and E) The protein expression levels of CHOP and PARP were detected by western blotting upon RES administration for 48 h. Data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001. RES, resveratrol; DDP, cisplatin; CCK-8, Cell Counting Kit-8; PARP, poly-ADP-ribose polymerase; CHOP, CCAAT/enhancer binding protein homologous protein.
RES and DDP synergistically inhibit AGS cell viability
To investigate the growth-inhibitory effect of RES/DDP cotreatment on AGS cells, the cells were exposed to different doses of RES (0, 5, 10, 20 and 40 µM) and DDP (0, 0.5, 1 and 2 µg/ml) alone or in combination for 48 h. The combination treatment of RES (20 µM) and DDP (1 µg/ml) significantly suppressed viability of AGS cells compared to the viability observed under either RES treatment or DDP treatment alone (P<0.01; Fig. 2A). Therefore, 20 µM and 1 µg/ml were selected as the concentrations for RES and DDP, respectively, for the subsequent experiments. Next, cell viability was determined following administration of RES (20 µM) and DDP (1 µg/ml) either individually or in combination for 24, 48, 72 and 96 h. Although exposure to RES (20 µM) or DDP (1 µg/ml) for 24 h did not significantly inhibit cell viability, it was determined that RES and DDP cotreatment for 48, 72 and 96 h significantly inhibited AGS cell viability compared with that of either single-agent treatment (P<0.05; Fig. 2B). As shown in Fig. 2C, RES and DDP cotreatment notably decreased the number of cells compared to the number observed under either single-agent treatment alone. Besides, cell shrinkage, detachment and reduced cytoplasm were evident in the cells under RES/DDP combination treatment (Fig. 2C). In addition, the IC50 value against AGS cells decreased from 4.779 µg/ml under DDP-only treatment to 3.154 µg/ml under combination DDP/RES (20 µM) treatment (data not shown). Furthermore, the analysis of CI values by CompuSyn software at ED50, ED75 and ED90 (Table I) indicated that RES and DDP have synergistic inhibitory effects on AGS cell viability (22). The results of colony-forming assays indicated that the combination treatment significantly suppressed AGS cell viability over that attained by RES or DDP treatment alone (P<0.001; Fig. 2D and E). Collectively, the present results demonstrated that RES and DDP synergistically inhibit AGS cell viability.
Table I.
Combination index values of RES and DDP combination treatment at ED50, ED75 and ED90.
| RES (µM)/DDP (µg/ml) | ED50 | ED75 | ED90 |
|---|---|---|---|
| 25:1 | 0.931 | 0.867 | 0.910 |
| 12.5:1 | 0.911 | 0.853 | 0.862 |
RES, resveratrol; DDP, cisplatin; ED, effective dose.
RES sensitizes AGS cells to DDP by inducing cell apoptosis
FITC-Annexin V/PI double-staining assays were conducted to assess apoptotic cells after cotreatment with RES (20 µM) and DDP (1 µg/ml) for 48 and 72 h (Fig. 3A). The present results demonstrated that RES/DDP combination treatment for 48 h induced a higher apoptotic rate compared with that of DDP treatment alone (P<0.01; Fig. 3B). In particular, when the cotreatment time was extended to 72 h, the apoptotic rate was increased and was significantly higher than that under either RES or DDP administration alone (P<0.01; Fig. 3C). To investigate the molecular mechanisms underlying the proapoptotic effects of the RES/DDP combination treatment, apoptosis-related proteins were analyzed by western blotting. The results revealed that RES/DDP cotreatment upregulated the levels of the proapoptotic protein Bax and the cleaved form of PARP compared with the levels under DDP treatment alone, whereas expression of the antiapoptotic protein Bcl-2 was downregulated under cotreatment relative to DDP-only treatment (P<0.05; Fig. 3D and E). Collectively, the results indicated that RES enhanced the antitumor effect of DDP on cell apoptosis.
RES/DDP combination treatment activates ER stress-mediated apoptotic signaling pathways
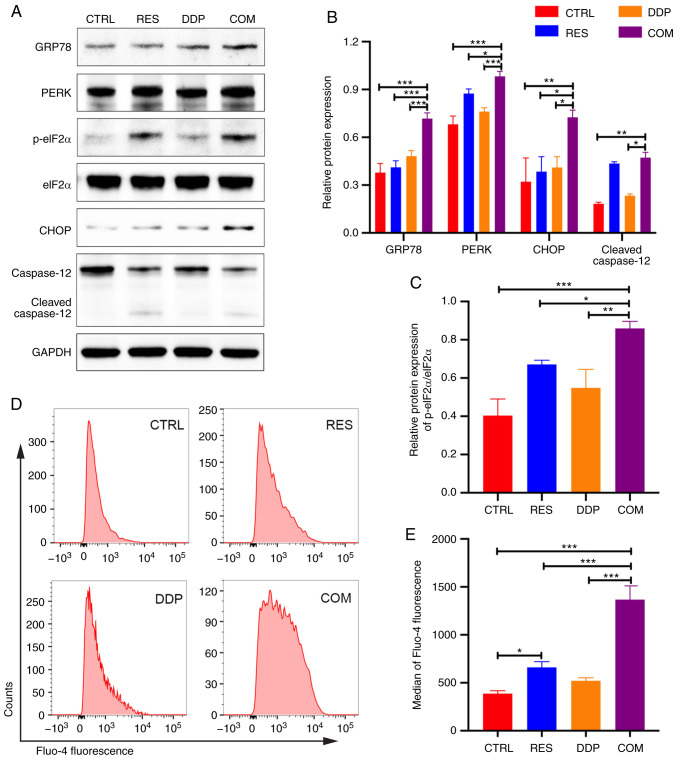
To better understand the molecular mechanisms underlying the proapoptotic effects of the RES/DDP combination, western blotting was performed. The expression of the ER stress chaperone GRP78 was significantly upregulated under RES/DDP combination treatment compared to the expression under either single-agent treatment alone (P<0.001; Fig. 4A and B). Moreover, cotreatment with RES and DDP significantly increased PERK, p-eIF2α and CHOP protein levels but had no significant effect on the expression of eIF2α, indicating that the PERK/eIF2α/ATF4/CHOP signaling pathway was activated by cotreatment (P<0.05; Fig. 4A-C). In addition, cleaved caspase-12 was upregulated upon RES and DDP combination treatment compared with its expression under DDP treatment alone (P<0.05; Fig. 4A and B). The enhancement of intracellular Ca2+ levels induces ER stress and apoptosis (25). Herein, Ca2+ probes and flow cytometry were applied to evaluate intracellular Ca2+ levels. It was revealed that exposure to RES (20 µM) alone for 48 h caused an increase in Ca2+ levels compared with levels in untreated cells (P<0.05; Fig. 4D and E). However, the RES/DDP combination treatment significantly enhanced the cytosolic Ca2+ levels and exhibited the greatest increase compared with the levels under either single-agent treatment alone (P<0.001; Fig. 4D and E). These results demonstrated that RES/DDP cotreatment activated ER stress-mediated apoptotic signaling pathways in AGS cells.
Figure 4.
RES/DDP combination treatment activates endoplasmic reticulum stress-mediated apoptotic signaling pathways. (A-C) The protein expression levels of GRP78, PERK, p-eIF2α, eIF2α, CHOP and caspase-12 were determined by western blotting. (D) Fluo-4 AM probe and flow cytometry were used to detect intracellular Ca2+ concentrations after RES (20 µM) and DDP (1 µg/ml) cotreatment for 48 h. (E) The median Fluo-4 fluorescence intensities are presented. The data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001. RES, resveratrol; DDP, cisplatin; GRP78, glucose-regulated protein 78; PERK, PRKR-like ER kinase; p-eIF2α, phosphorylated eukaryotic translation initiation factor 2α; CHOP, CCAAT/enhancer binding protein homologous protein; CTRL, control; COM, combination treatment.
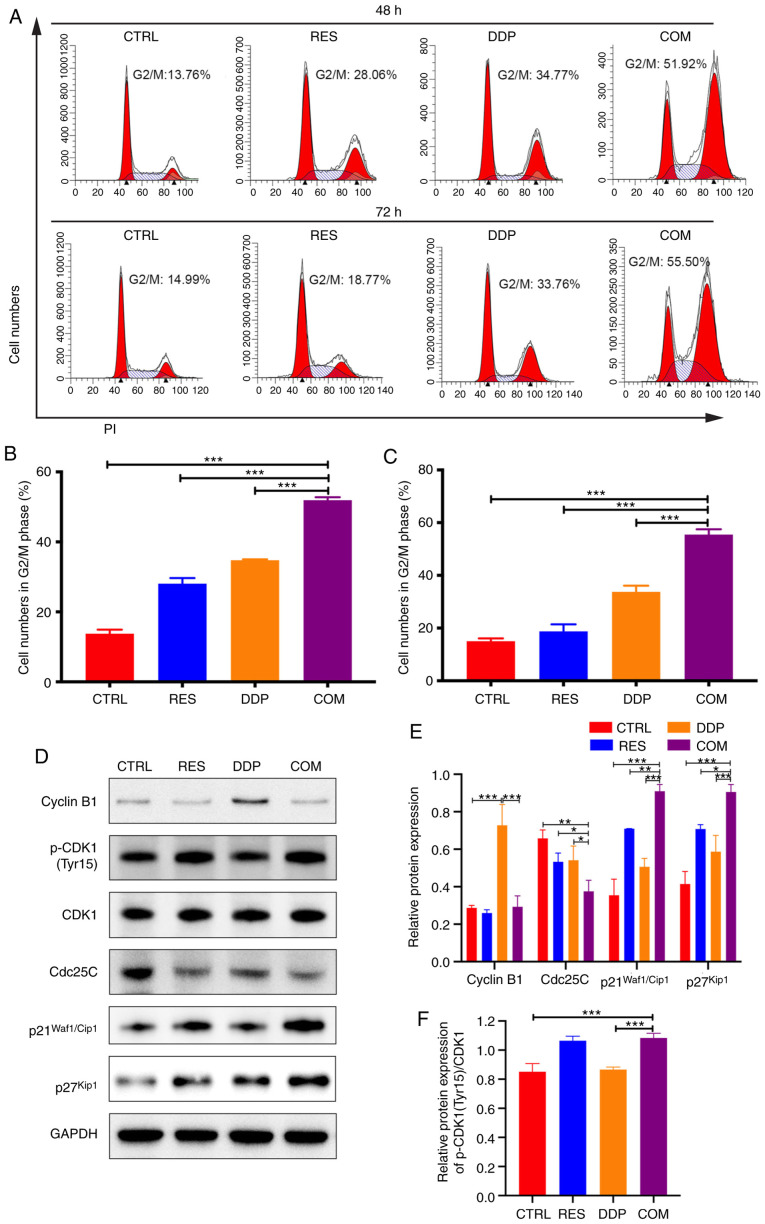
RES sensitizes AGS cells to DDP by inducing G2/M phase arrest
To identify the effect of RES/DDP combination treatment on cell cycle progression, PI staining assays and flow cytometry were performed. As revealed in Fig. 5A-C, RES/DDP cotreatment significantly increased the number of cells in the G2/M phase compared to the number observed under either single-agent treatment alone (P<0.001). Moreover, the expression levels of cell cycle progression regulators were detected by western blotting. The present results indicated that upon RES/DDP combination treatment, the phosphorylation level of CDK1 (Tyr15) and the protein levels of p21Waf1/Cip1 and p27Kip1 were significantly increased, whereas Cdc25C protein levels were downregulated (P<0.05; Fig. 5D-F). In addition, it was determined that DDP administration significantly upregulated cyclin B1 protein expression, while combination treatment of DDP and RES significantly decreased the protein level of cyclin B1 (P<0.001; Fig. 5D and E). These results demonstrated that RES sensitizes AGS cells to DDP by inducing G2/M phase arrest and by inactivating the CDK1-cyclin B1 complex and upregulating p21Waf1/Cip1 and p27Kip1 expression.
Figure 5.
RES sensitizes AGS cells to DDP by inducing G2/M cell cycle arrest. (A) PI staining and flow cytometry were performed to determine cell cycle progression after RES (20 µM) and DDP (1 µg/ml) cotreatment for 48 and 72 h. (B and C) The percentage of cells in the G2/M phase was examined. (D-F) After RES (20 µM) and DDP (1 µg/ml) combination treatment for 48 h, the protein levels of cyclin B1, p-CDK1 (Tyr15), CDK1, Cdc25C, p21Waf1/Cip1 and p27Kip1 were detected by western blotting. All data are presented as the mean ± SD (n=3). *P<0.05, **P<0.01 and ***P<0.001. RES, resveratrol; DDP, cisplatin; p-CDK1, phosphorylated cyclin-dependent kinase 1; CTRL, control; COM, combination treatment.
Discussion
Severe side effects and resistance to chemotherapy have been pressing issues hampering the success of GC treatment (26). Hence, new effective chemotherapeutic strategies are urgently required. RES has been revealed to act as an antineoplastic drug in numerous types of cancers, including GC (12–15,27). However, whether RES and DDP have synergistic effects against GC, and if so, what mechanisms underlie these effects are unknown. In the present study, evidence was provided that RES and DDP synergistically inhibited cell viability, prompted cell apoptosis and induced G2/M cell cycle arrest in gastric adenocarcinoma AGS cells. Furthermore, ER stress-induced apoptosis signaling pathways were significantly activated and the CDK1-cyclin B1 complex was inactivated under RES/DDP combined treatment.
The combination of naturally occurring agents with conventional chemotherapeutic drugs has been applied in the treatment of various types of cancers and can improve therapeutic efficiency and reduce dosages to alleviate toxicity (28). Despite recent advances in molecular targeted therapy and immunotherapy, DDP remains an important component of chemotherapeutic regimens for GC (5,26). However, its systemic toxicity and drug resistance impede its use. RES, a natural polyphenol compound found in plants, is considered to have potent anticancer activity against various types of cancers (12–15,27). Moreover, RES has been reported to act as a chemical sensitizer and to enhance the anticancer effect of DDP in prostate cancer (29), lung cancer (30,31) and hepatoma (32). Herein, it was revealed that RES exerted antineoplastic effects against the GC cell line AGS by suppressing cell viability and promoting cell apoptosis in a dose-dependent manner. Furthermore, the present results demonstrated that RES and DDP synergistically inhibited cell viability and promoted apoptosis of AGS cells, revealing RES as a promising effective chemical sensitizer for DDP treatment in GC patients.
Adverse conditions in the tumor microenvironment, such as nutrient deprivation, oxidative stress, energy perturbations and hypoxia, dysregulate proteostasis of the ER, resulting in a cellular state termed ‘ER stress’ (33). ER stress triggers the UPR, which subsequently induces prosurvival programs to maintain homeostasis or activates apoptosis when damage is irreversible (34). The UPR is vital for cancer cell survival in adverse environments and induces drug resistance to conventional chemotherapy. Furthermore, blocking the adaptive pathway or promoting the apoptotic pathway of the UPR is a potential antineoplastic strategy since ER stress acts as a ‘double-edged sword’ during carcinogenesis (18). Previous studies have reported that RES promotes ER stress-mediated apoptosis in colon cancer (19), ovarian cancer (20), and malignant melanoma (21,35). However, the role of RES in regulating the UPR in GC cells is unknown. In the present study, it was revealed that RES/DDP combination treatment significantly upregulated the expression of the ER stress hallmark GRP78 and significantly increased cytoplasmic Ca2+ levels in AGS cells, indicating the occurrence of ER stress under cotreatment.
The PERK/eIF2α/ATF4/CHOP signaling pathway is an important modulator of ER stress-mediated apoptosis (16). As a UPR signal transducer on the ER membrane, upon activation, PERK phosphorylates eIF2α at Ser51 to decrease global protein translation but increase ATF4 mRNA expression, thereby upregulating CHOP expression (36). CHOP is an ER stress-associated apoptosis marker that further upregulates the expression of proapoptotic proteins such as death receptor 5 (DR5) and Bim and inhibits the expression of the antiapoptotic protein Bcl-2 (16). Caspase-12 is an ER membrane-resident caspase that is upregulated only upon ER stress, and its activation leads to cleavage of caspase-9 and caspase-3, resulting in apoptosis (37). Recently, it was reported that RES promotes ER stress-mediated apoptosis in numerous types of cancer cells, such as colon cancer (19), ovarian cancer (20), lung cancer (38), malignant melanoma (21) and nasopharyngeal carcinoma (39). The mechanisms included upregulation of CHOP and activation of PERK and caspase-12. Herein, it was revealed that RES administration upregulated CHOP expression in a dose-dependent manner. Moreover, RES combined with DDP treatment significantly activated the PERK/eIF2α/ATF4/CHOP signaling pathway and induced caspase-12 cleavage in AGS gastric adenocarcinoma cells. Therefore, it is suggested that RES/DDP combination treatment promotes ER stress-mediated apoptosis in the GC cell line AGS by activating the PERK/eIF2α/ATF4/CHOP signaling pathway and caspase-12.
Dysregulation of cell cycle progression is a hallmark of tumor development, and targeting the cell cycle is an effective antineoplastic strategy (40). Unlike previous studies that revealed that RES blocked GC cells at the G0/G1 phase (41), the present results revealed that RES/DDP combination treatment significantly induced G2/M cell cycle arrest. Moreover, Suttie et al (42) demonstrated that a low dose (0.156 µg/ml, LD10) of DDP arrested AGS cells in G0/G1 phase, whereas a high dose (5 µg/ml, LD50) of DDP induced G2/M phase arrest. In the present study, it was similarly revealed that exposure to low dose of DDP (0.5 µg/ml) for 72 h induced G0/G1 phase arrest in AGS cells (data not shown). However, DDP treatment (1 µg/ml) for 48 or 72 h led to G2/M cell cycle arrest. Based on the previous literature and our experimental results, we speculate that differences in cell cycle phase arrest in AGS cells are associated with differences in the dose of DDP. In addition, the proteins regulating cell cycle progression at the G2/M checkpoint were detected by western blotting. The CDK1-cyclin B1 complex plays a critical role in the G2/M transition (43). The downregulation or inactivation of either CDK1 (also known as Cdc2) or cyclin B1 blocks cell cycle progression to the G2 phase. Furthermore, the activity of CDK1 is regulated by Cdc25C, a phosphatase, which dephosphorylates CDK1 at Thr14 and Tyr15 residues, leading to the activation of the CDK1-cyclin B1 complex (44). p21Waf1/Cip1 and p27Kip1 are cyclin-dependent kinase inhibitors (CKIs) that reduce the activity of CDK1 (45), and both are thought to be tumor suppressive. In the present study, it was determined that RES/DDP combination treatment upregulated the protein levels of p-CDK1 (Tyr15), p21Waf1/Cip1 and p27Kip, downregulated Cdc25C expression, and reduced cyclin B1 protein expression (which was increased by DDP administration alone), leading to G2/M phase arrest.
There are certain limitations to the present study worth mentioning. The primary limitation is that the target protein and molecular mechanisms are currently not fully defined and further research is required. In this study, we only presented the possible molecular mechanisms underlying the antineoplastic effects for RES/DDP cotreatment by activating ER stress-mediated apoptosis and suppressing the activity of CDK1-cyclin B1 complex. Further exploration of the specific mechanisms would be addressed in a future study. In addition, we may also perform rescue experiments to validate the specificity of the signaling pathways. Since increase of Ca2+ during ER stress has been revealed to be mainly associated with inositol trisphosphate receptors (IP3R) (46), future experiments such as blocking or downregulating IP3R are required to clarify the relationship between RES/DDP combination treatment and Ca2+ levels.
In conclusion, it was revealed that RES and DDP synergistically inhibited cell growth of the GC cell line AGS by inducing ER stress-mediated apoptosis and G2/M phase arrest. Further experiments revealed that the PERK/eIF2α/ATF4/CHOP signaling pathway and caspase-12 were activated by RES/DDP cotreatment. Additionally, RES/DDP combination treatment suppressed the activity of the CDK1-cyclin B1 complex and upregulated p21Waf1/Cip1 and p27Kip1 expression to arrest AGS cells in G2/M phase. These findings identify RES as a promising adjuvant for GC chemotherapy.
Supplementary Material
Acknowledgements
The authors would like to thank the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases of the First Affiliated Hospital of Zhejiang University for excellent technical assistance.
Funding
The present study was supported by Zhejiang Traditional Chinese Medicine Science and Technology Project (grant no. 2017ZZ010), the Key Research and Development Program of Zhejiang Province (grant no. 2019C03031), and the Zhejiang Medical Science and Technology Program (grant no. 2018266817).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors contributions
MR and XZ contributed equally to this study. MR, XZ and MG designed and performed the experiments. WJ and JY performed part of the experiments. MY, YW and SL contributed to the data analysis. MR wrote the manuscript. XZ and MG reviewed and edited the manuscript. FJ conceived the study and was responsible for the revision of the manuscript and final decision to submit the article for publication. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Ca Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, et al. The Chinese society of clinical oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26:141–148. doi: 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 6.Quintanilha JCF, Saavedra KF, Visacri MB, Moriel P, Salazar LA. Role of epigenetic mechanisms in cisplatin-induced toxicity. Crit Rev Oncol Hematol. 2019;137:131–142. doi: 10.1016/j.critrevonc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis Oncol. 2017;1:35. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnefont-Rousselot D. Resveratrol and cardiovascular diseases. Nutrients. 2016;8:250. doi: 10.3390/nu8050250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed T, Javed S, Javed S, Tariq A, Šamec D, Tejada S, Nabavi SF, Braidy N, Nabavi SM. Resveratrol and Alzheimers disease: Mechanistic insights. Mol Neurobiol. 2017;54:2622–2635. doi: 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 10.Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: The evidence so far. Nutr Res Rev. 2018;31:85–97. doi: 10.1017/S095442241700021X. [DOI] [PubMed] [Google Scholar]
- 11.Wong RHX, Howe PRC. Resveratrol counteracts insulin resistance-potential role of the circulation. Nutrients. 2018;10:1160. doi: 10.3390/nu10091160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang Z, Chen K, Cheng L, Yan B, Qian W, Cao J, Li J, Wu E, Ma Q, Yang W. Resveratrol and cancer treatment: Updates. Ann N Y Acad Sci. 2017;1403:59–69. doi: 10.1111/nyas.13466. [DOI] [PubMed] [Google Scholar]
- 13.Zulueta A, Caretti A, Signorelli P, Ghidoni R. Resveratrol: A potential challenger against gastric cancer. World J Gastroenterol. 2015;21:10636–10643. doi: 10.3748/wjg.v21.i37.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol. 2005;11:280–284. doi: 10.3748/wjg.v11.i2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z, Xie Q, Chen Z, Ni H, Xia L, Zhao Q, Chen Z, Chen P. Resveratrol suppresses the invasion and migration of human gastric cancer cells via inhibition of MALAT1-mediated epithelial-to-mesenchymal transition. Exp Ther Med. 2019;17:1569–1578. doi: 10.3892/etm.2018.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 17.Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69:169–181. doi: 10.1016/j.molcel.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients. 2018;10:1021. doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JW, Woo KJ, Lee JT, Lim JH, Lee TJ, Kim SH, Choi YH, Kwon TK. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–1273. [PubMed] [Google Scholar]
- 20.Gwak H, Kim S, Dhanasekaran DN, Song YS. Resveratrol triggers ER stress-mediated apoptosis by disrupting N-linked glycosylation of proteins in ovarian cancer cells. Cancer Lett. 2016;371:347–353. doi: 10.1016/j.canlet.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 21.Heo JR, Kim SM, Hwang KA, Kang JH, Choi KC. Resveratrol induced reactive oxygen species and endoplasmic reticulum stressmediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int J Mol Med. 2018;42:1427–1435. doi: 10.3892/ijmm.2018.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 23.Gu ML, Wang YM, Zhou XX, Yao HP, Zheng S, Xiang Z, Ji F. An inhibitor of the acetyltransferases CBP/p300 exerts antineoplastic effects on gastrointestinal stromal tumor cells. Oncol Rep. 2016;36:2763–2770. doi: 10.3892/or.2016.5080. [DOI] [PubMed] [Google Scholar]
- 24.Wang YM, Gu ML, Meng FS, Jiao WR, Zhou XX, Yao HP, Ji F. Histone acetyltransferase p300/CBP inhibitor C646 blocks the survival and invasion pathways of gastric cancer cell lines. Int J Oncol. 2017;51:1860–1868. doi: 10.3892/ijo.2017.4176. [DOI] [PubMed] [Google Scholar]
- 25.Bahar E, Kim H, Yoon H. ER stress-mediated signaling: Action potential and ca(2+) as key players. Int J Mol Sci. 2016;17:1558. doi: 10.3390/ijms17091558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin SR, Chang CH, Hsu CF, Tsai MJ, Cheng H, Leong MK, Sung PJ, Chen JC, Weng CF. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br J Pharmacol. 2020;177:1409–1423. doi: 10.1111/bph.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Martínez D, Soto A, Gil-Araujo B, Gallego B, Chiloeches A, Lasa M. Resveratrol promotes apoptosis through the induction of dual specificity phosphatase 1 and sensitizes prostate cancer cells to cisplatin. Food Chem Toxicol. 2019;124:273–279. doi: 10.1016/j.fct.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Li W, Shi Y, Wang R, Pan L, Ma L, Jin F. Resveratrol promotes the sensitivity of small-cell lung cancer H446 cells to cisplatin by regulating intrinsic apoptosis. Int J Oncol. 2018;53:2123–2130. doi: 10.3892/ijo.2018.4533. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Li W, Wang R, Nan Y, Wang Q, Liu W, Jin F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int J Oncol. 2015;47:1460–1468. doi: 10.3892/ijo.2015.3124. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Peng Q, Li Y, Gao Y. Resveratrol enhances cisplatin-induced apoptosis in human hepatoma cells via glutamine metabolism inhibition. BMB Rep. 2018;51:474–479. doi: 10.5483/BMBRep.2018.51.9.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706. doi: 10.1016/j.cell.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corazzari M, Gagliardi M, Fimia GM, Piacentini M. Endoplasmic reticulum stress, unfolded protein response, and cancer cell fate. Front Oncol. 2017;7:78. doi: 10.3389/fonc.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limonta P, Moretti RM, Marzagalli M, Fontana F, Raimondi M, Montagnani Marelli M. Role of endoplasmic reticulum stress in the anticancer activity of natural compounds. Int J Mol Sci. 2019;20:961. doi: 10.3390/ijms20040961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urra H, Dufey E, Avril T, Chevet E, Hetz C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer. 2016;2:252–262. doi: 10.1016/j.trecan.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 38.Gu S, Chen C, Jiang X, Zhang Z. ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction underlie apoptosis induced by resveratrol and arsenic trioxide in A549 cells. Chem Biol Interact. 2016;245:100–109. doi: 10.1016/j.cbi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Chow SE, Kao CH, Liu YT, Cheng ML, Yang YW, Huang YK, Hsu CC, Wang JS. Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis. 2014;19:527–541. doi: 10.1007/s10495-013-0945-0. [DOI] [PubMed] [Google Scholar]
- 40.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin KO, Park NY, Seo CH, Hong SP, Oh KW, Hong JT, Han SK, Lee YM. Inhibition of sphingolipid metabolism enhances resveratrol chemotherapy in human gastric cancer cells. Biomol Ther (Seoul) 2012;20:470–476. doi: 10.4062/biomolther.2012.20.5.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suttie SA, Park KG, Smith TA. [18F]2-fluoro-2-deoxy-D-glucose incorporation by AGS gastric adenocarcinoma cells in vitro during response to epirubicin, cisplatin and 5-fluorouracil. Br J Cancer. 2007;97:902–909. doi: 10.1038/sj.bjc.6603971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satyanarayana A, Kaldis P. Mammalian cell-cycle regulation: Several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene. 2009;28:2925–2939. doi: 10.1038/onc.2009.170. [DOI] [PubMed] [Google Scholar]
- 44.Perdiguero E, Nebreda AR. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle. 2004;3:733–737. doi: 10.4161/cc.3.6.906. [DOI] [PubMed] [Google Scholar]
- 45.Hu X, Moscinski LC. Cdc2: A monopotent or pluripotent CDK? Cell Prolif. 2011;44:205–211. doi: 10.1111/j.1365-2184.2011.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.