Abstract
Background
The aim of this study was to investigate the optimal order of radiation therapy in patients affected by stage IIIA pathologic N2 (IIIA/N2) non-small-cell lung cancer (NSCLC) and to identify its potential risk factors.
Methods
17,654 (8786 men and 8868 women) diagnosed with NSCLC stage IIIA-N2 from 2004 to 2015 patients were identified in the Surveillance, Epidemiology, and End Results (SEER) database. Among the relevant clinical parameters, we evaluated overall survival (OS), lung cancer-specific survival (LCSS) and other variables such as age, sex and tumor size in patients who were treated with different combinations of surgery and radiotherapy strategies.
Results
We discovered that surgery benefit in younger IIIA/N2 NSCLC patients (age ≤ 75), and compared with surgery only, preoperative radiotherapy significantly improved the survival rate most (p < 0.001). When we performed the OS and LCSS analysis in the subgroup of patients’ age > 75 years old, who underwent postoperative radiotherapy (PORT) had the highest survival rate (p < 0.001). Multivariate analyses showed that the following parameters had a negative impact on survival: female sex, older age, no chemotherapy, large tumor size, high tumor grade, no surgery or radiotherapy.
Conclusions
In IIIA/N2 NSCLC patients, surgery, radiotherapy and chemotherapy were associated with improved OS and LCSS. Younger patients underwent surgical resection and chemotherapy, the main population we studied, benefited most from preoperative radiotherapy in all orders with radiation therapy (p < 0.001). In patients more than 75 years old, there was no clear benefit from only surgery, and PORT was recommended in case of having surgery.
Keywords: Non-small-cell lung carcinoma, Survival, Radiotherapy, Surgery, SEER
Background
Lung cancer is the leading cause of cancer-related mortality worldwide [1]. Lung cancer includes small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), the major type of NSCLC are adenocarcinoma (AD) and squamous cell carcinoma (SQCC). In patients diagnosed with lung cancer, 15% are stage IIIA NSCLC [2–4], while stage IIIA pathologic N2 (IIIA/N2) account for 50% of the locally advanced NSCLCs cases [5–7]. NSCLC patients in IIIA stage having a tumor size T1–T2 (T2: tumor > 3 cm and ≤ 5 cm) and M0 (without distant metastasis), along with ipsilateral mediastinal and/or subcarinal lymph node (N2), are diagnosed as IIIA/N2 NSCLC according to the 8th edition TNM Stage Classification [8]. N2 are classified into three different groups: occult N2, resectable N2, and non-resectable N2 [9]. Therefore, the optimal treatment for IIIA/N2 NSCLC is still controversial because stage IIIA/N2 NSCLC patients form a very broad and diverse population [10, 11].
Currently, surgery is still the standard treatment of early-stage NSCLC, but the 5 year survival rate is only 50 to 60% [6], with a risk of locoregional recurrence of 20–40% in node-positive patients [12]. Thus, radiotherapy and/or chemotherapy combined with surgery represent the current therapeutic options for these patients. Adjuvant chemotherapy was considered to enhance survival in IIIA NSCLC patients with surgery [13, 14], but 20–40% of patients still had a local tumor failure [12]. Thus, radiotherapy, including preoperative radiotherapy and postoperative radiotherapy (PORT) is important and necessary.
Since tumor size, lymph node involvement, and comorbidities can widely vary among patients, the idea of having a universal treatment plan for stage IIIA/N2 NSCLC patients seems not feasible. While some studies confirmed that preoperative radiotherapy significantly improve survival [15, 16], other studies showed PORT demonstrated better survival instead [17, 18]. Thus, by retrospectively studying the outcomes of IIIA/N2 NSCLC patients that underwent surgery with either pre- or post-operative radiotherapy or both, we sought to answer the question of which strategy is ideal. Since prospective clinical study are lacking, we perform a retrospective study by using data from the Surveillance, Epidemiology, and End Results (SEER) database to determine which clinical parameters have an impact in the therapy outcome and to provide clinicians and patients more information to make an informed decision.
Methods
Data source
We used the US National Cancer Institute’s SEER database, which contain data from 18 registered cancer institute covering nearly 26% of the total US population [19]. The database coverage which is considered an accurate statistical representation of the U.S. population affected with cancer [20]. SEER*-Stat software, version 8.3.2, was used to extract data from the database.
Cohort
The cohort include patients who were pathologically diagnosed with lung adenocarcinoma (AD) (histological codes 8244, 8245, 8250–8255, 8260, 8290, 8310, 8323, 8333, 8480, 8481, 8490, 8507, 8550, 8570, 8571, 8574, and 8576), squamous cell carcinoma (SQCC) (histologic codes 8052, 8070–8075, 8083, 8084, 8123), and large cell carcinoma (LCC) (histological codes 8012–8014, 8046, 8050, 8003, 8004, 8022, 8031–8035, 8082, 8200, 8240, 8249, 8430, 8560, 8562, 8980) during a 10-year period, from 2004 to 2015. Patients graded as stage T1–2 and N2 were included in this study, while those having a previous malignant disease or distant metastasis were excluded, along with patients who died within 30 days after surgery. Another exclusion criteria was the lack of complete information for the following parameters: age, complete staging, tumor size and location, regional LN examination results, histology, differentiation grade, cause of death, and survival period. Figure 1 showed the detailed case selection process.
Fig. 1.
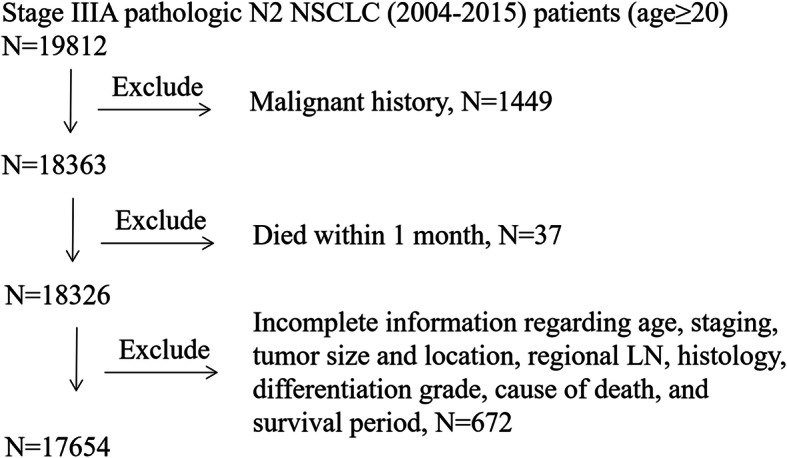
Patient selection for this study
Covariates
Demographic parameters included age, sex, race, insurance coverage, marital status, years of diagnosis, region, education, and median household income. Tumor characteristics included size, histology, T stage (based on the Eighth Edition Lung Cancer Stage Classification), primary site, and pathologic differentiation grade and laterality.
Statistical analysis
Pearson’s chi-square test was used to assess the baseline parameters and to evaluate the association between the groups. The Kaplan-Meier method was used to generate survival curves, the log-rank test was used to examine the differences in survival among subgroups and multivariate Cox Proportional Hazards Analysis was used to examine the effects of multiple potential prognostic factors on survival. Overall survival (OS) and lung cancer-specific survival (LCSS) were the endpoint measurements. OS was calculated from diagnosis to death from any cause, while LCSS was calculated from the time of diagnosis to death from lung cancer. OS and LCSS were estimated using follow-up data through 2017 and compared in different groups by using the Kaplan-Meier method. All tests were two-sided and p < 0.05 was considered to be significant. All analyses were performed using the SPSS software, version 22.0 (SPSS Inc. Chicago, IL).
Results
Baseline cohort characteristics
Based on the inclusion criteria, this study cohort was formed by 17,654 IIIA/N2 NSCLC patients, of which 8786 males and 8868 females. Among the patients, 5512 (31.22%) were treated neither with surgery or radiotherapy, 7184 (40.69%) received surgery only, 652 (3.69%) were given preoperative radiotherapy, 4206 (23.82%) were given PORT, and 100 (0.57%) were treated with radiotherapy both before and after surgery. The demographic and clinical parameter of patients are listed in Table 1. The tumor size and age were discretized and segmentation points were generated according to TNM stages (T1: tumor ≤3 cm T2: tumor > 3 cm and ≤ 5 cm) and by Youden index maximization, respectively.
Table 1.
Demographic and Clinical Parameters of patients with IIIA/N2 NSCLC
| Characteristics | No surgery or Radiotherapy (n = 5512) |
Surgery only (n = 7184) |
Preoperative radiotherapy (n = 652) |
Postoperative radiotherapy (PORT, n = 4206) |
Radiotherapy both before and after surgery(n = 100) | P value for X2 |
|---|---|---|---|---|---|---|
| Gender | <0.001 | |||||
| Male | 2572(46.66%) | 3680(51.22%) | 329(50.46%) | 2152(51.17%) | 53(53.00%) | |
| Female | 2940(53.34%) | 3504(48.78%) | 323(49.54%) | 2054(48.83%) | 47(47.00%) | |
| Age | <0.001 | |||||
| < =75 | 3893(70.63%) | 4940(68.76%) | 608(93.25%) | 3567(84.81%) | 92(92.00%) | |
| > 75 | 1619(29.37%) | 2244(31.24%) | 44(6.75%) | 639(15.19%) | 8(8.00%) | |
| Histologic type | <0.001 | |||||
| Adenocarcinoma | 2081(37.75%) | 3831(53.33%) | 342(52.45%) | 2486(59.11%) | 65(65.00%) | |
| Squamous | 2118(38.43%) | 1922(26.75%) | 173(26.53%) | 1003(23.85%) | 19(19.00%) | |
| Adenosquamous | 67(1.22%) | 158(2.20%) | 17(2.61%) | 88(2.09%) | 1(1.00%) | |
| Large cell | 144(2.61%) | 201(2.80%) | 23(3.53%) | 129(3.07%) | 1(1.00%) | |
| OTHER | 1102(19.99%) | 1072(14.92%) | 97(14.88%) | 500(11.89%) | 14(14.00%) | |
| Tumor Size (cm) | <0.001 | |||||
| ≤ 3 | 2318(42.05%) | 3765(52.41%) | 295(45.25%) | 2483(59.03%) | 47(47.00%) | |
| 3–5 | 3194(57.95%) | 3419(47.59%) | 357(54.85%) | 1723(40.97%) | 53(53.00%) | |
| Race | <0.001 | |||||
| White | 4444(80.62%) | 5810(80.87%) | 544(83.44%) | 3436(81.69%) | 78(78.00%) | |
| Black | 763(13.84%) | 802(11.16%) | 68(10.43%) | 482(11.46%) | 9(9.00%) | |
| Others | 297(5.39%) | 553(7.70%) | 40(6.13%) | 281(6.68%) | 13(13.00%) | |
| Unknown | 8(0.15%) | 19(0.27%) | 0(0.00%) | 7(0.17%) | 0(0.00%) | |
| Primary Site | <0.001 | |||||
| Upper lobe | 3454(62.66%) | 4302(59.88%) | 447(68.56%) | 2628(62.48%) | 65(65.00%) | |
| Middle lobe | 241(4.37%) | 326(4.54%) | 34(5.21%) | 226(5.37%) | 5(5.00%) | |
| Lower lobe | 1337(24.26%) | 2141(29.80%) | 138(21.17%) | 1098(26.11%) | 27(27.00%) | |
| NOS | 155(2.81%) | 195(2.71%) | 9(1.38%) | 123(2.92%) | 0(0.00%) | |
| Overlapping lesion | 33(0.60%) | 64(0.89%) | 6(0.92%) | 28(0.67%) | 2(2.00%) | |
| Main bronchus | 292(5.30%) | 156(2.17%) | 18(2.76%) | 103(2.45%) | 1(1.00%) | |
| Grade | <0.001 | |||||
| Grade I | 154(2.79%) | 327(4.55%) | 23(3.53%) | 138(3.28%) | 3(3.00%) | |
| Grade II | 900(16.33%) | 1935(26.93%) | 138(21.17%) | 1021(24.27%) | 26(26.00%) | |
| Grade III | 1722(31.24%) | 2427(33.78%) | 278(42.64%) | 1361(32.36%) | 39(39.00%) | |
| Grade IV | 83(1.51%) | 122(1.70%) | 5(0.77%) | 62(1.47%) | 3(3.00%) | |
| unknown | 2653(48.13%) | 2373(33.03%) | 208(31.90%) | 1624(38.61%) | 29(29.00%) | |
| Laterality | 0.001 | |||||
| Right-origin of primary | 3421(62.06%) | 4316(60.08%) | 405(62.12%) | 2631(62.55%) | 67(67.00%) | |
| Left-origin of primary | 2063(37.43%) | 2840(39.53%) | 246(37.73%) | 1531(36.40%) | 33(33.00%) | |
| Paired sit | 23(0.42%) | 20(0.28%) | 1(0.15%) | 38(0.90%) | 0(0.00%) | |
| Only one side - side unspecified | 4(0.07%) | 6(0.08%) | 0(0.00%) | 4(0.10%) | 0(0.00%) | |
| Not a paired site | 1(0.02%) | 2(0.03%) | 0(0.00%) | 2(0.05%) | 0(0.00%) | |
| Insurance status | <0.001 | |||||
| Medicaid | 594(10.78%) | 768(10.70%) | 45(6.90%) | 399(9.49%) | 7(7.00%) | |
| Uninsured | 101(1.83%) | 118(1.65%) | 10(1.53%) | 59(1.40%) | 2(2.00%) | |
| Unknown | 1316(23.88%) | 1858(25.86%) | 180(27.61%) | 847(20.14%) | 29(29.00%) | |
| Insured | 3501(63.52) | 4440(61.80%) | 417(63.96%) | 2901(68.97%) | 62(62.00%) | |
| Marital status | <0.001 | |||||
| Married | 2812(51.02%) | 3644(50.72%) | 393(60.28%) | 2440(58.01%) | 58(58.00%) | |
| Single | 671(12.17%) | 887(12.35%) | 76(11.66%) | 467(11.10%) | 12(12.00%) | |
| Divorced | 681(12.35%) | 903(12.57%) | 93(14.26%) | 540(12.84%) | 12(12.00%) | |
| widowed | 1079(19.6%) | 1401(19.50%) | 63(9.66%) | 574(13.65%) | 11(11.00%) | |
| Unknown | 181(3.28%) | 262(3.65%) | 20(3.07%) | 131(3.11%) | 1(1.00%) | |
| Unmarried or domestic partner | 6(0.11%) | 12(0.17%) | 1(0.15%) | 12(0.29%) | 0(0.00%) | |
| Separated | 82(1.49%) | 75(1.04%) | 6(0.92%) | 42(1.00%) | 6(6.00%) | |
| Year of diagnosis | <0.001 | |||||
| 2004–2007 | 1756(31.86%) | 2399(33.39%) | 241(36.96%) | 1105(26.27%) | 37(37.00%) | |
| 2008–2011 | 1982(35.96%) | 2437(33.92%) | 216(33.13%) | 1326(31.53%) | 35(35.00%) | |
| 2012–2017 | 1774(32.18%) | 2348(32.68%) | 195(29.91%) | 1775(42.20%) | 28(28.00%) | |
| Region | <0.001 | |||||
| EAST | 2733(49.58%) | 3243(45.14%) | 283(43.40%) | 2148(51.07%) | 38(38.00%) | |
| NORTHWEST | 1965(35.65%) | 3082(42.90%) | 265(40.64%) | 1417(33.69%) | 50(50.00%) | |
| SOUTHWEST | 661(11.99%) | 647(9.01%) | 86(13.19%) | 561(13.34%) | 10(10.00%) | |
| Pacific Coast | 153(2.78%) | 212(2.95%) | 18(2.76%) | 80(1.90%) | 2(2.00%) | |
| High school education | <0.001 | |||||
| ≥ 21 | 977(17.72%) | 1524(21.21%) | 102(15.64%) | 590(14.03%) | 19(19.00%) | |
| 13–20 | 1823(33.07%) | 2293(31.92%) | 159(24.39%) | 1199(28.51%) | 32(32.00%) | |
| 7–12.99 | 2363(42.87%) | 2968(41.31%) | 344(52.76%) | 2101(49.95%) | 41(41.00%) | |
| < 7 | 349(6.33%) | 399(5.55%) | 47(7.21%) | 316(7.51%) | 8(8.00%) | |
| Median household income, in tens | <0.001 | |||||
| < 38,000 | 422(7.66%) | 570(7.927%) | 28(4.29%) | 290(6.89%) | 5(5.00%) | |
| 38,000–47,999 | 1080(19.59%) | 1097(15.27%) | 88(13.50%) | 731(17.38%) | 14(14.00%) | |
| 48,000–62,999 | 2064(37.45%) | 2842(39.56%) | 276(42.33%) | 1588(37.76%) | 40(40.00%) | |
| > 63,000 | 1946(35.30%) | 2675(37.24%) | 260(39.88%) | 1597(37.97%) | 41(41.00%) | |
| Chemotherapy | <0.001 | |||||
| Yes | 4221(76.58%) | 3184(44.32%) | 631(96.78%) | 3791(90.13%) | 96(96.00%) | |
| no | 1291(23.42%) | 4000(55.68%) | 21(3.22%) | 415(9.87%) | 4(4.00%) | |
Abbreviations: NSCLC Non-small cell lung cancer, IIIA/N2 Stage IIIA pathologic N2
There were statistically significant differences in all the baseline parameters between groups (p < 0.001). Patients that underwent surgery only (40.69%) constitute the vast majority of patients included in the study, while those treated with radiotherapy both before and after surgery (0.57%) were the least representative, especially in elderly patients. PORT with surgery constituted an increasing proportion of therapeutic procedures during the period considered (26.27% from 2004 to 2007, 31.53% from 2008 to 2011, and 42.20% from 2012 to 2017), whereas preoperative radiotherapy with surgery decreased (36.96% from 2004 to 2007, 33.13% from 2008 to 2011, and 29.91% from 2012 to 2017) in the same period (Fig. 2), which means during this period, PORT became more and more popular than preoperative radiotherapy. The majority of patients over 75 years of age refused radiotherapy (84.83%) regardless of whether they underwent surgery, while the refusal rate was only at 67.43% in patients younger than 75 years. Additionally, patients who underwent radiotherapy combined with surgery (over 90%) were more likely to receive chemotherapy than those who only had surgery (44.32%). Moreover, patients who refused radiotherapy were older (30.43% vs 2.32% over 75 years old) and less treated with chemotherapy than others (58.33% vs 91.13%). Insured, married, high median household income people were more likely to have surgery and surgery combined with radiotherapy than uninsured, unmarried, and lower median household income people.
Fig. 2.
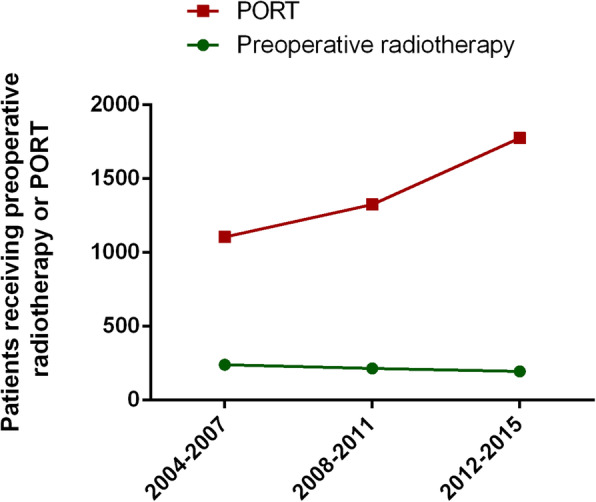
Number of IIIA/N2 NSCLC patients underwent preoperative radiotherapy and PORT from 2004 to 2015. There were 241, 216, 195 IIIA/N2 NSCLC patients received preoperative radiotherapy with surgery in 2004–2007, 2008–2011 and 2012–2017, respectively. Whereas, the number of IIIA/N2 NSCLC patients who underwent PORT with surgery were 1105, 1326, 1775 in the same time periods as above. Abbreviations: NSCLC, non-small cell lung cancer; IIIA/N2, stage IIIA pathologic N2; PORT, postoperative radiotherapy
Univariate and multivariate analysis
In the univariate COX regression analysis of OS and LCSS, compared with patients underwent surgery only, the hazard ratio (HR), 95% confidence interval (CI) [HR(95% CI)] of patients that underwent preoperative radiotherapy was 0.477 (0.429–0.531) and 0.507 (0.452–0.568) for OS and LCSS, respectively. That of PORT patients was 0.632 (0.602–0.662) and 0.645 (0.612–0.679); of patients that underwent radiotherapy both before and after surgery was 0.593 (0.466–0.755) and 0.56 (0.426–0.736), and of patients that underwent neither radiotherapy nor surgery was 1.052 (1.010–1.095) and 1.057(1.011–1.104). All the p-values were less than 0.05. This analysis also showed that the following parameters are associated with a significantly shorter OS and LCSS: female sex, old age, not AD, no chemotherapy, larger tumor, higher grade, no surgery or radiotherapy, white ethnicity, earlier year of diagnosis, non-upper lobe primary lesion, higher grade, unmarried, low income.
According to the multivariate analysis, age, sex, tumor size, histology, laterality, primary site, pathologic differentiation grade, chemotherapy and radiotherapy with surgery variables were statistically significant (p < 0.001). The multivariate analysis showed that all the four combination of surgery and radiotherapy promoted a better survival than having neither surgery nor radiotherapy. Patients with only surgery were taken as the reference for the subsequent analysis. The HR (95% CI, p) of patients that underwent preoperative radiotherapy was 0.589 (0.529–0.657), p < 0.001 and 0.606 (0.539–0.681), p < 0.001 for OS and LCSS, respectively. For patients that underwent PORT was 0.775 (0.737–0.816), p < 0.001 and 0.772 (0.731–0.816), p < 0.001 and for patients that underwent radiotherapy both before and after surgery was 0.752 (0.590–0.957), p = 0.021 and 0.687 (0.522–0.904), p = 0.007. For patients who refused surgery or radiotherapy, p values were not significant. Results of the univariate and multivariate Cox regression of prognostic factors for OS and LCSS in IIIA/N2 NSCLC patients are shown in Table 2.
Table 2.
Multivariate COX hazards regression for OS and LCSS in IIIA/N2 NSCLC patients based on prognostic factors
| Variables | OS | LCSS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| HR(95% Cl) | P | HR(95% Cl) | P | HR(95% Cl) | P | HR(95% Cl) | P | |
| Gender | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 0.799(0.772,0.828) | <0.001 | 0.823(0.794,0.853) | <0.001 | 0.823(0.792,0.855) | <0.001 | 0.845(0.813,0.879) | <0.001 |
| Age | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| < 75 | Reference | Reference | Reference | Reference | ||||
| > =75 | 1.645(1.582,1.710) | <0.001 | 1.364(1.310,1.421) | <0.001 | 1.590(1.524,1.659) | <0.001 | 1.334(1.276,1.395) | <0.001 |
| Histology | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Adenocarcinoma | Reference | Reference | Reference | Reference | ||||
| Squamous | 1.429(1.372,1.489) | <0.001 | 1.228(1.177,1.281) | <0.001 | 1.388(1.328,1.451) | <0.001 | 1.193(1.139,1.250) | <0.001 |
| Adenosquamous | 1.159(1.018,1.319) | 0.026 | 1.114(0.977,1.269) | 0.106 | 1.138(0.988,1.312) | 0.073 | 1.087(0.943,1.254) | 0.25 |
| Large cell | 1.248(1.125,1.384) | <0.001 | 1.159(1.041,1.292) | 0.007 | 1.303(1.167,1.456) | <0.001 | 1.209(1.077,1.357) | 0.001 |
| OTHER | 1.395(1.328,1.464) | <0.001 | 1.187(1.128,1.250) | <0.001 | 1.411(1.339,1.488) | <0.001 | 1.196(1.132,1.265) | <0.001 |
| Chemotherapy | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.556(0.536,0.577) | <0.001 | 0.635(0.610,0.662) | <0.001 | 0.586(0.563,0.610) | <0.001 | 0.665(0.636,0.695) | <0.001 |
| Tumor Size | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| ≤ 3 | Reference | Reference | Reference | Reference | ||||
| 3--5 | 1.275(1.231,1.320) | <0.001 | 1.221(1.178,1.266) | <0.001 | 1.311(1.261,1.362) | <0.001 | 1.258(1.209,1.308) | <0.001 |
| Grade | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Grade I | Reference | Reference | Reference | Reference | ||||
| Grade II | 1.128(1.015,1.253) | 0.025 | 1.134(1.020,1.260) | 0.02 | 1.147(1.021,1.289) | 0.021 | 1.154(1.027,1.297) | 0.016 |
| Grade III | 1.34(1.210,1.485) | <0.001 | 1.334(1.203,1.480) | <0.001 | 1.399(1.249,1.567) | <0.001 | 1.386(1.235,1.554) | <0.001 |
| Grade IV | 1.593(1.351,1.880) | <0.001 | 1.512(1.275,1.794) | <0.001 | 1.604(1.336,1.926) | <0.001 | 1.495(1.237,1.806) | <0.001 |
| unknow | 1.569(1.417,1.737) | <0.001 | 1.496(1.348,1.660) | <0.001 | 1.627(1.453,1.821) | <0.001 | 1.544(1.377,1.732) | <0.001 |
| Radiation with surgery | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Only surgery | Reference | Reference | Reference | Reference | ||||
| Preoperative radiotherapy | 0.477(0.429,0.531) | <0.001 | 0.589(0.529,0.657) | <0.001 | 0.507(0.452,0.568) | <0.001 | 0.606(0.539,0.681) | <0.001 |
| postoperative radiotherapy | 0.632(0.602,0.662) | <0.001 | 0.775(0.737,0.816) | <0.001 | 0.645(0.612,0.679) | <0.001 | 0.772(0.731,0.816) | <0.001 |
| Raiotherapy both before and after sugery | 0.593(0.466,0.755) | <0.001 | 0.752(0.590,0.957) | 0.021 | 0.56(0.426,0.736) | <0.001 | 0.687(0.522,0.904) | 0.007 |
| No surgery or radiation | 1.052(1.010,1.095) | 0.015 | 1.035(0.991,1.082) | 0.121 | 1.057(1.011,1.104) | 0.014 | 1.023(0.975,1.073) | 0.356 |
| Primary Site | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Upper lobe | Reference | Reference | Reference | Reference | ||||
| Middle lobe | 1.026(0.943,1.116) | 0.557 | 1.009(0.926,1.099) | 0.833 | 1.053(0.962,1.153) | 0.26 | 1.026(0.935,1.124) | 0.592 |
| Lower lobe | 1.103(1.059,1.149) | <0.001 | 1.08(1.037,1.125) | <0.001 | 1.102(1.054,1.151) | <0.001 | 1.078(1.032,1.127) | 0.001 |
| NOS | 1.303(1.172,1.449) | <0.001 | 1.314(1.172,1.474) | <0.001 | 1.29(1.148,1.449) | <0.001 | 1.311(1.157,1.486) | <0.001 |
| Overlapping lesion | 0.985(0.808,1.199) | 0.877 | 1.024(0.840,1.248) | 0.814 | 0.922(0.738,1.153) | 0.478 | 0.945(0.756,1.182) | 0.62 |
| Main bronchus | 1.252(1.136,1.379) | <0.001 | 1.128(1.023,1.245) | 0.016 | 1.302(1.174,1.444) | <0.001 | 1.171(1.054,1.301) | 0.003 |
| Laterality | 0.001 | <0.001 | <0.001 | <0.001 | ||||
| Right-origin of primary | Reference | Reference | Reference | Reference | ||||
| Left-origin of primary | 0.932(0.899,0.966) | <0.001 | 0.926(0.893,0.961) | <0.001 | 0.907(0.872,0.944) | <0.001 | 0.904(0.868,0.941) | <0.001 |
| Paired sit | 0.836(0.633,1.104) | 0.206 | 0.717(0.532,0.966) | 0.029 | 0.809(0.595,1.100) | 0.177 | 0.694(0.500,0.964) | 0.029 |
| Only one side - side unspecified | 1.556(0.861,2.810) | 0.143 | 1.339(0.737,2.430) | 0.338 | 1.14(0.543,2.391) | 0.73 | 0.991(0.470,2.090) | 0.982 |
| Not a paired site | 1.266(0.408,3.927) | 0.683 | 1.371(0.438,4.288) | 0.587 | 1.532(0.494,4.752) | 0.46 | 1.647(0.526,5.158) | 0.392 |
Abbreviations: NSCLC Non-small cell lung cancer, IIIA/N2 Stage IIIA pathologic N2, OS Overall survival, LCSS Lung cancer-specific survival, HRs Hazard ratios
Survival outcomes
The median follow-up for the whole cohort was 39 months for OS and 48 months for LCSS. The median follow-up for the surgery only, preoperative radiotherapy with surgery, PORT with surgery, radiotherapy both before and after surgery, and no surgery or radiotherapy groups were 36, 66, 51, 55 and 31 months, respectively for OS and 45, 72, 59, 66 and 40 months for LCSS. Patients who received preoperative radiotherapy with surgery had the longest 5-year overall survival (42.84%) and lung cancer-specific survival (47.12%).
The Kaplan-Meier method was used to estimate the OS and LCSS, showing that preoperative radiotherapy was the optimal strategy among IIIA/N2 patients (p < 0.001). Moreover, patients that underwent surgery had better survival than who refused it (p < 0.001). The survival of patients that underwent surgery combined with radiotherapy was better than patients who underwent surgery only (p < 0.001). The Survival analysis was performed using the log-rank test, and showed that the pairwise difference between each groups were statistically significant (Fig. 3).
Fig. 3.
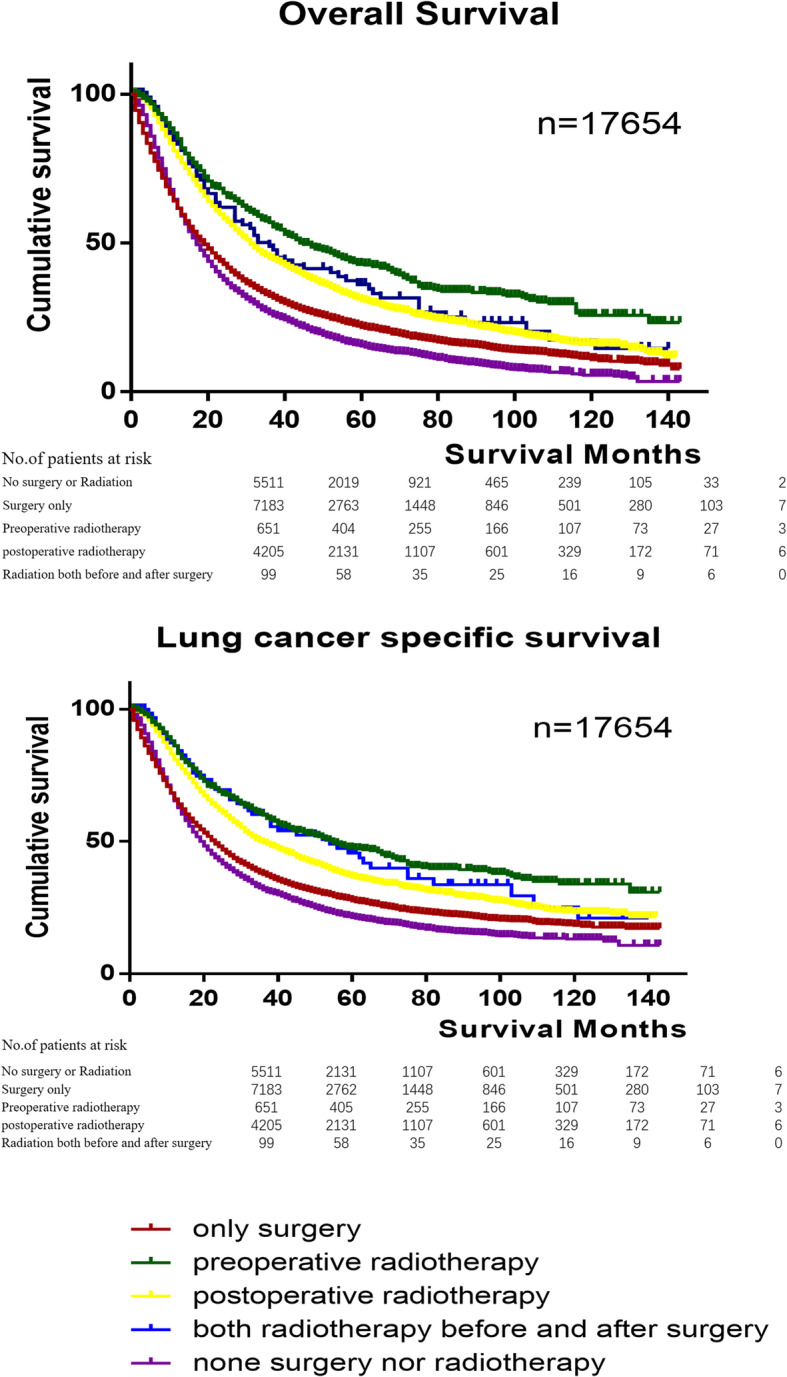
Kaplan–Meier analysis of different radiotherapy sequences on OS and LCSS of IIIA/N2 NSCLC patients. It showed that both in OS and LCSS of IIIA/N2 NSCLC patients, preoperative radiotherapy was the best strategy, and then were both preoperative and PORT, PORT, only surgery and neither surgery nor radiotherapy. Log Rank p < 0.001. The number of patients at risk in different time periods was under survival curves. Abbreviations: NSCLC, non–small cell lung cancer; IIIA/N2, stage IIIA pathologic N2; OS, Overall survival; LCSS, Lung cancer specific survival
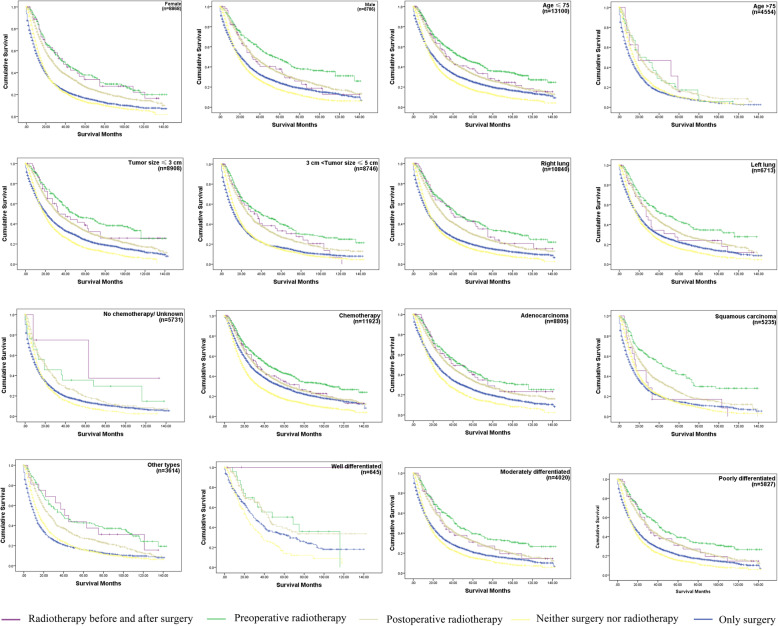
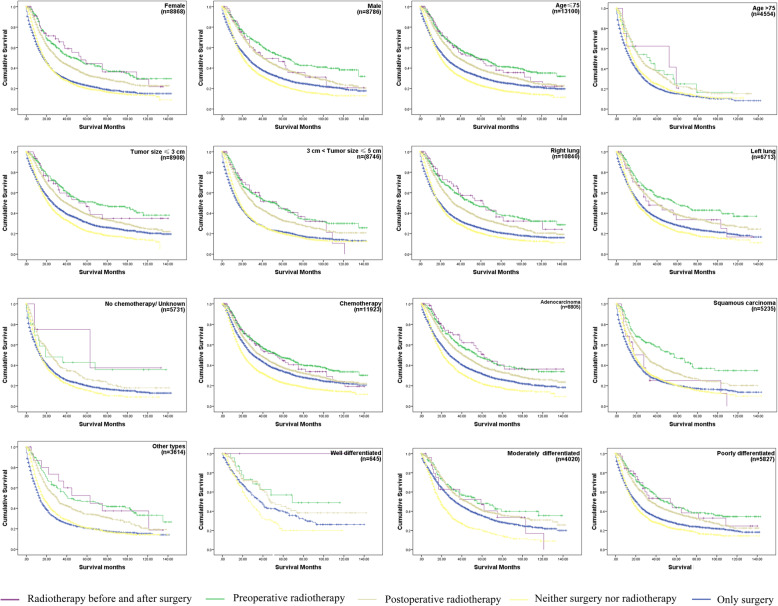
Similar results were observed in the subgroups (sex, age, tumor size, primary site, chemotherapy, histology, pathologic differentiation grade) analysis of the OS and LCSS Kaplan-Meier. Importantly, considering that most patients with IIIA/N2 NSCLC received chemotherapy in clinical, the chemotherapy subgroup was the most practical one. In chemotherapy subgroup, there were almost the same survival curves as overall survival curves and the survival rate of preoperative radiotherapy of both OS and LCSS were the highest in this subgroup. Interestingly, we also found that the optimal treatment for the subgroup of patients with > 75 years old was PORT, while in the subgroup of no chemotherapy the optimal treatment may be radiotherapy both before and after surgery (Fig. 4, Fig. 5). The OS and LCSS analysis showed that the survival rate of patients that underwent preoperative radiotherapy was not significantly different with patients who underwent PORT in the AD subgroup (p = 0.8274 and 0.7653 for OS and LCSS analysis, respectively). Moreover, there was no significant difference between the survival of patients who refused surgery and that of patients who received surgery only (p = 0.6848 and 0.5293 for OS and LCSS analysis, respectively) in the subgroup of patients with age > 75. Thus, the quality of life may be the main consideration for IIIA/N2 NSCLC patients with age > 75.
Fig. 4.
The OS curves in IIIA/N2 NSCLC subgroups with different surgery and radiotherapy combination. Log Rank p < 0.001 each subgroup. In subgroup age > 75, p- value of the OS of patients treated by preoperative radiotherapy compared to patients who underwent PORT was 0.6848. Each p-value < 0.05 in the pairwise comparison of other subgroups. Abbreviations: NSCLC, non-small cell lung cancer; IIIA/N2, stage IIIA pathologic N2; OS, Overall survival; PORT, postoperative radiotherapy. The number of patients at risk in different time periods was in Supplementary Table 1
Fig. 5.
The LCSS curves in IIIA/N2 NSCLC subgroups with different surgery and radiotherapy combination. Log Rank p < 0.001 in each subgroup. p-value of the lung cancer-specific survival of patients treated by preoperative radiotherapy compared to patients who underwent PORT was 0.5293. Each p value < 0.05 in the pairwise comparison of other subgroups. The number of patients at risk in different time periods was in Supplementary Table 1. Abbreviations: NSCLC, non-small cell lung cancer; IIIA/N2, stage IIIA pathologic N2; LCSS, Lung cancer-specific survival; PORT, postoperative radiotherapy
Discussion
In this study, we used the SEER Database to analyze the prognostic value of surgery and radiotherapy combinations and the relationship between therapeutic strategy with survival and HRs in IIIA/N2 NSCLC patients. Our data demonstrated that the use of surgery in IIIA/N2 NSCLC patients was effective in all the analyzed groups, except in patients older than 75 years old. In subgroup of age > 75, surgery did not improve survival, and PORT was more recommended if having surgery and radiation. For patients who underwent surgery combined with radiotherapy, the preoperative radiotherapy regimen led to the best result, while those who underwent neither surgery nor radiotherapy had the worst prognosis. Clinically, since adjuvant chemotherapy has become a standard treatment, the results of the chemotherapy subgroup deserve special attention, which were generally consistent with the overall OS and LCSS. The LCSS rate in the overall patients was similar to of the OS rate. In the Cox-regression analysis, the following parameters were associated with a higher risk of death: female sex, age > 75, SCC and other histologic types, no chemotherapy, poor differentiation, and larger tumor. With so many high-risk parameters, the results of survival analysis in subgroups were of great significance and we will further analyze the influence of age, gender, histologic types, chemotherapy, pathologic differentiation grade and tumor size on the survival of IIIA/N2 NSCLC patients in the future.
There was significant heterogeneity in the survival rate of IIIA/N2 NSCLC patients, for which the standard treatment had been debated for a long time [11, 21–23]. Although the optimal treatment approach in IIIA/N2 NSCLC patients remains undetermined, almost all studies confirmed the effectiveness of surgery. A recent review argued that in patients with IIIA/N2 NSCLC, radical resection with lymph node dissection is reasonable and early operation leads to a greater benefit [24]. Bryan DS et al. found that surgery improve survival in IIIA/N2 NSCLC, and in a survey, most thoracic surgeons recommended surgery as part of the therapy for IIIA/N2 NSCLC patients, because resection improves the rate of local control [25]. Consistently with this view, patients younger than 75 years old who underwent surgery showed an improved survival rate compared to those that do not in our study. However, there is still a lack of high-quality prospective evidence showing that how the different surgical procedures influence the survival rates.
Historically, surgery alone or combined with chemotherapy and radiotherapy has been the most common approach, and in recent decades there is a trend of performing radiotherapy before surgery. Previous studies reported that radiotherapy followed by surgery gave a survival benefit for IIIA/N2 NSCLC patients [26–29]. Several trials have been performed to determine the safety and efficacy of the combination of chemo- and radiotherapy [13, 27, 30–34]. Some of those reports demonstrated that chemotherapy combined with radiotherapy prolonged the survival of III/N2 NSCLC patients, as we found in this study [27, 30] while one clinical trial showed that radiotherapy combined with chemotherapy does not improve survival of IIIA/N2 NSCLC patients that underwent surgery [33]. Another study [35] suggested that surgery combined with chemoradiotherapy could improve the patients’ survival. Therefore, in previous studies there is no consensus for the optimal combination time of radiotherapy and chemotherapy [36]. In our study, we demonstrated that preoperative radiotherapy is the best strategy for approaches combining chemo and radiotherapy, consistently with a previous study based on SEER data [17], as well as another independent study [13].
The subgroup of patients with an age > 75 was different from the others, because surgery only did not appear to significantly improve survival in comparison to no surgery. PORT was the optimal treatment strategy in cases having surgery. These results can be explained by a worse treatment tolerance in this subgroup. In addition to that, these patients also underwent radiotherapy less frequently and had a higher death risk, consistently to a previous study [37]. Therefore, it is important to determine optimal treatment for the elderly population. However, older and frail patients are often excluded from clinical trials using strict eligibility criteria [38] and receive less standard treatment [39, 40]. Thus, future research should focus on the elderly population who could benefit from specific treatment regimens.
The SEER database includes a population size larger than other clinical trials [41], and the inclusion of the revision made by the TNM classification project makes it more reliable to predict the survival outcomes [42–44]. This study has several limitations: other than the intrinsic defects of retrospective study, important information, such as gene mutations, lymph nodes station, chemotherapy sequences, and extent of resection are not provided in the SEER database. Moreover, patients treated with radiotherapy are more likely to be treated with chemotherapy as well. As role of chemotherapy must be seriously taken into account [30], this study could have a bias favoring radiotherapy. Future prospective studies will need more detailed information on chemotherapy data.
Conclusions
According to this study, the different clinical outcomes between different strategies of surgery and radiotherapy combination may be useful to determine prognosis and to provide an important reference for clinicians and patients. Our data revealed that surgery, radiotherapy and chemotherapy were associated with improved OS and LCSS in patients with IIIA/N2 NSCLC. Younger patients underwent surgical resection and preoperative radiotherapy had the best chance of survival (p < 0.001). Based on our results, the rank of therapeutic strategies to improve OS and LCSS in IIIA/N2 NSCLC patients is the following: preoperative radiotherapy > radiotherapy both before and after surgery (considering the small number of people in this subgroup, we do not make a positive recommendation for this sequence, but this method should not be rejected or excluded, it worth more detailed relevant research in the future) > PORT > only surgery > neither surgery nor radiotherapy (p < 0.001). In conclusion, preoperative radiotherapy is worth attention. However, in patients with more than 75 years, there was no clear benefit from surgery. The quality of life should be the main consideration for them when making choice. Novel treatments like immunotherapy and targeting therapy as additional options will need to be evaluated in future studies.
Supplementary information
Additional file 1: Table S1. The number of patients at risk in different time periods in different subgroups.
Acknowledgments
We would like to thank all the staff of the National Cancer Institute for their efforts in the SEER program.
Abbreviations
- NSCLC
Non–small cell lung cancer
- IIIA/N2
Stage IIIA pathologic N2
- OS
Overall survival
- LCSS
Lung cancer-specific survival
- SEER
Surveillance, Epidemiology, and End Results registry
- HRs
Hazard ratios
- PORT
Postoperative radiotherapy
- AD
Adenocarcinoma
- SQCC
Squamous cell carcinoma
- LCC
Large cell carcinoma
Authors’ contributions
Guarantor of integrity of the entire study H.X.D, S.S.X, C.H.W. Study concepts and design H.X.D, S.S.X. Literature research H.X.D, S.S.X. Clinical studies S.S.X, L.L. Experimental studies / data analysis H.X.D. Statistical analysis H.X.D, L.L, S.S.X. Manuscript preparation H.X.D, S.S.X, C.H.W. Manuscript editing S.S.X, C.H.W. All authors had read and approved the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81802262). We also acknowledge the financial support of the Fundamental Research Funds for the Central Universities (No. 22120180584). Changhui Wang is the recipient of the foundations and was responsible for designing, guaranteeing of integrity of the entire study, editing manuscript in our study.
Availability of data and materials
All the data in the article was from SEER database at http://seer.cancer.gov/., which contain data from 18 registered cancer institute covering nearly 26% of the total US population.
Ethics approval and consent to participate
This research including formal consent was exempt by the ethics committee of the 10th People’s Hospital affiliated to Tongji University. All the data in the article was from SEER database, which was publicly available, and we received permission for using the data on non-commercial use.
Consent for publication
Not Applicable.
Competing interests
The authors declare no potential conflicts of interest related to this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuanshuan Xie, Email: xieshuanshuan@aliyun.com.
Changhui Wang, Email: 18717774084@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-07309-y.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Mountain CF. Staging classification of lung cancer. A critical evaluation. Clin Chest Med. 2002;23(1):103–121. doi: 10.1016/S0272-5231(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 3.Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K, R. Japanese Joint Committee for Lung Cancer Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229–1235. doi: 10.1097/JTO.0b013e318219aae2. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 5.Zeng X, Karnon J, Wang S, Wu B, Wan X, Peng L. The cost of treating advanced non-small cell lung cancer: estimates from the chinese experience. PLoS One. 2012;7(10):e48323. doi: 10.1371/journal.pone.0048323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L, C. International Association for the Study of Lung Cancer International Staging, and I. Participating The IASLC lung Cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Sun Y, Yu J, Ding C, Wang Z, Wang C, Wang D, Wang C, Wang Z, Wang M, Zhi X, Lu Y, Feng J, Liu Y, Liu X, Liu W, Wu G, Li X, Li K, Li E, Li W, Chen G, Chen Z, Yu P, Wu N, Wu M, Xiao W, Zhang L, Zhang Y, Zhang S, Yang S, Song X, Lin D, Luo R, Shan L, Zhou C, Zhou Z, Zhao Q, Hu C, Hu Y, Guo Q, Chang J, Huang C, Zeng X, Han B, Han X, Jia B, Han Y, Huang Y. China experts consensus on the diagnosis and treatment of advanced stage primary lung cancer (2016 Version) Zhongguo Fei Ai Za Zhi. 2016;19(1):1–15. doi: 10.3779/j.issn.1009-3419.2016.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung Cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Evison M, Clive A, Castle L, Powell H, Thomas R, Buttery R, Masani V, Harden S, West D, Woolhouse I. Resectable clinical N2 non-small cell lung Cancer; what is the optimal treatment strategy? An update by the British Thoracic Society lung Cancer specialist advisory group. J Thorac Oncol. 2017;12(9):1434–1441. doi: 10.1016/j.jtho.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24(19):2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 11.Bryan DS, Donington JS. The role of surgery in Management of Locally Advanced non-Small Cell Lung Cancer. Curr Treat Options in Oncol. 2019;20(4):27. doi: 10.1007/s11864-019-0624-7. [DOI] [PubMed] [Google Scholar]
- 12.Le Pechoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist. 2011;16(5):672–681. doi: 10.1634/theoncologist.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas M, Rube C, Hoffknecht P, Macha HN, Freitag L, Linder A, Willich N, Hamm M, Sybrecht GW, Ukena D, Deppermann KM, Droge C, Riesenbeck D, Heinecke A, Sauerland C, Junker K, Berdel WE, Semik M, G. German Lung Cancer Cooperative Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9(7):636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 14.Shah AA, Berry MF, Tzao C, Gandhi M, Worni M, Pietrobon R, D'Amico TA. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93(6):1807–1812. doi: 10.1016/j.athoracsur.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Sonett JR, Suntharalingam M, Edelman MJ, Patel AB, Gamliel Z, Doyle A, Hausner P, Krasna M. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann Thorac Surg. 2004;78(4):1200–1205. doi: 10.1016/j.athoracsur.2004.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Wang H, Song X, Yue J, Yu J. Preoperative radiation may improve the outcomes of resectable IIIA/N2 non-small-cell lung cancer patients: a propensity score matching-based analysis from surveillance, epidemiology, and end results database. Cancer Med. 2018;7(9):4354–4360. doi: 10.1002/cam4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang Z, Yang Y, Ding N, Huang C, Zhang T, Ni Y, Du J, Liu Q. Optimal managements of stage IIIA (N2) non-small cell lung cancer patients: a population-based survival analysis. J Thorac Dis. 2017;9(10):4046–4056. doi: 10.21037/jtd.2017.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Ma Z, Yang X, Wang Y, Xu Y, Xia W, Chen R, Qiu M, Jiang F, Yin R, Xu L, Xu K. Choice of postoperative radiation for stage IIIA pathologic N2 non-small cell lung cancer: impact of metastatic lymph node number. Radiat Oncol. 2017;12(1):207. doi: 10.1186/s13014-017-0946-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cronin KA, Ries LA, Edwards BK. The surveillance, epidemiology, and end results (SEER) program of the National Cancer Institute. Cancer. 2014;120(Suppl 23):3755–3757. doi: 10.1002/cncr.29049. [DOI] [PubMed] [Google Scholar]
- 20.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the surveillance, epidemiology, and end results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36(4):183–190. doi: 10.1016/j.currproblcancer.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Lorente D, Guzman R, Boada M, Guirao A, Carriel N, Molins L. N2 disease in non-small-cell lung cancer: straight to surgery? Future Oncol. 2018;14(6s):13–16. doi: 10.2217/fon-2017-0387. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, Nishiwaki Y. The prognosis of surgically resected N2 non-small cell lung cancer: the importance of clinical N status. J Thorac Cardiovasc Surg. 1999;118(1):145–153. doi: 10.1016/S0022-5223(99)70153-4. [DOI] [PubMed] [Google Scholar]
- 23.Regnard JF, Magdeleinat P, Azoulay D, Dartevelle P, Deneuville M, Rojas-Miranda A, Levasseur P. Results of resection for bronchogenic carcinoma with mediastinal lymph node metastases in selected patients. Eur J Cardiothorac Surg. 1991;5(11):583–586. doi: 10.1016/1010-7940(91)90224-8. [DOI] [PubMed] [Google Scholar]
- 24.Massard G, Renaud S, Reeb J, Santelmo N, Olland A, Falcoz PE. N2-IIIA non-small cell lung cancer: a plea for surgery! J Thorac Dis. 2016;8(Suppl 11):S849–S854. doi: 10.21037/jtd.2016.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veeramachaneni NK, Feins RH, Stephenson BJ, Edwards LJ, Fernandez FG. Management of stage IIIA non-small cell lung cancer by thoracic surgeons in North America. Ann Thorac Surg. 2012;94(3):922–926. doi: 10.1016/j.athoracsur.2012.04.087. [DOI] [PubMed] [Google Scholar]
- 26.Caglar HB, Baldini EH, Othus M, Rabin MS, Bueno R, Sugarbaker DJ, Mentzer SJ, Janne PA, Johnson BE, Allen AM. Outcomes of patients with stage III nonsmall cell lung cancer treated with chemotherapy and radiation with and without surgery. Cancer. 2009;115(18):4156–4166. doi: 10.1002/cncr.24492. [DOI] [PubMed] [Google Scholar]
- 27.Toyooka S, Kiura K, Shien K, Katsui K, Hotta K, Kanazawa S, Date H, Miyoshi S. Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg. 2012;15(6):954–960. doi: 10.1093/icvts/ivs412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshy M, Fedewa SA, Malik R, Ferguson MK, Vigneswaran WT, Feldman L, Howard A, Abdelhady K, Weichselbaum RR, Virgo KS. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol. 2013;8(7):915–922. doi: 10.1097/JTO.0b013e31828f68b4. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi M, Toyokawa G, Ohba T, Sasaki T, Kometani T, Hamatake M, Hirai F, Taguchi K, Yamanaka T, Seto T, Takenoyama M, Sugio K, Ichinose Y. Preoperative concurrent chemoradiotherapy of S-1/cisplatin for stage III non-small cell lung cancer. Ann Thorac Surg. 2013;96(5):1783–1789. doi: 10.1016/j.athoracsur.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2002;54(2):365–369. doi: 10.1016/S0360-3016(02)02943-7. [DOI] [PubMed] [Google Scholar]
- 31.Katakami N, Tada H, Mitsudomi T, Kudoh S, Senba H, Matsui K, Saka H, Kurata T, Nishimura Y, Fukuoka M. A phase 3 study of induction treatment with concurrent chemoradiotherapy versus chemotherapy before surgery in patients with pathologically confirmed N2 stage IIIA nonsmall cell lung cancer (WJTOG9903) Cancer. 2012;118(24):6126–6135. doi: 10.1002/cncr.26689. [DOI] [PubMed] [Google Scholar]
- 32.Pless M, Stupp R, Ris HB, Stahel RA, Weder W, Thierstein S, Gerard MA, Xyrafas A, Fruh M, Cathomas R, Zippelius A, Roth A, Bijelovic M, Ochsenbein A, Meier UR, Mamot C, Rauch D, Gautschi O, Betticher DC, Mirimanoff RO, Peters S, S.L.C.P. Group Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386(9998):1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 33.Yang CF, Gulack BC, Gu L, Speicher PJ, Wang X, Harpole DH, Onaitis MW, D'Amico TA, Berry MF, Hartwig MG. Adding radiation to induction chemotherapy does not improve survival of patients with operable clinical N2 non-small cell lung cancer. J Thorac Cardiovasc Surg. 2015;150(6):1484–1492. doi: 10.1016/j.jtcvs.2015.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Girard N, Mornex F, Douillard JY, Bossard N, Quoix E, Beckendorf V, Grunenwald D, Amour E, Milleron B. Is neoadjuvant chemoradiotherapy a feasible strategy for stage IIIA-N2 non-small cell lung cancer? Mature results of the randomized IFCT-0101 phase II trial. Lung Cancer. 2010;69(1):86–93. doi: 10.1016/j.lungcan.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Mirimanoff RO. Neoadjuvant chemoradiotherapy followed by surgery for stage IIIa and IIIb non-small-cell lung cancer (NSCLC): is it still justified? Chin Clin Oncol. 2015;4(4):49. doi: 10.3978/j.issn.2304-3865.2015.12.05. [DOI] [PubMed] [Google Scholar]
- 36.Rigotti NA. Strategies to help a smoker who is struggling to quit. JAMA. 2012;308(15):1573–1580. doi: 10.1001/jama.2012.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driessen EJM, Janssen-Heijnen MLG, Maas HA, Dingemans AC, van Loon JGM. Study protocol of the NVALT25-ELDAPT trial: selecting the optimal treatment for older patients with stage III non-small-cell lung Cancer. Clin Lung Cancer. 2018;19(6):e849–e852. doi: 10.1016/j.cllc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Schulkes KJ, Hamaker ME, van den Bos F, van Elden LJ. Relevance of a geriatric assessment for elderly patients with lung Cancer-a systematic review. Clin Lung Cancer. 2016;17(5):341–349. doi: 10.1016/j.cllc.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Mellemgaard A, Luchtenborg M, Iachina M, Jakobsen E, Green A, Krasnik M, Moller H. Role of comorbidity on survival after radiotherapy and chemotherapy for nonsurgically treated lung cancer. J Thorac Oncol. 2015;10(2):272–279. doi: 10.1097/JTO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 40.Semrau S, Zettl H, Hildebrandt G, Klautke G, Fietkau R. Older patients with inoperable non-small cell lung cancer: long-term survival after concurrent chemoradiotherapy. Strahlenther Onkol. 2014;190(12):1125–1132. doi: 10.1007/s00066-014-0710-5. [DOI] [PubMed] [Google Scholar]
- 41.Kelly RJ, Rajan A, Force J, Lopez-Chavez A, Keen C, Cao L, Yu Y, Choyke P, Turkbey B, Raffeld M, Xi L, Steinberg SM, Wright JJ, Kummar S, Gutierrez M, Giaccone G. Evaluation of KRAS mutations, angiogenic biomarkers, and DCE-MRI in patients with advanced non-small-cell lung cancer receiving sorafenib. Clin Cancer Res. 2011;17(5):1190–1199. doi: 10.1158/1078-0432.CCR-10-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A, 3rd, Goldstraw P, Rami-Porta R, S. International Association for Study of Lung Cancer, A.B.M. Prognostic Factors Committee, and I. Participating The IASLC lung Cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung Cancer. J Thorac Oncol. 2015;10(11):1515–1522. doi: 10.1097/JTO.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 43.Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, Goldstraw P, Committee IIS, Cancer R, Biostatistics, C. Observers to the, and I. Participating The IASLC lung Cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2(8):694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 44.Ball D, Mitchell A, Giroux D, Rami-Porta R, Committee IS, Participating I. Effect of tumor size on prognosis in patients treated with radical radiotherapy or chemoradiotherapy for non-small cell lung cancer. An analysis of the staging project database of the International Association for the Study of Lung Cancer. J Thorac Oncol. 2013;8(3):315–321. doi: 10.1097/JTO.0b013e31827dc74d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The number of patients at risk in different time periods in different subgroups.
Data Availability Statement
All the data in the article was from SEER database at http://seer.cancer.gov/., which contain data from 18 registered cancer institute covering nearly 26% of the total US population.